
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
1
Narrative
review
Expansion of Zika virus circulation from Africa to the
Americas, 1947-2018: a literature review*
Correspondence:
Gilmara de Souza Sampaio – Universidade Federal da Bahia, Hospital Universitário Edgard Santos, Laboratório de Pesquisa em
Infectologia, Rua João das Botas, No. 157b, Canela, Salvador, BA, Brazil. Postcode: 40110-160
E-mail: gilmara.s[email protected]
Gilmara de Souza Sampaio
1
– orcid.org/0000-0001-7299-3740
Carlos Brites
1
– orcid.org/0000-0002-4673-6991
Jan Felix Drexler
2
– orcid.org/0000-0002-3509-0232
Andres Moreira-Soto
2
– orcid.org/0000-0002-9224-5157
Fernanda Miranda
1
– orcid.org/0000-0002-7654-6443
Eduardo Martins Netto
1
– orcid.org/0000-0003-1691-6761
1
Universidade Federal da Bahia, Hospital Universitário Professor Edgard Santos, Salvador, BA, Brasil
2
Charité – Universitätsmedizin Berlin, Institut für Virologie, Berlim, Alemanha
Abstract
Objective: to describe the temporal and geographical expansion of Zika virus (ZIKV) circulation in countries and territories,
from the time it was first isolated until 2018. Methods: This was a non-systematic literature review covering the period from
1947 to 2018 using the MEDLINE database and World Health Organization estimates. Results: Since its isolation in 1947,
ZIKV circulation spread through Africa, Asia and the Pacific before reaching the Americas in 2013, causing serious clinical
manifestations; the highest seroprevalence rates were recorded in Yap (74%) and in Brazil (63%); genetic mutations, absence
of immunity and high vector susceptibility may have influenced ZIKV transmissibility and help to explain the magnitude of its
expansion. Conclusion: The spread of ZIKV circulation in the Americas was the most extensive recorded thus far, possibly
as a result of population and geographical characteristics of the sites where the virus circulated.
Keywords: Zika Virus, Flavivirus; Epidemiology; Epidemics; Congenital Abnormalities; Review Literature as Topic.
doi: 10.5123/S1679-49742019000200022
*Article derived from the Ph.D. dissertation entitled ‘The spread, prevalence and dynamics of Zika virus transmission, 1947-2018’,
defended by Gilmara de Souza Sampaio at the Health Sciences Postgraduate Program, Faculty of Medicine, Federal University of
Bahia, on March 21
st
2019.

2
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Expansion of Zika virus circulation: from Africa to the Americas
Introduction
Following its discovery in 1947, Zika virus (ZIKV) was
responsible for sporadic cases of mild infections that
were not cause for great concern and were limited to the
African and Asian continents.
1
This scenario changed with
effect from 2007, when the virus began to be considered
a pathogen capable of causing large epidemics after
having produced two large-scale outbreaks in the Pacific
Islands
2-4
and in French Polynesia.
4-6
ZIKV circulation
continued to expand (Figure 1A). Within a short time,
the virus was considered to be a Public Health concern
associated with a large number of microcephaly cases
that occurred in Brazil.
7,8
Concern as to the severity of the consequences of
infection led the Ministry of Health to declare a state
of Public Health Emergency of National Concern on
November 11
th
2015.
9
Following this declaration, the
Pan American Health Organization (PAHO) issued an
alert about the increased number of microcephaly cases
in Brazil’s Northeast region.
10
Just days afterwards, on
November 28
th
2015, despite there being little evidence,
the Ministry of Health confirmed the link between
ZIKV and the microcephaly outbreak.
11
On December
1
st
2015, the World Health Organization (WHO) and
PAHO issued an alert suggesting possible association
between ZIKV and the increase in cases of congenital
syndrome and Guillain-Barré syndrome.
12
On February
1
st
2016, WHO declared a Public Health Emergency of
International Concern.
13
The agility with which health
authorities and researchers acted enabled rapid proof
of the causal relationship between ZIKV infection and
the occurrence of microcephaly and other central
nervous system alterations. This position was endorsed
by WHO at a meeting of the Emergency Committee held
in June 2016
14
(Figure 1B).
The potential of the virus to give rise to a broad
spectrum of clinical manifestations was confirmed,
ranging from non-specific symptoms, easily
confused with other virus diseases, to neurological
manifestations and congenital malformations.
7,8,15-19
By the end of 2016, 2,366 Zika virus-associated
congenital abnormalities had been confirmed in
Brazil.
20
The emergence of ZIKV in the Americas and
the potential for its circulation to expand require better
comprehension of its epidemiological profile, in order
to facilitate understanding of the changes in infection
detected over time.
The objective of this literature review was to
describe the temporal and geographic expansion of
KIKV circulation in countries and territories, from the
time it was isolated up until 2018.
Methods
This is a narrative review of ZIKV circulation
expansion over the years in countries and territories
on diverse continents. We searched the MEDLINE
database (via PubMed), using the following keywords
retrieved from the Health Sciences Descriptors (DeCS)
virtual library: ‘zika’, ‘zika virus’, ‘flavivirus’ and
‘arbovirus’. Eligible articles were those that provided
microbiological evidence of ZIKV infection in humans
and non-humans published between 1947 and 2018, in
any region of the world, and which contained complete
information on how the study was conducted: period,
place, sample, type of test used for diagnosis and test
result. We excluded articles that did not present positive
laboratory test results for the virus in humans or where
virus isolation in non-humans was not conducted. No
restriction was made as to language or whether the
publication was free of charge or not.
We also used estimates, available on the official
website of the PAHO-WHO, of confirmed Zika cases or
confirmed cases of Zika infection-associated congenital
syndrome, occurring in countries or territories in
the Americas up until January 2018.
21
In order to
demonstrate the magnitude of infection in different
places, ZIKA infection seroprevalence in the samples
examined was calculated as follows: firstly we totaled the
positive test results and then divided the total number
of tests by the total number of samples in each study.
Global expansion of ZIKV circulation
1940s and 1950s
ZIKV is believed to have emerged in around
1920,
1
although it was only isolated in 1947 in a
study on yellow feber in Rhesus monkeys conducted
by researchers from the Virus Research Institute.
22
The term ‘Zika’ was borrowed from the name of the
By the end of 2016, 2,366 Zika virus-
associated congenital abnormalities
had been confirmed in Brazil.

3
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Gilmara de Souza Sampaio et al.
Emergence of the virus
around 1920
Virus isolated
in Aedes
First records of infection
on the Asian continent,
in Malaysia
Emergence of the virus
in the Pacific, on the
Micronesian island of Yap
Emergence of the virus on the
American continent
Records of Guillain-Barré
syndrome and microcephaly
cases
Virus isolated in rhesus
monkeys un Uganda
First cases of human
infection recorded
in Africa
First cases of infection by
Aedes aegypti recorded
in Malaysia
Virus emerges in French
Polynesia
Records of Guillain-Barré
syndrome cases
Ministry of Health declares
Public Health Emergency of
National Concern
Ministry of Health confirms
relationship between Zika
virus and microcephaly
WHO declares Public
Health Emergency of
International Concern
WHO declares end of
Public Health Emergency
of International Concern
PAHO issues alert about
increased microcephaly cases in
Northeast Brazil
WHO issues alert about possible
association between Zika
Virus and increased number of
microcephaly and Guillain-Barré
syndrome cases
WHO confirms association
between Zika virus and
microcephaly
Ministry of Health declares end
of Public Health Emergency of
National Concern
Notes:
A) Timeline of Zika virus circulation expansion up until 2015.
B) Measures adopted by health authorities (Brazilian Ministry of Health, Pan American Health Organization [PAHO] and World Health Organization [WHO]) in the face of the increased number of
microcephaly Guillain-Barré syndrome cases in Brazil.
Figure 1 – Timeline of Zika virus circulation expansion and measures adopted by health authorities
Zika forest, in Entebbe, Uganda, where the Institute
was based. Despite the study mentioned above being
focused on yellow feber and dengue, researchers found
evidence of Zika virus being a new pathogen.
22
In 1948, less than a year after its discovery, ZIKV was
isolated in the Aedes (Stegomyia) africanus vector,
22-24
even though at that time it was not known that it was
a ZIKV vector.
ZIKV spread following its original lineage, referred
to as African lineage Zika, and expanded over part
of the African continent.
1
In West Africa it was
introduced twice, at different times, and gave origin
to two African lineages.
14
The first human ZIKV infections were recorded in
1952, on the African continent, and were confirmed
by its presence in serum of ZIKA neutralizing
antibodies.
25,26
At that time there was no other way of
demonstrating ZIKV infection, since test results could
be biased because of a possible immune response
to yellow feber vaccination.
24
During the 1950s,

4
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Expansion of Zika virus circulation: from Africa to the Americas
studies identified the presence of these antibodies in
people living in North Africa,
27
West Africa,
25,28
East
Africa
22,26,29,30
and Central Africa,
31
as well as in South
Asia
32
and Southeast Asia
27,32-34
(Figure 2 and Figure
3A). The highest seroprevalence rates were recorded
in Malaysia and the Philippines: 50% and 36%,
respectively (Table 1).
Jaundice was found in the three human cases
identified in 1954, indicating that the virus could also
be viscerotropic.
28
Another characteristics described
was its affinity for nerve tissues, found in mice that
developed neurological diseases after being infected.
35
Aedes aegypti was found to be a ZIKV vector in 1956.
36
1960s to 1990s
The third ZIKV lineage, i.e. the Asian lineage,
originated from a strain isolated in Malaysia in around
1966.
37
It was here that the first Zika virus infection
attributed to the Aedes aegypti vector was recorded.
38
At that time, serological and virological evidence
indicated that it was circulating in almost all of Africa,
in its Northern,
39,40
Central,
40-43
Western
40,44-48
and
Eastern regions
39,49,50
(Figure 2 and Figure 3B). The
majority of the countries in these regions are located
in latitudes where the climate is tropical and this is
propitious for vector development. Joint circulation
of yellow feber virus and Zika virus was found in a
region of Ethiopia in 1968.
49
Studies indicate that ZIKV
antibodies can attenuate yellow feber virus infection
but do not interfere with its transmission.
51,52
In the
decades in question, the highest seroprevalence rates
were recorded in Burkina Faso (53%), Mali (52%)
and Benin (44%).
2000s
For more than half a century, ZIKV remained
confined to the African and Asian continents, before
emerging in the Pacific Islands in the late 2000s.
3
Between April and July 2007, the first large epidemic
caused by the virus occurred in Yap, an island in
Micronesia (Figure 2). Infection incidence was high
in this outbreak at around 74% (95% confidence
interval: 68-77).
3
Despite affecting the majority of
the population, only around 20% of total cases were
symptomatic and reported mainly having exanthema,
conjunctivitis and pain in their joints.
2,3
The serum
samples of the inhabitants of Yap were tested by means
of the enzyme-linked immunosorbent assay (ELISA),
the virus neutralization test to detect antibodies (NT)
and reverse transcription polymerase chain reaction
(RT-PCR). Phylogenetic analyses showed that a strain
of the Asian lineage of the virus was responsible for
this outbreak.
2
By the end of the 2000s, ZIKV had been isolated
in Ae. Aegypti, Ae. africanus, Ae. apicoargenteus,
Ae. luteocephalus, Ae. vitattus, Ae. Furcifer and Ae.
Hensilii species vectors,
36,38,48,51,53
whereby the latter
was the most frequent species in the Yap outbreak.
3
For a long time it was believed that the only form of
Zika virus transmission was by the bite of mosquitoes
of the Aedes genus.
2,23,29,38,53
In 2008, however, two
researchers were infected in Senegal and one of them
transmitted ZIKV to his wife, possibly through sexual
intercourse.
54
Other studies have corroborated this
hypothesis by indicating the possibility of this form
of transmission.
55,56
2010s
The 2010s were marked by important discoveries,
such as the possibility of mother-to-child Zika
virus transmission and clinical manifestations with
unprecedented association with Zika virus infection,
such as congenital abnormalities
17,57,58
and Guillain-
Barré syndrome.
5,15,16
At that time the virus caused a new epidemic that
affected a considerable part of the population of French
Polynesia with effect from 2013, with approximately
thirty thousand people infected
5,6,59
and 42 patients
with Guillain-Barré syndrome.
15,16
That epidemic was
the consequence of a new strain that appeared in the
same year and was genetically related to the strains
isolated in Yap in 2007 and in Cambodia in 2010.
4,6
It
is likely that its magnitude was the result of a population
with low levels of immunity, high vector density
59
and
genetic mutations in which important amino acids
were modified, increasing the capacity of Zika virus
transmission by the Aedes aegypti vector.
60
ZIKV continued to expand to other Pacific Ocean
Islands
4,5,59,61-64
and through Southeast Asia,
65-72
until
it was identified in the Americas in May 2015.
73-75
Between 2015 and 2016, seroprevalence in Brazil was
63% (Table 1).
The timeline of Zika virus circulation expansion is
shown in Figure 1A, while its geographic expansion is
shown in Figure 3, highlighted by period to demonstrate
its swiftness and extent.

5
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Gilmara de Souza Sampaio et al.
Continent/
region/year
Country or territory Type of test used
Continent/
region/year
Country or territory Type of test used
1940s 1970s
Africa Africa
1947 Uganda
22
IV 1972 Serra Leoa
132
HI
1948 Uganda
23
IV
1970-1972 Nigéria
53,128,133,134
NT, FC, HI
1950s 1971-1972 Angola
135
HI
Africa
1972 e 1975 Senegal
46
HI
1951 Nigéria
25
TPR 1976 Sudão
136
NT, HI
1952 Uganda
26
NT 1979 República Centro-Africana
137
HI
1952 Tanzânia
26
NT Asia
1953 Nigéria
28
NT 1970-1979 Indonésia
129,130
HI
1954 Chade
31
NT
1970-1979 Paquistão
131
FC
1954 Congo
31
NT 1980s
1954 Egito
27
NT
Africa
1955 Nigéria
25
TPR 1980 Nigéria
138
HI
1957 Moçambique
30
NT 1984 Uganda
139
HI
1958 Uganda
29
IV
1988 Senegal
47
ELISA
1960s Asia
Africa
1980-1983 Indonésia
129,130
HI
1960 Angola
41
HI
1980-1983 Paquistão
131
FC
1961–1962 República Centro-Africana
42
HI 1990s
1961–1964 Etiópia
49
HI
Africa
1962 Senegal
44
HI 1990-1991 Senegal
47
ELISA
1963–1964 República Centro-Africana
125
HI 1991-1992 Djibouti
40
ELISA
1963–1964 Burkina Faso
40
HI 1999 Costa do Marfim
48
ELISA
1963–1965 Costa do Marfim
126
HI Asia
1963–1965 Guiné-Bissau
45
HI
1996-1997 Malásia
141
NT
1964–1966 Togo
40
HI 2000s
1964-1966 Camarões
43
HI
Oceania
1964-1967 Mali
40
HI 2007 Ilha de Yap, Micronésia
3
ELISA, RT-PCR
1965 Níger
40
HI Africa
1967 Libéria
40
HI
2008 Senegal
54
NT, FC, HI
1967 Benin
40
HI 2010s
1967 Gabão
40
HI
Oceania
1966-1967 Uganda
39
HI 2013-2014 Ilha de Páscoa
62
RT-PCR
1966-1967 Quênia
50
HI 2013-2014 Ilhas Cook
63
ELISA, RT-PCR
1966-1967 Somália
39
HI 2013-2014 Nova Caledônia
64
RT-PCR
1966-1967 Marrocos
40
HI 2011-2014 Polinésia Francesa
4,5,59
ELISA
1967-1969 Uganda
39
HI 2015 Fiji
61
RT-PCR
1968 Quênia
127
HI 2015 Samoa
61
RT-PCR
1969 Nigéria
128
NT Africa
Asia 2010 Camarões
142
FC, HI
1969 Malásia
38
IV 2014 Zâmbia
143,144
ELISA
1969-1983 Indonésia
129,130
HI
2015 Cabo Verde
145
ELISA, RT-PCR
1969-1983 Paquistão
131
FC
Legend:
NT: virus neutralization test to detect antibodies.
ELISA: enzyme-linked immunosorbent assay.
CF: complement fixation test.
HI: hemagglutination inhibition test.
IMT: intracerebrally inoculated mice test.
VI: virus isolation.
PCR: polymerase chain reaction.
RT-PCR: reverse transcription polymerase chain reaction.
Figure 2 – Countries or territories recording Zika virus circulation between 1947 and 2018, listed by continent and
decade of occurrence
Continued on next page

6
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Expansion of Zika virus circulation: from Africa to the Americas
The seroprevalence tests used in the various
studies we consulted differ as to the method of
laboratory diagnosis used and, therefore, have
differing levels of sensitivity and specificity,
76,77
thus
compromising the comparability of the results of the
different methods applied.
RT-PCR was used in 31 studies and was the most
used test, followed by the hemagglutination inhibition
test (HI) in 24 studies, ELISA in 17 studies, NT in 13
studies and the complement fixation test (CF) in 4
studies. Some studies used more than one diagnosis
method. Cross-reactivity between ZIKV antibodies and
those of other flaviviruses may also have compromised
correct estimation of infection prevalence.
78,79
ZIKV in the Americas
The first genetic studies of the strain causing the
ZIKV epidemic on the American continent suggest that
it originated from a unique Asian genotype lineage,
introduced in Brazil between late 2013 and early 2014
and having come from French Polynesia.
6,80-83
Four
hypotheses have been raised regarding the introduction
Continent/
region/year
Country or territory Type of test used
Continent/
region/year
Country or territory Type of test used
2010s 2010s
Asia North America
2010-2015 Camboja
65
PCR 2016-2017 Belize
21
2010-2015 Indonésia
66,67
RT-PCR, PCR 2016-2017 Bonaire, Santo Eustáquio e Saba
21
2010-2015 Malásia
68
ELISA, PCR 2016-2017 Costa Rica
21
2010-2015 Filipinas
69
ELISA, RT-PCR 2016-2017 Cuba
21
2010-2015 Maldivas
70
RT-PCR 2016-2017 Granada
21
2012-2014 Tailândia
71,72
RT-PCR 2016-2017 Guadalupe
21,155
RT-PCR
North America 2016-2017 Ilhas Cayman
21
2015-2017 México
21,146
ELISA, RT-PCR 2016-2017 Ilhas Virgens (US)
21
2016-2017 Estados Unidos
21,147
NT, ELISA, RT-PCR 2016 Ilhas Virgens (UK)
21
Central American and Caribbean 2016-2017 Jamaica
21
2014-2016 Haiti
21,148
RT-PCR 2016-2017 Nicarágua
21,156
RT-PCR
2015-2016 Guiana
21
2016-2017 República Dominicana
21,157
ELISA, RT-PCR
2015-2016 Martinica
21,149
RT-PCR 2016-2017 San Martin
21
2015-2017 Barbados
21,150
RT-PCR 2016-2017 San Martin (parte holandesa)
21
2015-2017 Curaçao
21
2016 Santa Lúcia
21
2015-2017 El Salvador
21
2016 São Bartolomeu
21
2015-2017 Guatemala
21
2016-2017 São Cristóvão e Nevis
21
2015-2017 Panamá
21,151
ELISA, RT-PCR 2016 São Vicente e Granadinas
21
2015-2017 Guiana Francesa
21
2016-2017 Trindade e Tobago
21
2015-2017 Honduras
21
ELISA, RT-PCR 2016-2017 Ilhas Turcas e Caicos
21
2015-2017 Porto Rico
21,152
ELISA, RT-PCR South America
2015-2017 Suriname
21,153
RT-PCR 2015-2017 Brasil
21,73–75
RT-PCR
2016 Antígua e Barbuda
21
2015-2017 Colômbia
21,158
RT-PCR
2016 Dominica
21,154
RT-PCR 2015-2017 Venezuela
52,106
RT-PCR
2016 Guiné-Bissau
21
2016-2017 Argentina
21
2016 Montserrat
21
2016 Bolívia
21,159
NT, ELISA
2016-2017 Anguila
21
2016-2017 Equador
21,160
RT-PCR
2016-2017 Aruba
21
2015-2017 Paraguai
21
2016 Bahamas
21
2016-2017 Peru
21,161
RT-PCR
Legend:
NT: virus neutralization test to detect antibodies.
ELISA: enzyme-linked immunosorbent assay.
CF: complement fixation test.
HI: hemagglutination inhibition test.
IMT: intracerebrally inoculated mice test.
VI: virus isolation.
PCR: polymerase chain reaction.
RT-PCR: reverse transcription polymerase chain reaction.
Figure 2 – Countries or territories recording Zika virus circulation between 1947 and 2018, listed by continent and
decade of occurrence
7
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Gilmara de Souza Sampaio et al.
of the virus in Brazil. Initially it was thought that it
had happened in 2014, during the FIFA World Cup,
74
despite there being no Pacific countries among the
participating soccer teams. A second possibility was
that the virus was introduced during the Canoe Sprint
World Championship, held in August 2014 in Rio
de Janeiro, as there were teams from four Pacific
countries with registered Zika cases: French Polynesia,
New Caledonia, the Cook Islands and Easter Island.
84
However, the hypothesis of the virus having entered
Brazil via Rio de Janeiro does not help to explain why
such a high number of cases were concentrated in the
country’s Northeast region. The third hypothesis is that
the virus was introduced one year earlier, between July
and August 2013, during the Confederations Cup.
81
According to the fourth and final hypothesis, the virus
circulated in Oceania and Easter Island, spreading to
Central America and the Caribbean before arriving in
Brazil at the end of that year.
85
The only ancestor of
the cases studied was a strain from Haiti, indicating
that ZIKV could have been introduced in Brazil by
immigrants or Brazilian troops returning from that
country. However, recent studies indicate that the
virus followed a different route, namely from Brazil to
Central America, entering via Honduras, between July
and September 2014.
86
Phylogeographic analysis has
estimated that the virus arrived in Honduras from Brazil
and later spread to Guatemala, Nicaragua and southern
Mexico by early 2015. Another study corroborates
the hypothesis that the introduction of ZIKV in South
America occurred prior to its introduction in Central
America, possibly in the first half of 2013.
60
In Outober 2014, some municipalities of the
Brazilian states of Rio Grande do Norte, Paraíba and
Maranhão reported suspected cases of a viral disease
with presence of exanthema, mild feber, itching and
painful joints, these being symptoms not in keeping
with suspected cases of other exanthematous viral
infections, such as measles and dengue. Before long
a further six states reported exanthematous syndrome
cases between October 2014 and March 2015.
75
The intense and concomitant circulation of other
flaviviruses, together with similar clinical presentation,
low specificity of ELISA diagnostic tests for dengue
76
as
well as the fact of the presence of the pathogen in Brazil
still being unknown, led to ZIKV not being indicated
as the main suspect.
In March 2015, samples from the states of Rio
Grande do Norte and Bahia had positive ZIKV results
confirmed by RT-PCR.
73,74
It spread well beyond Brazil’s
borders. By late 2015, ten Central and Southern
American countries had recorded autochthonous
Dec. 2010
Dec. 2000
Dec. 60 a 90
Dec. 50
Uganda
No cases reported
N
1:275.207.309 1:275.207.309
1:275.207.309 1:275.207.309
A
C
B
D
Notes:
A) Countries or territories registering Zika virus circulation by the 1950s.
B) Countries or territories registering Zika virus circulation during the 1960s, 1970s, 1980s and 1990s.
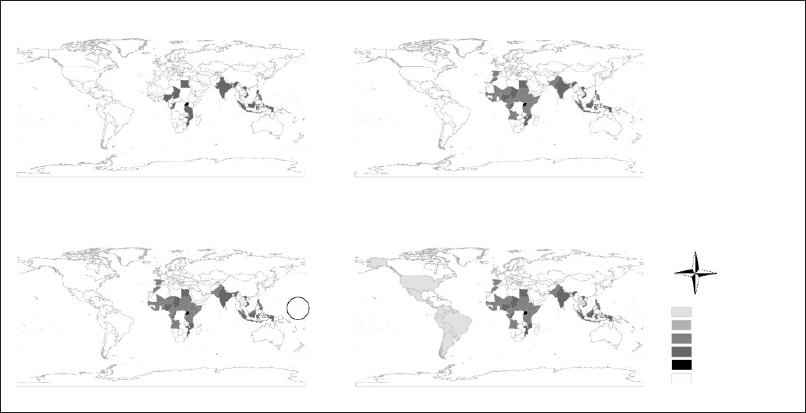
C) Countries or territories registering Zika virus circulation in the 2000s; the circle indicates the Pacific Islands.
D) All countries recording presence of Zika virus between 2010 and January 2018.
Figure 3 – Geographic and temporal expansion of Zika virus

8
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Expansion of Zika virus circulation: from Africa to the Americas
Table 1 – Zika virus seroprevalence in humans by affected country and period of infection, 1947-2016
Period Country/reference Type of test used No. of cases Total
Seroprevalence
(%)
1947-1984
a
Uganda
23,26,39,127,139
IMT, NT, HI 56 798 7
1947-1952 Tanzânia
26
IMT 6 36 17
1951-1975
b
Nigéria
25,28,40,53,128,132,138
NT,IMT,HI 1,090 3,018 36
1952 Índia
27
NT 33 196 17
1953 Filipinas
34
NT 19 53 36
1953, 1954 Malásia
27,33
NT 90 179 50
1954 Tailândia
33
NT 8 50 16
1954 Vietnã
33
NT 2 50 4
1954 Egito
27
NT 1 180 1
1957 Moçambique
30
NT 10 149 7
1960-1972
c
Angola
41,135
HI 202 5,082 4
1961-1979
d
República Centro-Africana
42,125
HI 186 1,177 16
1961-1964 Etiópia
49
HI 48 1,316 4
1962-1990
e
Senegal
44,46,47
HI, ELISA 203 1,292 16
1963,1964 Burkina Faso
40
HI 1,005 1,896 53
1963-1999
f
Costa do Marfim
48,126
HI, ELISA 393 906 43
1964,1965 Guiné-Bissau
45
HI 122 1,054 12
1964-1966 Togo
40
HI 401 1,294 31
1964-2010
g
Camarões
43,142
HI, CF 626 3,714 17
1964-1967 Mali
40
HI 1,232 2,369 52
1965 Níger
40
HI 55 308 18
1966 Somália
39
HI 3 242 1
1966-1968 Quênia
50,127
HI 509 3,134 16
1967 Benin
40
HI 108 244 44
1967 Gabão
40
HI 50 717 7
1972 Serra Leoa
132
HI 62 899 7
1983 Paquistão
131
CF 1 43 2
1983 Indonésia
129,130
HI 9 71 13
2007 Ilha de Yap, Micronésia
3
ELISA 414 557 74
2011-2013
h
Polinésia Francesa
59
ELISA 319 1,069 30
2014 Zâmbia
144
ELISA 217 3,625 6
2015-2016 Brasil
123
ELISA, PRNT 401 633 63
a) 1947-1952, 1966, 1967, 1984.
b) 1951-1952, 1955, 1965, 1966, 1967, 1969-1971, 1971-1975, 1972.
c) 1960, 1971, 1972.
d) 1961, 1962, 1963, 1964, 1979.
e) 1962, 1988, 1990.
f) 1963-1965, 1999.
g) 1964-1966, 2010.
h) 2011-2013, 2014.
Legend:
NT: virus neutralization test to detect antibodies.
ELISA: enzyme-linked immunosorbent assay.
CF: complement fixation test.
HI: hemagglutination inhibition test.
IMT: intracerebrally inoculated mice test.
PRNT: plaque reduction neutralization test.
9
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Gilmara de Souza Sampaio et al.
cases,
87
and by the beginning of the next year this
number had increased rapidly to 48 countries
88,89
(Figure 2 and Figure 3D). Only Canada and Bermuda,
both located in North America, and Chile and Uruguay,
in South America, did not have autochthonous cases.
Figure 4 shows the geographic and temporal spread
of ZIKV in the Americas, from the first recorded case
on the continent up until 2017.
Of all these countries, Brazil had the highest number
of ZIKV infections, totaling 137,288 confirmed cases
between 2015 and January 2018, followed by Puerto
Rico and Mexico, with 40,562 and 11,805 confirmed
cases, respectively.
21
In 2016 the total number of
cases reduced by more than 95% compared to the
previous year and on November 18
th
2016 WHO
stopped considering the virus as a Public Health
Emergency of International Concern.
90
In May 2017,
the Brazilian Health Ministry also declared the end of
the emergency.
91
In the first semester of 2015, a change was noted
in the pattern of the occurrence of Guillain-Barré
syndrome in two states of Brazil’s Northeast region,
namely Pernambuco and Bahia: in the former state
the number of cases tripled compared to the previous
year, with a peak in April, while Bahia recorded
a peak in occurrence of the syndrome between
June and July.
92,93
In the same period, four patients
undergoing solid organ transplantation were infected
with ZIKV, diagnosed by RT-PCR between June 2015
and January 2016.
94
In October of the same year, a
change was detected in the epidemiological pattern
of microcephaly occurrence, when Pernambuco state
health authorities informed the Ministry of Health about
a significant increase in the number of cases.
95
In late
November 2015, the Evandro Chagas Institute in the
state of Pará, a body linked to the Health Ministry’s
Health Surveillance Secretariat (IEC/SVS/MS), sent the
result of tests performed on a baby with microcephaly
to the Ministry: presence of ZIKV in blood and tissues.
This resulted in the Ministry of Health confirming the
relationship between ZIKV and microcephaly.
11
By the end of 2016, 22 countries had registered
cases of congenital syndrome associated with ZIKV
infection: a total of 2,525 reported cases, 2,289 (90%)
of which related to Brazil.
96
By December 2017, the 27
Brazilian states together recorded 3,071 microcephaly
cases, 2,004 (65%) of which occurred in the Northeast
region.
97
This number reduced significantly to 123
new cases between January and May 2018. In all,
there was a total of 3,194 records possibly associated
with ZIKV infection since these began to be counted
in 2015.
98
Zika virus, the cause of this pandemic,
had become a potential Public Health threat owing to
its association with neurological complications and
congenital malformations, which have been widely
documented.
7,8,12,18,19
The epidemics caused by the virus brought to light
a broad range of clinical manifestations; although it is
not possible to know the magnitude of the complications
related to ZIKV infection, which may possibly be greater
than those found so far. Little is known about issues such
as the severity of clinical presentation of ZIKV infection
or its viral load, nor about its influence on the clinical
spectrum found in different places and populations.
99
A descriptive study
100
assessed 1,950 confirmed
microcephaly cases in Brazil, 1,373 of which were
in the country’s Northeast region, using secondary
data obtained from the Health Ministry’s Notifiable
Diseases Information System (SINAN) relating to the
period between January 1
st
2015 and November 12
th
2016. Its results revealed two waves of ZIKV infection:
during the first wave, in 2015, there was a monthly
peak of microcephaly occurrence estimated at 50
cases per 10,000 live births, most of them (70% of the
total) living in the Northeast region; during the second
wave, occurrence was much lower, with monthly peaks
estimated as varying between 3 and 15 cases per 10,000
live births. The number of microcephaly cases related
to ZIKV infection following both outbreaks, or waves,
showed temporal variation according to the region of
the country; the reasons for these differences have,
however, yet to be enlightened.
A possible explanation for the result of these
estimates is the fact that, during the second wave
of infection, the relationship between ZKV infection
and the microcephaly cases in question was already
suspected and even supported by evidence, leading
the government to broadcast campaigns with the aim
of keeping the population informed. Pregnant women
began to take prevention measures in order to avoid
contact with the vector, such as using insect repellent,
bug screens and even delaying planned pregnancy.
100
Little is known about genetic variations between
ZIKV lineages and their ability to interfere with ZIKV
pathogenicity.
101
One experimental study that used
trophoblasts derived from human embryonic stem

10
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Expansion of Zika virus circulation: from Africa to the Americas
1:180.934.416 1:180.934.416 1:180.934.416
2014-2015
2016
2017
No cases reported
A B C
N
Notes:
A) Countries or territories registering Zika virus circulation between 2014 and 2015.
B) Countries or territories registering Zika virus circulation in 2016.
C) Countries or territories registering Zika virus circulation in 2017.
Figure 4 – Geographic and temporal expansion of Zika virus in the Americas, 2015- 2017
cells, found differences between the African and Asian
lineages with regard to their behavior in the placenta.
The occurrence of cell lysis – the process through
which a cell is destroyed or dissolved by plasma
membrane rupture – was only found in infections by
the African ZIKV lineage; however, no differences were
found in virus replication rates in relation to infection
caused by the two strains. This characteristic supports
the deduction that infection by an African strain at
the beginning of pregnancy would probably result in
miscarriage rather than congenital malformations.
102
It is possible that ZIKV’s pathogenic potential
depends on individual genetic variations, as indicated
by a comparative experimental study of three pairs of
dizygotic twins, when only one of each pair of twins
was diagnosed as having ZIKV congenital syndrome.
The study found that after infecting neural tissues, the
virus caused delayed development of the cells of the
twins who had the syndrome as well as increasing virus
replication. Transcriptome analysis results showed
a significant difference in DDIT4L mTOR protein
inhibitor levels between twins that had the syndrome
and twins that did not. The results found suggest the
existence of a relationship between individual genetic
disposition and increase in mTOR signaling and,
given that mTOR signaling pathways are critical for
autophagy mediated virus purification, ZIKV infections
are intensified in these people.
103
Discussion
The factors that led to large-scale and rapid
emergence, spread and apparent increase in Zika
virus pathogenicity in the Pacific and the Americas
are not yet totally understood. It is possible that there
are various mechanisms in operation, including virus
mutations that might increase transmission from
humans to mosquitoes, modulating the host immune
response.
104-107
Another factor lacking explanation is
the absence of large epidemics in Africa and Asia. A
hypothesis given for this fact is that it possibly reflects
higher levels of immunity provided by cross-protection
against other ZIKV-related flaviviruses ZIKV,
104
or that
epidemics that in fact existed may have been associated
with dengue due to the clinical similarity between
the two viruses dengue and due to their antibody
cross-reactivity.
37,108
Another study corroborated the
hypothesis that large outbreaks have not happened
in Africa because Aedes aegypti of African origin
may be less susceptible to virus strains than Aedes
aegypti that is not of African origin. Notwithstanding,
this characteristic has not helped to explain why there
have not been large outbreaks in Asia, different to the
Americas, given that the populations of both regions
had similar levels of susceptibility.
109
Intensity of ZIKV dissemination, viremia and clinical
symptoms in the Americas, including microcephaly,
11
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Gilmara de Souza Sampaio et al.
could have intensified owing to dengue virus immunity
existing in endemic regions.
110
However, a pediatric
cohort study conducted in Nicaragua between January
and February 2017 monitored 3,700 children aged
2 to 14 years and concluded that the opposite may
occur, i.e. ZIKV infection symptoms may be reduced
owing to prior dengue virus immunization.
111
This
hypothesis is corroborated by research involving an
experiment with mice that demonstrated the effect
of dengue infection on CD8+ T-cells in guaranteeing
cross-protection against ZIKV during pregnancy.
112
Another factor capable of intensifying dissemination
was a slight genetic alteration in ZIKV polyprotein
which happened before the 2013 outbreak and was
sufficient to permanently increase its infectivity in
human nerve cells.
105
This fact would help to explain
why microcephaly cases have been so numerous in the
Americas in comparison to other continents.
Other aspects to be considered are related to
Aedes aegypti and Aedes albopictus susceptibility, as
well as that of ZIKV strains, due to genetic differences
that influence vector response levels to infection and
consequent ZIKV transmission capacity.
113-120
The
American strain is more easily transmitted than the
Asian strain from which it is derived, this being an
hypothesis confirmed by analysis of vector saliva: only
the virus in the sample containing the American strain
remained viable after three days of infection, in addition
to having a higher infection rate in Aedes aegypti.
121
The highest number of Zika and microcephaly
cases was recorded in Northeast Brazil. Some reasons
indicated for this were presented in an ecological
analysis study: joint circulation of the virus that causes
Chikungunya feber, which exists in this region, could
increase the risk of other communicable diseases
being transmitted.
122
Another study found higher ZIKA
prevalence infection in poorer social classes.
123
The
fact that the first notifications of infections occurred in
Northeast Brazil, along with the massive media alerts
that followed, helped measures to be taken that may
possibly have contained greater spread of the virus in
other regions. The same reasoning could be applied to
Brazil, where the first infections were recorded, and to
the other Latin American countries where cases were
not numerous.
The Brazilian epidemic showed a sharp decline in
recorded infections with effect from 2017. A possible
cause of this may have been high seroprevalence in
the population, leading to immunity against ZIKV.
123,124
A study conducted in Bahia, a Northeastern Brazilian
state, assessed 633 individuals based on their serum
samples collected between 2015 and 2016. The
results of the analyses showed a rapid increase in ZIKV
infection seroprevalence in the population, reaching
63% in 2016.
123
It is possible that population and
geographic characteristics may have directly influenced
the pace of propagation, and the possible reasons
for this would be the variations in vector density,
immunity and changes in routine habits, as well as
high population mobility.
100,120,123
The outbreak in the Americas has ended.
Notwithstanding, a study supported by a stochastic
spatial model has indicated the possibility of the
emergence of new epidemics approximately every ten
years, this being the time needed for a new generation
of the population to become susceptible once more.
124
In conclusion, the spread of ZIKV circulation
seen in the last decade was the swiftest and largest
recorded thus far. Possible factors contributing to this
include genetic changes that increased its transmission
potential, together with favorable population and
geographic characteristics in the regions where the virus
circulated. Large numbers of microcephaly cases were
expected to occur in most countries in the Americas,
99
but in the end this expectation was not confirmed.
Similarly, the reasons why Brazil recorded such a higher
number of cases in comparison to other Latin American
countries has not been totally explained. Nor have the
reasons why most of the infections recorded in Brazil
were concentrated in its Northeastern region. This gives
rise to several unanswered questions as to increased
virus circulation and pathogenicity. Another limitation
of this literature review relates to the different methods
adopted in the studies consulted, thus hindering precise
seroprevalence rates from being obtained. Cross-
reactivity, caused by other flavivirus antibodies, was
a limiting impediment to the accuracy of the results
presented.
76
Further research is needed to overcome gaps in
knowledge about ZIKV pathogenesis and to assess
local risks; seroprevalence studies to identify regions
vulnerable to infection so as to foresee the potential
of future epidemics. A benefit of this work is that the
efforts of health authorities would be better directed,
so as to contribute to the development of effective Zika
virus infection control measures.

12
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Expansion of Zika virus circulation: from Africa to the Americas
Vaccine development also needs to be encouraged,
in order to interrupt the transmission chain and avoid
future outbreaks and epidemics.
Acknowledgement
We thank the Brazilian Hospital Services Company
(EBSERH), for its support in the development of
this study.
Authors’ contributions
Netto EM, Sampaio GS, Brites C, Moreira-Soto A and
Drexler JF took part in the conception and planning of the
study and writing and critical revision of the manuscript
contents. Sampaio GS and Miranda FL collected and
analyzed the study data. All the authors approved the
version to be published and take on responsibility for all
aspects of the work, including its accuracy and integrity.
1. Faye O, Freire CCM, Iamarino A, Faye O, Oliveira JVC,
Diallo M, et al. Molecular evolution of Zika Virus
during its emergence in the 20 th century. PLOS Negl
Trop Dis [Internet]. 2014 Jan [citado 2019 May 8];
8(1):e2636. Disponível em: https://journals.plos.org/
plosntds/article?id=10.1371/journal.pntd.0002636.
doi: 10.1371/journal.pntd.0002636
2. Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert
AJ, Johnson AJ, et al. Genetic and serologic properties
of Zika virus associated with an epidemic, Yap State,
Micronesia, 2007. Emerg Infect Dis [Internet]. 2008
Aug [citado 2019 May 8];14(8):1232-9. Disponível
em: https://wwwnc.cdc.gov/eid/article/14/8/08-0287_
article. doi: 10.3201/eid1408.080287
3. Duffy MR, Chen T-H, Hancock WT, Powers AM,
Kool JL, Lanciotti RS, et al. Zika virus outbreak on
Yap Island, Federated States of Micronesia. New
Engl J Med [Internet]. 2009 Jun [citado 2019 May
8];360(24):2536-43. Disponível em: https://www.
nejm.org/doi/full/10.1056/NEJMoa0805715. doi:
10.1056/NEJMoa0805715
4. Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread
of emerging Zika virus in the Pacific area. Clin
Microbiol Infect [Internet]. 2014 Oct [citado 2019
May 8];20(10):O595-6. Disponível em: https://
www.clinicalmicrobiologyandinfection.com/article/
S1198-743X(14)65391-X/fulltext. doi: 10.1111/1469-
0691.12707
5. Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S,
Benyon E, et al. Concurrent outbreaks of dengue,
chikungunya and Zika virus infections – an
unprecedented epidemic wave of mosquito-borne viruses
in the Pacific 2012 – 2014. Euro Surveill [Internet].
2014 Oct [citado 2019 May 8];19(41):pii:20929.
Disponível em: https://www.eurosurveillance.org/
content/10.2807/1560-7917.ES2014.19.41.20929
6. Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry
AL, Mallet HP, et al. Zika virus, French Polynesia,
South Pacific, 2013. Emerg Infect Dis [Internet]. 2014
Jun [citado 2019 May 8];20(6):1085-6. Disponível
em: https://wwwnc.cdc.gov/eid/article/20/6/14-0138_
article. doi: 10.3201/eid2006.140138
7. Martines RB, Bhatnagar J, Ramos AMO, Davi HOF,
Iglezias AD, Kanamura CT, et al. Pathology of
congenital Zika syndrome in Brazil: a case series.
Lancet [Internet]. 2016 Aug [citado 2019 May
8];388(10047):898-904. Disponível em: https://
www.thelancet.com/journals/lancet/article/PIIS0140-
6736(16)30883-2/fulltext. doi: 10.1016/S0140-
6736(16)30883-2
8. Costello A, Dua T, Duran P, Gülmezoglu M, Oladapo
OT, Perea W, et al. Defining the syndrome associated
with congenital Zika virus infection. Bull World
Health Organ [Internet]. 2016 Jun [citado 2019 May
8];94(6):406-406A. Disponível em: https://www.
ncbi.nlm.nih.gov/pmc/articles/PMC4890216/. doi:
10.2471/BLT.16.176990
9. Brasil. Ministério da Saúde. Portaria MS/GM no 1.813,
de 11 de novembro de 2015. Declara Emergência em
Saúde Pública de importância Nacional (ESPIN) por
alteração do padrão de ocorrência [Internet]. Diário
Oficial da União, Brasília (DF), 2015 nov 12; Seção
1:51. Disponível em: http://bvsms.saude.gov.br/bvs/
saudelegis/gm/2015/prt1813_11_11_2015.html
10. Pan American Health Organization. World Health
Organization. Epidemiological alert increase of
microcephaly in the northeast of Brazil [Internet].
2015 [citado 2019 May 8]. Disponível em: https://
www.paho.org/hq/dmdocuments/2015/2015-nov-17-
cha-microcephaly-epi-alert.pdf
11. Ministério da Saúde (BR). Ministério da Saúde
confirma relação entre vírus Zika e microcefalia
[Internet]. Brasília: Ministério da Saúde; 2015
[citado 2019 May 8]. Disponível em: http://www.blog.
saude.gov.br/index.php/combate-ao-aedes/50399-
ministerio-da-saude-confirma-relacao-entre-virus-
zika-e-microcefalia
References
13
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Gilmara de Souza Sampaio et al.
12. Pan American Health Organization. World Health
Organization. Neurological syndrome, congenital
malformations, and Zika virus infection. Implications
for public health in the Americas [Internet]. 2015
[citado 2019 May 8]. Disponível em: http://www.paho.
org/hq/index.php?option=com_docman&task=doc_vi
ew&Itemid=270&gid=32405&lang=en
13. World Health Organization. WHO Director-General
summarizes the outcome of the Emergency
Committee regarding clusters of microcephaly and
Guillain-Barré syndrome [Internet]. Geneva: World
Health Organization; 2016 [citado 2019 May 8].
Disponível em: http://www.who.int/mediacentre/
news/statements/2016/emergency-committee-zika-
microcephaly/en/
14. World Health Organization. WHO statement on the
first meeting of the International Health Regulations
(2005) (IHR (2005)) Emergency Committee on
Zika virus and observed increase in neurological
disorders and neonatal malformations [Internet].
Geneva: World Health Organization; 2016 [citado
2019 May 8]. Disponível em: http://www.who.int/
mediacentre/news/statements/2016/1st-emergency-
committee-zika/en/
15. Oehler E, Watrin L, Larre-Goffart P, Lastère S, Valour
F, Baudouin L, et al. Zika virus infection complicated
by Guillain-Barré syndrome – case report, French
Polynesia, December 2013. Euro Surveill [Internet].
2014 Mar[citado 2019 May 8];19(9):pii:20720.
Disponível em: https://www.eurosurveillance.org/
content/10.2807/1560-7917.ES2014.19.9.20720
16. Cao-lormeau VM, Blake A, Mons S, Lastere S, Roche
C, Vanhomwegen J, et al. Guillain-Barré Syndrome
outbreak caused by ZIKA virus infection in French
Polynesia. Lancet [Internet]. 2016 Apr [citado 2019
May 8];387(10027):1531-9. Disponível em: https://
www.ncbi.nlm.nih.gov/pmc/articles/PMC5444521/.
doi: 10.1016/S0140-6736(16)00562-6
17. Araújo TVB, Rodrigues LC, Ximenes RAA, Miranda
Filho DB, Montarroyos UR, Melo APL, et al.
Association between Zika virus infection and
microcephaly in Brazil, January to May, 2016:
preliminary report of a case-control study. Lancet
Infect Dis [Internet]. 2016 Dec [citado 2019 May
8];16(12):1356-63. Disponível em: https://www.
thelancet.com/journals/laninf/article/PIIS1473-
3099(16)30318-8/fulltext. doi: 10.1016/S1473-
3099(16)30318-8
18. Krauer F, Riesen M, Reveiz L, Oladapo OT, Porgo
TV, Haefliger A, et al. Zika Virus infection as a
cause of congenital brain abnormalities and
Guillain – Barre Syndrome: systematic review.
PLoS Med [Internet]. 2017 Jan [citado 2019 May
8];14(1):e1002203. Disponível em: https://journals.
plos.org/plosmedicine/article?id=10.1371/journal.
pmed.1002203. doi: 10.1371/journal.pmed.1002203
19. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR.
Zika Virus and Birth defects – reviewing the evidence
for causality. N Engl J Med [Internet]. 2016 May
[citado 2019 May 8];374(20):1981-7. Disponível
em: https://www.nejm.org/doi/full/10.1056/
NEJMsr1604338. doi: 10.1056/NEJMsr1604338
20. Ministério da Saúde (BR). Secretaria de Vigilância
em Saúde. Informe epidemiológico no 57 – semana
epidemiológica (SE) 52/2016 (25 a 31/12/2016)
- monitoramento dos casos de microcefalia no
Brasil [Internet]. Brasília: Ministério da Saúde;
2017 [citado 2019 maio 8]. Disponível em: http://
combateaedes.saude.gov.br/images/pdf/Informe-
Epidemiologico-n57-SE-52_2016-09jan2017.pdf
21. Pan American Health Organization. World Health
Organization. Zika suspected and confirmed
cases reported by countries and territories in the
Americas Cumulative cases, 2015-2017. Updated
as of 04 January 2018 [Internet]. Washington,
D.C.: Pan American Health Organization; 2017
[citado 2019 May 8]. Disponível em: https://
www.paho.org/hq/index.php?option=com_
docman&view=download&alias=43296-
zika-cumulative-cases-4-january-2018-
296&category_slug=cumulative-cases-pdf-
8865&Itemid=270&lang=en
22. Dick GW, Kitchen SF, Haddow AJ. Zika virus (I)
isolations and serological specificity. Trans R Soc
Trop Med Hyg [Internet]. 1952 Sep [citado 2019 May
8];46(5):509-20. Disponível em: https://www.ncbi.
nlm.nih.gov/pubmed/12995440
23. Dick GW. Zika virus (II). Pathogenicity and physical
properties. Trans R Soc Trop Med Hyg [Internet].
1952 Sep [citado 2019 May 8];46(5):521-34.
Disponível em: https://www.sciencedirect.com/
science/article/pii/0035920352900436. doi:
10.1016/0035-9203(52)90043-6
24. Dick GW. Epidemiological notes on some viruses
isolated in Uganda (Yellow fever, Rift Valley
fever, Bwamba fever, West Nile, Mengo, Semliki
forest, Bunyamwera, Ntaya, Uganda S and Zika
viruses). Trans R Soc Trop Med Hyg [Internet].
1953 Jan [citado 2019 May 8];47(1)13-48.
Disponível em: https://academic.oup.com/trstmh/
article/47/1/13/1901421. doi: 10.1016/0035-
9203(53)90021-2

14
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Expansion of Zika virus circulation: from Africa to the Americas
25. Macnamara FN, Horn DW, Porterfield JS. Yellow fever
and other arthropod-borne viruses. A consideration
of two serological surveys made in South Western
Nigeria. Trans R Soc Trop Med Hyg [Internet]. 1959
Mar [citado 2019 May 8];53(2):202-12. Disponível
em: https://www.ncbi.nlm.nih.gov/pubmed/13647627
26. Smithburn KC. Neutralizing antibodies against certain
recently isolated viruses in the sera of human beings
residing in East Africa. J Immunol [Internet]. 1952
Aug [citado 2019 May 8];69(2):223-34. Disponível
em: http://www.jimmunol.org/content/69/2/223.long
27. Smithburn KC. Neutralizing antibodies against arthropod-
borne viruses in the sera of long-time residents of Malaya
and borneo. Am J Hyg. 1954 Mar;59(2):157-63.
28. MacNamara FN. Zika virus: a report on three cases of
human infection during an epidemic of jaundice in
Nigeria. Trans R Soc Trop Med Hyg [Internet]. 1954
Mar [citado 2019 May 8];48(2):139-45. Disponível
em: https://www.sciencedirect.com/science/article/
pii/0035920354900061. doi: 10.1016/0035-
9203(54)90006-1
29. Weinbren MP, Williaams MC. Zika virus: further
isolations in the zka area, and some studies on the
strains isolated. Trans R Soc Trop Med Hyg. 1958
May;52(3):263-8.
30. Kokernot RH, Smithburn KC, Gandara AF, Mcintosh
BM, Heymann CS. Neutralization tests with sera from
individuals residing in Mozambique against specific
viruses isolated in Africa, transmitted by arthropods.
An Inst Med Trop (Lisb). 1960 Jan-Jun;17:201-30.
31. Pellissier A. Serological investigation on the incidence
of neurotropic viruses in French Equatorial Africa.
Bull Soc Pathol Exot Fil. 1954;47(2):223-27.
32. Smithburn KC, Kerr, JA,Gatne PB. Neutralizing
antibodies against certain viruses in the sera of
residents of India. J Immunol. 1954 Apr;72(4):248-57.
33. Pond WL. Arthropod-borne virus antibodies in
sera from residents of South-East Asia. Trans R
Soc Trop Med Hyg [Internet]. 1963 Sep [citado
2019 May 8];57(5):364-71. Disponível em:
https://www.sciencedirect.com/science/article/
pii/0035920363901007. doi: 10.1016/0035-
9203(63)90100-7
34. Hammon WM, Schrack Jr WD, Sather GE. Serological
survey for arthropod-borne virus infections in the
Philippines. Am J Trop Med Hyg [Internet]. 1958
May [citado 2019 May 8];7(3):323-8. Disponível
em: http://www.ajtmh.org/content/journals/10.4269/
ajtmh.1958.7.323. doi: 10.4269/ajtmh.1958.7.323
35. Regan RL, Brueckner AL. Comparison by electron
microscopy of the Ntaya and Zika viruses. Tex Rep
Biol Med. 1953;11(2):347-51.
36. Boorman JPT, Porterfield JS. A simple technique for
infection of mosquitoes with viruses transmission
of Zika virus. Trans R Soc Trop Med Hyg [Internet].
1956 Mar [citado 2019 May 8];50(3):238-42.
Disponível em: https://academic.oup.com/trstmh/
article-abstract/50/3/238/1899150?redirectedFrom=f
ulltext. doi: 10.1016/0035-9203(56)90029-3
37. Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang
V, Guzman H, et al. Genetic characterization of Zika
Virus strains: geographic expansion of the Asian
lineage. PLoS Negl Trop Dis [Internet]. 2012 [citado
2019 May 8];6(2):e1477. Disponível em: https://
journals.plos.org/plosntds/article?id=10.1371/journal.
pntd.0001477. doi: 10.1371/journal.pntd.0001477
38. Marchette NJ, Garcia R, Rudnick A. Isolation of Zika
virus from Aedes aegypti mosquitoes in Malaysia. Am J
Trop Med Hyg [Internet]. 1969 May [citado 2019 May
8];18(3):411-5. Disponível em: http://www.ajtmh.
org/content/journals/10.4269/ajtmh.1969.18.411.
doi: 10.4269/ajtmh.1969.18.411
39. Henderson BE, Metselaar D, Cahill K, Timms GL, Tukei
PM, Williams MC. Yellow fever immunity surveys in
northern Uganda and Kenya and Eastern Somalia, 1966-
67. Bull World Heal Organ [Internet]. 1968 [citado
2019 May 8];38(2):229-37. Disponível em: https://
www.ncbi.nlm.nih.gov/pmc/articles/PMC2554316/
40. Brès P. Données récentes apportées par les enquêtes
sérologiques sur la prévalence des arbovirus en
Afrique, avec référence spéciale à la fièvre jaune. Bull
World Heal Organ [Internet]. 1970 [citado 2019 May
8];43(2):223-67. Availble from: https://www.ncbi.
nlm.nih.gov/pmc/articles/PMC2427644/
41. Kokernot RH, Casaca VM, Weinbren MP, McIntosh BM.
Survey for antibodies against arthropod-borne viruses in
the sera of indigenous residents of Angola. Trans R Soc
Trop Med Hyg [Internet]. 1965 Sep [citado 2019 May
8];59(5):563-70. Disponível em: https://academic.oup.
com/trstmh/article-abstract/59/5/563/1913859?redirecte
dFrom=fulltext. doi: 10.1016/0035-9203(65)90159-8
42. Chippaux-Hyppolite C. Immunologic investigation
on the frequency of arbovirus in man in the Central
African Republic. Preliminary note. Bull Soc Pathol
Exot Filiales. 1965 Sep-Oct;58(5):812-20.
43. Salaun JJ, Brottes H. Arboviruses in Cameroun: serologic
investigation. Bull World Health Organ [Internet]. 1967
[Internet];37(3):343-61. Disponível em: https://www.
ncbi.nlm.nih.gov/pmc/articles/PMC2554275/
15
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Gilmara de Souza Sampaio et al.
44. Bres P, Lacan A, Diop I, Michel R, Peretti P, Vidal C.
Arborviruses in Senegal: serological survey. Bull Soc
Pathol Exot Fil. 1963 May-Jun;56:384-402.
45. Pinto MR. Survey for antibodies to arboviruses in the
sera of children in Portuguese Guinea. Bull World
Health Organ [Internet]. 1967 [citado 2019 May
8];37(1):101-8. Disponível em: https://www.ncbi.
nlm.nih.gov/pmc/articles/PMC2554218/
46. Renaudet J, Jan C, Ridet J, Adam C, Robin Y. A
serological survey of arboviruses in the human
population of Senegal. Bull Soc Pathol Exot Filiales.
1978 Mar-Apr;71(2):131-40.
47. Monlun E, Zeller H, Le Guenno B, Traoré-Lamizana M,
Hervy JP, Adam F, et al. Surveillance of the circulation
of arbovirus of medical interest in the region of eastern
Senegal. Bull Soc Pathol Exot. 1993;86(1):21-8.
48. Akoua-Koffi C, Diarrassouba S, Bénié VB, Ngbichi JM,
Bozoua T, Bosson A, et al. Investigation autour d’un
cas mortel de fièvre jaune en Côte d’Ivoire en 1999.
Bull Soc Pathol Exot [Internet]. 2001 Aug [citado
2019 May 8];94(3):227-30. Disponível em: https://
core.ac.uk/download/pdf/39846303.pdf
49. Sérié C, Casals J, Panthier R, Brès P, Williams MC.
Studies on yellow fever in Ethiopia. 2. Serological
study of the human population. Bull World
Health Organ [Internet]. 1968 [citado 2019 May
8];38(6):843-54. Disponível em: https://www.ncbi.
nlm.nih.gov/pmc/articles/PMC2554526/
50. Geser A, Henderson BE, Christensen S. A
multipurpose serological survey in Kenya. Bull World
Health Organ [Internet]. 1970 [citado 2019 May
8];43(4):539-52. Disponível em: https://www.ncbi.
nlm.nih.gov/pmc/articles/PMC2427766/
51. McCrae AW, Kirya BG. Yellow fever and Zika virus
epizootics and enzootics in Uganda. Trans R Soc Trop Med
Hyg [Internet]. 1982 [citado 2019 May 8];76(4):552-62.
Disponível em: https://www.sciencedirect.com/science/
article/pii/0035920382901614. doi: 10.1016/0035-
9203(82)90161-4
52. Haddow AJ, Williams MC, Woodall JP, Simpson DI, Goma
LK. Twelve isolations of zika virus from aedes (stegomyia)
africanus (theobald) taken in and above a uganda forest.
Bull World Health Organ [Internet]. 1964 [citado 2019
May 8];31:57-69. Disponível em: https://www.ncbi.nlm.
nih.gov/pmc/articles/PMC2555143/
53. Fagbami AH. Zika virus infections in Nigeria:
virological and seroepidemiological investigations in
Oyo State. J Hyg (Lond) [Internet]. 1979 Oct [citado
2019 May 8];83(2):213-9. Disponível em: https://
www.ncbi.nlm.nih.gov/pmc/articles/PMC2129900/
54. Foy BD, Kobylinski KC, Foy JLC, Blitvich BJ, Travassos
da Rosa A, Haddow AD, et al. Probable non-vector-
borne transmission of Zika virus, Colorado, USA.
Emerg Infect Dis [Internet]. 2011 May [citado 2019
May 8];17(5):880-2. Disponível em: https://www.
ncbi.nlm.nih.gov/pmc/articles/PMC3321795/. doi:
10.3201/eid1705.101939
55. Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-
Lormeau VM. Potential sexual transmission of Zika
virus. Emerg Infect Dis [Internet]. 2015 Feb [citado
2019 May 8];21(2):359-61. Disponível em: https://
www.ncbi.nlm.nih.gov/pmc/articles/PMC4313657/.
doi: 10.3201/eid2102.141363
56. Frank C, Cadar D, Schlaphof A, Neddersen N,
Günther S, Tappe D, et al. Sexual transmission of
Zika virus in Germany, April 2016. Euro Surveill
[Internet]. 2016 Jun [citado 2019 May 8];21(23).
Disponível em: https://www.eurosurveillance.org/
content/10.2807/1560-7917.ES.2016.21.23.30252.
doi: 10.2807/1560-7917.ES.2016.21.23.30252
57. Driggers RW, Ho C-Y, Korhonen EM, Kuivanen S,
Jääskeläinen AJ, Smura T, et al. Zika virus infection
with prolonged maternal viremia and fetal brain
abnormalities. N Engl J Med [Internet]. 2016
Jun [citado 2019 May 8];374(22):2142-51.
Disponível em: https://www.nejm.org/doi/10.1056/
NEJMoa1601824?url_ver=Z39.88-2003&rfr_
id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.
ncbi.nlm.nih.gov. doi: 10.1056/NEJMoa1601824
58. Brasil P, Pereira JP, Moreira ME, Nogueira RMR,
Damasceno L, Wakimoto M, et al. Zika virus
infection in pregnant women in Rio de Janeiro. N
Engl J Med [Internet]. 2016 Dec [citado 2019 May
8];375(24):2321-4. Disponível em: https://www.
nejm.org/doi/full/10.1056/NEJMoa1602412. doi:
10.1056/NEJMoa1602412
59. Aubry M, Finke J, Teissier A, Roche C, Broult J,
Paulous S, et al. Seroprevalence of arboviruses among
blood donors in French Polynesia, 2011–2013. Int
J Infect Dis [Internet]. 2015 Dec [citado 2019 May
8];41:11-2. Disponível em: https://www.ijidonline.
com/article/S1201-9712(15)00239-8/fulltext. doi:
10.1016/j.ijid.2015.10.005
60. Pettersson JHO, Bohlin J, Dupont-Rouzeyrol M,
Brynildsrud OB, Alfsnes K, Cao-Lormeau VM, et al.
Re-visiting the evolution, dispersal and epidemiology
of Zika virus in Asia article. Emerg Microbes Infect
[Internet]. 2018 May [citado 2019 May 8];7(1):79.
Disponível em: https://www.tandfonline.com/doi/
full/10.1038/s41426-018-0082-5. doi: 10.1038/
s41426-018-0082-5

16
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Expansion of Zika virus circulation: from Africa to the Americas
61. Sun J, Fu T, Mao H, Wang Z, Pan J, Rutherford S,
et al. A cluster of Zika virus infection in a Chinese
tour group returning from Fiji and Samoa. Sci Rep
[Internet]. 2017 Mar [citado 2019 May 8];7:43527.
Disponível em: https://www.ncbi.nlm.nih.gov/pmc/
articles/PMC5361211/. doi: 10.1038/srep43527
62. Tognarelli J, Ulloa S, Villagra E, Lagos J, Aguayo C, Fasce
R, et al. A report on the outbreak of Zika virus on Easter
Island, South Pacific, 2014. Arch Virol [Internet]. 2016
Mar [citado 2019 May 8];161(3):665-8. Disponível
em: https://link.springer.com/article/10.1007%2
Fs00705-015-2695-5. doi: 10.1007/s00705-015-2695-5
63. Pyke AT, Daly MT, Cameron JN, Moore PR, Taylor CT,
Humphreys JL, et al. Imported Zika virus infection
from the cook islands into Australia , 2014. PLoS Curr
[Internet]. 2014 Jun [citado 2019 May 8]; 2014;6:pii.
Disponível em: http://currents.plos.org/outbreaks/
index.html%3Fp=38348.html
64. Dupont-Rouzeyrol M, O'Connor O, Calvez E, Daures
M, John M, Grangeon J, et al. Co-infection with Zika
and dengue viruses in 2 patients, New Caledonia,
2014. Emerg Infect Dis [Internet]. 2015 Feb [citado
2019 May 8];21(2):381-2. Disponível em: https://
wwwnc.cdc.gov/eid/article/21/2/14-1553_article. doi:
10.3201/eid2102.141553
65. Heang V, Yasuda CY, Sovann L, Haddow AD, Travassos
da Rosa AP, Tesh RB, et al. Zika virus infection,
Cambodia, 2010. Emerg Infect Dis [Internet]. 2012
Feb [citado 2019 May 8];18(2):349-51. Disponível
em: https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC3310457/. doi: 10.3201/eid1802.111224
66. Kwong JC, Druce JD, Leder K. Case report : Zika virus
infection acquired during brief travel to Indonesia.
Am J Trop Med Hyg [Internet]. 2013 Sep [citado
2019 May 8];89(3):516-7. Disponível em: https://
www.ncbi.nlm.nih.gov/pmc/articles/PMC3771291/.
doi: 10.4269/ajtmh.13-0029
67. Perkasa A, Yudhaputri F, Haryanto S, Yohan B,
Myint KS, Ledermann JP, et al. Isolation of Zika
virus from febrile patient, Indonesia fatal. Emerg
Infect Dis [Internet]. 2016 May [citado 2019 May
8];22(5):924-5. Disponível em: https://wwwnc.cdc.
gov/eid/article/22/5/15-1915_article. doi: 10.3201/
eid2205.151915
68. Tappe D, Nachtigall S, Kapaun A, Schnitzler P, Günther
S, Schmidt-Chanasit J. Acute Zika virus infection after
travel to Malaysian Borneo, September 2014. Emerg
Infect Dis [Internet]. 2015 May [citado 2019 May
8];21(5):911-3. Disponível em: https://wwwnc.cdc.
gov/eid/article/21/5/14-1960_article. doi: 10.3201/
eid2105.141960
69. Alera MT, Hermann L, Tac-An IA, Klungthong C, Villa
D, Thaisomboonsuk B, et al. Zika virus infection,
Philippines, 2012. Emerg Infect Dis [Internet]. 2015
Apr [citado 2019 May 8];21(4):722-4. Disponível
em: https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC4378478/. doi: 10.3201/eid2104.141707
70. Korhonen EM, Huhtamo E, Smura T, Kallio-Kokko
H, Raassina M, Vapalahti O. Zika virus infection
in a traveller returning from the Maldives, June
2015. Euro Surveill [Internet]. 2016 [citado
2019 May 8];21(2). Disponível em: https://www.
eurosurveillance.org/content/10.2807/1560-7917.
ES.2016.21.2.30107. doi: 10.2807/1560-7917.
ES.2016.21.2.30107
71. Buathong R, Hermann L, Thaisomboonsuk B,
Rutvisuttinunt W, Klungthong C, Chinnawirotpisan P,
et al. Detection of Zika virus infection in Thailand
, 2012-2014. Am J Trop Med Hyg [Internet]. 2015
Aug [citado 2019 May 8];93(2):380-3. Disponível
em: https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC4530765/. doi: 10.4269/ajtmh.15-0022
72. Tappe D, Rissland J, Gabriel M, Emmerich P, Günther
S, Held G, et al. First case of laboratory-con fi rmed
Zika virus infection imported into Europe, November
2013. Euro Surveill [Internet]. 2014 Jan [citado 2019
May 8];19(4):pii: 20685. Disponível em: https://www.
eurosurveillance.org/content/10.2807/1560-7917.
ES2014.19.4.20685
73. Campos GS, Bandeira AC, Sardi SI. Zika virus
outbreak, Bahia, Brazil. Emerg Infect Dis [Internet].
2015 Oct [citado 2019 May 8];21(10):1885-
6. Disponível em: https://wwwnc.cdc.gov/eid/
article/21/10/15-0847_article. doi: 10.3201/
eid2110.150847
74. Zanluca C, Melo VC, Mosimann AL, Santos GI,
Santos CN, Luz K. First report of autochthonous
transmission of Zika virus in Brazil. Mem Inst
Oswaldo Cruz [Internet]. 2015 Jun [citado 2019
May 8];110(4):569-72. Disponível em: http://
www.scielo.br/pdf/mioc/v110n4/0074-0276-
mioc-0074-02760150192.pdf. doi: 10.1590/0074-
02760150192
75. Fantinato FFST, Araújo ELL, Ribeiro IG, Andrade
MR, Dantas ALM, Rios JMT, et al. Descrição dos
primeiros casos de febre pelo vírus Zika investigados
em municípios da região Nordeste do Brasil, 2015.
Epidemiol Serv Saúde [Internet]. 2016 out-dez
[citado 2019 maio 8];25(4):683-90. Disponível em:
http://www.scielo.br/pdf/ress/v25n4/2237-9622-
ress-S1679_49742016000400002.pdf. doi: 10.5123/
s1679-49742016000400002
17
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Gilmara de Souza Sampaio et al.
76. Felix AC, Souza NCS, Figueiredo WM, Costa AA,
Inenami M, Silva RMG, et al. Cross reactivity of
commercial anti-dengue immunoassays in patients
with acute Zika virus infection. J Med Virol [Internet].
2017 Aug [citado 2019 May 8];89(8)1477-9.
Disponível em: https://onlinelibrary.wiley.com/doi/
abs/10.1002/jmv.24789. doi: 10.1002/jmv.24789
77. Bozza FA, Moreira-Soto A, Rockstroh A, Fischer C,
Nascimento AD, Calheiros AS, et al. Differential shedding
and antibody kinetics of Zika and Chikungunya viruses,
Brazil. Emerg Infect Dis [Internet]. 2019 Feb [citado
2019 May 8];25(2):311-5. Disponível em: https://
wwwnc.cdc.gov/eid/article/25/2/18-0166_article
78. Waggoner JJ, Pinsky A. Zika virus : diagnostics
for an emerging pandemic threat. J Clin
Microbiol [Internet]. 2016 Apr [citado 2019 May
8];54(4)860-7. Disponível em: https://jcm.asm.org/
content/54/4/860.long. doi: 10.1128/JCM.00279-16
79. Center for Disease Control and Prevention (EUA). Revised
diagnostic testing for Zika, chikungunya, and dengue
viruses in US Public Health Laboratories [Internet]. 2016
[citado 2019 May 8]. Disponível em: http://www.cdc.gov/
zika/pdfs/denvchikvzikv-testing-algorithm.pdf
80. Yun S, Song B, Frank JC, Julander JG, Polejaeva
IA, Davies CJ, et al. Complete genome sequences
of three historically important, spatiotemporally
distinct, and genetically divergent strains of Zika
virus: MR-766, P6-740, and PRVABC-59. Genome
Announc [Internet]. 2016 Jul-Aug [citado 2019
May 8];4(4):e00800-16. Disponível em: https://
mra.asm.org/content/4/4/e00800-16. doi: 10.1128/
genomeA.00800-16
81. Faria NR, Azevedo RDSDS, Kraemer MUG, Souza R,
Cunha MS, Hill SC, et al. Zika virus in the Americas:
early epidemiological and genetic findings.
Science [Internet]. 2016 Apr [citado 2019 May
8];375(6283):345-9. Disponível em: https://science.
sciencemag.org/content/352/6283/345.long. doi:
10.1126/science.aaf5036
82. Metsky H, Matranga C, Wohl S, Schaffner S, Freije C,
Winnicki S, et al. Zika virus evolution and spread in
the Americas. Nature [Internet]. 2017 Jun [citado
2019 May 8];546(7658):411-5. Disponível em:
https://www.nature.com/articles/nature22402
83. Naccache SN, Thézé J, Sardi SI, Somasekar S,
Greninger AL, Bandeira AC, et al. Distinct zika
virus lineage in Salvador, Bahia, Brazil. Emerg
Infect Dis [Internet]. 2016 Oct [citado 2019 May
8];22(10):1788-92. Disponível em: https://wwwnc.
cdc.gov/eid/article/22/10/16-0663_article
84. Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus:
following the path of dengue and chikungunya?
Lancet [Internet]. 2015 Jul [citado 2019 May 8];
386(9990):243-4. Disponível em: https://www.
thelancet.com/journals/lancet/article/PIIS0140-
6736(15)61273-9/fulltext. doi: 10.1016/S0140-
6736(15)61273-9
85. Campos TDL, Durães-carvalho R, Rezende AM,
Carvalho OV, Kohl A, Wallau GL, et al. Revisiting key
entry routes of human epidemic arboviruses into
the Mainland Americas. Int J Genomics [Internet].
2018 Oct [citado 2019 May 8]; 2018:1-9.
Disponível em: https://www.hindawi.com/journals/
ijg/2018/6941735/cta/
86. Thézé J, Li T, Plessis L, Bouquet J, Kraemer
MUG, Somasekar S, et al. Genomic epidemiology
reconstructs the introduction and spread of Zika
virus in Central America and Mexico. Cell Host
Microbe [Internet]. 2018 Jun [citado 2019 May
8];23(6):855-64.e7. Disponível em: https://
www.cell.com/cell-host-microbe/fulltext/S1931-
3128(18)30218-X?_returnURL=https%3A%2F%2Fli
nkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1931
31281830218X%3Fshowall%3Dtrue. doi: 10.1016/j.
chom.2018.04.017
87. Pan American Health Organization. World Health
Organization. Zika epidemiological update: 22
September 2016 [Internet]. Washington, D.C.: Pan
American Health Organization; 2016 [citado 2019
May 8]. Disponível em: https://www.paho.org/hq/
dmdocuments/2016/2016-sep-22-cha-epi-update-
zika-virus.pdf
88. Pan American Health Organization. World Health
Organization. Zika epidemiological update: 29 December
2016 [Internet]. Washington, D.C.: Pan American Health
Organization; 2016 [citado 2019 May 8]. Disponível em:
https://www.paho.org/hq/dmdocuments/2016/2016-dec-
29-phe-epi-update-zika-virus.pdf
89. Ikejezie J, Shapiro CN, Kim J, Chiu M, Almiron M,
Ugarte C, et al. Zika virus transmission – region of the
Americas. MMWR Morb Mortal Wkly Rep [Internet].
2017 Mar [citado 2019 Ma 8];66(12):329-
34. Disponível em: https://www.cdc.gov/mmwr/
volumes/66/wr/mm6612a4.htm
90. World Health Organization. Fifth meeting of the
Emergency Committee under the International Health
Regulations (2005) regarding microcephaly, other
neurological disorders and Zika virus [Internet].
Geneva: World Health Organization; 2016 [citado
2019 May 8]. Disponível em: http://www.who.int/
mediacentre/news/statements/2016/zika-fifth-ec/en/

18
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Expansion of Zika virus circulation: from Africa to the Americas
91. Ministério da Saúde (BR). Ministério da Saúde
declara fim da emergência nacional para Zika
[Internet]. Brasília: Ministério da Saúde; 2017
[citado 2019 maio 8]. Disponível em: http://www.
brasil.gov.br/noticias/saude/2017/05/ministerio-da-
saude-declara-fim-da-emergencia-nacional-para-zika
92. Nóbrega MEB, Araújo ELL, Wada MY, Leite PL,
Percio J, Dimech GS. Surto de síndrome de Guillain-
Barré possivelmente relacionado à infecção
prévia pelo vírus Zika, Região Metropolitana do
Recife, Pernambuco, Brasil, 2015. Epidemiol
Serv Saúde [Internet]. 2018 [citado 2019 maio
8];27(2):e2017039. Disponível em: http://
www.scielo.br/pdf/ress/v27n2/2237-9622-ress-
27-02-e2017039.pdf. doi: 10.5123/s1679-
49742018000200016
93. Malta JM, Vargas A, Leite PL, Percio J, Coelho GE,
Ferraro AH, et al. Síndrome de Guillain-Barré e
outras manifestações neurológicas possivelmente
relacionadas à infecção pelo vírus Zika em
municípios da Bahia, 2015. Epidemiol Serv Saúde
[Internet]. 2017 jan-mar [citado 2019 maio
8];26(1):9-18. Disponível em: http://www.scielo.br/
pdf/ress/v26n1/2237-9622-ress-26-01-00009.pdf.
doi: 10.5123/s1679-49742017000100002
94. Nogueira ML, Estofolete CF, Terzian ACB, Mascarin do
Vale EPB, Silva RC, Silva RF, et al. Zika virus infection
and solid organ transplantation: a new challenge. Am
J Transplant [Internet]. 2017 Mar [citado 2019 May
8];17(3)791-5. Disponível em: https://onlinelibrary.
wiley.com/doi/full/10.1111/ajt.14047. doi: 10.1111/
ajt.14047
95. Vargas A, Saad E, Dimech GS, Santos RH, Sivini
MAVC, Albuquerque LC, et al. Características dos
primeiros casos de microcefalia possivelmente
relacionados ao vírus Zika notificados na região
metropolitana de Recife, Pernambuco. Epidemiol
Serv Saúde [Internet]. 2016 out-dez [citado
2019 maio 8];25(4):691-700. Disponível em:
http://www.scielo.br/pdf/ress/v25n4/2237-9622-
ress-S1679_49742016000400003.pdf. doi:
10.5123/s1679-49742016000400003
96. Pan American Health Organization. World Health
Organization. Zika suspected and confirmed
cases reported by countries and territories in the
Americas Cumulative cases, 2015-2016. Updated
as of 29 December 2016 [Internet]. Washington,
D.C.: Pan American Health Organization; 2016
[citado 2019 May 8]. Disponível em: https://www.
paho.org/hq/dmdocuments/2016/2016-dec-29-
phe-ZIKV-cases.pdf
97. Ministério da Saúde (BR). Secretaria de Vigilância
em Saúde. Monitoramento integrado de alterações
no crescimento e desenvolvimento relacionadas
à infecção pelo vírus Zika e outras etiologias
infecciosas, até a Semana 52 de 2017. Bol Epidemiol
[Internet]. 2018 [citado 2019 maio 8];49(6):1-10.
Disponível em: http://portalarquivos2.saude.gov.br/
images/pdf/2018/fevereiro/20/2018-003-Final.pdf
98. Ministério da Saúde (BR). Secretaria de Vigilância
em Saúde. Monitoramento integrado de alterações
no crescimento e desenvolvimento relacionadas
à infecção pelo vírus Zika e outras etiologias
infecciosas, até a Semana Epidemiológica 20 de
2018. Bol Epidemiol [Internet]. 2018 [citado
2019 maio 8];49(6). Disponível em: http://
portalarquivos2.saude.gov.br/images/pdf/2018/
junho/29/Monitoramento-integrado-de-alteracoes-
no-crescimento-e-desenvolvimento-relacionadas-a-
infeccao-pelo-virus-Zika.pdf
99. Rodrigues LC, Paixao ES. Risk of Zika-related
microcephaly: stable or variable? Lancet [Internet].
2017 Aug [citado 2019 May 8]; 390(10097):824-6.
Disponível em: https://www.thelancet.com/journals/
lancet/article/PIIS0140-6736(17)31478-2/fulltext.
doi: 10.1016/S0140-6736(17)31478-2
100. Oliveira WK, França GVA, Carmo EH, Duncan BB,
Souza Kuchenbecker R, Schmidt MI. Infection-
related microcephaly after the 2015 and 2016 Zika
virus outbreaks in Brazil: a surveillance-based
analysis. Lancet [Internet]. 2017 Aug [citado 2019
May 8];390(10097):861-70. Disponível em: https://
www.thelancet.com/journals/lancet/article/PIIS0140-
6736(17)31368-5/fulltext. doi: 10.1016/S0140-
6736(17)31368-5
101. Wang L, Valderramos SG, Wu A, Ouyang S, Li C, Brasil
P, et al. From mosquitos to humans: genetic evolution
of Zika virus. Cell Host Microbe [Internet]. 2016
May [citado 2019 May 8];19(5):561-5. Disponível
em: https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC5648540/. doi: 10.1016/j.chom.2016.04.006
102. Sheridan MA, Balaraman V, Schust DJ, Ezashi T,
Michael Roberts R, Franz AWE. African and Asian
strains of Zika virus differ in their ability to infect
and lyse primitive human placental trophoblast.
PLoS One [Internet]. 2018 [citado 2019 May
8];13(7):e0200086. Disponível em: https://www.
ncbi.nlm.nih.gov/pmc/articles/PMC6037361/. doi:
10.1371/journal.pone.0200086
103. Caires-Júnior LC, Goulart E, Melo US, Araujo BHS,
Alvizi L, Soares-Schanoski A, et al. Discordant
congenital Zika syndrome twins show differential in
19
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Gilmara de Souza Sampaio et al.
vitro viral susceptibility of neural progenitor cells.
Nat Commun [Internet]. 2018 Feb [citado 2019 May
8];Dec;9(1):475. Disponível em: https://www.nature.
com/articles/s41467-017-02790-9. doi: 10.1038/
s41467-017-02790-9
104. Pettersson JH, Eldholm V, Seligman SJ, Lundkvist A,
Falconar AK, Gaunt MW, et al. How did Zika virus
emerge in the Pacific Islands and Latin America?
MBio [Internet]. 2016 Oct [citado 2019 May
8];7(5):pii:e01239-16. Disponível em: https://mbio.
asm.org/content/7/5/e01239-16. doi: 10.1128/
mBio.01239-16
105. Yuan L, Huang XY, Liu ZY, Zhang F, Zhu XL, Yu JY, et
al. A single mutation in the prM protein of Zika virus
contributes to fetal microcephaly. Science [Internet].
2017 Nov [citado 2019 May 8];358(6365):933-
6. Disponível em: https://science.sciencemag.org/
content/358/6365/933. doi: 10.1126/science.aam7120
106. Li J, Xiong Y, Wu W, Liu X, Qu J, Zhao X, et al.
Zika virus in a traveler returning to China from
Caracas, Venezuela, February 2016. Emerg Infect
Dis [Internet]. 2016 Jun [citado 2019 May
8];22(6):1133-6. Disponível em: https://wwwnc.cdc.
gov/eid/article/22/6/16-0273_article. doi: 10.3201/
eid2206.160273
107. Xia H, Luo H, Shan C, Muruato AE, Nunes BTD,
Medeiros DBA, et al. An evolutionary NS1 mutation
enhances Zika virus evasion of host interferon
induction. Nat Commun [Internet]. 2018 Jan [citado
2019 May 8];9(1):414. Disponível em: https://www.
nature.com/articles/s41467-017-02816-2
108. Lanciotti RS, Lambert AJ, Holodniy M, Saavedra
S, Signor LC. Phylogeny of Zika virus in Western
hemisphere, 2015. Emerg Infect Dis [Internet].
2016 May [citado 2019 May 8];22(5):933-
5. Disponível em: https://wwwnc.cdc.gov/eid/
article/22/5/16-0065_article. doi: 10.3201/
eid2205.160065
109. Aubry F, Martynow D, Baidaliuk A, Merkling SH,
Dickson LB, Romero-Vivas CM, et al. Worldwide
survey reveals lower susceptibility of African Aedes
aegypti mosquitoes to diverse strains of Zika virus.
bioRxiv Prepr [Internet]. 2018 Jun [citado 2019
May 8];1-8. Disponível em: https://www.biorxiv.
org/content/early/2018/06/10/342741.full.pdf. doi:
10.1101/342741
110. Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere
JJ, Brown JA, et al. Enhancement of Zika virus
pathogenesis by preexisting antiflavivirus immunity.
Science [Internet]. 2017 Apr [citado 2019 May
8];356(6334):175-80. Disponível em: https://
science.sciencemag.org/content/356/6334/175.long.
doi: 10.1126/science.aal4365
111. Gordon A, Gresh L, Ojeda S, Katzelnick LC, Sanchez
N, Mercado JC, et al. Prior dengue virus infection
and risk of Zika: a pediatric cohort in Nicaragua.
PLoS Med [Internet]. 2019 Jan [citado 2019 May
8];16(1):e1002726. Disponível em: https://journals.
plos.org/plosmedicine/article?id=10.1371/journal.
pmed.1002726. doi: 10.1371/journal.pmed.1002726
112. Shresta S, Viramontes KM, Wang Y-T, Huynh A-T,
Diamond MS, Elong Ngono A, et al. Cross-reactive
Dengue virus-specific CD8+ T cells protect against
Zika virus during pregnancy. Nat Commun [Internet].
2018 Aug [citado 2019 May 8];9(1):3042.
Disponível em: https://www.nature.com/articles/
s41467-018-05458-0. doi: 10.1038/s41467-018-
05458-0
113. Weger-Lucarelli J, Rückert C, Chotiwan N, Nguyen
C, Garcia Luna SM, Fauver JR, et al. Vector
competence of American mosquitoes for three
strains of Zika virus. PLoS Negl Trop Dis [Internet].
2016 Oct [citado 2019 May 8];10(10):e0005101.
Disponível em: https://journals.plos.org/plosntds/
article?id=10.1371/journal.pntd.0005101. doi:
10.1371/journal.pntd.0005101
114. Ciota AT, Bialosuknia SM, Zink SD, Brecher M,
Ehrbar DJ, Morrissette MN, et al. Effects of Zika
virus strain and Aedes mosquito species on vector
competence. Emerg Infect Dis [Internet]. 2017 Jul
[citado 2019 May 8];23(7):1110-7. Disponível em:
https://wwwnc.cdc.gov/eid/article/23/7/16-1633_
article. doi: 10.3201/eid2307.161633
115. Azar SR, Roundy CM, Rossi SL, Huang JH, Leal
G, Yun R, et al. Differential vector competency of
aedes albopictus populations from the Americas
for Zika Virus. Am J Trop Med Hyg [Internet]. 2017
Aug [citado 2019 May 8];97(2):330-9. Disponível
em: https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC5544086/. doi: 10.4269/ajtmh.16-0969
116. Angleró-Rodríguez YI, MacLeod HJ, Kang S, Carlson
JS, Jupatanakul N, Dimopoulos G. Aedes aegypti
molecular responses to Zika virus: modulation of
infection by the toll and Jak/Stat immune pathways
and virus host factors. Front Microbiol [Internet].
2017 Oct [citado 2019 May 8];8:2050. Disponível
em: https://www.frontiersin.org/articles/10.3389/
fmicb.2017.02050/full. doi: 10.3389/
fmicb.2017.02050
117. Di Luca M, Severini F, Toma L, Boccolini D, Romi
R, Remoli ME, et al. Experimental studies of
susceptibility of Italian Aedes albopictus to Zika

20
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Expansion of Zika virus circulation: from Africa to the Americas
virus. Euro Surveill [Internet]. 2016 May [citado
2019 May 8];21(18). Disponível em: https://www.
eurosurveillance.org/content/10.2807/1560-7917.
ES.2016.21.18.30223. doi: 10.2807/1560-7917.
ES.2016.21.18.30223
118. Richard V, Paoaafaite T, Cao-Lormeau VM. Vector
competence of French Polynesian aedes aegypti
and aedes polynesiensis for Zika virus. PLoS Negl
Trop Dis [Internet]. 2016 Sep [citado 2019 May
8];10(9):e0005024. Disponível em: https://journals.
plos.org/plosntds/article?id=10.1371/journal.
pntd.0005024. doi: 10.1371/journal.pntd.0005024
119. Hall-Mendelin S, Pyke AT, Moore PR, Mackay IM,
McMahon JL, Ritchie SA, et al. Assessment of local
mosquito species incriminates aedes aegypti as the
potential vector of Zika virus in Australia. PLoS Negl
Trop Dis [Internet]. 2016 Sep [citado 2019 May
8];10(9):e0004959. Disponível em: https://www.
ncbi.nlm.nih.gov/pmc/articles/PMC5028067/. doi:
10.1371/journal.pntd.0004959
120. Chouin-Carneiro T, Vega-Rua A, Vazeille M,
Yebakima A, Girod R, Goindin D, et al. Differential
susceptibilities of aedes aegypti and aedes albopictus
from the Americas to Zika virus. PLoS Negl Trop
Dis [Internet]. 2016 Mar [citado 2019 May
8];10(3):e0004543. Disponível em: https://journals.
plos.org/plosntds/article?id=10.1371/journal.
pntd.0004543. doi: 10.1371/journal.pntd.0004543
121. Pompon J, Morales-Vargas R, Manuel M, Huat Tan
C, Vial T, et al. A Zika virus from America is more
efficiently transmitted than an Asian virus by aedes
aegypti mosquitoes from Asia. Sci Rep [Internet].
2017 Apr [citado 2019 May 8];7(1):1215. Disponível
em: https://www.nature.com/articles/s41598-017-
01282-6. doi: 10.1038/s41598-017-01282-6
122. Campos MC, Dombrowski JG, Phelan J, Marinho
CRF, Hibberd M, Clark TG, et al. Zika might not be
acting alone: using an ecological study approach
to investigate potential co-acting risk factors for an
unusual pattern of microcephaly in Brazil. PLoS One
[Internet]. 2018 Aug [citado 2019 May 8];13(8)
e0201452. Disponível em: https://journals.plos.org/
plosone/article?id=10.1371/journal.pone.0201452.
doi: 10.1371/journal.pone.0201452
123. Martins Netto E, Moreira-Soto A, Pedroso C, Höser
C, Funk S, Kucharski A, et al. High Zika virus
Seroprevalence in Salvador, Northeastern Brazil limits
the potential for further outbreaks. MBio [Internet].
2017 Nov [citado 2019 May 8];8(6):pii:e01390-17.
Disponível em: https://mbio.asm.org/content/8/6/
e01390-17. doi: 10.1128/mBio.01390-17
124. Ferguson NM, Cucunubá ZM, Dorigatti I, Nedjati-
gilani GL, Donnelly CA, Basáñez MG, et al.
Countering the Zika epidemic in Latin America.
Science [Internet]. 2016 Jul [citado 2019 May
8];353(6297):353-4. Disponível em: http://science.
sciencemag.org/content/353/6297/353/tab-pdf. doi:
10.1126/science.aag0219
125. Chippaux HC, Chippaux A. Yellow fever antibodies
in children in the Central African Republic. Bull
World Health Organ [Internet]. 1966 [citado
2019 May 8];34(1):105-11. Disponível em:
https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC2475960/
126. Robin Y, Brès P, Lartigue JJ, Gidel R, Lefèvre M,
Athawet B, Hery G. Arboviruses in Ivory Coast.
Serologic survey in the human population. Bull Soc
Pathol Exot Fil. 1968 Nov-Dec;61(6):833-45.
127. Henderson BE, Metselaar D, Kirya GB, Timms GL.
Investigations into yellow fever virus and other
arboviruses in the northern regions of Kenya. Bull
World Health Organ [Internet]. 1970 [citado 2019
May 8];42(5):787-95. Disponível em: https://www.
ncbi.nlm.nih.gov/pmc/articles/PMC2427481/
128. Fagbami AH, Fabiyi A, Monath TP. Dengue virus
infections in nigeria: a survey for antibodies in
monkeys and humans. Trans R Soc Trop Med Hyg
[Internet]. 1977 [citado 2019 May 8];71(1):60-5.
Disponível em: https://academic.oup.com/trstmh/
article-abstract/71/1/60/1934880?redirectedFrom=f
ulltext. doi: 10.1016/0035-9203(77)90210-3
129. Olson JG, Ksiazek TG, Suhandiman T. Zika virus, a
cause of fever in Central Java, Indonesia. Trans R Soc
Trop Med Hyg [Internet]. 1981 Jan [citado 2019 May
8];75(3):389-93. Disponível em: https://academic.
oup.com/trstmh/article-abstract/75/3/389/18816
99?redirectedFrom=fulltext. doi: 10.1016/0035-
9203(81)90100-0
130. Olson JG, Ksiazenk TG, Gubler DJ, Lubist SI,
Simanjuntak G, Lee VH, et al. A survey for arboviral
antibodies in sera of humans and animals in lombok,
Republic of Indonesia. Ann Trop Med Parasitol. 1983
Apr;77(2):131-7.
131. Darwish MA, Hoogstraal H, Roberts TJ, Ahmed
IP, Omar F. A sero-epidemiological survey for
certain in Pakistan arboviruses ( Togaviridae
). Trans R Soc Trop Med Hyg [Internet]. 1983
[citado 2019 May 8];77(4):442-5. Disponível em:
https://www.sciencedirect.com/science/article/
pii/0035920383901062. doi: 10.1016/0035-
9203(83)90106-2
21
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Gilmara de Souza Sampaio et al.
132. Robin Y, Mouchet J. Serological and entomological
study on yellow fever in Sierra Leone. Bull Soc Pathol
Exot Filiales. 1975 May-Jun;68(3):249-58.
133. Monath TP, Wilson DC, Casals J. The 1970 yellow
fever epidemic in Okwoga District, Benue Plateau
State, Nigeria. 3. Serological responses in persons
with and without pre-existing heterologous group
B immunity. Bull World Health Organ [Internet].
1973 [citado 2019 May 8];49(3):235-44. Disponível
em: https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC2481145/
134. Moore DL, Causey OR, Carey DE, Reddy S, Cooke
AR, Akinkugbe FM, et al. Arthropod-borne viral
infections of man in nigeria, 1964-1970. Ann Trop
Med Parasitol. 1975 Mar;69(1):49-64.
135. Filipe AR, Carvalho RG, Relvas A, Casaca V. Arbovirus
studies in Angola: serological survey for antibodies
to arboviruses. Am J Trop Med Hyg [Internet]. 1975
May [citado 2019 May 8];24(3):516-20. Disponível
em: http://www.ajtmh.org/content/journals/10.4269/
ajtmh.1975.24.516. doi: 10.4269/ajtmh.1975.24.516
136. Omer AH, McLaren ML, Johnson BK, Chanas AC,
Brumpt I, Gardner P, et al. A seroepidemiological
survey in the Gezira, Sudan, with special reference to
arboviruses. J Trop Med Hyg. 1981 Apr;84(2):63-6.
137. Gonzalez JP, Saluzzo JF, Hervé JP, Geoffroy B.
Serological survey on the prevalence of arboviruses
in man in forest and periforest environments of the
region of Lobaye (Central African Republic). Bull Soc
Pathol Exot Filiales. 1979 Sep-Dec;72(5-6):416-23.
138. Adekolu-John EO, Fagbami AH. Arthropod-borne
virus antibodies in sera of residents of Kainji
Lake Basin, Nigeria 1980. Trans R Soc Trop
Med Hyg [Internet]. 1983 Jan [citado 2019 May
8];77(2):149-51. Disponível em: https://academic.
oup.com/trstmh/article-abstract/77/2/149/19157
25?redirectedFrom=fulltext. doi: 10.1016/0035-
9203(83)90053-6
139. Rodhain F, Gonzalez JP, Mercier E, Helynck B,
Larouze B, Hannoun C. Arbovirus infections and viral
haemorrhagic fevers in Uganda: a serological survey
in Karamoja district, 1984. Trans R Soc Trop Med
Hyg [Internet]. 1989 Nov-Dec [citado 2019 May
8];83(6):851-4. Disponível em: https://academic.
oup.com/trstmh/article-abstract/83/6/851/19069
66?redirectedFrom=fulltext. doi: 10.1016/0035-
9203(89)90352-0
140. Rodier GR, Gubler DJ, Cope SE, Cropp CB, Soliman
AK, Polycarpe D, et al. Epidemic dengue 2 in the
city of Djibouti 1991-1992. Trans R Soc Trop Med
Hyg [Internet]. 1996 May-Jun [citado 2019 May
8];90(3):237-40. Disponível em: https://academic.
oup.com/trstmh/article-abstract/90/3/237/19029
58?redirectedFrom=fulltext. doi: 10.1016/S0035-
9203(96)90228-X
141. Wolfe ND, Kilbourn AM, Karesh WB, Rahman HA,
Bosi EJ, Cropp BC, et al. Sylvatic transmission
of arboviruses among bornean orangutans.
Am J Trop Med Hyg [Internet]. 2001 May-
Jun [citado 2019 May 8];64(5-6):310-6.
Disponível em: http://www.ajtmh.org/docserver/
fulltext/14761645/64/5/11463123.pdf?expires=155
8192456&id=id&accname=guest&checksum=887C
7DF8907D12064738B9F73377A09F
142. Fokam EB, Levai LD, Guzman H, Amelia PA, Titanji
VP, Tesh RB, et al. Silent circulation of arboviruses in
cameroon. East Afr Med J. 2010 Jun;87(6):262-8.
143. Babaniyi OA, Mwaba P, Songolo P, Mazaba-
Liwewe ML, Mweene-Ndumba I, Masaninga F, et
al. Seroprevalence of Zika virus infection specific
IgG in Western and North-Western Provinces of
Zambia. Int J Public Health [Internet]. 2015 Jan
[citado 2019 May 8];4(1):110-114. Disponível
em: http://internationalscholarsjournals.org/print.
php?article=seroprevalence-of-zika
144. Babaniyi OA, Chizema E, Eshetu-Shibeshi M,
Malama C, Masaninga F, Mazaba-Liwewe M, et al.
Risk assessment for yellow fever in western and
North-Western provinces of Zambia. J Glob Infect
Dis [Internet]. 2015 Jan-Mar [citado 2019 May
8];7(1):11-7. Disponível em: http://www.jgid.org/
article.asp?issn=0974-777X;year=2015;volume=7;
issue=1;spage=11;epage=17;aulast=Babaniyi. doi:
10.4103/0974-777X.150884
145. Lourenço J, Lourdes Monteiro M, Valdez T,
Monteiro Rodrigues J, Pybus O, Rodrigues Faria N.
Epidemiology of the Zika virus outbreak in the Cabo
Verde Islands, West Africa. PLoS Curr [Internet].
2018 Mar [citado 2019 May 8];10:pii. Disponível
em: http://currents.plos.org/outbreaks/article/
epidemiology-of-the-zika-virus-outbreak-in-the-cabo-
verde-islands-west-africa/
146. Guerbois M, Fernandez-Salas I, Azar SR, Danis-
lozano R, Alpuche-Aranda CM, Leal G, et al. Outbreak
of Zika virus infection, Chiapas State, Mexico, 2015,
and First con fi rmed transmission by aedes aegypti
mosquitoes in the Americas. J Infect Dis [Internet].
2016 Nov [citado 2019 May8];214(9):1349-56.
Disponível em: https://academic.oup.com/jid/
article/214/9/1349/2576416. doi: 10.1093/infdis/
jiw302

22
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Expansion of Zika virus circulation: from Africa to the Americas
147. Likos A, Griffin I, Bingham AM, Stanek D,
Fischer M, White S, et al. Local mosquito-borne
transmission of Zika virus - Miami-Dade and
Broward Counties, Florida, June-August 2016.
MMWR Morb Mortal Wkly Rep [Internet].
2016 Sep [citado 2019 May8];65(38):1032-
8. Disponível em: https://www.cdc.gov/mmwr/
volumes/65/wr/mm6538e1.htm. doi: 10.15585/
mmwr.mm6538e1
148. Lednicky J, Beau de Rochars VM, El Badry M, Loeb
J, Telisma T, Chavannes S, et al. Zika virus outbreak
in Haiti in 2014: molecular and clinical data. PLoS
Negl Trop Dis [Internet]. 2016 Apr [citado 2019
May 8];10(4):e0004687. Disponível em: https://
journals.plos.org/plosntds/article?id=10.1371/
journal.pntd.0004687. doi: 10.1371/journal.
pntd.0004687
149. Daudens-Vaysse E, Ledrans M, Gay N, Ardillon V,
Cassadou S, Najioullah F, et al. Zika emergence in
the French territories of America and description
of first confirmed cases of Zika virus infection on
Martinique, November 2015 to February 2016.
Euro Surveill [Internet]. 2016 Jul [citado 2019
May 8];21(28). Disponível em: https://www.
eurosurveillance.org/content/10.2807/1560-7917.
ES.2016.21.28.30285. doi: 10.2807/1560-7917.
ES.2016.21.28.30285
150. Reusken C, Pas S, GeurtsvanKessel C, Mögling R,
Kampen J, Langerak T, et al. Longitudinal follow-
up of Zika virus RNA in semen of a traveller
returning from Barbados to the Netherlands
with Zika virus disease, March 2016. Euro
Surveill [Internet]. 2016 Jun [citado 2019
May 8];21(23). Disponível em: https://www.
eurosurveillance.org/content/10.2807/1560-7917.
ES.2016.21.23.30251. doi: 10.2807/1560-7917.
ES.2016.21.23.30251
151. Araúz D, Urriola L, Jones J, Castillo M, Martínez A,
Murillo E, et al. Febrile or exanthematous illness
associated with Zika, dengue, and chikungunya.
Emerg Infect Dis [Internet]. 2016 Aug [citet 2019
May 8];22(8):1515-7. Disponível em: https://wwwnc.
cdc.gov/eid/article/22/8/16-0292_article. doi:
10.3201/eid2208.160292
152. Thomas DL, Sharp TM, Torres J, Armstrong PA,
Munoz-Jordan J, Ryff KR, et al. Local transmission of
Zika virus--Puerto Rico, November 23, 2015-January
28, 2016. MMWR Morb Mortal Wkly Rep [Internet].
2016 Feb [citado 2019 May 8];65(6):154-8.
Disponível em: https://www.cdc.gov/mmwr/
volumes/65/wr/mm6506e2.htm
153. Codrington J, Roosblad J, Baidjoe AY, Holband N,
Adde A, Kazanji M, et al. Zika virus outbreak in
Suriname, a report based on laboratory surveillance
data. PLoS Curr [Internet]. 2018 May [citado 2019
May 8];10:pii. Disponível em: http://currents.plos.
org/outbreaks/index.html%3Fp=75436.html
154. Ryan SJ, Carlson CJ, Stewart-Ibarra AM, Borbor-
Cordova MJ, Romero MM, Cox S, et al. Outbreak
of Zika virus infections, Dominica, 2016. Emerg
Infect Dis [Internet]. 2017 Nov [citado 2019 May
8];23(11):2016-7. Disponível em: https://wwwnc.
cdc.gov/eid/article/23/11/17-1140_article
155. Boyer Chammard T, Schepers K, Breurec S,
Messiaen T, Destrem AL, Mahevas M, et al. Severe
thrombocytopenia after Zika virus infection,
Guadeloupe, 2016. Emerg Infect Dis [Internet]. 2017
Apr [citado 2019 May 8];23(4):696-8. Disponível
em: https://wwwnc.cdc.gov/eid/article/23/4/16-1967_
article. doi: 10.3201/eid2304.161967
156. Waggoner JJ, Gresh L, Vargas MJ, Ballesteros
G, Tellez Y, Soda KJ, et al. Viremia and clinical
presentation in Nicaraguan patients infected with
Zika virus, chikungunya virus and dengue virus.
Clin Infect Dis [Internet]. 2016 Dec [citado 2019
May 8];63(12):1584-90. Disponível em: https://
academic.oup.com/cid/article/63/12/1584/2282806.
doi: 10.1093/cid/ciw589
157. Barzon L, Pacenti M, Berto A, Sinigaglia A, Franchin
E, Lavezzo E, et al. Isolation of infectious Zika virus
from saliva and prolonged viral RNA shedding in a
traveller returning from the Dominican Republic
to Italy, January 2016. Euro Surveill [Internet].
2016 [citado 2019 May 8];21(10):30159.
Disponível em: https://www.eurosurveillance.org/
content/10.2807/1560-7917.ES.2016.21.10.30159.
doi: 10.2807/1560-7917.ES.2016.21.10.30159
158. Pacheco O, Beltrán M, Nelson CA, Valencia D, Tolosa
N, Farr SL, et al. Zika virus disease in Colombia -
preliminary report. N Engl J Med [Internet]. 2016
Jun [citado 2019 May 8. Disponível em: http://www.
isags-unasul.org/uploads/biblioteca/6/bb[656]ling[3]
anx[2166].pdf. doi: 10.1056/NEJMoa1604037
159. Saba Villarroel PM, Nurtop E, Pastorino B, Roca Y,
Drexler JF, Gallian P, et al. Zika virus epidemiology
in Bolivia: a seroprevalence study in volunteer
blood donors. PLoS Negl Trop Dis [Internet].
2018 Mar [citado 2019 May 8];12(3):e0006239.
Disponível em: https://journals.plos.org/plosntds/
article?id=10.1371/journal.pntd.0006239. doi:
10.1371/journal.pntd.0006239

23
Epidemiol. Serv. Saude, Brasília, 28(2):e2018411, 2019
Gilmara de Souza Sampaio et al.
160. Villamil-Gómez WE, Rodríguez-Morales AJ, Uribe-
García AM, González-Arismendy E, Castellanos JE,
Calvo EP, et al. Zika, dengue, and chikungunya
co-infection in a pregnant woman from Colombia. Int
J Infect Dis [Internet]. 2016 Oct [citado 2019 May
8];51:135-8. Disponível em: https://www.ijidonline.
com/article/S1201-9712(16)31125-0/fulltext. doi:
10.1016/j.ijid.2016.07.017
161. Sánchez-Carbonel J, Tantaléan-Yépez D, Aguilar-Luis
MA, Silva-Caso W, Weilg P, Vásquez-Achaya F, et al.
Identification of infection by chikungunya, Zika, and
dengue in an area of the Peruvian coast. Molecular
diagnosis and clinical characteristics. BMC Res
Notes [Internet]. 2018 Mar [citado 2019 May
8];11(1):175. Disponível em: https://bmcresnotes.
biomedcentral.com/articles/10.1186/s13104-018-
3290-0. doi: 10.1186/s13104-018-3290-0
Received on 04/02/2019
Approved on 17/04/2019
Associate editor: Taís Freire Galvão – orcid.org/0000-0003-2072-4834
