
Recommendations for Improving the
Core Sets of Health Care Quality Measures
for Medicaid and CHIP
Summary of a Multistakeholder Review of the 2020 Child
and Adult Core Sets
Final Report
August 2019

2020 CHILD AND ADULT CORE SET STAKEHOLDER
WORKGROUP MEMBERS
Voting Members (Affiliation as of May 2019)
Gretchen Hammer, MPH, Co-chair
Public Leadership Consulting Group
David Kroll, MD
Department of Psychiatry, Brigham Health, Harvard Medical
School
David Kelley, MD, MPA, Co-chair
Pennsylvania Department of Human Services
Carolyn Langer, MD, JD, MPH
Fallon Health
Richard Antonelli, MD, MS
Boston Children’s Hospital
Lauren Lemieux
American College of Obstetricians and Gynecologists
Jill Arnold
Maternal Safety Foundation
Jill Morrow-Gorton, MD, MBA
University of Massachusetts Medical School
Lowell Arye, MS
Aging and Disability Policy and Leadership Consulting,
LLC
Amy Mullins, MD, CPE, FAAFP
American Academy of Family Physicians
Tricia Brooks, MBA
Georgetown University Center for Children and Families
Fred Oraene, MBA
Oklahoma Health Care Authority
Laura Chaise, MBA
Centene Corporation
Lisa Patton, PhD
IBM Watson Health
Lindsay Cogan, PhD, MS
New York State Department of Health
Jeff Schiff, MD, MBA
Minnesota Department of Human Services
James Crall, DDS, ScD, MS
UCLA School of Dentistry
Marissa Schlaifer, MS
OptumRx
Steve Groff
Delaware Department of Health and Social Services
Linette Scott, MD, MPH
California Department of Health Care Services
Kim Elliott, PhD, MA, CPHQ, CHCA
Health Services Advisory Group
Jami Snyder, MA
Arizona Health Care Cost Containment System
Tricia Elliott, MBA, CPHQ
The Joint Commission
Jennifer Tracey, MHA
Zero to Three
Shevaun Harris, MBA, MSW
Florida Agency for Health Care Administration
Sally Turbyville, DRPH, MS, MA
Children’s Hospital Association
Diana Jolles, PhD, CNM, FACNM
Frontier Nursing University
Bonnie Zima, MD, MPH
UCLA-Semel Institute for Neuroscience and Human
Behavior
Federal Liaisons (Non-voting)
Agency for Healthcare Research and Quality, HHS
Center for Clinical Standards & Quality, CMS, HHS
Centers for Disease Control and Prevention, HHS
Health Resources and Services Administration, HHS
Office of Infectious Disease and HIV/AIDS Policy (formerly National Vaccine Program Office), HHS
Office of the Assistant Secretary for Planning and Evaluation, HHS
Substance Abuse and Mental Health Services Administration, HHS
U.S. Department of Veterans Affairs
iii
ACKNOWLEDGEMENTS
This report was developed by Mathematica as part of the Technical Assistance and Analytic
Support for the Medicaid and CHIP Quality Measurement and Improvement Program, sponsored
by the Center for Medicaid and CHIP Services. The implementation of the 2020 Core Set
Review and production of the report was a team effort and we acknowledge the contributions of
the entire team.
Project director: Margo Rosenbach
Task leader: Bailey Orshan
Senior researchers: Rosemary Borck and Michaela Vine
Analysts: Allison Steiner, Chrissy Fiorentini, Ruth Hsu, and Steve Bruns
Task manager: Dayna Gallagher
Communications and administration: Christal Stone Valenzano, Brice Overcash, Brian
Willis, Fiona Shapiro, Derek Mitchell, Anthony Zampelli, Liah Caravalho, Autumn
Parker, Alyssa Smith, Shantal Alston James, Jess Coldren, and Colleen Fitts
Technical writers: Megan Thomas and Tanya Schwartz, Harbage Consulting
Mathematica would also like to acknowledge the contributions of the Workgroup members; each
member brought an invaluable perspective that informed the recommendations for the 2020
Child and Adult Core Sets. In particular, we thank the Workgroup co-chairs, Gretchen Hammer
and David Kelley, for their insightful facilitation and leadership.
In addition, we express our gratitude to the measure stewards contacted throughout the review
process. We appreciate the information they provided on the measures under consideration, and
for responding to questions from the Workgroup during the in-person meeting.
Finally, we thank the staff in the Division of Quality & Health Outcomes at the Center for
Medicaid and CHIP Services for their input and guidance.
iv
CONTENTS
ACRONYMS ................................................................................................................................................. v
EXECUTIVE SUMMARY ............................................................................................................................. vii
INTRODUCTION ........................................................................................................................................... 1
OVERVIEW OF THE CHILD AND ADULT CORE SETS ............................................................................. 2
DESCRIPTION OF THE 2020 CORE SET ANNUAL REVIEW PROCESS ................................................. 5
STATE PERSPECTIVES ON CORE SET REPORTING ............................................................................. 9
WORKGROUP RECOMMENDATIONS FOR IMPROVING THE 2020 CORE SETS ................................ 10
NEXT STEPS .............................................................................................................................................. 21
APPENDIX A: CHILD AND ADULT CORE SET MEASURES .................................................................. A.1
APPENDIX B: SUMMARY OF 2020 CORE SET ANNUAL REVIEW WORKGROUP
DISCUSSION OF MEASURES NOT RECOMMENDED FOR REMOVAL OR ADDITION ..................... B.1
APPENDIX C: PUBLIC COMMENTS ON THE DRAFT REPORT ............................................................ C.1
v
ACRONYMS
ABA-AD Adult Body Mass Index
Assessment
APC-CH Use of Multiple Concurrent
Antipsychotics in Children
and Adolescents
ACA Affordable Care Act
ACE Angiotensin Converting
Enzyme
BMI Body Mass Index
BRFSS Behavioral Risk Factor
Surveillance System
CAHPS Consumer Assessment of
Healthcare Providers and
Systems
CAP-CH Child and Adolescents’
Access to Primary Care
Practitioners
CCP-AD Contraceptive Care –
Postpartum Women Ages
21–44
CCP-CH Contraceptive Care –
Postpartum Women Ages
15–20
CCW Contraceptive Care – All
Women
CDC Centers for Disease Control
and Prevention
CHIP Children’s Health Insurance
Program
CHIPRA Children’s Health Insurance
Program Reauthorization Act
CLABSI-CH Pediatric Central Line–
Associated Bloodstream
Infections
CMCS Center for Medicaid and
CHIP Services
CMS Centers for Medicare &
Medicaid Services
CV Curriculum Vitae
ECDS Electronic Clinical Data
Systems
EHR Electronic Health Record
FFY Federal Fiscal Year
FVA-AD Flu Vaccinations for Adults
Ages 18 to 64
HA1C-AD Comprehensive Diabetes
Care: Hemoglobin A1c
(HbA1c) Testing
HCBS Home- and Community-
Based Services
HEDIS Healthcare Effectiveness
Data and Information Set
HHS U.S. Department of Health
and Human Services
HIV Human Immunodeficiency
Virus
HPC-AD Comprehensive Diabetes
Care: Hemoglobin A1c
(HbA1c) Poor Control (> 9.0
percent)
ACRONYMS (continued)
vi
HRSA Health Resources and
Services Administration
HSRI Human Services Research
Institute
HVL-AD HIV Viral Load Suppression
ICU Intensive Care Units
LTSS Long-Term Services and
Supports
MCO Managed Care Organization
MLTSS Managed Long-Term
Services and Supports
MPM-AD Annual Monitoring for
Patients on Persistent
Medications
MSC-AD Medical Assistance with
Smoking and Tobacco Use
Cessation
NCI
TM
National Core Indicators
TM
NCI-AD
TM
National Core Indicators for
Aging and Disabilities
TM
NCQA National Committee for
Quality Assurance
NQF National Quality Forum
OB/GYN Obstetrician/Gynecologist
OUD Opioid Use Disorder
PC01-AD PC-01: Elective Delivery
PC02-CH PC-02: Cesarean Birth
PCP Primary Care Practitioner
PDMP Prescription Drug Monitoring
Program
QMETRIC Quality Measurement,
Evaluation, Testing, Review,
and Implementation
Consortium
SUD Substance Use Disorder
TA/AS Technical Assistance and
Analytic Support Program
USPSTF U.S. Preventive Services
Task Force
WCC-CH Weight Assessment and
Counseling for Nutrition and
Physical Activity for
Children/Adolescents—Body
Mass Index Assessment for
Children/Adolescents

vii
EXECUTIVE SUMMARY
Medicaid and the Children’s Health Insurance Program (CHIP) provide health care coverage to
approximately 73 million people, including eligible children, pregnant women, low-income
adults, and individuals with disabilities.
1
The Centers for Medicare & Medicaid Services (CMS)
and its Center for Medicaid and CHIP Services (CMCS) use various tools to ensure that
Medicaid and CHIP beneficiaries receive health care coverage that promotes high quality care.
The Medicaid and CHIP Child and Adult Core Sets of health care quality measures (Core Sets)
are key components of this effort.
The Core Sets are used to assess the quality of care provided by states to Medicaid and CHIP
beneficiaries. The Core Sets are the mechanism for state reporting on a uniform set of measures
to facilitate state and national analyses, track performance over time, and use the results to drive
quality improvement in Medicaid and CHIP. Currently, state reporting on the Core Sets is
voluntary.
The Secretary of the U.S. Department of Health and Human Services is required to review and
update the Child and Adult Core Sets annually.
2
The annual Core Set review is designed to
identify gaps in existing quality measures and suggest updates to strengthen and improve the
Core Sets. The annual review includes input from numerous stakeholders, such as states, health
care providers, and quality experts.
CMCS contracted with Mathematica to convene the 2020 Child and Adult Core Set Annual
Review Stakeholder Workgroup (Workgroup). The Workgroup included 28 members, who
represented a diverse set of stakeholders based on affiliation, subject matter expertise, and
quality measurement and improvement experience (see inside front cover).
The Workgroup was charged with reviewing the 2019 Core Sets and recommending changes to
strengthen and improve the Core Sets for 2020. Workgroup members were asked to suggest
measures for removal from or addition to the Core Sets based on characteristics that support the
use of the Core Set measures for improving the quality of care for Medicaid and CHIP
beneficiaries. See Exhibit ES.1 for the characteristics Workgroup members considered during the
2020 Core Set review.
1
March 2019 Medicaid and CHIP Enrollment Data Highlights are available at
https://www.medicaid.gov/medicaid/program-information/medicaid-and-chip-enrollment-data/report-
highlights/index.html. Numbers reflect Medicaid and CHIP enrollment data as of March 2019, as reported by 50
states and the District of Columbia.
2
Annual updates to the Child Core Set are required under the Children’s Health Insurance Program Reauthorization
Act of 2009. Annual updates to the Adult Core Set are required under the Affordable Care Act.

viii
Exhibit ES.1. Characteristics Considered for Removal of Existing Measures and Addition
of New Measures
Characteristics Considered for Removal of Existing Measures
Actionability. Does the measure provide few useful or actionable results for state Medicaid and CHIP programs?
Clinical relevance. Does the measure no longer adhere to clinical evidence or guidelines?
Feasibility. Have states reported significant challenges to reporting the measure (such as barriers to accessing or
using data needed to report the measure)?
New or alternate measure. Is another measure being recommended to replace an existing Core Set measure?
Performance. Have states consistently reported a high level of performance on the measure, indicating little room
for improvement?
Characteristics Considered for Addition of New Measures
Actionability. Will the measure provide useful or actionable results for state Medicaid and CHIP programs?
Alignment. Is the measure used in other reporting programs?
Appropriateness for state-level reporting. Has the measure been validated and tested for state-level reporting? Is
it currently used by one or more states?
Feasibility. Will states be able to access the data needed to calculate the measure? Would technical assistance be
necessary or helpful to facilitate complete and accurate reporting of the measure by states?
Strategic priority. Does the measure fill a gap that has been identified in the Child or Adult Core Sets?
Workgroup members convened in person from May 7 to 9, 2019, to review 14 existing Core Set
measures suggested for removal from the 2020 Core Sets and 42 measures suggested for
addition. The 56 measures were presented, discussed, and voted on by domain.
3
To be
recommended for removal from or addition to the Core Sets, at least two-thirds of the eligible
Workgroup members were required to vote in favor of removal or addition. In summary, the
Workgroup recommended:
• Removal of 4 measures from the Child Core Set out of a total of 5 measures suggested for
removal
• Removal of 3 measures from the Adult Core Set out of a total of 9 measures suggested for
removal
• Addition of 5 measures to the Child and Adult Core Sets out of a total of 42 measures
suggested for addition
Exhibits ES.2 and ES.3 show the measures recommended for removal or addition, respectively.
3
The measures were organized by the following domains: Primary Care Access and Preventive Care, Maternal and
Perinatal Health, Care of Acute and Chronic Conditions, Behavioral Health Care, Dental and Oral Health
Services, Experience of Care: Patient-Reported Outcomes, Long-Term Services and Supports, and Other
Measures.

ix
Exhibit ES.2. Summary of Workgroup Recommendations of Measures to Remove from
the 2020 Core Sets
Measure Name Measure Steward NQF # (if endorsed)
Recommended for Removal from the Child Core Set
Child and Adolescents’ Access to Primary Care
Practitioners (CAP-CH)
National Committee for
Quality Assurance (NCQA)
Not endorsed
Weight Assessment and Counseling for Nutrition
and Physical Activity for Children/Adolescents—
Body Mass Index Assessment for
Children/Adolescents (WCC-CH)
NCQA 0024
Pediatric Central Line–Associated Bloodstream
Infections (CLABSI-CH)
Centers for Disease Control
and Prevention
0139
Use of Multiple Concurrent Antipsychotics in
Children and Adolescents (APC-CH)
a
NCQA Not endorsed
Recommended for Removal from the Adult Core Set
Adult Body Mass Index Assessment (ABA-AD) NCQA Not endorsed
Comprehensive Diabetes Care: Hemoglobin A1c
(HbA1c) Testing (HA1C-AD)
NCQA 0057
Annual Monitoring for Patients on Persistent
Medications (MPM-AD)
NCQA
2371
b
a
The Workgroup recommended that the APC-CH measure be replaced by another measure: Metabolic Monitoring for
Children and Adolescents on Antipsychotics.
b
This measure is no longer endorsed.
NQF = National Quality Forum.
Exhibit ES.3. Summary of Workgroup Recommendations of Measures to Add to the 2020
Core Sets
Measure Name Measure Steward NQF # (if endorsed)
Appropriate Antibiotic Prophylaxis for Children
with Sickle Cell Anemia
QMETRIC—University of Michigan 3166
Metabolic Monitoring for Children and
Adolescents on Antipsychotics
a
NCQA 2800
Use of Pharmacotherapy for Opioid Use Disorder CMS 3400
National Core Indicators (NCI)
Human Services Research Institute
(HSRI) and National Association of
State Directors of Developmental
Disabilities Services
Not endorsed
National Core Indicators for Aging and Disabilities
(NCI-AD) Adult Consumer Survey
HSRI and National Association of
States United for Aging and
Disabilities
Not endorsed
a
The Workgroup recommended that this measure replace the Use of Multiple Concurrent Antipsychotics in Children
and Adolescents (APC-CH) measure in the Child Core Set.
NQF = National Quality Forum.
This report summarizes the Workgroup’s review process and recommendations. It also includes
the public comments submitted on the draft report. CMCS will use the Workgroup’s
recommendations, as well as public comments, to inform decisions about how and whether to
modify the 2020 Core Sets. CMCS will release the 2020 Core Sets through a CMCS
Informational Bulletin by December 31, 2019.

1
INTRODUCTION
Medicaid and the Children’s Health Insurance Program (CHIP) provided health care coverage to
approximately 73 million people in March 2019, including eligible children, pregnant women,
low-income adults, and individuals with disabilities (Exhibit 1).
4
The Centers for Medicare &
Medicaid Services (CMS) and its Center for Medicaid and CHIP Services (CMCS) use various
tools to ensure that Medicaid and CHIP beneficiaries receive health care coverage that promotes
high quality care. The Medicaid and CHIP Child and Adult Core Sets (Core Sets) of health care
quality measures are key components of this effort.
The goal of the Core Sets is to encourage state
reporting on a uniform set of measures to facilitate
state and national analyses, track performance over
time, and use the results to drive quality
improvement in Medicaid and CHIP. Currently,
state reporting on the Core Sets is voluntary.
The Secretary of the U.S. Department of Health
and Human Services (HHS) is required to review
and update the Child and Adult Core Sets
annually.
5
The annual Core Set review is designed
to identify gaps in existing quality measures and
suggest updates to strengthen and improve the
Core Sets. The Child Core Set has undergone
annual reviews since January 2013 and the Adult
Core Set since January 2014.
CMCS contracted with Mathematica to convene the 2020 Child and Adult Core Set Annual
Review Stakeholder Workgroup (Workgroup). The Workgroup included 28 members, who
represented a diverse set of stakeholders based on their affiliation, subject matter expertise, and
quality measurement and improvement experience (see inside front cover). The Workgroup was
charged with assessing the 2019 Core Sets and recommending measures that should be removed
as well as new measures that should be added, in order to strengthen and improve the Core Sets
for 2020. The Workgroup was asked to focus on measures that were feasible for state reporting
and that could be used to meaningfully drive quality improvement in Medicaid and CHIP.
This report provides an overview of the Child and Adult Core Sets, describes the 2020 Core Set
annual review process, shares state perspectives on Core Set reporting, summarizes the
Workgroup recommendations for improving the Core Sets, and presents the public comments
submitted about the draft report. CMCS will use the Workgroup’s recommendations, as well as
4
March 2019 Medicaid and CHIP Enrollment Data Highlights are available at
https://www.medicaid.gov/medicaid/program-information/medicaid-and-chip-enrollment-data/report-
highlights/index.html. Numbers reflect Medicaid and CHIP enrollment data as of March 2019, as reported by 50
states and the District of Columbia.
5
The Children’s Health Insurance Program Reauthorization Act of 2009 (CHIPRA) calls for annual updates to the
Child Core Set. The Affordable Care Act calls for annual updates to the Adult Core Set.
Source: 2017 CMS Actuarial Report (2016 data).
Aged
(65+)
8%
Children
(nondisabled),
40%
Adults
(nondisabled),
37%
Individuals
with
disabilities,
15%
Exhibit 1. Distribution of Medicaid
Population, 2016
2
public comments, to inform decisions about how and whether to modify the 2020 Core Sets.
CMCS will release the 2020 Core Sets through a CMCS Informational Bulletin by December 31,
2019.
OVERVIEW OF THE CHILD AND ADULT CORE SETS
The Children’s Health Insurance Program Reauthorization Act of 2009 (CHIPRA) included
several provisions aimed at improving the quality of health care for children in Medicaid and
CHIP. CHIPRA required the HHS Secretary to identify and publish a core set of children’s
health care quality measures for voluntary use by state Medicaid and CHIP programs (referred to
as the Child Core Set). The initial Child Core Set, which was released in December 2009,
included 24 measures that covered both physical and mental health. The core set of health care
quality measures for adults covered by Medicaid (Adult Core Set) was established in 2010 under
the Patient Protection and Affordable Care Act (Affordable Care Act) in the same manner as the
Child Core Set. The initial Adult Core Set, which was released in January 2012, included 26
measures.
Appendix A contains tables showing the 2019 Child and Adult Core Set measures and the
history of measures included in the Child and Adult Core Sets from 2012 to 2019. Of the 26
measures in the 2019 Child Core Set, two-thirds were part of the initial Child Core Set.
Similarly, of the 33 measures in the 2019 Adult Core Set, two-thirds were part of the initial
Adult Core Set.
The 2019 Child Core Set
The 2019 Child Core Set includes 26 measures across six domains: (1) Primary Care Access and
Preventive Care, (2) Maternal and Perinatal Health, (3) Care of Acute and Chronic Conditions,
(4) Behavioral Health Care, (5) Dental and Oral Health Services, and (6) Experience of Care.
Nearly two-thirds of the 2019 Child Core Set measures fall into the Primary Care Access and
Preventive Care and Maternal and Perinatal Health domains (Exhibit 2). Seventy-three percent
are process measures and 85 percent can be calculated using administrative data only.

3
Exhibit 2. 2019 Child Core Set Measures, by Domain
For federal fiscal year (FFY) 2017 reporting, the most recent year for which data are publicly
available, all states voluntarily reported on at least one Child Core Set measure and 45 states
reported on at least half of the 26 measures in the 2017 Child Core Set. Twenty-one states
reported on more measures for FFY 2017 than for FFY 2016, and 47 states reported on both
Medicaid and CHIP populations. The median number of measures reported by states was 18.
Historically, the Child Core Set measures that are most frequently reported by states are related
to preventive dental services and primary care access and preventive care.
6
The 2019 Adult Core Set
The 2019 Adult Core Set includes 33 health care quality measures across five domains: (1)
Primary Care Access and Preventive Care, (2) Maternal and Perinatal Health, (3) Care of Acute
and Chronic Conditions, (4) Behavioral Health Care, and (5) Experience of Care. Two-thirds of
the measures are related to care of acute and chronic conditions and behavioral health care
(Exhibit 3). Seventy percent are process measures, and 88 percent can be calculated using
administrative data.
6
More information about the Child Core Set is available at https://www.medicaid.gov/medicaid/quality-of-
care/performance-measurement/child-core-set/index.html.
10
7
2
4
2
1
0
2
4
6
8
10
Primary Care Access
and Preventive Care
Maternal and Perinatal
Health
Care of Acute
and Chronic Conditions
Behavioral
Health Care
Dental and Oral Health
Services
Experience of Care
Core Set Domains

4
Exhibit 3. 2019 Adult Core Set Measures, by Domain
For FFY 2017 reporting, 45 states voluntarily reported on at least one Adult Core Set measure,
while 34 states reported on at least half of the 30 measures in the 2017 Adult Core Set. This
included 4 states that reported for the first time. Thirty-three states reported more measures for
FFY 2017 than for FFY 2016, with states reporting a median of 17 measures. Historically, the
Adult Core Set measures most frequently reported by states are spread across the domains.
7
Use of the Core Set for Quality Measurement and Improvement
CMCS and states use the Child and Adult Core Sets to monitor and improve the quality of care
provided to Medicaid and CHIP beneficiaries at the national and state levels and to measure
progress over time. CMCS publicly reports information on state performance on the Child and
Adult Core Sets annually through chart packs and other resources.
8
In addition, CMCS develops
initiatives to drive improvement in quality of care by using the Core Set measures—for example,
through its Maternal and Infant Health Initiative and Oral Health Initiative.
9
A subset of Core Set
7
More information about the Adult Core Set is available at https://www.medicaid.gov/medicaid/quality-of-
care/performance-measurement/adult-core-set/index.html.
8
Chart packs, measure-specific tables, fact sheets, and other information from annual Core Set reporting are
available at
https://www.medicaid.gov/medicaid/quality-of-care/performance-measurement/child-core-
set/index.html and https://www.medicaid.gov/medicaid/quality-of-care/performance-measurement/adult-core-
set/index.html.
9
More information about Medicaid and CHIP quality improvement initiatives is available at
https://www.medicaid.gov/medicaid/quality-of-care/index.html.

5
measures is also included in the Medicaid and CHIP Scorecard to increase public transparency in
state health system performance.
10
To support states and their partners in collecting, reporting, and using the Core Set measures to
drive improvement in Medicaid and CHIP, CMCS established a Technical Assistance and
Analytic Support (TA/AS) Program. The TA/AS program provides one-on-one assistance to
address technical issues related to collecting the Core Set measures, offers group trainings and
collaborative learning opportunities, prepares issue briefs and analytic reports, and helps states to
design and implement quality improvement initiatives that use the Core Set measures.
11
DESCRIPTION OF THE 2020 CORE SET ANNUAL REVIEW
PROCESS
This section describes the 2020 Core Set annual review process, including the call for
nominations for Workgroup members, the Workgroup composition, and the Workgroup timeline
and meetings.
Call for Nominations
Mathematica issued a call for nominations on December 14, 2018; nomination forms and a
resume or CV were due on January 11, 2019. Mathematica distributed the call for nominations
electronically to a wide range of state Medicaid and CHIP officials, health care provider
associations, and quality measurement experts. Mathematica received 64 nominations.
Nominations were reviewed to address legislative requirements for the Core Set annual review,
to ensure geographic distribution, and to represent diverse areas of expertise.
12
Workgroup members were required to submit a Disclosure of Interest form to report any
interests, relationships, or circumstances over the past four years that could give rise to a
potential conflict of interest or the appearance of a conflict of interest related to the Child and
Adult Core Set measures or measures reviewed during the Workgroup process. Workgroup
members who were deemed to have an interest in a measure recommended for consideration
were required to recuse themselves from voting on that measure.
10
More information about the Medicaid and CHIP Scorecard is available at https://www.medicaid.gov/state-
overviews/scorecard/index.html.
11
More information about the CMCS TA/AS Program is available at https://www.medicaid.gov/medicaid/quality-
of-care/downloads/tafactsheet.pdf.
12
The statute requires representation from states, medical and dental professionals (including members of allied
health professions), providers caring for children and families who live in medically underserved urban and rural
communities, national organizations serving children and those with chronic conditions, consumers and
purchasers of health care, and experts in quality measures, as well as voluntary consensus standards-setting
organizations and other organizations involved in the advancement of evidence-based measures of health care.

6
Workgroup Composition
The Workgroup included 28 voting members from state Medicaid agencies, professional
associations, universities, hospitals, and other organizations from across the country (the
Workgroup members are listed on the inside front cover of this report).
13
As a whole, the
Workgroup represented expertise in primary care access and preventive care, acute and chronic
conditions, maternal and perinatal health, behavioral health and substance use, dental and oral
health, long-term services and supports, disability, experience of care, patient safety, and health
disparities. Although some Workgroup members were nominated by an organization, all
Workgroup members were asked to participate as subject matter experts and consider what
measures would be best for improving the quality of care in Medicaid and CHIP overall, and not
to advocate on behalf of an organization or a specific interest.
The Workgroup also included non-voting federal liaisons, who represented eight federal agencies
(see front cover). The inclusion of federal liaisons reflects CMCS’s commitment to promoting
quality measurement alignment and working in partnership with other agencies to collect, report,
and use the Core Set measures to drive improvement in Medicaid and CHIP.
Workgroup Timeline and Meetings
As shown in Exhibit 4, Mathematica held two webinars in February and April 2019 to orient the
Workgroup members and to prepare for the in-person Workgroup meeting, which was convened
in May 2019. The two webinars and the in-person meeting were open to the public and public
comment was invited at multiple points.
The draft report was made available for public comment from July 8, 2019 through August 5,
2019. Forty public comments were submitted. See Appendix C for more information on the
public comments received on the draft report. CMCS will release the 2020 Core Sets by
December 31, 2019, after taking into account Workgroup recommendations and public
comments.
13
Three additional members were selected but were unable to participate due to conflicts with their schedules.
7
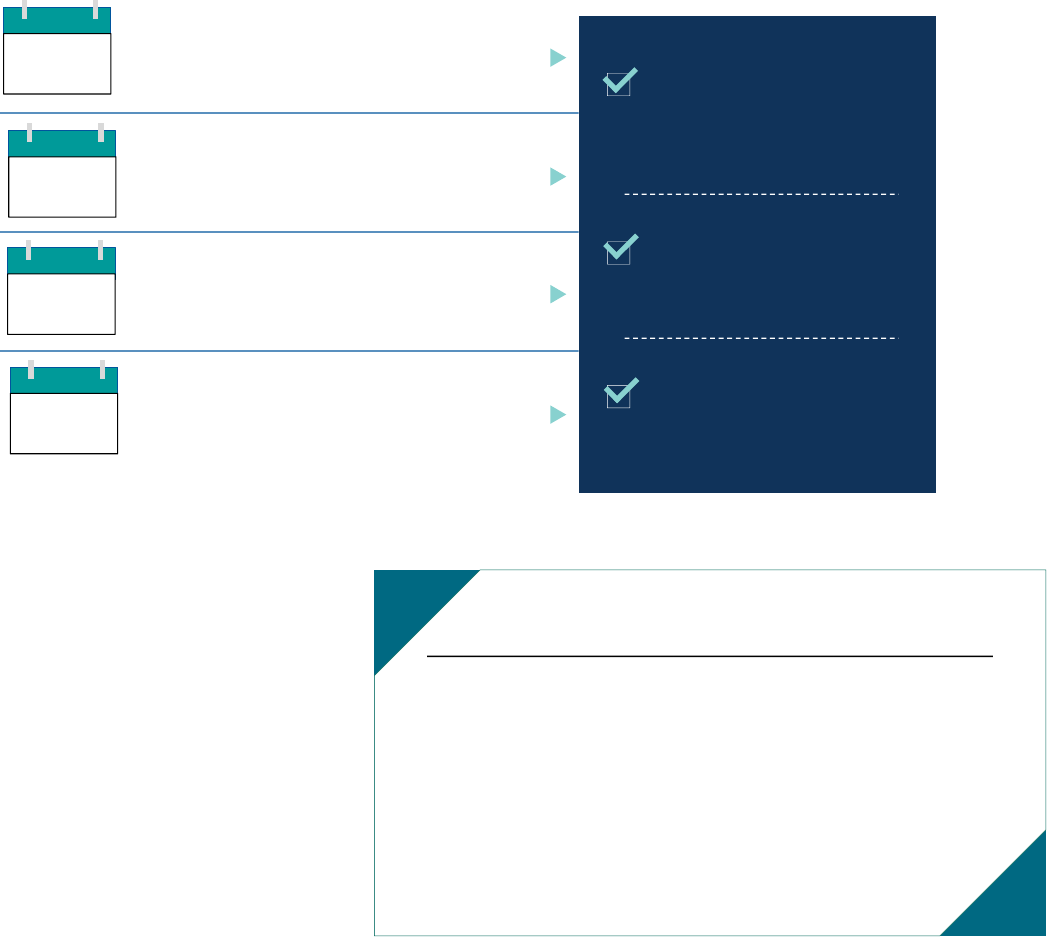
Exhibit 4. 2020 Core Set Annual Review Stakeholder Workgroup Timeline
Orientation Webinar
During the orientation webinar on
February 14, 2019, Mathematica
stated the Workgroup charge,
introduced the Workgroup members
and disclosure of interest process,
described the timeline for the 2020
annual review, and provided
background on the Core Sets. In
addition, CMCS outlined its goals
for state reporting of the Core Sets:
1. Increase the number of states
reporting the measures
2. Increase the number of measures
reported by each state
3. Improve the quality of the data reported by states
4. Streamline the Core Set data collection and reporting processes
5. Use the data to drive improvements in health care quality and outcomes
Mathematica explained the process for Workgroup members to suggest measures for removal
from or addition to the Child and Adult Core Sets. The Workgroup was charged with focusing on
measures that met the following criteria:
• Actionable. Results can be used to improve care delivery and health outcomes.
FEB
14
Orientation webinar
Measure recommendations due
Webinar to prepare for
in-person meeting
In-person meeting
July 2019:
Draft report made available
for public comment
August 2019:
Final report released
By December 31, 2019:
2020 Core Sets released
MAR
8
APR
23
M AY
7-9
Workgroup Charge
The Child and Adult Core Set Stakeholder Workgroup for the 2020
Annual Review is charged with assessing the 2019 Core Sets and
recommending measures for removal or addition, in order to
strengthen and improve the Core Sets for 2020.
The Workgroup should focus on measures that are actionable,
aligned, and appropriate for state-level reporting to ensure that the
measures can meaningfully drive improvement in quality of care
and outcomes in Medicaid and CHIP.

8
• Aligned. Measures are aligned with those used in other programs to minimize burden on states,
health plans, and providers where possible.
• Appropriate. The technical specifications, data collection methods, and data sources are
validated for state use or can be easily adapted by states.
Following the orientation meeting, Workgroup members were invited to suggest measures for
removal from or addition to the 2020 Core Sets. Workgroup members used an online tool to
provide their suggestions for removal or addition, including the rationale and whether measures
suggested for addition were intended to substitute for a current Core Set measure. Workgroup
members suggested the following:
• Fourteen measures for removal, including 5 of the 26 measures in the 2019 Child Core Set
and 9 of the 33 measures in the 2019 Adult Core Set
• Forty-two measures for addition across the six current Core Set domains,
14
as well as two
new domains related to Long-Term Services and Supports (LTSS) and Other Measures
Webinar to Prepare for the In-Person Meeting
The second webinar took place on April 23, 2019. To help Workgroup members prepare for the
discussion at the in-person meeting, Mathematica shared a list of the 14 measures suggested for
removal and the 42 measures suggested for addition. Mathematica provided guidance on how to
prepare for the measures discussion at the in-person meeting, including the criteria that
Workgroup members should consider for recommending measures for removal from or addition
to the Core Sets and the resources available to facilitate their review. These resources included
detailed measure information sheets, a worksheet to facilitate the review and record notes, and a
Medicaid and CHIP beneficiary profile. Workgroup members were responsible for reviewing all
materials related to the measures and coming to the meeting prepared to ask questions and
discuss the merits of each measure.
In-Person Meeting
The in-person meeting took place in Washington, D.C., May 7-9, 2019. Workgroup members,
federal liaisons, measure stewards, and members of the public attended the meeting. Measure
stewards and members of the public were also able to participate virtually via webinar.
Before discussing individual measures for removal from or addition to the Core Sets, the state
representatives serving on the Workgroup shared their experiences with Core Set reporting. The
discussion helped other Workgroup members better understand how states use the Core Set
measures and their approach to collecting data and calculating measures.
The discussion of measures was organized into eight domains: the six current Core Set domains
plus LTSS and Other Measures. For each domain, Mathematica described the measures
14
The current domains are Primary Care Access and Preventive Care, Maternal and Perinatal Health, Care of Acute
and Chronic Conditions, Behavioral Health Care, Dental and Oral Health Services, and Experience of Care.

9
suggested for removal or addition, highlighted the key technical specifications, and summarized
the rationale Workgroup members provided for suggesting the measures for removal or addition.
The Workgroup co-chairs facilitated the discussion of the measures. They sought technical
questions from Workgroup members and asked the measure stewards to clarify measure
specifications when needed. The Workgroup members then discussed the measures suggested for
removal or addition in each domain. The co-chairs accepted motions to vote on the measures in
each domain, and there were opportunities for public comment on the measures suggested for
removal or addition. Public comments were accepted in person and via telephone.
Mathematica facilitated the voting on the measures. Workgroup members voted by using
iClicker devices, with voting results presented in real time. For each measure suggested for
removal, Workgroup members could select either “A = Yes, I recommend removing this
measure from the Core Set” or “B = No, I do not recommend removing this measure from the
Core Set.” For each measure recommended for addition, Workgroup members could select either
“A = Yes, I recommend adding this measure to the Core Set” or “B = No, I do not recommend
adding this measure to the Core Set.” Measures were recommended for removal or addition if
two-thirds of the eligible Workgroup members voted yes.
15
STATE PERSPECTIVES ON CORE SET REPORTING
Mathematica invited the Workgroup member from New York’s Medicaid agency to present on
the state’s experience with collecting, reporting, and using the Core Set measures, as well as on
its performance measurement priorities. In addition to programming claims and administrative
measures internally, the state leverages managed care reporting and collates information from
managed care organizations (MCOs) to report almost all of the Core Set measures. Key themes
from the presentation included the following:
• Effort and resources. New York devotes a high level of effort and resources to implement,
report, and maintain Core Set measures, even administrative measures. It can take several
years to get new Core Set measures up and running; even small tweaks to existing measures
require substantial staff effort.
• Types of measures. The state uses Healthcare Effectiveness Data and Information Set
(
HEDIS®) measures to benchmark internal measure calculations. Measures that are not part
of the HEDIS measure set are more difficult to benchmark because they are not audited like
HEDIS measures are. The representative also noted that provider-based measures (such as
those developed for hospitals) are more difficult for the state to collect and report.
• Measure alignment. Aligning measures with other programs is important. The state looks
for measures that support its existing initiatives, such as Medicaid Section 1115
demonstrations, as well as measures that can be monitored across all types of health
insurance (commercial and public). Alignment helps to drive measure prioritization by the
state, health plans, and provider organizations.
15
Workgroup members who disclosed an interest in a measure were recused from voting on that measure, for
example, if they were a measure developer, a measure steward, or paid to promote a measure in some way.
10
• Future priorities. Moving forward, New York is looking to end medical record review and
to more fully integrate electronic data, such as measure results from health information
exchanges, into its efforts. Use of electronic data will facilitate the state’s focus on
population health management and clinical care.
The Workgroup included representatives from eight other state Medicaid/CHIP agencies:
Arizona, California, Delaware, Florida, Massachusetts, Minnesota, Oklahoma, and Pennsylvania.
They also shared their on-the-ground experiences with reporting the current Core Set measures,
monitoring other measures as part of their state quality improvement programs, and using this
information to inform programmatic and policy decisions. In this context, the states noted the
importance of using data to understand subpopulations, including age groups, racial and ethnic
groups, and rural versus urban experiences. State representatives also focused on the feasibility
and burden of collecting and reporting measures, particularly when there are substantive changes
from year to year.
The state perspectives provided important context for the Workgroup discussion of individual
measures. Non-state Workgroup members frequently called on state representatives for insights
about their experiences with measures suggested for removal or their assessment of the
feasibility and usability of measures suggested for addition.
WORKGROUP RECOMMENDATIONS FOR IMPROVING THE
2020 CORE SETS
Criteria Considered by the Workgroup
The 2020 Core Set Annual Review Workgroup considered 56 measures, including 14 measures
suggested for removal and 42 measures suggested for addition. To guide the discussion and
voting, Workgroup members were asked to consider the “fit” of each individual measure for the
Core Set according to a series of characteristics introduced in the orientation webinar (see
Exhibit 5). Additional principles that guided the discussion and voting for measure removal or
addition included the following:
• There is no target number, or a minimum or maximum number, of measures that should be
included in the Core Sets.
• States should have the capacity and data available to report the measures; otherwise, data will
be limited and incomplete if measures cannot be reported by a majority of states.
• The importance of each individual measure should be considered without regard to the
relative importance of measures within and across domains.
• The merits of each individual measure should be assessed based on the current technical
specifications. Voting was based on the current measure; no suggestions for modifications
were allowed.
• The measures should be assessed without regard to whether they will be in the Child Core
Set or the Adult Core Set or what domain they will be in, because these decisions will be
made by CMCS.

11
Exhibit 5. Characteristics Considered for Removal of Existing Measures and Addition of
New Measures
Characteristics Considered for Removal of Existing Measures
Actionability. Does the measure provide few useful or actionable results for state Medicaid and CHIP programs?
Clinical relevance. Does the measure no longer adhere to clinical evidence or guidelines?
Feasibility. Have states reported significant challenges to reporting the measure (such as barriers to accessing or
using data needed to report the measure)?
New or alternate measure. Is another measure being recommended to replace an existing Core Set measure?
Performance. Have states consistently reported a high level of performance on the measure, indicating little room
for improvement?
Characteristics Considered for Addition of New Measures
Actionability. Will the measure provide useful or actionable results for state Medicaid and CHIP programs?
Alignment. Is the measure used in other reporting programs?
Appropriateness for state-level reporting. Has the measure been validated and tested for state-level reporting? Is
it currently used by one or more states?
Feasibility. Will states be able to access the data needed to calculate the measure? Would technical assistance be
necessary or helpful to facilitate complete and accurate reporting of the measure by states?
Strategic priority. Does the measure fill a gap that has been identified in the Child or Adult Core Sets?
Summary of Workgroup Recommendations
The Workgroup recommended the removal of four measures from the Child Core Set, the
removal of three measures from the Adult Core Set (Exhibit 6), and the addition of five measures
to the Core Sets (Exhibit 7). This section summarizes the discussion and rationale for the
measures recommended for removal from or addition to the 2020 Core Sets. Additional
information on the measures not recommended for removal from or addition to the Core Sets is
included in Appendix B.
Exhibit 6. Summary of Workgroup Recommendations of Measures to Remove from the
2020 Core Sets
Measure Name Measure Steward NQF # (if endorsed)
Recommended for Removal from the Child Core Set
Child and Adolescents’ Access to Primary Care
Practitioners (CAP-CH)
National Committee for
Quality Assurance (NCQA)
Not endorsed
Weight Assessment and Counseling for Nutrition
and Physical Activity for Children/Adolescents—
Body Mass Index Assessment for
Children/Adolescents (WCC-CH)
NCQA 0024
Pediatric Central Line–Associated Bloodstream
Infections (CLABSI-CH)
Centers for Disease Control
and Prevention
0139
Use of Multiple Concurrent Antipsychotics in
Children and Adolescents (APC-CH)
a
NCQA Not endorsed

12
Measure Name Measure Steward NQF # (if endorsed)
Recommended for Removal from the Adult Core Set
Adult Body Mass Index Assessment (ABA-AD) NCQA Not endorsed
Comprehensive Diabetes Care: Hemoglobin A1c
(HbA1c) Testing (HA1C-AD)
NCQA 0057
Annual Monitoring for Patients on Persistent
Medications (MPM-AD)
NCQA
2371
b
a
The Workgroup recommended that the APC-CH measure be replaced by another measure: Metabolic Monitoring for
Children and Adolescents on Antipsychotics.
b
This measure is no longer endorsed.
NQF = National Quality Forum.
Exhibit 7. Summary of Workgroup Recommendations of Measures to Add to the 2020
Core Sets
Measure Name Measure Steward NQF # (if endorsed)
Appropriate Antibiotic Prophylaxis for Children
with Sickle Cell Anemia
QMETRIC— University of
Michigan
3166
Metabolic Monitoring for Children and
Adolescents on Antipsychotics
a
NCQA 2800
Use of Pharmacotherapy for Opioid Use
Disorder
CMS 3400
National Core Indicators (NCI)
Human Services Research
Institute (HSRI) and National
Association of State Directors of
Developmental Disabilities
Services
Not endorsed
National Core Indicators for Aging and
Disabilities (NCI-AD) Adult Consumer Survey
HSRI and National Association of
States United for Aging and
Disabilities
Not endorsed
a
The Workgroup recommended that this measure replace the Use of Multiple Concurrent Antipsychotics in Children
and Adolescents (APC-CH) measure in the Child Core Set.
NQF = National Quality Forum.
Measures Recommended for Removal from the Child Core Set
Child and Adolescents’ Access to Primary Care Practitioners (CAP-CH)
The CAP-CH measure assesses the percentage of children and adolescents who had a visit with a
primary care practitioner (PCP). Four rates are reported: children ages 12 to 24 months and 25
months to age 6 who had a visit with a PCP during the measurement year; and children ages 7 to
11 and adolescents ages 12 to 19 who had a visit with a PCP during the measurement year or the
year prior to the measurement year. Forty-eight states reported this measure for FFY 2017.
The Workgroup member who suggested the measure for removal indicated that the measure does
not provide useful or actionable results for state Medicaid and CHIP agencies; the measure uses
a very broad definition of primary care visits, which makes it more a utilization measure than a
quality measure. The member stated that true access to primary care involves a well-care visit,
which is already covered by three Child Core Set well-care measures.
Another Workgroup member noted that relatively high performance on the access to care

13
measure does not necessarily correlate with children actually receiving the recommended well-
child care. It was suggested that the three well-child visit measures in the 2019 Child Core Set
could serve as substitutes for this measure. Finally, the measure steward, the National Committee
for Quality Assurance (NCQA), proposed retiring the measure in 2018, which raised concerns
for the Workgroup about whether the measure would be maintained and updated if NCQA
retired the measure and it remained in the Core Set.
16
Weight Assessment and Counseling for Nutrition and Physical Activity for
Children/Adolescents—Body Mass Index Assessment for
Children/Adolescents (WCC-CH)
The WCC-CH measure assesses the percentage of children ages 3 to 17 who had a visit with a
PCP or OB/GYN practitioner and whose body mass index (BMI) percentile was documented in
the medical record. This measure documents evidence of BMI measurement only, and does not
include a counseling component. Thirty-seven states reported this measure for FFY 2017.
The Workgroup members who suggested the measure for removal described it as a
documentation measure that does not provide useful or actionable results for state Medicaid and
CHIP agencies. They further asserted that this measure does not reflect evidence-based practices
for interventions for children with or at risk of obesity. One Workgroup member also noted that
the data collection burden for this measure does not support its use, particularly because the
measure does not support an evidence-based practice.
17
Workgroup members also noted that, although state focus on childhood obesity is critical, the
clinical evidence to support the measure is lacking. One Workgroup member asserted that more
integrated and effective models to address obesity than screening alone, such as referrals to care,
should be prioritized in the Core Set. It was also noted that this measure is reported under the
Promoting Interoperability Program (formerly the Electronic Health Record [EHR] Incentive
Program), so removal of the measure from the Child Core Set would not disincentivize
physicians from conducting a BMI assessment.
One federal liaison voiced support for the child and adult BMI screening measures, noting there
is evidence to support BMI screening in the primary care setting and that BMI screening is part
of the U.S. Preventive Services Task Force (USPSTF) recommendations for both children and
adults. The commenter also cautioned about sending a signal about the low priority of this topic
if the WCC-CH measure is removed from the Child Core Set without a replacement.
Pediatric Central Line–Associated Bloodstream Infections (CLABSI-CH)
The CLABSI-CH measure assesses the number of CLABSIs in neonatal intensive care units
(ICUs) reported by acute care hospitals. The standardized infection ratios reported for each state
compare the observed number of infections reported during the measurement period to the
predicted number of infections for that period. Data for the measure are reported by hospitals to
16
Although the CAP measure was proposed for retirement from HEDIS 2018, the measure remains in HEDIS 2020
as a result of feedback through stakeholder discussions and public comments.
17
Due to limitations of claims data to calculate this measure, the hybrid data collection method, which uses a
combination of administrative and medical records, is typically required to produce accurate results.
14
the Centers for Disease Control and Prevention (CDC) in the National Health Care Safety
Network. Data reported to CDC are for all payers and not limited to Medicaid and CHIP.
Although the Core Set specifications include both neonatal and pediatric ICUs, CDC only reports
data for neonatal ICUs, so the Core Set data available for this measure include only neonatal
ICUs. CMCS obtains data for this measure directly from CDC each year.
The Workgroup member who suggested the CLABSI-CH measure for removal noted that the
measure does not provide useful or actionable results for state Medicaid and CHIP agencies.
Workgroup members discussed the value of measuring and tracking CLABSIs. One noted that
states have successfully worked across state agencies and with other states to use the data to
achieve reductions in pediatric CLABSIs. At the same time, Workgroup members questioned
whether the statewide data reported for the CLABSI measure were more actionable for state
departments of public health, which typically have regulatory authority over hospitals and
hospital-focused metrics, than for Medicaid agencies. Furthermore, this measure is not limited to
populations covered by Medicaid and CHIP. A Workgroup member from one state explained
that because the Medicaid agency does not have the raw data to focus on Medicaid beneficiaries
or review rates at the county or managed care plan level, it is challenging to use the measure to
drive quality improvement in the Medicaid or CHIP program. Workgroup members also
discussed how removing the measure from the Core Set would not necessarily undermine or
terminate the existing focus in states and departments of public health to continue to use
CLABSI data to improve hospital safety and quality and provide accountability at the state level.
Use of Multiple Concurrent Antipsychotics in Children and Adolescents
(APC-CH)
The APC-CH measure assesses the number of beneficiaries on two or more concurrent
antipsychotic medications for at least 90 consecutive days during the measurement year. Thirty-
seven states reported this measure for FFY 2017. The measure is currently included on the
Medicaid and CHIP Scorecard. This measure will be retired from HEDIS in 2020 and will no
longer be updated and maintained by the measure steward (NCQA).
The Workgroup member who suggested the measure for removal noted that state efforts have led
to high performance on this measure with little room for improvement. In 2017, the median rate
for this measure was 2.7 percent (lower rates are better). Moreover, the number of children in the
denominator has decreased over time, suggesting that the overall number of children on two or
more concurrent antipsychotic medications has decreased. Another measure of appropriate
antipsychotic treatment with a larger denominator, Metabolic Monitoring for Children and
Adolescents on Antipsychotics, was recommended as a replacement for this measure.
Workgroup members suggested that high performance on this measure may indicate that states
have achieved the appropriate level of utilization. Moreover, it was noted that there may be a
clinical justification for a small number of children to be prescribed these medications.
Workgroup members representing states commented that they would continue to track similar
measures, particularly for children in foster care, if this measure is removed from the Core Set.
15
Measures Recommended for Removal from the Adult Core Set
Adult Body Mass Index Assessment (ABA-AD)
The ABA-AD measure assesses the percentage of beneficiaries ages 18 to 74 who had an
outpatient visit and whose BMI was documented in the medical record. Thirty-two states
reported this measure for FFY 2017.
The rationale Workgroup members provided for suggesting removal of this measure was similar
to the rationale for removing the WCC-CH measure from the Child Core Set. As a measure of
documentation, rather than outcomes or evidence-based practices for combatting obesity, ABA-
AD does not assess whether a high BMI value resulted in follow-up services.
During the discussion, Workgroup members noted that this measure was routinely reported
under the Promoting Interoperability Program (formerly the EHR Incentive Program), as BMI is
often collected in EHRs. The Preventive Care and Screening: Body Mass Index Screening and
Follow-Up Plan measure was discussed as a replacement for this measure, which would move
the measure in the direction of treatment rather than documentation. However, this measure was
not ultimately recommended for addition because of concerns about states’ access to data to
calculate the measure, among other factors. One Workgroup member also noted that combatting
obesity may require a broader societal response than other health conditions, which makes it
more challenging for the health care system to address.
As mentioned earlier, one federal liaison voiced support for the child and adult BMI screening
measures, noting that there is evidence to support BMI screening in the primary care setting and
that BMI screening is part of American Academy of Pediatrics and USPSTF recommendations
for both children and adults. The commenter also cautioned about sending a signal about the low
priority of this topic if the ABA-AD measure were removed from the Adult Core Set without a
replacement.
Comprehensive Diabetes Care: Hemoglobin A1c (HbA1c) Testing (HA1C-AD)
The HA1C-AD measure assesses the percentage of beneficiaries ages 18 to 75 with diabetes
(types 1 and 2) who had a hemoglobin A1c (HbA1c) test. Thirty-eight states reported this
measure for FFY 2017.
The Workgroup members who suggested it for removal commented that the high performance on
the measure indicated that there was little room for improvement. They also noted that a measure
currently on the Core Set—Comprehensive Diabetes Care: Hemoglobin A1c (HbA1c) Poor
Control (> 9.0 percent) (HPC-AD)—is an outcome measure that also assesses whether testing is
being conducted. Removing the HA1C-AD measure would reduce state reporting burden without
losing the value of measuring diabetes control.
Two Workgroup members representing states noted that they no longer use this measure because
the HPC-AD measure includes a testing component; further, they want to hold plans accountable
for improved outcomes, rather than just testing. A Workgroup member also noted that the
HA1C-AD and HPC-AD measures are on the Core Set concurrently because not all states were
equipped to report on the HPC-AD measure when it was added. One member expressed concern
about removing this measure without knowing the screening rates in the 12 states that are not
reporting the measure.
16
Annual Monitoring for Patients on Persistent Medications (MPM-AD)
The MPM-AD measure assesses the percentage of beneficiaries age 18 and older who received at
least 180 treatment days of ambulatory medication therapy for a select therapeutic agent during
the measurement year and at least one therapeutic monitoring event for the therapeutic agent in
the measurement year. The therapeutics agents include angiotensin converting enzyme (ACE)
inhibitors or angiotensin receptor blockers (ARB) and diuretics. Thirty-six states reported on this
measure for FFY 2017. This measure will be retired from HEDIS in 2020 and will no longer be
updated and maintained by the measure steward (NCQA).
One Workgroup member recommended this measure for removal because states report high
performance rates on the measure, which indicates that there is little room for improvement. It
was also noted that the measure lost NQF endorsement in 2018.
During the Workgroup discussion, a Workgroup member representing a state noted that the high
performance rates have led them to remove this measure from their pay-for-performance
program. Another member described MPM-AD as a process measure that does not get to
outcomes.
Measures Recommended for Addition to the 2020 Core Sets
Appropriate Antibiotic Prophylaxis for Children with Sickle Cell Anemia
This measure assesses the percentage of children ages 3 months to 5 years who were identified as
having sickle cell anemia and who received appropriate antibiotic prophylaxis during the
measurement year.
One Workgroup member suggested this measure for addition because individuals with sickle cell
anemia, particularly infants and young children, are susceptible to life-threatening infections.
Antibiotic prophylaxis is a relatively easy and inexpensive care pathway that is underutilized.
During the discussion, the Workgroup compared this measure to another sickle cell measure
suggested for addition, Transcranial Doppler Ultrasonography Screening for Children with
Sickle Cell Anemia.
During the discussion, the Workgroup generally favored the antibiotic prophylaxis measure from
a clinical perspective, with members characterizing it as a measure of continuity of chronic
disease care that should be universally performed in all situations—compared to a transcranial
Doppler ultrasonography, which is a one-time screening that has to be linked to further
downstream processes. Workgroup members noted the disparities in the use of antibiotic
prophylaxis treatment and the opportunity for improvement. One Workgroup member also noted
that this was an administrative claims-based measure, so it was feasible for states to collect and
report. Finally, one Workgroup member noted that, because sickle cell anemia is a genetic
disease, the prevalence varies by state; therefore, this measure may or may not be a high priority
for states based on the size of their affected population.
Metabolic Monitoring for Children and Adolescents on Antipsychotics
This measure assesses the percentage of children and adolescents age 1 to 17 who had at least
two antipsychotic medication dispensing events of the same or different medication and had
monitoring for the development of abnormal cholesterol and blood sugar levels, which are
17
known side effects of these medications. An updated version of the measure is currently under
consideration that would combine the 1- to 5-year-old and 6- to 11-year-old age groups and add
separate rates for blood glucose and cholesterol. This measure was recommended to replace the
Use of Multiple Concurrent Antipsychotics in Children and Adolescents (APC-CH) measure,
which was recommended for removal from the Child Core Set.
The Workgroup member who suggested this measure noted that it would help states monitor
children on multiple concurrent antipsychotics (children previously identified by the APC-CH
measure) by identifying any gaps in their metabolic follow-up. The Workgroup member noted
that the Medicaid HEDIS national average for appropriate monitoring for children on these
medications was 34 percent in 2017, which suggests a gap in the quality of care provided to these
children.
One Workgroup member commented that this is one of the few measures that monitors
medication safety for children on psychotropic medications. In addition, the denominator for this
measure is larger than the denominator for APC-CH, which this measure was recommended to
replace.
Use of Pharmacotherapy for Opioid Use Disorder
This measure assesses the percentage of Medicaid beneficiaries ages 18 to 64 with an opioid use
disorder (OUD) who filled a prescription for, or were administered or ordered, a Food and Drug
Administration–approved medication for the disorder during the measurement year.
The Workgroup member who suggested this measure noted that it would fill a current gap in the
Core Sets by tracking the appropriate treatment of OUDs, which is a critical step in curbing the
national OUD epidemic.
One Workgroup member noted that while this measure does not assess treatment adherence, it
does provide information about the number of people initiating medication assistance treatment,
which is a good first step. Other members expressed that continuity of treatment is equally
important.
National Core Indicators (NCI) and National Core Indicators for Aging and
Disabilities (NCI-AD)
The NCI and NCI-AD assess the experience and outcomes of individuals with intellectual and
developmental disabilities and their families, and seniors and adults with physical disabilities,
respectively. Both are voluntary efforts undertaken by state developmental disabilities agencies
(NCI) and state Medicaid aging and disability agencies (NCI-AD).
• NCI surveys assess the experience of people who receive services from state developmental
disabilities agencies. It includes an in-person survey, family surveys for parents and
guardians of adults and children who receive supports, and a staff stability survey. Indicators
address key areas of concern in five domains: (1) individual outcomes; (2) health, welfare,
and rights; (3) system performance; (4) staff stability; and (5) family outcomes. Forty-six
states participate in the NCI program; 37 states collected data through NCI’s in-person
survey in 2018-2019.
18
• NCI-AD core indicators are standard measures used across states to assess the outcomes of
services provided to seniors and adults with physical disabilities. Indicators address 19 key
areas of concern including (1) service coordination, (2) rights and respect, (3) community
participation, (4) choice and decision-making, (5) health care, (6) safety, (7) relationships, (8)
satisfaction, (9) care coordination, (10) access to community, (11) access to needed
equipment, (12) wellness, (13) medications, (14) self-direction, (15) work, (16) everyday
living, (17) affordability, (18) control, and (19) person-centered planning. Seventeen states
collected NCI-AD data in 2018-2019.
The Workgroup member who suggested the measures noted that they would fill a gap in the Core
Sets related to LTSS for people with intellectual and developmental disabilities or for those who
use home- and community-based services (HCBS). Workgroup members acknowledged that
LTSS accounts for a substantial portion of Medicaid expenditures and that a large portion of
Medicaid beneficiaries use these services.
Workgroup members discussed the fact that many states are currently using the NCI and NCI-
AD measures; whereas other proposed measures, such as the Consumer Assessment of
Healthcare Providers and Systems Home and Community Based Services (HCBS CAHPS),
survey are newer. Workgroup members with experience using the NCI and NCI-AD measures in
their states articulated several advantages:
• Both sets of indicators have provided actionable results to states on beneficiary outcomes in
terms of function and well-being. In addition to calculating state-level rates, some states
oversample to assess performance for subpopulations within the state, including performance
by managed care plan, provider, region, and county. One Workgroup member reported that
her state was able to use the individual-level survey results to implement quality
improvement activities that made a difference in people’s lives.
• Both sets of indicators are aligned with measures used for other reporting programs and can
be used for both managed care and fee-for-service populations.
• Both sets of indicators have been tested and are believed to be valid and reliable, with strong
inter-rater reliability. In addition, technical assistance is available to states for implementing
the surveys. Multiple years of comparative data are available so that states can benchmark
their performance and progress.
Workgroup members acknowledged that adding new survey-based measures to the Core Set
would require states to either add requirements for these surveys to managed care contracts or to
field them directly. At the same time, measures from other data sources that assess the
experience of beneficiaries and their functional status and well-being are not currently available.
The NCI and NCI-AD surveys are accessible in multiple languages to people with disabilities
who are nonverbal, blind, deaf, or have other disabilities; both are also available in Spanish.
Cross-Cutting Themes in Measure Discussions
Several cross-cutting themes emerged from the Workgroup discussions about measures
suggested for removal or addition:
19
• Feasibility. One of the strongest considerations that Workgroup members expressed was the
feasibility for states to collect and report the measures. Throughout the meeting, Workgroup
members asked colleagues representing state Medicaid programs for their opinions on the
feasibility of measures. It was noted that feasibility varies by state, particularly related to
whether states have managed care delivery systems and are able to leverage MCOs to support
Core Set reporting. The Workgroup noted that measures that use already available
administrative data or measures used for other purposes (such as in pay for performance
programs) are more feasible. As part of this discussion, some Workgroup members
representing states expressed concern about survey-based measures due to the high level of
effort and resources required to administer surveys.
• Appropriateness. Workgroup members highlighted that the most appropriate measures for
the Core Sets are those in which state-to-state comparisons would be helpful in monitoring
the quality of care in Medicaid and CHIP. Given variations in state Medicaid programs and
delivery systems, the results of some measures may not be meaningful when compared
across states. Other measures may be more appropriate to monitor at the plan or provider
levels. Workgroup members repeatedly stressed that nothing about the value of the measure
or the importance of the topic area should be inferred from the decision not to recommend it
for addition to the Core Sets.
• Readiness. Workgroup members discussed whether measures were ready for implementation
in the Core Sets and for Medicaid and CHIP. For example, there were concerns about using
first-year HEDIS measures and measures that had not been tested for use in state Medicaid
programs. Workgroup members commented that the Core Sets are not the place to put new
measures or measures untested at the state level.
• Actionability. Workgroup members noted the importance of ensuring that Core Set measures
are actionable, that is, that CMCS and states can use the data to inform program and policy
decisions and to improve the quality of care for Medicaid and CHIP beneficiaries. There was
ample discussion in support of working toward moving from process to outcome measures as
they become feasible and ready. Outcome measures are necessary to more fully understand
the quality of care provided to Medicaid and CHIP beneficiaries.
Discussion of Core Set Measure Gaps
The Workgroup discussed improving the Core Sets by taking a holistic approach to measuring
the quality of care provided to diverse populations and subpopulations enrolled in Medicaid and
CHIP. Workgroup members frequently cited the need to address issues related to social
determinants of health as a gap area in the Core Sets, either as measures themselves or to risk
adjust measures for valid comparison. Workgroup members noted that the measures in the Core
Set are heavily focused on medical care, whereas Medicaid programs provide wraparound
services that are not being captured by the current Core Set measures. Workgroup members
acknowledged feasibility challenges for measuring and addressing the social determinants of
health; however, they suggested that CMCS, measure stewards, and states work together to
promote inclusion of such measures in quality measurement efforts.
Workgroup members expressed their preference for having a gap in the Core Set rather than
recommending measures that did not meet the specified criteria, and especially to avoid
increasing burden on states. In addition, because measures continue to be tested, the Workgroup

20
noted that many of the measures that were not recommended should be reconsidered in the
future. The Workgroup suggested potential gap areas that could be considered for future Core
Set measures (Exhibit 8).
Exhibit 8. Potential Gap Areas for Future Core Set Measures
Populations Health Areas
Health Care Delivery
Other Measure
Attributes
• Adolescent and young
adults
• Children in foster care
• Maternal health
• Men’s health
• Individuals with multiple
chronic conditions
• Elderly individuals,
including those who are
Medicare-Medicaid dual
eligibles
• Individuals of all ages
with disabilities,
including access to
services and supports to
assist them with living
and participating in the
community safely
• Immunizations
(prenatal, adult)
• Obesity
• Adverse childhood
experiences
• Child social and
emotional development
• LTSS (including
rebalancing)
• Oral health beyond
prevention
• Access to oral health
care for individuals with
special needs
• Behavioral health
integration in acute
medical settings
• Depression
• Suicide
• Trauma-informed care
• Rare diseases
• Follow-up on referrals
• Care transitions (e.g.,
from hospital or nursing
home to the community)
• Appropriateness of
care—underutilization
and overutilization
• Workforce and caregiver
supports
• Provider accountability
• Network adequacy
• Stratification by race
and ethnicity
• Measures addressing
social determinants of
health
• Measures with life
course potential
• Measures that cut
across Medicaid and
Medicare
Additional Workgroup Suggestions for Improving the Core Sets
In addition to making recommendations for specific measures, the Workgroup members
discussed improvements for the Core Sets and quality measurement more broadly.
Considering the Various Uses of Quality Measures in Medicaid and CHIP
Workgroup members representing state Medicaid and CHIP agencies noted that they use quality
measures for various purposes and indicated that not all measures are appropriate for the Core
Sets. Several state representatives, for example, expressed enthusiasm for taking some of the
measures back to their state, including some that the Workgroup did not recommend for addition
to the Core Set. State representatives noted that there are a lot of good measures that may not be
appropriate for the Core Set but that would be useful to states as part of their own quality
monitoring activities, such as evaluation of Medicaid Section 1115 and other waivers, managed
care oversight, and value-based purchasing.
Integrating Health Information Technology
The Workgroup stressed the importance of integrating health information technology, including
EHRs and electronic data extraction, into quality reporting efforts. Like New York, other state
representatives noted they were on a path toward integrating electronic data into reporting
efforts. One Workgroup member noted that states have made substantial investments in
hospitals, health systems, and providers to report electronic measures but that many states are not
21
yet yielding the value of these investments because reporting is difficult. To get to true
meaningful use, several members recommended that federal agencies work together to direct
resources and attention to electronic initiatives to assist states in collecting electronic quality
measures.
Creating Shared Learning and Technical Assistance Opportunities
Several Workgroup members suggested that federal agencies, including CMCS, provide shared
learning opportunities for states in the following areas:
• Racial and ethnic stratification. Several Workgroup members suggested providing
technical assistance to help states better understand the racial and ethnic makeup of their
Medicaid and CHIP population and the experiences of different racial and ethnic groups in
the health care system. This includes assistance in standardizing the collection of data on race
and ethnicity makeup.
• Data linkages. Workgroup members noted the need to link data for families in Medicaid to
better understand their needs and service patterns. For example, one Workgroup member
noted data challenges with pairing mothers and their babies in order to monitor the link
between perinatal services and child health outcomes.
• Medicaid and immunization registry coordination. A Workgroup member recommended
that CMCS consider an affinity group or grant opportunity to help drive state coordination
between Medicaid and public health registries, particularly immunization registries.
• State quality staff connections. A Workgroup member recommended convening an affinity
group for state quality staff to help them understand their work across states. They noted that
connecting with other staff working on the same issues could help with staff morale and
retention.
NEXT STEPS
The 2020 Core Set Annual Review Workgroup considered 14 measures for removal from the
Core Sets and 42 measures for addition. Workgroup members recommended the removal of 7
measures and the addition of 5 measures to the 2020 Core Sets. For the first time, the Workgroup
recommended adding 2 measures on LTSS and one measure on the treatment of opioid abuse.
The Workgroup considered such characteristics as the feasibility, appropriateness, readiness, and
actionability of measures for the Core Sets. Workgroup members discussed whether measures
were ready for implementation in the Core Sets and for Medicaid and CHIP. Workgroup
members commented that the Core Sets are not the place to put new or untested measures.
Workgroup members also repeatedly stressed that nothing about the value of the measure or the
importance of the topic area should be inferred from the decision not to recommend a measure
for addition to the Core Sets.
The draft report was available for public comment from July 8, 2019 through August 5, 2019.
Forty public comments were submitted. CMCS will use the Workgroup recommendations and
the public comments to inform decisions about how and whether to modify the Core Sets for
2020. CMCS will release the 2020 Core Sets through a CMCS Informational Bulletin by
December 31, 2019.
Appendix A
Child and Adult Core Set Measures
This page has been left blank for double-sided copying.

A.3
Exhibit A.1. 2019 Core Set of Children’s Health Care Quality Measures for Medicaid and
CHIP (Child Core Set)
NQF #
Measure
steward Measure name Data collection method
Primary Care Access and Preventive Care
0024 NCQA
Weight Assessment and Counseling for Nutrition and
Physical Activity for Children/Adolescents – Body Mass
Index Assessment for Children/Adolescents (WCC-CH)
Administrative, hybrid, or
EHR
0033 NCQA Chlamydia Screening in Women Ages 16–20 (CHL-CH) Administrative or EHR
0038 NCQA Childhood Immunization Status (CIS-CH)
Administrative, hybrid, or
EHR
0418/0418e CMS
Screening for Depression and Follow-Up Plan: Ages
12–17 (CDF-CH)
Administrative or EHR
1392 NCQA
Well-Child Visits in the First 15 Months of Life (W15-
CH)
Administrative or hybrid
1407 NCQA Immunizations for Adolescents (IMA-CH) Administrative or hybrid
1448* OHSU
Developmental Screening in the First Three Years of
Life (DEV-CH)
Administrative or hybrid
1516 NCQA
Well-Child Visits in the Third, Fourth, Fifth, and Sixth
Years of Life (W34-CH)
Administrative or hybrid
Not endorsed NCQA Adolescent Well-Care Visits (AWC-CH) Administrative or hybrid
Not endorsed NCQA
Children and Adolescents’ Access to Primary Care
Practitioners (CAP-CH)
Administrative
Maternal and Perinatal Health
0139 CDC
Pediatric Central Line-Associated Bloodstream
Infections (CLABSI-CH)
Medical records (CDC’s
NHSN)
0471 TJC PC-02: Cesarean Birth (PC02-CH) Hybrid
1360 CDC
Audiological Diagnosis No Later Than 3 Months of Age
(AUD-CH)
EHR
1382 CDC
Live Births Weighing Less Than 2,500 Grams (LBW-
CH)
State vital records
1517* NCQA
Prenatal and Postpartum Care: Timeliness of Prenatal
Care (PPC-CH)
Administrative or hybrid
2902 OPA
Contraceptive Care – Postpartum Women Ages 15–20
(CCP-CH)
Administrative
2903/2904 OPA
Contraceptive Care – All Women Ages 15–20 (CCW-
CH)
Administrative
Care of Acute and Chronic Conditions
1800 NCQA Asthma Medication Ratio: Ages 5–18 (AMR-CH) Administrative
Not endorsed NCQA
Ambulatory Care: Emergency Department (ED) Visits
(AMB-CH)
Administrative
Behavioral Health Care
0108 NCQA
Follow-Up Care for Children Prescribed Attention-
Deficit/Hyperactivity Disorder (ADHD) Medication
(ADD-CH)
Administrative or EHR
0576 NCQA
Follow-Up After Hospitalization for Mental Illness: Ages
6–17 (FUH-CH)
Administrative

Exhibit A.1. (continued)
A.4
NQF #
Measure
steward Measure name Data collection method
2801 NCQA
Use of First-Line Psychosocial Care for Children and
Adolescents on Antipsychotics (APP-CH)
Administrative
Not endorsed NCQA
Use of Multiple Concurrent Antipsychotics in Children
and Adolescents (APC-CH)
Administrative
Dental and Oral Health Services
2508*
DQA
(ADA)
Dental Sealants for 6–9 Year-Old Children at Elevated
Caries Risk (SEAL-CH)
Administrative
Not endorsed CMS
Percentage of Eligibles Who Received Preventive
Dental Services (PDENT-CH)
Administrative (Form
CMS-416)
Experience of Care
Not endorsed NCQA
Consumer Assessment of Healthcare Providers and
Systems (CAHPS®) Health Plan Survey 5.0H – Child
Version Including Medicaid and Children with Chronic
Conditions Supplemental Items (CPC-CH)
Survey
More information on 2019 Updates to the Child and Adult Core Health Care Quality Measurement Sets is available at
https://www.medicaid.gov/federal-policy-guidance/downloads/cib112018.pdf.
*This measure is no longer endorsed by NQF.
CDC = Centers for Disease Control and Prevention; CHIP = Children's Health Insurance Program; CMS = Centers for
Medicare & Medicaid Services; DQA (ADA) = Dental Quality Alliance (American Dental Association); EHR =
Electronic Health Record; NCQA = National Committee for Quality Assurance; NHSN = National Healthcare Safety
Network; NQF = National Quality Forum; OHSU = Oregon Health and Science University; OPA = U.S. Office of
Population Affairs; TJC = The Joint Commission.

A.5
Exhibit A.2. 2019 Core Set of Adult Health Care Quality Measures for Medicaid (Adult
Core Set)
NQF #
Measure
steward Measure name Data collection method
Primary Care Access and Preventive Care
0032 NCQA Cervical Cancer Screening (CCS-AD)
Administrative, hybrid, or
EHR
0033 NCQA Chlamydia Screening in Women Ages 21–24 (CHL-AD) Administrative or EHR
0039 NCQA Flu Vaccinations for Adults Ages 18 to 64 (FVA-AD) Survey
0418/0418e CMS
Screening for Depression and Follow-Up Plan: Age 18
and Older (CDF-AD)
Administrative or EHR
2372 NCQA Breast Cancer Screening (BCS-AD) Administrative or EHR
Not endorsed NCQA Adult Body Mass Index Assessment (ABA-AD) Administrative or hybrid
Maternal and Perinatal Health
0469/0469e TJC PC-01: Elective Delivery (PC01-AD) Hybrid or EHR
1517* NCQA
Prenatal and Postpartum Care: Postpartum Care (PPC-
AD)
Administrative or hybrid
2902 OPA
Contraceptive Care – Postpartum Women Ages 21–44
(CCP-AD)
Administrative
2903/2904 OPA
Contraceptive Care – All Women Ages 21–44 (CCW-
AD)
Administrative
Care of Acute and Chronic Conditions
0018 NCQA Controlling High Blood Pressure (CBP-AD)
Administrative, hybrid, or
EHR
0057 NCQA
Comprehensive Diabetes Care: Hemoglobin A1c
(HbA1c) Testing (HA1C-AD)
Administrative or hybrid
0059 NCQA
Comprehensive Diabetes Care: Hemoglobin A1c
(HbA1c) Poor Control (>9.0%) (HPC-AD)
Administrative, hybrid, or
EHR
0272 AHRQ
PQI 01: Diabetes Short-Term Complications Admission
Rate (PQI01-AD)
Administrative
0275 AHRQ
PQI 05: Chronic Obstructive Pulmonary Disease
(COPD) or Asthma in Older Adults Admission Rate
(PQI05-AD)
Administrative
0277 AHRQ PQI 08: Heart Failure Admission Rate (PQI08-AD) Administrative
0283 AHRQ
PQI 15: Asthma in Younger Adults Admission Rate
(PQI15-AD)
Administrative
1768 NCQA Plan All-Cause Readmissions (PCR-AD) Administrative
1800 NCQA Asthma Medication Ratio: Ages 19–64 (AMR-AD) Administrative
2082/3210e HRSA HIV Viral Load Suppression (HVL-AD) Administrative or EHR
2371* NCQA
Annual Monitoring for Patients on Persistent
Medications (MPM-AD)
Administrative
Behavioral Health Care
0004 NCQA
Initiation and Engagement of Alcohol and Other Drug
Abuse or Dependence Treatment (IET-AD)
Administrative or EHR
0027 NCQA
Medical Assistance with Smoking and Tobacco Use
Cessation (MSC-AD)
Survey

Exhibit A.2. (continued)
A.6
NQF #
Measure
steward Measure name Data collection method
0105 NCQA Antidepressant Medication Management (AMM-AD) Administrative or EHR
0576 NCQA
Follow-Up After Hospitalization for Mental Illness: Age
18 and Older (FUH-AD)
Administrative
1932 NCQA
Diabetes Screening for People With Schizophrenia or
Bipolar Disorder Who Are Using Antipsychotic
Medications (SSD-AD)
Administrative
2605 NCQA
Follow-Up After Emergency Department Visit for
Alcohol and Other Drug Abuse or Dependence (FUA-
AD)
a
Administrative
2605 NCQA
Follow-Up After Emergency Department Visit for Mental
Illness (FUM-AD)
a
Administrative
2607 NCQA
Diabetes Care for People with Serious Mental Illness:
Hemoglobin A1c (HbA1c) Poor Control (>9.0%)
(HPCMI-AD)
Administrative or hybrid
2940 PQA
Use of Opioids at High Dosage in Persons Without
Cancer (OHD-AD)
Administrative
Not
endorsed**
NCQA
Adherence to Antipsychotic Medications for Individuals
with Schizophrenia (SAA-AD)
Administrative
3389 PQA
Concurrent Use of Opioids and Benzodiazepines (COB-
AD)
Administrative
Experience of Care
Not
endorsed***
NCQA
Consumer Assessment of Healthcare Providers and
Systems (CAHPS®) Health Plan Survey 5.0H, Adult
Version (Medicaid) (CPA-AD)
Survey
More information on 2019 Updates to the Child and Adult Core Health Care Quality Measurement Sets is available at
https://www.medicaid.gov/federal-policy-guidance/downloads/cib112018.pdf.
*This measure is no longer endorsed by NQF.
**The Adult Core Set includes the NCQA version of the measure, which is adapted from the CMS measure (NQF
#1879).
***The Adult Core Set includes the NCQA version of the measure, which is adapted from the AHRQ measure (NQF
#0006).
a
The FUA-AD and FUM-AD measures were previously included in the Adult Core Set as a single measure
(FUA/FUM-AD). For the 2019 Adult Core Set, they are included as two separate measures.
AHRQ = Agency for Healthcare Research & Quality; CMS = Centers for Medicare & Medicaid Services; EHR =
Electronic Health Record; HRSA = Health Resources and Services Administration; NCQA = National Committee for
Quality Assurance; NQF = National Quality Forum; OPA = U.S. Office of Population Affairs; PQA = Pharmacy Quality
Alliance; TJC = The Joint Commission.

A.7
Exhibit A.3. Core Set of Children’s Health Care Quality Measures for Medicaid and CHIP (Child Core Set), 2012–2019
NQF #
Measure
Steward
Measure Name 2012 2013 2014 2015 2016 2017 2018 2019
Primary Care Access and Preventive Care
0024 NCQA
Weight Assessment and Counseling for Nutrition
and Physical Activity for Children/Adolescents –
Body Mass Index Assessment for
Children/Adolescents (WCC-CH)
X X X X X X X X
0033 NCQA
Chlamydia Screening in Women Ages 16–20
(CHL-CH)
X X X X X X X X
0038 NCQA Childhood Immunization Status (CIS-CH) X X X X X X X X
0418/0418e CMS
Screening for Depression and Follow-Up Plan:
Ages 12–17 (CDF-CH)
a
-- -- -- -- -- -- X X
1392 NCQA
Well-Child Visits in the First 15 Months of Life
(W15-CH)
X X X X X X X X
1407 NCQA Immunizations for Adolescents (IMA-CH) X X X X X X X X
1448* OHSU
Developmental Screening in the First Three
Years of Life (DEV-CH)
X X X X X X X X
1516 NCQA
Well-Child Visits in the Third, Fourth, Fifth and
Sixth Years of Life (W34-CH)
X X X X X X X X
1959 NCQA
Human Papillomavirus Vaccine for Female
Adolescents (HPV-CH)
b
-- X X X X -- -- --
NA NCQA Adolescent Well-Care Visits (AWC-CH) X X X X X X X X
NA NCQA
Child and Adolescents’ Access to Primary Care
Practitioners (CAP-CH)
X X X X X X X X
Maternal and Perinatal Health
0139 CDC
Pediatric Central Line-Associated Bloodstream
Infections (CLABSI-CH)
X X X X X X X X
0471 TJC PC-02: Cesarean Birth (PC02-CH)
c
X X X X X X X X
1360 CDC
Audiological Diagnosis No Later Than 3 Months
of Age (AUD-CH)
d
-- -- -- -- X X X X

Exhibit A.3. (continued)
A.8
NQF #
Measure
Steward
Measure Name 2012 2013 2014 2015 2016 2017 2018 2019
1382 CDC
Live Births Weighing Less Than 2,500 Grams
(LBW-CH)
X X X X X X X X
1391* NCQA Frequency of Ongoing Prenatal Care (FPC-CH)
e
X X X X X X -- --
1517* NCQA
Prenatal and Postpartum Care: Timeliness of
Prenatal Care (PPC-CH)
X X X X X X X X
2902 OPA
Contraceptive Care – Postpartum Women Ages
15–20 (CCP-CH)
f
-- -- -- -- -- X X X
2903/2904 OPA
Contraceptive Care – All Women Ages 15–20
(CCW-CH)
g
-- -- -- -- -- -- X X
NA
No current
measure
steward
Behavioral Health Risk Assessment (for
Pregnant Women) (BHRA-CH)
h
-- X X X X X -- --
Care of Acute and Chronic Conditions
0002* NCQA
Appropriate Testing for Children with Pharyngitis
(CWP-CH)
i
X X -- -- -- -- -- --
0060* NCQA
Annual Pediatric Hemoglobin A1C Testing
(PA1C-CH)
j
X X -- -- -- -- -- --
0657 AAOH-HNSF
Otitis Media with Effusion –Avoidance of
Inappropriate Systemic Antimicrobials in
Children: Ages 2-12 (OME-CH)
k
X -- -- -- -- -- -- --
1381*
Alabama
Medicaid
Annual Percentage of Asthma Patients 2
Through 20 Years Old with One of More
Asthma-Related Emergency Room Visits
(ASMER-CH)
l
X X -- -- -- -- -- --
1799* NCQA
Medication Management for People with Asthma
(MMA-CH)
m
-- X X X X X -- --
1800 NCQA
Asthma Medication Ratio: Ages 5–18 (AMR-
CH)
m
-- -- -- -- -- -- X X
NA NCQA
Ambulatory Care: Emergency Department (ED)
Visits (AMB-CH)
X X X X X X X X

Exhibit A.3. (continued)
A.9
NQF #
Measure
Steward
Measure Name 2012 2013 2014 2015 2016 2017 2018 2019
Behavioral Health Care
0108 NCQA
Follow-Up Care for Children Prescribed
Attention- Deficit/Hyperactivity Disorder (ADHD)
Medication (ADD-CH)
X X X X X X X X
0576 NCQA
Follow-Up After Hospitalization for Mental
Illness: Ages 6–17 (FUH-CH)
n
X X X X X X X X
1365 PCPI
Child and Adolescent Major Depressive
Disorder: Suicide Risk Assessment (SRA-CH)
o
-- -- -- X X X -- --
2801 NCQA
Use of First-Line Psychosocial Care for Children
and Adolescents on Antipsychotics (APP-CH)
p
-- -- -- -- -- X X X
NA NCQA
Use of Multiple Concurrent Antipsychotics in
Children and Adolescents (APC-CH)
q
-- -- -- -- X X X X
Dental and Oral Health Services
2508*
DQA
(ADA)
Dental Sealants for 6–9 Year-Old Children at
Elevated Caries Risk (SEAL-CH)
r
-- -- -- X X X X X
NA CMS
Percentage of Eligibles Who Received
Preventive Dental Services (PDENT-CH)
X X X X X X X X
NA CMS
Percentage of Eligibles That Received Dental
Treatment Services (TDENT-CH)
s
X X X -- -- -- -- --
Experience of Care
NA NCQA
Consumer Assessment of Healthcare Providers
and Systems (CAHPS®) Health Plan Survey
5.0H – Child Version Including Medicaid and
Children with Chronic Conditions Supplemental
Items (CPC-CH)
X X X X X X X X
X = Included in Child Core Set; -- = Not Included in Child Core Set.
AAO-HNSF = American Academy of Otolaryngology-Head and Neck Surgery; AMA = American Medical Association; CDC = Centers for Disease Control and
Prevention; CMS = Centers for Medicare & Medicaid Services; DQA (ADA) = Dental Quality Alliance (American Dental Association); NA = Measure is not NQF
endorsed; NCQA = National Committee for Quality Assurance; NQF = National Quality Forum; OHSU = Oregon Health and Science University; OPA = U.S. Office
of Population Affairs; PCPI = Physician Consortium for Performance Improvement; TJC = The Joint Commission.
More information on 2019 Updates to the Child and Adult Core Health Care Quality Measurement Sets is available at https://www.medicaid.gov/federal-policy-
guidance/downloads/cib112018.pdf.
*This measure is no longer endorsed by NQF.
Exhibit A.3. (continued)
A.10
a
The Screening for Depression and Follow-Up Plan: Ages 12 –17 measure was added to the 2018 Child Core Set to align with the Adult Core Set and replace the
Child and Adolescent Major Depressive Disorder: Suicide Risk Assessment measure as a broader measure of behavioral health.
b
The stand-alone HPV Vaccine for Female Adolescents measure was retired by the measure steward, and added to the Immunizations for Adolescents measure
beginning with the 2017 Child Core Set.
c
The California Maternal Quality Care Collaborative Cesarean Rate for Nulliparous Singleton Vertex measure was replaced by The Joint Commission PC-02:
Cesarean Birth measure beginning with the 2014 Child Core Set.
d
The Audiological Diagnosis No Later Than 3 Months of Age measure was added to the 2016 Child Core Set due to opportunities for quality improvement on the
measure and its alignment with the electronic health record incentive program.
e
The Frequency of Ongoing Prenatal care measure was retired from the Child Core Set in 2018 because it does not assess the content of the prenatal care visit.
f
The Contraceptive Care – Postpartum Women Ages 15–20 measure was added to the 2017 Child Core Set because it measures the provision of contraception to
mothers in the postpartum period, which can help women space pregnancies to their desired interpregnancy interval and help to improve future birth outcomes.
g
The Contraceptive Care – All Women Ages 15–20 measure was added to the 2018 Child Core Set to assess access to contraceptive care, which has an
important role in promoting health equity.
h
The Behavioral Health Risk Assessment (for Pregnant Women) measure was removed from the Child Core Set in 2018 due to implementation and data collection
challenges. AMA-PCPI was the measure steward for the 2013-2016 Child Core Sets; the measure had no steward for the 2017 Child Core Set.
i
The Appropriate Testing for Children with Pharyngitis measure was retired from the Child Core Set in 2014 because the clinical evidence for the measure is
obsolete.
j
The Annual Pediatric Hemoglobin A1C Testing measure was retired from the Child Core Set in 2014 because it affects a small number of children, has a weak
evidence base, and was approaching the improvement ceiling.
k
The Otitis Media with Effusion – Avoidance of Inappropriate Systemic Antimicrobials in Children (ages 2-12) measure was retired from the Child Core Set in 2013
because of significant state reporting challenges. AMA-PCPI was the measure steward for the 2012 Child Core Set.
l
The Annual Percentage of Asthma Patients 2 Through 20 Years Old with One or More Asthma-Related Emergency Room Visits measure was retired from the
Child Core Set in 2014 due to data quality concerns and the lack of a measure steward.
m
Beginning with the 2018 Child Core Set, the Asthma Medication Ratio: Ages 5–18 measure replaces the Medication Management for People with Asthma
measure, which was included in the 2013-2017 Child Core Sets.
n
The age group for the Follow-Up After Hospitalization for Mental Illness measure changed from ages 6 to 20 to ages 6 to 17 for the 2019 Child Core Set.
o
The Child and Adolescent Major Depressive Disorder: Suicide Risk Assessment measure was added to the 2015 Child Core Set to target a high prevalence
mental health condition that has severe consequences without appropriate treatment. The measure was removed from the Child Core Set in 2018 because of the
need for a broader measure of behavioral health.
p
The Use of First-Line Psychosocial Care for Children and Adolescents on Antipsychotics measure was added to the 2017 Child Core Set to promote the use of
nonpharmacologic, evidence-informed approaches to the treatment of mental and behavioral health problems of Medicaid and CHIP insured children on
psychotropic medications.
q
The Use of Multiple Concurrent Antipsychotics in Children and Adolescents measure was added to the 2016 Child Core Set to target inappropriate prescribing of
antipsychotic medications, which may have adverse health effects.
r
The Dental Sealants for 6–9 Year-Old Children at Elevated Caries Risk measure was added to the 2015 Child Core Set because it is linked to improved oral
health outcomes and responds to a legislative mandate to measure the use of dental sealants in this age group.
s
The Percentage of Eligibles That Received Dental Treatment Services measure was retired from the Child Core Set in 2015 because it is not an effective tool for
quality improvement; it is unclear if an increase or a decrease in the rate is desirable, and therefore the results are not actionable.
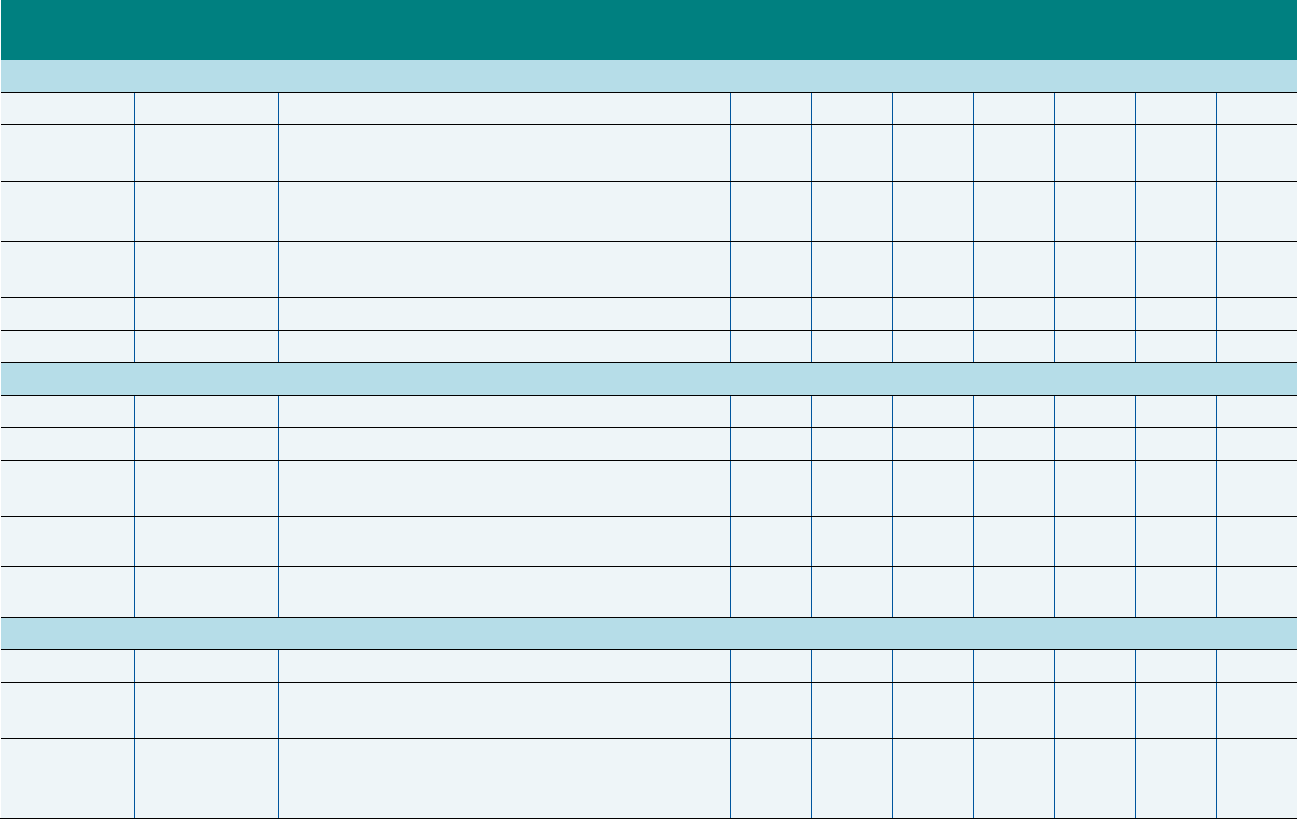
A.11
Exhibit A.4. Core Set of Adult Health Care Quality Measures for Medicaid (Adult Core Set), 2013–2019
NQF #
Measure
Steward
Measure Name 2013 2014 2015 2016 2017 2018 2019
Primary Care Access and Preventive Care
0032 NCQA Cervical Cancer Screening (CCS-AD) X X X X X X X
0033 NCQA
Chlamydia Screening in Women Ages 21–24 (CHL-
AD)
X X X X X X X
0039 NCQA
Flu Vaccinations for Adults Ages 18 to 64
(FVA-AD)
X X X X X X X
0418/0418e CMS
Screening for Depression and Follow-Up Plan: Age 18
and Older (CDF-AD)
X X X X X X X
2372 NCQA Breast Cancer Screening (BCS-AD) X X X X X X X
NA NCQA Adult Body Mass Index Assessment (ABA-AD) X X X X X X X
Maternal and Perinatal Health
0469/0469e TJC PC-01: Elective Delivery (PC01-AD) X X X X X X X
0476 TJC PC-03: Antenatal Steroids (PC03-AD)
a
X X X X X X --
1517* NCQA
Prenatal and Postpartum Care: Postpartum Care
(PPC-AD)
X X X X X X X
2902 OPA
Contraceptive Care – Postpartum Women
Ages 21–44 (CCP-AD)
b
-- -- -- -- X X X
2903/2904 OPA
Contraceptive Care – All Women Ages 21–44
(CCW-AD)
c
-- -- -- -- -- X X
Care of Acute and Chronic Conditions
0018 NCQA Controlling High Blood Pressure (CBP-AD) X X X X X X X
0057 NCQA
Comprehensive Diabetes Care: Hemoglobin
A1c (HbA1c) Testing (HA1C-AD)
X X X X X X X
0059 NCQA
Comprehensive Diabetes Care: Hemoglobin
A1c (HbA1c) Poor Control (>9.0%) (HPC-
AD)
d
-- -- X X X X X

Exhibit A.4. (continued)
A.12
NQF #
Measure
Steward
Measure Name 2013 2014 2015 2016 2017 2018 2019
0063* NCQA
Comprehensive Diabetes Care: LDL-C
Screening (LDL-AD)
d
X X -- -- -- -- --
0272 AHRQ
PQI 01: Diabetes Short-Term Complications
Admission Rate (PQI01-AD)
X X X X X X X
0275 AHRQ
PQI 05: Chronic Obstructive Pulmonary
Disease (COPD) or Asthma in Older Adults
Admission Rate (PQI05-AD)
X X X X X X X
0277 AHRQ
PQI 08: Heart Failure Admission Rate
(PQI08-AD)
X X X X X X X
0283 AHRQ
PQI 15: Asthma in Younger Adults Admission
Rate (PQI15-AD)
X X X X X X X
0403* NCQA Annual HIV/AIDS Medical Visit (HMV-AD)
e
X -- -- -- -- -- --
1768 NCQA Plan All-Cause Readmissions (PCR-AD) X X X X X X X
1800 NCQA
Asthma Medication Ratio: Ages 19–64
(AMR-AD)
f
-- -- -- -- -- X X
2082/3210e HRSA HIV Viral Load Suppression (HVL-AD) -- X X X X X X
2371* NCQA
Annual Monitoring for Patients on Persistent
Medications (MPM-AD)
X X X X X X X
Behavioral Health Care
0004 NCQA
Initiation and Engagement of Alcohol and Other Drug
Abuse or Dependence Treatment
(IET-AD)
X X X X X X X
0027 NCQA
Medical Assistance with Smoking and Tobacco
Use Cessation (MSC-AD)
X X X X X X X
0105 NCQA
Antidepressant Medication Management (AMM-
AD)
X X X X X X X
0576 NCQA
Follow-Up After Hospitalization for Mental Illness: Age
18 and Older (FUH-AD)
g
X X X X X X X
1932 NCQA
Diabetes Screening for People With Schizophrenia
or Bipolar Disorder Who Are Using Antipsychotic
Medications (SSD-AD)
h
-- -- -- X X X X
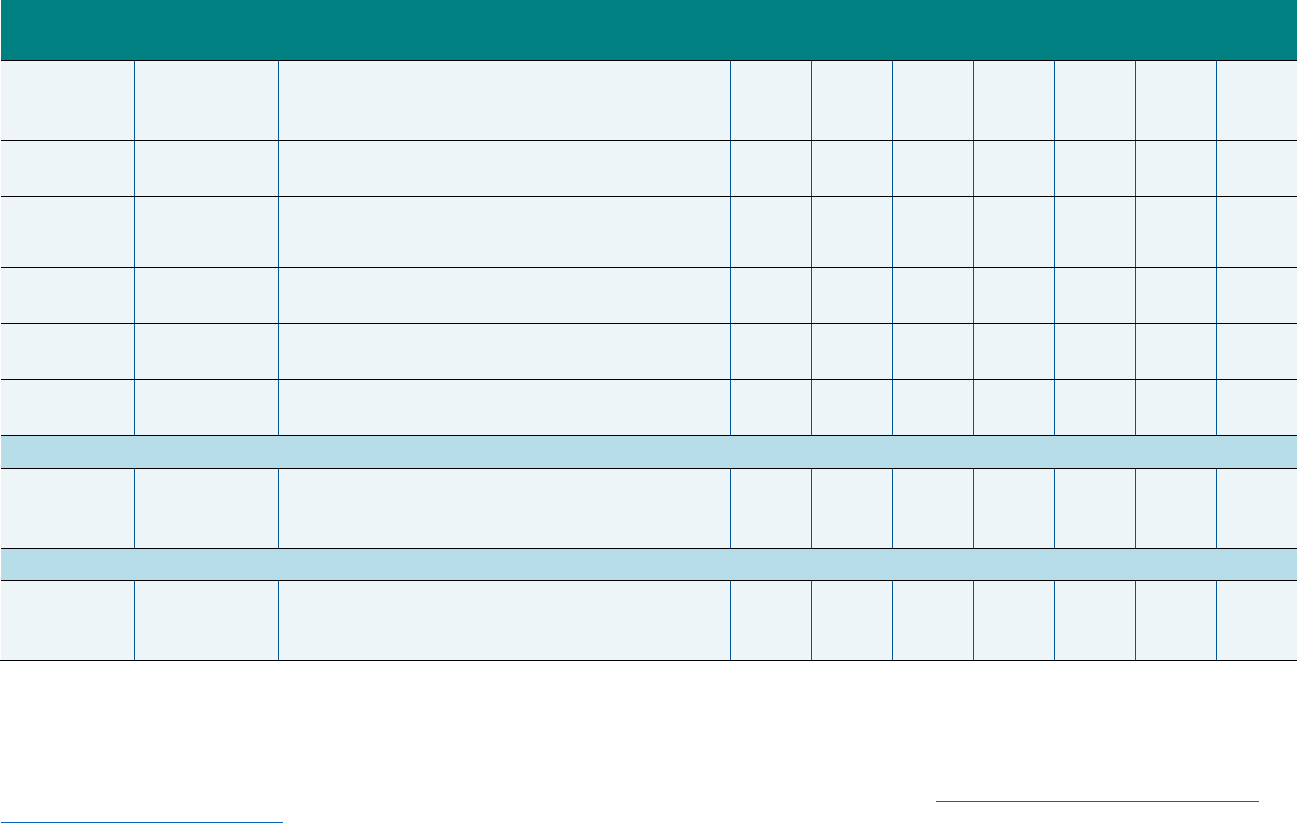
Exhibit A.4. (continued)
A.13
NQF #
Measure
Steward
Measure Name 2013 2014 2015 2016 2017 2018 2019
2605 NCQA
Follow-Up After Emergency Department Visit for
Alcohol and Other Drug Abuse or Dependence
(FUA -AD)
i
-- -- -- -- X X X
2605 NCQA
Follow-Up After Emergency Department Visit for
Mental Illness (FUM-AD)
i
-- -- -- -- X X X
2607 NCQA
Diabetes Care for People with Serious Mental
Illness: Hemoglobin A1c (HbA1c) Poor Control
(>9.0%)
(HPCMI-AD)
j
-- -- -- -- X X X
2940 PQA
Use of Opioids at High Dosage in Persons Without
Cancer (OHD-AD)
h
-- -- -- X X X X
NA NCQA
Adherence to Antipsychotic Medications for Individuals
with Schizophrenia (SAA-AD)
k
X X X X X X X
3389 PQA
Concurrent Use of Opioids and Benzodiazepines
(COB-AD)
l
-- -- -- -- -- X X
Care Coordination
0648* AMA-PCPI
Timely Transmission of Transition Record
(Discharges from an Inpatient Facility to Home/Self
Care or Any Other Site of Care) (CTR-AD)
m
X X X X -- -- --
Experience of Care
NA NCQA
Consumer Assessment of Healthcare Providers and
Systems (CAHPS®) Health Plan Survey 5.0H, Adult
Version (Medicaid) (CPA-AD)
n
X X X X X X X
X = Included in Adult Core Set; -- = Not Included in Adult Core Set.
AHRQ = Agency for Healthcare Research & Quality; AMA-PCPI = American Medical Association-Physician Consortium for Performance Improvement; CMS =
Centers for Medicare & Medicaid Services; HRSA = Health Resources and Services Administration; NA = Measure is not NQF endorsed; NCQA = National
Committee for Quality Assurance; NQF = National Quality Forum; OPA = U.S. Office of Population Affairs; PQA = Pharmacy Quality Alliance; TJC = The Joint
Commission.
More information on 2019 Updates to the Child and Adult Core Health Care Quality Measurement Sets is available at
https://www.medicaid.gov/federal-policy-
guidance/downloads/cib112018.pdf.
*This measure is no longer endorsed by NQF.
a
The Antenatal Steroids measure was retired from the Adult Core Set in 2019 due to the low number of states reporting this measure and the challenges states
have described in collecting it.
Exhibit A.4. (continued)
A.14
b
The Contraceptive Care – Postpartum Women Ages 21–44 measure was added to the 2017 Adult Core Set because it measures the provision of contraception
to mothers in the postpartum period, which can help women space pregnancies to their desired interpregnancy interval and help to improve future birth outcomes.
c
The Contraceptive Care – All Women Ages 21–44 measure was added to the 2018 Adult Core Set to assess access to contraceptive care, which has an
important role in promoting health equity.
d
The Comprehensive Diabetes Care: LDL-C Screening measure was replaced by the Comprehensive Diabetes Care: Hemoglobin A1c (HbA1c) Poor Control
(>9.0%) measure beginning with the 2015 Adult Core Set. The Comprehensive Diabetes Care: LDL-C Screening measure was retired from the Adult Core Set
because clinical guidelines underpinning this measure were in flux and because NCQA removed it from HEDIS 2015. The Comprehensive Diabetes Care:
Hemoglobin A1c Poor Control (>9.0%) measure addresses the prevalent condition of diabetes and facilitates state efforts to drive quality improvement on the risk
factor of poor HbA1c control.
e
The Annual HIV Medical Visit measure was replaced by the HIV Viral Load Suppression measure beginning with the 2014 Adult Core Set. The Annual HIV
Medical Visit measure lost NQF endorsement after the 2013 Adult Core Set was published. The HIV Viral Load Suppression measure is a regularly collected
clinical indicator that is predictive of overall outcomes.
f
The Asthma Medication Ratio: Ages 19–64 measure was added to the 2018 Adult Core Set and aligns with changes made to the 2018 Child Core Set.
g
The age group for the Follow-Up After Hospitalization for Mental Illness measure changed from age 21 and older to age 18 and older for the 2019 Adult Core Set.
h
Two measures focused on quality of care for adults with substance use disorders and/or mental health disorders were added to the 2016 Adult Core Set: (1)
Diabetes Screening for People With Schizophrenia or Bipolar Disorder Who Are Using Antipsychotic Medications focuses on the identification of cardiovascular
disease, a leading cause of morbidity and mortality in this population; and (2) Use of Use of Opioids at High Dosage in Persons Without Cancer is a measure of
potential overuse that addresses the epidemic of narcotic morbidity and mortality.
i
The Follow-Up After Emergency Department Visit for Mental Illness or Alcohol and Other Drug Abuse or Dependence (FUA/FUM-AD) measure was added to the
2017 Adult Core Set because it addresses priority areas of access and follow-up of care for adults with mental health or substance use disorders. In the 2017 and
2018 Adult Core Sets, this was included as a single measure (FUA/FUM-AD). For the 2019 Adult Core Set, Follow-Up After Emergency Department Visit for
Alcohol and Other Drug Abuse or Dependence (FUA-AD) and Follow-Up After Emergency Department Visit for Mental Illness (FUM-AD) are included as two
separate measures.
j
The Diabetes Care for People with Serious Mental Illness: Hemoglobin A1c (HbA1c) Poor Control (>9.0%) measure was added to the 2017 Adult Core Set
because it addresses chronic disease management for people with serious mental illness, and assesses integration of medical and behavioral services by
reinforcing shared accountability and linkage of medical and behavioral healthcare services.
k
The Adult Core Set includes the NCQA version of the Adherence to Antipsychotic Medications for Individuals with Schizophrenia measure, which is adapted from
the CMS measure (NQF #1879).
l
The Concurrent Use of Opioids and Benzodiazepines measure was added to the 2018 Adult Core Set because it addresses early opioid use and polypharmacy.
m
The Timely Transmission of Transition Record measure was retired from the Adult Core Set in 2017 due to the low number of states reporting this measure, a
decrease in the number of states reporting over time, and the challenges states have described in collecting it.
n
The Adult Core Set includes the NCQA version of the CAHPS® Health Plan Survey 5.0H, Adult Version (Medicaid) measure, which is adapted from the AHRQ
measure (NQF #0006).
Appendix B
Summary of 2020 Core Set Annual Review Workgroup
Discussion of Measures Not Recommended
For Removal or Addition
This page has been left blank for double-sided copying.

B.3
This appendix summarizes the discussion of measures suggested but not recommended for
removal from or addition to the 2020 Child and Adult Core Sets. The discussion took place
during the in-person Workgroup meeting May 7-9. The summary is organized by domain. For
more information about the measures discussed but not recommended for removal or addition,
please refer to Exhibit B.1 at the end of this appendix, including the measure name, measure
steward, NQF # (if endorsed), measure description, and data collection method.
Primary Care Access and Preventive Care
In the Primary Care Access and Preventive Care domain, the Workgroup first discussed
immunization measures, including the Flu Vaccinations for Adults Ages 18 to 64 (FVA-AD)
measure suggested for removal from the Adult Core Set and four immunization measures
proposed for addition (Flu Vaccinations for Adults Age 65 and Older; Influenza Immunization;
Adult Immunization Status; Prenatal Immunization Status). The FVA-AD measure was
suggested for removal because of the high cost of the CAHPS survey and the fact that it only
covers flu vaccinations while other measures include additional immunizations and wider age
ranges. The Influenza Immunization measure was suggested to replace the FVA-AD measure
because it is lower cost and more comparable across diverse populations, according to the
Workgroup member who suggested it. The Adult Immunization Status measure was suggested
for addition because it includes more vaccines than the current immunization measure (FVA-
AD) and would help states monitor appropriate adult immunization use beyond influenza.
Workgroup members suggested adding the Prenatal Immunization Status measure because
vaccinations for this population are not currently being measured in the Core Set, and there are
substantial disparities in prenatal immunization levels.
The Workgroup discussed the accuracy and reliability of the data needed for the immunization
measures, many of which rely on patient recall or administrative data that may be incomplete for
people who cycle in and out of Medicaid plans. Furthermore, because influenza vaccines can be
administered in a variety of settings, data on them might be incomplete. Workgroup members
noted that while all states have immunization registries, there is considerable variability in their
completeness. In the case of the Prenatal Immunization Status measure, Workgroup members
acknowledged its importance and strong connection to improved outcomes, but had concerns
about the feasibility of the new data collection method
18
and were reluctant to recommend a first-
year HEDIS measure that might not be ready for state reporting.
For the Lead Screening in Children and HIV Screening measures, Workgroup members
deliberated whether these measures were more appropriate for public health surveillance
programs rather than for Medicaid quality measurement. Data completeness concerns were also
raised for both measures, especially in states where there is no linkage between state public
health and Medicaid data. For the Body Mass Index Screening and Follow-Up Plan and Follow-
Up with Patient Family After Developmental Screening measures, Workgroup members
acknowledged these are areas of high interest but had concerns whether the proposed measures
would promote quality improvement. In addition, there were concerns about the burden of the
18
The Prenatal Immunization Status and Adult Immunization Status measures are specified for the Electronic
Clinical Data Systems (ECDS) data collection method, which includes data from administrative claims, electronic
health records, case management systems, and health information exchanges/clinical registries.
B.4
medical record reviews required to calculate these measures. For the Colorectal Cancer
Screening measure, Workgroup members acknowledged that such screenings are highly effective
and associated with reduced costs. However, they also restated concerns about recommending a
HEDIS measure that had not yet been used for the Medicaid population and raised concerns
about the measure’s extended look-back period.
Maternal and Perinatal Health
A Workgroup member suggested the PC-01: Elective Delivery (PC01-AD) measure for removal
from the Adult Core Set and suggested an existing Child Core Set measure, PC-02: Cesarean
Birth (PC02-CH), as a substitute. The Workgroup acknowledged that early elective induction, in
the absence of medical necessity, is a driver of cesarean rates and neonatal intensive care unit
utilization. Workgroup members commented that few states are reporting the PC01-AD measure
and state representatives noted the challenges with collecting the measure, including that the
numerator is not available in claims data, so medical record review or vital records linkage is
required (and some have found that vital records linkage does not provide the information
needed to calculate the measure). Some Workgroup members questioned whether reporting on
the rate of elective deliveries in the Core Set allows for action by states, and furthermore,
whether outliers on the measure should be regulated outside a quality measurement program.
They also noted that other perinatal measures have demonstrated more unwarranted variation and
impact a greater number of beneficiaries. However, several Workgroup members shared a
concern of slippage in performance if the PC01-AD measure is removed from the Adult Core Set
and noted that because Medicaid pays for such a high percentage of births, measures are an
important indicator of priorities for quality of care, and this issue is a high priority.
A Workgroup member suggested removal of the Contraceptive Care – Postpartum Women Ages
21–44 (CCP-AD) measure because another measure in the Core Set, the Contraceptive Care –
All Women Ages 15–20 (CCP-CH), addresses the same measure concept. It was noted that only
the ages 21–44 portion of the measure was suggested for removal and not the age 15–20
measure, which was concerning to the Workgroup. It was also clarified that the postpartum
population cannot be parsed out from the Contraceptive Care – All Women (CCW-CH/AD)
measure in the Child and Adult Core Sets. The Workgroup felt it important to have a measure for
postpartum women, as effective postpartum contraception is a method to increase birth spacing,
which is related to low birthweight and other poor outcomes. A Workgroup member also noted
that maintaining the measure in the Core Set could drive states to resolve payment issues around
insertion of long-acting reversible methods of contraception. A public commenter noted that
CMCS, CDC, and the Office of Population Affairs partnered to support states in calculating,
reporting, and using the Contraceptive Care measures to track access and drive improvements.
The PC-05: Exclusive Breast Milk Feeding measure was suggested for addition to the Core Sets
as there is evidence that breast milk feeding improves life course and reduces disparities. The
Workgroup member noted that the measure can be used to hold systems accountable with the
understanding that the goal is not a rate of 100 percent. Exclusive breastfeeding is a goal of the
World Health Organization, U.S. Department of Health and Human Services, American
Academy of Pediatrics, and the American College of Obstetricians and Gynecologists.
Workgroup members noted that it is a non-medical measure that can be used to address
disparities and capture data about intended breastfeeding, allowing states to see where
B.5
interventions are needed for certain hospitals and populations. Workgroup members questioned
whether there is anything built into the measure to take into account cultural preferences,
medications the mother is taking, or breastfeeding attempts, which may limit a mother’s ability
to breastfeed exclusively or at all in the first few days of life. The Workgroup shared concerns
about the title of the measure and the signal it would send if it was added to the Core Set. Finally,
one Workgroup member noted that the data collection method is medical record review, which
could make it difficult for states to report.
Workgroup members suggested the Prenatal Depression Screening and Follow-Up measure for
addition to the Core Sets because prenatal depression can be treated successfully if treated early;
the measure could be used to assess the content of prenatal care and to improve outcomes for
mothers and babies. A Workgroup member suggested addition of the Postpartum Depression
Screening and Follow-Up measure to capture maternal well-being and newborn development.
The measures address a gap area, could address disparities, and might incentivize meeting
minimum thresholds for screening. It was noted that these measures are particularly important
because (1) access to behavioral health care for the Medicaid population is essential; and (2)
women are especially vulnerable for depression in the perinatal period, which can have a large
impact on their lives and life of their child. It was also noted that some women do not return for
their postpartum appointment, so a Workgroup member noted that the postpartum measure will
pick up screens done at newborn appointments, which might be the only opportunity to reach the
mother.
The Prenatal Depression Screening and Follow-Up and Postpartum Depression Screening and
Follow-Up measures are proposed for addition to HEDIS 2020 and are specified for the ECDS
data collection method. Workgroup members shared concern with the measures being new and
untested at the state level as well as using a new data collection method. Although Workgroup
members noted the appeal and importance of having a measure that incentivizes documenting
postpartum screening in the mother’s chart rather than the infant’s, there was also concern about
being able to capture the infant’s date of birth without linking claims or vital records to the
mother’s record.
Care of Acute and Chronic Conditions
A Workgroup member suggested removal of the HIV Viral Load Suppression (HVL-AD)
measure from the Adult Core Set given the low uptake by states. Workgroup members
acknowledged the challenges of obtaining access to laboratory data on viral load suppression,
which one member attributed in part to the stigmatization of HIV. They expressed concerns that
dropping the measure might signal that CMCS is deprioritizing HIV and serve only to increase
stigmatization. Workgroup members discussed state progress on developing mechanisms to
report the measure, including through a learning collaborative jointly sponsored by CDC, HRSA,
and CMCS, which may increase the number of states able to report the measure in the future.
The Avoidance of Antibiotic Treatment for Acute Bronchitis/Bronchiolitis and Appropriate
Treatment for Upper Respiratory Infection measures assess appropriate use of antibiotics for
respiratory infections with the goal of improving patient safety. Workgroup members noted the
importance of these measures for combating inappropriate antibiotic use, which recently has
been affected by the rise in telemedicine. However, Workgroup members raised concerns about
B.6
the measure methodologies, including whether these conditions are coded accurately in
administrative data, and whether changes in coding practices could be mistaken for quality
improvement.
The Transcranial Doppler Ultrasonography Screening for Children with Sickle Cell Anemia
measure was one of two measures suggested for improving quality of care for children with
sickle cell anemia. This measure was suggested because it has the potential to address disparities
for a population at early risk for stroke. When comparing the two sickle cell measures,
Workgroup members felt that the Appropriate Antibiotic Prophylaxis measure was the more
actionable of the two and had more opportunities for improvement.
The Proportion of Days Covered: Antiretroviral Medications measure was suggested for
addition because viral load can be reduced if antiretrovirals are taken regularly, whereas lack of
compliance can lead to antiretroviral resistance. Workgroup members raised questions about how
the specifications handle pre-exposure prophylaxis and whether an HIV diagnosis is required for
an individual to be included in the measure-eligible population. The measure steward clarified
that the measure is not intended to capture prophylaxis adherence. Although it was suggested to
replace the HIV Viral Load Suppression measure, Workgroup members did not recommend it as
a replacement measure.
The Statin Therapy for the Prevention and Treatment of Cardiovascular Disease measure was
suggested for addition given the high prevalence of cardiovascular disease and the relative
availability and affordability of statins. Discussion of the measure centered on concerns that the
measure assessed whether a statin was ordered, rather than whether it was filled or taken. As
with other EHR and registry-based measures, Workgroup members also raised concerns about
feasibility due to limited access to the necessary data.
Behavioral Health Care
A Workgroup member suggested removal of the Medical Assistance with Smoking and Tobacco
Use Cessation (MSC-AD) measure from the Adult Core Set, because of low state uptake and the
high cost of conducting the CAHPS survey. Workgroup members noted that there are other
surveys and measures that monitor tobacco use. One concern with the MSC-AD measure is that
it does not ask about vaping, but rather leaves the question open for interpretation. The Tobacco
Use: Screening and Cessation Intervention measure was suggested as a potential replacement for
the MSC-AD measure, but Workgroup members raised similar concerns about the absence of
vaping from the measure specifications. Other members acknowledged that tobacco use is a large
public health issue, and that the Workgroup should not reject these measures solely because they
do not currently include vaping. Workgroup members also emphasized that tobacco cessation
education or other activities might occur outside the primary care setting, and that the MSC-AD
measure might give a broader perspective on those services.
A Workgroup member suggested the addition of the Preventive Care and Screening: Unhealthy
Alcohol Use: Screening and Brief Counseling measure to address gaps in assessing alcohol
screening and brief intervention among non-alcohol-dependent adults. This topic is a high
priority for some states, because it addresses gaps in alcohol screening, which is especially
relevant for pregnant women. Workgroup members noted that this measure is important because
B.7
there is a lack of accountability for alcohol screening among providers. However, Workgroup
members cited lack of specificity related to the screening tool as a weakness of the measure.
Further, one Workgroup member noted that the field of addiction medicine is moving away from
labeling people as having “problematic alcohol use.”
The Workgroup discussed, but did not recommend, three measures related to opioid use: (1) Use
of Opioids from Multiple Providers in Persons without Cancer, (2) Continuity of
Pharmacotherapy for Opioid Use Disorder, and (3) Pharmacotherapy for Opioid Use Disorder.
The first measure was suggested because it could be used to assess the effectiveness of state
initiatives to address the opioid epidemic. However, Workgroup members raised concerns about
underreporting, as individuals might pay out-of-pocket for opioids. One state representative
noted that they are currently calculating the measure but not releasing the results because the
data are unreliable. The Workgroup member who suggested the Continuity of Pharmacotherapy
for OUD measure noted that it was a first step in measuring recovery and health outcomes in a
population at high risk for overdose and death. It could be used to address the gap in assessing
retention in care, which can serve as a proxy for recovery. Workgroup members noted that
measuring continuity of medication assisted treatment is important; however, it was noted that the
measure does not incorporate a therapy component. The Pharmacotherapy for OUD measure was
suggested because of evidence that pharmacotherapy can improve outcomes for individuals with
OUD. This measure looks only at new episodes, which differentiates it from the Continuity of
Pharmacotherapy measure. Workgroup members deliberated whether measuring the first
appointment versus continuity of care was more valuable for the Core Set, with some Workgroup
members saying both are critical to measure. Workgroup members deliberated the merits of each
of the measures and called on the measure stewards and technical experts to differentiate the two
measures for future consideration.
A Workgroup member suggested the Query of Prescription Drug Monitoring Program measure
to address gaps in tracking the use of Prescription Drug Monitoring Programs (PDMPs), which
can improve prescribing of controlled substances, a key step in controlling the opioid epidemic.
According to the Workgroup member, PDMP implementation is associated with decreased
opioid-related overdose deaths. Several Workgroup members from state Medicaid agencies
raised concerns about state laws preventing health plans from accessing the PDMP data. Another
member noted that as part of the Substance Use-Disorder Prevention That Promotes Opioid
Recovery and Treatment for Patients and Communities (SUPPORT) Act, there will be reporting
requirements related to PDMP beginning in 2023.
A Workgroup member suggested addition of the Follow-Up After High-Intensity Care for
Substance Use Disorder measure to address a gap in tracking receipt of follow-up care for SUD
treatment services. The member who suggested this measure noted that nationally, there is
greater investment in inpatient services for SUD, and less emphasis on continuity of care after
receiving inpatient services. Workgroup members expressed concern that because this measure
was proposed for HEDIS 2020, it was not yet ready for the Core Set; however, one member
noted that the concept of follow-up care had been tested for other measures.
B.8
Dental and Oral Health Services
The Workgroup discussion about the three dental and oral health measures considered for
addition to the Core Sets focused on whether the measures were ready for implementation by
state Medicaid programs. The Workgroup discussion on the Ambulatory Care Sensitive
Emergency Department Visits for Dental Caries in Children and the Follow-Up After Emergency
Department Visits for Dental Caries in Children focused on the results from states that have
tested or implemented the measure, as well as the relationship between the proposed measures
and the two existing dental measures in the Child Core Set. The Workgroup also raised questions
about the measure technical specifications, the sources of data needed to calculate the measure,
and whether this information would be readily available to all state Medicaid programs
(especially those with dental carve-outs). Although the Workgroup noted that the Adults with
Diabetes – Oral Evaluation measure would fill a gap in the Adult Core Set and is feasible
(having been implemented in one state’s incentive program), some members expressed concern
that the measure was still undergoing testing and that it might be more related to diabetes (for
which there are several other Adult Core Set measures) than oral health care. Nevertheless, states
expressed considerable interest in the three measures and some indicated they were planning to
share the measures with their staff.
Experience of Care
A Workgroup member suggested removing both CAHPS measures (CAHPS Health Plan Survey
5.0H –Child Version [CPC-CH] and CAHPS Health Plan Survey 5.0H – Adult Version [CPA-
AD]), citing poor state response rates, the high cost of administering the surveys, and the fact
that results may not be comparable across diverse populations. Many Workgroup members noted
that CAHPS is valuable because analysis of the data helps understand how patients experience
the care they receive. State representatives commented that they analyze CAHPS data, including
by health plan in managed care states, publicly post the findings, and use the results to inform
system and health plan performance improvement. Workgroup members were interested in
learning more about how CMCS uses the CAHPS data that states report for the Core Sets.
Two measures were considered for addition to the Core Set: Child Hospital CAHPS Survey and
Healthy Days Core Module – Health-Related Quality of Life. The Workgroup member who
suggested the Child Hospital CAHPS Survey measure noted that it would fill a gap by measuring
the experience of health care for children in hospitals. This measure has been considered in the
past, and it was recommended for additional testing (which is in process). A Workgroup member
noted that states are not actively using the measure. In addition, the survey is currently conducted
for all children and would need to be modified to be specific to the Medicaid population.
Furthermore, a Workgroup member noted that Medicaid programs have limited oversight over
hospital care, which may make it less appropriate for the Child Core Set.
The Workgroup member who suggested the Healthy Days Core Module – Health-Related
Quality of Life measure noted that although there is robust dialogue on how to measure and
improve an individual’s or a community’s social determinants of health, few measures have been
used or tested. This measure, however, has been available in the Behavioral Risk Factor
Surveillance System (BRFSS) since 1993 and is on the core module for that surveillance system.
Workgroup members noted concerns about the feasibility of reporting this measure specifically
B.9
for Medicaid beneficiaries because questions on respondents’ insurance status (including
Medicaid coverage) are optional in BRFSS and are not asked by all states.
Long-Term Services and Supports
Workgroup members discussed six LTSS measures that were suggested but not recommended
for addition to the 2020 Core Sets. All six measures were suggested to fill a gap in the 2019 Core
Sets, which contain no LTSS-focused measures. Workgroup members noted the importance of
adding LTSS measures, as this population comprises a large and growing share of Medicaid
beneficiaries and Medicaid expenditures and existing measures do not capture the unique needs
and experiences of this population.
Workgroup members discussed four measures as a group: (1) LTSS: Successful Transition After
Long-term Institutional Stay, (2) LTSS: Comprehensive Assessment and Update, (3) LTSS:
Comprehensive Care Plan and Update, and (4) LTSS: Reassessment/Care Plan Update After
Inpatient Discharge. These measures were developed on behalf of CMS as part of a suite of
LTSS measures and were designed specifically for states with managed LTSS delivery systems
(currently about 24 states). Measure developers noted that the measures could potentially be
adapted for use in states with fee-for-service delivery of LTSS. Workgroup members raised
concerns about the feasibility of collecting the data at the state level, especially because three of
the four measures require a case management record review. Workgroup members noted that the
first measure, LTSS: Successful Transition After Long-Term Institutional Stay, is an outcome
measure designed to assess progress in transitioning people to the community. However, the
other three measures focus on processes rather than on outcomes, such as completing
assessments, care plans, and care plan updates. For the two measures related to care plans, the
Workgroup questioned how the care plan elements were selected. Some states and home and
community based service (HCBS) waiver programs already have their own approaches to care
planning, and Workgroup members suggested that it could be difficult or undesirable to mandate
a single federal approach. Other Workgroup members responded that although the measures are
not perfect and might not exclusively represent outcomes, LTSS is a noted gap area, and there is
value in beginning to assess LTSS across states. They pointed out that states could use these four
measures to compare results across LTSS plans and care management entities to identify issues.
Additionally, there was discussion about the potential for using these four measures in the Health
Home Core Set or in Medicaid MLTSS contracts if they are not appropriate for the Child and
Adult Core Sets.
The next LTSS measure suggested but not recommended for addition to the 2020 Core Sets was
the Consumer Assessment of Healthcare Providers and Systems Home and Community Based
Services (HCBS CAHPS) survey, a cross-disability survey of the experience of HCBS
beneficiaries receiving LTSS. It is designed to facilitate comparisons across state Medicaid
HCBS programs throughout the country and is available for voluntary use as part of quality
assurance and improvement activities and public reporting. The survey instrument is designed to
be accessible to all populations of beneficiaries with disabilities, including individuals who are
nonverbal. The measure steward noted that 17 states have used the survey, including states
participating in the Testing Experience & Functional Tools (TEFT) demonstration and MLTSS
states. Because this is a new survey and the platform is still under development (scheduled to
become available in January 2020), Workgroup members voiced concern about adding it to the
B.10
2020 Core Sets. Members noted that data collection would need to be built into requirements for
MLTSS plans or fielded and funded by state Medicaid programs, which might be costly. Some
members also expressed concern about the survey length and how it could affect response rates,
since the survey covers 21 different areas and would likely take 30 minutes to an hour or more to
complete.
The final LTSS measure discussed but not recommended by the Workgroup was the Personal
Outcome Measures, a tool designed to ensure that services and supports are person-centered.
During a Personal Outcome Measures interview, 21 indicators are used to understand the
presence, importance, and achievement of outcomes involving choice, health, safety, social
capital, relationships, rights, goals, dreams, employment, and more. Measure developers noted
that people have been trained to use the tool in 45 states, and it is available for public use online.
Some states already incorporate the tool into person-centered plans and others use it with a
sample of their clients. Workgroup members voiced concerns about the high cost and time
intensiveness of collecting this data via in-depth interviews.
As part of the discussion on both the HCBS CAHPS survey and the Personal Outcome Measures
tool, Workgroup members agreed that, although collecting in-depth information is challenging at
the state level, it is important to find a way to better understand the experiences of people
receiving LTSS. Likewise, in light of the significant resources that states invest in LTSS, it is
important to provide feedback to the Medicaid program. One member suggested that to
accommodate the variety of data collection options states are exploring, CMCS could give states
flexibility in choosing which tool to use to assess LTSS. States could explain which tool they
used when reporting to CMCS.
Other Measures
Workgroup members suggested two other measures that were discussed but not recommended
for addition to the 2020 Core Sets. The Workgroup member who suggested the Continuity of
Insurance: Informed Participation measure for consideration noted that duration of coverage is a
current gap in the Core Sets and that it affects the completeness of other measures in
understanding the experience of all Medicaid and CHIP beneficiaries. Workgroup members
asked questions about the measure technical specifications, which were answered by the measure
steward, and noted that the Core Set may not be the appropriate place for this measure. One
Workgroup member noted that this measure has not been used extensively, so it could be
beneficial for states to try it and see how it could be used for quality measurement and
improvement.
The Workgroup member who suggested the Health-Related Social Needs Screening measure
noted the growing evidence that addressing health-related social needs can help improve overall
health and well-being. The member commented that few measures are being used or tested to
enable state Medicaid programs to measure social needs. Many Workgroup members
emphasized the importance of measuring social determinants of health. However, it was noted
that CMS’s Center for Medicare & Medicaid Innovation is currently testing this measure, and as
a result, some Workgroup members were concerned that it is not ready for use in the Core Set.
There was also a question about whether states would want to use this tool or identify other tools
that achieve similar aims.

B.11
Exhibit B.1. Measures discussed by the 2020 Core Set Annual Review Workgroup but not recommended for removal or
addition, by domain
Measure name
Measure
steward NQF # Measure description
Data collection
method
Primary Care Access and Preventive Care
Discussed but not recommended for removal from the 2020 Core Set
Flu Vaccinations for Adults
Ages 18 to 64 (FVA-AD)
NCQA 0039
Percentage of beneficiaries ages 18 to 64 who received a
flu vaccination between July 1 of the measurement year
and the date when the Consumer Assessment of
Healthcare Providers and Systems (CAHPS) 5.0H Adult
Medicaid Survey was completed.
Survey (CAHPS 5.0H
Adult Medicaid
Survey)
Discussed but not recommended for addition to the 2020 Core Set
Lead Screening in
Children
NCQA Not endorsed
Percentage of children 2 years of age who had one or
more capillary or venous lead blood test for lead
poisoning by their second birthday.
Administrative or
hybrid
Follow-Up with Patient
Family After
Developmental Screening
AHRQ,
PMCoE
Not endorsed
Percentage of patients aged 6 months to 36 months
whose family received a follow-up discussion of
developmental screening results on the same day of the
screening visit.
EHR or medical record
review
Prenatal Immunization
Status
NCQA Not endorsed
Percentage of deliveries in the measurement period in
which women received influenza and tetanus, diphtheria
toxoids, and acellular pertussis (Tdap) vaccinations.
Three rates are reported: influenza, Tdap, and a
combination rate.
ECDS
a
Colorectal Cancer
Screening
NCQA 0034
Percentage of patients 50–75 years of age who had
appropriate screening for colorectal cancer.
Administrative or
hybrid
Flu Vaccinations for Adults
Age 65 and Older
NCQA 0039
Percentage of Medicare members 65 years of age and
older who received a flu vaccination between July 1 of
the measurement year and the date when the Medicare
CAHPS survey was completed.
Survey (this measure
is derived from the
Medicare CAHPS
Survey)
B..12
Measure name
Measure
steward NQF # Measure description
Data collection
method
Preventive Care and
Screening: Body Mass
Index Screening and
Follow-Up Plan
CMS 0421/0421e
Percentage of patients age 18 years and older with a
body mass index (BMI) documented during the current
encounter or during the previous 12 months AND with a
BMI outside of normal parameters, a follow-up plan is
documented during the encounter or during the previous
12 months of the current encounter. Normal Parameters:
Age 18 years and older BMI ≥ 18.5 and < 25 kg/m
2
.
Administrative or EHR
Adult Immunization Status NCQA Not endorsed
Percentage of adults 19 years and older who are up to
date on recommended routine vaccines for influenza;
tetanus and diphtheria (Td) or tetanus, diphtheria and
acellular pertussis (Tdap); herpes zoster; and
pneumococcal.
ECDS
a
HIV Screening CDC Not endorsed
Percentage of patients ages 15–65 who have been
tested for HIV within that age range.
EHR
Influenza Immunization PCPI 0041/0041e
Percentage of patients age 6 months and older seen for
a visit between October 1 and March 31 who received an
influenza immunization OR who reported previous receipt
of an influenza immunization.
Administrative or EHR
Maternal and Perinatal Health
Discussed but not recommended for removal from the 2020 Core Set
PC-01: Elective Delivery
(PC01-AD)
TJC 0469/0469e
Percentage of women with elective vaginal deliveries or
elective cesarean sections at ≥ 37 and < 39 weeks of
gestation completed. Lower rates are better for this
measure.
Hybrid or EHR
Contraceptive Care –
Postpartum Women Ages
21–44 (CCP-AD)
OPA 2902
Among women ages 21–44 who had a live birth, the
percentage that: (1) were provided a most effective or
moderately effective method of contraception within 3
and 60 days of delivery; (2) were provided a long-acting
reversible method of contraception within 3 and 60 days
of delivery.
Administrative

B.13
Measure name
Measure
steward NQF # Measure description
Data collection
method
Discussed but not recommended for addition to the 2020 Core Set
PC-05: Exclusive Breast
Milk Feeding
TJC 0480/0480e
Percentage of newborns that were exclusively fed breast
milk during the newborn’s entire hospitalization.
“Exclusive breast milk feeding” is defined as a newborn
receiving only breast milk and no other liquids or solids
except for drops or syrups consisting of vitamins,
minerals, or medicines.
EHR or chart review
Prenatal Depression
Screening and Follow-Up
NCQA Not endorsed
Percentage of deliveries in which women were screened
for clinical depression while pregnant and, if screened
positive, received follow-up care. Two rates are reported:
(1) depression screening: the percentage of deliveries in
which women were screened for clinical depression using
a standardized tool during pregnancy; and (2) follow-up
on positive screen: the percentage of deliveries in which
pregnant women received follow-up care within 30 days
of screening positive for depression.
ECDS
a
Postpartum Depression
Screening and Follow-Up
NCQA Not endorsed
Percentage of deliveries in which women were screened
for clinical depression during the postpartum period, and
if screened positive, received follow-up care. Two rates
are reported: (1) depression screening: percentage of
deliveries in which women were screened for clinical
depression using a standardized tool within 12 weeks (84
days) post-delivery; and (2) follow-up on positive screen:
percentage of deliveries in which women received follow-
up care within 30 days of screening positive for
depression.
ECDS
a
Care of Acute and Chronic Conditions
Discussed but not recommended for removal from the 2020 Core Set
HIV Viral Load
Suppression (HVL-AD)
HRSA 2082/3210e
Percentage of beneficiaries age 18 and older with a
diagnosis of human immunodeficiency virus (HIV) who
had an HIV viral load less than 200 copies/mL at last HIV
viral load test during the measurement year.
Administrative or EHR

B.14
Measure name
Measure
steward NQF # Measure description
Data collection
method
Discussed but not recommended for addition to the 2020 Core Set
Transcranial Doppler
Ultrasonography
Screening for Children
with Sickle Cell Anemia
QMETRIC–
University
of Michigan
2797
Percentage of children ages 2 through 15 years during
the measurement year and identified as having sickle cell
anemia who received at least one Transcranial Doppler
ultrasonography screening within a year.
Administrative
Proportion of Days
Covered: Antiretroviral
Medications
PQA Not endorsed
Percentage of individuals 18 years and older who met the
Proportion of Days Covered threshold of 90% for ≥ 3
antiretroviral medications during the measurement year.
Administrative
Statin Therapy for the
Prevention and Treatment
of Cardiovascular Disease
CMS Not endorsed
Percentage of the following patients—all considered at
high risk of cardiovascular events—who were prescribed
or were on statin therapy during the measurement period:
(1) adults age ≥ 21 years who were previously diagnosed
with or currently have an active diagnosis of clinical
atherosclerotic cardiovascular disease; OR (2) adults age
≥ 21 years who have ever had a fasting or direct low-
density lipoprotein cholesterol (LDL-C) level ≥ 190 mg/dL
or were previously diagnosed with or currently have an
active diagnosis of familial or pure hypercholesterolemia;
OR (3) adults ages 40–75 with a diagnosis of diabetes
with a fasting or direct LDL-C level of 70–189 mg/dL.
EHR or registry
Avoidance of Antibiotic
Treatment for Acute
Bronchitis/Bronchiolitis
NCQA 0058
Percentage of episodes for members age 3 months and
older with a diagnosis of acute bronchitis/bronchiolitis
that did not result in an antibiotic dispensing event.
Administrative or EHR
Appropriate Treatment for
Upper Respiratory
Infection
NCQA 0069
Percentage of episodes for members 3 months of age
and older with a diagnosis of upper respiratory infection
that did not result in an antibiotic dispensing event.
Administrative or EHR

B.15
Measure name
Measure
steward NQF # Measure description
Data collection
method
Behavioral Health Care
Discussed but not recommended for removal from the 2020 Core Set
Medical Assistance with
Smoking and Tobacco
Use Cessation (MSC-AD)
NCQA 0027
The three components of this measure assess different
facets of providing medical assistance with smoking and
tobacco use cessation: (1) advising smokers and tobacco
users to quit: a rolling average represents the percentage
of beneficiaries age 18 and older who were current
smokers or tobacco users and who received advice to
quit during the measurement year; (2) discussing
cessation medications: a rolling average represents the
percentage of beneficiaries age 18 and older who were
current smokers or tobacco users and who discussed or
were recommended cessation medications during the
measurement year; and (3) discussing cessation
strategies: a rolling average represents the percentage of
beneficiaries age 18 and older who were current smokers
or tobacco users and who discussed or were provided
cessation methods or strategies during the measurement
year.
Survey (CAHPS 5.0H
Adult Medicaid
Survey)
Discussed but not recommended for addition to the 2020 Core Set
Tobacco Use: Screening
and Cessation Intervention
PCPI 0028/0028e
Percentage of patients age 18 and older who were
screened for tobacco use one or more times within 24
months AND who received tobacco cessation
intervention if identified as a tobacco user.
Administrative or EHR
Preventive Care and
Screening: Unhealthy
Alcohol Use: Screening &
Brief Counseling
PCPI 2152
Percentage of patients age 18 years and older who were
screened for unhealthy alcohol use using a systematic
screening method at least once within the last 24 months
AND who received brief counseling if identified as an
unhealthy alcohol user.
EHR or registry
Use of Opioids from
Multiple Providers in
Persons Without Cancer
PQA 2950
Percentage of individuals age 18 and older without
cancer who received prescriptions for opioids from four or
more prescribers AND four or more pharmacies within
less than or equal to 180 days. Lower rates are better for
this measure.
Administrative

B.16
Measure name
Measure
steward NQF # Measure description
Data collection
method
Continuity of
Pharmacotherapy for
Opioid Use Disorder
USC 3175
Percentage of adults 18–64 years of age with
pharmacotherapy for opioid use disorder (OUD) who
have at least 180 days of continuous treatment.
Administrative or EHR
Pharmacotherapy for
Opioid Use Disorder
NCQA Not endorsed
Percentage of new pharmacotherapy treatment episodes
that resulted in 180 or more covered treatment days
among members 16 years of age and older with a
diagnosis of OUD.
Administrative or EHR
Query of Prescription Drug
Monitoring Program
CMS Not endorsed
For at least one Schedule II opioid electronically
prescribed using Certified Electronic Health Records
Technology (CEHRT) during the performance period, the
Merit-based Incentive Payment System eligible clinician
uses data from CEHRT to conduct a query of a
Prescription Drug Monitoring Program for prescription
drug history, except where prohibited and in accordance
with applicable law.
Administrative or EHR
Follow-Up After High-
Intensity Care for
Substance Use Disorder
NCQA Not endorsed
Percentage of acute inpatient hospitalizations, residential
treatment, or detoxification visits for a diagnosis of
substance use disorder that result in a follow-up visit or
service for substance use disorder among individuals 13
years of age and older. Two rates are reported: (1)
percentage of visits or discharges for which the individual
received follow-up for substance use disorder within the
30 days after the visit or discharge, and (2) percentage of
visits or discharges for which the individual received
follow-up for substance use disorder within the 7 days
after the visit or discharge.
Administrative
Dental and Oral Health Services
Discussed but not recommended for addition to the 2020 Core Set
Ambulatory Care Sensitive
Emergency Department
Visits for Dental Caries in
Children
ADA/
DQA
2689
Number of emergency department (ED) visits for caries-
related reasons per 100,000 member months for all
enrolled children. Rates are stratified by age and by ED
visit disposition (visits resulting in an inpatient admission
and those not resulting in an inpatient admission). Lower
rates are better for this measure.
Administrative

B.17
Measure name
Measure
steward NQF # Measure description
Data collection
method
Follow-Up After
Emergency Department
Visits for Dental Caries in
Children
ADA/
DQA
2695
Percentage of caries-related ED visits among children 0
through 20 years in the reporting period for which the
member visited a dentist within (1) 7 days and (2) 30
days of the ED visit.
Administrative
Adults with Diabetes –
Oral Evaluation
ADA/
DQA
Not endorsed
Percentage of enrolled adults with diabetes who received
a comprehensive or periodic oral evaluation or a
comprehensive periodontal evaluation within the
measurement year.
Administrative
Experience of Care
Discussed but not recommended for removal from the 2020 Core Set
Consumer Assessment of
Health Care Providers and
Systems (CAHPS) Health
Plan Survey 5.0H – Child
Version (Medicaid) (CPC-
CH)
NCQA Not endorsed
This measure provides information on parents’
experiences with their child’s health care and gives a
general indication of how well the health care meets their
expectations. Results summarize children’s experiences
through ratings, composites, and individual question
summary rates. The Child Core Set measure includes the
Children with Chronic Conditions Supplemental Items.
Survey
Consumer Assessment of
Health Care Providers and
Systems (CAHPS) Health
Plan Survey 5.0H – Adult
Version (Medicaid) (CPA-
AD)
NCQA Not endorsed
This measure provides information on beneficiaries’
experiences with their health care and gives a general
indication of how well the health care meets the
beneficiaries’ expectations. Results summarize
beneficiaries’ experiences through ratings, composites,
and individual question summary rates.
Survey
Discussed but not recommended for addition to the 2020 Core Set
Child Hospital Consumer
Assessment of Healthcare
Providers and Systems
(CAHPS) Survey
AHRQ 2548
This measure asks parents and guardians of children
under 18 years old to report on their and their child’s
experiences with inpatient hospital care. Results consist
of 39 items organized by overarching groups into 18
composite and single-item measures. The domains
include: Communication with Parent, Communication
with Child, Attention to Safety and Comfort, Hospital
Environment, and Global Rating.
Survey

B.18
Measure name
Measure
steward NQF # Measure description
Data collection
method
Healthy Days Core
Module – Health-Related
Quality of Life
CDC Not endorsed
The four Health-Related Quality of Life Healthy Days
Core Module (HRQOL-4) items ask about self-rated
general health and the number of days when a person
was physically unhealthy, mentally unhealthy, or limited
in usual activities within the previous 30 days. A
summary measure combines physically and mentally
unhealthy days. The module was developed for national
and state surveillance surveys, including the state-based
Behavioral Risk Factor Surveillance System (BRFSS),
the National Health and Nutrition Examination Survey,
and the Medicare Health Outcomes Survey.
Survey
Long-Term Services and Supports (LTSS)
Discussed but not recommended for addition to the 2020 Core Set
Consumer Assessment of
Healthcare Providers and
Systems (CAHPS) Home
and Community Based
Services (HCBS) Survey
CMS 2967
(19 HCBS
CAHPS
measures are
endorsed)
The HCBS CAHPS is a cross-disability survey of the
experience of HCBS beneficiaries receiving LTSS. It is
designed to facilitate comparisons across state Medicaid
HCBS programs that target adults with disabilities,
including frail elderly, individuals with physical disabilities,
persons with developmental or intellectual disabilities,
those with acquired brain injury, and persons with severe
mental illness. The HCBS CAHPS Survey is available for
voluntary use in HCBS programs as part of quality
assurance and improvement activities and public
reporting.
Survey
LTSS: Successful
Transition After Long-
Term Institutional Stay
CMS Not endorsed
Proportion of long-term institutional facility stays among
Medicaid Managed LTSS (MLTSS) plan members age 18
and older, which result in successful transitions to the
community (community residence for 60 or more days).
This measure is reported as an observed rate and a risk-
adjusted rate.
Administrative

B.19
Measure name
Measure
steward NQF # Measure description
Data collection
method
LTSS: Comprehensive
Assessment and Update
CMS Not endorsed
Percentage of Medicaid MLTSS plan members 18 years
of age and older who have documentation of a
comprehensive assessment in a specified time frame that
includes documentation of core elements. The following
rates are reported: (1) assessment of core elements:
MLTSS plan members who had a comprehensive LTSS
assessment with nine core elements documented within
90 days of enrollment (for new members) or annually;
and (2) assessment of supplemental elements: MLTSS
plan members who had a comprehensive LTSS
assessment with nine core elements and at least 12
supplemental elements documented within 90 days of
enrollment (for new members) or annually. In addition,
two rates of required exclusions should be reported: (1)
member could not be contacted for care planning; and (2)
member refused to participate in care planning.
Case management
record review
LTSS: Comprehensive
Care Plan and Update
CMS Not endorsed
Percentage of Medicaid MLTSS plan members 18 years
of age and older who have documentation of a
comprehensive LTSS care plan in a specified time frame
that includes documentation of core elements. The
following rates are reported: (1) care plan with core
elements documented: MLTSS plan members who had a
comprehensive LTSS care plan with nine core elements
documented within 120 days of enrollment (for new
members) or annually; and (2) care plan with
supplemental elements documented: MLTSS plan
members who had a comprehensive LTSS care plan with
nine core elements and at least four supplemental
elements documented within 120 days of enrollment (for
new members) or annually.
Case management
record review

B.20
Measure name
Measure
steward NQF # Measure description
Data collection
method
LTSS: Reassessment/
Care Plan Update After
Inpatient Discharge
CMS Not endorsed
Percentage of discharges from inpatient facilities for
Medicaid MLTSS plan members 18 years of age and
older for whom a reassessment and care plan update
occurred within 30 days of discharge. Two performance
rates are reported: (1) reassessment after inpatient
discharge: percentage of discharges from inpatient
facilities resulting in a LTSS reassessment within 30 days
of discharge; and (2) reassessment and care plan update
after inpatient discharge: percentage of discharges from
inpatient facilities resulting in an LTSS reassessment and
care plan update within 30 days of discharge. In addition,
two rates of required exclusions should be reported: (1)
member could not be contacted for assessment and/or
care planning; and (2) member refused to participate in
assessment and/or care planning.
Case management
record review
Personal Outcome
Measures
CQL Not endorsed
Personal Outcome Measures is a tool designed to ensure
that services and supports are person-centered. In a
Personal Outcome Measures interview, 21 indicators are
used to understand the presence, importance and
achievement of outcomes involving choice, health,
safety, social capital, relationships, rights, goals, dreams,
employment, and more. Measures are organized into five
topic areas: human security, community, relationships,
choices, and goals.
In-depth interview
Other Measures
Discussed but not recommended for addition to the 2020 Core Set
Continuity of Insurance:
Informed Participation
CHOP 3154
This measure assesses the continuity of enrollment of
children in publicly financed insurance programs
(Medicaid and CHIP), as defined by the ratio of enrolled
months to eligible months over an 18-month period
(called an “observation window”). The measure uses a
natural experiment based on the random event of
appendicitis to “inform” the estimate of coverage in a
given state.
Administrative

B.21
Measure name
Measure
steward NQF # Measure description
Data collection
method
Health-Related Social
Needs (HRSN) Screening
CMS Not endorsed
A 10-item screening tool designed to identify patient
needs in 5 domains that can be addressed through
community services (housing instability, food insecurity,
transportation difficulties, utility assistance needs, and
interpersonal safety).
Survey
a
ECDS data collection method includes data from administrative claims, electronic health records, case management systems, and health information
exchanges/clinical registries.
ADA = American Dental Association; AHRQ = Agency for Healthcare Research and Quality; CDC = Centers for Disease Control and Prevention; CHIP =
Children’s Health Insurance Program; CHOP = Children's Hospital of Philadelphia; CMCS = Centers for Medicaid and CHIP Services; CMS = Centers for Medicare
& Medicaid Services; CQL = Council on Quality and Leadership; DQA = Dental Quality Alliance; ECDS = Electronic Clinical Data System; EHR = Electronic Health
Record; HRSA = Health Resources and Services Administration; NA = Measure is not NQF endorsed; NCQA = National Committee for Quality Assurance; NQF =
National Quality Forum; OPA = Office of Population Affairs; PCPI = Physician Consortium for Performance Improvement; PMCoE = Pediatric Measurement
Center of Excellence; PQA = Pharmacy Quality Alliance; QMETRIC = Quality Measurement, Evaluation, Testing, Review, and Implementation Consortium; TJC =
The Joint Commission; USC = University of Southern California.
This page has been left blank for double-sided copying.
Appendix C
Public Comments on the Draft Report
This page has been left blank for double-sided copying.

C.1
The draft report was available for public review and comment from July 8, 2019 through August
5, 2019 and stakeholders were invited to submit comments via email. A total of 40 public
comments were received. Commenters included state and federal agencies, professional
associations, stakeholder organizations, research firms, and health plans. Mathematica
appreciates the time and effort taken by commenters to prepare and submit their comments on
the draft report.
Exhibit C.1 categorizes the public comments received on the draft report by the following topics:
general comments, measures recommended for removal from or addition to the Core Sets,
measures discussed but not recommended for removal or addition, and gap areas. Many
comments addressed more than one topic. The verbatim public comments are included after the
exhibit, organized in alphabetical order by commenter name (agency/organization or individual
last name).
In summary, public comments were submitted on all seven measures the Workgroup
recommended for removal from the Core Sets, and all five measures recommended for addition.
Comments were also received on 12 measures considered by the Workgroup, but not
recommended for removal from or addition to the 2020 Core Sets.
Exhibit C.1. Summary of Public Comments by Topic and Commenter
Topic Commenter
General Comments
• Adult Vaccine Access Coalition
• American Academy of Pediatrics
• American Association on Health and Disability and the
Lakeshore Foundation
• American Society of Hematology
• Anthem
• Association for Community Affiliated Plans
• California Department of Health Care Services
• Children's Dental Health Project
• Children's Hospital Association
• CVS Health
• Kaiser Permanente
• National Association of State Directors of Developmental
Disability Services
• Novo Nordisk
• Office of Infectious Disease and HIV/AIDS Policy
• YMCA of the USA
Measures Recommended for Removal from the Child Core Set
Children and Adolescents’
Access to Primary Care
Practitioners (CAP-CH)
• Association for Community Affiliated Plans
• California Department of Health Care Services

C.2
Topic Commenter
Weight Assessment and
Counseling for Nutrition and
Physical Activity for
Children/Adolescents – Body
Mass Index Assessment for
Children/Adolescents (WCC-
CH)
• American Academy of Pediatrics
• Association for Community Affiliated Plans
• Christopher Bolling
• California Department of Health Care Services
• Children’s Health Integrated Program in Childhood Obesity
• CVS Health
• Healthy Weight Partnership Inc.
• Kentucky Department for Medicaid Services
• Michael & Susan Dell Center for Healthy Living
• National Association of Community Health Centers
• New Balance Foundation for Obesity Prevention Center,
Boston Children’s Hospital
• Novo Nordisk
• Obesity Care Advocacy Network
• Redstone Center, Milken Institute School of Public Health
• Trust For America’s Health
• University of Texas School of Public Health
• YMCA of the USA
Pediatric Central Line-
Associated Bloodstream
Infections (CLABSI-CH)
• Association for Community Affiliated Plans
• California Department of Health Care Services
Use of Multiple Concurrent
Antipsychotics in Children
and Adolescents (APC-CH)
• Anthem
• Association for Community Affiliated Plans
• California Department of Health Care Services
Measures Recommended for Removal from the Adult Core Set
Adult Body Mass Index
Assessment (ABA-AD)
• Association for Community Affiliated Plans
• Christopher Bolling
• California Department of Health Care Services
• Kentucky Department for Medicaid Services
• Novo Nordisk
• Obesity Care Advocacy Network
• Redstone Center, Milken Institute School of Public Health
• Trust For America’s Health
• University of Texas School of Public Health
• YMCA of the USA
Comprehensive Diabetes
Care: Hemoglobin A1c
(HbA1c) Testing (HA1C-AD)
• Association for Community Affiliated Plans
• California Department of Health Care Services
• District of Columbia Department of Health Care Finance
• Novo Nordisk
• YMCA of the USA
Annual Monitoring for
Patients on Persistent
Medications (MPM-AD)
• Association for Community Affiliated Plans
• California Department of Health Care Services

C.3
Topic Commenter
Measures Recommended for Addition to the 2020 Core Sets
Appropriate Antibiotic
Prophylaxis for Children with
Sickle Cell Anemia
• American Board of Pediatrics
• American Society of Hematology
• Anthem
• Association for Community Affiliated Plans
• California Department of Health Care Services
• Children's Hospital Association
• CVS Health
• District of Columbia Department of Health Care Finance
• Kaiser Permanente
• Kentucky Department for Medicaid Services
Metabolic Monitoring for
Children and Adolescents on
Antipsychotics
• Anthem
• Association for Community Affiliated Plans
• California Department of Health Care Services
• Kaiser Permanente
Use of Pharmacotherapy for
Opioid Use Disorder
• Anthem
• Association for Community Affiliated Plans
• California Department of Health Care Services
• CVS Health
• District of Columbia Department of Health Care Finance
• Kaiser Permanente
National Core Indicators
• American Association on Health and Disability and the
Lakeshore Foundation
• Anthem
• Association for Community Affiliated Plans
• California Department of Health Care Services
• Connecticut Department of Developmental Services
• District of Columbia Department of Health Care Finance
• Human Services Research Institute
• Indiana Division of Disability and Rehabilitative Services
• Kaiser Permanente
• Kentucky Department for Medicaid Services
• Maryland Department of Health
• Minnesota Department of Human Services
• Missouri Division of Developmental Disabilities
• National Association of State Directors of Developmental
Disability Services
National Core Indicators for
Aging and Disabilities
• American Association on Health and Disability and the
Lakeshore Foundation
• Anthem
• Association for Community Affiliated Plans
• California Department of Health Care Services
• District of Columbia Department of Health Care Finance
• Human Services Research Institute
• Kaiser Permanente
• Kentucky Department for Medicaid Services
• Minnesota Department of Human Services

C.4
Topic Commenter
Measures Considered and Not Recommended for Addition by Domain
Primary Care Access and
Preventive Care Domain
• Adult Vaccine Access Coalition
• Biotechnology Innovation Organization
• Connecticut Children’s Office for Community Child Health/Help
Me Grow National Center
• Kaiser Permanente Washington Health Research Institute
Maternal and Perinatal Health
Domain
• Connecticut Children’s Office for Community Child Health/Help
Me Grow National Center
Care of Acute and Chronic
Conditions Domain
• American Board of Pediatrics
• Children's Hospital Association
• CVS Health
Behavioral Health Care
Domain
• CVS Health
Dental and Oral Health
Services Domain
• American Academy of Pediatrics
• Children's Dental Health Project
• Kaiser Permanente
Long Term Services and
Supports Domain
• Aging and Disability Policy and Leadership Consulting, LLC
• American Association on Health and Disability and the
Lakeshore Foundation
• Anthem
• Human Services Research Institute
• Indiana Division of Disability and Rehabilitative Services
• Maryland Department of Health
• Minnesota Department of Human Services (Missouri Division of
Developmental Disabilities
Gap Areas
• Adult Vaccine Access Coalition
• American Association on Health and Disability and the
Lakeshore Foundation
• Biotechnology Innovation Organization
• Children's Dental Health Project
• Connecticut Children’s Office for Community Child Health/Help
Me Grow National Center
• Allison LaRussa
C.5
PUBLIC COMMENTS LISTED ALPHABETICALLY BY
AGENCY/ORGANIZATION NAME OR INDIVIDUAL
COMMENTER’S LAST NAME
Adult Vaccine Access Coalition (Abby Bownas)
The Adult Vaccine Access Coalition (AVAC) appreciates the opportunity to comment on
Mathematica’s Summary of a Multi-stakeholder Review of the 2020 Child and Adult Core Sets.
We appreciate the Workgroup’s interest in strengthening and improving the Medicaid Child and
Adult Health Care Quality Core Sets for 2020, and their recognition of the importance of quality
measures to improve the health of individuals and entire communities.
AVAC encourages the Workgroup take a focused, concerted approach to adult immunizations
as a means of improving population health as well as the overall health of Medicaid patients.
We hope that as part of your final recommendations, the Workgroup will reconsider adoption of
two recent HEDIS immunization measures.
• Adult Immunization Status (AIS). Add the new Adult Immunization Status (AIS) measure, to
replace the current adult influenza vaccine measure based on Consumer Assessment of
Healthcare Providers and Systems (CAHPS) surveys. The new AIS measure is a composite
of the age-recommended vaccines for adults, including influenza vaccine.
• Prenatal Immunization Status. Add the new Prenatal Immunization Status, which measures
prenatal immunizations of Tdap and influenza. Retain the other two current immunization
measures: Childhood immunization status (CIS) and Immunization of Adolescents (IMA).
AVAC consists of over 50 organizational leaders in health and public health that are committed
to addressing the range of barriers to adult immunization and to raising awareness of the
importance of adult immunization. AVAC works towards common legislative and regulatory
solutions that will strengthen and enhance access to adult immunization across the health care
system. Our priorities and objectives are driven by a consensus process with the goal of
enabling the range of stakeholders to have a voice in the effort to improve access and utilization
of adult immunizations.
Potential Gap Areas for Future Core Set Measures
We appreciate the draft Workgroup report noted gaps in terms of immunization quality
measures, particularly with regard to prenatal and adult populations. Significant racial and ethnic
disparities currently exist in adult immunization
1
coverage rates and the failure to improve these
rates among the Medicaid population only exacerbates these disparities. Opportunities to
assess the immunization status of Medicaid beneficiaries, particularly pregnant women and
medically vulnerable adults with chronic conditions such as diabetes and heart disease, should
be done by the range of clinicians who care for them, including primary care and specialty
providers. Taking advantage of each and every patient encounter to facilitate counseling and
education on vaccines, based on their age and health status, and to offer a strong provider
recommendation have been found to improve the likelihood of a patient being immunized.
C.6
Published literature indicates that integrating immunization assessment and additional providers
offering these critical preventive services will result in greater opportunities for immunization.
2
The National Vaccine Advisory Committee’s (NVAC) Adult Immunization Standards call for all
providers caring for adult patients to assess, recommend, vaccinate or refer, and document
vaccinations. Immunization quality measures are a crucial tool for health care quality
improvement and have demonstrated effectiveness in improving immunization coverage across
adult populations. Quality measures, such as the adult immunization status measure and the
prenatal immunization status measure can help to fill gaps while eliminating disparities around
adult immunization moving forward.
Adult Immunization Status Measure
AVAC recommends that the Workgroup replace the current adult influenza vaccine measure
based on Consumer Assessment of Healthcare Providers and Systems (CAHPS) surveys. The
new AIS measure is a composite of several age-recommended vaccines for adults, including
the influenza vaccine. An adult immunization composite measure would provide a sound,
reliable and comprehensive means to assess the receipt of routine adult vaccinations
recommended by the Advisory Committee on Immunization Practices (ACIP). The Adult
Immunization Status (AIS) measure should be adopted as a final recommendation of the
Workgroup because it includes more vaccines than the current immunization measure (FVA-
AD) and would help states monitor recommended adult immunization use beyond influenza.
Many adult patients are not being assessed and offered important ACIP-recommended
vaccines, resulting in poor adult immunization coverage rates nationally.
3
Despite the clear harm
from influenza, as witnessed during the 2017-2018 influenza season, immunization coverage
rates continue to lag behind Healthy People 2020 goals. While the benefits of pneumococcal
vaccination of adults with certain chronic high-risk conditions are well documented, only about
20% of these persons are vaccinated. Adults over the age of 65 are especially vulnerable to
complications from vaccine preventable diseases and thus are recommended for vaccinations
including influenza, pneumococcal, and zoster. Unfortunately, even in this most vulnerable
population, vaccination coverage rates are below national goals.
In the Value and Imperative of Quality Measures for Adult Vaccines, renowned vaccine experts
explain how quality measures that capture and create incentives for appropriate adult
vaccinations can prevent illness and death, reduce caregiving demands, avoid unnecessary
healthcare spending, and set the foundation for healthy aging.
4
There is evidence that a
composite measure of the adult immunization schedule, such as those demonstrated by the
Northwest Tribal Epidemiology Center and by the National Nursing Home Quality Care
Collaborative, can improve patient health outcomes
5
. Adoption of an AIS measure would put
vaccination coverage rates into a larger context and encourage a more systematic approach for
all vaccines. Additionally, the HHS Office of Infectious Disease and HIV/AIDS Policy (OIDP) and
the Centers for Disease Control and Prevention (CDC) in collaboration with the National Adult
Immunization and Influenza Summit Quality Working group were instrumental in spearheading
the development and testing of a the AIS measure, along with the composite measure for
prenatal immunization, which has been adopted as part of HEDIS. We understand that the
C.7
Workgroup expressed concerns with states’ ability to accurately and reliably report the
immunization measures. The measures draw from Electronic Clinical Data Systems (ECDS),
which include immunization information systems (IIS), case management registries, claims, and
electronic health records (EHRs). We understand that while state Medicaid and CHIP programs
can access member claims, encounters, and the corresponding state/local Immunization IIS, it
may be more of a challenge for many state Medicaid agencies to capture EHR data. Therefore,
states could choose to assess different models of data capture, such as encouraging providers
to report to a community, regional or state-based health information exchange, in order to build
capacity for measures that rely on electronic clinical information. The National Committee for
Quality Assurance (NCQA) tested both measures in Medicaid and commercial health plans,
concluding that while the specifications are new and innovative, they are feasible to implement.
In addition, workgroup members representing state Medicaid programs expressed their
commitment to establishing the infrastructure by which to report these new data sources.
Because reporting measures within the Adult Core Set is currently voluntary, inclusion of these
new immunization measures would signal prioritization of this critical public health gap, while
allowing states to work on this new method of data collection and measure reporting. AVAC
supports the Workgroup member recommendation that CMCS consider an affinity group or
grant opportunity to help drive state coordination between Medicaid and public health registries,
particularly immunization registries. Immunization information systems, or registries are an
essential tool in managing immunization record data that enables providers to have accurate
information regarding a Medicaid beneficiary’s immunization status.
Prenatal Immunization Status Measure
AVAC urges the Workgroup to reconsider adoption of the prenatal immunization status
measure, which includes Tdap and influenza for 2020. Maternal and perinatal health has been
identified in prior reviews as an area to strengthen in the quality measure sets.
Like the AIS, the Prenatal Immunization Status measure will help to address substantial
disparities in prenatal immunization rates. Immunizing mothers during their third trimester
protects 9 in 10 babies from pertussis infections serious enough to need treatment in a
hospital.
6
Currently, prenatal immunization levels are lower among Medicaid members
compared to privately insured women. Getting a flu shot reduces a pregnant woman's risk of
hospitalization by 40% and helps protect the newborn before he/she is old enough to be
vaccinated. We appreciate that the Workgroup members acknowledged the importance of the
prenatal immunization status measure and its strong connection to improved health outcomes
for young infants. However, we respectfully disagree with the decision not to adopt the measure
because of data collection concerns.
The development and implementation of two new HEDIS 2019 measures—and adult
immunization composite measure comprising influenza, pneumococcal, zoster, and Tdap
vaccines and a prenatal (maternal) immunization measure comprising Tdap and influenza
vaccines—illustrates the recognition of the importance of adult immunization in protecting health
and the preventing disease in these medically vulnerable groups.
7
Adoption of these two quality
measures would provide useful and actionable results for state Medicaid and CHIP programs,
C.8
especially if they publicly post results and require reporting by Medicaid managed care plans.
Such performance assessment and feedback can drive quality improvement efforts to raise
immunization coverage rates. At the same time, the addition of these two new measures to the
Adult and Child Core set is critical to improving the health of adult and prenatal populations.
Again, thank you for the opportunity to share our perspective on this draft report. AVAC looks
forward to working with Mathematica on this important endeavor. Please contact an AVAC
Coalition Manager at (202) 540-1070 or in[email protected] if you wish to further
discuss our comments. To learn more about the work of AVAC visit www.adultvaccinesnow.org.
Citations
1
Williams, W.W. et al. MMWR Surveillance Summary 2017;66(11):1–28.
2
Quilici et al. “Role of vaccination in economic growth.” J Mark Access Health Policy; (2015)
3:10.3402/jmahp.v3.27044.
3
National Vaccine Advisory Committee. 2014. Public Health Rep. 2014 Mar-Apr; 129(2): 115–
123.
4
https://dev-adultvaccinesnow.pantheonsite.io/wp-content/uploads/2016/07/AVN-White-Paper-
FINAL.pdf.
5
https://www.hhs.gov/sites/default/files/tab_10.05_weiser_adult_iz_composite-measures.pdf.
6
https://www.cdc.gov/pertussis/pregnant/mom/vacc-effectiveness.html.
7
https://www.ncqa.org/news/ncqa-updates-quality-measures-for-hedis-2019/.
C.9
Aging and Disability Policy and Leadership Consulting, LLC (Lowell Arye)
I am writing to provide comments on the 2020 Core Set Review. As a member of the
Workgroup, I want to thank you for all the work you did in facilitating the meetings (both face to
face and the webinars). I also believe that the draft report does a good job in providing a
synopsis of our discussions and our recommendations.
My only comment is that I believe there needs to be more specificity included in some of the
reasons for the additions/deletions which were recommended. Specifically, in the area of Long
Term Services and Supports (LTSS), I believe that it would be useful to explain that
approximately 32% of all Medicaid expenditures are for LTSS and that it the workgroup found it
important to include measurements in the Core Set for almost a third of all Medicaid
expenditures. Similar information could be useful for the other additions as well.
I believe that inclusion of this information provides the case to CMS for inclusion of these
measurements in the Core Set. Thank you for all of your work. I look forward to the final report.
C.10
American Academy of Pediatrics (Kyle E Yasuda)
Thank you for the opportunity to review and provide comment on the Recommendations for
Improving the Core Sets of Health Care Quality Measures for Medicaid and CHIP Summary of a
Multistakeholder Review of the 2020 Child and Adult Core Sets. The American Academy of
Pediatrics (AAP) is an organization of 67,000 pediatricians, pediatric medical sub-specialists,
and pediatrics surgical specialists dedicated to the health, safety, and wellbeing of infants,
children, adolescents, and young adults. We have a long history of supporting our members to
ensure that “every child gets the right care every time” through a range of programs, activities,
and resources.
The development and implementation of national pediatric measures has moved considerably
slower than those of adults due to lack of evidence, risk adjustment, unreliable data sources,
and small patient populations for chronic pediatric conditions. Despite these challenges, the
AAP has remained a strong advocate supporting the harmonization of national quality
measurement efforts that promote child health and can be used for value-based payment. The
AAP promotes quality measures that: 1) Have a meaningful impact on child health and promote
the health of every child, 2) Utilize an evidence based or evidence informed approach when
determining impact on child health and development, 3) Are feasible for pediatricians and those
who care for children to collect and, 4) Reflect the diversity of pediatric care by covering the
broad range and complexity of pediatrics within a social determinants context.
Overall, the Academy agrees with the goals set forth by the workgroup to focus on measures
that are actionable, aligned, and appropriate for state-level reporting to drive improvement in the
quality of care and health outcomes. We strongly agree with the need to address gaps in the
core set pertaining to the social determinants of health. We also applaud Mathematica’s
transparency around the criteria for the recommended removal and additions from the CHIPRA
core set; as they are mostly in alignment with the Academy’s criteria for impacting child health.
We do however offer several suggestions for improvement on the process for assessing
measures as well as specific comments on several measure removals.
Regarding improvements on the process of measure assessment, we encourage Mathematica
to examine measures within and across domains for children. Children differ from adults and
models for pediatric quality measurement should take this diversity into account. Unique
differences between children and adults are often described in the literature include
development, dependency, differential epidemiology, demographics, and dollars. Children have
an upward developmental trajectory, with needs and abilities changing over time. Effectively
measuring children’s health requires more of a systems approach and examination of measures
across domain areas with further consideration on how these measures interact as a core set.
The types of care (prevention/wellness, acute care, mental/behavioral health), sites of care,
(inpatient, outpatient, school-based), healthy behaviors, overuse and appropriate treatment,
family and community engagement should all be considered.
The Academy is concerned about the removal of measures pertaining to oral health and body
mass index (BMI). Dental caries is the most common chronic condition of childhood –
C.11
disproportionally affecting children in Medicaid/CHIP and has implications on pain, Emergency
Department use, school (individual and group success), and long-term systemic health. The oral
health measures were removed without replacement, speaking against the 4th characteristic
(new or alternate measure) as mentioned in Exhibit ES.1. The AAP recommends that the oral
health measures should remain on the table for consideration in future sets due to the
importance of children’s oral health to overall health.
We also urge you to reconsider the removal of the Weight Assessment and Counseling in
Children. While we understand that the high performance of this measure is the reason for
removal, it is because of the current requirement, pediatricians across the country now check
BMI at every visit. Furthermore, children with disabilities have many competing demands during
their visits, and healthy weight counseling often gets overlooked. With decreased emphasis on
BMI measurement, children and teens that are just starting to increase their BMI and may miss
the opportunity for early intervention.
The AAP recognizes the effort Mathematica has put into the review process and the
development of the report. We applaud you for your commitment to improving health outcomes.
Thank you again for considering comments from the Academy. Please feel free to contact
Vanessa Shorte, Senior Director of Quality, at v[email protected] should you have questions.
C.12
American Association on Health and Disability and the Lakeshore
Foundation (Clarke Ross)
The American Association on Health and Disability and the Lakeshore Foundation appreciate
the opportunity to provide comments.
The American Association on Health and Disability (AAHD) (www.aahd.us) is a national non-
profit organization of public health professionals, both practitioners and academics, with a
primary concern for persons with disabilities. The AAHD mission is to advance health promotion
and wellness initiatives for persons with disabilities.
The Lakeshore Foundation (www.lakeshore.org) mission is to enable people with physical
disability and chronic health conditions to lead healthy, active, and independent lifestyles
through physical activity, sport, recreation and research. Lakeshore is a U.S. Olympic and
Paralympic Training Site; the UAB/Lakeshore Research Collaborative is a world-class research
program in physical activity, health promotion and disability linking Lakeshore’s programs with
the University of Alabama, Birmingham’s research expertise.
Overview
As a member of various related National Quality Forum committees since 2012 and as an
observer of this CMS-Mathematica Core Set Workgroup considerations, we find the report an
accurate and thoughtful description and summary of the workgroup’s discussions and decisions.
Thank you.
We reinforce the page 28 (pages when viewing the document from a web browser) Theme:
The Workgroup focuses on “a holistic approach to measuring the quality of care provided to
diverse populations and subpopulations enrolled in Medicaid and CHIP.” Numerous NQF
meetings and reports have emphasized the importance of the populations and subpopulations
when discussing quality and the Medicaid program. Thank you.
In discussing the Theme and overall approach, it might be helpful to remind the Medicaid and
CHIP audiences of the National Quality Strategy “Triple Aim” as a reference guide to the
nation’s approach to quality measurement in health and related services and supports. The
Triple Aim priorities are: (1) Improving the Patient Experience of Care; (2) Improving the health
of the population; and (3) Reducing the per capita cost of health care.
Long-Term Services and Supports: Overview
We appreciate the Appendix, page 54 (pages when viewing the document from a web browser)
concluding observation: “Workgroup members noted the importance of adding LTSS measures,
as this population comprises a large and growing share of the Medicaid beneficiaries and
Medicaid expenditures and existing measures do note capture the unique needs and
experiences of this populations.”
C.13
Further, the Appendix, page 55 states: Workgroup members recognize that “it is important to
find a way to better understand the experiences of people receiving LTSS.” And further on page
55: “In light of the significant resources that states invest in LTSS, it is important to provide
feedback to the Medicaid program.”
We recommend that these observations be moved from the Appendix discussion of particular
LTSS proposed measures to the actual text of the report.
And, we concur and support the comments submitted on the draft report for public comment by
Workgroup member Lowell Arye: “There needs to be more specificity included in some of the
reasons for the additions/deletions which were recommended. Specifically, in the area of Long
Term Services and Supports (LTSS), ….it would be useful to explain that approximately 32% of
all Medicaid expenditures are for LTSS and that the workgroup found it important to include
measurements in the Core Set for almost a third of all Medicaid expenditures. Similar
information could be useful for the other additions as well…..Inclusion of this information
provides the case to CMS for inclusion of these measurements in the Core Set.”
We appreciate the page 28 Theme observation that Medicaid wraparound services are not
being captured by the current core set.
Long-Term Services and Supports (LTSS): National Core Indicators and National Core
Indicators-Aging and Disability
We are delighted that the Workgroup voted to recommend to CMS the addition to the 2020 Core
Measure Set: both NCI and NCI-AD (pages 9, 21, 26, 27) (pages when viewing the document
from a web browser). We reinforce the need and importance of such recommendations.
Long Term Services and Supports (LTSS): CAHPS HCBS Experience Survey and Personal
Outcome Measures
The draft report for public comment Appendix discussion of CAHPS HCBS and POM is accurate
and helpful (pages 54, 55, 63, and 65). Missing from the report is the recognition that the
CAHPS HCBS failed recommendation for inclusion into the 2020 core set by one vote. This
recognition would enhance the significance of this measure compared with the many other
measures not endorsed for 2020 inclusion.
The Appendix discussion for both CAHPS HCBS and POM emphasize Workgroup concern with
the high-cost and time intensiveness of collecting data through these mechanisms. This is an
accurate reporting. We suggest that the report reference the NQF HCBS final report; home and
community-based services and supports are person-centered and highly individualized, a
reason for time intensive individual engagements.
Gaps
Thank you for the very helpful identification of measure gaps on page 29. To AAHD and the
Lakeshore Foundation, we particularly reinforce: individuals of all ages with disabilities including
C.14
living and participating in the community; LTSS including rebalancing; behavioral health
integration with primary care and physical health; care transitions; and addressing social
determinants of health.
Thank you for the opportunity to comment. If you have any questions please contact Clarke
Ross at [email protected].
C.15
American Board of Pediatrics (David Nichols)
The American Board of Pediatrics strongly supports the inclusion of the two core set measures
related to sickle cell disease:
1. Appropriate Antibiotic Prophylaxis for Children with Sickle Cell Anemia (NQF #3166)
2. Transcranial Doppler Ultrasonography Screening for Children with Sickle Cell Anemia (NQF
#2797)
The percentage of children with sickle cell disease who receive recommended antibiotic
prophylaxis and transcranial doppler screening is persistently low and in some cases, even
falling. The inclusion of these measures would have an immediate and dramatic impact of
focusing pediatricians on the importance of these care elements. This simple step would go a
long way in reducing a glaring health care disparity in the United States. Other chronic illnesses
of childhood (e.g., cystic fibrosis, inflammatory bowel disease) have seen dramatic
improvements in care, but sickle cell disease has not, in part because of the absence of
validated measures.
We urge the adoption of these core set measures in the strongest possible terms.
C.16
American Society of Hematology (Emily Cahill)
The American Society of Hematology (ASH) strongly supports the draft report
Recommendations for Improving the Core Sets of Health Care Quality Measures for Medicaid
and Chip: Summary of a Multistakeholder Review of the 2020 Child and Adult Core Sets,
including the inclusion of the following core set measure related to sickle cell disease:
Appropriate Antibiotic Prophylaxis for Children with Sickle Cell Anemia (NQF #3166)
With this inclusion, ASH commends the Workgroup for formally recognizing the marked quality
gap in clinical care provided for children with sickle cell disease. The antibiotic prophylaxis
measure, characterized as a continuity of care measure that should be universally applied and
is captured by claims data, is an appropriate addition to the 2020 Child Core Set. ASH
appreciates the opportunity to comment on this important work and to highlight this great
opportunity to drive improvement in the clinical care of children with sickle cell disease.
ASH is committed to addressing the burden of sickle cell disease (SCD) and is in the midst of a
multifaceted initiative to improve outcomes for individuals with the disease both in the United
States and globally. ASH is leading a number of activities to ensure that individuals with SCD
have access to high quality of care, including the development of new clinical practice
guidelines on the management of acute and chronic complications of SCD, and expanded SCD-
focused education and training.
Please let me know if you have any questions or would like to discuss further.
C.17
Anthem (Anthony Mader)
Anthem, Inc. (Anthem) appreciates this opportunity to comment on Mathematica’s draft report
“Recommendations for Improving the Core Sets of Health Care Quality Measures for Medicaid
and CHIP,” released in July 2019.
We appreciate Mathematica’s convening of the 2020 Child and Adult Core Set Annual Review
Stakeholder Workgroup (Workgroup) and the Workgroup’s thoughtful assessment of the
Centers for Medicare & Medicaid Services’ (CMS) Medicaid and CHIP Child and Adult Core
Sets (Core Sets) of healthcare quality measures. Anthem largely supports the Workgroup’s
recommended changes to the Core Sets for 2020 based on its review of the 2019 Core Sets.
However, we ask that the Workgroup consider the comments provided below as the Workgroup
finalizes its recommendations.
Detailed Comments
Workgroup Recommendations of Measures to Remove from the 2020 Core Sets
Anthem generally agrees with the Workgroup’s recommendations to remove certain measures
from the 2020 Core Set. In cases where the measure is due to be retired by its steward, it is
logical to remove it from the Core Set. However, we recommend the Use of Multiple Concurrent
Antipsychotics in Children and Adolescents (ACP-CH) be retained in the Child Core Set. The
Workgroup framed this measure as potentially “topped out,” but we believe there is still both
value and room for improvement, particularly for the Medicaid population, including children and
youth supported by the child welfare system.
The Workgroup recommended that the measure be replaced by Metabolic Monitoring for
Children and Adolescents on Antipsychotics. While we recommend that Use of Multiple
Concurrent Antipsychotics in Children and Adolescents (ACP-CH) be retained, we also support
adding this new measure, as both would add value to the Child Core Set.
Workgroup Recommendations of Measures to Add to the 2020 Core Sets
The Workgroup proposes to add two National Core Indicators (NCI) measure sets. We support
the concept of adding more quality measures pertaining to individuals requiring Long-Term
Services and Supports (LTSS). However, we are concerned that these survey measures were
not designed for use as health plan quality measures. The measure specifications are not in the
public domain and they would rely on time-intensive in-person interviews. If the individuals
conducting the in-person interviews have not had adequate training in interview techniques, the
validity of these surveys may be questionable. In addition, the sample size is small, so although
these interviews can provide valuable insights into the experiences of individuals, the
information may be limited. There are more appropriate ways for CMS to encourage states’ use
of the NCI and NCI-Aging and Disabilities (NCI-AD) instruments, and we recommend CMS
consult with Human Services Research Institute (HSRI) about the potential difficulties of
C.18
individual health plans using these measure sets before proceeding with adding them to the
Core Set(s).
Understanding that it may be too late in the process to recommend alternative measures for
2020, we instead recommend considering alternative measures for 2021 that would still achieve
the goal of measuring quality for the LTSS Medicaid population, while also being more broadly
impactful. For example, earlier this year, CMS, with help from Mathematica and the National
Committee for Quality Assurance (NCQA), released technical specifications for measures being
developed specifically for individuals accessing LTSS through Managed Care Organizations
(MCOs), some of which are aligned with Healthcare Effectiveness Data and Information Set
(HEDIS) measures. Data collection for these measures can be collected through case
management record review or administratively, making them likely less burdensome as well.
We are also concerned with the addition of the Appropriate Antibiotic Prophylaxis for Children
with Sickle Cell Anemia and the Use of Pharmacotherapy for Opioid Use Disorder measures,
specific to states that carve-out pharmacy from the medical benefit. Health plans operating in
carve-out markets have little opportunity to improve quality on these measures because we do
not directly manage the benefit. The ability for MCOs to provide whole-person care for its
members by fully integrating medical and pharmacy care is extremely helpful in maximizing the
quality of care and services for our members. Anthem is invested in whole-person care to
achieve optimal health outcomes, and a carve-out limits our ability to holistically coordinate and
manage care for our members.
We welcome the opportunity to discuss our recommendations to ensure the delivery of robust
benefits and access to quality care and services via the Medicaid and CHIP programs. Should
you have any questions or wish to discuss our comments further, please contact Lisa Watkins at
(202) 508-7889, or [email protected].
C.19
Association for Community Affiliated Plans (Margaret A Murray)
The Association for Community Affiliated Plans (ACAP) appreciates the opportunity to submit
comments on proposed changes to Child and Adult Core Measures. ACAP is an association of
66 nonprofit and community-based Safety Net Health Plans located in 29 states. Collectively,
ACAP health plans provide coverage to 20 million individuals enrolled in Medicaid, the
Children’s Health Insurance Program (CHIP), Medicare Special Needs Plans for dually-eligible
individuals, and Qualified Health Plans (QHPs) serving the health insurance Marketplaces.
ACAP plans are members of their communities, partnering with states to improve the health and
well-being of their members who rely upon Medicaid and CHIP as well as other publicly-
supported programs. We first will provide some general comments and then respond to specific
measure recommendations below.
General Comments
Overall Criteria: ACAP member plans agree with overarching criteria for removing measures in
which performance is going well and focusing on areas of known needed improvement. We also
support the use of measures where they are populated via administrative data
(encounters/claims) versus manual file review. Finally, we support selecting measures that are
impactable and would encourage the use of strategic workgroups that include the provider
community to be engaged with improving outcomes for select measures that remain challenging
year-over-year.
Outcomes vs Process Measures: In general, ACAP understands and appreciates the interest in
moving from process measures to outcomes measures. However, we know that peer-reviewed
publications are providing increasing evidence that there are confounding variables (beyond the
scope of influence by plans and providers) that impact outcomes measures more than they
impact process measures. These confounding variables are issues related to social
determinants of health (SDoHs). While Safety Net Health Plans (and other health plans) are
moving into the realm of addressing SDoHs, it is uncharted territory. Until this new evidence
matures, and interventions that effectively impact SDoHs are funded by state and federal
sources, we are concerned that replacing all process measures with outcomes measures does
not sufficiently recognize those SDoH-related confounding variables that may impact outcomes
rates due to issues not in control of health plans.
Proposed Measures for Removal
Child and Adolescents’ Access to Primary Care Practitioners (CAP-CH)
Support.
Weight Assessment and Counseling for Nutrition and Physical Activity for
Children/Adolescents— Body Mass Index Assessment for Children/Adolescents (WCC-CH)
Support with Concern.
C.20
While ACAP supports the removal of this measure in general based on many of its limitations
noted in the report, we remain concerned about the absence of a replacement measure that
addresses obesity. We understand that simple measurement without a planned, evidence-
based intervention may seem less impactful, but obesity is the major health problem in the U.S.
and is increasing. Measuring BMI signals to primary care providers the importance of the issue
and marks a place to start. As noted in the report, a federal liaison voicing support remarked
that “…there is evidence to support BMI screening in the primary care setting and that BMI
screening is part of American Academy of Pediatrics and USPSTF recommendations for both
children and adults.” Indeed, this report, as evidenced by Exhibit 8, notes that “Obesity” is a
potential gap area for future core set measures.
Pediatric Central Line–Associated Bloodstream Infections (CLABSI-CH)
Support.
Use of Multiple Concurrent Antipsychotics in Children and Adolescents (APC-CH)
Support.
Adult Body Mass Index Assessment (ABA-AD)
Support with Concern.
While ACAP supports the removal of this measure in general based on many of its limitations
noted in the report, we remain concerned about the absence of a replacement measure that
addresses obesity. We understand that simple measurement without a planned, evidence-
based intervention may seem less impactful, but obesity is the major health problem in the U.S.
and is increasing. Measuring BMI signals to primary care providers the importance of the issue
and marks a place to start. As noted in the report, a federal liaison voicing support remarked
that “…there is evidence to support BMI screening in the primary care setting and that BMI
screening is part of American Academy of Pediatrics and USPSTF recommendations for both
children and adults.” Indeed, this report, as evidenced by Exhibit 8, notes that “Obesity” is a
potential gap area for future core set measures.
Comprehensive Diabetes Care: Hemoglobin A1c (HbA1c) Testing (HA1C-AD)
Support with Concern.
While ACAP supports the removal of this measure in general based on many of its limitations
noted in the report, this is an example of replacing a process measure with an outcomes
measure (Comprehensive Diabetes Care: Hemoglobin A1c [HbA1c] Poor Control) where we
have concerns as noted above. A valid hypothesis is that process measures may better
measure the quality of the care provided, while outcomes measures are influenced by social
determinants of health (SDoHs). It is important to measure SDoHs and develop interventions to
address them, but until meaningful progress is made in addressing SDoHs, it is also desirable to
minimize confounding variables in measuring the quality of care provided. Keeping the
C.21
Comprehensive Diabetes Care: Hemoglobin A1c (HbA1c) Testing (HA1C-AD) alongside the
Diabetes Care: Hemoglobin A1c (HbA1c) Poor Control (HPC-AD) could allow comparison of the
process and outcome measures which can help inform a better understanding of the effects of
SDoHs. If the hypothesis proves true, the other unintended consequence of removing the
process measure will be to penalize providers who provide care for the most needy and
underserved (e.g., FQHCs) and could result in providers or managed care plans ‘cherry picking’
patients with fewer SDoHs in order to achieve better scores. This concern of possible
confounding influence of SDoHs warrants analysis of process and outcomes measures to
ensure they are measuring the factors they are intended to measure.
Annual Monitoring for Patients on Persistent Medications (MPM-AD)
Support.
Proposed Measures for Addition
Appropriate Antibiotic Prophylaxis for Children with Sickle Cell Anemia
Support.
Metabolic Monitoring for Children and Adolescents on Antipsychotics
Support.
Use of Pharmacotherapy for Opioid Use Disorder
Do not support.
ACAP is concerned that plans will not be able to access full data relating to this measure. First,
plans may have trouble identifying plan enrollees with opioid use disorders, as services for
those members are carved out to the state, county, or subcontracted managed behavioral
health/substance use disorder organizations in some jurisdictions. For the same reason, it may
not be possible for plans to track the full range of services provided. While in these jurisdictions
some medication assisted treatment may be provided by the plan, it would be difficult or
impossible for those plans to collect and deliver a full picture of the data required under this
proposed measure.
Second, regardless of whether behavioral health and substance use disorder services are
carved in or out of a Medicaid managed care plan, outdated federal regulations that pre-date
current models of care create significant barriers to holistic care for people with SUD and impact
the ability for health plans to capture the data needed to inform measures related to that care.
These barriers – found in 42 CFR Part 2 and requiring individualized and specific patient
consent before providers and plans can disclose a SUD to coordinate care – undermine efforts
to integrate behavioral and physical health services for people with SUD, ultimately leading to
worse health outcomes. We harbor concerns that the prohibitions on sharing data in 42 CFR
C.22
Part 2 will severely hinder plans’ efforts to report on any measure related to opioid overuse
treatment or any other SUD treatment.
National Core Indicators (NCI)
Do not support.
While ACAP supports the eventual addition of these indicators, our plans are concerned about
their ability to immediately adhere to this measure. Given that these measures are collected via
a survey, they are time-intensive for the Medicaid beneficiary and expensive to conduct. In
addition, the implementation of this survey may involve contract modifications between state
Medicaid agencies and health plans. Overall, we would recommend a staged addition of these
measures would be preferable to their proposed immediate inclusion.
We do note that these surveys are currently being conducted in a substantial number of states.
If the surveys were to be administered through other mechanisms, organizations, or agencies
rather than through Medicaid managed care plans, we would withdraw our “Do not support”
position as that position is primarily based on financial, operational, and timeline concerns.
While not of direct concern with regard to the use by the CMCS of the NCI survey to evaluate
the state, our plans would like further clarification on its potential impact on them and how the
state Medicaid agencies may use the results of that survey in their evaluation of the MCOs—we
understand this may be a state-by-state concern.
National Core Indicators for Aging and Disabilities Adult Consumer Survey (NCI-AD)
Do not support.
While ACAP supports the eventual addition of these indicators, our plans are concerned about
their ability to immediately adhere to this measure. Given that these measures are collected via
a survey, they are time-intensive for the Medicaid beneficiary and expensive to conduct. In
addition, the implementation of this survey may involve contract modifications between state
Medicaid agencies and health plans. Overall, we would recommend a staged addition of these
measures would be preferable to their proposed immediate inclusion.
We do note that these surveys are currently being conducted in a substantial number of states.
If the surveys were to be administered through other mechanisms, organizations, or agencies
rather than through Medicaid managed care plans, we would withdraw our “Do not support”
position as that position is primarily based on financial, operational, and timeline concerns.
While not of direct concern with regard to the use by the CMCS of the NCI-AD survey to
evaluate the state, our plans would like further clarification on its potential impact on them and
how the state Medicaid agencies may use the results of that survey in their evaluation of the
MCOs—we understand this may be a state-by-state concern.
Additional Comments: Other Measures Discussed but Not Recommended for Addition
C.23
Two other measures discussed by the Workgroup but ultimately not recommended for adoption
included Continuity of Insurance: Informed Participation and Health-Related Social Needs
(HRSN) Screening. Upon review of the discussion text, we understand and appreciate the
concerns raised by numerous Workgroup members.
ACAP member plans continue to be interested in being able to track issues related to coverage
churn and being able to measure continuity of insurance, including Medicaid coverage. ACAP
believes it is critical that some measure of the churning issue be included in the measurement
set as soon as possible. Churning has a direct impact on quality and the potential success of
quality improvement efforts. We would urge CMS and AHRQ to specifically undertake a study of
the impact of churning on the reliability and state-to-state comparability of the measurement set.
In addition, our plans see the value in better measuring the screening and assessment of
members’ social determinants of health. As such, we would urge CMCS to encourage measure
developers to continue to work on improving potential measures that address these two issues
with an expectation that they may be considered for future inclusion in the Core Measures.
Again, we thank you for this opportunity to comment on these important proposed modifications
7509), or Enrique Martinez-Vidal, Vice President for Quality and Operations (emartinez-
greater depth.
C.24
Biotechnology Innovation Organization (Phyllis Arthur)
The Biotechnology Innovation Organization (BIO) appreciates the opportunity to provide
comments on Mathematica’s Summary of a “Multi-stakeholder Review of the 2020 Child and
Adult Core Sets.”
BIO is the world's largest trade association representing biotechnology companies, academic
institutions, state biotechnology centers, and related organizations across the United States and
in more than 30 other nations. BIO’s members develop medical products and technologies to
treat patients afflicted with serious diseases, to delay the onset of these diseases, or to prevent
them in the first place. In that way, our members’ novel therapeutics, vaccines, and diagnostics
not only have improved health outcomes, but also have reduced healthcare expenditures due to
fewer physician office visits, hospitalizations, and surgical interventions. BIO membership
includes biologics and vaccine developers and manufacturers who work closely with myriad
stakeholders, including the public health and advocacy communities, to support policies that
help ensure access to innovative and life-saving medicines and vaccines for all individuals. BIO
appreciates the Workgroup’s recognition of the importance of quality measures in improving
patient heath as well as its efforts to strengthen the Medicaid Adult and Child Health Care
Quality Core Sets for 2020. We encourage the Workgroup to consider adoption of two recent
Healthcare Effectiveness Data and Information Set (HEDIS) immunization measures
1
:
1. Add the new Adult Immunization Status (AIS) measure, to replace the current adult
influenza vaccine measure based on Consumer Assessment of Healthcare Providers and
Systems (CAHPS) surveys. The new AIS measure is a composite of the age-recommended
vaccines for adults, including influenza vaccine.
2. Add the new Prenatal Immunization Status (PIS), which measures prenatal immunizations of
Tdap (tetanus, diphtheria, and acellular pertussis) and influenza.
With the addition of the AIS and PIS, the two immunization measures currently within the Child
Core Set, Childhood Immunization Status (CIS) and Immunization of Adolescents (IMA), should
be retained.
Potential Gap Areas for Future Core Set Measures
The Advisory Committee for Immunization Practices (ACIP) and Centers for Disease Control
and Prevention (CDC) guidelines for routine vaccination are evidence-based and developed to
improve the health of the U.S. population by preventing disease. Despite this evidence-based
guidance, many adults are not being assessed for and offered important ACIP-recommended
vaccines, resulting in poor adult immunization coverage rates nationally. Additionally, there are
significant ethnic and racial disparities within this cohort of the beneficiary population.
2,3
BIO appreciates that the Workgroup identified gaps in immunization quality measures,
particularly in prenatal and adult populations. Opportunities to assess the immunization status of
Medicaid beneficiaries, especially pregnant women and medically vulnerable adults with chronic
conditions such as diabetes and heart disease, should be done by the range of clinicians who
care for them. The National Vaccine Advisory Committee’s (NVAC) Adult Immunization
C.25
Standards call for all providers caring for adult patients to assess, recommend, vaccinate or
refer, and document vaccinations.
4
Leveraging each and every patient encounter to facilitate
education and provider recommendations for vaccines has been found to improve the likelihood
of a patient being immunized.
5
Immunization quality measures are a crucial tool for health care quality improvement and have
demonstrated effectiveness in improving immunization coverage across adult populations to
prevent illness and death, reduce caregiving demands, avoid unnecessary healthcare spending,
and set the foundation for healthy aging.
6
Quality measures, such as the AIS and PIS measures
can help to fill gaps and eliminate disparities in immunization.
Adult Immunization Status Measure
BIO recommends that the Workgroup replace the current adult influenza vaccine measure,
based upon Consumer Assessment of Healthcare Providers and Systems (CAHPS) surveys,
with the AIS measure. The AIS measure is a composite of several age-recommended vaccines
for adults, including the currently assessed influenza vaccine, and provides a sound, reliable,
and comprehensive means to assess the receipt of routine ACIP-recommended adult
vaccinations. It would enable states to monitor recommended adult immunization use beyond
influenza.
The Indian Health Service (IHS) Northwest Tribal Epidemiology Center and the National Nursing
Home Quality Care Collaborative first demonstrated that the AIS is an effective tool that can
improve patient health outcomes.
7
This measure, along with another composite measure for
prenatal immunization (see below), subsequently has been adopted as part of HEDIS through
efforts of the Department of Health and Human Services (HHS) Office of Infectious Disease and
HIV/AIDS Policy (OIDP) [formerly the National Vaccine Program Office (NVPO)] and the CDC in
collaboration with the National Adult Immunization and Influenza Summit Quality Working
Group. Additionally, the AIS was recently proposed for inclusion in the Medicare Shared
Savings Program, starting in performance year 2020, and the Medicare Merit-based Incentive
Payment System (MIPS), starting in performance year 2022. Within MIPS, inclusion was
proposed in several measure sets: allergy/immunology, family medicine, internal medicine,
obstetrics/gynecology, otolaryngology, preventive medicine, nephrology, general surgery,
oncology/hematology, infectious disease, rheumatology, geriatrics, skilled nursing facility, and
endocrinology. Alignment and harmonization of Medicare and Medicaid quality measures in this
way would further the federal government’s goals of consistency across quality programs.
We understand that the Workgroup did not recommend AIS for inclusion in the 2020 Medicaid
Adult Core Set of measures due to concerns about states’ ability to accurately and reliably
report this measure. The National Committee for Quality Assurance (NCQA) tested both
measures in Medicaid and commercial health plans. NCQA concluded that the fact that the
measures are drawn from Electronic Clinical Data Systems (ECDS), which include immunization
information systems (IIS), case management registries, claims, and electronic health records
(EHRs), makes them feasible to implement.
C.26
While we understand State Medicaid programs and Children’s Health Insurance Program
(CHIP) can access member claims, encounters, and the corresponding state/local IIS, many
state Medicaid agencies face new challenges to capture EHR data. NQCA continues to work
closely with health plans to provide technical support and to identify approaches to collect this
data – states could choose to assess different models of data capture, such as encouraging
providers to report to a community, regional or state-based health information exchange, in
order to build capacity for measures that rely on electronic clinical information.
As the Adult Core Set is voluntary, inclusion of the AIS would signal a prioritization of adult
vaccination while allowing states to further develop approaches to data collection and reporting
before all reporting for the Adult Core Set is required in 2024. BIO supports the Workgroup
recommendation that CMS consider an affinity group or grant opportunity to help drive state
coordination between Medicaid and public health registries, particularly immunization registries,
to help support uptake and use of the AIS.
BIO acknowledges that implementation of a quality measure often precedes health system and
health plan focus. While reporting challenges remain, they risk being unaddressed if adult
immunization is not prioritized and remains unmeasured.
Prenatal Immunization Status Measure
BIO also recommends the Workgroup reconsider adoption of the Prenatal Immunization Status
measure for 2020, which includes Tdap and influenza vaccination status. Maternal and prenatal
health has been identified in prior reviews as an area to strengthen in the quality measure sets.
Like the Adult Immunization Status measure, the Prenatal Immunization Status measure will
help to address substantial disparities in prenatal immunization rates. While maternal
immunization in the third trimester protects 9 out of 10 babies from pertussis infections serious
enough to require hospitalization, prenatal immunization rates are lower among Medicaid
members than those privately insured.
8
For pregnancies ending in 2016, in the commercial
cohort 50% of women received Tdap vaccination and 40% received influenza vaccination
compared to 30% for Tdap and 25% for influenza of mothers in the Medicare population.
9,10
BIO
appreciates the Workgroup’s acknowledgment of the importance of a prenatal immunization
status measure and its strong connection to improved health outcomes for young infants.
BIO notes the Workgroup cites similar concerns on data collection challenges with the AIS in its
decision to recommend the Prenatal Immunization Status measure. BIO affirms that, as with the
AIS, adoption of this voluntary measure would set a priority for immunization while state CHIP
and Medicare programs begin to work with NCQA and other bodies to work towards addressing
these challenges.
Adoption of these two quality measures would provide useful and actionable results for state
Medicaid and CHIP programs, especially if they publicly post results and require reporting by
Medicaid managed care plans. Such performance assessment and feedback can drive quality
improvement efforts to raise immunization coverage rates. At the same time, the addition of
C.27
these two new measures to the Adult and Child Core sets is critical to improving the health of
adult and prenatal populations.
Conclusion
BIO appreciates the opportunity to offer feedback on this draft report and looks forward to
working with Mathematica on this critical topic. Please do not hesitate to reach out to Greg
questions.
Citations
1
https://www.ncqa.org/news/ncqa-updates-quality-measures-for-hedis-2019/.
2
National Vaccine Advisory Committee. 2014. Public Health Rep. 2014 Mar-Apr; 129(2): 115–
123.
3
CDC. MMWR Morb Mortal Wkly Rep. 2017 May 5;66(11):1-28.
4
https://www.cdc.gov/vaccines/hcp/adults/for-practice/standards/index.html.
5
Quilici et al. “Role of vaccination in economic growth.” J Mark Access Health Policy; (2015)
3:10.3402/jmahp.v3.27044.
6
https://dev-adultvaccinesnow.pantheonsite.io/wp-content/uploads/2016/07/AVN-White-Paper-
FINAL.pdf.
7
https://www.hhs.gov/sites/default/files/tab_10.05_weiser_adult_iz_composite-measures.pdf.
8
CDC. https://www.cdc.gov/pertussis/pregnant/mom/vacc-effectiveness.html.
9
CDC. MMWR Morb Mortal Wkly Rep. 2013 Feb 22;62(7):131-5.
10
Harper SA et al. MMWR Recomm Rep. 2005 Jul 29;54(RR-8):1-40.
C.28
Christopher Bolling
Please know that as a practitioner of general pediatrics and a provider of pediatric obesity
treatment, I am opposed to dropping BMI assessment of children and adults as a HEDIS
measure. Removal of this requirement will reduce emphasis on and urgency around the public
health crisis of overweight and obesity. Obesity remains a threat to our nation's health and
healthcare system. Lessening the focus on overweight and obesity will result less scrutiny of
weight status in clinical settings and reduced opportunities to promote healthy weight.
C.29
California Department of Health Care Services (Lisa Albers)
We would like to offer the following comments on the draft recommendations for Improving the
Core Sets of Health Care quality Measures for Medicaid and CHIP.
• First of all, we would like to echo points put forth by the committee member from New York’s
Medicaid Agency on core set reporting. In particular, California also uses HEDIS measures
to set benchmarks for internal quality calculations and to hold health plans accountable.
Measures that are not part of the HEDIS measure set are more difficult to benchmark.
Additionally, we agree that provider-based measures, such as those developed for
hospitals, are more difficult for health plans and the state to collect and report.
• Regarding the specific measure recommendations, we agree with all of the measures
recommended for removal from the Core Sets, namely: Child and Adolescents’ Access to
Primary Care Practitioners (CAP-CH), Weight Assessment and Counseling for Nutrition and
Physical Activity for Children/Adolescents—Body Mass Index Assessment for
Children/Adolescents (WCC-CH), Pediatric Central Line–Associated Bloodstream Infections
(CLABSI-CH), Use of Multiple Concurrent Antipsychotics in Children and Adolescents (APC-
CH), Adult Body Mass Index Assessment (ABA-AD), Comprehensive Diabetes Care:
Hemoglobin A1c (HbA1c) Testing (CDC-HT), and Annual Monitoring for Patients on
Persistent Medications (MPM-AD).
- In particular, we support the removal of the CAP measure and the CLABSI measure
from the Child Core Set, and the removal of CDC-HT and MPM from the Adult Core Set.
The CAP measure is too broad in its definition of a primary care visit, so it is really just a
utilization measure, and the CLABSI measure, being a hospital-based measure, is
difficult for the health plans and state to report on as well as take action on, given that
the Dept. of Health Care Services (DHCS) holds contracts with its managed care health
plans, rather than hospitals.
- We also support the removal of CDC-HT, given that CDC-H9, an outcome measure,
remains on the Core Set and also includes A1c testing.
- Finally, we also support the removal of MPM as this is a measure with high performance
nationally, making it difficult for health plans and states to improve on, and the NCQA
has announced the retirement of MPM from its HEDIS measure set.
• Regarding the measures recommended for addition to the Core Sets, we support the
addition of Metabolic Monitoring for Children and Adolescents on Antipsychotics and the
Use of Pharmacotherapy for Opioid Use Disorder. In particular, the latter measure is a
particularly good choice given the underutilization of medication assistant treatment
nationally, and in California.
• While the Appropriate Antibiotic Prophylaxis for Children with Sickle Cell Anemia does
potentially represent a measure that targets continuity of care for chronic disease, the
population that would be targeted by the measure is quite small and rather specialized for
the Core Sets.
C.30
• While the National Core Indicators (NCI) and National Core Indicators for Aging and
Disabilities Adult Consumer Survey (NCI-AD) provide important information about members’
experiences, they are in person and family based surveys, which are labor and resource
intensive to collect and report on for states and health plans, and would require many states
to implement a new requirement of their health plans. These measures do not seem to meet
the feasibility and appropriateness for state level reporting criteria outlined by the Core Set
workgroup.
Thank you for providing the opportunity to comment.
C.31
Children's Dental Health Project (Colin Reusch)
The Children’s Dental Health Project (CDHP) appreciates the opportunity to comment on the
draft report titled “Recommendations for Improving the Core Sets of Health Care Quality
Measures for Medicaid and CHIP.” CDHP applauds the efforts of the Core Set Workgroup in
reviewing and recommending changes to the Medicaid and CHIP Child and Adult Core Sets. As
the national organization dedicated to eliminating dental disease as a barrier to child and family
success, CDHP recognizes the importance of meaningful measurement in driving care delivery
and evaluating the impact of public coverage programs. CDHP has long advocated for the
development and implementation of oral health quality measures to track both access to oral
health care and improvements in oral health status as a result of coverage and care delivery.
1
While we are disappointed that the 2020 Core Set annual review did not result in the addition of
new oral health quality measures, we appreciate the Workgroup’s careful deliberation and
establishment of a framework for measure evaluation. CDHP recognizes the need to weigh the
feasibility of and data sources for each measure. At the same time, we encourage the
Workgroup to be ambitious in advancing measures that have the greatest opportunity for
improving the health outcomes of children and adults served by Medicaid and CHIP. As such,
we hope that during the next review cycle the Workgroup will reconsider the measures related
to emergency department visits for dental caries and follow-up care after emergency
department visits.
In general, CDHP agrees with the Workgroup’s assessment of potential gap areas for future
Core Set measures. In particular, we agree that maternal health is an especially glaring gap in
the existing measure sets given the importance of health and oral health care during pregnancy
— both for women and infants. We also agree that there is a need to implement Core Set
measures related to oral health beyond prevention, as the Workgroup indicates in exhibit 8 in
the draft report. Moreover, we encourage the Workgroup to consider gaps for oral health
measurement with regard to follow-up on referrals, appropriateness of care, and network
adequacy. In addition, we suggest that the Workgroup consider how measure attributes, such
as stratification by race and ethnicity, as well as how the social determinants of health may be
applicable to oral health measures in future iterations of the Core Sets.
CDHP encourages the Workgroup to place considerable emphasis on the degree to which new
measures could fill gaps in the existing Core Sets, especially with regard to oral health.
Currently there are no oral health measures in the Adult Core Set despite the impact of oral
health on other chronic diseases, employment, and earning potential.
2
In addition, recent
research suggests that parents’ access to oral health care increases the likelihood that children
will access care.
3
The two oral health measures in the Child Core Set are focused on utilization
and process for dental services, leaving gaps with regard to appropriateness of care,
improvements in oral health status, and oral health care delivered in the primary care setting.
We encourage the Workgroup to seek input from leading oral health measurement experts,
including the Dental Quality Alliance, as they continue to conceptualize, develop, and test new
oral health quality measures for both children and adults.
C.32
Finally, we strongly support the Workgroup’s recommendation that federal agencies, including
CMCS, provide technical assistance to states for the purposes of improving data
standardization; establishing data linkages across care domains as well as between parents
and children; and providing state quality staff with opportunities to learn from one another.
CDHP would welcome such efforts. We urge that any technical assistance on quality
measurement and data collection in Medicaid and CHIP address oral health measurement and
associated data systems.
We look forward to engaging with the Workgroup as they continue to review and recommend
changes to the Child and Adult Core Sets. Please do not hesitate to CDHP’s Director of Policy,
Colin Reusch for additional information at: creus[email protected].
Citations
1
Children’s Dental Health Project, Association of State and Territorial Dental Directors, and
DentaQuest Foundation (December 2017). Making Oral Health Count: Toward a
Comprehensive Oral Health Measurement System. Available at:
https://www.astdd.org/docs/dqf-astdd-cdhp-measurement-brief.pdf.
2
Children’s Dental Health Project (August 2018). Meeting children’s and families’
comprehensive health needs: Building two-generation models that incorporate oral health.
Available at: https://s3.amazonaws.com/cdhp/Family-Centered/Fact+Sheet+-
+Family+centered+efforts+%26+OH_sm+endnotes.pdf.
3
Lipton, BJ (April 2019). Adult Medicaid Benefit Generosity and Receipt of Recommended
Health Services among Low-Income Children: The Spillover Effects of Medicaid Adult
Dental Coverage Expansions. Available at SSRN: https://ssrn.com/abstract=3364028
.
C.33
Children’s Health Integrated Program in Childhood Obesity (Sarah Barlow)
I strongly recommend against the removal of weight assessment and nutrition and physical
activity counseling from the core quality measures for children.
Quality measures in to address obesity should be retained and strengthened.
Obesity in childhood is highly prevalent, increasing in severity, and leads to high medical costs.
Removal of measures related to this health crisis implies lack of importance. Clinicians find
addressing this problem difficult, and they may turn their attention away from this problem if this
attention is not recognized as important.
Work led by my collaborator, Christy Turer, MD, MS, (in press) demonstrates that EHR markers
of clinician attention to BMI and obesity co-morbidities in primary care visits of children with
overweight and obesity results in improvement in relative BMI.
Rather than removing these quality markers, this committee should recommend improved
markers in this area; the goal should be evidence of clinician attention and action, including
offering evidence-based behavior-based interventions.
C.34
Children's Hospital Association (Kate Conrad)
On behalf of the nations’ children’s hospitals, the Children’s Hospital Association (CHA)
applauds the Workgroup’s thoughtful review and deliberations of the 2020 Core Sets for
Medicaid and CHIP, and largely supports the recommendations of changes to the measures set
that will improve the quality of care and health outcomes for Medicaid and CHIP beneficiaries.
We celebrate the addition of a measure that is widely endorsed to improve the quality of care for
children with sickle cell disease (SCD) – a grossly underserved patient population.
• Appropriate Antibiotic Prophylaxis for Children with Sickle Cell Anemia (NQF# 3166).
As most children with SCD are covered by Medicaid, this measure is highly relevant and meets
all criteria for measure inclusion: actionable, aligned, and appropriate.
Additionally, we encourage the Workgroup to consider another SCD measure in the 2020 Child
Core Set under the same rationale above.
• Transcranial Doppler Ultrasonography Screening for Children with Sickle Cell Anemia (NQF
#2797)
This measures also supports the assessment needs in the Sickle Cell Disease and Other
Heritable Blood Disorders Act of 2018, and are instrumental in preventing sepsis and stroke in
patients with SCD.
Children’s hospitals support inclusion of both SCD measures, and thanks to the Workgroup for
the opportunity to comment.
C.35
Connecticut Children’s Office for Community Child Health/Help Me Grow
National Center (Paul H Dworkin and Erin Cornell)
Connecticut Children’s Office for Community Child Health is a national leader in addressing
critical contemporary issues that have the potential to adversely affect children’s health and
development. The Office not only serves as a critical community resource, but also cultivates
innovative and cost-effective solutions to address existing gaps in our health care and child
service systems. The Office oversees a variety of community-oriented programs that address a
wide range of factors that influence children’s health, development, and well-being. One of
those programs is the Help Me Grow National Center, which is a system model that promotes
integrated, cross-sector collaboration to build efficient and effective early childhood systems that
mitigate the impact of adversity and support protective factors among families. Through model
implementation in communities and states across the country, Help Me Grow advances early
detection and intervention for at-risk children, so all children can grow and thrive to their full
potential.
Our efforts within The Office and the Help Me Grow National Center frequently focus on the
critical role of the child health provider in promoting the optimal health and well-being of young
children and families. This experience has reinforced both the importance and relative scarcity
of data that inform us as to the quality and impact of efforts to strengthen child health services in
support of children’s optimal healthy development. For example, while universal developmental
screening by child health providers is recommended, existing data suggests uptake among child
health providers is still far below this target, and there is little to no available data to supplement
screening rate data, such as whether such screening serves to identify and respond to need or
families’ experience with the screening, referral, and linkage process. The Child Core Set
measures provide a critical opportunity to expand our access to knowledge about national
health care quality for children served by Medicaid or CHIP, to leverage such knowledge as a
baseline around which states can design future efforts to improve performance, and as a way to
enable further investigation into the types of practice transformation strategies that are most
effective in increasing quality. We appreciate the opportunity to provide specific feedback on the
proposed Medicaid and CHIP Child Core Set:
While the inclusion of developmental and maternal depression screenings is encouraging, we
believe it is critical to track the number of children and mothers who are successfully connected
to follow-up services to ensure detection leads to assessment and intervention. Excluding
follow-up and other metrics that speak to quality of care significantly undermines the potential
benefit of screening tools to children and families and risks communicating to providers that
because linkage is not measured, it is not essential. In our experience, documentation of
successful linkage can be challenging, given the complexity of care coordination activities and
the number of transitions families may experience across settings. To circumvent this in our own
work, we track instead the proportion of families with concerns for which a provider documents
making any type of referral. While referral does not equate with successful linkage, this is
arguably a more substantive indicator of quality of service than screening conducted in isolation.
It is Help Me Grow’s experience that connecting patients to community-based programs and
services increases efficiencies by decreasing service duplication and ensuring support for all
C.36
children and families, not just those that are high-risk or with specific delays or disorders, given
the availability of tertiary intervention for the limited proportion of families with such needs. This
also increases the capacity of systems that serve the most vulnerable for adverse health,
developmental, and behavioral outcomes.
If factors such as feasibility of collecting the measures prevent the Core Set measures to
expand to capture activities beyond screening, we would at a minimum strongly recommend
that standard measures of not only developmental screening in the first three years of life, but
also social emotional screening be considered as a proposed new measure. While such a
measure risks the same limitations as developmental or maternal screening in isolation,
expanding to measure social emotional screening in primary care will provide the field with
actionable data about the degree to which pediatric primary care providers are responding to
the call to action to address children’s social emotional development during the early years of
life.
The inclusion of screenings for social determinants of health that influence the need for medical
care services is an important area of exploration for future health care quality measures. While
the core set workgroup has commented that measures around social determinants of health are
too new to implement, linking families to community-based resources and capturing the return
on investment for doing so contributes to a strong evidence base that may highlight important
gaps in the service delivery continuum. This is data that could lead to future actionable outcome
measures in the core set and provide a stronger representation of Medicaid services and their
utilization. Further, future measures could consider the degree to which such screening reflects
a patient/family-led agenda and priority setting; as above, screening for social determinants in a
way that reflects family-driven priorities will go further in measuring not just quantity of
screenings, but also quality.
Finally, though it is beyond the scope of this review and solicited public comment, we regularly
seek to promote visibility of emerging paradigms in how we measure and reflect upon our
success in strengthening child and family well-being. We are encouraged by trends such as
state-level efforts to measure parental resilience, knowledge of parenting and child
development, as well as reporting of population-based measures such as kindergarten
readiness. Such measures show us what is possible; we hope they continue to be considered
as potential future measures for targeted efforts to strengthen payer-based quality measures in
the health care setting.
If you have any questions or require additional information, please contact Ms. Erin Cornell,
Associate Director of the Help Me Grow National Center, at [email protected]
or 860-837-5756.
C.37
Connecticut Department of Developmental Services (Jordan A Scheff)
Thank you for the opportunity to comment on the report titled “Recommendations for Improving
the Core Sets of Health Care Quality Measures for Medicaid and CHIP.” The report succinctly
and clearly describes the discussions of the work group and provides helpful background
information.
I am writing these comments in my role as the Commissioner of the Department of
Developmental Services in Connecticut; the state of Connecticut Department of Developmental
Services (CT DDS) has participated in NCI for the past 7 consecutive years and plans to
participate again this year. As the Commissioner, discussions of reporting on measures in
Medicaid Long Term Services and Supports are of significant interest and concern.
Of note, there were 42 distinct measures suggested for inclusion in either the Child or Adult
Core Set. Of those 42, only 5 were identified by the workgroup as substantially meeting the
characteristics set forth as important for the measure to be recommended. That NCI was among
the measures recommended comes as no surprise, with its long history and reputation for
providing valid, reliable and most importantly relevant and actionable measures. NCI is well
known and heavily used in the national DD system.
I strongly encourage CMS to take up the recommendations of the workgroup to include NCI to
fill the gap in LTSS measures for the Medicaid Adult Core Measure set in 2020. To understand
how NCI meets the characteristics set forth by CMS and Mathematica, I would like to comment
on the specific characteristics for consideration of a New Measure as described in the report.
Characteristics Considered for Addition of a New Measure:
Actionability. Will the measure provide useful or actionable results for state Medicaid and CHIP
programs?
The NCI provides a multitude of data points that inform an understanding of what support
individuals are receiving in CT, an outline of their experiences, and level of satisfaction.
Additionally, these data stories provide the evidence for new agency initiatives, necessary policy
changes, and adjustments to current CT DDS procedures. The most significant information the
NCI provides is how agency policy impacts the individuals supported by the state of Connecticut
Department of Developmental Services.
Alignment. Is the measure used in other reporting programs?
The information gathered in the NCI is invaluable as it provides the CT DDS the opportunity to
collect information that in some cases is unavailable elsewhere. While the CT DDS data
systems collect information about an individual, supports, waiver enrollment, and a variety of
other variables, the NCI has been used to help with proxy information that CT DDS simply does
not have in other data sources. Legislative requests for information can be challenging, due to
the information available. The NCI provides a cornucopia of additional variables that provide
C.38
context into many aspects of an individual’s life. Additionally, NCI data has been used to
respond to queries from the general public, nonprofits, and other state or federal agencies.
CT DDS recently began using a new Individual Plans (IPs) at the beginning of FY19. The IP
was altered to increase the person-centered part of planning. As part of the IP Coding Project
CT DDS made the decision to test the hypothesis that the changes would allow the individuals
input in the plan. The IP Coding Project entails reviewing IPs to collect specific data about the
type of goals and social support individuals have, and if the goals listed in the IP match what the
individual has expressed interest in achieving within the year. Comparisons about individuals
wanting to live somewhere else and about employment match with the percentages expressed
in the NCI. Additionally, when the collected data from the new IPs is aggregated by residence,
the variation mimics the distribution in the NCI. This provides CT DDS with confidence that the
concepts used in the new IP Coding are valid.
Appropriateness for state-level reporting. Has the measure been validated and tested for state-
level reporting? Is it currently used by one or more states?
The NCI allows for analytical comparison between states. While the number of states using the
NCI varies by year, 46 states have participated in the NCI at some time.
The NCI is one of the only times CT DDS is able to hear from our individuals regarding their
experiences and satisfaction. State trends are invaluable and have spurred a variety of
initiatives and adjustments to policy as expressed above. The publically available chart
generator on the NCI website allows a review of a state to national trends, while the full In
Person Survey National Report Part I: Data (found here:
https://www.nationalcoreindicators.org/upload/core-indicators/17-
18_IPS_National_Report_PART_I_3_20_19.pdf) provides everything necessary to compare
between states. Additionally, the In Person Survey National Report Part II: History,
Methodology, Appendices provides detailed statistical information required to statistically
evaluate the data in Part I.
Feasibility. Will states be able to access the data needed to calculate the measure? Would
technical assistance be necessary or helpful to facilitate complete and accurate reporting of the
measure by states?
Currently the CT DDS gathers the requisite information for the NCI using the following
methodology. The data is collected from administrative data sets, case notes, and in person
survey with individuals served and/or someone that know the individual. Survey portions of the
NCI are completed by state staff and administrative staff enters the information into the provided
data collection website. The NCI occurs annually.
Further, CT DDS has begun to utilize the tools available to reduce the burden additional
assessments add. By prefilling portions of the NCI Adult In Person Survey and additional
training to expand the surveyor pool, data collection has become more efficient. We don’t plan
to stop there. This year the ability to upload some of the survey was utilized and CT DDS is
C.39
planning to use this advantage. HSRI has been responsive in providing instruction and clarifying
questions and inquiries about the ability to upload data. In January of 2019, the HSRI staff came
to Connecticut for in person training of over 30 new trainers.
Strategic priority. Does the measure fill a gap that has been identified in the Child or Adult Core
Sets?
NCI provides a Long Term Services and Supports measures needed by state Medicaid
agencies to understand the experience of people with developmental disabilities who access
and utilize home and community based services. The NCI measure set provides insight,
including the data cleaning, analysis, and summary report, which publicly compares all
participating state results. The public reporting of NCI results provides a level of accountability
needed by CT DDS in order to understand where we have improvement opportunities or need
to re-evaluate the effectiveness of our policies or practices.
We agree with the summary of the workgroup discussion, indicating the usefulness of the NCI
measures to our state, the flexibility and adaptability to be used by people with multiple types of
disabilities. From its inception in 1998, NCI has remained committed to channeling direct input
from people with I/DD to the state agency responsible for the administration of the service
delivery system. NCI assures the voice of the people is heard directly regarding the supports
and services so critical to their day to day well-being. This crucial aspect of NCI – the
importance to measure – weighs equally in Connecticut’s decision to continue using NCI.
C.40
CVS Health (Kevin Teel)
CVS Health is a healthcare innovation company helping people on their path to better health.
We appreciate the consideration of measures to strengthen Medicaid quality for children and
adults and are pleased to provide comments. Pharmacy benefit management organizations,
pharmacies, and pharmacists play an integral role in health quality outcomes, and yet there are
relatively few quality measures today that are specifically tied to the appropriate administration
of medication (e.g., Antidepressant Medication Management [AMM-AD]). Prescription
medications, medication therapy management, and pharmacy counseling can drive meaningful
results and should play a larger roles in the adult and child core set of measures.
With regard to specific measures for inclusion, CVS Health strongly supports the
recommendation to include Use of Pharmacotherapy for Opioid Use Disorder. As the U.S.
opioid abuse crisis grows worse, CVS Health has added new programs and redoubled our
efforts around education, proper medication disposal, utilization management, increased access
to naloxone, and ongoing advocacy for legislative solutions. We are enhancing our enterprise
initiatives to address prescription opioid misuse and abuse that will be supported by all parts of
the company, including our CVS Pharmacy retail presence in nearly 10,000 communities across
the country and CVS Caremark, our PBM that manages medications for more than 90 million
plan members. We plan to reach patients, providers, payers, advocacy organizations, elected
leaders and community health advocates. In addition to the current core set measures of Use of
Opioids at High Dosage (OHD-AD) and Concurrent Use of Opioids and Benzodiazepines (COB-
AD), the Use of Pharmacotherapy for Opioid Use Disorder will go far to establish the number of
people initiating medication assistance treatment.
Additionally, CVS Health supports the recommendation to include Appropriate Antibiotic
Prophylaxis for Children with Sickle Cell Anemia. Children with complex medical conditions
often rely on specialty drugs for treatment and management of their diseases. While there are
many promising new specialty drugs in the pipeline, costs are expected to increase at a rapid
pace over the next several years. As the nation’s largest specialty pharmacy, CVS Health had
developed solutions that improve patient care while helping to control costs. Prescription of
antibiotic prophylaxis, and its favorability over more invasive treatment, is well supported by
available research and the workgroup members. With the opportunity to provide comprehensive
care for children with genetic disorders and avoid costly hospitalizations for infection
management, the Appropriate Antibiotic Prophylaxis measure will make an important addition to
the Child Core Set.
CVS Health continues to support the addition of pharmacy-focused measures that were
discussed but not recommended for inclusion, such as Proportion of Days Covered:
Antiretroviral Medications, Statin Therapy for the Prevention and Treatment of Cardiovascular
Disease, and Tobacco Use: Screening and Cessation Intervention.
CVS Health is also concerned about the removal of the Weight Assessment and Counseling for
Nutrition and Physical Activity for Children/Adolescents measure while there is still an absence
of a measure in the Child Core set that indicates physician use of interventions and referrals for
C.41
children with or at risk of obesity. Current data suggests that documentation of an assessment is
still not common practice, and may also suggest lack of physician prioritization of obesity. We
agree with prior comments that caution against sending a signal about the low priority of this
topic if the WCC-CH measure is removed from the Child Core Set without a replacement, and
we recommend maintaining the measure until a suitable replacement is found.
Thank you for the opportunity to provide comments in support of these recommendations.

C.42
District of Columbia Department of Health Care Finance (Abby Kahn)
Below are DC’s comments:
ADDITIONS (5)
Measure Name DHCF Comments Rationale
Metabolic Monitoring for Children and
Adolescents on Antipsychotic
Medications
No comment
Use of Pharmacotherapy for Opioid
Use Disorder
Support increasing treatment for OUD is a
priority issue for DC, will initiate
enhanced monitoring due to new 1115
demo
Appropriate Antibiotic Prophylaxis for
Children with Sickle Cell Disease
Oppose Not a priority issue for DC, will maintain
routine monitoring
National Core Indicators Oppose NCI survey is administered by a
separate DC agency, DHCF does not
have access to data, data not
comparable year over year, no national
benchmarks exist
National Core Indicators for Aging
and Disabilities Adult Consumer
Survey
Oppose NCI survey is administered by a
separate DC agency, DHCF does not
have access to data, data not
comparable year over year, no national
benchmarks exist
REMOVALS (7)
Child Core DHCF Comments Rationale
Child and Adolescents’ Access to
Primary Care Practitioners (CAP-CH)
No comment
Weight Assessment and Counseling
for Nutrition and Physical Activity for
Children/Adolescents—Body Mass
Index Assessment for
Children/Adolescents (WCC-CH)
No comment
Pediatric Central Line–Associated
Bloodstream Infections (CLABSI-CH)
No comment
Use of Multiple Concurrent
Antipsychotics in Children and
Adolescents (APC-CH)
No comment
Adult Core DHCF Comments Rationale
Adult Body Mass Index Assessment
(ABA-AD)
No comment
Comprehensive Diabetes Care:
Hemoglobin A1c (HbA1c) Testing
(HA1C-AD)
Oppose This is a MY2018 MCO PIP measure,
MCOs are currently not meeting District
goal
Annual Monitoring for Patients on
Persistent Medications (MPM-AD)
No comment
C.43
Healthy Weight Partnership Inc. (Teresa Earle)
We note that you recommend the Weight Assessment and Counselling for Nutrition and
Physical Activity for Children / Adolescents – Body Mass Index Assessment for Children /
Adolescents (WCC-CH) measure for removal from the 2020 Core Set.
As an organization that is focused on prevention and management of child obesity, and on
behalf of a team that has been engaged in such work for almost 20 years in the USA and
internationally, we strongly oppose removing this measure from the Child Core Set in the
absence of including an improved measure. Specifically:
1. We agree with the federal liaison who noted that there is evidence to support BMI screening
in the primary care setting
2. BMI screening is part of the USPSTF recommendations for both children and adults
3. Removal of this measure will send a strong signal about the low priority of this topic if there
is no improved replacement. Something which burgeoning chronic illness in our country
cannot afford.
4. Whatever the beneficial impact of it, in and of itself, BMI screening in primary care is a
fundamental precursor to effective intervention. If there is no routine measurement for
individual patients there will be no trigger for discussion about an intervention to address the
issue. This will inevitably result in a reduced referral rate to such interventions
5. BMI screening at an individual level is one enabler of comparative effectiveness of different
interventions – both short and longer term
6. The evidence and our own observations indicate that patient interest in, and engagement
with interventions is greater if they are aware that they need to address an issue. If the
measure is removed and /or deprioritised fewer children and parents will be aware easily
that there is an issue and engage with evidence-based interventions
7. Work is taking place at a national level (HCP bodies and CDC-funded collaboratives) to
codify, pay for and prioritize evidence-based SCALABLE child weight management
interventions with a long overdue view to scaling up such activity – both across states and
nationally. This work will be hamstrung by removing this measure.
To be clear, we are not opposed to the removal of the measure per se, AS LONG AS IT IS
REPLACED IMMEDIATELY BY AN IMPROVED MEASURE, such as referral to evidence-based
care (of which there are sufficient interventions available nationally).
C.44
Human Services Research Institute (Julie Bershadsky)
First, thank you for the opportunity to review and comment on the draft report resulting from the
work of 2020 Child and Adult Core Set Review Workgroup. I was able to attend the in-person
meeting on May 9th and was thoroughly impressed with the thoughtful discussion of the
Workgroup.
As director of NCI-AD, I am heartened by the Workgroup’s recognition of the critical role of
LTSS in so many people lives’ and the importance of having valid, reliable and well-recognized
measures, keyed to outcomes important to recipients of LTSS and developed with their input.
As we have learned, developing and implementing meaningful LTSS measures is not easy, and
the importance of technical assistance for implementing them and using resultant data cannot
be overstated.
The NCI-AD Adult Consumer Survey has all the above characteristics, which, as evidenced by
the program’s rapid growth and expansion, are recognized and valued by participating states
and state agencies. While participation is voluntary, in 2018-2019, 17 states collected NCI-AD
Adult Consumer Survey data; in 2019-2020, we estimate 20-22 surveying states; that number is
expected to continue growing every year. Furthermore, the number of surveys conducted in
many states is also growing. Current sample sizes range from 400 to almost 4000 surveys per
state; many states oversample to target individual programs and various subpopulations within
those programs – for example, MCOs, service settings, geographic regions, and, in at least one
state, service providers. NCI-AD’s feasibility is well-established, and the technical assistance,
oversight, project management and centralized data analysis provided by HSRI and its partner,
NASUAD, contribute greatly to the program’s recognition and expansion. The importance of
these elements was evident in the Workgroup members discussion and are reflected in the draft
report.
There are a few clarifications and edits to the draft report we would like to suggest:
Page VI: Abbreviation “NCI-AD” stands for “National Core Indicators for Aging and Disabilities”,
without the “Adult Consumer Survey” at the end.
Page IX, Exhibit ES.3: Similarly, it would be more correct to refer to NCI-AD as “National Core
Indicators for Aging and Disabilities (NCI-AD) Adult Consumer Survey”.
Page 11, Exhibit 7: Same comment as right above.
Page 16, heading “National Core Indicators (NCI) and National Core Indicators for Aging and
Disabilities Adult Consumer Survey (NCI-AD)”: Same comment as above.
Page 16, first paragraph under NCI & NCI-AD: Should read “The NCI and NCI-AD measures
assess the experience and outcomes of individuals with intellectual and developmental
disabilities and their families, and seniors and adults with physical disabilities, respectively”.
C.45
Page 16, third paragraph under NCI & NCI-AD: Should read “NCI-AD is a voluntary effort by
state Medicaid aging and disability agencies to measure and track their performance. The core
indicators are standard measures used across states to assess the outcomes of services
provided to seniors and adults with physical disabilities. Indicators address nineteen key areas
of concern including (1) service coordination, (2) rights and respect, (3) community participation,
(4) choice and decision-making, (5) health care, (6) safety, (7) relationships, (8) satisfaction, (9)
care coordination, (10) access to community, (11) access to needed equipment, (12) wellness,
(13) medications, (14) self-direction, (15) work, (16) everyday living, (17) affordability, (18)
control, and (19) person-centered planning. Seventeen states collected NCI-AD data in 2018
and 2019.”
Page 17, last paragraph under NCI & NCI-AD: Should read “Workgroup members
acknowledged that adding new survey-based measures to the Core Set would require states to
either add requirements for these surveys to managed care contracts or to field them directly. At
the same time, measures from other data sources that assess the experience of beneficiaries
and their functional status and well-being are not currently available. The NCI and NCI-AD
surveys are accessible in multiple languages to people with disabilities who are nonverbal,
blind, deaf, or have other disabilities; both are also available in Spanish.”
Again, I appreciate the opportunity to comment and thank the Workgroup for its time and efforts.
C.46
Human Services Research Institute (Alexandra Bonardi)
Thank you for the opportunity to review and comment on the draft 2020 Child and Adult Core
Set Review Workgroup. Having attended the in-person discussion of the Workgroup during its
discussions of the measures considered in the Long Term Supports and Services domain, I
commend the workgroup for its consideration and thoughtful discussion of the measures.
I am pleased to have this opportunity to reflect on this report from my perspective as the
Director of National Core Indicators at Human Services Research Institute, and to provide some
important additional content and clarification specific to the National Core Indicators.
As states continue to support people with complex and long-term support needs, it is critical to
have recognized, valid measures against which to benchmark and improve outcomes. I support
the identification of LTSS measurement as a strategic priority for states.
The National Core Indicators (NCI) have demonstrated feasibility, with 46 states participating. In
2018-2019, 37 states collected data through the NCI’s In-Person Survey. While individual states
voluntarily participate, collect data, and are the owners of the data collected analyzed, results
are made public through web-based reports generated by HSRI and posted on the
NationalCoreIndicators.org website. As partners with states in this data collection effort, HSRI
and the National Association of State Directors, support states through robust training support to
ensure reliable data collection, direct and ongoing communication with all states to ensure
samples produce valid and comparable results, a standardized electronic data collection
platform, support to NCI coordinators in all states, and an annual meeting to develop states
capacity to collect and use the NCI data. We believe the ongoing participation of states
demonstrates that the effort to train surveyors and collect data yields valuable information on
quality and outcomes; information that cannot be collected by a means other than a direct
survey to service recipients. This was reflected in the workgroup members discussion.
States routinely provide examples of how NCI data is used. The summary data can be
downloaded from reports. NCI also provides a chart generator
(https://www.nationalcoreindicators.org/charts/) which allows visitors to the NCI website to
generate basic charts from the in-person survey results on a range of indicators in the
population receiving services from their Developmental Disabilities Agency. The NCI staff
stability survey is used to objectively examine issues in the direct support workforce across
agencies including wages, benefits, staff retention and turnover. Staff stability results have been
used to monitor the effects of policy and payment initiatives to increase worker wages, for
example.
Finally, there are a few clarifications we would like to offer for your consideration in the draft
report:
• Page 16 of the report includes the following description. “The NCI and NCI-AD measures
assess the experience and outcomes of individuals with intellectual and developmental
disabilities and their families and individuals with physical disabilities and their families,
respectively.” To clarify, NCI surveys are intended to provide state agencies and the public
C.47
information about the experience of people who receive services from state developmental
disabilities agency. It provides an In-Person Survey, Family Surveys for parents and
guardians of adults and children who receive supports and a Staff Stability Survey. NCI-AD
is intended to provide information from people who are receiving age-related support
services and people with physical disabilities through the NCI-AD Adult Consumer Survey
which is delivered in-person.
• The first bullet references the staff suitability survey, this should be changed to reflect the
tool’s name: the Staff Stability Survey.
Thank you again for this opportunity to offer comment.
C.48
Indiana Division of Disability and Rehabilitative Services (Kylee B Hope)
Thank you for the opportunity to comment on the report titled "Recommendations for Improving
the Core Sets of Health Care Quality Measures for Medicaid and CHIP." The report succinctly
and clearly describes the discussions of the work group and provides helpful background
information.
I am writing these comments in my role as the State Director of Developmental Disability
services in the state of Indiana; Indiana has participated in the National Core Indicators (NCI)
project for the past seven (7) years and is gearing up for year eight. As the Director, discussions
of reporting on measures in Medicaid Long Term Services and Supports is of significant interest
and concern. It is extremely important to distinguish between measures of acute or rehabilitative
long term care, which typically take place in a facility or institutional setting, from those which
are services or supports for daily life, which typically take place in home and community based
settings. This distinction is extremely important to make, and would be helpful if described in the
report.
Of note, there were 42 distinct measures suggested for inclusion in either the Child or Adult
Core Set. Of those 42, only 5 were identified by the workgroup as substantially meeting the
characteristics set forth as important for the measure to be recommended. That NCI was among
the measures recommended comes as no surprise, with its long history and reputation for
providing valid, reliable and most importantly relevant and actionable measures. NCI is well
known and heavily used in the national DD system.
I strongly encourage CMS to take up the recommendations of the workgroup to include NCI to
fill the gap in LTSS measures for the Medicaid Adult Core Measure set in 2020. To understand
how NCI meets the characteristics set forth by CMS and Mathematica, I would like to comment
on the specific characteristics for consideration of a New Measure as described in the report.
Characteristics Considered for Addition of a New Measure:
Actionability. Will the measure provide useful or actionable results for state Medicaid and CHIP
programs?
Indiana has utilized NCI data in a variety of ways. NCI data was used as initial assessment data
for Indiana's Statewide Transition Plan for compliance with the HCBS Settings Rule. The data
has been shared with legislatures, advocacy groups, and disability tasks forces to affect policy
and administrative changes. NCI data is being used as performance measure data for HCBS
waivers.
Additionally, NCI data will be used as an assessment of the quality of life for individuals with
disabilities living in the community through Indiana's Living Well Grant.
Alignment. Is the measure used in other reporting programs?
C.49
On a quarterly and annual basis, Indiana's NCI data is compiled and reviewed for several of the
performance measures within Indiana's two HCBS waivers. Indiana is also incorporating NCI
data into the Living Well Grant as part of the assessment and evaluation process. The data is
included in the annual reports to the grant funder, the Administration for Community Living.
Appropriateness for state-level reporting. Has the measure been validated and tested for state-
level reporting? Is it currently used by one or more states?
Indiana has participated in the NCI with 45 other states and the District of Columbia for several
years. The high state participation rate and the information to make comparisons between
states of similar size or states within our same geographic region is extremely helpful to
establish the framework of our system. Additionally, Indiana is able to study and analyze the
NCI indicators to reveal how our full system is functioning. As the State Director, this ability is
vitally important in my role. Indiana does have processes in place to measure individual
providers and individual lives, but NCI is the only systemic measurement system that provides
me with already cleaned, analyzed, and publicly reported data. The ability to have a nationally
recognized data set that is transparent is a profound step towards effective trust-building and
collaboration with our stakeholders. NCI provides a core effort in our stakeholder engagement
process.
Feasibility. Will states be able to access the data needed to calculate the measure? Would
technical assistance be necessary or helpful to facilitate complete and accurate reporting of the
measure by states?
Indiana employs an outside vendor to conduct a statistically valid random sample for each of the
two HCBS waivers. The vendor generates the random sample from the state's records,
populates the background information, conducts the face-to-face interviews, and enters the
information into the NCI system. Indiana invests substantial resources, including financial and
personnel, to obtain reliable and valid information. Indiana has been investing in NCI for over
seven years because the information obtained through the survey is invaluable. NCI data
provides insight into the functionality of the entire system as well as how Indiana compares to
similar states. Through this data, we can determine areas of strength and weakness, as well as
influence system improvements and regulatory changes.
Strategic priority. Does the measure fill a gap that has been identified in the Child or Adult Core
Sets?
NCI provides Indiana with information necessary to understand the experience of individuals
with intellectual and developmental disabilities who are accessing and utilizing Indiana's HCBS
waivers. NCI data is cleaned, analyzed, and a comprehensive report is publicly shared which is
not available in any other measure set. Because NCI data is publicly reported, my state agency
is accountable for the results. Through the NCI results, Indiana analyzes the information to
identify opportunities for improvement and/or evaluation of policies or practices.
C.50
Indiana agrees with the summary of the workgroup discussion which indicates the usefulness of
the NCI measures to our states and the flexibility and adaptability to be used by people with
multiple types of disabilities. Since NCI's inception in 1998, NCI has been steadfast in funneling
direct input from individuals with intellectual and developmental disabilities to the state agency
responsible for the administration of the service delivery system. NCI assures the voice of the
people is heard directly through face-to-face interviews regarding the supports and services so
essential to their day to day well-being. This fundamental aspect of NCI – the importance to
measure – weighs equally in Indiana's decision to continue using NCI.
C.51
Kaiser Permanente (Deborah Espinal)
Kaiser Permanente ("KP") appreciates the opportunity to review and comment on the 2020 Core
Set Review ("Review") for public comment. KP has the following comments/questions on the
Review for consideration:
1. KP notes that there are several measures which include services that are often provided by
other entities, separate from providers that directly contract with the managed care health
plan. For example, in California, the added measures of "Appropriate Antibiotic Prophylaxis
for Children with Sickle Cell Anemia" includes services that may be carved out of managed
care to California Children’s Services and "Metabolic Monitoring for Children and
Adolescents on Antipsychotics" includes services that are typically carved out of managed
care to the county mental health system. In addition, measures which fall under "Dental and
Oral Health Services" include dental care which is often entirely carved out of managed
care. While we understand that each state varies in its service delivery arrangement, it is
beneficial to acknowledge in the Review that managed care plans do not always have
access to all of the data required to report on every measure and that the state must clearly
communicate how and from whom the data should be reported.
2. In addition to the benefit of acknowledging the varying service providers that impact the
different measures and determining reporting responsibility, KP would like to comment on
the administrative burden managed care plans must undertake to report on these measures.
The level of this burden will be greatly increased depending on how the individual state
program chooses to administer the reporting program and responsibilities.
3. The Review references alignment with other measures as a main priority
(pg. 8 and Exhibit
ES.1) and also that the members of the Review Workgroup commented that the Core Sets
are not the place to put new or untested measures (pg. 20).
2
In response, KP would like to
note that there are measures in the 2020 Core Set that have an established, well-tested
National Commission on Quality Assurance (NCQA) option, yet were not used. The Review
also does not provide any rationale for not using the NCQA measure. Some of these
measures include "Screening for Depression and Follow-up Plan" at different ages and "Use
of Pharmacotherapy for Opioid Use Disorder," which both cite the CMS rather than the
NCQA measure. NCQA measures are trusted and well-tested and typically have already
been operationalized by managed care plans for easy reporting and more accurate data. KP
recommends that where measures can clearly be aligned with NCQA, the NCQA measure is
chosen.
4. Finally, KP would like to comment on the election of the two Human Services Research
Institute (HSRI), National Core Indicator (NCI) measures to be added for the 2020 Core Set
Measures. First, it is difficult to understand what would be required of managed care plans
to administer and report on these measures. Second, it is not clear that these measures
would provide meaningful information beyond what will already be collected through other
measures. Further rationale and basic technical guidance is needed for state programs as
well as managed care plans to understand the reasoning and benefit of utilizing these two
new measures.
C.52
KP appreciates the opportunity to provide comments on the 2020 Core Set Review for Public
Comment. Please feel free to reach out to KP with any questions.
C.53
Kaiser Permanente Washington Health Research Institute (Beverly B
Green)
2020 Core Set Review Public Comment: In support of adding colorectal cancer screening as a
core set measure.
Colorectal cancer (CRC) screening decreases mortality and incidence by over 50%
1
and may
be cost-saving because of the high costs of CRC treatment.
2
However, screening rates are low
especially among Medicaid insured adults (46%, compared to 70% in Medicare insured adults,
HEDIS 2017)
3
, a disparity that we believe is in part to the fact that Medicaid plans are not
required to report CRC rates. In contrast, Medicare plans are required to report CRC screening
rates. Medicaid enrollees are also 50% more likely to present with late stage colon cancer or die
from it, than those with commercial or Medicare insurance.
4
Over 75% of CRC deaths occur in
adults who are not up to date for CRC screening.
5
Nationally, the Medicaid population includes
about 1.8 million adults overdue for CRC screening.
4
Direct mailing of fecal immunochemical test (FITs) is low-cost evidence-based effective strategy
for increasing CRC screening, with meta-analyses demonstrating 22% increase in screening
rates.
6
Kaiser Permanente Northern California mails over 700,000 FIT kits to adult patients over-
due for CRC screening annually to achieve CRC screening rates of over 87%. CRC mortality
rates have dropped by 55% since the program began in 2006.
7
BeneFIT is a Centers for Disease Control and Prevention funded mailed FIT program
(U48DP005013) that was implemented in two Medicaid managed care insurance plans,
8
one in
Oregon and one in Washington state. The plans used claims data to identify enrollees overdue
for CRC screening and vendors to mail FIT kits directly to over 10,000 enrollees, with over 17%
completing FIT within 6 months. In year 2 of the program, the Washington health plan decided
to limit the program to only special needs enrollees (those with both Medicaid and Medicare
insurance), while the Oregon plan expanded its program. Oregon is one of 4 states that requires
insurance plans to report Medicaid CRC screening rates, Washington state does not have a
reporting requirement, a key factor in their decision to only offer the program to special needs
enrollees.
While a Medicaid CRC quality reporting metric is not enough on its own to decrease Medicaid
screening and outcome disparities, it is a necessary first step. Without it plans cannot track
progress, learn from best practices, and are less motivated to support community clinic efforts to
increase screening rates. The Health Resources and Services Administration (HRSA) require
Federally Qualified Health Centers (FQHCs) to report CRC screening rates,
9
with results
publicly available. In Washington state, some FQHCs, with grant support, have successfully
implemented their own mailed FIT programs. Medicaid plan support would help them to sustain
these programs long-term. Oregon state also includes Medicaid CRC screening rates as one of
its incentivized metrics. Since the program began in 2014 CRC screening rates have increased
by 11% (from 46.2% to 57.2%).
10
In contrast Washington, a state that does not required plans
to report Medicaid CRC screening, rates have only increased by 3% (from 43% to 46%) over
the same time period.
11

C.54
In the draft report two reasons were given for not adding CRC screening to the adult core set.
We address each below:
1. The long look back period for screening colonoscopy. This barrier also applies to Medicare
and commercially insured patients, but there are solutions. In BeneFIT, clinics review health
plan lists to identify people who do not need mailed FITs because of a colonoscopy, and to
update documentation.
8
Patients are also asked in when they are sent an introductory letter
prior to FIT mailings (and a number to call if they have had a colonoscopy in the prior 10
years). OCHIN, a nonprofit organization that provides electronic health records (EHR) and
support to over 500 health care organizations (mostly FQHCs) in 47 states has deployed
EHR reminders and embedded registries to identify age-eligible patients overdue for CRC
screening. Provider so providers can order screening tests or update records. These
interventions were successfully deployed to over 40,000 low-income patients in the STOP
Colon Cancer Trial (Coronado, JAMA Internal Medicine 2018).
12
2. Lack of testing of the colorectal cancer screening metric. Medicaid health insurance plans
already collect HEDIS Medicare CRC rates for special needs patients (adults with both
Medicaid and Medicare). Four states, including Oregon require Medicaid health plans to
report HEDIS colorectal cancer screening rates.
Below is a review the criteria for adding a new metric to the Medicaid and CHIP Child and Adult
Core set of quality indicators. As discussed above, all of the criteria are met.
Actionability: Will the measure
provide useful or actionable
results for state Medicaid
programs?
Yes, effective low-cost strategies exist for increasing CRC
screening uptake. Reporting allows plans to track their progress
and identify plans with best practices.
Alignment: Is the measure used
in other reporting programs?
CRC screening reporting is required by:
• CMS Medicare - HEDIS
• NCQA accreditation - HEDIS
• HRSA grantees (Federally Qualified Health Centers)
Appropriateness: for state-level
reporting: Has the measure been
validated and tested for state-
level reporting in one or more
states?
Four states have tested and require reporting of HEDIS Medicaid
CRC screening. The long look back for screening colonoscopy is a
challenge for all insurance plans, but there are solutions. Better
documentation decreases under and overuse of screening and
follow-up testing.
Feasibility: Will states be able to
access the data needed to
calculate the measure?
Health plans already collect this data for Medicare and
commercially insured enrollees. The CRC screening HEDIS metric
is a hybrid measure (claims data and/or chart audit). Audits done
for other HEDIS hybrid metrics could include CRC screening.
Strategic Priority: Does the
measure fill a gap area in the
Child and/or the Adult Core Set?
Yes, breast and cervical cancer are already included in the Adult
Core Set. Colorectal cancer screening is not included, even though
screening rates are lower and disparities larger.
C.55
To, summarize, Medicaid patients experience CRC screening and outcome disparities. Adding
the HEDIS CRC screening to the core metric is a necessary first step for change.
Citations
1
Zhang J, Cheng Z, Ma Y, He C, Lu Y, Zhao Y, Chang X, Zhang Y, Bai Y, Cheng N.
Effectiveness of Screening Modalities in Colorectal Cancer: A Network Meta-Analysis. Clin
Colorectal Cancer. 2017;16:252-63.
2
Ran T, Cheng CY, Misselwitz B, Brenner H, Ubels J, Schlander M. Cost-Effectiveness of
Colorectal Cancer Screening Strategies-A Systematic Review. Clin Gastroenterol Hepatol.
2019.
3
de Moor JS, Cohen RA, Shapiro JA, Nadel MR, Sabatino SA, Robin Yabroff K, Fedewa S, Lee
R, Paul Doria-Rose V, Altice C, Klabunde CN. Colorectal cancer screening in the United
States: Trends from 2008 to 2015 and variation by health insurance coverage. Prev Med.
2018;112:199-206. PMCID: PMC6202023.
4
Medicaid and CHIP Payment and Access Commission. MACStats: Medicaid and CHIP Data
Book. 2017 [updated 2017; cited August 3 2019]. Available from:
https://www.macpac.gov/wp-content/uploads/2015/12/MACStats-Medicaid-CHIP-Data-
Book-December-2017.pdf.
5
Doubeni CA, Fedewa SA, Levin TR, Jensen CD, Saia C, Zebrowski AM, Quinn VP, Rendle
KA, Zauber AG, Becerra-Culqui TA, Mehta SJ, Fletcher RH, Schottinger J, Corley DA.
Modifiable failures in the colorectal cancer screening process and their association with risk
of death. Gastroenterology. 2019;156:63-74.e6. PMCID:
C.56
Kentucky Department for Medicaid Services (Angela W Parker)
I agree with the Workgroup’s characteristics for removing or adding measures. However, I am
not sure all characteristics were taken into account with some of the recommendations.
I disagree with removing the Weight Assessment and Counseling for Nutrition and Physical
Activity for Children/Adolescents-BMI assessment (WCC-CH) and Adult BMI Assessment (ABA-
AD) from the Core Set for both children and adults. Obesity is an epidemic. I agree with the
federal liaison who voiced support for the measures. I am unsure what clinical evidence to
support the measure is lacking, but the answer is to improve on the clinical evidence, not take
away the focus. There are multiple health risks associated with obesity.
I disagree with the addition of the antibiotic prophylaxis measure for sickle cell anemia. It is
limited in scope due to the prevalence variability by state due to the rarity of the disease. The
focus should be on educating the providers who care for these patients and the consumers who
have this unfortunate disease.
National Core Indicators (NCI) and National Core Indicators for Aging and Disabilities Adult
Consumer Survey (NCI-AD) addition will be a challenge for those states not currently using and
could be an administrative burden. It does provide additional information that may be of use for
care management programs. However, most Medicaid Managed Care Organizations have tools
for identification for those populations.
C.57
Allison LaRussa
With thanks to the Center for Medicaid and CHIP Services (CMCS) for the opportunity to
comment, I would like to offer the following in response to the draft report “Recommendations
for Improving the Core Sets of Health Care Quality Measures for Medicaid and CHIP.” The draft
report states that the Child and Adult Core Set Stakeholder Workgroup for the 2020 Annual
Review is charged with assessing the 2019 Core Sets with focus on measurement that is
actionable, aligned, and appropriate. Actionable measures are defined as those whose “results
can be used to improve care delivery and health outcomes.” By including measures that have
the potential to spur improved quality of care, the Core Set is positioned as a valuable tool for
CMCS and participating states.
One integral and actionable aspect of health care quality, equity, was not emphasized in the
draft report. Reducing disparities in health care delivery and outcomes between subpopulations
is critical to improving quality of care and health care organizations across the country are
striving to close gaps in care related to social characteristics. Through the Core Set, CMCS can
encourage these efforts by supporting measurement that demonstrates the degree of equity in
care. Selecting measures for the Core Set that are sensitive to disparities related to gender,
race, ethnicity, geographic location, and socioeconomic status could highlight areas in which
state Medicaid and CHIP programs can take action to create more equitable health systems.
Disparities sensitivity should be one of the Core Set’s “characteristics considered for removal of
existing measures and addition of new measures,” and measures that are sensitive to
disparities should be prioritized for inclusion. Disparities sensitive measures may be those that
are prevalent among particular subpopulations or those that have been found to demonstrate
gaps in quality between subpopulations. Of note, the Workgroup did recommend to include the
disparities sensitive measure Appropriate Antibiotic Prophylaxis for Children with Sickle Cell
Anemia for the 2020 Core Set. However, the draft report does not indicate that disparities
sensitivity was systematically accounted for in Workgroup recommendations for each measure
under consideration. In evaluating measures under consideration for disparities sensitivity, the
Workgroup could employ a protocol such as that developed by the National Quality Forum
(NQF) in 2012 in which NQF-endorsed measures are identified as being disparities sensitive
after assessment using standard criteria.
1
Shared use of the Core Set presents an opportunity for shared focus on promoting health equity
and action toward reducing health disparities. In Exhibit 8 of the draft report, “stratification by
race and ethnicity” is noted as a gap area for future core measure sets. Further, “racial and
ethnic stratification” is listed in the draft report as a suggested area for shared learning
opportunities for states. Pursuit of these ideas dovetails with the practice of routinely and
systematically considering disparities sensitivity in evaluating measures for inclusion in the Core
Set. This approach brings equity to the forefront of quality measurement for Medicaid and CHIP,
creating opportunity for widespread data-driven improvements in care that are urgently needed.
C.58
Citation
1
National Quality Forum (2012). Healthcare Disparities and Cultural Competency Consensus
Standards: Disparities Sensitive Measure Assessment.
https://www.qualityforum.org/projects/Healthcare_Disparities_and_Cultural_Competency.as
px.
C.59
Maryland Department of Health (Bernard Simons)
Thank you for the opportunity to comment on the report titled “Recommendations for Improving
the Core Sets of Health Care Quality Measures for Medicaid and CHIP.” The report succinctly
and clearly describes the discussions of the work group and provides helpful background
information.
I am writing these comments in my role as the State Director of Developmental Disability
services for The Maryland Developmental Disabilities Administration. Maryland has participated
in NCI for the past several years. As the Director, discussions of reporting on measures in
Medicaid Long Term Services and Supports is of significant interest and concern. It is extremely
important to distinguish between measures of acute or rehabilitative long-term care, which
typically take place in a facility or institutional setting, from those, which are services or supports
for daily life, which typically take place in home, and community-based settings. This distinction
is extremely important to make and would be helpful if described in the report.
Of note, there were 42 distinct measures suggested for inclusion in either the Child or Adult
Core Set. Of those 42, only five were identified by the workgroup as substantially meeting the
characteristics set forth as important for the measure to be recommended. That NCI was among
the measures recommended comes as no surprise, with its long history and reputation for
providing valid, reliable and most importantly relevant and actionable measures. NCI is well
known and heavily used in the national DD system.
I strongly encourage CMS to take up the recommendations of the workgroup to include NCI to
fill the gap in LTSS measures for the Medicaid Adult Core Measure set in 2020. To understand
how NCI meets the characteristics set forth by CMS and Mathematica, I would like to comment
on the specific characteristics for consideration of a New Measure as described in the report.
Characteristics Considered for Addition of a New Measure:
Actionability. Will the measure provide useful or actionable results for state Medicaid and CHIP
programs?
Maryland has been using the NIC data to make policy decision and provide education and
information to our stakeholders based on the trends of our data collection.
Alignment. Is the measure used in other reporting programs?
The Maryland Developmental Disabilities Administration has been using the NCI data in our
quarterly reporting to the quality advisory committee to improve our performance measures and
basic HCBS waiver performance measures. We have been able to use the data to inform
stakeholders of areas where we need to improve and were we are doing outstanding. The data
has also been used to improve the health and safety of the person we support.
Appropriateness for state-level reporting. Has the measure been validated and tested for state-
level reporting? Is it currently used by one or more states?
C.60
NCI has been utilized by our quality assurance department, nurse and incident reporting across
four regions for the past several years. NCI indicators demonstrate how our full system is
functioning, which is critically important in my role as the State Director. We have mechanisms
in place to measure individual providers and individual lives, but NCI is the only systemic
measurement system that provides me with already cleaned, analyzed and publicly reported
data. This transparency of data forms a significant step towards effective trust-building and
collaboration with our stakeholders. NCI provides a core effort in our stakeholder engagement
process.
Feasibility. Will states be able to access the data needed to calculate the measure? Would
technical assistance be necessary or helpful to facilitate complete and accurate reporting of the
measure by states?
The Developmental Disabilities Administration is in the process of enhancing its service delivery
model and has created a new data based platform that will be use to collect NCI data that
focuses on critical information to know to be able to make policy decision as well as funding
decisions.
Strategic priority. Does the measure fill a gap that has been identified in the Child or Adult Core
Sets?
The NCI provides Maryland critical information to be able to understand the experience of
people with developmental disabilities who access and utilize home and community-based
services and their families. There is no other measure set which provides this insight. The public
reporting of NCI results provides a level of accountability needed by my state agency in order to
understand where we have improvement opportunities or need to re-evaluate the effectiveness
of our policies or practices.
We agree with the summary of the workgroup discussion, indicating the usefulness of the NCI
measures to our states, the flexibility and adaptability to be used by people with multiple types
of disabilities. From its inception in 1998, NCI has remained committed to channeling direct
input from people with I/DD to the state agency responsible for the administration of the service
delivery system. NCI assures the voice of the people is heard directly regarding the supports
and services so critical to their day-to-day well-being. This crucial aspect of NCI – the
importance to measure – weighs equally in state’s decision to continue using NCI.
C.61
Michael & Susan Dell Center for Healthy Living (Deanna M Hoelscher)
I am providing public comment on the report, Recommendations for Improving the Core Sets of
Health Care Quality Measures for Medicaid and CHIP: Summary of a Multi-Stakeholder Review
of the 2020 Child and Adult Core Sets. In particular, I would like to comment on the proposed
changes for the core indicator, Weight Assessment and Counselling for Nutrition and Physical
Activity for Children / Adolescents – Body Mass Index Assessment for Children / Adolescents
(WCC-CH). As a child obesity researcher for more than 25 years, this measurement has been
essential to our work in low-income communities, and the omission of this measure (without a
suitable substitute) would make it difficult for us to: (1) determine the extent of the child obesity
problem in Medicaid and CHIP participants, as well as to (2) document any changes that occur
through individual and environmental-level interventions.
My specific objections to the removal of this core indicator are as follows:
1. The U.S. Preventive Services Task Force (USPSTF) recommends that children and
adolescents 6 years and older be screened by clinicians, who should also offer behavioral
counselling or refer them to a comprehensive, behavioral intervention.
1
This
recommendation is graded B, based on the evidence, which indicates that the “net benefit is
moderate or there is moderate certainty that the net benefit is moderate to substantial”. This
was noted by one of the federal liaisons in the report. Thus, this measure (or an appropriate
replacement) should be implemented in Medicaid and CHIP populations.
2. Documentation of high levels of children with obesity is necessary to justify the development
or implementation of effective interventions in the clinical or community settings. For
example, in our study, the Texas Childhood Obesity Research Demonstration (TX CORD)
study, we found a decrease in the %BMI
p95
, a measure of severe obesity, in children who
participated in the CORD intervention.
2
This is consistent with other reviews that have
shown the benefit of lifestyle-based interventions for weight management among children
and adolescents that have at least 26 hours or more of contact time.
3
3. Measuring obesity rates in Medicaid or CHIP populations is also essential to monitor
progress in obesity prevention efforts that might be occurring via intensive community-based
efforts or through natural experiments in specific regions or states. The utility of these types
of measurements are clearly outlined in the Institute of Medicine’s (now the National
Academy of Science, Engineering, and Medicine) report on Evaluating Obesity Prevention
Efforts.
4
As the lead author of the chapter on the National Obesity Evaluation Plan,
surveillance through existing systems, such as the Medicaid/CHIP core indicators, was
considered an essential part of monitoring efforts.
4. Measuring obesity in the physician’s office can be an effective way to bring awareness to
parents about their child’s health. Using tools, such as the Next Steps materials from the
American Academy of Pediatrics, can help the clinician effectively communicate with the
family.
As can be seen, there are several compelling and evidence-based reasons to support the
measurement of body mass index in children in Medicaid and CHIP. I strongly encourage you to
C.62
re-consider the removal of this core indicator. Please let me know if you need further
documentation to support this request.
Citations
1
https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatement
Final/obesity-in-children-and-adolescents-screening1; JAMA 2017.
2
Butte et al., Childhood Obesity, 2015
3
O’Connor et al., JAMA, 2017
4
IOM (Institute of Medicine), 2013.
C.63
Minnesota Department of Human Services (Alex E Bartolic)
Thank you for the opportunity to comment on the report titled “Recommendations for Improving
the Core Sets of Health Care Quality Measures for Medicaid and CHIP.” The report succinctly
and clearly describes the discussions of the work group and provides helpful background
information.
I am writing these comments in my role as the State Director of Disability services in Minnesota.
Minnesota has actively participated in the NCI suite of surveys for the past five years. As the
Director, discussions of reporting on measures in Medicaid Long Term Services and Supports is
of significant interest and concern. It is extremely important to distinguish between measures of
acute or rehabilitative long term care, which typically take place in a facility or institutional
setting, from those which are services or supports for daily life, which typically take place in
home and community based settings. This distinction is extremely important to make, and would
be helpful if described in the report.
Of note, there were 42 distinct measures suggested for inclusion in either the Child or Adult
Core Set. Of those 42, only 5 were identified by the workgroup as substantially meeting the
characteristics set forth as important for the measure to be recommended. The NCI-Aging and
Disabilities (AD) Survey and NCI-In Person Survey (IPS) was among the measures
recommended, and that comes as no surprise. It has a long history and reputation for providing
valid, reliable and most importantly relevant and actionable measures. NCI is well known and
heavily used in the national disability services system.
Minnesota participates in the NCI surveys to learn how well home and community-based
services are supporting people. The NCI surveys is one way DHS hears directly from people
about how well the services and supports they receive help them live, work, and engage in their
community.
I strongly encourage CMS to take up the recommendations of the workgroup to include the NCI
surveys to fill the gap in LTSS measures for the Medicaid Adult Core Measure set in 2020. To
understand how NCI meets the characteristics set forth by CMS and Mathematica, I would like
to comment on the specific characteristics for consideration of a New Measure as described in
the report.
Characteristics Considered for Addition of a New Measure:
Actionability. Will the measure provide useful or actionable results for state Medicaid and CHIP
programs?
In recent years, Minnesota has used NCI data to monitor and improve services. An example of
this is how Minnesota has used NCI data on community inclusion to help identify and measure
progress towards meeting Olmstead goals. Multiple initiatives were developed as part of
Minnesota’s Olmstead Plan and NCI results are reported annually to the Minnesota Olmstead
Subcabinet to track progress.
C.64
Alignment. Is the measure used in other reporting programs?
NCI indicators are used in strategic plan measures, policy proposals, and other high-level
planning efforts. NCI indicators are used because they reflect people’s quality of life and
experience with long-term services and supports. The NCI indicators are used for a number of
reasons, including:
• Indicators touch on domains of people’s lives that Minnesota does collect elsewhere.
• Surveys are independently administered.
• Surveys gather feedback directly from people.
Appropriateness for state-level reporting. Has the measure been validated and tested for state-
level reporting? Is it currently used by one or more states?
The NCI-IPS has been utilized by 46 states and the District of Columbia for several years. The
comparisons between states of similar size, states within our same geographic region, and
similar LTSS service structure are helpful. In addition, the NCI indicators demonstrate how our
full system is functioning, which is critically important in my role as the State Director. We have
mechanisms in place to measure individual providers and individual lives, but NCI is the only
systemic measurement system that provides Minnesota with already cleaned, analyzed and
publicly reported data.
Feasibility. Will states be able to access the data needed to calculate the measure? Would
technical assistance be necessary or helpful to facilitate complete and accurate reporting of the
measure by states?
Minnesota already has implemented a system to collect and analyze state-level NCI data on a
yearly basis. We rely on our national partners National Association of State Directors of
Developmental Disabilities Services (NASDDDS), the National Association of States United for
Aging and Disabilities (NASUAD), and Human Services Research Institute (HSRI) to develop
the survey, training materials, and keep us informed of changes to the survey. We contract with
Vital Research to hire, train, implement the interviews, and compile/report data back to our
national partners for analysis.
Strategic priority. Does the measure fill a gap that has been identified in the Child or Adult Core
Sets?
NCI provides the Long Term Services and Supports measures needed by state Medicaid
agencies to understand the experience of people with physical and developmental disabilities
who access and utilize home and community based services. There is no other measure set
which provides this insight, including the data cleaning, analysis, and summary report which
publicly compares all participating state results. The public reporting of NCI results provides a
level of accountability needed by my state agency in order to understand where we have
improvement opportunities or need to re-evaluate the effectiveness of our policies or practices.
C.65
We agree with the summary of the workgroup discussion, indicating the usefulness of the NCI
measures to our states, the flexibility and adaptability to be used by people with multiple types
of disabilities. From its inception in 1998, NCI has remained committed to channeling direct
input from people with physical disabilities and I/DD to the state agency responsible for the
administration of the service delivery system. NCI assures the voice of the people is heard
directly regarding the supports and services so critical to their day to day well-being. This crucial
aspect of NCI – the importance to measure – weighs equally in state’s decision to continue
using NCI.
C.66
Missouri Division of Developmental Disabilities (Valerie Huhn)
Thank you for the opportunity to comment on the report titled “Recommendations for Improving
the Core Sets of Health Care Quality Measures for Medicaid and CHIP.” The report succinctly
and clearly describes the discussions of the work group and provides helpful background
information.
I am writing these comments in my role as the State Director of Developmental Disability
services in Missouri. As the Director, discussions of reporting on measures in Medicaid Long
Term Services and Supports is of significant interest and concern. It is extremely important to
distinguish between measures of acute or rehabilitative long term care, which typically take
place in a facility or institutional setting, from those which are services or supports for daily life,
which typically take place in home and community based settings. This distinction is extremely
important to make, and would be helpful if described in the report.
Of note, there were 42 distinct measures suggested for inclusion in either the Child or Adult
Core Set. Of those 42, only 5 were identified by the workgroup as substantially meeting the
characteristics set forth as important for the measure to be recommended. That NCI was among
the measures recommended comes as no surprise, with its long history and reputation for
providing valid, reliable and most importantly relevant and actionable measures. NCI is well
known and heavily used in the national DD system.
I strongly encourage CMS to take up the recommendations of the workgroup to include NCI to
fill the gap in LTSS measures for the Medicaid Adult Core Measure set in 2020. To understand
how NCI meets the characteristics set forth by CMS and Mathematica, I would like to comment
on the specific characteristics for consideration of a New Measure as described in the report.
Characteristics Considered for Addition of a New Measure:
Actionability. Will the measure provide useful or actionable results for state Medicaid and CHIP
programs?
Missouri uses NCI data for a variety of reasons. Based on the data, the Division of
Developmental Disabilities will address areas of enhancements to services and supports
through policies and practices, with the goal of providing continuous improvement for people
with developmental disabilities. See below to understand how NCI data drives decisions around
the states four I/DD Medicaid waivers.
Empowering Through Employment
In October 2016, the Division of Developmental Disabilities (DDD) launched the Empowering
through Employment Initiative to assist the growing number of individuals who express an
interest in community-based employment. According to National Core Indicators, 51% of
individuals receiving home and community-based services express an interest to work in the
community; however, fewer than 25% had a goal in their annual plan to support this interest and
only 3% of individuals on a monthly basis have services authorized to assist with their
C.67
employment pathway. NCI data helped launch this initiative, Missouri understood that people
wanted to work. Since Missouri started this effort, there has been a steady increase in the
number of individuals accessing employment related services. Today, over 1,000 individuals in
Missouri have employment services authorized. A substantial increase over when the state
started this effort when only 369 individuals were accessing employment.
Missouri Quality Outcomes
NCI data is used to measure the Missouri Quality Outcomes. The Missouri Quality Outcomes
are intended to be a guide to assist the user with facilitating discussion around key areas of
importance to the individual and supporting their personal goals, dreams and other areas of
interest to the individual that defines quality of life. Improving quality requires continuous efforts
on getting to know the person in the settings and situations where they are supported, as well
as, consistent interaction and involvement with the individual and their support systems for on-
going assessment of their quality of life. Here is a link to the full report:
https://dmh.mo.gov/dd/docs/ncimissouriqualityoutcomes-fullreport.pdf, and an additional link to
an At-A-Glance report summarizing safety and security:
https://dmh.mo.gov/dd/docs/qualityoutcomesafetyandsecurity.pdf.
Alignment. Is the measure used in other reporting programs?
Missouri Quality Outcomes
NCI data is used to measure the Missouri Quality Outcomes. The Missouri Quality Outcomes
are intended to be a guide to assist the user with facilitating discussion around key areas of
importance to the individual and supporting their personal goals, dreams and other areas of
interest to the individual that defines quality of life. Improving quality requires continuous efforts
on getting to know the person in the settings and situations where they are supported, as well
as, consistent interaction and involvement with the individual and their support systems for on-
going assessment of their quality of life. Here is a link to the full report:
https://dmh.mo.gov/dd/docs/ncimissouriqualityoutcomes-fullreport.pdf, and an additional link to
an At-A-Glance report summarizing safety and security:
https://dmh.mo.gov/dd/docs/qualityoutcomesafetyandsecurity.pdf.
NCI – State Budget Request
Missouri also uses NCI data to support state budget measures. Missouri received an in-state
performance management award for inclusion of NCI measures in our budget submissions. The
division is the only state division to use consumer satisfaction measures in multiple categories.
NCI measures are used to demonstrate consumer satisfaction and support quality measures in
the annual budget submission. In the link following, pages 934-936 are examples of how NCI
measures are incorporated into the division’s budget request.
https://oa.mo.gov/sites/default/files/FY_2020_Mental_Health_Budget_Gov_Rec_Book_3.pdf.
Appropriateness for state-level reporting. Has the measure been validated and tested for state-
level reporting? Is it currently used by one or more states?
C.68
NCI has been utilized by 46 states and the District of Columbia for several years. The
comparisons between states of similar size, or states within our same geographic region, is
extremely helpful to set the context of our system. In addition, the NCI indicators demonstrate
how our full system is functioning, which is critically important in my role as the State Director.
We have mechanisms in place to measure individual providers and individual lives, but NCI is
the only systemic measurement system that provides me with already cleaned, analyzed and
publicly reported data. This transparency of data forms a significant step towards effective trust-
building and collaboration with our stakeholders. NCI provides a core effort in our stakeholder
engagement process. The NCI Staff Stability Survey utilized in conjunction with the NCI Adult
Consumer survey are helping Missouri make our case for identifying ways to address the direct
support professional turnover issue. The Adult Consumer Survey tells us if individuals in
services believe staff have the right training to meet their needs, while the Staff Stability Survey
tells us our state turnover rate. Stakeholders (state government, local government, providers)
then utilize this data to help determine the rate of pay for direct support professionals.
Feasibility. Will states be able to access the data needed to calculate the measure? Would
technical assistance be necessary or helpful to facilitate complete and accurate reporting of the
measure by states?
The NCI Team produces reports that inform state efforts to strengthen policy, inform quality
improvement activities, evaluate programs and policies, and compare their performance with
national norms. Occasionally, NCI will release case examples that examine a hypothetical
process and course of action taken by a state to address specific topics. One of these case
examples addressed abuse and neglect and gave a scenario of how a Quality Advisory Council
used NCI data to propose quality improvement initiatives in their state. Missouri used this to
build our own report around safety.
Strategic priority. Does the measure fill a gap that has been identified in the Child or Adult Core
Sets?
NCI measures are needed by state Medicaid agencies to understand the experience of people
with developmental disabilities who access and utilize home and community based services.
This measure set helps Missouri get data out about what people in services really want,
sometimes that gets lost with guardian involvement and just generally acceptance of past
practices. There is no other measure set which provides this insight, including the data cleaning,
analysis, and summary report which publicly compares all participating state results. The public
reporting of NCI results provides a level of accountability needed by my state agency in order to
understand where we have improvement opportunities or need to re-evaluate the effectiveness
of our policies or practices.
We agree with the summary of the workgroup discussion, indicating the usefulness of the NCI
measures to our states, the flexibility and adaptability to be used by people with multiple types
of disabilities. From its inception in 1998, NCI has remained committed to channeling direct
input from people with I/DD to the state agency responsible for the administration of the service
delivery system. NCI assures the voice of the people is heard directly regarding the supports
C.69
and services so critical to their day to day well-being. This crucial aspect of NCI – the
importance to measure – weighs equally in state’s decision to continue using NCI.
C.70
National Association of Community Health Centers (Ron Yee)
We are pleased to provide comments in response to Recommendations for Improving the 2020
Core Sets of Health Care Quality Measures for Medicaid and CHIP.
NACHC strongly recommends continuation of the Weight Assessment and Counseling for
Nutrition and Physical Activity for Children/Adolescents Body Mass Index measure to improve
health outcomes.
1. Our nation is in the midst of an obesity epidemic, and BMI screening is a component of the
USPSTF recommendations for both children and adults.
2. Screening is essential for early diagnosis and intervention, leading to improved health
outcomes.
3. Food insecurity and poverty adversely affect health center patients leading to obesity.
4. In 2018, health centers screened, counseled on nutrition and physical activity, and referred
over 3,549,030 3-17 year old patients with a BMI greater than or equal to the 85th
percentile.
5. In addition, health centers referred over 10,860,741 patients 18 years of age and older due
to their BMI results.
6. These core sets allow for tracking of pediatric obesity over time for Medicaid and other
funders, helping to identify gaps in care and access points.
7. Documenting the BMI using the Medicaid Child Core measure incentivizes clinicians to
reduce pediatric obesity and improve health outcomes.
Health centers appreciate Mathematica and the Multi-Stakeholder reviewers. Thank you for the
opportunity to comment on this proposed rule. NACHC and its member organizations are willing
to provide clarification or answer any follow up information on our comments, please contact
C.71
National Association of State Directors of Developmental Disability
Services (Mary Lou Bourne)
Thank you for the opportunity to comment on the report titled “Recommendations for Improving
the Core Sets of Health Care Quality Measures for Medicaid and CHIP.” The report conveys
the commitment made by the workgroup to understand a broad spectrum of measures important
to State Medicaid agencies. The report also clearly captures the thorough and well organized
approach used to consider measures nominated for removal and for addition.
As the Steward for National Core Indicators (NCI), we would like to correct the description of
NCI contained on page 16 of the report. Specifically, we would recommend rewriting the first
bulleted paragraph under NCI as noted below to ensure factual accuracy:
NCI survey measures are standardized indicators used across by 46 states and the District of
Columbia to assess the statewide system performance within a state’s LTSS for individuals age
18 and older with intellectual and developmental disabilities who receive at minimum one
service in addition to case management, and their families. The survey’s instruments include in-
person surveys, family surveys, and a staff suitability stability survey. Indicators address key
areas of concern in five domains: (1) individual outcomes; (2) health, welfare, and rights; (3)
system performance; (4) staff stability; and (5) family outcomes. NCI has been used since its
launch in 1998, is completely voluntary and was designed by and for State Developmental
Disability systems. Forty-six states and the District of Columbia participate in the NCI program.
More than 20,000 surveys were entered into the NCI data base in the past data cycle.
On Page 17, the statement “Both sets of indicators have been tested and are believed to be
valid and reliable, with strong inter-rater reliability” seems to suggest that validity and reliability
has not been demonstrated. We would suggest this be changed to say “NCI indicators have had
validity and reliability demonstrated in various ways throughout its 22 year history. Further
details on testing including inter-rater reliability and other fidelity analyses, are available through
the measure stewards.”
Beyond the factual clarification for direct reference to NCI in the report, please allow us to
demonstrate how NCI meets the characteristics used to determine if a measure’s conditions
would merit addition to the Adult Core Measure Set. While we believe NCI could meet both the
criteria for patient experience and LTSS, we do agree that it fits well as a demonstration of
LTSS Medicaid services. Specifically, NCI currently demonstrates these characteristics in the
following examples.
Actionability. Will the measure provide useful or actionable results for state Medicaid and CHIP
programs?
States use the NCI data for Waiver Assurance and sub-assurance data, in their QIS models,
and to identify areas for improvement on an annual basis. States such as Massachusetts,
Missouri, Washington and Arizona have used NCI data to prioritize system efforts for many
years.
C.72
Alignment. Is the measure used in other reporting programs?
State Medicaid I/DD operating agencies align and utilize NCI data in many additional reporting
programs. Some states use NCI data within the structure of their 1915(c) Home and Community
Based Services (HCBS) Waiver Assurances and Sub Assurance performance measures. Other
states utilize NCI as a keystone of their quality improvement system as described in Appendix H
of their 1915(c) waiver applications to CMS; states also use NCI as a key stakeholder reporting
mechanism, and to inform their improvement or change strategies during waiver re-design
activities. States use the NCI annually to inform legislative and budget discussions, thus
providing context and outcome based understanding of their large and complex systems.
Additionally, states routinely look to NCI data to determine the impact of policy decisions on the
lives of people directly affected. Finally, the unparalleled length of time that states have been
using NCI provides an unequivocal longitudinal data set, enabling states to view the long-range
impacts of systems change(s) and policy determinations over time.
Appropriateness for state-level reporting. Has the measure been validated and tested for state-
level reporting? Is it currently used by one or more states?
NCI has been utilized by 46 states and the District of Columbia for several years. The
comparisons between states of similar size, or states within the same geographic region, is
extremely helpful to set the context of state systems. In addition, the NCI indicators demonstrate
how the full system is functioning, thus providing state I/DD agencies with mechanisms to
measure systemic performance and identify statewide system improvements. In addition, NCI
reporting provides states with cleaned, analyzed and publicly reported data. The transparency is
highly valued by many stakeholders.
Feasibility. Will states be able to access the data needed to calculate the measure? Would
technical assistance be necessary or helpful to facilitate complete and accurate reporting of the
measure by states?
With 46 States plus the District of Columbia already collecting the data, the feasibility is
demonstrated each year, with sustainability demonstrated by the 18 states that have used NCI
for more than 10 years, and an additional 17 having used NCI for more than 5 years. It is fair to
note that in any given year a state may choose to collect data through face to face data
collection, through mail-out data collection, or through a direct upload link. While the majority of
states collect face-to-face data annually, some states choose to alternate between a data
collection year and a data utilization year.
Strategic priority. Does the measure fill a gap that has been identified in the Child or Adult Core
Sets?
Medicaid is the largest payer for LTSS in this country. Total Medicaid LTSS expenditures were
approximately $167 billion in FY 2016 and comprised more than 30% of total Medicaid
expenditures. Heretofore, there has been a gap in measures on this critically important and
sizable portion of the Medicaid program. NCI provides the Long Term Services and Supports
C.73
measures needed by state Medicaid agencies to understand the experience of people with
developmental disabilities who access and utilize home and community based services. There
is no other measure set which provides this insight, including the data cleaning, analysis, and
summary report which publicly compares all participating state results. The public reporting of
NCI results provides a level of accountability welcomed by state agencies in order to identify
improvement opportunities or to re-evaluate the effectiveness of policies or practices.
Thank you again for the opportunity to provide public comment on this report, and many thanks
to the workgroup for their many hours of dedication to the task. We are happy to answer any
questions and as the measure stewards, we look forward to providing Person Reported
Outcome Measures to the LTSS area of the Medicaid Adult Core Measure Set.
C.74
New Balance Foundation for Obesity Prevention Center, Boston Children’s
Hospital (Cara Ebbeling)
Tracking body mass index (BMI) percentile over time provides a reasonable method for
screening, monitoring changes in weight status, and identifying children who are at risk for
obesity-related morbidities. However, a key question is: Why measure an “outcome” (i.e., BMI
percentile) when the “treatment” in primary care settings (i.e., brief counseling for nutrition and
physical activity during office visits) is known to be largely ineffective?
When considering effectiveness, the importance of intervention intensity to promote healthful
lifestyle behaviors has been highlighted in several literature reviews. Based on a meta-analysis
of brief primary care interventions for pediatric weight management, Sim et al.
1
argued that such
interventions have only a marginal effect on BMI and emphasized the need for intensive
behavioral interventions. In a narrative review of interventions conducted in primary care clinics,
Lenders et al.
2
surmised that increasing intervention intensity could lead to improved weight
outcomes. After conducting an evidence review, the US Preventive Services Task Force
3,4
concluded that comprehensive, intensive interventions result in improved weight status among
children with obesity. Using contact hours as an indicator of intensity, the Task Force noted that
at least 26 contact hours (e.g., bi-weekly 1-hour visits for 12 months) are necessary to promote
weight loss. Behavioral interventions with 52 or more contact hours promote even greater
weight loss and some improvements in risk factors for metabolic diseases. In summary, simply
measuring BMI percentile and providing brief counseling for nutrition and physical activity is not
an evidence-based approach for treating children with obesity.
However, there is reason for optimism as models of integrated care hold promise for enhancing
intervention intensity.
5-8
These models rely on active and measurable care coordination
involving clinicians, nonclinical professionals (e.g., staff at community organizations which
provide afterschool programming), and family members with explicit roles for care planning and
coordination.
8,9
Appropriate measures to assess care coordination and intervention intensity are
essential to models of integrated care. Until systems for implementing such models are in place
nationally, to achieve effective intervention intensity for treating children with obesity, BMI
percentile as an outcome measure should be removed from the core set.
Thank you for the opportunity to provide comment.
Citations
1
Sim LA, Lebow J, Wang Z, Koball A, Murad MH. Brief primary care obesity interventions: a
meta-analysis. Pediatrics. 2016;138:e20160149. PMID: 27621413.
2
Lenders CM, Manders AJ, Perdomo JE, Ireland KA, Barlow SE. Addressing pediatric obesity
in ambulatory care: where are we and where are we going? Curr Obes Rep. 2016;5:214-
240. PMID: 27048522.
C.75
3
O'Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and
intervention for weight management in children and adolescents: evidence report and
systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427-2444.
PMID: 28632873.
4
Grossman DC, Bibbins-Domingo K, Curry SJ, et al. Screening for obesity in children and
adolescents: US Preventive Services Task Force recommendation statement. JAMA.
2017;317:2417-2426. PMID: 28632874.
5
Dietz WH, Solomon LS, Pronk N, et al. An integrated framework for the prevention and
treatment of obesity and its related chronic diseases. Health Aff (Millwood). 2015;34:1456-
1463. PMID: 26355046.
6
Fleischman A, Hourigan SE, Lyon HN, et al. Creating an integrated care model for childhood
obesity: a randomized pilot study utilizing telehealth in a community primary care setting.
Clin Obes. 2016;6:380-388. PMID: 27863024.
7
Wilfley DE, Staiano AE, Altman M, et al. Improving access and systems of care for evidence-
based childhood obesity treatment: Conference key findings and next steps. Obesity (Silver
Spring). 2017;25:16-29. PMID: 27925451.
8
Ebbeling CB, Antonelli RC. Primary care interventions for pediatric obesity: need for an
integrated approach. Pediatrics. 2015;135:757-758. PMID: 25825541.
9
Singer SJ, Burgers J, Friedberg M, Rosenthal MB, Leape L, Schneider E. Defining and
measuring integrated patient care: promoting the next frontier in health care delivery. Med
Care Res Rev. 2011;68:112-127. PMID: 20555018.
C.76
Novo Nordisk (Todd M Hobbs)
Novo Nordisk is pleased to provide the following comments on the draft report of the 2020 Child
and Adult Core Set Review Workgroup: Recommendations for Improving the Core Set of Health
Care Quality Measures for Medicaid and CHIP. Novo Nordisk is a global healthcare company
with 95 years of innovation and leadership in diabetes care. This heritage has given us
experience and capabilities that also enable us to help people defeat other serious chronic
conditions: rare bleeding disorders, growth hormone-related disorders, and obesity. As an
organization, we are also committed to ensuring patients have access to high-quality, affordable
health care. We support the Centers for Medicare and Medicaid Services’ continued efforts to
transform the health care delivery system through competition and innovation to provide
patients with better value and outcomes.
We appreciate the need to streamline measure systems and reduce reporting burdens on health
care providers. However, we urge CMS to retain the Core measures related to care for both
children and adults living with diabetes and obesity. Specifically, we recommend that CMS
retain the following measures:
• Weight assessment and counseling for nutrition and physical activity for children/
adolescents - Body mass index assessment for children/adolescents
• Adult Body Mass Index Assessment
• Comprehensive Diabetes Care: Hemoglobin A1C (HbA1c) Testing
Given the burden of obesity and diabetes on our health care system, patients, and on our
society, we believe it is vital that CMS continue to focus on measuring and improvement care for
patients with these conditions.
Discussion
As our healthcare system continues to move towards value-based care arrangements that
encourage providers to improve patient outcomes and manage overall costs, it is critical to have
the right quality measures in place. Novo Nordisk supports CMS’s Patients over Paperwork and
Meaningful Measure initiatives that reduce clinician burden of data collection and support
measures that improve health outcomes and quality of care for patients. However, there are
several measures that are suggested for removal from the Medicaid and CHIP Child and Adult
Core Sets that Novo Nordisk believes are crucial to promote good quality of care for Medicaid
beneficiaries.
Obesity and type 2 diabetes (T2D) are two of the most prevalent and costly chronic conditions in
the United States. The prevalence of obesity among adults is 39.6% and 18.5% among youth
(aged 2-19), with rates continuing to increase year after year
1
, costing the U.S. health care
system at least $147 billion each year.
2
Diabetes affects 30.3 million people in the U.S., with
direct and indirect costs around $245 billion per year.
3
Diabetes and obesity are also cost
drivers for Medicaid.
4
In 2015, diabetes was among the leading causes of death in the United
States, with over 79,000 deaths directly attributed to diabetes as the underlying cause.
5
In
C.77
addition, T2D increases the risk of cardiovascular disease, cancer, and other closely related co-
morbidities.
6
Im-proving overall quality and outcomes is essential for patients with these two
conditions.
Novo Nordisk strongly urges CMS to reconsider the removal of the measure Weight
assessment and counseling for nutrition and physical activity for children/adolescents—Body
mass index assessment for children/adolescents (WCC-CH) from the Child Core Set. We
strongly urge CMS to reconsider removal of the measure Adult Body Mass Index Assessment
(ABA-AD) from the Adult Core Set. It is imperative that the U.S. continue to make improvements
in quality of care, treatment, and outcomes for those with obesity. It is a costly and sometimes
debilitating disease that affects a large percentage of the U.S. population, and its impact and
prevalence continue to rise.
Screening is the first step for preventing the disease itself, as well as the many associated
complications. Currently, many guidelines and recommendations recognize this and include
screening for overweight and obesity:
• Recommendations from the United States Preventative Services Task Force (USPSTF) for
both children
7
and adults
8
• Joint statement from American College of Cardiology (ACC), American Heart Association
(AHA) and The Obesity Society (TOS)
9
• Practice guidelines from the Department of Veterans Affairs (VA) and the Department of
Defense (DoD)
10,
and;
• Clinical guidelines from the American Association of Clinical Endocrinologists (AACE) and
American College of Endocrinology (ACE)
11
.
Removal of the BMI index assessment measures would mean that the Medicaid and CHIP Child
and Adult Core Sets of Health Care Quality Measures would be misaligned with current
guidelines and recommendations and would signal that screening is considered unimportant or
is topped out without room for improvement in the Medicaid and CHIP programs—when in fact
neither is the case. State mean performance in 2017 was only 52.3% in the Child Core Set
12
and 62.9% in the Adult Core Set
13
, suggesting these measures need to be maintained in the
Core Sets and additional initiatives should be taken to increase provider performance for
screening for obesity.
Novo Nordisk also strongly disagrees with the removal of the measure Comprehensive Diabetes
Care: Hemoglobin A1C (HbA1c) Testing (HA1C-AD) from the Adult Core Set. The majority of
Workgroup members believe that this measure has either topped out with “little room for
improvement” or that it is duplicative of a measure currently on the Core Set—Comprehensive
Diabetes Care: Hemoglobin A1c (HbA1c) Poor Control (> 9.0 percent) (HPC-AD), which is an
outcome measure that also assesses whether testing is being conducted. Novo Nordisk
disagrees with this recommendation and assessment for the following reasons:
• Nearly 25% of the 30.3 million people in the US with diabetes are undiag-nosed.
14
C.78
• Nearly 34% of US adults have prediabetes and less than 12% are aware of it.
12
• The current USPSTF guideline for screening for abnormal blood glucose recommends
screening for abnormal blood glucose as part of a cardiovascular risk assessment in adults
aged 40 to 70 years who are overweight or obese. The guideline, which is a comprehensive
screening guideline, further states that clinicians should consider screening individuals with
one or more of the following characteristics: History of gestational diabetes or polycystic
ovarian syndrome, family history of diabetes, or are members of a racial/ethnic group
disproportionately impacted by the disease. Furthermore, the guideline suggests that
clinicians offer or refer patients with abnormal blood glucose to intensive behavioral
counseling interventions to promote a healthful diet and physical activity.
15
Novo Nordisk believes that the Hemoglobin A1c (HbA1c) Poor Control (> 9.0 percent) (HPC-
AD) is an important measure but that it is primarily focused on adults who have already been
diagnosed with diabetes. It is a performance measure that holds health plans accountable for
both testing and improved outcomes in this population. The Hemoglobin A1C (HbA1c) Testing
(HA1C-AD) measure is an important and distinct measure intended to identify those who have
prediabetes who can then be appropriately referred to an evidence-based diabetes prevention
program to prevent their progression to full blown type 2 diabetes; and to identify those with
diabetes who have not yet been diagnosed. Removal of this measure from the Adult Core Set
will send a message that early diagnosis and prevention strategies for type 2 diabetes and its
associated complications in this population are not a priority.
Potential New Measures
It is clear there is a need to measure processes and outcomes so that we can drive towards
improvements in care for patients with obesity and diabetes – two of the costliest and prevalent
chronic conditions in the U.S. Given that future quality measure work will largely focus on
outcome measures such as BMI reduction or maintenance, a process measure such as BMI
screening, which is currently in the Medicaid/CHIP Core Quality Set, is the first step before an
outcome measure can be developed. Novo Nordisk would like to share information on pipeline
obesity measure development efforts underway through a partnership between the AMGA,
Discern Health, and the National Quality Forum (NQF)
16
:
In 2016, National Quality Forum (NQF), in collaboration with the STOP Obesity Alliance,
convened a roundtable discussion on system-level accountability in treating individuals with
obesity. Following this meeting, an NQF Measure Incubator® strategy session was held in early
2017 to further refine the measure concepts proposed in the initial discussion. Participants
included experts in obesity care, population health, and measure development and
implementation, along with patients and patient advocates. Key recommendations included a
greater focus on the clinical treatment of obesity, coupled with population- and community-
based approaches to address the obesity epidemic. The Expert Panel prioritized two measure
concepts for further development: 1) an outcome measure focused on serial body mass index
(BMI) reduction or maintenance; and, 2) a shared decision-making (SDM) measure that focuses
on patient-centered communication and clinician action to guide obesity care.

C.79
Initial development and testing of these measures began in 2018 as part of a broader subset of
obesity measures used in the AMGA Obesity Care Model Collaborative, a 3-year collaborative
to define, pilot, and evaluate a framework and necessary components to address obesity in
multispecialty medical groups and integrated health systems. Discern Health is the lead
developer for four obesity quality measures for the adult population:
1. Documentation of obesity diagnosis;
2. Weight change over time;*
3. Evidence-based treatment for obesity; and,
4. Obesity quality of life patient-reported outcome performance measure (PRO-PM).
*,#
* Modified version of prioritized measure concept from the NQF-convened 2017 strategy
session.
#
Initial measure testing focused on early feasibility assessment only.
Through the AMGA, Discern and NQF obesity measurement project,
17
the team is specifying
and testing the following measures:
Measure Title Description Numerator Denominator
Documentation of
obesity diagnosis
Percent of patients with
documentation of
obesity diagnosis (ICD-
10 or notation in EHR
problem list).
Number of patients with
a documented BMI, and
if >30, a corresponding
obesity diagnosis.
Patients aged 18–79, as
of the first day of the
reporting period, with 1
or more ambulatory
visits/ encounters during
the reporting period.
Weight change over time Percent of patients with
an initial BMI ≥ 25 who
have achieved at least a
5 percent reduction in
weight during the
measurement year.
Number of patients who
achieved 5 percent or
more weight reduction
over a 12-month period.
Note: The pilot test will
explore percent weight
loss stratification, as well
as a maintenance
indicator.
Patients aged 18–79, as
of the first day of the
reporting period,
indicating a BMI ≥ 25
with: 1 or more
ambulatory
visits/encounters in the
reporting period AND
weight associated with
any visit at least 9
months but no longer
than 12 months earlier.
Pilot testing through large health systems/provider practices is wrapping up and we anticipate
having finalized measure specifications and more information on the feasibility and scientific
acceptability (reliability and validity) of these measures in late 2019. We would welcome the
opportunity to discuss CMS’ consideration of use of any or all for the Medicaid Adult or Child
Core Set.
C.80
Summary
Diabetes and obesity are among the most important health management challenges facing the
U.S. It is vital that CMS continue to send a strong signal to providers that they should screen for
and manage these conditions so that patients get the best care and achieve the best outcomes.
Removing the BMI and A1c measures will send the wrong signal that obesity and diabetes are
not a top priority for CMS.
Thank you for this opportunity to comment on the draft report of the 2020 Child and Adult Core
Set Review Workgroup. Novo Nordisk will continue to work towards improving care. If you have
any questions about our comments, please do not hesitate to reach out to me at
Citations
1
Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth:
United States, 2015–2016. NCHS data brief, no 288. Hyattsville, MD: National Center for
Health Statistics. 2017. https://www.cdc.gov/nchs/data/databriefs/db288.pdf.
2
Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to
obesity: payer- and service-specific estimates. Health Aff 2009;28(5):w822-31.
https://www.ncbi.nlm.nih.gov/pub-med/19635784.
3
American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes
Care. 2013;36(4):1033–1046. https://www.ncbi.nlm.nih.gov/pubmed/23468086.
4
Brookings Institute, Obesity Costs Evident at the State Level. December 12, 2014. Available at
https://www.brookings.edu/blog/up-front/2014/12/12/obesity-costs-evident-at-the-state-
level/. Accessed August 5, 2019. Garfield RL and Damico A. Medicaid Expansion under
Health Reform may Increase Service Use and Improve Access for Low Income Adults with
Diabetes. Health Affairs 31. No 1(2012): 159-167. Ng BP, Shrestha SS, Lanza A, Smith B,
Zhang P. Medical Expenditures Associated With Diabetes Among Adult Medicaid Enrollees
in Eight States. Prev Chronic Dis 2018;15:180148. DOI:
https://doi.org/10.5888/pcd15.180148.
5
Centers for Disease Control and Prevention. About Underlying Cause of Death 1999–2015.
CDC WONDER Database. http://wonder.cdc.gov/ucd-icd10.html.
6
Weng W, Tian Y, Kong S, Ganguly R, Hersloev M, Brett J, Hobbs T. The prevalence of
cardiovascular dis-ease and antidiabetes treatment characteristics among a large type 2
diabetes population in the US. Endo-crinology, Diabetes & Metabolism. Vol 2, issue 3, July
2019. Zalenko Z and Gallagher EJ. Diabetes and Cancer. Endocrinology and Metabolism
Clinics. March 2014. Vol 43, Issue 1. Pp 167-185.
7
USPSTF (2017). Screening for Obesity in Children and Adolescents US Preventive Services
Task Force Recommendation Statement. JAMA, 317(23):2417-2426.
https://jamanetwork.com/journals/jama/fullarti-cle/2632511.
C.81
8
USPSTF (2012). Screening for and management of obesity in adults; U.S. Preventative
Services Task Force Recommendations Statement. Ann Intern Med, 157:373-378.
https://annals.org/aim/fullarti-cle/1355696/screening-management-obesity-adults-u-s-
preventive-services-task-force.
9
AHA/ACC/TOS (2013). 2013 AHA/ACC/TOS Guideline for the Management of Overweight and
Obesity in Adults: A Report of the American College of Cardiology/American Heart
Association Task Force on Practice Guidelines and The Obesity Society. Circulation,
129[suppl 2]:S102-138. https://www.ahajour-
nals.org/doi/pdf/10.1161/01.cir.0000437739.71477.ee.
10
VA/DoD (2014). VA/DoD Clinical practice guidelines for screening and management of
overweight and obesity.
https://www.healthquality.va.gov/guidelines/CD/obesity/CPGManagementOfOverweightAnd
Obesi-tyFINAL041315.pdf.
11
AACE/ACE (2016). AACE/ACE Clinical Practice Guidelines for Comprehensive Medical Care
of Patients with Obesity. https://journals.aace.com/doi/pdf/10.4158/EP161365.GL.
12
Performance on the Child Core Set Measures, FFY 2017. Retrieved from: https://www.medi-
caid.gov/medicaid/quality-of-care/performance-measurement/child-core-set/index.html.
13
Performance on the Adult Core Set Measure, FFY 2017. Retrieved from:
https://www.medicaid.gov/medi-caid/quality-of-care/performance-measurement/adult-core-
set/index.html.
14
CDC, National Diabetes Statistics Report 2017. Available at:
https://www.cdc.gov/diabetes/index.html. Accessed August 4, 2019.
15
USPSTF, Abnormal Blood Glucose and Type 2 Diabetes Mellitus: Screening. Available at:
https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/scre
ening-for-ab-normal-blood-glucose-and-type-2-diabetes. Accessed August 4, 2019.
16
This work is supported by Novo Nordisk Inc.
17
Funding for this work was provided by Novo Nordisk Inc.
C.82
Obesity Care Advocacy Network (Jeanne Blankenship, Meredith Dyer, and
Joe Nadglowski)
The Co-Chairs of the Obesity Care Advocacy Network (OCAN) are pleased to echo the
comments of the Sumner M. Redstone Global Center for Prevention and Wellness regarding the
July 2019 draft report, “Recommendations for Improving the Core Sets of Health Care Quality
Measures for Medicaid and CHIP: Summary of a Multi-Stakeholder Review of the 2020 Child
and Adult Core Sets.”
OCAN’s mission is to unite and align key obesity stakeholders and the larger obesity community
around key obesity-related education, policy and legislative efforts in order to elevate obesity on
the national agenda. The primary goals of OCAN are to: prevent disease progression; improve
access to evidence-based treatments for obesity; improve standards of quality care in obesity
management; eliminate weight bias; and foster innovation in future obesity treatments.
For these reasons, OCAN is extremely concerned that CMS is proposing to remove “Weight
Assessment and Counseling for Nutrition and Physical Activity for Children/Adolescents-Body
Mass Index Assessment for Children/Adolescents (WCC-CH) and Adult Body Mass Index
Assessment (ABA-AD) from the 2020 Child and Adult Core Sets.
Weight Assessment and Counseling for Nutrition and Physical Activity for Children/Adolescents-
Body Mass Index Assessment for Children/Adolescents (WCC-CH)
Eighteen percent of U.S. children and adolescents have obesity. Screening for BMI is a critical
initial step in the process of identifying and referring a child with obesity to the comprehensive
and intensive behavioral interventions necessary to improve weight and reduce comorbidities
associated with obesity. The United States Preventive Services Task Force (USPSTF)
recommends screening using BMI for children and adolescents and provides the
recommendation with a “B” grade, reflecting a “high certainty that the net benefit is moderate or
there is a moderate certainty that the net benefit is moderate to substantial.” Similarly, the
American Academy of Pediatrics (AAP) recommends screening for obesity using BMI so that
children and adolescents can be effectively treated for obesity. Screening is the initial step in the
process of treatment.
The summary for the work group recommendation to remove WCC-CH states that the measure,
“does not provide useful or actionable results for state Medicaid and CHIP agencies.” Further,
members, “asserted that this measure does not reflect evidence-based practices for
interventions for children with or at risk of obesity.” The conclusion that BMI screening does not
reflect evidence-based practices is contradicted by both the USPSTF and AAP
recommendations which find that screening is a critical part of evidence-based practice for
treating obesity in children and adolescents. In addition, we contend that this measure does
provide actionable information for Medicaid and CHIP agencies by providing important data
related to the extent to which healthy weight is being discussed with patients. While we agree
with the work group member who suggested that developing a measure that also examines
interventions, such as referrals to care, would be more useful, simply removing the WCC-CH
C.83
measure with no replacement is a step backward in improving obesity care and treatment for
children and adolescents.
The work group summary also notes that some members asserted that because BMI is
recorded under the Promoting Interoperability Program (formerly the Electronic Health Records
Incentive Program), most physicians would not be disincentivized from conducting the BMI
screening. Unfortunately, we do not have evidence that this is the case. Removing the measure
from the core set with no replacement sends the message that obesity identification and
treatment is unimportant.
We urge that the final recommendations continue to include the WCC-CH measure to maintain
consistency with both the USPSTF and AAP recommendations, align with evidence-based
treatment standards for obesity, and ensure that addressing obesity remains a priority.
Adult Body Mass Index Assessment (ABA-AD)
OCAN also opposes the removal of the ABA-AD measurement for the same reasons stated
above. The USPSTF also includes BMI screening for adults in their recommendations as part of
an evidence-based treatment plan for adults with obesity. In in the case of the WCC-CH
recommendation, removal of the ABA-AD measure with no replacement both contradicts the
USPSTF recommendation and risks lowering the priority of obesity treatment among the health
care community.
The workgroup summary for this section indicated that one member noted that obesity may
require a broader societal response than other health conditions, which makes it more
challenging for the health care system to address. It is certainly true that obesity is a complex
disease and that prevention efforts should focus on societal issues, such as access to nutritious
food and opportunities to be physically active. However, there are evidence-based interventions
and treatments for obesity (community-based programs such as the Diabetes Prevention
Program, intensive behavioral therapy, pharmacotherapy and surgery) just as there are for other
complex chronic diseases that may be related to broader societal drivers and singling out
obesity as requiring a uniquely non-clinical response is not supported by research.
Thank you again for your consideration of these comments, should you have any questions,
please feel free to contact OCAN Washington Coordinator Chris Gallagher at 571-235-6475 or
via email at [email protected].
C.84
Office of Infectious Disease and HIV/AIDS Policy (Alice Tsai)
Thank you for your notification and a minor erratum on page 4 (December 14, 2018?) for
consideration.
Also, as my organization has undergone a change in June from the National Vaccine Program
Office to the Office of Infectious Disease and HIV/AIDS Policy, can you please reflect that on
the report? An example is provided below:
Office of Infectious Disease and HIV/AIDS Policy (formerly National Vaccine Program Office)
C.85
Redstone Center, Milken Institute School of Public Health (Jeff Hild)
The Sumner M. Redstone Global Center for Prevention and Wellness (Redstone Center) at the
Milken Institute George Washington University School of Public Health appreciate the
opportunity to submit comments to the draft “Recommendations for Improving the Core Sets of
Health Care Quality Measures for Medicaid and CHIP.” Specifically, we are opposed to the
recommended removal of the Weight Assessment and Counseling for Nutrition and Physical
Activity for Children/Adolescents-Body Mass Index Assessment for Children/Adolescents
(WCC-CH) in the Child Core Set and the Adult Body Mass Index Assessment (ABA-AD) in the
Adult Core Set.
The Redstone Center is one of the leading sources in the United States for promising and
evidence-based nutrition and physical activity strategies for the prevention and control of
obesity. The Redstone Center is also the home of the Strategies to Overcome and Prevent
Obesity Alliance (STOP), a collaboration of consumer, provider, government, labor, health
insurers and quality of care organizations working together to drive innovative and practical
strategies to combat obesity. The Redstone Center is chaired by Dr. William Dietz, a national
expert on obesity prevention and care, a consultant to the Roundtable on Obesity Solutions at
the National Academy of Medicine, and the former Director of the Division of Nutrition, Physical
Activity, and Obesity at the Centers for Disease Control.
Weight Assessment and Counseling for Nutrition and Physical Activity for Children/Adolescents-
Body Mass Index Assessment for Children/Adolescents (WCC-CH)
The WCC-CH measure assesses the percentage of children ages 3 to 17 who have visited a
primary care provider or OB/GYN practitioner and were screened for body mass index (BMI).
The report notes that 37 states reported this measure in Fiscal Year 2017.
We oppose the recommended removal of WCC-CH from the core set. Eighteen percent of U.S.
children and adolescents have obesity. Screening for BMI is a critical initial step in the process
of identifying and referring a child with obesity to the comprehensive and intensive behavioral
interventions necessary to improve weight and reduce comorbidities associated with obesity.
The United States Preventive Services Task Force (USPSTF) recommends screening using
BMI for children and adolescents and provides the recommendation with a “B” grade, reflecting
a “high certainty that the net benefit is moderate or there is a moderate certainty that the net
benefit is moderate to substantial.” Similarly, the American Academy of Pediatrics (AAP)
recommends screening for obesity using BMI so that children and adolescents can be
effectively treated for obesity. Screening is the initial step in the process of treatment.
The summary for the work group recommendation to remove WCC-CH states that the measure,
“does not provide useful or actionable results for state Medicaid and CHIP agencies.” Further,
members, “asserted that this measure does not reflect evidence-based practices for
interventions for children with or at risk of obesity.” The conclusion that BMI screening does not
reflect evidence-based practices is contradicted by both the USPSTF and AAP
recommendations which find that screening is a critical part of evidence-based practice for
treating obesity in children and adolescents. In addition, we contend that this measure does
C.86
provide actionable information for Medicaid and CHIP agencies by providing important data
related to the extent to which healthy weight is being discussed with patients. While we agree
with the work group member who suggested that developing a measure that also examines
interventions, such as referrals to care, would be more useful, simply removing the WCC-CH
measure with no replacement is a step backward in improving obesity care and treatment for
children and adolescents.
The work group summary also notes that some members asserted that because BMI is
recorded under the Promoting Interoperability Program (formerly the Electronic Health Records
Incentive Program), most physicians would not be dis-incentivized from conducting the BMI
screening. Unfortunately, we do not have evidence that this is the case. Removing the measure
from the core set with no replacement sends the message that obesity identification and
treatment is unimportant.
We urge that the final recommendations continue to include the WCC-CH measure to maintain
consistency with both the USPSTF and AAP recommendations, align with evidence-based
treatment standards for obesity, and ensure that addressing obesity remains a priority.
Adult Body Mass Index Assessment (ABA-AD)
We oppose the removal of the ABA-AD measurement for the same reasons stated above. The
USPSTF also includes BMI screening for adults in their recommendations as part of an
evidence-based treatment plan for adults with obesity. In in the case of the WCC-CH
recommendation, removal of the ABA-AD measure with no replacement both contradicts the
USPSTF recommendation and risks lowering the priority of obesity treatment amongst the
health care community.
The workgroup summary for this section indicated that one member noted that obesity may
require a broader societal response than other health conditions, which makes it more
challenging for the health care system to address. It is certainly true that obesity is a complex
disease and that prevention efforts should focus on societal issues, such as access to nutritious
food and opportunities to be physically active. However, there are evidence-based interventions
and treatments for obesity, including pharmacotherapy, intensive behavioral therapy, and
surgery, just as there are for other complex chronic diseases that may be related to broader
societal drivers and singling out obesity as requiring a uniquely non-clinical response is not
supported by research.
Again, we urge that the final recommendations continue to include ABA-AD as part of the core
set.
Thanks for the opportunity to comment. Please contact Dr. William Dietz
(mailto:[email protected]) or Jeff Hild (jeffhild@gwu.edu) with any questions about the
comment.
C.87
Trust For America’s Health (John Auerbach)
We are writing you to express our concern for the proposed removal of two quality measures
from the 2020 Core Sets of Health Care Quality Measures for Medicaid and CHIP. Trust for
America’s Health (TFAH) is a non-profit, non-partisan public health organization that promotes
optimal health for every person and community and makes the prevention of illness and injury a
national priority. We believe that addressing obesity in the United States is a key component of
improving public health more broadly. As we noted in our 2018 “The State of Obesity: Better
Policies for a Healthier America” report, obesity rates continue to be alarmingly high, for both
youth and adults.
1
Obesity costs the United States $149 billion in medical expenses annually –
with about half of those expenses paid by Medicare and Medicaid.
2,3,4
Because obesity is such a pervasive, and costly, national epidemic, all efforts to track and
assess weight in Medicaid and CHIP must be maintained and protected, and that removal of
these quality measures would harm public health efforts. Health care quality measures for
obesity are already limited in use,
5
and removal of these two measures could seriously hinder
wider implementation of healthcare-based efforts to prevent and treat obesity. We hope that the
stakeholder workgroup reassesses its preliminary recommendation to remove the weight-
related quality measures from the 2020 Core Sets of Health Care Quality Measures for
Medicaid and CHIP.
Quality Measure: Weight Assessment and Counseling for Nutrition and Physical Activity for
Children/Adolescents – Body Mass Index Assessment for Children/Adolescents (WCC-CH)
Children who are overweight or have obesity are more likely to have obesity as adults.
6
It is
important that efforts to track body mass index (BMI) are protected.
TFAH appreciates the workgroup’s rationale for removing the measures – namely, “that more
integrated and effective models to address obesity than screening along, such as referrals to
care, should be prioritized in the Core Set.” Measuring BMI is not enough to address obesity;
however, how obesity is addressed should be determined on a case-by-case basis. Some
cases of childhood obesity may necessitate referrals to community or clinical interventions,
while other cases may require ongoing consultation with parents and the patient. The National
Quality Forum’s own rationale for inclusion of this measure states “for children who are
overweight or obese, obesity in adulthood is likely to be more severe. Children’s weight status is
an important thing to monitor. Children need guidance on maintaining healthy eating and
exercising habits.”
7
Likewise, the U.S. Preventive Services Task Force (USPSTF), an
independent panel that makes evidence-based recommendations about clinical preventive
services, has recommended that clinicians screen for obesity in children and adolescents 6
years and older.
8
Lastly, the American Academy of Pediatrics (AAP) also recommends
screening for obesity using BMI so that children and adolescents can be effectively treated for
obesity. Screening is the initial step in the process of treatment.
We urge the workgroup to consider how to expand this measure to include screening and
referral, in lieu of eliminating it altogether.
C.88
Quality Measure: Adult Body Mass Index Assessment (ABA-AD)
As of 2016, the national adult obesity rate was 39.6 percent, after holding at around 34-35
percent between 2005 and 2012.
9,10
In 2000, no state had an obesity rate over 20 percent.
11
In
2016, Colorado, the state with the lowest obesity rate, had an obesity rate of 22.6 percent.
12
In
just 16 years, the ceiling has become the floor.
Now, more than ever, tracking adult BMI is of the utmost importance for demonstrating the
need, improving patient health knowledge, and improving care. Many Americans who are
overweight or obese do not realize they are overweight or obese. According to a 2016 Gallup
poll, while 70.4 percent of American adults are obese or overweight, only 36 percent of those
polled thought they had a weight problem.
13
Although not a perfect measure of health, BMI is
widely used as the measurement for CDC thresholds, is an inexpensive screening tool to serve
as population-level assessment of obesity prevalence, and is used within clinical practice
guidelines for recommending obesity treatment options.
14
In addition, measuring BMI enables
providers to track weight over time, counsel those at risk for developing obesity, and ultimately
prevent the progression of the condition and its comorbidities.
Similarly, to the workgroup’s rationale for removal of the WCC-CH Quality Measure, TFAH
acknowledges that the current measure is “a measure of documentation, rather than outcomes
or evidence-based practices for combatting obesity.” However, no meaningful alternative is
offered that would justify removal of the measure. Until a more effective measure is offered, we
urge the workgroup to maintain the current measure.
TFAH recognizes that chronic disease, like obesity, may require actions beyond the scope of a
health care provider. On top of quality care, obesity requires public health interventions that
target social and environmental factors. In order to align health care provider responsibility with
meaningful outcomes, a measure should take into account both measurement and referral.
However, there are evidence-based interventions and treatments for obesity (community-based
programs such as the Diabetes Prevention Program, intensive behavioral therapy, etc.) just as
there are for other complex chronic diseases that may be related to broader societal drivers and
singling out obesity as requiring a uniquely non-clinical response is not supported by research.
The working group does not include any recommended addition that would address this
concern.
It is important to note that the USPSTF has recommended that clinicians offer or and refer
adults with a BMI of 30 or higher to intensive, multicomponent behavioral interventions.
15
TFAH urges the workgroup to keep or expand this measure to include screening and referral, in
lieu of eliminating it altogether.
Thank you for your attention on this matter. We stand ready to work with stakeholders and
workgroup members to ensure that these quality measures remain included, as they are
important to the health and wellbeing of the public. If you have any questions, please contact
Dara Lieberman, TFAH’s Director of Government Relations at [email protected].
C.89
Citations
1
Warren, M., Beck, S., & Rayburn, J. (2018). The State of Obesity: Better Policies for a
Healthier America.
2
Obesity Prevention Source. “Economic Costs: Paying the Price for Those Extra Pounds.”
Harvard T.H. Chan School of Public Health. https://www.hsph.harvard.edu/obesity-
prevention-source/obesity-consequences/economic/ (accessed April 11, 2018).
3
Kim DD, Basu A. “Estimating the medical care costs of obesity in the United States:
Systematic review, meta-analysis, and empirical analysis.” Value Health, 19(5): 602–613,
2016. doi: 10.1016/j.jval.2016.02.008.
4
Finkelstein EA, Trogdon JG, Cohen JW, et al. “Annual medical spending attributable to
obesity: payer-and service-specific estimates.” Health Affairs, 28(5): w822-31, 2009. doi:
10.1377/hlthaff.28.5. w822.
5
Zvenyach T and Pickering MK. Health Care Quality: Measuring Obesity in Performance
Frameworks. Obesity (2017) 25, 1305-1312.
https://onlinelibrary.wiley.com/doi/pdf/10.1002/oby.21884.
6
Frieden T, Dietz W, Collins S. “Reducing Childhood Obesity Through Policy Change: Acting
Now To Prevent Obesity.” Health Affairs, 29(3), 2010.
https://doi.org/10.1377/hlthaff.2010.0039.
7
National Quality Forum. NQF #0024 Weight Assessment and Counseling for Nutrition and
Physical Activity for Children/Adolescents, 2012. http://www.qualityforum.org/Projects/n-
r/Population_Health_Measures/0024_BMI_Child_NCQA_051112.aspx (accessed August 5,
2019).
8
Final Update Summary: Obesity in Children and Adolescents: Screening. U.S. Preventive
Services Task Force. June 2017.
https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obes
ity-in-children-and-adolescents-screening1.
9
Hales CM, Carroll MD, Fryar CD, et al. “Prevalence of Obesity Among Adults and Youth:
United States, 2015–2016.” National Center for Health Statistics, Data Brief 288, October
2017. https://www.cdc.gov/nchs/data/databriefs/ db288.pdf (accessed April 17, 2018).
10
Hales CM, Fryar CD, Carroll MD, et al. “Trends in Obesity and Severe Obesity Prevalence in
US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016.” Journal of the American
Medical Association, 319(16): 1723-1725, 2018. JAMA.doi:10.1001/ jama.2018.3060.
https://jamanetwork.com/ journals/jama/fullarticle/2676543 (accessed April 1, 2018).
11
“F as in Fat: How Obesity Threatens America’s Future—2011.” Trust for America’s Health and
Robert Wood Johnson Foundation, 2011. http:// www.tfah.org/report/88/ (accessed July 18,
2017). (Based on data using the previous BRFS methodology in use from 2008–2010.)
12
Behavioral Risk Factor Surveillance System, 2017. Centers for Disease Control and
Prevention. https://www.cdc.gov/brfss/annual_data/ annual_2017.html (September 6,
2018).
C.90
13
Ingraham, C. “Nearly half of America’s overweight people don’t realize they’re overweight.”
Washington Post, December 1, 2016.
https://www.washingtonpost.com/news/wonk/wp/2016/12/01/nearly-half-of-americas-
overweight-people-dont-realize-theyre-overweight/?utm_term=.7f5346b48096 (accessed
August 5, 2019).
14
Zvenyach T and Pickering MK. Health Care Quality: Measuring Obesity in Performance
Frameworks. Obesity (2017) 25, 1305-1312.
https://onlinelibrary.wiley.com/doi/pdf/10.1002/oby.21884.
15
Final Update Summary: Weight Loss to Prevent Obesity-Related Morbidity and Mortality in
Adults: Behavioral Interventions. U.S. Preventive Services Task Force. September 2018.
https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obes
ity-in-adults-interventions1.
C.91
University of Texas School of Public Health (Belinda M Reininger)
Thank you for the opportunity to provide input into the Core Set Measures for Children and
Adults. I would like to focus my comments specifically on the recommendation to remove weight
assessment and counseling for nutrition and physical activity for children / adolescents – Body
Mass Index Assessment for Children / Adolescents WCC-CH and the measure of Adult Body
Mass Index Assessment (ABA-AD).
As the Regional Dean for the University of Texas School of Public Health in Brownsville and the
lead investigator for multiple programs addressing adult and childhood obesity over the last 18
years in partnership with clinics, hospitals and schools, I am completely shocked that these
Health Care Quality Measures would be proposed for removal. There are numerous reasons
why these measures are necessary to remain in the Core Set of Measures:
1. Low literacy populations need an objective view about their weight and its relationship to
chronic diseases. I work in a low income, low literacy population. There are many elements of
health care that occur in a clinic visits that are not understood by people living in our region of
the country (although I would argue that this is likely true for low literacy populations across the
country). However, when a provider conducts a weight assessment and counsels the patient
about the importance of maintaining a healthy weight through physical activity and nutrition, this
information is often understood. The relationship between weight and disease is becoming
better understood. With that being said, an individual’s perception of their own weight may not
be accurately categorized as “overweight” or “obese”. When the provider gives information on
BMI based on growth charts or BMI charts, the patient obtains an objective view of their risk for
disease through counseling. Many low income, low literacy populations do not have easy
access to scales or these charts, so a provider’s assessment and counseling is the essential
information to understand the importance of a normal BMI.
2. There is no doubt that assessment and information sharing is only part of the solution to our
national obesity crisis. We absolutely need additional Quality Measures about referrals to and
follow-up from evidence-based interventions for both children and adults. But, an important part
of the solution still remains in the screening for overweight and obesity and providing counseling
to motivate behavior change. Providers have to remain part of the solution and removing these
measures from children and adults dismisses the provider role in the overall crisis.
3. BMI is arguably the most important predictor of youth onset of chronic disease and adult
chronic disease. As a healthcare system, fully aware of the obesity epidemic in our country, we
must remain vigilant and expect all providers in all states to screen and counsel patients on
BMI. Removing these measures signals politicians, providers, community leaders and beyond
that we are either ignorant of the research or dismissive of its importance in driving health care
costs in the US.
4. Data analyses on health outcomes, without BMI, becomes less useful. BMI is an important
co-variate in data analysis of health outcome data. If we do not report BMI on youth and children
it becomes even more difficult to examine and explain changing trends in chronic disease rates.
In fact, not only is BMI an important variable in health outcomes we expect it is also an
C.92
important variable in health outcomes we may not expect. In our past analysis, BMI was an
important predictor of risk for H1N1, for extended hospital visits, and for conversion from
prediabetes to diabetes.
5. BMI is an important measure for other conditions and is used to monitor changes in health
status. Swings in weight may be predictive of cancer, congestive heart failure, kidney disease,
etc. BMI should remain a measure so as to not impact other disease monitoring.
There are more reasons that BMI should absolutely not be removed from the quality core
measures for children and adults and I would be glad to discuss further if that would be helpful.
As a final point, based on the draft report for comment I would like to note that within your expert
panel there was mention made of a replacement measure for the current adult BMI assessment
(ABA-AD). While this may be a point for further exploration, the recommendation was not put in
place. I believe there should be extreme concern about dismantling an important measure
without having a replacement measure already launched and tested.
Again thank you for the opportunity to comment.
C.93
YMCA of the USA (Katie Adamson)
YMCA of the USA, the national resource office for the nation’s 2,700 YMCAs, is pleased to
provide comments on the draft Recommendations for Improving the Core Set of Health Care
Quality Measures for Medicaid and CHIP. The Y believes that all people—regardless of age,
income or background—should be able to live life to its fullest, healthiest potential. As one of the
nation’s leading nonprofits strengthening communities through youth development, healthy
living, and social responsibility, the Y engages 21 million people in more than 10,000
communities across the country.
The Y’s reach into communities makes the organization an ideal service network for programs
like the YMCA’s Diabetes Prevention Program and the YMCA’s Healthy Weight and Your Child
program. These programs address both living with prediabetes and obesity and children and
adolescents living with obesity.
Based on the importance of screening and diagnostic measures in the delivery of these
evidence-based programs, we strongly recommend that CMS retain the Core Measures related
to care for diabetes and obesity, namely:
• the Comprehensive Diabetes Care: Hemoglobin A1c (HbA1c) Testing (HA1C-AD)
• the Adult Body Mass Index Assessment
• the Body Mass Index Assessment for Children/Adolescents
Obesity and type 2 diabetes are two of the most prevalent and costly chronic conditions in our
nation. Removing these three Core Measures could negatively impact both Medicaid and CHIP
recipients by acting as a disincentive for physicians to screen for BMI and HbA1c. Maintaining
these measures will continue to incentivize physicians in Medicaid and CHIP to screen, identify
and refer patients to evidence-based programs in the community that address prediabetes,
adult obesity and childhood obesity.
The YMCA’s Diabetes Prevention Program helps adults at high risk of developing type 2
diabetes reduce their risk for developing the disease by taking steps that will improve their
overall health and well-being. Research by the National Institutes of Health has shown that
programs like the YMCA’s Diabetes Prevention Program can reduce the number of new cases
of type 2 diabetes by 58%, and 71% in adults over the age of 60.
To qualify for the YMCA’s Diabetes Prevention Program an Adult 18+ must be:
Overweight (BMI >25)*
At risk for or have been diagnosed with prediabetes via a blood test with one of the following
results:
• Fasting Plasma Glucose between 100–125 mg/dL
• 2-hour Plasma Glucose between 140–199 mg/dL
C.94
• A1c between 5.7% and 6.4%
• Or have a previous diagnosis of gestational diabetes
• If a blood test is not available, a qualifying risk score based on a combination of risk
factors— family history, age, etc.
As of March 2019, the YMCA’s Diabetes Prevention Program has served over 64,000
participants at over 1,100 sites in 42 states across the country. The YMCA’s Diabetes
Prevention Program uses a CDC-approved curriculum and is part of the CDC-led National
Diabetes Prevention Program, and is available to all individuals who qualify, regardless of
insurance status or Y membership. Almost 20% of those served were low-income and 20% of
those in Y programs were referred by a physician or as the result of a screening program.
Referrals to the program are essential and the Y has closely partnered with the CDC and the
American Medical Association to help increase screenings, identification of prediabetes and
referrals to the program.
The Y was the awardee for the demonstration program from the Centers for Medicare and
Medicaid Innovation that resulted in the establishment of the Medicare Diabetes Prevention
Program (MDPP) expanded model. Many states are considering including the National Diabetes
Prevention Program as covered service in their Medicaid program and a few states have
already advanced coverage (e.g. California). As more states move to include the National
Diabetes Prevention Program for their Medicaid recipients, both the Adult Body Mass Index and
the A1c measure for prediabetes become essential measurements.
Despite the availability and success of this evidence-based health intervention to address
prediabetes, many providers and patients are still unaware of prediabetes as a condition.
According to CDC, more than 30% of U.S. adults have prediabetes, and less than 12% are
aware of it.
Screening, identification and referral modalities are cornerstones in providing quality care
through programs like the National Diabetes Prevention Program and the Medicare Diabetes
Prevention Program. We urge CMCS to follow the USPSTF recommendations (cited below)
regarding both the Comprehensive Diabetes Care: Hemoglobin A1c (HbA1c) Testing (HA1C-
AD) and the Adult Body Mass Index (BMI) Assessment and to maintain the Core Measures
related to care for diabetes and obesity.
The USPSTF Recommendation Statement for Abnormal Blood Glucose and Type 2 Diabetes
Mellitus Screening national data estimates from 2012, states that approximately 86 million
Americans aged 20 years or older have IFG or IGT.
1
Approximately 15% to 30% of these
persons will develop type 2 diabetes within 5 years if they do not implement lifestyle changes to
improve their health.
2
The USPSTF concludes with moderate certainty that there is a moderate
net benefit to measuring blood glucose to detect IFG, IGT, or diabetes and implementing
intensive lifestyle interventions for persons found to have abnormal blood glucose.
3
The
USPSTF previously found adequate evidence that intensive behavioral counseling interventions
for persons at increased risk for CVD have moderate benefits in lowering CVD risk.
4
Populations
in which these benefits have been shown, include persons who are obese or overweight and
C.95
have hypertension, hyperlipidemia or dyslipidemia, and/or IFG or IGT.
5
Benefits of behavioral
interventions include reductions in blood pressure, glucose and lipid levels, and obesity and an
increase in physical activity. USPSTF further cited that studies that specifically treat persons
who have IFG or IGT with intensive lifestyle interventions to prevent the development of
diabetes consistently show a moderate benefit in reducing progression to diabetes. And
USPSTF adds that lifestyle interventions have greater effects on reducing progression to
diabetes than metformin or other medications.
6
In the USPSTF Final Recommendation for Weight Loss to Prevent Obesity-Related Morbidity
and Mortality in Adults: Behavioral Interventions, the USPSTF recommends that clinicians offer
or refer adults with a body mass index (BMI) of 30 or higher to intensive, multicomponent
behavioral interventions.
7
Those recommended interventions specifically highlight the Diabetes
Prevention Program. Removing the Adult Body Mass Index Assessment from the Core Set
Measurement does not maintain consistency with USPSTF recommendations or align with
evidence-based treatment standards for obesity and will very likely send the message that
obesity identification and treatment is unimportant.
In addition to the burden of prediabetes and obesity among adults, childhood Obesity is a
growing problem in the United States. More than one third of children and teenagers, ages 2 to
19, are obese or overweight, and that rate has tripled in the past 30 years. Childhood obesity
can have a harmful effect on the body in several ways, putting children at high risk to develop
cardiovascular disease, diabetes, sleep apnea, asthma, joint problems, heartburn, and social
and psychological problems. Obese children are more likely to become obese adults, leading to
more serious health conditions including heart disease, diabetes and some cancers.
The YMCA’s Healthy Weight and Your Child is a 25-session evidence-based program for
children with obesity. The program includes a Family Information Session followed by 25
sessions delivered over four months. The family-based weight-management program focuses
on nutrition education and physical activity to encourage healthier eating habits and an active
lifestyle to reach a healthy weight. The Healthy Weight and Your Child curriculum is adapted
from the most widely disseminated and extensively evaluated child weight management
program in the world (also known as MEND). Studies have shown the program model is
effective in reducing a child’s body mass index and waist circumference, reducing sedentary
behaviors, increasing physical activity, and improving self-esteem.
The Y’s Healthy Weight and Your Child program is designed to support youth and families as
they seek to achieve positive change including:
• a reduction in the child’s body mass index (BMI);
• the adoption of healthier eating habits by the family;
• an increase in daily physical activity; and
• improved self-esteem for participants
Youth must meet the following criteria to qualify to participate in the program:
C.96
• The child must be 7 to 13 years old at the start of a group class.
• The child must carry excess weight, with a body mass index of the 95th percentile or higher.
• The child must receive clearance from a health care provider or school nurse to participate
in physical activity.
The YMCA’s Healthy Weight and Your Child program was only recently launched, but today
already more than 100 Ys in 37 states offer the Healthy Weight and Your Child program and
have served 1,822 children. More than 60% of the children served were eligible for free or
reduced-priced lunches and 57% of referrals have come from a doctor or health care
professional.
As the YMCA’s Diabetes Prevention Program, which is part of the National Diabetes Prevention
Program, became an expanded model program in Medicare, it is our long-term goal to work with
partners in the physician and the patient advocacy community to advance state Medicaid
coverage of the Healthy Weight and Your Child Program. To remove the BMI assessment for
children/adolescents measurement as we are beginning to scale the Healthy Weight and Your
Child program with health care providers, including the American Academy of Pediatrics, would
hinder efforts to improve the lives of children and adolescents living with obesity.
We want to support a comment of one of your reviewers/panelists who noted that the Final
Recommendation Statement of the USPSTF was that clinicians screen for obesity in children
and adolescents 6 years and older and offer or refer them to comprehensive, intensive
behavioral interventions to promote improvements in weight status.
8
Body mass index is the
recommended screening test for obesity by the USPSTF and they recognize that “identifying
obesity in children and how to address it are important steps in helping children and families
obtain the support they need.”
9
In closing, we hope that Mathematica’s Technical Assistance and Analytical Support Team and
Workgroup, and thus CMCS, will give serious consideration to our comments and retain the
Core Set Measurements (BMI for adults and children/adolescents and A1c) that encourage and
help providers screen, identify and refer low-income individuals in Medicaid and CHIP to
programs that can address prediabetes, adult obesity and childhood/adolescent obesity,
improve health outcomes, quality of care, and save substantial medical costs. The
recommendations to remove these Core Set Measurements are not in agreement with the latest
recommendations by the USPSTF and will hinder providers’ ability to provide life-saving
interventions to children, families and individuals at-risk of diabetes and obesity and related
complications.
Citations
1
U.S. Preventative Services Task Force: Screening for Abnormal Blood Glucose and Type 2
Diabetes. The USPSTF website:
https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatemen
tFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes
2
Ibid.
C.97
3
Ibid.
4
Ibid.
5
Ibid.
6
Ibid.
7
U.S. Preventative Services Task Force. Final Recommendation Statement: Weight Loss to
Prevent Obesity-Related Morbidity and Mortality in Adults. The USPSTF website:
https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatemen
tFinal/obesity-in-adults-interventions1
8
U.S. Preventative Services Task Force. Final Recommendation Statement Obesity in Children
and Adolescents: Screening. The USPSTF website:
https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatemen
tFinal/obesity-in-children-and-adolescents-screening1
9
Ibid.

Mathematica
Princeton, NJ • Ann Arbor, MI • Cambridge, MA
Chicago, IL • Oakland, CA • Seattle, WA
Tucson, AZ • Woodlawn, MD • Washington, DC
EDI Global, a Mathematica Company
Bukoba, Tanzania • High Wycombe, United Kingdom
mathematica-mpr.com
