
U.S. DEPARTMENT OF
Energy Efficiency &
ENERGY EDUCATION AND WORKFORCE DEVELOPMENT
Renewable Energy
ENERGY
Alternative Fuels Used in
Transportation
Grades: 5-8
Topic: Hydrogen and Fuel Cells, Vehicles, Biomass
Owner: National Renewable Energy Laboratory
This educational material is brought to you by the U.S. Department of Energy’s
Office of Energy Efficiency and Renewable Energy.

This lesson plan may contain links to other resources,
including suggestions as to where to purchase materials.
These links, product descriptions, and prices may change over time.
A
lternative Fuels Used in Trans
p
ortation
For the Teacher:
The use of energy is a factor in all our
lives, and that is why it is important for
us teachers to have our students learn
about the energy we use now and the
new forms of energy that are becoming
available. Non-renewable energy
sources are diminishing everyday, and it
is vital that students learn about
renewable energy sources to help them
as they grow to become better informed
and more responsible about the energy
resources they use.
The use of gasoline for
transportation is the most commonly
used fuel. However, there are multiple
alternative fuels that are making their
ways to the market. These alternative
fuels include such things as propane,
natural gas, electric hybrids, hydrogen
fuel cells, and biodiesel. Students will
probably have heard of some of these
alternative fuels, but they may not
understand how and why they are
better then ordinary gasoline. The
projects included in this section are
designed to give students the
opportunity to create their own
investigation and test alternative fuels
and their relation to transportation.
The projects included will fit
easily with regular classroom lessons
surrounding scientific inquiry and the
scientific method. The projects have
of the capability to cross multiple
education disciplines such as chemistry,
physics, economics, and marketing and
they involve social interaction as well as
group learning.
Alternative fuels are being
researched by top scientists every day
at NREL to discover which fuel methods
work best, how well they work and how
easily they can be distributed to the
public. The authors of this section are
studying the emissions released from
large trucks running on different
biodiesel fuels to compare which blends
create lower emissions.
National Science Education
Standards by the National Academy
of Sciences:
Science Content Standards: 5-8
Science as Inquiry
- Content Standard A:
“Abilities necessary to do scientific
inquiry”
“Understandings about scientific
inquiry”
Physical Science
- Content Standard B:
“Properties and changes of
properties in matter”
“Transfer of energy”
216

This lesson plan may contain links to other resources,
including suggestions as to where to purchase materials.
These links, product descriptions, and prices may change over time.
Earth and Space Science
- Content Standard D:
“Structure of the earth system”
Science and Technology
- Content Standard E:
“Abilities of technological design”
“Understandings about science and
technology”
Science in Personal and Social
Perspectives
- Content Standard F:
“Populations, resources, and
environments”
“Science and technology in society”
Technolo
g
y Description:
Transportation by cars and trucks
radically changed the face of our
country over the last hundred years,
with petroleum providing the fuel for
our vehicles. We use about 13 million
barrels of oil each day to keep us on the
move. Americans drive their personal
vehicles about 2.3 trillion miles a year
with 98 percent of our vehicles running
on petroleum or diesel fuels. United
States imports two-thirds of all the
petroleum we use; therefore, cheaper
and renewable alternative fuels would
be desirable to reduce our dependence.
In addition to the dependence factor,
one also needs to consider that the
emissions from gasoline-powered
vehicles are fairly extensive and include
CO, CO
2
, NOx, SOx, VOCs, OH
-
, and PM.
Some of these emissions are known or
probable human carcinogens, including
benzene (known), formaldehyde,
acetaldehyde, and 1,3-butadiene
(probable). Gasoline can also impact the
environment if spilled, since it spreads
on water surfaces and quickly
penetrates porous soils and
groundwater.
The idea of alternative fuels has
been around about as long as there
been vehicles. In the 1880s, Henry
Ford built one of his first automobiles to
run on ethanol. The alternative fuels
that are being actively explored by the
Department of Energy include:
methanol; propane; ethanol;
compressed and liquefied natural gas;
electricity; hybrid electricity; biodiesel;
and hydrogen fuel cells. Factors such as
cost, fuel distribution, emissions, vehicle
systems analysis, energy storage, power
and propulsion systems, and advanced
power electronics are just some of the
considerations in phasing in alternative
fuels and advanced vehicle design.
Complete Combustion
Fuel (hydrocarbons) + Air (O
2
& N)
⇒ CO
2
+ H
2
O + N
Typical Engine Combustion
Fuel + Air Unburned Hydrocarbons
+ NO
x
+ CO + CO
2
+ H
2
O
Improving fuel economy, cost,
availability, and emissions are the
primary goals of research into
alternative fuels and transportation.
Alternative Fuels-
Methanol
Methanol, or wood alcohol, is a
colorless, odorless, toxic liquid.
Methanol is the simplest alcohol
(CH
3
OH), produced by replacing one
217

This lesson plan may contain links to other resources,
including suggestions as to where to purchase materials.
These links, product descriptions, and prices may change over time.
hydrogen atom of methane with a
hydroxyl radical (OH). Methanol can be
produced from natural gas, coal,
residual oil, or biomass.
Although vehicles can operate on pure
methanol fuel (M100), methanol
blended with 15 percent unleaded
gasoline–M85- is more practical for real
world applications. Because methanol is
a liquid fuel, it does not require major
changes in the distribution system or in
car engines, but no major auto
manufacturers offer M85 compatible
vehicles at this time. The cost of M85 is
equal to or slightly higher than premium
blends. M85 has a lower energy
content per gallon, so mileage is lower;
but power, acceleration and payload
capacity are comparable to gasoline.
Vehicles using methanol, however, must
use a special, expensive lubricant.
Propane
Propane is an energy-rich fossil fuel
often called liquefied petroleum gas
(LPG). It is colorless and odorless; an
odorant called mercaptan is added to
serve as a warning agent. Propane is a
by-product of petroleum refining and
natural gas processing. And, like all
fossil fuels, it is nonrenewable. The
chemical formula for propane is C
3
H
8
.
Under normal atmospheric
pressure and temperature, propane is a
gas. Under moderate pressure and/or
lower temperature, however, propane
can easily be changed into a liquid and
stored in pressurized tanks. Propane is
270 times more compact in its liquid
state than it is as a gas, making it a
portable fuel.
Propane has been used as a
transportation fuel for more than half a
century and is the most widely used and
most accessible alternative fuel. Today
about three percent of total propane
consumption is used to fuel 270,000
vehicles, mostly in fleets. For fleet
vehicles, the cost of using propane is 5
to 30 percent less than for gasoline.
Ethanol
Ethanol is a clear, colorless
alcohol fuel made by fermenting the
sugars found in grains—such as corn
and wheat—as well as potato wastes,
cheese whey, corn fiber, rice straw,
urban wastes, and yard clippings. There
are several processes that can produce
alcohol (ethanol) from biomass. The
most commonly used processes today
use yeast to ferment the sugars and
starch in the feedstock to produce
ethanol. A new process uses enzymes
to break down the cellulose in woody
fibers, making it possible to produce
ethanol from trees, grasses, and crop
residues.
In the 1970s, the oil embargoes
revived interest in ethanol as an
alternative fuel. Today, more than fifty
ethanol plants, mostly in the Midwest,
produce over a billion gallons of ethanol.
Gasoline containing ten percent
218

This lesson plan may contain links to other resources,
including suggestions as to where to purchase materials.
These links, product descriptions, and prices may change over time.
ethanol—E10—is widely used in urban
areas that fail to meet standards for
carbon monoxide and ozone. Since
ethanol contains oxygen, using it as a
fuel additive results in up to 25 percent
fewer carbon monoxide emissions than
conventional gasoline. E10 is not
considered an alternative fuel under
EPACT, but a replacement fuel.
Methane
Methane, the natural gas we use
for heating, cooking, clothes drying, and
water heating, can also be a clean
burning transportation fuel when
compressed (CNG) or liquefied (LNG).
Compressed natural gas (CNG) vehicles
emit 85-90 percent less carbon
monoxide, 10-20 percent less carbon
dioxide, and 90 percent fewer reactive
non-methane hydrocarbons than
gasoline-powered vehicles. (Reactive
hydrocarbon emissions produce ozone,
one of the components of smog that
causes respiratory problems.) These
favorable emission characteristics result
because natural gas is 25 percent
hydrogen by weight; the only
combustion product of hydrogen is
water vapor. Natural gas is usually
placed in pressurized tanks when used
as a transportation fuel. Even
compressed to 2,400-3,600 pounds per
square inch (psi), it still has only about
one-third as much energy per gallon as
gasoline. As a result, natural gas
vehicles typically have a shorter range,
unless additional fuel tanks are added,
which can reduce payload capacity. With
an octane rating of 120+, power,
acceleration and cruise speed are
comparable.
Electricity
In 1891, William Morrison of Des
Moines, Iowa, developed the first
electric car. By the turn of the century,
dedicated electric vehicles (EVs)
outnumbered their gasoline-powered
counterparts by two-to-one. Today
there are about 10,500 dedicated EVs in
use in the United States, mostly in the
West and South. Researchers are still
working on the same problem that
plagued those early dedicated EVs: the
need for an efficient battery. The
batteries limit the range of a dedicated
EV, which is determined by the amount
of energy stored in its battery pack. The
more batteries a dedicated EV can carry,
the more range it can attain, to a point.
Too many batteries can weigh down a
vehicle, reducing its load-carrying
capacity and range, and causing it to
use more energy. The typical dedicated
EV can only travel 50 to 130 miles
between charges. This driving range
assumes perfect driving conditions and
vehicle maintenance. Weather
conditions, terrain, and some
accessories use can significantly reduce
the range.
219

This lesson plan may contain links to other resources,
including suggestions as to where to purchase materials.
These links, product descriptions, and prices may change over time.
Hybrid Electricity
Hybrid Electric Vehicles (HEVs) may be
the best alternative vehicle for the near
future, especially for the individual
consumer. HEVs offer many of the
energy and environmental advantages
of the dedicated electric vehicle without
the drawbacks. Hybrids are powered by
two energy sources: an energy
conversion unit (such as a combustion
engine or fuel cell) and an energy
storage device (such as battery,
flywheel, or ultracapacitor). The energy
conversion unit can be powered by
gasoline, methanol, compressed natural
gas, hydrogen, or other alternative
fuels. HEVs have the potential to be
two to three times more fuel-efficient
than conventional vehicles. An HEV
battery doesn’t have to be recharged. It
has a generator powered by the internal
combustion engine to recharge the
batteries whenever they are low. A
regenerative braking system captures
excess energy when the brakes are
engaged. The recovered energy is also
used to recharge the batteries.
Gas-Electric Hybrids
Biodiesel
Biodiesel is a fuel made by
chemically reacting alcohol with
vegetable oils, fats, or greases, such as
recycled restaurant greases. It is most
often used in blends of two percent or
20 percent (B20) biodiesel. It can also
be used as neat biodiesel (B100).
Biodiesel fuels are compatible with and
can be used in unmodified diesel
engines with the existing fueling
infrastructure. It is the fastest growing
alternative transportation fuel in the
U.S. Biodiesel contains virtually no
sulfur, so it can reduce sulfur levels in
the nation’s diesel fuel supply.
Removing sulfur from petroleum-based
diesel results in poor lubrication.
Biodiesel is a superior lubricant and can
restore the lubricity of diesel fuel in
blends of only one or two percent.
Biodiesel can also improve the smell or
diesel fuel, sometimes smelling like
french fries.
Berkeley Curbside Recycling Trucks Now Fueled
by Recycled Vegetable Oil
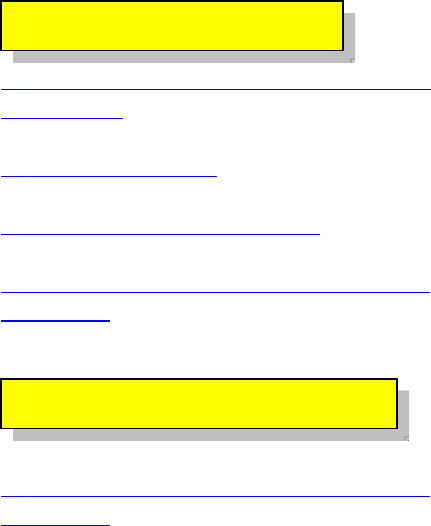
Hydrogen Fuel Cell
In the future, hydrogen may
provide a significant contribution to the
alternative fuel mix. The space shuttles
use hydrogen for fuel. Fuel cells use
hydrogen and oxygen to produce
electricity without harmful emissions;
water is the main by-product. Hydrogen
is a gas at normal temperatures and
pressures, which presents greater
transportation and storage hurdles than
liquid fuels. No distribution system
currently exists. Today, the
predominant method of producing
hydrogen is steam reforming of natural
220

This lesson plan may contain links to other resources,
including suggestions as to where to purchase materials.
These links, product descriptions, and prices may change over time.
gas, although biomass and coal can also
be used as feedstocks.
SunLine Transit Agency
Hydrogen Fuel Cell Bus
Resources:
Energy Efficiency and Renewable Energy
http://www.fueleconomy.gov/
National Energy Educational
Development
http://www.need.org
Energy Efficiency and Renewable Energy
http://www.eere.energy.gov
Kentucky Clean Fuels Coalition
www.kentuckycleanfuels.org
U.S. Environmental Protection Agency
www.epa.gov
National Renewable Energy Laboratory -
Department of Energy
www.nrel.gov
Alternate Transportation Fuels
http://www.need.org/needpdf/Alternativ
eFuels.pdf
Energy Correlation to National Science
Education Content Standards
http://www.need.org/needpdf/Correlatio
ns.pdf
Pro
j
ect Ideas
1 What is the heat content of
two alternative fuels?
Learning Objective: You will be able
to measure the amount of heat
absorbed by water during the
combustion of methanol and ethanol in
a calorimeter.
Controls and Variables: Volume of
water, temperature change in heat sink
(100 ml of water), mass of fuel used,
and heat content of two fuels.
Materials and Equipment: Economy
calorimeter; alcohol burners; ethanol;
methanol; & thermometers.
Safety and Environmental
Requirements: Safety glasses should
be worn at all times. The frame of the
economy calorimeter retains heats and
care must be taken when moving after
testing.
Suggestions:
• Determine the number of chemical
bonds in methanol (5) and ethanol
(8).
221

This lesson plan may contain links to other resources,
including suggestions as to where to purchase materials.
These links, product descriptions, and prices may change over time.
• Correlate the heat content of each
fuel related to the number of
chemical bonds.
• Students explore why the obtained
heat values fall below the actual heat
values (ethanol = 7089 cal/g and
methanol = 5426 cal/g).
• Do a balance of equations for the
combustion of methanol
(2CH
3
OH + 3 O
2
⇒ 2CO
2
+ 4H
2
O +
energy) and ethanol (C
2
H
5
OH + 3 O
2
⇒ 2CO
2
+ 4H
2
O + energy).
• Determine the oxygen to fuel ratio
for each fuel and how this ratio
would change the volume of carbon
dioxide produced.
Places To Purchase:
Calorimeter:
https://www1.fishersci.com/wps/portal/
HOME
($25.60, economy food calorimeter)
https://www.freyscientific.com
($21.95, economy food calorimeter)
A homemade calorimeter can be
made by using two different size cans
with holes through the top of both cans
to suspend the smaller can over the
flame with glass stir rod. The larger can
is a tube and the smaller can opens only
on top, to hold water and take
temperature readings (Note diagram
below).
Wickless Leakproof Burner
https://www1.fishersci.com/wps/portal/
HOME
($14.00, holds 100cc of fuel)
https://www.freyscientific.com
($13.65, holds 100cc of fuel)
Flint Glass Alcohol Lamp
https://www.freyscientific.com
($6.25, 8 oz. capacity)
https://www1.fishersci.com/wps/portal/
HOME
($6.35, 8 oz. capacity)
Ethanol
https://www1.fishersci.com/wps/portal/
HOME
($10.80, 1 liter)
https://www.freyscientific.com
($8.35, 1 liter)
Methanol
https://www1.fishersci.com/wps/portal/
HOME
($5.15, 500 ml)
https://www.freyscientific.com
($6.65, 500 ml)
2 What is the economically
best choice between
purchasing a hybrid or a
typical gasoline engine
automobile?
Learning Objective: You will be able
to show which type of engine is the
most economical in the long run
between a hybrid and typical gasoline
engine.
222
This lesson plan may contain links to other resources,
including suggestions as to where to purchase materials.
These links, product descriptions, and prices may change over time.
Controls and Variables: The different
variables for each automobile will be the
initial cost, the operating cost (gasoline
price), and the miles per gallon of fuel.
Constants should be the amount of
driving that would be done in each
automobile.
Materials and Equipment: Pen, paper
and access to the Internet for research.
Safety and Environmental
Requirements: None
Suggestions:
• The payback period is the length of
time you must own an energy-
efficient vehicle before the decreased
operational costs make up for the
difference in initial purchase price.
Calculate the payback period for a
Honda Civic (Hybrid) vs. a Honda
Civic (Gasoline) using the following
figures:
Honda Civic (Hybrid)
Initial Cost: $19,650
Tax Incentive: $1500
Miles per Gallon: 48 mpg
Honda Civic (Gasoline)
Initial Cost: $17,260
Tax Incentive: $0
Miles per Gallon: 40 mpg
Hybrid Gasoline
Initial Cost
$19,650 $17,260
Tax Incentive
$1,500 $0
Fuel Economy
48 mpg 40 mpg
Difference in
Initial cost
(+) $2390
Difference in cost
after tax incentive
(+) $890
Fuel economy at a
gas rate of $2.10
per gallon for
one year at 10,000
$525/yea
miles
$438/year r
A
mount of time till
hybrid savings
exceed gasoline
10.2
initial savings
years
• Investigate the rate at which hybrid
vehicles have been decreasing in
initial cost. What might be some
factors for this decrease? Do they
expect hybrid vehicles to someday
have a lower initial cost then
gasoline engine vehicles?
• Research other types of alternative
fuels. Is a hybrid more cost
efficient then compressed natural
gas (CNG), hydrogen fuel cells,
propane, or biodiesel?
3 Determine a plan for large
number fleets of automobiles
to transition for traditional
gasoline engines to
alternative fuel engines.
The mayor of a large city in your
area has asked your class to develop a
plan to reduce emissions created by his
fleet, including school buses, public
223
4
This lesson plan may contain links to other resources,
including suggestions as to where to purchase materials.
These links, product descriptions, and prices may change over time.
buses, sanitation trucks, police,
emergency vehicles, and the city fleet of
automobiles. Divide the project into six
parts and in each part develop a plan to
present to the mayor, listing
recommendations and costs for each
type of vehicle and the rationale for
each recommendation.
List the recommendations of each
part by vehicle category. Where there
are several recommendations, debate
and defend recommendations until a
consensus is reached.
Learning Objective: You will
understand different forms of alternative
fuels as well as a greater understanding
of citywide economics.
Controls and Variables: None
Materials and Equipment: Pen, paper
and access to the Internet for research.
Safety and Environmental
Requirements: None
Suggestions: Invite area experts to
visit the classroom to discuss alternative
fuel vehicles.
Present your findings in a formal report
to be sent to your local mayor.
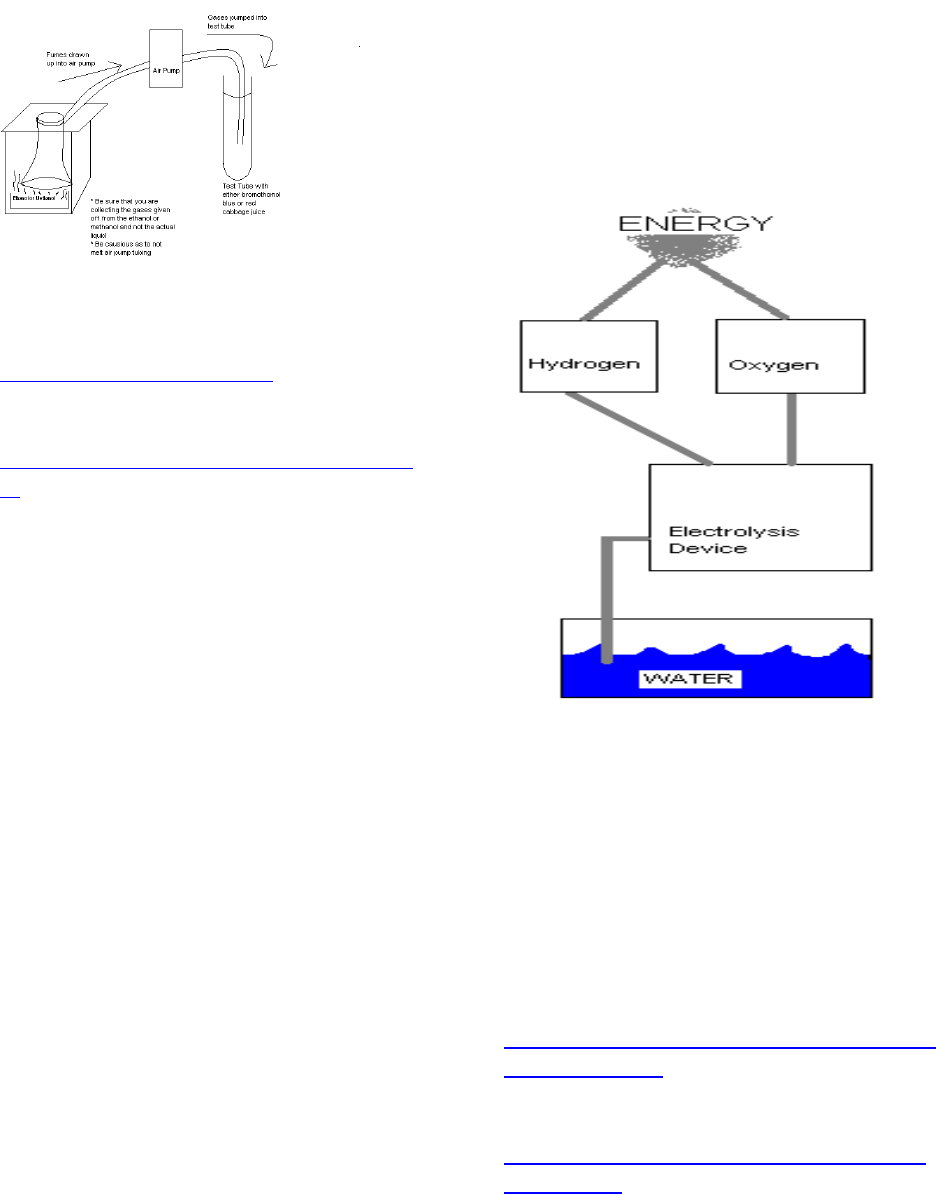
Quantify the relative amount
of CO
2
given off by the
methanol vs. ethanol during
the combustion process.
Utilize a fish aquarium pump to
pull samples of exhaust fumes from
above the calorimeter experiments
(done in project idea one) and let it
pump the collected gases into a test
tube of bromothymol blue solution or
red cabbage juice to determine the
relative CO
2
content. (Bromothymol
blue will change from blue to green to
yellow in the presence of CO
2
.)
Learning Objective: You will be able
to test for the presence of CO
2
in the
combustion of ethanol and methanol
and qualitatively compare the two
amounts.
Controls and Variables: The different
amounts of ethanol and methanol that
are used can either be held constant or
varied depending on the experiment.
Hold the solution of bromothymol blue
or red cabbage juice constant through
each test.
Materials and Equipment: A fish
aquarium air pump with tubing, air
pump in sealed container with inlet and
outlet air tubes, two test tubes, scale to
determine mass of fuel consumed,
material from project #1, bromothymol
blue solution or red cabbage juice, and
stopwatch.
Safety and Environmental
Requirements: Safety wear (goggles,
lab apron, heat resistant gloves), well
ventilated area for burning, and waste
container. (Do not pour ethanol or
methanol down the drain or into
garbage.)
224
5
This lesson plan may contain links to other resources,
including suggestions as to where to purchase materials.
These links, product descriptions, and prices may change over time.
Places to Purchase:
Bromothymol Blue, 0.04% in Ethanol
http://www.baddley.com/ ($12.00 – 125
ml)
http://www.clarksonlab.com/salesaz.ht
m ($14.95 - 5 g powder reagent)
What goes into building a
hydrogen fuel cell car?
There are many different
available models of hydrogen fuel cell
cars that can be bought. Try just using
the basic parts to build a unique
hydrogen fuel cell vehicle.
Learning Objective: You will be able
to understand the hydrogen fuel cell
process of how water (through
electrolysis) is turned into power to run
a motor.
Controls and Variables: A simple
hydrogen fuel cell vehicle kit will give all
the components necessary; everything
else is up to you.
Materials and Equipment: Hydrogen
fuel cell vehicle kit, some power tools
may be necessary when building custom
vehicle bodies. You will also need
smooth surface to run the vehicles on.
Safety and Environmental
Requirements: Safety protection
should be taken when building the cars
from scratch. You also require
protection from small electrical circuits
and a moving motor.
Suggestions:
1. See who can build the fastest
vehicle by changing wheels and axis,
body types, and gear sizes.
2. Determine the efficiency of the
hydrogen fuel cell.
Places to Purchase:
Hydrogen Fuel Cell Kits
http://electronickits.com/kit/complete/so
lar/fuelcell.htm
(~ $125)
http://sciencekit.com/category.asp_Q_c
_E_427448
(~ $220)
225
This lesson plan may contain links to other resources,
including suggestions as to where to purchase materials.
These links, product descriptions, and prices may change over time.
References:
http://www.nap.edu/readingroom/books
/nses/html/
http://www.nrel.gov
http://www.fueleconomy.gov/
http://www.need.org/needpdf/Alternativ
eFuels.pdf
Photo References:
http://www.need.org/needpdf/Alternativ
eFuels.pdf
226
