
1199
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
A systematic literature review and meta-analyses (where
appropriate) were performed and the GRADE approach was
used to update the previous American Academy of Sleep
Medicine Practice Parameters on the treatment of intrinsic
circadian rhythm sleep-wake disorders. Available data
allowed for positive endorsement (at a second-tier degree of
con dence) of strategically timed melatonin (for the treatment
of DSWPD, blind adults with N24SWD, and children/
adolescents with ISWRD and comorbid neurological disorders),
and light therapy with or without accompanying behavioral
interventions (adults with ASWPD, children/adolescents with
DSWPD, and elderly with dementia). Recommendations
against the use of melatonin and discrete sleep-promoting
medications are provided for demented elderly patients, at a
second- and rst-tier degree of con dence, respectively. No
recommendations were provided for remaining treatments/
populations, due to either insuf cient or absent data. Areas
where further research is needed are discussed.
Keywords: circadian rhythms, DSWPD, ASWPD, N24SWD,
ISWRD
Citation: Auger RR, Burgess HJ, Emens JS, Deriy LV,
Thomas SM, Sharkey KM. Clinical practice guideline for the
treatment of intrinsic circadian rhythm sleep-wake disorders:
advanced sleep-wake phase disorder (ASWPD), delayed
sleep-wake phase disorder (DSWPD), non-24-hour sleep-
wake rhythm disorder (N24SWD), and irregular sleep-wake
rhythm disorder (ISWRD). An update for 2015. J Clin Sleep
Med 2015;11(10):1199 –1236 .
pii: jc-00358-15
http://dx.doi.org/10.5664/jcsm.5100
SUMMARY
Purpose
The present document replaces/updates the previous Ameri-
can Academy of Sleep Medicine (AASM) Practice Parameters
pertaining to the intrinsic CRSWDs (i.e., ASWPD, DSWPD,
N24SWD, and ISWRD). The treatment of remaining CRSWDs
is not addressed.
Methodology
The AASM commissioned a Task Force (TF) of 4 members
with expertise in the eld of CRSWDs, appointed a Board of
Directors (BOD) liaison, and assigned a Science and Research
Department staff member to manage the project. PICO (Pa-
tient, Population or Problem, Intervention, Comparison, and
Outcomes) questions were developed by the TF and approved
by the BOD. Extensive literature searches were performed to
identify articles of interest, and relevant data were extracted by
Clinical Practice Guideline for the Treatment of Intrinsic
Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-
Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase
Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder
(N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD).
An Update for 2015
An American Academy of Sleep Medicine Clinical Practice Guideline
R. Robert Auger, MD
1
; Helen J. Burgess, PhD
2
; Jonathan S. Emens, MD
3
; Ludmila V. Deriy, PhD
4
; Sherene M. Thomas, PhD
4
;
Katherine M. Sharkey, MD, PhD
5
1
Mayo Center for Sleep Medicine, Rochester, MN;
2
Rush University Medical Center, Chicago, IL;
3
Portland VA Medical Center,
Portland, OR;
4
American Academy of Sleep Medicine, Darien, IL;
5
Brown University, Providence, RI
the TF. The TF developed consensus-based relevant outcomes,
rated their relative importance, and determined clinical signi -
cance thresholds. Extracted data were pooled across studies
for each outcome measure in accordance with PICO questions,
and based upon CRSWD diagnosis, study design, patient pop-
ulation, outcome of interest, and method of derivation. Statis-
tical analyses were performed using dedicated software, and
meta-analyses were completed when applicable. The GRADE
(Grading of Recommendations Assessment, Development,
and Evaluation) approach was used to develop recommenda-
tion statements and to determine the direction and strengths of
these recommendations based upon a composite assessment of
evidence quality, bene ts versus harms analyses, and patient
values and preferences.
Findings
Available data allowed for positive endorsement (at a sec-
ond-tier degree of con dence) of strategically timed melatonin
(for the treatment of DSWPD, blind adults with N24SWD, and
S P E C I A L A RT I C L E S
1200
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
children/adolescents with ISWRD and comorbid neurologi-
cal disorders), and light therapy with or without accompany-
ing behavioral interventions (adults with ASWPD, children/
adolescents with DSWPD, and elderly with dementia and
ISWRD). Recommendations against the use of melatonin and
discrete sleep-promoting medications are provided for de-
mented elderly patients, at a second- and rst-tier degree of
condence, respectively. No recommendations were provided
for remaining treatments/populations, due to either insufcient
or absent data.
Recommendations are as Follows
ASWPD
5.1.4a The TF suggests that clinicians treat adult ASWPD
patients with evening light therapy (versus no treatment).
[WEAK FOR]
DSWPD
5.2.6.1a The TF suggests that clinicians treat DSWPD in
adults with and without depression with strategically timed
melatonin (versus no treatment). [WEAK FOR]
5.2.6.2.1a The TF suggests that clinicians treat children
and adolescents with DSWPD (and no comorbidities) with
strategically timed melatonin (versus no treatment). [WEAK
FOR]
5.2.6.2.2a The TF suggests that clinicians treat children
and adolescents with DSWPD comorbid with psychiatric
conditions with strategically timed melatonin (versus no
treatment). [WEAK FOR]
5.2.9.2a The TF suggests that clinicians treat children
and adolescents with DSWPD with post-awakening light
therapy in conjunction with behavioral treatments (versus no
treatment). [WEAK FOR]
N24SWD
5.3.6.1a The TF suggests that clinicians use strategically
timed melatonin for the treatment of N24SWD in blind adults
(versus no treatment). [WEAK FOR]
ISWRD
5.4.4a The TF suggests that clinicians treat ISWRD in
elderly patients with dementia with light therapy (versus no
treatment). [WEAK FOR]
5.4.5a The TF recommends that clinicians avoid the use
of sleep-promoting medications to treat demented elderly
patients with ISWRD (versus no treatment). [STRONG
AGAINST]
5.4.6.1a The TF suggests that clinicians avoid the use of
melatonin as a treatment for ISWRD in older people with
dementia (versus no treatment). [WEAK AGAINST]
5.4.6.2a The TF suggests that clinicians use strategically
timed melatonin as a treatment for ISWRD in children/
adolescents with neurologic disorders (versus no treatment).
[WEAK FOR]
5.4.9.1a The TF suggests that clinicians avoid the use
of combined treatments consisting of light therapy in
combination with melatonin in demented, elderly patients
with ISWRD (versus no treatment). [WEAK AGAINST]
Conclusion
Use of the GRADE system for this updated Clinical Prac-
tice Guideline represents a major change. This update should
provide clinicians with heightened condence with respect to
prescribing select treatments and, equally importantly, should
serve as a roadmap for future studies that will propel higher
qualit y, more sophisticated therapies for the i nt rinsic CRSWDs.

1201
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
1.0 INTRODUCTION
The American Academy of Sleep Medicine (AASM) pro-
duced the rst Practice Parameters (and associated reviews) for
the evaluation and treatment of circadian rhythm sleep-wake
disorders (CRSWDs) in 2007.
1–3
The purpose of the present
publication is to provide an evidence-based update of existing
recommendations for the treatment of the intrinsic CRSWDs:
advanced sleep-wake phase disorder (ASWPD), delayed sleep-
wake phase disorder (DSWPD), non-24-hour sleep-wake
rhythm disorder (N24SWD), and irregular sleep-wake rhythm
disorder (ISWRD). The extrinsic or predominantly environ-
mentally inuenced CRSWDs, namely shift work and jet lag
disorder, are not addressed in this paper.
2.0 BACKGROUND
Reviewed studies that included patients with an explicitly
stated CRSWD predominantly utilized the International Classi-
cation of Sleep Disorders, Second Edition (ICSD-2)
4
diagnostic
criteria, despite the fact that International Classication of Sleep
Disorders, Third Edition (ICSD-3)
5
nomenclature is referenced
throughout the manuscript. Important modications to the In-
ternational Classication of Sleep Disorders include incorpora-
tion of the word “wake” (the ICSD-2 referred solely to circadian
rhythm sleep disorders), which highlights the signicant impair-
ments these conditions exert on daytime functioning. Caregiver
input is also emphasized in the ICSD-3, particularly with respect
to diagnostic assessments among cognitively impaired and pedi-
atric patients. Other major changes include the recommendation
that CRSWD diagnoses are ascertained via actigraphy derived
data when possible (with inclusion of both work/school and
free days), to provide objective longitudinal documentation of
sleep-wake patterns. Consistent with this emphasis on objective
measures, circadian phase assessments (e.g., dim light melato-
nin onset, or DLMO) are also recommended, if feasible. Other
changes include a de-emphasis on “conventional” and “socially
acceptable” clock times (recognizing the relative nature of these
terms, and instead highlighting patients’ subjective concerns),
extensive additions to the “Pathology and Pathophysiology” and
“Polysomnographic and Other Objective Findings” sections, and
new descriptions of “Developmental Issues” and “Clinical and
Pathophysiologic Subtypes.”
5
In many instances, this review incorporated trials with par-
ticipants who were not recruited in strict accordance with In-
ternational Classication of Sleep Disorders criteria, but who
nonetheless described symptoms consistent with a CRSWD
(based upon Task Force consensus). Examples include pediat-
ric/adolescent patients with “idiopathic sleep-onset insomnia,”
whose symptoms were consistent with DSWPD, as well as
select populations of institutionalized elderly patients, among
whom varied descriptions of insomnia, nighttime wakefulness,
and daytime napping selectively appeared to be representative
of ISWRD, despite the fact that this condition was not named
explicitly. A similar approach was taken for this latter group of
patients during literature review and development of the previ-
ous Practice Parameters.
1,3
A brief scientic background is required. The two-process
model for sleep regulation delineates two principle mechanisms
for the governance of sleep and wakefulness: “Process S” and
“Process C.”
6
The homeostatic drive to sleep (Process S) is pro-
portional to the duration of wakefulness. In contrast, Process C
creates a drive for wakefulness that variably opposes Process S
and is dependent upon circadian (“approximately daily”) rhythms
intrinsic to the individual. Master coordination of this sleep/wake
rhythm is provided by the neurons of the suprachiasmatic nuclei
located within the hypothalamus.
7–10
As this intrinsic period is
typically slightly longer than 24 hours in humans
11,12
synchroni-
zation to the 24-hour day (entrainment) is accomplished by vari-
ous environmental inputs, the most important of which is light
and dark exposure.
13
Failure to synchronize can alter the phase
relationships between internal rhythms and the light/dark cycle,
which may manifest in the form of circadian rhythm sleep-wake
disorders (CRSWDs). The intrinsic CRSWDs refer to those con-
ditions that are thought to exist predominantly due to innate phe-
nomena, although exogenous components contribute to varying
degrees in all of these disorders.
The intrinsic CRSWDs are briey characterized as follows.
DSWPD manifests as a delay of the major sleep episode with
respect to the patient’s desired timing or the timing required to
attend to social, educational, and/or occupational demands. Pa-
tients report extreme difculty both with falling asleep at bed-
times considered typical among their peers, and with waking at
the required or desired times, but sleep quality is typically re-
ported as normal when the individual sleeps at the delayed times.
In contrast, an advance of the major sleep episode with respect
to the patient’s desired or required sleep-wake times character-
izes ASWPD. ASWPD patients report extreme difculty staying
awake during evening hours and frequently note falling asleep
before completion of pertinent work, social, or family obligations.
In addition, wake time is undesirably early, and considered atypi-
cal in comparison to peers. For both conditions, symptoms must
be present for at least 3 months and schedules need to be docu-
mented with sleep diaries and/or wrist actigraphy for a period of
at least 7 days.
N24SWD is diagnosed when patients fail to entrain to the
24-hour light-dark cycle and clock times. Thus, patients exhibit
sleep-wake patterns that show a progressive delay (usually) or
advance, depending upon the period length (tau) of their en-
dogenous circadian rhythms. During a symptomatic period, the
time of high sleep propensity gradually shifts, such that patients
experience daytime hypersomnolence and nighttime insomnia.
Most patients with N24SWD are totally blind, but this disorder
also occurs among sighted individuals. In contrast to the other
CRSWDs, a N24SWD diagnosis requires at least 14 days of doc-
umentation of progressively shifting sleep-wake times with sleep
diaries and/or actigraphy.
Patients with ISWRD lack a clear circadian pattern of sleep-
wake behavior. Thus, aficted individuals experience pro-
longed periods of wakefulness during the typical nocturnal
sleep episode in addition to excessive sleepiness and prolonged
sleep bouts during daytime hours. Sleep is fragmented and
frequently insufcient. ISWRD is observed more commonly
among patients with neurodevelopmental or neurodegenera-
tive disorders, and can pose particular challenges for caregiv-
ers. Documentation (sleep diaries and/or actigraphy) of multiple
non-circadian sleep-wake bouts for a period of at least 7 days is
required for diagnosis.

1202
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
Interventions for CRSWDs can be broadly categorized as fol-
lows: (1) prescribed timing of sleep-wake and/or physical activity/
exercise, (2) strategic receipt and/or avoidance of light, (3) use of
medications and/or supplements to phase shift and/or to promote
sleep or wakefulness, and (4) alternate interventions that exert ef-
fects by altering bodily functions to impact sleep/wake behaviors
(i.e., somatic interventions).
Light is strategically timed according to phase response
cur ves (PRCs).
2
In brief, light can suppress melatonin secretion
14
and phase shift circadian timing in humans,
15
leading to the use
of timed light exposure as a treatment for CRSWDs. Light timed
in the evening and before the core body temperature minimum
(CBT
min
) leads to phase delays, and light timed after the CBT-
min
in the morning leads to phase advances.
15
Larger effects are
observed with greater intensities of light and longer durations
of light, but the increases are nonlinear.
16,17
Additionally, the re-
sponse to light is modied by prior exposure to light or “light
histor y,”
18,19
such that a history of less light exposure leads to a
greater response to light. Just as light exposure can shift circa-
dian timing, so too can the strategic avoidance or reduction of
light.
20,21
Finally, the human circadian system is most sensitive
to short wavelength blue light (~480 nm),
22,23
although at bright
intensities phase shifts to white broad spectrum light and blue
enriched light are similar, presumably due to a saturation of
photoreceptors.
24,25
Less is known about the variables contributing to melatonin
response.
2
The melatonin PRC is approximately 180 degrees out
of phase with the light PRC, such that dosing in the afternoon/
evening shifts rhythms earlier and dosing in the morning shifts
rhythms later. As the CBT
min
serves as the “inection point” be-
tween delaying and advancing effects for light, the DLMO serves
as the approximate inection point for advancing and delaying
effects of melatonin. Optimal dosing of melatonin for circadian
effects remains unclear, and studies suggest that timing is more
important than dose (PRCs for doses above 5 mg have not been
published). In addition to phase shifting effects, melatonin may
also have direct soporic effects, particularly at higher doses.
3.0 METHODS
3.1 Expert Task Force
In order to develop these Clinical Practice Guidelines, the
American Academy of Sleep Medicine (AASM) commissioned
a Task Force (TF) of four members with expertise in the eld of
CRSWDs, appointed an AASM Board of Directors (BOD) liai-
son, and assigned an AASM Science and Research Department
staff member to manage the project. Prior to appointment, the
content experts were required to disclose all potential conicts
of interest according to AASM policy. None were declared. The
TF performed an extensive review of the scientic literature
and assessed the available evidence employing the methodol-
ogy of evidence-based medicine (specically, meta-analysis
and the Grading of Recommendations Assessment, Develop-
ment and Evaluation system, or GRADE) to draft recommenda-
tions. The present paper was approved by the AASM BOD and
replaces the previous Practice Parameters.
1
The AASM expects
these guidelines to have a positive impact on clinical decision-
making and patient outcomes. These recommendations reect
the state of knowledge at the time of publication and will be
revised when the availability of new information necessitates.
3.2 PICO Questions
Eight PICO (Patient, Population or Problem, Intervention,
Comparison, and Outcomes) questions were developed, based
on both the inquiries raised in the previous AASM publica-
tions
1,3
and an investigation of systematic reviews, meta-anal-
yses, and guidelines published subsequently (Table 1). The
AASM BOD ultimately approved these questions. In addition,
combination treatments were also reviewed for the four intrin-
sic CRSWDs included in this guideline.
3.3 Literature Searches
Literature search #1 was performed in PubMed using broad
terms (see Appendix), in order to identify systematic reviews,
meta-analyses or relevant practice guidelines published sub-
sequent to availability of the previous AASM Practice Pa-
rameters. Examination of discovered papers (n = 93) enabled
elucidation of Practice Parameter recommendations requir-
ing revisions, and also assisted with further renement of the
PICO questions. The next literature search (#2) targeted treat-
ment trials involving intrinsic CRSWDs that addressed at least
one PICO question. This search utilized PubMed, Embase, and
PsycInfo databases.
At least two TF members carefully assessed the abstract of
each retrieved article (n = 2,063), to determine whether the
publication should be included for further consideration. The
following list of general exclusion criteria was used:
Table 1—PICO question parameters.
Population Intervention Comparison Outcomes
Patients diagnosed
with intrinsic
CRSWDs
(ASWPD, DSWPD,
N24SWD, ISWRD)
1. Prescribed sleep-wake scheduling
2. Timed physical activity/exercise
3. Strategic avoidance of light (e.g., with the use of eyewear)
4. Light therapy
5. Sleep-promoting medications (hypnotics/sedatives/
neuroleptics/other novel agents)
6. Timed oral administration of melatonin or agonists
7. Wakefulness-promoting medications (e.g., modanil,
traditional stimulants)
8. Other somatic interventions
9. Combination treatments
Control group, those
treated with placebo
or, when a comparison
group was not available,
measurements performed
“before” (baseline) and
“after” treatment
Physiologic circadian phase
markers (DLMO [saliva/plasma],
urinary melatonin metabolite,
constant routine CBT
Min
)
Total sleep time (TST)
Initial sleep latency (ISL)
Sleep onset time (SOT)
Sleep offset time (SOffT )

1203
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
1. Diagnosis or not treatment
2. Not CRSWD
3. Not intrinsic CRSWD (shift work or jet-lag disorder)
4. Wrong publication type (review, editorial, etc.)
5. Not human subjects
6. Mechanistic or methodological study
7. Study was published before October 2006
When there were questions or disagreements, the full text
of the article was reviewed in detail until consensus was
reached. The same search terms, databases and inclusion/ex-
clusion criteria were used for literature search #3, although
new date limitations were applied (June, 2012–March, 2014),
with the intention to capture articles published after comple-
tion of search #2. Four hundred fty-three additional publica-
tions were retrieved, and TF assessments occurred in the same
manner described above. Finally, TF members selected several
literature reviews (by consensus), and screened reference lists
to identify other articles of potential interest. This “pearling”
process served as a “spot control” for the previous searches,
and ensured that important articles were not missed. All dupli-
cate references were eliminated.
Since new inclusion/exclusion criteria were used in this
project, investigations cited in the previous Practice Param-
eters
1
were not necessarily incorporated into the current analy-
sis. Studies that did not meet inclusion criteria were selectively
used for discussion purposes, but were neither included in the
GRADE reports nor used as a basis for recommendations. The
TF made a particular effort to discuss those studies (contain-
ing either patients or healthy subjects) that might spur and/or
improve future clinical research for the reviewed CRSWDs.
A nal PubMed search was conducted to identify harms
or adverse effects attributed to the relevant interventions:
light therapy (PICO 4), hypnotics (PICO 5), and melatonin
(PICO 6) (see Appendix). Limitations were imposed to select
for English-language “meta-analyses” and “systematic re-
views” pertaining to human subjects. The titles and abstracts
of articles produced by these searches were reviewed for rel-
evance, and pertinent publications were examined. Other cited
articles from the “Harms and Adverse Effects” section were
culled from prior searches (but deemed ineligible for quantita-
tive analysis) or were provided via TF members’ preemptive
awareness and consensus regarding relevancy. Adverse effects
of combined treatments were addressed based on the singular
components of combinations.
3.4 Treatment Efficacy Outcomes
During the process of data extraction, the TF developed
a list of patient-oriented clinically relevant outcomes and
rated their relative importance. Physiologic circadian phase
markers, total sleep time (TST), initial sleep latency (ISL),
sleep onset time (SOT), and sleep offset time (SOffT) were
deemed CRITICAL for making recommendations, and a sig-
nicance threshold was dened for each outcome based upon
consensus (Table 2), as no published standards exist. Sleep
parameters were alternately evaluated with polysomnogra-
phy (PSG), wrist actigraphy, or sleep diaries. Although both
wakefulness after sleep onset and sleep efciency were also
commonly reported, the two variables were rated as IMPOR-
TANT (but not CRITICAL) by the TF. As such, related data
did not factor into clinical recommendations. A unique sce-
nario arose for N24SWD, for which entrainment status (i.e.,
whether the biological clock is synchronized to the 24-hour
day) was solely utilized as a CRITICAL outcome measure,
as it denes this CRSWD physiologically (see section 5.3 in
this paper).
3.5 Extraction of Evidence
Quantitative data pertaining to the outcomes of interest as
well as information necessary for systematic evaluation and
grading of the evidence were extracted from accepted articles
using a dedicated spreadsheet. Articles determined to lack
quantitative data or with data presented in a format incompat-
ible with desired statistical analyses were rejected at this stage,
but used selectively for further discussion. In instances where
desired data were available but not presented in the desired for-
mat, the authors were contacted, and raw data were acquired if
possible. Data were pooled across the studies for each outcome
measure in accordance with PICO questions and based on
diagnosis, study design, patient population, clinical outcome
of interest, and method of derivation (e.g., PSG derived data
were analyzed separately from data derived from actigraphy
or sleep diaries).
3.6 Statistical Analyses
Meta-analyses were completed (in the few instances pos-
sible) using the random effects model. All computations were
performed using the Review Manager software,
26
and included
calculations of the mean difference (MD) ± standard deviation
(SD) for CRITICAL outcomes. Values analyzed in this manner
are displayed to the hundredths place. Age demographics and
information regarding melatonin doses are presented in the
format provided by the associated study (mean ± SD if avail-
able) but, in an effort to maintain consistency, are displayed
only to the tenths place in instances where the authors pro-
vided values with numerical place values of lower hierarchy.
The results of meta-analyses are depicted in gures within the
Table 2—CRITICAL outcomes and their TF-dened clinical signicance thresholds.
Diagnosis
Clinical Signicance Thresholds
Circadian Phase
(change in minutes)
TST (change in
minutes)
ISL (change in
minutes)
SOT (change in
minutes)
SOffT (change in
minutes)
Entrainment
Status
ASWPD 30 30 15 15 15 N/A
DSWPD 30 30 15 15 15 N/A
ISWRD 30 30 15 15 15 N/A
N24SWD N/A N/A N/A N/A N/A Yes/No

1204
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
text, in association with a “forest plot.” Summary of ndings
tables for all investigations are presented in the Appendix.
When studies contained placebo/control groups, the evalu-
ation of the effect of treatment was performed by comparison
of averaged posttreatment and averaged post-placebo/control
group values, regardless of the authors’ approaches. In studies
with crossover or “before-after” designs where there was no
placebo/control group, posttreatment values were compared to
baseline values. Our use of this methodology occasionally pro-
duced results that differed from those reported in the original
publications (e.g.
27–29
).
3.7 Interpretation of Clinical Significance of Results
Interpretation of clinical signicance was ascertained via
comparisons with predened thresholds (see Table 2 and
Figure 1). For meta-analyses, the pooled MD (black diamond)
on “forest plots” depicts the average response or magnitude of
effect across all studies, the width of the diamond represents
the associated 95% condence interval (CI), and the “no ef-
fect” line represents nil benet from the intervention. The dot-
ted lines on the left and right sides (equidistant from the “no
effect” line) represent clinical decision thresholds dened by
the TF (Figures 1A, 1B, and 1C). The right side represents an
increase in the outcome measure, while the left represents a
decrease. An increase in some outcome measures, such as TST,
represents improvement. If the black diamond of TST data lies
beyond the clinical signicance threshold on the right side, the
result of a treatment is interpreted as a clinically signicant
improvement (Figure 1A). If, however, the diamond lies to the
left of the “clinical signicance” line, the decrease is regarded
as a clinically signicant undesired outcome, and the treatment
is deemed contraindicated. When improvement is signied by
a decrease in the outcome measure (e.g., ISL), the interpreta-
tion is reversed.
When the condence interval crosses the clinical signi-
cance threshold on one side, the evidence is graded one level
down (Figure 1B) for “serious imprecision.” When the con-
dence interval crosses the clinical signicance threshold on
both sides of the no effect line, the evidence is graded two lev-
els down for “very serious imprecision” (Figure 1C). Since
the Review Manager software does not operate with clinical
signicance thresholds, these dotted lines are not depicted in
the gures associated with the actual data. The interpretation
of clinical signicance from results of individual studies was
accomplished in the same manner, but forest plots were not
created.
3.8 Quality of Evidence
The GRADE approach was used for the assessment of qual-
ity of evidence
30–37
(also see: http://www.gradeworkinggroup.
org/publications/JCE_series.htm). In contrast to other meth-
ods, an estimate of effect is generated for critical outcomes
across studies, as opposed to an evaluation of individual stud-
ies. Multiple aspects of quality of evidence are assessed, with
downgrading of evidence as applicable (see Table 3).
GRADE assigns high quality to evidence from randomized
controlled trials while evidence from observational studies is
considered low quality. However, high quality evidence can
be graded down, and low quality evidence can be graded up,
based upon the factors described below (see Table 3). The
analysis of risk of bias includes review of the presence/ab-
sence of blinding, allocation concealment, loss to follow up,
or selective outcome reporting. Indirectness occurs when
the question being addressed is different than the available
evidence in terms of population, intervention, comparator, or
outcome. There is inconsistency when there is unexplained
heterogeneity of the results. Imprecision is described in sec-
tion 3.7 in this paper.
In GRADE, there are 4 specic categories for assessing the
quality of a body of evidence.
High: corresponds to a high level of certainty that the true
effect lies close to that of the estimate of the effect.
Moderate: corresponds to a moderate level of certainty in
the effect estimate; the true effect is likely to be close to
the estimate of the effect, but there is a possibility that it
is substantially different.
A
No effect line
No effect line
No effect line
Clinical significance
threshold defined by the TF
B
Clinical significance
threshold defined by the TF
C
Clinical significance
threshold defined by the TF
Figure 1—Guide for interpretation of clinical signicance of the results.
Examples of (A) clinically signicant improvement; (B) “serious” imprecision, grade one level down; (C) “very serious” imprecision, grade two levels down.

1205
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
Low: corresponds to a low level of certainty in the effect
estimate; the true effect may be substantially different
from the estimate of the effect.
Very low: corresponds to very little certainty in the effect
estimate; the true effect is likely to be substantially
different from the estimate of effect.
The body of evidence for each outcome was assessed and
graded, taking into account quality considerations based on
the quantitative analysis and other major factors described
above. CRITICAL outcome results are presented as summary
of ndings tables organized by PICO question and patient pop-
ulation (see Appendix, Tables S1–S12).
A cumulative quality grade for a particular PICO question
and patient population is predicated upon the lowest level of
evidence assigned to one of the CRITICAL outcomes. Thus, if
a recommendation was based upon two outcomes, one of mod-
erate and one of low quality, the overall grade would be low.
3.9 Strength of Recommendations
The TF developed recommendation statements and deter-
mined the strengths of these recommendations based on the
balance of the following major factors:
1. Level of evidence—based on an assessment of the
quality of evidence using GRADE criteria (see Table 3),
the TF categorized the evidence as:
a. High
b. Moderate
c. Low
d. Ver y Low
2. Benets vs. Harms—based upon CRITICAL outcomes
and analysis of any harms/side effects, the TF assessed
whether:
a. Benets outweighed harms
b. Benets equaled harms
c. Harms outweighed benets
d. The balance between benets and harms was unclear
3. Patient values and preferences—based on the clinical
expertise of the TF and relevant published data,
including discussion in the referenced papers about
tolerability, compliance, and patients’ experiences with
the treatments in question, the TF judged whether:
a. The vast majority of well-informed patients (> 90%)
would most likely use this patient-care strategy,
compared to alternative patient-care strategies or no
treatment
b. The majority of well-informed patients would most
likely use this patient-care strategy, compared to
alternative patient-care strategies or no treatment
c. The majority of well-informed patients would most
likely NOT use this patient-care strategy, compared
to alternative patient-care strategies or no treatment
d. The vast majority of patients (> 90%) would most
likely NOT use this patient-care strategy, compared
to alternative patient-care strategies or no treatment
Taking these variables into consideration, each recommen-
dation statement was given a “strength value” of Strong For,
Weak For, Weak Against or Strong Against (see Table 4). As
an example, a body of evidence could be rated VERY LOW,
while another could be rated MODERATE, and yet both could
generate a WEAK FOR recommendation, based upon the
above mentioned factors.
There were multiple cases when the TF chose to make “No
Recommendation,” which reects either a complete lack of
available evidence (no studies were published) or situations
when evidence was available but either did not meet review
inclusion criteria or was considered insufcient to support a
recommendation (see Appendix, Tables S5 and S6). It needs
to be emphasized that “No Recommendation” does not mean
that treatments should not be tried but that, in the absence of
sufcient and conclusive evidence, clinicians should use their
best judgment to decide in each particular case whether or not
a treatment should be used. At the step of review of the ex-
tracted evidence, the TF made a decision to exclude studies
with fewer than 10 subjects if the study constituted a single
source of evidence, as it was felt that afliated data were insuf-
cient to support a recommendation.
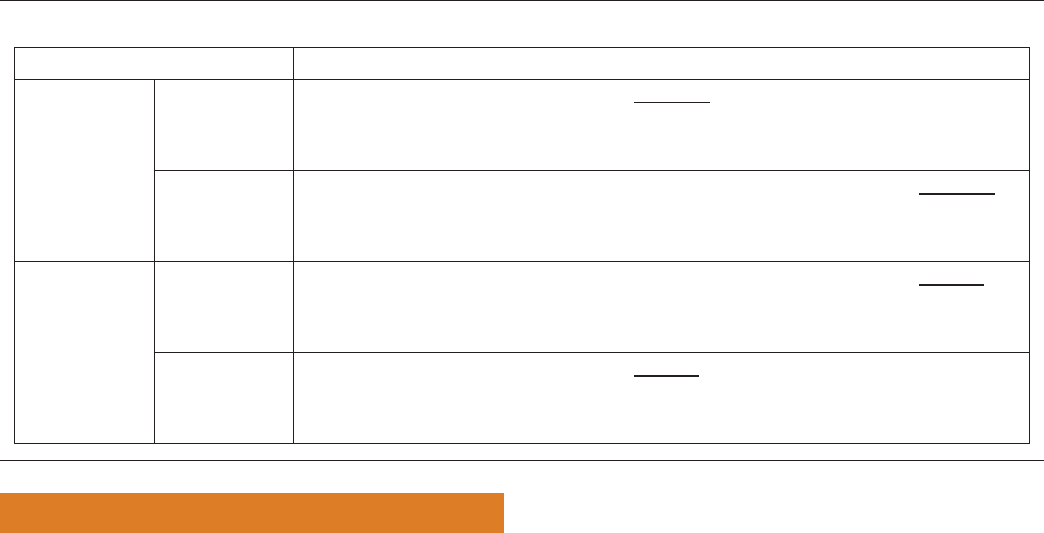
Table 3—Summary of GRADE approach to rating quality of evidence (from Guyatt et al.
30
).
Study Design
Initial Quality of a
Body of Evidence Downgrade if Upgrade if Quality of a Body of Evidence
Randomized trials High → Risk of bias
−1 Serious
−2 Very serious
Inconsistency
−1 Serious
−2 Very serious
Indirectness
−1 Serious
−2 Very serious
Imprecision
−1 Serious
−2 Very serious
Publication bias
−1 Serious
−2 Very serious
Large effect
+1 Large
+2 Very large
Dose response
+1 Evidence of a
gradient
All plausible residual
confounding
+1 Would reduce
a demonstrated
effect
+1 Would suggest
a spurious
effect if no
effect was
observed
High (four plus:
⊕⊕⊕⊕)
Moderate (three plus: ⊕⊕⊕⊝)
Observational studies Low → Low (two plus: ⊕⊕⊝⊝)
Very Low (one plus: ⊕⊝⊝⊝)
1206
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
4.0 HARMS AND ADVERSE EFFECTS
4.1 Light Therapy
No studies/reviews were identied that specically ad-
dressed potential harms among patients with CRSWDs. In
their Cochrane Systematic Review for the treatment of non-
seasonal depression, Tuunainen and colleagues
38
found that
hypomania was the sole side effect that was more common
among patients receiving light therapy versus controls (Rela-
tive Risk 4.91 [CI 1.66–4.46]). Nevertheless, light treatment
has been safely used for the treatment of bipolar depression,
with careful monitoring.
39
Other commonly described side effects include eyestrain,
nausea, and agitation, albeit with predominant spontaneous
remission. Treatment-emergent headaches also commonly re-
mit,
40
but light therapy can induce migraines in approximately
one-third of those susceptible.
41
Finally, although commercially available products do not
emit ultraviolet light, patients with eye disease and/or those
using photosensitizing medications should only use light
therapy with periodic ophthalmological and/or dermatologi-
cal monitoring of the underlying condition.
40,42,43
Reassuringly,
one study reported no changes in extensive ophthalmologic ex-
aminations among seasonal affective disorder patients without
preexisting conditions after up to 6 years of daily use in the fall
and winter months.
42
4.2 Melatonin
Melatonin is considered a dietary supplement, and is there-
fore not subject to the scrutiny afforded to United States Food
and Drug Administration (FDA)-approved medications. Con-
cerns have been raised about the purity of available prepara-
tions, as well as the reliability of stated doses. Formulations
that are United States Pharmacopeial Convention Veried can
be considered most reliable in this regard.
Few identied papers addressed risks or side effects speci-
cally among patients with CRSWDs. In general, melatonin is
associated with a lack of reported serious adverse effects.
44–48
A
review by the National Academy of Sciences stated that short-
term use of ≤ 10 mg/daily (higher than typical chronobiotic
doses) appears to be safe in healthy adults but recommended
caution in children/adolescents and women of reproductive
age (see further below). Adverse effects such as headaches,
somnolence, hypotension, hypertension, gastrointestinal up-
set, and exacerbation of alopecia areata have been reported
at higher melatonin doses in healthy adults, and the same
effects have been reported at lower doses among those with
relevant preexisting conditions. Melatonin has also been asso-
ciated with an increase in depressive symptoms,
49
and caution
is advised when prescribing to patients taking warfarin and to
patients with epilepsy, as a result of various case reports sub-
mitted to the World Health Organization.
45
A recent publica-
tion described impairment in glucose tolerance among healthy
women
50
subsequent to acute melatonin administration.
Studies that address long-term effects are scarce, as are stud-
ies that specically involve pediatric/adolescent populations.
A randomized, placebo-controlled trial that investigated the
toxicology of a 28-day treatment with 10 mg melatonin (solely
comprised of healthy male adult participants) revealed no
group differences with respect to adverse effects on polysom-
nographically recorded sleep, subjective sleepiness, numerous
clinical laboratory examinations, or other subjectively recorded
events.
51
Similarly, in a meta-analysis that reviewed controlled
trials with melatonin (n = 10 studies, over 200 subjects) used
for ≤ 3 months, there were few reports of adverse events.
48
A long-term follow-up study of pediatric patients with
DSWPD + attention decit hyperactivity disorder (ADHD)
who utilized melatonin doses up to 10 mg (mean follow-up time
of approximately 4 years) detected no serious adverse events
as a result of serial interviews with the children’s parents, and
65% of participants continued to use the medication daily.
52
A
follow-up open-label prospective study of subjects with neu-
rodevelopmental disabilities comorbid with DSWPD who re-
ceived controlled-release melatonin (max dosage 15 mg) up to
3.8 years similarly described no adverse events.
53,54
Patients and
Table 4—Denitions of AASM strengths of recommendations.
AASM Strength of Recommendation Example Characteristics Guiding Recommendation
FOR
STRONG
• There is a high degree of clinical certainty in the net benets of this patient-care strategy.
• The vast majority of well-informed patients would most likely choose this patient-care strategy, compared to
alternative patient-care strategies or no treatment.
WEAK
• There is a lower degree of clinical certainty in the balance between benets vs. harms (e.g., net benets) of
this patient-care strategy.
• The majority of well-informed patients would most likely choose this patient-care strategy, compared to
alternative patient-care strategies or no treatment.
AGAINST
WEAK
• There is a lower degree of clinical certainty in the balance between benets vs. harms (e.g., net harms) of
this patient-care strategy.
• The majority of well-informed patients would most likely not choose this patient-care strategy, compared to
alternative patient-care strategies or no treatment.
STRONG
• There is a high degree of clinical certainty in the net harms of this patient-care strategy.
• The vast majority of well-informed patients would most likely not choose this patient-care strategy,
compared to alternative patient-care strategies or no treatment.

1207
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
caregivers are nevertheless frequently wary to use this supple-
ment, due to concerns related to potential adverse effects on
growth hormone regulation (10 mg dose),
55
and on reproductive
function/development (3 mg dose).
56
Possibly relevant to the
latter concern, Tanner stages were assessed serially in a ques-
tionnaire-based study involving children/adolescents (mean du-
ration ~3 years), in an effort to compare pubertal development
among those using melatonin (mean dose ~3 mg) during pre-
puberty to non-melatonin users in the general Dutch population
(controls).
57
No signicant group differences were detected.
4.3 Hypnotics
General adversities attributed to sedative-hypnotics (though
not specically among patients with CRSWDs) include in-
creased risks for falls, headaches, nausea, medication-med-
ication interactions, and drug dependence,
58,59
with elderly
patients at specic high risk.
60–62
Data regarding the use of
hypnotics specically among demented older adults (a popu-
lation of interest for this review, see section 5.4 in this paper)
are scarce,
63
but their cognitive and other vulnerabilities would
appear to place them at even greater risk than non-demented
elderly adults.
Benzodiazepines in particular are associated with an in-
creased incidence of confusion, impaired motor performance,
anterograde amnesia, daytime sleepiness, and physiologic
dependence.
63
The newer generation nonbenzodiazepine ben-
zodiazepine receptor agonists (e.g., zolpidem, zaleplon, eszop-
iclone) have shorter half-lives and fewer overall side effects,
but high quality data to support their use with demented older
adults are nonexistent.
63,64
Commonly used over-the-counter
antihistamines have very high rates of side effects, includ-
ing cognitive impairment, daytime somnolence, and anticho-
linergic responses.
63
Trazodone, a sedating antidepressant, is
widely used off-label as a hypnotic, despite the fact that there
is virtually no evidence-based data to support its efcacy
with older adults.
65
Moreover, it is associated with signicant
risks, including priapism, orthostatic hypotension, and car-
diac arrhythmias.
65
Finally, the off-label use of neuroleptics
for dementia-related behavioral disturbances (including sleep
disturbances) is associated with a “black box” warning, due to
increased mortality risks (approximately twofold higher than
that associated with placebo-treated patients), mostly due to
cardiovascular or infectious causes.
66
5.0 RECOMMENDATIONS FOR TREATMENTS OF
INTRINSIC CRSWDs
There were 8 different treatments, in addition to combi-
nation treatments, that were reviewed for the four intrinsic
CRSWDs included in this guideline. The strengths of the rec-
ommendations, for those intrinsic CRSWD treatments that
have recommendations, are shown in Table 5. The full recom-
mendation statements, including all GRADE components that
were considered when determining the direction and strength
of the recommendation, are shown in Table 6.
5.1 Recommendations for the Treatment of ASWPD
ASWPD is characterized by a stable advance (earlier tim-
ing) of the major sleep episode, such that habitual sleep onset
and offset typically occur two or more hours prior to required
or desired sleep times. Affected individuals complain of early
morning or maintenance insomnia and excessive evening
sleepiness. When allowed to maintain an advanced schedule,
sleep quality and quantity are improved.
5
5.1.1 Prescribed Sleep-Wake Scheduling for Patients with
ASWPD
Sleep-wake scheduling has only been described in one case
report, but favorably affected the predened CRITICAL out-
comes (SOT and SOffT, both subjective) in ASWPD.
67
No new
studies were identied.
In the report of a 62-year-old male, sleep times were ad-
vanced 3 hours every 2 days for 2 weeks and then stabilized at
the desired times, which were maintained at 5 months follow-
up. This treatment was designated as an OPTION in the 2007
Practice Parameters, but the current TF did not regard a single
case report as sufcient evidence for a recommendation.
There is insufcient evidence to support the use of pre-
scribed sleep-wake scheduling as a treatment for patients
with ASWPD (versus no treatment). No recommendation.
5.1.2 Timed Physical Activity/Exercise for Patients with
ASWPD
No recommendation was made in the 2007 Practice Param-
eters, and no new studies were identied.
There is no evidence to support the use of timed physi-
cal activity or exercise as a treatment for patients with
ASWPD. No recommendation.
5.1.3 Strategic Avoidance of Light for Patients with ASWPD
No recommendation was made in the 2007 Practice Param-
eters, and no new studies were identied.
There is no evidence to support the use of strategic avoid-
ance of light as a treatment for patients with ASWPD. No
recommendation.
5.1.4 Light Therapy for Patients with ASWPD
No treatment trials of light therapy in ASWPD have been
published since the 2007 Practice Parameters, which recom-
mended this therapy as an OPTION. Most of the previously
referenced studies
68–71
were not included for the current
analysis, as participants were not clearly diagnosed with
ASWPD. Discrete benets from this treatment are difcult
to ascertain given methodological limitations within the
two reviewed studies
72,73
(discussed further below), and the
cumulative level of evidence was VERY LOW (Appendix,
Table S1).
Only one randomized Advanced Sleep Phase Disorder
(ASPD)/ASWPD treatment trial was identied (also refer-
enced in the previously published Practice Parameters).
72
A
parallel group design was used to test 28 days of daily evening
exposure to a white broad-spectrum light (~265 lux) versus a
dim red light control (~2 lux) among 47 patients (mean age
70.0 ± 6.4 years). The light source consisted of a rice paper
shade over a vertical light tube, and the protocol instructed
subjects to sit within 1 meter of the light source, for 2–3 hours
in duration, ending 1 hour before habitual bedtime. There
were no signicant posttreatment group differences with

1208
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
respect to most predened CRITICAL outcomes, namely
circadian phase (urinary 6-sulfatoxymelatonin [aMT6s] acro-
phase), ISL (actigraphy and subjective), SOT (actigraphy and
subjective), SOffT (actigraphy and subjective), and TST (sub-
jective). Total sleep time (actigraphy) signicantly decreased
posttreatment within the active treatment group (mean differ-
ence 34.62 minutes [CI −0.96 to −68.28]), possibly due to a
nonsignicant delay in SOT. Importantly, while photosensors
attached to the light source indicated good compliance with
the scheduled light therapy on and off times, light exposure
data on wrist worn photometers suggested that, on average,
patients were only adjacent to the light source for about half
of the scheduled treatment duration. Nonetheless, the treat-
ment was well tolerated, and the majority of the patients who
received the white broad spectrum light source asked to keep
it for long-term personal use.
Given the low intensity of light used in this study, the TF
questioned the applicability of the results, and elected to ex-
pand the GRADE analysis to include a non-randomized trial
(Appendix, Table S1). A parallel group design study by
Campbell and colleagues
73
(also referenced in the previous
Practice Parameters) tested 12 days of a daily evening expo-
sure to bright white light (4,000 lux) versus a dim red light (~50
lux) control in 16 patients with ASWPD (mean age 70.4 ± 4.9
years). The light source consisted of two light boxes (proxim-
ity to source not specied), and therapy was for 2 hours in
duration, between 20:00 and 23:00 (ending before habitual
bedtime). The treatment signicantly delayed circadian phase
(CBT
Min
, mean difference 141.00 minutes [CI 26.10–255.90]),
and increased TST (PSG, mean difference 51.30 minutes [CI
2.69–99.91]), but both values crossed the threshold of the pre-
determined clinically signicant minimal change (see Table 2
and Appendix, Table S1). There were no signicant changes
in ISL, SOT, or SOffT (PSG) posttreatment relative to the con-
trol condition (Appendix, Table S1).
The results of studies that tested evening light therapy in
patients complaining of insomnia (not eligible for the current
review, as subjects were not discreetly diagnosed with AS-
WPD) are generally favorable. For example, in studies of pa-
tients with early-morning awakenings, evening light delayed
circadian timing,
70,71
delayed SOffT
71
and increased TST,
70,71
although positive effects were not always observed.
69
Eve-
ning light therapy also phase delayed circadian timing and
SOT in patients with sleep maintenance insomnia.
68
In other-
wise healthy older adults with sleep complaints, evening light
therapy delayed the DLMO, but there was no observed effect
on ISL and TST, while sleep timing remained xed by the
study protocol.
74
Table 5 —Overview of AASM recommendation status for Intrinsic CRSWD treatments
Treatment ASWPD DSWPD N24SWD ISWRD
Prescribed sleep-wake
scheduling
No Recommendation No Recommendation No Recommendation No Recommendation
Timed physical activity/
exercise
No Recommendation No Recommendation No Recommendation No Recommendation
Strategic avoidance of light No Recommendation No Recommendation No Recommendation No Recommendation
Light therapy 5.1.4a WEAK FOR (adults) No Recommendation No Recommendation
5.4.4a WEAK FOR
(elderly with dementia)
Sleep-promoting
medications
No Recommendation No Recommendation No Recommendation
5.4.5a STRONG AGAINST
(elderly with dementia)
Timed oral administration of
melatonin or agonists
No Recommendation
5.2.6.1a WEAK FOR
(adults with and without
depression)
5.2.6.2.1a WEAK FOR
(children/adolescents
without comorbidities)
5.2.6.2.2a WEAK FOR
(children/adolescents with
psychiatric comorbidities)
5.3.6.1a WEAK FOR
(blind adults)
No Recommendation
(sighted)
5.4.6.1a WEAK AGAINST
(elderly with dementia)
5.4.6.2a WEAK FOR
(children/adolescents with
neurologic disorders)
Wakefulness-promoting
medications
No Recommendation No Recommendation No Recommendation No Recommendation
Other somatic interventions No Recommendation No Recommendation No Recommendation No Recommendation
Combination treatments No Recommendation
No Recommendation
(adults)
5.2.9.2a WEAK FOR (light
therapy + multicomponent
behavioral interventions for
children/adolescents)
No Recommendation
5.4.9.1a WEAK AGAINST
(combination treatment
of light and melatonin for
demented, elderly patients)

1209
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
Table 6—Recommendation statements for treatment of patients with intrinsic CRSWDs.
Treatment
(PICO Question) Recommendation Statement
Direction and
Strength of
Recommendation
Quality of
Evidence
Benets/
Harms
Assessment
Patients’ Values and
Preferences
Advanced Sleep-Wake Phase Disorder (ASWPD)
5.1.4 Light
therapy
(PICO Question 4)
5.1.4a The TF suggests that clinicians treat
adult ASWPD patients with evening light
therapy (versus no treatment)
WEAK FOR VERY LOW Benets
closely
balanced with
harms
The majority of patients
would use this treatment.
Delayed Sleep-Wake Phase Disorder (DSWPD)
5.2.6 Timed oral
administration
of melatonin or
agonists
(PICO Question 6)
5.2.6.1a The TF suggests that clinicians
treat DSWPD in adults with and without
depression with strategically timed melatonin
(versus no treatment)
WEAK FOR LOW Uncertainty in
the estimates
of benets/
harms
The majority of patients
would use this treatment.
5.2.6.2.1a The TF suggests that clinicians
treat children and adolescents with DSWPD
(and no comorbidities) with strategically timed
melatonin (versus no treatment)
WEAK FOR MODERATE Uncertainty in
the estimates
of benets/
harms
The majority of patients
would use this treatment,
with appropriate informed
consent from the patient
and caregiver.
5.2.6.2.2a The TF suggests that clinicians
treat children and adolescents with DSWPD
comorbid with psychiatric conditions with
strategically timed melatonin (versus no
treatment)
WEAK FOR LOW Uncertainty in
the estimates
of benets/
harms
The majority of patients
would use this treatment,
with appropriate informed
consent from the patient
and caregiver.
5.2.9 Combination
Treatments
5.2.9.2a The TF suggests that clinicians treat
children/adolescents with DSWPD with post-
awakening light therapy in conjunction with
behavioral treatments (versus no treatment)
WEAK FOR LOW Benets
outweigh
harms
The majority of patients
would use this treatment,
particularly with active
caregiver support.
Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD)
5.3.6 Timed oral
administration
of melatonin or
agonists
(PICO Question 6)
5.3.6.1a The TF suggests that clinicians
use strategically timed melatonin for the
treatment of N24SWD in blind adults (versus
no treatment)
WEAK FOR LOW Benets
outweigh
harms
The majority of patients
would use this treatment.
Irregular Sleep-Wake Rhythm Disorder (ISWRD)
5.4.4 Light
Therapy
(PICO Question 4)
5.4.4a The TF suggests that clinicians treat
ISWRD in elderly patients with dementia with
light therapy (versus no treatment)
WEAK FOR VERY LOW Benets
closely
balanced with
harms
The majority of well-
informed patients and/or
caregivers of would elect to
use this treatment.
5.4.5 Sleep-
promoting
medications
(PICO Question 5)
5.4.5a The TF recommends that clinicians
avoid the use of sleep-promoting medications
to treat demented elderly patients with
ISWRD (versus no treatment)
STRONG
AGAINST
NONE* Harms
outweigh
benets
The vast majority of well-
informed patients and/or
caregivers would NOT elect
to use this treatment.
5.4.6 Timed oral
administration
of melatonin or
agonists
(PICO Question 6)
5.4.6.1a The TF suggests that clinicians
avoid the use of melatonin as a treatment for
ISWRD in older people with dementia (versus
no treatment)
WEAK AGAINST LOW Harms
outweigh
benets
The majority of patients
and/or caregivers would
NOT elect to use this
treatment.
5.4.6.2a The TF suggests that clinicians use
strategically timed melatonin as a treatment
for ISWRD in children/adolescents with
neurologic disorders (versus no treatment)
WEAK FOR MODERATE Benets
outweigh
harms
The majority of patients
and/or caregivers would
elect to use this treatment.
5.4.9 Combination
treatments
5.4.9.1a The TF suggests that clinicians
avoid the use of light therapy combined with
melatonin in demented, elderly patients with
ISWRD (versus no treatment)
WEAK AGAINST VERY LOW Harms
outweigh
benets
The majority of patients
and/or caregivers would
NOT elect to use this
treatment.
* Although no randomized controlled trials have examined sleep-promoting medications for the treatment of ISWRD, other extant literature indicates that
administration of hypnotics to demented elderly patients increases risks of falls and other untoward outcomes (see separate “Harms and Adverse Effects”
section).
1210
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
5.1.4a The TF suggests that clinicians treat adult ASWPD
patients with evening light therapy (versus no treatment).
[WEAK FOR]
Summary: No treatment trials of light therapy in ASWPD
have been published since the 2007 Practice Parameters, which
recommended this therapy as an OPTION. The largest effects
were seen after a 12-day treatment of 2 hours of bright white
broad spectrum light (~4,000 lux) from 2 light boxes (proxim-
ity to source not specied), timed to occur daily between 20:00
and 23:00, and ending before habitual bedtime.
73
Nevertheless,
the overall quality of evidence derived from the analyses of
two publications
72,73
was VERY LOW (Appendix, Table S1),
with potential benets of light therapy closely balanced with
the harm/burden. Associated risks are minimal, as detailed
separately in the “Harms and Adverse Effects” section. Pa-
tients report reasonable compliance and high satisfaction with
this treatment,
72
and light boxes are available over the counter
in the U.S., at relatively affordable prices. Thus, the majority
of well-informed patients would choose light therapy versus
no treatment.
5.1.5 Sleep-Promoting Medications for Patients with ASWPD
No recommendation was made in the 2007 Practice Param-
eters, and no new studies were identied.
There is no evidence to support the use of sleep-promot-
ing medications as a treatment for patients with ASWPD.
No recommendation.
5.1.6 Timed Oral Administration of Melatonin or Agonists for
Patients with ASWPD
The administration of a low dose of melatonin after early
morning awakenings and upon nal arising in the morning
was indicated as an OPTION for ASWPD in the 2007 Practice
Parameters, based on expert consensus alone. No new studies
were identied.
There is no evidence to support the use of melatonin
or agonists as a treatment for patients with ASWPD. No
recommendation.
5.1.7 Wakefulness-Promoting Medications for Patients with
ASWPD
No recommendation was made in the 2007 Practice Param-
eters, and no new studies were identied.
There is no evidence to support the use of wakefulness-
promoting medications as a treatment for patients with
ASWPD. No recommendation.
5.1.8 Other Somatic Interventions for Patients with ASWPD
No recommendation was made in the 2007 Practice Param-
eters, and no new studies were identied.
There is no evidence to support the use of other somatic
interventions as a treatment for patients with ASWPD. No
recommendation.
5.1.9 Combination Treatments for Patients with ASWPD
No recommendation was made in the 2007 Practice Param-
eters, and no new studies were identied.
There is no evidence to support the use of combination
treatments for patients with ASWPD. No recommendation.
5.2 Recommendations for the Treatment of DSWPD
DSWPD is characterized by habitual sleep-wake timing that
is delayed, usually greater than two hours, relative to conven-
tional or socially acceptable timing. Affected individuals com-
plain of difculty falling asleep at a time required to obtain
sufcient sleep duration on a school or work night, and experi-
ence difculties arising at the required times. When allowed to
follow his or her preferred schedule, sleep quality and quantity
are typically reported as normal.
5
5.2.1 Prescribed Sleep-Wake Scheduling for Patients with
DSWPD
The previous recommendation was designated as an OP-
TION (specically with respect to chronotherapy, a prescribed
progressive delay of the sleep/wake schedule until the desired
schedule is reached), based upon two studies with adult partici-
pants.
75,76
The 1993 study by Ito and colleagues,
75
reviewed pre-
viously, was not included in the current analysis, as it did not
investigate discrete sleep outcomes, but instead incorporated
subjective assessments of global functioning. The 1981 study
by Czeisler and colleagues
76
was excluded due to a low number
of subjects (n = 5). There is one report of an adult DSWPD
patient who developed free-running circadian rhythms after
engaging in this treatment.
77
Although ineligible for current analyses, 3 studies were
published subsequent to availability of the previous Practice
Parameters that may bear relevance to the use of prescribed
sleep-wake scheduling (other than chronotherapy) as a ther-
apy for adolescent patients.
78–80
Two groups
79,80
described
potentially meaningful outcomes, but the parameters of inter-
est were different from those specically dened by the TF,
and, in the case of the de Sousa study,
80
all participants were
healthy adolescents (i.e., not aficted with DSWPD). Similarly,
the Sharkey group (2011) studied a cohort with subthreshold
DSWPD (the general CRSWD social/occupational impairment
criterion was not applied), such that participants did not meet
inclusion criteria for this review.
78
While a 6-day prescribed
advanced sleep schedule (with adjunctive strategic avoidance
of evening light) resulted in concomitant advances in DLMO,
the majority of participants exhibited decreased TST.
There is insufcient evidence to support prescribed sleep-
wake scheduling as a stand-alone treatment (versus no
treatment) for patients with DSWPD. No recommendation.
5.2.2 Timed Physical Activity/Exercise for Patients with
DSWPD
No recommendation was made in the 2007 Practice Param-
eters, and no new studies were identied.
There is no evidence to support the use of timed physi-
cal activity or exercise as a treatment for patients with
DSWPD. No recommendation.
5.2.3 Strategic Avoidance of Light for Patients with DSWPD
No recommendation was made in the 2007 Practice Parameters.
A relevant open-label study was published subsequently, but
it did not meet inclusion criteria for the present analysis, as pre-
dened critical outcomes were not employed (i.e., solely submea-
sures from the Pittsburgh Sleep Quality Index were utilized).
81
Adult subjects with DSWPD + ADHD were instructed to wear
1211
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
amber glasses that blocked wavelengths ≤ 530 nm “…from sun-
down until bedtime every evening,” for a minimum of 3 hours,
and for a period of 2 weeks. Concomitant instructions included
the use of only oor and table lamps (i.e., avoidance of overhead
lights) during the evening. If a participant awoke during the
night, he/she was instructed to don the glasses prior to light
exposure. In addition, subjects were given specic instructions
to avoid/minimize caffeine, nicotine, and alcohol. Outcomes
were compared to a weeklong baseline assessment period. As
determined by the Pittsburgh Sleep Quality Index, signicant
improvements in TST, ISL, and sleep quality were noted. In a
separate study potentially related to the treatment of DSWPD,
adult insomnia patients who wore “blue blocker” (< 550 nm)
glasses during the 3 hours prior to habitual bedtime demon-
strated improved subjective sleep quality
82
compared with the
placebo intervention (yellow-tinted glasses that blocked only
ultraviolet light). Importantly, there are no tangible risks asso-
ciated with these interventions.
There is insufcient evidence to support the use of stra-
tegic avoidance of light as a treatment for patients with
DSWPD (versus no treatment). No recommendation.
5.2.4 Light Therapy for Patients with DSWPD
The previously published recommendation was designated
as a GUIDELINE and was based on two studies.
28,83
The
Rosenthal study was not analyzed for current purposes due
to a lack of rigorously reported sleep-related outcomes.
83
The
Cole study
28
is included in a separate section (see Combination
Treatments, below). A separate open-label light therapy trial
was identied,
84
but ultimately rejected due to a small number
of subjects (n = 6). Only one study pertaining to adult DSWPD
populations has been published subsequent to the release of the
previous CRSWD Practice Parameters in 2007 (also reviewed
in the Combination Treatments section below).
85
There is insufcient evidence to support efcacy of post-
awakening light therapy (monotherapy) as a treatment for
DSWPD (versus no treatment). No recommendation.
5.2.5 Sleep-Promoting Medications for Patients with DSWPD
No recommendation was made in the 2007 Practice
Parameters.
There are isolated reports regarding the use of hypnotics
in DSWPD (typically as an adjunctive treatment with chrono-
therapy), but there is insufcient rigor in methodology for pur-
poses of evidence analysis.
75,86
Two reports describe DSWPD
patients’ resistance to the effects of traditional hypnotics.
87,88
Nevertheless, a laboratory-based study that imposed a 4-hour
phase advance on healthy subjects described sleep-related ben-
ets (PSG and subjective measures) with zolpidem.
89
There is insufcient evidence to support the use of sleep-
promoting medications as a treatment for patients with
DSWPD (versus no treatment). No recommendation.
5.2.6 Timed Oral Administration of Melatonin or Agonists for
Patients with DSWPD
5.2.6.1 Melatonin for adult patients with dswpd
The previously published recommendation was designated
as a GUIDELINE, and was supported by four studies.
27,90 –92
Two of these publications
91,92
did not meet inclusion criteria
for the present review, due to an insufcient number of sub-
jects,
91
or due to design and reporting limitations
92
that hin-
dered comparisons with the included investigations. One study
was published subsequent to availability of the previous Prac-
tice Parameters.
93
The three reviewed investigations provide
contradictory information regarding sleep/circadian-related
effects of melatonin among adults with DSWPD. Level of re-
viewed evidence: LOW (Appendix, Table S2).
In a randomized double-blinded placebo-controlled study
(parallel design, n = 11, mean age 28.2 ± 5.7 years
27
), Mundey
and colleagues utilized either 0.3 or 3.0 mg melatonin (versus
placebo) between 15:00–21:30 (1.5–6.5 hours before baseline
DLMO), with an advance in timing of 1 hour after 2 weeks, for
a total treatment duration of 29 days. No improvements in ac-
tigraphically determined sleep parameters were observed, and
our analysis demonstrated no group difference with respect
to the timing of DLMO
27
(Appendix, Table S2). As the pres-
ent review did not analyze outcomes relative to the timing of
melatonin administration, however, it is important to note that
the authors reported an inverse relationship between the tim-
ing of melatonin administration (irrespective of dose) and the
magnitude of DLMO phase advance, such that earlier timing
of the former (in relation to DLMO) resulted in greater phase
advances. No such correlation was identied with respect to
CBT
Min
(assessed only within the active treatment groups).
The Kayumov and Rahman studies (same investigative
group) demonstrated substantial select PSG-measured ben-
ets in sleep parameters (TST, ISL), but an afliated circadian
phase marker was not employed.
90,93
Solely the Mundey group
included such a measure. The Rahman study
93
(n = 20, ran-
domized, double-blind, placebo-controlled, crossover design,
mean age 30.8 ± 12.4 and 35.6 ± 14.0 years for females and
males, respectively) utilized 5 mg melatonin administered
between 19:00–21:00, for a period of 28 days. The Kayumov
study
90
(n = 20, same design/age distribution) used the same
dose, but scheduled it at 19:00 the rst week, between 19:00–
21:00 during the second and third weeks (according to sub-
jects’ preferences), and at a consistent time during the fourth
week (average chosen time 21:00). Analyses among a subset of
depressed patients from both studies (n = 28) demonstrated a
statistically signicant increase in TST (mean difference 41.44
minutes, [CI 13.19–69.70]), but the level of evidence was down-
graded due to inconsistency and imprecision (see Figure 2 and
Tables 2 and 3). More denitive results were obtained from an
analysis of a subset of non-depressed patients from the Rah-
man study (Appendix, Table S2) (n = 12, TST = 56.00 minutes
[CI 48.51–63.49]).
ISL was polysomnographically assessed with the same
subcategorization of groups. Among the depressed subgroup
(n = 28), sleep latency decreased by 43.52 minutes [CI −34.45
to −52.60] (see Figure 3 and Appendix, Table S2). Among
the non-depressed subjects from the Rahman study (n = 12),
sleep latency decreased by 37.70 minutes [CI −31.75 to −43.65]
(Appendix, Table S2).
5.2.6.1a The TF suggests that clinicians treat DSWPD
in adults with and without depression with strategically
timed melatonin (versus no treatment). [WEAK FOR]

1212
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
Summary: The previously published recommendation was
designated as a GUIDELINE. The overall quality of evidence
from the analyses of the three accepted/reviewed studies
27,90,93
was LOW (Figures 2 and 3 and Appendix, Table S2), and
data regarding the sleep/circadian-related effects of melatonin
were contradictory. Positive results were obtained with a 5 mg
dose timed between 19:00–21:00 (no circadian-based timing),
for a period of 28 days.
90,93
The Rahman study
93
was the sole
study identied subsequent to publication of the previous Prac-
tice Parameters. Taking into account the discussion regarding
potential safety/adverse effects of melatonin (see separate
“Harms and Adverse Effects” section), the benets/harms ratio
remains uncertain, but clinical experience suggests frequent
acceptance of this treatment among adults versus no treatment.
5.2.6.2. Melatonin for children/adolescents with dswpd
5.2.6.2.1 Melatonin for children/adolescents with DSWPD and no
comorbidities
No studies were previously reviewed which directly ad-
dressed the pediatric/adolescent population. Strategically
timed melatonin at the dose specied below is effective for
children/adolescents with DSWPD. Level of reviewed evi-
dence: MODERATE (Appendix, Table S3).
One randomized, placebo-controlled double-blinded study
was reviewed (unpublished raw data provided courtesy of au-
thor).
29
Participants ranged in age from 6–12 years. The three
active treatment groups received melatonin at dosages of 0.05
mg/kg, 0.1 mg/kg, and 0.15 mg/kg, with respective mean doses
of 1.6 ± 0.4 mg (n = 16), 2.9 ± 0.9 mg (n = 19), and 4.4 ± 1.0 mg
(n = 18). Seventeen participants were allocated to the placebo
group. The duration of treatment was 6 nights, with instruc-
tions to take melatonin 1.5–2.0 hours prior to habitual bedtime
(unclear if equivalent to habitual sleep onset time), with con-
sistent nightly timing.
The data of 64 participants were utilized for actigraphy/
sleep-related analyses. With respect to CRITICAL outcomes,
sleep onset time favorably advanced in comparison to placebo
among the 0.1 and 0.15 mg/kg groups (mean difference −33.70
minutes [CI −10.95 to −56.46] and −42.77 minutes [CI −21.77
to −63.78], respectively), but the condence interval of the
value associated with the lower dose crossed the threshold of
the predetermined clinically signicant minimal change (see
Table 2 and Appendix, Table S3). Statistical differences were
not observed with the 0.05 mg/kg group. Nevertheless, sleep
latency improved among all three groups (statistical and clini-
cally signicant differences) in comparison to placebo (mean
difference −38.39 minutes [CI −18.24 to −58.53], −44.24 min-
utes [CI −24.04 to −64.44], and −43.80 minutes [CI −24.06 to
−63.54], respectively, in order of ascending dosage).
With respect to DLMO analyses, no signicant differences
were noted among any of the three treatment groups in com-
parison to placebo. However, the authors separately calculated
individualized outcomes based upon melatonin use in relation
to both DLMO (circadian) and clock time of administration
(TOA). These analyses were not compatible with the Review
Manager software used in this project. Data were depicted
solely within gures (i.e., no raw representation). As with the
previously discussed Mundey study,
27
a positive relationship
between DLMO phase advances and an earlier circadian TOA
was described (no relationship observed with respect to clock
TOA), but no differences were observed between the various
melatonin dosage groups. On the contrary, no advantages be-
tween clock versus circadian TOA were demonstrated in rela-
tion to sleep onset and initial sleep latency times.
5.2.6.2.1a The TF suggests that clinicians treat children
and adolescents with DSWPD (and no comorbidities)
with strategically timed melatonin (versus no treatment).
[WEAK FOR]
Figure 2—Meta-analysis of data for PSG determined TST in response to melatonin treatment of adult patients with DSWPD
and comorbid depression.
Figure 3—Meta-analysis of data for PSG determined ISL in response to melatonin treatment of adult patients with DSWPD
and comorbid depression.

1213
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
Summary: This is a new recommendation in comparison to
the prior Practice Parameter, as no studies were previously re-
viewed which directly addressed the pediatric/adolescent pop-
ulation. The level of reviewed evidence from a singular study
29
was MODERATE (Appendix, Table S3). Optimal results
were obtained with a dose of 0.15 mg/kg, taken 1.5–2.0 hours
prior to habitual bedtime, for 6 nights. Although no serious
adverse reactions have been described in relation to melatonin
use to date, relevant concerns have been raised by select stud-
ies with respect to the pediatric/adolescent population,
94
and
rigorous long-term data are lacking (see separate “Harms and
Adverse Effects” section). As such, the benets/harms assess-
ment is uncertain. Clinical experience nevertheless supports
frequent acceptance of this therapy versus no treatment, with
appropriate informed consent from the patient and caregiver.
5.2.6.2.2 Melatonin for children/adolescents with DSWPD and psychi-
atric comorbidities
This is a new recommendation in comparison to the previ-
ous Practice Parameters, as no studies specically addressed
this patient population. Strategically timed fast-release mela-
tonin at dosages ranging from 3–5 mg may be effective for
children/adolescents with DSWPD and psychiatric comorbidi-
ties. Level of reviewed evidence: LOW (Appendix, Table S4)
Two randomized, placebo-controlled studies by the same
group examined the use of melatonin for DSWPD among chil-
dren/adolescents with various psychiatric comorbidities (all
were diagnosed with ADHD).
95,96
Participants aged 6–12 years
received fast-release melatonin (one of several instances where
melatonin formulation was specied) for 4 weeks at dosages
of 3 or 5 mg, at either 18:00 or 19:00. The more recent study
95
based dosage on weight (3 mg if < 40 kg; 5 mg if > 40 kg,
taken at 19:00), while the earlier protocol
96
uniformly provided
5 mg at 18:00. Combined analyses (n = 132) revealed an ad-
vance in DLMO of nearly 1 hour in comparison to placebo
(mean difference −54.22 minutes [CI −31.67 to −76.78]) (see
Figure 4). Actigraphically assessed sleep-onset time (n = 130)
also advanced (mean difference −36.57 minutes [CI −16.96 to
−56.18]) (see Figure 5). Other actigraphically derived sleep pa-
rameters were obtained only in the more recent study (n = 105).
A signicant decrease in ISL was detected (mean difference
−18.70 minutes [CI −7.01 to −30.39], but the condence interval
crossed the threshold of the predetermined clinically signi-
cant minimal change (see Table 2 and Appendix, Table S4).
No signicant group differences were noted with respect to
TST.
95
Previously published subjective assessments failed to
demonstrate signicant group differences in TST (n = 31), ISL
(n = 33), or sleep-onset (n = 33) or offset (n = 32) times.
96
Although not accepted for analysis for this review due to con-
cerns regarding patient heterogeneity, a randomized placebo-
controlled study by Wasdell and colleagues (which contained
an admixture of DSWPD and insomnia patients) explored the
effects of 5 mg controlled-release melatonin (10 days) among
approximately 50 children with neurodevelopmental disabili-
ties (ages 2–18, mean 7.4 years).
53
Melatonin was administered
20–30 minutes prior to the caregiver determined desired time
of sleep onset. Signicant improvements were observed in sub-
jectively and actigraphically recorded TST, ISL, and clinician-
and caregiver-assessed overall sleep disorder severity and
other functional and health dimensions. During the 3-month
open-label phase of the trial, escalation of the melatonin dos-
age provided no denitive benets. A separate follow-up open-
label prospective study (duration up to 3.8 years) demonstrated
continued caregiver-reported benets on sleep, overall health,
behavior, education, and learning.
54
5.2.6.2.2a The TF suggests that clinicians treat children
and adolescents with DSWPD comorbid with psychiatric
conditions with strategically timed melatonin (versus no
treatment). [WEAK FOR]
Figure 5—Meta-analysis of data for actigraphically-determined SOT in response to melatonin treatment of children/
adolescents with DSWPD and comorbid psychiatric conditions.
Figure 4—Meta-analysis of data for DLMO in response to melatonin treatment of children/adolescents with DSWPD and
comorbid psychiatric conditions.
1214
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
Summary: This is a new recommendation in comparison to
the previous Practice Parameters, as no studies specically ad-
dressed this patient population. The overall quality of evidence
from the analyses of the two reviewed studies
95,96
was LOW
(see Figures 4 and 5 and Appendix, Table S4). A fast-release
formulation of melatonin was utilized, with dosages ranging
from 3–5 mg, taken between 18:00–19:00 (no circadian-based
timing), for 4 weeks. In the pooled analysis, actigraphically
assessed sleep onset time advanced in conjunction with an
advance in the circadian phase marker (DLMO). Although no
serious adverse reactions have been described in relation to
melatonin use to date, relevant concerns have been raised by
select studies with respect to the pediatric/adolescent popu-
lation, and rigorous long-term data are lacking (see separate
“Harms and Adverse Effects” section). As such, the benets/
harms assessment is uncertain. Clinical experience neverthe-
less supports frequent acceptance of this therapy versus no
treatment, with appropriate informed consent from the patient
and caregiver.
5.2.7 Wakefulness-Promoting Medications for Patients with
DSWPD
No recommendation was made in the 2007 Practice Param-
eters, and no new studies were identied.
There is no evidence to support the use of wakefulness-
promoting medications as a treatment for patients with
DSWPD. No recommendation.
5.2.8 Other Somatic Interventions for Patients with DSWPD
In the previous Practice Parameters, oral vitamin B12 ther-
apy was described as “not indicated,” designated at the GUIDE-
LINE level. No subsequent relevant studies (involving B12 or
separate somatic interventions) have been published. There
is no clear evidence of benet from oral vitamin B12 among
adults with DSWPD. Level of reviewed evidence: VERY LOW
(Appendix, Table S5).
In a multicenter study
97
that contained predominantly adult
DSWPD patients (n = 50, mean age 26.8 ± 1.3 years), partic-
ipants received 3 mg total daily dose of vitamin B12 (three
times daily divided dosing) versus placebo (double-blinded)
for a period of 4 weeks. No clinically signicant differences
were observed with respect to subjectively assessed TST and
SOT (see Table 2 and Appendix, Table S5). Remaining sleep
parameters were not compatible with the TF’s predened clini-
cal outcomes (ISL was evaluated according to a 5-point Likert
scale).
There is insufcient evidence to support the use of oral
vitamin B12 (and no evidence to support alternate somatic
interventions) among patients with DSWPD (versus no
treatment). No recommendation.
5.2.9 Combination Treatments for Patients with DSWPD
Combination treatments for DSWPD were not addressed
separately in the previous Practice Parameters and associated
review papers, but one of the reviewed studies
28
was addressed
in the singular light therapy category. The combination treat-
ments sections throughout the paper contain studies that ex-
plicitly denote dual treatment interventions, although the
TF acknowledges that other reviewed studies may have also
employed multicomponent treatment strategies, but failed to
mention or emphasize their nature in the published method-
ologies or to include proper controls for all components of the
treatment.
5.2.9.1. light/coMbination treatMents for adults with
dswpd
There is no evidence to support efcacy of light therapy
(provided by means other than a “light box”) in association
with concomitant behavioral instructions among adults. Level
of reviewed evidence: VERY LOW (Appendix, Table S6).
In a randomized parallel clinical trial (n = 54) involving
adults (mean age 25 ± 6 years) with DSWPD, Cole and col-
leagues (2002)
28
used a light mask that exposed participants to
2700 lux of white broad spectrum light on closed eyelids (about
57 lux at the cornea) for a period of 26 days, and compared it
to an inactive condition (0.1 lux of red light, estimated corneal
illuminance = 0.007 lux). Light therapy commenced (at an in-
tensity of < 0.01 lux) 4 hours before the scheduled rise time,
proceeded with ramped intensity for 1 hour, and remained at
full brightness until arising. Volunteers were not informed of
the hypotheses of the study and were blinded to the treatment
received by the other group. The study personnel who inter-
acted with participants and scored data were also blind to treat-
ment assignments.
Both groups received concomitant behavioral instructions.
Subsequent to the rst 2 treatment days, subjects were asked to
advance both bedtime and time of arising (in equal measure) on
a daily basis, to achieve a cumulative advance of at least 1 hour
per week (≤ 15 minutes daily, unless he/she experienced spon-
taneous awakening > 15 minutes ahead of schedule 2 days in a
row). Subjects were also asked to stop advancing sleep if he/she
achieved a preselected target wakeup time, but were allowed
to change the target time if he/she wished. Participants were
urged to avoid naps during the treatment interval, and were
provided specic instructions to minimize late-afternoon and
evening light exposure on all treatment days. Specic interven-
tions included shading of windows between 17:00 and dusk and
utilization of dark wraparound form-tting goggles (2% trans-
mittance) if necessary to go outdoors during this time interval.
Subjects were also instructed to use the minimum practical
amount of articial room light from 17:00 to bedtime.
As determined by the acrophase of aMT6s excretion, there
were no group phase differences. Similarly, there were no dif-
ferences in (predominantly actigraphically measured, n = 2
sleep logs) sleep onset or offset times. When the group was
subdivided according to whether the baseline acrophase was
later or earlier than 06:00 (n = 23 and n = 22, respectively, used
as a measure to divide groups by the degree to which they were
physiologically delayed), signicant differences were noted
solely for the former group, but the condence interval crossed
the threshold of the predetermined clinically signicant mini-
mal change (mean difference −83.00 minutes in comparison to
the inactive condition [CI −8.09 to −157.91]) (see Table 2 and
Appendix, Table S6). No signicant differences were noted
with respect to actigraphically determined sleep onset and off-
set times among either subgroup. The light mask was never-
theless described as well tolerated, with little (actigraphically
measured) sleep disturbance.
1215
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
A separate group
85
performed a randomized controlled trial
of 7 days duration (n = 18, mean age 28.2 ± 10.6 years) using
post-awakening morning blue light in association with a grad-
ually advancing sleep/wake schedule. The portable source con-
tained light-emitting diodes (LEDs; 470 nm peak wavelength
with irradiance of 65 μW/cm
2
) attached to the lower rims of
spectacle frames, approximately 15 mm from the cornea, for
a duration of 2 hours, immediately after arising. While a bed-
time was not prescribed, subjects were instructed to advance
wake times by 30 minutes daily. The presence or absence of
blinding procedures was not specied. As compared to the
control group (lighting exposure unspecied), DLMO ad-
vanced by 2.53 hours (reported verbally and depicted in a g-
ure). However, there were no group differences in subjectively
assessed TST, SOT, or SOffT. Among other design limitations,
many details were missing with respect to methodology, and
there was high imprecision in the reported results.
Despite the present uncertainty as to benets, there are few
major risks associated with a trial of one of these “non-light
box” interventions. A review of adverse effects associated
with light interventions in general is addressed elsewhere (see
“Harms and Adverse Effects” section). It is interesting that the
light mask worn during the sleep episode in the Cole protocol
28
was well tolerated, with little (actigraphically measured) sleep
disturbance, as compliance with post-awakening light therapy
can be poor.
98
A laboratory-based study from Figueiro and Rea shows
promise for future “light masks” in the treatment of CRSWDs,
based upon optimal eyelid transmittance using green LEDs
(max wavelength of 527 nm) and individualized dose assess-
ments to predict optimal circadian benet.
99
Particularly rel-
evant to the discussion of combination therapies, at least three
laboratory-based studies among healthy adults describe a
synergistic effect (with respect to circadian phase advances)
when strategically timed light and melatonin therapy are used
together.
100–102
In addition, although physiologic phase assess-
ments were not employed, a separate case series described suc-
cess with this treatment combination among DSWPD patients
in the eld (age range 15–60 years). Three milligrams melatonin
was taken 2 hours prior to desired bedtime in conjunction with
either outdoor or 5000 lux white broad spectrum light exposure,
for a minimum of 30 minutes, between 06:00–08:00. During a
median follow-up period of 6.4 weeks, 82% reported improve-
ments of sleep patterns, with a mean sleep phase advance of ap-
proximately 2 hours.
103
Reported outcomes were unfortunately
not compatible with those selected by the TF for this review.
There is insufcient evidence to support the use of novel
forms of light therapy (i.e., via means other than a “light
box”), in association with concomitant behavioral instruc-
tions among adults with DSWPD (versus no treatment). No
recommendation.
5.2.9.2 light/coMbination treatMents for children/
adolescents with dswpd
Post-awakening light therapy in conjunction with behav-
ioral instructions is effective among adolescents with DSWPD,
but a physiologic circadian correlate is lacking. Level of re-
viewed evidence: LOW (Appendix, Table S7). This is a new
recommendation, based both upon the novel cohort (solely
children/adolescents) and light/behavioral multicomponent
interventions.
One non-blinded randomized controlled trial
104
was identi-
ed (n = 40), composed of adolescents aged 13–18 (mean ages
14.7 ± 1.7 and 14.7 ± 1.8 years in the active and control groups,
respectively). The minority of participants (20%) had mental
health comorbidities, and was spread evenly across the two
groups. Over a period of 8 weeks, active subjects (n = 23) were
exposed to either post-awakening natural sunlight (when avail-
able) or a broad spectrum lamp (~1000 lux, proximity to source
not specied) for ≥ 30 minutes (2 hours maximum), with the
time of administration advanced 30 minutes daily from “natu-
ral” wake time, until a target time of 06:00 was reached (3–5
weeks). Light therapy was subsequently discontinued and a reg-
ular rise time between 06:30–07:30 was advised. Concomitant
multicomponent behavioral education/interventions (includ-
ing instructions to “reduce evening light”) were provided in
six 45–60 minute sessions (with parental involvement), either
weekly (rst 4 sessions) or biweekly (last 2 sessions). Compli-
ance was monitored with sleep diaries, but afliated data were
not provided. An objective measure was not employed to mea-
sure light therapy compliance specically. The control group
was designated to a waitlist (n = 17).
Solely completed participants’ data were analyzed (i.e. an
intention-to-treat analysis was not undertaken). With respect
to CRITICAL outcomes during weekday assessments, sig-
nicant differences were detected with respect to subjective
TST (mean difference 72.00 minutes [CI 37.35 to 106.65]), and
ISL (mean difference −43.10 minutes [CI −22.46 to −63.74]).
Sleep onset and offset times also demonstrated signicant
group differences, but the condence interval crossed the
threshold of the predetermined clinically signicant minimal
change (mean difference −42.00 minutes [CI −2.74 to −81.26]
and −23.00 minutes [CI −0.87 to −45.13], respectively) (see
Table 2 and Appendix, Table S7). Weekend assessments
demonstrated benecial signicant differences for sleep onset
times (mean difference −93.9 minutes [CI −49.09 to −138.71]).
Signicant favorable differences were also noted with respect
to ISL and sleep offset times, but the condence interval again
overlapped the threshold of the predetermined clinically sig-
nicant minimal change (mean difference −26.5 minutes [CI
−4.37 to −48.63] and −51.0 minutes [CI −10.82 to −91.18], re-
spectively). No weekend differences were noted for TST. With
the exception of weekday sleep offset times, 6-month follow-
up assessments (n = 15) revealed no signicant differences in
comparison to the values observed immediately posttreatment.
5.2.9.2a The TF suggests that clinicians treat children
and adolescents with DSWPD with post-awakening light
therapy in conjunction with behavioral treatments (versus
no treatment). [WEAK FOR]
Summary: This is a new recommendation, based both upon
the novel cohort (solely children/adolescents) and light/behav-
ioral multicomponent interventions. The level of reviewed evi-
dence
104
was LOW (Appendix, Table S7), and solely weekday
data were considered with respect to determination of the rec-
ommendation, as this information is presumably most relevant
in the clinical setting. Light therapy occurred via exposure to
natural sunlight (when available), or with use of a white broad
1216
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
spectrum lamp (~1000 lux, proximity to source not specied),
for ≥ 0.5 hours (2 hours maximum), with the time of adminis-
tration advanced by 0.5 hours daily from “natural” wake time,
until a target time of 06:00 was reached. Light therapy was sub-
sequently discontinued, and behavioral interventions ensued.
Follow-up data are promising. Overall, a benets/harms ratio
analysis favors a trial of treatment, as children/adolescents with
DSWPD represent a particularly challenging patient population
(for a multitude of reasons), and the suggested interventions
pose no apparent safety concerns (see separate “Harms and Ad-
verse Effects” section). Clinical experience suggests that mo-
tivated patients would accept this treatment option versus no
treatment, particularly with active caregiver support.
5.3 Recommendations for the Treatment of N24SWD
N24SWD occurs when the hypothalamic circadian pace-
maker fails to entrain (synchronize) to the 24-hour day. As a
result, individuals can suffer from periodic nighttime insom-
nia and daytime somnolence as the circadian rhythms of sleep
propensity and alertness drift in and out of synchrony with the
24-hour day.
5
The condition primarily occurs in blind individu-
als, and at least 50% of the totally blind (i.e., those with no light
perception) are thought to suffer from the disorder.
105
While the
etiology in the blind is a loss of photic input to the pacemaker,
106
the pathophysiology among sighted individuals is unknown.
As stated above, entrainment status was uniquely utilized as
an outcome measure, as it physiologically denes this disorder
(just as elevated blood pressures characterize essential hyper-
tension). This was the sole outcome variable rated as CRITI-
CAL for N24SWD.
5.3.1 Prescribed Sleep-Wake Scheduling for Patients with
N24SWD
The previously published Practice Parameters
1
recom-
mended prescribed sleep-wake scheduling at an OPTION
level, noting that it may be a useful method to improve the
entrainment of circadian rhythms among sighted patients with
N24SWD. However, this guideline was created in the absence
of discrete evidence, as there have been no published trials
among sighted or blind patients.
There is some evidence that sleep timing (independent of
the timing of light exposure) is able to reset the circadian pace-
maker in humans,
20,107–109
and prescribed sleep-wake schedul-
ing therefore represents a potential therapeutic intervention
for N24SWD. However, this evidence is indirect, as data were
culled from basic science experiments conducted among non-
clinical populations.
There is no evidence to support the use of prescribed
sleep-wake scheduling in patients with N24SWD. No
recommendation.
5.3.2 Timed Physical Activity/Exercise for Patients with
N24SWD
There was no recommendation in the previous Practice Param-
eters
1
regarding timed physical activity/exercise as a treatment
for patients with N24SWD. Physical activity has been demon-
strated to reset the timing of the circadian pacemaker among
healthy subjects
110
and therefore represents another potential
therapy. Indeed, the circadian pacemaker can be entrained in
blind individuals in the absence of circadian photoreception.
111
As with prescribed sleep-wake scheduling, however, there have
not been any published trials. The evidence is therefore indirect
and insufcient to serve as the basis of a recommendation.
There is no evidence to support the use of timed physi-
cal activity or exercise in patients with N24SWD. No
recommendation.
5.3.3 Strategic Avoidance of Light for Patients with N24SWD
There is one case report that includes strategic avoidance of
light, and it is included below in the combination treatments
section.
112
The previous Practice Parameters
1
provided no per-
tinent recommendations.
There is no evidence to support the use of strategic avoid-
ance of light (as monotherapy) in patients with N24SWD.
No recommendation.
5.3.4 Light Therapy for Patients with N24SWD
The previous Practice Parameters recommended this treat-
ment at an OPTION level (for sighted individuals), based on
5 case series/reports,
112–116
all composed of 1–2 subjects. The
small numbers of study participants prevented inclusion for the
present report, and no studies have been published subsequently.
There is some experimental evidence that light is capable of
resetting the circadian pacemaker in the absence of conscious
light perception,
117
and most blind individuals who retain such
photic input to the circadian pacemaker would not be expected
to have N24SWD. However, some such individuals may have
insufcient exposure to light to maintain entrainment and
therefore timed light exposure may be a potential therapeu-
tic intervention in a subset of blind individuals with N24SWD.
However, as noted, there have been no studies that examined
this question.
There is insufcient evidence to support the use of light
therapy in patients with N24SWD (versus no treatment).
No recommendation.
5.3.5 Sleep-Promoting Medications for Patients with N24SWD
The previous Practice Parameters did not make any rec-
ommendation for the use of sleep-promoting medications in
N24SWD, and no new studies were identied.
There is no evidence to support the use of sleep-
promoting medications in patients with N24SWD. No
recommendation.
5.3.6 Timed Oral Administration of Melatonin or Agonists for
Patients with N24SWD
5.3.6.1 Melatonin for blind adult patients with n24swd
The previously published recommendation was designated
at the GUIDELINE level,
1
based upon 4 case reports
118–121
and
5 small observational studies.
122–126
Some of these studies, and
others, have examined important treatment parameters and
outcomes, including dose,
125,127,128
phase angle of entrainment,
126
and circadian time of administration,
129
but many were small,
often uncontrolled trials. Three placebo-controlled, crossover
studies from the previous Practice Parameters were eligible for
the current review,
122–124
and, despite the relatively large odds
ratio, the overall level of evidence for the CRITICAL outcome

1217
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
(circadian entrainment) was LOW (Appendix, Table S8), as
the data are from small observational investigations.
Sack and colleagues (n = 7, mean age 47.3 ± 5.0 years) ad-
ministered 10 mg of melatonin 1 hour prior to preferred bed-
time,
123
while Lockley et al. (n = 7, mean age 44.6 ± 8.4 years)
122
and Hack et al. (n = 10, mean age 48.2 ± 12.5 years)
124
adminis-
tered 5 mg and 0.5 mg of melatonin, respectively, at 21:00. The
duration of treatment for these studies was 26–81 days. Re-
sults (excluding subjects without placebo control or complete
CRITICAL outcome data) were combined for meta-analysis
of entrainment as a dichotomous outcome (Yes/No), using the
Mantel-Haezel test (see Figure 6 and Appendix, Table S8).
The odds ratio for entrainment was 21.18 [CI 3.22–39.17]. In
other words, the likelihood of entrainment with melatonin
was ~21 times higher in comparison to placebo. Although the
evidence level can only be graded as LOW due to the fact that
these are small observational studies, this is the best evidence
to date that melatonin is an effective treatment for N24SWD.
Taken together, these placebo-controlled studies represent the
largest number of N24SWD patients whose entrainment status
was assessed subsequent to melatonin treatment.
5.3.6.1a The TF suggests that clinicians use strategically
timed melatonin for the treatment of N24SWD in blind
adults (versus no treatment). [WEAK FOR]
Summary: This recommendation was designated at the
GUIDELINE level (for the blind) in the previous Practice Pa-
rameters.
1
Only 3 studies
122–124
met inclusion criteria for the
present analysis and the level of evidence from these small
trials was LOW (Figure 6 and Appendix, Table S8). Doses
ranged between 0.5–10.0 mg, and were administered either
1 hour prior to preferred bedtime, or at a xed clock hour
(21:00), for a period of 26–81 days. Patient preference would
be expected to favor the use of easily obtained and inexpensive
melatonin that requires once daily dosing. No serious adverse
reactions to melatonin have been described to date (see sepa-
rate “Harms and Adverse Effects” section), and therefore the
benets of use appear to outweigh any potential harms. A ma-
jority of well-informed patients and caregivers would therefore
accept this treatment option versus no treatment.
5.3.6.2 Melatonin for sighted patients with n24swd
The recommendation for sighted individuals was provided
at an OPTION level in the previous Practice Parameters,
1
based upon 4 case series.
115,130–132
None of these studies were
eligible for the current review, based upon insufcient num-
bers of subjects.
There is insufcient evidence to support the use of mela-
tonin among sighted patients with N24SWD (versus no
treatment). No recommendation.
5.3.7 Wakefulness-Promoting Medications for Patients with
N24SWD
The previous Practice Parameters did not make any per-
tinent recommendations regarding the use of wakefulness-
promoting medications in N24SWD, and no new studies were
identied.
As noted above, both sleep and activity have the potential
to reset the circadian pacemaker and therefore it is reasonable
to think that medications that promote either sleep or wakeful-
ness might be useful in the treatment of this disorder, either by
entrainment of the biological clock or by improving alertness
and sleep when individuals are sleeping out of synchrony with
the circadian pacemaker. Similar to sleep-wake scheduling
and physical activity however, there have not been any studies
of either of these approaches in N24SWD.
There is no evidence to support the use of wakefulness-
promoting medications in patients with N24SWD. No
recommendation.
5.3.8 Other Somatic Interventions for Patients with N24SWD
The previous Practice Parameters cited “insufcient evi-
dence” to support the use of oral vitamin B12 for use in sighted
individuals with N24SWD (OPTION), based on 2 open-label
case reports.
133,134
No studies have since been published per-
taining to the use of vitamin B12 (or other alternate somatic
interventions) for N24SWD.
There is insufcient evidence to support the use of oral
vitamin B12 (and no evidence to support alternate somatic
interventions) among patients with N24SWD (versus no
treatment). No recommendation.
5.3.9 Combination Treatments for Patients with N24SWD
The previous Practice Parameters did not discuss the use of
combination treatments in N24SWD. There are 4 uncontrolled
case reports involving a combination of treatments in sighted
individuals with N24SWD.
112,116,135,136
However, these studies
did not meet inclusion criteria for the present review.
Figure 6—Meta-analysis of evidence for entrainment as a result of melatonin treatment of blind adult patients with N24SWD.
1218
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
There is insufcient evidence to support the use of com-
bination treatments in patients with N24SWD (versus no
treatment). No recommendation.
5.4 Recommendations for the Treatment of ISWRD
ISWRD is diagnosed when patients exhibit no clear cir-
cadian pattern of sleep-wake behavior. Aficted individuals
demonstrate wakefulness during conventional sleeping hours
and bouts of sleep during the day.
5
The condition is observed
most commonly among patients with neurodevelopmental or
neurodegenerative disorders, and can pose particular chal-
lenges for caregivers.
5.4.1 Prescribed Sleep-Wake Scheduling for Patients with
ISWRD
There was no recommendation in the previous Practice
Parameters regarding prescribed sleep-wake scheduling as a
stand-alone treatment for patients with ISWRD, and no new
studies were identied. However, prescribed schedules have
been tested in combination with other modalities (see section
5.4.9, “Combination Treatments”).
There is no evidence to support the use of prescribed
sleep-wake scheduling as a stand-alone treatment for pa-
tients with ISWRD. No recommendation.
5.4.2 Timed Physical Activity/Exercise for Patients with
ISWRD
There was no recommendation in the previous Practice Pa-
rameters regarding timed physical activity/exercise as a sole
treatment for patients with ISWRD, and no new studies were
identied. However, scheduled activity has been used in com-
bination with other modalities (see section 5.4.9, “Combination
Treatments”).
There is no evidence to support the use of timed physical
activity or exercise as a stand-alone treatment for patients
with ISWRD. No recommendation.
5.4.3 Strategic Avoidance of Light for Patients with ISWRD
There was no recommendation in the previous Practice Pa-
rameters regarding strategic avoidance of light as a treatment
for patients with ISWRD, and no new studies were identied.
There is no evidence to support the use of strategic avoid-
ance of light as a treatment for patients with ISWRD. No
recommendation.
5.4.4 Light Therapy for ISWRD in Elderly Patients with
Dementia
In contrast to the present review, the 2007 Practice Param-
eters
1
did not separate ISWRD treatment recommendations by
patient subgroup. The section that follows (and other sections)
is subcategorized as warranted.
The recommendation in the 2007 Practice Parameters was
designated as an OPTION, but only one
137
of the previously
reviewed studies
138–145
and one new study
146
met inclusion cri-
teria for the current document. Although neither demonstrated
improvements in CRITICAL outcomes, one
137
showed that
light therapy improved behavioral symptoms, an area of sig-
nicant clinical importance. The cumulative level of analyzed
evidence was VERY LOW (Appendix, Table S9).
Both reviewed light therapy studies examined institutional-
ized elderly subjects with dementia.
137,146
In their randomized
clinical trial of 50 nursing home patients (mean age 86 ± 8
years), Dowling and colleagues compared 60 minutes of light
therapy scheduled from 09:30–10:30 (1.5 hours after the nurs-
ing home’s set rise time) 5 days per week for a period of 10
weeks, to a dim-light control condition consisting of indoor
light of 150–200 lux.
146
Bright light was delivered via white
broad-spectrum light boxes, positioned on tables and located
approximately 30 to 34 inches from participants’ eyes. Light
levels in the direction of gaze were monitored for each partici-
pant during each treatment session to maintain levels > 2500
lux. At the end of the intervention, average actigraphically
measured TST did not differ between active and control groups.
A second open-label study tested light therapy of 2 hours du-
ration between 09:00–11:00 among 14 patients (mean age 75
years, range 61–83) with sleep disturbances. Patients were
treated for 4 weeks with white broad-spectrum tabletop light
boxes that produced 3000–5000 lux and were positioned at a
distance of 1 meter from the participants. As with the Dowl-
ing study, no improvements in TST (subjective) were demon-
strated, but the study did show improvement of caregiver-rated
behavioral symptoms, including decreases in wandering, vio-
lent behavior, restlessness, and symptoms of delirium.
137
Other research that was ineligible for this review may none-
theless inform clinicians’ decision-making regarding the use
of light therapy among demented elderly patients with ISWRD.
Riemersma van der Lek and colleagues
147
randomized twelve
assisted-living facilities in the Netherlands to common areas
that were lit with ceiling xtures that emitted either bright
white broad spectrum (1000 lux) or dim light (300 lux). Par-
ticipants in the bright light facilities had lower TST compared
to participants living in a dim light facility at two follow-up
assessments (6 weeks and 6 months after the change in facil-
ity light levels). Other outcomes, including cognitive decits
and depression, were improved in association with the light
intervention, however. Additional studies cited in the previous
Practice Parameters and associated review
1,3
treated subjects
with white broad-spectrum articial light or outdoor light at
levels ranging from 1000–8000 lux. Durations of daily light
exposure ranged from 45–120 minutes and treatment dura-
tions lasted from 10 days to 11 weeks.
138–145
Although presently
dened CRITICAL sleep outcomes were not analyzed, some
of these studies showed positive effects of light exposure on
24-hour rest-activity rhythms, with more consolidated rest pe-
riods at night and more activity and fewer naps during the day.
5.4.4a The TF suggests that clinicians treat ISWRD in
elderly patients with dementia with light therapy (versus
no treatment). [WEAK FOR]
Summary: This recommendation was designated as an
OPTION in the 2007 Practice Parameters, and only one sub-
sequent study has been published that met inclusion criteria
for the current document.
146
The cumulative level of reviewed
evidence (2 studies)
137,146
was VERY LOW (Appendix, Table
S9), and none of the TF-dened CRITICAL outcomes showed
improvement. Behavioral symptoms nevertheless improved in
the sole study that measured this outcome.
137
The interventions
consisted of white broad spectrum light therapy, 2500–5000
1219
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
lux (~1 meter from participants), 1–2 hours in duration, be-
tween 09:00–11:00, for a period of 4–10 weeks.
137,146
Benets of
treatment are closely balanced with harm/burden. In addition
to the general side effects reported in the “Harms and Adverse
Effects” section, other side effects in this population range from
complaints of eye irritation
138
to agitation and confusion,
141
and
these potential drawbacks should be considered when recom-
mending treatment. Furthermore, depending on the method
and setting of light delivery, treatment may be labor intensive,
and modest improvements in outcomes may not justify associ-
ated costs. Nevertheless, clinical experience suggests that the
majority of well-informed patients and/or caregivers of elderly,
demented patients with ISWRD would choose light therapy in
comparison to no intervention.
5.4.5 Sleep-Promoting Medications for ISWRD in Elderly
Patients with Dementia
There was no recommendation in the previous Practice Pa-
rameters regarding sleep-promoting medications for patients
with ISWRD, and no new studies have been published. Various
investigations have concluded, however, that hypnotic medica-
tions increase risks of adverse events within this population
(see “Harms and Adverse Effects” section).
5.4.5a The TF recommends that clinicians avoid the use
of sleep-promoting medications to treat demented elderly
patients with ISWRD (versus no treatment). [STRONG
AGAINST]
Summary: This is a new recommendation in comparison
to the previous Practice Parameters, which did not address the
use of sleep-promoting medications (other than melatonin) for
ISWRD. Although no relevant subsequent studies have been
published, other extant literature indicates that administration
of hypnotics to demented elderly patients increases risks of
falls and other untoward outcomes Altered pharmacokinetics
observed with aging may be one mechanism by which hypnot-
ics increase adverse events in older adults.
148
Risk appears to
be increased even further in elderly patients with dementia,
149
particularly when used in combination with other medica-
tions
150
(also see separate “Harms and Adverse Effects” sec-
tion). Thus, the risk of harm from use of hypnotics in demented
elderly patients with ISWRD outweighs potential positive ef-
fects. As such, the vast majority of well-informed patients and/
or caregivers would not select this treatment.
5.4.6 Timed Oral Administration of Melatonin or Agonists for
Patients with ISWRD
5.4.6.1 Melatonin for elderly patients with deMentia and
iswrd
In the 2007 Practice Parameters, melatonin was deemed
“not indicated” for this specic population, based upon two
studies,
151,152
only one of which was eligible for the current re-
view.
151
No subsequently published relevant studies were iden-
tied. Melatonin administration did not signicantly improve
the predened CRITICAL outcome of TST. Level of evidence:
LOW (Appendix, Table S10).
Twenty-ve patients with dementia and ISWRD (mean age
84.2 ± 7.6 years) were enrolled in a double-blind crossover trial
of 6 mg slow-release melatonin versus placebo.
151
Participants
were studied for a 2-week baseline period and were then ran-
domized to receive either 6 mg slow-release melatonin or pla-
cebo for 2 weeks at their usual bedtimes, followed by a 1-week
washout period and crossover to the second study period.
Mean TST estimated with actigraphy did not differ between
the two groups.
Other research may inform clinical decision-making in this
area. Singer and colleagues examined the effects of treatment
with 2.5 mg slow-release or 10 mg immediate-release melatonin
administered 1 hour before bedtime (versus placebo) on acti-
graphically estimated TST in patients with Alzheimer’s disease
and sleep disturbance.
152
The patients in this study did not have
discreet diagnoses of ISWRD and data were therefore not eli-
gible for inclusion in the present review. Nevertheless, in keeping
with the results of Serfaty and colleagues,
151
this large, well-
designed trial also failed to show an improvement in TST with
either dose of melatonin compared to placebo. Riemersma van
der Lek and colleagues
147
published another potentially relevant
combination study among dementia patients in assisted living in
whom ISWRD was not specically identied. In contrast to the
other studies, the melatonin-only arm (2.5 mg immediate-release
formulation administered approximately one hour before bed-
time) demonstrated decreased actigraphic ISL and increased TST
compared to placebo. However, detrimental effects of melatonin
on mood and daytime functioning were also observed.
5.4.6.1a The TF suggests that clinicians avoid the use of
melatonin as a treatment for ISWRD in older people with
dementia (versus no treatment). [WEAK AGAINST]
Summary: Melatonin was deemed “not indicated” for the
treatment of ISWRD in older people with dementia (OPTION)
in the previous Practice Parameters. The present recommen-
dation against melatonin treatment is based on one reviewed
study that failed to show benet with respect to the CRITICAL
outcome of TST (Level of evidence: LOW (Appendix, Table
S10).
151
Furthermore, there is evidence that melatonin could be
harmful in this population.
147
Thus, the risk-benet ratio sug-
gests that the potential for harms outweighs the possibility for
benets. Clinical experience therefore dictates that the ma-
jority of older patients with dementia and/or their caregivers
would not favorably accept a trial of melatonin.
5.4.6.2 Melatonin in children/adolescents with iswrd
and neurologic disorders
Melatonin was recommended at the OPTION level in the
previous Practice Parameters for children with various neuro-
logic disorders,
1
based upon four studies,
153–156
none of which
was eligible for the current review, due to an insufcient num-
ber of participants or grouping of participants with different
CRSWDs. One new eligible study was identied,
157
and mela-
tonin was shown to improve select predened CRITICAL out-
comes (TST, ISL), although the condence interval associated
with both values crossed the threshold of the predetermined
clinically signicant minimal change (see Table 2). The level of
reviewed evidence was MODERATE (Appendix, Table S11).
Wright and colleagues
157
performed a double-blind, ran-
domized, controlled, crossover trial in 16 children (mean age
9 ± 2.9 years) with autism spectrum disorder. The protocol
1220
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
consisted of 1-month baseline, 3-months melatonin vs. placebo,
1-month washout, 3-months melatonin vs. placebo (cross-
over), and 1-month medication free. Melatonin dosing (using
a “standard release” formulation administered 30–40 minutes
before planned bedtime) started at 2 mg, and parents were
given the option of increasing the dose by 2 mg every 3 days,
until 50% or more improvement in sleep was observed, up
to 10 mg. The average nal dose during the melatonin arm
was 7 ± 3.0 mg (range 2–10 mg). Parents’ subjective reports of
ISL and TST were improved, although both values crossed the
threshold of the predetermined clinically signicant minimal
change (mean difference −51.71 minutes [CI −13.49 to −89.93]
and 48.45 minutes [CI 6.29–90.61 minutes], respectively) (see
Table 2).
Studies that were not eligible for analysis may nonetheless
provide additional relevant clinical information. In an open-
label observational trial in ve children with severe develop-
mental disabilities and disrupted sleep-wake patterns, Pillar
and colleagues
153
found that 3 mg melatonin administered
at 18:30 increased actigraphically recorded TST. Other trials
have tested the effects of melatonin for nocturnal sleep distur-
bance in children with a range of neurodevelopmental difcul-
ties.
154 –156
Many of the children included in these studies had
at least one comorbid disability (e.g., epilepsy, blindness), and
all had disturbed sleep-wake patterns. Although considerable
inter-individual variability was observed in the response to
melatonin, all of the studies showed signicant improvements
in at least one sleep measure. Another important nding from
these studies is the relative safety of melatonin in this popula-
tion as no signicant side effects were observed.
5.4.6.2a The TF suggests that clinicians use strategically
timed melatonin as a treatment for ISWRD in children/
adolescents with neurologic disorders (versus no
treatment). [WEAK FOR]
Summary: This recommendation was designated as an OP-
TION in the 2007 Practice Parameters, but none of the reviewed
studies were eligible for the current analysis. One subsequently
published eligible study was identied, with a MODERATE
level of evidence
157
(Appendix, Table S11). The data indicate
that melatonin administration of 2–10 mg during the hour be-
fore planned bedtime may improve CRITICAL sleep outcomes
in children/adolescents with neurologic disorders and ISWRD;
although condence intervals associated with positive values
crossed the threshold of the predetermined clinically signi-
cant minimal change (see Table 2). Another caveat is that this
recommendation is culled from a small sample of patients
with a range of developmental disorders. As such, it may not
generalize to all children/adolescents with ISWRD/neurologic
disorders. Although no serious adverse reactions have been
described in relation to melatonin use to date, relevant con-
cerns have been raised by select studies with respect to the
pediatric/adolescent population,
94
and rigorous long-term data
are lacking (see separate “Harms and Adverse Effects” sec-
tion). Nevertheless, clinical experience suggests that a majority
of patients and caregivers would accept this treatment option
(versus no treatment), particularly taking into account signi-
cant burdens associated with the neurologic disabilities and
severe associated sleep disturbances.
5.4.7 Wakefulness-Promoting Medications for Patients with
ISWRD
There was no recommendation in the previous Practice Pa-
rameters regarding wakefulness-promoting medications as a
treatment for patients with ISWRD, and no new studies were
published.
There is no evidence to support the use of wakefulness-
promoting medications as a treatment for patients with IS-
WRD. No recommendation.
5.4.8 Other Somatic Interventions for Patients with ISWRD
There was no recommendation in the previous Practice Pa-
rameters regarding other somatic interventions as a treatment
for patients with ISWRD, and no subsequent relevant studies
have been published.
There is no evidence to support the use of other somatic
interventions for the treatment of patients with ISWRD.
No recommendation.
5.4.9 Combination Treatments in Demented Elderly Adults
with ISWRD
This recommendation was designated as a GUIDELINE
in the previous Practice Parameters,
1
based upon two stud-
ies, neither of which were included in the present analysis, ei-
ther because participants did not clearly have a diagnosis of
ISWRD
158
or because predened CRITICAL outcomes were
not measured.
159
One randomized controlled trial pertaining to
the treatment of demented elderly patients with ISWRD
146
was
published subsequent to 2007, and did not demonstrate benet
with respect to predened CRITICAL outcomes. The level of
reviewed evidence was VERY LOW (Appendix, Table S12).
Dowling and colleagues
146
examined sleep-related out-
comes from the combination of light treatment (> 2500 lux
white broad spectrum light delivered by light boxes at a dis-
tance of 30–34 inches from the eye between 09:30–10:30 for 10
weeks) and either melatonin (5 mg immediate-release between
17:00–18:00) or placebo among 32 nursing home patients with
Alzheimer’s disease (mean age 86 ± 8 years). The dim-light
control condition was exposed to indoor light of 150–200 lux
(see additional study details in section 5.4.4.1 in this paper).
The intervention did not signicantly improve actigraphically
estimated TST, the sole investigated CRITICAL outcome.
Various other studies were ineligible for the current analy-
sis, but nonetheless bear potential relevance to clinicians. Two
non-blinded, randomized trials examined multimodal treat-
ments that included daytime activity, bright light exposure,
and sleep scheduling in community-dwelling elderly patients
with dementia.
158,160
The results demonstrated signicant de-
creases in nighttime wakefulness, and greater adherence to the
intervention was associated with more improvement. Combi-
nation treatments involving prescribed sleep-wake scheduling,
light exposure and increased daytime activity have also been
examined in two 5-day studies among demented, elderly nurs-
ing home residents.
159,161
Participants were required to be out of
bed from 08:00 to 20:00, had scheduled low-intensity physical
activity 3 times per day, and exposure to at least 30 minutes of
outdoor sunlight daily. Other procedures included implemen-
tation of a structured nighttime routine by caregivers, and min-
imization of nighttime noise and interventions. Although these

1221
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
studies did not measure outcomes dened by the TF, the multi-
modal interventions signicantly improved daytime function-
ing and amplitude of rest-activity rhythms. The investigations
were restricted by constraints inherent to the nursing home
environment (e.g., high dropout rate, inability to blind raters
to condition), yet both had relatively large sample sizes. An
additional caveat is that many more participants were screened
than were eligible for participation, such that the results may
not generalize to all patients with dementia.
5.4.9.1a The TF suggests that clinicians avoid the use
of combined treatments consisting of light therapy in
combination with melatonin in demented, elderly patients
with ISWRD (versus no treatment). [WEAK AGAINST]
Summary: This recommendation was designated as a
GUIDELINE in the previous Practice Parameters. One rel-
evant randomized controlled trial
146
was published subsequent
to 2007. The level of reviewed evidence from this single study
was VERY LOW (Appendix, Table S12). Including melatonin
as part of a combination treatment with light therapy does not
appear to confer additional benet
146
and may increase the po-
tential for harms.
147
Clinical experience suggests that patients/
caregivers would carefully consider the risks of depression
and withdrawn behaviors with treatments that include melato-
nin. Thus, the majority of patients/caregivers would not accept
combination treatments consisting of melatonin and bright
light (versus no treatment). Other combination treatments (e.g.,
bright light, scheduled sleep-wake, and physical activity) are
worthy of further investigation.
5.4.9.2 coMbination treatMents for children/adolescents
with iswrd
The previous Practice Parameters recommendation was des-
ignated at the OPTION level, based upon the results of one
study.
162
No new studies were identied. The previously cited
investigation was an open trial in children with moderate-to-
severe mental retardation and associated nocturnal sleep dis-
turbances, and employed combination treatment with light
therapy, prescribed sleep-wake schedules, and timed daytime
activit y.
162
Five of 14 patients showed improvements in TST
measured on sleep diaries (completed by parents). However, as
it was not clear that these patients met diagnostic criteria for
ISWRD, this study was not eligible for analysis in the present
review.
There is insufcient evidence to support the use of com-
bination treatments in children/adolescents with ISWRD
(versus no treatment). No recommendation.
6.0 CONCLUSIONS AND FUTURE DIRECTIONS
Circadian-based basic science developments continue to
outpace clinical research pertaining to CRSWDs. Since pub-
lication of the prior Practice Parameters,
1
relatively few new
studies have emerged, although it is encouraging that inves-
tigations specically oriented to the pediatric/adolescent and
other “special” populations have been published. The major
change with the present review is the use of the GRADE sys-
tem of analysis. While more rigorous in many respects than the
previously employed evidence-based assessment, derived data
is designed to be more clinically relevant, as the GRADE sys-
tem requires a combined consideration of strength of evidence,
in conjunction with risk/benet analyses and determination
of patient values and preferences. As such, many previously
endorsed practice recommendations have been negated, and
numerous PICO questions remain unanswered. While this cer-
tainly points out the signicant gaps with respect to the clinical
CRSWD research (highlighted by the prior Standards of Prac-
tice group),
1
these updated Practice Parameters are intended
to provide clinicians with heightened condence in prescrib-
ing treatments and, equally importantly, they should serve as
a roadmap for future studies that will propel higher quality,
more sophisticated CRSWD therapies.
Generally speaking, larger more rigorously designed stud-
ies (randomized placebo-controlled trials) with ICSD-3 de-
ned CRSWDs
5
are required, and replication of results from
separate centers is essential. Clinical research pertaining to
ASWPD and ISWRD in particular has suffered signicantly
due to a lack of adherence to International Classication of
Sleep Disorders diagnostic criteria. In the specic case of AS-
WPD, one can only conjecture that the results of light therapy
studies that address sleep maintenance/early-morning awak-
ening difculties are applicable to the treatment of ASWPD,
and uncertainty will remain unless strict terminology are used.
Others have raised concerns about the frequency at which one
actually encounters patients with this condition in the clinical
arena,
163
a topic that is beyond the scope of this discussion. As
for ISWRD, the term is rarely used in the medical literature,
presumably because the comorbid disorders that frequently
overlap (e.g., dementia, developmental disabilities) tend to
overshadow the CRSWD. Nevertheless, sleep disturbances are
among the most onerous of difculties for caregivers of these
patients. Thus, lack of consideration of the formal diagnosis of
ISWRD in studies of sleep in these populations makes it dif-
cult to identify effective treatments for this important clinical
problem.
More specically, future studies could advance the eld by
including detailed therapeutic information, such as the method
and means of treatment delivery (e.g., protective eyewear vs. vo-
litional avoidance of light, light therapy intensity/wavelength/
proximity, continuous versus pulsed light administration [in-
cluding gradually versus abruptly changing illumination
164
],
melatonin formulation, etc.), relationship of treatment timing
with respect to a dened physiologic circadian phase marker or
other sleep parameter, inclusion/exclusion of prescribed sleep/
wake schedules or other behavioral interventions, and study en-
vironment (laboratory vs. non-laboratory). Regarding the latter
factor, eld-based studies are sorely needed, and one must be
cautious not to let tightly controlled bench research prema-
turely dictate clinical treatment. As a prime example, there are
currently no data to support devices that solely deliver blue
short wavelength light in the treatment of CRSWDs, and two
laboratory-based studies that describe no additional benet
with blue-enriched bright light,
24,25
despite the fact that these
wavelengths have been identied as especially important for
circadian phase resetting in non-clinical experiments.
2
More
importantly, compliance with post-awakening “light boxes” in
the eld is very poor,
98
and studies that examine the bypassing
of this compliance barrier are particularly intriguing.
28,99,165,166
1222
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
Future research should address “dose” of light including lux
level and duration,
17
and should also consider season
167
and
other environmental factors that affect overall light exposure
histor y.
19
Finally, more such studies specically targeting
CRSWD populations are desired.
From the standpoint of outcomes, similar clinically relevant
sleep-related measures will be required for inter-study com-
parative purposes (PSG vs. actigraphy vs. subjective reports,
physiologic or nonphysiologic circadian marker), along with
systematic measures of treatment compliance, to accurately
inform clinical practice. In the instance of ISWRD, it should
be determined whether separate outcome measures (e.g., circa-
dian amplitude, rest-activity cycle variations) may be superior
indicators of treatment efcacy. For all CRSWDs, measures
of daytime functioning/alertness could enhance clinical
relevance.
Inter-study medication comparisons will require equivalent
dosing (analyzed melatonin study doses ranged from 0.3–10.0
mg), timing (with respect to clock time, typical sleep onset
time or other physiologic/non-physiologic circadian marker),
and treatment durations, to accurately gauge benet. This
guideline does not address specic medication/supplement
doses (but rather states those cited in the literature). Optimal
dosing may vary between CRSWDs. Furthermore, signicant
inter-individual differences may also exist within singular
CRSWDs, as exemplied by a study of N24SWD subjects that
showed a tenfold variation in entraining doses.
136
The issue of
formulation may also be relevant in melatonin studies (regu-
lar- vs. sustained-release vs. sublingual, etc.), and one group
suggested that slow exogenous melatonin metabolism could be
responsible for a lack of sustained effect in select instances.
168
Taking into account melatonin safety concerns (particularly
among children and those of reproductive age), future properly
powered studies should be performed to identity the lowest
effective melatonin dosage and duration of treatment (acute
and maintenance). Long-term physiologic studies are needed
to accurately ascertain any serious chronic risks, particularly
as melatonin supplements are not subject to FDA oversight.
94
In January of 2014, the FDA approved the melatonin ago-
nist Hetlioz
TM
(tasimelteon) for the treatment of N24SWD
among the blind. This is the rst FDA-approved drug for any
CRSWD. Since the last date of our latest literature search, the
results of a peer-reviewed, randomized, placebo-controlled
study were published.
169
This is the largest treatment study
with N24SWD patients, and it demonstrated that Tasimelteon
safely and effectively treated aficted patients: entrainment
occurred in 20% (8 of 40) of patients receiving the drug com-
pared with 3% (1 of 38) receiving placebo. This entrainment
rate is lower than the 67% entrainment rate (12 of 18) found in
the current meta-analysis of melatonin treatment in N24SWD,
but this may have been a product of the short duration of treat-
ment with Tasimelteon prior to the assessment of entrain-
ment, as noted by the authors. Consistent with this hypothesis,
higher rates of entrainment were found during longer, open-
label treatment with tasimelteon. Direct comparisons between
Tasimelteon and melatonin have yet to be conducted. At least
two other investigations (involving ramelteon) also sug-
gest a potential future CRSWD treatment role for melatonin
agonists.
170,171
Related to long-term risks of circadian-based interventions
in general, research is needed to determine the minimum re-
quired duration of specic treatments (or to determine that
they are required indenitely), and/or to determine mainte-
nance treatment schedules. Further studies that investigate
multi-modal or combination therapies are needed to determine
whether combinations may prove to be synergistic, and/or to
extricate independent effects of treatment modalities, so that
relative successes and failures can be exploited for differ-
ing clinical scenarios. With respect to the latter point, in the
previously cited Gradisar study (involving adolescents with
DSWPD),
104
light therapy was discontinued (and apparently
not required) once a target wake time was reached, at which
time solely behavioral interventions ensued. It is not clear to
what degree this treatment could be generalized to all DSWPD
populations.
Demonstration of superiority (or lack thereof) of circadian
versus clock-hour TOA for interventions should engender
studies that aim to explore demonstrable benets of phase as-
sessments in the clinical setting, which in turn could serve to
delineate relative chronobiotic versus hypnotic effects of medi-
cations/supplements. Some of the reviewed interventions dem-
onstrated successful sleep-related outcomes without changes
in the circadian phase marker and vice versa. In the instance
that the importance of circadian TOA is demonstrated, it will
be necessary to determine light and melatonin phase PRCs for
adult populations aficted with CRSWDs (as they may dif-
fer from normal populations
15,172
) and to determine the same
for both aficted and healthy pediatric/adolescent popula-
tions. Complicating matters, alterations in phase relationships
between the circadian timing system and the timing of sleep
among those with CRSWDs could impact the ability of inter-
ventions to exert benets, even with knowledge of the PRC.
For example, longer intervals from various endogenous mela-
tonin parameters
173
and CBT
min
174 –176
to sleep offset have fre-
quently been described among adult patients with DSWPD
as compared to controls. However, this nding has not been
demonstrated among protocols in which subjects are forced to
maintain a more conventional sleep/wake schedule,
27,177,178
sug-
gesting that this observation may simply be a consequence of
longer habitual TST. Greater elucidation is required. On a sep-
arate note, effective treatments may need to address concomi-
tant impairment of homeostatic sleep processes in CRSWDs,
as has been demonstrated in DSWPD and among adolescents
in general.
179,180
Whether hypnotics have a role in this setting
deserves to be further explored.
89
Present guidelines predominantly reect biological under-
pinnings associated with CRSWDs. Studies are needed to
investigate and understand predominant exogenous and en-
dogenous contributors to the development and perpetuation
of CRSWDs, so that different subtypes (and possibly differ-
ent treatment/prophylactic regimens) can be identied. In the
case of adolescents/young adults (and, to a lesser degree, other
adults
181
), numerous exogenous factors, such as increased auton-
omy with respect to sleep time, employment, and involvement
in extracurricular activities have been identied as variables
contributing to the generally observed delay in sleep/wake pat-
terns,
182
but have not been studied among adolescent DSWPD
cohorts specically.
183,184
Additionally, repeated exposure to

1223
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
frustrations at not being able to fall asleep at a desired time
can lead to the development of a concomitant conditioned in-
somnia, which can perpetuate sleep difculties. Exposure to
indoor lighting during evening hours
185 –188
and/or delays in
weekend wake times
189–191
have also been implicated as con-
tributors to persistently delayed sleep/wake times, but have not
been specically implicated in adolescent DSWPD.
192
Some
have urged that school lighting environments be optimized for
maximal circadian benets.
193
In the case of N24SWD, it may
be that the exogenous and endogenous contributors to the dis-
order differ between blind and sighted individuals and that this
may similarly necessitate different treatment regimens. Iden-
tication and manipulation of exogenous variables in trials of
CRSWDs may prove fruitful.
The associated development of clinical proles would en-
able clinicians to better ascertain which patients might respond
to suggested treatments, and related research is encouraged.
In the Gradisar study involving adolescents with DSWPD,
104
school non-attendance, unrestricted sleep during vacation pe-
riods, and (not surprisingly) amotivation were all noted to be
barriers to successful outcomes with light therapy. Patients t-
ting this prole are perhaps better suited to less complex inter-
ventions. In a separate study involving young adult subjects
with DSWPD and N24SWD receiving melatonin, a higher
response rate correlated indirectly with shorter habitual TST,
as well as a later age of onset.
131
Information such as this may
eventually allow clinicians to optimally tailor treatment.
In select cases, accommodation to a CRSWD patient’s cir-
cadian preference may be most practical, and further studies
examining implementation of such schedules are desirable.
Believing that some CRSWD cases are refractory to treat-
ment, Dagan and Abadi (2001)
194
recommended foregoing
therapy (specically among DSWPD patients), and instead
urged implementation of rehabilitation and accommodation to
the preferred sleep/wake schedule in select instances, includ-
ing support for disability from duties that require strict sleep/
wake schedules, and encouragement to pursue endeavors with
more exible scheduling. The benets of such accommodation
were demonstrated in a separate military-based study, with
evidence of superior performance and mood among those en-
abled to adapt a relatively delayed sleep/wake schedule, which
correlated with increased TST.
195
A later school start time may
be sought for adolescents, if practical and available. This inter-
vention alone can signicantly increase TST and mitigate as-
sociated impairments.
196 –199
Unfortunately, the implementation
of this policy change frequently encounters staunch political
resistance and is presently available in select regions only.
200
In sum, although much work remains, signicant progress
has been made in the recognition/treatment of CRSWDs since
the inception of Sleep Medicine as a distinct medical disci-
pline. Our aim with the present guidelines is to provide clini-
cians with immediate access to up-to-date information in order
to make properly informed treatment decisions. The circadian
system impacts a wide range of physiology, and treatment of
CRSWDs would therefore be expected to exert a wide-ranging
impact on manifold aspects of health. This publication should
serve as an impetus to address clinical research deciencies
and to promote novel inquiries for treatments of these chal-
lenging and interesting conditions.
ABBREVIATIONS
ADHD, attention decit hyperactivity disorder
aMT6s, 6-sulfatoxymelatonin (urinary metabolite of
melatonin)
CBT
Min
, core body temperature minimum
DLMO, dim light melatonin onset
GRADE, grading of recommendations assessment,
development and evaluation
ISL, initial sleep latency
PSG, polysomnography
SOT, sleep onset time
SOffT, sleep offset time
TF, task force
TOA, time of administration
TST, total sleep time
REFERENCES
1. Morgenthaler TI, Lee-Chiong T, Alessi C, et al. Practice parameters for the
clinical evaluation and treatment of circadian rhythm sleep disorders. An
American Academy of Sleep Medicine report. Sleep 2007;30:1445–59.
2. Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part
I, basic principles, shift work and jet lag disorders. An American Academy of
Sleep Medicine review. Sleep 2007;30:1460–83.
3. Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part
II, advanced sleep phase disorder, delayed sleep phase disorder, free-running
disorder, and irregular sleep-wake rhythm. An American Academy of Sleep
Medicine review. Sleep 2007;30:1484–501.
4. American Academy of Sleep Medicine. International classication of sleep
disorders, 2nd ed. Westchester, IL: American Academy of Sleep Medicine,
2005.
5. American Academy of Sleep Medicine. International classication of sleep
disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine, 2014.
6. Borbely AA. A two process model of sleep regulation. Hum Neurobiol
1982;1:195–204.
7. Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm
following suprachiasmatic lesions in the rat. Brain Res 1972;42:201–6.
8. Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor
activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U
S A 1972;69:1583–6.
9. Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic
nucleus determines circadian period. Science 1990;247:975–8.
10. Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons
dissociated from rat suprachiasmatic nucleus express independently phased
circadian ring rhythms. Neuron 1995;14:697–706.
11. Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-
hour period of the human circadian pacemaker. Science 1999;284:2177–81.
12. Burgess HJ, Eastman CI. Human tau in an ultradian light-dark cycle. J Biol
Rhythms 2008;23:374–6.
13. Dunlap JC, Loros JL, DeCoursey PJ. Chronobiology: biological timekeeping.
Sinauer Associates, Inc., 2004.
14. Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses
melatonin secretion in humans. Science 1980;210:1267–9.
15. St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW.
Human phase response curve to a 1 h pulse of bright white light. J Physiol
2012;590:3035–45.
16. Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the
human circadian pacemaker to nocturnal light: melatonin phase resetting and
suppression. J Physiol 2000;526 Pt 3:695–702.
17. Chang AM, Santhi N, St Hilaire M, et al. Human responses to bright light of
different durations. J Physiol 2012;590:3103–12.
18. Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on
the suppression of melatonin by light in humans. J Pineal Res 2002;33:198–
203.
19. Chang AM, Scheer FA, Czeisler CA. The human circadian system adapts to
prior photic history. J Physiol 2011;589:1095–102.
1224
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
20. Buxton OM, L’Hermite-Baleriaux M, Turek FW, van Cauter E. Daytime naps in
darkness phase shift the human circadian rhythms of melatonin and thyrotropin
secretion. Am J Physiol Regul Integr Comp Physiol 2000;278:R373–82.
21. Burgess HJ, Molina TA. Home lighting before usual bedtime impacts circadian
timing: a eld study. Photochem Photobiol 2014;90:723–6.
22. Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression:
evidence for a novel non-rod, non-cone photoreceptor system in humans.
J Physiol 2001;535:261–7.
23. Brainard GC, Hanin JP, Greeson JM, et al. Action spectrum for melatonin
regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci
2001;21:6405–12.
24. Smith MR, Eastman CI. Phase delaying the human circadian clock with blue-
enriched polychromatic light. Chronobiol Int 2009;26:709–25.
25. Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian
clock with blue-enriched polychromatic light. Sleep Med 2009;10:287–94.
26. RevMan 5.2. The Nordic Cochrane Center, The Cochrane Collaboration.
Copenhagen, 2012.
27. Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC. Phase-
dependent treatment of delayed sleep phase syndrome with melatonin. Sleep
2005;28:1271–8.
28. Cole RJ, Smith JS, Alcala YC, Elliott JA, Kripke DF. Bright-light mask treatment
of delayed sleep phase syndrome. J Biol Rhythms 2002;17:89–101.
29. van Geijlswijk IM, van der Heijden KB, Egberts AC, Korzilius HP, Smits
MG. Dose nding of melatonin for chronic idiopathic childhood sleep onset
insomnia: an RCT. Psychopharmacology 2010;212:379–91.
30. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-
GRADE evidence proles and summary of ndings tables. J Clin Epidemiol
2011;64:383 –94.
31. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating
the quality of evidence. J Clin Epidemiol 2011;64:401–6.
32. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality
of evidence--study limitations (risk of bias). J Clin Epidemiol 2011;64:407–15.
33. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the
quality of evidence--publication bias. J Clin Epidemiol 2011;64:1277–82.
34. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality
of evidence--imprecision. J Clin Epidemiol 2011;64:1283–93.
35. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality
of evidence--inconsistency. J Clin Epidemiol 2011;64:1294–302.
36. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality
of evidence--indirectness. J Clin Epidemiol 2011;64:1303–10.
37. Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the
quality of evidence. J Clin Epidemiol 2011;64:1311–6.
38. Tuunainen A, Kripke DF, Endo T. Light therapy for non-seasonal depression.
Cochrane Database System Rev 2004:CD004050.
39. Dauphinais DR, Rosenthal JZ, Terman M, DiFebo HM, Tuggle C, Rosenthal
NE. Controlled trial of safety and efcacy of bright light therapy vs. negative
air ions in patients with bipolar depression. Psychiatry Res 2012;196:57–61.
40. Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression:
efcacy, protocol, safety, and side effects. CNS Spectrums 2005;10:647–63;
quiz 72.
41. Ulrich V, Olesen J, Gervil M, Russell MB. Possible risk factors and precipitants
for migraine with aura in discordant twin-pairs: a population-based study.
Cephalalgia 2000;20:821–5.
42. Gallin PF, Terman M, Reme CE, Rafferty B, Terman JS, Burde RM.
Ophthalmologic examination of patients with seasonal affective disorder,
before and after bright light therapy. Am J Ophthalmol 1995;119:202–10.
43. Reme CE, Rol P, Grothmann K, Kaase H, Terman M. Bright light therapy
in focus: lamp emission spectra and ocular safety. Technol Health Care
1996;4:403–13.
44. Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the
treatment of primary sleep disorders. PloS One 2013;8:e63773.
45. Herxheimer A, Petrie KJ. Melatonin for the prevention and treatment of jet lag.
Cochrane Database Systematic Rev 2002:CD001520.
46. Spadoni G, Bedini A, Rivara S, Mor M. Melatonin receptor agonists: new options
for insomnia and depression treatment. CNS Neurosci Ther 2011;17:733–41.
47. Committee on the Framework for Evaluating the Safety of the Dietary
Supplements FaNB, Board on Life Sciences, Institute of Medicine and
National Research Council of the National Academies. Dietary supplements:
a framework for evaluating safety. Washington, DC: The National Academies
Press, 2005.
48. Buscemi N, Vandermeer B, Hooton N, et al. The efcacy and safety of
exogenous melatonin for primary sleep disorders. A meta-analysis. J Gen
Intern Med 2005;20:1151–8.
49. Werneke U, Turner T, Priebe S. Complementary medicines in psychiatry:
review of effectiveness and safety. Br J Psychiatry 2006;188:109–21.
50. Rubio-Sastre P, Scheer FA, Gomez-Abellan P, Madrid JA, Garaulet M. Acute
melatonin administration in humans impairs glucose tolerance in both the
morning and evening. Sleep 2014;37:1715–9.
51. Seabra ML, Bignotto M, Pinto LR Jr., Tuk S. Randomized, double-blind
clinical trial, controlled with placebo, of the toxicology of chronic melatonin
treatment. J Pineal Res 2000;29:193–200.
52. Hoebert M, van der Heijden KB, van Geijlswijk IM, Smits MG. Long-term
follow-up of melatonin treatment in children with ADHD and chronic sleep
onset insomnia. J Pineal Res 2009;47:1–7.
53. Wasdell MB, Jan JE, Bomben MM, et al. A randomized, placebo-controlled trial
of controlled release melatonin treatment of delayed sleep phase syndrome
and impaired sleep maintenance in children with neurodevelopmental
disabilities. J Pineal Res 2008;44:57–64.
54. Carr R, Wasdell MB, Hamilton D, et al. Long-term effectiveness outcome of
melatonin therapy in children with treatment-resistant circadian rhythm sleep
disorders. J Pineal Res 2007;43:351–9.
55. Valcavi R, Zini M, Maestroni GJ, Conti A, Portioli I. Melatonin stimulates
growth hormone secretion through pathways other than the growth hormone-
releasing hormone. Clin Endocrinol 1993;39:193–9.
56. Luboshitzky R, Shen-Orr Z, Nave R, Lavi S, Lavie P. Melatonin administration
alters semen quality in healthy men. J Androl 2002;23:572–8.
57. van Geijlswijk IM, Mol RH, Egberts TC, Smits MG. Evaluation of sleep,
puberty and mental health in children with long-term melatonin treatment
for chronic idiopathic childhood sleep onset insomnia. Psychopharmacology
2011;216:111–20.
58. Ilomaki J, Paljarvi T, Korhonen MJ, et al. Prevalence of concomitant use of
alcohol and sedative-hypnotic drugs in middle and older aged persons: a
systematic review. Ann Pharmacother 2013;47:257–68.
59. Buscemi N, Vandermeer B, Friesen C, et al. The efcacy and safety of drug
treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen
Intern Med 2007;22:1335–50.
60. Peron EP, Gray SL, Hanlon JT. Medication use and functional status decline
in older adults: a narrative review. Am J Geriatr Pharmacother 2011;9:378–91.
61. Woolcott JC, Richardson KJ, Wiens MO et al. Meta-analysis of the impact
of 9 medication classes on falls in elderly persons. Arch Intern Med
2009;169:1952–60.
62. Bakken MS, Engeland A, Engesaeter LB, Ranhoff AH, Hunskaar S, Ruths S.
Risk of hip fracture among older people using anxiolytic and hypnotic drugs:
a nationwide prospective cohort study. Eur J Clin Pharmacol 2014;70:873–80.
63. Deschenes CL, McCurry SM. Current treatments for sleep disturbances in
individuals with dementia. Curr Psychiatry Reps 2009;11:20–6.
64. Hajak G, Muller WE, Wittchen HU, Pittrow D, Kirch W. Abuse and dependence
potential for the non-benzodiazepine hypnotics zolpidem and zopiclone: a
review of case reports and epidemiological data. Addiction 2003;98:1371–8.
65. James SP, Mendelson WB. The use of trazodone as a hypnotic: a critical
review. J Clin Psychiatry 2004;65:752–5.
66. Stone M. Mortality and antipsychotic drug use in dementia-related behavioral
disorders. US Department of Health and Human Services, Food and Drug
Administration, Center for Drug Evaluation and Research, 2005.
67. Moldofsky H, Musisi S, Phillipson EA. Treatment of a case of advanced sleep
phase syndrome by phase advance chronotherapy. Sleep 1986;9:61–5.
68. Suhner AG, Murphy PJ, Campbell SS. Failure of timed bright light exposure
to alleviate age-related sleep maintenance insomnia. J Am Geriatr Soc
2002;50:617–23.
69. Pallesen S, Nordhus IH, Skelton SH, Bjorvatn B, Skjerve A. Bright light
treatment has limited effect in subjects over 55 years with mild early morning
awakening. Percept Motor Skills 2005;101:759–70.
70. Lack L, Wright H, Kemp K, Gibbon S. The treatment of early-morning
awakening insomnia with 2 evenings of bright light. Sleep 2005;28:616–23.
71. Lack L, Wright H. The effect of evening bright light in delaying the circadian
rhythms and lengthening the sleep of early morning awakening insomniacs.
Sleep 1993;16:436–43.
72. Palmer CR, Kripke DF, Savage HC Jr., Cindrich LA, Loving RT, Elliott JA.
Efcacy of enhanced evening light for advanced sleep phase syndrome.
Behav Sleep Med 2003;1:213–26.
73. Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance
insomnia with timed exposure to bright light. J Am Geriatr Society
1993;41:829 –36.
74. Munch M, Scheuermaier KD, Zhang R, et al. Effects on subjective and
objective alertness and sleep in response to evening light exposure in older
subjects. Behav Brain Res 2011;224:272–8.
1225
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
75. Ito A, Ando K, Hayakawa T, et al. Long-term course of adult patients with
delayed sleep phase syndrome. Jpn J Psychiatry Neurol 1993;47:563–7.
76. Czeisler CA, Richardson GS, Coleman RM, et al. Chronotherapy: resetting
the circadian clocks of patients with delayed sleep phase insomnia. Sleep
1981;4:1–21.
77. Oren DA, Wehr TA. Hypernyctohemeral syndrome after chronotherapy for
delayed sleep phase syndrome. N Engl J Med 1992;327:1762.
78. Sharkey KM, Carskadon MA, Figueiro MG, Zhu Y, Rea MS. Effects of an
advanced sleep schedule and morning short wavelength light exposure
on circadian phase in young adults with late sleep schedules. Sleep Med
2011;12:685 –92.
79. Iwamitsu Y, Ozeki Y, Konishi M, Murakami J, Kimura S, Okawa M. Psychological
characteristics and the eicacy of hospitalization treatment on delayed sleep
phase syndrome patients with school refusal. Sleep Biol Rhythms 2007;5:15–
22.
80. de Sousa IC, Araújo IC, de Azevedo CV. The effect of a sleep hygiene
education program on the sleep-wake cycle of Brazilian adolescent students.
Sleep Biol Rhythms 2007;5:251–58.
81. Fargason RE, Preston T, Hammond E, May R, Gamble KL. Treatment of
attention decit hyperactivity disorder insomnia with blue wavelength light-
blocking glasses. Chronophysiol Ther 2013:1–8.
82. Burkhart K, Phelps JR. Amber lenses to block blue light and improve sleep: a
randomized trial. Chronobiol Int 2009;26:1602–12.
83. Rosenthal NE, Joseph-Vanderpool JR, Levendosky AA, et al. Phase-shifting
effects of bright morning light as treatment for delayed sleep phase syndrome.
Sleep 1990;13:354–61.
84. Watanabe T, Kajimura N, Kato M, Sekimoto M, Takahashi K. Effects of
phototherapy in patients with delayed sleep phase syndrome. Psychiatry Clin
Neurosci 1999;53:231–3.
85. Lack L, Bramwell T, Wright H, Kemp K. Morning blue light can advance the
melatonin rhythm in mild delayed sleep phase syndrome. Sleep Biol Rhythms
2007:78–80.
86. Ohta T, Iwata T, Kayukawa Y, Okada T. Daily activity and persistent sleep-
wake schedule disorders. Progr Neuropsychopharmacol Biol Psychiatry
1992;16:529 – 37.
87. Mizuma H, Miyahara Y, Sakamoto T, Kotorii T, Nakazawa Y. Two cases
of delayed sleep phase syndrome (DSPS). Jpn J Psychiatry Neurol
1991;45:163–64.
88. Yamadera H, Takahashi K, Okawa M. A multicenter study of sleep-wake
rhythm disorders: therapeutic effects of vitamin B12, bright light therapy,
chronotherapy and hypnotics. Psychiatry Clin Neurosci 1996;50:203–9.
89. Walsh JK, Deacon S, Dijk DJ, Lundahl J. The selective extrasynaptic
GABAA agonist, gaboxadol, improves traditional hypnotic efcacy measures
and enhances slow wave activity in a model of transient insomnia. Sleep
2007;30:593–602.
90. Kayumov L, Brown G, Jindal R, Buttoo K, Shapiro CM. A randomized, double-
blind, placebo-controlled crossover study of the effect of exogenous melatonin
on delayed sleep phase syndrome. Psychosom Med 2001;63:40–8.
91. Dahlitz M, Alvarez B, Vignau J, English J, Arendt J, Parkes JD. Delayed sleep
phase syndrome response to melatonin. Lancet 1991;337:1121–4.
92. Dagan Y, Yovel I, Hallis D, Eisenstein M, Raichik I. Evaluating the role of
melatonin in the long-term treatment of delayed sleep phase syndrome
(DSPS). Chronobiol Int 1998;15:181–90.
93. Rahman SA, Kayumov L, Shapiro CM. Antidepressant action of melatonin in
the treatment of delayed sleep phase syndrome. Sleep Med 2010;11:131–6.
94. Kennaway DJ. Potential safety issues in the use of the hormone melatonin in
paediatrics. J Paediatr Child Health 2015;51:584–9.
95. Van der Heijden KB, Smits MG, Van Someren EJ, Ridderinkhof KR, Gunning
WB. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic
sleep-onset insomnia. J Am Acad Child Adolesc Psychiatry 2007;46:233–41.
96. Smits MG, Nagtegaal EE, van der Heijden J, Coenen AM, Kerkhof GA.
Melatonin for chronic sleep onset insomnia in children: a randomized placebo-
controlled trial. J Child Neurol 2001;16:86–92.
97. Okawa M, Takahashi K, Egashira K, et al. Vitamin B12 treatment for delayed
sleep phase syndrome: a multi-center double-blind study. Psychiatry Clin
Neurosci 1997;51:275–9.
98. Bjorvatn B, Pallesen S. A practical approach to circadian rhythm sleep
disorders. Sleep Med Rev 2009;13:47–60.
99. Figueiro MG, Rea MS. Preliminary evidence that light through the eyelids can
suppress melatonin and phase shift dim light melatonin onset. BMC Res Notes
2012;5:221.
100. Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CI. Advancing
human circadian rhythms with afternoon melatonin and morning intermittent
bright light. J Clin Endocrinol Metab 2006;91:54–9.
101. Burke TM, Markwald RR, Chinoy ED, et al. Combination of light and
melatonin time cues for phase advancing the human circadian clock. Sleep
2013;36:1617–24.
102. Paul MA, Gray GW, Lieberman HR. et al. Phase advance with separate
and combined melatonin and light treatment. Psychopharmacology
2011;214:515–23.
103. Samaranayake CB, Fernando A, Warman G. Outcome of combined melatonin
and bright light treatments for delayed sleep phase disorder. Royal Aust NZ J
Psychiatry 2010;44:676.
104. Gradisar M, Dohnt H, Gardner G, et al. A randomized controlled trial of
cognitive-behavior therapy plus bright light therapy for adolescent delayed
sleep phase disorder. Sleep 2011;34:1671–80.
105. Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H. Circadian rhythm
abnormalities in totally blind people: incidence and clinical signicance. J Clin
Endocrinol Metab 1992;75:127–34.
106. Czeisler CA, Shanahan TL, Klerman EB, et al. Suppression of melatonin
secretion in some blind patients by exposure to bright light. N Engl J Med
1995;332:6–11.
107. Danilenko KV, Cajochen C, Wirz-Justice A. Is sleep per se a zeitgeber in
humans? J Biol Rhythms 2003;18:170–8.
108. Gordijn MC, Beersma DG, Korte HJ, van den Hoofdakker RH. Effects of light
exposure and sleep displacement on dim light melatonin onset. J Sleep Res
1999;8:163–74.
109. Hoban TM, Lewy AJ, Sack RL, Singer CM. The effects of shifting sleep two
hours within a xed photoperiod. J Neural Transm 1991;85:61–8.
110. Buxton OM, Lee CW, L’Hermite-Baleriaux M, Turek FW, Van Cauter E.
Exercise elicits phase shifts and acute alterations of melatonin that vary with
circadian phase. Am J Physiol Regul Integr Comp Physiol 2003;284:R714–24.
111. Klerman EB, Rimmer DW, Dijk DJ, Kronauer RE, Rizzo JF 3rd, Czeisler CA.
Nonphotic entrainment of the human circadian pacemaker. Am J Physiol
1998;274:R991–6.
112. Oren DA, Giesen HA, Wehr TA. Restoration of detectable melatonin
after entrainment to a 24-hour schedule in a ‘free-running’ man.
Psychoneuroendocrinology 1997;22:39–52.
113. Hoban TM, Sack RL, Lewy AJ, Miller LS, Singer CM. Entrainment of a free-
running human with bright light? Chronobiol Int 1989;6:347–53.
114. Watanabe T, Kajimura N, Kato M, Sekimoto M, Hori T, Takahashi K. Case of a
non-24 h sleep-wake syndrome patient improved by phototherapy. Psychiatry
Clin Neurosci 2000;54:369–70.
115. Okawa M, Uchiyama M, Ozaki S, et al. Melatonin treatment for circadian
rhythm sleep disorders. Psychiatry Clin Neurosci 1998;52:259–60.
116. Hayakawa T, Kamei Y, Urata J, et al. Trials of bright light exposure and
melatonin administration in a patient with non-24 hour sleep-wake syndrome.
Psychiatry Clin Neurosci 1998;52:261–2.
117. Klerman EB, Shanahan TL, Brotman DJ, et al. Photic resetting of the human
circadian pacemaker in the absence of conscious vision. J Biol Rhythms
2002;17:548–55.
118. Arendt J, Aldhous M, Wright J. Synchronisation of a disturbed sleep-wake
cycle in a blind man by melatonin treatment. Lancet 1988;1:772–3.
119. Folkard S, Arendt J, Aldhous M, Kennett H. Melatonin stabilises sleep onset
time in a blind man without entrainment of cortisol or temperature rhythms.
Neurosci Lett 1990;113:193–8.
120. Lapierre O, Dumont M. Melatonin treatment of a non-24-hour sleep-wake
cycle in a blind retarded child. Biol Psychiatry 1995;38:119–22.
121. Tzischinsky O, Pal I, Epstein R, Dagan Y, Lavie P. The importance of timing in
melatonin administration in a blind man. J Pineal Res 1992;12:105–8.
122. Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin
administration can entrain the free-running circadian system of blind subjects.
J Endocrinol 2000;164:R1–6.
123. Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running
circadian rhythms by melatonin in blind people. N Engl J Med 2000;343:1070–7.
124. Hack LM, Lockley SW, Arendt J, Skene DJ. The effects of low-dose 0.5-mg
melatonin on the free-running circadian rhythms of blind subjects. J Biol
Rhythms 2003;18:420–9.
125. Lewy AJ, Bauer VK, Hasler BP, Kendall AR, Pires ML, Sack RL. Capturing the
circadian rhythms of free-running blind people with 0.5 mg melatonin. Brain
Res 2001;918:96–100.
126. Lewy AJ, Hasler BP, Emens JS, Sack RL. Pretreatment circadian period
in free-running blind people may predict the phase angle of entrainment to
melatonin. Neurosci Lett 2001;313:158–60.
1226
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
127. Lewy AJ, Emens JS, Sack RL, Hasler BP, Bernert RA. Low, but not high,
doses of melatonin entrained a free-running blind person with a long circadian
period. Chronobiol Int 2002;19:649–58.
128. Lewy AJ, Emens JS, Leer BJ, Yuhas K, Jackman AR. Melatonin entrains
free-running blind people according to a physiological dose-response curve.
Chronobiol Int 2005;22:1093–106.
129. Lewy AJ, Emens JS, Bernert RA, Leer BJ. Eventual entrainment of the human
circadian pacemaker by melatonin is independent of the circadian phase of
treatment initiation: clinical implications. J Biol Rhythms 2004;19:68–75.
130. Siebler M, Steinmetz H, Freund HJ. Therapeutic entrainment of circadian
rhythm disorder by melatonin in a non-blind patient. J Neurol 1998;245:327–8.
131. Kamei Y, Hayakawa T, Urata J, et al. Melatonin treatment for circadian rhythm
sleep disorders. Psychiatry Clin Neurosci 2000;54:381–2.
132. McArthur AJ, Lewy AJ, Sack RL. Non-24-hour sleep-wake syndrome in a
sighted man: circadian rhythm studies and efcacy of melatonin treatment.
Sleep 1996;19:544–53.
133. Okawa M, Mishima K, Nanami T, et al. Vitamin B12 treatment for sleep-wake
rhythm disorders. Sleep 1990;13:15–23.
134. Ohta T, Ando K, Iwata T, et al. Treatment of persistent sleep-wake schedule
disorders in adolescents with methylcobalamin (vitamin B12). Sleep
1991;14:414–8.
135. Kamgar-Parsi B, Wehr TA, Gillin JC. Successful treatment of human non-24-
hour sleep-wake syndrome. Sleep 1983;6:257–64.
136. Sugita Y, Ishikawa H, Mikami A, et al. Successful treatment for a patient with
hypernychthermal syndrome. Sleep Res 1987:642.
137. Mishima K, Okawa M, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Morning
bright light therapy for sleep and behavior disorders in elderly patients with
dementia. Acta Psychiatr Scand 1994;89:1–7.
138. Fetveit A, Bjorvatn B. The effects of bright-light therapy on actigraphical
measured sleep last for several weeks post-treatment. A study in a nursing
home population. J Sleep Res 2004;13:153–8.
139. Ancoli-Israel S, Gehrman P, Martin JL, et al. Increased light exposure
consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s
disease patients. Behav Sleep Med 2003;1:22–36.
140. Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR Effect of light
treatment on sleep and circadian rhythms in demented nursing home patients.
J Am Geriatr Soc 2002;50:282–9.
141. Skjerve A, Holsten F, Aarsland D, Bjorvatn B, Nygaard HA, Johansen IM.
Improvement in behavioral symptoms and advance of activity acrophase after
short-term bright light treatment in severe dementia. Psychiatry Clin Neurosci
2004;58:3 43 –7.
142. Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Van Someren
EJ. Effect of morning bright light treatment for rest-activity disruption in
institutionalized patients with severe Alzheimer’s disease. Int Psychogeriatr
2005;17:221–36.
143. Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. Indirect bright light
improves circadian rest-activity rhythm disturbances in demented patients.
Biol Psychiatry 1997;41:955–63.
144. Fetveit A, Skjerve A, Bjorvatn B. Bright light treatment improves sleep in
institutionalised elderly--an open trial. Int J Geriatr Psychiatry 2003;18:520–6.
145. Satlin A, Volicer L, Ross V, Herz L, Campbell S. Bright light treatment of
behavioral and sleep disturbances in patients with Alzheimer’s disease. Am J
Psychiatry 1992;149:1028–32.
146. Dowling GA, Burr RL, Van Someren EJ, et al. Melatonin and bright-light
treatment for rest-activity disruption in institutionalized patients with
Alzheimer’s disease. J Am Geriatr Soc 2008;56:239–46.
147. Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van
Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive
function in elderly residents of group care facilities: a randomized controlled
trial. JAMA 2008;299:2642–55.
148. Greenblatt DJ, Harmatz JS, Singh NN, et al. Pharmacokinetics of zolpidem
from sublingual zolpidem tartrate tablets in healthy elderly versus non-elderly
subjects. Drugs Aging 2014;31:731–6.
149. Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use
and hip fractures in nursing home residents. JAMA Intern Med 2013;173:754–
61.
150. Hartikainen S, Rahkonen T, Kautiainen H, Sulkava R. The use of psychotropics
and survival in demented elderly individuals. Int Clin Psychopharmacol
2005;20:227–31.
151. Serfaty M, Kennell-Webb S, Warner J, Blizard R, Raven P. Double blind
randomised placebo controlled trial of low dose melatonin for sleep disorders
in dementia. Int J Geriatr Psychiatry 2002;17:1120–7.
152. Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial
of melatonin for sleep disturbance in Alzheimer’s disease. Sleep 2003;26:893–
901.
153. Pillar G, Shahar E, Peled N, Ravid S, Lavie P, Etzioni A. Melatonin improves
sleep-wake patterns in psychomotor retarded children. Pediatr Neurol
2000;23:225–8.
154. Jan JE, Espezel H, Appleton RE. The treatment of sleep disorders with
melatonin. Dev Med Child Neurol 1994;36:97–107.
155. Jan JE, Hamilton D, Seward N, Fast DK, Freeman RD, Laudon M. Clinical trials
of controlled-release melatonin in children with sleep-wake cycle disorders.
J Pineal Res 2000;29:34–9.
156. McArthur AJ, Budden SS. Sleep dysfunction in Rett syndrome: a trial of
exogenous melatonin treatment. Dev Med Child Neurol 1998;40:186–92.
157. Wright B, Sims D, Smart S et al. Melatonin versus placebo in children with
autism spectrum conditions and severe sleep problems not amenable to
behaviour management strategies: a randomised controlled crossover trial. J
Autism Dev Disord 2011;41:175–84.
158. McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Nighttime insomnia
treatment and education for Alzheimer’s disease: a randomized, controlled
trial. J Am Geriatr Soc 2005;53:793–802.
159. Alessi CA, Martin JL, Webber AP, Cynthia Kim E, Harker JO, Josephson KR.
Randomized, controlled trial of a nonpharmacological intervention to improve
abnormal sleep/wake patterns in nursing home residents. J Am Geriatr Soc
2005;53:803–10.
160. McCurry SM, Pike KC, Vitiello MV, Logsdon RG, Larson EB, Teri L. Increasing
walking and bright light exposure to improve sleep in community-dwelling
persons with Alzheimer’s disease: results of a randomized, controlled trial.
J Am Geriatr Soc 2011;59:1393–402.
161. Martin JL, Marler MR, Harker JO, Josephson KR, Alessi CA. A multicomponent
nonpharmacological intervention improves activity rhythms among nursing
home residents with disrupted sleep/wake patterns. J Gerontol A Biol Sci Med
Sci 2007;62:67–72.
162. Guilleminault C, McCann CC, Quera-Salva M, Cetel M. Light therapy
as treatment of dyschronosis in brain impaired children. Eur J Pediatr
1993;152:754–9.
163. Auger RR. Advance-related sleep complaints and advanced sleep phase
disorder. Sleep Med Clin 2009;4:219–27.
164. Kondo M, Tokura H, Wakamura T, et al. Inuences of twilight on diurnal
variation of core temperature, its nadir, and urinary 6-hydroxymelatonin sulfate
during nocturnal sleep and morning drowsiness. Coll Antropol 2009;33:193–9.
165. Fromm E, Horlebein C, Meergans A, Niesner M, Randler C. Evaluation of a
dawn simulator in children and adolescents. Biol Rhythm Res 2011;42:417–25.
166. Terman M, Jiuan Su T. Circadian rhythm phase advance with dawn simulation
treatment for winter depression. J Biol Rhythms 2010;25:297–301.
167. Higuchi S, Motohashi Y, Ishibashi K, Maeda T. Less exposure to daily
ambient light in winter increases sensitivity of melatonin to light suppression.
Chronobiol Int 2007;24:31–43.
168. Braam W, van Geijlswijk I, Keijzer H, Smits MG, Didden R, Curfs LM. Loss of
response to melatonin treatment is associated with slow melatonin metabolism.
J Intellect Disabil Res 2010;54:547–55.
169. Lockley SW, Dressman MA, Licamele L, et al. Tasimelteon for non-24-hour
sleep-wake disorder in totally blind people (SET and RESET): two multicentre,
randomised, double-masked, placebo-controlled phase 3 trials. Lancet 2015
Aug 4. [Epub ahead of print].
170. Richardson GS, Zee PC, Wang-Weigand S, Rodriguez L, Peng X. Circadian
phase-shifting effects of repeated ramelteon administration in healthy adults.
J Clin Sleep Med 2008;4:456–61.
171. Rajaratnam SM, Polymeropoulos MH, Fisher DM, et al. Melatonin agonist
tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two
randomised controlled multicentre trials. Lancet 2009;373:482–91.
172. Burgess HJ, Revell VL, Molina TA, Eastman CI. Human phase response curves
to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocrinol Metab
2010;95:3325–31.
173. Shibui K, Uchiyama M, Okawa M. Melatonin rhythms in delayed sleep phase
syndrome. J Biol Rhythms 1999;14:72–6.
174. Ozaki S, Uchiyama M, Shirakawa S, Okawa M. Prolonged interval from
body temperature nadir to sleep offset in patients with delayed sleep phase
syndrome. Sleep 1996;19:36–40.
175. Watanabe T, Kajimura N, Kato M, et al. Sleep and circadian rhythm disturbances
in patients with delayed sleep phase syndrome. Sleep 2003;26:657–61.

1227
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
176. Uchiyama M, Okawa M, Shibui K, et al. Altered phase relation between
sleep timing and core body temperature rhythm in delayed sleep phase
syndrome and non-24-hour sleep-wake syndrome in humans. Neurosci Lett
2000;294:101–4.
177. Wyatt JK, Stepanski EJ, Kirkby J. Circadian phase in delayed sleep phase
syndrome: predictors and temporal stability across multiple assessments.
Sleep 2006;29:1075–80.
178. Chang AM, Reid KJ, Gourineni R, Zee PC. Sleep timing and circadian phase in
delayed sleep phase syndrome. J Biol Rhythms 2009;24:313–21.
179. Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in
adolescents. Sleep 2005;28:1446–54.
180. Uchiyama M, Okawa M, Shibui K et al. Poor compensatory function for sleep
loss as a pathogenic factor in patients with delayed sleep phase syndrome.
Sleep 2000;23:553–8.
181. Asaoka A, Fukuda K, Tsutsui Y, Yamazaki K. Does television viewing cause
delayed and/or irregularsleep–wake patterns? Sleep Biol Rhythms 2007;5:23–
27.
182. Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed
phase in adolescence. Sleep Med 2007;8:602–12.
183. Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician
1990;17:5–12.
184. Wyatt JK. Delayed sleep phase syndrome: pathophysiology and treatment
options. Sleep 2004;27:1195–203.
185. Peixoto CA, da Silva AG, Carskadon MA, Louzada FM. Adolescents living in
homes without electric lighting have earlier sleep times. Behav Sleep Med
2009;7:73–80.
186. Vollmer C, Michel U, Randler C. Outdoor light at night (LAN) is correlated with
eveningness in adolescents. Chronobiol Int 2012;29:502–8.
187. Cajochen C, Frey S, Anders D, et al. Evening exposure to a light-emitting
diodes (LED)-backlit computer screen affects circadian physiology and
cognitive performance. J Appl Physiol 2011;110:1432–8.
188. Figueiro MG, Rea MS. Evening daylight may cause adolescents to sleep less
in spring than in winter. Chronobiol Int 2010;27:1242–58.
189. Taylor A, Wright HR, Lack LC. Sleeping-in on the weekend delays circadian
phase and increases sleepiness the following week. Sleep Biol Rhythms
2008;6:172–79.
190. Burgess HJ, Eastman CI. A late wake time phase delays the human dim light
melatonin rhythm. Neurosci Lett 2006;395:191–5.
191. Crowley SJ, Carskadon MA. Modications to weekend recovery sleep delay
circadian phase in older adolescents. Chronobiol Int 2010;27:1469–92.
192. Auger RR, Burgess HJ, Dierkhising RA, Sharma RG, Slocumb NL. Light
exposure among adolescents with delayed sleep phase disorder: a prospective
cohort study. Chronobiol Int 2011;28:911–20.
193. Figueiro MG, Rea MS. Lack of short-wavelength light during the school
day delays dim light melatonin onset (DLMO) in middle school students.
Neuroendocrinol Lett 2010;31:92–6.
194. Dagan Y, Abadi J. Sleep-wake schedule disorder disability: a lifelong
untreatable pathology of the circadian time structure. Chronobiol Int
2001;18:1019–27.
195. Miller NL, Tvaryanas AP, Shattuck LG. Accommodating adolescent sleep-wake
patterns: the effects of shifting the timing of sleep on training effectiveness.
Sleep 2012;35:1123–36.
196. National Sleep Foundation. 2006 Sleep in America Poll Summary Findings,
Washington, DC: National Sleep Foundation, 2006.
197. Wahlstrom K. Changing times: ndings from the rst longitudinal study of later
school start times. NAASP Bull 2002;86:3–21.
198. Danner F, Phillips B. Adolescent sleep, school start times, and teen motor
vehicle crashes. J Clin Sleep Med 2008;4:533–5.
199. Owens JA, Belon K, Moss P. Impact of delaying school start time on adolescent
sleep, mood, and behavior. Arch Pediatr Adolesc Med 2010;164:608–14.
200. Wahlstrom K. The prickly politics of school starting times. Phi Delta Kappa
1999;80:344–47.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Ilene Rosen for serving as a board liaison for this
project and assuring that the guideline adhered to the standards set forth by the
Board of Directors of American Academy of Sleep Medicine. The authors also ac-
knowledge the contributions of the following individuals: Christine Stepanski, MS,
for performing literature searches #1 and #2; and Michelle M. Tangredi, PhD, for
managing the project at the initial steps. The authors are grateful to the organiza-
tions and individuals who provided their insights and suggestions during the public
comment period. Every comment was internally documented and addressed, and
decisions regarding corresponding amendments in the text were left to the TF’s
discretion. The project was funded by the American Academy of Sleep Medicine.
SUBMISSION & CORRESPONDENCE INFORMATION
Submitted for publication August, 2015
Accepted for publication August, 2015
Address correspondence to: R. Robert Auger, MD, Mayo Center for Sleep Medicine,
Rochester, MN; Email: research@aasmnet.org
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no nancial
conicts of interest. Drs. Deriy and Thomas are employed by the American Acad-
emy of Sleep Medicine.
APPENDIX
Search Terms and MeSH Used in the Literature
Search #1
Sleep disorders, circadian rhythm”[MeSH Terms] OR
(“sleep”[All Fields] AND “disorders”[All Fields] AND
“circadian”[All Fields] AND “rhythm”[All Fields])
OR “circadian rhythm sleep disorders”[All Fields] OR
(“circadian”[All Fields] AND “rhythm”[All Fields]
AND “sleep”[All Fields] AND “disorder”[All Fields]) OR
“circadian rhythm sleep disorder”[All Fields] OR (free[All
Fields] AND “running”[All Fields] AND “disorder”[All
Fields]) OR (irregular[All Fields] AND sleep-wake[All
Fields] AND rhythm[All Fields]) OR (non[All Fields] AND
24-hour[All Fields] AND (“sleep disorders”[MeSH Terms]
OR (“sleep”[All Fields] AND “disorders”[All Fields]) OR
“sleep disorders”[All Fields] OR (“sleep”[All Fields] AND
“disorder”[All Fields]) OR “sleep disorder”[All Fields])) OR
((“Blindness”[Mesh] OR (“blindness”[MeSH Terms] OR
“blindness”[All Fields] OR nonsighted[All Fields])) AND
(“sleep disorders”[MeSH Terms] OR (“sleep”[All Fields]
AND “disorders”[All Fields]) OR “sleep disorders”[All
Fields])) AND (Meta-Analysis[ptyp] OR Practice
Guideline[ptyp] OR systematic[sb]).
Terms for the Literature Search #2 in Pubmed
Database
((((circadian rhythm sleep disorders[tw] OR circadian rhythm
sleep disorder[tw] OR “Sleep Disorders, Circadian
Rhythm”[Mesh:noexp] OR “chronobiology disorders”[tw] OR
“Chronobiology Disorders”[Mesh:noexp] OR
hypernychthemeral[tw] OR circadian misalignment[tw] OR
circadian dysregulation[tw]) OR ((irregular[tw] OR non 24
hour[tw]) AND (sleep wake[tw] OR sleep-wake[tw])) OR
((advanced[tw] OR delayed[tw]) AND sleep phase[tw]) OR
(idiopathic[tw] AND chronic[tw] AND (sleep-onset[tw] OR
sleep onset[tw]) AND insomnia[tw]) OR ((nonentrained[tw] OR
non-entrained[tw]) AND (sleep[tw] OR “sleep”[mesh] OR
“Sleep Disorders”[Mesh:noexp] OR circadian[tw] OR
“Circadian Rhythm”[MeSH Terms])) OR ((DSPS[tw] OR
ASPS[tw] OR early morning awakening[tw] OR (phase
advance[All Fields] OR phase advance/delay[All Fields] OR
phase advanced[All Fields] OR phase advancement[All Fields]
1228
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
OR phase advancements[All Fields] OR phase advances[All
Fields]) OR (phase delay[All Fields] OR phase delay/arrest[All
Fields] OR phase delayed[All Fields] OR phase delaying[All
Fields] OR phase delays[All Fields]) OR (phase shift[All Fields]
OR phase shifted[All Fields] OR phase shifter[All Fields] OR
phase shifter/modulator[All Fields] OR phase shifters[All
Fields] OR phase shifting[All Fields] OR phase shiftkeying[All
Fields] OR phase shiftmeasurement[All Fields] OR phase
shifts[All Fields])) AND (sleep[tw] OR “sleep”[mesh] OR
“Sleep Disorders”[Mesh:noexp] OR circadian[tw] OR
“Circadian Rhythm”[MeSH Terms])) OR ((free running[tw]
AND (disorder[tw] OR rhythm[tw])) AND (sleep[tw] OR
“sleep”[mesh] OR “Sleep Disorders”[Mesh:noexp] OR
circadian[tw] OR “Circadian Rhythm”[MeSH Terms])) OR
((dark therapy[tw] OR light therapy[tw] OR amber lenses[tw]
OR Blue light[tw] OR bright light[tw] AND blue blockers[tw]
OR blue-blocker[tw] OR blue blocker[tw] OR blue-blocker[tw]
OR eyewear[tw] OR Phototherapy[tw] OR
“Phototherapy”[Mesh]) AND (sleep[tw] OR “sleep”[mesh] OR
“Sleep Disorders”[Mesh:noexp] OR circadian[tw] OR
“Circadian Rhythm”[MeSH Terms])) OR “sleep phase
chronotherapy”[MeSH Terms] OR ((chronotherapy[tw] OR
chronotype[tw] OR “Chronotherapy”[Mesh:noexp]) AND
(sleep[tw] OR “sleep”[mesh] OR “Sleep
Disorders”[Mesh:noexp] OR circadian[tw] OR “Circadian
Rhythm”[MeSH Terms])) OR ((melatonin receptor agonists[tw]
OR melatonin receptor agonist[tw] OR melatonin agonist[tw]
OR melatonin agonists[tw] OR melatonin receptor
antagonists[tw] OR melatonin receptor antagonist[tw] OR
melatonin antagonist[tw] OR melatonin antagonists[tw] OR
“Receptors, Melatonin/drug effects”[Mesh] OR “Receptors,
Melatonin/agonists”[Mesh] OR “Receptors, Melatonin/
antagonists and inhibitors”[Mesh]) AND (sleep[tw] OR
“sleep”[mesh] OR “Sleep Disorders”[Mesh:noexp] OR
circadian[tw] OR “Circadian Rhythm”[MeSH Terms])) OR
((melatonin[tw] OR “melatonin”[MeSH Terms] OR
ramelteon[tw] OR “ramelteon”[Substance] OR tasimelteon[tw]
OR “tasimelteon”[Supplementary Concept] OR
agomelatine[tw] OR “S 20098”[Supplementary Concept])
AND (sleep[tw] OR “sleep”[mesh] OR “Sleep
Disorders”[Mesh:noexp] OR circadian[tw] OR “Circadian
Rhythm”[MeSH Terms])) OR ((dim light melatonin onset[tw]
OR DLMO[tw]) AND (sleep[tw] OR “sleep”[mesh] OR “Sleep
Disorders”[Mesh:noexp] OR circadian[tw] OR “Circadian
Rhythm”[MeSH Terms])) OR ((Blindness[tw] OR blind
person[tw] OR blind people[tw] OR blind subject[tw] OR blind
subjects[tw] OR blind patient[tw] OR blind patients[tw] OR
“Visually Impaired Persons”[Mesh] OR enucleated[tw] OR
“Blindness”[Mesh] OR nonsighted[tw] OR (visual
impairement[All Fields] OR visual impairment[All Fields] OR
visual impairment/blindness[All Fields] OR visual
impairments[All Fields]) OR visually impaired[tw]) AND
(sleep[tw] OR “sleep”[mesh] OR “Sleep
Disorders”[Mesh:noexp] OR circadian[tw] OR “Circadian
Rhythm”[MeSH Terms])) OR ((“dementia”[MeSH Terms] OR
dementia[tw] OR “alzheimer disease”[MeSH Terms] OR
alzheimer[tw] OR alzheimers’s[tw]) AND (sleep[tw] OR
“sleep”[mesh] OR “Sleep Disorders”[Mesh:noexp] OR
circadian[tw] OR “Circadian Rhythm”[MeSH Terms])) OR
((owl[tw] OR owls[tw] OR lark[tw] OR larks[tw] OR
morningness[tw] OR eveningness[tw] OR morning types[tw]
OR morning type[tw] OR evening types[tw] OR evening
type[tw]) AND (sleep[tw] OR “sleep”[mesh] OR “Sleep
Disorders”[Mesh:noexp] OR circadian[tw] OR “Circadian
Rhythm”[MeSH Terms]))) AND ((randomized controlled
trial[pt] OR controlled clinical trial[pt] OR randomized[tiab]
OR placebo[tiab] OR “drug therapy”[Subheading] OR
randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT
(“animals”[MeSH Terms] NOT “humans”[MeSH Terms]))
AND (English[la] AND (“2006/10/01”[PDAT] :
“3000/12/31”[PDAT]) NOT (Comment[pt] OR Editorial[pt] OR
News[pt] OR Newspaper Article[pt] OR Letter[pt] OR Case
Reports[pt] OR Review[pt])))) NOT (((((circadian rhythm sleep
disorders[tw] OR circadian rhythm sleep disorder[tw] OR
“Sleep Disorders, Circadian Rhythm”[Mesh:noexp] OR
“chronobiology disorders”[tw] OR “Chronobiology
Disorders”[Mesh:noexp] OR hypernychthemeral[tw] OR
circadian misalignment[tw] OR circadian dysregulation[tw]))
OR (((irregular[tw] OR non 24 hour[tw]) AND (sleep wake[tw]
OR sleep-wake[tw]))) OR (((advanced[tw] OR delayed[tw])
AND (sleep phase[tw]))) OR ((idiopathic[tw] AND chronic[tw]
AND (sleep-onset[tw] OR sleep onset[tw]) AND insomnia[tw]))
OR (((nonentrained[tw] OR non-entrained[tw]) AND (sleep[tw]
OR “sleep”[mesh] OR “Sleep Disorders”[Mesh:noexp] OR
circadian[tw] OR “Circadian Rhythm”[MeSH Terms]))) OR
(((DSPS[tw] OR ASPS[tw] OR phase advance* OR phase delay*
OR phase shift*) AND (sleep[tw] OR “sleep”[mesh] OR “Sleep
Disorders”[Mesh:noexp] OR circadian[tw] OR “Circadian
Rhythm”[MeSH Terms]))) OR (((free running[tw] AND
(disorder[tw] OR rhythm[tw])) AND (sleep[tw] OR
“sleep”[mesh] OR “Sleep Disorders”[Mesh:noexp] OR
circadian[tw] OR “Circadian Rhythm”[MeSH Terms]))) OR
(((dark therapy[tw] OR light therapy[tw] OR amber lenses[tw]
OR Blue light[tw] OR bright light[tw] OR eyewear[tw] OR
Phototherapy[tw] OR “Phototherapy”[Mesh]) AND (sleep[tw]
OR “sleep”[mesh] OR “Sleep Disorders”[Mesh:noexp] OR
circadian[tw] OR “Circadian Rhythm”[MeSH Terms]))) OR
((Sleep Phase Chronotherapy[Mesh])) OR (((chronotherapy[tw]
OR “Chronotherapy”[Mesh:noexp]) AND (sleep[tw] OR
“sleep”[mesh] OR “Sleep Disorders”[Mesh:noexp] OR
circadian[tw] OR “Circadian Rhythm”[MeSH Terms]))) OR
(((melatonin receptor agonists[tw] OR melatonin receptor
agonist[tw] OR melatonin agonist[tw] OR melatonin
agonists[tw] OR melatonin receptor antagonists[tw] OR
melatonin receptor antagonist[tw] OR melatonin antagonist[tw]
OR melatonin antagonists[tw] OR melatonin receptor
inhibitor[tw] OR melatonin receptor inhibitors[tw] OR
melatonin inhibitor[tw] OR melatonin inhibitors[tw] OR
“Receptors, Melatonin/drug effects”[Mesh] OR “Receptors,
Melatonin/agonists”[Mesh] OR “Receptors, Melatonin/
antagonists and inhibitors”[Mesh]) AND (sleep[tw] OR
“sleep”[mesh] OR “Sleep Disorders”[Mesh:noexp] OR
circadian[tw] OR “Circadian Rhythm”[MeSH Terms]))) OR
(((melatonin[tw] OR “melatonin”[MeSH Terms] OR
ramelteon[tw] OR “ramelteon”[Substance] OR tasimelteon[tw]
OR “tasimelteon”[Supplementary Concept] OR
agomelatine[tw] OR “S 20098”[Supplementary Concept])
AND (sleep[tw] OR “sleep”[mesh] OR “Sleep
1229
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
Disorders”[Mesh:noexp] OR circadian[tw] OR “Circadian
Rhythm”[MeSH Terms]))) OR (((dim light melatonin onset[tw]
OR DLMO[tw]) AND (sleep[tw] OR “sleep”[mesh] OR “Sleep
Disorders”[Mesh:noexp] OR circadian[tw] OR “Circadian
Rhythm”[MeSH Terms]))) OR (((Blindness[tw] OR
“Blindness”[Mesh] OR nonsighted[tw] OR (visual impair*) OR
(visually impaired[tw])) AND (sleep[tw] OR “sleep”[mesh] OR
“Sleep Disorders”[Mesh:noexp] OR circadian[tw] OR
“Circadian Rhythm”[MeSH Terms]))) OR (((“dementia”[MeSH
Terms] OR dementia[tw] OR “alzheimer disease”[MeSH
Terms] OR alzheimer[tw] OR alzheimers’s[tw]) AND (sleep[tw]
OR “sleep”[mesh] OR “Sleep Disorders”[Mesh:noexp] OR
circadian[tw] OR “Circadian Rhythm”[MeSH Terms]))) OR
(((owl[tw] OR owls[tw] OR lark[tw] OR larks[tw] OR
morningness[tw] OR eveningness[tw] OR morning types[tw]
OR morning type[tw] OR evening types[tw] OR evening
type[tw]) AND (sleep[tw] OR “sleep”[mesh] OR “Sleep
Disorders”[Mesh:noexp] OR circadian[tw] OR “Circadian
Rhythm”[MeSH Terms]))))) AND ((randomized controlled
trial[pt] OR controlled clinical trial[pt] OR randomized[tiab]
OR placebo[tiab] OR “drug therapy”[Subheading] OR
randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT
(“animals”[MeSH Terms] NOT “humans”[MeSH Terms]))
AND (English[la] AND (“2006/10/01”[PDAT] :
“3000/12/31”[PDAT]) NOT (Comment[pt] OR Editorial[pt] OR
News[pt] OR Newspaper Article[pt] OR Letter[pt] OR Case
Reports[pt] OR Review[pt])))
Terms for the Literature Search #2 in Embase Database
(‘circadian rhythm sleep disorders’:de,ab,ti OR ‘circadian
rhythm sleep disorder’:de,ab,ti OR ‘circadian rhythm
sleep disorder’/exp OR ‘chronobiology disorders’:de,ab,ti
OR ‘hypernychthemeral’:de,ab,ti OR ‘circadian
misalignment’:de,ab,ti OR ‘circadian dysregulation’:de,ab,ti)
OR((‘advanced’:de,ab,ti OR ‘delayed’:de,ab,ti) AND
(‘sleep phase’:de,ab,ti)) OR ((‘irregular’:de,ab,ti OR
‘non 24 hour’:de,ab,ti) AND (‘sleep wake’:de,ab,ti
OR ‘sleep-wake’:de,ab,ti)) OR ((‘idiopathic’:de,ab,ti
AND ‘chronic’:de,ab,ti AND (‘sleep-onset’:de,ab,ti
OR ‘sleep onset’:de,ab,ti) AND ‘insomnia’:de,ab,ti)) OR
((‘nonentrained’:de,ab,ti OR ‘non-entrained’:de,ab,ti)
AND (‘sleep’:de,ab,ti OR ‘sleep’/exp OR ‘sleep disorder’/
de OR ‘circadian’:de,ab,ti OR ‘circadian rhythm’/exp)) OR
((‘DSPS’:de,ab,ti OR ‘ASPS’:de,ab,ti OR ‘early morning
awakening’:de,ab,ti OR phase advance* OR phase delay* OR
phase shift*) AND (‘sleep’:de,ab,ti OR ‘sleep’/exp OR ‘sleep
disorder’/de OR ‘circadian’:de,ab,ti OR ‘circadian rhythm’/
exp)) OR (((‘free running’:de,ab,ti AND (‘disorder’:de,ab,ti
OR ‘rhythm’:de,ab,ti)) AND (‘sleep’:de,ab,ti OR ‘sleep’/
exp OR ‘sleep disorder’/de OR ‘circadian’:de,ab,ti OR
‘circadian rhythm’/exp))) OR ((‘dark therapy’:de,ab,ti
OR ‘light therapy’:de,ab,ti OR ‘amber lenses’:de,ab,ti OR
‘blue blocker’:de,ab,ti OR ‘blue-blocker’:de,ab,ti OR ‘blue
blockers’:de,ab,ti OR ‘blue-blockers’:de,ab,ti OR ‘Blue
light’:de,ab,ti OR ‘bright light’:de,ab,ti OR ‘eyewear’:de,ab,ti
OR ‘Phototherapy’:de,ab,ti OR ‘phototherapy’/exp) AND
(‘sleep’:de,ab,ti OR ‘sleep’/exp OR ‘sleep disorder’/de
OR ‘circadian’:de,ab,ti OR ‘circadian rhythm’/exp)) OR
((‘chronotherapy’:de,ab,ti OR ‘chronotype’:de,ab,ti OR
‘chronotherapy’/exp) AND (‘sleep’:de,ab,ti OR ‘sleep’/exp
OR ‘sleep disorder’/de OR ‘circadian’:de,ab,ti OR ‘circadian
rhythm’/exp OR ‘sleep therapy’/exp)) OR ((‘melatonin receptor
agonists’:de,ab,ti OR ‘melatonin receptor agonist’:de,ab,ti
OR ‘melatonin agonist’:de,ab,ti OR ‘melatonin
agonists’:de,ab,ti OR ‘melatonin receptor antagonists’:de,ab,ti
OR ‘melatonin receptor antagonist’:de,ab,ti OR ‘melatonin
antagonist’:de,ab,ti OR ‘melatonin antagonists’:de,ab,ti
OR ‘melatonin receptor inhibitor’:de,ab,ti OR ‘melatonin
receptor inhibitors’:de,ab,ti OR ‘melatonin inhibitor’:de,ab,ti
OR ‘melatonin inhibitors’:de,ab,ti OR ‘melatonin
receptor’/exp/dd_dt OR (‘melatonin receptor’/exp
AND ‘agonist’/exp)) AND (‘sleep’:de,ab,ti OR ‘sleep’/
exp OR ‘sleep disorder’/de OR ‘circadian’:de,ab,ti OR
‘circadian rhythm’/exp)) OR ((‘melatonin’:de,ab,ti OR
‘melatonin’/exp OR ‘ramelteon’:de,ab,ti OR ‘ramelteon’/
exp OR ‘tasimelteon’:de,ab,ti OR ‘tasimelteon’/exp
OR ‘agomelatine’:de,ab,ti OR ‘agomelatine’/exp) AND
(‘sleep’:de,ab,ti OR ‘sleep’/exp OR ‘sleep disorder’/de
OR ‘circadian’:de,ab,ti OR ‘circadian rhythm’/exp)) OR
((‘dim light melatonin onset’:de,ab,ti OR ‘DLMO’:de,ab,ti)
AND (‘sleep’:de,ab,ti OR ‘sleep’/exp OR ‘sleep disorder’/
de OR ‘circadian’:de,ab,ti OR ‘circadian rhythm’/
exp)) OR ((‘Blindness’:de,ab,ti OR ‘blindness’/exp
OR ‘blind person’:de,ab,ti OR ‘blind people’:de,ab,ti
OR ‘blind subject’:de,ab,ti OR ‘blind subjects’:de,ab,ti
OR ‘blind patient’:de,ab,ti OR ‘blind patients’:de,ab,ti
OR ‘enucleated’:de,ab,ti OR ‘nonsighted’:de,ab,ti OR
(visual AND impair*) OR (‘visually impaired’:de,ab,ti))
AND (‘sleep’:de,ab,ti OR ‘sleep’/exp OR ‘sleep disorder’/
de OR ‘circadian’:de,ab,ti OR ‘circadian rhythm’/exp)) OR
((‘dementia’/exp OR ‘dementia’:de,ab,ti OR ‘Alzheimer
disease’/exp OR ‘alzheimer’:de,ab,ti OR alzheimer*)
AND (‘sleep’:de,ab,ti OR ‘sleep’/exp OR ‘sleep disorder’/
de OR ‘circadian’:de,ab,ti OR ‘circadian rhythm’/exp))
OR ((‘owl’:de,ab,ti OR ‘owls’:de,ab,ti OR ‘lark’:de,ab,ti
OR ‘larks’:de,ab,ti OR ‘morningness’:de,ab,ti OR
‘eveningness’:de,ab,ti OR ‘morning types’:de,ab,ti OR
‘morning type’:de,ab,ti OR ‘evening types’:de,ab,ti OR
‘evening type’:de,ab,ti) AND (‘sleep’:de,ab,ti OR ‘sleep’/exp
OR ‘sleep disorder’/de OR ‘circadian’:de,ab,ti OR ‘circadian
rhythm’/exp)) AND ((random* OR factorial* OR crossover*
OR cross NEXT/1 over* OR cross-over* OR placebo* OR
doubl* NEXT/1 blind* OR singl* NEXT/1 blind* OR assign*
OR allocat* OR volunteer* OR ‘crossover procedure’/de OR
‘double blind procedure’/de OR ‘randomized controlled trial’/
de OR ‘single blind procedure’/de) OR (‘clinical trial’/exp
OR ‘counterbalance’:de,ab,ti OR ‘counterbalanced’:de,ab,ti
OR ‘crossover’:de,ab,ti OR ‘trial’:de,ab,ti)) AND ((AND
[english]/lim AND [2006-2012]/py AND [embase]/lim NOT
[medline]/lim NOT (‘editorial’:it OR ‘conference review’:it
OR ‘conference paper’:it OR ‘note’:it OR ‘conference
abstract’:it OR ‘letter’:it OR ‘review’:it) NOT ([animals]/lim
NOT [humans]/lim))
Terms for the Literature Search # 2 in PsychInfo
Database
((TI “circadian rhythm sleep disorders” or TI “circadian
rhythm sleep disorder” or AB “circadian rhythm sleep
1230
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
disorders” or AB “circadian rhythm sleep disorder” or
TI “chronobiology disorders” or AB “chronobiology
disorders” OR TI “chronobiology disorder” or AB
“chronobiology disorder” or TI “hypernychthemeral” or AB
“hypernychthemeral” or TI “circadian misalignment” or AB
“circadian misalignment” or TI “circadian dysregulation” or
AB “circadian dysregulation”)) OR ((TI “advanced” or AB
“advanced” or TI “delayed” or AB “delayed”) and (TI “sleep
phase” or AB “sleep phase”)) OR ((TI “irregular” or AB
“irregular” or TI “non 24 hour” or AB “non 24 hour”) AND
(TI “sleep wake” or AB “sleep wake” or TI “sleep-wake” or
AB “sleep-wake”)) OR ((TI “idiopathic” or AB “idiopathic”)
and (TI “chronic” or AB “chronic” ) and (TI “sleep-onset” or
AB “sleep-onset” or TI “sleep onset” or AB “sleep onset”)
and (DE “Insomnia” or TI “insomnia” or AB “insomnia”))
OR ((TI “nonentrained” or AB “nonentrained” OR TI “non-
entrained” OR AB “non-entrained”) AND (TI “sleep” or
AB “sleep” or DE “Sleep” or DE “Sleep Disorders” or TI
“circadian” or AB “circadian” or DE “Human Biological
Rhythms” or DE “Sleep Wake Cycle”)) OR ((TI “DSPS”
or AB “DSPS” or TI “ASPS” or AB “ASPS” or TI “early
morning awakening” or AB “early morning awakening” or
TI “phase advance*” or AB “phase advance*” or TI “phase
delay*” or AB “phase delay*” or TI “phase shift*” or AB
“phase shift*”) and (TI “sleep” or AB “sleep” or DE “Sleep”
or DE “Sleep Disorders” or TI “circadian” or AB “circadian”
or DE “Human Biological Rhythms” or DE “Sleep Wake
Cycle”)) OR (((TI “free running” or AB “free running”)
and (TI “disorder” or AB “disorder” or TI “rhythm” or AB
“rhythm”)) and (TI “sleep” or AB “sleep” or DE “Sleep” or
DE “Sleep Disorders” or TI “circadian” or AB “circadian”
or DE “Human Biological Rhythms” or DE “Sleep Wake
Cycle”))) OR ((TI “dark therapy” or AB “dark therapy” or
TI “light therapy” or AB “light therapy” or TI “amber lenses”
or AB “amber lenses” or TI “blue blocker” or AB “blue
blocker” or TI “blue-blocker” or AB “blue-blocker” or TI
“blue blockers” or AB “blue blockers” or TI “blue-blockers”
or AB “blue-blockers” or TI “Blue light” or AB “Blue light”
or TI “bright light” or AB “bright light” or TI “eyewear” or
AB “eyewear” or DE “Optical Aids” or TI “Phototherapy”
or AB “Phototherapy” or DE “Phototherapy”) and (TI
“sleep” or AB “sleep” or DE “Sleep” or DE “Sleep Disorders”
or TI “circadian” or AB “circadian” or DE “Human
Biological Rhythms” or DE “Sleep Wake Cycle”)) OR ((TI
“chronotherapy” or AB “chronotherapy” or TI “chronotype”
or AB “chronotypes” or TI “chronotypes” or AB
“chronotypes” or TI “chronotherapy” or AB “chronotherapy”)
AND (TI “sleep” or AB “sleep” or DE “Sleep” or DE “Sleep
Disorders” or TI “circadian” or AB “circadian” or DE
“Human Biological Rhythms” or DE “Sleep Wake Cycle”))
OR ((TI “melatonin receptor agonists” or AB “melatonin
receptor agonists” or TI “melatonin receptor agonist” or AB
“melatonin receptor agonist” or TI “melatonin agonist” or
AB “melatonin agonist” or TI “melatonin agonists” or AB
“melatonin agonists” OR TI “melatonin receptor antagonists”
or AB “melatonin receptor antagonists” or TI “melatonin
receptor antagonist” or AB “melatonin receptor antagonist”
or TI “melatonin antagonist” or AB “melatonin antagonist” or
TI “melatonin antagonists” or AB “melatonin antagonists” or
TI “melatonin receptor inhibitor” or AB “melatonin receptor
inhibitor” or TI “melatonin receptor inhibitors” or AB
“melatonin receptor inhibitors” or TI “melatonin inhibitor”
or AB “melatonin inhibitor” or TI “melatonin inhibitors” or
AB “melatonin inhibitors”) AND (TI “sleep” or AB “sleep”
or DE “Sleep” or DE “Sleep Disorders” or TI “circadian”
or AB “circadian” or DE “Human Biological Rhythms” or
DE “Sleep Wake Cycle”)) OR ((TI “melatonin” OR AB
“melatonin” or DE “melatonin” OR TI “ramelteon” OR AB
“ramelteon” OR TI “tasimelteon” OR AB “tasimelteon” OR
TI “agomelatine” OR AB “agomelatine”) AND (TI “sleep”
or AB “sleep” or DE “Sleep” or DE “Sleep Disorders” or
TI “circadian” or AB “circadian” or DE “Human Biological
Rhythms” or DE “Sleep Wake Cycle”)) OR ((TI “dim light
melatonin onset” or AB “dim light melatonin onset” or TI
“DLMO” or AB “DLMO”) and (TI “sleep” or AB “sleep”
or DE “Sleep” or DE “Sleep Disorders” or TI “circadian”
or AB “circadian” or DE “Human Biological Rhythms”
or DE “Sleep Wake Cycle”)) OR ((TI “Blindness” or AB
“blindness” or TI “blind person” or AB “blind person” or
TI “blind people” or AB “blind people” or TI “blind subject”
or AB “blind subject” or TI “blind subjects” or AB “blind
subjects” or TI “blind patient” or AB “blind patient” or TI
“blind patients” or AB “blind patients” or TI “enucleated”
or AB “enucleated” or TI “nonsighted” or AB “nonsighted”
or (TI “visual” and TI “impair*”) or (AB “visual” and
TI “impair*”) or TI “visually impaired” or AB “visually
impaired” or DE “Vision Disorders” or DE “Balint’s
Syndrome” or DE “Blind” or DE “Eye Disorders” or DE
“Hemianopia”) and (TI “sleep” or AB “sleep” or DE “Sleep”
or DE “Sleep Disorders” or TI “circadian” or AB “circadian”
or DE “Human Biological Rhythms” or DE “Sleep Wake
Cycle”)) OR ((DE “Dementia” OR DE “AIDS Dementia
Complex” OR DE “Dementia with Lewy Bodies” OR DE
“Presenile Dementia” OR DE “Semantic Dementia” OR
DE “Senile Dementia” OR DE “Vascular Dementia” or DE
“Alzheimer’s Disease” OR TI “dementia” or AB “dementia”
or TI “Alzheimer*” or AB “Alzheimer*”) AND (TI “sleep”
or AB “sleep” or DE “Sleep” or DE “Sleep Disorders” or
TI “circadian” or AB “circadian” or DE “Human Biological
Rhythms” or DE “Sleep Wake Cycle”)) OR ((TI “owl” or AB
“owl” OR TI “owls” or AB “owls” or TI “lark” or AB “lark”
OR TI “larks” or AB “larks” or TI “morningness” or AB
“morningness” or TI “eveningness” or AB “eveningness” or
TI “morning types” or AB “morning types” or TI “morning
type” or AB “morning type” or TI “evening types” or AB
“evening types” or TI “evening type” or AB “evening type”
or TM “Horne and Ostberg Morningness-Eveningness
Questionnaire”) and (TI “sleep” or AB “sleep” or DE “Sleep”
or DE “Sleep Disorders” or TI “circadian” or AB “circadian”
or DE “Human Biological Rhythms” or DE “Sleep Wake
Cycle”)) AND ((randomized controlled trial[pt] OR controlled
clinical trial[pt] OR randomized[tiab] OR placebo[tiab]
OR “drug therapy”[Subheading] OR randomly[tiab] OR
trial[tiab] OR groups[tiab]) NOT (“animals”[MeSH Terms]
NOT “humans”[MeSH Terms])) AND ((English[la] AND
(“2006/10/01”[PDAT] : “3000/12/31”[PDAT]) NOT (Comment
[pt] OR Editorial [pt] OR News [pt] OR Newspaper Article
[pt] OR Letter [pt] OR Case Reports [pt] OR Review [pt]))

1231
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
Terms for the Literature Search for Harms/Adverse
Effects of light Treatment (Pubmed Database)
(harm* OR “side effect*” OR “adverse effect*”) and (Light*
OR “light therapy*” OR “light exposure*” OR “Light
treatment*”) and “sleep”
Terms for the Literature Search for Harms/Adverse
Effects of Treatment with Hypnotics (Pubmed
Database)
(harm* OR “side effect*” OR “adverse effect*”) and
(hypnotic*)
Terms for the Literature Search for Harms/Adverse
Effects of Melatonin Treatment (Pubmed Database)
(harm* OR “side effect*” OR “adverse effect*”) and
(melatonin*)
Limits Used for the Searches of Harms/Adverse
Effects of Treatments
Article types: meta-analysis, systematic review
Species: humans
Languages: English
Table S1—Light treatment compared to placebo for ASWPD in adults.
Bibliography:
A
Palmer et al., 2003;
B
Campbell et al., 1993.
1
CI of the absolute effect crosses the clinical signicance threshold (dened by the TF) on both
sides of the “no effect” line.
2
CI of the absolute effect crosses the clinical signicance threshold dened by the TF.
3
CI of the absolute effect crosses the clinical
signicance threshold (dened by the TF) and the “no effect” line. aMT6, 6-sulfatoxymelatonin (urinary metabolite of melatonin); TST, total sleep time; ISL,
initial sleep latency; SOT, sleep onset time; SOffT, sleep offset time; CBT
Min
, core body temperature minimum; CI, condence interval; min, minutes; PSG,
polysomnography; TF, task force.
Summary of Findings Tables (Tables S1–S12)

1232
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
Table S2—Melatonin treatment compared to placebo for DSWPD in adults with and without depression.
Bibliography:
A
Kayumov et al., 2001;
B
Mundey et al., 2005;
C
Rahman et al., 2010.
*
In studies with crossover design, where more than one measurements
were taken on the same patients, all calculations were made based on the number of measurements.
1
High level of heterogeneity.
2
CI of the absolute
effect crosses the clinical signicance threshold dened by the TF.
3
CI of the absolute effect crosses the clinical signicance threshold (dened by the TF)
and the “no effect” line.
4
CI of the absolute effect crosses the clinical signicance threshold (dened by the TF) on both sides of the “no effect” line. TST,
total sleep time; ISL, initial sleep latency; DLMO, dim light melatonin onset; SOT, sleep onset time; SOffT, sleep offset time; CI, condence interval; PSG,
polysomnography; TF, task force; min, minutes.
Table S3 —Melatonin treatment compared to placebo for DSWPD in children/adolescents with no comorbidities.
Bibliography: van Geijlswijk et al., 2010.
1
CI of the absolute effect crosses the clinical signicance threshold dened by the TF on both sides of the “no effect”
line.
2
CI of the absolute effect crosses the clinical signicance threshold (dened by the TF) and the “no effect” line.
3
CI of the absolute effect crosses the
clinical signicance threshold dened by the TF. DLMO, dim light melatonin onset; ISL, initial sleep latency; SOT, sleep onset time; CI, condence interval;
mg, milligrams; min, minutes; kg, kilograms; TF, task force.

1233
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
Table S4—Melatonin treatment compared to placebo for DSWPD in children/adolescents comorbid with psychiatric conditions.
Bibliography:
A
Smits et al., 2001;
B
van der Heijden et al., 2007.
1
CI of the absolute effect crosses the “no effect” line and the clinical signicance threshold
dened by the TF.
2
CI of the absolute effect crosses the clinical signicance threshold dened by the TF.
3
CI of the absolute effect crosses the clinical
signicance threshold (dened by the TF) on both sides of the “no effect” line. TST, total sleep time; ISL, initial sleep latency; DLMO, dim light melatonin
onset; SOT, sleep onset time; SOffT, sleep offset time; CI, condence interval; TF, task force; min, minutes.
Table S5—Vitamin B12 compared to placebo for DSWPD in adults.
Bibliography: Okawa et al., 1997.
1
CI of the absolute effect crosses the clinical signicance threshold dened by the TF. TST, total sleep time; SOT, sleep
onset time; CI, condence interval; TF, task force; min, minutes.

1234
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
Table S6 —Light/combination treatment compared to controls for DSWPD in adults.
Bibliography:
A
Cole et al., 2002;
B
Lack, Bramwell et al., 2007.
1
This is a short paper with lack of detail in methods and results; a lot of information is missing.
2
Condence interval of the absolute effect crosses the clinical signicance threshold (dened by the TF) on both sides of the “no effect line.”
3
Condence
interval of the absolute effect crosses the “no effect” line.
4
Condence interval of the absolute effect crosses the clinical signicance threshold dened by
the TF. TST, total sleep time; SOT, sleep onset time; SOffT, sleep offset time; aMT6, 6-sulfatoxymelatonin (urinary metabolite of melatonin); CI, condence
interval; TF, task force; min, minutes.
Table S7—Light/combination treatment (multicomponent behavioral interventions + light) compared to controls for DSWPD in
children/adolescents.
Bibliography: Gradisar et al., 2011.
1
Study was not blinded, and an intention to treat analysis was NOT utilized.
2
CI of the absolute effect does not reach
clinical signicance threshold dened by the TF. TST, total sleep time; ISL, initial sleep latency; SOT, sleep onset time; SOffT, sleep offset time; CI,
condence interval; TF, task force; min, minutes.

1235
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders
Table S8 —Melatonin treatment compared to placebo for N24SWD in blind adults.
Bibliography: Lockley et al., 2000; Sack et al., 2000; Hack et al., 2003. CI, condence interval; OR, odds ratio.
Table S9 —Light treatment compared to placebo for ISWRD in elderly with dementia.
Bibliography:
A
Dowling et al., 2008;
B
Mishima et al., 1994.
1
A. No intention-to-treat principle: only completed cases were analyzed. B. Selective reporting:
dropouts are not described and some actigraphy variables are not reported (sleep onset, offset, sleep efciency etc.).
2
CI of the absolute value crosses the
clinical signicance threshold (dened by the TF) on both sides of the “no effect” line.
3
A. No blinding, B. No intention-to-treat principle observed. C. Role
of control group is vaguely described.
3
CI of the absolute effect crosses the clinical signicance threshold (dened by the TF) on both side of the “no effect”
line. TST, total sleep time; CI, condence interval; TF, task force; min, minutes.
Table S10—Melatonin treatment compared to placebo for ISWRD in elderly with dementia.
Bibliography: Serfaty et al., 2002.
1
CI of the absolute effect crosses the clinical signicance threshold (dened by the TF) on both sides of “no effect” line.
TST, total sleep time; CI, condence interval; TF, task force; min, minute.
Table S11—Melatonin treatment compared to placebo for ISWRD in children/adolescents with neurologic disorders.
Bibliography: Wright et al., 2011.
1
CI of the absolute effect crosses the clinical signicance threshold dened by the TF. TST, total sleep time; ISL, initial
sleep latency; CI, condence interval; TF, task force; min, minutes.

1236
Journal of Clinical Sleep Medicine, Vol. 11, No. 10, 2015
RR Auger, HJ Burgess, JS Emens et al.
Table S12—Comparison of combination of light therapy and melatonin/placebo for ISWRD in elderly with dementia.
Bibliography: Dowling et al., 2008.
1
A. No intention-to-treat principle: only completed cases were analyzed. B. Selective reporting: dropouts are not
described and some actigraphy variables are not reported (sleep onset,/offset, etc.).
2
CI of the absolute effect crosses the clinical signicance threshold
(dened by the TF) on both sides of the “no effect” line. PSWS, prescribed sleep-wake scheduling; TST, total sleep time; CI, condence interval; TF, task
force; min, minutes.
