
Summary of EtR and proposed
recommendations for Pfizer’s MenABCWY
vaccine
Jennifer Collins MD, MSc
Interim Co-Lead, ACIP Meningococcal Vaccines Work Group
October 25, 2023
National Center for Immunization & Respiratory Diseases

ACIP Recommendations for Meningococcal Vaccines
▪ Routine schedule
– MenACWY: dose 1 at age 11–12 years, booster dose at age 16 years
– MenB (shared clinical decision-making): two doses at age 16–23 years (preferred age 16–18 years)
▪ Special situations
Indication
MenACWY
(age ≥2 months)
MenB
(age ≥10 years)
Medical conditions
Asplenia X X
Complement Deficiency X X
Complement inhibitor use X X
HIV infection X
Other
Some microbiologists X X
Exposure during an outbreak X X
Travel to hyperendemic areas X
First-year college students X
Military recruits X
2

Meningococcal vaccines licensed and
available in the United States
▪ MenACWY vaccines are interchangeable
▪ MenB vaccines are NOT interchangeable
Vaccine Trade Name Manufacturer Minimum age
MenACWY-CRM Menveo GSK 2 months
MenACWY-TT MenQuadfi Sanofi Pasteur 2 years
3
Vaccine Trade Name Manufacturer Minimum age
MenB-4C Bexsero GSK 10 years
MenB-FHbp Trumenba Pfizer 10 years

Pfizer’s MenABCWY Vaccine
▪ Licensed as a 2-dose series (6-month interval) for individuals aged 10–25 years
▪ Comprised of Trumenba (serogroup B) and Nimenrix (serogroups ACWY)
– Trumenba
• Consists of two purified recombinant lipidated FHbp antigens, one from
each FHbp subfamily (A and B)
• Currently licensed and available in U.S. (10–25 years)
– Nimenrix
• Meningococcal group A, C, W, and Y polysaccharide tetanus toxoid
conjugate vaccine
• Not licensed in U.S. but used extensively in Europe and elsewhere for more
than a decade
4

Policy Questions for 3 PICOs
▪ Should the pentavalent vaccine be included as an option for
MenACWY/MenB vaccination in people currently recommended to
receive both vaccines?
▪ Should the pentavalent vaccine be included as an option for people
currently recommended to receive MenACWY only?
▪ Should the pentavalent vaccine be included as an option for people
currently recommended to receive MenB only?
PICO 1
PICO 2
PICO 3
5

GRADE Table 1: Combined Policy Question and PICO
Policy Question
Should the pentavalent vaccine be included as an option for people currently recommended to
receive
MenACWY and MenB, MenACWY only, or MenB only?
Population
All individuals aged 10 years or older currently recommended to receive
MenACWY+MenB,
MenACWY, or MenB vaccine
Intervention
Vaccination with Pfizer’s pentavalent (
MenABCWY) vaccine
Comparison
Vaccination with currently licensed
MenACWY+MenB, MenACWY, or MenB vaccine
Outcomes
•
Meningococcal disease caused by serogroups A, B, C, W, and Y (as appropriate by PICO)
•
Short-term immunity
•
Persistent immunity
•
Interference with other recommended vaccines administered concurrently
•
Serious adverse events
•
Non-serious adverse events
6

How PICOs Translate into Schedule Options for Healthy
Adolescents – assuming MenB #1 at age 16 years
Legend
Q = MenACWY (quadrivalent)
B = MenB
P = MenABCWY (pentavalent)
Options
11–12 year
old dose
16 year old
dose #1
16 year old
dose #2
Standard of care (MenACWY only)
Q Q –
Standard of care (MenACWY + MenB)
Q Q+B B
PICO 1 (MenABCWY as option for MenACWY + MenB)
Q P B
PICO 2 (MenABCWY as option for MenACWY)
P P ±B
PICO 3 (MenABCWY as option for MenB)
Q P P
Combination of all 3 PICOs
P P P
7

Schedule options presented in June
Legend
Q = MenACWY (quadrivalent)
B = MenB
P = MenABCWY (pentavalent)
Options
11–
12 year
old dose
16 year old
dose #1
16 year old
dose #2
WG
Proposal
Standard of care (MenACWY only)
Q Q – N/A
Standard of care (MenACWY + MenB)
Q Q+B B N/A
PICO 1 (MenABCWY as option for MenACWY + MenB)
Q P B
PICO 2 (MenABCWY as option for MenACWY)
P P B
PICO 3 (MenABCWY as option for MenB)
Q P P
Combination of all 3 PICOs
P P P
8

Since June, the WG has refined the EtR and further
considered possible implications of each PICO
(especially PICO 3) based on
▪ ACIP members’ concerns raised during the June meeting
– Cost effectiveness
– Concerns about increasing exposure to B component related to reactogenicity, low
burden of disease, and limitations to protection
– Optimal timing of B component is often not age 16 years
– Fidelity to clinical trial data and licensure
– Stocking and administration considerations
▪ Cost effectiveness analysis
– Updates to quoted price of the pentavalent vaccine
– Refinements to the CDC model
9

Summary of updated EtR

PUBLIC HEALTH PROBLEM
Is meningococcal disease a problem of public health importance?
▪ Incidence of meningococcal
disease is low and decreasing
▪ Causes very severe disease
▪ Poor outcomes even with
treatment
– Case fatality 10–15%
– 10–20% of survivors have
permanent sequelae
11
WG interpretation
PICO 1
MenABCWY vs. MenACWY + MenB
PICO 2
MenABCWY vs. MenACWY
PICO 3
MenABCWY vs. Men B
Yes Yes Yes

BENEFITS & HARMS
▪ Three randomized control trials studied
– MenABCWY 2 doses (0, 6 months and 0, 12 months) vs. MenACWY-CRM 1 dose + MenB-FHbp 2 doses
(0, 6 months)
– Among ACWY-naïve and ACWY-primed participants
– Available data facilitated assessment of select outcomes through GRADE
• Short-term immunity
• Persistent immunity
• Serious adverse events
• Non-serious adverse events
▪ Other important benefits and harms were not assessed through GRADE but factored into WG
interpretations
– Increased reactogenicity of MenB relative to MenACWY
– Limitations to B protection
• Low VE expected following a single dose
• Rapidly waning protection following 2-dose series
• Multiple studies demonstrating MenB vaccination has no effect on meningococcal carriage
12

BENEFITS AND HARMS: Summary of GRADE
Type Outcome Importance
Design
(# studies)
Findings
Evidence type*
Healthy
Increased risk
Benefits
Meningococcal disease
caused by serogroups, A, B,
C, W, and Y
Critical n/a
No data available ND ND
Short-term immunity Critical
RCT (1)
Serogroup-specific seroresponses one month after
the first trial dose of ACWY- or B-
containing vaccine
occurred as often or more often in the pentavalent
group compared with the control group
Moderate Low
Persistent immunity Important
RCT (2)
Seroresponse rates by serogroup were similar:
- 48 months after 2 doses pentavalent vs. 54
months after 1 dose MenACWY-CRM
- 48 months after 2 doses pentavalent vs. 2 doses
MenB-FHbp
Low─
moderate
Low
Harms
Serious adverse events Critical
RCT (3)
Significantly more SAEs occurred in the pentavalent
group vs. comparison group; none were attributed
to the vaccine
Low Very low
Non-serious adverse
events
Important
RCT (3)
Significantly more non-serious adverse events
occurred in the pentavalent group vs. comparison
group
Low Very low
Interference with other
recommended vaccines
administered concurrently
Important
n/a No data available ND ND
13
*Downgrades primarily related to indirectness of intervention and comparison groups relative to PICOs, people at increased risk not being included, and wide confidence intervals for adverse events

BENEFITS & HARMS – Work Group interpretations
14
Question
PICO 1
MenABCWY vs. MenACWY
+
MenB
PICO 2
MenABCWY vs.
MenACWY
PICO 3
MenABCWY vs. Men B
How substantial are the
desirable anticipated
effects?
Small
Minimal, small, or
moderate
Minimal
How substantial are the
undesirable anticipated
effects
Small Minimal or small Minimal or small
Do the desirable effects
outweigh the
undesirable effects?
Favors intervention
Favors intervention,
comparison, or both
Favors intervention or
comparison
What is the overall
certainty?
Varies by group Varies by group Varies by group

15
VALUES
▪ Limited data were available
– Among adolescents during 2021, vaccination coverage of at least 1 dose
• 89% for MenACWY
• 31% for MenB
– Limited data are available on vaccine uptake in other individuals recommended to receive
MenACWY or MenB vaccine
▪ Use of combination vaccines can reduce number of injections and is generally preferred over
separate injections of the equivalent component vaccines
1,2
13
1 General Best Practice Guidelines for Immunization. Best Practice Guidance of the ACIP. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf
2 American Academy of Pediatrics. Red Book 2018. Report of the Committee on Infectious Diseases. 31
st
Ed. https://seciss.facmed.unam.mx/wp-content/uploads/2021/02/Red-Book-31th-Edition.pdf
15
Question
PICO 1
MenABCWY vs. MenACWY + MenB
PICO 2
MenABCWY vs. MenACWY
PICO 3
MenABCWY vs. Men B
Does the target population feel
that desirable effects are large
relative to undesirable effects?
Probably yes Probably yes
Probably yes or don’t
know
Important uncertainty or
variability in how much people
value the main outcomes?
Probably no Probably yes Probably yes

16
ACCEPTABILITY
Is the intervention acceptable to key stakeholders?
▪ Limited data were available
▪ Acceptability likely depends on PICO and balance of stakeholder values
– Health care providers likely supportive of options that allow stocking fewer vaccines
1,2
– Potential to increase vaccination rates against serogroup B disease
– Reduces number of injections from 4 to 3 for some patients
– Potential to incentivize MenB administration at age 16 years with waning immunity by peak risk for some
patients
• Many vaccine providers prefer waiting until closer to exposure to congregate settings (college/military)
– Concerns about increasing exposure to MenB (which is more reactogenic than MenACWY) when burden of
MenB disease is already low despite low vaccine coverage
• 31% single dose
• <12% second dose
1
CDC. Timing and Spacing of Immunobiologics: General Best Practice Guidelines for Immunization. ACIP Timing and Spacing Guidelines for Immunization | CDC.
2
Hall E, Odafe S, Madden J, Schillie S. Qualitative Conceptual Content Analysis of COVID-19 Vaccine Administration Error Inquiries. Vaccines. 2023; 11(2):254.
16
WG interpretation
PICO 1
MenABCWY vs. MenACWY + MenB
PICO 2
MenABCWY vs. MenACWY
PICO 3
MenABCWY vs. Men B
Probably yes or yes Probably yes or yes Don’t know

RESOURCE USE
Is the intervention a reasonable and efficient allocation of resources?
▪ All proposed meningococcal
vaccine strategies are
expensive, including currently
recommended options for
adolescents (QQ and QQBB)
▪ With new price estimates, QPP
is the most cost-effective
option when MenB protection
is desired
17
WG interpretation
PICO 1
MenABCWY vs. MenACWY + MenB
PICO 2
MenABCWY vs. MenACWY
PICO 3
MenABCWY vs. Men B
Probably yes or yes Probably no or no Probably yes or yes
Strategy
Cost/person
Public sector
QQ
241.2
QQBB
554.88
QPB
479.94
QPP
465.6
QQPP
586.2
Private sector
QQ
372.0
QQBB
854.64
QPB
707.32
QPP
666.0
QQPP
852.0

EQUITY
What would be the impact on health equity?
▪ Limited data were available
▪ The pentavalent vaccine is not expected to negatively impact equity
▪ It could potentially reduce disparities among those who might be interested in being
vaccinated against serogroup B but who might not receive clinical care that includes
discussion of the MenB vaccine
▪ Possible risk of clinics not stocking monovalent B vaccines with some policy options, which
could affect availability for
– Outbreaks
– People at increased risk recommended to receive 3 doses of MenB-FHbp
18
WG interpretation
PICO 1
MenABCWY vs. MenACWY + MenB
PICO 2
MenABCWY vs. MenACWY
PICO 3
MenABCWY vs. Men B
Probably no impact or varies
Probably increased, varies, or
don’t know
Don’t know

FEASIBILITY
Is the intervention feasible to implement?
▪ Challenges with insurance coverage specific to the pentavalent vaccine not expected
▪ Substantial financial burdens for providers or health systems not expected
▪ Pentavalent vaccine would provide additional option in current schedule and may reduce
number of doses for some people
▪ Administration requires reconstitution, which may lead to administration errors
1
▪ Stocking three different meningococcal vaccine types may be prohibitive for some
providers
▪ Lack of B vaccines interchangeability complicates stocking considerations
19
WG
interpretation
PICO 1
MenABCWY vs. MenACWY + MenB
PICO 2
MenABCWY vs. MenACWY
PICO 3
MenABCWY vs. Men B
Probably yes or yes Probably yes or yes Probably yes or yes
1
https://www.cdc.gov/mmwr/volumes/65/wr/mm6506a4.htm
20
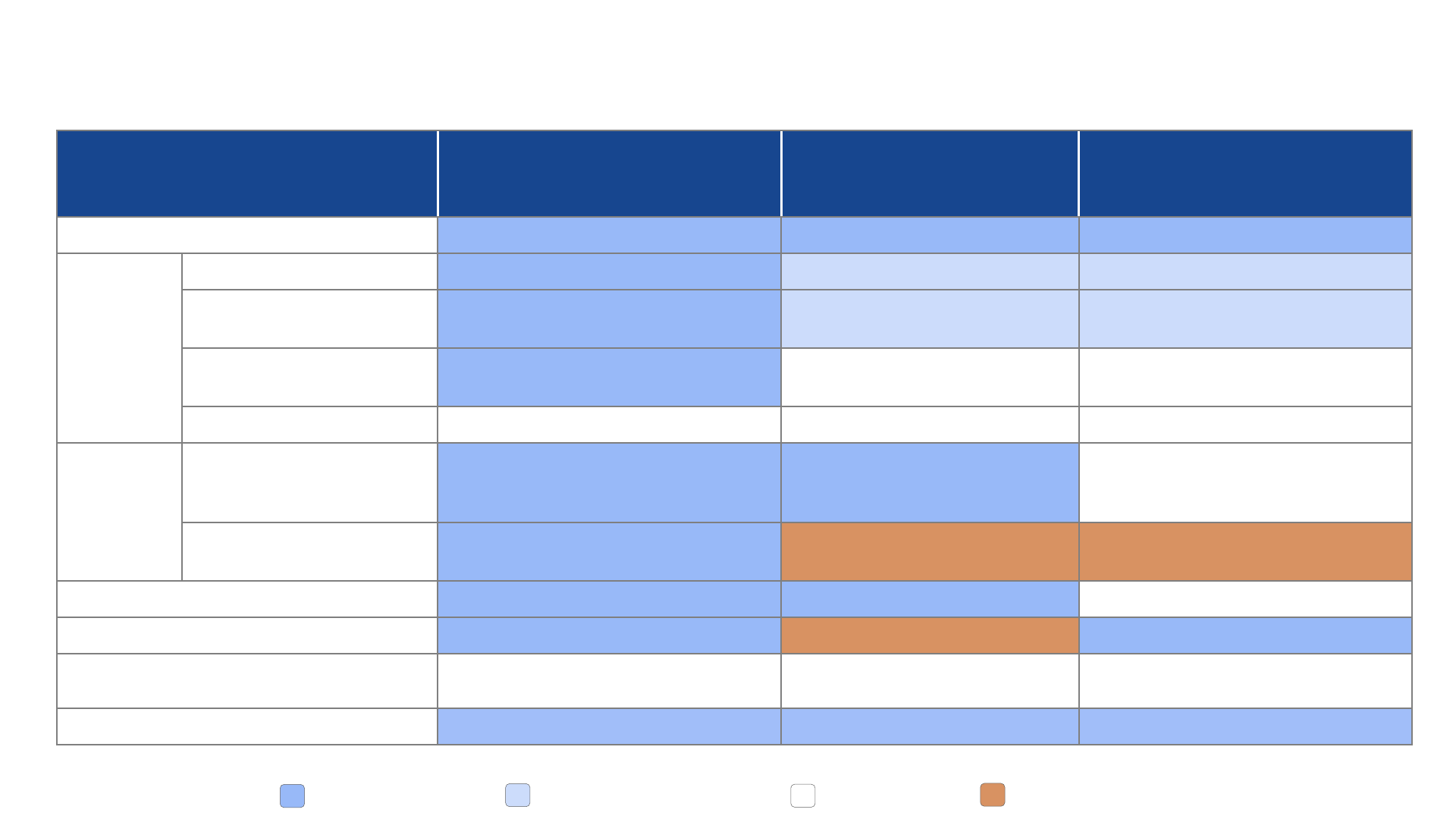
Domain
PICO 1
MenABCWY vs. MenACWY + MenB
PICO 2
MenABCWY vs. MenACWY
PICO 3
MenABCWY vs. Men B
Public health problem Yes Yes Yes
Benefits
&
harms
Desirable anticipated effects Small Minimal, small, or moderate Minimal
Undesirable anticipated
effects
Small Minimal or small Minimal or small
Desirable effects > undesirable
effects?
Favors intervention
Favors intervention, comparison, or
both
Favors intervention or comparison
Overall certainty Varies by group Varies by group Varies by group
Values
Are desirable effects large
relative to undesirable
effects?
Probably yes Probably yes Probably yes or don’t know
Important uncertainty or
variability?
Probably no Probably yes Probably yes
Acceptability Probably yes or yes Probably yes or yes Don’t know
Resource use Probably yes or yes Probably no or no Probably yes or yes
Equity Probably no impact or varies
Probably increased, varies, or don’t
know
Don’t know
Feasibility Probably yes or yes Probably yes or yes Probably yes or yes
EtR summary – all 3 PICOs
20
Favorable Somewhat favorable Uncertain Unfavorable

Summary of work group consensus and
debate
21
▪ Strong consensus in favor of PICO 1: MenABCWY as an option for MenACWY + MenB (QPB)
▪ Strong consensus against PICO 2: MenABCWY as an option for MenACWY only (PPB)
▪ Limited consensus regarding PICO 3: MenABCWY as an option for MenB only
▪ Options debated for PICO 3
Option
Preference
A
Reject outright
B
Accept with limitations (i.e., QPP only)
C
Accept fully (i.e., QPP, QQPP, QQPB)

Q
11–12 16 17 18 19 20 21 22 23
Age (years)
BB
Q
Yes
(age >16
years)
22
Existing recommendations for routine schedule
incorporating SCDM
QBB
Yes
(age 16 years)
Serogroup B
vaccine
desired based
on shared
clinical
decision-
making?
No
Q

Q
11–12 16 17 18 19 20 21 22 23
Age (years)
BB
Q
Yes
(age >16
years)
23
QBB
Yes
(age 16 years)
Serogroup B
vaccine
desired based
on shared
clinical
decision-
making?
No
Q
PB
B
P
Option A adds QPB to the existing options
Lack of data
Neither option is consistent
with licensure (i.e., 2-dose
MenABCWY series)

Q
11–12 16 17 18 19 20 21 22 23
Age (years)
BB
Q
Yes
(age >16
years)
24
QBB
Yes
(age 16 years)
Serogroup B
vaccine
desired based
on shared
clinical
decision-
making?
No
Q
PB
B
P
Option B adds QPP to Option A
PP

Q
11–12 16 17 18 19 20 21 22 23
Age (years)
BB
Q
Yes
(age >16
years)
25
QBB
Yes
(age 16 years)
Serogroup B
vaccine
desired based
on shared
clinical
decision-
making?
No
Q
PB
B
P
Option C adds QQPP and QQPB to option B
PP
Q
Q
PP
PB
• Higher cost
• Lack of data

26
Summary of routine schedule interpretation
for 3 options
26
All options would permit current standard of care (i.e., QQ vs. QQBB under SCDM)
Option*
Preference for PICO
3
Schedule options incorporating
SCDM for
MenB
A
Reject outright
QPB
B
Accept with limitations
QPB +
QPP
C
Accept fully
QPB + QPP +
QQPP + QQPB
*All options include a recommendation in favor of PICO 1 and against PICO 2

27
WG deliberations regarding 3 most favored options
CONSIDERATION
Option A
PICO 1 (QPB)
Option B
PICO 1 + PICO 3 (QPP only)
Option C
PICO 1 + PICO 3 (QPP, QQPP, QQPB)
CLINICAL
Alignment with clinical trial data
Not directly assessed; however,
second pentavalent dose is
primarily for additional B
protection
Directly assessed in clinical trial (6
-
or 12
- month interval between
pentavalent doses)
Options with additional antigenic
exposures for which safety and
immunogenicity have not been assessed
(QQPP, QQPB)
Alignment with licensure Off-label
Yes
Yes
Excess doses for ≥1 serogroup No
Yes (1 dose)
Yes (multiple doses)
STOCKING AND ADMINISTRATION
Flexibility (especially for under-
resourced clinics)
Least
Intermediate
Most
Minimum # vaccines to stock if using
MenABCWY for routine indications*
3
2
2
ECONOMIC
Projected cost effectiveness Unclear cost effectiveness
Most cost
-effective option based
on recent price update from Pfizer
Includes more expensive options not
assessed in CE model (e.g., QQPP)
Potential for insurance reimbursement
issues
Yes
No
No
Most favorable Somewhat favorable Least favorable
27
*All options would require stocking 3 vaccines for special situations if using MenABCWY. Minimum number of vaccines to stock will remain 2 (MenACWY, MenB) if not using MenABCWY.

28
WG deliberations regarding 3 most favored options
CONSIDERATION
Option A
PICO 1 (QPB)
Option B
PICO 1 + PICO 3 (QPP only)
Option C
PICO 1 + PICO 3 (QPP, QQPP, QQPB)
CLINICAL
Alignment with clinical trial data
Not directly assessed; however,
second pentavalent dose is
primarily for additional B
protection
Directly assessed in clinical trial (6
-
or 12
- month interval between
pentavalent doses)
Options with additional antigenic
exposures for which safety and
immunogenicity have not been assessed
(QQPP, QQPB)
Alignment with licensure Off-label
Yes
Yes
Excess doses for ≥1 serogroup No
Yes (1 dose)
Yes (multiple doses)
STOCKING AND ADMINISTRATION
Flexibility (especially for under-
resourced clinics)
Least
Intermediate
Most
Minimum # vaccines to stock if using
MenABCWY for routine indications*
3
2
2
ECONOMIC
Projected cost effectiveness Unclear cost effectiveness
Most cost
-effective option based
on recent price update from Pfizer
Includes more expensive options not
assessed in CE model (e.g., QQPP)
Potential for insurance reimbursement
issues
Yes
No
No
Most favorable Somewhat favorable Least favorable
28
*All options would require stocking 3 vaccines for special situations if using MenABCWY. Minimum number of vaccines to stock will remain 2 (MenACWY, MenB) if not using MenABCWY.

29
WG deliberations regarding 3 most favored options
CONSIDERATION
Option A
PICO 1 (QPB)
Option B
PICO 1 + PICO 3 (QPP only)
Option C
PICO 1 + PICO 3 (QPP, QQPP, QQPB)
CLINICAL
Alignment with clinical trial data
Not directly assessed; however,
second pentavalent dose is
primarily for additional B
protection
Directly assessed in clinical trial (6
-
or 12
- month interval between
pentavalent doses)
Options with additional antigenic
exposures for which safety and
immunogenicity have not been assessed
(QQPP, QQPB)
Alignment with licensure Off-label
Yes
Yes
Excess doses for ≥1 serogroup No
Yes (1 dose)
Yes (multiple doses)
STOCKING AND ADMINISTRATION
Flexibility (especially for under-
resourced clinics)
Least
Intermediate
Most
Minimum # vaccines to stock if using
MenABCWY for routine indications*
3
2
2
ECONOMIC
Projected cost effectiveness Unclear cost effectiveness
Most cost
-effective option based
on recent price update from Pfizer
Includes more expensive options not
assessed in CE model (e.g., QQPP)
Potential for insurance reimbursement
issues
Yes
No
No
Most favorable Somewhat favorable Least favorable
29
*All options would require stocking 3 vaccines for special situations if using MenABCWY. Minimum number of vaccines to stock will remain 2 (MenACWY, MenB) if not using MenABCWY.

30
WG deliberations regarding 3 most favored options
CONSIDERATION
Option A
PICO 1 (QPB)
Option B
PICO 1 + PICO 3 (QPP only)
Option C
PICO 1 + PICO 3 (QPP, QQPP, QQPB)
CLINICAL
Alignment with clinical trial data
Not directly assessed; however,
second pentavalent dose is
primarily for additional B
protection
Directly assessed in clinical trial (6
-
or 12
- month interval between
pentavalent doses)
Options with additional antigenic
exposures for which safety and
immunogenicity have not been assessed
(QQPP, QQPB)
Alignment with licensure Off-label
Yes
Yes
Excess doses for ≥1 serogroup No
Yes (1 dose)
Yes (multiple doses)
STOCKING AND ADMINISTRATION
Flexibility (especially for under-
resourced clinics)
Least
Intermediate
Most
Minimum # vaccines to stock if using
MenABCWY for routine indications*
3
2
2
ECONOMIC
Projected cost effectiveness Unclear cost effectiveness
Most cost
-effective option based
on recent price update from Pfizer
Includes more expensive options not
assessed in CE model (e.g., QQPP)
Potential for insurance reimbursement
issues
Yes
No
No
Most favorable Somewhat favorable Least favorable
30
*All options would require stocking 3 vaccines for special situations if using MenABCWY. Minimum number of vaccines to stock will remain 2 (MenACWY, MenB) if not using MenABCWY.

31
Balance of Consequences — PICO 1
MenABCWY as an option for MenACWY+MenB
Undesirable
consequences
clearly outweigh
desirable
consequences in
most settings
Undesirable
consequences
probably outweigh
desirable
consequences in
most settings
The balance
between
desirable and
undesirable
consequences is
closely balanced
or uncertain
Desirable
consequences
probably outweigh
undesirable
consequences in
most settings
Desirable
consequences
clearly outweigh
undesirable
consequences in
most settings
There is
insufficient
evidence to
determine the
balance of
consequences
31
Majority of WG members think desirable consequences probably
or clearly outweigh undesirable consequences in most settings
Most common 2
nd
most common 3
rd
most common

32
Most common 2
nd
most common
Work Group Interpretation — PICO 1
We do not recommend the intervention, but it may be used within FDA licensed indications
We recommend the intervention for individuals based on shared clinical decision
-making
We recommend the intervention
Should the pentavalent vaccine be included as an option for
MenACWY/MenB vaccination in people currently recommended to
receive both vaccines?
32
Majority of WG members favored recommending the intervention

33
Balance of Consequences — PICO 2
MenABCWY as an option for MenACWY
Undesirable
consequences
clearly outweigh
desirable
consequences in
most settings
Undesirable
consequences
probably outweigh
desirable
consequences in
most settings
The balance
between
desirable and
undesirable
consequences is
closely balanced
or uncertain
Desirable
consequences
probably outweigh
undesirable
consequences in
most settings
Desirable
consequences
clearly outweigh
undesirable
consequences in
most settings
There is
insufficient
evidence to
determine the
balance of
consequences
33
Most common 2
nd
most common 3
rd
most common
Majority of WG members think undesirable consequences probably or
clearly outweigh desirable consequences in most settings

34
Work Group Interpretation — PICO 2
We do not recommend the intervention, but it may be used within FDA licensed indications
We recommend the intervention for individuals based on shared clinical decision
-making
We recommend the intervention
Should the pentavalent vaccine be included as an option for
people currently recommended to receive MenACWY only?
34
Most common 2
nd
most common
Majority of WG members favored not recommending the intervention

35
Most common 2
nd
most common
Balance of Consequences — PICO 3
MenABCWY as an option for MenB
Undesirable
consequences
clearly outweigh
desirable
consequences in
most settings
Undesirable
consequences
probably outweigh
desirable
consequences in
most settings
The balance
between
desirable and
undesirable
consequences is
closely balanced
or uncertain
Desirable
consequences
probably outweigh
undesirable
consequences in
most settings
Desirable
consequences
clearly outweigh
undesirable
consequences in
most settings
There is
insufficient
evidence to
determine the
balance of
consequences
35
The WG did not reach a majority consensus on the balance of
consequences

36
Work Group Interpretation — PICO 3
We do not recommend the intervention, but it may be used within FDA licensed indications
We recommend the intervention for individuals based on shared clinical decision
-making
We recommend the intervention but only in certain circumstances (i.e., QPP)
We recommend the intervention in all circumstances
Should the pentavalent vaccine be included as an option for
people currently recommended to receive MenB only?
36
Added an additional option because some WG members favored QPP only

37
Work Group Interpretation — PICO 3
We do not recommend the intervention, but it may be used within FDA licensed indications
We recommend the intervention for individuals based on shared clinical decision
-making
We recommend the intervention but only in certain circumstances (i.e., QPP)
We recommend the intervention in all circumstances
Should the pentavalent vaccine be included as an option for
people currently recommended to receive MenB only?
▪ WG was divided regarding PICO 3
▪ Majority favored PICO 3 in some form
▪ Substantial minority of work group members favored not recommending the intervention
37
Most common 2
nd
most common 3
rd
most common 4
th
most common

38
38
PICO 1 (QPB)
PICO 2 (PPB)
PICO 3 (QPP only)
Pfizer’s MenABCWY vaccine may be used when both MenACWY and MenB are indicated at the
same visit.* If MenABCWY is administered in this way, a second dose of MenABCWY may be
administered 6 months later to complete the series.
*1) Healthy individuals aged 16–23 years (routine schedule) when shared clinical decision-
making favors administration of MenB vaccination, 2) individuals aged 10 years and older at
increased risk of meningococcal disease (e.g., due to persistent complement deficiencies,
complement inhibitor use, or functional or anatomic asplenia) due for both vaccines.
▪ Remarks:
• for Pfizer’s MenABCWY vaccine, data are not available regarding safety or immunogenicity of dosing intervals exceeding
12 months
• the licensed B component vaccines are not interchangeable by manufacturer. Administration of a B component vaccine
(MenB or MenABCWY) requires that subsequent B component vaccine doses be from the same manufacturer
• the minimum interval for Pfizer’s MenABCWY vaccine is 6 months. Individuals at increased risk of meningococcal disease
who are recommended to receive additional doses of MenACWY and MenB less than 6 months after a dose of
pentavalent meningococcal vaccine should instead receive separate MenACWY and MenB-FHbp vaccines
Combined draft proposal for option B

39
Rationale in favor of combined draft proposal
▪ Aligns with clinical trial data and licensure
▪ Allows for fewer injections than QQBB
▪ Provides flexibility with vaccine inventory, including for clinics that prefer to stock
2 vaccines for routine indications
▪ Stocking fewer vaccines may increase equity (e.g., if under-resourced clinics are
less likely to stock 3 vaccines)
▪ Most cost-effective option based on recent price update from Pfizer
39

40
Rationale against combined draft proposal
▪ Unnecessary ACWY antigen exposure for second pentavalent dose in routine schedule
(i.e., when only MenB is indicated)
▪ Not as much flexibility for providers as Option 3
General considerations (all options):
▪ Potential to incentivize MenB at age 16 years with waning immunity by peak risk (i.e.,
college/military) for some patients
▪ Uncertainty regarding cost estimates
▪ If using MenABCWY, it will be necessary to stock 3 vaccines to cover all indications
(routine schedule + special situations), which may be challenging for some vaccine
providers
40

Acknowledgments
▪ ACIP Members on the WG
– Kathy Poehling (Chair)
– Lynn Bahta
– Jamie Loehr
▪ Ex Officio WG Members
– Margaret Bash (FDA)
– Mark Connelly (FDA)
– Francisco Leyva (NIH)
▪ WG Liaisons and Consultants
– Amra Resic (AAFP)
– Samir Shah (AAP)
– Sharon McMullen (ACHA)
– Cacky Tate / Karyn Lyons (AIM)
– Paul Cieslak (CSTE)
– Kathy Hsu (IDSA)
– Joseline Zafack (NACI)
– Jeff Goad (NFID)
– Jessica Cataldi (PIDS)
– Amy Middleman (SAHM)
– David Stephens (Emory)
▪ CDC Contributors
– Sam Crowe (DBD/NCIRD)
– Lucy McNamara (DBD/NCIRD)
– Ismael Ortega-Sanchez (DVD/NCIRD)
– Andrew Leidner (ISD/NCIRD)
– LeAnne Fox (DBD/NCIRD)
– Susan Hariri (DBD/NCIRD)
– Amy Rubis (DBD/NCIRD)
– Noele Nelson (DBD/NCIRD)
– Alison Albert (DBD/NCIRD)
– Angela Jiles (DBD/NCIRD)
– Jonathan Duffy (DHQP/NCEZID)
– Tanya Myers (DHQP/NCEZID)
– Liz Velazquez (ISD/NCIRD)
– Jessica MacNeil (ACIP Secretariat)
– Melinda Wharton (ACIP Secretariat)
▪ GRADE/EtR Support
– Doug Campos-Outcalt (Arizona)
– Rebecca Morgan (Case Western Reserve)
41

Thank you!
Questions?
