
The
EMBO
Journal
vol.
13
no.
1
pp.
168
-
179,
1994
spalt
encodes
an
evolutionarily
conserved
zinc
finger
protein
of
novel
structure
which
provides
homeotic
gene
function
in
the
head
and
tail
region
of
the
Drosophila
embryo
Ronald
P.Kuhnlein,
Gotz
Frommer,
Markus
Friedrich1,
Marcos
Gonzalez-Gaitan,
Alexander
Weber1,
Juliane
F.Wagner-Bernholz2,
Walter
J.Gehring2,
Herbert
Jackle
and
Reinhard
Schuh3
Max-Planck-Institut
fur
biophysikalische
Chemie,
Abteilung
Molekulare
Entwicklungsbiologie,
Am
Fassberg,
37077
Gottingen,
lInstitut
fur
Genetik
und
Mikrobiologie
der
Universitiit
Miinchen,
Maria-Ward-Str.
la,
80638
Miinchen,
Germany
and
2Biozentrum
der
Universitat
Basel,
Abteilung
Zellbiologie,
Klingelbergstrasse
70,
CH-4056
Basel,
Switzerland
3Corresponding
author
Communicated
by
H.Jackle
The
region
specific
homeotic
gene
spalft
(sal)
of
Drosophila
melanogaster
promotes
the
specification
of
terminal
pattern
elements
as
opposed
to
segments
in
the
trunk.
Our
results
show
that
the
previously
reported
sal
transcription
unit
was
misidentified.
Based
on
P-element
mediated
germ
line
transformation
and
DNA
sequence
analysis
of
sal
mutant
alleles,
we
identified
the
transcription
unit
that
carries
sal
function.
sal
is
located
close
to
the
misidentified
transcription
unit,
and
it
is
expressed
in
similar
temporal
and
spatial
patterns
during
embryogenesis.
The
sal
gene
encodes
a
zinc
finger
protein
of
novel
structure
composed
of
three
widely
spaced
'double
zinc
finger'
motifs
of
internally
conserved
sequences
and
a
single
zinc
finger
motif
of
different
sequence.
Antibodies
produced
against
the
sal
protein
show
that
sal
is
first
expressed
at
the
blastoderm
stage
and
later
in
restricted
areas
of
the
embryonic
nervous
system
as
well
as
in
the
developing
trachea.
The
antibodies
detect
sal
homologous
proteins
in
corresponding
spatial
and
temporal
patterns
in
the
embryos
of
related
insect
species.
Sequence
analysis
of
the
sal
gene
of
Drosophila
viruis,
a
species
which
is
phylogenetically
separated
by
-
60
million
years,
suggests
that
the
sal
function
is
conserved
during
evolution,
consistent
with
its
proposed
role
in
head
formation
during
arthropod
evolution.
Key
words:
Drosophila
embryogenesis/homeotic
genes/spalt
gene/transcription
factors/zinc
finger
proteins
Introduction
Specification
of
segment
identity
in
the
trunk
region
of
the
Drosophila
melanogaster
embryo
requires
the
activity
of
homeotic
selector
genes
located
within
the
Antennapedia
(ANT-C)
and
the
Bithorax
(BX-C)
complexes
(Lewis,
1978;
Kaufman
et
al.,
1980).
Expression
of
the
homeotic
selector
genes
is
initiated
under
the
control
of
the
segmentation
gene
cascade
and
spatially
delimited
by
negative
regulatory
interactions
between
the
different
homeotic
genes
168
themselves,
all
of
which
encode
homeodomain
proteins
likely
to
act
as
transcription
factors
(for
reviews,
see
Gehring
and
Hiromi,
1986;
Akam,
1987;
Affolter
et
al.,
1990).
The
normal
function
of
the
Antennapedia
(Antp)
and
BX-C
genes
depends
on
the
activity
of
the
gene
teashirt
(tsh)
which
is
globally
required
for
segmental
identity
throughout
the
entire
trunk
region
(Roder
et
al.,
1992).
At
later
stages
of
development
the
spatial
expression
domains
of
homeotic
selector
genes
are
maintained
through
the
activity
of
members
of
the
Polycomb
(Pc)
group
of
genes
(Lewis,
1978;
Jiirgens,
1985).
An
additional
class
of
homeotic
genes,
the
'region
specific
homeotic
genes',
acts
in
the
terminal
regions
of
the
embryo,
specifying
pattern
elements
in
both
the
head
and
tail
regions.
spalt
(sal)
andforkhead
(jkh),
the
two
members
of
this
class
of
homeotic
genes,
are
located
on
different
chromosomes
outside
the
homeotic
selector
gene
complexes
(Jiirgens,
1988;
Jurgens
and
Weigel,
1988).
Infkh
mutants,
ectodermal
parts
of
the
gut,
i.e.
the
foregut
and
the
hindgut,
both
develop
as
ectopic
head
structures.
This
suggests
that
the
flh
gene
promotes
terminal
as
opposed
to
segmental
development
(Jurgens
and
Weigel,
1988).
Mutations
in
the
sal
gene
lead
to
incomplete
transformations
of
pattern
elements
of
the
posterior
head
and
the
anterior
tail
towards
the
trunk,
i.e.
structures
which
are
characteristic
of
the
prothorax
develop
in
the
head,
and
structures
of
the
eighth
abdominal
segment
are
formed
in
the
tail
region.
These
phenotypic
effects
within
the
head
and
the
tail
region
of
sal
mutants
seem
to
be
very
different,
but
double
mutant
analysis
of
sal
and
the
homeotic
selector
gene
Abdominal-B
(AbdB)
shows
that
sal
activity
promotes
head
as
opposed
to
trunk
development,
i.e.
AbdB/sal
double
mutants
develop
thoracic
structures
in
place
of
the
ectopic
head
structures
found
in
the
tail
region
of
AbdB
single
mutant
embryos
(Jiirgens,
1988).
Furthermore,
sal
mutations
cause
inappropriate
expression
of
the
homeotic
selector
gene
Ultrabithorax
(Ubx)
(Casanova,
1989)
and
hence
sal
may
participate
in
the
cross-regulatory
interactions
typical
among
other
homeotic
genes.
At
the
molecular
level
flh
has
been
shown
to
encode
a
DNA
binding
protein
which
is
likely
to
act
as
a
transcription
factor
with
a
conserved
DNA
binding
motif,
the
forkhead-
domain'
(Weigel
et
al.,
1989;
Weigel
and
Jackle,
1990).
The
gene,
previously
identified
to
carry
sal
function,
encodes
a
small
protein
of
142
amino
acids
which
lacks
any
known
protein
motif
(Frei
et
al.,
1988).
While
jkh-related
coding
sequences
and
homeodomain
proteins
in
particular
have
been
identified
in
other
insects
as
well
as
in
vertebrates,
the
coding
sequence
of
the
previously
identified
sal
gene
was
found
to
be
conserved
only
in
closely
related
Drosophila
species
(Reuter
et
al.,
1989).
However,
a
basic
genetic
function
which
contributes
to
the
separation
of
head
and
trunk
segments
should
be
conserved
throughout
insect
evolution,
since
the
basic
separation
of
a
primitive
head
from
the
segmented
body
region
must
have
already
occurred
in
myriapod-like
ancestors
of
the
recent
insects
(Jiirgens,
1988).
©
Oxford
University
Press

Molecular
genetics
of
the
Drosophila
spalt
gene
Based
on
this
phylogenetic
argument,
the
previously
identified
142
amino
acid
protein
was
thought
to
have
an
accessory
or
modulating
function
for
head
development
rather
than
representing
the
decisive
gene
product
required
to
separate
the
terminal
regions
from
the
trunk
(Reuter
et
al.,
1989).
Here
we
show
that,
in
fact,
sal
function
is
not
associated
with
the
142
amino
acid
protein.
Based
on
sal
mutant
rescue
by
a
transgene
and
sal
mutant
associated
alterations
of
protein
coding
sequences,
we
present
evidence
that
a
zinc
finger-
type
protein
of
novel
structure
provides
sal
function
in
D.
melanogaster.
The
sal
gene
product
and
its
expression
pattern
are
conserved
in
other
dipteran
species.
Results
sal
function
has
been
mapped
within
120
kb
of
DNA
encompassing
the
chromosomal
region
32F/33A
on
the
left
arm
of
the
second
chromosome
(Frei
et
al.,
1988;
Jiirgens,
1988),
and
it
has
been
assigned
to
a
small
transcription
unit
within
a
15
kb
genomic
DNA
fragment
(Frei
et
al.,
1988).
In
order
to
identify
molecular
lesions
associated
with
sal
loss-
of-function
mutations,
we
analysed
the
various
sal
alleles
in
molecular
detail.
In
all
of
the
five
sal
loss-of-function
alleles
(Jiirgens,
1988;
this
work,
see
Materials
and
methods),
wild-type
levels
of
transcripts in
the
correct
spatial
and
temporal
expression
patterns
were
observed
(data
not
shown).
This
suggested
that
the
molecular
lesions
causing
the
loss-of-function
mutations
may
reside
within
the
coding
sequences
of
the
transcript.
However,
DNA
sequence
analysis
revealed
the
wild-type
coding
sequence
in
all
of
the
three
sal
alleles
examined
(data
not
shown).
Thus,
a
different
transcript
from
the
previously
identified
one
is
likely
to
be
essential
for
sal
function.
To
search
for
additional
transcribed
DNA
sequences
we
examined
the
region
encompassing
the
15
kb
DNA
fragment
of
the
rescuing
transgene
by
Northern
blot
analysis.
No
additional
transcripts
or
different
splicing
forms
of
the
previously
identified
transcript
could
be
detected
(data
not
shown).
These
results
left
severe
doubts
concerning
the
assignment
of
sal
gene
function
to
the
previously
identified
transcription
unit,
consistent
with
the
observation
that
the
primary
protein
sequence
encoded
by
this
transcript
is
not
conserved
during
insect
evolution
(Reuter
et
al.,
1989).
For
this
reason,
we
repeated
the
P-element
transformation
experiments
involving
the
15
kb
genomic
DNA
as
reported
previously
(Frei
et
al.,
1988).
In
the
previous
rescue
experiments,
the
P-element
construct
containing
15
kb
of
DNA
of
the
sal
region
was
injected
directly
into
embryos
of
the
salIA55
cn
bw
sp/CyO;
ry-506/ry5O6
genotype,
and
single
eclosed
males
or
females
were
mated
with
salIIB57
cn
bw
sp/CyO;iy5-6/ry506
flies.
Two
independent
ry+
transgenic
lines
were
analysed
in
more
detail
and
they
suggested
a
rescue
of
the
embryonic
sal
phenotype
due
to
the
integrated
DNA.
To
exclude
an
experimental
artifact
or
error
in
this
experimental
design
as
a
source
of
the
'rescuing
activity',
we
altered
the
experi-
mental
design
by
injecting
the
P-element
construct
into
ry56l/ry506
embryos.
Two
transgenic
lines
were
established
and
the
gene
activity
of
the
transgene
was
analysed
in
sal
lack
of
function
mutant
background.
No
signs
of
rescuing
activity
coming
from
the
transgene
could
be
observed
in
these
lines.
These
experiments
strongly
suggest
that
the
previously
identified
sal
transcript
does
not
carry
sal
function
as
defined
by
the
mutant
phenotype.
For
reasons
described
below
we
refer
to
it
as
the
sal
adjacent
(sala)
transcript.
Identification
of
the
sal
transcription
unit
In
search
of
a
transcription
unit
that
encodes
sal
function
we
examined
DNA
fragments
encompassing
the
120
kb
sal
region
by
in
situ
hybridization
to
whole
mount
Drosophila
embryos.
Close
to
the
chromosomal
break
point
delimiting
the
sal
region
proximally,
we
found
a
transcript
encoded
by
F4.5
DNA
(Figure
1;
see
also
Frei
et
al.,
1988)
which
is
expressed
in
spatial
and
temporal
patterns
similar
to
those
of
sala.
As
shown
in
Figure
lBa,
F4.5
expression
is
found
in
three
distinct
regions
of
cellular
blastoderm
embryos.
Transcripts
are
forming
an
anterior
(60-70%
of
egg
length)
and
a
posterior
(12-20%
of
egg
length)
stripe
in
positions
corresponding
to
the
precursors
of
the
pattern
elements
which
are
affected
in
sal
mutant
embryos
(Jiirgens,
1988),
and
in
a
dorsally
localized
'horse-shoe
domain'
in
the
presumptive
pregnathal
head
region
(80-86%
of
egg
length).
A
first
hint
that
the
F4.5
transcript
may
carry
sal
function
is
derived
from
examination
of
the
lacZ
enhancer-detection
strain
A405.
1M2
(Bellen
et
al.,
1989).
In
this
strain,
the
DNA
of
the
enhancer
trap
construct
resides
within
DNA
sequences
corresponding
to
clone
F4.5
(Wagner-Bernholz
et
al.,
1991).
Embryos
containing
this
lacZ
reporter
gene
show
localized
,3-galactosidase
expression
in
patterns
that
correspond
to
the
patterns
of
F4.5
expression
(Beilen
et
al.,
1989).
Furthermore
the
A405.
1M2
lacZ
chromosome
failed
to
complement
sal
lack
of
function
mutations
(Wagner-
Bernholz
et
al.,
1991).
To
test
whether
the
lacZ
insertion
has
caused
the
sal
mutation
we
performed
P-element
'jump-out
experiments'
(Cooley
et
al.,
1988).
The
removal
of
the
P-element
from
its
site
of
insertion
resulted
in
a
reversion
of
the
sal
allele
to
wild-type
(data
not
shown),
indicating
that
the
lacZ
insertion
has
caused
the
sal
mutation
which
we
refer
to
as
saVA405.
In
order
to
identify
the
F4.5
transcription
unit
as
the
one
that
carries
sal
function,
we
employed
a
P-element
mediated
germ
line
transformation
and
sequence
analysis
of
DNA
encoding
the
F4.5
transcript
of
sal
mutant
alleles.
A
26
kb
DNA
fragment
that
contains
the
F4.5
transcription
unit
and
16
kb
of
non-transcribed
sequences
(Figure
1A)
was
used
to
generate
a
P-element
construct,
termed
P[C20-sa126].
When
inserted
into
the
fly
germ
line,
the
P[C20-sa126]
transgene
rescues
the
allelic
combination
sal/4405salIIBS7that
produces
a
weak
sal
phenotype
in
fertile
flies.
Furthermore,
embryos
homozygous
for
a
sal
loss-of-
function
mutation,
such
as
salIIB57(JUrgens,
1988),
develop
a
normal
head
region,
and
the
tail
phenotype
is
partially
rescued
in
response
to
the
P[C20-sa126]
transgene
(for
details,
see
Figure
2).
These
results
indicate
that
the
P[C20-sa126]
transgene
contains
sal
function.
We
also
analysed
F4.5
expression
and
the
sequence
of
the
F4.5
coding
region
of
three
different
sal
loss-of-function
alleles
of
known
genetic
origin
(see
Materials
and
methods).
In
embryos
homozygous
for
the
alleles
sal445
(Jiirgens,
1988),
sal16
and
sal65
(this
work,
see
Materials
and
methods),
the
F4.5
transcript
is
expressed
in
patterns
and
at levels
indistinguishable
from
wild-type
(data
not
shown).
However,
such
embryos
lack
the
expression
of
the
corresponding
protein
as
revealed
by
specific
antibody
stainings
(see
below).
These
observations
suggest
that
each
of
the
three
independent
sal
mutations
resides
within
the
169
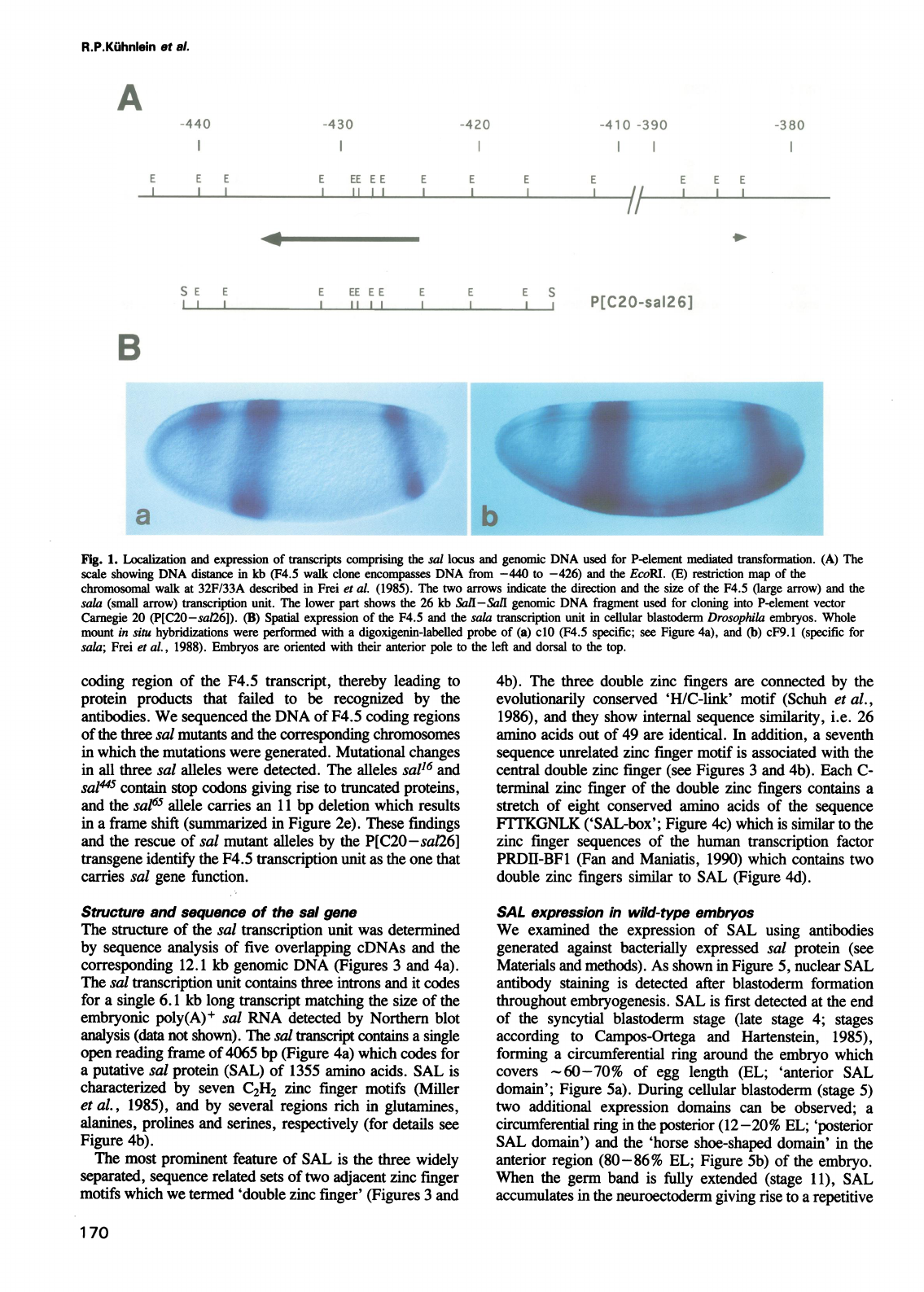
-430
420
E
EE
E
E
E
E
E
E
II
E
EE
EE
E
E
E
S
I
II
P[C20-sal26]
Fig.
1.
Localization
and
expression
of
ftransripts
comprising
the
sal
locus
and
genomic
DNA
used
for
P-element
mediated
transformation.
(A)
The
scale
showing
DNA
distance
in
kb
(F4.5
walk
clone
encompasses
DNA
from
-440
to
-426)
and
the
EcoRI.
(E)
restriction
map
of
the
chromosomal
walk
at
32F/33A
described
in
Frei
et
aL.
(1985).
The
two
arrows
indicate
the
direction
and
the
size
of
the
F4.5
(large
arrow)
and
the
sala
(small
arrow)
ftrascription
unit.
The
lower
part
shows
the
26
kb
Sail-Sall
genomic
DNA
fragment
used
for
cloning
into
P-element
vector
Carnegie
20
(P[C20-sa126]).
(B)
Spatial
expression
of
the
F4.5
and
the
sala
transription
unit
in
cellular
blastoderm
Drosophila
embryos.
Whole
mount
in
situ
hybridizations
were
performed
with
a
digoxigenin-labelled
probe
of
(a)
cdO
(F4.5
specific;
see
Figure
4a),
and
(b)
cF9.
1
(specific
for
sala;
Frei
et
al.,
1988).
Embryos
are
oriented
with
their
anterior
pole
to
the
left
and
dorsal
to
the
top.
coding
region
of
the
F4.5
transcript,
thereby
leading
to
protein
products
that
failed
to
be
recognized
by
the
antibodies.
We
sequenced
the
DNA
of
F4.5
coding
regions
of
the
three
sal
mutants
and
the
corresponding
chromosomes
in
which
the
mutations
were
generated.
Mutational
changes
in
all
three
sal
alleles
were
detected.
The
alleles
sal16
and
sal445
contain
stop
codons
giving
rise
to
truncated
proteins,
and
the
sal65
allele
carries
an
11
bp
deletion
which
results
in
a
frame
shift
(summarized
in
Figure
2e).
These
findings
and
the
rescue
of
sal
mutant
alleles
by
the
P[C20-sal26]
transgene
identify
the
F4.5
transcription
unit
as
the
one
that
carries
sal
gene
function.
Structure
and
sequence
of
the
sal
gene
The
structure
of
the
sal
transcription
unit
was
determined
by
sequence
analysis
of
five
overlapping
cDNAs
and
the
corresponding
12.1
kb
genomic
DNA
(Figures
3
and
4a).
The
sal
transcription
unit
contains
three
introns
and
it
codes
for
a
single
6.1
kb
long
transcript
matching
the
size
of
the
embryonic
poly(A)+
sal
RNA
detected
by
Northern
blot
analysis
(data
not
shown).
The
sal
transcript
contains
a
single
open
reading
frame
of
4065
bp
(Figure
4a)
which
codes
for
a
putative
sal
protein
(SAL)
of
1355
amino
acids.
SAL
is
characterized
by
seven
C2H2
zinc
finger
motifs
(Miller
et
al.,
1985),
and
by
several
regions
rich
in
glutamines,
alanines,
prolines
and
serines,
respectively
(for
details
see
Figure
4b).
The
most
prominent
feature
of
SAL
is
the
three
widely
separated,
sequence
related
sets
of
two
adjacent
zinc
finger
motifs
which
we
termed
'double
zinc
finger'
(Figures
3
and
4b).
The
three
double
zinc
fingers
are
connected
by
the
evolutionarily
conserved
'H/C-link'
motif
(Schuh
et
al.,
1986),
and
they
show
internal
sequence
similarity,
i.e.
26
amino
acids
out
of
49
are
identical.
In
addition,
a
seventh
sequence
unrelated
zinc
finger
motif
is
associated
with
the
central
double
zinc
finger
(see
Figures
3
and
4b).
Each
C-
terminal
zinc
finger
of
the
double
zinc
fingers
contains
a
stretch
of
eight
conserved
amino
acids
of
the
sequence
FTTlKGNLK
('SAL-box';
Figure
4c)
which
is
similar
to
the
zinc
finger
sequences
of
the
human
transcription
factor
PRDII-BFI
(Fan
and
Maniatis,
1990)
which
contains
two
double
zinc
fingers
similar
to
SAL
(Figure
4d).
SAL
expression
in
wild-type
embryos
We
examined
the
expression
of
SAL
using
antibodies
generated
against
bacterially
expressed
sal
protein
(see
Materials
and
methods).
As
shown
in
Figure
5,
nuclear
SAL
antibody
staining
is
detected
after
blastoderm
formation
throughout
embryogenesis.
SAL
is
first
detected
at
the
end
of
the
syncytial
blastoderm
stage
(late
stage
4;
stages
according
to
Campos-Ortega
and
Hartenstein,
1985),
forming
a
circumferential
ring
around
the
embryo
which
covers
-60-70%
of
egg
length
(EL;
'anterior
SAL
domain';
Figure
5a).
During
cellular
blastoderm
(stage
5)
two
additional
expression
domains
can
be
observed;
a
circumferential
ring
in
the
posterior
(12-20%
EL;
'posterior
SAL
domain')
and
the
'horse
shoe-shaped
domain'
in
the
anterior
region
(80-86%
EL;
Figure
Sb)
of
the
embryo.
When
the
germ
band
is
fully
extended
(stage
11),
SAL
accumulates
in
the
neuroectoderm
giving
rise
to
a
repetitive
170
R.P.Kuhnlein
et
a!.
A
-440
E
E
E
l
-4
1
0
-3
90
-3
8()
S
E
E
I
I
I
7E
11i
B

Molecular
genetics
of
the
Drosophila
spalt
gene
on
CCG
CTG
CGA
wt
P
L
R
328
CCG
CTG
TGA
sal
65
P
L
Stop
t-
GAT
CAA
ATG
TCG
CCC
ACG
GAT
AGC
D
Q
S
P
T
D
S
528
GAT
CA-
---
---
---
-C
GGA
TAG
D
H
G
Stop
on
COOH
I
wt
TCG
CCT
CAA
s
P
Q
595
sal
445
TCG
CCT
TAA
S P
Stop
Fig.
2.
Rescue
of
sal
mutant
embryos
by
germ
line
transformation
and
molecular
lesions
in
the
DNA
of
amorphic
sal
mutant
alleles.
Comparison
of
dark-field
cuticle
preparations
of
wild-type
(a),
amorphic
sallIB57/sallIB57
(b),
hypomorphic
salIIB57/sa,405
(c)
and
P-element
transformed
salJIB57/salJIB57;P[C20-sal26]
(d)
larvae.
(c)
shows
a
hypomorphic
salIIB57/salA405
larva
with
an
extreme
sal
mutant
head
similar
to
(b)
and
a
wild-
type
tail
similar
to
(a).
In
contrast
to
the
amorphic
phenotype
(b)
the
head
of
the
transformed
larvae
(d)
is
almost
normal,
whereas
the
tail
of
the
transformed
larvae
(d)
shows
a
somewhat
shortened
and
reduced
pair
of
Filzk6rper
compared
with
wild-type
(a).
Note
that
the
P-element
transformed
salJIB57/salA405;P[C20-sa126]
embryos
develop
into
viable
and
fertile
flies,
indicating
a
complete
rescue
up
to
adulthood
of
the
otherwise
lethal
salJJBS7/salA405
(c)
transheterozygotes
by
the
P-element
P[C20-sat26].
(e)
The
nucleotide
sequences
and
the
deduced
amino
acid
sequences
of
wild-
type
and
mutant
sal
alleles
are
compared.
The
numbers
refer
to
wild-type
SAL
amino
acid
sequence
(see
Figure
3).
Nucleotide
changes
in
the
mutant
DNA
are
indicated
in
bold.
Arrowheads
facing
the
scheme
of
the
sal
protein
show
the
positions
of
the
stop
codons
in
the
allelic
DNA.
Ovals
symbolize
the
location
of
the
seven
zinc
fingers
within
the
sal
protein.
pattern
in
the
central
nervous
system
(Figure
5f
and
g).
During
stages
15-17
of
embryogenesis,
SAL
is
predominantly
expressed
in
both
the
central
nervous
system
and
in
the
tracheal
system
(Figure
5g
and
h).
In
order
to
localize
the
early
SAL
domains
with
respect
to
segment
primordia,
we
used
antibodies
directed
against
the
protein
encoded
by
the
segment
polarity
gene
engrailed
(en)
to
mark
the
anterior
margins
of
each
of
the
parasegments
(Martinez-Arias
and
Lawrence,
1985).
As
defined
by
en
expression,
SAL
expression
in
the
anterior
SAL
domain
spreads
over
parasegments
1-3
and
fades
to
barely
detectable
levels
in
parasegment
4
(Figure
6).
Thus,
it
covers
the
anlagen
of
the
maxillary
and
the
labial
segments,
as
well
as
the
posterior
part
of
the
mandibular
segment
and
the
anterior
part
of
the
first
thoracic
segment.
The
posterior
SAL
domain
spans
parasegments
14
and
15
as
well
as
the
primordium
of
the
hindgut
up
to
the
Malpighian
tubule
anlagen.
The
posterior
borders
of
the
SAL
domains
are
fuzzy
while
the
anterior
borders
coincide
cell-by-cell
with
parasegmental
boundaries
(Figure
6).
sal
is
conserved
in
higher
Diptera
In
order
to
see
whether
sal
function
is
conserved
during
insect
evolution
at
the
molecular
level,
we
analysed
the
SAL
expression
pattern
in
Drosophila
virilis
(D.
virilis)
and
Drosophila
pseudoobscura
(D.pseudoobscura)
embryos,
and
in
embryos
of
the
more
distantly
related
dipteran
species
Musca
domestica
(M.domestica).
We
used
the
anti-SAL
antibodies
to
examine
whether
SAL
homologous
protein
is
expressed
in
those
embryos.
As
shown
in
Figure
7,
the
SAL
antibody
staining
pattern
in
D.
virilis
and
D.pseudoobscura
corresponds
both
spatially
and
temporally
to
the
expression
pattern
observed
in
D.
melanogaster
embryos.
In
M.domestica,
however,
SAL
antibody
staining
corres-
ponding
to
the
D.
melanogaster
pattern
is
first
detectable
at
the
germ
band
extension
stage
(Figure
7e;
see
also
Discus-
171
NH2
wt
e
sal
16
%.ff
A A
A

R.P.Kuhnlein
et
al.
1
gaattccccgataaaaaggggggttttatacaaacaatagcgaagaacacaaaatcttataaaccgaaattaaaaaaggcaacaacaacgctcataactgcgctgccaagcgagatggcg
121
ggc
gtggaagcgaga
ggagtgagtgagtgagcgaagag
gcgcaacaaatagcagactctt
acgctagag
241
ccctcccataaattatgataaattattaatcgagagagcgagcgagatggggcattacaacaacaatagcgcaaaggtgagccgcaaaagacagaaggaagtctcgctgcttctcttt
g
361
tatgttctcatt
caAgdatggcccgagageaaagagagagaggQgtaaagaagagcagctcataat
cgccgaaca,agtltcataaacgtc
ctcaggtaaaaaragcgartgcgac
g
481
agcatcacgaggcaaaagtagaagpaaaaaag
ccagg
gagc
ggccggcaaagtaacagtaacagcaatttaataaaattatgatgagagcgagcgagaggcscatatgaa
601
aaataatatgaaatgcaacaaa
catgcatgt
agagt
aacacaagataaattggctgagtgatagtgattftcagaatataataacacacacacacacacatacacaaac
721
c
attctcac
aaagcagccgaacaaagccgaacfcccacaacttct
cctattattcta
cattt
ttttgtcactcttatttGGACGCGCGTTGCTGMCGTTCG
841
CGCGACACTCGA
TC
TCT
AGTGCC
TCG
TMCACG
aGCGTATCGTAACGCGCACT'CA;TTCTGTTTTMGGCGAGTTTMCCATCGATTTACACAAGATAMCCCCCAAGTG
961
CCGCGAMAATTACCACTTACGATGACAATCAACGGTCAG,TTG
1
GCCAACAGCTAGATACGATAAATTAGCTAAACACGCGCAAAGGCCAGCGGTACTACGCGMCCCACTG
1081
CCGACGGCAAAACACAAAAGTGCTACAAGTMCTAAAMAAGTTAITTTCCTCACAAAATAAACCAMAAAAACATAGTGCAATGATCAAGCTGAGTTMTGAAATCMACCA
1201
AAAACACACTCACAAAAAGTGCTAACTAAAG6TTCTCTTGATGAAAAATCACCTGTCCAACGTTCTGTGTGCGATGCGTAGTGACTTCAAGGATMTCACCAGGAGACCATCAATAAA
M
K
N
H
L
S
N
V
L
C
A
M
R
S
D
F
K
D
N
H
Q
E
T
I
N
K
M
1321
TGATACAAMGTACAGTGAATGCTGTCA
AACAGCTG
AAGGATCGCGCTCGCAGCGCCGACAAAGgtgagctgaaaatctatttgccaagoaaa..........
I
Q
F
G
T
V
K
Y
G
I
V
K
Q
L
K
D
R
A
R
S
A
D
K
A
..........
..
aactcgtatcctaaataatttgagaatgttgttttctgtgttttcagACAITCGGGTAsGCGOATCAG
EGAAEGGG
GCSPLTGCTCGCACTA-CG
6241
AACCACTACGGCCAGTCCCAGCCGAAGTCCCGAGCCGGAGGAGGAGCAGCCCGAGGAGCAGAGCACTTCAGAGCAGAGCATACCAGAGCAAAGCACACCAGACCACCAACTCGAGAACGA
T T
T
A
S P
S
R
S
P
E P
E
E
E
Q
P E
E
0
S
T
S
E
Q
S
I
P
E
0S
T
P
D
H
0
L
E
N
D
6361
TATCAAATCCGAGGCGMATCAGAGATAGAGCCCGTTGAGGATMCAACAACAGAGTGGCGATGACAAAGCCCAGTTCCGAGGAGCGGGAACCGAATGCCAGTGGCTCCATGCCGAGTTC
I
K
S
E
A
K
S
E
I
E P
V
E
D
N
N
N
R V
A
M
T
K
P
S
S
E
E
R
E P
N
A
5
G
S
M
P
S
S
6481
CCCAGTGGCGGAGGCCAGTGCCGAGGAGGCGGCCACCGAGAGGACGCCGGAAAAGGAGAAGGAGAAGGACGTGGAGGTCGATGTGGAGATGCCCGATGAGGCACCCAGCAGTGCGGTGCC
P
V
A
E
A
S
A
E
E
A
A
T
E
R
T
P
E
K
E
K
E
K
D
V
E
V
D
V
E
M
P
D
E
A
P
S
S
A
V
P
6601
CTCGACTGAGGTMCTCTGCCGGGCGGAGCAGGAGCACCGGTCACCCTGGAGGCCATCCAAAATATGCAAAT6GCCATT6CCCAGMGCGGCCAAGACCATTGCGAATGGTTCCAATGG
S
T
E
V
T
L
P
G G
A
G
A
P
V
T
L
E
A
I
Q
N
M
0
M
A
I
A
Q
F
A
A
K
T
I
A
N
G
S
N
G
6721
AGCCGACAATGAGGCTGCCATGAAGCAGTTGGCCTTCCTTCAGCAAACCCTCTTCAATCTGCAGCAACAGCAGCTCTTCCAGATCCAGCTGATCCAACAGCTCCAGTCGCAGCTGGCGCT
A
D
N
E
A
A
M
K
0
L
A
F
L
Q
Q
T
L
F
N
L
0
0
0 0
L
F
0I0LI
0
0
L
Q
S
Q
L
A
L
6841
CAATCAGGCGAAACAGGAAGAGGATACCGA,GGAGGATGCGGATCAGGAGCAAGATCAGGAACAGG
A
CAGATACCTAGAGAG
GAGGACGCATCGCCGATATGGAACTGCGCCAGAA
N
Q
A
K
Q
E
E
D
T
E
E
D
A
D
Q
E
Q
D
Q
E
Q
E
T
D
T
Y
E E
E
E
R
I
A
D
M
E
L
R
Q
K
6961
GGCGGAGGCCAGAATGGCGGAGGCTAAAGCGCGTCAGCATCTTAAAMTGCTGGTGTTCCGCTGCGAGAGTCCTCCGGTTCTCCAGCTGAATCTCTGAAGCGAAGACGTGAGCATGATCA
A
E
A
R
M
A
E
A
K
A
R
H
L
I
N
A
G
V
P
L
R
E
S
S
G
S P
A
E
S
L
K
R
R
R
E
H
D
H
7081
CGAATCCCAGCCAAATCGTAGAACGAGMGGATMACACACACAAAGCAGATACGGCGCAGGATGCGCTGGCCAAGTTAAAGGAAATGGAGAACACACCACTGCCCTTCGGTTCCGATCT
E
S
Q
P
N
R
R
T
S
L
D
N
T
H
K
A
D
T
A
Q
D
A
L
A
K
L
K
E
M
E N
T
P
L
P
F
G
S
D
L
7201
GGCTTCCAGCATTATCACCAACCATGATGATCTGCCCGAGCCGAATTCCCTGGACCTGCTCCAGAAACGT6CCCAGGA6GTGCTGGACTCCGCGTCGCAG6GGATCCTGGCCAACAGCAT
A
S
S
I
I
T
N
H
D D
L
P
E P N
S
L
D
L
L
0
K
R
A
0
E
V
L
D
S
A
S
0
G
I
L
A
N
S
M
7321
GGCTGACGACM
GCCTTCGGTGAGAAATCGGGTGAGGGAAAGGGTCGCAATGAGCCGTTCTTCAAGCACCGCTGCAGGTACTGCGGGAA6GTCM
GGCTCGGACTCGGCGCTCCAGAT
A
D D
F
A
F
G
E
K
S
G
E
G
K
G
R
N
E
P
F F
K
H
R C
R
Y
C
G
K
V
F
G
S
D
S
A
L
0
I
7441
CCACATAAGATCGCATACTGGCGAGCGGCCCMAAGTGCAATGTGTGCGGCAGTCGGTTCACCACCAAGGGCAACCTTAAGGTTCACMCAGCGGCATGCCCAMAG6TTCCCCCATGT
H
I
R
S
H
T
G
E
R
P
F
K
C
N
V C
G
S
R
F
T
T
K
G
N
L
K
V
H
F
Q
R
H
A
QC
K
F
P
H
V
7561
GCCCATGAATGCCACGCCCATTCCGGAGCACATGGACAAGMCATCCGCCGCTGCTGGATCMATGTCGCCCACGGATA6CTCTCCCMATCATTCCCC6CCGCCGCCCCCATTGGGCTC
P
M
N
A
T
P
I
P
E
H
M
D
K
F
H
P
P
L
L
D
0
M
S
P
T
D
S
S
P N H
S P
A
P
P
P
L
G
S
7681
TGCTCCGGCATCCMCCGCCCGCCTTCCCTGGCCTTCAGAATCTCTATCGCCCGCCTAT6GGATCCTTAMAAATCTTGGAGCCGCTGCGCCGCACCAATACTTCCCTCAGGAGTTGCC
A
P
A
S
F P
P
A
F
P
G
L
Q
N
L
Y
R
P P
M
E
I
L
K
S
L
G
A
A
A
P
H
Q
Y
F
P
Q
E
L
P
7801
CACGGATCTGAGAAACCCTCGCCTCAATTGGATGAGGATGAGCCGCAGGTTAA6AGAACUCCGTCGAAGAGMGGACAGCGGGAGGAGCATGAACAGGAGATGGCAGAGTGCTCAGA
T D
L
R
K
P S P
Q
L
D
E
D
E
P
Q
V
K
N E
P
V
E
E
K
D
Q
R
E
E
H
E
0E
M
A
E
C
S
E
7921
GCCCGAGCCGGAACCGCTGCCCCTAGAGTGCGCATCAAGGAGGAGCGTGTGGAGGAGCAGGAACAGGTTAAACAGGAGGACCATCGCATAGAGCCACGTAGGACACCCTCTCCTTCATC
P
E
P
E
P
L
P
L
E
V
R
I
K
E
E
R
V
E
E
Q
E
Q
V
K
0
E
D
H
R
I
E
P
R
R
T
P
S
P
S
S
8041
AGAGCACCGCTCCCCGCACCACCACCGTCACAGCCACTGGGCTATCCACCAGTGGTGCAGCCCATCCAACCGGCCGCACTTATGCATCCGCAATCTTCGCCGGGCTCGCAATCCCACCT
E
H
R
S P
H
H
H
R
H
S
H
M
G
Y
P
P
V V
0
P
I
Q
P
A
A
L
M
H P
Q
S
S
P
G
S
Q
S
H
L
8161
GGATCACCTGCCCACGCCGGGGCAATTGCCACCCCGCGAAGAMCTTCGCTGAGCGMTCCCCCTTMACMACCACCGCCAA6ATGCTATCACCCGAACACCACTCTCCAGTAAGATC
D
H
L
P
T
P
G
L
P
P
R
E
D
F F
A
E
R
F P
L
N
F
T
T
A
K
M
L
S
P
E
H
H
S
P
V
R
S
8281
GCCCGCTGGCGGAGCACTTCCACCGGGTGTTCCACCACCACCGCACCACCACCCGCACCACATGGCCAGATCGCCGTTCMAACCCCATCAAGCACGAGATGGCCGCACTACTGCCCCG
P
A
G
G
A
L
P
P
G
V
P
P
P
P
H
H
H
P
H
H
M
A
R
S
P
F
F
N
P
I
K
H
E
M
A A
L
L
P
R
8401
CCCGCAlAC4AG
CGATAACTCGTGGGAGAACTTCATCGAGGMCGACACCTGTGAG
ACCATGMGTAAGGACTAGAGA6ACAAGMGATACGATCCCMATCAGTGTGTGGT
P
H S
N
D
N
S
W
E
N
F
I
E
V
S
N
T
C
E
T
M K
L
K
E
L
M
K
N
K
K
I
S
D
P
N
Q
C V
V
8521
CTGTGATCGGGTGTTATCCTGCAAGAGTGCCCTCCAGATGCACTACCGAACCCACACCGGTGAGCGCCCATTCAAGTGCAGGATCTGCGGCAGGGCATTCACCACCAAGGGCAACCTAAA
C
D
R
V
L
S
C K
S
A
L
Q
M
H
Y
R
T
H
T
G
E
R
P
F
K
C R
I
C
G
R
A
F
T
T K
G
N
L
K
8641
GACCCACATGGCTGTGCACAAGATTCGTCCGCCGATGAGAMCTTCCACCAGTGCCCCGMGCCACAAGAAGTACTCGAATGCCCTGGTCCTGCAGCAGCACATCCGATTGCATACGGG
T
H
M
A
V
H
K
I
R
P
P
M
R
N
F
H
Q
C
P
V C
H
K
K
Y
S
N
A
L
V
L
0 0
H
I
R
L
H
T
G
8761
TGAGCCCACTGATCTGACGCCGGAGCAAATCCAGGCGGCCGAGATCAGGGACCCGCCACCTTCGATGATGCCCGGTCACMATGAATCCCTTCGCAGCGGCTGCCTTCCAMCGGTGC
E
P
T
D
L
T
P
E
0
I
Q
A
A
E
I
R
D
P
P
P
S
M
M
P
G
H
F
M
N
P
F
A
A A
A
F
H
F
G
A
8881
TCTTCCCGGCGGTCCAGGTGGTCCTCCGGGTCCGAATCATGGTGCCCACAATGGCGCCTTGGGATCGGAGTCGTCGCAGGGCGATATGGATGATMTATGGACTGCGGCGAGGACTACGA
L
P
G
G
P
G
G
P P
G
P
N
H
G
A
H
N
G
A
L
G
S
E
S
S
Q
G
D
M
D
D
N
M
D
C
G
E
D
Y
D
9001
CGATGATGTGTCGTCGGAGCACCTCTCGAATAGTMTCTCGAGCAGGAGGGCGACAGATCGCGCTCTGGTGATGACTTCAAGTCCCTGTTGTTCGAGCAAAGCTGAGAATTGATGCCAC
D
D
V
S
S
E
H
L
S
N
S
N
L
E
CQC
E
G
D
R
S
R
S
G
D
D
F
K
S
L
L
F
E
Q
K
L
R
I
D
A
T
9121
CGGTGTGGTTAACACGAACCCCGTAAGACCGCGTTCCTCCGC
GCAGTCATGGCCATTCGGTGGGCTCCACCTCTGCGCCCACCTCGCCCAGCGTA
ATGCATCATCCCAGGTTATCAA
G
V
V
N
T
N
P
V
R
P
R
S
S
A
S
S
H
G
H
S
V
G
S
T
S
A
P
T
S
P
S
V
H
A
SS0V
I
K
9241
GCGCAGCTCTTCGCCCGCTCGTTCAGAGGCTTCTCAGGGAGCCCTGGACTTGACGCCCU6TGCTGCCCCCACATCGA6TTCCAGTTCGCGTTCTCCCCTGCCA
6~~CCAGTCAG
R
S
S
S
P
A
R
S
E
A
S
0
G
A
L
D
L
T
P
R
A
A
P
T
S
S
S
S
S
R
S
P
L P
K
E
K
P
V
S
9361
TCCGCCCAGCTTGCCTAGGAGTCCCAGTGGTTCTAGCCACGCCTCCGCCAACATACTGACCTCACCCCTGCCGCCCACCGTGGGCATTGACTGCTTGCCTAAGAC6TGCAACACCA
M
P
P
S
L
P
R
S P
S
G
S
S H
A
S
A
N
I
L
T
S
P
L
P
P
T
V
G
I
D
C
L
P
K
G
L
H
H L
9481
6G=CAGCAG6CAGCATCACCT11TbATbGWCACAAAGCGCAGTGGCAGCGGCAGCAGCTGCGCAGCACCATCATCACCAGCAAATGGCTGCACTCGATCAGCACCAGAGACTGCGTCG
00H0
H
L
M
0
0
0
A
A
V
A
A
A
A
A
A
Q
H
H H
H
0
0
M
A
A
L
D
0
H
0
E
0
L
R R
9601
C&AGCO.oTH6AACGCAGCAAAAGGCCGCAGCAGCTGCTGCAGACGGCCGCAGCAGCCGCGGCCCAGCGACAAACACCTCCGCAAGCCCGTGATCAGCGGCAGGAAGGGGACC6GG
E
A
A
E
A
0
0
K
A
A
A
A
A
A
A
A
A
A
A
A
A
A
0
R
0
T
P
P
Q
A
R
D
0
R
Q
E
G
G
P
G
9721
AGCGGGACCGCCGCCCAATCCGTTGATGGGCGCCCGCCCGCCCTTCGGCATGTTCCCCAACCTGCCGCTCTTCCCCCCCGCCACCACCCAGAACATGTGCAATGCGATGAACCAGATCGC
A
G
P
P
P
N
P
L
H
G
A
R
P P
F
G
M
F
P
N
L
P
L
F
P
P
A
T
T
N
M
C
N
A
M
N
Q
I
A
9841
CCAGTCCGTAATGCCGGCGGCTCCATCACCACGCCCTCA
GCGGT
GTTCGCGGCAGTACCACCTGCG6CATCT6CTACAAGACATTCCCCTGCCACTCGGCGCTGGAGATCCACTA
QSV
M
P
A
A
P
F
N
P
L
A
L
S
G
V R
G
S
T
T
C
G
I
C
Y
K
T
F P
C
H
S
A
L
E
I
H
Y
9961
CGGSAGCCACACC^AAAGG6CGGCCATTCAAGTGCAGCATCTGTGATCGCGGCTTTACACCAAGgtgagctatagttacttctattctgaatttattggggggttttctaacggtgccta
R
S H
T
K
E
R
P
F
K
C
S
I
C
D
R
G
F
T
T
K
10081,
cacttaaaacaaaatttaaaccaaaaaactoatoaaaaatttcctttttttttcatttattttccaaGG6AACCTGAAGCAACACATGCTAACTCATAAAATCCGCGATATGGAGCAAGa
96O
a;aacvv;aacyaLaywa; s; ;;;;S; aS;
;auv1
l l l u uu
27
51
68
106
148
1"
28
we
348
38
428
468
508
7Us
548
828
708
748
788
m
908
948
1028
1086
1108
1148
llU
118
1228
1286
1308
133
6
N
L
K
Q
H
M
L
T
H
K
I
R
D
N
E
Q
E
134
10201
MCCTTCAGAAATCGTGCCGTAAAgtatgtaagtcttccaatatcacccatcccgtcctgtccttttcattcctattcataaatcccsttagtttgcttttaccaactcttcttatttctt
T
F
R
N R
A
V
K
1S
10321
atggcactttttctttacgatgatttatacatcttttaacaagttatattatcagtagtttatagattttggagacactatasatacttccctatagataattgttcctatgcccctaat
10441
gaccatcttattaaatacattaatcatttcacttttactaaacaatccacatctttttgctctttcccat
gcagAT6A6TGAGTGGAACMAAGTCCGAATAAGTAAACACTCTACAC
10561
TACCACGATTACGATGCAGATGGCTTMTCCGCTATCAAGATCACTTGACCCCCGGAAMMGMGGCGATCGCAGTCCAGACCCAGTCMGATGCAATCGATTCTCGCAACCAAATGA
10681
TCTCMGTMTAGAGCGAMTGAGGGGGAGAAAGAGGACGTGACAGGTCCGGTGAAGiATCGGTCGTGTAACAAATCMATAMATMAATTGTTGCCCACMTATTACTTG
10801
ATTGTTGTTCCAAGCGAMGGAAAAGTMM6CCMACTGCAMAATGGGCTGATCGATGATCGATTATGTTCCCGGGCTCGGGCCAACmAAMeMlATGATCACCGGGGMAT
10921
TAACGGGGG6AATGCCGAGCACACGTACACCCATACTAAGGTGGGATCATGAMACGTATCCAMAGATGCATCAAAAGCGAM6TGTTCCAGCTAMCMATCGAAAGATCTGCTGATCTG
11041
GAACCAAAGCTGCTTGGTATGGAGAGACTGATGGCGMCATGTTCCACAMACTGAGAACGGAATCTAAACTAAATCCAAATCCGTATCCGGACTCGTAMGTATCCATATTCGMAT
11161
ACGAGTCCGAATCCGAGGCAGCTGATGAA6CGCAG6TGAAGGCGTAGTAAMATCAAMATTCGAAMAAAGCM
ATCTTMAGGTATATGCTMAAA
IAAAAMTTGTACCT
11281
MGCGAGACATGTGTACATACGTATATATsAMTATATATGATATATTATAACCAAATCCCAAGAACGCATACGCATACACGTTMTMATCTAMGGAATGTGCAATMTATGA
11401
CATGCTAAAM
AGATGCATCGCCGAGCGCA
GGCTTMGMCCTACTACTCGTMATTAGAMATMTCCACMCTGTACATACTTCGTATATAAGCACCCACACTCGCACAC
11521
ACTTATATATCATAACACACACMGACMCGCTTTGC
MCGAGMGGGTTACGAGCTAA
AAACGAAATAAMAATCTAAAAATTTAGGATTGTAT
11641
ATTAAATGTAWAAACGAAATACAMTTACGATTGCAGTGGCCGGGGGAMTCGAAGCCCCCCMATCGMGCCCCAGCAAMATGCMCCATATCACAGATGAAGAACCACMAAAGATAT
11761
CTAACATTCATAGTTMAMAGTTGTTAGCCGAGGAATCCCCGACCCACAACCCAAMACCCC_AA_ACCCGCTCACACTCTMMCACTTCATTGGMA
11881
GGAAGGATTAACCCCTTAGCGATAAGTAAGTCTATGAGCGATCGTACATGTATTGATTACCACMATTAMTTATACACGGATGCCAATGTATCCCACTCTGTTCGTAAGCATTMGCATA
12001
GTCTCATMMATTACGCMGCCCAAGC
CGAA6AAAMCAGAAACTAAAAA
TGCAMTAAMT
TCAGGAAGT
AACATAAATATTGTAMMGTTATGA
12121
AAAATTaatoaacc
tctcclagttttctttcggatctacggat
->
poly(g)
tall
Flg.
3.
Nucleotide
and
deduced
amino
acid
sequences
of
the
sal
gene.
The
sequence
of
12
164
bp
of
the
genornic
sal
region
(available
under
accession
No.
X75541),
excluding
DNA
sequences
of
the
first
intron,
is
presented
and
numbered
on
the
left
side.
The
DNA
sequence
of
the
coding
strand
of
a
composite
sal
cDNA
is
indicated
by
upper
case
type.
Introns
and
genomic
sequences
not
represented
in
the
cDNA
are
in
lower
case
type.
The
predicted
amino
acid
sequence
is
shown
below
the
nucleotide
sequence
and
numbered
on
the
right
side.
The
protein
sequence
shown
is
a
conceptual
translation
of
the
longest
open
reading
frame
within
the
cDNA
sequences.
It
begins
at
the
fifth
ATG
of
the
cDNA
and
ends
at
a
TGA
triplet
indicated
by
asterisks.
Two
putative
polyadenylation
signals
at
the
3'end
of
the
transcription
unit
are
double
underlined
and
the
poly(A)
tail
of
a
sal
cDNA
clone
is
indicated
by
an
arrowhead.
The
P-insertion
site
of
the sal
mutant
salA405
is
at
nucleotide
position
480.
172

Molecular
genetics
of
the
Drosophila
spalt
gene
-430
I
E
E
E
E
I
-435
E
clQ
c6
c3-1
0
-
--I~~~~~~n
n
Icr
2
9c
c
.I
II%
I
1
1
1
I
u
Stop
AAUAAA
I
II
I I
I
w
ANk
Aft
---------I
I
-.
C
OO
H
m
JRYFGKVF
GSDSALQ
II
S
R
TGERP
FF
3G
S
RPVTTGN=V@FQ
R
VN7DRVLSCKSALQRTGE
R
P
Ft
I[
G
NLXIPAA
C
UJGIYRYKTF
PCHSALE
I
YR.SrKERPFKS
IADRG?TTXGNLKQpML
t
Yg3NRACAKPSVLLKJI
RSrGERPYP>VTDGFS
FKTKSSLY
KKKSHAz
&E3GIRC
KKP
SMLKKI
R7DVRPYHEYF
SFXXOGLTBTKSA
Fig.
4.
Structural
organization
of
the
sal
gene,
the
putative
sal
protein
and
its
similarity
with
PRDII-BF1.
(a)
Molecular
EcoRI
(E)
restriction
map
of
the
genomic
region
containing
the
sal
gene
(upper
part)
and
the
location
of
five
cDNA
clones.
The
scale
refers
to
DNA
distance
(in
kb)
as
described
in
Frei
et
al.
(1985)
(see
also
Figure
1).
The
composite
molecular
structure
of
the
sal
transcription
unit
is
presented
below.
The
translational
start
(AUG),
the
end
of
the
open
reading
frame
(Stop)
and
the
poly(A)
signal
(AAUAAA)
are
indicated.
Dotted
lines:
intronic
sequences
not
present
in
the
cDNA
clones.
Black
bar:
longest
open
reading
frame
(4065
bp)
of
the
sal
transcript.
Open
bar:
untranslated
region
of
the
sal
transcript.
(b)
Diagram
showing
structural
features
of
the
predicted
sal
protein.
The
seven
ovals
indicate
the
localization
of
the
seven
zinc
finger
motifs
within
the
protein
(filled
ovals
symbolize
the
double
zinc
fingers).
Regions
enriched
for
certain
amino
acids
are
shown
as
boxes
with
different
shadings.
Black
boxes:
regions
with
38%
(N-terminal)
and
27%
(C-terminal)
glutamine
residues,
respectively.
Open
box:
region
with
53%
alanine
residues.
Hatched
box:
region
with
33%
proline
residues.
Stippled
box:
region
with
31%
serine
residues.
(c
and
d)
The
invariant
positions
of
the
cysteines
and
histidines
of
SAL
(c)
and
PRDII-BFI
(d)
double
zinc
fingers
are
boxed.
Identical
H/C-link
amino
acids
in
the
SAL
double
zinc
fingers
(c)
are
underlined
whilst
other
identical
amino
acids
are
shown
with
dark
background.
Note
the
seven
identical
amino
acids
in
the
C-terminal
zinc
finger
of
the
SAL
double
finger
structures
referred
to
as
the
'SAL-box'.
Amino
acid
positions
of
PRDII-BF1
double
zinc
fingers
(d)
shared
by
all
SAL
double
zinc
fingers
are
shown
with
dark
background
or,
in
the
case
of
the
H/C-link
are
underlined.
sion).
These
findings
suggest
that
SAL
corresponding
protein
is
functionally
conserved
and
required
in
the
same
anlagen
as
SAL
in
D.
melanogaster.
In
order
to
show
the
degree
of
molecular
identity
between
SAL
and
the
proposed
SAL
encoding
gene
of
another
Drosophila
species,
we
cloned
and
sequenced
the
DNA
of
the
sal
homologue
of
D.
virilis,
a
species
that
is
sufficiently
diverged
from
D.
melanogaster
to
allow
only
functionally
meaningful
protein
regions
to
be
conserved
(Kassis
et
al.,
1986;
Treier
et
al.,
1989).
In
both
D.melanogaster
and
D.
virilis,
the
positions
of
the
exon-intron
boundaries
of
the
sal
transcription
unit
are
conserved
(data
not
shown).
The
putative
protein
sequences
shown
in
Figure
7f
indicate
that
the
two
proteins
contain
three
zinc
finger
groups
of
almost
complete
sequence
identity
in
the
same
relative
positions.
Sequences
at
each
side
of
the
three
zinc
finger
groups
show
a
higher
degree
of
sequence
similarity
than
the
in-between
regions.
Within
those,
islands
of
10-30
conserved
amino
acids
are
found.
The
longest
detectable
open
reading
frame
of
both
genes
has
a
common
conserved
initiation
codon
at
the
N-terminus,
although
this
initiation
codon
is
preceded
by
another
in-frame
initiation
codon
which
adds
11
amino
acids
to
the
N-terminus
of
SAL
from
D.melnogaster.
These
results
suggest
that
sal
function
is
conserved
both
functionally
and
molecularly
in
Drosophila
and
probably
also
in
other
diptera.
173
-425
E
AUG
1
kb
a
b
NH2
d

e
f
9
h
Fig.
5.
sal
protein
expression
during
Drosophila
embryonic
development.
Whole
mount
preparations
of
wild-type
embryos
were
stained
with
anti-
SAL
antibodies.
Stages
are
described
according
to
Campos-Ortega
and
Hartenstein
(1985).
(a)
Early
stage
5,
expression
of
the
anterior
SAL
domain.
(b)
Late
stage
5,
cellular
blastoderm.
The
posterior
SAL
domain
and
'horse-shoe
domain'
become
visible
(see
text).
(c)
Stage
8,
germ
band
extension.
The
posterior
domain
moves
cephalad
during
the
phase
of
germ
band
elongation.
(d)
Stage
9,
stomodeal
plate
formation.
The
anterior
SAL
domain
starts
to
fade
out,
whilst
the
posterior
domain
persists.
(e)
Stage
10,
fully
extended
germ
band.
(f)
Stage
12,
germ
band
retraction.
Strong
expression
within
the
region
of
the
developing
posterior
spiracles.
Segmentally
repeated
SAL
expression
in
restricted
parts
of
the
ventral
cord
and
the
procephalic
neurogenic
region.
(g)
Stage
14,
beginning
of
head
involution.
SAL
expression
in
the
tracheal
system
(h)
Stage
14.
Focus
on
lateral
epidermis.
Staining
in
the
oenocytes,
bilateral
groups
of
cells
in
abdominal
segments
1-7
(Hartenstein
and
Jan,
1992)
and
parts
of
the
tracheal
system
becomes
visible.
Discussion
Our
results
show
that
the
region
specific
homeotic
gene
sal
encodes
an
evolutionarily
conserved
zinc
finger
protein.
The
identification
of
the
sal
gene
is
based
on
two
independent
lines
of
evidence.
A
transgene
that
contains
a
single
transcription
unit
rescues
sal
mutant
embryos,
and
molecular
lesions
were
found
in
the
sequence
of
all
sal
alleles
analysed.
These
findings
are
in
contrast
to
the
previous
assignment
of
the
sal
gene
which,
as
is
shown
here,
is
based
on
an
experimental
artifact
or
an
experimental
error.
With
respect
to
its
chromosomal
location
next
to
sal,
we
rename
this
gene
as
sal
adjacent
(sala).
SAL
is
expressed
in
the
segment
anlagen
affected
by
sal
mutant
embryos
sal
mutations
affect
posterior
head
and
anterior
tail
segments.
In
the
head
region
sal
mutants
cause
partial
transformation
of
maxillary
and
labial
segments
to
develop
prothoracic
structures
(Jiirgens,
1988).
In
accordance
with
this
mutant
phenotype,
SAL
is
expressed
in
parasegments
1-3
which
include
the
primordia
of
both
the
maxillary
and
labial
R.P.Kuhnlein
et
al.
a
b
c
d
174
-cgmmb,-
...
.1
"W,
I..
..r
k
fv#.
j
10
mppl-
1.
.4..
is
4.
-At
-,.,
.*.-
A*
40,
-*-
.4."-
At
t.

Molecular
genetics
of
the
Drosophila
spalt
gene
Fig.
6.
sal
protein
expression
with
respect
to
parasegmental
boundaries.
Whole
mount
preparations
of
wild-type
fully
extended
germ
band
embryos
double
stained
with
antibodies
against
sal
(blue)
and
engrailed
proteins
(brown)
(a-c);
single
staining
against
sal
protein
(brown)
(d).
(a
and
b)
show
the
anterior
SAL
expression
domain
(a,
lateral
view:
dorsal
up,
anterior
left,
b,
ventral
view).
The
mandibular
engrailed
stripe
(in
PS
1)
marks
the
anterior
boundary
of
the
SAL
expression;
both
limits
coincide
cell-by-cell.
The
SAL
expression
in
the
anterior
prothoracic
compartment
(posterior
PS
3)
is
weak
and
very
weak
expression
is
also
detectable
in
PS
4.
(c
and
d)
show
the
posterior
SAL
expression
domain
(c:
ventral
view.
d:
lateral
view;
dorsal
up,
anterior
left).
The
anterior
limit
of
the
posterior
SAL
expression
domain
coincides
with
engrailed
expression
in
abdominal
segment
8
(in
PS
14).
The
posterior
boundary
coincides
with
the
posterior
tip
of
the
hindgut,
demarcated
by
the
Malpighian
tubules
primordium
that
separates
ectodermal
hindgut
from
endodermal
midgut
(d).
Arrowheads
indicate
parasegmental
boundaries
and
numbers
refer
to
parasegments.
Abbreviations:
PS,
parasegment;
hg,
hindgut;
mt,
Malpighian
tubules
primordium;
mg,
midgut.
segments.
However,
very
weak
SAL
expression
is
also
detectable
posterior
to
parasegment
3.
Therefore,
the
parasegmental
nature
of
the
posterior
boundary
of
the
anterior
SAL
domain
remains
an
open
question.
In
the
posterior
domain,
SAL
expression
is
found
in
parasegments
14
and
15,
which
are
homeotically
transformed
in
the
sal
mutants
(Jiirgens,
1988),
but
it
also
fades
into
the
primordium
of
the
hindgut
whose
metameric
nature
is
unclear
(Jiirgens
and
Weigel,
1988).
Therefore
early
SAL
expression
is
similar
to
the
restricted
parasegmental
expression
that
has
been
observed
with
the
region
specific
homeotic
genefkh
and
the
homeotic
selector
genes
(Akam,
1987;
Ingham,
1988;
Weigel
et
al.,
1989).
However,
the
barely
detectable
SAL
expression
in
parasegment
4
may
also
be
of
functional
importance,
since
Ubx
gene
product
(which
is
restricted
to
parasegments
5-13
in
wild-type
embryos)
expands
into
parasegments
3
and
4
of
sal
mutants
(Casanova,
1989).
This
suggests
that
low
levels
of
SAL
are
able
to
repress
Ubx
in
the
wild-type
embryo
either
by
its
direct
interaction
with
Ubx
cis-regulatory
elements
(see
below)
or
indirectly
through
as
yet
unknown
factors.
Widely
spaced
double
zinc
finger
motifs
in
the
sal
protein
The
sal
protein
(SAL)
contains
seven
zinc
finger
motifs
of
the
C2H2-type
first
identified
in
the
transcription
factor
TFIIA
(Miller
et
al.,
1985).
Their
arrangement
in
three
sets
of
highly
conserved
and
widely
spaced
double
zinc
fingers
suggests
that
they
may
have
derived
from
an
ancestral
gene
encoding
a
single
double
zinc
finger
motif,
through
sequence
duplication
or
intragenic
conversion.
Among
the
double
zinc
fingers
the
most
striking
amino
acid
homology
is
found
in
two
boxes,
the
H/C-link
(Schuh
et
al.,
1986)
and
an
array
of
eight
identical
amino
acids,
termed
'SAL-box',
which
are
found
in
the
C-terminal
finger
motif
of
the
double
zinc
fingers.
Interestingly,
two
pairs
of
widely
spaced
and
conserved
finger
pairs
have
also
been
observed
in
a
human
transcription
factor,
PRDII-BF1
(Fan
and
Maniatis,
1990).
In
addition
to
the
structural
conservation,
PRDII-BF1
and
SAL
show
a
significant
degree
of
sequence
similarity
within
the
pairs
of
zinc
fingers
suggesting
that
widely
spaced
and
sequence
related
'double
zinc
fingers'
may
define
a
conserved
subfamily
of
zinc
finger
proteins.
Zinc
finger
motifs
are
characteristic
of
a
distinct
class
of
nucleic
acid
binding
proteins.
Molecular
modelling
(Berg,
1988;
Gibson
et
al.,
1988)
and
two-dimensional
NMR
(Lee
et
al.,
1989)
have
led
to
a
proposal
for
the
three-dimensional
structure
of
C2H2-type
zinc
fingers:
the
Cys
-Cys
loop
forms
an
antiparallel
(3-sheet
followed
by
an
a-helical
region
through
the
His
-His
loop
which
contacts
in
the
major
groove
of
DNA
(Pavletich
and
Pabo,
1991).
The
'SAL-box'
extends
into
the
proposed
helical
region.
Thus,
the
three
sets
175

b
d
e
r
1
9
S
2
3
9
.2
34
9
772
r
2
~
..f.6
,~
~
~ ~ ~
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
C.
6
1225
772
135
.2
-
-
~~~~~~~~~~~~~~~35
f
Fig.
7.
Early
sal
protein
expression
in
different
dipteran
embryos
and
comparison
of
the
putative
sal
proteins
of
D.
melanogaster
and
D.
virilis.
Embryos
of
D.pseudoobscura
(a
and
b),
D.virilis
(c
and
d)
and
M.domestica
(e)
are
stained
with
anti-SAL
antibodies.
(a
and
c)
cellular
blastoderm
stage;
(b,d
and
e)
germ
band
extension
stage.
(f)
Sequence
comparison
of
sal
protein
of
D.melanogaster
(upper
sequence;
numbered
in
bold
type)
and
D.
virilis
(lower
sequence;
numbered
in
plain
type).
Vertical
dashes
represent
identities;
horizontal
dashes
represent
gaps
in
the
sequence.
The
zinc
finger
regions
are
shown
with
dark
background.
Alignment
conditions
using
Mac
Molly
Tetra,
Version
1.2
from
Soft
Gene
GmbH:
minimal
window
size
20;
number
of
mismatches
5;
gap
penalty
5;
mismatch
penalty
4.
DNA
sequence
of
the
D.virilis
sal
is
available
under
accession
No.
Z27444).
176
R.P.Kuhnlein
at
a!.
a
C
-z'
14
"..
.-M.%F

Molecular
genetics
of
the
Drosophila
spalt
gene
of
double
zinc
fingers
in
SAL
may
each
recognize
the
same
DNA
target
sites
as
shown
for
each of
two
double
zinc
fingers
of
PRDII-BF1
(Fan
and
Maniatis,
1990).
The
role
of
sal
The
C2H2-type
of
zinc
fingers
can
be
grouped
in
different
functional
classes.
They
might
act
as
DNA
binding
transcriptional
regulators
and/or
bind
to
RNA.
Other
zinc
finger
proteins
are
integral
components
of
chromatin,
or
provide
the
nuclear
transport
of
cytoplasmic
components
(reviewed
in
El-Baradi
and
Pieler,
1991).
Zinc
finger
containing
transcription
factors
have
been
shown
to
contain
diagnostic
domains
such
as
proline-
and
glutamine-rich
regions
which
are
thought
to
function
as
activator
sequences
(Courey
and
Tjian,
1988;
Mermod
et
al.,
1989),
or
alanine-
and
proline-rich
regions
required
for
repressor
function
(Licht
et
al.,
1990;
Han
and
Manley,
1993).
SAL,
which
accumulates
in
the
nuclei,
contains
both
types
of
sequence.
Thus,
it
may
function
as
a
transcriptional
activator
or
repressor
of
target
gene
expression.
A
possible
target
gene
of
SAL
is
tsh,
a
unique
homeotic
gene
which
defines
the
ground
state
of
the
trunk
(R6der
et
al.,
1992).
In
addition,
tsh
is
essential
for
specifying
the
identity
of
the
anterior
prothorax
by
acting
in
concert
with
Sex
combs
reduced
(Scr).
In
the
absence
of
sal,
the
labial
segment
is
partially
transformed
to
anterior
prothorax,
although
Scr
expression
in
this
segment
is
not
altered
in
sal
mutants
(Casanova,
1989).
However,
tsh
expression
expands
towards
the
anterior
in
such
a
way
that
Scr
and
tsh
expression
coexist
in
the
labial
anlagen
which
then
gains
anterior
prothoracic
identity
(R6der
et
al.,
1992).
Thus,
sal
activity
inhibits
tsh
expression
in
the
wild-type
embryo,
and
thereby
prevents
trunk
development.
This
observation
is
consistent
with
the
hypothesis
that
SAL
is
a
transcriptional
repressor
of
the
tsh
gene
and
that
its
interaction
with
tsh
prevents
trunk
development
within
the
head
and
the
tail
regions.
Antp
and
tsh
activities
combine
for
mesothorax,
the
segment
in
which
mesothoracic
leg
and
wing
imaginal
discs
normally
form.
Struhl
(1981)
observed
that
some
cells
in
the
mesothorax
leg
disc
which
lack
the
expression
of
Antp
form
patches
of
antenna-like
cells
in
the
adult
and
based
on
this
finding
he
suggested
that
Antp
normally
represses
the
activity
of
'head
forming
genes'
in
the
leg
disc.
It
has
recently
been
shown
that
sal
expression
occurs
in
antennal
but
not
in
leg
discs,
and
that
Antp
activity
serves
as
a
strong
repressor
of
sal
(Wagner-Bernholz
et
al.,
1991).
Although
we
do
not
yet
know
whether
sal
plays
a
decisive
role
in
antennal
development,
it
will
be
interesting
to
determine
whether
sal
may
carry
the
function
proposed
by
Struhl
(1981).
The
early
period
of
antennal
disc
expression
of
sal
is
at
the
time
in
development
when
ectopic
expression
of
Antp
is
required
to
induce
antenna-to-leg
transformations.
This
SAL
expression
is
entirely
repressed
by
Antp
activity
and
leg
instead
of
antennal
structures
are
formed
(Wagner-Bemholz
et
al.,
1991).
Since
sal
activity
represses
tsh
expression,
and
Antp
enhances
tsh
activity
in
the
blastoderm
(Roder
et
al.,
1992),
it
might
be
that
sal
activity
represses
tsh
expression
in
the
wild-type
antennal
discs,
and
ectopic
expression
of
Antp
might
then
act
as
an
antagonist
of
sal
i.e.
repressing
the
tsh
repressor
and
enhancing
tsh
activity
at
the
same
time.
Obviously,
these
proposed
interactions
need
to
be
elucidated
by
molecular
means
in
order
to
establish
the
regulatory
circuitry
that
leads
to
the
homeotic
antenna-to-leg
transformation
in
the
fly.
Conservation
of
SAL
The
overall
sequence
conservation
between
SAL
of
D.
melanogaster
and
its
homologue
in
D.
virilis
is
close
to
70%
amino
acid
identity,
although
the
two
species
are
-
60
million
years
of
evolution
apart
from
each
other.
This
degree
of
overall
sequence
conservation
is
within
the
range
described
for
several
other
developmentally
important
genes
(Kassis
et
al.,
1986;
Treier
et
al.,
1989;
Michael
et
al.,
1990).
The
double
zinc
fingers
are
almost
completely
conserved
in
sequence,
and
a
high
degree
of
conservation
is
found
within
the
glutamine-rich
regions
in
front
of
the
first
and
third
double
zinc
finger.
This
implies
that
those
regions,
which
might
be
required
for
DNA
binding
and
transcriptional
activation
(Courey
and
Tjian,
1988),
respectively,
are
essential
for
SAL
function.
In
contrast
to
these
conserved
and
diagnostic
protein
motifs,
no
sequence
similarity
with
known
protein
modules
has
been
detected
for
the
conserved
N-terminal
region
of
SAL,
and
thus
the
significance
of
the
conservation
remains
unclear.
Contrary
to
the
frequent
occurrence
of
cryptic
simplicity
in
other
regulatory
genes
(Colot
et
al.,
1988;
Treier
et
al.,
1989)
the
sal
proteins
lack
such
sequences.
Thus,
slippage-like
processes
may
not
be
involved
in
the
evolution
of
the
sal
proteins.
Instead,
the
highly
diverged
sequences
could
be
explained
by
insertions
and
deletions
of
blocks
of
30-60
bp
fragments
within
the
sal
transcription
unit.
However,
those
alterations
do
not
affect
the
relative
distance
between
the
three
double
zinc
finger
motifs,
suggesting
evolutionary
constraints
concerning
the
spacing
of
these
protein
domains.
The
argument
that
the
two
proteins
carry
corresponding
biological
functions
during
the
development
of
the
two
Drosophila
species
is
consistent
with
the
finding
that
the
SAL
antigene
is
found
in
corresponding
patterns
in
the
blastoderm
of
both
D.melnogaster
and
D.
virilis,
and
during
gastrulation
of
M.
domestica,
a
dipteran
species
which
is
-100
million
years
separated
from
D.
melanogaster
(Hennig,
1981).
The
relatively
late
appearance
of
SAL
antigene
in
M.domestica
embryos
may
be
explained
by
a
weak
cross-reactivity
which
requires
high
amounts
of
the
homologous
protein
to
be
visualized
by
the
SAL
antibody.
Alternatively,
SAL
homologous
expression
might
be
delayed
in
M.
domestica
as
has
been
observed
with
various
segmentation
genes
that
are
expressed
in
the
terminal
regions
of
those
embryos,
which
would
imply
that
the
mode
of
terminal
development
differs
between
Musca
and
Drosophila
(Sommer
and
Tautz,
1991).
It
had
been
proposed
that
sal
activity
plays
a
conserved
role
in
head
formation
during
arthropod
development,
an
evolutionary
event
leading
to
the
organizational
level
of
myriapods
through
a
process
in
which
anterior
trunk
segments
of
annelid-like
ancestors
were
integrated
into
a
primitive
head
(Jiirgens,
1988).
As
a
first
step
towards
a
critical
test
of
this
hypothesis
we
have
shown
SAL
conservation
among
higher
dipteran
species.
Since
SAL
homologous
sequences
have
already
been
identified
in
vertebrates
such
as
Xenopus
laevis
(R.Stick,
personal
communication)
and
mouse
(G.
Schiitz,
personal
communication),
we
expect
SAL
to
be
conserved
throughout
the
animal
kingdom
in
a
manner
preceded
by
the
genes
of
the
homeotic
selector
gene
complexes.
177

R.P.Kuhnlein
et
al.
Materials
and
methods
Isolation,
sequencing
and
analysis
of
wild-type
and
mutant
DNA
Isolation
of
sal
cDNAs,
preparation
of
DNA,
Southern
blot
analysis
and
handling
of
DNA
were
done
by
standard
methods
(Sambrook
et
al.,
1989).
DNA
sequencing
was
performed
using
the
USB
Sequenase
2.0
Sequencing
Kit
based
on
the
chain
termination
procedure
(Sanger
et
al.,
1977).
Single-
suanded
DNA
templates
were
generated
using
M
13
vectors
(Yanisch-Perron
et
al.,
1985).
The
sequences
of
the
wild-type
genomic
DNA
and
cDNAs
were
determined
on
subcloned
restriction
fragments.
Sequence
analysis
and
comparison
of
the
predicted
sal
protein
were
performed
on
the
SwissProt
database
using
the
HUSAR
program
package
(based
on
the
GCG
package)
of
the
DKFZ,
Heidelberg.
To
analyse
the
DNA
from
mutant
sal
alleles
we
amplified
genomic
sal
DNA
from
single
mutant
embryos
by
PCR
as
described
in
Hulskamp
(1991).
An
identified
mutation
was
confirmed
by
DNA
sequence
analysis
of
at
least
one
additional
mutant
embryo
and
the
parental
DNA
for
control.
Isolation
of
sal
alleles
The
two
sal
alleles,
sal16
and
sal65,
were
induced
by
30
mM
EMS
fed
to
b
pr
cn
wxwxt
bw
males.
They
were
mated
to
CyO
balancer
females
for
3
days.
The
Fl
male
progeny
were
individually
crossed
with
sallIB57
cn
bw
sp/CyO
females.
In
6800
lines
two
putative
sal
alleles
were
found
by
the
lack
of
homozygous
cn,
bw
(white
eyed)
F2
progeny.
Both
lines,
sal16
and
sal65,
were
test-crossed
with
different
sal
alleles
and
checked
for
their
embryonic
phenotype
and
lethality.
sal16
and
sal65
do
not
complement
salIIB57,
salIL4SS,
sal445
(Jurgens
1988)
and
salA405
(Bellen
et
al.,
1989;
see
also
Results),
and
cuticle
preparations
of
the
various
mutant
combinations
indicate
that
both
sal16
and
sal65
homozygous
embryos
show
the
sal
lack
of
function
phenotype
described
by
Jiurgens
(1988).
Generation
of
antisera
and
antibody
purification
The
10.1
cDNA
coding
for
the
amino
acids
403-763
of
the
predicted
sal
protein
was
subcloned
into
pUR
vector
(Ruther
and
Muller-Hill,
1983)
and
pATH
vector
(Koemer
et
al.,
1991)
to
generate
pUR-
10.1
(acZ-sal
fusion
protein)
and
pATH-
10.1
(trpE-sal
fusion
protein).
The
purification
of
fusion
proteins,
generation
of
antisera
in
rabbits
and
the
purification
of
10.1
antibodies
were
performed
according
to
Gaul
et
al.
(1987)
with
minor
modifications.
In
contrast
to
their
procedure
we
used
the
trpE
-sal
fusion
protein
to
generate
antisera.
For
affinity
purification
of
antibodies
directed
against
the
sal
part
of
the
trpE-sal
fusion
protein
an
Affigel
10/15
lacZ-sal
fusion
protein
column
was
used.
Purified
antibodies
were
checked
for
activity
against
sal
specific
protein
sequences
by
Western
blot
analysis
and
whole
mount
antibody
staining
of
embryos.
DNA
sequence
analysis
of
strong
sal
mutants
(sal445,
sal65
and
sal16)
reveals
that
these
embryos
are
only
able
to
express
truncated
sal
protein
lacking
major
parts
of
the
wild-type
protein.
Embryos
derived
from
heterozygous
parents
of
these
sal
mutants
were
stained
with
the
affinity
purified
anti-sal
antibodies
and
antibodies
against
Kr
protein
(Gaul
et
al.,
1987)
as
an
internal
control.
One
quarter
of
these
embryos
showed
only
Kr
protein
staining
(data
not
shown),
indicating
that
the
anti-sal
antibodies
fail
to
recognize
antigens
in
homozygous
sal
mutants.
Therefore
our
purified
antibodies
detect
the
sal
protein
specifically.
Developmental
expression
analysis
Whole
mount
preparations
of
embryos
were
antibody
stained
using
the
VECTASTAIN
Elite
ABC-peroxidase
system
(Vector
Laboratories)
with
DAB
as
substrate
under
the
conditions
described
(Macdonald
and
Struhl,
1986).
In
situ
hybridizations
of
whole
mount
embryos
using
digoxigenin-
labelled
DNA
probes
were
done
as
published
(Tautz
and
Pfeifle,
1989).
Germ-line
transformation
and
genetic
analysis
The
26
kb
SalI-SalI
fragment
was
subcloned
from
a
genomic
cosmid
into
SalI-digested
Carnegie
20
vector
(C20-sal26)
(Rubin
and
Spradling,
1983).
About
1000
embryos
of
the
ry506/,y506
stock
were
injected
with
the
recombinant
and
the
helper
plasmid
as
published
(Spradling,
1986).
Two
out
of
400
GO
flies
produced
ry+
Fl
flies
indicating
a
transformation
of
two
independent
fly
lines.
The
fly
stocks
P(C20-sal26)ry+A
(insertion
mapped
to
the
second
chomosome)
and
P(C20-sal26)ry+B
(insertion
mapped
to
the
third
chromosome)
were
established.
Using
P(C20-sal26)ry+A
and
the
jump-start
technique
(Cooley
et
al.,
1988;
Robertson
et
al.,
1988)
two
additional
fly
strains
could
be
obtained
that
had
the
P
element
inserted
on
the
third
chromosome.
The
following
experiments
were
performed
independently
with
the
three
P
element
lines
inserted
on
the
third
chromosome.
To
analyse
the
transformed
chromosomes
in
a
salb
background
we
crossed
sallIB57cn
bw
sp/CyO;ry50/ry506
males
with
+
1+
;,ySO6
[P(C20-sal26)ry+]/ry5O6
females.
salllB57cn
bw
sp/+
ryS06/ry506
[P(C20-sal26)ry+]
males
were
backcrossed
with
sallB57cn
bw
sp/CyO;ry5O6/rySO6
females
and
from
their
progeny
salIJB57cn
bw
sp/CyO;ry5O6/,ySO6
[P(C20-sal26)ry+]
males
and
females
were
collected
and
crossed
to
establish
fly
lines.
To
analyse
the
sal
mutant
rescue
capacity
of
P(C20-sal26)
we
crossed
sallJB57cn
bw
sp/CyO;ry506/rySO6
[P(C20-sat26)ry+]
males
with
females
carrying
the
hypomorphic
sal
allele
cn
pr/CyO
Pry+[salI405];ry5O6/ry5O6.
This
cross
produced
salIIB57cn
bw
sp/CyO
Pry+[salA405];ry5O6/ry5O6
[P(C20-sal26)ry+]
adult
flies
showing
a
rescue
to
viability
of
the
otherwise
embryonic
lethal
sal405/salllB57mutant
combination.
A
stock
of
salIJB57cn
bw
sp/CyO
Pry+[saA405S];,y5O6/ry5O6
[P(C20-sal26)ry+]
was
established
and
analysed
in
detail.
The
embryonic
progeny
of
the
stock
were
collected
and
embryos
were
stained
for
(3-
galactosidase
activity
(Bellen
et
al.,
1989)
to
distinguish
between
embryos
carrying
the
CyO
Pry+[salA405]
chromosome
which
expresses
,-
galactosidase
from
the
sallJB57cn
bw
sp
homozygous
ones.
Among
the
unstained
embryos
(homozygous
salliB57
embryos)
some
show
the
amorphic
sal
phenotype
while
the
majority
develop
a
wild-type
head
but
show
a
shortening
of
the
normally
stretched
posterior
spiracles.
Those
embryos
die
during
first
instar
larval
development.
The
same
phenotype
was
observed
among
transheterozygous
salIIB57/salILASS
(or
sal445,
sal65
or
sal16
)
embryos.
These
rescue
results
were
confirmed
independently
with
the
three
P-element
lines
inserted
on
the
third
chromosome.
Screening
and
sequencing
of
the
sal
gene
from
D.
virilis
About
80
000
plaques
of
a
genomic
D.virilis
library
were
screened
as
described
(Treier
et
al.,
1989),
using
the
sal
10.1
cDNA.
One
positive
clone
was
identified
and
shown
to
contain
the
sal
gene
of
D.
virilis.
The
gene
was
analysed
by
restriction
analysis,
hybridization
and
DNA
sequencing.
DNA
sequence
analysis
was
predominantly
done
of
the
coding
sequences
and
the
exon
-intron
boundaries.
Acknowledaements
We
thank
R.Kemler
and
D.Vestweber
for
helping
with
the
antibody
production
and
R.Sommer
for
providing
the
M.domestica
embryos.
We
also
thank
D.Tautz
for
providing
the
D.virilis
library
and
B.Hovemann
for
the
cDNA
library.
Many
thanks
go
to
T.Berleth,
S.Cohen,
G.Jurgens
and
G.Schutz
for
critical
reading
of
the
manuscript.
This
work
was
supported
by
the
Deutsche
Forschungsgemeinschaft
(DFG
grant
Schu
683/1-2
and
SFB
236).
References
Affolter,M.,
Schier,A.
and
Gehring,W.J.
(1990)
Curr.
Opin.
Cell
Biol.,
2,
485-495.
Akam,M.
(1987)
Development,
101,
1-22.
Bellen,H.J.,
O'Kane,C.,
Wilson,C.,
Grossniklaus,U.,
Pearson,R.K.
and
Gehring,W.J.
(1989)
Genes
Dev.,
3,
1288-1300.
Berg,J.M.
(1988)
Proc.
Natl
Acad.
Sci.
USA,
85,
99-102.
Campos-Ortega,J.
and
Hartenstein,V.
(1985)
The
Embryonic
Development
of
Drosophila
melanogaster.
Springer-Verlag,
Berlin.
Casanova,J.
(1989)
Wilhelm
Roux's
Arch.
Dev.
Biol.,
198,
137-140.
Colot,H.V.,
Hall,J.C.
and
Rosbash,M.
(1988)
EMBO
J.,
7,
3929-3937.
Cooley,L.,
Kelley,R.
and
Spradling,A.
(1988)
Science,
239,
104-210.
Courey,A.J.
and
Tjian,R.
(1988)
Cell,
55,
887-898.
El-Baradi,T.
and
Pieler,T.
(1991)
Mech.
Dev.,
35,
155-169.
Fan,C.-M.
and
Maniatis,T.
(1990)
Genes
Dev.,
4,
29-42.
Frei,E.,
Baumgartner,S.,
Edstrom,J.-E.
and
Noll,M.
(1985)
EMBO
J.,
4,
979-987.
Frei,E.,
Schuh,R.,
Baumgartner,S.,
Burri,M.,
Noll,M.,
Jurgens,G.,
Seifert,E.,
Nauber,U.
and
Jackle,H.
(1988)
EMBO
J.,
7,
197-204.
Gaul,U.,
Seifert,E.,
Schuh,R.
and
Jackle,H.
(1987)
Cell,
50,
639-647.
Gehring,W.J.
and
Hiromi,Y.
(1986)
Annu.
Rev.
Genet.,
20,
147-173.
Gibson,T.J.,
Postma,J.P.M.,
Brown,R.S.
and
Argos,P.
(1988)
Protein
Engng,
2,
209-218.
Han,K.
and
Manley,J.L.
(1993)
Genes
Dev.,
7,
491-503.
Hartenstein,V.
and
Jan,Y.N.
(1992)
WIlhe1n
Roux
'sArch.
Dev.
Biol.,
201,
194-220.
Hennig,W.
(1981)
Insect
Phylogeny.
Wiley,
Chichester,
UK.
Hulskamp,M.
(1991)
PhD
Thesis.
University
of
Tiubingen,
Tubingen,
Germany.
178

Molecular
genetics
of
the
Drosophila
spalt
gene
Ingham,P.
(1988)
Nature,
335,
25-33.
Jurgens,G.
(1985)
Nature,
316,
153-155.
Juirgens,G.
(1988)
EMBO
J.,
7,
189-196.
Juirgens,G.
and
Weigel,D.
(1988)
Wilhelm
Roux's
Arch.
Dev.
Biol.,
197,
345-354.
Kassis,A.J.,
Poole,S.J.,
Wright,D.K.
and
O'Farell,P.H.
(1986)
EMBO
J.,
5,
3583-3589.
Kaufman,T.C.,
Lewis,R.A.
and
Wakimoto,B.T.
(1980)
Genetics,
94,
115-
133.
Koerner,T.J.,
Hil,J.E.,
Myers,A.M.
and
Tzagoloff,A.
(1991)
Methods
Enzymol.,
194,
477-490.
Lee,M.S.,
Gippert,G.P.,
Soman,K.W.,
Case,D.A.
and
Wright,P.E.
(1989)
Science,
245,
635-637.
Lewis,E.B.
(1978)
Nature,
276,
565-570.
Licht,J.D.,
Grossel,M.J.,
Figge,J.
and
Hansen,U.M.
(1990)
Nature,
346,
76-79.
Macdonald,P.M.
and
Struhl,G.
(1986)
Nature,
324,
537-545.
Martinez-Arias,A.
and
Lawrence,P.A.
(1985)
Nature,
313,
639-642.
Mermod,N.,
O'Neill,E.A.,
Kelly,T.J.
and
Tjian,R.
(1989)
Cell,
58,
741
-753.
Michael,W.M.,
Bowtell,D.D.L.
and
Rubin.,G.M.
(1990)
Proc.
NatlAcad.
Sci.
USA,
87,
5351-5353.
Miller,J.,
McLachlan,A.D.
and
Klug,A.
(1985)
EMBO
J.,
4,
1609
-1614.
Pavletich,N.P.
and
Pabo,C.O.
(1991)
Science,
252,
809-817.
Reuter,D.
,Schuh,R.
and
Jackle,H.
(1989)
Proc.
Natl
Acad.
Sci.
USA,
86,
5483-5486.
Robertson,H.M.,
Preston,C.R.,
Phillis,R.W.,
Johnson-Schlitz,D.M.,
Benz,W.K.,
Engels,W.R.
(1988)
Genetics,
118,
461-470.
Roder,L.,
Vola,C.
and
Kerridge,S.
(1992)
Development,
115,
1017-1033.
Rubin,G.M.
and
Spradling,A.C.
(1982)
Science,
218,
348-353.
Ruther,U.
and
Muller-Hill,B.
(1983)
EMBO
J.,
2,
1792-1794.
Sambrook,J.,
Fritsch,E.F.
and
Maniatis,T.
(1989)
Molecular
Cloning:
A
Laboratory
Manual.
Cold
Spring
Harbor
Laboratory
Press,
Cold
Spring
Harbor,
NY.
Sanger,F.,
Nicklen,S.
and
Coulson,A.R.
(1977)
Proc.
NatlAcad.
Sci.
USA,
74,
5463-5467.
Schuh,R.,
Aicher,W.,
Gaul,U.,
Cote,S.,
Preiss,A.,
Maier,D.,
Seifert,E.,
Nauber,U.,
Schroder,C.,Kemler,R.
and
Jackle,H.
(1986)
Cell,
47,
1025-1032.
Spradling,A.C.
(1986)
In
Roberts,D.B.
(ed.),
Drosophila:
A
Practical
Approach.
IRL
Press,
Oxford,
pp.
175-197.
Struhl,G.
(1981)
Nature,
292,
335-338.
Sommer,R.
and
Tautz,D.
(1991)
Development,
113,
419-430.
Tautz,D.
and
Pfeifle,C.
(1989)
Cell,
98,
81-85.
Treier,M.,
Pfeifle,C.
and
Tautz,D.
(1989)
EMBO
J.,
8,
1517-1525.
Wagner-Bernholz,J.T.,
Wilson,C.,
Gibson,G.,
Schuh,R.
and
Gehring,W.J.
(1991)
Genes
Dev.,
5,
2467-2480.
Weigel,D.
and
Jackle,H.
(1990)
Cell,
63,
455-456.
Weigel,D.,
Jurgens,G.,
Kuttner,F.,
Seifert,E.
and
Jackle,H.
(1989)
Cell,
57,
645-658.
Yanisch-Perron,C.,
Vieira,J.
and
Messing,J.
(1985)
Gene,
33,
103-119.
Received
on
August
16,
1993;
revised
on
October
11,
1993
179
