CLINICIAN’S CORNER
Optimizing orthodontic treatment in patients
taking bisphosphonates for osteoporosis
James J. Zahrowski
Tustin, Calif
Bisphosphonates have unique pharmacological characteristics unlike those of any other drug group. Millions
of adults take oral bisphosphonates for long-term treatment of osteoporosis and osteopenia; some of these
people will most likely also seek orthodontic treatment. Adverse dental effects from bisphosphonates have
been reported, including decreased tooth movement, impaired bone healing, and osteonecrosis in the man-
dible and the maxilla. Osteonecrosis has been rarely observed after bisphosphonate use for osteoporosis.
However, adverse drug effects might occur more frequently in orthodontic patients, and they would probably
be noted before the end-stage pathology of osteonecrosis. Adverse effects during orthodontic treatment,
including decreased tooth movement, could last for years after the drug therapy is stopped. Successful ortho-
dontic treatment requires optimal bone healing to prevent excessive tooth mobility. Bisphosphonates appear
to have 2 bone elimination rates—a fast elimination of weeks from the bone surface and a slow elimination of
years after incorporation into the bone structure. This article presents methods to clinically and radiographi-
cally monitor orthodontic patients who are taking oral bisphosphonates. Efforts to minimize adverse effects
and optimize orthodontic procedures with physician-approved drug holidays are discussed. The orthodontic
treatment results of 3 patients who received bisphosphonate therapy are reported. (Am J Orthod Dentofacial
Orthop 2009;135:361-74)
B
isphosphonate (BP) is used for the long-term
treatment of osteoporosis and osteopenia by mil-
lions of adults who might also seek orthodontic
treatment.
1-4
Approximately 1.5 million osteoporotic
fractures occur annually in the United States, with the
incidence projected to rise.
5
Osteoporotic fractures are
a principal cause of disability and death.
6
Although
a higher fracture risk is noted in those with osteoporosis
than osteopenia, an estimated 33 million people in the
United States—80% of them women—have osteopenia
with more fracture risk than the normal population.
7
Af-
ter menopause, decreased estrogen secretion leads to
relatively increased osteoclastic activity and increased
bone resorption.
3,4
The internal cross-links break be-
cause of more resorption in trabecular bone than in
cortical bone.
8
The destabilized bone structure allows
more fractures to occur in the hip and the lumbar verte-
bral regions.
8,9
Bone density in these regions is usually
measured by dual-energy x-ray absorptiometry.
4
Osteo-
penia is defined as decreased bone density of 1 to 2.5 SD
below the mean. Osteoporosis is defined as a further de-
crease of bone density more than 2.5 SD below the
mean. Patients with severe osteoporosis and a previous
fragility fracture are at higher risk for future fractures.
Oral BP treatment has been related to a 50% decrease
of bone fractures in the hips and the vertebrae.
3
Intrave-
nous BP, such as zoledronic acid given yearly or ibandr-
onate given every 3 months, have been recommended
for osteoporosis treatment by increasing compliance
and decreasing fractures up to 70%.
10,11
Oral BP is 1
of the 15 most prescribed drugs in the United States
and a primary long-term osteoporosis treatment that re-
duces morbidity and mortality with few adverse medical
effects.
2,7
The BP pharmacological site of action is in the oste-
oclast, which removes the outer ruffled border, inacti-
vates function, and decreases the lifespan of the
cell.
12-14
The drug enters the osteoclast through endo-
cytic vacuoles.
15
Commonly used BP, containing a nitro-
gen group, primarily inhibits farnesyl pyrophosphate
synthetase and geranylgeranyl pyrophosphatase.
15,16
Enzyme inhibition causes a decrease of proteins respon-
sible for cytoskeletal integrity and intracellular signal-
ing.
12,16
There is some evidence that this drug group
might also inhibit osteoclast precursors and osteoblast
communication with osteoclasts.
15,16
Absorption of
BP through the small intestine is low. Approximately
0.06% of the oral dose reaches the bloodstream as
opposed to 100% when given intravenously.
13,17
Once
the drug is in the bloodstream, approximately 50% is
excreted within hours by the kidneys, and the other
Private practice, Tustin, Calif.
Reprint requests to: James J. Zahrowski, 13372 Newport Ave E, Suite E, Tustin,
Submitted, April 2008; revised and accepted, August 2008.
0889-5406/$36.00
Copyright Ó 2009 by the American Association of Orthodontists.
doi:10.1016/j.ajodo.2008.08.017
361

half is preferentially bound to the surfaces of high bone
turnover.
12
Preferential drug binding was documented
by a 3-times higher alendronate concentration in trabec-
ular bone, which has a 3-times greater bone turnover
rate than cortical bone.
8
Various locations in the body
have different bone repair rates. It was reported that al-
veolar bone has up to a 10-times greater bone turnover
rate than skeletal bones because of the constant mastica-
tory forces.
18
After BP is incorporated into the bone,
drug elimination occurs slowly and is regulated by the
physiologic rate of bone turnover.
9,17,19
Since high
bone turnover occurs during orthodontic treatment,
more BP might be bound and incorporated around the
teeth than in other bone areas of the body.
After 3 months of oral BP use, bone resorption de-
creased by 50% to75% as measured by osteoclastic sys-
temic bone markers, carbon or nitrogen telopeptide.
20
After 6 months of oral drug use, bone formation also de-
creased by 50% as measured by an osteoblastic systemic
bone marker, bone-specific alkaline phosphatase.
21
The
resultant decrease in bone formation was thought to be
indirectly caused by intercellular osteoclastic mediators
suppressing osteoblastic activity. The systemic bone
markers stabilized at these levels and did not decrease
further after long-time oral BP use.
22,23
One study noted
that nonhealing skeletal fractures occurred after many
years of continuous oral BP use.
24
Fracture site biopsies
showed a 95% decrease in bone formation, whereas the
systemic marker of bone formation decreased by only
50%. Therefore, systemic bone function tests might
not accurately describe locally decreased bone function
around the teeth caused by BP.
Adverse dental effects of BP were reported to de-
crease tooth movement, impair bone healing, and induce
osteonecrosis in the mandible and the maxilla.
25-27
This
drug group causes decreased tooth movement rapidly
and was reported to interfere with orthodontic re-
sults.
28,29
Optimal tooth movement and bone healing,
which are dependent upon osteoclastic and osteoblas-
tic activity, are important for a successful orthodontic
treatment result. Although BP-induced osteonecrosis
was first reported in 2003, similar nonhealing extrac-
tion sites with jaw necroses was reported in 19th cen-
tury factory workers who were overexposed to white
phosphorus.
30,31
BP-induced osteonecrosis is currently
defined as exposed necrotic bone in the mandible or
the maxilla for at least 8 weeks with prior BP use
and no history of radiation treatment to the jaws.
32
The soft-tissue exposure of necrotic bone usually
was observed after extractions but also was noted
with periodontal disease and periodontal surgery, or
occurred spontaneously over tori or posterior lingual
of the mandible.
33
Untreatable jaw osteonecrosis
was reported with an incidence of 4% to 10% in
bone cancer patients who received continuous large
intravenous doses of BP, zoledronic acid or pamidro-
nate.
27,33-36
The reported high incidence and severity
of osteonecrosis is probably due to the 12 to 50 times
greater systemic BP dose given to treat bone cancer
than osteoporosis.
37,38
Bone cancers, such as multiple
myeloma or metastatic breast cancer, treated with BP
given intravenously usually contraindicate any ortho-
dontic or elective dental surgery procedures.
25,33
BP
pharmacology is important for the orthodontist to un-
derstand to evaluate adverse drug effects to the bone
around the teeth.
BP STRUCTURE RELATES TO PHARMACOLOGICAL
ACTIVITY
BP has a chemical structure change in which car-
bon, substituted for oxygen in pyrophosphate, is be-
tween 2 phosphates as shown in Figure 1.
12
Pyrophosphate, rapidly inactivated into 2 phosphates
by tissue alkaline phosphatase, is secreted by the
smooth muscle and can prevent vascular or soft-tissue
calcification.
39
BP affects bone regulation because of
the structural carbon change.
9,12
The drug cannot be
metabolized by the tissue or the liver and can only be
eliminated through the kidneys. BP has R1 and R2
groups attached to the carbon; these increase bone af-
finity and drug potency, respectively (Fig 1). The com-
mon medical uses, bone affinity, potency, and R group
structures of the BP types are shown in Table I.A
greater affinity to human bone was found when a hy-
droxyl group (OH) was present in the R1 group, as
Fig 1. BP structure. Carbon between the phosphate
groups allows no metabolism in the body (pyrophos-
phate contains oxygen between phosphate groups).
Pharmacology of different types depends on R1 and
R2 groups attached to carbon. R1 group increases bind-
ing to bone (calcium), especially when OH is present; R2
group increases potency, especially when nitrogen is
present.
362 Zahrowski
American Journal of Orthodontics and Dentofacial Orthopedics
March 2009

demonstrated by the 15 times stronger affinity of
alendronate to bone than clodronate.
40
The BP types
used for osteoporosis have strong affinities to bone
with smaller variations, since they all have an OH group
in R1. Alendronate has the strongest affinity to bone,
30% stronger than risedronate or zoledronic acid and
almost 2 times stronger than ibandronate. Since these
drugs have a high affinity to calcium, they are quickly
targeted and bound to the exposed hydroxyapatite of ac-
tively resorbing bone in the body.
12
When nitrogen was
present in the R2 group, drug potency was in-
creased.
12,13,17,41
Increased potency corresponds to
a smaller amount of the drug needed to suppress osteo-
clastic function. Zoledronic acid is the most potent type
due to a cyclic nitrogen group in R2. The relative sys-
temic effects per dose for the BP types used in osteopo-
rosis are compared in Table II. Higher-potency drug
types are usually given at lower dosages to provide sim-
ilar medical treatment efficacy and fewer adverse ef-
fects. Similar systemic effect doses for alendronate,
risedronate, and ibandronate are noted when comparing
daily dosages. A higher systemic dose is given as the
time between doses is increased. This can be noted
when comparing the increased weekly dose of alendro-
nate or risedronate to the respective daily dose. The
150-mg monthly dose of ibandronate is given at twice
the expected oral dose from a simple calculation of
the daily dose multiplied by 30 days. A larger monthly
dose is needed for effectiveness, probably due to
ibandronate’s lower bone affinity that allows the active
drug on the bone surface to leave more rapidly and be
eliminated. Although this article discusses the entire
BP drug group, the severity of adverse dental effects
might be different for each BP type based on differ-
ences in systemic effective dose, bone affinity, and
other inherent characteristics.
Table I. Comparison of BP types
Common use
1
Types
1
Cancer Osteoporosis Pagets, hypercalcemia R1 group*
12
Bone affinity
40
† R2 group
12
‡ Potency
3,17,41
x
Alendronate -OH 1.5 -(CH2)3-NH2 700
(Fosamax [Merck,
Whitehouse Station,
NY], oral)
X
Risedronate -OH 1.1
-CH2
N
2000
(Actonel [Proctor &
Gamble, Cincinnati,
Ohio], oral)
X
Ibandronate -OH 0.8
-(CH
2
)
2
– N – CH
3
(CH
2
)
4
- CH
3
4000
(Boniva [Roche, Basel,
Switzerland], oral
and IV)
X
Zoledronic acid -OH 1.1
-CH2 – N
N
10,000
(Reclast [Novartis, Basel,
Switzerland], IV)
X
(Zometa [Novartis], IV) X
Pamidronate -OH 1.1 -(CH2)2-NH2 325
(Aredia [Novartis], IV) X
Etidronate -OH 1.0 -CH3 1
(Didronel [Proctor &
Gamble], oral and IV)
X
Tiludronate -H 0.5
-S CI
10
(Skelid [Sanofi-Aventis,
Paris, France], oral)
X
Clodronate -CI 0.1 -CI 10
(Bonefos [Berlex Inc,
Schering, NJ], oral)
X
IV, Intravenous; Ki, human bone affinity.
*R1 group: hydroxyl (OH) increases binding to bone; †Bone affinity: relative bone affinity compared to etidronate as 1 (etidronate Ki divided by type
Ki); ‡R2 group: nitrogen (N) increases drug potency; xPotency: relative drug potency compared to etidronate as 1.
American Journal of Orthodontics and Dentofacial Orthopedics
Zahrowski 363
Volume 135, Number 3
BP HAS FAST AND SLOW ELIMINATION RATES
FROM BONE
The bone elimination half-lives for BP have been re-
ported over an extremely wide range from several days
for ibandronate to over 10 years for alendronate.
13,17
However, the different methodologies of the elimination
studies are often overlooked. The BP drug group appears
to have 2 bone elimination rates: fast and slow.
13,17,37,42
The short BP half-life was documented by short-
term blood studies of ibandronate (37-157 hours), zole-
dronic acid (146 hours), and risedronate (224-561
hours).
11,13,43,44
Alendronate was observed to have
a similar short half-life when compared with risedronate
over a 30-day period.
42
The documented short half-lives
for the BP types provide additional information for the
entire drug group regarding bone surface elimination.
By pharmacokinetic principle, drug concentration
would be estimated to decrease 94% after the drug is
discontinued for a time period of 4 half-lives.
45
Most ac-
tive BP, that is on the bone surface, should be eliminated
rapidly after the drug is stopped for a period of 4 half-
lives. A biphasic bone elimination of alendronate was
reported in rats with approximately 40% of the drug
leaving in 30 days, and the rest leaving at a much slower
rate.
8
The blood concentration decreased rapidly and
could not be accurately measured 30 days after drug dis-
continuation. A biphasic bone elimination rate was also
established for osteoporosis patients taking alendro-
nate.
46
Forty percent of alendronate bound to the skele-
ton was rapidly excreted in the urine during a 3-month
period. The rapid elimination rate was interpreted to
be alendronate leaving the bone surfaces before bone in-
corporation. The rest of the alendronate was estimated
to be slowly excreted over decades after bone incorpo-
ration. Therefore, stopping oral BP for 3 months would
appear to decrease the active drug to a minimum level
on the bone surface and in the blood.
A long BP half-life was documented by long-term
urine collections of alendronate and pamidronate.
13,47
It is believed that the BP drug group, once incorporated
in the bone as an inactive drug, would be released
slowly as an active drug during normal bone repair.
8,9,19
Since the active drug release would slow bone turnover,
it would also slow its own elimination from the bone.
This could explain the estimated long drug elimination
half-life of more than 10 years.
48
The BP, incorporated
into the bone, continued to decrease skeletal fractures
for 5 years after drug discontinuation.
23
Orthodontic
treatment, as teeth are moved into the BP incorporated
bone, might be adversely affected years after the drug
is stopped.
OSTEONECROSIS OCCURS RARELY AND MIGHT
BE PREVENTABLE
During oral BP treatment for osteoporosis, osteonec-
rosis has been noted rarely and is usually treatable. The
length of continuous oral BP use and type of dental pro-
cedure are important to note. Most osteonecrosis cases
were discovered in patients who had taken oral BP con-
tinuously for more than 3 years and had extractions.
33
Other factors that increase osteonecrosis risk might be
diabetes, periodontal disease, glucocorticoids, alcohol,
and smoking. No large prospective study has carefully
evaluated the incidence of osteonecrosis after long-
term continuous oral BP treatment for osteoporosis.
Osteonecrosis incidence was first reported: 0.7 cases
per 100,000 patient years of drug exposure.
49
Some
investigators suggested that this incidence was underre-
ported.
26,50,51
Recent reports suggested that the osteo-
necrosis incidence of approximately 1:5000 occurs
after 2 to 3 years of continuous oral BP use with in-
creased incidence after extractions, as shown in Table
III. In a large Australian survey of patients taking weekly
oral alendronate for more than 2 years, an osteonecrosis
Table II. Comparisons of BP types used for osteoporosis treatment
Types
1,10,11,43
Potency
13,17,41
Dose (interval)
1,10,11,43
Systemic absorption
13,17
Relative systemic
effect per dose*
Oral treatment for osteoporosis
Alendronate (Fosamax) 700 10 mg (daily) 0.6% 1
70 mg (weekly) 0.6% 7
Risedronate(Actonel) 2,000 5 mg (daily) 0.6% 1.4
35 mg (weekly) 0.6% 10
Ibandronate (Boniva) 4,000 2.5 mg (daily) 0.6% 1.4
150 mg (monthly) 0.6% 90
Intravenous treatment for osteoporosis
Ibandronate (Boniva) 4,000 3 mg (3 months) 100% 286
Zoledronic acid (Reclast) 10,000 5 mg (12 months) 100% 1190
Higher systemic effect dose is given as dosage interval increased.
*Potency x dose x systemic absorption and compared to alendronate as 1.
364 Zahrowski American Journal of Orthodontics and Dentofacial Orthopedics
March 2009
incidence of 1:2300 to 1:8500 was reported; it increased
up to 1:300 when extractions were performed.
50
A retro-
spective institutional study reported osteonecrosis from
oral bisphosphonates occurred frequently after extrac-
tions, with a high incidence of 1:20.
51
This study also re-
ported that no osteonecrosis was found after 4384
extractions in patients never using oral bisphosphonates.
Osteonecrosis has been successfully treated with ne-
crotic bone removal and bone grafting after an oral
drug holiday of 6 months, with the drug restarted 3
months after the surgery.
26,32
Chlorhexidine 0.12%
rinses and appropriate antibiotics were used to help con-
trol secondary infections.
The American Association of Oral Maxillofacial
Surgeons (AAOMS) recommends osteonecrosis preven-
tion through a drug holiday if oral BP has been taken
continuously for more than 3 years or less than 3 years
with glucocorticoids, such as prednisone, as shown in
Table III.
32
After physician approval, a drug holiday is
requested 3 months before and 3 months after elective
dental surgery. The AAOMS recommendations for os-
teonecrosis prevention were based on successful treat-
ment of BP-induced osteonecrosis in osteoporosis
patients after an oral drug holiday. A drug holiday is
not needed before routine root canal treatment, root scal-
ing, or tooth restorative procedures.
49
A study of 98 pub-
lished cases of oral BP-induced osteonecrosis found that
50% of the patients were concurrently taking glucocorti-
coids, which might be a contributing factor.
52
Long-term
glucocorticoids are used to treat chronic inflammatory
conditions and might chemically induce osteoporosis,
which is commonly treated with oral BP.
53
Glucocorti-
coids directly decrease osteoblastic activity and increase
the oral absorption of alendronate by 20% to 44%.
48,53
Most BP-induced necroses commonly seem to in-
volve the bone surrounding the teeth with later progres-
sion into the alveolar bone in the jaws.
26
BP might also
inhibit normal vascularization at high concentrations
found in bone.
54
These reports support a theory that
greater adverse effects of BP occur in areas of high
bone repair. An exaggerated cycle of the active BP be-
ing bound and released might decrease cellular bone
function more in high bone-turnover areas than in low
areas.
25
Nontooth-bearing areas having lower bone
turnover might explain why prospective and retrospec-
tive studies of implant placements have not reported ad-
verse effects from oral BP use.
55,56
However, caution is
needed, since implant failures have been reported after
long oral BP use.
26,57,58
Dental procedures that involve
the bone around the teeth, such as extractions, periodon-
tal surgery, and tooth movement, appear to be more sus-
ceptible to adverse BP effects and are commonly
included in orthodontic treatment plans.
ADVERSE EFFECTS OF INTRAVENOUS BP
The osteonecrosis incidence after long-term BP
given intravenously for osteoporosis treatment is
unknown but presumed to be rare. In a 3-year, dou-
ble-blind study, 3889 women were given 5 mg of zole-
dronic acid yearly for osteoporosis intravenously and
3876 were given a placebo. Two osteonecrosis cases
were found and treated successfully. One osteonecrosis
patient was found with zoledronic acid treatment and
the other with a placebo.
59
The study’s methodology
contained no routine dental screenings and no com-
ments about prior BP use for the placebo patient.
The osteonecrosis incidence might have increased if
the study had been longer than 3 years, since almost
all osteonecrosis cases from oral BP were noted after
3 years of continuous use.
33
Although the systemic ef-
fect per dose is much higher from intravenous when
compared with oral usage, the time interval between
doses is longer (Table II). The long dosage interval
Table III. Osteonecrosis incidence and prevention during oral BP treatment for osteoporosis
Osteonecrosis incidence
50,51
(after 2-3 years continuous oral BP use)
No drug holiday Drug holiday
Usually rare, extractions may dramatically
increase incidence
Decreased drug: PDL bone surface and blood
Higher risk with glucocorticoid use, diabetes,
PDL radiographic changes
Less risk of osteoneocrosis,
Optimized bone healing
Osteonecrosis prevention (AAOMS guidelines)
32
(continuous BP more than 3 years or less than 3 years with glucocorticoid use)
Drug holiday 3 months before and 3 months after elective dental alveolar surgery
Any drug holiday must be done with the knowledge and consent of the prescribing physician
If drug holiday not authorized: osteonecrosis risk explained, usually treated successfully if occurs
No drug holiday needed for routine root canals, root scaling, or restorative procedures
49
American Journal of Orthodontics and Dentofacial Orthopedics Zahrowski 365
Volume 135, Number 3
should result in a low active drug level retained on the
bone surfaces and allow a more normal cellular func-
tion to return between doses. Until 5- to 10-year stud-
ies with routine dental screenings are performed, both
intravenous and oral BP used for osteoporosis treat-
ment are presumed to have similar rare occurrences
of osteonecrosis.
Orthodontists should proceed with caution regard-
ing decreased tooth movement and bone formation after
intermittent intravenous BP administration for osteopo-
rosis with ibandronate (Boniva) and zoledronic acid
(Reclast). Ibandronate is more frequently administered
probably because of lower bone affinity and effective
systemic dose than zoledronic acid (Tables I and II).
Higher effective systemic doses noted from intravenous
than from oral administration could greatly inhibit tooth
movement (Table II). During orthodontic treatment,
concurrent intravenous BP could highly elevate the
bone surface levels around teeth and lead to more
drug incorporation.This might limit present and future
tooth movement more rapidly and profoundly. Ibandro-
nate at the 3-mg dose given intravenously every 3
months and perhaps the monthly 150-mg oral dose
would lead to higher initial bone surface levels that
might remain elevated enough to slow tooth movement
until the next dose is given. Zoledronic acid, at a 5-mg
dose intravenously every 12 months, should immedi-
ately increase the lamina dura surface level but decrease
3 months after the initial dose. Intuitively, a limited or-
thodontic treatment plan might be successful if started 3
to 6 months after the previous dose and finished before
the next dose is given. Since 1 dose of zoledronic acid
sustains a 12-month reduction of bone turnover, it is un-
certain how much tooth movement or bone healing
would occur between doses. A small amount of a highly
potent BP, remaining on the bone surface or released
from the bone could be enough to interfere with ortho-
dontic treatment.
RADIOGRAPHIC CHANGES: SIGN OF DECREASED
BONE FUNCTION DURING BP USE
A radiographic hyper-mineralized area might sig-
nify osteoclastic activity that has been dramatically de-
creased from BP use.
26
The sclerotic areas might not
have enough osteoclastic activity to remove diseased
bone and form proper vascular structures. BP slows
bone formation, but mineralization is unaffected.
19,21
Sclerosis was reported as a beginning BP toxicity in
alveolar bone before osteonecrosis.
26,33,60
Sclerotic
bone was observed when no orthodontic tooth move-
ment occurred during BP use.
29
The sclerotic areas
can appear around teeth or obscure the periodontal liga-
ment (PDL) space.
33
A widened PDL space might be
a sign of decreased bone formation before osteonecro-
sis.
26
The lamina dura around the PDL and the PDL
space should be closely examined in initial and progress
radiographs, especially in the mandibular molar regions.
The bone surrounding the mandibular molars might be
more susceptible to adverse BP effects because posterior
occlusal forces cause higher bone turnover, and the man-
dible has a lower vascular supply than the maxilla.
26,27
After long-term continuous BP use, radiographi-
cally obscured PDL space and sclerosis of the lamina
dura were noted in the left posterior region, signifying
possible local BP toxicity (Fig 2). Osteonecrosis was
observed after the extraction of a painful mandibular
molar on the contralateral side.
PATIENT 1: COMPROMISED NONEXTRACTION
RESULT WITH ORAL BP USE
Oral BP use during orthodontic treatment would
sustain a high blood concentration with presumably
more active drug bound and incorporated into the
bone surrounding teeth. Progressively slower tooth
movement could occur with continued BP administra-
tion. Slow tooth movement can continue years after
stopping the drug.
A 60-year-old woman, completing nonextraction,
nonsurgical orthodontic treatment that lasted 4.5 years
with compromised results, requested a second opinion
(Fig 3). Her concerns were uneven posterior occlusion,
heavy occlusal contact on anterior bridge, minor poste-
rior spacing, slow tooth movement, long treatment time,
and reports of BP inducing osteonecrosis. Before her or-
thodontic treatment, she had a significant right posterior
open bite and used alendronate for osteoporosis for 18
months. No signs or symptoms of osteonecrosis were
apparent from a clinical exam or history. The beginning
panoramic radiograph showed mild sclerosis within
normal limits on the mandibular right second molar,
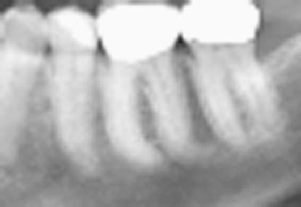
Fig 2. Obscured PDL with sclerosis might signify early
BP toxicity. After long-term continuous BP use, osteo-
necrosis presented on the contralateral side following
a molor extraction.
366 Zahrowski
American Journal of Orthodontics and Dentofacial Orthopedics
March 2009

a small left condyle, and a periodontal defect between
the maxillary left first and second molars (Fig 3, A).
After 2 years of orthodontic treatment and concurrent
alendronate use, pronounced sclerotic bone around the
teeth and widened PDL spaces were observed, espe-
cially in the mandible (Fig 3, B). The physician, un-
aware of the changed radiographic signs, stopped
alendronate and started teriparatide treatment to achieve
higher bone density for her osteoporosis treatment. Af-
ter 3.5 years of orthodontic treatment, widened PDL
spaces and diffuse sclerotic areas were present (Fig 3,
C). After 4.5 years of orthodontic treatment, diffuse
sclerosis and widened PDL spaces were present after
alendronate was stopped for 2.5 years (Fig 3, D). Ortho-
dontic treatment was discontinued by the orthodontist
because of decreased tooth movement and fear of osteo-
necrosis. No root resorption was noted when comparing
the initial and final periapical radiographs.
PATIENT 2: COMPROMISED EXTRACTION RESULT
WITH CONCURRENT ORAL BP
During orthodontic extraction treatment, BP could
incorporate in the extraction site and around the teeth
being moved. Root translation into the extraction site
might be slowed after stopping the drug. Decreased
bone formation and excessive tooth mobility can
occur.
A 50-year-old woman came with a Class II (3 mm
left molar) malocclusion, 3 missing first premolars,
moderate mandibular incisor crowding, lower midline
to the right, periodontal bone loss, and no tooth mobil-
ity (Fig 4). After periodontal treatment, comprehensive
orthodontic treatment was started with extraction of
the remaining mandibular left first premolar. Ortho-
dontic space closure was extremely slow. The patient
had started taking alendronate approximately 6 months
before the extraction and stopped 12 months later be-
cause of esophagitis, a common adverse side effect.
9
She did not report alendronate use in her medical his-
tory because she did not believe it was an orthodontic
concern. Space closure was difficult, and divergent
roots were noted in the extraction site (Fig 4, B).
Less inhibition of tooth movement was noted after
the alendronate was discontinued for 6 months. The or-
thodontic appliances were repositioned to obtain paral-
lel roots. After 7 months, little root movement
occurred and a hyper-mineralized area was observed
within the extraction site (Fig 4, C and D). Alendro-
nate use had been stopped for 13 months. Excessive
mobility and widened PDL spaces were noted. No
traumatic occlusion or change in periodontal status
was present. Orthodontic movement of the incisors
Fig 3. Patient 1: 60-year-old woman requested a second
opinion after compromised nonextraction result with con-
current BP use. A, Initial panoramic radiograph shows peri-
odontal bone loss between the maxillary left first and
second molars and a small left condyle. Prior continuous
BP use of 18 months. Sclerosis in mandibular region is
within normal limits. B, Profound sclerotic areas (greater
in the mandible) surrounding the PDL and widened PDL
spaces are present after 2 years of orthodontic treatment
and concurrent BP use (3.5 years total). C, Diffuse sclerosis
and widened PDL spaces are present after 3.5 years of or-
thodontic treatment. BP was discontinued 18 months ear-
lier. D, Diffuse sclerosis and widened PDL spaces are noted
after 4.5 years of orthodontic treatment. Decreased move-
ment was noted after BP was discontinued 2.5 years ago.
American Journal of Orthodontics and Dentofacial Orthopedics Zahrowski 367
Volume 135, Number 3

was difficult, even though there was mobility. Slow
tooth movement, mandibular incisor mobility, and
compromised parallel roots were observed throughout
the orthodontic treatment.
PATIENT 3: OPTIMIZED EXTRACTION RESULT
WITH AN ORAL BP HOLIDAY
After stopping oral BP for 3 months, a minimum ac-
tive drug level should be present on bone surfaces and in
the blood. A BP holiday throughout orthodontic treat-
ment should sustain a low drug level with less drug in-
corporation into the bone surrounding the teeth.
A 74-year-old woman presented with a Class I oc-
clusion, severe periodontal bone loss, routine periodon-
tal maintenance, severe mandibular incisor crowding,
and a recently fractured mandibular left central incisor
that was clinically unrestorable (Fig 5). She had been
taking oral alendronate continuously for 3 years. Her
intraoral examination was normal, without tooth pain
or exposed necrotic bone. An orthodontic treatment
plan was requested before further dental treatment.
The beginning occlusal photograph showed the frac-
tured incisor temporarily bonded (Fig 5, A). The initial
panoramic radiograph showed mild sclerosis around the
mandibular molars (Fig 5, B). The furcal radiolucency
of the mandibular right first molar was under periodon-
tal observation and present before alendronate use. The
initial mandibular anterior periapical radiographs
showed obscured PDL spaces (Fig 5, C). Continuous
alendronate use for 3 years and the obscured PDL
were interpreted as possible decreased bone function.
An osteonecrosis risk was noted for the planned incisor
extraction. Since there were no signs of infection, im-
mediate extraction was thought to be unnecessary. An
immediate temporary root canal was deemed unneces-
sary because of the obliterated root canal. The physi-
cian stopped the alendronate for 3 months before the
incisor extraction according to the AAOMS oral BP
prevention guidelines (Table III). The physician de-
cided that the bone density goal was reached, and no in-
creased patient morbidity was expected during the
extended drug holiday throughout orthodontic treat-
ment. After evaluation of the orthodontic records, lim-
ited braces were placed between the mandibular first
premolars after consideration of a functional posterior
occlusion, severe periodontal bone loss, age, and the pa-
tient’s request not to treat the maxillary incisors. The
limited orthodontic treatment was successful with nor-
mal extraction healing, space closure, and acceptable
parallel roots within 14 months. Acceptable parallel
roots, although not ideal, were somewhat slow to
Fig 4. Patient 2: compromised extraction result with
concurrent oral BP therapy. A, Initial panoramic radio-
graph of 50-year-old woman. BP use started 6 months
prior to extraction of left mandibular bicuspid and ortho-
dontic treatment. B, Slow space closure and divergent
roots are noted in extraction site at 12 months of treat-
ment. Mandibular incisors had excessive mobility, in-
creased PDL spaces and decreased movement. BP
was discontinued 6 months prior (12 months total use).
C, Nonparallel roots in extraction site after brace reposi-
tioning (7 months prior) is noted at 19 months of treat-
ment. Incisor mobility and increased PDL spaces still
present. D, Sclerotic area is noted within the extraction
site at 19 months of treatment.
368 Zahrowski
American Journal of Orthodontics and Dentofacial Orthopedics
March 2009
obtain. The final photograph showed successful align-
ment and space closure (Fig 5, D). Debonding of the
fixed retainer was noted and repaired. The final pano-
ramic radiograph showed no sclerotic changes, PDL
spaces within normal limits, and mild root resorption
on lower central incisor (Fig 5, E). The final mandibular
incisor periapical radiographs showed mild sclerosis
and PDL spaces. No mobility was noted on the mandib-
ular incisors. Mild root resorption was noted on the
mandibular incisors. The patient was pleased with the
limited extraction treatment result after prior counsel-
ing about possible adverse effects. The alendronate
was planned to be restarted by the physician after the
orthodontic treatment with no expected adverse dental
effects. A bonded retainer was used, although BP has
been reported to help decrease orthodontic relapse.
28
The general dentist and the periodontist were asked to
monitor for future radiographic signs after 3 years of
continued alendronate use.
DISCUSSION
The orthodontic treatment in patient 1 could have
been stopped at 2 years after excessive sclerosis was
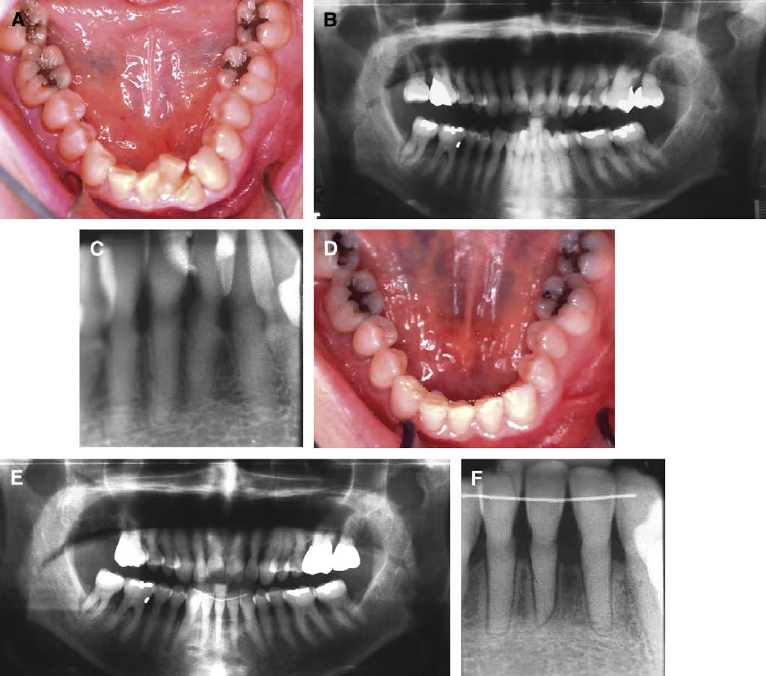
Fig 5. Patient 3: optimized extraction result with an oral BP holiday. A, Initial mandibular occlusal
photograph shows the fractured unrestorable left central incisor. B, Initial panoramic radiograph
shows mild sclerosis and severe periodontal bone loss. Patient had taken alendronate for 3 years.
C, Initial mandibular incisor periapical radiograph. Obscured PDL is a possible sign of decreased
bone function; the obliterated root canals are from age. D, Final occlusal photograph. The patient’s
physician authorized a drug holiday 3 months before and during orthodontic treatment. E, Final pan-
oramic radiograph shows no sclerotic changes and acceptable parallel roots. This limited extraction
treatment was successful in 14 months. F , Final lower incisor periapical radiograph shows PDL
spaces and sclerosis within normal limits. Mild root resorption is noted.
American Journal of Orthodontics and Dentofacial Orthopedics Zahrowski 369
Volume 135, Number 3
noted around the PDL, since little tooth movement
would be anticipated (Fig 3, B). An osteonecrosis risk
was present after 3 years of continuous alendronate
use and a changed radiographic sign denoting possible
local drug toxicity. Higher osteonecrosis risk and less
bone healing would be expected if extractions or peri-
odontal surgery had been performed.
26
This was impor-
tant, since a periodontal defect was noted between the
left first and second molars. After alendronate was
stopped for 3 to 6 months, the osteonecrosis risk should
have decreased closer to a normal range.
26,32
After the
BP is stopped for 3-6 months, a consideration to con-
tinue orthodontics can be made if clinical and radio-
graphic signs begin to decrease. The length of
orthodontic continuation is a clinical judgement based
on the occlusal improvement that can be achieved. Mi-
nor tooth movement can usually be accomplished, how-
ever major tooth movement might be slowed for years
without normal bone healing, especially if sclerosis is
still present (Fig 3, C and D). Ideally, initiating ortho-
dontic treatment in patient 1 could have been delayed
until 3 months after the physician discontinued the
alendronate and chose an alternate osteoperosis treat-
ment, teriparatide. Teriparatide, a recombinant parathy-
roid hormone, causes more bone formation by
increasing osteoblastic activity. Animal experiments
have shown that parathyroid hormone reverses BP de-
pression of osteoclastic activity.
61
Teriparatide was
used to treat a rare osteonecrosis case that did not heal
after a 6-month oral BP holiday.
62
Since teriparatide in-
creases osteoclastic activity, the osteonecrosis risk
should decrease, and orthodontic tooth movement
should increase. In this case, tooth movement continued
to be inhibited from excessive bone incorporation of BP
during the concurrent drug use with orthodontic treat-
ment. Although the sclerosis appeared to be decreasing,
it was observable years after the alendronate was
stopped (Fig 3, C and D). Sclerosis might have a variable
duration, since, in another patient, the sclerosis disap-
peared within a year after the alendronate was stopped.
29
Concurrent BP use during orthodontic extractions
allows the drug to integrate into the healing bone. Ex-
traction site closure would cause active BP release
from bone resorption, decrease osteoclastic function,
and inhibit further tooth movement. The incorporated
BP might stay in the extraction site for years after
drug is discontinued and continue to slow tooth move-
ment. Slow tooth movement and nonparallel roots in
the extraction site with concurrent BP use has been re-
ported.
29
Hyper-mineralized lines were observed after
tooth extractions with concurrent BP use.
26,60
Hyper-
mineralized brittle bones were found in children given
long-term BP.
63
In patient 2, the hyper-mineralized ex-
traction site might signify decreased bone formation
from BP that was not sufficient to affect healing (Fig
4, D). Inhibited movement, excessive mobility, and in-
creased PDL spaces were noted during and after space
closure. The BP, bound and incorporated into bone,
could have decreased bone formation and inhibited
new tooth movement even after the drug was stopped.
A 95% decrease in bone formation was reported after
alendronate use.
24
Widened PDL spaces were noted as
a possible local sign of BP toxicity.
26
Subnormal bone
formation, a decrease of 75% to 95%, would presum-
ably occur sooner and more frequently than an end-
stage necrosis or no bone formation. Detection of early
warning signs can be beneficial to provide better ortho-
dontic care and prevent end-stage pathology in patients
taking oral BP.
A drug holiday was used in patient 3 to decrease the
osteonecrosis risk before extraction. If the physician
had decided not to stop the alendronate, the oral surgeon
would have informed the patient of the osteonecrosis
risk. Without a drug holiday, compromised extraction
space closure would have been expected. In this case,
the extraction site was closed satisfactorily. Orthodontic
treatment was optimized by sustaining minimum BP
concentrations on the bone surface and in blood. Mild
root resorption was noted after orthodontic treatment.
BP has been shown to decrease root resorption.
64
Since
the active drug on the bone surface would decrease after
a drug holiday, the pharmacological protection against
root resorption should also decrease. Even though
a drug holiday might optimize orthodontic treatment,
tooth movement still might not be ideal with extended
oral BP use. Longer use will cause more BP to incorpo-
rate in trabecular bone and remain for many years. Slow
drug release from the skeleton into the bloodstream
would allow more BP to bind during tooth movement.
Drug accumulation can be significant in patients who
have been taking oral BP for long periods. It has been
estimated that 25% of a daily dose is released from
the skeleton daily after 10 years of continuous oral ad-
ministration.
1
BP might also decrease the activity of os-
teoclastic progenitor cells that could contribute to long-
term adverse effects.
16,65
Research is needed to under-
stand the long-term adverse effects of BP in orthodon-
tics.
An orthodontic screening should ask about prior BP
use. A detailed history is needed to establish the specific
oral or intravenous BP preparations, the duration of use,
and the medical purpose of treatment (osteopenia, oste-
oporosis, or severe osteoporosis with prior fragility frac-
tures). Patients with severe osteoporosis might have
a greater skeletal fracture risk during a drug holiday.
A notation should be made of any patient given
370 Zahrowski American Journal of Orthodontics and Dentofacial Orthopedics
March 2009

intravenous BP (zoledronic acid or pamidronate) for
cancer treatment, since orthodontic or elective dental
surgery should be contraindicated, and drug holidays
do not apply.
25,27,33
Intravenous zoledronic acid is
used to treat both osteoporosis and cancer patients by
changing the time intervals between doses. Zoledronic
acid (Reclast), 5 mg intravenously, is given every 12
months for osteoporosis treatment.
11
Zoledronic acid
(Zometa), 4 mg intravenously, is given every 3 to 4
weeks for bone cancer treatment.
1
Bone cancer treat-
ment requires frequent administration of zoledronic
acid to sustain high surface bone levels to limit the can-
cer’s detrimental effect on skeletal bones.
The suggested guidelines, shown in Table IV, are to
optimize orthodontic treatment through appropriate
drug holidays and monitor adverse dental effects during
oral BP treatment of osteoporosis. The guidelines do not
guarantee ideal orthodontic results but should lessen
nonideal results and minimize adverse effects with sound
clinical treatment plans and continued patient monitor-
ing. The guidelines are not meant to be the standard of
care and should be reevaluated as future studies dictate.
The orthodontist should have the patient read and sign
the American Association of Orthodontists’ informed
consent for BP before orthodontic treatment. Changing
clinical and radiographic signs should be carefully mon-
itored especially prior to a drug holiday, since they might
suggest decreased alveolar bone function and early local
drug toxicity. If positive signs occur, orthodontic treat-
ment might need to be discontinued, even if treatment
goals are not achieved. Clinical monitoring should in-
clude changes in decreased movement and increased mo-
bility. Radiographic monitoring should include changes
of sclerotic or radiolucent bone surrounding teeth. Ra-
diographic changes can be caused by other pathologies,
such as infection, prior accidents, periodontal disease,
or occlusal trauma. Careful evaluation of trabecular var-
iations, lamina dura, and PDL space in routine orthodon-
tic patients will give proper comparisons of radiographic
changes caused by BP use. AAOMS osteonecrosis pre-
vention should be considered for any extraction or peri-
odontal surgery planned in an area of decreased bone
function. After 2 to 3 years of oral BP administration,
the differential diagnosis of tooth pain should include os-
teonecrosis. If routine dental procedures, such as root ca-
nal therapy or periodontal scaling, do not relieve
symptoms, then osteonecrosis should be considered
strongly in the differential diagnosis.
49
The orthodontist
should not act unilaterally if any adverse drug effects are
observed. The physician should be informed of any
changed clinical or radiographic signs that indicate
a BP drug holiday may be of value to help orthodontic
treatment or decrease osteonecrosis risk.
Radiographic signs are not diagnostic for osteonec-
rosis but can suggest a higher risk. The clinical osteo-
necrosis definition, requiring tissue exposure of
necrotic bone, might be a late diagnosis. Osteonecrosis
has been reported before clinical signs of exposed
Table IV. Suggested orthodontic optimization guidelines during BP treatment for osteoporosis
Drug holiday 3 months before and during orthodontic tooth movement
(decreases and maintains low active drug level: blood and PDL bone surfaces)
No drug holiday Drug holiday
Orthodontic risks
Progressively slower tooth movement Closer to normal range
More tooth mobility
Teeth less likely to move in future
Orthodontic treatment suggestions
Consider: delay untill drug stopped or alternative drug Optimized treatment
(especially extraction cases)
Consider: limited, nonextraction cases with caution
BP effect accumulates with continued use Long prior BP use might
BP bone incorporation might limit future movement slow tooth movement after holiday
Monitoring signs: BP decreased bone function
Clinical signs: slow movement, excessive mobility
Radiographic signs: sclerosis around teeth, obscured PDL, or excessive PDL space
(Rule out trauma, infection, periodontal causes, and normal bone variations)
If signs present, consider careful monitoring, drug holiday, and delay or discontinue orthodontics
The suggested guidelines do not guarantee ideal results but are meant to lessen nonideal results
with sound orthodontic treatment plans and continuous monitoring for adverse effects.
Drug holiday must be done with the knowledge and consent of the prescribing physician
American Journal of Orthodontics and Dentofacial Orthopedics
Zahrowski 371
Volume 135, Number 3
necrotic bone.
66
Magnetic resonance imaging (MRI)
has shown all BP-induced osteonecrosis lesions before
they were clinically present.
67
MRI found all necrotic
bone lesions from other causes better than bone scans
or computerized tomography when compared with his-
tologic diagnosis in the hip, knee, ankle, and shoulder.
68
MRI should be considered for early recognition of os-
teonecrosis in the alveolar bone. MRI might have signif-
icant value, since histologic diagnosis cannot be made
in a suspected BP-induced osteonecrosis for fear of
worsening the situation.
The successful clinical outcome of osteonecrosis
treatment from oral BP has been reported to be related
to a serum C telopeptide level (CTX) level when it is
greater than 150 ng per milliliter.
69
The CTX level, a sys-
temic osteoclastic bone marker, is obtained from a fast-
ing, early-morning blood draw. The use of the CTX is
controversial, since it measures systemic osteoclastic ac-
tivity from the entire skeleton and does not specifically
measure the local osteoclastic function in the alveolar
bone. However, the CTX level might be a sensitive mea-
sure of altered bone function caused from the active drug
residing on the bone surfaces. Although controversial,
the CTX level can be considered after a 3- to 6-month
drug holiday to confirm that systemic osteoclastic func-
tion is normal before major dental surgical procedures
when BP was taken continuously for more than 5 years.
Ideally, the orthodontist should contact the prescrib-
ing physician to discuss the planned orthodontic proce-
dure, expected risks, and possible optimization with
a drug holiday. Optimizing orthodontic treatment after
BP use has different requirements than osteonecrosis
prevention (Tables III and IV). Two bone elimination
rates have been reported for the BP drug group. A
3-month drug holiday should lower the active drug con-
centration in the blood and on the bone surfaces around
the teeth to lower the risk of osteonecrosis. An extended
BP holiday during orthodontic treatment should sustain
low active drug levels, lessen drug incorporation sur-
rounding teeth, and optimize tooth movement and
bone healing. During tooth movement, bone turnover
will slowly release any previously incorporated inactive
drug as an active drug. BP incorporated in the bone,
which can remain for many years, should be minimized
in areas where teeth are planned to be moved.
The patient’s treatment goal for osteoporosis might
have been reached, and the physician could decide that
no further oral BP is needed during orthodontic treat-
ment. A randomized, double-blind, multicenter study
of 6459 patients concluded that there was no difference
in the 10-year fracture rate comparing a group taking
alendronate for 5 years with another group taking the
drug continuously for 10 years.
23
However, it was
concluded that high-risk vertebral fracture patients can
benefit from longer 10-year treatment.
An alternate osteoporosis treatment can be started
by the physician because the treatment goal has not
been reached. It is the physician’s responsibility to sug-
gest alternative osteoporosis treatments, even though
alternate medications might have fewer adverse dental
effects. Alternate treatments such as raloxifene, a selec-
tive estrogen receptor modulator, and estrogen both
have short half-lives with no bone accumulation, and
should affect tooth movement less than BP. However,
alternate drugs could be less effective for osteoporosis.
Treatment alternatives can also have a greater incidence
of serious adverse medical effects.
7
Estrogen can in-
crease the risk of breast cancer, deep venous thrombo-
sis, and stroke. Raloxifene might increase the risk of
deep venous thrombosis and stroke. Teriparatide is an
effective treatment for vertebral osteoporosis with
shortcomings of high expense, daily subcutaneous in-
jections, duration of treatment not to exceed 2 years,
and another osteoporosis medication needed after 2
years to retain the increased bone density.
5
It is not appropriate for all patients to have a BP hol-
iday, and the physician might decide that the fracture
risk is too high. The orthodontist can consider not treat-
ing or delaying treatment, especially in elective extrac-
tion patients, until the BP can be stopped or an
alternative medication is appropriate. A consideration
to treat limited, nonextraction patients could be made
with the following precautions. Concurrent BP use
might cause drug accumulation and progressively
slower tooth movement. Slow tooth movement can con-
tinue for years after the drug is discontinued. Orthodon-
tic treatment might have to be discontinued before the
treatment goals have been accomplished. No patient
should stop oral BP medication without the knowledge
and consent of the primary prescribing physician be-
cause bone density might not have risen sufficiently to
prevent hip and vertebral fractures. Orthodontists
should understand that prevention of hip or vertebral
fractures takes priority over an elective orthodontic pro-
cedure. Physicians should understand that the patient’s
desired orthodontic result might not be achieved if BP
is continued, especially if the medication is not cur-
rently beneficial. After discussing the medical and or-
thodontic risks and benefits, a mutual decision can be
made in the patient’s best interest.
CONCLUSIONS
This article was written to begin parameters to min-
imize adverse effects and optimize orthodontic treat-
ment in the millions of patients taking oral BP for
372 Zahrowski American Journal of Orthodontics and Dentofacial Orthopedics
March 2009
osteoporosis. Orthodontists clearly need to understand
the pharmacology and adverse effects of this unique
drug group to evaluate early warning signs of decreased
bone function before progression to necrosis. Tooth
movement, tooth mobility, and radiographic changes
of the lamina dura and the PDL spaces need to be eval-
uated and monitored in patients taking oral BP. This
drug group has many medical benefits, and the in-
creased orthodontic risks might be small compared
with the increased medical risks if the medication is
stopped. No patient should discontinue BP without the
knowledge and consent of the primary prescribing phy-
sician. Osteonecrosis occurs rarely during BP treatment
for osteoporosis, and the AAOMS guidelines for osteo-
necrosis prevention and treatment should be reviewed.
Ideally, the orthodontist and the physician should dis-
cuss the patient’s risks and benefits of BP treatment ac-
cording to the severity of the osteoporosis, the projected
risks to orthodontic procedures, and the appropriateness
of a drug holiday to optimize orthodontic treatment and
minimize adverse dental effects.
REFERENCES
1. Burnham TH, Wickersham RM, editors. Drug facts and compar-
isons. 57th ed. St Louis: Wolter Kluwer; 2003. p. 336-49.
2. Ukens C. The top 200 brand drugs in 2005. Drug Topics 2006;150:
25.
3. Lindsay R, Cosman F. Osteoporosis. In: Braunwald E, Hauser SL,
Fauci AS, Longo DL, Kasper DL, Jameson JL, editors. Harrison’s
principles of internal medicine. 15th ed. New York: McGraw-Hill;
2001. p. 2226-36.
4. Raisz LG. The osteoporosis revolution. Ann Intern Med 1997;
126:458-62.
5. Rosen C. Postmenopausal osteoporosis. N Engl J Med 2005;353:
595-603.
6. Raisz LG. Screening for osteoporosis. N Engl J Med 2005;353:
164-71.
7. Khosla S, Melton LJ. Osteopenia. N Engl J Med 2007;356:
2293-300.
8. Lin JH, Russell G, Gertz B. Pharmacokinetics of alendronate: an
overview. Int J Clin Pract 1999;101(Suppl):18-26.
9. Watts NB. Treatment of osteoporosis with bisphosphonates. En-
docrinol Metab Clin North Am 1998;27:419-39.
10. Prescribing information for Boniva, ibandronate intravenous.
Nutley, NJ: Roche Therapeutics; February 2007.
11. Prescribing information for Reclast, zoledronic acid intravenous.
East Hanover, NJ: Novartis Pharmaceuticals; August 2007.
12. Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP,
Monkkonen J, et al. Cellular and molecular mechanisms of action
of bisphosphonates. Cancer 2000;88(12 Suppl):2961-78.
13. Licata AA. Discovery, clinical development, and therapeutic uses
of bisphosphonates. Ann Pharmacother 2005;39:668-77.
14. Masarachia P, Weinreb M, Balena R, Rodan GA. Comparison of
the distribution of 3H-alendronate and 3H-etidronate in rat and
mouse bones. Bone 1996;19:281-90.
15. Rogers MJ. New insights into the molecular mechanisms of action
of bisphosphonates. Curr Pharm Des 2003;9:2643-58.
16. Van Beek ER, Lowik CWGM, Papapoulos SE. Bisphosphonates
suppress bone resorption by a direct effect on early osteoclast pre-
cursors without affecting the osteoclastogenic capacity of osteo-
genic cells: the role of protein geranylgeranylation in the action
of nitrogen-containing bisphosphonates or osteoclast precursors.
Bone 2002;30:64-70.
17. Lin JH. Bisphosphonates: a review of the pharmacokinetic prop-
erties. Bone 1996;18:75-85.
18. Dixon RB, Tricker ND, Garetto LP. Bone turnover in elderly ca-
nine mandible and tibia. J Dent Res 1997;76:336 (IADR abstract
2579).
19. Rodan G, Reszka A, Golub E, Rizzoli R. Bone safety of long-term
bisphosphonate treatment. Curr Med Res Opin 2004;20:
1291-300.
20. Rocha ML, Malacara JM, Sanchez-Marin FJ, Vazquez de la
Torre CJ, Fajardo ME. Effect of alendronate on periodontal dis-
ease in postmenopausal women: a randomized placebo-controlled
trial. J Periodontol 2004;75:1579-85.
21. Meunier PJ, Arlot M, Chavassieux P, Yates J. The effects of
alendronate on bone turnover and bone quality. Int J Clin Pract
1999;101(Suppl):14-7.
22. Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T,
Hochberg MC, et al. Fracture risk reduction with alendronate in
women with osteoporosis: the Fracture Intervention Trial. FIT re-
search group. J Clin Endocrinol Metab 2000;85:4118-24.
23. Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S,
Quandt SA. Effects of continuing or stopping alendronate after
5 years of treatment. J Am Med Assoc 2006;296:2927-38.
24. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA,
Pak CY. Severely suppressed bone turnover: a potential complica-
tion of alendronate therapy. J Clin Endocrinol Metab 2005;90:
1294-301.
25. Zahrowski JJ. Bisphosphonate treatment: an orthodontic concern
calling for a proactive approach. Am J Orthod Dentofacial Orthop
2007;131:313-22.
26. Marx RE. Oral and intravenous bisphosphonate-induced osteo-
necrosis of the jaws. Hanover Park, Illinois: Quintessence; 2007.
27. Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonec-
rosis of the jaws associated with the use of bisphosphonates: a re-
view of 63 cases. J Oral Maxillofac Surg 2004;62:527-34.
28. Igarashi K, Mitani H, Adachi H, Shinoda H. Anchorage and reten-
tive effects of a bisphosphonate (AHBuBP) on tooth movement in
rats. Am J Orthod Dentofacial Orthop 1994;106:279-89.
29. Rinchuse DJ, Rinchuse DJ, Sosovicka MF, Robison JM,
Pendleton R. Orthodontic treatment of patients using bisphospho-
nates: a report of 2 cases. Am J Orthod Dentofacial Orthop 2007;
131:321-6.
30. Marx RE. Pamidronate (aredia) and zoledronate (zometa) induced
avascular necrosis of the jaws: a growing epidemic. J Oral Max-
illofac Surg 2003;61:1115-7.
31. Hellstein JW, Marek CL. Bisphosphonate osteochemonecrosis
(bis-phossy jaw): is this phossy jaw of the 21st century? J Oral
Maxillofac Surg 2005;63:682-9.
32. AAOMS position paper. American Association of Oral and Max-
illofacial Surgeons Position Paper on Bisphosphonate-Related
Osteonecrosis of the Jaws. J Oral Maxillofac Surg 2007;65:
369-76.
33. Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-in-
duced exposed bone (osteonecrosis/osteopetrosis) of the jaws:
risk factors, recognition, prevention, and treatment. J Oral Maxil-
lofac Surg 2005;63:1567-75.
34. Guarneri V, Donati S, Nicolini M, Givannelli S, D’Amico R,
Conte PF. Renal safety and efficacy of I.V. bisphosphonates in pa-
tients with skeletal metastases treated for up to 10 years. Oncolo-
gist 2005;10:842-8.
American Journal of Orthodontics and Dentofacial Orthopedics
Zahrowski 373
Volume 135, Number 3
35. Durie B, Katz M, Crowley J. Osteonecrosis of the jaw and
bisphosphonates (letter). New Engl J Med 2005;353:99-102.
36. Schwarz HC. Bisphosphonate-associated osteonecrosis of jaws.
J Oral Maxillofac Surg 2005;63:1555-6.
37. Zahrowski JJ. Comment on the American Association of Oral
Maxillofacial Surgeons statement on bisphosphonates. J Oral
Maxillofac Surg 2007;65:1440-1.
38. Woo SB, Hellstein JW, Kalmar JR. Systemic review: bisphosph-
onates and osteonecrosis of the jaws. Ann Intern Med 2006;144:
753-61.
39. Lomashvili KA, Cobbs S, Hennigar RA, Hardcastle KI,
O’Neill WC. Phosphate-induced vascular calcification: role of py-
rophosphate and osteopontin. J Am Soc Nephrol 2004;15:
1392-401.
40. Leu C, Luegmayr E, Freedman LP, Rodan GA, Reszka AA. Rel-
ative binding affinities of bisphosphonates for human bone and
relationship to antiresorptive efficacy. Bone 2006;38:628-36.
41. Muhlbauer RC, Bauss F, Schenk R, Janner M, Bosies E, Strein K,
et al. BM21.0955, a potent new bisphosphonate to inhibit bone re-
sorption. J Bone Miner Res 1991;6:1003-11.
42. Porras A, Denker A, Santhanagopal A, Daifotis AG. Alendronate
and risedronate have similar pharmacokinetic half-lives when an-
alyzed over the same time interval. J Bone Miner Res 2003;
18(Suppl 2):S371.
43. Prescribing information of Boniva, ibandronate tablets. Nutley,
NJ: Roche Therapeutics; August 2006.
44. Prescribing information for Actonel, risedronate tablets. North
Norwich, NY: Proctor & Gamble Pharmaceuticals; April 2008.
45. Wilkinson GR. Pharmokinetics. In: Hardman JG, Limbird LE,
editors. Goodman & Gilman’s the pharmacological basis of ther-
apeutics. 10th ed. New York: McGraw-Hill; 2001. p. 3-29.
46. Khan SA, Kanis JA, Vasikaran S, Kline WF, Matuszewski BK,
McCloskey EV, et al. Elimination and biochemical responses to
intravenous alendronate in postmenopausal osteoporosis. J Bone
Miner Res 1997;12:1700-7.
47. Papapoulos SE, Cremers SC. Prolonged bisphosphonate release
after treatment in children. N Engl J Med 2007;356:1075-6.
48. Prescribing information for Fosamax, alendronate tablets. White-
house Station, NJ: Merck; 2006.
49. Edwards BJ, Hellstein JW, Jacobsen PL, Kaltman S, Mariotti A,
Migliorati CA. ADA report of the Council on Scientific Affairs:
dental management of patients receiving oral bisphosphonate
therapy—expert panel recommendations. J Am Dent Assoc
2006;137:1144-50.
50. Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency
of bisphosphonate-associated osteonecrosis of the jaws in Aus-
tralia. J Oral Maxillofac Surg 2007;65:415-23.
51. Sedghizadeh PP, Stanley K, Caligiuri M, Hofkes S, Lowry B,
Shuler CF. Oral bisphosphonate use and the prevalence of osteo-
necrosis of the jaw. JADA 2009;140:61-6.
52. Hess LM, Jeter JM, Berham-Hutchins M, Alberts DS. Factors as-
sociated with osteonecrosis of the jaw among bisphosphonate
users. Am J Med 2008;121:475-83.
53. Schimmer BP, Parker KL. Adrenocorticotropic hormone; adreno-
cortical steroids and their synthetic analogs; inhibitors of the syn-
thesis and actions of andrenocortical hormones. In: Hardman JG,
Limbird LE, editors. Goodman & Gilman’s the pharmacological
basis of therapeutics. 10th ed. New York: McGraw-Hill; 2001.
p. 1649-77.
54. Wood J, Bonjean K, Ruetz S. Novel antiangiogenic effects of the
bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther
2002;302:1055-61.
55. Fugazzotto PA, Lightfoot WS, Jaffin R, Kumar A. Implant place-
ment with or without simultaneous tooth extraction in patients
taking oral bisphosphonates: postoperative healing, early fol-
low-up, and incidence of complications in two private practices.
J Periodontol 2007;78:1664-9.
56. Jeffcoat MK. Safety of oral bisphosphonates: controlled studies
on alveolar bone. Int J Oral Maxillofac Implants 2006;21:349-53.
57. Wang HL, Weber D, McCauley LK. Effect of long-term oral bi-
sphosphonates on implant wound healing: literature review and
a case report. J Periodontol 2007;78:584-94.
58. Starck WJ, Epker BN. Failure of osseointegrated dental implants
after diphosphonate therapy for osteoporosis: a case report. Int J
Oral Maxillofac Implants 1995;10:74-8.
59. Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA,
et al. Once-yearly zoledronic acid for treatment of postmeno-
pausal osteoporosis. N Engl J Med 2007;356:1809-22.
60. Markewicz MR, Margarone JE 3rd, Campbell JH, Aguirre A. Bi-
sphosphonate-associated osteonecrosis of the jaws: a review of
current knowledge. J Am Dent Assoc 2005;136:1669-74.
61. van der Pluijm G, Lowik CW, de Groot H, Alblas MJ, van der
Wee-Pals LJ, Bijvoet OLM, Papapoulos SE. Modulation of
PTH-stimulated osteoclastic resorption by bisphosphonates in
fetal mouse bone explants. J Bone Miner Res 1991;6:1203-10.
62. Harper RP, Fung E. Resolution of bisphosphonate-associated os-
teonecrosis of the mandible: possible application for intermittent
low-dose parathyroid hormone [rhPTH(1-34)]. J Oral Maxillofac
Surg 2007;65:573-80.
63. Marini JC. Do bisphosphonates make children’s bones better or
brittle? N Engl J Med 2003;349:423-6.
64. Igarashi K, Adachi H, Mitani H, Shinoda H. Inhibitory effect of
the topical administration of a bisphosphonate (risedronate) on
root resorption incident to orthodontic tooth movement in rats.
J Dent Res 1996;75:1644-9.
65. Sahni M, Guenther HL, Fleisch H, Collin P, Martin TJ. Bi-
sphosphonates act on rat bone resorption through the mediation
of osteoblasts. J Clin Invest 1993;91:2004-11.
66. Junquera L, Gallego L. Nonexposed bisphosphonate-related os-
teonecrosis of the jaws: another clinical variant? J Oral Maxillofac
Surg 2008;66:1516-7.
67. Garcia-Ferrer L, Bagan JV, Martinez-Sanjuan V, Hernandez-
Bazan S, Garcia R, Jimenez-Soriano, et al. MRI of mandibular os-
teonecrosis secondary to bisphosphonates. Am J Roentgenol
2008;190:949-55.
68. Mont MA, Ulrich SD, Seyler TM, Smith JM, Marker DR,
McGrath MS, et al. Bone scanning of limited value for diagnosis
of symptomatic oligofocal and multifocal osteonecrosis. J Rheu-
matol 2008;35:1629-34.
69. Marx RE, Cillo JE, Ulloa JJ. Oral bisphosphonate-induced osteo-
necrosis: risk factors, prediction of risk using serum CTX testing,
prevention, and treatment. J Oral Maxillofac Surg 2007;65:
2397-410.
374 Zahrowski American Journal of Orthodontics and Dentofacial Orthopedics
March 2009
