
1
Primary Care Respiratory
Update
PCRS position on FeNO testing for
asthma diagnosis
The fractional exhaled nitric oxide (FeNO) test
measures the level of NO in the exhaled breath
and provides an indication of eosinophilic inflam-
mation in the lungs. For the diagnosis of asthma,
the British Thoracic Society (BTS) and the Scot-
tish Intercollegiate Guidelines (SIGN) position
FeNO testing after the objective evaluation of air-
ways obstruction and alongside other potential
tests for inflammation such as determination of
blood eosinophil levels, IgE skin-prick test to
detect atopy, and tests for variability (reversibility,
peak expiratory flow [PEF] charting and challenge
tests).
1
Patients with a history and clinical char-
acteristics that support a high probability of
asthma and who have had an objective measure
of reversible airways obstruction do not need
FeNO before progressing to a trial of treatment.
1
Additional objective evidence including FeNO is
recommended as an optional investigation as a
test for eosinophilic asthma for those considered
to have an intermediate probability of asthma.
1
The current PCRS position aligns with the guid-
ance issued by BTS/SIGN. This article reviews
the evidence base and clinical guidelines upon
which the PCRS position is based.
Background
Asthma is a heterogeneous condition character-
ized by respiratory symptoms (wheeze, cough,
breathlessness, chest tightness and pain) asso-
ciated with variable airflow obstruction, hyper-
responsiveness and often an underlying inflam-
mation. There is no single defining feature or
symptom of asthma, however variability is at its
core, so diagnosis is achieved through a holistic
evaluation of patient symptoms over time along-
side repeated physiologic evaluation of lung func-
tion, and assessment of response to trials of
treatment. Pathologic evaluations including tests
for eosinophilic airway inflammation, and other
investigations, may sometimes be needed.
Nitric oxide (NO) is produced in the lungs and
so can be detected in the exhaled breath and
elevated exhaled NO levels are thought to be
related to eosinophilic lung inflammation.
2
Frac-
tional exhaled NO (FeNO) testing is quantitative,
noninvasive, simple and safe and elevated FeNO
may be supportive of a diagnosis of asthma in
untreated individuals presenting with respiratory
symptoms.
3
However, while suggestive, a posi-
tive FeNO test is not conclusive evidence of
asthma. Indeed, eosinophilic lung inflammation
has been suggested to be a contributing factor
to asthma in approximately 50% of cases, with
the remaining 50% of cases not showing evi-
dence of eosinophilic lung inflammation.
4,5
There has been considerable discussion in
recent years regarding the relevance of FeNO
testing in the diagnostic workup of patients pre-
senting with respiratory symptoms for whom a
diagnosis of asthma is suspected. Here we
review the current recommendations for the role
of FeNO testing in the diagnosis of asthma and
explore the benefits, limitations and challenges of
utilising this test in the primary care setting.
How is FeNO testing conducted?
The FeNO test measures the level of NO in the
exhaled breath. FeNO testing is conducted using
a handheld device into which the patient blows
FeNO Testing For Asthma Diagnosis -
A PCRS Consensus
FeNO Testing For Asthma Diagnosis - A PCRS Consensus was commissioned to set out
the PCRS position on the role of FeNO testing within the context of asthma diagnosis.
Carol Stonham Vice chair, Primary Care Respiratory Society and
NHS Gloucestershire CCG and Noel Baxter Chair PCRS Executive
Feno article2.qxp_Layout 1 28/06/2019 09:33 Page 1

2
Primary Care Respiratory
Update
for 10 seconds at 60 litres a minute. A shorter test is available for
children. The result is provided within approximately 1 minute with
a FeNO level ≥35 ppb as a positive test in children and a level
≥40 ppb as a positive test in adults.
6
What are the current recommendations for FeNO
testing for asthma diagnosis?
NICE
In November 2017 the National Institute for Health and Care
Excellence (NICE) issued guidance for the diagnosis, monitoring
and management of asthma.
6
The guidance focuses on objective
testing for the diagnosis of asthma and suggests FeNO evalua-
tion be considered as an objective test alongside spirometry and
peak expiratory flow (PEF) at initial presentation if equipment is
available, and as part of the diagnostic algorithm for both children
over 5 and adults with respiratory symptoms suggestive of
asthma (Box 1).
BTS/SIGN
The 2016 BTS/SIGN guidance takes a pragmatic approach to
asthma diagnosis and recommends that for patients with a
high probability of asthma, a trial of treatment is appropriate.
1
The guideline incorporates FeNO testing as part of the
diagnostic algorithm only for patients with an intermediate
probability of asthma where further evidence is required
(Box 2). Unlike the current NICE guidance, the principle inves-
tigation is to test for airway obstruction and bronchodilator
reversibility on spirometry. FeNO testing is positioned after
spirometric evaluation as an optional investigation to test for
eosinophilic inflammation along side determination of blood
eosinophil level, IgE skin-prick test for detection of atopy, and
tests for variability (reversibility, PEF charting and challenge
tests)
1
if the results of spirometric evaluation are not clear. A
positive FeNO increases the probability of asthma but a
negative test does not exclude a diagnosis of asthma.
Box 1: NICE guidance for the role of FeNO is the evaluation and diagnosis of asthma in children over
5 and adults
6
FeNO in the diagnosis of asthma in children
• Consider a FeNO test in children and young people (aged 5-16 years) if there is diagnostic uncertainty after initial
assessment and they have either:
o Normal spirometry or
o Obstructive spirometry with a negative bronchodilator reversibility test. Regard a FeNO level of ≥35 ppb as
a positive test
•
Suspect asthma in children and young people (aged 5-16 years) if they have symptoms suggestive of asthma and:
o A FeNO level ≥35 ppb with normal spirometry and negative peak flow variability or
o A FeNO level ≥35 ppb with obstructive spirometry but negative bronchodilator reversibility and no variability in
peak flow or
o Normal spirometry, a FeNO level ≤34 ppb and a positive peak flow variability
• Diagnose asthma in children and young people (aged 5-16 years) if they have symptoms suggestive of asthma
and:
o A FeNO ≥35 ppb with normal spirometry and negative peak flow variability or
o Obstructive spirometry and positive bronchodilator reversibility
FeNO in the diagnosis of asthma in adults
• Offer a FeNO test to adults (aged >17 years) if a diagnosis of asthma is being considered. Regard a FeNO level
of ≥40 ppb as a positive test
• Suspect asthma in adults (aged >17) with symptoms suggestive of asthma, obstructive spirometry and:
o Negative bronchodilator reversibility, and either a FeNO level ≥40 ppb, or a FeNO levels between 25 and
39 ppb and positive peak flow variability, or
o
Positive bronchodilator reversibility, a FeNO level between 25 and 39 ppb and negative peak flow variability
• Diagnose asthma in adults (>17 years) if they have symptoms suggestive of asthma and:
o A FeNO ≥40 ppb with either positive bronchodilator reversibility or positive peak flow variability or bronchial
hyperreactivity or
o A FeNO between 25 and 39 ppb and a positive bronchial challenge test or
o Positive bronchodilator reversibility and positive peak flow variability irrespective of FeNO level
Feno article2.qxp_Layout 1 28/06/2019 09:33 Page 2
3
Primary Care Respiratory
Update
Benefits of FeNO testing as part of the diagnostic
workup for asthma
Reliance on physiologic measures of lung function is a point-
in-time measure and as such if patients are asymptomatic on
the day they attend for testing the results may be negative. A
negative result may also be delivered if the test did not achieve
optimal quality due to operational or patient factors. FeNO is
an objective measure of eosinophilic lung inflammation which
is likely to persist even in the absence of overt respiratory
symptoms on a given day.
7-9
At present, data on the cost-effectiveness of FeNO testing in
the primary care setting is limited but early indications suggest
this may be favourable.
10
Although an initial investment in equip-
ment and training is required with ongoing consumable costs,
there may be cost savings associated with correct diagnosis,
reduced referrals to secondary care and reductions in emergency
primary care and accident and emergency visits.
11-14
While not currently recommended in clinical practice guide-
lines, evidence suggests FeNO testing may also be informative
for the ongoing monitoring of patients with asthma with poor
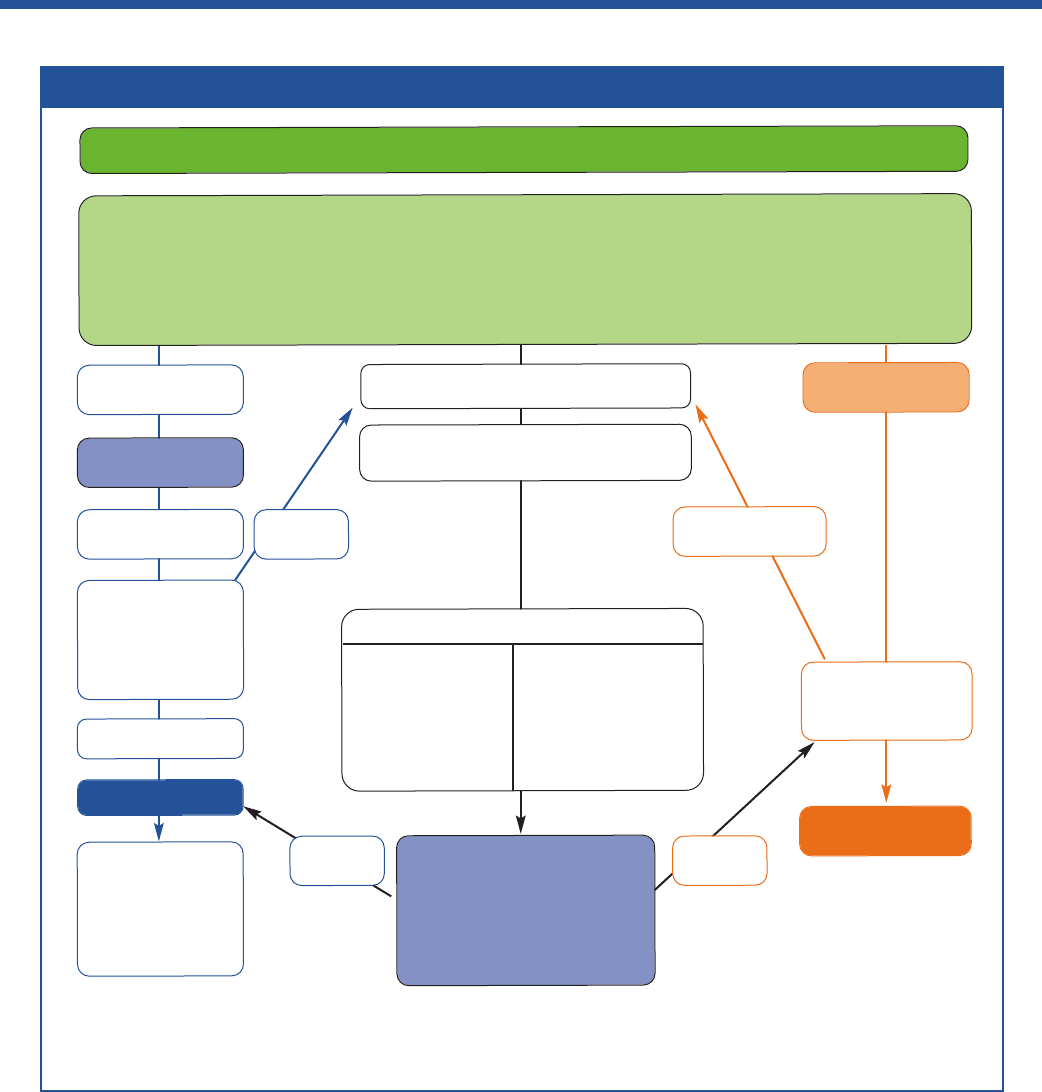
Box 2: The BTS/SIGN diagnostic algorithm for patients presenting with respiratory symptoms
1
Presentation with respiratory symptoms: wheeze, cough, breathlessness, chest tightness*
Structured clinical assessment (from history and examination of previous medical records)
Look for
• recurrent episodes of symptoms
• symptom variability
• absence of symptoms of alternative diagnosis
• recorded observation of wheeze
• personal history of atopy
• historical record of variable PEF or FEV
1
Adjust maintenance
dose. Provide self-
management advice
Arrange on-going
review
High probability
of asthma
Code as:
suspected asthma
Poor
response
Initiation of
treatment
Assess response
objectively
(lung function/
validated symptom
score)
Good response
Asthma
Good
response
Poor
response
Other diagnosis
confirmed
Investigate/treat for
other more likely
diagnosis
Low probability
of asthma
Other diagnosis
unlikely
Intermediate probability of asthma
Test for variability:
• reversibility
• PEF charting
• challenge tests
Test for eosinophilic
inflammation or
atopy:
• FeNO
• blood eosinophils
• skin-prick test, IgE
Options for investigations are:
Test for airway obstruction
spirometry + bronchodilator reversibility
Suspected asthma:
Watchful waiting
(if symptomatic)
or
Commence treatment and
assess response objectively
* In children under 5 years and others unable to undertake spirometry in whom there is a high or intermediate probability of asthma, the options are
monitored initiation of treatment or watchful waiting according to the assessed probability of asthma
Feno article2.qxp_Layout 1 28/06/2019 09:33 Page 3

4
Primary Care Respiratory
Update
control, providing an objective measure of steroid responsiveness
and providing an alert of persistent lung inflammation even in the
absence of evidence of airway obstruction.
9,15
Changes in FeNO
levels may be useful to guide step up and step down of anti-
inflammatory medication and may prompt an evaluation of
adherence and inhaler technique. Having an objective test result
may facilitate opening up a conversation about adherence and
inhaler technique that may be otherwise difficult to approach or
forgotten.
Challenges and limitations of FeNO testing
There is some overlap between FeNO levels among individuals
with and without asthma.
3
An evaluation of the results of eight
studies among adults within the secondary care setting sug-
gested that around 1 in 5 individuals with a positive FeNO test
will not have asthma (false positive) and around 1 in 5 people with
a negative FeNO test will have asthma (false negatives).
1
Data
are lacking for primary care populations. However, as a general
rule, a FeNO level ≥40 ppb is regarded as positive in adults with
a level of ≥35 ppb regarded as positive in children.
1
A variety of factors not related to the pathology of asthma
can result in increased and decreased levels of FeNO, confound-
ing the utility of this test in supporting a diagnosis of asthma
(Box 3).
Understanding these potentially confounding factors and the
potential for false positive and false negative results is essential
to the proper utilization of FeNO testing as part of the diagnostic
workup of patients presenting with respiratory symptoms.
In the general practice setting cost may be a barrier to the
routine use of FeNO testing as part of the work up of patients
presenting with respiratory symptoms suggestive of asthma. The
introduction of Primary Care Networks and new ways of working
with larger populations offers opportunity in primary care beyond
practice level. FeNO is not currently widely available in the UK
and if this test is to be a required component of the diagnostic
workup primary care networks will be required to invest in the
necessary equipment, training (usually provided by the manu-
facturer of the equipment required) and consumables or rely
on referrals to secondary care. Routine FeNO testing for all
patients with asthma may not be a practical approach for all
primary care practices at this time. The NICE 2017 guideline
recommends a FeNO test for all adults presenting with acute
respiratory symptoms suggestive of asthma if equipment is
available and if testing will not compromise treatment of the
acute episode. However, treatment can be initiated for patients
who are acutely unwell at presentation if waiting for objective
tests may compromise treatment of the acute episode.
Objective tests should then be carried out once the acute
symptoms have been controlled. Referral to secondary care
may be made in cases of diagnostic uncertainty. An alternative
to investment in FeNO testing by individual primary care prac-
tices may be a locality-based approach whereby primary care
practices in a given locality or Primary Care Network pool
resources to invest in a FeNO testing service. This approach
is currently being trialled in the UK.
6,16
Conclusions
FeNO testing is a quantitative, non-invasive, simple and safe test
making it suitable for use in the primary care setting with appro-
priate training of health care professional with responsibility for
delivering and interpreting the results.
3
The benefits to patients
are that they do not need to be referred to secondary care for
additional testing as a positive FeNO test alongside respiratory
symptoms and lung function tests suggestive of asthma supports
a diagnosis. However, concerns remain over the necessity for
FeNO testing in every asthma diagnosis and its cost-effective-
ness, and it has been suggested the FeNO testing is more
appropriately placed in diagnostic centres within the community,
intermediate or secondary care setting. Given the current limita-
tions of extending FeNO testing to all patients presenting with
symptoms suggestive of asthma, the current PCRS position
aligns with the guidance issued by BTS/SIGN namely the use of
Box 3: Confounding factors that may result in an increased or decreased FeNO level
1
Confounding factors that may INCREASE
FeNO levels
FeNO levels may be higher than population norms in:
• Men, tall individuals and those consuming a diet high
in nitrates
FeNO levels may be elevated in:
• Patients with allergic rhinitis exposed to an allergen
even in the absence of respiratory symptoms
• Patients with active rhinovirus infection
Confounding factors that may DECREASE
FeNO levels
FeNO levels may be lower than population norms in:
• Children (a lower reference range must be used)
FeNO levels may be reduced in:
• Cigarette smokers
• Patients recently treated with inhaled or oral
corticosteroids
Feno article2.qxp_Layout 1 28/06/2019 09:33 Page 4

5
Primary Care Respiratory
Update
FeNO testing as an optional investigation to test for eosinophilic
inflammation where there is diagnostic uncertainty.
Acknowledgements
We gratefully acknowledge the considered review of this document provided by
our colleagues Hetal Dhruve (Community Pharmacist, London), Deborah Leese
(Pharmacist, Chesterfield) and Laura Rush (Practice Respiratory Lead, Somerset).
Editorial support was provided by Dr Tracey Lonergan.
References
1. BTS/SIGN. SIGN 153. British guideline on the management of asthma.
Published September 2016. Available at: https://www.brit-thoracic.org.uk/docu-
ment-library/clinical-information/asthma/btssign-asthma-guideline-2016/.
Accessed March 2019.
2. Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, et al.
Pathological features and inhaled corticosteroid response of eosinophilic and
non-eosinophilic asthma. Thorax 2007;62:43-9.
https://doi.org/10.1136/thx.2006.073429
3. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al.
An official ATS clinical practice guideline: interpretation of exhaled nitric oxide
levels (FeNO) for clinical applications. Am J Respir Crit Care Med 2011;184:
602-15. https://doi.org/10.1164/rccm.9120-11ST
4. Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma:
important and possible mechanisms. Thorax 2002;57:643-8.
https://thorax.bmj.com/content/57/7/643
5. McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Rand Sutherland E, Chinchilli
VM, et al. Asthma Clinical Research Network of the National Heart, Lung, and
Blood Institute: a large subgroup of mild-to-moderate asthma is persistently
noneosinophilic. Am J Respir Crit Care Med 2012;185:612-9.
https://doi.org/10.1164/rccm.201109-1640OC
6. NICE. Asthma: diagnosis, monitoring and chronic asthma management. NICE
guideline ng80. Published 29 November 2017. Available at:
https://www.nice.org.uk/guidance/ng80. Accessed March 2019.
7. Boulet LP, Turcotte H, Plante S, Chakir J. Airway function, inflammation and
regulatory T cell function in subjects in asthma remission. Can Respir J 2012;
19:19-25. http://dx.doi.org/10.1155/2012/347989
8. Cano-Garcinuno A, Carvajal-Urena I, Diaz-Vazquez CA, Dominguez-
Aurrecoechea B, Garcia-Merino A, Molla-Caballeron de Rodas P, et al.
Clinical correlates and determinants of airway inflammation in pediatric
asthma. J Investig Allergol Clin Immunol 2010;20:303-10.
http://www.jiaci.org/summary/vol20-issue4-num604
9. Paro-Heitor ML, Bussamra MHCF, Saraiva-Romanholo BM, Martins MA,
Okay TS, Rodrigues JC. Exhaled nitric oxide for monitoring childhood asthma
inflammation compared to sputum analysis, serum interleukins and pulmonary
function. Pediatr Pulmonol 2008;43:134-41. https://doi.org/10.1002/ppul.20747
10. Harnan SE, et al. Measurement of exhaled nitric oxide concentration in asthma:
a systematic review and economic evaluation of NIOX, MINO, NIOZ VERO and
NObreath. Health Technology Assessment 2015;19.82. Available at:
https://www.ncbi.nlm.nih.gov/books/NBK321828/. Accessed March 2019.
11. Calhoun WJ, Ameredes BT, King TS, Icitovic N, Blkeecker ER, Castro M, et al.
Comparison of physician-, biomarker-, and symptom-based strategies for
adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT
randomized controlled trial. JAMA 2012;308:987-97.
https://doi.org/10.1001/2012.jama.10893
12. Peirsman EJ, Carvelli TJ, Hage PY, Hanssens LS, Pattyn L, Raes MM, et al.
Exhaled nitric oxide in childhood allergic asthma management: a randomised
controlled trial. Pediatr Pulmonol 2014;49:624-31.
https://doi.org/10.1002/ppul.22873
13. Powell H, Murphy VE, Taylor DR, Hensley MJ, McCaffery K, Giles W, et al.
Management of asthma in pregnancy guided by measurement of fraction of
exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet 2011;
378:983-90. https://doi.org/10.1016/S0140-6736(11)60971-9
14. Syk J, Malinovschi A, Johansson G, Unden AL, Andreasson A, Lekander M, et al.
Anti-inflammatory treatment of atopic asthma guided by exhaled nitric oxide: a
randomized, controlled trial. J Allergy Clin Immunol Pract 2013; 1:639-48.
https://doi.org/10.1016/j.jaip.2013.07.013
15. LaForce C, Brooks E, Herje N, Dorinsky P, Rickard K. Impact of exhaled nitric
oxide measurements on treatment decisions in an asthma specialty clinic. Ann
Allergy Asthma Immunol 2014; 113:619-23.
https://doi.org/10.1016/j.anai.2014.06.013
16. Metting EI, Riemersma RA, Kocks JH, Piersma-Wichers MG, Sanderman R,
van der Molen T. Feasibility and effectiveness of an asthma/COPD service for
primary care: a cross-sectional baseline description and longitudinal results.
NPJ primary care respiratory medicine 2015;25.
https://www.nature.com/articles/npjpcrm2014101
Date of Preparation:
June 2019 Version 1
Feno article2.qxp_Layout 1 28/06/2019 09:33 Page 5
