Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Centers for Disease Control and Prevention
National Center for Immunization and Respiratory Diseases
Photographs and images included in this presentation are licensed solely for CDC/NCIRD online and presentation use. No rights are implied or extended for use in printing or any use by other CDC CIOs or any external audiences.
Pneumococcal Disease and Pneumococcal Vaccines
Andrew Kroger, M.D., M.P.H.
Medical Educator
Pink Book Webinar Series
September 24, 2018

Second most common cause of vaccine-preventable death in the U.S.
Major clinical syndromes
– Pneumonia
– Bacteremia
– Meningitis
Pneumococcal Disease

Invasive Pneumococcal Disease
Incidence by Age Group–2013*
*CDC Active Bacterial Core surveillance 2009 report:
http://www.cdc.gov/abcs/reports-findings/survreports/spneu13.html
0
5
10
15
20
25
30
35
<1 1 2-4 5-17 18-34 35-49 50-64 65+
Rate/100,000 pop.
Age Group (Yrs)
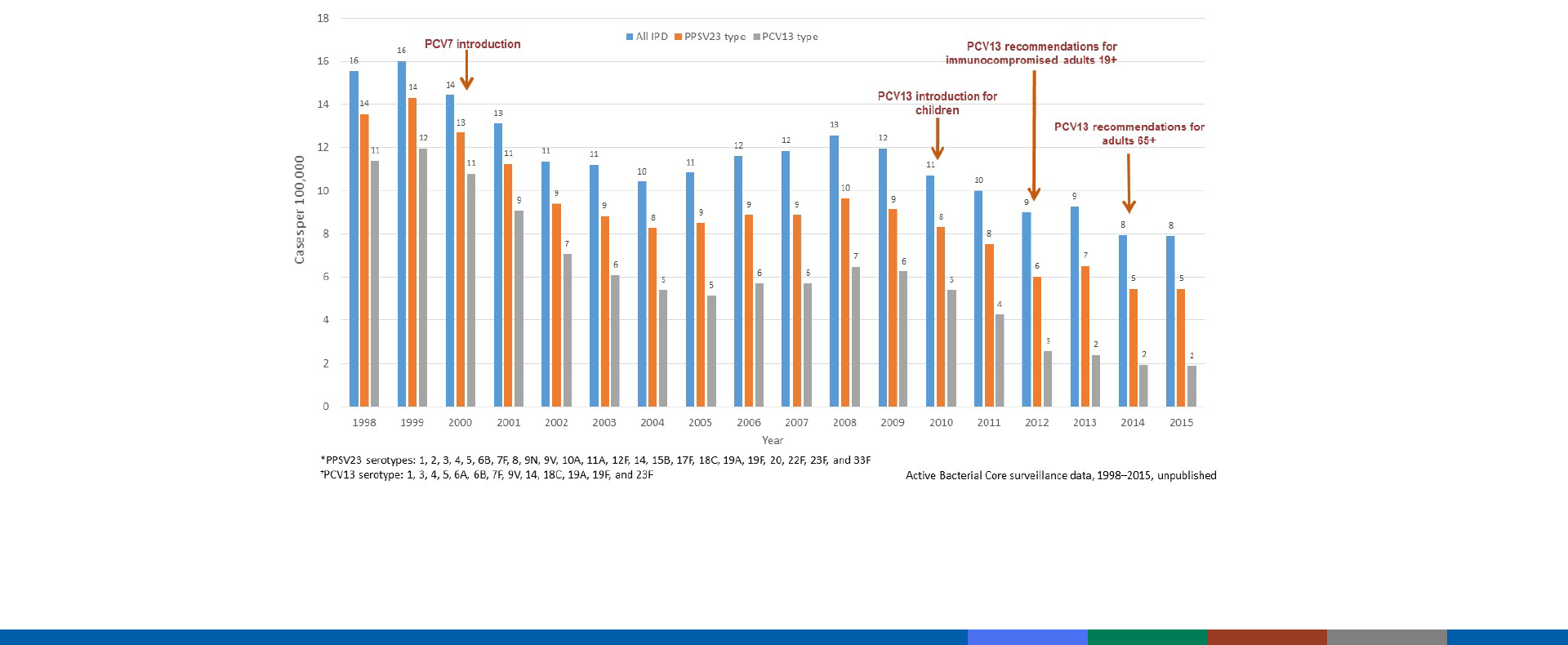
Trends in Invasive Pneumococcal Disease Among
Adults 19–64 Years of Age, 1998–2015
http://www.cdc.gov/abcs/reports-findings/survreports/spneu-types.html

Functional or anatomic asplenia, including sickle-cell disease
Altered immunocompetence
Underlying medical conditions, including chronic renal disease,
nephrotic syndrome, and CSF leak
Cigarette smoking (adults 19 years and older)
Cochlear implant
Risk Factors for Invasive
Pneumococcal Disease

Reservoir
Human carriers
Transmission
Respiratory and autoinoculation
Temporal pattern
Winter and early spring
Communicability
Unknown; probably as long as
organism in respiratory secretions
Pneumococcal Disease Epidemiology

1977
14-valent polysaccharide vaccine licensed
1983
23-valent polysaccharide vaccine licensed
(PPSV23)
2000
7-valent polysaccharide conjugate vaccine
licensed (PCV7)
2010
13-valent polysaccharide conjugate
vaccine licensed (PCV13)
Pneumococcal Vaccines

Purified capsular polysaccharide antigen from 23 types of
pneumococcus
Not effective in children younger than 2 years
Pneumococcal Polysaccharide Vaccine (PPSV23)
Characteristics

Contains 13 serotypes of S. pneumoniae conjugated to nontoxic
diphtheria CRM197 carrier protein
Approval based on demonstration of immunologic noninferiority to
PCV7 rather than clinical efficacy
Pneumococcal Conjugate Vaccine (PCV13)
Characteristics
MMWR 2010;59(No. 9):258-61

PCV7 introduced into routine schedule 2000
PCV7 Introduction Among U.S. Children and
its Impact on Invasive Pneumococcal Disease
Moore, IDSA, 2009 and CDC, unpublished data
0
10
20
30
40
50
60
70
80
90
100
1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009
Cases per 100,000
Year
Rates of IPD Among Children <5 yrs old
Overall -76 (-79,-73)
PCV7 type -100 (-100,-99)
PCV7 Introduction

Pneumococcal Conjugate Vaccine
(PCV13) in Children
In 2008, 61% of invasive pneumococcal disease cases among children younger than
5 years were attributable to the serotypes included in PCV13

In 2013, 20%-25% of invasive pneumococcal disease cases among
adults 65 years old and older were attributable to PCV13 serotypes
10 percent of community-acquired pneumonia in adults due to PCV13
serotypes (Pfizer urine studies)
Pneumococcal Conjugate Vaccine (PCV13)
in Adults

Most estimates range between 60%-70% effective against invasive
disease among immunocompetent older persons and adults with
underlying illnesses
Effectiveness among immunocompromised or very old persons not
demonstrated
Pneumococcal Polysaccharide Vaccine
(PPSV23)Immunogenicity/Effectiveness

Highly immunogenic in infants and young children, including those with
high-risk medical conditions
PCV7 was 97% effective against invasive disease caused by vaccine
serotypes (presumably PCV13 as well)
Pneumococcal Conjugate Vaccine (PCV13)
Immunogenicity/Efficacy

New Evidence Supporting PCV13 use among Adults,
CAPiTA Results
CAPiTA, ACIP, June 2014
Study/Population Endpoint
Vaccine Efficacy
(95% CI)
CAPiTA
~85,000 Adults
65+
Netherlands
PCV13-
serotype IPD
75% (41%, 91%)
PCV13-
serotype
nonbacteremic
pneumonia
45% (14%, 65%)


PCV13 is approved by the Food and Drug Administration for:
– Children 6 weeks through 17 years of age
– Adults 50 years of age and older
ACIP recommended use of PCV13 for immunocompromised persons 6
years and older (2012, 2013)
ACIP recommended use of PCV13 for all adults 65 years or older in
2014
PCV13 Licensure

PCV13 in Children

Routine vaccination recommendation for children 2–59 months of age
– 4 doses at 2, 4, 6, and 12 to 15 months
– Fewer doses if series started at 7 months of age or older
Children who have received 1 or more doses of PCV7 should complete
the immunization series with PCV13
ACIP Recommendations for PCV13
MMWR 2010;59(No. 9):258-61

Age at First Dose # of Doses Booster
7-11 months 2 doses Yes
12-23 months 2 doses* No
24-59 months 1 dose No
24-71 months, medical conditions** 2 doses* No
Pneumococcal Conjugate Vaccine Schedule for
Unvaccinated Older Children–Primary Series
*Separated by at least 8 weeks; see
MMWR
2010;59(RR-11):1–19
**Chronic heart, lung disease, diabetes, CSF leak, cochlear implant, sickle cell disease, other
hemoglobinopathies, functional or anatomic asplenia, HIV infection, immunocompromising conditions

A single supplemental dose of PCV13 is recommended for children who
have received a complete age-appropriate series of PCV7:
– Healthy children 14 through 59 months
– Children 14 through 71 months with an underlying medical condition (including
those who have already received a dose of PPSV)
ACIP Recommendations for PCV13
Supplemental Dose
MMWR 2010;59(No. 9):258-61

Children aged 24–71 months with underlying medical conditions who
received an incomplete schedule of PCV7 should receive 2 doses of
PCV13 (8 weeks apart)
ACIP Recommendations for PCV13
Children

A dose of PCV13 should be administered to children 6 through 18 years
of age who are at increased risk for invasive pneumococcal disease*
(and no prior PCV13 doses)
– Functional or anatomic asplenia, including sickle cell disease
– HIV infection and other immunocompromising conditions
– Cochlear implant
– CSF leak
Regardless of previous history of PCV7 or PPSV vaccine
ACIP Recommendations for
PCV13 Dose
*Off-label recommendation, ACIP vote, February 20, 2013

PCV13 Use in Adults

Licensed for use among adults >50 years old on 12/30/11
FDA approved under the Accelerated Approval Pathway
Based on noninferior immunogenicity compared to PPSV23
Postapproval condition of licensure:
– Randomized controlled trial of PCV13 against pneumococcal pneumonia among
adults >65 years old in the Netherlands
PCV13 for Adults

ACIP now recommends PCV13 for adults 65 years old and older
Some adults have received PCV13 already
PCV13 for Adults (2014)

Incidence of IPD in Adults Aged 18-64 Years with Selected
Underlying Conditions, United States, 2009
8
26
28
32
41
52
59
173
186
0
20
40
60
80
100
120
140
160
180
200
HEALTHY CVD DIABETES PULMONARY KIDNEY LIVER ALCOHOL HIV/AIDSHEMATOLGICAL
CANCER
Cases per 100,000 persons
3-7 fold
increased risk
20 fold
increased risk
Unpublished data, Active Bacterial Core surveillance, 2009
HEMATOLOGICAL
CANCER

Adults 19 years of age or older with:
– Immunocompromising conditions
– Functional or anatomic asplenia
– CSF leaks
– Cochlear implants
Those who have not previously received PCV13 or PPSV23
should receive a single dose of PCV13 followed by a dose of PPSV23 at
least 8 weeks later, with a booster dose of PPSV23 5 or more years
later
PCV13 for Immunocompromised Adults*
*MMWR. October 12, 2012 ; 61(40):816-819

PPSV23 Use in Children and Adults

Persons 2 years and older with normal immune systems who have
chronic illness including:
Persons in environments or settings with increased risk
Pneumococcal Polysaccharide Vaccine Recommendations
Cardiovascular disease Alcoholism
Pulmonary disease (asthma if 19 years
old or older)
Smoking (19 years old or older)
Diabetes CSF leak
Liver disease Cochlear implant

Persons 2 years and older who are immunocompromised (due to
disease or treatment)
– Asplenia (functional or anatomic)
– Chronic renal failure
– Nephrotic syndrome
– Hodgkin disease
– Lymphoma and leukemia
– Multiple myeloma
– Organ transplant
– HIV infection
Pneumococcal Polysaccharide Vaccine Recommendations

Routine revaccination of immunocompetent persons is not
recommended
Revaccination recommended for persons 2-64 years of age who are at
highest risk of serious pneumococcal infection
Pneumococcal Polysaccharide Vaccine Revaccination
MMWR 2010;59(No.34):1102-5

5-year interval (2-64 years) with additional dose after 65th birthday, 5
years after previous dose:
– Functional or anatomic asplenia (including sickle cell disease)
– Immunosuppression (including HIV infection)
– Transplant
– Chronic renal failure
– Nephrotic syndrome
1 dose is recommended after the 65th birthday, but only 1 dose
recommended after 65th birthday
Pneumococcal Polysaccharide Vaccine
Candidates for Revaccination
MMWR 2010;59(No.34):1102-5 and 2010;59(RR-11)

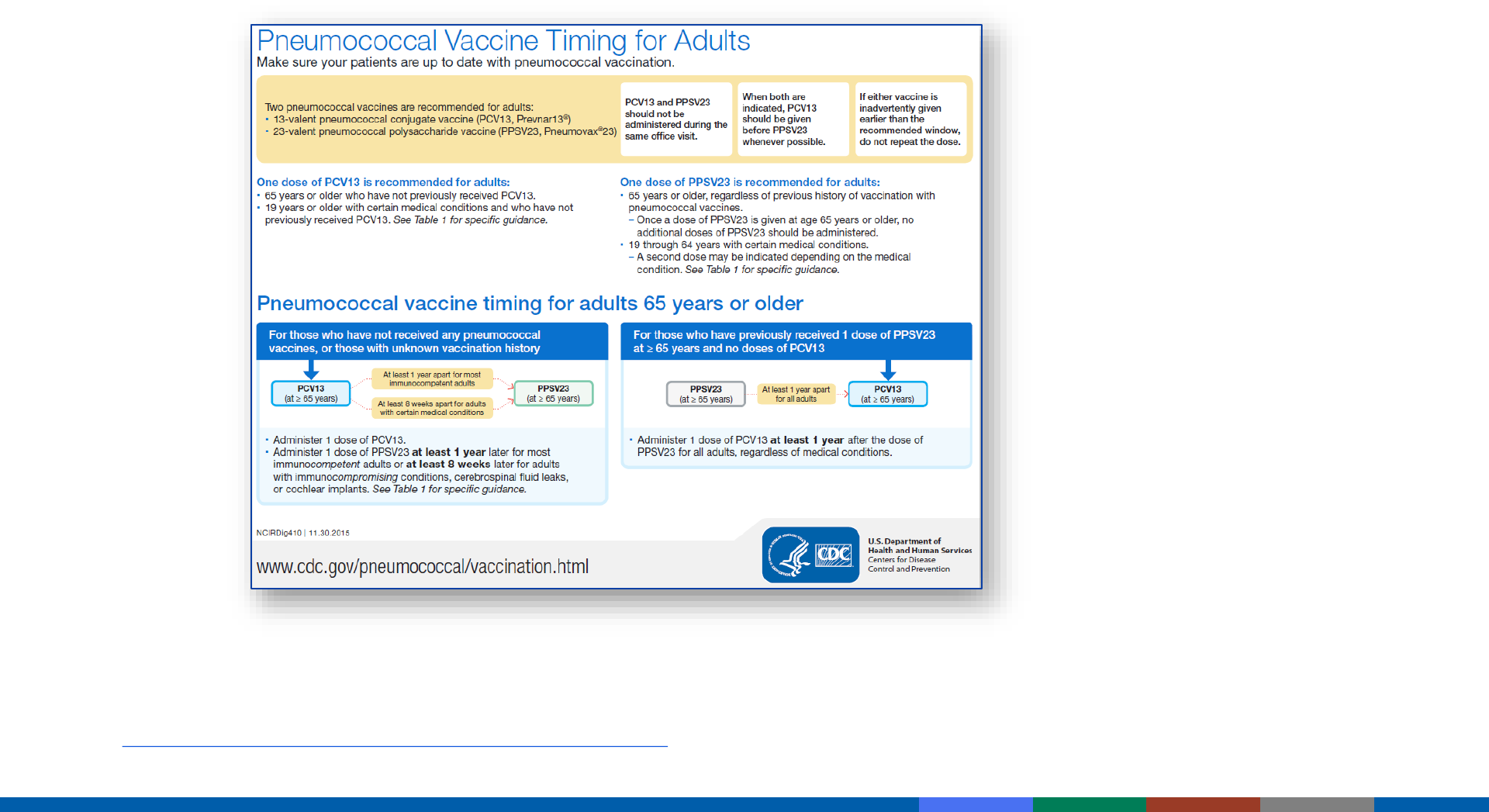
PCV13 and PPSV23 should not be administered during the same clinic
visit
– Either vaccine may be administered simultaneously with influenza vaccine
Administer PCV13 before PPSV23 whenever possible
Administering PCV13 and PPSV23 Vaccines
General Rules

PCV13 and PPSV23 for Adults 19 – 64 Years
Immunocompromised, asplenic (sickle cell, hemoglobinopathy),
CSF leaks, cochlear implants who are pneumococcal-naive
>8 weeks
PCV13
PPSV23
+ + PPSV23*
PPSV23
(@ >65 years)
+
>5 years
>5 years
*Second PPSV23 dose before age 65 years NOT recommended for adults with CSF leaks or
those with cochlear implants
*ACIP off-label recommendation for PCV13 for adults 19 through 49 years of age

PCV13 and PPSV23 for Adults 19 – 64 Years
Immunocompromised, asplenic (sickle cell, hemoglobinopathy),
CSF leaks, cochlear implants
who have previously received PPSV23
>1 year
PPSV23
PCV13
+ + PPSV23*
PPSV23
(@ >65 years)
+
>8 weeks
>5 years
*Second PPSV23 dose before age 65 years NOT recommended for adults with CSF leaks or
those with cochlear implants
*ACIP off-label recommendation for PCV13 for adults 19 through 49 years of age
>5 years
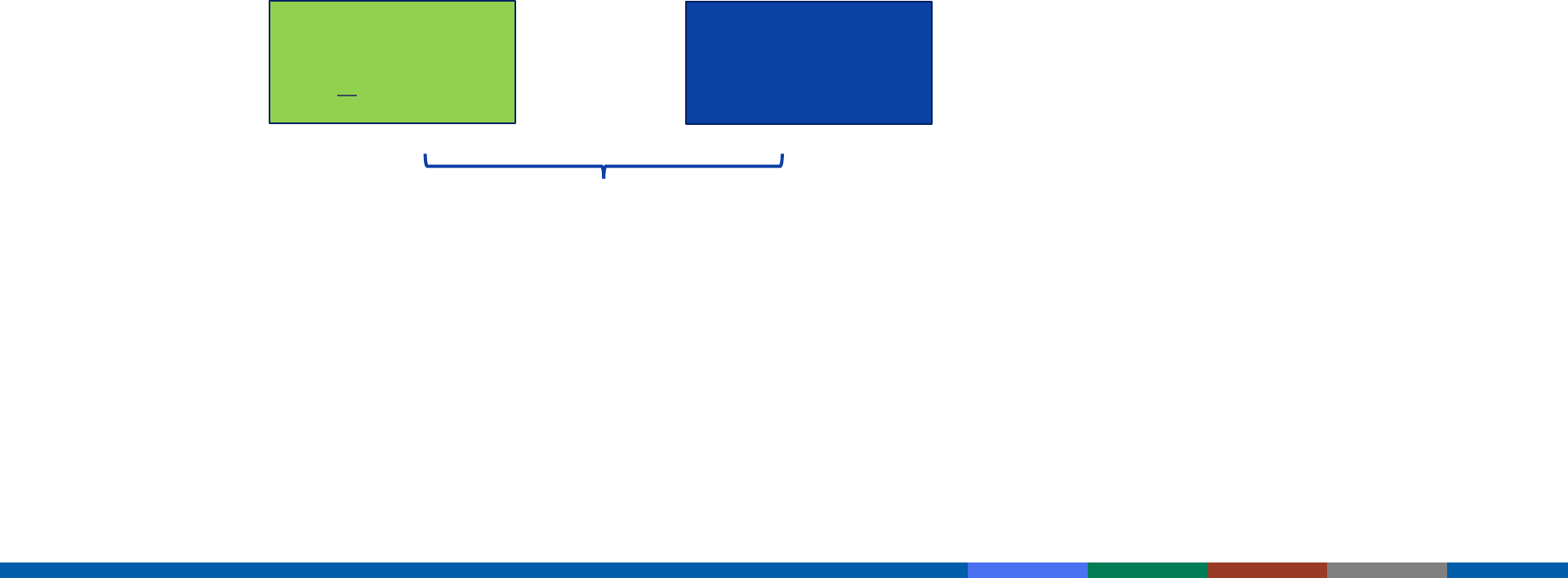
PCV13 and PPSV23 for Adults 65 Years and Older
Pneumococcal-naïve or unknown vaccination history
Healthy adult
12 months
PCV13
(@ >65 years)
PPSV23
+
If PPSV23 cannot be given at 12 months later, it should be
given during the next visit

PCV13 and PPSV23 for Adults 65 Years and Older
Pneumococcal-naïve or unknown vaccination history
High-risk immunocompromised adult
8 weeks
PCV13
(@ >65 years)
PPSV23
+

PCV13 and PPSV23 for Adults 65 Years and Older
Previously received 1 or more doses of PPSV23
High-risk immunocompromised adult
> 1 year
PPSV23*
(@ > 65 years)
PCV13
+
PPSV23*
(@ < 65 years)
+
PCV13
(@ > 65 years)
PPSV23*
(@ > 65 years)
+
> 1 year
> 8 weeks
> 5 years
*Doses already administered

Severe allergic reaction to vaccine component or following prior dose
of vaccine
Moderate or severe acute illness
Pneumococcal Vaccines
Contraindications and Precautions

Administer PCV13 vaccine via intramuscular (IM) injection
– Needle gauge: 22–25 gauge
– Needle length*: 5/8 – 1.5 inch depending on the patient’s age and/or weight
– Site*:
• Birth–11 months: Vastus lateralis muscle is preferred
• 1–2 years: Vastus lateralis muscle is preferred; deltoid muscle may be used if the
muscle mass is adequate
• 3 years and older: Deltoid muscle is preferred; vastus lateralis muscle may be
used
Administer at the same medical visit as other vaccines, except Men
ACWY-D in asplenic persons (others, OK to administer)
Pneumococcal Conjugate (PCV13)
Vaccine Administration
*Professional judgement should be used to determine the proper needle length and site. Factors influencing site including local reaction, number of vaccine to be
administered age and muscle mass

PPSV23 maybe be administered by IM or subcutaneous injection
– IM injection
• Needle gauge: 22–25 gauge
• Needle length*: 1–1.5 inch depending on the patient’s age and/or weight
• 2 years: Vastus lateralis muscle is preferred; deltoid muscle may be used if the
muscle mass is adequate
• 3 years and older: Deltoid muscle is preferred; vastus lateralis muscle may be
used
– Subcutaneous injection:
• Needle gauge/length: 23–25 gauge; 5/8
th
inch needle in the upper outer triceps
area
Vaccine Administration
PPSV23
*Professional judgement should be used to determine the proper needle length and site. Influencing factors include injection technique, local reaction, number of vaccines to
be administered, patient age, size and muscle mass

PPSV23 PCV
Local reactions 30%-50% 5%-49%
Fever, myalgia <1% 24-35%
Febrile seizures ---
Rare:
1-14/100,000; with IIV 4 -45/ 100,000
Severe adverse reactions rare 8% (local)
Pneumococcal Vaccines
Adverse Reactions