
gsk.com
31 January 2024
Full-year and Q4 2023 results
Conference call and webcast for investors and analysts

2
This presentation may contain forward-looking statements. Forward-looking statements give the Group’s current expectations or forecasts of future events. An investor can identify these
statements by the fact that they do not relate strictly to historical or current facts. They use words such as ‘anticipate’, ‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’, ‘target’ and
other words and terms of similar meaning in connection with any discussion of future operating or financial performance. In particular, these include statements relating to future actions,
prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the outcome of contingencies such as legal
proceedings, dividend payments and financial results.
Other than in accordance with its legal or regulatory obligations (including under the Market Abuse Regulations, UK Listing Rules and the Disclosure Guidance and Transparency Rules of
the Financial Conduct Authority), the Group undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
Investors should, however, consult any additional disclosures that the Group may make in any documents which it publishes and/or files with the US Securities and Exchange Commission
(SEC). All investors, wherever located, should take note of these disclosures. Accordingly, no assurance can be given that any particular expectation will be met and investors are
cautioned not to place undue reliance on the forward-looking statements.
Forward-looking statements are subject to assumptions, inherent risks and uncertainties, many of which relate to factors that are beyond the Group’s control or precise estimate. The
Group cautions investors that a number of important factors, including those in this presentation, could cause actual results to differ materially from those expressed or implied in any
forward-looking statement. Such factors include, but are not limited to, those discussed under Item 3.D ‘Risk factors’ in the Group’s Annual Report on Form 20-F for the full year (FY) 2022.
Any forward-looking statements made by or on behalf of the Group speak only as of the date they are made and are based upon the knowledge and information available to the
Directors on the date of this presentation.
A number of adjusted measures are used to report the performance of our business, which are non-IFRS measures. These measures are defined and reconciliations to the nearest IFRS
measure are available in the Q4 2023 earnings release and Annual Report on Form 20-F for FY 2022.
All guidance, outlooks and expectations regarding future performance and the dividend should be read together with the section “Guidance and outlooks, assumptions and cautionary
statements” on pages 54 to 55 of GSK’s full year and Q4 2023 stock exchange announcement and “Guidance and outlooks, assumptions and basis of preparation related to 2024
guidance, 2021-26 and 2031 outlooks" in the appendix of this presentation.
Basis of preparation: On 18 July 2022, GSK plc separated its Consumer Healthcare business from the GSK Group to form Haleon, an independent listed company. Comparative figures
have been restated on a consistent basis. Earnings per share, Adjusted earnings per share and Dividends per share have been adjusted to reflect the GSK Share Consolidation on 18 July
2022.
Cautionary statement regarding forward-looking statements

3
Agenda
Delivering commitments and upgrading outlooks
Emma Walmsley
2023 performance and guidance for 2024
Luke Miels, Deborah Waterhouse and Julie Brown
Outlooks for 2026
-2031
Emma Walmsley and Julie Brown
Q&A
Emma Walmsley, Tony Wood, Luke Miels, Deborah Waterhouse,
Julie Brown, and David Redfern

4
Delivering commitments and upgrading outlooks
Emma Walmsley, Chief Executive Officer

5
Sales growth
1
•
2021: 1%
•
2022: 10%
•
2023: 14%
Launches since 2021
1
• Arexvy
• Apretude
• Cabenuva
• Jemperli
• Ojjaara
Vaccines and Specialty
1
•
2021: 58% of sales
•
2022: 62% of sales
•
2023: 66% of sales
Significant delivery and improved performance since 2021
commitments
Business Development
7
Adj. operating margin
1
•
2021: 25.6%
•
2022: 28.5%
•
2023: 28.6%
Acquisitions:
Affinivax
Aiolos Bio
8
BELLUS Health
Sierra Oncology
Alliances:
Alector
Arrowhead
Halozyme
Hansoh Pharma
Ideya
Janssen Pharma
Mersana
Scynexis
Spero Therapeutics
Springworks
Vir Biotechnology
Wuxi Biologics
2023 Sales
£2.3bn
>16
Acquisitions
& Alliances
R&D late-stage pipeline
2021 Investor Update
Arexvy
MenABCWY
gepotidacin
Apretude
depemokimab
Jemperli
−
Zejula
−
Blenrep
−
Jesduvroq
x otilimab
Progressed/acquired since 2021
MAPS
2
24v/30+v
mRNA
3
influenza
TH HSV
4
bepivirosen
Brexafemme
tebipenem
HIV LA
5
camlipixant
Jemperli LCI
6
CD226
Absolute values at AER; changes at CER, unless stated otherwise
1. All values excl. COVID-19 solutions and on a continuing basis 2. Multiple Antigen Presenting System 3. Messenger RiboNucleic Acid 4. Therapeutic herpes simplex virus 5. Long acting 6. Lifecycle
innovation 7. Select publicly announced acquisitions or alliances that GSK has entered into since June 2021 8. Subject to customary conditions, including applicable regulatory agency clearances under the Hart-
Scott-Rodino Act in the US

6
Strong 2023
performance
Sales
£30.3bn, +5%
+14%
1
Delivered 14%
1
sales growth,
16%
1
adj. operating profit growth
Profitable growth across portfolio:
• Vaccines 24%
1
• Specialty Medicines 15%
1
• General Medicines 5%
New products launched since 2017
2
delivered £ 11 billion sales
Trust/ESG progress sustained:
• Sector leadership recognised by S&P
3
• Low-carbon Ventolin inhaler
programme advances
• Leadership diversity ambitions
achieved
4
Adj. operating profit
£8.8bn, +12%
+16%
1
Adj. EPS
155.1p, +16%
+22%
1
Dividend per share
58p
Highlights
Absolute values at AER; changes at CER, unless stated otherwise 1. Excluding COVID-19 solutions 2. Products include: Zejula, Trelegy, Shingrix,
Juluca, Dovato, Duvroq, Rukobia, Blenrep, Cabenuva, Jemperli, Apretude, Arexvy, Ojjaara 3. S&P Global, The Sustainability Yearbook - 2023
Rankings 4. Ambition to increase female representation at VP level and above roles to at least 45% by 2025 and target at least 30% and 18%
ethnically diverse leaders by the end of 2025 is the US and UK, respectively

7
Delivering on our commitments and upgrading our outlooks
2024 Guidance
•
Sales growth: 5-7%
•
Adj. OP growth: 7-10%
•
Adj. EPS growth: 6-9%
2021-2026 New Outlook
• >7% Sales CAGR
1
• >11% Adj. operating profit CAGR
1
• >31% Adj. operating profit
margin
• >£10bn CGFO
2
2026-2031 New Outlook
• >£38bn sales by 2031
• Continued focus on margin
improvement, with broadly
stable OP
4
margin through
dolutegravir loss of exclusivity
5
2031 Previous Ambition
3
• >£33bn sales
2021-2026 Previous Outlook
3
• >5% Sales CAGR
• >10% Adj. operating profit CAGR
• 30% Adj. operating profit margin
• >10bn CGFO
All guidance, outlooks and expectations regarding future performance should be read together with the section “Guidance and outlooks, assumptions and cautionary statements” on pages 54 to 55 of GSK’s full-year
and Q4 2023 stock-exchange announcement. 2024 guidance growth at CER, unless stated otherwise. All outlook statements are given on a CER basis and use 2023 average exchange rates as a base. All values
excluding COVID-19 solutions 1. Compound annual growth rate 2. Cash flow generated from operations 3. Investor Update June 2021 4. Adj. operating profit excl. COVID-19 solutions.
5. Loss of exclusivity in the US and EU is expected in 2028-2030

8
2023 performance and guidance for 2024
Luke Miels, Chief Commercial Officer
Deborah Waterhouse, CEO, ViiV Healthcare and President, Global Health

9
Strong growth in 2023 for all product areas and regions
Absolute values at AER; changes at CER, unless stated otherwise
1. Excluding COVID-19 solutions
0
5,000
10,000
15,000
20,000
25,000
30,000
35,000
FY 2022 FY 2023
Sales (£m)
Sales contribution by product area
1
0
5,000
10,000
15,000
20,000
25,000
30,000
35,000
FY 2022 FY 2023
Sales (£m)
Sales contributions by region
1
+16%
+8%
+15%
+24%
+15%
+5%
+14% +14%
+10% +10%
Vaccines Specialty Medicines General Medicines
US Europe International

10
Vaccines: +24%
1
with outstanding
Arexvy
launch
Absolute values at AER; changes at CER for full year, unless stated otherwise
1. Excluding COVID-19 solutions 2. Messenger ribonucleic acid 3. Multiple Antigen Presenting System 4. FY 2023 global sales 5. United States Census Bureau, International Database, Year 2023
6. 9 October 2023: GSK and Zhifei announce exclusive strategic vaccine partnership in China 7. Centers for Disease Control and Prevention
0
2,000
4,000
6,000
8,000
10,000
12,000
FY 2022 FY 2023
Sales (£m)
Sales contribution by disease area
1
Further progress expected for mRNA
2
, MAPS
3
, HSV, MenABCWY and Arexvy in 2024
+17%
+7%
+14%
-29%
+24%
+17%
n/a
RSV Shingles Meningitis Influenza Established vaccines
RSV (
Arexvy
) £1,238m
4
• 11% of 60+ population in US vaccinated against RSV; 68% vaccinated
with Arexvy
Shingles (
Shingrix
) +17%
• 35% of >120m US adults
5
recommended to receive Shingrix now
vaccinated
• In 40 countries with <4% penetration in majority of markets
• Partnership with Zhifei
6
in China progressing well
• Confident in delivering >£4bn in peak year sales
Meningitis +14%
•
Bexsero
+14% driven by strong growth in Europe and International
•
Menveo
+12% driven by US CDC
7
replenishment and Brazil performance
Influenza (
Fluarix/FluLaval
) -29%
• Performance in line with expectations of decreased demand and
commoditised market
Established vaccines +7%
2024: expect increase high-single digit to low-double digit %
1
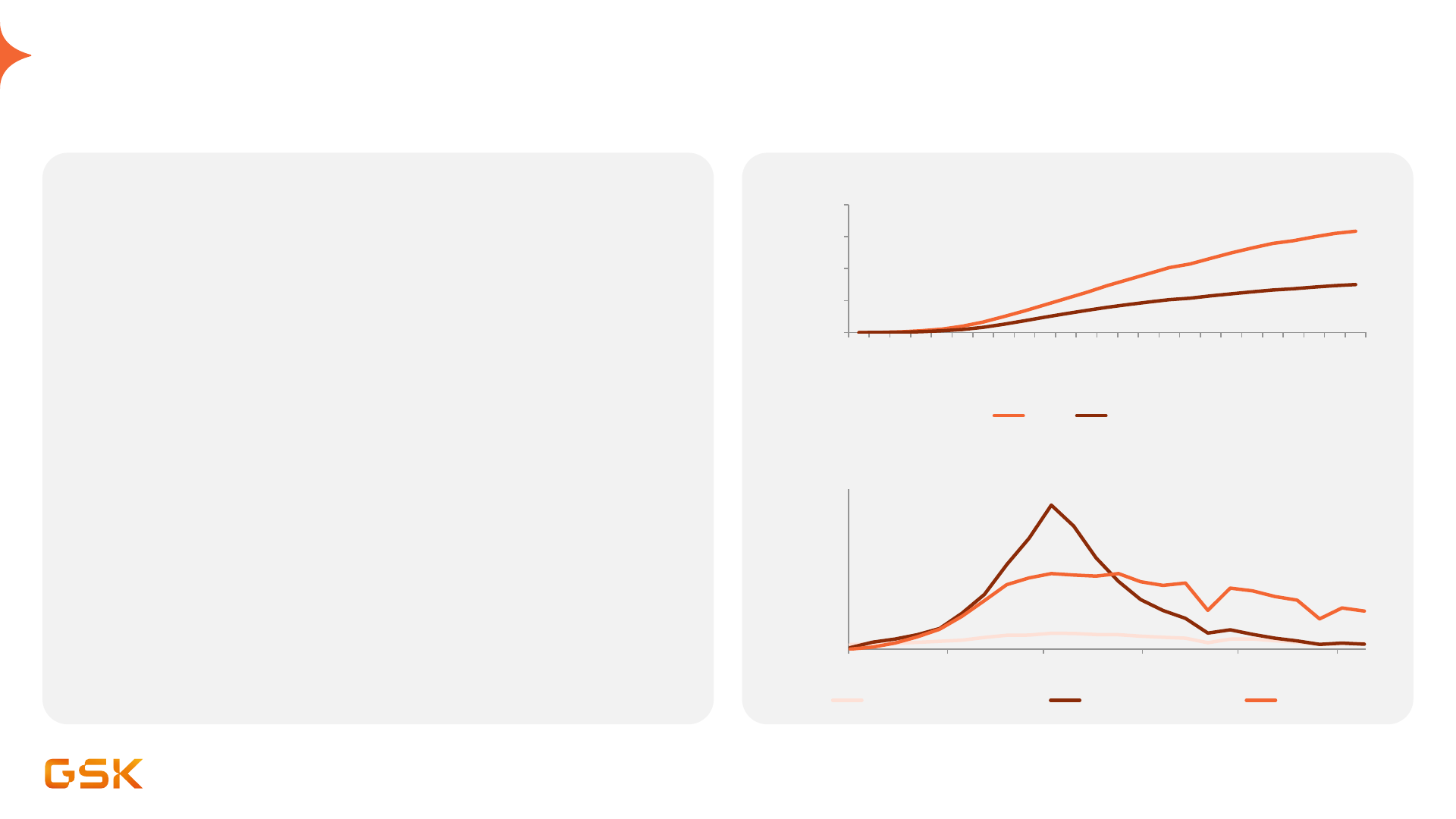
11
Arexvy
launch dynamics
Success in 2023, continued growth in 2024
1. GSK respiratory syncytial virus healthcare professional awareness trial and usage study, December 2023 2. 2023 US Census population data 3. Note: This information is an estimate derived from the use of
information under license from the following IQVIA information service: IQVIA – NPA for the period August 2023 – January 2024. IQVIA expressly reserves all rights, including rights of copying, distribution and
republication
US sets up success for global expansion
• 94.6% efficacy in comorbid population resonating well
• ~2/3 of healthcare professionals prefer Arexvy
1
• Strong position in all major pharmacies
Approved in 39 countries in 2023
• 1
st
entrant in US, Canada, EU and Japan
• Expect additional market approvals and reimbursements in 2024
Continued evidence generation
• 3
rd
year efficacy data in H1 2024
• Expand the market for at risk individuals aged 50 to 59
• 15m people 50-59 with high-risk comorbidities for more severe
RSV infections in the US
2
0.0
2.0
4.0
6.0
8.0
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
Total prescriptions (millions)
Number of weeks
Weekly TRx since launch
3
Arexvy Competitor
0.0
0.4
0.8
1.2
Aug/2023 Sep/2023 Oct/2023 Nov/2023 Dec/2023 Jan/2024
Total prescription (millions)
Vaccine seasonality by weekly US TRx
3
Pneumococcal vaccine brand HD QIV flu vaccine brand RSV Total

12
Specialty: +15%
1
with double-digit growth in all product areas
Absolute values at AER; changes at CER for full year, unless stated otherwise
1. Excluding COVID-19 solutions 2. Chronic obstructive pulmonary disease 3. Severe eosinophilic asthma 4. national reimbursement drug list 5. Eosinophilic granulomatosis with polyangiitis
6. Kidney Disease Improving Global Outcomes 7. Lupus nephritis 8. FY 2023 global sales 9. First line 10. 11 December 2023: Jemperli plus chemo approved as first and only frontline IO
treatment in EU for dMMR/MSI-H primary advanced or recurrent endometrial cancer
0
2,000
4,000
6,000
8,000
10,000
12,000
FY 2022 FY 2023
Sales (£m)
Sales contribution by disease area
1
+13%
+18%
+15%
+15%
+23%
Depemokimab and Nucala COPD
2
data in 2024
HIV Respiratory/Immunology Oncology
HIV +13%
• 2023 growth driven by oral two-drug regimen and long-acting portfolio
Respiratory/Immunology +18%
•
Nucala
+18%: strong growth in all geographies and indications driven
by new patient growth and US performance. China SEA
3
approval,
launching in private markets; NRDL
4
submission planned for
H2 2024 based on experience with EGPA
5
•
Benlysta
+19%: growth in major markets and continued market
expansion; updated KDIGO
6
guidelines in LN
7
now recommend use at
initial therapy driving further penetration with only ~25% use in the class
Oncology +23%
•
Ojjaara
£33m
8
: strong launch building momentum, first and only
treatment indicated for myelofibrosis patients with anaemia in US
•
Jemperli
£141m
8
: continued momentum and growth in 1L
9
endometrial
cancer in the US; EU 1L endometrial cancer approval in Dec 2023
10
•
Zejula
+15%: driven by US tablet introduction, global expansion and
new patient starts
•
Blenrep
-69%: positive phase III interim analysis of DREAMM-7 head-to-
head with daratumumab, DREAMM-8 phase III data expected H2 2024
2024: expect increase low-double digit %
1

13
General Medicines: +5% driven by
Trelegy
in respiratory
Absolute values at AER; changes at CER for full year, unless stated otherwise
IQVIA MIDAS® monthly data, since launch up to (and including) August 2023, reflecting estimates of real-world activity
1. Chronic obstructive pulmonary disease 2. GSK Annual Reports 2020-23; FactSet major competitor sales in GBP 3. Single inhaler triple therapy 4. Inhaled corticosteroid 5. Long-acting beta-agonists
6. Global Initiative for Chronic Obstructive Lung Disease 7. Average Manufacturer Price
0
2,000
4,000
6,000
8,000
10,000
12,000
FY 2022 FY 2023
Sales (£m)
Sales contribution by disease area
+6%
+2%
+5%
+1%
Respiratory Other General Medicines
Respiratory +6%
•
Trelegy
+29%: Top-selling brand in asthma and COPD
1
market
worldwide; delivering >£2bn in 2023
• Benefit of Arexvy co-promotion and prevention/treatment approach
leading to longer sales calls
• SITT
3
Class displacing ICS
4
/LABA
5
with GOLD
6
guidelines and early
optimisation message accelerating growth
Other General Medicines +2%
•
Augmentin
+17%
• Emerging Markets +15%
• Up to $700m sales exposure related to US AMP
7
Cap removal; Q4 2023
accrued $150m related to rebates and inventory burn
2024: expect decrease mid-single digit %
0
500
1,000
1,500
2,000
2,500
2020 2021 2022 MAT SEPT 23 MAT DEC 23
Sales (£m)
2
Trelegy Seretide/Advair Major competitor

14
HIV: 13% growth in 2023 driven by oral two-drug regimen (2DR) and
long-acting (LA) portfolio
Strong execution across portfolio
Growth driven by oral 2DR and LA
injectables
• FY 2023 sales of £6.4bn driven by oral 2DR and LA
injectable regimens
• Oral 2DR and LA regimens >50% of total HIV portfolio
• Dovato sales of £1.8bn - leading oral 2DR
• Cabenuva sales of £708m - growing >100% vs 2022
• Apretude sales of £149m - potential to transform PrEP
1
• Pipeline: Data to be presented at CROI
2
in March
Target dosing intervals for LA regimens extended to
every four months in treatment and prevention with
roadmap to reach every six months by end of decade
1. Pre-Exposure Prophylaxis 2. Conference on Retroviruses and Opportunistic Infections
25%
26%
28%
33%
36%
39%
42%
46%
47%
51%
53%
55%
0%
10%
20%
30%
40%
50%
60%
0
200
400
600
800
1,000
1,200
Q1
2021
Q2
2021
Q3
2021
Q4
2021
Q1
2022
Q2
2022
Q3
2022
Q4
2022
Q1
2023
Q2
2023
Q3
2023
Q4
2023
HIV Innovation sales as a % of total HIV sales
Sales (£m)
Dovato Cabenuva Juluca Apretude Oral 2DR & LA

15
2023 performance and guidance for 2024
Julie Brown, Chief Financial Officer

16
Delivered a step-change in financial performance in 2023
2022 2023 AER CER Key commentary on CER basis
Adjusted results
£m £m % %
Sales
29,324 30,328 3 5
Sales grew +14% (excl. COVID
-19
solutions) with strong growth in
Vaccines (
Arexvy
and
Shingrix
) and HIV
Cost of sales (8,741) (7,716) (12) (11) Benefit from lower sales of lower margin Xevudy and favourable mix
Gross profit
20,583 22,612 10 12
Gross profit margin 70.2% 74.6% +440 bps +460 bps Improved +20 bps (excl. COVID-19 solutions)
SG&A (8,128) (9,029) 11 13 Increased investment for growth in Vaccines and HIV
Research and development (5,062) (5,750) 14 14 Increased investment in late-stage Vaccines and new product launches
Royalties 758 953 26 26 Benefit from Gardasil, Kesimpta and Biktarvy
Operating profit
8,151 8,786 8 12
Grew +16% (excl. COVID
-19 solutions)
Operating profit margin 27.8% 29.0% +120 bps +180 bps Improved + 60 bps (excl. COVID-19 solutions)
Earnings per share
139.7p 155.1 11 16
EPS grew +22% (excl. COVID
-19 solutions)
2022 2023 AER CER
Total results
£m £m % %
Total operating profit 6,433 6,745 5 10
Total operating profit margin 21.9% 22.2% +30 bps +100bps
Total earnings per share 110.8p 121.6p 10 16
Adjusted results for continuing operations unless stated otherwise; some figures may not sum due to rounding. See page 20 of GSK’s full-year and Q4 2023 stock-exchange announcement for a full reconciliation of
Total to Adjusted results

Improved 2023 adjusted operating margin
0.2%
0.2%
0.3%
0.6%
COGS SG&A
0.0%
R&D Royalties FY 23
Margin
at 22 FX
Currency FY 23
margin
at 23 FX
28.5%
29.1%
28.6%
FY 22
Margin
+60bps
Including COVID-19 solutions +180 bps CER
Excluding COVID-19 solutions +60 bps CER
4.6%
1.9%
1.4%
0.5%
0.6%
FY 22 margin COGS SG&A R&D Royalties FY 23
margin at
22 FX
Currency FY 23
margin at
23 FX
27.8%
29.6%
29.0%
+180bps
Margin benefit driven by decline in lower margin
Xevudy and increased royalties
Margin benefits driven by improved productivity
and increased royalties
1
1
Note: Chart may not sum due to rounding
1. The dilutive effect of Covid-19 solutions reduced from 2022 to 2023 due to decreasing Covid-19 sales and a favorable product mix towards higher margin pandemic sales

18
2023 free cash flow of £3.4bn
Cash generated from operations of £8.1bn
£m £m
2022 2023
Adj. operating profit 8,151 8,786
Decrease/(Increase) in working
capital
67 (1,233)
Gilead Science, Inc. settlement
income
927 -
Other CGFO
1
(1,201) 543
Cash generated from operations
7,944 8,096
Taxation paid (1,310) (1,328)
Net capex
2
(1,916) (2,304)
Other
3
(1,370) (1,055)
Free cash flow
3,348 3,409
Net debt
17,197 15,040
Key drivers of cash flow
Full year
Cash generated from operations increased despite annualising the
Gilead Science, inc settlement in 2022 (£0.9bn) due to:
• Higher Adjusted Operating profit
• Favourable timing of Xevudy cashflows
• Lower UK special pension contributions
• Partly offset by higher receivables from Arexvy sales
Net capital investment increased primarily due to lower proceeds from
asset disposals than in 2022
Q4 2023
Extremely strong quarter, delivering £3.7bn cash from operations versus
£2.1bn in 2022, including benefit from Arexvy collections from Q3
launch
1. Cash generated from operations, including changes in returns and rebates, significant legal payments and operating contingent consideration liability payments 2. Net Capex includes purchases less disposals of
property, plant and equipment and intangibles 3. Other includes net interest paid and dividends to Non-Controlling Interests

19
Capital deployment supports business growth and shareholder
returns
1. Free Cash Flow (FCF) is £3.4bn, including the capital expenditure net of disposal proceeds for plant, property & equipment and intangibles of £1.3bn and £1.0bn, respectively (included in targeted business
development above) 2. Targeted BD in the above chart includes net intangible capex, net purchase of businesses, and net equity investment 3. Other includes dividend and distribution income, exchange on net
debt and other financing items
5.7
1.3
2.5
2.2
0.7
1.8
2022
Net debt
FCF ex-Capex Capex - PP&E Targeted BD Dividends to
shareholders
Other Haleon stake
monetisation
2023
Net debt
17.2
15.0
-£2.2bn
Invest for growth
Shareholder
distributions
£bn
1
2
3

Sales
• Vaccines: high single- to low double-digit % increase
• Specialty Medicines
1
: low double-digit % increase
• HIV: high single- to low double-digit % increase
• General Medicines: mid-single digit % decrease
P&L
• SG&A: low single-digit increase
• R&D: increase broadly in line with sales to support growth of pipeline
• Royalties: £500-£550m; minimal Gardasil royalties (2023: £472m)
• Net finance expense: slightly lower than 2023
• Adjusted Tax rate: around 17%
20
2024 guidance at CER (excl. COVID-19 solutions)
2024 modelling considerations
Guidance
Sales: 5% to 7% growth
Adj. operating profit: 7% to 10% growth
Adj. earnings per share: 6% to 9% growth
All guidance, outlooks, and expectations should be read together with the guidance, outlooks, assumptions, and cautionary statements in GSK’s full-year and Q4 2023 stock-exchange announcement.
1. including HIV.

21
Outlooks for 2026-2031
Emma Walmsley, Chief Executive Officer
Julie Brown, Chief Financial Officer

22
Delivering profitable growth 2026-2031
2026 2031
Build from existing strong performance momentum
With upside opportunity from:
• Early-stage pipeline data
readouts
• Targeted business development
Early-stage pipeline with key inflections and launches
+ targeted business development
Phase II: 30 vaccines and medicines in development
10
Phase I: 23 vaccines and medicines in development
10
>£38 billion RA
9
Innovation-led growth
• Significant growth portfolio with
increasing sales from Vaccines and
Specialty medicines
• >12 major product launches from
2025
Sales >£38bn RA
9
Planned launches ≥£2bn
5
PYS
1
Marketed Growth Drivers ≥£2bn PYS
1
Sales >7% CAGR
4
Arexvy
Shingrix
Meningococcal
vaccines
2
Cabenuva/Apretude
Nucala
Benlysta
Oncology
3
Meningococcal
vaccines
2
mRNA influenza
HIV PrEP
6
depemokimab
camlipixant
Anti-infectives
7
MAPS 24v/30v+
TH HSV
bepirovirsen
HIV treatment
6
Jemperli LCI
8
CD226
All outlook statements are given on a constant currency basis and use 2023 average exchange rates as a base. Pipeline sales are risk-adjusted and include anticipated sales of new products and lifecycle innovation. COVID therapeutic and vaccines solutions sales and profits (2021-
2023) are excluded from the above. See "Guidance and outlooks, assumptions and basis of preparation related to 2024 guidance, 2021-26 and 2031 outlooks" in Appendix of this presentation. 1. Peak year sales 2. Meningococcal vaccines (Bexsero and MenABCWY) 3. Includes
Zejula, Jemperli, and Ojjaara 4. Compound annual growth rate 5. Does not include Blenrep 6. Every-four-month ultra long-acting dosing. ≥£2bn applies to the aggregation of sales of PrEP and treatment. 7. Includes gepotidacin, tebipenem HBr and Brexafemme 8. Lifecycle
innovation 9. Risk-adjusted sales 10. See the appendix for a full list of vaccines and medicines in clinical development.
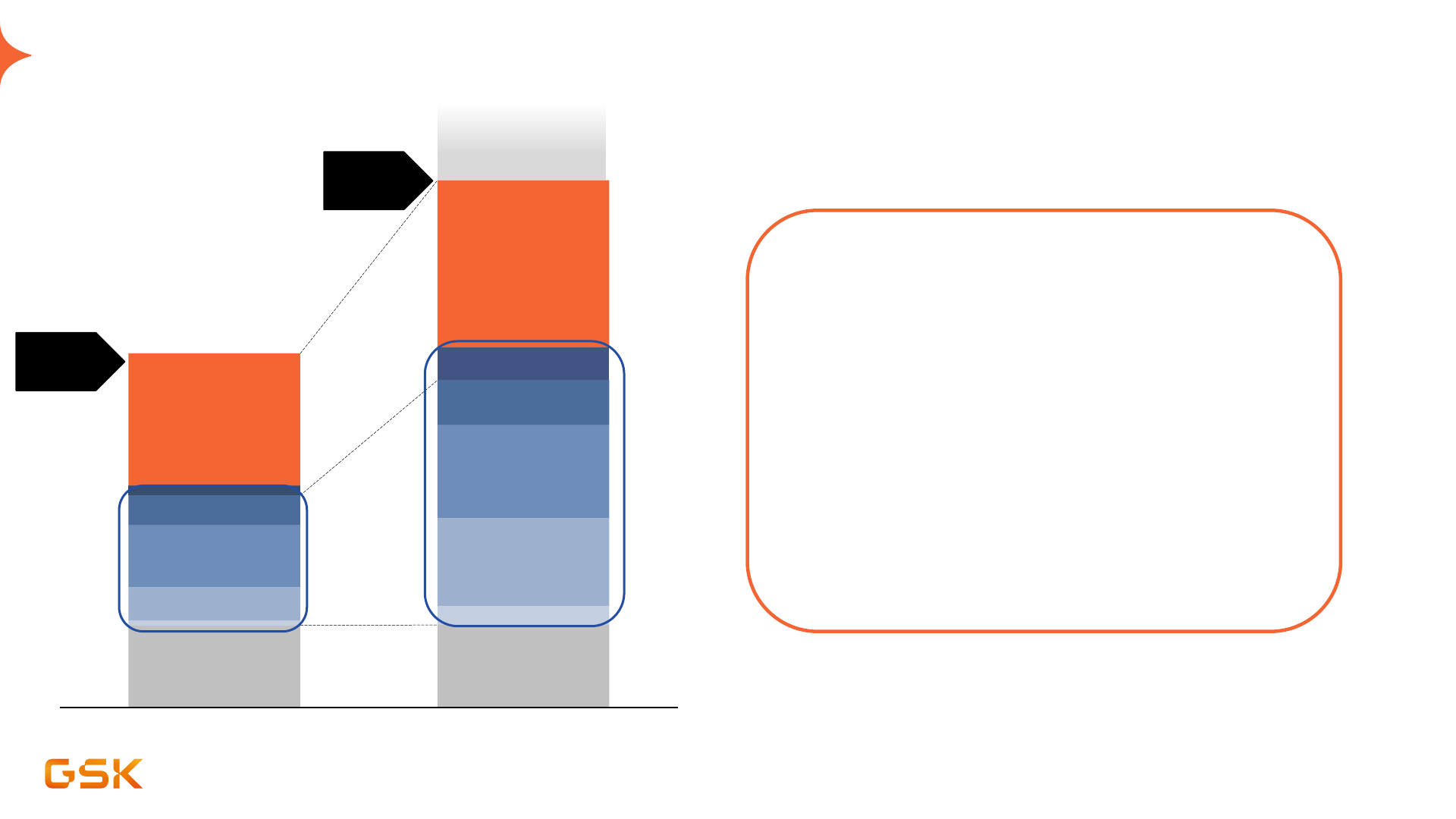
2031 sales outlook: high potential and attractive risk profile
2031 risk adjusted sales 2031 non risk adjusted sales
HIV
Vaccines
General
Medicines
Other Spec
Oncology
1
Respiratory/
Immunology
Vaccines
Respiratory/
Immunology
Oncology
1
Other Spec
General
Medicines
Sales risk
adjusted
> £38bn
Infectious Diseases
Infectious Diseases
23
HIV
Early-stage pipeline +
targeted BD
Sales
Non risk
adjusted
Marketed
• 2031 sales outlook of >£38bn provided on risk
adjusted basis
• More than 90% of sales supporting 2031
outlook of >£38 bn come from products
already approved, or from planned launches
≥£2bn, with majority planned for launch in
next four years
• Significant potential upside with successful
development outcomes and targeted BD
Pipeline opportunity
Charts are schematic and not to scale. All outlook and ambition statements are given on a constant currency basis and use 2023 average exchange rates as a base. Pipeline sales are risk-adjusted and include
anticipated sales of new vaccines and medicines and life cycle innovation. See "Guidance and outlooks, assumptions and basis of preparation related to 2024 guidance, 2021-26 and 2031 outlooks" in appendix.
1. Does not include Blenrep

General Medicines
Significant future pipeline value "unlocks" in 2024-2026
24
Launch year and NRA PYS
1
potential of major launches 2026-31
2025+ Anti-infectives
2
~£2bn
in peak year sales
1
Vaccines
2026+ mRNA seasonal flu
and combinations –
Influenza
>£3bn
in peak year sales
1
2027+ MAPs -
pneumococcal disease
24/30+
>£4bn
in peak year sales
1
2026 camlipixant -
refractory chronic cough
>£2.5bn
in peak year sales
1
Specialty Medicines
2025+ depemokimab / IL5
–
Nucala
COPD
>£4bn
in peak year sales
1
2026+ HIV Q4M ULA
prevention and treatment
>£3bn
in peak year sales
1
2027 bepirovirsen -
hepatitis B virus
>£2bn
in peak year sales
1
2031 CD226 – exploratory
oncology indications
>£2bn
in peak year sales
1
2025+
MenABCWY/
Bexsero
–
Meningococcal vaccines
~£2bn
in peak year sales
1
2028 TH HSV
3
– suppress
recurrence of genital
herpes
>£2bn
in peak year sales
1
2027+
Jemperli
LCI - lung,
colorectal and head &
neck
>£2bn
in peak year sales
1
Scale opportunities in all core product areas
All outlook statements are given on a constant currency basis and use 2023 average exchange rates as a base. See "Guidance and outlooks, assumptions and basis of preparation related to 2024 guidance, 2021-26
and 2031 outlooks" in appendix. 1. Non-risk adjusted peak year sales potential 2. Anti-infectives includes gepotidacin, tebipenem HBr and
Brexafemme
3. Therapeutic herpes simplex virus
25
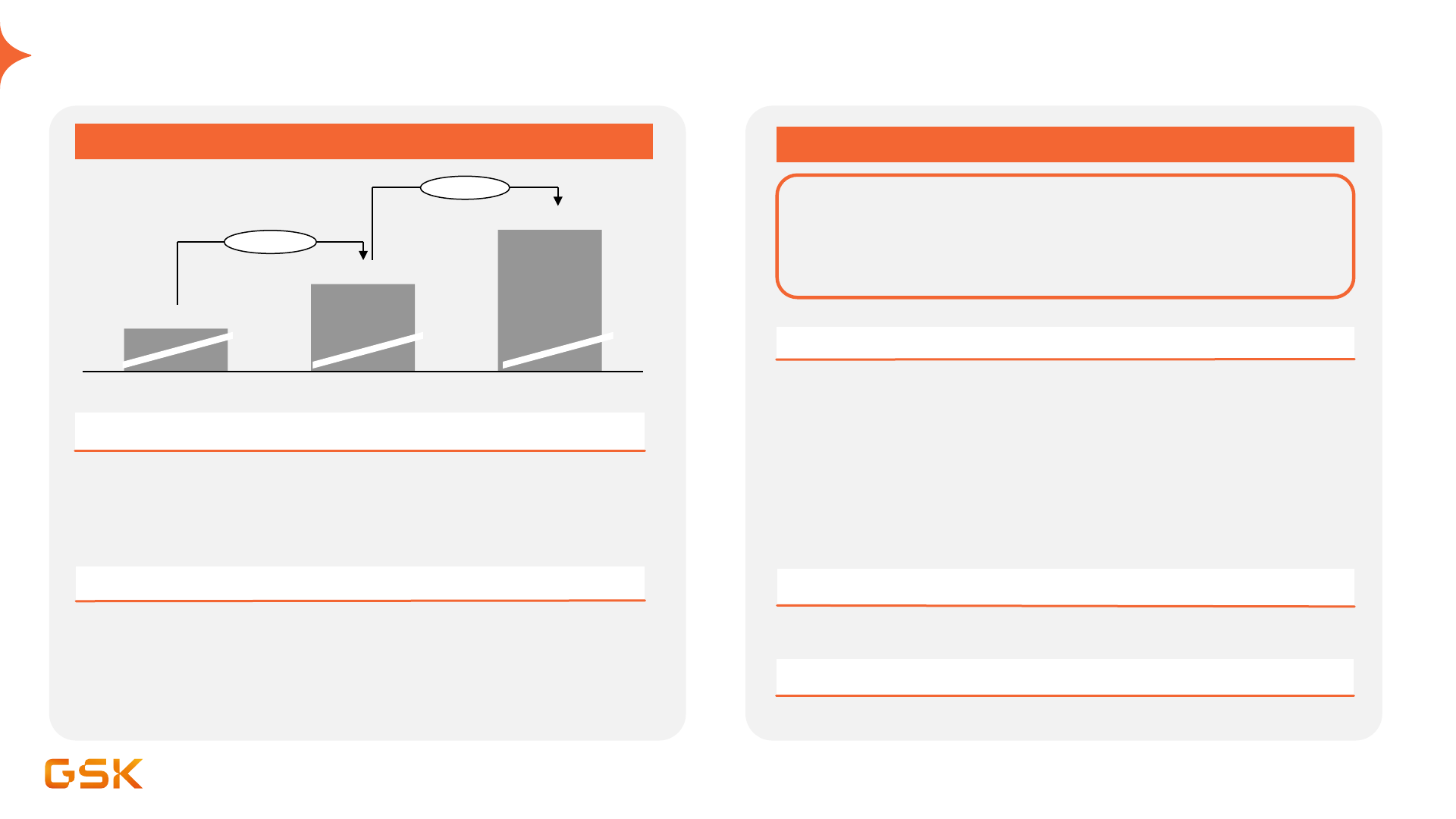
Sustained focus on operating margin improvement
2021 – 2026
• Gardasil royalties end in 2023
Headwinds
• Sales mix shift to Vaccines and Specialty Medicines
• Productivity across supply chain, commercial and functions
• Major restructuring complete
Key drivers
2026 – 2031
Continued focus on margin improvement,
with broadly stable OP margin through
dolutegravir loss of exclusivity (2028-2030)
1
• Dolutegravir LOE (from 2028-2030 (US/EU) with majority impact
2029-2030
Headwinds
Key drivers
2021 2023 2026
25.6%
28.6%
>31%
+290 bps
>240 bps
• Early-stage pipeline progression and targeted BD
Upside opportunity through additional revenue growth
• HIV portfolio transition (~40% of HIV business in long-acting
therapies anticipated at start of loss of exclusivity in US/EU)
• Operating margin mix benefit from Vaccine and Specialty
Medicines marketed and new pipeline products
• >12 major product launches planned 2026-31
• Expected productivity gains in supply chain and SG&A, with
increased use of AI and analytics
All outlook statements are given on a constant currency basis and use 2023 average exchange rates as a base. See "Guidance and outlooks, assumptions and basis of preparation related to 2024 guidance, 2021-
26 and 2031 outlooks" in appendix. Operating margin bridge excludes COVID-19 solutions; FX: 2021 reported margin at 2021 average exchange rates. 2023 and 2026 at 2023 average exchange rates. Basis point
movement at CER. 1. Anticipated loss of exclusivity from 2028-2030 in US/EU

26
Capital allocation framework to support investment and returns
Priority is to invest for growth, coupled with attractive shareholder returns
+8%
+11%
+17%
+15%
+12%
+8%
Sustainable, profitable growth and cash generation
Attractive and growing shareholder returns
Invest for growth
Pipeline (organic and targeted BD)
New product launches
1
Shareholder distributions
Progressive dividend (40-60% pay-out ratio)
Excess cash returns
2
Underpinned by strong balance sheet with strong investment grade credit rating
2022
1
dividend
2023 dividend
2024 dividend
55p/share
expect 58p/share
expect 60p/share
1. GSK group dividend 2022; GSK related only and excludes dividend related to Consumer in H1-2022; FY 2022 dividend 61.25p/share

27
IR Roadmap 2024 to 2025
H1 2024 H2 2024
*
2025
Execution
• Full year 2023 results
• Guidance 2024
• Q1 2024 results
• Half-year 2024 results
• Q3 2024 results
• Full-year 2024 results
• Guidance 2025
• Q1 2025 results
• Half-year 2025 results
• Q3 2025 results
Pipeline
Phase III and
regulatory decisions
2
Regulatory Decisions
• Ojjaara/Omjjara:
MOMENTUM, myelofibrosis (JP)
• Ojjaara/Omjjara: MOMENTUM,
myelofibrosis (EU)
• Nucala: severe asthma (CN)
• Arexvy, RSV, 50-59 YoA (US, EU, JP)
• Nucala, nasal polyposis (JP)
• gepotidacin: EAGLE
• MenABCWY 1
st
gen
(US, EU)
4
•
Jemperli
RUBY (Part 1) EC
3
1L (US)
•
Blenrep
, DREAMM-7/82 L+ MM
1, 8
(US, EU, CN, JP)
• depemokimab ANCHOR-1/2 CRSwNP
9
, (US)
• depemokimab SWIFT-1/2 SEA
16
(US)
• gepotidacin uUTI
10
, GC
11
(US)
• Nucala MATINEE COPD
12
(EU, CN)
• Nucala CRSwNP
9
(CN)
• Zejula ZEAL, 1L maintenance NSCLC (US)
• linerixibat cholestatic pruritus (US, EU, CN, JP)
Phase III readouts
• gepotidacin EAGLE-1, GC
11
• depemokimab SWIFT-1/2, SEA
16
• Zejula FIRST 1L maintenance OC
•
Jemperli
RUBY, 1L dMMR/MSI-H EC
3
(EU)
•
Jemperli
RUBY Part 1L OS
5
EC
3
•
Jemperli
RUBY Part 2, 1L EC
3
•
Blenrep
DREAMM-7, 2L+MM
1, 8
• depemokimab ANCHOR-1/2, CRSwNP
• Nucala MATINEE, COPD
•
Blenrep
DREAMM-8, 2L+ multiple
myeloma
1
• cobolimab COSTAR, 2L NSCLC
6
• Zejula ZEAL, 1L maintenance NSCLC
6
• linerixibat GLISTEN, PBC
7
• depemokimab OCEAN, EGPA
13
• camlipixant CALM 1/2, RCC
14
• tebipenem PIVOT-PO, cUTI
15
Capital Allocation
• Full-year 2023 dividend declaration
• Dividend expectation 2024
• Full-year 2024 dividend declaration
• Dividend expectation 2025
Investor
engagement
• Meet the management, Oncology • Meet the management, Early pipeline
Roadshows and Medical congresses
* HIV long-acting combination decision 1. Not included in the updated outlook 2. Includes phase III data readouts and regulatory decisions with the applicable geography denoted in brackets 3. Endometrial cancer 4. Regulatory submission and acceptance 5. Overall survival
overall population Phase III data readout ; 6. Non-Small Cell Lung Cancer 7. Cholestatic pruritus in primary biliary cholangitis 8 Multiple Myeloma; 9 Chronic rhinosinusitis with nasal polyps 10 Uncomplicated Urinary Tract Infections 11. Urogenital gonorrhoea 12 Chronic Obstructive
Pulmonary Disease 13 Eosinophilic granulomatosis with polyangiitis 14. Refractory Chronic Cough 15. Complicated urinary tract infection 16. Severe eosinophilic asthma

28
R&D based on science of
the immune system and
use of new platform and
data technologies
Leaders in development of
new Vaccines and
Specialty Medicines, for
Infectious Diseases, HIV
Respiratory/Immunology
and Oncology
Products that improve
the health of millions of
people, and sector
leaders in ESG
performance
Strong momentum and
improving outlook for
sustained growth through
the decade
Focused on prevention and changing the course of disease
Ahead Together

29

30
Appendix

31
2024 full year outlook considerations to support modelling
2023 growth
excl Covid
2024 Guidance 2024 Assumptions
2021 – 2026
BIU 2021
2021 – 2026
BIU 2024
Turnover +14% 5-7% >5% CAGR >7% CAGR
- Vaccines +24% HSD – LDD HSD CAGR LDD CAGR
- Specialty +15% LDD DD CAGR DD CAGR
- HIV +13% HSD – LDD MSD CAGR 6-8%
1
- Gen Meds +5% MSD decr
Largely Amp Cap removal
Broadly Stable Broadly Stable
Adj. Operating Profit +16% 7-10%
SG&A: LSD increase
R&D: increase broadly in line with sales
Royalties: £500-£550m; minimal
Gardasil royalties
>10% CAGR >11% CAGR
Adj. Op. Profit margin 28.6% n/a >30% >31%
Adj EPS + 22% 6-9%
Interest: slightly lower than 2023
Tax rate: around 17%
Non-controlling interest: ViiV is main
ongoing NCI
Dividend 58p 60p
All guidance, outlooks and expectations regarding future performance should be read together with the section “Guidance and outlooks, assumptions and cautionary statements” on pages 54 to 55 of of GSK’s full-
year and Q4 2023 stock-exchange announcement.. 2024 guidance growth at CER, unless stated otherwise. All outlook statements are given on a CER basis and use 2023 average exchange rates as a base. All values
excluding COVID-19 solutions. CAGR is compound annual growth rate. 1. As per HIV Meet The Management event, 28 September 2023

32
2023 Total to adjusted operating profit reconciliation
2022 2023 Key commentary on CER basis
Operating profit
(£m)
Operating profit
(£m)
Total results
6,431
6,745
+10% at CER
Intangible amortisation 739 719
Intangible impairment 296 398
Major restructuring 321 382
~£1.1bn benefits to date
1
Transaction-related 1,750 572
ViiV CCL
2
movements
Divestments, significant
legal and other
(1,388) (30)
Receipt of dividend and distribution income from investments, legal
fees and settlements
Adjusted
results 8,151 8,786
+ 12%
at CER
Table may not sum due to rounding. See page 20 of GSK’s full-year and Q4 2023 stock-exchange announcement for a full reconciliation 1. Separation Preparation restructuring programme initiated in 2020
2. Contingent consideration liabilities

33
Improved adj. earnings per share with +16% growth at CER
2022 2023
Key commentary on CER basis
£m £m
Adj. operating
profit (OP) 8,151 8,786
+12% incl. COVID; +16% excl. COVID
-19 solutions
Net finance expense (791) (669) Lower bond interest costs and higher interest income
Share of associates (2) (5)
Tax (1,138) (1,257)
Tax rate 15.5% 15.5% In-line with guidance
Non-controlling interests (595) (572) Lower net profits in some Group entities
Adj. Profit attributable to shareholders
5,625 6,283
+17% incl. COVID
Adj. earnings per share (EPS)
139.7p 155.1p
+16% incl. COVID, +22% excl. COVID
-19 solutions
Total EPS
110.8p 121.6p
+16% at CER
Weighted average number of shares (millions) 4,026 4,052
Adjusted results and for continuing operations unless stated otherwise.

34
Quarterly summary of results
2022 2023
Q1 Q2 Q3 Q4 FY Q1 Q2 Q3 Q4 FY
Including COVID
-19 solutions
Sales (£m) 7,190 6,929 7,829 7,376 29,324 6,951 7,178 8,147 8,052 30,328
Operating profit (£m) 1,943 2,008 2,605 1,595 8,151 2,092 2,170 2,772 1,752 8,786
Operating margin 27.0% 29.0% 33.3% 21.6%
27.8%
30.1% 30.2% 34.0% 21.8%
29.0%
Earnings per share (pence) post-share
consolidation
32.3 34.7 46.9 25.8
139.7 37.0 38.8 50.4 28.9 155.1
COVID
-19 solutions impact
Sales (£m) 1,307 466 417 183 2,373 132 41 1 20 194
Operating profit (£m) 194 58 141 69 462 118 57 (4) 9 179
Earnings per share (pence) post-share
consolidation
4.1 1.2 2.9 1.5
9.7 2.5 1.2 (0.1) 0.2 3.8
Excluding COVID
-19 solutions impact
Sales (£m) 5,883 6,463 7,412 7,193 26,951 6,819 7,137 8,146 8,032 30,134
Operating profit (£m) 1,749 1,950 2,464 1,526 7,689 1,974 2,113 2,776 1,743 8,607
Operating margin 29.7% 30.2% 33.2% 21.2%
28.5%
28.9% 29.6% 34.1% 21.7%
28.6%
Earnings per share (pence) post-share
consolidation
28.2 33.5 44.0 24.3
130.0 34.5 37.6 50.5 28.7 151.3
Adjusted results and for continuing operations; some figures may not sum due to rounding.

35
Currency
1. Based on 2023 GSK continuing operations, including COVID-19 solutions 2. The other currencies that each represent more than 1% of GSK sales include Australian Dollar, Brazilian Real, Canadian Dollar, Chinese Yuan and Indian
Rupee. In total, they accounted for 9% of GSK revenues in 2023. 3. If exchange rates were to hold at the closing rates on 24 January 2024 ($1.27/£1, €1.17/£1 and Yen 188/£1) for the rest of 2024, the estimated impact on 2024
Sterling turnover growth for GSK would be -3% and if exchange gains or losses were recognised at the same level as in 2023, the estimated impact on 2024 Sterling Adjusted Operating Profit growth for GSK would be -5%.
2023 currency sales exposure
1
2024 adj. operating profit
3
US $ 52% US $: 10 cents movement in the average exchange rate for full year
impacts adj. operating profit by approx. +/- 9.0%
Euro € 19% Euro €: 10 cents movement in the average exchange rate for full year
impacts adj. operating profit by approx. +/- 0.5%
Japanese ¥ 4% Japanese ¥: 10 Yen movement in the average exchange rate for full
year impacts adj. operating profit by approx. +/- 1.0%
Other
2
25%
2022 2023
Historical average exchange rates
quarterly
Q1 Q2 Q3 Q4 FY 22 Q1 Q2 Q3 Q4 FY 23
US $
1.34 1.26 1.18 1.19 1.24 1.22 1.25 1.26 1.25 1.24
Euro €
1.19 1.18 1.16 1.15 1.17 1.14 1.15 1.16 1.15 1.15
Japanese ¥
156 162 161 165 161 162 173 182 183 175
Historical period end exchange rates
US $
1.31 1.21 1.11 1.20 1.24 1.26 1.23 1.27
Euro €
1.18 1.16 1.13 1.13 1.14 1.17 1.16 1.15
Japanese ¥
160 165 160 159 165 183 183 180
36
Investor roadmap highlights progress of key events
Q2 2023
Q3 2023 Q4 2023
H1 2024
H2 2024
Execution
• Q2 and Half-year
2023 results
• Full-year 2023
upgraded guidance
• Q3 and Year-to-date
2023 results
• Full-year and Q4 2023
results
• Performance vs BIU
2021
1
• Full-year 2024 guidance
• Q1 2024 results
• Q2 and Half-year 2024 results
• Q3 and Year-to-date 2024 results
• Full-year and Q4 2024 results
• Performance vs BIU 2021
1
• Guidance 2025
Pipeline
Phase III and
regulatory
decisions
2
• Therapy Area
Strategy
• R&D priorities
• Arexvy US
regulatory approval
• Arexvy second
season data
• BELLUS Health, Inc.
acquisition
completed
• SCYNEXIS, Inc.
exclusive license
completed
• Arexvy RSV, ≥60 YoA (JP)
• Arexvy, RSV, 50-59 YoA
• Apretude, HIV pre-exposure (EU)
• Vocabria, HIV treatment (CN)
• Ojjaara, MOMENTUM, myelofibrosis (US)
• Jemperli RUBY, 1L dMMR/MSI-H EC
3
(US)
• Jemperli: RUBY, 1L dMMR/MSI-H
EC
3
(EU)
• Ojjaara: MOMENTUM, myelofibrosis
(EU)
• Ojjaara: MOMENTUM, myelofibrosis
(JP)
• Blenrep: DREAMM-7, 2L+ MM
• gepotidacin: EAGLE-1, GC
• depemokimab: SWIFT-1/2, asthma
• Jemperli: RUBY (Part 2), 1L EC
3
• Jemperli: RUBY (Part 1) 1L OS
4
EC
3
• Zejula: FIRST, 1L maintenance OC
ovarian cancer
• Arexvy: RSV, 50-59 YoA (US, EU, JP)
• Nucala: CRSwNP (JP)
• Nucala: severe asthma (CN)
• depemokimab: ANCHOR-1/2,
CRSwNP
• Nucala MATINEE, COPD
• cobolimab: COSTAR, 2L NSCLC
• Blenrep: DREAMM-8, 2L MM
• Zejula: ZEAL, 1L maintenance NSCLC
• linerixibat: GLISTEN, PBC
5
Capital
Allocation
• Capital allocation
• R&D and BD
priorities
• TA priorities
• Full-year 2023
dividend
declaration
• Full-year 2024 dividend declaration
Investor
Engagement
Roadshows
Meet the
management,
Infectious Diseases
Meet the management,
HIV
Meet the
management,
Respiratory
Meet the management, Oncology
Medical congresses
1. June 2021 Investor Update 2. Includes phase III data readouts and regulatory decisions with the applicable geography denoted in brackets 3. Endometrial cancer 4. Overall survival population
5. cholestatic pruritus in primary biliary cholangitis

37
Upcoming pipeline catalysts: 2024 and 2025
H1 2024 H2 2024 2025
Regulatory
decision
Ojjaara/Omjjara: MOMENTUM, myelofibrosis JP Arexvy: 50-59 YoA
10
US, EU, JP
gepotidacin: EAGLE-2/3, uUTI
11
US
Nucala: CRSwNP
1
JP
gepotidacin: EAGLE-1, GC
5
US
MenABCWY vaccine 1st Gen
US, EU
depemokimab: SWIFT-1/2, asthma
US
depemokimab: ANCHOR-1/2, CRSwNP
1
US
Nucala: CRSwNP
1
CN
Nucala: MATINEE, COPD
12
US
Blenrep: DREAMM-7/8, 2L+ MM
7
US, EU, CN, JP
Jemperli
2
: RUBY (Part 1)
3,
1L EC
4
US
linerixibat: GLISTEN, cholestatic pruritus in PBC
14
US
Regulatory
submission and
acceptance
MenABCWY vaccine 1st Gen
US
gepotidacin: EAGLE-2/3, uUTI
11
US
gepotidacin: EAGLE-1, GC
5
US
Nucala: CRSwNP
1
CN
MenABCWY vaccine 1st Gen
EU
tebipenem pivoxil: PIVOT-PO, cUTI
15
US
Jemperli
2
: RUBY (Part 1)
3
, 1L EC
4
US
depemokimab: SWIFT-1/2, asthma
US
camlipixant: CALM-1/2, RCC
16
US, EU
depemokimab: ANCHOR-1/2, CRSwNP
1
US
Nucala: MATINEE, COPD
12
EU, CN
Nucala: MATINEE, COPD
12
US Blenrep: DREAMM-7/8, 2L+ MM
7
US, EU, CN, JP
cobolimab
2
: COSTAR, 2L NSCLC
13
US, EU
linerixibat: GLISTEN, cholestatic pruritus in PBC
14
US, EU, CN, JP
Late
-
stage phase
III and phase II
readouts
gepotidacin: EAGLE-1, GC
5
depemokimab: ANCHOR-1/2, CRSwNP
1
tebipenem pivoxil: PIVOT-PO, cUTI
15
depemokimab: SWIFT-1/2, asthma Nucala: MATINEE, COPD
12
camlipixant: CALM-1/2, RCC
16
Blenrep: DREAMM-7
6
, 2L+ MM
7
Blenrep: DREAMM-8, 2L+ MM
7
depemokimab: OCEAN, EGPA
17
Zejula
2
: FIRST, 1L maintenance OC
8
cobolimab
2
: COSTAR, 2L NSCLC
13
Zejula
2
: ZEAL, 1L maintenance NSCLC
13
mRNA Seasonal flu
9
linerixibat: GLISTEN, cholestatic pruritus in PBC
14
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven
1. Chronic rhinosinusitis with nasal polyps 2. Tesaro asset 3. Overall population 4. Endometrial cancer 5. Urogenital gonorrhoea 6. Overall survival 7. Multiple myeloma 8. Ovarian cancer 9. Phase II 10. Years of age 11. Uncomplicated urinary tract infection 12. Chronic
obstructive pulmonary disorder 13. Non-small cell lung cancer 14. Treatment of cholestatic pruritus in primary biliary cholangitis 15. Complicated urinary tract infection 16. Refractory chronic cough 17. Eosinophilic granulomatosis with polyangiitis

38
12 major planned launches
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven
MenABCWY
mRNA influenza
Anti-infectives (gepotidacin, tebipenem and Brexafemme)
MAPs 24v/30+v
TH HSV (herpes simplex virus)
bepirovirsen
HIV PrEP
HIV treatment
depemokimab
camlipixant
Jemperli LCI
CD226
1. Every-four-month ultra long-acting dosing

39
71 potential new vaccines and medicines in pipeline
Phase III / Registration – 18 assets
Arexvy (RSV vaccine) Recombinant protein, adjuvanted* RSV older adults (50-59 YoA)^
gepotidacin (2140944) BTI inhibitor* Uncomplicated UTI**
bepirovirsen (3228836) Antisense oligonucleotide* Chronic HBV infection**
Bexsero (MenB vaccine) Recombinant protein, OMV Meningitis B (infants US)
MenABCWY vaccine (3536819) Recombinant protein, OMV, conjugated vaccine MenABCWY, 1
st
Gen
tebipenem pivoxil (3778712) Antibacterial carbapenem* Complicated UTI
ibrexafungerp (5458448) Antifungal glucan synthase inhibitor* Invasive candidiasis
Nucala (mepolizumab) Anti-IL5 antibody COPD
depemokimab (3511294) Long-acting anti-IL5 antibody* Asthma**
latozinemab (4527223) Anti-sortilin antibody* Frontotemporal dementia
1
**
camlipixant (5464714) P2X3 receptor antagonist Refractory chronic cough
Low carbon version of MDI
2
, Ventolin (salbutamol)
Beta 2 adrenergic receptor agonist Asthma
3
Ojjaara/Omjjara (momelotinib) JAK1, JAK2 and ACVR1 inhibitor* Myelofibrosis^
4
Jemperli (dostarlimab) Anti-PD-1 antibody* Endometrial cancer^**
Zejula (niraparib) PARP inhibitor* Ovarian cancer**
Blenrep (belantamab mafodotin) Anti-BCMA ADC* Multiple myeloma
cobolimab (4069889) Anti-TIM-3 antibody* Non-small cell lung cancer
linerixibat (2330672) IBAT inhibitor Cholestatic pruritus in primary biliary cholangitis
*In-license or other alliance relationship with third party ** Additional indications or candidates also under investigation ^ In registration
1. Phase III trial in patients with progranulin gene mutation 2. Metered dose inhaler 3. Phase III start expected in 2024 4. Approved in US and EU 5. In phase I/II study 6. Phase II study start imminent 7. Transition activities
underway to enable further progression by partner 8. GSK has an exclusive global license option to co-develop and commercialise the candidate
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven

*In-license or other alliance relationship with third party ** Additional indications or candidates also under investigation ^ In registration
1. Phase III trial in patients with progranulin gene mutation 2. Metered dose inhaler 3. Phase III start expected in 2024 4. Approved in US and EU 5. In phase I/II study 6. Phase II study start imminent 7. Transition activities
underway to enable further progression by partner 8. GSK has an exclusive global license option to co-develop and commercialise the candidate
40
71 potential new vaccines and medicines in pipeline
3437949 Recombinant protein, adjuvanted* Malaria fractional dose
4406371 Live, attenuated MMRV new strain
3536852 GMMA* Shigella
3528869 Viral vector with recombinant protein, adjuvanted* Chronic HBV infection
5
**
4023393 Recombinant protein, OMV, conjugated vaccine MenABCWY, 2
nd
Gen
5
4178116 Live, attenuated Varicella new strain
5101956 MAPS* Adult pneumococcal disease, 24-valent
5101955 MAPS* Paediatric pneumococcal disease, 24-valent
4106647 Recombinant protein, adjuvanted* Human papillomavirus
5
4348413 GMMA Gonorrhoea
5
4382276 mRNA* Seasonal flu
4396687 mRNA* COVID-19
3993129 Adjuvanted recombinant subunit Cytomegalovirus
5
3943104 Recombinant protein, adjuvanted* Therapeutic herpes simplex virus
5
5637608 Hepatitis B virus-targeted siRNA* Chronic HBV infection
4077164 Bivalent GMMA Invasive non-typhoidal salmonella**
3036656 Leucyl t-RNA synthetase inhibitor* Tuberculosis
sanfetrinem cilexetil (GV118819) Serine beta lactamase inhibitor* Tuberculosis
BVL-GSK098 Ethionamide booster* Tuberculosis
3810109 Broadly neutralizing antibody* HIV
3739937 Maturation inhibitor HIV
4004280 Capsid protein inhibitor HIV
4011499 Capsid protein inhibitor HIV
4524184 Integrase inhibitor* HIV
6
Benlysta (belimumab) Anti-BLys antibody Systemic sclerosis associated interstitial lung disease
3858279 Anti-CCL17 antibody* Osteoarthritis pain**
1070806 Anti-IL18 antibody Atopic dermatitis
4527226 (AL-101) Anti-sortilin antibody* Alzheimer’s disease
6
belrestotug (4428859) Anti-TIGIT antibody* Non-small cell lung cancer**
4532990 HSD17B13 siRNA* Non-alcoholic steatohepatitis
Phase II – 30 assets
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven

41
71 potential new vaccines and medicines in pipeline
Phase I – 23 assets
*In-licence or other alliance relationship with third party ** Additional indications or candidates also under investigation ^ In registration
1. Phase III trial in patients with progranulin gene mutation 2. Metered dose inhaler 3. Phase III start expected in 2024 4. Approved in US and EU 5. In phase I/II study 6. Phase II study start imminent 7. Transition activities
underway to enable further progression by partner 8. GSK has an exclusive global license option to co-develop and commercialise the candidate
3536867 Bivalent conjugate* Salmonella (typhoid + paratyphoid A)
2556286 Mtb cholesterol dependent inhibitor* Tuberculosis
3186899 CRK-12 inhibitor*
7
Visceral leishmaniasis
3494245 Proteasome inhibitor* Visceral leishmaniasis
3772701 P. falciparum whole cell inhibitor* Malaria
4024484 P. falciparum whole cell inhibitor* Malaria
3882347 FimH antagonist* Uncomplicated UTI
3923868 PI4K beta inhibitor Viral COPD exacerbations
3965193 PAPD5/PAPD7 inhibitor Chronic HBV infection
5
5251738 TLR8 agonist* Chronic HBV infection
cabotegravir (1265744) Integrase inhibitor HIV
3888130 Anti-IL7 antibody* Autoimmune disease
3915393 TG2 inhibitor* Pulmonary fibrosis
3862995 Anti-IL33 antibody COPD
5462688 RNA-editing oligonucleotide* Alpha-1 antitrypsin deficiency
4347859 Interferon pathway modulator Systemic lupus erythematosus
4381562 Anti-PVRIG antibody* Cancer
6097608 Anti-CD96 antibody* Cancer
XMT-2056
9
(wholly owned by Mersana Theraprutics)
STING agonist ADC* Cancer
belantamab (2857914) Anti-BCMA antibody Multiple myeloma
4524101 DNA polymerase theta inhibitor* Cancer
5
5733584 (HS-20089) ADC-targeting B7-H4* Gynecologic malignancies
4172239 DNMT1 inhibitor* Sickle cell disease
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven

42
Changes since Q3 2023
Changes on pipeline Achieved pipeline catalysts
Regulatory decisions
Nucala – severe asthma CN
Jemperli
1
– RUBY, dMMR/MSI-H 1L endometrial cancer EU
Omjjara: MOMENTUM, myelofibrosis EU
Regulatory submission acceptances
Arexvy – 50-59 YoA EU, JP
Other events
Blenrep – DREAMM-7, 2L+ MM – Positive headline phase III data
Jemperli
1
– RUBY (Part 2), 1L EC – Positive phase III data readout
New to Phase I
4024484 – P. falciparum whole cell inhibitor, malaria
3862995 – Anti-IL33 antibody, COPD
5462688 – RNA-editing oligonucleotide, Alpha-1 antitrypsin deficiency
4347859 – Interferon pathway modulator, systemic lupus erythematosus
5733584 – ADC targeting B7-H4, gynecologic malignancies
New to Phase II
3943104 – Recombinant protein, adjuvanted, Therapeutic HSV
5637608 – HBV-targeted siRNA sequential combination, chronic HBV infection
4077164 – Bivalent GMMA, Invasive non-typhoidal salmonella**
4524184 – Integrase inhibitor, HIV
New to Phase III
Low carbon version of MDI, Ventolin – Beta 2 adrenergic receptor agonist, asthma
Removed from Phase I
4429016 – Bioconjugated recombinant protein, adjuvanted, K. pneumoniae
4182137 (VIR7832) – Anti-spike protein antibody, COVID-19
Removed from Phase II
VIR2482 – Neutralising monoclonal antibody, influenza
Infectious diseases
HIV (ViiV)
Respiratory/Immunology
Oncology
Opportunity driven
** Additional indications or candidates also under investigation
1. Tesaro asset

43
ADC Antibody drug conjugate EGPA Eosinophilic granulomatosis with polyangiitis NSCLC Non-small cell lung cancer
AE Adverse event FVC Forced vital capacity OMV Outer membrane vesicle
AESI Adverse event of special interest GC Urogenital gonorrhea ORR Overall response rate
AUC Area under curve GMMA Generalised Modules for Membrane Antigens OS Overall surival
BCMA B-cell maturation antigen GSI Gamma secretase inhibitor PBC Primary biliry cholangitis
BICR Blinded Independent Central Review HA Healthy adults PFS Progression-free survival
BRCA Breast cancer HBV Hepatitis B virus PFS2 Time to second disease progression or death
CAE Corneal adverse events HES Hypereosinophilic syndrome PK Pharmacokinetic
CBR Clinical benefit rate Hgb Hemoglobin PMF
Primary myelofibrosis
cCR Complete clinical response hSBA Human serum bactericidal assay Post-PV/ET MF Post-essential thrombocythemia myelofibrosis
CKD Chronic kidney disease HZ Herpes zoster RCC Refractory chronic cough
CfB Change from baseline IC Immunocompromised RL Repeat dose level
CMV Cytomegalovirus ICR Independent central review RRMM Relapsed/refractory multiple myeloma
CN China iNTS Invasive non-typhoidal salmonella RSV Respiratory syncytial virus
COPD Chronic obstructive pulmonary disease ITT Intention-to-treat SAD Single ascending dose
CP Cholestatic pruritus JP Japan SAE Serious adverse event
CRR Complete response rate LLOQ Lower limit of quantitation siRNA Small interfering RNA
CRSwNP Chronic rhinosinusitis with nasal polyps LRTS Lower respiratory tract symptoms SoC Standard of care
cUTI Complicated urinary tract infection MAD Multiple ascending dose SSc-ILD
Systemic sclerosis associated interstitial lung disease
CV Cardiovascular MAE Medical attended events TOC Test of cure
DDI Drug-drug interaction MDI Metered dose inhaler TTBR Time to best response
DFS Disease-freee survival MAPS Mulitple Antigen Presenting System TTD Time to treatment discontinuation
DL Dose level MM Multiple myeloma TTP Time to tumour progression
DLT Dose-limiting toxicity MMR Measles, mumps and rubella TTR Time to treatment response
dMMR Deficient mismatch repair MMRV Measles, mumps, rubella and varicella UTI Urinary tract infection
DoR Duration of response MRD Multiple rising dose uUTI Uncomplicated urinary tract infection
DPNP Diabetic peripheral neuropathic pain MSI-H Microsatellite instability high VGPR Very good partial remission
EASI Eczema Area and Severity Index NASH Nonalcoholic steatohepatitis VSP Vital sign parameters
EGPA Eosinophilic granulomatosis with polyangiitis NRS Numeric Rating Scale YoA Years of age
Glossary

44
In outlining the guidance for 2024 and outlooks for the period 2021-2026 and for 2031, the Group has made certain assumptions about the macro-economic environment, the
healthcare sector (including regarding existing and possible additional governmental legislative and regulatory reform), the different markets and competitive landscape in which the
Group operates and the delivery of revenues and financial benefits from its current portfolio, its development pipeline and restructuring programmes.
2024 Guidance
These planning assumptions as well as operating profit and earnings per share guidance and dividend expectations assume no material interruptions to supply of the Group’s
products, no material mergers, acquisitions or disposals, no material litigation or investigation costs for the Company (save for those that are already recognised or for which provisions
have been made) and no change in the Group’s shareholdings in ViiV Healthcare. The assumptions also assume no material changes in the healthcare environment or unexpected
significant changes in pricing as a result of government or competitor action. The 2024 guidance factors in all divestments and product exits announced to date.
2021-26 and 2031 outlooks
The assumptions for GSK’s updated revenue, operating profit, operating margin and cash flow outlooks, 2031 revenue outlook and margin expectations through dolutegravir loss of
exclusivity assume the delivery of revenues and financial benefits from its current and development pipeline portfolio of drugs and vaccines (which have been assessed for this purpose
on a risk-adjusted basis, as described further below); regulatory approvals of the pipeline portfolio of drugs and vaccines that underlie these expectations (which have also been
assessed for this purpose on a risk-adjusted basis, as described further below); no material interruptions to supply of the Group’s products; successful delivery of the ongoing and
planned integration and restructuring plans; no material mergers, acquisitions or disposals or other material business development transactions; no material litigation or investigation
costs for the company (save for those that are already recognised or for which provisions have been made); no share repurchases by the company; and no change in the
shareholdings in ViiV Healthcare. GSK assumes no premature loss of exclusivity for key products over the period.
The assumptions for GSK’s updated revenue, operating profit, operating margin and cash flow outlooks, 2031 revenue outlook and margin expectations through dolutegravir loss of
exclusivity also factor in all divestments and product exits announced to date as well as material costs for investment in new product launches and R&D. Risk-adjusted sales includes
sales for potential planned launches which are risk adjusted based on the latest internal estimate of the probability of technical and regulatory success for each asset in development.
Potential future sales contribution from Blenrep have not been included.
Notwithstanding these outlooks and expectations, there is still uncertainty as to whether our assumptions, outlooks and expectations will be achieved, including based on the other
assumptions outlined above.
All outlooks statements are given on a constant currency basis and use 2023 average exchange rates as a base (£1/$1.24, £1/€1.15, £1/Yen 175). 2021-2026 outlook refers to the 5 years
to 2026 with 2021 as the base year.
Assumptions and basis of preparation related to 2024 guidance, 2021-26 and 2031 outlooks

45
Use of GSK conference call, webcast and presentation slides
The GSK plc webcast, conference call and presentation slides (together the ‘GSK materials’) are for your personal, non-commercial use only. You may not copy,
reproduce, republish, post, broadcast, transmit, make available to the public, sell or otherwise reuse or commercialise the GSK materials in any way. You may not edit,
alter, adapt or add to the GSK materials in any way, nor combine the GSK materials with any other material. You may not download or use the GSK materials for the
purpose of promoting, advertising, endorsing or implying any connection between you (or any third party) and us, our agents or employees, or any contributors to the
GSK materials. You may not use the GSK materials in any way that could bring our name or that of any Affiliate into disrepute or otherwise cause any loss or damage to
us or any Affiliate. GSK plc, 980 Great West Road, Brentford, Middlesex, TW8 9GS, United Kingdom. Telephone +44 20 8047 5000, www.gsk.com

gsk.com
