
25.2.21
T: Can I describe the properties of
solids, liquids and gases?

Properties of Materials: Solids
These items
are all solids!
What do they
have in
common?
Materials in a solid state keep their shape unless a
force is applied to them.
Solids can be cut, squashed or twisted. They will
not change shape on their own.
Solid materials always take up the same amount of
space. They do not spread out or flow. Solids do not
have to be hard. They can be squashy or soft.

Properties of Materials: Liquids
These items
are all liquids!
What do they
have in
common?
Materials in a liquid state take the shape of the
container they are in.
Although liquids can change shape, they do not
change their volume. This means they still take
up the same amount of space.
Liquids are pulled down to the bottom of a
container by gravity.
Liquids can flow or be poured.

Properties of Materials: Gases
These items
are all gases!
What do they
have in
common?
Materials in a gaseous state can spread out to
completely fill the container or room they are in.
Gases have weight.
Gases can be squashed.
Gases do not keep their shape.

What are particles
We can explain the differences
between solids, liquids and
gases by knowing what they
are made of.
Scientists have found out that
all materials are made of very
tiny particles. These particles
are so small that we cannot see
them with our eyes, or even
with a microscope!
The position and behaviour of
the particles is different in
solids, liquids and gases.
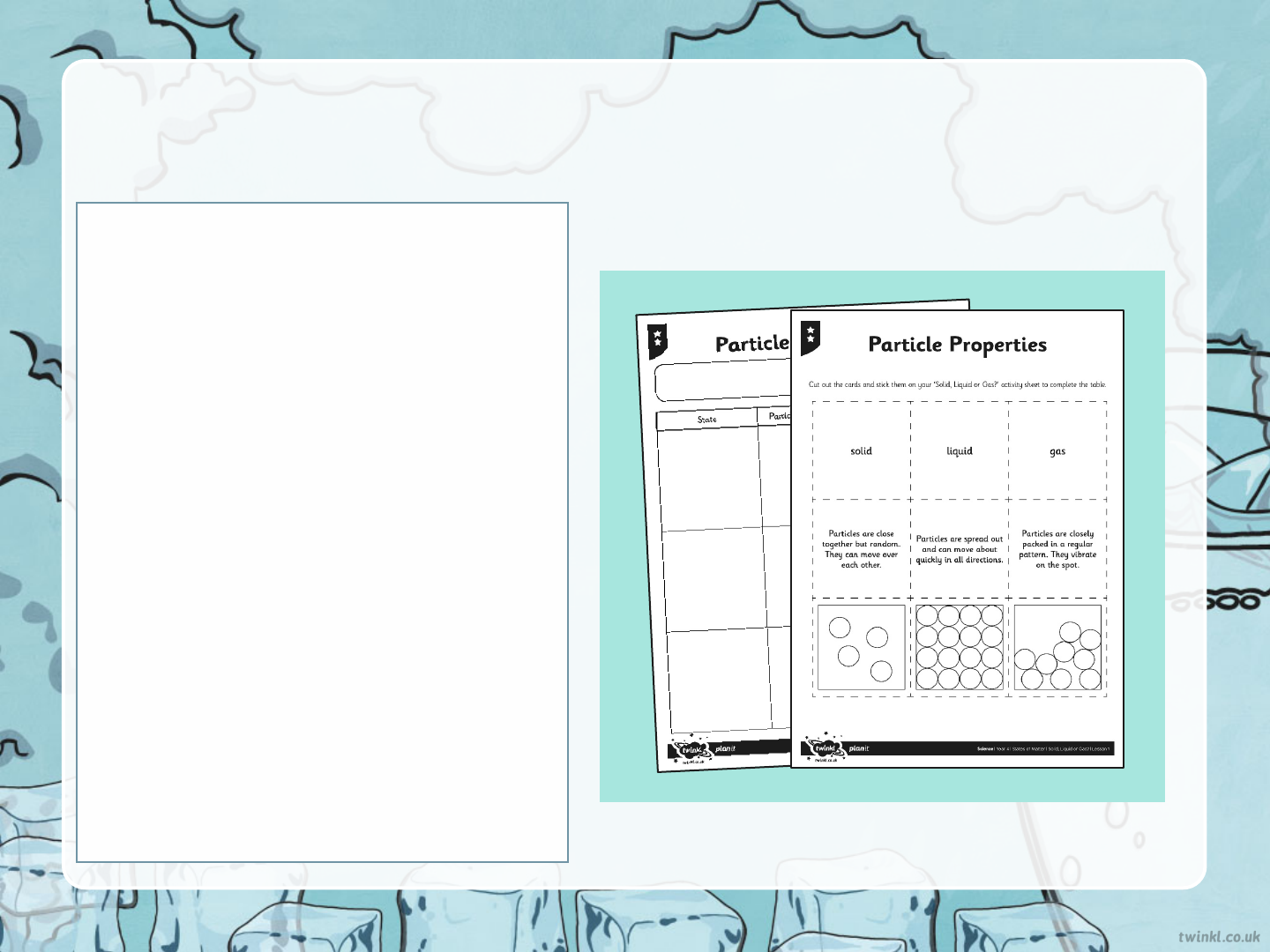
Today’s Task
For today’s task, you will need
glue and a pair of scissors (If
you do not have these at home,
you can draw your answers on
a piece of paper).
Your task today is to match the
state of matter to the correct
property and picture of the
particles. There are three
worksheets for you to choose
from: one star, two stars and
three stars. The more stars you
choose, the more challenging
your task will be.

Success Criteria
Aim
• I can sort and describe materials.
• I can sort materials into solids, liquids or gases.
• I can describe the properties of solids, liquids and gases.
• I can show the difference between the particles in solids, liquids and gases.


