
Vaccines Investor Event
June 29, 2023
Innovation to drive
sustainable growth
in Vaccines
Part 1

This document contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended.
Forward-looking statements are statements that are not historical facts. These statements include projections and estimates and their
underlying assumptions, statements regarding plans, objectives, intentions and expectations with respect to future financial results, events,
operations, services, product development and potential, and statements regarding future performance. Forward-looking statements are
generally identified by the words “expects”, “anticipates”, “believes”, “intends”, “estimates”, “plans” and similar expressions. Although Sanofi’s
management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that
forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally
beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or
projected by, the forward-looking information and statements. These risks and uncertainties include among other things, the uncertainties
inherent in research and development, future clinical data and analysis, including post marketing, decisions by regulatory authorities, such as
the FDA or the EMA, regarding whether and when to approve any drug, device or biological application that may be filed for any such product
candidates as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such
product candidates, the fact that product candidates if approved may not be commercially successful, the future approval and commercial
success of therapeutic alternatives, Sanofi’s ability to benefit from external growth opportunities, to complete related transactions and/or obtain
regulatory clearances, risks associated with intellectual property and any related pending or future litigation and the ultimate outcome of such
litigation, trends in exchange rates and prevailing interest rates, volatile economic and market conditions, cost containment initiatives and
subsequent changes thereto, and the impact that pandemics or other global crises may have on us, our customers, suppliers, vendors, and
other business partners, and the financial condition of any one of them, as well as on our employees and on the global economy as a whole.
The risks and uncertainties also include the uncertainties discussed or identified in the public filings with the SEC and the AMF made by Sanofi,
including those listed under “Risk Factors” and “Cautionary Statement Regarding Forward-Looking Statements” in Sanofi’s annual report on
Form 20-F for the year ended December 31, 2022. Other than as required by applicable law, Sanofi does not undertake any obligation to update
or revise any forward-looking information or statements.
Brand names appearing in this presentation are trademarks of Sanofi and/or its affiliates. Not all trademarks related to products under
development have been approved as of the date of this presentation by the relevant health authorities.
2
Forward-looking statements
Vaccines Investor Event

3:00-3:20
3:20-3:40
3
Agenda
Vaccines Investor Event
Vaccines Investor Event, June 29, 2023
Introduction
Expand leadership
- Deliver Best-in-Class RSV franchise
- Win in Influenza
Q&A
Break
2:00-2:10
2:10-3:00
New growth areas in vaccines
- Enter multi-billion PCV market
- Establish Best-in-Class meningitis portfolio
- Leverage leading-edge mRNA platform
- New frontiers
Concluding remarks
Q&A
3:40-4:30
4:40-5:00
4:30-4:40
3

Introduction
Paul Hudson
Chief Executive Officer

5
Dupixent® is a product in collaboration with Regeneron 1. Sales CAGR from 2018 base to 2025
Dupixent
®
Maximize patient benefits
with ambition to achieve
>€13bn peak sales across
type 2 inflammatory diseases
[COPD not included]
Pipeline
Prioritize and accelerate
portfolio of potentially
transformative therapies
Vaccines
Expected mid-to-high
single-digit growth
1
, through
differentiated products,
market expansion, launches
€8.3bn
sales in 2022,
+43.8%
5 years after
launch
6 . 3%
growth in 2022
84
projects in clinical
development
Driving growth with strategic choices
Vaccines Investor Event

6
Strategic transformation delivered first set of guidance targets
1. 2018 proforma BOI margin of 24.6% without equity investment in Regeneron sold in May 2020, excluding IFRS16 impacts.
Strong cash flow
Ahead of guidance
EPS growth
30%
BOI margin
~16%
growth at CER
10 consecutive quarters of
growth
540bps BOI
margin improvement
from 2019 to 2022
1
€2.7bn
cost savings re-invested
in growth drivers
>25
value-creating BD and M&A deals
Accelerating
digitalization
: use of AI and
data science at scale
2020 - 2022
Vaccines Investor Event

7
Launches
Pivotal
readouts
27 readouts
in immunology,
vaccines, neurology,
rare diseases,
and oncology
Early to
mid-stage pipeline
Expansion into COPD
tolebrutinib (BTKi)
Relapsing MS
Hemophilia A/B
fitusiran
Powerful business and pipeline momentum in 2023
Baring unforeseen events.
Vaccines Investor Event

8
Strong positive pipeline news flow in H1 2023
Vaccines Investor Event
Submissions
Dupixent
®
CSU
US
300,000 people with CSU inadequately
controlled by antihistamines
Read
-outs
Dupixent
®
COPD
Phase 3
Around 900,000 patients in G7
itepekimab (IL
-33)
COPD
Phase 3 IA
Around 1.8m patients in G7
amlitelimab (OX40L)
AD
Phase 2b
Moving in phase 3
frexalimab (CD40L)
MS
Phase 2b
Moving in phase 3
SAR’765
(IL
-13/TSLP)
Asthma
Phase 1b
Moving in phase 2b
SAR’566 (oral TNFi)
Psoriasis
Phase 1b
Moving in phase 2b
Barring unforeseen events. Dupixent is not yet approved neither in CSU nor COPD by any regulatory authority; itepekimab, amlitelimab, frexalimab, SAR’765 and SAR’566 are still under investigation and not yet approved.

Play to Win: Leverage innovation to drive next growth chapter
Barring unforeseen events. 1. Sales from 2018.
2020-2022
Toda
y
2023-2025
Guidance of
BOI margin of
>32%
by 2025
Refocus with
decisive actions
Growth through
winning assets
Margin expansion
Transformative
launches
Agile and efficient
resource deployment
Leading R&D
productivity
2026-2030
Industry leader in immunology
with >€22bn sales by 2030
Doubling vaccines sales by 2030
1
No meaningful LOE
Ambition to launch
3-5 new products
with
€2-5bn peak sales potential each
9 Vaccines Investor Event

Expand leadership
in vaccines
Thomas Triomphe
Head of Vaccines GBU
Jean-François Toussaint
Head of Vaccines R&D

11
Our ambition in Vaccines
1. Vs. 2018, risk adjusted, internal estimate
Unlock the potential
of mRNA in Vaccines with
Next-Generation platform
Continued strong growth
driven by four core
franchises: Influenza,
Meningitis, PPH &
Boosters, RSV
Build an industry
leading pipeline to
address unmet needs
More than double Vaccine sales by 2030
1
Vaccines Investor Event

Focus on growth Lead with innovation Accelerate efficiency Reinvent how we work
Sales growth
2018-2022 CAGR
Vaccines reached
blockbuster status
- Fluzone HD
- Penta/Hexaxim
Countries with
Beyfortus licenses
New phase 1/2
programs over
2022-2023
Vaccines profitability
from 2018 to 2022
Merged Pharma &
Vaccines manufacturing
& supply, 2 Evolutive
Facilities on track for
2025 operation
TBio experts retained
across mRNA Center
of Excellence 2 years
post-acquisition
Female senior leaders
12 Vaccines Investor Event
Execution of Play To Win strategy in Vaccines
+8%
2
32
6
1
+6pts
45%
+90%

R&D transformation has started to deliver strong results
State-of-the-art immunology
& antigen design
Selecting the best technology
platform for each target
Innovative antigens designed, including
mRNA-encoded bacterial vaccine approach
High throughput translational science &
proprietary MIMIC
®
technology introduced
9 vaccine technologies employed across the pipeline
Leading-edge mRNA platform added
Expanding into new
infectious diseases
Chlamydia final antigens selected
Acne mechanism of action validated
Additional new research programs initiated
13 Vaccines Investor Event
* Compared to Dec 2021

2021 2023e 2025e
14 Vaccines Investor Event
Phase 3 &
Registration
Pre-clinical
3 new products registered
6 new phase 1/2 programs
mRNA Flu QIV
RSV Older Adult
RSV OA/PIV/hMPV
MenPenta
Acne
NextGen Flu
2022-2023 progress Pipeline moving at pace
Phase 1/2
Beyfortus
At least 5 new FiC / BiC programs expected to enter phase 3
by 2025

Recent highlights from our leading-edge mRNA platform
AI/ML augmented
mRNA Workforce
15 Vaccines Investor Event
>600 experts and more than
30 collaborations across all
aspects of the platform
Proprietary generative
modeling for mRNA and lipid
design
Next generation
mRNA products
As many as 5 distinct LNPs
clinically tested by 2023
4 mRNA enhancement
features for next clinical
candidate
Rapid deployment
across the pipeline
Pivot to modified mRNA and
clinical validation in 9 months
7 phase 1/2 launched since
2022

R&D toolbox
9 vaccine technologies
employed across the pipeline
Industrial powerhouse
Ability to deliver at scale
Extensive medical expertise
Innovative approaches to
generate impactful real-world
evidence
Commercial strength
Engagement of strong
stakeholder networks
We have what it takes
to win in protection
against preventable
diseases
16 Vaccines Investor Event
Sanofi Vaccines is built on strong foundations

Affordable
access
R&D for unmet
needs
Planet care In and beyond
the workplace
Ensuring access to
medicines for the poorest
countries
Acting for the most
vulnerable communities
Building sustainability
for a healthy planet
Building an inclusive
workplace
Blister-free
vaccine
Yellow Fever
vaccine
17 Vaccines Investor Event
Sanofi societal commitments embedded in our business

109,000 severe infections
and 51,000 deaths in 2018
worldwide
>500 million doses distributed
worldwide since 1953
Major partner and supplier of
UNICEF, committed to stay
ready to respond to outbreaks
Positive phase 2 results of our
next generation vaccine
18 Vaccines Investor Event
Yellow Fever program with thorough Global Access Plan
South & Central
America: 13 countries
Africa: 29
countries/regions

19 Vaccines Investor Event
Ambition to manufacture 100% blister-free packaging by 2027
Saving ~330 tons of plastic per year
Reducing the amount of microplastics in the environment
Up to 50% reduction of transported pallets
Reducing the need for cold chain space and transport by ~1/3rd
30% reduction in distribution costs
40% of blister-free syringes by end of 2023, 100% by 2027

20 Vaccines Investor Event
Fluzone HD
Influenza QIV
mRNA
Next-gen mRNA
Flu vaccine
PCV21 Chlamydia
Acne
MenQuadfi
MenB
MenPenta
Beyfortus
RSV toddler
RSV older
adult (OA)
Influenza
Deepen our leadership in existing franchises
New growth areas
Meningitis
Travel & Endemic
RSV Pneumo New frontiers
Fluzone HD
pediatric
Pandemic
Influenza
Next-gen
Yellow fever
Next-gen rabies
RSV OA
respiratory
combo
Data to be shared today
New data from 12 assets featured today

Deliver Best-in-Class
RSV franchise
Kimberly Tutwiler
Head of RSV Franchise
Jean-François Toussaint
Head of Vaccines R&D

Infant
30%
Toddler
10%
Older Adult
60%
RSV Market
~€8bn
2030
Beyfortus:
Best-in-Class immunization for All Infant
Protection in first season
RSV Toddler
SP0125: First-in-Class vaccine for
protection from second season onwards
RSV Older Adult
SP0256: First-in-Class RSV-hMPV-PIV
combination
22
Ambition to lead in RSV across all target populations
Vaccines Investor Event
Source: Sanofi internal forecast

23
U.S. Advisory committee votes 21-0 in favor of nirsevimab
Vaccines Investor Event
ACIP meeting anticipated before the RSV season
Unanimously voted in favor for 1
st
season
- Favorable benefit/risk profile for prevention of
RSV LRTD in newborns & infants born during or
entering 1
st
season
Voted 19-2 in favor for 2
nd
season
- Favorable benefit/risk profile for prevention of
RSV LRTD in children up to 24 months of age who
remain vulnerable

1 year
follow-up
Participants:
8,000+ infants
≥29 weeks
gestational age
1
No intervention
N=4,021
Beyfortus
N = 4,037
Days 1 181
1
st
visit:
randomization
Monthly
follow up
Primary
completion
366
Primary endpoint
Reduction of hospitalization
due to RSV Lower-
Respiratory-Tract-Infection
(LRTI)
Study objectives
Showcase seamless
implementation in real
world setting
Enrich hospitalization data
in France, Germany and UK
Confirm safety profile
in large population
24
HARMONIE study confirms pivotal
trial data in real world setting
Vaccines Investor Event
SB Drysdale, (2023, May 8–12). A Phase 3 randomized open-label study of nirsevimab (versus no intervention) in preventing hospitalizations due to respiratory syncytial
virus (RSV) in infants (HARMONIE) [Oral presentation]. ESPID 2023: Lisbon, Portugal. 1. Not eligible for palivizumab

25 Vaccines Investor Event
SB Drysdale, (2023, May 8–12). A Phase 3 randomized open-label study of nirsevimab (versus no intervention) in preventing hospitalizations due to respiratory syncytial virus (RSV) in infants (HARMONIE) [Oral presentation]. ESPID 2023: Lisbon, Portugal.
Adverse Events Category
Adverse Events type
Nirsevimab
(N=4,016) N, (%)
No Intervention
(N=4,020) N, (%)
Any treatment emergent adverse event (TEAE) 1,479 (36.8) 1,326 (33.0)
Leading to discontinuation of study 1 (< 0.1) 1 (< 0.1)
Leading to death 0 (0.0) 0 (0.0)
Grade 1 severity 1,171 (29.2) 1,014 (25.2)
Grade 2 severity 462 (11.5) 436 (10.8)
Grade 3 severity 48 (1.2) 46 (1.1)
Unknown 67 (1.7) 56 (1.4)
Any study treatment related TEAE 86 (2.1) 0 (0.0)
Leading to discontinuation of study 0 (0.0) 0 (0.0)
Leading to death 0 (0.0) 0 (0.0)
Grade 1 severity 68 (1.7) 0 (0.0)
Grade 2 severity 21 (0.5) 0 (0.0)
Grade 3 severity 1 (< 0.1) 0 (0.0)
Unknown 1 (< 0.1) 0 (0.0)
Any serious TEAE 89 (2.2) 67 (1.7)
Leading to discontinuation of study 1 (< 0.1) 0 (0.0)
Leading to death 0 (0.0) 0 (0.0)
Excellent safety and tolerability profile confirmed

26 Vaccines Investor Event
Incidence of RSV-LRTI hospitalization
Impressive 83% reduction of RSV-LRTI hospitalizations
confirmed in real world setting
RSV is the leading cause of
hospitalization in infants
Efficacy of Beyfortus has been
consistent across all studies, and
is maintained for 5 months to cover
the duration of the RSV season
No intervention
n=4,021
Beyfortus
n=4,037
1.5%
0.3%
83.2%
Relative Risk Reduction
(95% CI: 67.8-92.0;
p<0.001)
1. SB Drysdale, (2023, May 8–12). A Phase 3 randomized open-label study of nirsevimab (versus no intervention) in preventing hospitalizations due to respiratory syncytial virus (RSV) in infants (HARMONIE) [Oral presentation]. ESPID 2023: Lisbon, Portugal.
27
Nirsevimab expected to prevent 3x more RSV events than maternal vaccine
Key input nirsevimab Maternal Vaccine
Efficacy RSV MA-LRTI
79.5% 51.3%
(success criteria not met)
Reduction of RSV MA-LRTI
hospitalization
83.2% 69.4%
% reduction of RSV-related events
in babies born before season
50.5% 12.7%
% reduction of RSV-related events
in babies born preterm
60.6% 4.1%
Immunization coverage rate
80% 50%
Efficacy all-cause LRTI
hospitalizations
58%
2.5%
1
for MA-LRTI all-cause
(success criteria not met)
291,320
90,173
0
50,000
100,000
150,000
200,000
250,000
300,000
350,000
nirsevimab RSV maternal PreF vaccine
Prevented RSV-related Events:
Hospitalizations, Office and Emergency Room Visits
3x
Modeled impact of nirsevimab and maternal immunization in a U.S. birth cohort for first RSV season
Source Notes: Model- Kieffer A, Beuvelet M, Sardesai A, et al. Expected Impact of Universal Immunization With Nirsevimab Against RSV-Related Outcomes and Costs Among All US Infants in Their First RSV Season: A Static Model. J Infect Dis. 2022;226(Supplement_2):S282-s292. Inputs:
Hospitalizations: CDC New Vaccine Surveillance Network (NVSN) hospitalization rates for children under 2 years of age from December 2016 to September 2020. Primary care & ER :visits: Lively JY, Curns AT, Weinberg GA, et al. Respiratory Syncytial Virus-Associated Outpatient Visits
Among Children Younger Than 24 Months. J Pediatric Infect Dis Soc. 2019;8(3):284-286. RSV season: National Respiratory and Enteric Virus Surveillance System (NREVSS) (2015-2019). Immunization rates pediatric CDC National Center for Health Statistics, DTaP
https://www.cdc.gov/nchs/fastats/immunize.htm Immunization Rates Maternal: CDC FluVaxView Flu, Tdap, Covid Vaccination coverage among pregnant women April 2022 https://www.cdc.gov/flu/fluvaxview/pregnant-women-apr2022.htm . Efficacy nirsevimab: Simões EAF, Lancet Child
Adolesc Health. 2023 Mar;7(3):180-189. Drysdale S, 41st Annual Meeting of the European Society for Paediatric Infectious Diseases (Lisbon). Griffin MP, et al. N Engl J Med. 2020;383(5):415-425. Hammitt LL, et al. N Engl J Med. 2022;386(9):837-846. Beyfortus. EU Summary of Product
Characteristics (SmPC). Efficacy Maternal RSV preF vaccine: Kampmann B, Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med 2023;388:1451-64
1. Included RSV MA LRTI all cause (2.5%) in the absence of data for all cause LRTI hospitalization to compare
[Modelled with clinical data & assumptions from CDC
comparison; not from head-to-head studies]
Vaccines Investor Event

28 Vaccines Investor Event
Priority review granted
Production is already underway
Stakeholders fully engaged
License, ACIP recommendation and VFC
inclusion expected soon
Contracting and reimbursement underway
Licensed in EU, Great Britain, Canada
Broad population programs expected in
Spain and France
Ready to launch Beyfortus in the 2023 season

29 Vaccines Investor Event
1. Hall CB, et al. Pediatrics. 2013;132(2):e341-e348. 2. Hall CB, et al. N Engl J Med. 2009;360(6):588-598. 2. Taylor S, 2016 Modelling estimates of the burden of respiratory syncytial virus infection in children in the UK | BMJ Open
0
50
100
150
200
6-11 months 12-23 months
Incidence Rates per 1000
Bronchiolitis
16.1
6.9
4.4
3.7
Antibiotic
prescription
Respiratory
disease
Acute respiratory
disease
Pneumonia
High rate of GP consultations due to
diseases caused by RSV (0 to 5 years of age)
RSV in toddlers: significant burden in 2
nd
season and beyond
Hospitalization visits Emergency room visits
Pediatric office visits
Influenza RSV
RSV Burden in Toddlers
0.6

Live attenuated vaccine uniquely designed to ensure safety and maximize immunogenicity
Intranasal delivery design for
complete toddler protection
- RSV inhibition at its point of entry
- Broad protection against both upper
and lower respiratory tract disease
URTD
LRTD
30 Vaccines Investor Event
SP0125 is the first RSV vaccine designed to protect all toddlers

31 Vaccines Investor Event
Beyfortus administered
ahead or during first season
1
st
dose of
RSV Toddler
vaccine
2
nd
dose of
RSV Toddler
vaccine
To be given during existing
routine visits
Beyfortus and RSV Toddler vaccine provide continuous protection
First RSV Season
(October – March)
April - September
Second RSV Season
(October – March)
Protected for first
RSV season
Protected from
second RSV season
onwards

n=61
Placebo
RSVt Dose A 2x
n=57
n=61
Toddlers
6-18 mo
Days
RSVt - High dose
RSVt - Low dose
1 8456
32 Vaccines Investor Event
Safety
Adverse events following
vaccination
Immunogenicity
Neutralizing antibody
responses
Vaccine response rate
Composite endpoint
factoring immunogenicity
and vaccine virus
replication
Live attenuated vaccine (SP0125) Phase 1/2 design

* Based on investigator assessment. AE of special interest: acute otitis media, upper and lower respiratory infections.
Participants experiencing at least one
unsolicited AE within 28 days after vaccination
RSVt LD
(n=61)
RSVt HD
(n=57)
Placebo
(n=61)
RSVt LD
(n=48)
RSVt HD
(n=48)
Placebo
(n=54)
n (%) n (%) n (%) n (%) n (%) n (%)
Not related to vaccination
37 (60.7) 30 (52.6) 38 (62.3) 22 (45.8) 17 (35.4) 23 (42.6)
Related to vaccination
5 (8.2) 6 (10.5) 4 (6.6) 4 (8.3) 3 (6.3) 2 (3.7)
AE of special interest*
15 (24.6) 8 (14.0) 15 (24.6) 7 (14.6) 5 (10.4) 6 (11.1)
Medically attended AE
28 (45.9) 23 (40.4) 26 (42.6) 19 (39.6) 14 (29.2) 17 (31.5)
AE leading to study discontinuation
0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0)
Serious AE
1 (1.6) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0)
Death
0 (0) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0)
First administration Second administration
33 Vaccines Investor Event
SP0125 demonstrated safety profile similar to placebo

34 Vaccines Investor Event
* Absence of prior exposure to RSV was determined by measuring serum IgA before vaccination
vaccine
response
rate (%) in
subjects
never
exposed
to RSV*
80
40
0
20
60
100
First
administration
Two dose
regimen
RSVt
LD
PlaceboRSVt
HD
RSVt
LD
PlaceboRSVt
HD
Strong vaccine response observed with SP0125
Strong (93%) vaccine
response after two
administrations of the High
dose formulation
Marginal difference
between the Low and High
dose formulations
35
Both formulations induced a robust immune response
Vaccines Investor Event
1. Absence of prior exposure to RSV was determined by measuring serum IgA before vaccination 2. Karron et.al. Am J Respir Crit Care Med Vol 203:5, 2021
% volunteers
Serum neutralizing antibody levels
Neutralizing Titre (1/dil)
100
80
60
40
20
0
1 10 100 1000 10000
RSV low dose at D0
RSV high dose at D0
Placebo at D0
RSV low dose at D84
RSV high dose at D84
Placebo at D84
Robust neutralizing antibody
response in toddlers not previously
exposed to RSV
1
Similar immune response
observed for the Low and High dose
formulations
Immune response in line with
prior studies that showed
reduction of RSV-medically attended
disease
2
Move to phase 3 in H1 2024

36 Vaccines Investor Event
Limited antigenic drift of RSV, hMPV
and PIV removes need for annual
vaccination
8
Estimated burden in US >65 population:
1-6
RSV Older Adult: addressing important unmet need with the
most compelling respiratory combination vaccine
1. Widmer et al., 2012; 2. Russell et al., 2019 (62% of RSV); 3. Colosia et al., 2017; 4. Using RSV rate from Colosia 2017 as proxi. 5. https://www.cdc.gov/rsv/research/us-surveillance.html 6.Compilated data from CDC, 9 seasons from 2010-2011 to
2018-2019 https://www.cdc.gov/flu/about/burden/index.html 7. Burden in already vaccinated pop 8. Assuming vaccine durability >1 year
Disease burden from RSV-hMPV-PIV similar to Influenza

SP0256 Phase 1/2 trial design of mono vaccine in older adults
mRNA Dose 1 ; LNP #1
mRNA Dose 2 ; LNP #1
mRNA Dose 3 ; LNP #2
Placebo
mRNA Dose 3 ; LNP #1
mRNA Dose 2 ; LNP #2
mRNA Dose 1 ; LNP #2
n= 10 & 100
n= 10 & 100
n= 10 & 100
n= 10 & 100
n= 10 & 100
n= 10 & 100
n= 10 & 100
29
1
Days
37 Vaccines Investor Event
Safety
Adverse events following
vaccination
Immunogenicity
Serum neutralizing antibody
response measured by
plaque reduction
neutralization assay
Adults
18-49
&
≥60 yo

*RSV-A neutralizing antibodies Geometric Mean Titer ratio (D29 vs baseline D1
38
mRNA RSV OA vaccine
was well tolerated
mRNA RSV OA vaccine
significantly boosted RSV
neutralizing antibody
responses
38
Boosted RSV-A Neutralizing
Antibodies (selected formulation)
Reactogenicity
(selected formulation)
% participants
with
solicited
reactions
D7
Positive phase 1/2 results support SP0256 as the backbone for
the combo respiratory vaccine
Vaccines Investor Event
Vaccine
Older
Adults >60
Vaccine
Younger
Adults 18-49
Saline
Older
Adults >60
Vaccine
Older
Adults >60
Vaccine
Younger
Adults 18-49
Saline
Older
Adults >60
Grade 1 Grade 2 Grade 3
100
80
60
40
20
Neut Ab GMTR (D29/D1)*
5
0
20
10
15
25
5.4
1.0
11.5

39
Only Sanofi has the potential to offer Best-in-Class protection
for all targeted ages
Vaccines Investor Event
Beyfortus SP0125 SP0256
PROFILE
Best-in-Class for All Infant
Protection in first season
First-in-Class vaccine for
second season onwards
First-in-Class with RSV-hMPV-
PIV mRNA combination
NEXT STEPS
Ready for launch
Phase 3 start in H1 2024
Target submission in 2026
Phase 2b RSV & Phase 1/2
combo start in 2023
Target submission
for combo in 2026+

Win in Influenza
Bill Averbeck
Head of Influenza Franchise
Saranya Sridhar
Head of Translational Medicine

Pioneered the
transition to
quadrivalent
flu vaccines
Established
Protection Beyond
Flu as the new
standard of care
Pursuing the
next chapter in
flu with mRNA
technology
Leading with innovation rooted in Protection Beyond Flu
Worldwide
market leader
with €3bn sales in
2022
41 Vaccines Investor Event
Sanofi is the global leader in Influenza vaccines

Three attributes imperative for winning in seasonal flu
42 Vaccines Investor Event
Protection Beyond Flu
Safety & tolerability
Administration
Demonstrated efficacy in hospitalization and infection reduction
through high quality / consistent data – not just immunogenicity
Excellent tolerability profile
Fully liquid formulation, pre-filled syringe
Shelf life covering duration of flu season at refrigerator
temperature (2-8°C)

Immunogenicity
Protection Beyond Flu
Superior Efficacy
43 Vaccines Investor Event
Source: Sanofi internal analysis
Fluzone HD share of U.S. 65+ years old flu market value, $bn
2016
0.5
20132010 2011 20202012 2014 20172015 2018 2019 2021 2022
0.3
0.4
0.3
0.3
0.6
0.7
0.8
1.0
1.1
1.4
1.6
1.7
It takes Protection Beyond Flu to win
Fluzone HD and Flublok in
CDC preferential
recommendation for 65+
Fluzone HDOther
PBF

44
DANFLU-1
1
DANFLU-2
2
Objective Impact of QIV HD vs SD on
pneumonia and influenza
(P&I) and other
hospitalizations
Impact of QIV HD vs SD on
P&I and other hospitalizations
Design Randomized real-world study
12k subjects 65-79
Randomized real-world study
Target 208k subjects 65+
Outcome /
next steps
64.4% reduction in P&I
hospitalization
Presented at ESC 2022,
accepted in NEJM Evidence
19k randomized to date
Started in 22/23 season
Outstanding results confirmed in most recent randomized
real-world studies
PBF
Fluzone High-Dose/Efluelda set the bar high in 60/65+
1. Johansen ND, et al. NEJM Evidence. 2023. 2. Clinicaltrials.gov: NCT05517174.
Vaccines Investor Event
Driving global expansion
Recommendations or
preferential reimbursement in
10+ key markets

Increasing reactogenicity
% Preference Share
HCPs Consumers
Increasing reactogenicity
% Preference Share
-69%
-69%
SoC
mRNA vaccine with
2x Grade 2 side
effects vs. SoC
mRNA vaccine with
3x Grade 3 side
effects vs. SoC
SoC
mRNA vaccine with
2x Grade 2 side
effects vs. SoC
mRNA vaccine with
3x Grade 3 side
effects vs. SoC
Tolerability
45 Vaccines Investor Event
Source: Based on quantitative and qualitative conjoint analysis market research. Q4 2022. US, UK, DE, & AU. Quantitative: 2180 consumers, 501 HCPs. Qualitative: 72 consumers, 94 HCPs.
HCPs and consumers are unlikely to use vaccines with
3x severe side effect burden compared to Standard Dose

Flu vaccination networks set
up to maximize access;
unfit to manage ultra-cold
chains and short shelf life
Doctor’s
office
School
Pharmacy
Drive-
through
Workplace
Lack of refrigerator-stable,
full-season product
could decrease HCP
uptake/use by
-37%
46 Vaccines Investor Event
Administration
Source: Based on quantitative and qualitative conjoint analysis market research. Q4 2022. U.S., UK, Germany and Australia. Quantitative: 2180 consumers, 501 HCPs. Qualitative: 72 consumers, 94 HCPs.
HCPs do not accept administration hurdles for flu vaccines

47 Vaccines Investor Event
SP0273
mRNA Flu QIV
mRNA neuraminidase (NA)
n=65 & 65
mRNA Flu QIV Dose 1
mRNA Flu QIV Dose 3
Fluzone High-Dose
Flublok
Fluzone Standard Dose
mRNA Flu QIV Dose 2
n=65 & 65
n=65 & 65
n=60 & 60
n=60 & 60
n= 0 & 60
291 Days
n=30 & 30
mRNA NA Dose 1
mRNA NA Dose 3
Fluzone High-Dose
mRNA NA Dose 2
n=30 & 30
n=30 & 30
n=30 & 30
Phase 1/2 study
Flu QIV (modified mRNA)
- Safety and immunogenicity
with 3 different LNPs
Neuraminidase (unmodified
mRNA and LNP#1)
- Pilot study to test
neuraminidase
immunogenicity
Comprehensive mRNA flu vaccine program
&
Adults
≥65 yo
Adults
18-64 yo
&
Adults
≥65 yo
Adults
18-64 yo
291 Days

48 Vaccines Investor Event
FlublokSP0273 mRNA Flu QIV Fluzone SD
1
2
4
8
16
32
GMTR D29/D01
A/H3N2 A/H1N1 B/YAM B/VIC
0
20
40
60
80
100
% Seroconversion
A/H3N2 A/H1N1 B/YAM B/VIC
SP0273 results
- Immune response for A
strains comparable to SoC
- Immune responses for B
strains trend lower than
SoC
Strong immune responses against A strains
Hemagglutination inhibition titers in 18-64 years old

Hemagglutination inhibition titers
Sanofi
18-64 years
49 Vaccines Investor Event
Immune response in line with other mRNA flu vaccine program
1. Moderna Third Annual Vaccines Day March 24th, 2022. Phase 2, Age group 18 y and older. DISCLAIMER: data from separate studies should be interpreted with care.
SP0273 mRNA Flu QIV
Fluzone SD
0
20
40
60
80
100
% Seroconversion
A/H3N2 A/H1N1 B/YAM B/VIC
1
2
4
8
16
32
GMTR D29/D01
A/H3N2 A/H1N1 B/YAM B/VIC
Competitor data
1
>18years
mRNA flu QIV results
In both mRNA trials:
- A strain results similar to
comparator
- Low B response is a class
effect across mRNA
platforms

SP0273 reactogenicity compares favorably to other mRNA trial
8
3
0
7
0
5
10
15
20
Reactogenicity in 18-64 years
% Any Grade 3 solicited
injection site reactions
% Any Grade 3 solicited
systemic reactions
mRNA-1010
Moderna
1
9
0 0
15.8
0
5
10
15
20
50 Vaccines Investor Event
FlublokFluzone
Standard
Dose
SP0273
mRNA
Flu QIV
SP0273 results
- Reactogenicity higher
compared to current
licensed flu vaccines
- Systemic reactions lower
than in a comparator
mRNA vaccine in a
different trial
1
mRNA-1010
Moderna
1
FlublokFluzone
Standard
Dose
SP0273
mRNA
Flu QIV
1. Data collected by Moderna in 18-49 years volunteers in a separate phase 2 trial. Moderna Third Annual Vaccines Day March 24th, 2022. DISCLAIMER: data from separate studies should be interpreted with care.

51 Vaccines Investor Event
Protection Beyond Flu is the centerpiece of clinical efforts
Utilize advanced computational techniques
to optimize strain selection
Machine learning
Focus on neuraminidase to improve
vaccine effectiveness
Antigen composition
Ambition to match our Standard of Care in influenza with
Sanofi’s next-generation mRNA vaccine

ML offers meaningful advances in strain selection process as demonstrated now also for H1 strains
ML model robustly selects strains
with greater breadth
1
ML-strains cover broader H1 Seasonal
Influenza Space vs WHO strain
2
-8.00
-4.00
0.00
4.00
8.00
6B.1A.5a.2
6B.1A.5a.2
6B.1A.5a.1
6B.1A.5a.1
6B.1A.5a.1
6B.1A.5b
6B.1A.1
6B.1
6B.1A
WHO strain
ML1 strain
ML2 strain
Antigenic space 1
Antigenic space 2
ML SelectionWHO strain Breadth of coverage
52 Vaccines Investor Event
1. Theoretical representation for illustrative purposes 2. Log2 fold change of mNT titers compared to WHO strain. Color boxes represent different H1 sequence clades from Nexstrain
Proof of concept achieved for H3 & H1 strains
Potential to improve coverage with Machine Learning
53 Vaccines Investor Event
SP0273
mRNA Flu QIV
mRNA neuraminidase (NA)
n=65 & 65
mRNA Flu QIV Dose 1
mRNA Flu QIV Dose 3
Fluzone High-Dose
Flublok
Fluzone Standard Dose
mRNA Flu QIV Dose 2
n=65 & 65
n=65 & 65
n=60 & 60
n=60 & 60
n= 0 & 60
291 Days
n=30 & 30
mRNA NA Dose 1
mRNA NA Dose 3
Fluzone High-Dose
mRNA NA Dose 2
n=30 & 30
n=30 & 30
n=30 & 30
Phase 1/2 study
Flu QIV (modified mRNA)
- Safety and immunogenicity
with 3 different LNPs
Neuraminidase (unmodified
mRNA and LNP#1)
- Pilot study to test
neuraminidase
immunogenicity
Comprehensive mRNA flu vaccine program
&
Adults
≥65 yo
Adults
18-64 yo
&
Adults
≥65 yo
Adults
18-64 yo
291 Days

mRNA neuraminidase immunogenicity as strong as Fluzone HD
mRNA neuraminidase results
- Immune responses comparable
to Fluzone HD
- NB: Fluzone HD has 2.5 to 3
times higher NA concentrations
than SD vaccines and sets the
bar for future vaccines
1
- Good tolerability and safety,
comparable to Fluzone HD
2
54 Vaccines Investor Event
1. Gao Z, Robinson K, Skowronski DM, De Serres G, Withers SG. Vaccine. 2020 Jan 22;38(4):715-718. doi: 10.1016/j.vaccine.2019.11.041 2. Data on file
Neuraminidase inhibition titers (N2)
mRNA NA
Fluzone HD
1
2
4
8
18-64
years
≥ 65
years
GMTR D29/D01
% Seroconversion
0
20
40
60
80
100
18-64
years
≥ 65
years

Offering superior flu protection for key age groups at risk
SP0273 Next-generation mRNA flu
Enhance B strain immune response, improve immunogenicity, upgrade
antigen design & optimize strain selection via machine learning
Acceptable tolerability and thermostability
PBF SoC
55 Vaccines Investor Event
Vaxigrip Tetra / Fluzone SD
Flublok / Supemtek
Fluzone HD / Efluelda

Q&A session Part 1
Kimberly Tutwiler
Head of RSV Franchise
Bill Averbeck
Head of Influenza Franchise
Saranya Sridhar
Head of Translational Medicine
Thomas Triomphe
Head of Vaccines GBU
Jean-François Toussaint
Head of Vaccines R&D

Vaccines Investor Event
June 29, 2023
Innovation to drive
sustainable growth
in Vaccines
Part 2

3:00-3:20
3:20-3:40
58
Agenda
Vaccines Investor Event
Vaccines Investor Event, June 29, 2023
Introduction
Expand leadership
- Deliver Best-in-Class RSV franchise
- Win in Influenza
Q&A
Break
2:00-2:10
2:10-3:00
New growth areas in vaccines
- Enter multi-billion PCV market
- Establish Best-in-Class meningitis portfolio
- Leverage leading-edge mRNA platform
- New frontiers
Concluding remarks
Q&A
3:40-4:30
4:40-5:00
4:30-4:40
58

Enter multi-billion
PCV market
Thomas Grenier
Head of Franchise & Product Strategy
Jean-François Toussaint
Head of Vaccines R&D

~80%
~20%
Large pneumococcal vaccine market
Pediatric
Adult / Elderly
€6.8bn
(Global, 2022)
Focus on pediatric development
First-in-Class PCV20+ in pediatric population
Synergy with Sanofi pediatric vaccine portfolio
Strong collaboration with SK Bioscience
PCV21: growth driver with strong portfolio fit
60 Vaccines Investor Event
Source: Pfizer, GSK, Merck 2022 Annual Reports; Sanofi estimates
Drive growth with PCV21 in attractive pediatric market

Serotype composition per vaccine
Significant residual burden
in U.S. pediatrics < 5 years
2
- ~1,500 cases IPD
- 1.5m Acute Otitis Media cases
- 270k cases of pneumonia
9N serotype provides ~5-7%
pts gain in IPD coverage
across all ages
1,3
IPD incremental coverage
rate in all ages
1
First PCV21 pediatric vaccine extends protection against disease
Sanofi PCV21
Pfizer PCV20
Merck PCV15
Pfizer PCV13
26%
29%
14%
9%
17%
31%
6%
5%
37%
27%
PCV13 non PCV10
PCV15 non PCV13
PCV20 non PCV15
PCV21 non PCV20
NVT/Others
61 Vaccines Investor Event
US
2019+2020
EU
2019+2020
NVT: Non-vaccine type NT: Non-typable IPD: Invasive pneumococcal disease
1. All age groups – US ABC data and ECDC Surveillance Atlas 2. Internal model 3. Tiley KS, J Infect Dis 2022; Plainvert C, Infect Dis Now 2022; Ekinci E, Front in Pediatr 2021.
1 3 4 5
6A
6B
7F
9V
14
18C
19A
19F
23F
22F
33F
8
10A
11A
12F
15B
9N
2
17F
20

62 Vaccines Investor Event
Study design
PCV21 Formulation #1
PCV21 Formulation #2
PCV21 Formulation #3
PCV13
n= 175
n= 175
n= 175
n= 175
13-162 4 6 12 - 157
4-dose
regimen
Months
Infants
2 Months
PCV21 (SP0202) Phase 2 designed to enable pivotal program
Safety
Immunogenicity
- Post-dose 3 IgG geometric mean
concentration and seroresponse
- Post-dose 4 IgG geometric mean
concentration
=> Standard evaluation criteria for
pivotal trials and registration
Select formulation for pivotal program

Solicited injection site reactions
0
20
40
60
80
100
Dose 1 Dose 2 Dose 3 Dose 4
PCV21 PCV13
Solicited systemic reactions
0
20
40
60
80
100
Dose 1 Dose 2 Dose 3 Dose 4
% of participants
reporting
% of participants
reporting
Safety profile comparable with PCV13 across all 4 doses
63 Vaccines Investor Event
PCV21 (SP0202) well-tolerated in pediatric population
PCV21 selected formulation for next phase PCV21_Phase II [NCT04398706]

-30 -10 10 30
0,25 0,5 1 2 4
0,25 0,5 1 2 4
IgG GMC ratio and difference % seroresponse vs PCV13
Post Dose 3: IgG GMC ratio Post Dose 4: IgG GMC ratioPost Dose 3: Difference seroresponse
1
3
4
5
6A
6B
7F
9V
14
18C
19A
19F
23F
1
3
4
5
6A
6B
7F
9V
14
18C
19A
19F
23F
1
3
4
5
6A
6B
7F
9V
14
18C
19A
19F
23F
64 Vaccines Investor Event
PCV21_Phase II [NCT04398706] Seroresponse: IgG concentration ≥0.35 µg/mL for all serotypes
PCV20_Phase III [NCT04382326] Seroresponse: IgG concentration ≥0.35 µg/mL for all serotypes except ≥ 0.23 µg/mL, ≥0.10 µg/mL and ≥ 0.12 µg/mL for serotypes 5, 6B and 19A respectively
PCV21 selected formulation for next phase
DISCLAIMER: data from separate studies should be interpreted with care.
Registration
criteria based
on PCV13
PCV20 Phase 3
PCV21 Phase 2
Favorable PCV21 immune responses when compared to PCV20
Serotypes shared by PCV13, PCV20 and PCV21

65 Vaccines Investor Event
PCV21_Phase II [NCT04398706] Seroresponse: IgG concentration ≥0.35 µg/mL for all serotypes
PCV20_Phase III [NCT04382326] Seroresponse: IgG concentration ≥0.35 µg/mL for all serotypes except ≥ 0.23 µg/mL, ≥0.10 µg/mL and ≥ 0.12 µg/mL for serotypes 5, 6B and 19A respectively
Note: difference (% and GMC ratio) vs lowest serotype in PCV 13
PCV21 selected formulation for next phase DISCLAIMER: data from separate studies should be interpreted with care.
0,25 1 4 16
-30 -10 10 30
0,25 1 4 16
8
9N
10A
11A
12F
15B
22F
33F
8
9N
10A
11A
12F
15B
22F
33F
8
9N
10A
11A
12F
15B
22F
33F
0.5
0.5
Favorable PCV21 immune responses when compared to PCV20
IgG GMC ratio and difference % seroresponse vs lowest in PCV13 group
Post Dose 3: IgG GMC ratio Post Dose 4: IgG GMC ratioPost Dose 3: Difference seroresponse
Registration
criteria based
on PCV13
PCV20 Phase 3
PCV21 Phase 2
Serotypes shared with PCV20 or unique to PCV21

66 Vaccines Investor Event
PCV21_Phase II _ [NCT04398706] Seroresponse: IgG concentration ≥0.35 µg/mL for all serotypes
PCV20_Phase III_ [NCT04382326] Seroresponse: IgG concentration ≥0.35 µg/mL for all serotypes except ≥ 0.23 µg/mL, ≥0.10 µg/mL and ≥ 0.12 µg/mL for serotypes 5, 6B and 19A respectively
Note: for serotypes 15B and 22F, difference (% and GMC ratio) vs lowest serotype in Prevnar 13
DISCLAIMER: data from separate studies should be interpreted with care.
Robust performance of the 4 serotypes
conjugated to TT
Innovative carrier to break serotype composition ceiling
Introducing new carrier for 4 serotypes to improve
performance
Carrier protein TT Carrier protein CRM
197
1 3 4 5
6A
6B
7F
9V
14
18C
19A
19F
23F
8
9N
10A
11A
12F
15B
22F
33F
PCV13
PCV21
Post Dose 4: IgG GMC ratio
0,25
1
4
16
1 5 15B 22F
GMC ratio vs
Prevnar
13
PCV20 Phase 3PCV21 Phase 2

Boosting effect is comparable to
PCV13 for common serotypes
Robust immune response for
the additional serotypes
Single
booster
dose
n= 35
n= 35
n= 35
n= 35
67 Vaccines Investor Event
0,1
1
10
100
1 3 4 5 6A 6B 7F 9V 14 18C 19A 19F 23F
GMC µg /
mL
0,1
1
10
100
8 9N 10A 11A 12F 15B 22F 33F
GMC µg /
mL
PCV21 Formulation #1
300
PCV21 Formulation #2
PCV21 Formulation #3
PCV13
primed with 3
doses of
PCV13 at 2, 4, 6
months
PCV21 PCV13
Phase 2 interchangeability data support PCV21 as booster
Days
Toddlers
12-15 Mo

68
Phase 3 starts in H1 2024
Expected submission in 2027
Initiating development of
next generation PCV21+ vaccines
Vaccines Investor Event
Ambitious program with first pediatric PCV20+ vaccine; clear
blockbuster potential

Establish Best-in-Class
meningitis portfolio
Thomas Grenier
Head of Franchise & Product Strategy
Saranya Sridhar
Head of Translational Medicine

Best-in-Class MenACWY profile
- Novel serogroup-specific design,
unique chemical and structural
features
- Higher serogroup C responses
- Fully-liquid presentation
- Broad age-indication
- Up to 7 years persistence in
different age groups
Leadership in the U.S. with >60% MS
70 Vaccines Investor Event
International roll-out ongoing
- MenQuadfi registered in 53 countries and expanding
- WHO pre-qualified
source: Sanofi DDD CDC & others
Consolidate MenQuadfi market leadership

71 Vaccines Investor Event
Adolescents (10-17 years)
Higher or comparable immune response vs.
Pfizer’s ACWY in adolescents
Infants & Toddlers (2-12 months)
Higher immune response with 3 doses of MenQuadfi
vs. 4 doses of GSK’s ACWY
New clinical evidence reinforces MenQuadfi’s Best-in-Class profile
0
50
100
150
200
A W Y
C
log2 hSBA GMTs
A C W Y
Booster
1
4
16
64
256
1024
Primary vaccination
Immune response vs. competition
3000
2000
1000
0
GMTs
GMTs
MenQuadfi (N=159) Pfizer’s ACWY (N=161)
GMTs
MenQuadfi
®
(N=176)
(2, 6 months old)
GSK’s ACWY (N=81)
(2, 4, 6 months old)
MenQuadfi
®
(N=126)
(12 months old)
GSK’s ACWY (N=60)
(12 months old)
A C W Y
Comparison of hSBA GMT responses 30 days after vaccination
1
hSBA GMTs
Comparison of hSBA GMT responses 30 days after vaccination
2
2: Sanofi data on file (MET33)1. Sanofi data on file (MEQ71)
Source: Sanofi data on file
MenQuadfi first and only ready-to-use syringe
~80% preference by U.S. HCPs
1
when
ready-to-use syringe option is available
Unique presentation competitive
advantage: no other ACWY syringe
available
U.S. FDA submission in July 2023,
available early 2024
72 Vaccines Investor Event

73
MenQuadfi addresses current recommendation for
quadrivalent MenACWY immunization in most markets
Vaccines Investor Event
1. Published routine vaccination policies 2. In the U.S., MenB vaccination for 16- to 23-year-old people is a shared clinical decision
Complex and various routine recommendations
1
due to different IMD
incidence by serogroup, age, geography
MenQuadfi currently has the
most complete product profile
Immunization programs
expected to evolve over time,
including serogroup B adoption
- Many countries still transitioning
from C to ACWY
- Pace of ACWY switch to
pentavalent highly dependent
on schedule compatibility, cost
effectiveness and impact on
public budget
Infants Toddlers Adolescents
U.S.
MenACWY (11&16 yrs)
France
MenB (3 mo)
MenC+B (5 mo)
MenC+B (12 mo)
Germany
MenC (12/23 mo)
Italy
MenB (3,4,6 mo) MenACWY (13-15mo) MenACWY (12/18 yrs)
Spain
MenC (4 mo) MenC (12 mo) MenACWY (12 yrs)
UK
MenB (2,4 mo) MenC+B (12 mo) MenACWY (13/15 yrs)
Australia
MenACWY (12 mo) MenACWY (14-16 yo)
Saudi Arabia
MenACWY (9 mo) MenACWY (12 mo) MenACWY (18 yrs)

74 Vaccines Investor Event
MenB antigen formulation
Phase 1/2 clinical study design
• 4 major antigens used to cover broad
diversity and variable strain expression
Novel MenB formulation (SP0230) to provide optimal protection
MenB Formulation #1
MenB Formulation #5
Bexsero (MenB GSK)
Adolescents
10-17 yo
Adults
18-55 yo
MenB Formulation #2
MenB Formulation #3
MenB Formulation #4
MenB Formulation #6
Trumenba (MenB Pfizer)
n=72
n=72
n=72
n=72
n=72
n=72
n=72
n=72
61
1
Days
31
Non-Lipidated
fHBP A
NadA
Outer
Membrane
Vesicle
Non-Lipidated
fHBP B
fHBP A: factor-H binding protein subfamily A; fHBP B: factor-H binding protein subfamily B; NadA: Neisserial adhesin A

75 Vaccines Investor Event
hSBA seroresponse rate
1
– Sanofi MenB vs Bexsero
²
Vaccine strains
2
Cross-protection against strains
not in the vaccine
3
MenB Formulation 1
MenB Formulation 3
hSBA seroresponse rate
1
– Sanofi MenB vs Trumenba
Vaccine strains
2
Cross-protection against strains
not in the vacine
3
MenB Formulation 1
MenB Formulation 3
Higher point estimates (>+15%) similar (+/- 15%) Lower (<15%)
1. hSBA seroresponse - % of participants with ≥ 4-fold rise of antibody titer from baseline 2. Tested strains exhibiting one of the Sanofi vaccine antigen 3. Tested strains exhibiting different antigens from the Sanofi vaccine
MenB strong phase 1/2 results demonstrate competitiveness
and support move to next phase
Sanofi formulations were
well tolerated
All antigens are immunogenic
Breadth of protection
reaching expected level

76
Strong preclinical data support advancement of MenPenta
program in ready-to-use syringe to phase 1/2 in H2 2023
Vaccines Investor Event
Source: Sanofi data on file
No immune interference between MenPenta
components (rabbit model)
Vaccines
MenB MenPenta MenQuadfi
B Vaccine
strains
1 100 85 0
2 100 100 0
3 100 100 0
4 100 100 0
A, C, W, Y
vaccine
strains
A 100 100 85
C 85 100 100
W 0 100 100
Y 0 100 100
% of responders demonstrating a 4-fold increase
between D0 and D42 in a serum bactericidal assay
Liquid MenPenta stability data give
high confidence in PFS formulation
% of Free Polysaccharide ACWY of MenPenta
20
10
0
0 2 4 6 8 10
No immune interference
between MenB and
MenQuadfi antigens
Good stability of the
fully-liquid formulation
Advancing MenPenta
liquid formulation to
phase 1/2 in H2 2023
Antigen Contents of MenPenta by RP-LC
0 3 6 9
200
150
100
50
0
fHBP A
fHBP B
NadA
dOMV
Months
0 2 4 6 8 10
Months

77 Vaccines Investor Event
Comprehensive and competitive meningococcal portfolio
provides new source of growth
MenQuadfi Best-in-Class MenACWY vaccine
MenB formulation demonstrates strong potential
for cross-protection across B strains
Advancing MenPenta development in ready-to-use
syringe with expected U.S. submission in 2027

Leverage leading-edge
mRNA platform
Jean-François Toussaint
Head of Vaccines R&D
Frank DeRosa
Head of Research for mRNA CoE

- 7 mRNA Phase 1/2 clinical trials: Flu, RSV,
platform, 3 LNPs screened
- >600 dedicated employees, of which >250
new recruits
- Extensive external network of academia,
industry and government partnerships
Execution
- Innovative antigen, mRNA and LNP designs
across viral and bacterial targets
- Highly competitive LNP selected for improved
immunogenicity and better tolerability
- Developed high-throughput translational
science model with proprietary MIMIC
®
system
to predict clinical outcomes
Built a leading-edge mRNA platform in just 18 months
Innovation
79 Vaccines Investor Event

80
Accelerated learnings from holistic data integration leveraging
AI/ML models
Vaccines Investor Event
mRNA design space
(Coding and non-coding
Sequences)
5 generative and active
learning ML models
Multi features optimization
Predictive and generative
models developed
1
st
version of predictive modeling
for reactogenic signatures
Rationally designed for high
immunogenicity and stability
deep understanding
Platform evolution
Immunogenicity
& durability
Adverse
events
Inflammatory
profiling
Clinical profile
preclinical prediction
Mechanism of
action
Clinical profile
AI/ML models
Antigen
design
mRNA
design
LNP
Optimization
Translational
models
81 Vaccines Investor Event
Robust preclinical antibodies titers across many target antigens (viral & bacterial)
rProt mRNA Negative
control
1
10
100
Seroneutralization titers
rProt mRNA Negative
control
10
3
10
4
10
5
10
6
10
7
OPK titers
ELISA titer (log10EU)
rProt
0
2
4
6
8
mRNA
Negative
control
Bacterial
Source: Data on file. rProt = recombinant Protein
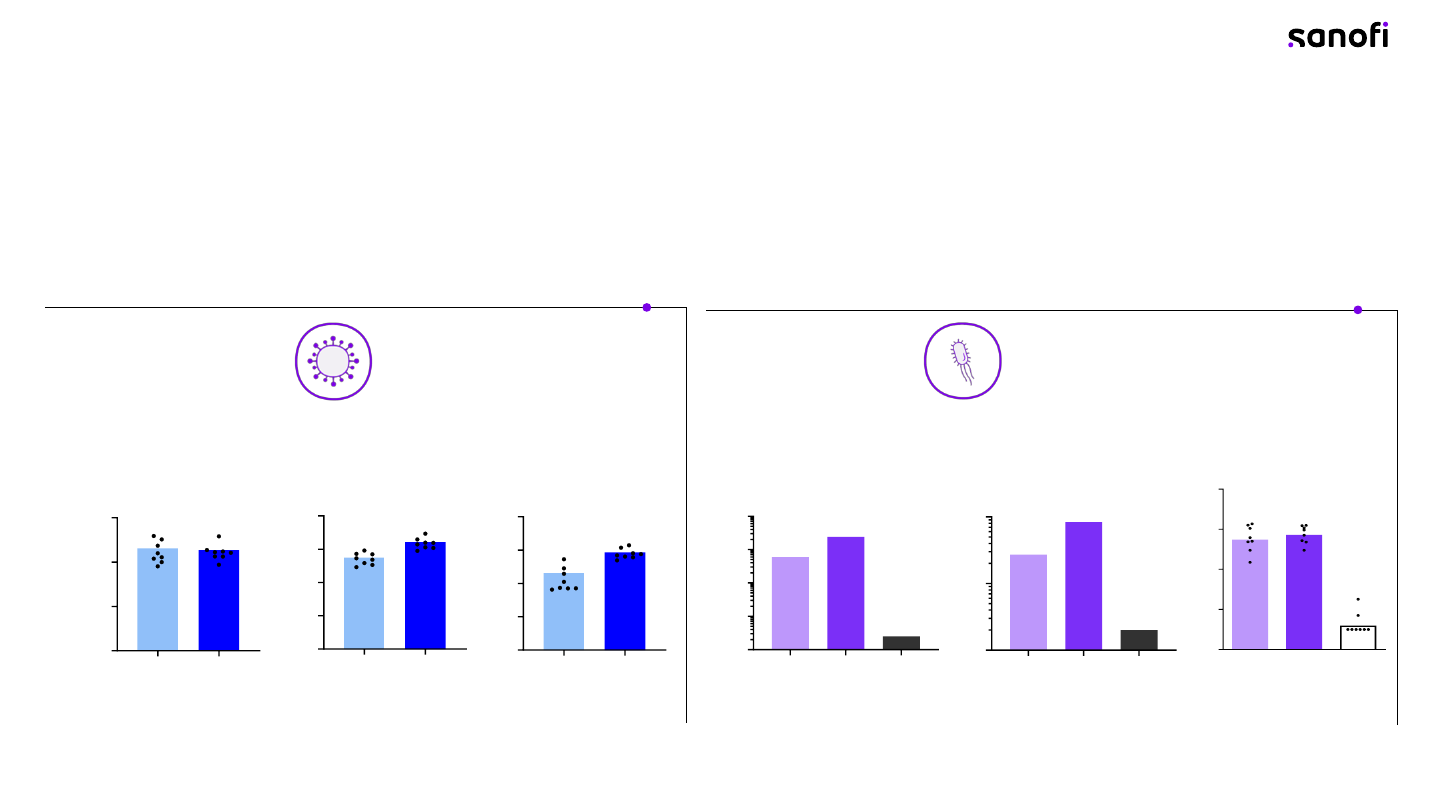
Acne 1 Acne 2 Chlamydia
Platform now includes both viral and bacterial protein targets
rProt mRNA
2
0
2
5
2
10
2
15
Neutralizing titer
-1
(log
2
)
rProt mRNA
2
0
2
5
2
10
2
15
2
20
Neutralizing titer
-1
(log
2
)
rProt mRNA
2
0
2
5
2
10
2
15
2
20
Neutralizing titer
-1
(log
2
)
Viral
RSV hMPV PIV3
82
Leverage leading-edge mRNA platform for Best-in-Class /
First-in-Class mRNA vaccines and therapeutics
Vaccines Investor Event
Target
balanced efficacy
and tolerability
Generate
enhanced HCP and
patient experience
Utilize
broad spectrum
of applications
Reactogenicity ThermostabilityPotency Viral Targets Bacterial Targets

83 Vaccines Investor Event
0
5
10
15
mRNA copies (%)
Control sequence
Codon optimized
Control
Sequence
Codon
Optimized
0
10
20
30
40
50
mRNA Translationally
Active Proportion (%)
✱✱
mRNA Translation
Increased Translation Efficiency
(Polysome Profiling)
1
Antigen
(HA)
~ 1000
Sequences
tested in-vitro
~ 500 000
sequences
generated in-silico
30+
Sequences
tested in-vivo
10
20
30
40
50
60
70
80
90
100
110
120
130
140
150
160
170
180
190
200
210
220
230
240
250
260
270
280
0
1
2
3
4
Optimized Construct #
Expression relative to benchmark
Codon 1
Codon 2
Codon 3
Codon 4
Codon 5
Codon 6
5’ cap 5’ UTR
Coding Region
3’ UTR Poly (A) Tail
Start Codon Stop Codon
mRNA Sequence Optimization Process
Increased Protein Expression
(Immunofluorescence)
Control
Sequence
Codon
Optimized
0
10000
20000
30000
40000
Target Protein Expression
✱✱✱
Codon Optimized
Control Sequence
** p < 0.01
*** p < 0.001
Source: Data on file
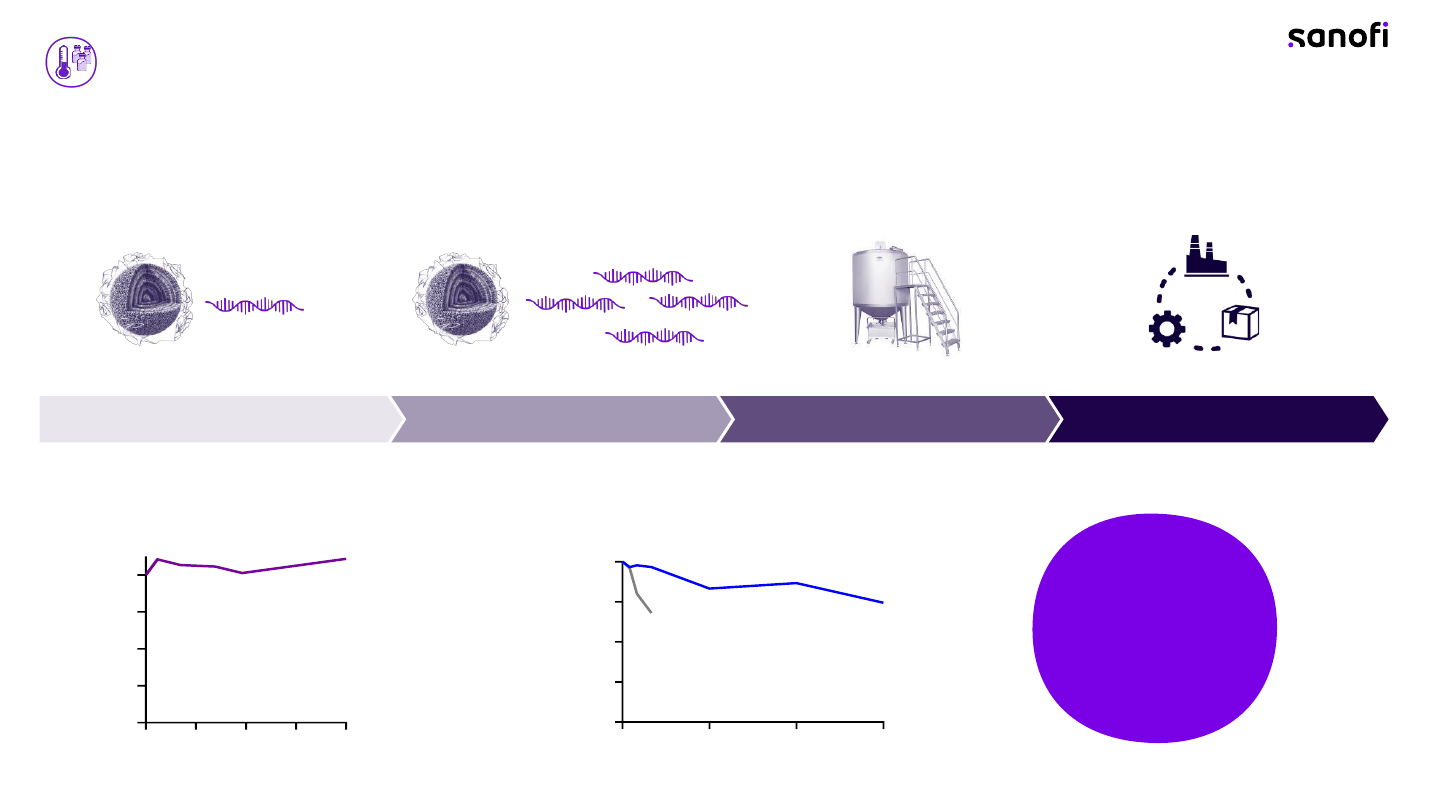
Our new platform enables improved mRNA performance
AUG GA G CUU CGG AGC UAG
Potency

+
Ionizable lipid
Helper lipid
Cholesterol
PEGylated lipid
The helper lipid helps create the lipid membrane of
the LNP, and it allows for the LNP to easily fuse to the
mRNA’s target cell and endosomal membrane
Cholesterol enhances the stability of the LNP and ensures
it is sturdy and rigid. This assists with the introduction of
the mRNA into the cells
Polyethylene glycol, or a PEG lipid, is what helps maintain
the overall physical nanostructure of the LNP and protects
the mRNA nanoparticles from the body’s natural clearance
mechanisms
The ionizable lipid wraps around the mRNA and helps
transport and release it to the targeted cell
Potency
84 Vaccines Investor Event
All four LNP components leave significant room for optimization

85 Vaccines Investor Event
Potency
Extensive ionizable libraries developed for improved
potency for multiple routes of administration
Ionizable Lipid
+
0
20
40
60
80
100
Protein (ng/ml)
MC3
SM-102
Intramuscular
Novel helper lipids demonstrating significant
improvements in potency (~2-3x)
LNP1
Control
LNP1
Novel
Helper
LNP2
Control
LNP2
Novel
Helper
LNP3
Control
LNP3
Novel
Helper
0
2 0
4 0
6 0
Protein (ng/mL)
6 h
24 h
LNP3
LNP2
LNP1
Helper Lipid
(1)
(1)
(1)
Novel sterols demonstrating significant
improvements in potency (~3-4x)
1 2 3 4 5 6
0
50
100
150
LNP1
Control
LNP1
Sterol
LNP2a
Sterol
LNP2b
Sterol
LNP2
Control
50
100
150
Protein (ng/mL)
Sterol
(1)
(1)
(3)
Novel 5
th
Excipient
Significant potency boost with excipient (~4x)
LNP Control
Excip #1
Excip #2
Excip #3
Excip #4
Excip #5
Excip #6
Excip #7
Excip #8
0
20
40
60
80
100
Protein (ng/ml)
(3)
(2)
(1) p < 0.0001
(2) P < 0.001
(3) P < 0.01
Sanofi novel science supports improved LNP and better potency
Novel PEG alternatives maintaining performance in vivo
Alt. PEG1 Alt. PEG2 Alt. PEG3 Alt. PEG4 PEG Control Buffer
10
100
1000
10000
HA Neutralizing Titers
LLOQ
PEGylated Lipid

LNP with unmodified mRNA
LNP with modified mRNA
86 Vaccines Investor Event
Unmodified vs modified
Mono- vs multi-valent
Multiple targets
Multiple LNPs (4+)
Reactogenicity
Immunogenicity
LNP3
(RSV)
LNP2
(RSV)
LNP2
(Flu)
LNP3
(Flu)
LNP1
(Flu)
LNP1
(Flu)
LNP2
(Flu)
Fast learnings from diverse clinical trials with mRNA and LNP
Clinical trials
Reactogenicity
Multiple Sanofi LNP candidates

87
Sanofi’s proprietary MIMIC
®
system to increase efficiency of
mRNA screening
Vaccines Investor Event
Reactogenicity
Preclinical MIMIC Prediction
2021 2023
-10
0
10
20
30
40
50
log2 fold
-change in cytokines
LNP 2
(Modified RNA)
LNP 3
(Modified RNA)
LNP 2
(Unmodified RNA)
0
20
40
60
Solicited Systemic
Reactions (%)
Grade 1
Grade 2
Grade 3
Fever Headache ArthralgiaChills MyalgiaMalaise
Modified
LNP
2
LNP
3
LNP
2
LNP
3
LNP
2
LNP
3
LNP
2
LNP
3
LNP
2
LNP
3
LNP
2
LNP
3
Clinical Outcomes
0
20
40
60
80
100
Solicited Systemic Reactions (%)
Grade 1-2
Grade 3
Unmodified
Modified
rHA Dose
Level 1
Dose
Level 2
Dose
Level 3
Dose
Level 1
Dose
Level 2
Dose
Level 3
Dose
Level 4
Dose
Level 5
IIV4
comparator
0 3 6 9
0
25
50
75
100
Change in % mRNA Integrity
Months
QIV LNP candidate
+ Excipient
Liquid LNP
(Control)
Prototype LNP demonstrating
12-months+ stability as 2-8
o
C liquid
0 3 6 9 12
0
25
50
75
100
Months
Change in %mRNA Integrity
Prototype LNP
88 Vaccines Investor Event
Multivalent LNP
Thermostability
Scale-up Industrial environmentMonovalent LNP
QIV LNP demonstrating
~9 months stability as 2-8
o
C liquid
Significant progress toward improved thermostability
Increasing complexity
Next step:
Achieve large
scale batches

89 Vaccines Investor Event
Viral Targets Bacterial TargetsReactogenicity ThermostabilityPotency
Competitive platform in just 18
months with 7 clinical trials…
LNP
Optimization
mRNA
Optimization
…innovating across technology… and biology
Novel Antigen
Design
…to cross new frontiers
Our leading-edge mRNA platform is poised to break grounds in
vaccine innovation

New frontiers
Dr William Geisler, MD, MPH
Professor of Medicine and Epidemiology,
University of Alabama at Birmingham
Sally Mossman
Head of Vaccine Research Portfolio Strategy

Innovation to address unmet needs in infectious diseases
91 Vaccines Investor Event
Chlamydia
- Dr William Geisler on the burden of chlamydia
disease
- Positive data enable selection of final vaccine
candidate
Acne
- Key preclinical data support clinical evaluation
of therapeutic vaccine candidate
- GMP production to enable clinical evaluation
ongoing

Chlamydia

Dr William Geisler, MD, MPH
Professor of Medicine and Epidemiology,
University of Alabama at Birmingham

94
Chlamydia Burden
and Need for a Vaccine

95
Chlamydia is the most common bacterial STI worldwide
(~129 million new cases annually)
Van Gerwen, et al. Nat Microbiol. 2022
2020: WHO estimated 377 million
new cases of four STIs
• Chlamydia: 129 million new cases
• Gonorrhea: 82 million new cases
• Syphilis: 7.1 million new cases
• Trichomoniasis: 156 million new cases
These numbers represent incident cases of chlamydia, gonorrhea, trichomoniasis and syphilis in 2016.

96
Chlamydia is a chronic infection and most infected
persons do not have symptoms or signs of infection
Farley et al. Prev Med. 2003
Natural Course of Chlamydia in 83 Asymptomatic
Colombian Women
~50% of infections
persist at 1 year
Women Men
Molano et al. J Infect Dis. 2005
<25%
>75%
>50%<50%
Asymptomatic Symptomatic

Causes upper genital tract inflammation in 10%-15%
of women (PID),
1,2
which may be complicated by:
• Infertility (up to 18%)
1
• Chronic pelvic pain (up to 33%)
3
• Ectopic pregnancy (3-fold risk)
4
Associated with adverse pregnancy outcomes
4
• Miscarriage, stillbirth, premature birth, and low birth weight
(1.5-5-fold risk)
• Infection in newborns (eye and lung infection)
Increases risk for HIV acquisition (1.5-2-fold)
5-6
97
Chlamydia has important health consequences,
and the burden of morbidity is greater in women
1. Haggerty, et al. J Infect Dis. 2010 2. Oakeshott, et al. BMJ. 2010 3. Ness, et al. Am J Obstet Gynecol. 2002 4. Tang, et al. Sex Transm Infect. 2020 5. Barker, et al. Sex Transm Dis. 2022 6. Malekinejad, et al. Sex Transm Dis. 2022

Chlamydia Prevention Measures
• Abstinence
• Sexual health education
• Barrier methods (e.g., condoms)
• NO VACCINE AVAILABLE
Chlamydia Testing (with highly accurate NAAT)
• Routine screening in young women, MSM, and other women and men at risk
• Diagnostic testing with symptoms/signs
Chlamydia Treatment
• Treatment of patient and partner(s)
• Doxycycline x 7d or azithromycin x 1 effective
• No antibiotic resistance concerns
98
Chlamydia control programs provide a comprehensive
approach to preventing and treating chlamydia
2021 CDC STIs Treatment Guidelines

99
Control programs have been ineffective in decreasing
chlamydia rates, justifying need for a preventative vaccine
1. Hosenfeld, et al. Sex Transm Dis. 2009 2. Brunham and Rekart. Sex Transm Dis. 2008 3. Kreisel, et al. Sex Transm Dis. 2021
Rates of Reported Chlamydia Cases, U.S., 1984–2021*
(* Per 100,000)
• Many chlamydia cases go
undetected and untreated
• Natural infection does not
elicit long-lived protective
immunity in most
– Reinfection occurs in up to 20%
within one year
1
• Treatment early in course
of infection may impair
immunity
2
CDC STD Surveillance, 2021
- 1,644,416 cases reported
- Estimated ~4 million new cases
3


Chlamydia next to HPV in terms of costs in STIs
HBV
$46 M
HSV-2
$91 M
Trichomoniasis
$144 M
Syphilis
$174 M
Gonorrhea
$271 M
Chlamydia
$691 M
HPV
$775 M
HIV
$13.7 B
Direct medical costs by infection in U.S.
Queensland government in Australia fully
recognizes the burden of disease in chlamydia
Supporting our vaccine development through
the Translational Science Hub in Queensland
101 Vaccines Investor Event
Significant direct medical costs in STI attributed to chlamydia
Source: CDC’s Sexually Transmitted Disease Surveillance, 2021, sexually Transmitted Disease Surveillance, 2021, accessed May 10
Chlamydia trachomatis (CT) is a bacterium
that lives inside human cells
- Antibodies and CD4 T cell responses
- Recognition of multiple chlamydia
serovars (serotypes)
- Broad population coverage (HLA: Human
Lymphocyte Antigen)
Targeted immune profile
CD4 T
cells
Anti-
bodies
IFN
102 Vaccines Investor Event
Antibodies may
contribute to
protection
IFN- producing
CD4+T cells are
the primary
mediators of
protection
EB (elementary body)
RB (reticulate body)
Inclusion
Chlamydia biology requires sophisticated vaccine design

1
10
100
1000
10000
2
4
6
8
103
Innovative multi-antigen vaccine candidate achieves targeted
immune profile, moving to phase 1/2 in 2024
Vaccines Investor Event
Serovar equates to serotype terminology in chlamydia field
2
3
4
5
6
Serovar D
Serovar E
Serovar F
Serovar G
Antigen A
LNP only
IFN spots/ 10
6
splenocytes
ELISA titer anti
-EB (log10EU)
ELISA titer anti
-EB (log10EU)
Antigen B
LNP only
Antigen C
LNP only
Antigen D
Broad cross-serovar T cell
responses
Cross-serovar antibodies recognizing
native elementary bodies
Spleen cells secreting Interferon-gamma
in mice immunized with mRNA encoding
Antigen A, or empty LNP control
Elementary body (EB) binding antibodies in sera from mice immunized
with mRNA encoding Antigen B, C, D or empty LNP control
Serovar D
Serovar E
Serovar G
Serovar D
Serovar E
Serovar G
1
10
100
1000
10000
2
3
4
5
6
2
4
6
8

Acne

105 Vaccines Investor Event
Mild
acne
Moderate
acne
Severe
acne
High burden of disease
- Chronic nature of condition
- Psycho-social impact on patients
- Contribution to antimicrobial resistance
- Economic impact of treatment
- Unmet needs with current treatments
Source: IHME/GBD 2019 estimates. Available at https://vizhub.healthdata.org/gbd-compare/
Chen H. et al. Magnitude and temporal trend of acne vulgaris burden in 204 countries and territories from 1990 to 2019: an analysis from the Global Burden of Disease Study 2019
Layton A. M. et al. Reviewing the global burden of acne: how could we improve care to reduce the burden?
Acne is chronic inflammatory skin disease and the 8
th
most
common medical condition globally
Incidence and prevalence significant and
increasing
- 8.6 million prevalent cases in U.S.
- 18.3 million prevalent cases in EU

106 Vaccines Investor Event
Desired Acne
Treatment
Attributes
Need for improvement vs standard of care
on all dimensions
Reliable
efficacy
Easy
administration
and monitoring
No serious
side effects
Suitable for
long-term use
No skin
sensitivity
“Isotretinoin has the efficacy, but it’s complicated
and has risks – none of the options we have give
us everything we need in one treatment’’
– Dermatologist, Germany
“Acne is very hard on patients because it is a
disease that everyone can see;…I don’t take it lightly
because I know it can have psychological and
social ramifications”
– Dermatologist, US
Recent market research points to gaps in treatment landscape
driving a significant need for novel approaches
Sanofi internal HCP market research, 1Q23

107 Vaccines Investor Event
Targeted intervention designed to restore a healthy
skin microbiome
Leveraging antigens from Origimm acquisition,
enhanced with additional antigen
Critical functional assays developed and running
Synergy between Sanofi Vaccines and Pharma
Immunology Franchise
Full speed development of mRNA-based candidate
Recombinant protein antigens obtained
through Origimm acquisition validated
with strong proof of mechanism data
OPK: opsonophagocytic killing of C. acnes bacteria
Our ambitious approach in the acne immunotherapeutic space
Antigen 1 Antigen 2 Negative
control
OPK titers
10
3
10
4
10
5
10
6
10
2

Bacterial Virulence Factors
Keratinocytes Immune Cells Sebocytes
Corneocyte
Sebum
C.acnes
C. acnes
Acne vulgarum: skin dysbiosis
driven by outgrowth of pathogenic
Cutibacterium acnes
108 Vaccines Investor Event
Inflammatory cascade
Origimm Antigens
- Expressed on surface of pathogenic
C. acnes strains
- Induce opsonophagocytic killing
antibodies to reduce bacterial burden
- Essential adhesin and iron uptake
functionality
Additional Vaccine Antigen
- Based on key bacterial
virulence factor
- Induces antibodies that
neutralize the virulence factor,
interrupting the inflammatory
cascade that drives disease
Therapeutic vaccine addressing multiple pathogenic mechanisms
Pharmaceutics 2019, 11(10), 490; https://doi.org/10.3390/pharmaceutics11100490 Targeted Topical Delivery of Retinoids in the Management of Acne Vulgaris: Current Formulations and Novel Delivery Systems by Gemma Latter
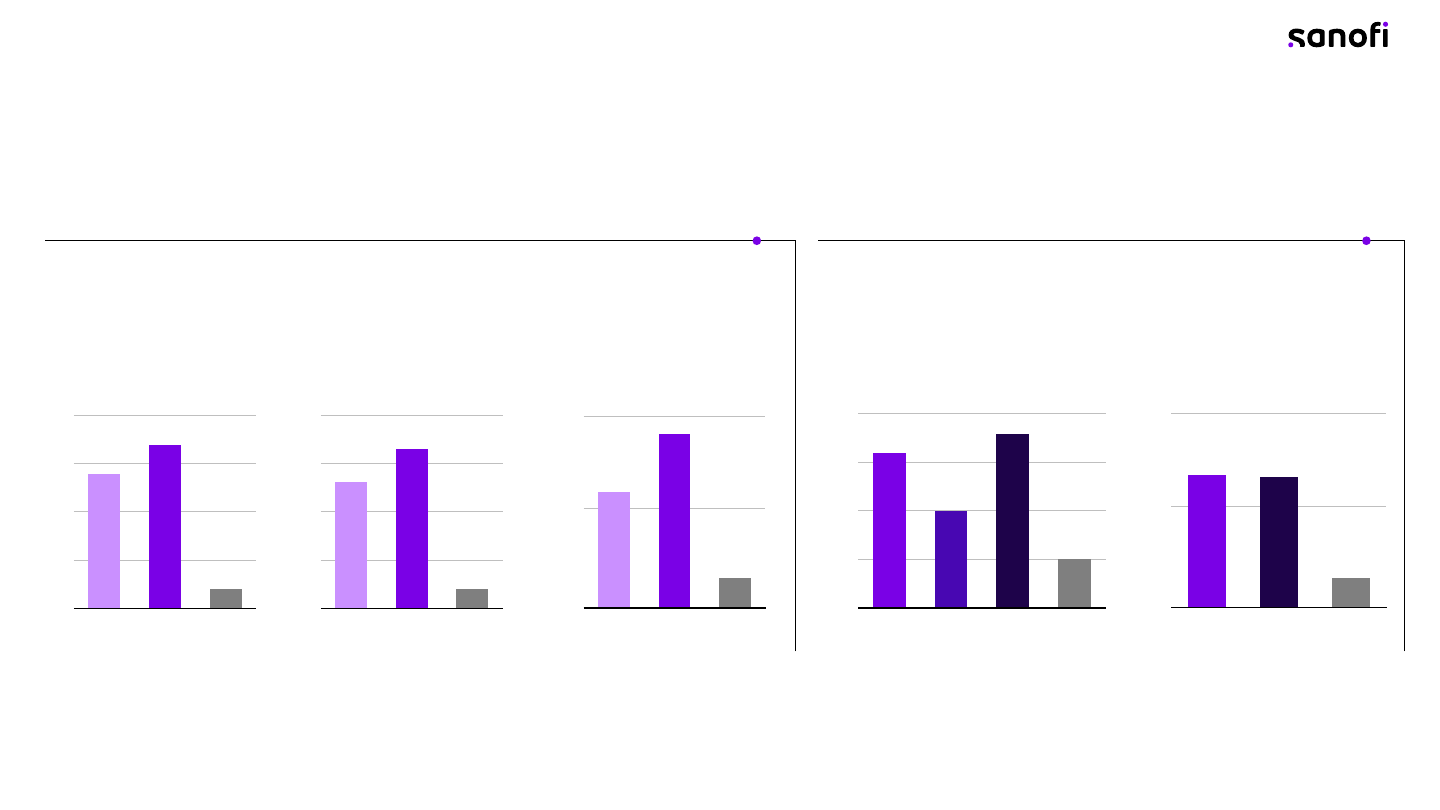
mRNA expression of bacterial antigens induces functional
immune responses at least equivalent to recombinant proteins
10
3
10
4
10
5
10
6
10
7
OPK titers
10
3
10
4
10
5
10
6
10
7
OPK titers
1
100
10
Seroneutralization
titers
rProt mRNA Negative
control
rProt mRNA Negative
control
rProt mRNA Negative
control
1
100
10
Seroneutralization
titers
mRNA
Antigen
3
Negative
control
No interference in induced responses with
combination of all three mRNAs
10
3
10
4
10
5
10
6
10
7
OPK titers
mRNA
Antigen
2
mRNA
Antigens
1+2+3
Negative
control
mRNA
Antigen
1
Antigen 1 Antigen 2 Antigen 3
mRNA
Antigens
1+2+3
OPK: opsonophagocytic killing
OPK: opsonophagocytic killing
Neutralization of
inflammatory factor
Neutralization of
inflammatory factor
109 Vaccines Investor Event
Positive pre-clinical data support move to phase 1/2 in 2023

Expanding to
new disease areas
Therapeutic or prophylactic
Association of infectious agents with chronic diseases
Microbiome
Addressing
unmet needs
Leveraging the right
technological solutions
110 Vaccines Investor Event
Moving at pace to unlock new areas in infectious disease

Conclusion
Thomas Triomphe
Head of Vaccines GBU

Sanofi drives innovation with BiC/FiC vaccines pipeline
3
New products approved since Vaccines Investor Event
in December 2021
mRNA
Leading-edge mRNA platform to lift our influenza standard
of care and deliver innovation to address unmet needs
6
New vaccine candidates expected in phase 1/2 trial in
2022/23
First-in-Class / Best-in-Class vaccine candidates expected
in phase 3 by 2025 across diverse preventative and
therapeutic areas
At least 5
112 Vaccines Investor Event

Sanofi
Vaccines sales
>€10bn
by 2030
1
113 Vaccines Investor Event
1. At 2023 rate
Launch Beyfortus blockbuster and build BiC RSV franchise
Continue to win in Influenza
Enter Pneumococcal market with PCV blockbuster candidate
Sustain growth of established business
Introduce our new mRNA vaccines to market
On a clear path to generate >€10bn sales by 2030

Thomas Triomphe
Head of Vaccines GBU
Jean-François Toussaint
Head of Vaccines R&D
Frank DeRosa
Head of Research for
mRNA CoE
Thomas Grenier
Head of Vaccines F&PS
Dr William Geisler, MD, MPH
Professor of Medicine & Epidemiology
University of Alabama at Birmingham
Sally Mossman
Head of Vaccine Research
Portfolio Strategy
Q&A session Part 2
Saranya Sridhar
Head of Translational Medicine

Appendices

Vaccines Investor Event
Collaborations
116
Name Developed in collaboration with…
Beyfortus
®
AstraZeneca
Dupixent
®
itepekimab (IL-33)
Regeneron
frexalimab
ImmuNext
VidPrevtyn
®
Beta
GSK and with funding from Biomedical Advanced Research and Development Authority (BARDA)
SP0202 SK Bioscience

Vaccines Investor Event
Abbreviations
ACIP Advisory Committee on Immunization Practices
AE Adverse Event
AI Artificial Intelligence
BTK Bruton’s Tyrosine Kinase
CD Cluster of Differentiation
CDC Centers for Disease Control and Prevention
COPD Chronic Obstructive Pulmonary Disease
CT Chlamydia Trachomatis
dOMV detoxified Outer Membrane Vesicles
EB Elementary Body
ELISA Enzyme-Linked Immunosorbent Assay
ESPID European Society for Paediatric Infectious Diseases
FDA Food and Drug Administration
fHBP factor-H Binding Protein
GMC Geometric Mean Concentration
GMP Good Manufacturing Practice
GMT Geometric Mean Titers
GP General Practitioner or Glycoprotein
HA Hemagglutinin
HBV Hepatitis B Virus
HCP Healthcare Professionals
HD High Dose
HIV Human Immunodeficiency Virus
117
HLA Human Lymphocyte Antigen
hMPV Human Metapneumovirus
HPV Human Papillomavirus
HSBA Human Serum Bactericidal Activity
HSV-2 Herpes Simplex Virus type 2
IFN Interferon
IgA Immunoglobulin A
IgG Immunoglobulin G
IL-13 Interleukin 13
IMD Invasive Meningococcal Disease
IPD Invasive Pneumococcal Disease
LNP Lipid Nanoparticle
LRTD Lower Respiratory Tract Disease
LRTI Lower Respiratory Tract Infection
MA-LRTI Medically Attended LRTI
ML Machine Learning
mNT micro Neutralization Test
MoA Mode of Action
mRNA messenger RNA
MS Multiple Sclerosis
NA Neuraminidase
NadA Neisserial adhesin A
NT Non-typable
NVT Non-vaccine type
OA Older Adults
OPK Opsonophagocytic killing
P&I Pneumonia and Influenza
PBF Protection Beyond Flu
PCV Pneumococcal Conjugate Vaccine
PEG PEGylated
PFS Pre-filled Syringe
PIV Parainfluenza Virus
QIV Quadrivalent Influenza Vaccine
RP-LC
Reversed Phase Liquid Chromatography
rProt recombinant Protein
RSV Respiratory Syncytial Virus
SD Standard Dose
SoC Standard of Care
STI Sexually Transmitted Infection
TEAE Treatment Emergent Adverse Event
TNFI Tumor Necrosis Factor Inhibitor
TSLP Thymic Stromal Lymphopoietin
TT Tetanus Toxoid
URTD Upper Respiratory Tract Disease
UTR Untranslated Region
VFC Vaccines for Children
WHO World Health Organization

