
National Center for Immunization & Respiratory Diseases
Update on HPV Vaccination Policy, 2019
Current Issues in Immunization Webinar
December 11, 2019
Lauri Markowitz, MD
Team Lead and Associate Director for Science, HPV
Division of Viral Diseases

Changes in HPV vaccination
policy, 2019
Harmonization of catch-up
recommendations through age 26
years for all persons
Shared clinical decision-making for
adults age 27 through 45 years
2
MMWR 2019; 68; 698-702

Recommendations for HPV vaccination in the United
States - before June 26, 2019
Routine vaccination
– Age 11 or 12 years
– Vaccination can be started at age 9 years
Catch-up vaccination
– Females through age 26 years
– Males through age 21 years
– Certain populations through age 26 years*
Males aged 22 through 26 years may be vaccinated
*Men who have sex with men, transgender persons, and persons with certain immunocompromising conditions
MMWR 2014;63 (RR05) MMWR 2015;64:300-4 MMWR 2016; 65:2105-8
3

Current recommendations for HPV vaccination in the
United States
Routine vaccination
– Age 11 or 12 years
– Vaccination can be started at age 9 years
Catch-up vaccination
– Through age 26 years, if not adequately vaccinated
MMWR 2019; 68; 698-702
4

Current recommendations for HPV vaccination in the
United States
Routine vaccination
– Age 11 or 12 years
– Vaccination can be started at age 9 years
Catch-up vaccination
– Through age 26 years, if not adequately vaccinated
Shared clinical decision-making
– Some adults age 27 through 45 years, if not adequately vaccinated
MMWR 2019; 68; 698-702
5

2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019
Recommendation for Females
Routine
: 11 or 12 years
Catch-up: through 26 years
3-dose schedule
Recommendation for Males
Routine
: 11 or 12 years
Catch-up: through 21 years
3-dose schedule
2-dose schedule
if first dose
age <15 years
Catch-up: through 26
Shared clinical decision-
making
: some adults
27 through 45 years
Evolution of HPV vaccination recommendations –
United States
6

2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019
Recommendation for Females
Routine
: 11 or 12 years
Catch-up: through 26 years
3-dose schedule
Recommendation for Males
Routine
: 11 or 12 years
Catch-up: through 21 years
3-dose schedule
7
2-dose schedule
if first dose
age <15 years
Catch-up: through 26
Shared clinical decision-
making
: some adults
27 through 45 years
Quadrivalent Vaccine
Bivalent Vaccine
9-valent Vaccine
2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019
Evolution of HPV vaccination recommendations,
vaccine availability and use – United States

HPV vaccines licensed and age ranges, United States
Before October 2018
After the end of 2016, only 9vHPV has been distributed in the United States
In April 2018, 9vHPV manufacturer filed an application to expand age indication through
age 45 years
8
Vaccine HPV types Licensure ages
Bivalent (2vHPV) 16,18 Females 9–25 yrs
Quadrivalent (4vHPV) 6,11,16,18 Females and males 9–26 yrs
9-valent (9vHPV) 6,11,16,18,
31,33,45,52,58
Females and males 9–26 yrs

HPV vaccines licensed and age ranges, United States
Since October 2018
After the end of 2016, only 9vHPV has been distributed in the United States
HPV vaccines have been licensed through age 45 years or older in other countries
9
Vaccine HPV types Licensure ages
Bivalent (2vHPV) 16,18 Females 9–25 yrs
Quadrivalent (4vHPV) 6,11,16,18 Females and males 9–26 yrs
9-valent (9vHPV) 6,11,16,18,
31,33,45,52,58
Females and males 9–45 yrs

Licensure of 9vHPV for use in expanded age range
FDA Summary Basis for Regulatory Action
Results of a randomized, double-blind, placebo-controlled trial (base study) of
4vHPV that included women aged 27─45 years
Observational follow-up through 10 years in a subset of women in the base study
A cross-study immunogenicity analysis showing non-inferiority of immune
responses to 4vHPV in males aged 27─45 years vs aged 16─26 years
Extrapolation of data to 9vHPV in individuals aged 27─45 years
10
Munoz et al. Lancet 2009; Castellsague et al. Br J Cancer 2011 (end of study results); Luna et al. PLoS One 2013 (6 year follow-up); Luxembourg (10 year follow-up
presented at ACIP June 2018); Giuliano et al. Vaccine 2015; Giuliano et al. N Engl J Med 2011; Palefsky et al. N Engl J Med 2011
https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM622941.pdf
ACIP uses Evidence to Recommendations framework
PICO question and background
Problem
Benefits and harms
Values
Acceptability
Resource use
Feasibility of implementation
Balance of consequences
Type of recommendation and
recommendation text
Evidence to Recommendations
Framework
Recommendation
options
https://www.cdc.gov/vaccines/acip/recs/grade/downloads/ACIP-evidence-rec-frame-508.pdf
11
PICO: population, intervention, comparison, outcomes

Evidence to Recommendations: Benefits and Harms
4vHPV efficacy trial in women ages 24–45 years (n=3,819)
– Efficacy against endpoint of persistent HPV infection, extragenital lesions, CIN1+
• Per-protocol efficacy: 88.7% (95% CI: 78.1–94.8)
• Intention-to-treat efficacy: 47.2% (95% CI: 33.5–58.2)
9vHPV immunogenicity trial in women ages 27–45 years (n=640)
– Antibody titers non-inferior compared to women ages 16–26 years
– >99% of women in both age groups seroconverted to all 9vHPV types
12
Castellsagué X et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24-45
years of age. Br J Cancer 2011
Luxembourg A. 9vHPV immunogenicity and safety trial in mid-adult females. Presentation to ACIP, Atlanta, GA. June 26, 2019.
CIN, cervical intraepithelial neoplasia

Evidence on benefits:
– Efficacy: 3 RCTs of 4vHPV and/or 2vHPV
– Immunogenicity: 3 RCTs, 6 observational trials
Evidence on harms:
– Safety: 5 RCTs, 4 observational trials
GRADE for HPV Vaccination of Mid-Adults http://www.cdc.gov/vaccines/acip/recs/grade/table-refs.html.
RCTs, randomized controlled trials
Evidence to Recommendations: Benefits and Harms
13

Conceptual model of HPV infection leading to
cervical cancer
First HPV infection occurs soon
after onset of sexual activity
HPV infection highest in late
teens/early 20s
Most infections clear or become
undetectable within 1-2 years
Many precancers clear
Precancers can progress to cancer
after many years/decades
Schiffman et al, Nature Reviews 2016
14

Understanding the burden of disease due to incident
HPV infection in adults
HPV incidence highest in late teens and early twenties
New HPV infections do occur in mid-adults
– New partner is risk factor; new partners decrease with increasing age
Epidemiology of HPV infection differs for males and females
Some uncertainty about immunity after clearance of natural infection
– Immunity thought to be low; higher for females than males
Progression to cancer occurs over years/decades
– Some high risk HPV types more likely to progress to cancer
Rodriquez et al. JNCI 2010; Winer et al. CEBP 2011; Winer JID 2016; Beachler et al. JID 2016; Kahn et al. JNCI 2005; Giuliano et al. Int J Cancer 2015

Evidence to Recommendations: Resource use
5 health economic models of HPV vaccination in the U.S. were reviewed
– The cost-effectiveness ratio for the current HPV vaccination program ranged
from cost-saving to about $35,000 per QALY gained
– In the context of the existing program, expanding vaccination through age 30, 35,
40 or 45 years would provide relatively small additional health benefits
– The incremental cost per QALY for also vaccinating adults through age 30 years
exceeded $300,000 in 4 of 5 models
– Variation in results across models was due to factors such as uncertainties about
HPV natural history
QALY, quality-adjusted life year
Chesson HW. Overview of Health Economic Models for HPV Vaccination of Mid-Adults. Presentation to ACIP, Atlanta, GA. June 2019.
16

Percent change in incidence
Current recommendation
Current recommendation plus adults through age 45 years
32,000,000 cases prevented
Cervical cancer
CIN 2/3
Years since start of vaccination program
Other HPV cancers
Anogenital warts
56,000 additional cases prevented
13,000,000 cases prevented
3,000 additional cases prevented
653,000 cases prevented
124,000 additional cases prevented
4,000 additional cases prevented
769,000 cases prevented
CIN, cervical intraepithelial neoplasia; Median estimates generated by 50 best fitting parameter sets
Brisson, Presentation to ACIP, Atlanta, GA. February 2019
Estimated impact of HPV vaccination: HPV-ADVISE results
• In the context of the existing HPV
vaccination program, expanding
vaccination to adults through age 45
years would produce relatively small
additional health benefits and less
favorable cost-effectiveness ratios
17

Estimated number needed to vaccinate
HPV vaccines are most effective when given before exposure to HPV
Population benefit would be minimal, yet some individuals in this age range
might be able to benefit from vaccination
Estimated number needed to vaccinate (NNV) to prevent one case of
anogenital warts, cervical precancer, or cancer, is:
Cervical precancer is CIN2+
NNV results from HPV-ADVISE, per Chesson HW, Overview of Health Economic Models for HPV Vaccination of Mid-Adults, presentation to ACIP, June 2019
NNV with existing vaccination
program
9, 22, and 202
NNV with vaccination through age
45 years
120, 800, and 6,500

Evidence to Recommendations framework
PICO question and background
Problem
Benefits and harms
Values
Acceptability
Resource use
Feasibility of implementation
Balance of consequences
Type of recommendation and
re
commendation text
Evidence to Recommendations
Framework
https://www.cdc.gov/vaccines/acip/recs/grade/downloads/ACIP-
evidence-rec-frame-508.pdf
Recommendation options
– Recommend the intervention
– Recommend for individuals based
on
shared clinical decision-making
(formerly “permissive” or Category B)
– Do not recommend the
intervention
19
ACIP recommends intervention
for individuals based on
shared clinical decision-making
Recommendation relies
upon guidance of clinician in
the context of individual
clinician-patient interactions
to determine whether or
not vaccination is
appropriate for a patient
ACIP does not
recommend the
intervention
Vaccination is not
recommended
https://www.cdc.gov/vaccines/acip/recs/grade/etr.html
ACIP recommends the
intervention
Vaccination
recommended for all
persons in the age
group or group at
increased risk for
vaccine preventable
disease
Types of ACIP recommendations
20

Changing ACIP terminology over time for similar
type of recommendation
Permissive
Recommendation
2009 HPV vaccine for
boys
“HPV4 may be given to
males aged 9 through 26
years…”
Category B Rec/
Clinical Decision Making
2015 Men B for
adolescents and young
adults
“…adolescents and young
adults may be vaccinated
with a serogroup B
meningococcal (MenB)
vaccine…”
Shared Clinical
Decision-Making
2019 HPV vaccine for
adults age 27-45 years
“…shared clinical decision-
making regarding HPV
vaccination is recommended
for some adults aged 27
through 45 years who are
not adequately vaccinated”

Routine HPV vaccination of adults 27 through 45 years
was not brought to ACIP for consideration
ACIP recommends intervention
for individuals based on
shared clinical decision-making
Recommendation relies
upon guidance of clinician in
the context of individual
clinician-patient interactions
to determine whether or
not vaccination is
appropriate for a patient
ACIP does not
recommend the
intervention
Vaccination is not
recommended
https://www.cdc.gov/vaccines/acip/recs/grade/etr.html
ACIP recommends the
intervention
Vaccination
recommended for all
persons in the age
group or group at
increased risk for
vaccine preventable
disease
22

Shared clinical decision making category addresses situations where
–
vaccination may benefit some individuals, but
– will have relatively minimal population-level impact
Identifying who will benefit from vaccination is not always straight forward
Shared clinical decision-making
23

Shared clinical decision-making for HPV vaccination of
adults age 27 through 45 years
MMWR 2019; 68; 698-702
HPV vaccination does not need to be discussed with most adults aged >26
years
For adults aged 27 through 45 years who are not adequately vaccinated,
clinicians can consider discussing HPV vaccination with persons who are most
likely to benefit
Ideally, vaccination should be given in early adolescence because vaccination is
most effective before exposure to HPV through sexual activity
24

HPV is a very common sexually transmitted infection. Most HPV infections are
transient and asymptomatic and cause no clinical problems.
Although new HPV infections are most commonly acquired in adolescence and
young adulthood, some adults are at risk for acquiring new HPV infections. At any
age, having a new sex partner is a risk factor for acquiring a new HPV infection.
Persons who are in a long-term, mutually monogamous sexual partnership are not
likely to acquire a new HPV infection.
Most sexually active adults have been exposed to some HPV types, although not
necessarily all of the HPV types targeted by vaccination.
No clinical antibody test can determine whether a person is already immune or
still susceptible to any given HPV type.
Considerations for shared clinical decision-making for
HPV vaccination of adults age 27 through 45 years
MMWR 2019; 68; 698-702
25

HPV vaccine efficacy is high among persons who have not been exposed to
vaccine-type HPV before vaccination.
Vaccine effectiveness might be low among persons with risk factors for HPV
infection or disease (e.g., adults with multiple lifetime sex partners and likely
previous infection with vaccine-type HPV), as well as among persons with certain
immunocompromising conditions.
HPV vaccines are prophylactic (i.e., they prevent new HPV infections). They do not
prevent progression of HPV infection to disease, decrease time to clearance of HPV
infection, or treat HPV-related disease.
Considerations for shared clinical decision-making for
HPV vaccination of adults age 27 through 45 years (con’t)
MMWR 2019; 68; 698-702
26

2020 Adult Immunization Schedule – Draft

Administration. Dosing schedules, intervals, and definitions of persons considered
adequately vaccinated have not changed.
No prevaccination testing (e.g., Pap or HPV testing) is recommended to establish
the appropriateness of HPV vaccination.
Cervical cancer screening. Cervical cancer screening guidelines and
recommendations should be followed.
https://www.cdc.gov/cancer/cervical/pdf/guidelines.pdf
Pregnancy. For persons who are pregnant, HPV vaccination should be delayed until
after pregnancy; however, pregnancy testing is not needed before vaccination.
Other recommendations have not changed
MMWR 2019; 68; 698-702 MMWR Recomm Rep 2014;63(No. RR-05)
28

Recommended number of HPV vaccine doses
and dosing schedule, United States
Population
Number of
vaccine doses
Interval between
doses
Persons initiating vaccination at 9 through 14
years, except immunocompromised persons
2 0, 6–12 months
Persons in the recommended age groups
initiating vaccination at age 15 or older and
persons with immunocompromising conditions
3 0, 1–2, 6 months
• No maximum interval between doses; schedule does not need to be restarted
if there is longer than recommended number of months between doses
29
In a 2-dose schedule of HPV vaccine, the minimum interval between first and second doses is 5 months. In a 3-dose schedule of HPV vaccine, the minimum intervals are 4
weeks between the first and second doses, 12 weeks between the second and third doses, and 5 months between the first and third doses

Countries with HPV vaccine in the
national immunization program, 2019

Current global HPV vaccine
demand/supply imbalance
World Health Organization recommendations
– 2009 - HPV vaccination of girls for single age cohort of girls
– 2016 - Multi-age cohort vaccination (age 9-14 years in first year)
– Increased vaccine demand
HPV vaccine demand/supply imbalance
– Projected to last 3-5 years
– Delay introduction in some countries
– Prevent multi-age cohort vaccination
No HPV vaccine shortage anticipated in United States
WHO has issued recommendations for more equitable,
global allocation of the limited HPV vaccine supply
https://www.who.int/immunization/programmes_systems/procurement/v3p/platform/module2/WHO_HPV_market_study_public_summary.pdf
https://www.who.int/wer/2019/wer9447/en/

Summary
Adolescents remain the focus of US HPV vaccination program.
HPV vaccination is most effective when given before exposure to any HPV
Changes in recommendations in 2019 include:
Catch-up harmonized across genders through age 26 years.
– Simplifies the immunization schedule and may be more feasible to implement.
Shared clinical decision-making for some persons aged 27 through 45 years.
– Providers do not need to discuss HPV vaccination with most adults > age 26 years.
– CDC is not actively promoting vaccination of adults > 26 years.
32

Future considerations
ACIP reviews relevant data as they become available and updates vaccine
policy as needed.
33

Questions?

For more information, contact CDC
1-800-CDC-INFO (232-4636)
TTY: 1-888-232-6348 www.cdc.gov
The findings and conclusions in this report are those of the authors and do not necessarily represent the
official position of the Centers for Disease Control and Prevention.
Thank You
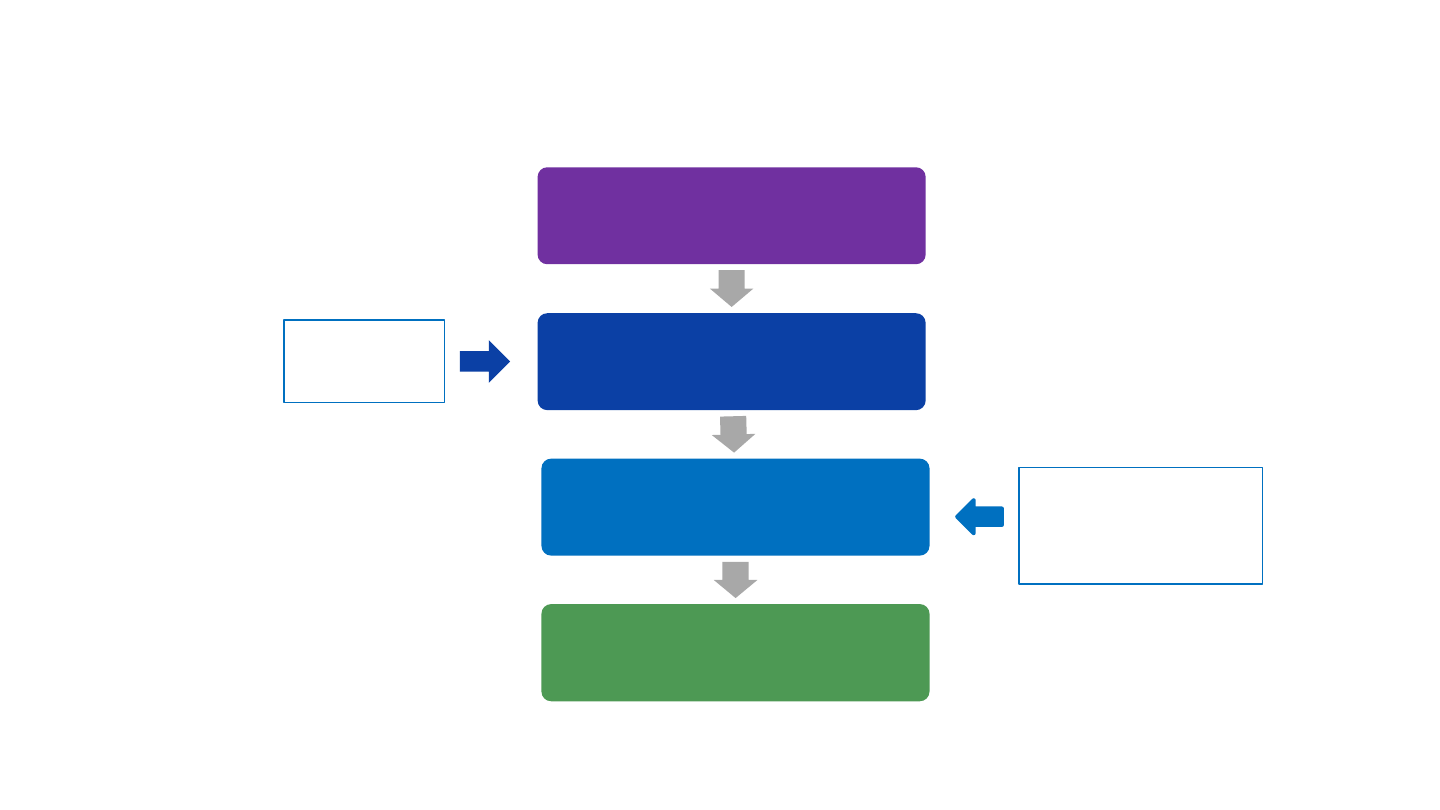
Vaccine regulatory approval and recommendations
Data from clinical trials
submitted to FDA
FDA licensure
Advisory Committee on
Immunization Practices (ACIP)
recommendations
CDC acceptance and
publication in MMWR
• Efficacy
• Safety
Evidence to
Recommendations
Framework
FDA, Food and Drug Administration; MMWR, Morbidity and Mortality Weekly Report
36
