
Page Left Blank Intentionally

Table of Contents
Preface...........................................................................................................................5
Map of the Mississippi Public Health Districts ............................................................. 6
Reportable Disease List.................................................................................................7
Vaccine Preventable Diseases.................................................................................... 9
Haemophilus influenzae type b (Hib), invasive......................................................9
Hepatitis B, acute ....................................................................................................10
Influenza...................................................................................................................13
Measles ....................................................................................................................17
Meningococcal disease, invasive ........................................................................19
Mumps...................................................................................................................... 21
Pertussis ....................................................................................................................22
Pneumococcal disease, invasive..........................................................................25
Rubella......................................................................................................................27
Varicella................................................................................................................... 29
Sexually Transmitted Diseases................................................................................... 31
Chlamydia ............................................................................................................... 31
Gonorrhea................................................................................................................34
HIV Disease..............................................................................................................38
Syphilis......................................................................................................................42
Tuberculosis.................................................................................................................48
Enteric Diseases ..........................................................................................................52
Campylobacteriosis................................................................................................52
Cryptosporidiosis.....................................................................................................54
E. coli O157:H7/ HUS................................................................................................56
Hepatitis A................................................................................................................ 59
Listeriosis................................................................................................................... 60
Salmonellosis ........................................................................................................... 62
Shigellosis................................................................................................................. 66
Vibrio disease .......................................................................................................... 69
Zoonotic Diseases.......................................................................................................71
Arboviral Infections (mosquito-borne).................................................................. 71
Eastern Equine Encephalitis (EEE) .....................................................................72
LaCrosse Encephalitis ........................................................................................73
St. Louis Encephalitis ..........................................................................................74
West Nile Virus ....................................................................................................75
Lyme Disease...........................................................................................................78
Rabies....................................................................................................................... 79
Rocky Mountain spotted fever...............................................................................82
Reportable Disease Statistics.....................................................................................84
List of Contacts, Editors and Contributors ................................................................. 86
General References....................................................................................................87

Annual Summary 2009
5
Preface
Public health surveillance involves the systematic collection, analysis and dissemination
of data regarding adverse health conditions. The data are used to monitor trends and
identify outbreaks in order to assess risk factors, target disease control activities,
establish resource allocation priorities and provide feedback to the medical community
and the public. These data support public health interventions for both naturally
occurring and intentional spread of disease.
Statistics incorporated into tables, graphs and maps reflect data reported from health
care providers who care for Mississippi residents. Cases counted have met the
surveillance case definitions of the CDC and the Council of State and Territorial
Epidemiologists (CSTE). Unless otherwise noted all rates are per 100,000 population.
Data are based on “event” date of the case with the exception of TB in which the case
confirmation date is used. The “event” date is defined as the earliest known date
concerning a case and is hierarchical (onset, diagnosis, laboratory date or date of
report to the health department).
Mississippi law (Section 41-3-17, Mississippi Code of 1972 as amended) authorized the
Mississippi State Board of Health, under which MSDH operates, to establish a list of
diseases which are reportable. The reportable disease list and the Rules and
Regulations Governing Reportable Diseases and Conditions may be found online at
http://www.msdh.state.ms.us/msdhsite/_static/14,0,194.html
. Class 1 diseases,
reportable by telephone at first knowledge or suspicion, are those to which the MSDH
responds immediately to an individual case; Class 2 diseases, those reportable within a
week of diagnosis, and Class 3 diseases, reportable only by laboratories, do not
necessitate an immediate response to an individual case.
To report a case of any reportable disease or any outbreak, please call 601-576-7725
during working hours in the Jackson area, or 1-800-556-0003 outside the Jackson area.
For reporting tuberculosis, you also may call 601-576-7700, and for reporting STD’s or
HIV/AIDS, you may call 601-576-7723. For emergency consultation or reporting Class 1
diseases or outbreaks nights and weekends please call 601-576-7400.
The data included in the following document have come from physicians, nurses,
clinical laboratory directors, office workers and other health care providers across the
state who called or sent in reports. Without these individuals, public health surveillance
and response would be incapacitated. For your dedication to this important part of
public health information, we thank you.
Paul Byers, MD
Acting State Epidemiologist

Annual Summary 2009
6
Mississippi Public Health Districts
Public Health
Districts
Northwest Public Health
District I
Dr. Alfio Rausa*
662-563-5603
Northeast Public Health
District II
Dr. Jessie Taylor*
662-841-9015
Delta/Hills Public Health
District III
Dr. Alfio Rausa*
662-453-4563
Tombigbee Public Health
District IV
Dr. Jessie Taylor*
662-323-7313
West Central Public Health
District V
Dr. Mary Gayle Armstrong*
601-978-7864
East Central Public Health
District VI
Dr. Rebecca James*
601-482-3171
Southwest Public Health
District VII
Dr. Thomas Dobbs*
601-684-9411
Southeast Public Health
District VIII
Dr. Thomas Dobbs*
601-544-6766
Coastal Plains Public Health
District IX
Dr. Robert Travnicek*
228-831-5151
*District Health Officer

Annual Summary 2009
7
Reportable Disease List
Mississippi State Department of Health
2008 List of Reportable Diseases and Conditions
Reporting Hotline: 1-800-556-0003
Monday - Friday, 8:00 am - 5:00 pm
To report inside Jackson telephone area or for consultative services
Monday - Friday, 8:00 am - 5:00 pm: (601) 576-7725
Class 1 Conditions may be reported nights, weekends and holidays by calling:
(601) 576-7400
Class 1: Diseases of major public health importance which shall be reported directly to the
Mississippi State Department of Health (MSDH) by telephone within 24 hours of first
knowledge or suspicion. Class 1 diseases and conditions are dictated by requiring an
immediate public health response. Laboratory directors have an obligation to report
laboratory findings for selected diseases (refer to Appendix B of the Rules and
Regulations Governing Reportable Diseases and Conditions).
Any Suspected Outbreak (including foodborne and waterborne outbreaks)
(Possible biological weapon agents appear in bold italics)
Anthrax Glanders Smallpox
Arboviral infections including but Hemolytic uremic syndrome (HUS), Staphylococcus aureus,
not limited to those due to: post-diarrheal vancomycin resistant
California encephalitis virus Hepatitis A (VRSA) or vancomycin
Eastern equine encephalitis virus HIV infection, including AIDS intermediate (VISA)
LaCrosse virus Influenza-associated pediatric Syphilis (including
Western equine encephalitis
virus
mortality, in patients <18 years of congenital)
St. Louis encephalitis virus age Tuberculosis
West Nile virus
Invasive disease
†‡
due to:
Tularemia
Botulism (including foodborne, Neisseria meningitidis or Typhoid fever
infant or wound) Haemophilus influenzae type b Typhus fever
Brucellosis Measles Varicella Infection,
Chancroid Melioidosis primary, in patients >15
Cholera Pertussis years of age
Creutzfeldt-Jakob disease, Plague Viral hemorrhagic fevers
including new variant Poliomyelitis (filoviruses [e.g., Ebola,
Diphtheria Psittacosis Marburg] and
Escherichia coli O157:H7 Q fever arenaviruses [e.g.,
Encephalitis (human) Ricin intoxication (castor Lassa, Machupo])
beans) Yellow fever
Any unusual disease or manifestation of illness, including but not limited to the appearance of a novel or
previously controlled or eradicated infectious agent, or biological or chemical toxin.

Annual Summary 2009
8
Class 2: Diseases or conditions of public health importance of which individual cases shall be
reported by mail, telephone or electronically, within 1 week of diagnosis. In outbreaks
or other unusual circumstances they shall be reported the same as Class 1. Class 2
diseases and conditions are those for which an immediate public health response is not
needed for individual cases.
Chlamydia trachomatis, genital Malaria Spinal cord injuries
infection Meningitis other than Streptococcus
Dengue meningococcal or H. influenzae pneumoniae,
Ehrlichiosis type b
invasive disease
‡
,
Enterococcus, invasive infection
‡
,
Mumps Antibiotic resistant
vancomycin resistant M. tuberculosis infection (positive Streptococcus
Gonorrhea TST) in children < 15 years of age pneumoniae,
Hepatitis (acute, viral only) Note- Noncholera vibrio disease
invasive disease
‡
in
Hepatitis A requires Class 1 Poisonings* (including elevated children < 5 years of age
Report blood lead levels**) Tetanus
Legionellosis Rocky Mountain spotted fever Trichinosis
Listeriosis Rubella (including congenital) Viral encephalitis in horses
Lyme disease Salmonellosis and ratites
Shigellosis
†
Usually presents as menin
g
itis or septicemia, or less commonly as cellulitis, epi
g
lottitis, osteomyelitis,
pericarditis or septic arthritis.
‡
Specimen obtained from a normally sterile site.
*Reports for poisonings shall be made to Mississippi Poison Control Center, UMMC 1-800-222-1222.
**Elevated blood lead levels (as designated below) should be reported to the MSDH Lead Program at
(601) 576-7447.
Blood lead levels (venous) of >
10 µg/dL in children less than 16 years of age
Blood lead levels (venous) of >
25 µg/dL in those 16 years or older
Except for rabies and equine encephalitis, diseases occurring in animals are not required to be reported to
the MSDH.
Class 3: Laboratory based surveillance. To be reported by laboratories only. Diseases or
conditions of public health importance of which individual laboratory findings shall be
reported by mail, telephone, or electronically within one week of completion of
laboratory tests (refer to Appendix B of the Rules and Regulations Governing Reportable
Diseases and Conditions).
All blood lead test results Cryptosporidiosis Histoplasmosis
Blastomycosis Hansen disease (Leprosy) Nontuberculous
Campylobacteriosis Hepatitis C infection mycobacterial disease
Class 4: Diseases of public health importance for which immediate reporting is not necessary for
surveillance or control efforts. Diseases and conditions in this category shall be reported
to the Mississippi Cancer Registry within six months of the date of first contact for the
reportable condition.
The National Program of Cancer Registries at the Centers for Disease Control and Prevention
requires the collection of certain diseases and conditions. A comprehensive reportable list
including ICD9CM codes is available on the Mississippi Cancer Registry website,
http://mcr.umc.edu/documents/Reportablecasesafter1006.pdf
.
Each record shall provide a minimum set of data items which meets the uniform standards
required by the National Program of Cancer Registries and documented in the North
American Association of Central Cancer Registries (NAACCR).

Annual Summary 2009
9
Vaccine Preventable Diseases
Haemophilus influenzae type b (Hib), invasive
2009 Case Total 0 2009 rate/100,000 0.0
2008 Case Total 4 2008 rate/100,000 0.1
Clinical Features
Haemophilus influenzae, type b (Hib) is an invasive bacterial disease, particularly
among infants, that can affect many organ systems. Invasive disease usually begins as
a bloodstream infection, with bacteria spreading to distant sites. Epiglottitis,
pneumonia, septic arthritis, and septicemia are other forms of invasive disease. Hib
meningitis presents with fever, decreased mental status and nuchal rigidity. Neurologic
sequelae can occur in 15-30% of survivors, with hearing impairment the most common.
Case fatality rate is 2-5% even with antimicrobial therapy. Peak incidence is usually in
infants 6-12 months of age; Hib disease rarely occurs beyond 5 years of age. In the
prevaccine era, meningitis accounted for 50-60% of all cases of invasive disease. Since
the late 1980’s, with the licensure of Hib conjugate vaccines, Hib meningitis has
essentially disappeared in the U.S.
Infectious Agent
Haemophilus influenzae type b, a gram-negative encapsulated bacterium.
Reservoir
Humans, asymptomatic carriers.
Transmission
Respiratory droplets and contact with nasopharyngeal secretions during the infectious
period.
Incubation
Uncertain; probably short, 2-4 days.
Period of Communicability
As long as organisms are present and up to 24-48 hours after starting antimicrobial
therapy.
Methods of Control
Two Hib conjugate vaccines are licensed for routine childhood vaccination. The
number of doses in the primary series is dependent on the type of vaccine used. A
primary series of PRP-OMP (PedvaxHIB®) vaccine is two total doses, at 2 and 4 months

Annual Summary 2009
10
of age; the primary series with PRP-T (ActHIB®) requires three total doses, given at 2, 4
and 6 months of age. A booster dose at 12-15 months of age is recommended
regardless of which vaccine is used for the primary series. Vaccination with Hib
containing vaccines may decrease the carriage rate, decreasing the chances of
infection in unvaccinated in children. Immunization is not recommended for children
over 5 years of age.
The Mississippi State Department of Health (MSDH) investigates all reported suspected
Hib cases and provides prophylactic antibiotics (rifampin) for all household contacts
with one or more children under one year old or in households with children 1-3 years
old who are inadequately immunized. During investigation, contacts are often treated
before the isolate’s serotype is known. MSDH requests that all Haemophilus influenzae
isolates be sent to the Public Health Laboratory (PHL) for serotyping.
Reporting Classification
Class 1.
Epidemiology and Trends
Prior to the development and widespread use of Hib conjugate vaccines in the late
1980’s and early 1990’s, Hib was the most common cause of bacterial meningitis in
children < 5 years of age. In Mississippi, conjugate vaccine was first offered to 18 month
olds in 1989, to 15 month olds in 1990, and as a primary series, starting at 2 months of
age, with a 12-15 month booster, in January 1991. With the institution of vaccination, the
number of reported cases of invasive disease dropped from 82 in 1989, to 5 by 1994.
There have been less than 5 cases per year since 1995.
In 2009, there were no reported cases of Hib.
Hepatitis B, acute
2009 Case Total 32 2009 rate/100,000 1.1
2008 Case Total 58 2008 rate/100,000 2.0
Clinical Features
An acute viral illness characterized by the insidious onset of anorexia, abdominal
discomfort, nausea and vomiting. Clinical illness is often unrecognized because
jaundice occurs in only 30-50% of adults and fewer than 10% of children. Approximately
5% of all acute cases progress to chronic infection. Younger age at infection is a risk
factor for becoming a chronic carrier with 90% of perinatally infected infants becoming
chronic carriers. Chronic cases may have no evidence of liver disease, or may develop
clinical illness ranging from chronic hepatitis, to cirrhosis, liver failure or liver cancer.
Hepatitis B infections are the cause of up to 80% of hepatocellular carcinomas
worldwide.

Annual Summary 2009
11
Infectious Agent
Hepatitis B virus, a hepadnavirus.
Reservoir
Humans.
Transmission
Transmission occurs through parenteral or mucosal exposure to body fluids of hepatitis B
surface antigen (HBsAg) positive persons, such as perinatal exposure, through contact
with contaminated needles, or through sexual contact. Blood and blood products,
saliva, semen and vaginal secretions are known to be infectious. The three main groups
at risk for hepatitis B infection are heterosexuals with infected or multiple partners,
injection-drug users, and men who have sex with men.
Incubation
45-180 days, average 60-90 days.
Period of Communicability
As long as HBsAg is present in blood. In acute infections, surface Ag can be present 1-2
months after onset of symptoms.
Methods of Control
Routine hepatitis B vaccination series is recommended for all children beginning at
birth, with catch-up at 11-12 years of age if not previously vaccinated. The usual three
dose schedule is 0, 1-2, and 6-18 months. Vaccination is also recommended for high risk
groups, including those with occupational exposure, household and sexual contacts of
HBsAg positive individuals (both acute and chronic infections), and injecting drug users.
Transmission of hepatitis B can be interrupted by identification of susceptible contacts
and HBsAg positive pregnancies, and the timely use of post-exposure prophylaxis with
vaccine and/or immune globulin.
Perinatal transmission is very efficient in the absence of post-exposure prophylaxis, with
an infection rate of 70-90% if the mother is both HBsAg and hepatitis B e antigen
(HBeAg) positive. The risk of perinatal transmission is about 10% if the mother is only
HBsAg positive. MSDH, through the Perinatal Hepatitis B Program, tracks HBsAg positive
pregnant women, provides prenatal HBsAg testing information to the delivery hospitals
when available, and monitors infants born to infected mothers to confirm completion of
the vaccine series by 6 months of age, and then tests for post-vaccine response and for
possible seroconversion at 9-12 months of age. Post-exposure prophylaxis is highly
effective in preventing hepatitis B vertical transmission, therefore, testing of all pregnant
women for HBsAg is recommended with each pregnancy.
Reporting Classification
Class 2.

Annual Summary 2009
12
Epidemiology and Trends
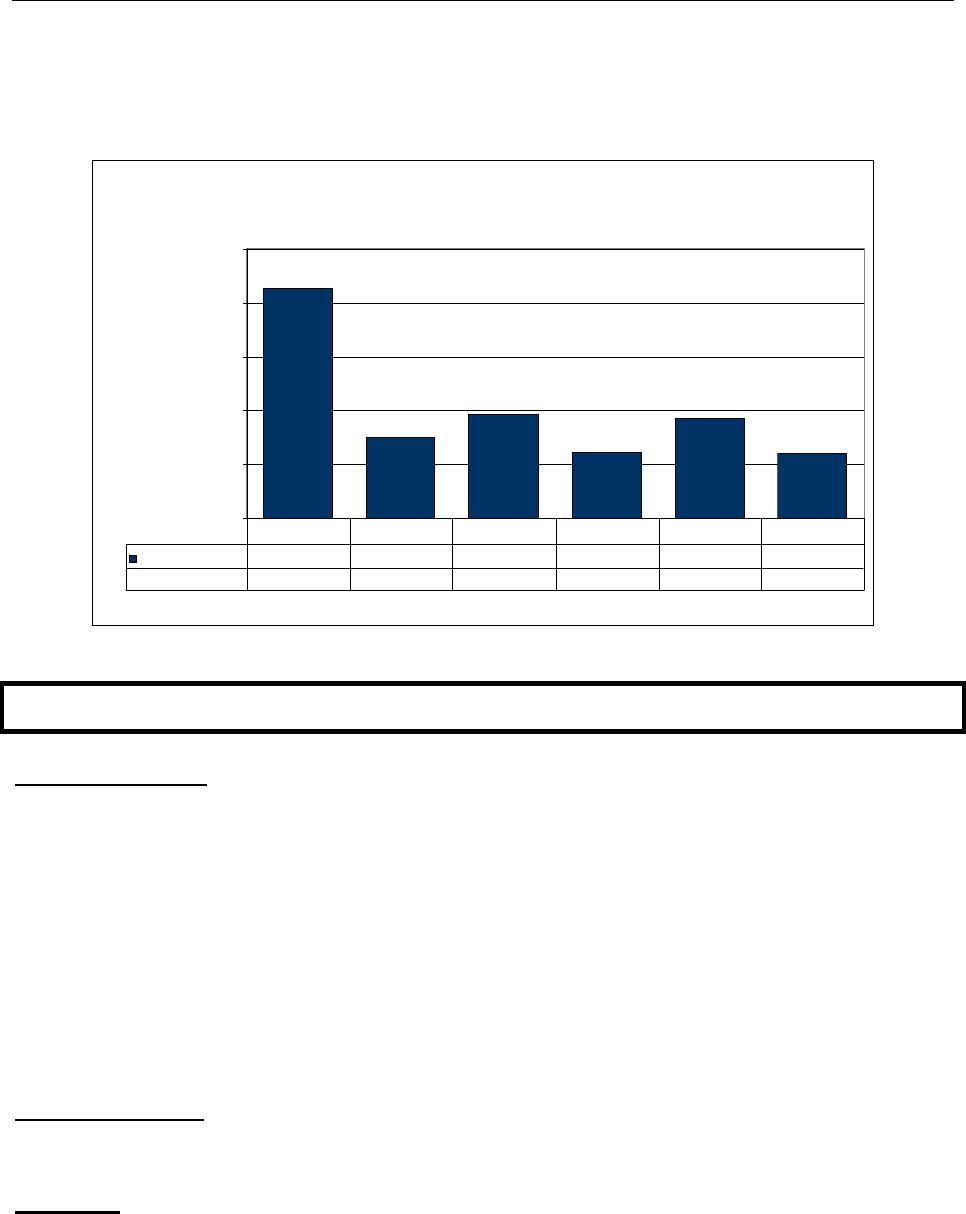
In 2009, 32 cases of acute hepatitis B were reported. This was lower than the 58 cases
reported in 2008, and the three year average (2006-2008) of 37 cases reported annually
(Figure 1). Twenty (63%) of the 32 reported cases occurred in individuals aged 15-34
years. Overall, the cases ranged in age from 16 to 64 years old (Figure 2).
Figure 1
Hepatitis B, Acute, Rates by Year, United States and Mississippi, 2000-2009
0.0
1.0
2.0
3.0
4.0
5.0
6.0
Incidence per 100,000 population
Hepatitis B, acute Rate (U.S.)
2.8 2.8 2.8 2.6 2.1 1.7 1.6 1.5 1.3
Hepatitis B, acute Rate (MS)
3.5 3.2 3.3 2.3 3.5 1.8 0.4 1.4 2.0 1.1
Hepatitis B, acute Cases (MS)
99 91 95 65 102 52 13 41 58 32
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009*
*2009 U.S. data not available.
Figure 2
Hepatitis B, acute, Cases by Age Group, Mississippi, 2009
0
1
2
3
4
5
6
7
0-4 5-9 10-14 15-19 20-24 25-29 30-34 35-39 40-44 45-49 50-54 55-59 60-64 65+
Age Group
Number of Cases

Annual Summary 2009
13
In 2009, 82 HBsAg positive pregnant women were reported to the Perinatal Hepatitis B
Program. This is lower than the 102 reported in 2008 and the three year average of 104
(Figure 3). There were no reported cases of HBsAg-positive infants born to HBsAg
positive mothers in 2009. This was similar to 2008; however, in 2007 there were two cases
of perinatal transmission.
Figure 3
HBsAg-positive Pregnant Women, Mississippi, 2000-2009
0
20
40
60
80
100
120
Number of cases
HBsAg-positive
Pregnant Women
78 42 56 72 105 104 108 101 102 82
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Influenza
Clinical Features
An acute viral infection of the respiratory tract characterized by sudden onset of fever,
often with chills, headache, malaise, diffuse myalgia, and nonproductive cough. In a
typical influenza season, persons aged 65 years and older, young children, pregnant
and postpartum women, and persons at any age with chronic underlying illnesses are
at highest risk of complications. In the recent pandemic caused by 2009 H1N1, the
highest risk of complications were in pregnant and postpartum women, young children
and persons of any age with chronic underlying illnesses. Pneumonia due to secondary
bacterial infections is the most common complication of any influenza infection. In a
recent report issued by the CDC, during the period 1976—2007, estimated influenza
deaths ranged from a low of 3,349 to a high of 48,614 per year in the United States.
Infectious Agent
Influenza is caused by an RNA virus. There is usually one predominant subtype of
influenza virus causing the majority of infection each influenza season; however
influenza A subtypes and influenza B can circulate causing infection each season.

Annual Summary 2009
14
Reservoir
Humans.
Transmission
Transmission occurs person to person by direct or indirect contact with virus laden
droplets or respiratory secretions.
Incubation
The incubation period usually is 1 to 4 days, with a mean of 2 days.
Period of Communicability
From 1 day before clinical onset through 3-5 days from clinical onset in adults, and up
to 7-10 days in young children.
Methods of Control
Yearly vaccination is recommended with either trivalent inactivated vaccine (TIV) or
live attenuated influenza vaccine (LAIV). A separate vaccine was developed for the
pandemic strain, 2009 H1N1, but was not available in adequate quantities for
distribution to the general public until after the 2009 peak incidence of disease in
Mississippi (see Epidemiology and Trends). Education on basic personal hygiene,
specifically transmission from unprotected coughs and sneezes and from hand to
mucous membrane is highly important in preventing or slowing transmission of influenza.
Antivirals, amantadanes (amantadine and rimantadine) and neuraminidase inhibitors
(oseltamivir and zanamivir), can also be used to prevent and treat influenza. High
levels of resistance to the adamantanes persist among influenza A (2009 H1N1) and A
(H3N2) viruses circulating globally. The adamantanes are not effective against
influenza B viruses. Fortunately, influenza A (2009 H1N1) and A (H3N2), as well as
influenza B viruses continue to be sensitive to the neuraminidase inhibitors. Consult the
Centers for Disease Control and Prevention (CDC) MMWR July 31, 2009, Vol. 58, No.
RR—08, “Prevention and Control of Influenza: Recommendations of the Advisory
Committee on Immunization Practices (ACIP), 2009” for vaccine recommendations and
guidelines.
Reporting Classification
Class 1: Influenza associated pediatric deaths (<18 years of age).
Epidemiology and Trends
Influenza activity usually occurs from December through March or April each season,
but infections can be seen earlier or later. Peak activity is typically from February to
March. The risk of complications depends on many factors, including age and
underlying medical conditions. Vaccination status and the match of vaccine to
circulating viruses affect both the susceptibility to infection and the possibility of
complications. Outbreaks can occur in group settings, such as nursing homes.

Annual Summary 2009
15
MSDH monitors seasonal influenza activity statewide through an active syndromic
surveillance program reported by sentinel providers. In the 2009-2010 influenza season,
70 sentinel providers in 42 counties were enrolled in this system, representing hospital
emergency departments, urgent care and primary care clinics, and college and
university student health centers. These providers reported weekly numbers of
nontrauma patient visits consistent with an influenza-like illness (ILI), defined as fever >
100ºF and cough and/or sore throat in the absence of a known cause other than
influenza. MSDH uses this information to estimate the magnitude of the state’s weekly
influenza activity. These data are also used to estimate the geographic spread of
influenza within the state, ranging from no activity to widespread activity. This
terminology represents a geographic estimate rather than an indication of severity of
the season. ILI providers are also supplied with kits for PCR influenza testing at the Public
Health Laboratory (PHL).
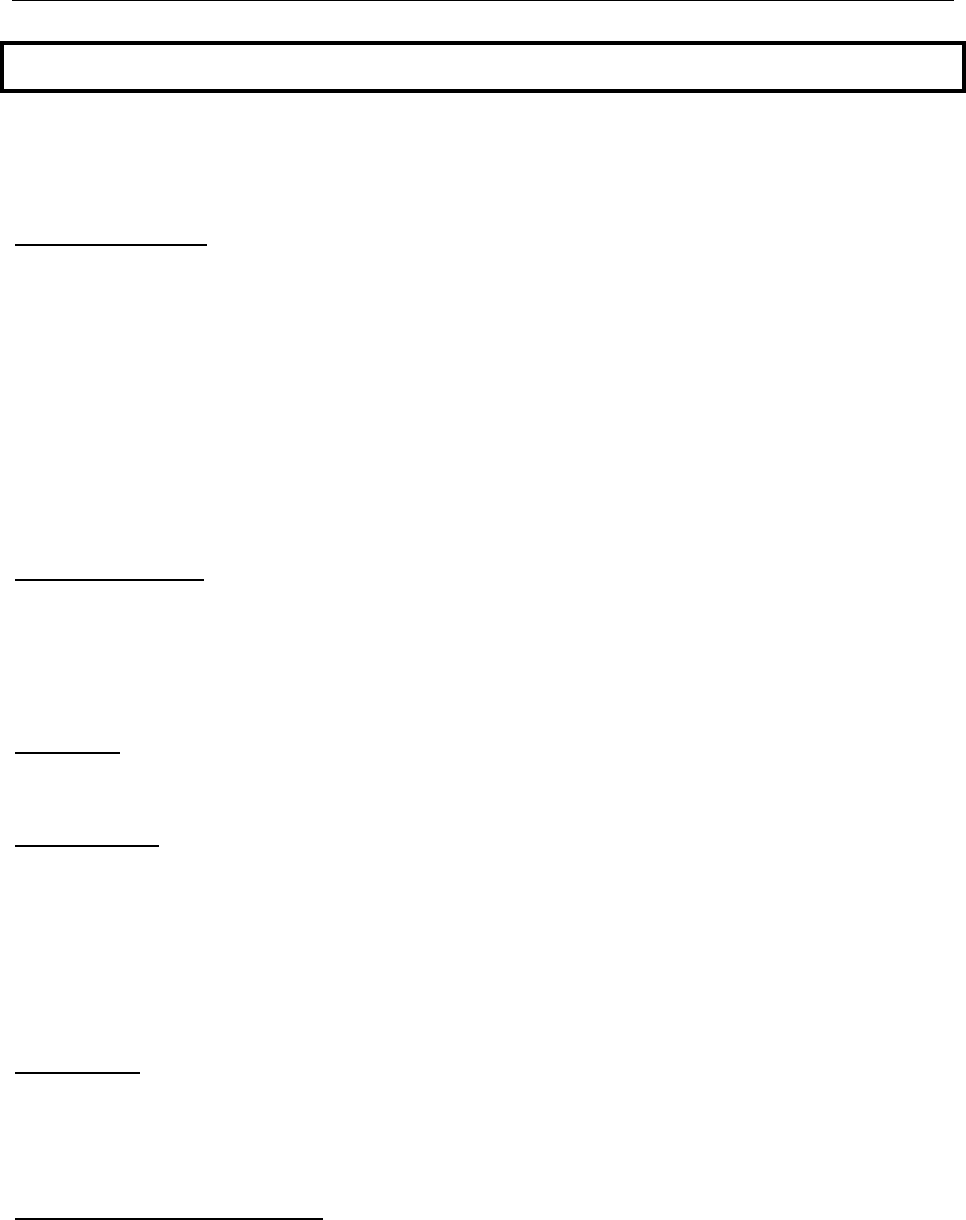
2009 was unusual in that the usual 2008-2009 influenza season was followed by a
pandemic of influenza A (2009 H1N1). The first confirmed case of 2009 H1N1 in Mississippi
occurred on May 15, 2009, however, influenza activity remained low in Mississippi until
the week ending 8/22/2009, approximately one week after the opening of schools in
most parts of the state. Then ILI activity increased and peaked during the week ending
9/5/2009. It then declined and remained lower than the 3 year average for the
remainder of the 2009-2010 season (Figure 4).
Figure 4
Comparison of 3-year ILI Baseline with the 2009-2010
Mississippi Influenza Season Statewide ILI Rate, CDC Weeks 30-30 (2010)
0.0
2.0
4.0
6.0
8.0
10.0
12.0
14.0
16.0
18.0
AUG
1
A
U
G
22
SEPT 12
O
CT 3
OCT 24
NOV 14
D
EC 5
DEC 26
JAN 9
JAN
30
FEB
20
MAR 13
APR 3
APR
2
4
MAY 15
J
UNE
5
JUNE
26
J
UL
Y 17
Week Ending
Percent of Non-Trauma Patients with presenting
with URI/ILI Symptoms
3-Year Avg.
2009-2010
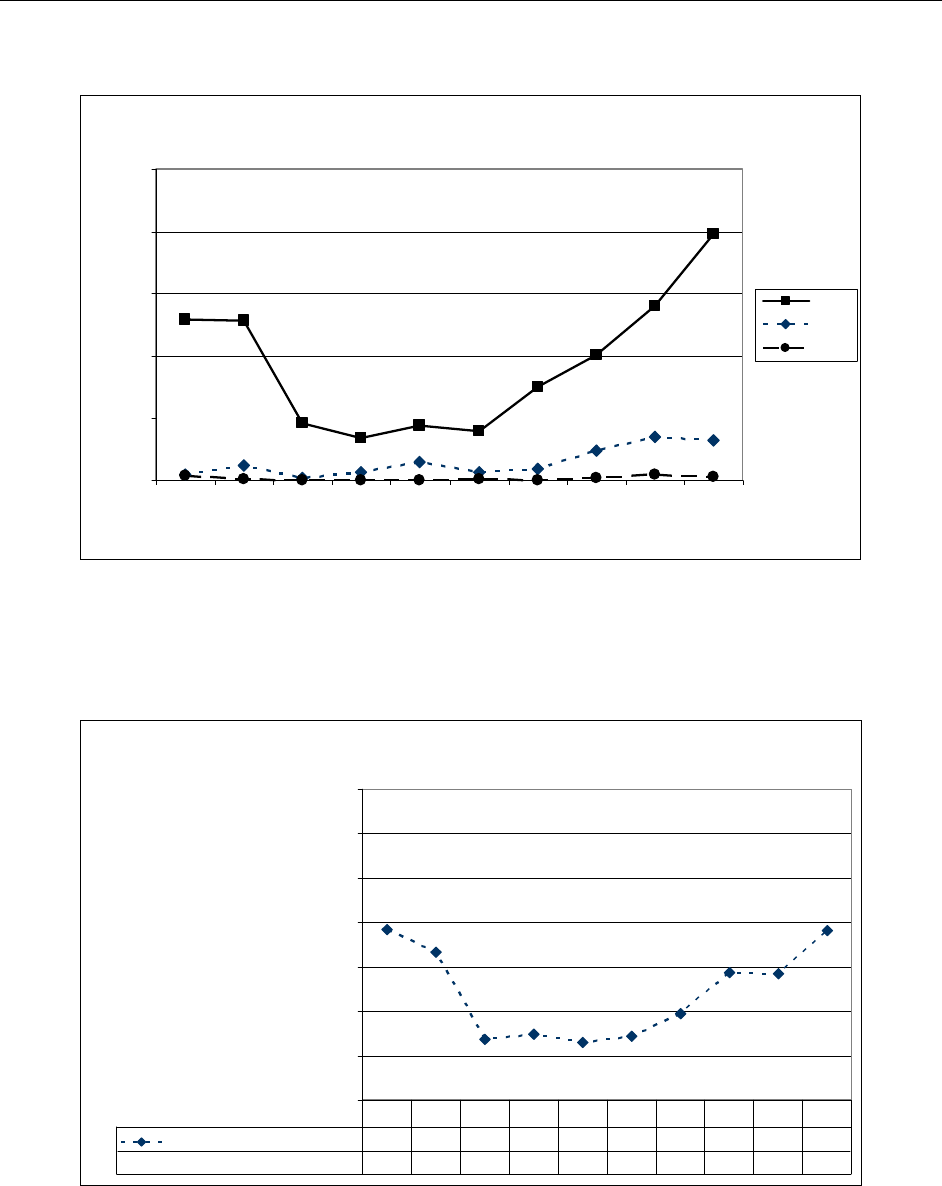
Positive PCR samples were reported throughout the state, with a mixture of influenza A
(H1N1-seasonal), influenza A (H3N2) and influenza B occurring through May 2009 (Figure
5). Beginning in May 2009, Pandemic influenza A (2009 H1N1) appeared with increasing
frequency and became the dominant subtype by June (Figure 6). From July through

Annual Summary 2009
16
the end of 2009, greater than 98% of sub-typeable virus specimens were 2009 H1N1
Pandemic Influenza A.
Figure 5
Comparison of Statewide ILI Rate to Positive Influenza Isolates by Subtype, Mississippi,
CDC Weeks 30-16, 2009-2010
0
50
100
150
200
250
CDC Week
Number of positive influenza isolates
0.0
2.0
4.0
6.0
8.0
10.0
12.0
14.0
16.0
18.0
Percent of non-trauma patients presenting
with URI/ILI symptoms
A (2009 H1N1)
42 47 49 106 129178113 49 60 68 42 29 21 14 12 7 11 2 3 5 3 7 4 1 6 12 4 13 15 15 18 26 13 6 2 2 2 0 0
A (S eason a l H3)
110010000000000000000000000000000000000
A (unknow n subtype)
03447271226230121000000002000000001100000
B
000010110000000000000000000000021000001
ILI rate
3.2 3.6 4.3 8.7 13. 15. 14. 13. 12. 10. 7.7 7.7 6.9 6.9 7.1 6.8 6.7 6.9 6.4 5.5 6.4 6.6 6.4 5.6 4.5 4.7 5.0 5.4 5.8 5.9 5.7 6.5 6.3 5.2 3.9 3.7 3.7 3.6 4.2
30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
Figure 6
Comparison of Statewide ILI Rate to Positive Isolates by Subtype, Mississippi,
CDC Weeks 17-34, 2009
0
20
40
60
80
100
120
140
Number of positive influenza isolates
0.0
2.0
4.0
6.0
8.0
10.0
12.0
14.0
16.0
Percent of non-trauma patients presenting with URI/ILI
symptoms
A (2009 H1N1)
0 0 4 6 24 25 20 45 33 34 24 36 44 42 47 49 106 129
A (S eason a l H 1)
570000000000000000
A (S eason a l H 3)
100600201000011001
B
8175791000010000001
ILI rate
6.7 5.2 4.5 5.2 4.2 3.9 4.2 3.2 3.2 3.2 3.4 3.1 3.0 3.5 3.9 4.6 9.3 13.6
17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34
The age groups most affected by Pandemic influenza A (2009 H1N1) differed from
those seen with seasonal influenza. Seasonal influenza has its greatest impact in those
aver age 65 and in children under age 4. However, persons over age 65 appeared to
be less susceptible to infection with 2009 Pandemic influenza A (H1N1), so that rates of
Annual Summary 2009
17
hospitalization with Pandemic influenza A (2009 H1N1) were greatest in young people
(Figure 7).
Figure 7
Cumulative Lab Confirmed Influenza-Associated Hospitalization Rates
by Age Group, Mississippi, August 30, 2009-May 22, 2010
0.0
5.0
10.0
15.0
20.0
25.0
Age group
Hospitalization Rate per 100,000 Population
Rate per 100,000
21.3 7.4 9.6 6.1 9.2 5.9
Hospitalizations
47 44 25 59 48 22
0-4 5-18 19-24 25-49 50-64 65+
Measles
Clinical Features
Measles is a highly contagious viral illness characterized
by cough, coryza, conjunctivitis
(3 C’s), fever, an erythematous maculopapular
rash, and a pathognomonic
enanthema (Koplik spots). Complications are seen more frequently in children younger
than 5 years of age and in adults 20 years of age and older. Diarrhea, pneumonia and
encephalitis are the most common complications seen. The risk of death is higher in
these age groups as well; the most common cause of death is pneumonia in children,
and acute encephalitis in adults. Subacute sclerosing panencephalitis is a rare
degenarative central nervous system disease that is thought to be due to persistent
measles infection of the brain, and typically presents approximately 7 years after initial
infection.
Infectious Agent
Measles virus, in the paramyxovirus family.
Reservoir
Humans.

Annual Summary 2009
18
Transmission
Transmitted by direct contact with large infectious droplets or, less commonly, by
airborne spread. Measles is highly contagious, and all persons without previous disease
or vaccination are susceptible.
Incubation
Eight to ten days.
Period of Communicability
Three to five days before to four days after rash onset.
Methods of Control
Measles, mumps and rubella (MMR) vaccine is recommended for all children at 12 to15
months of age with a second dose at school entry (4 to 6 years of age). Appropriate
two dose vaccination induces immunity in 99% of individuals.
MSDH investigates all reported cases and provides prophylaxis for all contacts as
appropriate. Measles vaccine administered within 72 hours of exposure may provide
protection in some cases. Immunoglobulin, given within six days of exposure, can
prevent or modify measles in susceptible persons who are at high risk for complications.
Reporting Classification
Class 1.
Epidemiology and Trends
Measles occurs throughout the world with peak incidence usually in late winter and
spring. There have been no reported cases of measles in Mississippi since 1992, when
there were 17 reported cases. Fifteen of those cases were associated with an outbreak
at the University of Mississippi and the index case’s infection in that outbreak was traced
to an exposure in Europe. Following this outbreak, a history of 2 doses of MMR was
required to attend public universities in Mississippi.
Widespread measles immunization has led to the interruption of endemic transmission
of measles in the United States and Mississippi. However, measles continues to be
endemic or has become endemic again in several countries, particularly in Europe, due
in part to dropping immunization rates. Sporadic outbreaks are reported in the U.S. and
are largely due to imported cases. Transmission from these cases easily occurs in
communities with high numbers of unvaccinated persons. Continued high vaccine
rates in the U.S. and in Mississippi are important to provide appropriate population
immunity and decrease the risk to those who are too young to receive vaccine or have
medical contraindications to vaccination.

Annual Summary 2009
19
Meningococcal disease, invasive
2009 Case Total 5 2009 rate/100,000 0.2
2008 Case Total 12
2008 rate/100,000
0.4
Clinical Features
Invasive meningococcal disease is an acute bacterial illness characterized by
meningitis and/or meningococcemia that may rapidly progress to purpura fulminans,
shock and death. Symptoms include rapid onset of fever, severe headache, stiff neck,
nausea and vomiting, and possibly a petechial rash. The case fatality rate, even with
the use of antibiotics and improved supportive measures, remains high at 8-15%. Long
term sequelae occur in 10-20% of survivors and include hearing loss and mental
retardation.
Infectious Agent
Neisseria meningitidis (N. meningitidis), a gram negative aerobic diplococcus. The most
common serogroups in the United States are B, C, W-135, and Y. Licensed vaccines are
not protective against serogroup B.
Reservoir
Humans. Up to 5-10% of the population may be asymptomatic carriers.
Transmission
Transmission of N. meningitidis is person to person by direct contact with respiratory
droplets from the nose and throat of infected individuals or carriers. Less than 1% of
colonized individuals will progress to invasive disease.
Incubation
The incubation period is 2-10 days, commonly 3-4 days.
Period of Communicability
Individuals remain contagious until meningococci are no longer present in nasal or
throat secretions, usually 24 hours after antibiotic treatment has begun.
Methods of Control
Vaccination and post-exposure prophylaxis are effective in preventing invasive
meningococcal disease. Routine vaccination with the quadrivalent meningococcal
conjugate vaccine (MCV4) is recommended for all children aged 11-12 years, children
aged 13-18 years not previously vaccinated, and any person aged 2-55 years with
increased risk for meningococcal disease (terminal complement deficiencies,
functional or anatomic asplenia, college freshman living in dormitories, and travelers to
countries in which N. meningitidis is hyperendemic or epidemic). Use of the

Annual Summary 2009
20
meningococcal polysaccharide vaccine (MPSV) should be limited to persons older
than 55 years of age, or used when MCV4 is not available.
MSDH investigates each reported case and provides prophylactic antibiotics (rifampin)
for household contacts and other appropriate close contacts. Health care workers are
not usually at risk unless there is direct contact with nasopharyngeal secretions (mouth-
to-mouth resuscitation).
Reporting Classification
Class 1.
Epidemiology and Trends
In 2009, there were five reported cases of invasive meningococcal disease. This is a
decline of more than half the cases from 2008. Typically, over the last decade, 7-24
cases are reported annually in Mississippi (Figure 8). Nationally, infants less than 12
months of age have the highest incidence of invasive disease. In the U.S., rates of
disease decline in early childhood, increase during adolescence and early adulthood,
then decrease again in older adults. The 2009 MS cases ranged in age from 15 months
to 63 years, with 60% of cases in the adolescent years and younger (Figure 9).
MSDH requests that all isolates be submitted to the PHL for typing. One of the
confirmed cases in 2009 was typed as serogroup Y. The serogroups for the other four
cases were unknown.
In total, rifampin prophylaxis was provided for 37 contacts of meningococcal disease
cases in 2009. There were no confirmed deaths reported in 2009 from meningococcal
disease.

Annual Summary 2009
21
Figure 8
Meningococcal Disease Rates by Year, United States and Mississippi,
2000-2009
0.0
0.2
0.4
0.6
0.8
1.0
1.2
Incidence per 100,000 population
Meningococcal Rate (U.S.)
0.80.80.60.60.50.40.40.40.4
Meningococcal Rate (MS)
0.50.60.70.80.70.20.20.40.40.2
Meningococcal Cases (MS)
15 18 20 24 20 7 7 12 12 5
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009*
*2009 U.S. data not available.
Figure 9
Meningococcal Disease by Age Group, Mississippi, 2009
0
1
2
3
4
5
< 1 1-4 5-9 10-14 15-19 20-24 25-29 30-34 35-39 40-44 45-49 50-54 55-59 60-64 65+
Age Group
Number of Cases
Mumps
Clinical Features
A viral illness with acute onset of fever, tenderness and swelling in one or more of the
salivary glands. Parotitis is the most common presentation, but asymptomatic infections

Annual Summary 2009
22
do occur. Symptoms typically resolve within 7-10 days. Orchitis in postpubertal males
and oophoritis in postpubertal females are the most frequent complications.
Infectious Agent
Mumps virus, in the paramyxovirus family.
Reservoir
Humans.
Transmission
Spread through airborne transmission or by direct contact with infected droplet nuclei
or saliva.
Incubation
About 16 – 18 days (range 14 – 25).
Period of Communicability
Three days before to four days after onset of symptomatic disease. Virus has been
isolated from saliva up to 7 days before and 9 days after onset of parotitis.
Method of Control
Measles, mumps and rubella (MMR) vaccine routinely given at 12 – 15 months of age
with a second dose at 4 – 6 years. Immunization of susceptible contacts may be helpful
in prevention of infection.
Reporting Classification
Class 2.
Epidemiology and Trends
In Mississippi, there are typically 1-2 cases reported annually. In 2009 there was one
reported mumps case, compared to zero cases in 2008.
Pertussis
2009 Case Total 80 2009 rate/100,000 2.7
2008 Case Total 104
2008
rate/100,000
3.5
Clinical Features
An acute bacterial disease of the respiratory tract distinguished by prolonged
paroxysmal coughing with a characteristic inspiratory “whoop.” There are three clinical
stages: catarrhal stage, paroxysmal cough stage, and a convalescent stage. Post-
tussive vomiting is common in the paroxysmal stage. Infants under 6 months of age,

Annual Summary 2009
23
vaccinated children, adolescents and adults often do not have whoop or paroxysms.
Pneumonia is the most frequent complication; the majority of fatalities occur in children
under 6 months of age. Adults and adolescents may have a mild illness which often is
undiagnosed, but serve as a source of infection for unvaccinated or incompletely
vaccinated children.
Infectious Agent
Bordetella pertussis, an aerobic gram negative rod.
Reservoir
Humans. Adolescents and adults are reservoirs for B. pertussis and are often the source
of infection in infants.
Transmission
Direct contact with respiratory secretions by airborne route, probably via droplets.
Incubation
Average 9-10 days. (Range 6-20 days).
Period of Communicability
Most transmissible in the catarrhal stage (which lasts about 1 week) and then during the
first 2 weeks after onset of paroxysmal cough, or a total of 21 days after symptom onset.
Communicability then gradually decreases and becomes negligible. Individuals are no
longer considered contagious after 5 days of antibiotic treatment.
Methods of Control
Vaccination and post-exposure prophylaxis are effective in preventing pertussis.
Pertussis vaccine is combined with diphtheria and tetanus toxoids (DTaP); the primary
series consists of four doses given between the ages of 2 months and 18 months, with a
booster at 4-6 years of age.
Pertussis immunity wanes 5-10 years after the booster vaccine, leaving adolescents and
adults more vulnerable to infection. A pertussis containing vaccine (Tdap) was recently
approved for the vaccination of adolescents and adults. Adolescents and adults
should receive a single dose of Tdap to replace a single dose of tetanus (Td).
MSDH investigates each reported case and provides prophylactic antibiotics
(erythromycin, azithromycin) for all household contacts where there is a child less than
one year of age or a pregnant woman in the last three weeks of her pregnancy in the
home.
Reporting Classification
Class 1.

Annual Summary 2009
24
Epidemiology and Trends
Among the diseases for which universal childhood vaccination is recommended,
pertussis is consistently the one that has the highest number of cases annually.
Susceptibility of nonimmunized persons is universal.
In 2009, there were 80 reported cases of pertussis infections. This is a slight decrease
from 2008, with 104 reported cases. The three year average for 2006-2008 was 132
cases (Figure 10).
Over half of the cases in 2009 were among children under 12 months of age (Figure 11).
No pertussis deaths were reported in 2009.
Figure 10
Pertussis Rates by Year, United States and Mississippi, 2000-2009
0.0
1.0
2.0
3.0
4.0
5.0
6.0
7.0
8.0
9.0
10.0
Incidence per 100,000 population
Pertussis Rate (U.S.)
2.8 2.7 3.4 4.0 8.8 8.7 5.2 3.5 4.4
Pertussis Rate (MS)
0.2 0.2 0.7 0.5 0.6 2.1 1.3 8.8 3.5 2.7
Pertussis Cases (MS)
5 6 19 14 18 60 37 256 104 80
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009*
*2009 U.S. data not available.

Annual Summary 2009
25
Figure 11
Pertussis Cases by Age Group, Mississippi, 2009
0
5
10
15
20
25
30
35
40
45
<1 1-4 5 - 9 10 -
14
15 -
19
20 -
24
25 -
29
30 -
34
35 -
39
40 -
44
45 -
49
50 -
54
55 -
59
60 -
64
65+
Age Group
Number of Cases
Pneumococcal disease, invasive
Clinical Features
An acute bacterial infection with two clinical invasive syndromes: septicemia and
meningitis. Septicemia is the most common clinical presentation, with a case fatality
rate as high as 60% among the elderly. Pneumococcal meningitis has a case-fatality
rate of 30%, but may be as high as 80% in elderly persons. Symptoms of meningitis
include abrupt onset of high fever, headache, lethargy, vomiting, irritability, and nuchal
rigidity. It is the leading cause of bacterial meningitis in children less than 5 years of
age. Neurologic sequelae are common among meningitis survivors.
Infectious Agent
Streptococcus pneumoniae, a gram-positive diplococcus. Most strains causing severe
forms of disease are encapsulated; there are 90 known capsular serotypes.
Reservoir
The nasopharynx of asymptomatic human carriers. Carriage is more common in
children than adults.
Transmission
Droplet spread and contact with respiratory secretions.
Incubation
Unknown; probably short, 1-4 days.

Annual Summary 2009
26
Period of Communicability
Period of communicability is unknown, but it is presumed that transmission can occur as
long as S. pneumoniae occurs in respiratory secretions.
Methods of Control
Conjugate and polysaccharide vaccines are available for the prevention of
pneumococcal disease. The conjugate vaccine (PCV7) is approved for children
younger than 24 months of age and children 24-59 months of age at risk for invasive
disease. PCV7 is administered at 2, 4, 6, and 12-15 months of age. The polysaccharide
vaccine (PPV23) is recommended for all adults 65 years of age and older and any
person 2 years of age or older at high risk for invasive pneumococcal disease (chronic
disease such as cardiovascular disease, pulmonary disease or diabetes, and individuals
with cochlear implants).
Reporting Classification
Class 2; invasive disease in children less than 5 years of age and all antibiotic resistant
invasive disease.
Epidemiology and Trends
In 2009 there were 28 reported cases of invasive disease caused by S. pneumoniae in
children less than 5 years of age. This was comparable to the 27 reported cases in 2008
(Figure 12). Of these 28 cases, 24 manifested as septicemia, two had meningitis, and
two had S. pneumoniae isolated from pleural fluid. Ages ranged from 1 month to 4
years of age. Twelve of the 28 invasive S. pneumoniae cases were antibiotic resistant.
Of those 12 cases, 10 of the invasive infections were infected with organisms that
exhibited resistance to one or more antibiotics.
A total of 56 cases of antibiotic resistant invasive S. pneumoniae infections were
reported in 2009, compared to 29 cases reported in 2008. This total included 12 children
less than 5 years of age with drug resistant invasive disease. Of the 56 cases in 2009, 48
(86%) were septic, six cases (11%) had meningitis, and two (4%) had S. pneumoniae
isolated from pleural fluid. Reported cases of antibiotic resistant invasive disease ranged
in age from 2 months to 96 years, with 37 cases (66%) occurring in individuals age 40 or
older (Figure 13). Antibiotic resistance to penicillin was documented in 80%; resistance
to trimethoprim/sulfamethoxazole and erythromycin (61% and 66%, respectively) were
also noted. One S. pneumoniae meningitis death was reported in a 73 year old male.
The antibiotic resistance pattern of this case had intermediate resistance to penicillin.

Annual Summary 2009
27
Figure 12
Streptococcus pneumoniae
, Invasive Disease, Children less
than 5 Years of Age, by Age Group and Clinical Presentation,
Mississippi, 2009
0
2
4
6
8
10
12
14
<11234
Age Group
Number of Cases
Meningitis
Septicemia
Other
Figure 13
Streptococcus pneumoniae
, Invasive Disease, Antibiotic Resistant Cases by
Age Group and Clinical Presentation, Mississippi, 2009
0
5
10
15
20
25
<112345-910-
14
15-
19
20-
24
25-
29
30-
34
35-
39
40-
44
45-
49
50-
54
55-
59
60-
64
65+
Age Group
Number of Cases
Meningitis
Septicemia
Other
Rubella
Clinical Features
A mild, febrile viral disease characterized by a 3 day maculopapular rash. Children
often have few signs or symptoms other than the rash. The rash, typically fainter than a
measles rash, appears on the face initially and progresses distally. Adults may have a
febrile prodrome and lymphadenopathy. Up to 50% of all rubella infections are

Annual Summary 2009
28
subclinical or asymptomatic. Complications occur most often in adults and include
arthritis and encephalitis. Infection during pregnancy, especially in the first trimester,
may result in congenital rubella syndrome (CRS), causing fetal death, prematurity or
birth defects.
Infectious Agent
Rubella virus is classified as a togavirus, genus Rubivirus.
Reservoir
Humans.
Transmission
Direct contact with nasopharyngeal secretions of infected persons or by droplet
spread. Rubella is moderately contagious. Maternal-fetal transmission causes CRS.
Incubation
Usually 14 days, with a range of 12-23 days.
Period of Communicability
The period of communicability is about 1 week before and up to 5-7 days after onset of
the rash. Infants with congenital rubella syndrome may shed the virus for months after
birth.
Methods of Control
Vaccination is the most effective method in preventing rubella. Rubella vaccine is
available combined with measles and mumps vaccines as MMR. The first dose of MMR
is recommended at 12-15 months, followed by a second dose at 4-6 years. All
susceptible adolescents and adults, especially women of child bearing age, should be
vaccinated with MMR vaccine.
Reporting Classification
Class 2.
Epidemiology and Trends
There were no reported cases of rubella in Mississippi in 2009. The last reported case in
the state, in a 4 year old, was in 1986.

Annual Summary 2009
29
Varicella
2009 Case Total 5 2009 rate/100,000 0.2
2008 Case Total 14 2008 rate/100,000 0.5
Clinical Features
An acute viral disease with primary infection (chickenpox) characterized by a
generalized pruritic rash that progresses rapidly from macules to papules to vesicular
lesions before crusting. The rash will be seen in various stages of development, usually
appears first on the head, and is more highly concentrated on the trunk rather than
extremities. Adults may have 1-2 days of fever and discomfort prior to rash onset, but
the rash is frequently the first sign of disease in children. Adults may have more severe
disease and have a higher incidence of complications (secondary bacterial infections,
pneumonia, aseptic meningitis and encephalitis). Herpes zoster is a localized
manifestation of latent varicella infection, with incidence increasing with age. Lesions
usually follow unilateral dermatomal patterns, but can be widespread or disseminated.
Postherpetic neuralgia occurs in up to 15% of zoster patients.
Infectious Agent
Varicella zoster virus, a member of the herpes virus group.
Reservoir
Humans.
Transmission
Person to person transmission by airborne droplet or direct contact with the lesions.
Indirect spread can occur through contact with articles freshly soiled by vesicular or
respiratory secretions. Maternal-fetal transmission also occurs. Susceptible contacts to
localized herpes zoster may develop chickenpox by direct contact with fluid from the
lesions, but respiratory transmission can occur in disseminated zoster.
Incubation
The incubation period is 14-16 days with a range of 10-21 days.
Period of Communicability
The period of communicability can be up to 5 days before onset of the rash (usually 2
days) and continues until all lesions are crusted (about 5 days).
Methods of Control
The live attenuated varicella vaccine is effective in preventing chickenpox. Routine
vaccination is recommended at 12 months with a second dose at 4-6 years of age. Two
doses of vaccine are recommended for all susceptible healthcare workers. The

Annual Summary 2009
30
vaccine can also be used to prevent disease, or at least modify severity of illness, in
susceptible persons if give within 3 days of exposure to an infected individual.
In 2006, FDA approved herpes zoster vaccine for persons 60 years of age and older.
Clinical trials indicate vaccine efficacy of 64%, with less severe disease in those who
developed zoster, and 66% less postherpetic neuralgia.
MSDH investigates outbreaks of varicella and vaccine is recommended after exposure
if there is no evidence of prior disease or vaccination. The vaccine is 70% - 100%
effective in preventing or attenuating disease if given within 72 hours of exposure.
Reporting Classification
Class 1; varicella infection, primary, in patients >15 years of age.
Epidemiology and Trends
In 2009, there were five reported cases of varicella infection in patients 15 years of age
or older. The cases ranged in age from 15-52 years. None of these cases were
epidemiologically linked. The three year average from 2006 to 2008 was six cases of
varicella per year.

Annual Summary 2009
31
Sexually Transmitted Diseases
Chlamydia
2009 Case Total 23,592 2009 rate/100,000 799.2
2008 Case Total 21,261 2008 rate/100,000 723.5
Clinical Features
A sexually transmitted bacterial infection causing urethritis in males and cervicitis in
females. Urethritis in men presents with scant to moderate mucopurulent urethral
discharge, urethral itching, and dysuria. Cervicitis presents as a mucopurulent
endocervical discharge, often with endocervical bleeding. The most significant
complications in women are pelvic inflammatory disease and chronic infections, both
of which increase the risk of ectopic pregnancy and infertility. Perinatal transmission of
chlamydia occurs when an infant is exposed to the infected cervix during birth resulting
in chlamydial pneumonia or conjunctivitis. Asymptomatic infection may be found in 1%-
25% of sexually active men. Up to 70% of sexually active women with chlamydial
infections may also be asymptomatic.
Infectious Agent
Chlamydia trachomatis, an obligate intracellular bacteria. Immunotypes D through K
have been identified in 35-50% of nongonococcal urethritis.
Reservoir
Humans.
Transmission
Transmitted primarily through sexual contact.
Incubation
Incubation period is poorly defined, ranging from 7 to 14 days or longer.
Period of Communicability
Unknown.
Methods of Control
Prevention and control of chlamydia are based on behavior change, effective
treatment, and mechanical barriers. Condoms and diaphragms provide some degree
of protection from transmission or acquisition of chlamydia. Effective treatment of the
infected patient and their partners, from 60 days prior to the onset of symptoms, is
recommended.

Annual Summary 2009
32
Reporting Classification
Class 2.
Epidemiology and Trends
Chlamydia is the most frequently reported bacterial sexually transmitted disease in the
United States and in Mississippi. In 2009, 23,592 cases of chlamydia were reported in
Mississippi, an increase of 11% from 2008 (21,261). Mississippi has reported case rates
higher than the United States average (Figure 14) for several years, and when
compared to other states, Mississippi has the country’s highest rate. The overall
increase in cases can be partially attributed to aggressive statewide screening for
chlamydia in all MSDH STD, family planning, and prenatal clinics beginning April 2004.
Figure 14
Chlamydia Rates by Year, United States and Mississippi, 2000-2009
0.0
100.0
200.0
300.0
400.0
500.0
600.0
700.0
800.0
Incidence per 100,000 population
Chlamydia Rate (U.S.)
252.1 278.3 289.4 301.7 316.5 329.4 347.8 370.2 401.3 409.2
Chlamydia Rate (MS )
451.0 414.0 410.9 423.2 649.8 727.7 652.8 745.1 723.5 799.2
Chlamydia Cases (MS)
12,828 11,777 11,799 12,193 18,863 21,258 19,001 21,686 21,261 23,592
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Chlamydia was reported in every public health district, with the highest incidence rates
noted in Public Health District III (Figure 15).
Implementation
of statewide
screening

Annual Summary 2009
33
Figure 15
Chlamydia Incidence by Public Health District, Mississippi, 2009
Chlamydia infections were reported over a range of age groups, but the largest
proportion was reported among 15-24 year olds, accounting for 76% of the reported
cases (Figure 16). African Americans accounted for 83% of the reported cases in which
race was known (Figure 17). In 2009, the rate of chlamydia infections for African
Americans (1399.6) was nine times the rate for whites (150.3).
Figure 16
Chlamydia Cases by Age Group, Mississippi, 2009
0
1,000
2,000
3,000
4,000
5,000
6,000
7,000
8,000
9,000
10,000
0-4 5-9 10-14 15-19 20-24 25-29 30-34 35-39 40-44 45-49 50-54 55-59 60+
Age Group
Number of cases
District Cases Rate*
I 2615
826.2
II 1849
524.5
III 3236
1426.7
IV 1757
717.9
V 6217
979.3
VI 2042
832.6
VII 1554
891.0
VIII 2221
728.9
IX 2101
464.6
State 23,592
799.2
*per 100,000 population

Annual Summary 2009
34
Figure 17
Chlamydia Cases by Race, Mississippi, 2000-2009
0
2000
4000
6000
8000
10000
12000
14000
16000
18000
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Year
Number of cases
Black
White
Other
Gonorrhea
2009 Case Total 7,241 2009 rate/100,000 245.3
2008 Case Total 7,497 2008
rate/100,000
255.1
Clinical Features
A bacterial infection associated primarily with infection of the urogenital tract
producing symptoms of discharge and dysuria. Other less common sites of infection
include: pharynx, rectum, conjunctiva, and blood.
Complications associated with gonorrhea infection in men consist of epididymitis,
penile lymphangitis, penile edema, and urethral strictures. The primary complication
associated with gonorrhea infection in women is pelvic inflammatory disease, which
produces symptoms of lower abdominal pain, cervical discharge, and cervical motion
pain. Asymptomatic infections do occur. Pregnant women infected with gonorrhea
may transmit the infection to their infants during a vaginal delivery. Infected infants can
develop conjunctivitis leading to blindness if not rapidly and adequately treated.
Septicemia can also occur in infected infants.
Infectious Agent
Neisseria gonorrhoeae, an intracellular gram-negative diplococcus.

Annual Summary 2009
35
Reservoir
Humans.
Transmission
Gonorrhea is transmitted primarily by sexual contact, but transmission from the infected
cervix to an infant during birth occurs.
Incubation
In men, the incubation period is primarily 2-5 days, but may be 10 days or longer. In
women, it is more unpredictable, but most develop symptoms less than 10 days after
exposure.
Period of Communicability
In untreated individuals, communicability can last for months; but if an effective
treatment is provided communicability ends within hours.
Methods of Control
Prevention and control of gonorrhea are based on education, effective treatment, and
mechanical barriers. Condoms and diaphragms provide some degree of protection
from transmission or acquisition of gonorrhea. Effective treatment of the infected
patient and their partners from 60 days prior to the onset of symptoms is recommended.
Reporting Classification
Class 2.
Epidemiology and Trends
Gonorrhea is the second most commonly reported notifiable disease in the United
States. In Mississippi, from 2003-2007, the number of gonorrhea cases increased 31.4%,
from 6,328 to 8,315 cases (Figure 18). Although there was a slight decrease in cases in
2008 and 2009, Mississippi had the highest case rate of gonorrhea in the United States.
Gonorrhea was reported in every public health district, with the highest incidence
noted in Public Health District III (Figure 19).
Although the burden of disease impacted individuals in most of the age groups, 69% of
reported cases were among 15-24 year olds (Figure 20). African Americans accounted
for 91% of the reported cases in which race was known (Figure 21). In 2009, the rate of
gonorrhea infections for African Americans (488.7) was eighteen times the rate of
whites (27.4).

Annual Summary 2009
36
Figure 18
Gonorrhea Rates by Year, United States and Mississippi, 2000-2009
0.0
50.0
100.0
150.0
200.0
250.0
300.0
350.0
400.0
450.0
Incidence per 100,000 population
Gonorrhea Rate (U.S.)
129.0 128.5 122.0 115.2 112.4 114.6 120.9 118.9 111.6 99.1
Gonorrhea Rate (MS)
326.5 272.7 239.4 219.6 246.7 245.5 258.0 285.7 255.1 245.3
Gonorrhea Cases (MS)
9,287 7,756 6,875 6,328 7,163 7,170 7,510 8,315 7,497 7,241
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Figure 19
Gonorrhea Incidence by Public Health District, Mississippi, 2009
District Cases Rate*
I 652
206.0
II 470
133.3
III 1012
446.2
IV 496
202.7
V 2115
333.2
VI 737
300.5
VII 413
236.8
VIII 739
242.5
IX 607
134.2
State 7,241
245.3
*per 100,000 population

Annual Summary 2009
37
Figure 20
Gonorrhea Cases by Age Group, Mississippi, 2009
0
500
1,000
1,500
2,000
2,500
3,000
0-4 5-9 10-14 15-19 20-24 25-29 30-34 35-39 40-44 45-49 50-54 55-59 60+
Age Group
Number of cases
Figure 21
Gonorrhea Cases by Race, Mississippi, 2000-2009
0
1000
2000
3000
4000
5000
6000
7000
8000
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Year
Number of cases
Black
White
Other
Annual Summary 2009
38
HIV Disease
2009 Case Total 610 2009 rate/100,000 20.7
2008 Case Total 606 2008
rate/100,000
20.6
Clinical Features
The clinical spectrum of human immunodeficiency virus (HIV) infection varies from
asymptomatic infections to advanced immunodeficiency with opportunistic
complications. One half to two thirds of recently infected individuals have
manifestations of an infectious mononucleosis-like syndrome in the acute stage. Fever,
sweats, malaise, myalgia, anorexia, nausea, diarrhea, and non-exudative pharyngitis
are prominent symptoms in this stage. Constitutional symptoms of fatigue and wasting
may occur in the early months or years before opportunistic disease is diagnosed. Over
time, HIV can weaken the immune system, lowering the total CD4 count and leading to
opportunistic infections and the diagnosis of Acquired Immunodeficiency syndrome
(AIDS).
Infectious Agent
Human immunodeficiency virus is a retrovirus with two known types, HIV-1 and HIV-2.
These two types are serologically distinct and have a different geographical
distribution, with HIV-1 being primarily responsible for the global pandemic and the
more pathogenic of the two.
Reservoir
Humans.
Transmission
HIV infection can be transmitted from person to person during sexual contact, by blood
product transfusion, sharing contaminated needles or infected tissue or organ
transplant. Transmission by contact with body secretions like urine, saliva, tears or
bronchial secretions has not been recorded. Without appropriate prenatal treatment,
15-30% of infants born to HIV positive mothers are infected. Breast feeding is also a
known cause of mother to infant transmission of HIV.
Incubation
The period from the time of infection to the development of AIDS ranges from 1 year up
to 15 years or longer. The availability of effective anti-HIV therapy has greatly reduced
the development of AIDS in the U.S.
Period of Communicability
Individuals become infectious shortly after infection and remain infectious throughout
the course of their lives.

Annual Summary 2009
39
Methods of Control
Abstinence is the only sure way to avoid sexual HIV transmission; otherwise mutual
monogamy with partners known to be uninfected and the use of latex condoms are
known to reduce the risk of infection. Abstinence and a mutually monogomas
relationship with an uninfected partner are the only ways to completely avoid risk of
sexual HIV transmission. Consistent and correct condom use and as well as treatment
of HIV infected individuals to reduce the viral load, are good risk reduction methods.
Confidential HIV testing and counseling and testing of contacts, prenatal prevention by
counseling and testing all pregnant women, and early diagnosis and treatment with
appropriate anti-retroviral therapy can reduce transmission. Post-exposure prophylaxis
for health care workers exposed to blood or body fluids suspected to contain HIV is an
important worksite preventive measure. MSDH performs contact investigation,
counseling and testing around each reported case of HIV infection.
Reporting Classification
Class 1.
Epidemiology and Trends
Both HIV infection and AIDS are reportable at the time of diagnosis, so many patients
will be reported twice (once at first diagnosis of HIV infection, and again when
developing an AIDS defining illness). The epidemiologic data that follows is regarding
the initial report of HIV disease, whether first diagnosed as HIV infection or AIDS. Over
the past few years, there has been little change in HIV disease trends. There were 610
cases of HIV disease reported in 2009, a less than1% increase from 2008 (606) (Figure
22).
Figure 22
HIV Disease Rates by Year, Mississippi, 2000-2009
0.0
5.0
10.0
15.0
20.0
25.0
30.0
35.0
Incidence per 100,000 population
HIV Disease Rate (MS)
21.8 25.2 21.7 21.7 20.9 19.8 20.6 21.0 20.6 20.7
HIV Disease Cases (MS)
620 716 623 625 607 577 599 611 606 610
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009

Annual Summary 2009
40
Individuals from every Public Health District were impacted by this disease. Public
Health District V reported the highest case rate, statewide, followed by District III (Figure
23).
Figure 23
HIV Incidence by Public Health District, Mississippi, 2009
HIV disease was reported in all age groups, with 58% of the cases reported among 20-
39 year olds (Figure 24). African Americans were disproportionately impacted by HIV
disease. In 2009, 78% of new cases were among African Americans in which race was
known (Figure 25).
Figure 24
HIV Disease Cases by Age Group, Mississippi, 2009
0
20
40
60
80
100
120
0-4 5-9 10-14 15-19 20-24 25-29 30-34 35-39 40-44 45-49 50-54 55-59 60+
Age Group
Number of cases
District Cases Rate*
I 62
19.6
II 27
7.7
III 67
29.5
IV 33
13.5
V 218
34.3
VI 36
14.7
VII 28
16.1
VIII 63
20.7
IX 76
16.8
State 610
20.7
*per 100,000 population

Annual Summary 2009
41
Figure 25
HIV Disease Cases by Race, Mississippi, 2000-2009
0
100
200
300
400
500
600
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Year
Number of cases
Black
White
Other
Additional References:
CDC. Guidelines for national immunodeficiency virus case surveillance, including
monitoring for human immunodeficiency virus infection and acquired
immunodeficiency syndrome. MMWR 1999/48(RR13;1-28.
Sterling, T. R. & Chaisson, R. E. (2005). General Manifestations of Human
Immunodeficiency Virus. In G. L. Mandell, J. E. Bennett, and R. Dolin (Eds.),
Mandell, Douglas, and Bennet’s Principles and Practice of Infectious Diseases (6
th
ed.). (Vols.1-2). (pp. 1548-1549). Philadelphia, PA: Elsevier Churchill Livingstone.

Annual Summary 2009
42
Syphilis
Primary and Secondary Syphilis
2009 Case Total 235 2009 rate/100,000 8.0
2008 Case Total 185 2008
rate/100,000
6.3
Total Early Syphilis
2009 Case Total 562 2009 rate/100,000 19.0
2008 Case Total 418 2008 rate/100,000 14.2
Clinical Features
Syphilis is a bacterial infection that has three stages: primary, secondary, and tertiary.
The primary lesion (chancre) is a painless indurated ulcer that develops at the sight of
initial infection, usually on the external genitalia. Even without treatment, this lesion
resolves in 4-6 weeks. Secondary syphilis may then develop and is characterized by a
generalized symmetrical maculopapular rash that often involves the soles and palms. It
may be accompanied by generalized lymphadenopathy, fever, malaise, sore throat,
headache and arthalgia. Clinical manifestations of secondary syphilis usually resolve
without treatment in weeks to months. Tertiary syphilis will develop years later in 15-40% if
untreated, primarily as cardiovascular or neurosyphilis, or as skin, bone, visceral or
mucosal surface gummas. Latent syphilis, a period of seroreactivity without clinical
disease, is classified as early (infection acquired within the preceding year) or late
(infection of more than a year’s duration).
Fetal transmission occurs through the placenta in untreated women with early syphilis,
resulting in congenital syphilis. Congenital syphilis can lead to abortions, stillbirths or
death shortly after birth. An infected infant may be asymptomatic for the first few weeks
of life; however, late manifestations may occur resulting in CNS involvement or other
conditions such as Hutchinson teeth, saddlenose, periostitis, interstitial keratitis or
deafness.
Infectious Agent
Treponema pallidum, a spirochaete.
Reservoir
Humans.
Transmission
Syphilis is transmitted primarily by sexual contact with an infected individual with early
syphilis (the first year of infection), especially during primary and secondary syphilis.
Transplacental infection of the fetus occurs during the pregnancy of an infected

Annual Summary 2009
43
woman, resulting in congenital syphilis. Transmission can also result from a blood
transfusion if the donor is in the early stages of infection.
Incubation
The average incubation period for syphilis before clinical manifestations is 3 weeks but
ranges from 3 – 90 days.
Period of Communicability
In untreated individuals, communicability can last for up to two years. Syphilis is most
communicable during the primary and secondary stages. Maternal-fetal transmission is
more likely in early syphilis, but may occur at any stage.
Methods of Control
Mechanical barriers, early detection, and effective treatment of the patient and their
partners are effective methods in prevention and control of syphilis. MSDH performs
contact investigation and treatment for each reported case of syphilis.
Reporting Classification
Class 1.
Epidemiology and Trends
Mississippi had a decline in primary and secondary (P&S) syphilis from 1997 through
2003, and since then, has had an increase in rates. Although P&S syphilis rates
remained below the national average from 2002 through 2006, in 2009, MS ranked fifth
nationally (Figure 26).
Figure 26
Primary and Secondary Syphilis Rates by Year, United States and
Mississippi, 2000-2009
0.0
2.0
4.0
6.0
8.0
10.0
12.0
Incidence per 100,000 population
P&S Syphilis Rate (U.S.)
2.1 2.2 2.4 2.5 2.7 2.9 3.3 3.8 4.5 4.6
P&S Syphilis Rate (MS)
4.9 5.0 1.7 1.4 2.0 1.6 3.0 4.5 6.3 8.0
P&S Syphilis Cases (MS)
138 141 48 40 59 47 87 132 185 235
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009

Annual Summary 2009
44
Districts VIII, V, and IX had the highest incidence of P&S syphilis (Figure 27). Seventy-
seven percent of P&S syphilis cases occurred among 15-39 year olds (Figure 28) and
85% were among African Americans in which race was known (Figure 29).
Figure 27
Primary and Secondary Syphilis Incidence by Public Health District,
Mississippi, 2009
Figure 28
Primary and Secondary Syphilis Cases by Age Group,
Mississippi, 2009
0
10
20
30
40
50
60
70
0-4 5-9 10-14 15-19 20-24 25-29 30-34 35-39 40-44 45-49 50-54 55-59 60+
Age Group
Number of cases
District Cases Rate*
I 14
4.4
II 6
1.7
III 23
10.1
IV 7
2.9
V 75
11.8
VI 8
3.3
VII 9
5.2
VIII 44
14.4
IX 49
10.8
State 235
8.0
*per 100,000 population
Annual Summary 2009
45
Figure 29
Primary and Secondary Syphilis Cases by Race, Mississippi, 2000-2009
0
50
100
150
200
250
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Year
Number of cases
Black
White
Other
In 2009, Mississippi reported 562 cases of total early syphilis (first year of infection). There
has been an increase in cases reported since 2004 (Figure 30).
Figure 30
Total Early Syphilis Rates by Year, Mississippi, 2000-2009
0.0
5.0
10.0
15.0
20.0
25.0
30.0
35.0
Incidence per 100,000 population
Total Early Syphilis Rate (MS)
19.2 16.6 6.9 7.4 6.5 7.2 9.8 14.3 14.2 19.0
Total Early Syphilis Cases (MS)
546 473 197 213 188 209 284 417 418 562
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Total early syphilis was reported in every district. District VIII had the highest case rate in
the state (Figure 31).

Annual Summary 2009
46
Figure 31
Total Early Syphilis Incidence by Public Health District,
Mississippi, 2009
Forty-one percent of reported cases were among 20-29 year olds (Figure 32). African
Americans are disproportionately affected, accounting for 82% of cases for which race
was known (Figure 33).
Figure 32
Total Early Syphilis Cases by Age Group, Mississippi, 2009
0
20
40
60
80
100
120
140
160
0-4 5-9 10-14 15-19 20-24 25-29 30-34 35-39 40-44 45-49 50-54 55-59 60+
Age Group
Number of cases
District Cases Rate*
I 40
12.6
II 12
3.4
III 52
22.9
IV 13
5.3
V 195
30.7
VI 25
10.2
VII 17
9.7
VIII 109
35.8
IX 99
21.9
State 562
19.0
*per 100,000 population

Annual Summary 2009
47
Figure 33
Total Early Syphilis Cases by Race, Mississippi, 2000-2009
0
100
200
300
400
500
600
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Year
Number of cases
Black
White
Other

Annual Summary 2009
48
Tuberculosis
Tuberculosis
2009 Case Total 121 2009 rate/100,000 4.2
2008 Case Total 117 2008 rate/100,000
4.0
Clinical Features
Pulmonary tuberculosis (TB) is the most common form of active TB disease, but disease
can be extrapulmonary, involving many organ systems. Symptoms are dependent on
the site of infection, but pulmonary TB generally presents with cough (dry and later
productive), pleuritic chest pains, hemoptysis, shortness of breath, fever, malaise,
weakness, night sweats, and anorexia and weight loss. Latent tuberculosis infections
(LTBI) occur and are asymptomatic.
Infectious Agent
Mycobacterium tuberculosis complex, an acid fast bacillus.
Reservoir
Primarily humans, rarely primates; in some areas, diseased cattle.
Transmission
Exposure to tubercle bacilli in airborne droplet nuclei, 1 to 5 microns in diameter. The risk
of infection with the tubercle bacillus is directly related to the degree of exposure.
Incubation
Tuberculin skin test conversion or positive BAMT, indicating LTBI, occurs 2-10 weeks after
exposure to active TB disease. Ten percent of persons with LTBI will develop clinically
active disease with the first 12-24 months after infection constituting the most hazardous
period. HIV infection increases the risk and shortens the interval for development of
active disease following infection with TB. Children under 5 years of age have the
highest risk of developing active disease.
Period of Communicability
The degree of communicability depends on the number of bacilli discharged, virulence
of the bacilli, adequacy of ventilation, exposure of bacilli to sun or UV light, and
opportunities for aerosolization. Antimicrobial chemotherapy usually eliminates
communicability within 2-4 weeks. Children with primary tuberculosis are generally not
infectious. LTBI is not infectious.

Annual Summary 2009
49
Methods of Control
Prompt identification, diagnosis and treatment of potentially infectious patients with TB
disease. MSDH performs contact investigation, TB screenings in high risk areas, and
provides treatment for all active and latent TB infections.
Reporting Classification
Class 1.
Epidemiology and Trends
Mississippi had a consistent decline in TB morbidity from 1989 through 2005 and TB rates
were below the national average in each of the 2001-2006 reporting periods. However,
from a low of 103 cases in 2005, reported cases increased in 2006 (115), 2007 (137), 2008
(117) and 2009 (121). The case rate was above the national average in 2007 (4.7), and
again in 2009 (4.2) (Figure 34).
Figure 34
Tuberculosis Rates by Year, United States and Mississippi, 2000-2009
0
1
2
3
4
5
6
7
8
9
10
Incidence per 100,000 population
TB Rate (US)
5.8 5.6 5.2 5.1 4.9 4.8 4.6 4.4 4.2 3.8
TB Rate (MS)
6.1 5.4 4.7 4.4 4.1 3.5 4.0 4.7 4.0 4.2
TB Cases (M S)
173 154 134 128 119 103 115 137 117 121
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Geographically, TB was reported in every public health district, with the highest
incidence noted in Public Health Districts V and III (Figure 35).

Annual Summary 2009
50
Figure 35
Tuberculosis Incidence by Public Health District, Mississippi, 2009
Disease occurred in all age ranges, with the majority (75%) of cases reported in
individuals 40 years of age and older (Figure 36). Disease in the African American
population routinely accounts for approximately two-thirds of morbidity (Figure 37).
There has also been a rise in TB cases among patients co-infected with HIV over the
past few years (Figure 38).
Figure 36
Tuberculosis Cases by Age Group, Mississippi, 2009
0
5
10
15
20
25
30
<1 1-4 5-9 10-
14
15-
19
20-
24
25-
29
30-
34
35-
39
40-
44
45-
49
50-
54
55-
59
60-
64
65+
Age Group
Number of Cases
District Cases Rate*
I 8 2.5
II 8 2.3
III 11 4.8
IV 8 3.3
V 59 9.3
VI 8 3.3
VII 5 2.9
VIII 4 1.3
IX 10 2.3
State 121 4.2
*per 100,000 population

Annual Summary 2009
51
Figure 37
Tuberculosis Cases by Race, Mississippi, 2000-2009
0
20
40
60
80
100
120
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Year
Number of cases
Black
White
Other
Figure 38
Tuberculosis and HIV Coinfections, Mississippi, 2000-2009
0.0
2.0
4.0
6.0
8.0
10.0
12.0
14.0
16.0
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Year
Percentage of TB cases Coinfected with
HIV

Annual Summary 2009
52
Enteric Diseases
Campylobacteriosis
2009 Case Total 110 2009 rate/100,000 3.7
2008 Case Total 115 2008 rate/100,000
3.9
Clinical Features
Campylobacteriosis is a zoonotic bacterial disease of variable severity ranging from
asymptomatic infections to clinical illness presenting with diarrhea, abdominal pain,
fever, and nausea and vomiting. Symptoms typically resolve after one week, but may
persist for weeks if untreated. Rare post-infectious syndromes include reactive arthritis
and Guillain-Barre syndrome (GBS).
Infectious Agent
Campylobacter jejuni (C. jejuni) causes most cases of diarrheal illness in humans.
Reservoir
Commonly present in cattle and poultry.
Transmission
Transmission mainly occurs through ingestion of undercooked meat, usually poultry, but
occasionally contaminated food or water or raw milk. The number of organisms
required to cause infection is low.
Incubation
Average incubation is 2-5 days, with a range from 1-10 days.
Period of Communicability
Person to person transmission does not typically occur, though the infected individual
may shed organisms for up to 7 weeks without treatment.
Methods of Control
Disease prevention includes promotion of proper food handling, good hand washing,
particularly after handling raw meats, and after contact with feces of dogs and cats.
Pasteurizing milk and chlorinating water are also important. Symptomatic individuals
should be excluded from food handling or care of patients in hospitals or long term
care facilities.
Reporting Classification
Class 3.

Annual Summary 2009
53
Epidemiology and Trends
In 2009, there were 110 reported cases of campylobacteriosis in Mississippi, comparable
to the 115 cases reported in 2008 and the three-year (2006-2008) average of 107 cases
(Figure 39).
Figure 39
Campylobacteriosis Rates and Cases by Year, Mississippi, 2000-2009
0.0
1.0
2.0
3.0
4.0
5.0
6.0
Incidence per 100,000 Population
Campylobacteriosis Rate
3.4 4.4 3.7 3.8 3.8 3.2 2.7 4.4 3.9 3.7
Campylobacteriosis Cases
97 126 107 109 110 93 79 128 115 110
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Campylobacter infections are typically more common in the warmer months, as are
many enteric illnesses, with 42% of the total 2009 cases occurring in June, July, and
August, however cases are reported to MSDH year round (Figure 40). The highest rates
of infection are in children less than five years of age. In 2009, 25% of all reported cases
were in children younger than five years of age (Figure 41).
Figure 40
Campylobacteriosis Cases by Month, Mississippi, 2009
0
2
4
6
8
10
12
14
16
18
20
January
F
ebr
uary
March
April
M
ay
June
July
A
u
gust
September
October
N
ovember
D
ece
mber
Month
Number of Cases
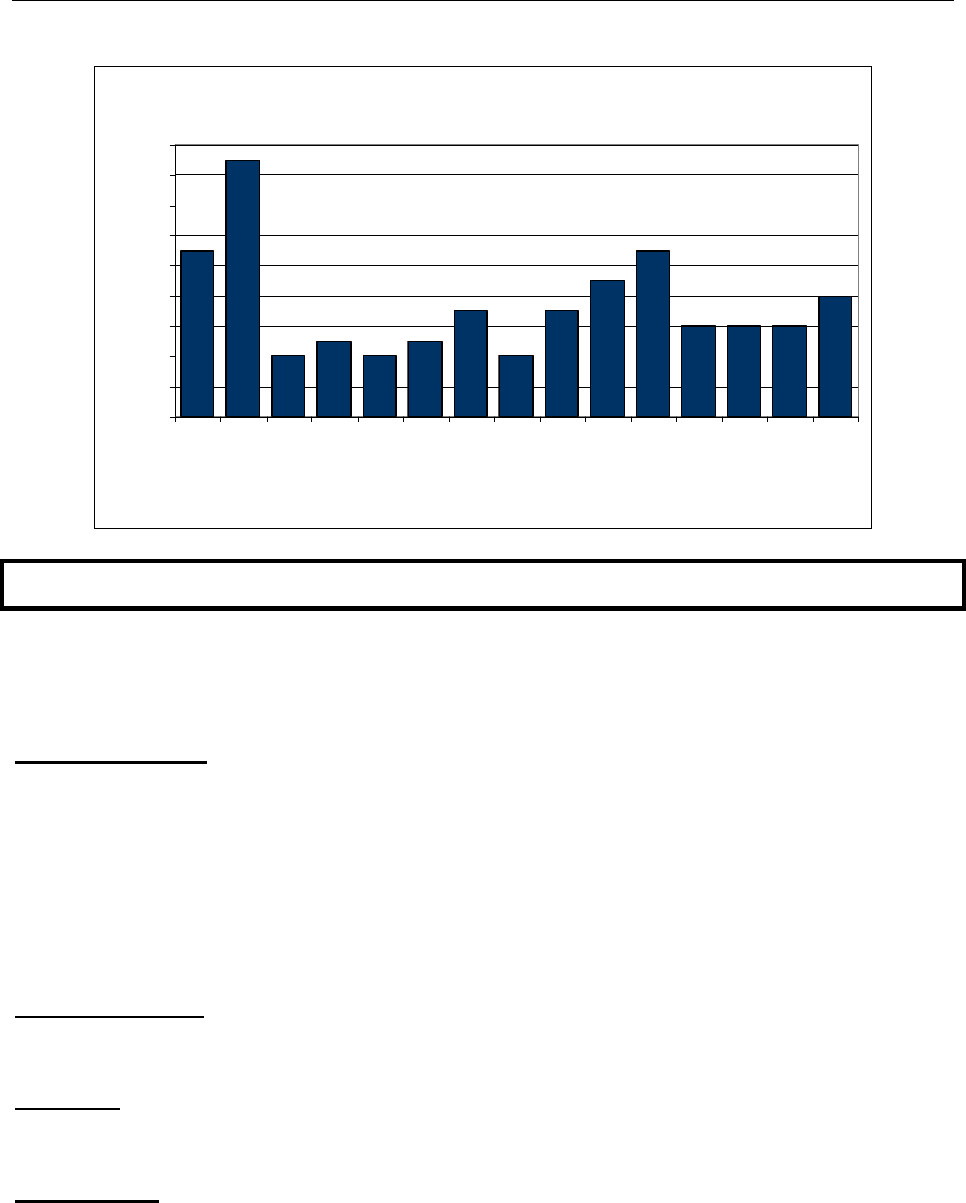
Annual Summary 2009
54
Figure 41
Campylobacteriosis Cases by Age Group, Mississippi, 2009
0
2
4
6
8
10
12
14
16
18
<1 1-4 5-9 10-
14
15-
19
20-
24
25-
29
30-
34
35-
39
40-
44
45-
49
50-
54
55-
59
60-
64
65+
Age Group
Number of Cases
Cryptosporidiosis
2009 Case Total 19 2009 rate/100,000 0.6
2008 Case Total 17 2008 rate/100,000
0.6
Clinical Features
A parasitic infection characterized by profuse, watery diarrhea associated with
abdominal pain. Symptoms include anorexia, weight loss, fever, and nausea and
vomiting less frequently. Symptoms often wax and wane and but generally disappear in
30 days or less in healthy people. Asymptomatic infections do occur. The disease may
be prolonged and fulminant in immunodeficient individuals unable to clear the
parasite. Children under 2, animal handlers, travelers, men who have sex with men, and
close personal contacts of infected individuals are more prone to infection.
Infectious Agent
Cryptosporidium parvum, a coccidian protozoan, is associated with human infection.
Reservoir
Humans, cattle and other domesticated animals.
Transmission
Fecal-oral, which includes person-to-person, animal-to-person, waterborne (including
recreational use of water) and foodborne transmission. Oocysts are highly resistant to
chemicals used to purify drinking water and recreational water (swimming pools, water
parks). The infectious dose can be as low as 10 organisms.

Annual Summary 2009
55
Incubation
1 to 12 days (average 7 days).
Period of Communicability
As long as oocysts are present in the stool. Oocysts may be shed in the stool from the
onset of symptoms to several weeks after symptoms resolve.
Methods of Control
Education of the public regarding appropriate personal hygiene, including
handwashing. Symptomatic individuals with a diagnosis of cryptosporidiosis should not
use public recreational water (eg, swimming pools, lakes, ponds) while they have
diarrhea and for at least 2 weeks after symptoms resolve. It is recommended that
infected individuals be restricted from handling food, and symptomatic children be
restricted from attending daycare until free of diarrhea. Prompt investigation of
common food or waterborne outbreaks is important for disease control and prevention.
Reporting Classification
Class 3.
Epidemiology and Trends
There were 19 reported cases of cryptosporidiosis in 2009, which is comparable to 2008
with 17 reported cases. In a typical year, usually between 3-29 cases are reported
(Figure 42). The reported cases ranged in age from 3 to 66 years (Figure 43).
Figure 42
Cryptosporidiosis Rates by Year, United States and Mississippi, 2000-2009
0.0
1.0
2.0
3.0
4.0
5.0
Incidence per 100,000 population
Cryptosporidiosis Rate (US)
1.2 1.3 1.1 1.2 1.2 1.9 2.1 3.7 3.0
Cryptosporidiosis Rate (MS)
0.5 0.5 0.4 0.3 1.0 0.1 0.9 3.5 0.6 0.6
Cryptosporidiosis Cases (MS)
15 15 12 10 29 3 25 103 17 19
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009*
*2009 U.S. data not available.

Annual Summary 2009
56
Figure 43
Cryptosporididosis by Age Group, Mississippi, 2009
0
1
2
3
4
5
< 1 1-4 5 - 9 10 -
14
15 -
19
20 -
24
25 -
29
30 -
34
35 -
39
40 -
44
45 -
49
50 -
54
55 -
59
60 -
64
65+
Age Group
Number of Cases
E. coli O157:H7/ HUS
2009 Case Total 6 2009 rate/100,000 0.2
2008 Case Total 5 2008 rate/100,000 0.2
Clinical Features
Escherichia coli (E. coli) O157:H7 is the most virulent serotype of the Shiga toxin-
producing E. coli (STEC), and is associated with diarrhea, hemorrhagic colitis, hemolytic-
uremic syndrome (HUS), and postdiarrheal thrombotic thrombocytopenic purpura (TTP).
Symptoms often begin as nonbloody diarrhea but can progress to diarrhea with occult
or visible blood. Severe abdominal pain is typical, and fever is usually absent. The very
young and the elderly are more likely to develop severe illness and HUS, defined as
microangiopathic hemolytic anemia, thrombocytopenia, and acute renal dysfunction.
HUS is a complication in about 8% of E. coli O157:H7 infections. Supportive care is
recommended as antibiotic use may increase the risk of progression to HUS.
Infectious Agent
E. coli are gram negative bacilli. E. coli O157:H7 is thought to cause more than 90% of
all diarrhea-associated HUS.
Reservoir
Cattle, to a lesser extent other animals, including sheep, deer, and other ruminants.
Humans may also serve as a reservoir for person-to-person transmission.

Annual Summary 2009
57
Transmission
Mainly through ingestion of food contaminated with ruminant feces, usually
inadequately cooked hamburgers; also contaminated produce or unpasteurized milk.
Direct person-to-person transmission can occur in group settings. Waterborne
transmission occurs both from contaminated drinking water and from recreational
waters.
Incubation
2-10 days, with a median of 3-4 days.
Period of Communicability
Duration of excretion is typically 1 week or less in adults but can be up to 3 weeks in
one-third of children. Prolonged carriage is uncommon.
Methods of Control
Education regarding proper food preparation and handling and good hand hygiene is
essential in prevention and control. Pasteurization of milk and juice is important.
MSDH investigates all reported cases of HUS and E. coli O157:H7 infections. All isolates
should be submitted to the Public Health Laboratory (PHL) for molecular subtyping, or
DNA “fingerprinting”, with pulsed-field gel electrophoresis (PFGE). Isolate information is
submitted to a national tracking system (PulseNet), a network of public health and food
regulatory agencies coordinated by the CDC. This system facilitates early detection of
common source outbreaks, even if the affected persons are geographically far apart,
and assists in rapidly identifying the source of outbreaks.
Reporting Classification
Class1.
Epidemiology and Trends
In 2009, six E. coli O157:H7 infections were reported to MSDH; two of which resulted in
HUS. On average, eight infections have been reported annually over the past three
years (2006-2008) (Figure 44). The six cases in 2009 were not related to any outbreaks
and were not epidemiologically linked. There were no deaths reported in Mississippi in
2009. Of the 30 cases of E. coli O157:H7/HUS that were reported to MSDH between 2006
and 2009, 20% occurred in children less than 10 years of age (Figure 45).

Annual Summary 2009
58
Figure 44
E. coli
O157:H7/HUS Rates by Year, United States and Mississippi, 2000-2009
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
1.6
1.8
2.0
Incidence per 100,000 population
E. coli O157:H7/HUS Rate (US)
1.6 1.2 1.3 0.9 0.9 0.9 1.5 1.6 1.7
E. coli O157:H7/HUS Rate
(MS)
1.20.40.50.30.60.20.40.30.20.2
E. coli O157:H7 cases (MS)
35 11 15 9 16 7 11 8 5 6
2000 2001 2002 2003 2004 2005 2006* 2007 2008 2009**
* 2006 U.S. rate includes E. coli O157:H7; shiga toxin positive, serogroup non-O157; and shiga toxin positive, not serogrouped.
**2009 U.S. data not available.
Figure 45
E. coli
O157:H7/HUS, by Age Group, Mississippi, 2006-2009
0
1
2
3
4
5
6
7
<1 1-4 5-9 10-
14
15-
19
20-
24
25-
29
30-
34
35-
39
40-
44
45-
49
50-
54
55-
59
60-
64
65+
Age Group
Number of Cases
2009
2006-2008

Annual Summary 2009
59
Hepatitis A
2009 Case Total 9 2009 rate/100,000 0.3
2008 Case Total 7 2008 rate/100,000 0.2
Clinical Features
Hepatitis A is a viral illness with an abrupt onset of fever, malaise, anorexia, nausea,
vomiting, and abdominal pain, followed by jaundice in a few days. The disease varies
in intensity from a mild illness of 1-2 weeks, to a severe disease lasting several months.
Most cases among children are asymptomatic and the severity of illness increases with
age; the case fatality rate is low—0.1%-0.3%. No chronic infection occurs.
Infectious Agent
Hepatitis A virus (HAV), an RNA virus.
Reservoir
Humans, rarely chimpanzees and other primates.
Transmission
Transmission occurs through the fecal-oral route either by person to person contact or
ingestion of contaminated food or water. Common source outbreaks may be related
to infected food handlers. Many younger children are asymptomatic, but shed virus
and are often sources of additional cases.
Incubation
Average 28-30 days, (range 15-50 days).
Period of Communicability
Infected persons are most likely to transmit HAV 1-2 weeks before the onset of
symptoms and in the first few days after the onset of jaundice, when viral shedding in
the stool is at its highest. The risk of transmission then decreases and becomes minimal
after the first week of jaundice.
Methods of Control
In the prevaccine era, hygienic measures and post-exposure immune globulin were the
primary means of preventing infection. Vaccine was first introduced in 1995, and
following successful vaccination programs in high incidence areas, the Advisory
Committee on Immunization Practices (ACIP) recommended routine vaccination for all
children in 2005. Children aged 12-23 months of age should receive one dose of
hepatitis A vaccine followed by a booster 6-18 months later, with catch up vaccination
for children not vaccinated by 2 years of age.
Post-exposure prophylaxis is recommended, within two weeks of exposure, for all
susceptible individuals who are close personal contacts of, or attend daycare with
infected individuals, or are exposed to hepatitis A virus through common source

Annual Summary 2009
60
outbreaks. Hepatitis A vaccine (with completion of the series) is recommended for
post-exposure prophylaxis for all healthy persons aged 12 months to 40 years. Immune
globulin should be considered for children less than 12 months of age, adults over 40
years of age, and those in whom vaccination is contraindicated. Use of both
simultaneously can be considered with higher risk exposures. Post-exposure prophylaxis
is not generally indicated for healthcare workers unless epidemiological investigation
indicates ongoing hepatitis A transmission in the facility.
Reporting Classification
Class 1.
Epidemiology and Trends
There were nine hepatitis A cases reported in Mississippi in 2009. This was comparable to
the seven cases reported in 2008 and to the three year (2006-2008) average of eight
annual cases (Figure 46). The 2009 cases ranged in age from 49 years to 91 years; none
were related to a common source outbreak.
Figure 46
Hepatitis A, acute, Rates by Year, United States and Mississippi, 2000-2009
0.0
2.0
4.0
6.0
8.0
10.0
Incidence per 100,000 population
Hepatitis A, acute Rate (U.S.)
4.9 3.8 3.1 2.7 2.0 1.5 1.2 1.0 0.9
Hepatitis A, acute Rate (MS)
4.6 1.5 2.2 0.6 0.8 0.7 0.3 0.3 0.2 0.3
Hepatitis A, acute Cases (MS)
132 42 63 16 24 19 9 8 7 9
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009*
*2009 U.S. data not available.
Listeriosis
2009 Case Total 5 2009 rate/100,000 0.2
2008 Case Total 6 2008 rate/100,000
0.2
Clinical Features
A bacterial illness that in immunocompetent adults may present as an acute, mild
febrile illness. In the elderly, immunocompromised persons, diabetics, alcoholics and in
newborns, illness may present as meningoencephalitis and/or septicemia. The onset of

Annual Summary 2009
61
meningoencephalitis can be sudden with fever, intense headache, nausea, vomiting
and signs of meningeal irritation. Infected pregnant women may be asymptomatic or
experience only a mild febrile illness; however, infection during pregnancy can lead to
miscarriage or stillbirth, premature delivery, or infection of the newborn. The case
fatality rate is as high as 30-50% in newborns.
Infectious Agent
Listeria monocytogenes, a gram-positive, rod-shaped bacterium.
Reservoir
Mainly occurs in soil, forage, water, mud and silage. Animal reservoirs include domestic
and wild mammals, fowl and people. Asymptomatic fecal carriage is as high as 10% in
humans.
Transmission
Ingestion of unpasteurized or contaminated milk and soft cheeses, as well as
vegetables and ready-to-eat meats, such as deli meats or hot dogs. Unlike most other
foodborne pathogens, Listeria tends to multiply in contaminated foods that are
refrigerated. In neonates, infection can be transmitted in utero or by passage through
the infected birth canal.
Incubation
Variable, estimated median incubation is 3 weeks (range 3-70 days)
Period of Communicability
Mothers of infected newborns can shed the bacterium in vaginal discharges and urine
for 7-10 days post delivery. Infected individuals can shed the bacteria in their stools for
several months.
Methods of Control
Education for proper food handling and preparation. Avoid unpasteurized (raw) milk or
foods made from unpasteurized milk, such as soft cheeses, which can support the
growth of organisms during ripening. Consume perishable and ready-to-eat foods as
soon as possible after purchase, and cook hot dogs thoroughly before consumption.
These recommendations are especially important during pregnancy. MSDH investigates
all reported cases for rapid identification of common source outbreaks.
Reporting Classification
Class 2.
Epidemiology and Trends
There were five reported cases of listeriosis in Mississippi in 2009, which was comparable
to 2008 and with the average number of cases reported for the past three years. The
incidence rate in Mississippi has remained below national rates since Listeria was added
to the National Notifiable Disease List in 2000 (Figure 47).

Annual Summary 2009
62
Figure 47
Listeriosis Rates by Year, United States and Mississippi, 2000-2009
0.0
0.1
0.2
0.3
0.4
0.5
0.6
Incidence per 100,000 population
Listeriosis Rate (U.S.)
0.3 0.2 0.2 0.2 0.3 0.3 0.3 0.3 0.2
Listeriosis Rate (MS)
0.0 0.0 0.1 0.1 0.1 0.2 0.1 0.1 0.2 0.2
Listeriosis Cases (MS)
0023252365
2000* 2001 2002 2003 2004 2005 2006 2007 2008 2009**
*Added to National Notifiable Disease List in 2000.
**2009 U.S. data not available.
Two neonatal infections (0-19 days) were reported in 2009. Three additional cases were
reported in individuals ranging in age from 41 to 69 years old. One death was reported
in a 57 year old. None of the infections were epidemiologically linked or associated
with common source outbreaks.
Salmonellosis
2009 Case Total 901 2009 rate/100,000 30.5
2008 Case Total 1079 2008 rate/100,000
36.7
Clinical Features
Salmonellosis is a bacterial disease that commonly presents as acute enterocolitis, with
sudden onset of headache, abdominal pain, diarrhea, nausea and sometimes
vomiting. Fever is almost always present. Dehydration may occur in infants and the
elderly, and septicemia occasionally results from infection.
Infectious Agent
Salmonella organisms are gram negative bacilli. The genus Salmonella is divided into
two species: S. enterica (divided into six subspecies) and S. bongori. Subspecies are
further divided into multiple serotypes. Almost all of the serotypes pathogenic for
humans are in one subspecies of S. enterica. Currently, there are more than 2460
identified Salmonella serotypes. The predominant isolates in Mississippi are Salmonella
serotypes Javianna, Mississippi, Newport and Typhimurium.

Annual Summary 2009
63
Reservoir
Domestic and wild animals, including poultry, swine, cattle, and rodents, and many
reptiles. Humans are also reservoirs, especially in mild and unrecognized cases. Chronic
carriers are prevalent in animals and birds.
Transmission
Salmonella is transmitted through ingestion of organisms in food derived from infected
animals or food or water contaminated by feces from an infected animal. Person to
person transmission by fecal oral route also occurs. Although S. serotype Enteritidis is not
commonly seen in Mississippi, this serotype can be passed trans-ovarially from infected
hens to their eggs and transmission can then occur when eggs are not fully cooked.
Incubation
From 6 to 72 hours, usually about 12-36 hours.
Period of Communicability
Throughout the course of infection; extremely variable, several days to several weeks.
A temporary carrier state occasionally continues for months, especially in infants.
Methods of Control
Transmission of Salmonella can be controlled with proper food preparation and sanitary
measures for food processing, proper hand hygiene, and clean water supplies. MSDH
investigates all possible common source food or waterborne outbreaks. The Public
Health Laboratory (PHL) requests isolate submission for molecular subtyping with pulsed-
field gel electrophoresis (PFGE). The DNA pattern, or “fingerprint”, is submitted to
PulseNet, a national tracking network coordinated by the CDC. This system facilitates
early detection of common source outbreaks, even if the affected persons are
geographically far apart, often allowing the source to be more rapidly identified.
Reporting Classification
Class 2.
Epidemiology and Trend
In Mississippi, 901 cases of salmonellosis were reported to MSDH in 2009. This marked a
decrease in the rate and number of reported cases in Mississippi (Figure 48). In 2009,
the Salmonella serotypes Typhimurium, Newport, Mississippi and Javiana accounted for
over 51% of the isolates seen in Mississippi.

Annual Summary 2009
64
Figure 48
Salmonellosis Rates by Year, United States and Mississippi, 2000-2009
0.0
10.0
20.0
30.0
40.0
Incidence per 100,000 population
Salmonellosis Rate (U.S.)
14.5 14.4 15.7 15.2 14.5 15.4 15.5 16.0 16.9
Salmonellosis Rate (MS)
23.1 31.7 41.2 36.3 31.2 30.9 27.1 35.9 36.7 30.5
Salmonella Cases (MS)
657 907 1183 1047 907 904 789 1049 1079 901
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009*
*2009 U.S. data not available.
Infections occur in people of all ages, but there is higher incidence in infants and small
children. In 2009, 415 (46%) of the cases were in children less than 5 years of age (Figure
49).
Figure 49
Salmonellosis Cases by Age Group, Mississippi, 2009
0
50
100
150
200
250
300
< 1 1-4 5-9 10-
14
15-
19
20-
24
25-
29
30-
34
35-
39
40-
44
45-
49
50-
54
55-
59
60-
64
65-
69
70-
74
75-
79
80-
84
85+
Age Group
*Age unknown for one case
Number of Cases
In 2009 MSDH investigated a large outbreak of Salmonella that occurred in an adult
detention center located in Public Health District IX (Gulf Coast region). On February
12, 2009, MSDH was first notified that an inmate at the facility had a stool specimen

Annual Summary 2009
65
positive for Salmonella, Group D, and that 40 or 50 inmates had exhibited symptoms of
gastroenteritis on February 8
and 9, 2009.
An initial environmental evaluation revealed that the facility was built for a capacity of
approximately 200 inmates, but was currently housing more than 380 individuals. In
addition to overcrowded conditions, there was also limited access to bathrooms,
showers and sinks. All meals for the inmates were prepared in a central kitchen,
operated by a private company that provided four supervisory staff overseeing 22-24
inmates who prepared the actual meals. The kitchen was designed to prepare meals
for 80-100 inmates, but was serving upwards of 400 individuals. The kitchen staff was
also interviewed, and food samples were collected from the stock food that was used
to prepare meals between February 4 through February 7.
To find cases and define the scope of the outbreak, 319 inmates were interviewed with
an in depth questionnaire to determine food history over the last 72 hours, symptom
assessment and onset dates. In addition, stool samples were obtained to confirm the
etiologic agent.
Cases were defined as anyone with diarrhea and two or more of the following
symptoms: nausea, vomiting, bloody stools, cramps, fever, chills or headache. A total
of 184 individuals were identified as cases. The predominant symptoms were diarrhea
(100%), abdominal cramps (90%), nausea (81%) and headache (81%). Onset dates of
illness ranged from February 4, 2009 to February 17, 2009 (Figure 50). The median
duration of symptoms was seven days. An analysis of the food consumption
questionnaires did not reveal any food item served that was associated with illness.
Figure 50
Onset Dates for the Adult Detention Center
Salmonella
Outbreak,
Public Health District IX, Mississippi, February 2009
0
5
10
15
20
25
30
35
40
2
/2/09
2/3/09
2
/
4/
09
2
/
5/
09
2
/6/09
2
/7/09
2/8/09
2
/
9/
09
2/10/09
2
/
11/09
2
/
12/
0
9
2
/1
3/
09
2/14/09
2/15/09
2
/
16/09
2
/
17/09
2
/1
8/
09
2
/19/09
Date of Onset
Number of Cases
Seventeen stool samples were obtained from ill individuals. Thirteen of the samples
were positive for Salmonella, Group D. Twelve of the isolates were sent to the PHL for
serotyping and PFGE. All isolates were identified as Salmonella enteritidis, and PFGE
analysis showed a 100% match for all the isolates. One of the positive samples was in
an ill inmate kitchen worker, who had an unknown onset of illness.

Annual Summary 2009
66
Seven stock food samples used to prepare meals served from February 4-7 (collard
greens, lettuce, dry milk, mayonnaise, pasteurized eggs and ground chicken) were
tested for Salmonella. Only the ground chicken tested positive for Salmonella, but was
Group B instead of the outbreak Group D.
Although a single point source was not identified in this outbreak, several risk factors
were identified that likely contributed to rapid person to person transmission once the
infectious agent was introduced, possibly from an infected kitchen worker. The facility
was overcrowded with a lack of sanitary conditions for inmates, and the kitchen was
not an adequate size to cook, serve and clean for the number of inmates in the
detention center.
To prevent further outbreaks, it was recommended that overcrowding and sanitation
issues be addressed. Follow up with the facility indicated that no further inmates or staff
became ill after February 17, 2009.
Shigellosis
2009 Case Total 52 2009 rate/100,000 1.8
2008 Case Total 290
2008 rate/100,000
9.9
Clinical Features
An acute bacterial illness characterized by loose, often bloody stools (dysentery), fever,
and nausea with vomiting, cramps and tenesmus. Asymptomatic infections occur.
Illness is usually self-limited, lasting an average of 4-7 days; however infection with
Shigella dysenteriae (S. dysenteriae) is often associated with severe illness with a case
fatality rate of 20% among hospitalized patients. All age groups are susceptible, with the
peak incidence in 1-4 year olds. Children in daycares, persons in institutions, and in
facilities where adequate hand washing is difficult to maintain are at high risk for
outbreaks of shigellosis.
Infectious Agent
Genus Shigella, a gram negative bacterium comprising four serogroups: Group A, S.
dysenteriae; Group B, S. flexneri; Group C, S. boydii; and Group D, S. sonnei.
Predominant isolates in Mississippi are Group D, S. sonnei.
Reservoir
Humans are the primary reservoir.
Transmission
Primarily person to person by direct and indirect fecal oral contact. Infection may also
occur after ingestion of contaminated food or water. The infective dose can be as low
as 100-200 organisms.
Incubation
Ranges from 12 hours to 7 days, with an average of 2-4 days.

Annual Summary 2009
67
Methods of Control
Disease prevention includes promotion of good hand washing, exclusion from work for
food handlers or from school or daycare for children until symptom free for at least 24
hours. MSDH performs prompt investigation of common source food or waterborne
outbreaks, and investigates all reported infections in children less than 5 years of age.
Reporting Classification
Class 2.
Epidemiology and Trends
There were 52 cases of Shigellosis reported to MSDH during 2009, a marked decrease
from 2008 (Figure 51). There have been cyclic increases every 6-8 years since 1992, with
a peak of 1426 cases in 2007 associated with a large outbreak that occurred in the
Jackson metropolitan area and along the Gulf Coast. Although Shigellosis is usually a
summer month illness with 46% of the cases reported between May and August, cases
are reported to MSDH year round (Figure 52). The reported cases ranged in age from 3
months to 87 years, with 58% occurring in children less than 10 years of age (Figure 53).
Figure 51
Shigellosis Rates by Year, United States and Mississippi, 2000-2009
0.0
10.0
20.0
30.0
40.0
50.0
60.0
Incidence per 100,000 population
Shigellosis Rate (U.S.)
8.1 7.1 8.2 8.1 5.0 5.5 5.2 6.6 7.4
Shigellosis Rate (MS)
7.5 20.6 12.1 6.0 1.9 3.6 4.6 48.9 9.9 1.8
Shigella Cases (MS)
214 590 347 174 54 106 133 1426 290 52
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009*
*2009 U.S. data not available.

Annual Summary 2009
68
Figure 52
Shigellosis Cases by Month of Onset, Mississippi, 2009
0
1
2
3
4
5
6
7
8
9
Jan Feb Mar Apr May June July Aug Sept Oct Nov Dec
Month
Number of Cases
Figure 53
Shigellosis Cases by Age Group, Mississippi, 2009
0
2
4
6
8
10
12
14
16
18
<1 1-4 5-9 10-
14
15-
19
20-
24
25-
29
30-
34
35-
39
40-
44
45-
49
50-
54
55-
59
60-
64
65+
Age Group
Number of Cases

Annual Summary 2009
69
Vibrio disease
2009 Case Total 11 2009 rate/100,000 0.4
2008 Case Total 7 2008 rate/100,000
0.2
Clinical Features
Several noncholera Vibrio species can cause illness in humans, usually wound infections,
septicemia or gastroenteritis. Vibrio vulnificus and Vibrio parahaemolyticus are the two
most frequently reported species in Mississippi.
V. vulnificus causes sepsis 12 hours to 3 days after ingestion of contaminated seafood,
usually raw oysters, especially among people with chronic liver disease, alcoholism, or
immunosuppression. These same groups are at risk for severe wound infections from
contact with coastal waters. V. vulnificus sepsis is characterized by fever, chills, blistering
skin lesions, shock and death. The case fatality rate is over 50% when septicemia occurs.
V. parahaemolyticus infection typically causes gastroenteritis with watery diarrhea with
abdominal cramps, nausea, vomiting and fever; less commonly wound infections.
Infectious Agent
Anaerobic, gram-negative halophilic (salt requiring) bacteria found naturally in marine
and estuarine environments. Vibrio vulnificus and Vibrio parahaemolyticus are the two
most frequently reported species in Mississippi. Other species common to Mississippi are
V. mimicus, V. hollisae, and V. fluvialis. Nontoxigenic Vibrio cholerae serogroups (non-
O1/non-O139) are also reported.
Reservoir
Found free living in warm coastal waters, and in fish and shellfish, particularly oysters.
Transmission
Ingestion of the organisms in raw, undercooked, or contaminated fish and shellfish, or
any food or water contaminated with raw seafood. Wound infections with V. vulnificus
occur when wounds are exposed to estuarine waters.
Incubation
Median incubation period of 23 hours, with a range of 5-92 hours.
Period of Communicability
Not typically transmitted person to person.
Methods of Control
Seafood should be cooked adequately. Wounds exposed to seawater (either
occupational or accidental) should be rinsed with clean fresh water. All children and
immunocompromised individuals, especially alcoholics or individuals with liver disease,

Annual Summary 2009
70
should avoid eating raw seafood, especially oysters. MSDH investigates all reported
cases to determine the source of infection and possible risk factors of the case.
Reporting Classification
Class 2.
Epidemiology and Trends
In 2009, there were 11 reported Vibrio infections. This was close to twice the number of
reported cases in 2008 (7) and higher than the three year average of 7 cases for 2006-
2008 (Figure 54).
Of the 11 reported cases, five were due to V. vulnificus (3 isolated from blood cultures
and 2 from wound cultures), two were due to V. parahaemolyticus (1 isolated from a
stool culture and the other from an unknown site), four were due to Non-O1 V. cholerae
(two isolated from stool cultures, 1 isolated from a wound culture, and one from an
unknown site). There was one reported death attributed to V. vulnificus in 2009 in a 59
year old man with an underlying history of alcoholic cirrhosis.
Figure 54
Non-Cholera Vibrio Cases by Year, Mississippi,
2000-2009
0
5
10
15
20
25
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Year
Number of Cases

Annual Summary 2009
71
Zoonotic Diseases
Arboviral Infections (mosquito-borne)
Background
Arthropod-borne viral (arboviral) diseases in Mississippi are limited to a few types
transmitted by mosquitoes. In this state, there are four main types of arboviral
infections that have been reported: West Nile virus (WNV), St. Louis encephalitis (SLE),
eastern equine encephalitis (EEE), and LaCrosse encephalitis (LAC). WNV and SLE are
members of the Flavivirus genus, while EEE is an Alphavirus, and LAC is in the California
virus group of Bunyaviruses.
Infections do not always result in clinical disease. When illness occurs, symptoms can
range from a mild febrile illness to more severe cases of neuroinvasive disease with
symptoms of encephalitis and/or meningitis. Neuroinvasive disease can result in long
term residual neurological deficits or death. The proportion of infected persons who
develop symptoms depends largely on the age of the persons and the particular virus
involved.
Mosquito borne arboviral infections are typically more common in the warmer months
when mosquitoes are most active, but WNV cases have been reported year round. All
are transmitted by the bite of an infected mosquito, but the mosquito vectors and their
habitats differ. Infections are not transmitted by contact with an infected animal or
other person; humans and horses are “dead end” or incidental hosts. Rare instances of
WNV transmission have occurred through transplanted organs, blood transfusions, and
transplacentally.
Methods of Control
The methods of controlling mosquito-borne infections are essentially the same for all the
individual diseases. The best preventive strategy is to avoid contact with mosquitoes.
Reduce time spent outdoors, particularly in early morning and early evening hours
when mosquitoes are most active; wear light-colored long pants and long-sleeved
shirts; and apply mosquito repellant to exposed skin areas. Reduce mosquito breeding
areas around the home and workplace by eliminating standing or stagnant water.
Larvacides are effe
ctive when water cannot be easily drained.
Mosquito Surveillance
Mosquitoes are collected throughout the state for West Nile and other arboviral testing
to provide information regarding the burden and geographic distribution of infected
vectors. Mosquitoes are collected by local mosquito programs and MSDH personnel
and submitted as pools of 5-50 mosquitoes for testing. In 2009, 439 mosquito pools were
submitted to MSDH PHL for WNV, SLE, and EEE testing.

Annual Summary 2009
72
Arboviral Testing
The Public Health Laboratory (PHL) performs an arboviral panel consisting of IgM testing
for WNV and SLE, and, for patients under 25 years of age, LAC IgM. Clinicians are
encouraged to call MSDH Epidemiology or the PHL for specifics and indications for
arboviral testing.
Please refer to the individual disease summaries for information and epidemiology of
each specific arbovirus.
Eastern Equine Encephalitis (EEE)
Clinical Features
Clinical illness is associated with symptoms that can range from a mild flu-like illness
(fever, headache, muscle aches) to seizures and encephalitis progressing to coma and
death. The case fatality rate is 30-50%. Fifty percent of those persons who recover from
severe illness will have permanent mild to severe neurological damage. Disease is more
common in young children and in persons over the age of 55.
Infectious Agent
Eastern equine encephalitis virus, a member of the genus Alphavirus.
Reservoir
Maintained in a bird-mosquito cycle. Humans and horses are incidental hosts.
Transmission
Through the bite of an infected mosquito, usually Coquilletidia perturbans. This
mosquito, known as the salt and pepper or freshwater marsh mosquito, breeds mainly in
marshy areas.
Incubation
3-10 days (generally within 7 days).
Reporting Classification
Class 1.
Epidemiology and Trends
Human cases are relatively infrequent largely because primary transmission takes place
in and around marshy areas where human populations are generally limited. There
were no reported cases of EEE in Mississippi in 2009. The last two reported cases of EEE
occurred in October 2002.
Horses also become ill with EEE and are dead end hosts. Infected horses can serve as
sentinels for the presence of EEE, and can indicate an increased risk to humans. The
Mississippi Board of Animal Health reports equine infections to MSDH, and in 2009, 40
horses tested positive for EEE. The EEE-positive horses were located throughout the state

Annual Summary 2009
73
with 65% of the horses reported from District IX and District VIII (14 and 12 cases,
respectively). District VI reported eight EEE-positive horses, while Districts II, III, and IV
each reported two cases. There were no reported EEE positive mosquito pools in 2009.
LaCrosse Encephalitis
2009 Case Total 0 2009 rate/100,000 0.0
2008 Case Total 3 2008 rate/100,000 0.1
Clinical Features
Clinical illness occurs in about 15% of infections. Initial symptoms of LaCrosse
encephalitis infection include fever, headache, nausea, vomiting and lethargy. More
severe symptoms usually occur in children under 16 and include seizures, coma, and
paralysis. The case fatality rate for clinical cases of LaCrosse encephalitis is about 1%.
Infectious Agent
LaCrosse encephalitis virus, in the California serogroup of Bunyaviruses.
Reservoir
Chipmunks and squirrels.
Transmission
Through the bite of an infected Ochlerotatus triseriatus mosquito (commonly known as
the tree-hole mosquito). This mosquito is commonly associated with tree holes and most
transmission tends to occur in rural wooded areas. However, this species will also breed
in standing water in containers or tires around the home.
Incubation
7-14 days.
Reporting Classification
Class 1.
Epidemiology and Trends
Reported LaCrosse encephalitis remains relatively rare in Mississippi, with 15 reported
cases since 1999. There were no reported cases of LaCrosse encephalitis in 2009.
Of the 15 total cases since 1999, 53% were in females. The ages ranged from 3 months
to 78 years of age, with 93% of the cases being under the age of 15.
Another Bunyavirus in the California group, Jamestown Canyon encephalitis virus, has
also been seen in Mississippi, with one reported case in 1993, one in 2006, and one in
2008. There were no reported cases of Jamestown Canyon encephalitis virus in 2009.

Annual Summary 2009
74
St. Louis Encephalitis
2009 Case Total 2 2009 rate/100,000 0.1
2008 Case Total 0 2008 rate/100,000 0.0
Clinical Features
Less than 1% of infections result in clinical illness. Individuals with mild illness often have
only a headache and fever. The more severe illness, meningoencephalitis, is marked by
headache, high fever, neck stiffness, stupor, disorientation, coma, tremors, occasional
convulsions (especially in infants) and spastic (but rarely flaccid) paralysis. The mortality
rate from St. Louis encephalitis (SLE) ranges from 5 to 30%, with higher rates among the
elderly.
Infectious Agent
St. Louis encephalitis virus, a member of the genus Flavivirus.
Reservoir
Maintained in a bird-mosquito cycle. Infection does not cause a high mortality in birds.
Transmission
Through the bite of an infected mosquito generally belonging to genus Culex (Culex
quinquefasciatus, Culex pipiens), the southern house mosquito. This mosquito breeds in
standing water high in organic materials, such as containers and septic ditches near
homes.
Incubation
5-15 days.
Reporting Classification
Class 1.
Epidemiology and Trends
The number of reported SLE cases fluctuates annually. There were no cases reported in
2004, 2006, or 2008, but there were nine cases with one death reported in 2005 and two
reported cases in 2007. There were no deaths due to SLE in 2007.
Mississippi had two reported cases of SLE in 2009. Both cases presented as
neuroinvasive, were in individuals greater than 65 years of age, and were from District
VIII. There were no deaths due to SLE in 2009. No positive SLE mosquito pools were
reported in 2009.
Annual Summary 2009
75
West Nile Virus
2009 Case Total 53 2009 rate/100,000 1.8
2008 Case Total 65 2008 rate/100,000 2.2
Clinical Features
Clinical illness occurs in approximately 20% of infected individuals. Most with clinical
manifestations will develop the milder West Nile fever, which includes fever, headache,
fatigue, and sometimes a transient rash. About 1 in 150 infected persons develop more
severe West Nile neuroinvasive disease ranging from symptoms compatible with
meningitis to encephalitis. Encephalitis is the most common form of severe illness and is
usually associated with altered consciousness that may progress to coma. Focal
neurological deficits and movement disorders may also occur. West Nile poliomyelitis, a
flaccid paralysis syndrome, is seen less frequently. The elderly and immunocompromised
are at highest risk of severe disease.
Infectious Agent
West Nile virus, a member of the genus Flavivirus.
Reservoir
WNV is maintained in a bird mosquito cycle, has been detected in more than 317
species of birds, particularly crows and jays.
Transmission
Primarily through the bite of an infected southern house mosquito (Culex
quinquefasciatus). This mosquito breeds in standing water with heavy organic matter.
Incubation
3-15 days.
Reporting Classification
Class 1.
Epidemiology and Trends
In Mississippi, West Nile virus was first isolated in horses in 2001 followed by human
infections in 2002 with 192 cases reported. The years following saw a decrease in the
number of reported infections; however in 2006, there was a resurgence of 184 cases
(Figure 55). In 2009, there were 53 reported cases with 5 deaths.

Annual Summary 2009
76
Figure 55
West Nile Virus Rates by Year, United States and Mississippi, 2000-2009
0.0
1.0
2.0
3.0
4.0
5.0
6.0
7.0
Incidence per 100,000 population
WNV Rate (U.S.)*
0.0 0.0 1.4 3.4 0.9 1.0 1.4 1.2 0.4 0.2
WNV Rate (MS)
0.0 0.0 6.7 2.9 1.8 2.4 6.3 4.7 2.2 1.8
WNV Cases (MS)
0 0 192 83 52 71 184 136 65 53
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
*U.S. data: 21 cases in 2000; 66 cases in 2001.
WNV is now thought to be endemic in Mississippi, and the mosquito vector is present the
entire year. Human illness can occur year round, but is most prevalent from July to
October. August and September are usually the peak months (Figure 56).
Figure 56
West Nile Virus Cases by Month of Onset, Mississippi, 2009
0
5
10
15
20
25
January
Fe
b
r
u
ary
March
A
p
r
i
l
May
June
July
August
Se
p
t
e
mbe
r
Oc
t
o
b
er
N
o
ve
m
ber
D
e
ce
mb
er
Month
Number of Cases
Of these 53 cases, 42% were classified as WNV fever and 58% were encephalitis. The
cases ranged in age from 10 months to 89 years. Nearly 68% were 50 years or older
(Figure 57).

Annual Summary 2009
77
Figure 57
West Nile Virus Cases by Age Group, Mississippi, 2009
0
2
4
6
8
10
12
14
16
18
20
0-4 5-9 10-14 15-19 20-24 25-29 30-34 35-39 40-44 45-49 50-54 55-59 60-64 65+
Age Group
Number of Cases
WNV infection can occur in any part of the state, and since 2001, activity (human
cases, positive mosquito pools, horses or birds) has been reported in every Mississippi
County except Issaquena. Approximately 62% of the cases in 2009 occurred in Forrest,
Hinds, Rankin, and Harrison counties (Figure 58).
A total of five mosquito pools tested positive for WNV. Horses may also become ill with
WNV and can act as sentinels for the presence of infected mosquitoes. The Mississippi
Board of Animal Health reports equine infections to MSDH. In 2009, eleven horses tested
positive for WNV with about half of the cases being in District IX.
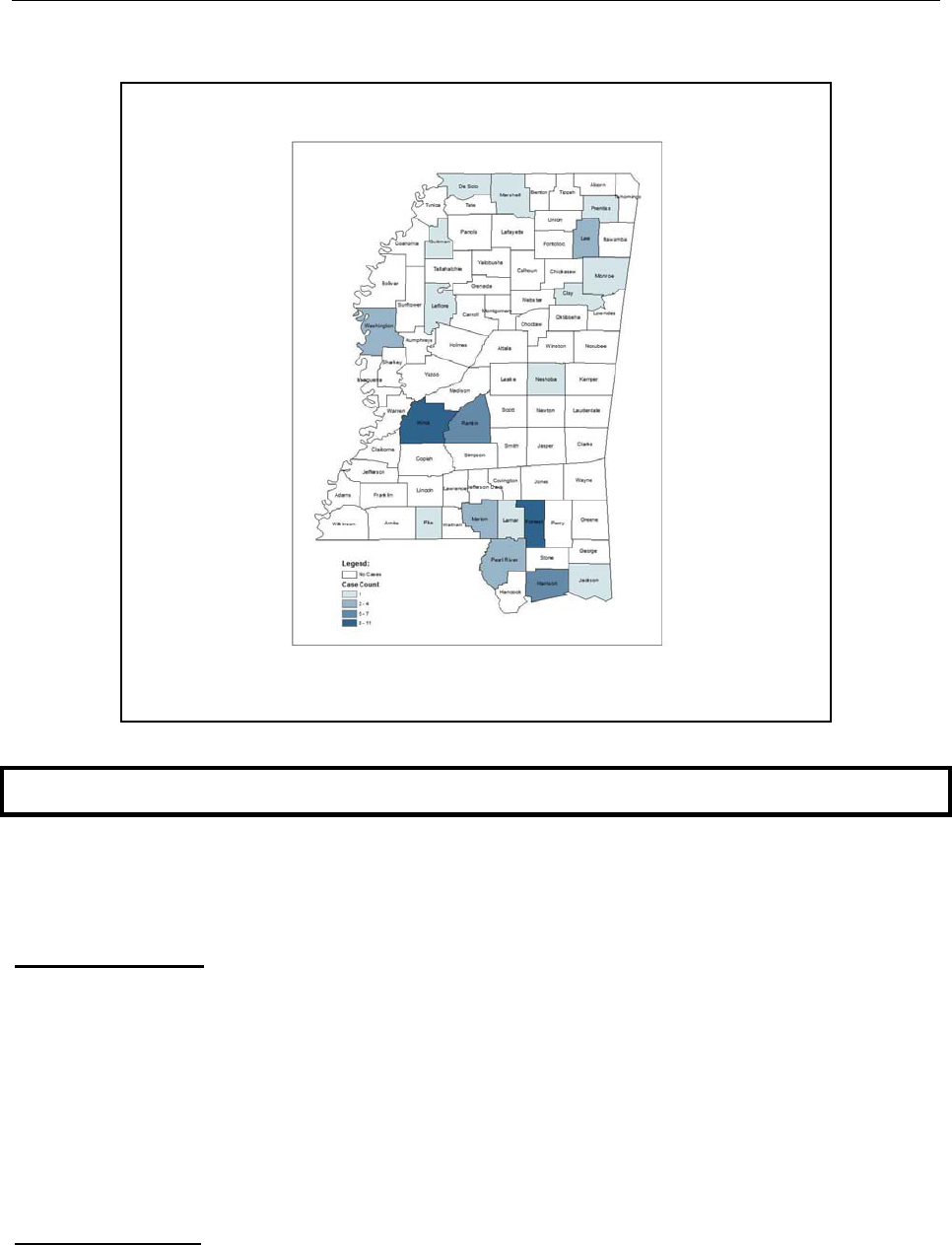
Annual Summary 2009
78
Figure 58
West Nile Virus Cases by County, Mississippi, 2009
Lyme Disease
2009 Case Total 0 2009 rate/100,000 0.0
2008 Case Total 0 2008 rate/100,000 0.0
Clinical Features
A tick-borne bacterial disease characterized primarily by a distinct “bull’s-eye” rash
(erythema migrans) in the early stage of the infection. The rash is present in up to 60%-
80% of patients. Accompanying symptoms may include malaise, fever, headache, stiff
neck, myalgias, migratory arthralgias and/or lymphadenopathy. In untreated patients,
chronic or late manifestations may include musculoskeletal symptoms (joint swelling or
chronic arthritis), neurological manifestations (aseptic meningitis, cranial neuritis, facial
palsy, rarely encephalomyelitis), and cardiac abnormalities (specifically 2nd or 3rd
degree atrioventricular conduction defects).
Infectious Agent
Borrelia burgdorferi, a spirochete.

Annual Summary 2009
79
Reservoir
Small mammals, mainly mice. Deer are efficient maintenance hosts and play an
important role in transporting ticks.
Transmission
Transmission occurs through the bite of an infected Ixodes scapularis tick (black-legged
tick). Nymphs are more likely to transmit disease, and they feed primarily on small
mammals. Studies indicate the tick usually must be attached 24 hours or longer to
efficiently transmit the bacteria. No person to person transmission or maternal fetal
transmission has been confirmed.
Incubation
2-30 days after tick exposure for erythema migrans, however, early infection may be
inapparent and patients may present weeks to months after exposure with late
manifestations.
Methods of Control
Avoid tick infested areas when possible. When unavoidable, use tick repellant and
measures to decrease tick exposure. After leaving tick prone areas examine body well
and remove any ticks. It is important to promptly remove any attached ticks; it is not
necessary to remove the head.
Reporting Classification
Class 2.
Epidemiology and Trends
Most cases occur in late spring and summer. Lyme disease is not considered endemic
in Mississippi, although the vector is present in the state. Since 2004 the number of
annual reported cases has ranged from 0-3. There were no confirmed cases reported
in 2009, but there were two cases in 2007.
Rabies
Clinical Features
Rabies is an acute fatal progressive disease that affects the central nervous system.
Early signs include anxiety, discomfort or paresthesia at the site of the bite of an
infected animal, primarily raccoons and bats in the U.S. Progression to symptoms of
cerebral dysfunction such as confusion, agitation, delirium, hallucinations, and insomnia
occurs within a few days of symptom onset. This is followed by generalized paralysis,
coma and death within 2 to 10 days.
Infectious Agent
Lyssavirus, family Rhabdoviridae; an RNA virus. Variants occur among animal species
and geographic location, but all of the members of the genus are antigenically
related.

Annual Summary 2009
80
Reservoir
Rabies has an urban and a wild cycle. The urban cycle (maintained by rabid dogs) has
been reduced greatly in the U.S., but carnivores (primarily raccoons, wild canids, and
skunks) and several species of insectivorous bats maintain the wild cycle in areas of the
U.S. Currently, only bats maintain the cycle in Mississippi.
Transmission
The most common mode of rabies virus transmission is through the bite of an infected
host. All mammals are susceptible to varying degrees. Transmission has also been
documented through organ transplantation, specifically corneal transplants, from a
donor dying of undiagnosed rabies.
Incubation
The incubation period can be up to six months or longer. The incubation period is longer
the farther away the bite is from the CNS.
Period of Communicability
Rabies is transmissible once it reaches the CNS and can be found in the salivary glands.
The animal is usually exhibiting abnormal behavior and other clinical signs by this time.
Methods of Control
The best method of control is prevention. Domestic animal rabies vaccination
programs, as well as pre- and post-exposure rabies vaccination in humans have
significantly decreased the human risk and deaths from rabies in the United States.
People who are bitten by animals that are known reservoirs of rabies exhibiting
abnormal behavior, such as unprovoked aggressiveness, increased drooling or paralysis
should be considered at higher risk, and consideration should be given to the use of
post-exposure vaccination.
Recommendations for preventing and controlling rabies in animals can be found in the
Compendium of Animal Rabies Prevention and Control, at
http://www.nasphv.org/Documents/RabiesCompendium.pdf
.
Recommendations for prevention of rabies in humans can be found in the document
by the Advisory Committee on Immunization Practices entitled Human Rabies
Prevention—United States, 2008 http://www.cdc.gov/mmwr/pdf/rr/rr57e507.pdf
.
Reporting Classification
Class 1 (human or animal).
Epidemiology and Trends
In the U.S. in the 1940s and 1950s, canines were the predominant reservoir and cause of
human rabies. By 2006, however, approximately 92% of animal rabies cases were in
wildlife, and only 8% were in domestic animals. This change is attributed to concerted,
targeted rabies vaccination campaigns and stray animal control that have reduced
the number of canine rabies cases from 6,947 in 1947 to 79 in 2006. Currently, most

Annual Summary 2009
81
human cases in the United States are caused by bat strains of rabies. In the U.S., bats
are now the second most reported rabid animal behind raccoons.
The MSDH PHL is the only laboratory in Mississippi that tests for rabies in animals. Since
1962, bats are the only animals that have tested positive for rabies in Mississippi. Usually
between 2-11 bats test positive each year. There were 4 positive bats out of 75 tested in
the PHL in 2009. Since 1999, there has been a wide geographic distribution of positive
bats, with 52 reported positives in 25 counties (Figure 59). There has not been an
indigenous terrestrial animal (land) rabies case reported in Mississippi since 1961,
however, rabies occurs in terrestrial animals annually in states that border Mississippi
(Arkansas, Alabama, Louisiana, and Tennessee).
Mississippi reported a human case of rabies due to a bat strain in a 10 year old boy in
2005. Prior to this 2005 human case, the last reported human rabies case in Mississippi
was in 1953 and this was transmitted by a terrestrial animal.
Figure 59
Rabies in Bats by County, Mississippi, 2000-2009
County with at least one
positive bat

Annual Summary 2009
82
Rocky Mountain spotted fever
2009 Case Total 9 2009 rate/100,000 0.3
2008 Case Total 12 2008 rate/100,000 0.4
Clinical Features
A rickettsial illness with acute onset of fever, severe headache, malaise, myalgia,
nausea, vomiting, and may include a macular or maculopapular rash on the
extremities, including the palms and soles, which usually spreads over the entire body.
A petechial rash often follows. In untreated cases and those with delayed recognition,
fatality occurs in 13-25% of the cases. Early stages of Rocky Mountain spotted fever
(RMSF) are often confused with ehrlichiosis and meningococcemia.
Infectious Agent
Rickettsia rickettsii, a gram-negative coccobacillus.
Reservoir
Small rodents (chipmunks, squirrels, white-footed mice).
Transmission
Through bite of an infected Dermacentor variabilis tick (American dog tick). A 4-6 hour
attachment is required for transmission.
Incubation
3-14 days (most occurring between 5-7 days).
Period of Communicability
No evidence of person to person transmission.
Methods of Control
Avoid tick infested areas when possible. When unavoidable, use tick repellant and
measures to decrease tick exposure. After leaving tick prone areas, examine body well
and remove any ticks; removing the embedded head of the tick is not necessary.
Reporting Classification
Class 2.
Epidemiology and Trends
In 2009, there were nine cases of Rocky Mountain spotted fever reported in Mississippi.
This is lower than the three year (2006-2008) average of 14 cases (Figure 60). The cases
ranged in age from 28 to 67 years of age. There were no reported deaths.

Annual Summary 2009
83
Figure 60
Rocky Mountain Spotted Fever Rates by Year, United States and Mississippi,
2000-2009
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
1.6
1.8
2.0
Incidence per 100,000 population
RMSF Rate (U.S.)
0.2 0.3 0.4 0.4 0.6 0.7 0.8 0.7 0.8
RMSF Rate (MS)
0.3 0.6 1.1 1.8 1.0 0.6 0.3 0.7 0.4 0.3
RMSF Cases (MS)
9 1732533018102012 9
2000 2001 2002 2003 2004 2005 2006 2007 2008 2009*
*2009 U.S. data not available.

Annual Summary 2009
84
Reportable Disease Statistics
Mississippi Reportable Disease Statistics
2009
Public
Health District
I
II
III
IV
V
VI
VII
VIII
IX
State Total
Primary & Secondary Syphilis
14 6 23 7 75 8 9 44 49 235
Total Early Syphilis
40 12 52 13 195 25 17 109 99 562
Gonorrhea
652
470 1,012
496 2,115
737 413 739 607 7,241
Chlamydia
2,615
1,849
3,236
1,757
6,217
2,042
1,554
2,221 2,101
23,592
Sexually
Transmitted
Diseases
HIV Disease
62 27 67 33 218 36 28 63 76 210
Pulmonary Tuberculosis (TB)
6 5 11 7 50 5 4 3 10 101
Extrapulmonary TB
1 2 1 1 9 2 2 2 0 20
Myco-
bacterial
Diseases
Mycobacteria Other Than TB
30 20 10 17 94 27 21 36 42 297
Diphtheria
0 0 0 0 0 0 0 0 0 0
Pertussis
10 4 12 3 10 5 15 8 13 80
Tetanus
0 0 0 0 0 0 0 0 0 0
Poliomyelitis
0 0 0 0 0 0 0 0 0 0
Measles
0 0 0 0 0 0 0 0 0 0
Mumps
0 0 0 0 1 0 0 0 0 1
Hepatitis B (acute)
2 6 3 2 6 5 0 2 6 32
Invasive H. influenzae b
disease
0 0 0 0 0 0 0 0 0 0
Vaccine
Preventable
Diseases
Invasive Meningococcal
disease
0 0 1 1 1 1 0 0 1 5
Hepatitis A (acute)
0 0 1 0 2 1 1 4 0 9
Salmonellosis
61 141 31 71 221 84 68 103 107 901
Shigellosis
14 19 0 6 5 3 1 1 1 52
Campylobacteriosis
7 16 18 7 18 13 6 12 12 110
Enteric
Diseases
E. coli O157:H7/HUS
1 0 0 1 3 0 0 0 1 6
Animal Rabies (bats)
0 0 0 1 2 0 0 0 1 4
Lyme disease
0 0 0 0 0 0 0 0 0 0
Rocky Mountain spotted fever
0 2 1 1 1 1 0 0 3 9
Zoonotic
Diseases
West Nile virus
2 5 3 2 15 1 1 14 10 53

Annual Summary 2009
85
Mississippi
Provisional Reportable Disease Statistics
November 2010
Figures for the current month are provisional
Public
Health District
State
Totals*
I
II
III
IV
V
VI
VII
VIII
IX
Nov
2010
Nov
2009
YTD
2010
YTD
2009
Primary & Secondary Syphilis
1 0 0 1 4 2 2 1 5 16 12 199 195
Total Early Syphilis
2 3 3 3 18 7 3 4 7 50 31 549 496
Gonorrhea
57 31 81 54 173 47 21 49 50 563 553 5,615
6,771
Chlamydia
217 145 230
147
500 163
127
132 139 1,800 1,863
19,613
21,813
Sexually
Transmitted
Diseases
HIV Disease
4 4 5 2 16 5 2 6 4 48 55 491 507
Pulmonary Tuberculosis (TB)
4 1 0 0 4 1 1 0 1 12 7 90 91
Extrapulmonary TB
0 1 0 1 0 0 0 0 0 2 1 10 19
Myco-
bacterial
Diseases
Mycobacteria Other Than TB
5 1 2 0 5 4 1 2 4 24 25 362 264
Diphtheria
0 0 0 0 0 0 0 0 0 0 0 0 0
Pertussis
0 1 0 1 0 3 0 0 2 7 10 76 73
Tetanus
0 0 0 0 0 0 0 0 0 0 0 0 0
Poliomyelitis
0 0 0 0 0 0 0 0 0 0 0 0 0
Measles
0 0 0 0 0 0 0 0 0 0 0 0 0
Mumps
0 0 0 0 0 0 0 0 0 0 0 0 1
Hepatitis B (acute)
0 0 0 0 0 0 1 0 0 1 3 35 31
Invasive H. influenzae b
disease
0 0 0 0 0 0 0 0 0 0 0 0 0
Vaccine
Preventable
Diseases
Invasive Meningococcal
disease
0 0 0 0 0 0 0 0 0 0 0 5 4
Hepatitis A (acute)
0 0 0 0 0 0 0 0 0 0 0 2 8
Salmonellosis
8 16 1 4 21 2 3 5 8 70** 59 1160
869
Shigellosis
1 0 1 0 2 1 2 0 0 7 2 51 45
Campylobacteriosis
1 3 0 0 1 0 3 1 4 13 7 125 104
Enteric
Diseases
E. coli O157:H7/HUS
0 0 0 13 0 0 0 0 0 13 0 24 6
Animal Rabies (bats)
0 0 0 0 0 0 0 0 0 0 0 0 4
Lyme disease
0 0 0 0 0 0 0 0 0 0 0 0 0
Rocky Mountain spotted fever
0 0 0 0 0 0 0 0 1 1 0 16 9
Zoonotic Diseases
West Nile virus
0 0 0 0 0 0 0 0 0 0 0 9 52
*Totals include reports from Department of Corrections and those not reported from a specific District.
**
Address unknown for two cases.

Annual Summary 2009
86
List of Contacts, Editors and Contributors
Office of the State Health Officer 601.576.7634
Mary Currier, MD, MPH
State Health Officer
Office of Communicable Diseases/Epidemiology 601.576.7725
Paul Byers, MD
Acting State Epidemiologist
Joy Sennett, MHS
Director
Immunization Program 601.576.7751
Tammy Clark, RN
Director
STD/HIV/AIDS Program
Nicholas Mosca, DDS 601.576.7723
Director
Tuberculosis Program 601.576.7700
J.M. Holcombe, MPPA
Director
Editors
Theresa Kittle, MPH
Epidemiologist
Office of Communicable Diseases/Epidemiology
Melanie Vail Fuller
Special Projects Officer
Office of Communicable Diseases/Epidemiology
Contributors
Cindy Allard, RN Malorie Givan, MPH
Jannifer Anderson, RN Sheryl Hand, RN
Bruce Brackin , MPH Carl Haydel, MS
Alisha Brinson, MS Kendra Johnson, MPH
Kristina Clarke, MPH Leandro Mena, MD, MPH
Monique Drake Barry Mullins, MPH
Brigid Elchos, RN, DVM, DACVPM Steve Quilter, MPH
Sandor Feldman, MD Wendy Varnado, MS

Annual Summary 2009
87
General References
• Heymann D, ed. Control of Communicable Diseases Manual. 19th ed.
Washington, D.C.: American Public Health Association; 2008.
• CDC. Epidemiology and Prevention of Vaccine-Preventable Diseases, 2009. 11
th
ed.
• Pickering LK, ed. Red Book: 2006 Report of the Committee on Infectious
Diseases. 27
th
ed. Elk Grove Village, IL: American Academy of Pediatrics; 2006.
• CDC. Sexually Transmitted Disease Surveillance 2009; November 2010.
• CDC. Diseases and Conditions A-Z Index. Available online at:
http://www.cdc.gov/DiseasesConditions/az/H.html
.
• CDC. Case Definitions for Nationally Notifiable Infectious Diseases. Available
online at: http://www.cdc.gov/epo/dphsi/nndsshis.htm
.
• CDC. MMWR: Summary of Notifiable Diseases, United States, 1999-2008. Available
online at: http://www.cdc.gov/mmwr/summary.html
.

