
White Paper
ZEISS Lightsheet Z.1
Sample Preparation

White Paper
2
ZEISS Lightsheet Z.1
Sample Preparation
Author: Flood P.M., Kelly R., Gutiérrez-Heredia L.
and E.G. Reynaud
School of Biology and Environmental Science,
University College Dublin, Ireland
Date: Sept 2013
This paper describes theoretical and practical aspects of sample preparation for Light Sheet Fluorescence
Microscopy (LSFM). We present general rules for sample handling and mounting, as well as guidelines with
respect to the best preparative technique to use, taking into account sample type, structure and properties.
Step by step protocols and recommended materials for ZEISS Lightsheet Z.1 samples are included.
These protocols cover sample preparation ranging from micrometer-sized fluorescent beads to millimeter-sized
insects, providing detailed information relating to preparation and observation techniques. Finally, this paper
identifies the main artifacts and problems that can result from the preparation techniques.
CONTENTS (click text to go to page):
1 INTRODUCTION
2 SAMPLE MOUNTING FOR LSFM
2.1 The perfect Sample for LSFM
2.2 Holding the Sample
2.2.1 Embedded Samples
2.2.2 Hanging Samples
2.2.3 Enclosed Samples
2.2.4 FEP Tubing
2.3 Materials and Equipment
2.3.1 Sample Chambers
2.3.2 Molding and Mounting Supports
2.3.3 Sample Holder
2.3.4 Gels and Polymers
2.3.5 Hydrogel Preparation
2.4 Fixation and Fixatives
2.5 Stains and Staining
2.5.1 Choosing a Fluorescent Label
2.6 Antifading Agents
2.7 Cleaning, Labelling and Storing Samples
3
5
5
6
8
12
13
15
15
15
16
18
18
19
20
20
20
21
21
22
22
23
24
24
25
26
27
28
28
28
30
31
33
3
SPECIFIC EXAMPLES OF SAMPLE
PREPARATION
3.1 Preparation of Fluorescent Beads
3.2 Preparation of a Medaka Fish Embryo
(Oryza latypes)
3.3 Preparation of a Fly Pupa
(Drosophila melanogaster)
3.4 Preparation of a Plant Root
(Arabidopsis thaliana)
3.5 Imaging Cell Cysts in an Extracellular Matrix Gel
3.6
Immunostaining and Preparation of
MDCK Cell Cysts
3.7 Preparation of a Whole Mount of a Mosquito
(Anopheles gambiae)
4 TIPS, TROUBLESHOOTING AND
ADDITIONAL INFORMATION
4.1 Tips
4.2 Troubleshooting
4.3 Suggested Additional Sources of Information
4.4 References and Further Reading
5 Index

White Paper<< Back to contents
3
1. INTRODUCTION
A microscope generally performs best on suitable samples,
and when the samples are optimally prepared for the imag-
ing method and microscope type. In Light Sheet Fluorescence
Microscopy (LSFM), the sample is commonly mounted in a
liquid filled chamber and can be rotated easily. It is scanned
through a sheet of light which illuminates the focal plane of
a perpendicularly mounted objective lens. The resulting image
of an optical section is observed through the objective and
is usually detected on a camera-based detector. Since the
geometry of the optical beam paths and the optics differ
significantly from the conventional inverted and upright
compound microscopes, the sample mounting protocols
also differ significantly.
If the sample is perfectly transparent, like a block of 1%
agarose with beads inside, the light sheet can penetrate
deeply and does not change its properties and shape along
the illumination axis. Also, the fluorescent signal can reach
the detector unperturbed by scattering effects in the specimen
or hydrogel. However, if the sample is slightly opaque and
diffracts or scatters the light sheet heavily (lipids, lipid vesicles
or dense collagen fiber arrays that scatter light strongly) then
the well-defined shape and thickness of the sectioning light
sheet degrades along the illumination axis. In a second effect,
the detected image from a well illuminated sample might
still be degraded by such a poorly transparent sample.
These effects can contribute independently to the final image
quality of a LSFM and can be minimized or worked around by
careful sample positioning in the microscope as well as by an
optimized sample preparation protocol.
Ultimately, a fully opaque sample that can completely block
the penetration of light and a light sheet (insect cuticular
structures, bones…) will limit the imaging capabilities of
Light Sheet Fluorescence Microscopes and Lightsheet Z.1
to its surface.
Furthermore, the image quality in Fluorescence Microscopy
in general – and in LSFM in particular – is not only deter-
mined by sample transparency that can be optimized by
choosing a suitable model (transparent fish like Medaka),
suitable growth conditions (no phenol red in the growth
media to avoid autofluorescence) or, potentially, a clearing
treatment (not suitable for Lightsheet Z.1). It is also important
to have a homogenous signal from a homogeneously
labelled sample. Antibodies, for example, are rather large
molecules that cannot penetrate deeply into tissue so it is
difficult to image a complete juvenile fish after antibody as
only the first 50 μm to 100 μm will be labelled, the interior
showing reduced signal levels due to the limited diffusion
of the antibody.
Samples must be carefully considered when using LSFMs
such as Lightsheet Z.1 as well as the label or dye used must
be carefully chosen. In planning an experiment, it should be
kept in mind that most labelling and imaging protocols have
been developed for thin specimens and therefore many
aspects are not adapted to imaging larger samples such as
embryos, tissue slices or complete mosquitoes.
Many organisms have been imaged using Light Sheet
Fluorescence Microscopy (Table 1) and you may want to
read further specific papers to clarify sample preparation
issues.

White Paper<< Back to contents
4
Topic Subtopic Sample/Model Organism Technique/ LSFM
implementation
Reference
Physics Technical set up of MISERB
Structured illumination
Light Sheet Characteristics
Image formation
Image View fusion
Fluorescent beads
Mouse cochlea
Fluorescent beads
Caenorhabditis elegans
live sea urchin embryo, live
Danio rerio embryo
MISERB
sTSLIM
SPIM
DSLM
LSFM
Fahrback et al, 2010
Schroter et al, 2011
Ritter et al, 2008
Olarte et al, 2012
Rubio-Guivernau et al, 2012
Biochemistry Laser Microsurgery
Microtubule dynamic instability
mRNA nuclear export
Heterochromatin dynamics
Imaging of engineered gene
expression
In vitro microtubules
In vitro microtubules
Chironomus tentans Salivary
Glands
MCDK cells, Drosophila
melanogaster
Drosophila melanogaster
SPIM
SPIM
SPIM
LSFM (FCS)
SPIM
Engelbrecht et al, 2007
Keller et al, 2008
Siebrasse et al, 2012
Capoulade, 2011
Ejsmont et al, 2009
Microbiology Marine microbiology Various bacteria, protozoa etc. LSFM Fuchs et al, 2002
Cell biology Adaptive optics to improve
imaging performance
Intracellular imaging
Nuclear protein localisation
Imaging large living samples
Tumour spheroids
Mammalian cell organelles
Cellular spheroids
MCDK cell cysts
waoSPIM
Bessel beam plane illumination
SPIM
SPIM
Jorand et al, 2012
Planchon et al, 2011
Zanacchi et al, 2011
Verveer et al, 2007
Plant Biology Live imaging of root growth
Consecutive imaging of vertically
growing root
Arabidopsis thaliana
Arabidopsis thaliana
DSLM
SPIM
Maizel et al, 2011
Sena et al, 2011
Developmental Biology Imaging of developing organs
Embryogenesis visualisation
Zebrafish development
Cell identity lineaging and
neurodevelopmental imaging
Gene Expression: hour glass
model verification
Danio rerio heart valve
Drosophila embryo
Danio rerio
Caenorhabditis elegans
Drosophila melanogaster
SPIM
SPIM
mSPIM
iSPIM
SPIM
Scherz et al, 2008
Huisken et al, 2004
Kaufmann et al, 2012
Wu et al, 2011
Kalinka et al, 2010
Physiology Middle ear structure
3D reconstruction of inner ear
Brain in vivo imaging
3D reconstruction for
morphological analysis
Scan of whole brain
Neural network imaging
Sectioning of thick tissues
Imaging neuronal activity
Imaging of immunolabelled
receptors
Optical sectioning
Plastic Phantom Structure
Cavia porcellus
Microspheres
Bast‘s valve
Mouse brain
Mouse brain
Mouse cochlea/zebrafish inner
ear, brain/ rat brain
Mouse vomeronasal cells
Mouse
Meriones unguiculatus
cochlea, Hippocampus reidi
head, Xenopus laevis
(HR) OPFOS
OPFOS; LSFM
miniSPIM
OPFOS
LSFM
Ultramicroscope
TSLIM
OCPI
SPIM
OPFOS
Buytaert et al, 2007
Hofman et al, 2009
Engelbrecht et al, 2010
Hofman et al, 2007
Mertz and Kim, 2010
Dodt et al, 2007
Santi et al, 2009
Holekamp et al, 2008
Klohs et al, 2008
Buytaert et al, 2012
Large organism
general biology
Whole organism 3D reconstruction
Whole organism 3D reconstruction
Imaging copepod gut contents
Whole organism 3D reconstruction
Ormia ochracea;
Emblemasoma auditrix
Drosophila melanogaster
Calanus pacificus
Thermocyclops consimilis
LSP
Ultramicroscope
PLIF
LSFM
Huber et al, 2001
Jahrling et al, 2010
Jaffe et al, 2009
Boistel et al. 2011

White Paper<< Back to contents
5
2. SAMPLE MOUNTING FOR LSFM
• Lightsheet Z.1 is optimized for gel embedded samples.
The sample chamber must be filled with a watery solution
(refractive index of 1.33) at all times, to ensure optimal
image quality.
• Lightsheet Z.1 is not designed for the use with clearing
reagents. Lightsheet Z.1 is built for aqueous media. Also
the sample chamber and the system cavity for the sample
chamber and sample holder are not compatible with
aggressive chemicals.
2.1 The perfect Sample for LSFM
Prior to observing a sample using a microscope, a preparation
step is usually necessary. The sample properties and the
microscope characteristics provide guidelines and also
limitations to sample preparation and imaging. The classic
method of mounting an object for microscopy requires the
use of a slide and coverslip that in turn limits access to the
sample from one side (Fig. 1/A and B). The sample is not
touching the objective lens and the number of refractive
barriers is at least two (coverslip, mounting medium) and
can increase further if immersion oil is needed.
Fig. 1 Relations between the sample and the objective in microscopy. Samples are traditionally isolated from the objective by a glass coverslip (A and B)
limiting access to one side only. (C). In LSFM, the illumination is positioned at 90° compared to the detection axis and can be set up in a sideways geometry
(“horizontal microscope”).
The depth of the field of view is dependent on the type of
objective lens and the sample properties, and will deterio-
rate with the thickness of the sample. Light Sheet Fluores-
cence Microscopy (LSFM) utilizes illumination along an axis
that is perpendicular to the detection axis (Fig. 1/C). More-
over, it usually allows sample rotation to record multiple im-
age stacks that are acquired independently along different
directions. To allow the high level of sample mobility re-
quired for such Multiview imaging, the sample is inserted in
a sample holder from above. The sample holder is hanging
in and linked to an x, y, z, and alpha positioning motor
stage, allowing complete three dimensional translations and
free rotation around an axis parallel to gravity. This configu-
ration leaves the illumination and the detection paths com-
pletely open but requires the preparation of a sample that
can be held from above or below in a medium-filled chamber.
Such geometry goes hand in hand with the convenient use
of water dipping or air objective lenses. As mentioned
already in the introduction, another important aspect of
sample preparation is the transparency of the specimen,
especially when imaging large objects. Ideally, the light sheet
penetrates as deeply as possible into the sample. Any obstacle
or opaque medium will limit the light sheet penetration depth,
generating shadows that will be read out as artifacts on the
final image.

White Paper<< Back to contents
6
In LSFM, the sample is usually imaged in a water-based
buffer. Generally, it can be kept dry and imaged in air but
this has extensive limitations like diffraction due to the
significant jump in refractive index from air to the sample
material. This has several consequences for sample preparation.
First, the refractive index of the mounting medium should
be close to that of the sample buffer. The mounting medium
should not scatter the illumination or the detection light.
Second, the mounting medium should not dissolve in water.
Third, its diffusive properties should be close to those of
water/medium. Fourth, the medium should be non-toxic
for live samples. Fifth, the medium should be flexible to
allow the sample to develop. Finally, it should not change
its mechanical properties during a period of observation
(72 hrs and more).
The following part of this section will deal with sample as a
general term but we have devised them in four main classes
(Fig. 2) and you can check their size relationship (Fig. 2) and
keep that in mind as different samples of different sizes will
mean different sample preparation approaches and handling.
2.2 Holding the Sample
In LSFM, the detection axis is at 90° from the illumination
axis. There are two main approaches to design such an optical
configuration: horizontal or vertical, with respect to the detec-
tion axis. In both cases, the sample must be positioned at the
intersection between the two axes in order to be observed.
Lightsheet Z.1 is a horizontal LSFM implementation and so the
sample is presented from above, hanging along the gravitation
axis to be scanned through the light sheet in order to acquire
stacks of optical section images. Several possibilities exist to
hold the sample in such an optical configuration.
In a vertical configuration, the simplest way is to place the
sample on a slide or a cuvette filled with medium underneath
the objective (Dodt et al., 2007), alternatively the sample can
be embedded in a gel rod that can be rotated. In a horizontal
configuration, like Lightsheet Z.1, the sample can be either
embedded in a stiff gel (Fig. 3/A) (Huisken et al., 2004)
hooked and positioned in front of the objective (Fig. 3/B)
(Engelbrecht et al., 2007), placed in a container (Fig. 3/C)
(Engelbrecht et al., 2007, Kaufmann et al., 2012, Pampaloni
et al., 2007) or placed on a slide and positioned at a 45° angle
(Fig. 3/D). Alternatively, some investigators are using a system
presenting the sample from underneath for better stability
(Huber et al., 2001).
• Lightsheet Z.1 is not designed to support
mounting on a coverslip (Fig. 3/D).
Fig. 2 Different sample types. (A) very large objects (cm), (B) large objects (mm), (C) medium size samples (100 μm) and small samples (10 μm) (D).
Each is represented in relation to the following one to allow size comparison.

White Paper<< Back to contents
7
Every mounting technique has some advantages and disad-
vantages. Here, we would like to mention one important
parameter: the position of the sample relative to the objective
lens. Gel embedding (Fig. 3/A and E) is usually safe but the
capillary that holds the gel can potentially touch the detection
objective. Such collisions are even more likely for hook
(Fig. 3/B and F) and coverslip (Fig. 3/D and H) mountings.
It is important to remember that in Lightsheet Z.1 the sample
can interact with the detection lens as well as the walls of
the sample chamber and this could affect the imaging process.
One of the important advantages of the LSFM optics geometry
is that it allows so-called Multiview imaging. In this case the
sample must be mounted to support the required positioning.
One approach used in Lightsheet Z.1 to support this experi-
mental paradigm is to place the object in a gel rod that can
be rotated (Fig. 3/A and E) in front of the objective. The hy-
drogel cylinder must be sufficiently stable to avoid movement
during rotation. Typical preparation protocols use 0.8 % to
1.0 % agarose (see below in this section) to take this into
account. The following sections will address the four main
types of sample preparation that can be used: embedding,
hanging, enclosing or flattening.
Fig. 3 Sample positioning in LSFM. The sample can be held in front of the objective (A) embedded in gel, (B) by a clip, (C) in a container, or on a coverslip
(D; Note: ZEISS Lightsheet Z.1 is not designed to support this way of mounting the sample). (E to H) show an eye bird view of the mounting (A to D).

White Paper<< Back to contents
8
2.2.1 Embedded Samples
Embedding objects in plastic materials is a routine procedure
widely used in the preparation of samples for electron
microscopy. In the case of sample preparation for LSFM
however, the immobilization of hydrated biological materials
must not impair biological activity. It is necessary to keep
the object we wish to observe in a perfect condition.
In the case of LSFM, it is also necessary to contain the
sample in such a way that it can be positioned and rotated
in front of the objective. Furthermore, transparency of the
mounting medium is essential to allow imaging. A basic
technique of mounting objects for the LSFM is to shape
them into a cylinder of gel (for example agarose, see also
section 2.3.4 Gels and Polymers) that can then be mounted
on a dedicated holder. Lightsheet Z.1 package provides four
capillaries sizes adapted to the sample holder to embed
objects of various sizes. The special sample holder of
Lightsheet Z.1 adapts to hold these capillaries for precise
positioning (translation and rotation) of the cylinder-shaped
object for observation through the detection optics. The used
gel such as agarose behaves like mechanically stabilized water,
supporting the object. It can be easily molded and the gel
chosen should have an optical (refraction) index close to that
of water. The object can be any size, as the gel can be molded
accordingly (Fig. 4/A to D). The various gelling agents and
polymers that can be used are discussed in greater detail in
section 2.3 Materials and Equipment. The preparation of
embedded samples requires a container suitable for molding
the gel. The simplest approach is to use any cylinder with a
tight-fitting plunger to pump the molten gel into it and let it
polymerize inside before pushing it out. The cylinder can be
a syringe, a capillary or even a pipette.
In the case of Lightsheet Z.1, the Sample Starter Kit provides
four types of color coded capillaries and a specific sample
mounting device with color coded sleeves to fit each type of
capillary perfectly to the sample holder. Moreover, a syringe
sample holder is provided.
Above the preparation of a syringe for large sample and a
basic protocol for capillary mounting are discussed.
Fig. 4 Embedded samples. Large samples, such as an adult Drosophila melanogaster, can be embedded in a large gel tube or a cut 1 ml syringe (A),
intermediate size samples, such a Medaka or Zebrafish embryo can be prepared by using either a cut 1 ml plastic syringe (see also Fig. 5) or a glass
capillary (C and D) and small samples such as Drosophila melanogaster embryos or early stage cell clusters can be prepared using a smaller capillary (D).

White Paper<< Back to contents
9
An example of how to tailor a syringe for Lightsheet Z.1
sample preparation is illustrated in Fig. 5. The tip of the
syringe is cut off to create an even cylinder, and the gel
solution is pumped in using the plunger. The sample is
then positioned precisely within the gel.
• After the gel has polymerized, the plunger is used to
push out the specimen prior to imaging. Imaging is not
done through the syringe or capillary, since this would
impair the image quality due to the optical properties of
the material.
For smaller samples, a capillary can be used as a sample em-
bedding container. There are several commercial companies
that provide glass capillaries with specific Teflon plunger.
Lightsheet Z.1 sample preparation kit comes with four sizes
of capillaries and their specific plunger for this purpose.
• Make sure you use the right capillary for your sample.
The sample size should not be more than 2/3 of the final
agarose diameter and no less than 1/3. You should also
ensure that you use the right plunger for you particular
capillary. Finally, the Teflon plunger should be handled
carefully and checked regularly for integrity to avoid leaks
that will lead to sliding of the gel rod.
The important points to consider are that the materials used
do not interfere with the gel, the object to image or the
sample preparation (chemical compatibility, melting point,
transparency etc.), it must be easily prepared or easily
purchased, it must be compatible to the LSFM sample holder
as well as the x, y, z stage, it must fit to the sample chamber
and should not cause damage to the objective lens once
rotated or moved. It is also reasonable to consider reusable
sample holders to limit waste. We have found that home-
made sample embedding container using 1 ml syringes
(BD Biosciences, Braun, Terumo or your local laboratory plastic
ware supplier), 1 ml plastic pipettes (see your local laboratory
plastic ware supplier such as Falcon or VWR), and glass capillar-
ies (Brand, Sutter Instruments or check your local glassware
specialist, see also section 4.3 Suggested Additional Sources
of Information) (Fig. 6) are particularly effective. The plungers
usually come with the cylinders or can be made using metal
rods, plastic or metal wires of an appropriate diameter. Once
the sample embedding container is prepared, the sample
preparation can begin. The first step consists of preparing the
supporting agent at a suitable concentration and temperature.
The gelling agent is usually a 0,7 to 1 % solution of low
melting agarose in water or PBS, depending of the sample to
be embedded (fixed, living, sensitivity to osmotic pressure
etc.). If the sample needs to be maintained in a drop of solution
or contains water or buffer it is advisable to use a higher con-
centration of agarose to obtain a final concentration of 1%
once the sample is embedded. The use of low melting-point
agarose is recommended (Roth, n° 6351.1) as its melting
temperature is only approx. 60 °C and it can be maintained
liquid at just above 37 °C prior to embedding.
There are two principal methods of embedding an object.
The first is to directly mix the object with the agarose then
Fig. 5 Preparing a sample embedding container with a syringe. Many tubular objects can be used to make embedded samples. A simple technique is il-
lustrated here. The tip of a syringe is cut away (A, B), agarose can then be easily pumped in using the plunger (C), and the sample can be positioned within the
agarose tube (D). After polymerization the sample can be pushed out of the syringe for imaging (E).

White Paper<< Back to contents
10
pump it into the sample embedding cylinder. This is a conve-
nient way of embedding very small objects such as pollen
grains (Swoger et al., 2007); yeast (Taxis et al., 2006) or cell
clusters (Pampaloni et al., 2007) or even large objects like
fish embryos. The action of pumping in the sample with the
agarose results in a self-alignment of the specimen within
the tube (Fig. 7/A, E and F).
The second method is to fill the sample embedding container
with the gelling agent, then to place the object within the gel
using a needle or forceps (Fig. 7/A, B, C and D). This approach
is more suitable for those samples that cannot be easily aligned
using the first technique.
In some cases, it may still be challenging to align the specimen
in the most suitable way for imaging. The orientation of the
sample must then be optimized, so that interesting details are
facing the surface of the agarose cylinder with as little material
as possible in the optical path. One solution is to fill a syringe
with agarose and allow it to cool until it solidifies. The agarose
is then pushed out of the syringe (Fig. 8/A and B). A small
V-shaped groove can be cut into the gel and the sample then
positioned in the V-groove.
The gel can be cut into various shapes depending on the
needs (cylinder, hole). Afterwards the gel with the specimen
is pulled back into the syringe and is covered with more molten
agarose. The agarose is allowed to cool and solidify, this
time period can be shortened by cooling the whole sample.
For example the housing of the sample can be rinsed with
cold water, although care must be taken to ensure that the
polymer does not come into contact with the water, other-
wise the cooling agarose would become diluted and lose its
stability necessary for holding the sample. After polymerization,
the sample is ready for imaging.
Fig. 6 Preparing a sample embedding container with a capillary. A glass capillary can be used. For ZEISS Lightsheet Z.1 they come from the manufacturer
(Brand) in just the right length (A). Other capillaries can be cut to an appropriate length (B), the agarose with the object can be easily pumped in using the
suitable plungers with Teflon tips (C). If no such plunger is available it can be made, for example, from a piece of electrical wire (D). To avoid leakage, such a
plunger can be sealed with nail polish (E) once the sample is pushed out. The sample can then be imaged (F).

White Paper<< Back to contents
11
Fig. 7 Basic principles of sample embedding. A cylinder with a suitable plunger is used as a mounting device (A). The 1 % low melting-point agarose is
melted, then brought to 37 °C, then pumped into the cylinder. (B) The object is then introduced to the agarose with a needle or forceps. (C) Once solidified,
the embedded sample can be pushed out and imaged (D). Alternatively, the object, devoid of water, or other solution, is added to a solution of 1 % low
melting-point agarose at just above gelling temperature (typically 40 °C) and sucked into the cylinder (E) and then allowed to polymerize. The embedded
sample can then be pushed out and imaged (F).
Fig. 8 Aligning an embedded sample. The sample can be aligned in a particular orientation to allow the details of interest to be close to the outer surface of
the agarose. The solidified agarose is pushed out the syringe a few millimeters and a small v-groove is cut into the cylinder to take up the sample (A).
The sample is placed into the v-groove (B). The sample on the agarose is pulled back into the syringe and more agarose is added (C). After the cylinder has
completely solidified the sample is pushed out of the syringe allowing free sight on to the sample (D). The same approach can be used to carve a central tunnel
in the middle of the agarose to align the sample along the agarose tube axis.
White Paper<< Back to contents
12
2.2.2 Hanging Samples
• Lightsheet Z.1 is optimized for gel embedding
samples The sample chamber must be filled with a
watery solution (refractive index of 1.33) at all times,
to ensure optimal image quality.
• This mounting technique can also be used, but will
require some initial adaptations to the sample holder.
An intuitive way of imaging an object is to simply take it as
it is and place it in front of an objective. In an LSFM, this can
be done by hanging the object in front of the objectiv where
the axis of rotation and gravity are parallel. This can be
achieved using a simple hook made of glass, stainless steel
or plastic (Fig. 9/A). This mounting technique can be used
for large samples such as organs (for example the brain) or
complete organisms (insect, fish). One main drawback is the
fact that the hook will partially damage the object and may
also interfere with the field of view.
• Lightsheet Z.1 has a maximum Field of View of approx.
2.5 mm (depending on zoom settings). This and the
dimensions of the sample chamber might limit the size
of the sample.
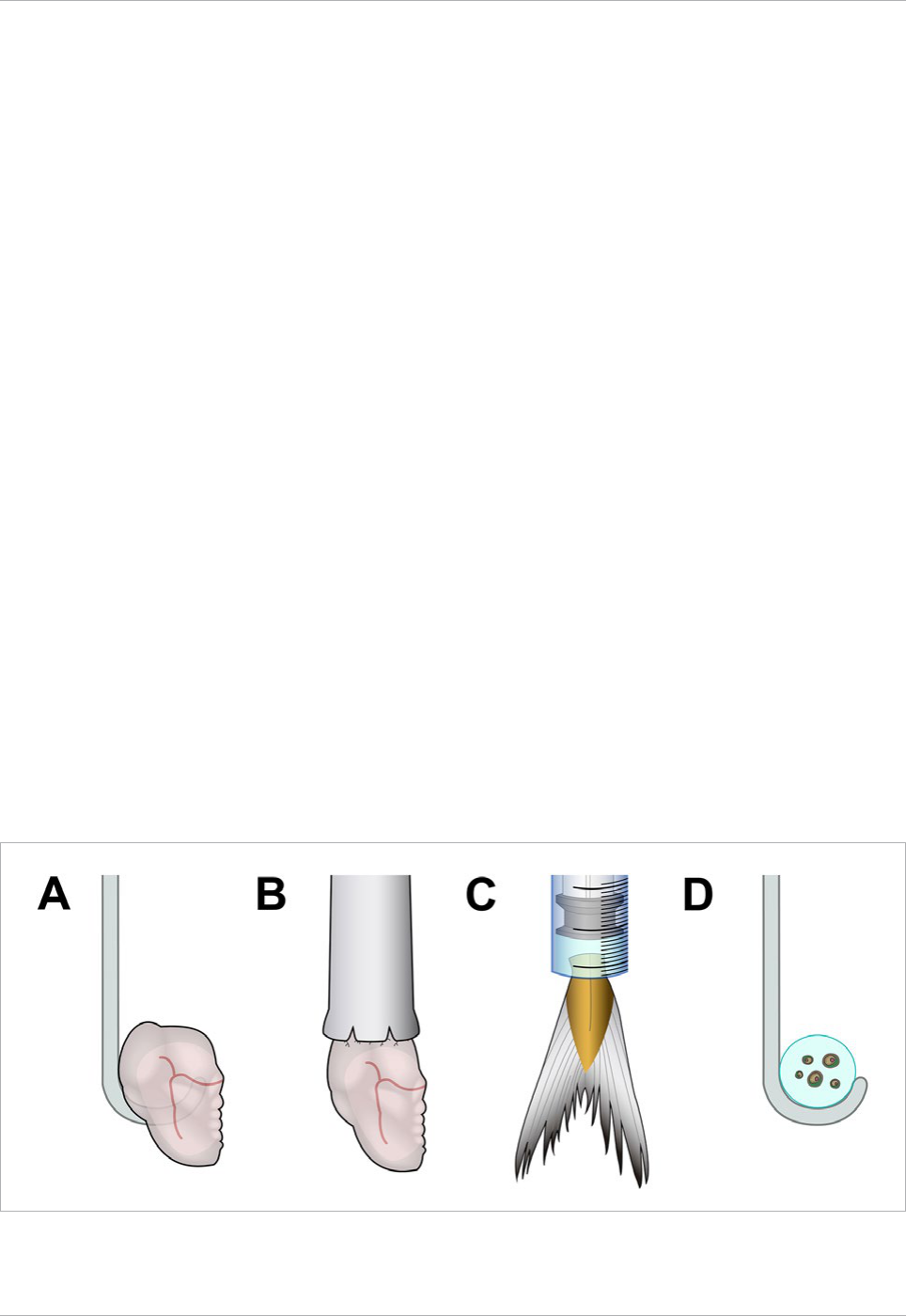
Fig. 9 Different ways of hanging a sample. Samples can be either hooked or deposited on a bent glass capillary (A, D), glued to a rod or capillary (B),
or clamped on a syringe tip using the plunger and the syringe body as a holder (C).
Interestingly, such a hook can also be tailored to mount
small objects embedded in agarose. The drop of agarose is
more stable as it is closely held by the hook. This is very
important when imaging at very high magnification (100x).
The hanging method has been successfully used for imaging
single Saccharomyces cerevisae (Taxis et al., 2006) and holding
fish fins during laser nanosurgery (Engelbrecht et al., 2007).

White Paper<< Back to contents
13
2.2.3 Enclosed Samples
• Lightsheet Z.1 is optimized for gel embedding
samples The sample chamber must be filled with a
watery solution (refractive index of 1.33) at all times,
to ensure optimal image quality.
• This mounting technique can also be used, but will
require some initial adaptations to the sample holder.
The last important technique of holding samples to be men-
tioned in this section is to create a container that can hold
the object in front of the objective lens. This technique is
particularly suitable for specimens that should not be
embedded (for example due to temperature, physical
constraints etc.) or that need to be constantly maintained
in a specific buffer (for example in vitro assays, or living
cells). The container must be suitable for LSFM imaging.
It must be basically transparent and be suitable for the
object but also for the imaging chamber and the sample
Fig. 10 Enclosed chambers for LSFM. Incubation chambers can be made by molding an agarose beaker that can be mounted on a simple plastic holder and
loaded with the sample prior to imaging (A). Another solution is to create a chamber with a specific polymer with a refractive index close to water (for example
PFTE or FPE) using heat or glue to seal the chamber to the needed size and volume, and attach it to a suitable holder(syringe, capillary etc…) (B). A PDMS,
FPE tube or glass chamber can also be considered (C).
holder. It can be hooked or clipped using specific holders.
There are two main methods of generating such containers,
using gelling agent to shape out a container (Fig. 10/A) or
using polymers such as PTFE (Polytetrafluorethylen, Teflon)
or FPE (Fluorinated Ethylene Propylene) to make it (Fig. 10/B
and C).
The container can be easily molded using a gelling agent specifi-
cally chosen for its stiffness and transparency. The custom-made
molding system is made from a syringe where the plunger has
been modified to hold a cylinder of smaller diameter. This system
allows tailoring of the size of the container wall and is easy to
use for molding (Fig. 11/A). The plunger is pulled into the syringe
body and filled with molten gelling agent. The lunger is further
pulled to create the bottom part of the container (Fig. 11/B and
C). Alternatively, the system can be used to generate a hollow
tube that can be subsequently sealed (Fig. 11/D). The gelling
agent is left to polymerize and then the tube is removed from
the modified plunger. The containers can be used directly or
Fig. 11 Making and mounting an agarose incubation chamber. A modified syringe plunger is made by inserting a smaller diameter cylinder on to the
plunger (A, B). The tube is molded by simply pouring the molten agent into this device (C). Once removed, the open end of the container (D) can be closed with
agarose (E).

White Paper<< Back to contents
14
kept in a water-based buffer for later use. The gelling agent
must be transparent, and although the use of agarose is pos-
sible, the concentration will depend on the size of the container
walls and the inside chamber. It is recommended to use a high-
er concentration of gelling agent to ensure the stability of the
container. We have used a 1ml syringe as a molding system and
a concentration of 1.5 % agarose for the container molding.
The stability is good and the degradation of the optical path
is minimal. Higher agarose concentrations may generate aber-
rations. Another possibility is to use a polymer to make the
chamber.
The polymer, similar to the gelling agent, must be transparent
or at least have an optical index as close as possible to water
or the buffer used during the experiment. The polymer is usu-
ally used as a sheet that can be formed as required. The other
possibility is to approach commercial manufacturers to make
polymer chambers at the specific sizes and lengths required.
Fusing polymer sheets can be done using a welding iron with
controlled temperature or a welding device use for melting
together plastic bags. As described in Fig. 12, the polymer foil
is folded to an appropriate size. This can be made easier by
using a guide or template, in this case a micropipette.
The polymer is fused together. The tube generated is finally
fused together on the other side to make a complete container.
The polymer chamber can be easily mounted on the LSFM by
using a clip, a slotted metal capillary or glued to a micropi-
pette. However, the last two options have the disadvantage
of partially obscuring the field of view.
This technique has been successfully used to image living cells
(Engelbrecht et al., 2007) and cell clusters (Pampaloni et al.,
2007).
Fig. 12 Making and mounting an incubation polymer foil chamber. A piece of polymer foil (A) is folded and either heat- or glue-sealed to generate a
tube of a predefined size (B). Excess foil can be removed or used to glue the tube on to a specific holder (capillary,thread, metal rod…) (D). One side of the tube
can be then glued or heat-sealed to close the chamber (C). The polymer used must be suitable for microscopy and easy to seal. The chamber can be glued to a
support, held by forceps, or inserted into a slit rod.

White Paper<< Back to contents
15
2.2.4 FEP Tubing
More recently, the availability of Fluorinated Ethylene Propylene
(FEP) tube of different diameters has been successfully used for
long term imaging of Zebrafish embryos (Kaufman et al., 2012).
Here, we refer only to the paper by Anna Kaufmann, Michaela
Mickoleit, Michael Weber and Jan Huisken in Development 139,
3242-3247 (2012) (“Multilayer mounting enables long-term im-
aging of zebrafish development in a light sheet microscope”) and
emphasize the fact that the mounting method described in this
article is fully compatible with Lightsheet Z.1.
2.3 Materials and Equipment
This section gives an overview on the materials and equipment
for sample mounting and the sample chambers of Lightsheet
Z.1. The generalization of the concept is also mentioned.
2.3.1 Sample Chambers
In Lightsheet Z.1, the sample is positioned within a hamber
containing an aqueous solution. This chamber is tailored
with O-rings to tightly fit the detection optics and avoid
leakage. The upper part of the chamber is open to allow
introduction of the sample. The bottom can be equipped
with a Peltier Block or a Heatingblock (optional incubation).
The remaining three sides are made in such a way that glass
coverslips can be fixed allowing entrance of the light sheets
from two sides and observation of the object by the user
during the different steps of imaging using the appropriate
software feature. The original chamber is made of medical
steel, however, depending on the buffer used (salt, pH etc.),
the experiment being performed (time lapse, live cell imaging
etc.), there might be a need for more specific chambers.
Carl Zeiss Microscopy provides the technical drawing of the
sample chamber for Lightsheet Z.1 so that users can develop
their specific sample chamber
1
.
You can refer to the sample chamber section of this manual
for further information.When designing a chamber for your
particular application you must take into account the following
points:
Transparency: user visual access, light sheet entry and exit
routes, the distance of the coverslips for the light sheet –
and the water filled space in between – are a crucial measure
in the optics calculation of Lightsheet Z.1 system. To ensure
the functionality of the system these have to be maintained
when a custom made chamber is designed.
• Temperature control: heating devices, cooling devices
• Volume: size of the sample, buffer used (cost), drug
treatment (cost)…
• Fitting: objective, illumination position, stage, heaters…
• Material: buffer, heater, sterilization, UV protection…
• Flow: flow entry and exit
• Size
• Cost
1
Carl Zeiss Microscopy GmbH (hereinafter “we”) hereby informs you that we will warrant the specified and agreed performance of Lightsheet Z.1 system only if sample
chambers are applied and used that either are delivered or explicitly approved by us.
The sample chamber design has been optimized to ensure the most established applications of Light Sheet Fluorescence Microscopy. Exceptional applications may
require a slightly modified sample chamber design. In order to enable customized modifications of the existing sample chamber we also provide the corresponding
CAD file and a technical drawing. We explicitly advise you that already minor deviations of the dimensions and tolerances specified in these documents will cause a
significant loss of image quality and can potentially result in a liquid leakage. Therefore, you will not hold us or one of our affiliates liable for any damages caused
by the employment of self-built or third-party-built sample chambers, the use of such self-built or third-party-built sample chambers will be solely on your own risk.
Furthermore we want to inform you, that we will not render any assistance relating to the production and application of such self-built or third-party-built sample
chambers.

White Paper<< Back to contents
16
1. Front system cavity door
2. Upper sample opening
3. Upper system cavity door
4. Securing screw
5. Sample chamber grip
6. Sample chamber
7. Guide rails/sample chamber mount
8. Connections for incubation
9. Hose and syringe
Fig. 13 Removing and inserting the sample chamber. The chamber has five entry points allowing the positioning of the objective, the sample holder, the light
sheet and the observation by the user. Heated chamber. The chamber can be equipped (optional) with a Peltier Block that can be tuned according to needs or a
Heatingblock. For further details on the sample chamber handling, accessories, its cleaning and assembly please read the corresponding chapters of this manual.
2.3.2 Molding and Mounting Supports
As described previously, there are several options to prepare a
sample and therefore several options to manipulate and mount
it. Initially, readily available products found in cell biology
laboratories: syringes, capillaries or pipettes were used to
mount samples. These components are all commercially avai-
lable, cheap and convenient for LSFM sample preparation.
However, they still need to be prepared for the specific needs.
Plastic syringes exist in various sizes (0.2 ml, 0.3 ml, 0.5 ml,
or 1 ml) and have tight plungers that easily allow pumping
and movement of the agarose rod used to embed the sample.
They can also be used to hang the sample by effectively
using the plunger and syringe body as forceps. The sample
holder disc for syringes (Fig. 14/I and K) provided should be
used in this case. Moreover, they can be purchased sterile for
single use applications.
In the case of Lightsheet Z.1, the sample kit is provided with
four types of color coded capillaries with matching plungers
(Fig. 14/A and B) and color coded sleeves to fit perfectly
each type of capillary to the sample holder (Fig. 14/C).
The typical protocol of sample mounting is a two-step process
of choosing carefully your sample mounting system based
on your sample (size, agarose/sample ratio…) then to as-
semble it (e.g. plunger+tip+capillary) beforehand. Prepare it
(e.g. sample + agarose) and insert it in the upper sample
opening in Lightsheet Z.1 (Fig. 14/G and H).
White Paper<< Back to contents
17
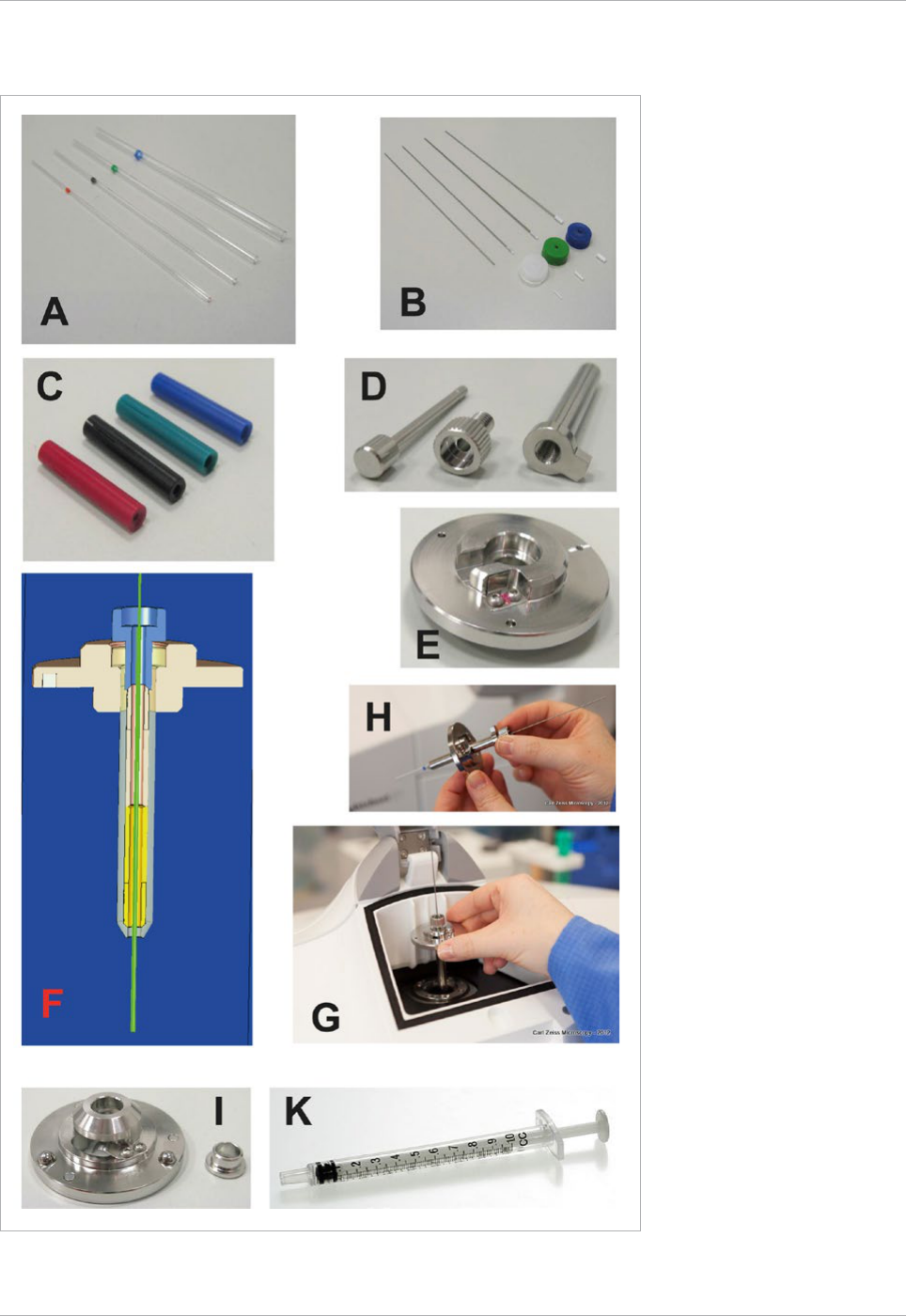
Fig. 14 Sample mounting accessories
as part of the sample chamber and
holder starter kit.
A. Capillaries (inner diameter of capillary
size 1 / ~0.68 mm, size 2 / ~1 mm,
size 3 / ~1.5 mm, size 4 / ~2.15 mm)
B. Specific plungers and Teflon tips for
each capillary.
C. Specific color coded sleeves to adapt
each capillary to the sample holder (F).
D. Sample holder stem for capillaries,
clamp screw, ejection tool.
E. Sample holder disc for capillaries
F. Sample Holder diagram showing the
capillary, the stem and disc of the
sample holder.
G + H. Sample holder handling and
insertion in Lightsheet Z.1.
I. Sample holder disc for syringes,
adapter ring.
K. Syringe (1 ml).

White Paper<< Back to contents
18
A few points must be taken into account when choosing a
particular mount:
• Compatibility. This is a crucial issue. The mount must be
compatible with the object you want to image (chemistry,
temperature etc.), but it must also be compatible with the
stage holder.
• Stability (mechanical, optical, chemical).
• Tightness. In the case of embedded samples, once the
• gel has solidified, the cylinder of gelling agent is pushed
through the capillary out of the distal end by a plunger
fitting into the capillary. The system must be air tight to
avoid air entry leading to a displacement of the gel rod.
The plunger can be sealed with a drop of wax, acrylamide
or nail polish, i.e. anything that prevents the plunger and
hence the agarose containing the sample from moving.
• Cost.
2.3.3 Sample Holder
Once the specimen is prepared and properly labeled, it is ready
to be imaged. While in conventional imaging there is a suitable
platform on which to place the glass slide or the chamber, in
LSFM the object must be held from above via the sample hold-
er. Depending on the size of the sample there are two different
types of sample holders available: sample holder for capillaries
and syringes (Fig. 14/C-F and I). Always use the minimal cylinder
diameter necessary for your specimen size to avoid excessive
amounts of agarose. The largest sample holder has been de-
signed to accommodate a 1ml syringe that can be inserted
from the top with a plunger that can be operated once the
sample holder is mounted on the stage. Once inserted, the
syringe is perfectly fitted to the sample holder as the two flaps
used for injection fit the upper part of the holder. In this way
the object support is well maintained, an essential issue for
imaging and multiview imaging as the object is moved through
the light sheet by the stage. Capillaries have been extensively
used to image small embedded objects, as hooks for very large
objects, and as support for enclosed objects, so the capillary
has become commonly used for LSFM sample embedding.
Capillaries are made of glass. They can break. They
will slide when wet. Please handle them with care and
dispose of them properly.
2.3.4 Gels and Polymers
Gelling agents are commonly used for preparing semi-solid
or solid tissue culture media. Gels provide support to tissues
growing in static conditions. The gelling agent usually has
several properties. In particular, it does not react with media
constituents, is not digested by enzymes, and remains stable
at all incubation temperatures. Gelling agents are very versatile
and useful tools in LSFM as they allow easier sample prepa-
ration. This section will present in more detail the properties,
advantages and disadvantages of two well-described gelling
agents and provides an additional list of gelling agents.
Agarose
Agarose is a complex carbohydrate polymer material, generally
extracted from seaweed. It is used in chromatography and
electrophoresis as a medium through which a substance can
be analyzed by separating it into its components. The mole-
cules are extremely water-soluble due to their large number of
hydroxy groups, and solutions tend to be low-melting point
aqueous gels. A wide range of different agaroses, of varying
molecular weights and properties are commercially available.
These include low melting types, (for example, Agarose Type
VII, low melting temperature: gelling temperature below 30 °C,
melting temperature above 65 °C) which can be used if the
sample is sensitive to high temperatures. Interestingly, the re-
fractive index of the low melting type is lower than that of
normal agarose. However, to obtain the same strength, a
higher concentration needs to be used. With a concentration
of 1 % (w/w) the low melting point agarose has the same
stability as a 0.5 % agarose (normal). The refractive index at
this concentration is still lower than that of normal agarose,
minimizing distortions when imaging. In our laboratory,
we preferentially work with agarose as it is easy to handle,
has good optical properties and is not expensive.
Gelrite
Gelrite gellan gum is a self-gelling hydrocolloid that forms
rigid, brittle, transparent gels in the presence of soluble salts.
Chemically, it is a polysaccharide comprised of uronic acid,
rhamnose, and glucose. It is produced by the bacterial strain
S-60 of Pseudomonas elodea. Gelrite is a trademark of Merck
and Co, Inc (Rahway, NJ), Kelco Division, USA. One advantage
of Gelrite is the lower scattering of light compared to an aga-
rose gel with the same stability. It has a higher index of refrac-
tion but less scattering compared to agarose. Gelrite has a
!

White Paper<< Back to contents
19
consistent batch-to-batch quality due to a stringent control of
the fermentation process. Only half the amount of Gelrite is
required for the same purpose. It hydrates rapidly and gel set-
ting can be easily controlled. The stability of the gel depends
on the concentration of divalent cations (Mg2+, Ca2+) there-
fore a gel made with Gelrite and pure water is unstable com-
pared to a PBS- (buffer) based gel. Polymerisation is faster com-
pared to agarose, which might be advantageous for some
applications. The temperatures for gelling and remelting are
similar to that of agarose. Additional list of gelling agents:
• Galactan
• Agar
• Gelatin
• Carrageenan
• Alginate
• Phytagel™
• Agargel™
• Transfergel™
2.3.5 Hydrogel Preparation
Every gelling agent is prepared following specific protocols
that vary widely from supplier to supplier, and from labora-
tory to laboratory depending on the final application.
We will not try to cover every single one of these but rather
give a simple protocol that we have been using in our labo-
ratory to prepare embedded samples in agarose as a gelling
agent. The preparation is done as follows:
1) Preparing a 1 % low melting agarose gel.
Weigh 1 gram of low melting point (“low gelling”) agarose
(“Agarose Low Melt” (no 6351.1 from http://www.carlroth.
com) and place it in a flask. Add 100 ml of solvent (water,
PBS) to the flask. Swirl to mix the solution. Place the flask in
the microwave. Heat above 95 °C until the solution is com-
pletely clear and no small floating particles are visible. Do not
allow the agarose to boil over as this will affect the final aga-
rose concentration. Swirl the flask frequently to mix the solu-
tion, prevent the agarose from burning, and prevent boiling
retardation.
Wear heat-protective gloves when handling the flask.
The agarose can be also sterilized for sterile use
(cell culture). It is also possible to remove dissolved
air bubbles using a vacuum pump.
2) Cooling the gel
Once molten the gel is left to cool to 37 °C (or just above
gelling temp - read the material properties sheet) in a water
bath or on a heating plate. It is very important, especially for
sensitive samples, to ensure that the agarose is at 37 °C
before use.
Note: Alternatively, you can aliquot your agarose solution
into 1 ml or 2 ml Eppendorf tubes for later use. Label them
and store them in a cool and dry place. In this case, each
aliquot can be liquefied using a heating block (80 °C – 90 °C)
then transferred to a heating block at 37 °C.
3) Using the gel
At this stage follow the examples described in the section
dealing with embedded sample preparation. Avoid bubble
formation during handling and pipetting as they will impair
the embedding process. Work quickly as the low melting
point agarose will polymerize rapidly as it was kept at 37 °C
close to the gelling temperature.
4) Polymerization of the gel
Let the gel polymerize. Avoid contact with any water-based
solution as it will dilute the gelling agent solution. The process
of polymerization can be accelerated by cooling down the
embedded sample (cold water, fridge…) – but keep in mind
that this might affect viability of a living sample.
5) Using the prepared sample
Once fully polymerized, the embedded specimen can be
manipulated, but keep in mind that it is a gel and therefore
fragile. Avoid any kind of friction, or shock etc. As it is a
water-based object it must be kept wet at all times to avoid
drying out and damaging the sample. Moreover, many types
of gel may change their properties over time (e.g. swelling),
and this can result in loosening of the gel in the support.
It is therefore important to use your sample as soon as
possible and monitor its quality over time if you plan to
reuse it.
!

White Paper<< Back to contents
20
2.4 Fixation and Fixatives
Many experimental samples will require fixation prior to imag-
ing. The goal of fixation is to maintain cellular structure as
close as possible to the native state. Proper fixation typically
facilitates immunohistochemical analyses if desired, and is an
important step prior to further processing. Specialized fixation
procedures and processing may be required for certain tissues
(e.g. bone de-calcification) or preserving specific target anti-
gens. The processing of most samples begins with fixation to
preserve morphology. A fixation method must take into
account two things: the preservation of cellular 3-D structure
and maintenance of good access to antigenic sites. The goal
is to preserve sufficient cellular organization to allow identifi-
cation of the features of interest, but not to destroy the anti-
genicity of the target. Fixation is also frequently combined
with permeabilization to allow the staining solutions used in
later steps access to the cellular interior. Commonly used
histological methods of fixation and permeabilization often
consist of treating the cells with solvents, such as methanol.
While these methods are rapid-acting precipitating fixatives,
they are also good permeabilizing agents, but have one
significant negative consequence: cellular shrinkage. The de-
gree of shrinkage may be almost insignificant for monolayers
of cells, but will distort tissue samples dramatically. To take full
advantage of the three-dimensional reconstruction capability
of the LSFM microscope, the use of a fixative that does not
destroy in vivo structure and organization is imperative.
It is important to remember that different specimens may
require different fixation methods. Testing and optimizing
for each new sample type will ensure that the best balance
between preservation and labeling is obtained. Fixing and
permeabilizing your cells affects the cell morphology and the
availability of the antigen you are trying to detect. You may
get different results with different reagents, times and
concentrations, hence the need for protocol optimization.
The distortion of cell morphology is something to bear in
mind when interpreting the images.
2.5 Stains and Staining
In LSFM, like in any microscopy technique using fluorescence,
the sample can be labeled using specific fluorescent dyes,
fluorescent proteins or fluorescently coupled antibodies.
Two basic techniques are generally used: direct labeling and
indirect labeling. Both labeling methods are suitable for
LSFM microscopy. Direct labeling consists of using fluorescent
proteins, fluorescently labeled primary antibody or a dye that
cause the structure of interest to become fluorescent.
Advantages of this method include speed and ease of appli-
cation. A potential disadvantage is lack of sensitivity (low
signal intensity). The indirect method involves binding a
primary antibody to the epitope of interest, followed by a
fluorescently labeled secondary antibody. The main advan-
tage of using this technique is the great amplification of
signal possible through an antibody cascade. The disadvan-
tages include increased complexity, the method is more time
consuming, and there are often problems with non-specific
antibody reactions.
2.5.1 Choosing a Fluorescent Label
The choice of label depends upon the available equipment
on your LSFM set-up (lasers, filters) and the availability of
certain fluorescent protein variants, fluorochromes conjugated
to required antibodies for use in multiple labeling schemes.
In general, the laser lines available dictate which fluoro-
phores or fluorescent proteins can be used. Recent advances
in biochemistry have created new families of fluorophores
with very favorable signal-to-noise and quantum efficiency
(QE) properties. Similarly, many laboratories have developed
a wide variety of fluorescent proteins that span the spectra
from GFP
2
to Plum.
2
Flood P.M., Kelly R., Gutiérrez-Heredia L. and E.G. Reynaud
School of Biology and Environmental Science, University College Dublin, Belfield, Dublin 4, Dublin, Ireland

White Paper<< Back to contents
21
2.6 Antifading Agents
Fluorescently labeled cells and tissues exhibit a characteristic
photobleaching curve in response to excitation by the light.
Much of the photobleaching can be attributed to the gen-
eration of free radicals. The use of free radical scavengers
has been shown to decrease the rate of photobleaching.
Common scavengers include n-propyl gallate, p-phenylene-
diamine and DABCO (1,4-diazobicyclo-(2,2,2)-octane).
Live systems have been reported to reduce photobleaching
in the presence of vitamin C or Trolox. As the LSFM technol-
ogy reduces greatly the phototoxicity and photobleaching
effects during imaging, we never encounter samples that
require the use of antifading agents so far. However, some
applications may require the use of radical scavengers during
long time imaging of GFP expressing samples as repeated
exposure may lead to a regular increase of the free radical
contents, which might affect its behavior over time.
2.7 Cleaning, Labelling and Storing Samples
One important point about samples is their handling. In the
case of LSFM, all the samples are three dimensional objects
that are mounted to be imaged in a chamber containing
water based medium. They must then be maintained in a
moist environment. Once prepared and prior to imaging,
the samples can be held in a filled beaker or Falcon tube
filled with the appropriate medium, e.g. water, PBS (Fig. 15).
One simple solution we have developed in the laboratory is
to use a beaker filled with the right buffer. The samples are
maintained by using plasticine on the beaker border.
An alternative is to cover the top of the beaker with an
aluminum foil and accommodate the sample holders such as
the 1 ml syringe by drilling a hole in the foil. This handling
technique limits evaporation. More advanced holders can be
designed and manufactured according to need. You will find
a couple of examples that we have made in our laboratory2
to handle various size of sample embedding containers.
They include a water tank that keeps the samples moisture
at all time. They are stable and can be easily move from the
laboratory to the microscope as well as stored in the fridge.
As in LSFM there is no need for oil or any specific chemical
for imaging, the cleaning of samples is not necessary. How-
ever, you can rinse the sample within the capillary or syringe
with water or your specific buffer after imaging if the chamber
was containing particles, bacteria or other chemicals (dyes,
drugs etc.).
Labeling the samples can be an issue as it can be tricky to
mark the name of every sample on the embedding container
(capillary, syringe…). A simple marking technique is to use
tape roll around the syringe plunger or the capillary. This
must not affect the handling of the sample on the microscope.
Fig. 15 Supports for sample embedding containers. Support are used to hold three dimensional objects that cannot be held flat easily. Moreover,
embedded samples need to be kept in buffer to avoid gel shrinkage or sample damage. A simple system is to use a beaker filled with PBS and place plasticine on
the upper border to support the sample embedding container (A). More elaborate supports can be made using clips of different sizes for holding syringes (B) or
even capillaries (C).

White Paper<< Back to contents
22
Another approach is to number the sample and to register
the detail on a lab book. However, this can be a problem if
you store many sets of samples in the same fridge day after
day.
Sample preparation techniques usually allow long time storage
(paraffin embedding, slides…). As long as a few basic rules
are followed (keeping away from light, temperature…) they
can be kept up to years. In the case of LSFM, the samples are
imaged in a water environment and must be always kept wet,
even for long time storage. This can be a challenge. Usually,
we keep fixed samples in the fridge using a sample embedding
container support and we refill the buffer tank from time to
time. However, we never kept samples for more than a month
under such conditions. A longer storage possibility is to use a
water tight container where the samples are kept with enough
water not to dry out. One point to consider is the way the
sample was prepared. Embedded samples may weaken with
time as some gels may not maintain their strength over time
at 4 °C. Hooked samples may as well be loosening and fall
from their support. It may be better to unhook them and store
them in a different type of container.
3 SPECIFIC EXAMPLES OF SAMPLE PREPARATION
In order to make this sample preparation section as useful as
possible the following pages describe mounting techniques
for specific samples, in particular describing the equipment
needed, step by step protocols and illustrations based on our
own laboratory
3
experiences.
• Fluorescent beads are used for later Landmark Registration
processing of acquired Multiview data, and should be
included during embedding of samples of interest.
Prepare agarose as described in section 3.1 Preparation
of Fluorescent Beads accordingly.
3.1 Preparation of Fluorescent Beads
Samples with fluorescent beads are often used to characterize
the imaging properties of a microscope such as the LSFM. Using
a reproducible sample is an important tool to calibrate the instru-
ment. This protocol describes how to handle fluorescent beads
and to prepare optimal concentrations to image with an LSFM.
Equipment and reagents:
• Fluorescent beads
• 1 % Low Melting Point (LMP) Agarose in deionised water
• Capillary (Size 4, Blue, #701910, BRAND GmbH)
• Sonicator
• Heating block- Vortex
Method
1. Vortex the bead solution to make a homogeneous dispersion.
2. Dilute a small volume of the bead dispersion in deionized
or distilled water to a concentration 100x higher than
the one desired for the specimen. Depending on the size
of the beads and the magnification required it is first
necessary to calculate the bead-agarose ratio (see below).
3. Sonicate the dilution for 5 minutes at maximum power.
4. Prepare a liquid agarose solution of a chosen concentra-
tion (0.5 % - 1 %) and cool it down to just above the
gelling point (usually 38-40 °C).
5. Mix diluted fluorescent beads with the agarose in ratio
1:100 and vortex the mixture. Use a pipette or a capillary
(by sucking in and out the liquid agarose several times)
to mix the bead solution and the agarose thoroughly.
6. Insert an appropriate plunger and Teflon tip.
7. Push the plunger through the capillary, so the front end
of the plunger is sticking out of the capillary by a bit
before entering the liquid agarose and sucking the
agarose in. This will avoid air bubble formation at the
plunger.
8. Suck in the agarose/beads by pulling the opposite end of
the wire/plunger.
9. Let the gel polymerize (approx. 5 minutes) before imag-
ing.
10. Make sure that only a very short part of the agarose
lock is pulled out of the glass capillary during image
acquisition.
11. When multiple views are recorded, it is best to image
from the centre of the agarose block.
Beads should be fluorescent in the part of the spectrum
you would like to analyze for 1 channel systems.
With Lightsheet Z.1, a two channel system, one can use one
channel for the beads (e.g., red) and one channel for the
specimen label (e.g, GFP.) Fluorescent beads covering whole
3
Flood P.M., Kelly R., Gutiérrez-Heredia L. and E.G. Reynaud
School of Biology and Environmental Science, University College Dublin, Belfield, Dublin 4, Dublin, Ireland

White Paper<< Back to contents
23
visible spectrum are nowadays easily available from various
different suppliers (e.g. Polysciences, Invitrogen, Estapor/
Merck etc.). In our case, the density of the fluorescent beads
is chosen to end up with several hundred beads in the imaged
volume. For example, for a 40x magnification lens the volume
of interest is around (200 μm)
3
= 8*10
-6
ml. If the fluorescent
beads are shipped as a solution of 5*10
13
particles/ml, you
have to dilute them 1:10
6
in agarose to have approximately
400 particles in the volume of interest. Having too few of
them (less than 100) in the three-dimensional image will give
you no or poor processing results, while too many of them
(more than 1000) might considerably increase processing time
without a significantly improving the final results.
Moreover, a gel with sufficient stiffness but minimal impact
on the image has to be used to immobilize the beads. 1% low-
melting agarose (Sigma, Type VII) is being routinely applied
for lenses with numerical apertures up to 0.8 NA. For 1.0 NA
objective lenses and above, a more diluted (e.g. 0.5 %) gel
must be used to minimize gel-caused image aberration.
3.2 Preparation of a Medaka Fish Embryo
(Oryza latypes)
Embryos have been extensively used for many decades to
study developmental mechanisms as well as diseases. They
can range from micrometers to centimeters depending on
the species used (frog, fish, fly, worm, etc.). This protocol
applies to most embryos. The important point is the temper-
ature. The embryo must not be damaged by temperature
shock during embedding. Moreover, the embryo should not
be constraint by the stiffness of the gel. This may impair its
normal development.
Equipment and reagents
• 1.5 % Low Melting Point (LMP) Agarose in E3 (Fish buffer)
• Mesab/Tricaine 0.4 % stock (3-Aminobenzoic Acid Ethyl Ester)
• Capillary (Size 4, Blue, #701910, BRAND GmbH)
• Electrical thread (1,6 mm) or plunger
• Heating block
Method
1. Select embryos for imaging, dechorionate. Melt 1.5 %
LMP agarose, aliquot 0.5 ml into a 1.5 ml Eppendorf
tube. Add 150 ml of Mesab to ensure that the embryos
do not move during imaging. Invert the tube to mix and
allow agarose to cool to 40º C.
2. Add the embryo to the tube containing agarose using a
Pasteur pipette, transferring as little buffer as possible.
• Add the embryo to the Eppendorf tube as a drop on
the tube wall. If necessary remove the extra buffer
with a yellow tip before dropping the embryo into
the agarose. Or transfer the embryo to an empty
Eppendorf tube, remove all medium and add the
liquid agarose.
3. Let the embryo fall to bottom of the Eppendorf tube.
Insert a capillary into the tube and suck the embryo into
it by pulling out the thread or plunger like a syringe piston.
• When sucking up the agarose, make sure that ini-
tially the plunger is sticking out of the capillary with-
in the liquid agarose, to avoid air bubble formation.
Furthermore leave some space between the plunger
and the sample (see Fig. 16/D)
4. Allow the agarose to harden and place the capillary in a
stand in water or PBS.
5. Mount on the capillary sample holder prior to imaging.
Fig. 16 Mounting an Oryza latypes embryo. (A) The embryo is prepared (labelling, drug treatment, dissected...) (B) The embryo is deposited on the side of the Eppen-
dorf tube and the excess of water is removed with a pipette. (C) The embryo is dropped into the agarose and pumped into the capillary. (D) The embryo can be imaged.

White Paper<< Back to contents
24
3.3 Preparation of a Fly Pupa (Drosophila melanogaster)
Some type of embryos are hydrophobic once dissected and
cannot be mounted using the technique described above as
they will float on the agarose and will be impossible to embed.
The following protocol is suitable for this type of embryo
such as fly embryos or pupas.
Equipment and reagents
• Drosophila melanogaster pupa or embryo
• 1 % Low Melting Point (LMP) Agarose in water or PBS
• Capillary (Size 4, Blue, #701910, BRAND GmbH)
• Heating block (90 °C and 40 °C)
Method (see Figure 17)
1. Choose a pupa Drosophila melanogaster. Melt 1 % LMP
agarose, aliquot 0.5 ml into a 1.5 ml Eppendorf tube.
Invert the tube to mix and allow agarose to cool to 40º C.
2. To allow sample preparation the pupa must be sub-
merged in agarose by pouring it directly on top of it in
a large drop of molten low melting point agarose.
3. The pupa can then be pumped into a capillary as previ-
ously described.
4. The insect can then be imaged.
3.4 Preparation of a Plant Root (Arabidopsis thaliana)
Plant research is an important field of investigation using
plants as model systems. They are threedimensional objects
that are difficult to image fully and are usually dissected and
sliced before being imaged and analyzed. This protocol has
been used to mount complete young Arabidopsis thaliana
plants for imaging root development directly on the micro-
scope.
Equipment and reagents
• 1 % Low Melting Point (LMP) agarose in plant buffer
• 1 ml syringes
• Arabidopsis thaliana seeds
• Heating block
Method (see Figure 18)
1. Several agarose beakers are prepared as described in
the enclosed sample section.
2. Instead of pushing out the plunger to extract the aga-
rose beaker, the plunger is pulled in to the end of the
syringe where it can be released leaving the beaker
inside the syringe.
• You need to make a long walled beaker to avoid
inconvenient breakages and leakages that may be
caused by the following steps.
3. A seed of Arabidopsis thaliana is put at the bottom of
the beaker.
4. The beakers are kept in the syringe in a humidified and
well lit chamber to allow seed germination.
5. Once the root is visible within the bottom part of the
beaker, a normal plunger is inserted in the top part of the
syringe, where the open part of the beaker is present.
6. Push down the beaker into the syringe until the root
can be seen outside of the syringe cylinder
7. The beaker is mounted in the sample chamber filled
with distilled water or plant growth media at room
temperature.
• As the plant depends on light to grow long-term
imaging must take into account the illumination of
the leaves between imaging sessions.

White Paper<< Back to contents
25
3.5 Imaging Cell Cysts in an Extracellular Matrix Gel
Live imaging of cells has been a major tool in cell biology.
For this, cells must be maintained in optimal conditions during
the complete time of the experiment. The incubation options
for the Lighsheet Z.1 are described in another section of this
manual (CHAPTER 1 HARDWARE). However, cells must be
mounted in a way that allows them to hang in front of the
objective from above. This protocol describes one way of
imaging MDCK cells that naturally form cysts when grown in
an extracellular matrix.
Equipment and reagents
• MDCK cells grown in an extracellular matrix (Matrigel,
ExtraCell etc.)
• 1.5 % Low Melting Point (LMP) Agarose in PBS
• Modified plunger
• Sealing device
• Slitted capillary
• 1 ml syringe
• Capillary holder
• Heating block
• Polytetrafluorethylene foil or FPE tube
Method
1. An agarose beaker or a polymer foil chamber is prepared
as previously described in the section 2.2.3 Enclosed
Samples.
2. MDCK cysts are grown in an extracellular matrix gel.
3. Cells can be stained at this stage with live markers
(nuclear, mitochondrial, lysosomal etc.) before mounting.
Fig. 17 Mounting a Drosophila pupa. (A) The pupa is prepared (labeling, drug treatment, dissected...) (B) The pupa is deposited in a watch glass and
covered by melted agarose to embed the hydrophobic pupa. (C) The pupa is then pumped into the capillary. (D) The pupa can be imaged.
Fig. 18 Mounting an Arabidopsis thaliana root. (A) An Arabidopsis thaliana seed is positioned at the bottom of an agarose cylinder (B).
After a few days of development, the root can be seen in the agarose cylinder bottom. (C) The agarose cylinder is pushed out of the syringe for imaging.

White Paper<< Back to contents
26
4. Cells within the gel are transferred into the chosen
chamber (agarose, polymer) and mounted in a 37 °C
and CO2 chamber on the LSFM using a cut tip to limit
shearing damage.
• If cells are grown in a different manner it is possible
to mix them with a supporting gel prior to loading
into a chamber. They can also be grown within the
gel already present in an incubation chamber. This
limits damage, shear and temperature changes dur-
ing sample preparation and handling.
5. The agarose incubation chamber is mounted on a specific
holder. The polymer chamber can be either clipped or
glued to a supportive holder.
• Eukaryotic cells are highly sensitive to environmental
change (temperature, pH, osmotic pressure etc.).
The transfer steps must be rapid and carried out in a
sterile manner (wherever possible) especially for
long term time lapse experiments. It is important to
be gentle and use cut tips and pre-warmed materials
at all times, including the sample chamber.
6. Monitor the cell status during imaging to check viability
and changes.
3.6 Immunostaining and Preparation of MDCK Cell Cysts
Immunofluorescence allows highlighting of specific proteins
or structures using specific antibodies. This protocol is used
to perform immunofluorescence on cysts which are three-
dimensional cell structures that can be grown in extracellular
matrix gel such as collagen.
Equipment and reagents
• 1.5 % Low Melting Point (LMP) agarose in water or PBS
• Capillary (Size 4, Blue, #701999, BRAND GmbH)
• Electrical thread (1.6 mm) or plunger
• 4 % paraformaldehyde solution
• Antibodies (primary and secondary)
• PBS
• Triton X-100
• Bovine Serum Albumin (BSA) or Foetal Calf Serum (FCS)
• Heating block
Method
1. MDCK cell cysts grown in extracellular matrix are collected
and centrifuged at 500-1000g to pellet the cysts with the
gel.
2. The supernatant is removed and replaced with 4 % para-
formaldehyde and incubated for 15 minutes on a wheel
or rocker to efficiently mix the gel pellet within the fixative.
3. The gel is pelleted and the supernatant is replaced by
0.1M glycine to quench the paraformaldehyde, and then
incubated for 10 minutes.
4. The gel pellet is washed twice with PBS (500-1000 g,
5 minutes).
5. The pelleted cysts are permeabilized with PBS/1 % Triton
X-100 for 10 minutes on a wheel orrocker to efficiently
mix the gel pellet.
6. The gel pellet is washed twice with PBS (500-1000 g,
5 minutes).
7. The gel is incubated for 10 minutes in PBS/1 % FCS on
a wheel or a rocker to block the extra epitopes and
efficiently mix the gel pellet.
8. The gel pellet is incubated with the primary antibodies
at the concentration indicated by the supplier, using a
wheel or rocker to efficiently mix the gel pellet.
9. The gel pellet is washed twice with PBS (500-1000g,
5 minutes).
10. The gel pellet is incubated with the secondary antibodies
at the concentration indicated by the supplier,
using a wheel or a rocker to efficiently mix the gel pellet.
11. The gel pellet is washed twice with PBS (500-1000 rpm, 5
minutes).
12. The cysts can be stained at this stage with Hoechst to la-
bel the nuclei.
13. The gel is pelleted and as much of the supernatant as
possible is removed.
14. The gel pellet is mixed with low melting point
agarose, mixed and pumped into a capillary.
• The extracellular gel tends to clump once fixed and
may stay as one piece when mounting. Care should
be taken to quickly but efficiently resuspend the gel.
15. Allow the agarose to polymerize.
16. Fill the chamber with PBS prior to introduce the sample
for imaging.

White Paper<< Back to contents
27
3.7 Preparation of a Whole Mount of a Mosquito
(Anopheles gambiae)
Adult insects are very large objects that can be up to 5 cm
long. Moreover, they have an exoskeleton made of chitin
which is hydrophobic and autofluorescent. This characteristic
allows imaging without labeling by simply using the chitin
autofluorescence to image the insect surface. This protocol
applies to every type of adult insects as well as for various
type of plankton.
Equipment and reagents:
• An adult Anopheles gambiae
• 1.5 % Low Melting Point (LMP) agarose in water or PBS
• 1 ml syringe (BD Biosciences)
• Ethanol (70 %)
• Glycerol (50 %)
• Sucrose
• Heating block
Method
1. Choose an adult Anopheles gambiae and immobilize
it by cold treatment. Melt 1.5 % LMP agarose, aliquot
0.5 ml into a 1.5 ml Eppendorf tube. Invert the tube to
mix and allow agarose to cool to 40º C.
2. To avoid bubble formation on the insect surface that will
affect imaging, the insect must be treated either with
ethanol 70 % (animal death) or using 50 % glycerol or
1 M sucrose to cover the hydrophobic chitin surface prior
to embedding (Fig. 19/B).
3. The syringe is prepared as previously described and filled
with molten low melting point agarose (40 °C).
4. The insect can then be inserted into the agarose cylinder
and aligned using a needle or forceps (Fig. 19/C).
5. The insect can then be imaged (Fig. 19/D).
This technique can be applied to any insect or similar type of
organism possessing an exoskeleton. Depending on the animal
part to be observed the insect can be aligned differently or
dissected prior to embedding (head, wings, guts, salivary
glands…).
Fig. 19 Mounting a complete adult Anopheles gambiae. (A) The insect is paralyzed. (B) The chitin surface is treated using 70 % ethanol. (C) The insect is
positioned within the melted agarose. (D) The mounted insect is ready for imaging.

White Paper<< Back to contents
28
4 TIPS, TROUBLESHOOTING AND
ADDITIONAL INFORMATION
4.1 Tips
Protocols
As a general rule, follow the protocol carefully and try it out
well in advance as you may encounter difficulties and missing
parts (chemicals…) that may hinder you to use the sample
straight away. The protocols presented in this section are not
the ultimate solution for every problem. They can be improved
and adapted to your need. If you encounter a problem, check
the scientific literature and the other protocols presented and
see if you can find a tip that you may apply to solve your prob-
lem. Be creative.
Safety
Please refer to the safety instructions provided with
Lightsheet Z.1.
Setup Requirements and Maintenance
For optimal performance of Lightsheet Z.1, please check the
Setup Requirements information delivered with the system.
For information about maintenance and cleaning any part of
Lightsheet Z.1, including the optics, please refer to section
2.7 Cleaning, Labelling and Storing Samples.
Refractive index mismatch
Light is refracted when it crosses the interface between two
media of differing refractive indices (RI). Mismatching the
refractive index of the objective immersion medium and
mounting medium is one of the main causes of image degra-
dation in microscopy. Refractive index mismatch results in
stretching/compression of the z-axis. Also, spherical aberration
is worsened by axial spreading of the point-spread function
(PSF) resulting in reduced axial resolution. This phenomenon
is exacerbated with depth and with a high numerical aperture
objective, serious problems arise when imaging deeper than
10 μm into an aqueous sample. The mounting medium and
the immersion medium should be matched. It is not a major
issue in LSFM but this as to be considered when filling the
imaging chamber in regard to the sample preparation tech-
nique, especially for embedding as gelling agents are used.
4.2 Troubleshooting
Common problems
LSFM is a fluorescence microscopy technique so many trouble-
shooting guidelines from other microscopy techniques apply
here as well. Do not hesitate to ask experts in the field, check
the literature, as well as internet resources that may provide
you with a more detailed solution to the problem you have
encountered. Also check with your Lightsheet Z.1 application
specialist from ZEISS for FAQs and tips for troubleshooting.
Sample image is unclear, blurred or has insufficient
contrast.
• This can be a simple optical problem: objectives or filters
are dirty. Clean them accordingly. You can also check
that all the components are well in place and aligned.
• Your light sheet might not be properly aligned. For
Lightsheet Z.1, please realign the light sheet using the
Light sheet auto-adjust function of the ZEN software.
• You could be imaging through an additional layer of
material: glass, plastic... that belong to the mounting
support and not the sample itself. Please, check the
sample position or move it around to see if another angle
solve the problem as a piece of glue, additional agarose
or part of the mounting material is having a blurring
effect.
• In the case of sample embedding it may occur that the
gelling media is of low quality or badly polymerized.
This leads to an uneven polymerization of the media that
modify the optical path. Try again.
• The light sheet comes from the side and any obstacle
modifies its quality. Check the illumination axis for any
obstacle (capillary, objects…).
• Check if the medium level in the sample chamber has
dropped below the imaging level. The specimen has to
be fully immersed for good image quality.
Sample image is partially obscured or unevenly
illuminated.
• This can be a simple optical problem: objectives or filters
are dirty. Clean them accordingly. You can also check
that all the components are well in place and aligned.
• Your light sheet might not be properly aligned. For
Lightsheet Z.1, please realign the light sheet using the
Light sheet auto-adjust function of the ZEN software.

White Paper<< Back to contents
29
• The light sheet comes from the side and any obstacle
modifies its quality. Check the illumination axis for any
obstacle (capillary, objects…).
Sample signal is weak.
• Human eyes have trouble quickly adjusting to the dark
and it will be hard to discern a very dim fluorescent speci-
men immediately after darkening the room. You may
want to check your sample using another microscope or
a stereomicroscope equipped with a fluorescent lamp.
• It could be an optical problem, e.g. extra filter in the
optical path, misaligned illumination or dying laser. You
can also increase excitation energy (laser). However, the
risk of bleaching and signal saturation will increase.
• In the case of immunofluorescence, you should increase
antibody concentration or incubation time but this might
in turn increase nonspecific background signal.
• In the case of GFP signal, the expression level might be
too low or you have photobleached or damaged the GFP
signal during sample preparation (fixation, ethanol treat-
ment, excessive illumination during dissection...).
High background signal within the sample.
• In the case of immunofluorescence, you should decrease
antibody concentration or incubation time
• but this may decrease the overall signal. You can also use
blocking steps during the immunofluorescence (eg. BSA,
FCS…). If preparing tissue section, you should increase
the stringency of the washing steps.
• The imaging chamber may be dirty as well as the media
inside. This contamination affects the quality of the light
sheet and scattering occurs.
• You can apply deconvolution to your stacks afterwards.
The sample is moving.
• If you are imaging live samples, it may be simply due to
life. The sample is moving so you may want to increase
the anesthetic concentration or the agarose concentra-
tion to restrain any movement.
• The mounting is unstable. This occurs if the chosen
material is not properly maintained (bad tweezers,
leaking plunger, badly polymerized agarose…).
For embedded sample preparation, you can improve the
stability by limiting the amount of agarose emerging out
of the syringe or the capillary, as the longer is the agarose
tube the more unstable it will be. You can also tighten
the plunger by sealing it with nail polish to avoid air leak-
age that will lead to gliding of the agarose tube.
• The system table might not be a float. Check that it is
connected to the pneumatic supply and the air-dampen-
ing is active.
• The cables are not properly secured with the cable holders
at the system table.
• Other instruments that produce vibrations, not completely
dampened by the system table, are in close proximity
(e.g. fridges, centrifuges, etc.).
• The stage is not properly fitted or damaged and prone to
vibrations. This includes the sample holder and the imag-
ing chamber support.
Optical aberrations
• As in any optical technique, the LSFM has advantages
and disadvantages. Some optical aberrations are more
generals and can be dealt with easily. Do not hesitate to
ask experts in the field, check the literature as well as
Internet resources that may provide a more detailed
solution to the problem you have encountered.
• A few optical aberrations are, however, typical for LSFM,
as the optical axis are at a 90 degrees angles. Lines and
stripes occur as any objects blocking the light sheet will
reduce the light intensity leading to a discrepancy along
the illuminated plane. This is often a problem with big
samples, highly scattering samples or sample which have
absorbing structures at the surface of the specimen vol-
ume. Rotating the sample to give a better path for the
light sheet should be considered first. Dual Side illumina-
tion and/or Pivot scanning of the light sheet can often
eliminate these effects (available in Lightsheet Z.1)
Second, the sample could be orientated differently
during mounting or partially dissected to limit obstacles.
The concentration of the objects can also be a problem
especially with samples with optical properties (beads,
tubes, glass capillaries…). For example, if you image
large number of cell clusters, you may have a few of
them in the light sheet path. By reducing the amount
of objects, you will automatically reduce the lines and
stripes.
• The use of image processing methods such as deconvo-
lution may help to get rid of those aberrations.

White Paper<< Back to contents
30
4.3 Suggested Additional Sources of Information
Chemicals
• Agarose:
− Molecular biology grade, for routine use, SIGMA,
Ref. A-9539
− Type VII, low gelling temperature, SIGMA,
Ref. A-4018-50G
− “Agarose Low Melt” (no 6351.1) from Carl Roth
www.carlroth.com (from the US please contact
Brunschwig Chemie B.V., Amsterdam, NL, e-Mail:
• Gelrite Gellan Gum, SIGMA, Ref. G-1910
• Glycerine anhydrous, AppliChem, Ref. A3552,1000
• Nail Polish, any cosmetic shop near you
• PBS, local supplier
• Distilled water, local supplier
• Ethanol, local supplier
• Companies:
− Sigma- Aldrich (http://www.sigmaaldrich.com/)
− Applichem (http://www.applichem.de/)
− MP BIomedicals (http://www.mpbio.com/)
− Merck KGa (http://www.merck.de/)
Materials
•Capillaries
− 100 μl, color code Blue, Brand GmbH, Ref. 7087 45
− 200 μl, color code Red, Brand GmbH, Ref. 7087 57
•Companies:
− Brand GmbH (http://www.brand.de/en/home/)
− SpectraGlass (http://www.spectraglass.com/)
− Harvard Apparatus (http://www.harvardapparatus.com/)
•Syringes
− 1 ml, BD Plastipak, BD Biosciences, Ref.300013
− 0.5 ml, BD Microfine Insulin U100 Syringe, 29 g,
Ref. PLA257L
− 0.3 ml, BD Microfine Insulin U100 Syringe, 30 g,
Ref. PLA470U
− Braun Omnifix F Solo 1 ml Syringe (PZN 0569881,
Ref. 61706)
− Terumo 1 ml Syringe (Ref. BS-01T)
Pipettes
− 2 ml serological pipette, FALCON, BD Labware,
Ref.35 7507
− 1 ml serological pipette, FALCON, BD Labware,
Ref.35 7521
Equipment
For the following, please refer to your local lab supply
companies:
• Heating blocks
• Stereomicroscope
• Scalpels
• Tweezers
• Dissection Needles
• Watch glass
• Sonicator

White Paper<< Back to contents
31
4.4 References and Further Reading
• Buytaert, J.A.N. et al., 2011. The OPFOS Microscopy Family: High-Resolution Optical Sectioning of Biomedical Specimens.
Anatomy research international, 2012.
• Capoulade, J. et al., 2011. Quantitative fluorescence imaging of protein diffusion and interaction in living cells.
Nature biotechnology, 29(9), pp.835–839.
• Dodt, H.U. et al., 2007. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain.
Nature methods, 4(4), pp.331–336.
• Ejsmont, R.K. et al., 2009. A toolkit for high-throughput, cross-species gene engineering in Drosophila. Nature methods.
• Engelbrecht, C.J. et al., 2007. Three-dimensional laser microsurgery in light-sheet based microscopy (SPIM).
Optics Express, 15(10), pp.6420–6430.
• Engelbrecht, C.J., Voigt, F. & Helmchen, F., 2010. Miniaturized selective plane illumination microscopy for high-contrast
in vivo fluorescence imaging. Optics letters, 35(9), pp.1413–1415.
• Fahrbach, F.O. & Rohrbach, A., 2010. A line scanned light-sheet microscope with phase shaped selfreconstructing beams.
Optics Express, 18(23), pp.24229–24244.
• Fuchs, E. et al., 2002. Thin laser light sheet microscope for microbial oceanography. Optics Express, 10(2), p.145.
• Hama H, Kurokawa H, Kawano H, Ando R, Shimogori T, Noda H, Fukami K, Sakaue-Sawano A, Miyawaki A. 2011.
Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci.
14(11):1481-8.
• Hofman, R. et al., 2008. Morphology and function of Bast’s valve: additional insight in its functioning using
D-reconstruction. European Archives of Oto-Rhino-Laryngology, 265(2), pp.153–157.
• Hofman, R., Segenhout, J. & Wit, H., 2009. Three-dimensional reconstruction of the guinea pig inner ear, comparison of
OPFOS and light microscopy, applications of 3D reconstruction. Journal of microscopy, 233(2), pp.251–257.
• Holekamp, T.F., Turaga, D. & Holy, T.E., 2008. Fast three-dimensional fluorescence imaging of activity in neural populations
by objective-coupled planar illumination microscopy. Neuron, 57(5), pp.661–672.
• Huber, D., Keller, M. & Robert, D., 2001. 3D light scanning macrography. Journal of microscopy, 203(2), pp.208–213.
• Huisken, J. et al., 2004. Optical sectioning deep inside live embryos by selective plane illumination microscopy.
Science, 305(5686), pp.1007–1009.
• Jährling, N. et al., 2010. Three-dimensional reconstruction and segmentation of intact Drosophila by ultramicroscopy.
Frontiers in systems neuroscience, 4.

White Paper<< Back to contents
32
• Kalinka, A.T. et al., 2010. Gene expression divergence recapitulates the developmental hourglass model.
Nature, 468(7325), pp.811–814.
• Karaköylü, E.M. et al., 2009. Copepod feeding quantified by planar laser imaging of gut fluorescence.
Limnology and Oceanography: Methods, 7, pp.33–41.
• Kaufmann, A. et al., 2012. Multilayer mounting enables long-term imaging of zebrafish development in a light sheet
microscope. Development, 139(17), pp.3242–3247.
• Keller, P.J. et al., 2008. Three-Dimensional Microtubule Behavior in Xenopus Egg Extracts Reveals Four Dynamic States
and State-Dependent Elastic Properties. Biophysical journal, 95(3), pp.1474–1486.
• Lorenzo, C. et al., 2011. Live cell division dynamics monitoring in 3D large spheroid tumor models using light sheet microscopy.
Cell Division, 6(1), p.22.
• Maizel, A. et al., 2011. High-resolution live imaging of plant growth in near physiological bright conditions using light sheet
fluorescence microscopy. The Plant Journal, 68(2), pp.377–385.
• Mertz, J. & Kim, J., 2010. Scanning light-sheet microscopy in the whole mouse brain with HiLo background rejection.
Journal of biomedical optics, 15(1).
• Olarte, O.E. et al., 2012. Image formation by linear and nonlinear digital scanned light-sheet fluorescence microscopy
with Gaussian and Bessel beam profiles. Biomedical Optics Express, 3(7), pp.1492–1505.
• Pampaloni, F., Reynaud, E.G. & Stelzer, E.H.K., 2007. The third dimension bridges the gap between cell culture and
live tissue. Nature reviews Molecular cell biology, 8(10), pp.839–845.
• Ritter, J.G. et al., 2008. High-contrast single-particle tracking by selective focal plane illumination microscopy.
Optics express, 16(10), pp.7142–7152.
• Rubio-Guivernau, J.L. et al., 2012. Wavelet-based image fusion in multi-view three-dimensional microscopy.
Bioinformatics, 28(2), pp.238–245.
• Santi, P.A. et al., 2009. Thin-sheet laser imaging microscopy for optical sectioning of thick tissues.
Biotechniques, 46(4), p.287.
• Scherz, P.J. et al., 2008. High-speed imaging of developing heart valves reveals interplay of morphogenesis and function.
Development, 135(6), pp.1179–1187.
• Schröter, T.J. et al., 2012. Scanning thin-sheet laser imaging microscopy (sTSLIM) with structured illumination and
HiLo background rejection. Biomedical Optics Express, 3(1), pp.170–177.

White Paper<< Back to contents
33
21
15
20
22
15
16
18
18
19
6
22
23
24
24
8
12
13
21
20
5 Index
A
Antifading Agents
F
FEP Tubing
Fixation and Fixatives
L
LSFM
Sample Mounting
M
Materials and Equipment
Sample Chambers
Molding and Mounting Supports
Sample Holder
Gels and Polymers
Hydrogel Preparation
S
Sample
Holding
Sample Preparation
Preparation of Fluorescent Beads
Preparation of a Medaka Fish Embryo
Preparation of a Fly Pupa
Preparation of a Plant Root
Samples
Embedded
Hanging
Enclosed
Cleaning, Labelling, and Storing
Stains and Staining

