
Marketing and
Regulatory
Programs
Agricultural
Marketing
Service
Fruit and
Vegetable
Program
Specialty
Crops
Inspection
Division
General Procedures
Manual
July 2013
i
General Procedures Manual
Table of Contents
Introduction .................................................................................................................................. 1
Guide for Electronic Usage .......................................................................................................... 1
Appeal Inspections ....................................................................................................................... 1
Formal Appeal Inspections .................................................................................................. 1
Bulk Containers ........................................................................................................................... 6
Frozen Products Packed in Used Containers ............................................................................... 6
Tote Bins ...................................................................................................................................... 6
Correspondence............................................................................................................................ 8
Correspondence to the National Office................................................................................ 8
Fee Computation ........................................................................................................................ 12
Current Fee Documents ..................................................................................................... 12
Fees for Lot Inspection grading service ............................................................................. 12
Table I Product Grouping .......................................................................................................... 13
Table II Fees for Product Grouping ........................................................................................... 14
Table III Fees for Dried Fruits and Processed Raisins (Excluding Figs & Dates) .................... 14
Table IV Fees for Coffee, Tea, and Sugar Products .................................................................. 15
Table V Fees for Samples Submitted by the Applicant (Unofficial Samples) .......................... 16
Table VI Fees for Update Samples ............................................................................................ 16
Table VII .................................................................................................................................... 17
Fees for Special Agreements for Lot Inspection ........................................................................ 17
Fees for Continuous Inspection and Pack Certification contracts ..................................... 17
Fees for chemical and microbiological analyses ............................................................... 18
Fees for Dried Dates .......................................................................................................... 20
Fees for letter contracts for unofficial samples .................................................................. 20
Certification Fees ....................................................................................................................... 21
Certify to a U.S. Grade ..................................................................................................... 21
Export Certificates restricted to product condition ........................................................... 21
Export Verification Service .............................................................................................. 23
Dairy Verification Service ................................................................................................ 23
Microanalysis .................................................................................................................... 24
Futures........................................................................................................................................ 25
Review Program for Concentrated Orange Juice for Manufacturing (OM) and Pasteurized
Orange Juice (POJ) for Futures Contracts ................................................................................. 26
ii
Futures Review Sample ............................................................................................................. 28
Review Program for Apple Juice Concentrate (AJC) Futures ................................................... 32
General Inspection ..................................................................................................................... 39
Initial Grading Procedures ......................................................................................................... 39
Determining Net Contents ......................................................................................................... 40
Definitions......................................................................................................................... 40
Scales ................................................................................................................................ 40
Rules for Reading Scales .................................................................................................. 40
Taking Net Weights .......................................................................................................... 40
Measuring Net Contents .................................................................................................. 41
Determining Tare Weight .......................................................................................................... 41
Determining Drained Weight ..................................................................................................... 43
Grade or Compliance Determination ......................................................................................... 43
Deviants/Worse than Deviants ................................................................................................... 46
Multiple Deviants on a Single Sample Unit ..................................................................... 46
Administrative Allowances for “Worse than Deviants” ................................................... 46
Scoring Individual Quality Factors ............................................................................................ 49
Alien Vegetables ........................................................................................................................ 49
Inspection Procedures for Fruit Concentrates, Nectars, and Purees .......................................... 50
Products Packed with Sauce, Garnish, or Seasonings ............................................................... 53
Emerson Good Samaritan Food Donation Act .......................................................................... 55
Disposition of Samples .............................................................................................................. 55
Donated/Returned Product Disposition Log .............................................................................. 56
In-Plant Inspection ..................................................................................................................... 57
Types of In-Plant inspection ...................................................................................................... 57
Continuous Inspection ...................................................................................................... 57
Plant-Assisted Continuous Inspection (PAC) ................................................................... 57
Pack Certification.............................................................................................................. 61
The Role of In-Plant inspection ................................................................................................. 62
Services provided by the Applicant under In-Plant inspection .................................................. 62
Basic Facility Requirements ...................................................................................................... 64
The SCI Inspector ...................................................................................................................... 69
Service versus Regulatory Authority ................................................................................ 69
Competence....................................................................................................................... 69
Decisions ........................................................................................................................... 69
iii
Uniformity......................................................................................................................... 69
Demeanor of Inspectors .................................................................................................... 70
Inspector’s Guidelines for Acceptable Behavior ............................................................... 71
Management of Inspection Costs ....................................................................................... 75
Constructive Suggestions and Advice ............................................................................... 76
Prompt Reporting ............................................................................................................... 76
Authority of SCI Inspection Staff .............................................................................................. 77
Treatment of SCI Inspection Staff ............................................................................................. 78
Automatic Samplers ................................................................................................................... 80
In-Plant Line Check Guidelines for Inspectors .......................................................................... 81
Line checks for grade determination of finished product ................................................. 81
Line checks of unprocessed product ................................................................................. 82
Incomplete Grade .............................................................................................................. 83
Inspection of Sort-Out Fruit ....................................................................................................... 84
Reporting Results ....................................................................................................................... 84
Reporting Inspection Results ............................................................................................ 84
Reporting Special Cases ................................................................................................... 85
Other In-Plant Inspection Duties ............................................................................................... 85
Sanitation and Safety ........................................................................................................ 85
Inspection of Products Processed Elsewhere .................................................................... 86
In-Plant tanker inspection and sealing .............................................................................. 86
Syrup Designations ........................................................................................................... 90
In-Plant Inspectors Filing System .............................................................................................. 94
Yearly (Y) File ........................................................................................................................... 95
Permanent (P) File ..................................................................................................................... 96
In-Plant Label Review and Approved Identification ................................................................. 97
Label Control - Official Marks ......................................................................................... 98
Label Control Sheet ................................................................................................................. 100
Labels for Products Not Under Contract ........................................................................ 101
Labels for Products Not Covered by U.S. Grade Standards ........................................... 101
Maintain a Permanent File .............................................................................................. 101
Division Action on the Misuse of Approved Identification............................................ 102
Misbranding Report ................................................................................................................. 103
Forms and Reports ................................................................................................................... 106
Signing and Initialing Completed Forms ........................................................................ 106
iv
Daily Inspection Report (DIR) (Form FV-416) .............................................................. 107
Daily Pack Report ........................................................................................................... 113
Request for Certificate .................................................................................................... 115
Certificate Request Worksheet ................................................................................................ 116
Hold and Release Forms ................................................................................................. 117
Annual Report ................................................................................................................. 120
Labeling ................................................................................................................................... 131
Definition of Labels ........................................................................................................ 131
Classification of Labels................................................................................................... 132
Approved Identification .................................................................................................. 133
Official Grade and Inspection Marks .............................................................................. 133
Marks Not Considered Official ....................................................................................... 133
Questionable Marks ........................................................................................................ 134
Division Policy on Use of Approved Identification ....................................................... 134
Misrepresentation by Unfair Labeling Practices ............................................................. 137
Metrication ............................................................................................................................... 144
Numerical Values............................................................................................................ 144
Rounding ......................................................................................................................... 145
Systems ........................................................................................................................... 146
Domestic and metric measures ....................................................................................... 146
Non-Manipulation Certification Inspection Service ................................................................ 148
Applicant responsibilities: .............................................................................................. 148
SCI field office responsibilities: ..................................................................................... 150
Inspection procedures ..................................................................................................... 152
Product Origin Requirements .................................................................................................. 158
Regulations .............................................................................................................................. 159
Re-Inspection ........................................................................................................................... 160
Classification of Inspection............................................................................................. 160
Review Programs ..................................................................................................................... 161
Review Program for Processed Raisins .......................................................................... 161
Review Programs for Flavor Evaluation of Canned Ripe Olives ................................... 162
Product Grade Review Procedures for Products Not Covered by Formal Review Programs
(Monthly and Four Month Cycle Review Program) ........................................................ 165
Score Sheet/Tally Sheet Completion ....................................................................................... 176
Completing standardized score sheets and tally sheets ................................................... 176
Completing the universal score sheet, form FV-364 ...................................................... 186
v
No Applicable Grade Products ....................................................................................... 187
Quality Level Descriptions and Abbreviations ........................................................................ 189
Updating Previously Inspected Product .......................................................................... 189
Guidelines for the Development of Plant Score Sheets .................................................. 190
Optional Check List for Completion of Score Sheets and Tally Sheets .................................. 191
Attachments ....................................................................................................................... 192

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 1 of 192
INTRODUCTION
This manual is designed for Specialty Crops Inspection (SCI) Division (SCI) employees of the
U.S. Department of Agriculture (USDA). Its purpose is to provide background information and
guidelines to assist in the uniform inspection of processed fruit and vegetable commodities and
in the performance of general inspection duties. The procedures contained in this manual are an
integral part of Division services. If needed, contact your immediate supervisor for any situation
not addressed in this manual.
This manual contains links to various internal and external sources of information. For
inspection personnel without internet or intranet access, please contact your immediate
supervisor to obtain hard copies of documents as needed.
GUIDE FOR ELECTRONIC USAGE
The Administrative, Inspection, and Management (AIM) System of instructional manuals is
available electronically in Adobe Acrobat Portable Document Format (PDF) at the following
intranet address: http://agnis/sites/FV/PPB/AIM/default.aspx
.
When accessed electronically, AIM materials have hyperlinks and hypertext (visible as
underlined blue text) available to the PDF user. Clicking on a hyperlink takes the reader to a
web site with information relating to the subject. Hypertext will link the reader to a different
page within the current manual - or even a different manual - with information relating to the
subject. For example, the hypertext in the Table of Contents allows a reader to go directly to the
section of interest in the manual by clicking on the section title within the Table of Contents.
PDF offers a variety of tools depending on the Adobe version the reader has. The newer the
version, the more tools available. To learn about the variety of PDF search options available:
• Click on the “Help” tab on the top of this page,
• Then click on the “Adobe Acrobat Help” bar,
• Type the word “Search” in the “Search” box, and click on the “Search” button,
• A series of options will become available,
• Click on the “Access Search Features” link and follow the instructions for the type of
search you are interested in.
This document format allows a PDF user to easily search for content within a document, or within
multiple documents.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 1 of 192
APPEAL INSPECTIONS
Processed products are re-inspected in order to issue either an up-to-date certificate or an appeal
certificate. Frequently the applicant doesn’t question the quality and condition of the lot, but
needs a current certificate in order to satisfy a buyer-seller contract. Occasionally the quality of
previously inspected merchandise is questioned by a financially interested party, and they
request an appeal inspection.
Inspection certificates are accepted as prima facie evidence of the facts contained within.
Although it is Division policy to assign only one grade representing a specific lot as a whole,
there have been cases involving the Perishable Agricultural Commodities Act (PACA) in which
both the plaintiff and defendant produced USDA certificates, each with a different grade. It is
very difficult to settle such claims unless the grade on one certificate can be reversed.
There should be no conflicting certificates on the lot unless the quality of the lot has changed due
to age, storage, handling, or other condition factors. If such a change has taken place, the
original certificate is valid for the date of original inspection, and the later certificate is also valid
for the date of the later inspection. These instructions will address such situations.
A. Formal Appeal Inspections
If possible, formal appeal inspections should be discouraged. An appeal may
often be avoided by having the inspector or supervisor review a few samples of
the product with the applicant. He or she can explain why the sample units were
assigned a certain grade, or why certain factors were evaluated as they were.
If the applicant requests a formal appeal, the inspection should be handled as
outlined in the Regulations for appeal inspections. These instructions supplement
the Regulations.
1. Filing for Appeal
An appeal inspection may be requested by any financially interested party
who is not satisfied with the results of the inspection as represented by a
certificate on the lot in question. They may file for an appeal, provided:
a. the lot of processed products can be identified as the same lot
previously inspected;
b. the request is within 30 days of the previous inspection (this period
may be extended by the Regional or National offices); and
c. the complaint concerns a factor(s) which would not be a result of a
change in a condition factor(s) since the previous inspection. See
Section 2a below.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 2 of 192
The Division will issue an appeal certificate when it has been established that the
previous inspection is in error. The original certificate is reversed and copies of
the appeal certificate are sent to all financially interested parties.
2. Denying Request for Appeal
Requests for an appeal inspection may be denied under the following
circumstances:
a. The complaint concerns a factor which may have undergone a
change of condition since the original inspection, such as:
(1) Oxidation of frozen fruits,
(2) Severe de-tinning of apple sauce or other canned product
(3) Hydrogen swells or other types of spoilage,
(4) Frozen broccoli or asparagus spears that are shattered due
to possible mishandling,
(5) Flavor deterioration of canned citrus juice due to long
storage at high temperatures, and
(6) Severe dehydration of frozen vegetables.
b. The lot cannot be identified.
c. The first inspection certification was based on restricted sampling.
For an example of a certificate showing restricted sampling, see
the AIM Inspection Series, Certification Manual.
d. Code marks do not agree with the original inspection.
e. Count does not agree with the original certificate. There are
certain exceptions to this provision. If the original certificate
covered a large lot, and the re-inspection covers a substantial
quantity of merchandise, an appeal on a portion of the original
certificate may be granted, provided that there is no question about
identity. However, an appeal would not be granted on a very small
segment of the original quantity; for example if the quantity would
be substantially less than the quantity represented by a single
sample unit on the original inspection.
f. Lot is not accessible for proper sampling.
g. If there has been a revision to the grade standards between the two

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 3 of 192
inspections, and such revision would affect the grade assigned to
the product.
h. The original certificate is Grade Not Certified (GNC) account
unsanitary conditions of the plant, or use of unsound or
unwholesome raw material.
For example, a frog or mouse may be found in a container during
the original inspection. Plant conditions may warrant a GNC
certificate even though this incident is strictly accidental. It would
be most unlikely that a re-inspection of the lot would find another
frog or mouse. However the Division would not deem it advisable
to completely ignore previous production or inspection history and
reverse the original inspection certificate.
i. Reasons for appeal are inadequate or frivolous, for example - "I
just don't trust the inspectors in the (location) office."
j. When it is not in the interest of USDA to perform the appeal. This
is a broad category and the National office would need to evaluate
all facts in order to render an appropriate decision.
Even if denied an appeal inspection due to any of these reasons, the
applicant may request a re-inspection or an up-to-date inspection on the lot
in question. The result of this new inspection would establish the grade of
the lot except that no grade would be assigned if USDA records show that
the original certificate was GNC account unsanitary processing conditions,
or use of unsound or unwholesome raw material (see 2 h. above).
3. Performing the Appeal
a. Information Required
The application may be made orally or in writing. If made orally,
it must be confirmed in writing. It must include:
(1) The location of the lot,
(2) The reason(s) for the appeal,
(3) The date and serial number of the certificate covering the
original inspection of the product, and
(4) The name of issuing office.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 4 of 192
b. Sampling
The sampling rate on an appeal inspection should be at least the
next sample size larger than the normal sampling rate for the lot
size, based on the single sampling plan. In other words, if the
sample size in the Regulations would normally be 13 for the size of
lot involved, at least 21 sample units would be drawn on the same
lot for an appeal inspection.
If the applicant merely requests an up-to-date inspection and it is
not known until the sample is examined that an appeal is in order
(as determined by the Division), the certificate should not be
issued until additional sample units are drawn.
Since stratification can occur in large containers, at least a portion
of samples of bulk container lots (such as 30 pound frozen) should
be full containers. For example, frozen strawberries may contain
rocks that would tend to settle to the bottom of the can during
handling prior to freezing. A subsample from the top or center of
the can is not likely to reveal the presence of this type of foreign
material.
c. Who may perform the Appeal inspection?
Any inspector other than the inspector who performed the original
inspection may perform an appeal inspection. Whenever practical,
two inspectors who are thoroughly familiar with the product
should be assigned to conduct the appeal.
If one inspector starts the inspection, and the need to issue an
appeal inspection certificate doesn’t become evident until the
inspection is partially complete, a second inspector should be
called in to confirm the results.
d. Notify the Regional and National offices
If a formal appeal is filed, or if during a routine re-inspection
procedure it appears that an appeal is in order, notify the Regional
and National offices. They may require a few samples to verify
the new inspection results.
e. Examination of Product
Because of the economic significance of the appeal, all samples of
the product should be very carefully graded and checked for all
factors. If objective tests are available, they should be used.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 5 of 192
f. GNC
Ordinarily an appeal would not be granted because of unsanitary
plant or product conditions where the original inspection findings
make an appeal unjustified. See 2 h. on page 3.
4. Fees
a. Appeal fees are as follows:
(1) Grade Sustained - charge regular fee plus expenses.
(2) Grade Reversed - no charge for inspection, but charge
applicant for expenses incurred in sampling or shipping
samples.
Note: An appeal certificate reversing the original inspection can only be
issued after receiving authority from the National office. If the
inspection results indicate that this is the case, submit details to the
Regional office. The Regional office will confer with the National
office before results are reported or a certificate is issued.
5. Certification
See the AIM Inspection Series, Certification Manual for specific
information related to the certification of appeals and re-inspections.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 6 of 192
BULK CONTAINERS
Frozen Products Packed in Used Containers
Used 30-pound metal and/or plastic containers with friction-type lids are refilled by some
processors of frozen foods. Reused containers must be clean at time of refilling. Obtain a letter
of conformance from the processor stating that the containers were not previously used for non-
food products. Products filled into unsanitary containers are to be classed as Grade Not
Certified (GNC).
It may be difficult to accurately describe reused containers. Some of these may have been used
several times and appear to be old, abused, or otherwise damaged. They may bear an assortment
of code marks and label statements. Certification statements regarding used containers may be
found in the AIM Inspection Series, Certification Manual.
Note: Reused containers are not acceptable for government purchases (state, Federal, and
Department of Defense) unless specified in the contract or solicitation. Do not certify
without a written waiver unless specified in the contract.
Tote Bins
Many packers use large bulk containers called "tote-bins" for frozen vegetables. These bins are
used during the packing season to store frozen vegetables for repack at a later date. The bins
vary in size and construction but can hold as much as a ton of product.
For financial reasons, users of tote-bins need to know whether or not the product is free flowing
or frozen in a solid mass. The type of pack should always be shown in the Body of the
Inspection Certificate.
A. Free flowing describes a pack in which the individual units of the pack are not
frozen together in a solid mass, but may be easily separated into individual units.
There may be some crusting on the interior surface of the tote-bin or a few clumps
of units frozen together within the container, but these can be separated with
moderate pressure.
B. Solid mass describes a pack in which the individual units of the product in the
container are predominantly or entirely frozen together in a solid mass. Such
product will require additional cost in repacking the product due a special "break-
up" step in the process.
For the purpose of inspection and certification, a lot can be a group of tote-bins or a single tote-
bin. Tote-bins must be properly identified for future re-inspection, segregation, etc. Codes
should be at least ½ inch in height, and stamped on the tote bins or on tags placed in upper right
or left-hand corner of the side normally facing the fork lift driver. The area for applying the
codes may be painted to facilitate application and legibility.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 7 of 192
The coding system may include the following:
• Plant (when more than 1 plant is operated by the same company)
• Quality designation
• Date of Pack (day, month, and year)
• Product, type style, size, etc. (when not readily apparent, such as covered bins or
numerous product styles)
• Period, shift, or lot number
• Bin numbers
• Special notation of abnormal quality variations (i.e., Grade B for color only, etc.)
• Different colored tape, tags, or marking ink for different quality levels, or other
special notations.
To determine the sampling rate for an item packed in tote-bins or bulk storage, the poundage is
converted to institutional size (2 ½ pound) containers. Using the number of 2 ½ pound
containers the lot would represent, refer to the Regulations or the AIM Inspection Series,
Sampling Manual.
When sampling tote bins, use a sample scoop and dig into the product as far as possible in at
least 3 locations. The sample drawn from each of the three locations may be considered as a
sample unit for inspection and certification if each tote-bin is a separate lot. It may be
composited as one sample unit if the lot consists of a group of tote-bins. Sampling may be more
representative with the use of a trier, or thief, which will penetrate deeply into each bin. This
implement should be constructed to permit sampling of the product from all levels in the bin. If
the product is a solid mass, or if representative portions of the bin cannot be sampled, the
certificate should be flagged to indicate the extent of restricted sampling. For restricted
certification of tote-bins, see the AIM Inspection Series, Certification Manual.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 8 of 192
CORRESPONDENCE
The cost for mailing personal items, including personal letters, notices of group social functions,
holiday greetings, or job applications, must be paid for by the employee regardless of any
association of the contents to official business.
With the use of personal computers and the electronic media, all letterheads and memos
produced locally should be reviewed by a supervisor before use. Correspondence directed to
parties outside the Department must be cleared with the Officer-in-Charge (OIC) before mailing.
Approved USDA/Agricultural Marketing Service (AMS) letterhead for communications of any
official nature should always be used, whether within the Department or to outside parties.
Approved AMS letterhead may be found at the AMS Correspondence and Writing Guidelines
site at the following intranet address under “Templates”:
http://agnis/sites/compliance/PAD/PerAcc/AMSCorrGuid/default.aspx
Inspectors with an AMS network ID and AMS electronic mail (e-mail) address can use this as an
effective means of communication. In-plant inspectors may access this e-mail through the
plant’s network, as long as the plant agrees and the AMS activity meets AMS security
requirements. It is not appropriate to use plant stationery for official correspondence, even if
used internally. An exception would be to use e-mail to stop shipment initially and place non-
conforming product on hold. However, the proper official forms must be prepared and executed
according to instructions when finalizing.
A. Correspondence to the National Office
1. Identification of the sender
Return addresses on envelopes consist of the inspector’s name, title, and
field office address.
2. Identification of the receiver - Incoming mail
To facilitate the sorting of incoming mail, mark all items with the last
name of the recipient if the envelope contains items for more than one
person.
When the entire contents of the envelope is destined to go to a specific
recipient, mark the outside of the envelope to identify the delivery point
(i.e., Inspection Branch, Division Director, etc).
a. Envelopes and packages
Envelopes and packages that are sent through the U.S. Postal
Service (including MRE, Tray Pack, and USDA review samples)
shall be addressed as shown on the following page:
USDA AMS FV Specialty Crops Inspection (SCI) Division

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 9 of 192
STOP 0247
1400 Independence Ave. SW
Washington, DC 20250-0247
Refer to the applicable sections in the AIM Inspection Series,
USDA Purchases Manual, or the Contract Service Section (CSS)
SharePoint site located at the following intranet address:
http://agnis/sites/FV/PPB/DCIS/default.aspx for instructions on
marking shipping cases for review samples under CSS and USDA
inspection programs.
b. Confidential correspondence
Envelopes containing correspondence of a confidential nature shall
be opened only by the addressee, and be identified as follows:
FOR THE PERSONAL ATTENTION OF
or
TO BE OPENED BY ADDRESSEE ONLY
These statements are to be used only when mailing information
that calls for confidentiality, such as employee data subject to the
privacy act, or material of a sensitive nature.
3. Courtesy copies
The original and all copies of Division correspondence sent through the
U.S. postal system shall note where courtesy copies were sent. When
transmitting an electronic message, a courtesy copy shall be sent to the
appropriate mailboxes. Employees shall use discretion and recognize that
sensitive materials should not be transmitted electronically.
Distribution of courtesy copies will be based on the originating location.
Use the following guide:
a. Regional offices
A copy of all typed and/or electronic correspondence that concerns
policy or procedure, or other subjects that may have an effect on
Division programs will be forwarded to the Division Director.
b. Field offices
(1) A copy to the Regional office in the sending and receiving
area, if applicable.
(2) When appropriate, a copy to the Division Director and

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 10 of 192
Chief of the applicable Branch.
"When appropriate" refers to items of a sensitive nature,
such as correspondence related to personnel issues, or
briefs that pertain to industry representatives, trade
associations, the news media, or other governmental
agencies. Material of this nature shall not be released
unless it has been cleared by the Regional Section Head.
c. Inspection points and plant inspectors
(1) A copy to the field office.
(2) When appropriate, a copy to the Regional office.
(3) When appropriate, a copy to the Division Director and
Chief of the applicable Branch.
4. Correspondence or samples shipped by express delivery
a. Delivery by special carrier
When sending correspondence that must be delivered overnight, or
when shipping packages of canned or frozen review samples that
are handled by any express company (such as Federal Express),
DO NOT use the National office STOP 0247 address. The
National office address for deliveries by an express carrier is:
USDA AMS FV Specialty Crops Inspection (SCI) Division
1400 Independence Avenue SW
Room 0726 South Building
Washington, DC 20250-0001
The carrier has to guarantee in house delivery during the posted
office hours. Destination delivery charges must be prepaid, or the
sender's account number used for payment.
Refer to the applicable sections in the AIM Inspection Series,
Inspections for Operational Rations Purchased by the Department
of Defense Manual, or USDA Purchases Manual for instructions
on marking shipping cases for review samples under DCIS and
USDA inspection programs.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 11 of 192
b. Samples submitted for special review
Duplicate sample units will be drawn for the field and Regional
offices when submitting samples to the National office for
evaluation of quality factors. Inspectors will complete three score
sheets/tally sheets, including the reason for submission either on
the inspection document or on a Sample Review Form. Send one
copy of the applicable score/tally sheet with the sample(s) to the
National office, and similar sets to the field and Regional offices.
Product evaluation results will be returned through the Regional
office.
When sending samples for special review, mark the shipping case
SPECIAL REVIEW, and notify the appropriate section by
telephone or fax of the approximate delivery date. This should
prevent any delay in evaluation.
c. Samples for Inspection Branch (IB)
Samples submitted for Inspection purposes are usually requested
by a Marketing Specialist of the IB. A memo should be enclosed
with the sample to indicate the purpose for submitting the sample,
and the shipping case should be marked to the attention of the IB.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 12 of 192
FEE COMPUTATION
A. Current Fee Documents
The current fees for various inspection services are located on the USDA, SCI home page
at the following internet address:
http://www.ams.usda.gov/AMSv1.0/processedinspection by clicking on the “SCI News”
link found in the “SCI Services” block.
B. Fees for Lot Inspection grading service
Table I Product grouping.
Table II Fees for product grouping.
Table III Fees for dried fruits and processed raisins (excluding figs & dates).
Table IV Fees for coffee, tea, and sugar products.
Table V Fees for samples submitted by the applicant.
Table VI Fees for update samples.
Table VII Fees for special agreements for lot inspection.
The hours in Tables II, III, and IV are for grading the product. This includes time
for set-up, clean-up, typing, and other time spent in normal inspection and
certification, as well as analysis (including all analyses called for in the AIM
Inspection Series, Foreign Material Manual). The hours do not include travel and
sampling time, nor do they include special requirements, such as vitamin C
analysis on fortified products. Fees for sampling, condition of container,
checkloading, and case stamping must be charged at a minimum of one-half hour
for each task.
If the fees computed using the applicable tables are determined to be inadequate
or excessive for the time involved, bill for the actual time required for the
inspection. However, prior to billing clear this with the Regional Section Head,
who will inform the National office of the circumstances.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 13 of 192
TABLE I
PRODUCT GROUPING
(Use with Tables II & III)
GROUP I
Includes all non-standardized products and products covered by U.S. Grade Standards, except for those
products which are listed in Groups II - IV, and Table IV.
GROUP II
CANNED FROZEN
Asparagus Olive Oil and
Corn, W. Kernel Olive-Pomace Oil
Cranberry Juice Cocktail Peaches
Cranberry Sauce Pears
Fruit Butters Pie Filling
Fruit Concentrates Pineapple (all styles)
Fruits for Salad Pineapple Juice
Fruit Nectars Plums
Fruit Purees Preserves
Green Beans RTP Cherries
Green Olives Ripe Olives
Mushrooms Sweet Potatoes
Wax Beans
Asparagus Onion Rings
Broccoli Potatoes, Fr. Fried
Brussels Sprouts Potatoes Hash Br.
Conc. Apple Juice RTP Cherries
Corn (all styles) Succotash
Cranberries Turnip Greens
Cauliflower with Turnips
Green Beans Wax Beans
GROUP III
CANNED FROZEN
Barbecue Sauce Raspberries
Berries Salsa
Blueberries Sauerkraut
Chili Sauce Spaghetti Sauce
Conc. Tomato Juice Spinach
Fruit Cocktail Three Bean Salad
Leafy Greens Tomatoes (all styles)
Mixed Vegetables Tomato Catsup
Peas Tomato Juice
Peas and Carrots Tomatoes and Okra
Pickles & Relish Tomato Puree
Tomato Sauce
Berries
Blueberries
Leafy Greens
Mixed Vegetables
Peas
Peas and Carrots
Raspberries
Strawberries
DEHYDRATED PRODUCTS
Dehydrated Fruits
Dehydrated Potatoes
GROUP IV
CANNED FROZEN
Tomato Paste
All Citrus Juices
Peanut Butter
Frozen Concentrated Grape Juice
All Citrus Juices
DEHYDRATED PRODUCTS
Dehydrated Grape Crystals
Dehydrated Citrus Crystals

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 14 of 192
TABLE II
FEES FOR PRODUCT GROUPING
(Use with Table I)
Sample Size
3
6
13
21
29
38 1/
Group I Products
Hours
3
3.5
4.75
5.75
7
9.25
Group II Products
Hours
3.5
4.5
6.5
8.25
10.5
14.25
Group III Products
Hours
4
5.5
8
11.5
15
20.25
Group IV Products
Hours
4.5
6.5
10
15
20
26.5
1/ For re-inspection only, when lot originally required 29 sample units.
TABLE III
FEES FOR DRIED FRUITS AND PROCESSED RAISINS
(EXCLUDING FIGS & DATES)
(Use with Table I)
Sample Size
6
12
18
24
30
Composite(s)
1
2
3
4
5
Total Hours For Grading
Including One Microanalysis
4
7
10
13
16
Total Hours For Grading With
More Than One Microanalysis
5.5
10
14.5
19
23.5

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 15 of 192
TABLE IV
FEES FOR COFFEE, TEA, AND SUGAR PRODUCTS
Molasses, Syrup, Honey
For 3 samples units, or less
3 hours
For each additional sample unit
0.25 hour
Green Coffee
Green bean grade, first sample
1 hour
For each additional sample
0.5 hour
Cup test, first sample
1 hour
For each additional sample
0.25 hour
Combination green bean grade and cup test, first sample
1.5 hours
For each additional sample
0.75 hour
Coffee and Tea - bid samples 2/
Cup test, first sample
1 hour
For each additional sample
0.25 hour
Coffee, Instant Coffee, Tea, Instant Tea 2/
1-10,000 lbs.
1 hour
10,001-20,000 lbs.
2 hours
20,001-30,000 lbs.
3 hours
Each additional 10,000 lbs.
0.5 hour
2/ Charge additional time for analysis at the rate specified in section D. “Fees for
chemical and microbiological analyses”.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 16 of 192
Guidelines for fees for grading services performed on samples submitted by the applicant shall
be based on the hours specified below. Product groupings are as shown in Table I of this
instruction.
TABLE V
FEES FOR SAMPLES SUBMITTED BY THE APPLICANT
(UNOFFICIAL SAMPLES)
Product Group
Two sample units or less of
the same product 3/
Each additional sample unit
Group I, Hours
2.5
0.5
Group II, Hours
3
0.75
Group III, Hours
3.5
1
Group IV, Hours
4.0
1.25
Coffee, tea, and sugar products should be billed in accordance with Table IV.
3/ For products graded on attributes standards, see the
AIM Inspection Series, Sampling
Manual for special procedures for unofficial samples.
TABLE VI
FEES FOR UPDATE SAMPLES
Sample Size (Range)
1 - 3
4 - 6
7 - 13
Total Hours For Update
1.5
2.5
3.5

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 17 of 192
TABLE VII
FEES FOR SPECIAL AGREEMENTS FOR LOT INSPECTION
Sample Size
3
6
13
21
29
Group I Hours
1.75
2.25
3.25
4.25
5.25
Group II Hours
2.25
3
4.25
6.5
8
Group III Hours
2.5
3.25
4.75
7
9.25
Group IV Hours
3
4
5
8
10
Note: Special agreements for lot inspection service should be initiated when the
volume of the total pack suggests that increased personnel efficiency
would result. See the AIM Management Site
regarding contractual
agreements.
C. Fees for Continuous Inspection and Pack Certification contracts
All charges will be in accordance with the current rate as shown in the Code of
Federal Regulations (CFR) 7 CFR 52.41 through 52.52 found at the following
internet address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CFR.
Holiday pay includes those holidays in Section 6103 (a) Title 5 U.S. Code, found
at the following internet address: http://uscode.house.gov/search/criteria.shtml.
For Pack Certification Contracts, no travel expenses, mileage and/or per diem
shall be charged on any year-round or less than year-round contracts. Only the
hours actually worked on the assignment are charged. Intermittent contracts
(minimum of 8 hours in lieu of 40) shall be billed in accordance with
7 CFR 52.51. Applicants will be billed for travel time as well as hours worked on
the assignment; no mileage and/or per diem expenses are charged.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 18 of 192
D. Fees for chemical and microbiological analyses
The following table contains the hours to charge for chemical and microbiological
analyses that are made at the request of the applicant, or that are required because
of specification or program requirements (such as vitamin C analysis on fortified
products). Do not assess a fee for analytical tests when routine tests such as Brix
or acidity are associated with quality grading. The applicable fee shown for the
various groupings in the previous section “B. Fees - Lot Inspection Grading
Service” incorporates these charge(s) for analysis.
The hourly charge shown includes normal sample preparation time. When the
nature of the product requires more than normal sample preparation time, the
applicant shall be charged for the extra time on an hourly basis to the nearest
quarter hour. The charge for this preparation time shall be recorded so that it is
separately identifiable from the analysis fee.
This fee structure was established to promote uniformity in the charges assessed
for chemical and bacteriological tests. When the hour(s) shown for a single
analysis is found to be grossly inadequate or excessive for the time involved for a
particular test, the fee assessed may be based on the actual time it takes to perform
the test(s). However any modification or variation to the hours shown must be
cleared with the Regional Section Head.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 19 of 192
Type of Test Analysis Hours for Single Analysis Hours for Each Additional 1/
Absorbency (grape juice) ½ ½
Acidity ¼ ¼
Alcohol Insoluble Solids (AIS) 1 ½
Ascorbic acid (titration) 1 ¼
Brix (direct) ¼ ¼
Brix (dilution) ½ ½
Catalase Test ½ ½
Color (honey etc.) ½ ¼
Consistency, Bostwick ¼ ¼
Fiber Test (green & wax beans) 1 1
Filth, heavy ½ ½
Filth, light (macro) ½ ½
Filth, light (micro) 1 1
Fly Egg and Maggot ½ ¼
Insect Fragments 1 ¾
Insoluble Solids ½ ½
Maggot/Larvae/Worm Count ½ ½
Maggot Count (Mushrooms only) 1 ½
Methyl Anthranilate 1 ½
Microscopic Examination 1 1
Moisture (oven) ½ ¼
Mold Count (direct smear) ½ ¼
Mold Count (centrifuge) ¾ ½
Mold Count (pulping) 1 ¾
Naringin 1 ¼
Peroxidase Test ½ ¼
Potassium Sorbate 1 ½ 1
pH ¼ ¼
Recoverable Oil (citrus) 1 ½
Salt (back titration) ¾ ¾
Salt (potentiometric) ½ ¼
Sand Test (raisins) 1 1
Sieve Test ¼ ¼
Soluble Solids (refractometer) ¼ ¼
Specific Gravity 1 1
Sulfur Dioxide (Monier-Williams) 1 ½ 1
Titer 1 1
Total Solids (oven drying) ½ ¼
Tough String Test (green/wax beans) ½ ½
Viscosity 1 ½ 1
Vitamin C (see Ascorbic Acid)
Water Insoluble Inorganic Residues 2 1 ½
(WIIR)
Wrapper Adherence Test ½ ¼
1/ If no charge is listed for an additional analysis, charge at the same rate as a single analysis.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 20 of 192
E. Fees for Dried Dates
In order to provide uniformity in the assessment of fees, the schedule as outlined
here shall be observed unless circumstances are so unusual as to justify using the
hourly rate.
In calculating the fee, when multiple code marks are offered for inspection, bill
and certify each code mark or lot separately.
Charge for driving and sampling time at the current hourly rate.
FEE SCHEDULE for DRIED DATES
Sample
Size
3
8
14
26
36
44
56
68
82
Hours
2
2 3/4
4 1/2
6 1/2
8 1/2
10 3/4
13 1/4
16 1/4
19 1/4
F. Fees for letter contracts for unofficial samples
The following instructions outline requirements for special letter contracts for
unofficial samples. This service may be used by any applicant that does not need
official sampling.
The following fees and conditions are applicable to products covered by U.S.
Grade Standards, although products not covered by U.S. Grade Standards may be
included. Please check with the National Office for the appropriate fee.
1. A sample unit is a container and/or its entire contents, a portion of the
contents of one or more containers, or other unit of commodity, or a
composite mixture of a product used for inspection. (For products graded
on attribute standards, see the AIM Inspection Series, Sampling Manual).
2. A sample is any number of sample units to be used for inspection.
3. A minimum of six sample units shall be used per submittal. Sample units
may consist of multiples of one or more products.
4. A minimum of 50 sample units will be submitted per year.
5. Unofficial grading will not be performed on products packed under in-
plant inspection, or on lots that have previously been officially inspected.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 21 of 192
6. Restrictive grading or analysis
a. The applicant may request the grading to be restricted to any
quality or non-quality factor, and/or analysis. The fee for
restrictive grading (excluding micro, chemical, or analytical work)
will be in accordance with the fee for product Group I. The micro,
chemical, or analytical fee will be in accordance with the
appropriate fee for that test.
Charges for Inspection
Product
Two sample units or
less of same product
Each additional sample
unit of same product
Group I* Hours
1.5 0.25
Group II* Hours
2 0.5
Group III* Hours
2.5 0.75
Group IV* Hours
3 1.0
∗ Product grouping as shown in B. Table I, Fees for Lot Inspection Grading Service
Certification Fees
A. Certify to a U.S. Grade
The applicable fee shall be in accordance with the current SCI Fees-Lot
Inspection Grading Service found at the following internet address:
http://www.ams.usda.gov/processedinspection, under “SCI Services” click on the
“SCI News” link, under “SCI Services and Fees” click on the “Current User Fee
Information” link.
B. Export Certificates restricted to product condition
The fee for product that is restricted to organoleptic evaluations and certified for
export shall be in accordance with the current SCI Fees-Lot Inspection Grading
Service found at the following internet address:
http://www.ams.usda.gov/AMSv1.0/processedinspection. The number of hours to
charge is shown in the following examples:
1. Sample units submitted by an applicant
Unofficial sample units submitted at one time by an applicant may consist
of more than one item, including any number of sample units per item.
Charge one hour for the first sample unit, and ¼ hour for each additional
sample unit.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 22 of 192
Example: An applicant requests export certification for 20 items and each item
consists of two sample units.
20 items x 2 sample units = 40 sample units
First sample unit = 1 sample x 1 hour = 1 hour charged at the current lot
rate.
39 additional sample units = 39 samples x ¼ hour = a charge of 9 ¾ hours
at the current lot rate.
Total Cost = 10 ¾ hours charged at the current lot rate.
2. Officially Drawn Sample
When an applicant requests that sample units be drawn from a lot or more
than one lot at the same time, the lot or lots shall be sampled in accordance
with SCI Division sampling plans. Charge 1 ½ hours for the first three
sample units and ¼ hour for each additional sample unit.
Example: An applicant requests export certification for four lots of product. One lot
of canned green beans - 150 cases; one lot of canned pears - 150 cases;
one lot of canned corn - 150 cases; and one lot of canned beets - 150
cases. Each lot requires a minimum of 3 sample units. The total number
of sample units evaluated is 12. Charge as follows:
4 lots x 3 sample units = 12 sample units
First 3 sample units charge 1 ½ hours at the current lot rate.
9 additional sample units = 9 samples x 1/4 hour = 2 ¼ hours charged at
the current lot rate.
Total Cost = 3 ¾ hours charged at the current lot rate.
Example: An applicant requests export certification for three lots of product. One lot
of canned peaches - 650 cases; one lot of canned pears - 200 cases; and
one lot of canned cherries - 200 cases. The lot of canned peaches requires
a minimum of 13 sample units. Each lot of pears and cherries requires a
minimum of 6 sample units. The total number of sample units evaluated is
25.
Charge as follows:
First 3 sample units charge 1 ½ hours at the current lot rate.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 23 of 192
22 additional sample units = 22 samples x ¼ hour = 5 ½ hours charged at
the current lot rate.
Total Cost = 7 hours charged at the current lot rate.
Note: The applicant shall be charged for travel time, sampling time, condition of
container inspection, etc., for officially drawn samples.
When the certificate is restricted to organoleptic evaluation, and includes
sample units either submitted by an applicant or officially drawn samples,
the hours charged include the cost of issuing certificates. Do not charge
an applicant for additional certificates, even though the applicant may
request that each item be certified separately.
C. Export Verification Service
Assess the applicant at the hourly rate currently in effect in accordance with the
Regulations, 7 CFR 52.42 which may be found at the following internet address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CFR.
The fee shall cover the time for travel, the verification inspection, and the
certificate preparation. Charge one hour for the first certificate on an application.
For additional certificates requested by the same applicant at the same time,
charge one half hour per certificate.
D. Dairy Verification Service
The applicant will be charged at the current hourly rate in effect in accordance
with the Regulations, 7 CFR 52.42 which may be found at the following internet
address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CFR.
Charges will be assessed to cover the time for travel, verification inspection
procedure, and preparation of the certificate.
Example: Various Processed Products Offered
One on-site verification is performed to cover four dairy items, and
the applicant submits five samples of processed fruit and vegetable
products. Charges to assess are as follows:
1.0 Hour driving time;
2.5 Hours verification inspection and preparation of the dairy
export Certificate; and
2.0 Hours for product condition evaluation of the five
processed fruit and vegetable items.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 24 of 192
5.5 Total hours assessed at current hourly rate.
E. Microanalysis
When such tests are requested by the applicant, or when required by an FDA
defect action level or Division guideline, charges for microanalysis will be in
accordance with section D. “Fees for chemical and microbiological analyses”.
SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 25 of 192
FUTURES
The following instructions are for sampling orange juice products packed in tanker trucks for
futures contracts when facilities have not contracted with SCI for in-plant continuous inspection
services, but are contracted under an alternate Intercontinental Exchange (ICE) approved SCI
inspection program. ICE has changed the requirements for delivery and warehousing of futures.
Continuous Inspection is no longer required for Frozen Concentrated Orange Juice (FCOJ). In
addition, Not from Concentrate (NFC) orange juice has been added to futures contracts and does
not require Continuous Inspection. Lot inspection as described below is acceptable for futures
contracts.
When applicants request lot inspection of FCOJ or NFC loaded into tanker trucks for futures
contracts, follow these procedures:
A. Schedule inspection personnel to perform sanitation inspection of the processing
facility and the bulk container(s) prior to loading. Complete and retain the
Sanitation Score sheet FV 416-3 which can be found on the AMS Forms Catalog
at the following intranet address:
http://agnis/AMSFormsCatalog/Forms/AllItems.aspx.
Follow instructions in the AIM Inspection Series, Sanitation and Safety Manual
for recording, following up, and reporting deficiencies.
B. Draw samples of the product during loading into the tanker truck(s). Sampling of
pre-loaded tankers is not authorized.
C. Draw samples using one of the following procedures:
1. Automatic sampler - Use automatic samplers to obtain representative
samples. Follow Division Instructions for set up and use of automatic
samplers. (See the Automatic Samplers section in this Manual.)
2. Manually drawn samples - Draw representative, individual sub-samples
randomly during the filling process. The frequency will be determined by
the time it takes to fill the container. Draw the number of samples as
specified in the Regulations, 7 CFR 52.38 Table III, Group IV, which may
be found at the following internet address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=C
FR.
The “on-line” rate is authorized.
D. Analyze and record the results for each individual sample at the facility.
E. Bill applicants in accordance with Section A of this manual, under “Fee
Computation”.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 26 of 192
Review Program for Concentrated Orange Juice for Manufacturing (OM) and Pasteurized
Orange Juice (POJ) for Futures Contracts
To ensure uniform grading of Futures deliveries, these instructions provide procedures for the
review of product samples of OM that represent deliveries of Frozen Concentrated Orange Juice
(FCOJ) Futures, and POJ that represent deliveries of Not From Concentrate (NFC) Futures.
In January 2007, Intercontinental Exchange (ICE), Inc. acquired the New York Board of Trade
(NYBOT), which was previously known as Citrus Associates of the New York Cotton
Exchange, Inc. NYBOT is now known as ICE Futures US. ICE Futures US is regulated by the
Commodity Futures Trading Commission, and has been designated as a marketer for contracts in
FCOJ and NFC Futures buying and options on Futures. OM is produced to meet the
requirements of an FCOJ Futures contract. POJ is produced to meet the requirements of an NFC
Futures contract. ICE Rules Chapters 7, 13, and 25 are the source of most of the requirements
for these commodities.
The U.S. Standards for Grades of Orange Juice effective January 10, 1983, are used as the
standards to determine the grade and quality delivered on contracts, per ICE rules 13.02 and
25.02. A USDA certificate is issued at the time of grading. In addition, ICE Futures US
procedures require USDA, AMS, FV, SCI to perform a “grade review” for each lot SCI grades
for ICE Futures US. Any delivery of FCOJ or NFC futures which fails the grade review is
subject to penalties or remedies per ICE Rule 7.70. ICE Rules 13.02, 13.03, and 7.64 of the
FCOJ rules state:
“For FCOJ-A and FCOJ-B: “U.S. Grade A” with a Brix value of not less than 62.5 degrees,
having a Brix value to acid ratio of not less than 14.0 to 1 nor more than 19.0 to 1 and a
minimum score of 94 points, with the minimums for the component factors fixed at 37 points for
color, 37 points for flavor, and 19 points for defects.
The maximum amount of bottom (sinking) pulp shall be twelve percent, and the percentage of
recoverable oil shall not be less than 0.005 percent nor more than 0.020 percent. The maximum
temperature allowed at tanker shipment is plus 20 degrees Fahrenheit.”
Effective with the July 2009 delivery month, the country of origin requirements stipulate that the
OM be produced from product of the United States, Brazil, Mexico, Costa Rica, or any blend
thereof.
For NFC, the following is in accordance with Rules 25.02, 25.03, and 7.64 of the ICE Futures
US:
“U.S. Grade A” Pasteurized Orange Juice conforming to Food and Drug Administration
(FDA), 21 CFR 146.140, but without the addition of any of the optional concentrated
orange juice ingredients referenced in CFR 146.140(b) and without the addition of
optional sweetening ingredients referenced in CFR 146.140(c), with a Brix value of 11.5
to 12.5 degrees having a Brix value to acid ratio of not less than 14.0 to 1 nor more than
20.5 to 1 and a minimum score of 92 points, with the minimums for the component
factors fixed at 36 for color, 36 points for flavor, and 18 points for defects; product shall

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 27 of 192
be 100 percent Florida origin. The maximum amount of bottom (sinking) pulp shall be
fifteen percent and the percentage of recoverable oil shall not be greater than 0.035
percent. The maximum temperature allowed at tanker shipment is plus 35 degrees
Fahrenheit.
ICE Futures US rules require USDA inspection for grade at the time of Futures delivery,
as well as specifying a review program to monitor the quality requirements. The
following reviews were put in place to meet these requirements:
1. Effective January 1992, all deliveries for FCOJ Futures will be reviewed
by the Winter Haven Area office, (WHAO) Winter Haven, Florida.
2. Effective January 2007, all deliveries for NFC Futures will be reviewed by
the WHAO, Winter Haven, Florida.
Note: Product declared by applicants as “Futures Quality” is not considered
Futures product.
3. SCI Responsibilities
a. Inform the applicant(s) about sampling, grading, billing, and
review procedures;
b. Select and draw official sample units of product as it is being
loaded for delivery;
c. Inspect and grade OM/POJ, issue score sheets, distribute
certificates, and retain a portion of the sample for review; and
d. Conduct product reviews to assure uniformity of grading and
inspection at all facilities.
4. Grade reviews
SCI will conduct grade reviews as follows:
a. All sample units for review shall be officially drawn. Unofficial
sample units submitted by the applicant will not be accepted.
b. Unless otherwise specified, all grade reviews will be conducted by
the WHAO, on behalf of the ICE Futures US Inc.
c. The origin and facility identity will be unknown to the SCI
reviewers.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 28 of 192
5. Sampling procedure for review samples
Retain 2 six ounce (minimum) samples for each OM/FCOJ delivery (each
mobile tanker) and retain two 24 to 32 ounce (minimum) samples for each
POJ/NFC delivery. Of the two samples drawn, retain one sample. Submit
the other sample to the WHAO for review. The sample MUST be
delivered or sent into the WHAO for next day delivery, and should arrive
at the WHAO Monday thru Friday, 0800 to 1630, not including holidays.
Do not ship on Friday. Samples shall be adequately packed in insulated
containers with sufficient cool packs to preserve product quality. Samples
can be shipped in plastic bottles, or any other container that can be safely
shipped without breakage or leakage. Taped screw cap lids or sealed cans
are preferred. Each sample shall be identified as indicated in Section 6
below, and shall be accompanied by a score sheet, certificate (FV-149 or
FV-146 as applicable), and the bill of lading. Associated submittal
numbers shall be recorded on all paper work submitted with the samples.
Futures Sampling Reminder:
The sample must be representative of the lot. This will be the actual juice
sample taken from the tanker. The sample must not be pulled from a
blend tank or from a blend of storage tanks. It must also be the same
sample which was graded and recorded on the score sheet.
6. Identification of review samples for submittal to the WHAO
Mark all review samples with labels taped to each sample container.
Submittal numbers are to be assigned to each review sample based on the
fiscal year beginning with the number 1 and continuing with consecutive
numbers throughout the fiscal year. An example of a label for marking the
first submitted review sample of the fiscal year is as follows:
Futures Review Sample
Plant Name: John Doe and Company
Facility No: 6000
Date Inspected: 04/01/10
BL/Manifest No: B58505
Submittal No: 001

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 29 of 192
7. Notification of WHAO
The field office providing the inspection service will notify the WHAO of
futures deliveries via email on a weekly basis. The message shall include
the following information for each delivery:
• Facility No.
• BL/Manifest No.
• Submittal No.
• Certificate No.
• Date Inspected
8. Sample control procedure
The WHAO OIC will designate a control official for handling samples
during the receipt and review process. When a Futures FCOJ/NFC sample
arrives in the WHAO, the designated control official will record the
sample identification in a review log and assign a control number. The
identification label will be removed to ensure that the origin and
production facility are not disclosed during the review process. Only the
control official will know the sample origin and production facility - he or
she will not participate as a member of the review panel to determine
compliance with ICE Futures US.
9. Review procedure
The grade review will evaluate the product in accordance with quality
requirements of the U.S. Standards for Grades of Orange Juice and the
ICE Futures US Rules as follows:
a. FCOJ Futures (Graded as OM)
(1) Reconstitution
The review sample will be reconstituted to a corrected Brix
of 11.8 degrees.
(2) Color
The quality factor of color will be determined using a
USDA approved colorimeter. The minimum color score
for FCOJ Futures is 37 points.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 30 of 192
(3) Defects
The quality factor for defects will be determined by visual
examination. The minimum defect score for FCOJ Futures
is 19 points.
(4) Flavor
The quality factor for flavor will be determined
organoleptically. The minimum flavor score for FCOJ
Futures is 37 points.
(5) Documentation
The reviewers will report the score points for each quality
factor for each assigned control number to the control
official. The control official will record the results of the
flavor panel scores and other results in the review log as
final documentation.
(6) Notification of Review Results
Results of the review in relationship to the production
facility and origin are confidential and will be available
only to authorized ICE Futures US representatives. A
summary will be provided quarterly to the Division
Director.
b. NFC Futures (Graded as POJ)
(1) Color
The quality factor of color will be determined using a
USDA approved colorimeter. The minimum color score
for NFC Futures is 36 points.
(2) Defects
The quality factor for defects will be determined by visual
examination. The minimum defect score for NFC Futures
is 18 points.
(3) Flavor
The quality factor for flavor will be determined
organoleptically. The minimum flavor score for NFC
Futures is 36 points.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 31 of 192
(4) Documentation
The reviewers will report the score points for each quality
factor for each assigned control number to the control
official. The control official will record the results of the
flavor panel scores and other results in the review log as
final documentation.
(5) Notification of Review Results
Results of the review in relationship to the production
facility and origin are confidential and will be available
only to authorized ICE Futures representatives. A
summary will be provided quarterly to the Division
Director.
10. Billing for review samples
The Winter Haven Area office will bill the applicant one hour for each
review sample at the current hourly rate shown in the Regulations,
7 CFR 52.42 which may be found at the following internet address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=C
FR.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 32 of 192
Review Program for Apple Juice Concentrate (AJC) Futures
The AJC sampling and testing process begins when a Minneapolis Grain Exchange (MGEX)
Regular warehouse contacts a Specialty Crops Inspection (SCI) Division field office to schedule
sampling of AJC product. The SCI scheduler must collect the warehouse name and the number
of test lots as well as any other information that is required by SCI to schedule a test. Sampling
appointments must fall within three business days of the initial notification from the warehouse.
Note: A Regular warehouse is a warehouse authorized by MGEX to participate in the AJC
Futures Program. A warehouse contact list can be found in Appendix 1 of the Apple Juice
Concentrate Manual at www.mgex.com/ajc/ .
Procedures
A. At the time of scheduling, the warehouse must provide the Certificate of Analysis
(COA), the production manifest provided by the producer, and a Warehouse
Management System (WMS) report listing the individual drums/bins contained in
each test lot to the SCI field office by their preferred method (fax or email). The
warehouse email address/fax number must be able to receive the completed test
results from SCI as well. Prior to making the sample selection(s) SCI must ensure
that the WMS report of the test lot provided by the warehouse clearly identifies
the COA lot number (identifying a unique production run) listed on the COA
and/or the production manifest.
B. Upon receipt of the documentation described in A., SCI must verify that all
product specification(s) listed on the documentation received from the warehouse
meets MGEX delivery specification requirements as shown on the following
page. If any contract specification is not met, the warehouse must be contacted
and the sampling appointment canceled. If the sampling appointment is cancelled
subsequent to this verification process, SCI may assess an administrative charge
of two hours or actual time, whichever is greater, at the current lot rate.
C. If no link between the WMS Report, the COA and the manifest can be
established, SCI must contact the warehouse to notify them of the issue. Unless
the issue can be promptly resolved by the warehouse, SCI must cancel the
sampling appointment until the warehouse submits the proper documentation.
D. SCI must identify its random test sample drum(s)/bin(s) and notify the appropriate
warehouse no later than the close of business on the following business day that
the documents described in A. are received. After receipt of this information, the
warehouse will prepare the drum/bin for testing.
E. The SCI inspector must complete or review an applicant completed FV-356
Application for Inspection and Certificate of Sampling. The form must identify
the test lot number, test lot size (number of drums/bins), the entire COA number,
the type of container (drums/bins), and the number of samples drawn from the lot.
SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 33 of 192
MGEX Apple Juice Concentrate Futures Contract
Specifications
Brix (degrees)
Minimum 70.0°
Acid (Malic, weight/weight, %) 1.0 - 2.2
Color (transmittance @ 440
nm)
> 40 %
Turbidity (NTU) ≤ 3 NTU
Thermoduric Acidophilic Bacilli
(TAB)
< 1 cfu/10 mL
Total Plate Count (TPC) < 1,000 cfu/mL
Patulin @ single strength ≤ 50 ppb
Yeast < 500 cfu/ml
Flavor
Characteristic of ripe, mature
apples with no off flavors or
aromas.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 34 of 192
Sampling
A. On the day of sampling, the SCI field inspectors must arrive at the warehouse at
the scheduled time and notify the appropriate contact person at the regular
warehouse upon arrival. The SCI field office will charge the warehouse for travel
time to and from the warehouse at the current lot rate.
B. Prior to the AJC sampling, the SCI inspectors must ensure that the label of the
drum/bin selected for sampling clearly identifies the COA lot number listed on the
COA and manifest. If any other information is on the label, it must meet MGEX
contract specifications. If the COA lot number is not listed on the label, the SCI
inspector must ensure that the producer manifest clearly links the drum/bin to the
proper COA/production run. The production label or WMS label must identify
the correct drum/bin that was selected for sampling. If the drum or bin is not
labeled, the SCI inspector must verify with the warehouse that the proper drum or
bin was pulled as requested.
C. The warehouse must assist SCI in all matters required for sampling such as
providing a sanitary sampling environment. The SCI inspector must also observe
the unsealing of the container.
D. Using sanitary utensils and containers, supplied by the warehouse, the SCI
inspector will pull:
• one (1) eight ounce sample for SCI,
• three (3) eight ounce samples for Science and Technology (S&T), and
• one (1) eight ounce retain sample for a total of five (5) samples per production lot.
If the warehouse does not supply appropriate containers, both cups and lids, the
SCI office may charge the warehouse for containers supplied by the USDA.
E. Immediately after drawing the samples, the SCI inspector should label all five (5)
samples with the test lot number. The SCI inspector shall place the samples in a
cooler with adequate cool packs for transport to the respective testing locations.
SCI inspectors will have an appropriate number of coolers depending on the
sample size taken.
F. After the samples have successfully been drawn from the container, the SCI
inspector must affix a “USDA TESTED” label, supplied by the warehouse, on the
sampled drum/bin. The inspector must also observe the warehouseman re-sealing
(closing) the drum/bin using the appropriate warehouse method.
G. If there is any noncompliance with the sampling procedures above, SCI must
cancel the sampling appointment unless the issue can be promptly resolved by the
warehouse. Should the product owner wish to continue the testing process even

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 35 of 192
though the issue is not resolved, the MGEX USDA Letter Report (see example
Letter Report) must not be used to issue testing results.
H. Form FV-637 Laboratory Sample Submittal Sheet must be completed for S&T
and for SCI. The SCI inspector must submit the appropriate paperwork with the
samples. The FV-637 must contain the test lot number in the “Lot No.” field and
the four requested analyses, TAB, TPC, Patulin and Yeast in the “Analysis
Requested” field.
I. The SCI inspector shall mark the sample to be shipped for grading by SCI with
the test lot number and drum/bin number and SCI on the lid and cup.
J. S&T receives three (3) samples. The SCI inspector should mark each cup and lid
with S&T (for Science and Technology), plus the test lot number and drum/bin
number. Indentify the specific analysis each sample is to be tested for by marking
one sample cup TPC (total plate count) and Yeast, the second sample cup Patulin
and the remaining cup TAB (Thermoduric Acidophilic Bacilli).
K. The retained sample shall be marked SCI, the test lot number, the drum/bin
number as well as the word “retain”.
L. The sampling office shall keep the retained sample for a minimum of thirty (30)
days from the date of sampling. Any retained samples must be stored below 32
degrees Fahrenheit within 6 hours of sampling.
M. Samples shall be adequately packed in insulated containers with sufficient cool
packs to preserve product quality. If at any time the samples are not stored
correctly or the SCI security strips are broken other than by the sampling/grading
office, the sample is void and unusable.
N. All samples must be properly packed (as stated in G. above), and shipped
overnight to the appropriate labs for testing on the same business or the next
available business day following when the sample was taken. If, due to the timing
of sampling, same day shipping cannot be performed, the sampling office must
store the samples below 32 degrees Fahrenheit within 6 hours of sampling and
ship the samples on the following business day. The SCI office will charge the
warehouse actual shipping expense and preparation time to ship samples to the
appropriate location.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 36 of 192
Issuing a Letter Report
Upon receiving test results from the samples taken, the SCI office issuing the Letter Report must
ensure the following when issuing a USDA Letter Report for delivery against an MGEX futures
contract to the warehouse. (USDA Report FV- ####):
A. The USDA Letter Report must contain the following:
1. Unique USDA Letter Report Number
2. The name and address of the warehouse storing the tested AJC
3. Date in which the AJC was sampled
4. Date in which the associated AJC was produced (from COA)
5. The Test Lot number identified by the warehouse requesting the sampling
6. The drum/bin numbers sampled by the SCI inspectors
7. Product specifications for MGEX delivery listed on the COA must be
identified under the Applicant’s COA Results: column
8. USDA test results must be listed under USDA Analysis: column of the
Letter Report
9. The Letter Report must also identify whether product tested meets or fails
MGEX contract specifications as noted in the “Meets/Fails” column of the
Letter Report.
B. All Letter Reports must be issued to the warehouse within two (2) business days
from the completion of all tests or a maximum of fifteen (15) business days from
sampling. The Letter Report must be issued by email. The letter may be faxed if
the inspection office is unable to issue the letter by email
C. SCI must keep copies of issued Letter Reports for a minimum of five (5) years
and have the ability to re-issue a previously issued Letter Report should the
original be lost or misplaced.
D. Any re-issued Letter Report issued as a result of the scenario described in C.
above, must be clearly marked “Duplicate.” Any re-issue will result in an
administrative charge of one hour at the current lot rate.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 37 of 192
Example Letter Report
WHL – (####) (Issue Date)
(Warehouse Operator)
(Warehouse Address)
(Warehouse Address)
Dear: (Warehouse Operator),
This report details the results of the analysis on one sampling of Apple Juice Concentrate (AJC) that our department drew on
(sample date). The samples were drawn from (drum/bin) number (###) from Test Lot number (###) as established by the
warehouse. The Certificate of Analysis (COA) presented to our department at the time of sampling represents lot number
(###) and production date (###) establishing the applicant’s connection to the COA and the production run established by the
applicant’s lot.
In conducting the analysis, our department verified that all AJC product characteristics listed on any document obtained in the
sampling process meets or exceeds Minneapolis Grain Exchange, Inc. (MGEX) AJC futures contract specifications. The test
results are provided below and are used to provide verification that AJC product represented by Test Lot number (###) meets
or fails MGEX AJC futures contract specifications.
DATE SAMPLED:(sample date)
APPLICANT (Warehouse Operator)
Test LOT # (See above)
DRUM/BIN (See above)
MGEX AJC Futures Contract
Specifications
Applicant’s
COA Results
USDA Analysis Meets/Fails
Brix (degrees)
Min 70.0
70
71.0
Meets
Acid (Malic, weight/weight,%)
1.0 - 2.2
1.86
1.92
Meets
Color (transmittance@ 440 nm)
> 40%
50 %
68%
Meets
Turbidity
≤ 3 NTU
1 NTU
≤ 3 NTU
Meets
Thermoduric Acidophilic Bacilli (TAB)
< 1 cfu/10 ml
< 1 cfu/10 ml
< 1 cfu/10 ml
Meets
Total Plate Count (TPC)
< 1,000 cfu/ml
< 1,000 cfu/ml
< 1,000 cfu/ml
Meets
Patulin @ single strength
≤ 50 ppb
10 ppb
≤ 50 ppb
Meets
Yeast
≤ 500 cfu/ml
300 cfu/ml
≤ 500 cfu/ml
Meets
Flavor
Characteristic of mature
apples with no off flavors or
aromas.
mature
Typical
Meets
Based on the test results listed above, the sample (Meets or Fails) the MGEX AJC Futures specifications.
Sincerely,
(NAME, TITLE)
USDA-AMS-FV-PPD
123 Third Street, S.E.
Juiceville, FL 12345
cc: ____________
123 Third Street, S.E.
Juiceville, Fl 12345

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 38 of 192
Billing
The SCI field office performing the sampling duties for the AJC Futures verification program
will charge the warehouse for travel time to and from the sampling location, shipping expense
and preparation time to ship the samples to the appropriate location. SCI may also charge for
sampling containers, if not supplied by the warehouse, and any cancellation charges that may
apply. If a warehouse(s) requests sampling in more than one location in the same day, please see
you immediate supervisor for clarification of billing charges.
The grading office shall charge three (3) hours per verification sample(s) tested.
Science and Technology Division (S&T) will be responsible for billing the warehouse for the
analysis S&T performs.
Shipping Samples
The sampling field office shall ship the SCI sample, completed FV-356 Application for
Inspection and Certificate of Sampling, and a copy of the FV-637 Laboratory Sample Submittal
Sheet for S&T and COA (s) to the Winter Haven Office:
ATTN: Officer in Charge
USDA-AMS-FV-SCI
98 Third Street, S.W.
Winter Haven, Fl 33880
Office: (863) 294-7416
Fax: (863) 294-5219
The S&T samples, and the FV-637 Laboratory Sample Submittal Sheet and COA(s) shall be
shipped to the National Science Lab:
Roger Simonds, Laboratory Manager
USDA-AMS-National Science Laboratory
801 Summit Crossing Place, Suite B
Gastonia, NC 28054
Office: (704) 867-3873
Fax: (704) 853-2800

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 39 of 192
GENERAL INSPECTION
Initial Grading Procedures
The order of examination for quality, analytical and microanalyses is much the same for most
products. Generally factors that are affected by exposure to the air are evaluated first, such as
Color. For some products such as jams, jellies and applesauce, Consistency is the first
consideration.
Another general rule is to make the physical and organoleptic examination before analytical and
micro tests, cooking or any other tests that do not require the product to be intact or that would
not be affected by the results of other testing.
Familiarity with the product, its grade standard, and any additional grading instructions is
essential in starting grading procedures.
The color factor is more critical in some products such as mushrooms and sweet-potatoes, but in
all cases there is a tendency for product to become dull and somewhat darkened on exposure to
air. Color should be evaluated soon as possible after the container is opened.
After obtaining net weights, be alert for any abnormal product or container characteristics upon
opening containers.
A. In the case of dried fruits, look for darkening, sugaring, etc.
B. For jams and jellies, check for mold in the head space area. In low moisture fruits
and fruit juice crystals (or powder), look for "caking".
C. For frozen product, look for abnormal appearance, color and/or odor. Observe
amount of frost, ice crystals, layer of ice, dehydration, shattered or broken units
(as in broccoli and asparagus).
D. For canned product, look for darkening in the head space area, particularly, with
light colored products such as applesauce.
E. Also visually check the head space of canned fruits and vegetables. Often the
actual measurement is unnecessary since the can is obviously filled to more than
90 percent of its capacity. As a general rule, check the headspace of any sample
units that have a low net weight, or appear to have a low content level. This is
done easily with a head space gauge.
F. After emptying cans to determine fill of container and/or drained weight, check
metal cans for internal defects such as severe detinning or rust.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 40 of 192
Determining Net Contents
A. Definitions
"Gross weight" is the weight of the packaged unit including both the food and the
primary container.
"Tare" or "tare weight" is the weight of the empty, clean, dry primary container in
which the food is packaged.
"Net weight" is the difference between the gross weight and the tare weight. It is
the weight of the food itself, including any packing medium
B. Scales
Scale accuracy and sensitivity to one tenth of an ounce is adequate for most
grading purposes. In some instances greater sensitivity may be necessary. For
example it may be necessary to weigh small packages in gram units rather than
ounces. Individual procedures for specific products will incorporate such
requirements.
Check scales frequently to verify that they are accurate and in proper working
order. A standardized weight or set of calibrated weights may be used for this
purpose. These weights must be kept clean and damage free, and not used for
routine weighing operations.
C. Rules for Reading Scales
Tares - Electronic scales can zero tare weights exactly. If using a mechanical
scale, read to the higher mark. For example, if the scale is graduated in tenths of
an ounce and the weight of the tare can is between 1.3 and 1.4 ounces, read the
weight as 1.4 ounces.
When the tare has been established, the tare bar may be adjusted accordingly and
the net weights can be read directly. If there is no predetermined tare, take gross
weights first and determine the tare weight after containers are empty, clean and
dry. Record net weight by subtracting the tare weight from the gross weight.
Gross, Net and Drained Weight - Read to the lower mark. For example, if the
scale is graduated in tenths of an ounce and if a package of asparagus weights
between 10.3 and 10.4 ounces, read as 10.3 ounces.
D. Taking Net Weights
Place containers on the scale in the center of the pan for an accurate reading. If
there is any residue on the containers such as frost on the exterior surfaces of
frozen food packages, remove such material before weighing.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 41 of 192
E. Measuring Net Contents
When the product is fluid in nature use the following methods to determine net
contents or volume.
1. Convert Net Weight to Fluid Ounces
After determining net weights as indicated above, convert to volume
measurement with the following formula:
Net Weight (oz) X 0.9614
Fill (fl. oz.) = Specific Gravity @ 20 degrees C.
Example -- Tomato Juice No. 3 cylinder
Net Weight - 49.2 ounces
Refractive Index (20 degrees C) - 1.3415 (See AIM Inspection Series,
Technical Procedures Manual, Sucrose Conversion Table to obtain
Specific Gravity) Specific Gravity - 1.02289
(49.2) (0.9614) 47.3
Fill = 1.02289 = 1.02289 = 46.24 fl. oz.
2. Measuring Flask
Glass flasks are available that are accurately calibrated for measuring
volume of liquids. The flask is calibrated for volume of a liquid at a
standard temperature, usually 20 degrees C. The legend is etched on each
flask. The fluid product being measured must be at this temperature for
accuracy. It is also important to avoid incorporating air into the liquid
when filling the flask. To do so, always pour the liquid slowly down the
side of the flask. Heavier products such as tomato juice, nectars, etc.,
must stand after filling the flask to permit any trapped air to escape.
Determining Tare Weight
These procedures applies to all types of primary containers -- cans, glass, fiber, etc., except
polyethylene or other thin plastic film when the weight of the primary container is quite uniform
and is a very small percentage of the total gross weight. In such uniform light weight poly-bags,
a single bag from the lot may be used as the tare.
When the tare involves cartons, the liquid from frozen products such as frozen sliced
strawberries soaks into the fiber during the freezing period. Rather than using new or air-dried
cartons, use soaked cartons wiped with a dry towel to determine the tare for such a lot.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 42 of 192
A. Field Office Procedures, Option 1 - Average Tare
Wash, dry and weigh 3 empty containers from the lot. Subtract the smallest
weight from the largest. Using this number, determine the minimum total number
of containers to be weighed for the tare according to the Table below. The larger
the variations between containers the more samples are needed to determine the
average tare for the lot.
Range of First Three Minimum Number Containers
Container Weights to Determine Tare
Ounces Grams Containers Needed
0 to .10 0 to 2.83 3
.10 to .20 2.84 to 5.67 6
.20 to .25 5.69 to 7.08 9
.25 to .30 7.09 to 8.50 15
.30 to .35 8.51 to 9.92 20
.35 or greater 9.53 or greater 25
B. Field Office Procedures, Option 2 - Two Heaviest
If it is impractical or impossible to weigh the number of containers called for in
the above Table, or if the applicant desires, use the average weight of the 2
heaviest of the 3 containers as the tare.
C. In-Plant Inspection, Option 1 - Average Tare
Select at random 15 empty containers and lids, and weigh them (collectively, if
possible). Use the average weight from these 15 containers as the tare. Make this
tare determination at least once per day, normally early in the shift and again at
any time there is a change in the supply of containers.
D. In-Plant Inspection, Option 2 – Pre-weighed Tare
Weigh the tare containers, and mark these containers with their respective
weights; or identify the containers with the tare weight recorded on the worksheet.
Pass these pre-weighed tare containers through the filler, and capture them after a
point where there is no further chance to alter the fill. Subtract the appropriate
tare weight from the gross weight to determine the net weight of each container.
E. In-Plant Inspection, Option 3 - Heaviest Tare
In some instances the plant may wish to use the heaviest of the 15 weighed cans
as tare. Or if in line control is based on subgroups of, for example 5 cans, the
heaviest of the 5 may be used as the tare. This option should only be used when
the plant requests such restrictive control.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 43 of 192
Determining Drained Weight
Tare a dry, empty 8 mesh sieve (except use a 2 mesh sieve for Canned Tomatoes). Use the 8
inch diameter sieve for smaller capacity containers, and the 12 inch diameter sieve for larger
containers. Rest one edge of the tared sieve on the side of a grading tray approximately 2 ½
inches deep. This will produce a sieve slope angle of about 15 to 17 degrees. Distribute the
product evenly on the screen, except for canned spinach which is not spread on the screen. Drain
for two minutes without disturbing the product, except to invert halves of fruit that are obviously
holding pockets of syrup.
At the end of two minutes, place the sieve on the scale and read the drained weight directly. To
expedite grading and examination of the product, it is ideal to have several sieves standardized to
a particular weight. This can be done through careful selection of sieves, and the addition of
solder or stainless steel washers and wire to increase the lighter sieves to the weight of the
heaviest. By using a single tare on the scale, standardized sieve weights will allow multiple
drained weight samples to be weighed sequentially at the end of the two minute draining period.
Do not re-tare the sieves after the first weighing. Any slight residue remaining on the mesh is
considered a part of drained weight. Re-taring the sieve would offset this small increment of
weight. However, the sieve should be washed if there is excessive build-up of material and
syrup. This is often seen when draining Canned Apples, for example.
Grade or Compliance Determination
When inspection for quality is based on any U.S. grade standard with a scoring system, there is
an allowance for samples that fall below the quality of the indicated grade. Lot sizes range from
3 sample units to 29 sample units, based on the type of containers and the size of the lot. Apply
the following acceptance numbers (the number of sample units that may fall outside the indicated
grade). If these numbers are not exceeded, the lot as a whole can meet the grade. (See the AIM
Inspection Series, Sampling Manual.)
LOT INSPECTION:
Sample Size (No. of Sample Units)
Acceptance Number 1/
3
0
6
1
13
2
21
3
29
4
ON-LINE IN-PLANT
INSPECTION:
Sample Size (No. of Sample Units)
Acceptance Number 1/
3
0
6
1
6
1
13
2
21
3
1/ Indicates the number of grade deviants allowed for the sample size of the lot

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 44 of 192
However there are other considerations before a final grade for a lot can be determined. See A.
through F. below.
The Regulations, 7 CFR 52.13, which may be found at the following internet address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CFR, states in part:
“ Inspection service shall be performed on the basis of the appropriate United States standards
for grades of processed products, Federal, Military, Veterans Administration or other
government agency specifications, written contract specifications, or any written specification or
instruction which is approved by the Administrator.”
Unless otherwise approved by the Administrator, compliance with such grade standards,
specifications, or instructions shall be determined by evaluating the product, or sample, in
accordance with the requirements of such standards, specifications or instructions: Provided,
that when inspection for quality is based on any U.S. grade standard which contains a scoring
system, the grade to be assigned to a lot is the grade indicated by the average of the total of the
scores of the respective sample units: Provided further, That -
A. Such sample complies with the applicable standards of quality promulgated under
the Federal Food, Drug, and Cosmetic Act;
B. Such sample complies with the product description;
C. Such sample meets the indicated grade with respect to factors of quality which are
not rated by score points; and
D. With respect to those factors of quality which are rated by score points, each of
the following requirements is met:
1. None of the sample units falls more than one grade below the indicated
grade because of any quality factor to which a limiting rule applies;
2. None of the sample units falls more than 4 score points below the
minimum total score for the indicated grade;
3. The number of deviants does not exceed the applicable acceptance number
indicated in the sampling plans contained in Sec. 52.38 (“deviants”, as
used in this paragraph, means sample units that fall into the next grade
below the indicated grade but do not score more than 4 points below the
minimum total score for the indicated grade);
E. If any of the provisions contained in paragraphs (b) (3) and (4) of this section are
not met, the grade is determined by considering such provisions in connection
with lower grades in succession until the grade of the lot, if assignable, is
established; and
F. When it is determined that a portion of a lot bearing a particular identification
mark is of lower quality or deficient in other factors, the grade or compliance of

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 45 of 192
the lot shall be no higher than that of the portion bearing the particular
identification mark.
The Regulations also state in 7 CFR 52.38 that: Except as otherwise provided for in this
section in connection with in-plant inspection and unless otherwise approved by the
Administrator, samples shall be selected from each lot in the exact number of sample
units indicated for the lot size in the applicable sampling plans. The lot size is to
correspond to a sample size with a maximum of 29 sample units: Provided, that at the
discretion of the inspection service, the number of sample units selected may be increased
to the exact number of sample units indicated for any one of the larger sample sizes
provided for in the appropriate plans. The samples size may be increased beyond 29
sample units in accordance with the following sampling plan:
Sample Size 38 48 60
Acceptance Number 5 6 7
SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 46 of 192
Deviants/Worse than Deviants
A. Multiple Deviants on a Single Sample Unit
Many U.S. Standards provide allowance for several kinds of deviations within a
single quality factor. Related deviations on a single unit should be counted only
once. Unrelated deviations should be counted for each deviation. This procedure
should be followed whether the product has a score point or an attribute standard.
Examples:
Related defects ("off-suture" and “partially detached piece") on a single peach
half:
Count the unit against partially detached pieces. Don't count the unit
again under off-suture.
Unrelated defects ("off-suture" and "poor color") on a single peach half:
Count the half twice, against both off-suture and poor color.
B. Administrative Allowances for “Worse than Deviants”
This instruction outlines the Division policy of accepting “worse than deviants”
for quality. This does not apply to sample units containing foreign material
(GNC).
The Division Regulations do not provide any tolerance or acceptance criteria to
cover sample units that are “worse than deviants.” However, administrative
discretion permits an infrequent occurrence of a sample unit that is “worse than a
deviant” with respect to quality factors.
1. “Worse than Deviant” Allowance
An infrequent sample unit - 1 in 48 - will be permitted to be “worse than
deviant.”
A sample size of 48 is not typically graded during in-plant inspection, and
the maximum sample size for lot inspection is 29. So a “worse than a
deviant” would not be acceptable under normal circumstances. However
the applicant may request that additional samples be drawn from the lot to
make up to 48 samples. If these additional samples contain no further
“worse than deviant” units, the lot is considered acceptable.
2. Application of Allowance
If a lot meets the 1 in 48 infrequent occurrence criteria, the offending
factor is scored at the lower limit permitted for a deviant for the grade, and

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 47 of 192
included with other deviants for lot acceptance and certification.
Example:
If a sample unit in a Grade “A” lot is “Substandard” account maturity, it is
considered a “worse than deviant.” If a total of 48 units are graded and
contain no additional “worse than deviant” samples, the offending sample
unit is scored at the lower limit of the Grade B range for maturity, and
included with other deviants for lot acceptance and certification.
3. Segregation and re-inspection
a. In-Plant
If an item fails by having two or more sample units in 48 that are
“worse than deviants,” the packer may segregate the lot and offer it
for re-inspection if:
(1) It is consecutive production not containing any “worse than
deviants” and
(2) The portion is separately identified by sub-code from any
portions containing “worse than deviants” or
(3) It is a sub-code with only one “worse than deviant.”
Example:
21 sample units were graded from a product consisting of 8 sub-
codes. The item fails because it contains a “worse than deviant” in
sub-code 7.
If the applicant requests, a total of 48 samples may be graded. If
no additional “worse than deviants” are found, the lot is accepted.
If another “worse than deviant” is found in the additional sample
units, for example in sub-code 2, the lot fails, but may be
segregated and re-inspected as follows:
Assume 6 sample units have been graded from each sub-code.
• No sub-codes may be passed without further examination.
• Sub-codes 3, 4, 5, and 6 may be combined per a. (1) and (2)
above. Twenty-four more sample units (to total 48) from these
four codes must be graded.
• Since they are not consecutive sub-codes per a. (1) above, 1

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 48 of 192
and 8 must be examined separately, with an additional 42
sample units checked from each sub-code.
• Sub-codes 2 and 7 must also be examined separately, each with
an additional 42 sample units graded. No additional “worse
than deviant” is permitted in either of the two codes.
b. Lot Inspection
If a lot fails by having 2 or more sample units in 48 that are “worse
than deviant,” the applicant may segregate the lot and offer it for
re-inspection if:
(1) It consists of code marks not containing any “worse than
deviants”, or
(2) It is a code mark with only one “worse than deviant.”
Example:
21 sample units were graded from a lot consisting of 6 code marks.
The lot fails because one “worse than deviant” is found in code A.
If the applicant requests, a total of 48 samples may be graded. If
no additional “worse than deviants” are found, the lot is accepted.
If in the 27 additional sample units any additional “worse than
deviants” are found (for example, one in code A and one in code
C), the lot fails, but may be segregated and re-inspected as follows:
Assume 8 sample units have been graded from each code.
• No codes may be passed without further examination.
• Codes B, D, E, and F may be combined per b. (1) above.
Sixteen more sample units (to total 48) from the 4 codes must
be graded.
• Code C must be examined separately with an additional 40
sample units graded. No additional “worse than deviants” are
permitted.
• Code A may not be re-inspected, as it already has 2 “worse
than deviants” in 48 sample units.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 49 of 192
Scoring Individual Quality Factors
Most score point grade standards evaluate and score each quality factor independently. Some of
these factors are closely related. Most attributes grade standards evaluate the quality factors
collectively. However, some factors (e.g., color in frozen field peas) are set to separate sampling
plans in attribute standards.
Unless otherwise cited in the standards or grading manuals, evaluate each quality factor without
consideration of other quality factors.
Alien Vegetables
The following guidelines provide for the uniform classification of small units of "alien"
vegetables (e.g., diced turnips in diced carrots, corn in peas, lima beans in speckled butter beans,
etc.) when accidentally included with other vegetables.
The standard sample unit size shall be 10 ounces (284 grams), or multiples of 10 ounces. If
multiples are used, divide the number of 10 ounce increments to obtain the average number of
"alien" units per 10 ounces.
Unless the U.S. Standards for Grades provide for "dissimilar varieties" (e.g., frozen field peas
and frozen black-eyed peas), classify each offending alien unit as minor, major or severe.
A. Minor
Similar in size, shape, and color, such as lima beans in butter beans, chopped kale
in chopped collards, diced parsnips in diced turnips.
B Major
Similar in size and shape, but different in color, such as diced carrots in diced
turnips, lima beans in speckled butter beans.
C. Severe
Different in size, shape or color, such as corn in peas, diced carrots in cut green
beans, black-eyed peas in lima beans.
Products that contain alien vegetables shall be considered as meeting the labeled component(s) if
all the allowances in the U.S. Standards for Grades (if any) are satisfied, and the tolerances in the
table below are met.
Total (Minor + Major + Severe)
Major Severe
Average number per 10 ounces
6 2 1

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 50 of 192
Inspection Procedures for Fruit Concentrates, Nectars, and Purees
This instruction provides general guidelines for the uniform inspection of fruit concentrates,
nectars, and purees. Similar techniques may also be applied to vegetable purees, which may not
be covered by a U.S. Standards for Grades. However, there are Commercial Item Descriptions
(CID's) that have established salient characteristics and product requirements related to these
products. CIDs may be found on the USDA, SCI home page at the following internet address:
http://www.ams.usda.gov/AMSv1.0/processedinspection. From the SCI homepage, in the “I
Want To” box on the top right of the screen, click on the “Find a Standard or CID” link. This
will take you to the current CIDs available.
A. Definitions
1. Fruit Concentrate
Pulped and/or chopped fruit from which a substantial portion of the water
has been removed. The Brix reading of the product is at least two times
that of the juice of the fruit.
2. Fruit Nectar
A pulpy fruit drink prepared by blending fruit juice and/or pureed fruit
pulp or whole fruit equivalent with water or syrup to a specified fruit
content by weight, usually in the range of one-half by weight of fruit pulp.
3. Fruit Puree
Pulped and screened fruit which may or may not have a portion of the
water removed. The Brix reading of the product is usually less than two
times that of the juice of the fruit.
B. In-plant inspection of raw materials
Observe the quality and condition of the raw product used to prepare the finished
product. Good initial product wholesomeness should lead to good processed
product wholesomeness.
Observe the effectiveness of the washing process in removing dirt and other loose
material, and the effectiveness of the trimming and removal of decayed and
unsound fruit. Report any deficiencies to the appropriate plant contact person.
C. Non-Quality Factors
1. Fill of Container
Be sure to measure the headspace of any containers with low net contents
to determine if they meet the recommended headspace allowance.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 51 of 192
2. Brix or Refractive Index
Determine the refractive index on all quality samples.
3. Total Acidity
The total acidity (anhydrous citric acid) may be important to the end user.
When total acidity is determined for concentrates and purees, express the
result as wt/wt; when determined for nectars, express the result as wt/vol.
D. Quality Factors
1. Color
Determine the factor for color on the finished product.
Procedure:
Nectars Pour a well mixed aliquot into a 1¼"-1½" diameter
glass cylinder for color evaluation.
Concentrates Dilute sample to same Brix used for defect
and Purees determination. Pour a carefully diluted aliquot into
a 1¼"-1½" diameter glass cylinder. Consider both
the diluted aliquot and the finished product when
making color determination.
"Good color" means that the color is bright and typical, and is free from
any slight brown or dull color due to oxidation, scorching, or other causes.
"Fairly good color" means that the color is typical and may have a slight
brown color due to oxidation, or may be slightly dull.
"Poor color" means the product is off color for any reason.
Color descriptions for "Good" color (also see applicable CID for color):
Apricot A bright orange color, free from brown cast.
Peach A bright color ranging from light-yellow to orange-yellow,
free from any material dullness or green tinge.
Pear A color ranging from yellow-white to beige-white. A slight
dull tinge is typical. The color shall be free from any
material dullness or dark cream tinge.
Apple A color ranging from a whitish to a golden yellow, or may
show a slight green tinge characteristic of the variety. A
good color is normally slightly translucent and shiny.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 52 of 192
2. Defects
a. Sample Size
Defects in these products usually originate in the fruit pulp. They
may be caused by poor trimming of the fruit, or from faulty
machinery such as broken screens, or improperly set brushes or
paddles in the finishers. Other defects such as dark specks may
come from burned coils or improperly cleaned tanks or machinery.
For all nectars, use a 200 gram sample of the finished product for
defect determination. For all concentrates and purees, use a 100
gram sample after dilution to the following Brix level for defect
determination.
Apricot 14.5 ± 0.5 degree brix
Peach 11.0 ± 0.5 degree brix
Pear 13.5 ± 0.5 degree brix
Apple 14.0 ± 0.5 degree brix
Nectarine 11.0 ± 0.5 degree brix
Prune 19.0 ± 0.5 degree brix
b. Evaluation
Pour the sample into an approximately 12x19 inch large, shallow,
white bottom tray, and spread the material evenly over the bottom
surface. Use the table below as a guide to evaluating defects.
Defects Practically Free 1/ Reasonably Free 2/
Particles of pit exceeding
1
/
16
1 3
inch in greatest dimension
Dark specks exceeding
1
/
16
but 1 4
not
1
/
8
inch in greatest dimension
Dark specks exceeding
1
/
8
but not 0 1
1
/
4
inch in greatest dimension
Total of above, plus dark specks 12 36
1
/
32
to
1
/
16
inch, light specks
exceeding
1
/
32
inch, and particles
of pit
1
/
16
inch or less
1/ Total of above, plus any other defects that do not materially affect appearance
2/ Total of above, plus any other defects that do not seriously affect appearance

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 53 of 192
3. Flavor and Odor
Good flavor and odor means that the finished product shall have a normal
flavor and odor, characteristic of ripe and properly processed fruit, and
shall be free from any objectionable flavors and odors including traces of
bitter or immature flavor.
E. Analysis
Prepare the sample for mold analysis in accordance with instructions in the AIM
Inspection Series, Foreign Material Manual.
Products Packed with Sauce, Garnish, or Seasonings
The following instructions concern Division policy on the applicability of the U. S. Standards to
products which have added sauce, garnish, or seasonings, whether or not specifically mentioned
in the standard. The same principles apply to all methods of processing.
Examples:
Frozen Peas with Petite Onions
Frozen Broccoli with Cheese Sauce
Canned Asparagus with Butter Sauce
Because the U. S. Standards do not generally include sauces and garnishes as a part of product
description, misunderstandings have led to incorrect certification of such products as “No
Applicable Grade.”
A. Policy
Division policy is to consider the U. S. Standards for the principal item as
applicable to those products packed "with" or "in" or "seasoned with" another
ingredient. Consider the U. S. Standards for both items as applicable to those
products packed "and” another ingredient.
Examples:
For “Frozen Peas with Onions” the primary ingredient would be considered peas,
and the U. S. Standards for Frozen Peas would be applicable. Onions would be
considered a garnish.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 54 of 192
For “Canned Peas and Onions” the product is considered a “mixture” of two
principal items packed together. In this case, each component should be graded
on its applicable standard. In this example, the product would be certified as:
PRODUCT: CANNED PEAS AND ONIONS
GRADE: PEAS – U. S. GRADE A or U. S. FANCY
ONIONS – U. S. GRADE B or U. S. EXTRA STANDARD
B. Inspection procedure
It may be difficult to grade certain products because of the presence of a sauce,
seasoning or garnish. In general, these should be removed by washing and
draining from the main product prior to grading. Then the principal ingredient
can be evaluated in accordance with its U. S. Standard. Observing the quality of
various ingredients prior to mixing can be helpful under in-plant inspection.
Consideration should be given to the effect of the garnish or seasoning on the
appearance and eating quality of the end item. For example, if lima beans are
packed in butter sauce that has a rancid flavor which carries over into the end
product, certify the product as substandard.
Example:
GRADE: SUBSTANDARD account off-flavor (rancid flavor in butter sauce)
Some sauces may contribute a flavor that one inspector finds distasteful, such as a
pungent or sharp cheese sauce. This would be normal for the particular sauce and
should not be "flagged" because of the inspector’s personal likes or dislikes.
C. Official marks
Our policy permits grade labeling and use of official marks on products
considered to be covered by the applicable U. S. Standard for the principal
ingredient.
Those using official marks on such products need to furnish the Division with
some indication that the ingredients used are wholesome and of good quality.
Under in-plant inspection, it is frequently possible for the inspector to verify that
the in-going ingredient is of acceptable quality. In some instances it may be
necessary to secure a grading certificate or other official document from another
commodity program (e.g. Dairy) indicating that the added ingredient is a
wholesome product.
SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 55 of 192
Emerson Good Samaritan Food Donation Act
The Emerson Act is Public Law 104 – 210. By giving the Act the full force and effect of law, it
serves to encourage the donation of food and grocery products to nonprofit organizations for
distribution to needy. The full text of the Emerson Good Samaritan Food Donation Act may be
found at the following internet address: http://www.gpo.gov/fdsys/pkg/PLAW-
104publ210/html/PLAW-104publ210.htm.
Disposition of Samples
Division employees must exercise ethical standards and proper conduct in the disposition of
samples of processed products. Failure to comply with product disposition procedures as
described in these instructions may result in appropriate disciplinary action.
Under no circumstance shall any Division employee be permitted to remove any product
for personal use from any processing plant, grading office, inspection point, or from any
other site where official grading is performed. This includes product from containers that
have been opened as well as product from unopened containers.
The Regulations Governing Inspection and Certification of Processed Fruits and Vegetables and
Related Products found in 7 CFR 52.12 (which may be found at the following internet address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CFR) states "Any
sample of a processed product that has been used for inspection may be returned to the applicant,
at his request and expense; otherwise it shall be destroyed or disposed of to a charitable
institution."
The area field office supervisor will make sure that all product is returned to the applicant or
disposed of in accordance with Division policy. A product disposition log will be established for
all grading sites when product samples are returned to the applicant or donated to a charitable
organization. Product disposition records shall be maintained for a period of three years.
The product disposition log may consist of entries made in a spiral notebook, or on sheets of
paper in a 3-ring binder. The log shall name the grading site and must include the following:
A. Date product returned/donated;
B. Product (example: canned green beans);
C. Quantity (example: 6 cases - various size containers);
D. Name of organization receiving samples; and
E. Signature of individual accepting the samples.
Contact your supervisor with any questions regarding the procedure for recording the disposition
of samples or the method used to dispose of samples. An example guide is shown on the
following page.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 56 of 192
Donated/Returned Product Disposition Log
Date Product Quantity
Organization
Receiving Samples
Signature of
Receiving Individual

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 57 of 192
IN-PLANT INSPECTION
In-Plant Inspection is the term applied to the type of inspection service in which a USDA
inspector is assigned to a specific plant under a formal contract. The Inspector will establish the
grade and condition of all products covered by the agreement, and performs other duties as
required by the contract or by administrative approval of the Division. In-Plant Inspection is
further designated as either Continuous Inspection (Type 1 or 2) or Pack Certification.
Note: The Quality Assurance Program (QAP) is a separate inspection program. See the
AIM Inspection Series, QAP Manual for QAP-specific information.
Types of In-Plant inspection
A. Continuous Inspection
Continuous Inspection (Type 1) is the type of In-Plant Inspection in which an
inspector is stationed in the plant during all stages of food preparation and
processing, during any re-packing operations, and during any operations which
will remove or change the original code placed on containers (e.g., re-labeling
frozen products when the code is perforated in the label). Aside from product
grading and certification, In-plant duties include:
1. Monitoring the plant’s Sanitation Standard Operating Procedure (SSOP) to
assure the sanitation policies are satisfactory and executed effectively;
2. Case stamping, or checkloading and condition of container when requested
by the management for certification;
3. If removal of “Approved Identification” marks is required, verify their
removal from container labels.
B. Plant-Assisted Continuous Inspection (PAC)
The PAC program (also known as Type 2 Continuous Inspection) is a voluntary
program designed to give facilities under continuous inspection an alternative
means of utilizing SCI personnel. The PAC program requires that at least one
SCI inspector be present in a facility whenever the facility is running. In this
respect, it is the same as Type 1 Continuous Inspection. However, PAC is
designed to utilize plant-supplied personnel to perform certain inspection duties
while under the direction of SCI personnel.
SCI inspectors are assigned to processing facilities with continuous year-round,
in-plant contracts after the plant meets the Plant Survey requirements listed in the
Regulations, 7 CFR 52.81, which may be found at the following internet address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CFR. A
SCI Plant Systems Audit is acceptable in lieu of the SCI Plant Survey for the PAC
program.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 58 of 192
This program will enable a processor to use SCI in-plant inspection procedures,
sampling plans, instructions, and acceptance and rejection criteria. Designated
grade marks and the “OFFICIALLY SAMPLED” stamp may also be used.
1. Guidelines for Development of a PAC Program
a. Letter Agreement
SCI and Plant management establish a mutual agreement on plant
assigned personnel and their duties. This agreement will detail the
assigned plant personnel’s specific job requirements and
responsibilities while under SCI direction. The details will be
outlined in a signed Letter Agreement, which will be maintained
on file by SCI. The original will be filed at the plant, and a copy
filed at both the area field office and the Regional offices.
b. Training
The plant provides employees with the basic training necessary to
perform their job responsibilities.
SCI will train and test plant personnel on performing specific tasks
according to guidelines in SCI instructions and procedures. The
results of the training and testing program will be used to evaluate
the plant employee’s performance and competency. We then
designate which job functions the plant employee may perform.
We work alongside plant employees to assure satisfactory
completion of assigned duties. We provide feedback to
employees’ supervisors, and may provide plant employees with
additional training.
We maintain records on plant personnel participating in the
program, and inform plant management if a plant employee
demonstrates unacceptable performance. Plant management may
select another employee to perform required tasks, and we will
assist with training of the new employee. The Letter Agreement
will be updated with the new employee’s name and his or her
assigned tasks.
c. Certification
Only SCI inspectors may sign USDA Certificates of Quality and
Condition, and USDA Certificates of Loading.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 59 of 192
2. Letter Agreement
A Letter Agreement will be prepared for the signature of the SCI Officer-
in-Charge and plant management. This will show the name of each plant
employee assigned to assist the SCI inspector, and each employee’s job
function(s) under PAC. An example of an acceptable Letter Agreement is
shown below. The tasks contained in the Letter Agreement will indicate a
brief description of the job function. The task may be unique to the
operation in a specific plant and for a particular product. Tasks for a plant
employee assigned to assist the SCI inspector in sampling and other
routine tasks may include but are not limited to the following:
a. Start of shift and during shift:
Sanitize equipment (food contact surfaces).
Draw samples from lines per SCI instructions.
Records time sample is drawn and container code mark.
Determines and records net weight of each container.
Records vacuum and quality score point values as directed.
Prepares sample for mold count as directed.
b. End of shift:
Cleans laboratory and grading equipment
Sanitize food contact surfaces.
The SCI Inspector-in-Charge shall file the original Letter Agreement with
the in-plant contract, and provide a photocopy to the area field office and
to plant management. The Letter Agreement must be updated whenever a
plant employee not listed on the document is trained and assigned a task to
assist the Inspector-in-Charge.
SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 60 of 192
United States Department of Agriculture
Agricultural Marketing Service
Fruit and Vegetable Program
Specialty Crops Inspection (SCI) Division
Letter Agreement – Plant Assisted Continuous Inspection Program
Plant Assigned Personnel
I (We) ____________________________, located at ___________________________________
Plant Official(s) printed name (Plant name and address)
_______________________________ hereby agree to assign the plant personnel shown below
to assist the Specialty Crops Inspection (SCI) Division inspector in performing certain routine
tasks as listed:
NAME TASKS DATE
ASSIGNED
EXAMPLE
The above employees will be under the direct supervision of the SCI Inspector-in-Charge in the
performance of the above tasks. The plant employee will have access to all written instructions
describing the procedure or method that is relevant to the job function, and if applicable, a job
instruction sheet will be prepared to describe the tasks.
_____________________________________ ______________________________________
By (Print & Sign Name of Plant Management) By (Print and Sign Name of Officer-In-Charge
_____________________________________ _______________________________________
Title Title
_____________________________________ _______________________________________
Date Date
EXAMPLE

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 61 of 192
3. Reporting performance
If the performance of a plant employee assigned to assist SCI under a
Letter Agreement is satisfactory, the Inspector-in-Charge can inform plant
management verbally. For any occasion that plant employee performance
is unacceptable, plant management should be notified in writing. A
memorandum should be prepared stating the name of the employee, the
task(s) that the employee is assigned to perform, and deficiencies in the
employee’s performance of a particular task. Recommendations for
achieving acceptable performance, such as additional training, should be
included. If it is necessary to remove the employee from an area, the
Letter Agreement should be revised immediately to remove the employee,
and a courtesy copy of the revised Letter Agreement sent to the Officer-in-
Charge. If plant officials refuse to remove an employee who has
demonstrated repeated unacceptable performance, contact your supervisor.
C. Pack Certification
Pack Certification is another contracted service of inspection and grading in an
approved plant. For this service, one or more inspectors may make inspections of
the preparation and processing of products under contract, but are not required to
be present at all times the plant is in operation.
1. Under a Designated Lot contract, inspectors will sample, grade, and
certify only those lots designated by the applicant.
2. Under a Quality Assurance (QA) contract, inspectors use information
available from the applicant’s quality control records to certify lots, as
requested. The inspector(s) grade lots at random as often as necessary to
verify the reliability of the applicant’s quality control system.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 62 of 192
The Role of In-Plant inspection
The primary purpose of in-plant inspection is to inspect and certify the quality of all products
covered by the agreement. Formal certificates are issued on those lots specifically requested by
the applicant or contractor. Other advantages of in-plant inspection include:
A. Rapid grading and certification on the applicant’s premises. Since inspectors are
available to certify designated lots upon request, results may be obtained quickly,
which is a significant advantage.
B. Assisting the applicant in maintaining desired quality levels. Although USDA
inspectors are not to function primarily as quality control personnel, their duties
are often closely integrated with the plant quality control program. Their
constructive suggestions may assist in achieving and maintaining good quality.
C. Assuring compliance with specifications or contract requirements for those
factors that can only be readily checked by in-plant observation. Such factors
include the proportion of fruit in preserves and jellies, the time and temperature of
blanch, the fruit-sugar ratio in frozen fruits, the fill weights for canned fruits, and
domestic origin requirements.
D. Maintaining regular checks on plant sanitation and condition of raw materials.
E. Assisting in compliance with Food and Drug Administration (FDA) regulations
and requirements of state and municipal regulatory agencies.
In addition to the above, plants operating under an In-Plant Inspection Contract may use
approved inspection and/or grade marks on their labels and in their advertising programs, as
specified in the Regulations.
Services provided by the Applicant under In-Plant inspection
In-plant inspection services provided by the applicant include but are not limited to the
following:
A. Adequate product identity and quality segregation, maintained from the plant to
warehouse by means of proper coding and warehousing.
B. Adequate office space adjacent to the laboratory facilities with suitable desks and
office equipment for the proper care and security of inspection records.
Equipment shall include a lockable drawer, file cabinet, or locker.
C. Access to office equipment to maintain or communicate records, and clerical
assistance as needed to issue certificates. If the plant has a computer network
established within the premises, USDA inspectors should be permitted network
access to facilitate communication between plant management and USDA.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 63 of 192
D. Access to processing, packaging, and production reports. These are vital to
sampling and preparation of daily inspection reports and certificates.
E. Janitorial service for the laboratory and office. This may include the washing of
pans and other inspection equipment. The inspector should encourage this as
standard practice.
Note: Laundry service is sometimes provided by plants, which may include furnishing
towels or lab smocks and coats. However, this is not considered a required
service. The inspector is responsible for his or her own proper attire.
F. As appropriate, provide extra assistance to the inspector.
There may be circumstances under which the inspector requires temporary help
during an in-plant assignment. A request for a plant employee assigned to
perform specific tasks for the SCI inspector may be made to plant management.
Plant assigned employees must have a clear understanding of their job functions,
be well trained, and have access to required written procedures, methods, and
instructions related to job functions. Plant assigned assistance is a good
management tool that can enhance SCI's efficiency.
Assigned plant employees are under the direct supervision of the SCI inspector in
charge. To verify accurate performance of duties, the Inspector must observe the
plant employee performing designated tasks several times daily. If a task is
performed incorrectly, the inspector in charge will document the error and the
time it occurred on the reverse side of the score sheet, tally sheet, or on a separate
memo. The Inspector will inform the area supervisor of the problem, and counsel
the plant employee and/or provide additional training to ensure the task will be
performed properly in the future.
If the assigned task is performed incorrectly after the employee has been
counseled and/or trained, advise plant management of the situation and request
that they select another person capable of performing the required task(s).
Note: These instructions are not applicable to the assignment of plant personnel
under the Quality Assurance Program (QAP) and other Division Verification
(Audit) Programs.
A letter agreement will be prepared for the signature of the plant official to show
the name of each plant employee assigned to assist the SCI inspector and the
employee's job function(s). Please see “Section B, Part 2, PAC, Letter
Agreement” under “Types of Pack” for examples and descriptions.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 64 of 192
Basic Facility Requirements
The minimum, basic facilities for adequately performing inspection duties are required for all
types of In-Plant Inspection services. These requirements vary between plants, depending on
plant size, quality and quantity of production, nature of the products packed, and length of
packing operation. A plant under a seasonal contract may not be as elaborately equipped as a
plant under year-round inspection, but the basic needs still must be met. The lab should be well
organized and large enough and to adequately house the equipment needed to perform the
required inspection procedures.
The plant shall provide the following equipment and materials as needed:
1. Grading table with adequate and approved lighting.
2. Grading trays of sufficient size.
3. Accurate scales.
4. Potable water source.
5. All necessary lab supplies, such as glassware, chemicals, and handling
equipment.
6. Any other miscellaneous equipment such as specifications and inspection
aids.
7. The lab should have adequate counter space to perform all required
inspection duties, and be designed to be cleanable.
A. Approved Lighting
When grading processed fruit and vegetable products, approved lighting sources
are used to make critical color evaluations and comparisons. USDA approved
lighting sources include the following (other sources may be used if the alternate
sources are equal to or better than those listed; and approved through the National
Office):
• Macbeth Examolite Model TC-440 B (no longer available; replaced by
Examolite SD-840)
• Macbeth Examolite EBA-220 Light Box (no longer available; replaced
by Macbeth Spectralight II 75B no longer available; replaced by
Macbeth Spectralight II); and
• Macbeth Munsell Disk Colorimeter (no longer available)

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 65 of 192
1. Macbeth Examolite Model TC-440 B
Properly installed, the Macbeth Examolite Model TC-440 B is designed to
provide a color and spectral quality of illumination close to north sky
daylight at 7500 degrees Kelvin. These approved lighting fixtures deliver
approximately 100 foot candles of intensity, and are suitable for table
lighting of most routine grading activities. Two diffusing glass
replacement panels are available; the water white prismatic tempered glass
panel and the optional matte white, UV stabilized, 1/8-inch acrylic panel.
a. Installation
Ideally, two or more units are suspended from the ceiling,
approximately 4 feet above the surface of the table. It is
recommended that light from the fixtures overlap the table by 1 to
2 feet on all sides. If this is not feasible, you should confine your
color judging to the area of the table that is well illuminated by the
light source.
b. Operation
The walls and ceiling of the grading room should be painted a light
neutral gray, preferably to match Munsell N8. Color chips
matching this color can be obtained from the Macbeth Corporation,
or paint matching this color can be purchased from your local paint
supplier. Walls should be painted with a flat or eggshell luster.
Interfering sources of artificial light should be turned off, and
direct rays from the sun should be avoided when using the
Examolite. If the room contains windows adjacent to the grading
table, the light from the windows should be sealed off by either
painting the panes or using tight fitting light gray colored shades,
venetian blinds, or drapes.
c. Maintenance
The Macbeth Examolite fixture has been purposely designed
without a ventilation system, and is not absolutely dust tight. Dust
and lint will change both the intensity and the color of the lighting.
Therefore the entire unit, reflector, lamps, tubes and diffusing glass
should be cleaned at least every six months, and more often under
dusty conditions. To clean, the diffusing glass is removed and
washed with soap and water, then thoroughly rinsed and dried so
that no film of soap remains on the glass.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 66 of 192
The interior of the Macbeth Examolite fixture is painted with the
same baked finish that is used on a refrigerator. Fixture surfaces
should be washed with soap and water at the same time as the
lamps and tubes are cleaned.
The Macbeth Examolite fluorescent tubes maintain their intensity
and color better than other fluorescent tubes, and have a life of
approximately 7,500 hours. However after 6,500 hours, there is a
considerable change in all fluorescent tubes. The special
Examolite incandescent lamps have a life of approximately 5,000
hours. To maintain the standard color quality and intensity of
illumination, all tubes and lamps must be operating. Any burned
out lamps and/or fluorescent tubes must be replaced before
operating.
Depending on usage, all fluorescent tubes and incandescent lamps
should be replaced at least every 2 years. Keep a log card or
attached sticker on each unit showing when the fixture was last re-
lamped so the maintenance schedule can be maintained.
d. Supplies
(1) Fluorescent lamps - 7500K (CIE D75)
40-watt rapid-start Macbeth F40T12/EX
Part No. 59006230
(2) Incandescent lamps -
33-watt Macbeth 33WA19/EX long-life
Part No. 59006240
(3) Prismatic "Water white glass" diffuser
Part No. 27005110
(4) Matte White UV Stabilized Acrylic diffuser
Part No. 27004410
Available from:
X-Rite Incorporated, Munsell Color Services www.xrite.com
4300 44th Street SE, Grand Rapids, MI 49512
Telephone: (877) 888-1720, Fax: (616) 803-2076, (800)-248-9748
The following list of fluorescent lamps are designed to provide
color and spectral quality of lighting equal to or better than the
Macbeth lamps for official grading of processed fruit and
vegetable products. If it becomes necessary to replace the
fluorescent lamps in the Macbeth fixture with lamps other than

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 67 of 192
Macbeth, remove the incandescent lamps from the fixture. It is not
necessary to remove the lighting diffuser from the Macbeth fixture.
(1) For lamps using T12 and T16 electronic ballasts:
Color Classer 75 http://www.durotest.com/fluor.CM.html
12401 McNulty Road, Suite # 101
Philadelphia, PA 19154
Sales: 1-800-289-3876 Ext. 4269, Fax: 1-888-959-7250
Customer Service: 1-800-289-3876 Ext. 2117
E-mail: info@duro-test.com
(2) For lamps using T8 electronic ballasts:
F32T8/75 (48”, 32 watts, 7500K)
F25T8/75 (36”, 25 watts, 7500K)
F17T8/75 (24”, 17 watts, 7500K)
Visual Color Systems, Inc.
http://visualcolorsystems.com/index.html
PO Box 93, Mountaindale, New York 12763
Phone: 1-845-434-2646, Fax: 1-845-434-6115
(3) General Electric Chroma 75 or Sylvania Designer 7500K
Purchase at a local G.E. lighting supplier
(4) Ballast (if needed):
Purchase at a local electrical supply house
Match to lamps being used (T8, T12, etc.)
2. Macbeth Examolite EBA-220 Light Box (no longer available) and
Macbeth Munsell Disk Colorimeter (no longer available)
The Macbeth Examolite Light Box Model EBA-220 and the Macbeth
Munsell Disk Colorimeters are designed to deliver in excess of 150 foot
candles of intensity. These units are used to make critical color
evaluations and comparisons.
a. Installation
The unit should be installed in a place that is relatively free from
most extraneous light. If this is not possible, temporarily close out
daylight, or turn off other inside lighting when the unit is in use.
Place the lighting unit on a shelf or a table at a height that will
allow easy viewing by the inspector. The unit should be plugged
into an alternating current receptacle that will deliver from 105 to
115 volts without radical fluctuation. Before installation, select a
receptacle that is not on the same circuit with equipment requiring
a heavy intermittent load.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 68 of 192
b. Maintenance
These units may deliver varying degrees of illumination due to
deterioration. To minimize deterioration, use the following
schedule:
(1) Completely re-lamp the unit at the beginning of each new
processing season;
(2) Remove the diffusing glass and carefully wash with soap
and water. Make sure the glass is reinstalled in the unit
with the waffle pattern UP, the fluted pattern DOWN and
the ribs of the flutes PARALLEL to the fluorescent tubes.
(3) Over the years, the gray paint on the sample holders, and
perhaps the box holders, will wear. Repaint them as
needed. Use the same paint color instructions as previously
described in "Operation" under the Macbeth Examolite TC-
440 B section.
Use extreme care while handling and cleaning the two
Corning blue glass filters of the Disk Colorimeter. These
filters provide the proper color temperature as well as the
required foot candle intensity.
c. Supplies
(1) Fluorescent lamps for EBA-220; 20 watt, 120 volt
F20T12 CEWX
(2) Incandescent lamps for EBA-220; 15 watt, 120 volt, inside
frost 15A-15IF
(3) Incandescent lamps for Disk Colorimeter
R46-300 watt reflector flood lamps
The above supplies may be available from your local electrical or
lighting company.
3. Ordering supplies
Take advantage of available discounts when purchasing the fluorescent
tubes and incandescent lamps noted above. These can often be purchased
on a carton basis from your local electrical supply house or lighting fixture
company. However replacement diffuser glasses are available only from
the supplier of the lighting fixture.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 69 of 192
The SCI Inspector
Since the SCI Inspection Service is a service organization, there are certain expectations of SCI
Inspectors working under In-Plant assignments.
A. Service versus Regulatory Authority
SCI inspectors do not have regulatory authority. Our organization does not
“shut down the plant.” This role is in the hands of various State agencies and the
FDA. SCI inspectors will perform their duties within the scope of their authority.
As a service provider, the Division serves to assist processors and other applicants
by offering third party, independent grading and certification services. An
inspector may diplomatically offer suggestions and alert processors to the
existence of regulatory issues. However, the inspector’s primary function is to
inform the applicant of the quality and condition of products inspected, and to
issue various reports as needed.
B. Competence
Inspectors are expected to be well versed on official procedures relevant to the
needs of the plant, and be able to provide accurate information to plant
management. They must keep up-to-date on instructions issued by the Division,
and be completely familiar with the appropriate U.S. Standards, U.S. Code of
Federal Regulations, applicable state statutes and other rules, regulations, and
specifications pertinent for the particular plant. The inspector must have a good
knowledge of the functions of plant equipment, operations, and procedures
C. Decisions
The decisions made by inspectors may affect the methods of operations and have
a financial impact on the applicant. These decisions should be timely and limited
to the grading and inspection service performed. Every effort should be made to
ensure that an assigned grade is correct. When monitoring plant operations,
inspectors should seek to identify production problems such as mislabeling,
incorrect codes and under-filling of containers as soon as possible. Many
situations can be addressed by applying current instructions. However, if
circumstances that are not addressed arise, contact your supervisor immediately.
D. Uniformity
A processor expects to receive the same service - impartial, uniform interpretation
and application of U.S. Standards, specifications, and regulations - as any other
applicant operating under In-Plant Inspection. Inspectors are to promote a “level
playing field” by applying current guidelines equitably to all applicants.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 70 of 192
E. Demeanor of Inspectors
Inspectors shall:
1. Demonstrate tact and a reasonable, level temperament;
2. Maintain confidentiality of company processes, formulations, operations
and other proprietary information; and
3. Present a neat, professional appearance.
F. Appropriate Workplace Attire Policy
The purpose of this policy is to clearly define the standard of attire expected of
SCI employees.
SCI employees need to project a professional image in keeping with our standing
in the food industry. Clothing should be clean, neat, modest, and contribute to a
professional appearance. Footwear should closed-toed with closed heels. In
certain working conditions, inappropriate clothing and footwear can pose safety
hazards to employees. It is therefore particularly important that attire be
appropriate for the employee's work environment without hindering the
performance of their duties.
Inappropriate attire is any clothing that is unclean, unkempt, torn, frayed, or does
not project a professional image as normally acceptable in the workplace. In
particular, any clothing that reveals underwear or personal body areas (i.e.,
midriffs, cleavage, buttocks, bare shoulders, underarms etc.) is inappropriate.
Some examples of inappropriate attire include sweatpants, pajamas, exercise
pants, spandex, shorts, see-through clothing, short or tight skirts, spaghetti strap
dresses/tops, tank tops, muscle shirts, t-Shirts (except as stated below), fishnet-
type shirts, sports jerseys, beach-type clothing, jeans with holes, excessively
baggy clothing, and flip-flops.
Because of the requirements of their duties, there are additional guidelines for
inspectors performing in-plant assignments. In plant inspectors are required to
wear white from the waist up, or a white lab coat or smock. Inspectors shall not
wear t-shirts unless required by the type of work environment (e.g., incoming
raisin inspection), or mandatory plant-specific policies (e.g., no clothing with
buttons allowed in the plant). Under these circumstances, t-shirts worn must not
display writing or graphics, with the exception of an official USDA logo. In a
facility that maintains a no-button policy, the inspector is still required to wear a
smock or lab coat.
While working lot inspection or in-plant, inspectors shall follow Food and Drug
Administration, current Good Manufacturing Practices. This includes wearing
bump hats or hard hats, and following proper practices regarding hair restraints
(hairnets and beard nets, if applicable), jewelry, and nail polish. No open-heeled

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 71 of 192
or sandal-type shoes shall be worn. Inspectors shall also follow any additional
plant-specific policies as appropriate.
Remember, our customers are paying for our services. They deserve service
provided by clean, well-groomed, properly-dressed professional USDA
employees. We need to be the ones setting a good example - anything less is
unacceptable. If you have any questions concerning this memorandum, feel free
to contact your supervisor or the Regional/National offices.
G. Inspector’s Guidelines for Acceptable Behavior
The following provides guidance and reminders to inspectors to ensure effective
delivery of service:
Above all, BE COURTEOUS.
Keep matters on a businesslike basis.
Be prompt in reporting to duty. It is always a safe practice to be 15 minutes early.
Be friendly, but not overly so.
Know the rules for ethical conduct and ensure they are followed. Refrain from
inappropriate language, gestures, and actions.
Do not visit with inspectors in other plants unless you have business to discuss.
Avoid non-professional behavior.
As a representative of the Department, be aware of how your official activity is
perceived when in plant. Avoid watching television, reading a newspaper or
magazine, using cell phones for non-emergency personal calls, operating personal
electronic equipment, and other activities which are not service oriented unless
special circumstances exist.
If you are in a situation which could cause embarrassment to you and/or the
Department, please remove yourself from it and/or contact your immediate
supervisor for assistance.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 72 of 192
Points of Emphasis
DO
Do establish with plant management the chain of command of individuals to
whom information pertaining to our inspection service is to be given and the
specific information to be given each such individual.
Do report the failure of machinery or equipment to the attention of plant
management---immediately.
Do report to the plant management marked changes in any of the quality factors
or in such things as fill, net weight, vacuum, labeling issues, and syrup densities.
Do report to the plant management any sanitation shortcomings. Follow through
for prompt plant action since monitoring of sanitation for compliance with GMPs
is one of the principal functions of In-Plant Inspection Service. Document all
Sanitation deficiencies and corrective actions. Unsatisfactory conditions at a
facility are very serious matters and can result in a recommendation of withdrawal
of service if not properly addressed by plant management.
Do be familiar with U.S. Grade Standards, applicable Commodity Specifications,
FDA regulations, and State requirements.
Do be diplomatic when talking with plant personnel. Use an organized approach
to communicate issues and problems to plant management. Use tact when
making any suggestion or constructive criticism with management. Choose a
time which will not interfere with plant operations and will not cause personal
irritations. TACT, when dealing with plant management and personnel, cannot be
over stressed.
Do develop communication channels, which may include established times and/or
means, which facilitate efficient sharing of information with appropriate plant
contacts.
Do deal with people with whom you come into contact as a result of your official
duties with dignity, courtesy, and respect.
Do inform the IIC or your supervisor of any significant plant issues whether they
are resolved or unresolved.
Do accompany FDA inspectors on plant tours. Avoid becoming involved in
controversies between FDA and plant management. Maintain a neutral but
cooperative attitude. Thoroughly familiarize yourself with current instructions
pertaining to FDA plant inspections. Notify your supervisor when you become
aware of an upcoming visit by FDA to the plant.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 73 of 192
Do ask qualified plant personnel to open complicated equipment, if necessary for
you to draw samples or check sanitation, rather than to open it yourself. (Follow
Company Lock Out-Tag Out Procedures)
Do follow all company safety and operating procedures and policies.
Do ensure the accuracy of all official documents
DO NOT
Do not make recommendations, suggestions, or criticisms of any degree or kind to
the employees of the plant. These should be made only to the plant management
or designated personnel as appropriate.
Do not incorporate recommendations, suggestions, or criticisms in the DIR.
Do not give instructions to the plant management on changes in the packing
operations that you believe should be done. Instead, offer these as suggestions for
their consideration. Their decisions shall be considered as final.
Do not give instructions to any plant employees except assigned plant assistants
who work under your direction.
Do not take it upon yourself to stop, slow down, or speed up the operations of
production equipment for any reason. Explain the situation to the management
and leave that decision to them.
Do not assert that you have regulatory or “police” authority.
Do not repair or adjust production equipment for any reason. Explain the
situation to the management and leave that decision to them.
Do not engage in arguments. If you disagree with someone on a particular matter,
you may make your opinion known, if appropriate with regard to the
circumstances, but it must be done in such a way that it does not alienate or
intimidate the other party.
Do not enter into discussions between the processors and growers regarding the
quality of the raw product or other matters.
Do not punch a plant time clock. Follow set T&A procedures established by the
Division.
Do not accept gratuities or bribes. Follow Division/Program/Agency/Department
steps to report such activity.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 74 of 192
Do not give information acquired through your official duties at a plant to anyone
not connected with the plant except your supervisors. Maintaining confidentiality
of plant information is required under the Trade Secrets Act.
Do not allow visitors to interfere with your work.
Do not smoke in the plant or warehouse, only smoke in designated areas.
Do not bring visitors, outside inspectors, or family members into the plant unless
plant management is consulted and approval is given. Be sure work will be
carried on in your absence before taking time to show them through the plant.
Do not make statements without being sure the information is correct.
Do not show official documents to non-designated plant personnel or leave such
documents where they might be seen by anyone other than designated plant
personnel unless authorized to do so by plant management.
Do not display or leave unattended information restricted to USDA personnel.
Confidential guides or correspondence should not be placed on desks or in other
places where they may be observed or copied by unauthorized persons.
Do not allow unauthorized access to USDA computers or computer equipment.
Do not share your USDA computer password(s) with others.
Do not use phones, including cell phones, for personal calls, including cell
phones, unless necessary and within Division guidelines.
Do not use personal electronic devices unless their use is in the delivery of
service.
Do not discuss plant business such as (but not limited to) finances, plant
processes, and production figures with anyone except designated plant personnel.
Do not conduct personal business during duty hours.
Do not abuse break privileges.
Do not conduct activities related to personal hygiene (e.g., shaving, brushing your
teeth, etc.), in the plant lab.
Do not wear unsecured jewelry or other similar objects.
Do not sleep at the plant, even during break periods.
Do not enter into controversial arguments regarding procedures or discuss plant
quality with outside buyers.
Do not enter into contentious discussions with plant personnel concerning
controversial topics (e.g., politics, religion, divisive current news events, etc.).

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 75 of 192
H. Current Good Manufacturing Practices (GMPs)
It is important to remember that while inspectors are working on an in-plant
assignment, they must follow all of the GMPs for the plant. This includes, but is
not limited to, such things as: the use of hair nets (and proper covering for
beards/moustaches if applicable); fingernails should be clean; nail polish should
not be worn; jewelry should not be worn. Pens, pencils, and other items should
not be in a shirt or smock pocket. The inspector should not create any other
condition or engage in any other practice that would be considered unacceptable
under the GMPs.
I. Employee Signature of a Firm’s Hygiene, Health and Confidentiality Statements
Specialty Crop Inspection (SCI) Division employees and Federal-State
Inspection Program (FSIP) cooperators while performing inspection and audit
activities must comply with a firm’s employee and visitor hygiene/sanitation
policies (i.e., use of hair, beard or head coverings, no jewelry, and wear coveralls
or protective clothing and boots as required). If required, you may read, fill out,
and certify a firm’s visitor hygiene/sanitation/safety policy statement.
We have been informed through our general counsel that we are not to complete
questionnaires or sign releases for health information. SCI Division policy
regarding personnel is in accordance with the Food and Drug Administration,
current Good Manufacturing Practices, 21 CFR 110:
Disease Control Any person who by medical examination or supervisory
observation is shown to have, or appears to have, an illness, open lesion,
including boils, sores, or infected wounds, or any abnormal source of
microbial contamination by which there is a reasonable possibility of food,
food-contact surfaces, or food packaging material becoming contaminated
shall be excluded from any operations which may be expected to result in
such contamination until the condition is corrected. Personnel shall be
instructed to report such health conditions to their supervisors.
SCI Division employees should not sign confidentiality statements from
applicants. We are not in a position to commit the government to confidentiality
statements. SCI Division has an established history of providing inspection
service and audit programs that have maintained the confidentiality of an
applicant’s information. Federal employees are generally prohibited from
disclosing confidential information by “United States Code: Title 18 – Crimes and
Criminal Procedure: Part 1 – Crimes; Chapter 93-Public Officers and Employees;
Section 1905, disclosure of confidential Information Generally. The penalties for
not adhering to this regulation are imprisonment, monetary fines and removal
from service.”
SCI Division policy is that that company plans and relevant company documents
reviewed during the audit will not be removed from the facility. Also documents
sent to SCI Division for review are to be stamped as "CONFIDENTIAL" and

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 76 of 192
remain the property of the company. When the stated purpose of the document
has been fulfilled the company can request that the documents be returned or
destroyed.
J. Management of Inspection Costs
Inspectors shall report their time and attendance and plant billing records
accurately, and take any steps needed to clarify and resolve questions that may be
posed by the applicant.
Inspectors should use Division and applicant resources efficiently. This applies to
supplies and equipment as well as personnel and staffing. Avoid wasting
supplies, samples from “cut-outs,” and the time of plant officials and employees.
Inspectors must be prudent, but also exercise judgment concerning equipment and
assistance needs. Consider plant resources and specific inspection needs before
requesting plant provided assistance.
During the course of the packing season, there may be slow shifts or periods at the
plant. The effective use of compensatory time or annual leave can help prevent
overstaffing with SCI personnel at these times. To help plan for such occasions,
inspectors should frequently check with production managers and stay informed
of any scheduling changes.
J. Constructive Suggestions and Advice
Inspectors may offer observations to processors on possible changes affecting
product quality, sanitation, and compliance with various contract requirements.
However, inspectors must maintain the confidentiality of the processor’s
proprietary information. All observations provided by an inspector are to be
limited to the activities of the plant, with no reference to or use of information
originating from another plant. Failure to do this is a breach of the Trade Secrets
Act, 18 U.S.C. § 1905, which can be found at the following internet address:
http://frwebgate.access.gpo.gov/cgi-
bin/usc.cgi?ACTION=BROWSE&TITLE=18USCPI&PDFS=YES.
K. Prompt Reporting
Inspectors should minimize any delays in reporting inspection results to the plant.
Sanitation issues and any other issues affecting the quality of product should be
reported immediately. However, some other operational concerns should be
reported to plant management within 24 hours from the time of observation.
Basic responsibilities under both Pack Certification and Continuous Inspection
are the same, with an emphasis on plant sanitation and product inspection. Under
a Quality Assurance type contract, emphasis is placed on verification of the
plant=s ability to assess product quality and sanitation.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 77 of 192
Authority of SCI Inspection Staff
The inspector is not in a position to require the processor or plant personnel to perform any
action. For example, the inspector cannot require the labeling crew to change labels or have a
line operator change codes. In areas concerning sanitation, approved identification, and direction
of SCI-assigned line check personnel or laboratory assistants, the inspector does exercise some
authority. However even in these areas, needs for desirable changes should be expressed
diplomatically, and the facts appropriately reported to the designated contact person.
• Inspectors are not authorized to start, stop, or otherwise “operate” plant
machinery.
• Inspectors must not threaten to withdraw inspection from the plant because of
nonconformance with contract requirements. Situations such as these should be
reported to the inspector’s supervisor.
• Inspectors should not overlook unsanitary conditions or practices that are illegal
or irregular. Inspectors should solicit the help of a supervisor in correcting such
deficiencies as needed.
• Inspectors shall not become involved with the hiring, firing, or supervision of
plant personnel. If plant personnel are assigned to assist the inspector, they shall
be directed only to the extent necessary to ensure that proper USDA inspection
procedures are being followed.
• Inspectors must not become involved in discussions or negotiations with plant
personnel, labor unions, government labor relations boards, packer-growers
relations, and similar groups or organizations.
• Except for others within the Department, inspectors shall not become socially
involved with people with whom they have an official relationship. Social
activities with plant personnel, such as luncheons or dinner entertainment should
be avoided. It is also inappropriate to date a plant employee, as this could be
perceived as a loss of impartiality. If you are in a situation that could cause
embarrassment to you and/or the Division, please remove yourself from it and
contact your immediate supervisor for assistance.
• An inspector may observe a plant pack and subsequently ship a product which is
Substandard, fails mandatory standards, or is GNC. The Division does not
condone this, but has no authority to restrict shipments unless approved
identifications are involved. Note that Division procedures for monitoring
disposition of any product considered adulterated must be followed, including
notification of FDA as applicable.
• The inspector must not attempt to grade the fresh or raw products used at the
plant, as this is the function of the Fresh Products Division. However, the

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 78 of 192
inspector should check the raw product for wholesomeness and presence of
foreign material, and may report these findings to the plant. If there is a
consistent problem with the wholesomeness of the raw product, the inspector
should contact his or her supervisor.
• The inspector must not order a plant to dump or refuse to accept unsatisfactory
raw material. This decision rests with plant management. However, this does not
preclude cautioning the plant that processing such material may result in an
unsatisfactory product.
Despite these limitations on the inspector’s authority, the inspector can exercise tremendous
influence on the operation of the plant. By being aware of unsatisfactory conditions and making
appropriate, timely reports and suggestions, the inspector can generally obtain prompt remedial
action. Inspection certificates and Daily Inspection Reports (DIRs) may be necessary for some
plants to promote sales and secure operating capital. These reports reflect the ability of the plant
to meet acceptable standards and specifications. Through these functions, the inspector has
considerable impact on plant operations.
Treatment of SCI Inspection Staff
As a representative of the Federal Government, and by demonstrating fairness, honesty, and
initiative, the inspector can expect to be treated with respect. The inspector must not be pushed
around or treated disrespectfully by plant personnel. If you are subject to unacceptable conduct
by a service user, contact your supervisor. Information on the situation must be provided
through proper Division channels to determine the appropriate course of action to take.
A. SCI stands behind its position regarding discrimination, unsafe working
conditions, intimidation, or manipulation by applicants during inspection
procedures. SCI will not tolerate actions or language from an applicant or an
applicant's agent that is intended to intimidate, threaten, interfere with, or harass an
inspector, or alter the course of an inspection. The subjective nature of these
situations requires each case to be considered on an individual basis to determine if
an action or statement is unacceptable, and if there is cause to deny grading or
inspection service.
The Regulations, 7 CFR 52.54, which may be found at the following internet
address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CFR,
states in part: "The following acts or practices, or the causing thereof, may be
deemed sufficient cause for the debarment, by the Administrator, of any person
including any agents, officers, subsidiaries, or affiliates of such person, from any
or all benefits of the act for a specified period. (3). Any interference with,
obstruction of, or attempted interference with, or attempted obstruction of any
inspector, inspector's aid, or licensed sampler in the performance of his duties by
intimidation, threat, assault, bribery, or any other means -- real or imagined.”
The Regulations, 7 CFR 52.11 states that: "The Director may withdraw grading

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 79 of 192
or inspection service from a person for a correctable cause. Such service, after
appropriate corrective action is taken, will be restored immediately, or as soon
thereafter as a grader or inspector can be made available."
B. Service may be denied for any of the following reasons:
1. Discrimination:
Derogatory remarks made to or about an inspector, or adverse actions that
are clearly taken by an applicant or an applicant's agent because of the
inspector's race, color, religion, sex, national origin, handicapped
condition, or age will be considered discrimination.
2. Unsafe working conditions:
A physical assault by an applicant or an applicant's agent toward an
inspector, or other working conditions that jeopardize an inspector's health
or well-being will be considered unsafe working conditions.
3. Intimidation:
Any undue pressure, influence, or action, or any verbal threat made by the
applicant or an applicant's agent to the inspector will be considered
intimidation.
4. Manipulation:
Any action by the applicant or the applicant's agent that is intended to alter
the outcome of an inspection by misrepresenting the facts will be
considered manipulation.
If an inspector encounters a situation where a reasonable person would assume
that any of the above conditions exist, the inspector should immediately leave the
work area. The Inspector’s immediate supervisor or someone in the chain of
command should be contacted immediately for further instructions. All pertinent
information shall then be relayed by the supervisor to the Regional Section Head
and/or the National office. The National office shall determine whether service
should be denied, or other action should be taken.
On all applications for inspection and in-plant assignments, the Officer-in-Charge,
Assistant Officer-in-Charge, or Sub-Area Supervisor shall base his/her decision
on which inspector to assign solely on the inspector's qualifications and
experience with the commodity or type of inspection to be performed. The
decision shall never be based on an applicant's stated or implied desire for or
against having certain inspectors on their premises.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 80 of 192
Automatic Samplers
The following guidelines provide for the uniform use of automatic samplers to draw
representative samples of juices and similar products of a comminuted, fluid, or homogeneous
nature. The main objective of the sampling process is to get the best possible representation of
product into the composite sample. Follow the guidelines below when using automatic samplers.
• The sampler shall be installed at a location where no further changes to product
will occur. It should be installed adjacent to either the loading or off-loading
location.
• It must be maintained in sanitary condition.
• The device is confirmed to sample from the production line.
• The sample size shall approximate a one quart composite, with a minimum of
twenty four fluid ounces.
• When sampling for a truck tanker (which will hold approximately 5,000 gallons),
set the sampling device to take a minimum of 13 sample units per composite
sample.
• When product is moved to a tank farm, set the sampling device to take a
minimum of 60 sample units per hour. This shall be done for every composite
sample.
• If the automatic sampler fails to operate properly, manually draw samples of the
product. Follow the on-line sampling rate in the AIM Inspection Series, Sampling
Manual.
• Manually drawn samples shall not be added to a composite sample from an
automatic sampler. These samples shall stand on their own as official samples.
• The score sheet for product sampled by approved automatic samplers must
contain the statement: “Sampled by Approved Automatic Sampler.”

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 81 of 192
In-Plant Line Check Guidelines for Inspectors
Line checks for the purpose of grade determination are sample units selected from the processing
line at a point where the physical characteristics and quality will not be altered by any further
hand or mechanical means, but may be subject to further processing such as final freezing. Line
checks for grade determination are considered official samples. They provide a record of what
has happened during the packing shift. They keep the inspector and the processor informed of
conditions during processing, and helps the processor keep quality at the desired level. The
number of line check samples examined will depend on the sampling plan in effect at a particular
processing plant. See the AIM Inspection Series, Sampling Manual for on-line sampling
procedures.
Use approved score sheets and tally sheets for recording inspection results. The AMS Forms
Catalog (which may be found at the following intranet address:
http://agnis/AMSFormsCatalog/Forms/AllItems.aspx) contains a list of approved score sheets
and tally sheets. See the “Score sheet and Tally sheet” section of this manual for guidelines on
the development, approval, and completion of plant specific score sheets.
Whenever possible, make samples available for examination by plant officials and supervisors
after grading. This affords the inspector an opportunity to discuss any pertinent observations or
changes needed during production, and encourages communication between all parties, the plant,
supervisors, and subordinate inspectors.
A. Line checks for grade determination of finished product
Samples of the finished product are taken after processing is complete and no
further change in quality is likely to occur. Samples are selected on a random
basis so that each sample unit has an equal chance of being selected. If the
production rate is reasonably uniform, spaced sampling intervals during a set
period of packing time will generally produce good lot representation. If the rate
of production fluctuates considerably, the sampling interval should be adjusted
accordingly.
Regardless of production patterns, inspectors must not be too predictable in their
sampling intervals. Such predictability may allow for processor manipulation
timed to temporarily improve the product at the anticipated sample selection time.
This may be more of a problem if the grading site is located far away from the
processing lines and in view of the production workers. Line check personnel
would be seen while on their way to draw samples in time for workers to intensify
their efforts to improve quality. The inspector’s goal is to draw samples that
accurately reflect the quality of the product, and such samples are not
representative of the lot.
Samples of finished product should receive all the same processing and handling
as the lot which they represent, and should ideally be taken from the location of
the stored lot. If this is not practical due to the size of the facility or location of
storage, consult your supervisor for guidance.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 82 of 192
B. Line checks of unprocessed product
Line checks of samples prior to final processing give the inspector and the
processor an immediate picture of the likely finished product quality. The
processor can promptly implement correction procedures if needed to improve
subsequent production. These line checks are generally considered official
samples that are used to help determine the final grade of the product.
Since they are not processed, all unprocessed line check sample units from the
canning line must be opened immediately after the sample units are drawn.
Samples drawn at this stage are limited in the extent to which they fully reflect
finished product. Inspectors must examine samples of the finished product before
assigning a final grade, regardless of the number of line check samples evaluated.
Samples of the finished product should be taken from the same location as the
stored lot to ensure that they received the same processing and handling as the lot
they represent. In some cases this will not be practical, especially in large
canneries. If not, finished samples may be taken at the end of the processing line
at proper intervals. If needed, consult your supervisor for guidance.
For a Quality Assurance or QAP type contract, only finished product is used for
verifying reliability.
There are many important reasons for examining the finished product:
• The lot is subject to further handling and processing after line checks are
examined. “Character” in many products may be adversely affected by
rough handling or overcooking. Canned foods may be overcooked or
undercooked. Frozen foods may not be completely frozen.
• The color of the product may deteriorate because of improper processing
or may be improved by heat exchange and equalization.
• Oxidation and “pinking” will sometimes be prominent in certain products
after processing even though not apparent in the raw product.
• Bruises in the raw product often diminish or disappear after heat
processing.
• Frozen fruits may develop considerable oxidation between the line check
and freezing operation.
• Adulteration by foreign material is possible. If various fruit products are
held too long in warm weather after sugaring and packaging, higher mold
counts may be found in samples after freezing than were found in line

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 83 of 192
check samples. Objectionable material may be introduced where packing
medium is added at a point after line checks are taken.
• Improper cooling may cause external rust on canned foods soon after
processing.
• Cooker “jams” or rough handling may dent many cans in the lot. Under
certain conditions, cans may “panel” or “buckle” after improper cooling.
• After freezing and the resulting expansion, frozen foods in packing media
may show poor condition because of “leakers.” Liquid may soak through
and discolor primary containers of some frozen foods if held too long in
the containers before freezing.
• Dried foods may be excessively moist in containers with faulty seals.
It may be necessary to disregard line check indications and base the final grade of
the product entirely on examination of finished product samples. In this situation,
the line check score sheets should include an explanation about why they were
considered unreliable.
C. Incomplete Grade
A final grade cannot be determined without finished product inspection. If
inspection results are only available from unprocessed line checks, an
“incomplete grade” (IG) may be established by supplementing line check results
with any other factors affecting quality of which the inspector is aware.
Experience with the product is the key to determining accurate incomplete grades.
Products bearing approved identification must be completely inspected and
assigned a final grade.
Issue only “incomplete grades” on lots that have been shipped before finished
product samples can be drawn, unless official inspection can be completed at the
point where the lots arrive. Never issue a final grade based on a few cases left
from a lot that has been shipped unless the certificate covers only those few cases
remaining. If part of a lot is shipped before samples can be drawn, assign an
incomplete grade to the shipped portion and a final grade to the remaining
portion, noting complete details on the score sheets and Daily Inspection Reports
involved.
If sufficient finished product sample units are later examined from a lot
previously assigned an incomplete grade, and if they indicate lot quality to be
higher or lower than the incomplete grade, the final grade is based on the
examination of finished product samples.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 84 of 192
Inspection of Sort-Out Fruit
SCI occasionally receives requests to make official inspections of product that has been sorted
out of the regular pack. This is particularly true with RTP cherries, strawberries and other
berries. Although we do not wish to encourage this type of inspection, we also have no grounds
for refusing to inspect a product according to U.S. Standards.
Quite often the question is whether the grade should be substandard or whether the product is
GNC. Or there may be a Buyer Specification, and we need to determine if the product meets that
specification. For these situations, use the following guidelines:
A. Be sure to check the product for foreign material as indicated in the AIM
Inspection Series, Foreign Material Manual.
B. Even if the product meets the Buyer's Specification, it should also be on "Hold
For Re-Examination, account foreign material" where examination for filth
indicates the product might exceed the guidelines. Then proceed in accordance
with AIM Inspection Series, Foreign Material Manual
.
C. If the product is frozen in bulk such as barrels, certification and inspection should
be restricted to that portion of the containers from which the sample is drawn.
D. Do not show mold counts or rot counts or actual numerical results of any filth
examination on the certificate.
E. Do not restrict foreign material examination to one factor such as mold count,
leaving other applicable filth analyses undetermined.
Reporting Results
A. Reporting Inspection Results
It’s crucial to report result to the plant in a timely manner. To the processor, this
is one of the main advantages of having in-plant inspection. Plant employees
designated to receive reports must be identified for new inspectors.
An individual line check sample that is below desired quality should be reported
immediately to the designated plant contact so he or she may take any necessary
corrective action. This person is usually a QA or QC manager or technician. It’s
a good policy to note on the line check sheet both the name of the plant contact
and the time the notification was given.
After verbal reporting, written notification is usually provided to the plant in three
ways:
1. Notification to the designated plant contact person immediately after
results are obtained. The inspector should establish a daily routine for
SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 85 of 192
reporting grading results to the person responsible for placing lots into
storage, for example writing the grade of each item packed on the
warehouse manager’s copy of the pack report or plant production report.
2. Copies of the score sheets (if desired).
3. Copies of the Daily Inspection Report.
B. Reporting Special Cases
Products that are Grade Not Certified, or fail FDA Standards, or fail requirements
pertaining to the use of “Approved Identification” on the label marks, all require
special reporting procedures.
The plant will want to prevent recurrence and to make sure these items are not
inadvertently shipped or mixed with like items in the warehouse.
A letter should be written to the responsible plant official according to Division
regulations. The designated plant individual responsible for HOLD disposition
should sign the letter and enter the date received. Four copies of the letter should
be made, with the plant retaining the original. The inspector shall make the
prescribed departmental distribution on the remainder of the copies. See “Hold
and Release” instructions in this manual. In the case of “Approved Identification”
label marks, these must be removed under our direct supervision within a
reasonable length of time, and before leaving the plant.
Other In-Plant Inspection Duties
A. Sanitation and Safety
Monitoring sanitation is one of the principal functions of the Division’s In-Plant
Inspection Service. Promptly report any sanitation shortcomings to the
designated plant management representative. It is essential to follow-up on
reported deficiencies to verify that corrective actions have been taken in a
reasonable amount of time.
An unsatisfactory condition in the plant is considered sufficient reason for
affected product to be GNC. In such instances, first contact your immediate
supervisor to explain the circumstances and determine the appropriate steps to
take. See the AIM Inspection Series, Sanitation and Safety Manual for detailed
procedures.
Make sanitation tours in accordance with the AIM Inspection Series, Sanitation
and Safety Manual. Observe the beginning of processing operations. Note any
adverse conditions and notify appropriate plant personnel. Check the first few
containers of finished product from all processing lines for contamination from
improperly flushed pipelines or other equipment. Use GMPs as well as any plant

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 86 of 192
Sanitation Standard Operating Procedures (SSOP) as guidelines for determining
deficiencies.
Maintain a clean laboratory throughout the day. Follow proper sanitation
guidelines concerning the sanitation of workplace and equipment. Sampling must
always be performed using proper GMPs.
Processing plants generally have a safety manual geared to their own processing
facility. Some plants may have a plant-specific USDA safety manual. Review
the contents of both (if available) to become familiar with plant personnel and the
processing plant, including evacuation routes and emergency procedures. Be
aware of available information which could also contain pertinent plant safety
requirements and Material Safety Data Sheets (MSDS) for all chemicals used in
the plant. This information will increase your awareness of your surroundings,
and help you handle an emergency if it should arise at the plant.
Become familiar with SCI Safety instructions. Review the
AIM Inspection
Series, Sanitation and Safety Manual. Ask qualified plant personnel to open
complicated equipment when sampling and sanitation requirements dictate.
Follow the plant’s procedures for “Lock Out-Tag Out” procedures, and do not
place your hands or any body part within the confines of operating equipment.
AT ALL TIMES, SAFETY FIRST
B. Inspection of Products Processed Elsewhere
There may be times when plant management purchases a processed fruit or
vegetable for resale or further processing. The inspector should honor an
occasional request by the plant to inspect and/or certify such a product, provided
that the product was not officially inspected before. There should be no
additional charge for this service unless the inspector’s workday is lengthened in
the process. However, if the plant inspector is not proficient in grading the
product, or if the plant facilities lack necessary inspection equipment, plant
management should be advised to request that the product be inspected under lot
inspection (on a fee basis) through the area OIC.
C. In-Plant tanker inspection and sealing
A good sanitation and sealing program is crucial to maintaining the quality level
and integrity of the product. The processor/shipper is responsible for the sanitary
conditions of tankers, product loading, off-loading, and sealing. SCI
responsibilities are limited to grading and related inspection activities. This
includes monitoring tanker sanitation, assigning product grade, assisting in proper
sealing, and documentation of inspection results.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 87 of 192
1. Plant's role
a. Notify inspector if tanker sealing is requested, and if any sealing
requirements are specified.
b. Provide adequate lighting and the tools necessary to provide tanker
inspection.
c. Provide a sanitary loading facility, and a clean tanker capable of
being "properly" sealed.
d. Make valves accessible for inspection, properly load the tanker,
insure proper sealing, and provide acceptable seals. USDA seals
may be used in lieu of company seals.
e. Determine "acceptable packer's risk" in respect to loading tanker
based on tankers back-haul record.
f. Ensure that Occupational Safety and Health Administration
(OSHA) requirements are addressed.
2. SCI’s role
a. Monitor and conduct sanitation checks of equipment and tankers
for proper sanitation.
b. Document and report unacceptable sanitation conditions to
management.
c. Notify management of tankers that cannot be properly sealed.
3. Tanker condition and loading sites
Although not required, it is recommended that the loading area be
sheltered from the weather. In unsheltered locations the load may be
contaminated by objects falling into the product, or insects and foreign
objects intruding into uncovered loading nozzles.
Product shall not be loaded unless the tanker has been inspected. The
loading equipment must be clean. Verification of these conditions is a
responsibility jointly shared by both processor and inspector.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 88 of 192
4. Prior to loading
The designated plant person should obtain a wash ticket from the transport
company prior to acceptance for loading. These are usually attached to
the rear port. The ticket may be examined to confirm acceptable previous
back-hauls, adequate washing, and trailer identification.
If the tanker is to be sealed, it should be determined that it can be properly
sealed during the pre-loading sanitation check. Properly sealed means that
the openings are sealed to prevent access to the product. Sealing requires
tight seals and secure fittings, and should meet customer specifications.
5. Tanker inspection
a. Safety precautions
USDA personnel should never enter the tanker. Entry into a closed
tanker requires a Confined Space Permit. See the Regulations,
29 CFR 1910.146, found at the following internet address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionC
ode=CFR. This is required by the Occupational Safety and Health
Administration (OSHA), and is the responsibility of the industry to
provide. When a tanker is presented for inspection, USDA
personnel shall allow plant personnel to break seals and open
lids before performing inspections. At no time will USDA
personnel enter a tanker.
b. Inspection of tanker parts
Use a flashlight to inspect the tanker. Visually inspect lids, valves
fittings, vent covers, gaskets and openings. Look inside the tank
both to the front and to the rear through the dome lid. Examine for
any product residue indicating an unclean surface.
Loose equipment and helmets should be secured or removed
before approaching any openings.
c. Wash water
When residual wash or rinse water is present, it should be drained
from the tanker. It may be checked for metal shavings, detergent
residue, and traces of product. Tankers are not expected to be
completely dry, and may contain some amount of clean water. In
the absence of residual water, the dry surface of the rear valve may
be checked for residues. A magnet may be used on the residue if
the presence of metal shavings is suspected.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 89 of 192
When unacceptable conditions exist (such as metal shavings,
detergent and/or residues, product and/or residues, and/or mold
residues) the tanker must be rejected until properly cleaned. If it is
determined that the tanker is not clean, any product loaded would
be Grade Not Certified.
d. Ability to seal
In controlled situations it may not be necessary to remove all seals.
Otherwise, remove the seals and open rear, side, or under tank
assemblies (dust cover, plunger and cap to product lines), top dome
lids and vent caps. All access areas should be inspected for the
ability to seal. When the tanker cannot be properly sealed, notify
the applicant.
Note: When the applicant has requested tanker sealing and the tanker
cannot be properly sealed, but the processor elects to ship without
seals, the appropriate inspection documents will show “TANKER
CANNOT BE PROPERLY SEALED".
6. Incoming tankers with product
Acceptable verification and product receiving procedures should be
established between in-plant inspectors and plant personnel. Verification
should include trailer identity and a review of inspection documents with
seal numbers when applicable.
Note: Acceptance or rejection of product is at the receiver's discretion. SCI
inspectors will consider the product as officially graded if the USDA
documents accompanying the load are consistent with the load identity
regardless of whether or not applied seals are intact.
7. Sealing loaded tankers
When sealing is requested and proper sealing is completed, seal numbers
shall be recorded on the score sheet and other appropriate inspection
documents.
All previous seals shall be replaced with new seals, except under
controlled situations when tankers are being used for continuous loading
and off-loading. For example, if the tanker remains within acceptable
conditions while operating back and forth between inspection sites, only
the seals necessary for on and off-loading need to be changed. This
decision is a joint responsibility between the shipper and the trucking
company. The inspector will monitor verification of seals that remain
intact throughout the operation.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 90 of 192
a. Dome Area
The top dome should be sealed with at least two seals placed on
opposite sides of the hatch/lid. If the hinge can be removed,
additional seals may be necessary.
b. Lower Areas
After the processor has properly secured valves and closed the
covers, the access door will be sealed on tankers that have all
valves inside a locked box.
After the processor has closed the valve and installed the dust
cover and retainer nut, the dust cover will be sealed on tankers
with rear or lower valves with dust covers.
c. Vents
To be considered “properly sealed,” all vents must be equipped so
that seals may be installed.
8. Other areas of concern
a. Temperature
Product temperatures can be verified during the loading process as
specified by standards or other applicable specifications. Results
will be recorded on appropriate inspection documents.
b. Score Sheets
The score sheet and certificate confirm that the product in the
tanker is the product that has been inspected
D. Syrup Designations
Syrup designation is based on Brix readings determined on “equalized” product;
that is, after the equalization of the natural sugars in the fruit and sugars in the in-
going packing medium. For the inspection purposes of canned fruits in syrup,
equalization is considered complete 15 days after packing. However rather than
delay inspection for the 15 day time needed for this process, equalization may be
achieved by comminuting all of the product and all of the packing medium
together. This is known as “simulated equalization.” See the Brix section in the
AIM Inspection Series, Technical Procedures Manual for additional information
on simulated equalization procedures.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 91 of 192
1. Simulated equalization
Compliance of each item with a specified syrup designation shall be
determined on a shift basis, on samples drawn at regular intervals. Entire
individual containers must be blended for each determination.
a. Sampling
Sample units may be drawn from the processing line at any point
after the seaming operation, and preferably where they emerge
from the cooking and cooling operation. Samples for simulated
equalization may be obtained as follows:
(1) The same container that is used for grade determination
may be used for Brix determination IF all fruit and syrup
can be retrieved for comminuting after quality factors have
been determined. Loss of either fruit or syrup component
will lead to inaccurate results.
(2) Separate containers may be drawn from the line for Brix
determination only.
b. Sampling frequency
The sampling frequency for Brix determination is based on the
required number of sample units needed to meet the on-line
sampling rate in the Regulations, and knowledge of previous
production. At least three determinations must be made. Refer to
the AIM Inspection Series, Sampling Manual for information
regarding time sampling plans that cover non-quality factors.
Sampling frequency shall be as follows:
Low Volume: One sample unit per 60 to 90 minutes.
Medium Volume: One sample unit per 45 minutes.
High Volume: One sample unit per 30 minutes.
No change in sampling frequency is necessary unless production
for three consecutive basic production periods (production shift)
indicates a change from one volume designation to another.
c. Sub-group samples
If the plant wants more than one determination each time (for
example, sub-groups of two), the number of sub-groups shall be
equal to or exceed one third of the number of sample units required

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 92 of 192
by the Regulations on-line sampling plan. Do not average the two
readings. Each container must meet the acceptance criteria as
shown in 3 Acceptance Criteria on the following page.
d. Lateral Samples
Lateral samples may be drawn at the option of the plant. However,
these samples may be used only for the plant's guidance, or to
confirm rejection of a lot. They may not be used to determine
compliance with the average specified as shown under Acceptance
Criteria.
e. Sampling "Failed Lots"
If a lot fails because of low average Brix, or insufficient sample
units (less than minimum number required), the lot may be
rejected. However the packer has the option to request that
additional containers be drawn throughout the lot to make up to at
least the minimum required for on-line in-plant inspection. If the
inspector elects to draw more than this minimum, it shall be in
increments as outlined in the Regulations, i.e., 3, 6, 13, 21, 29.
f. General Procedure
Record the net weights when line samples are in the laboratory.
This information may be valuable to the processor, since to some
extent low or high net weights influence the final Brix readings.
Cool the samples to approximately 70 degrees F before opening.
If they are available, use several blender bowls to save time in
comminuting multiple samples. Designate each bowl for use on
those products that are consistently close in degrees Brix. After
one sample unit is comminuted, it may be poured back into its
original container. The bowl should be completely drained before
another sample unit is comminuted. Continue this procedure until
all containers are blended, and the comminuted product(s) is ready
for refractometer readings. Leave the bowls inverted after Brix
measurements have been completed, or wash with hot water and
thoroughly dry before each use.
g. Communication
When a Brix determination is below the required value:
(1) Immediately notify the proper designated plant official.
Record the name of this plant employee and the time of
notification on work sheet or score sheet.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 93 of 192
(2) If requested, check additional blends and record these
additional values in a separate column on blend sheet or
work sheet. These would be considered "lateral samples"
and cannot be used to "erase" results of official samples.
2. 15-day Equalization
To bring a lot up-to-date fifteen days or more after pack, measure the Brix
on all sample units, using techniques described in the AIM Inspection
Series, Technical Procedures Manual.
Incorporate these readings with the original blend readings if they confirm
the original results. If these readings fail to confirm the original results,
make a full inspection for Brix at the appropriate single sampling rate and
certify on this basis, disregarding the readings obtained by simulated
equalization.
3. Acceptance Criteria
A code or group of codes may be considered separately from other codes
in a shift if it meets the full single sampling rate. To be considered
acceptable, the re-sampled portion must meet the requirements listed
below. Portions that fail to meet these requirements are rejected.
a. Simulated Equalization
(1) The average Brix of the sample must be within the range of
the specified syrup designation.
(2) Except as indicated in paragraph a. (4), no individual
sample unit may be below the range of the next lower or
above the next higher syrup designation. If no lower
designation exists, none may fall more than 2 degrees Brix
below the minimum of the specified syrup designation.
(3) Not more than three successive Brix readings on sample
units drawn at regular intervals may be below the minimum
or above the maximum for the specified designation.
(4) If an individual container fails paragraph a. (2), draw three
additional containers representing the production of
approximately the same time period. Record results on
blend or work sheet adjacent to the low sample unit. Use
these additional three test results to appraise the status of
the sub-group period as follows:

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 94 of 192
(a) If 2 or more of the additional three Brix readings are
below the minimum or above the maximum
requirement for the intended syrup designation,
reject the period code.
(b) If no more than one of these additional three Brix
readings is below the minimum or above the
maximum, the period is accepted or failed based on
evaluation of the entire shift.
b. 15-Day Equalization
(1) The average Brix must be within the range of the specified
syrup designation.
(3) No individual may be below the range of the next lower or
above the next higher syrup designation. If no lower
designation exists, none may fall more than 2 degrees Brix
below the minimum of the specified syrup designation.
In-Plant Inspectors Filing System
A list of key management and plant personnel names should be kept on file for ready access by
new inspection personnel. Since title and structure of management levels differ between plants,
the lists may vary accordingly. The form and style should be based on plant management
structure and “Chain of Command.” Include names of plant contact individuals on each shift.
Since many inspectors go from plant to plant, it is important that the filing systems be relatively
uniform to minimize confusion for inspectors. This system consists of two basic parts:
• A Yearly File; and
• A Permanent File.
In many plants, these files may be adequately housed in a single file drawer. Larger plants
requiring a greater number of records may need a three-drawer filing cabinet. Whatever type of
storage is used, it must be lockable. The IIC is allowed flexibility in establishing these files.
The inspector only needs to set up folders for those topics applying to the particular plant, and
arrange them in the given sequence. The letter and number of each folder enables the inspector
to return it to the current filing location easily. Tab the folders with the approximate terminology
as specified in the following:

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 95 of 192
YEARLY (Y) FILE
NUMBER TITLE
Y-0 In-Plant Contract
Y-1 In-Plant Billing Worksheet FV- 434 – Completed
Y-2 Certificate Ledger
Y-3 Unofficial Sample Requests
Y-4 Certificate Requests
Y-4a Certificate Requests - Completed
Y-5 Certificates of Loading B Copy
Y-6 Certificates Used______________ to _______________.
Y-6a (Insert proper serial numbers. Use 25 certificates
Y-6b or multiple thereof per folder. Add sub-letters to filed
numbers as needed.)
Y-7 FV-66's Used_________________ to _______________.
Y-7a Y-7a, Y-7b, etc. (File in same manner as Y-6).
Y-8 Daily Inspection Reports__________________________.
Y-8a (Insert month and year. Use one folder for each month of
Y-8b packing season or as desired. Staple pack report and
sanitation score sheet to DIR).
Y-9 Scoresheets. (Insert name of product. Use a folder for
Y-9a each commodity packed.)
Y-10 Line Checks_____________________. (Insert name of
Y-10a commodity. Folder for each commodity packed.)
Y-11 Division Activity Report
Plants under a QAP do not keep a file for Y-8, Y-9, and Y-10, except for the records used for
verification. Inspections for USDA contracts should maintain all original completed forms with
the contract folder, with copies in numbered files. See the AIM Inspection Series, USDA
Purchases Manual for coverage of USDA contracts.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 96 of 192
PERMANENT (P) FILE
NUMBER TITLE
P-0 Standard Operating Procedures
P-1 Key Plant Personnel List
P-2 Accountability Records
P-3 Confidential Annual Reports
P-4 Plant Audit Reports
P-5 Plant Survey Reports
P-6 Plant Coding System
P-7 Label Reviews
P-8 Report of Regulatory Agency Inspections - FV-425
P-9a Correspondence - Incoming
P-9b Correspondence - Outgoing
P-9c Memos from Field Office
P-10 Time and Attendance Reports
P-11 Power of Attorney
P-12 Proposals (for material such as standards and handbooks
under study)
P-13 Conference Questions (to be submitted)
P-14 Attachments - Washington Mail
P-15a Blanks - Loading Certificates
P-15b Blanks - Quality Certificates
P-15c Blanks - FV-66
P-15d Blanks - Certificate Worksheets
P-15e Blanks - Certificate of Date of Pack
P-15f Blanks - Certificate of Sampling
P-15g Contract Status Forms
P-15h Blanks - DIR
P-15j Blanks - Hold and Release
P-15k Blanks - Power of Attorney
P-15l Blanks - Sanitation Scoresheets

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 97 of 192
P-15m Blanks - Time and Attendance
P-15n Blanks - Travel and Expense Vouchers
P-15p Blanks - Scoresheets (folder for each commodity)
P-15q Blanks - Line Checks (folder for each commodity)
P-16 Official Stationery
P-17 Official Envelopes
If for any reason forms are kept elsewhere, a note should be placed in the
appropriate folder(s) in the file directing other inspectors to the location of these
materials. Unused Certificates of Quality and Condition must be kept in a
secure and locked location.
In-Plant Label Review and Approved Identification
This section addresses the review of labeling and advertising materials bearing official USDA
grade or inspection marks. When reviewing product manufacturer labels, the inspector must
check for all issues that are required under the Inspection Agreement With Industry Contract. If
a State or Federal statute requires certain label information, and requires that product meet
certain criteria for their “Special Mark,” the inspector’s review should include these criteria.
Any deviations should be reported to plant management. Inspectors should not enter into any
discussion or controversy over label statements that are under mandatory labeling regulations,
such as under the Federal Food, Drug, and Cosmetic Act, or applicable state laws and
regulations. However, inspectors should be aware of any incidences of potentially deceptive or
inaccurate labeling. If you are directly aware of such an incident through knowledge of the
plant=s processes, report it to your supervisor. If processors, distributors, or others in private
industry have questions about proper labeling, they should be tactfully referred to the agency
(usually FDA) with authority on the subject. For further Label information, see the “Labeling”
section of this manual.
Although the National office approves the use of official label marks by the applicant, the IIC of
a plant will be responsible for reviewing and verifying all labeling materials bearing approved
identification. Questions or problems that cannot be resolved are to be directed through the
normal chain of command.
When labels are completed and available at the plant, they should be reviewed as far in advance
of use as possible. The ideal situation would be to review preliminary label sketches, paste-ups,
or proofs of proposed labels before they are manufactured. This step could prevent significant
errors and save the firm considerable expense and inconvenience. Whether materials are
checked prior to manufacture or not, inspectors should be sure to verify finished labels because
there may have been changes since the initial review.
Products packed or certified under contract in-plant or lot inspection may bear certain types of
“Approved Identification.” These products must meet the requirements stated in the
Regulations, and additional qualifications set forth within in-plant contracts. Inspectors must

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 98 of 192
monitor and be alert for instances when or where these qualifications and requirements may be
violated by packers, distributors, advertisers, and/or other interested parties.
Packers under In-Plant Contract that use “Approved Identification” on their product containers
are required to submit proofs or samples of proposed labels to the plant IIC for review prior to
use. This does not apply to lot inspection and the use of labels with the prefix “U.S.”
A. Label Control - Official Marks
1. Year-round and seasonal inspection when a resident inspector is assigned
to the plant
a. The IIC and the area supervisor will review and approve all labels
bearing official marks prior to use. Questions or problems that
cannot be resolved by the IIC should be referred to the area
supervisor. If necessary, the area supervisor will contact the
Regional and/or National office.
b. Each label will be assigned a number to distinguish it from all
other labels. This number is assigned by the IIC or the area
supervisor, and will be used for label identity and inventory. It
will contain a letter prefix of three or more characters and be
numbered in consecutive order.
Example: America Better Foods Company
AGourmet@ label - Canned Peas 8 oz.
ABF1
AGourmet@ label - Canned Peas #303
ABF2
c. The IIC will maintain a permanent file for all approved labels
during the assignment. The file will include a copy of the label(s)
and a ledger for label identity and inventory.
d. The plant should notify the inspector when labeling product with
official marks.
e. The supervisor will periodically review the permanent file and the
plant’s label inventory control sheet.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 99 of 192
2. Seasonal Inspection when a resident inspector is not in the plant
a. It is the applicant’s responsibility to provide advance notification
to the area supervisor before using labels with approved marks.
b. The inspector or area supervisor will compare the labels with those
in the approved label file to determine if they have been approved.
The inspector will also check the USDA ledger inventory and the
plant’s label inventory. If there are discrepancies in the inventory,
the area supervisor will be notified.
c. The inspector shall check product codes against file records for
product and grade verification.
d. Inspectors and supervisors should be alert for discrepancies in
inventories which could indicate misuse or mishandling of labels.
(See example Label Control Sheet shown on the following page.)
e. Label inventory records will be made available to USDA during
plant visits. Additional label inventory checks may be requested at
the discretion of the area supervisor.
f. When applicable, removal of labels with “official grade marks”
shall be performed in accordance with the regulations.
See Section E.
on page 102.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 100 of 192
Label Control Sheet
Product
Container
Size
Label
Label No.
Previous
Balance
Newly
Purchased
Labels
Used
Current
Balance
Cases
Shipped
Cert. No.
Canned Peas (Sw)
8 oz.
Gourmet
ABF 1
100,000
-----
28,000
71,200
600
(48/8)
R3641
Canned Peas (Sw)
#303
Gourmet
ABF 1
750,000
200,000
50,000
900,000
2075
(24/303)
R3645
Canned Peas
(Ear.)
#10
“Garden
Fresh”
ABF 3
Corn
#303
French Style
Green Beans
8 oz.
Cut Green Beans
#303
Cut Green Beans
#10
Frozen Spinach
(W.L.)
12 oz.
Frozen Spinach
(Ch)
3 lb.
EXAMPLE
SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 101 of 192
B. Labels for Products Not Under Contract
Products that are not covered by the In-Plant Inspection Agreement shall not bear
the official “Approved Identification” marks on their labels. If such labels are
proposed or are being used inadvertently, the agreement must be amended to
include inspection of those products.
C. Labels for Products Not Covered by U.S. Grade Standards
Products for which there are no U.S. Grade Standards must not reference a U.S.
Grade on their labels. However, if the product is of acceptable commercial quality
and packed under a Continuous Inspection Contract, such products may bear
Inspection Marks without reference to quality.
D. Maintain a Permanent File
IICs will incorporate complete and records of all labels reviewed into a permanent
filing system. This file will be maintained in a binder categorized by the type of
product, such as:
1. Canned fruits
2. Canned vegetables
3. Frozen fruits
4. Frozen vegetables
5. Frozen potatoes
6. Miscellaneous
7. Citrus - all
8. Citrus - Florida only
a. Concentrates to include institutional pack
b. Single strength juices
c. Sections
d. Fresh unpasteurized juices
e. Beverage Bases in excess of 20 percent citrus
The basic categories have been established primarily for distributor’s labels;
however, packer’s private labels should also be included in the binders.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 102 of 192
The appropriate binders are assigned to the custody of the IIC at each plant. Once
assigned, the binders will remain with the USDA plant files regardless of the
movement of the inspectors. The binders assigned to each plant will cover only
the types of products the plant is capable of processing.
E. Division Action on the Misuse of Approved Identification
If the Regulations have been violated, approved Identification must be removed
from the containers. Noncompliance should be brought to the attention of your
immediate supervisor. After determining that the product is in violation of the
Regulations, the inspector will:
1. Inform the processor
After verbally informing the plant supervisor and warehouse contacts,
follow up with a written statement to avoid any misunderstandings.
The use of Hold and Release forms
or similar requests are acceptable for
warehouse communications on this subject. More information on this may
be found in “Forms and Reports” section “E” on 116.
The follow-up statement to the processor should be in a typed letter or
e-mail if the plant inspectors have access. A copy should be sent to the
area OIC, Regional Section Head, and Division Director.
An example of such a letter is shown on the following page and may be
used as a guide. A deadline for removal of approved identification should
be specified. This date is usually left to the discretion of the IIC, who
should factor in applicable conditions when setting a reasonable time frame
for completion.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 103 of 192
MISBRANDING REPORT
1. Certificate Number: _______________________________________________________
2. Date of Certificate: ________________________________________________________
3. Name of Division employee reporting the violation:
________________________________________________________________________
4. Office Address and Telephone Number:
________________________________________________________________________
5. Applicant: (name, address, and telephone number)
________________________________________________________________________
6. Receiver: (name, and address, if known)
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
7. Product Inspected:_________________________________________________________
8. Misbranding Factor: (grade, origin, weight, etc.)
________________________________________________________________________
9. Number of cases:__________________________________________________________
10. Principal Label (container) Marks: ____________________________________________
________________________________________________________________________
________________________________________________________________________
11. Shipping Container (case) Marks:
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
12. Location of Lot: (name and address)
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
REMARKS:
Fruit and Vegetable Program
Specialty Crops Inspection Division

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 104 of 192
2. Report incident on the DIR
Issue the DIR for Special Attention as mentioned in “Forms, Reports and
Correspondence”. Insert a footnote (such as 1/ or 2/) by the final grade
assigned to the item and briefly describe the violation under “Remarks.”
(for distribution, see Reports for Special Attention)
3. Supervise removal of approved identification
Either supervise the removal operation, or closely check the lot after
removal. Account for all cases or containers in the lot. Do not allow the
processor to circumvent removal by such actions as donating the product to
charity or selling it to plant workers or local stores.
Removal may be achieved in a variety of ways:
a. Blocking out with an ink stamp. Frozen containers may be treated
in this manner by wiping the spot with a dry rag and immediately
stamping with a quick-drying ink such as that used for felt-tipped
marking pens.
b. Blocking out with paper stickers.
c. Labeling over the offending label or overwrapping over the
offending overwrap. The label underneath must not show through.
d. Completely stripping the labels.
e. Replacing friction-type lids or screw closure lids.
f. In many instances of mislabeling, it is possible to correct the label
so that it complies with regulations and therefore eliminates the
need for removal of Approved Identification. The processor should
be encouraged to do so whenever possible. Examples of these
corrections are:
(1) Blocking out the word inaccurately describing a syrup
density, and ink stamping the correct word adjacent to the
block.
(2) Blocking out the inaccurate net weight and inserting the
correct net weight.
(3) Blocking out the word inaccurately describing style, and
inserting the correct word.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 105 of 192
4. Give the processor written release
Acknowledge the removal or label correction, and be sure to identify the lot
by cross referencing to the letter originally requesting label removal.
5. Report removal on DIR
Under “Remarks,” report the removal or label corrections on a DIR marked
for Special Attention and refer to the number of the DIR on which the
violation was originally reported. If the removal or correction is
accomplished in the “off-season” when DIRs are not being issued, write a
brief official memorandum to the area OIC, reporting this information.
Distribute copies to the Regional Section Headand the Division Director.
The deadline for removal of Approved Identification may be postponed to a
later date if requested by the processor and if the reasons for doing so are
valid. If the processor has a good reason for the request, the inspector may
allow postponement and write a letter to the processor stating:
a. Acknowledgment of the processor=s request and reason for
postponement;
b. Period of postponement; and
c. Reference to the exact lot affected and reference to the letter to the
processor that originally requested removal.
This letter shall be distributed in the same manner as the original letter
requesting removal.
6. If the processor does not receive a postponement and fails to comply with
the deadline date, the inspector shall write an official memorandum to the
OIC stating the facts. Copies of this memorandum shall be distributed to
the Regional Section Head and the National office. The OIC shall then
contact the processor and ask for immediate compliance. Should this fail,
the inspector will notify the Regional Section Head and the National office.
If the processor ships a lot which is mislabeled with respect to Approved
Identification after due notification, the processor is in violation of the
Regulations, 7 CFR 52.54, which may be found at the following internet
address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CF
R, and the In-Plant Inspection Contract. Penalties may range from a
written warning to cancellation of the inspection contract, or in extreme
cases, legal prosecution.
If a lot which is mislabeled with respect to Approved Identification is
shipped, the inspector will report the facts to his or her immediate

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 106 of 192
supervisor in writing. The inspector must be certain that the lot has
been shipped.
Copies of the completed report shall be submitted through the inspector’s
immediate supervisor and distributed as follows:
a. Reference the identity of the lot and previous correspondence;
b. If he or she chooses, include recommendations for appropriate
Division action; and
c. Distribute copies of the report to the Regional Section Headand the
National office.
Forms and Reports
A. Signing and Initialing Completed Forms
Some routine forms require the signature of the inspector on an in-plant
assignment, and some require only the inspector’s initials.
1. Inspector’s signature required
a. Completed sanitation score sheet or report.
b. All forms and documents that substantiate product inspection results
and which the inspector personally completes. This may include
such forms for official checks as fill weights, line checks, Brix
blends (simulated equalization), microanalysis, enzymatic activity,
fruit-sugar ratios, and others.
c. All Certificates of Quality and Condition (including Form FV-66),
Daily Inspection Reports, and product score sheets.
2. Inspector’s initials required
In many processing plants inspectors utilize the services of qualified plant
personnel who perform certain inspection duties under the inspector=s
technical supervision. These technicians are not allowed to fill out
sanitation score sheets. They may record information on product score
sheets, certificate worksheets, and Daily Inspection Report worksheets,
provided the inspector carefully checks their work. They do perform and
record results of many official routine duties, such as those mentioned
under 1 b. above.
These technicians must affix their signatures to forms on which they have
entered official information. During each workday the inspector must

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 107 of 192
exercise close supervision over technical personnel. The inspector’s
initials must be entered on line check forms which the inspector personally
reviewed, observed, or examined.
B. Daily Inspection Report (DIR) (Form FV-416)
The official electronic FV-416 is readily available and should be used at the plant.
This report summarizes the results of inspection activities and plant production for
each processing day. The FV-416 is available on the AMS Forms Catalog and
may be found at the following intranet address:
http://agnis/AMSFormsCatalog/Forms/AllItems.aspx. It also serves as:
1. A semi-formal certification of the quality and condition of each inspection
item;
2. An aid to the plant inspector when subsequently certifying products;
3. A source for compilation of data;
4. A basis for the processor or the processor’s sales representative to plan
sales and shipments; and
5. A form of communication within the Division.
The DIR is an official form and must be prepared with the same care as a
Certificate of Quality and Condition. Certification rules regarding appearance,
errors, and initialing of errors also apply to the DIR. See the AIM Inspection
Series, Certification Manual for additional information.
1. Completing the Daily Inspection Report
The following instructions correspond with the lettered items shown in the
example below.
a. Report Number - Chronologically number each report throughout
the packing year, starting with number one. Start renumbering on
January 1 or at the beginning of the packing season, whichever best
suits the particular plant’s operations.
b. Date - Packing day covered by the report. Do not cover more than
one day on one report.
c. Company - Name of the processing plant.
d. Location - City and State in which the plant is located.
e. Item No. - Number each item chronologically.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 108 of 192
f. Product - “X” appropriate box. List each item by commodity, type,
and style, using the same terminology as specified in instructions
for Certificates of Quality and Condition.
g. Container Size - Number as normally packed per case/size of
container. For example: “24/2½.”
h. Code or Other Marks - Code markings of primary containers. If
cases (secondary containers) are also coded, but the code differs
from that on the primary containers, insert a numbered footnote
(such as “1/” or “2/”) by the primary code, and list the case-code
under “Remarks.”
i. Number of Cases or Pounds - For canned product, insert the number
of cases packed. If the product is not cased, enter the equivalent
number of cases based on the amount of primary containers
normally packed per case. For frozen or dried products, insert the
number of pounds packed.
j. Packer’s Grade - State the letter-grade which the packer intended
for the item.
k. Incomplete Grade - This column is left blank unless a final grade
has not been established for the item.
l. Final Grade – Show the letter-grade established by inspection
results for the item. Include any necessary qualification symbols.
(See section B 7
)
m. Inspectors - List the names of the inspectors who were on duty
during the day covered. The inspector who checks the DIR for
errors shall sign the report.
n. Total Pack - Enter the total number of cases or pounds packed.
Repack should be entered on the DIR and included in the total pack.
o. Remarks - Indicate recommendations, reasons for downgraded
items, and other pertinent information not listed elsewhere. It is not
necessary to list upgraded items. Any listed recommendations
should have been previously discussed with plant officials.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 109 of 192
REPRODUCE LOCALLY. Include form number and edition date on all reproductions.
U.S. DEPARTMENT OF AGRICULTURE
AGRICULTURAL MARKETING SERVICE
DAILY INSPECTION REPORT
Report No.
(a)
Date
(b)
Page of
Company Name and Address (Include City, State, and Zip Code)
(c) (d)
Continuous Plant Inspection Other
IDENTIFICATION
GRADE
Item No.
(e)
Product
(f)
Canned
Frozen
Dried
Dehydrated
Container
Size
(g)
Code or Other
Mark
(h)
Number of
Cases
(i)
Packers
(j)
Incomplete
(k)
Final
(l)
Signature of Inspectors (Print and Sign Name)
(m)
Total
Pack
(n)
Cases
Pounds
Remarks
(o)
Form FV-416 Example

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 110 of 192
2. Reports for special attention
A DIR which contains information on a violation of Division Regulations
(e.g. mislabeling or misuse of approved identification) should be sent to the
field office, the Regional office, and the National office immediately. If
mailed, it should be sent separately from other material and marked
“Special Attention” to ensure that it is handled appropriately.
3. Corrected reports
When errors, omissions, or changes require issuing a corrected DIR, the
words “CORRECTED REPORT” should be typed or printed in ink in the
upper right corner. It is not necessary to repeat every item on the original
report, list only those that have changed. Attach the corrected report to the
originally issued version.
4. Supplementary reports
Some plant operations require supplementary DIRs; for example to
document drained weights after stabilization 30 days after packing for
Canned Mushrooms. Each supplementary report shall cover only one
original report. It shall bear the same report number and date as the
original, but the words “SUPPLEMENTARY REPORT” should be typed
or printed in ink in the upper right corner. It is not necessary to repeat
every item on the original report, just show items requiring supplemental
information. This information may be legibly handwritten in ink, or typed
in the body or under “Remarks” on the supplementary report. Include the
date the information was determined. Attach the report to the originally
issued DIR.
5. Last day of pack
The DIR representing the last day of pack for any single commodity should
state this in the “Remarks” section; for example, “Last day of freestone
peach pack.” Similarly, the report for the last packing day of the entire
packing season should state this in the “Remarks” section: “Last day of
packing season” and also include the total cases packed.
6. Distribution
a. DIRs - Original to Processor (and additional copies, if requested)
One copy to Field office
One copy to Inspector’s files

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 111 of 192
b. Reports for Special Attention - The same distribution as indicated
above for routine reports, except also send one copy to the Division
Director and the Regional office.
c. Frequency of Distribution - These reports shall be given to the
processor daily. Those copies destined for the various offices
within the Division shall be mailed at least once a week.
7. Symbol designations
The use of these symbols on the DIR promotes brief, uniform reporting of
lengthy descriptions sometimes necessary to qualify final grades. These
symbols are used only on the Daily Inspection Report, product score sheets,
daily pack reports, and other reports that circulate within the plant. Do not
use these symbols on Certificates of Quality and Condition or other forms.
Inspectors using these symbols shall ensure that all appropriate plant
personnel (such as sales manager, manager, and warehouseman) have
copies of the legend for the symbols.
Do not use symbols that are not listed. Upon request, the National office
will add symbols to this list. Although use of these symbols is not
mandatory, they are recommended for large plants and plants operating on
a year-round basis.
1a Low average drained weight.
1b Most containers fail drained weight.
1c Low drained weight. Individual containers below limits for good
commercial practice. 1/
2a Fails syrup requirements. Low average syrup. 1/
2b Fails syrup requirements. Brix on individual containers below the
limits for good commercial practice. 1/
2c Fails syrup requirements. Runs (simulated equalization).
3a Low average solids. 1/
3b Fails intended concentration account individual containers below
the limits for good commercial practice.
3c Mixed solids. Individual containers within limits for more than two
concentration ranges.
3d Average solids below processor’s declaration.
3e Fails intended concentration account individual containers exceed
limits for good commercial practice.
4a Percentage of heads below requirements. 1/
4b Fails count and/or percentage by weight requirements. 1/

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 112 of 192
5a Low net weight or net volume. Average below label declaration of
National Institute of Standards and Technology (NIST)
recommendation. 1/
5b Low net weight or net volume. Individual containers below the
limits for good commercial practice. 1/
6a Fails FDA Standard of Fill. Drained weight below FDA
requirements. 1/
6b Fails FDA Standard of Fill. Excessive headspace. 1/
7a Fails FDA Standard of Identity. 1/
7b Fails FDA Style or USDA Standard for product description. 1/
8a Fails FDA Standards of Quality.
9a Low average fill weight. 1/
9b Fails fill weight control limits account low subgroup average. 1/
9c Fails fill weight control limits account low individuals. 1/
9d Fails fill wt control limits account all Mi or
values below
min
10a Lot contains any zero vacuums.
10b Lot contains any reading 1 through 4 inches.
10c Incomplete number of vacuum readings taken for certification.
11 Insufficient containers examined for acceptance of Condition of
Container (interior or external).
12 Fails buyer’s specification or specific contract requirements. 1/
13 Inaccurate, partial, or illegible coding (embossing, stamping, or ring
marks).
14 Positive Catalase.
15 Positive Peroxidase.
16 Fails to meet declared fruit-sugar ratio. 1/
17 PACA label misrepresentation.
1/ Not to be mixed with lots meeting requirements for unqualified certification.
2/ NAG –No applicable U.S. Grade for this product. Put equivalent grade in
parenthesis if based on a U.S. Standard for a similar product (i.e., “NAG (B)”).

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 113 of 192
C. Daily Pack Report
The processor must provide the inspector a written report of the quantity of each
item packed and inspected. This information may be in the form of a penciled
note, or an efficient photocopied form that also serves as a worksheet for
completing the DIR.
Many processing plants produce a variety of items each day, and preparing the
DIR becomes a time-consuming job, perhaps requiring over an hour of an
inspector’s workday. With plant cooperation and the inspector’s ingenuity, pack
forms can be developed that cut down DIR preparation time significantly.
Note: If you have the pack report, it is not necessary to prepare a DIR. In large plants
this is a time saver that can be used to perform other necessary duties. The sample
report included here may serve as a guide for creating such a combination Pack
Report/DIR worksheet in any plant. The report is described as follows:
1. All items of each commodity normally packed by that plant are pre-
entered. Some lines are left blank so that special-pack items may be
entered. The static portions of the container codes are also included.
2. This is a fill-in type form that is initiated by the warehouse person each day.
All listed items are not necessarily packed each day. The inspector only
enters figures for those items packed during that particular day.
3. The inspector is given two copies of the semi-completed form. On both
copies, the inspector inserts pack figures, complete code marks, item
numbers, and the final grades for each item, including any necessary
symbol designation.
4. The names of all participating inspectors are written in, and any necessary
comments are entered under “Remarks.”
5. One copy of the completed worksheet is returned to the warehouse person
as a report of the day’s grades. This allows stock records to be completed
and finished product to be stacked in the proper location.
6. The other copy is given to administrative staff (if available) to be filed with
the inspector’s file copy of the DIR.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 114 of 192
DAILY PACK REPORT
REPORT DATE:1/1/2011
PAGE 1 OF 1 PAGES
COMPANY
ABC CANNING COMPANY
LOCATION
PERALTA, CALIFORNIA
IDENTIFICATION
GRADE
CANNED :X:
CODE MARK
ITEM:.
Y. CLINGSTONE
SIZE
M9
OVER
SIRUP
FORWARD
TOTAL
PACKER
FINAL
PEACHES, HALVED
48/8 OZ
B2P
HEAVY
B
PEACHES, SLICED
48/8 OZ
B2SP
HEAVY
B
PEACHES, HALVED
24/303
B2P
HEAVY
B
PEACHES, SLICED
24/303
B2SP
HEAVY
B
PEACHES, HALVED
24/303
C2P
LIGHT
C
PEACHES, HALVED
24/303
C3P
LIGHT
C
PEACHES, SLICED
24/303
C2SP
LIGHT
C
PEACHES, HALVED
24/2 1/2
A2P
EXTRA
HEAVY
A
PEACHES, HALVED
24/2 1/2
B2P
HEAVY
B
PEACHES, HALVED
24 2 1/2
B3P
HEAVY
B
PEACHES, HALVED
24/2 1/2
C1P
LIGHT
C
PEACHES, HALVED
24/2 1/2
C2P
LIGHT
C
INSPECTORS:
TODAYS
TOTAL
PACK
REMARKS:
Item Nos___Grade___to___account
Item Nos___Grade___to___account
Item Nos___Grade___to___account
Item Nos___Grade___to___account
Item Nos___Grade___to___account
NOTE: SEE SYMBOL DESIGNATION SHEET FOR SYMBOL MEANINGS.
EXAMPLE

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 115 of 192
D. Request for Certificate
Although not mandatory, a written request from the plant for a certificate is
recommended. A request worksheet providing all necessary information for
certification and distribution of certificate copies will help prevent
misunderstandings between the processor and the inspector. When properly filed
for later reference, copies of the request will also provide the processor and the
inspector a record of past certifications.
The example on the following page is a certificate request worksheet that may be
used as a guide in drafting an effective worksheet for any processing plant.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 116 of 192
CERTIFICATE REQUEST WORKSHEET
No.____________________________
Date ___________________________
To_____________________________
Plant___________________________
CERTIFICATE
NO
CASES
CAN
SIZE
ITEM
COUNT
SIZE
SOLIDS
SYRUP
Applicant______________________
Address_________________________
Shipper or
Seller_________________________
Address_________________________
Receiver or
Buyer__________________________
Address_________________________
Contract or
Order No.______________________
Grade According to
U.S. Standards_____________
Fed. Spec._________
Other___________
Check Certificate Required:
FV-146____ FV-147____ FV-149____
FV-66____ FV-67____
Day after Pack_________________
Up-to-Date______________________
Date Desired___________________
Signed__________________________
REMARKS: Applicant, shipper or seller and receiver or buyer may be any company or
individual designated.
ATTENTION WAREHOUSE:
The warehouse shall furnish a list of locations, with included codes, side marks and case counts
necessary for the above items on the reverse side of this form. This information must be dated
and signed by the warehouseperson and all copies for
warded to the USDA.
Date forwarded
by USDA_________________
Certificates
Original
Forwarded by USDA To:
Copies
Distributed by USDA:
Superintendent
Sales
Warehouse
File (USDA)
EXAMPLE

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 117 of 192
E. Hold and Release Forms
Occasionally it’s necessary to request that the processor hold a particular lot or
code, for example when it needs additional inspection performed to establish the
final grade, or because it requires removal of approved identification.
Inspectors should immediately inform plant management of such situations
verbally, and then follow up promptly with written notification. HOLD and
RELEASE forms can be developed and used in plants for this purpose. Proper use
of these documents can prevent costly errors due to misunderstandings and poor
communication. Completed copies are distributed to the warehouse manager and
plant superintendent. The inspector retains a copy after it has been signed by the
warehouse manager.
The IIC of the processing plant has the ultimate responsibility to make sure
offending product is identified and designated “ON HOLD.” The inspector must
verify that the product is not shipped, which can happen quickly with modern
electronic inventory and billing procedures.
The Hold and Release Form examples on the following pages may be used as
guides in preparing these documents.
Refer to the AIM Inspection Series, Foreign Material Manual
for “Hold”
procedures and forms concerning the presence of “foreign material.”

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 118 of 192
PLANT________________________________
DATE______________________
TO___________________________________
TIME______________________
FROM_________________________________
HOLD NO.__________________
HOLD
PRODUCT
NO. OF CASES
CONTAINER SIZE
CONTAINER CODES
LABORATORY REMARKS
THE ABOVE ITEMS ARE TO BE HELD IN THE WAREHOUSE UNTIL RELEASED FOR
SHIPMENT BY THE USDA.
SIGNED USDA
DISTRIBUTION BY USDA
WAREHOUSE
PLANT SUPERINTENDENT
FILE
Initials of Warehouseman

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 119 of 192
PLANT________________________________
DATE______________________
TO___________________________________
TIME______________________
FROM_________________________________
RELEASE FOR SHIPMENT
The items being held on HOLD NO. are released for shipment
LABORATORY REMARKS
SIGNED_______________________
Distribution by USDA
WAREHOUSE
PLANT SUPERINTENDENT
FILE

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 120 of 192
F. Annual Report
1. General
This report serves many purposes. It summarizes the highlights and
unusual situations occurring at the plant over the past year. It identifies
deficiencies in plant sanitation. It notes the cooperation and attitude of
plant management. This becomes a matter of Division record for
consideration in subsequent decisions that may affect the plant. The report
may also indicate the need for revisions of standards, new standards, or
instructions.
Each report must be complete in itself, not simply an update of the previous
report. It should be well-written and effectively communicate information
about the plant to Division supervisors up the chain of command. These
reports are read and used by more Division employees than inspectors may
realize. They are reviewed by the OIC, the Regional Section Head, the
Head of the Inspection Branch, and the Division Director.
2. Who submits the report
The annual report is the responsibility of the in-plant IIC. If a firm has
more than one plant under in-plant inspection, a separate report must be
submitted for each. Preparation of the report does not relieve the inspector
of the responsibility to immediately notify plant personnel of any
deficiency or condition requiring immediate action.
3. Frequency and distribution of reports
The report is to be completed at the end of each processing year or
processing season of 15 consecutive days or more, whichever is more
feasible. Copies of the completed report shall be submitted through the
inspector’s immediate supervisor and distributed as follows:
1 copy for Officer-in-Charge;
1 copy for Regional Section Head; and
1 copy for National office.
4. Instructions for preparation
The annual report shall be brief and contain concise and factual
information. It is “For Official Use Only” and is not for release to the
processor.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 121 of 192
Detailed summaries are not required, nor is it necessary to include
information that is available from other sources, such as cost of inspection,
plant lay-outs, detailed reports of volume or grades. The total inspection
hours worked are to be included, along with the names of personnel
assigned to the plant.
Although the IIC is ultimately responsible for the preparation of the final
report, each inspector shall contribute any key information based on
personal knowledge and notes accumulated throughout the assignment. If
an inspector leaves the assignment during the season, he or she may
complete a “partial summary” and leave this partial report with the
incoming inspector or supervisor to be integrated later into the final report.
With this information available, the report should not take more than two or
three hours to prepare.
Use the following outline as a guide in preparing the report. Include any
important points that may not be specifically mentioned. Do not answer
“yes,” “no,” or “none” to items in the outline without identifying the
subject, since those reading it may not have an outline for reference.
5. Outline for summary of In-Plant inspection
An electronic, fill-able version of the In-plant Annual Report “For Official
Use Only” is located on the AMS Forms Catalog at the following intranet
address: http://agnis/AMSFormsCatalog/Forms/AllItems.aspx. The
FV-433 is intended to be the preferred method of completing the in-plant
"For Official Use Only" annual report. Using the FV-433 contributes to
SCI's goal of uniformity. Other methods of completing the in-plant "For
Official Use Only" annual report are acceptable.
a. Cover Page.
The cover page should show in the upper right corner that it is
“FOR OFFICIAL USE ONLY” and include the following
information:
(1) Name of Firm:
(2) Plant Location: (include plant phone number and area code)
(3) Type of Inspection:
(Continuous, Designated Lot, Quality Assurance, etc.)

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 122 of 192
(4) Period Covered:
For year-round plants, use the fiscal year starting October 1
and ending September 30. For less than year-round, use the
actual starting and ending dates of service.
(5) Copies to:
Division Director
Regional Section Head
Officer-in-Charge
(6) Submitted By:
(Name)
(Title)
(7) Date:
(8) Total Inspection Hours:
(9) Total Pounds Inspected: (May be reported at the discretion
of the Officer-in-Charge)
b. Plant Management
(1) Personnel.
Show all key members of management, from quality control
personnel to the president of the company. List their first
and last names, being sure to spell them correctly. If the
firm is large and plant personnel are essentially responsible
for management, show plant management personnel
separately from top management at the firm’s headquarters.
In each report, always show the President, the Vice-
President, the Secretary, and headquarters address, even if
there is no change from the previous report.
(2) Cooperation.
Indicate briefly the degree of cooperation, whether good or
poor. Include any major deficiencies that were not brought
under control or corrected. If known, include fact-based
reasons for these developments. The inspector should also

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 123 of 192
include suggestions that could facilitate solving major
problems encountered at the plant.
c. Inspection personnel.
A list of all inspectors working at the plant during the packing year
should be included, showing the grade level and total hours worked
for each person. This should be listed in the lower right hand
corner of the cover page.
d. Products Processed.
Accurately list the following, including any pertinent facts of
interest on new or experimental products:
(1) Products packed during period of report, under headings of
“canned,” “frozen,” “dried,” or other classification.
(2) Any experimental packs.
(3) Products packed at facility with in-plant inspection but not
covered by current agreement.
(4) Products not specifically covered by agreements that are
expected to be packed in a future season.
It is not necessary to list the volume of pack or percentages based
on grade or container size. However, if the processor wants a report
showing this information, prepare one for the processor, and attach
a copy of it to the annual report for distribution to the OIC, the
Regional Section Head, and the National office.
Indicate briefly any major problems that caused a general lowering
of quality or conditions, such as unusual quality of raw materials
due to abnormal season, weather conditions, insect infestation, or
fruit deterioration.
e. Sanitation.
Indicate overall sanitation. Include issues such as unsatisfactory
storage of containers or ingredients, lack of adequate waste
disposal, or major breakdown of equipment or power. Particularly
note any effective measures used that led to improved conditions.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 124 of 192
f. Major changes of facilities and equipment.
(1) New or proposed buildings, processing units, or anticipated
expansion of facilities.
(2) New equipment installed; changes made (or anticipated) in
rebuilding equipment or processing facilities.
g. Coding, handling, or warehousing.
Briefly describe the coding system, and product handling and
warehousing. Include improvements and/or inadequacies as
applicable.
h. Application of U.S. Standards, Manuals, and Inspector’s
Instructions.
Cite any specific problems in use of these documents. Note if
problems are due to the standards (e.g., ambiguous), or to new
harvesting or processing methods (e.g., more HEM, more
breakage), or to the season (e.g., it was especially wet), or to the
plant facilities (e.g., old equipment), etc.
Describe any problems with existing inspection and visual aids.
Provide suggestions for any improvements that would help to meet
current inspection needs.
Indicate the approximate amount (volume) of products or styles
certified for products that are not covered by U.S. Standards. Note
any interest expressed by the plant regarding development of U.S.
grade standards for these items.
For copies distributed to the Regional and National offices,
supervisors must provide a written response indicating the steps
taken regarding concerns expressed about standards, manuals, and
instructions.
i. Miscellaneous.
Indicate anything that the inspector feels would be of particular
interest to the OIC or the Regional and National offices that is not
covered in other parts of the report.
j. Housing for Inspectors.
Indicate whether the per diem rate for the area is adequate to cover
expenses. Further indicate the availability of rooms, apartments
(furnished or unfurnished), or houses for rent, and satisfactory
eating-places, if relevant.
See an example of an acceptable Annual Report on the following pages.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 125 of 192
FOR OFFICIAL
USE ONLY
Name of Firm: ABC Canning Company
Plant Location: Westfield, California
Type of Inspection: Continuous Inspection
Period Covered: 10/01/2009 to 09/30/2010
Copies to: (Insert Name), Division Director
(Insert Name), Regional Section Head
Mr. William F. Blake, Officer-in-Charge
William F. Blake
Submitted by:
Kim S. Inspector
Kim S. Inspector
Agricultural Commodity Grader
October 2, 2010
Total Inspection Hours: 5,440
SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 126 of 192
I. Management
The Centerville Head Office and the Westfield Plant officials for the coming year are as
follows:
Head Office…………(Centerville, California)
Phone: 702-752-6214
1. General Manager…………………William A. Mann
2. Assistant Manager…………..……Kathy E. Fisher
3. Secretary-Treasurer………………Gwen O. Godwin
4. Supervisor Quality Control……….Ernest Seton
Cannery………………………………………..(Westfield, California)
Phone: 702-624-1625
1. Superintendent……………………..Frank Batuck
(Assumed position 6/10/10)
2. Assistant Superintendent………….Roberta L. Panker
II. Cooperation
Cooperation of the plant management with the inspector has been good throughout the year,
especially regarding suggestions about sanitation, safety and improving quality of product.
III. Inspection Personnel
One inspector is assigned on a year-round basis and during the peak of the canning season
additional inspectors are assigned.
Grade Hours
Inspector-in-Charge Kim S. Inspector GS-9 2,680
Temporary Inspector Harriet A. Jones GS-7 1,900
Temporary Inspector James W. Smith GS-7 60
Temporary Inspector Wendy A. Horner GS-7 250
Temporary Inspector Carl W. Blue GS-7 400
Temporary Inspector Ron J. Conte GS-5 50
Temporary Inspector Pat J. Darrin GS-5 100
TOTAL HOURS 5,440

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 127 of 192
IV. Products: Processed
ASPARAGUS
The cannery participated in the asparagus diversion program again this year, and began
operations March 18, 2010. With the converting of an existing warehouse to a ripening and
cold storage unit, the fresh asparagus could be held in an atmosphere of highly moisturized air
and at a temperature of less than 42 degrees F. This optimum condition prevented the rapid
deterioration of the product and enabled the cannery to better utilize and maintain the quality
and productive capacity of their plant. The month of April produced ideal growing
temperatures, but operations were curtailed in the month of May due to unsatisfactory growing
conditions imposed by cool and windy weather. Despite this development, the operations
progressed satisfactorily with the use of the molding room for the accumulation of the fresh
product.
A sharp 8-1/2 cent per pound increase was noted in this year’s field price over last year, with no
apparent rise in the important Midwest and Atlantic Coast producing areas. Contrary to earlier
expectations, all producing areas of the United States developed packs of Canned Asparagus
this year that exceeded the year 2009 output.
ELBERTA PEACHES
Because of large increase bearing acreage and better sizing, early season reports showed an
estimated total crop greater than any previous year. But due to an abnormally heavy green crop,
the Elberta tonnage picked out well below these pre-season estimates. However, overall quality
was substantially superior to last year’s and with significant changes in the processing facilities,
the pack reflects a much higher proportion of better grades. This same trend is indicated
generally from other canneries, and as a result the marketing of this year’s pack is not expected
to be as severely impacted by off-grade merchandise as last year’s. The market for Elbertas
continues to be unsatisfactory however, due largely to continued efforts to move the heavy
carry-overs of the year 2009 pack.
KADOTA FIGS
The canning operations on Kadota Figs commenced on October 16, 2009, but were curtailed by
difficulties in attending the harvest in the latter stages of the season. Lower than normal
temperatures and cool nights, together with water shortages, have resulted in a sharp overall
drop off in production. This in turn caused abnormally small sizes and below average quality.
As a result, the final production fell below pre-season expectations.
One problem encountered in the first weeks of processing was the heavy infestation of the raw
product by dried fruit beetles. This contamination was caused by poor field and harvesting
methods by the farmer. As a result of this condition, the inspector found it necessary to suggest
stringent changes in preliminary washing procedures. Management cooperated fully, and after
these improvements were made, there were no further problems with the finished product.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 128 of 192
IV. Products: Processed (cont.)
TOMATOES
Varying conditions in various producing areas of the state resulted in a rather confused picture
for early production prospects. It was evident that many of the older producing areas had
experienced substantial reductions in acreage, due largely to the unavailability or high rental of
suitable land. On the other hand, major acreage expansion was reported in the Westfield Valley,
where new land was being put into production.
The cannery started production on the first of September, but as the season progressed,
deliveries were not sufficient to permit continuous operations.
One of the features of the tomato operation was the 55-gallon drum filling installation. This
facility fills each of the four drums with approximately 528 pounds of 32 percent solid tomato
paste every 10 minutes.
V. Sanitation
General appearance and housekeeping the past year was exceptionally good. The usual
problems of running “around the clock” on certain products were encountered, but generally
speaking the sanitation and clean-up was good.
The plant improved the sanitation condition of the plant because of three factors. The first one
was the message passed down the chain of command concerning the crucial role of good plant
sanitation as well as the management of a plant sanitation program. An effective program was
implemented and plant employees did their parts to make it work. The second factor was the
installation and use of a multi-drop High Pressure-Low Volume Kobe pumping system
throughout the processing area. This helped keep hand-cleaning down to a minimum and also
reduced the cleaning time necessary for each area. The third factor was the two-shift,
approximately nine-hour schedule that was maintained during the fruit processing season. This
allowed a clean-up crew ample time to properly clean the plant.
VI. Major Changes of Facilities and Equipment
GENERAL
Installation of new boiler
Installation of three rotary type cookers
Installation of automatic can depalletizer and can handling system
A new fresh water well
Concrete filled floors in cookroom
New 400,000 case warehouse
Ripening and cold-storage room

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 129 of 192
ASPARAGUS PROCESSING
Two band saw cutters
Asparagus sizer
Spray washer and detergent tank
PEACH PROCESSING
Four slicers
Four sizers
TOMATO PROCESSING
Evaporating unit and flash cooler
Additional sorting belt
VII. Coding, Handling, and Warehousing
The coding method used by the company is by the embossed method on the lid end of the can.
Some monitoring of the legibility of the code was necessary, with adjustments made when
needed. The plant was made aware of the “inkjet type” system of coding to increase legibility.
This could be a more accurate method to help track inventory more precisely.
Handling and warehousing of the finished product was efficient. Even though the company
added warehouses two years ago, anticipation of a bumper crop and inventory called for the use
of outside storage of their product. Their storage and code tracking methods allow separate
tracking of all five individual production lines.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 130 of 192
VIII Application of U.S. Standards and Inspector’s Instructions
ASPARAGUS AND FIGS
No particular problem in applying the standards and instructions.
PEACHES
The standards are difficult to apply with respect to Grade D and Below Standards in Quality.
Specifically, application of the off-suture cut tolerance and uniformity of size rule are not
uniform and do not reflect as poor a quality as indicated. Instructions should provide for
standard sample sizes and uniform procedures.
TOMATOES
No problem with the standards except that no provisions are made for “stemmed.” Instructions
should increase the tolerance on “sprouted seeds,” on page 10. No standards for chopped
tomatoes with basil. Copy of buyer’s specifications for the product attached. Approximately
50,000 cases packed.
IX. Miscellaneous
With the introduction of the can dispensing units this year, the overall coding system for the
cannery was improved. By using a basic type die mark and different combinations of side
marks, the cook room could better utilize the full capacity of their cookers. It also proved to be
more efficient in the warehouse as a mere glance at the uncased merchandise could determine its
particular style, grade, etc.
X. Housing for Inspectors
The plant is located approximately 3 miles from the city limits, where there are several hotels
and restaurants available at moderate rates. The most affordable accommodations are at a motel
located a half-mile from the plant. The motel has been recently renovated and complies with the
Federal Fire and Safety Act. This proximity to the plant also saves mileage costs. This
availability makes the current per diem rate for this area adequate.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 131 of 192
LABELING
The labeling of processed food products is regulated by the FDA. The current FDA labeling
guidelines may be found at the following internet address:
http://www.fda.gov/Food/IngredientsPackagingLabeling/default.htm.
Under the Continuous Inspection program, labels bearing official marks are formally reviewed in
the National office. Possible violations of the Food and Drug Act on labels using official marks
will be brought to the processor’s attention, as will questionable labels observed in the course of
inspection. Depending upon the nature of the deviation, corrective action may be recommended
or required.
Various additives are sometimes used in the preparation of non-standardized processed foods.
These additives, such as acids, salt, sugar, approved colorings, calcium lactate, etc., are usually
used during the manufacturing process. For example, some frozen vegetables may pass through
brine separators during processing and accumulate trace amounts of salt in the finished product.
Similarly, citric acid may be used in the manufacture of canned apple sauce. Other examples
include additives such as sugar, coloring, and calcium lactate sometimes used during the
processing of French fried potatoes. The use of these substances can pose a labeling problem.
Because of the method of application, the additive(s) may carry over to the finished product in
negligible quantities. This may seem to be inconsequential. However, since many of these
products are not standardized, under Food and Drug labeling requirements it is necessary to
declare each ingredient, including additives when used.
It is the responsibility of the packer to conform to FDA requirements. SCI approval of labels with
official marks does not relieve the company of this responsibility. We will not permit the use of
labels with official marks when we have knowledge that undeclared additives are being used in
violation of Food and Drug labeling requirements. Contact your supervisor if you believe this to
be an issue at your in-plant assignment.
A. Definition of Labels
For purposes of this instruction, the term “labeling” refers to statements that
describe the product(s) involved, and which may appear on a variety of materials.
Labeling materials may include, but is not limited to:
1. Paper labels.
2. Spot labels or stickers (as affixed to metal or glass containers, e.g., Jams,
Olives, etc.)
3. Stickers in the shape of USDA shields separate from other kinds of
labeling.
4. Lids, caps, and tops of containers bearing official identification.
5. Printed overwraps (such as wax wraps in frozen foods).

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 132 of 192
6. Metal and fiber combination containers, as for frozen fruit and juices.
7. Insert packages (such as those containing separate sweetening ingredients
inside of another product).
8. Wax paper cartons, e.g., primary packages, such as fiber containers for
chilled juices.
9. Lithographed or otherwise coated or marked containers.
10. Transparent film packages.
11. Paper inserts which show through transparent overwraps.
12. Metal containers.
13. Shipping containers.
14. Stenciling or stamping.
15. Any form of label not mentioned that is used in conjunction with official
marks.
B. Classification of Labels
1. Packer’s Private Label
Illustration of statement to
identify the class of label
These labels show that the product was
packed or manufactured by the processor
.
These labels are for use only at the plants
operated by the firm named, whether or
not the plants are located at the main
headquarters. The location of plants need
not be shown on the label.
PACKED BY
X-Y-Z PROCESSING CO.
2. Packer’s Distributor Label
These labels show that the product was
packed or manufactured for or distributed
by the firm named at their own plants or
at plant locations of other processors.
PACKED FOR
X-Y-Z PROCESSING CO.
(OR)
DISTRIBUTED BY X-Y-Z
PROCESSING CO.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 133 of 192
3. Distributor’s Label
These labels show that the product was
packed or manufactured for or distributed
by a firm other than a processor engaged
in actual processing. These firms are
usually wholesale food concerns; or may
be a brokerage firm, cooperative sales
agency, or other marketing service group.
DISTRIBUTED BY
GOOD FOODS CO., INC.
(OR)
PACKED FOR GOOD
FOODS CO., INC.
(OR)
MANUFACTURED FOR
AND SOLD BY GOOD
FOODS CO., INC.
C. Approved Identification
The Regulations describes the use of approved identification in 7 CFR 52.3 and
52.53. Penalties for misuse of approved identification are in 52.54. Both sections
may be found at the following internet address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CFR. All
inspectors shall read and become familiar with this information.
D. Official Grade and Inspection Marks
This grouping includes:
1. “Grade Marks” that include the prefix “U.S.” regardless of the presence or
absence of a USDA shield on the container. Exceptions to this are quality
statements for honey and maple syrup. The “U.S.” prefix used in
conjunction with these products is exempt from approval and control by the
Division.
2. “Inspection Marks” include the USDA shield or statements that the
product(s) is “Packed Under Continuous Inspection (or the QAP) of the
U.S. Department of Agriculture.”
3. “Grade Marks” and “Inspection Marks” combined.
Note: Certain grade quality designations for some frozen products are in violation
of the Perishable Agricultural Commodity Act regulations if they do not
meet the labeled grade (such as Grade A or Grade B). See Section H for
more details on PACA misrepresentations and contact your immediate
supervisor as needed.
E. Marks Not Considered Official
1. Quality marks or statements which do not include the prefix “U.S.” (See
exception D 3. above).

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 134 of 192
2. Quality marks or statements that do not include the prefix “U.S.” and
which are not enclosed within the official shield.
3. Quality marks or statements that do not include the prefix “U.S.” and
which are not accompanied by an adjacent official shield either on the
primary container or on the cases.
4. Quality marks or statements that do not include the prefix “U.S.” and
which are not accompanied by a statement indicating USDA inspection on
the primary container or on the cases.
5. Quality marks or statements enclosed in designs that do not simulate the
USDA shield, such as triangles, circles, and rectangles.
6. Plain outline of shields embossed on metal container lids.
F. Questionable Marks
Certain marks similar to the design or statements of official marks are often used.
Examples:
Marks showing inspection or certification, such as:
“Inspected: This carton is one of a lot from which samples have been
inspected by the U.S. Department of Agriculture.” or
“USDA certificate covers contents” or
“Grade A, certificated quality”
Labels displaying similar marks or statements shall be submitted to the National
Office for review through the normal chain of command.
G. Division Policy on Use of Approved Identification
1. In-Plant or Approved Plant-Lot Inspection
Products packed under in-plant or approved plant-lot inspection may bear
“Official Marks” as defined in the regulations.
This means if any processing or repackaging operations (other than
re-labeling) take place on the product in a plant under in-plant inspection,
the product may bear approved identification. However, all component
products must either have been previously inspected, or must be examined
for wholesomeness prior to being used as the product or part of the product.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 135 of 192
All previously inspected items must be suitably identified and accompanied
by inspection reports. The receiving inspector will examine the lot and
verify its identification and condition.
All items not previously inspected must be examined for wholesomeness
according to the AIM Inspection Series, Foreign Material Manual. The
Manual also includes the sampling rate for these analyses and visual and
sensory examinations, which shall be in accordance with the applicable
table for lot inspection.
The following official marks may be used:
a. Continuous Inspection
Official marks shown as figures 1 through 14 of 7 CFR 52.53
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionC
ode=CFR
of the regulations, provided marks 9 and 10 are limited to plants
operating under QAP.
b. In-Plant Inspection (other than continuous)
Official marks shown as figures 5 through 14 of 7 CFR 52.53
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionC
ode=CFR
of the regulations, provided marks 9 and 10 are limited to plants
operating under QAP.
c. Plant Lot Inspection
Official marks shown as figures 11 through 14 of 7 CFR 52.53
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionC
ode=CFR
of the regulations may be used when the requirements in the
regulations are met.
2. Quality Limitations
Products bearing approved grade marks in conjunction with any statement
of quality must equal or exceed the stated quality.
Approved inspection marks (see 7 CFR 52.53 figures 3 and 4 of the
regulations) may be applied to products labeled under continuous
inspection for which there are no applicable U.S. standards for grades. To
do so, they must be of acceptable commercial quality and include no
implication of quality grade on the label. Approved grade marks,
see 7 CFR 52.53b, figures 1 and 2; 7 CFR 52.53c figures 5 and 6; and

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 136 of 192
7 CFR 52.53d, figures 11 and 12 of the Regulations may not be applied to
products for which there are no applicable U.S. standards for grades.
3. Fill Weight Criteria
Products failing USDA recommended fill weight criteria may not bear
approved identification, EXCEPT if the product fails the fill weight criteria
but subsequently meets the drained weight criteria.
4. Drained Weights
Products failing USDA recommended drained weight criteria may not bear
approved identification.
5. “Grade Not Certified,” Grade D, or Substandard
Products which are “Grade Not Certified,” Grade D, or Substandard for any
reason shall not bear approved identification.
6. Mislabeling and FDA Regulations
Products failing an FDA standard of identity, quality, or fill may not be
associated with approved identification.
Products that have false or misleading information on their labels may not
bear approved identification. Variations during packing can result in
mislabeled products. Examples of common types of mislabeling:
a. Product solids lower than stated or required by an FDA standard of
identity;
b. Net weight lower than stated;
c. Type or style different from stated or inferred by label graphic or
description;
d. Packing media densities different from stated;
e. Proportion of main ingredients different from that indicated by label
statements, graphics, or description;
f. Improper wording concerning the packer or distributor, such as:
“Packed by XYZ Distributors” when the statement should have
been “Packed for XYZ Distributors.”

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 137 of 192
7. Products normally inspected by another Department
SCI Inspectors may be required to inspect products not normally covered
by the U.S. Department of Agriculture. Inspection of fish or fish products
through an agreement with the U.S. Department of Commerce is one
example. The processor must coordinate with the other Department
concerning the proper use of their approved identification, if any.
H. Misrepresentation by Unfair Labeling Practices
The following instructions are for identifying and reporting potential and
fraudulent violations through unfair labeling practices. The Perishable
Agricultural Commodities Act (PACA) Division, USDA, AMS home page may be
found at the following internet address:
http://www.ams.usda.gov/AMSv1.0/PACA. PACA is assigned the responsibility
for administering PACA misrepresentation regulations covering certain frozen
fruits and vegetables. To ensure compliance, PACA Division representatives will
conduct on-site inspections at both shipping and receiving points. Depending on
the results of these inspections and the seriousness of the violation, appropriate
disciplinary action may be taken.
Misrepresentation - Definition
The PACA defines a misrepresentation as an unfair trade practice that may occur
by word, act, mark, stencil, label, statement or deed of the character, kind, grade,
quality, quantity, size, pack, weight, condition, degree of maturity, or State,
country, or region of origin of a particular frozen fruit or vegetable received,
shipped, sold, or offered to be sold in interstate or foreign commerce.
1. Products covered
PACA Division regulations cover most labeled frozen fruits and vegetables
grown and processed in or outside the United States that are transported in
interstate or foreign commerce
Exceptions: Frozen fruit juices;
Frozen fruit drinks;
Frozen fruit juice concentrates; and
Frozen French fried potatoes and potato products.
2. SCI responsibilities
SCI is responsible for providing the PACA Division Misbranding Officer
the necessary documents pertaining to all alleged and fraudulent
misrepresentation violations. The Misbranding Officer will examine the
documents, and may conduct an investigation prior to initiating regulatory
action.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 138 of 192
SCI employees are cautioned not to place a "HOLD" on the item(s) in
question or to refuse inspection service. Our function is only to document
the label misrepresentation, and to report the violation if the lot containing
the alleged violation is shipped.
The applicant has the option to rework a lot to resolve a label
misrepresentation. If a lot is reworked and the alleged violation is
corrected, do not submit any documents to the Misbranding Officer. A
PACA violation occurs only when the lot that contains the alleged violation
is shipped or transferred to another location.
3. In-Plant inspection service
Under in-plant situations, misrepresentations (whether intentional or
unintentional) are not considered potential or alleged violations as long as
the lot is still in the applicant's warehouse. As indicated above, when the
lot enters interstate or foreign commerce, the packer, shipper, or broker
may be in violation of PACA regulations. When the Daily Inspection
Report annotates an alleged label misrepresentation, it's the applicant's
responsibility to inform the Inspector-in-Charge when the particular lot will
be reworked or relabeled. The SCI inspector will sample the new lot to
confirm the alleged violation has been corrected.
a. Verbal reports
It may be necessary to telephone the PACA Division Misbranding
Officer to provide a verbal description of the information shown on
the Misbranding Report. If this is necessary, contact the PACA
Division Misbranding Officer at (202) 720-5073. Conditions that
will require a call to PACA Division are as follows:
(1) A lot that contains an alleged violation has been transferred
to another location; or
(2) A lot that contains an alleged violation continues to remain
in the applicant's warehouse, and in-plant inspection service
is no longer in effect.
b. Documentation
Quality and non-quality results that differ from what is declared on
the label shall be documented as follows:
(1) Daily Inspection Report (DIR) FV-416 or a plant's Daily
Pack Report. Use the number 17 as a symbol to designate a
particular line item that does not comply with PACA
regulations (see In-Plant Inspection, Daily Inspection Report

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 139 of 192
in this Manual);
(2) Certificate of Quality and Condition - Flag the grade
statement of the certificate in accordance with Division
instructions (commercial, state, Department of Defense, or
Federal procurement); and
(3) Complete the Misbranding Report,
c. Distribution of Documents
(1) To PACA Division
To take appropriate action, the PACA Division Misbranding
Officer must receive one copy of the applicable certificate of
quality and condition and the misbranding report. These
documents shall be promptly furnished to the Misbranding
Officer, either by Fax or by use of priority mail.
(a) Fax Number: (202) 690-4413
(b) Mailing Address:
USDA, AMS, FV Programs, PACA Division
1400 Independence Avenue SW
STOP 0242, Room 2095 South Building
Washington, D.C. 20250-0242
Attention: Misbranding Officer
(2) To SCI
The inspector-in-charge will provide the field office with
three copies each of the Certificate of Quality and
Condition, and the Misbranding Report. The Officer-in-
Charge will retain one copy of each document and distribute
the balance as follows:
(a) Regional Office: one copy of each document.
(b) National Office: one copy of each document.
Attach a note to each set of documents that reads: "PACA
Label Misrepresentation."

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 140 of 192
4. Lot inspection service, product not previously graded
The following procedures regarding verbal reports, documentation, and
distribution of documents are applicable when the applicant indicates the
inspection lot has not been previously graded, and the inspector-in-charge
is able to verify this.
a. Verbal reports
Contact the PACA Division Misbranding Officer at (202) 720-
5073.
Provide the PACA Division official with a verbal description of the
information on the Misbranding Report. The telephone call should
be made on the same day that the SCI inspector determines that the
alleged PACA Division label violation has occurred.
b. Documentation
(1) Certificate of Quality and Condition - flag the grade
statement of the certificate in accordance with Division
instructions (commercial, state, Department of Defense, or
Federal procurement); and
(2) Complete the Misbranding Report,
c. Distribution of documents
(1) To PACA Division
One copy of each of the applicable Certificate of Quality
and Condition, and the Misbranding Report shall be
promptly furnished to the PACA Division Misbranding
Officer, either by Fax or use of priority mail.
(a) Fax Number: (202) 690-4413
(b) Mailing Address:
USDA, AMS, FV Programs, PACA Division
1400 Independence Avenue SW
STOP 0242, Room 2095 South Building
Washington, D.C. 20250-0242
Attention: Misbranding Officer

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 141 of 192
(2) To SCI
The Officer-in-Charge shall make sure that the Certificate of
Quality and Condition, and the Misbranding Report are
distributed as follows:
(a) Regional Office: one copy of each document.
(b) National Office: one copy of each document.
Attach a note to each set of documents that reads "PACA
Label Misrepresentation."
(3) To U.S. Customs Service
When imported product is graded and a labeling
misrepresentation is found, provide the local U.S. Customs
Service agent with a copy of the Misbranding Report
.
5. Lot inspection service, product previously graded
When the applicant indicates the lot was previously graded, or another
office verifies that the lot was previously graded, proceed as follows:
a. Obtain a faxed copy of the original score sheet(s) or certificate, if
applicable, with the code mark(s) represented by the lot.
b. When a portion of a lot that was previously graded is resubmitted
(commercial, state, Department of Defense, or Federal
procurement), a labeling misrepresentation will be decided by the
results of the original inspection.
c. When inspection records indicate the origin inspection lot was
unlabeled at time of sampling, a labeling misrepresentation will be
decided by the results of the resubmitted lot.
d. Verbal reports, documentation, and distribution of documents will
be in accordance with the information shown in Section 4. above.
6. Alleged or suspected label misrepresentation
PACA Division will coordinate with the National office to establish a
program to sample product that is suspected of having an alleged label
misrepresentation violation. When a PACA label misrepresentation is
found, it will be at the discretion of the Misbranding Officer to contact the
shipper, broker, or distributor to initiate appropriate corrective or
disciplinary action.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 142 of 192
The PACA Division Misbranding Officer will make the request for official
lot inspection service through the National office. Information concerning
the request will be transmitted to the applicable Regional and field offices
by the Inspection Branch.
Note: Details concerning the lot(s) to sample will be coordinated between the
Misbranding Officer and the Officer-in-Charge. The Misbranding Officer
will contact the shipper, broker, or distributor to schedule an agreeable date
and time for product sampling.
a. Applicant Is PACA Division
When PACA Division is the applicant, the responsibilities of the
area field office are as follows:
(1) Select a Division representative to accompany the PACA
Division Misbranding Officer to the processor's warehouse,
commercial cold storage facility, or distribution center;
(2) Officially sample the appropriate number of sample units for
each lot in accordance with the Regulations Governing
Inspection and Certification of Processed Fruits and
Vegetables and Related Products;
(3) Inspect and grade all sample units for the appropriate quality
and non-quality factors, and examine product for the
presence of foreign material (see the AIM Inspection Series,
Foreign Material Manual);
(4) Transmit a facsimile of the completed Misbranding Report
to the PACA Misbranding Officer, and the Regional and
National offices.
(5) Distribute the grading and inspection documents as
indicated below. Show the applicant as the Perishable
Agricultural Commodities Act Division, and the applicant's
address as, Washington, D.C. 20250;
(a) PACA Division:
Original and two copies of the Certificate of Quality
and Condition (see 4. c. above for mailing address).

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 143 of 192
(b) Regional Office:
One copy of the Certificate of Quality and
Condition, and a one copy of the applicable score
sheet(s) or tally sheet(s).
(c) National Office:
One copy of the Certificate of Quality and
Condition, and a one copy of the applicable score
sheet(s) or tally sheet(s).
(6) Prepare and submit Form AD-742, Transfer and Adjustment
Voucher (which may be found on the Office of the Chief
Information Officer, Approved Computer Generated Forms
site at the following internet address:
http://www.ocio.usda.gov/sites/default/files/docs/2012/AD0
742-F-10-91.pdf),
to the Fruit and Vegetable Program Administrative Office,
Washington, D.C. to recover all costs of the inspection.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 144 of 192
METRICATION
To provide uniformity and simplify application and use of the metric system of measurements
(Systems International or SI), all Division personnel will utilize the following rules when
requested or when it is appropriate to apply the metric system to inspection and/or certification.
Symbols
For purposes of uniformity, the following symbols should be used in metric statements
where appropriate:
liter l meter m
milliliter ml millimeter mm
kilogram kg centimeter cm
gram g square meter m
2
milligram mg square centimeter cm
2
degree Celsius degree C kilopascal kpa
• Symbols are not capitalized unless the unit is derived from a proper name, e.g., degree
C, for degree Celsius.
• Periods are not to be used after the symbol.
• Symbols are always written in the singular form. Do not add "s" to express the plural
when the symbol is used.
• Square centimeter (cm
2
) refers to individual squares of 1 cm on an edge, e.g., 8 square
centimeters (8 cm
2
) means an area in the shape of a rectangle 1 cm by 8 cm.
• On the other hand, a square 8 cm on an edge is an area in the shape of a square 8 cm
by 8 cm for a total area of 64 cm
2
. When it is possible for the symbol to be misread, it
is recommended that the measurement be written out (8 cm
2
= 8 square centimeters, or
an area of 1 cm by 8 cm).
A. Numerical Values
1. Conversions:
For accurate of equivalency, multiply the specified quantity by the
conversion factor given in the table on the following page:

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 145 of 192
To convert from To Multiply by
ounce-(avoirdupois) kilograms-(kg) 0.0235
pound-(avoirdupois) kilograms-(kg) 0.0453592
fluid ounce-(U.S.) liter-(l) 0.029574
pint-(U.S. Liquid) liter-(l) 0.473176
quart-(U.S. Liquid) liter-(l) 0.946353
gallon-(U.S. Liquid) liter-(l) 3.785412
inch meter-(m) 0.0254
foot meter-(m) 0.3048
yard meter-(m) 0.9144
square inch centimeter
2
-(cm
2
) 6.4516
square foot meter
2
-(m
2
) 0.092903
cubic inch centimeter
3
-(cm
3
) 16.387064
cubic foot liter-(l) 28.316847
inch of mercury kilopascal 3.39
(vacuum)
Note that most of the above conversions result in kilograms, liters and meters. Changing
these results to submultiples requires only moving the decimal two places to the right for
centi-submultiples, and three places right for milli-submultiples.
B. Rounding
In all conversions, the number of significant digits retained should be such that
accuracy is neither sacrificed nor exaggerated. As a general rule, retain only the
first three digits. When converting minimum (not less than) tolerances, round
down to obtain the converted value, e.g., 196.4 g becomes 196 g or 1.759 m
becomes 1.75 m. Conversely, round up in the case of maximum (not more than)
tolerances, e.g., 196.4 g becomes 197 g, or 1.759 m becomes 1.76 m. When
converting values that are not limits, round in the normal manner, e.g., 196.4 g
becomes 196 g, or 1.759 m becomes 1.76 m.
1. Rule of 1000
Selected multiple and sub-multiple prefixes for units should result in
numerical values greater than 1 and less than 1000, e.g., 1.96 kg, not 1960
g, or 750 mm (75 cm), not 0.75 m.
2. Use only decimal fractions. Common fractions (½, ¼, etc.), are not
permitted.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 146 of 192
C. Systems
English System - Use of the English system without reference to Systems
International system.
Full Metric - Use Systems International units without reference to English units.
Soft Metric Conversion - Where the measurement is in English terms so that it
appears as a whole number, e.g., 1-lb (453.6 g).
Hard Metric Conversion - Where the measurement is made in SI terms so that it
appears as a round number, e.g., 450 g (0.99 lb.).
D. Domestic and metric measures
Domestic Weights
1 grain = 0.0001428 pounds
7,000 grains = 1 pound
16 ounces = 1 pound
2,000 pounds = 1 short ton
2,240 pounds = l long ton
0.892857 long tons = 1 short ton
1.12 short tons = 1 long ton
Metric Weights
1,000 micrograms (F) = 1 milligram (mg)
1,000 milligrams = 1 gram (g)
1,000 grams = 1 kilogram (kg)
1,000 kilograms = 1 metric ton
Domestic equivalents of metric measures
1 gram = 0.035274 ounces
1 kilogram = 2.204622 pounds
1 metric ton = 2,204.622 pounds
1 metric ton = 1.1023 short tons
1 metric ton = 0.9842 long tons
1 liter
liquid measure = 2.1134 pints,
1 liter
liquid measure = 1.05671 quarts,
1 liter
liquid measure = 0.26418 gallons,

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 147 of 192
Metric equivalents of domestic measures
1 grain = 64.799 mg
1 ounce = 28.3495 grams
1 pound, = 453.5924 grams
1 short ton = 907.185 kg
1 short ton = 0.9072 metric tons
1 long ton = 1,016.047kg
1 long ton = 1.016 metric tons
1 pint,
liquid measure = 0.47317 liters
1 quart,
liquid measure = 0.9463 liters
1 gallon,
liquid measure = 3.785 liters
Liquid measure: British-U.S. equivalents
1 British quart,
liquid measure = 1.2009 U.S. quarts
1 British gallon,
liquid measure = 1.20094 U.S. gallons

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 148 of 192
NON-MANIPULATION CERTIFICATION INSPECTION SERVICE
This instruction outlines procedures for performing Non-Manipulation inspection services and
issuing Non-Manipulation Certificates. Inspection for Non-Manipulation Certification combines
a Plant Survey, a Condition of Container (COC) examination, and a review of Product Identity
Documents. This last item involves tracing records for the product from its departure from the
country of origin through its present storage location. A Non-Manipulation Certificate is a Letter
Report provided to the applicant to certify that based on SCI inspection of records, COC
examination, and plant survey, the product being certified has been:
• Handled in accordance with Good Manufacturing Practices,
• Stored under appropriate conditions, and
• Has maintained its identity to assure continued suitability.
These procedures allow flexibility to meet the applicant’s needs. The following references are
found in the AIM Inspection Series of instructional manuals:
• General Procedures Manual, Regulations Section
• Sampling Manual, Sampling Procedures
• Condition of Food Container Manual, U.S. Standards for Condition of Food Containers
• Sanitation and Safety Manual, Plant Sanitation Requirements, and Good Manufacturing
Practices (GMP’s)
• Certification Manual
• Form FV-356 Application for Inspection and Certificate of Sampling which may be found
on the AMS Forms Catalog at the following internet address:
http://agnis/AMSFormsCatalog/Forms/AllItems.aspx.
A. Applicant responsibilities:
1. Request the Non-Manipulation Certification service
2. Provide information as requested for Non-Manipulation Certification
services - application for inspection and certificate of sampling form
FV-356; and
3. Provide information indicated on the “Documents required for
Non-Manipulation Certification” shown below to the appropriate SCI field
office.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 149 of 192
Documents required for Non-Manipulation Certification
It is the applicant’s responsibility to provide clear and concise documentation showing that the
product has not been tampered with or undergone reprocessing. To do so, documents must
provide uninterrupted documentation, in chronological order, tracing the identity of the product
from departure from the country of origin to its present location. Supporting documents are to be
submitted with an inventory listing them in chronological order for tracing purposes.
These documents may include, but are not limited to:
A. Export certificates from the country of origin;
B. Intermediary conveyance document;
C. Incoming bill of lading;
D. Incoming receipt documents, i.e., warehousing count sheets;
E. Inventory control documents; and
F. Warehouse documents indicating the date of storage, i.e., storage billing
documents.
The following are examples of inadequate documentation:
A. A document that cannot be linked to the previous document in the tracing
process.
B. A lapse in time or a change in location without documented possession of the
product would indicate missing documents or possible manipulation of the
product.
C. Illegible documents
D. Documents that show signs of having been tampered with or altered.
E. Documents showing intermediary storage within the United States where the
storage facility was not:
1. a Bonded Customs Storage facility; or
2. a storage facility where the product had previously been certified under
SCI Non-Manipulation Certification.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 150 of 192
B. SCI field office responsibilities:
1. Complete the application for inspection and certificate of sampling form
(FV-356);
2. Supply the applicant with a copy of “Documents required for
Non-Manipulation Certification” shown above;
3. Provide the applicant with an estimated fee. The current fees for various
inspection services are located on the USDA, SCI internet site. Click on the “SCI
News” link found in the “SCI Services” block at the following internet address:
http://www.ams.usda.gov/AMSv1.0/processedinspection, next click on the
“Current User Fee Information” link in the “SCI Services and Fees”
section, then click on the “Fee for Specialty Crops Inspection (SCI)
Division (SCI) Audit, Survey, and Verification Programs Not Covered by
Other Fee Announcements”;
4. Review the documents submitted by the applicant, and complete the
Product Identity Review Sheet shown below. If the documentation is
determined to be inadequate, the inspector will notify the applicant, and
request additional documents to complete the tracing process. See
examples of inadequate documentation in subsection d, numbers (1)
through (5) of these instructions;
5. Schedule product inspection at the warehouse or storage location. Request
that the product be staged prior to arrival for accessibility, and ask that
warehouse staff be available to assist as needed;
6. Provide inspection results to the applicant as follows:
a. Prepare the Non-Manipulation Certificate and send it to the
applicant at the address indicated on form FV-356; or
b. Inform the applicant of the failing portion of the inspection and that
a Non-Manipulation Certificate is not available;
7. Bill the applicant; and
8. File the inspection documents in the applicant’s file in the SCI field office.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 151 of 192
Sheet __ of ___
Product Identity Review Sheet Date: Print and Sign Name:
Title of
Document
Document
Number
Originator of
Document
Date on
Document
Product
Quantity and
Container Size
Label and Code
Markings
Document traces
with previous
document
Yes/No/NA
Comments:
Fruit and Vegetable Programs
Specialty Crops Inspection Division

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 152 of 192
C. Inspection procedures
1. Review
SCI inspection personnel will review the Product Identity documents
submitted by the applicant before performing the plant survey or condition
of container examination.
a. The following shall be recorded on the Product Identity Review
Sheet shown above for each document:
(1) Title of the document;
(2) Number of the document;
(3) Originator of the document;
(4) Date on the document ;
(5) Product indicated;
(6) Quantity and container size indicated;
(7) Label and code markings; and
(8) Determination of whether or not the document follows the
previous document in the tracing process.
b. Documents shall be recorded in chronological order as follows:
(1) Export certificate from the country of origin;
(2) Documents showing the mode of conveyance, vessel, etc;
(3) Any intermediary transport or storage facility documents;
(4) Transfer documents from each mode of conveyance;
(5) Documents showing receipt of the product at the location
where the inspection is being performed; and
(6) Inventory and/or storage records for the product at the
inspection location.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 153 of 192
c. Complete the Product Identity Review Sheet; a completed example
is shown following this section. Complete the following:
(1) Title of Document block to indicate the title of the
document being reviewed;
(2) Document Number block to indicate the document’s
identifying code number or serial number;
(3) Originator of Document block to indicate the name of the
person/organization that signed for or completed the
document;
(4) Date on Document block to indicate the date the document
was completed;
(5) Product block to indicate the name of the product as
identified on the document;
(6) Quantity and Container Size block to indicate the total
quantity, size, and type of container of product as identified
on the document;
(7) Label and Code Markings block to indicate the complete
label and code markings as identified on the document; and
(8) Document Traces with Previous Document block will be
completed with Yes, No, or NA (not applicable).
(a) “Yes” indicates the document can be traced to the
previous document in the tracing process by date,
location, and/or time;
(b) “No” indicates the document does not trace to the
previous document in the tracing process. A lapse of
time or change in location without documented
possession of the product leads to missing
documents, which could indicate possible
manipulation of product. If this happens, the
applicant will be required to provide additional
documentation to complete the tracing process
before the Non-Manipulation Certificate can be
issued;

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 154 of 192
(c) “NA” will indicate the document is the first in a
series of documents being traced, and has no prior
document to which to trace.
d. If the inspector finds inadequate documentation, the problem(s)
shall be described in the comments section of the Product Identity
Review Sheet. If additional space is required, the back of the form
may be referenced and used for continuation. The inspector shall
notify the applicant if inadequate documentation has been provided
and request additional documents to complete the tracing process.
The following are examples of inadequate documentation:
(1) A document that cannot be linked to the previous document
in the tracing process.
(2) A lapse in time or a change in location without documented
possession of the product.
(3) Illegible documents.
(4) Documents that show signs of tampering or alteration.
(5) Documents showing intermediary storage within the United
States where the storage facility was not a:
(a) Bonded Customs Storage facility; or
(b) Storage facility where the product had previously
been certified under SCI Non-Manipulation
Certification
2. Plant survey
A plant survey at the facility where the product is currently stored will be
completed in accordance with guidelines in the AIM Inspection Series,
Sanitation and Safety Manual. Only the front page, page 2, and section H
of the plant survey are completed. The temperature and other observations
(e.g., dry, humid) will be noted on the Non-Manipulation Certificate.
3. Condition of container
A condition of container inspection will be performed on the lot in
accordance with the AIM Inspection Series, Condition of Food Container
Manual.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 155 of 192
4. Certification
A letter report will be completed following the guidelines in The AIM
Inspection Series, Certification Manual. An example is shown below. This
document will be based on the following:
(a) Applicant information;
(b) Product identity review;
(c) Warehouse evaluation results; and
(d) Condition of container inspection.
5. Billing
Prepare billing in accordance with Division instructions. See section B. 3
on page 150.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 156 of 192
Example of Non-Manipulation Letter Report:
(Insert Current Letterhead)
January 25, 2011
YAK-002-11
Growers and Shippers Co.
101 Your Street
Anytown, USA 23456
Reference: Inspection performed on January 25, 2011.
We have completed the warehouse portion of the plant survey for warehouse XYZ located at123
North Warehouse Drive, Anytown, USA 23456. The warehouse conditions observed were
acceptable.
A condition of container inspection was performed on the lot. The lot was found to meet
applicable U.S. Standards for Condition of Food Containers. The sample units were labeled
Really Good Food Product, 25 kg. (all primary label information including code markings should
be included, as noted in the AIM Inspection Series, Certification Manual). Certificate number(s)
(ABC123, etc.) shows that (insert the product name) was imported into the USA from (insert
Country of origin) and stored at XYZ warehouse for a period of 9 months. Based on our
inspection of records we have determined that (insert the product name) was handled in
accordance with good manufacturing practices and stored under appropriate conditions to assure
its security and continued suitability.
Inspection of records shows these samples represent 208 pallets of 24/25 kg containers each and 1
pallet of 8/25 kg containers, weighing a total of 125,000 kilograms.
John Q. Public
Agricultural Commodity Grader

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 157 of 192
Sheet __ of ___
Product Identity Review Sheet Date: 11/25/2010 Print and Sign Name: John Q. Smith
John Q. Smith
Title of
Document
(as shown on
document)
Document
Number
(as shown on
document)
Originator of
Document
(as shown on document)
Date on
Document
(as shown on
document)
Product
(as shown on document)
Quantity and
Container Size
(as shown on document)
Label and Code
Markings
(as shown on document)
Document
traces with
previous
document
Yes/No/NA
Certificate from
(country of
origin)
ABC 123
Agricultural
Department
Country of Origin
A.B. Inspector
10/23/10
(Product as identified
on certificate)
125,000 kg.
5,000
25 kilogram
Bags
Really Good Food
Product
A123, B123, C123
N/A
Shipping Bill of
Lading
0001112223
33
Land or Sea Import
Company
Jack D. Shipper
10/25/10
(Product as identified
on shipping bill of
lading)
125,000 kg.
5,000
25 kg. Bags
Really Good Food
Product A123,
B123, C123
Yes
Incoming Port
Receipt
A1B2C3
Port of Entry
Mary L. Smith
10/27/10
(Product as identified
on incoming port
receipt)
125,000 kg
Really Good Food
Product A123,
B123, C123
Yes
Shipping Bill of
Lading
XYZ445566
Ship to You Inc.
J. Doe
10/29/10
(Product as identified
on incoming bill of
lading)
125,000 kg
5,000
25 kilogram
Bags
Really Good Food
Product A123,
B123, C123
Yes
Warehouse
Receiving Slip
09876
The Warehouse
Co.
I. M. Boss
10/30/10
(Product as identified
on incoming
Warehouse slip)
5,000
25 kilogram
Bags
Really Good Food
Product A123,
B123, C123
Yes
Warehouse
Storage Bill
987345000
The Warehouse
Co.
I. M. Boss
10/21/10
(Product as identified
on warehouse storage
bill)
5,000
25 kilogram
Bags
Really Good Food
Product A123,
B123, C123
Yes
Comments: Documentation acceptable.
Fruit and Vegetable Programs
Specialty Crops Inspection Division

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 158 of 192
PRODUCT ORIGIN REQUIREMENTS
SCI inspectors in certain situations may have responsibilities for monitoring and reporting the use
of foreign product or product components in processed foods under their inspection. Applicable
origin requirements are stated in the procurement documents such as contracts, solicitations,
announcements, invitations to bid, or in governing rules or regulations. In some instances the
contractor/producer may be required to provide the SCI inspector with a letter or certification of
conformance attesting to the product’s compliance with a product origin requirement.
If a product is to be of 100 percent domestic origin, the following examples illustrate products
that do NOT meet this requirement:
A. Imported pineapple tidbits to be used in a canned fruit cocktail.
B. A concentrated or single strength juice product that contains an imported juice
concentrate component.
C. Use of imported tomato paste in the production of canned vegetarian beans.
D. Production of frozen French fried potatoes from potatoes grown in Canada.
When present at the processing facility, the inspector should monitor product formulation and
verify that the product and product components are of the required origin. The inspector should
be familiar with and follow the appropriate verification instructions for the product inspected.
Discuss deviations with appropriate supervisory personnel, and notify the appropriate plant
official of any deviations as instructed.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 159 of 192
REGULATIONS
SCI provides an impartial, official inspection service for processed fruits and vegetables.
Applicants may use this service to obtain inspections of any of these products in which they have
a financial interest. The service is voluntary and self-supporting, and is offered on a fee-for-
service basis through the Agricultural Marketing Services, Fruit and Vegetable Program.
The inspection service facilitates the orderly marketing of products in many respects. It helps the
buyer or the seller determine if the terms of contracts or purchase orders have been met. It helps
establish loan values, and supplements in-plant quality control programs. Inspection helps in
settling claims for damage incurred in transit or storage.
The Regulations in the publication at the following internet site contain the current rules that
govern inspection and certification of processed products, including sampling, fees, sanitary
requirements for approved plants, and related matters.
The Regulations, 7 CFR 52, is available at the following internet address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CFR
.
The following is to provide clarification of the regulations governing inspection and certification
regarding the use of Approved Identification (7 CFR 52.53). The use of approved lot inspection
id requires lot inspection be performed.
A. The use of the grade shields depicted in 7 CFR 52.53(c) figures 5 & 6 and the
"PACKED UNDER INSPECTION OF THE U.S. DEPT. OF AGRICULTURE"
statement (with or without the shield) as depicted in figures 7 & 8, shall only be
used on product that is produced by a plant operating under USDA inspection
service contracts when an in-plant inspector is present during processing,
packaging, and packing.
B. Except for product packed under the Quality Assurance Program, any product that
is produced without an in-plant inspector present during processing, packaging,
and packing may only bear the marks covering lot inspection as depicted in 7 CFR
52.53(d) figures 11 through 14.
Example:
If a processing plant wants to use approved identification marks and has an inspector present
during its first shift, but runs a second shift with no inspector present, the plant may use the
approved marks depicted in 7 CFR 52.53(c) figures 5 through 8 for the product produced on first
shift only. If the plant wishes to use approved identification marks on second shift product, the
plant may only use the marks depicted in 7 CFR 52.53(d) figures 11 through 14. The use of any
other approved identification mark on the second shift product is not permitted.
SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 160 of 192
RE-INSPECTION
A. Classification of Inspection
When contacted by an applicant for inspection, the field office should inquire if the
product has been inspected before. If it has been inspected before and can be
identified, contact the original inspection office to obtain pertinent information
regarding the lot such as grade, condition, lot size, any deviations, etc.
1. If the re-inspection results agree with the original results, proceed with
certification, making no reference to the original certificate.
2. If the re-inspection results do not agree with the original results and it is
apparent that changes in quality are the results of condition factors, proceed
with certification using the word NOW preceding the grade statement.
(See AIM Inspection Series, Certification Manual.)
3. If the re-inspection results do not agree with the original results and it is
apparent that changes are not the results of condition factors, an appeal
inspection is in order. However before issuing the appeal certificate, do the
following:
a. Increase sample size, particularly when the first sampling is less
than 13.
b. Before certification, contact your immediate supervisor for further
instructions regarding the disposition of the inspection.
c. If possible, draw supplemental sample units for possible review by
the Regional or National offices.
4. Fees
a. If the inspection is a re-inspection to bring the lot up to date, the
usual fee rate will apply.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 161 of 192
REVIEW PROGRAMS
It is SCI policy to continually evaluate the adequacy and effectiveness of its services. An
effective means of so doing is a product review program to determine if offices are grading and
inspecting products uniformly.
The Division currently has several types of product review programs in effect.
A. Review Program for Processed Raisins
The processed raisin review covers the grading of processed raisins, and is
conducted monthly. All lots of raisins will be sampled and graded by the field
office using normal procedures. The review sample will be selected from the
remainder of the composite sample used to determine the quality grade of the lot.
It will be selected from the first lot of raisins sampled and graded each month, and
subsequently every tenth lot of raisins sampled and graded that month. At the
beginning of each month, the review program will begin again, starting with lot 1.
The following procedures shall be used by each field office, sub-office, or
inspection point grading processed raisins:
1. Sample and grade all lots in accordance with current instructions.
2. The review sample consists of 64 ounces of raisins. Select this 64 ounce
review sample from the remainder of the composite used to determine the
quality grade of the first lot graded, and subsequently every tenth lot graded
during each month.
3. Complete the score sheet and the micro analytical work sheet. In the
remarks section of the score sheet, note "Sample submitted on (date) for
National Processed Raisin Review".
4. Make two copies of the score sheet and the micro analytical work sheet.
6. Prepare a speed memo with the name of applicant, product name, lot
number, codes, field office, and sample number for the month (i.e., first
sample, second sample, etc.). Make two copies of the memo. Speed memos
(form AD 0311) may be found on the Office of the Chief Information
Officer, Approved Computer Generated Forms site at the following internet
address: http://www.ocio.usda.gov/sites/default/files/docs/2012/AD0311-F-
03-81-OCIO.PDF.
7. Mark the samples to clearly identify the applicant, lot number, and field
office. Also mark on the shipping container "National Processed Raisin
Review (insert month and year)."

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 162 of 192
7. Send the memo, a copy of the score sheet and micro analytical work sheet,
and the raisin review sample to the Fresno, CA field office within 5
working days after final grading.
8. The Fresno office will grade and perform microanalysis on review samples,
and record their results on official score sheets and micro worksheets.
Photocopies of these documents will be sent to the submitting field office
and the Regional offices within 15 working days after the end of the month.
Review results and follow up
1. The Regional offices and the National office will review the inspection
results with the Fresno office on a monthly basis.
2. The Regional offices will follow up on lots which show significantly
different results between the original lot and the review sample. The
Regional office will coordinate corrective action with the National office
and the area field office.
3. The Inspection Section will review reports quarterly. If corrective action is
needed, the Division Director and Regional Section Heads are notified.
B. Review Programs for Flavor Evaluation of Canned Ripe Olives
This instruction establishes two review programs for the evaluation of flavor in
canned ripe olives. The goal of these programs is to maintain uniform inspection
of flavor in domestic and imported canned ripe olives. One review will focus on
product found to have questionable or off-flavor; the other review will cover
canned ripe olives inspected by all SCI offices to establish uniformity.
SCI is authorized to grade and certify domestic and imported canned ripe olives
under the Regulations, 7 CFR, Parts 932 and 944, which also establish the
minimum quality requirements for these products. Canned ripe olives reviewed
under these programs will be graded according to the requirements specified in the
CFR. Each SCI area office will submit sample units from lots found to have off-
flavor for the Questionable or Off-Flavor Review. In addition, all area offices
involved in inspection of canned ripe olives will participate in the All-Office
Flavor Review for olives of all styles and container types.
1. Procedures for Questionable or Off-Flavor Review
If a questionable or off-flavor is detected during the process of inspecting a
lot of olives, the area office shall submit subsample units representing the
lot to the Western Regional office, Eastern Regional office, and Stockton
Area office. A final grade and score shall not be assigned to the lot
until notified by the applicable Regional office.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 163 of 192
Three subsamples of equal amounts will be taken from one of the
questionable or off-flavor samples inspected by the area office. The olives
submitted for review must be from the same sample container inspected by
the area office.
a. The subsamples of olives shall be put into 16-ounce plastic, wide
mouth jars with twist top lids. The jars shall be filled with brine,
sealed, and packaged into shipping cases with cold packs to
maintain the condition of the subsamples. Ship the samples by
overnight delivery.
b. Complete the Canned Ripe Olive Flavor Review sheet, form FV-
377 which may be found on the AMS Forms Catalog at the
following intranet address:
http://agnis/AMSFormsCatalog/Forms/AllItems.aspx. Include the
Customs Entry Number and shipping container number. Mark each
subsample with the shipping container number, and “(insert area
office) Questionable or Off-Flavor Review Sample(s).” Include the
FV-377 along with the subsample. Send an e-mail to the
supervisors in the three receiving offices to notify them that
samples are being sent.
c. Do not complete the flavor portion of the original score sheet until
the flavor review results have been received from your Regional
office. The Regional offices and Stockton Area office will
determine the flavor grade of the samples. The applicable Regional
office will send the results to the area office and the National office,
identifying the reports as “Questionable or Off-Flavor Review
Results”.
2. Procedures for All-Office Flavor Review
The All-Office Flavor Review can be activated or deactivated as needed.
The Division Director will determine the need for the review with input
from the Regional offices and Inspection and Standardization Section.
Regional offices will notify area offices by e-mail of flavor review
activation or deactivation status
When the program is in effect, each area office, including the Stockton
Area office, will select three additional sample units from lots sampled on a
monthly basis. For example, if the sampling plan requires 13 sample units,
collect 16 sample units from the lot. The three review samples should be
duplicate codes matching at least one of the samples drawn for inspection,
and all review samples must have the same production code. One way to
accomplish this is to pull four containers from a single case, and after

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 164 of 192
verifying that all four codes are the same, mark three containers as review
samples. The fourth will be included among the 13 samples for grading.
Area offices will submit one unopened sample from each lot to the Western
Regional office, one to the Eastern Regional office, and one to the
Stockton, CA Area office for review. In order to evaluate the effectiveness
of the inspection service provided, sample units must be reviewed from
various importers and countries of origin.
Note: The Regional and National offices may change the frequency and number
of samples selected at any time. Regional offices will notify field offices
of the sampling frequency by e-mail. Area offices will be asked to submit
sample units from lots sampled at one of the following rates:
a. From every lot,
b. From a predetermined reduced number of lots (such as drawing
review sample units from every fifth or tenth lot sampled), or
c. At a predetermined number of sample units per month (such as
submitting two sample units per month).
Depending on results of the All-Office Flavor Review, and /or
recommendations from the National and Regional offices, the Regional
offices may select one or more area offices to submit additional sample
units. The Regional offices will notify the selected area offices and the
National office by e-mail.
a. Immediately upon completion of inspection, the area office will
submit the review sample unit from each designated lot along with a
copy of the score sheets. Sample units will be marked, “(insert area
office) All-Office Flavor Review Sample).” Complete and submit
the Canned Ripe Olive Flavor Review sheet, form FV-377 which
may be found on the AMS Forms Catalog at the following intranet
address: http://agnis/AMSFormsCatalog/Forms/AllItems.aspx.
b. Both Regional offices and the Stockton Area office will determine
the flavor grade of the review samples. The evaluation of Stockton
Area office samples shall be performed by an inspector or
supervisor other than the original grader. The applicable Regional
office will send the results to the area office and the National office,
identifying the reports as “All-Office Flavor Review Results.”
c. The applicable Regional office will follow-up up on sample units
found to be different than the original grade of the lot, and will

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 165 of 192
coordinate corrective action with the National office and respective
area office.
C. Product Grade Review Procedures for Products Not Covered by Formal Review
Programs (Monthly and Four Month Cycle Review Program)
These instructions provide for a review of all products:
• For which USDA provides an official grade or determination (including
NAG, CID, purchase order or buyer’s specification).
• Which are not currently covered by a formal grading review program
Products for the following programs are not part of this review:
• USDA Purchases
• Operational Rations In-Plant Inspection Review Program
• Review Program for Concentrated Orange Juice for Manufacturing (OM)
for Futures Contracts,
• Review Program for Processed Raisins, and
• Review Program for Flavor Evaluation of Canned Ripe Olives.
1. Frequency of product reviews:
The Monthly Review Program is conducted every month by the supervisor
(Officer-in-Charge (OIC), assistant OIC, subarea supervisor), or an
individual officially acting in that capacity.
The Four Month Cycle Review Program is conducted once during period of
four-months, resulting in three review periods per fiscal year. The review
periods are from October 1 through January 31, February 1 through
May 31, and June 1 through September 30. Samples submitted for the Four
Month Cycle Review will be reviewed by both the area supervisor and the
National office.
Review sample submissions may be tracked on the optional worksheets
“Optional Monthly and Four Month Cycle Review Program Submission
Worksheet” and “Monthly Review Program Summary Sheet” included at
the end of this instruction.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 166 of 192
2. Submission of Monthly and Four Month Cycle Review Program samples:
a. Label each sample with the following information: field office,
applicant, date graded, grade assigned, and inspector.
b. Identify exterior of shipping container “Four Month Cycle Review,”
c. Identify on exterior of shipping case if frozen or chilled samples are
enclosed (Example: “Frozen samples, open upon receipt”) ,
d. Freeze samples of chilled product to preserve quality and ship
frozen samples to arrive the next day with sufficient dry ice or
freezer packs to maintain frozen condition of sample.
e. Refrigerate subsamples of canned product to maintain product
quality and safety and ship with sufficient freezer packs or dry ice
to keep the product cold.
f. Ship chilled or frozen samples to arrive the next business day; DO
NOT ship before weekends or holidays,
g. Seal and apply tamper evident tape,
h. Pack samples securely to prevent damage or deterioration,
i. Amount of sample to submit: For most products, the original
container or package will be adequate. For products with a specific
sample unit size, follow Division procedures to obtain sufficient
product for grading. In the case of bulk products, draw a two pound
(thirty two ounces) subsample (or a minimum of six fluid ounces
for juice concentrates). Submit a minimum of thirty two ounces of
single strength juices.
3. Procedure for Monthly Review Program:
a. Lot inspection:
The supervisor will develop a schedule to systematically review one
lot per month from the field office and each inspection point.
For each lot selected for review, one sample unit is drawn (two
samples if both Monthly and Four Month Cycle Reviews are
performed.) The supervisor will designate the lot to be reviewed
prior to sampling so that additional sample units can be drawn.
This also includes Child Nutrition (CN) products produced under
lot inspection.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 167 of 192
Complete the top portion of the Monthly and Four Month Cycle
Review Program Sample Submittal Sheet, Form FV-379 and
submit with the samples brought to the grading lab. The lot shall be
graded and certified as usual. The review sample shall not be
graded at this time.
(Form FV-379 can be found on the AMS Forms Catalog at the
following intranet address:
http://agnis/AMSFormsCatalog/Forms/AllItems.aspx)
The supervisor shall grade the review sample after the inspector has
completed grading the lot and issued the certificate (or other
inspection document) by completing a score sheet, or annotating the
score sheet issued by the inspector. The supervisor should compare
the grade of the review sample with the original score sheet(s) and
certificate. He or she will make any recommendations to the
inspector who carried out the original grading, and record remarks
in the “Area Office Review” section of the Monthly and Four
Month Cycle Review Program Sample Submittal Sheet, Form
FV-379. Alternately, the supervisor may create a “new review” in
the “Product Grade Review Program” database in Lotus Notes and
enter the information for the top of the form and the “Area Office
Review.”
A copy of the completed Monthly and Four Month Cycle Review
Program Sample Submittal Sheet, Form FV-379, (or a printed copy
of the “new review” from the Lotus Notes “Product Grade Review
Program” database) and copies of the score sheet(s) (including
reverse, if used, or copies of labels) and supporting documentation
are then sent to the appropriate Regional office.
The supervisor will record a summary of the review results on the
worksheet Monthly Review Program Summary Sheet. The original
is retained in the field office, and a copy is mailed to the Regional
office at the end of each month.
b. In plant, Child Nutrition (CN) in plant and Quality Assurance
Program (QAP) inspection:
In addition to the review for lot inspection, the supervisor will
develop a schedule to systematically review one lot per month from
each processing plant (including CN and QAP plants) in operation
during the month.
While on a supervisory visit, the supervisor will randomly designate
one lot to be used for the monthly product review. For QAP, this
lot must have been verified by the USDA grader.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 168 of 192
The supervisor shall draw one sample unit at random from the
designated lot in the warehouse/storage, and complete the top
portion of the FV-379. The supervisor shall grade the review
sample by completing a score sheet, or annotating the score sheet
issued by the inspector, and compare the grade of the review sample
with the original score sheet(s) and inspection records. He or she
will make any recommendations to the inspector who carried out
the original grading, and record remarks on the FV-379, or by
creating a “new review” in the “Product Grade Review Program”
database in Lotus Notes.
A copy of the completed FV-379 (or a printed copy from Product
Grade Review Program database in Lotus Notes) and copies of the
score sheet(s) (including reverse, if used, or copies of labels) and
supporting documentation are then sent to the appropriate Regional
office.
The supervisor will record a summary of the review results on the
Monthly Review Program Summary Sheet. The original is retained
in the field office, and a copy is mailed to the appropriate Regional
office at the end of each month.
4. Procedure for the Four Month Cycle Review Program:
a. Lot and In-plant Product Review
The supervisor will select one lot during each four month cycle
from each of the following:
• Lot inspections conducted in the field office;
• Lot inspections performed at each inspection point.
In addition, the supervisor will select one lot from each of the
processing plants, including CN and QAP in operation during the
four month cycle.
For example, a field office having two inspection points and four
processing plant should submit three samples for lot inspection (one
per field office and inspection point) and four samples representing
each of the processing facilities in operation during the period.
After the each lot has been graded by the inspector, the supervisor
shall grade one of each of the review samples by completing a score
sheet, or annotating the score sheet issued by the inspector, and
compare the grade of the review sample with the original inspection
records. He or she will make any recommendations to the inspector
who carried out the original grading, and record remarks on the FV-
379 (or by creating a “new review” in the “Product Grade Review

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 169 of 192
Program” database in Lotus Notes).
b. The second review sample will be submitted to the National office
with copies of:
• Score sheet(s), (including reverse, if used, or copies of labels),
• Supporting documentation (including certificate, letter report or
other document, if issued,
• Lab results,
• A copy of the FV-379 (or a printed copy from the Lotus Notes
“Product Grade Review Program” database) indicating the
results of the field office review, and
• Label and ship samples following instructions in C. 2.
Retain a completed copy of the FV-379 in the field office. The
supervisors shall complete the Monthly Review Program Summary
Sheet, retaining the original in the field office and mailing a copy to
the Regional office.
The National office shall grade the samples submitted and compare
the results. The reviewing official from the National office shall
record the grading results and any comments or recommendations
on the copy of the Monthly and Four Month Cycle Review Program
Sample Submittal Sheet and enter the grading results and any
comments or recommendations in the “Product Grade Review
Program” database in Lotus Notes. A summary of all findings will
be returned to the Regional Section Heads and will be entered into
the database used to track the consistency of grading. Field offices
may view the results for samples submitted in the “Product Grade
Review Program” database in Lotus Notes.
5. Cost of samples
The Regulations Governing Inspection and Certification of Processed
Fruits and Vegetables and Related Products provides the authority for the
agency to draw samples for review. All costs shall be borne by the
applicants.
6. Instructions for completing FV-379
Please see the example and instructions on the following pages.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 170 of 192
Date Submitted:
Area Office:
Inspection Point (if applicable):
Samples Submitted? Y or N
Type of Inspection
CN In-Plant CN Lot In-Plant Lot QAP
In-plant Inspection
1
st
Shift 2
nd
Shift 3
rd
Shift
Applicant Name:
Applicant Address:
Certificate No.
USDA Grade Assigned:
Date Graded:
Product:
Product Type:
Canned Chilled Frozen Dried
Code:
Inspectors Name (Print and Sign):
AREA OFFICE REVIEW
Results of Evaluation:
I agree with grader’s evaluation. Y or N
Comments:
Supporting documentation submitted Yes, No
Supervisory evaluation documentation attached Yes, No
Return cooler to field office Yes, No, Not Applicable
Return freezer packs to field office
Yes,
No,
Not Applicable
Signature (Print and Sign):
Date:
NATIONAL OFFICE REVIEW
Results of Evaluation:
Supporting documentation submitted Yes, No
Supervisory evaluation documentation attached Yes, No
I agree with the Grader’s evaluation Yes, No
I agree with the Area Office evaluation Yes, No
Signature (Print and Sign):
Date:
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
Monthly and Four Month Cycle Review Program Sample Submittal Sheet
FV-379 (EXAMPLE)

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 171 of 192
Open “Product Grade Review Program on amsnotes” and “Create” a “New Review,” or print off a
blank FV-379 from the AMS Forms catalog. The following items correspond to the numbered
sections on the previous page:
1. Enter the date the sample is mailed/shipped to field office.
2. Select the field office from Lotus Notes drop down list or complete using
locations listed in Organizational Directory.
3. Select inspection point from Lotus Notes drop down list or complete using
locations listed in Organizational Directory. If samples are not from an
inspection point, show “Not Applicable.”
4. If samples are submitted to the National Office (Four Month Cycle
Review), select “Yes.”
5. Select the type of inspection service. For Child Nutrition (CN) please
select “CN Lot” or “CN In-plant” inspection. For other inspections, select
the box for “Lot,” “In-plant” or “Quality Assurance Program” (“QAP”).
6. If In-plant inspection service is selected in block #5, select the shift
inspection was performed.
7. Enter the name of the applicant company as it appears in the billing
program.
8. Enter the city, state and zip code for the applicant company.
9. If a certificate is issued, record certificate (including letter report) number.
If no certificate is issued, enter N/A.
10. Enter grade assigned. For products with no applicable grade, select/enter
NAG.
11. Enter date product was graded (from score sheet, certificate, or other
inspection document.
12. Enter the product name as it appears on score sheet, certificate, or other
inspection document. For Lotus Notes, select from drop down list. All CN
products are grouped together and are identified by “CN” prefix. If the
product does not appear on the list, new products may be added by typing
in “New keyword.” For 100% juice blends, use “juice blends” or “juice
blends from concentrate” and add any other identifying information in the
“Comment” section under “Area Office Evaluation.”
13. Select product type: “Canned” (Heat process), “Chilled,” “Frozen” or
“Dried” (Dehydrated.)
14. Enter code marks as they appear on score sheet, tally sheet, certificate or

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 172 of 192
inspection documents.
15. Inspector should print and sign official name. (If QAP, indicate if the
individual is plant employee.)
16. Area Office review. This section should be completed by a supervisor or
someone officially acting in that capacity. If acting, this should be
reflected by “acting OIC,” “acting AOIC,” or “acting subarea supervisor”
next to the signature.
(a) Indicate if supervisor’s grading results agree with inspector’s results
by selecting Yes or No
(b) Comments: use this section to record:
(1) Security strip numbers.
(2) Any corrective actions and follow-up to errors in grading or
supporting documents.
(3) Please record grading results on inspector’s score sheet
adjacent to sample results, or on a separate score sheet
submitted with supporting documents. Identify as “see
score sheet attached” (Any grading results entered into this
section will need to be entered into the Lotus Notes
database.)
(4) Use this location to identify sample number or identification
(such as Winter Haven DP-4)
(5) Review supporting documents and indicate if they are
submitted (yes or no). Supporting documents include score
sheet or tally sheet (include reverse or attached information
if applicable), analytical results, certificate, requirements
(copy of buyer’s specification or purchase order), waivers,
certificate, results of CN review, and cooking or preparation
instructions (for CN products).
(6) Record if the supervisor’s grading results are attached (yes
or no).
(7) If samples were sent in a cooler, please indicate if the cooler
needs to be returned (yes or no).
(8) Please indicate if the freezer packs need to be returned (yes
or no).

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 173 of 192
17. Supervisor should print and sign name. If person is “acting,” this should be
indicated as John Smith, acting OIC.
18. Record date supervisor reviewed sample and supporting documents. If
using Lotus Notes, please “save” and “replicate” the form in the database.
Print a copy of the form, sign it and attach to supporting documents and
submit with sample.
19. National Office Review. Record yes or no for (a) through (d) of the
following:
(a) Inspector’s supporting documentation submitted (see 16 (b) (3)),
(b) Supervisory evaluation documentation attached (score sheet),
(c) I agree with the Grader’s evaluation,
(d) I agree with the Area Office evaluation.
(e) In the comment section, record the following information:
(1) Indicate if correct form versions are used,
(2) Indicate if any portions of the submittal sheet are
incomplete,
(3) Are statements on inspection documents sheets in
accordance with Division instructions,
(4) Are there errors on the supporting documents?
(5) Record any comments from panel, is flavor weak? Is it still
within the grade assigned by inspector/supervisor (but
should have been assigned a higher or lower score? Is the
sample received less uniform, more or less firm, more or
less uniform than results on supporting inspection
documents?
(6) Has the sample been submitted in a timely manner?
(f) Record name and signature of person conducting the review.
(g) Record date of review.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 174 of 192
Product
Applicant
or
Plant
Lot (L)
In-Plant (IP)
CN Lot (CN L)
CN In-Plant (CN IP)
QAP (QAP)
INSPECTION
POINT (name)
Inspector
Grade
Assigned
Field Office Review
Date
Reviewed
Grade
Assigned
Supervisor’s
Initials
Supporting
Documents
for
Inspector
Yes/No
Supporting
Documents
for
Supervisor?
Yes/No
Corrective
Action
Yes/No
Example:
Cnnd wk
corn
ABC
Packer
L
Annie
Jones
B
05/21/2012
B
JS
Yes
Yes
No
Monthly Review Program Summary Sheet for ___________________________ from ______________ to ______________
(Field Office) (Start Date) (End Date)
Page 1 of ____
Optional Monthly and Four Month Cycle Review Program Submission Worksheet

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 175 of 192
October - Cycle #1
Monthly Review
Four Month Cycle Review
November - Cycle #1
Monthly Review
Four Month Cycle Review
December - Cycle #1
Monthly Review
Four Month Cycle Review
January - Cycle #1
Monthly Review
Four Month Cycle Review
February - Cycle #2
Monthly Review
Four Month Cycle Review
March - Cycle #2
Monthly Review
Four Month Cycle Review
April - Cycle #2
Monthly Review
Four Month Cycle Review
May -Cycle #2
Monthly Review
Four Month Cycle Review
June - Cycle #3
Monthly Review
Four Month Cycle Review
July -Cycle #3
Monthly Review
Four Month Cycle Review
August - Cycle #3
Monthly Review
Four Month Cycle Review
September - Cycle #3
Monthly Review
Four Month Cycle Review

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 176 of 192
SCORE SHEET/TALLY SHEET COMPLETION
The purpose of this instruction is to provide guidelines for completing score sheets and tally
sheets. It is extremely important for score sheets and tally sheets to be complete, accurate,
and legible. Often a score sheet is the primary means by which SCI inspection results are
communicated. Copies of score sheets and tally sheets are often requested by applicants and
other financially interested parties, and are frequently requested through the Freedom of
Information Act.
SCI score sheets and tally sheets are official documents and may be introduced and accepted as
evidence in litigation. Score sheets and tally sheets must be prepared so that the information
shown is clear, complete, and accurate. Entries on score sheets and tally sheets must be based on
facts observed and test results. To preserve authenticity, only officially approved versions of
Fruit and Vegetable (FV) forms are to be used. Approved FV score sheets and tally sheets
may not be manipulated manually or electronically. Do not alter original format or data.
Approved versions of FV score sheets and tally sheets may be found in the AMS Forms Catalog
at the following intranet address: http://agnis/AMSFormsCatalog/Forms/AllItems.aspx
.
Each grader participating in the grading of a product is responsible for assuring that score/tally
sheets have all the necessary data entered correctly, including accurate computations; and
assuring that all tests have been completed. Each grader will print and sign his/her name on the
score sheet(s) or tally sheet(s).
The utmost care and diligence must be observed in the preparation of these official documents. In
the case of legal action, inspectors may be subpoenaed by the court to testify to the accuracy of
the score sheet, tally sheet, or resulting inspection certificate on which their signatures appear.
Everyone - from those who collect the data during inspection to those who are responsible for
reviewing the work - shares the responsibility for the accuracy and adequacy of these documents.
A. Completing standardized score sheets and tally sheets
SCI policy is to record in the appropriate blocks on the score sheet or tally sheet,
the results of quantitative examinations, analytical tests, and actual quality level(s)
observed during the grading of the product. In variable grade point standards,
score sheets are used to assess product. The overall quality factors are assigned a
number, and the final grade is determined. In attribute standards, a tally sheet is
used to assess product. Prerequisite quality factors are examined, quality factors are
counted, and the final grade is determined.
Note: When information that is common to both types of documents is described in
this instruction, the documents will be referred to as score/tally sheets.
A thoroughly completed score/tally sheet is one that has all the data required to
support the grade of the product. Report each value in the appropriate sample unit
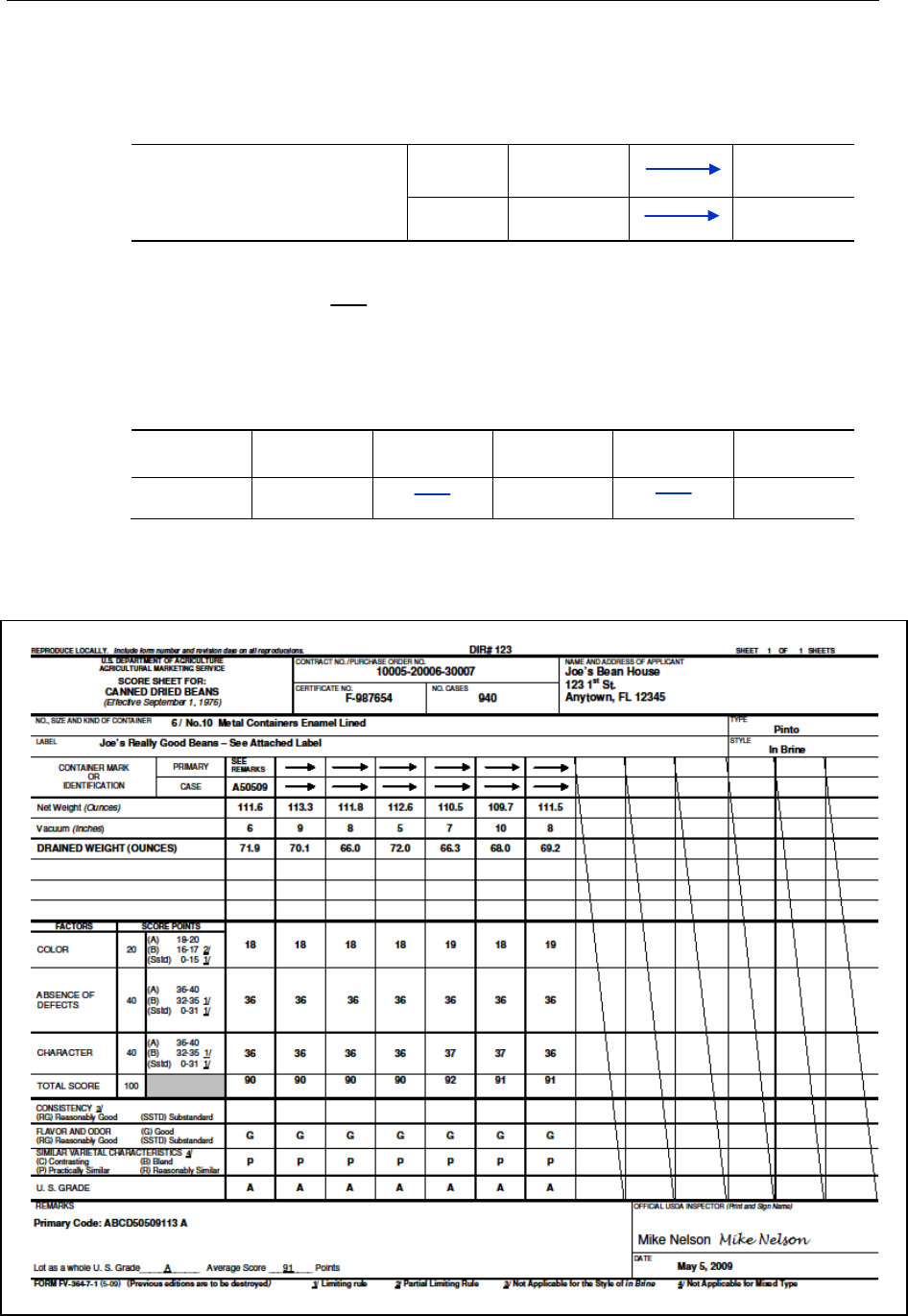
SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 177 of 192
box. Do not use symbols, such as ditto marks, check marks, or arrows to repeat a
value, except arrows may be used to show repeating codes. Example:
CONTAINER MARK
OR
IDENTIFICATION
PRIMARY
A157900 L2
B157900 L2
CASE
A157900
B157900
Do not leave empty boxes on the score/tally sheet unless the row is marked N/A.
A long dash such as ( ) will be used to indicate No Data when a test was not
performed on a sample unit. It is not necessary to put dashes in boxes that are
marked Type, Style, etc., when it is clear that no values are required to be entered
for each sample unit. Example:
STYLE
Sliced
COUNT
23
24
23
To reduce the likelihood of being altered, draw diagonal lines through remaining
empty columns/rows on the score/tally sheet after the final sample unit. Example:

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 178 of 192
Date score/tally sheets with the date the grading is performed. If more than one
date is required to complete the grading, more than one date must appear on the
score/tally sheet. Example:
OFFICIAL USDA INSPECTOR (Print and Sign Name)
John Doe John Doe
DATE
6/05/09, 6/06/09
Note: All entries on the score/tally sheet must be in permanent blue ink. Record
factual information; do not include any personal opinions or assumptions on
the score/tally sheet. Score/tally sheets must be COMPLETE, ACCURATE,
and LEGIBLE.
The first step in completing a score/tally sheet is locating the most up-to-date
version available. These forms are located in printable pdf format in the AMS
Forms Catalog. The AMS Forms Catalog is available at the following intranet
address: http://agnis/AMSFormsCatalog/Forms/AllItems.aspx
. Click on the
“view” box on the right of the screen for a drop down list, then click on “Fruit and
Vegetable” and scroll to the FV form number needed. Inspectors without intranet
access may contact their immediate supervisor for assistance as needed.
Note: Do not use the Universal Score Sheet, Form FV-364 when a product has a
current standardized score/tally sheet. Only use this form for products that
do not have an applicable grade standard, or for which a standardized
score/tally sheet is not currently available.
After selecting the appropriate score/tally sheet, enter the pre-grading information.
This information is located on the “Application for Inspection and Certificate of
Sampling” Form FV-356 or from in-plant sources under in-plant inspection. The
pre-grading information may include but is not limited to:
• Complete name and address of applicant,
• Contract or purchase order number,
• Case count,
• Size and kind of container,
• Pertinent label information, and
• Type, style, or variety

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 179 of 192
It is helpful to the grader and anyone reviewing the score/tally sheet to note the
target values concerning quantitative examinations and/or analytical tests for such
parameters as net weight, vacuum, drained weight, Brix, etc.
Example:
Net Weight (Ounces) (min 16.0)
16.2 16.1 16.2
1. Principal Label Marks
Record only essential label information on the score/tally sheet. Essential
label information includes:
• Brand name,
• Commodity name (including type, style, count, size, syrup designation),
• Quality statement (if any),
• All references to net weight or drained weight or net contents,
• Country of origin, and
• Packers and/or distributor’s name and address.
Additional printed data may be added to score/tally sheets, as long as the
original score/tally sheet data and format has not been altered. It may be
helpful to prepare a sort of “template” score/tally sheet with added
information for frequent applicants, labels, or other information. Verify
this with your supervisor before preparing. Photocopying and using these
“templates” with their pre-printed information can be a time-saver.
However, if the score/tally sheet has pre-printed information added,
this information must be verified before continuing. You must check to
make sure that none of the information has changed since the score/tally
sheet template was prepared
Label information should be recorded exactly as found on the label. If a
container has extensive label markings and it is not possible to attach a
label, label markings may be shown on the reverse of the score/tally sheet.
A notation must be made in the “label” section of the score/tally sheet
stating that label marks are listed on the reverse side. It is permissible to
write in the “label” section of the score/tally sheet the “brand name” as
shown on the label, followed by the statement “See Attached Label” and
attach a label in lieu of copying the label information onto the score/tally
sheet. See the following example:

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 180 of 192
Example:
NO., SIZE AND KIND OF CONTAINER 12/46 ounce, beaded body, enamel lined metal cans
LABEL Valley Brand Grape Juice – See Attached Label
CONTAINER MARK
OR
IDENTIFICATION
PRIMARY
A157900 L2
B157900 L2
CASE
A157900 B157900
2. Sample Codes
When grading lot inspection samples containing numerous mixed codes, it
is helpful to sort and arrange the sample units logically - alphabetically,
numerically, and/or chronologically. This ensures that like codes are
grouped together and codes are easier to track when transferred to the
score/tally sheet. Record code markings in the row titled “Container Mark
or Identification.” Within this row there are two separate rows identified as
“Primary” and “Case.” See the example above.
The U.S. Standards for Condition of Food Containers defines the “primary"
container as: “The immediate container in which the product is packaged
and which serves to protect, preserve, and maintain the condition of the
product. It may be metal, glass, fiber, wood, textile, plastic, paper, or any
other suitable type of material, and may be supplemented by liners,
overwraps, or other protective materials.” The “case” would then be
considered the container in which the “primary” container is packaged
In the case of bulk packed products, the case may be the primary container,
as for example, processed raisins in 50 pound cases. The case code marks
for such products would be recorded in the area designated for “Primary”
codes; there would be no entry for “Case” codes.
Accurately record the code markings on the score/tally sheet. Code
markings are to be recorded exactly as they are written on the container.
Refer to the AIM Inspection Series, Sampling Manual and Certification
Manual for examples of how to record code marks and allowable
exceptions. All marks and spaces have a significant meaning in the code,
and should be accurately recorded on the score/tally sheet. If a code
includes a time designation or marking, it must be recorded. If the color of
the ink used is part of the code marks, record the color of the ink.
When repeating identical code marks, use arrows to show sample units in
the lot that have the identical primary or case code markings. This is the
only area on the score/tally sheet that arrows may be used.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 181 of 192
When codes contain excessive numbers, letters, or symbols and are too
long to be legibly written within the space provided on the score/tally sheet,
the codes may be shown on the reverse side. Codes will be identified by
sample number, and listed left to right just as they would appear on the
front of the score/tally sheet in the row entitled “Container Mark or
Identification.”
If there is only one code in the lot that exceeds the space provided on the
score/tally sheet, the code may be written in the remarks section. Whenever
a code mark is written anywhere other than in the “Container Mark or
Identification” section of the score/tally sheet, a notation must be made in
that section indicating where the code(s) is listed.
Example:
CONTAINER MARK
OR
IDENTIFICATION
PRIMARY
Code marks on
reverse side of
score sheet
CASE
A157900
B157900
3. Corrections
When an entry on a score/tally sheet requires correction, the only approved
method is to draw a single line through the error, write the correct entry
next to the error, and initial the correction. White-out is not allowed. If
the date of the correction is different from the date of the score/tally sheet,
show the date of the correction.
Example:
4. Recording Data
Follow the directions as shown in AIM Inspection Series, Technical
Procedures Manual which references procedures for recording weights,
vacuum readings, and headspace.
When recording analytical data on the score/tally sheet, use the correct
units of measure. For example, if a U.S. grade standard states that drained
weight is recorded in ounces, then record the data onto the score/tally sheet
in ounces, rather than pounds or grams.
COLOR
27 28
TB
4/01/10
27
27
28
27

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 182 of 192
When rounding numbers, it is important to observe the significance of the
numbers being recorded. Score points on a score sheet are recorded in
whole numbers only. Score point averages are rounded to the nearest
whole number:
• Record 92.4 as 92
• Record 92.5 as 93
For specified defects, record only to the point of significant accuracy as
determined by the method and equipment used for the analysis. See the
AIM Inspection Series, Certification Manual for additional information on
recording analytical data. Unless otherwise specified, net contents, net
weights, drained weights, Brix readings, and other similar factors shall be
recorded to the first decimal place.
Example:
Net Weight (Ounces)
(min 16.0)
16.2 16.1 16.2
For analytical determinations in which the value is relatively low (usually
less than 1 percent), record to the second decimal place.
Example:
Total Acidity (As Malic) (g/100 ml)
0.47 0.41
Recoverable oil is recorded to the third decimal place.
Example:
Recoverable Oil (Percent by Volume)
0.017 0.018
5. Flagging and Optional Limiting Rule Symbols
Unless an applicant requests not to have non-conformances flagged, values
that are outside the required range specified in the U.S. Standards,
Commercial Item Description (CID), or other specification shall be flagged
to bring attention to the nonconformance.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 183 of 192
Example:
Net Weight (Ounces) (min 16.0)
16.2 16.1 16.2
Vacuum (Inches) (min. 5)
7 2 10
Quality factors subject to limiting rules are shown as part of the score sheet
for the product. As an option, during grading the inspector may use
symbols to identify or “flag” any score points causing a limiting rule to
affect the sample grade.
The following list of optional limiting rule symbols may be used to identify
score point results that cause a limiting rule to affect the sample unit grade.
Grade B Quality factor – a circle around a score point value is used
to identify a quality factor that requires the sample unit to be assigned
Grade B, even though the total score point value indicates Grade A.
Grade C Quality factor – a triangle around a score point value is
used to identify a quality factor that requires the sample unit to be assigned
Grade C, even though the total score point value indicates a higher grade.
Substandard Quality Factor – a square around a score point value
is used to identify a quality factor that requires the sample unit to be
assigned Substandard, even though the total score point value indicates a
higher grade.
Note: When any abbreviations or symbols are used to flag information on
the score/tally sheet, a key defining the abbreviations or symbols must
be shown in the Remarks section
Example:
TOTAL SCORE 100
93* 92 93* 91 93* 94
U.S. GRADE
A A A B
A
A
REMARKS Notified MB
Key: = Grade B limiting rule applies.
* = Cooked sample
FV 364-XX (06-09) 1/ Limiting Rule

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 184 of 192
6. Remarks Section
The “Remarks” section of the score/tally sheet is used to show information
that is needed to summarize the inspection. Essential information to be
shown in this section (including a key for any abbreviations or symbols):
• The lot grade, average score points, applicable ranges, averages;
• Compliance or non-compliance with specifications. A copy of the
specifications may be attached to the completed score/tally sheet.
• If grading frozen product, notation of which samples were cooked.
• Observations and other factual information that further explain the
grading of the lot.
When the average score for a lot is in a higher score point range than the
grade it is given (e.g., Grade A range score points, but Grade B because of
a limiting rule), show the reason (quality factor) that limited the lot grade.
Example:
COLOR
20
(A) 18-20
(B) 16-17 1/
(C) 14-15 1/
(SSTD) 0-13 1/
18
19
18
19
19
18
ABSENCE OF
DEFECTS
40
(A) 36-40
(B) 32-35 1/
(C) 28-31 1/
(SSTD)0-27 1/
37
35
38
35
36
36
CHARACTER
40
(A) 36-40
(B) 32-35 1/
(C) 28-31 1/
(SSTD)0-27 1/
36 37 36 36 37 37
TOTAL
SCORE
100
91 91 92 90 92 91
U.S. GRADE
A B A B A A
REMARKS Key: BL = Blemish Lot a
s a Whole U.S. Grade B or U.S. EXTRA STANDARD (account defects)
= Grade B limiting rule applies Average Score 91Points
Meets requirements for State of Ohio, Bid No. X9654123, Item No.3
FV 364-XX (06-09) 1/ Limiting Rule
BL
BL

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 185 of 192
Always write out the grade on a score/tally sheet and its corresponding
certificate exactly the way it is written in the U.S. standards for grades.
When a U.S. grade standard describes the grade with dual nomenclature
such as “U.S. Grade A or U.S. FANCY,” show both terms. If the standard
does not use dual nomenclature, show only the letter grade.
Some score/tally sheets may not have a “Remarks” section. When there is
not enough space, or no designated space for information such as sampling
plan used, final grade, case count, or other information that applies to the
entire lot, the top margin area of the score/tally sheet may be used.
Example:
SAMPLING PLAN:
Time Sampling FINAL GRADE: U.S. GRADE B (account defects)
Medium Volume Average Score 90 Points
REPRODUCE LOCALLY. Include form number and revision date on all reproductions. COLD STORAGE TEMPERATURE (
°
F) -10
U.S. DEPARTMENT OF AGRICULTURE
AGRICULTURAL MARKETING SERVICE
SCORE SHEET FOR:
FROZEN WHOLE KERNEL CORN
(Effective August 1, 1952)
CONTRACT NO. / P.O. NO.
CERTIFICATE NO.
NO. CASES
NO., SIZE AND KIND OF CONTAINER 1/30 Pound Corrugated Fiber Case with Blue Poly Liners
Sometimes a lot will have a mixture of sample unit grades. For grade or
compliance determination in such cases, refer to the Regulations,
7 CFR 52.13, located at the following internet address:
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CF
R.
7. Unfrozen sample units
Inspection results from unfrozen sample units drawn during production are
reported on score/tally sheets with the following additions, deletions, or
qualifications.
The score sheet or tally sheet will clearly indicate the extent of grading
performed on the lot. These procedures are intended to coincide with those
outlined in AIM Inspection Series, Technical Procedures Manual.
a. When products are intended to be frozen and frozen verification
sample units will be examined at a later date:
(1) Show name of product as "FROZEN ------------------."
1202XXX5551212
X-123456
1,008
SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 186 of 192
(2) In the "Remarks" section show the following:
"Grade (line or lot grade) Incomplete, Frozen Samples Not
Examined."
b. When products are intended to be frozen, and frozen verification
sample units will not be examined at request of the applicant:.
(1) Delete the word "FROZEN" in the title of the score sheet
and add the words "PREPARED FOR FREEZING."
(2) In the "Remarks" section show the following:
"NO APPLICABLE U.S. STANDARDS OF GRADES
FOR THIS PRODUCT."
8. The Completed Score/Tally Sheet
A properly completed score/tally sheet contains all data required to support
the grade of the product. If foreign material examination or outside
analytical results are required, the completed analytical forms shall be
attached to the score/tally sheet.
Before signing and submitting the score/tally sheet, verify that it is
complete. If you have any questions, compare against the Optional
Checklist for Completion of Score Sheets and Tally Sheets.
B. Completing the universal score sheet, form FV-364
Form FV-364, commonly referred to as the Universal score sheet, is used for
products that are not covered by a U.S. grade standard and do not have a
standardized score/tally sheet. It is also used for products that require only an
organoleptic examination. Such products may be for export certification and/or
condition inspections. (See the AIM Inspection Series, Certification Manual.)
Before completing the Form FV-364, the inspector must determine each of the
qualitative and quantitative inspection factors for the product. The quality factors
used to evaluate products that have no applicable grade (NAG) may be based on a
standardized product with similar characteristics, a buyer’s specification, or a CID.
After determining the quality factors for a NAG product, list all of them on the
Form FV-364. The quality level descriptions for NAG items are listed on the
following page. Do not use the quality level descriptions from standardized
products.
Exception: If the applicant requests grading of a NAG product based on a similar standardized
product, score points and/or quality level descriptions from the standardized

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 187 of 192
product may be used. However, the final grade for the product must be NAG. In
this case include the following statement in the remarks section of the FV-364:
“Product has no applicable grade. Score points based on the U.S. Standards for
Grades of (fill in name of product).”
The following items correspond to the numbered areas on the following page.
1. Product inspected,
2. Certificate number, if applicable,
3. Contract number or purchase order number, if applicable,
4. Number of cases in lot,
5. Name and address of applicant, including zip code,
6. Number, size, and kind of container,
7. Label nomenclature; label may be attached,
8. Container codes exactly as they appear on the container,
9. Non-quality factors, quantitative, and analytical data,
10. Quality factors,
11. Quality level descriptions and their abbreviations,
12. Remarks,
13. Lot as a Whole: U.S. Grade ___ , or Meets or Fails contract requirements,
14. Inspector’s printed name and signature, Date(s) of inspection.
C. No Applicable Grade Products
NAG products do not have U.S. standards for grades, so score points cannot be
used to indicate the quality level of the quality factors evaluated (see exception on
the previous page). Instead descriptive terms such as “practically free” from
defects, “reasonably good” color, etc., are used to record the quality level of each
factor. Abbreviations for these descriptive terms are used for recording the results
of the evaluation, as shown in the Quality Level Descriptions and Abbreviations
Table shown on page 187. In addition, the descriptive terms contained in the most
relevant grade standard, grading manual or instruction for each applicable factor
may be used. In this case include the following statement in the remarks section of
the FV-364: “Product has no applicable grade. Descriptive factors and terms
based on (fill in the name of the relevant instruction used).” Inspectors will use the
appropriate abbreviation to record the quality level for each grade factor
characteristic of the NAG product. Inspectors will include a key for the
abbreviations used on the score sheet.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 188 of 192
REPRODUCE LOCALLY. Include form number and revision date on all reproductions. SHEET OF SHEETS
U.S. DEPARTMENT OF AGRICULTURE
AGRICULTURAL MARKETING SERVICE
SCORE SHEET FOR:
1
CONTRACT NO. / PURCHASE OORDER NO.
3
NAME AND ADDRESS OF APPLICANT
5
CERTIFICATE NO.
2
NO. CASES
4
NO., SIZE AND KIND OF CONTAINER
6
LABEL
7
CONTAINER MARK
OR
IDENTIFICATION
PRIMARY
8
CASE
8
9
9
9
9
FACTORS
SCORE POINTS
10
11
10
11
10
11
10
11
TOTAL SCORE 100
U. S. GRADE / MEETS (M) OR FAILS (F)
REMARKS
12
Lot as a whole U. S. Grade ___13___ or MEETS or FAILS (Circle One).
OFFICIAL USDA INSPECTOR (Print and Sign Name)
14
DATE
FORM FV-364E Example (Previous editions are to be destroyed.) 1/ Limiting Rule 2/ Partial Limiting Rule

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 189 of 192
Quality Level Descriptions and Abbreviations
Color or Appearance Clarity
G=Good PC=Practically Clear
RG=Reasonably Good RC=Reasonably Clear
FG=Fairly Good FC=Fairly Clear
P=Poor P=Poor
Absence of Defects Flavor and Odor (or Aroma)
PF=Practically Free G=Good (Normal or Typical)
RF=Reasonably Free RG=Reasonably Good
FF=Fairly Free P=Poor (not typical for the product)
P=Poor
Uniformity of Size Overall Quality Level
PU=Practically Uniform in Size M=Meets
RU=Reasonably Uniform in Size F=Fails
FU=Fairly Uniform in Size
P=Poor
Character or Consistency or Texture
G=Good
RG=Reasonably Good
FG=Fairly Good
P=Poor
D. Updating Previously Inspected Product
When an applicant requests an up-to-date certificate, refer to the AIM Inspection
Series, Certification Manual for the correct procedures. Start a new score/tally
sheet and write “Updated Inspection” in the upper left margin. Attach a copy of
the previously completed score/tally sheet to this new score/tally sheet. Write the
previous inspection certificate number on the new sheet, and identify as “previous
certificate number”.
The lot may not have to be entirely re-graded, but ALWAYS evaluate flavor and
odor on re-inspected lots. If the lot is determined to be in good condition and there
is no apparent change in the product, a limited “verification” type of inspection is
appropriate. Write on the new score/tally sheet “No apparent change in grade” in
the sample unit boxes of the quality factors that have not changed since the
previous inspection. If quality factors have changed, re-grade the lot, assigning
score points for all factors. Consult your supervisor on how to recertify product
that is no longer of the same quality.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 190 of 192
E. Guidelines for the Development of Plant Score Sheets
It is essential that “company” or “applicant” documents used by the applicant
to record their results do not reference the Department of Agriculture or any
official marks. This will differentiate between official score/tally sheets and
applicant score/tally sheets. Unless other authorization is given, only approved FV
forms shall be used when providing inspection/grading services to applicants.
Applicants who contract for in-plant inspection may request using a score/tally
sheet developed for their facility. Requests for approval of automated
(computerized) score sheets shall be submitted through the Regional office to the
National office.
Guidelines for Approval of Applicant Score/Tally Sheets
The plant’s own score/tally sheets may be approved for SCI use by the
area supervisor if they comply with the following guidelines:
a. All score/tally sheets SHALL allow for recording all information
applicable to the standardized or non-standardized product,
b. The term “Official Inspector” may appear on the score/tally sheet;
USDA inspection personnel will print and sign their names in the
designated space and write “USDA” adjacent to their signatures,
c. “Lot Grade”____________ (and average score, when applicable)
shall appear in the “Remarks” section of the score/tally sheet,
d. Plant score/tally sheet form numbers are acceptable,
e. The original score/tally sheet may be white in color,
f. The term “Official USDA Inspector” SHALL NOT appear on the
score/tally sheet,
g. References to the United States Department of Agriculture (USDA),
official or approved identification marks SHALL NOT appear on
the score/tally sheet,
h. There SHALL BE NO certification statement,
i There SHALL BE NO reference to plant sanitation, and
j. When information is recorded on the score/tally sheet by trained
plant personnel, it SHALL be initialed by that individual.

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 191 of 192
Optional Check List for Completion of Score Sheets and Tally Sheets
1. Is the correct version of the score/tally sheet for the product being used?
2. Is the applicant information correct?
3. Are product and container size and type complete and correct?
4. If applicable, is contract or purchase order number documented?
5. Is the case count shown?
6. Are codes recorded properly?
7. Are principal label marks recorded, or is the label attached and noted?
8. Are any errors properly corrected (single line through, corrected and initialed)?
9. Are repeated values (except codes) written out, without the use of ditto marks,
arrows, or check marks?
10. Do non-quality factors meet?
11. If applicable, are limiting/partial limiting rules correctly applied?
12. Are all samples within the deviant range of indicated grade (i.e., no “worse than
deviants” present in lot, or within ranges established on page 43, Grade
Compliance)?
13. Are score points for each sample totaled correctly?
14. Is the grade for each sample unit shown?
15. Does the lot meet quality requirements by code (i.e., no “lower quality codes”
present in lot, as established in Regulations?
16. Is the grade of the lot shown? Is it correct?
17. Is there a key to explain abbreviations or symbols?
18. Is the average score of the lot shown? Is it correct?
19. If applicable, is compliance with specification noted?
20. If product is frozen, are cooked samples noted?
21. Is inspector’s name both printed and signed?
22. Is the score/tally sheet properly dated?

SCI Division Inspection Series General Procedures Manual
Effective Date: July 2013 Page 192 of 192
Attachments Version Date
(Printed for distribution)
7 CFR 52 Sections (circle as applicable): 3, 12, 13, 38, 41, 42, 43, 44, 45, 46, 47, __________
48, 49, 50, 51, 52, 53, 54, 81
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CFR.
29 CFR 1910.146: __________
http://www.gpo.gov/fdsys/browse/collectionCfr.action?collectionCode=CFR.
Application for Inspection and Certificate of Sampling, Form FV-356: __________
http://agnis/AMSFormsCatalog/Forms/AllItems.aspx.
Canned Ripe Olive Flavor Review Sheet, Form FV-377: __________
http://agnis/AMSFormsCatalog/Forms/AllItems.aspx.
Daily Inspection Report, FV- 416: __________
http://agnis/AMSFormsCatalog/Forms/AllItems.aspx
.
Emerson Good Samaritan Food Donation Act: __________
http://www.gpo.gov/fdsys/pkg/PLAW-104publ210/html/PLAW-104publ210.htm.
FDA Labeling Guidelines: __________
http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/default.htm
.
Fees - Current Fees for Various Inspection Services: __________
http://www.ams.usda.gov/AMSv1.0/processedinspection
.
Sanitation Score Sheet, FV- 416-3: __________
http://agnis/AMSFormsCatalog/Forms/AllItems.aspx.
Speed Memo, AD 0311: __________
http://www.ocio.usda.gov/sites/default/files/docs/2012/AD0311-F-03-81-OCIO.PDF.
Trade Secrets Act, 18 U.S.C. 1905: __________
http://frwebgate.access.gpo.gov/cgi-
bin/usc.cgi?ACTION=BROWSE&TITLE=18USCPI&PDFS=YES.
Transfer and Adjustment Voucher, Form AD-742: __________
http://www.ocio.usda.gov/sites/default/files/docs/2012/AD0742-F-10-91.pdf),
Checked Materials have been printed from the links in this Manual and included for reference.
