
1
Background
The most common human papillomavirus associated (HPVa) cancers are cervical cancer among
women and oropharyngeal cancer among men. In Illinois, non-Hispanic Black women have the
highest rate of new cervical cancer cases among all racial/ethnic groups, while non-Hispanic
White men have the highest rate of new oropharyngeal cancer cases. Further, rural populations
in Illinois have significantly higher rates of oropharyngeal and cervical cancer, when compared to
suburban and urban areas of Illinois. See HPV-Associated Cancers in Illinois – Part I (December
2020).
One of the most effective evidence-based interventions to reduce the incidence of HPVa cancers
is uptake of the HPV vaccine. Studies in the U.S. and globally have shown the HPV vaccination
prevents cancer-causing infections, including the HPV types that cause most genital warts.
1
,
2
In
fact, the HPV vaccination has the potential to prevent more than 90% of cancers caused by HPV.
3
,
4
The highly effective 9-valent HPV vaccine, Gardasil® 9, has been available for use in the United
States since late 2016 and protects against nine types of HPV (types 6, 11, 16, 18, 31, 33, 45, 52,
and 58);
5
the majority of HPVa cancers are caused by HPV 16 or 18. Today, Gardasil® 9 is the only
HPV vaccine available in the U.S. The HPV vaccine is an effective way to protect against HPV when
administered at the recommended age of 11 or 12 years (or can start at age 9) for both girls and
boys. In 2019, the Advisory Committee on Immunization Practices (ACIP) recommended the HPV
vaccine for adults 27 – 45 years of age using a shared clinical decision-making strategy to
determine if HPV vaccination for individuals within this age group is of benefit.
6
HPV Vaccines Recommended for Adolescents
Vaccines recommended for adolescents, 11 to 12 years of age, include tetanus-diphtheria-
pertussis (Tdap) booster vaccination, also referred to as Tdap, and the initiation of meningococcal
conjugate (MenACWY) vaccination and HPV vaccination.
7
HPV vaccine dosage is dependent on age of initiation. Most individuals who initiate vaccination
at ages 9 through 14 receive the vaccine as a two-dose series, and for those who initiate
vaccination at ages 15 through 26, a three-dose series (Figure 1) is administered. For
immunocompromised individuals ages 9-26 and individuals 27 years or older, a three-dose
vaccination series is recommended.
8
HPV-Associated
Cancers in Illinois
June 2021
Part II

2
In Illinois, HPV vaccination is not a requirement for adolescents entering school, whereas Tdap
and MenACWY are required, despite national recommendations from four leading national
medical associations since 2014.
9
,
10
Figure 1: Recommended Schedule for HPV Vaccination
†
(Source: CDC)
Since the HPV vaccine has been in use, rates of cancers and genital warts caused by HPV have
dropped 86% among adolescent girls.
11
Although screening is available for HPV infection and for
cervical cancer, no screening tests are available for the five other types of cancers caused by HPV:
oropharyngeal, anal, vulvar, vaginal, and penile cancers.
12
As a result, these types of cancer are
often detected at a later stage. HPV vaccination, however, can prevent these other HPV-related
cancers from developing.
7
Healthy People 2030 (HP2030) provides science-based, 10-year national objectives for improving
the health of all Americans by encouraging collaborations across communities and sectors,
empowering individuals toward making informed health decisions, and measuring the impact of
prevention activities. The HP2030 objective for HPV is to “increase the proportion of adolescents
ages 13 through 15 who receive recommended doses of the HPV vaccine.”
13
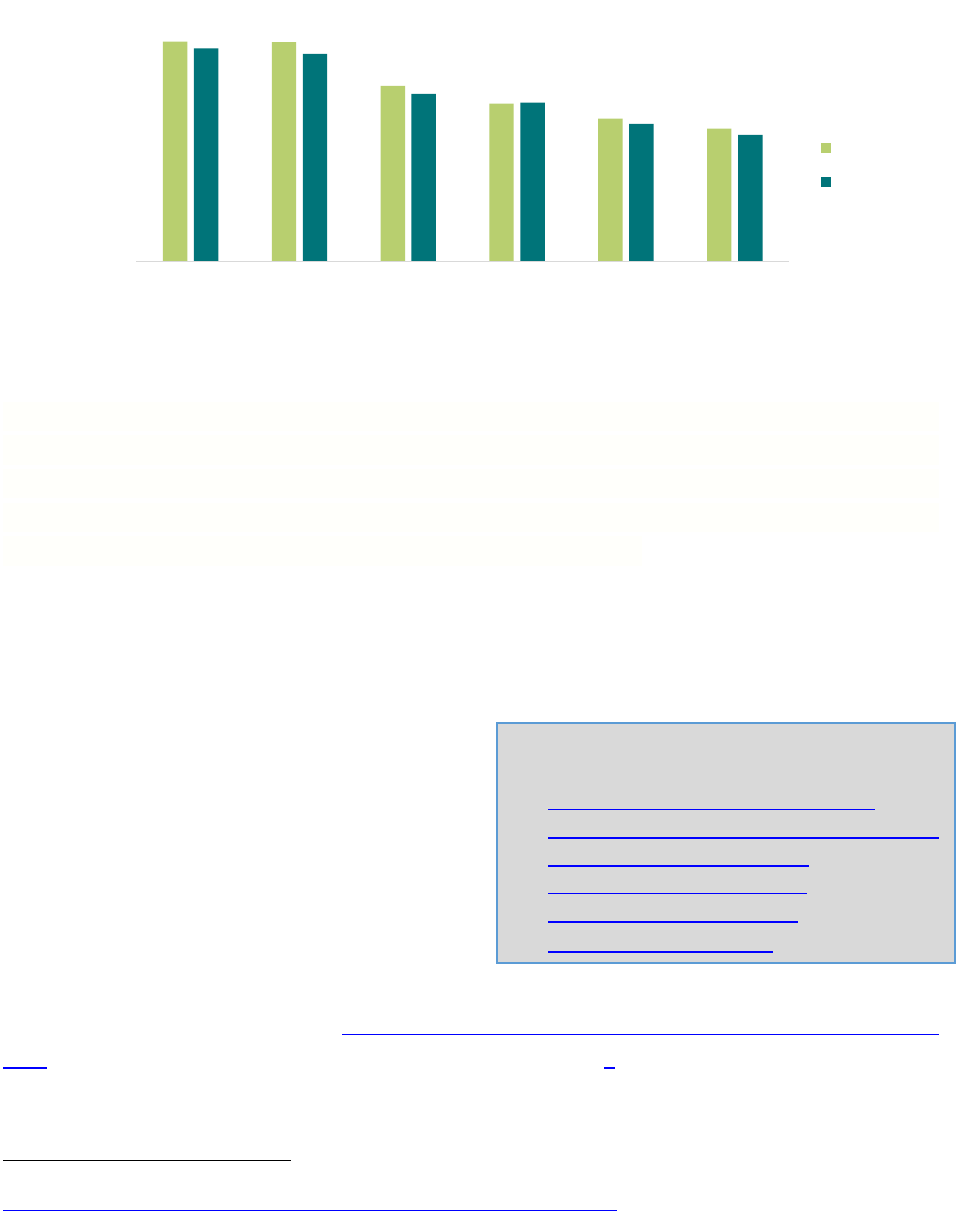
Although Illinois has
exceeded the HP2030 goal for Tdap (90%), the state has yet to reach the HP2030 goal of 80% for
HPV vaccination (Figure 2). In Illinois, the percentage of males and females who have initiated
and completed the HPV vaccination series is higher than the U.S., and females lead males in these
metrics.
†
HPV Vaccine Schedule and Dosing. Centers for Disease Control and Prevention. https://www.cdc.gov/hpv/hcp/schedules-
recommendations.html. Published August 15, 2019. Accessed July 23, 2020.
Signifies the ideal age range to initiate the HPV vaccine.
3
Figure 2: Coverage for Recommended Adolescent Vaccines, 13-17 years (2018)
‡
Programs and Policies to Increase HPV Vaccination
State and federally funded programs, laws, and regulations work to reduce the burden of
infectious diseases, such as HPV. U.S. policies that have been effective include financing to assure
access and availability of recommended vaccines
14
and ensuring a reliable and steady supply of
the HPV vaccine.
15
National and state efforts are being administered to increase the proportion
of adolescents receiving recommended doses of the HPV vaccine.
Specifically, the Illinois Department of Public Health’s (IDPH) Illinois Comprehensive Cancer
Control Program (ICCCP) works with statewide and community partners to reduce cancer
incidence and mortality by addressing areas across the cancer continuum, from primary
prevention to survivorship. One of the focus
areas within the ICCCP includes HPV-related
primary prevention strategies. One of the
primary functions of the ICCCP is collaboration
with the Illinois Cancer Partnership (ICP), which
includes public, private, and nonprofit sectors
partners that work together to establish,
promote, and implement the state’s cancer
control plan, which is updated every five years. Increasing HPV vaccination is identified as a
cancer prevention strategy in the Illinois Comprehensive Cancer Control Plan (2016-2021) and
the 2022-2027 Illinois Comprehensive Cancer Control Plan with the goal of increasing the
proportion of eligible adolescents who have completed the HPV vaccination series.
‡
Centers for Disease Control and Prevention TeenVax View. Data from 2018 National Immunization Survey—Teen (NIS-Teen).
https://www.cdc.gov/vaccines/imz-managers/coverage/teenvaxview/data-reports/index.html. Accessed November 2020.
92%
92%
73%
66%
60%
55%
89%
87%
70%
66%
57%
53%
0%
20%
40%
60%
80%
100%
TDAP MenACWY HPV ≥1
Female
HPV ≥1 Male HPV ≥2
Female
HPV ≥2 Male
IL
US
The IDPH ICCCP collaborates with the following
internal and external programs:
IDPH Vaccines for Children Program
Illinois Breast and Cervical Cancer Program
IDPH School Health Program
Illinois State Cancer Registry
IDPH Oral Health Programs
American Cancer Society

4
National HPV Vaccination Roundtable
The ICCCP actively participates in the Illinois HPV Advisory Group (National HPV Vaccination
Roundtable), which is a coalition of more than 70 organizations working at the intersection of
immunization and cancer prevention. Founded in 2014 by the American Cancer Society and the
Centers for Disease Control and Prevention (CDC), the mission of the roundtable is to convene,
communicate with, and catalyze member organizations and, by extension, the public to prevent
HPV cancers and raise HPV vaccination rates.
Comprehensive Cancer Control National Partnership (CCCNP)
Since 1998, the CDC’s National Comprehensive Cancer Control Program (NCCCP) has
provided funding, guidance, and technical assistance that programs across the country use
to develop, to implement, and to evaluate state-wide cancer control plans. NCCCP awardees
advance cancer control priorities, including increasing HPV vaccination and participating in the
Comprehensive Cancer Control National Partnership (CCCNP), where HPV vaccination remains a
top priority.
In addition to these state and federal public health initiatives, some states have passed legislation
and regulations to improve access to and uptake of the HPV vaccine as a part of their state’s
school attendance policies and cancer control efforts. Policy opportunities include legislative as
well as system level strategies to increase HPV vaccination (see Table 1). In Illinois, all students
entering sixth grade (and their parents or legal guardians) are required to be provided written
information about the link between HPV and certain types of cancers.
§
In addition, individuals 18
years and younger are eligible to receive the vaccine, as medically indicated, at no cost.
**
In 2014, four leading national medical associations — the American Academy of Family Physicians
(AAFP), the American Academy of Pediatrics (AAP), the American College of Physicians (ACP), and
the American College of Obstetricians and Gynecologists (ACOG) — together with the
Immunization Action Coalition and the CDC, have issued a call urging physicians across the United
States to educate patients about the HPV vaccine, and to strongly recommend HPV vaccination.
10
§
Illinois Senate Bill 2866 (2017)
**
Illinois Senate Bill 937 (2007)
5
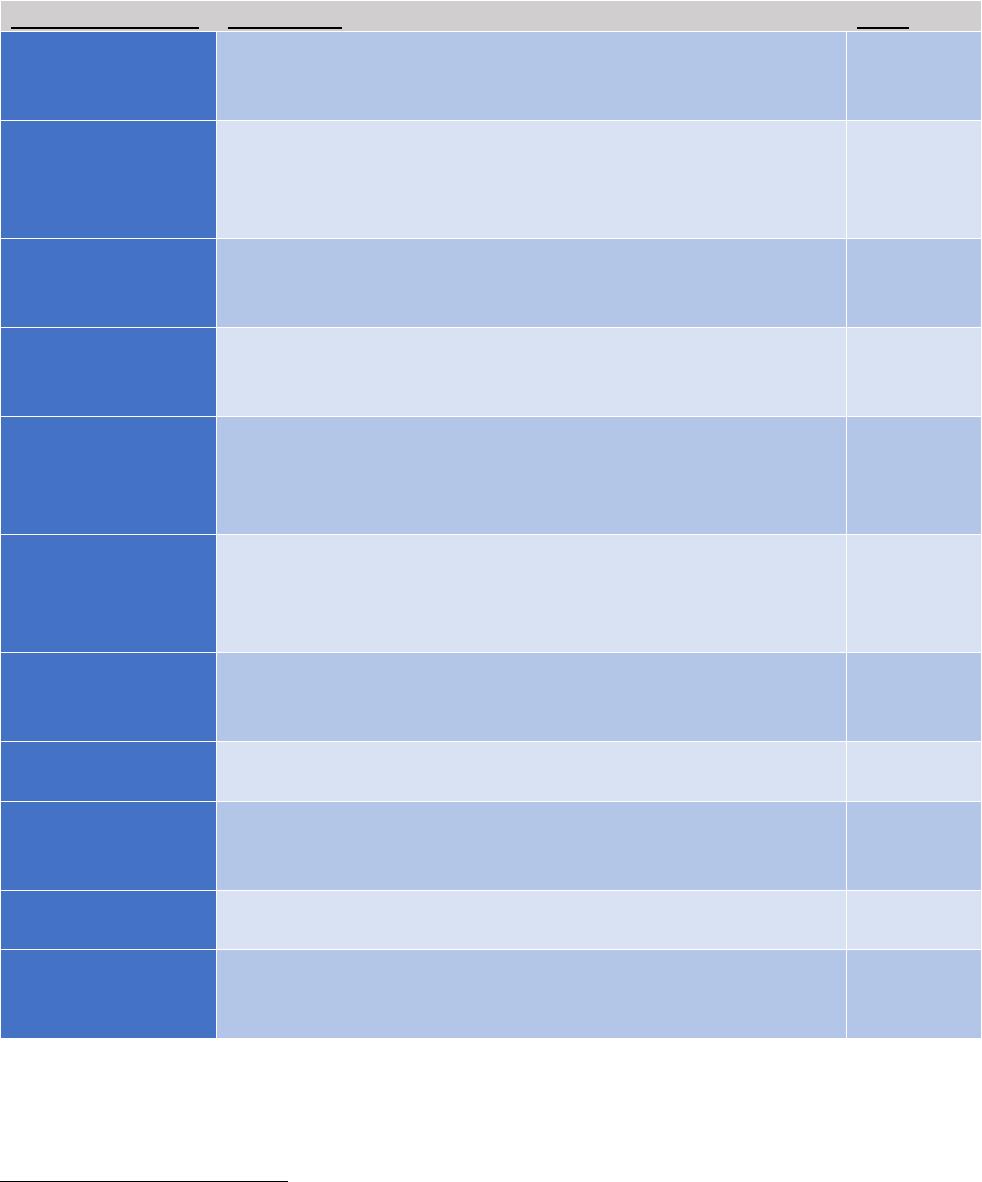
Table 1: Summary of Policy Opportunities to Increase HPV Vaccination
††
Policy Opportunity
Description
Level
Health care
provider
recommendation**
HPV vaccination recommendation to patients at each visit,
particularly when other vaccines are being administered;
decreases missed opportunities.
Provider
Reminder and
recall systems**
Reminders within the electronic medical record, prompting
providers to initiate HPV vaccination recommendation;
patient reminders to initiate and/or complete the HPV
vaccine series.
Clinic
State
immunization
registries*
Statewide registries in which all immunization records are
entered and maintained.
State
Standing orders**
Official clinic protocols that give clinical staff authorization to
complete immunizations for patients meeting recommended
guidelines.
Clinic
Provider
assessment and
feedback
evaluations**
Routine feedback to providers on patients’ HPV vaccination
series initiation and completion rates.
Clinic
Participation in
Vaccine for
Children (VFC)
Program**
Clinic approval and implementation of processes that allow
for participation in the VFC Program.
Clinic
Vaccination in
alternative
settings**
Providing HPV vaccination programs in schools, pharmacies,
mobile clinics, dental practices, and other community-based,
non-medical settings.
Clinic,
Community
Pharmacy-related
laws*
State-enacted laws allowing pharmacists to provide the HPV
vaccine series to youth and young adults.
State
School-entry
requirements*
State-enacted laws that require students to initiate and
complete the HPV vaccine series to maintain eligibility to
attend school.
State
Communication
campaigns**
Leveraging rural community partnerships and voices of local
residents to deliver positive HPV vaccination messaging.
Community
Rural HPV
vaccination
research*
Increased funding for interventional rural HPV vaccination
research (e.g., randomized controlled trials, quasi-
experimental studies, and pragmatic trials).
National
*”Big P” policies include legislative policies and/or other federal or state mandates
**”Little P” policies include local written policies and system level strateiges and processes
††
St. Jude Children’s Research Hospital: HPV Cancer Prevention Program (2021, April 27) HPV Vaccination: A Look at State Policy and A Path
Forward. [Virtual Seminar].

6
HPV Vaccination Coverage in Illinois
HPV vaccination rates in Illinois vary by select demographic characteristics (Figure 3). Slightly
more individuals living below the poverty line are up to date (UTD) on HPV vaccination compared
to those living at or above poverty for both males and females. With the exception of Black non-
Hispanic males, the percentage of HPV UTD is slightly higher in females than males. Hispanic
females have a higher percentage of HPV UTD than Black and White non-Hispanic females. The
percentage of HPV UTD in Illinois varies based on location with the highest percentage of HPV
UTD for both males and females in large cities.
16
Females in rural areas of the state report the
lowest HPV UTD percentage. Data on males in rural areas in the state has been suppressed due
to small sample size.
The Illinois Comprehensive Automated Immunization Registry Exchange (I-CARE) is a web-based
immunization record-sharing application developed by IDPH and is designed to record, to track,
and to report immunizations. I-CARE 2018 data was examined to determine county-level
variation in completion, also referred to as up to date (UTD), of the HPV vaccination series among
13- to 15-year-olds (Appendix A). In Illinois’s 102 counties, HPV vaccination (UTD) ranged from
5% to 54%. Generally, UTD HPV vaccination percentages were higher in more urban areas, such
as Cook County and Suburban Cook County, larger cities, including Chicago, Champaign, Peoria,
and Springfield, and suburban areas in northeastern and central Illinois. UTD HPV vaccination
percentages were lower in more rural regions in the state, particularly in northwestern and
southern counties of Illinois.
Figure 3: HPV Vaccination Up to Date Percentages, Ages 13-17 by Select Demographics,
Illinois (2018)
Challenges and Next Steps
51%
59%
54%
60%
56%
45%
0%
20%
40%
60%
80%
Black only
non-Hispanic
Hispanic White non-
Hispanic
Race/Ethnicity
Female Male
35%
56%
63%
52%
59%
0%
20%
40%
60%
80%
Rural Small City Large City
Location
56%
57%
50%
54%
0%
20%
40%
60%
80%
At or Above
Poverty
Below Povery
Poverty
Data Source: TeenVax View, Illinois. Centers for Disease Control. Data from 2018 National Immunization Survey—Teen (NIS-Teen).
https://www.cdc.gov/vaccines/imz-managers/coverage/teenvaxview/data-reports/index.html. Published July 22, 2019. Accessed June 25, 2020.
Note: UTD includes adolescents with three doses, and those with two doses (when the first HPV vaccine dose was initiated before age 15 years
and time between the first and the second dose was at least five months minus four days). Data on males in rural areas in the state has been
suppressed due to small sample size.
Black non-
Hispanic
Hispanic
White non-
Hispanic

7
Identifying and Addressing Challenges to HPV Vaccine Uptake
One of the primary reasons behind lower rates of vaccine uptake, for both vaccine initiation and
completion, ties back to parental intention to vaccinate their children. A 2020 study found the
most common reason for lack of intent or vaccine hesitancy among parents to initiate the vaccine
series for unvaccinated adolescents was safety concerns (23%).
17
Among parents of adolescents who
received only one HPV vaccine dose, lack of a recommendation from a health care provider (22%)
was the most frequently cited reason for absence of intent to complete the series
Recent studies to better understand vaccine hesitancy, related to the COVID-19 vaccine among
adolescents, aligns with similar themes to include safety, trust, and perceived risk of infection.
18
Additional barriers to HPV vaccine uptake include health care access, cost, caregiver support,
peer influence, school-based interventions, and provider/practice-based interventions.
19
Improving vaccine confidence remains a critical strategy to instill trust among patients, parents
or families, and providers, especially among rural populations in Illinois.
Clinician recommendation remains the number one reason parents decide to vaccinate;
providers can leverage their relationship with parents to provide education and to address
vaccine hesitancy. Provider resources have been developed by CDC, the American Academy of
Pediatrics (AAP), and the American Academy of Family Physicians (AAFP), collectively referred to
as Provider Resources for Vaccine Conversations with Parents.
Conclusion and Next Steps
HPVa cancer cases vary by demographic and geographic factors across Illinois. Several different
strategies can effectively address these variations. However, HPV vaccine uptake using evidence-
based strategies is a top national objective and has been a recommendation of the Community
Preventive Services Task Force since 2009. Changes in recommendations from the ACIP in 2019
include HPV vaccination catch-up among not only females, but also males, through age 26 years
and a simplified immunization schedule. However, adolescents are still the main focal point to
initiate the HPV vaccine to prevent HPV infection and reduce HPV-associated cancer rates.
To address disparities in vaccine uptake, the ICCCP and partners will need to focus efforts around
statewide polices to support vaccination among adolescents in parallel to addressing vaccine
hesitancy and other known barriers. Many statewide and community-driven strategies are being
implemented and evaluated to address vaccine access as well as hesitancy among adolescents
during the response to the COVID-19 pandemic. These promising practices and lessons learned

8
would serve well to inform future strategies to increase HPV vaccine uptake, especially among
males as well as rural populations across Illinois.
Acknowledgements
The authors would like to thank the following individuals for their review and input into the
development of this report:
Suzanne Elder, American Cancer Society
Lori Koch, Illinois State Cancer Registry

9
APPENDIX A: HPV Vaccination Series Up to Date Among 13 – 15 Year Olds in Illinois, By County (2019)
*
Data Limitations: I-CARE data source excludes deceased patients, as well as those patients that initiated one or more doses out
of state and completed their series in Illinois.
*
Source: Illinois Comprehensive Automated Immunization Registry Exchange (I-CARE) data. Unpublished data from 2019
Notes: HPV Immunization rates for Illinois’s 102 counties were grouped into quintiles
Percent of 13-15 year
olds with UTD HPV
vaccination series, 2019

10
Appendix B: Resource Guide (HPV Associated Cancers in Illinois – Part II)
HPV-Associated Cancers in Illinois – Part I (December 2020)
https://www.dph.illinois.gov/sites/default/files/publications/hpv-associated-cancers-illinois-part-1-final-
01282021.pdf
IDPH Vaccines for Children Program
http://dph.illinois.gov/topics-services/prevention-wellness/immunization/vfc-program
Illinois Breast and Cervical Cancer Program
http://dph.illinois.gov/topics-services/life-stages-populations/womens-health-services/ibccp
IDPH School Health Program
https://dph.illinois.gov/topics-services/life-stages-populations/maternal-child-family-health-
services/school-health
Illinois State Cancer Registry
https://www.dph.illinois.gov/data-statistics/epidemiology/cancer-registry
IDPH Oral Health Programs
https://dph.illinois.gov/topics-services/prevention-wellness/oral-health
American Cancer Society
https://www.cancer.org/health-care-professionals/hpv-vaccination-information-for-health-
professionals/our-hpv-vaccination-initatives.html
Illinois Comprehensive Cancer Control Plan (2016-2021)
https://dph.illinois.gov/sites/default/files/publications/state-cancer-plan-050818.pdf
National Vaccination HPV Roundtable
https://hpvroundtable.org/
Comprehensive Cancer Control National Partnership (CCCNP)
https://www.cccnationalpartners.org/about-us
Provider Resources for Vaccine Conversations with Parents
https://www.cdc.gov/vaccines/hcp/conversations/

11
References
1
Centers for Disease Control and Prevention. HPV Vaccine Safety and Effectiveness Data
https://www.cdc.gov/hpv/hcp/vaccine-safety-data.html. Accessed September 27, 2020.
2
Centers for Disease Control and Prevention. HPV Vaccine Information for Young Women
https://www.cdc.gov/std/hpv/stdfact-hpv-vaccine-young-women.htm. Accessed June 07, 2020.
3
Lei J, Ploner A, Elfström KM, Wang J, Roth A, Fang F, Sundström K, Dillner J, Sparén P. HPV Vaccination
and the Risk of Invasive Cervical Cancer. N Engl J Med. 2020 Oct 1;383(14):1340-1348. doi:
10.1056/NEJMoa1917338. PMID: 32997908
4
Centers for Disease Control and Prevention (CDC). Cancers caused by HPV are preventable.
https://www.cdc.gov/hpv/hcp/protecting-patients.html. Accessed June 11, 2021.
5
Basic Information about HPV and Cancer. Centers for Disease Control and Prevention.
https://www.cdc.gov/cancer/hpv/basic_info/index.htm. Published August 22, 2018. Accessed June 24,
2020.
6
Centers for Disease Control and Prevention (CDC). Grading of Recommendations Assessment,
Development and Evaluation (GRADE) for use of HPV vaccine in adults ages 27 through 45 years.
https://www.cdc.gov/vaccines/acip/recs/grade/HPV-adults.html. Accessed May 31, 2021.
7
Easy-to-read Immunization Schedule by Vaccine for Ages 7-18 Years. Centers for Disease Control and
Prevention. https://www.cdc.gov/vaccines/schedules/easy-to-read/adolescent-easyread.html.
Published February 3, 2020. Accessed August 4, 2020.
8
HPV Vaccine Administration. Centers for Disease Control and Prevention.
https://www.cdc.gov/vaccines/vpd/hpv/hcp/administration.html. Published March 17, 2020. Accessed
June 24, 2020.
9
Illinois Department of Public Health (IDPH). Minimum Immunization Requirements Entering a Child
Care Facility or School in Illinois, Fall 2019.
http://www.dph.illinois.gov/sites/default/files/publications/Minimum%20Immunization%20Requireme
nts.pdf Accessed May 31, 2021.
10
Leading Medical and Public Health Organizations Join Efforts Urging Physicians to Strongly
Recommend Human Papilloma Virus (HPV) Vaccination. AAFP Home. https://www.aafp.org/media-
center/releases-statements/all/2014/HPV-vaccination-dear-colleague-letter.html. Published February
12, 2014. Accessed August 4, 2020.
11
Reasons to Get Vaccinated Against HPV. Centers for Disease Control and Prevention.
https://www.cdc.gov/hpv/parents/vaccine/six-reasons.html, Published March 26, 2019. Accessed June
24, 2020.
12
HPV. Centers for Disease Control and Prevention. https://www.cdc.gov/hpv/hcp/protecting-
patients.html. Published November 13, 2019. Accessed June 24, 2020.

12
13
U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion.
Objectives and Data, Vaccination. https://health.gov/healthypeople/objectives-and-data/browse-
objectives/vaccination/increase-proportion-adolescents-who-get-recommended-doses-hpv-vaccine-iid-
08. Accessed March 21, 2021.
14
Institute of Medicine 2004. Financing Vaccines in the 21st Century: Assuring Access and Availability.
Washington, DC: The National Academies Press. https://doi.org/10.17226/10782
15
U.S. Department of Health and Human Services (HHS). 2010 National Vaccine Plan, Protecting the
Nation’s Health through Immunization. https://www.hhs.gov/sites/default/files/nvpo/vacc_plan/2010-
Plan/nationalvaccineplan.pdf. Accessed May 31, 2021.
16
MSA status was determined based on household reported city and county of residence and was
grouped into three categories: MSA principal city, MSA nonprincipal city, and non-MSA. MSA and
principal city were as defined by the U.S. Census Bureau (https://www.census.gov/programs-
surveys/metro-micro.htmlexternal icon). Non-MSA areas include urban populations not located within
an MSA as well as completely rural areas.
17
K. Sonawane, Y. Zhu, J. Montealegre, D. Lairson, C. Bauer, L. McGee, A. Giulian, and A. Deshmukh,
Parental intent to initiate and complete the human papillovavirus vaccine series in the USA; a
nationwide, cross-sectional survey. Vol. 5 (pp 484-492): Sept 2020. https://doi.org/10.1016/S2468-
2667(20)30139-0
18
Centers for Disease Control and Prevention. COVID-19 State of Vaccine Confidence Insights Report.
Rpt 8, May 24, 2021 (date range: April 27 – May 10, 2021).
19
Peterson CE, Silva A, Holt HK, Balanean A, Goben AH, Dykens JA. Barriers and facilitators to HPV
vaccine uptake among US rural populations: a scoping review. Cancer Causes Control. 2020 Sep
31(9):801-814. doi: 10.1007/s10552-020-01323-y. Epub 2020 Jun 14. PMID: 32537702.
