
State Operations Manual
Appendix A - Survey Protocol,
Regulations and Interpretive Guidelines for Hospitals
Table of Contents
(Rev. 220; Issued:04-19-24)
Transmittals for Appendix A
Survey Protocol
Introduction
Task 1 - Off-Site Survey Preparation
Task 2 - Entrance Activities
Task 3 - Information Gathering/Investigation
Task 4 - Preliminary Decision Making and Analysis of Findings
Task 5 - Exit Conference
Task 6 – Post-Survey Activities
Psychiatric Hospital Survey Module
Psychiatric Unit Survey Module
Rehabilitation Hospital Survey Module
Inpatient Rehabilitation Unit Survey Module
Hospital Swing-Bed Survey Module
Regulations and Interpretive Guidelines
§482.1 Basis and Scope
§482.2 Provision of Emergency Services by Nonparticipating Hospitals
§482.11 Condition of Participation: Compliance with Federal, State and Local Laws
§482.12 Condition of Participation: Governing Body
§482.13 Condition of Participation: Patient's Rights
§482.21 Condition of Participation: Quality Assessment and Performance Improvement
Program
§482.22 Condition of Participation: Medical staff
§482.23 Condition of Participation: Nursing Services
§482.24 Condition of Participation: Medical Record Services
§482.25 Condition of Participation: Pharmaceutical Services
§482.26 Condition of Participation: Radiologic Services
§482.27 Condition of Participation: Laboratory Services
§482.28 Condition of Participation: Food and Dietetic Services
§482.30 Condition of Participation: Utilization Review
§482.41 Condition of Participation: Physical Environment
§482.42 Condition of Participation: Infection Prevention and Control and Antibiotic
Stewardship Programs
§482.43 Condition of Participation: Discharge Planning
§482.45 Condition of Participation: Organ, Tissue and Eye Procurement
§482.51 Condition of Participation: Surgical Services
§482.52 Condition of Participation: Anesthesia Services
§482.53 Condition of Participation: Nuclear Medicine Services
§482.54 Condition of Participation: Outpatient Services
§482.55 Condition of Participation: Emergency Services
§482.56 Condition of Participation: Rehabilitation Services
§482.57 Condition of Participation: Respiratory Services
§482.60 Condition of Participation: Special provisions applying to psychiatric hospitals
§482.61 Condition of Participation: Special medical record requirements for psychiatric
hospitals
§482.62 Condition of Participation: Special staff requirements for psychiatric hospitals
Survey Protocol
Introduction
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
Hospitals are required to be in compliance with the Federal requirements set forth in the
Medicare Conditions of Participation (CoP) in order to receive Medicare/Medicaid
payment. The goal of a hospital survey is to determine if the hospital is in compliance with
the CoP set forth at 42 CFR Part 482. Also, where appropriate, the hospital must be in
compliance with the PPS exclusionary criteria at 42 CFR 412.20 Subpart B and the
swing-bed requirements at 42 CFR 482.66
Certification of hospital compliance with the CoP is accomplished through observations,
interviews, and document/record reviews. The survey process focuses on a hospital’s
performance of patient-focused and organizational functions and processes. The hospital
survey is the means used to assess compliance with Federal health, safety, and quality
standards that will assure that the beneficiary receives safe, quality care and services.
Regulatory and Policy Reference
• The Medicare Conditions of Participation for hospitals are found at 42CFR Part 482.
• Survey authority and compliance regulations can be found at 42 CFR Part 488
Subpart A.
• Should an individual or entity (hospital) refuse to allow immediate access upon
reasonable request to either a State Agency or CMS surveyor, the Office of the
Inspector General (OIG) may exclude the hospital from participation in all Federal
healthcare programs in accordance with 42 CFR 1001.1301.
• The regulatory authority for the photocopying of records and information during the
survey is found at 42 CFR 489.53(a)(13).
• The CMS State Operations Manual (SOM) provides CMS policy regarding survey
and certification activities.
Surveyors assess the hospital’s compliance with the CoP for all services, areas and
locations in which the provider receives reimbursement for patient care services billed
under its provider number.
Although the survey generally occurs during daytime working hours (Monday through
Friday), surveyors may conduct the survey at other times. This may include weekends and
times outside of normal daytime (Monday through Friday) working hours. When the
survey begins at times outside of normal work times, the survey team modifies the survey,
if needed, in recognition of patients’ activities and the staff available.
All hospital surveys are unannounced. Do not provide hospitals with advance notice of the
survey.
Tasks in the Survey Protocol
Listed below, and discussed in this document, are the tasks that comprise the survey
protocol for hospital.
Task 1
Off-Site Survey Preparation
Task 2
Entrance Activities
Task 3
Information Gathering/ Investigation
Task 4
Preliminary Decision Making and Analysis of Findings
Task 5
Exit Conference
Task 6
Post-Survey Activities
Survey Modules for Specialized Hospital Services
The modules for PPS-exempt units (psychiatric and rehabilitation), psychiatric hospitals,
rehabilitation hospitals and swing-bed hospitals are attached to this document. The survey
team is expected to use all the modules that apply to the hospital being surveyed. For
example, if the hospital has swing-beds, a PPS excluded rehabilitation unit, and a PPS
excluded psychiatric unit, the team will use those three modules in addition to this protocol
to conduct the survey. If the hospital is a rehabilitation hospital, the team will use the
rehabilitation hospital module in addition to this protocol to conduct the survey. If the
hospital is a psychiatric hospital and if the survey team will be assessing the hospital’s
compliance with both the hospital CoPs and psychiatric hospital special conditions, the
team will use the psychiatric hospital module in addition to this protocol to conduct the
survey.
Survey Team
Size and Composition
The SA (or the RO for Federal teams) decides the composition and size of the team. In
general, a suggested survey team for a full survey of a mid-size hospital would include two-
four surveyors who will be at the facility for 3 or more days. Each hospital survey team
should include at least one RN with hospital survey experience, as well as other surveyors
who have the expertise needed to determine whether the facility is in compliance. Survey
team size and composition are normally based on the following factors:
• Size of the facility to be surveyed, based on average daily census;
• Complexity of services offered, including outpatient services;
• Type of survey to be conducted;
• Whether the facility has special care units or off-site clinics or locations;
• Whether the facility has a historical pattern of serious deficiencies or complaints;
and
• Whether new surveyors are to accompany a team as part of their training.
Training for Hospital Surveyors
Hospital surveyors should have the necessary training and experience to conduct a hospital
survey. Attendance at a Basic Hospital Surveyor Training Course is suggested. New
surveyors may accompany the team as part of their training prior to completing the Basic
Hospital Surveyor Training Course.
Team Coordinator
The survey is conducted under the leadership of a team coordinator. The SA (or the RO for
Federal teams) should designate the team coordinator. The team coordinator is responsible
for assuring that all survey preparation and survey activities are completed within the
specified time frames and in a manner consistent with this protocol, SOM, and SA
procedures. Responsibilities of the team coordinator include:
• Scheduling the date and time of survey activities;
• Acting as the spokesperson for the team;
• Assigning staff to areas of the hospital or tasks for the survey;
• Facilitating time management;
• Encouraging on-going communication among team members;
• Evaluating team progress and coordinating daily team meetings;
• Coordinating any ongoing conferences with hospital leadership (as determined
appropriate by the circumstances and SA/RO policy) and providing on-going
feedback, as appropriate, to hospital leadership on the status of the survey;
• Coordinating Task 2, Entrance Conference;
• Facilitating Task 4, Preliminary Decision Making;
• Coordinating Task 5, Exit Conference; and
• Coordinating the preparation of the Form CMS-2567.
Task 1 - Off-Site Survey Preparation
General Objective
The objective of this task is to analyze information about the provider in order to identify
areas of potential concern to be investigated during the survey and to determine if those
areas, or any special features of the provider (e.g., provider-based clinics, remote locations,
satellites, specialty units, PPS-exempt units, services offered, etc.) require the addition of
any specialty surveyors to the team. Information obtained about the provider will also
allow the SA (or the RO for Federal teams) to determine survey team size and composition,
and to develop a preliminary survey plan. The type of provider information needed
includes:
• Information from the provider file (to be updated on the survey using the
Hospital/CAH Medicare Database Worksheet), such as the facility’s ownership, the
type(s) of services offered, any prospective payment system (PPS) exclusion(s),
whether the facility is a provider of swing-bed services, and the number, type and
location of any off-site locations;
• Previous Federal and state survey results for patterns, number, and nature of
deficiencies, as well as the number, frequency, and types of complaint investigations
and the findings;
• Information from CMS databases available to the SA and CMS. Note the exit date
of the most recent survey;
• Waivers and variances, if they exist. Determine if there are any applicable survey
directive(s) from the SA or the CMS Regional Office (RO); and
• Any additional information available about the facility (e.g., the hospital’s Web site,
any media reports about the hospital, etc).
Off-Site Survey Preparation Team Meeting
The team should prepare for the survey offsite so they are ready to begin the survey
immediately upon entering the facility. The team coordinator should arrange an off-site
preparation meeting with as many team members as possible, including specialty surveyors.
This meeting may be a conference call if necessary.
During the meeting, discuss at least the following:
• Information gathered by the team coordinator;
• Significant information from the CMS databases that are reviewed;
• Update and clarify information from the provider file so a surveyor can update the
Medicare database using the “Hospital/CAH Medicare Database Worksheet,”
Exhibit 286;
• Layout of the facility (if available);
• Preliminary team member assignments;
• Date, location and time team members will meet to enter the facility;
• The time for the daily team meetings; and
• Potential date and time of the exit conference.
Gather copies of resources that may be needed. These may include:
• Medicare Hospital CoP and Interpretive Guidelines (Appendix A);
• Survey protocol and modules;
• Immediate Jeopardy (Appendix Q);
• Responsibilities of Medicare Participating Hospitals in Emergency Cases
(Appendix V);
• Hospital Swing-Bed Regulations and Interpretive Guidelines (Appendix T);
• Hospital/CAH Medicare Database Worksheet, Exhibit 286;
• Exhibit 287, Authorization by Deemed Provider/Supplier Selected for
Accreditation Organization Validation Survey; and
• Worksheets for swing-bed, PPS exclusions, and restraint/seclusion death reporting.
Task 2 - Entrance Activities
General Objectives
The objectives of this task are to explain the survey process to the hospital and obtain the
information needed to conduct the survey.
General Procedures
Arrival
The entire survey team should enter the hospital together. Upon arrival, surveyors should
present their identification. The team coordinator should announce to the Administrator, or
whoever is in charge, that a survey is being conducted. If the Administrator (or person in
charge) is not onsite or available (e.g., if the survey begins outside normal daytime
Monday-Friday working hours), ask that they be notified that a survey is being conducted.
Do not delay the survey because the Administrator or other hospital staff is/are not on site
or available.
Entrance Conference
The entrance conference sets the tone for the entire survey. Be prepared and courteous, and
make requests, not demands. The entrance conference should be informative, concise, and
brief; it should not utilize a significant amount of time. Conduct the entrance conference
with hospital administrative staff that is available at the time of entrance. During the
entrance conference, the Team Coordinator should address the following:
• Explain the purpose and scope of the survey;
• Briefly explain the survey process;
• Introduce survey team members, including any additional surveyors who may join
the team at a later time, the general area that each will be responsible for, and the
various documents that they may request;
• Clarify that all hospital areas and locations, departments, and patient care settings
under the hospital provider number may be surveyed, including any contracted
patient care activities or patient services located on hospital campuses or hospital
provider based locations;
• Explain that all interviews will be conducted privately with patients, staff, and
visitors, unless requested otherwise by the interviewee;
• Discuss and determine how the facility will ensure that surveyors are able to obtain
the photocopies of material, records, and other information as they are needed;
• Obtain the names, locations, and telephone numbers of key staff to whom questions
should be addressed;
• Discuss the approximate time, location, and possible attendees of any meetings to be
held during the survey. The team coordinator should coordinate any meetings with
facility leadership; and
• Propose a preliminary date and time for the exit conference.
During the entrance conference, the Team Coordinator will arrange with the hospital
administrator, or available hospital administrative supervisory staff if he/she is unavailable
to obtain the following:
• A location (e.g., conference room) where the team may meet privately during the
survey;
• A telephone for team communications, preferably in the team meeting location;
• A list of current inpatients, providing each patient’s name, room number,
diagnosis(es), admission date, age, attending physician, and other significant
information as it applies to that patient. The team coordinator will explain to the
hospital that in order to complete the survey within the allotted time it is important
the survey team is given this information as soon as possible, and request that it be
no later than 3 hours after the request is made. SAs may develop a worksheet to give
to the facility for obtaining this information;
• A list of department heads with their locations and telephone numbers;
• A copy of the facility’s organizational chart;
• The names and addresses of all off-site locations operating under the same provider
number;
• The hospital’s infection control plan;
• A list of employees;
• The medical staff bylaws and rules and regulations;
• A list of contracted services; and
• A copy of the facility’s floor plan, indicating the location of patient care and
treatment areas;
Arrange an interview with a member of the administrative staff to complete the
Hospital/CAH Medicare Database Worksheet that will be used to update the provider’s file
in the Medicare database. The worksheet may not be given to hospital personnel for
completion.
Hospital Tours
Guided tours of the hospital are not encouraged and should be avoided. While a tour of a
small facility may take place in less than one-man hour, a tour of a large facility could
consume several man hours of allocated survey time and resources that are needed to
conduct the survey.
Initial On-Site Team Meeting
After the conclusion of the Entrance Conference, the team will meet in order to evaluate
information gathered, and modify surveyor assignments, as necessary. Do not delay the
continuation of the survey process waiting for information from the provider, but adjust
survey activities as necessary. During the on-site team meeting, team members should:
• Review the scope of hospital services;
• Identify hospital locations to be surveyed, including any off-site locations;
• Add survey protocol modules and adjust surveyor assignments, as necessary, based
on new information;
• Discuss issues such as change of ownership, sentinel events, construction activities,
and disasters, if they have been reported;
• Make an initial patient sample selection (The patient list may not be available
immediately after the entrance conference, therefore the team may delay completing
the initial patient sample selection a few hours as meets the needs of the survey
team); and
• Set the next meeting time and date.
Sample Size and Selection
To select the patient sample, review the patient list provided by the hospital and select
patients who represent a cross-section of the patient population and the services provided.
Patient logs (ER, OB, OR, restraint, etc) may be used in conjunction with the patient list to
assure the sample is reflective of the scope of services provided by the hospital.
Whenever possible and appropriate, select patients who are in the facility during the time of
survey (i.e., open records). Open records allow surveyors to conduct a patient-focused
survey and enable surveyors to validate the information obtained through record reviews
with observations and patient and staff interviews. There may be situations where closed
records are needed to supplement the open records reviewed (e.g., too few open records,
complaint investigation, etc), surveyors should use their professional judgment in these
situations and select sample that will enable them to make compliance determinations. If it
is necessary to remove a patient from the sample during the survey, (e.g., the patient refuses
to participate in an interview), replace the patient with another who fits a similar profile.
This should be done as soon as possible in the survey.
Select the number of patient records for review based on the facility’s average daily census.
The sample should be at least 10 percent of the average daily census, but not fewer than 30
inpatient records. For small general hospitals (this reduction does not apply to surgical or
other specialty hospitals) with an average daily census of 20 patients or less, the sample
should not be fewer than 20 inpatient records, provided that number of records is adequate
to determine compliance. Within the sample, select at least one patient from each nursing
unit (e.g., med/surg, ICU, OB, pediatrics, specialty units, etc). In addition to the inpatient
sample, select a sample of outpatients in order to determine compliance in outpatient
departments, services, and locations. The sample size may be expanded as needed to assess
the hospital’s compliance with the CoP.
If a complaint is being investigated during the survey, include patients who have been
identified as part of the complaint in the sample. Issues or concerns identified through
complaints may be an area of focus when selecting the patient sample.
Give each patient in the sample a unique identifier. Appropriate identifiable information
should be kept on a separate identifier list. Do not use medical record numbers, Social
Security numbers, care unit or billing record numbers to identify patients.
To conduct an initial survey of a hospital there must be enough inpatients currently in the
hospital and patient records (open and closed) for surveyors to determine whether the
hospital can demonstrate compliance with all the applicable CoP. The number of current
and discharged inpatients and outpatients in relation to the complexity of care provided to
patients and the length of stay of those patients needs to be large enough for surveyors to
evaluate the manner and degree to which the hospital satisfies all the standards within each
CoP including any CoP applying to optional services offered by the hospital. Utilize the
same sample size and selection methods as previously discussed.
Task 3 - Information Gathering/Investigation

General Objective
The objective of this task is to determine the hospital’s compliance with the Medicare CoP
through observations, interviews, and document review.
Guiding Principles
• Focus attention on actual and potential patient outcomes, as well as required
processes.
• Assess the care and services provided, including the appropriateness of the care and
services within the context of the regulations.
• Visit patient care settings, including inpatient units, outpatient clinics, anesthetizing
locations, emergency departments, imaging, rehabilitation, remote locations,
satellites, etc.
• Observe the actual provision of care and services to patients and the effects of that
care, in order to assess whether the care provided meets the needs of the individual
patient.
• Use the interpretive guidelines and other published CMS policy statements to guide
the survey.
• Use Appendix Q for guidance if Immediate Jeopardy is suspected.
General Procedures
Survey Locations
For hospitals with either no or a small number of off-campus provider-based locations,
survey all departments, services, and locations that bill for services under the hospital’s
provider number and are considered part of the hospital.
For hospitals with many provider-based locations survey:
• All hospital departments and services at the primary hospital campus and on the
campuses of other remote locations of the hospital;
• All satellite locations of the hospital;
• All inpatient care locations of the hospital;
• All out-patient surgery locations of the hospital;
• All locations where complex out-patient care is provided by the hospital; and
• Select a sample of each type of other services provided at additional provider-
based locations.
On any Medicare hospital survey, contracted patient care activities or patient services (such
as dietary services, treatment services, diagnostic services, etc.) located on hospital
campuses or hospital provider based locations should be surveyed as part of the hospital for
compliance with the conditions of participation.
During the Survey
• Observe what activities are taking place and assess the CoP that represent the scope
and complexity of the patient care services located at each location, as well as, any
other CoP that apply to those locations. Expand the survey activities as necessary.
• The SA and surveyors have discretion whether to allow, or to refuse to allow,
facility personnel to accompany the surveyors during a survey. Surveyors should
make a decision whether to allow facility personnel to accompany them based on
the circumstances at the time of the survey.
• The team should meet at least daily in order to assess the status of the survey,
progress of completion of assigned tasks, areas of concern, and to identify areas for
additional investigations. The team meetings should include an update by each
surveyor that addresses findings and areas of concern that have been identified. If
areas of concern are identified in the discussion, the team should coordinate efforts
to obtain additional information. Additional team meetings can be called at any time
during the survey to discuss crucial problems or issues.
• All significant issues or significant adverse events must be brought to the team
coordinator’s attention immediately.
• Maintain open and ongoing dialogue with the facility staff throughout the survey
process. Conferences with facility staff may be held in order to inform them of
survey findings. This affords facility staff the opportunity to present additional
information or to offer explanations concerning identified issues. Survey
information must not be discussed unless the investigation process and data
collection for the specific concerns is completed.
• Surveyors should always maintain a professional working relationship with facility
staff.
• Surveyors need to respect patient privacy and maintain patient confidentiality at all
times during the survey.
• Surveyors should maintain their role as representatives of a regulatory agency.
Although non-consultative information may be provided upon request, the surveyor
is not a consultant.
Patient Review
A comprehensive review of care and services received by each patient in the sample should
be part of the hospital survey. A comprehensive review includes observations of
care/services provided to the patient, patient and/or family interview(s), staff interview(s),
and medical record review. After obtaining the patient’s permission, observe each sample
patient receiving treatments (e.g., intravenous therapy, tube feedings, wound dressing
changes) and observe the care provided in a variety of treatment settings, as necessary, to
determine if patient needs are met.
Observations
Observations provide first-hand knowledge of hospital practice. The regulations and
interpretive guidelines offer guidance for conducting observations. Observation of the care
environment provides valuable information about how the care delivery system works and
how hospital departments work together to provide care. Surveyors are encouraged to
make observations, complete interviews, and review records and policies/procedures by
stationing themselves as physically close to patient care as possible. While completing a
chart review, for instance, it may be possible to also observe the environment and the
patients, as far as care being given, staff interactions with patients, safety hazards, and
infection control practices. When conducting observations, particular attention should be
given to the following:
• Patient care, including treatments and therapies in all patient care settings;
• Staff member activities, equipment, documentation, building structure, sounds and
smells;
• People, care, activities, processes, documentation, policies, equipment, etc., that are
present that should not be present, as well as, those that are not present that should
be present;
• Integration of all services, such that the facility is functioning as one integrated
whole;
• Whether quality assessment and performance improvement (QAPI) is a facility-
wide activity, incorporating every service and activity of the provider and whether
every facility department and activity reports to, and receives reports from, the
facility’s central organized body managing the facility-wide QAPI program; and
• Storage, security and confidentiality of medical records.
A surveyor should take complete notes of all observations and should document: the date
and time of the observation(s); location; patient identifiers, individuals present during the
observation, and the activity being observed (e.g., therapy, treatment modality, etc).
A surveyor should have observations verified by the patient, family, facility staff, other
survey team member(s), or by another mechanism. For example, when finding an out-dated
medication in the pharmacy, ask the pharmacist to verify that the drug is out-dated. In
addition, a surveyor should integrate the data from observations with data gathered through
interviews and document reviews.
Surveyors must not examine patients by themselves, although in certain circumstances, in
order to determine a patient’s health status and whether appropriate health care is being
provided, especially to ensure a patient’s welfare where he/she appears to be in immediate
jeopardy, it is permissible and necessary to examine the patient. After obtaining permission
from the patient, the surveyor should request that a staff member of the facility examine the
patient in the surveyor’s presence. The health and dignity of the patient is always of
paramount concern. A surveyor must respect the patient’s right to refuse to be examined.
Interviews
Interviews provide a method to collect information, and to verify and validate information
obtained through observations. Informal interviews should be conducted throughout the
duration of the survey. Use the information obtained from interviews to determine what
additional observations, interviews, and record reviews are necessary. When conducting
interviews, observe the following:
• Maintain detailed documentation of each interview conducted. Document the
interview date, time, and location; the full name and title of the person
interviewed; and key points made and/or topics discussed. To the extent
possible, document quotes from the interviewee.
• Interviews with facility staff should be brief. Use a few well-phrased questions
to elicit the desired information. For example, to determine if a staff member is
aware of disaster procedures and his/her role in such events, simply ask, “If you
smelled smoke, what would you do?”
• When interviewing staff, begin your interviews with staff that work most closely
with the patient.
• Conduct patient interviews regarding their knowledge of their plan of care, the
implementation of the plan, and the quality of the services received. Other
topics for patient or family interview may include patient rights, advanced
directives, and the facility’s grievance/complaint procedure.
• Interviews with patients must be conducted in privacy and with the patient’s
prior permission.
• Use open-ended questions during your interview.
• Validate all information obtained.
• Telephone interviews may be conducted if necessary, but a preference should be
made for in- person interviews.
• Integrate the data from interviews with data gathered through observations and
document reviews.
Staff interviews should gather information about the staff’s knowledge of the patient’s
needs, plan of care, and progress toward goals. Problems or concerns identified during a
patient or family interview should be addressed in the staff interview in order to validate the
patient’s perception, or to gather additional information.
Patient interviews should include questions specific to the patient’s condition, reason for
hospital admission, quality of care received, and the patients knowledge of their plan of
care. For instance, a surgical patient should be questioned about the process for preparation
for surgery, the patient’s knowledge of and consent for the procedure, pre-operative patient
teaching, post-operative patient goals and discharge plan.
Document Review
Document review focuses on a facility’s compliance with the CoP. When conducting a
document review, document the source and date of the information obtained. When making
document copies, identify the original date of the document and indicate the date and time
the copies were made. Once a document review is completed, integrate the data obtained
with data gathered through observations and interviews to decide if the hospital is in
compliance with the CoP. Documents reviewed may be both written and electronic and
include the following:
• Patient’s clinical records, to validate information gained during the interviews, as
well as for evidence of advanced directives, discharge planning instructions, and
patient teaching. This review will provide a broad picture of the patient’s care.
Plans of care and discharge plans should be initiated immediately upon admission,
and be modified as patient care needs change. The record review for that patient
who has undergone surgery would include a review of the pre-surgical assessment,
informed consent, operative report, and pre-, inter-, and post-operative anesthesia
notes. Although team members may have a specific area assigned during the
survey, the team should avoid duplication of efforts during review of medical
records and each surveyor should review the record as a whole instead of targeting
the assigned area of concern. Surveyors should use open patient records rather than
closed records, whenever possible;
• Closed medical records may be used to determine past practice, and the scope or
frequency of a deficient practice. Closed records should also be reviewed to provide
information about services that are not being provided by the hospital at the time of
the survey. For example, if there are no obstetrical patients in the facility at the
time of the survey, review closed OB records to determine care practices, or to
evaluate past activities that cannot be evaluated using open records. In the review of
closed clinical records, review all selected medical records for an integrated plan of
care, timelines of implementation of the plan of care, and the patient responses to
the interventions.
• Personnel files to determine if staff members have the appropriate educational
requirements, have had the necessary training required, and are licensed, if it is
required;
• Credential files to determine if the facility complies with CMS requirements and
State law, as well as, follows its own written policies for medical staff privileges
and credentialing;
• Maintenance records to determine if equipment is periodically examined and to
determine if it is in good working order and if environmental requirements have
been met;
• Staffing documents to determine if adequate numbers of staff are provided
according to the number and acuity of patients;
• Policy and procedure manuals. When reviewing policy and procedure manuals,
verify with the person in charge of an area that the policy and procedure manuals are
current; and
• Contracts, if applicable, to determine if patient care, governing body, QAPI, and
other CoP requirements are included.
Photocopies
Surveyors should make photocopies of all documents needed to support survey findings.
The surveyor needs access to a photocopier where he/she can make their own photocopies
of needed documents. If requested by the hospital, the surveyor should make the hospital a
copy of all items photocopied. All photocopies need to be dated and timed as to when
photocopied, and identified such as “hospital restraint policy- 2/17/04 page 3” or “Patient #
6, progress note- 2/17/04.”
Completion of Hospital/CAH Medicare Database Worksheet
Arrange an interview with a member of the administrative staff to update and clarify
information from the provider file. The Hospital/CAH Medicare Database Worksheet will
be used to collect information about the hospital’s services, locations, and staffing by
Medicare surveyors during hospital surveys. The worksheet will be completed by the
surveyors using observation, staff interviews, and document review. The worksheet will
not be given to hospital staff to complete. The worksheet is used to collect information that
will later be entered into the Medicare database. During the interview clarify any
inconsistencies from prior information or information gathered during the survey.
Task 4 - Preliminary Decision Making and Analysis of Findings
General Objectives
The general objectives of this task are to integrate findings, review and analyze all
information collected from observations, interviews, and record reviews, and to determine
whether or not the hospital meets the Conditions of Participation found at 42 CFR Part 482
and, as appropriate, the PPS exclusionary criteria at 42 CFR Part 412 Subpart B, and the
swing-bed requirements at 42 CFR 482.66. The team’s preliminary decision-making and
analysis of findings assist it in preparing the exit conference report. Based on the team’s
decisions, additional activities may need to be initiated.
General Procedures
Preparation
Prior to beginning this Task, each team member should review his/her notes, worksheets,
records, observations, interviews, and document reviews to assure that all investigations are
complete and organized for presentation to the team.
Discussion Meeting
At this meeting, the surveyors will share their findings, evaluate the evidence, and make
team decisions regarding compliance with each requirement. Proceed sequentially through
the requirements for each condition appropriate to the facility as they appear in regulation.
For any issues of noncompliance, the team needs to reach a consensus. Decisions about
deficiencies are to be team decisions, with each member having input. The team should
document their decisions, the substance of the evidence, and the numbers of patients
impacted, in order to identify the extent of facility noncompliance. The team must ensure
that their findings are supported by adequate documentation of observations, interviews and
document reviews and includes any needed evidence such as photocopies. Any additional
documentation or evidence needed to support identified non compliance should be gathered
prior to the exit conference but at a minimum, prior to exiting the hospital.
Determining the Severity of Deficiencies
The regulations at 42 CFR 488.26 state, “The decision as to whether there is compliance
with a particular requirement, condition of participation, or condition for coverage, depends
upon the manner and degree to which the provider or supplier satisfies the various standards
within each condition.” When noncompliance with a condition of participation is noted, the
determination of whether a lack of compliance is at the Standard or Condition level depends
upon the nature (how severe, how dangerous, how critical, etc.) and extent (how prevalent,
how many, how pervasive, how often, etc.) of the lack of compliance. The cited level of
the noncompliance is determined by the interrelationship between the nature and extent of
the noncompliance.
A deficiency at the Condition level may be due to noncompliance with requirements in a
single standard or several standards within the condition, or with requirements of
noncompliance with a single part (tag) representing a severe or critical health or safety
breach. Even a seemingly small breach in critical actions or at critical times can kill or
severely injure a patient, and represents a critical or severe health or safety threat.
A deficiency is at the Standard level when there is noncompliance with any single
requirement or several requirements within a particular standard that are not of such
character as to substantially limit a facility’s capacity to furnish adequate care, or which
would not jeopardize or adversely affect the health or safety of patients if the deficient
practice recurred.
When a deficient practice (noncompliance) is determined to have taken place prior to the
survey and the hospital states that it has corrected the deficient practice/issue
(noncompliance), issues for the survey team to consider would include:
• Is the corrective action superficial or inadequate, or is the corrective action adequate
and systemic?
• Has the hospital implemented the corrective intervention(s) or action(s)?
• Has the hospital taken a QAPI approach to the corrective action to ensure
monitoring, tracking and sustainability?
The survey team uses their judgment to determine if any action(s) taken by the hospital
prior to the survey is sufficient to correct the noncompliance and to prevent the deficient
practice from continuing or recurring. If the deficient practice is corrected prior to the
survey, do not cite noncompliance. However, if the noncompliance with any requirements
is noted during the survey, even when the hospital corrects the noncompliance during the
survey, cite noncompliance.
All noted noncompliance must be cited even when corrected on site during the survey.
Citing noncompliance at the appropriate level is important to the integrity of the survey
process. Citing too high a level is unfair to the hospital. Citing noncompliance at a level
below the noted degree and manner of the noncompliance does not ensure that the hospital
will develop acceptable plans of correction and implement corrective actions, and does not
depict accurately whether the care provided adversely affects the health and safety of
patients; and continued deficient practices may lead to adverse patient outcomes such as
injury or death.
Gathering Additional Information
If it is determined that the survey team needs additional information to determine facility
compliance or noncompliance, the team coordinator should decide the best way to conduct
the additional review.
Task 5 - Exit Conference
General Objective
The general objective of this task is to inform the facility staff of the team’s preliminary
findings.
Prior to the Exit Conference
• The team coordinator is responsible for organization of the presentation of the exit.
• The team determines who will present the findings.
• If the team feels it may encounter a problem during the exit, they should contact
their immediate supervisor.
Discontinuation of an Exit Conference
It is CMS’ general policy to conduct an exit conference at the conclusion of each survey.
However, there are some situations that justify refusal to continue or to conduct an exit
conference. For example:
• If the provider is represented by counsel (all participants in the exit conference
should identify themselves), surveyors may refuse to conduct the conference if the
lawyer tries to turn it into an evidentiary hearing; or
• Any time the provider creates an environment that is hostile, intimidating, or
inconsistent with the informal and preliminary nature of an exit conference,
surveyors may refuse to conduct or continue the conference. Under such
circumstances, it is suggested that the team coordinator stop the exit conference and
call the State agency for further direction.
Recording the Exit Conference
If the facility wishes to audio tape the conference, it must provide two tapes and tape
recorders, recording the meeting simultaneously. The surveyors should take one of the tapes
at the conclusion of the conference. Video taping is also permitted if it is not disruptive to
the conference, and a copy is provided at the conclusion of the conference. It is at the sole
discretion of the surveyor(s) to determine if video taping is permitted.
General Principles
The following general principles apply when conducting an exit conference:
• The facility determines which hospital staff will attend the exit conference.
• The identity of an individual patient or staff member must not be revealed in
discussing survey results. Identity includes not just the name of an individual
patient or staff member, but also includes any reference by which identity might be
deduced.
• Because of the ongoing dialogue between surveyors and facility staff during the
survey, there should be few instances in which the facility is unaware of surveyor
concerns or has not had an opportunity to present additional information prior to the
exit conference.
Exit Conference Sequence
The following discusses the sequence of events in conducting an exit conference.
Introductory Remarks:
• Thank everyone for cooperation during the survey.
• Introduce all team members, mentioning any that have concluded their portion of
the survey and have left the facility.
• Briefly mention the reason for the survey.
• Explain that the exit conference is an informal meeting to discuss preliminary
findings.
• Indicate that official findings are presented in writing on the Form CMS-2567.
Ground Rules
• Explain how the team will conduct the exit conference and any ground rules.
• Ground rules may include waiting until the surveyor finishes discussing each
deficiency before accepting comments from facility staff.
• State that the provider will have an opportunity to present new information after the
exit conference for consideration after the survey.

Presentation of Findings
• Avoid referring to data tag numbers.
• Present the findings of noncompliance, explaining why the findings are a violation.
If the provider asks for the regulatory basis, provide it.
• Refrain from making any general comments (e.g., “Overall the facility is very
good”). Stick to the facts. Do not rank findings. Treat requirements as equal as
possible.
• Do not identify unmet requirements as condition or standard level. Avoid
statements such as, “the condition was not met” or “the standard was not met.” It is
better to state “the requirement is not met.”
• If immediate jeopardy was identified, explain the significance and the need for
immediate correction. Follow instructions in Appendix Q.
• Assure that all findings are discussed at the exit conference.
Closure
• Explain that a statement of deficiencies (Form CMS-2567) will be mailed within 10
working days to the hospital.
• Explain that the Form CMS-2567 is the document disclosed to the public about the
facility’s deficiencies and what is being done to remedy them. The Form CMS-
2567 is made public no later than 90 calendar days following completion of the
survey. It documents specific deficiencies cited, the facility’s plans for correction
and timeframes, and it provides an opportunity for the facility to refute survey
findings and furnish documentation that requirements are met.
• Inform the facility that a written plan of correction must be submitted to the survey
agency within 10 calendar days following receipt of the written statement of
deficiencies.
• Explain the required characteristics of a plan of correction. The characteristics
include:
Corrective action to be taken for each individual affected by the deficient practice,
including any system changes that must be made;
• The position of the person who will monitor the corrective action and the
frequency of monitoring;
• Dates each corrective action will be completed;
• The administrator or appropriate individual must sign and date the Form
CMS-2567 before returning it to the survey agency; and
• The submitted plan of correction must meet the approval of the State agency,
or in some cases the CMS Regional Office for it to be acceptable.
• If the exit conference was audio or video taped, obtain a copy of the tape in its
entirety before leaving the facility.

All team members should leave the facility together immediately following the exit
conference. If the facility staff provides further information for review, the team
coordinator should decide the best way to conduct the further review. It is usually prudent
for at least two individuals to remain.
Task 6 – Post-Survey Activities
General Objective
The general objective of this task is to complete the survey and certification requirements,
in accordance with the regulations found at 42 CFR Part 488.
General Procedures
Each State agency and Federal Regional Office should follow directives in the State
Operations Manual. The procedures include:
• Timelines for completing each step of the process;
• Responsibilities of the team coordinator and other team members to complete the
Form CMS-2567, “Statement of Deficiencies,” following the “Principles of
Documentation”;
• Notification to the facility staff regarding survey results;
• Additional survey activities based on the survey results (e.g., revisit, forwarding
documents to the Regional Office for further action/direction);
• Completion of “Hospital Restraint/Seclusion Death Reporting Worksheet,” as
appropriate;
• Compilation of documents for the provider file;
• Signed Authorization by Deemed Provider/Supplier Selected for Accreditation
Organization Validation Survey is forwarded to RO; and
• Enter the information collected on the Hospital/CAH Medicare Database Worksheet
into the Medicare database.
Plan of Correction
Regulations at 42 CFR 488.28(a) allow certification of providers with deficiencies at the
Standard or Condition level “only if the facility has submitted an acceptable plan of
Correction [POC] for achieving compliance within a reasonable period of time acceptable
to the Secretary.” Failure to submit a POC may result in termination of the provider
agreement as authorized by 42 CFR 488.28(a) and §489.53(a)(1). After a POC is
submitted, the surveying entity makes the determination of the appropriateness of the POC.
Psychiatric Hospital Survey Module
Background
State survey agencies are given the responsibility for conducting surveys of psychiatric
hospitals to determine compliance with the two special conditions of participation.
However, most are not staffed with the personnel with the psychiatric expertise necessary to
conduct the surveys. In light of this, CMS has contracted out this function for many years,
and a panel of contracted psychiatric consultants has conducted the majority of surveys of
psychiatric hospitals.
General Procedures
When conducting a validation survey or other full survey of a psychiatric hospital using the
hospital A-tags located in Appendix A of the SOM, the SA is also expected to conduct a
review of the two special conditions of participation for psychiatric hospitals if:
• The State survey agency has the expertise (i.e., at a minimum a Masters prepared
psychiatric nurse or an RN with inpatient psychiatric nursing experience) to serve on
the team. The surveyors should conduct the survey of the two special conditions as
outlined in Appendix AA of the State Operations Manual.
It is strongly recommended that when the State survey agency does not have the clinical
expertise to conduct surveys of psychiatric hospitals, the SA should request, through its
regional office, the assistance of the contracted psychiatric surveyors.
Regulatory Authority and Requirements for Psychiatric Hospitals
The following regulations describe the special requirements for psychiatric hospitals:
• 42 CFR 482.60 -- Special provisions applying to psychiatric hospitals;
• 42 CFR 482.61 -- Special medical record requirements for psychiatric hospitals; and
• 42 CFR 482.62 -- Special staff requirements for psychiatric hospitals.
The focus of the survey is on the “outcome” experienced by the patient and how the
provider implements the plan of care. The survey process includes seven (7) tasks. When
these tasks are viewed in total, they give a clear indication of how the provider is meeting
or not meeting the requirements. Appendix AA of the State Operations Manual (SOM)
gives detailed instructions on how to perform these tasks. The seven tasks are:
• Representative sample of patients -- selection methodology;
• Record review of individuals in the sample;
• Other record review;
• Direct patient observation;
• Interviews;
• Visit to each area of the hospital serving certified patients; and
• Team assessment of compliance.
Survey Preparation When Surveying the Two Special Conditions
When the SA is preparing to perform a survey of the special conditions while conducting a
full survey (A-tags) of a psychiatric hospital, include in the preparation for the survey a
review of Appendix A of the SOM, “Interpretive Guidelines and Survey Procedures for
Hospitals,” and a review of Appendix AA, “Interpretive Guidelines and Survey Procedures
for Psychiatric Hospitals.” Appendix AA includes the following exhibits:

• Exhibit 1, “Medicare/Medicaid Psychiatric Hospital Survey Data,” Form CMS-724
(this form must be completed by the hospital and survey team.)
• Exhibit 2, “Surveyor Worksheet for Psychiatric Hospital Review: Two Special
Conditions,” Form CMS-725 (*optional)
• Exhibit 3, “CMS Death Record Review Data Sheet,” Form CMS-726 (*optional)
• Exhibit 4, “CMS Nursing Complement Data,” Form CMS-727 (*optional)
• Exhibit 5, “CMS Total Nursing Staff Data,” Form CMS-728 (*optional)
• Exhibit 6, “Data Collection Medical Staff Coverage,” Form CMS-729 (*optional)
*Although these forms are optional, they are useful for data collection purposes and will
assist in determining the hospital’s compliance status.
Identify all remote and satellite locations of the psychiatric hospital.
Survey Methods
Follow the interpretive guidelines and survey protocol in Appendix AA to determine
compliance with the two special conditions. As judged appropriate by the survey team,
patients selected for the survey of the two special conditions may be used to meet the
patient selection requirements in the hospital survey protocol. Surveyors should select
patients using the sample selection methodology in Appendix AA of the psychiatric
hospital survey protocol and, using the hospital survey protocol patient selection methods,
should select any remaining or additional patients needed to determine the hospital’s
compliance with the hospital CoP.
Identify any psychiatric hospital satellite(s), remote locations, or other provider based
location(s). Record the location, name, address, and telephone number for every satellite,
remote location, or provider based location on the Hospital/CAH Medicare Database
Worksheet for updating the Medicare database.
Post-Survey Activities
Follow the directions and procedures in the SOM for post-survey activities. The findings
for deficiencies noted for the A-tags and B-tags must be documented on separate Form
CMS-2567.
Psychiatric Unit Survey Module
When conducting a full survey of an accredited or non-accredited hospital that has a PPS
excluded psychiatric unit, conduct a survey of the psychiatric unit using the survey methods
in this module to assess the hospital’s compliance with the excluded psychiatric unit
requirements.
Excluded unit surveys utilizing these methods will count as annual validation compliance
surveys of the hospital’s self-attestation of compliance with the excluded psychiatric unit
requirements.
Background
The PPS excluded psychiatric unit is part of the hospital and is included as part of the
overall hospital survey. The term “exclusion” is a reimbursement term. Patient care in a

PPS excluded psychiatric unit is reimbursed at the PPS psychiatric unit excluded rate rather
than the hospital PPS rate. In order for a hospital to receive the excluded rate for
psychiatric care provided in its excluded unit, the unit must comply with the excluded
psychiatric unit requirements found at 42 CFR 412.27.
A PPS excluded psychiatric unit is regulated by both the hospital CoP at 42 CFR 482 (also
found in Appendix A of the SOM) and the PPS excluded psychiatric unit requirements at 42
CFR 412.27. The actual psychiatric unit requirements are based on the Special Conditions
of Participation for Psychiatric Hospitals found in §§482.60, 482.61, and 482.62.
Requirements for PPS Excluded Psychiatric Units
• 42 CFR 482 - Conditions of Participation for hospitals;
• 42 CFR 412.25 - Excluded hospital units: Common Requirements; and
• 42 CFR 412.27 - Excluded psychiatric units: Additional Requirements
Activities Conducted Prior to Psychiatric Unit Survey
• Contact the RO to determine if the hospital has approval for a PPS excluded
psychiatric unit.
• Contact the RO to determine the unit’s cost-reporting period.
• If possible, establish the location or locations of the psychiatric unit. Determine if
the unit has a satellite or satellites in other locations. Determination or verification
of this information may have to wait until the survey team is onsite.
• Do not conduct the survey of the PPS excluded psychiatric unit requirements within
90 days of the end of the hospital’s cost reporting period.
• Review the “Psychiatric Unit Criteria Worksheet,” Form CMS-437.
Survey Tool
The “Psychiatric Unit Criteria Worksheet,” Form CMS-437.
Survey Procedures for Determining Compliance with the PPS Excluded Psychiatric
Unit Requirements
• The surveyor of the psychiatric unit requirements should be an RN.
• Select 10 percent of the unit’s average daily census or a minimum of two patients
for the patient sample.
• The selected patients should be included in the patient sample used for the full
hospital survey.
• Hospital survey activities should be conducted concurrently with the survey of the
PPS excluded psychiatric unit requirements.
• Using the “Psychiatric Unit Criteria Worksheet,” Form CMS-437, verify whether
the requirements have been met by checking the appropriate boxes marked “YES”
and “NO.” Under the column “Explanatory Statement,” document specifics of the
findings. Additional findings can be documented in a narrative note that should be
attached to the worksheet.

• Select additional patients (open or closed records) as needed to determine
compliance with the excluded psychiatric unit requirements.
• If there are patients on the unit who were admitted the day before or on the day of
the survey, verify that all applicable requirements have been completed or are
already in place. These patients are in addition to the number of patients needed to
establish the minimum patient sample size.
• If there are no patients on the unit at the time the survey is conducted, review closed
patient records of unit patients treated within 6 months of the date of the survey.
• Identify if the psychiatric unit has a satellite or satellites. Record the location, name,
address, and telephone number for every satellite on the Hospital/CAH Medicare
Database Worksheet for updating the Medicare database.
Exit Conference
• Inform the hospital of findings of noncompliance with the excluded psychiatric unit
requirements.
• Inform the hospital that the SA will forward the completed CMS Form-437 to the
hospital at the same time as the completed CMS Form-2567.
Post-Survey Activities
• Do not include the survey findings of the excluded psychiatric unit requirements on
the Form CMS-2567 that is used to document the hospital survey findings.
• If there are requirements that have not been met, notify the RO. Document survey
findings of PPS psychiatric unit requirements on the Form CMS-437. Submit the
completed Form CMS-437 to the RO within the same time frame as the completion
of the Form CMS-2567 and at least 60 days prior to the end of the hospital’s cost
reporting period.
• Follow the procedures in the SOM for post-survey activities.
Rehabilitation Hospital Survey Module
When conducting a full survey of an accredited or non-accredited rehabilitation hospital,
conduct a survey of the hospital’s compliance with rehabilitation hospital excluded
requirements using the survey methods in this module.
Surveys of the PPS excluded rehabilitation hospital requirements utilizing these methods
will count as annual validation compliance surveys of the hospital’s self-attestation of
compliance with the excluded requirements.
Background
The term “exclusion” is a reimbursement term. Patient care in a PPS excluded
rehabilitation hospital is reimbursed at the PPS rehabilitation hospital excluded rate rather
than at the hospital PPS rate. In order for a hospital to receive the excluded rate for
rehabilitation care provided, the hospital must comply with the excluded requirements
found at 42 CFR 412.
A PPS excluded rehabilitation hospital is regulated by both the hospital CoP at 42 CFR 482

(also found in Appendix A of the SOM) and the PPS excluded rehabilitation hospital
requirements at 42 CFR 412.
Regulatory Authority and Requirements for PPS Excluded Rehabilitation Hospitals
• 42 CFR 482 - Conditions of Participation for Hospitals;
• 42 CFR 412.22 - Excluded hospitals and hospital units: General rules; and
• 42 CFR 412.23 - Excluded hospitals: Classifications
Activities Conducted Prior to a Rehabilitation Hospital Survey
• Contact the RO to determine if the hospital has approval for a PPS excluded
rehabilitation hospital.
• Contact the RO to determine the hospital’s cost reporting period.
• Do not conduct the survey of the PPS excluded rehabilitation hospital requirements
within 90 days of the end of the hospital’s cost reporting period.
• Identify any satellite locations of the hospital.
• Verify with the RO that the hospital is in compliance with the inpatient population
percent rule, and that each satellite, if any, is independently in compliance with the
inpatient population percent rule.
• Review the “Rehabilitation Hospital Criteria Worksheet,” Form CMS-437B.
Survey Tool
“Rehabilitation Hospital Criteria Worksheet,” Form CMS-437B.
Survey Procedures for Determining Compliance with the PPS Excluded
Rehabilitation Hospital Requirements
• Survey activities to determine hospital compliance with the PPS excluded
rehabilitation hospital requirements should be conducted concurrently with the full
survey of the hospital’s compliance with the hospital CoP.
• Using the “Rehabilitation Hospital Criteria Worksheet,” Form CMS-437B, verify
whether the requirements have been met by checking the appropriate box marked
“YES” or “NO.” Under the column “Explanatory Statement,” document specifics
about the findings. Additional findings can be documented in a narrative note that
should be attached to the worksheet.
• Select a minimum of two current inpatients for the patient sample.
• Select additional patients (open or closed records) as needed to determine
compliance with the excluded rehabilitation hospital requirements.
• The selected patients should be included in the patient sample used for the full
hospital survey.
• Identify if the rehabilitation hospital has remote locations, satellites, or other
provider based locations. Record the location, name, address and telephone number
for every remote location, satellite, or provider based location on the Hospital/CAH
Medicare Database Worksheet for updating the Medicare database.
Exit Conference
• Inform the hospital of findings of noncompliance with the excluded rehabilitation
hospital requirements.
• Inform the hospital that the SA will forward the completed Form CMS-437B to the
hospital at the same time as the completed Form CMS-2567.
Post Survey Activities
• Do not include the survey findings for the PPS excluded rehabilitation hospital
requirements on the Form CMS-2567.
• If there are PPS excluded hospital requirements that have not been met, notify the
RO. Document survey findings of the PPS excluded rehabilitation hospital
requirements on the CMS 437B. Submit the completed Form CMS-437B to the RO
within the same time frame as the completion of the Form CMS-2567 and at least 60
days prior to the end of the hospital’s cost reporting period.
• Follow the requirements in the SOM for post-survey activities.
Inpatient Rehabilitation Unit Survey Module
When conducting a full survey of an accredited or non-accredited hospital that has a PPS
excluded rehabilitation unit, conduct a survey of the rehabilitation unit using the survey
methods in this module to assess the hospital’s compliance with the excluded rehabilitation
unit requirements.
Surveys of the PPS excluded rehabilitation unit requirements utilizing these methods will
count as annual validation compliance surveys of the hospital’s self-attestation of
compliance with the excluded requirements.
Background
The PPS excluded rehabilitation unit is part of the hospital and is included as part of the
overall hospital survey. The term “exclusion” is a reimbursement term. Patient care in a
PPS excluded rehabilitation unit is reimbursed at the PPS excluded rehabilitation unit rate
rather than the hospital PPS rate. In order for a hospital to receive the excluded rate for
rehabilitation care provided in its excluded unit, the unit must comply with the excluded
rehabilitation unit requirements found at 42 CFR 412.
A PPS excluded rehabilitation unit is regulated by both the hospital CoP at 42 CFR 482
(also found in Appendix A of the SOM) and the PPS excluded rehabilitation unit
requirements at 42 CFR 412.
Requirements for PPS Excluded Rehabilitation Units
• 42 CFR 482 - Conditions of Participation for hospitals;
• 42 CFR 412.25 - Excluded hospital units: Common Requirements;
• 42 CFR 412.29 - Excluded rehabilitation units: Additional Requirements; and
• State Operations Manual, Chapter 3, §3100.
Activities Conducted Prior to Rehabilitation Unit Survey
• Contact the RO to determine if the hospital has approval for a PPS excluded
rehabilitation unit.
• Contact the RO to determine the unit’s cost-reporting period.
• Do not conduct the survey of the PPS excluded rehabilitation unit requirements
within 90 days of the end of the hospital’s cost reporting period.
• Verify with the RO that the hospital is in compliance with the inpatient population
percent rule for the unit and that each rehabilitation unit satellite, if any, is
independently in compliance with the inpatient population percent rule.
• If possible, establish the location or locations of the rehabilitation unit. Determine if
the unit has a satellite or satellites in other locations. Determination or verification
of this information may have to wait until the survey team is onsite.
• Review the “Rehabilitation Unit Criteria Worksheet,” Form CMS-437A.
Survey Tool
The “Rehabilitation Unit Criteria Worksheet,” Form CMS-437A.
Survey Procedures for Determining Compliance with the PPS Excluded
Rehabilitation Unit Requirements
• Survey activities to determine hospital compliance with the PPS excluded
rehabilitation unit requirements should be conducted concurrently with the full
survey of the hospital’s compliance with the hospital CoP.
• Using the “Rehabilitation Hospital Criteria Worksheet,” Form CMS-437A, verify
whether the requirements have been met by checking the appropriate box marked
“YES” or “NO.” Under the column “Explanatory Statement,” document specifics
about the findings. Additional findings can be documented in a narrative note that
should be attached to the worksheet.
• Select 10 percent of the unit’s average daily census or a minimum of two current
patients for the patient sample.
• The selected patients should be included in the patient sample used for the full
hospital survey.
• Select additional patients (open or closed records) as needed to determine
compliance with the excluded rehabilitation unit requirements.
• If there are no patients on the unit at the time the survey is conducted, review closed
patient records of unit patients treated within six months of the survey.
• Identify if the rehabilitation unit has a satellite or satellites. Record the location,
name, address and telephone number for every satellite on the Hospital/CAH
Medicare Database Worksheet for updating the Medicare database.
Exit Conference
• Inform the hospital of findings of noncompliance with the excluded rehabilitation
unit requirements.
• Inform the hospital that the SA will forward the completed Form CMS-437A to the
hospital at the same time as the completed Form CMS-2567.
Post Survey Activities
• Do not include the survey findings for the PPS excluded rehabilitation unit
requirements on the Form CMS-2567.
• If there are PPS excluded unit requirements that have not been met, notify the RO.
Document survey finding of the PPS rehabilitation unit requirements on the
CMS-437A. Submit the completed Form CMS-437A to the RO within the same
time frame as the completion of the Form CMS-2567 and at least 60 days prior to
the end of the hospital’s cost reporting period.
• Follow the requirements in the SOM for post-survey activities.
Hospital Swing-Bed Survey Module
When conducting a full survey of an accredited or unaccredited hospital that has swing-bed
approval, conduct a survey of the hospital swing-bed requirements found at 42 CFR 482.66.
These requirements, as well as interpretive guidelines, are found in Appendix T of the
SOM. An optional survey worksheet, also found in Appendix T, may be used.
Background
Swing-bed patients are hospital patients who are situated in the hospital but for whom the
hospital is receiving reimbursement for skilled nursing services, as opposed to acute-care
reimbursement. The reference to swing-bed is a patient care and reimbursement status and
has no relationship to geographic location in the facility. The patient may be in acute-care
status one day and change to swing-bed status the next day. It is not necessary for the
patient to change location in the hospital when the reimbursement status changes, but
moving to a different location is allowed. A 3-day qualifying stay for the same spell of
illness in any hospital or critical access hospital (CAH) is required prior to admission to
swing-bed status. The 3-day qualifying stay does not need to be from the same facility as
the swing-bed admission.
Regulatory Authority and Requirements for Hospital Providers of Extended Care
Services (“Swing-beds”)
Hospital swing-bed care is regulated by both the hospital requirements at 42 CFR Part 482
(reprinted at Appendix A of the SOM) and the swing-bed requirements at 42 CFR 482.66
(also in Appendix T, along with an optional surveyor worksheet). The actual swing-bed
survey requirements are referenced in the Medicare Nursing Home requirements at 42 CFR
Part 483.
Section 1883 of the Act authorizes payment under Medicare for post-hospital SNF services
provided by any hospital that meets certain requirements. By regulation, the Secretary has
specified these requirements at 42 CFR 482.66:
• The hospital has a Medicare provider agreement;
• The facility has fewer than 100 hospital beds, excluding beds for newborns and beds
in intensive care type inpatient units;

• The hospital is located in a rural area. This includes all areas not delineated as
“urbanized” areas by the Census Bureau, based on the most recent census;
• The hospital does not have in effect a 24-hour nursing waiver granted under 42 CFR
§488.54(c);
• The hospital has not had a swing-bed approval terminated within the two years
previous to application; and
• The hospital meets the swing-bed CoP on Resident Rights; Admission, Transfer,
and Discharge Rights; Resident Behavior and Facility Practices; Patient Activities;
Social Services; Discharge Planning; Specialized Rehabilitative Services; and
Dental Services.
Activities Conducted Prior to Swing-Bed Survey
Prior to conducting the swing-bed survey, verify the following:
• The hospital continues to be located in a rural census tract;
• The hospital does not have a 24-hour nursing waiver in place; and
• The hospital’s swing-bed approval is in effect and has not been terminated within
the two previous years.
Survey Procedures
In conducting the survey, verify that the hospital has fewer than 100 hospital beds,
excluding beds for newborns and beds in intensive care units. A hospital licensed for more
than 100 beds may be eligible for swing-bed approval if it utilizes and staffs for fewer than
100 beds. Count the staffed beds in each nursing unit. Do not count beds in recovery
rooms, intensive care units, operating rooms, newborn nurseries or stretchers in emergency
departments.
Assess the hospital’s compliance with the swing-bed requirements at 42 CFR 482.66,
found in Appendix T of the SOM, and the hospital survey methods contained in the hospital
survey protocol. Swing-bed requirements apply to any patient discharged from the hospital
and admitted to a swing-bed for skilled nursing services. The requirements for acute-care
hospitals also apply.
If swing-bed patients are present during the on-site inspection, conduct an open record
review and an environmental assessment. Include patient interviews and observations of
care and services. However, if no swing-bed patients are present during the on-site
inspection, review two closed records for compliance with swing-bed requirements. In all
cases, review policies, procedures, and contracted services to assure that the hospital has the
capability to provide the services needed.
It is important for surveyors to maintain on-going documentation of their findings during
the course of the survey for later reference. Surveyors may use the optional swing-bed
worksheet as a note-taking tool to document and record their findings on the survey.
Exit Conference
Any findings of noncompliance may be discussed during the time of the hospital exit
conference.
Post-Survey Activities
The findings for swing-bed deficiencies must be documented on a separate Form CMS-
2567, even though the swing-bed survey is being conducted simultaneously with the
hospital survey.
Regulations and Interpretive Guidelines
(Rev. 149, Issued: 10-09-15, Effective: 10-09-15, Implementation: 10-09-15)
NOTE: in the regulations or guidance which follow, in every instance where the
following terms appear:
• “spouse” means an individual who is married to another individual as a result of
marriage lawful where it was entered into, including a lawful same-sex marriage,
regardless of whether the jurisdiction where the hospital is located, or in which the
spouse lives, permits such marriages to occur or recognizes such marriages.
• “marriage” means a marriage lawful where entered into, including a lawful same-
sex marriage, regardless of whether the jurisdiction where the hospital is located,
or in which the spouse lives, permits such marriages to occur or recognizes such
marriages;
• “family” includes, but is not limited to, an individual’s “spouse” (see above); and
• “relative” when used as a noun, includes, but is not limited to, an individual’s
“spouse” (see above).
Furthermore, except where CMS regulations explicitly require an interpretation in
accordance with State law, wherever the text of a regulation or associated guidance
uses the above terms or includes a reference to a patient’s “representative,”
“surrogate,” “support person,” “next-of-kin,” or similar term in such a manner as
would normally implicitly or explicitly include a spouse, the terms are to be
interpreted consistent with the guidance above.
A hospital is expected to recognize all lawful marriages and spouses for
purposes of compliance with the Conditions of Participation, regardless of
any laws to the contrary of the state or locality or other jurisdiction where
the hospital is located or where the spouse lives.
A-0001
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.2 Provision of Emergency Services by Nonparticipating Hospitals
(a) The services of an institution that does not have an agreement to participate in the
Medicare program may, nevertheless, be reimbursed under the program if--
(1) The services are emergency services; and
(2) The institution meets the requirements of section 1861(e)(1) through (5) and (7)
of the Act. Rules applicable to emergency services furnished by non-
participating hospitals are set forth in subpart G of part 424 of this chapter.
(b) Section 440.170(e) of this chapter defines emergency hospital services for purposes
of Medicaid reimbursement.
Interpretive Guidelines §482.2
The statutory requirements that a hospital must meet are:
• The hospital is primarily engaged in providing, by or under the supervision of
MD/DOs, to inpatients, diagnostic services and therapeutic services for medical
diagnosis, treatment, and care of injured, disabled or sick persons, or rehabilitation
services for the injured, disabled, or sick persons;
• The hospital maintains clinical records on all patients;
• The hospital has medical staff bylaws;
• The hospital has a requirement that every Medicare patient must be under the care
of an MD/DO;
• The hospital provides 24-hour nursing services rendered or supervised by a
registered professional nurse and has a licensed, practical, or registered professional
nurse on duty at all times; and
• The hospital is licensed or is approved as meeting the standards for licensing, as a
hospital as defined by the State.
A-0008
(Rev.172, Issued: 11-17-17, Effective: 11-17-17, Implementation: 11-17-17)
§482.1 Basis and scope.
(a) Statutory basis. (1) Section 1861(e) of the [Social Security] Act provides that—
(i) Hospitals participating in Medicare must meet certain specified requirements; and
(ii) The Secretary may impose additional requirements if they are found necessary in
the interest of the health and safety of the individuals who are furnished services in
hospitals.
. . .
(b) Scope. Except as provided in subpart A of part 488 of this chapter, the provisions
of this part serve as the basis of survey activities for the purpose of determining whether a
hospital qualifies for a provider agreement under Medicare and Medicaid.
Interpretive Guidelines §482.1(a)(1)
Hospital Definition and Regulatory Enforcement Authorities
In order to qualify for a provider agreement as a hospital (other than a psychiatric hospital
as defined at section 1861(f) of the Act) under Medicare and Medicaid, an entity must meet
and continue to meet all of the statutory provisions of §1861(e) of the Act, including the
Condition of Participation (CoP) requirements. See also 42 CFR 488.3(a)(1) and 42 CFR
489.12. This means the entity must:
• Be primarily engaged in providing, by or under the supervision of physicians, to
inpatients (A) diagnostic services and therapeutic services for medical diagnosis,
treatment, and care of injured, disabled, or sick persons, or (B) rehabilitation services
for the rehabilitation of injured, disabled, or sick persons;
• Maintain clinical records on all patients[addressed in 42 CFR 482.24, Medical
Records];
• Have medical staff bylaws [42 CFR 482.12, Governing Body, and 42 CFR 482.22,
Medical Staff];

• Have a requirement that every patient with respect to whom payment may be made
under Title XVIII must be under the care of a physician except that a patient receiving
qualified psychologist services (as defined in section 1861(ii) of the Act) may be under
the care of a clinical psychologist with respect to such services to the extent permitted
under State law [42 CFR 482.12, Governing Body];
• Provide 24-hour nursing service rendered or supervised by a registered professional
nurse, and has a licensed practical nurse or registered professional nurse on duty at all
times…[42 CFR 482.23, Nursing Services];
• Have in effect a hospital utilization review plan which meets the requirements of section
1861(k) of the Act [42 CFR 482.30, Utilization Review];
• Have in place a discharge planning process that meets the requirements of section
1861(ee) of the Act [42 CFR 482.43, Discharge Planning];
• If located in a state in which state or applicable local law provides for the licensing of
hospitals, be licensed under such law or be approved by the agency of the State or
locality responsible for licensing hospitals as meeting the standards established for such
licensing [42 CFR 482.11, Compliance with Federal, State, and Local Laws];
• Have in effect an overall plan and budget that meets the requirements of section 1861(z)
of the Act [42 CFR 482.12, Governing Body]; and
• Meet any other requirements as the Secretary finds necessary in the interest of the
health and safety of individuals who are furnished services in the institution [42 CFR
Parts 482 and 489, among others].
Primarily Engaged
Generally, a hospital is primarily engaged in providing inpatient services under section
1861(e)(1) of the Act when it is directly providing such services to inpatients. Having the
capacity or potential capacity to provide inpatient care is not the equivalent of actually
providing such care. Inpatient hospital services are defined under section 1861(b) of the
Act and in the regulations at 42 CFR Part 409, Subpart B. CMS guidance describes an
inpatient as
“a person who has been admitted to a hospital for bed occupancy for purposes of receiving
inpatient hospital services …. Generally, a patient is considered an inpatient if formally
admitted as an inpatient with the expectation that he or she will require hospital care
that is expected to span at least two midnights and occupy a bed even though it later
develops that the patient can be discharged or transferred to another hospital and not
actually use a hospital bed overnight.” (Medicare Benefit Policy Manual, Chapter 1, §10,
(https://www.cms.gov/Regulations-and-
Guidance/Guidance/Manuals/Downloads/bp102c01.pdf)
The “expectation of a two midnight stay” for an inpatient is that the intent of the physician
was that the patient be admitted to the hospital for an inpatient stay as opposed to that of
observation status which is an outpatient service.
Therefore, an average length of stay (ALOS) of two midnights would be one of the
benchmarks considered for certification as a hospital.
• In making a determination of whether or not a facility is primarily engaged in
providing inpatient services and care to inpatients, CMS considers multiple
factors and will make a final determination based on an evaluation of the facility
in totality. Such factors include, but are not limited to, average daily census
(ADC), average length of stay (ALOS), the number of off-campus outpatient
locations, the number of provider based emergency departments, the number of
inpatient beds related to the size of the facility and scope of services offered,
volume of outpaient surgical procedures compared to inpatient surgical
procedures, staffing patterns, patterns of ADC by day of the week, etc.
Hospitals are not required to have a specific inpatient to outpatient ratio in order
to meet the definition of primarily engaged.
In order for surveyors to determine whether or not a hospital is in compliance with the
statutory and regulatory requirements of Medicare participation, including the definition of
a hospital, they must observe the provision of care. Medicare requirements at 42 CFR
488.26(c)(2) state that “The survey process uses resident and patient outcomes as the
primary means to establish the compliance process of facilities and agencies. Specifically,
surveyors will directly observe the actual provision of care and services to residents and/or
patients, and the effects of that care, to assess whether the care provided meets the needs of
individual residents and/or patients.”
Because §488.26(c)(2) and section 1861(e) of the Act refer to patients (plural) hospitals
must have at least two inpatients at the time of the survey in order for surveyors to conduct
the survey. However, just because a facility has two inpatients at the time of a survey does
not necessarily mean that the facility is primarily engaged in inpatient care and satisfies all
of the statutory requirements to be considered a hospital for Medicare purposes. Having
two patients at the time of a survey is merely a starting point in the overall survey and
certification process.
If a hospital does not have at least two inpatients at the time of a survey, a survey will not
be conducted at that time and an initial review of the facility’s admission data will be
performed by surveyors while onsite to determine if the hospital has had an ADC of at least
two and an ALOS of at least two midnights over the last 12 months. Average daily census
is calculated by adding the midnight daily census for each day of the 12 month period and
then dividing the total number by the number of days in the year. For facilities that have
multiple campuses operating under the same CMS Certification Number (CCN), the ADC
is not calculated individually at each campus. All locations make up the entire facility and
the ADC will be based on the total inpatient census from all campuses. This also includes
PPS excluded psychiatric and rehabilitation units that are part of the facility.
In order to be considered primarily engaged in providing inpatient services, prospective
hospital providers and currently participating hospitals should also be able to maintain an
ALOS of two midnights or greater. The ALOS is calculated by dividing the total number of
inpatient hospital days (day of admission to day of discharge, including day of death) by the
total number of discharges in the hospital over 12 months. For facilities that have not been
operating for 12 months at the time of the survey, an ADC calculated using 12 months as
the denominator may falsely result in an ADC of less than two. Therefore, facilities that
have been operating less than 12 months at the time of the survey, should calculate its ADC
based on the number of months the facility has been operational but no less than three
months. This does not mean that a facility must be operational for at least three months
before a survey can be completed. It merely means that the ADC cannot be calculated
using a denominator of less than three months.
• If the ADC and ALOS is two or more, the State Survey Agency (SA) or Accrediting
Organization (AO) makes the determination that a second survey will be attempted
at a later date.
• If the facility does not have a minimum ADC of two inpatients and an ALOS of two
over the last 12 months (or less than 12 months for facilities that have not been
operational for at least 12 months), the facility is most likely not primarily engaged
in providing care to inpatients and the SA or AO may not conduct the survey. The
SA or AO must immediately contact the RO to inform them that a survey could not
be completed and the CMS Regional Office will review additional information
provided by the SA or AO to determine whether a second survey should be
attempted.
• When the ADC and ALOS are NOT a minimum of two, the SA or AO do not make
the final determination whether a second survey will be attempted. Instead, the SA
or AO must obtain further information from the facility (other factors described
below), review the information and make a recommendation to the RO regarding
whether a second survey should be attempted. The SA or AO must provide its
recommendation in writing to the RO along with the supporting information used to
make the recommendation. The RO must review the recommendation and
information and make a determination on whether a second survey will be
conducted and communicate its decision to the SA or AO within seven business
days of receipt of the recommendation. AO communication to the RO must be via
the current established process used for all other written communication to the RO.
• If during a second survey attempt, the facility does not have two inpatients, the
survey will not be conducted and the SA or AO must cite condition level non-
compliance with §482.1. In addition, the SA or AO must immediately notify the RO
of the situation. The RO will then proceed with either denial of certification (for
initial applicants) in the Medicare program or termination of the provider agreement
(for currently participating hospitals). For currently participating hospitals, the RO
will base any termination action on the totality of the situation including
consideration of any access to care issues.
Other factors that the CMS Regional Office should consider in determining whether to (1)
conduct a second survey or (2) recommend denial of an initial applicant or termination of a
current provider agreement, include but are not limited to:
• The number of provider-based off-campus emergency departments (EDs). An
unusually large number of off-campus EDs may suggest that a facility is not
primarily engaged in inpatient care and is instead primarily engaged in providing
outpatient emergency services.
• The number of inpatient beds in relation to the size of the facility and services
offered.
• The volume of outpatient surgical procedures compared to inpatient surgical
procedures.
• If the facility considers itself to be a “surgical” hospital, are procedures mostly
outpatient?
• Does the information indicate that surgeries are routinely scheduled early in
the week and does it appear this admission pattern results in all or most
patients being discharged prior to the weekend (for example does the facility
routinely operate in a manner that its designated “inpatient beds” are not in
use on weekends)?
• Patterns and trends in the ADC by the day of the week. For example, does the ADC
consistently drop to zero on Saturdays and Sundays? Therefore suggesting that the
facility is not consistently and primarily engaged in providing care to inpatients.
• Staffing patterns. A review of staffing schedules should demonstrate that nurses,
pharmacists, physicians, etc. are scheduled to work to support 24/7 inpatient care
versus staffing patterns for the support of outpatient operations.
• How does the facility advertise itself to the community? Is it advertised as a
“specialty” hospital or “emergency” hospital? Does the name of the facility include
terms such as “clinic” or “center” as opposed to “hospital”?
The CMS RO should consider all of the above factors (and other factors as necessary) to
make a determination as to whether or not a facility is truly operating as a hospital for
Medicare purposes. A determination of non-compliance with § 482.1 will not be based on a
single factor, such as failing to have two inpatients at the time of a survey.
It is important to note that CMS has the final authority to make the determination of
whether or not a facility has met the statutory definition of a hospital after considering the
facility’s entire situation, the recommendations of the SA or AO surveyors as well as the
evidence submitted by the SAs and AOs. As stated previously, a facility that meets State
requirements for obtaining State status as a hospital is not automatically considered a
hospital for federal survey and certification purposes without further evaluation and
consideration of all relevant CMS requirements. In addition, approval by the Medicare
administrative contractor of an enrollment application does not convey hospital status for
CMS purposes. Hospital status is only conveyed and approved by the CMS RO after a
survey has been completed and the results clearly demonstrate that the facility has met all
the federal requirements, including the statutory definition.
Survey Procedures §482.1(a)(1)
• Verify there are at least two inpatients currently in the hospital at the time of survey
• If yes, proceed with evaluating the whether the hospital is primarily engaged in
providing the requisite services of a hospital, as well as in the Conditions of
Participation.
• If there are currently no inpatients in the hospital, no survey is to be conducted and
surveyors should ask to see the following, in order to make the proper determination
of the hospital’s status and to make the proper recommendations to the RO:
o ADC over the last 12 months (or less for facilities operational for less than
12 months)
Look for patterns and trends in the ADC by the day of the week.
o ALOS over the last 12 months (or less for facilities operational for less than
12 months)
o The number of provider-based off-campus emergency departments.
o The volume of outpatient surgical procedures compared to inpatient surgical
procedures
o Staffing schedules by day of week and shift over the last 12 months (or less
for facilities operational for less than 12 months)
o Verify the facility is providing the appropriate types and adequate numbers
of staff to support 24/7 inpatient services (i.e. nursing, pharmacy, physicians,
etc.)
o Review the number of inpatient beds in relation to the size of the facility and
services offered.
o Determine if the number of inpatient beds could support emergency or
unplanned admissions from the volumes of other services offered by the
facility, such as ED patients or outpatient surgery patients?
• If the initial review of the above information indicates that the facility is most likely
not providing care to inpatients, then a second survey will not be conducted.
However, if the review of the information indicates the facility has had an ADC and
ALOS of two over the last 12 months (or less for facilities operational for less than
12 months) and there are no other concerns regarding facility’s eligibility to be
surveyed as a hospital, then a second survey will be scheduled for a future
unannounced date after consulting with the RO.
• Whenever the SA or AO is unable to complete a survey because the hospital did not
have a sufficient number of inpatients that is a representative sample of the different
types of services and patient populations that are treated at that hospital, it must
immediately report this information to the RO.
• Determine through interview, observation, and record review that the hospital meets
the statutory requirements as defined by 1861(e), including the CoPs Verify the
facility does the following:
• Maintains clinical records on all patients;
• Has medical staff bylaws;
• Has a requirement that every patient with respect to whom payment may be
made under this title must be under the care of a physician except that a patient
receiving qualified psychologist services (as defined in section 1861(ii) of the
Act) may be under the care of a clinical psychologist with respect to such
services to the extent permitted under State law;
• Provides 24-hour nursing service rendered or supervised by a registered
professional nurse, and has a licensed practical nurse or registered professional
nurse on duty at all times…;
• Has in effect a hospital utilization review plan which meets the requirements of
section 1861(k) of the Act;
• Has in place a discharge planning process that meets the requirements of section
1861(ee) of the Act;
• If located in a state in which state or applicable local law provides for the
licensing of hospitals, be licensed under such law or be approved by the agency
of the State or locality responsible for licensing hospitals, as meeting the
standards established for such licensing;
• Has in effect an overall plan and budget that meets the requirements of section
1861(z) of the Act.
A-0020
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.11 Condition of Participation: Compliance with Federal, State and
Local Laws
Interpretive Guidelines §482.11
The hospital must ensure that all applicable Federal, State and local law requirements are
met.
A-0021
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.11(a) The hospital must be in compliance with applicable Federal
laws related to the health and safety of patients.
Survey Procedures §482.11(a)
Interview the CEO, or appropriate individual designated by the hospital, to determine
whether the hospital is in compliance with Federal laws related to patient health and safety.
(For example, ask if the hospital was cited since its last survey for any violation of Section
504 of the Rehabilitation Act of 1973 related to denying people with disabilities access to
care. If so, verify that satisfactory corrections have been made to bring the hospital into
compliance with that law.) Refer or report noted noncompliance with Federal laws and
regulations to the appropriate agency having jurisdiction (e.g., accessibility issues, blood-
borne pathogens, standard precautions, and TB control to OSHA; hazardous chemical/waste
issues to EPA; etc.)
A-0022
(Rev.172, Issued: 11-17-17, Effective: 11-17-17, Implementation: 11-17-17)
§482.11(b) The hospital must be--
(1) Licensed; or
(2) Approved as meeting standards for licensing established by the agency of the
State or locality responsible for licensing hospitals.
Interpretive Guidelines §482.11(b)
Hospitals applying for initial Medicare certification as a hospital or hospitals currently
participating in Medicare must, among other things, meet the statutory definition of a
hospital under section 1861(e) of the Act. Section 1861(e)(7) of the Act further requires
that a hospital located in a state which provides for the licensing of hospitals, the hospital
must be licensed in accordance with state law or approved as meeting standards for
licensing as established by the agency of the State or locality responsible for the licensing
of hospitals.
While a facility may have a license from a state to operate as a hospital or may have been
approved by a state as a hospital under state or local standards and authorities, that facility
may still not meet the Medicare definition of a hospital as per the Act. The criteria used by
a state to determine that a hospital meets the requirements for state licensure as a hospital is
not the same criteria used to define a hospital for the purpose of participation in Medicare,
and each state has its own criteria and standards for licensure.
The definition of a hospital and the issue of whether the facility is Primarily Engaged are
issues not applicable to a Critical Access Hospital (CAH).
Survey Procedures §482.11(b)
• Prior to the survey, determine whether the hospital has a current license by the state
or local authority in which it operates, or, if it is located within a
State that does not license hospitals, verify that the responsible State agency has
approved the hospital as meeting the State’s established standards for the licensing
of hospitals.
A-0023
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.11(c) The hospital must assure that personnel are licensed or meet
other applicable standards that are required by State or local laws.
Interpretive Guidelines §482.11(c)
All staff that are required by the State to be licensed must possess a current license. The
hospital must assure that these personnel are in compliance with the State’s licensure laws.
The laws requiring licensure vary from state to state. Examples of healthcare professionals
that a state may require to be licensed could include: nurses, MD/DOs, physician assistants,
dieticians, x-ray technologists, dentists, physical therapists, occupational therapists,
respiratory therapists and hospital administrators.
All staff must meet all applicable standards required by State or local law for hospital
personnel. This would include at a minimum:
• Certification requirements;
• Minimum qualifications;
• Training/education requirements; and
• Permits (such as food handlers permits).
When telemedicine is used and the practitioner and patient are located in different states,
the practitioner providing the patient care service must be licensed and/or meet the other
applicable standards that are required by State or local laws in both the state where the
practitioner is located and the state where the patient is located.
Survey Procedures §482.11(c)
• Verify for those personnel required to be licensed, certified, and/or permitted by the
State, that the hospital has established, and follows procedures for determining that
personnel are properly licensed, certified, and/or permitted.
• Verify that staff and personnel are licensed, certified, and/or permitted in
accordance with State and local requirements.
• Verify that staff and personnel meet all standards (such as continuing education,
basic qualifications, etc.) required by State and local laws or regulations. Verify
that the hospital has a mechanism established and enforced to ensure compliance.
• Review a sample of personnel files to verify that licensure and/or other required
credentials information is up to date. Verify State licensure compliance of the direct
care personnel as well as administrators and supervisory personnel.
A-0043
(Rev. 122, Issued: 09-26-14, Effective: 09-26-14, Implementation: 09-26-14)
§482.12 Condition of Participation: Governing Body
There must be an effective governing body that is legally responsible for the conduct
of the hospital. If a hospital does not have an organized governing body, the persons
legally responsible for the conduct of the hospital must carry out the functions
specified in this part that pertain to the governing body.
Interpretive Guidelines §482.12
The hospital must have a governing body which is effective in carrying out its
responsibilities for the conduct of the hospital. In the absence of an organized governing
body, there must be written documentation that identifies the individual or individuals that
are legally responsible for the conduct of the hospital operations.
If the hospital is part of a healthcare system that includes several separately certified
hospitals, each with its own Medicare provider agreement and CMS Certification Number,
the governing body of the healthcare system has the option to act as the governing body of
each separately certified hospital, unless doing so would conflict with State law. A hospital
system also has the option to form several governing bodies, each of which is responsible
for several separately certified hospitals. For example, a health system operating hospitals
in many States might choose to form regional sub-boards each responsible for the hospitals
in its region, or a health system that has a mixture of types of hospitals may choose to form
one sub-board responsible for its short-term acute care hospitals and another for its long
term care hospitals.
When deciding whether or not to exercise the option to have a single governing body for
multiple hospitals in the system, another factor for systems to consider might be Medicare
payment requirements at §§412.22(e) - (h) applicable to certain types of hospitals, i.e., non-
grandfathered Hospitals-within-Hospitals and Hospital Satellites. In such cases where the
hospital system owns both the tenant and the host hospital, using a single governing body
for both hospitals would jeopardize the payment status of a hospital that is being paid by
Medicare under a payment system excluded from the Hospital Inpatient Prospective
Payment System (IPPS). However, surveyors do not assess compliance with or enforce the
Medicare payment regulations that govern Hospitals-within-Hospitals or Hospital Satellites.
The Medicare program offers hospital facilities considerable flexibility regarding how they
choose to participate. Based on the geographic and other institutional limitations set out in
the “provider-based” regulation at §413.65, which addresses provider-based status for
hospital facilities in multiple locations, hospital governing bodies make business decisions
about how they want to participate in Medicare, and they indicate on their Medicare
enrollment application the choices they have made. It is not uncommon to find multiple
hospital campuses with one owner located in the same geographic area enrolled in Medicare
as one hospital. It is also not uncommon to see a hospital system choosing to enroll its
various facilities as separately certified hospitals. Various factors enter into consideration
when the governing body of a system makes these decisions.
For example, some governing bodies prefer to enroll various campuses as separate
hospitals, out of a concern that problems at one hospital’s campus might jeopardize the
Medicare participation of the other campuses if they were a multi-campus hospital covered
under one Medicare provider agreement. In other cases a governing body may see the
benefits of integrating clinical services on multiple campuses into one integrated hospital.
In still other cases, the deciding factor might be the implications for Medicare
reimbursement of graduate medical education, the ease of adding satellite locations, etc.
CMS defers to the governing bodies of hospitals to weigh the pertinent factors and
permissible options, and to make business decisions in their best interest when applying to
participate in Medicare. CMS’s hospital certification decisions and issuance of a provider
agreement and associated CCN follow from these business decisions by a hospital’s
governing body. But once the “hospital,” with whatever component parts, has been
certified, that hospital must independently demonstrate its compliance with the CoPs,
independent of any other facility. (77 FR 29040, May 16, 2012)
If a hospital system has chosen to have a one body act as the governing body for multiple
separately certified hospitals (i.e., a system governing body), this does not alter the fact that
each hospital must independently demonstrate compliance with the CoPs. Examples of
what this means include, but are not limited to, the following:
• Each separately certified hospital must be separately and independently assessed
for its compliance with the CoPs, through either State Survey Agency or
approved Medicare hospital accreditation program surveys. There is no survey
of a hospital “system,” since the Medicare provider agreement and its terms are
specific to each certified hospital.
• A system governing body may wish to adopt identical policies and procedures
for many aspects of a hospital’s operations across all of its hospitals within the
system. It has the flexibility to do so, but the documentation of such policies
and procedures must be clear that the governing body has chosen to apply them
to specifically named hospitals. Also, each hospital must be able to present for
inspection the system governing body policies and procedures that clearly apply
to that hospital. For example:
A document that says “XX Healthsystem has adopted the following policy” is not
acceptable. Instead, the document must be more specific, such as, “XX
Healthsystem adopts the following policy and procedure for Hospital A, Hospital B,
and Hospital C.” Furthermore, the names of each hospital (Hospitals A, B, and C in
this example) must correspond to the names used for their provider agreements. For
example, if Hospital C is one Medicare-certified hospital with two inpatient
campuses, one called “East” and one called “West,” it is not acceptable for the
policy document to state, “XX Healthsystem adopts the following policy and
procedure for Hospital A, Hospital B, and Hospital East and Hospital West.” It
would be acceptable to state, “XX Healthsystem adopts the following policy and
procedure for Hospital A, Hospital B, and Hospital C.”
It also is not acceptable for the policy document to state, “XX Healthsystem adopts
the following policy and procedure for Hospital A, Hospital B, and Hospital East,
but not Hospital West.” Since “Hospitals” East and West refer to separate campuses
of Hospital C, which participates in Medicare as one multi-campus hospital, it is not
appropriate to refer to these separate campuses of C as “hospitals,” since the XX
Healthsystem made a business decision to enroll them as parts of one multi-campus
hospital in Medicare. CMS recognizes that, depending on the particular policy
topic, it may be acceptable to have policies that vary by type of unit/department
within a hospital. The system governing body could achieve this as follows: “XX
Healthsystem adopts the following policy and procedure requiring that a physician
be on-site 24 hours per day, seven days per week on the inpatient campuses of
Hospital A and Hospital B, but within Hospital C, only for the East inpatient
campus.”
• Likewise, the minutes of the governing body must be written in such a manner
so that it is clear when the governing body has taken actions that apply to a
specific certified hospital.
• Departments of separately certified hospitals with one system governing body
cannot be operationally integrated. For example, if a system has chosen to
operate three separately certified hospitals in relatively close proximity to each
other rather than to have them certified as one multi-campus hospital, then each
hospital must have its own nursing service. It may not have one integrated
nursing service with one Director of Nursing who manages one nursing staff for
all three hospitals. The system cannot maintain one integrated schedule that
assigns nursing staff among the different hospitals. The system also cannot
move them back and forth between hospitals on an ad hoc, as needed basis, as if
they were one hospital.
On the other hand, the policies and procedures the governing body has adopted for the
nursing service in each hospital may be identical, so long as the services operate
separately. It is also permissible for the same individual to be the Director of Nursing
for each hospital, provided that he or she is able to carry out all of the duties of the
position in each hospital, such as managing each hospital's separate nursing staff. It is
also permissible for one nurse to work at multiple hospitals within the system, in the
same way that a nurse may work for multiple hospitals that do not share ownership, but
the nurse must have separate work schedules for each hospital. Such schedules cannot
overlap.
• Likewise, although the system may choose to operate a quality
assessment/performance improvement (QAPI) program at the system level
which standardizes indicators measured across system hospitals, each
separately-certified hospital in the system must have a QAPI program that is
specific to that hospital. This is required not only to demonstrate compliance,
but also for the governing body to function effectively, since reviewing QAPI
program results only at the system level would make it difficult for the
governing body to identify and act upon problems that are localized to one
hospital.
For example, the system may choose to use the same quality indicators or the same
methodology to track adverse events across all system hospitals. But each certified
hospital must have its own QAPI data with respect to these indicators and adverse
events. If a system is tracking readmission rates across all of its hospitals, it must be
able to separate out the hospital-specific results for the governing body’s review and
possible action.
The governing body must be functioning effectively and holds the ultimate responsibility
for the hospital’s compliance not only with the specific standards of the governing body
CoP, but also with all of the CoPs. This is the case regardless of whether the regulatory text
for a particular condition or standard within a condition specifically mentions
responsibilities of the governing body. Substantial, i.e., condition-level, non-compliance
with one of the other hospital CoPs may be an indicator that the governing body is not
functioning effectively. However, it is not the policy of CMS that condition-level
noncompliance with any other CoP automatically results in a condition-level citation of the
governing body CoP. Surveyors must consider whether the manner and degree of the other
deficiencies provide sufficient evidence to conclude that the governing body is not
functioning effectively.
Survey Procedures §482.12
• Verify that the hospital has an organized governing body or has written
documentation that identifies the individual or individuals that are
responsible for the conduct of the hospital operations.
• If the hospital is part of a hospital system which uses one governing body for
several of the hospital’s separately certified within the system:
• Review the governing body minutes to determine if it is clear which actions pertain
to which hospitals.
• Select for review several policy and procedure documents adopted by the system
governing body to determine if it is clear that they apply to the hospital being
surveyed.
A-0044
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(a) Standard: Medical Staff. The governing body must:
Interpretive Guidelines §482.12(a)
The governing body must ensure the medical staff requirements are met.
A-0045
(Rev. 122, Issued: 09-26-14, Effective: 09-26-14, Implementation: 09-26-14)
[The governing body must:]
§482.12(a)(1) Determine, in accordance with State law, which categories of
practitioners are eligible candidates for appointment to the medical staff;
Interpretive Guidelines §482.12(a)(1)
The governing body must determine, in accordance with State law, which categories of
practitioners are eligible for appointment to the medical staff.
Physicians
The medical staff must, at a minimum, be composed of doctors of medicine or doctors of
osteopathy. In addition, the medical staff may include other types of practitioners included
in the definition of a physician in Section 1861(r) of the Social Security Act:
• Doctor of dental surgery or of dental medicine;
• Doctor of podiatric medicine;
• Doctor of optometry; and
• a Chiropractor.
In all cases, the practitioner included in the definition of a physician must be legally
authorized to practice within the State where the hospital is located and providing services
within their authorized scope of practice. In addition, in certain instances the Social
Security Act and regulations attach further limitations as to the type of hospital services for
which a practitioner may be considered to be a “physician.” See 42 CFR 482.12(c)(1) for
more detail on these limitations.
The governing body has the flexibility, consistent with State law, to determine whether
practitioners included in the definition of a physician other than a doctor of medicine or

osteopathy are eligible for appointment to the medical staff.
For Information Only – Not Required/ Not to be Cited
CMS expects that all physician practitioners granted privileges are also appointed as
members of the medical staff. However, if State law limits the composition of the
hospital’s medical staff to certain categories of practitioners, e.g., only MDs or DOs,
there is nothing in the CoPs that prohibits hospitals and their medical staffs from
establishing certain practice privileges for other categories of physician practitioners
excluded from medical staff membership under State law, or from granting those
privileges to individual practitioners in those categories, as long as such privileges are
recommended by the medical staff, approved by the governing body, and in accordance
with State law. (79 FR 27114 - 27115, May 12, 2014)
For physician practitioners granted privileges only, the hospital’s governing body and its
medical staff must exercise oversight, such as through credentialing and competency
review, of those other physician practitioners to whom it grants privileges, just as it would
for those practitioners appointed to its medical staff.
Non-physician practitioners
Furthermore, the governing body has the authority, in accordance with State law, to grant
medical staff privileges and membership to non-physician practitioners. The
corresponding regulation at 42 CFR 482.22(a) allows hospitals and their medical staffs to
take advantage of the expertise and skills of all types of practitioners who practice at the
hospital when making decisions concerning medical staff privileges and membership.
Granting medical staff privileges and membership to non-physician practitioners is an
option available to the governing body; it is not a requirement.
For Information Only – Not Required/ Not to be Cited
CMS expects that all practitioners granted privileges are also appointed as members of
the medical staff. However, if State law limits the composition of the hospital’s medical
staff to certain categories of practitioners, e.g., only physician practitioners, there is
nothing in the CoPs that prohibits hospitals and their medical staffs from establishing
certain practice privileges for those specific categories of non-physician practitioners
excluded from medical staff membership under State law, or from granting those
privileges to individual practitioners in those categories, as long as such privileges are
recommended by the medical staff, approved by the governing body, and in accordance
with State law. (79 FR 27114 - 27115, May 12, 2014)
For non-physician practitioners granted privileges only, the hospital’s governing body and
its medical staff must exercise oversight, such as through credentialing and competency
review, of those non-physician practitioners to whom it grants privileges, just as it would
for those practitioners appointed to its medical staff.
Practitioners are described in Section 1842(b)(18)(C) of the Act as any of the following:
• Physician assistant (as defined in Section 1861(aa)(5) of the Act);
• Nurse practitioner (as defined in Section 1861(aa)(5) of the Act);
• Clinical nurse specialist (as defined in Section 1861(aa)(5) of the Act);
• Certified registered nurse anesthetist (as defined in Section 1861(bb)(2) of the
Act);
• Certified nurse-midwife (as defined in Section 1861(gg)(2) of the Act);
• Clinical social worker (as defined in Section 1861(hh)(1) of the Act;
• Clinical psychologist (as defined in 42 CFR 410.71 for purposes of Section
1861(ii) of the Act);
• Anesthesiologist’s Assistant (as defined at §410.69); or
• Registered dietician or nutrition professional.
Other types of licensed healthcare professionals have a more limited scope of practice and
usually are not eligible for hospital medical staff privileges, unless their permitted scope of
practice in their State makes them more comparable to the above listed types of non-
physician practitioners. Some examples of types of such licensed healthcare professionals
who might be eligible for medical staff privileges, depending on State law and medical staff
bylaws, rules and regulations include, but are not limited to:
• Physical Therapist (as defined at §410.60 and §484.4);
• Occupational Therapist (as defined at §410.59 and §484.4); and
• Speech Language Therapist (as defined at §410.62 and §484.4).
Furthermore, some States have established a scope of practice for certain licensed
pharmacists who are permitted to provide patient care, services that make them more like
the above types of non-physician practitioners, including the monitoring and assessing of
patients and ordering medications and laboratory tests. In such States, a hospital may grant
medical staff privileges to such pharmacists and/or appoint them as members of the medical
staff. There is no standard term for such pharmacists, although they are sometimes referred
to as “clinical pharmacists.”
Practitioners may be granted active, courtesy, emergency, temporary, etc. membership or
privileges in accordance with state law and as specified in the medical staff bylaws, rules,
and regulations.
Survey Procedures §482.12(a)(1)
Review documentation and verify that the governing body has determined and stated the
categories of physicians and practitioners that are eligible candidates for appointment to the
medical staff or to be granted medical staff privileges.
A-0046
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The governing body must:]
§482.12(a)(2) Appoint members of the medical staff after considering the
recommendations of the existing members of the medical staff;
Interpretive Guidelines §482.12(a)(2)
The governing body determines whether to grant, deny, continue, revise, discontinue, limit,
or revoke specified privileges, including medical staff membership, for a specific
practitioner after considering the recommendation of the medical staff. In all instances, the
governing body’s determination must be consistent with established hospital medical staff
criteria, as well as with State and Federal law and regulations. Only the hospital’s
governing body has the authority to grant a practitioner privileges to provide care in the
hospital.
Survey Procedures §482.12(a)(2)
• Review records of medical staff appointments to determine that the governing body
is involved in appointments of medical staff members.
• Confirm that there is evidence that the governing body considered recommendations
of the medical staff before making medical staff appointments.
A-0047
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The governing body must:]
§482.12(a)(3) Assure that the medical staff has bylaws;
Interpretive Guidelines §482.12(a)(3)
The governing body must assure that the medical staff has bylaws and that those bylaws
comply with State and Federal law and the requirements of the Medicare hospital
Conditions of Participation.
Survey Procedures §482.12(a)(3)
Verify that the medical staff operates under current bylaws that are in accordance with
Federal and State laws and regulations.
A-0048
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The governing body must:]
§482.12(a)(4) Approve medical staff bylaws and other medical staff rules
and regulations;
Interpretive Guidelines §482.12(a)(4)
The governing body decides whether or not to approve medical staff bylaws submitted by
the medical staff. The medical staff bylaws and any revisions must be approved by the
governing body before they are considered effective.
Survey Procedures and §482.12(a)(4)
• Verify that the medical staff operates under current bylaws, rules and policies that
have been approved by the governing body.
• Verify that any revisions or modifications in the medical staff bylaws, rules and
policies have been approved by the medical staff and the governing body, e.g.,
bylaws are annotated with date of last review and initialed by person(s) responsible.
A-0049
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The governing body must:]
§482.12(a)(5) Ensure that the medical staff is accountable to the governing
body for the quality of care provided to patients;
Interpretive Guidelines §482.12(a)(5)
The governing body must ensure that the medical staff as a group is accountable to the
governing body for the quality of care provided to patients. The governing body is
responsible for the conduct of the hospital and this conduct includes the quality of care
provided to patients.
All hospital patients must be under the care of a practitioner who meets the criteria of 42
CFR 482.12(c)(1)and who has been granted medical staff privileges, or under the care of a
practitioner who is directly under the supervision of a member of the medical staff. All
patient care is provided by or in accordance with the orders of a practitioner who has been
granted privileges in accordance with the criteria established by the governing body, and
who is working within the scope of those granted privileges.
Survey Procedures §482.12(a)(5)
• Verify that the governing body is periodically apprised of the medical staff
evaluation of patient care services provided hospital wide, at every patient care
location of the hospital.
• Verify that any individual providing patient care services is a member of the
medical staff or is accountable to a member of the medical staff qualified to evaluate
the quality of services provided, and in turn, is responsible to the governing body for
the quality of services provided.
A-0050
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The governing body must:]
§482.12(a)(6) Ensure the criteria for selection are individual character,
competence, training, experience, and judgment; and
Interpretive Guidelines §482.12(a)(6)
The governing body must assure that the medical staff bylaws describe the privileging
process to be used by the hospital. The process articulated in the medical staff bylaws,
rules, or regulations must include criteria for determining the privileges that may be granted
to individual practitioners and a procedure for applying the criteria to individual
practitioners that considers:
• Individual character;
• Individual competence;
• Individual training;
• Individual experience; and
• Individual judgment.
The governing body must ensure that the hospital’s bylaws governing medical staff
membership or the granting of privileges apply equally to all practitioners in each
professional category of practitioners.
Survey Procedures §482.12(a)(6)
• Verify that there are written criteria for appointments to the medical staff and
granting of medical staff privileges.
• Verify that granting of medical staff membership or privileges, both new and
renewal, is based upon an individual practitioner’s meeting the medical staff’s
membership/privileging criteria.
• Verify that, at a minimum, criteria for appointment to the medical staff/granting of
medical staff privileges are individual character, competence, training, experience,
and judgment.
A-0051
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The governing body must:]
§482.12(a)(7) Ensure that under no circumstances is the accordance of
staff membership or professional privileges in the hospital dependent
solely upon certification, fellowship or membership in a specialty body or
society.
Interpretive Guidelines §482.12(a)(7)
In making a judgment on medical staff membership, a hospital may not rely solely on the
fact that a MD/DO is, or is not, board-certified. This does not mean that a hospital is
prohibited from requiring board certification when considering a MD/DO for medical staff
membership, but only that such certification must not be the only factor that the hospital
considers. In addition to matters of board certification, a hospital must also consider other
criteria such as training, character, competence and judgment. After analysis of all of the
criteria, if all criteria are met except for board certification, the hospital has the discretion to
decide not to select that individual to the medical staff.
Survey Procedures §482.12(a)(7)
Verify that written criteria for appointment to the medical staff and granting of medical
staff privileges are not dependent solely upon certification, fellowship, or membership in a
specialty body or society.
A-0052
(Rev.78, Issued: 12-22-11, Effective/Implementation: 12-22-11)
[The governing body must:]
§482.12(a)(8) Ensure that, when telemedicine services are furnished to the hospital’s
patients through an agreement with a distant-site hospital, the agreement is written
and that it specifies that it is the responsibility of the governing body of the distant-site
hospital to meet the requirements in paragraphs (a)(1) through (a)(7) of this section
with regard to the distant –site hospital’s physicians and practitioners providing
telemedicine services. The governing body of the hospital whose patients are receiving
the telemedicine services may, in accordance with §482.22(a)(3) of this part, grant
privileges based on its medical staff recommendations that rely on information
provided by the distant-site hospital.
§482.12(a)(9) Ensure that when telemedicine services are furnished to the hospital’s
patients through an agreement with a distant-site telemedicine entity, the written
agreement specifies that the distant-site telemedicine entity is a contractor of services
to the hospital and as such, in accordance with §482.12(e), furnishes the contracted
services in a manner that permits the hospital to comply with all applicable conditions
of participation for the contracted services, including, but not limited to, the
requirements in paragraphs (a)(1) through (a)(7) of this section with regard to the
distant-site telemedicine entity’s physicians and practitioners providing telemedicine
services. The governing body of the hospital whose patients are receiving the
telemedicine services may, in accordance with §482.22(a)(4) of this part, grant
privileges to physicians and practitioners employed by the distant-site telemedicine
entity based on such hospital’s medical staff recommendations; such staff
recommendations may rely on information provided by the distant-site telemedicine
entity.
Interpretive Guidelines §482.12(a)(8)&(a)(9)
“Telemedicine,” as the term is used in this regulation, means the provision of clinical
services to patients by physicians and practitioners from a distance via electronic
communications. The distant-site telemedicine physician or practitioner provides clinical
services to the hospital patient either simultaneously, as is often the case with teleICU
services, for example, or non-simultaneously, as may be the case with many teleradiology
services. “Simultaneously” means that the clinical services (for example, assessment of the
patient with a clinical plan for treatment, including any medical orders needed) are provided
to the patient in “real time” by the telemedicine physician or practitioner, similar to the
actions of an on-site physician or practitioner. “Non-simultaneously” means that, while the
telemedicine physician or practitioner still provides clinical services to the patient upon a
formal request from the patient’s attending physician, such services may involve after-the-
fact interpretation of diagnostic tests in order to provide an assessment of the patient’s
condition and do not necessarily require the telemedicine practitioner to directly assess the
patient in “real time.” This would be similar to the services provided by an on-site
radiologist who interprets a patient’s x-ray or CT scan and then communicates his or her
assessment to the patient’s attending physician who then bases his or her diagnosis and
treatment plan on these findings. (See 76 FR 25551-25552, May 5, 2011)
A hospital may make arrangements through written agreements either with a distant-site
Medicare-participating hospital or a distant-site telemedicine entity for the provision of
telemedicine services to the hospital’s patients by physicians or practitioners who have been
granted privileges by the distant-site hospital or telemedicine entity. For the purposes of
this rule, a distant-site telemedicine entity is defined as an entity that -- (1) provides
telemedicine services; (2) is not a Medicare-participating hospital; and (3) provides
contracted services in a manner that enables a hospital using its services to meet all
applicable CoPs, particularly those requirements related to the credentialing and privileging
of practitioners providing telemedicine services to the patients of a hospital. A distant-site
telemedicine entity would include a distant-site hospital that does not participate in the
Medicare program that is providing telemedicine services to a Medicare-participating
hospital. (See 76 FR 25553, May 5, 2011)
If a hospital enters into an agreement for telemedicine services with a distant-site hospital
or telemedicine entity, the agreement must be in writing. Furthermore, the written
agreement must specify, in the case of a:
• Distant-site hospital, that it is the responsibility of the governing body of the
distant-site hospital to satisfy the requirements of §§482.12(a)(1) through (a) (7)
with respect to those physicians and practitioners at the distant-site hospital who
furnish telemedicine services under the agreement. Since the distant-site hospital
must also be a Medicare-participating hospital (see §482.22(a)(3)), it has an
independent obligation to comply with these governing body requirements
concerning medical staff membership and privileging. Nevertheless, the written
agreement between the hospital and the distant-site hospital must explicitly
include a provision addressing the distant-site hospital’s obligation to comply with
these provisions.
• Distant-site telemedicine entity, that the written agreement specifies that they
entity is a contractor providing telemedicine services to the hospital, and that, in
accordance with the requirements governing services under arrangement at
§482.12(e), the telemedicine entity furnishes the contracted telemedicine services
in a manner that permits the hospital to comply with the Conditions of
Participation, including, but not limited to, the governing body requirements of
§§482.12(a)(1) through (a) (7) with respect to those physicians and practitioners at
the distant-site telemedicine entity who furnish telemedicine services under the
agreement.
There are additional requirements for the content of the written agreement, specified at
§482.22(a)(3) and §482.22(a)(4) under the medical staff Condition of Participation, which
are discussed in the interpretive guidelines for those regulations.
The hospital’s governing body must grant privileges to each telemedicine physician or
practitioner providing services at the hospital under an agreement with a distant-site
hospital or telemedicine entity before they may provide telemedicine services. The scope
of the privileges in the hospital must reflect the provision of the services via a
telecommunications system. For example, a surgeon at a distant-site hospital may provide
telemedicine consultation services at a hospital under agreement, but obviously would not
be able to perform surgery by this means and must not have surgical privileges in the
hospital as part of his/her telemedicine services privileges. If the surgeon also periodically
performed surgery on-site at the hospital, then he or she would have to have privileges to do
so, granted in the traditional manner provided for at §482.12(a)(1) through §482.12(a)(7)
and §482.22(a)(1) and §482.22(a)(2).
In granting privileges to telemedicine physicians and practitioners, the hospital’s governing
body has the option of considering hospital medical staff recommendations that rely, in
accordance with §482.22(a)(3) and §482.22(a)(4), upon the credentialing and privileging
decisions of the distant-site hospital or telemedicine entity. With respect to the decisions of
a distant-site telemedicine entity, the regulation states that this streamlined privileging
option is available to the hospital for physicians and practitioners “employed” by the
distant-site telemedicine entity. We are interpreting “employed” in this context to mean
“utilized by” the distant-site telemedicine entity to provide telemedicine services to the
hospital under an agreement. Since it is common for telemedicine entities to contract with,
rather than employ, the physicians and practitioners it utilizes to provide telemedicine
services, it would not be reasonable or consistent with the regulatory intent to interpret
“employed” to mean that the physicians or practitioners are employees of the distant-site
telemedicine entity.
When the hospital’s governing body exercises the option to grant privileges based on its
medical staff recommendations that rely upon the privileging decisions of a distant-site
telemedicine hospital or entity, it may, but is not required to, maintain a separate file on
each telemedicine physician and practitioner, or may instead have a file on all telemedicine
physicians and practitioners providing services at the hospital under each agreement with a
distant-site hospital or telemedicine entity, indicating which telemedicine services
privileges the hospital has granted to each physician and practitioner on the list.
Relying upon the credentialing and privileging decisions of the distant-site hospital or
telemedicine entity is an option available to the hospital’s governing body, not a
requirement. A governing body may, if it so chooses, require its medical staff to
independently review the credentials of and make privileging recommendations for each
telemedicine physician and practitioner in accordance with §482.22(a)(1) and
§482.22(a)(2), rather than permit its medical staff to rely upon the privileging decisions of
the distant-site hospital or telemedicine entity. The agreement with the distant-site hospital
or telemedicine entity may not require the hospital to rely upon the distant-site
organization’s privileging decisions.
Survey Procedures §482.12(a)(8)&(a)(9)
• Ask the hospital’s leadership whether it uses telemedicine services. If yes:
• Ask to see a copy of the written agreement(s) with the distant-site hospital(s) or
telemedicine entity(ies). Does each agreement include the required elements
concerning credentialing and privileging of the telemedicine physicians and
practitioners?
• Does the hospital have documentation indicating that it granted privileges to each
telemedicine physician and practitioner?
• Does the documentation indicate that for each telemedicine physician and
practitioner there is a medical staff recommendation, including an indication of
whether the medical staff conducted its own review or relied upon the decisions of
the distant-site hospital or telemedicine entity?
A-0053
(Rev. 122, Issued: 09-26-14, Effective: 09-26-14, Implementation: 09-26-14)
[The governing body must:]
§482.12(a)(10) Consult directly with the individual assigned the responsibility for the
organization and conduct of the hospital’s medical staff, or his or her designee. At a
minimum, this direct consultation must occur periodically throughout the fiscal or
calendar year and include discussion of matters related to the quality of medical care
provided to patients of the hospital. For a multi-hospital system using a single
governing body, the single multi-hospital system governing body must consult directly
with the individual responsible for the organized medical staff (or his or her designee)
of each hospital within its system in addition to the other requirements of this
paragraph (a).
Interpretive Guidelines §482.12(a)(10)
In accordance with §482.22(b)(3), there must be an individual member of the hospital’s
medical staff who is assigned responsibility for the organization and conduct of the
medical staff (for purposes of this guidance, the “leader” of the medical staff).
§482.12(a)(10) requires that the governing body consult with this individual, or with
someone the leader of the medical staff has designated.
“Direct consultation” means that the governing body, or a subcommittee of the governing
body, meets with the leader(s) of the medical staff(s), or his/her designee(s) either face-to-
face or via a telecommunications system permitting immediate, synchronous
communication. (79 FR 27113, May 12, 2014)
This regulation does not preclude a hospital from having a member of the medical staff
serve as a member of the hospital’s governing body. However, membership on the
governing body by a medical staff member is not sufficient per se to satisfy the requirement
for periodic consultation. In such a situation the hospital meets the consultation
requirement only if the medical staff member serving on the governing body is the leader of
the medical staff, or his or her designee, and only if such membership includes meeting
with the board periodically throughout the fiscal or calendar year and discussing matters
related to the quality of medical care provided to patients of the hospital. If there were a
change in the medical staff leadership or his/her designee, and the bylaws governing terms
and conditions of governing body membership did not allow for substitution of the new
leader of the medical staff (or his or her designee) on the governing body, then the
governing body would be expected to engage in direct consultation with the new leader of
the medical staff, or his or her designee.
It should be noted that if a hospital chooses to have the leader of the medical staff, or his or
her designee, serve on the governing body, there is nothing in the regulation which
prohibits the hospital from also including other medical staff members on the governing
body in addition to the leader of the medical staff, or his or her designee.
In the case of a multi-hospital system that has one single governing body, the governing
body must consult with each separately certified hospital’s medical staff leader, or his/her
designee. The consultations do not have to be separate. For example, the system governing
body could periodically have a meeting that includes the leader of the medical staff, or
his/her designee, from each hospital within the system, so long as there is discussion of
matters related to the quality of medical care provided to the patients of each hospital.
If the medical staff members at separately certified hospitals in a multi-hospital system and
the hospital system’s governing body also have opted to have a unified medical staff (see
guidance for §482.22(b)(4)) for some or all of the hospitals in the system, then the
governing body must consult with the leader of the unified medical staff or his/her
designee. In this case, the leader of the unified medical staff, or the designee, as applicable,
is expected to be aware of the concerns/views of members of the medical staff practicing at
each separately certified hospital using the unified medical staff.
It is up to the governing body as to whether the leader of the medical staff must make the
designation in writing when he or she chooses to designate another individual for these
periodic consultations, or whether the leader of the medical staff may make informal, ad
hoc designations. It is also up to the governing body as to whether it wishes to establish
minimum advance notice of a designation from the leader of the medical staff to the
governing body.
The requirement for the governing body to consult periodically throughout the year leaves
some flexibility for the governing body to determine how often during the year its
consultations with the leader of the medical staff or designee would occur, but it is expected
that consultations occur at least twice during either a calendar or fiscal year. (“Fiscal year”
refers to the Medicare cost-reporting year for the hospital; in the case of a hospital system
with multiple, separately certified hospitals that have one single governing body and a
unified medical staff, it is possible that individual hospitals have separate fiscal years. In
this case, it would be more practical for the governing body to use a calendar year basis for
determining the frequency of consultation.)
The governing body is expected to determine the number of consultations needed based on
various factors specific to the hospital, or to each of the hospitals within a multi-hospital
system. These factors include, but are not limited to, the scope and complexity of hospital
services offered, specific patient populations served by a hospital, and any issues of patient
safety and quality of care that a hospital’s quality assessment and performance
improvement program might periodically identify as needing the attention of the governing
body in consultation with its medical staff. The hospital must also provide evidence that
the governing body is appropriately responsive to any periodic and/or urgent requests from
the leader of the medical staff or designee for timely consultation on issues regarding the
quality of medical care provided to patients of the hospital. (79 FR 27112, May 12, 2014).
The “year” referenced in the regulation may be either the calendar year or the hospital’s
fiscal year, as identified on its Medicare cost report. It is up to the hospital which approach
it will take, but it must document the approach selected and consistently apply it. For
example, if a hospital chooses to use the calendar year, and had only one consultation
during a calendar year, it could not then point out that it had had two meetings during the
time period covered by its fiscal year.
The required consultation must include discussion of matters related to the quality of
medical care provided to the hospital’s patients, or, in the case of a hospital system with one
single governing body and a unified medical staff, the quality of medical care provided to
each separately certified hospital’s patients.
The hospital’s governing body must adopt policies and procedures addressing how it
implements the requirement for periodic, direct consultation with the leader of the medical
staff, or the designee. The hospital must have evidence that the required consultations do
take place, such as meeting agendas and lists of attendees, or minutes taken of the
discussion, including who was present, etc., and that matters related to the quality of
medical care provided to patients of the hospital were discussed.
Survey Procedures §482.12(a)(10)
• Ask the hospital’s CEO how the hospital complies with the requirement for
periodic consultations by the governing body with the leader of the hospital’s
medical staff, or the leader’s designee. Can the CEO provide evidence that such
consultations have occurred, e.g., meeting agendas and lists of attendees,
meeting minutes, etc.
• Ask the CEO whether the hospital tracks these consultations by the calendar
year or its fiscal year; ask to see a copy of the policy that establishes this.
• Is there evidence that the consultations were “direct?”
• Is there evidence that the governing body met with the medical staff leader
or designee at least twice during the previous year?
• Is there evidence that the discussion concerned matters related to the quality
of medical care in the hospital?
• Ask the leader of the hospital’s medical staff, or his/her designee, whether he or
she has had meetings with either the whole governing body or a subcommittee
of it to discuss the quality of medical care in the hospital.
• Has the leader/designee ever requested a meeting in addition to those
regularly scheduled, to discuss a matter of urgent concern to the medical
staff? If yes, did the governing body respond by setting up a meeting?
If the hospital shares a unified medical staff with other separately certified hospitals in a
multi-hospital system, the interview with the leader of the medical staff, or designee, may
have to be conducted by telephone. Ask the leader/designee how he/she gathers
information about the concerns/views of members of the medical staff practicing at the
hospital being surveyed about the quality of medical care provided at that hospital.
A-0057
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(b) Standard: Chief Executive Officer
The governing body must appoint a chief executive officer who is responsible for
managing the hospital.
Interpretive Guidelines §482.12(b)
The Governing Body must appoint one chief executive officer who is responsible for
managing the entire hospital.
Survey Procedures §482.12(b)
• Verify that the hospital has only one chief executive officer for the entire hospital.
• Verify that the governing body has appointed the chief executive officer.
• Verify that the chief executive officer is responsible for managing the entire
hospital.
A-0063
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(c) Standard: Care of Patients
In accordance with hospital policy, the governing body must ensure that the following
requirements are met:
A-0064
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[…the governing body must ensure that the following requirements are met:]
§482.12(c)(1) Every Medicare patient is under the care of:
(i) A doctor of medicine or osteopathy. (This provision is not to be
construed to limit the authority of a doctor of medicine or osteopathy to
delegate tasks to other qualified health care personnel to the extent recognized
under State law or a State’s regulatory mechanism.);
(ii) A doctor of dental surgery or dental medicine who is legally authorized
to practice dentistry by the State and who is acting within the scope of his or
her license;
(iii)A doctor of podiatric medicine, but only with respect to functions which he
or she is legally authorized by the State to perform;
(iv) A doctor of optometry who is legally authorized to practice optometry
by the State in which he or she practices;
(v) A chiropractor who is licensed by the State or legally authorized to
perform the services of a chiropractor, but only with respect to treatment by
means of manual manipulation of the spine to correct a subluxation
demonstrated by x-ray to exist; and
(vi) A clinical psychologist as defined in §410.71 of this chapter, but only
with respect to clinical psychologist services as defined in §410.71 of this
chapter and only to the extent permitted by State law.
Interpretive Guidelines §482.12(c)(1)
Practitioners other than doctors of medicine or osteopathy may join the medical staff if the
practitioners are appropriately licensed and medical staff membership is in accordance with
State law.
Every Medicare or Medicaid patient must be under the care of a licensed practitioner as
defined in this requirement.
Survey Procedures §482.12(c)(1)
Verify that Medicare patients are under the care of a licensed practitioner as defined by
(c)(1).
A-0065
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[…the governing body must ensure that the following requirements are met:]
§482.12(c)(2) Patients are admitted to the hospital only on the recommendation of
a licensed practitioner permitted by the State to admit patients to a hospital.
Survey Procedures §482.12(c)(2)
• Verify that admitting privileges are limited to those categories of practitioners as
allowed by State law.
• Verify that patients are admitted only by those practitioners who are currently
licensed and have been granted admitting privileges by the governing body in
accordance with State laws and medical staff bylaws.
A-0066
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[…the governing body must ensure that the following requirements are met:]
§482.12(c)(2) (continued)
If a Medicare patient is admitted by a practitioner not specified in paragraph
(c)(1) of this section, that patient is under the care of a doctor of medicine or
osteopathy.
Interpretive Guidelines §482.12(c)(2)
CMS hospital regulations do permit licensed practitioners (e.g., nurse practitioners,

midwives, etc), as allowed by the State, to admit patients to a hospital, and CMS does not
require these practitioners be employed by a MD/DO. However, CMS regulations do
require that Medicare and Medicaid patients admitted by these practitioners be under the
care of an MD/DO. Evidence of being under the care of an MD/DO must be in the
patient’s medical record. If a hospital allows these practitioners to admit and care for
patients, as allowed by State law, the governing body and medical staff would have to
establish policies and bylaws to ensure that the requirements of 42 CFR §482 are met.
Midwife Patients
42 CFR 482.1(a)(5) states, "Section 1905(a) of the Act provides that 'medical assistance'
(Medicaid) payments may be applied to various hospital services. Regulations interpreting
those provisions specify that hospitals receiving payment under Medicaid must meet the
requirements for participation in Medicare (except in the case of medical supervision of
nurse midwife services. See §§440.10 and 440.165 of this chapter)."
Midwives are not specified at 42 CFR §482.12(c)(1).
Section 482.1(a)(5), when taken together with this requirement (42 CFR 482.12(c)(2))
means that in a State that permits midwives to admit patients (and in accordance with
hospital policy and practitioner privileges), CMS requires ONLY Medicare patients of a
midwife be under the care of a doctor of medicine or osteopathy. CMS DOES NOT require
Medicaid or other non-Medicare patients admitted by a midwife to be under the care of a
doctor of medicine or osteopathy.
Survey Procedures §482.12(c)(2)
If the hospital grants admitting privileges to these practitioners, select Medicare and
Medicaid patients (select only Medicare patients for midwives) that are admitted to the
hospital by these practitioners. Determine if the patient is/was under the care of an
MD/DO.
A-0067
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[…the governing body must ensure that the following requirements are met:]
§482.12(c)(3) A doctor of medicine or osteopathy is on duty or on call at all times.
Survey Procedures §482.12(c)(3)
• Verify the governing body has established and monitors the enforcement of policies
that ensure a doctor of medicine or osteopathy is on duty or on call at all times to
provide medical care and onsite supervision when necessary.
• Review the “call” register and documents that assure that a doctor of medicine or
osteopathy is on duty or on call at all times.
• Interview nursing staff. How do they know who is on call? Are they able to call the
on-call MD/DO and speak with him/her at all times? When appropriate, do on-call
MD/DOs come to the hospital to provide needed care.
A-0068
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[…the governing body must ensure that the following requirements are met:]
§482.12(c)(4) A doctor of medicine or osteopathy is responsible for the care of each
Medicare patient with respect to any medical or psychiatric problem that--
(i) Is present on admission or develops during hospitalization; and
(ii) Is not specifically within the scope of practice of a doctor of dental
surgery, dental medicine, podiatric medicine, or optometry; a
chiropractor; or clinical psychologist, as that scope is--
(A) Defined by the medical staff;
(B) Permitted by State law; and
(C) Limited, under paragraph (c)(1)(v) of this section, with respect to
chiropractors.
Interpretive Guidelines §482.12(c)(4)
CMS hospital regulations do permit licensed practitioners (i.e., doctors of dental surgery,
dental medicine, podiatric medicine, or optometry; chiropractors; or clinical psychologists),
as allowed by the State, to admit patients to a hospital. However, CMS does require that
Medicare and Medicaid patients who are admitted by a doctor of dental surgery, dental
medicine, podiatric medicine, or optometry; a chiropractor; or clinical psychologist be
under the care of a MD/DO with respect to any medical or psychiatric problem that is
present on admission or develops during hospitalization that is outside the scope of practice
of the admitting practitioner. If a hospital allows a doctor of dental surgery, dental
medicine, podiatric medicine, or optometry, a chiropractor or a clinical psychologist to
admit and care for patients, as allowed by State law, the governing body and medical staff
must establish policies and bylaws to ensure that the requirements of 42 CFR §482 are met.
As applicable, the patient’s medical record must demonstrate MD/DO responsibility/care.
Survey Procedures §482.12(c)(4)
• Verify that an assigned doctor of medicine or osteopathy is responsible for and is
monitoring the care of each Medicare or Medicaid patient with respect to all medical
or psychiatric problems during the hospitalization.
• If non-MD/DOs admit patients, verify that every Medicare/Medicaid patient is being
monitored by an MD/DO who is responsible for any medical or psychiatric problem
outside the scope of practice of the admitting practitioners.
A-0073
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(d) Standard: Institutional Plan and Budget
The institution must have an overall institutional plan that meets the following
conditions:
(1) The plan must include an annual operating budget that is prepared
according to generally accepted accounting principles.
(2) The budget must include all anticipated income and expenses. This
provision does not require that the budget identify item by item the components of
each anticipated income or expense.
(3) The plan must provide for capital expenditures for at least a 3-year period,
including the year in which the operating budget specified in paragraph (d)(2) of
this section is applicable.
(4) The plan must include and identify in detail the objective of, and the
anticipated sources of financing for, each anticipated capital expenditure in excess
of $600,000 (or a lesser amount that is established, in accordance with section
1122(g)(1) of the Act, by the State in which the hospital is located) that relates to
any of the following:
(i) Acquisition of land;
(ii) Improvement of land, buildings, and equipment; or
(iii)The replacement, modernization, and expansion of buildings and
equipment.
Survey Procedures §482.12(d)
Verify that an institutional plan and budget exist, includes items 1-4, and complies with all
items in this standard. Do not review the specifics or format in the institutional plan or the
budget.
A-0074
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(d)(5) The plan must be submitted for review to the planning agency
designated in accordance with section 1122(b) of the Act, or if an agency is not
designated, to the appropriate health planning agency in the State. (See Part 100 of
this title.)
Survey Procedures §482.12(d)(5)
Determine that the hospital’s plan for capital expenditures has been submitted to the
planning agency designated to review capital expenditures. In certain cases facilities used
by HMO and CMP patients are exempt from the review process.
A-0075
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(d)(5) (Continued)
A capital expenditure is not subject to section 1122 review if 75 percent of the
health care facility’s patients who are expected to use the service for which the
capital expenditure is made are individuals enrolled in a health maintenance
organization (HMO) or competitive medical plan (CMP) that meets the
requirements of section 1876(b) of the Act, and if the Department determines that
the capital expenditure is for services and facilities that are needed by the HMO or
CMP in order to operate efficiently and economically and that are not otherwise
readily accessible to the HMO or CMP because--
(i) The facilities do not provide common services at the same site;
(ii) The facilities are not available under a contract of reasonable duration;
(iii) Full and equal medical staff privileges in the facilities are not available;
(iv) Arrangements with these facilities are not administratively feasible; or
(v) The purchase of these services is more costly than if the HMO or CMP
provided the services directly.
A-0076
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(d)(6) The plan must be reviewed and updated annually
Survey Procedures §482.12(d)(6)
Verify that the plan and budget are reviewed and updated annually.
A-0077
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(d)(7) The plan must be prepared--
(i) Under the direction of the governing body; and
(ii) By a committee consisting of representatives of the governing body, the
administrative staff, and the medical staff of the institution.
Survey Procedures §482.12(d)(7)
Verify that the governing body, administrative staff, and medical staff have participated in
the development of the institutional plan and budget.
A-0083
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(e) Standard: Contracted Services
The governing body must be responsible for services furnished in the hospital whether
or not they are furnished under contracts. The governing body must ensure that a
contractor of services (including one for shared services and joint ventures) furnishes
services that permit the hospital to comply with all applicable conditions of
participation and standards for the contracted services.
Interpretive Guidelines §482.12(e)
The governing body has the responsibility for assuring that hospital services are provided in
compliance with the Medicare Conditions of participation and according to acceptable
standards of practice, irrespective of whether the services are provided directly by hospital
employees or indirectly by contract. The governing body must take actions through the
hospital’s QAPI program to: assess the services furnished directly by hospital staff and
those services provided under contract, identify quality and performance problems,
implement appropriate corrective or improvement activities, and to ensure the monitoring
and sustainability of those corrective or improvement activities. See §482.21 QAPI.
Survey Procedures §482.12(e)
Ascertain that all contractor services provided in the hospital are in compliance with the
Conditions of Participation for hospitals.
A-0084
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(e)(1) The governing body must ensure that the services performed under
a contract are provided in a safe and effective manner.
Interpretive Guidelines §482.12(e)(1)
Indirect arrangements may take into consideration services provided through formal
contracts, joint ventures, informal agreements, shared services, or lease arrangements. The
patient care services, and all other services, provided under contract are subject to the same
hospital-wide quality assessment and performance improvement (QAPI) evaluation as other
services provided directly by the hospital.
Survey Procedures §482.12(e)(1)
• Determine if the hospital has a mechanism to evaluate the quality of each contracted
service and ensures that each contracted service is provided in a safe and effective
manner.
• Review the QAPI plan to ensure that every contracted service is evaluated.
A-0085
§482.12(e)(2) The hospital must maintain a list of all contracted services, including
the scope and nature of the services provided.
Survey Procedures §482.12(e)(2)
Review the list of contracted services and verify that there is a delineation of contractor
responsibility.
A-0091
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(f) Standard: Emergency Services
Interpretive Guidelines §482.12(f )
The hospital must ensure the emergency services requirements are met.

A-0092
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(f)(1) If emergency services are provided at the hospital, the hospital must
comply with the requirements of §482.55.
A-0093
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(f)(2) If emergency services are not provided at the hospital, the governing
body must assure that the medical staff has written policies and procedures for
appraisal of emergencies, initial treatment, and referral when appropriate.
Interpretive Guidelines §482.12(f)(2)
This requirement applies hospital-wide (all on-campus and off-campus locations) to
hospitals that do not provide emergency services.
Hospitals without emergency departments must have appropriate policies and procedures in
place for addressing individuals’ emergency care needs 24 hours per day and 7 days per
week, including the following:
• Appraisal of Persons with Emergencies: A hospital must have medical staff
policies and procedures for conducting appraisals of persons with emergencies.
The policies and procedures must ensure that:
o As required by 42 CFR 482.23(b), an RN is immediately available, as needed, to
provide bedside care to any patient and that,
o Among such RN(s) who are immediately available at all times, there must be an
RN(s) who is/are qualified, through a combination of education, licensure, and
training, to conduct an assessment that enables them to recognize the fact that a
person has a need for emergency care.
The policies and procedures for appraisal should provide that the MD/DO (on-site
or on-call) would directly provide appraisals of emergencies or provide medical
direction of on-site staff conducting appraisals.
• Initial Treatment: A hospital must have medical staff policies and procedures for
providing the initial treatment needed by persons with emergency conditions.
Among the RN(s) who must be available at all times in a hospital as required by 42
CFR 482.23(b), there must be RN(s) who are qualified, through a combination of
education, licensure, and training, to provide initial treatment to a person
experiencing a medical emergency. The on-site or on-call physician could provide
initial treatment directly or provide medical oversight and direction to other staff.
This requirement, taken together with other hospital regulatory requirements,
suggests that a prudent hospital would evaluate the patient population the hospital
routinely cares for in order to anticipate potential emergency care scenarios and
develop the policies, procedures, and staffing that would enable it to provide safe
and adequate initial treatment of an emergency.
• Referral when Appropriate: A hospital must have medical staff policies and
procedures to address situations in which a person’s emergency needs may exceed
the hospital’s capabilities. The policies and procedures should be designed to
enable hospital staff members who respond to emergencies to: (a) recognize when
a person requires a referral or transfer, and (b) assure appropriate handling of the
transfer. This includes arrangement for appropriate transport of the patient. Further,
in accordance with the Discharge Planning CoP at 42 CFR 482.43(d), the hospital
must transfer patients to appropriate facilities, i.e., those with the appropriate
capabilities to handle the patient’s condition. The regulation also requires that
necessary medical information be sent along with the patient being transferred. This
enables the receiving hospital to treat the medical emergency more efficiently.
• Patient Transportation and Emergency Medical Services (EMS)
A hospital may arrange transportation of the referred patient by several methods,
including using the hospital’s own ambulance service, the receiving hospital’s
ambulance service, a contracted ambulance service, or, in extraordinary
circumstances, alerting EMS via calling 9-1-1. There is no specific Medicare
prohibition on a hospital with or without an emergency department calling 9-1-1 in
order to obtain transport of a patient to another hospital. Use of 9-1-1 to obtain
transport does not, however, relieve the hospital of its obligation to arrange for the
patient’s transfer to an appropriate facility and to provide the necessary medical
information along with the patient.
A hospital policy or practice that relies on calling 9-1-1 in order for EMS to
substitute its emergency response capabilities for those the hospital is required to
maintain, as described above, is not consistent with the Medicare CoPs. For
example, a hospital may not rely upon 9-1-1 to provide appraisal and initial
treatment of medical emergencies that occur at the hospital. Such policy or
practice should be considered as condition-level non-compliance with the
applicable CoP, 42 CFR 482.55 or 42 CFR 482.12(f).
Survey Procedures §482.12(f)(2)
• Verify that the medical staff has adopted written policies and procedures for the
management of medical emergencies.
• Review emergency care policies and procedures. Are they consistent with the
expectations articulated above for appraisal, initial treatment, and referral? Do they
address emergency procedures for all on-campus and off-campus locations?
• Interview hospital staff at various locations. Can they state their duties and what
they are to do if an individual seeks or needs emergency care at their location?
A-0094
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.12(f)(3) If emergency services are provided at the hospital but are not provided
at one or more off-campus departments of the hospital, the governing body of the
hospital must assure that the medical staff has written policies and procedures in
effect with respect to the off-campus department(s) for appraisal of emergencies and
referral when appropriate.
Interpretive Guidelines §482.12(f)(3)
This requirement applies to any off-campus hospital department/location that does not
qualify as a dedicated emergency department in accordance with 42 CFR 489.24(b) and is
part of a hospital that provides emergency services. Such departments/locations must have
and must implement medical staff policies and procedures for the appraisal of emergencies
and referral when appropriate.

• Appraisal of Persons with Emergencies: A hospital must have medical staff policies
and procedures for conducting appraisals of persons with emergencies at off-campus
departments/locations that are not dedicated emergency departments. The policies and
procedures must ensure that clinical personnel -- who are qualified, through a
combination of education, licensure, and training, to conduct an assessment that enables
them to recognize the fact that a person has a need for emergency care -- are available
during all hours of operation at the off-campus department/location.
• Referral when Appropriate: A hospital must have medical staff policies and
procedures to address situations in which a person’s emergency needs may exceed the
capabilities of the off-campus departments/locations that are not dedicated emergency
departments. The policies and procedures should be designed to enable staff members
at such locations to: (a) recognize when a person requires a referral or transfer, and (b)
assure appropriate handling of the transfer. This includes arrangement for appropriate
transport of the patient along with the transfer of the patient’s medical information so
that the receiving hospital may treat the medical emergency more efficiently.
• Initial Treatment: Although there is no specific regulatory requirement for such off-
campus departments or locations to provide initial treatment of emergencies,
nevertheless they are expected to provide treatment and stabilization consistent with the
complexity of services, the type and qualifications of clinical staff, and the resources
available at that location. This expectation is based on the requirements of the
Outpatient Services CoP that hospital outpatient services meet the needs of the patients
in accordance with acceptable standards of practice, outpatient services must be
appropriately organized and integrated with inpatient services, and outpatient services
must have appropriate professional and nonprofessional personnel available. For
example, an off-campus cardiac rehabilitation clinic would be expected to have the
appropriate qualified staff, equipment (such as a crash cart), and policies and procedures
in place to appropriately provide appraisal, initial interventions, and referral of a patient
who experiences a cardiac emergency.
• A hospital policy or practice that relies on calling 9-1-1 in order for EMS to substitute
its emergency response capabilities for those the hospital is required to maintain at its
off-campus departments/locations, as described above, is not consistent with the
Medicare CoPs. However, given the more limited emergency capabilities that may be
present in some off-campus departments or locations, calling 9-1-1 to respond to an
emergency might be appropriate.
See the hospital emergency services CoP (42 CFR 482.55) for the emergency requirements
for the hospital’s locations that provide emergency services.
Survey Procedures §482.12(f)(3)
• Review emergency care policies and procedures. Determine if they address
emergency procedures for all off-campus locations.
• Interview off-campus hospital department staff. Can they state their duties and what
they are to do if an individual seeks emergency care?
A-0115
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13 Condition of Participation: Patient's Rights

A hospital must protect and promote each patient’s rights.
Interpretive Guidelines §482.13
These requirements apply to all Medicare or Medicaid participating hospitals including
short-term, acute care, surgical, specialty, psychiatric, rehabilitation, long-term, children's
and cancer, whether or not they are accredited. This rule does not apply to critical access
hospitals. (See Social Security Act (the Act) §1861(e).)
These requirements, as well as the other Conditions of Participation in 42 CFR 482, apply
to all parts and locations (outpatient services, provider-based entities, inpatient services) of
the Medicare participating hospital.
Survey Procedures §482.13
Survey of the Patients’ Rights Condition of Participation (CoP) should be coordinated by
one surveyor. However, each surveyor, as he/she conducts his/her survey assignments,
should assess the hospital’s compliance with the Patients’ Rights CoP.
A-0116
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(a) Standard: Notice of Rights
Interpretive Guidelines §482.13(a)
The hospital must ensure the notice of rights requirements are met.
A-0117
(Rev. 75, Issued: 12-02-11, Effective: 12-02-11, Implementation: 12-02-11)
§482.13(a)(1) A hospital must inform each patient, or when appropriate, the patient’s
representative (as allowed under State law), of the patient’s rights, in advance of
furnishing or discontinuing patient care whenever possible.
Interpretive Guidelines §482.13(a)(1)
The hospital must inform each patient, or when appropriate, the patient’s representative as
allowed by State law, of the patient’s rights. Whenever possible, this notice must be
provided before providing or stopping care. All patients, inpatient or outpatient, must be
informed of their rights as hospital patients. The patient’s rights include all of those
discussed in this condition, as well as any other rights for which notice is required under
State or Federal law or regulations for hospital patients. (See 42 CFR 482.11.) The
patient’s rights should be provided and explained in a language or manner that the patient
(or the patient’s representative) can understand. This is consistent with the guidance related
to Title VI of the Civil Rights Act of 1964 issued by the Department of Health and Human
Services - “Guidance to Federal Financial Assistance Recipients Regarding Title VI
Prohibition Against National Origin Discrimination Affecting Limited English Proficient
Persons” (August 8, 2003, 68 FR 47311). In accordance with §482.11, hospitals are
expected to comply with Title VI and may use this guidance to assist it in ensuring patient’s
rights information is provided in a language and manner that the patient understands.
Surveyors do not assess compliance with these requirements on limited English proficiency,
but may refer concerns about possible noncompliance to the Office for Civil Rights in the
applicable Department of Health and Human Services Regional Office.
Hospitals are expected to take reasonable steps to determine the patient’s wishes concerning
designation of a representative. Unless prohibited by applicable State law:
• When a patient who is not incapacitated has designated, either orally to hospital staff or
in writing, another individual to be his/her representative, the hospital must provide the
designated individual with the required notice of patients’ rights in addition to the
patient. The explicit designation of a representative takes precedence over any non-
designated relationship and continues throughout the patient’s inpatient stay or
outpatient visit, unless expressly withdrawn, either orally or in writing, by the patient.
• In the case of a patient who is incapacitated, when an individual presents the hospital
with an advance directive, medical power of attorney or similar document executed by
the patient and designating an individual to make medical decisions for the patient when
incapacitated, then the hospital must, when presented with the document, provide the
required notice of its policies to the designated representative. The explicit designation
of a representative takes precedence over any non-designated relationship and continues
throughout the patient’s inpatient stay or outpatient visit, unless the patient ceases to be
incapacitated and expressly withdraws the designation, either orally or in writing.
• When a patient is incapacitated or otherwise unable to communicate his or her wishes,
there is no written advance directive on file or presented, and an individual asserts that
he or she is the patient’s spouse, domestic partner (whether or not formally established
and including a same-sex domestic partner), parent (including someone who has stood
in loco parentis for the patient who is a minor child), or other family member and thus is
the patient’s representative, the hospital is expected to accept this assertion, without
demanding supporting documentation, and provide the required notice to the individual,
unless:
• More than one individual claims to be the patient’s representative. In such cases, it
would be appropriate for the hospital to ask each individual for documentation
supporting his/her claim to be the patient’s representative. The hospital should
make its determination of who is the patient’s representative based upon the
hospital’s determination of who the patient would most want to make decisions on
his/her behalf. Examples of documentation a hospital might consider could include,
but are not limited to, the following: proof of a legally recognized marriage,
domestic partnership, or civil union; proof of a joint household; proof of shared or
co-mingled finances; and any other documentation the hospital considers evidence
of a special relationship that indicates familiarity with the patient’s preferences
concerning medical treatment;
• Treating the individual as the patient’s representative without requesting supporting
documentation would result in the hospital violating State law. State laws,
including State regulations, may specify a procedure for determining who may be
considered to be the incapacitated patient’s representative, and may specify when
documentation is or is not required; or
• The hospital has reasonable cause to believe that the individual is falsely claiming to
be the patient’s spouse, domestic partner, parent or other family member.
Hospitals are expected to adopt policies and procedures that facilitate expeditious and
non-discriminatory resolution of disputes about whether an individual is the patient’s
representative, given the critical role of the representative in exercising the patient’s
rights.
A refusal by the hospital of an individual’s request to be treated as the patient’s
representative, based on one of the above-specified familial relationships, must be

documented in the patient’s medical record, along with the specific basis for the refusal.
In addition, according to the regulation at 42 CFR 489.27(a), (which cross references the
regulation at 42 CFR 405.1205), each Medicare beneficiary who is an inpatient (or his/her
representative) must be provided the standardized notice, “An Important Message from
Medicare” (IM), within 2 days of admission. Medicare beneficiaries who have not been
admitted (e.g., patients in observation status or receiving other care on an outpatient basis)
are not required to receive the IM. The IM is a standardized, OMB-approved form and
cannot be altered from its original format. The IM is to be signed and dated by the patient
to acknowledge receipt. See Exhibit 16 for a copy of the IM. Furthermore, 42 CFR
405.1205(c) requires that hospitals present a copy of the signed IM in advance of the
patient’s discharge, but not more than two calendar days before the patient’s discharge. In
the case of short inpatient stays, however, where initial delivery of the IM is within 2
calendar days of the discharge, the second delivery of the IM is not required.
The hospital must establish and implement policies and procedures that effectively ensure
that patients and/or their representatives have the information necessary to exercise their
rights.
Survey Procedures §482.13(a)(1)
• Determine the hospital’s policy for notifying all patients of their rights, both inpatient
and outpatient;
• Determine that the hospital’s policy provides for determining when a patient has a
representative and who that representative is, consistent with this guidance and State
law.
• Determine that the information provided to the patients by the hospital complies with
Federal and State law;
• Review records and interview staff to examine how the hospital communicates
information about their rights to diverse patients, including individuals who need
assistive devices or translation services. Does the hospital have alternative means, such
as written materials, signs, or interpreters (when necessary), to communicate patients’
rights?
• Review records and interview staff and patients or patients’ representatives (as
appropriate) to examine how the hospital determines whether the patient has a
representative, who that representative is, and whether notice of patients’ rights is
provided as required to patients’ representatives.
• Ask patients to tell you what the hospital has told them about their rights;
• Does staff know what steps to take to inform a patient about their patients’ rights,
including those patients’ with special communication needs?; and
• Review a sample of inpatient medical records for Medicare beneficiaries, to determine
whether the records contain a signed and dated IM provided within 2 days of the
admission of the patient. For patients whose discharge occurred more than 2 days after
the initial IM notice was issued, determine whether the hospital provided another copy
of the IM to the patient prior to discharge in a timely manner.
A-0118
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)

§482.13(a)(2) The hospital must establish a process for prompt resolution of patient
grievances and must inform each patient whom to contact to file a grievance.
Interpretive guidelines §482.13(a)(2)
The patient should have reasonable expectations of care and services and the facility should
address those expectations in a timely, reasonable, and consistent manner. Although
482.13(a)(2)(ii) and (iii) address documentation of facility time frames for a response to a
grievance, the expectation is that the facility will have a process to comply with a relatively
minor request in a more timely manner than a written response. For example, a change in
bedding, housekeeping of a room, and serving preferred food and beverage may be made
relatively quickly and would not usually be considered a "grievance" and therefore would
not require a written response.
The hospital must inform the patient and/or the patient's representative of the internal
grievance process, including whom to contact to file a grievance (complaint). As part of its
notification of patient rights, the hospital must provide the patient or the patient's
representative a phone number and address for lodging a grievance with the State agency.
The hospital must inform the patient that he/she may lodge a grievance with the State
agency (the State agency that has licensure survey responsibility for the hospital) directly,
regardless of whether he/she has first used the hospital's grievance process.
A “patient grievance” is a formal or informal written or verbal complaint that is made to
the hospital by a patient, or the patient’s representative, regarding the patient's care (when
the complaint is not resolved at the time of the complaint by staff present), abuse or neglect,
issues related to the hospital's compliance with the CMS Hospital Conditions of
Participation (CoPs), or a Medicare beneficiary billing complaint related to rights and
limitations provided by 42 CFR 489.
• "Staff present" includes any hospital staff present at the time of the complaint or
who can quickly be at the patient's location (i.e., nursing, administration, nursing
supervisors, patient advocates, etc.) to resolve the patient's complaint.
• If a patient care complaint cannot be resolved at the time of the complaint by staff
present, is postponed for later resolution, is referred to other staff for later
resolution, requires investigation, and/or requires further actions for resolution, then
the complaint is a grievance for the purposes of these requirements. A complaint is
considered resolved when the patient is satisfied with the actions taken on their
behalf.
• Billing issues are not usually considered grievances for the purposes of these
requirements. However, a Medicare beneficiary billing complaint related to rights
and limitations provided by 42 CFR 489 is considered a grievance.
• A written complaint is always considered a grievance. This includes written
complaints from an inpatient, an outpatient, a released/discharged patient, or a
patient’s representative regarding the patient care provided, abuse or neglect, or the
hospital's compliance with CoPs. For the purposes of this requirement, an email or
fax is considered "written."
• Information obtained from patient satisfaction surveys usually does not meet the
definition of a grievance. If an identified patient writes or attaches a written
complaint on the survey and requests resolution, then the complaint meets the
definition of a grievance. If an identified patient writes or attaches a complaint to
the survey but has not requested resolution, the hospital must treat this as a
grievance if the hospital would usually treat such a complaint as a grievance.
• Patient complaints that are considered grievances also include situations where a
patient or a patient's representative telephones the hospital with a complaint
regarding the patient’s care or with an allegation of abuse or neglect, or failure of
the hospital to comply with one or more CoPs, or other CMS requirements. Those
post-hospital verbal communications regarding patient care that would routinely
have been handled by staff present if the communication had occurred during the
stay/visit are not required to be defined as a grievance.
• All verbal or written complaints regarding abuse, neglect, patient harm, or hospital
compliance with CMS requirements are considered grievances for the purposes of
these requirements.
• Whenever the patient or the patient's representative requests that his or her
complaint be handled as a formal complaint or grievance or when the patient
requests a response from the hospital, the complaint is considered a grievance and
all the requirements apply.
• Data collected regarding patient grievances, as well as other complaints that are not
defined as grievances (as determined by the hospital), must be incorporated in the
hospital's Quality Assessment and Performance Improvement (QAPI) Program.
Survey Procedures §482.13(a)(2)
• Review the hospital’s policies and procedures to assure that its grievance process
encourages all personnel to alert appropriate staff concerning any patient grievance.
Does the hospital adhere to its policy/procedure established for grievances?
• Interview patients or the patient’s legal representative to determine if they know
how to file a complaint (grievance) and who to contact if they have a complaint
(grievance).
• Is the hospital following its grievance policies and procedures?
• Does the hospital’s process assure that grievances involving situations or practices
that place the patient in immediate danger are resolved in a timely manner?
• Does the patient or the patient’s representative know that he/she has the right to file
a complaint with the State agency as well as or instead of utilizing the hospital’s
grievance process?
• Has the hospital provided the telephone number for the State agency to all
patients/patient representatives?
• Are beneficiaries aware of their right to seek review by the QIO for quality of care
issues, coverage decisions, and to appeal a premature discharge?
A-0119
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(a)(2) (Continued)
[The hospital must establish a process for prompt resolution of patient grievances and
must inform each patient whom to contact to file a grievance.] The hospital’s
governing body must approve and be responsible for the effective operation of the
grievance process, and must review and resolve grievances, unless it delegates the
responsibility in writing to a grievance committee.
Interpretive guidelines §482.13(a)(2)
The hospital's grievance process must be approved by the governing body. The hospital's
governing body is responsible for the effective operation of the grievance process. This
includes the hospital's compliance with all of the CMS grievance process requirements. The
hospital's governing body must review and resolve grievances, unless it delegates this
responsibility in writing to a grievance committee. A committee is more than one person.
The committee membership should have adequate numbers of qualified members to review
and resolve the grievances the hospital receives (this includes providing written responses)
in a manner that complies with the CMS grievance process requirements.
Survey Procedures §482.13(a)(2)
• Determine if the hospital’s governing body approved the grievance process.
• Is the governing body responsible for the operation of the grievance process, or has
the governing body delegated the responsibility in writing to a grievance
committee?
• Determine how effectively the grievance process works. Are patient's or the patient
representative’s concerns addressed in a timely manner? Are patients informed of
any resolution to their grievances? Does the hospital apply what it learns from the
grievance as part of its continuous quality improvement activities?
• Is the grievance process reviewed and analyzed through the hospital’s QAPI process
or some other mechanisms that provides oversight of the grievance process?
A-0120
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(a)(2) (Continued)
[The hospital must establish a process for prompt resolution of patient grievances and
must inform each patient whom to contact to file a grievance. The hospital’s
governing body must approve and be responsible for the effective operation of the
grievance process, and must review and resolve grievances, unless it delegates the
responsibility in writing to a grievance committee.] The grievance process must
include a mechanism for timely referral of patient concerns regarding quality of care
or premature discharge to the appropriate Utilization and Quality Control Quality
Improvement Organization. At a minimum:
Interpretive Guidelines §482.13(a)(2)
Quality Improvement Organizations (QIOs) are CMS contractors charged with reviewing
the appropriateness and quality of care rendered to Medicare beneficiaries in the hospital
setting. The QIOs are also tasked with reviewing utilization decisions. Part of this duty
includes reviewing discontinuation of stay determinations based upon a beneficiary’s
request. The regulations state the functions of the QIOs in order to make Medicare
beneficiaries aware of the fact that if they have a complaint regarding quality of care,
disagree with a coverage decision, or they wish to appeal a premature discharge, they may

contact the QIO to lodge a complaint. The hospital is required to have procedures for
referring Medicare beneficiary concerns to the QIOs; additionally, CMS expects
coordination between the grievance process and existing grievance referral procedures so
that beneficiary complaints are handled timely and referred to the QIO at the beneficiary’s
request.
This regulation requires coordination between the hospital’s existing mechanisms for
utilization review notice and referral to QIOs for Medicare beneficiary concerns (See
42 CFR Part 489.27). This requirement does not mandate that the hospital automatically
refer each Medicare beneficiary’s grievance to the QIO; however, the hospital must inform
all beneficiaries of this right, and comply with his or her request if the beneficiary asks for
QIO review.
Medicare patients have the right to appeal a premature discharge (see Interpretive
Guidelines for 42 CFR 482.13(a)). Pursuant to 42 CFR 412.42(c)(3), a hospital must
provide a hospital-issued notice of non-coverage (HINN) to any fee-for-service beneficiary
that expresses dissatisfaction with an impending hospital discharge. Medicare Advantage
(MA) organizations are required to provide enrollees with a notice of non-coverage, known
as the Notice of Discharge and Medicare Appeal Rights (NODMAR), only when a
beneficiary disagrees with the discharge decision or when the MA organization (or hospital,
if the MA organization has delegated to it the authority to make the discharge decision) is
not discharging the enrollee, but no longer intends to cover the inpatient stay.
Survey Procedures §482.13(a)(2)
• Review patient discharge materials. Is the hospital in compliance with 42 CFR
§489.27?
• Does the hospital grievance process include a mechanism for timely referral of
Medicare patient concerns to the QIO? What time frames are established?
• Interview Medicare patients. Are they aware of their right to appeal premature
discharge?
A-0121
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[At a minimum:]
§482.13(a)(2)(i) The hospital must establish a clearly explained procedure for the
submission of a patient’s written or verbal grievance to the hospital.
Interpretive Guidelines §482.13(a)(2)(i)
The hospital’s procedure for a patient or the patient’s representative to submit written or
verbal grievances must be clearly explained. The patient or patient’s representative should
be able to clearly understand the procedure.
Survey Procedures §482.13(a)(2)(i)
• Review the information provided to patients that explains the hospital’s grievance
procedures. Does it clearly explain how the patient is to submit either a verbal or
written grievance?
• Interview patients or patient representatives. Does the patient, or (if he/she is

incapacitated) his/her representative, know about the grievance process and how to
submit a grievance?
A-0122
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[At a minimum:]
§482.13(a)(2)(ii) The grievance process must specify time frames for review of the
grievance and the provision of a response.
Interpretive Guidelines §482.13(a)(2)(ii)
The hospital must review, investigate, and resolve each patient’s grievance within a
reasonable time frame. For example, grievances about situations that endanger the patient,
such as neglect or abuse, should be reviewed immediately, given the seriousness of the
allegations and the potential for harm to the patient(s). However, regardless of the nature of
the grievance, the hospital should make sure that it is responding to the substance of each
grievance while identifying, investigating, and resolving any deeper, systemic problems
indicated by the grievance.
Document when a grievance is so complicated that it may require an extensive
investigation. We recognize that staff scheduling as well as fluctuations in the numbers and
complexity of grievances can affect the timeframes for the resolution of a grievance and the
provision of a written response. On average, a time frame of 7 days for the provision of the
response would be considered appropriate. We do not require that every grievance be
resolved during the specified timeframe although most should be resolved. 42 CFR
482.13(a)(2)(iii) specifies information the hospital must include in their response.
If the grievance will not be resolved, or if the investigation is not or will not be completed
within 7 days, the hospital should inform the patient or the patient's representative that the
hospital is still working to resolve the grievance and that the hospital will follow-up with a
written response within a stated number of days in accordance with the hospital's grievance
policy. The hospital must attempt to resolve all grievances as soon as possible.
Survey Procedures §482.13(a)(2)(ii)
What time frames are established to review and respond to patient grievances? Are these
time frames clearly explained in the information provided to the patient that explains the
hospital’s grievance process? On average, does the hospital provide a written response to
most of its grievances within the timeframe specified in its policy?
A-0123
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[At a minimum:]
§482.13(a)(2)(iii) In its resolution of the grievance, the hospital must provide the
patient with written notice of its decision that contains the name of the hospital
contact person, the steps taken on behalf of the patient to investigate the grievance, the
results of the grievance process, and the date of completion.
Interpretive Guidelines §482.13(a)(2)(iii)
The written notice of the hospital’s determination regarding the grievance must be
communicated to the patient or the patient’s representative in a language and manner the
patient or the patient’s legal representative understands.
The hospital may use additional tools to resolve a grievance, such as meeting with the
patient and his family. The regulatory requirements for the grievance process are minimum
standards, and do not inhibit the use of additional effective approaches in handling patient
grievances. However, in all cases the hospital must provide a written notice (response) to
each patient’s grievance(s). The written response must contain the elements listed in this
requirement.
When a patient communicates a grievance to the hospital via email the hospital may
provide its response via email pursuant to hospital policy. (Some hospitals have policies
against communicating to patients over email.) If the patient requests a response via email,
the hospital may respond via email. When the email response contains the information
stated in this requirement, the email meets the requirement for a written response. The
hospital must maintain evidence of its compliance with these requirements.
A grievance is considered resolved when the patient is satisfied with the actions taken on
their behalf.
There may be situations where the hospital has taken appropriate and reasonable actions on
the patient's behalf in order to resolve the patient's grievance and the patient or the patient's
representative remains unsatisfied with the hospital's actions. In these situations, the
hospital may consider the grievance closed for the purposes of these requirements. The
hospital must maintain documentation of its efforts and demonstrate compliance with CMS
requirements.
In its written response, the hospital is not required to include statements that could be used
in a legal action against the hospital, but the hospital must provide adequate information to
address each item stated in this requirement. The hospital is not required to provide an
exhaustive explanation of every action the hospital has taken to investigate the grievance,
resolve the grievance, or other actions taken by the hospital.
Survey Procedures §482.13(a)(2)(iii)
Review the hospital’s copies of written notices (responses) to patients. Are all patients
provided a written notice? Do the notices comply with the requirements?
A-0129
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(b) Standard: Exercise of Rights
Interpretive Guidelines §482.13(b)
The hospital must ensure that the exercise of patients’ rights requirements are met.
A-0130
(Rev. 75, Issued: 12-02-11, Effective: 12-02-11, Implementation: 12-02-11)
§482.13(b)(1) The patient has the right to participate in the development and
implementation of his or her plan of care.
Interpretive Guidelines §482.13(b)(1)
This regulation requires the hospital to actively include the patient in the development,
implementation and revision of his/her plan of care. It requires the hospital to plan the
patient’s care, with patient participation, to meet the patient’s psychological and medical
needs.
The patient’s (or patient’s representatives, as allowed by State law) right to participate in
the development and implementation of his or her plan of care includes at a minimum, the
right to: participate in the development and implementation of his/her inpatient
treatment/care plan, outpatient treatment/care plan, participate in the development and
implementation of his/her discharge plan, and participate in the development and
implementation of his/her pain management plan.
Hospitals are expected to take reasonable steps to determine the patient’s wishes concerning
designation of a representative to exercise the patient’s right to participate in the
development and implementation of the patient’s plan of care. Unless prohibited by
applicable State law:
• When a patient who is not incapacitated has designated, either orally to hospital staff
or in writing, another individual to be his/her representative, the hospital must involve
the designated representative in the development and implementation of the patient’s
plan of care. The explicit designation of a representative by the patient takes
precedence over any non-designated relationship and continues throughout the
patient’s inpatient stay or outpatient visit, unless expressly withdrawn, either orally or
in writing, by the patient.
• In the case of a patient who is incapacitated, when an individual presents the hospital
with an advance directive, medical power of attorney or similar document executed by
the patient and designating an individual to make medical decisions for the patient
when incapacitated, the hospital, when presented with the document, must involve the
designated representative in the development and implementation of the patient’s plan
of care. The explicit designation of a representative takes precedence over any non-
designated relationship and continues throughout the patient’s inpatient stay or
outpatient visit, unless the patient ceases to be incapacitated and expressly withdraws
the designation, either orally or in writing.
• When a patient is incapacitated or otherwise unable to communicate his or her wishes,
there is no written advance directive on file or presented, and an individual asserts that
he or she is the patient’s spouse, domestic partner (whether or not formally established
and including a same-sex domestic partner), parent (including someone who has stood
in loco parentis for the patient who is a minor child) or other family member and thus
is the patient’s representative, the hospital is expected to accept this assertion, without
demanding supporting documentation, and must involve the individual as the patient’s
representative in the development and implementation of the patient’s plan of care,
unless:
• More than one individual claims to be the patient’s representative. In such cases, it
would be appropriate for the hospital to ask each individual for documentation
supporting his/her claim to be the patient’s representative. The hospital should
make its determination of who is the patient’s representative based upon the
hospital’s determination of who the patient would most want to make decisions on
his/her behalf. Examples of documentation a hospital might consider could include,
but are not limited to, the following: proof of a legally recognized marriage,
domestic partnership, or civil union; proof of a joint household; proof of shared or
co-mingled finances; and any other documentation the hospital considers evidence
of a special relationship that indicates familiarity with the patient’s preferences
concerning medical treatment;
• Treating the individual as the patient’s representative without requesting supporting
documentation would result in the hospital violating State law. State laws,
including State regulations, may specify a procedure for determining who may be
considered to be the incapacitated patient’s representative, and may specify when
documentation is or is not required; or
• The hospital has reasonable cause to believe that the individual is falsely claiming to
be the patient’s spouse, domestic partner, parent or other family member.
Hospitals are expected to adopt policies and procedures that facilitate expeditious and
non-discriminatory resolution of disputes about whether an individual is the patient’s
representative, given the critical role of the representative in exercising the patient’s
rights.
A refusal by the hospital of an individual’s request to be treated as the patient’s
representative, based on one of the above-specified familial relationships, must be
documented in the patient’s medical record, along with the specific basis for the refusal.
Survey Procedures §482.13(b)(1)
• Does the hospital have policies and procedures to involve the patient or the patient’s
representative (as appropriate) in the development and implementation of his/her
inpatient treatment/care plan, outpatient treatment/care plan, discharge plan, and
pain management plan?
• Review records and interview staff and patients, or patients’ representatives (as
appropriate), to determine how the hospital involves the patient or the patient’s
representative (as appropriate) in the development and implementation of his/her
plan of care?
• Does the hospital’s policy provide for determining when a patient has a
representative who may exercise the patient’s right to participate in developing and
implementing his/her plan of care, and who that representative is, consistent with
this guidance and State law?
• Is there evidence that the patient or the patient’s representative was included or
proactively involved in the development and implementation of the patient’s plan of
care?
• Were revisions in the plan of care explained to the patient and/or the patient’s
representative (when appropriate)?
A-0131
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
§482.13(b)(2) The patient or his or her representative (as allowed under State law) has
the right to make informed decisions regarding his or her care. The patient's rights
include being informed of his or her health status, being involved in care planning and
treatment, and being able to request or refuse treatment. This right must not be
construed as a mechanism to demand the provision of treatment or services deemed
medically unnecessary or inappropriate.
Interpretive Guidelines §482.13(b)(2)
The right to make informed decisions means that the patient or patient’s representative is
given the information needed in order to make "informed" decisions regarding his/her care.
Patient’s Representative:
A patient may wish to delegate his/her right to make informed decisions to another person
(as allowed under State law).
Hospitals are expected to take reasonable steps to determine the patient’s wishes concerning
designation of a representative. Unless prohibited by applicable State law:
• When a patient who is not incapacitated has designated, either orally to hospital
staff or in writing, another individual to be his/her representative, the hospital must
provide the designated individual with the information required to make an
informed decision about the patient’s care. The hospital must also seek the written
consent of the patient’s representative when informed consent is required for a care
decision. The explicit designation of a representative by the patient takes precedence
over any non-designated relationship and continues throughout the patient’s
inpatient stay or outpatient visit, unless expressly withdrawn, either orally or in
writing, by the patient.
• In the case of a patient who is incapacitated, when an individual presents the
hospital with an advance directive, medical power of attorney or similar document
executed by the patient and designating an individual to make medical decisions for
the patient when incapacitated, the hospital must, when presented with the
document, provide the designated individual the information required to make
informed decisions about the patient’s care. The hospital must also seek the consent
of the designated individual when informed consent is required for a care decision.
The explicit designation of a representative takes precedence over any non-
designated relationship and continues throughout the patient’s inpatient stay or
outpatient visit, unless the patient ceases to be incapacitated and expressly
withdraws the designation, either orally or in writing.
• When a patient is incapacitated or otherwise unable to communicate his or her
wishes, there is no written advance directive on file or presented, and an individual
asserts that he or she is the patient’s spouse, domestic partner (whether or not
formally established and including a same-sex domestic partner), parent (including
someone who has stood in loco parentis for the patient who is a minor child), or
other family member and thus is the patient’s representative, the hospital is expected
to accept this assertion, without demanding supporting documentation, and provide
the individual the information required to make informed decisions about the
patient’s care. The hospital must also seek the consent of the individual when
informed consent is required for a care decision. Hospitals are expected to treat the
individual as the patient’s representative unless:
• More than one individual claims to be the patient’s representative. In such
cases, it would be appropriate for the hospital to ask each individual for
documentation supporting his/her claim to be the patient’s representative.
The hospital should make its determination of who is the patient’s
representative based upon the hospital’s determination of who the patient
would most want to make decisions on his/her behalf. Examples of
documentation a hospital might consider could include, but are not limited
to, the following: proof of a legally recognized marriage, domestic
partnership, or civil union; proof of a joint household; proof of shared or co-
mingled finances; and any other documentation the hospital considers
evidence of a special relationship that indicates familiarity with the patient’s

preferences concerning medical treatment;
• Treating the individual as the patient’s representative without requesting
supporting documentation would result in the hospital violating State law.
State laws, including State regulations, may specify a procedure for
determining who may be considered to be the incapacitated patient’s
representative, and may specify when documentation is or is not required; or
• The hospital has reasonable cause to believe that the individual is falsely
claiming to be the patient’s spouse, domestic partner, parent or other family
member.
Hospitals are expected to adopt policies and procedures that facilitate
expeditious and non-discriminatory resolution of disputes about whether an
individual is the patient’s representative, given the critical role of the
representative in exercising the patient’s rights.
A refusal by the hospital of an individual’s request to be treated as the patient’s
representative, based on one of the above-specified familial relationships, must
be documented in the patient’s medical record must, along with the specific
basis for the refusal.
Informed Decisions
The right to make informed decisions regarding care presumes that the patient or the
patient’s representative has been provided information about his/her health status,
diagnosis, and prognosis. Furthermore, it includes the patient's or the patient’s
representative’s participation in the development of his/her plan of care, including
providing consent to, or refusal of, medical or surgical interventions, and in planning for
care after discharge from the hospital. The patient or the patient's representative should
receive adequate information, provided in a manner that the patient or the patient's
representative can understand, to assure that the patient or the patient’s representative can
effectively exercise the right to make informed decisions.
Hospitals must establish processes to assure that each patient or the patient's representative
is given information on the patient's health status, diagnosis, and prognosis.
Giving informed consent to a treatment or a surgical procedure is one type of informed
decision that a patient or patient's representative may need to make regarding the patient's
plan of care. Hospitals must utilize an informed consent process that assures patients or
their representatives are given the information and disclosures needed to make an informed
decision about whether to consent to a procedure, intervention, or type of care that requires
consent. See the guidelines for 42 CFR 482.51(b)(2) pertaining to surgical services
informed consent and the guidelines for 42 CFR 482.24(c)(2)(v) pertaining to medical
records for further detail.
Informed decisions related to care planning also extend to discharge planning for the
patient's post-acute care. See the guidelines at 42 CFR 482.43(c) pertaining to discharge
planning for discussion of pertinent requirements.
Hospitals must also establish policies and procedures that assure a patient's right to request
or refuse treatment. Such policies should indicate how the patient's request will be
addressed. However, hospitals are under no obligation to fulfill a patient's request for a
treatment or service that the responsible practitioner has deemed medically unnecessary or
even inappropriate.
Required Hospital Disclosures to Patients:

Physician Ownership
In addition, there are certain provisions of the Medicare provider agreement rules
concerning disclosures that certain hospitals are required to make which are enforced under
42 CFR 482.13(b)(2):
• 42 CFR 489.3 defines a “physician-owned hospital” as any participating hospital in
which a physician or immediate family member of a physician (as defined in §411.351)
has an ownership or investment interest in the hospital, except for those satisfying an
exception found at §411.356(a) or (b). Surveyors are not required to make an
independent determination regarding whether a hospital meets the Medicare definition
of “physician-owned,” but they must ask whether the hospital is physician-owned.
• 42 CFR 489.20(u)(1) requires that all physician-owned hospitals provide written notice
to their patients at the beginning of each patient’s hospital inpatient stay or outpatient
visit stating that the hospital is physician-owned, in order to assist the patient in making
an informed decision about his or her care, in accordance with the requirements of
§482.13(b)(2).
• A planned inpatient stay or outpatient visit which is subject to the notice
requirement begins with the provision of a package of information regarding
scheduled preadmission testing and registration for a planned hospital admission for
inpatient care or for an outpatient service subject to notice. An unplanned inpatient
stay or outpatient visit subject to the notice requirement begins at the earliest point
at which the patient presents to the hospital.
• The notice must disclose, in a manner reasonably designed to be understood by all
patients, that the hospital is physician-owned and that a list of owners or investors who
are physicians or immediate family members of physicians is available upon request. If
the patient (or someone on behalf of the patient) requests this list, the hospital must
provide it at the time of the request.
• However, the notice requirement does not apply to any physician-owned hospital
that does not have at least one referring physician (as defined at §411.351) who has
an ownership or investment interest in the hospital or who has an immediate family
member who has an ownership or investment interest in the hospital. In such cases,
the hospital must sign an attestation statement that it has no referring physician with
an ownership or investment interest or whose immediate family member has an
ownership or investment interest in the hospital. The hospital must maintain this
attestation in its records.
• 42 CFR 489.20(u)(2) provides that physician-owned hospitals must require each
physician owner who is a member of the hospital’s medical staff to agree, as a condition
of obtaining/retaining medical staff membership or admitting privileges, to disclose in
writing to all patients they refer to the hospital their ownership or investment interest in
that hospital or that of any immediate family member. The hospital must require that
this disclosure be made at the time of the referral and the requirement should be
reflected in the hospital’s policies and procedures governing privileges for physician
owners.
• The hospital may exempt from this disclosure requirement any physician owner
who does not refer any patients to the hospital.
• 42 CFR 489.12 permits CMS to refuse to enter into a provider agreement with a
physician-owned hospital applicant that does not have procedures in place to notify

patients of physician ownership in the hospital as required under §489.20(u).
• 42 CFR 489.53(c) permits CMS to terminate a provider agreement with a physician-
owned hospital if the hospital fails to comply with the requirements at §489.20(u).
MD/DO 24/7 On-Site Presence
42 CFR 489.20(w) mandates that if there is no doctor of medicine or osteopathy present in
the hospital 24 hours per day, seven days per week, the hospital must provide written notice
of this to all inpatients at the beginning of a planned or unplanned inpatient stay, and to
outpatients for certain types of planned or unplanned outpatient visits. The purpose of this
requirement is to assist the patient in making an informed decision about his/her care, in
accordance with 42 CFR 482.13(b)(2). Hospitals that have an MD/DO on-site 24/7
(including residents who are MDs or DOs) do not need to issue any disclosure notice about
emergency services capability.
• The notice must be provided to all inpatients and to those outpatients who are under
observation or who are having surgery or any other procedure using anesthesia.
• The notice must be provided at the beginning of the planned or unplanned inpatient
stay, or outpatient visit subject to notice.
• A planned inpatient stay or outpatient visit which is subject to the notice
requirement begins with the provision of a package of information regarding
scheduled preadmission testing and registration for a planned hospital admission for
inpatient care or for an outpatient service subject to notice. An unplanned inpatient
stay or outpatient visit which is subject to the notice requirement begins at the
earliest point at which the patient presents to the hospital.
• Individual notices are not required in the hospital’s dedicated emergency department
(DED) (as that term is defined in 42 CFR 489.24(b)), but the DED must post a notice
conspicuously, in a place or places likely to be noticed by all individuals entering the
DED. The posted notice must state that the hospital does not have a doctor of medicine
or a doctor of osteopathy present in the hospital 24 hours per day, 7 days per week, and
must indicate how the hospital will meet the medical needs of any patient with an
emergency medical condition, as defined in 42 CFR 489.24(b) [the EMTALA
definition], at a time when there is no doctor of medicine or doctor of osteopathy
present in the hospital. If an emergency department patient is determined to require
admission, then the individual notice requirements of 42 CFR 489.20(w) would apply to
that patient.
• Before admitting an inpatient or providing outpatient services requiring notice, the
hospital must obtain a signed acknowledgement from the patient stating that he/she
understands that a doctor of medicine or doctor of osteopathy may not be present at all
times services are furnished to him/her.
• In the event of an unplanned surgery or inpatient admission to treat an emergency
medical condition, it may in some cases be necessary in the interest of the patient’s
safety to proceed with treatment before the required notice can be given and
acknowledgement can be obtained. In such circumstances, the hospital must
provide notice and obtain acknowledgement as soon as possible after the patient’s
stay or visit begins.
• For a hospital that participates in Medicare with multiple campuses providing inpatient
services (e.g., a main provider campus and separate satellite, remote, and/or provider-
based locations) under one CMS Certification Number, a separate determination is

made for each campus or satellite location with inpatient services as to whether the
disclosure notice is required. For example, if a hospital has a main campus and a
satellite location and a physician is present 24/7 on the main campus but not at the
satellite location, the hospital is required to provide the disclosure notice only at the
satellite location. No notice is required for patients presenting to the main provider
campus in this case. In this same example, if the hospital also has a provider-based, off-
campus ambulatory (i.e., same-day) surgery department, no notice is required at that
off-campus surgery site, since the hospital’s main campus does have an MD/DO present
24/7.
• 42 CFR 489.53(c) permits CMS to terminate a provider agreement with a hospital if the
hospital fails to comply with the requirements at §489.20(w) when it does not have an
MD or DO on-site 24/ 7.
Survey Procedures §482.13(b)(2)
• Is there a hospital policy addressing the patient's or the patient’s representative (as
appropriate) right to make informed decisions?
• Does the hospital’s policy provide for determining when a patient has a
representative who may exercise the patient’s right to make informed decisions, and
who that representative is, consistent with this guidance and State law?
• Is there a hospital policy addressing the patient's right to have information on his/her
medical status, diagnosis, and prognosis? Does it articulate the hospital's process for
assuring that patients have this information?
• Is there a hospital policy addressing how the patient will be involved in his/her care
planning and treatment?
• Is there evidence that the hospital routinely complies with its policies? Evidence would
be obtained through review of medical records, interviewing current patients and/or
interviewing hospital personnel to determine their understanding of the hospital's
informed decision-making policies and how they are implemented. Review of evidence
would be designed to determine whether patients/patient representatives are provided
adequate information about the patient's medical status, diagnosis, and prognosis, and
then allowed to make informed decisions about their care planning and treatment.
Assessing Required Disclosures:
Physician Ownership
• If the hospital indicates that it is physician-owned but is exempt under §489.20(v) from
the disclosure requirement of §489.20(u)(2), ask to see the signed attestation that it does
not have any referring physicians with an ownership/investment interest or whose
immediate family member has an ownership/investment interest in the hospital. (As
with any other on-the-spot correction of a deficiency during a survey, creation of an
attestation at the time of a survey does not mean that there was no deficiency and that
the hospital would not be cited.)
• If the hospital is physician-owned but not exempt from the physician ownership
disclosure requirements:
• Verify that appropriate policies and procedures are in place to assure that necessary
written notices are provided to all patients at the beginning of an inpatient or
outpatient stay.
• Review the notice the hospital issues to each patient to verify that it discloses, in a
manner reasonably designed to be understood by all patients, that the hospital meets
the Federal definition of “physician-owned,” that a list of owners and investors who
are physicians or immediate family members of physicians is available upon
request, and that such a list is provided to the patient at the time the request is made
by or on behalf of the patient.
• Determine through staff interviews, observation, and a review of policies and
procedures whether the hospital furnishes its list of physician owners and investors
at the time a patient or patient’s representative requests it.
• Determine through staff interviews and review of policies, procedures, and staff
records whether a physician-owned hospital’s medical staff membership and
admitting privileging requirements include a requirement that, as a condition of
continued membership or admitting privileges, physician owners who refer patients
to the hospital agree to provide written disclosure of their own or any immediate
family member’s ownership or investment interest to all patients at the time of the
referral to the hospital.
MD/DO 24/7 On-Site Presence
• Determine through interviews, observation, and medical record review whether an
MD/DO is present in the hospital, at each campus or satellite location providing
inpatient services 24 hours/day, seven days/week.
• For each required location where an MD/DO is not present:
• Verify that the appropriate policies and procedures are in place to assure written
notices that an MD/DO is not present at all times are provided at the beginning of an
inpatient stay or outpatient stay to all inpatients and to all outpatients receiving
observation services, surgery or another procedure requiring anesthesia.
• Verify that there is signed acknowledgment by patients of such disclosure, obtained
by the hospital prior to the patient’s admission or before applicable outpatient
services were provided.
• Ask a sample of inpatients and affected outpatients whether they were provided
notice about an MD/DO not being present at all times in the hospital.
• Verify that the hospital’s emergency department has signage with the appropriate
disclosure information.
• Review the notice the hospital issues to verify that it indicates how the hospital will
meet the medical needs of any patient who develops an emergency medical
condition at a time when no physician is present at that hospital, including any
remote location or satellite.
A-0132
(Rev. 75, Issued: 12-02-11, Effective: 12-02-11, Implementation: 12-02-11)
§482.13(b)(3) The patient has the right to formulate advance directives and to have
hospital staff and practitioners who provide care in the hospital comply with these
directives, in accordance with §489.100 of this part (Definition), §489.102 of this part
(Requirements for providers), and §489.104 of this part (Effective dates).

Interpretive Guidelines §482.13(b)(3)
An advance directive is defined at §489.100 as “a written instruction, such as a living will
or durable power of attorney for health care, recognized under State law (whether statutory
or as recognized by the courts of the State), relating to the provision of health care when the
individual is incapacitated.” The patient (inpatient or outpatient) has the right to formulate
advance directives, and to have hospital staff implement and comply with their advance
directive. The regulation at 42 CFR 489.102 specifies the rights of a patient (as permitted
by State law) to make medical care decisions, including the right to accept or refuse
medical or surgical treatment and the right to formulate, at the individual’s option, advance
directives.
In the advance directive, the patient may provide guidance as to his/her wishes concerning
provision of care in certain situations; alternatively the patient may delegate decision-
making authority to another individual, as permitted by State law. (In addition, the patient
may use the advance directive to designate a “support person,” as that term is used in
§482.13(h), for purposes of exercising the patient’s visitation rights.) When a patient who
is incapacitated has executed an advance directive designating a particular individual to
make medical decisions for him/her when incapacitated, the hospital must, when presented
with the document, provide the designated individual the information required to make
informed decisions about the patient’s care. (See also the requirements at §482.13(b)(2).)
The hospital must also seek the consent of the patient’s representative when informed
consent is required for a care decision. The explicit designation of a representative in the
patient’s advance directive takes precedence over any non-designated relationship and
continues throughout the patient’s inpatient stay or, as applicable, outpatient visit, unless
the patient ceases to be incapacitated and expressly withdraws the designation, either orally
or in writing.
§489.102 also requires the hospital to:
• Provide written notice of its policies regarding the implementation of patients’ rights to
make decisions concerning medical care, such as the right to formulate advance
directives. If an individual is incapacitated or otherwise unable to communicate, the
hospital may provide the advance directive information required under §489.102 to the
individual’s “family or surrogate in the same manner that it issues other materials about
policies and procedures to the family of the incapacitated individual or to a surrogate or
other concerned persons in accordance with State law.”(§489.102(e)) The guidance
concerning the regulation at §482.13(a)(1) governing notice to the patient or the
patient’s representative of the patient’s rights applies to the required provision of notice
concerning the hospital’s advance directive policies. Although both inpatients and
outpatients have the same rights under §482.13(a)(1), §489.102(b)(1) requires that
notice of the hospital’s advance directive policy be provided at the time an individual is
admitted as an inpatient. However, in view of the broader notice requirements at
§482.13(a)(1), the hospital should also provide the advance directive notice to
outpatients (or their representatives) who are in the emergency department, who are in
an observation status, or who are undergoing same-day surgery. The notice should be
presented at the time of registration. Notice is not required for other outpatients, given
that they are unlikely to become incapacitated.
• The notice must include a clear and precise statement of limitation if the hospital cannot
implement an advance directive on the basis of conscience. At a minimum, a statement
of limitation should:
• Clarify any differences between institution-wide conscience objections and those
that may be raised by individual physicians or other practitioners;
• Identify the State legal authority permitting such an objection; and
• Describe the range of medical conditions or procedures affected by the conscience
objection.
It should be noted that this provision allowing for certain conscience objections to
implementing an advance directive is narrowly focused on the directive’s content
related to medical conditions or procedures. This provision would not allow a
hospital or individual physician or practitioner to refuse to honor those portions of
an advance directive that designate an individual as the patient’s representative
and/or support person, given that such designation does not concern a medical
condition or procedure.
Issuance of the written notice of the hospital’s advance directive policies to the
patient or the patient’s representative must be documented in the patient’s medical
record.
• Document in a prominent part of the patient’s medical record whether or not the
patient has executed an advance directive;
• Not condition the provision of care or otherwise discriminate against an individual
based on whether or not the individual has executed an advance directive;
• Ensure compliance with requirements of State law concerning advance directives
and inform individuals that complaints concerning the advance directive
requirements may be filed with the State survey and certification agency;
• Provide for the education of staff concerning its policies and procedures on advance
directives. The right to formulate advance directives includes the right to formulate
a psychiatric advance directive (as allowed by State law); and
• Provide community education regarding advance directives and the hospital must
document its efforts.
A psychiatric advance directive is akin to a traditional advance directive for health care.
This type of advance directive might be prepared by an individual who is concerned that at
some time he or she may be subject to involuntary psychiatric commitment or treatment.
The psychiatric advance directive may cover a range of subjects, and may name another
person who is authorized to make decisions for the individual if he or she is determined to
be legally incompetent to make his/her own choices. It may also provide the patient’s
instructions about hospitalization, alternatives to hospitalization, the use of medications,
types of therapies, and the patient’s wishes concerning restraint or seclusion. The patient
may designate who should be notified upon his/her admission to the hospital, as well as
who should not be permitted to visit him or her. State laws regarding the use of psychiatric
advance directives vary.
In accordance with State law, a psychiatric advance directive should be accorded the same
respect and consideration that a traditional advance directive for health care is given.
Hospitals should carefully coordinate how the choices of a patient balance with the rights of
other patients, staff, and individuals in the event that a dangerous situation arises.
However, even if State law has not explicitly spoken to the use of psychiatric advance
directives, consideration should be given to them inasmuch as this regulation also supports
the patient’s right to participate in the development and implementation of his or her plan of
care. When the patient is, for whatever reason, unable to communicate his/her wishes, the
preferences expressed in the psychiatric advance directive can give critical insight to the
MD/DOs, nurses, and other staff as they develop a plan of care and treatment for the
patient.
Survey Procedures §482.13(b)(3)
• Review the hospital’s advance directive notice. Does it advise inpatients or applicable
outpatients, or their representatives, of the patient’s right to formulate an advance
directive and to have hospital staff comply with the advance directive (in accordance
with State law)? Does it include a clear, precise and valid statement of limitation if the
hospital cannot implement an advance directive on the basis of conscience?
• Review the records of a sample of patients for evidence of hospital compliance with
advance directive notice requirements. Does every inpatient or applicable outpatient
record contain documentation that notice of the hospital’s advance directives policy
was provided at the time of admission or registration? Is there documentation of
whether or not each patient has an advance directive? For those patients who have
reported an advance directive, has a copy of the patient’s advance directive been
placed in the medical record?
• What mechanism does the hospital have in place to allow patients to formulate an
advance directive or to update their current advance directive? Is there evidence that
the hospital is promoting and protecting each patient’s right to formulate an advance
directive?
• Determine to what extent the hospital complies, as permitted under State law, with
patient advance directives that delegate decisions about the patient’s care to a
designated individual.
• Determine to what extent the hospital educates its staff regarding advance directives.
• Interview staff to determine their knowledge of the advance directives of the patients
in their care.
• Determine to what extent the hospital provides education for the patient population
(inpatient and outpatient) regarding one’s rights under State law to formulate advance
directives.
A-0133
(Rev. 75, Issued: 12-02-11, Effective: 12-02-11, Implementation: 12-02-11)
§482.13(b)(4) - The patient has the right to have a family member or representative of
his or her choice and his or her own physician notified promptly of his or her
admission to the hospital.
Interpretive Guidelines §482.13(b)(4)
Identifying Who Is to Be Notified
For every inpatient admission, the hospital must ask the patient whether the hospital should
notify a family member or representative about the admission. If the patient requests such
notice and identifies the family member or representative to be notified, the hospital must
provide such notice promptly to the designated individual. The explicit designation of a
family member or representative by the patient takes precedence over any non-designated
relationship.
The hospital must also ask the patient whether the hospital should notify his/her own
physician. In the case of scheduled admissions, the patient’s own physician likely is already
aware of the admission. However, if the patient requests notice to and identifies the
physician, the hospital must provide such notice promptly to the designated physician,
regardless of whether the admission was scheduled in advance or emergent.
When a patient is incapacitated or otherwise unable to communicate and to identify a
family member or representative to be notified, the hospital must make reasonable efforts to
identify and promptly notify a family member or patient’s representative. If an individual
who has accompanied the patient to the hospital, or who comes to or contacts the hospital
after the patient has been admitted, asserts that he or she is the patient’s spouse, domestic
partner (whether or not formally established and including a same-sex domestic partner),
parent (including someone who has stood in loco parentis for the patient who is a minor
child), or other family member, the hospital is expected to accept this assertion, without
demanding supporting documentation, and provide this individual information about the
patient’s admission, unless:
• More than one individual claims to be the patient’s family member or
representative. In such cases it would not be inappropriate for the hospital to ask
each individual for documentation supporting his/her claim to be the patient’s
family member or representative. The hospital should make its determination of
who is the patient’s representative based upon the hospital’s determination of who
the patient would most want to make decisions on his/her behalf. Examples of
documentation a hospital might consider could include, but are not limited to, the
following: proof of a legally recognized marriage, domestic partnership, or civil
union; proof of a joint household; proof of shared or co-mingled finances; and any
other documentation the hospital considers evidence of a special relationship that
indicates familiarity with the patient’s preferences concerning medical treatment ;
• Treating the individual as the patient’s family member or representative without
requesting supporting documentation would result in the hospital violating State
law. State laws, including State regulations, may specify a procedure for
determining who may be considered to be the incapacitated patient’s family member
or representative, and may specify when documentation is or is not required; or
• The hospital has reasonable cause to believe that the individual is falsely claiming to
be the patient’s spouse, domestic partner, parent or other family member.
Hospitals are expected to adopt policies and procedures that facilitate expeditious and non-
discriminatory resolution of disputes about whether an individual should be notified as the
patient’s family member or representative, given the critical role of the representative in
exercising the patient’s rights. Hospitals may also choose to provide notice to more than
one family member.
When a patient is incapacitated and the hospital is able through reasonable efforts to
identify the patient’s own physician – e.g., through information obtained from a family
member, or from review of prior admissions or outpatient encounters, or through access to
the patient’s records in a regional system of electronic patient medical records in which the
hospital participates – the hospital must promptly notify the patient’s physician of the
admission.
Prompt Notice
The hospital must provide the required notice promptly. “Promptly” means as soon as
possible after the physician’s or other qualified practitioner’s order to admit the patient has
been given. Notice may be given orally in person, by telephone, by e-mail or other
electronic means, or by other methods that achieve prompt notification. It is not acceptable
for the hospital to send a letter by regular mail.
Medical Record Documentation
The hospital must document that the patient, unless incapacitated, was asked no later than
the time of admission whether he or she wanted a family member/representative notified,
the date, time and method of notification when the patient requested such, or whether the
patient declined to have notice provided. If the patient was incapacitated at the time of
admission, the medical record must indicate what steps were taken to identify and provide
notice to a family member/representative and to the patient’s physician.
Survey Procedures §482.13(b)(4)
• Determine if the hospital has policies that address notification of a patient’s family or
representative and physician when the patient is admitted as an inpatient.
• Ask the hospital who is responsible for providing the required notice. Interview
person(s) responsible for providing the notice to determine how they identify the
persons to be notified and the means of notification. What do they do in the case of an
incapacitated person to identify a family member/representative and the patient’s
physician?
• Review a sample of inpatient medical records. Do the medical records provide
evidence that the patient was asked about notifying a family member/representative and
his/her physician? Is there a record of when and how notice was provided? Was notice
provided promptly? Is there a record of the patient declining to have notice provided to
a family member/representative and his/her physician? Is there documentation of
whether the patient was incapacitated at the time of admission, and if so, what steps
were taken to identify a family member/representative and the patient’s physician?
A-0142
§482.13(c) Standard: Privacy and Safety
Interpretive Guidelines §482.13(c)
The hospital must ensure the privacy and safety requirements are met.
A-0143
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
§482.13(c)(1) - The patient has the right to personal privacy.
Interpretive Guidelines §482.13(c)(1)
The underlying principle of this requirement is the patient’s basic right to respect, dignity,
and comfort while in the hospital.
Physical Privacy
“The right to personal privacy” includes at a minimum, that patients have physical privacy
to the extent consistent with their care needs during personal hygiene activities (e.g.,
toileting, bathing, dressing), during medical/nursing treatments, and when requested as
appropriate.
People not involved in the care of the patient should not be present without his/her consent
while he/she is being examined or treated. If an individual requires assistance during
toileting, bathing, and other personal hygiene activities, staff should assist, giving utmost
attention to the individual’s need for privacy. Privacy should be afforded when the MD/DO
or other staff visits the patient to discuss clinical care issues or conduct any examination or
treatment.
However, audio/video monitoring (does not include recording) of patients in medical-
surgical or intensive-care type units would not be considered violating the patient’s privacy,
as long as there exists a clinical need, the patient/patient’s representative is aware of the
monitoring and the monitors or speakers are located so that the monitor screens are not
readily visible or where speakers are not readily audible to visitors or the public. Video
recording of patients undergoing medical treatment requires the consent of the patient or
his/her representative.
A patient’s right to privacy may also be limited in situations where a person must be
continuously observed to ensure his or her safety, such as when a patient is simultaneously
restrained and in seclusion to manage violent or self-destructive behavior or when the
patient is under suicide precautions.
Protecting Patient Personal Information
The right to personal privacy also includes limiting the release or disclosure of patient
information. Patient information includes, but is not limited to, the patient’s presence or
location in the hospital; demographic information the hospital has collected on the patient,
such as name, age, address, income; or information on the patient’s medical condition.
Such patient information may not be disclosed without informing the patient or the patient’s
representative in advance of the disclosure and providing the patient or the patient’s
representative an opportunity to agree, prohibit, or restrict the disclosure. Below is a
summary of privacy issues that surveyors might encounter in hospital settings, and the
related privacy requirements.
Permitted Disclosures:
A hospital is permitted to use and disclose patient information, without the patient’s
authorization, in order to provide patient care and perform related administrative functions,
such as payment and other hospital operations.
• Payment operations include hospital activities to obtain payment or be reimbursed for
the provision of health care to an individual.
• Hospital operations are administrative, financial, legal, and quality improvement
activities of a hospital that are necessary to conduct business and to support the core
functions of treatment and payment. These activities include, but are not limited
to: quality assessment and improvement activities, case management and care
coordination; competency assurance activities, conducting or arranging for medical
reviews, audits, or legal services, including fraud and abuse detection and compliance
programs; business planning, development, management, and administration and certain
hospital-specific fundraising activities.
Hospitals must develop and implement policies and procedures that restrict access to and
use of patient information based on the specific roles of the members of their workforce.
These policies and procedures must identify the persons, or classes of persons, in the
workforce who need access to protected health information to carry out their duties and the
categories of protected health information to which access is needed.
One example of a permitted disclosure is a Facility Directory. It is common practice in
many hospitals to maintain a directory of patient contact information. The hospital must
inform the patient, or the patient’s representative, of the individual information that may be
included in a directory and the persons to whom such information may be disclosed. The
patient, or the patient’s representative, must be given the opportunity to restrict or prohibit
any or all uses and disclosures. The hospital may rely on a patient’s/representative’s
individual’s informal permission to list in its facility directory the patient’s name, general
condition, religious affiliation, and location in the provider’s facility.
The provider may
then disclose the patient’s condition and location in the facility to anyone asking for the
patient by name, and also may disclose religious affiliation to clergy. If the opportunity to
prohibit or restrict uses and disclosures cannot be provided due to the patient’s incapacity or
emergency treatment circumstance, and there is no patient representative available, the
hospital may disclose patient information for the facility’s directory if such disclosure is in
the patient’s best interest. The hospital must provide the patient or the patient’s
representative an opportunity to prohibit or restrict disclosure as soon as it becomes
practicable to do so. The hospital may use patient information to notify, or assist in the
notification of, a family member, a personal representative of the patient, or another person
responsible for the care of the patient of their location, general condition, or death. The
hospital must have procedures in place, in accordance with State law, to provide appropriate
information to patient families or others in those situations where the patient is unable to
make their wishes known.
Incidental Uses and Disclosures May be Acceptable:
An incidental use or disclosure is a secondary use or disclosure of patient information that
cannot reasonably be prevented, is limited in nature, and that occurs as a result of another
use or disclosure that is permitted. Many customary health care communications and
practices play an important role in ensuring the prompt delivery of effective care. Due to
the nature of these communications and practices, as well as of the hospital environment,
the potential exists for a patient’s information to be disclosed incidentally. For example, a
hospital visitor may overhear a health care professional’s confidential conversation with
another health care professional or the patient, or may glimpse a patient’s information on a
sign-in sheet or nursing station whiteboard. The regulation protecting patient privacy does
not impede these customary and essential communications and practices and, thus, a
hospital is not required to eliminate all risk of incidental use or disclosure secondary to a
permitted use or disclosure, so long as the hospital takes reasonable safeguards and
discloses only the minimum amount of personally identifiable information necessary. For
example, hospitals may:
• Use patient care signs (e.g. “falls risk” or “diabetic diet” ) displayed at the bedside
or outside a patient room;
• Display patient names on the outside of patient charts; or
• Use “whiteboards” that list the patients present on a unit, in an operating room suite,
etc.
Hospitals are expected to review their practices and determine what steps are reasonable to
safeguard patient information while not impeding the delivery of safe patient care or
incurring undue administrative or financial burden as a result of implementing privacy
safeguards.
Examples of reasonable safeguards could include, but are not limited to:
• Requesting that waiting customers stand a few feet back from a counter used for
patient registration;
• Use of dividers or curtains in areas where patient and physician or other hospital
staff communications routinely occur;
• Health care staff speaking quietly when discussing a patient’s condition or treatment
in a semi-private room;
• Utilizing passwords and other security measures on computers maintaining
personally identifiable health information; or
• Limiting access to areas where white boards or x-ray light boards are in use, or
posting the board on a wall not readily visible to the public, or limiting the
information placed on the board.
Survey Procedures §482.13(c)(1)
• Conduct observations/interview patients or their representatives to determine if
patients are provided reasonable privacy during examinations or treatments,
personal hygiene activities and discussions about their health status/care and other
appropriate situations.
• Review hospital policy and interview staff concerning their understanding of the use
of patient information in the facility directory. Does the policy address the
opportunity for the patient or patient’s representative to restrict or prohibit use of
patient information in emergent and non-emergent situations?
• Review hospital policy and conduct observations/interview staff to determine if
reasonable safeguards are used to reduce incidental disclosures of patient
information.
• If audio and/or visual monitoring is utilized in the med/surg or ICU setting, conduct
observations to determine that monitor screens and/or speakers are not readily
visible or audible to visitors or the public.
A-0144
(Rev. 216; Issued:07-21-23; Effective: 07-21-23; Implementation: 07-21-23)
§482.13(c)(2) - The patient has the right to receive care in a safe setting.
Interpretive Guidelines §482.13(c)(2)
The intention of this requirement is to specify that each patient receives care in an
environment that a reasonable person, similarly situated as the patient, would consider to be
safe. For example, hospital staff should follow current standards of practice for patient
environmental safety, infection control, and security. The hospital must protect vulnerable
patients, including newborns and children. Additionally, this standard is intended to provide
protection for the patient’s emotional health and safety as well as his/her physical safety.
Respect, dignity, and comfort would also be components of an emotionally safe
environment. To provide care in a safe setting, hospitals should identify patients at risk for
intentional harm to self or others, identify environmental safety risks for such patients, and
provide education and training for staff and volunteers. Patients at risk of suicide (or other
forms of self-harm), or who exhibit violent behaviors toward others, receive healthcare
services in both inpatient and outpatient locations of hospitals. Therefore, non-psychiatric
settings of all hospitals where patients with psychiatric conditions may be cared for should
also identify patients at risk for intentional harm to self or others and mitigate
environmental safety risks. Psychiatric patients requiring medical care in a non-psychiatric
setting (medical inpatient units, ED, ICU, etc.) must be evaluated, monitored, and cared for
appropriately when demonstrating suicidal ideation or potential harm to others. The care
could include, but not be limited to, utilizing safety measures such: as 1:1 monitoring with
continuous visual observation, removal of sharp objects from the room/area, or removal of
equipment that can be used as a weapon.
Although all risks cannot be eliminated, hospitals should be able to demonstrate how they
identify patients at risk of self-harm or harm to others and steps they are taking to minimize
those risks in accordance with nationally recognized standards and guidelines.
The potential risks include, but are not limited to, those from ligatures, sharps, harmful
substances, access to medications, breakable windows, accessible light fixtures, plastic bags
(for suffocation), oxygen tubing, bell cords, etc.
Hospitals may find the recommendations and resources in the 2018 report, Recommended
standard care for people with suicide risk: Making health care suicide safe, issued by the
National Action Alliance for Suicide Prevention (Action Alliance), to be highly useful in
developing the best practices for effective patient screening and assessment for those
patients at risk for harm to themselves, as well as for improving the care of patients at risk
of suicide.
1
The Action Alliance is the public-private partnership working to advance the
National Strategy for Suicide Prevention and reduce the suicide rate 20 percent by 2025.
Notably, the report advances two of the goals of the National Strategy for Suicide
Prevention (National Strategy): (a) Promote suicide prevention as a core component of
health care services; and (b) Promote and implement effective clinical and professional
practices for assessing and treating those identified as being at risk for suicidal behaviors.
(To download a copy of the National Strategy, please visit:
www.actionallianceforsuicideprevention.org).
Identifying Patients at Risk
There are numerous models and versions of patient risk assessment tools available to
identify patients at risk for harm to self or others. No one-size-fits-all tool is available.
Therefore, the type of patient risk assessment tool used should be appropriate to the patient
population, care setting, and staff competency. All hospitals should implement a patient risk
assessment strategy, but it is up to the individual hospital to implement the appropriate
strategies. For example, a patient risk assessment strategy in a post-partum unit would most
likely not be the same risk assessment strategy utilized in the emergency department.
Environmental Safety Risks
Just as all hospitals should implement a patient risk assessment strategy, all hospitals should
implement an environmental risk assessment strategy. Environmental risk assessment
strategies may not be the same in all hospitals or hospital units. The hospital implements
environmental risk assessment strategies appropriate to its specific care environment and
patient population. That does not mean that a unit which does not typically care for patients
with psychiatric conditions should not be conducting environmental risk assessments. It
means that the risk assessment must be appropriate to the unit and should consider the
possibility that the unit may sometimes care for patients at risk for harm to self or others.
While CMS does not require the use of an Environmental Risk Assessment Tool (e.g., the
Veteran’s Administration Environmental Risk Assessment Tool), the use of such tools may
be used as a way for the hospital to assess safety risks in all patient care environments to
minimize environmental risks and to document the assessment findings. Examples of
Environmental Risk Assessment Tool content may include prompts for staff to assess items
such as, but not limited to:
• Ligature risks such as handrails, doorknobs, door hinges, shower curtains, exposed
plumbing/pipes, soap, and paper towel dispensers on walls, power cords on medical
1
National Action Alliance for Suicide Prevention: Transforming Health Systems Initiative Work Group.
(2018). Recommended standard care for people with suicide risk: Making health care suicide safe.
Washington, DC: Education Development Center, Inc. (accessed at:
https://theactionalliance.org/sites/default/files/action_alliance_recommended_standard_care_final.pdf )
equipment or call bell cords, light fixtures, or projections from ceilings, etc.
• Unattended items such as utility or housekeeping carts that contain hazardous items
(mops, brooms, cleaning agents, hand sanitizers, etc.)
• Unsafe items brought to patients by visitors in locked psychiatric units of hospitals and
psychiatric hospitals
• Windows that can be opened or broken
• Unprotected lighting fixtures; and
• Inadequate staffing levels to provide appropriate patient observation and monitoring
Hospital staff should be trained to identify environmental safety risks regardless of whether
or not the hospital has chosen to implement the use of an environmental risk assessment
tool to identify potential or actual risks in the patient care environment.
Education and Training
Hospitals should provide the appropriate level of education and training to staff regarding
the identification of patients at risk of harm to self or others, the identification of
environmental patient safety risk factors, and mitigation strategies. Staff includes direct
employees, volunteers, contractors, per diem staff, and any other individuals providing
clinical care under arrangement. Hospitals have the flexibility to tailor the training to the
particular services staff provide and the patient populations they serve. Hospitals should
provide education and training to all new staff initially upon orientation and whenever
policies and procedures change. However, CMS recommends initial training and then
ongoing training at least every two years thereafter.
Correction of Environmental Risks
Cited noncompliance that include ligature risks, which do not pose an immediate jeopardy
situation or no longer pose an immediate jeopardy situation because the immediate threat to
patient health and safety has been removed by the hospital, or has been mitigated through
the implementation of appropriate interim patient safety measures, should be corrected as
soon as possible, but within the timeframe noted by the CMS Location, the SA, or the AO.
Interim patient safety measures are expected to be implemented as part of an acceptable
plan of correction to mitigate patient safety risks, as appropriate.
Interim patient safety measures to mitigate identified ligature or safety risks may include,
without limitation, continuous visual observation or 1:1 observation based on the type of
identified risk.
Survey Procedures §482.13(c)(2)
• Review and analyze patient and staff incident and accident reports to identify any
incidents or patterns of incidents concerning a safe environment. Expand your review if you
suspect a problem with safe environment in the hospitals.
• Interview staff in patient care areas to determine how the hospital has trained staff to
identify risks in the care environment and, if found, how staff report those findings.
• Observe and interview staff at units where infants and children are inpatients. Are
appropriate security protections (such as alarms, arm banding systems, etc.) in place? Are
they functioning?
• Review policy and procedures on what the hospital does to curtail unwanted visitors,
contaminated materials, or unsafe items that pose a safety risk to patients and staff.
• Access the hospital’s security efforts to protect vulnerable patients including newborns,
children, and patients at risk of suicide or intentional harm to self or others. Is the hospital
providing appropriate security to protect patients? Are appropriate security mechanisms in
place and being followed to protect patients? Security mechanisms are based on nationally
recognized standards of practice.
A-0145
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(c)(3) - The patient has the right to be free from all forms of abuse or
harassment.
Interpretive Guidelines §482.13(c)(3)
The intent of this requirement is to prohibit all forms of abuse, neglect (as a form of abuse)
and harassment whether from staff, other patients or visitors. The hospital must ensure that
patients are free from all forms of abuse, neglect, or harassment. The hospital must have
mechanisms/methods in place that ensure patients are free of all forms of abuse, neglect, or
harassment.
Abuse is defined as the willful infliction of injury, unreasonable confinement, intimidation,
or punishment, with resulting physical harm, pain, or mental anguish. This includes staff
neglect or indifference to infliction of injury or intimidation of one patient by another.
Neglect, for the purpose of this requirement, is considered a form of abuse and is defined as
the failure to provide goods and services necessary to avoid physical harm, mental anguish,
or mental illness.
The following components are suggested as necessary for effective abuse protection:
• Prevent. A critical part of this system is that there are adequate staff on duty,
especially during the evening, nighttime, weekends and holiday shifts, to take care
of the individual needs of all patients. (See information regarding meaning of
adequate at those requirements that require the hospital to have adequate staff.
Adequate staff would include that the hospital ensures that there are the number and
types of qualified, trained, and experienced staff at the hospital and available to
meet the care needs of every patient.)
• Screen. Persons with a record of abuse or neglect should not be hired or retained as
employees.
• Identify. The hospital creates and maintains a proactive approach to identify events
and occurrences that may constitute or contribute to abuse and neglect.
• Train. The hospital, during its orientation program, and through an ongoing
training program, provides all employees with information regarding abuse and
neglect, and related reporting requirements, including prevention, intervention, and
detection.
• Protect. The hospital must protect patients from abuse during investigation of any
allegations of abuse or neglect or harassment.
• Investigate. The hospital ensures, in a timely and thorough manner, objective
investigation of all allegations of abuse, neglect or mistreatment.
• Report/Respond. The hospital must assure that any incidents of abuse, neglect or
harassment are reported and analyzed, and the appropriate corrective, remedial or
disciplinary action occurs, in accordance with applicable local, State, or Federal law.
As a result of the implementation of this system, changes to the hospital’s policies and
procedures should be made accordingly.
Survey Procedures §482.13(c)(3)
• Examine the extent to which the hospital has a system in place to protect patients
from abuse, neglect and harassment of all forms, whether from staff, other patients,
visitors or other persons. In particular, determine the extent to which the hospital
addresses the following issues.
o Are staffing levels across all shifts sufficient to care for individual patient’s
needs?
o Does the hospital have a written procedure for investigating allegations of
abuse and neglect including methods to protect patients from abuse during
investigations of allegations?
o How does the hospital substantiate allegations of abuse and neglect?
o Do incidents of substantiated abuse and neglect result in appropriate action?
o Has the hospital implemented an abuse protection program? Does it comply
with Federal, State and local laws and regulations? Is it effective?
o Are appropriate agencies notified in accordance with State and Federal laws
regarding incidents of substantiated abuse and neglect?
o Can staff identify various forms of abuse or neglect?
o Do staff members know what to do if they witness abuse and neglect?
o What evidence is there that allegations of abuse and neglect are thoroughly
investigated?
o Does the hospital conduct criminal background checks as allowed by State
law for all potential new hires?
o Is there evidence the hospital employs people with a history of abuse,
neglect or harassment?
A-0146
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(d) Standard: Confidentiality of Patient Records
Interpretive Guidelines §482.13(d)
The hospital must ensure the confidentiality of patient records requirements are met.
A-0147
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
§482.13(d)(1) - The patient has the right to the confidentiality of his or her clinical
records.
Interpretive Guidelines §482.13(d)(1)
The right to confidentiality of the patient’s medical record means the hospital must
safeguard the contents of the medical record, whether it is in paper or electronic format, or a
combination of the two, from unauthorized disclosure. Confidentiality applies wherever the
record or portions thereof are stored, including but not limited to central records, patient
care locations, radiology, laboratories, record storage areas, etc.
A hospital is permitted to disclose patient information, without a patient’s authorization, in
order to provide patient care and perform related administrative functions, such as payment
and other hospital operations.
• Payment operations include hospital activities to obtain payment or be reimbursed for
the provision of health care to an individual.
• Hospital operations are administrative, financial, legal, and quality improvement
activities of a hospital that are necessary to conduct business and to support the core
functions of treatment and payment. These activities include, but are not limited
to: quality assessment and improvement activities, case management and care
coordination; competency assurance activities, conducting or arranging for medical
reviews, audits, or legal services, including fraud and abuse detection and compliance
programs; business planning, development, management, and administration and
certain hospital-specific fundraising activities.
The hospital must develop policies and procedures that reasonably limit disclosures of
information contained in the patient’s medical record to the minimum necessary, even when
the disclosure is for treatment or payment purposes, or as otherwise required by State or
Federal law.
When the minimum necessary standard is applied, a hospital may not disclose the entire
medical record for a particular purpose, unless it can specifically justify that the whole
record is the amount reasonably needed for the purpose.
A hospital may make an authorized disclosure of information from the medical record
electronically, and may also share an electronic medical record system with other health
care facilities, physicians and practitioners, so long as the system is designed and operated
with safeguards that ensure that only authorized disclosures are made.
The hospital must obtain the patient’s, or the patient’s representative’s, written
authorization for any disclosure of information in the medical record when the disclosure is
not for treatment, payment or health care operations.
Survey Procedures §482.13(d)(1)
• Verify that the hospital has policies and procedures addressing the protecting of
information in patients’ medical record from unauthorized disclosures.
• Observe locations where medical records are stored to determine whether
appropriate safeguards are in place to protect medical record information.
• Interview staff to determine their understanding of and compliance with the
hospital’s policies and procedures for protecting medical record information.
A-0148
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.13(d)(2) - The patient has the right to access their medical records, including
current medical records, upon an oral or written request, in the form or format
requested by the individual. If it is readily producible in such form and format
(including in an electronic form or format when such medical records are maintained
electronically); or if not, in a readable hard copy form or such other form or format as
agreed by the facility and the individual, and within a reasonable timeframe. The
hospital must not frustrate the legitimate efforts of individuals to gain access to their
own medical records and must actively seek to meet these requests as quickly as its
record keeping system permits.
Interpretive Guidelines §482.13(d)(2)
Guidance is pending and will be updated in future release.
Survey Procedures §482.13(d)(2)
• Does the hospital promote and protect the patient’s right to access information
contained in his/her clinical record?
• Does the hospital have a procedure for providing records to patients within a
reasonable time frame?
• Does the hospital’s system frustrate the legitimate efforts of individuals to gain
access to their own medical record?
• Does the procedure include the method to identify what documents were not
provided and the reason?
A-0154
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e) Standard: Restraint or seclusion.
All patients have the right to be
free from physical or mental abuse, and corporal punishment. All patients have the
right to be free from restraint or seclusion, of any form, imposed as a means of
coercion, discipline, convenience, or retaliation by staff. Restraint or seclusion may
only be imposed to ensure the immediate physical safety of the patient, a staff
member, or others and must be discontinued at the earliest possible time.
Interpretive Guidelines §482.13(e):
The intent of this standard is to identify patients’ basic rights, ensure patient safety, and
eliminate the inappropriate use of restraint or seclusion. Each patient has the right to
receive care in a safe setting. The safety of the patient, staff, or others is the basis for
initiating and discontinuing the use of restraint or seclusion. Each patient has the right to be
free from all forms of abuse and corporal punishment. Each patient has the right to be free
from restraint or seclusion, of any form, imposed as a means of coercion, discipline,
convenience, or retaliation by staff. Restraint or seclusion may not be used unless the use
of restraint or seclusion is necessary to ensure the immediate physical safety of the patient,
a staff member, or others. The use of restraint or seclusion must be discontinued as soon as
possible based on an individualized patient assessment and re-evaluation. A violation of
any of these patients’ rights constitutes an inappropriate use of restraint or seclusion and
would be subject to a condition level deficiency.
The patient protections contained in this standard apply to all hospital patients when the
use of restraint or seclusion becomes necessary, regardless of patient location. The
requirements contained in this standard are not specific to any treatment setting within the
hospital. They are not targeted only to patients on psychiatric units or those with
behavioral/mental health care needs. Instead, the requirements are specific to the patient
behavior that the restraint or seclusion intervention is being used to address.
In summary, these restraint and seclusion regulations apply to:
• All hospitals (acute care, long-term care, psychiatric, children's, and cancer);
• All locations within the hospital (including medical/surgical units, critical care
units, forensic units, emergency department, psychiatric units, etc.); and
• All hospital patients, regardless of age, who are restrained or secluded (including
both inpatients and outpatients).
The decision to use a restraint or seclusion is not driven by diagnosis, but by a
comprehensive individual patient assessment. For a given patient at a particular point in
time, this comprehensive individualized patient assessment is used to determine whether the
use of less restrictive measures poses a greater risk than the risk of using a restraint or
seclusion. The comprehensive assessment should include a physical assessment to identify
medical problems that may be causing behavior changes in the patient. For example,
temperature elevations, hypoxia, hypoglycemia, electrolyte imbalances, drug interactions,
and drug side effects may cause confusion, agitation, and combative behaviors. Addressing
these medical issues may eliminate or minimize the need for the use of restraints or
seclusion.
Staff must assess and monitor a patient’s condition on an ongoing basis to ensure that the
patient is released from restraint or seclusion at the earliest possible time. Restraint or
seclusion may only be employed while the unsafe situation continues. Once the unsafe
situation ends, the use of restraint or seclusion should be discontinued. However, the
decision to discontinue the intervention should be based on the determination that the need
for restraint or seclusion is no longer present, or that the patient’s needs can be addressed
using less restrictive methods.
Hospital leadership is responsible for creating a culture that supports a patient’s right to be
free from restraint or seclusion. Leadership must ensure that systems and processes are
developed, implemented, and evaluated that support the patients’ rights addressed in this
standard, and that eliminate the inappropriate use of restraint or seclusion. Through their
QAPI program, hospital leadership should:
• Assess and monitor the use of restraint or seclusion in their facility;
• Implement actions to ensure that restraint or seclusion is used only to ensure the
physical safety of the patient, staff and others; and
• Ensure that the hospital complies with the requirements set forth in this standard as
well as those set forth by State law and hospital policy when the use of restraint or
seclusion is necessary.
Patients have a right to receive safe care in a safe environment. However, the use of
restraint is inherently risky. When the use of restraint is necessary, the least restrictive
method must be used to ensure a patient’s safety. The use of restraint for the management
of patient behavior should not be considered a routine part of care.

The use of restraints for the prevention of falls should not be considered a routine part of a
falls prevention program. Although restraints have been traditionally used as a falls
prevention approach, they have major, serious drawbacks and can contribute to serious
injuries. There is no evidence that the use of physical restraint, (including, but not limited
to, raised side rails) will prevent or reduce falls. Additionally, falls that occur while a
person is physically restrained often result in more severe injuries.
2
In fact in some instances reducing the use of physical restraints may actually decrease the
risk of falling.
3
Consider, for example, a patient who is displaying symptoms of Sundowner’s Syndrome, a
syndrome in which a patient's dementia becomes more apparent at the end of the day than at
the beginning of the day. The patient is not acting out or behaving in a violent or self-
destructive manner. However, the patient has an unsteady gait and continues to get out of
bed even after staff has tried alternatives to keep the patient from getting out of bed. There
is nothing inherently dangerous about a patient being able to walk or wander, even at night.
Under the provisions of this regulation, the rationale that the patient should be restrained
because he “might” fall does not constitute an adequate basis for using a restraint for the
purposes of this regulation. When assessing a patient’s risk for falls and planning care for
the patient, staff should consider whether the patient has a medical condition or symptom
that indicates a current need for a protective intervention to prevent the patient from
walking or getting out of bed. A history of falling without a current clinical basis for a
restraint intervention is inadequate to demonstrate the need for restraint. It is important to
FOOTNOTES
2
- American Geriatrics Society, British Geriatrics Society, and American Academy of
Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in
older persons. Journal of the American Geriatrics Society. 49(5):664-72, 2001 May.
- Neufeld RR, Libow LS, Foley WJ, Dunbar JM, Cohen C, Breuer B. Restraint reduction
reduces serious injuries among nursing home residents. J Am Geriatr Soc 1999; 47:1202-
1207.
- Si M, Neufeld RR, Dunbar J. Removal of bedrails on a short-term nursing home
rehabilitation unit. Gerontologist 1999; 39:611-614.
- Hanger HC, Ball MC, Wood LA. An analysis of falls in the hospital: can we do without
bedrails? J Am Geriatr Soc 1999; 47:529-531.
- Tinetti ME, Liu YB, Ginter S. Mechanical restraint use and fall related injuries among
residents of skilled nursing facilities. Ann Intern Med 1992; 116:369-374.
- Capezuti E, Evans L, Strumpf N, Maislin G. Physical restraint use and falls in nursing
home residents. J Am Geriatr Soc 1996; 44:627-633.
- Capezuti E, Strumpf NE, Evans LK, Grisso JA, Maislin G. The relationship between
physical restraint removal and falls and injuries among nursing home residents. J
Gerontol A Biol Sci Med Sci 1998; 53:M47-M52.
3
University of California at San Francisco (UCSF)-Stanford University Evidence-based
Practice Center Subchapter 26.2. Interventions that Decrease the Use of Physical
Restraints” of the Evidence Report/Technology Assessment, No. 43 entitled, “Making
Health Care Safer: A Critical Analysis of Patient Safety Practices.” The full report can be
accessed at: http://www.ahrq.gov/qual/errorsix.htm
note that the regulation specifically states that convenience is not an acceptable reason to
restrain a patient. In addition, a restraint must not serve as a substitute for the adequate
staffing needed to monitor patients.
An individualized patient assessment is critical. In this example, an assessment should
minimally address the following questions:
• Are there safety interventions or precautions (other than restraint) that can be taken
to reduce the risk of the patient slipping, tripping, or falling if the patient gets out of
bed?
• Is there a way to enable the patient to safely ambulate?
• Is there some assistive device that will improve the patient’s ability to self
ambulate?
• Is a medication or a reversible condition causing the unsteady gait?
• Would the patient be content to walk with a staff person?
• Could the patient be brought closer to the nurse’s station where he or she could be
supervised?
If an assessment reveals a medical condition or symptom that indicates the need for an
intervention to protect the patient from harm, the regulation requires the hospital to use the
least restrictive intervention that will effectively protect the patient from harm. Upon
making this determination, the hospital may consider the use of a restraint; however, that
consideration should weigh the risks of using a restraint (which are widely documented in
research) against the risks presented by the patient’s behavior. If the hospital chooses to
use the restraint, it must meet the requirements contained in this standard.
In addition, a request from a patient or family member for the application of a restraint,
which they would consider to be beneficial, is not a sufficient basis for the use of a restraint
intervention. A patient or family member request for a restraint intervention, such as a vest
restraint or raising all four side rails, to keep the patient from getting out of bed or falling
should prompt a patient and situational assessment to determine whether such a restraint
intervention is needed. If a need for restraint is confirmed, the practitioner must then
determine the type of restraint intervention that will meet the patient's needs with the least
risk and most benefit to the patient. If restraint (as defined by the regulation) is used, then
the requirements of the regulation must be met.
Patient care staff must demonstrate through their documentation in the patient’s medical
record that the restraint intervention used is the least restrictive intervention that protects the
patient’s safety, and that the use of restraint is based on individual assessments of the
patient. The assessments and documentation of those assessments must be ongoing in order
to demonstrate a continued need for restraint. Documentation by the physician or other
staff once a day may not be adequate to support that the restraint intervention needs to
continue and may not comply with the requirement to end the restraint as soon as possible.
A patient’s clinical needs often change over time.
CMS does not consider the use of weapons in the application of restraint or seclusion as a
safe, appropriate health care intervention. For the purposes of this regulation, the term
“weapon” includes, but is not limited to, pepper spray, mace, nightsticks, tazers, cattle
prods, stun guns, and pistols. Security staff may carry weapons as allowed by hospital
policy, and State and Federal law. However, the use of weapons by security staff is
considered a law enforcement action, not a health care intervention. CMS does not support
the use of weapons by any hospital staff as a means of subduing a patient in order to place
that patient in restraint or seclusion. If a weapon is used by security or law enforcement
personnel on a person in a hospital (patient, staff, or visitor) to protect people or hospital

property from harm, we would expect the situation to be handled as a criminal activity and
the perpetrator be placed in the custody of local law enforcement.
The use of handcuffs, manacles, shackles, other chain-type restraint devices, or other
restrictive devices applied by non-hospital employed or contracted law enforcement
officials for custody, detention, and public safety reasons are not governed by this rule.
The use of such devices are considered law enforcement restraint devices and would
not be considered safe, appropriate health care restraint interventions for use by
hospital staff to restrain patients. The law enforcement officers who maintain custody
and direct supervision of their prisoner (the hospital’s patient) are responsible for the use,
application, and monitoring of these restrictive devices in accordance with Federal and
State law. However, the hospital is still responsible for an appropriate patient assessment
and the provision of safe, appropriate care to its patient (the law enforcement officer’s
prisoner).
Survey Procedures §482.13(e)
• Review hospital restraint and seclusion policies and procedures to determine if they
address, at a minimum:
o Who has the authority to discontinue the use of restraint or seclusion (based
on State law and hospital policies); and
o Circumstances under which restraint or seclusion should be discontinued.
(Also see §482.13(e)(3)).
• Review a sample of medical records of patients for whom restraints were used to
manage non-violent, non-self-destructive behavior, as well as a sample of medical
records of patients for whom restraint or seclusion was used to manage violent or
self-destructive behavior;
• Include in the review patients who are currently in restraint or seclusion, as well as
those who have been in restraint or seclusion during their hospital stay (include both
violent or self-destructive patients as well as non-violent, non-self-destructive
patients).
• What evidence is there that hospital staff identified the reason for the restraint or
seclusion, and determined that other less restrictive measures would not be effective
before applying the restraint?
• Interview staff who work directly with patients to determine their understanding of
the restraint and seclusion policies. If any patients are currently in restraint or
seclusion, ascertain the rationale for use and when the patient was last monitored
and assessed.
• Is the actual use of restraints or seclusion consistent with hospital restraint and
seclusion policies and procedures, as well as CMS requirements?
• Review incident and accident reports to determine whether patient injuries occurred
proximal to or during a restraint or seclusion intervention. Are incidents and
accidents occurring more frequently with restrained or secluded patients?
• If record review indicates that restrained or secluded patients sustained injuries,
determine what the hospital did to prevent additional injury. Determine if the
hospital investigated possible changes to its restraint or seclusion policies.
• Obtain data on the use of restraint and seclusion for a specified time period (e.g., 3
months) to determine any patterns in their use for specific units, shifts, days of the
week, etc.
• Does the number of patients who are restrained or secluded increase on weekends,
on holidays, at night, on certain shifts; where contract nurses are used; in one unit
more than other units? Such patterns of restraint or seclusion use may suggest that
the intervention is not based on the patient’s need, but on issues such as
convenience, inadequate staffing or lack of staff training. Obtain nursing staffing
schedules during time periods in question to determine if staffing levels impact the
use of restraint or seclusion.
• Interview a random sample of patients who were restrained to manage non-violent,
non-self-destructive behavior. Were the reasons for the use of a restraint to manage
non-violent, non-self-destructive behavior explained to the patient in understandable
terms? Could the patient articulate his/her understanding?
A-0159
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e) (1) Definitions. (i) A restraint is—
(A) Any manual method, physical or mechanical device, material, or
equipment that immobilizes or reduces the ability of a patient to move his or
her arms, legs, body, or head freely; or
Interpretive Guidelines §482.13(e)(1)(i)(A)
This restraint definition applies to all uses of restraint in all hospital care settings. Under
this definition, commonly used hospital devices and other practices could meet the
definition of a restraint, such as:
• Tucking a patient’s sheets in so tightly that the patient cannot move;
• Use of a “net bed” or an “enclosed bed” that prevents the patient from freely exiting
the bed. EXCEPTION: Placement of a toddler in an "enclosed" or "domed" crib;
• Use of "Freedom" splints that immobilize a patient's limb;
• Using side rails to prevent a patient from voluntarily getting out of bed; or
• Geri chairs or recliners, only if the patient cannot easily remove the restraint
appliance and get out of the chair on his or her own.
NOTE: Generally, if a patient can easily remove a device, the device would not be
considered a restraint. In this context, “easily remove” means that the manual
method, device, material, or equipment can be removed intentionally by the
patient in the same manner as it was applied by the staff (e.g., side rails are put
down, not climbed over; buckles are intentionally unbuckled; ties or knots are
intentionally untied; etc.) considering the patient’s physical condition and
ability to accomplish objective (e.g., transfer to a chair, get to the bathroom in
time).
Survey Procedures §482.13(e)(1)(i)(A)
• Determine whether the hospital’s policy and procedures employ a definition or
description of what constitutes a restraint that is consistent with the regulation.
• While touring hospital units look for restraints in use. Where a restraint is in use,
check the medical record for appropriate documentation.
•
Interview hospital staff to determine whether they know the definition of a restraint.
A-0160
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e)(1)(i)(B) [A restraint is - ] A drug or medication when it is used as a
restriction to manage the patient's behavior or restrict the patient's freedom of
movement and is not a standard treatment or dosage for the patient's condition.
Interpretive Guidelines §482.13(e)(1)(i)(B)
Drugs or medications that are used as part of a patient's standard medical or psychiatric
treatment, and are administered within the standard dosage for the patient’s condition,
would not be subject to the requirements of standard (e). These regulations are not intended
to interfere with the clinical treatment of patients who are suffering from serious mental
illness and who need therapeutic doses of medication to improve their level of functioning
so that they can more actively participate in their treatment. Similarly, these regulations are
not intended to interfere with appropriate doses of sleeping medication prescribed for
patients with insomnia, anti-anxiety medication prescribed to calm a patient who is anxious,
or analgesics prescribed for pain management. The regulatory language is intended to
provide flexibility and recognize the variations in patient conditions.
Whether or not an order for a drug or medication is PRN (Latin abbreviation for pro re nata
- as needed; as circumstances require) or a standing-order does not determine whether or
not the use of that drug or medication is considered a restraint. The use of PRN or
standing-order drugs or medications is only prohibited if the drug or medication meets the
definition of a drug or medication used as a restraint.
Criteria used to determine whether the use of a drug or medication, or combination of drugs
or medications is a standard treatment or dosage for the patient's condition includes all of
the following:
• The drug or medication is used within the pharmaceutical parameters approved by
the Food and Drug Administration (FDA) and the manufacturer for the indications
that it is manufactured and labeled to address, including listed dosage parameters;
• The use of the drug or medication follows national practice standards established or
recognized by the medical community, or professional medical associations or
organizations; and,
• The use of the drug or medication to treat a specific patient’s clinical condition is
based on that patient's symptoms, overall clinical situation, and on the physician's or
other licensed independent practitioner’s (LIP) knowledge of that patient's expected
and actual response to the medication.
Another component of “standard treatment or dosage” for a drug or medication is the
expectation that the standard use of a drug or medication to treat the patient's condition
enables the patient to more effectively or appropriately function in the world around them
than would be possible without the use of the drug or medication. If the overall effect of a
drug or medication, or combination of drugs or medications, is to reduce the patient's ability
to effectively or appropriately interact with the world around the patient, then the drug or
medication is not being used as a standard treatment or dosage for the patient's condition.
As with any use of restraint or seclusion, staff must conduct a comprehensive patient
assessment to determine the need for other types of interventions before using a drug or
medication as a restraint. For example, a patient may be agitated due to pain, an adverse
reaction to an existing drug or medication, or other unmet care need or concern.
There are situations where the use of a drug or medication is clearly outside the standard for
a patient or a situation, or a medication is not medically necessary but is used for patient
discipline or staff convenience (neither of which is permitted by the regulation).
• EXAMPLE 1: A patient has Sundowner's Syndrome, a syndrome in which a
patient's dementia becomes more apparent at the end of the day rather than at the
beginning of the day. The patient may become agitated, angry, or anxious at
sundown. This may lead to wandering, pacing the floors, or other nervous
behaviors. The staff finds the patient’s behavior bothersome, and asks the physician
to order a high dose of a sedative to “knock out” the patient and keep him in bed.
The patient has no medical symptoms or condition that indicates the need for a
sedative. In this case, for this patient, the sedative is being used inappropriately as a
restraint for staff convenience. Such use is not permitted by the regulation.
A drug or medication that is not being used as a standard treatment for the patient’s
medical or psychiatric condition, and that results in restricting the patient’s freedom
of movement would be a drug used as a restraint.
In addition, the regulation does not permit a drug or medication to be used to
restrain the patient for staff convenience, to coerce or discipline the patient, or as a
method of retaliation. While drugs or medications can be a beneficial part of a
carefully constructed, individualized treatment plan for the patient, drug and
medication use should be based on the assessed needs of the individual patient, and
the effects of drugs and medications on the patient should be carefully monitored.
• EXAMPLE 2: A patient is in a detoxification program. The patient becomes
violent and aggressive. Staff administers a PRN medication ordered by the patient’s
physician or other LIP to address these types of outbursts. The use of the
medication enables the patient to better interact with others or function more
effectively. In this case, the medication used for this patient is not considered a
“drug used as a restraint.” The availability of a PRN medication to manage
outbursts of specific behaviors, such as aggressive, violent behavior is standard for
this patient’s medical condition (i.e., drug or alcohol withdrawal). Therefore, this
patient’s medication does not meet the definition of “drug used as a restraint” since
it is a standard treatment or dosage for the patient’s medical or psychiatric
condition. The use of this medication for this patient is not affected by standard (e).
If a drug or medication is used as a standard treatment (as previously defined) to
address the assessed symptoms and needs of a patient with a particular medical or
psychiatric condition, its use is not subject to the requirements of this regulation.
However, the patient would still need to receive assessments, monitoring,
interventions, and care that are appropriate for that patient’s needs.
The regulation supports existing State laws that provide more vigorous promotion of the
patient’s choice and rights. Therefore, when a State’s law prohibits the administration of
drugs against the wishes of the patient without a court order, the State law applies.
Survey Procedures §482.13(e)(1)(i)(B)
• Determine whether the hospital’s policies and procedures employ a definition or
description of what constitutes the use of drugs or medications as a restraint that is
consistent with the regulation.
•
Interview hospital staff to determine whether they can identify when the use of a
drug or medication is considered a chemical restraint.
A-0161
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e)(1)(i)(C) - A restraint does not include devices, such as orthopedically
prescribed devices, surgical dressings or bandages, protective helmets, or other
methods that involve the physical holding of a patient for the purpose of conducting
routine physical examinations or tests, or to protect the patient from falling out of bed,
or to permit the patient to participate in activities without the risk of physical harm
(this does not include a physical escort).
Interpretive Guidelines §482.13(e)(1)(i)(C)
The devices and methods listed here would not be considered restraints, and, therefore, not
subject to these requirements. These devices and methods are typically used in medical-
surgical care.
Use of an IV arm board to stabilize an IV line is generally not considered a restraint.
However, if the arm board is tied down (or otherwise attached to the bed), or the entire limb
is immobilized such that the patient cannot access his or her body, the use of the arm board
would be considered a restraint.
A mechanical support used to achieve proper body position, balance, or alignment so as to
allow greater freedom of mobility than would be possible without the use of such a
mechanical support is not considered a restraint. For example, some patients lack the
ability to walk without the use of leg braces, or to sit upright without neck, head, or back
braces.
A medically necessary positioning or securing device used to maintain the position, limit
mobility, or temporarily immobilize the patient during medical, dental, diagnostic, or
surgical procedures is not considered a restraint.
Recovery from anesthesia that occurs when the patient is in a critical care or
postanesthesia care unit is considered part of the surgical procedure; therefore, medically
necessary restraint use in this setting would not need to meet the requirements of the
regulation. However, if the intervention is maintained when the patient is transferred to
another unit, or recovers from the effects of the anesthesia (whichever occurs first), a
restraint order would be necessary and the requirements of standard (e) would apply.
Many types of hand mitts would not be considered restraint. However, pinning or
otherwise attaching those same mitts to bedding or using a wrist restraint in conjunction
with the hand mitts would meet the definition of restraint and the requirements would
apply. In addition, if the mitts are applied so tightly that the patient's hand or fingers are
immobilized, this would be considered restraint and the requirements would apply.
Likewise, if the mitts are so bulky that the patient's ability to use their hands is significantly
reduced, this would be considered restraint and the requirements would apply.
NOTE: Because this definition of physical restraint does not name each device and

situation that can be used to immobilize or reduce the ability of the patient to
move his or her arms, legs, body or head freely, it promotes looking at each
patient situation on a case-by-case basis.
In addition, if a patient can easily remove a device, the device would not be considered a
restraint. In this context, “easily remove” means that the manual method, device, material,
or equipment can be removed intentionally by the patient in the same manner as it was
applied by the staff (e.g., side rails are put down, not climbed over; buckles are intentionally
unbuckled; ties or knots are intentionally untied; etc.) considering the patient’s physical
condition and ability to accomplish the objective (e.g., transfer to a chair, get to the
bathroom in time).
Age or developmentally appropriate protective safety interventions (such as stroller
safety belts, swing safety belts, high chair lap belts, raised crib rails, and crib covers) that a
safety-conscious child care provider outside a health care setting would utilize to protect an
infant, toddler, or preschool-aged child would not be considered restraint or seclusion for
the purposes of this regulation. The use of these safety interventions needs to be addressed
in the hospital’s policies or procedures.
Physical Escort
A physical escort would include a “light” grasp to escort the patient to a desired location. If
the patient can easily remove or escape the grasp, this would not be considered physical
restraint. However, if the patient cannot easily remove or escape the grasp, this would be
considered physical restraint and all the requirements would apply.
Physical holding
The regulation permits the physical holding of a patient for the purpose of conducting
routine physical examinations or tests. However, patients do have the right to refuse
treatment. See §482.13(b)(2). This includes the right to refuse physical examinations or
tests. Holding a patient in a manner that restricts the patient's movement against the
patient’s will is considered restraint. This includes holds that some member of the medical
community may term “therapeutic holds.” Many deaths have occurred while employing
these practices. Physically holding a patient can be just as restrictive, and just as
dangerous, as restraining methods that involve devices. Physically holding a patient during
a forced psychotropic medication procedure is considered a restraint and is not included in
this exception.
For the purposes of this regulation, a staff member picking up, redirecting, or holding an
infant, toddler, or preschool-aged child to comfort the patient is not considered restraint.
Physical Holding for Forced Medications
The application of force to physically hold a patient, in order to administer a medication
against the patient’s wishes, is considered restraint. The patient has a right to be free of
restraint and, in accordance with §482.13(b)(2), also has a right to refuse medications,
unless a court has ordered medication treatment. A court order for medication treatment
only removes the patient’s right to refuse the medication. Additionally, in accordance with
State law, some patients may be medicated against their will in certain emergency
circumstances. However, in both of these circumstances, health care staff is expected to use
the least restrictive method of administering the medication to avoid or reduce the use of
force, when possible. The use of force in order to medicate a patient, as with other restraint,
must have a physician’s order prior to the application of the restraint (use of force). If
physical holding for forced medication is necessary with a violent patient, the 1-hour face-
to-face evaluation requirement would also apply.
In certain circumstances, a patient may consent to an injection or procedure, but may not be
able to hold still for an injection, or cooperate with a procedure. In such circumstances, and
at the patient’s request, staff may “hold” the patient in order to safely administer an
injection (or obtain a blood sample, or insert an intravenous line, if applicable) or to
conduct a procedure. This is not considered restraint.
Side rails
A restraint does not include methods that protect the patient from falling out of bed.
Examples include raising the side rails when a patient is: on a stretcher, recovering from
anesthesia, sedated, experiencing involuntary movement, or on certain types of therapeutic
beds to prevent the patient from falling out of the bed. The use of side rails in these
situations protects the patient from falling out of bed and, therefore, would not be subject to
the requirements of standard (e).
However, side rails are frequently not used as a method to prevent the patient from falling
out of bed, but instead, used to restrict the patient’s freedom to exit the bed. The use of side
rails to prevent the patient from exiting the bed would be considered a restraint and would
be subject to the requirements of standard (e). The use of side rails is inherently risky,
particularly if the patient is elderly or disoriented. Frail elderly patients may be at risk for
entrapment between the mattress or bed frame and the side rail. Disoriented patients may
view a raised side rail as a barrier to climb over, may slide between raised, segmented side
rails, or may scoot to the end of the bed to get around a raised side rail and exit the bed.
When attempting to leave the bed by any of these routes, the patient is at risk for
entrapment, entanglement, or falling from a greater height posed by the raised side rail, with
a possibility for sustaining greater injury or death than if the patient had fallen from the
height of a lowered bed without raised side rails. In short, the patient may have an
increased risk for a fall or other injury by attempting to exit the bed with the side rails
raised. The risk presented by side rail use should be weighed against the risk presented by
the patient's behavior as ascertained through individualized assessment.
When the clinician raises all four side rails in order to restrain a patient, defined in this
regulation as immobilizing or reducing the ability of a patient to move his or her arms, legs,
body, or head freely to ensure the immediate physical safety of the patient, then the
requirements of this rule apply. Raising fewer than four side rails when the bed has
segmented side rails would not necessarily immobilize or reduce the ability of a patient to
move freely as defined in the regulation. For example, if the side rails are segmented and
all but one segment are raised to allow the patient to freely exit the bed, the side rail is not
acting as a restraint and the requirements of this rule would not apply. Conversely, if a
patient is not physically able to get out of bed regardless of whether the side rails are raised
or not, raising all four side rails for this patient would not be considered restraint because
the side rails have no impact on the patient’s freedom of movement. In this example, the
use of all four side rails would not be considered restraint. Therefore, the requirements of
this rule would not apply.
When a patient is on a bed that constantly moves to improve circulation or prevents skin
breakdown, raised side rails are a safety intervention to prevent the patient from falling out
of bed and are not viewed as restraint.
When a patient is placed on seizure precautions and all side rails are raised, the use of side
rails would not be considered restraint. The use of padded side rails in this situation should
protect the patient from harm; including falling out of bed should the patient have a seizure.
Placement in a crib with raised rails is an age-appropriate standard safety practice for every
infant or toddler. Therefore, placement of an infant or toddler in the crib with raised rails
would not be considered restraint.
If the patient is on a stretcher (a narrow, elevated, and highly mobile cart used to transport
patients and to evaluate or treat patients), there is an increased risk of falling from a
stretcher without raised side rails due to its narrow width, and mobility. In addition,
because stretchers are elevated platforms, the risk of patient injury due to a fall is
significant. Therefore, the use of raised side rails on stretchers is not considered restraint
but a prudent safety intervention. Likewise, the use of a seat belt when transporting a
patient in a wheelchair is not considered restraint.
Survey Procedures §482.13(e)(1)(i)(C)
• Determine whether the hospital’s policies and procedures employ a definition or
description of what constitutes a restraint that is consistent with the regulation.
• While touring hospital units look for bed side rail use to determine whether it is
consistent with the definition of a restraint. Where bed side rails are being used as a
restraint, check the medical record for appropriate documentation.
•
Interview hospital staff to determine whether they know the definition of a restraint,
particularly with respect to use of bed side rails.
A-0162
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e)(1)(ii) - Seclusion is the involuntary confinement of a patient alone in a room
or area from which the patient is physically prevented from leaving. Seclusion may
only be used for the management of violent or self-destructive behavior.
Interpretive Guidelines §482.13(e)(1)(ii)
Seclusion may only be used for the management of violent or self-destructive behavior that
jeopardizes the immediate physical safety of the patient, a staff member, or others.
Seclusion is not just confining a patient to an area, but involuntarily confining the patient
alone in a room or area where the patient is physically prevented from leaving. If a patient
is restricted to a room alone and staff are physically intervening to prevent the patient from
leaving the room or giving the perception that threatens the patient with physical
intervention if the patient attempts to leave the room, the room is considered locked,
whether the door is actually locked or not. In this situation, the patient is being secluded.
A patient physically restrained alone in an unlocked room does not constitute seclusion.
Confinement on a locked unit or ward where the patient is with others does not constitute
seclusion.
Timeout is not considered seclusion. Timeout is an intervention in which the patient
consents to being alone in a designated area for an agreed upon timeframe from which the
patient is not physically prevented from leaving. Therefore, the patient can leave the
designated area when the patient chooses.
Survey Procedures §482.13(e)(1)(ii)
•
Determine whether the hospital’s policy and procedures employ a definition or
description of what constitutes seclusion that is consistent with the regulation.
• While touring hospital units look for cases where a patient is in seclusion.

• Interview hospital staff to determine whether they know the definition of seclusion.
A-0164
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e)(2) - Restraint or seclusion may only be used when less restrictive
interventions have been determined to be ineffective to protect the patient, a staff
member, or others from harm.
Interpretive Guidelines §482.13(e)(2)
A comprehensive assessment of the patient must determine that the risks associated with the
use of the restraint or seclusion is outweighed by the risk of not using the restraint or
seclusion. Less restrictive interventions do not always need to be tried, but less restrictive
interventions must be determined by staff to be ineffective to protect the patient or others
from harm prior to the introduction of more restrictive measures. Alternatives attempted or
the rationale for not using alternatives must be documented.
The underpinning of this regulation is the concept that safe patient care hinges on looking at
the patient as an individual and assessing the patient’s condition, needs, strengths,
weaknesses, and preferences. Such an approach relies on caregivers who are skilled in
individualized assessment and in tailoring interventions to the individual patient’s needs
after weighing factors such as the patient’s condition, behaviors, history, and environmental
factors.
Resources are available to assist clinicians in identifying less restrictive interventions. For
example, the American Psychiatric Association (APA), American Psychiatric Nurses
Association (APNA), and the National Association of Psychiatric Health Systems
(NAPHS), with support from the American Hospital Association (AHA), have sponsored
the publication of a document entitled, “Learning from Each Other—Success Stories and
Ideas for Reducing Restraint/Seclusion in Behavioral Health.” This document, published in
2003, was developed through dialogue with clinicians in the field and included extensive
input from behavioral healthcare providers throughout the country who have been working
to reduce the use of restraint and seclusion and to improve care within their facilities. To
access this document and other useful resources, visit the web sites of the sponsoring
organizations: http://www.naphs.org; http://www.psych.org; http://www.apna.org;
http://www.aha.org.
Survey Procedures §482.13(e)(2)
• Do physician’s or other LIP's orders specify the reason for restraint or seclusion, the
type of restraint, and the duration of restraint or seclusion?
•
Does the severity of the behavior justify seclusion or restraint usage by identifying an
immediate and serious danger to the physical safety of the patient or others?
• Is there evidence that the hospital considers factors other than the individual patient in
determining causes for the need for restraints or seclusion (i.e., environmental factors)?
• Does the medical record include documentation of an individual patient assessment and
a revision of the plan of care?
• Does the medical record reflect changes in behavior and staff concerns regarding safety

risks to the patient, staff, or others prompting use of seclusion or restraints?
• Did the patient’s behavior place the patient or others at risk for harm? Was the patient’s
behavior violent or self-destructive?
• Were other, less restrictive interventions tried and documented, or is there evidence that
alternatives were considered and determined to be insufficient?
A-0165
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e)(3) - The type or technique of restraint or seclusion used must be the least
restrictive intervention that will be effective to protect the patient, a staff member, or
others from harm.
Interpretive Guidelines §482.13(e)(3)
Resources are available to assist clinicians in identifying less restrictive restraint or
seclusion interventions. For example, the American Psychiatric Association (APA),
American Psychiatric Nurses Association (APNA), and the National Association of
Psychiatric Health Systems (NAPHS), with support from the American Hospital
Association (AHA), have sponsored the publication of a document entitled, “Learning from
Each Other—Success Stories and Ideas for Reducing Restraint/Seclusion in Behavioral
Health.” This document, published in 2003, was developed through dialogue with the field
and extensive input from behavioral healthcare providers throughout the country who have
been working to reduce the use of restraint and seclusion and to improve care within their
facilities. To access this document and other useful resources, visit the web sites of the
sponsoring organizations: http://www.naphs.org; http://www.psych.org;
http://www.apna.org; http://www.aha.org
Survey Procedures §482.13(e)(3)
• Is there clear documentation in the patient’s medical record describing the steps or
interventions used prior to the use of the needed restraint or seclusion? That is, what
documentation is in the medical record to explain the rationale for the use of restraint or
seclusion?
• Is there documentation that less restrictive measures were tried or considered?
• Is the restraint or seclusion intervention the least restrictive intervention that meets
the patient’s clinical needs and protects the safety of the patient, staff, or others?
• Did the staff determine that less restrictive alternatives would not meet the patient’s
clinical needs, or protect the patient’s safety or the safety of others?
• Do ongoing documented assessments demonstrate that the restraint or seclusion
intervention is needed at this time (or at a time in the past) and that the restraint or
seclusion intervention remains the least restrictive way to protect the patient’s
safety?
• If the time of restraint or seclusion use is lengthy, look for evidence that the
symptoms necessitating the use of restraint or seclusion have persisted. Is there
evidence to indicate that the staff have evaluated whether or not the restraint or
seclusion can be safely discontinued?
A-0166
§482.13(e)(4) - The use of restraint or seclusion must be --
(i) in accordance with a written modification to the patient's plan of care.
Interpretive Guidelines §482.13(e)(4)(i)
The use of restraint or seclusion (including drugs or medications used as restraint as well as
physical restraint) must be documented in the patient’s plan of care or treatment plan. The
use of restraint or seclusion constitutes a change in a patient’s plan of care.
The regulation does not require that a modification to the patient’s plan of care be made
before initiating or obtaining an order for the use of restraint or seclusion. The use of a
restraint or seclusion intervention should be reflected in the patient’s plan of care or
treatment plan based on an assessment and evaluation of the patient. The plan of care or
treatment plan should be reviewed and updated in writing within a timeframe specified by
hospital policy.
Survey Procedures §482.13(e)(4)(i)
• Determine whether the hospital’s procedures are consistent with the requirements of
this regulation. Does the plan of care or treatment reflect a process of assessment,
intervention, and evaluation when restraint or seclusion is used?
• Is there evidence of assessment of the identified problem or of an individual patient
assessment?
• Does the patient’s plan of care reflect that assessment?
• What was the goal of the intervention?
• What was the described intervention?
• Who is responsible for implementation?
• Was the patient informed of the changes in his or her treatment plan or plan of care?
• Did the physician or other LIP write orders that included a time limit? Were these
orders incorporated into the plan of care?
• After the discontinuation of the restraint or seclusion intervention, was this
information documented in an update of the plan of care or treatment plan?
A-0167
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The use of restraint or seclusion must be --]
§482.13(e)(4)(ii) - implemented in accordance with safe and appropriate restraint and
seclusion techniques as determined by hospital policy in accordance with State law.
Interpretive Guidelines §482.13(e)(4)(ii)
Restraint or seclusion must be implemented appropriately and safely, and reflect hospital
policy in accordance with State law.
The use of restraint or seclusion must never act as a barrier to the provision of other
interventions to meet the patient’s needs.
Survey Procedures §482.13(e)(4)(ii)
• Review the hospital’s policies and procedures to determine if they reflect current
standards of practice regarding safe and appropriate restraint and seclusion
techniques. Are there any references to State law statutes or any indication State
laws were reviewed and incorporated?
• Review a sample of patient medical records that include patients who required the
use of restraint or seclusion for the management of both violent, self-destructive
behaviors, and non-violent, non-self-destructive behaviors.
• After restraints were applied, was an assessment immediately made to ensure that
restraints were properly and safely applied?
• Were the hospital policies and procedures followed?
• Was the use of restraint or seclusion effective in achieving the purpose for which it
was ordered? If not, were timely changes made?
• Was there any evidence of injury to the patient?
A-0168
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.13(e)(5) - The use of restraint or seclusion must be in accordance with the order
of a physician or other licensed practitioner who is responsible for the care of the
patient and authorized to order restraint or seclusion by hospital policy in accordance
with State law.
Interpretive Guidelines §482.13(e)(5)
Hospitals must have policies and procedures for the initiation of restraint or seclusion that
identify the categories of licensed practitioners (LPs) that are permitted to order restraint or
seclusion in that hospital, consistent with State law.
The regulation requires that a physician or other LP responsible for the care of the patient to
order restraint or seclusion prior to the application of restraint or seclusion. In some
situations, however, the need for a restraint or seclusion intervention may occur so quickly
that an order cannot be obtained prior to the application of restraint or seclusion. In these
emergency application situations, the order must be obtained either during the emergency
application of the restraint or seclusion, or immediately (within a few minutes) after the
restraint or seclusion has been applied. The failure to immediately obtain an order is
viewed as the application of restraint or seclusion without an order. The hospital should
address this process in its restraint and seclusion policies and procedures. The policies and
procedures should specify who can initiate the emergency application of restraint or
seclusion prior to obtaining an order from a physician or other LP.
Licensed Practitioner (LP)
For the purpose of ordering restraint or seclusion, an LP is any practitioner permitted by
State law and hospital policy as having the authority to order restraints or seclusion for
patients.
A resident who is authorized by State law and the hospital’s residency program to practice
as a physician can carry out functions reserved for a physician or LP by the regulation. A
medical school student holds no license, and his/her work is reviewed and must be
countersigned by the attending physician; therefore, he or she is not licensed or
independent. A medical school student is not a LP.
Protocols
A protocol cannot serve as a substitute for obtaining a physician's or other LP’s order prior
to initiating each episode of restraint or seclusion use. If a hospital uses protocols that
include the use of restraint or seclusion, a specific physician or LP order is still required for
each episode of restraint or seclusion use. The philosophy that serves as a foundation for
the regulation is that restraint or seclusion use is an exceptional event, not a routine
response to a certain patient condition or behavior. Each patient must be assessed, and
interventions should be tailored to meet the individual patient’s needs. The creation of a
protocol can run counter to this philosophy if it sets up the expectation that restraint or
seclusion will be used as a routine part of care. The use of restraint or seclusion is a last
resort when less restrictive measures have been determined ineffective to ensure the safety
of the patient, staff or others, should not be a standard response to a behavior or patient
need.
Survey Procedures §482.13(e)(5)
• Review hospital policies and medical staff by-laws to ascertain clinical practice
guidelines that describe the responsibilities of medical staff and clinicians who are
privileged to order restraint and seclusion.
• Do the hospital’s written policies identify what categories of practitioners the State
recognizes as an LP or as having the authority to order restraint and seclusion?
• Does the hospital have written policies indicating which practitioners are permitted
to order restraint or seclusion in the facility?
• Do the hospital’s written policies conform to State law?
• Does the hospital have established policies for who can initiate restraint or
seclusion?
• Does the hospital utilize protocols for the use of restraint or seclusion? If so, is the
use of protocols consistent with the requirements of the regulation?
• Do the medical records reviewed identify the physician or LP who ordered each use
of restraint or seclusion?
• During the medical record review, verify that a physician or LP order was obtained
prior to the initiation of restraint or seclusion. When emergency application of
restraint or seclusion was necessary, verify that a physician or LP order was
obtained immediately (within a few minutes) after application of the restraint or
seclusion.
A-0169
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e)(6) - Orders for the use of restraint or seclusion must never be written as a

standing order or on an as needed basis (PRN).
Interpretive Guidelines §482.13(e)(6)
This regulation prohibits the use of standing or PRN (Latin abbreviation for pro re nata - as
needed; as circumstances require) orders for the use of restraint or seclusion. The ongoing
authorization of restraint or seclusion is not permitted. Each episode of restraint or
seclusion must be initiated in accordance with the order of a physician or other LIP. If a
patient was recently released from restraint or seclusion, and exhibits behavior that can only
be handled through the reapplication of restraint or seclusion, a new order would be
required. Staff cannot discontinue a restraint or seclusion intervention, and then re-start it
under the same order. This would constitute a PRN order. A “trial release” constitutes a
PRN use of restraint or seclusion, and, therefore, is not permitted by this regulation.
When a staff member ends an ordered restraint or seclusion intervention, the staff member
has no authority to reinstitute the intervention without a new order. For example, a patient
is released from restraint or seclusion based on the staff’s assessment of the patient’s
condition. If this patient later exhibits behavior that jeopardizes the immediate physical
safety of the patient, a staff member, or others that can only be handled through the use of
restraint or seclusion, a new order would be required.
NOTE: A temporary, directly-supervised release, however, that occurs for the purpose
of caring for a patient's needs (e.g., toileting, feeding, or range of motion
exercises) is not considered a discontinuation of the restraint or seclusion
intervention. As long as the patient remains under direct staff supervision, the
restraint is not considered to be discontinued because the staff member is present
and is serving the same purpose as the restraint or seclusion.
The use of PRN orders for drugs or medications is only prohibited when a drug or
medication is being used as a restraint. A drug or medication is deemed to be a restraint
only if it is not a standard treatment or dosage for the patient’s condition, and the drug or
medication is a restriction to manage the patient’s behavior or restricts the patient’s freedom
of movement Using a drug to restrain the patient for staff convenience is expressly
prohibited.
EXCEPTIONS
• Geri chair. If a patient requires the use of a Geri chair with the tray locked in place
in order for the patient to safely be out of bed, a standing or PRN order is permitted.
Given that a patient may be out of bed in a Geri chair several times a day, it is not
necessary to obtain a new order each time.
• Raised side rails. If a patient's status requires that all bedrails be raised (restraint)
while the patient is in bed, a standing or PRN order is permitted. It is not necessary
to obtain a new order each time the patient is returned to bed after being out of bed.
• Repetitive self-mutilating behavior. If a patient is diagnosed with a chronic
medical or psychiatric condition, such as Lesch-Nyham Syndrome, and the patient
engages in repetitive self-mutilating behavior, a standing or PRN order for restraint
to be applied in accordance with specific parameters established in the treatment
plan would be permitted. Since the use of restraints to prevent self-injury is needed
for these types of rare, severe, medical and psychiatric conditions, the specific
requirements (1-hour face-to-face evaluation, time-limited orders, and evaluation
every 24 hours before renewal of the order) for the management of violent or self-
destructive behavior do not apply.
Survey Procedures §482.13(e)(6)
Review a random sample of medical records for patients that have been restrained or
secluded. Review orders, progress notes, flow sheets, and nursing notes to:
• Verify that there is a physician or other LIP order for each episode of restraint or
seclusion;
• Evaluate patterns of use and verify that orders were obtained when necessary;
• Verify that the documentation specifically addresses the patients’ behaviors or
symptoms; and,
• Determine if restraint or seclusion is being improperly implemented on a PRN basis.
A-0170
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e)(7) - The attending physician must be consulted as soon as possible if the
attending physician did not order the restraint or seclusion.
Interpretive Guidelines §482.13(e)(7)
The attending physician is the MD/DO who is responsible for the management and care of
the patient. Hospital medical staff policies determine who is considered the attending
physician. The intent of this requirement is to ensure that the physician who has overall
responsibility and authority for the management and care of the patient is aware of the
patient’s condition and is aware of the restraint or seclusion intervention. It is important to
consult with the attending physician to promote continuity of care, to ensure patient safety,
and to elicit information that might be relevant in choosing the most appropriate
intervention for the patient. The attending physician may have information regarding the
patient’s history that may have a significant impact on the selection of a restraint or
seclusion intervention or an alternative intervention, and the subsequent course of
treatment. Therefore, consultation should occur as soon as possible. Hospital policies and
procedures should address the definition of “as soon as possible” based on the needs of their
particular patient population(s). However, any established time frames must be consistent
with “as soon as possible.”
The hospital CoPs do permit the patient to be under the care of a treating LIP other than a
physician. Section 482.12(c)(1) requires every Medicare patient to be under the care of
a doctor of medicine or osteopathy; or, within the scope of their respective licenses, a
doctor of dental surgery or dental medicine, a doctor of podiatry, chiropractor, or clinical
psychologist. The individual overseeing the patient’s care may be the attending physician
or a health professional practicing with the delegated authority or supervision of a doctor of
medicine or osteopathy as permitted by State law and hospital policy.
When the attending physician of record is unavailable, responsibility for the patient must be
delegated to another physician, who would then be considered the attending physician.
This provision does not specify that consultation with the attending physician be face-to-
face. The consultation can occur via telephone.
Survey Procedures §482.13(e)(7)
• Review the patient’s medical record for documentation that the attending physician was
notified immediately if the attending physician did not order the restraint or seclusion.
Was the attending physician notified “as soon as possible?”
• Review the hospital’s policies and procedures regarding consultation with the attending
physician if the attending physician did not order the restraint or seclusion.
• Interview staff to determine if actual practice is consistent with written hospital policies
and procedures.
A-0171
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e)(8) - Unless superseded by State law that is more restrictive --
(i) Each order for restraint or seclusion used for the management of violent or self-
destructive behavior that jeopardizes the immediate physical safety of the patient,
a staff member, or others may only be renewed in accordance with the following
limits for up to a total of 24 hours:
(A) 4 hours for adults 18 years of age or older;
(B) 2 hours for children and adolescents 9 to 17 years of age; or
(C) 1 hour for children under 9 years of age; and
Interpretive Guidelines §482.13(e)(8)(i)
Patients of all ages are vulnerable and at risk when restrained or secluded to manage violent
or self-destructive behavior. Therefore, time limits have been established for each order for
restraint or seclusion used to manage violent or self-destructive behavior. State law may
require more restrictive time limits. These time limits do not apply to orders for restraint
used to manage non-violent or non-self-destructive behavior. However, the requirement
that restraint use be ended at the earliest possible time applies to all uses of restraint.
In the final rule on the use of restraint or seclusion, CMS did not include specific criteria for
differentiating between emergency situations where the patient’s behavior is violent or self-
destructive and jeopardizes the immediate physical safety of the patient, a staff member, or
others, and non-emergency use of restraint. Clinicians are adept at identifying various
behaviors and symptoms, and can readily recognize violent and self-destructive behavior
that jeopardizes the immediate physical safety of the patient, a staff member, or others.
Asking clinicians to act based on an evaluation of the patient’s behavior is no different than
relying on the clinical judgment that they use daily in assessing the needs of each patient
and taking actions to meet those individual needs.
The regulation identifies maximum time limits on the length of each order for restraint or
seclusion based on age. The physician or other LIP has the discretion to write the order for
a shorter length of time. The length-of-order requirement identifies critical points at which
there is mandatory contact with a physician or other LIP responsible for the care of the
patient. In addition, the time limits do not dictate how long a patient should remain in
restraint or seclusion. Staff is expected to continually assess and monitor the patient to
ensure that the patient is released from restraint or seclusion at the earliest possible time.
Restraint or seclusion may only be employed while the unsafe situation continues. Once
the unsafe situation ends, the use of restraint or seclusion should be discontinued. The
regulation explicitly states that the intervention must be discontinued at the earliest possible
time, regardless of the length of time identified in the order. For example, if a patient’s
behavior is no longer violent or self-destructive 20 minutes after the intervention is
initiated, then the restraint or seclusion should be discontinued, even if the order was given
for up to 4 hours. If restraint or seclusion is discontinued prior to the expiration of the
original order, a new order must be obtained prior to reinitiating the use of restraint or
seclusion.

At the end of the time frame, if the continued use of restraint or seclusion to manage violent
or self-destructive behavior is deemed necessary based on an individualized patient
assessment, another order is required. When the original order is about to expire, an RN
must contact the physician or other LIP, report the results of his or her most recent
assessment and request that the original order be renewed (not to exceed the time limits
established in the regulation). Whether or not an onsite assessment is necessary prior to
renewing the order is left to the discretion of the physician or other LIP in conjunction with
a discussion with the RN who is over-seeing the care of the patient. Another 1-hour face-
to-face patient evaluation (see §482.13(e)(12) and the related interpretive guidance) is not
required when the original order is renewed.
The original restraint or seclusion order may only be renewed within the required time
limits for up to a total of 24 hours. After the original order expires, a physician or other LIP
must see and assess the patient before issuing a new order.
EXCEPTION: Repetitive self-mutilating behaviors – see interpretive guidance for
§482.13(e)(6).
Survey Procedures §482.13(e)(8)(i)
• When restraint or seclusion is used to manage violent or self-destructive behavior, do
orders contain the appropriate time frames based on the patient’s age? Does the total
number of hours covered by an order or its renewal exceed 24 hours?
• If more restrictive State laws apply, are they being followed?
• Is the renewal order for restraint or seclusion based on a comprehensive individual
patient assessment?
• Is there evidence in the patient’s medical record that the symptoms necessitating the
continued use of restraint or seclusion have persisted?
A-0172
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[Unless superseded by State law that is more restrictive --]
§482.13(e)(8)(ii) - After 24 hours, before writing a new order for the use of restraint or
seclusion for the management of violent or self-destructive behavior, a physician or
other licensed practitioner who is responsible for the care of the patient and
authorized to order restraint or seclusion by hospital policy in accordance with State
law must see and assess the patient..
Interpretive Guidelines §482.13(e)(8)(ii)
At a minimum, if a patient remains in restraint or seclusion for the management of violent
or self-destructive behavior 24 hours after the original order, the physician or other LP must
see the patient and conduct a face-to-face re-evaluation before writing a new order for the
continued use of restraint or seclusion. Twenty-four hours of restraint or seclusion for the
management of violent or self-destructive behavior is an extreme measure with the potential
for serious harm to the patient.
State laws may be more restrictive and require the physician or other LP to conduct a face-
to-face re-evaluation within a shorter timeframe.

When the physician or other LP renews an order or writes a new order authorizing the
continued use of restraint or seclusion, there must be documentation in the patient’s medical
record that describes the findings of the physician's or other LPs re-evaluation supporting
the continued use of restraint or seclusion.
EXCEPTION: Repetitive self-mutilating behaviors – see interpretive guidance for
§482.13(e)(6).
Survey Procedures §482.13(e)(8)((ii)
• If restraint or seclusion is used to manage violent or self-destructive behavior for
longer than 24 hours, is there documentation of a new written order, patient
assessments, and a re-evaluation by a physician or other LP in the medical record?
Does the documentation provide sufficient evidence to support the need to continue
the use of restraint or seclusion? Is there evidence in the medical record that the
symptoms necessitating the continued use of restraint or seclusion have persisted?
• Does the patient’s plan of care or treatment plan address the use of restraint or
seclusion?
• What is the patient’s documented clinical response to the continued need for
restraint and seclusion?
A-0173
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[Unless superseded by State law that is more restrictive --]
§482.13(e)(8)(iii) - Each order for restraint used to ensure the physical safety of the
non-violent or non-self-destructive patient may be renewed as authorized by hospital
policy.
Interpretive Guidelines §482.13(e)(8)(iii)
Hospitals have the flexibility to determine time frames for the renewal of orders for
restraint of the non-violent, non-self-destructive patient. These time frames should be
addressed in hospital policies and procedures.
Survey Procedures §482.13(e)(8)(iii)
• Review the hospital policy on renewal of restraint orders for the management of
non-violent, non-self-destructive patient behavior.
• Interview staff and review the medical record documentation to ensure that practice
is consistent with the hospital policy.
A-0174
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e)(9) - Restraint or seclusion must be discontinued at the earliest possible
time, regardless of the length of time identified in the order.
Interpretive Guidelines §482.13(e)(9)
Restraint or seclusion may only be employed while the unsafe situation continues. Once
the unsafe situation ends, the use of restraint or seclusion must be discontinued.
Staff members are expected to assess and monitor the patient’s condition on an ongoing
basis to determine whether restraint or seclusion can safely be discontinued. The regulation
requires that these interventions be ended as quickly as possible. However, the decision to
discontinue the intervention should be based on the determination that the patient’s
behavior is no longer a threat to self, staff members, or others. When the physician or LIP
renews an order or writes a new order authorizing the continued use of restraint or
seclusion, there must be documentation in the medical record that describes the patient’s
clinical needs and supports the continued use of restraint or seclusion.
The hospital policies and procedures should address, at a minimum:
• Categories of staff that the hospital authorizes to discontinue restraint or seclusion in
accordance with State law; and
• The circumstances under which restraint or seclusion is to be discontinued.
Survey Procedures §482.13(e)(9)
• Does the hospital have policies and procedures for ending restraint or seclusion? Do
the policies include a requirement to end the restraint or seclusion as soon as is
safely possible?
• Does the medical record contain evidence that the decision to continue or
discontinue the use of restraint or seclusion was based on an assessment and re-
evaluation of the patient’s condition?
• Interview staff to determine whether they are aware that use of a restraint or
seclusion must be discontinued as soon as is safely possible.
A-0175
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.13(e)(10) - The condition of the patient who is restrained or secluded must be
monitored by a physician, other licensed practitioner or trained staff that have
completed the training criteria specified in paragraph (f) of this section at an interval
determined by hospital policy.
Interpretive Guidelines §482.13(e)(10)
Ongoing assessment and monitoring of the patient's condition by a physician, other LP or
trained staff is crucial for prevention of patient injury or death, as well as ensuring that the
use of restraint or seclusion is discontinued at the earliest possible time. Hospital policies
are expected to guide staff in determining appropriate intervals for assessment and
monitoring based on the individual needs of the patient, the patient's condition, and the type
of restraint or seclusion used. The selection of an intervention and determination of the
necessary frequency of assessment and monitoring should be individualized, taking into
consideration variables such as the patient’s condition, cognitive status, risks associated
with the use of the chosen intervention, and other relevant factors. In some cases, checks
every 15 minutes or vital signs taken every 2 hours may not be sufficient to ensure the
patient’s safety. In others, it may be excessive or disruptive to patient care (e.g., it may be
unnecessary to mandate that a patient with wrist restraints, and who is asleep, be checked
every 15 minutes and awakened every 2 hours to take the patient’s vital signs). Similarly,

depending on the patient’s needs and situational factors, the use of restraint or seclusion
may require either periodic (e.g., every 15 minutes, every 30 minutes, etc.) or continual
(i.e., moment to moment) monitoring and assessment.
Hospital policies should address: frequencies of monitoring and assessment; assessment
content (e.g., vital signs, circulation, hydration needs, elimination needs, level of distress
and agitation, mental status, cognitive functioning, skin integrity, etc.); providing for
nutritional needs, range of motion exercises, and elimination needs; and mental status and
neurological evaluations.
With the exception of the simultaneous use of restraint and seclusion, one-to-one
observation with a staff member in constant attendance is not required by this regulation
unless deemed necessary based on a practitioner’s clinical judgment. For example, placing
staff at the bedside of a patient with wrist restraints may be unnecessary. However, for a
more restrictive or risky intervention and/or a patient who is suicidal, self injurious, or
combative, staff may determine that continual face-to-face monitoring is needed. The
hospital is responsible for providing the level of monitoring and frequency of reassessment
that will protect the patient's safety.
Hospitals have flexibility in determining which staff performs the patient assessment and
monitoring. This determination must be in accordance with the practitioner’s scope of
clinical practice and State law. For example, assessment and monitoring are activities
within a registered nurse’s scope of practice. However, some trained, unlicensed staff may
perform components of monitoring (e.g., checking the patient's vital signs, hydration and
circulation; the patient’s level of distress and agitation; or skin integrity), and may also
provide for general care needs (e.g., eating, hydration, toileting, and range of motion
exercises). Section 482.13(f) requires that before applying restraints, implementing
seclusion, or performing associated monitoring and care tasks, staff must be trained and
able to demonstrate competency in the performance of these actions.
Survey Procedures §482.13(e)(10)
• Review hospital policies regarding assessment and monitoring of a patient in
restraint or seclusion.
o What evidence do you find that the hospital’s monitoring policies are put into
practice for all restrained or secluded patients?
o Do hospital policies identify which categories of staff are responsible for
assessing and monitoring the patient?
o Do hospital policies include time frames for offering fluids and nourishment,
toileting/elimination, range of motion, exercise of limbs and systematic release
of restrained limbs? Is this documented in the patient’s medical record?
• Review patient medical records:
o Was there a valid rationale for the decision regarding the frequency of patient
assessment and monitoring documented in the medical record?
o Was documentation consistent, relevant, and reflective of the patient’s
condition?
o Are time frames described for how often a patient is monitored for vital signs,
respiratory and cardiac status, and skin integrity checks?
o Is there documentation of ongoing patient monitoring and assessment (e.g., skin
integrity, circulation, respiration, intake and output, hygiene, injury, etc)?
o Is the patient’s mental status assessed? Is this documented in the medical
record?
o Is the patient assessed regarding continued need for the use of seclusion or
restraint?
o Is there adequate justification for continued use and is this documented?
o Is the level of supervision appropriate to meet the safety needs of the patient
who is at a higher risk for injury (e.g., self-injurious, suicidal)?
A-00176
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.13(e)(11) - Physician and other licensed practitioner training requirements must
be specified in hospital policy. At a minimum, physicians and other licensed
practitioners authorized to order restraint or seclusion by hospital policy in
accordance with State law must have a working knowledge of hospital policy
regarding the use of restraint or seclusion.
Interpretive Guidelines §482.13(e)(11)
At a minimum, physicians and other LPs authorized to order restraint and seclusion must
have a working knowledge of hospital policy regarding the use of restraint and seclusion.
Hospitals have the flexibility to identify training requirements above this minimum
requirement based on the competency level of their physicians and other LPs, and the needs
of the patient population(s) that they serve. Physicians receive training in the assessment,
monitoring, and evaluation of a patient’s condition as part of their medical school
education. However, physicians generally do not receive training regarding application of
restraint or implementation of seclusion as part of their basic education. Depending on the
level and frequency of involvement that a physician or other LP has in the performance of
these activities, additional training may or may not be necessary to ensure the competency
of these individuals in this area. The hospital is in the best position to determine if
additional physician or other LP training is necessary based on the model of care, level of
physician competency, and the needs of the patient population(s) that the hospital serves.
Survey Procedures §482.13(e)(11)
• Review the hospital policy regarding restraint and seclusion training requirements
for physicians and other LPs. Are the minimum training requirements addressed?
• Review medical staff credentialing and privileging files to determine if physicians
or other LPs involved in restraint and seclusion activities have completed the
required training.
A-0178
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.13(e)(12) - When restraint or seclusion is used for the management of violent or
self-destructive behavior that jeopardizes the immediate physical safety of the patient,
a staff member, or others, the patient must be seen face-to-face within 1 hour after the
initiation of the intervention --
(i) By a –
(A) Physician or other licensed practitioner; or
(B) Registered nurse who has been trained in accordance with the
requirements specified in paragraph (f) of this section.
Interpretive Guidelines §482.13(e)(12)(i)
When restraint or seclusion is used to manage violent or self-destructive behavior, a
physician or other LP, or a registered nurse (RN) trained in accordance with the
requirements specified under §482.13(f), must see the patient face-to-face within 1-hour
after the initiation of the intervention. This requirement also applies when a drug or
medication is used as a restraint to manage violent or self-destructive behavior.
The 1-hour face-to-face patient evaluation must be conducted in person by a physician or
other LP, or trained RN. A telephone call or telemedicine methodology is not permitted.
If a patient’s violent or self-destructive behavior resolves and the restraint or seclusion
intervention is discontinued before the practitioner arrives to perform the 1-hour face-to-
face evaluation, the practitioner is still required to see the patient face-to-face and conduct
the evaluation within 1 hour after the initiation of this intervention. The fact that the
patient’s behavior warranted the use of a restraint or seclusion indicates a serious medical
or psychological need for prompt evaluation of the patient behavior that led to the
intervention. The evaluation would also determine whether there is a continued need for
the intervention, factors that may have contributed to the violent or self-destructive
behavior, and whether the intervention was appropriate to address the violent or self-
destructive behavior.
EXCEPTION: Repetitive self-mutilating behaviors – see interpretive guidance for
§482.13(e)(6).
Survey Procedures §482.13(e)(12)(i)
• Review the hospital policy regarding the 1-hour face-to-face evaluation.
• What categories of practitioners does the hospital policy authorize to conduct the 1-
hour face-to-face evaluation?
• Interview staff to determine if practice is consistent with hospital policy.
A-0179
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[the patient must be seen face-to-face within 1 hour after the initiation of the
intervention -- ]
§482.13(e)(12)(ii)To evaluate –
(A) The patient's immediate situation;
(B) The patient's reaction to the intervention;
(C) The patient's medical and behavioral condition; and
(D) The need to continue or terminate the restraint or seclusion.
Interpretive Guidelines §482.13(e)(12)(ii)
The 1-hour face-to-face evaluation includes both a physical and behavioral assessment of
the patient that must be conducted by a qualified practitioner within the scope of their
practice. An evaluation of the patient’s medical condition would include a complete review
of systems assessment, behavioral assessment, as well as review and assessment of the
patient’s history, drugs and medications, most recent lab results, etc. The purpose is to
complete a comprehensive review of the patient’s condition to determine if other factors,
such as drug or medication interactions, electrolyte imbalances, hypoxia, sepsis, etc., are
contributing to the patient’s violent or self-destructive behavior.
Training for an RN or PA to conduct the 1-hour face-to-face evaluation would include all of
the training requirements at §482.13(f) as well as content to evaluate the patient's
immediate situation, the patient's reaction to the intervention, the patient's medical and
behavioral condition (documented training in conducting physical and behavioral
assessment); and the need to continue or terminate the restraint or seclusion.
Survey Procedures §482.13(e)(12)(ii):
• Was the 1-hour face-to-face evaluation conducted by a practitioner authorized by
hospital policy in accordance with State law to conduct this evaluation?
• If the 1-hour face-to-face evaluations are conducted by RNs who are not advanced
practice nurses (APN), verify that those RNs have documented training that
demonstrates they are qualified to conduct a physical and behavioral assessment of
the patient that addresses: the patient’s immediate situation, the patient’s reaction to
the intervention, the patient’s medical and behavioral condition, and the need to
continue or terminate the restraint or seclusion.
• Does documentation of the 1-hour face-to-face evaluation in the patient’s medical
record include all the listed elements of this requirement?
• Did the evaluation indicate whether changes in the patient’s care were required, and,
if so, were the changes made?
• Is practice consistent with hospital policy and State law?
A-0180
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e)(13) - States are free to have requirements by statute or regulation that are
more restrictive than those contained in paragraph (e)(12)(i) of this section.
Interpretive Guidelines §482.13(e)(13)
States are free to have requirements that are more restrictive regarding the types of
practitioners who may conduct the 1-hour face-to-face evaluation. Generally, States may
have more restrictive requirements as long as they do not conflict with Federal
requirements.
Survey Procedures §482.13(e)(13):
• When preparing for the hospital survey, determine whether there are State
provisions governing the use of restraint or seclusion that are more restrictive than
those found in this section.
• When State requirements are more restrictive, apply those requirements instead of
those found in this section.
A-0182
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.13(e)(14) - - If the face-to-face evaluation specified in paragraph (e)(12) of this
section is conducted by a trained registered nurse, the trained registered nurse must
consult the attending physician or other licensed practitioner who is responsible for
the care of the patient soon as possible after the completion of the 1 hour face-to-face
evaluation.
Interpretive Guidelines §482.13(e)(14)
When a trained RN conducts the required face-to-face evaluation, he or she must consult
the attending physician or other LP responsible for the patient’s care as soon as possible
after the completion of the evaluation. Hospital policy should address the expected time
frame for and the components of the consultation with the attending physician or other LP
consistent with “as soon as possible.” This consultation should include, at a minimum, a
discussion of the findings of the 1-hour face-to-face evaluation, the need for other
interventions or treatments, and the need to continue or discontinue the use of restraint or
seclusion. A consultation that is not conducted prior to a renewal of the order would not be
consistent with the requirement, “as soon as possible.”
Survey Procedures §482.13(e)(14):
• Review the relevant hospital restraint and seclusion policy.
• Does the hospital policy clarify expectations regarding the requirement, “as soon as
possible”?
• Does documentation in the patient’s medical record indicate consultation with the
attending physician or other LP when the 1-hour face-to-face evaluation was
conducted by a trained RN?
• Is practice consistent with hospital policy?
A-0183
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e)(15) - All requirements specified under this paragraph are applicable to the
simultaneous use of restraint and seclusion. Simultaneous restraint and seclusion use
is only permitted if the patient is continually monitored –
(i) Face-to-face by an assigned, trained staff member; or
(ii) By trained staff using both video and audio equipment. This monitoring
must be in close proximity to the patient.
Interpretive Guidelines §482.13(e)(15)
When the simultaneous use of restraint and seclusion is employed, there must be adequate
documentation that justifies the decision for simultaneous use as well as vigilance in
continuously monitoring the patient so that the patient’s care needs are met.
All requirements specified under standard (e) apply to the simultaneous use of restraint and
seclusion. The simultaneous use of restraint and seclusion is not permitted unless the
patient is continually monitored by trained staff, either through face-to-face observation or

through the use of both video and audio equipment. Monitoring with video and audio
equipment further requires that staff perform the monitoring in close proximity to the
patient. For the purposes of this requirement, “continually” means ongoing without
interruption. The use of video and audio equipment does not eliminate the need for
frequent monitoring and assessment of the patient.
An individual who is physically restrained alone in his or her room is not necessarily being
simultaneously secluded. The individual’s privacy and dignity should be protected to the
extent possible during any intervention. In fact, the purpose of restraining a patient alone in
his or her room may be to promote privacy and dignity versus simultaneously using
seclusion and restraint. While this distinction may be difficult to make, it is helpful to
consider whether the patient would, in the absence of the physical restraint, be able to
voluntarily leave the room. If so, then the patient is not also being secluded. However, if
the physical restraint was removed and the patient was still unable to leave the room
because the door was locked or staff were otherwise physically preventing the patient from
doing so, then the patient is also being secluded.
Staff must take extra care to protect the safety of the patient when interventions that are
more restrictive are used. Monitoring must be appropriate to the intervention chosen, so
that the patient is protected from possible abuse, assault, or self injury during the
intervention.
Survey Procedures §482.13(e)(15):
• Review the hospital’s policy regarding simultaneous use of restraint and seclusion to
determine whether it provides for continual monitoring and otherwise complies with
all requirements of §482.13.
• Conduct document review and staff interviews to determine if practice is consistent
with the hospital policy and required uninterrupted audio and visual monitoring is
provided as required.
• Is the staff member monitoring the patient with video and audio equipment trained
and in close proximity to ensure prompt emergency intervention if a problem arises?
• Does the video equipment cover all areas of the room or location where the patient
is restrained or secluded?
• Is the audio and video equipment located in an area that assures patient privacy?
• Is the equipment appropriately maintained and in working condition?
A-0184
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(e)(16) - When restraint or seclusion is used, there must be documentation in
the patient's medical record of the following:
(i) The 1-hour face-to-face medical and behavioral evaluation if restraint or
seclusion is used to manage violent or self-destructive behavior;
Interpretive Guidelines §482.13(e)(16)(i)
When restraint or seclusion is used to manage violent or self-destructive behavior, the 1
hour face-to-face medical and behavioral evaluation must be documented in the patient’s
medical record.
Survey Procedures §482.13(e)(16)(i)
Does the patient’s medical record include documentation of the1 hour face-to-face medical
and behavioral evaluation when restraint or seclusion is used to manage violent or self-
destructive behavior?
A-0185
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[When restraint or seclusion is used, there must be documentation in the patient's
medical record of the following:]
§482.13(e)(16)(ii) - A description of the patient's behavior and the intervention used.
Interpretive Guidelines §482.13(e)(16)(ii)
Documentation that must be included in the patient’s medical record when the patient is
restrained or secluded includes a description of the patient’s behavior and the intervention
used. The patient’s behavior should be documented in descriptive terms to evaluate the
appropriateness of the interventions used. The documentation should include a detailed
description of the patient’s physical and mental status assessments, and of any
environmental factors (e.g., physical, milieu, activities, etc.) that may have contributed to
the situation at the time of the intervention.
Survey Procedures §482.13(e)(16(ii)
• Does the patient’s medical record include a clear description of the patient’s
behavior that warranted the use of restraint or seclusion?
• Was the intervention employed appropriate for the identified behavior?
• What was the patient’s clinical response to the intervention(s)?
A-0186
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[When restraint or seclusion is used, there must be documentation in the patient's
medical record of the following:]
§482.13(e)(16)(iii) - Alternatives or other less restrictive interventions attempted (as
applicable).
Interpretive Guidelines §482.13(e)(16)(iii)
The use of restraint or seclusion must be selected only when less restrictive measures have
been judged to be ineffective to protect the patient or others from harm. It is not always
appropriate for less restrictive alternatives to be attempted prior to the use of restraint or
seclusion. When a patient’s behavior presents an immediate and serious danger to his- or
herself, or others, immediate action is needed. For example, when a patient physically
attacks someone, immediate action is needed. While staff should be mindful of using the
least intrusive intervention, it is critical that the intervention selected be effective in
protecting the patient or others from harm.
Survey Procedures §482.13(e)(16)(iii):
• Does the patient’s medical record document any alternatives or less restrictive
interventions attempted by staff, if appropriate?
• What was the effect of less restrictive interventions, if attempted by staff?
• Were the interventions selected appropriate to the targeted patient behaviors?
• When an immediate and serious danger to the patient or others occurred, was the
more restrictive intervention(s) effective? Could a less restrictive intervention have
been used to ensure the safety of the patient, staff or others?
A-0187
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[When restraint or seclusion is used, there must be documentation in the patient's
medical record of the following:]
§482.13(e)(16)(iv) - The patient's condition or symptom(s) that warranted the use of
the restraint or seclusion.
Interpretive Guidelines §482.13(e)(16)(iv)
A comprehensive, individualized patient assessment is necessary to identify the most
appropriate intervention to effectively manage a patient’s condition or symptom(s). When
using a restraint or seclusion intervention, the patient’s condition or symptom(s) must be
identified and documented in the patient’s medical record.
Survey Procedures §482.13(e)(16)(iv):
Does the patient’s medical record include descriptions of the patient’s condition or
symptom(s) that warranted the use of restraint or seclusion?
A-0188
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[When restraint or seclusion is used, there must be documentation in the patient's
medical record of the following:]
§482.13(e)(16)(v) - The patient's response to the intervention(s) used, including the
rationale for continued use of the intervention.
Survey Procedures §482.13(e)(16)(v):
• Does the patient’s medical record include descriptions of the impact of the
intervention on the patient behavior that resulted in the use of restraint or seclusion?
• Does the patient's medical record include a detailed assessment of the patient's
response to the intervention and a well-reasoned plan for the continued use of
restraint or seclusion?

A-0194
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(f) Standard: Restraint or seclusion: Staff training requirements.
The patient has the right to safe implementation of restraint or seclusion
by trained staff.
Interpretive Guidelines §482.13(f)
Without adequate staff training and competency, the direct care staff, patients, and others
are placed at risk. Patients have a right to the safe application of restraint or seclusion by
trained and competent staff. Staff training and education play a critical role in the reduction
of restraint and seclusion use in a hospital.
Survey Procedures §482.13(f)
• Determine whether the hospital has staff training and education program that
protects the patient’s right to safe implementation of restraint or seclusion.
•
Observe patients in restraint or seclusion to verify safe application of the restraint or
seclusion.
A-0196
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(f)(1) Training Intervals - Staff must be trained and able to demonstrate
competency in the application of restraints, implementation of seclusion, monitoring,
assessment, and providing care for a patient in restraint or seclusion –
(i) Before performing any of the actions specified in this paragraph;
(ii) As part of orientation; and
(iii) Subsequently on a periodic basis consistent with hospital policy.
Interpretive Guidelines §482.13(f)(1)(i) - (iii)
All staff designated by the hospital as having direct patient care responsibilities, including
contract or agency personnel, must demonstrate the competencies specified in standard (f)
prior to participating in the application of restraints, implementation of seclusion,
monitoring, assessment, or care of a patient in restraint or seclusion. These competencies
must be demonstrated initially as part of orientation and subsequently on a periodic basis
consistent with hospital policy. Hospitals have the flexibility to identify a time frame for
ongoing training based on the level of staff competency, and the needs of the patient
population(s) served.
Training for an RN or PA to conduct the 1-hour face-to-face evaluation would include all of
the training requirements at §482.13(f) as well as content to evaluate the patient's
immediate situation, the patient's reaction to the intervention, the patient's medical and
behavioral condition, and the need to continue or terminate the restraint or seclusion. An
evaluation of the patient’s medical condition would include a complete review of systems
assessment, behavioral assessment, as well as review and assessment of the patient’s
history, medications, most recent lab results, etc. The purpose of the 1-hour face-to-face
evaluation is to complete a comprehensive review of the patient’s condition and determine
if other factors, such as drug or medication interactions, electrolyte imbalances, hypoxia,
sepsis, etc., are contributing to the patient’s violent or self-destructive behavior.

Once initial training takes place, training must be provided frequently enough to ensure that
staff possesses the requisite knowledge and skills to safely care for restrained or secluded
patients in accordance with the regulations. The results of skills and knowledge
assessments, new equipment, or QAPI data may indicate a need for targeted training or
more frequent or revised training.
Hospitals are required to have appropriately trained staff for the proper and safe use of
seclusion and restraint interventions. It would not be appropriate for a hospital to routinely
call upon a law enforcement agency or agencies as a means of applying restraint or
initiating seclusion. If hospital security guards, or other non-healthcare staff, as part of
hospital policy, may assist direct care staff, when requested, in the application of restraint or
seclusion, the security guards, or other non-healthcare staff, are also expected to be trained
and able to demonstrate competency in the safe application of restraint and seclusion (in
accordance with §482.13(f))
Survey Procedures §482.13(f)(1)(i) - (iii)
• Does the hospital have a documented training program for the use of restraint and
seclusion interventions employed by the hospital?
• Does the hospital have documented evidence that all levels of staff, including
agency or contract staff, that have direct patient care responsibilities and any other
individuals who may be involved in the application of restraints (e.g., security
guards) have been trained and are able to demonstrate competency in the safe use of
seclusion and the safe application and use of restraints?
• Review and verify restraint and seclusion education staff training documentation for
all new employees and contract staff.
• Does the training include demonstration of required competencies?
• What areas were included in this training program?
A-0199
§482.13(f)(2) Training Content. - The hospital must require appropriate staff to have
education, training, and demonstrated knowledge based on the specific needs of the
patient population in at least the following:
(i) Techniques to identify staff and patient behaviors, events, and
environmental factors that may trigger circumstances that require the use
of a restraint or seclusion.
Interpretive Guidelines §482.13(f)(2)(i)
The term “appropriate staff” includes all staff that apply restraint or seclusion, monitor,
assess, or otherwise provide care for patients in restraint or seclusion.
All staff, including contract or agency personnel, designated by the hospital as having direct
patient care responsibilities are required to receive training in the areas of clinical
techniques used to identify patient and staff behaviors, events and environmental factors
that may trigger circumstances that require the use of restraint or seclusion. This training
should be targeted to the specific needs of the patient populations being served, and to the
competency level of staff.

Staff needs to be able to employ a broad range of clinical interventions to maintain the
safety of the patient and others. The hospital is expected to provide education and training
at the appropriate level to the appropriate staff based upon the specific needs of the patient
population being served. For example, staff routinely providing care for patients who
exhibit violent or self-destructive behavior that jeopardizes the immediate physical safety of
the patient, a staff member, or others (such as in an emergency department or on a
psychiatric unit) generally require more in-depth training in the areas included in the
regulation than staff routinely providing medical/surgical care. Hospitals may develop and
implement their own training programs or use an outside training program. However,
standard (f) specifies that individuals providing staff training must be qualified as
evidenced by education, training, and experience.
Hospitals have the flexibility to develop their own training program to meet the staff
training requirements at §482.13(f) or purchase a training program from the outside. CMS
does not specify that any particular outside vendor must be used to provide the required
training. Each hospital must assess the learning needs and competency of their staff to
determine how extensive periodic training and staff competency demonstration must be
subsequent to initial training. The training program must be provided to all appropriate
staff. Any person monitoring or providing care to a restrained patient must demonstrate the
knowledge and abilities required by the regulations.
At a minimum, physicians and other LIPs authorized to order restraint or seclusion by
hospital policy in accordance with State law must have a working knowledge of hospital
policy regarding the use of restraint and seclusion. Hospitals have the flexibility to identify
training requirements above this minimum based on the competency level of their
physicians and other LIPs and the needs of the patient population that they serve.
Survey Procedures §482.13(f)(2)(i):
• Does the hospital educational program include techniques related to the specific
patient populations being served?
• Does the hospital educational program include techniques to identify staff and
patient behaviors, events, and environmental factors that may trigger circumstances
that require the use of restraint or seclusion?
• Does the hospital educational program provide more in-depth training in the areas
included in the regulation for staff members who routinely provide care to patients
who exhibit violent or self-destructive behavior (e.g., staff who work in the
emergency department or psychiatric unit)?
• Interview staff to assess their knowledge of the restraint and seclusion techniques
addressed in this requirement.
A-0200
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The hospital must require appropriate staff to have education, training, and
demonstrated knowledge based on the specific needs of the patient population in at
least the following:]
§482.13(f)(2)(ii) - The use of nonphysical intervention skills.
Interpretive Guidelines §482.13(f)(2)(ii)

Although we recognize that there may be circumstances in which the use of restraint or
seclusion may be necessary to prevent a patient situation from escalating, staff often
skillfully intervene with alternative techniques to redirect a patient, engage the patient in
constructive discussion or activity, or otherwise help the patient maintain self-control and
avert escalation.
The use of nonphysical intervention skills does not mean attempting a complex series of
interventions or a lengthy checklist of steps to initiate before restraining or secluding a
patient. Rather, a whole toolbox of possible interventions can be implemented during the
course of a patient’s treatment based upon the assessment of an individual patient’s
responses.
Survey Procedures §482.13(f)(2)(ii)
• Does the hospital educational program address the use of nonphysical intervention
skills?
• Does the hospital’s training program comply with the regulatory requirements?
• Interview staff to assess their non-physical intervention skills.
A-0201
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The hospital must require appropriate staff to have education, training, and
demonstrated knowledge based on the specific needs of the patient population in at
least the following:]
§482.13(f)(2)(iii) - Choosing the least restrictive intervention based on an
individualized assessment of the patient's medical, or behavioral status or condition.
Interpretive Guidelines §482.13(f)(2)(iii)
The underpinning of this regulation is the concept that safe patient care hinges on looking at
the patient as an individual and assessing the patient’s condition, needs, strengths,
weaknesses, and preferences. Such an approach relies on caregivers who are skilled in
individualized assessment and in tailoring interventions to individual patient’s needs after
weighing factors such as the patient’s condition, behaviors, history, and environmental
factors.
Resources are available to assist clinicians in identifying less restrictive interventions. For
example, the American Psychiatric Association (APA), American Psychiatric Nurses
Association (APNA), and the National Association of Psychiatric Health Systems
(NAPHS), with support from the American Hospital Association (AHA), have sponsored
the publication of a document entitled, “Learning from Each Other—Success Stories and
Ideas for Reducing Restraint/Seclusion in Behavioral Health.” This document, published in
2003, was developed through dialogue with the field and extensive input from behavioral
healthcare providers throughout the country who have been working to reduce the use of
restraint and seclusion, and to improve care within their facilities. To access this document
and other useful resources, visit the web sites of the sponsoring organizations:
http://www.naphs.org; http://www.psych.org; http://www.apna.org; http://www.aha.org
Survey Procedures §482.13(f)(2)(iii)
• Does the hospital educational program address choosing the least restrictive
intervention based on an individualized assessment of the patient’s medical or
behavioral status or condition?
• Does the hospital educational program address how to conduct an assessment of a
patient’s medical and behavioral conditions?
• Does the hospital educational program address types of interventions appropriate to
the specific needs of the patient population(s) served and ranging from less to more
restrictive?
• Interview staff to determine if they are able to demonstrate the abilities addressed in
this requirement.
A-0202
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The hospital must require appropriate staff to have education, training, and
demonstrated knowledge based on the specific needs of the patient population in at
least the following:]
§482.13(f)(2)(iv) - The safe application and use of all types of restraint or seclusion
used in the hospital, including training in how to recognize and respond to signs of
physical and psychological distress (for example, positional asphyxia).
Interpretive Guidelines §482.13(f)(2)(iv)
Patients have a right to the safe application of restraint or seclusion by trained and
competent staff.
Survey Procedures §482.13(f)(2)(iv)
• Is all staff, including contract or agency personnel, identified by the hospital as
direct caregivers trained and able to demonstrate competency in the safe use of all
types of restraints or seclusion used in the hospital?
• Does the hospital educational program address recognition and response to patient
signs of physical and psychological distress?
• Is staff able to identify signs of physical and psychological distress in a timely
manner?
• Is staff able to respond to and appropriately treat signs of physical and psychological
distress?
• Review hospital data (i.e., incident reports, patient injury or death reports, etc.) to
identify any patterns of patient injuries or death that may indicate that staff are not
adequately trained to recognize and respond to patient signs of physical and
psychological distress.
A-0204
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The hospital must require appropriate staff to have education, training, and
demonstrated knowledge based on the specific needs of the patient population in at
least the following:]
§482.13(f)(2)(v) - Clinical identification of specific behavioral changes that indicate
that restraint or seclusion is no longer necessary.
Interpretive Guidelines §482.13(f)(2)(v)
The use of restraint or seclusion must be ended at the earliest possible time regardless of the
length of time identified in the order. Staff must be trained and demonstrate competency in
their ability to identify specific patient behavioral changes that may indicate that restraint or
seclusion is no longer necessary and can be safely discontinued.
Survey Procedures §482.13(f)(2)(v)
Does the hospital educational program address identification of specific behavioral changes
that may indicate that restraint or seclusion is no longer necessary?
Interview staff to determine if they are able to demonstrate the abilities addressed in this
requirement.
A-0205
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The hospital must require appropriate staff to have education, training, and
demonstrated knowledge based on the specific needs of the patient population in at
least the following:]
§482.13(f)(2)(vi) - Monitoring the physical and psychological well-being of the patient
who is restrained or secluded, including but not limited to, respiratory and circulatory
status, skin integrity, vital signs, and any special requirements specified by hospital
policy associated with the 1-hour face-to-face evaluation.
Interpretive Guidelines §482.13(f)(2)(vi)
Staff must be trained and demonstrate competency in monitoring the physical and
psychological well-being of a patient who is restrained or secluded, including but not
limited to, respiratory and circulatory status, skin integrity, vital signs, and as well as any
special requirements specified by hospital policy associated with the 1-hour face-to-face
evaluation.
Survey Procedures §482.13(f)(2)(vi)
• Does the hospital educational program address monitoring the physical and
psychological needs of patients who are restrained or secluded, including but not
limited to, respiratory and circulatory status, skin integrity, vital signs, and any
special requirements specified by hospital policy associated with the 1-hour face-to-
face evaluation?
• Does the hospital educational program address the specific requirements for the
training of RNs and PAs that the hospital authorizes to conduct the 1-hour face-to-
face evaluation?
• Interview staff to determine if they are able to demonstrate the competencies
addressed in this regulation.
A-0206
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The hospital must require appropriate staff to have education, training, and
demonstrated knowledge based on the specific needs of the patient population in at
least the following:]
§482.13(f)(2)(vii) - The use of first aid techniques and certification in the use of
cardiopulmonary resuscitation, including required periodic recertification.
Interpretive Guidelines §482.13(f)(2)(vii)
Hospitals are required to provide a safe environment for the patients in their care. When
restraint or seclusion techniques are used, patients are placed at a higher risk for injuries or
even death. Hospitals must require appropriate staff (all staff who apply restraint or
seclusion, monitor, access or provide care for a patient in restraint or seclusion) to receive
education and training in the use of first aid techniques as well as training and certification
in the use of cardiopulmonary resuscitation.
Hospitals are not required to use any particular recognized first aid course. Additionally,
such courses may not adequately address the immediate interventions, the “first aid”, that
needs to be rendered to a restrained or secluded patient who is in distress or injured. The
goal is for staff to be able to render the appropriate “first aid” required if a restrained or
secluded patient is in distress or injured. For example, a patient is found hanging in a vest
restraint, a restrained patient is choking on food, a secluded suicidal patient is found
hanging, a secluded suicidal patient has cut himself, etc. Hospital staff need to assess their
patient population and identify likely scenarios, develop a first aid training that addresses
those scenarios, and provide that “first aid” training to all staff that care for restrained or
secluded patients.
Survey Procedures §482.13(f)(2)(vii)
• Does the hospital educational program address first aid techniques?
• Is appropriate staff certified in cardiopulmonary resuscitation?
• Does the hospital educational program include, or provide for, staff training and
certification in cardiopulmonary resuscitation (including provisions for
recertification)?
A-0207
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(f)(3) Trainer Requirements. - Individuals providing staff training must be
qualified as evidenced by education, training, and experience in techniques used to
address patients' behaviors.
Interpretive Guidelines §482.13(f)(3)
There is no requirement that training be obtained from Federally-specified programs.
Hospitals may develop and implement their own training programs or use an outside
training program. However, individuals providing the training must be qualified as
evidenced by education, training and experience in techniques used to address patients’
behaviors for the patient populations being served. Trainers should demonstrate a high
level of knowledge regarding all the requirements of these regulations as well as the
hospital’s policies and procedures that address these requirements.
Survey Procedures §482.13(f)(3)
Review personnel files of individuals responsible for providing staff education and training.
Do the individuals providing the education and training possess education, training, and
experience to qualify them to teach the staff? Are they qualified to identify and meet the
needs of the patient population(s) being served?
Does the hospital have a system for documenting and ensuring that the individuals
providing education and training have the appropriate qualifications required by this
regulation?
A-0208
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.13(f)(4) Training Documentation. - The hospital must document in the staff
personnel records that the training and demonstration of competency were
successfully completed.
Interpretive Guidelines §482.13(f)(4)
Staff personnel records must contain documentation that the training and demonstration of
competency were successfully completed initially during orientation and on a periodic basis
consistent with hospital policy.
Survey Procedure §482.13(f)(4)
• Review a sample of staff personnel records, including contract or agency staff, to
determine if the training and demonstration of competency have been completed
during orientation and on a periodic basis consistent with hospital policy.
A-0213
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
§482.13(g) Standard: Death Reporting Requirements: - Hospitals must report deaths
associated with the use of seclusion or restraint.
(1) With the exception of deaths described under paragraph (g)(2) of this section, the
hospital must report the following information to CMS by telephone, facsimile, or
electronically, as determined by CMS, no later than the close of business on the next
business day following knowledge of the patient’s death:
(i) Each death that occurs while a patient is in restraint or seclusion.
(ii) Each death that occurs within 24 hours after the patient has been removed from
restraint or seclusion.
(iii) Each death known to the hospital that occurs within 1 week after restraint or
seclusion where it is reasonable to assume that use of restraint or placement in seclusion
contributed directly or indirectly to a patient's death, regardless of the type(s) of restraint
used on the patient during this time. “Reasonable to assume” in this context includes, but is
not limited to, deaths related to restrictions of movement for prolonged periods of time, or

death related to chest compression, restriction of breathing or asphyxiation….
(3) The staff must document in the patient’s medical record the date and time the death
was:
(i) Reported to CMS for deaths described in paragraph (g)(1) of this section; or….
Interpretive Guidelines §482.13(g)(1) & (3)(i)
The hospital must report to its CMS Regional Office each death that occurs:
• While a patient is in restraint or in seclusion, except when no seclusion has been
used and the only restraint used was a soft, cloth-like two-point wrist restraints;
• Within 24 hours after the patient has been removed from restraint or seclusion,
except when no seclusion has been used and the only restraint used was a soft, two-
point wrist restraint; or,
• Within 1 week after use of restraint or seclusion where the death is known to the
hospital and it is reasonable to assume that the use of restraint or seclusion
contributed directly or indirectly to the patient’s death, regardless of the type(s) of
restraint used on the patient during this time.
o “Reasonable to assume” applies only to those deaths that occur on days 2-7
after restraint or seclusion has been discontinued.
o This criterion applies regardless of the type of restraint that was used on the
patient. In other words, it applies to all uses of restraint or seclusion where
the patient has died on days 2-7 after the restraint or seclusion was
discontinued, and it is reasonable to assume the use of the restraint or
seclusion contributed to the patient’s death. In a case where only two-point
soft wrist restraints were used and there was no seclusion, it may reasonably
be presumed that the patient’s death was not caused by the use of restraints.
o In cases involving death within one week after use of restraint or seclusion
where the intervention may have contributed to the patient’s death, it is
possible that the patient’s death might occur outside the hospital and that the
hospital might not learn of the patient’s death, or that there might be a delay
in the hospital’s learning of the patient’s death.
See the guidance for §482.13(g)(2) for handling of deaths while a patient was in, or within
24 hours after removal of a soft, two-point wrist restraint, when no other restraint or
seclusion was used.
The reports required under §482.13(g)(1) must be submitted to the CMS Regional Office by
telephone, facsimile, or electronically, as determined by the Regional Office no later than
close of the next business day following the day in which the hospital knows of the
patient’s death. The report must include basic identifying information related to the
hospital, the patient’s name, date of birth, date of death, name of attending
physician/practitioner, primary diagnosis(es), cause of death (preliminary, in case a final,
official cause of death is not yet available), and type(s) of restraint or seclusion used. CMS
makes a standard form available for hospitals to use in submitting the required reports.
Hospitals must document in the patient’s medical record the date and time each reportable
death associated with the use of restraint or seclusion was reported to the CMS Regional
Office.
After reviewing the submitted information, the Regional Office will determine whether an
on-site investigation of the circumstances surrounding the patient’s death is warranted and
will direct the State Survey Agency to conduct a survey if applicable.
Survey Procedures §482.13(g)(1) & (3)(i):
• Does the hospital have restraint/seclusion death reporting policies and procedures
that address responsibilities and systems for identifying restraint/seclusion-
associated deaths reportable to CMS and for implementing the reporting and
recordkeeping requirements?
• Can the hospital provide examples of restraint/seclusion-associated deaths that were
reported to CMS?
o If yes, review the report and medical records to determine whether:
• the reports met the criteria for reporting to CMS;
• were submitted timely to CMS;
• were complete; and
• the date and time the death reported to CMS was entered into the
patient’s medical record.
o If no:
• Ask the hospital how it ensures that there were no reportable
restraint/seclusion-associated deaths.
• If the hospital’s system relies upon staff identification of reportable
deaths, interview several applicable staff members to determine
whether they are aware of the hospital’s policy and know when and
where to report internally a restraint/seclusion-associated death. Ask
if there have been any patient deaths that meet the reporting
requirements.
• Interview staff in various types of inpatient units, including a psychiatric unit if
applicable, to determine whether they are aware of any patients who died while in
restraints or seclusion or within one day of restraint or seclusion discontinuation,
excluding cases involving only the use of two-point soft wrist restraints and no
seclusion. If yes, check whether the hospital has any evidence that these cases were
reported to CMS.
A-0214
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
l§482.13(g) Standard: Death Reporting Requirements: [- Hospitals must report
deaths associated with the use of seclusion or restraint.]
(2) When no seclusion has been used and when the only restraints used on the
patient are those applied exclusively to the patient’s wrist(s), and which are composed
solely of soft, non-rigid, cloth-like materials, the hospital staff must record in an
internal log or other system, the following information:
(i) Any death that occurs while a patient is in such restraints.
(ii) Any death that occurs within 24 hours after a patient has been removed from
such restraints.
(3) The staff must document in the patient’s medical record the date and time the
death was:
(ii) Recorded in the internal log or other system for deaths described in
paragraph (g)(2) of this section.
(4) For deaths described in paragraph (g)(2) of this section, entries into the log or
other system must be documented as follows:
(i) Each entry must be made not later than seven days after the date of death of the
patient.
(ii) Each entry must document the patient’s name, date of birth, date of death, name
of attending physician or other licensed practitioner who is responsible for the care of
the patient medical record number, and primary diagnosis(es).
(iii) The information must be made available in either written or electronic form to
CMS immediately upon request.
Interpretive Guidelines §482.13(g)(2), (3)(ii), & (4)
Hospitals must maintain an internal log or other type of tracking system for recording
information on each death that occurs:
• While a patient is in only 2-point soft, cloth-like non-rigid wrist restraints and there
is no use of seclusion; and
• Within 24 hours of the patient being removed from 2-point soft, cloth-like non-rigid
wrist restraints where there was no use of any other type of restraint or seclusion.
Use of the log or tracking system is limited only to patient deaths meeting one of these two
criteria. Examples of patient deaths associated with restraints that must still be reported to
CMS include:
• Deaths occurring during or within 24 hours of discontinuation of 2-point soft, cloth-
like non-rigid wrist restraints used in combination with any other restraint device or
with seclusion; or
• Deaths associated with the use of other types of wrist restraints, such as 2-point rigid
or leather wrist restraints.
These cases would not be included in this internal log or tracking system and would require
reporting the death to CMS using telephone, fax, or electronically.
The two-point soft wrist restraint death report must be entered into the internal log or
tracking system within 7 days of the patient’s death.
The death report log or tracking system entry must include:
• The patient’s name;
• Patient’s date of birth;
• Patient’s date of death;
• Name of the attending physician or other licensed practitioner who is responsible for
the care or the patient;
• Patient’s medical record number; and
• Primary diagnosis(es).
Depending on the size and nature of the patient population the hospital serves and the types
of services it provides, there will likely be variations in the frequency of restraint use as
well as in the incidence of patient deaths. Surveyors should adjust their expectations for the
volume of log or tracking system entries accordingly. For example, hospitals with intensive
care units might be more likely to use both soft, 2-point wrist restraints and to have
seriously ill patients who die as a result of their disease while such restraints are being used
or within 24 hours after their discontinuance. On the other hand, a rehabilitation hospital
would be expected to use such restraints less frequently, and to have patients who die less
frequently while hospitalized.
The log or tracking system must be available in written, i.e., hard copy, or electronic form
immediately upon CMS’s request. CMS will specify the form in which the information is
to be provided. Generally CMS would request access to the log or tracking system during
an on-site survey by CMS staff or State surveyors acting on CMS’s behalf when assessing
compliance with restraint/seclusion requirements. However, CMS may also request that a
copy of portions or the entire log or tracking system be provided, even though no survey is
in progress. Accreditation organizations conducting hospital inspections in accordance with
a CMS-approved Medicare hospital accreditation program are also entitled to immediate
access to the log or tracking system.
The hospital is not required to make the contents of the log or tracking system available to
any other outside parties, unless required to do so under other Federal or State law.
The hospital must document in the patient’s medical record the date and time the death
report entry was made into the log or tracking system.
Survey Procedures §482.13(g)(2), (3)(ii), & (4)
• Does the hospital have restraint/seclusion death reporting policies and procedures
that address responsibilities and systems for identifying restraint/seclusion-
associated deaths that must be recorded in an internal hospital log/tracking system,
and for implementing the reporting and recordkeeping requirements?
• Ask the hospital how it ensures that each death that must be captured in the
log/tracking system is identified and entered.
• Interview inpatient unit staff to determine whether they have had patients who die
while 2-point soft wrist restraints are being used without seclusion or within 24
hours of their discontinuance. If yes, ask the hospital to demonstrate that it has
recorded such deaths.
• If the hospital’s log or tracking system relies upon staff identification of reportable
deaths, interview several applicable staff members to determine whether they are
aware of the hospital’s policy and know when and where to report internally a
restraint/seclusion-associated death.
• Review the log/tracking system for patient deaths associated with use of only 2-
point soft wrist restraints to determine if:
• Each entry was made within 7 days of the patient’s death; and
• Each entry contains all the information required under the regulation.
• Is the hospital able to make the log or tracking system available immediately on
request?
• Review a sample of medical records of patients whose deaths were entered in the
log or tracking system.
Does the medical record indicate that only soft, 2-point wrist restraints were used?
• Is there documentation in the medical record of the entry into the log or tracking
system?
A-0215
(Rev. 75, Issued: 12-02-11, Effective: 12-02-11, Implementation: 12-02-11)
§482.13(h) Standard: Patient visitation rights. A hospital must have written policies
and procedures regarding the visitation rights of patients, including those setting forth
any clinically necessary or reasonable restriction or limitation that the hospital may
need to place on such rights and the reasons for the clinical restriction or limitation
Interpretive Guidelines, §482.13(h)
Visitation plays an important role in the care of hospital patients. An article published in
2004 in the Journal of the American Medical Association (Berwick, D.M., and Kotagal, M.:
“Restricted visiting hours in ICUs: time to change.” JAMA. 2004; Vol. 292, pp. 736-737)
discusses the health and safety benefits of open visitation for patients, families, and
intensive care unit (ICU) staff and debunks some of the myths surrounding the issue
(physiologic stress for the patient; barriers to provision of care; exhaustion of family and
friends). The article ultimately concluded that “available evidence indicates that hazards
and problems
regarding open visitation are generally overstated and manageable,” and that
such visitation policies “do not harm patients but rather may help them by providing a
support system and shaping a more familiar environment” as they “engender trust in
families, creating a better
working relationship between hospital staff and family members.”
Hospitals that unnecessarily restrict patient visitation often miss an opportunity to gain
valuable patient information from those who may know the patient best with respect to the
patient’s medical history, conditions, medications, and allergies, particularly if the patient
has difficulties with recall or articulation, or is totally unable to recall or articulate this vital
personal information. Many times visitors who may know the patient best act as an
intermediary for the patient, helping to communicate the patient’s needs to hospital staff.
Although visitation policies are generally considered to relate to visitors of inpatients,
“visitors” also play a role for outpatients who wish to have a support person present during
their outpatient visit. For example, a same-day surgery patient may wish to have a support
person present during the pre-operative patient preparation or post-operative recovery. Or
an outpatient clinic patient may wish to have a support person present during his or her
examination by a physician. Accordingly, hospital visitation policies must address both the
inpatient and outpatient settings.
Hospitals are required to develop and implement written policies and procedures that
address the patient’s right to have visitors. If the hospital’s policy establishes restrictions or
limitations on visitation, such restrictions/limitations must be clinically necessary or
reasonable. Furthermore, the hospital’s policy must include the reasons for any
restrictions/limitations. The right of a patient to have visitors may be limited or restricted
when visitation would interfere with the care of the patient and/or the care of other patients.
The regulation permits hospitals some flexibility, so that health care professionals may
exercise their best clinical judgment when determining when visitation is, and is not,
appropriate. Best clinical judgment takes into account all aspects of patient health and
safety, including the benefits of visitation on a patient’s care as well as potential negative
impacts that visitors may have on other patients in the hospital.
Broad examples of circumstances reasonably related to the care of the patient and/or the
care of other patients that could provide a basis for a hospital to impose restrictions or
limitations on visitors might include (but are not limited to) when:
• there may be infection control issues;
• visitation may interfere with the care of other patients;
• the hospital is aware that there is an existing court order restricting contact;
• visitors engage in disruptive, threatening, or violent behavior of any kind;
• the patient or patient’s roommate(s) need rest or privacy;
• in the case of an inpatient substance abuse treatment program, there are protocols
limiting visitation; and
• the patient is undergoing care interventions. However, while there may be valid reasons
for limiting visitation during a care intervention, we encourage hospitals to try to
accommodate the needs of any patient who requests that at least one visitor be allowed
to remain in the room to provide support and comfort at such times.
It may also be reasonable to limit the number of visitors for any one patient during a
specific period of time, as well as to establish minimum age requirements for child visitors.
However, when a hospital adopts policies that limit or restrict patients’ visitation rights, the
burden of proof is upon the hospital to demonstrate that the visitation restriction is
reasonably necessary to provide safe care.
Hospitals are expected to provide a clear explanation in their written policy of the clinical
rationale for any visitation restrictions or limitations reflected in that policy. Hospitals are
not required, however, to delineate each specific clinical reason for policies limiting or
restricting visitation, given that it is not possible to anticipate every instance that may give
rise to a clinically appropriate rationale for a restriction or limitation. If visitation policies
differ by type of unit, e.g., separate policies for intensive care units, or for newborn
nurseries, the hospital policy must address the clinical rationale for this differentiation
explicitly.
The hospital’s policies and procedures are expected to address how hospital staff who play
a role in facilitating or controlling visitor access to patients will be trained to assure
appropriate implementation of the visitation policies and procedures and avoidance of
unnecessary restrictions or limitations on patients’ visitation rights.
Survey Procedures §482.13(h)
• Verify that the hospital has written policies and procedures that address the right of
patients to have visitors.
• Review the policy to determine if there are limitations or restrictions on visitation. If
there are, does the policy explain the clinical rationale for the restrictions or limitations?
Is the rationale clear and reasonably related to clinical concerns?
• Is there documentation of how the hospital identifies and trains staff who play a role in
facilitating or controlling access of visitors to patients?
• Are hospital staff aware of the visitation policies and procedures? Can staff on a given
unit correctly describe the hospital’s visitation policies for that unit?
A-0216
(Rev. 75, Issued: 12-02-11, Effective: 12-02-11, Implementation: 12-02-11)
§482.13(h) Standard: Patient visitation rights. A hospital must have written policies
and procedures regarding the visitation rights of patients, including those setting forth
any clinically necessary or reasonable restriction or limitation that the hospital may
need to place on such rights and the reasons for the clinical restriction or limitation. A
hospital must meet the following requirements:
(1) Inform each patient (or support person, where appropriate) of his or her
visitation rights, including any clinical restriction or limitation on such rights, when he
or she is informed of his or her other rights under this section.
(2) Inform each patient (or support person, where appropriate) of the right,
subject to his or her consent, to receive the visitors whom he or she designates,
including, but not limited to, a spouse, a domestic partner (including a same-sex
domestic partner), another family member, or a friend, and his or her right to
withdraw or deny such consent at any time.
Interpretive Guidelines §482.13(h)(1)&(2)
Hospitals are required to inform each patient (or the patient’s support person, where
appropriate) of his/her visitation rights. A patient’s “support person” does not necessarily
have to be the same person as the patient’s representative who is legally responsible for
making medical decisions on the patient’s behalf. A support person could be a family
member, friend, or other individual who supports the patient during the course of the
hospital stay. Not only may the support person visit the patient, but he or she may also
exercise a patient’s visitation rights on behalf of the patient with respect to other visitors
when the patient is unable to do so. Hospitals must accept a patient’s designation, orally or
in writing, of an individual as the patient’s support person.
When a patient is incapacitated or otherwise unable to communicate his or her wishes and
an individual provides an advance directive designating an individual as the patient’s
support person (it is not necessary for the document to use this exact term), the hospital
must accept this designation, provide the required notice of the patient’s visitation rights,
and allow the individual to exercise the patient’s visitation rights on the patient’s behalf.
When a patient is incapacitated or otherwise unable to communicate his or her wishes, there
is no advance directive designating a representative on file, and no one has presented an
advance directive designating himself or herself as the patient’s representative, but an
individual asserts that he or she, as the patient’s spouse, domestic partner (including a
same-sex domestic partner), parent or other family member, friend, or otherwise, is the
patient’s support person, the hospital is expected to accept this assertion, without
demanding supporting documentation, provide the required notice of the patient’s
visitation rights, and allow the individual to exercise the patient’s visitation rights on the
patient’s behalf. However, if more than one individual claims to be the patient’s support
person, it would not be inappropriate for the hospital to ask each individual for
documentation supporting his/her claim to be the patient’s support person.
• Hospitals are expected to adopt policies and procedures that facilitate expeditious
and non-discriminatory resolution of disputes about whether an individual is the
patient’s support person, given the critical role of the support person in exercising
the patient’s visitation rights.
• A refusal by the hospital of an individual’s request to be treated as the patient’s
support person with respect to visitation rights must be documented in the patient’s
medical record, along with the specific basis for the refusal.
Consistent with the patients’ rights notice requirements under the regulation at
§482.13(a)(1), the required notice of the patient’s visitation rights must be provided,
whenever possible, before the hospital provides or stops care. The notice to the patient, or
to the patient’s support person, where appropriate, must be in writing. If the patient also
has a representative who is different from the support person, the representative must also
be provided information on the patient’s visitation rights, in addition to the support person,
if applicable. In the event that a patient has both a representative and a support person who
are not the same individual, and they disagree on who should be allowed to visit the patient,
the hospital must defer to the decisions of the patient’s representative. As the individual
responsible for making decisions on the patient’s behalf, the patient’s representative has the
authority to exercise a patient’s right to designate and deny visitors just as the patient would
if he or she were capable of doing so. The designation of, and exercise of authority by, the
patient’s representative is governed by State law, including statutory and case law. Many
State courts have addressed the concept of substituted judgment, whereby the patient’s
representative is expected to make medical decisions based on the patient’s values and
interests, rather than the representative’s own values and interests. State courts have also
developed a body of closely related law around the matter of a representative acting in the
patient’s best interest. Such case law regarding substituted judgment and best interest may
be a resource for hospitals on how to address such conflict situations as they establish
visitation policies and procedures. Hospitals may also choose to utilize their own social
work and pastoral counseling resources to resolve such conflicts to assure the patient’s
well-being.
The required visitation rights notice must address any clinically necessary or reasonable
limitations or restrictions imposed by hospital policy on visitation rights, providing the
clinical reasons for such limitations/restrictions, including how they are aimed at protecting
the health and safety of all patients. The information must be sufficiently detailed to allow
a patient (or the patient’s support person) to determine what the visitation hours are and
what restrictions, if any, apply to that patient’s visitation rights.
The notice must also inform the patient (or the patient’s support person, where appropriate)
of the patient’s right to:
• Consent to receive visitors he or she has designated, either orally or in writing,
including but not limited to, a spouse, a domestic partner (including a same-sex
domestic partner), another family member, or a friend;
• Receive the visitors he or she has designated, including but not limited to, a spouse,
a domestic partner (including a same-sex domestic partner), another family member,
or a friend; and
• Withdraw or deny his/her consent to receive specific visitors, either orally or in
writing.
The medical record must contain documentation that the required notice was provided to
the patient or, if appropriate, the patient’s support person.
Survey Procedures §482.13(h)(1)&(2)
• Determine whether the hospital’s visitation policies and procedures require
providing notice of the patient’s visitation rights to each patient or, if appropriate, to
a patient’s support person and/or, as applicable, the patient’s representative.
• Review the hospital’s standard notice of visitation rights. Does it clearly explain
the:
• hospital’s visitation policy, including any limitations or restrictions, such as
visiting hours, numbers of visitors, unit-specific restrictions, etc., and the
clinical rationale for such limitations or restrictions?
• right of the patient to have designated visitors, including but not limited to, a
spouse, a domestic partner (including a same-sex domestic partner), another
family member, or a friend, and the right to withdraw or deny consent to
visitation?
• Review a sample of medical records to determine if there is documentation that the
required notice was provided.
• Ask the hospital to identify how the required notice is provided. Ask staff
responsible for providing the notice how they accomplish this. Ask the staff if they
are familiar with the concept of a patient’s “support person” and what it means.
• Ask a sample of current hospital patients or patients’ support persons (where
appropriate) whether they were provided notice of their right to have visitors. Ask if
they were able to have visitors when they wanted to. If not, verify whether the
restriction/limitation on visitors was addressed in the hospital’s visitation policies
and notice, and does not violate the regulations at §482.13(h)(3)&(4). (See
interpretive guidelines for the latter provisions.)
• Ask a sample of current hospital patients or patients’ support persons (where
appropriate) whether the hospital did not limit some or all visitors, contrary to the
patient’s wishes.
A-0217
(Rev. 75, Issued: 12-02-11, Effective: 12-02-11, Implementation: 12-02-11)
§482.13(h) Standard: Patient visitation rights. A hospital must have written policies
and procedures regarding the visitation rights of patients, including those setting forth
any clinically necessary or reasonable restriction or limitation that the hospital may
need to place on such rights and the reasons for the clinical restriction or limitation. A
hospital must meet the following requirements:
(3) Not restrict, limit, or otherwise deny visitation privileges on the basis of
race, color, national origin, religion, sex, gender identity, sexual orientation, or
disability.
(4) Ensure that all visitors enjoy full and equal visitation privileges consistent
with patient preferences.
Interpretive Guidelines §482.13(h)(3)&(4)
The hospital’s visitation policies and procedures may not use the race, color, national
origin, religion, sex, gender identity, sexual orientation, or disability of either the patient (or
the patient’s support person ore representative, where appropriate) or the patient’s visitors
(including individuals seeking to visit the patient) as a basis for limiting, restricting, or
otherwise denying visitation privileges.
The hospital’s policies and procedures must ensure that all visitors (including individuals
seeking to visit the patient) enjoy full and equal visitation privileges, consistent with the
preferences the patient (or, where appropriate, the patient’s support person) has expressed
concerning visitors. In other words, it is permissible for the patient (or the patient’s support
person, where appropriate) to limit the visiting privileges of his/her visitors, including
providing for more limited visiting privileges for some visitors than those for others. But it
is not permissible for the hospital, on its own, to differentiate among visitors without any
clinically necessary or reasonable basis. This includes visitors designated by the patient
who have characteristics not addressed specifically in §482.13(h)(3), when those
characteristics do not reasonably relate to a clinically reasonable basis for limiting or
denying visitation. For example, it would not be appropriate to prohibit a designated visitor
based on that individual’s style of dress, unless there was a clinically reasonable basis for
doing so.
The hospital is responsible for ensuring that hospital staff treat all individuals seeking to
visit patients equally, consistent with the preferences of the patient (or, where appropriate,
the patient’s support person) and do not use the race, color, national origin, religion, sex,
gender identity, sexual orientation, or disability of either the patient(or the patient’s support
person or representative, where appropriate) or the patient’s visitors (including individuals
seeking to visit the patient) as a basis for limiting, restricting, or otherwise denying
visitation privileges. Hospitals are expected to educate all staff who play a role in
facilitating or controlling visitors on the hospital’s visitation policies and procedures, and
are responsible for ensuring that staff implement the hospital’s policies correctly. Hospitals
are urged to develop culturally competent training programs designed to address the range
of patients served by the hospital.
Survey Procedures §482.13(h)(3)&(4)
• Review the hospital’s visitation policies and procedures to determine whether they
restrict, limit, or otherwise deny visitation to individuals on a prohibited basis.
• Ask the hospital how it educates staff to assure that visitation policies are
implemented in a non-discriminatory manner.
• Ask hospital staff who play a role in facilitating or controlling visitors to discuss
their understanding of the circumstances under which visitors may be subject to
restrictions/limitations. Are the restrictions/limitations appropriately based on the
hospital’s clinically-based policies?
• Ask hospital patients (or patients’ support persons, where appropriate) whether the
hospital has restricted or limited visitors against their wishes. If yes, verify whether
the restriction/limitation on visitors was addressed in the hospital’s visitation
policies and in the patient notice, and whether it was appropriately based on a
clinical rationale rather than impermissible discrimination.
A-0263
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.21 Condition of Participation: Quality Assessment and Performance
Improvement Program
The hospital must develop, implement, and maintain an effective, ongoing, hospital-
wide, data-driven quality assessment and performance improvement program. The
hospital’s governing body must ensure that the program reflects the complexity of the
hospital’s organization and services; involves all hospital departments and services
(including those services furnished under contract or arrangement); and focuses on
indicators related to improved health outcomes and the prevention and reduction of
medical errors. The hospital must maintain and demonstrate evidence of its QAPI
program for review by CMS.
A-0273
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
Data Collection & Analysis
§§482.21(a), 482.21(b)(1), 482.21(b)(2)(i), & 482.21(b)(3)
§482.21(a) Standard: Program Scope
(1) The program must include, but not be limited to, an ongoing program that shows
measurable improvement in indicators for which there is evidence that it will improve
health outcomes….
(2) The hospital must measure, analyze, and track quality indicators…and other
aspects of performance that assess processes of care, hospital service and operations.
§482.21(b) Standard: Program Data.
(1) The program must incorporate quality indicator data including patient care
data, and other relevant data such as data submitted to or received from Medicare
quality reporting and quality performance programs, including but not limited to data
related to hospital readmissions and hospital-acquired conditions.
(2) The hospital must use the data collected to--
(i) Monitor the effectiveness and safety of services and quality of care; and…
(3) The frequency and detail of data collection must be specified by the hospital’s
governing body.
A-0283
(Rev. 105, Issued: 03-21-14, Effective: 03-21-14, Implementation: 03-21-14)
Quality Improvement Activities
§§482.21(b)(2)(ii), 482.21(c)(1) & 482.21 (c)(3)
§482.21(b)(2) Standard: Program Data
The hospital must use the data collected to--…
(ii) Identify opportunities for improvement and changes that will lead to
improvement.
§482.21(c) Standard: Program Activities
(1) The hospital must set priorities for its performance improvement activities that--
(i) Focus on high-risk, high-volume, or problem-prone areas;
(ii) Consider the incidence, prevalence, and severity of problems in those
areas; and
(iii) Affect health outcomes, patient safety, and quality of care.
(3) The hospital must take actions aimed at performance improvement and, after
implementing those actions, the hospital must measure its success, and track
performance to ensure that improvements are sustained.
A-0286
(Rev. 105, Issued: 03-21-14, Effective: 03-21-14, Implementation: 03-21-14)
Patient Safety, Medical Errors & Adverse Events
§§482.21(a)(1), 482.21(a)(2), 482.21(c)(2), & 482.21(e)(3)
§482.21(a) Standard: Program Scope.
(1) The program must include, but not be limited to, an ongoing program that shows
measurable improvement in indicators for which there is evidence that it will …
identify and reduce medical errors.
(2) The hospital must measure, analyze, and track…adverse patient events….
§482.21(c) Standard: Program Activities…
(2) Performance improvement activities must track medical errors and adverse
patient events, analyze their causes, and implement preventive actions and
mechanisms that include feedback and learning throughout the hospital.
§482.21(e) Standard: Executive Responsibilities. The hospital’s governing body (or
organized group or individual who assumes full legal authority and responsibility for
operations of the hospital), medical staff, and administrative officials are responsible
and accountable for ensuring the following:…
(3) That clear expectations for safety are established.
A-0297
(Rev. 105, Issued: 03-21-14, Effective: 03-21-14, Implementation: 03-21-14)
Performance Improvement Projects
§482.21(d) Standard: Performance Improvement Projects.
As part of its quality assessment and performance improvement program, the hospital
must conduct performance improvement projects.
(1) The number and scope of distinct improvement projects conducted annually must
be proportional to the scope and complexity of the hospital’s services and operations.
(2) A hospital may, as one of its projects, develop and implement an information
technology system explicitly designed to improve patient safety and quality of care.
This project, in its initial stage of development, does not need to demonstrate
measurable improvement in indicators related to health outcomes.
(3) The hospital must document what quality improvement projects are being
conducted, the reasons for conducting these projects, and the measurable progress
achieved on these projects.
(4) A hospital is not required to participate in a QIO cooperative project, but its own
projects are required to be of comparable effort.
A-0308
(Rev. 105, Issued: 03-21-14, Effective: 03-21-14, Implementation: 03-21-14)
Standard Tag for requirements found only in Condition stem statement
§482.21 Condition of Participation: Quality Assessment and Performance
Improvement Program
… The hospital’s governing body must ensure that the program reflects the
complexity of the hospital’s organization and services; involves all hospital
departments and services (including those services furnished under contract or
arrangement)… The hospital must maintain and demonstrate evidence of its QAPI
program for review by CMS.
A-0309
(Rev. 105, Issued: 03-21-14, Effective: 03-21-14, Implementation: 03-21-14)
Executive Responsibilities
§482.21(e) Standard: Executive Responsibilities
The hospital’s governing body (or organized group or individual who assumes full
legal authority and responsibility for operations of the hospital), medical staff, and
administrative officials are responsible and accountable for ensuring the following:
(1) That an ongoing program for quality improvement and patient safety, including
the reduction of medical errors, is defined, implemented, and maintained.
(2) That the hospital-wide quality assessment and performance improvement efforts
address priorities for improved quality of care and patient safety and that all
improvement actions are evaluated…
(5) That the determination of the number of distinct improvement projects is
conducted annually.
A-0315
(Rev. 105, Issued: 03-21-14, Effective: 03-21-14, Implementation: 03-21-14)
Providing Adequate Resources
§482.21(e) Standard: Executive Responsibilities
[§482.21(e) The hospital’s governing body (or organized group or individual who
assumes full legal authority and responsibility for operations of the hospital), medical
staff, and administrative officials are responsible and accountable for ensuring the
following:]
(4) That adequate resources are allocated for measuring, assessing, improving, and
sustaining the hospital’s performance and reducing risk to patients.
A-0320
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.21(f) Standard: Unified and integrated QAPI program for multi-hospital
systems.
If a hospital is part of a hospital system consisting of multiple separately certified
hospitals using a system governing body that is legally responsible for the conduct of
two or more hospitals, the system governing body can elect to have a unified and
integrated QAPI program for all of its member hospitals after determining that such a
decision is in accordance with all applicable State and local laws. The system
governing body is responsible and accountable for ensuring that each of its separately
certified hospitals meets all of the requirements of this section. Each separately
certified hospital subject to the system governing body must demonstrate that:
Interpretive Guidelines §482.21(f)
Guidance is pending and will be updated in future release.
A-0321
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.21(f)(1) The unified and integrated QAPI program is established in a manner
that takes into account each member hospital's unique circumstances and any
significant differences in patient populations and services offered in each hospital; and
Interpretive Guidelines §482.21(f)(1)
Guidance is pending and will be updated in future release.
A-0322
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.21(f)(2) The unified and integrated QAPI program establishes and implements
policies and procedures to ensure that the needs and concerns of each of its separately
certified hospitals, regardless of practice or location, are given due consideration, and
that the unified and integrated QAPI program has mechanisms in place to ensure that
issues localized to particular hospitals are duly considered and addressed.
Interpretive Guidelines §482.21(f)(2)
Guidance is pending and will be updated in future release.
A-0338
(Rev. 122, Issued: 09-26-14, Effective: 09-26-14, Implementation: 09-26-14)
§482.22 Condition of Participation: Medical Staff
The hospital must have an organized medical staff that operates under bylaws
approved by the governing body, and which is responsible for the quality of medical
care provided to patients by the hospital.
Interpretive Guidelines §482.22
The hospital must have one medical staff for the entire hospital (including all campuses,
provider-based locations, satellites, remote locations, etc.). For example, a multi-campus
hospital may not have a separately organized medical staff for each campus. On the other
hand, in the case of a hospital system, it is permissible for the system to have a unified and
integrated medical staff (hereafter referred to as a “unified medical staff”) for multiple,
separately certified hospitals. The medical staff must be organized and integrated as one
body that operates under one set of bylaws approved by the governing body. These medical
staff bylaws must apply equally to all practitioners within each category of practitioners at
all locations of the hospital and to the care provided at all locations of the hospital. The
medical staff is responsible for the quality of medical care provided to patients by the
hospital.
Survey Procedures §482.22
Surveyors assess the manner and degree of noncompliance with the standards within this
condition to determine whether there is condition-level noncompliance.
A-0339
(Rev. 122, Issued: 09-26-14, Effective: 09-26-14, Implementation: 09-26-14)
§482.22(a) Standard: Eligibility and Process for Appointment to Medical
Staff
The medical staff must be composed of doctors of medicine or osteopathy. In
accordance with State law, including scope-of-practice laws, the medical staff may also
include other categories of physicians (as listed at §482.12(c)(1)) and non-physician
practitioners who are determined to be eligible for appointment by the governing

body.
Interpretive Guidelines §482.22(a)
The hospital’s governing body has the responsibility, consistent with State law, including
scope-of-practice laws, to determine which types/categories of physicians and, if it so
chooses, non-physician practitioners or other licensed healthcare professionals (collectively
referred to in this guidance as “practitioners”) may be privileged to provide care to hospital
patients. All practitioners who require privileges in order to furnish care to hospital patients
must be evaluated under the hospital’s medical staff privileging system before the hospital’s
governing body may grant them privileges. All practitioners granted medical staff
privileges must function under the bylaws, regulations and rules of the hospital’s medical
staff. The privileges granted to an individual practitioner must be consistent with State
scope-of-practice laws.
Physicians:
The medical staff must at a minimum be composed of doctors of medicine or doctors of
osteopathy. In addition, the medical staff may include other types of practitioners included
in the definition in Section 1861(r) of the Social Security Act of a “physician:”
• Doctor of dental surgery or of dental medicine;
• Doctor of podiatric medicine;
• Doctor of optometry; and a
• Chiropractor.
In all cases, the practitioner included in the definition of a physician must be legally
authorized to practice within the State where the hospital is located and providing services
within their authorized scope of practice. In addition, in certain instances the Social
Security Act and regulations attach further limitations as to the type of hospital services for
which a practitioner may be considered to be a “physician.” See §482.12(c)(1) for more
detail on these limitations.
The governing body has the flexibility to determine, consistent with State law, whether
practitioners included in the definition of a physician, other than doctors of medicine or
osteopathy, are eligible for appointment to the medical staff.
For Information Only – Not Required/ Not to be Cited
CMS expects that all physician practitioners granted privileges are also appointed as
members of the medical staff. However, if State law limits the composition of the
hospital’s medical staff to certain categories of practitioners, e.g., only MDs or DOs,
there is nothing in the CoPs that prohibits hospitals and their medical staffs from
establishing certain practice privileges for other categories of physician practitioners
excluded from medical staff membership under State law, or from granting those
privileges to individual practitioners in those categories, as long as such privileges are
recommended by the medical staff, approved by the governing body, and in accordance
with State law. (79 FR 27114 - 27115, May 12, 2014)
For physician practitioners granted privileges only, , the hospital’s governing body and its
medical staff must exercise oversight, such as through credentialing and competency
review, of those other physician practitioners to whom it grants privileges, just as it would
for those practitioners appointed to its medical staff.
Non-physician practitioners

Furthermore, the governing body has the authority, in accordance with State law, to grant
medical staff privileges and membership to non-physician practitioners. The regulation
allows hospitals and their medical staffs to take advantage of the expertise and skills of all
types of practitioners who practice at the hospital when making recommendations and
decisions concerning medical staff privileges and membership.
For Information Only – Not Required/ Not to be Cited
CMS expects that all practitioners granted privileges are also appointed as members of
the medical staff. However, if State law limits the composition of the hospital’s medical
staff to certain categories of practitioners, e.g., only physician practitioners, there is
nothing in the CoPs that prohibits hospitals and their medical staffs from establishing
certain practice privileges for those specific categories of non-physician practitioners
excluded from medical staff membership under State law, or from granting those
privileges to individual practitioners in those categories, as long as such privileges are
recommended by the medical staff, approved by the governing body, and in accordance
with State law. (79 FR 27114 - 27115, May 12, 2014)
For non-physician practitioners granted privileges only, the hospital’s governing body and
its medical staff must exercise oversight, such as through credentialing and competency
review, of those non-physician practitioners to whom it grants privileges, just as it would
for those practitioners appointed to its medical staff.
Practitioners are described in Section 1842(b)(18)(C) of the Act as any of the following:
• Physician assistant (as defined in Section 1861(aa)(5) of the Act);
• Nurse practitioner (as defined in Section 1861(aa)(5) of the Act);
• Clinical nurse specialist (as defined in Section 1861(aa)(5) of the Act);
• Certified registered nurse anesthetist (as defined in Section 1861(bb)(2)
of the Act);
• Certified nurse-midwife (as defined in Section 1861(gg)(2) of the Act);
• Clinical social worker (as defined in Section 1861(hh)(1) of the Act);
• Clinical psychologist (as defined in 42 CFR 410.71 for purposes of
Section 1861(ii) of the Act);
• Anesthesiologist’s Assistant (as defined in §410.69); or
• Registered dietician or nutrition professional.
Other types of licensed healthcare professionals have a more limited scope of practice and
usually are not eligible for hospital medical staff privileges, unless their permitted scope of
practice in their State makes them more comparable to the above types of non-physician
practitioners. Some examples of types of such licensed healthcare professionals who might
be eligible for medical staff privileges depending on State law and medical staff bylaws,
rules and regulations include, but are not limited to:
• Physical Therapist (as defined at §410.60 and §484.4);
• Occupational Therapist (as defined at §410.59 and §484.4); and
• Speech Language Therapist (as defined at §410.62 and §484.4).
Furthermore, some States have established a scope of practice for certain licensed
pharmacists who are permitted to provide patient care services that make them more like
the above types of non-physician practitioners, including the monitoring and assessing of
patients and ordering medications and laboratory tests. In such States, a hospital may grant
medical staff privileges to such pharmacists and/or appoint them as members of the medical
staff. There is no standard term for such pharmacists, although they are sometimes referred
to as “clinical pharmacists.”
Practitioners may be granted active, courtesy, emergency, temporary, etc. membership or
privileges in accordance with state law and as specified in the medical staff bylaws, rules,
and regulations.
Survey Procedures §482.22(a)
• Ask the hospital and medical staff leadership to describe the categories of
practitioners who are members of the medical staff or who may be granted
medical staff privileges. Ask for documentation that supports their response.
• If the hospital grants medical staff privileges and/or membership to physicians
who are not MDs/DOs or to non-physician practitioners, ask the hospital and
medical staff leadership to describe the process the hospital uses to ensure that
any privileges granted are consistent with State law. Ask for documentation that
supports their response.
Ask the hospital and medical staff leadership to describe the process by which they
exercise oversight of practitioners granted privileges only.
A-0340
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.22(a)(1) - The medical staff must periodically conduct appraisals of its members.
Interpretive Guidelines §482.22(a)(1)
The medical staff must at regular intervals appraise the qualifications of all practitioners
appointed to the medical staff/granted medical staff privileges. In the absence of a State
law that establishes a timeframe for periodic reappraisal, a hospital’s medical staff must
conduct a periodic appraisal of each practitioner. CMS recommends that an appraisal be
conducted at least every 24 months for each practitioner.
The purpose of the appraisal is for the medical staff to determine the suitability of
continuing the medical staff membership or privileges of each individual practitioner, to
determine if that individual practitioner’s membership or privileges should be continued,
discontinued, revised, or otherwise changed.
The medical staff appraisal procedures must evaluate each individual practitioner’s
qualifications and demonstrated competencies to perform each task or activity within the
applicable scope of practice or privileges for that type of practitioner for which he/she has
been granted privileges. Components of practitioner qualifications and demonstrated
competencies would include at least: current work practice, special training, quality of
specific work, patient outcomes, education, maintenance of continuing education,
adherence to medical staff rules, certifications, appropriate licensure, and currency of
compliance with licensure requirements.
In addition to the periodic appraisal of members, any procedure/task/activity/privilege
requested by a practitioner that goes beyond the specified list of privileges for that
particular category of practitioner requires an appraisal by the medical staff and approval by
the governing body. The appraisal must consider evidence of qualifications and
competencies specific to the nature of the request. It must also consider whether the
activity/task/procedure is one that the hospital can support when it is conducted within the
hospital. Privileges cannot be granted for tasks/procedures/activities that are not conducted
within the hospital, regardless of the individual practitioner’s ability to perform them.
After the medical staff conducts its reappraisal of individual members, the medical staff
makes recommendations to the governing body to continue, revise, discontinue, limit, or
revoke some or all of the practitioner’s privileges, and the governing body takes final
appropriate action.
A separate credentials file must be maintained for each medical staff member. The hospital
must ensure that the practitioner and appropriate hospital patient care areas/departments are
informed of the privileges granted to the practitioner, as well as of any revisions or
revocations of the practitioner’s privileges. Furthermore, whenever a practitioner’s
privileges are limited, revoked, or in any way constrained, the hospital must, in accordance
with State and/or Federal laws or regulations, report those constraints to the appropriate
State and Federal authorities, registries, and/or data bases, such as the National Practitioner
Data Bank.
Survey Procedures §482.22(a)(1)
• Determine whether the medical staff has a system in place that is used to reappraise
each of its current members and their qualifications at regular intervals, or, if
applicable, as prescribed by State law.
• Determine whether the medical staff by-laws identify the process and criteria to be
used for the periodic appraisal.
• Determine whether the criteria used for reevaluation comply with the requirements
of this section, State law and hospital bylaws, rules, and regulations.
• Determine whether the medical staff has a system to ensure that practitioners seek
approval to expand their privileges for tasks/activities/procedures that go beyond the
specified list of privileges for their category of practitioner.
Determine how the medical staff conducts the periodic appraisals of any current member of
the medical staff who has not provided patient care at the hospital or who has not provided
care for which he/she is privileged to patients at the hospital during the appropriate
evaluation time frames. Is this method in accordance with State law and the hospital’s
written criteria for medical staff membership and for granting privileges?
A-0341
(Rev. 122, Issued: 09-26-14, Effective: 09-26-14, Implementation: 09-26-14)
§482.22(a)(2) - The medical staff must examine the credentials of all eligible
candidates for medical staff membership and make recommendations to the governing
body on the appointment of the candidates in accordance with State law, including
scope-of-practice laws, and the medical staff bylaws, rules, and regulations. A
candidate who has been recommended by the medical staff and who has been
appointed by the governing body is subject to all medical staff bylaws, rules, and
regulations, in addition to the requirements contained in this section.
Interpretive Guidelines §482.22(a)(2)
There must be a mechanism established to examine credentials of individual prospective
members (new appointments or reappointments) by the medical staff. The individual’s
credentials to be examined must include at least:
• A request for clinical privileges;
• Evidence of current licensure;
• Evidence of training and professional education;
• Documented experience; and
• Supporting references of competence.
The medical staff may not make its recommendation solely on the basis of the presence or
absence of board certification, but must consider all of the elements above. However, this
does not mean that the medical staff is prohibited from requiring in its bylaws board
certification when considering a MD/DO for medical staff membership or privileges; only
that such certification may not be the only factor that the medical staff considers.
The medical staff makes recommendations to the governing body for each candidate for
medical staff membership/privileges that are specific to type of appointment and extent of
the individual practitioner’s specific clinical privileges, and then the governing body takes
final appropriate action.
Each practitioner who is a member of the medical staff or who holds medical staff
privileges is subject to the medical staff’s bylaws, rules, and regulations, in addition to all
the requirements of the Medical Staff Condition of Participation. The medical staff and the
governing body must enforce its medical staff requirements and take appropriate actions
when individual members or other practitioners with privileges do not adhere to the medical
staff’s bylaws, regulations, and rules. They must likewise afford all members/practitioners
who hold privileges the protections and due process rights provided for in the bylaws, rules
and regulations.
A separate credentials file must be maintained for each individual medical staff member or
applicant. The hospital must ensure that the practitioner and appropriate hospital patient
care areas/departments are informed of the privileges granted to the practitioner.
Survey Procedures §482.22(a)(2)
• Determine whether the medical staff bylaws identify the process and criteria
to be used for the evaluation of candidates for medical staff
membership/privileges.
• Determine whether the criteria used for evaluation comply with the
requirements of this section, State law, and hospital bylaws, rules, and
regulations.
• Determine whether the medical staff has a system to ensure that practitioners
seek approval to expand their privileges for tasks/activities/procedures that
go beyond the specified list of privileges for their category of practitioner.
Ask the leadership of the medical staff what methods are used to ensure that
all medical staff members and non-member practitioners who hold privileges
adhere to the medical staff bylaws, rules and regulations and are afforded the
protections and due process rights provided for under the bylaws, rules and
regulations. Ask for specific examples of actions taken.
When interviewing practitioners during the survey, ask how they are made aware of
their rights and responsibilities with respect to medical staff bylaws, rules and
regulations.
A-0342
(Rev. 78, Issued: 12-22-11, Effective/Implementation: 12-22-11)
§482.22(a)(3) When telemedicine services are furnished to the hospital’s patients
through an agreement with a distant-site hospital, the governing body of the hospital
whose patients are receiving the telemedicine services may choose, in lieu of the
requirements in paragraphs (a)(1) and (a)(2) of this section, to have its medical staff
rely upon the credentialing and privileging decisions made by the distant-site hospital
when making recommendations on privileges for the individual distant-site physicians
and practitioners providing such services, if the hospital’s governing body ensures,
through its written agreement with the distant-site hospital, that all of the following
provisions are met:
(i) The distant-site hospital providing the telemedicine services is a Medicare-
participating hospital.
(ii) The individual distant-site physician or practitioner is privileged at the distant-site
hospital providing the telemedicine services, which provides a current list of the
distant-site physician’s or practitioner’s privileges at the distant-site hospital.
(iii) The individual distant-site physician or practitioner holds a license issued
or recognized by the State in which the hospital whose patients are receiving the
telemedicine services is located.
(iv) With respect to a distant-site physician or practitioner, who holds current
privileges at the hospital whose patients are receiving the telemedicine services, the
hospital has evidence of an internal review of the distant-site physician’s or
practitioner’s performance of these privileges and sends the distant-site hospital such
performance information for use in the periodic appraisal of the distant-site physician
or practitioner. At a minimum, this information must include all adverse events that
result from the telemedicine services provided by the distant-site physician or
practitioner to the hospital’s patients and all complaints the hospital has received
about the distant-site physician or practitioner.
Interpretive guidelines §482.22(a)(3)
The hospital’s governing body has the option, when considering granting privileges to
telemedicine physicians and practitioners, to have the hospital’s medical staff rely upon the
credentialing and privileging decisions of the distant-site hospital for these physicians and
practitioners. This process would be in lieu of the traditional process required under
§482.22(a)(1) and §482.22(a)(2), whereby the hospital’s medical staff conducts its own
review of each telemedicine physician’s or practitioner’s credentials and makes a
recommendation based on that individualized review.
In order to exercise this alternative credentialing and privileging option, the hospital’s
governing body must ensure through its written agreement with the distant-site hospital that
all of the following requirements are met:
• The distant-site hospital participates in the Medicare program. If the distant-site
hospital’s participation in Medicare is terminated, either voluntarily or involuntarily,
at any time during the agreement, then, as of the effective date of the termination,
the hospital may no longer receive telemedicine services under the agreement;
• The distant-site hospital provides to the hospital a list of all its physicians and
practitioners covered by the agreement, including their privileges at the distant-site
hospital. The list may not include any physician or practitioner who does not hold
privileges at the distant-site hospital. The list must be current, so the agreement
must address how the distant-site hospital will keep the list current;
• Each physician or practitioner who provides telemedicine services to the hospital’s
patients under the agreement holds a license issued or recognized by the State where
the hospital (not the distant-site hospital) is located. States may have varying
requirements as to whether they will recognize an out-of-state license for purposes
of practicing within their State, and they may also vary as to whether they establish
different standards for telemedicine services. The licensure requirements governing
in the State where the hospital whose patients are receiving the telemedicine
services is located must be satisfied, whatever they may be; and
• The hospital has evidence that it reviews the telemedicine services provided to its
patients and provides feedback based on this review to the distant-site hospital for
the latter’s use in its periodic appraisal of each physician and practitioner providing
telemedicine services under the agreement. At a minimum, the hospital must review
and send information to the distant-site hospital on all adverse events that result
from a physician or practitioner’s provision of telemedicine services under the
agreement and on all complaints it has received about a telemedicine physician or
practitioner covered by the agreement.
Survey Procedures §482.22(a)(3)
• If the hospital provides telemedicine services to its patients under an agreement with
a distant-site hospital, ask whether the hospital’s governing body has exercised the
option to have the medical staff rely upon the credentialing and privileging
decisions of the distant-site hospital in making privileging recommendations on
telemedicine physicians and practitioners. If yes, ask to see:
• The written agreement with the distant-site hospital. Does the agreement
address the required elements concerning the distant-site hospital’s Medicare
participation, licensure of telemedicine physicians and practitioners, current list
of telemedicine physicians and practitioners with privileges, and review by the
hospital of the telemedicine physicians’ and practitioners’ services and provision
of information based on its review to the distant-site hospital?
• The list provided by the distant-site hospital of the telemedicine physicians and
practitioners, including their current privileges and pertinent licensure
information.
Evidence that the hospital reviews the services provided by the telemedicine physicians and
practitioners, including any adverse events and complaints, and provides feedback to the
distant-site hospital.
A-0343
(Rev. 78, Issued: 12-22-11, Effective/Implementation: 12-22-11)
§482.22(a)(4) When telemedicine services are furnished to the hospital’s patients
through an agreement with a distant-site telemedicine entity, the governing body of
the hospital whose patients are receiving the telemedicine services may choose, in lieu
of the requirements in paragraphs (a)(1) and (a)(2) of this section, to have its medical
staff rely upon the credentialing and privileging decisions made by the distant- site
telemedicine entity when making recommendations on privileges for the individual
distant-site physicians and practitioners providing such services, if the hospital’s
governing body ensures, through its written agreement with the distant-site
telemedicine entity, that the distant-site telemedicine entity furnishes services that, in
accordance with §482.12(e), permit the hospital to comply with all applicable
conditions of participation for the contracted services. The hospital’s governing body
must also ensure, through its written agreement with the distant-site telemedicine
entity, that all of the following provisions are met:
(i) The distant-site telemedicine entity’s medical staff credentialing and privileging
process and standards at least meet the standards at §482.12(a)(1) through (a)(7) and
§482.22(a)(1) through (a)(2).
(ii) The individual distant-site physician or practitioner is privileged at the distant-site
telemedicine entity providing the telemedicine services, which provides the hospital
with a current list of the distant-site physician’s or practitioner’s privileges at the
distant-site telemedicine entity.
(iii) The individual distant-site physician or practitioner holds a license issued or
recognized by the State in which the hospital whose patients are receiving such
telemedicine services is located.
(iv) With respect to a distant-site physician or practitioner, who holds current
privileges at the hospital whose patients are receiving the telemedicine services, the
hospital has evidence of an internal review of the distant-site physician’s or
practitioner’s performance of these privileges and sends the distant-site telemedicine
entity such performance information for use in the periodic appraisal of the distant-
site physician or practitioner. At a minimum, this information must include all
adverse events that result from the telemedicine services provided by the distant-site
physician or practitioner to the hospital’s patients, and all complaints the hospital has
received about the distant-site physician or practitioner.
Interpretive guidelines §482.22(a)(4)
For the purposes of this rule, a distant-site telemedicine entity is defined as an entity that --
(1) provides telemedicine services; (2) is not a Medicare-participating hospital; and (3)
provides contracted services in a manner that enables a hospital using its services to meet
all applicable Conditions of Participation, particularly those requirements related to the
credentialing and privileging of practitioners providing telemedicine services to the patients
of a hospital. A distant-site telemedicine entity would include a distant-site hospital that
does not participate in the Medicare program that is providing telemedicine services to a
Medicare-participating hospital. (See 76 FR 25553, May 5, 2011)
The hospital’s governing body has the option, when considering granting privileges to
telemedicine physicians and practitioners, to have the hospital’s medical staff rely upon the
credentialing and privileging decisions of the distant-site telemedicine entity for these
physicians and practitioners. This process would be in lieu of the traditional process
required under §482.22(a)(1) and §482.22(a)(2), whereby the medical staff conducts its
own review of each telemedicine physician’s or practitioner’s credentials and makes a
recommendation based on that individualized review.
In order to exercise this alternative credentialing and privileging option, the hospital’s
governing body must ensure that its written agreement with the distant-site hospital enables
the hospital, as required under the regulation at §482.12(e) governing services provided
under arrangement, to comply with all applicable hospital Conditions of Participation. In
particular, the written agreement between the hospital and the distant-site telemedicine
entity must ensure that all of the following requirements are met:
• The distant-site telemedicine entity utilizes a medical staff credentialing and
privileging process and standards that at least meets the standards for the medical
staff of a hospital established at §482.12(a)(1) through (a)(7) and §482.22(a)(1)
through (a)(2);
• The distant-site telemedicine entity provides a list to the hospital of all physicians
and practitioners covered by the agreement, including their privileges at the distant-
site telemedicine entity. The list may not include any physician or practitioner who
does not hold privileges at the distant-site telemedicine entity. The list must be
current, so the agreement must address how the distant-site telemedicine entity will
keep the list current;
• Each physician or practitioner who provides telemedicine services to the hospital’s
patients under the agreement holds a license issued or recognized by the State where
the hospital is located. States may have varying requirements as to whether they
will recognize an out-of-state license for purposes of practicing within their State,
and they may also vary as to whether they establish different standards for
telemedicine services. The licensure requirements governing in the State where the
hospital whose patients are receiving the telemedicine services is located must be
satisfied, whatever they may be; and
• The hospital has evidence that it reviews the telemedicine services provided to its
patients and provides a written copy of this review to the distant-site telemedicine
entity for the latter’s use in its periodic appraisal of the physicians and practitioners
providing telemedicine services under the agreement. At a minimum, the hospital
must review and send information to the distant-site telemedicine entity on all
adverse events that result from a physician or practitioner’s provision of
telemedicine services and on all complaints it has received about a telemedicine
physician or practitioner.
Survey Procedures 482.22(a)(4)
• If the hospital provides telemedicine services to its patients under an agreement with
a one or more distant-site telemedicine entities, ask whether the hospital’s governing
body has exercised the option to have the medical staff rely upon the credentialing
and privileging decisions of the distant-site telemedicine entity in making
privileging recommendations on telemedicine physicians and practitioners. If yes,
ask to see:
• The written agreement(s) with the distant-site telemedicine entity(ies). Does
each agreement address the required elements concerning the distant-site
telemedicine entity’s utilization of a medical staff credentialing and privileging
process that meets the requirements of the hospital CoPs, appropriate licensure
of telemedicine physicians and practitioners, current list of telemedicine
physicians and practitioners specifying their privileges, and written review by
the hospital of the telemedicine physicians’ and practitioners’ services and
provision of information based on its review to the distant-site hospital?
• The list provided by the distant-site telemedicine entity of the telemedicine
physicians and practitioners covered by the agreement, including their current
privileges and pertinent licensure information.
• Evidence that the hospital reviews the services provided by the telemedicine
physicians and practitioners, including any adverse events and complaints, and
provides written feedback to the distant-site telemedicine entity.
• Ask the hospital how it verifies that the telemedicine entity employs a
credentialing and privileging process that meets or exceeds what is required for
hospitals under the Medicare CoPs? (Surveyors do not attempt to independently
verify whether or not the distant-site telemedicine entity’s credentialing and
privileging process fulfills the regulatory requirements. Surveyors focus only on
whether the hospital takes steps to ensure that the distant-site telemedicine entity
complies with the terms of the written agreement.)
A-0347
(Rev. 122, Issued: 09-26-14, Effective: 09-26-14, Implementation: 09-26-14)
§482.22(b) Standard: Medical Staff Organization and Accountability
The medical staff must be well organized and accountable to the governing body for the
quality of the medical care provided to the patients.
(1) The medical staff must be organized in a manner approved by the governing body.
(2) If the medical staff has an executive committee, a majority of the members of the
committee must be doctors of medicine or osteopathy.
(3) The responsibility for organization and conduct of the medical staff must be
assigned only to one of the following:
(i) An individual doctor of medicine or osteopathy.
(ii) A doctor of dental surgery or dental medicine, when permitted by State
law of the State in which the hospital is located.
(iii) A doctor of podiatric medicine, when permitted by State law of the
State in which the hospital is located.
Interpretive Guidelines §482.22(b)(1) – (3)
The conditions of participation create a system of checks and balances within an overall
framework of collaboration between the governing body and the medical staff (and, to a
certain degree, also between an individual practitioner and the hospital’s medical staff and
governing body). Each has its own areas of authority. The medical staff has oversight of
all practitioners practicing in the hospital through processes such as peer review and making
recommendations concerning privileging and re-privileging. The governing body has the
authority to establish the categories of healthcare professionals (regardless of the terms used
to describe those categories) who are eligible for privileges and medical staff appointment.
However, the governing body must rely on the medical staff to apply the criteria for
privileging and appointment to those eligible candidates and to make their
recommendations before the governing body makes a final decision to appoint or not
appoint a practitioner to the medical staff. (77 FR 29042 May 16, 2012).
If the hospital uses a unified medical staff that it shares with other hospitals that are part of
a multi-hospital system, this does not change the requirement for the medical staff to be
well organized and accountable to the system’s governing body for the quality of care in
each separately certified hospital.
Leadership of the medical staff
The members of the hospital’s medical staff must select, in accordance with the medical
staff bylaws, rules or regulations approved by the governing body, a single individual to
lead the medical staff and be responsible for the organization and conduct of the medical
staff. This individual must be a doctor of medicine or osteopathy, or, if permitted by State

law where the hospital is located, a doctor of dental surgery, dental medicine, or podiatric
medicine. Removal of the leader of the medical staff may only occur in accordance with
medical staff bylaws, rules or regulations.
If the hospital uses a unified medical staff, only one individual may be responsible for the
organization and conduct of the unified medical staff; that individual may or may not hold
privileges and practice at the hospital being surveyed. When the individual does not
practice at the hospital being surveyed and it is necessary to interview this individual as part
of a survey, a telephone interview must be arranged.
Executive Committee
The medical staff bylaws, rules and regulations may provide for the members of the
medical staff to select a smaller executive committee to which it delegates many of the
functions of the medical staff, in order to increase the efficiency of its operations. If the
medical staff has an executive committee, the majority of the voting members must be
doctors of medicine (MDs) or osteopathy (DOs).
For Information Only – Not Required/ Not to be Cited
A hospital is not required to have an executive committee. However, use of an executive
committee may facilitate efficient and effective functioning of the medical staff in
hospitals systems that use a unified medical staff, particularly if the executive committee
includes members from each hospital that shares the unified medical staff.
Accountability of the medical staff
The medical staff must be accountable to the hospital’s governing body for the quality of
medical care provided to the patients. The medical staff demonstrates its accountability
through its exercise of its duties related to appointment of members of the medical staff, its
conduct of reappraisals, including peer reviews, its approval of policies and procedures as
required under other conditions of participation and its leadership participation in the
organization and implementation of the hospital’s quality assessment and performance
improvement program required in accordance with §482.21.
If the hospital uses a unified medical staff, the medical staff continues to be accountable for
the quality of care in each separately certified hospital that uses the unified medical staff.
Survey Procedures §482.22(b)(1) – (3)
• Verify that the medical staff has a formal, organized structure reflected in the
medical staff bylaws, rules and regulations and that functions and
responsibilities within the medical staff and with respect to the governing
body and other parts of the hospital are reflected.
• If there is a medical staff executive committee, verify that a majority of the
members are doctors of medicine or osteopathy.
• Verify that an individual doctor of medicine or osteopathy, or if permitted by
State law, a doctor of dental surgery, dental medicine, or podiatric medicine,
selected by the medical staff, is responsible for the conduct and organization
of the medical staff.
• Ask the CEO and medical staff leadership to describe the mechanisms by
which the medical staff fulfills its responsibility to be accountable for the
quality of medical care in the hospital.

• Interview several members of the medical staff, including both practitioners who
hold leadership or executive committee positions and ones who do not. Ask them
what their medical staff duties and responsibilities are and how they perform them.
Ask them to describe how the medical staff is accountable for the quality of medical
care provided to patients.
A-0348
(Rev. 122, Issued: 09-26-14, Effective: 09-26-14, Implementation: 09-26-14)
§482.22(b)(4) - If a hospital is part of a hospital system consisting of multiple
separately certified hospitals and the system elects to have a unified and integrated
medical staff for its member hospitals, after determining that such a decision is in
accordance with all applicable State and local laws, each separately certified hospital
must demonstrate that:….
Interpretive Guidelines §482.22(b)(4)
A hospital that is part of a system consisting of multiple separately certified hospitals may
use a single unified and integrated medical staff (hereafter referred to as a “unified medical
staff”) that is shared with one or more of the other hospitals in the system. In other words,
as long as the requirements of §482.22(b)(4) are met, it is not necessary for each separately-
certified hospital within the system to have its own distinct medical staff organization and
structure, including hospital-specific medical staff bylaws, rules and requirements, hospital-
specific medical staff leadership, hospital-specific credentialing and peer review, etc.
Instead, it may use one medical staff organization and structure for multiple hospitals, so
long as all of the requirements of this section are met. However, separately certified
hospitals which share a single unified and integrated medical staff must also share a system
governing body, in accordance with the provisions of §482.12, since only one governing
body may carry out the governing body’s medical staff responsibilities for a unified medical
staff.
Note that a multi-campus hospital that has several inpatient campuses that are provider-
based, remote locations of the hospital is not a multi-hospital system. A multi-campus
hospital is one certified hospital, not several separately certified hospitals. A multi-campus
hospital may not have separate medical staffs at each campus, since each hospital must have
no more than one medical staff. A multi-campus hospital with one medical staff separate
from that of other certified hospitals is not employing a unified medical staff as that term is
used in this regulation. However, a multi-campus hospital that is part of a hospital system
consisting of multiple separately certified hospitals may share a unified medical staff with
other separately certified hospitals within the system.
It should also be noted that a hospital system that includes certain types of hospitals, i.e.,
Hospitals-within-Hospitals or Hospital Satellites, that are being paid under a Medicare
payment system other than the Hospital Inpatient Prospective Payment System (IPPS)
might jeopardize the Medicare payment status of those excluded hospitals if it owns both
the tenant and host hospitals and uses a unified medical staff for both. This is the case even
if the requirements of §482.22(b)(4) are met. However, surveyors do not assess compliance
with or enforce the Medicare payment regulations that govern Hospitals-within-Hospitals or
Hospital Satellites.
When granting practitioners privileges to provide patient care, a hospital’s governing body
must specify those hospitals in the system where the privileges apply, since, in addition to
the qualifications of individual practitioners, the services provided at each hospital must be
considered when granting privileges. For example, psychiatric hospitals do not offer
surgical services, labor and delivery services, nuclear medicine, etc., so it would not be
appropriate for practitioners practicing in these areas to hold privileges at psychiatric
hospitals in a multi-hospital system that uses a unified medical staff. Likewise if a multi-
hospital system covers a wide geographic area, many of its practitioners may have no
interest in practicing on site at hospitals that are distant from their usual practice location(s).
In addition, in order for the acceptance or opt-out provisions of §482.22(b)(4)(i) and (ii) to
be workable, privileges must be granted on a hospital-specific basis to practitioners who
actually practice or are likely to practice at the hospital.
The governing body in a multi-hospital system must elect to exercise this option. Since a
number of hospital systems interpreted the Medical Staff CoP to permit a unified and
integrated medical staff prior to publication of the final rule at §482.22(b)(4) on May 12,
2014 or its effective date on July 11, 2014, the existence of a unified medical staff prior to
July 11, 2014 is considered evidence of the hospital’s governing body’s election of this
option.
• This does not relieve the governing body of the responsibility to conduct a
review of all applicable State and local laws, including regulations, and
make a determination that use of a unified medical staff that is shared by
multiple hospitals does not conflict with those laws. The hospital must
maintain documentation of this determination by its governing body.
• Nor does it relieve the governing body of the obligation to inform the
medical staff of the right to vote to opt out of a unified medical staff
arrangement. (See discussion of §482.22(b)(4)(ii), which requires
notification of all members of this right. Failure to comply would be cited
under the tag for §482.22(b)(4)(ii).)
If a hospital is part of a multi-hospital system that wishes to establish a unified medical staff
for some or all of its separately certified hospitals after the July 11, 2014 effective date of
the final rule at §482.22(b)(4), then the hospital’s system governing body must document in
writing its decision to elect to use the unified medical staff option, conditioned upon
acceptance of a unified medical staff by the hospital’s medical staff in accordance with
§482.22(b)(4)(i).The governing body must also document its determination that such
election does not conflict with State or local laws, including regulations.
Surveyors are not expected, as part of their assessment of compliance with the Medicare
CoPs, to evaluate whether the governing body’s determination of compliance with State and
local law is accurate. This would be handled by the appropriate State or local authorities,
or, if the State Survey Agency is the appropriate authority, under its State licensure or other
authority and not as part of a Federal survey.
Survey Procedures §482.22(b)(4)
• Ask the hospital and medical staff leadership if the hospital is part of a multi-
hospital system of separately certified hospitals. If yes, ask if the hospital also
shares its governing body and medical staff with one or more other separately-
certified hospitals in the system.
• If yes:
• Does the use of the unified medical staff predate July 11, 2014? If yes,
ask for documentation of the governing body’s determination that use of
a unified medical staff does not conflict with State or local law.
• Did the use of the unified medical staff start after July 11, 2014? If yes,
ask for documentation of the governing body’s decision to elect use of a
unified medical staff and of its determination that use of a unified
medical staff does not conflict with State or local law.

• Can the hospital produce documentation that practitioners who practice
at the hospital have been granted privileges by the hospital’s governing
body that specify the practitioner’s privileges apply to specific
hospital(s), which include the hospital being surveyed?
A-0349
(Rev. 122, Issued: 09-26-14, Effective: 09-26-14, Implementation: 09-26-14)
[§482.22(b)(4) - If a hospital is part of a hospital system consisting of multiple
separately certified hospitals and the system elects to have a unified and integrated
medical staff for its member hospitals, after determining that such a decision is in
accordance with all applicable State and local laws, each separately certified hospital
must demonstrate that:]
(i) The medical staff members of each separately certified hospital in the system (that
is, all medical staff members who hold specific privileges to practice at that hospital)
have voted by majority, in accordance with medical staff bylaws, either to accept a
unified and integrated medical staff structure or to opt out of such a structure and to
maintain a separate and distinct medical staff for their respective hospital;
Interpretive Guidelines §482.22(b)(4)(i)
The decision for a particular certified hospital in a multi-hospital system to use a unified
medical staff is a joint one arrived at by the:
• Election of the unified medical staff option by the hospital’s governing body;
and
• Acceptance by a majority of the medical staff members who hold privileges to
practice at that particular hospital, voting in accordance with the medical staff
bylaws.
The medical staff of each hospital also has the option to opt out of an existing unified
medical staff, when a majority of the medical staff members who hold privileges to practice
at that particular hospital, voting in accordance with the medical staff bylaws, vote to do so.
For purposes of voting on whether to accept or opt out of a unified medical staff, the term
“privileges to practice at that particular hospital” is interpreted to mean only those
practitioners who hold privileges to practice on-site at the hospital. Practitioners who hold
only telemedicine privileges at a hospital are not to be included when identifying which
practitioners are eligible to vote nor what constitutes a majority of the practitioners holding
privileges at the hospital.
A hospital that is part of a hospital system is expected to have medical staff bylaws, rules
and requirements that address the regulatory requirements of §482.22(b)(4)(i) – (iv) related
to using a unified medical staff, including the processes under the bylaws for voting to
accept or opt out of a unified medical staff. This is the case even if the hospital currently
does not use a unified medical staff.
If the hospital uses a unified medical staff, depending on State law requirements, the unified
medical staff bylaws, rules and requirements required at §482.22(b)(4)(ii) may substitute
for hospital-specific medical staff bylaws, rules and requirements. However, CMS
recognizes that the process of amending bylaws can be a lengthy one. Hospitals that were
part of a hospital system using a unified medical staff as of July 11, 2014 are expected to
have initiated the process before December 31, 2014 to effect the necessary amendments,
even if the process is not completed until after that date. Likewise, when a hospital is
acquired by a system but maintains separate participation in Medicare, if the hospital’s
governing body elects to use a unified medical staff and the medical staff accepts such
election, the hospital is expected to initiate the necessary changes to its medical staff
bylaws, rules and requirements no later than six months after the effective date of its
acquisition.
In establishing medical staff bylaws governing medical staff voting on the questions of
acceptance of, or opting out of, a unified medical staff, the medical staff and the governing
body, which must approve the revised bylaws in accordance with §482.12(a)(4), have the
flexibility to determine the details of the voting process, such as how an acceptance or opt-
out vote can be requested; whether all categories of members holding privileges to practice
on-site at the hospital are afforded medical staff voting rights; whether voting will be in
writing and open or by secret ballot, etc. However, a hospital may not set up bylaws that
unduly restrict the rights of medical staff members when voting on the issue of accepting or
opting out of a unified medical staff structure. For example:
Hospitals may not establish different criteria as to which categories of medical staff
members have voting rights with respect to a vote to accept or opt out of a unified
medical staff than are used for other amendments to the medical staff’s bylaws,
except as required under the regulation at §482.22(b)(4) that only members holding
privileges to practice at the hospital may vote. (See also the discussion below
concerning delegation of authority to the medical staff executive committee.)
Hospitals may not require as a condition for holding an opt-out vote that there be a
petition signed by the same number of voting members as would be required for a
successful vote to opt out.
Hospitals may require for a successful acceptance or opt-out vote a
“supermajority”, that is, a majority that is greater than a simple majority of more
than fifty percent of the medical staff members with voting rights holding privileges
to practice at the hospital, so long as the same type of supermajority is otherwise
generally required to amend the medical staff’s bylaws, rules and requirements.
In the case where a hospital system has a unified medical staff and members of the
staff at a hospital in the system exercise their right to hold a vote on the question of
opting out, the hospital may not permit delegation of an opt-out decision to the
unified medical staff’s executive committee. This is the case even when the
executive committee is otherwise delegated authority to amend unified medical staff
bylaws, rules and requirements that it recommends for approval to the governing
body. In cases where the bylaws permit such delegation to the unified medical
staff’s executive committee for other purposes, a “majority” for purposes of
conducting a vote on whether to opt out of a unified medical staff consists of a
simple majority, that is, any number which is greater than fifty percent, of the
medical staff members practicing at the hospital who have voting privileges.
o On the other hand, in the case where a hospital that is part of a hospital system
but has a separate medical staff is holding a vote on whether to accept
participating in a unified medical staff, a hospital may permit a vote by members
of the hospital’s medical staff executive committee only, if this is consistent
with the hospital’s medical staff bylaws governing amendments in effect at the
time of the vote.
A hospital may establish a minimum interval between acceptance or opt-out votes,
such as not permitting a vote more than once every two years. However, a
minimum interval between votes longer than two years might unduly restrain the
rights of the members of the medical staff and would not be permissible.
It is not expected that the medical staff bylaws, rules and requirements that were in effect as
of July 11, 2014 would address the issue of a unified medical staff, nor the process of
voting by medical staff members at each hospital to accept or opt out of a unified medical
staff. Although it is expected that the medical staff bylaws, rules and requirements of
hospitals that are part of a hospital system will be amended in a timely fashion as discussed
above, this does not mean that a vote to accept or opt out of a unified medical staff may not
take place prior to enactment of such amendments.
Voting is governed by the hospital’s medical staff bylaws in effect at the time of the vote,
except that only voting members of the medical staff who hold privileges to practice on-site
at that hospital may participate in the vote. With respect to what constitutes a “majority,”
the provisions of the bylaws governing voting rights and voting procedures at the time of
the vote apply. However, as discussed above, in the case of a vote to opt-out of a unified
medical staff, the vote may not be delegated to the executive committee of the unified
medical staff.
Since a number of hospital systems interpreted the Medical Staff CoP to permit a unified
medical staff prior to publication of the final rule at §482.22(b)(4) on May 12, 2014 or its
effective date of July 11, 2014, in the case of a hospital’s use of a unified medical staff
which began prior to the latter date, it is not necessary for the hospital to hold a vote among
the members of the medical staff who hold privileges at that hospital to determine whether
the majority accepts the continued use of a unified medical staff. However, the governing
body is expected to formally notify the medical staff practicing at each hospital of its
preference to continue using a unified medical staff arrangement, as well as of the right of
the medical staff holding privileges at each hospital to vote to opt out of the unified medical
staff.
If the system governing body of a hospital that is part of the multi-hospital system but
which has a separate medical staff elects after July 11, 2014 to create a system unified
medical staff structure and/or to include the hospital’s medical staff in an already existing
unified medical staff structure, the hospital must arrange for a vote by medical staff
members, in accordance with the medical staff bylaws, on whether or not to accept use of a
unified medical staff for their hospital. The hospital may not use a unified medical staff
unless a majority of its medical staff members holding voting rights vote, in accordance
with the hospital’s medical staff bylaws, to accept a unified medical staff.
Even if a majority of a hospital’s medical staff has voted to use a unified medical staff in
the past, the members of the unified medical staff with voting rights and holding privileges
to practice on-site at that hospital still retain the right to hold a vote to opt out of the unified
medical staff structure at a future date. If a majority of the staff with voting rights and
holding privileges at that hospital vote, in accordance with the unified medical staff’s
bylaws, to opt out, then the hospital must establish a separate medical staff.
Survey Procedures §482.22(b)(4)(i)
• Assess compliance with this regulation if the hospital is part of a system that
consists of more than one separately certified hospital, regardless of whether it
uses a unified medical staff at the time of survey or not. (See survey procedures
for §482.22(b)(4) above.)
• If the hospital uses a unified medical staff, ask the hospital’s leadership when it
began to do so. Is there any documentation to support the response?
• If the hospital began using a unified medical staff after July 11, 2014, is
there evidence that a majority of the medical staff holding privileges at the
hospital at the time of the vote voted in accordance with medical staff
bylaws to accept using a unified medical staff?
• If the hospital uses a unified medical staff, do the medical staff bylaws clearly
describe a process by which a vote to opt out of using a unified medical staff
may be requested and conducted?
• Are there provisions that are described in the guidance above as unduly
limiting the rights of medical staff members to vote on whether to accept or
opt out of a unified medical staff?
• If there are other requirements in the voting process that appear to limit opt-
out voting, ask the medical staff leadership to explain why the limitations are
reasonable and not unduly restrictive.
• Ask the hospital and members of the medical staff whether there has ever
been a vote on the question of opting out. If yes, ask the hospital to produce
evidence that a majority of the practitioners holding privileges at the hospital
voted against opting out.
• Can the hospital readily identify the medical staff members who are eligible to
vote whether to accept or to opt out of a unified medical staff?
A-0350
(Rev. 122, Issued: 09-26-14, Effective: 09-26-14, Implementation: 09-26-14)
[§482.22(b)(4) - If a hospital is part of a hospital system consisting of multiple
separately certified hospitals and the system elects to have a unified and integrated
medical staff for its member hospitals, after determining that such a decision is in
accordance with all applicable State and local laws, each separately certified hospital
must demonstrate that:]
(ii) - The unified and integrated medical staff has bylaws, rules, and requirements that
describe its processes for self-governance, appointment, credentialing, privileging, and
oversight, as well as its peer review policies and due process rights guarantees, and
which include a process for the members of the medical staff of each separately
certified hospital (that is, all medical staff members who hold specific privileges to
practice at that hospital) to be advised of their rights to opt out of the unified and
integrated medical staff structure after a majority vote by the members to maintain a
separate and distinct medical staff for their hospital;
Interpretive Guidelines §482.22(b)(4)(ii)
A hospital that uses a unified medical staff must ensure that the unified medical staff has
one set of bylaws, rules and requirements that describe the medical staff’s processes for
self-governance, appointment, credentialing, privileging, oversight, peer review, and due
process rights guarantees. Consistent with the requirements for a system governing body in
§482.12, the documentation of the bylaws, rules and requirements that apply to the unified
medical staff must identify each separately certified hospital that has elected to use a
unified medical staff and which, therefore, is covered by the unified medical staff bylaws,
rules and regulations. Depending on State law requirements, the unified medical staff
bylaws, rules and requirements may be in addition to or instead of hospital-specific medical
staff bylaws, rules and requirements. The unified medical staff’s bylaws, rules and
requirements must not conflict with any of the specific requirements for medical staff found
elsewhere in §482.12 or §482.22, or under any other hospital CoPs which assign
responsibilities to the hospital’s medical staff.
The unified medical staff’s bylaws, rules and requirements addressing its self-governance
processes must provide for a process by which members of the unified medical staff
holding privileges to practice on site at each separately certified hospital are advised that
they have the right to vote on whether to opt out of participation in the unified medical
staff, and that if a majority vote to opt out, then the hospital must establish a separate
medical staff. At a minimum, the hospital must advise medical staff members in writing of
their right to vote by majority to opt out when medical staff membership is first granted,
and when it is renewed.
The bylaws must address the process by which a vote to opt out of the unified medical staff
is conducted. In establishing the unified medical staff bylaws governing opting out, the
unified medical staff, and the system governing body, which must approve the medical
staff’s bylaws, rules or regulations in accordance with §482.12(a)(4), have the flexibility to
determine the details of the voting process, such as how an acceptance or opt-out vote can
be requested; whether all categories of members holding privileges to practice on-site at the
hospital are afforded medical staff voting rights; whether voting will be in writing and open
or by secret ballot, etc. However, the unified medical staff and system governing body may
not set up bylaws that unduly restrict the rights of medical staff members at each separately
certified hospital to vote whether to accept or opt out of a unified medical staff structure.
For example:
The bylaws, rules and requirements may not establish different criteria as to which
categories of medical staff members have voting rights with respect to a vote to
accept or opt out of a unified medical staff than are used for any other type of voting
the medical staff engages in, except as required under the regulation at
§482.22(b)(4) that only members holding privileges to practice at the hospital may
vote. (See also the discussion below concerning delegation of authority to the
medical staff executive committee.)
The bylaws, rules and requirements may not require as a condition for holding an
opt-out vote that there be a petition signed by the same number of voting members
as would be required for a successful vote to opt out.
The bylaws, rules and requirements may require for a successful acceptance or opt-
out vote a “super-majority,” that is, a majority that is greater than a simple majority
of more than fifty percent of the medical staff members with voting rights holding
privileges to practice at the hospital, so long as the same type of supermajority is
otherwise required to amend the unified medical staff’s bylaws, rules and
requirements.
In the case where a hospital system has a unified medical staff and members of the
staff at a hospital in the system exercise their right to hold a vote on the question of
opting out, the unified medical staff bylaws may not permit delegation of an opt-out
decision to the unified medical staff’s executive committee. This is the case even
when the executive committee is otherwise delegated authority to amend unified
medical staff bylaws, rules and requirements that it recommends for approval to the
governing body. In cases where the bylaws permit such delegation to the unified
medical staff’s executive committee for other purposes, a “majority” for purposes of
conducting a vote on whether to opt out of a unified medical staff consists of a
simple majority, that is, any number which is greater than fifty percent of the
medical staff members practicing at the hospital who have voting privileges.
The bylaws, rules and requirements may establish a minimum interval between
acceptance or opt-out votes, such as not permitting a vote more than once every two
years. However, minimum interval between votes longer than two years might
unduly restrain the rights of the members of the medical staff and would not be
permissible.
Survey Procedures §482.22(b)(4)(ii)
• Assess compliance with this regulation only if the hospital uses a unified
medical staff. (See survey procedures for §482.22(b)(4) above)
• Ask the hospital’s leadership for evidence that the unified medical staff’s
bylaws, rules and requirements are readily available, and that it is clear that they
apply to that hospital.
• Ask the hospital’s leadership to provide evidence that the unified medical staff
bylaws, rules or requirements address the rights of members holding privileges
and voting rights at the hospital to vote to opt out of using the unified medical
staff, including notification of these rights.
• Ask how the unified medical staff bylaws define a majority for the purpose of an
opt-out vote. If the unified medical staff bylaws require a super-majority, ask
for evidence that this is consistent with the way “majority” is defined for other
amendments to the bylaws.
• Do the bylaws, rules or requirements clearly describe how and when voting
members holding privileges at the hospital are advised of their rights?
• Can the hospital readily identify the members of the unified medical staff
practicing at the hospital who are eligible to vote to opt out and therefore must
be advised of their rights?
• Do the credentialing and privileging files of members of the medical staff have
any evidence of their being notified of their right to vote by majority to opt out?
• Interview several members of the medical staff to determine if they recall being
notified of their right to vote by majority to opt out.
A-0351
(Rev.122, Issued: 09-26-14, Effective: 09-26-14, Implementation: 09-26-14)
[§482.22(b)(4) - If a hospital is part of a hospital system consisting of multiple
separately certified hospitals and the system elects to have a unified and integrated
medical staff for its member hospitals, after determining that such a decision is in
accordance with all applicable State and local laws, each separately certified hospital
must demonstrate that:]
(iii) - The unified and integrated medical staff is established in a manner that takes
into account each member hospital’s unique circumstances and any significant
differences in patient populations and services offered in each hospital; and….
Interpretive Guidelines §482.22(b)(4)(iii)
The separately certified hospitals belonging to a multi-hospital system and using a single
unified medical staff may be very different from each other, presenting different needs and
challenges for the medical staff. As a result, the unified medical staff is expected to take
these differences into account rather than using a one-size-fits-all approach for all of its
policies and procedures. For example, a multi-hospital system may:
• Consist of a mixture of different types of hospitals, such as short-term acute care
hospitals, psychiatric hospitals, rehabilitation hospitals, children’s hospitals, and
long-term care hospitals. As a result, they would offer different types of
services to different patient populations. This could have implications for
medical staff functions such as the periodic review of credentials and privileges
and ongoing peer review of the quality of medical care. It could also have
implications for other responsibilities the medical staff has under various CoPs.
For example, the medical staff has a key role in the development and oversight
of the use of standing orders/protocols, but these orders/protocols may need to
be specific to each hospital, reflecting the types of services a hospital offers and
its patient population;
Consist of hospitals that differ in size, ranging from comparatively small hospitals in
rural areas, or which provide specialized rehabilitation or long term care hospital
services, to very large short term acute care service hospitals. Such differences
could have implications for various medical staff requirements, such as on-call
requirements.
Consist of hospitals that differ as to whether they are teaching hospitals or not,
which would have implications for policies concerning the roles and supervision of
residents.
Consist of hospitals that are located in different states which have different licensure
requirements affecting the organization and composition of the medical staff. For
example, in one state it might be permissible for non-physician practitioners to be
members of the medical staff, while in another the medical staff is limited to
physicians.
On the other hand, a multi-hospital system may have a conscious strategy of having
hospitals that are very similar to each other in terms of size, services, patient populations
served, and type of location. In this case, the unified medical staff would have fewer
challenges in addressing the needs of each hospital, and might have more policies that are
uniform across the medical staff.
In all cases the hospital’s leadership and the medical staff leadership must be able to explain
how the way in which the unified medical staff is organized and functions takes account of
and responds to the unique circumstances of the hospital that is being surveyed.
Survey Procedures §482.22(b)(4)(iii)
• Assess compliance with this regulation only if the hospital uses a unified
medical staff. (See survey procedures for §482.22(b)(4) above)
Ask the hospital’s and medical staff’s leadership to describe the other types of
hospitals in the system with which it shares a unified medical staff, and how the
hospital’s unique circumstances are addressed. For example, how does the unified
medical staff assure that:
o Standing orders it has approved are also approved by the nursing and pharmacy
leadership in each separately certified hospitals? (see §482.24(c)(3)(i));
o Policies and procedures developed by the medical staff to minimize drug errors,
if this function has not been delegated to the hospital’s pharmaceutical service,
take into account any unique hospital circumstances? (see §482.25);
o The formulary system established by the medical staff takes into account any
unique hospital circumstances? (See §482.25(b)(9));
o The medical staff’s specification of procedures and treatments requiring a
properly executed informed consent reflects any unique hospital circumstances?
(see §482.24(c)(4)(v));
o The medical staff carries out its joint responsibility with the CEO and Director
of nursing for ensuring that hospital-specific infection control problems
identified by the hospital’s infection control officer(s) are addressed in the
hospital’s QAPI and training programs? (see §482.42(b));
o The medical staff fulfills its joint executive responsibilities, along with the
hospital’s governing body and administrative officials, for ensuring that the
hospital-specific QAPI program is:
Ongoing, defined, implemented and maintained;
Addresses hospital-specific priorities for improved quality of care and patient
safety, and that all improvements are evaluated;
Establishes clear expectations for safety in the hospital;
Allocates adequate resources for the hospital-specific QAPI program; and
Determines annually the number of distinct improvement projects conducted in
the hospital?
(See §482.21(e))
o Medical staff policies governing ordering of outpatient services address any unique
hospital circumstances? (See §482.54(c)(4))
o Medical staff policies and recommendations governing which practitioners may be
authorized to write orders and be responsible for the care of the patient conform to
State law, including scope of practice law, for the State in which the hospital is
located? (multiple citations in various CoPs)
A-0352
(Rev.122, Issued: 09-26-14, Effective: 09-26-14, Implementation: 09-26-14)
[§482.22(b)(4) - If a hospital is part of a hospital system consisting of multiple
separately certified hospitals and the system elects to have a unified and integrated
medical staff for its member hospitals, after determining that such a decision is in
accordance with all applicable State and local laws, each separately certified hospital
must demonstrate that:]
(iv) The unified and integrated medical staff establishes and implements policies and
procedures to ensure that the needs and concerns expressed by members of the
medical staff, at each of its separately certified hospitals, regardless of practice or
location, are given due consideration, and that the unified and integrated medical staff
has mechanisms in place to ensure that issues localized to particular hospitals are duly
considered and addressed.
Interpretive Guidelines §482.22(b)(4)(iv)
The hospital’s unified medical staff must have written policies and procedures that address
how it considers and addresses needs and concerns expressed by members who practice at
the hospital. This provision is not about an individual medical staff member’s concerns
with privileges granted or not granted to him/her, peer review results, due process issues,
etc., since these matters are addressed under the requirements at §482.22(a) and (c) as well
as §482.22(b)(4)(ii). Instead, this provision addresses a requirement for the unified medical
staff to consider and address concerns that practitioners have concerning their own
hospital’s needs. For example, physicians practicing in a children’s hospital may have
concerns about having protocols for medication administration that reflect specific pediatric
patient concerns, or physicians practicing in a small rural hospital may have concerns about
how to get timely telemedicine consults from their colleagues in urban areas.
The medical staff has flexibility in establishing its written policies and procedures for
addressing these local concerns, but at a minimum they must cover the following:
A process by which members who practice at a hospital can raise their local
concerns and needs with the unified medical staff’s leadership;
How members are informed of the process by which they can raise their local
concerns and needs;
A process for referring the concerns and needs raised to the appropriate committee
or other group within the medical staff for due consideration; and
Documentation of the outcome of the medical staff’s review of the concerns and
needs raised.
Survey Procedures §482.22(b)(4)(iv)
• Assess compliance with this regulation only if the hospital uses a unified
medical staff. (See survey procedures for §482.22(b)(4) above)
Determine that the unified medical staff has policies and procedures addressing how
members can raise local concerns and needs. Do the written policies and procedures
cover the minimum elements?
Ask the hospital and the medical staff leadership whether any members practicing at
the hospital have raised concerns or needs. If yes, ask for documentation on how
the concern/need was considered and addressed by the unified medical staff.
Ask members of the medical staff if they are aware they can raise local concerns or needs
with the leadership of the unified medical staff.
A-0353
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.22(c) Standard: Medical Staff Bylaws
The medical staff must adopt and enforce bylaws to carry out its responsibilities. The
bylaws must:
Interpretive Guidelines §482.22(c)
The medical staff must regulate itself by bylaws that are consistent with the requirements of
this and other CoPs that mention medical staff bylaws, as well as State laws. The bylaws
must be enforced and revised as necessary.
Survey Procedures §482.22(c)
• Verify that the medical staff have bylaws that comply with the CoPs and State law.
• Verify that the bylaws describe a mechanism for ensuring enforcement of its
provisions along with rules and regulations of the hospital.
• Verify that the medical staff enforce the bylaws.
A-0354
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The bylaws must:]
§482.22(c)(1) - Be approved by the governing body.
Interpretive Guidelines §482.22(c)(1)
Medical staff bylaws and any revisions of those bylaws must be submitted to the governing
body for approval. The governing body has the authority to approve or disapprove bylaws
suggested by the medical staff. The bylaws and any revisions must be approved by the
governing body before they are considered effective.
Survey Procedures §482.22(c)(1)
Verify that the medical staff bylaws have been approved by the medical staff and the
governing body.
A-0355
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The bylaws must:]
§482.22(c)(2) - Include a statement of the duties and privileges of each category of
medical staff (e.g., active, courtesy, etc.)
Interpretive Guidelines §482.22(c)(2)
The medical staff bylaws must state the duties and scope of medical staff privileges each
category of practitioner may be granted. Specific privileges for each category must clearly
and completely list the specific privileges or limitations for that category of practitioner.
The specific privileges must reflect activities that the majority of practitioners in that
category can perform competently and that the hospital can support.
Although the medical staff bylaws must address the duties and scope for each category of
practitioner, this does not mean that each individual practitioner within the category may
automatically be granted the full range of privileges. It cannot be assumed that every
practitioner can perform every task/activity/privilege that is specified for the applicable
category of practitioner. The individual practitioner’s ability to perform each
task/activity/privilege must be individually assessed.
Survey Procedures §482.22(c)(2)
Determine whether the bylaws specify the duties and scope of medical staff privileges for
each category of practitioner eligible for medical staff membership or privileges.
A-0356
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The bylaws must:]
§482.22(c)(3) - Describe the organization of the medical staff.
Interpretive Guidelines §482.22(c)(3)
The medical staff bylaws must describe the organizational structure of the medical staff,
and lay out the rules and regulations of the medical staff to make clear what are acceptable
standards of patient care for all diagnostic, medical, surgical, and rehabilitative services.
Survey Procedures §482.22(c)(3)
• Verify that the bylaws specify the organization and structure of the medical staff,
and a mechanism that delineates accountability to the governing body.
• Verify that the bylaws describe who is responsible for regularly scheduled review
and evaluation of the clinical work of the members of the medical staff and describe
the formation of medical staff leadership.
A-0357
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
[The bylaws must:]
§482.22(c)(4) - Describe the qualifications to be met by a candidate in order for the
medical staff to recommend that the candidate be appointed by the governing body.
Interpretive Guidelines §482.22(c)(4)
The medical staff bylaws must describe the qualifications to be met by a candidate for
medical staff membership/privileges in order for the medical staff to recommend the
candidate be approved by the governing body. The bylaws must describe the privileging
process to be used in the hospital. The process articulated in the medical staff bylaws must
include criteria for determining the privileges that may be granted to individual
practitioners and a procedure for applying the criteria to individual practitioners that
considers:
• Individual character;
• Individual competence;
• Individual training;
• Individual experience; and
• Individual judgment.
The medical staff may not rely solely on the fact that a MD/DO is, or is not, board-certified
in making a judgment on medical staff membership. This does not mean that the medical
staff is prohibited from requiring board certification when considering a MD/DO for
medical staff membership; only that such certification is not the only factor that the hospital
considers. After analysis of all of the criteria, if all criteria are met except for board
certification, the medical staff has the discretion to not recommend that individual for
medical staff membership/privileges.
The bylaws must apply equally to all practitioners in each professional category of
practitioners.
The medical staff then recommends individual candidates that meet those requirements to
the governing body for appointment to the medical staff.
Survey Procedures §482.22(c)(4)
• Verify that there are written criteria for appointments to the medical staff and
granting of medical staff privileges.
• Verify that granting of medical staff membership or privileges, is based upon an
individual practitioner’s meeting the medical staff’s membership/privileging
criteria.
• Verify that at a minimum, criteria for appointment to the medical staff/granting of
medical staff privileges are individual character, competence, training, experience,
and judgment.
• Verify that written criteria for appointment to the medical staff and granting of
medical staff privileges are not dependent solely upon certification, fellowship, or
membership in a specialty body or society.
A-0358
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[The bylaws must:]
§482.22(c)(5) Include a requirement that --
(i) A medical history and physical examination be completed and documented
for each patient no more than 30 days before or 24 hours after admission or
registration, but prior to surgery or a procedure requiring anesthesia services, and
except as provided under paragraph (c)(5)(iii) of this section. The medical history and
physical examination must be completed and documented by a physician (as defined
in section 1861(r) of the Act), an oral and maxillofacial surgeon, or other qualified
licensed individual in accordance with State law and hospital policy
Interpretive Guidelines §482.22(c)(5)(i)
The purpose of a medical history and physical examination (H&P) is to determine whether
there is anything in the patient's overall condition that would affect the planned course of
the patient's treatment, such as a medication allergy, or a new or existing co-morbid
condition that requires additional interventions to reduce risk to the patient.
The Medical Staff bylaws must include a requirement that an H&P be completed and
documented for each patient no more than 30 days prior to or 24 hours after hospital
admission or registration, but prior to surgery or a procedure requiring anesthesia services
except when the patient is receiving an outpatient surgical or procedural services and when
the medical staff has developed and maintained a policy (in accordance with
§482.22(c)(5)(v)) that identifies specific patients that do not require a comprehensive
medical H&P, or any update to it, prior to the specific outpatient surgery or procedure.
The H&P may be handwritten or transcribed, but always must be placed within the patient’s
medical record within 24 hours of admission or registration, or prior to surgery or a
procedure requiring anesthesia services, whichever comes first.
An H&P is required prior to surgery and prior to procedures requiring anesthesia services,
regardless of whether care is being provided on an inpatient or outpatient basis. (71 FR
68676) An H&P that is completed within 24 hours of the patient’s admission or
registration, but after the surgical procedure, procedure requiring anesthesia, or other
procedure requiring an H&P would not be in compliance with this requirement.
The medical history and physical examination must be completed and documented by a
physician (as defined in section 1861(r) of the Act), oral and maxillofacial surgeon, or other
qualified licensed individual in accordance with State law and hospital policy.
Section 1861(r) defines a physician as a:
• Doctor of medicine or osteopathy;
• Doctor of dental surgery or of dental medicine;
• Doctor of podiatric medicine;
• Doctor of optometry; or a
• Chiropractor.
In all cases the practitioners included in the definition of a physician must be legally
authorized to practice within the State where the hospital is located and providing services
within their authorized scope of practice. In addition, in certain instances the Social
Security Act attaches further limitations as to the type of hospital services for which a
practitioner is considered to be a “physician.” For example, a chiropractor is considered a
physician only with respect to treatment by means of manual manipulation of the spine (to
correct a subluxation).
Other qualified licensed individuals are those licensed practitioners who are authorized in
accordance with their State scope of practice laws or regulations to perform an H&P and
who are also formally authorized by the hospital to conduct an H&P. Other qualified
licensed practitioners could include nurse practitioners and physician assistants.
More than one qualified practitioner can participate in performing, documenting, and
authenticating an H&P for a single patient. When performance, documentation, and
authentication are split among qualified practitioners, the practitioner who authenticates the
H&P will be held responsible for its contents. (71 FR 68675)
A hospital may adopt a policy allowing submission of an H&P prior to the patient’s hospital
admission or registration by a physician who may not be a member of the hospital's medical
staff or who does not have admitting privileges at that hospital, or by a qualified licensed
individual who does not practice at that hospital but is acting within his/her scope of
practice under State law or regulations. Generally, this occurs where the H&P is completed
in advance by the patient’s primary care practitioner. (71 FR 68675)
When the H&P is conducted within 30 days before admission or registration, an update
must be completed and documented by a licensed practitioner who is credentialed and
privileged by the hospital’s medical staff to perform an H&P. (71 FR 68675) (See
discussion of H&P update requirements at 42 CFR 482.22(c)(5)(ii).)
Surveyors should cite noncompliance with the requirements of 42 CFR
482.22(c)(5) for failure by the hospital to comply with any of this standard's components.
Survey Procedures §482.22(c)(5)(i)
• Review the medical staff bylaws to determine whether they require that a physical
examination and medical history be done for each patient no more than 30 days before
or 24 hours after admission or registration by a physician (as defined in section 1861(r)
of the Act), an oral and maxillofacial surgeon, or other qualified licensed individual in
accordance with State law and hospital policy. Verify whether the bylaws require the
H&P be completed prior to surgery or a procedure requiring anesthesia services.
• Review the hospital’s policy, if any, to determine whether other qualified licensed
individuals are permitted to conduct H&Ps to ensure that it is consistent with the State’s
scope of practice law or regulations.
• Verify that non-physicians who perform H&Ps within the hospital are qualified and
have been credentialed and privileged in accordance with the hospital’s policy.
• Review a sample of inpatient and outpatient medical records that include a variety of
patient populations undergoing both surgical and non-surgical procedures to verify that:
o There is an H&P that was completed no more than 30 days before or 24 hours
after admission or registration, but, in all cases, prior to surgery or a procedure
requiring anesthesia services, except when an assessment is completed and
documented pursuant to §482.22(c)(5)(iii); and
o The H&P was performed by a physician, an oral and maxillofacial surgeon, or
other qualified licensed individual authorized in accordance with State law and
hospital policy.
A-0359
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[The bylaws must:]
§482.22(c)(5) - Include a requirement that --
(ii) An updated examination of the patient, including any changes in the
patient's condition, be completed and documented within 24 hours after
admission or registration, but prior to surgery or a procedure requiring
anesthesia services, when the medical history and physical examination are
completed within 30 days before admission or registration, and except as
provided under paragraph (c)(5)(iii) of this section. The updated
examination of the patient, including any changes in the patient's
condition, must be completed and documented by a physician (as defined
in section 1861(r) of the Act), an oral and maxillofacial surgeon, or other
qualified licensed individual in accordance with State law and hospital
policy.
Interpretive Guidelines §482.22(c)(5)(ii)
The Medical Staff bylaws must include a requirement that when a medical history and
physical examination has been completed within 30 days before admission or registration,
an updated medical record entry must be completed and documented in the patient's
medical record within 24 hours after admission or registration, except when the patient is
receiving an outpatient surgical or procedural services and when the medical staff has
developed and maintained a policy (in accordance with §482.22(c)(5)(v)) that identifies
specific patients that do not require a comprehensive medical H&P, or any update to it,
prior to the outpatient surgery or procedure.
The examination must be conducted by a licensed practitioner who is credentialed and
privileged by the hospital’s medical staff to perform an H&P. In all cases, the update must
take place prior to surgery or a procedure requiring anesthesia services. The update note
must document an examination for any changes in the patient's condition since the patient's
H&P was performed that might be significant for the planned course of treatment. The
physician or qualified licensed individual uses his/her clinical judgment, based upon his/her
assessment of the patient’s condition and co-morbidities, if any, in relation to the patient’s
planned course of treatment to decide the extent of the update assessment needed as well as
the information to be included in the update note in the patient’s medical record.
If, upon examination, the licensed practitioner finds no change in the patient's condition
since the H&P was completed, he/she may indicate in the patient's medical record that the
H&P was reviewed, the patient was examined, and that "no change" has occurred in the
patient's condition since the H&P was completed (71 FR 68676). Any changes in the
patient’s condition must be documented by the practitioner in the update note and placed in
the patient’s medical record within 24 hours of admission or registration, but prior to
surgery or a procedure requirement anesthesia services. Additionally, if the practitioner
finds that the H&P done before admission is incomplete, inaccurate, or otherwise
unacceptable, the practitioner reviewing the H&P, examining the patient, and completing
the update may disregard the existing H&P, and conduct and document in the medical
record a new H&P within 24 hours after admission or registration, but prior to surgery or a
procedure requiring anesthesia.
Survey Procedures §482.22(c)(5)(ii)
• Review the medical staff bylaws to determine whether they include provisions
requiring that, when the medical history and physical examination was completed
within 30 days before admission or registration, an updated medical record entry
documenting an examination for changes in the patient's condition was completed
and documented in the patient's medical record within 24 hours after admission or
registration.
• Determine whether the bylaws require that, in all cases involving surgery or a
procedure requiring anesthesia services, the update to the H&P must be completed
and documented prior to the surgery or procedure.
• In the sample of medical records selected for review, look for cases where the
medical history and physical examination was completed within 30 days before
admission or registration. Verify that an updated medical record entry documenting
an examination for any changes in the patient's condition was completed and
documented in the patient's medical record within 24 hours after admission or
registration. Verify that in all cases involving surgery or a procedure requiring
anesthesia services, the update was completed and documented prior to the surgery
or procedure.
A-0360
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[The bylaws must:]
§482.22(c)(5) - Include a requirement that --
(iii) An assessment of the patient (in lieu of the requirements of paragraphs
(c)(5)(i) and (ii) of this section) be completed and documented after
registration, but prior to surgery or a procedure requiring anesthesia
services, when the patient is receiving specific outpatient surgical or
procedural services and when the medical staff has chosen to develop and
maintain a policy that identifies, in accordance with the requirements at
paragraph (c)(5)(v) of this section, specific patients as not requiring a
comprehensive medical history and physical examination, or any update to
it, prior to specific outpatient surgical or procedural services. The
assessment must be completed and documented by a physician (as defined
in section 1861(r) of the Act), an oral and maxillofacial surgeon, or other
qualified licensed individual in accordance with State law and hospital
policy.
Interpretive Guidelines §482.22(c)(5)(iii)
Guidance is pending and will be updated in future release.
A-0361
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[The bylaws must:]
§482.22(c)(5) - Include a requirement that --
(iv) The medical staff develop and maintain a policy that identifies those
patients for whom the assessment requirements of paragraph (c)(5)(iii) of
this section would apply. The provisions of paragraphs (c)(5)(iii), (iv), and
(v) of this section do not apply to a medical staff that chooses to maintain a
policy that adheres to the requirements of paragraphs of (c)(5)(i) and (ii) of
this section for all patients.
Interpretive Guidelines §482.22(c)(5)(iv)
Guidance is pending and will be updated in future release.
A-0362
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[The bylaws must:]
§482.22(c)(5) - Include a requirement that --
(v) The medical staff, if it chooses to develop and maintain a policy for the
identification of specific patients to whom the assessment requirements in
paragraph (c)(5)(iii) of this section would apply, must demonstrate
evidence that the policy applies only to those patients receiving specific
outpatient surgical or procedural services as well as evidence that the
policy is based on:
(A) Patient age, diagnoses, the type and number of surgeries and
procedures scheduled to be performed, comorbidities, and the level of
anesthesia required for the surgery or procedure.
(B) Nationally recognized guidelines and standards of practice for
assessment of specific types of patients prior to specific outpatient
surgeries and procedures.
(C) Applicable state and local health and safety laws.
Interpretive Guidelines §482.22(c)(5)(v)
Guidance is pending and will be updated in future release.
A-0363
(Rev.78, Issued: 12-22-11, Effective/Implementation: 12-22-11)
[The bylaws must:]
§482.22(c)(6) - Include criteria for determining the privileges to be granted to
individual practitioners and a procedure for applying the criteria to individuals
requesting privileges. For distant-site physicians and practitioners requesting
privileges to furnish telemedicine services under an agreement with the hospital, the
criteria for determining privileges and the procedure for applying the criteria are also
subject to the requirements in §482.12(a)(8) and (a)(9), and §482.22(a)(3) and (a)(4).
Interpretive Guidelines §482.22(c)(6)
All patient care is provided by or in accordance with the orders of a physician or
practitioner who meets the medical staff criteria and procedures for the privileges granted,
who has been granted privileges in accordance with those criteria by the governing body,
and who is working within the scope of those granted privileges.
Privileges are granted by the hospital’s governing body to individual practitioners based on
the medical staff’s review of that individual practitioner’s qualifications and the medical
staff’s recommendations for that individual practitioner to the governing body. However,
in the case of telemedicine physicians and practitioners providing telemedicine services
under an agreement, the governing body has the option of having the medical staff rely
upon the credentialing and privileging decisions of the distant-site hospital or telemedicine
entity with which the hospital has entered into an agreement. When the governing body has
exercised this option, the medical staff’s bylaws must include a provision allowing the
medical staff to rely upon the credentialing and privileging decisions of a distant-site
hospital or telemedicine entity when that distant-site hospital or entity is required under the
terms of its agreement with the hospital to employ a credentialing and privileging process
that conforms to the provisions of §482.12(a)(8) and (a)(9), and §482.22(a)(3) and (a)(4).
Survey Procedures §482.22(c)(6)
• Verify that the medical staff bylaws contain criteria for granting, withdrawing, and
modifying clinical privileges to individual physicians and practitioners of the
medical staff and that a procedure exists for applying these criteria.
• In the case of telemedicine physicians and practitioners providing telemedicine
services under an agreement with the hospital where the hospital’s governing body
has opted to have the medical staff rely upon the credentialing and privileging
decisions of the distant-site hospital or telemedicine entity, verify that the bylaws
include a provision permitting such reliance.
• Verify that physicians and practitioners who provide care to patients are working
within the scope of the privileges granted by the governing body.
A-0385
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.23 Condition of Participation: Nursing Services
The hospital must have an organized nursing service that provides 24-hour nursing
services. The nursing services must be furnished or supervised by a registered nurse.
Interpretive Guidelines §482.23
The hospital must have an organized nursing service and must provide on premise nursing
services 24 hours a day, 7 days a week with at least 1 registered nurse (RN) furnishing or
supervising the service 24 hours a day, 7 days a week (Exception: small rural hospitals
operating under a waiver as discussed in §482.23(b)(1)).
The Social Security Act (SSA) at §1861(b) states that nursing services must be furnished to
inpatients and furnished by the hospital. The SSA at §1861(e) further requires that the
hospital have a RN on duty at all times (except small rural hospitals operating under a
nursing waiver).
The nursing service must be a well-organized service of the hospital and under the direction
of a registered nurse.
The nursing service must be integrated into the hospital-wide QAPI program.
Survey Procedures §482.23
• Determine if the nursing service is integrated into the hospital-wide QAPI program.
• Interview the director of the service. Request the following items:
○ Organizational chart(s) for nursing services for all locations where the hospital
provides nursing services;
○ Job or position descriptions for all nursing personnel including the director’s
position description.
• Select at least one patient from every inpatient care unit. Observe the nursing care
in progress to determine the adequacy of staffing and to assess the delivery of care.
Other sources of information to use in the evaluation of the nursing services are:
nursing care plans, medical records, patients, family members, accident and
investigative reports, staffing schedules, nursing policies and procedures, and QAPI
activities and reports. Interview patients for information relative to the delivery of
nursing services.
A-0386
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.23(a) Standard: Organization
The hospital must have a well-organized service with a plan of administrative
authority and delineation of responsibilities for patient care. The director of the
nursing service must be a licensed registered nurse. He or she is responsible for the
operation of the service, including determining the types and numbers of nursing
personnel and staff necessary to provide nursing care for all areas of the hospital.
Interpretive Guidelines §482.23(a)
The hospital may have only one nursing service hospital-wide and the single nursing
service must be under the direction of one RN.
The director of the nursing service must be a currently licensed RN and he/she is
responsible for the operation of the nursing service. The operation of the nursing service
would include the quality of the patient care provided by the nursing service.
The director of the nursing service must determine and provide the types and numbers of
nursing care personnel necessary to provide nursing care to all areas of the hospital.
The organization will include various configurations of the following hospital personnel as
determined necessary by the hospital and the Director of Nursing:
• Assistant/Associate Director(s);
• Supervisors/Coordinators;
• Head Nurses/Nurse Managers;
• Staff Nurses;
• Unit Secretaries/Clerks;
• Nurses Aide/Orderlies.
Survey Procedures §482.23(a)
• Review the organizational chart or plan for nursing services. Determine that the
organizational chart(s) displays lines of authority that delegates responsibility within
the department.
• Read the position description for the director of nursing (DON) to determine that it
delegates to the DON specific duties and responsibilities for operation of the
service.
• Verify that the director is currently licensed in accordance with state licensure
requirements.
• Verify that the DON is involved with or approved the development of the nursing
service staffing policies and procedures.
• Verify that the DON approves the nursing service patient care policies and
procedures.
A-0392
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.23(b) Standard: Staffing and Delivery of Care
The nursing service must have adequate numbers of licensed registered nurses,
licensed practical (vocational) nurses, and other personnel to provide nursing care to
all patients as needed. There must be supervisory and staff personnel for each
department or nursing unit to ensure, when needed, the immediate availability of a
registered nurse for care of any patient.
Interpretive Guidelines §482.23(b)
The nursing service must ensure that patient needs are met by ongoing assessments of
patients’ needs and provides nursing staff to meet those needs. There must be sufficient
numbers, types and qualifications of supervisory and staff nursing personnel to respond to
the appropriate nursing needs and care of the patient population of each department or
nursing unit.
There must be a RN physically present on the premises and on duty at all times. Every
inpatient unit/department/location within the hospital-wide nursing service must have
adequate numbers of RNs physically present at each location to ensure the immediate
availability of a RN for the care of any patient.
A RN would not be considered immediately available if the RN were working on more than
one unit, building, floor in a building, or provider (distinct part SNF, RHC, excluded unit,
etc.) at the same time.
Staffing schedules must be reviewed and revised as necessary to meet the patient care needs
and to make adjustments for nursing staff absenteeism.
Survey Procedures §482.23(b)
• Determine that there are written staffing schedules which correlate to the number
and acuity of patients. Verify that there is supervision of personnel performance
and nursing care for each department or nursing unit. To determine if there are
adequate numbers of nurses to provide nursing care to all patients as needed, take
into consideration:
o Physical layout and size of the hospital;
o Number of patients;
o Intensity of illness and nursing needs;
o Availability of nurses’ aides and orderlies and other resources for nurses,
e.g., housekeeping services, ward clerks etc.;
o Training and experience of personnel;
• Review medical records to determine if patient care that is to be provided by nurses
is being provided as ordered.
A-0393
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.23(b)(1) - The hospital must provide 24-hour nursing services furnished or
supervised by a registered nurse, and have a licensed practical nurse or registered
nurse on duty at all times, except for rural hospitals that have in effect a 24-hour
nursing waiver granted under §488.54(c)of this chapter.
Interpretive Guidelines §482.23(b)(1)
The hospital must provide nursing services 24 hours a day, 7 days a week. An LPN can
provide nursing services if an RN, who is immediately available for the bedside care of
those patients, supervises that care.
EXCEPTION: Section 488.54(c) sets forth certain conditions under which rural
hospitals of 50 beds or fewer may be granted a temporary waiver of the
24-hour registered nurse requirement by the regional office.
Rural is defined as all areas not delineated as “urbanized” areas by the Census Bureau, in
the most recent census. Temporary is defined as a one year period or less and the waiver
cannot be renewed.
Survey Procedures §482.23(b)(1)
• Review the nurse staffing schedule for a one-week period. If there are concerns
regarding insufficient RN coverage, review the staffing schedules for a second week
period to determine if there is a pattern of insufficient coverage. Document daily
RN coverage for every unit of the hospital. Verify that there is at least one RN for
each unit on each tour of duty, 7 days a week, 24 hours a day. Additional nurses

may be required for vacation or absenteeism coverage.
• EXCEPTION: If the hospital has a temporary waiver of the 24-hour RN
requirement in effect, verify and document the following:
o 50 or fewer inpatient beds;
o The character and seriousness of the deficiencies do not adversely affect the
health and safety of patients
o The hospital meets all the other statutory requirements in §1861(e)(1-8).
o The hospital has made and continues to make a good faith effort to comply
with the 24 hour nursing requirement. Determine the recruitment efforts and
methods used by the hospitals’ administration by requesting copies of
advertisements in newspapers and other publications as well as evidence of
contact with nursing schools and employment agencies. Document that the
salary offered by the hospital is comparable to three other hospitals, located
nearest to the facility.
o The hospital’s failure to comply fully with the 24 hour nursing requirement
is attributable to a temporary shortage of qualified nursing personnel in the
area in which the hospital is located.
o A registered nurse is present on the premises to furnish the nursing service
during at least the daytime shift, 7 days a week.
o On all tours of duty not covered by a registered nurse, a licensed practical
(vocational) nurse is in charge.
A-0394
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.23(b)(2) - The nursing service must have a procedure to ensure that hospital
nursing personnel for whom licensure is required have valid and current licensure.
Interpretive Guidelines §482.23(b)(2)
The hospital’s procedure must ensure that all nursing personnel have valid and current
licensure that complies with State licensure laws. Furthermore, the Condition of
Participation (CoP) Compliance with Federal, State and local laws (42 CFR 482.11)
requires the hospital to assure that personnel meet applicable standards (such as continuing
education, certification or training) required by State or local law.
Survey Procedures §482.23(b)(2)
• Review hospital personnel records or records kept by the nursing service to
determine that RNs, LPNs, and other nursing personnel for whom licensure is
required have current valid licenses.
• Review the nursing service licensure verification policies and procedures. Is
licensure verified for each individual nursing services staff person for whom
licensure is required?
A-0395
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.23(b)(3) - A registered nurse must supervise and evaluate the nursing care for
each patient.
Interpretive Guidelines §482.23(b)(3)
A RN must supervise the nursing care for each patient. A RN must evaluate the care for
each patient upon admission and when appropriate on an ongoing basis in accordance with
accepted standards of nursing practice and hospital policy. Evaluation would include
assessing the patient’s care needs, patient’s health status/conditioning, as well as the
patient’s response to interventions.
Survey Procedures §482.23(b)(3)
• Review staffing schedules and assignments.
• Determine that a RN is assigned to supervise and evaluate the nursing care furnished
to each patient.
A-0396
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.23(b)(4) - The hospital must ensure that the nursing staff develops and keeps
current a nursing care plan for each patient that reflects the patient’s goals and the
nursing care to be provided to meet the patient’s needs. The nursing care plan may be
part of an interdisciplinary care plan.
Interpretive Guidelines §482.23(b)(4)
Nursing care planning starts upon admission. It includes planning the patient’s nursing care
to meet the patient’s needs and interventions toward meeting patient treatment goals while
in the hospital as well as planning for discharge to meet post-hospital needs. A nursing care
plan is based on assessing the patient’s nursing care needs (not solely those needs related to
the admitting diagnosis). The assessment considers the patient’s treatment goals and, as
appropriate, physiological and psychosocial factors and patient discharge planning. The
plan develops appropriate nursing interventions in response to the identified nursing care
needs. The nursing care plan is kept current by ongoing assessments of the patient’s needs
and of the patient’s response to interventions, assessment of patient treatment goals, and
updating or revising the patient’s nursing care plan in response to assessments. The nursing
care plan is part of the patient’s medical record and must comply with the medical records
requirements at §482.24.
Hospitals have the flexibility of developing the nursing care plan as part of a larger,
coordinated interdisciplinary plan of care. This method may serve to promote
communication among disciplines and reinforce an integrated, multi-faceted approach to a
patient’s care, resulting in better patient outcomes. The interdisciplinary plan of care does
not minimize or eliminate the need for a nursing care plan. It does, however, serve to
promote the collaboration between members of the patient’s health care team.
The required documentation for the nursing component of an interdisciplinary care plan
remains the same. For other components, the hospital should follow the current
documentation policies that it uses to document services provided by other disciplines, such
as services provided by physical therapists, occupational therapists, speech-language
pathologists, and others. Documentation should follow the standards of practice for those
disciplines in addition to any specific requirements that the hospital might want to establish.
The documentation must also comply with the requirements of the medical records
requirement at §482.24. (77 FR 29049, May 16, 2012)
Survey Procedures §482.23(b)(4)
Select a sample of nursing or interdisciplinary care plans. Approximately 6-12 plans should
be reviewed. For each plan reviewed, with respect to the nursing care component:
• Was the plan initiated as soon as possible after admission for each patient?
• Does the plan describe and reflect patient goals as part of the patient’s nursing care
assessment and, as appropriate, physiological and psychosocial factors and patient
discharge planning?
• Is the plan consistent with the plan for medical care of the practitioner responsible
for the care of the patient?
• Is there evidence of reassessment of the patient’s nursing care needs and response to
nursing interventions and, as applicable, revisions to the plan?
• Was the plan implemented in a timely manner?
A-0397
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.23(b)(5) - A registered nurse must assign the nursing care of each patient to other
nursing personnel in accordance with the patient’s needs and the specialized
qualifications and competence of the nursing staff available.
Interpretive Guidelines §482.23(b)(5)
A RN must make all patient care assignments. The director of the nursing service and the
hospital are to ensure that nursing personnel with the appropriate education, experience,
licensure, competence and specialized qualifications are assigned to provide nursing care
for each patient in accordance with the individual needs of each patient.
Survey Procedures §482.23(b)(5)
• Review the nursing assignments. Did an RN make the assignments? Determine
that the assignments take into consideration the complexity of patient’s care needs
and the competence and specialized qualifications of the nursing staff.
• Ask a charge nurse what considerations are necessary when making staff
assignments. Answers should include:
o Patient needs;
o Complexity of patients;
o Any special needs of individual patients;
o Competence of nursing personnel;
o Qualifications of nursing personnel;
o Education of nursing personnel; and
o Experience of nursing personnel.
A-0398
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.23(b)(6) - All licensed nurses who provide services in the hospital must adhere to
the policies and procedures of the hospital. The director of nursing service must
provide for the adequate supervision and evaluation of all nursing personnel which
occur within the responsibility of the nursing service, regardless of the mechanism
through which those personnel are providing services (that is, hospital employee,
contract, lease, other agreement, or volunteer).
Interpretive Guidelines §482.23(b)(6)
The hospital must ensure that there are adequate numbers of clinical nursing personnel to
meet its patients nursing care needs. In order to meet their patient’s needs the hospital may
supplement their hospital employed licensed nurses with volunteer and or contract licensed
nurses.
The hospital and the director of the nursing service are responsible for the clinical activities
of all nursing personnel regardless of whether they are hospital employees, contracted staff,
or volunteers.
All licensed nurses who are working at the hospital must adhere to the policies and
procedures of the hospital. The hospital and the director of the nursing service are
responsible for ensuring that licensed nursing personnel know the hospital’s policies and
procedures in order to adhere to those policies and procedures.
The hospital and the director of the nursing service ensure that nursing care staff person is
adequately supervised and that their clinical activities are evaluated. This supervision and
evaluation of the clinical activities of each non-employee nursing staff person must be
conducted by an appropriately qualified hospital-employed RN.
Survey Procedures §482.23(b)(6)
• Review the method for orienting all licensed nurses to hospital policies and
procedures. The orientation should include at least the following:
o The hospital and the unit;
o Emergency procedures;
o Nursing services policies and procedures; and
o Safety policies and procedures.
• Determine if all nursing personnel are appropriately oriented prior to providing care.
• Confirm with the director of nurses that all nurses performance are evaluated by the
hospital at least once a year. If the performance evaluation is not considered
confidential, review two evaluations.
A-0399
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)

§482.23(b)(7) - The hospital must have policies and procedures in place establishing
which outpatient departments, if any, are not required under hospital policy to have a
registered nurse present. The policies and procedures must:
(i) Establish the criteria such outpatient departments must meet, taking into
account the types of services delivered, the general level of acuity of patients served
by the department, and the established standards of practice for the services
delivered;
(ii) Establish alternative staffing plans;
(iii) Be approved by the director of nursing;
(iv) Be reviewed at least once every 3 years
Interpretive Guidelines §482.23(b)(7)
Guidance is pending and will be updated in future release.
A-0405
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.23(c) Standard: Preparation and Administration of Drugs.
(1) Drugs and biologicals must be prepared and administered in accordance with
Federal and State laws, the orders of the practitioner or practitioners responsible for
the patient’s care, and accepted standards of practice.
(i) Drugs and biologicals may be prepared and administered on the orders of other
practitioners not specified under §482.12(c) only if such practitioners are
acting in accordance with State law, including scope of practice laws, hospital
policies, and medical staff bylaws, rules, and regulations….
(2) All drugs and biologicals must be administered by, or under supervision of,
nursing or other personnel in accordance with Federal and State laws and regulations,
including applicable licensing requirements, and in accordance with the approved
medical staff policies and procedures.
Interpretive Guidelines §§482.23(c)(1), (c)(1)(i) and (c)(2)
According to the Institute of Medicine of the National Academies, medication errors are
among the most common medical errors, harming at least 1.5 million people each year.
4
It
has been estimated that drug-related adverse outcomes were noted in nearly 1.9 million
inpatient hospital stays (4.7 percent of all stays), and 838,000 treat-and-release ED visits
(0.8 percent of all visits).
5
Although technological advances in electronic order entry,
medication administration, and electronic medical records hold a great deal of promise for
decreasing medication errors, there are a multitude of human and environmental factors that
will impact their success. The increasing complexity of medical care and patient acuity
present significant challenges that require an approach to medication administration that
takes advantage of available technology while recognizing that it must be integrated into the
medication administration work processes in a manner that meets the needs of patients and
promotes their safety.
4
Institute of Medicine. Preventing Medication Errors. Washington DC: The National Academies Press, 2007.
5
Lucado, Jennifer, et al, Medication-Related Adverse Outcomes in U.S. Hospitals and Emergency
Departments. Statistical Brief #109, April, 2011. Healthcare Cost and Utilization Project, Agency For
Healthcare Research and Quality, Rockville, MD.
The regulations at §482.23(c) and §482.23(c)(1) promote safety in the preparation and
administration of drugs and biologicals to hospital patients by requiring preparation and
administration in accordance with:
• Federal and State law;
• Accepted standards of practice;
• Orders of the practitioner(s) responsible for the patient’s care, as permitted under
State law, hospital policy and medical staff bylaws, rules and regulations; and
• Medical staff-approved policies and procedures.
Federal and State Law
Federal law regulates the approval and classification of drugs and biologicals. Individual
States establish laws and regulations which specify the scope of practice for various types
of licensed healthcare professionals, including which medications they may prescribe and
administer, including controlled substances.
Accepted Standards of Practice
Hospital policies and procedures for the preparation and administration of all drugs and
biologicals must not only comply with all applicable Federal and State laws, but also must
be consistent with accepted standards of practice based on guidelines or recommendations
issued by nationally recognized organizations with expertise in medication preparation and
administration. Examples of such organizations include, but are not limited to:
• American Society of Health-System Pharmacists (http://www.ashp.org/default.aspx)
• Infusion Nurses Society (http://www.ins1.org)
• Institute for Safe Medication Practices (www.ismp.org)
• National Coordinating Council for Medication Error Reporting and Prevention
(www.nccmerp.org)
• U.S Pharmacopeia (www.usp.org)
Orders of an authorized practitioner
Drugs must be administered in response to an order from a practitioner, or on the basis of a
standing order which is appropriately authenticated subsequently by a practitioner. (See
§482.23(c)(1) (ii) concerning standing orders.) Generally, the ordering practitioner is the
practitioner(s) responsible for the care of the patient. However, other practitioners not
specified under §482.12(c) may write orders for the preparation and administration of drugs
and biologicals, if they are acting in accordance with State law, including scope of practice
laws, hospital policies and procedures, and medical staff bylaws, rules and regulations.
This includes practitioners ordering outpatient services who do not have privileges in the
hospital but who are permitted under their State scope of practice and authorized by
hospital and medical staff policy to order outpatient services.
In accordance with standard practice, all practitioner orders for the administration of drugs
and biologicals must include at least the following:
• Name of the patient;
• Age and weight of the patient, to facilitate dose calculation when applicable. Policies
and procedures must address weight-based dosing for pediatric patients as well as in
other circumstances identified in the hospital’s policies. (Note that dose calculations
are based on metric weight (kg, or g for newborns). If a hospital permits practitioners
to record weight in either pounds or using metric weight, the opportunity for error
increases, since some orders would require conversion while others would not.
Accordingly, hospitals must specify a uniform approach to be used by prescribing
practitioners. For example, a hospital could require all prescribers to use pounds or
ounces and have the electronic ordering system or the pharmacy convert to metric);
• Date and time of the order;
• Drug name;
•
Dose, frequency, and route;
• Dose calculation requirements, when applicable;
•
Exact strength or concentration, when applicable;
• Quantity and/or duration, when applicable;
• Specific instructions for use, when applicable; and;
• Name of the prescriber.
Medical Staff Approved Policies and Procedures
The hospital’s medical staff must approve policies and procedures for medication
administration, consistent with the requirements of Federal and State law and accepted
standards of practice. It is recommended that the medical staff consult with nurses,
pharmacists, Quality Assessment and Performance Improvement program staff, and others
in developing these policies and procedures. The adopted policies and procedures must
address key issues related to medication administration, which include but are not limited
to:
Personnel authorized to administer medication
§482.23(c)(2) requires that all drugs and biologicals are administered by, or under the
supervision of, nursing or other personnel, in accordance with Federal or State law and
approved medical staff policies and procedures. State law requirements include licensure
requirements. Policies and procedures must identify categories of licensed personnel and
the types of medications they are permitted to administer, in accordance with state laws.
The policies and procedures must also address education and training for all personnel
administering drugs and biologicals.
Medication administration education and training is typically included in hospital
orientation or other continuing education for nursing staff and other authorized healthcare
personnel. Training or continuing education topics regarding medication administration
may include but are not limited to the following:
• Safe handling and preparation of authorized medications;
• Knowledge of the indications, side effects, drug interactions, compatibility,
and dose limits of administered medications;
• Equipment, devices, special procedures, and/or techniques required for
medication administration;
Policies and procedures must address the required components of the training and if the
training provided during hospital orientation imparts sufficient education or whether
ongoing in-services or continuing education will be required to demonstrate competence.

Basic safe practices for medication administration
The hospital’s policies and procedures must reflect accepted standards of practice that
require the following be confirmed prior to each administration of medication (often
referred to as the “five rights” of medication administration practice):
• Right patient: the patient’s identity— acceptable patient identifiers include, but are
not limited to: the patient’s full name; an identification number assigned by the
hospital; or date of birth. Identifiers must be confirmed by patient wrist band, patient
identification card, patient statement (when possible) or other means outlined in the
hospital’s policy. The patient’s identification must be confirmed to be in agreement
with the medication administration record and medication labeling prior to medication
administration to ensure that the medication is being given to the correct patient.
• Right medication: the correct medication, to ensure that the medication being given to
the patient matches that prescribed for the patient and that the patient does not have a
documented allergy to it;
• Right dose: the correct dose, to ensure that the dosage of the medication matches the
prescribed dose, and that the prescription itself does not reflect an unsafe dosage level
(i.e., a dose that is too high or too low);
• Right route: the correct route, to ensure that the method of administration – orally,
intramuscular, intravenous, etc., is the appropriate one for that particular medication
and patient; and
• Right time: the appropriate time, to ensure adherence to the prescribed frequency and
time of administration.
NOTE: the “5 rights” focus specifically on the process of administering medications.
The medication process is generally recognized as consisting of five stages:
ordering/prescribing; transcribing and verifying; dispensing and delivering;
administering; and monitoring/reporting. Errors may occur in other components of the
process, even when there is strict adherence to the “5 rights” of medication
administration, for example when there has been a prescribing or a dispensing error.
Hospitals are also expected to comply with requirements under the Pharmaceutical
Services CoP at §482.25 and the patient safety requirements under the Quality
Assessment and Performance Improvement CoP at §482.21, using a comprehensive
systems approach to all components of the medication process.
For Information – Not Required/Not to be Cited
Recent literature
*
identifies up to nine “rights” of medication administration including:
Right patient
Right drug
Right route
Right time
Right dose
Right documentation
Right action (appropriate reason)
Right form
Right response

However, other sources refer to 8 or 10 “rights, and some of these topics, such as right action, appear to
involve prescribing and/or dispensing. Accordingly, there does not (yet) appear to be consensus about
expanding beyond the 5 “rights.”
*Reference: Elliott, M. and Lis, Y. (2010). The Nine Rights of Medication Administration: An Overview. British
Journal of Nursing, Vol. 19, 5, 300-305.
Hospitals are encouraged to promote a culture in which it is not only acceptable, but also
strongly encouraged, for staff to bring to the attention of the prescribing practitioner
questions or concerns they have regarding medication orders. Any questions about orders
for drugs or biologicals are expected to be resolved promptly, whether they arise prior to
the preparation, dispensing, or administration of the medication.
Hospitals must also ensure staff adherence to accepted standards of practice required to
prevent healthcare-associated infections related to medication preparation and/or
administration, including compounded sterile preparations (CSPs). Adherence to these
standards is assessed under the infection control CoP at 42 CFR 482.42.
A drug or biological is outdated after its expiration date, which is set by the manufacturer
based on stability testing under specified conditions as part of the U.S. Food and Drug
Administration’s (FDA) approval process. It should be noted that a drug or biological may
become unusable prior to its expiration date if it has been subjected to conditions that are
inconsistent with the manufacturer’s approved labeling.
A drug or biological is also outdated after its “beyond-use date” (BUD), which may be
reached before the expiration date, but never later. The BUD is the date and time after
which the medication must not be used, stored or transported. The BUD takes into account
the specific conditions and potential for deterioration and microbial growth that may occur
during or after the original container is opened, while preparing the medication for
dispensing and administration, and/or during the compounding process if it is a
compounded medication.
The BUD is to be based on information provided by the manufacturer, whenever such
information is available. The hospital must maintain and implement policies and
procedures that provide clear and consistent direction to pharmacy staff regarding how to
determine a BUD when complete BUD information is not available from the manufacturer.
Timing of Medication Administration
Appropriate timing of medication administration must take into account the complex nature
and variability among medications; the indications for which they are prescribed; the
clinical situations in which they are administered; and the needs of the patients receiving
them. The chemical properties, mechanism of action, or therapeutic goals of some
medications require administration at the exact time prescribed, or within a narrow window
of its prescribed scheduled time, to avoid compromising patient safety or achievement of
the intended therapeutic effect. However, the therapeutic effect of many other medications
is uncompromised by a much broader window of time for administration. Consequently,
the application of a uniform required window of time before or after the scheduled time for
the administration of all medications, without regard to their differences, could undermine
the ability of nursing staff to prioritize nursing care activities appropriately. This could also
result in staff work-arounds that jeopardize patient safety due to the imposition of
unrealistic or unnecessary time constraints for medication administration. Instead, hospital
policies and procedures must specifically address the timing of medication administration,
based on the nature of the medication and its clinical application, to ensure safe and timely
administration. The policies and procedures must address at least the following:
• Medications not eligible for scheduled dosing times;

• Medications eligible for scheduled dosing times;
• Administration of eligible medications outside of their scheduled dosing times and
windows; and
• Evaluation of medication administration timing policies, including adherence to them.
Medications or categories of medication not eligible for scheduled dosing times
The policies and procedures must identify medications or categories of medication which
are not eligible for scheduled dosing times, either in general or in specific clinical
applications. These are medications that require exact or precise timing of administration,
based on diagnosis type, treatment requirements, or therapeutic goals. The policies and
procedures must reflect consideration of factors including, but not limited to, the
pharmacokinetics of the prescribed medication; specific clinical applications; and patient
risk factors. Examples of medications that hospitals may choose to identify as not eligible
for scheduled dosing times may include, but are not limited to:
• Stat doses (immediate);
• First time or loading doses (initial large dose of a drug given to bring blood, tissue or
fluid levels to an effective concentration quickly);
• One-time doses; doses specifically timed for procedures;
• Time-sequenced doses; doses timed for serum drug levels;
• Investigational drugs; or
• Drugs prescribed on an as needed basis (prn doses).
The policies and procedures must ensure timely administration of such medications. In
addition they must specify if the policy for the administration of these medications will be
applied hospital-wide or only for specific diagnosis types, hospital units or clinical
situations.
Medications eligible for scheduled dosing times
Medications eligible for scheduled dosing times are those prescribed on a repeated cycle of
frequency, such as once a day, BID (twice a day), TID (three times a day), hourly intervals
(every 1, 2, 3 or more hours), etc. The goal of this scheduling is to achieve and maintain
therapeutic blood levels of the prescribed medication over a period of time. Medication
administration policies and procedures typically establish standardized dosing times for the
administration of all ‘scheduled’ medications. For example, medications prescribed for BID
(twice a day) administration might, under a given hospital’s policies and procedures, be
scheduled to be administered at 8am and 8pm. Another hospital might choose to schedule
BID medications at 7:30 am and 7:30 pm. Use of these standardized times facilitates the
medication administration process, e.g., by providing to the hospital’s pharmacy that
morning doses of all BID drugs must be dispensed and delivered to patient units in time for
the scheduled administration. For the nursing staff, the scheduled administration time might
prompt prioritization of additional activities that may be required, in the case of particular
drugs, such as vital sign assessment or the collection and review of blood work, to ensure
safe and timely medication administration.
Policies and procedures for medications eligible for scheduled dosing times must also
address: first dose medications, including parameters within which nursing staff are allowed
to use their own judgment regarding the timing of the first and subsequent doses, which
may fall between scheduled dosing times; retiming of missed or omitted doses; medications
that will not follow scheduled dosing times; and patient units that are not subject to
following the scheduled dosing times.
Time-critical scheduled medications
Time-critical scheduled medications are those for which an early or late administration of
greater than thirty minutes might cause harm or have significant, negative impact on the
intended therapeutic or pharmacological effect. Accordingly, scheduled medications
identified under the hospital’s policies and procedures as time-critical must be administered
within thirty minutes before or after their scheduled dosing time, for a total window of 1
hour.
It is possible for a given medication to be time- critical for some patients, due to diagnosis,
clinical situation, various risk factors, or therapeutic intent, but not time-critical for other
patients. Therefore, hospital policies and procedures must address the process for
determining whether specific scheduled medications are always time-critical, or only under
certain circumstances, and how staff involved in medication administration will know when
a scheduled medication is time-critical. Examples of time-critical scheduled
medications/medication types may include, but are not limited to:
• Antibiotics;
• Anticoagulants;
•
Insulin;
• Anticonvulsants;
• Immunosuppressive agents;
• Pain medication (non-IV);
• Medications prescribed for administration within a specified period of time of
the medication order;
• Medications that must be administered apart from other medications for
optimal therapeutic effect; or
• Medications prescribed more frequently than every 4 hours.
Non-time-critical scheduled medications
Non-time critical scheduled medications are those for which a longer or shorter interval of
time since the prior dose does not significantly change the medication’s therapeutic effect
or otherwise cause harm. For such medications greater flexibility in the timing of their
administration is permissible. Specifically:
Medications prescribed for daily, weekly or monthly administration may be within 2 hours
before or after the scheduled dosing time, for a total window that does not exceed 4 hours.
• Medications prescribed more frequently than daily but no more frequently than every
4 hours may be administered within 1 hour before or after the scheduled dosing time,
for a total window that does not exceed 2 hours.
Missed or late administration of medications
The hospital’s policies and procedures must address the actions to be taken when
medications eligible for scheduled dosing times are not administered within their permitted
window of time. This includes doses which may have been missed due to the patient being
temporarily away from the nursing unit, for example, for tests or procedures; patient
refusal; patient inability to take the medication; problems related to medication availability;
or other reasons that result in missed or late dose administration. Likewise, policies and
procedures must also outline guidelines for the administration and timing of new
medications which are initiated between standardized dosing times.
These policies and procedures must identify parameters within which nursing staff are
allowed to use their own judgment regarding the rescheduling of missed or late doses and
when notification of the physician or other practitioner responsible for the care of the
patient is required prior doing so. In either case, the reporting of medication errors that are
the result of missed or late dose administration must be reported to the attending physician
in accordance with requirements at §482.25(b)(6). See interpretive guidance at
§482.25(b)(6) for more details on internal reporting requirements
Evaluation of medication administration timing policies
Hospitals must periodically evaluate their medication administration timing policies,
including staff adherence to the policies, to determine whether they assure safe and
effective medication administration. Consistent with the QAPI requirements at 42 CFR
482.21(c)(2), medication errors related to the timing of medication administration must be
tracked and analyzed to determine their causes. Based on the results of the evaluations of
the policies and the medication administration errors, the medical staff must consider
whether there is a need to revise the policies and procedures governing medication
administration timing.
Assessment/Monitoring of Patients Receiving Medications
Observing the effects medications have on the patient is part of the multi-faceted
medication administration process. Patients must be carefully monitored to determine
whether the medication results in the therapeutically intended benefit, and to allow for early
identification of adverse effects and timely initiation of appropriate corrective action.
Depending on the medication and route/delivery mode, monitoring may need to include
assessment of:
• Clinical and laboratory data to evaluate the efficacy of medication therapy, to
anticipate or evaluate toxicity and adverse effects. For some medications, including
opioids, this may include clinical data such as respiratory status, blood pressure, and
oxygenation and carbon dioxide levels;
• Physical signs and clinical symptoms relevant to the patient’s medication therapy,
including but not limited to, somnolence, confusion, agitation, unsteady gait,
pruritus, etc.
Certain types of medications are considered inherently high risk for adverse drug events.
Although mistakes may or may not be more common with these drugs, the consequences of
errors are often harmful, sometimes fatal, to patients. (See also the discussion of high-risk
medications (typically referred to as “high-alert” medications) in the guidance for
§482.25(a)(1))
For Information – Not Required/Not to be Cited
The Institute for Safe Medication Practices (ISMP) makes available a list of high
alert medications, which it defines as those medications that bear a heightened risk
of causing significant patient harm when they are used in error. The current list may
be found at:
http://www.ismp.org/Tools/highAlertMedicationLists.asp
In addition, certain factors place some patients at greater risk for adverse effects of
medication. Factors including, but not limited to, age, altered liver and kidney function, a
history of sleep apnea, patient weight (obesity may increase apnea or smaller patients may
be more sensitive to dose levels of medications), asthma, history of smoking, drug-drug
interactions, and first-time medication use may contribute to increased risk.
Consideration of patient risk factors as well as the risks inherent in a medication must be
taken into account when determining the type and frequency of monitoring. Further, to
enhance continuity of care/safe medication administration, it is essential to communicate all
relevant information regarding patients’ medication risk factors and monitoring
requirements during hand-offs of the patient to other clinical staff, such as when patients are
transferred internally from one unit to another, during shift report at change of shift, etc.
This would apply to hand-offs involving not only to nursing staff, but also to any other
types of staff who administer medications, e.g., respiratory therapists.
Adverse patient reactions, such as anaphylaxis or opioid-induced respiratory depression,
require timely and appropriate intervention, per established hospital protocols, and must
also be reported immediately to the practitioner responsible for the care of the patient. (See
the guidance for §482.23(c)(5) and §482.25(b)(6), concerning reporting of adverse
medication-related events.)
An example of vigilant post-medication administration monitoring in the case of a high-
alert medication where patient factors may increase risk would be regularly checking vital
signs, oxygen level via pulse oximetry, and sedation levels of a post-surgical patient who is
receiving pain medication via a patient controlled analgesia (PCA) pump. Narcotic
medications, such as opioids, are often used to control pain but also have a sedating effect.
Patients can become overly sedated and suffer respiratory depression or arrest, which can
be fatal. Timely assessment and appropriate monitoring is essential in all hospital settings
in which opioids are administered, to permit intervention to counteract respiratory
depression should it occur. (See also the discussion of the requirements for intravenous
medications at §482.23(c)(4)).
As part of the monitoring process, staff are expected to include the patient’s reports of
his/her experience of the medication’s effects. Further, when monitoring requires
awakening the patient in order to assess effects of the medications, the patient and/or the
patient’s representative must be educated about this aspect of the monitoring process. In
addition, hospitals are encouraged to educate the patient and his/her representative and/or
family members about notifying nursing staff promptly when there is difficulty breathing or
other changes that might be a reaction to medication.
Hospital policies and procedures are expected to address how the manner and frequency of
monitoring, considering patient and drug risk factors, are determined, as well as the
information to be communicated at shift changes, including the hospital’s requirements for
the method(s) of communication.
Documentation
Note that documentation of medication administration is addressed in the Medical Records
CoP, at §482.24(c), which specifies the required content of the medical record. Within this
regulation §482.24(c)(vi) requires that the record contain: “All practitioners’ orders,
nursing notes, reports of treatment, medication records, radiology, and laboratory reports,
and vital signs and other information necessary to monitor the patient’s condition.”
Documentation is expected to occur after actual administration of the medication to the
patient; advance documentation is not only inappropriate, but may result in medication
errors. Proper documentation of medication administration actions taken and their
outcomes is essential for planning and delivering future care of the patient. See the
guidance for the various parts of §482.24(c) concerning documentation in the medical
record. Deficiencies in documentation would be cited under the applicable Medical
Records regulation.
Survey Procedures §§482.23(c)(1), (c)(1)(i), and (c)(2)
Verify that there is an effective method for the administration of drugs. Use the following
indicators for assessing drug administration:
• Verify that there are policies and procedures approved by the medical staff and
governing body concerning ordering of drugs and biologicals by practitioners.
• Verify that there are policies and procedures approved by the medical staff covering
who is authorized to administer medications, and that the policies are followed.
• Verify nursing staff authorized to administer drugs and biological are
practicing within their State-permitted scope of practice.
• Are personnel other than nursing personnel administering drugs or
biologicals? If yes, determine if those personnel are administering drugs or
biologicals in accordance with Federal and State laws and regulations,
including scope of practice laws, hospital policy, and medical staff by-laws,
rules and regulations. Use the above procedures to determine compliance.
• Verify that there are policies and procedures approved by medical staff addressing the
timing of medication administration.
• Verify that the hospital has, consistent with its policies, identified medications: which
are:
• not eligible for scheduled dosing times;
• Eligible for scheduled dosing times and are time-critical; and
• Eligible for scheduled dosing times and are not time-critical.
• Verify the hospital has established total windows of time that do not exceed the
following:
• 1 hour for time-critical scheduled medications;
• 2 hours for medications prescribed more frequently than daily, but no more
frequently than every 4 hours; and
• 4 hours for medications prescribed for daily or longer administration intervals.
• Verify that the hospital’s policy describes requirements for the administration of
identified time-critical medications. Is it clear whether time-critical medications or
medication types are identified as such for the entire hospital or are unit-, patient
diagnosis-, or clinical situation- specific?
• Review a sample of medical records to determine whether medication administration
conformed to an authorized practitioner’s order, i.e., that there is an order from an
authorized practitioner, or an applicable standing order, and that the correct medication
was administered to the right patient at the right dose via the correct route, and that
timing of administration complied with the hospital’s policies and procedures. Check
that the practitioner’s order was still in force at the time the drug was administered.
• Observe the preparation of drugs and their administration to patients [medication
pass] in order to verify that procedures are being followed
• Is the patient’s identity confirmed prior to medication administration?
• Are procedures to assure the correct medication, dose, and route followed?
• Are drugs administered in accordance with the hospital’s established policies
and procedures for safe and timely medication administration?
• Does the nurse remain with the patient until oral medication is taken?
• Are patients assessed by nursing and/or other staff, per hospital policy, for their
risk to their prescribed medications?
• Are patients who are at higher risk and/or receiving high-alert medications
monitored for adverse effects?
• Are staff knowledgeable about intervention protocols when patients experience
adverse medication-related events?
• Interview personnel who administer medication to verify their understanding of the
policies regarding timeliness of medication administration.
• Are they able to identify time-critical and non-time-critical scheduled
medications? Medications not eligible for scheduled dosing times?
• Are they able to describe requirements for the timing of administration of time
critical and non-time critical medications in accordance with the hospital’s
policies?
A-0406
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
§482.23(c)(1) (ii)– Drugs and biologicals may be prepared and administered on the
orders contained within pre-printed and electronic standing orders, order sets, and
protocols for patient orders only if such orders meet the requirements of §482.24(c)(3).
§482.23(c)(3) - With the exception of influenza and pneumococcal polysaccharide
vaccines, which may be administered per physician-approved hospital policy after an
assessment of contraindications, orders for drugs and biologicals must be documented and
signed by a practitioner who is authorized to write orders in accordance with State law and
hospital policy, and who is responsible for the care of the patient as specified under
§482.12(c)…
§482.23(c)(3)(iii) Orders for drugs and biologicals may be documented and signed by
other practitioners not specified under §482.12(c) only if such practitioners are acting
in accordance with State law, including scope of practice laws, hospital policies, and
medical staff bylaws, rules, and regulations.
Interpretive Guidelines §482.23(c)(1) (ii ),(c)(3) and (c)(3)(iii)
All orders for drugs and biologicals, with the exception of influenza and pneumococcal
vaccines, must be documented and signed by a practitioner who is responsible for the care
of the patient, as specified under §482.12(c), or who is another practitioner who is
authorized by hospital policy and medical staff bylaws, rules and regulations, and who is
acting in accordance with State law, including scope of practice laws.
Flu and pneumonia vaccines
Influenza and pneumococcal vaccines may be administered per physician-approved hospital
policy, i.e., hospital policy approved by the physician members of the medical staff. There
must be an assessment of contraindications prior to administration of the vaccine(s). There
is no requirement for authentication by a practitioner when influenza and pneumococcal
vaccines are administered to a patient in accordance with hospital policy and State law.
Standing orders
Nurses or other personnel authorized by hospital policy and in accordance with State law
may administer drugs and biologicals in accordance with pre-printed and electronic
standing orders, order sets, and protocols for patient orders, collectively referred to in this
guidance as “standing orders,” to address well- defined clinical scenarios involving
medication administration. The requirements governing the hospital’s development and
use of standing orders are found at the Medical Records CoP, under §482.24(c)(3). For the
nursing services requirement under§482.23(c)(1) (ii), compliance assessment focuses on
whether nurses comply with the hospital’s established standing orders policies and
procedures when administering drugs or biological in accordance with a standing order.
Survey Procedures §482.23(c)(1) (ii ), (c)(3) and (c)(3)(iii)
• Review the hospital’s policy for drug and biological orders. Does it require that all
administration of drugs or biologicals be based on either an applicable standing
order or the order of a practitioner who is responsible for the care of the patient or
otherwise authorized by hospital and medical staff policy and in accordance with
State law to write orders?
• Interview nursing staff to determine whether they initiate medications in accordance
with standing orders. Are they familiar with the hospital’s policies and procedures
for using standing orders? Are they following the policies and procedures? Ask to
see the protocol for a standing order used by nursing staff, and ask nursing staff to
explain how their practice conforms to the protocol.
• Review a sample of open and closed patient medical records. Although the
regulation applies to both inpatient and outpatient medical records, the sample
should be weighted to include more inpatient records.
• Determine whether all orders for drugs and biologicals, with the exception of
influenza and pneumococcal vaccines, are included in the patient’s medical record
and authenticated by a practitioner who is authorized to write orders by hospital and
medical staff policy and in accordance with State law and who is responsible for the
care of the patient.
• Determine whether all standing orders which were initiated by a nurse were
authenticated by an authorized practitioner.
• Determine whether all orders for drugs and biologicals contain the required
elements.
A-0407
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
§482.23(c)(3)(i) - If verbal orders are used, they are to be used infrequently.
Interpretive Guidelines §482.23(c)(3)(i)
Verbal orders, if used, must be used infrequently. This means that the use of verbal orders
must not be a common practice. Verbal orders pose an increased risk of miscommunication
that could contribute to a medication or other error, resulting in a patient adverse event.
Verbal orders should be used only to meet the care needs of the patient when it is
impossible or impractical for the ordering practitioner to write the order or enter it into an
electronic prescribing system without delaying treatment. Verbal orders are not to be used
for the convenience of the ordering practitioner. (71 FR 68679)
Hospitals are expected to develop appropriate policies and procedures that govern the use of
verbal orders and minimize their use, such as policies which:
• Describe situations in which verbal orders may be used as well as limitations or
prohibitions on their use;
• Provide a mechanism to establish the identity and authority of the practitioner
issuing a verbal order;
• List the elements required for inclusion in the verbal order process;
● Establish protocols for clear and effective communication and verification of verbal
orders.
The content of verbal orders must be clearly communicated. CMS expects nationally
accepted read-back verification practice to be implemented for every verbal order. (71 FR
68680) As required by §482.24(b), all verbal orders must be promptly documented in the
patient’s medical record by the individual receiving the order.
Survey Procedures §482.23(c)(3)(i)
• Are there policies and procedures in place to minimize the use of verbal orders?
• Interview direct care staff to determine whether actual practice is consistent with
verbal order policies and procedures.
• Review both open and closed patient medical records for the use of verbal orders.
• Were the policies and procedures for the use of verbal orders followed?
• Does the number of verbal orders found in the sampled records suggest routine use,
which the regulations do not permit? The number of verbal orders is not in itself
evidence of noncompliance, but should result in more focused analysis. For
example:
• Is there a pattern to the use of verbal orders? Some patterns might make sense –
e.g., for orders entered between midnight and 7:00 a.m., it might be plausible
that it was impossible for the prescribing practitioner to write/computer-enter the
order. On the other hand, if one patient care unit has a high proportion of verbal
orders, while another does not, this might be a flag for inconsistent
implementation of the hospital’s policies and procedures for verbal orders.
• Are verbal orders used frequently for certain types of situations, and if so, is it
reasonable to assume that it is impossible or impractical for the prescribing
practitioners to write/enter the orders in such situations?
• Do certain practitioners use verbal orders frequently? From the limited number
of records sampled it may be difficult to detect trends related to specific
practitioners, but if a surveyor finds such evidence, further investigation is
warranted to determine if it is evidence of noncompliance.
A-0408
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
§482.23(c)(3)(ii) - When verbal orders are used, they must only be accepted by persons
who are authorized to do so by hospital policy and procedures consistent with Federal
and State law.
Interpretive Guidelines §482.23(c)(3)(ii)
A verbal order for drugs and biologicals may only be accepted by an individual who is
permitted by Federal and State law and hospital policy to accept verbal orders. Consistent
with the requirements of §482.24(b), the person who received the verbal order must
promptly document it in the medical record.
Survey Procedures §482.23(c)(3)(ii)
• Determine whether the hospital has policies and procedures, consistent with Federal
and State law, governing who is authorized to accept verbal orders.
• Review open and closed patient medical records containing verbal orders for drugs
and biologicals. Determine whether the orders were accepted and documented by
authorized hospital personnel.
• Interview several direct care staff to determine if they are permitted to take verbal
orders for drugs and biologicals, and determine whether such staff have been
authorized to do so in accordance with hospital policy.
A-0409
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.23(c)(3)(iii) - Orders for drugs and biologicals may be documented and signed by
other practitioners, only if such practitioners are acting in accordance with State law,
including scope of practice laws, hospital policies, and medical staff bylaws, rules, and
regulations.
Interpretive Guidelines §482.23(c)(3)(iii)
All orders for drugs and biologicals, with the exception of influenza and pneumococcal
vaccines, must be documented and signed by a practitioner who is responsible for the care
of the patient or who is another practitioner who is authorized by hospital policy and
medical staff bylaws, rules and regulations, and who is acting in accordance with State law,
including scope of practice laws.
Flu and pneumonia vaccines
Influenza and pneumococcal vaccines may be administered per physician-approved hospital
policy, i.e., hospital policy approved by the physician members of the medical staff. There
must be an assessment of contraindications prior to administration of the vaccine(s). There
is no requirement for authentication by a practitioner when influenza and pneumococcal
vaccines are administered to a patient in accordance with hospital policy and State law.
Standing orders
Nurses or other personnel authorized by hospital policy and in accordance with State law
may administer drugs and biologicals in accordance with pre-printed and electronic
standing orders, order sets, and protocols for patient orders, collectively referred to in this
guidance as “standing orders,” to address well- defined clinical scenarios involving
medication administration. The requirements governing the hospital’s development and
use of standing orders are found at the Medical Records CoP, under §482.24(c)(3). For the
nursing services requirement under§482.23(c)(1) (ii), compliance assessment focuses on
whether nurses comply with the hospital’s established standing orders policies and
procedures when administering drugs or biological in accordance with a standing order.
Survey Procedures §482.23(c)(3)(iii)
• Review the hospital’s policy for drug and biological orders. Does it require that all
administration of drugs or biologicals be based on either an applicable standing
order or the order of a practitioner who is responsible for the care of the patient or
otherwise authorized by hospital and medical staff policy and in accordance with
State law to write orders?
• Interview nursing staff to determine whether they initiate medications in accordance
with standing orders. Are they familiar with the hospital’s policies and procedures
for using standing orders? Are they following the policies and procedures? Ask to
see the protocol for a standing order used by nursing staff, and ask nursing staff to
explain how their practice conforms to the protocol.
• Review a sample of open and closed patient medical records. Although the
regulation applies to both inpatient and outpatient medical records, the sample
should be weighted to include more inpatient records.
• Determine whether all orders for drugs and biologicals, with the exception of
influenza and pneumococcal vaccines, are included in the patient’s medical record
and authenticated by a practitioner who is authorized to write orders by hospital and
medical staff policy and in accordance with State law and who is responsible for the
care of the patient.
• Determine whether all standing orders which were initiated by a nurse were
authenticated by an authorized practitioner.
• Determine whether all orders for drugs and biologicals contain the required
elements.
A-0410
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.23(c)(4) - Blood transfusions and intravenous medications must be administered
in accordance with State law and approved medical staff policies and procedures.
Interpretive Guidelines §482.23(c)(4)
Intravenous (IV) medications and blood transfusions must be administered in accordance
with State law and approved medical staff policies and procedures. Further, many of the
medications included in the high-alert categories are administered intravenously. (See also
the discussion of high-risk/high-alert medications in the guidance for §482.25(b).) Hospital
policies and procedures for blood transfusions and IV medications must be based on
accepted standards of practice, and must address at least the following:
Vascular Access Route
Patients may require a form of vascular access to deliver blood or medications, either
venous or arterial, based on the desired treatment plan. Safe administration of blood
transfusions and IV medications includes the correct choice of vascular access. IV
medications, such as fluids, antibiotics, and chemotherapy, may require specific types of
access, such as peripheral or central catheters versus implanted port devices, based on the
medication’s chemical properties or safety concerns. Hospital policies and procedures must
address which medications can be given intravenously via what type of access.
Other Patient Safety Practices
In addition to the basic safe practices that apply to all medication administration (See the
discussion of safe medication administration practices, and medication administration in
general, at §482.23(c)), there are additional safe practices specific to IV medication
administration that require consideration, including but not limited to, the following:
• Tracing invasive lines and tubes prior to administration to ensure the medication is to be
administered via the proper route (for example, peripheral catheter versus epidural
catheter connections);
• Avoiding forcing connections when the equipment offers clear resistance;
• Verifying proper programming of infusion devices (concentrations, flow rate, dose
rate).
Patient Monitoring
As discussed in the medication administration guidance for §§482.23(c)(1), (c)(1)(i) and
(c)(2), patients must be monitored for the effects of medications. To the extent that IV
medications have a more rapid effect on the body, it is important that staff administering
medications understand each medication and its monitoring requirements. Policies and
procedures for IV medication administration must address appropriate IV medication
monitoring requirements, including assessment of patients for risk factors that would
influence the type and frequency of monitoring.
For example: a 50 year old patient with a history of renal failure is receiving IV
vancomycin to treat a wound infection. The hospital policy for IV antibiotics, including
vancomycin, requires the patient’s kidney function to be monitored daily with blood
draws. Based on review of the lab results, a practitioner responsible for the care of the
patient would be expected to determine on a timely basis whether or not the antibiotic
dose needs to be adjusted to protect kidney function or prevent drug toxicity while
achieving the desired therapeutic effects. Staff administering the medication would be
expected to review the lab results as well, and to raise with a practitioner responsible for
the care of the patient any concerns they might have about whether an adjustment in the

medication is needed.
Hospital policies and procedures related to monitoring patients receiving IV medications
are expected to address, but are not limited to, the following:
• Monitoring for Fluid & Electrolyte Balance
Whenever IV medications and blood transfusions are administered, the patient may
become at risk for fluid and electrolyte imbalance. Hospital policies and procedures
must address monitoring and treatment for fluid and electrolyte imbalances that may
occur with blood transfusions and IV medications.
• Monitoring Patients Receiving High-alert Medications, Including IV Opioids
Policies and procedures related to IV medication administration must address those
medications the hospital has identified as high-alert medications and the monitoring
requirements for patients receiving such drugs intravenously.
At a minimum, hospitals are expected to address monitoring for over-sedation and
respiratory depression related to IV opioids for post-operative patients
Opioids are a class of medication used frequently in hospitals to treat pain. The
sedating effects of opioids make it difficult at times to properly assess the patient’s level
of sedation. It can be erroneously assumed that patients are asleep when they are
actually exhibiting progressive symptoms of respiratory compromise - somnolence,
decreased respiratory rate, and decrease in oxygen levels. These symptoms, if
unrecognized, can progress to respiratory depression and even death.
Certain characteristics, in addition to those discussed in the medication administration
guidance for §§482.23(c)(1), (c)(1)(i) and (c)(2), place patients receiving opioids at
higher risk for oversedation and respiratory depression. These additional factors
include, but are not limited to
6
:
• Snoring or history of sleep apnea
• No recent opioid use or first-time use of IV opioids
• Increased opioid dose requirement or opioid habituation
• Longer length of time receiving general anesthesia during surgery
• Receiving other sedating drugs, such as benzodiazepines, antihistamines, sedatives,
or other central nervous system depressants
• Preexisting pulmonary or cardiac disease
• Thoracic or other surgical incisions that may impair breathing
Of particular concern are patients receiving IV opioids post-operatively. The
effects of IV opioids in post-operative patients must be monitored vigilantly via serial
assessments of pain, respiratory status, and sedation levels.
Hospitals must have policies and procedures related to the use of high-alert medications,
including IV opioids for post-operative patients. Policies and procedures must address,
at a minimum, the process for patient risk assessment, including who conducts the
assessments, and, based on the results of the assessment, monitoring frequency and
duration, what is to be monitored, and monitoring methods. The policies and
procedures must also address whether and under what circumstances practitioners
6
Jarzyna D., Junquist C., Pasero C., et al. American Society for Pain Management Nursing - Guidelines on
Monitoring for Opioid-Induced Sedation and Respiratory Depression. Pain Management Nursing, Vol 12,
No. 3 (September), 2011: pp 118-145
prescribing IV opioids are allowed to establish protocols for IV opioid administration
and monitoring that differ from the hospital-wide policies and procedures.
The frequency of the serial assessments and duration of the monitoring timeframe for
post-operative patients receiving IV opioids must be determined based on at least the
following considerations:
• Patient risk for adverse events;
• Opioid dosing frequency and IV delivery method. (push or patient-controlled
analgesia (PCA));
• Duration of IV opioid therapy.
Regardless of the above factors, at a minimum monitoring must include the following:
• Vital signs (blood pressure, temperature, pulse, respiratory rate)
• Pain level;
• Respiratory status;
• Sedation level; sedation levels are important indicators for the clinical effects of
opioids. Sedation is a useful assessment parameter to observe the effects of opioids
since sedation typically precedes respiratory depression
7
. See the blue box below
for information on sedation assessment methods.
In addition to vigilant nursing assessment at appropriate intervals, hospitals may choose to
use technology to support effective monitoring of patients’ respiratory rate and oxygen
levels.
For additional information regarding recommendations of expert organizations on post-
operative opioid monitoring, including technology-supported monitoring, see blue boxes
below. The practices described in the blue boxes below are not required under the
regulations.
The assessment and monitoring process must be explained to the patient and/or the patient's
representative, to communicate the rationale for vigilant monitoring, including that it might
be necessary to awaken the patient in order to assess effects of the medications. In
addition, hospitals are encouraged to educate the patient and his/her representative and/or
family members about notifying nursing staff promptly when there is difficulty breathing or
other changes that might be a reaction to medication.
7
Institute for Safe Medication Practices (ISMP), Medication Safety Alert – Fatal PCA Adverse Events
Continue to Happen…Better Patient Monitoring is Essential to Prevent Harm. May 30, 2013
For Information – Not Required/Not to be Cited
In addition to assessing risk for respiratory depression, the Institute for
Safe Medication Practices recommends hospitals use a standard sedation
scale when assessing patients receiving PCA. Scales such as the Richmond
Agitation Sedation Scale, Pasero, Ramsey, or Glasgow Coma Scale are
useful in assessing sedation.
Institute for Safe Medication Practices (ISMP), Medication Safety Alert – Fatal PCA Adverse
Events Continue to Happen…Better Patient Monitoring is Essential to Prevent Harm. May 30, 2013
For Information – Not Required/Not to be Cited
Institute for Safe Medication Practices Guidelines for PCA Monitoring
Assessment of Opioid
Tolerance
Vital
Signs
Pain
Sedation
Respiratory
Rate
Quality
SPO
2
*
&/or
ETCO
2
**
Baseline Assessment
before PCA
X
X
X
X
X
X
PCA Initiation or
Change in Drug/Syringe
Q 15 minutes x 1
hour
Q 1 hour x 4 hours
Then Q 2 hours
X
X
X
X
X
X
PCA Dose Change or
Bolus
Q 1 hour x 4 hours
Then Q 2 hours
X
X
X
X
X
X
Adverse Event or
Patient Deterioration
(e.g., adverse change in
sedation score)
Q 15 minutes x 1
hour
Q 1 hour x 4 hours
Then Q 2 hours
X
X
X
X
X
X
Hand-offs/Shift Change
X
X
X
X
X
X
Institute for Safe Medication Practices (ISMP), Medication Safety Alert – Fatal PCA
Adverse Events Continue to happen…Better Patient Monitoring is Essential to Prevent
Harm. May 30, 2013 ISMP adapted these recommendations from the San Diego
Patient Safety Council
* SPO
2
: Saturation of peripheral oxygen via pulse oximetry
** ETCO
2
: End-tidal carbon dioxide via capnography
For Information – Not Required/Not to be Cited
The Patient Safety Movement Foundation
PSMF recommends all patients receiving IV opioids have continuous measure-
through motion and low perfusion pulse oximetry, and that patients on
supplemental oxygen also have continuous respiration rate monitoring. It also
calls for the monitoring system to be linked with a notification system to clinical
staff who can respond immediately. It calls for an escalation protocol so that if a
staff person does not acknowledge the alert in 60 seconds a second person will be
notified.
The Patient Safety Movement Foundation - Actionable Patient Safety Solution
(APSS) #1: Failure to Rescue: Post-Operative Respiratory Depression. January 13,
2013
Adverse patient reactions require timely and appropriate intervention, per established
protocols, and must also be reported immediately to the practitioner responsible for the care
of the patient. (See the guidance for §482.23(c)(5) and §482.25(b)(6), concerning reporting
of adverse medication-related events.)
Blood Components and Blood Administration Procedures
According to the U.S. Department of Health and Human Services, 13,785,000 units of
whole blood and red blood cells were transfused in the United States in 2011
8
. The
collection, testing, preparation, and storage of blood and blood components are regulated by
the Food and Drug Administration. However, administration of blood products via
transfusion is governed by §482.23(c)(4). Blood transfusions can be life-saving. However,
8
The 2011 National Blood Collection and Utilization Survey Report. Retrieved September 27,2013 from
http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf
For Information – Not Required/Not to be Cited
Anesthesia Patient Safety Foundation
• APSF calls for every patient receiving postoperative opioid analgesics to be managed
based on the following clinical considerations*:
• Individualize the dose and infusion rate of opioid while considering
the unique aspects of each patient’s history and physical status.
• Make continuous monitoring of oxygenation (pulse oximetry) the
routine rather than the exception.
• Assess the need for supplemental oxygen, especially if pulse
oximetry or intermittent nurse assessment are the only methods of
identifying progressive hypoventilation.
• When supplemental oxygen is indicated, monitoring of ventilation
may warrant the use of technology designed to assess breathing or
estimate arterial carbon dioxide concentrations. Continuous
monitoring is most important for the highest risk patients, but
depending on clinical judgment, should be applied to other
patients.
APSF also has issued a video on opioid induced ventilatory impairment:
http://apsf.org/resources_video4.php
*Stoelting, RK.,Weinger MB. Dangers of postoperative opioids: Is there a Cure? APSF Newsletter
2009;24:2.
like IV medications, blood transfusions are not without risk of harm to patients.
Transfusion reactions and/or errors can be fatal.
In addition to the safe practices and other safety considerations that apply to all IV
medication administration, policies and procedures must address blood administration
procedures that are consistent with accepted standards of transfusion practice, including but
not limited to:
• Confirming the following prior to each blood transfusion:
• the patient’s identity
• verification of the right blood product for the right patient
The standard of practice calls for two qualified individuals, one of whom will be
administering the transfusion, to perform the confirmation.
• Requirements for patient monitoring, including frequency and documentation of
monitoring
• How to identify, treat, and report any adverse reactions the patient may experience
during or related to transfusion.
Staff Training and Competencies
Intravenous (IV) medications and blood transfusions must be administered by qualified
personnel, regardless of whether they are practitioners or non-practitioners. Generally IV
medications and blood transfusions are administered to patients by registered nurses (RNs),
consistent with State law governing scope of practice, and approved medical staff policies
and procedures.
Among other things, personnel must be able to demonstrate competency in venipuncture, in
accordance with State law and hospital policy. If other types of vascular access are utilized,
staff must have demonstrated competency in appropriate usage, care, and maintenance.
Staff must also be trained in early detection of and timely intervention for IV opioid-
induced over-sedation and respiratory depression.
Education and training regarding these procedures are typically included in the nurse’s
hospital orientation. Nursing staff who receive training for intravenous medication
administration and/or blood transfusion administration during hospital orientation or during
other continuing education programs would meet the requirements of this regulation.
Content of the training must address each required component of the approved medical staff
policies and procedures.
Other non-practitioners, for example, licensed practical nurses or licensed vocational
nurses, with demonstrated competence may also administer IV medications and blood
transfusions if they are acting in accordance with State law, including scope of practice law,
and the hospital’s approved medical staff policies and procedures. (77 FR 29050, May 16,
2012)
For non-practitioners, the appropriate competencies must be documented in the qualified
staff person’s employee record.
All State law and scope of practice requirements must be met regarding the administration
of intravenous medications and blood transfusions, as applicable.
Survey Procedures §482.23(c)(4)
• Interview nursing staff on different units who administer IV medications and blood
transfusions. Are staff knowledgeable with respect to:
• Venipuncture techniques;
• Safe medication administration practices, including general practices applying to
all types of medications and practices concerning IV tubing and infusion
pumps;
• Maintaining fluid and electrolyte balance;
• Patient assessment for risk related to IV medications and appropriate
monitoring;
• Early detection and intervention for IV opioid-induced respiratory depression in
post-operative patients;
• With respect to blood transfusions:
• Blood components;
• Process for verification of the right blood product for the right patient;
and
• Transfusion reactions: identification, treatment, and reporting
requirements.
• Review the files for a sample of staff who administer blood products and IV
medications, for evidence that competency was assessed and training was provided as
appropriate.
• If able, observe blood transfusion and IV medication administration to assess staff
adherence to accepted standards of practice.
o Were safe medication administration practices used?
o Was the transfused patient correctly identified and matched to the correct blood
product prior to administration?
o Was the appropriate access used for IV medications?
o Were appropriate steps taken with regard to IV tubing and infusion pumps?
o Are patients being monitored post-infusion for adverse reactions?
• If staff appear to not be following accepted standards of practice for patient risk
assessment related to IV medications, particularly opioids, and appropriate monitoring
of patients receiving IV medications and/or blood transfusions, review policies and
procedures for IV medication administration and blood transfusion to determine if they
address safe practices considerations.
• Review a sample of medical records.
o Are blood transfusions and IV medications administered in accordance with
State law and approved medical staff policies and procedures?
• Are blood transfusions and IV medications administered by personnel who are
working within their scope of practice in accordance with State law and
approved medical staff policies?
A-0411
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.23(c)(5) - There must be a hospital procedure for reporting transfusion reactions,
adverse drug reactions, and errors in administration of drugs.
Interpretive Guidelines §482.23(c)(5)
Adverse drug reactions and drug administration errors
There is a similar but more detailed and prescriptive requirement concerning internal
hospital reporting of adverse drug reactions, drug administration errors and
incompatibilities under the Pharmaceutical Services CoP at §482.25(b)(6). Therefore, it is
not necessary for hospitals to establish a different procedure in the case of adverse drug
reactions and drug administration errors for such events when nurses administer drugs or
transfusions. Consult the guidance for §482.25(b)(6) to see what must be reported, to
whom, and in what timeframe. Failure to make required reports concerning adverse drug
reactions and errors in administration of drugs should be cited under §482.23(c)(5) when
the drug was administered by a nurse, as well as under §482.25(b)(6).
Transfusion reactions
Transfusion reactions can occur during or after a blood transfusion. A patient’s immune
system recognizes the foreign blood product and attempts to destroy the transfused cells.
Incompatible blood products are typically the cause of transfusion reactions. Symptoms
may include back pain, bloody urine, hives, chills, fainting, dizziness, fever, flank pain, and
skin flushing. More serious complications may include acute kidney failure, anemia,
respiratory distress, shock and even death.
Transfusion reactions are serious and can be life-threatening. The hospital must have
policies and procedures in place for the internal reporting of transfusion reactions. The
policies must include procedures for reporting transfusion reactions immediately to the
practitioner responsible for the care of the patient. The transfusion reaction must also be
reported to the hospital-wide quality assessment performance improvement program as an
adverse event, in accordance with the QAPI CoP at 42 CFR 482.21(c)(2). The transfusion
reaction must be documented in the patient’s medical record, including the prompt
notification of the responsible practitioner.
Survey Procedures §482.23(c)(5)
• For adverse drug events and medication administration errors, follow the survey
procedures for §482.25(b)(6). Deficiencies are to be cited under both
§482.23(c)(5)and §482.25(b)(6) when the drug or transfusion related to an adverse
drug reaction, transfusion reaction or medication administration error relates to a
drug or transfusion administered by a nurse.
• Request the hospital policy and procedure for internal reporting of transfusion
reactions.
• Interview nursing staff responsible for administering blood transfusions to
determine whether they are familiar with and comply with the hospital’s policies.
• Ask to see if there are any transfusion-related incident reports. Is there evidence
that the transfusion reaction was reported immediately to the practitioner
responsible for the patient’s care? Was it reported to the hospital’s QAPI program?
A-0412
(Rev. 116, Issued: 06-06-14 Effective: 06-06-14, Implementation 06-06-14)
§482.23(c)(6) The hospital may allow a patient (or his or her caregiver/support person
where appropriate) to self -administer both hospital-issued medications and the
patient’s own medications brought into the hospital, as defined and specified in the
hospital’s policies and procedures.
(i) If the hospital allows a patient to self-administer specific hospital-issued
medications, then the hospital must have policies and procedures in place to:
(A) Ensure that a practitioner responsible for the care of the patient has issued an
order, consistent with hospital policy, permitting self-administration.
(B) Assess the capacity of the patient (or the patient’s caregiver/support person where
appropriate) to self-administer the specified medication(s).
(C) Instruct the patient (or the patient’s support person where appropriate) in the safe
and accurate administration of the specified medication(s).
(D) Address the security of the medication(s) for each patient.
(E) Document the administration of each medication, as reported by the patient (or the
patient’s caregiver/support person where appropriate), in the patient’s medical
record.
Interpretative Guidelines §482.23(c)(6)(i)
Hospitals have the option of establishing a program for self-administration by patients, or,
when applicable, patient caregivers or support persons, of hospital-issued medications. The
existence of this regulatory option does not mean that a hospital must offer medication self-
administration programs or that a patient has a right to self-administer their medications.
A hospital program for patient self-administration of hospital-issued medications could be
beneficial for the appropriate patients if the proper precautions are taken in designing and
implementing such a program. Generally such a program would apply only to inpatients,
but there may be circumstances under which a hospital finds it appropriate to permit self-
administration of hospital-issued medications by outpatients or their caregivers/support
persons.
Among the potential benefits of medication self-administration, teaching patients or their
caregivers/support persons adherence to the proper medication regimen could reduce
hospital inpatient length of stay and also might have a positive effect on continued
compliance with the regimen after discharge, potentially avoiding an emergency department
visit or inpatient readmission secondary to post-hospital patient medication administration
errors and noncompliance.
Hospitals have the discretion to establish policies providing for different levels of patient
self-administration, and may make these levels across-the-board, patient-specific, or
medication-specific. For example, a hospital may choose whether or not a nurse must be
present to supervise the self-administration, and whether this supervision requirement could
vary according to the type of medication or the capacity of the individual patient (or the
patient’s caregiver/support person). A hospital may also determine through its policies and
procedures whether supervision requirements must be addressed in the practitioner’s order
or whether this may be left to the discretion of the nurse who assesses the patient. A
hospital may choose to exclude certain medications from patient self-administration, for
example, because they pose too great a medication security challenge, or because the
manner in which they must be administered does not lend itself to safe self-administration.
(77 FR 29052, May 16, 2012) It must be clear in the hospital’s policies and procedures
whether it has established such a policy and what kind of limitations it has established for
its program of patient self-administration of hospital-issued medications.
It is expected that the medical staff, nursing and pharmacy departments are to collaborate in
developing policies and procedures governing self-administration of hospital-issued
medications which are approved by the governing body.
Required elements of a self-administration program:
If the hospital chooses to develop programs for self-administration of hospital-issued
medications by patients (and/or their caregiver/support persons), the following must be in
place:
• An order allowing the patient to administer hospital-issued medications. The
order must be consistent with the hospital’s policy concerning self-administration of
hospital-issued medications and be written by a practitioner who is responsible for
the care of the patient and who is authorized to order medications, in accordance
with hospital policies and procedures, State law, including scope of practice laws,
and medical staff by-laws, rules, and regulations.
• A documented assessment of the capacity of the patient (or their
caregiver/support person) to successfully administer medications for which
self-administration has been authorized. Nurses are expected to exercise their
clinical judgment and to inform the practitioner responsible for the care of the
patient about any reservations the nurse might have about an individual patient’s (or
caregiver/support person’s) capacity to safely self-administer medications. The
assessment must be documented and must highlight the findings that are affirmative
– i.e., support patient-self-administration – and negative – i.e., call into question
patient self-administration. The nurse is also expected to document any discussions
with the practitioner responsible for the care of the patient regarding the nurses’
concerns about patient’s (or caregiver/support person’s) capacity to safely self-
administer medications. Hospitals may, as a matter of policy, permit a nurse to
return to nurse administration for particular doses of a medication for which there is
a self-administration order, without a discussion with the responsible practitioner if,
based on the nurse’s assessment, the patient’s capacity has been temporarily
diminished and there is no caregiver/support person who is assisting the patient with
self-administration of medication. For example, a patient who has just had an
invasive test or procedure may not be fully alert for a period thereafter, or the parent
of a minor patient, who is administering medications to the patient may for whatever
reasons not be available and a scheduled medication dose is close to being overdue.
• Instruction in self-administration. As part of the assessment of the patient’s self-
administration capacity, nurses are expected to identify the patient’s (or the patient’s
caregiver/support person’s) education and/or training needs. These needs may be
related to type of medication, unique individual medication requirements, delivery
route, dosage and scheduling, equipment (e.g. syringes, pill-cutters, measuring
containers, etc.) intravenous access, potential adverse side effects and what to do if
they occur, infection control measures, storage, medication disposal, among
others. Education and training needs, and how they were addressed, must be
documented in the medical record.
• Security of the self-administered medications. The security of a patient’s self-
administered medications is extremely important, but does not lend itself well to a
one-size-fits-all regulatory requirement. There are Federal and State laws, including
the Pharmaceutical Services CoP, which require a higher level of security for certain
medications (for example, controlled substances). Hospitals are expected to comply
with these already-established requirements and laws, and generally should not
include such medications as part of a patient self-administration program.
• Note that Patient-controlled Analgesia (PCA) pumps are a special variant of patient
self-administration. Such pumps allow patients, within tightly controlled, pre-
determined parameters with respect to dosage and minimum time intervals between
doses, to release an intravenous dose of a controlled substance pain medication that
has been pre-loaded into the PCA pump in a manner that prevents tampering by an
unauthorized person. PCA pumps are considered secure despite their use of
controlled substances.
PCA pumps allow for the self-administration of intravenous (IV) medications to
patients. See the interpretive guidelines for §482.23(c)(4) concerning assessment and
monitoring requirements for post-surgical patients receiving IV opioids, including via
patient-controlled analgesia (PCA) pumps, in and out of the post-anesthesia care and
intensive care units.
Hospitals are also free to exclude other medications besides controlled substances from
their patient self-administered medication programs when the hospital has concerns
over its capacity to address the safety and security of these other medications for
patients.
A hospital may choose to have a policy where it maintains a list of medications that it
excludes from self-administration entirely, due to security concerns. It may choose to
have a policy that addresses the security of a particular medication on a patient-by-
patient basis. Or it may establish a policy that is a combination of both of these
approaches to medication security. (77 FR 29052, May 16, 2012)
• Documentation of medication administration. Under the regulation, a nurse must
document the self-administration of a medication. In cases where the nurse directly
supervised the self-administration, the nurse is expected to indicate that the
medication administration was observed and confirmed. On the other hand, where
direct nurse supervision is not required, the nurse is required to document only what
the patient, or the patient’s caregiver/support person, reports to the nurse as to the
time and amount of medication administered. Nurses are expected to assess whether
the reports of the patient or patient’s caregiver/support person indicate, with respect
to timing and dosage, that the patient is receiving the medication as ordered.
Survey Procedures §482.23(c)(6)(i)
If the hospital permits patient self-administration of hospital-issued medications:
• Ask the hospital to identify current inpatients for whom self-administration of
hospital-issued medications is permitted.
• Interview of several of these patients (or their caregivers/support persons when
applicable) to verify that they received instruction on how to administer their
medications
• Interview nurses caring for the selected patients. Ask them:
• What the applicable hospital policies and procedures are regarding supervision
of self-medication.
• How they assess a patient’s (or patient’s caregiver/support person’s) capacity to
self-administer medication. If they have concerns, how do they communicate
them to the responsible practitioner? Does their hospital permit nurses to return
to nurse administration of medications in response to temporary reduction in
patient capacity or absence of the patient’s caregiver/support person? If so, how
do the nurses make this assessment?
• How they instruct a patient (or patient’s caregiver/support person’s) in
medication self-administration.
• How self-administered medications are secured.
• How they document self-administration of medications.
• To provide a copy of the hospital’s policies and procedures. Are they following
the policies and procedures?
• Review the medical records for the selected patients. Is there documentation of:
• An order for self-administration of specific medication(s).
• A nurse assessment of the patient’s (or patient’s caregiver/support person’s)
capacity to self-administer medication.
• Documentation of nurse instruction to the patient or (or patient’s
caregiver/support person) in safe and appropriate techniques for self-
administration of medication.
• Documentation of self-administration times and doses, as reported by the patient
or (or patient’s caregiver/support person) or directly observed by a nurse.
• Do the hospital’s policies and procedures for self-administration of hospital-issued
medications address:
• Limitations on medications not eligible for self-administration or patient
conditions which exclude self-administration;
• Orders for self-administration of medication;
• Requirements, if any, for supervision of self-administration;
• Assessment of self-medication capacity;
• Instruction in self-medication;
• Security of self-administered medications; and
• Documentation of self-administration.
A-0413
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
[§482.23(c)(6) The hospital may allow a patient (or his or her caregiver/support
person where appropriate) to self -administer both hospital-issued medications and
the patient’s own medications brought into the hospital, as defined and specified in the
hospital’s policies and procedures.]
§482.23(c)(6)(ii) If the hospital allows a patient to self-administer his or her own
specific medications brought into the hospital, then the hospital must have policies and
procedures in place to:
(A) Ensure that a practitioner responsible for the care of the patient has issued an
order, consistent with hospital policy, permitting self-administration of
medications the patient brought into the hospital.
(B) Assess the capacity of the patient (or the patient’s caregiver/support person where
appropriate) to self-administer the specified medication(s) and also determine if
the patient (or the patient’s caregiver/supplier person where appropriate) needs
instruction in the safe and accurate administration of the specified medication(s).
(C) Identify the specified medication(s) and visually evaluate the medication(s) for
integrity.
(D) Address the security of the medication(s) for each patient.
(E) Document the administration of each medication, as reported by the patient (or the
patient’s caregiver/support person where appropriate), in the patient’s medical
record.
Interpretative Guidelines §482.23(c)(6)(ii)
Hospitals have the option of establishing a program for self-administration by patients, or,
when applicable, patient caregivers or support persons, of medications the patient brings
himself or herself to the hospital. The existence of this regulatory option does not mean
that a hospital must offer medication self-administration programs or that a patient has a
right to retain and self-administer medications they bring with them from home.
A hospital program for patient self-administration of medications the patient brings from
home could be beneficial for the appropriate patients if the proper precautions are taken in
designing and implementing such a program. Generally such a program would apply only
to inpatients, but there may be circumstances under which a hospital finds it appropriate to
permit self-administration of medications that outpatients or their caregivers/support
persons bring with them.
Among the potential benefits of permitting self-administration of medications the patient
brings from home is that problems are avoided related to the hospital’s formulary not
including a particular medication that a patient needs to continue to take during his/her
hospital stay, and the patient prefer to avoid medication substitution. The hospital also
gains an opportunity to identify suboptimal patient medication administration techniques
for these drugs and to provide instruction designed to ensure that the patient is
administering his/her medications properly.
Hospitals have the discretion to establish policies providing for different levels of patient
self-administration, and may make these levels across-the-board, patient-specific, or
medication-specific. For example, a hospital may choose whether or not a nurse must be
present to supervise the self-administration, and whether this supervision requirement could
vary according to the type of medication or the capacity of the individual patient (or the
patient’s caregiver/support person). A hospital may also determine through its policies and
procedures whether supervision requirements must be addressed in the practitioner’s order
or whether this may be left to the discretion of the nurse who assesses the patient. A
hospital may choose to exclude certain medications from patient self-administration, for
example, because they pose too great a medication security challenge. It must be clear in
the hospital’s policies and procedures whether it has established such a policy and what
kind of limitations it has established for its program of patient self-administration of
medications the patient brings from home.
It is expected that the medical staff, nursing and pharmacy departments are to collaborate in
developing policies and procedures for self-administration of medications the patient brings
from home which are approved by the governing body.
Required elements of a self-administration program:
If the hospital chooses to develop programs for self-administration of medications brought
from home by patients (and/or their caregiver/support persons), the following must be in
place:
• An order allowing the patient to administer medications brought from home. The
order must be consistent with the hospital’s policy concerning self-administration of
medications brought from home and be written by a practitioner who is responsible for
the care of the patient and who is authorized to order medications, in accordance with
hospital policies and procedures, State law, including scope of practice laws, and
medical staff by-laws, rules, and regulations.
• A documented assessment of the capacity of the patient (or their caregiver/support
person) to successfully administer the medication(s) specified in the order,
including a determination whether the patient (or their caregiver/support person)
needs instruction in the safe and accurate administration of the specified
medication(s). Nurses are expected to exercise their clinical judgment and to inform
the practitioner responsible for the care of the patient about any reservations the nurse
might have about an individual patient’s (or caregiver/support person’s) capacity to
safely self-administer medications. The assessment must be documented and must
highlight the findings that are affirmative – i.e., support patient-self-administration –
and negative – i.e., call into question patient self-administration. The nurse is also
expected to document any discussions with the practitioner responsible for the care of
the patient regarding the nurses’ concerns about patient’s (or caregiver/support
person’s) capacity to safely self-administer medications. (77 FR 29052, May 16, 2012)
Hospitals may, as a matter of policy, permit a nurse to return to nurse administration for
particular doses of a medication for which there is a self-administration order, without a
discussion with the responsible practitioner if, based on the nurse’s assessment, the
patient’s capacity has been temporarily diminished and there is no caregiver/support
person who is assisting the patient with self-administration of medication. For
example, a patient who has just had an invasive test or procedure may not be fully alert
for a period thereafter, or the parent of a minor patient, who is administering
medications to the patient may for whatever reasons not be available and a scheduled
medication dose is close to being overdue.
As part of the assessment of the patient’s self-administration capacity, nurses are
expected to identify whether the patient (or the patient’s caregiver/support person)
needs instruction in the safe and accurate administration of the specified medication(s).
Even though the patient has been taking the medication at home, the patient (or the
patient’s caregiver/support person) may not be using optimal administration
techniques. Patient needs may be related to type of medication, unique individual
medication requirements, delivery route, dosage and scheduling, equipment (e.g.
syringes, pill-cutters, measuring containers, etc.) intravenous access, potential adverse
side effects and what to do if they occur, infection control measures, storage,
medication disposal, among others. Education and training needs identified, and how
they were addressed, must be documented in the medical record.
• Identification/visual evaluation for integrity. Hospitals must have policies and
procedures addressing how they will identify the medications the patient has brought
from home. Identification is important because the label on the patient’s medication
container may not accurately reflect the contents. Further, the medication might have
expired or have not been stored correctly in the patient’s home, requiring hospitals to at
least conduct a visual inspection to see if the medication appears to have retained its
integrity. It is recognized that a visual inspection for integrity may not be definitive,
but the regulation does not require use of more complex methods.
• Security of the self-administered medications. The security of a patient’s self-
administered medications is extremely important, but does not lend itself well to a one-
size-fits-all regulatory requirement. There are Federal and State laws, including the
Pharmaceutical Services CoP, which require a higher level of security for certain
medications (for example, controlled substances). Hospitals are expected to comply
with these already-established requirements and laws, and generally should not include
such medications as part of a patient self-administration program.
Hospitals are also free to exclude other medications besides controlled substances from
their patient self-administered medication programs when the hospital has concerns
over its capacity to address the security of these other medications for patients.
A hospital may choose to have a policy where it maintains a list of medications brought
from home that it excludes from self-administration entirely, due to security concerns.
It may choose to have a policy that addresses the security of a particular medication on
a patient-by-patient basis. Or it may establish a policy that is a combination of both of
these approaches to medication security.
• Documentation of medication administration. Under the regulation, a nurse must
document the self-administration of a medication. In cases where the nurse directly
supervised the self-administration, the nurse is expected to indicate that the medication
administration was observed and confirmed. On the other hand, where direct nurse
supervision is not required, the nurse is required to document only what the patient, or
the patient’s caregiver/support person, reports to the nurse as to the time and amount of
medication administered. Nurses are expected to assess whether the reports of the
patient or patient’s caregiver/support person indicate, with respect to timing and dosage,
that the patient is receiving the medication as ordered.
Survey Procedures §482.23(c)(6) and (c)(6)(i)
If the hospital permits patient self-administration of medications brought from home:
• Ask the hospital to identify current inpatients for whom self-administration of
medications brought from home is permitted.
• Interview of several of these patients (or their caregivers/support persons when
applicable) to ask if that they received instruction on how to self-administer their
medications consistent with hospital policy.
• Interview nurses caring for the selected patients. Ask them:
• What the applicable hospital policies and procedures are regarding supervision
of self-medication.
• How they assess a patient’s (or patient’s caregiver/support person’s) capacity to
self-administer medication. If they have concerns, how do they communicate
them to the responsible practitioner? Does their hospital permit nurses to return
to nurse administration of medications in response to temporary reduction in
patient capacity or absence of the patient’s caregiver/support person? If so, how
do the nurses make this assessment?
• How they instruct a patient (or patient’s caregiver/support person’s) in safe and
proper medication self-administration when educational needs have been
identified.
• How self-administered medications are secured.
• How they document self-administration of medications.
• To provide a copy of the hospital’s policies and procedures. Are they following
the policies and procedures?
• Review the medical records for the selected patients. Is there documentation of:
• An order for self-administration of specific medication(s).
• A nurse assessment of the patient’s (or patient’s caregiver/support person’s)
capacity to self-administer medication and identification of whether or not there
are educational needs that have been met.
• Documentation of the identification and visual assessment of medications
brought from home.
• Documentation of self-administration times and doses, as reported by the patient
or (or patient’s caregiver/support person) or directly observed by a nurse.
• Do the hospital’s policies and procedures for self-administration of medications
brought from home address, consistent with the regulatory requirements, the
following:
• Limitations on medications eligible for self-administration or patient conditions
which exclude self-administration;
• Orders for self-administration of medications brought from home;
• Requirements, if any, for supervision of self-administration;
• Assessment of self-medication capacity, including identification of educational
needs and how they are to be met;
• Identification and visual inspection for integrity of self-administered
medications brought from home;
• Security of self-administered medications; and
• Documentation of self-administration in the medical record?
A-0431
§482.24 Condition of Participation: Medical Record Services
The hospital must have a medical record service that has administrative responsibility
for medical records. A medical record must be maintained for every individual
evaluated or treated in the hospital.
Interpretive Guidelines §482.24
The term “hospital” includes all locations of the hospital.
The hospital must have one unified medical record service that has administrative
responsibility for all medical records, both inpatient and out patient records. The hospital
must create and maintain a medical record for every individual, both inpatient and out
patient evaluated or treated in the hospital.
The term “medical records” includes at least written documents, computerized electronic
information, radiology film and scans, laboratory reports and pathology slides, videos,
audio recordings, and other forms of information regarding the condition of a patient.
Survey Procedures §482.24
• Review the organizational structure and policy statements and interview the person
responsible for the medical records service to ascertain that the service is structured
appropriately to meet the needs of the hospital and the patients.
• Review a sample of active and closed medical records for completeness and
accuracy in accordance with Federal and State laws and regulations and hospital
policy. The sample should be 10 percent of the average daily census and be no less
than 30 records. Additionally, select a sample of outpatient records in order to
determine compliance in outpatient departments, services, and locations.
A-0432
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.24(a) Standard: Organization and Staffing
The organization of the medical record service must be appropriate to the scope and
complexity of the services performed. The hospital must employ adequate personnel
to ensure prompt completion, filing, and retrieval of records.
Interpretive Guidelines §482.24(a)
The medical records service must be organized, equipped, and staffed in accordance with
the scope and complexity of the hospital’s services and in such a manner as to comply with
the requirements of this regulation and other Federal and State laws and regulations.
There must be an established medical record system that is organized and employs adequate
personnel to ensure prompt:
• Completion of medical records;
• Filing of medical records; and
• Retrieval of medical records.
The term “employs adequate personnel” includes:
• That medical record personnel are employees of the hospital;
• That the hospital employs an adequate number of medical record personnel,
employs adequate types of medical record personnel, and employs personnel who
possess adequate education, skills, qualifications and experience to ensure the
hospital complies with requirements of this regulation and other Federal and State
laws and regulations.
Survey Procedures §482.24(a)
• Verify that there is an established system that addresses at least the following
activities of the medical records services:
o Timely processing of records;
o Coding/indexing of records;
o Retrieval of records;
o Compiling and retrieval of data of quality assurance activities.
• Verify that the system is reviewed and revised as needed.
• Interview staff, if needed, review written job descriptions and staffing schedules to
determine if staff is carrying out all designated responsibilities.
• Verify that the hospital employs adequate medical record personnel as previously
described.
• Are medical records promptly completed in accordance with State law and hospital
policy?
• Select a sample of past patients of the hospital (inpatient and/or outpatient).
Request those patients’ medical records. Can the hospital promptly retrieve those
records?
A-0438
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.24(b) Standard: Form and Retention of Record
The hospital must maintain a medical record for each inpatient and outpatient.
Medical records must be accurately written, promptly completed, properly filed and
retained, and accessible. The hospital must use a system of author identification and
record maintenance that ensures the integrity of the authentication and protects the
security of all record entries.
Interpretive Guidelines §482.24(b)
The hospital must maintain a medical record for each inpatient and outpatient evaluated or
treated in any part or location of the hospital.
All medical records must be accurately written. The hospital must ensure that all medical
records accurately and completely document all orders, test results, evaluations, care plans,
treatments, interventions, care provided and the patient’s response to those treatments,
interventions and care.
All medical records must be promptly completed. Every medical record must be complete
with all documentation of orders, diagnosis, evaluations, treatments, test results, care plans,
discharge plans, consents, interventions, discharge summary, and care provided along with
the patient’s response to those treatments, interventions, and care. The record must be
completed promptly after discharge in accordance with State law and hospital policy but no
later than 30 days after discharge.
The medical record must be properly filed and retained. The hospital must have a
medical record system that ensures the prompt retrieval of any medical record, of any
patient evaluated or treated at any location of the hospital within the past 5 years. [
§482.24(b)(1) addresses the 5 year medical record retention requirement]
The medical record must be accessible. The hospital must have a medical record system
that allows the medical record of any patient, inpatient or outpatient, evaluated and/or
treated at any location of the hospital within the past 5 years to be accessible by appropriate
staff, 24 hours a day, 7 days a week, whenever that medical record may be needed.
Medical records must be properly stored in secure locations where they are protected from
fire, water damage and other threats.
Medical information such as consultations, orders, practitioner notes, x-ray interpretations,
lab test results, diagnostic test results, patient assessments and other patient information
must be accurately written, promptly completed and properly filed in the patients’ medical
record, and accessible to the physicians or other care providers when needed for use in
making assessments of the patient’s condition, decisions on the provision of care to the
patient, and in planning the patient’s care. This requirement applies to the medical records
of current inpatients and outpatients of the hospital.
The hospital must have a system of author identification and record maintenance that
ensures the integrity of the authentication and protects the security of all record entries.
The medical record system must correctly identify the author of every medical record entry
and must protect the security of all medical record entries. The medial record system must
ensure that medical record entries are not lost, stolen, destroyed, altered, or reproduced in
an unauthorized manner. Locations where medical records are stored or maintained must
ensure the integrity, security and protection of the records. These requirements apply to
both manual and electronic medical record systems.
Survey Procedures §482.24(b)
• Determine the location(s) where medical records are maintained.
• Verify that a medical record is maintained for each person treated or receiving care.
The hospital may have a separate record for both inpatients and outpatients.
However, when two different systems are used they must be appropriately cross
referenced and accessible.
• Verify that procedures ensure the integrity of authentication and protect the security
of patient records.
• Verify that medical records are stored and maintained in locations where the records
are secure, that protects them from damage, flood, fire, etc., and limits access to
only authorized individuals.
• Verify that records are accurate, completed promptly, easily retrieved and readily
accessible, as needed, in all locations where medical records are maintained.
A-0439
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.24(b)(1) - Medical records must be retained in their original or legally
reproduced form for a period of at least 5 years.
Interpretive Guidelines §482.24(b)(1)
Medical records are retained in their original or legally reproduced form in hard copy,
microfilm, computer memory, or other electronic storage media. The hospital must be able
to promptly retrieve the complete medical record of every individual evaluated or treated in
any part or location of the hospital within the last 5 years.
In accordance with Federal and State law and regulations, certain medical records may have
retention requirements that exceed 5 years (for example: FDA, OSHA, EPA).
Survey Procedures §482.24(b)(1)
• Determine that records are retained for at least 5 years, or more if required by State
or local laws.
• Select a sample of patients, both inpatient and outpatient who were patients of the
hospital between the previous 48-60 months. Request their medical record. Is it
promptly retrieved? Is it complete? Is it in original or in a legally reproduced form?
A-0440
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.24(b)(2) - The hospital must have a system of coding and indexing medical
records. The system must allow for timely retrieval by diagnosis and procedure, in
order to support medical care evaluation studies.
Survey Procedures §482.24(b)(2)
Verify that the hospital uses a coding and indexing system that permits timely retrieval of
patient records by diagnosis and procedures.
A-0441
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
§482.24(b)(3) - The hospital must have a procedure for ensuring the confidentiality of
patient records. Information from or copies of records may be released only to
authorized individuals, and the hospital must ensure that unauthorized individuals
cannot gain access to or alter patient records. Original medical records must be
released by the hospital only in accordance with Federal or State laws, court orders,
or subpoenas.
Interpretive Guidelines §482.24(b)(3)
Release of Information from or Copies of Records:
The hospital must have a procedure to ensure the confidentiality of each patient’s medical
record, whether it is in paper or electronic format, or a combination of the two, from
unauthorized disclosure. Confidentiality applies wherever the record or portions thereof
are stored, including but not limited to central records, patient care locations, radiology,
laboratories, record storage areas, etc.
A hospital is permitted to disclose medical record information, without a patient’s
authorization, in order to provide patient care and perform related administrative functions,
such as payment and other hospital operations.
• Payment operations include hospital activities to obtain payment or be reimbursed for
the provision of health care to an individual.
• Health care operations are administrative, financial, legal, and quality improvement
activities of a hospital that are necessary to conduct business and to support the core
functions of treatment and payment. These activities include, but are not limited
to: quality assessment and improvement activities, case management and care
coordination; competency assurance activities, conducting or arranging for medical
reviews, audits, or legal services, including fraud and abuse detection and compliance
programs; business planning, development, management, and administration and
certain hospital-specific fundraising activities.
The hospital must develop policies and procedures that reasonably limit disclosures of
information contained in the patient’s medical record to the minimum disclosure necessary,
except when the disclosure is for treatment or payment purposes, or as otherwise required
by State or Federal law.
When the minimum necessary standard is applied, a hospital may not disclose the entire
medical record for a particular purpose, unless it can specifically justify that the whole
record is the disclosure amount reasonably required for the purpose.
A hospital may disclose information from the medical record electronically, and may also
share an electronic medical record system with other health care facilities, physicians and
practitioners, so long as the system is designed and operated with safeguards that ensure
that only authorized disclosures are made.
The hospital must obtain written authorization from the patient or the patient’s
representative for any other disclosure of medical record information.
Preventing Unauthorized Access
The hospital must ensure that unauthorized individuals cannot gain access to patient
records. This applies to records in electronic as well as hard copy formats. Patient records
must be secure at all times and in all locations. This includes open patient records for
patients who are currently inpatients in the hospital and outpatients in outpatient clinics.
For hard copy records, techniques such as locked cabinets or file rooms and limiting access
to keys or pass codes may be employed. For electronic records technical safeguards, such
as business rules that limit access based on need to know, passwords, or other control
mechanisms must be in place. When disposing of copies of medical records, physical
safeguards might include first shredding documents containing confidential information,
taking appropriate steps to erase information from media used to store electronic records,
etc.
Release of Original Records
The hospital must not release the original of a medical record that exists in a hard copy,
paper version only, unless it is required to do so in response to a court order, a subpoena, or
Federal or State laws. For electronic records, the hospital must ensure that the media or
other mechanism by which the records are stored electronically is not removed in such a
way that all or part of the record is deleted from the hospital’s medical record system. The
hospital must have policies and procedures that address how it assures that retains its
“original” medical records, unless their release is mandated by law/court order/subpoena.
Survey Procedures §482.24(b)(3)
• Verify that policies are in place that limit access to, and disclosure of, medical
records to permitted users and uses, and that require written authorization for other
disclosures. Are the policies consistent with the regulatory requirements?
• Observe whether patient records are secured from unauthorized access at all times
and in all locations.
• Ask the hospital to demonstrate what precautions are taken to prevent physical or
electronic altering of content previously entered into a patient record, or to prevent
unauthorized disposal of patient records.
• Verify that patient medical record information is released only as permitted under
the hospital’s policies and procedures.
• Conduct observations and interview staff to determine what safeguards are in place
or precautions are taken to prevent unauthorized persons from gaining physical
access or electronic access to information in patient records.
• If the hospital uses electronic patient records, is access to patient records controlled
through standard measures, such as business rules defining permitted access,
passwords, etc.?
• Do the hospital’s policies and procedures provide that “original” medical records are
retained, unless their release is mandated under Federal or State law, court order or
subpoena? Interview staff responsible for medical records to determine if they are
aware of the limitations on release of “original” medical records.
A-0449
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.24(c) Standard: Content of Record
The medical record must contain information to justify admission and continued
hospitalization, support the diagnosis, and describe the patient’s progress and
response to medications and services.
Interpretive Guidelines §482.24(c)
The medical record must contain information such as notes, documentation, records,
reports, recordings, test results, assessments etc. to:
• Justify admission;
• Justify continued hospitalization;
• Support the diagnosis;
• Describe the patient’s progress;
• Describe the patient’s response to medications; and
• Describe the patient’s response to services such as interventions, care, treatments,
etc.
The medical record must contain complete information/documentation regarding
evaluations, interventions, care provided, services, care plans, discharge plans, and the
patient’s response to those activities.
Patient medical record information, such as, laboratory reports, test results, consults,
assessments, radiology reports, dictated notes, etc. must be promptly filed in the patient’s
medical record in order to be available to the physician and other care providers to use in
making assessments of the patient’s condition, to justify continued hospitalization, to
support the diagnosis, to describe the patient’s progress, and to describe the patient’s
response to medications, interventions, and services, in planning the patient’s care, and in
making decisions on the provision of care to the patient.
A-0450
(Rev. 47, Issued: 06-05-09, Effective/Implementation: 06-05-09)
§482.24(c)(1) - All patient medical record entries must be legible, complete, dated,
timed, and authenticated in written or electronic form by the person responsible for
providing or evaluating the service provided, consistent with hospital policies and
procedures.
Interpretive Guidelines §482.24(c)(1)
All entries in the medical record must be legible. Orders, progress notes, nursing notes, or
other entries in the medical record that are not legible may be misread or misinterpreted and
may lead to medical errors or other adverse patient events.
All entries in the medical record must be complete. A medical record is considered
complete if it contains sufficient information to identify the patient; support the
diagnosis/condition; justify the care, treatment, and services; document the course and
results of care, treatment, and services; and promote continuity of care among providers.
With these criteria in mind, an individual entry into the medical record must contain
sufficient information on the matter that is the subject of the entry to permit the medical
record to satisfy the completeness standard.
All entries in the medical record must be dated, timed, and authenticated, in written or
electronic form, by the person responsible for providing or evaluating the service provided.
• The time and date of each entry (orders, reports, notes, etc.) must be accurately
documented. Timing establishes when an order was given, when an activity
happened or when an activity is to take place. Timing and dating entries is
necessary for patient safety and quality of care. Timing and dating of entries
establishes a baseline for future actions or assessments and establishes a timeline of
events. Many patient interventions or assessments are based on time intervals or
timelines of various signs, symptoms, or events. (71 FR 68687)
• The hospital must have a method to establish the identity of the author of each entry.
This would include verification of the author of faxed orders/entries or computer
entries.
• The hospital must have a method to require that each author takes a specific action
to verify that the entry being authenticated is his/her entry or that he/she is
responsible for the entry, and that the entry is accurate.

The requirements for dating and timing do not apply to orders or prescriptions that are
generated outside of the hospital until they are presented to the hospital at the time of
service. Once the hospital begins processing such an order or prescription, it is responsible
for ensuring that the implementation of the order or prescription by the hospital is promptly
dated, and timed in the patient’s medical record.
When a practitioner is using a preprinted order set, the ordering practitioner may be in
compliance with the requirement at §482.24(c)(1) to date, time, and authenticate an order if
the practitioner accomplishes the following:
• Last page: Sign, date, and time the last page of the orders, with the last page also
identifying the total number of pages in the order set.
• Pages with Internal Selections: Sign or initial any other (internal) pages of the
order set where selections or changes have been made.
o The practitioner should initial/sign the top or bottom of the pertinent
page(s);and
o The practitioner should also initial each place in the preprinted order set
where changes, such as additions, deletions, or strike-outs of components
that do not apply, have been made.
• It is not necessary to initial every preprinted box that is checked to
indicate selection of an order option, so long as there are no changes
made to the option(s) selected.
In the case of a pre-established electronic order set, the same principles would apply, so that
the practitioner would date, time and authenticate the final order that resulted from the
electronic selection/annotation process, with the exception that pages with internal changes
would not need to be initialed or signed if they are part of an integrated single electronic
document.
Authentication of medical record entries may include written signatures, initials, computer
key, or other code. For authentication, in written or electronic form, a method must be
established to identify the author. When rubber stamps or electronic authorizations are used
for authentication, the hospital must have policies and procedures to ensure that such
stamps or authorizations are used only by the individuals whose signature they represent.
There shall be no delegation of stamps or authentication codes to another individual. It
should be noted that some insurers and other payers may have a policy prohibiting the use
of rubber stamps as a means of authenticating the medical records that support a claim for
payment. Medicare payment policy, for example, no longer permits such use of rubber
stamps. Thus, while the use of a rubber stamp for signature authentication is not prohibited
under the CoPs and analysis of the rubber stamp method per se is not an element of the
survey process, hospitals may wish to eliminate their usage in order to avoid denial of
claims for payment.
Where an electronic medical record is in use, the hospital must demonstrate how it prevents
alterations of record entries after they have been authenticated. Information needed to
review an electronic medical record, including pertinent codes and security features, must
be readily available to surveyors to permit their review of sampled medical records while
on-site in the hospital.
When State law and/or hospital policy requires that entries in the medical record made by
residents or non-physicians be countersigned by supervisory or attending medical staff
members, then the medical staff rules and regulations must address counter-signature
requirements and processes.
A system of auto-authentication in which a physician or other practitioner authenticates an
entry that he or she cannot review, e.g., because it has not yet been transcribed, or the
electronic entry cannot be displayed, is not consistent with these requirements. There must
be a method of determining that the practitioner did, in fact, authenticate the entry after it
was created. In addition, failure to disapprove an entry within a specific time period is not
acceptable as authentication.
The practitioner must separately date and time his/her signature authenticating an entry,
even though there may already be a date and time on the document, since the latter may not
reflect when the entry was authenticated. For certain electronically-generated documents,
where the date and time that the physician reviewed the electronic transcription is
automatically printed on the document, the requirements of this section would be satisfied.
However, if the electronically-generated document only prints the date and time that an
event occurred (e.g., EKG printouts, lab results, etc.) and does not print the date and time
that the practitioner actually reviewed the document, then the practitioner must either
authenticate, date, and time this document itself or incorporate an acknowledgment that the
document was reviewed into another document (such as the H&P, a progress note, etc.),
which would then be authenticated, dated, and timed by the practitioner.
Survey Procedures §482.24(c)(1)
Review a sample of open and closed medical records.
• Determine whether all medical record entries are legible. Are they clearly written in
such a way that they are not likely to be misread or misinterpreted?
• Determine whether orders, progress notes, nursing notes, or other entries in the
medical record are complete. Does the medical record contain sufficient
information to identify the patient; support the diagnosis/condition; justify the care,
treatment, and services; document the course and results of care, treatment, and
services; and promote continuity of care among providers?
• Determine whether medical record entries are dated, timed, and appropriately
authenticated by the person who is responsible for ordering, providing, or evaluating
the service provided.
• Determine whether all orders, including verbal orders, are written in the medical
record and signed by the practitioner who is caring for the patient and who is
authorized by hospital policy and in accordance with State law to write orders.
• Determine whether the hospital has a means for verifying signatures, both written
and electronic, written initials, codes, and stamps when such are used for authorship
identification. For electronic medical records, ask the hospital to demonstrate the
security features that maintain the integrity of entries and verification of electronic
signatures and authorizations. Examine the hospital’s policies and procedures for
using the system, and determine if documents are being authenticated after they are
created.
A-0454
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
§482.24(c)(2) - All orders, including verbal orders, must be dated, timed, and
authenticated promptly by the ordering practitioner or by another practitioner who is
responsible for the care of the patient only if such a practitioner is acting in
accordance with State law, including scope-of-practice laws, hospital policies, and
medical staff bylaws, rules, and regulations.
Interpretive Guidelines §482.24(c)(2)
The hospital must ensure that all orders, including verbal orders, are dated, timed, and
authenticated promptly. The Merriam-Webster online dictionary defines “prompt” as
performed readily or immediately.
Verbal orders are orders for medications, treatments, interventions or other patient care that
are transmitted as oral, spoken communications between senders and receivers, delivered
either face-to-face or via telephone.
The receiver of a verbal order must date, time, and sign the verbal order in accordance with
hospital policy. CMS expects hospital policies and procedures for verbal orders to include
a read-back and verification process.
The prescribing practitioner must verify, sign, date and time the order as soon as possible
after issuing the order, in accordance with hospital policy, and State and Federal
requirements.
Authentication of a verbal order may be written, electronic, or faxed. The hospital must
have a method for establishing the identity of the practitioner who has given a verbal order,
including verification of the author of faxed verbal orders or computer entries.
In some instances, the ordering practitioner may not be able to authenticate his or her order,
including a verbal order (e.g., the ordering practitioner gives a verbal order which is written
and transcribed, and then is “off duty” for the weekend or an extended period of time). In
such cases it is acceptable for another practitioner who is responsible for the patient’s care
to authenticate the order, including a verbal order, of the ordering practitioner as long as it
is permitted under State law, hospital policies and medical staff bylaws, rules, and
regulations. Hospitals may choose in their policies to restrict which practitioners it would
authorize to authenticate another practitioner’s orders. For example, a hospital could
choose to restrict authentication of orders for pediatric patients to practitioners who are
privileged to provide pediatric care. (77 FR 29053, May 16, 2012)
• All practitioners responsible for the patient’s care are expected to have knowledge of
the patient’s hospital course, medical plan of care, condition, and current status.
• When a practitioner other than the ordering practitioner authenticates an order, that
practitioner assumes responsibility for the order as being complete, accurate and final.
• A qualified non-physician practitioner, such as a physician assistant (PA) or nurse
practitioner (NP), who is responsible for the care of the patient may authenticate a
physician’s or other qualified non-physician practitioner’s order only if the order is
within his/her scope of practice.
If State law requires that the ordering practitioner authenticate his/her own orders, or his/her
own verbal orders, then a practitioner other than the prescribing practitioner would not be
permitted to authenticate the verbal order in that State.
(71 FR 68682 and 77 FR 29053, May 16, 2012)
NOTE CONCERNING VERBAL ORDERS FOR LABORATORY TESTS:
The requirement to authenticate promptly a verbal order applies to verbal orders associated
with both inpatients and outpatients. It is possible that a hospital verbal order for a
laboratory test could be authenticated in compliance with the Clinical Laboratory
Improvement Amendment (CLIA) regulatory standard of authentication, i.e., within 30
days, but nonetheless be out of compliance with the hospital Medical Records Services
requirement for prompt authentication of all orders, including verbal orders. Because CLIA
laboratories – even if physically situated in a hospital – are surveyed for compliance only
with CLIA regulations, the laboratory would not be cited for a deficiency by a CLIA survey
team. However, hospital surveyors conducting a survey would cite the hospital’s inpatient
or outpatient recordkeeping for deficiencies under the Medical Record Services CoP if the
lab order originated for a patient during a hospital inpatient stay or hospital outpatient clinic
visit and the order was not authenticated promptly.
Survey Procedures §482.24(c)(2)
Does the hospital have policies and procedures requiring prompt authentication of all
orders, including verbal orders, by the ordering practitioner or, if permitted under State
law, hospital policy and medical staff bylaws, rules and regulations, another practitioner
responsible for the care of the patient?
• Do the hospital's policies and procedures for verbal orders include a "read back and
verify" process where the receiver of the order reads back the order to the ordering
practitioner to verify its accuracy?
Review orders, including verbal orders, in a sample of medical records. Have orders
been dated, timed, and authenticated promptly by the ordering practitioner or, if
permitted under State law, hospital policy and medical staff bylaws, rules and
regulations, another practitioner who is responsible for the care of the patient?
• Has the receiver of a verbal order dated, timed, and signed the order according to
hospital policy?
A-0457
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
§482.24(c) (3) Hospitals may use pre-printed and electronic standing orders, order
sets, and protocols for patient orders only if the hospital:
(i) Establishes that such orders and protocols have been reviewed and approved by the
medical staff and the hospital’s nursing and pharmacy leadership;
(ii) Demonstrates that such orders and protocols are consistent with nationally
recognized and evidence-based guidelines;
(iii) Ensures that the periodic and regular review of such orders and protocols is
conducted by the medical staff and the hospital’s nursing and pharmacy leadership to
determine the continuing usefulness and safety of the orders and protocols; and
(iv) Ensures that such orders and protocols are dated, timed, and authenticated promptly
in the patient’s medical record by the ordering practitioner or another practitioner
responsible for the care of the patient only if such a practitioner is acting in accordance with
State law, including scope-of-practice laws, hospital policies, and medical staff bylaws,
rules, and regulations.
Interpretive Guidelines §482.24(c)(3)
What is covered by this regulation?
There is no standard definition of a “standing order” in the hospital community at large (77
FR 29055, May 16, 2012), but the terms “pre-printed standing orders,” “electronic standing
orders,” “order sets,” and “protocols for patient orders” are various ways in which the term
“standing orders” has been applied. For purposes of brevity, in our guidance we generally
use the term “standing order(s)” to refer interchangeably to pre-printed and electronic
standing orders, order sets, and protocols. However, we note that the lack of a standard
definition for these terms and their interchangeable and indistinct use by hospitals and
health care professionals may result in confusion regarding what is or is not subject to the
requirements of §482.24(c)(3), particularly with respect to “order sets.”
• Not all pre-printed and electronic order sets are considered a type of “standing order”
covered by this regulation. Where the order sets consist solely of menus of treatment or
care options designed to facilitate the creation of a patient-specific set of orders by a
physician or other qualified practitioner authorized to write orders, and none of the
treatment choices and actions can be initiated by non-practitioner clinical staff before
the physician or other qualified practitioner actually creates the patient-specific
order(s), such menus would not be considered “standing orders” covered by this
regulation. We note in such cases the menus provide a convenient and efficient method
for the physician/practitioner to create an order, but the availability of such menu
options does not create an “order set” that is a “standing order” subject to the
requirements of this regulation. The physician/practitioner may, based on his/her
professional judgment, choose to: use the available menu options to create an order;
not use the menu options and instead create an order from scratch; or modify the
available menu options to create the order. In each case the physician/practitioner
exercises his privileges to prescribe specific diagnosis and/or treatment activities that
are to be implemented for a patient.
• On the other hand, in cases where hospital policy permits treatment to be initiated, by a
nurse, for example, without a prior specific order from the treating
physician/practitioner, this policy and practice must meet the requirements of this
regulation for review of standing orders, regardless of whether it is called a standing
order, a protocol, an order set, or something else. Such treatment is typically initiated
when a patient’s condition meets certain pre-defined clinical criteria. For example,
standing orders may be initiated as part of an emergency response or as part of an
evidence-based treatment regimen where it is not practical for a nurse to obtain either a
written, authenticated order or a verbal order from a physician or other qualified
practitioner prior to the provision of care.
• Hybrids, where a component for non-practitioner-initiated treatment is embedded within
a menu of options for the physician or other qualified practitioner, still require
compliance with the requirements for a standing order for that component. For
example, if an order set includes a protocol for nurse-initiated potassium replacement,
that protocol must be reviewed under the requirements of this regulation before it may
become part of a menu of treatment options from which a physician or other qualified
practitioner would select treatments for a particular patient.
Requirements for “Standing Orders”
Hospitals have the flexibility to use standing orders to expedite the delivery of patient care
in well-defined clinical scenarios for which there is evidence supporting the application of
standardized treatments or interventions.
Appropriate use of standing orders can contribute to patient safety and quality of care by
promoting consistency of care, based on objective evidence, when orders
may be initiated as part of an emergency response or as part of an evidence-based treatment
regimen where it is not practicable for a nurse or other non-practitioner to obtain a verbal or
authenticated written order from a physician or other practitioner responsible for the care
of the patient prior to the provision of care.
In all cases, implementation of a standing order must be medically appropriate for the
patient to whom the order is applied.
Much of the evidence on the effectiveness of standing orders in hospitals has been narrowly
focused on aspects of their use by Rapid Response Teams addressing inpatient emergencies.
However, standing orders may also be appropriate in other clinical circumstances,
including, but not limited to:
• Protocols for triaging and initiating required screening examinations and stabilizing
treatment for emergency department patients presenting with symptoms suggestive of
acute asthma, myocardial infarction, stroke, etc. (This does not relieve a hospital of its
obligations under the Emergency Medical Treatment and Labor Act (EMTALA) to have
qualified medical personnel complete required screening and, when applicable,
stabilizing treatment in a timely manner.)
• Post-operative recovery areas.
• Timely provision of immunizations, such as certain immunizations for newborns, for
which there are clearly established and nationally recognized guidelines.
Standing orders may not be used in clinical situations where they are specifically
prohibited under Federal or State law. For example, the hospital patient’s rights
regulation at §482.13(e)(6) specifically prohibits the use of standing orders for
restraint or seclusion of hospital patients.
When deciding whether to use standing orders, hospitals should also be aware that,
although use of standing orders is permitted under the hospital Conditions of Participation,
some insurers, including Medicare, may not pay for the services provided because of the
use of standing orders. (77 FR 29056)
Minimum requirements for standing orders. Hospitals may employ standing orders only
if the following requirements are met for each standing order for a particular well-defined
clinical scenario:
• Each standing order must be reviewed and approved by the hospital’s medical staff and
nursing and pharmacy leadership before it may be used in the clinical setting. The
regulation requires a multi-disciplinary collaborative effort in establishing the protocols
associated with each standing order.
• The hospital’s policies and procedures for standing orders must address the process
by which a standing order is developed; approved; monitored; initiated by
authorized staff; and subsequently authenticated by physicians or other practitioners
responsible for the care of the patient.
• For each approved standing order, there must be specific criteria clearly identified in
the protocol for the order for a nurse or other authorized personnel to initiate the
execution of a particular standing order, for example, the specific clinical situations,
patient conditions, or diagnoses by which initiation of the order would be justified.
Under no circumstances may a hospital use standing orders in a manner that requires
any staff not authorized to write patient orders to make clinical decisions outside of
their scope of practice in order to initiate such orders.
Since residents are physicians, this regulation does not require specific criteria for a
resident to initiate the execution of a particular standing order. However, there may
be State laws governing the practice of residents in hospitals that are more
restrictive; if so, the hospital is expected to comply with the State law. Likewise,
the hospital may choose through its policies and medical staff bylaws, rules and
regulations to restrict the role of residents with respect to standing orders.
• Policies and procedures should also address the instructions that the medical,
nursing, and other applicable professional staff receive on the conditions and criteria
for using standing orders as well as any individual staff responsibilities associated
with the initiation and execution of standing orders. An order that has been initiated
for a specific patient must be added to the patient’s medical record at the time of
initiation, or as soon as possible thereafter.
• Likewise, standing order policies and procedures must specify the process whereby
the physician or other practitioner responsible for the care of the patient
acknowledges and authenticates the initiation of all standing orders after the fact,
with the exception of influenza and pneumococcal vaccines, which do not require
such authentication in accordance with § 482.23(c)(2).
(76 FR 65896, October 24, 2011 & 77 FR 29056, May 16, 2012)
• The hospital must be able to document that the standing order is consistent with
nationally recognized and evidence-based guidelines. This does not mean that there
must be a template standing order available in national guidelines which the hospital
copies, but rather that the content of each standing order in the hospital must be
consistent with nationally recognized, evidence-based guidelines for providing care.
The burden of proof is on the hospital to show that there is a sound basis for the
standing order.
• Each standing order must be subject to periodic and regular review by the medical staff
and the hospital’s nursing and pharmacy leadership, to determine the continuing
usefulness and safety of the orders and protocols. At a minimum, an annual review of
each standing order would satisfy this requirement. However, the hospital’s policies
and procedures must also address a process for the identification and timely completion
of any requisite updates, corrections, modifications, or revisions based on changes in
nationally recognized, evidence-based guidelines. The review may be prepared by the
hospital’s QAPI program, so long as the medical staff and nursing and pharmacy
leadership read, review, and, as applicable, act upon the final report. Among other
things, reviews are expected to consider:
• Whether the standing order’s protocol continues to be consistent with the latest
standards of practice reflected in nationally recognized, evidence-based guidelines;
• Whether there have been any preventable adverse patient events resulting from the
use of the standing order, and if so, whether changes in the order would reduce the
likelihood of future similar adverse events. Note that the review would not be
expected to address adverse events that are a likely outcome of the course of
patient’s disease or injury, even if the order was applied to that patient, unless there
is concern that use of the standing order exacerbated the patient’s condition; and
• Whether a standing order has been initiated and executed in a manner consistent
with the order’s protocol, and if not, whether the protocol needs revision and/or staff
need more training in the correct procedures.
• An order that has been initiated for a specific patient must be added to the patient’s
medical record at the time of initiation, or as soon as possible thereafter. The hospital
must ensure each standing order that has been executed is dated, timed, and
authenticated promptly in the patient’s medical record by the ordering practitioner or
another practitioner responsible for the care of the patient. Another practitioner who is
responsible for the care of the patient may date, time and authenticate the standing order
instead of the ordering practitioner, but only if the other practitioner is acting in
accordance with State law, including scope of practice laws, hospital policies, and
medical staff bylaws, rules and regulations.
The hospital’s standing orders policies and procedures must specify the process
whereby the responsible practitioner, or another authorized practitioner, acknowledges
and authenticates the initiation of each standing order after the fact, with the exception
of standing orders for influenza and pneumococcal vaccines, which do not require such
authentication. Further, the responsible practitioner must be able to modify, cancel,
void or decline to authenticate orders that were not medically necessary in a particular
situation. The medical record must reflect the physician’s actions to modify, cancel,
void or refusal to authenticate a standing order that the physician determined was not
medically necessary. (76 FR 65896, October 24, 2011)
Survey Procedures §482.24(c)(3)
• Ask the hospital’s medical staff and its nursing and pharmacy leadership whether
standing orders are used. If yes, ask them to describe how a standing order is
developed and monitored, and their role in the process.
• Ask to see an example of one or more standing orders, including documentation on
the development of the order, including:
• Reference to the evidence-based national guidelines that support it;
• Participation of medical staff and nursing and pharmacy leadership in the
review and approval of the standing order;
• Description of the protocol to be followed when initiating the execution of
the order, including description of the roles and responsibilities of various
types of staff;
• Description of the process for authenticating the order’s initiation by the
practitioner responsible for the care of the patient, or another authorized
practitioner;
• Evidence of training of personnel on the order’s protocol; and
• Evidence of periodic evaluation and, if needed, modification of the standing
order, including whether the order remains consistent with current evidence-
based national guidelines, staff adherence to the protocol for initiation and
execution, and whether there have been any preventable adverse events
associated with the order.
• Ask staff providing clinical services in areas of the hospital where standing orders
might be typically used, including but not limited to, the emergency department,
labor and delivery units, and inpatient units, whether standing orders are used. If
they say yes, ask them:
o To describe a typical scenario where a standing order would be used, and
what they would do in that case.
o For a copy of the protocol for that standing order. Does their description
conform to the protocol?
• Review a sample of medical records of patients where a nurse-initiated standing
order was used and verify that the order was documented and authenticated by a
practitioner responsible for the care of the patient.
A-0458
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.24(c)(4) - All records must document the following, as appropriate:
(i) Evidence of--
(A) A medical history and physical examination completed and
documented no more than 30 days before or 24 hours after admission
or registration, but prior to surgery or a procedure requiring
anesthesia services, and except as provided under paragraph
(c)(4)(i)(C) of this section. The medical history and physical
examination must be placed in the patient’s medical record within 24
hours after admission or registration, but prior to surgery or a
procedure requiring anesthesia services.
Interpretive Guidelines §482.24(c)(4)(i)(A)
Guidance is pending and will be updated in future release.
A-0461
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.24(c)(4) - [All records must document the following, as appropriate:
(i) Evidence of --]
(B) An updated examination of the patient, including any changes
in the patient’s condition, when the medical history and physical
examination are completed within 30 days before admission or
registration , and except as provided under paragraph (c)(4)(i)(C) of
this section. Documentation of the updated examination must be
placed in the patient's medical record within 24 hours after
admission or registration, but prior to surgery or a procedure
requiring anesthesia services.
Interpretive Guidelines §482.24(c)(4)(i)(B)
Guidance is pending and will be updated in future release.
A-0462
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.24(c)(4) - All records must document the following, as appropriate:
(i) Evidence of --
(C) An assessment of the patient (in lieu of the requirements of paragraphs
(c)(4)(i)(A) and (B) of this section) completed and documented after
registration, but prior to surgery or a procedure requiring anesthesia
services, when the patient is receiving specific outpatient surgical or
procedural services and when the medical staff has chosen to develop and
maintain a policy that identifies, in accordance with the requirements at §
482.22(c)(5)(v), specific patients as not requiring a comprehensive medical
history and physical examination, or any update to it, prior to specific
outpatient surgical or procedural services.
Interpretive Guidelines §482.23(c)(4)(i)(C)
Guidance is pending and will be updated in future release.
A-0463
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
[All records must document the following, as appropriate:]
§482.24(c)(4)(ii) - Admitting diagnosis.
Interpretive Guidelines §482.24(c)(4)(ii)
All inpatient medical records must contain the admitting diagnosis.
Survey Procedures §482.24(c)(4)(ii)
Verify in a sample of medical records that the patient’s admitting diagnosis is documented
in each medical record.
A-0464
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
[All records must document the following, as appropriate:]
§482.24(c)(4)(iii) - Results of all consultative evaluations of the patient and
appropriate findings by clinical and other staff involved in the care of the patient.
Interpretive Guidelines §482.24(c)(4)(iii)
All patient records, both inpatient and outpatient, must contain the results of all consultative
evaluations of the patient and appropriate findings by clinical and other staff involved in the
care of the patient. This information must be promptly filed in the patient’s medical record
in order to be available to the physician or other care providers to use in making
assessments of the patient’s condition, to justify treatment or continued hospitalization, to
support or revise the patient’s diagnosis, to support or revise the plan of care, to describe
the patient’s progress and to describe the patient’s response to medications, treatments, and
services.
Survey Procedures §482.24(c)(4)(iii)
Review a sample of medical records of patients who have orders for consultative
evaluations. Are the results/reports and other clinical findings of those consultative
evaluations included in the patient’s medical record?
A-0465
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
[All records must document the following, as appropriate:]
§482.24(c)(4)(iv) - Documentation of complications, hospital acquired infections, and
unfavorable reactions to drugs and anesthesia.
Interpretive Guidelines §482.24(c)(4)(iv)
All patient medical records, both inpatient and outpatient, must document:
• Complication;
• Hospital-acquired infections;
• Unfavorable reactions to drugs; and
• Unfavorable reactions to anesthesia.
Survey Procedures §482.24(c)(4)(iv)
Through observations, interviews, and reviews of hospital reports and documentation,
determine if patient complications, hospital-acquired infections, unfavorable reactions to
drugs/anesthesia have been documented in the applicable patient’s medical record.
A-0466
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
[All records must document the following, as appropriate:]
§482.24(c)(4)(v) - Properly executed informed consent forms for procedures and
treatments specified by the medical staff, or by Federal or State law if applicable, to
require written patient consent.
Interpretive Guidelines §482.24(c)(4)(v)
Informed consent is discussed in three locations in the CMS Hospital CoPs. See also the
guidelines for 42 CFR 482.13(b)(2) pertaining to patients' rights, and the guidelines for 42
CFR 482.51(b)(2), pertaining to surgical services.
The medical record must contain a document recording the patient’s informed consent for
those procedures and treatments that have been specified as requiring informed consent.
Medical staff policies should address which procedures and treatments require written
informed consent. There may also be applicable Federal or State law requiring informed
consent. The informed consent form contained in the medical record should provide
evidence that it was properly executed.
Informed Consent Forms
A properly executed informed consent form should reflect the patient consent process.
Except as specified for emergency situations in the hospital’s informed consent policies, all
inpatient and outpatient medical records must contain a properly executed informed consent
form prior to conducting any procedure or other type of treatment that requires informed
consent. An informed consent form, in order to be properly executed, must be consistent
with hospital policies as well as applicable State and Federal law or regulation. A properly
executed informed consent form contains the following minimum elements:
• Name of the hospital where the procedure or other type of medical treatment is to
take place;
• Name of the specific procedure, or other type of medical treatment for which
consent is being given;
• Name of the responsible practitioner who is performing the procedure or
administering the medical treatment;
• Statement that the procedure or treatment, including the anticipated benefits,
material risks, and alternative therapies, was explained to the patient or the patient’s
legal representative; (Material risks could include risks with a high degree of
likelihood but a low degree of severity, as well as those with a very low degree of
likelihood but high degree of severity. Hospitals are free to delegate to the
responsible practitioner, who uses the available clinical evidence as informed by the
practitioner’s professional judgment, the determination of which material risks,
benefits and alternatives will be discussed with the patient.)
• Signature of the patient or the patient’s legal representative; and
• Date and time the informed consent form is signed by the patient or the patient’s
legal representative.
If there is applicable State law governing the content of the informed consent form, then the
hospital’s form must comply with those requirements.
A well-designed informed consent form might also include the following additional
information:
• Name of the practitioner who conducted the informed consent discussion with the
patient or the patient’s representative.
• Date, time, and signature of the person witnessing the patient or the patient’s legal
representative signing the consent form.
• Indication or listing of the material risks of the procedure or treatment that were
discussed with the patient or the patient’s representative;
• Statement, if applicable, that physicians other than the operating practitioner,
including but not limited to residents, will be performing important tasks related to
the surgery, in accordance with the hospital’s policies and, in the case of residents,
based on their skill set and under the supervision of the responsible practitioner.
• Statement, if applicable, that qualified medical practitioners who are not physicians
who will perform important parts of the surgery or administration of anesthesia will
be performing only tasks that are within their scope of practice, as determined under
State law and regulation, and for which they have been granted privileges by the
hospital.
Survey Procedures §482.24(c)(4)(v)
• Verify that the hospital has assured that the medical staff has specified which
procedures and treatments require written patient consent.
• Verify that the hospital’s standard informed consent form contains the elements
listed above as the minimum elements of a properly executed informed consent.
• Compare the hospital’s standard informed consent form to the hospital’s policies on
informed consent, to verify that the form is consistent with the policies. If there is
applicable State law, verify that the form is consistent with the requirements of that
law.
• Review a minimum of six random medical records of patients who have, are
undergoing, or are about to under a procedure or treatment that requires informed
consent. Verify that each medical record contains informed consent forms.
• Verify that each completed informed consent form contains the information for each
of the elements listed above as the minimum elements of a properly executed
informed consent, as well as any additional elements required by State law and/or
the hospital’s policy.
A-0467
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
[All records must document the following, as appropriate:]
§482.24(c)(4)(vi) - All practitioners’ orders, nursing notes, reports of treatment,
medication records, radiology, and laboratory reports, and vital signs and other
information necessary to monitor the patient’s condition.
Interpretive Guidelines §482.24(c)(4)(vi)
The requirement means that the stated information is necessary to monitor the patient’s
condition and that this and other necessary information must be in the patient’s medical
record. In order for necessary information to be used it must be promptly filed in the
medical record so that health care staff involved in the patient’s care can access/retrieve this
information in order to monitor the patient’s condition and provide appropriate care.
The medical record must contain:
• All practitioner’s orders (properly authenticated);
• All nursing notes (including nursing care plans);
• All reports of treatment (including complications and hospital-acquired infections);
• All medication records (including unfavorable reactions to drugs);
• All radiology reports;
• All laboratory reports;
• All vital signs; and
• All other information necessary to monitor the patient’s condition.
Survey Procedures §482.24(c)(4)(vi)
• Verify that the patient records contain appropriate documentation of practitioners’
orders, interventions, findings, assessments, records, notes, reports and other
information necessary to monitor the patient’s condition.
• Is this information included in patient records in a prompt manner so that health care
staff involved in the care of the patient have access to the information necessary to

monitor the patient’s condition?
A-0468
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
[All records must document the following, as appropriate:]
§482.24(c)(4)(vii) - Discharge summary with outcome of hospitalization, disposition of
case, and provisions for follow-up care.
Interpretive Guidelines §482.24(c)(4)(vii)
All patient medical records must contain a discharge summary. A discharge summary
discusses the outcome of the hospitalization, the disposition of the patient, and provisions
for follow-up care. Follow-up care provisions include any post hospital appointments, how
post hospital patient care needs are to be met, and any plans for post-hospital care by
providers such as home health, hospice, nursing homes, or assisted living.
The MD/DO or other qualified practitioner with admitting privileges in accordance with
State law and hospital policy, who admitted the patient is responsible for the patient during
the patient’s stay in the hospital. This responsibility would include developing and entering
the discharge summary.
Other MD/DOs who work with the patient’s MD/DO and who are covering for the patient’s
MD/DO and who are knowledgeable about the patient’s condition, the patient’s care during
the hospitalization, and the patient’s discharge plans may write the discharge summary at
the responsible MD/DO’s request.
In accordance with hospital policy, and 42 CFR Part 482.12(c)(1)(i) the MD/DO may
delegate writing the discharge summary to other qualified health care personnel such as
nurse practitioners and MD/DO assistants to the extent recognized under State law or a
State’s regulatory mechanism.
Whether delegated or non-delegated, we would expect the person who writes the discharge
summary to authenticate, date, and time their entry and additionally for delegated discharge
summaries we would expect the MD/DO responsible for the patient during his/her hospital
stay to co-authenticate and date the discharge summary to verify its content.
The discharge summary requirement would include outpatient records. For example:
• The outcome of the treatment, procedures, or surgery;
• The disposition of the case;
• Provisions for follow-up care for an outpatient surgery patient or an emergency
department patient who was not admitted or transferred to another hospital.
Survey Procedures §482.24(c)(4)(vii)
• Verify that a discharge summary is included to assure that proper continuity of care
is required.
• Verify that a final diagnosis is included in the discharge summary.
A-0469
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
[All records must document the following, as appropriate:]
§482.24(c)(4)(viii) - Final diagnosis with completion of medical records within 30 days
following discharge.
Interpretive Guidelines §482.24(c)(4)(viii)
All medical records must contain a final diagnosis. All medical records must be complete
within 30 days of discharge or outpatient care.
Survey Procedures §482.24(c)(4)(viii)
Select a sample of patients who have been discharged for more than 30 days. Request their
medical records. Are those records complete? Does each record have the patient’s final
diagnosis?
A-0489
(Rev. 151, Issued: 11-20-15, Effective: 11-20-15, Implementation: 11-20-15)
§482.25 Condition of Participation: Pharmaceutical Services.
The hospital must have pharmaceutical services that meet the needs of the patients.
The institution must have a pharmacy directed by a registered pharmacist or a drug
storage area under competent supervision. The medical staff is responsible for
developing policies and procedures that minimize drug errors. This function may be
delegated to the hospital’s organized pharmaceutical service.
Interpretive Guidelines §482.25
A hospital must provide pharmaceutical services that meet the needs of its patients. The
services must include either a pharmacy that is directed by a pharmacist, or, when
appropriate, a drug storage area that is competently supervised. The hospital’s medical staff
is responsible for developing pharmaceutical policies and procedures that minimize the
potential for medication errors, but may delegate this function to the pharmaceutical
service.
The manner or degree of noncompliance with the requirements of this Condition and its
component standards must be evaluated to determine whether there is substantial
noncompliance with the Condition, warranting a Condition-level citation.
A-0490
(Rev. 151, Issued: 11-20-15, Effective: 11-20-15, Implementation: 11-20-15)
Standard-level Tag for
§482.25 Condition of Participation: Pharmaceutical Services.
The hospital must have pharmaceutical services that meet the needs of the patients….
Interpretive Guidelines §482.25
What is included in pharmaceutical services?
Pharmaceutical services encompass the functions of procuring, storing, compounding, re-
packaging, and dispensing all medications, biologicals, chemicals and medication-related
devices within the hospital. They also include providing medication-related information to
care professionals within the hospital, as well as direct provision of medication-related care.
Meeting patient needs
Hospitals must provide pharmaceutical services that meet the needs of their patients. The
scope and complexity of pharmaceutical services available in the hospital must be consistent
with
the volume and types of patients the hospital serves. Except in unusual circumstances,
the pharmaceutical service is expected to make available in a timely manner the volume and
types of medications typically needed. These would be those medications typically
prescribed by the hospital’s practitioners for hospital patients receiving inpatient services,
surgical services, diagnostic services involving medications as a component of testing, and
outpatient drug therapies administered while the patient is in the hospital.
Not every hospital is expected to offer the same level of pharmaceutical services. For
example:
• It would not be uncommon for a psychiatric hospital to maintain a relatively limited
pharmaceutical service, due to minimal need for compounding, and/or dispensing
multiple types and forms of medications and biologicals.
• On the other hand, a short-term acute care hospital with a busy oncology outpatient
service and other complex medical and surgical departments would be expected to
provide a wider range of pharmaceutical services that are ready to be furnished
when needed.
Survey Procedures §482.25
• Ask the hospital for evidence of the scope and complexity of its pharmaceutical
services.
• Ask how the hospital has determined that the services meet the needs of its patients.
• Ask unit nursing staff if prescribed medications are routinely available and timely.
If there are reports of frequent delays or other problems, probe further with the
director of pharmaceutical services.
A-0491
(Rev. 151, Issued: 11-20-15, Effective: 11-20-15, Implementation: 11-20-15)
[§482.25 Condition of Participation: Pharmaceutical Services
…..The medical staff is responsible for developing policies and procedures that
minimize drug errors. This function may be delegated to the hospital’s organized
pharmaceutical service.]
§482.25(a) Standard: Pharmacy Management and Administration
The pharmacy or drug storage area must be administered in accordance with
accepted professional principles.
Interpretive Guidelines §482.25(a)
Pharmaceutical services must be administered in accordance with accepted professional
principles. Accepted professional principles includes compliance with applicable Federal
and State laws, regulations, and guidelines governing pharmaceutical services, as well as,
standards or recommendations promoted by nationally recognized professional
organizations, such as those found in the U.S. Pharmacopeia/National Formulary
(USP/NF).
The hospital’s pharmacy service must ensure safe and appropriate procurement, storage,
preparation, dispensing, use, tracking and control, and disposal of medications and
medication-related devices throughout the hospital, for both inpatient and outpatient
services.
Hospitals may choose how to set up the pharmaceutical services utilizing various methods
including, but not limited to:
• a unit dose system (i.e.; single unit package, dispensed in most ready to administer
form possible),
• individual prescription (i.e.; instruction for a single patient, written by a medical
practitioner for a medication or treatment),
• floor stock system (i.e.; storage of pharmaceutical and over-the-counter drugs on the
patient care unit), or
• a combination of these systems, as long as they are properly stored.
However, hospitals with only a drug storage area must only use drugs that are pre-packaged
and need no further preparation beyond that required at the point of care.
The hospital must develop, implement and periodically review and revise as needed policies
and procedures governing provision of pharmaceutical services. The regulation makes the
hospital’s medical staff responsible for the policies and procedures, but also permits the
medical staff to delegate this function to the hospital’s pharmaceutical services. The
policies and procedures must reflect accepted professional pharmacy principles, and the
pharmacy director must be able to identify the source(s) used when developing and
adopting the policies and procedures. There must also be a process to train staff on the
applicable policies and procedures and to monitor their adherence.
Policies and Procedures for Minimizing Drug Errors
Medication errors are a substantial source of morbidity and mortality risk in the hospitalized
setting. Therefore, hospitals must take steps to prevent, identify, and minimize these errors.
These steps must be based on accepted professional principles. This includes not only
ensuring that the pharmacy processes conform to of accepted standards of pharmacy
practice but also proactively identifying and reviewing Adverse Drug Events (ADE) that
occur. Pharmacies also need to be aware of external alerts to real or potential pharmacy-
related problems in hospitals.
The pharmaceutical services policies and procedures must be designed to minimize drug
errors and are expected to address:
• High-alert medications - are considered inherently high risk for adverse drug events.
High alert drugs may include controlled medications, medications not on the approved
FDA list, medications with a narrow therapeutic range, psychotherapeutic medications,
look-alike/sound-alike medications and those new to the market or new to the hospital.
Although mistakes may or may not be more common with these drugs, the
consequences of errors are often harmful, sometimes fatal, to patients. Examples of
ways to minimize high alert medication errors include, but are not limited to, the
following: dosing limits, administration guidelines, packaging, labeling and storage.
• Investigational medications - hospitals that conduct research involving investigational
medications must have a policy and procedure in place to ensure that investigational
medications are safely controlled and administered. Procedures for the use of
investigational medications include, but are not limited to, the following: A written
process for reviewing, approving, supervising and monitoring investigational
medications specifying that when pharmacy services are provided, the pharmacy
controls the storage, dispensing, labeling, and distribution of the investigational
medication.
• Adherence to professional standards of practice for all compounding, packaging
dispensing and drug disposal activities;
• Standardizing medication-related devices and equipment where feasible. For example,
limit the types of general-purpose infusion pumps to one or two;
• Availability of up-to-date medication information and pharmacy expertise on-call when
pharmacy does not operate 24 hours a day;
• Standardization of prescribing and communication practices to include:
o Avoidance of dangerous abbreviations;
o All elements of the order – dose, strength, units (metric), route, frequency, and rate;
o Alert systems for look-like and sound-alike drug names;
o Use of facility approved pre-printed order sheets whenever possible.
o Prohibition of orders to “resume previous orders;”
• Availability of patient-specific information to all individuals involved in provision of
pharmaceutical services. The patient information must be sufficient to properly order,
prepare, dispense, administer and monitor medications as appropriate;
• Identification of when weight-based dosing for pediatric populations is required; and
• A voluntary, non-punitive, reporting system to monitor and report adverse drug events
(including medication errors and adverse drug reactions);
• Monitoring drug alerts and/or recalls. The hospital should have a means to incorporate
external alerts and/or recommendations from national associations and governmental
agencies for review and facility policy and procedure revision consideration. National
associations could include Institute for Safe Medications Practice and National
Coordinating Council for Medication Error Reporting and Prevention. Governmental
agencies may include: Food and Drug Administration, Med Watch Program; and
• The hospital’s pharmacy services must be integrated into its hospital-wide QAPI
program and therefore, it is important to flag new types of mistakes and continually
improve and refine policies and procedures as a result of analyses of errors and adverse
events.
Survey Procedures §482.25(a)
• Is the hospital’s organized pharmaceutical services responsible for the procurement,
distribution and control of all medication products used in the hospital (including
medication-related devices) for inpatient and outpatient care?
• If the hospital has a drug storage area instead of a pharmacy, does it use only drugs
that are pre-packaged and need no further preparation beyond that required at the
point of care?
• Is there evidence that the hospital’s medical staff has either adopted pharmaceutical
services policies and procedures, or has delegated this task to the pharmaceutical
services?
• Can the pharmacy director provide evidence that the policies and procedures are
consistent with accepted professional principles?
• Can the pharmacy director provide evidence that policies and procedures address key
areas to prevent medication errors?
• Is there evidence of training staff on applicable pharmaceutical policies and
procedures?
• Is there a process in place to monitor adherence to policies and procedures?
A-0492
(Rev. 151, Issued: 11-20-15, Effective: 11-20-15, Implementation: 11-20-15)
[§482.25 Condition of Participation: Pharmaceutical Services
The hospital…. must have a pharmacy directed by a registered pharmacist or a drug
storage area under competent supervision….
§482.25(a)(1) - A full-time, part-time, or consulting pharmacist must be responsible
for developing, supervising, and coordinating all the activities of the pharmacy
services.
Interpretive Guidelines §482.25 and §482.25(a)(1)
Pharmaceutical services offered throughout the hospital must be under the direction of a
pharmacist, who may be full-time, part-time, or consulting. This is required even in the
case of a hospital that has a drug storage area instead of a pharmacy. The director must
have documented training or expertise in hospital pharmacy practice and management. The
hospital must have written criteria for the qualifications of the pharmacy director in
accordance with the scope of services provided.
The extent of pharmaceutical services provided by the hospital determines whether a part-
time director of the services is sufficient. Depending on the volume and complexity of the
hospital’s services, oversight may not require full-time on-site management at the hospital’s
pharmacy, but may be accomplished through regularly scheduled visits, and/or use of
telecommunications in accordance with Federal and State law and accepted professional
principles. If the hospital does not have a full-time pharmacist, it must be able to provide
evidence of how a part-time or consulting pharmacist is able to perform all functions
relating to developing, supervising and coordinating all pharmacy services activities.
In general, hospital pharmacies are staffed with registered pharmacists and pharmacy
technicians who perform various functions, including, but not limited to, compounding,
labeling, and dispensing of various drugs and biologicals.
There may be instances of small hospitals that do not have a pharmacy but utilize a drug
storage area for dispensing pre-packaged drugs only. If the hospital has a drug storage area
in lieu of a pharmacy, the day-to-day operations of pharmaceutical services must be under
the supervision of an individual who, if not a pharmacist, nevertheless has documented
competency to oversee compliance with all the pharmaceutical services regulatory
requirements (e.g., security, access to locked areas, etc.). The hospital must establish in
writing the qualifications of the drug storage area supervisor.
The job description or the written agreement for the responsibilities of the pharmacist
director should be clearly defined and include development, supervision and coordination
of all the activities of pharmacy services, including active leadership of those committees
responsible for establishing medication-related policies and procedures.
Survey Procedures §482.25 and §482.25(a)(1)
• Does the hospital have a pharmacist who has been appointed to direct the
pharmaceutical services?
• Are there written criteria for the qualifications of the pharmacist director?
• Is there evidence in the pharmacist’s file that he/she satisfies the criteria?
• If the hospital has a drug storage area in lieu of a pharmacy, is there evidence the
storage area is under competent supervision?
• Review the pharmaceutical services Director’s file to verify that he or she meets the
qualifications established by the medical staff and has been granted privileges as a
pharmacist.
• If the Director is a part-time employee or consultant, ask him/her how much
time/week is spent on developing, supervising and coordinating pharmaceutical
services.
• Review the implementation of the pharmacy director’s responsibilities by:
• Reviewing minutes of meetings (if any) with facility staff regarding
pharmaceutical services;
• Reviewing the job description or the written agreement to see that the
responsibilities of the pharmacist are clearly defined and include development
supervision and coordination of all the activities of pharmacy services;
• Determining whether the pharmacy director/manager routinely evaluates the
performance and competency of pharmacy personnel?
• Ask the pharmacy director to describe how policies and procedures related to
pharmaceutical services are developed, approved, and implemented. What is
his/her role in this process?
• Is there any evidence of problems within the pharmaceutical services that
suggest lack of supervision?
A-0493
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.25(a)(2) - The pharmaceutical service must have an adequate number of
personnel to ensure quality pharmaceutical services, including emergency services.
Interpretive Guidelines §482.25(a)(2)
There must be sufficient personnel to respond to the pharmaceutical needs of the patient
population being served.
The pharmaceutical services staff must be sufficient in types, numbers, and training to
provide quality services, including 24 hour, 7-day emergency coverage, or there is an
arrangement for emergency services, as determined by the needs of the patients and as
specified by the medical staff.
The number of pharmacists and/or the number of hours of services provided by pharmacists
at the hospital, or at each location of the hospital that provides pharmaceutical services,
must meet and be in accordance with the needs of its patients and accepted professional
principles (as previously defined), and reflect the scope and complexity of the hospital’s
pharmaceutical services.
There must be sufficient numbers and types of personnel to provide accurate and timely
medication delivery, ensure accurate and safe medication administration and to provide
appropriate clinical services as well as the participation in continuous quality improvement
programs that meet the needs of the patient population being served.
Survey Procedures §482.25(a)(2)
• Determine that the pharmaceutical services staff is sufficient in number and training
to provide quality services, including 24 hour, 7-day emergency coverage, or there
is an arrangement for emergency services, as determined by the needs of the patients
and as specified by the medical staff.
• Determine if there are sufficient personnel to provide accurate and timely
medication delivery, ensure accurate and safe medication administration and to
provide appropriate clinical services as well as the participation in continuous
quality improvement programs that meet the needs of the patient population being
served.
A-0494
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.25(a)(3) - Current and accurate records must be kept of the receipt and
disposition of all scheduled drugs.
Interpretive Guidelines §482.25(a)(3)
Components of a record system to maintain current and accurate records of the receipt and
disposition of scheduled drugs would include:
• Accountability procedures to ensure control of the distribution, use, and disposition
of all scheduled drugs.
• Records of the receipt and disposition of all scheduled drugs must be current and
must be accurate.
• Records trace the movement of scheduled drugs throughout the service.
• The pharmacist is responsible for determining that all drug records are in order and
that an account of all scheduled drugs is maintained and reconciled.
• The record system, delineated in policies and procedures, tracks movement of all
scheduled drugs from the point of entry into the hospital to the point of departure
either through administration to the patient, destruction or return to the manufacture.
This system provides documentation on scheduled drugs in a readily retrievable
manner to facilitate reconciliation of the receipt and disposition of all scheduled
drugs.
• All drug records are in order and an account of all scheduled drugs is maintained
and any discrepancies in count are reconciled promptly.
• The hospital system is capable of readily identifying loss or diversion of all
controlled substances in such a manner as to minimize the time frame between the
actual loss or diversion to the time of detection and determination of the extent of
loss or diversion?
• Facility policies and procedures should minimize scheduled drug diversion.
Survey Procedures §482.25(a)(3)
• Determine if there is a record system in place that provides information on
controlled substances in a readily retrievable manner.
• Review the records to determine that they trace the movement of scheduled drugs
throughout the service.
• Determine if there is a system, delineated in policies and procedures, that tracks
movement of all scheduled drugs from the point of entry into the hospital to the
point of departure either through administration to the patient, destruction or return
to the manufacture. Determine if this system provides documentation on scheduled
drugs in a readily retrievable manner to facilitate reconciliation of the receipt and
disposition of all scheduled drugs.
• Determine if the pharmacist is responsible for determining that all drug records are
in order and that an account of all scheduled drugs is maintained and periodically
reconciled.
• Is the hospital system capable of readily identifying loss or diversion of all
controlled substances in such a manner as to minimize the time frame between the
actual losses or diversion to the time of detection and determination of the extent of
loss or diversion?
• Determine if facility policy and procedures minimize scheduled drug diversion.
A-0500
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.25(b) Standard: Delivery of Services
In order to provide patient safety, drugs and biologicals must be controlled and
distributed in accordance with applicable standards of practice, consistent with
Federal and State law.
Interpretive Guidelines §482.25(b)
Drugs and biologicals must be controlled and distributed in accordance with applicable
Federal and State laws and regulations, and in accordance with applicable standards of
practice. Applicable standards of practice include compliance with all Federal and State
laws, regulations, and guidelines. The procedures established to prevent unauthorized
usage and distribution must provide for an accounting of the receipt and disposition of
drugs subject to the Comprehensive Drug Abuse Prevention and Control Act of 1970.
Other sources of additional guidelines could include, but are not limited to: American
Society of Health-System Pharmacists, American College of Clinical Pharmacy, American
Pharmacists Association, United States Pharmacopeia, etc.
The hospital must have a process in place for medication orders to be received in the
pharmacy and dispensed in a safe and timely manner. Safe dispensing of medications must
be in accordance with accepted standards of practice and includes, but is not limited to, the
following:
• Implementing systems such as dose limits, pre-printed orders, special labeling, or
double checks to minimize adverse drug events, especially for high alert
medications;
• Reviewing all medication orders (except in emergency situations) for
appropriateness by a pharmacist before the first dose is dispensed. A process is
established for resolving questions with the prescribing practitioner and the
discussion and outcome are documented in the patient’s medical record or pharmacy
copy of the prescriber’s order;
This review should include:
• Therapeutic appropriateness of a patient’s medication regimen;
• Therapeutic duplication in the patient’s medication regimen;
• Appropriateness of the drug, dose, frequency, and route of administration;
• Real or potential medication-medication, medication-food, medication-
laboratory test and medication-disease interactions;
• Real or potential allergies or sensitivities; and
• Other contraindications.
• Medications dispensed by the hospital are retrieved when recalled or discontinued
by the manufacturer or the Food and Drug Administration (FDA) for safety reasons;
• Policies and procedures that address the use of medications brought into the hospital
by patients or their families when self-administration of medications is permitted by
hospital policy; and
• Having a system in place to reconcile medications that are not administered (e.g.,
left in the patient’s medication drawer) when the pharmacy inventories patient
medications or restocks patient medications. For example, did the patient refuse the
medication, was there a clinical or treatment reason the medication was not used, or
was the medication not used due to an error?
Monitoring the Effects of Medications
The pharmaceutical service may be responsible for monitoring the effects of medication(s)
specified per hospital policy to assure medication therapy is appropriate and minimizes the
occurrence of adverse events. Typically this occurs with anticoagulant therapy and
antibiotics prescribed for the pharmacy to establish or adjust the dosage (i.e.; “pharmacy to
dose” order). In such cases, the pharmacy’s monitoring process includes:
• Clinical and laboratory data to evaluate the efficacy of medication therapy to
anticipate or evaluate toxicity and adverse effects;
• Physical signs and clinical symptoms relevant to the patient’s medication therapy;
• Assessing the patient’s own perceptions about side effects, and, when appropriate,
perceived efficacy.
(See also the Nursing CoP discussion regarding monitoring of patients at §482.23(c)(4)).
Survey Procedures §482.25(b)
• Are medication orders routinely reviewed by the pharmacy before the first dose?
What evidence can the hospital present that such reviews take place?
• Are questions regarding medication orders resolved with the prescriber and a written
notation of these discussions documented in the patient’s medical record or
pharmacy copy of the prescriber’s order?
• Does the hospital pharmacy have a system for monitoring the effects of medication
therapies for cases specified per hospital policy?
• Does the hospital retrieve and remove medications available or patient use when the
hospital has been informed of a drug recall?
A-0501
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.25(b)(1) - All compounding, packaging, and dispensing of drugs
and biologicals must be under the supervision of a pharmacist and
performed consistent with State and Federal laws.
Interpretive Guidelines §482.25(b)(1)
All pharmaceutical services involving compounding, packaging, or dispensing of drugs
and biologicals, must be conducted by or under the supervision of a pharmacist and
performed consistent with State and Federal laws. The hospital must adopt and
implement written policies and procedures to ensure all medications are prepared by
authorized personnel.
Compounded Preparations
Hospitals use many medications that need to be reconstituted, mixed or which otherwise
may be considered “compounded” preparations. Some may be compounded in the
hospital pharmacy and/or the hospital may obtain some or all from external sources. The
external sources could include:
(1)
Manufacturers;
(2)
registered outsourcing facilities, and/or compounding pharmacies.
Regardless of the source, if accepted standards for safe compounding are not met,
compounded medications may contain less or more than the intended dose and/or may be
chemically or microbiologically contaminated, with potentially devastating or even lethal
consequences for the patients who receive them.
Use of Registered Outsourcing Facilities
The Drug Quality and Security Act (DQSA), signed into law on November 27, 2013,
contains provisions relating to the oversight of compounding of human drugs. The DQSA
created a new section 503B in the FDCA under which a compounder may elect to
become an “outsourcing facility.” The law defines an “outsourcing facility” as a facility
at one geographic location or address that is engaged in the compounding of sterile drugs;
has elected to register as an outsourcing facility; and complies with all of the
requirements of section 503B of the FDCA. Facilities that elect to register as outsourcing
facilities, per section 503B:
•
Must comply with the FDA’s Current Good Manufacturing Practice (CGMP)
requirements, which contain minimum requirements for the methods, facilities,
and controls used in manufacturing, processing, and packing of a drug product.
The CGMP requirements make sure that a product is safe for use, and that it has
the ingredients and strength it claims to have. The FDA’s publishes the most
current versions of its draft and final regulations and guidance related to
compounding on its website:
http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Pharmacy
Compounding/default.htm ;
•
Will be inspected by FDA according to a risk-based schedule; and
•
Must meet certain other conditions, such as reporting adverse events and
providing FDA with certain information about the products they compound.
In a January 2014 letter to purchasers of compounded medications (available at
http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/PharmacyCompo
unding/ucm380596.htm ), the Commissioner of the FDA encouraged the use of registered
outsourcing facilities and noted that,“[a]s a purchaser of compounded drugs, you can play
an important role in improving the quality of compounded drugs by requiring
compounding pharmacies that supply drugs to your facility to register as outsourcing
facilities. Once they register, you and the patients you serve can be assured that FDA will
inspect these facilities on a risk-based schedule, hold them to CGMP requirements,
monitor the adverse event reports they are required to submit to the agency, and require
appropriate labeling.”
FDA has posted a list of Registered Human Drug Compounding Outsourcing Facilities,
including the end date of the last FDA inspection related to compounding, whether
investigators observed any significant objectionable conditions, and whether other
FDA actions were taken based on the last inspection, at:
http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/pharmacycompou
n ding/ucm378645.htm
Note that these registered outsourcing facilities are also popularly referred to as “503B
pharmacies.”
Use of Compounding Pharmacies
Compounding pharmacies, not registered as an outsourcing facility with the FDA, are
popularly referred to as “503A pharmacies” and generally are subject to oversight only
by their State pharmacy board. If a hospital obtains compounded medications from a
compounding pharmacy rather than a manufacturer or a registered outsourcing facility,
then the hospital must demonstrate how it assures that the compounded medications it
receives under this arrangement have been prepared in accordance with accepted
professional principles for compounded drugs as well as applicable State or Federal
laws or regulations. For example, does the contract with the vendor include provisions:

•
Ensuring that the hospital has access to quality assurance data verifying that
the vendor is adhering to current standards of practice for compounding
medications and can the hospital document that it obtains and reviews such
data?
•
Requiring the vendor to meet the requirements of Section 503A of the FDCA
concerning pharmacy compounding of human drug products?
Medications Compounded by the Hospital’s Pharmacy
Only the pharmacy compounds or admixes all sterile medications, intravenous admixtures,
or other drugs except in emergencies or when not feasible (for example, when there is a
need for emergency or immediate patient administration of a compounded sterile
preparation). In addition, all compounding of medications used or dispensed by the
hospital must be performed consistent with standards of safe practice applicable to both
sterile and non-sterile compounding.
Compounded medications, whether non-sterile or sterile, may be subject to physical and
chemical contamination and unintended variations in strength. Microbial contamination
and bacterial endotoxins are particularly hazardous with respect to compounded
medications that are intended to be sterile.
Packaging and Labeling of Medications
Safe medication use includes proper packaging and labeling to reduce the risk of error.
For individual drug containers: each floor stock drug container is expected to be labeled
with the name and strength of the drug, lot and control number equivalent, and expiration
date. Appropriate accessory and cautionary statements are included as well as the
expiration date and/or, if applicable, a beyond-use date (BUD). In addition, where
applicable, each patient’s individual drug container is expected to be labeled with the
patient’s full name and quantity of the drug dispensed.
If the unit dose system is utilized, each single unit dose package is expected to be labeled
with the name and strength of the drug, lot and control number equivalent, expiration date
and/or, if applicable, a BUD.
For Information – Not Required/Not to be Cited
ASHP Research and Education Foundation
TM
“Outsourcing Sterile
Products Preparation: Contractor Assessment Tool”
The ASHP Research and Education Foundation
TM
offers a tool that hospitals may find
useful for assessing vendors that provide compounded sterile preparations. The tool
can be found at:
http://www.ashpfoundation.org/MainMenuCategories/PracticeTools/SterileProductsTo
ol.aspx and click on "Start using Sterile Products Outsourcing Tool now."

Dispensing of Medications
Medications must be dispensed by the hospital in a manner that is safe and meets the
needs of the patient:
•
Quantities of medications are dispensed which minimize diversion and potential
adverse events while meeting the needs of the patient;
•
Medications are dispensed in a timely manner. The hospital must have a system
that ensures that medication orders get to the pharmacy and medications get back
to patients promptly;
•
Whenever possible, medications are dispensed in the most ready to administer
form available from the manufacturer or, if feasible, in unit dose that have been
repackaged by the pharmacy;
•
The hospital consistently uses the same dose packaging system, or, if a different
system is used, provides education about the use of the dose packaging system;
•
All concerns, issues or questions are clarified with the individual prescriber before
dispensing; and
•
Medications dispensed by the hospital are retrieved when recalled or discontinued
by the manufacturer or the Food and Drug Administration (FDA) for safety
reasons.
Medications must be available for administration to patients when needed, including
when the pharmacy is not open. Methods to accomplish this when the pharmacy is not
open could include, but are not limited to, one or more of the following: automated
dispensing units outside the pharmacy, night cabinets, contracted services after hours via
telepharmacy contracting, on-call pharmacists, etc.
•
Automated Dispensing Cabinets (ADCs) for medications are a secure option for
medication storage since they ensure locked storage of medications and allow for
electronic tracking of controlled substances and other drugs. These cabinets often
have embedded security features, such as login and password or biometric
identification so that they can only by accessed by authorized personnel.
•
Policies and procedures must address who can access medications during after-
hours.
Survey Procedures §482.25(b)(1)
•
Determine that only pharmacists or pharmacist-supervised personnel compound,
package and dispense drugs or biologicals in accordance with State and Federal laws
and regulations and accepted standards of practice by:
For Information Only
Certain provisions of the FDCA address the labeling of prescription drugs generally
(e.g., section 503(b)(2) of the FDCA). Section 503B of the FDCA includes labeling
requirements for drugs compounded by registered outsourcing facilities (see section
503B(a)(10)). Although hospitals are expected to comply with these requirements,
surveyors conducting a Medicare survey do not assess compliance with other Federal
laws.
• Interviewing pharmacy and hospital staff to determine who prepares and
dispenses drugs and biologicals;
• Observing on site preparation and dispensing operations;
• Inspecting drug storage areas.
•
Can the hospital demonstrate that compounded medications used and/or dispensed
by the hospital are being compounded consistent with standard operating
procedures and quality assurance practices? Can the pharmacy director provide
evidence that compounded medications used and/or dispensed by the hospital are
being compounded consistent with standard operating procedures and quality
assurance practices?
• If the hospital obtains compounded products from external compounding sources,
are the external source(s) registered with the FDA as outsourcing facilities? If not,
can the hospital demonstrate that it systematically evaluates and monitors whether
the outside compounding pharmacy adheres to accepted standards for safe
compounding?
• Can the pharmacy director explain the risk level(s) of the CSPs being produced in-
house and/or obtained from external sources?
• If any CSPs are produced in the hospital:
• Ask for one or more examples of situations in which a BUD had to be
determined for a compounded sterile medication (CSP) based on the policy.
Interview pharmacy personnel assigned to carry out this function within the
hospital and/or to assess how this is done by external source(s) of CSPs. Is
there evidence that the BUDs are determined consistent with the hospital’s
policies and procedures?
• Interview staff who engage in sterile and non-sterile compounding. Are they
knowledgeable about applicable levels of aseptic practices?
• Ask the pharmacy director to demonstrate how the following are
accomplished to ensure that sterile compounding practices are consistent
with standards for the risk level(s) of CSPs being produced for/dispensed to
hospital patients:
• Verification of compounding accuracy and sterility;
• Environmental quality and controls, including environmental
sampling; testing and monitoring; and cleaning and disinfection;
• Personnel training and competency assessment, including but not
limited to accuracy/precision in identifying and measuring ingredients;
cleansing and garbing; aseptic manipulation skills; environmental
quality and disinfection; appropriate work practices within and
adjacent to the direct compounding area; verification/calibration of
equipment; sterilization; and post-production quality checks.
• Review the hospital’s procedures for maintaining the quality of CSPs during
storage, transport and dispensing. Are CSPs packaged in a manner to protect
package integrity and sterility? How are CSP-specific requirements with respect
to motion, light exposure, temperature and potentially hazardous contents
addressed? How does the hospital ensure that such information is effectively
conveyed to non-pharmacy health care personnel and/or to patients/caregivers, if
applicable?
• Can the hospital document that it is systematically monitoring and tracking
adherence to all of the quality assurance and personnel training and competency
standards described above? Have any problems or risks been identified? If so,
did the hospital take effective action to protect patients, if relevant, and to
effectively remedy the problem/risk?
A-0502
(Rev. 151, Issued: 11-20-15, Effective: 11-20-15, Implementation: 11-20-15)
§482.25(b)(2)(i) - All drugs and biologicals must be kept in a secure area,
and locked when appropriate.
Interpretive Guidelines §482.25(b)(2)(i)
A secure area means that drugs and biologicals are stored in a manner to prevent
unmonitored access by unauthorized individuals. Drugs and biologicals must not be stored
in areas that are readily accessible to unauthorized persons. For example, if medications are
kept in a private office, or other area where patients and visitors are not allowed without the
supervision or presence of a health care professional (for example, ambulatory infusion),
they are considered secure. Areas restricted to authorized personnel only would generally
be considered “secure areas.” If there is evidence of tampering or diversion, or if
medication security otherwise becomes a problem, the hospital is expected to evaluate its
current medication control policies and procedures, and implement the necessary systems
and processes to ensure that the problem is corrected, and that patient health and safety are
maintained. (71 FR 68689)
All controlled substances must be locked. Hospitals are permitted flexibility in the storage
of non-controlled drugs and biologicals when delivering care to patients, and in the
safeguarding of drugs and biologicals to prevent tampering or diversion. An area in which
staff are actively providing care to patients or preparing to receive patients, i.e., setting up
for procedures before the arrival of a patient, would generally be considered a secure area.
When a patient care area is not staffed, both controlled and non-controlled substances are
expected to be locked.
Generally labor and delivery suites and critical care units are staffed and actively providing
patient care around the clock, and, therefore, considered secure. However, hospital policies
and procedures are expected to ensure that these areas are secure, with entry and exit
limited to appropriate staff, patients and visitors.
The operating room suite is considered secure when the suite is staffed and staff are actively
providing patient care. When the suite is not in use (e.g., weekends, holidays and after
hours), it would not be considered secure. A hospital may choose to lock the entire suite,
lock non-mobile carts containing drugs and biologicals, place mobile carts in a locked
room, or otherwise lock drugs and biologicals in a secure area. If an individual operating
room is not in use, the hospital is expected to lock non-mobile carts, and ensure mobile
carts are in a locked room. (71 FR 68689)
This regulation gives hospitals the flexibility to integrate patient self-administration of non-
controlled drugs and biologicals into their practices as appropriate. When a hospital allows
a patient to self-administer selected drugs and biologicals, the hospital authorizes the patient
to have access to these medications. This regulation is consistent with the current practice
of giving patients access at the bedside to urgently needed medications, such as
nitroglycerine tablets and inhalers. It supports the current practice of placing selected
nonprescription medications at the bedside for the patient’s use, such as lotions and creams,
and rewetting eye drops. Hospitals are expected to address patient self-administration of
non-controlled drugs and biologicals in their policies and procedures (see self-
administration discussion at §§482.23(c)(6)(i) and 482.23(c)(6)(ii)). This regulation
supports hospital development, in collaboration with the medical staff and the nursing and
pharmacy departments, of formal patient medication self-administration programs for select
populations of patients, including hospital policies and procedures necessary to ensure
patient safety and security of medications. The policies and procedures are expected to
include measures to ensure the security of bedside drugs and biologicals. They are also
expected to address both the competence of the patient to self-administer drugs and
biologicals as well as patient education regarding self-administration of drugs and
biologicals. (71 FR 68689)
Due to their mobility, mobile nursing medication carts, anesthesia carts, epidural carts and
other medication carts containing drugs or biologicals (hereafter, all referred to as “carts”)
must be locked in a secure area when not in use. Hospital policies and procedures are
expected to address the security and monitoring of carts, locked or unlocked, containing
drugs and biologicals in all patient care areas to ensure their safe storage and to ensure
patient safety. (71 FR 68689)
Medication automated distribution units with security features, such as logon and password
or biometric identification, are considered to be locked, since they can only be accessed by
authorized personnel who are permitted access to the medications. Such units must be
stored in a secure area.
Survey Procedures §482.25(b)(2)(i)
• Review hospital policies and procedures governing the security of drugs and
biologicals to determine whether they provide for securing and locking as
appropriate;
• Review hospital policies and procedures governing patient self-administration of
drugs and biologicals;
• Observe whether medications in various areas of the hospital are stored in a secure
area, and locked when appropriate. Are medication storage areas periodically
inspected by pharmacy staff to make sure medications are properly stored?
• Determine that security features in automated medication distribution units are
implemented and actively maintained, e.g., that access authorizations are regularly
updated to reflect changes in personnel, assignments, etc.
• Interview staff to determine whether policies and procedures to restrict access to
authorized personnel are implemented and effective;
• If patient self-administration of drugs and biologicals is permitted, interview
patients and staff to determine whether policies and procedures are implemented and
effective.
A-0503
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.25(b)(2)(ii) - Drugs listed in Schedules II, III, IV, and V of the Comprehensive
Drug Abuse Prevention and Control Act of 1970 must be kept locked within a secure
area.
Interpretive Guidelines §482.25(b)(2)(ii)
All Schedule II, III, IV, and V drugs must be kept locked within a secure area. A secure
area means the drugs and biologicals are stored in a manner to prevent unmonitored access
by unauthorized individuals. Medication automated distribution units with logon and
password/biometric identification are considered to be locked, since they can only be
accessed by authorized personnel who are permitted access to Schedule II – V medications.
Mobile nursing medication carts, anesthesia carts, epidural carts and other medication carts
containing Schedule II, III, IV, and V drugs must be locked within a secure area.
Survey Procedures §482.25(b)(2)(ii)
• Determine whether there is a hospital policy and procedure that requires Schedule
II, III, IV, and V drugs to be kept in a locked storage area.
• Observe in various parts of the hospital whether Schedule II, III, IV and V drugs are
locked and stored in a secure area..
• Determine whether security features in automated medication distribution units are
implemented and actively maintained, e.g., that access authorizations are regularly
updated to reflect changes in personnel, assignments, etc.
• Interview staff to determine whether policies and procedures to restrict access to
authorized personnel are implemented and effective.
A-0504
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.25(b)(2)(iii) - Only authorized personnel may have access to locked areas.
Interpretive Guidelines §482.25(b)(2)(iii)
The hospital must assure that only authorized personnel may have access to locked areas
where drugs and biologicals are stored.
A hospital has the flexibility to define which personnel have access to locked areas, based
on the hospital’s needs as well as State and local law. For example, a hospital could include
within its definition of “authorized personnel” ancillary support personnel, such as
engineering, housekeeping staff, orderlies and security personnel as necessary to perform
their assigned duties. The hospital’s policies and procedures must specifically address how
“authorized personnel” are defined for purposes of this section. It is not necessary for the
policy to name specific authorized individuals, but the policy should be clear in describing
the categories of personnel who have authorized access, as well as whether there are
different levels of access authorized in different areas of the hospital, or at different times of
day, or for different classes of drugs and biologicals, etc.
The hospital’s policies and procedures must also address how it prevents unauthorized
personnel from gaining access to locked areas where drugs and biologicals are stored.
Whenever unauthorized personnel have access, or could gain access, to those locked areas,
the hospital is not in compliance with this requirement and is expected to re-evaluate and
tighten its security measures.
Survey Procedures §482.25(b)(2)(iii)
• Determine whether there is a hospital policy and procedure defining authorized
personnel that are permitted access to locked areas where drugs and biologicals are
stored.
• Determine whether there is a hospital policy and procedure for limiting access to
locked storage areas to authorized personnel only.
• Observe whether or not access to locked storage areas is limited to personnel
authorized by the hospital’s policy.
A-0505
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.25(b)(3) - Outdated, mislabeled, or otherwise unusable drugs and biologicals
must not be available for patient use.
Interpretive Guidelines §482.25(b)(3)
The hospital must have a pharmacy labeling, inspection, and inventory management system
that ensures that outdated, mislabeled, or otherwise unusable drugs and biologicals are not
available for patient use. This would include drugs that are the subject of a manufacturer’s
recall.
A drug or biological is outdated after its expiration date, which is set by the manufacturer
based on stability testing under specified conditions as part of the FDA approval process. It
should be noted that a drug or biological may become unusable prior to its expiration date if
it has been subjected to conditions that are inconsistent with the manufacturer’s approved
labeling.
A drug or biological is also outdated after its “beyond-use date” (BUD), which may be
reached before the expiration date, but never later. The BUD takes into account the specific
conditions and potential for deterioration and microbial growth that may occur during or
after the original container is opened, while preparing the medication for dispensing and
administration, and/or during the compounding process if it is a compounded medication.
The BUD is to be based on information provided by the manufacturer, whenever such
information is available. The hospital must maintain and implement policies and
procedures that provide clear and consistent direction to pharmacy staff regarding how to
determine a BUD when complete BUD information is not available from the manufacturer.
The policies and procedures must be based on accepted professional principles.
For individual drug containers: each floor stock drug container is expected to be labeled
with the name and strength of the drug, lot and control number equivalent, and expiration
date. Appropriate accessory and cautionary statements are included as well as the
expiration date and/or, if applicable, a BUD. In addition, where applicable, each patient’s
individual drug container is expected to be labeled with the patient’s full name and quantity
of the drug dispensed.
If the unit dose system is utilized, each single unit dose package is expected to be labeled
with the name and strength of the drug, lot and control number equivalent, expiration date
and/or, if applicable, a BUD.
Survey Procedures §482.25(b)(3)
• Spot-check the labels of individual drug containers to verify that they conform to
Federal and State laws, and/or contain the following minimal information:
• Each patient’s individual drug container bears his/her full name, and strength
and quantity of the drug dispensed. Appropriate accessory and cautionary
statements are included as well as the expiration date and/or, if applicable, a
BUD;
• Each floor stock container bears the name and strength of the drug, lot and
control number of equivalent, expiration date;
• If the unit dose system is utilized, verify that each single unit dose package bears
name and strength of the drug, lot and control number equivalent, expiration date
and/or, if applicable, a BUD;
• Inspect patient-specific and floor stock medications to identify expired, mislabeled or
unusable medications.
A-0506
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.25(b)(4) - When a pharmacist is not available, drugs and biologicals must be
removed from the pharmacy or storage area only by personnel designated in the
policies of the medical staff and pharmaceutical service, in accordance with Federal
and State law.
Interpretive Guidelines §482.25(b)(4)
Routine after-hours access to the pharmacy by non-pharmacists for access to medication
should be minimized and eliminated as much as possible. The use of well-designed night
cabinets, after-hours medication carts, and other methods may preclude the need for non-
pharmacist to enter the pharmacy. Policies and procedures should be consistent with
Federal and State Law.
If an urgent or emergent patient need occurs, the hospital must be able to provide
medications to the patients in its facility.
The hospital must have a process for providing medications to meet patient needs when the
pharmacy is closed.
When non-pharmacist health care professionals are allowed by law and regulation to obtain
medications after the pharmacy is closed, the following safeguards are applied:
• Access is limited to a set of medications that has been approved by the hospital.
These medications can be stored in a night cabinet, automated storage and
distribution device, or a limited section of the pharmacy.
• Only trained, designated prescribers and nurses are permitted access to medications.
• Quality control procedures (such as an independent second check by another
individual or a secondary verification built into the system, such as bar coding) are
in place to prevent medication retrieval errors.
• The hospital arranges for a qualified pharmacist to be available either on-call or at
another location (for example, at another organization that has 24-hour pharmacy
service) to answer questions or provides medications beyond those accessible to
non-pharmacy staff.
• This process is evaluated on an on-going basis to determine the medications
accessed routinely and the causes of accessing the pharmacy after hours.
• Changes are implemented as appropriate to reduce the amount of times non-
pharmacist health care professionals are obtaining medications after the pharmacy is
closed.
Survey Procedures §482.25(b)(4)
• Determine through pharmacy records that when the pharmacist is not available,
drugs are removed from the pharmacy (drug storage area) only by a designated
individual (in accordance with State law if applicable) and only in amounts
sufficient for immediate therapeutic needs.
• Review policies and procedures to determine who is designated to remove drugs and
biologicals from the pharmacy or storage area and the amount a non-pharmacist
may remove in the absence of a pharmacist. The individual(s) designated should be
identified by name and qualifications.
• Determine that a system is in place that accurately documents the removal of
medications (type and quantity) from either the pharmacy or the after hours supply.
• Determine that the pharmacist reviews all medication removal activity and
correlates the removal with current medication orders in the patient medication
profile.
• Determine if the pharmacist routinely reviews the contents of the after-hours supply
to determine if it is adequate to meet the after-hours needs of the hospital.
A-0507
(Rev. 151, Issued: 11-20-15, Effective: 11-20-15, Implementation: 11-20-15)
§482.25(b)(5) - Drugs and biologicals not specifically prescribed as to time
or number of doses must automatically be stopped after a reasonable time
that is predetermined by the medical staff.
Interpretive Guidelines §482.25(b)(5)
In accordance with accepted standards of practice, the medical staff, in coordination and
consultation with the pharmacy service, determines and establishes the reasonable time to
automatically stop orders for drugs and biologicals not specifically prescribed as to time or
number of doses. The hospital must implement, monitor, and enforce this automatic stop
system.
It is important to note that hospitals with an electronic health record (EHR) system may
have time and dose parameters automatically built into computerized provider order entry
(CPOE) screens. These may be part of the hospital’s plan for addressing automatic stop
orders.
Survey Procedures §482.25(b)(5)
• Review policies and procedures to determine that there is a protocol established by
the medical staff to discontinue and review patients’ medical records to determine
compliance with stop-order policy;
• Ask unit staff what happens in the case of drugs with no stop date or prescribed
number of doses. Are they aware of the automatic stop policy? Can they describe
how it is enforced?
A-0508
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
§482.25(b)(6) - Drug administration errors, adverse drug reactions, and
incompatibilities must be immediately reported to the attending physician and, if
appropriate, to the hospital’s quality assessment and performance improvement
program .
Interpretive Guidelines §482.25(b)(6)
Hospitals are required to ensure that the attending physician is made immediately aware of
drug administration errors, adverse drug reactions, and incompatibilities. When the
attending physician is unavailable, the covering physician must be notified. When the
covering physician must be notified, the patient’s attending physician must be notified as
soon as he/she is available. In addition, when appropriate, such events must also be
reported to the hospital-wide Quality Assessment and Performance Improvement (QAPI)
program.
The hospital must adopt policies and procedures that identify the types of events that must
be reported immediately to the attending physician, as well as those to be reported to the
QAPI program.
• Drug administration error:
The National Coordinating Council Medication Error Reporting and Prevention
definition of a medication error is “Any preventable event that may cause or lead to
inappropriate medication use or patient harm while the medication is in the control
of the health care professional, patient, or consumer. Such events may be related to
professional practice, health care products, procedures, and systems, including
prescribing; order communication; product labeling, packaging, and nomenclature;
compounding; dispensing; distribution; administration; education; monitoring; and
use.” In the context of this regulation, however, “drug administration error” is
limited to those errors in administration that actually reach the patient, i.e., a
medication actually is administered to a patient when it should not be, or the wrong
dose is administered, or the wrong root of administration is used, etc., or a
medication that should have been administered to the patient has not been
administered in a timely manner, as discussed in the medication administration
standard at 42 CFR 482.23(c).
• Adverse drug reaction:
The American Society of Health-System Pharmacists (ASHP) defines an adverse
drug reaction (ADR) as “Any unexpected, unintended, undesired, or excessive
response to a drug that:
1. Requires discontinuing the drug (therapeutic or diagnostic)
2. Requires changing the drug therapy
3. Requires modifying the dose (except for minor dosage adjustments)
4. Necessitates admission to a hospital
5. Prolongs stay in a health care facility
6. Necessitates supportive treatment
7. Significantly complicates diagnosis
8. Negatively affects prognosis, or
9. Results in temporary or permanent harm, disability, or death.
Consistent with the definition, an allergic reaction (an immunologic hypersensitivity
occurring as the result of unusual sensitivity to a drug) and an idiosyncratic reaction
(an abnormal susceptibility to a drug that is peculiar to the individual) are also
considered ADRs.”
• Drug incompatibilities
A drug incompatibility occurs when drugs interfere with one another chemically or
physiologically. Drugs known to be incompatible must not be mixed, administered
together, or administered within a timeframe where they will interfere with each
other.
When IV medications are administered with known incompatibilities, an error has
occurred and it needs to be reported to the attending physician immediately. Any
unexpected reaction that occurs between IV medications not previously identified as
incompatible also needs to be reported.
Hospitals can minimize the risk of administering incompatible medications by
making available pertinent resources, such as drug incompatibility charts and online
incompatibility references. The incompatibility information needs to be readily
available to staff administering medications. The information needs to be kept up-
to-date as the information is frequently updated by drug manufacturers.
The immediate reporting requirement applies to drug administration errors, adverse drug
reactions or incompatibilities that have harmed or have the potential to harm the patient. If
the outcome of the drug administration error is unknown, the physician must also be
notified without delay.
Drug administration errors that result in no or insignificant harm to the patient must also be
documented in the medical record but do not require immediate reporting to the attending
physician. For example, if an analgesic dose is missed during the night shift, it can be
reported first thing in the morning. Hospital staff is expected to use their clinical judgment,
based on patient presentation and assessment in accordance with hospital policy and
procedures, to determine whether immediate reporting is required.
On the other hand, for purposes of reporting to the hospital’s QAPI program, hospitals
must, in accordance with the requirements of the QAPI CoP at 42 CFR 482.21(c)(2), track
and report not only the errors that cause or risk harm to the patient, but also those which do
not. Such “near misses” and suspected ADRs may reveal important information about
systems vulnerabilities that the hospital should address in order to avoid events that result in
harm.
Hospitals must establish policies and procedures for reporting of medication errors, ADRs,
and incompatibilities, and ensure that staff is aware of the reporting process. For those
events that require immediate reporting, the hospital’s policies must establish timeframes
for reporting that are based on the clinical effect of the error on the patient.
To improve staff willingness to report medication error incidents, hospitals are encouraged
to adopt a non-punitive approach that focuses on system issues rather than individual health
care professionals. A non-punitive approach is likely to encourage reporting by those who
otherwise may fear retribution or hospital disciplinary action.
In addition to employing broad definitions of medication errors and ADRs for QAPI
tracking purposes and encouraging the reporting of medication errors, ADRs and drug
incompatibilities, the hospital must take additional steps to identify these events as part of
its QAPI program where medical errors and adverse patient events are measured, analyzed
and tracked. Reliance solely on incident reporting fails to identify the majority of errors
and adverse reactions. Proactive identification includes observation of medication passes,
concurrent and retrospective review of a patient’s clinical records, ADR surveillance team,
implementation of medication usage evaluations for high-alert drugs, and identification of
indicator drugs that, when ordered, automatically generate a drug regimen review for a
potential adverse drug event.
The hospital must have a method by which to measure the effectiveness of its systems for
identifying and reporting to the QAPI program medication errors and ADRs. Such methods
could include use of established benchmarks for the size and scope of services provided by
the hospital, or studies on reporting rates published in peer-reviewed journals. Hospitals are
encouraged, and may be required by State law, to participate in statewide and national
reporting of drug administration errors, adverse drug reactions, and incompatibilities.
National organizations include, but are not limited to, the Food and Drug Administration’s
(FDA) MedWatch Reporting Program and the Institute for Safe Medication Practices
(ISMP) Medication Errors Reporting Program.
Survey Procedures §482.25(b)(6)
• Does the hospital have policies and procedures that define medications errors,
ADRs, and drug incompatibilities? Do they address the circumstances under which
they must be reported immediately to the attending physician, as well as to the
hospital’s QAPI program? Do they address how reporting is to occur?
• Are all medication errors and suspected ADRs promptly recorded in the patient’s
medical record, including those not subject to immediate reporting?
• If upon review of a sample of records, a suspected ADR or medication error is
identified, determine if it was reported immediately to the attending or covering
physician, in accordance with the hospital’s written policies and procedures. If it is
reported to a covering physician, determine if it was also reported to the attending
physician when he/she became available.
• Ask hospital staff what they do when they become aware of a medication error,
ADR or drug incompatibility. Are staff aware of and do they follow the hospital’s
policy and procedures?
• Ask hospital staff how they manage drug incompatibilities. What tools do they use
in the clinical setting to minimize the risk of incompatibilities? How is the
information related to drug incompatibilities made available to the clinical staff
administering IV medications (posters, online tools, etc.)? How often is the
information updated to ensure accuracy?
• Interview hospital staff to ascertain awareness of the hospital’s policy on reporting
and documentation of medication errors and adverse drug reactions.
• How does information regarding medication errors, adverse drug reactions, and
incompatibilities get reported to the hospital QAPI program? Ask staff to speak to
the process.
• For QAPI reporting purposes, is the hospital’s definition of an ADR and medication
error based on national standards?
A-0509
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.25(b)(7) - Abuses and losses of controlled substances must be reported, in
accordance with applicable Federal and State laws, to the individual responsible for
the pharmaceutical service, and to the chief executive officer, as appropriate.
Survey Procedures §482.25(b)(7)
• Interview the pharmacists, or pharmacy employees to determine their understanding
of the controlled drug policies.
• Conduct a spot check of drug use and other inventory records to ensure that drugs
are properly accounted for.
• Review reports of pharmaceutical services to determine if there are reported
problems with controlled drugs and what actions have been taken to correct the
situation.
• Interview the Pharmacy Director, pharmacist and pharmacy employees to determine
their understanding of the controlled drug policies. Is there a policy and procedure
for handling controlled drug discrepancies?
• Review reports of pharmaceutical services to determine if there are reported
problems with controlled drugs and what actions have been taken to correct the
situation.
• Determine if controlled drug losses were reported to appropriate authorities in
accordance with State and Federal laws.
A-0510
(Rev. 151, Issued: 11-20-15, Effective: 11-20-15, Implementation: 11-20-15)
§482.25(b)(8) - Information relating to drug interactions and information
of drug therapy, side effects, toxicology, dosage, indications for use, and
routes of administration must be available to the professional staff.
Interpretive Guidelines §482.25(b)(8)
The pharmacy must be a resource for medication-related information to the hospital’s
health-care practitioners and other health care personnel to optimize therapeutic outcomes
and minimize adverse drug events. Information must be available concerning drug
interactions and information of drug therapy, side effects, toxicology, dosage, indications
for use, and routes of administration. The pharmacy may also assist other health care
professionals with the following medication-related functions:
• Collection and organization of patient-specific information (height, weight,
allergies);
• Identification of the presence of medication-therapy problems, both potential and
actual, such as drug-drug interactions, excessive doses;
• Identification and specification of pharmaco-therapeutic goals;
• Implementation of a monitoring plan in collaboration with the patient, if applicable,
and other health-care professionals;
• Monitoring the effects of the pharmacotherapeutic regimen – could include
adjusting doses based on lab values (i.e.: Coumadin dosing); or
• Redesigning the regimen and monitoring plan as indicated.
For example, practitioners may write an order for “pharmacy to dose” an antibiotic. The
pharmacist would then take patient-specific information, review the patient’s current
medication therapies for any problems, and then calculate the dose required to meet
therapeutic goals.
Increasingly, as hospitals move to computerized physician-order entry (CPOE) of
medication orders, much of this consultation function (e.g.; dosage, path of administration,
drug-drug interactions and other contraindications, etc.) is built in to the electronic health
record (EHR) system. However, the pharmacy service remains responsible for the
provision of accurate, up-to-date information to meet the needs of the hospital’s
practitioners, nursing staff and patients.
The hospital must also have immediately available sufficient up-to-date reference material
on drug therapy, whether in electronic or hard copy format. A pharmacist also should be
readily available by telephone or other means to respond to questions from practitioners and
nursing personnel.
Survey Procedures §482.25(b)(8)
• Is drug information readily available to nurses and practitioners, whether in hard
copy or electronic format?
• If drug information is built in to the hospital’s EHR system, ask the pharmacy
director how the hospital ensures that the information is accurate and up-to-date;
• Ask practitioners whether needed reference information is available to them when
prescribing drugs;
• Ask nursing staff whether needed reference information is available to them when
administering drugs or biologicals and when monitoring patients for effects of
medication therapies.
A-0511
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.25(b)(9) - A formulary system must be established by the medical staff to assure
quality pharmaceuticals at reasonable costs.
Interpretive Guidelines §482.25(b)(9)
The medical staff must establish a formulary system. The formulary lists medications for
dispensing or administration that the hospital maintains or that are readily available. In
accordance with accepted standards of practice, the medical staff, in consultation with the
pharmacy service, should develop written criteria for determining what medications are
available for dispensing or administration. At a minimum, the criteria include the
indication for use, effectiveness, risks (including propensity for medication errors, abuse
potential, and sentinel events), and costs.
Processes and mechanisms should be established to monitor patient responses to a newly
added medication before the medication is made available for dispensing or administration
within the hospital.
Medications designated as available for dispensing or administration are reviewed
periodically based on emerging safety and efficacy information.
The hospital should have processes to approve and procure medications that are not on the
hospital’s medication list.
The hospital should have processes to address medication shortages and outages, including
the following:
• Communicating with appropriate prescribers and staff;
• Developing approved substitution protocols;
• Educating appropriate LIPs, appropriate health care professionals, and staff about
these protocols; and
• Obtaining medications in the event of a disaster.
Survey Procedures §482.25(b)(9)
• Interview the pharmacist to determine that the medical staff has established a
formulary that lists drugs that actually are available in the hospital.
• Interview the Pharmacy Director to determine that there is a process for creation and
periodic review of a formulary system.
• Determine that the formulary lists drugs that are available.
______________________________________________________________________
A-0528
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.26 Condition of Participation: Radiologic Services
The hospital must maintain, or have available, diagnostic radiologic services. If
therapeutic services are also provided, they, as well as the diagnostic services, must
meet professionally approved standards for safety and personnel qualifications.
Interpretive Guidelines §482.26
Hospitals must offer diagnostic radiologic services and may also offer therapeutic
radiologic services. No matter where they are furnished in the hospital (including all
departments on all campuses and off-site locations) radiologic services must satisfy
professionally approved standards for safety and personnel qualifications. Hospitals are
expected to take a consistent approach in their policies and procedures for radiologic
services safety and personnel qualifications throughout the hospital. This may be
accomplished in several ways, including by having one organized radiologic service under
the direction of the radiologist who supervises all ionizing radiology services (see
§482.26(c)(1)), or by the governing body ensuring a uniform approach to radiologic
services that are offered in multiple departments of the hospital. .
The elements of the Condition’s regulatory language (the Condition “stem” statement) are
close, but not identical, to those found in the standards at §§482.26(a) and (b). We have,
therefore, repeated elements of this Condition regulatory language in the Tags for both

§§482.26(a) and (b), in order to permit citation of deficiencies that are specific to
requirements found in the Condition stem statement at either the standard or condition
level, as appropriate. The manner or degree of noncompliance with the requirements of
this Condition and its component standards must be evaluated to determine whether there is
substantial noncompliance with the Condition, warranting a Condition-level citation.
What is included in Radiologic Services?
Radiologic services encompass many different modalities used for the purpose of diagnostic
or therapeutic medical imaging and radiation therapy. Each type of technology yields
different information about the area of the body being studied or treated, related to possible
disease, injury, or the effectiveness of medical treatment. All the modalities use some form
of radiation, which is a term for energy waves or particles that pass through a medium, such
as light or radio signals through the air. Some of these modalities (radiography, computed
tomography, fluoroscopy) utilize ionizing radiation, which has enough energy to potentially
cause damage to DNA, while others (ultrasound, magnetic resonance imaging) use other
forms of non-ionizing radiation to view the human body in order to diagnose, monitor, or
treat medical conditions.
Most of the definitions and terms referred to in this guidance are based on technical
information available on the U.S. Food and Drug Administration’s (FDA) website, located
at http://www.fda.gov/Radiation-EmittingProducts/default.htm or from the Radiologic
Society of North America’s (RSNA) website, located at http://www.radiologyinfo.org .
Diagnostic & Therapeutic Radiologic Services
Diagnostic and therapeutic radiologic services may use the same modalities, but for
different purposes. Diagnostic services are performed to determine a specific cause of the
medical problem with which the patient presents (e.g., fractured bone, occluded artery,
tumor), while therapeutic services are performed to treat a specific problem (e.g., stenting
of an artery or embolization of a blood vessel, lithotripsy of a renal stone, external beam
radiation therapy to a cancerous tumor). Regardless of the purpose of the radiologic
services, the risks to the patient and staff, if applicable, depend on the modality used, the
length of the study/procedure, the size of the patient, the specifics of the device being used,
and other factors.
Modalities that use Ionizing Radiation
Radiography (X-rays) is a technique for generating and recording an x-ray pattern for the
purpose of providing the user with a static image(s) after termination of the exposure.
During a radiographic procedure, an x-ray beam is passed through the body. A portion of
the x-ray is absorbed or scattered by the body’s internal structure and the remaining x-ray
pattern is transmitted to a detector, so that an image may be recorded for later evaluation.
The recording of the pattern may occur on film or through electronic means (digital). X-
rays are used to diagnose or treat patients by displaying images of the internal structure(s)
of the body to assess the presence or absence of disease, foreign objects, and structural
damage or anomaly.
Some common examples include:
• Verification of correct placement of invasive catheters, tubes, or devices;
• Orthopedic evaluations for fractured or dislocated bones;
• Chest x-ray to identify common conditions, such as congestive heart failure or
pneumonia;
• Evaluations of radio-opaque foreign bodies in soft tissues; and
• Mammography.
Dual-energy X-ray absorptiometry (DEXA) is a form of medical imaging that uses very
small amounts of ionizing radiation to measure bone mineral density and determine an
individual’s risk for bone fractures or establish the diagnosis of osteoporosis. The amount
of radiation used is less than one-tenth the dose of a traditional chest X-ray and less than
one day’s exposure to natural radiation.
Computed Tomography (CT) scanning, also called computerized axial tomography
(CAT) scanning, is a medical imaging procedure that uses x-rays to show cross-sectional
images of the body. A CT imaging system produces cross-sectional images or "slices" of
areas of the body, like the slices in a loaf of bread. During a CT scan, a patient undergoes
several consecutive and simultaneous X-rays that can be configured as a three dimensional
reconstruction of the part of the body that is being imaged. Thus, a CT scan delivers more
ionizing radiation to the patient than radiography. CTs are better able to distinguish
between different types of tissues in the body than radiography and, given its ability to
image large areas over a short period of time, CT offers significantly improved resolution of
many different structures in a variety of spatial configurations. Often a CT scan will be
performed using x-ray dye or contrast agent, which can be administered by mouth or by
vein. This technique further helps to identify the intestines or vasculature, which can assist
with the diagnosis of disease or injury.
Some common examples include:
• CT of the brain to distinguish between an ischemic or hemorrhagic stroke;
• CT of the abdomen and pelvis to evaluate for internal bleeding following trauma;
• CT of the chest to determine the presence of a pulmonary embolus; and
• CT of the aorta with intravenous contrast agent to determine a ruptured aneurysm.
Fluoroscopy is a type of medical imaging that shows a continuous x-ray image on a
monitor, much like an x-ray movie. It is used to diagnose or treat patients by displaying the
movement of a body part, or of an instrument or x-ray dye (contrast agent) through the
body.
Fluoroscopy is used in many types of examinations and procedures. Some examples
include:
• Barium upper GI (gastrointestinal) series and enemas (to view movement through the
GI tract);
• Catheter insertion (to direct the placement of a catheter in a blood vessel);
• Orthopedic surgery (to view fracture treatments); and
• Angiography (to determine if there are blockages in arteries).
The amount of ionizing radiation that a patient and the medical staff receive during the
procedure depends on the procedure’s length and complexity.
Radiation Therapy
Ionizing radiation can also be used for therapeutic purposes, in which the energy is utilized
to directly kill cancerous cells.
External beam therapy (EBT) is a method to deliver a beam of high-energy x-rays to a
patient’s tumor. The beam is generated outside the patient and is targeted at the tumor site.
The goal is to deposit the energy to kill the cancer cells while sparing the normal tissue.
EBT is often used to treat cancers of the breast, head and neck, prostate, lung, and brain. It
also can be used to provide palliative care for painful sites of metastases to bone.
Brachytherapy is a type of radiation therapy in which radioactive material is placed
directly inside or next to the tumor. This type of therapy allows for a higher dose of
radiation to treat a smaller area and in a shorter time than with EBT. It can be either
temporary, in which the radioactive material is placed inside or near a tumor for a specified
amount of time, often via a catheter; or permanent, in which radioactive seeds or pellets are
placed near or inside a tumor and left there permanently, eventually decaying so that the
radioactivity diminishes to nothing. Brachytherapy is often used to treat solid tumors,
including prostate, breast, and gallbladder cancer.
Radiologic Services modalities that do not use ionizing radiation
Ultrasound
Ultrasound imaging (sonography) uses high-frequency sound waves to view soft tissues,
such as muscles and internal organs. Because ultrasound images are captured in real-time,
they can show movement of the body's internal organs as well as blood flowing through
blood vessels. This imaging modality has no documented evidence of dangers to the patient
or staff administering it, however, caution about the frequency of use has been encouraged,
particularly in the imaging of fetuses. Ultrasound imaging is used in many types of
examinations and procedures. Some examples include:
• Doppler ultrasound (to visualize blood flow through a blood vessel);
• Echocardiogram (to view the heart);
• Fetal ultrasound (to view the fetus in pregnancy);
• Ultrasound-guided biopsies of suspicious masses;
• Doppler fetal heart rate monitors (to listen to the fetal heart beat); and
• Lithotripsy to break up kidney stones; this procedure uses high energy sound waves
(shock waves), but there is minimal risk to the patient and staff from this form of
energy. Pre- and post-procedure radiographs are taken of the patient, which confer the
same risk as a standard X-ray of that part of the body.
Magnetic resonance imaging (MRI) is a medical imaging procedure that uses strong
magnetic fields and radio waves to produce cross-sectional images of organs and internal
structures in the body. Because the signal detected by an MRI machine varies depending on
the water content and local magnetic properties of a particular area of the body, different
tissues or substances can be distinguished from one another in the study image.
MRI can give different information about structures in the body than can be obtained using
a standard x-ray, ultrasound, or computed tomography (CT) exam. For example, an MRI
study of a joint can provide detailed images of ligaments and cartilage, which are not
visible using other modalities. In some cases, an MRI contrast agent is given by vein to
show internal structures or abnormalities more clearly.
In most MRI devices, an electric current is passed through coiled wires to create a
temporary magnetic field in a patient’s body. (In open-MRI devices, permanent magnets are
used.) Radio waves are sent from and received by a transmitter/receiver in the machine, and
these signals are used to produce digital images of the area of interest.
MRI scans facilitate diagnosis or monitoring of treatments for a variety of medical
conditions, including:
• Abnormalities of the brain and spinal cord;
• Tumors, cysts, and other abnormalities in various parts of the body;
• Injuries or abnormalities of the joints;
• Certain types of heart problems;
• Diseases of the liver and other abdominal organs;
• Causes of pelvic pain in women (e.g., fibroids, endometriosis); and
• Suspected uterine abnormalities in women undergoing evaluation for infertility.
A-0529
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
[§482.26 Condition of Participation: Radiologic Services
The hospital must maintain, or have available, diagnostic radiologic services...]
§482.26(a) Standard: Radiologic Services
The hospital must maintain, or have available, radiologic services according to the
needs of the patients.
Interpretive Guidelines §482.26 (a)
Mandatory and Optional Radiologic Services
The hospital must maintain, or have available, diagnostic radiological services according to
the needs of the volume and types of patients the hospital serves. “Maintain” in this context
means furnishing radiologic services on-site, while having them available means providing
access to radiologic services even when they are not furnished on-site. For example:
• It would not be uncommon for a psychiatric hospital to maintain on-site relatively
limited or no radiologic services, while making more extensive diagnostic services
available under arrangement, at a site outside the psychiatric hospital.
• On the other hand, a short-term acute care hospital with a busy emergency department
that handles trauma, stroke, and other complex medical and surgical cases would be
expected to maintain on-site a wider range of diagnostic radiologic services that are
ready to be furnished when needed.
A hospital’s diagnostic radiologic services must be maintained or available at all times.
Multi-campus hospitals must have diagnostic radiologic services that can be furnished when
needed in a clinically appropriate timeframe for each location providing inpatient, same-day
surgery, and emergency services. The scope and complexity of diagnostic radiological
services maintained or available must be specified in writing, in order to demonstrate how
the hospital meets the needs of its patients.
Therapeutic radiologic services are optional, but if they are offered, must also comply with
the Radiologic Services requirements.
Radiological services may be provided by the hospital directly utilizing its own staff, or
through a contractual arrangement. The hospital is responsible for ensuring that the
services meet all the requirements of this regulation, regardless of whether they are
provided directly or under arrangement. Diagnostic radiologic services provided under
arrangement may be provided either on the hospital’s campus or in an adjacent or other
nearby, readily accessible facility so long as the services, including those required on an
urgent or emergent basis, can be furnished within clinically appropriate timeframes.
Increasingly, hospitals are also separating the performance of radiologic studies, which may
be done on-site or at a readily accessible facility off the hospital’s campus, from the
interpretation of the studies, which can be performed remotely by a teleradiology
practitioner in a timely fashion. This practice is acceptable, so long as the teleradiology
practitioner is privileged in accordance with the requirements of the Governing Body
(§482.12) and Medical Staff (§482.22) CoPs.
Survey Procedures §482.26(a)
• With respect to assessing whether the diagnostic radiologic services meet the needs of
the hospital’s patients:
• Ask the hospital for evidence of the scope and complexity of its diagnostic
radiologic services.
• Ask how the hospital has determined that the services meet the needs of its patients.
• Verify that the hospital either maintains or makes available diagnostic radiologic
services that can be provided promptly when needed.
• If the hospital has an emergency department, are diagnostic radiologic services
maintained or available at all times to support the emergency department?
• If the diagnostic radiologic services are not on the same campus as the hospital’s
emergency department, same-day surgery, inpatient locations, or other areas where
services dependent upon radiologic services are provided, ask the hospital how it
ensures that services are furnished within clinically required timeframes. Does the
hospital have an arrangement with an off-site facility to furnish diagnostic services
when needed?
• How does the hospital ensure that staff authorized to interpret diagnostic studies are
ready to furnish services within clinically required timeframes, either on-site or
through telecommunications media that permit remote review and interpretation of
studies?
A-0535
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
[§482.26 Condition of Participation: Radiologic Services
…. If therapeutic services are also provided, they, as well as the diagnostic services,
must meet professionally approved standards for safety and personnel qualifications.]
§482.26(b) Standard: Safety for Patients and Personnel
The radiologic services, particularly ionizing radiology procedures, must be free from
hazards for patients and personnel.
Interpretive Guidelines §482.26(b)

The hospital must adopt and implement radiologic services policies and procedures that
provide safety for affected patients and hospital personnel and which are consistent with
accepted professional standards for radiologic services.
Ionizing Radiology Procedures
Radiologic services modalities that use ionizing radiation have increased the ability to
detect disease or injury early enough for a medical problem to be managed, treated, or
cured. When applied and performed appropriately, these radiologic studies or procedures
can maintain or improve health and save lives.
X-ray energy used in radiologic services also has a potential to harm living tissue. The most
significant risks are:
• Cataracts and skin damage, but only at very high levels of radiation exposure; and
• An increase in the possibility that a person exposed to x-ray energy will develop cancer
later in life. The risk of developing cancer from radiologic services radiation exposure
is generally very small, and it depends on at least three factors—the amount of the
radiation dose, the age of the person exposed, and the sex of the person exposed:
• The lifetime risk of cancer increases the larger the dose and the more x-ray studies
or procedures a patient undergoes;
• The lifetime risk of cancer is larger for a patient who received x-rays at a younger
age than for one who receives them at an older age; and.
• Women are at a somewhat higher lifetime risk than men for developing radiation-
associated cancer after receiving the same exposures at the same ages.
MRI:
MRIs are useful when a soft tissue injury or disease process is suspected and are generally
considered at low risk of causing harm to patients or staff. However, they also are not
entirely risk-free. Potential risks include projectile risk of magnetic objects being sucked
into the main magnet, thermal injury and burns, adverse effects on devices and leads
implanted in patients, and hearing damage.
Provision of services in accordance with professionally approved standards for safety
All radiological services provided by the hospital, including both diagnostic and, if offered,
therapeutic services, must be provided in accordance with acceptable standards of practice,
including standards for safety.
Professionally approved standards include maintaining compliance with applicable Federal
and State laws and regulations governing radiological services, including, but not limited to,
facility licensure and/or certification requirements.
Professionally approved standards also include the recommendations or guidelines
promulgated by expert governmental agencies, such as the U.S. Food and Drug
Administration, as well as those issued by nationally recognized professional organizations,
such as the American Medical Association, American College of Radiology, Radiological
Society of North America, The Alliance for Radiation Safety in Pediatric Imaging,
American Society of Radiologic Technologists, the American College of Cardiology, the
American College of Neurology, the American College of Physicians, etc.
Generally, there are different standards for different imaging modalities used to provide
radiologic services; there may also be different standards for diagnostic versus therapeutic
uses, as well as for pediatric versus adult patients, etc. For example, the American College
of Radiology has separate diagnostic radiology guidance documents for general radiology,
CT, MRI, and ultrasound, among others. The hospital must be able to document the source
standards that form the basis for its policies and procedures for each of its radiologic
services modalities and/or settings. For example, if one organization’s standards are used
for mammography services, another’s for CT services, another’s for MRI, and another’s for
pediatric X-rays, this must be clearly indicated.
In order to ensure safety and freedom from hazards, the hospital’s radiologic services
policies and procedures must include, but are not limited to, provisions addressing the
following:
• For ionizing radiation services, application of the fundamental principle of As Low as
Reasonably Achievable or ALARA, which is defined by the U.S. Environmental
Protection Agency (EPA) as “A principle of radiation protection philosophy that
requires that exposures to ionizing radiation be kept as low as reasonably achievable,
economic and social factors being taken into account. The protection from radiation
exposure is ALARA when the expenditure of further resources would be unwarranted
by the reduction in exposure that would be achieved.” (Federal Guidance Report No.
14, Radiation Protection Guidance for Diagnostic and Interventional X-ray Procedures,
p. 100, November, 2014) Although CMS does not interpret or enforce EPA guidance,
the ALARA principle is considered an accepted standard of practice for ionizing
radiation services to which hospitals must adhere.
• Written protocols developed or approved by the radiologist responsible for the
radiologic services, in conjunction with other qualified radiologic services personnel
(e.g., a medical physicist, radiologic technologists, patient safety officers, etc.) designed
to ensure that diagnostic studies and therapeutic procedures are routinely performed in a
safe manner, utilizing parameters and specifications that are appropriate to the ordered
study/procedure. The hospital must ensure that protocols for the various types of
ionizing radiation diagnostic or therapeutic imaging modalities are designed to
minimize the amount of radiation while maximizing the yield and producing
diagnostically acceptable image quality. Existing protocols must be reviewed
periodically and updated as needed. The rationale and details for changes to technical
parameters must be documented.
For Information Only – Not Required/Not to be Cited
Hospitals are encouraged to follow the recommendation in the EPA’s Guidance
Report No. 14 concerning patient radiation dosage. The report says “As the ICRP
[International Commission on Radiological Protection] has stated, ‘Provided that the
medical exposures of patients have been properly justified and that the associated
doses are commensurate with the medical purpose, it is not appropriate to apply dose
limits or dose constraints to the medical exposure of patients, because such limits or
constraints would often do more harm than good’ (ICRP 2007b). While dose limits
do not apply to medical exposures, radiation doses to patients should always be
optimized. All responsible parties should always strive to minimize patient
irradiation to the dose that is necessary to perform the procedure with adequate image
quality. The recommendation against establishing absolute dose limits should
not discourage a facility from implementing diagnostic reference levels for
imaging and interventional procedures. Exceeding these levels should prompt a
review of practice at the facility as a quality assurance measure. Dose
notification and alert values for CT, notification levels for use during
interventional procedures, and trigger levels for follow-up after interventional
procedures are also appropriate QA measures [emphasis added]...(EPA Guidance
Report No. 14, p.6)
• Policies and protocols to identify patients at high risk for adverse events for whom the
radiologic study or procedure might be contraindicated, e.g., pregnant women,
individuals with known allergies to contrast agents, individuals with implanted devices,
etc. Policies would address the steps to be taken, and by which personnel, if an order is
written for a radiologic study or procedure for an individual identified in the radiologic
services policies as potentially at high risk (e.g., notify the ordering physician, cancel
the procedure personally, etc.).
• Specific requirements related to procedures to mitigate radiation hazards are discussed
in the guidance for §482.26(b)(1).
• Procedures to address risks associated with modalities that do not use ionizing radiation.
For example, with respect to MRI:
• Measures to prevent magnetic materials from being closer than is safe to the MRI
suite, per nationally recognized guidelines;
• If equipment and supplies, such as fire extinguishers and oxygen tanks, are
located in the MRI area, they are MR-safe, i.e., they are non-ferromagnetic;
• Provision of adequate and effective hearing protection to patients, staff and others
who might be in the MRI suite while the scans are taking place; and
• Measures to reduce the risk of thermal injuries/burns during MRI. This would
include, but is not limited to, screening patients to identify those who may have
metallic tattoos or metal in them, proper patient positioning, ensuring implants are
MR Conditional, checking for electrically conductive materials that might be in
close proximity to the patient and taking the appropriate precautions, and instructing
the patient to immediately report any burning sensations experienced during the
scan.
• Training required by personnel permitted to enter areas where radiologic services are
provided.
• Training and, as applicable, qualifications, required for personnel who perform
diagnostic imaging studies or therapeutic procedures utilizing radiologic services
equipment. This includes proper operation of equipment per manufacturer’s
instructions and hospital policy.
• Areas where radiologic services are provided must be equipped with the necessary
equipment or materials to immediately respond to potential adverse events. This could
include, but is not limited to, things like a crash cart, emergency stop mechanisms,
cleaning and decontamination agents if applicable, etc.
For Information Only – Not Required/Not to be Cited
Hospitals are encouraged to also address the following in their Radiologic Services:
• Encouraging physicians and other practitioners with privileges to order radiologic
studies or procedures that utilize ionizing radiation to consider both the benefits and
risks of the procedures.
• Recording and tracking the dosing patients receive. There are several nationally
recognized quality assurance programs designed to assist health care providers in
developing and maintaining this data, including, but not limited to:
• The Alliance for Safety in Pediatric Imaging (www.Imagegently.org)
• The Conference of Radiation Control Program Directors
• The American College of Radiology data registry (http://nrdr.acr.org)
• The Nationwide Evaluation of X-ray Trends (NEXT program)
Further, although the EPA’s Guidance Report No. 14 was developed by an Interagency
Working Group on Medical Radiation specifically to provide guidance to Federal
facilities that use diagnostic and interventional X-ray equipment, it should also be useful
to non-Federal medical facilities and hospitals are encouraged to review it. The
Guidance Report addresses the following topics:
• Radiation Safety Standards and General Concerns
• Structural Shielding and Door Interlock Switches
• Requesting and Performing Studies Involving X-rays
• Technical Quality Assurance
• General Guidelines for Clinical Imaging, organized into separate sections for Medical
and Dental, and further broken down by modality
• Imaging Informatics
• Recommendations for Facility Action
Medical Physicists
According to the American Association of Physicists in Medicine, the practice of Medical
Physics means the use of principles and accepted protocols of physics to ensure the correct
quality, quantity, and placement of radiation during the performance of a radiological
procedure. Hospitals are not required under the regulations to have a medical physicist on
staff or under contract. However, since radiologic services are required to be free from
hazards to patients and hospital personnel, hospitals must ensure that qualified personnel,
whether or not they are medical physicists, develop and carry out protocols and test,
calibrate, and maintain radiologic services equipment and that there is a reliable means to
validate the results.
For Information Only - Not Required/Not to be Cited
Definition of a Medical Physicist
An example of a definition of and qualifications for a medical physicist is provided by
the American Association of Physicists in Medicine:
“For the purpose of providing clinical professional services, a Qualified Medical
Physicist (QMP) is an individual who is competent to independently provide clinical
professional services in one or more of the subfields
1
of medical physics. The subfields of
medical physics are:
• Therapeutic Medical Physics
• Diagnostic Medical Physics
• Nuclear Medicine Physics
• Medical Health Physics
…. A Qualified Medical Physicist meets each of the following credentials:
• Has earned a master’s and/or doctoral degree in physics, medical physics, biophysics,
radiological physics, medical health physics, or equivalent disciplines from an
accredited college or university; and
• Has been granted certification in the specific subfield(s) of medical physics with its
associate medical health physics aspects by an appropriate national certifying body
and abides by the certifying body’s requirements for continuing education.”
http://www.aapm.org/org/default.asp
For Information Only - Not Required/Not to be Cited
The responsibilities of the medical physicist in a hospital may include:
• protection of the patient and others from potentially harmful or excessive radiation;
• establishment, with the approval of the Director of Radiologic Services, of adequate
protocols to ensure accurate patient dosimetry;
• measurement and characterization of radiation;
• determination of delivered dose;
• promotion of procedures necessary to ensure image quality;
• development and direction of quality assurance programs; and assistance to other
healthcare professionals in optimizing the balance between the beneficial and
deleterious effects of radiation.
Hospitals are also encouraged to involve a medical physicist in the calibration of the
radiologic services equipment and monitoring of radiation dosage exposures to staff.
Consistent with the requirements under the Quality Assessment and Performance
Improvement (QAPI) CoP at 42 CFR 482.21, the hospital must monitor the quality and
safety of radiologic services.
Examples of indicators of potential quality and safety problems could include, but are not
limited to:
• Improper patient preparation, such as inadequate intravenous access or lack of pre-
medication, such that procedures must be cancelled or reordered;
• Repeats of the same studies in the hospital for the same patient within a short time
span, which may be an indicator of poor image quality; or
• Diagnostic imaging studies or therapeutic procedures performed in a manner
inconsistent with the applicable hospital written protocol.
Under the QAPI CoP, hospitals are required to undertake improvement activities in areas
that represent high risk, high volume, or problem-prone areas. Problems identified in
radiologic services may meet these criteria. In addition, adverse events related to radiologic
services must be analyzed for their causes, and preventive actions must then be undertaken.
Deficiencies identified related to tracking, analyzing, and addressing adverse event and
quality indicator data and performance improvement activities must be cited under the
applicable QAPI standards.
Survey Procedures §482.26(b)
• Verify that there are written hospital policies and procedures and protocols for specific
radiologic services modalities that are based on identified professionally approved
standards, and which address the ALARA principle as well as the other safety and risk-
reduction measures discussed in the guidance.
• Ask for evidence that safety protocols are reviewed periodically and, if applicable,
updated.
• Determine if the radiologic services staff are familiar with the policies and procedures
related to safety in general and specific clinical protocols.
• Observe whether the policies and procedures are followed when radiologic services are
delivered to patients. Ask for the protocol(s) for one or more studies/procedure(s) you
observed and check if they were followed.
• Verify that radiologic services staff are trained at appropriate intervals to ensure that
they are operating the equipment according to manufacturer’s instructions and hospital
policy
• Verify that radiologic services staff know how to respond to adverse events.
• Confirm that areas where radiologic services are provided are equipped with the
equipment or materials to immediately respond to an adverse event.
• Ask the radiologist who supervises ionizing radiologic services how the hospital
monitors the quality and safety of radiologic services.
• Verify that adverse events are analyzed for their causes and that preventive actions are
taken (deficiencies to be cited both here and under the applicable QAPI citation).
A-0536
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.26(b)(1) - Proper safety precautions must be maintained against radiation
hazards. This includes adequate shielding for patients, personnel, and facilities, as well
as appropriate storage, use and disposal of radioactive materials.
Interpretive Guidelines §482.26(b)(1)
The hospital must adopt and implement written policies and procedures to ensure safety
from radiation hazards. The policies and procedures must include, but are not limited to,
consideration of the following:
• Clear and easily recognizable signage identifying hazardous radiation areas;
• Limitations on access to areas containing radiologic services equipment.
• Appropriate use of shielding, including:
• Types of personal protective shielding (e.g., lead aprons, lead gloves, protective
eyewear, thyroid shields, portable individualized lead panels, stationary barriers) to
be used, under what circumstances, for patients, including high-risk patients as
identified in radiologic services policies and procedures, patient family members or
support persons who may be needed to be with the patient during a study or
procedure, and hospital personnel;
• Lead and concrete barriers built into the walls and other structures of the imaging
areas;
• Identification and use of appropriate containers to be used for various radioactive
materials, if applicable, when stored, in transport between locations within the hospital,
in use, and during/after disposal.
For Information Only - Not Required/ Not to be Cited
The Occupational Health and Safety Administration (OSHA) has requirements for
protecting hospital staff from radiation exposure, some of which are summarized below:
• “For information about exposure limits see: 29 CFR 1910.1096, Ionizing Radiation
Standard. The standard also requires:
• Each radiation area shall be conspicuously posted with a sign or signs bearing the
radiation caution symbol, with the wording ‘Caution Radiation Area’ [29 CFR
1910.1096(e)(2)]”
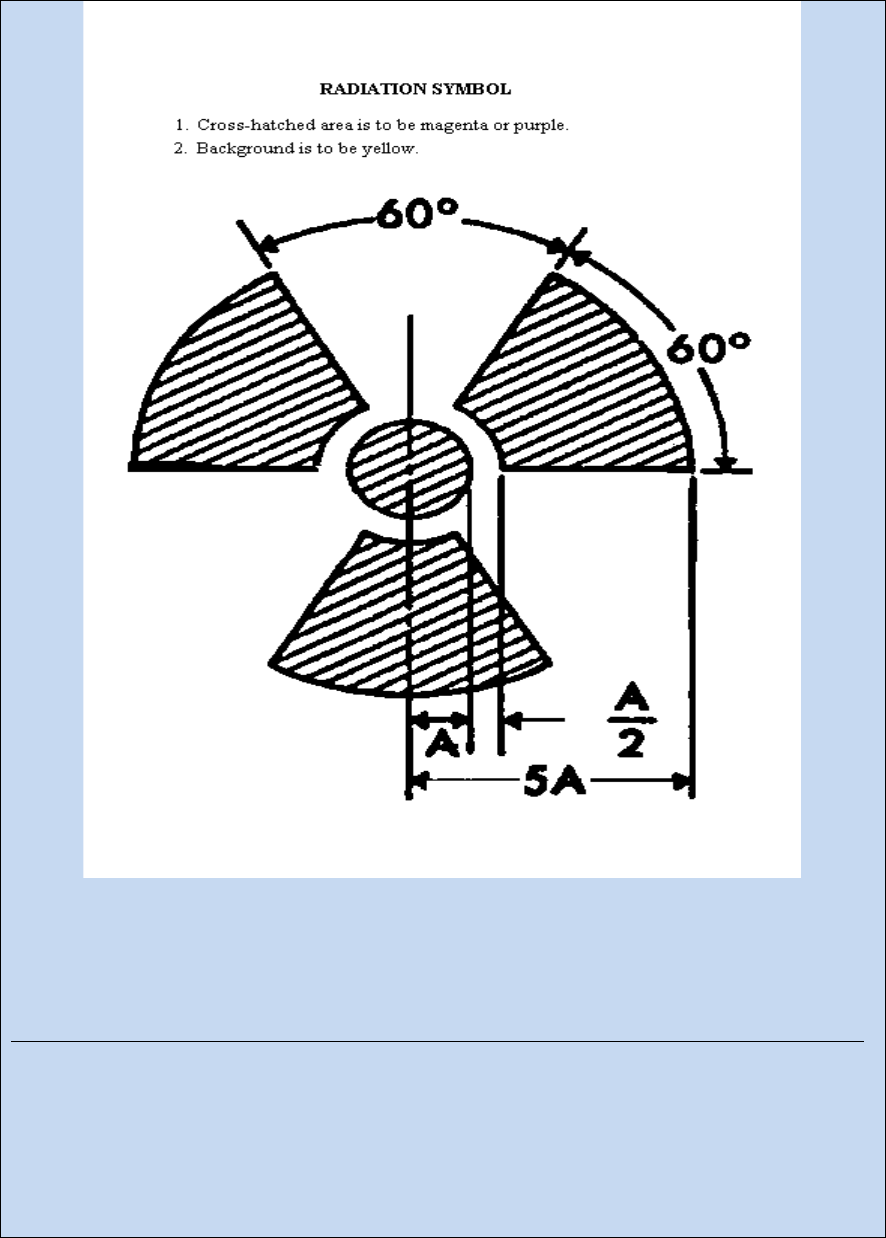
This document also discusses other tools to prevent radiation exposure.
See:
https://www.osha.gov/SLTC/etools/hospital/clinical/radiology/radiology.html#Radiation
As a reminder, although hospitals are required to comply with applicable OSHA
requirements, surveyors conducting surveys on behalf of CMS do not interpret or assess
compliance with the requirements of OSHA or other Federal Agencies. Surveyors do
assess compliance with Medicare requirements that may overlap or duplicate OSHA
requirements.
Survey Procedures §482.26(b)(1)
• Verify that personal shielding, supplies and equipment are properly maintained and
routinely inspected by the hospital.
• Determine if the proper shielding is applied to a patient who is undergoing a procedure
using ionizing radiation.
• Determine if staff members appropriately extricate themselves from the immediate
exposure field while performing a study or procedure using ionizing radiation.
• Determine if staff wear shielding as appropriate, per hospital policy.
• Verify that any hazardous radiation materials are clearly labeled, properly stored in a
safe manner in the requisite containers, and disposed of in the appropriate manner.
A-0537
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.26(b)(2) - Periodic inspection of equipment must be made and hazards identified
must be properly corrected.
Interpretive Guidelines §482.26(b)(2)
The hospital must have policies and procedures in place to ensure that periodic inspections
of radiology equipment are conducted, current and that problems identified are corrected in
a timely manner. Equipment includes not only devices used to deliver diagnostic or
therapeutic radiologic services, but also exposure meters, badges, or personal radiation
monitoring devices used by staff, as well as equipment the hospital uses to inspect or
calibrate devices used to deliver diagnostic or therapeutic radiologic services. The hospital
must ensure that equipment is inspected in accordance with manufacturer’s instructions and
Federal and State laws, regulations, and guidelines, and hospital policy, as applicable.
Inspections and maintenance, including correction of identified hazards, must be performed
by qualified employees (e.g., medical physicists, qualified biomedical technicians, etc.) or
through contractual arrangements with vendors with appropriate expertise.
Hospitals must follow the manufacturer’s instructions as to how to inspect and maintain
radiologic equipment. This includes acceptance testing (i.e., upon initial installation and
after major upgrades) as well as ongoing inspection and maintenance. Documentation of
preventive maintenance, quality control tests, service records, and major software/hardware
upgrades must be maintained by the hospital and be readily available for inspection.
The hospital must also have a system in place, to identify and remedy equipment hazards in
a timely manner. This system must include, but is not limited to: periodic and consistent
calibration of equipment and, for equipment using ionizing radiation, monitoring of
dosimetry parameters with phantoms to ensure that an accurate dose of radiation is
delivered per the applicable protocol. In addition, hospitals must also have a system to
track all modifications made to the equipment that would significantly impact the accuracy
of the dosage delivered. Any adverse events related to over- or under-dosing must be
identified and addressed.

For Information Only - Not Required/Not to be Cited
Requirements for Manufacturers Re: Instructions that Must be Made Available
Below is an FDA summary of its requirements for manufacturers of x-ray systems to
make available to purchasers and, upon request, to other parties, information related to
maintenance of the following types of systems:
• For all diagnostic x-ray systems, manufacturers are required to provide to purchasers,
and, upon request, to others at a cost not to exceed the cost of publication and
distribution, manuals or instruction sheets that include technical and safety
information (21 CFR 1020.30(h). This information must include a schedule of the
maintenance necessary to keep the equipment in compliance with §§1020.30,
1020.31, 1020.32, and 1020.33 (21 CFR 1020.30(h)(1)(ii)). Manufacturers are also
required to provide to assemblers, and, upon request, to others at a cost not to exceed
the cost of publication and distribution, instructions for assembly, installation,
adjustment, and testing of specified components of diagnostic x-ray systems adequate
to ensure that the products will comply with applicable provisions of §§1020.30,
1020.31, 1020.32, and 1020.33, when assembled, installed, adjusted, and tested as
directed (21 CFR 1020.30(g)).
• In addition to the requirements applicable to all diagnostic x-ray systems, there are
also other requirements for specific systems:
• Manufacturers of fluoroscopic x-ray systems manufactured on or after June 10,
2006 are required to provide a schedule of maintenance for any system
instrumentation associated with the display of air kerma information necessary to
maintain the displays of air kerma rate and cumulative air kerma within the limits
of allowed uncertainty specified by 21 CFR 1020.32(k)(6). And, if the capability
for user calibration of the display is provided, adequate instructions for such
calibration must be supplied (21 CFR 1020.30(h)(6)(i)).
• Manufacturers of computed tomography (CT) systems are required to provide a
specific phantom or phantoms for quality assurance testing of specific system
parameters on these systems (21 CFR 1020.33(d)(1)), and instructions on the use
of the phantom(s), including a schedule of testing appropriate for the system and
allowable variations for the indicated parameters (21 CFR 1020.33(d)(2)).
• Manufacturers of cabinet x-ray systems are required to provide purchasers, and
others, upon request, at a cost not to exceed the cost of preparation and
distribution, manuals and instructions. These documents must include, among
other technical and safety information, a schedule of maintenance necessary to
keep the system in compliance with 21 CFR 1020.40 (21 CFR 1020.40(c)(9)(i)).
Cabinet x-ray systems that are intended to be assembled or installed by the
purchaser must be accompanied by instructions for assembly, installation,
adjustment and testing of the cabinet x-ray adequate to ensure that the system is in
compliance with the applicable provisions of 21 CFR 1020.40 when assembled,
installed, adjusted and tested as directed (21 CFR 1020.40(c)(9)(ii)).
Survey Procedures §482.26(b)(2)
• Review the inspection records (logs) to verify that periodic inspections are conducted in
accordance with Federal and State laws, regulations and manufacturer’s instructions.
• Verify that inspection and maintenance activities were performed by qualified
individuals
• Verify that the maintenance logs show documentation of the calibration upon
installation and after major upgrades or servicing.
• Review with the appropriate personnel the inspection schedule and the mechanism for
identifying hazards, including accurate dosimetry determinations with phantom patients,
as applicable.
• Determine that any problems identified through the testing and maintenance
program are properly corrected in a timely manner and the correction is maintained
over time.
A-0538
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.26(b)(3) - Radiation workers must be checked periodically, by the use of
exposure meters or badge tests, for amount of radiation exposure.
Interpretive Guidelines §482.26(b)(3)
This requirement applies to radiologic services personnel, as well as other hospital
employees who may be regularly exposed to radiation due to working near radiation
sources. This could include certain nursing and maintenance staff. The types or locations of
employees who require monitoring for radiation exposure must be identified in the policies
and procedures for the radiologic services developed or approved by the radiologist who
supervises the services, in conjunction with the appropriately qualified radiation safety
personnel. The monitoring of staff exposure must be documented by qualified personnel.
Hospitals are expected to educate staff who are monitored for radiation exposure about the
appropriate use of the monitoring meters or badges (or through use of a “personal radiation
monitoring device,” which employs modern technology for the same measurement
purpose). Hospitals must educate staff on the importance of tracking their radiation
exposure over various timeframes, such as the most recent month and year, as well as their
cumulative exposure through work. Staff also must be educated about the appropriate
storage of the meters and/or badges as well as the procedures to follow if the exposure
device exceeds cumulative dosage parameters specified per hospital policy. The hospital is
expected to proactively monitor staff cumulative dosage and take appropriate steps if an
individual staff member’s cumulative dosage level exceeds parameters specified per
hospital policy.
For Information Only – Not Required/Not to be Cited
The Occupational Safety and Health Administration (OSHA) has requirements for
protecting hospital staff from radiation exposure, some of which are summarized below:
• “For information about exposure limits see: 29 CFR 1910.1096, Ionizing Radiation
Standard. The standard also requires:
• …Every employer shall supply appropriate personnel monitoring equipment, such
as film badges, pocket chambers, pocket dosimeters, or film rings, and shall
require the use of such equipment [29 CFR 1910.1096(d)(2)]
• Employers shall maintain records of the radiation exposure of all employees for
whom personnel monitoring is required under paragraph (d) of this section and
advise each employee of his individual exposure at least yearly…
See:
https://www.osha.gov/SLTC/etools/hospital/clinical/radiology/radiology.html#Radiation
As a reminder, although hospitals are required to comply with applicable OSHA
requirements, surveyors conducting surveys on behalf of CMS do not interpret or
assess compliance with the requirements of OSHA or other Federal Agencies.
Surveyors do assess compliance with Medicare requirements that may overlap or
duplicate OSHA requirements.
Survey Procedures §482.26(b)(3)
• Verify that staff being monitored have been trained about the appropriate use and
storage of their badges/meters. Are staff knowledgeable about their personal radiation
exposure over various timeframes?
• Observe whether staff in categories or locations identified for monitoring have
radiation-detecting meters or badges and that they appropriately wear and store them.
• Review records to verify that monitoring of staff exposure is documented.
• Ask the hospital what steps it takes if staff exposure exceeds parameters established
per hospital policy. Can the hospital provide examples, or, if it asserts there have
been no cases in the prior 12 – 24 months, do its records support this?
A-0539
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.26(b)(4) - Radiologic services must be provided only on the order of practitioners
with clinical privileges or, consistent with State law, of other practitioners authorized
by the medical staff and the governing body to order the services.
Interpretive Guidelines §482.26(b)(4)
The medical staff and the governing body determine the necessary qualifications and
clinical privileges that practitioners must have to order diagnostic radiologic studies or
therapeutic procedures.
For outpatient services, the governing body and medical staff may also authorize
practitioners who do not have hospital privileges to order such studies or procedures, as
permitted under State law. For example, a hospital may decide that it will routinely accept
orders from physicians in the communities it services for outpatient diagnostic studies,
regardless of whether those physicians have privileges to practice in the hospital. See the
guidance for §482.54(c) for more information on requirements related to outpatient orders
from practitioners who do not hold privileges to practice at the hospital.
The order must include information for the radiologic technologist about the study or
procedure to be performed, and the technologist is expected to review this information prior
to implementing the order.
For Information Only – Not Required/Not to be Cited
Hospitals are strongly encouraged, but not required, to develop standard formats for
practitioner’s orders for radiologic services that clearly document the diagnostic or
therapeutic purpose of the study/procedure, as well as any other pertinent information
that may lead to altering the dose of radiation, including, but not limited to:
• Indication (reason) for the study/procedure
• Previous imaging studies of the body part(s) under investigation;
• Additional relevant radiation exposure; and
• Previous adverse events (e.g., over- or underexposure of dosing, allergic reaction to
contrast dye) during radiologic procedures.
In addition, hospitals are encouraged to adopt policies to ensure that the radiation
technologist performing the study/procedure confirms the order with the ordering
practitioner if there are any concerns about its appropriateness.
Survey Procedures §482.26(b)(4)
• Review medical records to determine that there is an order for all radiologic services,
and that the order was dated/timed and authenticated by an authorized practitioner prior
to the diagnostic study or therapeutic procedure being performed.
Observe whether a radiologic technologist confirms that there is an order from an
authorized practitioner and reviews information included in the order before beginning a
study or procedure.
A-0546
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.26(c) – Standard: Personnel
(1) A qualified full-time, part-time or consulting radiologist must supervise the
ionizing radiology services and must interpret only those radiologic tests that are
determined by the medical staff to require a radiologist’s specialized knowledge. For
purposes of this section, a radiologist is a doctor of medicine or osteopathy who is
qualified by education and experience in radiology.
Interpretive Guidelines §482.26(c)(1)
The regulation defines a radiologist as a doctor of medicine (MD) or doctor of osteopathy
(DO) who is qualified by education and experience in radiology. The medical staff must
establish the specific criteria related to education and experience that must be met in order
to be privileged as a radiologist in the hospital.
Ionizing radiologic services offered throughout the hospital must be under the supervision
of a radiologist, who may be part-time, full-time, or consulting. This may be accomplished
in several ways, including by having one organized radiologic service under the direction of
the supervising radiologist, or by the governing body ensuring a uniform approach to
ionizing radiologic services that are offered in multiple, separately organized departments
of the hospital which collaborate with the supervising radiologist in developing their
department-specific protocols for ensuring that these services are free from hazards for
patients and personnel.
The supervising radiologist, including, if applicable, a consultant who provides such
supervision, must be privileged as a radiologist at the hospital. The extent of radiologic
services provided by the hospital determines whether the supervising radiologist must carry
out these responsibilities full or part-time.
For diagnostic radiologic services using ionizing radiation, policies and procedures must, in
addition to the requirements addressed in other portions of the radiologic services CoP,
identify which types of radiologic tests require interpretation by a radiologist, as opposed to
another type of practitioner holding privileges; the hospital’s medical staff must approve
this policy.
When interpretation of radiologic tests (studies) is provided via telemedicine, the
radiologist interpreting the radiological test must be licensed and/or meet the other
applicable standards that are required by State or local laws in the state where the hospital
(and, therefore, the patient) is located. The requirements concerning granting of privileges
to teleradiologists are addressed in the medical staff (§482.22) and governing body
(§482.12) Conditions of Participation.
Survey Procedures §482.26(c)(1)
• Review the medical staff privileging criteria for a radiologist. Review the
credentialing and privileging file of the supervising radiologist to verify that he or
she meets the qualifications established by the medical staff and has been granted
privileges as a radiologist.
• If the supervising radiologist is a part-time employee or consultant, ask him/her
how much time/week is spent on supervising ionizing radiologic services.
•
Is there any evidence of problems within the radiologic services that suggest
lack of supervision?
• Determine whether the medical staff has reviewed and approved a policy identifying
the types of diagnostic radiologic tests (studies) that require interpretation by a
radiologist. Review records to determine that a radiologist interprets those tests
(studies) that have been designated.
A-0547
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.26(c)(2) - Only personnel designated as qualified by the medical staff may use the
radiologic equipment and administer procedures.
Interpretive Guidelines §482.26(c)(2)
The medical staff must develop policies, consistent with State law, that govern the
designation of all personnel who are qualified to use the radiologic equipment and perform
diagnostic or therapeutic studies or procedures. Qualifications must include appropriate
training and demonstrated competence in the use of equipment and administration of
procedures prior to being designated as qualified. Only designated individuals may use the
equipment and perform studies or procedures.
The use of the radiologic equipment includes, but is not limited to, functions such as
operating the equipment according to the manufacturer’s instructions and hospital policy,
and interfacing with specialized technology as needed.
In addition to a radiologist, and although not specifically mentioned in the regulations,
radiologic technologists are typically involved in the delivery of radiologic services in a
hospital. Radiologic technologists are medical personnel who typically perform diagnostic
imaging examinations and administer radiation therapy treatments, as permitted under State
law. They are educated in anatomy, patient positioning, examination techniques, equipment
protocols, radiation safety, radiation protection, and basic patient care. All radiologic
technologists using radiologic equipment or performing studies/procedures must be
designated to do so.
Personnel also need to know how to respond to adverse events that may occur during a
radiologic study or procedure.
Hospitals are expected to regularly reassess staff competency and to provide periodic
training needed to keep staff skills up-to-date. The hospital must document training
completion dates and evidence of satisfactory competence. Staff that complete training but
cannot demonstrate satisfactory competence must not be permitted to use radiologic
equipment and/or administer procedures.
Survey Procedures §482.26(c)(2)
• Verify that the medical staff established criteria for personnel who use radiologic
services equipment and perform studies or procedures.
• Determine which staff are using which pieces of radiological equipment. Review their
personnel folders to determine if they meet the qualifications established by the medical
staff for the tasks they perform.
• Verify that radiologic services staff are periodically trained and reassessed for
competence to ensure that they are operating the equipment according to manufacturer
instructions and hospital policy and know how to respond to adverse events related to
their use of the equipment.
A-0553
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.26(d) Standard: Records
Records of radiologic services must be maintained.
(1) - The radiologist or other practitioner who performs radiology services must sign
reports of his or her interpretations.
(2) - The hospital must maintain the following for at least 5 years:
(i) Copies of reports and printouts.
(ii) Films, scans, and other image records, as appropriate.
Interpretive Guidelines §482.26(d)
The hospital must maintain records for all radiologic procedures performed. At a
minimum, the records must include the orders for the services, copies of reports and
printouts, and any films, scans, digital or other image records, as appropriate.
Radiology films, image records, scans, digital files, reports, and printouts must be secure
and properly stored for at least five years. If State law requires a longer period, the hospital
must comply, but surveyors do not assess compliance with State law requirements as part of
the Federal survey. Patient radiologic services records are considered patient medical
records and the hospital must comply with the requirements of the medical records CoP
(§482.24). All reports of studies must be signed by the radiologist or other authorized
practitioner (in the case of studies not designated as requiring a radiologist to interpret
them) who reads and evaluates the findings of the study. Acceptable forms of signature
include paper signatures as well as electronic signatures.
Survey Procedures §482.26(d)
• Determine whether the hospital maintains radiologic services records for at least 5 years
after the study or procedure. (Assess them for compliance with the Medical Records
CoP at §482.24 at the same time, but make sure to cite general medical record
noncompliance under that CoP.)
• Request records for all different imaging modalities furnished by the hospital, to
determine if the procedure for maintaining the records is consistent among all the
radiologic services.
• Review radiologic records to determine that reports of studies are signed by the
radiologist or other authorized practitioner (in the case of studies not designated as
requiring a radiologist to interpret them ) who read and evaluated the findings of the
study
A-0576
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.27 Condition of Participation: Laboratory Services
The hospital must maintain, or have available, adequate laboratory services to meet
the needs of its patients. The hospital must ensure that all laboratory services
provided to its patients are performed in a facility certified in accordance with Part
493 of this chapter.
Interpretive Guidelines §482.27
The hospital must maintain or have available laboratory services whenever its patients need
those services. The hospital may make laboratory services available directly, through
contractual agreements, or through a combination of direct and contractual services. The
scope and complexity of the hospital laboratory service must be adequate to meet the needs
of its patients. The hospital must maintain, or have available, adequate laboratory services
to meet the needs of its patients at each campus or off-campus location of the hospital. All
laboratory services, whether direct or contractual, whether conducted in a lab or in another
location, must be provided in accordance with Clinical Laboratory Improvement Act
(CLIA) requirements. Every hospital laboratory service must be operating under a current
CLIA certificate appropriate to the level of services performed.
The hospital’s laboratory services, including any contracted services, must be integrated
into its hospital-wide QAPI program.
Patient laboratory results and all other laboratory clinical patient records are considered
patient medical records and the hospital must comply with the requirements of the Medical
Records CoP.
Survey Procedures §482.27
• Determine the total number of laboratories, the location of each laboratory, and
every location where laboratory procedures are performed.
• Verify that the laboratory service and all laboratory locations are integrated into the
hospital-wide QAPI program.
• If laboratory services are contracted, verify that the review of the quality of those
services is integrated into the hospital-wide QAPI program.
A-0582
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.27(a) Standard: - Adequacy of Laboratory Services
The hospital must have laboratory services available, either directly or through a
contractual agreement with a certified laboratory that meets requirements of Part 493
of this chapter.
Interpretive Guidelines §482.27(a)
The CLIA certification may be accomplished by having one certificate for the entire
hospital’s laboratory services, by having one certificate for each laboratory, or by the
hospital having a mixture. Whatever the arrangement, all laboratory services must be
provided in accordance with CLIA requirements and under a current CLIA certificate, even
when those laboratory services take place outside of a lab.
Survey Procedures §482.27(a)
• Determine which services are provided directly by the facility and which are
provided through contractual agreements.
• Determine if the referral laboratory is CLIA certified for the appropriate test
specialty.
• If the hospital provides laboratory services in multiple locations, verify that all
laboratory services are operating under a current CLIA certificate.
• Examine records and determine if the services, including emergency services, are
provided in accordance with the hospital’s policies.
A-0583
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.27(a)(1) - Emergency laboratory services must be available 24 hours a day.
Interpretive Guidelines §482.27(a)(1)
The hospital must provide emergency laboratory services 24 hours a day, 7 days a week.
These onsite emergency services may be provided directly by the hospital or through onsite
contracted laboratory services. Emergency lab services include collection, processing, and
provision of results to meet a patient's emergency laboratory needs.
In a hospital with multiple hospital campuses, these emergency laboratory services must be
available onsite 24/7 at each campus.
The medical staff must determine which laboratory services are to be immediately available
to meet the emergency laboratory needs of patients who may be currently at the hospital or
those patients who may arrive at the hospital in an emergency condition. The emergency
laboratory services (procedures, tests, personnel) available should reflect the scope and
complexity of the hospital’s operation and be provided in accordance with Federal and State
law, regulations and guidelines and acceptable standards of practice.
At a hospital with off-campus locations the medical staff must determine which, if any,
laboratory services must be immediately available to meet the emergency laboratory needs
of the patients who are likely to seek care at each off-campus location. The emergency
laboratory services available must reflect the scope and complexity of the hospital's
operations at the location and be provided in accordance with Federal and State law,
regulations and guidelines and acceptable standards of practice. The services must be
available during the hours of operation of that location.
Survey Procedures §482.27(a)(1)
Review the written description of the emergency laboratory services. Review records
(including accession records, worksheets, and test reports) to verify the 24-hour availability
of emergency services and that those services are provided when required.
A-0584
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.27(a)(2) - A written description of services provided must be available to the
medical staff.
Survey Procedures §482.27(a)(2)
• Verify the existence of a written description of the laboratory services provided,
including those furnished on routine and stat basis (either directly or under an
arrangement with an outside facility).
• Verify that the description of services is accurate and current.
A-0585
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.27(a)(3) - The laboratory must make provision for proper receipt and reporting
of tissue specimens.
Interpretive Guidelines §482.27(a)(3)
The laboratory must have written instructions for the collection, preservation,
transportation, receipt, and reporting of tissue specimen results.
Survey Procedures §482.27(a)(3)
Review tissue records (accession records, worksheets, and test reports) to determine
whether the laboratory follows the written protocol.
A-0586
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.27(a)(4) - The medical staff and a pathologist must determine which tissue
specimens require a macroscopic (gross) examination and which require both
macroscopic and microscopic examinations.
Interpretive Guidelines §482.27(a)(4)
Laboratory written policies, approved by the medical staff and a pathologist, must state
which tissue specimens require a macroscopic examination and which tissue specimens
require both macroscopic and microscopic examination.
Survey Procedures §482.27(a)(4)
• Verify that the hospital has a written policy for examination requirements.
• Review the written policies and tissue reports to assure that tissue specimens are
examined in accordance with the written policies.
• Verify that the policies are in accordance with these requirements and other Federal
and State laws, regulations, and guidelines.
A-0592
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.27(b) Standard: - Potentially Infectious Blood and Blood
Components
(1)Potentially human immunodeficiency virus (HIV) infectious blood and blood
components. Potentially HIV infectious blood and blood components are prior
collections from a donor –
(i) Who tested negative at the time of donation but tests reactive for
evidence of HIV infection on a later donation;
(ii) Who tests positive on the supplemental (additional, more specific) test or
other follow-up testing required by FDA; and
(iii) For whom the timing of seroconversion cannot be precisely estimated.
(2) Potentially hepatitis C virus (HCV) infectious blood and blood components.
Potentially HCV infectious blood and blood components are the blood and
blood components identified in 21 CFR 610.47.
(3) Services furnished by an outside blood collecting establishment. If a hospital
regularly uses the services of an outside blood collecting establishment, it must
have an agreement with the blood collecting establishment that governs the
procurement, transfer, and availability of blood and blood components. The
agreement must require that the blood collecting establishment notify the
hospital --
(i) Within 3 calendar days if the blood collecting establishment supplied
blood and blood components collected from a donor who tested negative
at the time of donation but tests reactive for evidence of HIV or HCV
infection on a later donation or who is determined to be at increased risk
for transmitting HIV or HCV infection;
(ii) Within 45 days of the test, of the results of the supplemental (additional,
more specific) test for HIV or HCV, as relevant, or other follow-up testing
required by FDA;
(iii) Within 3 calendar days after the blood collecting establishment supplied
blood and blood components collected from an infectious donor,
whenever records are available, as set forth at 21 CFR 610.48(b)(3).
(4) Quarantine of blood and blood components pending completion of testing. If the
blood collecting establishment (either internal or under an agreement) notifies
the hospital of the reactive HIV or HCV screening test results, the hospital must
determine the disposition of the blood or blood component and quarantine all
blood and blood components from previous donations in inventory.
(i) If the blood collecting establishment notifies the hospital that the result of
the supplemental (additional, more specific) test or other follow-up testing
required by FDA is negative, absent other informative test results, the
hospital may release the blood and blood components from quarantine.
(ii) If the blood collecting establishment notifies the hospital that the result of
the supplemental (additional, more specific) test or other follow-up testing
required by FDA is positive, the hospital must –
(A) Dispose of the blood and blood components; and
(B) Notify the transfusion recipients as set forth in paragraph
(b)(6) of this section.
(iii) If the blood collecting establishment notifies the hospital that the result of
the supplemental (additional, more specific) test or other follow-up testing
required by FDA is indeterminate, the hospital must destroy or label
prior collections of blood or blood components held in quarantine as set
forth at 21 CFR 610.46(b)(2), 610.47(b)(2), and 610.48(c)(2).
(5) Recordkeeping by the hospital. The hospital must maintain --
(i) Records of the source and disposition of all units of blood and blood
components for at least 10 years from the date of disposition in a manner
that permits prompt retrieval; and
(ii) A fully funded plan to transfer these records to another hospital or
other entity if such hospital ceases operation for any reason.
(6) Patient notification. If the hospital has administered potentially HIV or HCV
infectious blood or blood components (either directly through its own blood
collecting establishment or under an agreement) or released such blood or blood
components to another entity or appropriate individual, the hospital must take
the following actions:
(i) Make reasonable attempts to notify the patient, or to notify the
attending physician who ordered the blood or blood component and ask
the physician to notify the patient, or other individual as permitted
under paragraph (b)(10) of this section, that potentially HIV or HCV
infectious blood or blood components were transfused to the patient and
that there may be a need for HIV or HCV testing and counseling.
(ii) If the physician is unavailable or declines to make the notification,
make reasonable attempts to give this notification to the patient, legal
guardian or relative.
(iii) Document in the patient’s medical record the notification or attempts to
give the required notification.
(7) Time frame for notification. For donors tested on or after February 20, 2008.
For notifications resulting from donors tested on or after February 20, 2008 as set
forth at 21 CFR 610.46 and 21 CFR 610.47 the notification effort begins when the
blood collecting establishment notifies the hospital that it received potentially HIV or
HCV infectious blood and blood components. The hospital must make reasonable
attempts to give notification over a period of 12 weeks unless--
(i) The patient is located and notified; or
(ii) The hospital is unable to locate the patient and documents in the
patient’s medical record the extenuating circumstances beyond the
hospital’s control that caused the notification timeframe to exceed 12
weeks.
(8) Content of notification. The notification must include the following information:
(i) A basic explanation of the need for HIV or HCV testing and
counseling.
(ii) Enough oral or written information so that an informed decision can be
made about whether to obtain HIV or HCV testing and counseling.
(iii) A list of programs or places where the person can obtain HIV or HCV
testing and counseling, including any requirements or restrictions the
program may impose.
(9) Policies and procedures. The hospital must establish policies and procedures for
notification and documentation that conform to Federal, State, and local laws,
including requirements for the confidentiality of medical records and other patient
information.
(10) Notification to legal representative or relative. If the patient has been adjudged
incompetent by a State court, the physician or hospital must notify a legal
representative designated in accordance with State law. If the patient is competent,
but State law permits a legal representative or relative to receive the information on
the patient’s behalf, the physician or hospital must notify the patient or his or her
legal representative or relative. For possible HIV infectious transfusion recipients that
are deceased, the physician or hospital must inform the deceased patient’s legal
representative or relative. If the patient is a minor, the parents or legal guardian must
be notified.
Interpretive Guidelines §482.27(b)
This regulation requires the hospital to have a system in place to take appropriate action
when notified that blood or blood components it received are at increased risk of
transmitting HIV or HCV.
A-0593
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.27(c) Standard: General blood safety issues. For lookback activities only
related to new blood safety issues that are identified after August 24, 2007, hospitals
must comply with FDA regulations as they pertain to blood safety issues in the
following areas:
(1) Appropriate testing and quarantining of infectious blood and blood components.
(2) Notification and counseling of recipients that may have received infectious blood
and blood components.
A-0618
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.28 Condition of Participation: Food and Dietetic Services
The hospital must have organized dietary services that are directed and staffed by
adequate qualified personnel. However, a hospital that has a contract with an outside
food management company may be found to meet this Condition of Participation if
the company has a dietician who serves the hospital on a full-time, part-time, or
consultant basis, and if the company maintains at least the minimum standards
specified in this section and provides for constant liaison with the hospital medical
staff for recommendations on dietetic policies affecting patient treatment.
Interpretative Guidelines §482.28
The hospital’s food and dietetic services must be organized, directed and staffed in such a
manner to ensure that the nutritional needs of the patients are met in accordance with
practitioners’ orders and acceptable standards of practice.
The hospital should have written policies and procedures that address at least the following:
• Availability of a diet manual and therapeutic diet menus to meet patients’ nutritional
needs;
• Frequency of meals served;
• System for diet ordering and patient trays delivery;
• Accommodation of non-routine occurrences (e.g., parenteral nutrition (tube
feeding), total parenteral nutrition, peripheral parenteral nutrition, change in diet
orders, early/late trays, nutritional supplements, etc);
• Integration of the food and dietetic service into the hospital-wide QAPI and
Infection Control programs;
• Guidelines for acceptable hygiene practices of food service personnel; and
• Guidelines for kitchen sanitation.
The same standards apply whether the food and dietetic services are provided by the
hospital directly, through a contractual agreement, or by off-site vendor.
The hospital must be in compliance with Federal and State licensure requirements for food
and dietary personnel as well as food service standards, laws and regulations.
A-0619
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.28(a) Standard: Organization
Interpretive Guidelines §482.28(a):
The hospital must ensure that the specific food and dietetic services organization
requirements are met.
A-0620
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.28(a)(1) - The hospital must have a full-time employee who–
(i) Serves as director of the food and dietetic services;
(ii) Is responsible for daily management of the dietary services; and
(iii)Is qualified by experience or training.
Interpretive Guidelines §482.28(a)(1)
The service director must be a full-time employee who has been granted the authority and
delegated responsibility by the hospital’s governing body and medical staff for the
operation of the dietary services. This authority and delegated responsibility includes, the
daily management of the service, implementing training programs for dietary staff, and
assuring that established policies and procedures are maintained that address at least the
following:
• Safety practices for food handling;
• Emergency food supplies;
• Orientation, work assignments, supervision of work and personnel performance;
• Menu planning, purchasing of foods and supplies, and retention of essential records
(e.g., cost, menus, personnel, training records, QAPI reports, etc);
• Service QAPI program.
Additionally, the service director must demonstrate, through education, experience and/or
specialized training, the qualifications necessary to manage the service, appropriate to the
scope and complexity of the food service operations.
Survey Procedures §482.28(a)(1)
• Verify that the director of the food and dietetic services is a full-time employee.
• Review the service director’s job description to verify that it is position-specific and
that responsibility and authority for the direction of the food and dietary service has
been clearly delineated.
• Review the service director’s personnel file to verify that he/she has the necessary
education, experience, and training to manage the service, appropriate to the scope
and complexity of food service operations.
A-0621
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.28(a)(2) - There must be a qualified dietitian, full-time, part-time or on a
consultant basis.
Interpretive Guidelines §482.28(a)(2)
A qualified dietitian must supervise the nutritional aspects of patient care. Responsibilities
of a hospital dietitian may include, but are not limited to:
• Approving patient menus and nutritional supplements;
• Patient, family, and caretaker dietary counseling;
• Performing and documenting nutritional assessments and evaluating patient
tolerance to therapeutic diets when appropriate;
• Collaborating with other hospital services (e.g., medical staff, nursing services,
pharmacy service, social work service, etc) to plan and implement patient care as
necessary in meeting the nutritional needs of the patients;
• Maintaining pertinent patient data necessary to recommend, prescribe, or modify
therapeutic diets as needed to meet the nutritional needs of the patients.
Qualification is determined on the basis of education, experience, specialized training, State
licensure or registration when applicable, and maintaining professional standards of
practice.
If the qualified dietitian does not work full-time, and when the dietitian is not available, the
hospital must make adequate provisions for dietary consultation that meets the needs of the
patients. The frequency of consultation depends on the total number of patients, their
nutritional needs and the number of patients requiring therapeutic diets or other nutritional
supplementation.
Survey Procedures §482.28(a)(2)
• Review the dietitian’s personnel file to determine that he/she is qualified based on
education, experience, specialized training, and, if required by State law, is licensed,
certified, or registered by the State.
• If the dietitian is not full-time, determine that the number of hours spent working is
appropriate to serve the nutritional needs of the patients, and that the hospital makes
adequate provisions for a qualified consultant coverage when the dietitian is not
available.
A-0622
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.28(a)(3) - There must be administrative and technical personnel competent in
their respective duties.
Interpretive Guidelines §482.28(a)(3)
Administrative and technical personnel must be competent in their assigned duties. This
competency is demonstrated through education, experience and specialized training
appropriate to the task(s) assigned. Personnel files should include documentation that the
staff member(s) is competent in their respective duties.
Survey Procedures §482.28(a)(3)

Review personnel files for administrative and technical staff to determine they have
appropriate credentials as required and have received adequate training and are competent
in their respective duties.
A-0629
(Rev. 137, Issued: 04-01-15, Effective: 03-27-15, Implementation: 03-27-15)
§482.28(b) Menus must meet the needs of patients.
(1) - Individual patient nutritional needs must be met in accordance with recognized
dietary practices.
Interpretive Guidelines §482.28(b)(1)
Each hospital patient for whom the hospital is providing one or more meals or nutrition
must have their nutritional needs met in a manner that is consistent with recognized dietary
practices. Affected patients include all inpatients and those patients in outpatient status,
including the provision of observation services, whose stay is sufficiently long that they
must be fed. According to the U.S. Department of Agriculture’s (USDA) Food and
Nutrition Center the nationally recognized source for recommended dietary intakes
allowances is the Institute of Medicine Food and Nutrition Board’s Dietary Reference
Intakes (DRIs), which are designed to provide recommended nutrient intakes for use in a
variety of settings. The DRIs are a set of four reference values:
• Recommended Dietary Allowance (RDA) is the average daily dietary intake
of a nutrient that is sufficient to meet the requirement of nearly all (97-98%)
healthy persons.
• Adequate Intake (AI) for a nutrient is similar to the Estimated Safe and
Adequate Daily Dietary Intakes (ESADDI) and is only established when an
RDA cannot be determined. Therefore a nutrient either has an RDA or an
AI. The AI is based on observed intakes of the nutrient by a group of healthy
persons.
• Tolerable Upper Intake Level (UL) is the highest daily intake of a nutrient
that is likely to pose no risks of toxicity for almost all individuals. As intake
above the UL increases, risk increases.
• Estimated Average Requirement (EAR) is the amount of a nutrient that is
estimated to meet the requirement of half of all healthy individuals in the
population.
USDA provides access to an interactive DRI tool and DRI tables at
http://fnic.nal.usda.gov/dietary-guidance/dietary-reference-intakes
Meeting individual patient nutritional needs may include the use of therapeutic diets.
Therapeutic diets refer to a diet ordered as part of the patient’s treatment for a disease or
clinical condition, to eliminate, decrease, or increase certain substances in the diet (e.g.,
sodium or potassium), or to provide mechanically altered food when indicated.
Patients must be assessed for their risk for nutritional deficiencies or need for therapeutic
diets and/or other nutritional supplementation.
Examples of patients who may have specialized dietary needs and may require a more
detailed nutritional assessment include, but are not limited to:
All patients requiring artificial nutrition by any means (i.e., enteral nutrition (tube
feeding), total parenteral nutrition, or peripheral parenteral nutrition);
Patients whose medical condition, surgical intervention, or physical status interferes
with their ability to ingest, digest or absorb nutrients;
Patients whose diagnosis or presenting signs/symptoms indicates a compromised
nutritional status (e.g., anorexia nervosa, bulimia, electrolyte imbalances, dysphagia,
malabsorption, end stage organ diseases, etc.);
Patients whose medical condition can be adversely affected by their nutritional
intake (e.g., diabetes, congestive heart failure, patients taking certain medications,
renal diseases, etc.).
Patients who refuse the food served should be offered substitutes that are of equal
nutritional value in order to meet their basic nutritional needs.
The care plan for patients identified as having specialized nutritional needs must address
those needs as well as monitoring of their dietary intake and nutritional status. The
methods and frequency of monitoring could include one or more of the following, as well
as other methods:
• Patient weight (BMI, unintended weight loss or gain)
• Intake and output
• Lab values
Survey Procedures §482.28(b)(1)
• Can the dietician demonstrate how the menus meet the nutritional needs of
patients. For example, does the service rely upon DRIs, including RDAs, in
developing menus?
• Can the dietician demonstrate patients are assessed for special nutritional needs
and how the hospital assures the needs of those with specialized needs are met?
• When observing care in inpatient units (or observation units where meals are
provided) ask staff how patients are assessed for nutritional needs.
o Ask them how they monitor patients identified as having specialized
needs.
o Is there evidence that therapeutic diets are provided as ordered?
Does the sample of patient records being reviewed include patients identified with special
nutritional needs? If not, ask to see records for several such patients. Determine if there is
evidence of monitoring the dietary intake and nutritional status of patients identified as
having special nutritional needs.
A-0630
(Rev. 137, Issued: 04-01-15, Effective: 03-27-15, Implementation: 03-27-15)
§482.28(b)(2) - All patient diets, including therapeutic diets, must be ordered by a
practitioner responsible for the care of the patient, or by a qualified dietitian or
qualified nutrition professional as authorized by the medical staff and in accordance
with State law governing dietitians and nutrition professionals.
Interpretive Guidelines §482.28(b)(2)
Patient diets, including therapeutic diets, must be provided in accordance with orders from a
practitioner responsible for the care of the patient, or by a qualified dietitian or qualified
nutrition professional who is permitted to order diets under State law and authorized to do
so by the medical staff.
Diets must be based on an assessment of the patient’s nutritional and therapeutic needs and
documented in the patient’s medical record (including documentation about the patient’s
tolerance to any therapeutic diet ordered).
The hospital’s governing body may choose, when permitted under State law and upon
recommendation of the medical staff, to grant qualified dietitians or qualified nutrition
professionals diet-ordering privileges. In many cases State law determines what criteria an
individual must satisfy in order to be a “qualified dietician;” State law may define the term
to mean a “registered dietician” registered with a private organization, such as the
Commission on Dietetic Registration, or State law may impose different or additional
requirements. Terms such as “nutritionists,” “nutrition professionals,” “certified clinical
nutritionists,” and “certified nutrition specialists” are also used to refer to individuals who
are not dieticians, but who may also be qualified under State law to order patient diets. It is
the responsibility of the hospital to ensure that individuals are qualified under State law
before appointing them to the medical staff or granting them privileges to order diets.
If the hospital chooses not to grant diet-ordering privileges to dietitians or other nutrition
professionals, even when permitted under State law, the patient’s diet must be prescribed by
a practitioner responsible for the patient’s care. In this situation, a dietitian or nutrition
professional who does not have privileges to order diets may nevertheless assess a patient’s
nutritional needs and provide recommendations or consultations for patients to a
practitioner responsible for the care of the patient.
Survey Procedures §482.28(b)(2)
• Review patient records to verify that diet orders are provided as prescribed
by the practitioner(s) responsible for the care of the patient, a qualified
dietitian, or qualified nutrition professional.
• If diet orders are prescribed by a dietitian or other nutrition professional,
review their records to verify that he or she was appointed to the medical
staff with diet-ordering privileges, or was granted diet-ordering privileges
without being appointed to the medical staff.
o Ask the hospital how it determines whether the dietician/nutrition
professional is qualified under state law. Review staff records to verify
that dieticians/nutrition professionals demonstrate the required
qualifications.
A-0631
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.28(b)(3) - A current therapeutic diet manual approved by the dietitian and
medical staff must be readily available to all medical, nursing, and food service
personnel.
Interpretive Guidelines §482.28(b)(3)
The therapeutic diet manual must be approved by the dietitian and the medical staff. The
publication or revision date of the approved therapeutic diet manual must not be more than
5 years old. The therapeutic diet manual (or copies of it) must be available to all medical,
nursing and food service personnel.
Survey Procedures §482.28(b)(3)
• Determine that the therapeutic diet manual is current, and:
o Has been approved by both the medical staff and a qualified dietitian;
o Is readily available to MD/DOs, nursing and food service personnel;
o Is in accordance with the current national standards, such as RDA or DRI;
o Includes the different types of therapeutic diets routinely ordered at the hospital;
and
o Is consistently used as guidance for ordering and preparing patient diets.
A-0652
(Rev. 137, Issued: 04-01-15, Effective: 03-27-15, Implementation: 03-27-15)
§482.30 Condition of Participation: Utilization Review
The hospital must have in effect a utilization review (UR) plan that provides for
review of services furnished by the institution and by members of the medical staff to
patients entitled to benefits under the Medicare and Medicaid programs.
Interpretive Guidelines §482.30
If the hospital does not satisfy one of the exception criteria at §482.30(a), it must have a UR
plan in effect which provides for review of services provided to Medicare and Medicaid
beneficiaries.
Survey Procedures §482.30
The manner and degree of noncompliance with one or more of the UR standards is
considered when determining whether there is condition-level compliance or non-
compliance.
A-0653
(Rev. 137, Issued: 04-01-15, Effective: 03-27-15, Implementation: 03-27-15)
§482.30(a) Standard: Applicability
The provisions of this section apply except in either of the following
circumstances:
(1) A Utilization and Quality Control Quality Improvement Organization (QIO)
has assumed binding review for the hospital.
(2) CMS has determined that the UR procedures established by the State under
title XIX of the Act are superior to the procedures required in this section, and has
required hospitals in that State to meet the UR plan requirements under §§456.50
through 456.245 of this chapter.

Interpretive Guidelines §482.30(a)
The regulation permits two exceptions to the requirement for a hospital UR plan: (1) where
the hospital has an agreement with a QIO under contract with the Secretary to assume
binding review for the hospital or; (2) where CMS has determined that UR procedures
established by the State under Medicaid are superior to the UR requirements for the
Medicare program and has required hospitals in that State to meet the UR requirements for
the Medicaid program at 42 CFR 456.50 through 456.245.
According to the regulation at 42 CFR 476.86(e), QIO review and monitoring activities
fulfill the requirements for compliance activities of State Survey Agencies under §1861(k)
of the Social Security Act (Act). The statutory requirements for utilization review at
§1861(k) of the Act are reiterated in the UR CoP at 42 CFR 482.30. Therefore, a hospital
meets the exception requirements of 42 CFR 482.30 if a QIO has assumed binding review
for the hospital. (The hospital may not make requests for work to be performed by the QIO
that goes beyond the scope of the QIO’s contract with the Secretary.)
The regulation at 42 CFR 489.20(e) requires a hospital to maintain an agreement with a
QIO to review the admissions, quality, appropriateness, and diagnostic information related
to inpatient services for Medicare patients, if there is a QIO with a contract with CMS in the
area where the hospital is located.
CMS anticipates that most hospitals comply with the UR CoP by means of the QIO
exception.
With regard to the second exception, CMS would have to determine that UR procedures
established by a State under Medicaid are superior to the UR requirements for Medicare.
Currently no UR plans established by a State under Medicaid have been approved as
exceeding the requirements under Medicare and required for hospital compliance with the
Medicare UR CoP within that State. In the event that CMS approves a State’s Medicaid UR
process for compliance with the Medicare UR CoP, CMS will advise the affected State
Survey Agency.
Survey Procedures §482.30(a)
Surveyors are to verify either that the hospital:
• Has its own UR plan in place and that it meets the regulatory requirements; or
• If it does not have its own UR plan, that it has an agreement with the QIO that
provides for binding UR review. Surveyors should ask to see the signed, dated
agreement. If the hospital has an agreement with a QIO, it is not necessary for
surveyors to assess the remaining UR standards.
It is not necessary for SAs to conduct routine surveys for compliance with the provider
agreement requirement to have a QIO agreement. However, a hospital that does not satisfy
the UR CoP through either its own program or a QIO agreement may be cited for violating
the UR CoP at the condition level.
A-0654
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§82.30(b) Standard: Composition of Utilization Review Committee
A UR committee consisting of two or more practitioners must carry out the UR
function. At least two of the members of the committee must be doctors of medicine
or osteopathy. The other members may be any of the other types of practitioners
specified in §482.12(c)(1).
(1) Except as specified in paragraphs (b)(2) and (3) of this section, the UR
committee must be one of the following:
(i) A staff committee of the institution;
(ii) A group outside the institution--
(A) Established by the local medical society and some or all of the
hospitals in the locality; or
(B) Established in a manner approved by CMS.
(2) If, because of the small size of the institution, it is impracticable to have a
properly functioning staff committee, the UR committee must be established as
specified in paragraph (b)(1)(ii) of this section.
(3) The committee or group’s reviews may not be conducted by any individual
who--
(i) Has a direct financial interest (for example, an ownership interest) in
that hospital; or
(ii) Was professionally involved in the care of the patient whose case is
being reviewed.
Survey Procedures §482.30(b)
• Determine the composition of the UR committee;
• Determine that the governing body has delegated to the UR committee the authority
and responsibility to carry out the UR function;
• Verify that small hospitals delegate the UR function to an outside group if it is
impractical to have a staff committee;
• Ascertain that committee members are not financially involved in the hospital
(ownership of 5 percent or greater) nor participants in the development or execution
of the patient’s treatment plan.
A-0655
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.30(c) Standard: Scope and Frequency of Review
(1) The UR plan must provide for review for Medicare and Medicaid patients with
respect to the medical necessity of--
(i) Admissions to the institution;
(ii) The duration of stays; and
(iii) Professional services furnished including drugs and biologicals.
(2) Review of admissions may be performed before, at, or after hospital admission.
(3) Except as specified in paragraph (e) of this section, reviews may be conducted
on a sample basis.
(4) Hospitals that are paid for inpatient hospital services under the prospective
payment system set forth in Part 412 of this chapter must conduct review of
duration of stays and review of professional services as follows:
(i) For duration of stays, these hospitals need review only cases that they
reasonably assume to be outlier cases based on extended length of stay, as
described in §412.80(a)(1)(i) of this chapter; and
(ii) For professional services, these hospitals need review only cases that they
reasonably assume to be outlier cases based on extraordinarily high costs, as
described in §412.80(a)(1)(ii) of this chapter.
Interpretive Guidelines §482.30(c)
Admissions may be reviewed before, during, or after hospital admission as stated in the
hospital’s UR plan.
Reviews may be conducted on a sample basis, except for reviews of extended stay cases.
In an Inpatient Prospective Payment System (IPPS) hospital, to determine outlier review
compliance, “reasonably assumes” is a good faith test. The question to ask is whether the
hospital is reviewing outlier cases. In instances where there was no other review of outlier
cases, the question is whether it was reasonable for the hospital not to have known that the
cases were in fact outliers. Some medical judgment might be required to determine whether
it is reasonable for the hospital to have assumed that a patient fell into a DRG other than the
one eventually assigned by the intermediary. This would be an issue in long stay outlier
cases where the hospital did not review because the hospital erroneously assumed that the
patient was in a DRG under which the case would not have been an outlier.
Survey Procedures §482.30(c)
• Examine the UR plan and other documentation to determine that the medical
necessity for Medicare and Medicaid patients is reviewed with respect to admission,
duration of the stay, and the professional services furnished.
• Determine if the hospital is reimbursed under IPPS. This requirement does not
apply to IPPS excluded hospitals or units.
• Verify that in an IPPS hospital the following are being reviewed:
o Duration of stay in cases reasonably assumed to be outlier cases; and
o Professional services in cases reasonably assumed to be outlier cases.
A-0656
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.30(d) Standard: Determination Regarding Admissions or Continued
Stays
(1) The determination that an admission or continued stay is not medically
necessary-
(i) May be made by one member of the UR committee if the practitioner or
practitioners responsible for the care of the patient, as specified of §482.12(c),
concur with the determination or fail to present their views when afforded the
opportunity; and
(ii) Must be made by at least two members of the UR committee in all other
cases.
(2) Before making a determination that an admission or continued stay is not
medically necessary, the UR committee must consult the practitioner or
practitioners responsible for the care of the patient, as specified in §482.12(c), and
afford the practitioner or practitioners the opportunity to present their views.
(3) If the committee decides that admission to or continued stay in the hospital is
not medically necessary, written notification must be given, no later than 2 days
after the determination, to the hospital, the patient, and the practitioner or
practitioners responsible for the care of the patient, as specified in §482.12(c);
Interpretive Guidelines §482.30(d)
When other than a doctor of medicine or osteopathy makes an initial finding that the written
criteria for extended stay are not met, the case must be referred to the committee, or
subgroup thereof which contains at least one physician. If the committee or subgroup
agrees after reviewing the case that admissions, or extended stay is not medically necessary
or appropriate, the attending physician is notified and allowed an opportunity to present his
views and any additional information relating to the patient’s needs for admissions or
extended stay. When a physician member of the committee performs the initial review
instead of a non-physician reviewer, and he finds that admissions or extended stay is not
necessary no referral to the committee or subgroup is necessary and he may notify the
attending practitioner directly.
If the attending practitioner does not respond or does not contest the findings of the
committee or subgroup or those of the physician who performed the initial review, then the
findings are final.
If the attending physician contests the committee or subgroup findings, or if he presents
additional information relating to the patient’s need for extended stay, at least one
additional physician member of the committee must review the case. If the two physician
members determine that the patient’s stay is not medically necessary or appropriate after
considering all the evidence, their determination becomes final. Written notification of this
decision must be sent to the attending physician, patient (or next of kin), facility
administrator, and the single State agency (in the case of Medicaid) no later than 2 days
after such final decision and in no event later than 3 working days after the end of the
assigned extended stay period.
There are only 5 working days in a given week. Normally these days are Monday through
Friday, however, the institution has the option to establish 5 other days as working days.
When a holiday falls on a working day, that day is not counted as a working day.
In no case may a non-physician make a final determination that a patient’s stay is not
medically necessary or appropriate.
If, after referral of a questioned case to the committee or subgroup thereof, the physician
reviewer determines that an admission or extended stay is justified, the attending physician
shall be so notified and an appropriate date for subsequent extended stay review will be
selected and noted on the patient’s record.
Written notification of this final determination must be sent to the attending physician, the
patient (or next of kin), the facility administrator and the single State agency (in the case of

Medicaid) no later than 2 days after such final determination and in no event later than 3
working days after the end of the assigned extended stay period.
Where possible, the written notification should be received by all involved parties within
the stated time period. Where appropriate and desired, verbal notification may precede
written notification.
Survey Procedures §482.30(d)
• Review a sample of “medically unnecessary” decisions involving admissions or
continued stay that are not medically necessary and determine that these decisions
are made by:
o One member of the UR committee, if the practitioner(s) responsible for the
patient’s care concurs with the determination or fails to present his/her views.
The practitioner must be one of those specified in §482.12(c), or
o At least two members of the UR committee in all cases not qualified under the
above.
• Review a sample of “medically unnecessary” decisions and verify that the physician
or practitioners, as specified in §482.12(c), were informed of the committees
expected decision and were given an opportunity to comment.
• Review a sample of “medically unnecessary” cases and verify that all involved
parties are notified of the decision that care is medically not necessary no later than
two days following the decision.
A-0657
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.30(e) Standard: Extended Stay Review
(1) In hospitals that are not paid under the prospective payment system, the UR
committee must make a periodic review, as specified in the UR plan, or each
current inpatient receiving hospital services during a continuous period of
extended duration.
The scheduling of the periodic reviews may--
(i) Be the same for all cases; or
(ii) Differ for different classes of cases.
(2) In hospitals paid under the prospective payment system, the UR committee
must review all cases reasonably assumed by the hospital to be outlier cases
because the extended length of stay exceeds the threshold criteria for the diagnosis,
as described in §412.80(a)(1)(i). The hospital is not required to review an extended
stay that does not exceed the outlier threshold for the diagnosis.
(3) The UR committee must make the periodic review no later than 7 days after
the day required in the UR plan.
Survey Procedures §482.30(e)
• Review the facility’s definition of extended stay in the UR plan.
• Verify that the hospital’s UR plan requires a periodic review of each current
Medicare/Medicaid inpatient receiving hospital services of extended duration and
that the review is carried out at the specified time stated in the facility’s UR plan.
• The review may be the same for all cases or be different for different classes of care.
• If the committee uses a different number of days for different diagnosis or
functional categories for the period of extended stay, the surveyor must verify that
there is a written list with lengths of stay designated for each diagnosis of functional
category.
• Determine if the hospital is under IPPS. Hospitals under IPPS need only review
cases reasonably assumed to be outlier cases, and extended stay that exceeds the
outlier threshold for the diagnosis.
• Review minutes of the UR committee. Determine that the periodic reviews of
extended stay are carried out on or before the expiration of the stated period or no
later than 7 days after the day required in the hospital’s plan.
A-0658
(Rev. 137, Issued: 04-01-15, Effective: 03-27-15, Implementation: 03-27-15)
§482.30(f) Standard: Review of Professional Services
The committee must review professional services provided, to determine medical
necessity and to promote the most efficient use of available health facilities and
services.
Interpretive Guidelines §482.30(f)
“Professional” services means the services provided by practitioners, including both
physicians and non-physician practitioners.
The review includes medical necessity and efficient use of available health facilities and
services. Examples of topics a committee may review are:
• Availability and use of necessary services - underused, overuse, appropriate use
• Timeliness of scheduling of services - operating room, diagnostic
• Therapeutic procedures
Survey Procedures §482.30(f)
Determine that the committee performs a review of professional services.
A-0700
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.41 Condition of Participation: Physical Environment
The hospital must be constructed, arranged, and maintained to ensure the safety of
the patient, and to provide facilities for diagnosis and treatment and for special
hospital services appropriate to the needs of the community.
Interpretive Guidelines §482.41
This CoP applies to all locations of the hospital, all campuses, all satellites, all provider-
based activities, and all inpatient and outpatient locations.
The hospital’s Facility Maintenance and hospital departments or services responsible for the
hospital’s buildings and equipment (both facility equipment and patient care equipment)
must be incorporated into the hospital’s QAPI program and be in compliance with the
QAPI requirements.
Survey Procedures §482.41
Survey of the Physical Environment CoP should be conducted by one surveyor. However,
each surveyor as he/she conducts his/her survey assignments should assess the hospital’s
compliance with the Physical Environment CoP. The Life Safety Code survey may be
conducted separately by a specialty surveyor.
A-0701
(Rev. 176, Issued: 12-29-17, Effective: 12-29-17, Implementation: 12-29-17)
§482.41(a) Standard: Buildings
The condition of the physical plant and the overall hospital environment must be
developed and maintained in such a manner that the safety and well-being of patients
are assured.
Interpretive Guidelines §482.41(a)
The hospital must ensure that the condition of the physical plant and overall hospital
environment is developed and maintained in a manner to ensure the safety and well-being
of patients. This includes ensuring that routine and preventive maintenance and testing
activities are performed as necessary, in accordance with Federal and State laws,
regulations, and guidelines and manufacturer’s recommendations, by establishing
maintenance schedules and conducting ongoing maintenance inspections to identify areas
or equipment in need of repair. The routine and preventive maintenance and testing
activities should be incorporated into the hospital’s QAPI plan.
The hospital must be constructed and maintained to ensure risks are minimized for patients
as well as for employees and visitors. Hospitals are expected to demonstrate how they are
addressing important safety features in accordance with nationally recognized standards.
Although the following items are expected to be addressed when applicable, the list is not
all-inclusive.
Accessibility
• The hospital must ensure all buildings at all locations of the certified hospital meet
State and Federal accessibility standards (e.g. Office of Civil Rights requirements).
The requirements apply to the interior and exterior of all buildings.
Age-related safety features
• Hospitals are expected to address safety hazards and risks related to age-related
factors. Healthcare provided to neonatal, pediatric, and geriatric patients must be in
accordance with nationally recognized standards. Age-related risks may include
items such as security of inpatient and outpatient locations, access to medications,
cleaning supplies and other hazardous materials, furniture and other medical
equipment, and increased chance of falls.
Security
• To minimize the risk of unauthorized access to or inappropriate departure from
secured healthcare units, hospitals must demonstrate security features in accordance
with nationally recognized standards to ensure the safety of vulnerable patients.
This includes, but is not limited to, patients such as newborn (e.g. infant abduction),
pediatric, behavioral health, those with diminished capacity and
dementia/Alzheimer’s.
Access to non-clinical rooms identified as hazardous locations must be secured to
prevent patient and visitor entry. Examples include electrical rooms and heat,
ventilation, air conditioning (HVAC) rooms.
Ligature risk
•
The presence of unmitigated ligature risks in a psychiatric hospital or psychiatric
unit of a hospital is an immediate jeopardy situation. Additionally, this also
includes any location where patients at risk of suicide are identified. Ligature risk
findings must be referred to the health and safety surveyors for further evaluation
and possible citation under Patients’ Rights.
Weather-related exterior issues
•
Although hospitals cannot address all weather-related issues, they are expected to
address potential safety hazards specific to weather on both the exterior and interior
locations in accordance of nationally recognized standards. Areas of risk include
driveways, garages, entry points, walkways, etc.
Life Safety Code surveyors assess the use of power strips in healthcare facilities. However,
the following guidance is provided as reference for healthcare surveyors as they survey
physical environment along with other CoP requirements. Any observed power strip
deficiencies should be conveyed to the LSC surveyors for citation.
If line-operated medical equipment is used in a patient care room/area, inside the patient
care vicinity:
• UL power strips would have to be a permanent component of a rack-, table-,
pedestal-, or cart-mounted & tested medical equipment assembly
• Power strips providing power to medical equipment in a patient care room/area must
be UL 1363A or UL 60601-1
• Power strips cannot be used for non-medical equipment
If line-operated medical equipment is used in a patient care room/area, outside the patient
care vicinity:
• UL power strips could be used for medical & non-medical equipment with
precautions as described in the memo
• Power strips providing power to medical equipment in a patient care room/area must
be UL 1363A or UL 60601-1
• Power strips providing power to non-medical equipment in a patient care room/area
must be UL 1363
If line-operated medical equipment is not used in a patient care room/area, inside and
outside the patient care vicinity:
• UL power strips could be used with precautions
Power strips providing power to non-medical equipment in a patient care room/area must be
UL 1363. In non-patient care areas/rooms, other UL strips could be used with the general
precautions.
Survey Procedures §482.41(a)
• Verify that the condition of the hospital is maintained in a manner to assure the
safety and well-being of patients (e.g., condition of ceilings, walls, and floors,
presence of patient hazards, etc.).
• Review the hospital’s routine and preventive maintenance schedules to determine
that ongoing maintenance inspections are performed and that necessary repairs are
completed.
• Review a copy of the most recent environmental risk assessment to determine if the
hospital has identified any accessibility, age-related, security, suicide and/or weather
related risks or concerns. If environmental safety concerns have been identified in
this assessment, what plans have been implemented by the hospital to ensure
patient/staff safety?
• Refer any potential power strip use deficiencies to Life Safety Code surveyors.
Communicate findings with health and safety surveyors as appropriate.
A-0702
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.41(a)(1) - There must be emergency power and lighting in at least the operating,
recovery, intensive care, and emergency rooms, and stairwells. In all other areas not
serviced by the emergency supply source, battery lamps and flashlights must be
available.
Interpretive Guidelines §482.41(a)(1)
The hospital must comply with the applicable provisions of the Life Safety Code, National
Fire Protection Amendments (NFPA) 101, 2000 Edition and applicable references, such as,
NFPA-99: Health Care Facilities, for emergency lighting and emergency power.
Survey Procedures §482.41(a)(1)
Use the Life Safety Code Survey Report Form (CMS-2786) to evaluate compliance with
this item.
A-0703
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.41(a)(2) - There must be facilities for emergency gas and water supply.
Interpretive Guidelines §482.41(a)(2)
The hospital must have a system to provide emergency gas and water as needed to provide
care to inpatients and other persons who may come to the hospital in need of care. This
includes making arrangements with local utility companies and others for the provision of
emergency sources of water and gas. The hospital should consider nationally accepted
references or calculations made by qualified staff when determining the need for at least
water and gas. For example, one source for information on water is the Federal Emergency
Management Agency (FEMA).
Emergency gas includes fuels such as propane, natural gas, fuel oil, liquefied natural gas, as
well as any gases the hospital uses in the care of patients such as oxygen, nitrogen, nitrous
oxide, etc.
The hospital should have a plan to protect these limited emergency supplies, and have a
plan for prioritizing their use until adequate supplies are available. The plan should also
address the event of a disruption in supply (e.g., disruption to the entire surrounding
community).
Survey Procedures §482.41(a)(2)
• Review the system used by hospital staff to determine the hospital’s emergency
needs for gas and water. Verify that the system accounts for not only inpatients, but
also staff and other persons who come to the hospital in need of care during
emergencies.
• Determine the source of emergency gas and water, both the quantity of these
supplies readily available at the hospital, and that are needed within a short time
through additional deliveries.
• Verify that arrangements have been made with utility companies and others for the
provision of emergency sources of critical utilities, such as water and gas.
A-0709
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.41(b) Standard: Life Safety from Fire
The hospital must ensure that the life safety from fire requirements are met.
A-0710
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(b)
(1) Except as otherwise provided in this section—
(i) The hospital must meet the applicable provisions and must proceed in
accordance with the Life Safety Code (NFPA 101 and Tentative Interim
Amendments TIA 12–1, TIA 12–2, TIA 12–3, and TIA 12–4.) Outpatient
surgical departments must meet the provisions applicable to Ambulatory
Health Care Occupancies, regardless of the number of patients served.
(ii) Notwithstanding paragraph (b)(1)(i) of this section, corridor doors and
doors to rooms containing flammable or combustible materials must be
provided with positive latching hardware. Roller latches are prohibited on such
doors.
(2) In consideration of a recommendation by the State survey agency or
Accrediting Organization or at the discretion of the Secretary, may waive, for
periods deemed appropriate, specific provisions of the Life Safety Code, which
would result in unreasonable hardship upon a hospital, but only if the waiver
will not adversely affect the health and safety of the patients.
(3) The provisions of the Life Safety Code do not apply in a State where CMS finds
that a fire and safety code imposed by State law adequately protects patients in
hospitals.
Interpretive Guidelines §482.41(b)(1)–(3)
Guidance is pending and will be updated in future release.
A-0713
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(b)(4) - The hospital must have procedures for the proper routine storage and
prompt disposal of trash.
Interpretive Guidelines §482.41(b)(4)
Guidance is pending and will be updated in future release.
A-0714
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(b)(5) - The hospital must have written fire control plans that contain
provisions for prompt reporting of fires; extinguishing fires; protection of patients,
personnel and guests; evacuation; and cooperation with fire fighting authorities.
Survey Procedures §482.41(b)(5)
Guidance is pending and will be updated in future release.
A-0715
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(b)(6) - The hospital must maintain written evidence of regular inspection and
approval by State or local fire control agencies.
Interpretive Guidelines §482.41(b)(6)
Guidance is pending and will be updated in future release
A-0716
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(b)(7) - A hospital may install alcohol-based hand rub dispensers in its facility
if the dispensers are installed in a manner that adequately protects against
inappropriate access.
Interpretive Guidelines §482.41(b)(7):
Guidance is pending and will be updated in future release
A-0717
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(b)(8) When a sprinkler system is shut down for more than 10 hours, the
hospital must:
(i) Evacuate the building or portion of the building affected by the system outage until
the system is back in service, or
(ii) Establish a fire watch until the system is back in service.
Interpretive Guidelines §482.41(b)(8):
Guidance is pending and will be updated in future release.
A-0718
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(b)(9) Buildings must have an outside window or outside door in every sleeping
room, and for any building constructed after July 5, 2016 the sill height must not
exceed 36 inches above the floor. Windows in atrium walls are considered outside
windows for the purposes of this requirement.
(i) The sill height requirement does not apply to newborn nurseries and rooms
intended for occupancy for less than 24 hours.
(ii) The sill height in special nursing care areas of new occupancies must not exceed 60
inches.
Interpretive Guidelines §482.41(b)(9):
Guidance is pending and will be updated in future release.
A-0720
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(c) Standard: Building Safety
Except as otherwise provided in this section, the hospital must meet the applicable
provisions and must proceed in accordance with the Health Care Facilities Code
(NFPA 99 and Tentative Interim Amendments TIA 12–2, TIA 12–3, TIA 12–4, TIA
12–5 and TIA 12–6).
(1) Chapters 7, 8, 12, and 13 of the adopted Health Care Facilities Code do not apply
to a hospital.
(2) If application of the Health Care Facilities Code required under paragraph (c) of
this section would result in unreasonable hardship for the hospital, CMS may waive
specific provisions of the Health Care Facilities Code, but only if the waiver does not
adversely affect the health and safety of patients.
Interpretive Guidelines §482.41(c)(1) and (2):
Guidance is pending and will be updated in future release.
A-0722
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(d) Standard: Facilities
The hospital must maintain adequate facilities for its services.
Interpretive Guidelines §482.41(d)
Adequate facilities means the hospital has facilities that are:
• Designed and maintained in accordance with Federal, State and local laws,
regulations and guidelines; and
• Designed and maintained to reflect the scope and complexity of the services it offers
in accordance with accepted standards of practice.
Survey Procedures §482.41(d)
• Observe the facility layout and determine if the patient’s needs are met. Toilets,
sinks, specialized equipment, etc. should be accessible.
• Review the facility’s water supply and distribution system to ensure that the water
quality is acceptable for its intended use (drinking water, irrigation water, lab water,
etc.). Review the facility water quality monitoring and, as appropriate, treatment
system.
A-0723
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(d)(1) - Diagnostic and therapeutic facilities must be located for the safety of
patients.
Interpretive Guidelines §482.41(d)(1)
Guidance is pending and will be updated in future release.
A-0724
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(d)(2) - Facilities, supplies, and equipment must be maintained to ensure an
acceptable level of safety and quality.
Interpretive Guidelines §482.41(d)(2)
Guidance is pending and will be updated in future release.
A-0725
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(d)(3) - The extent and complexity of facilities must be determined by the
services offered.
Interpretive Guidelines §482.41(d)(3)
Physical facilities must be large enough, numerous enough, appropriately designed and
equipped, and of appropriate complexity to provide the services offered in accordance with

Federal and State laws, regulations and guidelines and accepted standards of practice for
that location or service.
Survey Procedures §482.41(d)(3)
Verify through observation that the physical facilities are large enough and properly
equipped for the scope of services provided and the number of patients served.
0726
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(d)(4) - There must be proper ventilation, light, and temperature controls in
pharmaceutical, food preparation, and other appropriate areas.
Interpretive Guidelines §482.41(d)(4)
Guidance is pending and will be updated in future release.
A-0730
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.41(e)
The standards incorporated by reference in this section are approved for
incorporation by reference by the Director of the Office of the Federal Register in
accordance with 5 U.S.C. 552(a) and 1 CFR part 51. You may inspect a copy at the
CMS Information Resource Center, 7500 Security Boulevard, Baltimore, MD or at the
National Archives and Records Administration (NARA). For information on the
availability of this material at NARA, call 202–741–6030, or go to:
http://www.archives.gov/federal_register/code_of_federalregulations/ibr_locations.ht
ml. If any changes in this edition of the Code are incorporated by reference, CMS will
publish a document in the Federal Register to announce the changes.
(1) National Fire Protection Association, 1 Batterymarch Park, Quincy, MA 02169,
www.nfpa.org, 1.617.770.3000.
(i) NFPA 99, Standards for Health Care Facilities Code of the National Fire Protection
Association 99, 2012 edition, issued August 11, 2011.
(ii) TIA 12–2 to NFPA 99, issued August 11, 2011.
(iii) TIA 12–3 to NFPA 99, issued August 9, 2012.
(iv) TIA 12–4 to NFPA 99, issued March 7, 2013.
(v) TIA 12–5 to NFPA 99, issued August 1, 2013.
(vi) TIA 12–6 to NFPA 99, issued March 3, 2014.
(vii) NFPA 101, Life Safety Code, 2012 edition, issued August 11, 2011;
(viii) TIA 12–1 to NFPA 101, issued August 11, 2011.
(ix) TIA 12–2 to NFPA 101, issued October 30, 2012.
(x) TIA 12–3 to NFPA 101, issued October 22, 2013.
(xi) TIA 12–4 to NFPA 101, issued October 22, 2013.
(2) [Reserved]
A-0747
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.42 Condition of Participation: Infection Prevention and Control and
Antibiotic Stewardship Programs
The hospital must have active hospital-wide programs for the surveillance, prevention,
and control of HAIs and other infectious diseases, and for the optimization of
antibiotic use through stewardship. The programs must demonstrate adherence to
nationally recognized infection prevention and control guidelines, as well as to best
practices for improving antibiotic use where applicable, and for reducing the
development and transmission of HAIs and antibiotic resistant organisms. Infection
prevention and control problems and antibiotic use issues identified in the programs
must be addressed in collaboration with the hospital-wide quality assessment and
performance improvement (QAPI) program.
Interpretive Guidelines §482.42
Guidance is pending and will be updated in future release.
A-0748
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.42(a) Standard: Infection prevention and control program
organization and policies. The hospital must demonstrate that:
(1) An individual (or individuals), who is qualified through education, training,
experience, or certification in infection prevention and control, is appointed by the
governing body as the infection preventionist(s)/infection control professional(s)
responsible for the infection prevention and control program and that the
appointment is based on the recommendations of medical staff leadership and nursing
leadership;
Interpretive Guidelines §482.42(a)(1)
Guidance is pending and will be updated in future release.
A-0749
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.42(a)(2) The hospital infection prevention and control program, as documented
in its policies and procedures, employs methods for preventing and controlling the
transmission of infections within the hospital and between the hospital and other
institutions and settings;
Interpretive Guidelines §482.42(a)(2)
Guidance is pending and will be updated in future release.
A-0750
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.42(a)(3) The infection prevention and control program includes surveillance,
prevention, and control of HAIs, including maintaining a clean and sanitary
environment to avoid sources and transmission of infection, and addresses any
infection control issues identified by public health authorities; and
Interpretive Guidelines §482.42(a)(3)
Guidance is pending and will be updated in future release.
A-0751
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.42(a)(4) The infection prevention and control program reflects the scope and
complexity of the hospital services provided.
Interpretive Guidelines §482.42(a)(4)
Guidance is pending and will be updated in future release.
A-0770
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.42(c)(1) Standard: Leadership responsibilities
(1) The governing body must ensure all of the following:
(i) Systems are in place and operational for the tracking of all infection
surveillance, prevention, and control, and antibiotic use activities, in order to
demonstrate the implementation, success, and sustainability of such activities.
Interpretive Guidelines §482.42(c)(1)(i)
Guidance is pending and will be updated in future release.
A-0771
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[§482.42(c)(1) The governing body must ensure all of the following:]
(ii) All HAIs and other infectious diseases identified by the infection
prevention and control program as well as antibiotic use issues identified by the
antibiotic stewardship program are addressed in collaboration with hospital
QAPI leadership.
Interpretive Guidelines §482.42(c)(1)(ii)
Guidance is pending and will be updated in future release.
A-0772
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.42(c)(2) Standard: Leadership responsibilities
(2) The infection preventionist(s)/infection control professional(s) is responsible for:
(i) The development and implementation of hospital-wide infection
surveillance, prevention, and control policies and procedures that adhere to
nationally recognized guidelines.
Interpretive Guidelines §482.42(c)(2)(i)
Guidance is pending and will be updated in future release.
A-0773
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[§482.42(c)(2) The infection preventionist(s)/infection control professional(s) is
responsible for:]
(ii) All documentation, written or electronic, of the infection prevention and
control program and its surveillance, prevention, and control activities.
Interpretive Guidelines §482.42(c)(2)(ii)
Guidance is pending and will be updated in future release.
A-0774
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[§482.42(c)(2) The infection preventionist(s)/infection control professional(s) is
responsible for:]
(iii) Communication and collaboration with the hospital’s QAPI program on
infection prevention and control issues.
Interpretive Guidelines §482.42(c)(2)(iii)
Guidance is pending and will be updated in future release.
A-0775
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[§482.42(c)(2) The infection preventionist(s)/infection control professional(s) is
responsible for:]
(iv) Competency-based training and education of hospital personnel and staff,
including medical staff, and, as applicable, personnel providing contracted
services in the hospital, on the practical applications of infection prevention
and control guidelines, policies, and procedures.
Interpretive Guidelines §482.42(c)(2)(iv)
Guidance is pending and will be updated in future release.
A-0776
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[§482.42(c)(2) The infection preventionist(s)/infection control professional(s) is
responsible for:]
(v) The prevention and control of HAIs, including auditing of adherence to
infection prevention and control policies and procedures by hospital personnel.
Interpretive Guidelines §482.42(c)(2)(v)
Guidance is pending and will be updated in future release.
A-0777
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[§482.42(c)(2) The infection preventionist(s)/infection control professional(s) is
responsible for:]
(vi) Communication and collaboration with the antibiotic stewardship
program.
Interpretive Guidelines §482.42(c)(2)(vi)
Guidance is pending and will be updated in future release.
A-0778
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.42(c)(3) Standard: Leadership responsibilities
(3) The leader(s) of the antibiotic stewardship program is responsible for:
(i) The development and implementation of a hospital-wide antibiotic
stewardship program, based on nationally recognized guidelines, to monitor
and improve the use of antibiotics.
Interpretive Guidelines §482.42(c)(3)(i)
Guidance is pending and will be updated in future release.
A-0779
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[§482.42(c)(3) The leader(s) of the antibiotic stewardship program is responsible for:]
(ii) All documentation, written or electronic, of antibiotic stewardship
program activities.
Interpretive Guidelines §482.42(c)(3)(ii)
Guidance is pending and will be updated in future release.
A-0780
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[§482.42(c)(3) The leader(s) of the antibiotic stewardship program is responsible for:]
(iii) Communication and collaboration with medical staff, nursing, and
pharmacy leadership, as well as with the hospital’s infection prevention and
control and QAPI programs, on antibiotic use issues.
Interpretive Guidelines §482.42(c)(3)(iii)
Guidance is pending and will be updated in future release.
A-0781
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
[§482.42(c)(3) The leader(s) of the antibiotic stewardship program is responsible for:]
(iv) Competency-based training and education of hospital personnel and staff,
including medical staff, and, as applicable, personnel providing contracted
services in the hospital, on the practical applications of antibiotic stewardship
guidelines, policies, and procedures.
Interpretive Guidelines §482.42(c)(3)(iv)
Guidance is pending and will be updated in future release.
A-0785
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.42(d) Standard: Unified and integrated infection prevention and control and
antibiotic stewardship programs for multi-hospital systems.
If a hospital is part of a hospital system consisting of multiple separately certified
hospitals using a system governing body that is legally responsible for the conduct of
two or more hospitals, the system governing body can elect to have unified and
integrated infection prevention and control and antibiotic stewardship programs for
all of its member hospitals after determining that such a decision is in accordance with
all applicable State and local laws. The system governing body is responsible and
accountable for ensuring that each of its separately certified hospitals meets all of the
requirements of this section. Each separately certified hospital subject to the system
governing body must demonstrate that:
Interpretive Guidelines §482.42(d)
Guidance is pending and will be updated in future release.
A-0786
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.42(d)(1) Standard: Unified and integrated infection prevention and control and
antibiotic stewardship programs for multi-hospital systems.
(1) The unified and integrated infection prevention and control and antibiotic
stewardship program sare established in a manner that takes into account each
member hospital's unique circumstances and any significant differences in patient
populations and services offered in each hospital;
Interpretive Guidelines §482.42(d)(1)
Guidance is pending and will be updated in future release.
A-0787
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.42(d)(2) The unified and integrated infection prevention and control and
antibiotic stewardship programs establish and implement policies and procedures to
ensure that the needs and concerns of each of its separately certified hospitals,
regardless of practice or location, are given due consideration;
Interpretive Guidelines §482.42(d)(2)
Guidance is pending and will be updated in future release.
A-0788
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.42(d)(3) The unified and integrated infection prevention and control and
antibiotic stewardship programs have mechanisms in place to ensure that issues
localized to particular hospitals are duly considered and addressed; and
Interpretive Guidelines §482.42(d)(3)
Guidance is pending and will be updated in future release.
A-0789
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.42(d)(4) A qualified individual (or individuals) with expertise in infection
prevention and control has been designated at the hospital as responsible for
communicating with the unified infection prevention and control and antibiotic
stewardship programs, for implementing and maintaining the policies and procedures
governing infection prevention and control and antibiotic stewardship as directed by
the unified infection prevention and control and antibiotic stewardship programs, and
for providing education and training on the practical applications of infection
prevention and control and antibiotic stewardship to hospital staff.
Interpretive Guidelines §482.42(d)(4)
Guidance is pending and will be updated in future release.
A-0799
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43 Condition of Participation: Discharge Planning
The hospital must have in effect a discharge planning process that focuses on the
patient goals and treatment preferences and includes the patient and his or her
caregivers support person(s) in the discharge planning for post-discharge care. The
discharge planning process and the discharge plan must be consistent with the
patient’s goals for care and his or her treatment preferences, ensure an effective
transition of the patient from hospital to post-discharge care, and reduce the factors
leading to a preventable hospital readmissions.
Interpretive Guidelines §482.43
Guidance is pending and will be updated in future release.
A-0800
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(a) Standard: Discharge Planning Process
(a) The hospital’s discharge planning process must identify at an early stage of
hospitalization those patients who are likely to suffer adverse health consequences
upon discharge in the absence of adequate discharge planning and must provide a
discharge planning evaluation for those patients so identified as well as for other
patients upon the request of the patient, patient’s representative, or patient’s
physician.
Interpretive Guidelines §482.43(a)
Guidance is pending and will be updated in future release.
A-0801
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(a)(4) Standard: Discharge Planning Process
(4) Upon the request of a patient’s physician, the hospital must arrange for the
development and initial implementation of a discharge plan for the patient.
Interpretive Guidelines §482.43(a)(4)
Guidance is pending and will be updated in future release.
A-0802
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(a)(6) Standard: Discharge Planning Process
(6) The hospital’s discharge planning process must require regular re-evaluation of
the patient’s condition to identify changes that require modification of the discharge
plan. The discharge plan must be updated, as needed, to reflect these changes.
Interpretive Guidelines §482.43(a)(6)
Guidance is pending and will be updated in future release.
A-0803
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(a)(7) Standard: Discharge Planning Process
(7) The hospital must assess its discharge planning process on a regular basis. The
assessment must include ongoing, periodic review of a representative sample of
discharge plans, including those patients who were admitted within 30 days of a
previous admission, to ensure that the plans are responsive to the patient post-
discharge needs.
Interpretive Guidelines §482.43(a)(7)
Guidance is pending and will be updated in future release.
A-0804
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(a)(8) Standard: Discharge Planning Process
(8) The hospital must assist patients, their families, or the patient’s representative in
selecting a post-acute care provider by using and sharing data that includes, but not
limited to, HHA, SNF, IRF, or LTCH data on quality measures and data on resource
use on measures. The hospital must ensure that the post-acute care data on quality
measures and data on resource measures is relevant and applicable to the patient’s
goals and treatment preferences.
Interpretive Guidelines §482.43(a)(8)
Guidance is pending and will be updated in future release.
A-0805
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(a)(1) Standard: Discharge Planning Evaluation
(1) Any discharge planning evaluation must be made in a timely basis to ensure the
appropriate arrangements for post-hospital care will be made before discharge and to
avoid unnecessary delays in discharge.
Interpretive Guidelines §482.43(a)(1)
Guidance is pending and will be updated in future release.
A-0806
(Rev. 87, Issued: 07-19-13, Effective: 07-19-13, Implementation: 07-19-13)
§482.43(b) Standard: Discharge Planning Evaluation
(1) The hospital must provide a discharge planning evaluation to the patients
identified in paragraph (a) of this section, and to other patients upon the patient’s
request, the request of a person acting on the patient’s behalf, or the request of the
physician.
(3) - The discharge planning evaluation must include an evaluation of the likelihood of
a patient needing post-hospital services and of the availability of the services.
(4) - The discharge planning evaluation must include an evaluation of the likelihood of
a patient’s capacity for self-care or of the possibility of the patient being cared for in
the environment from which he or she entered the hospital.
Interpretive Guidelines §482.43(b)(1), §482.43(b)(3) & §482.43(b)(4)
For every inpatient identified under the process required at §482.43(a) as at potential risk of
adverse health consequences without a discharge plan, a discharge planning evaluation
must be completed by the hospital. In addition, an evaluation must also be completed if the
patient, or the patient’s representative, or the patient’s attending physician requests one.
Unless the hospital has adopted a voluntary policy of developing an evaluation for every
inpatient, the hospital must also have a process for making patients, including the patient’s
representative, and attending physicians aware that they may request a discharge planning
evaluation, and that the hospital will perform an evaluation upon request. Hospitals must
perform the evaluation upon request, regardless of whether the patient meets the hospital’s
screening criteria for an evaluation.
In contrast to the screening process, the evaluation entails a more detailed review of the
individual patient’s post-discharge needs, in order to identify the specific areas that must be
addressed in the discharge plan.
§482.43(b)(4) requires that the evaluation include assessment of the patient’s capacity for
self-care or, alternatively, to be cared for by others in the environment, i.e., the setting, from
which the patient was admitted to the hospital. In general, the goal upon discharge is for a
patient to be able to return to the setting in which they were living prior to admission. This
may be the patient’s home in the community or residence in a nursing home. In the case of
transfer from another hospital, generally the preferred goal is to return the patient to the
setting from which he/she presented to the transferring hospital.
The evaluation must consider what the patient’s care needs will be immediately upon
discharge, and whether those needs are expected to remain constant or lessen over time. If
the patient was admitted from his/her private residence, the evaluation must include an
assessment of whether the patient is capable of addressing his/her care needs through self-
care. The evaluation must include assessment of whether the patient will require
specialized medical equipment or permanent physical modifications to the home, and the
feasibility of acquiring the equipment or the modifications being made. If the patient is not
able to provide some or all of the required self-care, the evaluation must also address
whether the patient has family or friends available who are willing and able to provide the
required care at the times it will be needed, or who could, if willing, be trained by the
hospital sufficiently to provide the required care.
§482.43(b)(3) requires the evaluation to consider the patient’s likelihood of needing post-
hospital services and the availability of such services.
If neither the patient nor the patient’s family or informal caregiver(s) are able to address all
of the required care needs, then the evaluation must determine whether there are
community-based services that are available to meet the patient’s needs while allowing the
patient to continue living at home.
Such health care services include, but are not limited to:
• Home health, attendant care, and other community-based services;
• Hospice or palliative care;
• Respiratory therapy;
• Rehabilitation services (PT, OT, Speech, etc.);
• End Stage Renal Dialysis services;
• Pharmaceuticals and related supplies;
• Nutritional consultation/supplemental diets; and/or
• Medical equipment and related supplies.
However, services may also include those that are not traditional health care services, but
which may be essential to a patient’s ongoing ability to live in the community, including,
but not limited to:
• Home and physical environment modifications;
• Transportation services;
• Meal services; and/or
• Household services, such as housekeeping, shopping, etc.
Some of the information related to needed services will emerge from the required
evaluation of the patient’s ability to receive care in the home, either as self-care or provided
by someone else. All patients, even those with a high capability for self-care, are likely to
require some follow-up ambulatory health care services, e.g., a post-discharge appointment
with their surgeon, specialist or primary care physician, or a series of appointments for
physical or occupational therapy. Some patients might have more complex care needs
which nevertheless may still be met in the home setting, depending on the specific clinical
needs and the services available in the patient’s community.
For example, some patients require wound care that exceeds the capabilities of their family
or others who act as informal caregivers. But they may be able to receive sufficient care in
the home setting through a home health service, if such services are available. Some
patients with chronic conditions may prefer to remain in their home and would be able to do
so using available community-based services, but also require financial supports, such as
Medicaid-financed home and community-based waiver services. If such supports are not
immediately available at the time of discharge while an application for waiver services is
pending, the evaluation should consider the availability of other short term supports that
would allow the patient to be discharged home.
If the result of the evaluation is that the patient cannot receive required care if he/she
returns to home, then an assessment must be made of options for transfer to another
inpatient or residential health care facility that can address the patient’s needs, including
other types of hospitals, such as rehabilitation hospitals; skilled nursing facilities; assisted
living facilities; nursing homes; or inpatient hospice facilities.
If prior to the hospital admission the patient was a resident in a facility that he or she wishes
to return to, such as an assisted living or nursing facility or skilled nursing facility, the
evaluation must address whether that facility has the capability to provide the post-hospital
care required by the patient. The post-discharge care requirements may be different than
the care that was previously provided. This requires dialogue and cooperation between
hospitals and post-hospital care facilities in the area served by the hospital, as well as with
the physicians who provide care to patients in either or both of these settings.
Long term care facilities often express concern that hospitals discharge patients to their
facilities with care needs that exceed their care capabilities, necessitating sending the
patient to the emergency department for care and possible readmission. On the other hand,
hospitals often express concern that long term care facilities send patients to the emergency
department with ambulatory care-sensitive conditions, i.e., conditions that either do not
require an acute level of care, or which could have been prevented from escalating to an
acute level had appropriate primary care been provided in a timely manner.
While hospitals cannot address these concerns in isolation, they are expected to be
knowledgeable about the care capabilities of area long term care facilities and to factor this
knowledge into the discharge planning evaluation.
Hospitals are expected to have knowledge of the capabilities and capacities of not only of
long term care facilities, but also of the various types of service providers in the area where
most of the patients it serves receive post-hospital care, in order to develop a discharge plan
that not only meets the patient’s needs in theory, but also can be implemented. This
includes knowledge of community services, as well as familiarity with available Medicaid
home and community-based services (HCBS), since the State’s Medicaid program plays a
major role in supporting post-hospital care for many patients.
If the hospital is one with specialized services that attract a significant number of patients
who will receive their post-hospital care in distant communities, the hospital is expected to
take reasonable steps to identify the services that will be available to the patient.
Once the determination has been made that services will be necessary post-discharge, the
team must then determine availability of those services or identify comparable
substitutions. Included in the evaluation is coordination with insurers and other payors,
including the State Medicaid agency, as necessary to ensure resources prescribed are
approved and available.
For Information– Not Required/Not to be Cited
Although not required under the regulations, hospitals would be well advised to develop
collaborative partnerships with post-hospital care providers to improve care transitions of
care that might support better patient outcomes. This includes not only skilled nursing
facilities and nursing facilities, but also providers of community-based services. For
example, Centers for Independent Living (CIL) and Aging and Disability Resource
Centers (ADRC) are resources for community-based services and housing available to
persons with disabilities and older adults. Hospitals can find local CIL’s at
http://www.ilru.org/html/publications/directory/index.html and ADRC’s and other
resources at http://www.adrc-tae.org/tiki-index.php?page=HomePage.
The ability to pay out of pocket for services must also be discussed with the family or other
support persons. Although hospitals are not expected to have definitive knowledge of the
terms of any given patient’s insurance coverage or eligibility for community-based services,
or for Medicaid coverage, they are expected to have a general awareness of these matters
and their impact on the patient’s post-discharge needs and prospects for recovery. For
example, if the patient is a Medicare beneficiary, the hospital is expected to be aware of
Medicare coverage requirements for home health care or admission to a rehabilitation
hospital, a skilled nursing facility, or a long term care hospital, etc. and to make the
beneficiary aware that they may have to pay out of pocket for services not meeting the
coverage requirements.
Similarly, for Medicaid, they should know coverage options for home health, attendant
care, and long term care services or have contacts at the State Medicaid agency that can
assist with these issues. As noted above, hospitals are also expected to have knowledge of
community resources to assist in arranging services. Some examples include Aging and
Disability Resource Centers and Centers for Independent Living (see box above).
For Information– Not Required/Not to be Cited
Providing a discharge planning tool to patients and their family or other support persons
may help to reinforce the discharge plan. Use of the tools may encourage patients’
participation in developing the plan as well as provide them an easy-to-follow guide to
prepare them for a successful transition from the hospital. The tool should be given to
patients on admission, reviewed throughout their stay, and updated prior to discharge.
Examples of available tools include:
• Medicare’s “Your Discharge Planning Checklist,” (available at
http://www.medicare.gov/publications/pubs/pdf/11376.pdf)
• Agency for Healthcare, Research and Quality’s (AHRQ) “Taking Care of Myself:
A Guide For When I Leave the Hospital,” (available at
http://www.ahrq.gov/qual/goinghomeguide.pdf)
• Consumers Advancing Patient Safety (CAPS) “Taking Charge of Your
Healthcare: Your Path to Being an Empowered Patient Toolkit” (available at
http://www.patientsafety.org/page/transtoolkit/).
The hospital CoP governing patients’ rights at §482.13(b) provides that “The patient has the
right to participate in the development and implementation of his or her plan of care.”
(CMS views discharge planning as part of the patient’s plan of care). “The patient or his/her
representative (as allowed under State law) has the right to make informed decisions
regarding his/her care” and “The patient’s rights include...being involved in care planning
and treatment.” Accordingly, hospitals are expected to engage the patient, or the patient’s
representative, actively in the development of the discharge evaluation, not only as a source
of information required for the assessment of self-care capabilities, but also to incorporate
the patient’s goals and preferences as much as possible into the evaluation. A patient’s
goals and preferences may be, in the hospital’s view, unrealistic. Identifying divergent
hospital and patient assessments of what is realistic enables a discussion of these
differences and may result in an assessment and subsequent development of a discharge
plan that has a better chance of successful implementation.
For Information – Not Required/Not to be Cited
If a patient exercises the right to refuse to participate in the discharge planning
evaluation, documentation of the refusal is recommended in the medical record.
Survey Procedures §482.43(b)(1), §482.43(b)(3) & §482.43(b)(4)
• In every unit with inpatient beds surveyed, is there evidence of discharge planning
evaluation activities?
• Are staff members who are responsible for discharge planning evaluation correctly
following the hospital’s policies and procedures?
• If the hospital does not require a discharge planning evaluation for all inpatients:
• Does the hospital have a standard process for notifying patients, their representative,
and physicians that they may request a discharge planning evaluation and that the
hospital will conduct an evaluation upon request?
• Can discharge planning and unit nursing staff describe the process for a patient or
the patient’s representative to request a discharge planning evaluation?
• Interview patients and their representatives. If they say they were not aware they
could request a discharge planning evaluation, can the hospital provide evidence
they received notice of their right?
• Interview attending physicians to see if they are aware they can request a discharge
planning evaluation. If they are not aware, can the hospital provide evidence of how
they inform the medical staff about this?
• Review a sample of cases to determine if the discharge planning evaluation documents
the patient’s (or the patient’s representatives) goals and preferences for post-discharge
placement and care.
• Review a sample of cases to determine if the discharge planning evaluation includes an
assessment of:
• The patient’s post-discharge care needs being met in the environment from which
he/she entered the hospital? What the patient’s care needs will be immediately upon
discharge, and whether those needs are expected to remain constant or lessen over
time?
• The patient’s insurance coverage (if applicable) and how that coverage might or
might not provide for necessary services post-hospitalization?
• For patients admitted from home --
• Whether the patient can perform activities of daily living (personal
hygiene and grooming, dressing and undressing, feeding, voluntary
control over bowel and bladder, ambulation, etc.)?
• The patient’s or family/other support person’s ability to provide self-
care/care?
• Whether the patient will require specialized medical equipment or
home modification?
• If yes, did the evaluation include an assessment of whether the
equipment is available or if the modifications can be made to
safely discharge the patient to that setting?
• If the patient or family/support person is unable to meet care needs or
there are additional care needs above their capabilities, did the
evaluation include an assessment of available community-based
services to meet post-hospital needs?
• For patients admitted from a nursing facility, skilled nursing facility or assisted
living facility did the evaluation assess whether the prior facility has the
capability to provide necessary post-hospital services to the patient (i.e. is the
same, higher, or lower level of care required and can those needs be met?)If yes,
is there any documentation that the patient’s care needs fall within the
capabilities of the facility?
• Are the results of the discharge planning evaluation documented in the medical
record?
A-0807
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(a)(2) Standard: Discharge Planning Evaluation
(2) A discharge planning evaluation must include an evaluation of a patient’s likely
need for appropriate post-hospital services, including, but not limited to hospice care
services, post-hospital extended care services, home health services, and non-health
care services and community based care providers, and must also include a
determination of the availability of the appropriate services as well as of the patient’s
access to those services.
Interpretive Guidelines §482.43(a)(2)
Guidance is pending and will be updated in future release.
A-0808
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(a)(3) Standard: Discharge Planning Evaluation
(3) The discharge planning evaluation must be included in the patient’s medical
record for use in establishing an appropriate discharge plan and the results of the
evaluation must be discussed with the patient (or the patient’s representative).
Interpretive Guidelines §482.43(a)(3)
Guidance is pending and will be updated in future release.
A-0809
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(a)(5) – Any discharge planning evaluation or discharge plan under this
paragraph must be developed by or under the supervision of a registered nurse, social
worker, or other appropriately qualified personnel.
Interpretive Guidelines §482.43(a)(5)
Guidance is pending and will be updated in future release.
A-0810
(Rev. 87, Issued: 07-19-13, Effective: 07-19-13, Implementation: 07-19-13)
§482.43(b)(5) - The hospital personnel must complete the evaluation on a timely basis
so that appropriate arrangements for post-hospital care are made before discharge,
and to avoid unnecessary delays in discharge.
Interpretive Guidelines §482.43(b)(5)
After a patient has been identified as needing an evaluation, or after a request for an
evaluation has been made by the physician, patient and/or patient’s representative, the
evaluation must be completed timely. This means there must be sufficient time after
completion to allow arrangements for post-hospital care to be made, without having to
delay the patient’s discharge in order to do so, or requiring the patient to transfer to a
different setting from where he/she was admitted from primarily due to the delay in making
appropriate arrangements. The comparatively short average length of stay of a short term
acute care hospital inpatient necessitates prompt attention to patients’ discharge planning
needs in that type of hospital. Failure to complete the evaluation in a timely manner could
make it more difficult to implement the patient’s final discharge plan, and/or may cause an
unnecessary delay in the patient’s discharge from the hospital. While other types of
hospitals with a longer average length of stay may be able to complete the evaluation at a
later point after admission, they too must complete it on a timely basis to avoid delays in
discharge.
Where a team approach is utilized by the hospital in developing the discharge planning
evaluation, there must be a process to promote efficient collaboration among team members
to complete the evaluation in a timely manner. Changes in patient condition throughout the
hospitalization warrant adjustments to the discharge plan.
Survey Procedures §482.43(b)(5)
• Review a sample of cases to determine if the discharge planning evaluation was
completed on a timely basis to allow for appropriate arrangements to be made for post-
hospital care and to avoid delays in discharge. In order to assess this:
• Determine when the discharge planning evaluation was initiated. If the evaluation
was not begun within 24 hours of the request or identification of the need for an
evaluation, ask why.
• Is there a pattern of delayed start or completion of the evaluation? If so, is the delay
due to circumstances beyond the hospital’s control (e.g., inability to reach the
beneficiary’s support person(s), continuing changes in the patient’s condition)
and/or is the delay due to the hospital’s failure to develop timely discharge planning
evaluations?
A-0811
(Rev. 87, Issued: 07-19-13, Effective: 07-19-13, Implementation: 07-19-13)
§482.43(b)(6) - The hospital … must discuss the results of the evaluation with the
patient or individual acting on his or her behalf.
Interpretive Guidelines §482.43(b)(6)
The results of the discharge planning evaluation must be discussed with the patient or the
patient’s representative. Documentation of this communication must be included in the
medical record, including if the patient rejects the results of the evaluation. It is not
necessary for the hospital to obtain a signature from the patient (or the patient’s
representative, as applicable) documenting the discussion.
The patient or the patient’s representative must be actively engaged in the development of
the plan, so that the discussion of the evaluation results represents a continuation of this
active engagement. It would not be appropriate for a hospital to conduct an evaluation
without the participation of the patient or the patient’s representative, and then present the
results of the evaluation to the patient as a finished product, since this would place the
patient in a passive position that is not consistent with the requirements of the patients’
rights CoP at §482.13(b).
Survey Procedures §482.43(b)(6)
• Review a sample of cases to determine if the discharge planning evaluation results were
discussed with the patient or the patient’s representative.
A-0812
(Rev. 87, Issued: 07-19-13, Effective: 07-19-13, Implementation: 07-19-13)
§482.43(b)(6) – [The hospital must] include the discharge planning evaluation in the
patient’s medical record for use in establishing an appropriate discharge plan….
Interpretive Guidelines §482.43(b)(6)
The hospital must include the discharge planning evaluation in the patient’s medical record
in order for it to guide the development of the patient’s discharge plan. Timely placement
of the evaluation in the medical record facilitates communication among members of the
patient’s healthcare team who should participate in a multidisciplinary process to develop
and implement the discharge plan. The evaluation and subsequent planning process may be
a continuous one and hospitals may choose not to divide the process into distinct
documents. The key requirement is that the evaluation results are included in the patient’s
medical record and are used in the development of the features of the discharge plan.
Survey Procedures §482.43(b)(6)
• Review a sample of cases to determine if the discharge planning evaluation results are
included in the medical record.
A-0813
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(b) Standard: Discharge of the patient and the provision and transmission of
the patient’s necessary medical information.
The hospital must discharge the patient , and also transfer or refer the patient where
applicable, along with all necessary medical information pertaining to the patient’s
current course of illness and treatment, post-discharge goals of care, and treatment
preferences, at the time of discharge, to the appropriate post-acute care service
providers and suppliers, facilities, agencies, and other outpatient service providers and
practitioners responsible for the patient’s follow-up or ancillary care.
Interpretive Guidelines §482.43(b)
Guidance is pending and will be updated in future release.
A-0814
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(c) Standard: Requirements related to post-acute care services.
For those patients discharged to home and referred for HHA services, or for those
patients transferred to a SNF for post-hospital extended care services, or transferred
to an IRF or LTCH for specialized hospital services, the following requirements apply,
in addition to those set out at paragraphs (a) and (b) of this section:
Interpretive Guidelines §482.43(c)
Guidance is pending and will be updated in future release.
A-0815
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(c)(1) –The hospital must include the discharge planning a list of HHA’s,
SNF’s, IRF’s, or LTCH’s that are available to the patient, that are participating in the
Medicare program, and that serve the geographic area (as defined by the HHA) in
which the patient resides, or in the case of a SNF, IRF, or LTCH, in the geographic
area requested by the patient. HHAs must request to be listed by the hospital as
available.
(i) The list must only be presented to patients for whom home health care post hospital
extended care services, SNF, IRF, or LTCH services are indicated and appropriate as
determined by the discharge planning evaluation.
(ii) For patients enrolled in managed care organizations, the hospital must
make the patient aware of the need to verify with their managed care
organization which practitioners, providers or certified suppliers are in the
network of the patient’s managed care organization, it must share this with the
patient or the patient’s representative.
(iii) [The hospital must] document in the patient’s medical record that the list
was presented to the patient or to the patient’s representative.….
Interpretive Guidelines §482.43(c)(1)(i)-(iii)
Guidance is pending and will be updated in future release.
A-0816
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(c)(2) The hospital , as part of the discharge planning process, must inform the
patient or the patient’s representative of their freedom to choose among participating
Medicare providers and suppliers of the post-discharge services and must, when
possible, respect the patient’s or the patient’s representative goals of care and
treatment preferences, as well as other preferences they express. The hospital must
not specify or otherwise limit the qualified providers or suppliers that are available to
the patients.
Interpretive Guidelines §482.43(c)(2)
Guidance is pending and will be updated in future release.
A-0817
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.43(c)(3) The discharge plan must identify any HHA or SNF to which the patient
is referred in which the hospital has a disclosable financial interest, as specified by the
Secretary, and any HHA or SNF that has a disclosable financial interest in a hospital
under Medicare. Financial interests that are disclosable under Medicare are
determined in accordance with the provisions of part 420, subpart C, of this chapter.
Interpretive Guidelines §482.43(c)(3)
Guidance is pending and will be updated in future release.
A-0818
(Rev. 87, Issued: 07-19-13, Effective: 07-19-13, Implementation: 07-19-13)
§482.43(c) Standard: Discharge Plan
(1) - A registered nurse, social worker, or other appropriately qualified personnel
must develop, or supervise the development of, a discharge plan if the discharge
planning evaluation indicates a need for a discharge plan.
Interpretive Guidelines §482.43(c)(1)
The discharge plan that is based on the findings of the discharge planning evaluation must
be developed by a registered nurse, social worker, or other appropriate qualified personnel,
or by a person who is supervised by such personnel. State law governs the qualifications
required to be considered a registered nurse or a social worker. The hospital’s written
discharge planning policies and procedures must specify the qualifications for personnel
other than registered nurses or social workers who develop or supervise the development of
the plan.
The qualifications should include such factors as previous experience in discharge planning,
knowledge of clinical and social factors that affect the patient’s functional status at
discharge, knowledge of community resources to meet post-discharge clinical and social
needs, and assessment skills. All personnel performing or supervising development of
discharge plans, including registered nurses and social workers, must have knowledge of
clinical, social, insurance/financial and physical factors that must be considered when
evaluating how a patient’s expected post-discharge care needs can be met.
For Information – Not Required/Not to be Cited
A well designed discharge planning process uses a multidisciplinary team approach.
Team members may include representatives from nursing, case management, social
work, medical staff, pharmacy, physical therapy, occupational therapy, respiratory
therapy, dietary, and other healthcare professionals involved with the patient’s care. The
team approach helps to ensure all of the patient’s post-discharge care needs are addressed
in the plan, increasing the likelihood of successful recovery and avoidance of
complications and readmissions.
The hospital CoP governing patients’ rights at §482.13(b) provides that “The patient has the
right to participate in the development and implementation of his or her plan of care.”
(CMS views discharge planning as part of the patient’s plan of care). “The patient or his/her
representative (as allowed under State law) has the right to make informed decisions

regarding his/her care” and “The patient’s rights include...being involved in care planning
and treatment.” Accordingly, hospitals are expected to engage the patient, or the patient’s
representative, actively in the development of the discharge plan, not only to provide them
the necessary education and training to provide self-care/care, but also to incorporate the
patient’s goals and preferences as much as possible into the plan. A patient will be more
likely to cooperate in the implementation of a discharge plan that reflects his/her
preferences, increasing the likelihood of a successful care transition and better health
outcomes.
A patient’s goals and preferences may be, in the hospital’s view, unrealistic. A hospital is
not obligated to develop a discharge plan that cannot be implemented. However, the fact
that a plan incorporating the patient’s goals and preferences might be more time-consuming
for the hospital to develop and implement than another alternative does not make the
patient’s preferred plan unrealistic.
For Information – Not Required/Not to be Cited
If a patient exercises the right to refuse to participate in discharge planning or to
implement a discharge plan, documentation of the refusal in the medical record is
recommended.
Survey Procedures §482.43(c)(1)
• Review a sample of cases to determine if the discharge plan was developed by an RN,
Social Worker, or other qualified personnel, as defined in the hospital discharge
planning policies and procedures, or someone they supervise? In order to assess this:
• Review the hospital’s written policy and procedure governing who is responsible for
developing or supervising the development of the discharge plan. Does the policy
permit someone other than a RN or social worker to be responsible for developing
or supervising development of such plans? If yes, does the policy specify the
qualifications of the personnel other than a RN or social worker to perform this
function?
• Determine which individual(s) are responsible for developing or supervising the
development of discharge plans. Review their personnel folders to determine if they
are a RN, social worker, or meet the hospital’s criteria for developing/supervising
the discharge plan. If they are not, are they supervised by an individual who is an
RN, social worker or qualified according to the hospital’s policies? Are their
discharge plans reviewed by their supervisor before being finalized?
• Ask personnel who supervise or develop discharge plans to give examples
illustrating their knowledge of healthcare and other resources available in the
community that could be utilized to meet patients’ expected post-discharge care
needs.
• Ask the discharge planner how the patient or patient’s representative is engaged to
participate in the development of the discharge plan. Does the discharge plan identify
the patient’s or patient’s representative discharge preferences?
• Does the discharge plan match the identified needs as determined by the discharge
planning evaluation?
A-0819
(Rev. 87, Issued: 07-19-13, Effective: 07-19-13, Implementation: 07-19-13)
§482.43(c)(2) In the absence of a finding by the hospital that a patient needs a
discharge plan, the patient’s physician may request a discharge plan. In such a case,
the hospital must develop a discharge plan for the patient.
Interpretive Guidelines §482.43(c)(2)
If a patient is not identified through the hospital’s discharge planning evaluation process as
requiring a discharge plan, the patient’s physician may nevertheless request a discharge
plan. The hospital must develop a discharge plan when requested to do so by the patient’s
physician.
If the hospital’s policies and procedures call for a discharge plan for every hospital
inpatient, then it is not necessary to include a separate provision in those policies requiring
development of a plan upon physician request, since such a provision would be superfluous.
Survey Procedures §482.43(c)(2)
• Review the hospital’s discharge planning policies and procedures to determine whether
it requires the development of a discharge plan for all inpatients, or only for those
identified as needing a plan through a risk-based identification and evaluation process.
• If the hospital does not require a discharge planning evaluation for all inpatients:
• Does the hospital have a standard process for notifying physicians that they may
request a discharge plan evaluation and that the hospital will develop a plan upon
request?
• Interview attending physicians to see if they are aware they can request a discharge
plan. If they are not aware they can request a discharge plan, can the hospital
provide evidence of how they inform the medical staff about this?
A-0820
(Rev. 87, Issued: 07-19-13, Effective: 07-19-13, Implementation: 07-19-13)
§482.43(c)(3) - The hospital must arrange for the initial implementation of the
patient’s discharge plan….
§482.43(c)(5) - As needed, the patient and family members or interested persons must
be counseled to prepare them for post-hospital care.
Interpretive Guidelines §482.43(c)(3) & §482.43(c)(5)
The hospital is required to arrange for the initial implementation of the discharge plan. This
includes providing in-hospital education/training to the patient for self-care or to the
patient’s family or other support person(s) who will be providing care in the patient’s home.
It also includes arranging:
• Transfers to rehabilitation hospitals, long term care hospitals, or long term care
facilities;
• Referrals to home health or hospice agencies;
• Referral for follow-up with physicians/practitioners, occupational or physical
therapists, etc.;
• Referral to medical equipment suppliers; and
• Referrals to pertinent community resources that may be able to assist with financial,
transportation, meal preparation, or other post-discharge needs.
(See §482.43(d) for more discussion about the hospital’s transfer or referral obligations
and the initial implementation of the plan relating to transfer/referral.)
The discharge planning process is a collaborative one that must include the participation of
the patient and the patient’s informal caregiver or representative, when applicable. In
addition, other family or support persons who will be providing care to the patient after
discharge need to be engaged in the process. Keeping the patient, and, when applicable,
the patient’s representative and other support persons informed throughout the development
of the plan is essential for its success. Providing them with information on post-discharge
options, what to expect after discharge and, as applicable, instruction and training in how to
provide care is essential. The patient needs clear instructions regarding what to do when
concerns, issues, or problems arise, including who to call and when they should seek
emergency assistance. Although it may be an important component of the discharge
instructions, it is not acceptable to only advise a patient to “return to the ED” whenever
problems arise.
There are a variety of tools and techniques that have focused on improving the support
provided to patients who are discharged back to their homes. A comprehensive approach
employing combinations of these techniques has been found to improve patient outcomes
and reduce hospital readmission rates, including, but not limited to:
• Improved education) to patients and support persons regarding disease processes,
medications, treatments, diet and nutrition, expected symptoms, and when and how
to seek additional help. Teaching methods must be based on recognized
methodologies. CMS does not prescribe any specific methodologies, but examples
include the teach-back, repeat-back approach and simulation laboratories;
• Written discharge instructions, in the form of checklists when possible, that are
legible, in plain language, culturally sensitive and age appropriate;
• Providing supplies, such as materials for changing dressings on wounds, needed
immediately post-discharge; and
• A list of all medications the patient should be taking after discharge, with clear
indication of changes from the patient’s pre-admission medications;
The education and training provided to the patient or the patient’s caregiver(s) by the
hospital must be tailored to the patient’s identified needs related to medications, treatment
modalities, physical and occupational therapies, psychosocial needs, appointments, and
other follow-up activities, etc. Repeated review of instructions with return demonstrations
and/or repeat-backs by the patient, and their support persons will improve their ability to
deliver care properly. This includes providing instructions in writing as well as verbally
reinforcing the education and training.
It is also necessary to provide information to patients and their support persons when the
patient is being transferred to a rehabilitation or a long term care hospital, or to a long term
care setting, such as a skilled nursing facility or nursing facility. The information should
address questions such as: the goal of treatment in the next setting and prospects for the
patient’s eventual discharge home.
The hospital must document in the patient’s medical record the arrangements made for
initial implementation of the discharge plan, including training and materials provided to
the patient or patient’s informal caregiver or representative, as applicable.
For Information – Not Required/Not to be Cited
Additional actions hospitals might consider taking to improve the patient’s post-
discharge care transition:
• Scheduling follow-up appointments with the patient’s primary care
physician/practitioner and in-home providers of service as applicable;
• Filling prescriptions prior to discharge;
• If applicable, arranging remote monitoring technologies, e.g., pulse oximetry and
daily weights for congestive heart failure (CHF) patients; pulse and blood
pressure monitoring for cardiac patients; and blood glucose levels for diabetic
patients; and
• Follow-up phone calls within 24 -72 hours by the hospital to the patient after
discharge.
The communication with the patient to ensure implementation of the discharge plan does
not stop at discharge. An initiative showing significant success in reducing preventable
readmissions involves the hospital contacting the patient by phone in the first 24 to 72
hours after discharge. The phone contact provides an opportunity for the patient to pose
questions and for the hospital to address any confusion related to medications, diet,
activity, etc., and to reinforce the education/instruction that took place in the hospital
prior to discharge. This also helps to reduce patient and family member anxieties as they
manage post-hospital care needs.
Hospital staff placing the calls should be familiar with the patient’s discharge plan and
qualified to address typical questions that might be expected. They should also be
knowledgeable about when to instruct the patient to seek a more immediate evaluation,
including where to go for such evaluation. Although this follow-up phone call can serve
as a customer service initiative for the hospital, the primary intent would be to provide an
opportunity for questions and to reduce or eliminate any confusion or concerns regarding
post-hospital care.
Survey Procedures §482.43(c)(3) & §482.43(c)(5)
• Review cases of discharged patients to determine if the hospital arranges initial
implementation of the discharge plan by providing:
• For patients discharged to home:
• In-hospital training to patient and family/support persons, using recognized
methods;
• Written discharge instructions that are legible and use non-technical language;
• A legible, complete, reconciled medication list that highlights changes from the post
hospital regimen;
• Referrals as applicable to specialized ambulatory services, e.g. physical therapy,
occupational therapy, home health, hospice, mental health, etc.;
• Referrals as applicable to community-based resources other than health services,
e.g. Departments of Aging, elder services, transportation services, Centers for
Independent Living, Aging and Disability Resource Centers, etc.;
• Arranging essential durable medical equipment, e.g. oxygen, wheel chair, hospital
bed, commode, etc.;
• Sending necessary medical information to providers that the patient was referred to
prior to the first post-discharge appointment or within 7 days of discharge,
whichever comes first; and
• For patients transferred to another inpatient facility, was necessary medical
information ready at time of transfer and sent to the receiving facility with the
patient?
• Were there portions of the plan the hospital failed to begin implementing, resulting in
delays in discharge?
A-0821
(Rev. 87, Issued: 07-19-13, Effective: 07-19-13, Implementation: 07-19-13)
§482.43(c)(4) - The hospital must reassess the patient’s discharge plan if there are
factors that may affect continuing care needs or the appropriateness of the discharge
plan.
Interpretive Guidelines §482.43(c)(4)
Changes in a patient’s condition may warrant adjustments to the discharge plan. Hospitals
must have in place either a routine reassessment of all plans or a process for triggering a
reassessment of the patient’s post-discharge needs, capabilities and discharge plan when
significant changes in the patient’s condition or available supports occur.
Survey Procedures §482.43(c)(4)
• Review a sample of cases to determine if any significant changes in the patient’s
condition were noted in the medical record that changed post-discharge needs, and if the
discharge plan was updated accordingly.
• In making this determination, ask staff responsible for discharge planning when and
how they reassess a patient’s discharge plan. If none of the records being used for the
tracers suggest a need to revise the discharge plan, ask staff to present one or more
clinical records that document reassessment.
A-0823
(Rev. 87, Issued: 07-19-13, Effective: 07-19-13, Implementation: 07-19-13)
§482.43(c)(6) - The hospital must include in the discharge plan a list of HHAs or SNFs
that are available to the patient, that are participating in the Medicare program, and
that serve the geographic area (as defined by the HHA) in which the patient resides, or
in the case of a SNF, in the geographic area requested by the patient. HHAs must
request to be listed by the hospital as available.
(i) - This list must only be presented to patients for whom home health care or post-
hospital extended care services are indicated and appropriate as determined by the
discharge planning evaluation.
(ii) - For patients enrolled in managed care organizations, the hospital must indicate
the availability of home health and post-hospital extended care services through
individuals and entities that have a contract with the managed care organizations.
(iii) The hospital must document in the patient's medical record that the list was
presented to the patient or to the individual acting on the patient's behalf.
§482.43(c)(7) The hospital, as part of the discharge planning process, must inform the
patient or the patient's family of their freedom to choose among participating
Medicare providers of post-hospital care services and must, when possible, respect
patient and family preferences when they are expressed. The hospital must not
specify or otherwise limit the qualified providers that are available to the patient.
§482.43(c)(8) The discharge plan must identify any HHA or SNF to which the patient
is referred in which the hospital has a disclosable financial interest, as specified by the
Secretary, and any HHA or SNF that has a disclosable financial interest in a hospital
under Medicare. Financial interests that are disclosable under Medicare are
determined in accordance with the provisions of Part 420, Subpart C, of this chapter.
Interpretative Guidelines §482.43(c)(6), §482.43(c)(7) & §482.43(c)(8)
The hospital must include a list of Medicare-participating home health agencies (HHAs)
and skilled nursing facilities (SNFs) in the discharge plan for those patients for whom the
plan indicates home health or post-hospital extended care services are required.
• “Extended care services” are defined at sections 1861(h) and (i) of the Social
Security Act as items or services furnished in a skilled nursing facility (SNF). SNFs
included on the list must be located in a geographic area that the patient or patient’s
representative indicated he/she prefers.
• For Home Health Agencies (HHAs) the list must consist of Medicare-participating
HHAs that have requested the hospital to be listed and which serve the geographic
area where the patient lives. Hospitals may expect the HHA to define its
geographic service area when it submits its request to be listed.
During the discharge planning process the hospital must inform the patient of his/her
freedom to choose among Medicare-participating post-hospital providers and must not
direct the patient to specific provider(s) or otherwise limit which qualified providers the
patient may choose among. Hospitals have the flexibility either to develop their own lists
or to print a list of skilled nursing facilities and home health agencies in the applicable
geographic areas from the CMS websites, Nursing Home Compare
(www.medicare.gov/NHcompare) and Home Health Compare
(www.medicare.gov/homehealthcompare). If hospitals develop their own lists, they are
expected to update them at least annually. (69 FR 49226, August 11, 2004)
For Information – Not Required/Not to be Cited
Hospitals may also refer patients and their families to the Nursing Home Compare and
Home Health Compare websites for additional information regarding Medicare-certified
skilled nursing facilities and home health agencies, as well as Medicaid-participating
nursing facilities.
The data on the Nursing Home Compare website include an overall performance rating,
nursing home characteristics, performance on quality measures, inspection results, and
nursing staff information.
Home Health Compare provides details about every Medicare-certified home health
agency in the country. Included on the website are quality indicators such as managing
daily activities, managing pain and treating symptoms, treating wounds and preventing
pressure sores, preventing harm, and preventing unplanned hospital care.
The hospital might also refer the patient and their representatives to individual State
agency websites, Long-Term Care Ombudsmen Program, Protection and Advocacy
Organizations, Citizen Advocacy Groups, Area Agencies on Aging, Centers for
Independent Living, and Aging and Disability Resource Centers for additional
information on long term care facilities and other types of providers of post-hospital care.
Having access to the information found at these sources may assist in the decision
making process regarding post-hospital care options.
If the patient is enrolled in a managed care insurance program that utilizes a network of
exclusive or preferred providers, the hospital must make reasonable attempts, based on
information from the insurer, to limit the list to HHAs and SNFs that participate in the
insurer’s network of providers.
If the hospital has a disclosable financial interest in a HHA or SNF on a patient’s list, or an
HHA or SNF on the list has a disclosable financial interest in the hospital, these facts must
also be stated on the list provided to the patient. Surveyors are not expected to know the
requirements for a disclosable financial interest under Part 420, Subpart C, but hospitals are
expected to know and comply with these requirements, and to identify for the surveyor
whether there are such disclosable financial interests between the hospital and any specific
HHAs or SNFs to which they refer/transfer patients.
When the patient or the patient’s family has expressed a preference, the hospital must
attempt to arrange post-hospital care with an HHA or SNF, as applicable, which meets
these preferences. If the hospital is unable to make the preferred arrangement, e.g., if there
is no bed available in the preferred SNF, it must document the reason the patient’s
preference could not be fulfilled and must explain that reason to the patient.
Survey Procedures §482.43(c)(6), §482.43(c)(7) & §482.43(c)(8):
• Review a sample of cases of patients discharged to HHAs or SNFs to determine if,
when applicable, the hospital provided the patient with lists of Medicare-
participating HHAs or SNFs. In making this determination:
• Is there documentation of a list of multiple HHAs or SNFs being provided
(including electronically) to the patient? If not, is there documentation for an
acceptable rationale for providing only one option, e.g., the patient’s home is
included in the service area of only one Medicare-participating HHA that
requested to be included on hospital lists, or there is only one Medicare-
participating SNF in the area preferred by the patient?
• Ask to see examples of lists of HHAs and SNFs provided to patients prior to
discharge.
• Ask the hospital if it has any disclosable financial interests in any HHA or SNF on
its lists, or if an HHA or SNF has a disclosable financial interest in the hospital. If
yes, is this stated clearly on the lists?
• Interview staff members involved with the discharge planning process. Ask them to
describe how patient preferences are taken into account in the selection of post-
hospital HHA or SNF services.
• Ask the hospital to identify current patients for whom HHA or SNF services are
planned. Interview the patient or the patient’s family to ask them:
• Were they presented with a list of HHAs or SNFs, as applicable, to choose
from?
• Did the hospital emphasize their freedom of choice?
• Did the hospital arrange for their referral/transfer to an HHA or SNF reflecting
their preferences? If not, did the hospital explain why their choice was not
feasible?
• If applicable, were they made aware of disclosable financial interest?
A-0837
(Rev. 87, Issued: 07-19-13, Effective: 07-19-13, Implementation: 07-19-13)
§482.43(d) Standard: Transfer or Referral
The hospital must transfer or refer patients, along with necessary medical
information, to appropriate facilities, agencies, or outpatient services, as needed, for
follow-up or ancillary care.
Interpretive Guidelines §482.43(d)
The hospital must take steps to ensure that patients receive appropriate post-hospital care by
arranging, as applicable, transfer to appropriate facilities or referrals to follow-up
ambulatory care services.
“Appropriate facilities, agencies, or outpatient services” refers to entities such as skilled
nursing facilities, nursing facilities, home health agencies, hospice agencies, mental health
agencies, dialysis centers, suppliers of durable medical equipment, suppliers of physical and
occupational therapy, physician offices, etc. which offer post-acute care services that
address the patient’s post-hospital needs identified in the patient’s discharge planning
evaluation. The term does not refer to non-healthcare entities, but hospitals also are
encouraged to make appropriate referrals to community-based resources that offer
transportation, meal preparation, and other services that can play an essential role in the
patient’s successful recovery.
“Appropriate facilities” may also include other hospitals to which a patient is transferred for
follow-up care, such as rehabilitation hospitals, long term care hospitals, or even other
short-term acute care hospitals.
Necessary medical information must be provided not only for patients being transferred, but
also for those being discharged home, to make the patient’s physician aware of the outcome
of hospital treatment or follow-up care needs. This is particularly important since the
increasing use of hospitalists in the inpatient hospital setting means the patient’s physician
may have had no interaction with the patient throughout the hospital stay. When the
hospital provides the patient’s physician with necessary medical information promptly,
among other things, this provides an opportunity for the patient’s physician to discuss with
the hospital care team changes to the patient’s preadmission medication regimen or other
elements of the post-discharge care plan about which the physician may have questions.
Facilitating opportunities for such communication and dialogue enhances the likelihood of
better patient outcomes after discharge.

The “medical information” that is necessary for the transfer or referral includes, but is not
limited to:
• Brief reason for hospitalization (or, if hospital policy requires a discharge summary
for certain types of outpatient services, the reason for the encounter) and principal
diagnosis;
• Brief description of hospital course of treatment;
• Patient’s condition at discharge, including cognitive and functional status and social
supports needed;
• Medication list (reconciled to identify changes made during the patient’s
hospitalization) including prescription and over-the-counter medications and herbal.
(Note, an actual list of medications needs to be included in the discharge
information, not just a referral to an electronic list available somewhere else in the
medical record.);
• List of allergies (including food as well as drug allergies) and drug interactions;
• Pending laboratory work and test results, if applicable, including information on
how the results will be furnished;
• For transfer to other facilities, a copy of the patient’s advance directive, if the
patient has one; and
• For patients discharged home:
• Brief description of care instructions reflecting training provided to patient
and/or family or other informal caregiver(s);
• If applicable, list of all follow-up appointments with practitioners with which
the patient has an established relationship and which were scheduled prior to
discharge, including who the appointment is with, date and time.
• If applicable, referrals to potential primary care providers, such as health
clinics, if available, for patients with no established relationship with a
practitioner.
The regulation requires transfer or referral “along” with necessary medical information. In
the case of a patient being transferred to another inpatient or residential health care facility,
the necessary information must accompany the patient to the facility. However, in the case
of a patient discharged home who is being referred for follow-up ambulatory care, the
transmittal of the information to the patient’s physician may take place up to 7 days after
discharge or prior to the first appointment for ambulatory care services that may have been
scheduled, whichever comes first. If the patient’s physician is not yet able to accept the
information electronically from the hospital, the hospital may provide the information to the
patient with instructions to give this information to the physician at their next appointment.
For Information – Not Required/Not to be Cited
Scheduling of follow-up appointments for ambulatory care services by the hospital prior
to discharge has been found to be an effective tool to ensure prompt follow-up and reduce
the likelihood of a preventable readmission. This follow-up visit shortly after discharge
provides an opportunity for the patient to address any issues or concerns experienced

after the inpatient stay. It also provides an opportunity for the primary care physician or
practitioner to review and reinforce the post-hospital plan of care with the patient, for
rehabilitation therapy to begin in a timely manner, to clarify any concerns related to
medication reconciliation or other adjustments to the patient’s pre-hospital regimen, etc.
It is recognized that hospitals have certain constraints on their ability to accomplish patient
transfers and referrals:
• They must operate within the constraints of their authority under State law;
• A patient may refuse transfer or referral; or
• There may be financial barriers limiting a facility’s, agency’s, or ambulatory care
service provider’s willingness to accept the patient. In such cases the hospital does
not have financial responsibility for the post-acute care services. However, hospitals
are expected to be knowledgeable about resources available in their community to
address such financial barriers, such as Medicaid services, availability of Federally
Quality Health Centers, Area Agencies on Aging, etc., and to take steps to make
those resources available to the patient. For example, in most states hospitals work
closely with the Medicaid program to expedite enrollment of patients eligible for
Medicaid.
Survey Procedures §482.43(d)
• Review a sample of records for discharged patients who had a discharge plan to
determine if:
• For patients discharged home:
• Necessary medical information was sent to a practitioner with which the patient
has an established relationship prior to the first post-discharge appointment or
within 7 days of discharge, whichever comes first;
• For patients without an established relationship with a practitioner, information
was provided on potential primary care providers, such as health clinics, if
available.
• For patients transferred to another inpatient facility, was necessary medical
information ready at time of transfer and sent to the receiving facility with the
patient?
• When applicable, there is documentation in the medical record of providing the
results of tests, pending at time of discharge, to the patient and/or post-hospital
provider of care?
A-0843
(Rev. 87, Issued: 07-19-13, Effective: 07-19-13, Implementation: 07-19-13)
§483.43(e) Standard: Reassessment
The hospital must reassess its discharge planning process on an on-going basis. The
reassessment must include a review of discharge plans to ensure that they are
responsive to discharge needs.
Interpretive Guidelines §483.43(e)
The hospital must reassess the effectiveness of its discharge planning process on an ongoing
basis. Since the QAPI CoP at §482.21 requires the QAPI program to be hospital-wide, the
discharge planning reassessment process is considered an integral component of the overall
hospital QAPI program.
The hospital must have a mechanism in place for ongoing reassessment of its discharge
planning process. The reassessment process must include a review of discharge plans in
closed medical records to determine whether they were responsive to the patient’s post-
discharge needs. One indicator of the effectiveness of the discharge plan is whether or not
the discharge was followed by a preventable readmission. Accordingly, hospitals are
expected to track their readmission rates and identify potentially preventable readmissions.
Typically readmissions at 7, 15, 30 days, or even longer, after discharge are tracked by
analysts studying readmissions to short-term acute care hospitals. Hospitals must choose at
least one interval to track. Since there are National Quality Forum-endorsed readmissions
measures that use a 30-day interval, and since such measures are permitted by law to be
used by CMS for payment-related purposes, it might be prudent for a hospital to track its
30-day readmissions rate, but other intervals are permissible. It is understood that
information on post-discharge admissions to other hospitals may not be not readily
available to hospitals, but all hospitals are expected to track readmissions to their own
hospital, and to do so on an ongoing basis, i.e., at least quarterly. Hospitals may employ
various methodologies to identify potentially preventable readmissions. There are
proprietary products that, for example, use claims data to identify such cases. Hospitals are
expected to document their methodology for tracking their readmissions rates.
Once the hospital has identified potentially preventable readmissions, it is expected to
conduct an in-depth review of the discharge planning process for a sample of such
readmissions (at least 10% of potentially preventable readmissions, or 15 cases/quarter,
whichever is larger is suggested but not required) in order to determine whether there was
an appropriate discharge planning evaluation, discharge plan, and implementation of the
discharge plan.
Hospitals are also expected to follow up on trends identified through analysis of their
readmissions, such as a concentration of readmissions related to post-surgical infections,
discharges from a particular service or unit, discharges to a particular extended care facility
or home health agency, discharges with the same primary diagnosis on the first admission,
etc. Such clustering or concentration may identify areas requiring more follow-up analysis
and potential remedial actions.
Having identified factors that contribute to preventable readmissions, hospitals are expected
to revise their discharge planning and related processes to address these factors. Consistent
with the requirements under the QAPI CoP, the hospital’s governing body, medical
leadership and administrative leadership are all expected to ensure that identified problems
are addressed, with further ongoing reassessment to achieve improvement.
Survey Procedures §482.43(e)
• Review hospital policies and procedures to determine whether the discharge planning
process is reassessed on an ongoing basis, i.e., at least quarterly.
• Does the hospital’s discharge planning reassessment policy include tracking and
analysis of readmissions?
• Do staff know how to obtain data on readmissions that enables them to review the
discharge plans for the initial admission? Ask them to identify medical records for
patients who were readmitted and to show you the documentation of the review of
the discharge planning process for the initial admission.

• Does the assessment of readmissions include an evaluation of whether the
readmissions were potentially preventable?
• Is there evidence of in-depth analysis of a sample of discharge plans in cases where
preventable readmissions were identified?
• Is there evidence that the hospital took action to address factors identified as
contributing to preventable readmissions?
A-0884
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.45 Condition of Participation: Organ, Tissue and Eye Procurement
Interpretive Guidelines §482.45:
The hospital must ensure the specific organ, tissue, and eye procurement requirements are
met.
A-0885
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
The hospital must have and implement written protocols that:
Interpretive Guidelines §482.45(a)
The hospital must have written policies and procedures to address its organ procurement
responsibilities.
A-0886
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.45(a)(1) - Incorporate an agreement with an OPO designated under part 486 of
this chapter, under which it must notify, in a timely manner, the OPO or a third party
designated by the OPO of individuals whose death is imminent or who have died in
the hospital. The OPO determines medical suitability for organ donation and, in the
absence of alternative arrangements by the hospital, the OPO determines medical
suitability for tissue and eye donation, using the definition of potential tissue and eye
donor and the notification protocol developed in consultation with the tissue and eye
banks identified by the hospital for this purpose;
Interpretive Guidelines §482.45(a)(1)
The hospital must have a written agreement with an Organ Procurement Organization
(OPO), designated under 42 CFR Part 486. At a minimum, the written agreement must
address the following:
• The criteria for referral, including the referral of all individuals whose death is
imminent or who have died in the hospital;
• Includes a definition of “imminent death”;
• Includes a definition of “timely notification”;
• Addresses the OPO’s responsibility to determine medical suitability for organ
donation;
• Specifies how the tissue and/or eye bank will be notified about potential donors
using notification protocols developed by the OPO in consultation with the hospital-
designated tissue and eye bank(s);
• Provides for notification of each individual death in a timely manner to the OPO (or
designated third party) in accordance with the terms of the agreement;
• Ensures that the designated requestor training program offered by the OPO has been
developed in cooperation with the tissue bank and eye bank designated by the
hospital;
• Permits the OPO, tissue bank, and eye bank access to the hospital’s death record
information according to a designated schedule, e.g., monthly or quarterly;
• Includes that the hospital is not required to perform credentialing reviews for, or
grant privileges to, members of organ recovery teams as long as the OPO sends only
“qualified, trained individuals” to perform organ recovery; and
• The interventions the hospital will utilize to maintain potential organ donor patients
so that the patient organs remain viable.
Hospitals must notify the OPO of every death or imminent death in the hospital. When
death is imminent, the hospital must notify the OPO both before a potential donor is
removed from a ventilator and while the potential donor’s organs are still viable. The
hospital should have a written policy, developed in coordination with the OPO and
approved by the hospital’s medical staff and governing body, to define “imminent death.”
The definition for “imminent death” should strike a balance between the needs of the OPO
and the needs of the hospital’s care givers to continue treatment of a patient until brain
death is declared or the patient’s family has made the decision to withdraw supportive
measures. Collaboration between OPOs and hospitals will create a partnership that furthers
donation, while respecting the perspective of hospital staff.
The definition for “imminent death” might include a patient with severe, acute brain injury
who:
• Requires mechanical ventilation;
• Is in an intensive care unit (ICU) or emergency department; AND
• Exhibits clinical findings consistent with a Glascow Coma Score that is less than or
equal to a mutually-agreed-upon threshold; or
• MD/DOs are evaluating a diagnosis of brain death; or
• An MD/DO has ordered that life sustaining therapies be withdrawn, pursuant to the
family’s decision.
Hospitals and their OPO should develop a definition of “imminent death” that includes
specific triggers for notifying the OPO about an imminent death.
In determining the appropriate threshold for the Glascow Coma Score (GCS), it is
important to remember that if the threshold is too low, there may be too many “premature”
deaths or situations where there is a loss of organ viability. Standards for appropriate GCS
thresholds may be obtained from the hospital’s OPO or organizations such as The
Association of Organ Procurement Organizations.
Note that a patient with “severe, acute brain injury” is not always a trauma patient. For
example, post myocardial infarction resuscitation may result in a patient with a beating
heart and no brain activity.
The definition agreed to by the hospital and the OPO may include all of the elements listed
above or just some of the elements. The definition should be tailored to fit the particular
circumstances in each hospital.
Hospitals may not use “batch reporting” for deaths by providing the OPO with periodic lists
of patient deaths, even if instructed to do so by the OPO. If the patient dies during a
transfer from one hospital to another, it is the receiving hospital’s responsibility to notify
the OPO.
“Timely notification” means a hospital must contact the OPO by telephone as soon as
possible after an individual has died, has been placed on a ventilator due to a severe brain
injury, or who has been declared brain dead (ideally within 1 hour). That is, a hospital must
notify the OPO while a brain dead or severely brain-injured, ventilator-dependent
individual is still attached to the ventilator and as soon as possible after the death of any
other individual, including a potential non-heart-beating donor. Even if the hospital does
not consider an individual who is not on a ventilator to be a potential donor, the hospital
must call the OPO as soon as possible after the death of that individual has occurred.
Referral by a hospital to an OPO is timely if it is made:
• As soon as it is anticipated that a patient will meet the criteria for imminent death
agreed to by the OPO and hospital or as soon as possible after a patient meets the
criteria for imminent death agreed to by the OPO and the hospital (ideally, within
one hour); AND
• Prior to the withdrawal of any life sustaining therapies (i.e., medical or
pharmacological support).
Whenever possible, referral should be made early enough to allow the OPO to assess the
patient’s suitability for organ donation before brain death is declared and before the option
of organ donation is presented to the family of the potential donor. Timely assessment of
the patient’s suitability for organ donation increases the likelihood that the patient’s organs
will be viable for transplantation (assuming there is no disease process identified by the
OPO that would cause the organs to be unsuitable), assures that the family is approached
only if the patient is medically suitable for organ donation, and assures that an OPO
representative is available to collaborate with the hospital staff in discussing donation with
the family.
It is the OPO’s responsibility to determine medical suitability for organ donation, and, in
the absence of alternative arrangements by the hospital, the OPO determines medical
suitability for tissue and eye donation, using the definition of potential tissue and eye donor
and the notification protocol developed in consultation with the tissue and eye banks
identified by the hospital for this purpose.
Survey Procedures §482.45(a)(1)
• Review the hospital’s written agreement with the OPO to verify that it addresses all
required information.
• Verify that the hospital’s governing body has approved the hospital’s organ
procurement policies.
• Review a sample of death records to verify that the hospital has implemented its
organ procurement policies.
• Interview the staff to verify that they are aware of the hospital’s policies and
procedures for organ, tissue and eye procurement.
• Verify that the organ, tissue and eye donation program is integrated into the
hospital’s QAPI program.
A-0887
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.45(a)(2) - Incorporate an agreement with at least one tissue bank and at least one
eye bank to cooperate in the retrieval, processing, preservation, storage and
distribution of tissues and eyes, as may be appropriate to assure that all usable tissues
and eyes are obtained from potential donors, insofar as such an agreement does not
interfere with organ procurement;
Interpretative Guidelines §482.45(a)(2)
The hospital must have an agreement with at least one tissue bank and at least one eye
bank. The OPO may serve as a “gatekeeper” receiving notification about every hospital
death and should notify the tissue bank or eye bank chosen by the hospital about potential
tissue and eye donors.
It is not necessary for a hospital to have a separate agreement with a tissue bank if it has an
agreement with its OPO to provide tissue procurement services; nor is it necessary for a
hospital to have a separate agreement with an eye bank if its OPO provides eye
procurement services. The hospital is not required to use the OPO for tissue or eye
procurement but is free to have an agreement with the tissue bank or eye bank of its choice.
The tissue banks and eye banks define “usable tissues” and “usable eyes.”
The requirements of this regulation may be satisfied through a single agreement with an
OPO that provides services for organ, tissue and eye, or by a separate agreement with
another tissue and/or eye bank outside the OPO, chosen by the hospital. The hospital may
continue current successful direct arrangements with tissue and eye banks as long as the
direct arrangement does not interfere with organ procurement.
Survey Procedures §482.45(a)(2)
Verify that the hospital has an agreement with at least one tissue bank and one eye bank that
specifies criteria for referral of all potential tissue and eye donors, or an agreement with an
OPO that specifies the tissue bank and eye bank to which referrals will be made. The
agreement should also acknowledge that it is the OPO’s responsibility to determine medical
suitability for tissue and eye donation, unless the hospital has an alternative agreement with
a different tissue and/or eye bank.
A-0888
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.45(a)(3) - Ensure, in collaboration with the designated OPO, that the family of
each potential donor is informed of its options to donate organs, tissues, or eyes, or to
decline to donate.
Interpretive Guidelines §482.45(a)(3)
It is the responsibility of the OPO to screen for medical suitability in order to select
potential donors. Once the OPO has selected a potential donor, that person’s family must
be informed of the family’s donation options.
Ideally, the OPO and the hospital will decide together how and by whom the family will be
approached.
Survey Procedures §482.45(a)(3)
• Verify that the hospital ensures that the family of each potential donor is informed
of its options to donate organs, tissues, or eyes, including the option to decline to
donate.
• Does the hospital have QAPI mechanisms in place to ensure that the families of all
potential donors are informed of their options to donate organs, tissues, or eyes, or
to decline to donate?
A-0889
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.45(a)(3) - (Continued)
The individual designated by the hospital to initiate the request to the family must be
an organ procurement representative or a designated requestor. A designated
requestor is an individual who has completed a course offered or approved by the
OPO and designed in conjunction with the tissue and eye bank community in the
methodology for approaching potential donor families and requesting organ or tissue
donation;
Interpretive Guidelines §482.45(a)(3)
The individual designated by the hospital to initiate the request to a family must be an organ
procurement representative, an organizational representative of a tissue or eye bank, or a
designated requestor. Any individuals involved in a request for organ, tissue, and eye
donation must be formally trained in the donation request process.
The individual designated by the hospital to initiate the request to the family must be an
OPO, tissue bank, or eye bank representative or a designated requestor. A “designated
requestor” is defined as a hospital-designated individual who has completed a course
offered or approved by the OPO and designed in conjunction with the tissue and eye bank
community.
Ideally, the OPO and the hospital will decide together how and by whom the family will be
approached. If possible, the OPO representative and a designated requestor should
approach the family together.
The hospital must ensure that any “designated requestor” for organs, tissues or eyes has
completed a training course either offered or approved by the OPO, which addresses
methodology for approaching potential donor families.
Survey Procedures §482.45(a)(3)
• Review training schedules and personnel files to verify that all designated requestors
have completed the required training.
• How does the hospital ensure that only OPO, tissue bank, or eye bank staff or
designated requestors are approaching families to ask them to donate?
A-0890
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.45(a)(4) - Encourage discretion and sensitivity with respect to the circumstances,
views, and beliefs of the families of potential donors;
Interpretive Guidelines §482.45(a)(4)
Using discretion does not mean a judgment can be made by the hospital that certain families
should not be approached about donation. Hospitals should approach the family with the
belief that a donation is possible and should take steps to ensure the family is treated with
respect and care. The hospital staff’s perception that a family’s grief, race, ethnicity,
religion or socioeconomic background would prevent donation should never be used as a
reason not to approach a family.
All potential donor families must be approached and informed of their donation rights.
Survey Procedures §482.45(a)(4)
• Interview a hospital-designated requestor regarding approaches to donation requests.
• Review the designated requestor training program to verify that it addresses the use
of discretion.
• Review the hospital’s complaint file for any relevant complaints.
A-0891
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.45(a)(5) - Ensure that the hospital works cooperatively with the designated OPO,
tissue bank and eye bank in educating staff on donation issues;
Interpretive Guidelines §482.45(a)(5)
Appropriate hospital staff, including all patient care staff, must be trained on donation
issues. The training program must be developed in cooperation with the OPO, tissue bank
and eye bank, and should include, at a minimum:
• Consent process;
• Importance of using discretion and sensitivity when approaching families;
• Role of the designated requestor;
• Transplantation and donation, including pediatrics, if appropriate;
• Quality improvement activities; and
• Role of the organ procurement organization.
Training should be conducted with new employees annually, whenever there are
policy/procedure changes, or when problems are determined through the hospital’s QAPI
program.
Those hospital staff who may have to contact or work with the OPO, tissue bank and eye
bank staff must have appropriate training on donation issues including their duties and
roles.
Survey Procedures §482.45(a)(5)
• Review in-service training schedules and attendance sheets.
• How does the hospital ensure that all appropriate staff has attended an educational
program regarding donation issues and how to work with the OPO, tissue bank, and
eye bank?
A-0892
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.45(a)(5) - (Continued)
[Ensure that the hospital works cooperatively with the designated OPO, tissue bank
and eye bank in educating staff on…] reviewing death records to improve
identification of potential donors, and
Interpretive Guidelines §482.45(a)(5)
Hospitals must cooperate with the OPOs, tissue banks and eye banks in regularly or
periodically reviewing death records. This means that the hospital must develop policies
and procedures which permit the OPO, tissue bank, and eye bank access to death record
information that will allow the OPO, tissue bank and eye bank to assess the hospital’s donor
potential, assure that all deaths or imminent deaths are being referred to the OPO in a timely
manner, and identify areas where the hospital, OPO, tissue bank and eye bank staff
performance might be improved. The policies must address how patient confidentiality will
be maintained during the review process.
Survey Procedures §482.45(a)(5)
• Verify by review of policies and records that the hospital works with the OPO,
tissue bank, and eye bank in reviewing death records.
• Verify that the effectiveness of any protocols and policies is monitored as part of the
hospital’s quality improvement program.
• Validate how often the reviews are to occur. Review the protocols that are in place
to guide record reviews and analysis.
• Determine how confidentiality is ensured.
A-0893
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.45(a)(5) - (Continued)
[Ensure that the hospital works cooperatively with the designated OPO, tissue bank
and eye bank in educating staff on…] maintaining potential donors while necessary
testing and placement of potential donated organs, tissues, and eyes take place.
Interpretive Guidelines §482.45(a)(5)
The hospital must have policies and procedures, developed in cooperation with the OPO,
that ensure that potential donors are maintained in a manner that maintains the viability of
their organs. The hospital must have policies in place to ensure that potential donors are
identified and declared dead within an acceptable time frame by an appropriate practitioner.
Survey Procedures §482.45(a)(5)
• Determine by review, what policies and procedures are in place to ensure that
potential donors are identified and declared dead by an appropriate practitioner
within an acceptable timeframe.
• Verify that there are policies and procedures in place to ensure the coordination
between facility staff and OPO staff in maintaining the potential donor.
A-0899
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.45(b) Standard: Organ Transplantation Responsibilities
(1) A hospital in which organ transplants are performed must be a member of
the Organ Procurement and Transplantation Network (OPTN) established and
operated in accordance with section 372 of the Public Health Service (PHS) Act (42
U.S.C. 274) and abide by its rules. The term “rules of the OPTN” means those
rules provided for in regulations issued by the Secretary in accordance with
section 372 of the PHS Act which are enforceable under 42 CFR 121.10. No
hospital is considered to be out of compliance with section 1138(a)(1)(B) of the Act,
or with the requirements of this paragraph, unless the Secretary has given the
OPTN formal notice that he or she approves the decision to exclude the hospital
from the OPTN and has notified the hospital in writing.
(2) For purposes of these standards, the term “organ” means a human kidney,
liver, heart, lung, or pancreas.
(3) If a hospital performs any type of transplants, it must provide organ
transplant related data, as requested by the OPTN, the Scientific Registry, and the
OPOs. The hospital must also provide such data directly to the Department when
requested by the Secretary.
Interpretive Guidelines §482.45(b)(1) –(3)
If you have questions concerning the facility membership in the Organ Procurement and
Transplantation Network; you may verify the membership by contacting the CMS regional
office or by calling the United Network for Organ sharing (UNOS) at 1-804-330-8500.
Survey Procedures §482.45(b)(1) – (3)
Verify by review, one year of reports submitted by the facility to the OPTN, the Scientific
Registry, the OPOs, and any data submitted to the Department per request of the Secretary.
A-0940
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.51 Condition of Participation: Surgical Services
If the hospital provides surgical services, the services must be well organized and
provided in accordance with acceptable standards of practice. If outpatient surgical
services are offered the services must be consistent in quality with inpatient care in
accordance with the complexity of services offered.
Interpretive Guidelines §482.51
The provision of surgical services is an optional hospital service. However, if a hospital
provides any degree of surgical services to its patients, the hospital must comply with all
the requirements of this Condition of Participation (CoP).
What constitutes “surgery”?
For the purposes of determining compliance with the hospital surgical services CoP, CMS
relies, with minor modification, upon the definition of surgery developed by the American
College of Surgeons. Accordingly, the following definition is used to determine whether or
not a procedure constitutes surgery and is subject to this CoP:
Surgery is performed for the purpose of structurally altering the human body by the
incision or destruction of tissues and is part of the practice of medicine. Surgery also
is the diagnostic or therapeutic treatment of conditions or disease processes by any
instruments causing localized alteration or transposition of live human tissue which
include lasers, ultrasound, ionizing radiation, scalpels, probes, and needles. The
tissue can be cut, burned, vaporized, frozen, sutured, probed, or manipulated by
closed reductions for major dislocations or fractures, or otherwise altered by
mechanical, thermal, light-based, electromagnetic, or chemical means. Injection of
diagnostic or therapeutic substances into body cavities, internal organs, joints,
sensory organs, and the central nervous system also is considered to be surgery (this
does not include the administration by nursing personnel of some injections,
subcutaneous, intramuscular, and intravenous, when ordered by a physician). All of
these surgical procedures are invasive, including those that are performed with
lasers, and the risks of any surgical procedure are not eliminated by using a light
knife or laser in place of a metal knife, or scalpel. Patient safety and quality of care
are paramount and, therefore, patients should be assured that individuals who
perform these types of surgery are licensed physicians (physicians as defined in
482.12(c)(1)) who are working within their scope of practice, hospital privileges,
and who meet appropriate professional standards.
If surgical services are provided, they must be organized and staffed in such a manner to
ensure the health and safety of patients.
Acceptable standards of practice include maintaining compliance with applicable Federal
and State laws, regulations and guidelines governing surgical services or surgical service
locations, as well as, any standards and recommendations promoted by or established by
nationally recognized professional organizations (e.g., the American Medical Association,
American College of Surgeons, Association of Operating Room Nurses, Association for
Professionals in Infection Control and Epidemiology, etc.)
Outpatient surgical services must be in compliance with all hospital CoPs including the
surgical services CoP. Outpatient surgical services must be provided in accordance with
acceptable standards of practice. Additionally, the hospital’s outpatient surgical services
must be consistent in quality with the hospital’s inpatient surgical services. Post-operative
care planning, coordination for the provision of needed post-operative care and appropriate
provisions for follow-up care of outpatient surgery patients must be consistent in quality
with inpatient care in accordance with the complexity of the services offered and the needs
of the patient.
The hospital’s inpatient and outpatient surgical services must be integrated into its hospital-
wide QAPI program.
Survey Procedures §482.51
Inspect all inpatient and outpatient operative rooms/suites. Request the use of proper attire
for the inspection. Observe the practices to determine if the services are provided in
accordance with acceptable standards of practice. Observe:
• That access to the operative and recovery area is limited to authorized personnel and
that the traffic flow pattern adheres to accepted standards of practice;
• The conformance to aseptic and sterile technique by all individuals in the surgical
area;
• That there is appropriate cleaning between surgical cases and appropriate terminal
cleaning applied;
• That operating room attire is suitable for the kind of surgical case performed, that
persons working in the operating suite must wear only clean surgical costumes, that
surgical costumes are designed for maximum skin and hair coverage;
• That equipment is available for rapid and routine sterilization of operating room
materials;
• That equipment is monitored, inspected, tested, and maintained by the hospital’s
biomedical equipment program and in accordance with Federal and State law,
regulations and guidelines and manufacturer’s recommendations;
• That sterilized materials are packaged, handled, labeled, and stored in a manner that
ensures sterility e.g., in a moisture and dust controlled environment and policies and
procedures for expiration dates have been developed and are followed in accordance
with accepted standards of practice.
• That temperature and humidity are monitored and maintained within accepted
standards of practice;
• That medical/surgical devices and equipment are checked and maintained routinely
by clinical/biomedical engineers.
• Verify that all surgical service activities and locations are integrated into the
hospital-wide QAPI program.
A-0941
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.51(a) Standard: Organization and Staffing
The organization of the surgical services must be appropriate to the scope of the
services offered.
Interpretive Guidelines §482.51(a)
When the hospital offers surgical services, the hospital must provide the appropriate
equipment and the appropriate types and numbers of qualified personnel necessary to
furnish the surgical services offered by the hospital in accordance with acceptable standards
of practice.
The scope of surgical services provided by the hospital should be defined in writing and
approved by the medical staff.
Survey Procedures §482.51(a)
Review the hospital’s organizational chart displaying the relationship of the operating room
service to other services. Confirm that the operating room’s organization chart indicates
lines of authority and delegation of responsibility within the department or service.
A-0942
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.51(a)(1) - The operating rooms must be supervised by an experienced registered
nurse or a doctor of medicine or osteopathy.
Interpretive Guidelines §482.51(a)(1)
The operating room (inpatient and outpatient) must be supervised by an experienced RN or
MD/DO. The RN or MD/DO supervising the operating room must demonstrate appropriate
education, background working in surgical services, and specialized training in the
provision of surgical services/management of surgical service operations. The hospital
should address its required qualifications for the supervisor of the hospital’s operating
rooms in its policies and the supervisor’s personnel file should contain information
demonstrating compliance with the hospital’s established qualifications.
Survey Procedures §482.51(a)(1)
• Verify that an RN or a doctor of medicine or osteopathy is assigned responsibility
for supervision of the operating rooms.
• Request a copy of the supervisor’s position description to determine that it specifies
qualifications, duties and responsibilities of the position. Verify that the supervisor
is experienced and competent in the management of surgical services.
A-0943
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.51(a)(2) - Licensed practical nurses (LPNs) and surgical technologists (operating
room technicians) may serve as “scrub nurses” under the supervision of a registered
nurse.
Interpretive Guidelines §482.51(a)(2)
If the hospital utilizes LPN or operating room technicians as “scrub nurses,” those
personnel must be under the supervision of an RN who is immediately available to
physically intervene and provide care.
Survey Procedures §482.51(a)(2)
• Determine that an RN is available for supervision in the department or service.
Validate the availability by requesting and reviewing a staffing schedule for the OR.
• Review staffing schedules to determine adequacy of staff and RN supervision.
A-0944
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.51(a)(3) - Qualified registered nurses may perform circulating duties in the
operating room. In accordance with applicable State laws and approved medical staff
policies and procedures, LPNs and surgical technologists may assist in circulatory
duties under the supervision of a qualified registered nurse who is immediately
available to respond to emergencies.
Interpretive Guidelines §482.51(a)(3)
The circulating nurse must be an RN. An LPN or surgical technologist may assist an RN in
carrying out circulatory duties (in accordance with applicable State laws and medical-staff
approved hospital policy) but the LPN or surgical technologist must be under the
supervision of the circulating RN who is in the operating suite and who is available to
immediately and physically respond/intervene to provide necessary interventions in
emergencies. The supervising RN would not be considered immediately available if the
RN was located outside the operating suite or engaged in other activities/duties which
prevent the RN from immediately intervening and assuming whatever circulating
activities/duties that were being provided by the LPN or surgical technologist. The
hospital, in accordance with State law and acceptable standards of practice, must establish
the qualifications required for RNs who perform circulating duties and LPNs and surgical
technologists who assist with circulating duties.
Survey Procedures §482.51(a)(3)
• If LPNs and surgical technologists (STs) are assisting with circulating duties, verify
that they do so in accordance with applicable State laws and medical-staff approved
policies and procedures.
• Verify in situations where LPNs and STs are permitted to assist with circulating
duties that a qualified RN supervisor is immediately available to respond to
emergencies.
• Verify that RNs working as circulating nurses are working in accordance with
applicable State laws and medical-staff approved policies and procedures.
A-0945
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.51(a)(4) - Surgical privileges must be delineated for all practitioners performing
surgery in accordance with the competencies of each practitioner. The surgical
service must maintain a roster of practitioners specifying the surgical privileges of
each practitioner.
Interpretive Guidelines §482.51(a)(4)
Surgical privileges should be reviewed and updated at least every 2 years. A current roster
listing each practitioner’s specific surgical privileges must be available in the surgical suite
and area/location where the scheduling of surgical procedures is done. A current list of
surgeons suspended from surgical privileges or whose surgical privileges have been
restricted must also be retained in these areas/locations.
The hospital must delineate the surgical privileges of all practitioners performing surgery
and surgical procedures. The medical staff is accountable to the governing body for the
quality of care provided to patients. The medical staff bylaws must include criteria for
determining the privileges to be granted to an individual practitioner and a procedure for
applying the criteria to individuals requesting privileges. Surgical privileges are granted in
accordance with the competencies of each practitioner. The medical staff appraisal
procedures must evaluate each individual practitioner’s training, education, experience, and
demonstrated competence as established by the hospital’s QAPI program, credentialing
process, the practitioner’s adherence to hospital policies and procedures, and in accordance
with scope of practice and other State laws and regulations.
The hospital must specify the surgical privileges for each practitioner that performs surgical
tasks. This would include practitioners such as MD/DO, dentists, oral surgeons, podiatrists,
RN first assistants, nurse practitioners, surgical physician assistants, surgical technicians,
etc. When a practitioner may perform certain surgical procedures under supervision, the
specific tasks/procedures and the degree of supervision (to include whether or not the
supervising practitioner is physically present in the same OR, in line of sight of the
practitioner being supervised) be delineated in that practitioner’s surgical privileges and
included on the surgical roster.
If the hospital utilizes RN First Assistants, surgical PA, or other non-MD/DO surgical
assistants, the hospital must establish criteria, qualifications and a credentialing process to
grant specific privileges to individual practitioners based on each individual practitioner’s
compliance with the privileging/credentialing criteria and in accordance with Federal and
State laws and regulations. This would include surgical services tasks conducted by these
practitioners while under the supervision of an MD/DO.
When practitioners whose scope of practice for conducting surgical procedures requires the
direct supervision of an MD/DO surgeon, the term “supervision” would mean the
supervising MD/DO surgeon is present in the same room, working with the same patient.
Surgery and all surgical procedures must be conducted by a practitioner who meets the
medical staff criteria and procedures for the privileges granted, who has been granted
specific surgical privileges by the governing body in accordance with those criteria, and
who is working within the scope of those granted and documented privileges.
Survey Procedures §482.51(a)(4)
• Review the hospital’s method for reviewing the surgical privileges of practitioners.
This method should require a written assessment of the practitioner’s training,
experience, health status, and performance.
• Determine that a current roster listing each practitioner’s specific surgical privileges
is available in the surgical suite and the area where the scheduling of surgical
procedures is done.
• Determine that a current list of surgeons suspended from surgical privileges or who
have restricted surgical privileges is retained in these areas/locations.
A-0951
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.51(b) Standard: Delivery of Service
Surgical services must be consistent with needs and resources. Policies governing
surgical care must be designed to assure the achievement and maintenance of high
standards of medical practice and patient care.
Interpretive Guidelines §482.51(b)
Policies governing surgical care should contain:
• Aseptic and sterile surveillance and practice, including scrub techniques;
• Identification of infected and non-infected cases;
• Housekeeping requirements/procedures;
• Patient care requirements:
o Preoperative work-up;
o Patient consents and releases;
o Clinical procedures;
o Safety practices;
o Patient identification procedures;
• Duties of scrub and circulating nurse;
• Safety practices;
• The requirement to conduct surgical counts in accordance with accepted standards
of practice;
• Scheduling of patients for surgery;
• Personnel policies unique to the O.R.;
• Resuscitative techniques;
• DNR status;
• Care of surgical specimens;
• Malignant hyperthermia;
• Appropriate protocols for all surgical procedures performed. These may be
procedure-specific or general in nature and will include a list of equipment,
materials, and supplies necessary to properly carry out job assignment;
• Sterilization and disinfection procedures;
• Acceptable operating room attire;
• Handling infections and biomedical/medical waste; and
• Outpatient surgery post-operative care planning and coordination, and provisions for
follow-up care.
Policies and procedures must be written, implemented and enforced. Surgical services’
policies must be in accordance with acceptable standards of medical practice and surgical
patient care.
NOTE: Use of Alcohol-based Skin Preparations in Anesthetizing Locations. Alcohol-
based skin preparations are considered the most effective and rapid-acting skin
antiseptic, but they are also flammable and contribute to the risk of fire.
It is estimated that approximately 100 surgical fires occur each year in the United States,

resulting in roughly 20 serious patient injuries, including one to two deaths annually.
(ECRI, “Surgical Fire Safety,” Health Devices 35 no 2 (February, 2006) 45-66)) Fires
occur when an ignition source, a fuel source, and an oxidizer come together. Heat-
producing devices are potential ignition sources, while alcohol-based skin preparations
provide fuel. Procedures involving electro-surgery or the use of cautery or lasers involve
heat-producing devices. There is concern that an alcohol-based skin preparation, combined
with the oxygen-rich environment of an anesthetizing location could ignite when exposed to
a heat-producing device in an operating room. Specifically, if the alcohol-based skin
preparation is improperly applied, the solution may wick into the patient’s hair and linens or
pool on the patient’s skin, resulting in prolonged drying time. Then, if the patient is draped
before the solution is completely dry, the alcohol vapors can become trapped under the
surgical drapes and channeled to the surgical site. (ECRI for Pennsylvania Patient Safety
Advisory 2, No. 2 (June, 2005) 13)
On the other hand, surgical site infections (SSI) also pose significant risks to patients;
according to the Centers for Disease Control and Prevention (CDC), such infections are the
third most commonly reported hospital-acquired infections. Although the CDC has stated
that there are no definitive studies comparing the effectiveness of the different types of skin
antiseptics in preventing SSI, it also states that “Alcohol is readily available, inexpensive,
and remains the most effective and rapid-acting skin antiseptic.” (CDC Hospital Infection
Control Practices Advisory Committee, “Guideline for Prevention of Surgical Site
Infection, 1999,” Infection Control and Hospital Epidemiology April 1999 (Vol 20 No. 4)
251, 257) Hence, in light of alcohol’s effectiveness as a skin antiseptic, there is a need to
balance the risks of fire related to use of alcohol-based skin preparations with the risk of
surgical site infection.
The use of an alcohol-based skin preparation in inpatient or outpatient anesthetizing
locations is not considered safe, unless appropriate fire risk-reduction measures are taken,
preferably as part of a systematic approach by the hospital to preventing surgery-related
fires. A review of recommendations produced by various expert organizations concerning
use of alcohol-based skin preparations in anesthetizing locations indicates there is general
consensus that the following risk reduction measures are appropriate:
• Using skin prep solutions that are: 1) packaged to ensure controlled delivery to the
patient in unit dose applicators, swabs, or other similar applicators; and 2) provide
clear and explicit manufacturer/supplier instructions and warnings. These
instructions for use should be carefully followed.
• Ensuring that the alcohol-based skin prep solution does not soak into the patient’s
hair or linens. Sterile towels should be placed to absorb drips and runs during
application and should then be removed from the anesthetizing location prior to
draping the patient.
• Ensuring that the alcohol-based skin prep solution is completely dry prior to
draping. This may take a few minutes or more, depending on the amount and
location of the solution. The prepped area should be inspected to confirm it is dry
prior to draping.
• Verifying that all of the above has occurred prior to initiating the surgical procedure.
This can be done, for example, as part of a standardized pre-operative “time out”
used to verify other essential information to minimize the risk of medical errors
during the procedure.
Hospitals that employ alcohol-based skin preparations in anesthetizing locations should
establish appropriate policies and procedures to reduce the associated risk of fire. They
should also document the implementation of these policies and procedures in the patient’s
medical record.
Failure by a hospital to develop and implement appropriate measures to reduce the risk of
fires associated with the use of alcohol-based skin preparations in anesthetizing locations
should be cited as condition-level noncompliance.
Survey Procedures §482.51(b)
• Review policies and procedures to determine whether they address the elements
specified in the interpretive guidelines. If the hospital uses alcohol-based skin
preparations in anesthetizing locations, determine whether it has adopted policies
and procedures to minimize the risk of surgical fires.
• Interview surgical services staff to determine whether they are aware of and follow
hospital policies and procedures.
A-0952
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.51(b)(1) - Prior to surgery or a procedure requiring anesthesia services and
except in the case of emergencies:
(i) A medical history and physical examination must be completed and
documented no more than 30 days before or 24 hours after admission or registration,
and except as provided under paragraph (b)(1)(iii) of this section.
Interpretive Guidelines §482.43(b)(1)(i)
There must be a complete history and physical examination (H & P), and an update, if
applicable, in the medical record of every patient prior to surgery, or a procedure requiring
anesthesia services, except in emergencies and, under §482.51(b)(1)(iii), for those specific
patients that are not required to have a comprehensive medical history and physical
examination, or any update to it, prior to specific outpatient surgical or procedural services
as determined by medical staff policy.
The H&P must be conducted in accordance with the requirements of 42 CFR 482.22(c)(5).
• The H&P must be completed and documented no more than 30 days before or 24 hours
after admission or registration. In all cases when it is determined that an H&P is
required, except for emergencies, the H&P must be completed and documented before
the surgery or procedure takes place, even if that surgery or procedure occurs less than
24 hours after admission or registration.
• If the H&P was completed within 30 days before admission or registration, then an
updated examination must be completed and documented within 24 hours after
admission or registration. In all cases when it is determined that an H&P is required,
except for emergencies, the update must be completed and documented before the
surgery or procedure takes place, even if that surgery or procedure occurs less than 24
hours after admission or registration.
Survey Procedures §482.51(b)(1)(i)
Review a sample of open and closed medical records of patients (both inpatient and
outpatient) who have had surgery or a procedure requiring anesthesia.
• Determine whether an H&P, if required was conducted and documented in a timely
manner.
• Determine whether the H&P, if required, was conducted in accordance with the
requirements of 42 CFR 482.22(c)(5).
• Determine whether the records of patients who are required to have an H&P, did not
have an H&P in a timely manner or update indicate that the surgery or procedure was
conducted on an emergency basis.
A-0953
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.51(b)(1) - Prior to surgery or a procedure requiring anesthesia services and
except in the case of emergencies:
(ii) An updated examination of the patient, including any changes in the
patient’s condition, must be completed and documented within 24 hours after
admission or registration when the medical history and physical examination are
completed within 30 days before admission or registration, and except as provided
under paragraph (b)(1)(iii) of this section.
Interpretive Guidelines §482.51(b)(1)(ii)
Guidance is pending and will be updated in future release.
A-0954
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.51(b)(1) - Prior to surgery or a procedure requiring anesthesia services and
except in the case of emergencies:
(iii) An assessment of the patient must be completed and documented after
registration (in lieu of the requirements of paragraphs (b)(1)(i) and (ii) of this section)
when the patient is receiving specific outpatient surgical or procedural services and
when the medical staff has chosen to develop and maintain a policy that identifies, in
accordance with the requirements at § 482.22(c)(5)(v), specific patients as not
requiring a comprehensive medical history and physical examination, or any update
to it, prior to specific outpatient surgical or procedural services.
Interpretive Guidelines §482.51(b)(1)(iii)
Guidance is pending and will be updated in future release.
A-0955
(Rev. 220; Issued: 04-19-24; Effective: 04-19-24; Implementation: 04-19-24)
§482.51(b)(2) - A properly executed informed consent form for the operation must
be in the patient’s chart before surgery, except in emergencies.
Interpretive Guidelines §482.51(b)(2)
Informed consent is addressed in two other portions of the CMS Hospital CoPs and the
SOM. Surveyors should review the guidelines for §482.13(b)(2) under Patients' Rights
and the guidelines for §482.24(c)(2)(v) under Medical Records to understand all
requirements related to informed consent.
The primary purpose of the informed consent process for surgical services is to ensure
that the patient, or the patient’s representative, is provided information necessary to
enable him/her to evaluate a proposed surgery before agreeing to the surgery. Typically,
this information would include potential short- and longer-term risks and benefits to the
patient of the proposed intervention, including the likelihood of each, based on the
available clinical evidence, as informed by the responsible practitioner’s professional
judgment. Informed consent must be obtained, and the informed consent form must be
placed in the patient’s medical record, prior to surgery, except in the case of emergency
surgery.
Hospitals must assure that the practitioner(s) responsible for the surgery obtain informed
consent from patients in a manner consistent with the hospital’s policies governing the
informed consent process.
It should be noted that there is no specific requirement for informed consent within the regulation at
§482.52 governing anesthesia services. However, given that surgical procedures generally entail use
of anesthesia, hospitals may wish to consider specifically extending their informed consent policies
to include obtaining informed consent for the anesthesia component of the surgical procedure.
Surgical Informed Consent Policy
The hospital’s surgical informed consent policy should describe the following:
(3)
Who may obtain the patient’s informed consent;
(4)
Which procedures require informed consent;
(5)
The circumstances under which surgery is considered an emergency, and may be undertaken
without an informed consent;
(6)
The circumstances when a patient’s representative, rather than the patient, may give
informed consent for a surgery;
(7)
The content of the informed consent form and instructions for completing it;
(8)
The process used to obtain informed consent, including how informed consent is to be
documented in the medical record;
(9)
Mechanisms that ensure that the informed consent form is properly executed and is in the
patient’s medical record prior to the surgery (except in the case of emergency surgery); and
(10)
If the informed consent process and informed consent form are obtained outside the
hospital, how the properly executed informed consent form is incorporated into the patient’s
medical record prior to the surgery.
If there are additional requirements under State law for informed consent, the hospital must
comply with those requirements.
Example of a Well-Designed Informed Consent Process
A well-designed informed consent process would include discussion of the following elements:
• A description of the proposed surgery, including the anesthesia to be used;
• The indications for the proposed surgery;
•
Material risks and benefits for the patient related to the surgery and anesthesia, including the
likelihood of each, based on the available clinical evidence, as informed by the responsible
practitioner’s clinical judgment. Material risks could include risks with a high degree of
likelihood but a low degree of severity, as well as those with a very low degree of likelihood but
high degree of severity;
•
Treatment alternatives, including the attendant material risks and benefits;
•
The probable consequences of declining recommended or alternative therapies;
•
Who will conduct the surgical intervention and administer the anesthesia;
• Whether physicians other than the operating practitioner, including, but not limited to, residents,
medical, advanced practice provider (such as nurse practitioners and physician assistants), and
other applicable students, will be performing important tasks related to the surgery, or
examinations or invasive procedures for educational and training purposes, in accordance with
the hospital’s policies. Important surgical tasks include: opening and closing, dissecting tissue,
removing tissue, harvesting grafts, transplanting tissue, administering anesthesia, implanting
devices, and placing invasive lines. Examinations or invasive procedures conducted for
educational and training purposes include, but are not limited to, breast, pelvic, prostate, and
rectal examinations, as well as others specified under state law.
• For surgeries in which residents will perform important parts of the surgery, discussion is
encouraged to include the following:
• That it is anticipated that physicians who are in approved post graduate residency
training programs will perform portions of the surgery, based on their availability and
level of competence;
• That it will be decided at the time of the surgery which residents will participate and their
manner or participation, and that this will depend on the availability of residents with the
necessary competence; the knowledge the operating practitioner/teaching surgeon has of
the resident’s skill set; and the patient’s condition;
• That residents performing surgical tasks will be under the supervision of the operating
practitioner/teaching surgeon; and
• Whether, based on the resident’s level of competence, the operating
practitioner/teaching surgeon will not be physically present in the same operating
room for some or all of the surgical tasks performed by residents.
NOTE: A “moonlighting” resident or fellow is a postgraduate medical trainee who is
practicing independently, outside the scope of his/her residency training
program and would be treated as a physician within the scope of the privileges
granted by the hospital.
Whether, as permitted by State law, qualified medical practitioners who are not
physicians will perform important parts of the surgery or administer the
anesthesia, and if so, the types of tasks each type of practitioner will carry out;
and that such practitioners will be performing only tasks within their scope of
practice for which they have been granted privileges by the hospital.
Informed Consent Forms
See the guidelines for §482.24(c)(2)(v) under Medical Records for discussion of the
content of a properly executed informed consent form.
Survey Procedures §482.51(b)(2)
•
Verify that the hospital has assured that the medical staff has specified which
procedures are considered surgery and, thus, are those that require a properly
executed informed consent form.
•
Verify that the hospital’s informed consent policies address the
circumstances when a surgery would be considered an emergency and thus
not require an informed consent form be placed in the medical record prior
to surgery.
•
Review a minimum of six medical records of surgical patients and verify that
they did not involve emergency surgery and that they contain informed consent
forms that were executed prior to the surgery. When possible, review medical
records of patients who are about to undergo surgery, or who are located in a
surgical recovery area.
• Interview two or three post-surgical patients, as appropriate based on their ability
to provide a cogent response, or the patients’ representatives to see how satisfied
they are with the informed consent discussion prior to their surgery.
A-0956
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.51(b)(3) - The following equipment must be available to the operating room
suites: call-in system, cardiac monitor, resuscitator, defibrillator, aspirator, and
tracheotomy set.
Survey Procedures §482.51(b)(3)
• Check to determine that the operating room suite has available the items listed:
o On-call system;
o Cardiac monitor;
o Resuscitator;
o Defibrillator;
o Aspirator (suction equipment); and
o Tracheotomy set (a cricothyroidotomy set is not a substitute).
Verify that all equipment is working and, as applicable, in compliance with the hospital’s
biomedical equipment inspection, testing, and maintenance program.
A-0957
(Rev. 116, I ssued: 06-06-14 Effective: 06-06-14, Implementation 06-06-14)
§482.51(b)(4) - There must be adequate provisions for immediate post-operative
care.
Interpretive Guidelines §482.51(b)(4)
Adequate provisions for immediate post-operative care means:
• Post-operative care must be provided to all surgical patients, including same-
day surgery patients, in accordance with acceptable standards of practice.
• A post-operative care area, usually referred to as the post-anesthesia care unit
(PACU), is a separate area of the hospital. Access is limited to authorized
personnel.
• Policies and procedures specify transfer requirements to and from the PACU.
Depending on the type of anesthesia and length of surgery, the post-operative
check before transferring the patient from the PACU includes, but is not
limited to:
o Level of activity;
o Respirations;
o Blood pressure;
o Level of consciousness;
o Level of pain;
o Patient color; and

• If a patient is not transferred to the PACU, determine that provisions are made
for close observation until the patient has regained consciousness, e.g., direct
observation by a qualified RN.
Post-operative Monitoring
Hospitals are expected to develop and implement policies and procedures addressing the
minimum scope and frequency of patient monitoring in post-PACU care settings,
consistent with accepted standards of practice.
Patients receiving post-operative intravenous (IV) opioid medications are of particular
concern, due to the higher risk for oversedation and respiratory depression
9
. Once out of
the PACU, patients receiving IV opioid medication may be placed on units where vital
signs and other monitoring traditionally has not been done as frequently as in the PACU
or intensive care units, increasing the risk that patients may develop respiratory
compromise that is not immediately recognized and treated. (See the interpretive
guidelines at §482.23(c)(4)). When post-surgical patients are transferred out of the
PACU to another area of the hospital but continued on IV opioid medications, they need
vigilant monitoring, even if post-PACU care is not typically referred to as “immediate”
post-operative care. Opioid- induced respiratory compromise has resulted in inpatient
deaths that might have been prevented with appropriate assessment and vigilant
monitoring of respiration and sedation levels.
10
Survey Procedures §482.51(b)(4)
• Verify that the hospital has provisions for post-operative care.
• Observe care provided to patients in a PACU to determine whether patients
are monitored and assessed appropriately prior to transfer or discharge (in the
case of same-day surgery patients) from the PACU.
• Does the hospital have a system for identifying and addressing the monitoring
needs of post-operative patients transferred from the PACU to other areas of
the hospital?
• Ask staff in the PACU and in units who receive patients from the PACU how
the needs of post-operative patients for vigilant monitoring is addressed when
the patients are transferred from the PACU to other areas of the hospital.
A-0958
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
See for example:
9
Institute for Safe Medication Practices (ISMP), Medication Safety Alert- High Alert
Medication Feature Reducing Patient Harm from Opiates. February 22, 2007.
10
Institute for Safe Medication Practices (ISMP), Medication Safety Alert – Fatal PCA Adverse Events
Continue to Happen…Better Patient Monitoring is Essential to Prevent Harm. May 30, 2013
§482.51(b)(5) - The operating room register must be complete and up-to-date.
Interpretive Guidelines §482.51(b)(5)
The register includes at least the following information:
• Patient’s name;
• Patient’s hospital identification number;
• Date of the operation;
• Inclusive or total time of the operation;
• Name of the surgeon and any assistant(s);
• Name of nursing personnel (scrub and circulating);
• Type of anesthesia used and name of person administering it;
• Operation performed;
• Pre and post-op diagnosis; and
• Age of patient.
Survey Procedures §482.51(b)(5)
Examine the OR register or equivalent record which lists all surgery performed by the
surgery service. Determine that the register includes items specified in the interpretive
guidelines.
A-0959
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.51(b)(6) - An operative report describing techniques, findings, and tissues
removed or altered must be written or dictated immediately following surgery and
signed by the surgeon.
Interpretive Guidelines §482.51(b)(6)
The operative report includes at least:
• Name and hospital identification number of the patient;
• Date and times of the surgery;
• Name(s) of the surgeon(s) and assistants or other practitioners who performed
surgical tasks (even when performing those tasks under supervision);
• Pre-operative and post-operative diagnosis;
• Name of the specific surgical procedure(s) performed;
• Type of anesthesia administered;
• Complications, if any;
• A description of techniques, findings, and tissues removed or altered;
• Surgeons or practitioners name(s) and a description of the specific significant
surgical tasks that were conducted by practitioners other than the primary
surgeon/practitioner (significant surgical procedures include: opening and
closing, harvesting grafts, dissecting tissue, removing tissue, implanting devices,
altering tissues); and
• Prosthetic devices, grafts, tissues, transplants, or devices implanted, if any.
Survey Procedures §482.51(b)(6)
Review a minimum of six random medical records of patients who had a surgical
encounter. Verify that they contain a surgical report that is dated and signed by the
responsible surgeon and includes the information specified in the interpretive guidelines.
A-1000
(Rev. 74, Issued: 12-02-11, Effective: 12-02-11, Implementation: 12-02-11)
§482.52 Condition of Participation: Anesthesia Services
If the hospital furnishes anesthesia services, they must be provided in a well-
organized manner under the direction of a qualified doctor of medicine or
osteopathy. The service is responsible for all anesthesia administered in the
hospital.
Interpretive Guidelines §482.52
The provision of anesthesia services is an optional hospital service. However, if a
hospital provides any degree of anesthesia service to its patients, the hospital must
comply with all the requirements of this Condition of Participation (CoP).
“Anesthesia” involves the administration of a medication to produce a blunting or loss of:
• pain perception (analgesia);
• voluntary and involuntary movements;
• autonomic function; and
• memory and/or consciousness,
depending on where along the central neuraxial (brain and spinal cord) the medication is
delivered.
In contrast, “analgesia” involves the use of a medication to provide relief of pain through
the blocking of pain receptors in the peripheral and/or central nervous system. The patient
does not lose consciousness, but does not perceive pain to the extent that may otherwise
prevail.
Anesthesia exists along a continuum. For some medications there is no bright line that
distinguishes when their pharmacological properties bring about the physiologic
transition from the analgesic to the anesthetic effects. Furthermore, each individual
patient may respond differently to different types of medications. The additional
definitions below illustrate distinctions among the various types of “anesthesia services”
that may be offered by a hospital. These definitions are generally based on American
Society of Anesthesiologists definitions found in its most recent set of practice guidelines
(Anesthesiology 2002; 96:1004-17).
• General anesthesia: a drug-induced loss of consciousness during which patients are
not arousable, even by painful stimulation. The ability to independently maintain
ventilatory support is often impaired. Patients often require assistance in maintaining
a patent airway, and positive pressure ventilation may be required because of
depressed spontaneous ventilation or drug-induced depression of neuromuscular
function. Cardiovascular function may be impaired. For example, a patient
undergoing major abdominal surgery involving the removal of a portion or all of an
organ would require general anesthesia in order to tolerate such and extensive
surgical procedure. General anesthesia is used for those procedures when loss of
consciousness is required for the sage and effective delivery of surgical services;
• Regional anesthesia: the delivery of anesthetic medication at a specific level of the
spinal cord and/or to peripheral nerves, including epidurals and spinals and other
central neuraxial nerve blocks, is used when loss of consciousness is not desired but
sufficient analgesia and loss of voluntary and involuntary movement is required.
Given the potential for the conversion and extension of regional to general anesthesia
in certain procedures, it is necessary that the administration of regional and general
anesthesia be delivered or supervised by a practitioner as specified in 42 CFR
482.52(a).
• Monitored anesthesia care (MAC): anesthesia care that includes the monitoring of
the patient by a practitioner who is qualified to administer anesthesia as defined by

the regulations at §482.52(a). Indications for MAC depend on the nature of the
procedure, the patient’s clinical condition, and/or the potential need to convert to a
general or regional anesthetic. Deep sedation/analgesia is included in MAC.
- Deep sedation/analgesia: a drug-induced depression of consciousness during
which patients cannot be easily aroused but respond purposefully following
repeated or painful stimulation. The ability to independently maintain
ventilatory function may be impaired. Patients may require assistance in
maintaining a patent airway, and spontaneous ventilation may be inadequate.
Cardiovascular function is usually maintained. Because of the potential for the
inadvertent progression to general anesthesia in certain procedures, it is
necessary that the administration of deep sedation/analgesia be delivered or
supervised by a practitioner as specified in 42 CFR 482.52(a).
• Moderate sedation/analgesia: (“Conscious Sedation”): a drug-induced depression
of consciousness during which patients respond purposefully to verbal commands,
either alone or accompanied by light tactile stimulation. No interventions are
required to maintain a patent airway, and spontaneous ventilation is adequate.
Cardiovascular function is usually maintained. CMS, consistent with ASA
guidelines, does not define moderate or conscious sedation as anesthesia (71 FR
68690-1).
• Minimal sedation: a drug-induced state during which patients respond normally to
verbal commands. Although cognitive function and coordination may be impaired,
ventilator and cardiovascular functions are unaffected. This is also not anesthesia.
• Topical or local anesthesia; the application or injection of a drug or combination of
drugs to stop or prevent a painful sensation to a circumscribed area of the body where
a painful procedure is to be performed. There are generally no systemic effects of
these medications, which also are not anesthesia, despite the name.
Rescue Capacity: As stated above, because the level of sedation of a patient receiving
anesthesia services is a continuum, it is not always possible to predict how an individual
patient will respond. Further, no clear boundary exists between some of these services.
Hence, hospitals must ensure that procedures are in place to rescue patients whose level
of sedation becomes deeper than initially intended, for example, patients who
inadvertently enter a state of Deep Sedation/Analgesia when Moderate Sedation was
intended. “Rescue” from a deeper level of sedation than intended requires an
intervention by a practitioner with expertise in airway management and advanced life
support. The qualified practitioner corrects adverse physiologic consequences of the
deeper-than-intended level of sedation and returns the patient to the originally intended
level of sedation. (Rescue capacity is not only required as an essential component of
anesthesia services, but is also consistent with the requirements under the Patients’ Rights
standard at §482.13(c)(2), guaranteeing patients care in a safe setting.)
Anesthesia services throughout the hospital (including all departments in all campuses
and off-site locations where anesthesia services are provided) must be organized into one
anesthesia service.
Areas where anesthesia services are furnished may include (but are not limited to):
• Operating room suite(s), both inpatient and outpatient;
• Obstetrical suite(s);
• Radiology department;
• Clinics;
• Emergency department;
• Psychiatry department;
• Outpatient surgery areas;
• Special procedures areas (e.g., endoscopy suite, pain management clinic, etc.)
The anesthesia services must be under the direction of one individual who is a qualified
doctor of medicine (MD) or doctor of osteopathy (DO). Consistent with the requirement
at §482.12(a)(4) for it to approve medical staff bylaws, rules and regulations, the
hospital’s governing body approves, after considering the medical staff’s
recommendations, medical staff rules and regulations establishing criteria for the
qualifications for the director of the anesthesia services. Such criteria must be consistent
with State laws and acceptable standards of practice.
As previously mentioned, there is often no bright line, i.e., no clear boundary, between
anesthesia and analgesia. This is particularly the case with moderate versus deep
sedation, but also with respect to labor epidurals. However, the anesthesia services CoP
establishes certain requirements that apply only when anesthesia is administered.
Consequently, each hospital that provides anesthesia services must establish policies and
procedures, based on nationally recognized guidelines that address whether specific
clinical situations involve anesthesia versus analgesia. (It is important to note that
anesthesia services are usually an integral part of “surgery,” as we have defined that term
in our guidance. Because the surgical services CoP at §482.51 requires provision of
surgical services in accordance with acceptable standards of practice, this provides
additional support for the expectation that anesthesia services policies and procedures
concerning anesthesia are based on nationally recognized guidelines. ) We encourage
hospitals to address whether the sedation typically provided in the emergency department
or procedure rooms involves anesthesia or analgesia. In establishing such policies, the
hospital is expected to take into account the characteristics of the patients served, the skill
set of the clinical staff in providing the services, as well as the characteristics of the
sedation medications used in the various clinical settings.
The regulation at 42 CFR 482.52(a) establishes the qualifications and, where applicable,
supervision requirements for personnel who administer anesthesia. However, hospital
anesthesia services policies and procedures are expected to also address the minimum
qualifications and supervision requirements for each category of practitioner who is
permitted to provide analgesia services, particularly moderate sedation. This expectation
is consistent not only with the requirement under this CoP to provide anesthesia services
in a well-organized manner, but also with various provisions of the Medical Staff CoP at
§482.22 and the Nursing Services CoP at §482.23 related to qualifications of personnel
providing care to patients. Taken together, these regulations require the hospital to assure
that any staff administering drugs for analgesia must be appropriately qualified, and that
the drugs are administered in accordance with accepted standards of practice.
Specifically:
• The Medical Staff CoP at §482.22(c)(6) requires the medical staff bylaws,
“Include criteria for determining the privileges to be granted to individual
practitioners and a procedure for applying the criteria to individuals requesting
privileges.”
• The Nursing Services CoP requires at:
• §482.23(b)(5) that nursing personnel be assigned to provide care based on
“the specialized qualifications and competence of the nursing staff available.”
• §482.23(c) that, “Drugs and biologicals must be prepared and administered in
accordance with Federal and State laws, …and accepted standards of
practice.” And
• §482.23(c)(3) , “… If … intravenous medications are administered by
personnel other than doctors of medicine or osteopathy, the personnel must
have special training for this duty.”
Finally, it is expected that the anesthesia services policies and procedures will undergo
periodic re-evaluation that includes analysis of adverse events, medication errors and
other quality or safety indicators related not only to anesthesia, but also to the
administration of medications in clinical applications that the hospital has determined
involve analgesia rather than anesthesia. This expectation is also supported by the
provisions of the Quality Assessment and Performance Improvement (QAPI) CoP at
§482.21, which requires the hospital to ensure its QAPI program, “…involves all hospital
departments and services…”; “…focuses on indicators related to improved health
outcomes and the prevention and reduction of medical errors....”; “…track[s] quality
indicators, including adverse patient events…”; “… use[s] the data collected to monitor
the effectiveness and safety of the services and quality of care…”; and “…take[s] actions
aimed at performance improvement…”
Hospitals are free to develop their own specific organizational arrangements in order to
deliver all anesthesia services in a well-organized manner. Although not required under
the regulation to do so, a well-organized anesthesia service would develop the hospital’s
anesthesia policies and procedures in collaboration with several other hospital disciplines
(e.g., surgery, pharmacy, nursing, safety experts, material management, etc.) that are
involved in delivering these services to patients in the various areas in the hospital.
A well-organized anesthesia service must be integrated into the hospital’s required
Quality Assessment/Performance Improvement program, in order to assure the provision
of safe care to patients.
Survey Procedures §482.52
• Request a copy of the organizational chart for anesthesia services.
• Determine that a doctor of medicine or osteopathy has the authority and
responsibility for directing all anesthesia services throughout the hospital.
• Look for evidence in the director’s file of the director’s appointment privileges
and qualifications, consistent with the criteria adopted by the hospital’s governing
body. Review the position description. Confirm that the director’s responsibilities
include at least the following:
- Planning, directing, and supervising all activities of the service;
- Evaluating the quality and appropriateness of the anesthesia services provided
to patients as part of the hospital’s QAPI program;
• Request a copy of and review the hospital’s anesthesia services policies and
procedures.
- Do they apply in all hospital locations where anesthesia services are provided?
- Do they indicate the necessary qualifications that each clinical practitioner
must possess in order to administer anesthesia as well as moderate sedation or
other forms of analgesia?
- Do they address what clinical applications are considered to involve analgesia,
in particular moderate sedation, rather than anesthesia, based on identifiable
national guidelines? What are the national guidelines that they are following
and how is that documented?
• Does the hospital have a system by which adverse events related to the
administration of anesthesia and analgesia, including moderate sedation, are
tracked and acted upon?
A-1001

(Rev. 59, Issued: 05-21-10, Effective/Implementation: 05-21-10)
§482.52(a) Standard: Organization and Staffing
The organization of anesthesia services must be appropriate to the scope of the
services offered. Anesthesia must be administered only by --
(1) A qualified anesthesiologist;
(2) A doctor of medicine or osteopathy (other than an anesthesiologist);
(3) A dentist, oral surgeon, or podiatrist who is qualified to administer
anesthesia under State law;
(4) A certified registered nurse anesthetist (CRNA), as defined in §410.69(b) of
this chapter, who, unless exempted in accordance with paragraph (c) of this
section, is under the supervision of the operating practitioner or of an
anesthesiologist who is immediately available if needed; or
(5) An anesthesiologist’s assistant, as defined in Sec. 410.69(b) of this chapter,
who is under the supervision of an anesthesiologist who is immediately
available if needed.
§482.52(c) Standard: State Exemption
(1) A hospital may be exempted from the requirement for MD/DO supervision
of CRNAs as described in paragraph (a)(4) of this section, if the State in
which the hospital is located submits a letter to CMS signed by the
Governor, following consultation with the State’s Boards of Medicine and
Nursing, requesting exemption from MD/DO supervision of CRNAs. The
letter from the Governor must attest that he or she has consulted with State
Boards of Medicine and Nursing about issues related to access to and the
quality of anesthesia services in the State and has concluded that it is in the
best interests of the State’s citizens to opt-out of the current MD/DO
supervision requirement, and that the opt-out is consistent with State law.
(2) The request for exemption and recognition of State laws, and the
withdrawal of the request may be submitted at any time, and are effective
upon submission.
Interpretive Guidelines §482.52(a) and (c)
Who May Administer Anesthesia
Topical/local anesthetics, minimal sedation, moderate sedation
The requirements at §482.52(a) concerning who may administer anesthesia do not apply
to the administration of topical or local anesthetics, minimal sedation, or moderate
sedation. However, the hospital must have policies and procedures, consistent with State
scope of practice law, governing the provision of these types of anesthesia services.
Further, hospitals must assure that all anesthesia services are provided in a safe, well-
organized manner by qualified personnel.
General anesthesia, regional anesthesia and monitored anesthesia, including deep
sedation/analgesia, may only be administered by:
• A qualified anesthesiologist;
• An MD or DO (other than an anesthesiologist);
• A dentist, oral surgeon or podiatrist who is qualified to administer anesthesia
under State law;
• A CRNA who is supervised by the operating practitioner or by an anesthesiologist
who is immediately available if needed; or
• An anesthesiologist’s assistant under the supervision of an anesthesiologist who is
immediately available if needed.
Administration by an MD/DO/dentist/oral surgeon/podiatrist
The hospital’s anesthesia services policies must address the circumstances under which
an MD or DO who is not an anesthesiologist, a dentist, oral surgeon or podiatrist is
permitted to administer anesthesia. In the case of a dentist, oral surgeon or podiatrist,
administration of anesthesia must be permissible under State law and comply with all
State requirements concerning qualifications. Hospitals should conform to generally
accepted standards of anesthesia care when establishing policies governing anesthesia
administration by these types of practitioners as well as MDs or DOs who are not
anesthesiologists.
Administration by a CRNA
Unless the hospital is located in a State that has chosen to opt out of the CRNA
supervision requirements, a CRNA administering general, regional and monitored
anesthesia must be supervised either by the operating practitioner who is performing the
procedure, or by an anesthesiologist who is immediately available.
Hospitals should conform to generally accepted standards of anesthesia care when
establishing policies for supervision by the operating practitioner. An anesthesiologist is
considered “immediately available” when needed by a CRNA under the
anesthesiologist’s supervision only if he/she is physically located within the same area as
the CRNA, e.g., in the same operative/ procedural suite, or in the same labor and delivery
unit, and not otherwise occupied in a way that prevents him/her from immediately
conducting hands-on intervention, if needed.
If the hospital is located in a State where the Governor has submitted a letter to CMS

attesting that he or she has consulted with State Boards of Medicine and Nursing about
issues related to access to and the quality of anesthesia services in the State and has
concluded that it is in the best interests of the State’s citizens to opt-out of the current
physician supervision requirement, and that the opt-out is consistent with State law, then
a hospital may permit a CRNA to administer anesthesia without operating practitioner or
anesthesiologist supervision. (A list of States that have opted out of the CRNA
supervision requirement may be found at
http://www.cms.hhs.gov/CFCsAndCoPs/02_Spotlight.asp)
A CRNA is defined in §410.69(b) as a “registered nurse who:
(1) Is licensed as a registered professional nurse by the State in which the nurse
practices;
(2) Meets any licensure requirements the State imposes with respect to non-
physician anesthetists;
(3) Has graduated from a nurse anesthesia educational program that meets the
standards of the Council on Accreditation of Nurse Anesthesia Programs, or such other
accreditation organization as may be designated by the Secretary; and
(4) Meets the following criteria:
(i) Has passed a certification examination of the Council on Certification of
Nurse Anesthetists, the Council on Recertification of Nurse Anesthetists, or any other
certification organization that may be designated by the Secretary; or
(ii) Is a graduate of a program described in paragraph (3) of this definition
and within 24 months after that graduation meets the requirements of paragraph (4)(i) of
this definition.”
Administration by an Anesthesiologist’s Assistant
An anesthesiologist’s assistant may administer anesthesia when under the supervision of
an anesthesiologist. The anesthesiologist must be immediately available if needed. An
anesthesiologist is considered “immediately available” to assist the anesthesiologist’s
assistant under the anesthesiologist’s supervision only if he/she is physically located
within the same area as the anesthesiologist’s assistant, e.g., in the same
operative/procedural suite, or in the same labor and delivery unit, and not otherwise
occupied in a way that prevents him/her from immediately conducting hands-on
intervention, if needed.
An anesthesiologist’s assistant is defined at §410.69(b) as a “person who-
(1) Works under the direction of an anesthesiologist;
(2) Is in compliance with all applicable requirements of State law, including any
licensure requirements the State imposes on nonphysician anesthetists; and
(3) Is a graduate of a medical school-based anesthesiologist’s assistant education
program that –
(a) Is accredited by the Committee on Allied Health Education and
Accreditation; and
(b) Includes approximately two years of specialized basic science and clinical
education in anesthesia at a level that builds on a premedical undergraduate science
background.”
Anesthesia Services Policies
The medical staff bylaws or rules and regulations must include criteria for determining
the anesthesia service privileges to be granted to an individual practitioner and a
procedure for applying the criteria to individuals requesting privileges, as required by the
regulations at §482. 22(c)(6) for any type of anesthesia services, including those not
subject to the anesthesia administration requirements at §482.52(a). The hospital’s
governing body must approve the specific anesthesia service privileges for each
practitioner who furnishes anesthesia services, addressing the type of supervision, if any,
required. The privileges granted must be in accordance with State law and hospital
policy. The type and complexity of procedures for which the practitioner may administer
anesthesia must be specified in the privileges granted to the individual practitioner.
Deficiencies related to these requirements should be cited under §482. 22(c)(6).
When a hospital permits operating practitioners to supervise a CRNA administering
anesthesia, the medical staff bylaws or rules and regulations must specify for each
category of operating practitioner, the type and complexity of procedures that category of
practitioner may supervise. However, individual operating practitioners do not need to be
granted specific privileges to supervise a CRNA.
Survey Procedures §482.52(a) and (c)
• Review the qualifications of individuals authorized to administer general
anesthesia, regional anesthesia and monitored anesthesia, including deep
sedation/analgesia to determine if they satisfy the requirements at §482.52(a) and
(c).
• Determine that there is documentation of current licensure and, as applicable,
current certification for all persons administering anesthesia.
• Determine if the state is an “opt-out state” and therefore permits CRNAs to
administer anesthesia without supervision in accordance with 482.52(c).
• Review the hospital’s policies and procedures governing supervision of CRNA’s
and anesthesiologist’s assistants, and determine whether they comply with the
regulatory requirements. and
• Review the qualifications of individuals authorized to furnish other anesthesia
services, to determine if they are consistent with the hospital’s anesthesia service
policies.
A-1002
(Rev. 59, Issued: 05-21-10, Effective/Implementation: 05-21-10)
§482.52(b) Standard: Delivery of Services
Anesthesia services must be consistent with needs and resources. Policies on
anesthesia procedures must include the delineation of preanesthesia and
postanesthesia responsibilities. The policies must ensure that the following are
provided for each patient:
Interpretive Guidelines §482.52(b)
Anesthesia services must be delivered in a manner that is consistent with the needs and
the resources of the hospital. Anesthesia policies at a minimum must address:
• How the hospital’s anesthesia services needs will be met;
• Delivery of anesthesia services consistent with recognized standards for
anesthesia care. A well-designed anesthesia services policy would address issues
such as:
o Patient consent;
o Infection control measures;
o Safety practices in all anesthetizing areas;
o Protocol for supportive life functions, e.g., cardiac and respiratory
emergencies;
o Reporting requirements;
o
Documentation requirements;
o Equipment requirements, as well as the monitoring, inspection, testing, and
maintenance of anesthesia equipment in the hospital’s biomedical equipment
program.
o Delineation of pre- and post-anesthesia staff responsibilities
Survey Procedures §482.52(b)
Review the policies developed on anesthesia procedures. Determine whether the
anesthesia service policies for delivery of care address the issues identified in interpretive
guidelines.
A-1003
(Rev. 74, Issued: 12-02-11, Effective: 12-02-11, Implementation: 12-02-11)
[The policies must ensure that the following are provided for each patient:]
§482.52(b) (1) - A pre-anesthesia evaluation completed and documented by an
individual qualified to administer anesthesia, as specified in paragraph (a) of this
section, performed within 48 hours prior to surgery or a procedure requiring
anesthesia services.
Interpretive Guidelines §482.52(b)(1)
A pre-anesthesia evaluation must be performed for each patient who receives general,
regional or monitored anesthesia. While current practice dictates that the patient
receiving moderate sedation be monitored and evaluated before, during, and after the
procedure by trained practitioners, a pre-anesthesia evaluation performed by someone
qualified to administer anesthesia as specified in §482.52(a) is not required because
moderate sedation is not considered to be “anesthesia”, and thus is not subject to that
requirement under this regulation.
The evaluation must be performed by someone qualified to administer anesthesia as
specified in §482.52(a), i.e., only by:
• A qualified anesthesiologist;
• A doctor of medicine or osteopathy (other than an anesthesiologist);
• A dentist, oral surgeon, or podiatrist who is qualified to administer anesthesia
under State law;
• A certified registered nurse anesthetist (CRNA), who, unless exempted in
accordance with paragraph (c) of this section, is under the supervision of the
operating practitioner or of an anesthesiologist who is immediately available if
needed; or
• An anesthesiologist’s assistant who is under the supervision of an anesthesiologist
who is immediately available if needed.
Although §482.12 (c)(1)(i) generally provides broad authority to physicians to delegate
tasks to other qualified medical personnel, the more stringent requirements at
§482.52(b)(1) do not permit delegation of the pre-anesthesia evaluation to practitioners
who are not qualified to administer anesthesia.
The pre-anesthesia evaluation must be completed and documented within 48 hours
immediately prior to any inpatient or outpatient surgery or procedure requiring anesthesia
services. The delivery of the first dose of medication(s) for the purpose of inducing
anesthesia, as defined above, marks the end of the 48 hour time frame.
In accordance with current standards of anesthesia care, some of the individual elements
contributing to the pre-anesthesia evaluation may be performed prior to the 48-hour
timeframe. However, under no circumstances may these elements be performed more
than 30 days prior to surgery or a procedure requiring anesthesia services. Review of
these elements must be conducted, and any appropriate updates documented, within the
48-hour timeframe.
The pre-anesthesia evaluation of the patient includes, at a minimum:
Elements that must be performed within the 48-hour timeframe:
• Review of the medical history, including anesthesia, drug and allergy history; and
• Interview, if possible given the patient’s condition, and examination of the
patient.
Elements that must be reviewed and updated as necessary within 48 hours, but which
may also have been performed during or within 30 days prior to the 48-hour time period,
in preparation for the procedure:
• Notation of anesthesia risk according to established standards of practice (e.g.,
ASA classification of risk);
• Identification of potential anesthesia problems, particularly those that may suggest
potential complications or contraindications to the planned procedure (e.g.,
difficult airway, ongoing infection, limited intravascular access);
• Additional pre-anesthesia data or information, if applicable and as required in
accordance with standard practice prior to administering anesthesia (e.g., stress
tests, additional specialist consultation);
• Development of the plan for the patient’s anesthesia care, including the type of
medications for induction, maintenance and post-operative care and discussion
with the patient (or patient’s representative) of the risks and benefits of the
delivery of anesthesia.
Survey Procedures §482.52(b)(1)
• Review a sample of inpatient and outpatient medical records for patients who had
surgery or a procedure requiring administration of anesthesia.
• Determine whether each patient had a pre-anesthesia evaluation by a practitioner
qualified to administer anesthesia.
• Determine whether each patient’s pre-anesthesia evaluation included at least the
elements described above.
• Determine that the pre-anesthesia evaluation was updated, completed and
documented within 48 hours prior to the delivery of the first dose of
medication(s) given for the purpose of inducing anesthesia for the surgery or a
procedure requiring anesthesia services.
A-1004
(Rev. 59, Issued: 05-21-10, Effective/Implementation: 05-21-10)
[The policies must ensure that the following are provided for each patient:]
§482.52(b)(2) - An intraoperative anesthesia record.
Interpretive Guidelines §482.52(b)(2)
There must be an intraoperative anesthesia record or report for each patient who receives
general, regional or monitored anesthesia. While current practice dictates that the patient
receiving moderate sedation be monitored and evaluated before, during, and after the
procedure by trained practitioners, an intraoperative anesthesia report is not required
because, as explained above , moderate sedation is not “anesthesia”. Current standard of
care stipulates that an intraoperative anesthesia record, at a minimum, includes:
• Name and hospital identification number of the patient;
• Name(s) of practitioner(s) who administered anesthesia, and as applicable, the
name and profession of the supervising anesthesiologist or operating practitioner;
• Name, dosage, route and time of administration of drugs and anesthesia agents;
• Techniques(s) used and patient position(s), including the insertion/use of any
intravascular or airway devices;
• Name and amounts of IV fluids, including blood or blood products if applicable;
• Timed-based documentation of vital signs as well as oxygenation and ventilation
parameters; and
• Any complications, adverse reactions, or problems occurring during anesthesia,
including time and description of symptoms, vital signs, treatments rendered, and
patient’s response to treatment.
Survey Procedures §482.52(b)(2)
Review records to determine that each patient has an intraoperative anesthesia record that
includes the elements described above.
A-1005
(Rev. 74, Issued: 12-02-11, Effective: 12-02-11, Implementation: 12-02-
11)
[The policies must ensure that the following are provided for each patient:]
482.52(b)(3) - A postanesthesia evaluation completed and documented by an
individual qualified to administer anesthesia, as specified in paragraph (a) of this
section, no later than 48 hours after surgery or a procedure requiring anesthesia
services. The postanesthesia evaluation for anesthesia recovery must be completed
in accordance with State law and with hospital policies and procedures that have
been approved by the medical staff and that reflect current standards of anesthesia
care.
Interpretive Guidelines §482.52(b)(3)
A postanesthesia evaluation must be completed and documented no later than 48 hours
after surgery or a procedure requiring anesthesia services. The evaluation is required any
time general, regional, or monitored anesthesia has been administered to the patient.
While current practice dictates that the patient receiving moderate sedation be monitored
and evaluated before, during, and after the procedure by trained practitioners, a
postanesthesia evaluation performed by someone qualified to administer anesthesia as
specified in §482.52(a) is not required under this regulation. (71 FR 68691)
The postanesthesia evaluation must be completed and documented by any practitioner
who is qualified to administer anesthesia; this need not be the same practitioner who
administered the anesthesia to the patient. In accordance with §482.52(a), anesthesia
must be administered only by:
• A qualified anesthesiologist;
• A doctor of medicine or osteopathy (other than an anesthesiologist);
• A dentist, oral surgeon, or podiatrist who is qualified to administer anesthesia
under State law;
• A certified registered nurse anesthetist (CRNA), who, unless exempted in
accordance with paragraph (c) of this section, is under the supervision of the
operating practitioner or of an anesthesiologist who is immediately available if
needed; or
• An anesthesiologist’s assistant who is under the supervision of an anesthesiologist
who is immediately available if needed.
Although §482.12(c)(1)(i) provides broad authority to physicians to delegate tasks to
other qualified medical personnel, the more stringent requirements of §482.52(b)(3) do
not permit delegation of the postanesthesia evaluation to practitioners who are not
qualified to administer anesthesia.
The calculation of the 48-hour timeframe begins at the point the patient is moved into the
designated recovery area. The evaluation generally should not be performed immediately
at the point of movement from the operative area to the designated recovery area. Rather,
accepted standards of anesthesia care indicate that the evaluation should not begin until
the patient is sufficiently recovered from the acute administration of the anesthesia so as
to participate in the evaluation, e.g., answer questions appropriately, perform simple
tasks, etc. While the evaluation should begin in the PACU/ICU or other designated
recovery location, it may be completed after the patient is moved to another inpatient
location or, for same day surgeries, if State law and hospital policy permits, after the
patient is discharged, so long as it is completed within 48 hours. The 48 hour timeframe
for completion and documentation of the postanesthesia evaluation is an outside
parameter. Individual patient risk factors may dictate that the evaluation be completed
and documented sooner than 48 hours. This should be addressed by hospital policies and
procedures (71 FR 68690).
For those patients who are unable to participate in the postanesthesia evaluation (e.g.,
post-operative sedation, mechanical ventilation, etc.), a postanesthesia evaluation should
be completed and documented within 48 hours with notation that the patient was unable
to participate. This documentation should include the reason for the patient’s inability to
participate as well as expectations for recovery time, if applicable. For those patients
who require long-acting regional anesthesia to ensure optimum medical care of the
patient, whose acute effects will last beyond the 48-hour timeframe, a postanesthesia
evaluation must still be completed and documented within 48 hours. However, there
should be a notation that the patient is otherwise able to participate in the evaluation, but
full recovery from regional anesthesia has not occurred and is not expected within the
stipulated timeframe for the completion of the evaluation.
The elements of an adequate post-anesthesia evaluation should be clearly documented
and conform to current standards of anesthesia care, including:
• Respiratory function, including respiratory rate, airway patency, and oxygen
saturation;
• Cardiovascular function, including pulse rate and blood pressure;
• Mental status;
• Temperature;
• Pain;
• Nausea and vomiting; and
• Postoperative hydration.
Depending on the specific surgery or procedure performed, additional types of
monitoring and assessment may be necessary.
Survey Procedures §482.52(b)(3)
• Review a sample of medical records for patients who had surgery or a procedure
requiring general, regional or monitored anesthesia to determine whether a post
anesthesia evaluation was written for each patient.
• Determine whether the evaluation was conducted by a practitioner who is
qualified to administer anesthesia.
• Determine whether the evaluation was completed and documented within 48
hours after the surgery or procedure.
• Determine whether the appropriate elements of a postanesthesia evaluation are
documented in the medical record.
A-1025
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.53 Condition of Participation: Nuclear Medicine Services
If the hospital provides nuclear medicine services, those services must meet the
needs of the patients in accordance with acceptable standards of practice.
Interpretative Guidelines §482.53
This is an optional hospital service. However, if a hospital provides any nuclear
medicine services to its patients, it must comply with the requirements of this Condition
of Participation.
The manner or degree of noncompliance with the requirements of this Condition and its
component standards must be evaluated to determine whether there is substantial
noncompliance with the Condition, warranting a Condition-level citation. However, the
regulatory language concerning provision of nuclear medicine services in a manner that
meets the needs of the patients in accordance with acceptable standards of practice
appears only in the condition “stem” statement of this CoP. This does not mean,
however, that deficiencies related to these requirements must automatically be cited at the
condition level. To facilitate, when appropriate, citation of deficiencies associated with
these requirements at the appropriate level, Tag A-1025 must be used for condition-level
citations, while Tag A-1026 must be used for standard-level citations related to the stem
statement language.
What is Nuclear Medicine and what is it used for?
Nuclear medicine uses radioactive material to diagnose or treat a variety of diseases and
conditions.
Diagnostic Nuclear Medicine
When a diagnostic nuclear medicine study is performed, a patient inhales, swallows, or is
injected with a small amount of a radiopharmaceutical that accumulates in a specific
organ or area of the body. A radiopharmaceutical is a drug that contains a radioactive
component. The energy emitted by the radioactive material is detected by a device,
processed and measured by a computer, and then displayed as an image on a screen or on
film that is then interpreted by a radiologist specially trained in nuclear medicine or
another type of physician with specialized training as a nuclear medicine physician. The
image(s) provide details on both the structure and function of organs and tissues.
For some studies, nuclear medicine techniques can be combined with other medical
imaging devices, such as CT scans or MRIs, in which the same machine can deliver,
detect, and process several types of images at the same time. The technique of
combining various imaging modalities is called hybrid imaging. Hybrid imaging can
provide more precise information and accurate diagnoses and is predominantly used in
the diagnosis and treatment of cancer.
Nuclear medicine diagnostic imaging scans are commonly performed to:
• Visualize heart blood flow and function, e.g., a cardiac stress test or myocardial
perfusion scan; this is the most frequent use of nuclear medicine diagnostic imaging.
• Diagnose blood clots in the lungs (pulmonary emboli) with a ventilation/perfusion
(V/Q) scan;
• Identify areas of infection, inflammation, or cancer metastases with a bone scan;
• Localize lymph nodes prior to surgery;
• Determine gastrointestinal tract muscle function by measuring time for swallowing
and emptying;
• Determine the functioning and perfusion of many other organs, including the thyroid
gland, kidneys, brain, and gall bladder
Therapeutic Nuclear Medicine
Nuclear medicine can also be used to treat various diseases and conditions. For these
types of procedures, a specific radiopharmaceutical agent is used to deliver a specific
amount of radioactivity to a targeted cell type or organ. The energy emitted by the
radioactive agent incapacitates or kills the diseased cells of that targeted tissue, and thus
limits the exposure of healthy tissue to radioactivity.
Examples of therapies that use nuclear medicine include (but are not limited to):
• Radioactive iodine to treat hyperthyroidism (Graves’ disease);
• Radioactive antibodies that target specific forms of lymphoma;
• Radioactive agents to relieve pain in areas of bony metastases.
A-1026
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
Standard-level Tag
§482.53 Condition of Participation: Nuclear Medicine Services
If the hospital provides nuclear medicine services, those services must meet the
needs of the patients in accordance with acceptable standards of practice.
Interpretative Guidelines §482.53
Nuclear medicine services must be provided in accordance with acceptable standards of
practice. Acceptable standards of practice include maintaining compliance with
applicable Federal and State law and regulations governing the use of nuclear medicine,
including facility licensure requirements, as well as standards and recommendations
promoted by nationally recognized professional organizations. Examples of nationally
recognized professional organizations in the area of nuclear medicine include, but are not
limited to, organizations such as the American College of Radiology, the Radiological
Society of North America, the Society of Nuclear Medicine and Molecular Imaging, the
American Society of Nuclear Cardiology, and the American Association of Physicists in
Medicine.
If nuclear medicine services are provided under arrangement, the governing body must,
in accordance with §482.12(e), ensure that the services are provided in a manner that
complies with the requirements of the nuclear medicine CoP.
Minimizing the risks of nuclear medicine
Nuclear medicine studies and procedures provide useful diagnostic information and
targeted therapies for patients. However, since they use radioactive materials that
produce high energy, there are also risks associated with the exposure to radioactivity.
Specifically, the risk involves exposure to ionizing radiation, which is a form of energy
given off by atomic particles that can cause damage to DNA in various living tissues.
The most significant risks include, but are not limited to:
• A small increase in the possibility that a person exposed to ionizing radiation will
develop cancer later in life.
The risk of developing cancer from nuclear medicine radiation exposure is generally
small and depends on at least three factors—the amount of the radiation dose, the age
of the patient or staff member at the time of the exposure, and the sex of the person
exposed:
• The lifetime risk of cancer increases the larger the dose and the greater the number of
studies or treatments involving radioactivity which he/she undergoes;
• The lifetime risk of cancer is larger for a patient who received exams that involve
radioactivity at a younger age, since less mature cells are more radiosensitive; and
• Women are at somewhat higher lifetime risk than men for developing radiation-
associated cancers after receiving the same exposures at the same age.
In order to minimize the risks of ionizing radiation and maximize patient safety during
nuclear medicine studies and procedures, hospitals are expected to apply the fundamental
principle of “As Low as Reasonably Achievable” or “ALARA,” which is defined by the
U.S. Environmental Protection Agency (EPA) as “A principle of radiation protection
philosophy that requires that exposures to ionizing radiation be kept as low as reasonably
achievable, economic and social factors being taken into account. The protection from
radiation exposure is ALARA when the expenditure of further resources would be
unwarranted by the reduction in exposure that would be achieved.” (Federal Guidance
Report No. 14, Radiation Protection Guidance for Diagnostic and Interventional X-ray
Procedures, p. 100, November, 2014)Although CMS does not interpret or enforce EPA
guidance, the ALARA principle is considered an accepted standard of practice for
nuclear medicine that hospitals must adhere to.
Hospitals are expected to be able to demonstrate how they incorporate ALARA into their
nuclear medicine services. They are also expected to have nuclear medicine policies and
procedures that take into consideration classes of patients who may be at higher risk for
over-exposure, as well as the radiation exposure of staff when preparing, storing,
transporting, administering and disposing of radioactive materials.
For Information Only – Not Required/Not to be Cited
Hospitals are encouraged to develop protocols for the use of radiopharmaceuticals
designed to achieve an optimal balance between minimizing the amount of radiation
exposure while maximizing the diagnostic image quality or therapeutic benefit.
The risk of excessive exposure for both patients and staff can be reduced by designing
and implementing nuclear medicine study protocols that:
• Minimize the distance between the source of radiation and its target; and
• Follow published guidelines for administered activity, i.e., the amount of
radiation administered by the radiopharmaceutical.
In addition, the hospital’s nuclear medicine services must be integrated into its hospital-
wide Quality Assessment and Performance Improvement (QAPI) program, as required by
§482.21. Consistent with these requirements, the hospital must monitor the quality and
safety of nuclear medicine services. Examples of nuclear medicine indicators of potential
quality and safety problems could include, but are not limited to:
• Incidents of improper patient preparation, such as inadequate intravenous access or
lack of pre-medication, such that procedures must be cancelled or reordered;
• Incidents of the wrong radiopharmaceutical being used, i.e., not the
radiopharmaceutical prescribed for the patient, or of the wrong dose of the prescribed
radiopharmaceutical being administered, or of use of the wrong route of
administration for the prescribed radiopharmaceutical.
• Repeats of the same diagnostic studies within a short time span, which may be an
indicator of poor image quality; or
• Diagnostic studies or therapeutic procedures performed in a manner inconsistent with
the applicable hospital written protocol.
In addition, the hospital is also required under the QAPI CoP to track medical errors and
adverse events related to nuclear medicine services. Adverse events related to nuclear
medicine services must be analyzed for their causes, and preventive actions must then be
undertaken. Deficiencies identified related to tracking, analyzing and addressing adverse
event and quality indicator data and performance improvement activities must be cited
under the applicable QAPI standards.
Survey Procedures §482.53
• If nuclear medicine services are offered, determine the type(s) of services provided
and the location where each service is provided.
• Ask the director of nuclear medicine services how the hospital ensures that the
services are provided in accordance with acceptable standards of practice.
o Can the director point to accepted guidelines or State or other Federal law that
support the hospital’s nuclear medicine policies and procedures?
o Can the director explain how the hospital’s policies, procedures, and protocols are
consistent with ALARA principles?
• Observe one or more nuclear medicine studies to determine whether the staff follows
the hospital’s protocols for that study. Ask the staff after the observation to show you
the applicable protocol and explain how they complied with it.
A-1027
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.53(a) Standard: Organization and Staffing
The organization of the nuclear medicine service must be appropriate to the scope
and complexity of the services offered.
(1) There must be a director who is a doctor of medicine or osteopathy qualified in
nuclear medicine.
(2) The qualifications, training, functions and responsibilities of the nuclear
medicine personnel must be specified by the service director and approved by the
medical staff.
Interpretive Guidelines §482.53(a)
The scope of nuclear medicine services offered by the hospital, including which types of
diagnostic studies and/or therapeutic procedures are provided, where they are provided in
the hospital, and the appropriately-trained staff and equipment needed to provide these
services must be specified in writing. Hospitals may choose to provide nuclear medicine
services in one location or at several different locations in the hospital, including, but not
limited to, inpatient and outpatient locations of the radiology, cardiology, and oncology
departments. The organization of the nuclear medicine service must encompass the full
scope and complexity of nuclear services offered throughout the hospital.
Nuclear Medicine Director
The hospital is required to have a director responsible for nuclear medicine services
offered throughout the hospital. The director must be a doctor of medicine (MD) or
osteopathy (DO) and must demonstrate through education, experience and specialized
training that he/she is qualified in nuclear medicine. Nuclear medicine physicians utilize
radioactive materials to diagnose and treat disease either by interpreting the images
created by radioisotopes or by prescribing and evaluating therapeutic interventions
involving radiopharmaceuticals. Typically these MDs/DOs initially specialize in
radiology or internal medicine and then complete subspecialty training in nuclear
medicine.
The hospital must describe in writing the qualifications it requires for the director of
nuclear medicine services.
Other Nuclear Medicine Personnel
Although not mentioned specifically in the regulation, there are several different
categories of personnel that may typically be involved in the provision of nuclear
medicine services, including (but not limited to):
• Nuclear medicine pharmacists: these individuals are pharmacists who specialize in
preparing, dispensing, and distributing radiopharmaceuticals;
• Nuclear medicine technologists: these individuals are trained to administer
radioactive materials and perform the specific imaging procedures and often process
the images for interpretation; and
• Nuclear medicine physicists.
The hospital must specify in writing the qualifications, training, functions and
responsibilities of each category of personnel used by the hospital, whether personnel are
employees or contractors, in the delivery of nuclear medicine services. The written
specifications must be developed by the Director and approved by the hospital’s medical
staff. Qualifications include at a minimum, job title, education, experience, specialized
training, and licensure/certification, consistent with any applicable Federal and State law.
The specifications must also address ongoing training for personnel.
Survey Procedures §482.53(a)
• Determine whether the scope of the nuclear medicine services offered is specified in
writing.
• Determine whether there are nuclear medicine policies developed by the director of
nuclear medicine governing provision of these services in every part of the hospital
offering nuclear medicine services.
• Verify that the hospital has a written description of the qualifications of the nuclear
medicine services director.
• Review the service director’s file to verify that he/she is a MD or DO and has the
necessary education, experience and specialized training in nuclear medicine, per the
hospital’s written policies.
• Verify that the qualifications, training, functions and responsibilities of the various
categories of nuclear medicine staff the hospital uses are specified by the director and
approved by the medical staff.
• Review personnel files for a sample of nuclear medicine staff to determine if they
meet the prescribed qualifications and have received ongoing training as required in
the hospital’s policies and procedures.
A-1035
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.53(b) Standard: Delivery of Service
Radioactive materials must be prepared, labeled, used, transported, stored, and
disposed of in accordance with acceptable standards of practice.
§482.53(b)(2) There is proper storage and disposal of radioactive material.
Interpretive Guidelines §482.53(b) and (b) (2)
The hospital must establish, in writing, and implement policies and procedures
addressing the use of radioactive materials within the hospital. The policies and
procedures must be based on acceptable standards of practice for the medical use of
radioactive materials and must address, at a minimum:
• Security of radioactive materials at every stage and location of their use within the
hospital, including determining who may have access to them, implementing
procedures to control access, and a system to track the receipt, usage and disposal of
all radioactive materials;
• Safe storage of radioactive materials, including radioactive waste awaiting disposal
outside the hospital;
• Clear, recognizable labeling of radioactive materials, waste, and hazardous areas in
all locations of the hospital, including during the preparation of such materials, if
applicable;
• Safe and secure transport of radioactive materials between locations within the
hospital;
• Safe handling with the appropriate personal and container protections, as applicable,
by personnel who prepare and administer radiopharmaceuticals within the hospital;
• Protection of patients from radiation hazards, including screening for high-risk
patients (for example, possible pregnancy, multiple nuclear medicine studies,
children, etc.);
• Maintenance and proper use of personal radiation monitoring devices (dosimeters) for
staff working in the vicinity of radiopharmaceuticals, according to manufacturer’s
instructions, particularly regarding the appropriate placement of the dosimeter on the
body, as indicated on the dosimeter;
• Safe and secure disposal of radioactive waste, including unused but unneeded
radioactive materials as well as, when extra precautions are applicable, human waste
products.
Survey Procedures §482.53(b)
• Verify through observation and document review that radioactive materials are
prepared, labeled, used, transported, stored, and disposed of in accordance with
hospital policies that are based on acceptable standards of practice.
• Ask the hospital to demonstrate how it limits access to radioactive materials at all
times.
o Verify that the hospital maintains accurate records of the receipt, distribution, and
disposal of radioactive materials, including radiopharmaceuticals.
o If the hospital prepares radiopharmaceuticals on site, observe the preparation to verify
that proper safety precautions are utilized to protect staff from excess radiation and
once prepared, stored in appropriate containers. If the radiopharmaceuticals are
obtained from an outside source, verify that the receipt and storage are appropriately
tracked.
o Verify that a clear, recognizable label for nuclear material is appropriately displayed
in all relevant areas throughout the hospital and on all radioactive materials.
o Verify that safety precautions are followed in the operations of the nuclear medicine
service and that personnel and patients maintain and wear appropriate body shielding
(e.g., lead aprons, lead gloves, thyroid shields) when appropriate.
Observe a staff member deliver a nuclear medicine procedure to a patient, paying
particular attention to adherence to hospital safety protocols during the delivery of the
radiopharmaceutical.
o Through interview and observation, determine if staff use their dosimeters according
to manufacturer’s instructions, particularly in the appropriate placement of the
dosimeter on the body, as indicated on the dosimeter.

o Ask the responsible staff to demonstrate how they ensure safe transport of radioactive
materials in the hospital.
o Interview the responsible staff to determine whether the appropriate container
protection devices—e.g., lead for gamma emitters—are being utilized for storage and
administration of radioactive materials.
o Ask staff to show the policy for disposal methods for radioactive waste or unused
material and to explain how they ensure that these procedures are followed.
A-1036
(Rev. 137, Issued: 04-01-15, Effective: 03-27-15, Implementation: 03-27-15)
§482.53(b)(1) In-house preparation of radio pharmaceuticals is by, or under the
supervision of, an appropriately trained registered pharmacist or doctor of
medicine or osteopathy.
Interpretive Guidelines §482.53(b)(1)
If the hospital prepares radio pharmaceuticals in-house, such preparation must be
performed by, or supervised by, a registered pharmacist or MD/DO who is qualified
through education, experience and training, in the preparation of radio pharmaceuticals,
consistent with Federal and State law. Hospitals must establish policies and procedures
for in-house preparation of radiopharmaceuticals, including by nuclear medicine
technologists under supervision.
The policies and procedures must identify the qualifications, roles and responsibilities of
staff preparing radiopharmaceuticals under supervision. The hospital must ensure that all
staff who are involved in the preparation and/or supervision of radiopharmaceuticals are
trained and demonstrate competencies in accordance with acceptable standards of
practice.
Hospitals are expected to develop policies and procedures with respect to supervision of
nuclear medicine technologists. CMS anticipates that most hospitals will follow the
supervision recommendations of the Society of Nuclear Medicine and Molecular
Imaging, but there is no mandate that every hospital does so. (79 FR 27120, May 12,
2014) Recommendations are available at:
http://www.snmmi.org/files/docs/NMT%20SOP%20Clinical%20Performance%20Standa
rds%20June%202013.pdf If a hospital is not following this guidance, then it must be
able to explain the basis for the supervision policies and procedures it has developed.
Survey Procedures §482.53(b)(1)
o If radio pharmaceuticals are prepared in-house, determine that the preparation
is performed by, or supervised by, a registered pharmacist or MD/DO.
• Review personnel records of pharmacists, MDs/DOs and nuclear medicine
personnel involved in the preparation and supervision of radio
pharmaceuticals to verify they have required qualifications per State law and
hospital policy.
• Verify that the hospital has policies regarding the supervision of nuclear
medicine personnel and the in-house preparation of radio pharmaceuticals.
• Ask the supervising pharmacist or MD/DO how technicians who prepare radio
pharmaceuticals are supervised. Are the supervision policies based on the
recommendations of the Society of Nuclear Medicine and Molecular
Imaging? If not, what is the basis for the supervision policies?
• Ask what policies/procedures the hospital uses to assure proper preparation.
• Ask what guidelines the hospital relies upon for radio pharmaceutical preparation.
A-1037
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.53(b)(2) - There is proper storage and disposal of radioactive material.
Survey Procedures §482.53(b)(2)
• Verify through observation and document review that radioactive materials,
including radioactive waste, have appropriate storage and disposal.
• Determine how the hospital disposes of unneeded radio nuclides and radio
pharmaceuticals.
o Are these methods in accordance with Federal and State laws, regulations and
guidelines?
o Are the methods described in hospital policy?
A-1038
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.53(b)(3) - If laboratory tests are performed in the nuclear medicine service, the
service must meet the applicable requirement for laboratory services specified in
§482.27.
Interpretive Guidelines §482.53(b)(3)
Any laboratory tests performed in connection with nuclear medicine services must
comply with the hospital Condition of Participation of laboratory services, including a
requirement to comply with 42 CFR Part 493, which establishes the Clinical Laboratory
Improvement Act (CLIA) requirements for laboratories.
Survey Procedures §482.53(b)(3)
The applicable survey procedures for the laboratory services Condition of Participation
must be used.
A-1044
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.53(c) Standard: Facilities
Equipment and supplies must be appropriate for the types of nuclear medicine
services offered and must be maintained for safe and efficient performance. The
equipment must be--
(1) Maintained in safe operating condition; and
(2) Inspected, tested and calibrated at least annually by qualified personnel.
Interpretive Guidelines §482.53(c)
The nuclear medicine service must use equipment and supplies that are designed and,
when applicable, approved to be used in conjunction with radioactive materials.
Equipment must be maintained so that it operates safely to minimize hazards to patients
and hospital personnel as much as possible. Accordingly, it must be inspected, tested,
and calibrated by personnel with the necessary qualifications. Personnel may be either
hospital employees or contracted. Personnel must follow the manufacturer’s quality
assurance instructions for acceptance testing (upon installation and after major upgrades)
and maintenance testing. Inspections, testing and calibration must occur at least annually
unless required to be more frequent according to the manufacturer’s instructions.
The hospital must have a policy and procedure for staff who operate the equipment to
follow if they suspect a malfunction.
Survey Procedures §482.53(c)
• Does the hospital have documentation that indicates that the equipment and supplies
it uses in nuclear medicine services are appropriate for use with radioactive materials?
• Is the hospital able to demonstrate how personnel, whether employees or contractors,
who inspect, test, calibrate, and maintain nuclear medicine services equipment are
qualified to do so?
• Review equipment maintenance records. Verify that equipment is tested, calibrated
and otherwise maintained at least annually, following the manufacturer’s
recommended procedures. Verify that if the manufacturer requires more frequent
than annual testing and maintenance that the hospital adheres to the manufacturer’s
prescribed schedule.
• Ask nuclear medicine services staff who operate equipment what they would do if
they suspected a malfunction. Does the hospital have a policy to address this and are
staff familiar with it?
A-1051
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.53(d) Standard: Records
The hospital must maintain signed and dated reports of nuclear medicine
interpretations, consultations, and procedures.
(1) The hospital must maintain copies of nuclear medicine reports for at least 5
years.
(2) The practitioner approved by the medical staff to interpret diagnostic
procedures must sign and date the interpretation of these tests.
Interpretive Guidelines §482.53(d)(1) & (2)
Nuclear medicine patient records, including interpretations, consultations, and procedures
are patient medical records and the hospital must comply with the Medical Records CoP
(§482.24).
Nuclear medicine patient records, like all patient medical records, must be maintained for
at least five years. If State law requires a longer period, the hospital must comply, but
surveyors do not assess compliance with State law requirements as part of the Federal
survey. Each report of an interpretation of a nuclear medicine diagnostic study must be
signed and dated by the practitioner who made the interpretation, as authorized by the
medical staff. Deficient nuclear medicine medical records practices related to these two
requirements must be cited under this regulation; depending on the specific facts, citation
under the Medical Records CoP might also be appropriate.
Survey Procedures §482.53(d)(1) & (2)
• Verify that copies of nuclear medicine reports are maintained for at least 5 years.
• Verify that reports of nuclear medicine interpretations are signed and dated only by
the practitioner who interpreted the study’s results, as authorized by the medical staff
to perform these interpretations
A-1054
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.53(d)(3) - The hospital must maintain records of the receipt and distribution of
radio-pharmaceuticals.
Interpretive Guidelines §482.53(d)(3)
Hospitals receive their radiopharmaceuticals from manufacturers, either for further in-
house preparation, or ready to use. Regardless of the source of the material, the hospital
must have records that track the movement of the radiopharmaceuticals upon receipt,
throughout the hospital. The records must specify:
• the type of radiopharmaceutical;
• the location in the hospital where it was received, stored and dispensed;
• the amount received or dispensed at each location;
• the staff member receiving or dispensing; and
• when applicable, how/when it is disposed of and by whom. This would also include,
when applicable, the type and amount of any radiopharmaceuticals returned to the
source vendor.
Additional information, including special transport instructions or precautions, may be
included.
The hospital must also have policies and procedures that address how often it reviews
these records and how it reconciles discrepancies between inventory on hand and records
of receipt, distribution, use, disposal and/or return to the source vendor.
Surveyor Procedures §482.53(d)(3)
• Ask the hospital to demonstrate how it maintains accurate records of the receipt and
distribution of radiopharmaceuticals at all locations throughout the hospital.
• Ask what the hospital’s policy is for frequency of review of the records; is there
evidence that the hospital complies with its policy?
• Ask the hospital to explain how it addresses discrepancies in the records.
• What actions does it take to determine whether there are errors in the records
versus unaccounted for loss of materials?
• If applicable, what further actions does it take to locate unaccounted for
radioactive materials?
• If applicable, what further actions does it take to prevent future recordkeeping
errors?
A-1055
(Rev. 141, Issued: 07-10-15, Effective: 07-10-15, Implementation: 07-10-15)
§482.53(d)(4) - Nuclear medicine services must be ordered only by practitioners
whose scope of Federal or State licensure and whose defined staff privileges allow
such referrals.
Interpretive Guidelines §482.53(d)(4)
Nuclear medicine services may only be ordered by practitioners holding privileges that
permit them to do so, consistent with State scope of practice law. However, for
outpatient services, consistent with the provisions of §482.54, the governing body and
medical staff may also authorize practitioners who do not have hospital clinical privileges
to order such studies or procedures, as permitted under State law.
Survey Procedures §482.53(d)(4)
• Verify that nuclear medicine services are ordered only by practitioners who have
privileges to do so or, for outpatient services when authorized consistent with the
provisions of §482.54, by other practitioners authorized to do so by the medical staff,
consistent with Federal and State law.
A-1076
(Rev. 137, Issued: 04-01-15, Effective: 03-27-15, Implementation: 03-27-15)
§482.54 Condition of Participation: Outpatient Services
If the hospital provides outpatient services, the services must meet the needs of the
patients in accordance with acceptable standards of practice.
Interpretive Guidelines §482.54
This is an optional hospital service; however, if a hospital provides any degree of
outpatient care to its patients, the hospital must comply with the requirements of this
Condition of Participation (CoP).
The Medicare Hospital CoP applies to both inpatient and outpatient services of the
hospital. The hospital must be in compliance with the CoP in 42 CFR §482 in all on-
campus and off-campus outpatient service locations.
Tag A-1081 permits standard-level citations for identified deficiencies and provides more
detailed guidance on the overall requirements for outpatient services.
The manner and degree of noncompliance identified in relation to Tags 1077 – 1080 may
result in substantial noncompliance with this CoP, requiring citation at the condition
level.
A-1077
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.54(a) Standard: Organization
Outpatient services must be appropriately organized and integrated with inpatient
services.
Interpretive Guidelines §482.54(a)
The organization of the hospital’s outpatient services must be appropriate to the scope
and complexity of services offered.
The hospital’s outpatient services, at all locations, must be integrated with inpatient
services (e.g., medical records, radiology, laboratory, surgical services, anesthesia
(including pain management) services, other diagnostic services, etc), as appropriate to
the outpatient services offered. The hospital must have written policies in place to assure
the integration of outpatient services, including an established method of communication
between outpatient service departments to corresponding inpatient services.
The hospital must coordinate the care, treatment and services provided to a patient. In
order to provide continuity of care, it should have an established method of
communication between inpatient services and outpatient care in order to provide
continuity of care to its patients.
Survey Procedures §482.53(a)
• Verify that the outpatient services are organized in a manner appropriate to the
scope and complexity of services offered.
• Verify that the hospital has an established method of communication and
established procedures to assure integration with inpatient services to provide
continuity of care.
• Review medical records of outpatients who were later admitted to the hospital in
order to determine that pertinent information from the outpatient record has been
included in the inpatient record.
• Determine that each outpatient service location is integrated with the appropriate
hospital inpatient services in accordance with the needs of the patient care
provided at each of those locations.
A-1079
(Rev. 95, Issued: 12-12-13, Effective: 06-07-13, Implementation: 06-07-13)
§482.54(b) Standard: Personnel
The hospital must --
(1) Assign one or more individuals to be responsible for outpatient services.
(2) Have appropriate professional and nonprofessional personnel available at each
location where outpatient services are offered, based on the scope and complexity of
outpatient services.
Interpretive Guidelines §482.54(b)
The hospital’s outpatient services may be directed by one or more individuals. Hospitals
have the flexibility to determine how best to organize their outpatient services, including
how direction will be provided. As services offered in outpatient departments become
more varied, complex and technologically advanced, hospitals may find it better to have
individuals with more specialized expertise providing direction for a specific type of
outpatient services.
Hospitals should define in writing the qualifications and competencies necessary for their
outpatient services department leader(s). These qualifications should include items such
as education, experience, and specialized training consistent with State law and
acceptable standards of practice.
The hospital should define in writing the qualifications and competencies necessary to
direct each outpatient service for which there is a separate director. Qualifications
include necessary education, experience and specialized training consistent with State
law and acceptable standards of practice.
Adequate types and numbers of qualified licensed healthcare professionals and other
personnel must be available to provide patients with the appropriate level of care for the
outpatient services offered by the hospital. The types and numbers of qualified personnel
required for area of the hospital’s main campus or for each provider-based off-site
location must be based on the scope and complexity of the outpatient services offered and
the number and types of patients treated as outpatients at each.
Survey Procedures 482.54(b)
• Ask the hospital how it has organized its outpatient services and to identify the
individual(s) responsible for providing direction for outpatient services.
• Review the organization’s policies and procedures to determine the person’s
responsibility.
• Review the position description and personnel file of the individual(s) responsible
for a selection of outpatient services to ensure that they are qualified, in
accordance with State law, acceptable standards of practice and hospital policy to
direct the service for which they are responsible.
• Visit several on- and off-campus locations where hospital outpatient services are
provided. Given the scope and complexity of the services being offered, are there
sufficient personnel with the appropriate education, experience, certifications,
current licensure where appropriate, and competencies for assigned
responsibilities?
A-1080
(Rev. 137, Issued: 04-01-15, Effective: 03-27-15, Implementation: 03-27-15)
§482.54(c) Standard: Orders for Outpatient Services
Outpatient services must be ordered by a practitioner who meets the
following conditions:
(1) Is responsible for the care of the patient.
(2) Is licensed in the State where he or she provides care to the patient.
(3) Is acting within his or her scope of practice under State law.
(4) Is authorized in accordance with State law and policies adopted by the medical
staff, and approved by the governing body, to order the applicable outpatient
services.
This applies to the following:
(i) All practitioners who are appointed to the hospital’s medical staff and
who have been granted privileges to order the applicable outpatient services.
(ii) All practitioners not appointed to the medical staff, but who satisfy the
above criteria for authorization by the medical staff and the hospital for ordering
the applicable outpatient services for their patients.
Interpretive Guidelines §482.54(c)
Orders for outpatient services may be made by any practitioner who is:
• Responsible for the care of the patient;
• Licensed in, or holds a license recognized in, the jurisdiction where he/she
provides care to the patient;
• Acting within his/her scope of practice under State law; and
• Authorized by the medical staff to order the applicable outpatient services
under a written hospital policy that is approved by the governing body.
This includes both practitioners who are on the hospital medical staff and
who hold medical staff privileges that include ordering the services, as
well as other practitioners who are not on the hospital medical staff, but
who satisfy the hospital’s policies for ordering applicable outpatient
services.
This regulation allows hospitals to accept orders for outpatient services both from
practitioners who hold hospital privileges as well as practitioners who do not, including
those who are not located in the hospital’s close geographic area.
It is not uncommon for individuals to obtain health care services in a variety of locations
from a variety of practitioners. Sometimes an individual elects to seek services from a
specialist in a tertiary setting removed from the area where the individual lives, but
prefers to get follow-up care, such as physical therapy after a surgery, closer to home.
Sometimes an individual may have multiple residences in different areas and may need to
continue care locally when moving between residences. Sometimes individuals receive
urgent or even emergent care while traveling. Accepting orders and referrals for
outpatient services from practitioners not on the medical staff or not holding privileges
enables a hospital to promotes ready access to care for patients in the area it serves.
Finally, sometimes a practitioner who does not practice in a local hospital may
nevertheless refer patients to that hospital for outpatient services, such as diagnostic
imaging, physical and occupational therapy, etc.
The authority to write orders for outpatient services is covered under the hospital’s
medical staff privileging process for members of the hospital’s medical staff and for
practitioners who have been granted privileges by the hospital without being appointment
to the medical staff.
For practitioners who do not hold hospital privileges the hospital’s medical staff policy
may permit them to refer patients to the hospital with orders for specific outpatient
services so long as all of the above criteria are met. The policy must address how the
hospital verifies the referring/ordering practitioner is appropriately licensed and acting
within his/her scope of practice. The regulation does not prescribe the details of the
licensure and scope of practice verification process but instead provides a hospital the
flexibility to accomplish this in the manner it finds efficient and effective. The hospital is
expected to ensure the verification process is followed for all outpatient services in all
hospital locations.
The policy must also make clear whether the policy applies to all hospital outpatient
services, or whether there are specific services for which orders may only be accepted
from practitioners with medical staff privileges. For example, a hospital may prefer not
to accept orders for a regimen of outpatient chemotherapy or outpatient therapeutic
nuclear medicine services from a referring physician who does not hold medical staff
privileges. In such cases, the hospital’s policy must make these exceptions clear to the
general authorization for accepting orders from referring practitioners.
Survey Procedures §482.54(c)
Survey a variety of settings that offer outpatient services. Ask department
staff whether orders or referrals for that type of outpatient service are
accepted from practitioners who do not hold hospital privileges. If yes:
•
Ask for evidence that the medical staff has adopted the policy.
• Ask how the hospital verifies that the order or referral comes from a
referring practitioner who is appropriately licensed in the jurisdiction
where he/she provides care to the patient and is practicing within
his/her scope of practice under State law to prescribe such orders. Ask
for documentation of such verification efforts.
• Ensure the same verification process is followed consistently in all outpatient
settings.
A-1081
(Rev. 137, Issued: 04-01-15, Effective: 03-27-15, Implementation: 03-27-15)
Standard-level Tag for
§482.54 Condition of Participation: Outpatient Services
If the hospital provides outpatient services, the services must meet the needs of the
patients in accordance with acceptable standards of practice.
Interpretive Guidelines §482.54
This is an optional hospital service; however, if a hospital provides any degree of
outpatient care to its patients, the hospital must comply with the requirements of this
Condition of Participation (CoP).
The Medicare Hospital CoP apply to both inpatient and outpatient services of the
hospital. The hospital must be in compliance with the CoP in 42 CFR §482 in all on-
campus and off-campus outpatient service locations.
All outpatient services provided by the hospital, both on campus and at any provider-
based clinics, must meet the needs of the patients, in accordance with acceptable
standards of practice. The hospital must ensure that services, equipment, staff, and
facilities are adequate to provide the outpatient services offered at each location in
accordance with acceptable standards of practice.
Acceptable standards of practice include standards that are set forth in Federal or State
laws, regulations or guidelines, as well as standards and recommendations promoted by
nationally recognized professional organizations (e.g., the American Medical
Association, American College of Radiology, American College of Surgeons, etc.).
If the hospital offers outpatient surgical services, the Surgical Services CoP (§482.5)
requires that the offered services must be consistent in quality with inpatient care in
accordance with the services offered.
The hospital’s outpatient services must be integrated into its hospital-wide QAPI
program.
Survey Procedures §482.54
• Verify that equipment, staff and facilities are adequate to provide the
outpatient services offered at each location are in accordance with acceptable
standards of practice.
• Verify that outpatient services at all locations are in compliance with the
hospital CoP.
• Determine locations and type(s) of outpatient services provided.
• Verify that the hospital’s outpatient services are integrated into its hospital-
wide QAPI program.
A-1100
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.55 Condition of Participation: Emergency Services
The hospital must meet the emergency needs of patients in accordance with
acceptable standards of practice.
A-1101
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.55(a) Standard: Organization and Direction. If emergency
services are provided at the hospital --
Interpretive Guidelines §482.55(a):
If emergency services are provided at the hospital, the hospital must ensure that specific
emergency services organization and direction requirements are met.
A-1102
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.55(a) - [If emergency services are provided at the hospital --]
(1) The services must be organized under the direction of a qualified member
of the medical staff;
Interpretive Guidelines §482.55(a)(1)
The hospital’s emergency services must be under the direction of a qualified member of
the hospital’s medical staff. The hospital’s medical staff establishes criteria for the
qualifications for the director of the hospital’s emergency services in accordance with
State law and acceptable standards of practice. A single emergency services director
must be responsible for the hospital’s emergency services.
Survey Procedures §482.55(a)(1)
Verify that emergency services are organized under the direction of a qualified member
of the medical staff.
A-1103
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.55(a) - [If emergency services are provided at the hospital --]
(2) The services must be integrated with other departments of the hospital;
Interpretive Guidelines §482.55(a)(2)
The hospital’s emergency service/department must be integrated with the other
departments of the hospital such as surgical services, lab, ICU, diagnostic services, etc.
The hospital must demonstrate that its emergency services are truly integrated into its
other departments. The integration must be such that the hospital can immediately make
available the full extent of its patient care resources to assess and render appropriate care
for an emergency patient.
Emergency Services integration would include at a minimum:
• Coordination and communication between the Emergency Department and other
hospital services/departments;
• Physical access for emergency department patients to the services, equipment,
personnel, and resources of other hospital departments/services;
• The immediate availability of services, equipment, personnel, and resources of
other hospital departments/services to emergency patients; and
• That the provision of services, equipment, personnel and resources of other

hospital departments/services to emergency department patients is within
timeframes that protect the health and safety of patients and is within acceptable
standards of practice, including:
o The length of time it takes to transport the emergency patient from the ED to
another hospital department where needed interventions or diagnostic services
will be rendered.
o The length of time it takes to deliver equipment or supplies, or for the staff
from other departments to travel from their location to the emergency
department in order to provide needed interventions, tests, care, or services.
Time is critical in the provision of emergency care. The hospital must be able to
demonstrate how the hospital’s other departments provide emergency patients the care
and services needed within safe and appropriate times.
In emergency care situations, the time needed to provide the patient with appropriate
diagnostic and care interventions can have a significant effect on the patient. Delays in
diagnosis and the provision of needed interventions is likely to adversely affect the health
and safety of patients who require emergency care. Therefore, a hospital that cannot
demonstrate integration of its emergency services with its other departments (including
radiological services, OR, intensive care, laboratory, etc.) would not be in compliance
with the Emergency Services CoP.
Many hospitals offer urgent care services on the hospital campus or in provider-based
clinics in the communities they serve. Those clinics must be in compliance with the
hospital CoP. Hospitals may organize their urgent care clinics as part of their outpatient
department or emergency services department. An urgent care clinic that:
• The hospital holds out to the public as providing only urgent care services and
possibly other services;
• Clearly advises the public that the urgent care clinic is not an emergency services
department; and
• Does not meet the EMTALA definition of dedicated emergency department;
would be evaluated for compliance with the integration requirement in the Outpatient
Services CoP (§482.54(a)) rather than the integration requirement in the Emergency
Services CoP. In most urgent care situations, the time, qualified personnel, equipment,
and other resources needed to provide the patient with appropriate diagnostic and care
interventions are less than needed in emergency situations.
Survey Procedures §482.55(a)(2)
Verify that there are established procedures to assure integration with either hospital
services including laboratory, radiology, and operating services to provide continuity of
care.
A-1104
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.55(a) - [If emergency services are provided at the hospital --]
(3) The policies and procedures governing medical care provided in the
emergency service or department are established by and are a continuing
responsibility of the medical staff.
Interpretive Guidelines §482.55(a)(3)
The hospital’s medical staff must establish policies and procedures governing the medical
care provided in the emergency service or emergency department. The medical staff
must have had ongoing/continuing assessment of the medical care provided in the
emergency service or department. Emergency service or emergency department policies
must be current and revised as necessary based on the ongoing monitoring conducted by
the medical staff and the emergency service or department QAPI activities.
Survey Procedures §482.55(a)(3)
Verify that procedures and policies for emergency medical services (including triage of
patients) are established, evaluated, and updated on an ongoing basis.
A-1110
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.55(b) Standard: Personnel
Interpretive Guidelines §482.55(b):
The hospital must ensure the emergency services personnel requirements are met.
A-1111
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.55(b)(1) - The emergency services must be supervised by a qualified member of
the medical staff.
Interpretive Guidelines §482.55(b)(1)
A qualified member of the medical staff must supervise the provision of emergency
services. Since §482.55(a)(1) requires that emergency services must be organized under
the direction of a qualified member of the medical staff, the requirement for supervision
at§482.55(b)(1) must be distinguished from the prior requirement. In this context,
“supervision” implies a more immediate form of oversight by a qualified member of the
medical staff during all times the hospital makes emergency services available. A
supervisor may be briefly absent from the emergency department, but is expected to be in
the hospital and immediately available to provide direction and/or direct care during the
operating hours of the emergency department.
The medical staff must establish criteria, in accordance with State law, regulations, and
guidelines, delineating the qualifications a medical staff member must possess in order to
be granted privileges for the supervision of the provision of emergency care services.
Qualifications include necessary education, experience and specialized training,
consistent with State law and acceptable standards of practice.
Survey Procedures §482.55(b)(1)
Verify that a qualified member of the medical staff is designated to supervise emergency
services.
A-1112
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.55(b)(2) - There must be adequate medical and nursing personnel qualified in
emergency care to meet the written emergency procedures and needs anticipated by
the facility.
Interpretive Guidelines §482.55(b)(2)
The hospital must staff the emergency department with the appropriate numbers and
types of professionals and other staff who possess the skills, education, certifications,
specialized training and experience in emergency care to meet the written emergency
procedures and needs anticipated by the facility.
The hospital must determine the categories and numbers of MD/DOs, specialists, RNs,
EMTs, and emergency department support staff the hospital needs to met its anticipated
emergency needs.
The medical staff must establish criteria, in accordance with State law and regulations
and acceptable standards of practice delineating the qualifications required for each
category of emergency services staff (e.g., emergency physicians, specialist MD/DO,
RNs, EMTs, mid-level practitioners, etc.).
As a suggested prudent practice the hospital should conduct periodic assessments of its
emergency needs in order to anticipate the policies, procedures, staffing, training, and
other resources that may be needed to address likely demands.
Additionally, the hospital should work cooperatively with Federal, State and local
emergency preparedness agencies and officials in order to identify likely risks to the
community (e.g., natural disasters, mass casualties, terrorist acts, etc.), to anticipate
demands and resources needed by the hospital emergency services, and to develop plans,
methods and coordinating networks to address those anticipated needs.
Survey Procedures §482.55(b)(2)
• Verify that there are sufficient medical and nursing personnel qualified in the
needs anticipated by the facility and that there are specific assigned duties for
emergency care personnel and a clear chain of command.
• Interview staff to determine that they are knowledgeable, within their own level
of participation in emergency care including:
o Parenteral administration of electrolytes, fluids, blood and blood components;
o Care and management of injuries to extremities and central nervous system;
o Prevention of contamination and cross infection.
A-1123
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.56 Condition of Participation: Rehabilitation Services
If the hospital provides rehabilitation, physical therapy, occupational therapy,
audiology, or speech pathology services, the services must be organized and staffed
to ensure the health and safety of patients.
Interpretive Guidelines §482.56
This is an optional hospital service. However, if a hospital provides any degree of
rehabilitative services to its patients, the hospital must comply with the requirements of
the Condition of Participation.
If rehabilitative services are provided, they must be organized and staffed in such a
manner to ensure the health and safety of patients. This includes providing rehabilitative
services in accordance with practitioner orders and acceptable standards of practice.
Acceptable standards of practice include compliance with any applicable Federal or State
laws, regulations or guidelines, as well as standards and recommendations promoted by
nationally recognized professional organizations (e.g., American Physical Therapy
Association, American Speech and Hearing Association, American Occupational
Therapy Association, American College of Physicians, American Medical Association,
etc.).
The hospital’s rehabilitation services must be integrated into its hospital-wide QAPI
program.
Survey Procedures §482.56
• Determine if the hospital provides any degree of rehabilitation services.
• Determine if the hospital’s rehabilitation services are integrated into its hospital-
wide QAPI program.
A-1124
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.56(a) Standard: Organization and Staffing
The organization of the service must be appropriate to the scope of the services
offered.
Interpretive Guidelines §482.56(a)
The hospital must provide the appropriate equipment and types and numbers of qualified
personnel necessary to furnish the rehabilitation services offered by the hospital in
accordance with acceptable standards of practice.
The scope of rehabilitation services offered by the hospital should be defined in written
policies and procedures, and approved by the Medical staff.
Each service, whether provided through a single discipline department or within a multi-
discipline department, must function with established lines of authority and responsibility
to ensure the health and safety of patients. There must be an adequate number of
qualified staff available when needed to evaluate each patient, initiate the plan of
treatment, and supervise supportive personnel when they furnish rehabilitation services.
The number of qualified staff is based on the type of patients treated and the frequency,
duration, and complexity of the treatment required.
Survey Procedures §482.56(a)
• Review the hospital’s policies and procedures to verify that the scope of
rehabilitation services offered is defined in writing.
• If services are provided under an arrangement, review policies and contracts.
• For each service, determine that adequate types and numbers of qualified staff are
available to ensure safe and efficient provision of treatment.
• Review medical records to verify that a qualified professional evaluates the
patient and initiates each treatment episode.
• Review a sample of personnel files to verify current licensure, certifications and
ongoing training, consistent with applicable State laws.
A-1125
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.56(a)(1) - The director of the services must have the necessary knowledge,
experience, and capabilities to properly supervise and administer the services.
Interpretive Guidelines §482.56(a)(1)
Each service must be accountable to an individual that directs the overall hospital-wide
operation of that service. An individual may serve as the director of a multi-service
department or as director of single service departments.
The service director must demonstrate through education, experience, and/or specialized
training that he/she has the necessary knowledge, experience and capabilities to properly
supervise and administer the service(s).
The director may be part-time or full time. In all situations the director retains
professional and administrative responsibility for personnel providing the service. If the
director is part-time, the time spent directing the service should be appropriate to the
scope of the services provided.
Survey Procedures §482.56(a)(1)
• Verify that each service is accountable to an individual who directs the overall
operation of that service.
• Review the service director’s position description to verify that he/she has been
granted the authority and responsibility for operation of the service, consistent
with hospital policies, State law, and accepted standards of practice.
• If the director does not work full-time, determine that the number of hours
(review timesheets) spent working is appropriate to the scope of services
provided.
• Review the director’s personnel file to determine that he/she has the necessary
education, experience and specialized training to properly supervise and
administer the service. This includes maintaining current licensure and
certifications as required by State law.
• Interview the director to determine if he/she has the necessary knowledge,
experience and capabilities to properly supervise and administer the service.
A-1126
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.56(a)(2) Physical therapy, occupational therapy, or speech-language pathology
or audiology services, if provided, must be provided by qualified physical therapists,
physical therapist assistants, occupational therapists, occupational therapy
assistants, speech-language pathologists, or audiologists as defined in part 484 of
this chapter.
A-1132
(Rev. 137, Issued: 04-01-15, Effective: 03-27-15, Implementation: 03-27-15)
§482.56(b) Standard: Delivery of Services
Services must only be provided under the orders of a qualified and licensed
practitioner who is responsible for the care of the patient, acting within his or her
scope of practice under State law, and who is authorized by the hospital’s medical
staff to order the services in accordance with hospital policies and procedures and
State laws.
Interpretive Guidelines §482.56(b)
Rehabilitation services must be ordered by a qualified and licensed practitioner who is
responsible for the care of the patient. The practitioner must have medical staff
privileges to write orders for these services or, for outpatient services, if hospital policy
permits acceptance of orders from outside practitioners, the practitioner’s order must
meet the requirements at §482.54(c).
For practitioners who have medical staff privileges, such privileges must be granted in a
manner consistent with the State’s scope of practice law, as well as with hospital policies
and procedures governing rehabilitation services developed by the medical staff.
Practitioners who may be granted privileges to order rehabilitation services include
physicians, and may also, in accordance with hospital policy, include Nurse Practitioners,
Physicians’ Assistants, and Clinical Nurse Specialists as long as they meet the parameters
of this requirement. Although the following licensed professionals are also considered
“practitioners” in accordance with Section 1842(b)(18)(C) of the Social Security Act,
they generally would not be considered responsible for the care of the patient with regard
to rehabilitation services or qualified to order rehabilitation services: Certified registered
nurse anesthetist (Section 1861(bb)(2) of the Act); Certified nurse-midwife (Section
1861(gg)(2) of the Act); Clinical social worker (Section 1861(hh)(1) of the Act); Clinical
psychologist (for purposes of Section 1861(ii) of the Act and as defined at 42 CFR
410.71); or registered dietician or nutrition professional.
Survey Procedures §482.56(b)
• Review medical records of patients receiving rehabilitation services. Determine
who wrote the orders for the rehabilitation services. Determine if the practitioner
is responsible for the care of the patient and privileged to write orders for
rehabilitation services. Verify the practitioner meets hospital medical staff policy
criteria to order services as well as State law for ordering rehabilitation services.
Does the hospital permit acceptance of orders from outside practitioners who do not
practice at the hospital? If so, evaluate for compliance with §482.54(c).
A-1133
(Rev. 72, Issued: 11-18-11, Effective: 11-18-11, Implementation: 11-18-11)
§482.56(b)(1) All rehabilitation services orders must be documented in the patient’s
medical record in accordance with the requirements at §482.24.
Interpretive Guidelines §482.56(b)(1)
The patient’s medical record must contain documentation of all rehabilitation services
ordered. The medical record entries must comply with regulations at §482.24.
Survey Procedures §482.56(b)(1)
Review a sample of patient medical records who received rehabilitation services.
Determine whether the rehabilitation service orders are legible, complete, dated, timed,
authenticated, and meet all other medical record requirements specified at §482.24.
A-1134
§482.56(b)(2) The provision of care and the personnel qualifications must be in
accordance with national acceptable standards of practice and must also meet the
requirements of §409.17 of this chapter.
Interpretive Guidelines §482.56(b)(2)
The provision of rehabilitation services care and development of the plan of care for
rehabilitation services can be initiated only after the order is written for services by a
qualified licensed practitioner responsible for the care of the patient. Physical therapy,
occupational therapy, or speech-language pathology must be furnished under a plan of
care. The regulation at 42 CFR 409.17 specifies the following rehabilitation services
plan of care requirements:
Establishment of the plan: “The plan must be established before treatment begins by one
of the following: (1) A physician. (2) A nurse practitioner, a clinical nurse specialist or a
physician assistant. (3) The physical therapist furnishing the physical therapy services.
(4) A speech-language pathologist furnishing the speech-language pathology services.
(5) An occupational therapist furnishing the occupational therapy services.”
Content of the plan: “The plan: (1) Prescribes the type, amount, frequency, and duration
of the physical therapy, occupational therapy, or speech-language pathology services to
be furnished to the individual; and (2) Indicates the diagnosis and anticipated goals.”
Changes in the plan: “Any changes in the plan are implemented in accordance with
hospital policies and procedures.”
Also in accordance with 42 CFR 409.17, rehabilitation services must be provided by
qualified physical therapists, physical therapy assistants, occupational therapists,
occupational therapy assistants, and/or speech-language pathologists who meet the
personnel qualifications defined in 42 CFR 484.4. Hospitals must have policies and
procedures consistent with State law.
Rehabilitation services must be provided according to national standards of practice as
established by professional organizations such as, but not limited to, the American
Physical Therapy Association, the American Occupational Therapy Association, and the
American Speech-Language-Hearing Association.
Survey Procedures §482.56(b)(2)
Review medical records of patients who received rehabilitation services. Determine
whether the required care plan was developed and implemented.
Review employee personnel files to verify the rehabilitation service providers (i.e.,
physical therapists, physical therapy assistants, occupational therapists, occupational
therapy assistants, and/or speech-language pathologists) have the necessary education,
experience, training, and documented competencies to provide rehabilitation services.
Ask the hospital what national standards of rehabilitation practice provide the basis for its
rehabilitation services. Is there supporting documentation?
A-1151
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.57 Condition of Participation: Respiratory Services
The hospital must meet the needs of the patients in accordance with acceptable
standards of practice. The following requirements apply if the hospital provides
respiratory care services.
Interpretive Guidelines §482.57
If a hospital provides care to patients who require respiratory care services, the hospital
must meet the needs of those patients, in accordance with acceptable standards of
practice. This is an optional hospital service. However, if a hospital provides any degree
of respiratory care to its patients, the hospital must comply with the requirements of this
Condition of Participation.
Acceptable standards of practice include compliance with applicable standards that are
set forth in Federal or State laws, regulations or guidelines, as well as standards and
recommendations promoted by nationally recognized professional organizations (e.g.,
American Medical Association, American Association for Respiratory Care, American
Thoracic Association, etc.).
The hospital’s respiratory services must be integrated into its hospital-wide QAPI
program.
Survey Procedures §482.57
• Determine if the hospital provides any degree of respiratory care services.
• Determine that the type and amount of respiratory care provided meets the needs
of the patients and is delivered in accordance with acceptable standards of
practice.
• Determine if the hospital’s respiratory services are integrated into its hospital-
wide QAPI program.
A-1152
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.57(a) Standard: Organization and Staffing
The organization of the respiratory care services must be appropriate to the scope
and complexity of the services offered.
Interpretive Guidelines §482.57(a)
The hospital must provide the appropriate equipment and types and numbers of qualified
personnel necessary to furnish the services offered by the hospital in accordance with
acceptable standards of practice.
The scope of diagnostic and/or therapeutic respiratory services offered by the hospital
should be defined in writing, and approved by the Medical staff.
Survey Procedures §482.57(a)
• Review the hospital’s organizational chart to determine the relationship of
respiratory care services to other services provided by the hospital.
• Review the hospital policies and procedures to verify that the scope of the
diagnostic and/or therapeutic respiratory care services provided is defined in
writing.
A-1153
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.57(a)(1) - There must be a director of respiratory care services who is a doctor
of medicine or osteopathy with the knowledge, experience and capabilities to
supervise and administer the service properly. The director may serve on either a
full-time or part-time basis.
Interpretive Guidelines §482.57(a)(1)
The service director must be a doctor of medicine or osteopathy and must demonstrate
through education, experience and specialized training that he/she has the qualifications
necessary to supervise and administer the service properly, appropriate to the scope and
complexity of services offered.
If the director serves on a part-time basis, the time spent directing the department should
be appropriate to the scope and complexity of services provided.
Survey Procedures §482.57(a)(1)
• Verify that a director has been appointed and that he/she has fixed lines of
authority and delegated responsibility for operation of the service.
• Interview staff regarding the role and oversight activities conducted by the
director.
• Review the service director’s credentialing file to determine that he/she is a M.D.
or D.O. and has the necessary education, experience and specialized training to
supervise and administer the service properly.
A-1154
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.57(a)(2) - There must be adequate numbers of respiratory therapists, respiratory
therapy technicians, and other personnel who meet the qualifications specified by the
medical staff, consistent with State law.
Interpretive Guidelines §482.57(a)(2)
There must be sufficient personnel available to respond to the respiratory care needs of
the patient population being served.
Survey Procedures §482.57(a)(2)
• Interview respiratory care staff regarding: services provided, schedules, and
availability of respiratory care staff throughout the day and week to determine that
the number and type of staff available is appropriate to the volume and types of
treatments furnished. If needed, review staffing and on-call schedules.
• Review a sample of personnel files for respiratory care staff to determine that the
personnel meet the qualifications specified by the medical staff, consistent with
State law.
A-1160
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.57(b) Standard: Delivery of Services
Services must be delivered in accordance with medical staff directives.
Interpretive Guidelines §482.57(b)
There should be written policies for the delivery of respiratory care services that are
developed and approved by the medical staff. Appropriate to the scope of services
provided, the written policies should address at least the following:
• Equipment assembly, operation, and preventive maintenance;
• Safety practices, including infection control measures for equipment, sterile
supplies, biohazardous waste, posting of signs, and gas line identification;
• Handling, storage, and dispensing of therapeutic gases to both inpatients and
outpatients;
• Cardiopulmonary resuscitation;
• Procedures to follow in the advent of adverse reactions to treatments or
interventions;
• Pulmonary function testing;
• Therapeutic percussion and vibration;
• Bronchopulmonary drainage;
• Mechanical ventilatory and oxygenation support;
• Aerosol, humidification, and therapeutic gas administration;
• Storage, access, control, administration of medications and medication errors; and
• Procedures for obtaining and analyzing blood samples (e.g., arterial blood gases).
A-1161
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.57(b)(1) - Personnel qualified to perform specific procedures and the amount
of supervision required for personnel to carry out specific procedures must be
designated in writing.
Interpretive Guidelines §482.57(b)(1)
The hospital must have written policies to address, at a minimum:
• Each type of respiratory care service provided by the hospital;
• The qualifications, including job title, licensure consistent with State law,
education, training and experience of personnel authorized to perform each type
of respiratory care service and whether they may perform it without supervision;
and
• The type of personnel qualified to provide the direct supervision.
Survey Procedures §482.57(b)(1)
• Review treatment logs, job descriptions of respiratory care staff, and policies and
procedures to determine the following:
o Duties and responsibilities of staff;
o Qualifications and education required, including licensure, consistent with
State law;
o Specialized training or experience needed to perform specific duties.
A-1162
(Rev. 37, Issued: 10-17-08; Effective/Implementation Date: 10-17-08)
§482.57(b)(2) - If blood gases or other clinical laboratory tests are performed in the
respiratory care unit, the unit must meet the applicable requirements for laboratory
services specified in §482.27.
Interpretive Guidelines §482.57(b)(2)
Refer to the guidelines under §482.27 for independent laboratory if blood gases and
laboratory tests are performed in the respiratory care unit.
A-1163
(Rev. 137, Issued: 04-01-15, Effective: 03-27-15, Implementation: 03-27-15)
§482.57(b)(3) - Services must only be provided under the orders of a qualified and
licensed practitioner who is responsible for the care of the patient, acting within his
or her scope of practice under State law, and who is authorized by the hospital’s
medical staff to order the services in accordance with hospital policies and
procedures and State laws.
Interpretive Guidelines §482.57(b)(3)
Respiratory care services must be ordered by a qualified and licensed practitioner who is
responsible for the care of the patient. The practitioner must have medical staff
privileges to write orders for these services or, for outpatient services, if hospital policy
permits acceptance of orders from outside practitioners, the practitioner’s order must
meet the requirements at §482.54(c).
For practitioners who have medical staff privileges, such privileges must be granted in a
manner consistent with the State’s scope of practice law, as well as with hospital policies
and procedures governing respiratory care services developed by the medical staff.
Practitioners who may be granted privileges to order respiratory care services include
physicians, and may also, in accordance with hospital policy, include Nurse Practitioners,
Physicians’ Assistants, Clinical Nurse Specialists, Certified Registered Nurse
Anesthetists, and Certified Nurse Midwives as long as they meet the parameters of this
requirement. Although the following licensed professionals are also considered
practitioners, in accordance with Section 1842(b)(18)(C) of the Social Security Act, they
generally would not be considered responsible for the care of the patient with regard to
respiratory care services or qualified to order respiratory care services: Clinical social
worker (Section 1861(hh)(1) of the Act and as defined in 42 CFR 410.71); Clinical
psychologist (for purposes of Section 1861(ii) of the Act); or registered dietician or
nutrition professional.
Survey Procedures §482.57(b)(3)
• Review medical records of patients receiving respiratory care
services. Determine who wrote the orders for the respiratory care
services. Determine if the practitioner is responsible for the care of the patient
and privileged to write orders for respiratory care services. Verify the practitioner
meets hospital medical staff policy criteria to order services as well as State law
for ordering respiratory care services.
Does the hospital permit acceptance of orders from outside practitioners who do not
practice at the hospital? If so, evaluate for compliance with §482.54(c).
A-1164
(Rev. 72, Issued: 11-18-11, Effective: 11-18-11, Implementation: 11-18-11)
§482.57(b)(4)- All respiratory care services orders must be documented in the
patient’s medical record in accordance with the requirements at §482.24.
Interpretive Guidelines §482.57(b)(4)
The patient’s medical record must contain documentation of all respiratory care services
ordered. The medical record entries must comply with regulations at 42 CFR 482.24.
Survey Procedures §482.57(b)(4)
Review a sample of patient medical records who received respiratory care services.
Determine whether the respiratory care services orders are legible, complete, dated,
timed, authenticated, and meet all other medical record requirements as specified at
§484.24.
A-1500
(Rev.183, Issued: 10-12-18, Effective: 10-12-18, Implementation: 10-12-18)
Condition of Participation: §482.58 Special requirements for hospital
providers of long-term care services (“swing-beds”)
A hospital that has a Medicare provider agreement must meet the following requirements
in order to be granted an approval from CMS to provide post-hospital extended care
services, as specified in §409.30 of this chapter, and be reimbursed as a swing-bed
hospital, as specified in §413.114 of this chapter:
Interpretive Guidelines §482.58
Swing-bed services are an optional service. The swing-bed concept allows a hospital to
use their beds interchangeably for either acute-care or post-acute care. A “swing-bed” is a
change in reimbursement status. The patient swings from receiving acute-care hospital
inpatient services and reimbursement to receiving post-acute care skilled nursing facility
(SNF) services and reimbursement. A psychiatric hospital is not allowed to have swing-
bed approval.
Allowing a hospital to operate swing-beds is done by issuing a “swing-bed approval”. If
the hospital fails to meet the swing-bed requirements (not the same as the hospital
conditions of participation (CoPs)), and the hospital does not implement a plan of
correction, they lose the approval to operate swing-beds and receive swing-bed
reimbursement. However, in such a situation, the hospital does not go on a termination
track. If the hospital continues to meet all other applicable hospital CoPs, it continues to
participate in Medicare, but loses its swing-bed approval.
Swing-beds do not have to be located in a special section of the hospital although a
hospital may choose to do so. The patient does not have to change locations in the
hospital merely because their admission status changes unless the hospital requires it. The
change in status from acute care to swing-bed status can occur within the same part of the
hospital or the patient can be moved to another part of the hospital for swing-bed
admission. Likewise, a patient may be discharged from one hospital and admitted in
swing bed status to another hospital that has swing bed approval.
Beds in a hospital IPPS-excluded rehabilitation or psychiatric unit, or a separately
certified co-located Medicare participating entity (e.g., a distinct part SNF/NF, another
hospital, or an inpatient hospice) cannot be used by the hospital for providing swing-bed
services.
There must be discharge orders from acute care hospital inpatient services and
subsequent admission orders for swing-bed services, the same as if the patient had been
transferred to a separately certified skilled nursing facility. The same clinical record may
be used for a swing-bed patient, but it must include discharge orders from acute care
hospital inpatient services and admission orders to swing-bed services, and the swing-bed
services (which may be SNF or NF level services) must be clearly delineated within the
clinical record.
There is no length of stay restriction for any hospital swing-bed patient. There is no
Medicare requirement to place a swing-bed patient in a nursing home and there are no
requirements for transfer agreements between hospitals and nursing homes. While there
is no length of stay limit for patients in swing-bed status, the intended use for swing beds
is for a transitional time period to allow the patient to fully recover to return home or
while awaiting placement into a nursing facility.
The Medicare statute and regulations require that, in order to be eligible for Medicare
coverage of post-hospital skilled nursing facility (SNF) or swing-bed care, a beneficiary
must have a qualifying 3-day inpatient stay in a participating or qualified hospital or
participating CAH prior to admission to a swing bed in a hospital, or admission to a SNF.
This requirement applies only to patients who are Medicare beneficiaries who seek
Medicare coverage of their SNF services. It is not evaluated or enforced through the
survey and certification process, since it is a coverage requirement.
In accordance with SOM Section 2037 hospitals seeking swing-bed approval are
screened prior to survey for their eligibility for swing beds. However, the CMS Regional
Office makes the determination whether the hospital has satisfied the eligibility criteria,
regardless of whether the State Survey Agency or Accrediting Organization, as
applicable, recommends approval of swing bed status.
NOTE: Swing-beds must not be confused with beds in a skilled nursing facility (SNF)
or nursing facility (NF), including a distinct part SNF/NF, that shares the same
building/campus as the hospital but is a separately certified provider with its own
Medicare provider agreement.
An onsite survey must be conducted and the hospital must meet all the requirements of
42 CFR 482.58 before the hospital can obtain swing bed approval. Surveyors assess the
manner and degree of non-compliance with the swing bed standards in determining
whether there is condition-level compliance or standard-level non-compliance.
A-1501
(Rev. 183, Issued: 10-12-18, Effective: 10-12-18, Implementation: 10-12-18)
§482.58 (a) Eligibility. A hospital must meet the following eligibility requirements:
(1) The facility has fewer than 100 hospital beds, excluding beds for
newborns and beds in intensive care type inpatient units (for eligibility of
hospitals with distinct parts electing the optional reimbursement method, see
§413.24(d)(5) of this chapter).
(2) The hospital is located in a rural area. This includes all areas not
delineated as “urbanized” areas by the Census Bureau, based on the most
recent census.
(3) The hospital does not have in effect a 24-hour nursing waiver granted
under §488.54(c) of this chapter.
(4) The hospital has not had a swing-bed approval terminated within the two
years previous to application.
Interpretive Guidelines §482.58(a)
Hospitals seeking swing-bed approval are screened prior to survey for their eligibility for
swing-beds. However, the CMS RO makes the determination whether the hospital has
satisfied the eligibility criteria, regardless of whether the SA or AO, as applicable,
recommends approval of swing-bed status (this responsibility may not be delegated to the
SA).
The eligibility criteria at 42 CFR 482.58(a) requires:
• The hospital has a Medicare provider agreement;
• An initial applicant hospital may seek swing-bed approval. If the
applicant hospital meets all Federal requirements for participation,
including those for swing-bed approval, the applicant hospital’s approval
for swing-bed services will be effective with the effective date of the
hospital’s Medicare participation agreement;
• The hospital has fewer than 100 maintained hospital beds, including any beds in
IPPS-excluded units, but excluding beds for newborns and beds in intensive care
type inpatient units;
• The bed-count will be evaluated by surveyors during the survey;
• Even though beds within a hospital’s IPPS-excluded psychiatric or
rehabilitation unit may not be used for the provision of swing bed services,
the beds maintained within those units are included with the number of
maintained beds within the hospital (that is because the §482.58(a)(1) does
not exclude those beds from the count);
• The bed-count is not based on the number of licensed or certified beds, but
rather the bed-count is based on maintained beds;
• Maintained beds are those patient beds within the Medicare certified
hospital that are present for use in providing inpatient services,
observation services, and/or swing-bed services.
• Maintained beds would include:
• Patient beds that are that located within nursing units of the
hospital;
• Established bed locations in patient rooms where the bed is
temporarily out of service or temporarily absent from the
location it routinely occupies; and
• Those beds that are located within nursing units that are
temporarily closed but are still included on the hospital’s
license and which can be brought into service when the
hospital chooses.
• Maintained beds would not include:
• Examination or procedure tables or stretchers located in
procedure rooms that are exclusively used for conducting
examinations or procedures; and
• Stretchers maintained in nursing units that are solely used for
patient transport;
Note: Maintained beds that are located within intensive care type
units and those beds that are maintained solely for the use of
newborns would not be included in the bed count.
• If a Medicare certified hospital has multiple inpatient locations such as
remote locations or satellites, all maintained beds at each location must be
combined into a single bed-count. The total count of maintained beds for
the Medicare certified hospital must be less than 100.
• The hospital is located in a rural area. This includes all areas not
delineated as “urbanized” areas by the Census Bureau, based on the most
recent census;
• The CMS RO Division of Survey and Certification (DSC) is responsible
for conducting the evaluation as to whether the hospital is located outside
of an urbanized area;
• The hospital must be located outside an urbanized area but may be located
in an urban cluster (the terms “urbanized area” and “urban cluster” are two
distinct classifications of population size used by the U.S. Census Bureau.
An urban cluster is not an “urbanized area”);
• The RO will utilize the U.S. Census Bureau’s most current edition of
American Factfinder to determine if the hospital is located outside of an
area designated as urbanized. See SOM §2037E for additional
instructions;
• In a situation where a hospital has multiple inpatient locations, such as a
multi-campus hospital (a hospital with remote locations), or a hospital
with satellites, each inpatient location must be individually evaluated to
determine if it is located outside an urbanized area. When any inpatient
location of the Medicare certified hospital is located within an urbanized
area the hospital does not qualify for swing-bed approval.
• A hospital’s swing bed approval must be terminated if the U.S. Census
Bureau delineates the hospital, or any inpatient location of the hospital, as
being located within an urbanized area.
• The hospital does not have in effect a 24-hour nursing waiver granted under 42
CFR 488.54(c);
• The RO must review the hospital’s ASPEN file to determine if the hospital
has in effect a 24-hour nursing waiver. A hospital with this waiver cannot
have swing-bed approval;
• A hospital that currently has swing-bed approval that seeks and is granted
a 24-hour nursing waiver under 42 CFR 488.54(c) must have its swing-
bed approval terminated;
• The hospital has not had a swing-bed approval terminated within the two years
previous to application;
• When a hospital is seeking initial swing-bed approval, the RO will review
the hospital’s ASPEN file to determine if the hospital previously had
swing-bed approval that was terminated within the two years previous to
the application; and
• A request for swing-bed approval will be denied if the hospital has had
swing-bed approval terminated within the previous two years. It does not
matter whether the termination was voluntary or involuntary.
Survey Procedures §482.58(a)
In conducting the survey, verify that the hospital has fewer than 100 hospital beds,
excluding beds for newborns and beds in intensive care units. A hospital licensed for
more than 100 beds may be eligible for swing-bed approval if it utilizes and staffs for
fewer than 100 beds. Surveyors are to count the beds in each nursing unit. Do not count
beds in recovery rooms, intensive care units, operating rooms, newborn nurseries, or
stretchers in emergency departments. However, do count the beds within IPPS-excluded
Rehabilitation and Psychiatric Units.
A-1562
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.58(b) Skilled nursing facility services. The facility is substantially in
compliance with the following skilled nursing facility requirements contained in
subpart B of part 483 of this chapter
§482.58(b)(1) Resident rights (§483.10(b)(7), (c)(1), (c)(2)(iii), (c)(6), (d), (e)(2), and
(4), (f)(4)(ii) and (iii), (h), (g)(8) and (17), and (g)(18) introductory text of this
chapter).
§483.10(b)(7): In the case of a resident adjudged incompetent under the laws of a
State by a court of competent jurisdiction, the rights of the resident devolve to and
are exercised by the resident representative appointed under State law to act on the
resident's behalf. The court-appointed resident representative exercises the
resident's rights to the extent judged necessary by a court of competent jurisdiction,
in accordance with State law.
In the case of a resident representative whose decision-making authority is limited
by State law or court appointment, the resident retains the right to make those
decision outside the representative's authority.
(ii)The resident's wishes and preferences must be considered in the exercise of rights
by the representative.
(iii)To the extent practicable, the resident must be provided with opportunities to
participate in the care planning process.
§483.10(c)(1): The right to be fully informed in language that he or she can
understand of his or her total health status, including but not limited to, his or her
medical condition.
§483.10(c)(2)(iii): The right to be informed, in advance, of changes to the plan of
care.
§483.10(c)(6): The right to request, refuse, and/or discontinue treatment, to
participate in or refuse to participate in experimental research, and to formulate an
advance directive.
§483.10(d): Choice of attending physician. The resident has the right to choose his
or her attending physician.
The physician must be licensed to practice, and
If the physician chosen by the resident refuses to or does not meet requirements
specified in this part, the facility may seek alternate physician participation as
specified in paragraphs (d)(4) and (5) of this section to assure provision of
appropriate and adequate care and treatment.
The facility must ensure that each resident remains informed of the name, specialty,
and way of contacting the physician and other primary care professionals
responsible for his or her care.
The facility must inform the resident if the facility determines that the physician
chosen by the resident is unable or unwilling to meet requirements specified in this
part and the facility seeks alternate physician participation to assure provision of
appropriate and adequate care and treatment. The facility must discuss the
alternative physician participation with the resident and honor the resident's
preferences, if any, among options.
(5) If the resident subsequently selects another attending physician who meets the
requirements specified in this part, the facility must honor that choice.
§483.10(e)(2):The right to retain and use personal possessions, including
furnishings, and clothing, as space permits, unless to do so would infringe upon the
rights or health and safety of other residents.
§483.10(e)(4): The right to share a room with his or her spouse when married
residents live in the same facility and both spouses consent to the arrangement.
§483.10(f)(4)(ii): The facility must provide immediate access to a resident by
immediate family and other relatives of the resident, subject to the resident's right
to deny or withdraw consent at any time.
§483.10(f)(4)(iii): The facility must provide immediate access to a resident by others
who are visiting with the consent of the resident, subject to reasonable clinical and
safety restrictions and the resident's right to deny or withdraw consent at any time.
§483.10(g)(8): The resident has the right to send and receive mail, and to receive
letters, packages and other materials delivered to the facility for the resident
through a means other than a postal service, including the right to:
Privacy of such communications consistent with this section; and
(ii) Access to stationery, postage, and writing implements at the resident's own
expense.
§483.10(g)(17): The facility must—
Inform each Medicaid-eligible resident, in writing, at the time of admission to the
nursing facility and when the resident becomes eligible for Medicaid of—
The items and services that are included in nursing facility services under the State
plan and for which the resident may not be charged;
Those other items and services that the facility offers and for which the resident
may be charged, and the amount of charges for those services; and
(ii) Inform each Medicaid-eligible resident when changes are made to the items and
services specified in §483.10(g)(17)(i)(A) and (B) of this section.
§483.10(g)(18):[introductory text]: The facility must inform each resident before, or
at the time of admission, and periodically during the resident's stay, of services
available in the facility and of charges for those services, including any charges for
services not covered under Medicare/Medicaid or by the facility's per diem rate.
§483.10(h): Privacy and confidentiality. The resident has a right to personal privacy
and confidentiality of his or her personal and medical records.
Interpretive Guidelines §482.58(b)(1)
Refer to Appendix PP of the State Operations Manual (SOM) for interpretive guidelines.
Survey Procedures §482.58(b)(1)
Refer to Appendix PP of the State Operations Manual (SOM) for survey procedures.
A-1564
(Rev. 183, Issued: 10-12-18, Effective: 10-12-18, Implementation: 10-12-18)
§482.58(b)(2) Admission, transfer, and discharge rights (§483.5
definition of transfer and discharge, §483.15(c)(1), (c)(2)(i), (c)(2)(ii),
(c)(3), (c)(4), (c)(5), and (c)(7))
• §483.5: definition of transfer and discharge: Transfer and discharge
includes movement of a resident to a bed outside of the certified facility
whether that bed is in the same physical plant or not. Transfer and discharge
does not refer to movement of a resident to a bed within the same certified
facility.
• §483.15(c)(1): Facility requirements—
(i) The facility must permit each resident to remain in the facility, and
not transfer or discharge the resident from the facility unless—
(A) The transfer or discharge is necessary for the resident's
welfare and the resident's needs cannot be met in the
facility;
(B) The transfer or discharge is appropriate because the
resident's health has improved sufficiently so the resident
no longer needs the services provided by the facility;
(C) The safety of individuals in the facility is endangered due
to the clinical or behavioral status of the resident;
(D) The health of individuals in the facility would otherwise be
endangered;
(E) The resident has failed, after reasonable and appropriate
notice, to pay for (or to have paid under Medicare or
Medicaid) a stay at the facility. Non-payment applies if the
resident does not submit the necessary paperwork for
third party payment or after the third party, including
Medicare or Medicaid, denies the claim and the resident
refuses to pay for his or her stay. For a resident who
becomes eligible for Medicaid after admission to a facility,
the facility may charge a resident only allowable charges
under Medicaid; or
(F) The facility ceases to operate.
(ii)The facility may not transfer or discharge the resident while the
appeal is pending, pursuant to §431.230 of this chapter, when a
resident exercises his or her right to appeal a transfer or discharge
notice from the facility pursuant to §431.220(a)(3) of this chapter,
unless the failure to discharge or transfer would endanger the
health or safety of the resident or other individuals in the facility.
The facility must document the danger that failure to transfer or
discharge would pose.
• §483.15(c)(2)(i) Documentation in the resident's medical record must
include:
(A) The basis for the transfer per paragraph (c)(1)(i) of this
section.
(B) In the case of paragraph (c)(1)(i)(A) of this section, the specific
resident need(s) that cannot be met, facility attempts to meet
the resident needs, and the service available at the receiving
facility to meet the need(s).
• §483.15(c)(2)(ii) The documentation required by paragraph (c)(2)(i) of this
section must be made by—
(A) The resident's physician when transfer or discharge is
necessary under paragraph (c)(1)(A) or (B) of this section; and
(B) A physician when transfer or discharge is necessary under
paragraph (c)(1)(i)(C) or (D) of this section.
• §483.15(c)(3): Notice before transfer. Before a facility transfers or discharges
a resident, the facility must—
(i) Notify the resident and the resident's representative(s) of the
transfer or discharge and the reasons for the move in writing and
in a language and manner they understand. The facility must send
a copy of the notice to a representative of the Office of the State
Long-Term Care Ombudsman.
(ii) Record the reasons for the transfer or discharge in the resident's
medical record in accordance with paragraph (c)(2) of this section;
and
(iii) Include in the notice the items described in paragraph (c)(5) of
this section.
• §483.15(c)(4): Timing of the notice. (i) Except as specified in paragraphs
(c)(4)(ii) and (8) of this section, the notice of transfer or discharge required
under this section must be made by the facility at least 30 days before the
resident is transferred or discharged.
(ii) Notice must be made as soon as practicable before transfer or
discharge when—
(A) The safety of individuals in the facility would be endangered
under paragraph (c)(1)(i)(C) of this section;
(B) The health of individuals in the facility would be endangered,
under paragraph (c)(1)(i)(D) of this section;
(C) The resident's health improves sufficiently to allow a more
immediate transfer or discharge, under paragraph (c)(1)(i)(B)
of this section;
(D) An immediate transfer or discharge is required by the
resident's urgent medical needs, under paragraph (c)(1)(i)(A)
of this section; or
(E) A resident has not resided in the facility for 30 days.
• §483.15(c)(5): Contents of the notice. The written notice specified in
paragraph (c)(3) of this section must include the following:
(i) The reason for transfer or discharge;
(ii) The effective date of transfer or discharge;
(iii)The location to which the resident is transferred or discharged;
(iv) A statement of the resident's appeal rights, including the name,
address (mailing and email), and telephone number of the entity
which receives such requests; and information on how to obtain an
appeal form and assistance in completing the form and submitting
the appeal hearing request;
(v) The name, address (mailing and email) and telephone number of
the Office of the State Long-Term Care Ombudsman;
(vi) For nursing facility residents with intellectual and developmental
disabilities or related disabilities, the mailing and email address
and telephone number of the agency responsible for the protection
and advocacy of individuals with developmental disabilities
established under Part C of the Developmental Disabilities
Assistance and Bill of Rights Act of 2000 (Pub. L. 106-402, codified
at 42 U.S.C. 15001 et seq.); and
(vii)For nursing facility residents with a mental disorder or related
disabilities, the mailing and email address and telephone number
of the agency responsible for the protection and advocacy of
individuals with a mental disorder established under the
Protection and Advocacy for Mentally Ill Individuals Act.
• §483.15(c)(7): Orientation for transfer or discharge. A facility must provide
and document sufficient preparation and orientation to residents to ensure
safe and orderly transfer or discharge from the facility. This orientation
must be provided in a form and manner that the resident can understand.
Interpretive Guidelines §482.58(b)(2)
Refer to Appendix PP of the State Operations Manual (SOM) for interpretive guidelines.
Survey Procedures §482.58(b)(2)
Refer to Appendix PP of the State Operations Manual (SOM) for survey procedures.
A-1566
(Rev. 183, Issued: 10-12-18, Effective: 10-12-18, Implementation: 10-12-18)
§482.58(b)(3) Freedom from abuse, neglect, and exploitation
(§483.12(a)(1), (a)(2), (a)(3)(i), (a)(3)(ii), (a)(4), (b)(1), (b)(2), (c))
• §483.12(a)(1): The facility must (1) Not use verbal, mental, sexual, or
physical abuse, corporal punishment, or involuntary seclusion.
• §483.12(a)(2): Ensure that the resident is free from physical or chemical
restraints imposed for purposes of discipline or convenience and that are not
required to treat the resident's medical symptoms. When the use of restraints
is indicated, the facility must use the least restrictive alternative for the least
amount of time and document ongoing re-evaluation of the need for
restraints.
• §483.12(a)(3)(i): Not employ or otherwise engage individuals who (i) Have
been found guilty of abuse, neglect, exploitation, misappropriation of
property, or mistreatment by a court of law.
• §483.12(a)(3)(ii): Have had a finding entered into the State nurse aide
registry concerning abuse, neglect, exploitation, mistreatment of residents or
misappropriation of their property.
• §483.12(a)(4): Report to the State nurse aide registry or licensing authorities
any knowledge it has of actions by a court of law against an employee, which
would indicate unfitness for service as a nurse aide or other facility staff.
• §483.12(b)(1): The facility must develop and implement written policies and
procedures that: (1)Prohibit and prevent abuse, neglect, and exploitation of
residents and misappropriation of resident property.
• §483.12(b)(2): Establish policies and procedures to investigate any such
allegations.
• §483.12(c): In response to allegations of abuse, neglect, exploitation, or
mistreatment, the facility must:
(1) Ensure that all alleged violations involving abuse, neglect,
exploitation or mistreatment, including injuries of unknown
source and misappropriation of resident property, are reported
immediately, but not later than 2 hours after the allegation is
made, if the events that cause the allegation involve abuse or result
in serious bodily injury, or not later than 24 hours if the events
that cause the allegation do not involve abuse and do not result in
serious bodily injury, to the administrator of the facility and to
other officials (including to the State Survey Agency and adult
protective services where state law provides for jurisdiction in
long-term care facilities) in accordance with State law through
established procedures.
(2) Have evidence that all alleged violations are thoroughly
investigated
(3) Prevent further potential abuse, neglect, exploitation, or
mistreatment while the investigation is in progress.
(4) Report the results of all investigations to the administrator or his
or her designated representative and to other officials in
accordance with State law, including to the State Survey Agency,
within 5 working days of the incident, and if the alleged violation
is verified appropriate corrective action must be taken.
Interpretive Guidelines §482.58(b)(3)
Refer to Appendix PP of the State Operations Manual (SOM) for interpretive guidelines.
Survey Procedures §482.58(b)(3)
Refer to Appendix PP of the State Operations Manual (SOM) for survey procedures.
A-1567
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.58(b)(4) Social services (§483.40(d) of this chapter).
• §483.40(d): The facility must provide medically-related social services to
attain or maintain the highest practicable physical, mental and psychosocial
well-being of each resident.
Interpretive Guidelines §482.58(b)(4)
Refer to Appendix PP of the State Operations Manual (SOM) for interpretive guidelines.
Survey Procedures §482.58(b)(4)
Refer to Appendix PP of the State Operations Manual (SOM) for survey procedures.
A-1568
(Rev. 183, Issued: 10-12-18, Effective: 10-12-18, Implementation: 10-12-18)
§482.58(b)(4) Patient activities (§483.24(c))
• §483.24(c): Activities
(1) The facility must provide, based on the comprehensive assessment
and care plan and the preferences of each resident, an ongoing
program to support residents in their choice of activities, both
facility-sponsored group and individual activities and independent
activities, designed to meet the interests of and support the
physical, mental, and psychosocial well-being of each resident,
encouraging both independence and interaction in the community.
(2) The activities program must be directed by a qualified professional
who is a qualified therapeutic recreation specialist or an activities
professional who—
(i) Is licensed or registered, if applicable, by the State in which
practicing; and
(ii) Is:
(A) Eligible for certification as a therapeutic recreation
specialist or as an activities professional by a recognized
accrediting body on or after October 1, 1990; or
(B) Has 2 years of experience in a social or recreational
program within the last 5 years, one of which was full-time
in a therapeutic activities program; or
(C) Is a qualified occupational therapist or occupational
therapy assistant; or
(D) Has completed a training course approved by the State.
Interpretive Guidelines §482.58(b)(4)
Refer to Appendix PP of the State Operations Manual (SOM) for interpretive guidelines.
Survey Procedures §482.58(b)(4)
Refer to Appendix PP of the State Operations Manual (SOM) for survey procedures.
A-1569
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.58(b)(5) Discharge summary (§483.20(l))
[Note: The regulations at §483.20(l) setting forth the requirements for a nursing
home resident discharge summary was revised and re-designated as §483.21(c)(2)
in 2016 (81 FR 68858, Oct. 4, 2016) which provides, “When the facility anticipates
discharge a resident must have a discharge summary that includes, but is not
limited to:
(i) A recapitulation of the resident’s stay that includes, but is not limited to,
diagnoses, course of illness/treatment or therapy, and pertinent lab, radiology, and
consultation results.
(ii) A final summary of the resident’s status to include items in paragraph (b)(2) of
§483.20, at the time of the discharge that is available for release to authorized
persons and agencies, with the consent of the resident or legal representative.
(iii) Reconciliation of all pre-discharge medications with the resident's post-
discharge medications (both prescribed and over-the-counter).
(iv) A post-discharge plan of care that is developed with the participation of the
resident and, with the resident’s consent, the resident representative(s), which will
assist the resident to adjust to his or her new living environment. The post-discharge
plan of care must indicate where the individual plans to reside, any arrangements
that have been made for the resident's follow up care and any post-discharge
medical and non-medical services.”]
A-1570
(Rev. 183, Issued: 10-12-18, Effective: 10-12-18, Implementation: 10-12-18)
§482.58(b)(5) Social services (§483.40(d) and 483.70(p))
• §483.40 (d): The facility must provide medically-related social services to
attain or maintain the highest practicable physical, mental and psychosocial
well-being of each resident.
• §483.70 (p): Social worker. Any facility with more than 120 beds must
employ a qualified social worker on a full-time basis. A qualified social
worker is:
(1) An individual with a minimum of a bachelor's degree in social
work or a bachelor's degree in a human services field including,
but not limited to, sociology, gerontology, special education,
rehabilitation counseling, and psychology; and
(2) One year of supervised social work experience in a health care
setting working directly with individuals.
Interpretive Guidelines §482.58(b)(5)
Refer to Appendix PP of the State Operations Manual (SOM) for interpretive guidelines.
Survey Procedures §482.58(b)(5)
Refer to Appendix PP of the State Operations Manual (SOM) for survey procedures.
A-1572
(Rev. 183, Issued: 10-12-18, Effective: 10-12-18, Implementation: 10-12-18)
§482.58(b)(6) Discharge planning (§483.20(e))
• §483.20(e) Coordination. A facility must coordinate assessments with the
preadmission screening and resident review (PASARR) program under
Medicaid in subpart C of this part to the maximum extent practicable to
avoid duplicative testing and effort. Coordination includes—
(1) Incorporating the recommendations from the PASARR level II
determination and the PASARR evaluation report into a resident's
assessment, care planning, and transitions of care.
(2) Referring all level II residents and all residents with newly evident
or possible serious mental disorder, intellectual disability, or a
related condition for level II resident review upon a significant
change in status assessment.
Interpretive Guidelines §482.58(b)(6)
Refer to Appendix PP of the State Operations Manual (SOM) for interpretive guidelines.
Survey Procedures §482.58(b)(6)
Refer to Appendix PP of the State Operations Manual (SOM) for survey procedures.
A-1573
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.58(b)(7) Dental services (§483.55(a)(2), (3), (4), and (5) and (b) of this chapter).
• §483.55 Dental services. The facility must assist residents in obtaining
routine and 24-hour emergency dental care.
(a) Skilled nursing facilities. A facility…
(2) May charge a Medicare resident an additional amount for routine and
emergency dental services;
(3) Must have a policy identifying those circumstances when the loss or
damage of dentures is the facility's responsibility and may not charge a
resident for the loss or damage of dentures determined in accordance
with facility policy to be the facility's responsibility;
(4)
Must if necessary or if requested, assist the resident—
(i) In making appointments; and
(ii) By arranging for transportation to and from the dental
services location; and
(5) Must promptly, within 3 days, refer residents with lost or damaged
dentures for dental services. If a referral does not occur within 3 days, the
facility must provide documentation of what they did to ensure the
resident could still eat and drink adequately while awaiting dental
services and the extenuating circumstances that led to the delay.
(b) Nursing facilities. The facility-
(1) Must provide or obtain from an outside resource, in accordance
with §483.70(g), the following dental services to meet the needs of
each resident:
(i) Routine dental services (to the extent covered under the State
plan); and
(ii) Emergency dental services;
(2) Must, if necessary or if requested, assist the resident—
(i) In making appointments; and
(ii) By arranging for transportation to and from the dental services
locations;
(3) Must promptly, within 3 days, refer residents with lost or
damaged dentures for dental services. If a referral does not occur
within 3 days, the facility must provide documentation of what
they did to ensure the resident could still eat and drink adequately
while awaiting dental services and the extenuating circumstances
that led to the delay;
(4) Must have a policy identifying those circumstances when the loss
or damage of dentures is the facility's responsibility and may not
charge a resident for the loss or damage of dentures determined in
accordance with facility policy to be the facility's responsibility;
and
(5) Must assist residents who are eligible and wish to participate to
apply for reimbursement of dental services as an incurred medical
expense under the State plan.
Interpretive Guidelines §482.58(b)(7)
Refer to Appendix PP of the State Operations Manual (SOM) for interpretive guidelines.
Survey Procedures §482.58(b)(7)
Refer to Appendix PP of the State Operations Manual (SOM) for survey procedures.
Special Provisions Applying to Psychiatric Hospitals
A-1574
(Rev. 183, Issued: 10-12-18, Effective: 10-12-18, Implementation: 10-12-18)
§482.58(b)(7) Specialized rehabilitative services (§483.65)
• §483.65(a) Provision of services. If specialized rehabilitative services such as
but not limited to physical therapy, speech-language pathology, occupational
therapy, respiratory therapy, and rehabilitative services for a mental
disorder and intellectual disability or services of a lesser intensity as set forth
at §483.120(c), are required in the resident's comprehensive plan of care, the
facility must—
(1) Provide the required services; or
(2) In accordance with §483.70(g), obtain the required services from
an outside resource that is a provider of specialized rehabilitative
services and is not excluded from participating in any federal or
state health care programs pursuant to section 1128 and 1156 of
the Act.
• §483.65(b) Qualifications. Specialized rehabilitative services must be
provided under the written order of a physician by qualified personnel.
Interpretive Guidelines §482.58(b)(7)
Refer to Appendix PP of the State Operations Manual (SOM) for interpretive guidelines.
Survey Procedures §482.58(b)(7)
Refer to Appendix PP of the State Operations Manual (SOM) for survey procedures.
A-1576
(Rev. 183, Issued: 10-12-18, Effective: 10-12-18, Implementation: 10-12-18)
§482.58(b)(8) Dental services (§483.55)
• §483.55 Dental services. The facility must assist residents in obtaining
routine and 24-hour emergency dental care.
(c) Skilled nursing facilities. A facility
(1) Must provide or obtain from an outside resource, in accordance
with §483.70(g), routine and emergency dental services to meet the
needs of each resident;
(2) May charge a Medicare resident an additional amount for routine
and emergency dental services;
(3) Must have a policy identifying those circumstances when the loss
or damage of dentures is the facility's responsibility and may not
charge a resident for the loss or damage of dentures determined in
accordance with facility policy to be the facility's responsibility;
(4) Must if necessary or if requested, assist the resident—
(iii)In making appointments; and
(iv) By arranging for transportation to and from the dental
services location; and
(5) Must promptly, within 3 days, refer residents with lost or damaged
dentures for dental services. If a referral does not occur within 3
days, the facility must provide documentation of what they did to
ensure the resident could still eat and drink adequately while
awaiting dental services and the extenuating circumstances that
led to the delay.
(d) Nursing facilities. The facility-
(1) Must provide or obtain from an outside resource, in accordance
with §483.70(g), the following dental services to meet the needs of
each resident:
(iii)Routine dental services (to the extent covered under the State
plan); and
(iv) Emergency dental services;
(2) Must, if necessary or if requested, assist the resident—
(i) In making appointments; and
(ii) By arranging for transportation to and from the dental services
locations;
(3) Must promptly, within 3 days, refer residents with lost or
damaged dentures for dental services. If a referral does not occur
within 3 days, the facility must provide documentation of what
they did to ensure the resident could still eat and drink adequately
while awaiting dental services and the extenuating circumstances
that led to the delay;
(4) Must have a policy identifying those circumstances when the loss
or damage of dentures is the facility's responsibility and may not
charge a resident for the loss or damage of dentures determined in
accordance with facility policy to be the facility's responsibility;
and
(5) Must assist residents who are eligible and wish to participate to
apply for reimbursement of dental services as an incurred medical
expense under the State plan.
Interpretive Guidelines §482.58(b)(8)
Refer to Appendix PP of the State Operations Manual (SOM) for interpretive guidelines.
Survey Procedures §482.58(b)(8)
Refer to Appendix PP of the State Operations Manual (SOM) for survey procedures.
A-1600
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.60-Special Provisions Applying to Psychiatric Hospitals - Psychiatric hospitals
must
Interpretive Guidelines §482.60
Guidance is pending and will be updated in future release
A-1601
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.60(a) Be primarily engaged in providing, by or under the supervision of a
doctor of medicine or osteopathy, psychiatric services for the diagnosis and
treatment of mentally ill persons.
Interpretive Guidelines §482.60
The hospital will be deemed to meet standard (a) if it meets standards (c) and (d).
A-1605
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.60(b) Meet the Conditions of Participation specified in§§482.1 through 482.23
and §§482.25 through 482.57;
Interpretive Guidelines §482.60(b)
Guidance is pending and will be updated in future release.
A-1610
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.60(c) Maintain clinical records on all patients, including records sufficient to
permit CMS to determine the degree and intensity of treatment furnished to
Medicare beneficiaries as specified in §482.61; and
Interpretive Guidelines §482.60(c)
Guidance is pending and will be updated in future release.
A-1615
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.60(d) Meet the staffing requirements specified in §482.62.
Interpretive Guidelines §482.60(d)
Guidance is pending and will be updated in future release.
A-1620
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61 Condition of Participation: Special Medical Record Requirements for
Psychiatric Hospitals
The medical records maintained by a psychiatric hospital must permit
determination of the degree and intensity of the treatment provided to individuals
who are furnished services in the institution.
Interpretive Guidelines §482.61
The clinical record should provide information that indicates need for admission and
treatment, treatment goals, changes in status of treatment and discharge planning, and
follow-up and the outcomes experienced by patients. The structure and content of the
individual patient’s record must be an accurate functional representation of the actual
experience of the individual in the facility. It must contain enough information to indicate
that the facility knows the status of the patient, has adequate plans to intervene, and
provides sufficient evidence of the effects of the intervention, and how their interventions
served as a function of the outcomes experienced. You must be able to identify this
through interviews with staff, and when possible with individuals being served, as well as
through observations.
A-1621
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(a) Standard: Development of Assessment/Diagnostic Data Medical records
must stress the psychiatric components of the record, including history of findings
and treatment provided for the psychiatric condition for which the patient is
hospitalized.
Interpretive Guidelines §482.61(a)
Guidance is pending and will be updated in future release.
A-1622
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(a)(1) The identification data must include the patient’s legal status.
Interpretive Guidelines §482.61(a)(1)
Definition: Legal Status is defined in the State statutes and dictates the circumstances
under which the patient was admitted and/or is being treated - i.e., voluntary, involuntary,
committed by court, evaluation and recertification are in accordance with state
requirements.
Determine through interview with hospital staff the terminology they use in defining
“legal status.” If evaluation and recertification is required by the State, determine that
legal documentation supporting this status is present. Changes in legal status should also
be recorded with the date of change.
A-1623
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(a)(2) A provisional or admitting diagnosis must be made on every patient at
the time of admission, and must include the diagnosis of intercurrent diseases as
well as the psychiatric diagnosis.
Interpretive Guidelines §482.61(a)(2)
There is an admission or working psychiatric diagnosis (including rule-out diagnoses)
written in the most current edition of the American Psychiatric Association’s Diagnostic
and Statistical Manual (DSM) or the approved International Classification of Diseases
(ICD) nomenclature. This diagnosis is made and entered into the chart of each patient at
the time of the admission examination. The final diagnosis may differ from the initial
diagnosis if subsequent evaluation and observation support a change.
If a diagnosis is absent, there must be justification for its absence. For example, if a
patient was psychotic on admission and was not accompanied by family or significant
others.
Intercurrent (other than psychiatric) diagnoses must be documented when they are made.
Attention should be paid to physical examination notes, including known medical
conditions, even allergies and recent exposure to infections, illness, or substance abuse,
and to available laboratory or test reports which identify abnormal findings to see that
these are reflected by appropriate diagnosis.
These diagnoses may be found in a variety of locations in the medical record, e.g., the
identification/face sheet, the finding of admission physical examination, the psychiatric
evaluation the “admission work up “ or the physician’s progress notes. Diagnostic
categories should include physical illness when present.
Survey Procedures §482.61(a)(2)
Are abnormal physical examination findings and/or laboratory findings justified by
further diagnostic testing and/or development of an intercurrent diagnosis, and, if so, was
such done?
If an identified physical illness requires immediate treatment, is the treatment being
given?
How will an identified physical illness be likely to impact on the patient’s eventual
outcome? To what extent has this potential impact been addressed by the team?
A-1624
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(a)(3) The reasons for admission must be clearly documented as stated by
the patient and/or others significantly involved.
Interpretive Guidelines §482.61(a)(3)
The purpose of this regulation is to provide an understanding of what caused the patient
to come to the hospital, and the patient’s response to admission.
The hospital records the statements and reason for admission given by family and by
others, as well as the patient (preferably verbatim), with informant identified, in a variety
of locations, e.g., in transfer and admission notes from the physician, nurses and social
workers.
Records should not contain vague, ill-defined reports from unknown sources. Records
should record “who,” “what,” “where,” “when,” and “why.”
Survey Procedures §482.61(a)(3)
Can the patient describe problems, stresses, situations experienced prior to hospitalization
or do they still exist?
Who is the informant?
Did the informant witness the patient’s behavior? If not, on what basis has the informant
come to know the patient’s behavior?
Has staff elicited whether the patient has exhibited similar behavior previously? If so,
what was different this time to make hospitalization necessary?
Were there other changes/events in the patient’s environment (death, separations of
significant others) which contributed to the need for hospitalization? If so, has staff
explored how these will impact in the patient’s treatment? Has this been addressed by the
treatment team?
Has there been an interruption or change in the patient’s medication which may have
been a factor in the patient’s hospitalization?
A-1625
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(a)(4) The social service records, including reports of interviews with
patients, family members, and others, must provide an assessment of home plans
and family attitudes, and community resource contacts as well as a social history.
Interpretive Guidelines §482.61(a)(4)
The purpose of the social work assessment is to determine the current baseline social
functioning (strengths and deficits) of the patient, from which treatment interventions and
discharge plans are to be formulated.
Patient length of stay is a key factor influencing hospital documentation policy, i.e.,
establishing timeframes for completion, documentation, and filing of the psychosocial
assessment, and treatment planning in the medical record.
A psychosocial history/assessment must be completed on all patients. Three key
components to be addressed:
A. Factual and Historical Information
1. Specific reasons for the patient’s admission or readmission;
2. A description of the patient’s past and present biopsychosocial functioning;3. Family
and marital history, dynamics, and patient’s relationships with family and significant
others;
4. Pertinent religious and cultural factors;
5. History of physical, sexual and emotional abuse;
6. Significant aspects of psychiatric, medical, and substance abuse history and treatment
as presented by family members and significant others;
7. Educational, vocational, employment, and military service history;
8. Identification of community resources including previously used treatment sources;
9. Identification of present environmental and financial needs.
B. Social Evaluation
1. Patient strength and deficits;
2. High risk psychosocial issues requiring early treatment planning and intervention - i.e.,
unattended child(ren) in home; prior noncompliance to specific treatment and/ or
discharge interventions; and potential obstacles to present treatment and discharge
planning.
C. Conclusions and Recommendations
Assessment of Sections A and B shall result in the development of (C) recommendations
related to the following areas:
1. Anticipated necessary steps for discharge to occur;
2. High risk patient and/or family psychosocial issues requiring early treatment planning
and immediate intervention regardless of the patient’s length of stay;3. Specific
community resources/ support systems for utilization in discharge planning - i.e.,
housing, living arrangements, financial aid, and aftercare treatment sources;
4. Anticipated social work role(s) in treatment and discharge planning.
Survey Procedures §482.61(a)(4)
Does the psychosocial history/assessment indicate:
1. Clear identification of the informants(s) and sources of information?
2. Whether information is considered reliable?
3. Patient participation to the extent possible in provision of data relative to treatment and
discharge planning?
4. Integration of significant data including identified high risk psychosocial issues
(problems) into the treatment plan?
5. How does the hospital insure the information is reliable?
A-1626
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(a)(5) When indicated, a complete neurological examination must be
recorded at the time of the admission physical examination.
Interpretive Guidelines §482.61(a)(5)
Upon admission the patient should receive a thorough history and physical examination
with all indicated laboratory examinations. These investigations must be sufficient to
discover all structural, functional, systemic and metabolic disorders. A thorough history
of the patient’s past physical disorders, head trauma, accidents, substance
dependence/abuse, exposure to toxic agents, tumors, infections, seizures or temporary
loss of consciousness, and headaches, will alert the physician to look for the presence of
continuing pathology or possible sequelae any of which may turn out to be significant
and pertinent to the present mental illness. Equally important is a thorough physical
examination to look for signs of any current illness since psychotic symptoms may be due
to a general medical condition or substance related disorder.
The screening neurological examination
As part of the physical examination, the physician will perform a “screening”
neurological examination. While there is no precise definition of a screening neurological
examination in medical practice such examination is expected to assess gross function of
the various divisions of the central nervous system as opposite to detailed, fine testing of
each division. Gross testing of Cranial Nerves II through XII should be included.
Statements such as “Cranial Nerves II to XII intact” are not acceptable. These areas may
be found in various parts of the physical examination and not just grouped specifically
under the neurological. In any case where a system review indicate positive neurological
symptomatology, a more detailed examination would be necessary, with neurological
work-up or consultation ordered as appropriate after the screening neurological
examination was completed.
Complete neurological examination.
A complete, comprehensive neurological examination includes a review of the patient’s
history, physical examination and for psychiatric patients, a review of the psychiatric
evaluation. The neurologist/psychiatrist himself/herself also takes a history to obtain the
necessary information not already available in the medical record or referral form. The
neurological examination is a detailed, orderly survey of the various sections of the
nervous system. As an example, whereas a simple reading of a printed page will be
sufficient to assess grossly the patient’s sight (cranial nerve II) in a complete neurological
examination, the neurologist may test visual acuity with a snellen chart, perform a
fundoscopic examination of both eyes (sometimes after dilating the pupils) and he/she
will examine the patient’s visual fields. In the examination of the motor system, the
power of muscle groups of the extremities, the neck and trunk are tested. Where an
indication of diminished strength is noted, testing of smaller muscle groups and even
individual muscles are tested. In a complete neurological examinations all the systems are
examined, but the physician will emphasize even more the areas pertinent to the problem
for which the examination was requested.
Survey Procedures §482.61(a)(5)
Did the presence of an abnormal physical finding or laboratory finding justify the need
for further diagnostic testing, or for the development of an intercurrent diagnosis? If the
finding justified further follow-up in either situation, was such follow-up done?
Is there evidence that a screening neurological examination was done and recorded at the
time of the physical examination?
Was the screening neurological or history indicative of possible involvement (tremors,
paralysis, motor weakness or muscle atrophy, severe headaches, seizures, head trauma?
If indicated, was a complete, comprehensive neurological exam ordered, completed and
recorded in the medical record in a timely manner?
A-1630
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(b) Standard: Psychiatric Evaluation. Each patient must receive a
psychiatric evaluation that must—
Interpretive Guidelines §482.61(b)
The psychiatric evaluation is done for the purpose of determining the patient’s diagnosis
and treatment and, therefore, it must contain the necessary information to justify the
diagnosis and planned treatment.
The psychiatric evaluation is a total appraisal or assessment of the patient’s illness. It is
the physician’s assessment of the contributing factors and forces in the evolution of the
patient’s illness including the patient’s perception of his or her illness. Through the
psychiatric evaluation the physician seeks to secure a biographical-historical perspective
of the patient’s personality, with a clear psychological picture of the patient as a specific
human being with his or her individual problems. While performing the psychiatric
evaluation, the physician reaches an understanding of the patient’s basic personality
structure, of the patient’s developmental period, of his or her value systems, of his or her
past medical history including surgical procedures and other treatments, his or her past
psychological traumatic experiences, his or her defense mechanisms, his or her
supporting systems, any precipitating factors and how all these may have impacted and
interplayed with each other to result in the present illness. In the psychiatric evaluation
the patient should emerge as a dynamic human being with a past, a present and a
potential future with a thread of logical continuity.
The psychiatric evaluation includes all the requirements described in this standard and the
information necessary to justify the diagnosis and treatment. A physician’s signature is
necessary. In those cases where the mental status portion of the psychiatric evaluation is
performed by a non-physician, there should be evidence that the person is credentialed by
the hospital, legally authorized by the State to perform that function, and a physician
review and countersignature is present, where required by hospital policy or State law.
In order to satisfy the requirements §482.61(b) (1-7) of this standard, and to meet the
standards of medical practice, the psychiatric evaluation should include the following
component parts:
Survey Procedures §482.61(b)
The patient’s chief complaints and/or reaction to hospitalization, recorded in patient’s
own words where possible. Why is the patient in the hospital? Was it his/her idea? (Does
he/she feel ill/disturbed/frightened?) Is the patient in the hospital against his/her will?
Who decided to hospitalize/why?
Past history of any psychiatric problems and treatment, including prior precipitating
factors, diagnosis, course and treatment. Has the patient been chronically ill?
Continuously/repeatedly? How severely has the past illness/treatment interfered with the
patient’s development and/or adjustment? Are there persistent symptoms/signs/behaviors
that must be addressed and treated in order to favorably impact on the future psychiatric
course? What medications or supports helped him/her improve in the past? Are the same
resources available to impact on the patient’s treatment during this episode?
Past family, educational, vocational, occupational and social history. To what extent, if
any, is there a presence or absence of familial predisposition? What is the patient’s
educational level? Was he/she a good student? Is he/she still interested in learning? What
jobs has the patient held? For how long? Is he/she now employed/unemployed? For how
long? Has he/she ever worked? How does the patient get along with people? As a child,
did he/she have friends? Does he/she have friends now? Within the psychiatric evaluation
does one find the specific signs and symptoms, and other factors, that justify the
diagnosis?
A-1631
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(b)(1) Be completed within 60 hours of admission;
Interpretive Guidelines §482.61(b)(1)
Guidance is pending and will be updated in future release
A-1632
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(b)(2) Include a medical history
Interpretive Guidelines §482.61(b)(2)
The psychiatric evaluation must include the non-psychiatric medical history including
physical disabilities, intellectual disabilities and treatment.
Survey Procedures §482.61(b)(2)
Does the evaluation include:
Relevant past surgery? Past medical conditions and disabilities especially those of a
chronic nature?
Have these contributed to the patient’s psychiatric condition? How?
Are any of these conditions still present to any significant degree? Are they likely to
impact on the patient’s recovery/remission? Should they be addressed immediately? Does
the facility have the capability to intervene? If not, how is the need to be met?
A-1633
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(b)(3) Contain a record of mental status;
Interpretive Guidelines §482.61(b)(3)
The mental status must describe the appearance and behavior, emotional response,
verbalization, thought content, and cognition of the patient as reported by the patient and
observed by the examiner at the time of the examination. This description is appropriate
to the patient’s condition.
Explore the mental status for descriptions of the patient’s presentation during the
examination that are relevant to the diagnosis and treatment of the patient. An example of
a portion of the patient interview: The patient periodically states the examiner’s name
correctly during this examination after hearing it once, accurately describes his past
history in great detail, precisely characterizes his present situation, can list events in
logical sequence that have led to his present illness, but believes that his pre-admission
insomnia, anorexia, and 35 pound weight loss over the past four months are totally the
result of his sexual promiscuity of ten years ago and have nothing to do with his
concurrent use of 50 to 60 mg. of Amphetamine daily.” From this information one can
conclude that the patient is oriented, his memory is intact, but that he has poor judgment
and no insight. It is not acceptable just to write “oriented, memory intact, judgment poor,
and insight nil,” without any supportive information.
A-1634
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(b)(4) Note the onset of illness and the circumstances leading to admission;
Interpretive Guidelines §482.61(b)(4)
In a hospitalized patient, the identified problem should be related to the patient’s need for
hospital admission. The psychiatric evaluation includes a history of present illness,
including onset, precipitating factors and reason for the current admission, signs and
symptoms, course, and the results of any treatment received.
Survey Procedures §482.61(b)(4)
How long has the patient been ill? Was it a gradual or sudden onset?
Is this a recurrence?
What were the precipitating factors? What happened?
What symptoms, signs, behaviors made this hospitalization necessary?
What treatment has the patient already received before coming to the hospital?
Is any medication he received listed?
A-1635
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(b)(5) Describe attitudes and behavior;
Interpretive Guidelines §482.61(b)(5)
The problem statement should describe behavior(s) which require change in order for the
patient to function in a less restrictive setting. The identified problems may also include
behavioral or relationship difficulties with significant others which require active
treatment in order to facilitate a successful discharge.
A-1636
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(b)(6) Estimate intellectual functioning, memory functioning and
orientation; and
Interpretive Guidelines §482.61(b)(6)
Refer to §482.61(b)(3)
A-1637
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(b)(7) Include an inventory of the patient’s assets in descriptive, not
interpretive fashion.
Interpretive Guidelines §482.61(b)(7)
Although the term strength is often used interchangeably with assets, only the assets that
describe personal factors on which to base the treatment plan or which are useful in
therapy represent personal strengths. Strengths are personal attributes i.e., knowledge,
interests, skills, aptitudes, personal experiences, education, talents and employment
status, which may be useful in developing a meaningful treatment plan. For purposes of
the regulation, words such as “youth,” “pretty,” “Social Security income,” and “has a
car” do not represent assets. (See also §482.61(c)(1).)
A-1640
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(c)(1) Standard Treatment Plan. Each patient must have an individualized,
comprehensive treatment plan based on an inventory of the patient’s strengths and
disabilities.
Interpretive Guidelines §482.61(c)(1)
The patient and treatment team collaboratively develop the patient’s treatment plan. The
treatment plan is the outline of what the hospital has committed itself to do for the
patient, based on an assessment of the patient’s needs. The facility selects its format for
treatment plans and treatment plan updates.
Survey Procedure §482.61(c)(1)
Determination of compliance regarding treatment plans is accomplished by the surveyor
using the following methods, and to the extent possible, the following order:
1. Observation of the patient and staff at planned therapies/meetings, in various settings
both on and off the patient units, in formal and informal staff-patient interactions and in a
variety of daily settings;
2. Interviews with patients, families, treatment staff and others involved directly or
indirectly with active treatment;
3. Reviews of scheduled treatment programs (individual, group, family meetings,
therapeutic activities, therapeutic procedures);
4. Attendance at multidisciplinary treatment planning meetings, if time permits; and
5. Medical record review.
Has the information gained from assessing/evaluating the patient been utilized to create
an individualized treatment plan?
A disability is any psychiatric, biopsychosocial problem requiring treatment/intervention.
The term disability and problem are used interchangeably. The treatment plan is derived
from the information contained in the psychiatric evaluation and in the
assessments/diagnostic data collected by the total treatment team. Based on the
assessment summaries formulated by team members of various disciplines, the treatment
team identifies which patient disabilities will be treated during hospitalization. Patient
strengths that can be utilized in treatment must be identified. (See also §482.61(b)(7).)
Treatment planning depends on several variables; whether the admission is limited to
crisis intervention, short-term treatment or long-term treatment. The briefer the hospital
stay, the fewer disciplines may be involved in the patient’s treatment.
There must be evidence of periodic review of the patient’s response and progress toward
meeting planned goals. If the patient has made progress toward meeting goals, or if there
is a lack of progress, the review must justify: (1) continuing with the current goals and
approaches; or (2) revising the treatment plan to increase the possibility of a successful
treatment outcome.
Consideration must be given to the type of psychiatric program(s) under review to
determine the timeframe for treatment plan review. The interval within which treatment
plan reviews are conducted is determined by the hospital, however, the hospital’s review
system must be sufficiently responsive to ensure the treatment plan is reviewed:
whenever a goal(s) has been accomplished; when a patient is regressing; when a patient is
failing to progress; or when a patient requires a new treatment goal. The facility is
expected to pursue aggressively the attendance of all relevant participants at the team
meetings. Question any routine and regular absences of individuals who would be
expected to attend.
Is the treatment plan individualized, i.e., patient-specific, or is there a predictable
sameness from plan to plan?
When packaged plans or programs are used, do staff include needed individual
adaptations in the plan?
Are the patient’s observed behaviors consistent with the problems and strengths
identified in the plan or update?
Have the views which the patient communicated to the surveyor regarding problems
which require treatment during hospitalization and plans for discharge, been incorporated
in the plan or update?
A-1641
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(c)(1)(i) The written plan must include—A substantiated diagnosis;
Interpretive Guidelines §482.61(c)(1)(i)
The substantiated diagnosis serves as the basis for treatment interventions. A
substantiated diagnosis is the diagnosis identified by the treatment team to be the primary
focus upon which treatment planning will be based. It evolves from the synthesis of data
from various disciplines.
At the time of admission, the patient may have been given an initial diagnosis or a rule-
out diagnosis. At the time of treatment planning, a substantiated diagnosis must be
recorded. It may be the same as the initial diagnosis, or, based on new information and
assessment, it may differ.
Rule-out diagnoses, by themselves are not acceptable as a substantiated diagnosis.
Data to substantiate the diagnosis may be found in, but is not limited to, the psychiatric
evaluation, the medical history and physical examination, laboratory tests, medical and
other psychological consults, assessments done by disciplines involved in patient
evaluations and information supplied from other sources such as community agencies and
significant others.
Survey Procedures §482.61(c)(1)(i)
What specific problems will be treated during the patient’s hospitalization?
Does the treatment plan identify and precisely describe problem behaviors rather than
generalized statements i.e., “paranoid,” “aggressive,” “depressed?” or generic
terminology i.e., “alteration in thought process,” “ineffective coping,” “alteration in
mood?”
Are physical problems identified and included in the treatment plan if they require
treatment, or interfere with treatment, during the patient’s hospitalization?
A-1642
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(c)(1)(ii) Short-term and long range goals;
Interpretive Guidelines §482.61(c)(1)(ii)
Based on the problems identified for treatment, short-term and long-range goals are
developed. Whether the use of short-term or a combination of short-term and long-range
goals is appropriate is dependent on the length of hospital stay.
Short-term and long-range goals include specific dates for expected achievement. As
goals are achieved, the treatment plan should be revised. When a goal is modified,
changed or discontinued without achievement, the plan should be reviewed for relevancy,
and updated as needed.
In crisis intervention and short-term treatment there may be only one timeframe for
treatment goals. As the length of hospital stay increases (often because of the long-term
chronic nature of the patient’s illness), both long-range and short-term goals are needed.
The long-range goal is achieved through the development of a series of short-term goals,
i.e., smaller, logical sequential steps which will result in reaching the long-range goal.
Both the short-term and long-range goals must be stated as expected behavioral outcomes
for the patient. Goals must be related to the problems identified for treatment. Goals must
be written as observable, measurable patient behaviors to be achieved. Discharge criteria
may be included as long-range goals.
Survey Procedures §482.61(c)(1)(ii)
How do treatment plan goals relate to the problems being treated?
Do goals indicate the outcomes to be achieved by the patient?
Are the goals written in a way that allow changes in the patient’s behavior to be
measured?
If not apparent, what criteria do staff use to measure success?
How relevant are the treatment plan goals to the patient’s condition?
A-1643
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(c)(1)(iii) The specific treatment modalities utilized;
Interpretive Guidelines §482.61(c)(1)(iii)
This requirement refers to all of the planned treatment modalities used to treat the patient
during hospitalization. Having identified the problems requiring treatment, and defining
outcome goals to be achieved, appropriate treatment approaches must be identified.
Modalities include all of the active treatment measures provided to the patient. It
describes the treatment that will be provided to the patient. It describes the treatment that
will be provided by various staff.
A daily schedule of unit activities does not, in itself, constitute planned modalities of
treatment. It is expected that when a patient attends various treatment
modalities/activities, it is a part of individualized planning with a specific purpose and
focus for that patient.
Simply “naming” modalities (i.e., individual therapy, group therapy, occupational
therapy, medication education) is not acceptable. The focus of the treatment must be
included.
Simply “stating” modality approaches (i.e., “set limits,” “encourage socialization,”
“discharge planning as needed”) is not acceptable. Modality approaches must be
specifically described in order to assure consistency of approach.
Observation of staff implementing treatment, both in structured and non-structured
settings, is a major criterion to determine whether active treatment is being provided in
accordance with planned treatment.
It must be clear to you that the active treatment received by the patient is internally
consistent and not simply a series of disconnected specific modalities delivered within
certain scheduled intervals.
Survey Procedures §482.61(c)(1)(iii)
Are qualified staff observed following the methods, approaches and staff intervention as
stated?
Can staff explain the focus of the modality they have provided?
Are observed treatment methods, approaches and interventions from all disciplines
included in the plan?
Do the pieces of the treatment plan work together to achieve the greatest possible gain for
the patient?
Does the hospital integrate its activities, therapies, treatments, and patient routines to
work for the patient’s therapeutic interest first, and its own convenience second?
Do the disciplines present at observed treatment planning meetings represent all of the
patient’s needs?
If the patient attends treatment planning, how do the staff prepare the patient to
participate?
If the patient does not attend, what reasons do staff give to explain the absence?
Is there a process to enable staff to reach a consensus regarding how treatment will be
carried out?
Is the patient included in the decision-making, whenever possible?
Are the final decisions regarding treatment approaches defined clearly by the end of the
discussion?
How does the patient get to know his/her treatment regime?
How does the treatment team encourage the patient to accept responsibility for engaging
in the treatment regime, rather than accepting it passively?
A-1644
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(c)(1)(iv) The responsibilities of each member of the treatment team; and
Interpretive Guidelines §482.61(c)(1)(iv)
There are no “correct” number of staff who comprise the treatment team. The disciplines
involved in the patient’s treatment depend upon the problems to be treated, the short-term
and long-range goals and the treatment approaches and modalities used to achieve the
goals.
The intent of the regulation is to insure that each individual on the treatment team who is
primarily responsible for ensuring compliance with particular aspects of the patient’s
individualized treatment program is identified. Identification of the staff should be
recorded in a manner that includes the name and discipline of the individual. If other
professionals or paraprofessionals provide care, the facility has the latitude to decide the
manner with which it will identify them on the treatment plan.
The patient, as well as family/significant others, should be aware of the staff responsible
for various aspects of treatment.
Survey Procedures §482.61(c)(1)(iv)
Are staff who are designated in the treatment plan observed carrying out treatment
activities and therapies? Is the information in the plan consistent with surveyor
observations?
Are the patients able to name the staff responsible for implementing their treatment? Is
this information consistent with the treatment plan?
A-1645
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(c)(1)(v) Adequate documentation to justify the diagnosis and the treatment
and rehabilitation activities carried out.
Interpretive Guidelines §482.61(c)(1)(v)
When the progress and treatment notes are reviewed, the content of the notes must relate
to the treatment plan. The notes must indicate what the hospital staff is doing to carry out
the treatment plan and the patient’s response to the interventions.
Survey Procedures §482.61(c)(1)(v)
Are the treatment notes relative to the identified problems?
Are the treatment notes indicative of the patient’s response to treatment?
Do the progress notes relate to specific patient problems or progress?
A-1650
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(c)(2) The treatment received by the patient must be documented in such a
way to assure that all active therapeutic efforts are included.
Interpretive Guidelines §482.61(c)(2)
Active treatment is an essential requirement for inpatient psychiatric care. Active
treatment is a clinical process involving ongoing assessment, diagnosis, intervention,
evaluation of care and treatment, and planning for discharge and aftercare, under the
direction of a psychiatrist. The patient is in the hospital because it has been determined
that the patient requires intensive, 24 hour, specialized psychiatric intervention that
cannot be provided outside the psychiatric hospital. The medical record must indicate that
the hospital adheres to the patient’s right to be counseled about medication, its intended
effects, and the potential side effects. If the patient requires, because of danger to self or
others, a more restrictive environment, the hospital must indicate that the staff attempted
to care for the patient in the least restrictive setting before progressing to a more
restrictive setting.
Through observation, look for evidence that each patient is receiving all the aspects of
treatment to which the hospital has committed itself based upon his/her assessment,
evaluation and plan of care. It is the hospital’s responsibility to provide those treatment
modalities with sufficient frequency and intensity to assure that the patient achieves
his/her optimal level of functioning.
Through observation and interviews, look for evidence that each patient’s rights are being
addressed and protected. There should be policies and procedures in place to address the
following areas: informed consent, confidentiality, privacy, and security. Expect to see
detailed policies and procedures regarding the therapeutic use of restrictions, such as
visitors, mail, and phone calls. Seclusion and restraint policies and procedures must
address patient protection and safety while in a restricted setting.
Clarification of the types of notes found in the medical record.
Treatment notes are recordings in the medical record that indicate provision of, and a
patient’s response to, a specific modality. This modality may be drug therapy, individual,
family, marital, or group therapy, art therapy, recreational therapy, and any specialized
therapy ordered by the physician or anyone credentialed by the facility, in accordance
with the State law, to write orders in the medical record.
A combined treatment and progress note may be written.
Progress notes are recordings in the medical record that are written by persons directly
responsible for the care and active treatment of the patient. Progress notes give a
chronological picture of how the patient is progressing toward the accomplishment of the
individual goals in the treatment plan. These are frequently shift notes, weekly notes, or
monthly notes.
Survey Procedures §482.61(c)(2)
Does the patient know his/her diagnosis?
What did the patient contribute to the formulation of the treatment plan? Goals of
treatment?
If the patient receives medication, does the patient understand the reason for the
medication? The name of the medication? The dose prescribed? The time of
administration? The desired effects? The potential side effects?
If medication is changed, is there a rationale for the change?
Are staff members recording their observations relative to the patient’s response to the
treatment modalities, including medication?
Is there evidence that the patient was afforded the opportunity to participate in his/her
plan of care?
What progress has the patient made? Has the patient achieved his/her optimal level of
functioning? If not, why? Are these reasons/barriers reflected in the current treatment
plan? Do treatment and progress notes support these insights?
Does the observed status of the patient in the various treatment modalities correspond to
the progress note reports of status?
Do all treatment team members document their observations and interventions so that the
information is available to the entire team?
If a restrictive procedure is used (e.g., restraint and/or seclusion), is there evidence that
attempts were made systematically to treat the patient in the least restrictive manner?
Is there evidence that the rights of the patient were protected while in the restrictive
setting in accordance with Federal and State law and accepted standards of practice?
A-1655
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(d) – Standard: Recording Progress.
Progress notes must be recorded by the physician(s), psychologists, or other licensed
independent practitioner(s) responsible for the care of the patient as specified in
§482.12(c); nurse, social worker and, when appropriate, others significantly
involved in active treatment modalities.
Interpretive Guidelines §482.61(d)
Refer to §482.61(c)(2) Interpretive Guidelines for clarification between treatment notes
and progress notes. The recording of progress is evidence of individual patient
performance. Specifically, the progress notes recorded by the professional staff, or others
responsible for the patient’s treatment, must give a chronological picture of the patient’s
progress or lack of progress towards attaining short and long-range goals outlined in the
individual treatment plan. Progress notes should relate to the goals of the treatment plan.
Notes that state, “patient slept well” or “no complaints” constitute observations and do
not indicate how the patient is responding to treatment and progressing towards set goals.
Frequency alone does not determine the adequacy of progress notes. Expect to see greater
frequency when patients are more acutely ill and/or in a crisis of some kind. Notes should
be dated and signed (signature and title or discipline).
Survey Procedures §482.61(d)
Are the physicians who are significantly involved in active treatment
modalities/interventions actually documenting progress?
Do the progress notes relate to the goals of the treatment plan? Do they include precise
statements of progress?
Is there a correlation between what is observed by the surveyor and what is described in
the notes?
Do the notes give a clear picture of the patient’s progress or lack thereof, during the
course of hospitalization?
In reviewing the patient’s progress, are aftercare/discharge plans being evaluated?
Are the nurses who are significantly involved in active treatment modalities/interventions
actually documenting progress?
Are the social workers that are significantly involved in active treatment
modalities/interventions plan actually documenting progress?
Are staff from other disciplines, i.e., rehabilitative therapy and psychology, which are
significantly involved in active treatment modalities/interventions actually documenting
progress?
A-1660
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(d) . . .The frequency of progress notes is determined by the condition of the
patient but must be recorded at least weekly for the first 2 months and at least once
a month thereafter . . .
Interpretive Guidelines §482.61(d)
Guidance is pending and will be updated in future release
Survey Procedures §482.61(d)
What is the frequency of progress notes in relation to the condition of the patient?
A-1661
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(d) . . .and must contain recommendations for revisions in the treatment
plan as indicated . . .
Interpretive Guidelines §482.61(d)
Guidance is pending and will be updated in future release
Survey Procedures §482.61(d)
Do the progress notes contain documentation substantiating changes/revisions in the
treatment plan and subsequent assessment of the patient’s responses and progress?
A-1662
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(d) . . . as well as [must contain] a precise assessment of the patient’s
progress in accordance with the original or revised treatment plan.
Interpretive Guidelines §482.61(d)
Guidance is pending and will be updated in future release
Survey Procedures §482.61(d)
Do the notes give a clear picture of the patient’s progress, or lack thereof, during the
course of hospitalization?
Are the progress notes related to the goals of the treatment plan?
A-1670
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(e) Standard: Discharge planning and discharge summary. The record of
each patient who has been discharged must have a discharge summary that includes
a recapitulation of the patient's hospitalization and…
Interpretive Guidelines §482.61(e)
The record of each patient who has been discharged should indicate the extent to which
goals established in the patient’s treatment plan have been met.
As part of discharge planning, staff consider the discharge alternatives addressed in the
psychosocial assessment and the extent to which the goals in the treatment plan have
been met.
The surveyor should refer to hospital policy for discharge timeframes.
The discharge summary should contain a recapitulation of the patient’s hospitalization,
which is a summary of the circumstances and rationale for admission, and a synopsis of
accomplishments achieved as reflected through the treatment plan. This summary
includes the reasons for admission, treatment achieved during hospitalization, a baseline
of the psychiatric, physical and social functioning of the patient at the time of discharge,
and evidence of the patient/family response to the treatment interventions.
A-1671
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(e) [The record of each patient who has been discharged must have a
discharge summary that includes] . . . recommendations from appropriate services
concerning follow-up or aftercare as well as …
Interpretive Guidelines §482.61(e)
The patient’s discharge summary should describe the services and supports that are
appropriate to the patient’s needs and that will be effective on the day of discharge.
Examples include:
• A complete description of arrangements with treatment and other community resources
for the provision of follow-up services. Reference should be made to prior verbal and
written communication and exchange of information;
• A plan outlining psychiatric, medical/physical treatment and the medication regimen as
applicable;
• Specific appointment date(s) and names and addresses of the service provider(s);
• Description of community housing/living arrangement;
• Economic/financial status or plan, i.e., supplemental security income benefits;
• Recreational and leisure resources; and A complete description of the involvement of
family and significant others with the patient after discharge.
Survey Procedures §482.61(e)
How does the discharge planning process verify appointment source(s), dates and
addresses?
How was the patient involved in the discharge and aftercare planning process?
Were discharge related documents made available to the patient, family, community
treatment source and/or any other appropriate sources?
Is there indication that the discharge planning process included the participation of
multidisciplinary staff and the patient? Have the results been communicated to the post-
hospital treatment entity?
Is there evidence that contact with the post-hospital treatment entity included
communication of treatment recommendations (including information regarding the
patient’s medications)?
Is a contact person named, and does the patient have a specific appointment date and time
for the initial follow-up visit?
A-1672
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.61(e) [The record of each patient who has been discharged must have a
discharge summary that includes] … a brief summary of the patient’s condition on
discharge.
Interpretive Guidelines §482.61(e)
The patient’s discharge planning process should address anticipated problems after
discharge and suggested means for intervention, i.e., accessibility and availability of
community resources and support systems including transportation, special problems
related to the patient’s functional ability to participate in aftercare planning.
The discharge summary and/or plan should contain information about the status of the
patient on the day of discharge, including psychiatric, physical and functional condition.
A-1680
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62 Condition of Participation: Special Staff Requirements for
Psychiatric Hospitals
The hospital must have adequate numbers of qualified professional and supportive
staff to evaluate patients, formulate written, individualized comprehensive
treatment plans, provide active treatment measures and engage in discharge
planning.
Interpretive Guidelines §482.62
The purpose of this Condition of Participation is to ensure that the psychiatric hospital is
adequately staffed with qualified mental health professionals and supportive staff to carry
out an intensive and comprehensive active treatment program and to protect and promote
the physical and mental health of the patients.
Through observation, interview and record review determine if numbers and/or
deployment of qualified staff is a concern. Review incident reports, medication error
reports, patient and staff injury reports, for indications that staffing is an issue.
Adequate numbers are defined to mean the numbers, and deployment, of staff with
qualifications to evaluate, plan, implement and document active treatment.
Do not look at numbers alone. The hospital is responsible for organizing its available
staff and administrative duties along with patient appointments, treatment plan meetings,
treatment sessions, activities, materials, equipment and patient assignments to wards and
groups in such a way that results in patients achieving the maximum therapeutic benefit.
Survey Procedure §482.62
Assess the adequacy of the Special Staffing Condition by:
1. Observing sampled patients and others during structured sessions and in unstructured
settings. You should be able to observe behavioral evidence of a rational organization
of resources.
2. Next, interview patients and staff to determine whether or not necessary treatment
modalities and other services are being provided in a timely manner.
3. Next review the medical records of patients in the sample to ascertain if necessary
active treatment assessments, treatments, evaluations and activities have been
conducted and documented.
4. Also, review other records such as restraint and seclusion records, incident reports,
medication error reports, reports of patient/staff injuries, etc., to determine the extent
to which staffing levels or deployment contributed to negative patient outcomes.
5. Evaluate all outcome data in light of the success or failure observed during the survey
relevant to each patient receiving active treatment, and achieving desired outcomes of
care. This is the primary basis for evaluating the adequacy of the hospital’s staffing
under this Special Condition.
A-1685
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(a)(1) Standard: Personnel.
The hospital must employ or undertake to provide adequate numbers of qualified
professional, technical, and consultative personnel to:
(1) Evaluate Patients.
Interpretive Guidelines §482.62(a)(1)
Guidance is pending and will be updated in future release
Survey Procedures §482.62(a)(1)
Is there adequate staff to assure that the admission work-ups (assessment, diagnostic data
gathering) are completed in a timely manner?
Is there evidence that there is continuing evaluation of the patient’s progress and response
to treatment?
Are evaluations delayed or absent?
A-1686
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(a)(2) [The hospital must employ or undertake to provide adequate numbers
of qualified professional, technical, and consultative personnel to:]
(2) Formulate written individualized, comprehensive treatment plans;
Interpretive Guidelines §482.62(a)(2)
Staffing must be sufficient so that members of the patient’s treatment team and others
responsible for evaluation and assessment can contribute their respective data for
consideration in the formulation of the treatment plan.
Survey Procedures §482.62(a)(2)
Was there sufficient discipline participation at the treatment team meeting to assure
formulation of a treatment plan that meets the patient’s individualized needs?
What problems prevent staff members from attending treatment meetings? Do they relate
to staffing?
Are the assessments/evaluations absent or delayed to the extent that they are not useful to
the treatment team for the purpose of planning individualized treatment?
A-1687
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(a)(3) [The hospital must employ or undertake to provide adequate numbers
of qualified professional, technical, and consultative personnel to:]
(3) Provide active treatment measures; and
Interpretive Guidelines §482.62(a)(3)
Active treatment occurs when the patient receives treatment interventions that are
delivered under the direction of a physician, and which are specific to patient strengths,
disabilities, and problems identified in the treatment plan. Treatment interventions and
other services are furnished in accordance with accepted standards of professional
practice. Although the active treatment process must be identifiable in documentation, it
must be first and foremost observable and evident in daily practice.
Treatment interventions need to be individualized, in that the patient receives assistance
with resolving or ameliorating the problems/circumstances that led to hospitalization.
Expect to see treatment focused on the unique needs of individual patients. For example,
several patients may be referred to “Anger Management Group,” but the focus of
discussion and therapeutic intervention may differ depending on the individual patient’s
particular issue regarding managing anger.
Whether structure must be imposed by staff or whether the patient can direct his or her
own activities for periods of time (without staff supervision), is based on the patient’s
ability to engage in constructive, appropriate behavior (without engaging in harm to self
or others). Be certain that the patient’s time on the unit is maximized toward the further
development of appropriate desired outcomes, including but not limited to leisure and
recreation.
Survey Procedures §482.62(a)(3)
Through observation, interviews and record reviews, can you determine that patients
receive active treatment?
Is the distribution of staff consistent with particular patient needs? Is appropriate staffing
sufficient to carry out treatment plans?
Does the patient attend therapies that are relevant to the identified problems that brought
the patient to the hospital?
Are staff absences and/or vacancies preventing the patient from receiving active
treatment? Are patients not attending therapeutic activities off the unit because there is no
staff to escort them? Are therapeutic groups not available on the unit for patients who are
not able to go off the unit?
Are patients observed not engaged in activities while staff attend to administrative tasks?
Are active treatment sessions or activities carried out at discrete time intervals
exclusively? Or is active treatment implemented as the patient’s needs emerge during the
course of the day, as well?
Does a review of quality assurance data reveal a pattern of serious incidents occurring on
particular shifts and/or days of the week?
What do patients report to the surveyor are their treatment modalities?
Do patient interviews indicate that patients believe the treatment being provided is
helpful?
Does the scheduling of activities and their content relate directly to the patient’s
treatment objectives or are the activities/content generalized, non-therapeutic “time-
fillers”?
Can staff describe how their activities relate to the patient’s treatment objectives?
At any point in time, in any of the patient’s experiences in the hospital is the thrust of the
patient’s treatment plan observable during the staff and/or patient interactions?
Is there a consistent, observable pattern of evidence that hospital staff provide, reinforce
and otherwise implement measures to achieve active treatment objectives?
A-1688
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(a)(4) [The hospital must employ or undertake to provide adequate numbers
of qualified professional, technical, and consultative personnel to:]
(1) Engage in discharge planning.
Interpretive Guidelines §482.62(a)(4)
The patient together with all relevant professionals caring for the patient should be
expected to participate in the discharge planning process. Staffing should be sufficient to
facilitate this outcome, to the maximum extent possible.
Survey Procedures §482.62(a)(4)
Do patients participate in their discharge planning process? If not, why?
Do staff interviews elicit information that staff working with patients are aware of the
discharge plans for those patients?
Do record review and interviews indicate that all relevant staff have participated in
discharge planning?
A-1690
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(b) Standard: Director of inpatient psychiatric services; medical staff.
Inpatient psychiatric services must be under the supervision of a clinical director,
service chief, or equivalent who is qualified to provide the leadership required for
an intensive treatment program…
Interpretive Guidelines §482.62(b)
Inpatient psychiatric services include the following functions: admission interviews,
assessments and evaluations; psychiatric and medical work-ups; treatment team
leadership; medication management; on-call provision of emergency psychiatric and
medical treatment; provision of individual, group and family therapies; provision of
clinical supervision to other professionals and paraprofessionals; provision of medical
and psychiatric educational workshops and conferences for all staff; and provision of
consultation to staff for clinical and/or administrative matters.
The clinical director is ultimately responsible for the medical and psychiatric care that is
provided to patients. The clinical director should ascertain that quality improvement
programs are in place to monitor all areas of patient care, and should implement
educational programs for all levels of staff.
Survey Procedure §482.62(b)
Just prior to the end of the survey, schedule a meeting with the clinical director. By the
time of this meeting, you should already have conducted required observation, interviews
and record reviews for at least a majority of the patients in the sample. Collect any
additional information that is necessary to consider in light of outcomes observed for
patients, including: the qualifications of the clinical director; the leadership exhibited for
the scope of psychiatric/medical treatment programs needed by patients; and the rationale
for medical staffing coverage. If necessary, follow-up on letters of complaint previously
reported serious problems, discrepancies with Data Collection Medical Staff Coverage
(CMS-729).
A-1691
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(b) …The number and qualifications of doctors of medicine and osteopathy
must be adequate to provide essential psychiatric services.
Interpretive Guidelines §482.62(b)
The number of full-time, part-time and consulting staff, who are board certified within
each category and their availability to the hospital must be adequate to provide
psychiatric services, as described above. Adequacy is considered in light of the
following:
1. Number of admissions, discharges and current patients by treatment units;
2. Size of the hospital;
3. Geographic proximity of the wards and units;
4. Organization and kinds of treatment services rendered to the patients;
5. Availability of the physician coverage on evening, nights and weekends;
6. Availability of physicians to participate in treatment planning;
7. Availability of psychiatrists to consult with non-psychiatric physicians about
psychotropic medication regimens; and
8. Availability of physicians to consult with multi-disciplinary staff about treatment
issues.
Survey Procedures §482.62(b)
How many staff are board certified? Fully trained? How many full-time/part-time
specialties are represented?
How are medical staff deployed? To what programs/units are they assigned? Why?
How much time do physicians spend on the units? Based on observations, interviews, and
medical record reviews is coverage adequate to meet the needs of sampled patients? To
meet the needs of other patients observed during the survey?
A-1692
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(b)(1) The clinical director, service chief or equivalent must meet the
training and experience requirements for examination by the American Board of
Psychiatry and Neurology, or the American Osteopathic Board of Neurology and
Psychiatry.
Interpretive Guidelines §482.62(b)(1)
A physician is qualified to take the examinations for board certification upon successful
completion of a psychiatric residency program approved by the American Board of
Psychiatry and Neurology and/or the American Osteopathic Board of Psychiatry and
Neurology.
Survey Procedures §482.62(b)(1)
Review the clinical director’s personnel folder or ask the clinical director if he/she has
one of the following:
a. Certification of the American Board of Psychiatry and Neurology and/or certification
of the American Osteopathic Board of Neurology and Psychiatry.
b. If no certification, evidence that the person took the Boards would satisfy that the
person had the training and equivalency to be admitted to the board examination.
c. If indicated, medical school and residency training
d. Length of time he has been employed at the facility; length of time he has been at his
position
To be admitted to the American Board Examinations the following conditions must be
met:
1. License without restrictions
2. Graduation from a medical school approved by either the Medical Osteopathic
Association or the American Medical Association.
3. A successful completion of an approved residency-training program for at least 3 years
before 1988 that the America Council on Graduate Medical Education (ACGME) approves.
After 1988, it has to be a four year accredited program.
A-1693
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(b)(2) The director must monitor and evaluate the quality and
appropriateness of services and treatment provided by the medical staff.
Interpretive Guidelines §482.62(b)(2)
Services and treatment prescribed to patients must be in accordance with appropriate and
acceptable standards of practice.
In states that allow psychologists to have admitting privileges, it is still the responsibility
of the clinical director to oversee the quality of the patient’s treatment.
Survey Procedures §482.62 (b)(2)
What mechanisms does the director use to monitor and evaluate the work of the medical
staff (personal interviews? Quality Improvement reports? incident reports?)?
When problems are discovered by the clinical director, how are they corrected?
Are services, notes, and reports timely?
Are medications used appropriately for the patient’s diagnosis?
A-1695
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(c) Standard: Availability of medical personnel.
Doctors of medicine or osteopathy and other appropriate professional personnel
must be available to provide necessary medical and surgical diagnostic and
treatment services. If medical and surgical diagnostic services and treatment are not
available within the institution, the institution must have an agreement with an
outside source of these services to ensure that they are immediately available or a
satisfactory agreement must be established for transferring patients to a general
hospital that participates in the Medicare program.
Interpretive Guidelines §482.62(c)
Contracts or other arrangements with individuals and/or providers assure that medical
and surgical services are available to meet the needs of the patients. Review the medical
and surgical services provided by the hospital during the interview with the clinical
director.
Discuss contract or arrangements with the clinical director for services provided off
grounds.
Survey Procedures §482.62(c)
How did the hospital meet the medical/surgical/diagnostic needs represented by each
patient in the sample? Were these done timely? Appropriately?
If contracts are not current or available, how are these services provided for the patient, if
needed? Is there evidence of negative outcomes as a result of these arrangements?
Are reports from other services such as pharmacy, radiology, and clinical laboratory
timely? Appropriate?
A-1700
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(d) Standard: Nursing services.
The hospital or unit must have a qualified director of psychiatric nursing services.
In addition to the director of nursing, there must be adequate numbers of registered
nurses, licensed practical nurses, and mental health workers to provide nursing care
necessary under each patient’s active treatment program and to maintain progress
notes on each patient.
Interpretive Guidelines §482.62(d)
Psychiatric nursing functions may include the following: supervision of paraprofessional
staff; assessment, planning, provision, and evaluation of psychiatric nursing care to
patients; medication teaching; management of the therapeutic milieu; provision of
mandatory and voluntary in-service training to all staff; and provision of specialized
treatments and therapies, such as individual, group and family therapies, that require the
clinical expertise of a professional psychiatric nurse.
Expect to see evidence of orientation programs as well as ongoing continuing education
programs for Licensed Practical Nurses and mental health workers that stress
individualized treatment interventions.
Determine that there is a qualified Director of Nursing (DON) providing the required
leadership and supervision for the psychiatric nursing department.
A-1701
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(d)(1) The director of psychiatric nursing services must be a registered nurse
who has a master’s degree in psychiatric or mental health nursing or its equivalent
from a school of nursing accredited by the National League for Nursing, or be
qualified by education and experience in the care of the mentally ill . . .
Interpretive Guidelines §482.62(d)(1)
During the interview with the DON, assess his/her educational background and
psychiatric nursing and leadership skills. If the DON has less than a Master’s Degree in
Psychiatric Nursing, expect to see evidence of experience and on-going training in
psychiatric nursing. Documented consultation from a nurse with a Master’s in Psychiatric
Nursing constitutes on-going training.
Survey Procedures §482.62(d)(1)
Are nursing assessments completed on all patients?
Do the multidisciplinary treatment plans reflect nursing input which include specific
nursing interventions for nursing problems (e.g. violence toward self/others,
physical/medical crises)?
Is nursing care evaluated by an R.N., with changes in the care based on the patient’s
progress or lack thereof?
Are intrusive techniques (e.g. seclusion, restraint, electroconvulsive therapy (ECT),
and/or medical procedures) and patient incidents (e.g. medication errors, patient falls,
patient-to-patient and patient-to-staff injuries) monitored in accordance with hospital
policy, State statutes and safe nursing practice?
Are nursing personnel observed relating to patients in a therapeutic manner?
A-1702
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(d)(1) . . . The director must demonstrate competence to participate in
interdisciplinary formulation of individual treatment plans; to give skilled nursing
care and therapy; and to direct, monitor, and evaluate the nursing care furnished.
Interpretive Guidelines §482.62(d)(1)
Based on structured observations of the patients in the sample and other patients in the
hospital, patient and staff interviews and medical record review, ascertain that nursing
services are provided in accordance with safe, acceptable standards of nursing practice.
Information obtained from the DON should include: implementation of continuous
quality improvement programs; provision of orientation, in-service and continuing
education programs for nursing personnel especially in the areas of psychiatric nursing,
nursing process, prevention and management of violence, CPR and Universal
Precautions.
A-1703
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(d)(2) The staffing pattern must ensure the availability of a registered nurse
24 hours each day. . . .
Interpretive Guidelines §482.62(d)(2)
Guidance is pending and will be updated in future release
A-1704
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(d)(2) . . .There must be adequate numbers of registered nurses, licensed
practical nurses, and mental health workers to provide the nursing care necessary
under each patient’s active treatment program.
Interpretive Guidelines §482.62(d)(2)
The evaluation of sufficient numbers and level of RNs, LPNs and mental health workers
is based on the patient characteristics as seen in structured observations of patients in the
sample and other patients in the hospital, patient interviews, and as evidenced in medical
records and other data related to patients (e.g. incident reports, seclusion/restraint
reports). Patient care assignments should be appropriate to the skills and qualifications of
the nursing personnel providing patient care.
There should be evidence that all nursing personnel have education, experience and/or
training in psychiatric care. Mental health workers spend the majority of their workday
interacting with patients. Expect to see evidence that they are receiving on-going
supervision and training. Mental health workers should be assigned patient care duties
and therapeutic modalities that reflect their educational level, psychiatric training, and
experience.
Survey Procedure §482.62(d)(2)
The nursing staffing patterns should be reviewed on a sample of approximately 25% of
the certified wards. The staffing, including levels of nursing personnel, should be
reviewed for the day(s) of the survey and evaluated based on the level of needs presented
by the patients. Additional staffing patterns shall be reviewed if a problem or concern is
evidenced. Decisions regarding extent of additional data (number of wards and dates) to
be reviewed shall be based on the degree of problem/concern. Patient need
assessment/patient acuity shall be reviewed for any wards as deemed necessary based on
problems/concerns found in the sampling review.
If your observations and/or interviews indicate a staffing problem, you may want to
consider the following variables in assessing adequacy of nursing personnel coverage:
1. Organization and types of services provided to patients by the nursing department;
2. Number and levels of nursing care needs of patients, including average length of stay,
acuity of patients and nursing care requirements;
3. Number and levels of nursing personnel based on the roles and functions required of
nursing;
4. Number of suicidal/assaultive patients;
5. Seclusion/restraint incidents;
6. Number of admissions and discharges;
7. Number and type of accidents and/or injuries;
8. Amount and complexity of medication regimens;
9. Medication errors;
10. Use of P.R.N. (as needed) medications;
11. Medical (physical) procedures;
12. Assignment and utilization of “pool” nursing personnel (those staff who are hired
through a contract service and are not employees of the hospital). Contractual staff should
receive orientation and training necessary for assigned functions, and should be
supervised by employees of the hospital;
13. Availability of RNs to supervise/consult with nursing/non-nursing personnel about
patient care;
14. Availability of RNs to assess and implement care in crisis situations;
15. Availability of RNs to interact with patients in structured activities; and
16. Involvement of patients with personnel.
A-1710
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(e) Standard: Psychological Services.
The hospital must provide or have available psychological services to meet the needs
of the patients.
Interpretive Guidelines §482.62(e)
Psychology services may include the following: diagnostic testing and diagnostic
formulations on request from physicians; provision of individual, group and family
therapies; participation in multi-disciplinary treatment conferences; and program
development and evaluation.
The number of full-time, part-time and consulting psychologists must be adequate to
provide necessary services to patients. Arrangements with outside resources must assure
that necessary patient services will be provided.
Survey Procedures §482.62(e)
Did the patients in the sample have a need for psychological services or testing? Were
they provided in a timely manner and with sufficient intensity?
Did any of the patients in the sample indicate a need for psychological services, but none
were requested?
What types of psychological services are offered? (e.g., assessments, therapy)
Do certain groups of patients receive testing routinely? Dementia?, Children?,
Adolescents? Why?
Once tests are performed, are results reported in sufficient time to be integrated in the
patient’s active treatment and treatment plan?
How does the hospital or Psychological Service Department determine whether or not: it
meets the needs of patients? Its services are underutilized or over-utilized?
Why have psychological services staff been deployed in the manner that they have?
A-1715
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(f) Standard: Social Services.
There must be a director of social services who monitors and evaluates the quality
and appropriateness of social services furnished. The services must be furnished in
accordance with accepted standards of practice and established policies and
procedures.
Interpretive Guidelines §482.62(f)
Social work functions may include the following functions: Intake or admission
screening, psychosocial assessment of a newly admitted patient; developing an update or
detailed re-assessment of the patient; high-social risk case finding; contact with family
and others significant in the patient’s life. Such functions may include patient and family
education, support, and advocacy; providing coordination/liaison with community-based
social and mental health agency(ies) regarding the pre-admission status of the patient;
participating as a member of the treatment team in development of treatment planning
and subsequent planned interventions (modalities). Such modalities may include
supportive, individual, couple, family, or group therapy, aimed at meeting specified goals
identified in the treatment plan.
Continuity of care is an important social work principle and may be demonstrated
through case management and a major role in discharge planning. Activities, in
conjunction with the patient wishes, may include contact with patient’s family,
identifying and assisting in referral of the patient to community-based agency(ies) at the
time of discharge. Finally, post-discharge follow-up may be done to assure that linkage of
the patient with community resources has occurred to reduce re-hospitalization.
Determine who completed the assessment required by §482.61(a)(4) and initiated
preliminary discharge planning. When staff other than a Social Worker perform these
duties, the Director of Social Work or a Master’s level social worker (MSW) qualified
supervisory staff member should be involved to oversee the quality and appropriateness
of service provided.
Patient and staff interviews, structured observations and review of selected medical
records yield the information necessary to determine how well social work has met the
needs of the patients. The surveyor should evaluate these data to determine adequacy of
qualified and support staff deployed to patient areas and their duties.
The social work policies for service provision to the patient should describe: the
organizational structure of the department (program) and the range of services performed
by the department.
Survey Procedure §482.62(f)
Just prior to the end of the survey, schedule a meeting with the Director of Social Work.
By the time of the meeting, you should already have conducted required observations,
interviews and record reviews for at least a majority of the patients in the sample. Collect
any additional information that is necessary to consider in light of outcomes observed for
patients, including: the qualifications of the director; the leadership exhibited for the
scope of services needed by the patient; and the rationale for social work staffing
coverage.
How does the director periodically audit the quality of social work services furnished?
What are the outcomes of audits conducted? What percentage of psychosocial
assessments was completed and available in written form at the time of the
interdisciplinary treatment plan? How does the patient’s social needs as addressed by the
social worker in the psychosocial assessment compare against the goals developed in the
interdisciplinary treatment plan?
Has social work staff provided active treatment in accordance with the patient’s treatment
plan?
Accepted standards of practice are based on policy statements adopted by the National
Association of Social Workers and a definition of social work practice in health care
adapted by the Consortium of Health Care Social Work Organizations. Staff should
adhere to the facility’s personnel requirements.
A-1716
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(f)(1) The director of the social work department or service must have a
master’s degree from an accredited school of social work or must be qualified by
education and experience in the social services needs of the mentally ill. If the
director does not hold a master’s degree in social work, at least one staff member
must have this qualification.
Interpretive Guidelines §482.62(f)(1)
The duties, functions, and responsibilities of the director of social services/social work
should be clearly delineated and documented in the facility’s policies and procedures. If
the director is not MSW qualified and at least one staff member is MSW qualified, verify
the duties, functions, and responsibilities of the MSW.
Survey Procedures §482.62(f)(1)
What are the director’s qualifications, experience and scope of duties within this
position?
If a MSW staff member, other than the director, is performing any of these duties, what
are this staff member’s experience and scope of duties performed? Why were these duties
delegated?
To what extent is the director’s knowledge of the social work needs of the various wards?
Why has the social work staff and services provided throughout the hospital been
deployed in the manner it has?
A-1717
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(f)(2) Social service staff responsibilities must include, but are not limited to,
participating in discharge, planning, arranging for follow-up care, and developing
mechanisms for exchange of appropriate information with sources outside the
hospital.
Interpretive Guidelines §482.62(f)(2)
Social work contact with the patient, family, and significant others should occur during,
or as soon as possible, after the admission. High-risk case finding should result in
significant data being available for early integration into the treatment plan and
subsequent social work action as indicated. The treatment team should consider, for
possible inclusion into the patient’s treatment plan, the anticipated social work role and
expected interventions as recommended in the psychosocial assessment . Treatment and
discharge planning activities, liaison/follow-up efforts should be based upon the goals,
including discharge goals, and staff responsibilities specified in the treatment plan.
Survey Procedures §482.62(f)(2)
Are social work staff routinely involved in providing services to the patient that are
identified in the treatment plan?
To what extent do social work staff provide discharge planning services to the patient in
the way of: supportive individual, couple, family, or group therapy focused on discharge
goals of the patient? Carrying out a liaison role with community resource providers?
Have social work staff assured that adequate information is provided to post-hospital
patient service providers?
A-1720
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(g) Standard: Therapeutic Activities.
T
he hospital must provide a therapeutic activities program.
Interpretive Guidelines §482.62(g)
A variety of therapeutic and rehabilitative activities are selectively used as therapeutic
tools in providing active treatment to the psychiatric patients. Therapeutic activities focus
upon the development and maintenance of adaptive skills that will improve the patient’s
functioning. In contrast, leisure activities provide the patient with individualized
opportunities to acquire knowledge, skills and attitudes about meaningful leisure
involvement and experiences. A patient may need treatment and/or remediation of
functional behavior(s) prior to leisure involvement. However, for some psychiatric
patients the priority need may be for leisure education and activities.
A-1725
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(g)(1) The program must be appropriate to the needs and interests of
patients and be directed toward restoring and maintaining optimal levels of physical
and psychosocial functioning.
Interpretive Guidelines §482.62(g)(1)
The hospital is responsible for ensuring consistent availability and provision of
individualized therapeutic activities and rehabilitative services based on patient needs.
The selection of individualized therapeutic and rehabilitative staff modalities should be
based on patient need and goals set in the patient’s treatment plan. Rehabilitative services
may include educational, occupational, recreational, physical, art, dance, music, and
speech therapies and vocational rehabilitation evaluation and counseling. There are other
disciplines that also serve patients. Consultants include but are not limited to the
following: educational instructors, registered occupational therapist/certified occupational
therapy assistant, certified therapeutic recreation specialist, certified therapeutic
recreation assistant, speech-language pathologist has certificate of clinical competence,
registered and certified music therapist, registered art therapist, and registered physical
therapist. The qualified vocational specialist may perform duties of a rehabilitation
counselor, vocational evaluator, or the work adjustment specialist.
A-1726
(Rev. 200, Issued: 02-21-20; Effective: 02-21-20, Implementation: 02-21-20)
§482.62(g)(2) The number of qualified therapists, support personnel, and
consultants must be adequate to provide comprehensive therapeutic activities
consistent with each patient’s active treatment program.
Interpretive Guidelines §482.62(g)(2)
Qualified staff should complete their respective discipline assessments for use in
multidisciplinary treatment planning. Specific role(s) and modalities to be implemented
by rehabilitative staff must be determined by goals set in the patient’s treatment plan.
Qualified therapists who provide clinical services and administrative staff should utilize
established monitoring and evaluation mechanisms to conduct consistent timely review of
the quality and appropriateness of therapeutic and rehabilitative services delivered to
patients.
Survey Procedures §482.62(g)(2)
Is there evidence that sampled patients and staff are familiar with the goals and staff
interventions described in the patient’s treatment plan? Are these observed interventions
being carried out? What is the patient’s response? Are these interventions and activities
of sufficient frequency and intensity to achieve maximum therapeutic benefit?
What are the qualifications, experience, duties and responsibilities of the Therapeutic
Activities Director and discipline supervisor(s)?
How is the program organized?
Did the patients in the sample have a need for any therapeutic activities? Were their
needs met?
Did any of the patients in the sample indicate a need for therapeutic activities, but none
were considered?
What kinds of services are provided to the patient population?
Are activity areas/sites accessible and available to meet the patient’s individual needs?
Are the facilities and resources adequate to enable implementation of goals set in the
patient’s treatment plan?
Does the program utilize available community resources to provide opportunities for
socialization, leisure, and therapeutic and/or rehabilitation activities for patients who can
participate outside the hospital setting?
Are current activity schedules clearly posted for patient and staff reference and use? Are
the scheduled activities related to the particular patient area and specific treatment needs
of patients?
Are patient needs met consistently at all times including evenings and weekends?
If a large number of patients are assigned to the same therapeutic activity, do patients
have individualized goals within their treatment plans?
Why have therapeutic activities staff been deployed in the manner they have?
Transmittals Issued for this Appendix
Rev #
Issue Date
Subject
Impl Date
CR#
R220SOMA
04/19/2024
Revisions to State Operations Manual
(SOM) Appendix A-Hospitals
04/19/2024
N/A
R216SOMA
07/21/2023
Revision to State Operations Manual
(SOM) Appendix A- Hospitals
07/21/2023
N/A
R200SOMA
02/21/2020
Revisions to the State Operations Manual
(SOM) Appendix A - Hospitals, Appendix
AA – Psychiatric Hospitals, Appendix B –
Home Health Agency, Appendix D - Portable
X-Ray, Appendix G - Rural Health
Clinics/Federally Qualified Health Centers,
Appendix H- End Stage Renal Disease
Facilities (ESRD), Appendix K –
Comprehensive Outpatient Rehabilitation
Facility, Appendix L - Ambulatory Surgical
Centers, Appendix M – Hospice, Appendix U
- Religious Nonmedical Healthcare
Institutions, Appendix W - Critical Access
Hospitals (CAHs), Appendix X - Organ
Transplant Program and Appendix Z -
Emergency Preparedness
02/21/2020
N/A
R183SOMA
10/12/2018
Revisions to Medicare State Operations
Manual (SOM) Table of Contents, Medicare
SOM Appendix, SOM Appendix A –
Survey
Protocol, Regulations and Interpretive
Guidelines for Hospitals, SOM Appendix T-
Regulations and Interpretive Guidelines for
Swing Beds in Hospitals, SOM Appendix
W- Survey Protocol, Regulations and
Interpretive Guidelines for Critical Access
Hospitals (CAHs) and Swing-Beds in CAHs.
10/12/2018
N/A
R176SOMA
12/29/2017
Revisions to State Operations Manual
(SOM) Appendix A – Survey Protocol,
Regulations and Interpretive Guidelines for
Hospitals
12/29/2017
N/A
R172SOMA
11/17/2017
Revision to State Operations Manual (SOM)
Appendix A – Survey Protocol, Regulations
and Interpretive Guidelines for Hospitals
11/17/2017
N/A
R170SOM
09/08/2017
Revisions to the State Operations Manual
(SOM) Appendix A – Survey Protocol,
Regulations and Interpretive Guidelines for
Hospitals- Rescinded and Replaced
Transmittal #172
09/08/2017
N/A
R151SOM
11/20/2015
Revisions to State Operations Manual (SOM),
Appendix A -Survey Protocol, Regulations and
Interpretive Guidelines for Hospitals
11/20/2015
N/A
R149SOM
10/09/2015
State Operations Manual (SOM) for All
Types of Providers and Suppliers Subject to
Certification
10/09/2015
N/A
R141SOM
07/10/2015
Revisions to the State Operations Manual
(SOM), Appendix A – Survey Protocol,
Regulations and Interpretive Guidelines for
Hospitals
07/10/2015
N/A
R137SOM
04/01/2015
Revisions to State Operations Manual
(SOM) Appendices A, G, L and T related to
Hospitals, Rural Health Clinics, Ambulatory
Surgical Centers and Swing Beds
03/27/2015
N/A
R122SOM
09/26/2014
Revisions to State Operations Manual
(SOM), Appendix A – Survey Protocol,
Regulations and Interpretive Guidelines for
Hospitals
09/26/2104
N/A
R116SOM
06/06/2014
Revised State Operations Manual (SOM)
Hospital Appendix A, Survey Protocol,
Regulations and Interpretive Guidelines for
Hospitals
06/06/2014
N/A
R105SOM
03/21/2014
Revised Appendix A, Interpretive
Guidelines for Hospitals, Condition of
Participation: Quality Assessment and
Performance Improvement
03/21/2014
N/A
R103SOM
02/14/2014
Revised State Operations Manual (SOM)
Hospital Appendix A
02/14/2014
N/A
R99SOM
01/31/2014
Revised State Operations Manual (SOM)
Appendices A, I, L, and W
01/31/2014
N/A
R95SOM
12/12/2013
Revised Appendix A, Interpretive
Guidelines for Hospitals, Appendix L,
Interpretive Guidelines for Ambulatory
Surgical Centers and Appendix W,
Interpretive Guidelines for Critical Access
Hospitals
06/07/2013
N/A
R89SOM
08/30/2013
Revised State Operations Manual (SOM)
Appendices A, I, L, and W – Rescinded and
replaced by Transmittal 99
08/30/2013
N/A
R87SOM
07/19/2013
Revised Appendix A, Interpretive
Guidelines for Hospitals, Condition of
Participation: Discharge Planning
07/19/2013
N/A
R84SOM
06/07/2013
Revised Appendix A, Interpretive
Guidelines for Hospitals, Appendix L,
Interpretive Guidelines for Ambulatory
Surgical Centers and Appendix W,
Interpretive Guidelines for Critical Access
Hospitals – Rescinded and replaced by
Transmittal 95
06/07/2013
N/A
R81SOM
03/23/2012
Revisions to State Operations Manual
(SOM), Appendix A, Hospitals
03/23/2012
N/A
R78SOM
12/22/2011
Revised Appendix A, Interpretive
Guidelines for Hospitals and Appendix W,
Interpretive Guidelines for Critical Access
Hospitals(CAH)
12/22/2011
N/A
R77SOM
12/22/2011
Revised Appendix A, “Interpretive
Guidelines for Hospitals”
12/22/2011
N/A
R75SOM
12/02/2011
Revised Appendix A, Interpretive
Guidelines for Hospitals and Appendix W,
Interpretive Guidelines for Critical Access
Hospitals(CAH)
12/02/2011
N/A
R74SOM
12/02/2011
Revised Appendix A, “Interpretive
Guidelines for Hospitals”
12/02/2011
N/A
R72SOM
11/18/2011
Revised Appendix A: Conditions of
Participation and Interpretive Guidelines for
Hospitals
11/18/2011
N/A
R59SOM
05/21/2010
Clarification of the Interpretive Guidelines
for the Anesthesia Services Condition of
Participation
05/21/2010
N/A
R47SOM
06/05/2009
Revised Appendix A, “Interpretive
Guidelines for Hospitals”
06/05/2009
N/A
R37SOM
10/17/2008
Revised Appendix A, “Interpretive
Guidelines for Hospitals”
10/17/2008
N/A
R01SOM
05/21/2004
Initial Release of Pub. 100-07
N/A
N/A
