
National Center for HIV, Viral Hepatitis, STD, and TB Prevention
Universal Adult Hepatitis B Vaccination:
Work Group Considerations
Division of Viral Hepatitis
LCDR Mark K. Weng, MD, MSc, FAAP
Hepatitis Vaccines Work Group, Advisory Committee on Immunization Practices
November 3, 2021

2
20,700 estimated acute HBV infections each year
(95% CI: 11,800–50,800)
1
> $1 billion spent on hepatitis B-related hospitalizations each
year (not including indirect costs)
2
Hepatitis B in the US
1) https://www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm; 2) Corte et al. J Gastroenterol Hepatol. 2014.

3
1.89 million persons living with chronic HBV (modeled
estimate; range, 1.49–2.40 million)
2
15-25% risk of premature death from cirrhosis or liver cancer
among people living with chronic HBV infection
3
Hepatitis B in the US
1) Wong et al. Am J Med. 2021 3) https://www.cdc.gov/std/treatment-guidelines/hbv.htm

Simplify a complex adult HepB vaccination schedule
• All infants
• Unvaccinated children aged <19 years
• Persons at risk for infection by sexual exposure
• Sex partners of hepatitis B surface antigen (HBsAg)–positive persons
• Sexually active persons who are not in a long-term, mutually monogamous relationship
(e.g., persons with more than one sex partner during the previous 6 months)
• Persons seeking evaluation or treatment for a sexually transmitted infection
• Men who have sex with men
• Persons at risk for infection by percutaneous or mucosal exposure to blood
• Current or recent injection-drug users
• Household contacts of HBsAg-positive persons
• Residents and staff of facilities for developmentally disabled persons
• Health care and public safety personnel with reasonably anticipated risk for exposure to
blood or blood-contaminated body fluids
• Hemodialysis patients and predialysis, peritoneal dialysis, and home dialysis patients
• Persons with diabetes aged 19–59 years; persons with diabetes aged ≥60 years at the
discretion of the treating clinician
• Others
• International travelers to countries with high or intermediate levels of endemic hepatitis
B virus (HBV) infection (HBsAg prevalence of ≥2%)
• Persons with hepatitis C virus infection
• Persons with chronic liver disease (including, but not limited to, persons with cirrhosis,
fatty liver disease, alcoholic liver disease, autoimmune hepatitis, and an alanine
aminotransferase [ALT] or aspartate aminotransferase [AST] level greater than twice the
upper limit of normal)
• Persons with HIV infection
• Incarcerated persons
•All other persons seeking protection from HBV infection
• All infants [No change]
• Unvaccinated children aged <19 years [No change]
All adults previously unvaccinated for hepatitis B
should receive hepatitis B vaccination
Existing Recommendations
New Recommendations (Proposed)
Persons recommended to receive hepatitis B vaccination
Schillie et al, 2018

5
HepB Immunization Strategy Evolves
Incidence, Hepatitis B
Year
HepB Recommendations and Est. Acute Hepatitis B Cases in the US, 1980─2019
Source: National Notifiable Diseases Surveillance System (NNDSS)
6
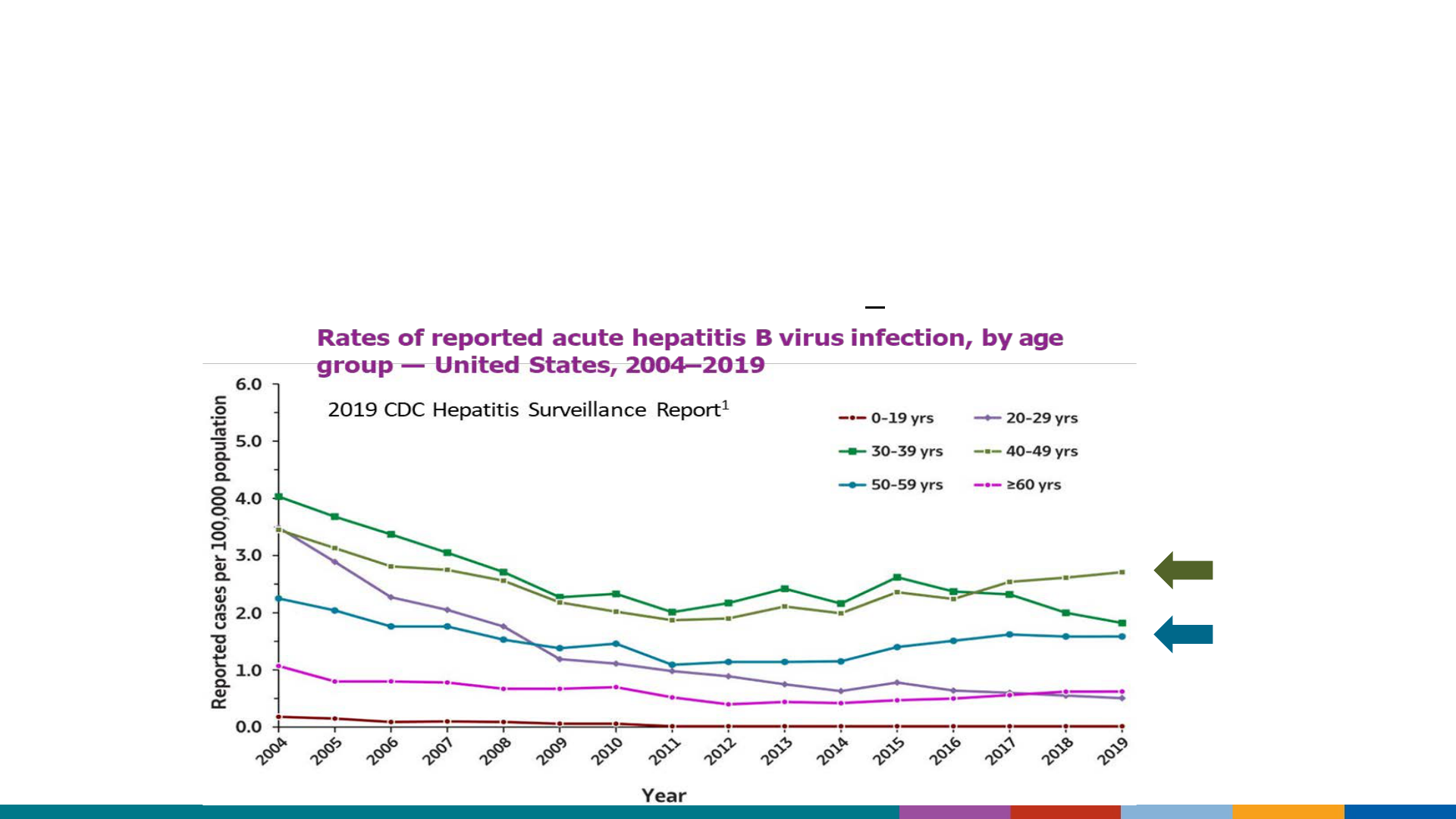
Initial decreases in new hep B infections plateaued 10 years ago
Rates are now highest among adults
Rates have increased among adults >40 years of age
Risk-based hepatitis B immunization among adults:
a partial success

7
Hepatitis B vaccine coverage (≥3 doses) among adults aged ≥19 years
*
30.0
38.9
33
67.2
33
15.3
0
10
20
30
40
50
60
70
80
90
100
Overall Travelers Chronic liver
conditions
Health care
personnel**
Diabetes
(19-59 years)
Diabetes (>60
years)
Percentage
* for adults with diabetes categories: 19-59 years and 60+ years
**Refers to health care personnel (HCP) overall; 75.3%
vaccination rate among HCP with direct patient care; 50.9%
among HCP without direct patient care
7
National Health Interview Survey (NHIS) – US, 2018
Lu et al. Vaccination Coverage Among Adults in the United States, National Health
Interview Survey, 2018. 2021 May 14;70(3):1-26. Surveillance of Vaccination
Coverage Among Adult Populations -United States, 2018 - PubMed (nih.gov)
8
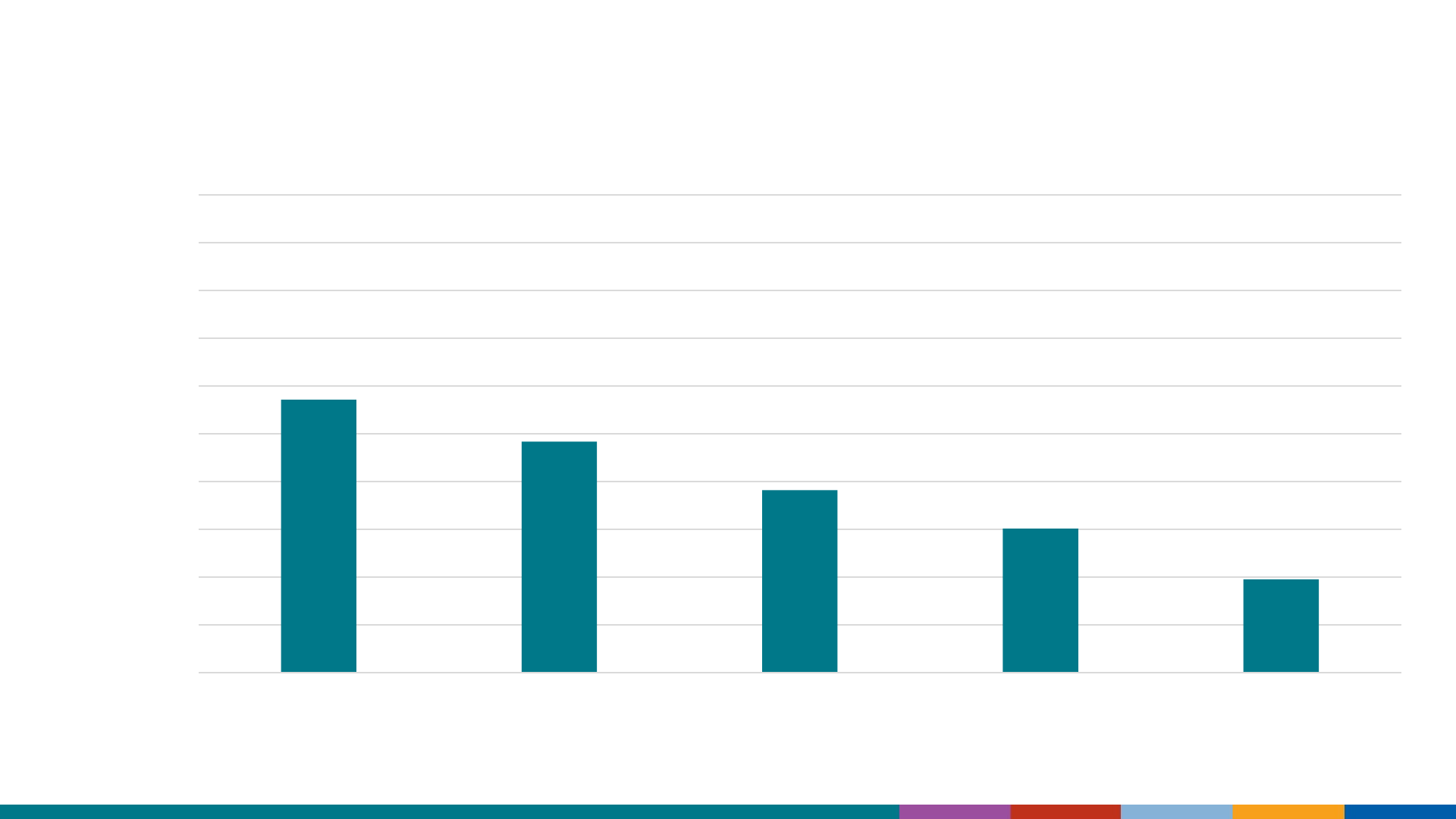
57.1
95% CI (53.7-60.4)
48.3
(45.3-51.3)
38.2
(35.2-41.2)
30.1
(27.6-32.8)
19.5
(18.0-21.1)
0
10
20
30
40
50
60
70
80
90
100
19-29 30-39 40-49 50-59 60+
Percentage
8
*Risk Factors: Diabetic, has chronic liver disease, OR traveled to HBV endemic country
Lu et al. National Health Interview Survey, 2018. Unpublished 2021.
Age (years)
HepB vaccination coverage decreased in older-aged adults
with ≥1 risk factor*

9
Limitations of risk-based approach
Availability of information regarding risk behaviors or exposures
associated with reported cases of acute hepatitis B virus infection
— US, 2019
2/3 of reported cases
were either missing
risk data or reported
no identified risk
Source: https://www.cdc.gov/hepatitis/statistics/2019surveillance/index.htm

10
Health equity: Disparities could be reduced with a
universal adult HepB recommendation
Rates of HBV infection for children and adolescents of all races/ethnicities converged to a
lower rate when a universal vaccination strategy was implemented for children ≤18y.
1, 2
Current rates among Black American adults are now up to 3x those of Asian/Pacific
Islander and Hispanic groups.
1
Racial/ethnic disparities remain in hepatitis B virus infections
Rates of reported
acute hepatitis B
virus infections, by
race/ ethnicity —
United States,
2004–2019
1. https://www.cdc.gov/hepatitis/stati
stics/2019surveillance/HepB.htm
2. Wasley et al. MMWR. 2008
3. Harris et al. MMWR. 2016

11
Health equity: Disparities could be reduced with a
universal adult HepB recommendation
Risk-based recommendations favor individuals with:
Consistent access to preventive health services
Trust to disclose potentially stigmatizing risk factor(s)
Awareness of risk (e.g., infected household contact or sex partner)
Health literacy

ACIP Hepatitis Work Group:
Responses to Committee Comments from Prior ACIP Meetings
1. Can universal recommendations increase vaccine
uptake among people with risk factors?
2. Is a universal HepB vaccination recommendation an
effective use of resources?
3. Should the proposed HepB recommendation include
adults of all ages?
• Compare with adding an upper age limit at ≤59 years and resuming
the existing risk-based recommendation for persons >59 years

1. Can universal recommendations increase vaccine
uptake among people with risk factors?
Risk-based Cohort “Universal” Cohort
Vaccine
Date of relevant recommendation
Coverage
(95% CI)
Coverage
(95% CI)
Flu
2010
25–64y +high risk
conditions
1
2009-10 season
28.6%
(±1.1)
51.0%
(± 1.4)
18−64 years +high risk conditions
1
2020-21 season
Pneumococcal
2012
19−64y at increased
risk
2
2018
23.3%
(22.0-24.6)
69.0%
(67.5-70.4)
≥65y
2
2018
HepB-BD
2005
Newborns
3
1/2003 – 6/2005
50.1%
(±1.1)
79.6%
(78-81)
birth year 2018
4
1
CDC FluVaxView
2
NHIS 2018. NHIS captures “any” pneumococcal vaccination; risk-based recommendation includes
groups with different pneumococcal recommendations.
3
Allred, NJ et al CDC MMWR 2008. Birth Dose, to 3 days from birth
4
CDC ChildVaxView, HepB Birth Dose by Age 0-3 Days

14
Advantages
Patient: Reduce stigma, barriers
• Remove need to disclose risk factors
Provider: Simpler recommendation;
easier implementation
Practice: Eliminate hepatitis B
nationally and globally
Advance health equity goals
Limitations
Level of future increased vaccine
uptake is not known
However, can infer magnitude from
public health experience with other
vaccines
1. Can universal recommendations increase vaccine
uptake among people with risk factors? Yes.

15
In a WG straw poll,
100% said “Yes”
1. Can universal recommendations increase vaccine
uptake among people with risk factors?

16
ICER: $153,000 per QALY gained
1
• ICER decreases as coverage improves in groups at higher risk*
Conservative economic model was presented, estimating health
improvements from universal adult HepB vaccination
• Reduce acute HBV infections by 24%
• Reduce HBV-related deaths by 23%
1
Hall et al, ACIP Presentation, Feb 2021. Assumptions: 3-dose vaccine; base case summary input of~30% coverage (based on 35.8%
protected, with varying age-group specific coverages among people with risk factors; 50% vaccination coverage in general population)
*With 20% additional coverage in high-risk groups, the $/QALY was $135,000, illustrating the benefits of increased access
2. Is a universal HepB vaccination
recommendation an effective use of resources?

17
In a WG straw poll,
70% said “Yes”
30% said “Probably Yes”
2. Is a universal HepB vaccination recommendation
an effective use of resources?

18
1
Hall et al 2021. Single model run applied to age ≤59y
2
Assumptions: 3-dose vaccine, base case: 50% vaccination coverage in general population; ~30% coverage (summary input based on
35.8% protected, with varying age-group specific coverages ) among people with risk factors
Subanalysis
1
(≤59y) Base Case
2
(all adults)
ICER per QALY gained $117,000 $153,000
Total incremental cost (2019 USD) ~$22 billion ~$32 billion
NNV to avert an acute infection 271 372
Doses given 298 million 352 million
Increase persons protected by 61% 89%
Reduce acute HBV infections by 23% 24%
3. Should the proposed recommendation include adults of all ages?
vs. including an upper age limit at ≤59 years

19
Advantages
HBV can still cause significant disease in
adults >59y
• Many adults will acquire risk factors as they
age (diabetes, renal disease)
• Immunize before acquiring comorbidities that
reduce response
Simplified implementation is likely to
be followed by patients, providers
Improve health equity across all ages
Limitations
Lower incidence among age >59y
(higher ICER for older populations)
Improved specificity with age limit
• Risk-based recommendation still needed
for adults >59y
Difficult to pinpoint future vaccine
uptake
3. Should the proposed recommendation include adults of all ages?
vs. including an upper age limit at ≤59 years

20
In a WG straw poll,
56% felt an age cut-off should
NOT be applied
3. Should the proposed recommendation include all ages?
vs. including an upper age limit at ≤59 years
• One-time HepB completion gives lifetime protection
• Mitigate dynamic risk
• Decreasing immune response at upper extremes of age

21
WG Summary
Preferred Adult HepB Recommendation
Universal 100%
Current
risk-based
0%

22
WG Summary:
HHS and NASEM
1
have called for viral hepatitis elimination
Evidence supports where universal recommendations are preferred
over risk-based vaccination approaches
More vaccine tools available than when risk-based policy was first
recommended
• Two 3-dose monovalent vaccines are available; safe, effective with long-term immunogenicity (>35 y)
• One 2-dose vaccine is available; safe and effective
• One vaccine in the pipeline
Universal hepatitis B vaccination recommendation among adults would
provide best chance of achieving HBV elimination goals
1. National Academies of Science, Engineering, and Medicine. https://www.nationalacademies.org/our-work/a-national-strategy-for-the-elimination-of-hepatitis-b-and-c

Proposed Recommendation
All adults previously unvaccinated for hepatitis B
should receive hepatitis B vaccination.

Thank you
24
The findings and conclusions in this report are those of the authors and do not necessarily represent the official
position of the Centers for Disease Control and Prevention.
