
____________________________________________________________________________________________________________________________________
HIGHLIGHTS OF PRESCRIBING INFORMATION
----------------------- WARNINGS AND PRECAUTIONS------------------------
These hig hlights do not include all the informatio n nee ded to use
XELODA
safely and effectively. See full prescribing information for
XELODA
.
XELODA
(capecitabine) tablets, for oral use
Initial U.S. Approval: 1998
WARNING: XELODA-WARFARIN INTERACTION
See full prescribing information for complete boxed warning.
Patients receiving concomitant XELODA and oral coumarin-derivative
anticoagulants such as warfarin and phe nprocoumo n should have their
anticoagulant response (INR or prothrombin time) monitored frequently
in order to adjust the anticoagulant dose accordingly. Altered coagulation
parameters and/or bleeding, including de ath, have been re ported during
concomitant use.
• Occurrence: Within several days and up to several mo nths after
initiating XELODA therapy; may also be seen within 1 month after
stopping XELODA
• Predisposing factors: age>60 and diagnosis of cancer
--------------------------RECENT MAJOR CHANGES-------------------------
Dosage and Administration (2.0) 10/2014
Contraindications (4.1) 02/2015
Warnings and Precautions (5.1, 5.2, 5.5, and 5.7) 10/2014
Warnings and Precautions (5.4) 02/2015
--------------------------- INDICATIONS AND USAGE ----------------------------
XELODA (capecitabine) is a nucleoside metabolic inhibitor with
antineoplastic activity indicated for:
• Adjuv ant Colon Cancer (1.1)
– Patients with Dukes’ C colon cancer
• Metastatic Colorectal Cancer (1.1)
– First-line as monotherapy when treatment with fluoropyrimidine
therapy alone is preferred
• Metastatic Breast Cancer (1.2)
– In combination with docetaxel after failure of prior anthracycline-
containing therapy
– As monotherapy in patients resistant to both paclitaxel and an
anthracycline-containing regimen
----------------------- DOSAGE AND ADMINISTRATION -----------------------
• Take XELODA with water within 30 min after a meal (2)
• Monotherapy: 1250 mg/m
2
twice daily orally for 2 weeks followed by a
one week rest period in 3-week cycles (2.1)
• Adjuvant treatment is recommended for a total of 6 months (8 cycles)
(2.1)
• In combination with docetaxel, the recommended dose of XELODA is
1250 mg/m
2
twice daily for 2 weeks followed by a 7-day rest period,
combined with docetaxel at 75 mg/m
2
as a 1-hour IV infusion every 3
weeks (2.1)
• XELODA dosage may need to be individualized to optimize patient
management (2.2)
• Reduce the dose of XELODA by 25% in patients with moderate renal
impairment (2.3)
--------------------- DOSAGE FORMS AND STRENGTHS----------------------
• Tablets: 150 mg and 500 mg (3)
------------------------------ CONTRAINDICATIONS ------------------------------
• Severe Renal Impairment (4.1)
• Hypersensitivity (4.2)
• Coagulopathy: May result in bleeding, death. Monitor anticoagulant
response (e.g., INR) and adjust anticoagulant dose accordingly. (5.1)
• Diarrhea: May be severe. Interrupt XELODA treatment immediately
until diarrhea resolves or decreases to grade 1. Recommend standard
antidiarrheal treatments. (5.2)
• Cardiotoxicity: Common in patients with a prior history of coronary
artery disease. (5.3)
• Increased Risk of Severe or Fatal Adverse Reactions in Patients
with Lo w or Absent Dihydropyrimidine Dehydrogenase (DPD)
Activity: Withhold or permanently discontinue XELODA in patients
with evidence of acute early-onset or unusually severe toxicity, which
may indicate near complete or total absence of DPD activity. No
XELODA dose has been proven safe in patients with absent DPD
activity. (5.4)
• Dehydratio n and Renal Failure: Interrupt XELODA treatment until
dehydration is corrected. Potential risk of acute renal failure secondary
to dehydration. Monitor and correct dehydration. (5.5).
• Pregnancy: Can cause fetal harm. Advise women of the potential risk to
the fetus. (5.6, 8.1)
• Mucocutaneous and Dermatologic Toxicity: Severe mucocutaneous
reactions, Steven-Johnson Syndrome (SJS) and Toxic Epidermal
Necrolysis (TEN), have been reported. XELODA should be permanently
discontinued in patients who experience a severe mucocutaneous
reaction during treatment. XELODA may induce hand-and-foot
syndrome. Interrupt XELODA treatment until the hand-and-foot
syndrome event resolves or decreases in intensity. (5.7)
• Hyperbilirubinemia: Interrupt XELODA treatment immediately until
the hyperbilirubinemia resolves or decreases in intensity. (5.8)
• Hematologic: Do not treat patients with neutrophil counts <1.5 x 10
9
/L
or thrombocyte counts <100 x 10
9
/L. If grade 3-4 neutropenia or
thrombocytopenia occurs, stop therapy until condition resolves. (5.9)
------------------------------ ADVERSE REACTIONS ------------------------------
Most common adverse reactions (≥30%) were diarrhea, hand-and-foot
syndrome, nausea, vomiting, abdominal pain, fatigue/weakness, and
hyperbilirubinemia. Other adverse reactions, including serious adverse
reactions, have been reported. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at
1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
------------------------------ DRUG INTERACTIONS-------------------------------
• Anticoagulants: Monitor anticoagulant response (INR or prothrombin
time) frequently in order to adjust the anticoagulant dose as needed. (5.2,
7.1)
• Phenytoin: Monitor phenytoin levels in patients taking XELODA
concomitantly with phenytoin. The phenytoin dose may need to be
reduced. (7.1)
• Leucovorin: The concentration of 5-fluorouracil is increased and its
toxicity may be enhanced by leucovorin. (7.1)
• CYP2C9 substrates: Care should be exercised when XELODA is
coadministered with CYP2C9 substrates. (7.1)
• Food reduced both the rate and extent of absorption of capecitabine. (2,
7.1, 12.3)
----------------------- USE IN SPECIFIC POPULATIONS -----------------------
• Nursing Mothers: Discontinue nursing when receiving XELODA
treatment. (8.3)
• Geriatric: Greater incidence of adverse reactions. Monitoring required.
(8.5)
• Hepatic Impairment: Monitoring is recommended in patients with mild
to moderate hepatic impairment. (8.6)
• Renal Impairme nt: Reduce XELODA starting dose in patients with
moderate renal impairment (2.3, 8.7, 12.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-
approved patient labeling
Revised: 03/2015
1
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: XELODA-WARFARIN INTERACTION
1 INDICATIONS AND USAGE
1.1 Colorectal Cancer
1.2 Breast Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Standard Starting Dose
2.2 Dose Management Guidelines
2.3 Adjustment of Starting Dose in Special Populations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Severe Renal Impairment
4.2 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Coagulopathy
5.2 Diarrhea
5.3 Cardiotoxicity
5.4 Dihydropyrimidine Dehydrogenase Deficiency
5.5 Dehydration and Renal Failure
5.6 Pregnancy
5.7 Mucocutaneous and Dermatologic Toxicity
5.8 Hyperbilirubinemia
5.9 Hematologic
5.10 Geriatric Patients
5.11 Hepatic Insufficiency
5.12 Combination With Other Drugs
6 ADVERSE REACTIONS
6.1 Adjuva nt Colon Ca ncer
6.2 Metastatic Colorectal Cancer
6.3 Breast Cancer
6.4 Clinically Relevant Adverse Events in <5% of Patients
7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions
7.2 Drug-Food Intera ction
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy: Category D
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Insufficiency
8.7 Renal Insufficiency
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharma cokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adjuva nt Colon Ca ncer
14.2 Metastatic Colorectal Cancer
14.3 Breast Cancer
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
*Sections or subsections omitted from the full prescribing information are not
listed.
2
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
FULL PRESCRIBING INFORMATION
WARNING: XELODA-WARFARIN INTERACTION
XELODA Warfarin Interaction: Patients receiving concomitant capecitabine and oral
coumarin-derivative anticoagulant therapy should have their anticoagulant response (INR
or prothrombin time) monitored frequently in order to adjust the anticoagulant dose
accordingly. A clinically important XELODA-Warfarin drug interaction was
demonstrated in a clinical pharmacology trial [see Warnings and Precautions (5.2) and
Drug Interactions (7.1)]. Altered coagulation parameters and/or bleeding, including death,
have been reported in patients taking XELODA concomitantly with coumarin-derivative
anticoagulants such as warfarin and phenprocoumon. Postmarketing reports have shown
clinically significant increases in prothrombin time (PT) and INR in patients who were
stabilized on anticoagulants at the time XELODA was introduced. These events occurred
within several days and up to several months after initiating XELODA therapy and, in a
few cases, within 1 month after stopping XELODA. These events occurred in patients with
and without liver metastases. Age greater than 60 and a diagnosis of cancer independently
predispose patients to an increased risk of coagulopathy.
1 INDICATIONS AND USAGE
1.1 Colorectal Cancer
• XELODA is indicated as a single agent for adjuvant treatment in patients with Dukes’ C
colon cancer who have undergone complete resection of the primary tumor when treatment
with fluoropyrimidine therapy alone is preferred. XELODA was non-inferior to 5-
fluorouracil and leucovorin (5-FU/LV) for disease-free survival (DFS). Physicians should
consider results of combination chemotherapy trials, which have shown improvement in DFS
and OS, when prescribing single-agent XELODA in the adjuvant treatment of Dukes’ C
colon cancer.
• XELODA is indicated as first-line treatment of patients with metastatic colorectal carcinoma
when treatment with fluoropyrimidine therapy alone is preferred. Combination chemotherapy
has shown a survival benefit compared to 5-FU/LV alone. A survival benefit over 5-FU/LV
has not been demonstrated with XELODA monotherapy. Use of XELODA instead of 5-
FU/LV in combinations has not been adequately studied to assure safety or preservation of
the survival advantage.
1.2 Breast Cancer
• XELODA in combination with docetaxel is indicated for the treatment of patients with
metastatic breast cancer after failure of prior anthracycline-containing chemotherapy.
• XELODA monotherapy is also indicated for the treatment of patients with metastatic breast
cancer resistant to both paclitaxel and an anthracycline-containing chemotherapy regimen or
resistant to paclitaxel and for whom further anthracycline therapy is not indicated (e.g.,
patients who have received cumulative doses of 400 mg/m
2
of doxorubicin or doxorubicin
equivalents). Resistance is defined as progressive disease while on treatment, with or without
an initial response, or relapse within 6 months of completing treatment with an anthracycline-
containing adjuvant regimen.
3
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

2 DOSAGE AND ADMINISTRATION
XELODA tablets should be swallowed whole with water within 30 minutes after a meal. Do not
crush or cut XELODA tablets. XELODA dose is calculated according to body surface area.
2.1 Standard Starting Dose
Monotherapy (Metastatic Colorectal Cancer, Adjuvant Colorectal Cancer, Metastatic Breast
Cancer)
The recommended dose of XELODA is 1250 mg/m
2
administered orally twice daily (morning
and evening; equivalent to 2500 mg/m
2
total daily dose) for 2 weeks followed by a 1-week rest
period given as 3-week cycles (see Table 1).
Adjuvant treatment in patients with Dukes’ C colon cancer is recommended for a total of 6
months [ie, XELODA 1250 mg/m
2
orally twice daily for 2 weeks followed by a 1-week rest
period, given as 3-week cycles for a total of 8 cycles (24 weeks)].
Table 1 XELODA Dose Calculation According to Body Surface Area
Dose Level 1250 mg/m
2
Twice a Day
Number of Tablets to be Taken at
Each Dose (Morning and Evening)
Surface Area
(m
2
)
Total Daily
Dose* (mg)
150 mg
500 mg
≤ 1.25
3000
0
3
1.26-1.37
3300
1
3
1.38-1.51
3600
2
3
1.52-1.65
4000
0
4
1.66-1.77
4300
1
4
1.78-1.91
4600
2
4
1.92-2.05
5000
0
5
2.06-2.17
5300
1
5
≥ 2.18
5600
2
5
*Total Daily Dose divided by 2 to allow equal morning and evening doses
In Combination With Docetaxel (Metastatic Breast Cancer)
In combination with docetaxel, the recommended dose of XELODA is 1250 mg/m
2
twice daily
for 2 weeks followed by a 1-week rest period, combined with docetaxel at 75 mg/m
2
as a 1-hour
intravenous infusion every 3 weeks. Pre-medication, according to the docetaxel labeling, should
be started prior to docetaxel administration for patients receiving the XELODA plus docetaxel
combination. Table 1 displays the total daily dose of XELODA by body surface area and the
number of tablets to be taken at each dose.
2.2 Dose Management Guidelines
General
XELODA dosage may need to be individualized to optimize patient management. Patients
should be carefully monitored for toxicity and doses of XELODA should be modified as
necessary to accommodate individual patient tolerance to treatment [see Clinical Studies (14)].
Toxicity due to XELODA administration may be managed by symptomatic treatment, dose
interruptions and adjustment of XELODA dose. Once the dose has been reduced, it should not be
4
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

increased at a later time. Doses of XELODA omitted for toxicity are not replaced or restored;
instead the patient should resume the planned treatment cycles.
The dose of phenytoin and the dose of coumarin-derivative anticoagulants may need to be
reduced when either drug is administered concomitantly with XELODA [see Drug Interactions
(7.1)].
Monotherapy (Metastatic Colorectal Cancer, Adjuvant Colorectal Cancer, Metastatic Breast
Cancer)
XELODA dose modification scheme as described below (see Table 2) is recommended for the
management of adverse reactions.
Table 2 Recommended Dose Modifications of XELODA
Toxicity
NCIC Grades* During a Course of Therapy
Dose Adjustment for Next
Treatment (% of starting dose)
Grade 1 Maintain dose level Maintain dose level
Grade 2
-1st appearance
Interrupt until resolved to grade 0-1
100%
-2nd appearance
75%
-3rd appearance
50%
-4th appearance
Discontinue treatment permanently
-
Grade 3
-1st appearance
Interrupt until resolved to grade 0-1
75%
-2nd appearance
50%
-3rd appearance
Discontinue treatment permanently
-
Grade 4
-1st appearance
Discontinue permanently
OR
If physician deems it to be in the
patient’s best interest to continue,
interrupt until resolved to grade 0-1
50%
*National Cancer Institute of Canada Common Toxicity Criteria were used except for the hand-and-foot syndrome
[see Warnings and Precautions (5)].
In Combination With Docetaxel (Metastatic Breast Cancer)
Dose modifications of XELODA for toxicity should be made according to Table 2 above for
XELODA. At the beginning of a treatment cycle, if a treatment delay is indicated for either
XELODA or docetaxel, then administration of both agents should be delayed until the
requirements for restarting both drugs are met.
The dose reduction schedule for docetaxel when used in combination with XELODA for the
treatment of metastatic breast cancer is shown in Table 3.
5
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
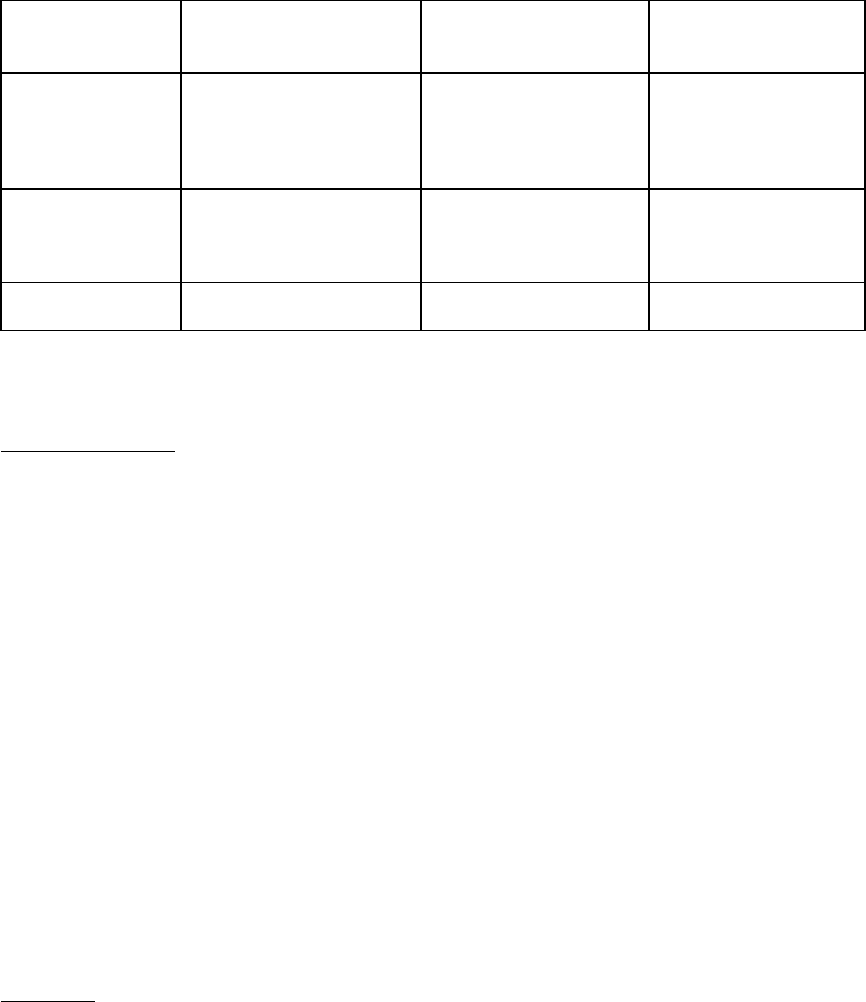
Table 3 Docetaxel Dose Reduction Schedule in Combination with XELODA
Toxicity
NCIC Grades*
Grade 2 Grade 3 Grade 4
1st appearance
Delay treatment until
resolved to grade 0-1;
Resume treatment with
original dose of 75 mg/m
2
docetaxel
Delay treatment until
resolved to grade 0-1;
Resume treatment at
55 mg/m2 of docetaxel.
Discontinue treatment
with docetaxel
2nd appearance
Delay treatment until
resolved to grade 0-1;
Resume treatment at
55 mg/m
2
of docetaxel.
Discontinue treatment
with docetaxel
-
3rd appearance
Discontinue treatment with
docetaxel
-
-
*National Cancer Institute of Canada Common Toxicity Criteria were used except for hand-and-foot syndrome [see
Warnings and Precautions (5)].
2.3 Adjustment of Starting Dose in Special Populations
Renal Impairment
No adjustment to the starting dose of XELODA is recommended in patients with mild renal
impairment (creatinine clearance = 51 to 80 mL/min [Cockroft and Gault, as shown below]). In
patients with moderate renal impairment (baseline creatinine clearance = 30 to 50 mL/min), a
dose reduction to 75% of the XELODA starting dose when used as monotherapy or in
combination with docetaxel (from 1250 mg/m
2
to 950 mg/m
2
twice daily) is recommended [see
Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)]. Subsequent dose
adjustment is recommended as outlined in Table 2 and Table 3 (depending on the regimen) if a
patient develops a grade 2 to 4 adverse event [see Warnings and Precautions (5.5)]. The starting
dose adjustment recommendations for patients with moderate renal impairment apply to both
XELODA monotherapy and XELODA in combination use with docetaxel.
Cockroft and Gault Equation:
(140 - age [yrs]) (body wt [kg])
Creatinine clearance for males = —————————————
(72) (serum creatinine [mg/dL])
Creatinine clearance for females = 0.85 x male value
Geriatrics
Physicians should exercise caution in monitoring the effects of XELODA in the elderly.
Insufficient data are available to provide a dosage recommendation.
3 DOSAGE FORMS AND STRENGTHS
XELODA is supplied as biconvex, oblong film-coated tablets for oral administration. Each light
peach-colored tablet contains 150 mg of capecitabine and each peach-colored tablet contains 500
mg of capecitabine.
6
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

4 CONTRAINDICATIONS
4.1 Severe Renal Impairment
XELODA is contraindicated in patients with severe renal impairment (creatinine clearance
below 30 mL/min [Cockroft and Gault]) [see Use in Specific Populations (8.7) and Clinical
Pharmacology (12.3)].
4.2 Hypersensitivity
XELODA is contraindicated in patients with known hypersensitivity to capecitabine or to any of
its components. XELODA is contraindicated in patients who have a known hypersensitivity to 5-
fluorouracil.
5 WARNINGS AND PRECAUTIONS
General
Patients receiving therapy with XELODA should be monitored by a physician experienced in the
use of cancer chemotherapeutic agents. Most adverse reactions are reversible and do not need to
result in discontinuation, although doses may need to be withheld or reduced [see Dosage and
Administration (2.2)].
5.1 Coagulopathy
Patients receiving concomitant capecitabine and oral coumarin-derivative anticoagulant therapy
should have their anticoagulant response (INR or prothrombin time) monitored closely with great
frequency and the anticoagulant dose should be adjusted accordingly [see Boxed Warning and
Drug Interactions (7.1)].
5.2 Diarrhea
XELODA can induce diarrhea, sometimes severe. Patients with severe diarrhea should be
carefully monitored and given fluid and electrolyte replacement if they become dehydrated. In
875 patients with either metastatic breast or colorectal cancer who received XELODA
monotherapy, the median time to first occurrence of grade 2 to 4 diarrhea was 34 days (range
from 1 to 369 days). The median duration of grade 3 to 4 diarrhea was 5 days. National Cancer
Institute of Canada (NCIC) grade 2 diarrhea is defined as an increase of 4 to 6 stools/day or
nocturnal stools, grade 3 diarrhea as an increase of 7 to 9 stools/day or incontinence and
malabsorption, and grade 4 diarrhea as an increase of ≥10 stools/day or grossly bloody diarrhea
or the need for parenteral support. If grade 2, 3 or 4 diarrhea occurs, administration of XELODA
should be immediately interrupted until the diarrhea resolves or decreases in intensity to grade 1
[see Dosage and Administration (2.2)]. Standard antidiarrheal treatments (eg, loperamide) are
recommended.
Necrotizing enterocolitis (typhlitis) has been reported.
5.3 Cardiotoxicity
The cardiotoxicity observed with XELODA includes myocardial infarction/ischemia, angina,
dysrhythmias, cardiac arrest, cardiac failure, sudden death, electrocardiographic changes, and
cardiomyopathy. These adverse reactions may be more common in patients with a prior history
of coronary artery disease.
7
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

5.4 Dihydropyrimidine Dehydrogenase Deficiency
Based on postmarketing reports, patients with certain homozygous or certain compound
heterozygous mutations in the DPD gene that result in complete or near complete absence of
DPD activity are at increased risk for acute early-onset of toxicity and severe, life-threatening,
or fatal adverse reactions caused by XELODA (e.g., mucositis, diarrhea, neutropenia, and
neurotoxicity). Patients with partial DPD activity may also have increased risk of severe, life-
threatening, or fatal adverse reactions caused by XELODA.
Withhold or permanently discontinue XELODA based on clinical assessment of the onset,
duration and severity of the observed toxicities in patients with evidence of acute early-onset or
unusually severe toxicity, which may indicate near complete or total absence of DPD activity.
No XELODA dose has been proven safe for patients with complete absence of DPD activity.
There is insufficient data to recommend a specific dose in patients with partial DPD activity as
measured by any specific test.
5.5 Dehydration and Renal Failure
Dehydration has been observed and may cause acute renal failure which can be fatal. Patients
with pre-existing compromised renal function or who are receiving concomitant XELODA with
known nephrotoxic agents are at higher risk. Patients with anorexia, asthenia, nausea, vomiting
or diarrhea may rapidly become dehydrated. Monitor patients when XELODA is administered to
prevent and correct dehydration at the onset. If grade 2 (or higher) dehydration occurs,
XELODA treatment should be immediately interrupted and the dehydration corrected. Treatment
should not be restarted until the patient is rehydrated and any precipitating causes have been
corrected or controlled. Dose modifications should be applied for the precipitating adverse event
as necessary [see Dosage and Administration (2.2)].
Patients with moderate renal impairment at baseline require dose reduction [see Dosage and
Administration (2.3)]. Patients with mild and moderate renal impairment at baseline should be
carefully monitored for adverse reactions. Prompt interruption of therapy with subsequent dose
adjustments is recommended if a patient develops a grade 2 to 4 adverse event as outlined in
Table 2 [see Dosage and Administration (2.2), Use in Specific Populations (8.7), and Clinical
Pharmacology (12.3)].
5.6 Pregnancy
XELODA may cause fetal harm when given to a pregnant woman. Capecitabine caused
embryolethality and teratogenicity in mice and embryolethality in monkeys when administered
during organogenesis. If this drug is used during pregnancy, or if a patient becomes pregnant
while receiving XELODA, the patient should be apprised of the potential hazard to the fetus [see
Use in Specific Populations (8.1)].
5.7 Mucocutaneous and Dermatologic Toxicity
Severe mucocutaneous reactions, some with fatal outcome, such as Stevens-Johnson syndrome
and Toxic Epidermal Necrolysis (TEN) can occur in patients treated with XELODA [see
Adverse Reactions (6.4)]. XELODA should be permanently discontinued in patients who
experience a severe mucocutaneous reaction possibly attributable to XELODA treatment.
8
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Hand-and-foot syndrome (palmar-plantar erythrodysesthesia or chemotherapy-induced acral
erythema) is a cutaneous toxicity. Median time to onset was 79 days (range from 11 to 360 days)
with a severity range of grades 1 to 3 for patients receiving XELODA monotherapy in the
metastatic setting. Grade 1 is characterized by any of the following: numbness,
dysesthesia/paresthesia, tingling, painless swelling or erythema of the hands and/or feet and/or
discomfort which does not disrupt normal activities. Grade 2 hand-and-foot syndrome is defined
as painful erythema and swelling of the hands and/or feet and/or discomfort affecting the
patient’s activities of daily living. Grade 3 hand-and-foot syndrome is defined as moist
desquamation, ulceration, blistering or severe pain of the hands and/or feet and/or severe
discomfort that causes the patient to be unable to work or perform activities of daily living. If
grade 2 or 3 hand-and-foot syndrome occurs, administration of XELODA should be interrupted
until the event resolves or decreases in intensity to grade 1. Following grade 3 hand-and-foot
syndrome, subsequent doses of XELODA should be decreased [see Dosage and Administration
(2.2)].
5.8 Hyperbilirubinemia
In 875 patients with either metastatic breast or colorectal cancer who received at least one dose
of XELODA 1250 mg/m
2
twice daily as monotherapy for 2 weeks followed by a 1-week rest
period, grade 3 (1.5-3 x ULN) hyperbilirubinemia occurred in 15.2% (n=133) of patients and
grade 4 (>3 x ULN) hyperbilirubinemia occurred in 3.9% (n=34) of patients. Of 566 patients
who had hepatic metastases at baseline and 309 patients without hepatic metastases at baseline,
grade 3 or 4 hyperbilirubinemia occurred in 22.8% and 12.3%, respectively. Of the 167 patients
with grade 3 or 4 hyperbilirubinemia, 18.6% (n=31) also had postbaseline elevations (grades 1 to
4, without elevations at baseline) in alkaline phosphatase and 27.5% (n=46) had postbaseline
elevations in transaminases at any time (not necessarily concurrent). The majority of these
patients, 64.5% (n=20) and 71.7% (n=33), had liver metastases at baseline. In addition, 57.5%
(n=96) and 35.3% (n=59) of the 167 patients had elevations (grades 1 to 4) at both prebaseline
and postbaseline in alkaline phosphatase or transaminases, respectively. Only 7.8% (n=13) and
3.0% (n=5) had grade 3 or 4 elevations in alkaline phosphatase or transaminases.
In the 596 patients treated with XELODA as first-line therapy for metastatic colorectal cancer,
the incidence of grade 3 or 4 hyperbilirubinemia was similar to the overall clinical trial safety
database of XELODA monotherapy. The median time to onset for grade 3 or 4
hyperbilirubinemia in the colorectal cancer population was 64 days and median total bilirubin
increased from 8 µm/L at baseline to 13 µm/L during treatment with XELODA. Of the 136
colorectal cancer patients with grade 3 or 4 hyperbilirubinemia, 49 patients had grade 3 or 4
hyperbilirubinemia as their last measured value, of which 46 had liver metastases at baseline.
In 251 patients with metastatic breast cancer who received a combination of XELODA and
docetaxel, grade 3 (1.5 to 3 x ULN) hyperbilirubinemia occurred in 7% (n=17) and grade 4 (>3 x
ULN) hyperbilirubinemia occurred in 2% (n=5).
If drug-related grade 3 to 4 elevations in bilirubin occur, administration of XELODA should be
immediately interrupted until the hyperbilirubinemia decreases to ≤3.0 X ULN [see
recommended dose modifications under Dosage and Administration (2.2)].
9
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
5.9 Hematologic
In 875 patients with either metastatic breast or colorectal cancer who received a dose of 1250
mg/m
2
administered twice daily as monotherapy for 2 weeks followed by a 1-week rest period,
3.2%, 1.7%, and 2.4% of patients had grade 3 or 4 neutropenia, thrombocytopenia or decreases
in hemoglobin, respectively. In 251 patients with metastatic breast cancer who received a dose of
XELODA in combination with docetaxel, 68% had grade 3 or 4 neutropenia, 2.8% had grade 3
or 4 thrombocytopenia, and 9.6% had grade 3 or 4 anemia.
Patients with baseline neutrophil counts of <1.5 x 10
9
/L and/or thrombocyte counts of <100 x
10
9
/L should not be treated with XELODA. If unscheduled laboratory assessments during a
treatment cycle show grade 3 or 4 hematologic toxicity, treatment with XELODA should be
interrupted.
5.10 Geriatric Patients
Patients ≥80 years old may experience a greater incidence of grade 3 or 4 adverse reactions. In
875 patients with either metastatic breast or colorectal cancer who received XELODA
monotherapy, 62% of the 21 patients ≥80 years of age treated with XELODA experienced a
treatment-related grade 3 or 4 adverse event: diarrhea in 6 (28.6%), nausea in 3 (14.3%), hand-
and-foot syndrome in 3 (14.3%), and vomiting in 2 (9.5%) patients. Among the 10 patients 70
years of age and greater (no patients were >80 years of age) treated with XELODA in
combination with docetaxel, 30% (3 out of 10) of patients experienced grade 3 or 4 diarrhea and
stomatitis, and 40% (4 out of 10) experienced grade 3 hand-and-foot syndrome.
Among the 67 patients ≥60 years of age receiving XELODA in combination with docetaxel, the
incidence of grade 3 or 4 treatment-related adverse reactions, treatment-related serious adverse
reactions, withdrawals due to adverse reactions, treatment discontinuations due to adverse
reactions and treatment discontinuations within the first two treatment cycles was higher than in
the <60 years of age patient group.
In 995 patients receiving XELODA as adjuvant therapy for Dukes’ C colon cancer after
resection of the primary tumor, 41% of the 398 patients ≥65 years of age treated with XELODA
experienced a treatment-related grade 3 or 4 adverse event: hand-and-foot syndrome in 75
(18.8%), diarrhea in 52 (13.1%), stomatitis in 12 (3.0%), neutropenia/granulocytopenia in 11
(2.8%), vomiting in 6 (1.5%), and nausea in 5 (1.3%) patients. In patients ≥65 years of age (all
randomized population; capecitabine 188 patients, 5-FU/LV 208 patients) treated for Dukes’ C
colon cancer after resection of the primary tumor, the hazard ratios for disease-free survival and
overall survival for XELODA compared to 5-FU/LV were 1.01 (95% C.I. 0.80 – 1.27) and 1.04
(95% C.I. 0.79 – 1.37), respectively.
5.11 Hepatic Insufficiency
Patients with mild to moderate hepatic dysfunction due to liver metastases should be carefully
monitored when XELODA is administered. The effect of severe hepatic dysfunction on the
disposition of XELODA is not known [see Use in Specific Populations (8.6) and Clinical
Pharmacology (12.3)].
5.12 Combination With Other Drugs
Use of XELODA in combination with irinotecan has not been adequately studied.
10
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates
observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials
of another drug and may not reflect the rates observed in practice.
6.1 Adjuvant Colon Cancer
Table 4 shows the adverse reactions occurring in ≥5% of patients from one phase 3 trial in
patients with Dukes’ C colon cancer who received at least one dose of study medication and had
at least one safety assessment. A total of 995 patients were treated with 1250 mg/m
2
twice a day
of XELODA administered for 2 weeks followed by a 1-week rest period, and 974 patients were
administered 5-FU and leucovorin (20 mg/m
2
leucovorin IV followed by 425 mg/m
2
IV bolus 5-
FU on days 1-5 every 28 days). The median duration of treatment was 164 days for capecitabine-
treated patients and 145 days for 5-FU/LV-treated patients. A total of 112 (11%) and 73 (7%)
capecitabine and 5-FU/LV-treated patients, respectively, discontinued treatment because of
adverse reactions. A total of 18 deaths due to all causes occurred either on study or within 28
days of receiving study drug: 8 (0.8%) patients randomized to XELODA and 10 (1.0%)
randomized to 5-FU/LV.
Table 5 shows grade 3/4 laboratory abnormalities occurring in ≥1% of patients from one phase 3
trial in patients with Dukes’ C colon cancer who received at least one dose of study medication
and had at least one safety assessment.
Table 4 Percent Incidence of Adverse Reactions Reported
in ≥5% of Patients Treated With XELODA or 5-FU/LV for
Colon Cancer in the Adjuvant Setting (Safety Population)
Adjuvant Treatment for Colon Cancer (N=1969)
XELODA
(N=995)
5-FU/LV
(N=974)
Body System/
Adverse Event
All Grades
Grade 3/4
All Grades
Grade 3/4
Gastrointestinal
Disorders
Diarrhea
47
12
65
14
Nausea
34
2
47
2
Stomatitis
22
2
60
14
Vomiting
15
2
21
2
Abdominal Pain
14
3
16
2
Constipation
9
-
11
<1
Upper Abdominal
Pain
7
<1
7
<1
Dyspepsia
6
<1
5
-
Skin and Subcutaneous
Tissue Disorders
Hand-and-Foot
Syndrome
60
17
9
<1
Alopecia
6
-
22
<1
11
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Adjuvant Treatment for Colon Cancer (N=1969)
XELODA
(N=995)
5-FU/LV
(N=974)
Body System/
Adverse Event
All Grades
Grade 3/4
All Grades
Grade 3/4
Rash
Erythema
7
6
-
1
8
5
-
<1
General Disorders and
Administration Site
Conditions
Fatigue
Pyrexia
Asthenia
Lethargy
16
7
10
10
<1
<1
<1
<1
16
9
10
9
1
<1
1
<1
Nervous System
Disorders
Dizziness
Headache
Dysgeusia
6
5
6
<1
<1
-
6
6
9
-
<1
-
Metabolism and
Nutrition Disorders
Anorexia
9
<1
11
<1
Eye Disorders
Conjunctivitis
5
<1
6
<1
Blood and Lymphatic
System Disorders
Neutropenia
2
<1
8
5
Respiratory Thoracic
and Mediastinal
Disorders
Epistaxis
2
-
5
-
12
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Table 5 Percent Incidence of Grade 3/4 Laboratory
Abnormalities Reported in ≥1% of Patients Receiving
XELODA Monotherapy for Adjuvant Treatment of
Colon Cancer (Safety Population)
Adverse Event
XELODA
(n=995)
Grade 3/4 %
IV 5-FU/LV
(n=974)
Grade 3/4 %
Increased ALAT (SGPT)
Increased calcium
Decreased calcium
Decreased hemoglobin
Decreased lymphocytes
Decreased neutrophils*
Decreased neutrophils/granulocytes
Decreased platelets
Increased bilirubin**
1.6
1.1
2.3
1.0
13.0
2.2
2.4
1.0
20
0.6
0.7
2.2
1.2
13.0
26.2
26.4
0.7
6.3
*The incidence of grade 3/4 white blood cell abnormalities was 1.3% in the XELODA arm and 4.9% in the IV 5-
FU/LV arm.
**It should be noted that grading was according to NCIC CTC Version 1 (May, 1994). In the NCIC-CTC Version 1,
hyperbilirubinemia grade 3 indicates a bilirubin value of 1.5 to 3.0 × upper limit of normal (ULN) range, and grade
4 a value of > 3.0 × ULN. The NCI CTC Version 2 and above define a grade 3 bilirubin value of >3.0 to 10.0 ×
ULN, and grade 4 values >10.0 × ULN.
6.2 Metastatic Colorectal Cancer
Monotherapy
Table 6 shows the adverse reactions occurring in ≥5% of patients from pooling the two phase 3
trials in first line metastatic colorectal cancer. A total of 596 patients with metastatic colorectal
cancer were treated with 1250 mg/m
2
twice a day of XELODA administered for 2 weeks
followed by a 1-week rest period, and 593 patients were administered 5-FU and leucovorin in the
Mayo regimen (20 mg/m
2
leucovorin IV followed by 425 mg/m
2
IV bolus 5-FU, on days 1-5,
every 28 days). In the pooled colorectal database the median duration of treatment was 139 days
for capecitabine-treated patients and 140 days for 5-FU/LV-treated patients. A total of 78 (13%)
and 63 (11%) capecitabine and 5-FU/LV-treated patients, respectively, discontinued treatment
because of adverse reactions/intercurrent illness. A total of 82 deaths due to all causes occurred
either on study or within 28 days of receiving study drug: 50 (8.4%) patients randomized to
XELODA and 32 (5.4%) randomized to 5-FU/LV.
13
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
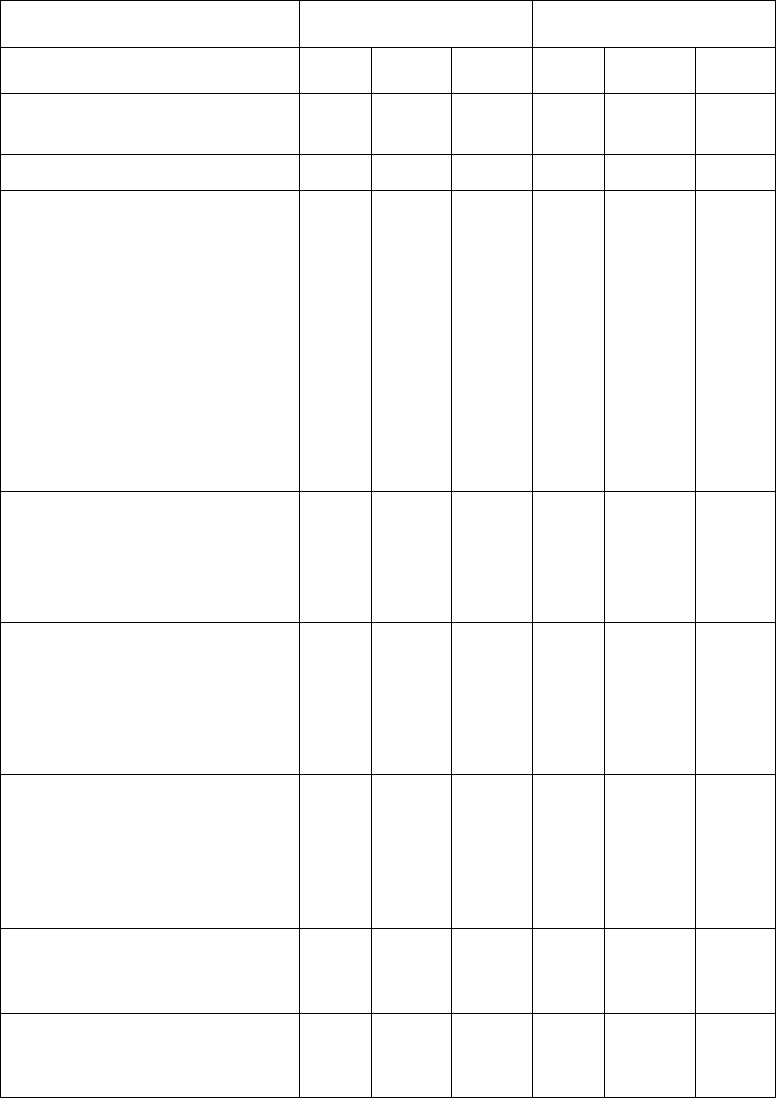
Table 6 Pooled Phase 3 Colorectal Trials: Percent Incidence
of Adverse Reactions in ≥5% of Patients
Adverse Event
XELODA
(n=596)
5-FU/LV
(n=593)
Total
%
Grade 3
%
Grade 4
%
Total
%
Grade 3
%
Grade 4
%
Number of Patients With
>
One
Adverse Event
96 52 9 94 45 9
Body System/Adverse Event
GI
Diarrhea
55
13
2
61
10
2
Nausea
43
4
–
51
3
<1
Vomiting
27
4
<1
30
4
<1
Stomatitis
25
2
<1
62
14
1
Abdominal Pain
35
9
<1
31
5
–
Gastrointestinal Motility Disorder
10
<1
–
7
<1
–
Constipation
14
1
<1
17
1
–
Oral Discomfort
10
–
–
10
–
–
Upper GI Inflammatory Disorders
8
<1
–
10
1
–
Gastrointestinal Hemorrhage
6
1
<1
3
1
–
Ileus
6
4
1
5
2
1
Skin and Subcutaneous
Hand-and-Foot Syndrome
54
17
NA
6
1
NA
Dermatitis
27
1
–
26
1
–
Skin Discoloration
7
<1
–
5
–
–
Alopecia
6
–
–
21
<1
–
General
Fatigue/Weakness
42
4
–
46
4
–
Pyrexia
18
1
–
21
2
–
Edema
15
1
–
9
1
–
Pain
12
1
–
10
1
–
Chest Pain
6
1
–
6
1
<1
Neurological
Peripheral Sensory Neuropathy
10
–
–
4
–
–
Headache
10
1
–
7
–
–
Dizziness*
8
<1
–
8
<1
–
Insomnia
7
–
–
7
–
–
Taste Disturbance
6
1
–
11
<1
1
Metabolism
Appetite Decreased
Dehydration
26
7
3
2
<1
<1
31
8
2
3
<1
1
Eye
Eye Irritation
Vision Abnormal
13
5
–
–
–
–
10
2
<1
–
–
–
14
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Adverse Event
XELODA
(n=596)
5-FU/LV
(n=593)
Total
%
Grade 3
%
Grade 4
%
Total
%
Grade 3
%
Grade 4
%
Respiratory
Dyspnea
Cough
Pharyngeal Disorder
Epistaxis
Sore Throat
14
7
5
3
2
1
<1
–
<1
–
–
1
–
–
–
10
8
5
6
6
<1
–
–
–
–
1
–
–
–
–
Musculoskeletal
Back Pain
Arthralgia
10
8
2
1
–
–
9
6
<1
1
–
–
Vascular
Venous Thrombosis
8
3
<1
6
2
–
Psychiatric
Mood Alteration
Depression
5
5
–
–
–
–
6
4
<1
<1
–
–
Infections
Viral
5
<1
–
5
<1
–
Blood and Lymphatic
Anemia
Neutropenia
80
13
2
1
<1
2
79
46
1
8
<1
13
Hepatobiliary
Hyperbilirubinemia
48
18
5
17
3
3
– Not observed
* Excluding vertigo
NA = Not Applicable
6.3 Breast Cancer
In Combination with Docetaxel
The following data are shown for the combination study with XELODA and docetaxel in
patients with metastatic breast cancer in Table 7 and Table 8. In the XELODA and docetaxel
combination arm the treatment was XELODA administered orally 1250 mg/m
2
twice daily as
intermittent therapy (2 weeks of treatment followed by 1 week without treatment) for at least 6
weeks and docetaxel administered as a 1-hour intravenous infusion at a dose of 75 mg/m
2
on the
first day of each 3-week cycle for at least 6 weeks. In the monotherapy arm docetaxel was
administered as a 1-hour intravenous infusion at a dose of 100 mg/m
2
on the first day of each 3-
week cycle for at least 6 weeks. The mean duration of treatment was 129 days in the combination
arm and 98 days in the monotherapy arm. A total of 66 patients (26%) in the combination arm
and 49 (19%) in the monotherapy arm withdrew from the study because of adverse reactions.
The percentage of patients requiring dose reductions due to adverse reactions was 65% in the
combination arm and 36% in the monotherapy arm. The percentage of patients requiring
treatment interruptions due to adverse reactions in the combination arm was 79%. Treatment
interruptions were part of the dose modification scheme for the combination therapy arm but not
for the docetaxel monotherapy-treated patients.
15
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Table 7 Percent Incidence of Adverse Events Considered Related
or Unrelated to Treatment in ≥5% of Patients Participating
in the XELODA and Docetaxel Combination vs Docetaxel
Monotherapy Study
Adverse Event
XELODA 1250 mg/m
2
/bid
With Docetaxel
75 mg/m
2
/3 weeks
(n=251)
Docetaxel
100 mg/m
2
/3 weeks
(n=255)
Total
%
Grade
3 %
Grade
4 %
Total
%
Grade
3 %
Grade
4 %
Number of Patients With at
Least One Adverse Event
99 76.5 29.1 97 57.6 31.8
Body System/Adverse Event
GI
Diarrhea
67
14
<1
48
5
<1
Stomatitis
67
17
<1
43
5
–
Nausea
45
7
–
36
2
–
Vomiting
35
4
1
24
2
–
Constipation
20
2
–
18
–
–
Abdominal Pain
30
<3
<1
24
2
–
Dyspepsia
14
–
–
8
1
–
Dry Mouth
6
<1
–
5
–
–
Skin and Subcutaneous
Hand-and-Foot Syndrome
63
24
NA
8
1
NA
Alopecia
41
6
–
42
7
–
Nail Disorder
14
2
–
15
–
–
Dermatitis
8
–
–
11
1
–
Rash Erythematous
9
<1
–
5
–
–
Nail Discoloration
6
–
–
4
<1
–
Onycholysis
5
1
–
5
1
–
Pruritus
4
–
–
5
–
–
General
Pyrexia
28
2
–
34
2
–
Asthenia
26
4
<1
25
6
–
Fatigue
22
4
–
27
6
–
Weakness
16
2
–
11
2
–
Pain in Limb
13
<1
–
13
2
–
Lethargy
7
–
–
6
2
–
Pain
7
<1
–
5
1
–
Chest Pain (non-cardiac)
4
<1
–
6
2
–
Influenza-like Illness
5
–
–
5
–
–
Neurological
Taste Disturbance
16
<1
–
14
<1
–
Headache
15
3
–
15
2
–
Paresthesia
12
<1
–
16
1
–
Dizziness
12
–
–
8
<1
–
Insomnia
8
–
–
10
<1
–
Peripheral Neuropathy
6
–
–
10
1
–
Hypoaesthesia
4
<1
–
8
<1
–
16
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Adverse Event
XELODA 1250 mg/m
2
/bid
With Docetaxel
75 mg/m
2
/3 weeks
(n=251)
Docetaxel
100 mg/m
2
/3 weeks
(n=255)
Total
%
Grade
3 %
Grade
4 %
Total
%
Grade
3 %
Grade
4 %
Metabolism
Anorexia
13
1
–
11
<1
–
Appetite Decreased
10
–
–
5
–
–
Weight Decreased
7
–
–
5
–
–
Dehydration
10
2
–
7
<1
<1
Eye
Lacrimation Increased
12
–
–
7
<1
–
Conjunctivitis
5
–
–
4
–
–
Eye Irritation
5
–
–
1
–
–
Musculoskeletal
Arthralgia
15
2
–
24
3
–
Myalgia
15
2
–
25
2
–
Back Pain
12
<1
–
11
3
–
Bone Pain
8
<1
–
10
2
–
Cardiac
Edema
33
<2
–
34
<3
1
Blood
Neutropenic Fever
16
3
13
21
5
16
Respiratory
Dyspnea
14
2
<1
16
2
–
Cough
13
1
–
22
<1
–
Sore Throat
12
2
–
11
<1
–
Epistaxis
7
<1
–
6
–
–
Rhinorrhea
5
–
–
3
–
–
Pleural Effusion
2
1
–
7
4
–
Infection
Oral Candidiasis
7
<1
–
8
<1
–
Urinary Tract Infection
6
<1
–
4
–
–
Upper Respiratory Tract
4
–
–
5
1
–
Vascular
Flushing
Lymphoedema
5
3
–
<1
–
–
5
5
–
1
–
–
Psychiatric
Depression
5
–
–
5
1
–
– Not observed
NA = Not Applicable
Table 8 Percent of Patients With Laboratory Abnormalities
Participating in the XELODA and Docetaxel Combination
vs Docetaxel Monotherapy Study
Adverse Event
XELODA 1250 mg/m
2
/bid
With Docetaxel
75 mg/m
2
/3 weeks
(n=251)
Docetaxel
100 mg/m
2
/3 weeks
(n=255)
Body System/Adverse Event
Total
%
Grade
3 %
Grade
4 %
Total
%
Grade
3 %
Grade
4 %
Hematologic
Leukopenia
91
37
24
88
42
33
17
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Adverse Event
XELODA 1250 mg/m
2
/bid
With Docetaxel
75 mg/m
2
/3 weeks
(n=251)
Docetaxel
100 mg/m
2
/3 weeks
(n=255)
Body System/Adverse Event
Total
%
Grade
3 %
Grade
4 %
Total
%
Grade
3 %
Grade
4 %
Neutropenia/Granulocytopenia
Thrombocytopenia
Anemia
Lymphocytopenia
86
41
80
99
20
2
7
48
49
1
3
41
87
23
83
98
10
1
5
44
66
2
<1
40
Hepatobiliary
Hyperbilirubinemia
20
7
2
6
2
2
Monotherapy
The following data are shown for the study in stage IV breast cancer patients who received a
dose of 1250 mg/m
2
administered twice daily for 2 weeks followed by a 1-week rest period. The
mean duration of treatment was 114 days. A total of 13 out of 162 patients (8%) discontinued
treatment because of adverse reactions/intercurrent illness.
Table 9 Percent Incidence of Adverse Reactions Considered Remotely,
Possibly or Probably Related to Treatment in ≥5% of
Patients Participating in the Single Arm Trial in Stage IV
Breast Cancer
Adverse Event
Phase 2 Trial in Stage IV Breast Cancer
(n=162)
Body System/Adverse Event
Total
%
Grade 3
%
Grade 4
%
GI
Diarrhea
57
12
3
Nausea
53
4
–
Vomiting
37
4
–
Stomatitis
24
7
–
Abdominal Pain
20
4
–
Constipation
15
1
–
Dyspepsia
8
–
–
Skin and Subcutaneous
Hand-and-Foot Syndrome
57
11
NA
Dermatitis
37
1
–
Nail Disorder
7
–
–
General
Fatigue
41
8
–
Pyrexia
12
1
–
Pain in Limb
6
1
–
18
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Adverse Event
Phase 2 Trial in Stage IV Breast Cancer
(n=162)
Body System/Adverse Event
Total
%
Grade 3
%
Grade 4
%
Neurological
Paresthesia
Headache
Dizziness
Insomnia
21
9
8
8
1
1
–
–
–
–
–
–
Metabolism
Anorexia
Dehydration
23
7
3
4
–
1
Eye
Eye Irritation
15
–
–
Musculoskeletal
Myalgia
9
–
–
Cardiac
Edema
9
1
–
Blood
Neutropenia
Thrombocytopenia
Anemia
Lymphopenia
26
24
72
94
2
3
3
44
2
1
1
15
Hepatobiliary
Hyperbilirubinemia
22
9
2
– Not observed
NA = Not Applicable
6.4 Clinically Relevant Adverse Events in <5% of Patients
Clinically relevant adverse events reported in <5% of patients treated with XELODA either as
monotherapy or in combination with docetaxel that were considered at least remotely related to
treatment are shown below; occurrences of each grade 3 and 4 adverse event are provided in
parentheses.
Monotherapy (Metastatic Colorectal Cancer, Adjuvant Colorectal Cancer, Metastatic Breast
Cancer)
Gastrointestinal: abdominal distension, dysphagia, proctalgia, ascites (0.1%), gastric ulcer
(0.1%), ileus (0.3%), toxic dilation of intestine, gastroenteritis (0.1%)
Skin & Subcutan.: nail disorder (0.1%), sweating increased (0.1%), photosensitivity reaction
(0.1%), skin ulceration, pruritus, radiation recall syndrome (0.2%)
General: chest pain (0.2%), influenza-like illness, hot flushes, pain (0.1%),
hoarseness, irritability, difficulty in walking, thirst, chest mass, collapse,
fibrosis (0.1%), hemorrhage, edema, sedation
19
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Neurological: insomnia, ataxia (0.5%), tremor, dysphasia, encephalopathy (0.1%),
abnormal coordination, dysarthria, loss of consciousness (0.2%), impaired
balance
Metabolism: increased weight, cachexia (0.4%), hypertriglyceridemia (0.1%),
hypokalemia, hypomagnesemia
Eye: conjunctivitis
Respiratory: cough (0.1%), epistaxis (0.1%), asthma (0.2%), hemoptysis, respiratory
distress (0.1%), dyspnea
Cardiac: tachycardia (0.1%), bradycardia, atrial fibrillation, ventricular
extrasystoles, extrasystoles, myocarditis (0.1%), pericardial effusion
Infections: laryngitis (1.0%), bronchitis (0.2%), pneumonia (0.2%),
bronchopneumonia (0.2%), keratoconjunctivitis, sepsis (0.3%), fungal
infections (including candidiasis) (0.2%)
Musculoskeletal: myalgia, bone pain (0.1%), arthritis (0.1%), muscle weakness
Blood & Lymphatic: leukopenia (0.2%), coagulation disorder (0.1%), bone marrow depression
(0.1%), idiopathic thrombocytopenia purpura (1.0%), pancytopenia (0.1%)
Vascular: hypotension (0.2%), hypertension (0.1%), lymphoedema (0.1%),
pulmonary embolism (0.2%), cerebrovascular accident (0.1%)
Psychiatric: depression, confusion (0.1%)
Renal: renal impairment (0.6%)
Ear: vertigo
Hepatobiliary: hepatic fibrosis (0.1%), hepatitis (0.1%), cholestatic hepatitis (0.1%),
abnormal liver function tests
Immune System: drug hypersensitivity (0.1%)
Postmarketing: hepatic failure, lacrimal duct stenosis, acute renal failure secondary to
dehydration including fatal outcome [see Warnings and Precautions (5.5)],
cutaneous lupus erythematosus, corneal disorders including keratitis, toxic
leukoencephalopathy, severe skin reactions such as Stevens-Johnson
Syndrome and Toxic Epidermal Necrolysis (TEN) [see Warnings and
Precautions (5.7)]
XELODA In Combination With Docetaxel (Metastatic Breast Cancer)
Gastrointestinal: ileus (0.4%), necrotizing enterocolitis (0.4%), esophageal ulcer (0.4%),
hemorrhagic diarrhea (0.8%)
Neurological: ataxia (0.4%), syncope (1.2%), taste loss (0.8%), polyneuropathy (0.4%),
migraine (0.4%)
Cardiac: supraventricular tachycardia (0.4%)
Infection: neutropenic sepsis (2.4%), sepsis (0.4%), bronchopneumonia (0.4%)
Blood & Lymphatic: agranulocytosis (0.4%), prothrombin decreased (0.4%)
Vascular: hypotension (1.2%), venous phlebitis and thrombophlebitis (0.4%),
postural hypotension (0.8%)
Renal: renal failure (0.4%)
Hepatobiliary: jaundice (0.4%), abnormal liver function tests (0.4%), hepatic failure
(0.4%), hepatic coma (0.4%), hepatotoxicity (0.4%)
Immune System: hypersensitivity (1.2%)
20
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions
Anticoagulants
Altered coagulation parameters and/or bleeding have been reported in patients taking XELODA
concomitantly with coumarin-derivative anticoagulants such as warfarin and phenprocoumon
[see Boxed Warning]. These events occurred within several days and up to several months after
initiating XELODA therapy and, in a few cases, within 1 month after stopping XELODA. These
events occurred in patients with and without liver metastases. In a drug interaction study with
single-dose warfarin administration, there was a significant increase in the mean AUC of
S-warfarin [see Clinical Pharmacology (12.3)]. The maximum observed INR value increased by
91%. This interaction is probably due to an inhibition of cytochrome P450 2C9 by capecitabine
and/or its metabolites.
Phenytoin
The level of phenytoin should be carefully monitored in patients taking XELODA and phenytoin
dose may need to be reduced [see Dosage and Administration (2.2)]. Postmarketing reports
indicate that some patients receiving XELODA and phenytoin had toxicity associated with
elevated phenytoin levels. Formal drug-drug interaction studies with phenytoin have not been
conducted, but the mechanism of interaction is presumed to be inhibition of the CYP2C9
isoenzyme by capecitabine and/or its metabolites.
Leucovorin
The concentration of 5-fluorouracil is increased and its toxicity may be enhanced by leucovorin.
Deaths from severe enterocolitis, diarrhea, and dehydration have been reported in elderly patients
receiving weekly leucovorin and fluorouracil.
CYP2C9 substrates
Other than warfarin, no formal drug-drug interaction studies between XELODA and other
CYP2C9 substrates have been conducted. Care should be exercised when XELODA is
coadministered with CYP2C9 substrates.
7.2 Drug-Food Interaction
Food was shown to reduce both the rate and extent of absorption of capecitabine [see Clinical
Pharmacology (12.3)]. In all clinical trials, patients were instructed to administer XELODA
within 30 minutes after a meal. It is recommended that XELODA be administered with food [see
Dosage and Administration (2)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy: Category D
XELODA can cause fetal harm when administered to a pregnant woman. Capecitabine at doses
of 198 mg/kg/day during organogenesis caused malformations and embryo death in mice. In
separate pharmacokinetic studies, this dose in mice produced 5’-DFUR AUC values about 0.2
times the corresponding values in patients administered the recommended daily dose.
Malformations in mice included cleft palate, anophthalmia, microphthalmia, oligodactyly,
polydactyly, syndactyly, kinky tail and dilation of cerebral ventricles. At doses of 90 mg/kg/day,
capecitabine given to pregnant monkeys during organogenesis caused fetal death. This dose
21
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
produced 5’-DFUR AUC values about 0.6 times the corresponding values in patients
administered the recommended daily dose.
There are no adequate and well controlled studies of XELODA in pregnant women. If this drug
is used during pregnancy, or if a patient becomes pregnant while receiving XELODA, the patient
should be apprised of the potential hazard to the fetus. Women should be advised to avoid
becoming pregnant while receiving treatment with XELODA [see Warnings and Precautions
(5.6)].
8.3 Nursing Mothers
Lactating mice given a single oral dose of capecitabine excreted significant amounts of
capecitabine metabolites into the milk. It is not known whether this drug is excreted in human
milk. Because many drugs are excreted in human milk and because of the potential for serious
adverse reactions in nursing infants from capecitabine, a decision should be made whether to
discontinue nursing or to discontinue the drug, taking into account the importance of the drug to
the mother.
8.4 Pediatric Use
The safety and effectiveness of XELODA in pediatric patients have not been established. No
clinical benefit was demonstrated in two single arm trials in pediatric patients with newly
diagnosed brainstem gliomas and high grade gliomas. In both trials, pediatric patients received
an investigational pediatric formulation of capecitabine concomitantly with and following
completion of radiation therapy (total dose of 5580 cGy in 180 cGy fractions). The relative
bioavailability of the investigational formulation to XELODA was similar.
The first trial was conducted in 22 pediatric patients (median age 8 years, range 5-17 years) with
newly diagnosed non-disseminated intrinsic diffuse brainstem gliomas and high grade gliomas.
In the dose-finding portion of the trial, patients received capecitabine with concomitant radiation
therapy at doses ranging from 500 mg/m
2
to 850 mg/m
2
every 12 hours for up to 9 weeks. After
a 2 week break, patients received 1250 mg/m
2
capecitabine every 12 hours on Days 1-14 of a 21-
day cycle for up to 3 cycles. The maximum tolerated dose (MTD) of capecitabine administered
concomitantly with radiation therapy was 650 mg/m
2
every 12 hours. The major dose limiting
toxicities were palmar-plantar erythrodysesthesia and alanine aminotransferase (ALT) elevation.
The second trial was conducted in 34 additional pediatric patients with newly diagnosed non-
disseminated intrinsic diffuse brainstem gliomas (median age 7 years, range 3-16 years) and 10
pediatric patients who received the MTD of capecitabine in the dose-finding trial and met the
eligibility criteria for this trial. All patients received 650 mg/m
2
capecitabine every 12 hours
with concomitant radiation therapy for up to 9 weeks. After a 2 week break, patients received
1250 mg/m
2
capecitabine every 12 hours on Days 1-14 of a 21-day cycle for up to 3 cycles.
There was no improvement in one-year progression-free survival rate and one-year overall
survival rate in pediatric patients with newly diagnosed intrinsic brainstem gliomas who received
capecitabine relative to a similar population of pediatric patients who participated in other
clinical trials.
22
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

The adverse reaction profile of capecitabine was consistent with the known adverse reaction
profile in adults, with the exception of laboratory abnormalities which occurred more commonly
in pediatric patients. The most frequently reported laboratory abnormalities (per-patient
incidence ≥40%) were increased ALT (75%), lymphocytopenia (73%), leukopenia (73%),
hypokalemia (68%), thrombocytopenia (57%), hypoalbuminemia (55%), neutropenia (50%), low
hematocrit (50%), hypocalcemia (48%), hypophosphatemia (45%) and hyponatremia (45%).
8.5 Geriatric Use
Physicians should pay particular attention to monitoring the adverse effects of XELODA in the
elderly [see Warnings and Precautions (5.11)].
8.6 Hepatic Insufficiency
Exercise caution when patients with mild to moderate hepatic dysfunction due to liver metastases
are treated with XELODA. The effect of severe hepatic dysfunction on XELODA is not known
[see Warnings and Precautions (5.12) and Clinical Pharmacology (12.3)].
8.7 Renal Insufficiency
Patients with moderate (creatinine clearance = 30 to 50 mL/min) and severe (creatinine clearance
<30 mL/min) renal impairment showed higher exposure for capecitabine, 5-DFUR, and FBAL
than in those with normal renal function [see Contraindications (4.2), Warnings and Precautions
(5.5), Dosage and Administration (2.3), and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
The manifestations of acute overdose would include nausea, vomiting, diarrhea, gastrointestinal
irritation and bleeding, and bone marrow depression. Medical management of overdose should
include customary supportive medical interventions aimed at correcting the presenting clinical
manifestations. Although no clinical experience using dialysis as a treatment for XELODA
overdose has been reported, dialysis may be of benefit in reducing circulating concentrations of
5’-DFUR, a low–molecular-weight metabolite of the parent compound.
Single doses of XELODA were not lethal to mice, rats, and monkeys at doses up to 2000 mg/kg
(2.4, 4.8, and 9.6 times the recommended human daily dose on a mg/m
2
basis).
11 DESCRIPTION
XELODA (capecitabine) is a fluoropyrimidine carbamate with antineoplastic activity. It is an
orally administered systemic prodrug of 5’-deoxy-5-fluorouridine (5’-DFUR) which is converted
to 5-fluorouracil.
The chemical name for capecitabine is 5’-deoxy-5-fluoro-N-[(pentyloxy) carbonyl]-cytidine and
has a molecular weight of 359.35. Capecitabine has the following structural formula:
23
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Capecitabine is a white to off-white crystalline powder with an aqueous solubility of 26 mg/mL
at 20ºC.
XELODA is supplied as biconvex, oblong film-coated tablets for oral administration. Each light
peach-colored tablet contains 150 mg capecitabine and each peach-colored tablet contains
500 mg capecitabine. The inactive ingredients in XELODA include: anhydrous lactose,
croscarmellose sodium, hydroxypropyl methylcellulose, microcrystalline cellulose, magnesium
stearate and purified water. The peach or light peach film coating contains hydroxypropyl
methylcellulose, talc, titanium dioxide, and synthetic yellow and red iron oxides.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Enzymes convert capecitabine to 5-fluorouracil (5-FU) in vivo. Both normal and tumor cells
metabolize 5-FU to 5-fluoro-2’-deoxyuridine monophosphate (FdUMP) and 5-fluorouridine
triphosphate (FUTP). These metabolites cause cell injury by two different mechanisms. First,
FdUMP and the folate cofactor, N
5-10
-methylenetetrahydrofolate, bind to thymidylate synthase
(TS) to form a covalently bound ternary complex. This binding inhibits the formation of
thymidylate from 2’-deoxyuridylate. Thymidylate is the necessary precursor of thymidine
triphosphate, which is essential for the synthesis of DNA, so that a deficiency of this compound
can inhibit cell division. Second, nuclear transcriptional enzymes can mistakenly incorporate
FUTP in place of uridine triphosphate (UTP) during the synthesis of RNA. This metabolic error
can interfere with RNA processing and protein synthesis.
12.3 Pharmacokinetics
Absorption
Following oral administration of 1255 mg/m
2
BID to cancer patients, capecitabine reached peak
blood levels in about 1.5 hours (T
max
) with peak 5-FU levels occurring slightly later, at 2 hours.
Food reduced both the rate and extent of absorption of capecitabine with mean C
ma x
and AUC
0-∞
decreased by 60% and 35%, respectively. The C
max
and AUC
0-∞
of 5-FU were also reduced by
food by 43% and 21%, respectively. Food delayed T
max
of both parent and 5-FU by 1.5 hours
[see Warnings and Precautions (5), Dosage and Administration (2), and Drug-Food Interaction
(7.2)].
The pharmacokinetics of XELODA and its metabolites have been evaluated in about 200 cancer
patients over a dosage range of 500 to 3500 mg/m
2
/day. Over this range, the pharmacokinetics of
XELODA and its metabolite, 5’-DFCR were dose proportional and did not change over time.
The increases in the AUCs of 5’-DFUR and 5-FU, however, were greater than proportional to
the increase in dose and the AUC of 5-FU was 34% higher on day 14 than on day 1. The
interpatient variability in the C
ma x
and AUC of 5-FU was greater than 85%.
Distribution
Plasma protein binding of capecitabine and its metabolites is less than 60% and is not
concentration-dependent. Capecitabine was primarily bound to human albumin (approximately
35%). XELODA has a low potential for pharmacokinetic interactions related to plasma protein
binding.
Bioactivation and Metabolism
24
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Capecitabine is extensively metabolized enzymatically to 5-FU. In the liver, a 60 kDa
carboxylesterase hydrolyzes much of the compound to 5’-deoxy-5-fluorocytidine (5’-DFCR).
Cytidine deaminase, an enzyme found in most tissues, including tumors, subsequently converts
5’-DFCR to 5’-DFUR. The enzyme, thymidine phosphorylase (dThdPase), then hydrolyzes 5’-
DFUR to the active drug 5-FU. Many tissues throughout the body express thymidine
phosphorylase. Some human carcinomas express this enzyme in higher concentrations than
surrounding normal tissues. Following oral administration of XELODA 7 days before surgery in
patients with colorectal cancer, the median ratio of 5-FU concentration in colorectal tumors to
adjacent tissues was 2.9 (range from 0.9 to 8.0). These ratios have not been evaluated in breast
cancer patients or compared to 5-FU infusion.
Metabolic Pathway of capecitabine to 5-FU
The enzyme dihydropyrimidine dehydrogenase hydrogenates 5-FU, the product of capecitabine
metabolism, to the much less toxic 5-fluoro-5, 6-dihydro-fluorouracil (FUH
2
).
Dihydropyrimidinase cleaves the pyrimidine ring to yield 5-fluoro-ureido-propionic acid (FUPA).
Finally, β-ureido-propionase cleaves FUPA to α-fluoro-β-alanine (FBAL) which is cleared in the
urine.
In vitro enzymatic studies with human liver microsomes indicated that capecitabine and its
metabolites (5’-DFUR, 5’-DFCR, 5-FU, and FBAL) did not inhibit the metabolism of test
substrates by cytochrome P450 isoenzymes 1A2, 2A6, 3A4, 2C19, 2D6, and 2E1.
Excretion
Capecitabine and its metabolites are predominantly excreted in urine; 95.5% of administered
capecitabine dose is recovered in urine. Fecal excretion is minimal (2.6%). The major metabolite
excreted in urine is FBAL which represents 57% of the administered dose. About 3% of the
administered dose is excreted in urine as unchanged drug. The elimination half-life of both
parent capecitabine and 5-FU was about 0.75 hour.
Effect of Age, Gender, and Race on the Pharmacokinetics of Capecitabine
25
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
A population analysis of pooled data from the two large controlled studies in patients with
metastatic colorectal cancer (n=505) who were administered XELODA at 1250 mg/m
2
twice a
day indicated that gender (202 females and 303 males) and race (455 white/Caucasian patients,
22 black patients, and 28 patients of other race) have no influence on the pharmacokinetics of 5’-
DFUR, 5-FU and FBAL. Age has no significant influence on the pharmacokinetics of 5’-DFUR
and 5-FU over the range of 27 to 86 years. A 20% increase in age results in a 15% increase in
AUC of FBAL [see Warnings and Precautions (5.11) and Dosage and Administration (2.3)].
Following oral administration of 825 mg/m
2
capecitabine twice daily for 14 days, Japanese
patients (n=18) had about 36% lower C
max
and 24% lower AUC for capecitabine than the
Caucasian patients (n=22). Japanese patients had also about 25% lower C
max
and 34% lower
AUC for FBAL than the Caucasian patients. The clinical significance of these differences is
unknown. No significant differences occurred in the exposure to other metabolites (5’-DFCR, 5’-
DFUR, and 5-FU).
Effect of Hepatic Insufficiency
XELODA has been evaluated in 13 patients with mild to moderate hepatic dysfunction due to
liver metastases defined by a composite score including bilirubin, AST/ALT and alkaline
phosphatase following a single 1255 mg/m
2
dose of XELODA. Both AUC
0-∞
and C
ma x
of
capecitabine increased by 60% in patients with hepatic dysfunction compared to patients with
normal hepatic function (n=14). The AUC
0-∞
and C
max
of 5-FU were not affected. In patients
with mild to moderate hepatic dysfunction due to liver metastases, caution should be exercised
when XELODA is administered. The effect of severe hepatic dysfunction on XELODA is not
known [see Warnings and Precautions (5.11) and Use in Special Populations (8.6)].
Effect of Renal Insufficiency
Following oral administration of 1250 mg/m
2
capecitabine twice a day to cancer patients with
varying degrees of renal impairment, patients with moderate (creatinine clearance = 30 to 50
mL/min) and severe (creatinine clearance <30 mL/min) renal impairment showed 85% and 258%
higher systemic exposure to FBAL on day 1 compared to normal renal function patients
(creatinine clearance >80 mL/min). Systemic exposure to 5’-DFUR was 42% and 71% greater in
moderately and severely renal impaired patients, respectively, than in normal patients. Systemic
exposure to capecitabine was about 25% greater in both moderately and severely renal impaired
patients [see Dosage and Administration (2.3), Contraindications (4.2), Warnings and
Precautions (5.5), and Use in Special Populations (8.7)].
Effect of Capecitabine on the Pharmacokinetics of Warfarin
In four patients with cancer, chronic administration of capecitabine (1250 mg/m
2
bid) with a
single 20 mg dose of warfarin increased the mean AUC of S-warfarin by 57% and decreased its
clearance by 37%. Baseline corrected AUC of INR in these 4 patients increased by 2.8-fold, and
the maximum observed mean INR value was increased by 91% [see Boxed Warning and Drug
Interactions (7.1)].
Effect of Antacids on the Pharmacokinetics of Capecitabine
When Maalox (20 mL), an aluminum hydroxide- and magnesium hydroxide-containing antacid,
was administered immediately after XELODA (1250 mg/m
2
, n=12 cancer patients), AUC and
C
ma x
increased by 16% and 35%, respectively, for capecitabine and by 18% and 22%,
26
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

respectively, for 5’-DFCR. No effect was observed on the other three major metabolites
(5’-DFUR, 5-FU, FBAL) of XELODA.
Effect of Capecitabine on the Pharmacokinetics of Docetaxel and Vice Versa
A Phase 1 study evaluated the effect of XELODA on the pharmacokinetics of docetaxel
(Taxotere
) and the effect of docetaxel on the pharmacokinetics of XELODA was conducted in
26 patients with solid tumors. XELODA was found to have no effect on the pharmacokinetics of
docetaxel (C
ma x
and AUC) and docetaxel has no effect on the pharmacokinetics of capecitabine
and the 5-FU precursor 5’-DFUR.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Adequate studies investigating the carcinogenic potential of XELODA have not been conducted.
Capecitabine was not mutagenic in vitro to bacteria (Ames test) or mammalian cells (Chinese
hamster V79/HPRT gene mutation assay). Capecitabine was clastogenic in vitro to human
peripheral blood lymphocytes but not clastogenic in vivo to mouse bone marrow (micronucleus
test). Fluorouracil causes mutations in bacteria and yeast. Fluorouracil also causes chromosomal
abnormalities in the mouse micronucleus test in vivo.
Impairment of Fertility
In studies of fertility and general reproductive performance in female mice, oral capecitabine
doses of 760 mg/kg/day (about 2300 mg/m
2
/day) disturbed estrus and consequently caused a
decrease in fertility. In mice that became pregnant, no fetuses survived this dose. The disturbance
in estrus was reversible. In males, this dose caused degenerative changes in the testes, including
decreases in the number of spermatocytes and spermatids. In separate pharmacokinetic studies,
this dose in mice produced 5’-DFUR AUC values about 0.7 times the corresponding values in
patients administered the recommended daily dose.
14 CLINICAL STUDIES
14.1 Adjuvant Colon Cancer
A multicenter randomized, controlled phase 3 clinical trial in patients with Dukes’ C colon
cancer (X-ACT) provided data concerning the use of XELODA for the adjuvant treatment of
patients with colon cancer. The primary objective of the study was to compare disease-free
survival (DFS) in patients receiving XELODA to those receiving IV 5-FU/LV alone. In this trial,
1987 patients were randomized either to treatment with XELODA 1250 mg/m
2
orally twice daily
for 2 weeks followed by a 1-week rest period, given as 3-week cycles for a total of 8 cycles (24
weeks) or IV bolus 5-FU 425 mg/m
2
and 20 mg/m
2
IV leucovorin on days 1 to 5, given as 4-
week cycles for a total of 6 cycles (24 weeks). Patients in the study were required to be between
18 and 75 years of age with histologically-confirmed Dukes’ stage C colon cancer with at least
one positive lymph node and to have undergone (within 8 weeks prior to randomization)
complete resection of the primary tumor without macroscopic or microscopic evidence of
remaining tumor. Patients were also required to have no prior cytotoxic chemotherapy or
immunotherapy (except steroids), and have an ECOG performance status of 0 or 1 (KPS ≥ 70%),
ANC ≥ 1.5x10
9
/L, platelets ≥ 100x10
9
/L, serum creatinine ≤ 1.5 ULN, total bilirubin ≤ 1.5 ULN,
AST/ALT ≤ 2.5 ULN and CEA within normal limits at time of randomization.
27
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

The baseline demographics for XELODA and 5-FU/LV patients are shown in Table 10. The
baseline characteristics were well-balanced between arms.
Table 10 Baseline Demographics
XELODA
(n=1004)
5-FU/LV
(n=983)
Age (median, years)
Range
62
(25-80)
63
(22-82)
Gender
Male (n, %)
Female (n, %)
542 (54)
461 (46)
532 (54)
451 (46)
ECOG PS
0 (n, %)
1 (n, %)
849 (85)
152 (15)
830 (85)
147 (15)
Staging – Primary Tumor
PT1 (n, %)
PT2 (n, %)
PT3 (n, %)
PT4 (n, %)
Other (n, %)
12 (1)
90 (9)
763 (76)
138 (14)
1 (0.1)
6 (0.6)
92 (9)
746 (76)
139 (14)
0 (0)
Staging – Lymph Node
pN1 (n, %)
pN2 (n, %)
Other (n, %)
695 (69)
305 (30)
4 (0.4)
694 (71)
288 (29)
1 (0.1)
All patients with normal renal function or mild renal impairment began treatment at the full
starting dose of 1250 mg/m
2
orally twice daily. The starting dose was reduced in patients with
moderate renal impairment (calculated creatinine clearance 30 to 50 mL/min) at baseline [see
Dosage and Administration (2.3)]. Subsequently, for all patients, doses were adjusted when
needed according to toxicity. Dose management for XELODA included dose reductions, cycle
delays and treatment interruptions (see Table 11).
Table 11 Summary of Dose Modifications in X-ACT Study
XELODA
N = 995
5-FU/LV
N = 974
Median relative dose intensity (%)
93
92
Patients completing full course of treatment (%)
83
87
Patients with treatment interruption (%)
15
5
Patients with cycle delay (%)
46
29
Patients with dose reduction (%)
42
44
Patients with treatment interruption, cycle delay,
or dose reduction (%)
57
52
The median follow-up at the time of the analysis was 83 months (6.9 years). The hazard ratio for
DFS for XELODA compared to 5-FU/LV was 0.88 (95% C.I. 0.77 – 1.01) (see Table 12 and
Figure 1). Because the upper 2-sided 95% confidence limit of hazard ratio was less than 1.20,
28
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

XELODA was non-inferior to 5-FU/LV. The choice of the non-inferiority margin of 1.20
corresponds to the retention of approximately 75% of the 5-FU/LV effect on DFS. The hazard
ratio for XELODA compared to 5-FU/LV with respect to overall survival was 0.86 (95% C.I.
0.74 – 1.01). The 5-year overall survival rates were 71.4% for XELODA and 68.4% for 5-
FU/LV (see Figure 2).
Table 12 Efficacy of XELODA vs 5-FU/LV in Adjuvant
Treatment of Colon Cancer
a
All Randomized Population
XELODA
(n=1004)
5-FU/LV
(n=983)
Median follow-up (months)
83
83
5-year Disease-free Survival Rates
(%)
b
59.1
54.6
Hazard Ratio
(XELODA/5-FU/LV)
(95% C.I. for Hazard Ratio)
p-value
c
0.88
(0.77 - 1.01)
p = 0.068
a
Approximately 93.4% had 5-year DFS information
b
Based on Kaplan-Meier estimates
c
Test of superiority of XELODA vs 5-FU/LV (Wald chi-square test)
Figure 1 Kaplan-Meier Estimates of Disease-Free Survival
(All Randomized Population)
a
a
XELODA has been demonstrated to be non-inferior to 5-FU/LV.
29
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Figure 2 Kaplan-Meier Estimates of Overall Survival
(All Randomized Population)
14.2 Metastatic Colorectal Cancer
General
The recommended dose of XELODA was determined in an open-label, randomized clinical
study, exploring the efficacy and safety of continuous therapy with capecitabine (1331
mg/m
2
/day in two divided doses, n=39), intermittent therapy with capecitabine (2510 mg/m
2
/day
in two divided doses, n=34), and intermittent therapy with capecitabine in combination with oral
leucovorin (LV) (capecitabine 1657 mg/m
2
/day in two divided doses, n=35; leucovorin 60
mg/day) in patients with advanced and/or metastatic colorectal carcinoma in the first-line
metastatic setting. There was no apparent advantage in response rate to adding leucovorin to
XELODA; however, toxicity was increased. XELODA, 1250 mg/m
2
twice daily for 14 days
followed by a 1-week rest, was selected for further clinical development based on the overall
safety and efficacy profile of the three schedules studied.
Monotherapy
Data from two open-label, multicenter, randomized, controlled clinical trials involving 1207
patients support the use of XELODA in the first-line treatment of patients with metastatic
colorectal carcinoma. The two clinical studies were identical in design and were conducted in
120 centers in different countries. Study 1 was conducted in the US, Canada, Mexico, and Brazil;
Study 2 was conducted in Europe, Israel, Australia, New Zealand, and Taiwan. Altogether, in
both trials, 603 patients were randomized to treatment with XELODA at a dose of 1250 mg/m
2
twice daily for 2 weeks followed by a 1-week rest period and given as 3-week cycles; 604
patients were randomized to treatment with 5-FU and leucovorin (20 mg/m
2
leucovorin IV
followed by 425 mg/m
2
IV bolus 5-FU, on days 1 to 5, every 28 days).
30
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

In both trials, overall survival, time to progression and response rate (complete plus partial
responses) were assessed. Responses were defined by the World Health Organization criteria and
submitted to a blinded independent review committee (IRC). Differences in assessments between
the investigator and IRC were reconciled by the sponsor, blinded to treatment arm, according to
a specified algorithm. Survival was assessed based on a non-inferiority analysis.
The baseline demographics for XELODA and 5-FU/LV patients are shown in Table 13.
Table 13 Baseline Demographics of Controlled Colorectal Trials
Study 1
Study 2
XELODA
(n=302)
5-FU/LV
(n=303)
XELODA
(n=301)
5-FU/LV
(n=301)
Age (median, years)
Range
64
(23-86)
63
(24-87)
64
(29-84)
64
(36-86)
Gender
Male (%)
Female (%)
181 (60)
121 (40)
197 (65)
106 (35)
172 (57)
129 (43)
173 (57)
128 (43)
Karnofsky PS (median)
Range
90
(70-100)
90
(70-100)
90
(70-100)
90
(70-100)
Colon (%)
Rectum (%)
222 (74)
79 (26)
232 (77)
70 (23)
199 (66)
101 (34)
196 (65)
105 (35)
Prior radiation therapy (%)
52 (17)
62 (21)
42 (14)
42 (14)
Prior adjuvant 5-FU (%)
84 (28)
110 (36)
56 (19)
41 (14)
The efficacy endpoints for the two phase 3 trials are shown in Table 14 and Table 15.
Table 14 Efficacy of XELODA vs 5-FU/LV in Colorectal Cancer
(Study 1)
XELODA
(n=302)
5-FU/LV
(n=303)
Overall Response Rate
(%, 95% C.I.)
21 (16-26)
11 (8-15)
(p-value)
0.0014
Time to Progression
(Median, days, 95% C.I.)
128 (120-136)
131 (105-153)
Hazard Ratio (XELODA/5-FU/LV)
95% C.I. for Hazard Ratio
0.99
(0.84-1.17)
Survival
(Median, days, 95% C.I.)
380 (321-434)
407 (366-446)
Hazard Ratio (XELODA/5-FU/LV)
95% C.I. for Hazard Ratio
1.00
(0.84-1.18)
31
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Table 15 Efficacy of XELODA vs 5-FU/LV in Colorectal Cancer
(Study 2)
XELODA
(n=301)
5-FU/LV
(n=301)
Overall Response Rate
(%, 95% C.I.)
21 (16-26)
14 (10-18)
(p-value)
0.027
Time to Progression
(Median, days, 95% C.I.)
137 (128-165)
131 (102-156)
Hazard Ratio (XELODA/5-FU/LV)
95% C.I. for Hazard Ratio
0.97
(0.82-1.14)
Survival
(Median, days, 95% C.I.)
404 (367-452)
369 (338-430)
Hazard Ratio (XELODA/5-FU/LV)
95% C.I. for Hazard Ratio
0.92
(0.78-1.09)
Figure 3 Kaplan-Meier Curve for Overall Survival of Pooled
Data (Studies 1 and 2)
XELODA was superior to 5-FU/LV for objective response rate in Study 1 and Study 2. The
similarity of XELODA and 5-FU/LV in these studies was assessed by examining the potential
difference between the two treatments. In order to assure that XELODA has a clinically
meaningful survival effect, statistical analyses were performed to determine the percent of the
survival effect of 5-FU/LV that was retained by XELODA. The estimate of the survival effect of
5-FU/LV was derived from a meta-analysis of ten randomized studies from the published
literature comparing 5-FU to regimens of 5-FU/LV that were similar to the control arms used in
these Studies 1 and 2. The method for comparing the treatments was to examine the worst case
(95% confidence upper bound) for the difference between 5-FU/LV and XELODA, and to show
that loss of more than 50% of the 5-FU/LV survival effect was ruled out. It was demonstrated
that the percent of the survival effect of 5-FU/LV maintained was at least 61% for Study 2 and
32
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

10% for Study 1. The pooled result is consistent with a retention of at least 50% of the effect of
5-FU/LV. It should be noted that these values for preserved effect are based on the upper bound
of the 5-FU/LV vs XELODA difference. These results do not exclude the possibility of true
equivalence of XELODA to 5-FU/LV (see Table 14, Table 15, and Figure 3).
14.3 Breast Cancer
XELODA has been evaluated in clinical trials in combination with docetaxel (Taxotere) and as
monotherapy.
In Combination With Docetaxel
The dose of XELODA used in the phase 3 clinical trial in combination with docetaxel was based
on the results of a phase 1 study, where a range of doses of docetaxel administered in 3-week
cycles in combination with an intermittent regimen of XELODA (14 days of treatment, followed
by a 7-day rest period) were evaluated. The combination dose regimen was selected based on the
tolerability profile of the 75 mg/m
2
administered in 3-week cycles of docetaxel in combination
with 1250 mg/m
2
twice daily for 14 days of XELODA administered in 3-week cycles. The
approved dose of 100 mg/m
2
of docetaxel administered in 3-week cycles was the control arm of
the phase 3 study.
XELODA in combination with docetaxel was assessed in an open-label, multicenter, randomized
trial in 75 centers in Europe, North America, South America, Asia, and Australia. A total of 511
patients with metastatic breast cancer resistant to, or recurring during or after an anthracycline-
containing therapy, or relapsing during or recurring within 2 years of completing an
anthracycline-containing adjuvant therapy were enrolled. Two hundred and fifty-five (255)
patients were randomized to receive XELODA 1250 mg/m
2
twice daily for 14 days followed by
1 week without treatment and docetaxel 75 mg/m
2
as a 1-hour intravenous infusion administered
in 3-week cycles. In the monotherapy arm, 256 patients received docetaxel 100 mg/m
2
as a 1-
hour intravenous infusion administered in 3-week cycles. Patient demographics are provided in
Table 16.
33
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Table 16 Baseline Demographics and Clinical Characteristics
XELODA and Docetaxel Combination vs Docetaxel in
Breast Cancer Trial
XELODA + Docetaxel
(n=255)
Docetaxel
(n=256)
Age (median, years)
52
51
Karnofsky PS (median)
90
90
Site of Disease
Lymph nodes
121 (47%)
125 (49%)
Liver
116 (45%)
122 (48%)
Bone
107 (42%)
119 (46%)
Lung
95 (37%)
99 (39%)
Skin
73 (29%)
73 (29%)
Prior Chemotherapy
Anthracycline
1
255 (100%) 256 (100%)
5-FU 196 (77%) 189 (74%)
Paclitaxel 25 (10%) 22 (9%)
Resistance to an Anthracycline
No resistance 19 (7%) 19 (7%)
Progression on anthracycline therapy
Stable disease after 4 cycles of anthracycline
65 (26%) 73 (29%)
therapy
Relapsed within 2 years of completion of
41 (16%) 40 (16%)
anthracycline-adjuvant therapy
Experienced a brief response to anthracycline
therapy, with subsequent progression while
78 (31%) 74 (29%)
on therapy or within 12 months after last dose 51 (20%) 50 (20%)
No. of Prior Chemotherapy Regimens for
Treatment of Metastatic Disease
0 89 (35%) 80 (31%)
1 123 (48%) 135 (53%)
2 43 (17%) 39 (15%)
3 0 (0%) 2 (1%)
1
Includes 10 patients in combination and 18 patients in monotherapy arms treated with an anthracenedione
XELODA in combination with docetaxel resulted in statistically significant improvement in time
to disease progression, overall survival and objective response rate compared to monotherapy
with docetaxel as shown in Table 17, Figure 4, and Figure 5.
34
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Table 17 Efficacy of XELODA and Docetaxel Combination vs
Docetaxel Monotherapy
Efficacy Parameter
Combination
Therapy
Monotherapy
p-value
Hazard
Ratio
Time to Disease
Progression
Median Days
95% C.I.
186
(165-198)
128
(105-136)
0.0001 0.643
Overall Survival
Median Days
95% C.I.
442
(375-497)
352
(298-387)
0.0126 0.775
Response Rate
1
32% 22% 0.009 NA
2
1
The response rate reported represents a reconciliation of the investigator and IRC assessments performed by the
sponsor according to a predefined algorithm.
2
NA = Not Applicable
Figure 4 Kaplan-Meier Estimates for Time to Disease
Progression XELODA and Docetaxel vs Docetaxel
35
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Figure 5 Kaplan-Meier Estimates of Survival XELODA
and Docetaxel vs Docetaxel
Monotherapy
The antitumor activity of XELODA as a monotherapy was evaluated in an open-label single-arm
trial conducted in 24 centers in the US and Canada. A total of 162 patients with stage IV breast
cancer were enrolled. The primary endpoint was tumor response rate in patients with measurable
disease, with response defined as a ≥50% decrease in sum of the products of the perpendicular
diameters of bidimensionally measurable disease for at least 1 month. XELODA was
administered at a dose of 1255 mg/m
2
twice daily for 2 weeks followed by a 1-week rest period
and given as 3-week cycles. The baseline demographics and clinical characteristics for all
patients (n=162) and those with measurable disease (n=135) are shown in Table 18. Resistance
was defined as progressive disease while on treatment, with or without an initial response, or
relapse within 6 months of completing treatment with an anthracycline-containing adjuvant
chemotherapy regimen.
36
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Table 18 Baseline Demographics and Clinical Characteristics
Single-Arm Breast Cancer Trial
Patients With
Measurable Disease
(n=135)
All Patients
(n=162)
Age (median, years)
55
56
Karnofsky PS
90
90
No. Disease Sites
1-2 43 (32%) 60 (37%)
3-4 63 (46%) 69 (43%)
>5
29 (22%)
34 (21%)
Dominant Site of Disease
Visceral
1
101 (75%) 110 (68%)
Soft Tissue 30 (22%) 35 (22%)
Bone
4 (3%)
17 (10%)
Prior Chemotherapy
Paclitaxel 135 (100%) 162 (100%)
Anthracycline
2
122 (90%) 147 (91%)
5-FU 110 (81%) 133 (82%)
Resistance to Paclitaxel 103 (76%) 124 (77%)
Resistance to an Anthracycline
2
Resistance to both Paclitaxel
55 (41%) 67 (41%)
and an Anthracycline
2
43 (32%)
51 (31%)
1
Lung, pleura, liver, peritoneum
2
Includes 2 patients treated with an anthracenedione
Antitumor responses for patients with disease resistant to both paclitaxel and an anthracycline
are shown in Table 19.
Table 19 Response Rates in Doubly-Resistant Patients Single-Arm
Breast Cancer Trial
Resistance to Both Paclitaxel and
an Anthracycline
(n=43)
CR
0
PR
1
11
CR + PR
1
11
Response Rate
1
(95% C.I.)
25.6%
(13.5, 41.2)
Duration of Response,
1
Median in days
2
(Range)
154
(63-233)
1
Includes 2 patients treated with an anthracenedione
2
From date of first response
37
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
For the subgroup of 43 patients who were doubly resistant, the median time to progression was
102 days and the median survival was 255 days. The objective response rate in this population
was supported by a response rate of 18.5% (1 CR, 24 PRs) in the overall population of 135
patients with measurable disease, who were less resistant to chemotherapy (see Table 18). The
median time to progression was 90 days and the median survival was 306 days.
15 REFERENCES
1. NIOSH Alert: Preventing occupational exposures to antineoplastic and other
hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human
Services, Public Health Service, Centers for Disease Control and Prevention,
National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication
No. 2004-165.
2. OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling
Occupational Exposure to Hazardous Drugs. OSHA, 1999.
http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
3. American Society of Health-System Pharmacists. ASHP Guidelines on Handling
Hazardous Drugs: Am J Health-Syst Pharm. 2006;63:1172-1193.
4. Polovich M., White JM, Kelleher LO (eds). Chemotherapy and biotherapy guidelines
and recommendations for practice (2nd ed.) 2005. Pittsburgh, PA: Oncology Nursing
Society.
16 HOW SUPPLIED/STORAGE AND HANDLING
150 mg
Color: Light peach
Engraving: XELODA on one side and 150 on the other
150 mg tablets are packaged in bottles of 60 (NDC 0004-1100-20).
500 mg
Color: Peach
Engraving: XELODA on one side and 500 on the other
500 mg tablets are packaged in bottles of 120 (NDC 0004-1101-50).
Storage and Handling
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled
Room Temperature]. KEEP TIGHTLY CLOSED.
Care should be exercised in the handling of XELODA. XELODA tablets should not be cut or
crushed. Procedures for the proper handling and disposal of anticancer drugs should be
considered. Any unused product should be disposed of in accordance with local requirements, or
drug take back programs. Several guidelines on the subject have been published.
1-4
17 PATIENT COUNSELING INFORMATION
Information for Patients (see FDA-approved Patient Labeling)
Patients and patients’ caregivers should be informed of the expected adverse effects of
XELODA, particularly nausea, vomiting, diarrhea, and hand-and-foot syndrome, and should
38
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

be made aware that patient-specific dose adaptations during therapy are expected and
necessary [see Dosage and Administration (2.2)]. As described below, patients taking
XELODA should be informed of the need to interrupt treatment and to call their physician
immediately if moderate or severe toxicity occurs. Patients should be encouraged to recognize
the common grade 2 toxicities associated with XELODA treatment See FDA-approved patient
labeling (Patient Information).
Dihydropyrimidine Dehydrogenase Deficiency
Patients should be advised to notify their healthcare provider if they have a known DPD
deficiency. Advise patients if they have complete or near complete absence of DPD activity
they are at an increased risk of acute early-onset of toxicity and severe, life-threatening, or
fatal adverse reactions caused by XELODA (e.g., mucositis, diarrhea, neutropenia, and
neurotoxicity) [see Warnings and Precautions (5.4)].
Diarrhea
Patients experiencing grade 2 diarrhea (an increase of 4 to 6 stools/day or nocturnal stools) or
greater or experiencing severe bloody diarrhea with severe abdominal pain and fever should be
instructed to stop taking XELODA and to call their physician immediately. Standard
antidiarrheal treatments (eg, loperamide) are recommended.
Dehydration
Patients experiencing grade 2 or higher dehydration should be instructed to stop taking
XELODA immediately and the dehydration corrected. Treatment should not be restarted until
the patient is rehydrated and any precipitating causes have been corrected or controlled.
Nausea
Patients experiencing grade 2 nausea (food intake significantly decreased but able to eat
intermittently) or greater should be instructed to stop taking XELODA immediately. Initiation of
symptomatic treatment is recommended.
Vomiting
Patients experiencing grade 2 vomiting (2 to 5 episodes in a 24-hour period) or greater should be
instructed to stop taking XELODA immediately. Initiation of symptomatic treatment is
recommended.
Hand-and-Foot Syndrome
Patients experiencing grade 2 hand-and-foot syndrome (painful erythema and swelling of the
hands and/or feet and/or discomfort affecting the patients’ activities of daily living) or greater
should be instructed to stop taking XELODA immediately. Initiation of symptomatic treatment is
recommended.
Stomatitis
Patients experiencing grade 2 stomatitis (painful erythema, edema or ulcers of the mouth or
tongue, but able to eat) or greater should be instructed to stop taking XELODA immediately and
to call their physician. Initiation of symptomatic treatment is recommended.
39
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Fever and Neutropenia
Patients who develop a fever of 100.5°F or greater or other evidence of potential infection should
be instructed to call their physician immediately.
XELODA
®
is a registered trademark of Hoffmann-La Roche, Inc.
© 2015 Genentech, Inc. All rights reserved.
40
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

Patient Information
XELODA
(zeh-LOE-duh)
(capecitabine) Tablets, Film Coated
What is the most important information I should know about XELODA?
XELODA can cause serious side effects, including:
• XELODA can interact with blood thinner medicines, such as warfarin
(COUMADIN
). Taking XELODA with these medicines can cause changes in how
fast your blood clots, and can cause bleeding that can lead to death. This can
happen as soon as a few days after you start taking XELODA, or later during
treatment, and possibly even within 1 month after you stop taking XELODA.
Your risk may be higher because you have cancer, and if you are over 60 years
of age.
• Before taking XELODA, tell your doctor if you are taking warfarin
(COUMADIN) or another blood thinner medicine.
• If you take warfarin (COUMADIN) or another blood thinner that is like
warfarin (COUMADIN) during treatment with XELODA, your doctor should do
blood tests often, to check how fast your blood clots during and after you
stop treatment with XELODA. Your doctor may change your dose of the blood
thinner medicine if needed.
See “What are the possible side effects of XELODA?” for more information
about side effects.
What is XELODA?
XELODA is a prescription medicine used to treat people with:
• cancer of the colon that has spread to lymph nodes in the area close to the
colon (Dukes’ C stage), after they have surgery.
• cancer of the colon or rectum (colorectal) that has spread to other parts of the
body (metastatic).
• breast cancer that has spread to other parts of the body (metastatic) together
with another medicine called docetaxel after treatment with certain other anti-
cancer medicines have not worked.
• breast cancer that has spread to other parts of the body and has not improved
after treatment with paclitaxel and certain other anti-cancer medicines, or who
cannot receive any more treatment with certain anti-cancer medicines.
It is not known if XELODA is safe and effective in children.
41
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Who should not take XELODA?
Do not take XELODA if you:
• have severe kidney problems.
• are allergic to capecitabine, 5-fluorouracil, or any of the ingredients in XELODA.
See the end of this leaflet for a complete list of ingredients in XELODA.
Talk to your doctor before taking XELODA if you are not sure if you have any of the
conditions listed above.
What should I tell my doctor before taking XELODA?
See “What is the most important information I should know about
XELODA?”.
Before you take XELODA, tell your doctor if you:
• have had heart problems.
• have kidney or liver problems.
• have been told that you lack the enzyme DPD (dihydropyrimidine
dehydrogenase)
• have any other medical conditions.
• are pregnant or plan to become pregnant. XELODA can harm your unborn baby.
You should not become pregnant during treatment with XELODA. Talk to your
doctor about birth control choices that may be right for you during treatment
with XELODA.
• are breastfeeding or plan to breastfeed. It is not known if XELODA passes into
your breast milk. You and your doctor should decide if you will take XELODA or
breastfeed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and over-
the-counter medicines, vitamins, and herbal supplements. XELODA may affect the
way other medicines work, and other medicines may affect the way XELODA works.
Know the medicines you take. Keep a list of them to show your doctor and
pharmacist when you get a new medicine.
How should I take XELODA?
• Take XELODA exactly as your doctor tells you to take it.
• Your doctor will tell you how much XELODA to take and when to take it.
• Take XELODA 2 times a day, 1 time in the morning and 1 time in the evening.
• Take XELODA within 30 minutes after finishing a meal. Swallow XELODA tablets
whole with water. Do not crush or cut XELODA tablets.
42
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
• Ask your healthcare provider or pharmacist how to safely throw away any
unused XELODA.
• If you have side effects with XELODA, if needed your doctor may decide to:
• change your dose of XELODA
• treat you with XELODA less often
• tell you to stop taking XELODA until certain side effects get better or go away
• stop your treatment with XELODA if you have certain side effects and they
are severe
• If you take too much XELODA, call your doctor or go to the nearest emergency
room right away.
What are the possible side effects of XELODA?
XELODA may cause serious side effects including:
See “What is the most important information I should know about
XELODA?”.
• diarrhea. Diarrhea is common with XELODA and can sometimes be severe.
Stop taking XELODA and call your doctor right away if the number of bowel
movements you have in a day increases by 4 or more than is usual for you. Ask
your doctor about what medicines you can take to treat your diarrhea. If you
have severe bloody diarrhea with severe abdominal pain and fever, call your
doctor or go to the nearest emergency room right away.
• heart problems. XELODA can cause heart problems including: heart attack
and decreased blood flow to the heart, chest pain, irregular heartbeats, changes
in the electrical activity of your heart seen on an electrocardiogram (ECG),
problems with your heart muscle, heart failure, and sudden death. Stop taking
XELODA and call your doctor right away if you get any of the following
symptoms:
o chest pain
o shortness of breath
o feeling faint
o irregular heartbeats or skipping beats
o sudden weight gain
o swollen ankles or legs
• unexplained tiredness
• loss of too much body fluid (dehydration) and kidney failure.
Dehydration can happen with XELODA and may cause sudden kidney failure that
43
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
can lead to death. You are at higher risk if you have kidney problems before
taking XELODA and also take other medicines that can cause kidney problems.
Nausea, and vomiting are common with XELODA. If you lose your appetite, feel
weak, and have nausea, vomiting, or diarrhea, you can quickly become
dehydrated.
Stop taking XELODA and call your doctor right away if you:
• vomit 2 or more times in a day.
• are only able to eat or drink a little now and then, or not at all due to nausea.
• have diarrhea. See “diarrhea” above.
• serious skin and mouth reactions.
• XELODA can cause serious skin reactions that may lead to death. Tell your
doctor right away if you develop a skin rash, blisters and peeling of your skin.
Your doctor may tell you to stop taking XELODA if you have a serious skin
reaction. Do not take XELODA again if this happens.
• XELODA can also cause “hand and foot syndrome.” Hand and foot syndrome
is common with XELODA and can cause you to have numbness and changes
in sensation in your hands and feet, or cause redness, pain, swelling of your
hands and feet. Stop taking XELODA and call your doctor right away if you
have any of these symptoms and you are not able to do your usual activities.
• you may get sores in your mouth or on your tongue when taking XELODA.
Stop taking XELODA and call your doctor if you get painful redness, swelling,
or ulcers in your mouth and tongue, or if you are having problems eating.
Tell your doctor if you have any side effect that bothers you or that does not
go away.
• increased level of bilirubin in your blood and liver problems. Increased
bilirubin in your blood is common with XELODA. Your doctor will check you for
these problems during treatment with XELODA.
• decreased white blood cells, platelets, and red blood cell counts. Your
doctor will do blood tests during treatment with XELODA to check your blood cell
counts.
If your white blood cell count is very low, you are at increased risk for infection.
Call your doctor right away if you develop a fever of 100.5
o
F or greater or have
other signs and symptoms of infection.
People 80 years of age or older may be more likely to develop severe or serious
side effects with XELODA.
The most common side effects of XELODA include:
• diarrhea
44
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda

• hand and foot syndrome
• nausea
• vomiting
• stomach-area (abdominal) pain
• tiredness
• weakness
• increased amounts of red blood cell breakdown products (bilirubin) in your blood
These are not all the possible side effects of XELODA. For more information, ask
your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects
to FDA at 1-800-FDA-1088.
How should I store XELODA?
• Store XELODA at room temperature between 68°F to 77°F (20°C to 25°C).
• Keep XELODA in a tightly closed container.
• Keep XELODA and all medicines out of the reach of children.
General information about the safe and effective use of XELODA.
Medicines are sometimes prescribed for conditions that are not mentioned in
patient information leaflets. Do not use XELODA for a condition for which it was not
prescribed. Do not give XELODA to other people, even if they have the same
symptoms you have. It may harm them.
You can ask your pharmacist or doctor for information about XELODA that is written
for health professionals.
For more information, go to http://www.gene.com/patients/medicines/xeloda or
call 1-877-436-3683.
What are the ingredients in XELODA?
Active ingredient: capecitabine
Inactive ingredients: anhydrous lactose, croscarmellose sodium, hydroxypropyl
methylcellulose, microcrystalline cellulose, magnesium stearate and purified water.
The peach or light peach film coating contains hydroxypropyl methylcellulose, talc,
titanium dioxide, and synthetic yellow and red iron oxides.
This Patient Information has been approved by the U.S. Food and Drug
Administration.
45
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
Revised: XXX 2015
XELODA
®
is a registered trademark of Hoffmann-La Roche, Inc.
© 2015 Genentech, Inc. All rights reserved.
46
Reference ID: 3718670
This label may not be the latest approved by FDA.
For current labeling information, please visit https://www.fda.gov/drugsatfda
