
DOH 505-038 July 2024
Certicate of Waiver Medical Test Site (MTS)
Application Packet
Contents:
1. 505-038 ....Certicate of Waiver Medical Test Site Application Index and Fee Information
.......................................................................................................................... 1 Page
2. 505-039 ....Certicate of Waiver Medical Test Site Application InstructionsChecklist
.......................................................................................................................... 3 Pages
3. 505-026 ....Certicate of Waiver Medical Test Site Application................................... 7 Pages
Important Information:
Laboratories licensed by the Washington Medical Test Site (MTS) licensure program are exempt from
the Clinical Laboratory Improvement Amendments of 1988 (CLIA). You do not need to apply to the
Centers for Medicare and Medicaid Services (CMS) for a CLIA number. Your MTS license will contain
both your MTS license number and your CLIA number.
If the application you are submitting is handwritten, please ensure the information is written clearly,
accurately, and legibly in order to ensure there is no delay in processing.
In order to process your request:
Return Completed Application (original copy) and fee in the form of check or money order
(made out to Department of Health) to:
Department of Health
P.O. Box 1099
Olympia, WA 98507-1099
Fee Information
Certicate of waiver MTS license applications received during the rst year of the state biennium
(7/01/2023 through 6/30/2024) are required to submit the full fee. Applications received during the
second year of the state biennium (7/01/2024 through 6/30/2025) are required to submit half of the
full fee. The corresponding fees are:
Certicate of waiver
Medical Test Site License Application $260 $130
To request this document in another format, call 1-800-525-0127. Deaf or hard of hearing customers, please call 711
(Washington Relay) or email [email protected].
Fee - Applies to applications
submitted during the rst
y e a r o f t h e b i e n n i u m
7/01/2023-6/30/2024
Fee - Applies to applications
submitted during the second
y e a r o f t h e b i e n n i u m
7/01/2024-6/30/2025
(This page intentionally left blank.)

DOH 505-039 July 2024 Page 1 of 3
When your application for a Medical Test Site is received by the Department of Health,
you will be notied in writing of any outstanding documentation needed to complete the
application process.
All information should be printed clearly in blue or black ink. It is your responsibility to
submit the required forms.
F Indicate type of application:
• New - Choose this option if the facility has never been issued an MTS license.
• Change of ownership - Choose this option if the facility was previously issued an
MTS license and is now under new ownership and/or has a new UBI number.
• Change of license type - Choose this option if the facility has previously
been issued a dierent type of MTS license, such as a Provider Performed
Microscopic Procedure (PPMP) MTS license, a categoried MTS license, or an
accredited MTS license.
F Check One:
Please check your legal owner/operator business structure type according to your
Washington State Master Business License.
F Section 1. Demographic Information:
Unied Business Identier Number (UBI #): Enter your Washington State UBI
#. All Washington State businesses must have a UBI #. City, county, and state
government departments also have UBI #s.
Federal Employer ID Number (FEIN): Enter your FEIN, if the business has been
issued one. If the facility FEIN is dierent than the Legal Owner FEIN, enter this
number on page two of the application under Facility Specic Federal Employer ID
Number (FEIN).
Legal Owner/Operator Entity Name: Enter the owner’s name as it appears on the
UBI/Master Business License.
Legal Owner Mailing Address: Enter the owner’s complete mailing address.
Phone and Fax: Enter the owner’s phone and fax numbers.
Email and Web Address: Enter the owner’s email and facility web addresses, if
applicable.
Facility Name: Enter the lab’s name as advertised on signs and web site.
Facility Specic Federal Employer ID Number (FEIN). Enter if dierent from the
Owner FEIN listed on page one of the application.
Physical Address: Enter the lab’s physical street location including city, state, zip
code, and county.
Certicate of Waiver
Application Instructions Checklist

DOH 505-039 July 2024 Page 2 of 3
Phone and Fax Numbers: Enter the lab’s phone and fax number.
Mailing Address: Enter the lab’s mailing address, if dierent than physical address.
F Section 2. Facility Specic Information:
Site Type: Please check one applicable site type.
Hours of Testing: List the days and hours of testing for this site.
Additional locations under this license: Attach a list of names, addresses and phone
numbers for additional locations, if applicable, and test(s) performed at each site.
F Section 3. Key Individuals:
Lab Director: Enter the lab director’s:
1. First name, last name, and Washington State professional license number, if applicable.
2. Email address.
3. If the director of this laboratory serves as the director for any separately licensed
laboratory, provide the name and CLIA number of the laboratory. Include laboratories
licensed in other states.
Lab Contact: Enter the lab contact’s:
1. First name, last name, and Washington State professional license number, if applicable.
2. Email address.
The lab contact will receive all information that we mail to your medical test site.
F Section 4. Additional Information—Waived Tests:
Fill in the test system and test manufacturer in the provided table for each
test your lab performs. Please verify the waived status of your test system at
https://www.accessdata.fda.gov.
If you perform any non-waived tests, do not complete this application. See the MTS
website to help you determine your correct license category or email the MTS Program at
F Section 5. Other Licensure, Certication, or Registration Information:
Legal Owner: List the names, titles, addresses, and phone numbers of the corporate
ocers, LLC members or manager, partners, etc. Attach additional pages, if necessary.
I n d i c a t e i f y o u w i s h t o r e t a i n t h e C L I A n u m b e r i f s w i t c h i n g t o n e w l i c e n s e t y p e .
Change of Ownership Information: If applicable, list the previous owner name, previous
name of facility, previous MTS license number, eective date of ownership change and
physical address. Indicate if you wish to retain the CLIA number if changing ownership.
F Section 6. Foreign Ownership: Complete if facility is owned fully or partially by foreign
entity.
F Signature:
The legal owner or authorized representative must sign and date the application. Print the
name and title of the legal owner or authorized representative.

You will receive a renewal notice for this license approximately 60 days before the expiration
date. The renewal will be mailed to the facility mailing address on le.
Please contact Facilities Customer Service at 360-236-4985 if you have any questions or
need assistance in completing the application form. Additional information is available on our
website at: https://doh.wa.gov/mts.
DOH 505-039 July 2024 Page 3 of 3
(This page intentionally left blank.)

DOH 505-026 July 2024 Page 1 of 7
Section 1. Demographic Information
Check One
UBI # Federal Employer ID Number (FEIN)
Legal Owner/Operator Entity Name (as it appears on the UBI/Master Business License)
Revenue: 0420030000
Phone (enter 10 digit #) Fax (enter 10 digit #)
Mailing Address
City State Zip Code County
Facility/Agency Name (Business name as advertised on signs or website)
City State Zip Code County
Physical Address
Mailing Address (if dierent than physical address)
Email Address Web Address
Certicate of Waiver Medical Test Site License Application
This is for: F New F Change of Ownership F Change of License Type
F Limited Partnership
F Municipality (City)
F Municipality (County)
F Non-Prot Corporation
F Association
F Corporation
F Limited Liability Company
F Limited Liability Partnership
F Partnership
F Sole Proprietor
F State Government Agency
F Trust
Date
Stamp
Here
Facility Phone (enter 10 digit #) Facility Fax (enter 10 digit #)
City State Zip Code County
P.O. Box 1099
Olympia, WA 98507-1099
360-236-4700
http://www.doh.wa.gov/mts
Medical Test Site # _______________________________CLIA # _____________________________
For Oce Use Only
Facility Specic Federal Employer ID Number (FEIN) (if dierent than one entered above.)

DOH 505-026 July 2024 Page 2 of 7
Section 3. Key Individuals
Lab Director (include MD, PhD, BS, etc. - if applicable. A professional license is not required to be a director for a
waived MTS license)
MTS Contact Person
Email Address
Email Address
Hours of Testing
Section 2. Facility Specic Information
___ 1 Ambulance
___ 2 Ambulatory Surgery Center
___ 3 Ancillary Test Site
___ 4 Assisted Living Facility
___ 5 Blood Banks
___ 6 Community Clinic
___ 7 Comprehensive Outpatient Rehab
___ 8 End Stage Renal Disease Dialysis
___ 9 Federally Qualied Health Center
___ 10 Health Fair
___ 11 Health Main. Organization
___ 12 Home Health Agency
___ 13 Hospice
___ 14 Hospital
___ 15 Independent Laboratory
___ 16 Industrial
___ 17 Insurance
___ 18 ICFMR
___ 19 Mobile Lab
___ 20 Pharmacy
___ 21 Physician Oce
___ 22 Other ____________________
___ 23 Prison
___ 24 Public Health Lab
___ 25 Rural Health Clinic
___ 26 Student Health Service
___ 27 Skilled Nursing Facility
___ 28 Tissue Bank/Repository
___ 29 Drug Treatment
___ 30 Clinic
___ 31 Adult Family Home
Site Type (check one only)
First Name
If you qualify as a not-for-prot laboratory or state or local government laboratory that performs limited public
health testing (total of 15 or less waived or moderate complexity tests) at dierent locations, you may apply for one
license.
This license will have additional locations under one license and the paragraph above applies: F Yes F No
If yes: Attach a list of names, addresses and phone numbers for each site that will be included under one license,
and a list of tests performed at each site. If any of the sites already have a MTS license, include the MTS and
CLIA numbers of the sites that will be consolidated under this license. If you are not a state or local government
laboratory, you must include a copy of your federal 501(c)(3) determination letter to be licensed in this manner.
Additional locations under this license
List days and times during which testing is performed. If testing 24/7 check here F
Sunday Monday Tuesday Wednesday Thursday Friday Saturday
From:
To:
Last Name WA State Professional License number
Does the director of this laboratory serve as director for any other laboratories that are separately licensed in
Washington or another state? F Yes F No
If yes, provide the name of the laboratory and CLIA number:
__________________________________________________________________________________________
First Name Last Name WA State Professional License number

DOH 505-026 July 2024 Page 3 of 7
Section 4. Additional Information—Waived Tests
Complete the table below for waived tests performed by the laboratory. Refer tot he Application Instructions Checklist,
Section 4, if you need assistance completing this table.
Test Name Test System (e.g. One Step Glucose) Test Manufacturer (e.g. ACME)
Adenovirus
Aerobic/Anaerobic Organisms -
Vaginal
Alanine Aminotransferase (ALT)
(SGPT)
Albumin
Albumin, Urinary
Alcohol, Saliva
Alkaline Phosphatase (ALP)
Amines
Amphetamines
Amylase
Aspartate Aminotransferase (AST)
(SGOT)
Bacteria Associated With Bacterial
Vaginosis
Barbiturates
Benzodiazepines
Bilirubin, Total
Bladder Tumor Associated Antigen
B-Type Natriuretic Peptide (BNP)
Buprenorphine
Calcium, Ionized
Calcium, Total
Cannabinoids (THC)
Carbon Dioxide, Total (CO2)
Catalase, Urine
Chlamydia
Chloride
Cholesterol
Cocaine Metabolites
Collagen Type I Crosslink,
N-Telopeptides (NTX)
Cotinine
Creatine Kinase (CK)
Creatinine
Eddp (Methadone Metabolite)
Erythrocyte Sedimentation Rate (ESR),
Nonautomated
Estrone-3 Glucuronide
Ethanol (Alcohol)
Fecal Occult Blood
Fentanyl
Fern Test, Saliva

Waived Tests (continued)
DOH 505-026 July 2024 Page 4 of 7
Follicle Stimulating Hormone (FSH)
Fructosamine
Gamma Glutamyl Transferase (GGT)
Gastric Occult Blood
Gastric pH
Glucose
Glycated Hemoglobin, Total
Glycosylated Hemoglobin (HGB A1C)
hCG, Urine
HDL Cholesterol
Helicobacter Pylori
Helicobacter Pylori Antibodies
Hematocrit
Hemoglobin
Hemoglobin By Copper Sulfate,
Nonautomated
Hepatitis C Virus Antibody
Herpes Simplex I And/Or II Antibodies
HIV-1 And HIV-2 Antibodies
HIV-1 And HIV-2 Antigens
Infectious Mononucleosis Antibodies
(Mono)
Inuenza (A/B)
Ketone, Blood
Ketone, Urine
Lactic Acid (Lactate)
LDL Cholesterol
Lead, Blood
Leukocyte Esterase, Urinary
Lithium
Luteinizing Hormone (LH)
Lyme Disease Antibodies (Borrelia
Burgdorferi Abs)
Matrix Metalloproteinases-9 (MMP-9)
Methadone
Methadone Metabolite (EDDP)
Methamphetamine
Methylenedioxymethamphetamine (MDMA)
Microalbumin
Morphine
Neisseria Gonorrhoeae
Neutrophil Percentage (Neut%)
Nicotine And/Or Metabolites
Nitrite, Urine
Norfentanyl
Nortriptyline

Waived Tests (continued)
DOH 505-026 July 2024 Page 5 of 7
Opiates
Osmolality, Tears
Ovulation Test (LH) By Visual Color
Comparison
Oxazepam
Oxycodone
pH
pH, Urine
Phencyclidine (PCP)
Phenobarbital
Phosphorus
Platelet Aggregation
Platelet Count
Potassium
Pregnanediol Glucuronide
Propoxyphene
Protein, Total
Prothrombin Time (PT)
Red Blood Cell Count (Erythrocyte
Count) (RBC)
Respiratory Bacterial Pathogens
Respiratory Syncytial Virus
Respiratory Viruses
SARS-CoV-2
SARS-CoV-2 And Other Respiratory
Viruses
Secobarbital
Semen
Sodium
Spun Microhematocrit
Streptococcus, Group A
Thyroid Stimulating Hormone (TSH)
Tramadol
Treponema Pallidum (Syphilis)
Antibodies
Trichomonas
Tricyclic Antidepressants
Triglyceride
Urea (BUN)
Uric Acid
Urinary Protein, Qualitative
Urine Dipstick Or Tablet Analytes,
Nonautomated
Urine hCG By Visual Color
Comparison Tests
Urinalysis
Vaginal pH
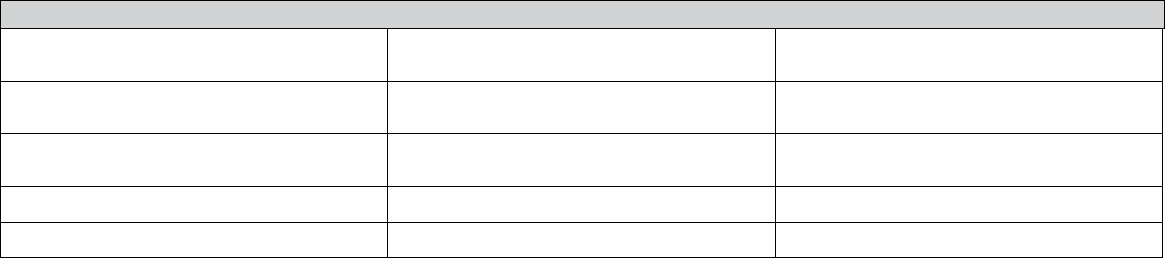
DOH 505-026 July 2024 Page 6 of 7
Waived Tests (continued)
White Blood Cell Count (Leukocyte
Count) (WBC)
White Blood Cell Dierential (WBC
Di)
Whole Blood Qualitative Dipstick
Glucose
Yeast, Candida Only
Other Waived Test(S) Not Listed
Provide an estimated total annual test volume for all waived tests performed: _________________________________

DOH 505-026 July 2024 Page 7 of 7
Section 5. Other Licensure, Certication or Registration Information
Name Address Phone # Title
Previous Name of Legal Owner
Previous Name of Facility Previous MTS License # Eective Date of Ownership
Change
Physical Address
Legal Owner Information–attach additional sheets as needed
Change of Ownership Information
List names, addresses, phone numbers, and titles of corporate ocers, partners, members, managers, etc.
Signature
I certify that I have received, read, understood, and agree to comply with state law and rule regulating this licensing
category. I also certify that the information herein submitted is true to the best of my knowledge and belief.
___________________________________________________________ _____________________________________
Signature of Owner/Authorized Representative of Medical Test Site Date
___________________________________________________________ _____________________________________
Print Name Print Title
City State
Zip Code
Section 6. Foreign Ownership
Does this facility have partial or full ownership by a foreign entity or foreign government? F Yes F N o
If yes, what is the country of origin for the foreign entity? _______________________________________
If changing license type, do you want the facility to keep the already assigned CLIA number? F Yes F N o
If yes, provide the CLIA number: _______________________________________
If changing ownership, do you want the facility to keep the already assigned CLIA number? F Yes F N o
If yes, provide the CLIA number: _______________________________________
