
Erratum. The following correction was made on 22 June 2016:
p. 7, second to last paragraph: ‘European Transport Networks’ Federation’ was changed to read ‘European Transport Workers’
Federation’
Suggested citation: European Centre for Disease Prevention and Control. Rapid Risk Assessment. Zika virus disease
epidemic: Sixth update, 20 May 2016. Stockholm: ECDC; 2016.
© European Centre for Disease Prevention and Control, Stockholm, 2016
3
Main conclusions and options for response
Given the continued spread of Zika virus in the Americas and Caribbean; the evidence of an association
between Zika virus infection during pregnancy and congenital CNS malformations; the association between
Zika virus infection and Guillain–Barré syndrome and the risk of local vector-borne transmission in Europe
during the 2016 summer season, EU/EEA Member States are recommended to consider a range of mitigation
measures.
The following uncertainties have been taken into consideration in developing the proposed options for
response:
There is growing evidence that Zika virus infection during the first and second trimester is associated with
increased risk for central nervous system malformation of the foetus. The risk associated with infection
during the third trimester is unknown. Therefore, Zika virus infection should be considered as a risk
throughout the entire duration of pregnancy.
The presence of viable Zika virus in semen has been detected up to 24 days after onset of Zika virus
infection symptoms. The longest interval reported between the onset of symptoms in a male and the
subsequent onset of the disease thought to be due to sexual transmission in a female partner is 19 days.
All the currently reported sexual transmission events are linked to symptomatic index cases. There is no
evidence of transmission by asymptomatic sexual partners.
Information for travellers to and EU citizens residing in areas with
active transmission
Travellers visiting countries where there is active transmission of Zika virus and EU citizens residing in
these countries should:
o be made aware of the ongoing outbreak of Zika virus infection and the fact that Zika virus is usually
transmitted by mosquito vectors but can be also transmitted by sexual intercourse;
o take measures to prevent mosquito bites indoors and outdoors, especially between sunrise and sunset
when
Aedes
mosquito vectors are most active and biting. These measures include:
− The use of mosquito repellent in accordance with the instructions indicated on the product label.
RAPID RISK ASSESSMENT
Zika virus disease epidemic
Sixth update, 20 May 2016

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
2
− Wearing long-sleeved shirts and long trousers, especially during the hours when the type of
mosquito that is known to transmit the Zika virus (
Aedes
) is most active.
− Sleeping or resting in screened or air-conditioned rooms or otherwise use mosquito nets, at
night and during the day.
Pregnant women and women who are planning to become pregnant and planning to travel to areas with
widespread transmission should postpone non-essential travel.
Pregnant women and women who are planning to become pregnant and planning to travel to areas with
sporadic transmission should consult their physician or a travel clinic and consider postponing non-
essential travel.
Pregnant women residing in countries with active transmission (sporadic and widespread) should consult
their healthcare providers for advice and follow strict measures to prevent mosquito bites.
Travellers with immune disorders or severe chronic illnesses should consult their doctor or seek advice
from a travel clinic - particularly with regard to effective prevention measures - before travelling to
countries with active transmission.
Travellers to countries with active Zika transmission and EU citizens residing there should be advised that
using condoms could reduce the risk of sexual transmission through semen.
Information for travellers returning from areas with active
transmission of Zika virus
Pregnant women who have travelled or resided in areas with active transmission should mention their
travel during antenatal visits in order to be assessed and monitored appropriately.
In order to protect the foetus, male travellers returning from areas with active transmission should
consider using a condom with a pregnant partner until the end of pregnancy.
Travellers returning from areas with ongoing Zika virus transmission should be advised to use a condom
for at least one month after returning, in order to reduce the potential risk of onward sexual transmission.
Travellers, including those with immune disorders or severe chronic illnesses, showing symptoms
compatible with Zika virus disease within two weeks of return from an area with active transmission are
advised to contact their healthcare provider and mention their recent travel.
Source and date of request
ECDC internal decision, 10 May 2016.
ECDC issues this risk assessment document according to Article 7(1) of Regulation (EC) No 851/2004 establishing a
European centre for disease prevention and control. In the framework of ECDC’s mandate, the specific purpose of
an ECDC risk assessment is to present different options on a certain matter. The responsibility for the choice of
which option to pursue and which actions to take lies exclusively with the EU/EEA Member States.
Public health issue
This document assesses the risks associated with the Zika virus epidemic in currently affected countries, in EU
Overseas Countries and Territories (OCTs) and Outermost Regions (OMRs) and in the EU Member States within
mainland Europe.
Since February 2014, ECDC has published seven risk assessments related to Zika virus epidemics [1-9].
Consulted experts
ECDC internal response team in alphabetical order: Sergio Brusin, Denis Coulombier, Niklas Danielsson, Dragoslav
Domanovic, Alastair Donachie, Kaja Kaasik Aaslav, Bertrand Sudre, Wim Van Bortel, Paula Vasconcelos and Hervé
Zeller.
Experts from the following institutions contributed to this risk assessment: WHO Regional Office for Europe, WHO
Regional Office for Western Pacific Region, WHO Regional Office for America/Pan American Health Organization
(PAHO) and WHO Headquarters.
ECDC acknowledges the valuable contributions of all experts. Although experts from the World Health Organization
(WHO) reviewed the risk assessment, the views expressed in this document do not necessarily represent the views
of WHO. All experts have submitted declarations of interest and a review of these declarations did not reveal any
conflicts of interest.

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
3
Disease background information
Zika virus disease is caused by an RNA virus transmitted to humans by
Aedes
mosquitoes, especially by the
Aedes
aegypti
species. More information about Zika virus disease can be found in the previous risk assessments [1-9] and
in the ECDC factsheet for health professionals. Two literature reviews have been published since the previous
ECDC risk assessment [10,11].
Highlights of recent scientific developments
Pathogen
Since the last Rapid Risk Assessment update, several studies have been published on the molecular evolution of
the virus and the structure of Zika virus, with possible implications for understanding the pathogenesis and
neurotropism [12-16]. A number of changes at the nucleotide and amino acid level were found between the
epidemic strains and the pre-epidemic strains isolated before 2007 [14]. It should be noted that the sequence
analyses and comparisons are made on a limited number of historical sequences and that the ancestral isolates
may have acquired mutations as a result of successive passages in mouse brain, potentially limiting the validity of
using these historical viral strains for such comparison. Further investigations are needed to determine to what
extent these nucleotide and subsequent amino acid substitutions are associated with changes in viral tropism,
transmissibility and fitness.
Zika virus is able to infect neural stem cells in vitro, which are progenitor cells of neurons and other brain cells. The
results strongly suggest that Zika virus abrogates neurogenesis during human brain development. Investigation by
immunohistochemistry, real-time RT-PCR analysis and serological assays involving five women infected with Zika
virus at different gestational time points provides additional evidence of placental and brain lesions associated with
Zika virus. Several recent publications based on animal models support an
in vivo
deleterious effect of Zika virus on
neural progenitor cells, leading to reduction of their proliferation and differentiation and increased apoptosis.
Clinical features and sequelae
Besides the known clinical features of Zika virus, meningoencephalitis has been reported in an 81-year-old man
[17]. Transient hearing loss for up to four weeks was reported in three adults [18]. Fatal outcome after Zika virus
infection remains a rare event. The role of comorbidities as a risk factor of fatal outcome is still to be investigated.
Intracranial calcifications in the periventricular, parenchymal, and thalamic regions were prominent findings in a
series of 23 infants from Pernambuco state (Brazil) with severe brain anomalies. The calcifications were similar to
those found in other congenital infections. Further studies confirmed the observed pattern, with calcifications in the
junction between cortical and subcortical white matter concomitant with congenital malformations during the
development of the cortex [19]. Additional findings included delayed myelination, ventriculomegaly, abnormalities
of corpus callosum and hypoplasia of the cerebellum and the brainstem.
Microcephaly and congenital central nervous system malformations
Accumulating data from scientific investigations add to the evidence that the emerging Zika virus strain from the
Asian lineage can cause transplacental infection and congenital CNS malformations in the developing brain [20,21].
However, the information available is still insufficient to quantify the potential co-factors and the frequency of
transplacental transmission. Therefore, the resulting risk of adverse pregnancy complications cannot be assessed.
In late March 2016, WHO reported that there is ‘
strong scientific consensus that Zika virus is a cause of GBS,
microcephaly and other neurological disorders based on observational, cohort and case–control studies currently
published’
[22]. Epidemiological observations are now supported by evidence from in vitro and in vivo laboratory
studies of the damaging effect of Zika virus on neuronal tissue, especially neural progenitor cells, therefore
possibly impairing the development of the foetus’ brain. These laboratory studies are strengthening the coherence
of the evidence and its consistency with Hill's criteria for causation.
The likelihood of neurological abnormalities in foetuses and neonates in symptomatic pregnant women is
substantial, based on the results of the study in Brazil [23].
Preliminary reports of a prospective case-control study conducted by Microcephaly Epidemic Research Group
(MERG) in the city of Recife, Brazil since January 2016 demonstrate a significant and strong association between
intra-uterine Zika virus infection during pregnancy and microcephaly in neonates. Final results are expected to be
published shortly [24].These preliminary results support the strength criteria of the Hill's criteria for causation.
It is probable that the risk of transplacental infection, as well as the risk of developing congenital CNS
malformations, depends on the gestational age at the time of infection. It is conceivable that other factors, such as
the mother’s age, genetic co-factors and her nutritional status among others, influence the risk of transplacental
transmission. Results from ongoing and further case–control and cohort studies are still required to more
accurately estimate the risk of microcephaly and other congenital CNS malformations linked with Zika virus
infection.

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
4
Severe congenital impairments reported in Brazil might represent the severe phenotype of a broader spectrum of
the Zika congenital syndrome. Further epidemiological studies on Zika virus in population will provide a better
understanding of the clinical spectrum of the Zika virus disease and the potential cognitive and functional
associated sequelae. In addition, further investigations are required into the mode of transmission and viral kinetics
in bodily fluids in order to adapt prevention and control measures.
Guillain–Barré syndrome and other post-infectious neurological syndromes
A case-control study in French Polynesia and recent observations support the role of Zika virus infection as a
presumptive infection event preceding Guillain-Barré syndrome [25]. Countries reporting an increase of Guillain-
Barré syndrome are affected by Zika viral strains from the Asian lineage.
Epidemiology
A retrospective study of acute undifferentiated febrile illness conducted in Haiti identified Zika virus among three
children from three different towns in rural Haiti (west of Port-au-Prince) in December 2014 [26]. These findings
suggest a circulation of Zika virus near Port-au-Prince already during late autumn 2014.
The first comprehensive study of the Zika virus outbreak in large urban areas (Rio de Janeiro, Brazil) conducted
during the first half of 2015 identified 364 suspected cases, 119 of which were laboratory confirmed [27]. The first
confirmed cases were retrospectively identified as having occurred in January 2015.
Zika epizootic infections among monkeys were reported in April 2016 for the first time in Brazil and Ecuador
[28,29]. The role of these nonhuman primates in the epidemiology of Zika disease, in particular mode of
transmission of the infection to monkey and potential sylvatic cycle in the Americas, is unknown.
Transmission
The first male-to-male sexual transmission of Zika virus was reported in April 2016 [30].
Zika virus was detected for the first time in
Aedes albopictus,
which was captured in the state of San Luis
Potosi, Mexico on 28 March 2016, and the virus was also detected and isolated from
Aedes aegypti
pools of
mosquitoes in Rio de Janeiro, Brazil.
Vector competence laboratory studies are providing new insights summarised as follow [31-34]:
Vector competence studies showed that the transmission rate of two populations of
Aedes aegypti
from
Madeira was around 20% at day nine after infection.
Aedes albopictus
populations from Europe were shown to be susceptible to Zika virus. A transmission rate
ranging from 0 to 50% at day 14 after infection was found for two populations of
Aedes albopictus
from
southern France using a Zika virus strain from New Caledonia (2014). These findings are in line with a
scientific communication that reported a transmission rate of 10% at day 10 after infection for an
Aedes
albopictus
population from Nice, France with Zika virus strain from French Polynesia. Furthermore, a
transmission rate of around 29% was found after 11 days post infection using an
Aedes albopictus
vector
population from Calabria, Italy with Zika virus strain from French Polynesia.
Diagnostics
Several Zika virus Quantitative PCR (qPCR) protocols assessed in a multicentric study show limited sensitivity and
incompatibility with Zika virus 2015-2016 outbreak strains [35].
The US Food and Drug Administration (FDA) has approved a new real-time reverse transcriptase PCR (rRT-PCR) for
detection of Zika virus in the blood [36].
CDC recommends that Zika virus RT-PCR detection (CDC Trioplex rRT-PCR assay authorised by the Food and Drug
Administration) be performed on urine collected <14 days after onset of symptoms in patients with suspected Zika
virus disease [37]. The molecular assay should be performed in conjunction with serum testing.
Event background information
Current situation worldwide
Autochthonous transmission of Zika virus was confirmed in Brazil in April 2015. Since January 2016 and as of week
16 of 2016, 120 161 probable cases (incidence rate 58.8 cases/100 000 inhabitants) of Zika virus infection have
been reported in 1 605 municipalities across 27 states, of which 39 993 were confirmed cases [38]. The mid-west
region has had the highest cumulative incidence rates since 2016 with 130.2 cases/100 000 inhabitants. The most
affected states were Mato Grosso (532.6 cases/100 000 inhabitants), Tocantins (238.4 cases/100 000 inhabitants),
Bahia (227 cases/100 000 inhabitants) and Rio de Janeiro (195.2 cases/100 000 inhabitants). In the state of Rio de
Janeiro between epidemiological week 13 and 16 there was an increase of more than 7 000 cases [39].
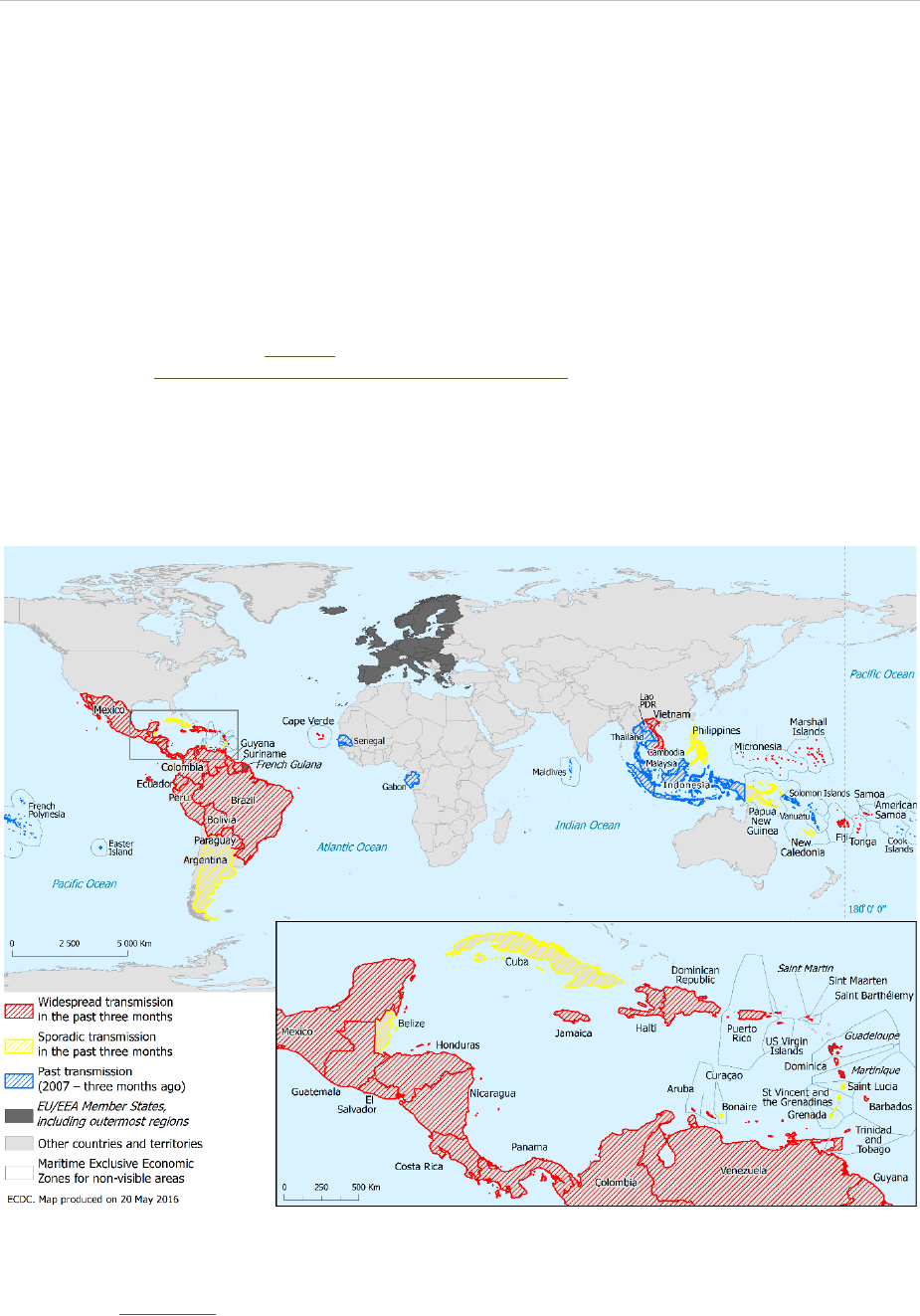
RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
5
Colombia remains the second most affected country in the Americas. Since October 2015 and as of 7 May 2016,
75 926 suspected and 4 867 confirmed cases have been reported nationally [40]. However, since the epidemic
reached its peak in week 5 of 2016, the number of suspected and confirmed cases has been steadily declining.
As of 19 May 2016, twenty-three cases of non-vector-borne transmission of Zika virus, most likely through sexual
transmission, have been reported in ten countries: Argentina (1), Canada (1), Chile (1), France (5), Italy (1),
Germany (1), New Zealand (1), Peru (1), Portugal (in the Autonomous Region of Madeira) (1) and the United
States of America (10).
Since the previous Rapid Risk Assessment published on 11 April 2016, five additional countries or territories
(Argentina, Peru, Saint-Barthélemy, Grenada and Belize) have reported autochthonous Zika virus transmission.
Over the past three months and as of 19 May 2016, autochthonous cases of Zika virus infection have been
reported from 50 countries or territories worldwide. In the past nine months, 51 countries or territories have
reported autochthonous cases of Zika virus infection. The latest information on the spread of the Zika virus
epidemic and an update on adverse pregnancy outcomes and post-infectious Guillain-Barré syndrome is available
through the ECDC Zika outbreak webpage [41]. Regular updates on the epidemiological situation are available on
an ECDC webpage Countries and territories with local Zika transmission [42].
WHO AFRO reported that the strain currently circulating in Cape Verde is genetically related to the strain circulating
in the Americas according to Institut Pasteur, Dakar [43]. Between week 41 in 2015 and week 17 in 2016,
7750 suspected Zika case were reported by the Ministry of Health in Cape Verde [44]. WHO and its partners are
continuing to support preparedness in the WHO African Region.
Figure 1. Countries and territories with reported confirmed autochthonous vector-borne transmission
of Zika virus infection in the past three months*, as of 20 May 2016
* As of week 17 in 2016, ECDC extended the period for classifying whether a country or territory has active local transmission
from two to three months. This change is based on the observation that previous Zika virus outbreaks usually lasted more than
two months. In addition, ECDC added a ‘countries and territories with past vector-borne transmission’ category for countries
having experienced transmission since 2007 and up to three months ago. More information about country classification is
available on the ECDC website.

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
6
Situation in the EU/EEA and EU outermost regions and
overseas countries and territories
As of 19 May 2016, no autochthonous vector-borne Zika virus transmission has been reported in the EU Member States
of mainland Europe. Since January 2016 and as of 19 May, ECDC has recorded 607 imported cases of Zika infection in 18
EU/EEA countries. The number is not based on systematic reporting through surveillance systems and hence cannot be
considered exhaustive. Two countries have reported the majority of the imported cases; France reported 317 cases of
Zika virus, which corresponds to an average of 20 cases per week since February 2016, and Spain reported 121 cases
since late January 2016 [45,46]. Thirty-four of the imported cases were among pregnant women.
Several EU Outermost Regions (OMR) and Overseas Countries and Territories (OCT) continue to report vector-
borne autochthonous Zika transmission: French Guiana, Guadeloupe, Martinique, Saint Martin, Saint-Barthélemy.
In addition, islands in the Dutch Antilles (Aruba, Bonaire, Curacao and Sint Maarten) continue to report
autochthonous transmission. According to the PAHO and WHO, the number of Zika cases is still increasing in the
EU Outermost Regions and Overseas Countries and Territories in the Caribbean [47].
Microcephaly and congenital central nervous system
malformations
As of 13 May 2016, congenital microcephaly, CNS malformations and other foetal malformations potentially
associated with Zika virus infection during pregnancy have been reported in eight countries or territories: Brazil,
Cape Verde, Colombia, French Polynesia, Martinique, Marshall Islands, Panama and Puerto Rico. Outside of the
epidemic area, two cases exposed during a longer stay in Brazil while pregnant have been diagnosed in Slovenia
and USA respectively [48,49], and two cases among travellers returning from the affected areas have been
reported, one from USA [49] and one from Catalonia, Spain [50].
Brazil
Since October 2015 and as of 14 May 2016, Brazil has reported 7 534 suspected cases of microcephaly from all
states and in the Federal District. Of these cases, 1 384 are reported as confirmed cases of microcephaly, 207 of
which had laboratory-confirmed presence of Zika virus infection. Of the remaining cases, 2 818 were investigated
and discarded as they did not fit the case definition, while 3 332 cases are still under investigation.
Among the 7 534 suspected cases of microcephaly, 273 intrauterine or neonatal deaths were reported. Of these,
59 cases were investigated and confirmed to have microcephaly or central nervous system malformations [51].
Colombia
Between weeks 1 and 18 in 2016, Colombia reported five confirmed cases of microcephaly associated with Zika
virus infection, 24 cases were investigated and discarded and 43 cases are still under investigation [40].
Guillain–Barré syndrome and other neurological syndromes
As of 12 May 2016 in the context of Zika virus circulation, 13 countries and territories worldwide have reported an
increased incidence of Guillain-Barré syndrome and/or laboratory confirmation of a Zika virus infection among GBS cases.
ECDC threat assessment for the EU
Since the Rapid Risk Assessment issued on 11 April 2016, the Zika epidemic remains of public health importance. The
epidemic continues to evolve and expand geographically across countries and territories in the Americas and Caribbean.
The outbreak is unprecedented and constitutes a significant development in the epidemiology of this emerging vector-
borne disease. The evolution of the Zika epidemic in the Americas demands close monitoring as it has a direct impact on
the risk of importation and possible occurrence of local transmission in the European Union.
The transmission season for dengue is starting in the Central American countries and Mexico and it is expected that the
transmission of Zika virus will increase there as well, because vector-borne transmission of Zika virus in these countries is
expected to mirror the seasonal pattern of dengue and chikungunya, two arboviruses transmitted by the same vectors
Aedes aegypti
and
Aedes albopictus
. The transmission season coincides with the summer holiday period in Europe and it
is expected that there will be an increase in the number of travellers returning to EU with Zika virus viraemia during this
period. This will increase the probability of onward transmission of Zika virus in receptive areas in Europe.
The occurrence of occasional cases of Zika virus in Asia is expected in view of the historical records of Zika virus
circulation, case reports of travel-related cases and previous sero-surveys.
On 18 April 2016, WHO’s Regional Office for Europe published an interim risk assessment on Zika for the WHO European
Region and concluded that on the island of Madeira (Portugal), and in the Black Sea coastal areas of Georgia and the
Russian Federation where
Aedes aegypti
is established, there is a high likelihood of local Zika virus transmission [52].

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
7
Travel-related risk for EU citizens
Travellers to countries where competent vectors are present and Zika virus circulation is ongoing are at risk of
becoming infected through mosquito bites. Due to the link between Zika virus infection and severe congenital
anomalies, pregnant women and women who are trying to become pregnant constitute a high-risk group with
regard to serious adverse outcomes of Zika virus infection.
Risk related to mass gatherings
The Rio de Janeiro 2016 Olympic Games (5–21 August 2016) and the Paralympic Games (7–18 September 2016)
are the two most prominent mass gathering events that will take place in the Americas in the coming months.
ECDC has published a specific risk assessment on these events, including an assessment for Zika virus infection
[53]. On 12 May, WHO published a statement on Zika virus and the Olympic and Paralympic Games Rio 2016,
providing specific advice for those participating and visiting Brazil for the Games [54]. ECDC is continuing to follow
the evolution of the Zika virus epidemic in order to assess and monitor the trends in Brazil [39].
Risk of importation and transmission in EU Outermost
Regions and Overseas Countries and Territories
Residents in EU Outermost Regions (OMR) and Overseas Countries and Territories (OCT) with competent and
active vectors are at increased risk of exposure to Zika virus.
Aedes aegypti
mosquitoes are present in the EU OCTs
and OMRs in the Americas and the Caribbean, and most of them have reported autochthonous transmission (see
Countries and territories with local Zika transmission) [42].
The risk associated with spread to as yet unaffected OCTs and OMRs in the area is significant because of the
immunologically naïve populations, the presence of competent vectors, the occurrence of prior outbreaks of
arboviruses transmitted by
Aedes
mosquitoes, the permissive climate and the movement of people in and between
countries and territories.
Other EU OMRs and OCTs outside of the Caribbean where mosquito vectors are present, such as
Aedes aegypti
in
Madeira and Mayotte or
Aedes albopictus
in
La Réunion, are at risk of local transmission should the virus be
introduced.
Madeira is of particular concern because of the presence of
Aedes aegypti
and the probability of transmission of
vector-borne pathogens is considered high during the summer months
.
The 2012 dengue epidemic demonstrated
the favourable conditions for mosquito-borne outbreaks during the summer season and the close relationship with
countries (such as Brazil and Venezuela) where Zika virus is currently circulating increases the risk of the virus
being imported [55].
According to the Interim Risk Assessment issued by WHO’s European Region, the capacity to contain Zika virus
transmission at an early stage is good for the countries of the WHO European Region overall [52].
Risk of importation and transmission in the continental EU
Zika virus circulation in the Americas and the Caribbean increases the risk of infection among travellers. Cases of
Zika virus infection arriving from countries with autochthonous transmission continue to be reported in the EU.
Based on modelling, the number of infections imported into Europe in 2016 from Brazil has been projected to
range between 508 and 1 778 in 2016, of which between 116 and 355 are expected to be symptomatic Zika
infections. The reported number of imported cases worldwide into the EU/EEA in 2016 already exceeds the lower
level of the modelling estimates [56].
There is no evidence to date of ‘airport transmission’ of mosquito-borne viral disease, similar to airport malaria
[57]. The risk of importation of Zika-infected mosquitoes inside aircraft cabins is low, and there is no evidence that
this plays a role in the transmission of arbovirus infections. WHO has issued specific guidance and
recommendations for aircraft disinsection [58,59]. On 21–22 April 2016, WHO organised an ad-hoc advisory group
to review the evidence on effectiveness of aircraft disinsection to prevent the international spread of mosquito-
borne disease, including Zika [60].
On 13 April the SHIPSAN Act Interim guidance on maritime transport and Zika virus disease [61] was updated. The
European Transport Workers’ Federation (EFT) and European Community Shipowners’ Association (ECSA)
acknowledged in a common statement the need to draw shipping companies’ and seafarer’s attention to the risks
of the Zika virus and to provide crew members on board ships calling at ports in affected countries with relevant
guidance to protect themselves [62].
The likelihood of mosquito-borne transmission of Zika virus infection in the EU is considered plausible only for
those areas where mosquitoes capable of carrying and transmitting the virus are present. The transmission
depends on several factors related to the mosquito, the virus and the environment [31,63]:

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
8
The introduction of the virus by a viraemic traveller during the summer season where
Aedes albopictus
is
established can be expected (see Situation in the EU/EEA and EU Outermost Regions and Overseas Countries
and Territories).
Aedes albopictus
is established around the Mediterranean basin. As part of the VectorNet
project, ECDC shows the current known distribution of invasive mosquito species in Europe at regional
administrative level
(NUTS 3) [64].
The suitable conditions for
Aedes albopictus
activity will increase progressively during the spring (April to
June) especially in southern Europe. By analogy with other mosquito-borne disease transmission, the
conditions for autochthonous Zika virus transmission will remain favourable in mainland Europe during
summer and autumn.
Factors such as survival, density, and biting behaviour of the vector species will determine the final
transmission potential of the vector species. Local vector-borne transmission in the EU is therefore not
excluded.
Given the low vector competence of the studied European populations of
Aedes albopictus
, the likelihood of local
vector-borne transmission in the EU is considered to be low to moderate.
Risk of Zika virus transmission via substances of human
origin
Zika virus RNA has been detected in blood, urine, saliva, seminal fluid and breast milk [65-69] (see Annex 1, Table 1).
People with asymptomatic infections and those who are viraemic in the incubation period of Zika disease could
potentially donate contaminated substances of human origin (SoHO) without their infections being recognised at
the time of donation. The virus can also be transmitted by SoHO from donors after clinical recovery from Zika virus
disease due to possible prolonged viraemia or a persistence of the virus in semen after viraemia has cleared. There
are no data available on the survival of Zika virus in processed and stored SoHO.
Assessing the risk of Zika virus transmission through contaminated SoHO is currently difficult because of the
paucity of data on the prevalence of Zika virus in the donor population and the limited number of case reports of
transmission via SoHO. According to Musso, et al, during the last Zika virus outbreak in French Polynesia, 42 of
1 505 (3%) blood donors, although asymptomatic at the time of donation, were found to be positive for the Zika
virus genome by RT-PCR, supporting a potential risk of transfusion-derived transmission [65,70]. The Brazilian
media reported possible cases of transfusion-transmitted Zika virus in March 2015 and February 2016 [71-73].
Several cases of sexual transmission from males to their partners have been reported (see ‘Risk of sexual
transmission’ below).
There are no documented transmissions of the virus via saliva, urine or breastfeeding. Cases of Zika virus
transmission through donated cells, tissues and organs have not been reported, but this possibility cannot be
excluded due to the confirmed presence of the virus in human blood and bodily fluids.
A recent case report of Zika congenital infection showed a prolonged detection of low viral level by quantitative RT-
PCR of Zika virus RNA in serum from the mother in weeks 16 and 20 of pregnancy; after termination of the
pregnancy, RT-PCR returned to negative. The kinetics of Zika virus RNA in the sera of infected pregnant women
are not yet well understood and would require assessment in larger studies [74].
The limited set of data indicates that there is a potential risk of Zika virus transmission through SoHO that may
cause serious consequences to the health of recipients. However, a scarcity of reported cases of donor-derived
Zika virus infection precludes a more accurate risk assessment. The evidence of association between Zika virus
infection and congenital malformations and Guillain-Barré syndrome justifies preventive measures to reduce the
risk of transmission via SoHO supply [75].
Risk of sexual transmission
Replicative Zika virus particles have been detected on two occasions in semen at 21 and 24 days after onset of
Zika symptoms [68,76]. Zika viral RNA has been reported in semen at 14 days [77], 21 days [68], 24 days [76]
and up to 62 days after clinical onset of disease [78].
Zika virus genome has also been detected in saliva during and after the acute phase of the disease and reported
from the symptom onset up to 29 days (see Annex 1, Table 1). A viral isolation from saliva was reported at day 6,
a second isolation is reported but the date on sampling is not available [79].
Reports of sexual transmission of Zika virus through contaminated male semen to a female partner or male partner
indicate the possible virus transmission through donated sperm [30,80-84].
In all published cases, males presented with a clinical illness compatible with Zika virus infection. The interval
between onset of symptoms in the man and in his female partner varies from four to 19 days. So far, no sexual
transmission of Zika virus from infected women to their partners has been reported.

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
9
Comprehensive data about the presence of viable virus, viral load or kinetics is not available, and at this point in
time the risk of transmission via saliva cannot be further assessed. Further comprehensive information about the
kinetics of the Zika virus in bodily fluids and the consequent clinical implication is required in order to adapt
prevention and control measures accordingly.
Conclusions and options for response
Given the continued spread of Zika virus in the Americas and Caribbean; the evidence of an association between
Zika virus infection during pregnancy and congenital CNS malformations; the association between Zika virus
infection and Guillain–Barré syndrome and the risk of local vector-borne transmission in Europe during the 2016
summer season, EU/EEA Member States are recommended to consider a range of mitigation measures.
The following uncertainties have been taken into consideration in developing the proposed options for response:
There is growing evidence that Zika virus infection during the first and second trimester is associated with
increased risk for central nervous system malformation of the foetus. The risk associated with infection during
the third trimester is unknown. Therefore, Zika virus infection should be considered as a risk throughout the
entire duration of pregnancy.
The presence of viable Zika virus in semen has been detected up to 24 days after onset of Zika virus infection
symptoms. The longest interval reported between the onset of symptoms in a male and the subsequent onset
of the disease thought to be due to sexual transmission in a female partner is 19 days.
All the currently reported sexual transmission events are linked to symptomatic index cases. There is no
evidence of transmission by asymptomatic sexual partners.
Information to travellers to and EU residents in areas with
active transmission
A list of countries and territories with active transmission (sporadic and widespread transmission) during the past
three months is available on the ECDC website.
Information for travellers to and EU citizens residing in areas with
active transmission
Travellers visiting countries where there is active transmission of Zika virus and EU citizens residing in these
countries should:
o be made aware of the ongoing outbreak of Zika virus infection and the fact that Zika virus is usually
transmitted by mosquito vectors but can be also transmitted by sexual intercourse.
o take measures to prevent mosquito bites indoors and outdoors, especially between sunrise and sunset
when
Aedes
mosquito vectors are most active and biting. These measures include:
− The use of mosquito repellent in accordance with the instructions indicated on the product label.
− Wearing long-sleeved shirts and long trousers, especially during the hours when the type of
mosquito that is known to transmit the Zika virus (
Aedes
) is most active.
− Sleeping or resting in screened or air-conditioned rooms, otherwise use mosquito nets, at night and
during the day.
Pregnant women and women who are planning to become pregnant and planning to travel to areas with
widespread transmission should postpone non-essential travel.
Pregnant women and women who are planning to become pregnant and planning to travel to areas with
sporadic transmission should consult their physician or a travel clinic and consider postponing non-essential
travel.
Pregnant women residing in countries with active transmission (sporadic and widespread) should consult their
healthcare providers for advice and follow strict measures to prevent mosquito bites.
Travellers with immune disorders or severe chronic illnesses should consult their doctor or seek advice from a
travel clinic - particularly on effective prevention measures - before travelling to countries with active
transmission.
Travellers to countries with active Zika transmission and EU citizens residing there should be advised that
using condoms could reduce the risk of sexual transmission through semen.

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
10
Information for travellers returning from areas with active
transmission of Zika virus
Pregnant women who have travelled or resided in areas with active transmission should mention their travel
during antenatal visits in order to be assessed and monitored appropriately.
In order to protect the foetus, male travellers returning from areas with active transmission should consider
using a condom with a pregnant partner until the end of pregnancy.
Travellers returning from areas with ongoing Zika virus transmission should be advised to use a condom for at
least one month after returning, in order to reduce the potential risk of onward sexual transmission.
Travellers, including those with immune disorders or severe chronic illnesses, showing symptoms compatible
with Zika virus disease within two weeks of return from an area with active transmission are advised to
contact their healthcare provider and mention their recent travel.
Surveillance of imported cases and monitoring of
transmission in the EU Member States of mainland Europe
Increase awareness among clinicians and travel health clinics of the evolution of the Zika virus outbreak and
the areas with active and past transmission (ECDC website) to allow them to consider Zika virus infection in
their differential diagnosis for travellers from those areas. Clinicians should be aware that Zika virus infection
can be pauci-symptomatic.
Enhance vigilance towards the early detection of imported cases of Zika virus infection into EU Member States,
EU OCTs and OMRs, in particular where Zika vectors are present, in order to reduce the risk of onward
autochthonous transmission.
Clusters of unexplained illness with a rash detected in receptive areas of mainland Europe between 1 May and
31 October should be investigated, and Zika virus infection should be considered as a possible cause.
Ensure timely reporting of autochthonous cases, in particular in receptive areas of EU Member States in
mainland Europe.
Strengthen laboratory capacity to confirm suspected Zika virus infections in the EU/EEA in order to
differentiate Zika virus infections from other arboviral infections (e.g. dengue, chikungunya).
Increase awareness among obstetricians, paediatricians and neurologists that Zika virus infections should be
investigated in patients presenting with congenital CNS malformations, microcephaly and Guillain-Barré
syndrome.
Information to healthcare providers in EU Member States
It is important to ensure that Zika virus-infected patients in areas with
Aedes
mosquito vectors avoid getting bitten
during the first week of illness (bed nets, screened doors and windows as recommended by PAHO/WHO).
Efforts should be made to increase awareness among health professionals who provide prenatal care of risk of
neurological congenital syndrome associated with Zika virus infection, especially during the two first trimesters,
and adapt prenatal monitoring in accordance with exposure to the vector [85,86]. ECDC maps showing Zika
transmission in the past nine months are provided to aid diagnosis of returning travellers, especially pregnant
women with travel history during pregnancy - returning from countries and territories that have recently- or are
currently experiencing local active Zika virus transmission.
In addition, due to the unprecedented size of the Zika virus epidemic, health services and practitioners should be
alerted to the possible occurrence of neurological syndromes (Guillain-Barré syndrome and other neurological
syndromes such as meningitis, meningoencephalitis and myelitis according to WHO/PAHO); potential disease
complications as yet described in the scientific literature and atypical clinical presentation among specific
populations (i.e. children, the elderly, immunocompromised individuals and those with sickle cell disease).
Safety of substances of human origin
Competent authorities, establishments and clinicians dealing with SoHO need to be vigilant and aware of the risk of
donor-derived Zika virus transmission through transfusion and transplantation. Measures to prevent Zika virus
transmission through SoHO should be taken in both affected and non-affected areas. Implementation of SoHO
safety measures should be defined by the risk assessment performed at the national level. The European
Commission’s Directorate General for health and food safety established a working group for the preparation of a
preparedness plan in Europe related to safety of substances of human origin in the event of a Zika virus outbreak.
ECDC will synchronise detailed SoHO safety measures, already described in Annex 1 of the risk assessment dated
9 March 2016 [8], with those defined in the SoHO safety preparedness plan at EU level.

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
11
Non-affected areas and areas with sporadic transmission
The primary measure to prevent Zika virus transmission in non-affected areas and areas with sporadic transmission
is the temporary deferral of donations from blood donors and living donors of cells and tissues who are at risk of
having been infected. When defining donors at risk criteria for consideration are:
A medical diagnosis of Zika virus disease;
Returning from areas with widespread transmission;
Reporting sexual intercourse with males diagnosed with Zika virus disease or who have returned from areas
with widespread transmission.
Based on the frequency of travel to currently affected areas, the Netherlands have assessed a risk of Zika virus
transmission by blood donors who have had sexual contact with a male returning from affected area as too small
to warrant their deferral [87]. Similarly, based on a risk assessment, Australia does not apply deferral to blood
donors who have had sexual contact with asymptomatic males returning from affected areas [88]. Thus, current
practice, as reported to date, is that the implementation of safety measures for this category of risk donors is being
considered and re-assessed as required, as part of the risk assessment for national preparedness plans.
Cells and tissues from deceased donors with a recent medical diagnosis of Zika virus infection should not be
accepted for donation. Periods defined for living SoHO donor deferral/acceptance should be set to provide a
sufficient safety margin for virus-free donation. This includes taking into account viral persistence in the particular
type of SoHO during and after the clinical course of Zika virus disease.
Areas with widespread transmission
Blood and tissue establishments may temporarily interrupt donations and import blood components or cells and
tissues from unaffected parts of the country and consider the use of pathogen inactivation for plasma, platelets
and some tissues. The screening of all donated blood and all donors of cells and tissues for the presence of Zika
virus RNA by nucleic acid testing (NAT) may be considered necessary to assure the safety and sustainability of
supply in areas with widespread transmission. A systematic review and pooled analysis to estimate the distribution
of times from Zika infection to symptom onset, seroconversion and viral clearance showed that symptom-based
screening reduces the risk of a positive Zika virus blood donation by 7% (RR 0.93, 95% CI 0.86–0.99), and
antibody screening by 29% (RR 0.71, 95% CI: 0.28–0.88) [89]. This estimate confirms that in areas with a high
incidence of Zika virus, blood establishments may consider NAT testing to identify lots safe for use in pregnant
women.
Test kits, registered/approved for use as screening tests, should be used for determining SoHO donor/donation
suitability. Commercial Zika tests for screening are still under development. Based on scientific data, SoHO
establishments and laboratories may develop in-house or adapt available commercial diagnostic test for screening
purposes. The use of such screening tests in the event of a Zika virus outbreak should be validated and approved
by the responsible national authority. Some blood establishments are gaining experience with in-house testing or
using adapted commercial tests. Semi-automated platforms for NAT screening using CE marked kits for diagnostics
were implemented for NAT screening in the French West Indies during the 2014 outbreak of chikungunya [90] and
are currently being implemented for NAT screening of blood donors for Zika virus in the French Antilles using the
RealStar RT-PRC Zika kit 1.0, Altona. At the end of March 2016, the US Food and Drug Administration approved
the use of an investigational test to screen blood donations for Zika virus under a new drug application being
investigated for areas with an active mosquito-borne transmission of Zika virus [91]. Those European Member
States that are most likely be impacted by a spread of the Zika virus infection could potentially use this test for
screening blood donations.
Irrespective of the presence of ongoing local virus transmission in the area, the risk of Zika virus transmission
through organs donated by living or deceased donors should be recognised and assessed during a pre-donation
evaluation and balanced against the benefits of the transplantation for each potential recipient.
Preparedness in the EU
Preparedness for the prevention and control of Zika virus infection in the EU/EEA will require capacities and
capabilities for early detection, response and communication. ECDC has published a preparedness planning guide
for diseases transmitted by
Aedes aegypti
and
Aedes albopictus
. The guide focuses on the main components that
should be considered when developing preparedness plans. Consistent with the evidence presented in this
document, the following components might be considered with regard to Zika virus preparedness [55,92-96].
Early detection mechanisms should ensure the following:
Rapid notification of human cases (imported and/or autochthonous).
Surveillance of those
Aedes
mosquito species that are vectors for Zika virus; this should include consideration
of entomological and environmental indicators. ECDC Guidelines for the surveillance of invasive mosquitoes in
Europe provide a useful overview of entomological surveillance at national and subnational levels [97].
Laboratory diagnosis capacity.

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
12
Response mechanisms should cover the following:
Organisational and planning mechanisms aimed at the prevention and control of mosquito-borne diseases.
Intersectoral and cross-disciplinary collaboration with all relevant partners.
Case management.
Safety of substances of human origin.
Gynaecological, obstetric and neonatal services to follow-up on infected pregnant women and to provide
reproductive health guidance.
Outbreak investigation capacity (including epidemiological, entomological and environmental aspects)
Rapid vector control measures against imported cases in areas with
Aedes
mosquito species that are vectors
for Zika virus.
Communication mechanisms:
Advice to travellers, with special focus on pregnant women.
Training of healthcare professionals on health impacts of Zika virus.
Community involvement in the control of mosquito populations through both individual and collective
preventive measures.
Involvement of mass media for communication purposes and to promote public awareness and protection.

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
13
Annex 1. Time of detection Zika virus in biological
samples
Table 1. Time of detection of Zika virus in human samples
Sample origin
Methods
Range of detection in days from onset of symptoms
Minimum (days)
Ref.
Maximum
(days)
Ref.
Blood
Molecular diagnostic
One day prior to
symptoms
[98]
14
[77]
Virus isolation
Urine
Molecular diagnostic
One day prior to
symptoms
[98]
15 to 29
[66,99-101]
Virus isolation
4
[102]
Saliva
Molecular diagnostic
1
[67]
29
[100]
Virus isolation
6
[100]
Seminal fluid
Molecular diagnostic
14
[77]
62
[78]
Virus isolation
21 to 24
[68,76]
Breast milk
Molecular diagnostic
3 (after delivery)
[69]
8 (after delivery)
[69]
Virus isolation
4 (after delivery)
[103]
According to communication during the international Zika summit which took place in Paris on 25–26 April 2016,
Zika virus RNA was detected in three asymptomatic individuals during a biological, clinical, serological and
virological follow-up of a military community exposed to Zika virus in Suriname over a two-week period (two urine
samples and one blood sample) [98].
The main features of detection Zika virus in human samples can be summarised as follow:
In the blood, Zika virus is usually detected between the day of onset of symptoms and five days after (up to
14 days) [77]. Molecular diagnostics were found positive the day prior the onset of symptoms and in one
asymptomatic patient [98]. It is estimated that Zika virus clearance in the blood takes on average 9.9 days
(95% CI: 6.8–21.4) [89].
In the urine, molecular diagnostic results were positive up to 29 days [100] and viral isolation at day four after
onset [102].
In the saliva, the Zika virus RNA was detected for up to 29 days [100]. Zika virus isolation from saliva has
been reported on day six after onset of a febrile illness in a patient returning from the Dominican Republic to
Italy [100]. Further investigation would be needed to evaluate the infectivity of Zika virus in saliva as a non-
vector-borne viral transmission mode of Zika disease.
In breast milk two studies have been published (see table above) along with a recent review [104].
Based on a modelling study, seroconversion occurs on average at 9.0 days (95% CI, 7.0–11.6) after infection but
serological results should be interpreted with caution due to cross-reactivity with other flaviviruses and depending
on the vaccination status against flaviviruses [89,105]. A recent case report of Zika congenital infection showed a
prolonged detection at low level by quantitative RT-PCR of Zika virus RNA in serum from the mother at between
weeks 16 and 20 of pregnancy and after termination of the pregnancy, RT-PCR returned to negative. The kinetics
of Zika virus RNA in the sera of infected pregnant women are not yet well understood and would require
assessment in larger studies [74].

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
14
References
1. European Centre for Disease Prevention and Control. Rapid risk assessment - Zika virus infection outbreak,
French Polynesia. 14 February 2014 [Internet]. Stockholm: ECDC; 2014. Available from:
http://ecdc.europa.eu/en/publications/Publications/Zika-virus-French-Polynesia-rapid-risk-assessment.pdf.
2. European Centre for Disease Prevention and Control. Rapid risk assessment - Zika virus infection outbreak,
Brazil and the Pacific region. 25 May 2015 [Internet]. Stockholm: ECDC; 2015. Available from:
http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-Zika%20virus-south-america-
Brazil-2015.pdf.
3. European Centre for Disease Prevention and Control. Rapid risk assessment - Microcephaly in Brazil
potentially linked to the Zika virus epidemic. 24 November 2015 [Internet]. Stockholm: ECDC; 2015.
Available from: http://ecdc.europa.eu/en/publications/Publications/zika-microcephaly-Brazil-rapid-risk-
assessment-Nov-2015.pdf.
4. European Centre for Disease Prevention and Control. Rapid risk assessment - Zika virus epidemic in the
Americas: potential association with microcephaly and Guillain-Barré syndrome. 10 December 2015
[Internet]. Stockholm: ECDC; 2015. Available from: http://ecdc.europa.eu/en/publications/Publications/zika-
virus-americas-association-with-microcephaly-rapid-risk-assessment.pdf.
5. European Centre for Disease Prevention and Control. Rapid risk assesment: Zika virus epidemic in the
Americas: potential association with microcephaly and Guillain-Barré syndrome. First update, 21 January
2016 [Internet]. Stockholm: ECDC; 2016. Available from:
http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-
2016.pdf.
6. European Centre for Disease Prevention and Control. Rapid risk assesment - Zika virus disease epidemic:
potential association with microcephaly and Guillain-Barré syndrome. Second update, 8 February 2016
[Internet]. Stockholm: ECDC; 2016. Available from: http://ecdc.europa.eu/en/publications/Publications/zika-
virus-rapid-risk-assessment-8-february-2016.pdf.
7. European Centre for Disease Prevention and Control. Rapid risk assessment - Zika virus disease epidemic:
potential association with microcephaly and Guillain-Barré syndrome. Third update, 23 February 2016
[Internet]. Stockholm: ECDC; 2016. Available from: http://ecdc.europa.eu/en/publications/Publications/zika-
virus-rapid-risk-assessment-23-february-2016.pdf.
8. European Centre for Disease Prevention and Control. Rapid risk assessment - Zika virus disease epidemic:
potential association with microcephaly and Guillain-Barré syndrome. Fourth update, 9 March 2016
[Internet]. Stockholm: ECDC; 2016. Available from: http://ecdc.europa.eu/en/publications/Publications/zika-
virus-rapid-risk-assessment-9-march-2016.pdf.
9. European Centre for Disease Prevention and Control. Rapid risk assessment - Zika virus disease epidemic:
potential association with microcephaly and Guillain-Barre syndrome. Fifth update, 11th April 2016.
[Internet]. Stockholm: ECDC; 2016. Available from: http://ecdc.europa.eu/en/publications/Publications/zika-
virus-rapid-risk-assessment-11-april-2016.docx.pdf
10. Malone RW, Homan J, Callahan MV, Glasspool-Malone J, Damodaran L, Schneider Ade B, et al. Zika Virus:
Medical Countermeasure Development Challenges. PLoS Negl Trop Dis. 2016 Mar;10(3):e0004530.
11. Plourde AR, Bloch EM. A Literature Review of Zika Virus. Emerg Infect Dis. 2016 Jul 15;22(7).
12. Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, et al. The 3.8 A resolution cryo-EM structure of
Zika virus. Science. 2016 Apr 22;352(6284):467-70.
13. Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, Ooi JS, et al. Structure of the thermally stable Zika
virus. Nature. Epub 2016 Apr 19.
14. Zhu Z, Chan JF, Tee KM, Choi GK, Lau SK, Woo PC, et al. Comparative genomic analysis of pre-epidemic
and epidemic Zika virus strains for virological factors potentially associated with the rapidly expanding
epidemic. Emerg Microbes Infect. 2016;5:e22.
15. Wang L, Valderramos SG, Wu A, Ouyang S, Li C, Brasil P, et al. From Mosquitos to Humans: Genetic
Evolution of Zika Virus. Cell Host Microbe. 2016 May 11;19(5):561-5.
16. Faria NR, Azevedo Rdo S, Kraemer MU, Souza R, Cunha MS, Hill SC, et al. Zika virus in the Americas: Early
epidemiological and genetic findings. Science. 2016 Apr 15;352(6283):345-9.
17. Carteaux G, Maquart M, Bedet A, Contou D, Brugieres P, Fourati S, et al. Zika Virus Associated with
Meningoencephalitis. N Engl J Med. 2016 Apr 21;374(16):1595-6.
18. Vinhaes E, Santos L, Dias L, Andrade N, Bezerra V, De Moraes L, et al. Transient hearing loss in adults
associated with Zika virus infection. In: International Zika Summit 2016; 25- 26 April 2016 [Internet]. Paris:
Institute Pasteur. Available from: http://www.zikasummit2016.org/images/Public/Zika-Abstracts.pdf.
19. de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, Coeli RR, Rocha MA, Sobral da Silva P, et al.
Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection
and microcephaly: retrospective case series study. BMJ. 2016;353:i1901.

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
15
20. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects - Reviewing the
Evidence for Causality. N Engl J Med. Epub 2016 Apr 13.
21. Broutet N, Krauer F, Riesen M, Khalakdina A, Almiron M, Aldighieri S, et al. Zika Virus as a Cause of
Neurologic Disorders. N Engl J Med. 2016 Apr 21;374(16):1506-9.
22. World Health Organization. Zika situation report: Zika virus, microcephaly and Guillain Barré syndrome. 31
March 2016 [Internet]. Geneva: WHO; 2016. Available from:
http://apps.who.int/iris/bitstream/10665/204718/1/zikasitrep_31Mar2016_eng.pdf?ua=1.
23. Brasil P, Pereira JP, Jr., Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro Nogueira RM, et al. Zika Virus
Infection in Pregnant Women in Rio de Janeiro - Preliminary Report. N Engl J Med. 2016 Mar 4.
24. Thalia VBA, Rodrigues L. Microcephaly and Zika infection: preliminary report of a case-control study. 2016.
In: International Zika Summit, 25-26 April 2016 [Internet]. Paris: Institute Pasteur. Available from:
http://www.zikasummit2016.org/images/Public/Zika-Abstracts.pdf
25. Cao-Lormeau V-M, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, et al. Guillain-Barré syndrome
outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016 (ePub:
29 February 2016).
26. Lednicky J, Beau De Rochars VM, El Badry M, Loeb J, Telisma T, Chavannes S, et al. Zika Virus Outbreak in
Haiti in 2014: Molecular and Clinical Data. PLoS Negl Trop Dis. 2016 Apr;10(4):e0004687.
27. Brasil P, Calvet GA, Siqueira AM, Wakimoto M, de Sequeira PC, Nobre A, et al. Zika Virus Outbreak in Rio de
Janeiro, Brazil: Clinical Characterization, Epidemiological and Virological Aspects. PLoS Negl Trop Dis.
2016;10(4):e0004636.
28. World Health Organization. Zika situation report: Zika virus, microcephaly and Guillain Barré syndrome. 14
April 2016 [Internet]. Geneva: WHO; 2016. Available from:
http://apps.who.int/iris/bitstream/10665/205189/1/zikasitrep_14Apr2016_eng.pdf?ua=1.
29. Favoretto S, Araujo D, Oliveira D, Duarte N, Mesquita F, Zanotto P, et al. First detection of Zika virus in
neotropical primates in Brazil: a possible new reservoir. bioRxiv [Internet]. 2016. Available from:
http://biorxiv.org/content/early/2016/04/20/049395.
30. Deckard TD, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, et al. Male-to-Male Sexual
Transmission of Zika Virus — Texas, January 2016. MMWR Morb Mortal Wkly Rep. 2016;65(14):372-74.
31. Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, et al. Differential
susceptibilities of
Aedes aegypti
and
Aedes albopictus
from the Americas to Zika Virus. PLoS Negl Trop Dis.
2016;10(3):e0004543.
32. Jupille H, Seixas G, Mousson L, Sousa CA, Failloux AB. Zika virus, a new threat for Europe? bioRxiv
[Internet]. 2016. Available from: http://biorxiv.org/content/biorxiv/early/2016/04/13/048454.full.pdf.
33. Ryckebusch F, Matondo M, Misse D, Choumet V. Infection of
Aedes albopictus
and
Aedes aegypti
with Zika
virus: perspectives for an emergence in Europe. 2016. In: International Zika Summit 2016; 25-26 April 2016
[Internet]. Paris: Institute Pasteur. Available from: http://www.zikasummit2016.org/images/Public/Zika-
Abstracts.pdf.
34. Di Luca M, Severini F, Toma L, Boccolini D, Romi R, Remoli ME, et al. Experimental studies of susceptibility
of Italian
Aedes albopictus
to Zika virus. Euro Surveill [Internet]. 2016 May 5; 21(18):[pii=30223 p.].
Available from: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=22468.
35. Corman VM, Rasche A, Baronti C, Aldabbagh S, Cadar D, Reusken CBEM, et al. Clinical comparison,
standardization and optimization of Zika virus molecular detection. Bull World Healt Organ [Internet].
Forthcoming. Available from: http://www.who.int/bulletin/online_first/16-175950.pdf.
36. U.S. Food and Drug Administration. Emergency use authorizations: Zika virus emergency use authorization
[Internet]. Silver Spring, MD: FDA; 2016. Available from:
http://www.fda.gov/MedicalDevices/Safety/EmergencySituations/ucm161496.htm#zika.
37. Centers for Disease Control and Prevention. Interim guidance for Zika testing of urine. MMWR Morb Mortal
Wkly Rep. Forthcoming.
38. Ministério da Saúde (Brasil). Monitoramento dos casos de dengue, febre de chikungunya e febre pelo vírus
Zika até a Semana Epidemiológica 16, 2016. Boletim Epidemiológico N° 20 - 2016 [Internet]: Ministério da
Saúde; 2016. Available from: http://portalsaude.saude.gov.br/images/pdf/2016/maio/17/2016-016---
Dengue-SE16-publica----o.pdf
39. Ministério da Saúde (Brazil). Monitoramento dos casos de dengue, febre de chikungunya e febre pelo virus
Zika ate a Semana Epidemiologica 13, Boletim Epidemiologico No 18 - 2016 [Internet]2016. Available from:
http://portalsaude.saude.gov.br/images/pdf/2016/abril/27/2016-014---Dengue-SE13-substitui----o.pdf
40. Instituto Nacional de Salud (Colombia). Semana epidemiológica número 18 de 2016 (01 may. al 07 may.).
Boletin epidemiologico semanal [Internet]. 2016. Available from: http://www.ins.gov.co/boletin-
epidemiologico/Boletn%20Epidemiolgico/2016%20Boletin%20epidemiologico%20semana%2018.pdf.

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
16
41. European Centre for Disease Prevention and Control. Zika outbreak in the Americas and the Pacific
[Internet]. Stockholm: ECDC; 2016. Available from:
http://ecdc.europa.eu/en/healthtopics/zika_virus_infection/zika-outbreak/Pages/zika-outbreak.aspx.
42. European Centre for Disease Prevention and Control. Countries and territories with local Zika transmission
[Internet]. Stockholm: ECDC; 2016. Available from:
http://ecdc.europa.eu/en/healthtopics/zika_virus_infection/zika-outbreak/Pages/Zika-countries-with-
transmission.aspx.
43. World Health Organization. WHO confirms Zika virus strain imported from the Americas to Cabo Verde. 20
may 2016. [Internet]. Geneva: WHO; 2016. Available from:
http://www.who.int/mediacentre/news/releases/2016/zika-cabo-verde/en/.
44. Direção-Nacional de Saúde (Cabo Verde)-Servico de Vigilancia intergrada e resposta a epidemias. Boletim
Informativo: Surto de casos suspeitos de infeção por Vírus Zika Ano 2016 [Internet]: Direção-Nacional de
Saúde 2016. Available from: http://www.minsaude.gov.cv/index.php/documentosite/zika-1/338-boletim-
informativo-surto-suspeito-zika-semana/file.
45. Institut de veille sanitaire. Zika - Données épidémiologiques [Internet]. 13 May 2016. Available from:
http://www.invs.sante.fr/fr/Dossiers-thematiques/Maladies-infectieuses/Maladies-a-transmission-
vectorielle/Zika/Donnees-epidemiologiques.
46. Ministerio de Sanidad SSeIE. Casos diagnosticados: 17 May 2016 [Internet]. Madrid: Ministerio de Sanidad;
2016. Available from:
http://www.msssi.gob.es/profesionales/saludPublica/zika/casosDiagnosticados/home.htm.
47. Pan American Health Organization / World Health Organization, Regional Office for the Americas. Suspected
and confirmed Zika cases reported by countries and territories in the Americas, 2015-2016 [Internet].
PAHO/WHO; 2016. Available from: http://ais.paho.org/phip/viz/ed_zika_epicurve.asp.
48. Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, et al. Zika Virus Associated with
Microcephaly. N Engl J Med. 2016 Mar 10;374(10):951-8.
49. World Health Organization. Zika situation report: Zika virus, microcephaly, and Guillain Barre syndrome. 12
May 2016 [Internet]. Geneva: WHO; 2016. Available from:
http://apps.who.int/iris/bitstream/10665/206311/1/zikasitrep_12May2016_eng.pdf?ua=1.
50. Generalitat de Catalunya. Comunicat sobre virus zika: 5 May 2016 [Internet]. Available from:
http://premsa.gencat.cat/pres_fsvp/AppJava/notapremsavw/292697/ca/comunicat-sobre-virus-zika.do.
51. Ministério da Saúde (Brazil). Ministério da Saúde confirma 1.384 casos de microcefalia no país. 14 May 2016
[Internet]. Ministerio da Saude (Brazil); 2016. Available from:
http://portalsaude.saude.gov.br/index.php/cidadao/principal/agencia-saude/23753-microcefalia-ministerio-
da-saude-confirma-1-384-casos-no-pais
52. World Health Organization. Zika virus technical report: interim risk assessment WHO European Region. May
2016 [Internet]. Geneva: WHO; 2016. Available from:
http://www.euro.who.int/__data/assets/pdf_file/0003/309981/Zika-Virus-Technical-report.pdf?ua=1.
53. European Centre for Disease Prevention and Control. Potential risks to public health related to
communicable diseases at the Olympics and Paralympics Games in Rio de Janeiro, Brazil 2016 [Internet].
Stockholm ECDC; 2016. Available from:
http://ecdc.europa.eu/en/publications/_layouts/forms/Publication_DispForm.aspx?List=4f55ad51-4aed-
4d32-b960-af70113dbb90&ID=1486
54. World Health Organization. Zika virus and the Olympic and Paralympic Games Rio 2016: 12 May 2016
[Internet]. Geneva: WHO. Available from: http://www.who.int/mediacentre/news/statements/2016/zika-
olympics/en/.
55. European Centre for Disease Prevention and Control. Dengue outbreak in Madeira, Portugal, March 2013.
[Internet]. Stockholm: ECDC; 2014. Available from:
http://ecdc.europa.eu/en/publications/Publications/dengue-madeira-ECDC-mission-2013.pdf.
56. Massad E, Tan S-H, Khan K, Wilder-Smith A. Estimated Zika virus importations to Europe by travellers from
Brazil. Global Health Action. 2016;9.
57. Gratz NG, Steffen R, Cocksedge W. Why aircraft disinsection? Bull World Health Organ. 2000;78(8):995-
1004.
58. World Health Organization. WHO statement on the first meeting of the International Health Regulations
(2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal
malformations. 1 February 2016 [Internet]. Geneva: WHO; 2016. Available from:
http://www.who.int/mediacentre/news/statements/2016/1st-emergency-committee-zika/en/.
59. International Programme on Chemical Safety (IPCS). Chemicals for aircraft disinsection [Internet]. Geneva
WHO; 2013. Available from: http://www.who.int/ipcs/assessment/aircraft_disinsection_review/en/.

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
17
60. World Health Organization. Report of the WHO Ad-hoc Advisory Group on aircraft disinsection for controlling
the international spread of vector-borne diseases [Internet]. Geneva: WHO; 2016. Available from:
http://apps.who.int/iris/bitstream/10665/205795/1/WHO_HSE_GCR_2016.12_eng.pdf?ua=1.
61. EU SHIPSAN ACT Joint Action. Interim guidance on maritime transport and Zika virus disease: update 13
April 2016 [Internet]. Shipsan; 2016. Available from: http://www.shipsan.eu/Home/Zikavirus.aspx.
62. European Transport Workers' Federation. ETF and ECSA joint declaration on the risks of the Zika virus and
guidance to stay safe for crews onboard ships calling in affected countries. 3 May 2016 [Internet]. Brussels:
ETF; 2016. Available from: http://www.etf-europe.org/etf-press-area.cfm/pressdetail/11349.
63. European Centre for Disease Prevention and Control. Mosquito maps: Current known distribution as of
October 2015 [Internet]. Stockholm: ECDC; 2015. Available from:
http://ecdc.europa.eu/en/healthtopics/vectors/vector-maps/Pages/VBORNET_maps.aspx.
64. European Centre for Disease Prevention and Control. VectorNet: A European network for sharing data on
the geographic distribution of arthropod vectors, transmitting human and animal disease agents [Internet].
Stockholm: ECDC; 2016. Available from:
http://ecdc.europa.eu/en/activities/diseaseprogrammes/emerging_and_vector_borne_diseases/Pages/VBOR
NET.aspx
65. Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, et al. Potential for Zika virus transmission through
blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014.
Euro Surveill [Internet]. 2014; 19(14):[pii=20761 p.]. Available from:
http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20761.
66. Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg
Infect Dis. 2015 Jan;21(1):84-6.
67. Musso D, Roche C, Tu-Xuan N, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin
Virol. 2015;68:53-5.
68. Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika
virus. Emerg Infect Dis. 2015 Feb;21(2):359-61.
69. Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus,
French Polynesia, December 2013 and February 2014. Euro Surveill [Internet]. 2014; 19(13):[pii=20751
p.]. Available from: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20751.
70. Aubry M, Finke J, Teissier A, Roche C, Broult J, Paulous S, et al. Seroprevalence of arboviruses among blood
donors in French Polynesia, 2011-2013. Int J Infect Dis. 2015 Oct 23;41:11-2.
71. Herriman R. Transfusion-associated Zika virus reported in Brazil. 18 December 2015 [Internet]. Outbreak
News Today; 2015. Available from: http://outbreaknewstoday.com/transfusion-associated-zika-virus-
reported-in-brazil-76935/.
72. Secretaria de Saúde de Campinas (Brasil), Hemocentro da Unicamp. Notícias: Campinas tem o primeiro caso
de Zika vírus confirmado. 2 February 2016 [Internet]. Campinas: Prefeitura de Campinas (Brasil); 2016.
Available from: http://www.campinas.sp.gov.br/noticias-integra.php?id=29241.
73. Souto L. São Paulo registra segundo caso de transmissão de zika por transfusão. 3 February 2016
[Internet]. O Globo; 2016. Available from: http://oglobo.globo.com/brasil/sao-paulo-registra-segundo-caso-
de-transmissao-de-zika-por-transfusao-18601427#ixzz3zBOmp9Nn
74. Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, et al. Zika Virus Infection with
Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med. Epub 2016 Mar 30.
75. World Health Organization. WHO Director-General summarizes the outcome of the Emergency Committee
on Zika. 1 February 2016 [Internet]. Geneva: WHO; 2016. Available from:
http://www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en/.
76. D'Ortenzio E, Matheron S, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, et al. Evidence of Sexual
Transmission of Zika Virus. N Engl J Med. Epub 2016 Apr 13.
77. Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, et al. Zika virus: high infectious
viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405.
78. Atkinson B, Hearn P, Afrough B, Lumley S, Carter D, Aarons. Emma J, et al. Detection of Zika virus in
semen. Emerg Infect Dis. 2016;22(5).
79. Bonaldo MC, Ribeiro IP, Lima NS, Santos AAC, Menezes LSR, Cruz SOD, et al. Isolation of infective Zika
virus from urine and saliva of patients in Brazil. bioRxiv [Internet]. 2016. Available from:
http://biorxiv.org/content/early/2016/03/24/045443.
80. Venturi G, Zammarchi L, Fortuna C, Remoli M, Benedetti E, Fiorentini C, et al. An autochthonous case of
Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill [Internet]. 2016;
21(8):[pii=30148 p.]. Available from: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21395.
81. Gobierno de la Provincia de Cordoba (Argentina). Confirman primer caso autóctono de zika en Córdoba
[Internet]. [Cordoba]: Gobierno de la Provincia de Cordoba; 2016. Available from:

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
18
http://prensa.cba.gov.ar/salud/confirman-primer-caso-autoctono-de-zika-por-probable-contagio-por-via-
sexual/.
82. France detects first sexually transmitted case of Zika virus [Internet]. [Paris]: France 24; 2016 [updated
2016 Feb 28]. Available from: http://www.france24.com/en/20160227-france-zika-first-sexually-
transmitted-case.
83. Ministry of Health (New Zealand). Media release: Possible case of sexual transmission of Zika virus. 3 March
2016 [Internet]. Wellington: MoH (New Zealand); 2016. Available from: http://www.health.govt.nz/news-
media/media-releases/possible-case-sexual-transmission-zika-virus.
84. Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-
vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011 May;17(5):880-2.
85. Petersen EE, Polen KND, Meaney-Delman D, Ellington SR, Oduyebo T, Cohn A, et al. Update: Interim
guidelines for health care providers caring for pregnant women and women of reproductive age with
possible Zika virus exposure — United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):315-22.
86. Olson CK, Iwamoto M, Perkins KM, Polen KND, Hageman J, Meaney-Delman D, et al. Preventing
transmission of Zika virus in labor and delivery settings through implementation of standard precautions —
United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(11):290-2.
87. Janssen MP. The risk of sexually transmitted Zika infection among Dutch blood donors [Internet]. Sanquin;
2016. Available from: http://www.sanquin.nl/repository/documenten/nl/413511/Mart_P._Janssen_-
_The_risk_of_sexually_acquired_Zika_infection_among_Dutch_blood_donors.pdf.
88. Australian Red Cross Blood Services. Rapid Risk Assessment:Zika virus risk assessment of donors who have
sexual contact with someone who has recently returned from an area with active on-going transmission of
Zika virus 2016.
89. Lessler J, Ott C, Carcelen A, Konikoff J, Williamson J, Bi Q, et al. Times to key events in the course of Zika
infection and their implications: a systematic review and pooled analysis. Bull World Health Organ
[Internet]. Epub 2016 Apr 1. Available from: http://www.who.int/bulletin/online_first/BLT.16.174540.pdf.
90. Gallian P, de Lamballerie X, Salez N, Piorkowski G, Richard P, Paturel L, et al. Prospective detection of
chikungunya virus in blood donors, Caribbean 2014. Blood. 2014 Jun 5;123(23):3679-81.
91. U.S. Food and Drug Administration. News release: FDA allows use of investigational test to screen blood
donations for Zika virus [Internet]. Silver Spring, MD: FDA; 2016. Available from:
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm493081.htm.
92. Special edition: Chikungunya and Zika virus, October 2014. Euro Surveill [Internet]. 2014. Available from:
http://www.eurosurveillance.org/images/dynamic/ET/V19N02/V19N02.pdf.
93. European Centre for Disease Prevention and Control. Dengue outbreak in Madeira, Portugal, October–
November 2012 [Internet]. Stockholm: ECDC; 2013. Available from:
http://ecdc.europa.eu/en/publications/publications/dengue-outbreak-madeira-mission-report-nov-2012.pdf.
94. European Centre for Disease Prevention and Control. Guidelines for the surveillance of invasive mosquitos in
Europe [Internet]. Stockholm: ECDC; 2012. Available from:
http://ecdc.europa.eu/en/publications/Publications/TER-Mosquito-surveillance-guidelines.pdf.
95. European Centre for Disease Prevention and Control. Guidelines for the surveillance of native mosquitoes in
Europe [Internet]. Stockholm: ECDC; 2014. Available from:
http://ecdc.europa.eu/en/publications/Publications/surveillance-of%20native-mosquitoes%20-
guidelines.pdf.
96. European Centre for Disease Prevention and Control. The climatic suitability for dengue transmission in
continental Europe [Internet]. Stockholm: ECDC; 2012. Available from:
http://ecdc.europa.eu/en/publications/publications/ter-climatic-suitablility-dengue.pdf.
97. European Centre for Disease Prevention and Control. Guidelines for the surveillance of invasive mosquitoes
in Europe [Internet]. Stockholm: ECDC; 2016. Available from:
http://ecdc.europa.eu/en/publications/Publications/TER-Mosquito-surveillance-guidelines.pdf.
98. De Laval F, Matheus S, Maquart M, Yvrard E, Guidez A, Barthes N, et al. Imported Zika virus outbreak in
French Guiana: new insights on epidemiology and diagnosis. In: International Zika Summit 2016; 25-26
April 2016 [Internet]. Paris: Institute Pasteur. Available from:
http://www.zikasummit2016.org/images/Public/Zika-Abstracts.pdf.
99. Rozé B, Najioullah F, Fergé J, Apetse K, Brouste Y, Cesaire R, et al. Zika virus detection in urine from
patients with Guillain-Barré syndrome on Martinique, January 2016. Euro Surveill [Internet]. 2016;
21(9):[pii=30154 p.]. Available from: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21400.
100. Barzon L, Pacenti M, Berto A, Sinigaglia A, Franchin E, Lavezzo E, et al. Isolation of infectious Zika virus
from saliva and prolonged viral RNA shedding in a traveller returning from the Dominican Republic to Italy,
January 2016. Euro Surveill [Internet]. 2016 10 March; 21(10):[pii=30159 p.]. Available from:
http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21409.

RAPID RISK ASSESSMENT Zika virus disease epidemic, sixth update – 20 May 2016
19
101. Bingham AM, Cone M, Mock V, Heberlein-Larson L, Stanek D, Blackmore C, et al. Comparison of test results
for Zika virus RNA in urine, serum, and saliva specimens from person with travel-associated Zika virus
disease - Florida, 2016. MMWR Morb Mortal Wkly Rep. Forthcoming.
102. Zhang F-C, Li X-F, Deng Y-Q, Tong Y-G, Qin C-F. Excretion of infectious Zika virus in urine. Lancet Infect
Dis. Epub 2016.
103. Dupont-Rouzeyrol M, Biron A, O'Connor O, Huguon E, Descloux E. Infectious Zika viral particles in
breastmilk. Lancet. 2016;387(10023):1051.
104. Colt S, Garcia-Casal MN, Pena-Rosas JP, Finkelstein JL, Rayco-Solon P, Weise Prinzo Z, et al. Transmission
of Zika virus through breast milk and other breastfeeding-related bodily-fluids: a systematic review. Bull
World Healt Organ [Internet]. Forthcoming. Available from: http://www.who.int/bulletin/online_first/16-
176677.pdf.
105. European Centre for Disease Prevention and Control. Interim guidance for healthcare providers and Zika
virus laboratory diagnosis [Internet]. Stockholm: ECDC; 2016. Available from:
http://ecdc.europa.eu/en/publications/Publications/zika-virus-guidance-healthcare-providers-and-laboratory-
diagnosis.pdf
