
2
1
G U I D A N C E
Guidance on labelling and packaging in
accordance with Regulation (EC) No 1272/2008
Version 4.2
March 2021

2
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Legal Notice
This document aims to assist users in complying with their obligations under the CLP
Regulation. However, users are reminded that the text of the CLP Regulation is the only
authentic legal reference and that the information in this document does not constitute
legal advice. Usage of the information remains under the sole responsibility of the user.
The European Chemicals Agency does not accept any liability with regard to the use that
may be made of the information contained in this document.
Guidance on labelling and packaging in accordance with Regulation (EC) No
1272/2008
Reference: ECHA-21-G-02-EN
Catalogue Number: ED-03-21-082-EN-N
ISBN: 978-92-9481-834-8
DOI: 10.2823/697587
Publication date: March 2021
Language: EN
© European Chemicals Agency, 2021
If you have questions or comments in relation to this document please, send them
(quote the reference and issue date) using the information request form. The
information request form can be accessed via the Contact ECHA page at:
https://echa.europa.eu/contact
European Chemicals Agency
Mailing address: P.O. Box 400, FI-00121 Helsinki, Finland
Visiting address: Annankatu 18, Helsinki, Finland

Guidance on Labelling and Packaging
Version 4.2 – March 2021
3
Document History
Version
Changes
Date
Version 1.0
(originally
unnumbered)
First edition
April 2011
Version 2.0
Full revision of the guidance addressing the content and structure.
Main changes in the guidance document include the following:
• Alignment with the 4
th
Adaptation to Technical Progress
(ATP) to the CLP Regulation (Commission Regulation (EU)
No 487/2013) bringing the CLP in line with the 4
th
revised
edition of the UN Globally Harmonised System (GHS);
• Addressing the provisions of the 5
th
ATP to the CLP
Regulation (Commission Regulation (EU) No 944/2013)
amending precautionary statement P210 to fully align it
with the changes arising from the 5
th
Revision of the UN
GHS;
• Addition of new section 3.5.1 on child-resistant fastening
(CRF) and tactile warnings of danger (TWD);
• Addition of new section 3.5.2 including information on
additional safety measures for liquid laundry detergents in
soluble capsules adopted by the Commission through
Regulation (EU) No 1297/2014;
• Addition of new sections 4.2.1 and 4.2.2 clarifying the
provisions of CLP Article 18(3) with regard to product
identifiers for substances and mixtures;
• Re-organisation of information in section 4.3 by inclusion of
new sections 4.3.1, 4.3.2, 4.3.3;
• Addition of new section 4.3.4 describing the issue of blank
pictograms;
• Re-organisation and clarification of information on
supplemental labelling in section 4.8 by inclusion of new
sections 4.8.1 and 4.8.2;
• Inclusion of clarification on the issue of “readability” and
“minimum letter size” in section 5.2;
• Re-organisation and update of the text in section 5.3 to
reflect the provisions of CLP Article 29 and sections 1.5.1
and 1.5.2 of Annex I to CLP;
• Inclusion of information on general and specific
requirements for fold-out labels in section 5.3.1.1;
• Section 6: Update of the labels and the text in examples in
line with the provisions of the 4
th
and 5
th
ATPs to CLP;
• Deletion of Example 6.6 (Single language label of a plant
protection product for supply & use in form of a fold-out
booklet);
• Inclusion of new Example 6 (fold-out label for a mixture
supplied to the general public);
September
2016

4
Guidance on Labelling and Packaging
Version 4.2 – March 2021
• Addition of section 6.1 separating the examples of labels on
packagings that are small or difficult to label;
• Addition of a new section 6.1 describing labelling of two-
component products;
• Clarification and extension of the text in section 7.2;
• Section 7.3: Update of the precautionary statements in
selection tables according to the provisions of the 4
th
and
5
th
ATPs to CLP;
• Section 7.4: Update of the practical examples in line with
the provisions of the 4
th
and 5
th
ATPs to CLP;
• Deletion of the outdated references to past deadlines and
to the DSD and DPD provisions thorough the whole
document;
• Alignment of the document with the latest ECHA corporate
image requirements.
Version 3.0
Full revision of the guidance. Main changes in the guidance
document include the following:
• Alignment with the 8
th
Adaptation to Technical Progress
(ATP) to the CLP Regulation (Commission Regulation (EU)
2016/918);
• Addition of a new section 5.4.2 clarifying the issue of
packaging used for consolidation of supply packaging
during transport;
• Update of the precautionary statements according to the
provisions of 8
th
ATP (section 6, section 7.3 and section
7.4).
July 2017
Version 4.0
Full revision of the guidance. Main changes in the guidance
document include the following:
• Alignment with Commission Regulation (EU) 2017/542,
which amends the CLP Regulation by adding an Annex on
harmonised information relating to emergency health
response;
• Addition of a new section 6.2 describing the labelling of
multi-component products with label examples;
• Deletion of the outdated paragraph “Limited derogation for
re-labelling and re-packaging” in Section 2.4 and deletion
of outdated section 3.4 on the “Differences between CLP
and DSD/DPD labelling rules”;
• Editorial changes and reformatting of the document;
• “Preamble” renamed “Preface” and moved before the table
of contents;
• Update of broken and outdated hyperlinks;
• Renumbering of sections, tables and figures.
March 2019
Version 4.1
Update via fast track to implement the amendment of the legal text
May 2020

Guidance on Labelling and Packaging
Version 4.2 – March 2021
5
Preface
This document describes specific provisions for the labelling and packaging of chemical
substances and mixtures under Titles III and IV of the Regulation (EC) No 1272/2008
1
(the CLP Regulation or “CLP”). The aim of this document is to assist manufacturers,
importers, downstream users and distributors of substances and mixtures in the effective
application of the CLP Regulation.
This guidance includes relevant amendments from the 2
nd
, 4
th
, 5
th
and 8
th
Adaptations to
Technical Progress (ATPs) to the CLP Regulation, as well as the changes brought about
by the ATP to the CLP Regulation related to labelling and packaging of liquid laundry
detergents in a soluble packaging for single use (Regulation (EU) No 1297/2014).
This document also includes relevant changes introduced by Commission Regulation (EU)
2017/542, which amends the CLP Regulation by adding Annex VIII on harmonised
information relating to emergency health response.
All current ECHA guidance documents are available on the ECHA website at:
https://echa.europa.eu/support/guidance.
1
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December
2008 on classification, labelling and packaging of substances and mixtures, amending and
repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006;
OJ L 353 31.12.2008, p. 1 (http://eur-lex.europa.eu/legal-
content/en/TXT/?uri=CELEX%3A02008R1272-20150601)
due to Commission Delegated Regulation 2020/11 of 29 October
2019. In particular:
• Clarification of labelling requirements with regards to the
UFI code in standard situations (in section 4.8.1.1);
• Clarification of labelling requirements with regards to the
UFI code in particular cases of fold-out labels, tie-on tags
or outer packaging (section 5.3.1);
• Minor changes and clarification in the labelling examples
concerning mixtures (section 6).
Version 4.2
Update to implement the amendment of the legal text due to
Commission Delegated Regulation 2020/1677 and Commission
Delegated Regulation 2020/1676 of 31 August 2020 (the
“workability amendments”) and limited to:
• Clarification of exemption from labelling requirements for
bespoke paints (new section 5.3.3);
• New example of application of the labelling requirements
for a bespoke paint (new section 6.3);
Minor changes and clarification in the rest of the document.
March 2021

6
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Table of Contents
PREFACE ............................................................................................................................ 5
1. INTRODUCTION ....................................................................................................... 10
1.1 Who should read this document?................................................................................ 10
1.2 What is in this document? ............................................................................................. 10
2. GENERAL OVERVIEW ............................................................................................. 11
2.1 Legal background .............................................................................................................. 11
2.2 Scope of labelling and packaging under the CLP Regulation .......................... 12
2.3 Derogations from labelling requirements for special cases ............................. 13
2.4 Timelines for classification, labelling, packaging and updating of CLP
hazard labels ............................................................................................................................... 13
3. REQUIREMENTS OF LABELLING AND PACKAGING IN ACCORDANCE
WITH THE CLP REGULATION ................................................................................... 14
3.1 General labelling rules ..................................................................................................... 14
3.2 Elements of the CLP hazard label ............................................................................... 14
3.3 Location of information on the CLP hazard label.................................................. 15
3.4 CLP rules on packaging of substances and mixtures .......................................... 17
3.4.1 Child-resistant fastening and tactile warnings of danger ......................................... 18
3.4.2 Liquid consumer laundry detergents in soluble packaging for single use.......... 21
4. RULES FOR THE APPLICATION OF THE CLP LABEL ELEMENTS ............... 23
4.1 Contact details of the supplier ..................................................................................... 23
4.2 Product identifiers ............................................................................................................. 23
4.2.1 Substances .................................................................................................................................. 23
4.2.2 Mixtures ........................................................................................................................................ 25
4.3 Hazard pictograms ............................................................................................................ 27
4.3.1 General information ................................................................................................................. 27
4.3.2 Shape, colour and dimensions ............................................................................................. 27
4.3.3 Precedence rules ....................................................................................................................... 28
4.3.4 Blank pictograms ...................................................................................................................... 29
4.4 Signal words ........................................................................................................................ 30
4.5 Hazard statements ............................................................................................................ 30
4.6 Precautionary statements .............................................................................................. 32
4.7 Codes for hazard and precautionary statements ................................................. 33
4.8 Supplemental labelling information ........................................................................... 33
4.8.1 Obligatory supplemental labelling information ............................................................ 34
4.8.1.1 Unique formula identifier (UFI) ............................................................................................................. 40
4.8.2 Non-obligatory supplemental labelling information ................................................... 43
5. GUIDANCE ON PARTICULAR ASPECTS OF CLP HAZARD LABELLING .... 43
5.1 Further aspects to consider for the CLP hazard label ......................................... 43
5.2 Size of the label and of the label elements ............................................................. 44
5.3 Exemptions from the labelling and packaging requirements .......................... 46

Guidance on Labelling and Packaging
Version 4.2 – March 2021
7
5.3.1 Use of fold-out labels, tie-on tags and outer packaging ........................................... 46
5.3.1.1 Fold-out labels and tie-on tags ............................................................................................................. 47
5.3.1.2 Outer packaging ...................................................................................................................................... 50
5.3.2 Omission of certain label elements.................................................................................... 50
5.3.2.1 Labelling of packages when the contents do not exceed 125 ml .................................................... 50
5.3.2.2 Labelling of soluble packaging for single use which does not exceed a volume of 25 ml........... 52
5.3.2.3 Labelling of inner packaging when the contents do not exceed 10 ml .......................................... 52
5.3.2.4 Unpackaged hazardous substances or mixtures supplied to the general public .......................... 52
5.3.2.5 Environmental labelling .......................................................................................................................... 53
5.3.3 Labelling exemptions for bespoke paints ........................................................................ 53
5.4 Interaction between the CLP and the transport labelling rules ..................... 54
5.4.1 Specific rules for labelling of outer packaging, inner packaging and single
packaging ................................................................................................................................................ 54
5.4.2 Packaging used for consolidation of supply packaging during transport ........... 55
6. EXAMPLE LABELS .................................................................................................... 58
Example 1: Single language label for a substance (not for the general public)
.......................................................................................................................................................... 58
Example 2: Multi-language label for a substance containing non-obligatory
supplemental information (not for the general public) ............................................ 60
Example 3: Single language label for a mixture containing both obligatory
and non-obligatory supplemental information (supplied to the general public)
.......................................................................................................................................................... 62
Example 4: Single language label for a substance containing supplemental
hazard statements (not for the general public) ........................................................... 64
Example 5: Multi-language label for a mixture containing both obligatory and
non-obligatory supplemental information (supplied to the general public) .... 65
Example 6: Fold-out label for a mixture (supplied to the general public) ........ 67
6.1 Packaging that is small or difficult to label ............................................................ 70
Example 7: Substance in a 8 ml bottle (not for the general public) ................................ 70
Example 8: Hazardous solid substance in a 100 ml bottle (not intended for the
general public) ...................................................................................................................................... 72
Example 9: Supply and transport label for a single package (not intended for the
general public) ...................................................................................................................................... 74
Example 10: Labelling for a mixture that is transported on land in outer and inner
packaging (not intended for the general public) ..................................................................... 76
Example 11: Labelling for a mixture that is transported on land in single packaging
(not intended for the general public) ........................................................................................... 77
6.2 Specific case: labelling of two-component products .......................................... 79
Example 12: Labelling of a two-component adhesive sold as a kit .................................. 79
Example 13: Labelling of a co-axial cartridge ........................................................................... 80
6.3 Specific case: labelling of a bespoke paint ............................................................. 83
Example 14: Labelling of a bespoke paint where colours are added on a tailor-made
basis at the point of sale ................................................................................................................... 83
7. GUIDANCE ON THE SELECTION OF PRECAUTIONARY STATEMENTS FOR
THE CLP HAZARD LABEL ............................................................................................ 84
7.1 Introduction ......................................................................................................................... 84
7.2 Methodology ........................................................................................................................ 85
7.3 Selection tables .................................................................................................................. 88
7.3.1 General precautionary statements .................................................................................... 90
7.3.2 Specific precautionary statements for physical hazards ........................................... 91
7.3.2.1 Explosives ................................................................................................................................................. 91
7.3.2.1 Explosives (continued) ........................................................................................................................... 93

8
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.1 Explosives (continued) ........................................................................................................................... 96
7.3.2.1 Explosives (continued) ........................................................................................................................... 98
7.3.2.2 Flammable gases (including chemically unstable gases) ............................................................... 101
7.3.2.2 Flammable gases (including chemically unstable gases) (continued) .......................................... 102
7.3.2.3 Aerosols .................................................................................................................................................. 103
7.3.2.3 Aerosols (continued) ............................................................................................................................. 104
7.3.2.4 Oxidising gases ...................................................................................................................................... 105
7.3.2.5 Gases under pressure ........................................................................................................................... 106
7.3.2.5 Gases under pressure (continued) ..................................................................................................... 107
7.3.2.6 Flammable liquids ................................................................................................................................. 108
7.3.2.7 Flammable solids ................................................................................................................................... 111
7.3.2.8 Self-reactive substances and mixtures .............................................................................................. 113
7.3.2.8 Self-reactive substances and mixtures (continued) ........................................................................ 115
7.3.2.8 Self-reactive substances and mixtures (continued) ........................................................................ 117
7.3.2.9 Pyrophoric liquids .................................................................................................................................. 119
7.3.2.10 Pyrophoric solids ................................................................................................................................. 121
7.3.2.11 Self-heating substances and mixtures ............................................................................................ 123
7.3.2.12 Substances and mixtures which, in contact with water, emit flammable gases ...................... 124
7.3.2.12 Substances and mixtures which, in contact with water, emit flammable gases (continued) 126
7.3.2.13 Oxidising liquids .................................................................................................................................. 127
7.3.2.13 Oxidising liquids (continued) ............................................................................................................. 129
7.3.2.14 Oxidising solids .................................................................................................................................... 130
7.3.2.14 Oxidising solids (continued) .............................................................................................................. 131
7.3.2.15 Organic peroxides ............................................................................................................................... 132
7.3.2.15 Organic peroxides (continued) ......................................................................................................... 134
7.3.2.15 Organic peroxides (continued) ......................................................................................................... 136
7.3.2.16 Corrosive to metals............................................................................................................................. 138
7.3.3 Specific precautionary statements for health hazards............................................. 139
7.3.3.1 Acute Toxicity – Oral ............................................................................................................................ 139
7.3.3.1 Acute Toxicity – Oral (continued) ....................................................................................................... 141
7.3.3.1 Acute Toxicity – Dermal ....................................................................................................................... 142
7.3.3.1 Acute Toxicity – Dermal (continued) ................................................................................................. 144
7.3.3.1 Acute Toxicity – Dermal (continued) ................................................................................................. 146
7.3.3.1 Acute Toxicity - Inhalation ................................................................................................................... 148
7.3.3.1 Acute Toxicity – Inhalation (continued) ............................................................................................ 150
7.3.3.1 Acute Toxicity – Inhalation (continued) ............................................................................................ 151
7.3.3.2 Skin corrosion/irritation ....................................................................................................................... 152
7.3.3.2 Skin corrosion/irritation (continued) .................................................................................................. 155
7.3.3.3 Serious eye damage - only .................................................................................................................. 157
7.3.3.3 Eye irritation – only .............................................................................................................................. 158
7.3.3.4 Respiratory sensitisation ...................................................................................................................... 159
7.3.3.4 Skin sensitisation .................................................................................................................................. 160
7.3.3.5 Germ cell mutagenicity ........................................................................................................................ 162
7.3.3.6 Carcinogenicity ...................................................................................................................................... 164
7.3.3.7 Reproductive toxicity ............................................................................................................................ 166
7.3.3.7 Reproductive toxicity (continued) ...................................................................................................... 168
7.3.3.8 Specific target organ toxicity after single exposure ........................................................................ 170
7.3.3.8 Specific target organ toxicity after single exposure (continued) .................................................. 172
7.3.3.8 Specific target organ toxicity after single exposure (continued) .................................................. 174
7.3.3.9 Specific target organ toxicity after repeated exposure ................................................................... 175
7.3.3.9 Specific target organ toxicity after repeated exposure (continued) ............................................. 177
7.3.3.10 Aspiration hazard ................................................................................................................................ 178
7.3.4 Specific precautionary statements for environmental hazards ............................ 179
7.3.4.1 Hazardous to the aquatic environment – short-term (acute) aquatic hazard ............................ 179
7.3.4.1 Hazardous to the aquatic environment – long-term (chronic) aquatic hazard ........................... 180
7.3.4.1 Hazardous to the aquatic environment – long-term (chronic) aquatic hazard (continued) ..... 181
7.3.5 Additional hazards.................................................................................................................. 182
7.3.5.1 Hazardous to the ozone layer ...................................................................................................... 182
7.4. Examples for the selection of precautionary statements for the label .... 183
Example A. Substance X assigned a physical and various health hazard
classifications ...................................................................................................................................... 183
Example B. Substance Y assigned a severe physical and health hazard classification
.................................................................................................................................................................. 185
Example C. Substance Z assigned physical, health and environmental classifications
.................................................................................................................................................................. 186
Example D. Mixture ABC for use by the general public ....................................................... 189

Guidance on Labelling and Packaging
Version 4.2 – March 2021
9
APPENDIX: GLOSSARY OF SELECTED TERMS USED IN THIS GUIDANCE
DOCUMENT ................................................................................................................... 191
Table of Figures
Figure 1: Blackened out empty diamonds ............................................................... 30
Figure 2: Readability ........................................................................................... 45
Figure 3: Decision flowchart for the application of CLP and transport labelling for single
packaging (left) and combination packaging (right) ................................................. 55
Figure 4: Application of CLP labelling on packaging used for supply and transport ....... 56
Table of Tables
Table 1: CLP labelling requirements versus discretion of the supplier ......................... 16
Table 2: The hazard classifications that trigger the CLP provisions for child-resistant
fastenings and/or tactile warnings ......................................................................... 20
Table 3: Substances that directly trigger the CLP provisions for child-resistant fastenings
and/or tactile warnings when they are contained in other substances or in mixtures at or
above the denoted concentration .......................................................................... 21
Table 4: Code ranges of hazard and precautionary statements under the CLP Regulation
......................................................................................................................... 33
Table 5: Obligatory supplemental labelling information pursuant to CLP Articles 25 and 32
......................................................................................................................... 36
Table 6: Minimum dimensions of labels and pictograms under the CLP Regulation ....... 44
Table 7: Labelling exemptions for packages of a capacity of 125 ml or less ................ 51

10
Guidance on Labelling and Packaging
Version 4.2 – March 2021
1. Introduction
1.1 Who should read this document?
This document is relevant for suppliers of chemical substances and mixtures, namely for:
• manufacturers and importers of substances;
• importers of mixtures;
• downstream users of substances and mixtures, including formulators;
• distributors of substances and mixtures, including retailers.
All suppliers must ensure that their substances and mixtures are labelled and packaged
in accordance with the provisions of the CLP Regulation (or CLP) before they are placed
on the EU market.
1.2 What is in this document?
This document provides guidance on the labelling and packaging requirements of
substances and mixtures set out in the CLP Regulation. The guidance opens in section 2
with a general overview, including legal background, scope of the CLP Regulation and
updating of CLP labels. That section also includes information about timelines for
classification, labelling, packaging and updating of CLP labels. The guidance continues in
section 3 and section 4 with an explanation of the requirements for labelling and
packaging and rules for the application of the CLP label elements. Section 5 provides
guidance on particular aspects of CLP hazard labelling (e.g. exemption from certain
labelling and packaging requirements, interaction between the CLP and transport
labelling rules, labelling requirements for specific cases of unique packaging). Finally,
section 6 and section 7 of the guidance provide practical examples illustrating different
situations that may be encountered when designing labels.
In particular, this guidance aims to clarify:
• what aspects to consider when estimating the label size needed;
• what types of supplemental information are possible, and where to place this
information on the label (section 4.8 of this guidance document);
• the conditions for small packaging exemptions;
• the interaction between CLP and the transport labelling rules;
• the technical requirements for liquid laundry detergents in a soluble packaging for
single use;
• how to select the most appropriate set of precautionary statements for the
label;
• how to structure the information on the label for appropriate readability.
For specific information on the application of the CLP criteria for physical, health and
environmental hazards, the reader is advised to consult the Guidance on the application
of the CLP criteria. For a general overview of basic features and procedures laid down in
the CLP Regulation, it might be useful to consult the Introductory Guidance on the CLP
Regulation.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
11
Note: All ECHA Guidance documents referred to in this document are available in the
support pages of the ECHA website at https://echa.europa.eu/guidance-
documents/guidance-on-clp
2. General overview
2.1 Legal background
The CLP Regulation is the EU Regulation on classification, labelling and packaging of
substances and mixtures. It is based on the United Nations Globally Harmonized System
of Classification and Labelling of chemicals (UN GHS). The CLP Regulation entered into
force on 20 January 2009 in the European Union and is now legally binding also in the
countries of the European Economic Area (EEA) (Norway, Iceland and Liechtenstein)
2
.
The CLP Regulation replaced the provisions of the Dangerous Substances Directive
67/548/EEC (DSD) and the Dangerous Preparations Directive 1999/45/EC (DPD) as of 1
June 2015 (see section 2.4 of this guidance document). The CLP Regulation is directly
applicable to suppliers in the EU who manufacture, import, use or distribute chemical
substances and mixtures.
This guidance explains the labelling and packaging rules of CLP and illustrates with
some examples how labels could be laid out.
In general, the CLP label must display certain label elements taken over from UN GHS,
including hazard pictogram(s), signal word, hazard and precautionary statements along
with supplemental information, where applicable, which reflect the assigned classification
of the substance or mixture. At the same time, the CLP Regulation retains some of the
labelling concepts of the DSD and DPD, such as the small packaging exemptions. In
order to accommodate certain hazard information not yet covered by the UN GHS, as
well as further label elements that are required by other EU legislation, the CLP
Regulation introduces the concept of “supplemental information” for the label.
All supplied substances and mixtures classified as hazardous and contained in a
packaging must be labelled in accordance with Title III (Hazard communication in the
form of labelling) and their packaging must be in accordance with Title IV (Packaging) of
the CLP Regulation.
In addition to the label, another key tool for hazard communication, intended for
professional/industrial users only, is the safety data sheet (SDS). The required SDS
format and content are defined in Article 31 and Annex II
3
to Regulation (EC) No
1907/2006 (REACH Regulation). These have been adapted to align them with the UN
GHS, as well as to be fully in line with the CLP Regulation. The information provided on
2
The CLP Regulation was incorporated in the EEA Agreement by Decision of the EEA Joint
Committee No 106/2012 of 15 June 2012 amending Annex II (Technical regulations, standards,
testing and certification) to the EEA Agreement (OJ L 309, 8.11.2012, p. 6–6).
3
Commission Regulations No 453/2010 and No 2015/830 have amended the REACH Regulation by
replacing Annex II to the REACH Regulation with the annexes to these regulations, to align the
requirements for safety data sheets with the rules for safety data sheets of the UN GHS, see:
http://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html.

12
Guidance on Labelling and Packaging
Version 4.2 – March 2021
the hazard label and in Section 2.2 of the SDS, for the same substance or mixture, must
be consistent
4
.
For further information on the compilation of the SDS, please consult the Guidance on
the compilation of safety data sheets.
2.2 Scope of labelling and packaging under the CLP Regulation
In general, substances and mixtures that are placed on the market are supplied in a
packaging with the necessary labelling information. A substance or mixture has to be
labelled according to the CLP rules where
• the substance or mixture is classified as hazardous;
• the mixture, even if not classified as hazardous, is addressed in CLP Article 25(6).
In this case the supplemental label elements as set out in Part 2 of Annex II to
CLP must be indicated together with the product identifier, name and telephone
number of the supplier.
In addition, an explosive article (i.e. an article containing one or more explosive
substances or mixtures) that meets the criteria as described in section 2.1 of Annex I to
CLP must be labelled according to the CLP rules.
Substances and mixtures within the scope of Regulation (EC) No 1107/2009
5
(Plant
Protection Products Regulation or PPPR) or Regulation (EU) No 528/2012 (Biocidal
Products Regulation or BPR) have to carry CLP labelling elements as appropriate.
Substances and mixtures within the scope of the PPPR also need to display the
supplemental statement EUH401 ‘To avoid risks to human health and the environment,
comply with the instructions for use’ (see CLP Article 25(2)). However, the labelling
provisions of these acts remain fully applicable to any product within their respective
scope (see Recital 47 of the CLP Regulation). For example, there are separate provisions
for updating labels for such substances and mixtures in these acts, and their suppliers
must apply these provisions instead of the CLP rules (see also CLP Article 30(3)).
Another deviation from the CLP Regulation is that different rules apply as to which
information may be presented in the form of a leaflet as an alternative way to
accommodate the required labelling information (see section 5.3.1.1 of this guidance
document).
The CLP Regulation also includes exemptions from labelling and packaging requirements,
for example for a packaging that is so small, or in such a shape that it is impossible to
meet the general rules for the application of labels (see section 5.3.1 of this guidance
document). In addition, the CLP Regulation allows suppliers to omit certain label
elements (see section 5.3.2 of this guidance document).
Certain substances and mixtures may also be supplied to the general public without
packaging, in which case a copy of the label elements is required to accompany the
substance or mixture, for example on an invoice. Currently, this only applies to
4
Note that there is no default requirement to place the Unique Formula Identifier (UFI) in the SDS
(except for unpackaged mixtures). When relevant, the UFI is to be included in Section 1.1 of the
SDS. For further details on UFI, please see section 4.8.1.1 of this guidance document.
5
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October
2009 concerning the placing of plant protection products on the market repeals Council
Directives 79/117/EEC and 91/414/EEC with effect from 14 June 2011. However, Article 80 of
Regulation (EC) No 1107/2009 specifies that Directive 91/414/EEC must continue to apply with
respect to active substances included in Annex I to that Directive for certain transitional periods.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
13
substances listed in Part 5 of Annex II to CLP (see section 5.3.2.4 of this guidance
document).
2.3 Derogations from labelling requirements for special cases
The CLP Regulation defines derogations from the CLP labelling requirements for special
cases and the conditions under which these derogations apply. One example of such a
special case is metals in massive form. CLP Article 23(d) provides that, in specific
cases, exemptions from the labelling requirements apply to: “metals in massive form,
alloys, mixtures containing polymers, mixtures containing elastomers”.
Section 1.3.4.1 of Annex I to CLP elaborates further on CLP Article 23 and gives
conditions when labelling is not required, namely: “if they do not present a hazard to
human health by inhalation, ingestion or contact with skin or to the aquatic environment
in the form in which they are placed on the market”.
The CLP legal text does not specify when a form of metal should be considered massive.
A default particle size limit cannot be specified to determine whether or not CLP Article
23 applies to any metal.
To apply the exemption from the labelling provisions, the manufacturer or supplier must
be able to demonstrate the lack of hazard in the form the metal or alloy is placed on the
market. Section 2.1 of the SDS must contain the classification of the metal and
information on the application of the labelling exemption for the form as placed on the
market.
In relation to the other cases described in CLP Article 23, please consult the Article and
section 1.3 of Annex I to CLP, as further guidance on these is not provided in this
document.
Another special case is that of bespoke paints. Annex VIII and Article 25(8) of CLP
provide for special provisions with regard to the Unique Formula Identifier (UFI) for
bespoke paints. More details are provided in sections 4.8.1.1 and 5.3.3.
2.4 Timelines for classification, labelling, packaging and updating
of CLP hazard labels
The CLP Regulation was introduced gradually before its full application as of 1 June
2015. During this transitional period, some of the rules of the CLP Regulation and the
previous legislation (DSD and DPD) were applicable in parallel to give companies time to
migrate to the CLP rules. However, companies were allowed to apply the CLP Regulation
in full on a voluntary basis, from its entry into force.
For substances, it has been obligatory to classify, label and package according to the CLP
Regulation since 1 December 2010. The same obligations have applied for mixtures since
1 June 2015. The transitional period for mixtures classified, labelled and packaged
according to DPD and already placed on the market before 1 June 2015 ended on 1 June
2017.
DSD and DPD are no longer applicable in any context and both substances
and mixtures must now be classified, labelled and packaged in accordance
with the CLP Regulation. This classification must be provided in the SDS for
substances and mixtures. There is no longer a requirement to provide either DSD
classifications of substances themselves or of component substances in mixtures or
the DPD classifications for mixtures in the SDS. Only the corresponding information

14
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Following any changes to the classification and labelling where the revised classification
is more severe or where new supplemental label elements are required, CLP Article 30
requires a supplier to update this information on the label without undue delay, i.e. as
soon as reasonably practicable.
Where labelling changes other than those described above are required (e.g. where the
revised classification will be less severe or the contact details of the supplier have
changed) the supplier has 18 months to update the label.
Where a new or updated harmonised classification arises from an Adaptation to Technical
Progress (ATP) to the CLP Regulation, the ATP provides the date of applicability.
Further label changes to be implemented within 18 months would also include the
update of labelling information for certain mixtures for which special rules for
supplemental labelling in accordance with Part 2 of Annex II to CLP apply.
However, there are separate provisions for updating labels in the BPR and the PPPR and
suppliers of substances or mixtures within the scope of these acts must apply these
provisions.
3. Requirements of labelling and packaging in
accordance with the CLP Regulation
3.1 General labelling rules
General and specific rules regarding the content and application of a CLP label are set
out in CLP Article 31.
The CLP Regulation requires that the labels are firmly affixed to one or more surfaces of
the immediate container of the substance or mixture and that they must be readable
horizontally when the package is set down normally. The label elements themselves, in
particular the hazard pictograms, must stand out clearly from the background.
Furthermore, all label elements must be of such size and spacing as to be easily read.
They must be clearly and indelibly marked. A physical label is not required when the
label elements are shown clearly on the packaging itself.
3.2 Elements of the CLP hazard label
According to CLP Article 17, a substance and mixture classified as hazardous must bear a
label including the following elements:
• Name, address and telephone number of the supplier(s);
• The nominal quantity of the substance or mixture in the package where this is
being made available to the general public, unless this quantity is specified
elsewhere on the package;
• Product identifiers;
• Hazard pictograms, where applicable;
• The relevant signal word, where applicable;
• Hazard statements, where applicable;
according to the CLP Regulation need be provided (see also the Guidance on the
compilation of safety data sheets).

Guidance on Labelling and Packaging
Version 4.2 – March 2021
15
• Appropriate precautionary statements where applicable;
• A section for supplemental information, where applicable.
According to Annex VIII to CLP
6
, a Unique Formula Identifier (UFI), if applicable, must
also be added to, i.e. printed on or affixed to, the label of mixtures falling under the
scope of CLP Article 45 and Annex VIII to CLP (see section 4.8.1.1 of this guidance
document).
It should be noted that for particular label elements precedence rules apply. These rules
are further explained in the sections below.
• The CLP Regulation requires the label to be written in the official language or
languages of the Member States where the substance or mixture is placed on the
market, unless the Member State concerned provides otherwise
7
. Suppliers may
accomplish this either by producing multi-language labels covering the official
languages of several of the countries where the substance or mixture is supplied,
or by producing separate labels for each country, each with the appropriate
language or languages.
Suppliers may use more languages than those required on their labels if they wish,
provided that the same details appear in all languages. However, this should not impact
the legibility of the obligatory labelling information, nor can it trigger exemptions from
the labelling requirements (see section 5.3.1 of this guidance document).
3.3 Location of information on the CLP hazard label
CLP Article 32 provides some limited rules that define the location of information on the
label. However, further details as to how label elements are arranged are left to the
discretion of the person responsible for compiling the label. As a general rule, the
information should be structured in a way that is easy to read and understand. Examples
are outlined in Table 1 below:
6
See Commission Regulation (EU) 2017/542.
7
Please consult the table “Languages required for labels and safety data sheets”, which is available
on the ECHA website web at: https://echa.europa.eu/regulations/clp/labelling.

16
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Table 1: CLP labelling requirements versus discretion of the supplier
CLP requirement (Article 32)
Example of decision left to the discretion
of the supplier
The hazard pictograms, signal word, hazard
statements and precautionary statements
must be kept together on the label.
The supplier is free to choose the
arrangement of the pictograms.
Hazard statements must be grouped together
on the label.
The supplier may choose the order of the
hazard statements.
The supplier may choose whether these
groups are to be presented on the left, on the
right or elsewhere on the label.
Precautionary statements must be grouped
together on the label.
The supplier may choose the order of the
precautionary statements, but should ensure
that they are grouped with the hazard
statements.
The supplier may choose whether these
groups are to be presented on the left, on the
right or elsewhere on the label.
In case more than one language is used on
the label, the hazard and precautionary
statements of the same language must be
grouped together on the label.
Where the supplier needs to use alternative
means to meet the requirements of CLP
Article 31 in relation to the language(s)
required in a particular Member State, he
may choose whether to accomplish this using
fold-out labels, tie-on tags or on an outer
packaging, in accordance with section 1.5.1 of
Annex I to CLP.
Any supplemental information as referred to
in CLP Article 25 must be included in the
section for supplemental labelling and placed
alongside the label elements referred to in
CLP Article 17(1)(a)–(g).
The supplier may choose how to visibly
separate this section from the section
containing the label elements referred to in
CLP Article 17(1)(a)-(g). He may also decide
to place this information in more than one
location on the label.
The label elements must be easily readable
(Article 31(3)).
It is recommended to keep full sentences
together and in one line, if possible. The font
size and spacing must be large enough and in
relation to the dimensions of the label.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
17
3.4 CLP rules on packaging of substances and mixtures
Before continuing to describe in more detail the CLP requirements for packaging, the
reader should be introduced to the three CLP definitions:
CLP Article 35 includes the requirements for packaging containing hazardous substances
or mixtures. These provisions are to ensure that:
• the packaging is designed, constructed and fastened so that the contents cannot
escape;
• the materials of the packaging and fastening are not damaged by the contents
and are not liable to form hazardous compounds with the contents;
• the packaging and fastenings are strong and solid throughout to ensure that they
will not loosen;
• packaging fitted with replaceable fastening devices is properly designed to allow
repeated refastening without the contents escaping;
• the packaging does not attract or arouse the curiosity of children or mislead the
consumer when supplied to the general public;
• the packaging does not have a similar presentation or a design used for foodstuff
or animal feed stuff or medicinal or cosmetic products which would mislead the
consumers.
Packaging that meets the requirements of the transport legislation is deemed to comply
with the requirements set out in the bullet points above (Note however that fulfilling the
conditions in the above bullet points alone is usually not enough to comply with the
requirements of the transport legislation).
For substances and mixtures to be supplied to the general public, the CLP Regulation
sets out rules for:
• the use of child-resistant fastening (CRF), also referred to as child-resistant
closure (see section 3.4.1 of this guidance document);
• the use of tactile warnings of danger (TWDs) (see section 3.4.1 of this guidance
document);
• liquid consumer laundry detergents in soluble packaging for single use (see
section 3.4.2 of this guidance document).
The first two provisions are triggered by either a specific hazard class/category or by the
concentration of specific substances contained in other substances or in mixtures (see
Tables 2 and 3 of this guidance document).
Article 2 (35): ‘package’ means the complete product of the packing operation,
consisting of the packaging and its contents;
Article 2 (36): ‘packaging’ means one or more receptacles and any other components
or materials necessary for the receptacles to perform their containment and other
safety functions;
Article 2 (37): ‘intermediate packaging’ means packaging placed between inner
packaging, or articles, and outer packaging;

18
Guidance on Labelling and Packaging
Version 4.2 – March 2021
3.4.1 Child-resistant fastening and tactile warnings of danger
The provisions described in this section apply only for product packaging intended for the
general public, for example: products on sale/offer at a retailer’s or an outlet where the
general public have open access to them, products sold to the general public through a
website.
The requirements for CRF and TWD do not apply to product packaging which is for
professional users only.
Child-resistant fastening
A child-resistant package
8
is a package consisting of a container and an appropriate
closure which is difficult to open (or gain access to the contents) for young children
under the age of fifty-two months, but which is not difficult for adults to use properly
9
.
Annex II to CLP refers to two types of CRF for packages:
• non-reclosable package - a package that, when all or part of the contents have
been removed, cannot be properly closed again, for example a blister pack or air
freshener refills;
• reclosable package - a package (for example a one litre bottle or a five litre
container) that, after it has been initially opened, can be reclosed and re-used
numerous times without loss of security.
For fastening of the above-mentioned packages, Annex II to CLP requires conformity
with the following standards, as amended:
• EN ISO 8317 (reclosable packages), and
• CEN EN 862 (non-reclosable packages).
Conformity with these standards may only be certified by laboratories that conform to
EN ISO/IEC 17025, as amended. The EN ISO/IEC 17025 standard relates to the
competence of testing laboratories and the requirements that they must meet to
demonstrate that they are technically competent and can generate technically valid
results. In specific cases referred to in section 3.1.4.2 of Annex II to CLP, i.e. if it seems
obvious that packaging is sufficiently safe for children because they cannot get access to
the contents without the help of a tool, the above tests on non-reclosable and reclosable
packages do not need to be performed
10
.
A packaging of whatever capacity supplied to the general public must be fitted with CRF
for substances or mixtures:
• classified for acute toxicity 1-3 – oral (H300 and H301), dermal (H310 and H311)
or inhalation (H330 and H331); STOT-SE 1 (H370); STOT-RE 1 (H372); skin
corrosion 1, subcategories 1A, 1B, 1C (H314), or
• classified as presenting an aspiration hazard (H304), with the exception of
8
Please note that the terminology differs between the CLP legal text and the EN standard. The CLP
Regulation refers to packaging fitted with child-resistant fastening, whereas EN ISO 8317 refers to
child-resistant packages.
9
According to EN ISO 8317.
10
See also the Report on the Forum pilot project on Child-resistant fastenings.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
19
substances and mixtures that are placed on the market in the form of aerosols or
in a container fitted with a sealed spray attachment, or
• containing methanol at a concentration greater or equal to 3% or
dichloromethane at a concentration greater or equal to 1% (see also Table 3 of
this guidance document).
Tactile warnings of danger (TWDs)
Packages provided with a TWD enables blind or visually impaired people to ascertain if
the packages contains a hazardous substance or mixture. A TWD must be placed on the
packaging, so that it can be felt before accessing the contents. The warning must be
located in such a way that any other embossed patterns do not cause confusion. The
exact location of the TWD must be according to EN ISO standard 11683.
The TWD must also remain tactile during the expected period of use of the package
under normal handling conditions. The TWD is not required on outer packaging such as
for example a cardboard box protecting a glass bottle
11
.
Annex II to CLP requires the TWD to conform to standard EN ISO 11683, as amended.
The required standard TWD symbol (the “normal” symbol under the ISO standard) is an
equilateral triangle. In exceptional cases (if the application of the normal symbol is not
physically possible), the three dots symbol may be used. If it is not physically possible to
even use the three dots symbol, the three mm symbol may be used
12
.
A packaging of whatever capacity supplied to the general public must be fitted with TWD
for substances or mixtures classified for:
• acute toxicity 1-4 – oral (H300, H301 and H302), dermal (H310, H311 and
H312) or inhalation (H330, H331 and H332);
• skin corrosion 1, subcategories 1A, 1B and 1C (H314);
• germ cell mutagenicity 2 (H341);
• carcinogenicity 2 (H351);
• reproductive toxicity 2 (H361);
• respiratory sensitisation 1, 1A and 1B (H334);
• STOT SE 1 or 2 (H370, H371);
• STOT RE 1 or 2 (H372 and H373);
• aspiration hazard 1 (H304);
• flammable gases 1 and 2 (H220 and H221);
• flammable liquids 1 and 2 (H224 and H225); or
• flammable solids 1 and 2 (H228).
According to section 3.2.1.2 of Annex II to CLP, a TWD is not required for transportable
gas receptacles. A TWD is also not required for aerosols and containers fitted with a
11
According to EN ISO 11683.
12
The arrangement and layout of the triangle, three dots as well as the three mm symbol are
specified in EN ISO 11683.

20
Guidance on Labelling and Packaging
Version 4.2 – March 2021
sealed spray attachment containing substances or mixtures classified as presenting an
aspiration hazard, unless they are classified for one or more of the other hazards
mentioned above.
Table 2 provides an overview of the hazard classifications triggering the CLP provisions
for CRF and/or TWD. Table 3 lists substances that can trigger the CLP provisions for CRF
and/or TWD if they are present in other substances or in mixtures at a certain
concentration.
Table 2: The hazard classifications that trigger the CLP provisions for child-
resistant fastenings and/or tactile warnings
Hazard Class, Category
Child-
resistant
Fastenings
Tactile
Warnings
Acute toxicity 1 to 3
✓
✓
Acute toxicity 4
✓
STOT SE 1
✓
✓
STOT SE 2
✓
STOT RE 1
✓
✓
STOT RE 2
✓
Skin corrosion (category 1, subcategories: 1A, 1B and 1C)
✓
✓
Respiratory sensitisation (category 1, subcategories: 1A and 1B)
✓
Aspiration hazard 1
Note that a CRF and TWD are not required if the substance or mixture
is supplied in the form of an aerosol or in a container fitted with a
sealed spray attachment and if the substance or mixture is not
classified for another hazard triggering CRF or TWD
✓
✓
Germ cell mutagenicity 2
✓
Carcinogenicity 2
✓
Reproductive toxicity 2
✓
Flammable gases 1 and 2
✓
Flammable liquids 1 and 2
✓
Flammable solids 1 and 2
✓

Guidance on Labelling and Packaging
Version 4.2 – March 2021
21
Table 3: Substances that directly trigger the CLP provisions for child-resistant
fastenings and/or tactile warnings when they are contained in other
substances or in mixtures at or above the denoted concentration
Identification of the substance
Concentration limit
Child-
resistant
Fastenings
Tactile
Warnings
Methanol
3%
✓
✓*
Dichloromethane
1%
✓
✓**
* It should be noted that above a certain concentration, methanol mixtures also need a tactile warning because
the mixtures would then have to be classified as flammable liquid category 2, STOT SE category 1 or 2.
** In addition, mixtures containing dichloromethane at a concentration above 1% would be classified as
carcinogenic category 2 and thereby need a tactile warning.
3.4.2 Liquid consumer laundry detergents in soluble packaging for
single use
Additional safety measures for liquid laundry detergents in soluble capsules are in place.
They aim to ensure better protection of the general public, especially young children who
can be tempted to put the capsules into their mouth.
These safety requirements make the packaging less attractive and more difficult to open
for children. In addition, the packaging is to display warnings to alert parents and child-
care providers that such products have to be kept out of reach of children.
Beside these specific rules, the supplier is responsible, according to CLP Article 35(2), for
taking all necessary steps to make sure that the design of the packaging is not attractive
to children, so that, for instance, it cannot be mistaken for foodstuff or toys.
A consumer laundry detergent is a detergent used for laundry, placed on the market for
use by non-professionals, including public launderettes
13
.
CLP Article 35(2) and section 3.3 of Annex II to CLP provide the following requirements
on packaging and labelling of liquid laundry detergents in dosages for single use
contained in a soluble packaging:
Obligation to market liquid consumer laundry detergents in an outer packaging
Liquid consumer laundry detergents contained in soluble packaging for single use (for
example liquid capsules or liquitabs for use in washing machines) must be contained in
an outer packaging. Failure to do so is considered as non-compliant with CLP Article
35(1) and section 3.3.1 of Annex II to CLP.
Provisions on the outer packaging
In order to reduce the attractiveness to children of liquid consumer laundry detergents
contained in soluble packaging for single use, the outer packaging must be opaque or
obscure (for example non-see through container of a block colour(s)) to prevent visibility
of the contents, i.e. the product or individual doses.
The outer packaging must bear precautionary statement P102 (“Keep out of reach of
children”) at a visible place and in a format that attracts attention.
13
Article 2(1a) of Regulation (EC) No 648/2004 on detergents.

22
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Furthermore, the outer packaging must be a self-standing container that is easily re-
closable, i.e. the pack closure must be easily re-closable in one single movement (for
example with one finger pressure for a tub packaging). This measure aims to avoid the
risk that the container will simply be left open if closing is too difficult.
As the main cause of incidents seems to be the easy access to the detergent capsules,
the outer packaging must be fitted with a closure that impedes the ability of young
children to open the packaging. Such a closure should require a coordinated action of
both hands with a certain strength that makes it difficult for young children to open it. It
should be noted that this requirement does not necessarily correspond with the closure
requirement for CRF described in section 3.4.1 of this guidance document.
In addition, the pack closure must be designed for repeated use to maintain its
functionality under conditions of repeated opening and closing for the entire life span of
the outer packaging.
Provisions on the soluble (inner) packaging
Additional technical requirements (mechanical resistance and water dissolution) were
introduced to make the soluble packaging more resistant.
In addition to the requirements for the outer packaging, the soluble packaging must
contain an aversive (e.g. bittering or other repulsive) agent against oral exposure. The
aversive agent must be added in a concentration that is safe and that causes oral
repulsive behaviour within a maximum time of six seconds.
The soluble film must also meet minimum mechanical and dissolution resistance criteria.
It must retain the liquid content for at least 30 seconds when placed in water at 20°C. It
must also resist mechanical compression of at least 300 N under standard test
conditions.
Soluble packaging for single use with a volume of contents equal to 25 ml or less may
benefit from a labelling exemption under the conditions specified in section 1.5.2.2 of
Annex I to CLP (see section 5.3.2.2 of this guidance document); the labelling
requirements of CLP Article 17 apply to soluble packaging where the volume of
contents is more than 25 ml.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
23
4. Rules for the application of the CLP label elements
4.1 Contact details of the supplier
According to CLP Article 17, the contact details of one or several suppliers must be
included on the label. In principle, there can be more than one supplier of the same
substance or mixture in the supply chain, e.g. in case a mixture has been supplied by
the formulator to a distributor who would supply it to third parties as well. However, CLP
Article 17 does not specify whether the contact details of both suppliers are needed in
such cases. Nor does it specify whether the contact details of one particular supplier
have precedence.
Following from CLP Article 4(4), each supplier must ensure that a hazardous substance
or mixture is labelled and packaged in accordance with Titles III and IV of the CLP
Regulation before it is placed on the market. On the way through the supply chain the
labelling for the same substance or mixture may vary depending on the volume of the
package or as a consequence of further layers of packaging (see section 5.2, section 5.3
and section 5.4 of this guidance document).
Where a supplier changes the packaging so that the label elements set out in CLP Article
17 have to be displayed differently than on the label/packaging supplied to him, they
take the responsibility for re-packaging and re-labelling and should add their own name
and contact information on the label. In this case, the supplier may also replace the
contact information of their supplier with their own contact details.
When the supplier does not change the packaging, they do not need to add their contact
details to the label or replace the contact information of their supplier with their own
contact details. They may do so if they wish to. In case the supplier changes the
languages(s) displayed on a label, they should add their contact details to the contact
details of the relevant supplier who issued the original label, as they are then responsible
for the correct translation of the label content.
4.2 Product identifiers
This section provides guidance on the requirements for the product identifiers for
substances (CLP Article 18(2)) and mixtures (CLP Article 18(3)). As a general rule, the
same product identifier(s) as selected for the label must be used in the SDS
14
for a
substance or mixture. Any product identifiers selected for the label must be written in
the official language(s) of the Member State(s) where the substance or mixture is placed
on the market, unless the Member State concerned provides otherwise (see CLP Article
17(2)).
4.2.1 Substances
The product identifier for a substance must consist of at least the following:
• a name and an identification number as given in Part 3 of Annex VI to CLP
14
For further information, please consult the Guidance on the compilation of safety data sheets.

24
Guidance on Labelling and Packaging
Version 4.2 – March 2021
The name can be any of the names stated as International Chemical Identification
in column 2 of the tables in Part 3 of Annex VI to CLP
15
. The identification number
is typically the Index number, the EC number or the CAS number. It is
recommended to use the number that warrants an unambiguous identification of
the substance; in some cases it may be warranted to use two numbers, e.g. the
CAS and the EC number. When translating the name of an Annex VI substance
into the required language(s), it may be useful to check whether an appropriate
translation is already available in a public database, for example in ECHA’s
Classification and Labelling (C&L) Inventory (see
https://echa.europa.eu/information-on-chemicals/cl-inventory-database). If there
is a translated name available in Annex VI to CLP or in the C&L Inventory, this
name should be given preference; or
• if the substance is not included in Part 3 of Annex VI to CLP, a name and an
identification number as they appear in the C&L Inventory.
The name is typically the IUPAC name
16
, the EC name or the CAS name. The
identification number must be the EC or the CAS number or the Index number
(originating from Table 3 of Annex VI to CLP). It is recommended to use the
number or numbers that warrant(s) an unambiguous identification of the
substance. The choice of an identifier such as (where applicable) the EC number
or CAS number is advisable to minimise the need for revision of the SDS; or
• if the substance is neither included in Part 3 of Annex VI to CLP nor in the C&L
Inventory database, the CAS number and the IUPAC name, or the CAS number
and another international chemical name, e.g. the name in INCI nomenclature
17
,
where applicable; or
• if no CAS number is available and none of the above apply, the IUPAC name or
another international chemical name, e.g. the name in INCI nomenclature, where
applicable.
15
Please note that Commission Regulation (EU) 2018/669 of 16 April 2018 (11
th
ATP to CLP)
introduces translations of the chemical names of substances subject to harmonised classification
and labelling listed in Table 3 of Annex VI to CLP in all languages. The 11
th
ATP was based on the
consolidated text of the CLP Regulation up to the 6
th
ATP, as in the later ATPs the chemical names
are already translated. All other information, apart from the chemical names, remains applicable
as stated in the relevant ATPs, in particular that related to classification and labelling, unless an
entry has been modified by an ATP that has been adopted after the 6
th
ATP and is already
applicable. The 11
th
ATP will apply from 1 December 2019 but can be used voluntarily ahead of
that date.
16
Where the IUPAC name exceeds 100 characters, suppliers can use one of the other names
(usual name, trade name or abbreviation) referred to in section 2.1.2 of Annex VI REACH provided
that a C&L notification to ECHA, in accordance with CLP Article 40(1)(b), includes both the IUPAC
name and the other name used.
17
The International Nomenclature Cosmetic Ingredients (INCI) name is mandatory in the
European Union (EU) according to Regulation (EC) No 1223/2009 for labelling the names of
ingredients on cosmetic products. The INCI system was introduced in the European Community in
1996/97 and is well established for cosmetic products. It is also used in many non-EU countries.
Since 2004, the INCI system is also mandatory in the EU for labelling of preservatives and
allergenic perfume ingredients according to the Detergents Regulation (EC) No 648/2004.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
25
4.2.2 Mixtures
The product identifiers for mixtures must include both:
• the trade name or the designation of the mixture; and
• the identity of all substances in the mixture that contribute to the classification of
the mixture as regards acute toxicity, skin corrosion or serious eye damage, germ
cell mutagenicity, carcinogenicity, reproductive toxicity, respiratory or skin
sensitisation, specific target organ toxicity (STOT), or aspiration hazard.
The CLP Regulation does not specify the type of chemical names
18
that should be used to
identify the chemical substances in the mixture. It only mentions the approach used for
identification of substances in the mixture that contribute to the classification of the
mixture (see CLP Article 18(3)(b) and the second paragraph of CLP Article 18(3)).
Nevertheless, when choosing a chemical name, it is recommended that the approach
outlined in CLP Article 18(2) is followed. On that basis, if a name of the substance is
shorter than other names available to the user/consumer or better recognised by the
user/consumer in the language of the Member State where the mixture is placed on the
market, this name should be used. This is often the case for common or basic
ingredients. Furthermore, if there is a translated name available in Annex VI to CLP
19
or
in the C&L Inventory, this name should be given preference.
In cases where another international chemical name (for example an INCI name) is
better known by the user/consumer, it is possible to deviate from the CLP Article 18(2)
approach. It is preferable to use the name that is regarded as well-known. The name of
the substance needs to unambiguously define its identity. Where an INCI name does not
sufficiently define the substance identity compared to, for example, the requirements of
CLP Article 18 (2) or the requirements for SDSs under the REACH Regulation, a clearer
identification should be preferred.
If the trade name or the designation of the mixture already includes the name(s) of the
substance(s) contributing to the classification of the mixture as defined in paragraph
3(b) of CLP Article 18, they do not need to be repeated. Moreover, if the supplemental
information on the label already contains the chemical name of the substance, e.g. in the
list of allergens and preservatives required by Regulation (EC) No 648/2004 on
detergents, it is advisable to use the same name. This approach should apply to both
consumer and professional products.
The selected chemical names must identify the substances primarily responsible for the
major health hazards that have caused the classification of the mixture and the
assignment of the corresponding hazard statements.
To reduce the number of substance (‘chemical’) names on the label, no more than four
names should be provided on the label for a mixture, unless necessary due to the nature
and severity of the hazards. This may be the case where a mixture contains more than
18
The terms used for identification of the mixture and the substances in the mixture must be the
same as those used in the safety data sheet.
19
Please note that Commission Regulation (EU) 2018/669 of 16 April 2018 (11
th
ATP to CLP)
introduces translations of the chemical names of substances subject to harmonised classification
and labelling listed in Table 3 of Annex VI to CLP in all languages. The 11
th
ATP was based on the
consolidated text of the CLP Regulation up to the 6
th
ATP, as in the later ATPs the chemical names
are already translated. All other information, apart from the chemical names, remains applicable
as stated in the relevant ATPs, in particular that related to classification and labelling, unless an
entry has been modified by an ATP that has been adopted after the 6
th
ATP and is already
applicable. The 11
th
ATP will apply from 1 December 2019 but can be used voluntarily ahead of
that date.

26
Guidance on Labelling and Packaging
Version 4.2 – March 2021
four substances present in significant concentrations and contributing to the
classification of the mixture for one or several of the hazards mentioned under CLP
Article 18(3)(b). As explained in CLP FAQ ID=1050 (available at
https://echa.europa.eu/support/qas-support/qas), there are no strict rules on how to
decide which substances should take precedence to be named on the label, but the
following may help in the selection. For non-additive health hazards (e.g. germ cell
mutagenicity, carcinogenicity, reproductive toxicity, respiratory or skin sensitisation and
specific target organ toxicity categories 1 and 2), all ingredients present in the mixture
at or above the generic concentration limit (GCL) or specific concentration limit (SCL)
should be considered as "primarily responsible for the major health hazards" within the
meaning of Article 18(3)(b) CLP and included on the label. For the additive health
hazards mentioned in Article 18 (3)(b) CLP (e.g. acute toxicity, skin corrosion, serious
eye damage, specific target organ toxicity category 3 and aspiration hazard), all
ingredients present in the mixture at or above the GCL or SCL should be included on the
label. However, where there are several ingredients contributing to classification for one
hazard endpoint, only the ingredients primarily contributing to the classification, for
example, those with the highest concentrations or closest to the GCL or SCL, need to be
included on the label, and therefore the names of other ingredients with limited
contribution to the classification are not required. In addition, specific labelling rules
apply to mixtures containing skin and respiratory sensitisers (see Table 3.4.3 of Annex I
to CLP and point 2.8 of Annex II to CLP).
Note that, although the UFI is an element of identification used for the purpose of Annex
VIII to CLP, it is not a product identifier within the meaning of CLP Article 18. The UFI is
part of the (obligatory) supplemental information (CLP Article 25(7)). Nevertheless
specific provisions may apply (see section 4.8.1.1 of this guidance document and the
Guidance on harmonised information relating to emergency health response – Annex
VIII to CLP).
The manufacturer, importer or downstream user of certain less hazardous substances
contained in a mixture may conclude that disclosing substance identifiers that are
required for the label or the SDS can put the confidential nature of his business or
intellectual property rights at risk. In such cases, he may submit a request to ECHA to be
granted permission to use an alternative chemical name in accordance with CLP Article
24. The alternative name should be a more general name identifying the most important
functional groups or an alternative designation. The conditions under which the use of an
alternative name may be granted are given in Part 1, section 1.4 of Annex I to CLP.
The above requests are subject to a fee, in accordance with Article 3 of Commission
Regulation (EU) No 440/2010 (the Fee Regulation). Where the request is submitted by a
micro, small or medium-sized enterprise (SME)
20
, ECHA will levy a reduced fee as set out
in Article 24(2) and Annex I to the Fee Regulation.
For more information on how to request the use of an alternative chemical name for a
substance in a mixture, please follow the technical instructions set out in the manual on
preparation of REACH and CLP dossiers: How to prepare a request for use of an
alternative chemical name for a substance in a mixture available in the Manuals section
of the ECHA website at https://echa.europa.eu/manuals. It is also advised to visit the
following section on the ECHA website: https://echa.europa.eu/support/dossier-
submission-tools/reach-it/requesting-an-alternative-chemical-name-in-mixtures.
20
SME is defined in Commission Recommendation 2003/361/EC.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
27
4.3 Hazard pictograms
4.3.1 General information
A hazard pictogram is a pictorial presentation to communicate information on the hazard
concerned (see also the definition provided in CLP Articles 2(3) and 31(2)). According to
CLP Article 19, the classification of a substance or mixture determines the hazard
pictograms that have to be displayed on a label. Information on the assignment of
hazard pictograms to specific hazard classes and categories/differentiations can also be
found in Annex V to CLP.
Currently, there are nine different pictograms. While normally only one pictogram is
assigned to an individual hazard class or category, a few hazard differentiations have to
carry two pictograms, namely substances and mixtures classified as self-reactive Type B
or as organic peroxide Type B (see also the below sections). It should also be noted that
some pictograms cover several hazard classes and categories.
4.3.2 Shape, colour and dimensions
The colour and presentation of a label must allow the hazard pictogram and its
background to be clearly visible. Hazard pictograms must be in the shape of a square set
at a point, i.e. they must appear as a diamond shape when the label is read horizontally,
and must have a black symbol on a white background with a red frame (see section
1.2.1 of Annex I to CLP). The exact type of red, i.e. the Pantone colour number, is not
defined, and labellers are free to use their discretion.
Each hazard pictogram must cover at least one fifteenth of the minimum surface area of
the label dedicated to the information required by CLP Article 17, but the minimum area
of the pictogram must not be less than 1 cm
2
. The minimum dimensions of labels and
pictograms are given in Table 1.3 of Annex I to CLP. For pictograms, these minimum
dimensions refer to the sides of the red frame of the pictogram itself, and not to the
sides of the virtual square within which the pictogram is placed:
Correct measurement Wrong measurement
Below is the exclamation mark (pictogram GHS07) as an example pictogram. It is
assigned to various health hazard classes and categories of lower severity (see Part 2 of
Annex V to CLP):
Printable pictograms are provided free of charge for download at
http://www.unece.org/trans/danger/publi/ghs/pictograms.html.

28
Guidance on Labelling and Packaging
Version 4.2 – March 2021
4.3.3 Precedence rules
For substances and mixtures classified for more than one hazard, several pictograms
may be required on the label. In such cases, the applicability of the precedence rules set
out in CLP Article 26 need to be checked. As a general rule, the pictograms that reflect
the most severe hazard category of each hazard class must be included on the label.
This would also apply where a substance has both a harmonised and a non-harmonised
(i.e. self-) classification (see CLP Article 26(2)).
Further to this, the CLP Regulation sets out precedence rules relating to particular hazard
pictograms and classifications:
• For physical hazards, if the label carries the pictogram GHS01 (exploding bomb),
then GHS02 (flame) and GHS03 (flame over circle) are optional …
mandatory optional optional
… except in cases where more than one pictogram is compulsory, namely for
substances and mixtures classified as self-reactive Type B or as organic peroxide
Type B (see Annex I to CLP);
• For physical and health hazards, if the label carries the pictogram GHS02 (flame)
or GHS06 (skull and crossbones), then GHS04 (gas cylinder) is optional
21
:
mandatory mandatory optional
• For health hazards, if the label carries the pictogram GHS06 (skull and
crossbones), then GHS07 (exclamation mark) must not appear:
21
This precedence rule was introduced by the Commission Regulation (EU) No 286/2011 of 10 March
2011 (2
nd
ATP to the CLP Regulation).
or

Guidance on Labelling and Packaging
Version 4.2 – March 2021
29
• For health hazards, if the label carries the pictogram GHS05 (corrosion), then
GHS07 (exclamation mark) must not be used for skin or eye irritation…
… but still has to be used for other hazards.
• For health hazards, if the label carries the pictogram GHS08 (health hazard) for
respiratory sensitisation, then GHS07 (exclamation mark) must not be used for skin
sensitisation or for skin or eye irritation …
… but still has to be used for other hazards.
In case a substance or mixture is assigned the supplemental hazard statement EUH071
(“Corrosive to the respiratory tract”), a corrosivity pictogram (GHS05) may be assigned
(see Note 1 of Table 3.1.3 in Annex I to CLP). Where this is done, the pictogram GHS07
(exclamation mark) for STOT SE category 3 (respiratory tract irritation) must be omitted
from the label, as well as the hazard statement H335 (“May cause respiratory
irritation”).
For substances and mixtures that have to be labelled in accordance with both the CLP
Regulation and the rules on the transport of dangerous goods, the CLP pictogram(s) may
be omitted from the label on the outer or single packaging when the CLP pictogram(s)
and the pictogram(s) for transport of dangerous goods relate to the same hazard (see
section 5.4 of this guidance document).
4.3.4 Blank pictograms
When preparing hazard labels, a common practice is to use pre-printed label stocks of
the diamonds (the label background is printed first before it is overprinted with the
specific label information). This may result in labels with a number of pre-printed empty
diamonds, not all of which may then be needed by a company that has purchased pre-
printed labels. In such a situation, one or more pre-printed diamonds may have to be
left empty.
The CLP Regulation does not explicitly forbid blank diamonds. However, any information
given in addition to the minimum mandatory labelling must not contradict or cast doubt
on the mandatory label information (CLP Article 25(3)), while empty red frames might
raise questions. If empty red frames are unavoidable, it is recommended to cover them
up with a solid overprint which blacks them out completely (see the example in Figure
1).

30
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Figure 1: Blackened out empty diamonds
Blacking-out of empty diamonds aims to avoid the impression that relevant hazard
symbols may have been left off the label through a printing mistake.
Please refer also to CLP FAQ ID=240 available at https://echa.europa.eu/support/qas-
support/qas.
4.4 Signal words
A signal word indicates the relative level of severity of a particular hazard. The label
must include the relevant signal word in accordance with the classification of the
hazardous substance or mixture: more severe hazards require the signal word ‘Danger’
while less severe hazards require the signal word ‘Warning’ (see CLP Article 20).
The signal word relevant for each specific classification is set out in the tables indicating
the label elements required for each hazard class as set out in Parts 2 to 5 of Annex I to
CLP. Some hazard categories, like explosives, division 1.6, do not have a signal word.
Where a substance or mixture is classified for more than one hazard, the label must only
bear one single signal word. In such cases, the signal word ‘Danger’ takes precedence
and the signal word ‘Warning’ must not appear.
4.5 Hazard statements
CLP hazard labels must also bear the relevant hazard statements describing the nature
and severity of the hazards of a substance or mixture (see CLP Article 21).
The hazard statements relevant for each hazard class and category/differentiation are
set out in the tables contained in Parts 2 to 5 of Annex I to CLP. An example is the

Guidance on Labelling and Packaging
Version 4.2 – March 2021
31
hazard statement H302 (“Harmful if swallowed”) assigned to acute oral toxicity, category
4. The wording for hazard statements is given in Tables 1.1, 1.2 and 1.3 of Annex III to
CLP.
In some cases, additional information to complement a hazard statement
22
may need to
be provided, such as the specification of the route of exposure or of the target organ for
certain health hazards, i.e. for the CMR, STOT SE (categories 1 and 2) and STOT RE
hazard classes. For example:
• for STOT RE category 1, the hazard statement H372 (“Causes damage to organs
through prolonged or repeated exposure”) must be complemented by the organs
affected if known and by the route of exposure if it is conclusively proven that no
other routes of exposure cause the hazard, e.g. H372 (“Causes damage to the
liver through prolonged or repeated dermal exposure”);
• for STOT SE category 1, the route of exposure or the target organ may have to
be included in the statement as well, e.g. H370 (“Causes damage to the liver via
ingestion”).
For reproductive toxicity, hazard statements H360 (“May damage fertility or the unborn
child”) and H361 (“Suspected of damaging fertility or the unborn child”) indicate a
general concern. These general hazard statements can be replaced by the hazard
statements indicating the specific effect of concern, if known, in accordance with section
1.1.2.1.2 of Annex VI to CLP (e.g. H360F “May damage fertility”, H361d “Suspected of
damaging the unborn child”, H360Df “May damage the unborn child. Suspected of
damaging fertility”).
If a substance classification is harmonised and included in Part 3 of Annex VI to CLP, the
corresponding hazard statement(s) relevant for this classification have to be used on the
label. Note that certain harmonised classifications marked with an asterisk in Part 3 of
Annex VI to CLP are minimum classifications and, based on available data, a more
severe classification as well as the corresponding hazard statement may need to be
assigned. Also, hazard statements may need to be included for the non-harmonised
parts of the classification of the same substance, i.e. for the hazard classes or
differentiations not covered in the Annex VI listing (see CLP Article 4(3)).
Table 1.2 of Annex III to CLP defines which combined hazard statements are allowed
23
.
Currently, combinations are allowed for acute toxicity hazard statements that relate to
different routes of exposure, but to the same category. Such statements can appear on
the label and in the SDS, for example for category 3 for the oral and dermal route
H301+H311 (“Toxic if swallowed or in contact with skin”).
If a substance or mixture is classified in several hazard classes or differentiations of a
hazard class, all hazard statements resulting from the classification must appear on the
label, unless there is evident duplication or redundancy (see CLP Article 27). For
example, if the hazard statement H314 (“Causes severe skin burns and eye damage”) is
assigned, H318 (“Causes serious eye damage”) may be omitted (see also section 3.3.4
of the Guidance on the application of the CLP criteria). Similarly, if the hazard statement
H410 (“Very toxic to aquatic life with long lasting effects”) is assigned, H400 (“Very toxic
to aquatic life”) may be omitted (see also section 4.1.6 of the Guidance on the
application of the CLP criteria). Duplication or redundancy should also be avoided for a
substance or mixture that is assigned the supplemental hazard statement EUH071
22
Please note that this does not constitute supplemental labelling information in the meaning of
CLP Article 25. It is rather additional hazard information that is required to be included within the
hazard statement itself, beyond the standardised wording.
23
Commission Regulation (EU) No 286/2011 of 10 March 2011.

32
Guidance on Labelling and Packaging
Version 4.2 – March 2021
(“Corrosive to the respiratory tract”)
24
. In this case, the hazard statement H335 (“May
cause respiratory irritation”) for STOT SE category 3 (respiratory tract irritation) should
be omitted from the label. Please note that the information provided on the hazard label
and in Section 2.2 of the SDS, for the same substance or mixture, must be consistent.
The correct wording of the hazard statements as it has to appear on the label is given in
Annex III to CLP, in all EU languages. The hazard statements of one language must be
grouped together with the precautionary statements of the same language on the label
(see section 3.3 of this guidance document).
4.6 Precautionary statements
CLP hazard labels must bear the relevant precautionary statements giving advice on
measures to prevent or minimise adverse effects to human health or the environment
arising from the hazards of a substance or mixture (see CLP Article 22). An example is
the precautionary statement P373 (“DO NOT fight fire when fire reaches explosives”).
The complete set of precautionary statements relevant for each hazard class and
category/differentiation is listed by alphanumeric code in the tables indicating the label
elements required for each hazard class in Parts 2 to 5 of Annex I to CLP.
Precautionary statements must be selected in line with the provisions set out in CLP
Articles 22 and 28 and with Part 1 of Annex IV to CLP: any selection must take into
account the hazard statements used, the intended or identified use(s) of the substance
or mixture, as well as the basic instructions specified in the “conditions for use” columns
in Tables 6.1 – 6.5 of Annex IV to CLP. Duplication and redundancy should be avoided.
Where the substance or mixture is supplied to the general public, one precautionary
statement addressing the disposal of that substance or mixture as well as the disposal of
packaging must in general
25
appear on the label (see CLP Article 28(2)). Normally, not
more than six precautionary statements must appear on the label, unless necessary to
reflect the nature and the severity of the hazards (see Example C in section 7.4 of this
guidance document).
For assistance with the selection of the most appropriate P-statements, please refer to
section 7 of this guidance document.
Part 2 of Annex IV to CLP lists, in all EU languages, the correct wording of the
precautionary statements as it must appear on a label. Where there are different
translations of P-statements, the translation in the national version of the CLP Regulation
usually gives the most relevant wording. The precautionary statements of one language
have to be grouped together with the hazard statements of the same language on the
label (see section 3.3 of this guidance document).
24
See also Note 1 of Table 3.1.3 in Annex I to CLP.
25
If it is clear that the disposal of the substance or mixture or the packaging does not present a
hazard to human health or the environment, a P-statement addressing disposal is not required.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
33
4.7 Codes for hazard and precautionary statements
Hazard and precautionary statements are codified using a unique alphanumerical code,
which consists of one letter and three numbers, as follows:
• the letter “H” for “hazard statement” or “P” or “precautionary statement”;
• for hazard statements, the first digit designating the type of hazard (2 for physical
hazards, 3 for health hazards and 4 for environmental hazards) and the next two
digits corresponding to the sequential numbering of hazards, as the codes from 200
to 210 for explosivity, the codes from 220 to 230 for flammability, etc.
• risk phrases carried through from the DSD and DPD, but which are not yet included
in the UN GHS, are codified as “EUH”;
• for precautionary statements, a digit reflecting one of the five types of statements,
namely general statements (1), prevention statements (2), response statements (3),
storage statements (4) and disposal statements (5), followed by two digits for the
sequential numbering of the statements themselves.
The code ranges for the hazard and precautionary statements under the CLP Regulation
are set out in Table 4 below:
Table 4: Code ranges of hazard and precautionary statements under the CLP
Regulation
Hazard Statements: H
Precautionary Statements: P
200 – 299 Physical hazard
100 – 199 General
300 – 399 Health hazard
200 – 299 Prevention
400 – 499 Environmental hazard
300 – 399 Response
400 – 499 Storage
500 – 599 Disposal
The codes of the hazard and precautionary statements and EUH statements are not
necessary for the label. The CLP Regulation only requires the actual phrasing of the
applicable statements on the label.
4.8 Supplemental labelling information
CLP Article 25 defines the concept of ‘supplemental information’ which is intended to
incorporate additional labelling information over and above that listed in CLP Article
17(a) to (g). This additional labelling information can be divided into two categories,
namely obligatory and non-obligatory information. Please note that, according to CLP
Article 25(6), supplemental labelling information might be obligatory for a mixture, even
if not classified as hazardous.
All ‘supplemental information’ must generally be located in the section for supplemental
information on the label. Both obligatory and non-obligatory supplemental information
have to appear in the same languages as the other CLP label elements.

34
Guidance on Labelling and Packaging
Version 4.2 – March 2021
As it is obligatory to place this information alongside the label elements required by CLP
Article 17(a) to (g), these supplemental label elements need to be considered carefully
as to the location and the space they need when preparing a CLP label for a substance or
mixture (see also Example 3 under section 6 of this guidance document).
Commission Regulation (EU) 2017/542 amended CLP to include the requirement for a
Unique Formula Identifier (UFI) as supplemental information on the label under Article
25(7) (see section 4.8.1.1 of this guidance document)
26
. However, there are no fixed
rules concerning the positioning of the UFI on the label: it can either be located in the
section for ‘supplemental information’ on the label, as described above, or be placed
(with the “UFI:” marker) in proximity of the product name or trade name. For practical
reasons, the UFI could also be printed on the packaging, as long as it remains in
proximity of the other labelling information. In any case, the UFI should be clearly visible
and easy to locate in case of an emergency (its main function is to help the emergency
responder in the identification of the mixture contained in the product). In the case of
bespoke paints for which a submission in accordance of Annex VIII has not been made
(and no UFI has been generated), the UFIs of the hazardous components have to be
included on the label (see section 5.3.3 for more details on special provisions for
bespoke paints).
4.8.1 Obligatory supplemental labelling information
Obligatory supplemental labelling information includes:
• Supplemental hazard statements relating to particular physical and health
properties. These are codified as “EUH” statements, e.g. EUH014 “Reacts
violently with water”. For some substances with harmonised classifications, the
supplemental hazard statements are included in Part 3 of Annex VI to CLP;
• Supplemental statements for certain mixtures, e.g. EUH204 “Contains
isocyanates. May produce an allergic reaction” (see Part 2 of Annex II to CLP).
These phrases are assigned EUH codes as well, to align their presentation with
the above supplemental hazard statements;
• The supplemental statement EUH401 “To avoid risks to human health and the
environment, comply with the instructions for use” for hazardous substances and
mixtures within the scope of Directive 91/414/EEC
27
(see Part 4 of Annex II to
CLP);
• Label elements resulting from other EU acts (see CLP Article 32(6)), for example:
– the authorisation number requested by the REACH Regulation;
26
Commission Regulation (EU) 2017/542 (as amended by Commission Delegated Regulation (EU)
2020/11 and Commission Delegated Regulation 2020/1677 and Commission Delegated Regulation
2020/1676 of 31 August 2020 (the “workability amendments”) also amended the CLP Regulation
by adding Annex VIII.
27
Repealed and replaced by Regulation (EC) No 1107/2009 of the European Parliament and of the
Council of 21 October 2009 concerning the placing of plant protection products on the market with
effect from 14 June 2011.
Obligatory supplemental information, when included, must be easy to identify
and read. Naturally, it has precedence over any non-obligatory supplemental
information if space on the label is limited.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
35
– the listing of surfactants and perfumes according to the Regulation (EC)
No 648/2004 on detergents, as amended;
– the authorisation number of the biocidal product according to the Biocidal
Products Regulation (EU) No 528/2012;
– the labelling provisions (i.a. flammability) of the Aerosol Dispensers
Directive 75/324/EEC (ADD), as amended; or
– the content of volatile organic compounds (VOCs) in accordance with
Directive 2004/42/EC
28
.
Further additional obligatory information can include:
• Specific response information as referred to in the brackets of the precautionary
statements P320 “Specific treatment is urgent (see … on this label)”, P321
“Specific treatment (see … on this label)” in Annex IV to CLP, e.g. “see
supplemental first aid instructions on this label” or “see supplemental instructions
on the administration of antidotes on this label”. See also Table 5 below and the
selection tables in section 7.3 of this guidance document;
• For mixtures containing components of unknown acute toxicity at a concentration
of 1% or greater, the statement “x percent of the mixture consists of
component(s) of unknown acute toxicity” (see section 3.1.3.6.2.2 of Annex I to
CLP). This statement has also to be included in the SDS, when this is provided
29
.
In addition, it may be appropriate to differentiate the hazard based on the route
of exposure, for example “x percent of the mixture consists of ingredient(s) of
unknown acute (oral/dermal/inhalation) toxicity”, in particular where the
substance is also classified for other hazards and where it is important to specify
the route of exposure (see also the Guidance on the application of the CLP
criteria);
• For mixtures for which no useable information on the short-term (acute) and/or
long-term (chronic) aquatic hazard is available for one or more of the relevant
components, the statement “Contains x percent of components with unknown
hazards to the aquatic environment” (see section 4.1.3.6.1 of Annex I to CLP).
This statement has to be included on the label and in the SDS;
• For mixtures subject to submission requirements under CLP Article 45 and Annex
VIII to CLP, a UFI, where applicable. Special provisions may apply to bespoke
paints. (see section 4.8.1.1 of this guidance document).
The CLP Regulation requires supplemental label information to be located in a specific
supplemental information section on the label. A supplier may also choose to place the
supplemental information in several locations, taking into account the requirements of
CLP Article 25 (see Example 3 and Example 5 in section 6 of this document).
Similarly, the section for supplemental label information should be visibly separated from
the labelling elements according to CLP Article 17(a) to (g), e.g. by placing it in another
section of the label, by putting it in a text box, using a different colour or using a
28
Directive 2004/42/EC of the European Parliament and of the Council of 21 April 2004 on the
limitation of emissions of volatile organic compounds due to the use of organic solvents in certain
paints and varnishes and vehicle refinishing products and amending Directive 1999/13/EC.
29
For further information on the compilation of the SDS, please consult the Guidance on the
compilation of safety data sheets.

36
Guidance on Labelling and Packaging
Version 4.2 – March 2021
different letter size. However, on a case-by-case basis, it may not be advisable to make
a visible differentiation between the CLP elements and obligatory supplemental labelling
information that is requested by another legislation, where the latter supports the safe
handling and use of a substance or mixture. For example, where additional EUH
statements express a warning similar to that contained in the hazard statements that
reflect a classification, it is even advisable to group both types of statements together on
the label so that they reinforce each other. For example, for a substance that is classified
as water-reactive category 1, the hazard statement EUH014 “Reacts violently with
water.” is very similar to H260 “In contact with water releases flammable gases which
may ignite spontaneously.” (see also Example 4 in section 6 of this guidance document).
In relation to readability, obligatory labelling information required by other EU legislation
(e.g. the content of volatile organic compounds as required by Directive 2004/42/EC or
the listing of specified constituents as required by Regulation (EC) No 648/2004) must
not be treated differently from other obligatory labelling information required by the CLP
Regulation itself. Obligatory information must be easy to identify and read and must take
precedence on the CLP label over any other non-obligatory supplemental information. An
overview of the obligatory supplemental label elements to be included in the section for
supplemental information on the label is provided in Table 5.
Table 5: Obligatory supplemental labelling information pursuant to CLP Articles
25 and 32
Legal Reference
Type and Applicability
Code
Content / Phrasing
CLP Article
25(1) and Annex
II, Part 1,
section 1.1
a) Supplemental hazard statements relating to certain physical
properties of substances and mixtures. They need to be assigned in
accordance with the conditions specified in Annex II to CLP when a
substance or mixture has already been classified on the basis of the
criteria in Annex I to CLP. For some substances with harmonised
classifications, supplemental hazard statements are included in Part 3
of Annex VI to CLP.
EUH014
‘Reacts violently with water’
EUH018
‘In use, may form flammable/
explosive vapour-air mixture’
EUH019
‘May form explosive peroxides’
EUH044
‘Risk of explosion if heated
under confinement’

Guidance on Labelling and Packaging
Version 4.2 – March 2021
37
Legal Reference
Type and Applicability
Code
Content / Phrasing
CLP Article
25(1) and Annex
II, Part 1,
section 1.2
b) Supplemental hazard statements relating to health properties of
substances and mixtures. They need to be assigned in accordance
with the conditions specified in Annex II to CLP, Part 1, section 1.2,
when a substance or mixture has already been classified on the basis
of the criteria in Annex I to CLP. For some substances with
harmonised classifications, supplemental hazard statements are
included in Part 3 of Annex VI to CLP. For EUH071, see also Annex I to
CLP, Table 3.1.3, Note 1.
EUH029
‘Contact with water liberates
toxic gas’
EUH031
‘Contact with acids liberates
toxic gas’
EUH032
‘Contact with acids liberates
very toxic gas’
EUH066
‘Repeated exposure may cause
skin dryness or cracking’
EUH070
‘Toxic by eye contact’
EUH071
‘Corrosive to the respiratory
tract’
CLP Article
25(6) and Annex
II, Part 2
Supplemental statements for certain mixtures. They need to be assigned to
mixtures in accordance with the conditions specified in Annex II to CLP, Part
2.
1. Mixtures containing lead
EUH201
‘Contains lead. Should not be
used on surfaces liable to be
chewed or sucked by children’
– for packaging content
less than 125 ml
EUH201A
‘Warning! Contains lead’
2. Mixtures containing
cyanoacrylates
EUH202
‘Cyanoacrylate.
Danger.
Bonds skin and eyes in
seconds.
Keep out of the reach of
children.’
3. Cement and cement
mixtures
EUH203
‘Contains chromium (VI). May
produce an allergic reaction’

38
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Legal Reference
Type and Applicability
Code
Content / Phrasing
4. Mixtures containing
isocyanates
EUH204
‘Contains isocyanates.
May produce an allergic
reaction’
5. Mixtures containing
epoxy constituents with
an average molecular
weight ≤ 700
EUH205
‘Contains epoxy constituents.
May produce an allergic
reaction’
6. Mixtures sold to the
general public which
contain active chlorine
EUH206
‘Warning!
Do not use together with other
products.
May release dangerous gases
(chlorine)’
7. Mixtures containing
cadmium (alloys) and
intended to be used for
brazing or soldering
EUH207
‘Warning!
Contains cadmium. Dangerous
fumes are formed during use.
See information supplied by the
manufacturer.
Comply with the safety
instructions.’
8. Mixtures not classified as
sensitising but
containing at least one
sensitising substance
30
EUH208
‘Contains (name of sensitising
substance). May produce an
allergic reaction’
9. Liquid mixtures
containing halogenated
hydrocarbons
EUH209
EUH209A
‘Can become highly flammable
in use
or
Can become flammable in use’
10. Mixtures not intended
for the general public
EUH210
‘Safety data sheet available on
request’
11. Aerosols
Aerosols are also subject to the
labelling provisions of Directive
75/324/EEC
30
According to the last paragraph of Section 2.8 of Annex II to CLP (introduced by Commission
Regulation (EU) No 286/2011 (2
nd
ATP to the CLP Regulation)), mixtures classified as sensitising
containing other substance(s) classified as sensitising (in addition to the one that leads to the
classification of the mixture) and present in a concentration equal to or greater than that specified
in Table 3.4.6 of Annex I to CLP must bear the name(s) of that/those substance(s) on the label.
This (these) name(s) should be placed together with the name(s) of the substance(s) relevant to
classification of the mixture. Note that EUH208 must be used when a mixture not classified as
sensitising contains sensitising substances. However, according to Commission Regulation (EU)
2016/918 (8
th
ATP to the CLP Regulation), where a mixture is labelled with EUH204 in accordance
with Section 2.4 of Annex II to CLP or EUH205 in accordance with Section 2.5 of Annex II, the
statement EUH208 may be omitted from the label when the only substances triggering EUH208
are isocyanates or epoxy constituents.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
39
Legal Reference
Type and Applicability
Code
Content / Phrasing
CLP Annex IV
Substances and mixtures
assigned the precautionary
statements:
• P320 - Specific treatment
is urgent (see … on this
label)
• P321 - Specific treatment
(see … on this label)
Supplemental first aid
instruction (e.g. administration
of an antidote or immediate
measures such as specific
cleansing agent) referred to in
the brackets of the
precautionary statements
CLP Annex I,
section
3.1.3.6.2.2
Mixture containing
ingredient(s) of unknown
acute toxicity at a
concentration at 1% or
greater
‘x percent of the mixture
consists of component(s) of
unknown acute toxicity’
(also for safety data sheet)
CLP Annex I,
section 4.1.3.6.1
Mixture where no useable
information on the
short-term (acute) and/or
long-term (chronic) aquatic
hazard is available for one
or more of the relevant
components
‘Contains x percent of
components with unknown
hazards to the aquatic
environment’.
(also for safety data sheet)
CLP Article
25(2)
Supplemental statement for
substances and mixtures
within the scope of Directive
91/414/EEC
31
EUH401
‘To avoid risks to human health
and the environment, comply
with the instructions for use’.
Label elements
resulting from
other
Community acts
pursuant to CLP
Article 32(6)
Examples:
Regulation (EC) No
1907/2006 (REACH)
Regulation (EC) No
648/2004 (detergents)
Directive 75/324/EEC on
aerosol dispensers (ADD)
authorisation number
labelling statements related
to restrictions in Annex XVII
of REACH, e.g. 'Restricted
to professional users'
listing of specified
constituents such as anionic
surfactants, oxygen
bleaching agents, enzymes,
disinfectants, optical
brighteners and perfumes
i.a. flammability labelling
content of volatile organic
compounds
31
Repealed by Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21
October 2009 concerning the placing of plant protection products on the market with effect from
14 June 2011.

40
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Legal Reference
Type and Applicability
Code
Content / Phrasing
Directive 2004/42/EC on
volatile organic
compounds (VOCs)
Biocidal Products
Regulation (EU) No
528/2012
for example: authorisation
number of the biocidal
product
CLP Article
25(7) and
Section 5, Part A
of Annex VIII
32
Unique formula identifier
(UFI) for mixtures classified
for health or physical effects
and subject to submission
requirements following CLP
Article 45 (see section
4.8.1.1 of this guidance
document)
n/a
Unique 16-digit alphanumeric
code
33
, for example:
UFI: VDU1-414F-1003-1862
CLP Article
25(8) and
Section 2.2.a,
Part B of Annex
VIII,
Unique formula identifier
(UFI) for mixture
components of bespoke
paints, subject to Article 45
and present in a
concentration exceeding
0.1% (see section 5.3.3 of
this Guidance document).
n/a
See above
4.8.1.1 Unique formula identifier (UFI)
A Unique Formula Identifier (UFI) is a unique alphanumeric code that links the
information on a mixture submitted under CLP Article 45 to a specific product placed on
the market (for further information, see ECHA’s Poison Centres website at:
https://poisoncentres.echa.europa.eu/).
The UFI is mandatory for all hazardous mixtures that require the submission of
information according to CLP Article 45, i.e. all the mixtures that are placed on the EU
market and classified under CLP as hazardous based on their health or physical effects.
Importers and downstream users are duty holders under Article 45 and are required to
include the UFI on the label of the mixture before placing it on the market. Distributors
may also have obligations under CLP Article 4(10), see section 3.1 of the Guidance on
harmonised information relating to emergency health response – Annex VIII to CLP.
Certain exemptions apply, more details are given in the same section of that guidance.
The UFI is considered as obligatory supplemental labelling information according to
Article 25(7) of the CLP Regulation, and should be included in the label section dedicated
to supplemental information. However, by way of derogation from Article 25(7) of the
32
See Commission Regulation (EU) 2017/542.
33
For more information, see the User guide for the UFI generator and additional support material
available on the ECHA Poison Centres website at at https://poisoncentres.echa.europa.eu/ufi-
generator.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
41
CLP Regulation, Article 29(4a)
34
allows to include the UFI in another way. The different
options are specified in Part A Section 5 of Annex VIII. Hence, while normally
supplemental labelling information should be located in the section for ‘supplemental
information’ on the label, the UFI can alternatively even be placed outside the label, by
being printed or affixed directly onto the inner packaging. The UFI must however be
located with the obligatory CLP label elements such as the product identifiers or hazard
information. The UFI should be clearly identifiable
35
. Where a UFI is added separately to
a label or packaging, the person responsible should ensure that it remains firmly
attached during normal handling and use.
When the inner packaging is in such a shape or so small that it is not possible to affix
the UFI on it, then the label elements including the UFI should be provided either on
fold-out labels, or tie-on tags, or an outer packaging (in accordance with section 1.5.1 of
Annex I to CLP, see section 5.3.1 of this guidance document), always with the other
label elements.
It should be noted that although CLP considers the UFI as supplemental information, it is
included in Section 1.1 (product identifier) of the SDS, in accordance with Annex II to
REACH, where applicable. Please note that the inclusion of the UFI in the SDS is only
required in specific cases, otherwise it is voluntary, see section 4.2 of the Guidance on
harmonised information relating to emergency health response – Annex VIII to CLP).
The UFI code must be preceded by the acronym “UFI:”, i.e. in capital letters, followed by
a colon.
The UFI must be clearly visible, legible and indelibly marked (see section 5.2 of this
guidance document where legibility, readability and size of the label elements are
described).
For mixtures not yet notified under national legislation, the use of a UFI will apply from 1
January 2021, in a stepwise manner, depending on the use of the mixture (see section
3.4.1 of the Guidance on harmonised information relating to emergency health response
– Annex VIII to CLP for more details on the compliance dates). If there is a need to
update an existing notification, please see section 3.5.2 of the Guidance on harmonised
information relating to emergency health response – Annex VIII to CLP.
Mixtures already notified under the national schemes where the submitted information
was not in accordance with Annex VIII, do not need to be re-labelled for the purpose of
including a UFI until 1 January 2025. However, if a submission update is needed before
that date, e.g. the mixture composition changes in accordance with certain criteria
(reasons for update are listed in Section 4.1, Part B of Annex VIII, see further details in
section 4.2.7 of Guidance on harmonised information relating to emergency health
response – Annex VIII to CLP), the company is required to comply with the Annex VIII
requirements and to re-label its mixtures with the UFI codes or affix the UFI codes to the
label (or on packaging right next to other obligatory CLP label elements) before placing
the mixtures, as changed, on the market. If a company voluntarily submits the
34
Regulation (EU) 2020/11 amended CLP by adding the new paragraph 4a to Article 29 (Exemption
from labelling and packaging requirements).
35
See specific section 5.3.1.1. for specific cases when the use of fold-out labels and tie-on tags are
allowed.

42
Guidance on Labelling and Packaging
Version 4.2 – March 2021
information in accordance with Annex VIII to CLP ahead of the applicable deadline
36
even
if has no obligation to do so, it is recommended to include the UFI on the label without
undue delay.
Please note that:
• For hazardous mixtures that are subject to the submission of information under
CLP Article 45, the UFI needs to be reflected on the label or on the packaging
located with other obligatory CLP labelling information.
• For hazardous mixtures used at industrial sites
37
, the UFI may alternatively be
indicated in Section 1.1 of the SDS.
• For hazardous mixtures sold unpackaged
38
, the UFI must be indicated in Section
1.1 (product identifier) of the SDS in accordance with Annex II of REACH
39
.
• For hazardous mixtures listed in Part 5 of Annex II to CLP the UFI must be
included in a copy of the label elements provided for in Article 29(3), e.g.
attached to the delivery note.
• For hazardous mixtures used for scientific research and development (SR&D), or
for product and process oriented research and development, a UFI is not required
as they are outside the scope of Annex VIII.
• For mixtures classified as ‘gases under pressure’ and/or ‘explosives (unstable
explosive and Divisions 1.1 to 1.6)’ only, a UFI is not required as these hazard
classes outside the scope of Annex VIII.
• For mixtures classified as hazardous to the environment only, a UFI is generally
not required as these hazard classes are outside the scope of CLP Article 45.
However, companies can voluntarily make a submission and include a UFI on the
label and are recommended to do so.
• In the case of multi-layered packaging, the UFI is allowed to be included on the
inner packaging only (by way of derogation from Article 25(7) of the CLP), on the
label or on the packaging located with the obligatory CLP label elements;
• A company may consider making submissions for mixtures that are outside the
scope of CLP Article 45 (for example, hazardous for the environment only). In
that case, the UFI may voluntarily be submitted and put on the label of these
mixtures (and it is recommended).
36
The readiness of each appointed body to receive the submission in the new format should be
checked with the relevant authority. ECHA has published on the Poison Centres website an
Overview of Member States decisions on implementing Annex VIII (at
https://poisoncentres.echa.europa.eu/echa-submission-portal).
37
Regardless of its possible inclusion downstream in mixtures intended for professional or
consumer uses.
38
e.g. mixtures listed in Part 5 of Annex II to CLP.
39
Annex II to REACH as amended by Commission Regulation (EU) 2020/878 which includes
reference to the UFI.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
43
An online tool to create and validate UFI codes, the UFI Generator, is available on the
Poison Centres website at https://poisoncentres.echa.europa.eu/ufi-generator. More
information and Manuals are available at https://poisoncentres.echa.europa.eu/ufi-
generator.
4.8.2 Non-obligatory supplemental labelling information
In some cases, suppliers may need to include certain elements on the label that are not
obligatory but are necessary for the handling and use of the product, for example
specific product information, basic instructions for use or P-statements that do not arise
directly from the classification of the product (e.g. “Read label before use” or “Do not get
in eyes” for eye irritant mixtures). Such non-obligatory supplemental labelling
information, the content of which is at the discretion of the supplier, is not part of the
labelling requirements under the CLP Regulation.
The need for non-obligatory information should also be taken into account when deciding
how to lay out the label. The non-obligatory supplemental information may also be
placed alongside the label elements required in CLP Article 17(a) to (g) and the
obligatory supplemental information, when applied. However, such information must not
be confusing to the user or contradict the obligatory label elements. It should also
provide further necessary details (see CLP Article 25(3)).
Additional labelling elements that come from the UN GHS but are not implemented in the
CLP Regulation may be included in the section for non-obligatory supplemental
information. These elements must not confuse the user.
In addition, any non-obligatory supplemental information, either included on the label or
on the packaging, must be consistent with the classification of the substance or mixture
(see CLP Article 25(4)). This means that statements such as ‘non-toxic’, ‘non-polluting’
or ‘ecological’, or other statements suggesting that the substance/mixture is not
hazardous or statements that are incompatible with the assigned classification must not
appear on the label or packaging of a classified substance or mixture.
5. Guidance on particular aspects of CLP hazard labelling
5.1 Further aspects to consider for the CLP hazard label
To enable the supplier to design labels in compliance with the CLP Regulation while at
the same time allowing for as much freedom in arranging labels as possible, further
labelling aspects should be considered.
• Label size: the CLP Regulation defines minimum dimensions for the size of the
label and some of its elements (see section 5.2 of this guidance document);
• Specific labelling rules that refer to specific labelling and packaging situations,
for example:
– a substance or mixture is contained in awkwardly shaped or small
packaging (see CLP Article 29),
– the packaging consists of multiple layers, and/or
– a substance or mixture is subject to the labelling provisions of the CLP
Regulation and to labelling provisions in accordance with the rules

44
Guidance on Labelling and Packaging
Version 4.2 – March 2021
on the transport of dangerous goods according to the UN
Recommendations on the Transport of Dangerous Goods – Model
Regulations (the so-called "Orange Book")
40
. The person responsible for
compiling a CLP label needs to consider all of these rules before making a
final decision on the label of the substance or mixture (see CLP Article 33);
• Selection of precautionary statements:
The selection of the most appropriate set of precautionary statements for the
label is largely at the discretion of the supplier. Please refer to section 7 of this
guidance document.
5.2 Size of the label and of the label elements
Section 1.2 of Annex I to CLP defines the label size, setting out minimum dimensions
for the label, with the pictogram size being linked to these minimum dimensions (see
also Table 6 below)
41
. Nevertheless, the label should be large enough to contain all the
label elements defined by the CLP Regulation while remaining legible. As a result, the
label may need to be larger than the minimum area specified.
Table 6: Minimum dimensions of labels and pictograms under the CLP
Regulation
Capacity of the package
Dimensions of the label
(in millimetres) for the
information required by CLP
Article 17
Dimensions of the
pictogram (in
millimetres)
≤ 3 litres
If possible, at least 52 x 74
Not smaller than 10 x 10
If possible, at least 16 x 16
> 3 litres but ≤ 50 litres
At least 74 x 105
At least 23 x 23
> 50 litres but ≤ 500 litres
At least 105 x 148
At least 32 x 32
> 500 litres
At least 148 x 210
At least 46 x 46
The CLP Regulation requires that the label elements as referred to in CLP Article 17(1)
be of such size and spacing as to be easily read.
Readability is determined by the combination of font size, letter spacing, spacing
between lines, stroke width, type colour, typeface, width-height ratio of the letters, the
surface of the material and significant contrast between the print and the background.
Some examples of the influence of these parameters on readability are shown in Figure 2
below.
40
Implemented in the EU through international modal agreements and Directive 2008/68/EC.
41
The size of the pictogram relates here to the dimensions of the pictogram itself, and not to the
size of the virtual square into which the pictogram is placed.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
45
Figure 2: Readability
A label may accommodate more language(s) than those required by the Member State
where the substance or mixture is placed on the market. As long as the label complies
with the (minimum) dimensions set out in Table 6 above and as long as legibility of the
text elements is warranted, the decision on the number of languages is at the discretion
of the respective supplier.
The exact size of the letters of the signal words, hazard statements, precautionary
statements and any supplemental information is not further defined in the legal text, i.e.
it is up to the supplier to determine the size of the letters that allows the label elements
to be easily read. However, the minimum letter size of 1.2 mm (‘x-height’) can be used
as a reference. A supplier may decide whether to increase the letter size with the overall
volume of the packaging and dimensions of the label, or to fix it more or less for all
volumes and labels.
Similarly, a supplier may decide whether to have larger letter sizes for certain label
elements while others are presented in smaller letters. Some practical options often
chosen are for example:
• providing the signal word “Danger” or “Warning” in larger letters on the label than
for the hazard and precautionary statements,
• presenting the obligatory label elements in larger letters than for the non-
obligatory labelling information.
Both of the above-mentioned options are in principle compatible with the CLP legal text
as long as the obligatory information on the label can be easily read.
The CLP Regulation links the size of the hazard pictograms to the minimum
dimensions of the label. Each hazard pictogram must cover at least one fifteenth of the
minimum surface area of the label dedicated to obligatory labelling information. The
minimum dimensions of labels and pictograms are given in Table 1.3 of Annex I to CLP.
The area of the pictogram for the smallest capacity of the package should be at least 16
mm x 16 mm, if possible, but must never be less than 1 cm
2
. The pictogram size should
be increased from the minimum dimensions where the actual label size allows this. The
idea behind this is that the label size and the size of the pictograms should remain
proportional to the size of the packaging.
A pictogram covering one fifteenth of the minimum surface area, obtained by multiplying
the dimensions as defined in Table 1.3 of Annex I to CLP, is considered to be legible. The
pictogram size has to be increased in all cases where it occupies less than one fifteenth
of the surface area of the label dedicated to the obligatory labelling information. For
small packaging, one fifteenth of the minimum size label is 16 mm x 16 mm. However,
sometimes even the minimum label size cannot be applied or the minimum size label can
only accommodate 10 mm x 10 mm pictograms (e.g. due to several pictograms). These
1-cm
2
pictograms are the smallest allowed and can be used only if there is no space for
the larger ones. A pictogram of at least 16 mm x 16 mm must always be used if
possible. “If possible” refers to the size of the label and thus if the label size allows for a
larger pictogram, then this must be used. However, where a supplier chooses to use a
label that is larger than the minimum dimensions for a certain capacity of the package, it

46
Guidance on Labelling and Packaging
Version 4.2 – March 2021
is not necessary to increase also the size of the pictogram, provided it covers one
fifteenth of the relevant minimum dimensions.
In principle, a label complying with the minimum dimensions set out above should be
large enough to contain all the label elements defined in CLP Article 17 while remaining
legible. Precedence must be given to the obligatory label elements and any obligatory
supplemental information required by the CLP Regulation and other EU legislation. If a
supplier chooses to add non-obligatory supplemental label elements, legibility may be
affected when more than just a small amount of such information is added. For larger
amounts of non-obligatory information the supplier should consider limiting this
information or increasing the size of the label. When the size of the label is increased,
the supplier should also consider increasing the size of the different obligatory label
elements. This should serve the purpose of facilitating their identification and
maintaining their legibility.
Any additional area gained by increasing the size of the label can be used for further
information which is considered important by the supplier. However, this should be
weighed against the requirement of CLP Article 25(3), namely that non-obligatory
supplemental information must not make it more difficult to identify the obligatory label
elements.
5.3 Exemptions from the labelling and packaging requirements
Not all packages allow the necessary labelling information on the label or on the
packaging to be displayed in line with the requirements of CLP Article 31.
CLP Article 29(1) and section 1.5.1 of Annex I to CLP provide derogations for a
packaging that is so small or in such a shape or form that it is impossible to meet the
requirements of CLP Article 31.
If the provisions of Article 29(1) cannot be applied, CLP Article 29(2) and section 1.5.2 of
Annex I to CLP allow the omission of certain label elements (see section 5.3.2 of this
guidance document).
5.3.1 Use of fold-out labels, tie-on tags and outer packaging
The packaging of a substance or mixture can be so small or in such a shape or form that
it is impossible to display the label elements in line with the requirements of CLP Article
31. This could be either because the Member State where the substance or mixture is
being placed on the market requires more than one language on the label, or simply
because the packaging is too small or difficult to label because of its form/shape so that
the full range of labelling elements even in a single language cannot be displayed.
Example:
For a container of a capacity > 50 litres, but ≤ 500 litres, the minimum size of a
pictogram must be 32 mm x 32 mm, which is one fifteenth of the area obtained by
multiplying the minimum dimensions (105 mm x 148 mm). (105 mm x 148 mm =
10.5 cm x 14.8 cm = 155.5 cm
2
. Then one fifteenth of 155 cm
2
= 10.36 cm2;
√10.36 cm
2
= 3.22 cm = 32.2 mm (rounded to 32 mm) for each dimension of each
pictogram). If the size of the label increases while the capacity of the container
remains the same (>50 litres, but ≤500 litres) the minimum size of each pictogram
should be at least one fifteenth of the minimum area related to obligatory
information required by CLP Article 17, i.e. 32 mm x 32 mm.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
47
In particular, it may be impossible for the label to be read horizontally when the package
is set down normally or the label elements are of insufficient size and spacing as to be
easily read.
In this situation the label elements defined in CLP Article 17 may be provided either on:
• fold-out labels; or
• tie-on tags; or
• outer packaging.
When one of the above-mentioned alternatives is used, the label on any inner packaging
or the part of the fold-out label that is directly attached to the packaging must contain at
least: the hazard pictogram(s), the product identifier referred to in CLP Article 18 and
the name and telephone number of the supplier of the substance or mixture. In this
case, the signal word, the hazard and precautionary statements as well as the
supplemental label information may be omitted. However, the use of these alternatives
is not allowed if a label becomes unreadable only because the supplier wishes to add
more languages on a label than are required in the Member State where the substance
or mixture is placed on the market.
5.3.1.1 Fold-out labels and tie-on tags
When a supplier recognises the need to use fold-out labels or tie-on tags, he should
consider the following aspects:
Compared to tie-on tags, the use of fold-out labels will probably be the preferred option
as this will offer most space for the label elements in many cases. Some information
relating to the content, quality and design of a fold-out label is given below. See also
Example 6 of this guidance document where a multilingual, fold-out label for a mixture
for supply and use is presented.
Fold-out labels can also be an option (and are in fact commonly used) when the amount
of obligatory supplemental labelling information required by other legislation would result
General requirements for fold-out labels and tie-on tags
The CLP Regulation does not foresee any separate provisions for tie-on tags or fold-
out labels. Both types of label must meet the same performance standards as any
other “normal” label, namely:
• the label elements (including the UFI, if applicable) must be indelible, easy to
read and stand out from the background;
• the size of the pictograms must be the same as the pictograms on the
equivalent, normal label.
The fold-out label or tie-on tag must be securely attached to the packaging, i.e. the
label remains attached to the packaging during reasonably expected handling of the
package.
At least the following CLP information must be firmly attached to the immediate
container:
• hazard pictograms,
• the product identifier,
• the name and telephone number of the supplier of the substance or mixture.
In addition, the UFI should be attached to the immediate container.

48
Guidance on Labelling and Packaging
Version 4.2 – March 2021
in a label that is too large for the packaging. Fold-out labels may help clearly structure
the labelling information by using different pages for different types of information (see
below).
Content, quality and design of a fold-out label
Content
A fold-out label generally consists of three parts, namely the front page (top leaf), inside
page(s) and the back page (firmly attached to the packaging).
The label elements and information required by CLP Articles 17 and 32(6) should be
included on the fold-out label as described below. In accordance with CLP Article 29(1),
the labelling information can only be provided using fold-out labels when it is not
possible to meet the requirements of CLP Article 31 for a label in the languages of the
Member State in which the substance or mixture is placed on the market.
• The front page must contain at least:
o the product identifier (CLP Article 18(2) for substances, CLP Article
18(3)(a) for mixtures); Please note that for mixtures, the product
identifier on the front and back page does not need to specify all the
components contributing to the classification of the mixture;
o hazard pictogram(s) (CLP Article 17(1)(d));
o signal words in all languages of the label (CLP Article 17(1)(e));
o nominal quantity (packages made available to the general public, unless
specified elsewhere in the package) (CLP Article 17(1)(b));
o contact details of supplier(s) (name, address and phone number) (CLP
Article 17(1)(a));
o a reference to the full safety information inside the fold-out label, for
example: “safety information, see inside” in all languages of the label or a
symbol to inform a user that the label can be opened and to illustrate that
additional information is available on inside pages (not in CLP Article
17(1));
o an abbreviation of the language (country code or language code) for all
the languages that are used in the inside pages; to avoid non-standard or
confusing abbreviations it is recommended to use the language code
according to e.g. ISO 639-1;
• Inside page(s) should contain:
o full labelling information (except for the hazard pictogram and the supplier
identification) as required by CLP Article 17(1) (including supplemental
information) for each language mentioned on the front page and grouped
by language, for example one language per page;
o an abbreviation of the language featured at the top of each of the inside
pages (country code or language code).
• The back page should repeat the information given on the front page, except for
the indication of the different languages in the inner layers.
Regarding the UFI, it is advised to include it on the back page (i.e. attached to the

Guidance on Labelling and Packaging
Version 4.2 – March 2021
49
immediate container) or affixed directly to the inner packaging right next to the other
labelling elements. This will allow the identification of the UFI in case when the other
pages of the fold-out label would for some reason no longer be attached.
In addition, it is recommended to include the UFI also on the front page to be easily
identifiable.
The use of several UFI codes on the same label is not recommended. A single UFI put on
the label should be notified in all MSs where the mixture is on the market. There is always
a possibility to notify a mixture in a “new” MS with a same “old” UFI which is already
notified in another MS, and this approach is highly recommended, giving possibility to
include only one UFI on the label. However, if different UFIs are used for different market
areas, the UFIs (with a country code in proximity) should be included in the inside pages
with the language or market area. The UFI relevant for each market area should be clearly
identifiable. It should be noted that no additional marker than “UFI:” should appear before
the actual UFI code. In exceptional circumstances, when the same label is used in different
countries where different UFIs are used, a country code should be used in proximity of the
UFI code.
Quality and design
There is no standard specified in CLP for label materials and performance of fold-out
labels. However, sufficient quality of the fold-out label needs to be ensured.
The exact manner in which this quality is ensured should be left to the discretion of the
supplier, but attention should be paid to the following aspects:
• Durability
Taking into account the different situations that may occur during normal
handling and use of the packaging (the contents of the package may dissolve the
printing or the users may read the label several times), it is clear that the fold-
out label must be sufficiently durable to maintain its functionality under repeated
use conditions (as applicable) for the entire life span of the product. This can be
achieved for example by protective coating of the label and using plasticised
pages.
The back page of a fold-out label should be firmly attached to the packaging to
resist normal handling and use. The pages should not be easily detachable from
each other.
• Readability
The information in the fold-out label should be easily read (see section 5.2 of this
guidance document). In the case of a booklet, page numbers can be considered.
The languages should be ordered in a logical way, e.g. alphabetically.
• Easy access to the information
The information on the fold-out label should be easily accessible by allowing easy
opening and reclosing of the label by the user. This can be ensured for example
by using a "Pull tab”, i.e. a small area of the label that allows lifting it easily from
its backing sheet. Easy access to the information (and readability) can also be
improved by featuring one language per inner page of the fold-out label.

50
Guidance on Labelling and Packaging
Version 4.2 – March 2021
5.3.1.2 Outer packaging
When packaging is too small or in such a form or shape that the labelling requirements
of CLP Article 31 cannot be met, one of the options provided by CLP Article 29(1) is to
provide limited labelling information on the inner packaging (i.e., according to section
1.5.1.2 of Annex I to CLP, at least: hazard pictograms, product identifier and name and
telephone number of the supplier of the substance or mixture) while the full labelling
information (including the UFI) is provided on outer packaging. This may be useful in the
case of many small units within one outer packaging. In such cases, the requirements
that normally apply to labels (see CLP Articles 31 and 32) will also apply to the label area
on the outer packaging. When the outer packaging option is used, a distributor or
retailer has to take care that all the label elements required by the CLP Regulation are
available when he places the single package units individually on the market.
5.3.2 Omission of certain label elements
In situations where it is impossible to meet the labelling requirements of CLP Article 31
(because of the small size, shape or form) and the full label information
42
cannot be
provided in fold-out labels, on tie-on tags or on an outer packaging, the label information
may be reduced subject to certain conditions specified in section 1.5.2 of Annex I to
CLP. This can be the case for:
• packages where contents do not exceed 125 ml and the substance or mixture is
classified in one of the hazard categories listed in Table 7 below – this also refers
to situations when a substance or mixture is re-filled into small volume bottles
(125 ml or less) that are marketed afterwards, or when small volume bottles (125
ml or less) are no longer sold in outer packaging, but individually (see also section
5.3.2.1 of this guidance document);
• soluble packaging for single use where contents do not exceed 25 ml (see also
section 5.3.2.2 of this guidance document).
Label information may also be adapted for:
• inner packaging of substances and mixtures for scientific research and
development or quality control analysis when the contents do not exceed 10 ml
(see also section 5.3.2.3 of this guidance document);
• unpackaged hazardous substances or mixtures supplied to the general public (see
also section 5.3.2.4 of this guidance document);
• environmental labelling (see also section 5.3.2.5 of this guidance document).
5.3.2.1 Labelling of packages when the contents do not exceed 125 ml
The label elements mentioned in column 2 of Table 7 may be omitted from the label of
packages that do not exceed 125 ml of capacity when the substance or mixture is
classified for the hazard classes or categories listed in column 1.
However, when the substance or mixture is classified for further hazard classes not listed
in column 1 of Table 7, the label elements related to these other hazard classes still need
to be included. Please refer also to section 1.5.2.1 of Annex I to CLP.
42
i.e. the information required by CLP Article 17.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
51
It should be noted that the exemptions regarding the labelling of small packages of
aerosols classified as flammable (Directive 75/324/EEC
43
) apply to aerosol dispensers.
Table 7: Labelling exemptions for packages of a capacity of 125 ml or less
Classification of the substance or mixture
Allowed omissions according to
section 1.5.2 of Annex I to CLP
Oxidising gases cat. 1 (H270)
Gases under pressure (H280, H281)
Flammable liquids cat. 2 or 3 (H225, H226)
Flammable solids cat. 1 or 2 (H228)
Self-reactive substances or mixtures, types C, D, E or F (H242)
Self-heating substances or mixtures, cat. 2 (H252)
Substances and mixtures which, in contact with water, emit
flammable gases, cat. 1, 2 or 3 (H260, H261)
Oxidising liquids cat. 2 or 3 (H272)
Oxidising solids cat. 2 or 3 (H272)
Organic peroxides, types C, D, E or F (H242)
Acute toxicity cat. 4 (H302, H312, H332)
(if the substance or mixture is not supplied to the general public)
Skin irritation cat. 2 (H315)
Eye irritation cat. 2 (H319)
STOT-SE cat. 2 or 3 (H371, H335, H336)
(if the substance or mixture is not supplied to the general public)
STOT-RE cat. 2 (H373)
(if the substance or mixture is not supplied to the general public)
Hazardous to the aquatic environment – short-term (acute)
aquatic hazard, cat. Acute 1 (H400)
Hazardous to the aquatic environment – long-term (chronic)
aquatic hazard, cat. Chronic 1 or 2 (H410 or H411)
hazard and precautionary
statements for the hazard classes
listed in column 1
comment: the hazard pictogram
and signal word are required for
the denoted hazard categories
Flammable gases cat.2 (H221)
Reproductive toxicity: effects on or via lactation (H362)
Hazardous to the aquatic environment – long-term (chronic)
aquatic hazard, cat. Chronic 3 or 4 (H412 or H413)
precautionary statements linked to
the hazard classes listed in column 1
comment: the hazard statements
and signal word must be provided
as no hazard pictogram is required
for the denoted hazard categories
Corrosive to metals (H290)
hazard pictogram, signal word,
hazard and precautionary
43
Council Directive 75/324/EEC of 20 May 1975 on the approximation of the laws of the Member
States relating to aerosol dispensers, as amended.

52
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Classification of the substance or mixture
Allowed omissions according to
section 1.5.2 of Annex I to CLP
statements for this hazard class
5.3.2.2 Labelling of soluble packaging for single use which does not
exceed a volume of 25 ml
The soluble packaging exemption applies to soluble packaging when the content does
not exceed a volume of 25 ml. For such packaging, the CLP label elements required by
CLP Article 17 may be omitted provided the packaging is intended for single use and it is
contained within an outer packaging that bears all the label elements required by CLP
Article 17.
The exemption applies in cases where the substance or mixture contained is classified
exclusively for one or more of the hazards categories in sections 1.5.2.1.1 (b), 1.5.2.1.2
(b) or 1.5.2.1.3 (b) of Annex I to CLP (see Table 7 above). However, this exemption
does not apply to substances and mixtures within the scope of Regulation (EC)
1107/2009 (for plant protection products) or Regulation (EU) No 528/2012 (for biocidal
products).
5.3.2.3 Labelling of inner packaging when the contents do not exceed 10
ml
The CLP label elements required by CLP Article 17 may be omitted from the inner
packaging provided that all the following conditions are met:
• the contents of inner packaging do not exceed a volume of 10 ml;
• the substance or mixture is placed on the market for supply to a distributor or
downstream user for scientific research and development (SR&D)
44
or quality
control analysis; and
• the inner packaging is contained within an outer packaging that contains all the
label elements required by CLP Article 17.
However, it should be noted that the label on inner packaging must contain the product
identifier and (if appropriate) the hazard pictograms; GHS01, GHS05, GHS06 and/or
GHS08. In case more than two pictograms are assigned, GHS06 and GHS08 may take
precedence over GHS01 and GHS05.
The exemption does not apply to substances and mixtures within the scope of Regulation
(EC) 1107/2009 (for plant protection products) or Regulation (EU) No 528/2012 (for
biocidal products).
5.3.2.4 Unpackaged hazardous substances or mixtures supplied to the
general public
Labelling information about unpackaged chemicals sold to the general public must be
made available to the customer, e.g. on an invoice or bill (see CLP Article 29(3)). When
the purchase of such substances or mixtures occurs at a different point in time than their
delivery to the customer, one might also consider providing a leaflet that contains the
44
For more information on substances manufactured, imported or used in scientific Research and
Development (SR&D) please consult ECHA Guidance on Scientific Research and Development
(SR&D) and Product and Process Orientated Research and Development (PPORD).

Guidance on Labelling and Packaging
Version 4.2 – March 2021
53
relevant labelling information when delivering the substance or mixture, or sending the
information electronically before or upon delivery. CLP Article 29(3) provisions apply to
substances listed in Part 5 of Annex II to CLP).
5.3.2.5 Environmental labelling
CLP includes the possibility to introduce exemptions from certain provisions on
environmental labelling for certain mixtures classified as hazardous to the environment
when it can be demonstrated that there would be a reduction in the environmental
impact (see CLP Article 29(4)). However, no such exemptions or specific provisions have
been agreed to date. Once determined in accordance with the procedure referred to in
CLP Articles 53 and 54, such exemptions or specific provisions would be defined in Part 2
of Annex II to CLP.
5.3.3 Labelling exemptions for bespoke paints
An exemption for bespoke paints is provided for in CLP Annex VIII, part A, point 2.2a.,
allowing formulators (when they have the submitters’ obligations under Article 45 and
Annex VIII for mixtures classified for health or physical hazards) of such mixtures, when
they wish so, to opt not to submit information under Annex VIII for the final paint as
supplied to consumers or professional users. Therefore, in these cases there is also no
obligation to create and include a UFI for the final paint on the label. See further details
in Guidance on harmonised information relating to emergency health response – Annex
VIII to CLP.
Bespoke paints, for the purposes of Annex VIII, are paints where the addition of colour,
i.e. tinting, takes place on demand (by a specific customer – consumer or professional
user) at the point of sale. The mixture used to form the foundation for a paint is the
‘paint base’ and the substance or mixture used to colour the paint is the ‘tinter’
45
.
When information has not been submitted on the final bespoke paint (and therefore no
UFI has been generated for this final mixture), the UFI of the paint base and of all the
tinter mixtures subject to Annex VIII and added to the bespoke paint are required to be
included on the label of the bespoke paint. There is no need to include the UFIs of tinter
mixtures present in the final paint below a concentration of 0.1%.
It should be noted that if the concentration of a mixture with a UFI (regardless of its
function, i.e. tinter or paint base) in the bespoke paint exceeds 5 %, the concentration
(exact value or range) of that mixture must also be indicated next to its UFI, in
accordance with section 3.4. of Part B of Annex VIII.
The UFIs of all the bespoke paint’s components must be included in the supplemental
information on the label and listed in descending order of concentration. If the final
bespoke paint has a different classification than the paint base, the label of the bespoke
paint has to be updated to reflect it.
For practical reasons, it is also allowed to include on the label of the final mixture (often
this is the same as that of the paint base) only the UFI of the paint base, as long as UFIs
of the tinters are shown on the container, close to the other CLP label information. The
paint base normally constitutes the main part of the final mixture and is likely to be the
most relevant for emergency response. The UFIs of all the tinters that are required to be
indicated, can be indicated in a space left for them on the label of the paint base, or on a
45
More details and definitions are provided in the Guidance on Annex VIII.

54
Guidance on Labelling and Packaging
Version 4.2 – March 2021
sticker placed on the container outside of the paint base label (see Example 14 in section
6.3 of this Guidance document). In practice, this means that it is possible to apply the
widely used practice of placing information on the colours used in the final paint on a
separate sticker, printed directly from the tinting machine, for including the relevant
UFIs of the used tinter mixtures on this same sticker. The sticker needs to be placed
next to the other label elements and in a location where the UFIs can easily be seen and
identified, also during the use of the product. In very specific situations, where the
container is too small to accommodate the information related to the tinters, and other
alternative options are not applicable (e.g. according to Article 29), it may be considered
to include the tinters’ UFIs on a sticker placed on the lid, as in such a situation
placement on the lid can also be considered as ‘right next to the other label elements’
and may be a better solution than covering other essential information. When using the
option of placing the tinter-information on the lid, it is strongly recommended to indicate
the colour code both on the container and on the lid, in order to allow the identification
of the correct lid in case the lid and the container get separated. This would reduce the
risk of providing misleading information to the emergency operator.
5.4 Interaction between the CLP and the transport labelling rules
5.4.1 Specific rules for labelling of outer packaging, inner packaging and
single packaging
Article 33 of the CLP Regulation sets out specific rules for situations where the packaging
of hazardous substances and mixtures is also required to meet the labelling provisions in
accordance with the rules on the transport of dangerous goods. The transport labelling
provisions are set out in the UN Recommendations on the Transport of Dangerous Goods
– Model Regulations. Transport labelling as referred to in CLP Article 33 includes all
labels and marks required by e.g. Directive 2008/68/EC
46
, for example the mark for
environmentally hazardous substances, elevated temperature marks and
limited/exempted quantities marks. A basic principle of the CLP Regulation is not to
override any labelling required by the transport rules while maintaining essential hazard
information on the relevant layer(s) of packaging.
When a package consists of an outer and an inner packaging, together with any
intermediate packaging, and the outer packaging meets the labelling provisions in
accordance with the rules on the transport of dangerous goods, the hazard pictograms
required by the CLP Regulation do not need to appear on the outer packaging. As
mentioned above, the limited/excepted quantity marks are considered as transport
46
Directive 2008/68/EC for the inland transport of dangerous goods (road and rail).
CLP labelling is normally required on every layer of a packaging intended for supply
and use.
Transport labelling will have to appear on the outer packaging of hazardous
substances and mixtures if these are “dangerous goods” according to the rules on the
transport of dangerous goods. In such cases, a CLP label may also appear on an outer
packaging.
Single packages need to carry both the CLP label and transport labelling. If a CLP
hazard pictogram on single or outer packaging relates to the same hazard as in the
rules for the transport of dangerous goods, the CLP pictogram may be omitted to
avoid unnecessary double labelling.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
55
labelling. Therefore, a CLP label is not required when those marks are carried on the
outer packaging. CLP labelling may however be used if desired, according to CLP Article
33(1).
Where the outer packaging is transparent, all CLP label elements can be omitted from it
where the CLP label beneath the transparent layer is clearly visible (CLP Article 33(2)).
The legal requirements of CLP Article 33 and the decisions involved when dealing with
them are depicted in Figure 3.
Figure 3: Decision flowchart for the application of CLP and transport labelling for
single packaging (left) and combination packaging (right)
5.4.2 Packaging used for consolidation of supply packaging during
transport
The CLP Regulation sets general packaging standards for suppliers to ensure the safe
supply of hazardous substances and mixtures.
‘Packaging’ is defined in the CLP Regulation as “one or more receptacles and any other
components or materials necessary for the receptacles to perform their containment and
other safety functions”. This means that the packaging of a substance or mixture can
comprise multiple layers, for instance a bottle and a box.
CLP rules apply to all layers of packaging used for supply purposes. Any further
packaging may then fall under the definition given in the transport legislation: “the
outer protection of a composite or combination packaging together with any absorbent
material, cushioning and any other components necessary to contain and protect inner
receptacles or inner packaging”. The function of outer packaging fulfilling this definition
will remain the same whether or not a transport label is affixed to it.
Transport
Inner (and any
intermediate) and outer
packaging
Transport
label
required?
No
Yes
Transport
label
required?
Transparent
outer
packaging?
No
Yes
Yes
No
Single packaging
CLP label
Transport label
and CLP label
CLP pictograms
covered by an
equivalent
transport
pictogram may
be omitted.
CLP label on
inner (and any
intermediate)
and outer
packaging (see
also section
5.4.2 below)
CLP label on
inner (and any
intermediate)
packaging
CLP label on
outer packaging
may be omitted.
Transport label on outer
packaging; CLP label on
inner (and any
intermediate)
packaging
The outer packaging
may additionally be CLP
labelled. If so, the CLP
pictograms covered by
an equivalent transport
pictogram may be
omitted.

56
Guidance on Labelling and Packaging
Version 4.2 – March 2021
CLP Article 33(2) should be interpreted as meaning that labelling according to CLP is
required for the outermost layer of packaging that remains when the transport
packaging is removed (and, as the case may be, to the inner and intermediate
packaging). This type of ‘outer’ packaging (illustration (b) in Figure 4) requires a CLP
label (see also section 5.3.1.2 and section 5.4.1 of this guidance document).
Figure 4: Application of CLP labelling on packaging used for supply and transport
Normally, suppliers, including distributors, use one and typically more additional layers of
packaging to make the transport of multiple chemicals more convenient and to ensure that
the correct products are delivered to each location in good condition. Such transport
packaging (illustration (c) on Figure 4), used for the purpose of:
• protection of supply packages during transport and handling, and/or
• consolidation (combining several supply packages into a larger load for
transport),
is thus outside the scope of the CLP Regulation and does not require a CLP label.
Where substances or mixtures are stored on site without being removed from their
transport packaging as they are awaiting further transport, other labelling
obligations outside the scope of CLP and the transport legislation may continue to apply,
for example, a workplace risk assessment under the scope of the worker protection
Framework Directive (89/391/EEC) and associated individual directives including the
Chemical Agents Directive (98/24/EC
47
), Carcinogens and Mutagens Directive
(2004/37/EC
48
) and, as appropriate, the safety and/or health signs according to
47
Council Directive 98/24/EC of 7 April 1998 on the protection of the health and safety of workers
from the risks related to chemical agents at work (OJ L 131, 5.5.1998, p. 11–23), amended by
Directive 2007/308/EC and Directive 2014/27/EU.
48
Directive 2004/37/EC of the European Parliament and the Council of 29 April 2004 on the
protection of workers from the risks related to exposure to carcinogens or mutagens at work (OJ L
158, 30.4.2004, p. 50) amended by Directive 2007/308/EC and Directive 2014/27/EU.
(a)
inner packaging
for supply
(b)
outer packaging
for supply
(c)
transport packaging

Guidance on Labelling and Packaging
Version 4.2 – March 2021
57
Directive 92/58/EC
49
. However, once the substances or mixtures are no longer in
transport they must be removed from the transport packaging to enable the CLP label
to be clearly seen, or a CLP label must be added to what was previously the transport
packaging.
49
Council Directive 92/58/EEC of 24 June 1992 on the minimum requirements for the provision of
safety and/or health signs at work (OJ L 245, 26.8.1992, p.23), amended by Directive
2007/308/EC and Directive 2014/27/EU.

58
Guidance on Labelling and Packaging
Version 4.2 – March 2021
6. Example labels
In this section, 13 examples are provided to illustrate different situations that may be
encountered when designing labels.
Please note that each of the labels below serves only as an example of how to arrange
the elements on the label in a given situation. The examples given are not exhaustive
or mandatory in all aspects and do not reflect specific uses. The dimensions of labels and
label elements shown below are not necessarily the actual dimensions.
Example 1: Single language label for a substance (not for the
general public)
This example represents a simple label for a substance for supply and use which takes
into account the CLP label elements only. It shows the CLP terminology and pictograms
in accordance with CLP Article 17(a) and (c) to (g), i.e. the product identifiers, the
identity of the supplier, the signal word, the hazard pictograms, the hazard and the
precautionary statements. As the substance is not supplied to the general public, the
nominal quantity of the substance contained in the package is not required on the label.
Highly flammable liquid and vapour. May be fatal if swallowed and
enters airways. Causes skin irritation. May cause drowsiness or
dizziness. Very toxic to aquatic life with long lasting effects.
Keep away from heat, hot surfaces, sparks, open flames and
other ignition sources. No smoking. Keep container tightly closed.
Store in a well-ventilated place. Avoid breathing mist/vapours.
Wear protective gloves and eye and face protection.
IF INHALED: Remove person to fresh air and keep comfortable
for breathing. Avoid release to the environment.
Hazard pictograms
Product identifiers
Signal word
Supplier identity
Hazard statements
Precautionary
statements

Guidance on Labelling and Packaging
Version 4.2 – March 2021
59
Considering the industrial/professional use, the combined statement P301 + P310 has
been omitted from the label. To further reduce the number of P-statements and the
amount of digestible information on the label, the statement P391 has also been omitted
from the label, as the prevention statements for the physical and health hazards appear
to contain the more urgent advice for the label. The final selection of the P-statements
resulted in six P-statements compared to the starting set of eight P-statements.
The selected P-statements would have to be included in the SDS, under heading 2.2
(“Label elements”). The de-selected statements can be introduced under the relevant
headings of the SDS to provide the industrial or professional user with sufficient
information to handle the substance safely.

60
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Example 2: Multi-language label for a substance containing non-
obligatory supplemental information (not for the general public)
The example label given below represents a multi-language label for supply and use. It
shows the CLP terminology and pictograms in accordance with CLP Article 17(a) and (c)
to (h), i.e. the product identifier, the identity of the supplier, the hazard pictograms, the
signal words and the hazard and precautionary statements in four languages.
As the substance is not supplied to the general public, the nominal quantity of the
substance contained in the package is not required on the label.
In accordance with CLP Article 32(3), the hazard and precautionary statements of one
language are located together on the label. A section for supplemental labelling is
included on the left-hand side of the label including non-obligatory supplemental
labelling information.
As to the lay-out, the label is an authentic label designed for a 2.5 litre bottle. Given that
the real dimensions are slightly larger than depicted here, there is still potential to
optimise the structuring of the information, e.g. by using a more prominent place for the
signal word or larger letters for H- and P-statements. Based on the minimum dimensions
for the label area, which would be at least 52 mm x 74 mm, the size of each of the
pictograms is supposed to be at least 257 mm
2
, corresponding to a side length of 16
mm, on the real label (see section 5.2 of this guidance document).
If the content of the part for supplemental labelling is increased (for example to
incorporate information related to the use of the substance), the overall area of the label
and the size of its elements may have to be increased as well, in particular the letter size
for the signal words, hazard and precautionary statements. Such an increase would
improve the legibility of the obligatory label information, which appears in multiple
languages. In this case, it may be wise also to increase the size of the pictograms.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
61
Hazard pictograms
Product identifiers
Signal word
Supplier identity
Section for
supplemental
labelling
information
(non-obligatory)
Hazard and
Precautionary
statements,
grouped by
language

62
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Example 3: Single language label for a mixture containing both
obligatory and non-obligatory supplemental information (supplied
to the general public)
The example label given below illustrates the supply and use label for a typical consumer
product (detergent).
All obligatory labelling information is shown, i.e. the product identifiers (trade name and
designation of the mixture; one of them would have been sufficient), the identity of the
supplier, the signal word, the hazard and precautionary statements and the obligatory
supplemental information, in accordance with Regulation (EC) No 648/2004 on
detergents, and including the UFI code. Please note that supplemental labelling
information according to CLP is grouped together whilst the other supplemental
information (in this case the bar code) is located in another place. The UFI can
alternatively be placed outside the label (e.g. printed or affixed on the inner packaging)
but right next to the other obligatory CLP label elements.
No P-statement on disposal is given as this is not required for a mixture classified as eye
irritant.
As the product is supplied to the general public, its nominal quantity is also provided on
the label. Beyond the obligatory supplemental information, also some non-obligatory
supplemental information is shown.
This label clearly separates the obligatory information as required by the CLP Regulation
and other Community legislation from the non-obligatory elements. The former is
delineated by two text boxes, with the “CLP box” being located in a central, eye-catching
position on the label. The non-obligatory label elements can be found in the lower part of
the label and in the upper part, under the headline “instructions for use”.
The label as depicted here has a real size of 165 mm x 72 mm; the area of the label that
contains the obligatory label elements, i.e. the two boxes and the nominal quantity, is
about 98 mm x 72 mm. In principle the area covered by the text block “For further
information visit …” must be subtracted; on the other hand, approximately the same
area covered by the line “trade name” should be added, so there is no change overall.
The label is larger than the minimum dimensions required by the CLP Regulation, which
is at least 52 mm x 74 mm for a 500 ml bottle. The pictogram complies with the
reference minimum area of 16 x 16 mm.
The label shown is primarily drafted for inner packaging. If the chemical is contained in
combination (= inner + outer) packaging, the same information has to be shown on the
outer packaging, unless the information on the inner packaging can be seen through the
outer packaging.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
63
Hazard pictogram
Product identifiers
(trade name and
designation of the
mixture)
Signal word
Supplier identity
Hazard statement
Precautionary
statements
Nominal quantity
Product identifier
(designation of the
mixture)
Non-obligatory
supplemental
information
(here: identified
uses)
Obligatory
supplemental
information
Non-obligatory
supplemental
information
UFI: VDU1-414F-1003-1862
UFI code
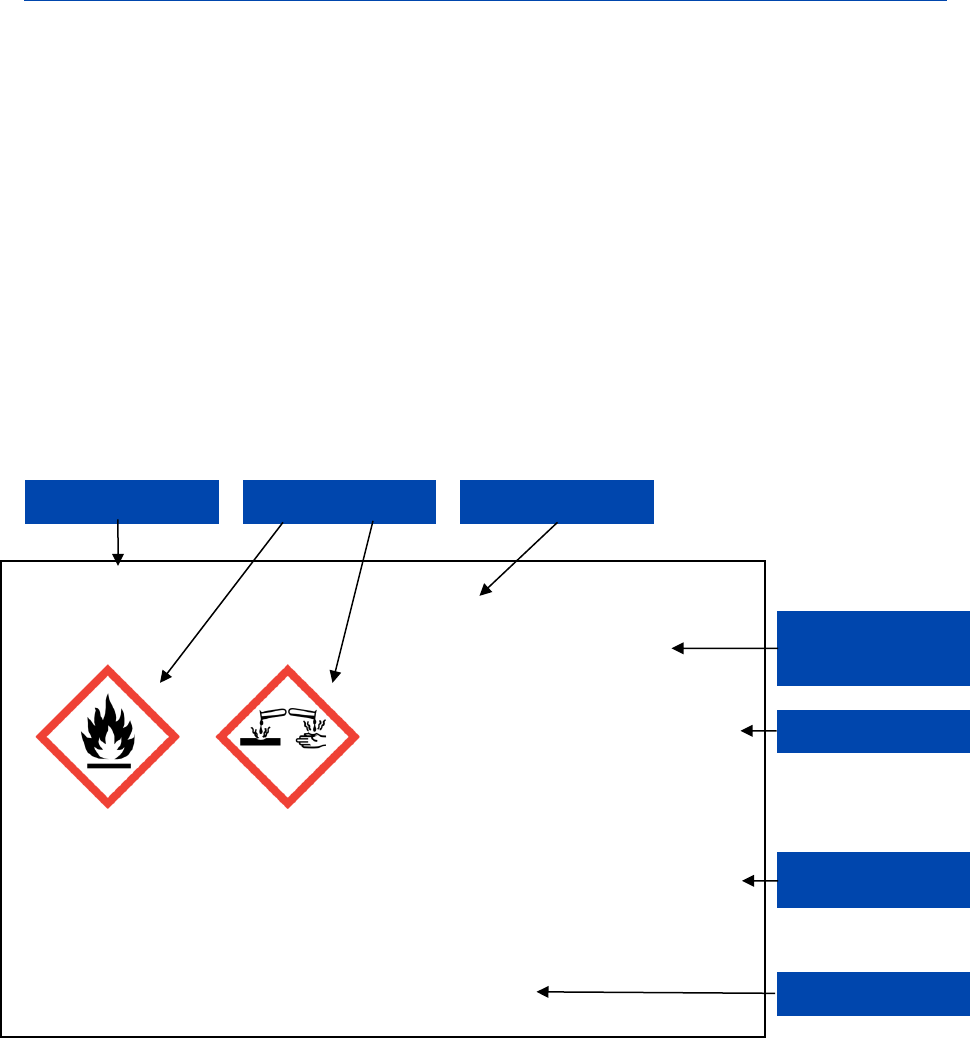
64
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Example 4: Single language label for a substance containing
supplemental hazard statements (not for the general public)
The example below illustrates a label for a substance for supply and use. A harmonised
classification (Water-react. cat. 1, Skin corr. cat. 1B) as well as the supplemental hazard
statement EUH014 are assigned through Annex VI to CLP. No other available, reliable
information was found that identified any further hazards. The substance is not intended
to be used by the general public; it is supplied in a 1 litre package.
All obligatory labelling information is shown, i.e. the product identifiers, the identity of
the supplier, the hazard pictograms, the signal word, the hazard and the supplemental
hazard statement EUH014, in accordance with Table 3 of Annex VI to CLP. Although
EUH014 is supposed to be supplemental information only, it is intentionally placed close
to the regular CLP hazard statements to reinforce the message provided by the latter.
Substance Z
EC No 123-123-1
Wear protective gloves / protective clothing / eye protection/face protection.
IF ON SKIN: Brush off loose particles from skin. Immerse in cool water. IF SWALLOWED:
Rinse mouth. Do NOT induce vomiting. Immediately call a POISON CENTER/doctor. IF
ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water
or shower. IF IN EYES: Rinse cautiously with water for several minutes. Remove contact
lenses, if present and easy to do. Continue rinsing.
Company X, Street Y, CITY ABC,
phone number: +49 (0) 0000 00 00 00.
Hazard pictograms
Signal word
Supplier identity
Hazard statements
Precautionary
statements
Supplemental
hazard statement
EUH014
Product identifiers
Danger
Reacts violently with water.
In contact with water releases
flammable gases which may ignite
spontaneously.
Causes severe skin burns and eye
damage.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
65
Example 5: Multi-language label for a mixture containing both
obligatory and non-obligatory supplemental information (supplied
to the general public)
Example 5 represents a draft multi-language label for a typical consumer chemical
(decorative paint) for supply and use.
All obligatory labelling information is shown, i.e. the product identifiers, the identity of
the supplier, the signal word, the hazard and precautionary statements and the
obligatory supplemental information, in particular information in accordance with
Directive 2004/42/EC on the limitation of emissions of volatile organic compounds
(VOCs) due to the use of organic solvents in certain paints and varnishes and vehicle
refinishing products, and including the UFI code (in this example, the same UFI code has
been used in the submission in each Member State). The UFI can alternatively be placed
outside of the label (e.g. printed or affixed on the inner packaging) but right next to the
other obligatory CLP label elements. As regards to the multi-language label in several
MSs, it should be clearly stated on the label to which country the specific UFI applies,
unless the same apply(ies) to all countries where the mixture is placed on the market. In
cases where a different UFI is used in each Member State (not recommended), it has to
be clear which UFI is relevant for each Member State (e.g. the relevant UFI with a
country code should be placed with the label elements of the applicable language(s) of
that Member State).
In accordance with CLP Article 32(3), the hazard and precautionary statements of one
language are located together on the label. As the chemical is supplied to the general
public, its nominal quantity is also provided on the label. Beyond the obligatory label
elements, non-obligatory supplemental information is shown.
This example label separates the CLP label elements from the supplemental information.
The CLP label elements are located in a more eye-catching position on the label while the
supplemental information can be found rather in the margins of the label. The texts
reflecting the supplemental information appear in slightly smaller letters than the CLP
label elements.
The size of this label is intended to be 125 mm x 150 mm when applied on the
packaging. This means that the real label will be considerably larger than the minimum
label size for a 1 litre package (52 x 74 mm) required under the CLP Regulation. The
pictogram size of 19 x 19 mm is less than 1/15
th
of the area of the whole label, but
greater than 1/15
th
of the area dedicated to the information required by CLP Article 17.

66
Guidance on Labelling and Packaging
Version 4.2 – March 2021
UFI code
Signal word, hazard
and precautionary
statements in three
languages
Hazard pictogram
Product identifiers
Non-obligatory
supplemental
information
Supplier identity
Nominal quantity
Non-obligatory
supplemental
information
Obligatory supplemental
information according to
the VOC Directive
Signal word
00000
00000
UFI: VDU1-414F-1003-1862

Guidance on Labelling and Packaging
Version 4.2 – March 2021
67
Example 6: Fold-out label for a mixture (supplied to the general
public)
The example below represents a multilingual, fold-out label for a mixture for supply and
use, intended for the general public.
The label for this mixture is required to bear a large number of obligatory CLP label
elements, namely three hazard pictograms, three hazard statements and numerous
precautionary statements subject to the principles of precedence. It was impossible to
put all these label elements on the immediate container due to its shape and size (plastic
container of 100 ml capacity). The supplier cannot accommodate on a standard label the
required information in the official language of the Member State where the product is
placed on the market (Poland). Because of this, the supplier has chosen to use a fold-out
label. This way, the supplier can also include the two additional languages they consider
necessary in this case. The label elements are included on the label in the following way:
Front page
• trade name or designation,
• hazard pictograms,
• signal words in all languages of the label,
• nominal quantity, as the mixture is made available to the general public,
• contact details of supplier,
• reference to the full safety information inside (in this case the front page contains
the symbol of an arrow to illustrate that the full safety information is available on
inside pages),
• country codes indicating which languages are covered by the label,
• UFI code (in this case, the same UFI code has been included in the submission in
each Member State). The UFI can alternatively be placed outside of the label (e.g.
printed or affixed on the inner packaging) but right next to the other obligatory
CLP label elements.
Inside pages
• full product identifier (including hazardous compounds A, B and C in this
particular case),
• signal word,
• hazard statements,
• precautionary statements,
The full safety information on the inside pages is given in each language mentioned
on the front page and also grouped by language. The country codes are featured on
the top of each inner page to enable the user to quickly identify his language.
Back page (attached to the immediate container)
• trade name or designation,
• hazard pictograms,
• signal word,
• nominal quantity,

68
Guidance on Labelling and Packaging
Version 4.2 – March 2021
• contact details of supplier,
• UFI code (only one code has been included in the submission in each Member
State).

Guidance on Labelling and Packaging
Version 4.2 – February 2021
69
UFI code
UFI: VDU1-414F-1003-1862
UFI: VDU1-414F-1003-1862
Signal
word
Supplier
identity
Hazard
statements
Trade name
Inside
pages
Language
code
Hazard
pictograms
Nominal
quantity
Precautionary
statements
Signal
word in all
languages of
the label
Language
codes
indicating
languages
covered by
the label
Front page
wrapped
around
the container
Symbol informing
the user that the label
can be opened and
indicating that the
additional information
is available on inside
pages
Product identifier
including three
hazardous
components that
contribute to the
classification of the
mixture
Back page
firmly affixed
to the
immediate
container
UFI code
UFI: VDU1-414F-1003-1862
UFI: VDU1-414F-1003-1862

70
Guidance on Labelling and Packaging
Version 4.2 – March 2021
6.1 Packaging that is small or difficult to label
The example labels in this section are authentic; they are applied on inner packaging
only because the package is transported in larger consignments with specific outside
labelling in accordance with the rules on the transport of dangerous goods. Please note
that the labelling exemptions only apply if the alternative labelling on fold-out labels, tie-
on tags or outer packaging is technically not feasible.
Example 7: Substance in a 8 ml bottle (not for the general public)
The example given below represents a two-language label in Finnish and Swedish for
small packaging for the substance. Both languages are required in Finland. According to
Annex VI to CLP, the substance is assigned the following classifications:
Flam. Liq. 2 H225 Highly flammable liquid and vapour
Repr. 2 H361 Suspected of damaging fertility or the unborn child (state
specific effect if known) (state route of exposure if it is conclusively
proven that no other routes of exposure cause the hazard))
Asp. Tox. 1 H304 May be fatal if swallowed and enters airways
STOT-RE 2 H373 May cause damage to organs (state all organs affected, if
known) through prolonged or repeated exposure (state route of
exposure if it is conclusively proven that no other routes of
exposure cause the hazard)
Skin Irrit. 2 H315 Causes skin irritation
STOT SE 3 H336 May cause drowsiness or dizziness
Aquatic Chronic 2 H411 Toxic to aquatic life with long lasting effects
Based on CLP Article 17, many labelling elements would be required. The bottle
containing the substance is placed on the market individually. Since it is assumed for
this example that the labelling information cannot be accommodated on a fold-out label,
tie-on tag or on outer packaging, the supplier is allowed to apply the small packaging
exemptions outlined in section 1.5.2 of Annex I to CLP.
Accordingly, the hazard and precautionary statements pertaining to the following hazard
classes and categories:
Flam. Liq. 2, STOT-RE 2, Skin Irrit. 2, STOT-SE 3 and Aquatic Chronic 2
may be omitted from the label. However, and in line with the CLP Regulation, the hazard
pictograms:
GHS02, GHS07, GHS08 and GHS09
were retained for these hazards.
No small packaging exemptions apply to the following hazards classes and categories:
Repr.2 and Asp. Tox. 1. This means that the pictograms and the hazard and
precautionary statements pertaining to these hazard classes and categories have been
retained.
The precautionary statements have obviously been reduced, following CLP Articles 22
and 28. For example, the statement P501 (“Dispose of contents/container to…”) was not
included because the substance is not supplied to the general public and there are no
specific disposal requirements above the normal expectation for the disposal of
chemicals (see also section 7 of this guidance document). Out of a set of originally 20
different precautionary statements, only one single (combination) statement, namely

Guidance on Labelling and Packaging
Version 4.2 – March 2021
71
P301+P310+P331 (“IF SWALLOWED: Immediately call a POISON CENTER/doctor. Do
NOT induce vomiting.”) finally remains on the label.
In accordance with CLP Article 32(3), the hazard statements of one language as well as
the precautionary statements, respectively, are located together on the label. Finally, the
signal word “Danger” (Finnish: Vaara; Swedish: Fara) was selected, in line with the
applicable precedence rule.
If the real dimensions of the label are 32 mm x 95 mm, it can accommodate four
pictograms of the required minimum size of 1 cm
2
. This may not always be possible for
even smaller packaging volumes, e.g. a bottle volume of 4 ml (see below). In order to
maintain the required minimum size of 1 cm
2
for the hazard pictograms in such cases,
either the size of the label or the volume of the bottle as such will have to be increased.
It may not be warranted to reduce the letter size of the texts as this will very probably
decrease their legibility.
Small packaging exemptions:
reduced set of hazard and
precautionary statements, grouped
together on the label by language
No omissions, but full range of
hazard pictograms
Due to space constraints on small volume
packaging, pictograms of the required
minimum size of 1 cm
2
cannot always be
accommodated. In this case, either the
size of the label or the volume of the
bottle will have to be increased.

72
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Example 8: Hazardous solid substance in a 100 ml bottle (not
intended for the general public)
This example represents a one-language label for small packaging for a solid substance
Y, which is assigned the following classifications:
Ox. Sol. 2 H272 May intensify fire; oxidiser
Carc. 1B H350 May cause cancer (state route of exposure if it is
conclusively proven that no other routes of exposure cause
the hazard)
Muta 1B H340 May cause genetic defects (state route of exposure if
it is conclusively proven that no other routes of exposure
cause the hazard)
Repr. 1B H360 May damage fertility or the unborn child (state specific
effect if known) (state route of exposure if it is conclusively
proven that no other routes of exposure cause the hazard)
Acute Tox. 2 (inhalation) H330 Fatal if inhaled
Acute Tox. 3 (oral) H301 Toxic if swallowed
STOT RE 1 H372 Causes damage to organs (state all organs affected, if
known) through prolonged or repeated exposure (state
route of exposure if it is conclusively proven that no other
routes of exposure cause the hazard)
Acute Tox. 4 (dermal) H312 Harmful in contact with skin
Skin Corr. 1B H314 Causes severe skin burns and eye damage
Resp. sens. 1 H334 May cause allergy or asthma symptoms or breathing
difficulties if inhaled
Skin sens. 1 H317 May cause an allergic skin reaction
Aquatic Acute 1 H400 Very toxic to aquatic life
Aquatic Chronic 1 H410 Very toxic to aquatic life with long lasting effects
Pursuant to CLP Article 17, a lot of labelling information would be required. Similarly to
the previous example, it is assumed that the supplier is allowed to use the small
packaging exemptions outlined in section 1.5.2 of Annex I to CLP.
Substance Y is not presumed to be listed in Annex VI to CLP, nor in the Classification and
Labelling Inventory. Therefore, only the product identifiers referred to in CLP Article
18(2)(c) need to be provided, i.e. the CAS number (if available, see CLP Article
18(2)(d)) and the IUPAC or another international name.
In accordance with the small packaging exemptions outlined in section 1.5.2 of Annex I
to CLP, only the hazard and precautionary statements pertaining to the following hazard
classes and categories:
Ox. Sol. 2, Acute Tox. 4, Aquatic Acute 1, and Aquatic Chronic 1
may be omitted from the label. This means that for all the other hazards listed above all
the label elements that are required under CLP Title II have to appear on the label.
The precautionary statements on the example label below start with “Obtain special
instructions before use.” A significant reduction has been performed for the
precautionary statements, based on CLP Articles 22 and 28. After application of the small
packaging exemptions and the selection of the most appropriate set of precautionary

Guidance on Labelling and Packaging
Version 4.2 – March 2021
73
statements, only five (combined) statements were chosen for the label, out of about 30
precautionary statements.
In addition to the hazard and precautionary statements, five different hazard pictograms
are required for the label, namely GHS03, GHS05, GHS06, GHS08 and GHS09.
Due to the severity of the hazards,
substantial reduction of the hazard
statements is not possible.
The number of the precautionary
statements however, has been
substantially reduced.

74
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Example 9: Supply and transport label for a single package (not
intended for the general public)
This example illustrates the provisions of CLP Article 33(3) and represents a label for a
hazardous mixture that is assigned the following classification:
Flam. Liq. 2 H225 Highly flammable liquid and vapour
Acute Tox. (dermal) 3 H311 Toxic in contact with skin
Skin Irrit. 2 H315 Causes skin irritation
STOT SE 3 H335 May cause respiratory irritation
STOT SE 3 H336 May cause drowsiness or dizziness
STOT RE 2 H373 May cause damage to organs (state all organs
affected, if known) through prolonged or repeated exposure
(state route of exposure if it is conclusively proven that no
other routes of exposure cause the hazard)
Asp. Tox. 1 H304 May be fatal if swallowed and enters airways
Aquatic Acute 1 H400 Very toxic to aquatic life
Aquatic Chronic 1 H410 Very toxic to aquatic life with long lasting effects
The mixture is intended to be supplied in single packaging, such as a 200-litre drum.
This means that both the CLP and the transport label elements must be shown on the
packaging. The mixture is for industrial use and not intended to be used by the general
public.
In this case, the supplier has chosen to include the transport label elements and marks
together with the CLP labelling elements on a joint label. This common label and the font
size used would be large enough to conform to the specifications set out in the European
Agreement concerning the International Carriage of Dangerous Goods by Road (ADR)
that has been implemented within the EU through Directive 2008/68/EC.
In relation to the CLP hazard pictograms GHS06 and GHS07, only GHS06 needs to be
displayed, in accordance with the precedence rule set out in CLP Article 26(1)(b).
However, the supplier has omitted the CLP hazard pictograms GHS06, GHS09 and
GHS02, as the underlying hazard classes and categories are already covered by the
corresponding transport pictograms.
In this example, the UFI is indicated on the label. However, for hazardous mixtures that
are subject to the submission of information under CLP Article 45, when they are
supplied for use at industrial sites the UFI can alternatively be indicated in the SDS only.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
75
TOXIFLAM
(Contains X, Y)
UFI: VDU1-414F-1003-1862
Danger
Highly flammable liquid and vapour.
Toxic in contact with skin.
Causes skin irritation.
May cause respiratory irritation.
May cause damage to liver, testis through
prolonged or repeated exposure.
May be fatal if swallowed and enters airways.
Very toxic to aquatic life with long lasting effects.
May cause drowsiness or dizziness.
Keep away from heat, hot surfaces, sparks,
open flames and other ignition sources.
No smoking. Wear protective gloves and clothing
and eye protection. IF SWALLOWED: Immediately
call a POISON CENTER/doctor. Do NOT induce vomiting.
Avoid release to the environment.
Dispose of container to the municipal collection point.
See safety data sheet for further details regarding safe use.
Manufactured by
Company X,
Street Y,
Town Z
Code 00000,
Tel: +49(0)0000000000.
UNXXXX
[Proper Shipping Name]
Product
identifier
CLP hazard
pictogram
Transport
labelling
Supplier
identity
Signal word
Hazard
statements
Precautionary
statements
Space for
further
supplemental
information
(e.g.
instructions
for use)
Product
identifier
including
substances
that
contribute to
the
classification
of the mixture
as acutely
toxic, STOT-
RE and toxic
by aspiration
UFI code

76
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Example 10: Labelling for a mixture that is transported on land in
outer and inner packaging (not intended for the general public)
This example illustrates the labelling of a transported mixture classified as:
Flam. Liq 3 H226 Flammable liquid and vapour
Acute Tox. 4 H312 Harmful in contact with skin
Acute Tox. 4 H332 Harmful if inhaled
Skin Irrit. 2 H315 Causes skin irritation
The mixture is contained in an inner packaging (bottles) that is in turn contained in an
outer packaging (box), which is not transparent. The mixture is for professional users
and not intended to be used by the general public. The UFI can alternatively be placed
outside of the label (e.g. printed or affixed on the inner packaging) but close to the other
obligatory CLP label elements.
Supplier
identity
Precautionary
statements
Hazard
statements
Signal word
Product
identifier
CLP hazard
pictograms
Inner
packaging
Outer
packaging
Only transport label required
(CLP labelling optional)
for outer packaging.
UFI: VDU1-414F
1003-1862
UFI code

Guidance on Labelling and Packaging
Version 4.2 – March 2021
77
Example 11: Labelling for a mixture that is transported on land in
single packaging (not intended for the general public)
This example illustrates the provisions related to the labelling of single packaging in
accordance with CLP Article 33(3). It is an example of a mixture that is classified and
labelled in accordance with the rules on the transport of dangerous goods and under the
CLP Regulation. The mixture is transported on land in single packaging (drum). It is not
intended to be used by the general public.
In this example, the full CLP labelling information is provided by means of a separate
label, in addition to the transport labelling information (version 1).
The CLP hazard pictogram GHS09 may be omitted from the packaging because it relates
to the same hazards as the “dead tree – dead fish” transport mark (version 2).
Since the mixture is hazardous to the environ ment only, a UFI is not required.
Version 1:
UN 3082 ENVIRONMENTALLY
HAZARDOUS SUBSTANCE,
LIQUID, N.O.S.
(Contains X)
1A2/Y1.2/100/08/NL/TDV441
U
N
Mixture Z
(Contains X)
Warning
Very toxic to aquatic life with long lasting
effects
Avoid release to the environment. Collect
spillage. Dispose of contents/container to local
waste disposal company.
VOC content: EU limit for this product is (cat. A/d):
300 g/l. This product contains max 299 g/l VOC.
Company X, Street Y, Town Z,
Code 00000,
Tel: +49(0)0000000000
Single packaging with the
transport labelling …
Obligatory supplemental
information according to
the VOC Directive
… and the CLP labelling
information

78
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Version 2:
1A2/Y1.2/100/08/NL/TDV
441
U
N
Mixture Z
(Contains X)
Warning
Very toxic to aquatic life with long lasting
effects
Avoid release to the environment. Collect
spillage. Dispose of contents/container to local
waste disposal company.
VOC content: EU limit for this product is (cat.
A/d): 300 g/l. This product contains max 299
g/l VOC.
Company X, Street Y, Town Z,
Code 00000,
Tel: +49(0)0000000000
UN 3082 ENVIRONMENTALLY
HAZARDOUS SUBSTANCE,
LIQUID , N.O.S.
(Contains X)
Single packaging with the
transport labelling …
… and the CLP labelling
information without the
CLP pictogram GHS09 for
the aquatic hazards
Obligatory supplemental
information according to
the VOC Directive

Guidance on Labelling and Packaging
Version 4.2 – March 2021
79
6.2 Specific case: labelling of two-component products
In certain specific cases the packaging of a product can be so unique that it is difficult to
meet the CLP labelling requirements. Examples of such situations are given below.
Please note that the examples only illustrate the general aspects of labelling of two-
component products and are not intended to present the correct selection of appropriate
label elements.
Example 12: Labelling of a two-component adhesive sold as a kit
The figure below shows an example of a popular two-component adhesive sold as a kit
consisting of two mixtures, namely an epoxy resin (Part A) and a hardener (Part B). The
two mixtures are placed in separate containers which are fixed together and sold as a kit
in transparent outer packaging. When used, the content of both containers is mixed after
or during extrusion. Part A and Part B react to produce a final mixture, which can be
used as an adhesive for a wide range of materials.
In this type of situation, two separate labels need to be affixed to the containers (one
label for each mixture (in a container)). The hazard information provided on the labels
must relate to the form/physical states in which both mixtures (Part A and Part B) are
placed on the market. On each label the UFI relevant for the specific mixture has to be
included. The outer packaging of the whole kit need not be labelled, as it is transparent
and permits the inner packaging (both containers) to be clearly seen.
If the product formed during end-use is hazardous (with different properties to the
mixtures in the containers), sufficient instructions to enable safe use must be provided
to the user. The instructions can for example be provided on the label or as a separate
leaflet in the package.
If such a product is not intended for the general public, two separate SDSs should be
provided to enable the users to meet their responsibilities in relation to the management
Please note:
A case-by-case judgement may be necessary when determining the labelling
requirements for similar, unique packaging. The information should not confuse the
user and the label should be easily understandable.
UFI: E600-30P1-S00Y-5079
UFI: VDU1-414F-1003-1862

80
Guidance on Labelling and Packaging
Version 4.2 – March 2021
of risks arising from the use of the reaction product that occur upon the end use of the
two mixtures (i.e. the adhesive).
As the adhesive in the example is also classified as hazardous, the relevant information
about the risk management measures should be provided in the SDSs.
Example 13: Labelling of a co-axial cartridge
A coaxial cartridge consists of a centre tube surrounded by an outer “doughnut” tube for
consistent two-component dispensing with a specified ratio of components (see figures
below). Normally, the two sections of the cartridge have their own moulded pistons. As
both pistons are pushed, the two components are pushed together to mix and react
through a static mix nozzle. A control valve located at the outlet prevents cross-
contamination. A divider plate keeps the components separate until they reach the
nozzle outlet.
In the case of a co-axial cartridge, there is an outer packaging – a single container
visible to the user. To ensure the safe use of the two-component product in the
cartridge, it should be labelled with one physical CLP label where the label elements for
each component mixture are clearly separated to differentiate between them.
The following mandatory elements of the CLP label should be shown (where applicable)
separately for each of the component mixtures:
• product identifier of the component mixture,
• hazard pictograms,
• signal word,
• hazard statements,
• precautionary statements,
• supplemental information specific to each mixture, including the UFI code.
Other mandatory elements of the CLP label, such as the identification of the supplier,
trade name and other supplemental information may be shown once on the label.
Coaxial outlet
Pistons
Component A
Component B
Centre tube
Cartridge body
Static mix
nozzle
Coaxial
cartridge

Guidance on Labelling and Packaging
Version 4.2 – March 2021
81
If the final blended mixture is not classified as hazardous, no additional information
needs to be included in the use instructions.
If the final blended mixture is more hazardous than the individual component mixtures,
or it has hazards not already addressed on the label, then information about this will
need to be included in the use instructions (e.g. on the label or provided inside an outer
packaging) and in Section 2.3 of the SDS(s).

82
Guidance on Labelling and Packaging
Version 4.2 – March 2021
00000
00000
Trade name
FAST CURING ANCHORING ADHESIVE
Contains: Substance X
. Flammable liquid and
vapour. May be fatal if swallowed and enters
airways. Causes skin irritation. Causes serious
eye irritation.
Keep away from heat, hot surfaces, sparks, open
flames and other ignition sources. No smoking.
Wear protective gloves/eye protection/face
protection. IF SWALLOWED: Immediately call a
POISON CENTER or doctor/physician. IF ON
SKIN (or hair): Take off immediately all
contaminated clothing. Rinse skin with water/
shower. Do NOT induce vomiting. In case of fire:
Use dry sand, dry chemical or alcohol-resistant
foam to extinguish.
Contains: Substance Y
. May cause an allergic
skin reaction. Causes serious eye irritation. Very
toxic to aquatic life with long lasting effects.
Avoid breathing vapours. Avoid release to the
environment. Wear protective gloves/eye
protection/face protection. If skin irritation or rash
occurs: Get medical advice/ attention. If eye
irritation persists: Get medical advice/attention.
Take off contaminated clothing and wash it before
reuse.
Consult most current local Product Data Sheet
and Safety Data Sheets prior to any use.
Temperature
Gel time
Curing time
+30 °C
4 min
35 min
+25 °C – +30 °C
4 min
40 min
+20 °C – +25 °C
5 min
50 min
+10 °C – +20 °C
6 min
85 min
+5 °C – +10 °C
10 min
145 min
+5 °C
18 min
145 min
–10 °C
1
30 min
24 h
1
Minimum cartridge temperature: +5 °C
Company X
Street Y
Town Z
Code 01234
Country
Tel: +01(0)234567890
www.companyx.com
300 ml
500 g
Best before end of XX/2019
Supplier
identity
Hazard
statements for
Component
mixture A
Trade name
Hazard
pictograms
for
Component
mixture A
Nominal
quantity
Precautionary
statements
For
Component
mixture A
Space for
further
supplemental
information
(e.g.
instructions for
use)
Hazard
statements for
Component
mixture B
Hazard
pictograms
for
Component
mixture B
Precautionary
statements
For
Component
mixture B
UFI code for
Component
mixture A
UFI code for
Component
mixture B
Signal word
for
Component
mixture A
Signal word
for
Component
mixture B
Product type
Component A
Danger
Component B
Warning
UFI: VDU1-414F-1003-1862
UFI: E600-30P1-S00Y-5079

Guidance on Labelling and Packaging
Version 4.2 – March 2021
83
6.3 Specific case: labelling of a bespoke paint
Example 14: Labelling of a bespoke paint where colours are added
on a tailor-made basis at the point of sale
The example below illustrates a label for a bespoke paint where colours are added to the
paint base on a tailor-made basis at the point of sale for supply and use. The product is
supplied in a one-litre paint can.
All obligatory labelling information of the paint base is shown on the paint base’s label.
Information on colours used are given on a separate sticker, printed directly from the
tinting machine, which includes the relevant UFIs (with concentrations if >5%) of the
used tinter mixtures on this same sticker. The sticker needs to be placed within the label
or in a location on the container in close proximity to the other label elements and where
the UFIs can be easily seen and identified, also during the use of the product.

84
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7. Guidance on the selection of precautionary statements
for the CLP hazard label
7.1 Introduction
Based on the UN GHS, the CLP Regulation assigns precautionary statements to all hazard
classes for the purpose of the safe supply and use of a substance or mixture. Based on
CLP Article 4, suppliers have to select precautionary statements for the CLP hazard label.
Suppliers can be the following:
• manufacturers or importers of substances,
• importers of mixtures;
• downstream users of substances or mixtures (including formulators),
• distributors (including retailers) of substances or mixtures, and/or
• producers or importers of explosive articles as defined in section 2.1 of Annex I to
CLP.
The selection of precautionary statements must be done based on CLP Articles 22 and 28
and CLP Annex IV:
Article 22
Precautionary statements
1. The label shall include the relevant precautionary statements.
2. The precautionary statements shall be selected from those set out in the tables in
Parts 2 to 5 of Annex I indicating the label elements for each hazard class.
3. The precautionary statements shall be selected in accordance with the criteria laid
down in Part 1 of Annex IV taking into account the hazard statements and the
intended or identified use or uses of the substance or the mixture.
4. The precautionary statements shall be worded in accordance with Part 2 of Annex
IV.
Article 28
Principles of precedence for precautionary statements
1. Where the selection of the precautionary statements results in certain precautionary
statements being clearly redundant or unnecessary given the specific substance,
mixture or packaging, such statements shall be omitted from the label.
2. Where the substance or mixture is supplied to the general public, one precautionary
statement addressing the disposal of that substance or mixture as well as the
disposal of packaging shall appear on the label, unless not required under Article
22. In all other cases, a precautionary statement addressing disposal shall not be
required, where it is clear that the disposal of the substance or mixture or the
packaging does not present a hazard to human health or the environment.
3. Not more than six precautionary statements shall appear on the label, unless
necessary to reflect the nature and the severity of the hazards.
Annex IV
“In selecting the precautionary statements in accordance with Articles 22 and 28(3),
suppliers may combine the precautionary statements in the tables [of Annex IV],
having regard to clarity and comprehensibility of the precautionary advice. (…).”

Guidance on Labelling and Packaging
Version 4.2 – March 2021
85
Neither the UN GHS nor the CLP Regulation provides for clear-cut rules on how to select
precautionary statements for the label (apart from the provisions of CLP Articles 22 and
28 and the basic instructions given in the columns specifying the conditions for use in
Tables 6.1-6.5 of Annex IV to CLP).
On the other hand, the number of precautionary statements under the CLP
Regulation/UN GHS has more than doubled when compared to the number of S-phrases
under the DSD. In a situation where selection rules are missing, an average hazardous
substance listed in Annex VI to CLP could easily be assigned more than 20 precautionary
statements on the label, based on the hazards of the substance (see section 3.4 of this
guidance document). The CLP Regulation requires that normally not more than six
precautionary statements must appear on the label, unless necessary to reflect the
nature and the severity of the hazards. Therefore, a substantial reduction of the number
of precautionary statements must be performed, based on effective selection rules.
7.2 Methodology
The selection of precautionary statements under the CLP Regulation is based on:
• the provisions set out in CLP Articles 22 and 28, and
• the basic instructions provided in the columns containing the conditions for use in
Tables 6.1-6.5 of Annex IV to CLP, and
• the instructions mentioned directly under the precautionary statements in the
selection tables (see section 7.3 of this guidance document).
The following approach was chosen for the selection of the precautionary statements
under the CLP Regulation:
• The P-statements
50
should be selected in accordance with the rules outlined in
CLP Article 28 and Part 1 of Annex IV to CLP;
• The selection of P-statements should take into account the underlying hazards
and identified or foreseen conditions for use of a substance or mixture;
• If the content of two P-statements is an obvious duplication, only the most
relevant statement should be selected;
• The P-statements assignment follows a “traffic light” system. The conditions for
use described in this guidance document distinguish between precautionary
statements that are “highly recommended”, “recommended”, “optional” and “not
to be used” for the hazard label;
• A particular recommendation should be seen in the light of the original CLP
conditions for use specified under the relevant precautionary statement in the
selection tables;
• Two target groups, i.e. the general public and the industrial/professional users,
are specified under the CLP Regulation. Where there is no explicit mention of the
target group, the conditions for use apply to both the general public and
industrial/professional users.
• Where the use of a particular precautionary statement is (highly) recommended
but some exemptions are indicated (“unless” condition), it should not be used
where the conditions specified in the “unless” clause apply:
50
Corresponding but not always identical to the former safety phrases (S-phrases) under the DSD.

86
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Vice versa, where a precautionary statement is only optional, it should be used
where the conditions specified in the “unless” clause apply:
• Similarly to the previous bullet point, where the use of a particular precautionary
statement is (highly) recommended under certain conditions
only, it should not be used where these conditions do not apply:
• For some hazards, the use of many specific precautionary statements will
normally have to be recommended. As a consequence, the number of
precautionary statements on the label will easily exceed the target number of six
even for simple substances.
On the other hand, the label, as compared to the SDS, is not always the only and
most appropriate means to convey a message to industrial/professional users,
e.g. for P241 (“Use explosion-proof electrical/ventilating/lighting/
…/equipment.”). In such cases, the guidance also refers to the SDS, typically by
phrasing a recommendation for both the label and the SDS. The recommendation
for inclusion on the label is then “weaker” than for the SDS, as for example P241
for flammable liquids or P373 (“DO NOT fight fire when fire reaches explosives”)
for explosive hazards. In some cases, it is even recommended to put the relevant
precautionary statements in the relevant section of SDS only;
• In relation to the physical hazards, it should always be determined whether
substances or mixtures displaying these hazards are supplied to or handled by
the general public. When this is not the case, the use of further precautionary
statements could be de-prioritised (“weaker” recommendation);
• For certain hazard classes listed in Table 6.5 of Annex IV, the CLP Regulation
requires at least one precautionary statement relating to disposal for substances
or mixtures supplied to the general public, as referred to under CLP Article 28(2);
For example:
P264 (“Wash … thoroughly after handling”) for the hazard class Skin
corrosion 1 should not be used for industrial/professional users where
P280 (“Wear protective gloves/protective clothing/eye protection/face
protection”) has already been selected for the hazard label of the
substance or mixture.
For example:
P410 (“Protect from sunlight”) for the hazard class Gases under pressure
should be applied in case the described gases are subject to (slow)
decomposition or polymerisation.
For example:
P260 (“Do not breathe dust/fume/gas/mist/vapours/spray”) would not be
recommended for skin corrosive substances or mixtures where inhalation
is unlikely to occur (e.g. for substances/mixtures that are not volatile and
where inhalable particles or mists do not occur during use).

Guidance on Labelling and Packaging
Version 4.2 – March 2021
87
• Where it is proposed to combine two or more precautionary statements that could
also be used on their own, the conditions of use specify “(highly) recommended,
in combination with Pxxx”:
Such combined statements should be counted as one P-statement.
• Additional guidance is provided for the application of the precautionary
statements P101 (“If medical advice is needed, have product container or label at
hand”), P102 (“Keep out of reach of children”) and P103 (“Read label before use”)
for hazardous substances and mixtures supplied to the general public (see table
in section 7.3.1 of this guidance document).
It should be noted that for substances and mixtures that are classified for physical,
health and environmental hazards, a selection based on the rules outlined in this
guidance document may still lead to a final set that significantly exceeds the target
number of six statements for the label (see Example C in section 7.4 of this guidance
document). Even if this can in principle be justified by CLP Article 28(3), the question
remains whether the extent of the labelling information is still digestible, in particular
where long combination statements appear.
Therefore, when verifying the set of P-statements selected on the basis of this guidance
document, it is proposed to take into account the following principles:
• certain prevention and response statements provide more urgent advice than
other statements, as rapid action may be crucial. Therefore, where similar P-
statements having different priorities are assigned because of different hazards,
the most stringent P-statement should be selected. This judgement can only be
done on a case-by-case basis and will strongly depend on the hazards involved:
• de-selecting statements that appear less urgent from the label and putting them
in the SDS would be a better option;
• to reduce the number of P-statements, the content of the hazard statements can
also be taken into account:
For example:
“Highly recommended, in combination with P302 + P352 (“IF ON SKIN: Wash
with plenty of water/…”) for P310 (“Immediately call a POISON
CENTER/doctor/...”) for the hazard class Acute Tox. 1 and 2 (dermal).
For example:
For a substance classified as acutely toxic and carcinogenic, the first aid
measures for acute toxicity will take precedence over the longer term effects,
i.e. P310 (“Immediately call a POISON CENTER/doctor/...”) will take precedence
over P311 (“Call a POISON CENTER/doctor/...”), P312 (“Call a POISON
CENTRE/doctor/…/if you feel unwell”) and P313 (“Get medical
advice/attention”).

88
Guidance on Labelling and Packaging
Version 4.2 – March 2021
When an SDS must be compiled, the precautionary statements selected for the CLP
hazard label have to be included in the SDS, under heading 2.2 “Label elements” (see
the Guidance on the compilation of safety data sheets). The de-selected statements can
be introduced under the relevant headings of the SDS instead, to provide the industrial
or professional user with sufficient information for handling the substance or mixture
safely.
7.3 Selection tables
The below selection tables (sections 7.3.1 to 7.3.5 of this guidance document) follow the
format as provided in Section 3 of Annex 3 to the UN GHS. The tables are arranged
according to the hazard class and category as appropriate.
This guidance document builds upon the generic provisions set out in CLP Articles 22 and
28, as well as the basic instructions provided in the columns containing the conditions for
use in Tables 6.1-6.5 of Annex IV to CLP. It takes into account i.a. the intended uses and
the physical properties of the substance or mixture.
The original CLP conditions for use are displayed in black colour under the relevant
precautionary statements in the selection tables below. In contrast, the conditions that
constitute EU guidance are marked with an asterisk () and in blue colour, in order to
distinguish them from the original CLP conditions for use (see also the columns
containing the conditions for use in Tables 6.1–6.5 of Annex IV to CLP).
When a forward slash or diagonal mark “/” appears in a precautionary statement
text, it indicates that a choice has to be made between the phrases it separates:
When three full stops “…” appear in the precautionary statement text, they indicate
that not all applicable conditions are listed. Therefore, the manufacturer or supplier
needs to add the required information as appropriate.
When square brackets “[…]” appear around some text in a precautionary statement,
they indicate that the text in square brackets is not appropriate in every case and should
For example:
P222 (“Do not allow contact with air”) for the hazard classes Pyrophoric liquids
and Pyrophoric solids can be omitted as the hazard statement H250 (“Catches fire
spontaneously if exposed to air”) is used.
For example:
P280 (“Wear protective gloves/protective clothing/eye protection/face
protection”) could read: “Wear eye protection” or “Wear eye and face
protection”.
For example:
In P312 (“Call a POISON CENTRE/doctor/…/if you feel unwell”), the use of “…”
indicates that any other choice needs to be specified by manufacturer or
supplier.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
89
be used only in certain circumstances. In these cases, conditions for use are included
explaining when the text should be used:
In selecting the precautionary statements in accordance with the conditions for use set
out in the tables, suppliers may combine these statements, having regard to clarity and
comprehensibility of the precautionary advice. In this case, the specific wording of the
component phrases must be retained in the combined phrases. The selection tables are
followed by four examples (A, B, C and D) of substances, illustrating the selection of
precautionary statements for the label (see section 7.4 of this guidance document).
For example:
P284 states: “[In case of inadequate ventilation] wear respiratory protection.”
This P-statement is given with the following condition for use: “- text in square
brackets may be used if additional information is provided with the chemical at
the point of use that explains what type of ventilation would be adequate for
safe use.”. The application of this condition should be interpreted as follows: if
additional information is provided with the chemical explaining what type of
ventilation would be adequate for safe use, the text in square brackets may be
used. In this case, P284 would read: “In case of inadequate ventilation wear
respiratory protection.” However, if the chemical is supplied without such
information, the text in square brackets should not be used, and P284 should
read: “wear respiratory protection”.

90
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.1 General precautionary statements
Precautionary Statement
P101
If medical advice is needed, have product container or label at hand.
- Consumer products
Highly recommended for all substances and mixtures classified for health hazards and that
are sold to the general public
P102
Keep out of reach of children.
- Consumer products
Highly recommended for substances and mixtures sold to the general public, except for
those only classified as hazardous to the environment
Applies also to packagings that are to be fitted with child resistant fastening (Annex II,
section 3.1.1.1)
P103
Read label before use.
- Consumer products
Optional, but may be required by other EU legislation

Guidance on Labelling and Packaging
Version 4.2 – March 021
91
7.3.2 Specific precautionary statements for physical hazards
7.3.2.1 Explosives
Hazard category Signal word Hazard statement
Unstable explosive Danger H200 Unstable explosive
Precautionary Statements
Prevention
Response
Storage
Disposal
P201
Obtain special instructions before
use.
Highly recommended
P250
Do not subject to
grinding/shock/friction/… .
if the explosive is mechanically
sensitive
…Manufacturer/supplier to specify
applicable rough handling.
Highly recommended if the
explosive is mechanically sensitive
Optional if the explosive is not
mechanically sensitive
P370 + P372 + P380 + P373
In case of fire:
Explosion risk.
Evacuate area. DO NOT fight fire
when fire reaches explosives.
Highly recommended
P401
Store in accordance with…
… Manufacturer/supplier to specify
local/regional/national/international
regulations as applicable.
Highly recommended for
inclusion in the safety data
sheet. Specify the applicable
regulation.
P501
Dispose of
contents/container to …
… in accordance with local/
regional/national/international
regulations (to be specified).
Manufacturer/supplier to
specify whether disposal
requirements apply to
contents, container or both.
Recommended for
inclusion in the safety data
sheet if there are specific
disposal requirements
above the normal
expectation for the
disposal of chemicals.

92
Guidance on Labelling and Packaging
Version 4.2 – March 2021
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Highly recommended to apply the
full wording of P280

Guidance on Labelling and Packaging
Version 4.2 – March 021
93
7.3.2.1 Explosives (continued)
Hazard category Signal word Hazard statement
Division 1.1 Danger H201 Explosive; mass explosion hazard
Division 1.2 Danger H202 Explosive; severe projection hazard
Division 1.3 Danger H203 Explosive; fire, blast or projection hazard
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces,
sparks, open flames and other
ignition sources. No smoking.
Highly recommended
P230
Keep wetted with …
- for substances and mixtures which
are wetted, diluted, dissolved or
suspended with a phlegmatiser in order
to reduce or suppress their explosive
properties (desensitized explosives)
… Manufacturer/supplier to specify
appropriate material.
Highly recommended
P234
Keep only in original packaging
Highly recommended
P370 + P372 + P380 + P373
In case of fire:
Explosion risk.
Evacuate area. DO NOT fight fire
when fire reaches explosives.
Highly recommended
P401
Store in accordance with…
… Manufacturer/supplier to specify
local/regional/national/international
regulations as applicable.
Highly recommended for
inclusion in the safety data
sheet. Specify the applicable
regulation.
P501
Dispose of
contents/container to …
… in accordance with local/
regional/national/international
regulations (to be specified).
Manufacturer/supplier to
specify whether disposal
requirements apply to
contents, container or both.
Recommended for inclusion
in the safety data sheet if
there are specific disposal
requirements above the
normal expectation for the
disposal of chemicals.
Specify the applicable
regulation.
Mandatory when supplied
to the general public
(where the Member State
allows such supply).

94
Guidance on Labelling and Packaging
Version 4.2 – March 2021
P240
Ground and bond container and
receiving equipment.
- if the explosive is electrostatically
sensitive.
Optional unless other conditions
deem it necessary
Recommended for inclusion in the
safety data sheet
P250
Do not subject to
grinding/shock/friction/… .
if the explosive is mechanically
sensitive
…Manufacturer/supplier to specify
applicable rough handling.
Highly recommended if the
explosive is mechanically sensitive
Optional if the explosive is not
mechanically sensitive

Guidance on Labelling and Packaging
Version 4.2 – March 021
95
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Protective gloves/protective
clothing/eye protection highly
recommended for
industrial/professional users
Face protection highly
recommended for
industrial/professional users where
articles are able to form hazardous
fragments
Recommended for explosives
supplied to the general public
(where Member States allows such
supply).

96
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.1 Explosives (continued)
Hazard category Signal word Hazard statement
Division 1.4 Warning H204 Fire or projection hazard
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces,
sparks, open flames and other ignition
sources. No smoking.
Highly recommended
P234
Keep only in original packaging
Highly recommended
P240
Ground and bond container and
receiving equipment.
- if the explosive is electrostatically
sensitive.
Optional unless other conditions deem it
necessary
Recommended for inclusion in the safety
data sheet
P370 + P372 + P380 + P373
In case of fire:
Explosion risk.
Evacuate area. DO NOT fight fire
when fire reaches explosives.
- except for explosives of division 1.4
(compatibility group S) in transport
packaging.
Highly recommended
P370 + P380 + P375
In case of fire: Evacuate area.
Fight fire remotely due to the risk
of explosion.
- for explosives of division 1.4
(compatibility group S) in transport
packaging.
Highly recommended
P401
Store in accordance with…
… Manufacturer/supplier to
specify
local/regional/national/internation
al regulations as applicable.
Highly recommended for
inclusion in the safety data
sheet. Specify the applicable
regulation.
P501
Dispose of
contents/container to …
… in accordance with local/
regional/national/internationa
l regulations (to be
specified).
Manufacturer/supplier to
specify whether disposal
requirements apply to
contents, container or both.
Recommended for
inclusion in the safety
data sheet if there are
specific disposal
requirements above the
normal expectation for
the disposal of chemicals.
Specify the applicable
regulation.
Mandatory when supplied
to the general public

Guidance on Labelling and Packaging
Version 4.2 – March 021
97
P250
Do not subject to
grinding/shock/friction/… .
if the explosive is mechanically sensitive
…Manufacturer/supplier to specify
applicable rough handling.
Highly recommended if the explosive is
mechanically sensitive
Optional if the explosive is not
mechanically sensitive
P280
Wear protective gloves/protective
clothing/ eye protection/ face
protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Protective gloves/protective clothing/eye
protection highly recommended for
industrial / professional users
Face protection highly recommended for
industrial / professional users where
articles are able to form hazardous
fragments
Recommended for explosives supplied to
the general public (where Member
States allows such supply).
(where the Member State
allows such supply).

98
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.1 Explosives (continued)
Hazard category Signal word Hazard statement
Division 1.5 Danger H205 May mass explode in fire
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces,
sparks, open flames and other
ignition sources. No smoking.
Highly recommended
P230
Keep wetted with …
- for substances and mixtures which are
wetted, diluted, dissolved or suspended
with a phlegmatiser in order to reduce
or suppress their explosive properties
(desensitized explosives)
… Manufacturer/supplier to specify
appropriate material.
Highly recommended
P234
Keep only in original packaging
Highly recommended
P370 + P372 + P380 + P373
In case of fire:
Explosion risk.
Evacuate area. DO NOT fight fire
when fire reaches explosives.
Highly recommended
P401
Store in accordance with…
… Manufacturer/supplier to specify
local/regional/national/international
regulations as applicable.
Highly recommended for
inclusion in the safety data
sheet. Specify the applicable
regulation.
P501
Dispose of
contents/container to …
… in accordance with local/
regional/national/internation
al regulations (to be
specified).
Manufacturer/supplier to
specify whether disposal
requirements apply to
contents, container or both.
Recommended for
inclusion in the safety
data sheet if there are
specific disposal
requirements above the
normal expectation for
the disposal of
chemicals. Specify the
applicable regulation.
Mandatory when
supplied to the general
public (where the
Member State allows
such supply).
No
additional
hazard
pictogram

Guidance on Labelling and Packaging
Version 4.2 – March 021
99
P240
Ground and bond container and
receiving equipment.
- if the explosive is electrostatically
sensitive.
Optional unless other conditions
deem it necessary
Recommended for inclusion in the
safety data sheet
P250
Do not subject to
grinding/shock/friction/… .
- if the explosive is mechanically
sensitive
…Manufacturer/supplier to specify
applicable rough handling.
Highly recommended if the explosive
is mechanically sensitive
Optional if the explosive is not
mechanically sensitive
P280
Wear protective gloves/protective
clothing/eye protection/ face
protection.
Manufacturer/supplier to specify the
appropriate type of equipment.

100
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Protective gloves/protective
clothing/eye protection highly
recommended for industrial /
professional users
Face protection highly recommended
for industrial / professional users
where articles are able to form
hazardous fragments
Recommended for explosives
supplied to the general public
(where Member States allows such
supply)
Notes on the labelling of Explosives
1) Unpackaged explosives or explosives repackaged in packaging other than the original or similar packaging must include all of the following label
elements:
a) the pictogram: exploding bomb;
b) the signal word “Danger”; and
c) the hazard statement: ‘Explosive; mass explosion hazard’
unless the hazard is shown to correspond to one of the hazard categories listed in Table 2.1.2 of Annex I to CLP, in which case the corresponding
symbol, the signal word and/or the hazard statement must be assigned.
2) Substances and mixtures, as supplied, with a positive result in Test Series 2 in Part I, Section 12, of the UN RTDG, Manual of Tests and Criteria,
which are exempted from classification as explosives (based on a negative result in Test Series 6 in Part I, Section 16 of the UN RTDG, Manual of
Test and Criteria) still have explosive properties. The user must be informed of these intrinsic explosive properties because they have to be
considered for handling – especially if the substance or mixture is removed from its packaging or is repackaged – and for storage. For this
reason, the explosive properties of the substance or mixture must be communicated in Section 2 and Section 9 of the safety data sheet and
other sections of the safety data sheet, as appropriate.

Guidance on Labelling and Packaging
Version 4.2 – March 021
101
7.3.2.2 Flammable gases (including chemically unstable gases)
Hazard category Signal word Hazard statement
1 Danger H220 Extremely flammable gas
2 Warning H221 Flammable gas
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces,
sparks, open flames and other
ignition sources. No smoking.
Highly recommended
P377
Leaking gas fire: Do not extinguish,
unless leak can be stopped safely.
Highly recommended
P381
In case of leakage, eliminate all
ignition sources.
Recommended
P403
Store in a well-ventilated
place.
Highly recommended
Pictogram
for hazard
category 1
only.

102
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.2 Flammable gases (including chemically unstable gases) (continued)
Hazard category Signal word Hazard statement
A No additional signal word H230 May react explosively even in the absence of air
B No additional signal word H231 May react explosively even in the absence of air
at elevated pressure and/or temperature
Precautionary Statements
Prevention
Response
Storage
Disposal
P202
Do not handle until all safety
precautions have been read and
understood.
Highly recommended
Note: This table lists only the precautionary statement that is assigned due to the chemical instability of the gas. For other precautionary
statements that are assigned based on the flammability see the respective table for flammable gases (of cat. 1 and 2) on the
previous page.
No
additional
hazard
pictogram

Guidance on Labelling and Packaging
Version 4.2 – March 021
103
7.3.2.3 Aerosols
Hazard category Signal word Hazard statement
1 Danger H222 Extremely flammable aerosol
H229 Pressurised container: May burst if heated
2 Warning H223 Flammable aerosol
H229 Pressurised container: May burst if heated
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces, sparks, open
flames and other ignition sources. No smoking.
Assigned in accordance with Directive 75/324/EEC
P211
Do not spray on an open flame or other ignition
source.
Assigned in accordance with Directive 75/324/EEC
P251
Do not pierce or burn, even after use.
Assigned in accordance with Directive 75/324/EEC
P410 + P412
Protect from sunlight. Do not
expose to temperatures exceeding
50 ºC/122 ºF.
Manufacturer/supplier to use
applicable temperature scale
Assigned in accordance with
Directive 75/324/EEC

104
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.3 Aerosols (continued)
Hazard category Signal word Hazard statement
3 Warning H229 Pressurised container: May burst if heated
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces, sparks, open
flames and other ignition sources. No smoking.
Assigned in accordance with Directive 75/324/EEC
P251
Do not pierce or burn, even after use.
Assigned in accordance with Directive 75/324/EEC
P410 + P412
Protect from sunlight. Do not
expose to temperatures exceeding
50 ºC/ 122ºF.
Manufacturer/supplier to use
applicable temperature scale
Assigned in accordance with
Directive 75/324/EEC
No
additional
hazard
pictogram

Guidance on Labelling and Packaging
Version 4.2 – March 021
105
7.3.2.4 Oxidising gases
Hazard category Signal word Hazard statement
1 Danger H270 May cause or intensify fire; oxidiser
Precautionary Statements
Prevention
Response
Storage
Disposal
P220
Keep away from clothing and other
combustible materials.
Highly recommended
P244
Keep valves and fittings free from oil and
grease.
Highly recommended
P370 + P376
In case of fire: Stop leak if safe to
do so.
Optional
Recommended for inclusion in the
safety data sheet.
P403
Store in a well-ventilated
place.
Highly recommended

106
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.5 Gases under pressure
Hazard category Signal word Hazard statement
Compressed gas Warning H280 Contains gas under pressure; may explode if heated
Liquefied gas Warning H280 Contains gas under pressure; may explode if heated
Dissolved gas Warning H280 Contains gas under pressure; may explode if heated
Precautionary Statements
Prevention
Response
Storage
Disposal
P410 + P403
Protect from sunlight. Store in a well-
ventilated place.
- P410 may be omitted for gases filled in
transportable gas cylinders in
accordance with packing instruction
P200 of the UN RTDG, unless those
gases are subject to (slow)
decomposition or polymerisation
Optional

Guidance on Labelling and Packaging
Version 4.2 – March 021
107
7.3.2.5 Gases under pressure (continued)
Hazard category Signal word Hazard statement
Refrigerated liquefied gas Warning H281 Contains refrigerated gas; may cause cryogenic burns or injury
Precautionary Statements
Prevention
Response
Storage
Disposal
P282
Wear cold insulating gloves and
either face shield or eye protection.
Highly recommended where liquid
splashes may occur, e.g. during
transfer of cryogenic liquids. In this
case the use of safety glasses with
side shields or a face shield should be
indicated in the safety data sheet.
P336 + P315
Thaw frosted parts with lukewarm
water. Do not rub affected area. Get
immediate medical advice/attention.
Recommended
P403
Store in a well-
ventilated place.
Optional

108
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.6 Flammable liquids
Hazard category Signal word Hazard statement
1 Danger H224 Extremely flammable liquid and vapour
2 Danger H225 Highly flammable liquid and vapour
3 Warning H226 Flammable liquid and vapour
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces, sparks,
open flames and other ignition sources. No
smoking.
Highly recommended
P233
Keep container tightly closed.
- if the liquid is volatile and may generate an
explosive atmosphere
Highly recommended for category 1, unless
P404 has already been assigned
Recommended for category 2, unless P404
has already been assigned
Optional for category 3
P303 + P361 + P353
IF ON SKIN (or hair): Take off
immediately all contaminated
clothing. Rinse skin with water [or
shower].
- text in square brackets to be included
where the manufacturer/supplier
considers it appropriate for the specific
chemical
Optional unless deemed necessary,
e.g. due to the risk of generating a
potentially explosive atmosphere
P370 + P378
In case of fire: Use … to extinguish.
- if water increases risk.
…Manufacturer/supplier to specify
appropriate media.
P403 + P235
Store in a well-
ventilated place.
Keep cool.
- for flammable
liquids Category 1 and
other flammable
liquids that are
volatile and may
generate an explosive
atmosphere.
Highly
recommended
P501
Dispose of
contents/container to …
… in accordance with
local/regional/
national/international regulations
(to be specified).
Manufacturer/supplier to specify
whether disposal requirements
apply to contents, container or
both.
Mandatory for the general
public if the substance /
mixture is subject to
legislation on hazardous
waste. It is recommended to
specify the site of disposal
while a reference to the
applicable legislation is not
necessary.
Recommended for industrial /
professional users if there

Guidance on Labelling and Packaging
Version 4.2 – March 021
109
P235
Keep cool.
- for flammable liquids category 1 and other
flammable liquids that are volatile and may
generate an explosive atmosphere
Highly recommended, unless P403 + P235 is
assigned.
P240
Ground and bond container and receiving
equipment.
- if the liquid is volatile and may generate an
explosive atmosphere
Optional unless other conditions deem it
necessary
Recommended for inclusion in the safety
data sheet
P241
Use explosion-proof
[electrical/ventilating/ lighting/…]
equipment.
- if the liquid is volatile and may generate an
explosive atmosphere
- text in square brackets may be used to specify
specific electrical, ventilating, lighting or other
equipment if necessary and as appropriate.
Optional unless other conditions deem it
necessary
Highly recommended if specific
extinguishing media are required or
appropriate
are specific disposal
requirements above the
normal expectation for the
disposal of chemicals.
It is recommended to specify
the site of disposal while a
reference to the applicable
legislation is not necessary.

110
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Recommended for inclusion in the safety
data sheet
P242
Use non-sparking tools.
- if the liquid is volatile and may generate an
explosive atmosphere and if the minimum
ignition energy is very low. (This applies to
substances and mixtures where the ignition
energy is <0.1 mJ, e.g. carbon disulphide).
Optional unless other conditions deem it
necessary
Recommended for inclusion in the safety
data sheet
P243
Take action to prevent static discharges.
- if the liquid is volatile and may generate an
explosive atmosphere
Optional unless other conditions deem it
necessary
Recommended for inclusion in the safety
data sheet
P280
Wear protective gloves/protective
clothing/eye protection/face protection.
Manufacturer/supplier to specify the appropriate
type of equipment.
Optional

Guidance on Labelling and Packaging
Version 4.2 – March 021
111
7.3.2.7 Flammable solids
Hazard category Signal word Hazard statement
1 Danger H228 Flammable solid
2 Warning H228 Flammable solid
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces, sparks,
open flames and other ignition sources. No
smoking.
Highly recommended
P240
Ground and bond container and receiving
equipment.
- if the solid is electrostatically sensitive
Optional unless other conditions deem it
necessary
Recommended for inclusion in the safety data
sheet
P370 + P378
In case of fire: Use … to extinguish.
- if water increases risk.
… Manufacturer/supplier to specify
appropriate media.
Highly recommended if specific
extinguishing media are required or
appropriate
112
Guidance on Labelling and Packaging
Version 4.2 – March 2021
P241
Use explosion-proof [electrical/ventilating/
lighting/…] equipment.
- if dust clouds can occur.
- text in square brackets may be used to specify
specific electrical, ventilating, lighting or other
equipment if necessary and as appropriate.
Optional unless other conditions deem it
necessary
Recommended for inclusion in the safety data
sheet
P280
Wear protective gloves/protective
clothing/eye protection/ face protection.
Manufacturer/supplier to specify the appropriate
type of equipment.
Optional

Guidance on Labelling and Packaging
Version 4.2 – March 021
113
7.3.2.8 Self-reactive substances and mixtures
Hazard category Signal word Hazard statement
Type A Danger H240 Heating may cause an explosion
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces,
sparks, open flames and other
ignition sources. No smoking.
Highly recommended
P234
Keep only in original packaging.
Highly recommended where the
packaging is important for preventing
or suppressing the effect of
dangerous reactions or explosion
P235
Keep cool.
- may be omitted if P411 is given on the
label
Recommended
P370 + P372 + P380 + P373
In case of fire:
Explosion risk. Evacuate area. DO
NOT fight fire when fire reaches
explosives
Highly recommended
P403
Store in a well-ventilated
place.
- except for temperature
controlled self-reactive
substances and mixtures or
organic peroxides because
condensation and consequent
freezing may take place
Highly recommended
P411
Store at temperatures not
exceeding …ºC/…ºF.
- if temperature control is
required (according to CLP
Annex I, section 2.8.2.4 or
2.15.2.3) or if otherwise
deemed necessary.
… Manufacturer/supplier to
specify temperature using the
applicable temperature scale.
P501
Dispose of
contents/container to …
… in accordance with local/
regional/national/international
regulations (to be specified).
Manufacturer/supplier to
specify whether disposal
requirements apply to
contents, container or both.
Recommended for inclusion
in the safety data sheet if
there are specific disposal
requirements above the
normal expectation for the
disposal of chemicals.
Specify the applicable
regulation.

114
Guidance on Labelling and Packaging
Version 4.2 – March 2021
P240
Ground and bond container and
receiving equipment.
- if electrostatically sensitive and able to
generate an explosive atmosphere
Optional unless other conditions deem
it necessary
Recommended for inclusion in the
safety data sheet
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Highly recommended
Highly recommended
P420
Store separately.
Recommended where
incompatible materials are
likely to produce a
particular risk. If this
statement is used, text
clarifying the incompatible
materials should be added
as supplemental
information.

Guidance on Labelling and Packaging
Version 4.2 – March 021
115
7.3.2.8 Self-reactive substances and mixtures (continued)
Hazard category Signal word Hazard statement
Type B Danger H241 Heating may cause a fire or explosion
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces,
sparks, open flames and other ignition
sources. No smoking.
Highly recommended
P234
Keep only in original packaging.
Highly recommended
P235
Keep cool.
- may be omitted if P411 is given on the label
Recommended
P240
P370 + P380 + P375 [+ P378]
51
In case of fire: Evacuate area.
Fight fire remotely due to the risk
of explosion. [Use … to
extinguish].
- text in square brackets to be used if
water increases risk.
…Manufacturer/supplier to specify
appropriate media.
Highly recommended
Text in square brackets is highly
recommended if specific
extinguishing media are required
or appropriate
P403
Store in a well-ventilated
place.
- except for temperature
controlled self-reactive
substances and mixtures or
organic peroxides because
condensation and consequent
freezing may take place
Highly recommended
P411
Store at temperatures not
exceeding …ºC/…ºF.
- if temperature control is
required (according to CLP
Annex I, section 2.8.2.4 or
2.15.2.3) or if otherwise deemed
necessary.
P501
Dispose of
contents/container to …
… in accordance with
local/regional/
national/international
regulations (to be
specified).
Manufacturer/supplier to
specify whether disposal
requirements apply to
contents, container or
both.
Mandatory for the
general public if the
substance / mixture is
subject to legislation
on hazardous waste. It
is recommended to
specify the site of
disposal while a
51
The use of square brackets is explained in section 7.3 of this guidance document.
116
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Ground and bond container and receiving
equipment.
- if electrostatically sensitive and able to
generate an explosive atmosphere
Optional unless other conditions deem it
necessary
Recommended for inclusion in the safety
data sheet
P280
Wear protective gloves/protective
clothing/eye protection/face protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Highly recommended
… Manufacturer/supplier to
specify temperature.
Highly recommended
P420
Store separately.
Recommended where
incompatible materials are
likely to produce a particular
risk. If this statement is
used, text clarifying the
incompatible materials
should be added as
supplemental information
reference to the
applicable legislation is
not necessary.
Recommended for
inclusion in the safety
data sheet if there are
specific disposal
requirements above
the normal expectation
for the disposal of
chemicals. Specify the
applicable regulation.
Guidance on Labelling and Packaging
Version 4.2 – March 021
117
7.3.2.8 Self-reactive substances and mixtures (continued)
Hazard category Signal word Hazard statement
Type C Danger H242 Heating may cause a fire
Type D Danger H242 Heating may cause a fire
Type E Warning H242 Heating may cause a fire
Type F Warning H242 Heating may cause a fire
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces,
sparks, open flames and other
ignition sources. No smoking.
Highly recommended
P234
Keep only in original packaging.
Highly recommended
P235
Keep cool.
- may be omitted if P411 is given on the
label
Recommended
P370 + P378
In case of fire: Use … to
extinguish.
- if water increases risk.
Manufacturer/supplier to specify
appropriate media.
Highly recommended if specific
extinguishing media are required
or appropriate
P403
Store in a well-ventilated
place.
- except for temperature
controlled self-reactive
substances and mixtures or
organic peroxides because
condensation and
consequent freezing may
take place
Highly recommended
P411
Store at temperatures not
exceeding …ºC/…ºF.
- if temperature control is
required (according to CLP
Annex I, section 2.8.2.4 or
2.15.2.3) or if otherwise
deemed necessary.
P501
Dispose of contents/container
to …
… in accordance with
local/regional/
national/international regulations
(to be specified).
Manufacturer/supplier to specify
whether disposal requirements
apply to contents, container or
both.
Mandatory for the general
public if the substance/mixture
is subject to legislation on
hazardous waste. It is
recommended to specify the
site of disposal while a
reference to the applicable
legislation is not necessary.
Recommended for inclusion in
the safety data sheet if there

118
Guidance on Labelling and Packaging
Version 4.2 – March 2021
P240
Ground and bond container and
receiving equipment.
- if electrostatically sensitive and able to
generate an explosive atmosphere
Optional unless other conditions deem
it necessary
Recommended for inclusion in the
safety data sheet
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Highly recommended
… Manufacturer/supplier to
specify temperature.
Highly recommended
P420
Store separately.
Recommended where
incompatible materials
are likely to produce a
particular risk. If this
statement is used, text
clarifying the
incompatible materials
should be added as
supplemental
information
are specific disposal
requirements above the
normal expectation for the
disposal of chemicals. Specify
the applicable regulation.
Guidance on Labelling and Packaging
Version 4.2 – March 021
119
7.3.2.9 Pyrophoric liquids
Hazard category Signal word Hazard statement
1 Danger H250 Catches fire spontaneously if exposed to air
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces, sparks,
open flames and other ignition sources. No
smoking.
Highly recommended
P222
Do not allow contact with air.
- if emphasis of the hazard statement is deemed
necessary
Optional
P231 + P232
Handle and store contents under inert
gas/…
Protect from moisture
…Manufacturer/supplier to specify appropriate
liquid or gas if “inert gas” is not appropriate.
Recommended
Highly recommended for inclusion in the
safety data sheet
P302 + P334
IF ON SKIN: Immerse in cool water or wrap
in wet bandages.
Highly recommended
P370 + P378
In case of fire: Use … to extinguish.
- if water increases risk.
…Manufacturer/supplier to specify appropriate
media.
Highly recommended if specific extinguishing
media are required or appropriate
120
Guidance on Labelling and Packaging
Version 4.2 – March 2021
P233
Keep container tightly closed
Highly recommended
P280
Wear protective gloves/protective
clothing/eye protection/face protection.
Manufacturer/supplier to specify the appropriate
type of equipment.
Highly recommended

Guidance on Labelling and Packaging
Version 4.2 – March 021
121
7.3.2.10 Pyrophoric solids
Hazard category Signal word Hazard statement
1 Danger H250 Catches fire spontaneously if exposed to air
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces, sparks,
open flames and other ignition sources. No
smoking.
Highly recommended
P222
Do not allow contact with air.
-if emphasis of the hazard statement is deemed
necessary
Optional
P231 + P232
Handle and store contents under inert
gas/…
Protect from moisture
…Manufacturer/supplier to specify appropriate
liquid or gas if “inert gas” is not appropriate.
Recommended
P302 + P335 + P334
IF ON SKIN: Brush off loose particles from
skin. Immerse in cool water or wrap in
wet bandages.
Highly recommended
P370 + P378
In case of fire: Use … to extinguish.
- if water increases risk.
…Manufacturer/supplier to specify appropriate
media.
Highly recommended if specific
extinguishing media are required or
appropriate

122
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Highly recommended for inclusion in the
safety data sheet
P233
Keep container tightly closed
Highly recommended
P280
Wear protective gloves/protective
clothing/eye protection/face protection.
Manufacturer/supplier to specify the appropriate
type of equipment.
Highly recommended
Guidance on Labelling and Packaging
Version 4.2 – March 021
123
7.3.2.11 Self-heating substances and mixtures
Hazard category Signal word Hazard statement
1 Danger H251 Self-heating; may catch fire
2 Warning H252 Self-heating in large quantities; may catch fire
Precautionary Statements
Prevention
Response
Storage
Disposal
P235
Keep cool.
- may be omitted if P413 is given on the
label
Highly recommended for the general
public
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Optional
P407
Maintain air gap between stacks or pallets.
Highly recommended
P413
Store bulk masses greater than … kg/…lbs at
temperatures not exceeding …ºC/…ºF.
… Manufacturer/supplier to specify mass and
temperature using applicable scale.
Highly recommended if the manufacturer has
specific information
P420
Store separately.
Recommended where incompatible materials are
likely to produce a particular risk. If this statement
is used, text clarifying the incompatible materials
should be added as supplemental information

124
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.12 Substances and mixtures which, in contact with water, emit flammable gases
Hazard category Signal word Hazard statement
1 Danger H260 In contact with water releases flammable gases which may
ignite spontaneously
2 Danger H261 In contact with water releases flammable gases
Precautionary Statements
Prevention
Response
Storage
Disposal
P223
Do not allow contact with water.
- if emphasis of the hazard statement is deemed
necessary
Optional
P231 + P232
Handle and store contents under inert gas/…
Protect from moisture.
- if the substance or mixture reacts readily with
moisture in air.
…Manufacturer/supplier to specify appropriate liquid
or gas if “inert gas” is not appropriate
Highly recommended where special emphasis is
required
P302 + P335 + P334
IF ON SKIN: Brush off
loose particles from skin.
Immerse in cool water.
Highly recommended
P370 + P378
In case of fire: Use … to
extinguish.
- if water increases risk.
… Manufacturer/supplier to
specify appropriate media.
Highly recommended if
specific extinguishing
media are required or
appropriate
P402 + P404
Store in a dry place. Store
in a closed container.
Recommended, unless
P231 has already been
assigned
Highly recommended for
inclusion in the safety data
sheet
P501
Dispose of
contents/container to …
… in accordance with
local/regional/
national/international
regulations (to be specified).
Manufacturer/supplier to
specify whether disposal
requirements apply to
contents, container or both.
Mandatory for the general
public if the substance /
mixture is subject to
legislation on hazardous
waste. It is recommended
to specify the site of
disposal while a reference
to the applicable legislation
is not necessary.

Guidance on Labelling and Packaging
Version 4.2 – March 021
125
P280
Wear protective gloves/protective clothing/eye
protection/face protection.
Manufacturer/supplier to specify the appropriate type
of equipment.
Recommended
Recommended for
inclusion in the safety data
sheet if there are specific
disposal requirements
above the normal
expectation for the
disposal of chemicals.
Specify the applicable
regulation.

126
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.12 Substances and mixtures which, in contact with water, emit flammable gases
(continued)
Hazard category Signal word Hazard statement
3 Warning H261 In contact with water releases flammable gases
Precautionary Statements
Prevention
Response
Storage
Disposal
P231 + P232
Handle and store contents under
inert gas/...
Protect from moisture.
- if the substance or mixture reacts
readily with moisture in air.
…Manufacturer/supplier to specify
appropriate liquid or gas if “inert gas”
is not appropriate
Highly recommended where special
emphasis is required
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Recommended
P370 + P378
In case of fire: Use … to
extinguish.
- if water increases risk.
… Manufacturer/supplier to specify
appropriate media.
Highly recommended if specific
extinguishing media are
required or appropriate
P402 + P404
Store in a dry place. Store in
a closed container.
Recommended, unless P231
has already been assigned
Highly recommended for
inclusion in the safety data
sheet
P501
Dispose of contents/container to
…
… in accordance with local/
regional/national/international
regulations (to be specified).
Manufacturer/supplier to specify
whether disposal requirements apply
to contents, container or both.
Mandatory for the general public if
the substance / mixture is subject
to legislation on hazardous waste.
It is recommended to specify the
site of disposal while a reference
to the applicable legislation is not
necessary.
Recommended for inclusion in the
safety data sheet if there are
specific disposal requirements
above the normal expectation for
the disposal of chemicals. Specify
the applicable regulation.
Guidance on Labelling and Packaging
Version 4.2 – March 021
127
7.3.2.13 Oxidising liquids
Hazard category Signal word Hazard statement
1 Danger H271 May cause fire or explosion; strong oxidizer
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot
surfaces, sparks, open flames
and other ignition sources. No
smoking.
Highly recommended
P220
Keep away from clothing and
other combustible materials.
Highly recommended
P280
Wear protective
gloves/protective clothing/eye
protection/ face protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Recommended
P306 + P360
IF ON CLOTHING: Rinse
immediately contaminated clothing
and skin with plenty of water
before removing clothes.
Recommended
P371 + P380 + P375
In case of major fire and large
quantities: Evacuate area. Fight fire
remotely due to the risk of
explosion.
Highly recommended
P370 + P378
In case of fire: Use … to extinguish.
- if water increases risk.
… Manufacturer/supplier to specify
appropriate media.
P420
Store separately.
Recommended where
incompatible materials are
likely to produce a
particular risk. If this
statement is used, text
clarifying the incompatible
materials should be added
as supplemental
information
Optional where P220 has
already been assigned
P501
Dispose of contents/container
to …
… in accordance with local/regional/
national/international regulations
(to be specified).
Manufacturer/supplier to specify
whether disposal requirements
apply to contents, container or both.
Mandatory for the general public
if the substance / mixture is
subject to legislation on
hazardous waste. It is
recommended to specify the site
of disposal while a reference to
the applicable legislation is not
necessary.
Recommended for inclusion in
the safety data sheet if there
are specific disposal
requirements above the normal
expectation for the disposal of
chemicals

128
Guidance on Labelling and Packaging
Version 4.2 – March 2021
P283
Wear fire resistant or flame
retardant clothing.
Recommended for inclusion in
the safety data sheet
Highly recommended if specific
extinguishing media are required or
appropriate

Guidance on Labelling and Packaging
Version 4.2 – March 021
129
7.3.2.13 Oxidising liquids (continued)
Hazard category Signal word Hazard statement
2 Danger H272 May intensify fire; oxidiser
3 Warning H272 May intensify fire; oxidiser
Precautionary Statements
Prevention
Response
Storage
P210
Keep away from heat, hot surfaces, sparks,
open flames and other ignition sources. No
smoking.
Highly recommended
P220
Keep away from clothing and other
combustible materials.
Highly recommended
P280
Wear protective gloves/protective
clothing/eye protection/ face protection.
Manufacturer/supplier to specify the appropriate
type of equipment.
Recommended
P370 + P378
In case of fire: Use … to
extinguish.
- if water increases risk.
… Manufacturer/supplier to
specify appropriate media.
Highly recommended if
specific extinguishing media
are required or appropriate
P501
Dispose of contents/container to
…
… in accordance with local/regional/
national/international regulations (to
be specified).
Manufacturer/supplier to specify
whether disposal requirements apply
to contents, container or both.
Mandatory for the general public
if the substance / mixture is
subject to legislation on
hazardous waste. It is
recommended to specify the site
of disposal while a reference to
the applicable legislation is not
necessary.
Recommended for inclusion in the
safety data sheet if there are
specific disposal requirements
above the normal expectation for
the disposal of chemicals. Specify
the applicable regulation.

130
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.14 Oxidising solids
Hazard category Signal word Hazard statement
1 Danger H271 May cause fire or explosion; strong oxidizer
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces, sparks,
open flames and other ignition sources. No
smoking.
Highly recommended
P220
Keep away from clothing and other combustible
materials.
Highly recommended
P280
Wear protective gloves/protective clothing/eye
protection/face protection.
Manufacturer/supplier to specify the appropriate type
of equipment.
Recommended
P283
Wear fire resistant or flame retardant clothing.
Recommended for inclusion in the safety data
sheet
P306 + P360
IF ON CLOTHING: Rinse
immediately contaminated clothing
and skin with plenty of water
before removing clothes.
Recommended
P371 + P380 + P375
In case of major fire and large
quantities: Evacuate area. Fight
fire remotely due to the risk of
explosion.
Highly recommended
P370 + P378
In case of fire: Use … to
extinguish.
- if water increases risk.
… Manufacturer/supplier to specify
appropriate media.
Highly recommended if specific
extinguishing media are required or
appropriate
P501
Dispose of contents/container
to …
… in accordance with local/regional/
national/international regulations
(to be specified).
Manufacturer/supplier to specify
whether disposal requirements
apply to contents, container or both.
Mandatory for the general public
if the substance / mixture is
subject to legislation on
hazardous waste. It is
recommended to specify the site
of disposal while a reference to
the applicable legislation is not
necessary.
Recommended for inclusion in
the safety data sheet if there
are specific disposal
requirements above the normal
expectation for the disposal of
chemicals. Specify the
applicable regulation.

Guidance on Labelling and Packaging
Version 4.2 – March 021
131
7.3.2.14 Oxidising solids (continued)
Hazard category Signal word Hazard statement
2 Danger H272 May intensify fire; oxidiser
3 Warning H272 May intensify fire; oxidiser
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces,
sparks, open flames and other ignition
sources. No smoking.
Highly recommended
P220
Keep away from clothing and other
combustible materials.
Highly recommended
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Recommended
P370 + P378
In case of fire: Use … to
extinguish.
- if water increases risk.
… Manufacturer/supplier to specify
appropriate media.
Highly recommended if specific
extinguishing media are required
or appropriate
P501
Dispose of contents/container to
…
… in accordance with
local/regional/national/
international regulations (to be
specified).
Manufacturer/supplier to specify
whether disposal requirements apply
to contents, container or both.
Mandatory for the general public if
the substance / mixture is subject
to legislation on hazardous waste.
It is recommended to specify the
site of disposal while a reference to
the applicable legislation is not
necessary.
Recommended for inclusion in the
safety data sheet if there are
specific disposal requirements
above the normal expectation for
the disposal of chemicals. Specify
the applicable regulation.

132
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.15 Organic peroxides
Hazard category Signal word Hazard statement
Type A Danger H240 Heating may cause an explosion
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces,
sparks, open flames and other
ignition sources. No smoking.
Highly recommended
P234
Keep only in original packaging.
Highly recommended where the
packaging is important for
preventing or suppressing the
effect of dangerous reactions or
explosion
P235
Keep cool
- may be omitted if P411 is given on
the label
Optional
P370 + P372 + P380 + P373
In case of fire:
Explosion risk.
Evacuate area. DO NOT fight
fire when fire reaches
explosives
Highly recommended
P403
Store in a well-ventilated place.
- except for temperature controlled
self-reactive substances and mixtures
or organic peroxides because
condensation and consequent freezing
may take place
Highly recommended, in
combination with P411 or P235
P410
Protect from sunlight.
Optional if P411 or P235 has
already been assigned
P411
Store at temperatures not
exceeding …ºC/…ºF.
- if temperature control is required
(according to CLP Annex I, section
2.15.2.3) or if otherwise deemed
necessary.
P501
Dispose of
contents/container to …
… in accordance with
local/regional/
national/international regulations
(to be specified).
Manufacturer/supplier to specify
whether disposal requirements
apply to contents, container or
both.
Recommended for inclusion
in the safety data sheet if
there are specific disposal
requirements above the
normal expectation for the
disposal of chemicals.
Specify the applicable
regulation.
Guidance on Labelling and Packaging
Version 4.2 – March 021
133
P240
Ground and bond container and
receiving equipment
- if electrostatically sensitive and able
to generate an explosive atmosphere
Optional unless other conditions
deem it necessary
Recommended for inclusion in the
safety data sheet
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Highly recommended
… Manufacturer/supplier to specify
temperature using the applicable
temperature scale.
Highly recommended
P420
Store separately.
Recommended where incompatible
materials are likely to produce a
particular risk. If this statement is
used, text clarifying the
incompatible materials should be
added as supplemental information

134
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.15 Organic peroxides (continued)
Hazard category Signal word Hazard statement
Type B Danger H241 Heating may cause a fire or explosion
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot surfaces,
sparks, open flames and other
ignition sources. No smoking.
Highly recommended
P234
Keep only in original packaging.
Highly recommended
P235
Keep cool
- may be omitted if P411 is given on the
label
Optional
P240
Ground and bond container and
receiving equipment
- if electrostatically sensitive and able to
generate an explosive atmosphere
P370 + P380 + P375 [+ P378]
In case of fire: Evacuate area.
Fight fire remotely due to the risk
of explosion. [Use … to
extinguish].
… Manufacturer/supplier to specify
appropriate media.
- text in square brackets to be used if
water increases risk.
Highly recommended
P403
Store in a well-ventilated place.
- except for temperature controlled
self-reactive substances and
mixtures or organic peroxides
because condensation and
consequent freezing may take place
Highly recommended, in
combination with P411 or P235
P410
Protect from sunlight.
Optional if P411 or P235 has
already been assigned
P411
Store at temperatures not
exceeding …ºC/…ºF.
- if temperature control is required
(according to CLP Annex I, section
2.15.2.3) or if otherwise deemed
necessary.
P501
Dispose of
contents/container to …
… in accordance with
local/regional/
national/international
regulations (to be specified).
Manufacturer/supplier to
specify whether disposal
requirements apply to
contents, container or both.
Mandatory for the general
public if the substance /
mixture is subject to
legislation on hazardous
waste. It is recommended
to specify the site of
disposal while a reference
to the applicable
legislation is not
necessary.
Recommended for
inclusion in the safety data

Guidance on Labelling and Packaging
Version 4.2 – March 021
135
Optional unless other conditions
deem it necessary
Recommended for inclusion in the
safety data sheet
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Highly recommended
… Manufacturer/supplier to specify
temperature using the applicable
temperature scale.
Highly recommended
P420
Store separately.
Recommended where
incompatible materials are likely
to produce a particular risk. If
this statement is used, text
clarifying the incompatible
materials should be added as
supplemental information
sheet if there are specific
disposal requirements
above the normal
expectation for the
disposal of chemicals.
Specify the applicable
regulation.

136
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.15 Organic peroxides (continued)
Hazard category Signal word Hazard statement
Type C Danger H242 Heating may cause a fire
Type D Danger H242 Heating may cause a fire
Type E Warning H242 Heating may cause a fire
Type F Warning H242 Heating may cause a fire
Precautionary Statements
Prevention
Response
Storage
Disposal
P210
Keep away from heat, hot
surfaces, sparks, open flames
and other ignition sources. No
smoking.
Highly recommended
P234
Keep only in original packaging.
Highly recommended
P235
Keep cool
- may be omitted if P411 is given on
the label
Optional
P370 + P378
In case of fire: Use … to
extinguish.
- if water increases risk.
… Manufacturer/supplier to
specify appropriate media.
Highly recommended if
specific extinguishing
media are required or
appropriate
P403
Store in a well-ventilated place.
- except for temperature controlled self-
reactive substances and mixtures or
organic peroxides because condensation
and consequent freezing may take place
Highly recommended, in combination
with P411 or P235
P410
Protect from sunlight.
Optional if P411 or P235 has already
been assigned
P411
Store at temperatures not exceeding
…ºC/…ºF.
- if temperature control is required
(according to CLP Annex I, section
2.15.2.3) or if otherwise deemed
necessary.
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to
be specified).
Manufacturer/supplier to specify
whether disposal requirements apply to
contents, container or both.
Mandatory for the general public if
the substance / mixture is subject
to legislation on hazardous waste.
It is recommended to specify the
site of disposal while a reference to
the applicable legislation is not
necessary.
Recommended for inclusion in the
safety data sheet if there are
specific disposal requirements
above the normal expectation for
the disposal of chemicals. Specify
the applicable regulation.

Guidance on Labelling and Packaging
Version 4.2 – March 021
137
P240
Ground and bond container and
receiving equipment
- if electrostatically sensitive and
able to generate an explosive
atmosphere
Optional unless other conditions
deem it necessary
Recommended for inclusion in
the safety data sheet
P280
Wear protective
gloves/protective clothing/eye
protection/face protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Highly recommended
… Manufacturer/supplier to specify
temperature using the applicable
temperature scale.
Highly recommended
P420
Store separately.
Recommended where incompatible
materials are likely to produce a
particular risk. If this statement is
used, text clarifying the incompatible
materials should be added as
supplemental information

138
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.2.16 Corrosive to metals
Hazard category Signal word Hazard statement
1 Warning H290 May be corrosive to metals
Precautionary Statements
Prevention
Response
Storage
Disposal
P234
Keep only in original packaging.
Recommended for the general public
Optional for industrial / professional
users
Recommended for inclusion in the
safety data sheet
P390
Absorb spillage to prevent
material damage.
Recommended
P406
Store in a corrosion resistant/…
container with a resistant inner
liner.
- may be omitted if P234 is given on the
label
… Manufacturer/supplier to specify
other compatible materials.
Optional
Do not use if P234 has already been
assigned

Guidance on Labelling and Packaging
Version 4.2 – March 021
139
7.3.3 Specific precautionary statements for health hazards
7.3.3.1 Acute Toxicity – Oral
Hazard category Signal word Hazard statement
1 Danger H300 Fatal if swallowed
2 Danger H300 Fatal if swallowed
3 Danger H301 Toxic if swallowed
Precautionary Statements
Prevention
Response
Storage
Disposal
P264
Wash … thoroughly after
handling.
Manufacturer/supplier to specify parts
of the body to be washed after
handling.
Highly recommended for the
general public
Recommended for industrial /
professional users
P270
Do not eat, drink or smoke when
using this product.
Highly recommended for the
general public for categories 1
and 2
P301 + P310
IF SWALLOWED: Immediately
call a POISON
CENTER/doctor/...
…Manufacturer/supplier to specify
the appropriate source of
emergency medical advice.
Highly recommended
P321
Specific treatment (see … on
this label).
- if immediate administration of
antidote is required.
… Reference to supplemental first
aid instruction.
P405
Store locked up.
Highly recommended for
the general public
Optional for industrial /
professional users unless
other conditions (Member
State legislation) deem it
necessary
P501
Dispose of contents/container to …
… in accordance with local/
regional/national/international
regulations (to be specified).
Manufacturer/supplier to specify
whether disposal requirements apply to
contents, container or both.
Mandatory for the general public if
the substance / mixture is subject
to legislation on hazardous waste. It
is recommended to specify the site
of disposal while a reference to the
applicable legislation is not
necessary.
Recommended for industrial /
professional users if there are
specific disposal requirements

140
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Recommended for the general
public for category 3
Optional for industrial /
professional users
Recommended for inclusion in the
safety data sheet
Highly recommended only in
exceptional cases where
specific treatment is known and
required
P330 in combination with P301
Rinse mouth.
Highly recommended for the
general public for categories 1
and 2 unless P301+P330+P331
is assigned
Recommended for the general
public for category 3 unless
P301+P330+P331 is assigned
Recommended for industrial /
professional users for
categories 1 and 2 unless
P301+P330+P331 is assigned
Optional for industrial /
professional users for category
3
above the normal expectation for
the disposal of chemicals. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not
necessary.

Guidance on Labelling and Packaging
Version 4.2 – March 021
141
7.3.3.1 Acute Toxicity – Oral (continued)
Hazard category Signal word Hazard statement
4 Warning H302 Harmful if swallowed
Precautionary Statements
Prevention
Response
Storage
Disposal
P264
Wash … thoroughly after handling.
Manufacturer/supplier to specify parts of
the body to be washed after handling.
Recommended
P270
Do not eat, drink or smoke when
using this product.
Recommended for the general public
Optional for industrial / professional
users
Recommended for inclusion in the
safety data sheet
P301 + P312
IF SWALLOWED: Call a
POISON CENTRE/doctor/…if
you feel unwell.
…Manufacturer/supplier to specify
the appropriate source of
emergency medical advice.
Optional
P330
Rinse mouth.
Optional
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified)
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to
legislation on hazardous waste. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not necessary.
Recommended for industrial /
professional users if there are specific
disposal requirements above the normal
expectation for the disposal of chemicals.
It is recommended to specify the site of
disposal while a reference to the
applicable legislation is not necessary.

142
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.1 Acute Toxicity – Dermal
Hazard category Signal word Hazard statement
1 Danger H310 Fatal in contact with skin
2 Danger H310 Fatal in contact with skin
Precautionary Statements
Prevention
Response
Storage
Disposal
P262
Do not get in eyes, on skin, or on
clothing.
Highly recommended
P264
Wash … thoroughly after handling.
Manufacturer / supplier to specify parts of
the body to be washed after handling.
Highly recommended
P270
Do not eat, drink or smoke when
using this product.
Highly recommended for the general
public
Optional for industrial / professional
users.
Recommended for inclusion in the
safety data sheet
P302 + P352
IF ON SKIN: Wash with plenty of
water/…
…Manufacturer/supplier may specify
a cleansing agent if appropriate, or
may recommend an alternative
agent in exceptional cases if water
is clearly inappropriate.
Recommended for the general
public
Recommended for inclusion in
the safety data sheet
P310
Immediately call a POISON
CENTER/doctor/...
…Manufacturer/supplier to specify
the appropriate source of
emergency medical advice.
Highly recommended, in
combination with P302+P352
P405
Store locked up.
Highly recommended for
the general public
Optional for
industrial/professional
users unless other
conditions (Member
State legislation) deem
it necessary
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify
whether disposal requirements apply to
contents, container or both.
Mandatory for the general public if
the substance / mixture is subject
to legislation on hazardous waste.
It is recommended to specify the
site of disposal while a reference to
the applicable legislation is not
necessary.
Recommended for inclusion in the
safety data sheet if there are
specific disposal requirements
above the normal expectation for
the disposal of chemicals. Specify
the applicable regulation.

Guidance on Labelling and Packaging
Version 4.2 – March 021
143
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
- Specify protective gloves/clothing.
Manufacturer/supplier may further specify
type of equipment where appropriate.
Highly recommended
P321
Specific treatment (see … on
this label).
- if immediate measures, such as
specific cleansing agent, are
advised
…Reference to supplemental first
aid instruction.
Highly recommended only in
exceptional cases where specific
treatment is known and
required
P361 + P364
Take off immediately all
contaminated clothing and wash
it before reuse
Recommended

144
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.1 Acute Toxicity – Dermal (continued)
Hazard category Signal word Hazard statement
3 Danger H311 Toxic in contact with skin
Precautionary Statements
Prevention
Response
Storage
Disposal
P280
Wear protective
gloves/protective
clothing/eye protection/face
protection.
- Specify protective
gloves/clothing.
Manufacturer/supplier may further
specify type of equipment where
appropriate.
Highly recommended
P302 + P352
IF ON SKIN: Wash with plenty of
water/…
…Manufacturer/supplier may specify a
cleansing agent if appropriate, or may
recommend an alternative agent in
exceptional cases if water is clearly
inappropriate.
Recommended for the general
public
Recommended for inclusion in the
safety data sheet
P312
Call a POISON
CENTRE/doctor/…if you feel
unwell.
…Manufacturer/supplier to specify the
appropriate source of emergency
medical advice.
Recommended
P405
Store locked up.
Highly recommended for
the general public
Optional for industrial /
professional users unless
other conditions (Member
State legislation) deem it
necessary
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to
legislation on hazardous waste. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not necessary.
Recommended for industrial /
professional users if there are specific
disposal requirements above the normal
expectation for the disposal of
chemicals. It is recommended to specify
the site of disposal while a reference to
the applicable legislation is not
necessary.

Guidance on Labelling and Packaging
Version 4.2 – March 021
145
P321
Specific treatment (see … on this
label).
- if immediate measures, such as
specific cleansing agent, are advised
…Reference to supplemental first aid
instruction.
Highly recommended only in
exceptional cases where specific
treatment is known and required
P361+P364
Take off immediately all
contaminated clothing and wash
it before reuse.
Recommended
146
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.1 Acute Toxicity – Dermal (continued)
Hazard category Signal word Hazard statement
4 Warning H312 Harmful in contact with skin
Precautionary Statements
Prevention
Response
Storage
Disposal
P280
Wear protective gloves/ protective
clothing /eye protection/face
protection.
- Specify protective gloves/clothing.
Manufacturer/supplier may further
specify type of equipment where
appropriate.
Recommended
P302 + P352
IF ON SKIN: Wash with plenty of
water/…
…Manufacturer/supplier may specify a
cleansing agent if appropriate, or may
recommend an alternative agent in
exceptional cases if water is clearly
inappropriate.
Optional
P312
Call a POISON CENTRE/doctor/…if
you feel unwell.
…Manufacturer/supplier to specify the
appropriate source of emergency medical
advice.
Recommended
P501
Dispose of contents/container to …
… in accordance with
local/regional/national/international
regulations (to be specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to
legislation on hazardous waste. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not necessary.
Recommended for industrial /
professional users if there are specific
disposal requirements above the
normal expectation for the disposal of
chemicals. It is recommended to
specify the site of disposal while a
reference to the applicable legislation
is not necessary.

Guidance on Labelling and Packaging
Version 4.2 – March 021
147
P321
Specific treatment (see … on this
label).
- if immediate measures, such as specific
cleansing agent, are advised.
…Reference to supplemental first aid
instruction.
Highly recommended only in
exceptional cases where specific
treatment is known and required
P362 + P364
Take off contaminated clothing and
wash it before reuse.
Optional

148
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.1 Acute Toxicity - Inhalation
Hazard category Signal word Hazard statement
1 Danger H330 Fatal if inhaled
2 Danger H330 Fatal if inhaled
Precautionary Statements
Prevention
Response
Storage
Disposal
P260
Do not breathe
dust/fume/gas/mist/vapours/
spray.
Manufacturer/supplier to specify
applicable conditions.
Highly recommended
P271
Use only outdoors or in a well-
ventilated area.
Highly recommended for the
general public
Optional for
industrial/professional users
P304 + P340
IF INHALED: Remove person
to fresh air and keep at rest
in a position comfortable for
breathing.
Highly recommended
P310
Immediately call a POISON
CENTER/doctor/…
…Manufacturer/supplier to
specify the appropriate source of
emergency medical advice.
Highly recommended, in
combination with P304+P340
P403 + P233
Store in a well-ventilated
place. Keep container tightly
closed.
- if the substance or mixture is
volatile and may generate a
hazardous atmosphere.
Highly recommended unless
P404 has already been
assigned
P405
Store locked up.
Highly recommended for the
general public
Optional for industrial /
professional users unless
other conditions (Member
State legislation) deem it
necessary
P501
Dispose of contents/container to …
… in accordance with
local/regional/national/international
regulations (to be specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to
legislation on hazardous waste. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not necessary.
Recommended for industrial /
professional users if there are specific
disposal requirements above the
normal expectation for the disposal of
chemicals. It is recommended to
specify the site of disposal while a
reference to the applicable legislation
is not necessary.

Guidance on Labelling and Packaging
Version 4.2 – March 021
149
P284
[In case of inadequate
ventilation] wear respiratory
protection.
- text in square brackets may be
used if additional information is
provided with the chemical at the
point of use that explains what type
of ventilation would be adequate for
safe use.
Manufacturer/supplier to specify
equipment.
Recommended for
industrial/professional users in
exceptional cases where
inadequate
ventilation/organisational
measures cannot sufficiently
prevent inhalation
Recommended for inclusion in the
safety data sheet
P320
Specific treatment is urgent
(see … on this label)
- if immediate administration of
antidote is required.
… Reference to supplemental
first aid instruction.
Highly recommended only in
exceptional cases where
specific treatment is known
and required

150
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.1 Acute Toxicity – Inhalation (continued)
Hazard category Signal word Hazard statement
3 Danger H331 Toxic if inhaled
Precautionary Statements
Prevention
Response
Storage
Disposal
P261
Avoid breathing
dust/fume/gas/mist/
vapours/spray.
- may be omitted if P260 is
given on the label.
Manufacturer/supplier to specify
applicable conditions.
Recommended
P271
Use only outdoors or in a
well-ventilated area.
Highly recommended for the
general public
Optional for
industrial/professional users
P304 + P340
IF INHALED: Remove person to fresh
air and keep comfortable for breathing.
Recommended
P311
Call a POISON CENTER/doctor/...
…Manufacturer/supplier to specify the
appropriate source of emergency medical
advice.
Recommended, in combination with
P304+P340
P321
Specific treatment (see … on this label)
- if immediate specific measures are
required.
…Reference to supplemental first aid
instruction.
Highly recommended only in exceptional
cases where specific treatment is known
and required
P403 + P233
Store in a well-ventilated
place. Keep container
tightly closed.
- if the substance or mixture
is volatile and may generate
a hazardous atmosphere.
Highly recommended
P405
Store locked up.
Highly recommended for
the general public
Optional for industrial /
professional users
unless other conditions
(Member State
legislation) deem it
necessary
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify
whether disposal requirements apply to
contents, container or both.
Mandatory for the general public if
the substance / mixture is subject to
legislation on hazardous waste. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not
necessary.
Recommended for industrial /
professional users if there are
specific disposal requirements above
the normal expectation for the
disposal of chemicals. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not
necessary.

Guidance on Labelling and Packaging
Version 4.2 – March 021
151
7.3.3.1 Acute Toxicity – Inhalation (continued)
Hazard category Signal word Hazard statement
4 Warning H332 Harmful if inhaled
Precautionary Statements
Prevention
Response
Storage
Disposal
P261
Avoid breathing
dust/fume/gas/mist/
vapours/spray.
- may be omitted if P260 is given on
the label.
Manufacturer/supplier to specify
applicable conditions.
Recommended
P271
Use only outdoors or in a well-
ventilated area.
Highly recommended for the general
public
Optional for industrial/professional
users
P304 + P340
IF INHALED: Remove person to
fresh air and keep comfortable for
breathing.
Optional
P312
Call a POISON CENTRE/doctor/…if
you feel unwell.
…Manufacturer/supplier to specify the
appropriate source of emergency
medical advice.
Recommended

152
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.2 Skin corrosion/irritation
Hazard category Signal word Hazard statement
Sub-categories 1A, 1B, 1C
and Category 1 Danger H314 Causes severe skin burns and eye damage
Precautionary Statements
Prevention
Response
Storage
Disposal
P260
Do not breathe dust/fume/gas/mist/
vapours/spray.
Manufacturer/supplier to specify
applicable conditions.
- specify do not breathe dusts or mists.
- If inhalable particles of dusts or mists
may occur during use.
Highly recommended
P264
Wash … thoroughly after handling.
Manufacturer/supplier to specify parts of
the body to be washed after handling.
Highly recommended for the general
public, unless P280 has already been
assigned
P301 + P330 + P331
IF SWALLOWED: Rinse mouth. Do
NOT induce vomiting.
Highly recommended for the general
public, provided that medical advice
indicates that the statement is
appropriate
Recommended for industrial /
professional users, provided that
medical advice indicates that the
statement is appropriate
P303 + P361 + P353
IF ON SKIN (or hair): Take off
immediately all contaminated
clothing. Rinse skin with water [or
shower].
- text in square brackets to be included
where the manufacturer/supplier
considers it appropriate for the specific
chemical.
P405
Store locked up.
Highly recommended for
the general public
Optional for industrial /
professional users unless
other conditions (Member
State legislation) deem it
necessary
P501
Dispose of
contents/container to …
…in accordance with
local/regional/
national/international
regulations (to be specified).
Manufacturer/supplier to specify
whether disposal requirements
apply to contents, container or
both.
Mandatory for the general
public if the substance /
mixture is subject to
legislation on hazardous
waste. It is recommended
to specify the site of
disposal while a reference to
the applicable legislation is
not necessary.
Recommended for industrial
/ professional users if there

Guidance on Labelling and Packaging
Version 4.2 – March 021
153
Highly recommended for industrial /
professional users, unless P280 has
already been assigned
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
- Specify protective gloves/clothing and
eye/face protection.
Manufacturer/supplier may further specify
type of equipment where appropriate.
Highly recommended
Highly recommended
P363
Wash contaminated clothing before
reuse.
Recommended for the general public
Recommended for inclusion in the
safety data sheet
P304 + P340
If INHALED: Remove person to fresh
air and keep comfortable for
breathing.
Optional
P310
Immediately call a POISON
CENTER/doctor/...
…Manufacturer/supplier to specify the
appropriate source of emergency
medical advice.
Highly recommended, in
combination with P303+P361+P353,
P305+P351+ P338 or P301 + P330
+ P331
P321
Specific treatment (see … on this
label).
…Reference to supplemental first aid
instruction.
Manufacturer/supplier may specify a
cleansing agent if appropriate.
are specific disposal
requirements above the
normal expectation for the
disposal of chemicals. It is
recommended to specify the
site of disposal while a
reference to the applicable
legislation is not necessary.

154
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Highly recommended only in
exceptional cases where specific
treatment is known and required
P305 + P351 + P338
IF IN EYES: Rinse cautiously with
water for several minutes. Remove
contact lenses, if present and easy
to do. Continue rinsing.
Highly recommended

Guidance on Labelling and Packaging
Version 4.2 – March 021
155
7.3.3.2 Skin corrosion/irritation (continued)
Hazard category Signal word Hazard statement
2 Warning H315 Causes skin irritation
Precautionary Statements
Prevention
Response
Storage
Disposal
P264
Wash … thoroughly after
handling.
… Manufacturer/supplier to specify
parts of the body to be washed after
handling.
Recommended
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
- Specify protective gloves.
Manufacturer/supplier may further
specify type of equipment where
appropriate.
Recommended
P302 + P352
IF ON SKIN: Wash with plenty of water/...
…Manufacturer/supplier may specify a cleansing
agent if appropriate, or may recommend an
alternative agent in exceptional cases if water is
clearly inappropriate.
Optional for the general public
Recommended for inclusion in the safety data
sheet
P321
Specific treatment (see … on this label).
…Reference to supplemental first aid instruction.
Manufacturer/supplier may specify a cleansing
agent if appropriate.
Recommended only in exceptional cases where
specific treatment is known and required

156
Guidance on Labelling and Packaging
Version 4.2 – March 2021
P332 + P313
If skin irritation occurs: Get medical
advice/attention.
- may be omitted when P333 + P313 is given on
the label.
Optional
P362 + P364
Take off contaminated clothing and wash it
before reuse.
Optional
Recommended for inclusion in the safety data
sheet

Guidance on Labelling and Packaging
Version 4.2 – March 021
157
7.3.3.3 Serious eye damage - only
52
Hazard category Signal word Hazard statement
1 Danger H318 Causes serious eye damage
Precautionary Statements
Prevention
Response
Storage
Disposal
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
- Specify eye/face protection.
Manufacturer/supplier may further
specify type of equipment where
appropriate.
Highly recommended
P305 + P351 + P338
IF IN EYES: Rinse cautiously with
water for several minutes. Remove
contact lenses, if present and easy to
do. Continue rinsing.
Highly recommended
P310
Immediately call a POISON CENTER/
doctor/...
…Manufacturer/supplier to specify the
appropriate source of emergency medical
advice.
Highly recommended, in combination
with P305+P351+P338
52
Where a chemical is classified as skin corrosion Sub-Category 1A, 1B, 1C or Category 1, labelling for serious eye damage/eye irritation can be omitted as this information
is already included in the hazard statement for skin corrosion Category 1 (H314).

158
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.3 Eye irritation – only
53
Hazard category Signal word Hazard statement
2 Warning H319 Causes serious eye irritation
Precautionary Statements
Prevention
Response
Storage
Disposal
P264
Wash … thoroughly after handling.
… Manufacturer/supplier to specify parts of the body to
be washed after handling.
Optional for the industrial/ professional users
Recommended for the general public
P280
Wear protective gloves/protective clothing/eye
protection/face protection.
- Specify eye/face protection.
Manufacturer/supplier may further specify type of
equipment where appropriate.
Recommended
P305 + P351 + P338
IF IN EYES: Rinse cautiously
with water for several
minutes. Remove contact
lenses, if present and easy to
do. Continue rinsing.
Recommended for the general
public
Recommended for inclusion in
the safety data sheet
P337 + P313
If eye irritation persists: Get
medical advice/attention.
Recommended
53
Where a chemical is classified as skin corrosion Sub-Category 1A, 1B, 1C or Category 1, labelling for serious eye damage/eye irritation can be omitted as this information
is already included in the hazard statement for skin corrosion Category 1 (H314).

Guidance on Labelling and Packaging
Version 4.2 – March 021
159
7.3.3.4 Respiratory sensitisation
Hazard category Signal word Hazard statement
1, 1A, 1B Danger H334 May cause allergy or asthma symptoms or breathing
difficulties if inhaled
Precautionary Statements
Prevention
Response
Storage
Disposal
P261
Avoid breathing dust/fume/gas/mist/
vapours/spray.
- may be omitted if P260 is given on the label.
Manufacturer/supplier to specify applicable conditions.
Highly recommended
P284
[In case of inadequate ventilation] wear
respiratory protection.
- text in square brackets may be used if additional
information is provided with the chemical at the
point of use that explains what type of ventilation
would be adequate for safe use.
Manufacturer/supplier to specify equipment.
Recommended for industrial/professional users in
exceptional cases where inadequate
ventilation/organisational measures cannot
sufficiently prevent inhalation
Recommended for inclusion in the safety data
sheet
P304 + P340
IF INHALED: Remove
person to fresh air and
keep comfortable for
breathing.
Highly recommended
P342 + P311
If experiencing
respiratory symptoms:
Call a POISON CENTER or
doctor/physician.
Highly recommended
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to
legislation on hazardous waste. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not necessary.
Recommended for industrial /
professional users if there are specific
disposal requirements above the
normal expectation for the disposal of
chemicals. It is recommended to
specify the site of disposal while a
reference to the applicable legislation
is not necessary.
160
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.4 Skin sensitisation
Hazard category Signal word Hazard statement
1, 1A, 1B Warning H317 May cause an allergic skin reaction
Precautionary Statements
Prevention
Response
Storage
Disposal
P261
Avoid breathing dust/fume/gas/mist/
vapours/spray.
- may be omitted if P260 is given on the
label.
Manufacturer/supplier to specify applicable
conditions.
Recommended
P272
Contaminated work clothing should not
be allowed out of the workplace.
Not intended to be used for the general
public
Optional for industrial/professional users
P302 + P352
IF ON SKIN: Wash with plenty of
water/...
…Manufacturer/supplier may specify a
cleansing agent if appropriate, or may
recommend an alternative agent in
exceptional cases if water is clearly
inappropriate.
Recommended for the general
public
Recommended for inclusion in the
safety data sheet
P333 + P313
If skin irritation or rash occurs: Get
medical advice/attention.
Recommended
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to
legislation on hazardous waste. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not necessary.
Recommended for industrial /
professional users if there are specific
disposal requirements above the
normal expectation for the disposal of
chemicals. It is recommended to
specify the site of disposal while a
reference to the applicable legislation
is not necessary.

Guidance on Labelling and Packaging
Version 4.2 – March 021
161
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
- Specify protective gloves.
Manufacturer/supplier may further specify
type of equipment where appropriate.
Highly recommended
P321
Specific treatment (see … on this
label)
… Reference to supplemental first aid
instruction.
Manufacturer/supplier may specify a
cleansing agent if appropriate.
Highly recommended only in
exceptional cases where specific
treatment is known and required
P362+P364
Take off contaminated clothing and
wash it before reuse.
Recommended

162
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.5 Germ cell mutagenicity
Hazard category Signal word Hazard statement
1A and 1B Danger H340 May cause genetic defects (state route of exposure if it is
conclusively proven that no other routes of exposure cause
the hazard)
2 Warning H341 Suspected of causing genetic defects (state route of exposure
if it is conclusively proven that no other routes of exposure cause
the hazard)
Precautionary Statements
Prevention
Response
Storage
Disposal
P201
Obtain special instructions before use.
Highly recommended for category 1A
and 1B
Recommended for category 2
P202
Do not handle until all safety
precautions have been read and
understood.
Optional where P201 is assigned
P308 + P313
IF exposed or concerned: Get
medical advice/attention.
Highly recommended for
category 1A and 1B
Recommended for category 2
P405
Store locked up.
Highly recommended for the
general public
54
Optional for
industrial/professional users
unless other conditions
(Member State legislation)
deem it necessary
P501
Dispose of contents/container to
…
… in accordance with local/regional/
national/international regulations (to
be specified).
Manufacturer/supplier to specify
whether disposal requirements apply
to contents, container or both.
Mandatory for the general public if
the substance / mixture is subject
to legislation on hazardous waste.
It is recommended to specify the
site of disposal while a reference
to the applicable legislation is not
54
Substances and mixtures which are listed in Appendix 1-6 of Annex XVII to Regulation (EC) No 1907/2006 (REACH) and which are assigned H340,
H350 or H360 are restricted to industrial / professional users and normally not supplied to the general public (see entry 28, 29 and 30 in Annex XVII to
REACH, as amended). The list of subsequent amendments of Annex XVII is accessible at http://echa.europa.eu/web/guest/regulations/reach/legislation.

Guidance on Labelling and Packaging
Version 4.2 – March 021
163
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Highly recommended
necessary.
Recommended for industrial /
professional users if there are
specific disposal requirements
above the normal expectation for
the disposal of chemicals. It is
recommended to specify the site
of disposal while a reference to
the applicable legislation is not
necessary.

164
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.6 Carcinogenicity
Hazard category Signal word Hazard statement
1A and 1B Danger H350 May cause cancer (state route of exposure if it is conclusively
proven that no other routes of exposure cause the hazard)
2 Warning H351 Suspected of causing cancer (state route of exposure if it is
conclusively proven that no other routes of exposure cause the
hazard)
Precautionary Statements
Prevention
Response
Storage
Disposal
P201
Obtain special instructions before
use.
Highly recommended for category
1A and 1B
Recommended for category 2
P202
Do not handle until all safety
precautions have been read and
understood.
Optional where P201 is assigned
P308 + P313
IF exposed or concerned: Get
medical advice/attention.
Highly recommended for
category 1A and 1B
Recommended for category
2
P405
Store locked up.
Highly recommended for the
general public
55
Optional for
industrial/professional users
unless other conditions
(Member State legislation)
deem it necessary
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if
the substance / mixture is subject to
legislation on hazardous waste. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not
necessary.
55
Substances and mixtures which are listed in Appendix 1-6 of Annex XVII to Regulation (EC) No 1907/2006 (REACH) and which are assigned H340, H350
or H360 are restricted to industrial / professional users and normally not supplied to the general public (see entry 28, 29 and 30 in Annex XVII to REACH
as amended). The list of subsequent amendments of Annex XVII is accessible at: http://echa.europa.eu/web/guest/regulations/reach/legislation.

Guidance on Labelling and Packaging
Version 4.2 – March 021
165
P280
Wear protective gloves/protective
clothing/eye protection/face
protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Highly recommended
Recommended for industrial /
professional users if there are specific
disposal requirements above the
normal expectation for the disposal of
chemicals. It is recommended to
specify the site of disposal while a
reference to the applicable legislation
is not necessary.

166
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.7 Reproductive toxicity
Hazard category Signal word Hazard statement
1A and 1B Danger H360 May damage fertility or the unborn child
(state specific effect if known)
(state route of exposure if it is conclusively proven
that no other routes of exposure cause the hazard)
2 Warning H361 Suspected of damaging fertility or the unborn child
(state specific effect if known)
(state route of exposure if it is conclusively proven that
no other routes of exposure cause the hazard)
Precautionary Statements
Prevention
Response
Storage
Disposal
P201
Obtain special instructions
before use.
Highly recommended for
category 1A and 1B
Recommended for category 2
P202
Do not handle until all safety
precautions have been read and
understood.
P308 + P313
IF exposed or concerned: Get
medical advice/attention.
Highly recommended for
category 1A and 1B
Recommended for category 2
P405
Store locked up.
Highly recommended
for the general
public
56
Optional for
industrial /
professional users
unless other
conditions (Member
State legislation)
deem it necessary
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to legislation
on hazardous waste. It is recommended to
specify the site of disposal while a reference
56
Substances and mixtures which are listed in Appendix 1-6 of Annex XVII to Regulation (EC) No 1907/2006 (REACH) and which are assigned H340,
H350 or H360 are restricted to industrial / professional users and normally not supplied to the general public (see entry 28, 29 and 30 in Annex XVII to
REACH as amended). The list of subsequent amendments of Annex XVII is accessible at ECHA website:
http://echa.europa.eu/web/guest/regulations/reach/legislation).

Guidance on Labelling and Packaging
Version 4.2 – March 021
167
Optional where P201 is assigned
P280
Wear protective
gloves/protective clothing/eye
protection/face protection.
Manufacturer/supplier to specify the
appropriate type of equipment.
Highly recommended
to the applicable legislation is not necessary.
Recommended for industrial / professional
users if there are specific disposal
requirements above the normal expectation
for the disposal of chemicals. It is
recommended to specify the site of disposal
while a reference to the applicable legislation
is not necessary.

168
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.7 Reproductive toxicity (continued)
Hazard category Signal word Hazard statement
Additional category for effects on
or via lactation No signal word H362 May cause harm to breast-fed children
Precautionary Statements
Prevention
Response
Storage
Disposal
P201
Obtain special instructions before use.
Highly recommended
P260
Do not breathe dust/fume/gas/mist/
vapours/spray.
Manufacturer/supplier to specify applicable
conditions.
- Specify do not breathe dusts or mists.
- if inhalable particles of dusts or mists may
occur during use.
Highly recommended
P263
Avoid contact during pregnancy and
while nursing.
Highly recommended
P308 + P313
IF exposed or concerned: Get
medical advice/attention.
Recommended
No hazard
pictogram

Guidance on Labelling and Packaging
Version 4.2 – March 021
169
P264
Wash … thoroughly after handling.
… Manufacturer / supplier to specify parts of
the body to be washed after handling.
Optional
P270
Do not eat, drink or smoke when using
this product.
Recommended for the general public
Optional for industrial / professional users
Recommended for inclusion in the safety
data sheet

170
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.8 Specific target organ toxicity after single exposure
Hazard category Signal word Hazard statement
1 Danger H370 Causes damage to organs (or state all organs affected, if known)
(state route of exposure if it is conclusively proven that no other
routes of exposure cause the hazard)
Precautionary Statements
Prevention
Response
Storage
Disposal
P260
Do not breathe
dust/fume/gas/mist/
vapours/spray.
Manufacturer/supplier to specify
applicable conditions.
Highly recommended where the
substance / mixture is volatile or a
gas or where exposure via
inhalation is possible, e.g. through
spraying or inhalable dust or in
case H370 indicates inhalation as a
route of exposure
P264
Wash … thoroughly after handling.
… Manufacturer / supplier to specify
parts of the body to be washed after
handling.
Optional
P308 + P311
IF exposed or concerned: Call
a POISON CENTER/doctor…
Manufacturer/supplier to specify
the appropriate source of
emergency medical advice.
Highly recommended
P321
Specific treatment (see … on
this label)
- if immediate measures are
required.
… Reference to supplemental first
aid instruction.
Highly recommended only in
exceptional cases where
specific treatment is known
and required
P405
Store locked up.
Highly recommended for
the general public
Optional for industrial /
professional users unless
other conditions (Member
State legislation) deem it
necessary
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to
legislation on hazardous waste. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not necessary.
Recommended for industrial /
professional users if there are specific
disposal requirements above the
normal expectation for the disposal of
chemicals. It is recommended to
specify the site of disposal while a
reference to the applicable legislation
is not necessary.
Guidance on Labelling and Packaging
Version 4.2 – March 021
171
P270
Do not eat, drink or smoke when
using this product.
Recommended for the general
public
Optional for industrial / professional
users
Recommended for inclusion in the
safety data sheet

172
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.8 Specific target organ toxicity after single exposure (continued)
Hazard category Signal word Hazard statement
2 Warning H371 May cause damage to organs (or state all organs affected, if
known)
(state route of exposure if it is conclusively proven that no other
routes of exposure cause the hazard)
Precautionary Statements
Prevention
Response
Storage
Disposal
P260
Do not breathe
dust/fume/gas/mist/
vapours/spray.
Manufacturer/supplier to specify
applicable conditions.
Highly recommended where the
substance / mixture is volatile or a
gas or where exposure via
inhalation is possible, e.g. through
spraying or inhalable dust or in
case H371 indicates inhalation as a
route of exposure
P264
Wash … thoroughly after handling.
… Manufacturer / supplier to specify
parts of the body to be washed after
handling.
Optional
P308 + P311
IF exposed or concerned: Call
a POISON CENTER/ doctor/...
Manufacturer/supplier to specify
the appropriate source of
emergency medical advice
Recommended
P405
Store locked up.
Highly recommended for
the general public
Optional for industrial /
professional users unless
other conditions (Member
State legislation) deem it
necessary
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to
legislation on hazardous waste. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not necessary.
Recommended for industrial /
professional users if there are specific
disposal requirements above the
normal expectation for the disposal of
chemicals. It is recommended to
specify the site of disposal while a
reference to the applicable legislation

Guidance on Labelling and Packaging
Version 4.2 – March 021
173
P270
Do not eat, drink or smoke when
using this product.
Recommended for the general
public
Optional for industrial / professional
users
Recommended for inclusion in the
safety data sheet
is not necessary.

174
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.8 Specific target organ toxicity after single exposure (continued)
Hazard category Signal word Hazard statement
3 Warning H335 May cause respiratory irritation; or
H336 May cause drowsiness or dizziness
Precautionary Statements
Prevention
Response
Storage
Disposal
P261
Avoid breathing
dust/fume/gas/mist/
vapours/spray.
- may be omitted if P260 is
given on the label.
Manufacturer/supplier to specify
applicable conditions.
Recommended
P271
Use only outdoors or in a
well-ventilated area.
Highly recommended for the
general public
Optional for industrial /
professional users
P304 + P340
IF INHALED: Remove person to
fresh air and keep comfortable
for breathing.
Optional
P312
Call a POISON
CENTRE/doctor/…if you feel
unwell.
…Manufacturer/supplier to specify
the appropriate source of
emergency medical advice.
Recommended
P403 + P233
Store in a well-ventilated
place. Keep container tightly
closed.
- if the substance or mixture is
volatile and may generate a
hazardous atmosphere.
Recommended unless P404 is
assigned
P405
Store locked up.
Highly recommended for the
general public
Optional for industrial /
professional users unless other
conditions (Member State
legislation) deem it necessary
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to
legislation on hazardous waste. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not necessary.
Recommended for industrial /
professional users if there are specific
disposal requirements above the normal
expectation for the disposal of
chemicals. It is recommended to specify
the site of disposal while a reference to
the applicable legislation is not
necessary.

Guidance on Labelling and Packaging
Version 4.2 – March 021
175
7.3.3.9 Specific target organ toxicity after repeated exposure
Hazard category Signal word Hazard statement
1 Danger H372 Causes damage to organs (state all organs affected, if known)
through prolonged or repeated exposure (state route of
exposure if it is conclusively proven that no other routes of
exposure cause the hazard)
Precautionary Statements
Prevention
Response
Storage
Disposal
P260
Do not breathe dust/fume/gas/mist/
vapours/spray.
Manufacturer/supplier to specify
applicable conditions.
Highly recommended where the
substance / mixture is volatile or a
gas or where exposure via inhalation
is possible, e.g. through spraying or
inhalable dust or in case H372
indicates inhalation as a route of
exposure
P264
Wash … thoroughly after handling.
… Manufacturer / supplier to specify parts
of the body to be washed after handling.
Optional
P314
Get medical advice/attention
if you feel unwell.
Recommended
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify whether disposal
requirements apply to contents, container or both.
Mandatory for the general public if the
substance / mixture is subject to legislation
on hazardous waste. It is recommended to
specify the site of disposal while a reference
to the applicable legislation is not necessary.
Recommended for industrial / professional
users if there are specific disposal
requirements above the normal expectation
for the disposal of chemicals. It is
recommended to specify the site of disposal
while a reference to the applicable legislation
is not necessary.

176
Guidance on Labelling and Packaging
Version 4.2 – March 2021
P270
Do not eat, drink or smoke when
using this product.
Recommended for the general public
Optional for industrial / professional
users
Recommended for inclusion in the
safety data sheet

Guidance on Labelling and Packaging
Version 4.2 – March 021
177
7.3.3.9 Specific target organ toxicity after repeated exposure (continued)
Hazard category Signal word Hazard statement
2 Warning H373 May cause damage to organs (state all organs affected, if
known) through prolonged or repeated exposure (state route of
exposure if it is conclusively proven that no other routes of
exposure
cause the hazard)
Precautionary Statements
Prevention
Response
Storage
Disposal
P260
Do not breathe
dust/fume/gas/mist/vapours/spray.
Manufacturer/supplier to specify
applicable conditions.
Highly recommended where the
substance / mixture is highly volatile
or a gas or where exposure via
inhalation is possible, e.g. through
spraying or inhalable dust or in case
H373 indicates inhalation as a route
of exposure
P314
Get medical advice/attention
if you feel unwell.
Recommended
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to
legislation on hazardous waste. It is
recommended to specify the site of
disposal while a reference to the
applicable legislation is not necessary.
Recommended for industrial /
professional users if there are specific
disposal requirements above the normal
expectation for the disposal of
chemicals. It is recommended to specify
the site of disposal while a reference to
the applicable legislation is not
necessary.

178
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.3.10 Aspiration hazard
Hazard category Signal word Hazard statement
1 Danger H304 May be fatal if swallowed and enters airways
Precautionary Statements
Prevention
Response
Storage
Disposal
P301 + P310
IF SWALLOWED: Immediately
call a POISON CENTER/
doctor/...
…Manufacturer/supplier to specify
the appropriate source of
emergency medical advice.
Highly recommended, in
combination with P331
P331
Do NOT induce vomiting.
Highly recommended, in
combination with P301 +P310
P405
Store locked up.
Highly recommended for the
general public
Optional for industrial /
professional users unless other
conditions (Member State
legislation) deem it necessary
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be specified).
Manufacturer/supplier to specify whether disposal
requirements apply to contents, container or both.
Mandatory for the general public if the
substance / mixture is subject to legislation on
hazardous waste. It is recommended to
specify the site of disposal while a reference to
the applicable legislation is not necessary.
Recommended for industrial / professional
users if there are specific disposal
requirements above the normal expectation
for the disposal of chemicals. It is
recommended to specify the site of disposal
while a reference to the applicable legislation
is not necessary.

Guidance on Labelling and Packaging
Version 4.2 – March 021
179
7.3.4 Specific precautionary statements for environmental hazards
7.3.4.1 Hazardous to the aquatic environment – short-term (acute) aquatic hazard
Hazard category Signal word Hazard statement
1 Warning H400 Very toxic to aquatic life
Precautionary Statements
Prevention
Response
Storage
Disposal
P273
Avoid release to the environment.
- if this is not the intended use.
Highly recommended
P391
Collect spillage.
Highly recommended
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to legislation
on hazardous waste. It is recommended to
specify the site of disposal while a reference
to the applicable legislation is not necessary.
Recommended for industrial / professional
users if there are specific disposal
requirements above the normal expectation
for the disposal of chemicals. It is
recommended to specify the site of disposal
while a reference to the applicable legislation
is not necessary.

180
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.4.1 Hazardous to the aquatic environment – long-term (chronic) aquatic hazard
Hazard category Signal word Hazard statement
1 Warning H410 Very toxic to aquatic life with long lasting effects
2 No signal word H411 Toxic to aquatic life with long lasting effects
Precautionary Statements
Prevention
Response
Storage
Disposal
P273
Avoid release to the environment.
- if this is not the intended use.
Highly recommended
P391
Collect spillage.
Highly recommended
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to legislation
on hazardous waste. It is recommended to
specify the site of disposal while a
reference to the applicable legislation is not
necessary.
Recommended for industrial / professional
users if there are specific disposal
requirements above the normal
expectation for the disposal of chemicals. It
is recommended to specify the site of
disposal while a reference to the applicable
legislation is not necessary.
Guidance on Labelling and Packaging
Version 4.2 – March 021
181
7.3.4.1 Hazardous to the aquatic environment – long-term (chronic) aquatic hazard (continued)
Hazard category Signal word Hazard statement
3 No signal word H412 Harmful to aquatic life with long lasting effects
4 No signal word H413 May cause long lasting harmful effects to aquatic life
Precautionary Statements
Prevention
Response
Storage
Disposal
P273
Avoid release to the environment.
- if this is not the intended use.
Recommended
P501
Dispose of contents/container to …
… in accordance with local/regional/
national/international regulations (to be
specified).
Manufacturer/supplier to specify whether
disposal requirements apply to contents,
container or both.
Mandatory for the general public if the
substance / mixture is subject to legislation
on hazardous waste. It is recommended to
specify the site of disposal while a reference
to the applicable legislation is not necessary.
Recommended for industrial / professional
users if there are specific disposal
requirements above the normal expectation
for the disposal of chemicals. It is
recommended to specify the site of disposal
while a reference to the applicable legislation
is not necessary.
No hazard
pictogram
is used

182
Guidance on Labelling and Packaging
Version 4.2 – March 2021
7.3.5 Additional hazards
7.3.5.1 Hazardous to the ozone layer
Hazard category Signal word Hazard statement
1 Warning H420 Harms public health and the environment by destroying ozone
in the upper atmosphere
Precautionary Statements
Prevention
Response
Storage
Disposal
P502
Refer to manufacturer or supplier for
information on recovery or recycling
Mandatory for the general public
Highly recommended for industrial /
professional users

Guidance on Labelling and Packaging
Version 4.2 – March 2021
183
7.4. Examples for the selection of precautionary statements for
the label
This section provides practical examples on how to select precautionary statements for
various model substances. The set of precautionary statements to be prioritised for the
label is highlighted in bold underlined (highly recommended) and underlined
(recommended), while the optional statements appear in normal letters (no highlighting)
and the statements not to be used/unless condition applies/ inclusion on safety data
sheet only are marked in grey colour.
Please note that even if a substance or mixture has the same hazards as one of the
following examples, another set of precautionary statements might be appropriate based
on the specific conditions for use given in the tables above.
Example A. Substance X assigned a physical and various health hazard
classifications
A. Classification and hazard statements:
Flam. Liq. 2 H225 Highly flammable liquid and vapour
Acute Tox. 3 (oral) H301 Toxic if swallowed
Acute Tox. 3 (dermal) H311 Toxic in contact with skin
Acute Tox. 3 (inhalation) H331 Toxic if inhaled
STOT-SE 1 H370 Causes damage to liver through dermal exposure
B. Further information:
Substance X is presumed to be volatile, but not so as to generate a potentially explosive
atmosphere.
There is possible exposure via inhalation.
Specific extinguishing media are not necessary. Specific treatment/measures is/are not
urgently required.
No specific disposal precautionary statements are required since the substance is not
intended to be used by the general public, but only by industrial/professional users.
C. Precautionary statements on the basis of the classification (see Annexes I and IV to
CLP) and according to this guidance document:
Acute Tox. 3
(Oral)
Acute Tox. 3
(Dermal)
Acute Tox. 3
(Inhalation)
STOT-SE 1
Flam. Liq. 2
P264
P270
P280
P261
P271
P260
P264
P270
P210
P233
P240
P241
P242
P243
P280

184
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Acute Tox. 3
(Oral)
Acute Tox. 3
(Dermal)
Acute Tox. 3
(Inhalation)
STOT-SE 1
Flam. Liq. 2
P301 + P310
P321
P330
P312
P321
P361 + P364
P363
P302 + P352
P304 + P340
P311
P321
P308 + P311
P321
P303 + P361 + P353
P370 + P378
P405
P405
P403 + P233
P405
P405
P403 + P235
P501
P501
P501
P501
P501
Explanation on use of bolding, underline and grey marker:
PXXX = highly recommended; PXXX = recommended; PXXX = optional; PXXX = not to be
used/unless condition applies/inclusion on safety data sheet only
D. Selection of highly recommended and recommended precautionary statements:
When the same statement is assigned to different hazards, but with a different priority,
the most conservative approach is taken. Where appropriate, precautionary statements
are combined into a single combination statement. Duplication of individual phrases is
avoided. The selection results in the following set of P-statements:
P210 Keep away from heat, hot surfaces, sparks, open flames and
other ignition sources. No smoking.
P260 Do not breathe dust/fume/gas/mist/vapours/spray.
P280 Wear protective gloves/protective clothing/eye
protection/face protection.
P301+P310 IF SWALLOWED: Immediately call a POISON
CENTER/doctor/…
P308+P311 IF exposed or concerned: Call a POISON CENTER/doctor/...
P304+P340 IF INHALED: Remove person to fresh air and keep comfortable for
breathing.
P403+P233 Store in a well-ventilated place. Keep container tightly
closed.
E. Result:
Selection in line with the guidance results in seven precautionary statements. A
substantial reduction is achieved compared to the starting set of potentially
applicable statements for the hazard label, assignable on the basis of the
underlying hazards. For example: P261 can be omitted, as P260 is already
assigned for the label.
The selected precautionary statements must be placed on the CLP hazard label. As an
SDS needs to be prepared, the statements would also have to be included in the SDS,
under heading 2.2 “Label elements” (see the Guidance on the compilation of safety data
sheets). The de-selected statements can be introduced under the relevant headings of
the SDS to provide the industrial or professional user with sufficient information to
handle the substance safely.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
185
Example B. Substance Y assigned a severe physical and health hazard
classification
A. Classification and hazard statements:
Ox. Sol. 1 H271 May cause fire or explosion; strong oxidiser
Skin Corr. 1A H314 Causes severe skin burns and eye damage
B. Further information:
Substance Y is a granular solid and is presumed to be non-volatile. Dust exposure during
handling and use is possible.
Specific extinguishing media are not necessary.
Specific treatment/measures is/are not urgently required. No specific disposal
precautionary statements are required since the substance is not intended to be used by
the general public, but only by industrial/professional users.
C. Precautionary statements on the basis of the classification (see Annexes I and IV to
CLP) and according to this guidance document:
Ox. Sol. 1
Skin Corr. 1A
P210
P220
P280
P283
P260
P264
P280
P306+P360
P371+P380+P375
P370+P378
P301+P330+P331
P303+P361+P353
P363
P304+P340
P310
P321
P305+P351+P338
-
P405
P501
P501
D. Selection of highly recommended and recommended precautionary statements:
When the same statement is assigned to different hazards, but with a different priority,
the most conservative approach is taken (i.e. the highest priority must be taken into
account). Where appropriate, precautionary statements are combined into a single
combination statement. Duplication of individual phrases is avoided. The selection results
in the following set of P-statements:

186
Guidance on Labelling and Packaging
Version 4.2 – March 2021
P210 Keep away from heat, hot surfaces, sparks, open
flames and other ignition sources. No smoking.
P220 Keep away from clothing and other combustible
materials
P260 Do not breathe
dust/fume/gas/mist/vapours/spray.
P280 Wear protective gloves/protective clothing/eye
protection/ face protection.
P301+P330+P331 IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
P303+P361+P353+310 IF ON SKIN (or hair): Take off immediately all
contaminated clothing. Rinse skin with water [or
shower]. Immediately call a POISON
CENTER/doctor/...
P305+P351+P338 IF IN EYES: Rinse cautiously with water for several
minutes. Remove contact lenses, if present and easy
to do. Continue rinsing.
P371+P380+P375 In case of major fire and large quantities: Evacuate
area. Fight fire remotely due to the risk of explosion.
E. Result:
Selection in line with this guidance document results in eight, mostly combined,
precautionary statements. A substantial reduction is achieved compared to the
starting set of potentially applicable statements for the CLP hazard label,
assignable on the basis of the underlying hazards.
The selected precautionary statements must be placed on the CLP hazard label. As an
SDS needs to be prepared, they would also have to be included in the SDS, under
heading 2.2 “Label elements” (see the Guidance on the compilation of safety data
sheets).
The de-selected statements can be introduced under the relevant headings of the SDS to
provide the industrial or professional user with sufficient information to handle the
substance safely.
Example C. Substance Z assigned physical, health and environmental
classifications
A. Classification and hazard statements:
Pyr. Liq. 1 H250 Catches fire spontaneously if exposed to air
Water-react. 1 H260 In contact with water releases flammable gases which may
ignite spontaneously
Skin Corr. 1B H314 Causes severe skin burns and eye damage
Aquatic Acute 1 H400 Very toxic to aquatic life
Aquatic Chronic 1 H410 Very toxic to aquatic life with long lasting effects

Guidance on Labelling and Packaging
Version 4.2 – March 2021
187
B. Further information:
Substance Z should be regarded as volatile. Therefore, there is a possible exposure via
inhalation. Specific extinguishing media are necessary, because water will increase the
risk when used for the extinguishing of fire.
As the disposal of the packaging presents a hazard to human health or the environment,
specific disposal precautionary statements are required (although the substance is not
intended to be used by the general public, but only by industrial/professional users). The
hazard statement H400 is omitted from the label to avoid duplication with H411.
C. Precautionary statements on the basis of the classification (see Annexes I and IV to
CLP) and according to this guidance document:
Pyr. Liq.1
Water-react. 1
Skin Corr. 1B
Aquatic
Acute 1
Aquatic
Chronic 1
P210
P222
P233
P280
P231+P232
P223
P231+P232
P280
P260
P264
P280
P273
P273
P302+P334
P370+P378
P302+P335+P334
P370+P378
P301+P330+P331
P303+P361+P353
P363
P304+P340
P310
P321
P305+P351+P338
P391
P391
P402+P404
P405
-
-
-
P501
P501
P501
P501
D. Selection of highly recommended and recommended precautionary statements:
When the same statement is assigned to different hazards, but with a different priority,
the most conservative approach is taken (i.e. the highest priority must be taken into
account). Where appropriate, precautionary statements are combined into a single
combination statement. Duplication of individual phrases is avoided.
P303+ P361+P353 IF ON SKIN (or hair): Take off immediately all
contaminated clothing. Rinse skin with water [or shower].
and

188
Guidance on Labelling and Packaging
Version 4.2 – March 2021
P302+P335+P334+P310 IF ON SKIN: Brush off loose particles from skin. Immerse
in cool water
57
. Immediately call a POISON
CENTER/doctor/…)
were merged into one single combination phrase:
P303+ P335+P334+P310+P361 where duplication of the message was avoided.
The selection results in the following set of P-statements:
P210 Keep away from heat, hot surfaces,
sparks, open flames and other ignition
sources. No smoking.
P260 Do not breathe dust/fume/gas/mist/
vapours/spray.
P273 Avoid release to the environment.
P280 Wear protective gloves/protective
clothing/eye protection/face protection.
P231+P232 Handle and store under inert gas. Protect
from moisture.
P301+P330+P331 IF SWALLOWED: rinse mouth. Do NOT induce
vomiting.
P303+ P335+P334+P310+P361 IF ON SKIN (or hair): Brush off loose
particles from skin. Immerse in cool
water
58
. Immediately call a POISON
CENTER/doctor/… Take off immediately
all contaminated clothing.
P305+P351+P338 IF IN EYES: Rinse cautiously with water
for several minutes. Remove contact
lenses, if present and easy to do.
Continue rinsing.
P370+P378 In case of fire: Use … to extinguish.
E. Result:
Selection in line with this guidance document results in nine, partly combined,
precautionary statements.
A substantial reduction is achieved compared to the starting set of potentially
applicable statements for the CLP hazard label, assignable on the basis of the
underlying hazards. For example, P264 has not been selected, because P280 is
more relevant.
To further reduce the number of the P-statements and the amount of digestible
information on the label, the statements P391 and P501 have been put in the
SDS, as the prevention and response statements for the physical and health
hazards appear to contain the more urgent advice for the label.
57
The sub-phrase of P334 “or wrap in wet bandages” is not to be used for water-reactive
substances and mixtures category 1 (Table 7.3.2.12 in section 7.3 of this guidance document).
58
The sub-phrase of P334 “or wrap in wet bandages” is not to be used for water-reactive
substances and mixtures category 1 (Table 7.3.2.12 in section 7.3 of this guidance document).

Guidance on Labelling and Packaging
Version 4.2 – March 2021
189
The selected precautionary statements must be placed on the CLP hazard label. As an
SDS needs to be prepared, they would also have to be included in the SDS, under
heading 2.2 “Label elements” (see the Guidance on the compilation of safety data
sheets). The de-selected statements can be introduced under the relevant headings of
the SDS to provide the industrial or professional user with sufficient information to
handle the substance safely.
Example D. Mixture ABC for use by the general public
A. Classification and hazard statements:
Flam. Liq. 2 H225 Highly flammable liquid and vapour
Acute Tox. 4 (oral) H302 Harmful if swallowed
Skin irrit. 2 H315 Causes skin irritation
B. Further information:
Mixture ABC is presumed to be volatile, but not so as to generate a potentially explosive
atmosphere. Specific extinguishing media are not necessary. Specific treatment is not
urgently required.
There are no specific disposal requirements. The mixture is intended to be used by the
general public.
C. Precautionary statements on the basis of the classification (see Annexes I and IV to
CLP) and according to this guidance document:
Flam. Liq. 2
Acute Tox. 4 (Oral)
Skin Irrit. 2
P101, P102
P210
P233
P240
P241
P242
P243
P280
P264
P270
P264
P280
P303 + P361 + P353
P370 + P378
P301+P312
P330
P302+P352
P321
P332+P313
P362+P364
P403 + P235
-
-
P501
P501
-
D. Selection of highly recommended and recommended precautionary statements:

190
Guidance on Labelling and Packaging
Version 4.2 – March 2021
When the same statement is assigned to different hazards, but with a different priority,
the most conservative approach is taken. Where appropriate, precautionary statements
are combined into a single combination statement. Duplication of individual phrases is
avoided. The selection results in the following set of P-statements:
P101 If medical advice is needed, have product container or label at hand.
P102 Keep out of reach of children.
P210 Keep away from heat, hot surfaces, sparks, open flames and other
ignition sources. No smoking.
P233 Keep container tightly closed.
P264 Wash … thoroughly after handling.
P280 Wear protective gloves.
P501 Dispose of contents/container to …
E. Result:
Selection in line with this guidance document results in seven precautionary
statements. A substantial reduction is achieved compared to the starting set of
potentially applicable statements for the CLP hazard label, assignable on the
basis of the underlying hazards.
The selected precautionary statements must be placed on the CLP hazard label. As an
SDS needs to be prepared, they would also have to be included in the SDS, under
heading 2.2 “Label elements” (see the Guidance on the compilation of safety data
sheets).
The de-selected statements can be introduced under the relevant headings of the SDS to
provide the industrial or professional user with sufficient information to handle the
substance safely.

Guidance on Labelling and Packaging
Version 4.2 – March 2021
191
Appendix: Glossary of selected terms used in this
guidance document
ADR the European Agreement concerning the
International Carriage of Dangerous Goods by
Road (concluded in Geneva on 30 September
1957) that has been implemented within the
EU through Directive 2008/68/EC;
Acute toxicity those adverse effects occurring following oral
or dermal administration of a single dose of a
substance or a mixture, or multiple doses
given within 24 hours, or an inhalation
exposure of 4 hours;
Acute aquatic toxicity the intrinsic property of a substance to be
injurious to an organism in a short term
exposure to that substance;
Aerosols this means aerosol dispensers, are any non-
refillable receptacles made of metal, glass or
plastics and containing a gas compressed,
liquefied or dissolved under pressure, with or
without a liquid, paste or powder, and fitted
with a release device allowing the contents to
be ejected as solid or liquid particles in
suspension in a gas, as a foam, paste or
powder or in a liquid state or in a gaseous
state;
Alloy a metallic material, homogeneous on a
macroscopic scale, consisting of two or more
elements so combined that they cannot be
readily separated by mechanical means;
alloys are considered to be mixtures for the
purposes of the CLP Regulation;
Article an object which during production is given a
special shape, surface or design which
determines its function to a greater degree
than does its chemical composition;
Aspiration the entry of a liquid or solid substance or
mixture directly through the oral or nasal
cavity, or indirectly from vomiting, into the
trachea and lower respiratory system;
BPR Regulation (EU) No 528/2012 of the European
Parliament and of the Council of 22 May 2012
concerning the making available on the
market and use of biocidal products (Biocidal
Products Regulation);
Carcinogen a substance or a mixture of substances which
induces cancer or increases its incidence;
CAS Chemical Abstract Service;

192
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Chemically unstable gas a flammable gas that is able to react
explosively even in the absence of air or
oxygen;
Chronic aquatic toxicity the intrinsic property of a substance to cause
adverse effects to aquatic organisms during
exposures which are determined in relation to
the life-cycle of the organism;
CLP or CLP Regulation Regulation (EC) No 1272/2008 on
Classification, Labelling and Packaging of
Substances and Mixtures;
CMR a substance or mixture which is carcinogenic,
mutagenic or toxic to reproduction;
Competent authority (CA) the authority or authorities or bodies
established by the member states to carry out
the obligations arising from the CLP
Regulation;
Corrosive to metals a substance or a mixture which by chemical
action will materially damage, or even destroy
metals;
CRC child-resistant closure;
CRF child-resistant fastening;
Distributor any natural or legal person established within
the Community, including a retailer, who only
stores and places on the market a substance,
on its own or in a mixture, for third parties;
Downstream user any natural or legal person established within
the Community, other than the manufacturer
or the importer, who uses a substance, either
on its own or in a mixture, in the course of his
industrial or professional activities. A
distributor or a consumer is not a downstream
user. A re-importer, exempted pursuant to
Article 2(7)(c) REACH Regulation, shall be
regarded as a downstream user;
DPD Dangerous Preparations Directive
(1999/45/EC);
DSD Dangerous Substances Directive
(67/548/EEC);
ECHA European Chemicals Agency or “the Agency,”
established under the REACH Regulation;
EU European Union;
Explosive article an article containing one or more explosive
substances or mixtures;
Explosive substance or mixtures a solid or liquid substance or mixture of
substances which is in itself capable by
chemical reaction of producing gas at such a
temperature and pressure and at such a

Guidance on Labelling and Packaging
Version 4.2 – March 2021
193
speed as to cause damage to the
surroundings. Pyrotechnic substances are
included even when they do not evolve gases;
Eye irritation the production of changes in the eye following
the application of test substance to the
anterior surface of the eye, which are fully
reversible within 21 days of application;
Flammable gas a gas or gas mixture having a flammable
range with air at 20 °C and a standard
pressure of 101.3 kPa;
Flammable liquid a liquid having a flash point of not more than
60°C;
Flash point the lowest temperature (corrected to a
standard pressure of 101.3 kPa) at which the
application of an ignition source causes the
vapours of a liquid to ignite under specified
test conditions;
Flammable solid a solid which is readily combustible, or may
cause or contribute to fire through friction.
Readily combustible solids are powdered,
granular, or pasty substances or mixtures
which are dangerous if they can be easily
ignited by brief contact with an ignition
source, such as a burning match, and if the
flame spreads rapidly;
GHS Globally Harmonised System of Classification
and Labelling of Chemicals developed within
the United Nations (UN) structure - the
international criteria agreed by the United
Nation Economic and Social Council (UN
ECOSOC) for the classification and labelling of
hazardous substances and mixtures;
Hazard category the division of criteria within each hazard
class, specifying hazard severity;
Hazard class the nature of the physical, health or
environmental hazard;
Hazard pictogram graphical composition that includes a symbol
plus other graphic elements, such as a border,
background pattern or colour that is intended
to convey specific information about the
hazard concerned;
Hazard statement a phrase assigned to a hazard class and
category that describes the nature of the
hazards of a hazardous substance or mixture,
including, where appropriate, the degree of
hazard;
Hazardous means fulfilling the criteria relating to physical
hazards, health hazards or environmental

194
Guidance on Labelling and Packaging
Version 4.2 – March 2021
hazards, laid down in Parts 2 to 5 of Annex I
to the CLP Regulation;
IMDG Code International Maritime Dangerous Goods Code
for the transport of dangerous goods by sea;
Import the physical introduction into the customs
territory of the Community;
Importer any natural or legal person established within
the Community who is responsible for import;
INCI International Nomenclature of Cosmetic
Ingredients;
Intermediate packaging packaging placed between inner packaging,
or articles, and outer packaging;
IUCLID International Uniform Chemical Information
Database;
IUPAC International Union of Pure and Applied
Chemistry;
Label an appropriate group of written, printed or
graphic information elements concerning a
hazardous substances or mixture, selected as
relevant to the target sector (s), that is
affixed to, printed on, or attached to the
immediate container of a hazardous
substance or mixture, or to the outside
packaging of a hazardous substances or
mixture (definition follows Chapter 1.2 of the
UN GHS);
Label element one type of information that has been
harmonised for use in a label, e.g. hazard
pictogram, signal word;
Manufacturer any natural or legal person established within
the Community who manufactures a
substance within the Community;
Manufacturing production or extraction of substances in the
natural state;
Mixture means a mixture or solution composed of two
or more substances. The UN GHS Chapter
1.2 includes the phrase, “in which they do
not react” at the end of an otherwise
identical definition;
Mutagen an agent giving rise to an increased
occurrence of mutations in populations of
cells and /or organisms;

Guidance on Labelling and Packaging
Version 4.2 – March 2021
195
Organic peroxides liquid or solid organic substances which
contain the bivalent -O-O- structure and may
be considered derivatives of hydrogen
peroxide, where one or both of the hydrogen
atoms have been replaced by organic
radicals. The term organic peroxide includes
organic peroxide mixtures (formulations)
containing at least one organic peroxide
Organic peroxides are thermally unstable
substances or mixtures, which can undergo
exothermic self-accelerating decomposition.
In addition, they can have one or more of the
following properties:
(i) be liable to explosive decomposition;
(ii) burn rapidly;
(iii) be sensitive to impact or friction;
(iv) react dangerously with other substances;
Oxidising gas any gas or gas mixture which may, generally
by providing oxygen, cause or contribute to
the combustion of other material more than
air does;
Oxidising liquid a liquid substance or mixture which, while in
itself not necessarily combustible, may,
generally by yielding oxygen, cause, or
contribute to, the combustion of other
material;
Oxidising solid a solid substance or mixture which, while in
itself not necessarily combustible, may,
generally by yielding oxygen, cause, or
contribute to, the combustion of other
material;
Package the complete product of the packing
operation, consisting of the packaging and its
contents;
Packaging one or more receptacles and any other
components or materials necessary for the
receptacles to perform their containment and
other safety functions;
Placing on the market supplying or making available, whether in
return for payment or free of charge, to a
third party. Import shall be deemed to be
placing on the market;
PPPR Regulation (EC) No 1107/2009 of the
European Parliament and of the Council of 21
October 2009 concerning the placing of plant
protection products on the market and
repealing Council Directives 79/117/EEC and
91/414/EEC;

196
Guidance on Labelling and Packaging
Version 4.2 – March 2021
Precautionary statement a phrase that describes recommended
measure(s) to minimise or prevent adverse
effects resulting from exposure to a
hazardous substance or mixture due to its
use or disposal;
Product identifier details permitting the identification of the
substance or mixture;
Pyrophoric liquid a liquid substance or mixture which, even in
small quantities, is liable to ignite within five
minutes after coming into contact with air;
Pyrophoric solid a solid substance or mixture which, even in
small quantities, is liable to ignite within five
minutes after coming into contact with air;
Pyrotechnic article an article containing one or more pyrotechnic
substances or mixtures;
Pyrotechnic substance or mixture a substance or mixture of substances
designed to produce an effect by heat, light,
sound, gas or smoke or a combination of
these as the result of non-detonative self-
sustaining exothermic chemical reactions;
REACH or REACH Regulation Regulation (EC) No 1907/2006 concerning
the Registration, Evaluation, Authorisation
and Restriction of Chemicals;
Registrant the manufacturer or the importer of a
substance or the producer or importer of an
article submitting a registration for a
substance under the REACH Regulation;
Reproductive toxicity includes adverse effects on sexual function
and fertility in adult males and females, as
well as developmental toxicity in the
offspring and effects on or via lactation;
Respiratory sensitiser a substance that will lead to hypersensitivity
of the airways following inhalation of the
substance;
SDS safety data sheet;
Self-heating substance or mixture a liquid or solid substance or mixture, other
than a pyrophoric liquid or solid, which, by
reaction with air and without energy supply,
is liable to self-heat; this substance or
mixture differs from a pyrophoric liquid or
solid in that it will ignite only when in large
amounts (kilograms) and after long periods
of time (hours or days);
Self-reactive substances or mixtures thermally unstable liquid or solid substances
or mixtures liable to undergo a strongly
exothermic decomposition even without
participation of oxygen (air). This definition
excludes substances and mixtures classified

Guidance on Labelling and Packaging
Version 4.2 – March 2021
197
according to the CLP Regulation as
explosives, organic peroxides or as oxidising;
Serious eye damage the production of tissue damage in the eye,
or serious physical decay of vision, following
application of a test substance to the anterior
surface of the eye, which is not fully
reversible within 21 days of application;
Signal word a word that indicates the relative level of
severity of hazards to alert the potential
reader of the hazard; the following two levels
are distinguished:
a) Danger means a signal word indicating the
more severe hazard categories; and
b) Warning means a signal word indicating
the less severe hazard categories;
Skin corrosion the production of irreversible damage to the
skin, namely visible necrosis through the
epidermis and into the dermis, following the
application of a test substance up to 4 hours;
Skin irritation the production of reversible damage to the
skin following the application of a test
substance for up to 4 hours;
Skin sensitiser a substance that will lead to an allergic
response following skin contact;
Specific target organ toxicity specific target organ toxicity, cf. STOT,
STOT-SE and STOT-RE;
STOT-SE specific, non lethal target organ toxicity
arising from a single exposure to a substance
or mixture;
STOT-RE specific, target organ toxicity arising from a
repeated exposure to a substance or
mixture;
Substance a chemical element and its compounds in the
natural state or obtained by any
manufacturing process, including any
additive necessary to preserve its stability
and any identified impurity deriving from the
process used, but excluding any solvent
which may be separated without affecting the
stability of the substance or changing its
composition;
Supplier any manufacturer, importer, downstream
user or distributor placing on the market a
substance, on its own or in a mixture, or a
mixture;
Trade name a designation under which a substance or
mixture is placed on the market;
TWD tactile warnings of danger;

198
Guidance on Labelling and Packaging
Version 4.2 – March 2021
UFI Unique Formula Identifier;
UN United Nations;
UN RTDG the United Nations Recommendations on the
Transport of Dangerous Goods;
Unstable explosive an explosive substance or mixture which is
thermally unstable and/or too sensitive for
normal handling, transport and use;
Use any processing, formulation, consumption,
storage, keeping, treatment, filling into
containers, transfer from one container to
another, mixing, production of an article or
any other utilisation.

EUROPEAN CHEMICALS AGENCY
ANNANKATU 18, P.O. BOX 400,
FI-00121 HELSINKI, FINLAND
ECHA.EUROPA.EU
Signal word Supplier
identity
Hazard
statements
Trade name Inside
pages
Language
code
Hazard
pictograms
Nominal
quantity
Precautiona
ry
statements
Signal
word in all
languages
of the label
Language
codes
indicating
languages
covered by
the label
Front page
wrapped
around
the
container
Symbol
informing
the user
that the
label can be
opened and
indicating
that the
additional
information
is available
on inside
pages
Product
identifier
including
three
hazardous
components
that
contribute
to the
classificatio
n of the
mixture
Back page
firmly
affixed to
the
immediate
container
UFI code
