
EN EN
EUROPEAN
COMMISSION
Brussels, 5.7.2023
SWD(2023) 412 final
COMMISSION STAFF WORKING DOCUMENT
IMPACT ASSESSMENT REPORT
Accompanying the document
Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE
COUNCIL
on plants obtained by certain new genomic techniques and their food and feed, and
amending Regulation (EU) 2017/625
{COM(2023) 411 final} - {SEC(2023) 411 final} - {SWD(2023) 411 final} -
{SWD(2023) 413 final}

i
Table of contents
1. Introduction: Scientific, Political, and Legal Context ....................................................................... 1
1.1. Scientific context ..................................................................................................................... 1
1.2. Political context ....................................................................................................................... 5
1.3. Legal context ........................................................................................................................... 9
1.4. International dimension ........................................................................................................ 10
1.5. Scope ..................................................................................................................................... 11
1.6. Interaction with existing legislation and upcoming initiatives .............................................. 12
1.7. Previous studies on the EU GMO legislation with relevance to NGTs .................................. 13
2. Problem definition ......................................................................................................................... 13
2.1. What are the problems? ....................................................................................................... 13
2.2. What is the size of the problem and who is affected? .......................................................... 16
2.3. What are the problem drivers? ............................................................................................. 18
2.4. How likely is the problem to persist? .................................................................................... 18
3. Why should the EU act?................................................................................................................. 19
3.1. Legal basis .......................................................................................................................... 19
3.2. Subsidiarity: Necessity of EU action .................................................................................. 20
3.3. Subsidiarity: Added value of EU action ............................................................................. 20
4. Objectives: What is to be achieved?............................................................................................. 20
4.1. General objectives ............................................................................................................. 20
4.2. Specific objectives ............................................................................................................. 21
5. What are the available policy options? ......................................................................................... 22
5.1. What is the baseline from which options are assessed? .................................................. 24
5.2. Description of the policy options ...................................................................................... 25
5.3. Options discarded at an early stage .................................................................................. 30
6. What are the impacts of the policy options? ................................................................................ 31
7. How do the options compare? ...................................................................................................... 58
8. Preferred Option ............................................................................................................................ 72
8.1. Description of the preferred option ...................................................................................... 72
8.2 Simplification and burden reduction, supporting the one-in-one-out approach ....................... 74
9. How will actual impacts be monitored and evaluated? ................................................................ 75
10. List of references ........................................................................................................................... 77
Annex 1: Procedural information .......................................................................................................... 91

ii
Annex 2: Stakeholder consultation (Synopsis report) ........................................................................... 97
Annex 2a: Summary report on feedback received on Inception Impact Assessment: Legislation for
plants produced by certain new genomic techniques ........................................................................ 111
Annex 3: Who is affected and how?.................................................................................................... 123
Annex 4: Analytical methods ............................................................................................................... 130
Annex 5: The techniques ..................................................................................................................... 148
Annex 6: Opinions of scientific advisory bodies and scientific organisations on NGTs ...................... 154
Annex 7: Applications of NGTs in plants and case studies on potential impacts ................................ 158
Annex 8: EU legal framework on GMOs .............................................................................................. 172
Annex 9: Problem Tree ........................................................................................................................ 177
Annex 10: Intervention logic ............................................................................................................... 178
Annex 11: Procedure for notification (Option 4) ................................................................................ 179
Annex 12: SME TEST ............................................................................................................................ 181

iii
Abbreviations
Term or acronym
Meaning or definition
CJEU
Court of Justice of the European Union
CD
Celiac Disease
DG SANTE
Directorate-General for Health and Food Safety
DUS
Distinctness, Uniformity and Stability
EASAC
European Academies’ Science Advisory Council
EFSA
European Food Safety Authority
ENGL
European Network of GMO (Genetically Modified Organisms)
Laboratories
ENSSER
European Network of Scientists for Social and Environmental
Responsibility
ERA
Environmental Risk Assessment
EU
European Union
EURL
European Union Reference Laboratory
EU-SAGE
European Sustainable Agriculture Through Genome Editing
FAO
Food and Agriculture Organization of the United Nations
F2F
Farm to Fork Strategy
FRM
Forest reproductive material
FSFS
Framework for a sustainable food system
FTE
Full Time Equivalent
GHG
Greenhouse gas
GMO
Genetically Modified Organism
GM
Genetically modified
HRI
Harmonised Risk Indicator
HT
Herbicide-tolerant
IIA
Inception impact assessment
IPR
Intellectual property rights
ISAA
International Service for the Acquisition of Agri-biotech Applications
JRC
European Commission’s Directorate-General Joint Research Center
LCA
Life-cycle assessment
MRIO
Multi-regional input-output modelling
NCWS
Non-celiac wheat sensitivity
NGT
New genomic techniques
OECD
Organization for Economic Co-operation and Development
PLB
Potato Late Blight
PRM
Plant reproductive material
QALY
Quality-adjusted life year
R&D
Research and development
RNQP
Regulated non-quarantine pest

iv
SAM HLG
Scientific Advice Mechanism High-Level Group
SDG
Sustainable Development Goal
SME
Small and medium-sized enterprise
SPS
Sanitary and phytosanitary measures
SUR
Sustainable use of plant protection products regulation
TFEU
Treaty on the Functioning of the European Union
UAA
Utilised Agricultural Area
UN
United Nations
UPOV
Union for the Protection of New Varieties of Plants
VCU
Value for Cultivation and Use
WTO
World Trade Organization
Glossary of scientific terms
Term or acronym
Meaning or definition
*
Agrobiodiversity
A measure of the number and relative abundance of
species and varieties in cultivation; it also takes into
account the genetic diversity within varieties
Breeders’ gene pool
The total genetic information available in one species and
other taxonomic species with which it can be cross-bred,
including by using advanced techniques such as embryo
rescue, induced polyploidy and bridge crosses.
Cisgenesis / intragenesis
Insertion of genetic material (e.g. a gene) into a recipient
organism from a donor that is sexually compatible
(crossable). The exogenous genetic material can be
introduced without (cisgenesis) or with
modifications/rearrangements (intragenesis).
Clustered regularly
interspaced short palindromic
repeat associated
nucleases/CRISPR associated
protein (CRISPR/Cas)
A family of SDNs (see definition below) that recognise a
precise position in the DNA where a cut will be effected.
Cover crop/cash cover crop
Plants planted to cover the soil. These crops are used to
manage, among others, soil erosion, soil fertility, soil
quality and weeds. Cash cover crops also fulfil this
function but also supply harvestable and marketable
products.
Desoxyribonucleic acid
(DNA)
The genetic material carrying the information for the
development, functioning, growth and reproduction of all
known organisms and many viruses.

v
Established genomic
techniques
Genetic modification techniques developed prior to 2001,
when the existing GMO legislation was adopted. In the
context of this impact assessment, the term does not
include random mutagenesis techniques, which are
exempted from the requirements of the GMO legislation.
Event-specific detection
method
A detection method that is capable to detect and identify a
specific transformation event. In the context of this impact
assessment, this means a method that is able to
differentiate a specific NGT product from conventional
ones that contain the same modification(s).
Exogenous DNA
DNA produced by whatever means outside a recipient
organism from a donor organism that can be sexually
compatible or not.
Foreign DNA
DNA produced by whatever means outside a recipient
organism from a donor organism that is sexually
incompatible (non-crossable).
Genetically Modified
Organism (GMO)
An organism, with the exception of human beings, in
which the genetic material has been altered in a way that
does not occur naturally by mating and/or natural
recombination.
Genome
The entire genetic material present in a cell of an
organism.
Genome editing (gene
editing) techniques
A subset of NGTs that allow precise modification of the
DNA in targeted positions in the genome. Genome editing
encompasses a variety of techniques, which may be
applied in mutagenesis, cisgenesis or transgenesis.
Mutagenesis
Creation of mutation(s) in an organism without insertion
of foreign genetic material.
New Genomic Techniques
(NGTs)
An umbrella term used to describe a variety of techniques
that can alter the genetic material of an organism and that
have emerged or have been developed since 2001, when
the existing legislation on the deliberate release of GMOs
into the environment was adopted.
Niche crops
Either neglected and underutilized crops cultivated in
previous centuries for food, feed and fibre uses, but which
have in recent times been reduced in importance and value
and for which hardly any breeding activity is ongoing, or
high value crops produced on relatively small volumes.
vi
Off-target effects
Unintended mutations that are introduced in locations of
the genome other than the intended one.
Oligonucleotide directed
mutagenesis (ODM)
A targeted mutagenesis technique by which
oligonucleotides (short pieces of DNA) are used to
introduce small, precise mutations in a genome.
Plant Reproductive Material
Any plant material (for example seeds, tubers, cuttings,
rootstocks, seedlings, young plants, fully grown trees) that
is used for the production of other plants.
Random mutagenesis
An umbrella term used to describe conventional breeding
techniques based on mutagenesis that have been used
since the 1950s; they involve irradiation or treatment with
chemicals in order to produce random mutations in a
genome, and typically involve screening of a large number
of mutants to select one with desirable properties.
Site-directed nucleases
(SDN)
Enzymes that cut DNA at precise and selected target
locations. SDNs use a guiding molecule to target the site
to be cut. Various SDNs exist, depending on the nature of
the guiding molecule and the type of enzyme, e.g. ZFNs,
TALENs, CRISPR/Cas. Depending on their type and
application, SDNs can be used for mutagenesis, cisgenesis
and transgenesis.
Site-directed nuclease type 1
(SDN-1)
A targeted mutagenesis technique using SDNs to
introduce small mutations in a specific location of the
genome. In SDN-1, no DNA template is provided, so the
type of mutation is random.
Site-directed nuclease type 2
(SDN-2)
A targeted mutagenesis technique using SDNs to
introduce small, precise mutations in a specific location of
the genome. In SDN-2, a DNA template is used to obtain a
pre-determined mutation.
Site-directed nuclease type 3
(SDN-3)
An application of SDNs that allows the introduction of
exogenous genetic material in a specific location of the
genome. If the inserted material comes from a donor
organism that is sexually compatible with the host
organism, the result is cisgenesis; if the inserted material
comes from a donor organism that is sexually
incompatible with the host organism, the result is
transgenesis.
Sustainability
The long-term ability of food systems to provide food
security in a way that does not compromise the economic,
social, and environmental bases that generate food security
vii
for future generation (HLPE 2020).
Targeted Mutagenesis
An umbrella term used to describe newer techniques of
mutagenesis that induce mutation(s) in selected target
locations of the genome without insertion of foreign
genetic material.
Transformation event
A genetically modified plant that has exogenous DNA
inserted in (a) certain location(s) of the genome
Transgenesis
Insertion of foreign genetic material (e.g. a gene) into a
recipient organism from a donor organism that is sexually
incompatible (non-crossable).
Unique alteration/
modification
An alteration/modification that is not present or unlikely to
be present in other GMOs or conventional/natural
varieties.
* For the purpose of this document

1
1. Introduction: Scientific, Political, and Legal Context
This impact assessment explores economic, environmental, social (including health) impacts,
as well as impacts on fundamental rights and administrative burden, of options for a legal
framework for the deliberate release, including placing on the market, of plants and their food
and feed products developed using certain new genomic techniques (NGTs), namely targeted
mutagenesis and cisgenesis
1
.
The term ‘new genomic techniques’ is specific to the EU regulatory environment and
encompasses genetic modification techniques that have emerged or have been developed
since 2001, when the centrepiece of the current EU legislation on genetically modified
organisms (GMOs) was adopted
2
(see section 1.1. below and Annex 5).
Plant breeding, the practice of changing and/or selecting the traits of plants in order to
produce desired characteristics, is a constantly evolving field. Humans have made use of
natural variation (i.e. mutations) since first cultivating land and breeding livestock around 13
000 years ago, selecting and retaining organisms suitable for agricultural use. For centuries,
breeders have used new scientific findings to increase the effectiveness and efficiency of their
efforts constantly adding new plant breeding techniques to their toolbox. During the 20
th
century a gradual shift took place from breeding being conducted mostly in public institutions
toward the private sector, mainly due to the introduction of hybrids. This led to consolidation
in the industry and the dominance of several key companies in the major field crops. The
emergence of biotechnology in agriculture in the 1980s led to a further reorganisation of the
sector moving plant breeding to a high-tech industry involving more and more trans-national
companies.
Today, the EU market for plant reproductive material (PRM) (agriculture and forestry) has an
estimated annual value of EUR 15 billion
3
.
The EU seed market is the third largest after the
USA and China and accounts for around 20% of the global market. Its value is estimated at
EUR 7-10 billion
4
and it comprises about 7 000 companies (most small and medium sized
enterprises, SMEs) with about 52.000 employees. EU companies are highly diverse as regards
size, portfolio of crops, geographical area and activities (plant breeding, maintenance of
varieties, multiplication, trade). The EU breeding sector is highly innovative and spends
around 15% of its annual turnover on R&D.
1.1. Scientific context
Development of the techniques
The advent of NGTs in the past two decades, associated to advances in the understanding of
how genes function and in genome sequencing techniques, provides new opportunities to alter
the genetic material of an organism allowing the rapid development of plant varieties with
specific characteristics. NGTs, as any breeding technique applicable in agriculture, make use
of genetic diversity either naturally occurring or resulting from human intervention, in order
to select or generate plants that feature desirable characteristics. NGTs constitute a diverse
group of techniques, each of which can be used in various ways to achieve different results
and products. NGT products may or may not contain foreign DNA; when they do, they may
result from the introduction into an organism of genetic material derived from the same
species or from other species, either crossable or non-crossable. In this respect, NGTs may
1
Unless specified, the term cisgenesis in this document encompasses also intragenesis.
2
Directive 2001/18/EC
3
Eurostat, see European Commission, 2023b. Annex 4.
4
Ragonnaud (2013)

2
produce modifications that could or could not be obtained in nature or by conventional
breeding. In most cases, these new techniques can lead to more targeted and precise changes
than conventional breeding or established genomic techniques.
Targeted mutagenesis and cisgenesis are considered NGTs and differentiated from established
genomic techniques because they have introduced novel features (e.g. higher precision and
speed, introduction of genetic material from a crossable species). They do not introduce
genetic material from a non-crossable species (transgenesis), which is the case with
established genomic techniques. In certain cases, genetic modifications introduced by NGTs
cannot be identified by analytical methods, while this is always possible for established
genomic techniques.
Targeted mutagenesis induces mutation(s) in selected target locations of the genome without
insertion of foreign genetic material and, in certain cases, can produce alterations of the
genetic material that can also occur naturally or that can be obtained by conventional
breeding. Cisgenesis includes the insertion of genetic material, by random or targeted
technologies
5
, into a recipient organism from a donor that is sexually compatible (i.e.
crossable) and produces alterations that in some cases can also be obtained by natural crossing
or conventional breeding. Both targeted mutagenesis and cisgenesis can also produce
alterations of the genome that are more complex and challenging to obtain through
conventional breeding.
Opinions of scientific bodies
Over the last decade, there have been numerous scientific opinions on NGTs (including on
targeted mutagenesis and cisgenesis) in the EU, e.g. by the Commission’s Scientific Advice
Mechanism High-Level Group (SAM HLG)
6
, the European Academies' Science Advisory
Council (EASAC)
7
, the Commission’s Joint Research Centre (JRC)
8
, and the European Food
Safety Authority (EFSA)
9
(see Annex 6). These reflect the majority positions on the relevant
scientific issues.
The above-mentioned bodies report on the increasing precision of certain NGTs compared to
conventional breeding approaches. Changes introduced with conventional breeding
techniques occur randomly, while certain NGTs can produce precisely located alterations to
DNA sequences. In view of their increased precision, such NGTs do not require the extensive
screening of large plant populations, necessary for conventionally bred plants, to select the
organism with the desired characteristics.
In addition, when changes are small and known in other organisms, the resulting products are
expected to display more predictable characteristics. For these reasons and for their increased
precision, many NGTs shorten the development time to obtain organisms with desired traits.
Since NGTs allow to target modifications, they generally result in fewer unintended genetic
modifications compared to conventional breeding techniques.
The European Group on Ethics in Science and New Technologies (EGE) published in 2021 an
opinion on the ethics of genome editing, which focuses on applications in the human, animal
5
Since the first discussions in the EU in 2007, cisgenesis and intragenesis have been included among the new
genomic techniques, regardless of the technology (random or targeted) used to produce them.
6
European Commission (2017)
7
EASAC (2017)
8
European Commission (2021a)
9
EFSA (2012, 2020, 2022a, 2022b, 2022c)

3
and plant domains
10
. Among its conclusions on plants, EGE recommends a systems approach
to evaluate costs and benefits in any future use and regulation proportional to the risk.
The European Union Reference Laboratory for GM food and feed (EURL GMFF) and the
European Network of GMO Laboratories (ENGL) issued in 2019 a report on the detection of
food and feed plant products obtained by new mutagenesis techniques
11
. They concluded that
it is not feasible to differentiate a specific NGT product from conventional ones that contain
the same modification(s). An updated report by the EURL-ENGL is expected in 2023
12
.
Overall, the above-mentioned opinions and reports recognise the variety of NGTs techniques,
and of the different products with different risk profiles they can generate. They note that
similar products obtained by different techniques are not expected to present significantly
different risks. Also, when the introduced changes are limited and not novel, the assessment
of potential risks might be facilitated.
Regarding targeted mutagenesis and cisgenesis techniques, EFSA and other scientific bodies
concluded that there are no new hazards specifically linked to the genomic modification
produced via these techniques as compared with conventional breeding or established
genomic techniques.
EFSA also considered that on a case-by-case basis, a lesser amount of data might be needed
for the risk assessment of plants produced by targeted mutagenesis and cisgenesis and
therefore there is a need for flexibility in the data requirements for risk assessments. EFSA
and other scientific bodies also concluded that, in targeted mutagenesis, off-target
modifications are fewer than those occurring with most mutagenesis techniques and, where
such changes occur, they are of the same types as those produced by conventional breeding.
The considerations above are shared by other major scientific bodies outside the EU,
including the US National Academies of Sciences, Engineering, and Medicine
13
, Health
Canada
14
, the UK Advisory Committee on Releases to the Environment
15
and the Norwegian
Biotechnology Advisory Board
16
. FAO acknowledges that issues of safety must be
considered
17
. FAO also considers that priorities can be established for regulatory interventions
including a food safety assessment while also recognizing that some of the food products
produced from gene editing could have food safety characteristics similar to foods with a long
history of safe use
18
.
However, some scientific organisations and agencies, such as the German Federal Agency of
Nature Conservation, the European Network of scientists for social and environmental
responsibility (ENSSER) and TestBiotech, disagree with the above opinions
19
. They raise the
concern that NGTs pose new and specific risks to the environment and human health
compared to previous applications of genetic engineering
20
. They consider that NGTs can be
used to achieve genomic changes beyond what is known from conventional breeding and can
10
https://ec.europa.eu/info/files/ethics-genome-editing_en
11
European Network of GMO Laboratories (ENGL) (2019)
12
European Network of GMO Laboratories (ENGL) (2023)
13
National Academies of Sciences, Engineering, and Medicine (2016)
14
Government of Canada (2022)
15
ACRE (2021)
16
Norwegian Biotechnology Advisory Board (2018)
17
FAO (2022)
18
FAO (2023)
19
Bundesamt für Naturschutz (2021); ENSSER (2021); Testbiotech (2022)
20
Kawall (2021)

4
alter the genome to a much greater extent with multiple modifications than with any previous
breeding method. Furthermore, they refer to unintended genetic changes that, according to
them, would be specific to the processes of NGTs and unlikely to occur via random processes
or conventional breeding. Furthermore, these organisations question that NGTs are precise,
controllable and predictable.
EFSA has evaluated the scientific literature provided by these organisations in the public
consultations conducted in the preparation of its opinions and considered that it does not
provide new evidence which would alter the validity of its scientific opinions. While
acknowledging that the application of NGTs can lead to a complexity of scenarios and that a
case-by-case approach is applicable to NGT plants, EFSA has confirmed that NGTs are more
precise, controllable and predictable than conventional methods and their precision is
continually increasing with technological progress. In addition, there are no unintended
modifications that would be specific for NGTs. EFSA has noted that introducing multiple
modifications is also not specific to NGT approaches; this can also be achieved by
conventional breeding approaches and established genomic techniques.
Research and development
Globally, the majority of the research on crops using NGTs is conducted in China (43%),
followed with some distance by the US (18%), with 14% of this research taking place in the
EU
21
. Research in the EU, also funded through the Horizon programme
22
, is mainly conducted
in France, Germany, Italy, Belgium and the Netherlands.
The type of plant applications that feature prominently in the research and development
pipeline, coupled with the fairly easy and speedy applicability of these new techniques, could
deliver benefits to farmers, consumers and to the environment. JRC
23
as well as a database of
available literature on the use of genome editing in crop plants maintained by the EU-SAGE
network
24
and a recent report from the Food and Agriculture Organization of the United
Nations (FAO)
25
, show that research using these techniques concerns a wider variety of crops
and traits compared to the transgenic organisms authorised in the EU or globally so far.
Examples include plants with improved tolerance or resistance to biotic stress (plant diseases
and pests), plants with improved tolerance or resistance to climate change effects in general
and abiotic stresses (environmental, e.g., temperature, drought), improved nutrient and water-
use efficiency in plants, and plants with improved agronomic (e.g., higher yield/input ratio
and improved resilience) and quality characteristics (e.g., taste, shelf-life). NGTs can
contribute to the development of new and improved plants and can further support the use of
underutilised, neglected and local crop species – this can also support the special needs in the
Outermost Regions. The research pipeline also includes applications of NGTs to develop the
type of traits that have been the most common in the GMOs authorised so far in the EU or in
other countries, such as herbicide-tolerance (see Annex 7).
21
https://www.eu-sage.eu/index.php/genome-search
22
E.g. GeneBEcon – (https://cordis.europa.eu/project/id/101061015). In addition, Horizon Europe includes a
forthcoming call HORIZON-CL6-2023-FARM2FORK-01-11: New detection methods on products derived from
new genomic techniques for traceability, transparency and innovation in the food system.
23
Parisi & Rodriguez Cerezo (2021)
24
https://www.eu-sage.eu/index.php/genome-search
25
FAO (2022)

5
NGTs have the potential to support the implementation of the UN 2030 Agenda for
Sustainable Development and the relevant Sustainable Development Goals (SDG)
26
, in
particular (see Annex 3.3):
SDG2 (End hunger, achieve food security and improved nutrition and promote
sustainable agriculture), by facilitating plant breeding applied to a wide variety of
crops and beneficial traits, thus contributing to three of the four components of food
security (food availability, stability and utilisation).
SDG3 (Good Health and Well-being), by facilitating the breeding of diverse nutritious
and healthy foods.
SDG9 (Industry, Innovation and Infrastructure), by promoting research in plant
breeding, strengthening the research capacity in plant biotechnology and facilitating
the development of innovative and sustainable products.
SDG12 (Responsible consumption and production), by contributing to the sustainable
management and efficient use of natural resources (e.g. nutrients and water).
SDG13 (Take urgent action to combat climate change and its impacts) by contributing
to resilience adaptive capacity to climate change, supporting sustainable farming
practices (no tilling), the development of crops not requiring land use change and land
saving.
1.2. Political context
GMOs today
GMOs have been a controversial topic in the EU since the adoption of the EU GMO
legislation. Since the entry into force of Regulation (EC) No 1829/2003
27
, no draft
Commission decision proposing the authorisation of GM food and feed has obtained a
favourable opinion by qualified majority of the Member State in the relevant Regulatory
Committee, notwithstanding favourable EFSA opinions. The Commission has authorised
284
28
GMOs for food and feed and 6 GMOs for non-food uses
29
, based on favourable EFSA
opinions. 20 of these have been renewed following a favourable EFSA opinion. All these
GMOs have been judged by EFSA as safe as their conventional counterparts. No regulatory
measures (to withdraw, suspend or amend an existing authorisation) have been taken based on
evidence of risks to human or animal health or to the environment.
In the EU, the only GMO authorised for cultivation is maize MON810. It is commercially
grown in Spain since 1995 and to lesser extent in Portugal. Since 2015, when a “cultivation
opt-out”
30
was introduced in the legislation, 18
31
of the 27 EU Member States
restricted/prohibited the cultivation of this GMO in all or part of their territories.
The number of field trials in the EU has decreased from 387 (between 2008 and 2014) to 63
(between 2015 and 2022)
32
.
26
https://www.un.org/sustainabledevelopment/sustainable-development-goals/
27
Regulation (EC) No 1829/2003
28
This number includes single events and stacked events including their sub-combinations authorised to be
placed on the market
29
Cut flowers (carnations)
30
Directive (EU) 2015/412
31
Austria, Belgium (Wallonia), Bulgaria, Cyprus, Croatia, Denmark, Germany, France, Greece, Hungary, Italy,
Latvia, Lithuania, Luxemburg, Malta, the Netherlands, Poland, Slovenia
32
Parisi & Rodriguez Cerezo (2021)

6
While the market for genetically modified (GM) food products in the EU is small, a
substantial market exists for GM feed. The EU is a major importer of high-protein content
agricultural commodities for feed use
33
, mainly from countries where cultivation is dominated
by GMOs. According to 2016 figures
34
, out of the 36 million tonnes of soybean equivalent
imported per year into the EU, around 30 million (i.e. 85%) was GM.
Whereas cultivation of GMOs in the EU is very limited, globally there is now experience of
more than 25 years since the first commercialisation of GM crops. Beyond the EU, in 2019,
27 countries grew around 190 million hectares of biotech crops. An additional 17 countries
imported biotech crops for food, feed, and processing. Thus, including the EU, a total of 71
countries have adopted biotech crops so far
35
. An extensive study by the US National
Academies of Sciences, Engineering and Medicine published in 2016 on GM crops reported
no observed adverse effects on health and the environment, while recognising the difficulty to
assess long-term environmental impacts
36
.
NGTs in the context of key EU strategies
The debate on NGTs is polarised in the EU, along similar lines as the debate on GMOs.
However, it takes place in a context that has changed significantly.
The double crisis of climate change and biodiversity loss have put the focus on long-term
resilience and the need for a transition to more sustainable agriculture and food systems. In
this context, the European Green Deal’s Farm to Fork Strategy presents the Commission’s
plan for a transition to a sustainable food system
37
. It specifically refers to biotechnology as a
possible tool for increasing sustainability, provided they are safe for consumers and the
environment while bringing benefits for society as a whole. The Biodiversity Strategy
38
aims
to support and incentivise the transition to fully sustainable agricultural practices, improving
the condition and diversity of agroecosystems.
In its 2022 Communication
39
“Safeguarding food security and reinforcing the resilience of
food systems”, the Commission has identified NGTs as potentially contributing to food
security. In this context, according to the FAO
40
, although the extent of the impact is still
speculative, the introduction of gene-editing technologies will have far-reaching implications
for agri-food and social systems in terms of their potential for improving and securing
production of food.
The Covid-19 pandemic and Russia’s war of aggression against Ukraine have also revealed
the EU’s external dependencies. While EU food security has not been at stake, a global spike
in prices of inputs needed for agri-food production, in particular energy, animal feed and feed
additives, and fertilisers has shown the EU’s vulnerability to price shocks. In its Trade Policy
Review Communication
41
, the Commission has stressed the role of trade openness within the
concept of “Open Strategic Autonomy”, notably recalling the importance of an open and fair
trade with well-functioning, diversified and sustainable global value chains.
33
EU Feed Protein Balance Sheet (November 2022), https://agriculture.ec.europa.eu/data-and-
analysis/markets/overviews/balance-sheets-sector/oilseeds-and-protein-crops_en
34
European Commission (2016)
35
ISAAA (2019)
36
National Academies of Sciences, Engineering, and Medicine (2016)
37
European Commission (2020a)
38
European Commission (2020b)
39
European Commission (2022a)
40
FAO (2022)
41
European Commission (2021b)

7
Strategic autonomy and resilience also depend on the diversification of the food system.
NGTs are applied to a far larger range of crop species than established techniques and can
thereby contribute, for example, to decreasing the EU’s dependence on imports of plant
proteins. NGTs and their technical accessibility could also support the diversification of
developers and users in the food systems, if access to and affordability of the technologies is
assured.
Under the European Climate Law
42
, the EU must continuously progress in enhancing adaptive
capacity, strengthening resilience and reducing vulnerability to climate change. In its
Communication ‘Forging a climate-resilient Europe - the new EU Strategy on Adaptation to
Climate Change’, the Commission acknowledged that making better use of genetic diversity
and non-harmful plant genetic resources for adaptation based on the latest science is among
the urgently needed solutions to help farmers and land managers tackle climate risks
43
.
The EU Bioeconomy Strategy
44
supports innovation at the service of the Green Deal
objectives, in particular the deployment of a circular bio-based industry contributing to the
replacement of fossil resources by sustainably generated bio-based materials and chemicals. It
recognises the potential of biotechnology and life sciences to achieve such objectives. The
Council's conclusions on the opportunities of the bioeconomy from March 2023
45
specifically
mentioned the importance of seizing the opportunities presented by the bioeconomy and
called on the Commission to enhance the integration of the bioeconomy into all policies and
ensure coherence among policies.
The European Parliament has adopted several resolutions
46
where it refers to new plant
breeding techniques and recognises the importance to develop and use such techniques which
respond to societal and agricultural demands and enhance the competitiveness of the
agriculture and horticulture sectors. In 2021 on a resolution
47
on Farm to Fork Strategy, the
European Parliament also referred to the Commission’s plans to initiate a regulatory policy
action plants derived from certain new genomic techniques highlighting the precautionary
principle and the need to ensure transparency and freedom of choice to farmers, processors
and consumers. Against this background, there is currently a strong demand by a range of
stakeholders including breeders, farmers and academia, to adapt the regulatory framework to
enable the development, marketing and use of NGTs as tools contributing to address current
challenges. Other stakeholders, however, consider that the benefits of NGTs are hypothetical
and achievable by means other than biotechnology. Some are concerned about safety (e.g.,
environmental organisations) and/or possible negative impacts on their business (organic and
GM-free sectors).
Other factors affecting the development and marketing of NGTs
There is widespread recognition that the development and marketing of NGTs, and the
realisation of their potential, depends on various factors. An appropriate regulatory framework
is important, but patents, access of farmers, public organisations and SMEs to the
42
Regulation (EU) 2021/1119
43
European Commission (2021d)
44
European Commission (2018)
45
Council of the European Union (2023)
46
https://www.europarl.europa.eu/doceo/document/TA-7-2014-0131_EN.html;
https://www.europarl.europa.eu/doceo/document/TA-8-2016-0251_EN.html;
https://www.europarl.europa.eu/doceo/document/TA-8-2016-0252_EN.html;
47
https://www.europarl.europa.eu/doceo/document/TA-9-2021-0425_EN.html

8
technologies and the market, and factors affecting consumer acceptance, will also have an
impact.
The intellectual property landscape regarding NGT plants is complex and rapidly evolving
48
.
Patents and access to affordable patent licenses for NGT technologies and for genetic material
obtained by these techniques will play a role in breeders’ (especially SMEs’) ability to
develop and market new plant varieties based on these techniques. Some plant breeders are
concerned that the cost of licences to access the technology can be prohibitive, notably for
small crops
49
. In addition, there are calls from some stakeholders that patentability should not
negatively impact the ability of farmers to choose, afford and propagate harvested NGT plant
seeds on their farms.
Consumer acceptance of NGTs has been the subject of several social research studies and
national/international surveys conducted across the EU (EU-wide and in Sweden, Norway,
Italy, the Netherlands, Belgium, Austria, Germany), as well as in other countries (such as the
UK, Switzerland, Canada, US, Australia, Japan, Korea and China), in the last 5 years.
Research so far is limited, and some studies involve limited participation. However, they
provide emerging evidence of factors that may drive consumer acceptance of NGTs. General
research on consumer perceptions and attitudes to food and food safety has also provided
insights into expected consumer views on NGTs.
Regarding citizens’ current perception of NGTs, there are studies reporting on the one hand
negative attitudes and on the other hand an increased acceptance of NGT food compared to
current perception of GMOs. Attitudes on GMOs in general and NGTs in particular are
closely connected to levels of knowledge and the perceived safety levels
50
. Several studies
indicate that the majority (60-64%) of Europeans have not heard of NGTs and that consumers'
knowledge on NGT organisms and GMOs is limited
51
. However, comparatively, at this stage
they appear to be more knowledgeable about GMOs generally than about NGTs
52
. The level
of awareness on NGTs varies among different countries but is generally low
53
.
EFSA has recently conducted research into food safety in general as well as on NGTs
concretely. Food safety is considered important by the majority (70%) of the EU population.
In terms of the most important concerns, 26% expressed concern about GM ingredients and
8% about NGT ingredients. Regarding NGTs, citizens are interested in possible risks (69%),
who benefits and who bears the risks (42%), regulation (40%), what benefits NGTs bring
(38%), consumers’ choice (38%), and the scientific process and techniques (31%). As regards
information on food risks, this research also showed high trust in scientists in all Member
States and in EU institutions in several Member States
54
.
Research conducted globally suggests that the majority of the respondents are receptive to the
use of NGT products, particularly in the agricultural sector, as long as they bring societal
48
Kock (2021)
49
See Annex 5, section 2.3.3. in Technopolis et al. (2023)
50
Strobbe et al. (2023), FSA. (2021), Sprink et al (2022), Son & Lim (2021), Bundesinstitut für
Risikobewertung (BfR) (2017), GENEinnovate. (2020), Beghin & Gustafson (2021)
51
European Commission (2021c), section 4.10.2, EFSA (November 2021). FSA (2021), Swedish Gene
Technology Advisory Board, Department of Plant biology (SLU), Novus. (December 2021), GENEinnovate.
(April 2020), The Greens/EFA (2021)
52
Hu et al. (2022)
53
Strobbe et al.(2023), Busch et al (2022), Kato-Nitta et al (2021), Beghin & Gustafson (2021)
54
EFSA. (November 2021), EFSA. (September 2022)

9
benefits and promote sustainability
55
, for example through traits adapted to climate change,
reduced pesticide use and improved nutritional content. A recent Eurobarometer on citizens
expectations on sustainability indicated that for consumers the most important characteristic
of sustainability are the health and nutritional aspects (41%), the absence of pesticides (32%)
and affordability (29%). 18% of the respondents correlated organic food with sustainability
56
.
The majority of respondents in research studies believe that labelling of NGT products is
necessary
57
. In some cases, there is also a preference for distinct labelling of GM and NGT
products. Research indicates that consumers may also desire information on the specific trait
that has been modified and which genetic technology has been used, to be provided on
product labels
58
.
In the context of this initiative, consumer trust is relevant not only as regards acceptance of
NGTs but also in relation to organic production. Research shows different results on the
importance of the absence of GMOs in the decision to purchase organic food. This decision
seems to depend on different factors, such as health awareness, food safety concerns,
environmentally friendly production practices, consumer knowledge of organic foods, animal
welfare, avoidance of chemical substances, perceived or subjective social norms and
availability of organic food options
59
.
In its 2021 opinion, EGE acknowledges the prevalence of public concern in relation to
GMOs, including the lack of public dialogue and informed debate, which in its view
accompanied the introduction of GMO products, and calls for more attention to public
dialogue on NGT plants
60
.
1.3. Legal context
In the EU, regulatory requirements for GMOs are enshrined in five main legislative acts
61
and
have two main objectives: to protect human and animal health and the environment and to
ensure the effective functioning of the internal market. They establish harmonised procedures
requiring an authorisation for the deliberate release of GMOs into the environment for
experimental purposes as well as for the placing on the market and cultivation of GMOs and
GM food and feed. This authorisation system is based on an assessment of the risks to human
and animal health and the environment, and includes requirements for post-authorisation
monitoring, labelling and traceability.
In the context of the Cartagena Protocol on Biosafety, the EU has made a number of
international commitments with respect to the safe transfer, handling and use of living
modified organisms resulting from modern biotechnology
62
.
55
Swedish Gene Technology Advisory Board (2021), GENEinnovate. (April 2020), Kato-Nitta et al. (2021),
Baum et al (2023), Sikkema (2021), Grain Club (2023), Rathenau Instituut (2023), Saleh et al. (2021)., Beghin
& Gustafson (2021)
56
Special Eurobarometer 505 – Wave EB93.2 – Kantar (December 2020)
57
YouGov (2022), Lindberg et al. (2023), The Greens/EFA (2021), Rathenau Instituut (2023)
58
GENEinnovate. (April 2020), Swedish Gene Technology Advisory Board (2021), YouGov (2022), Lindberg
et al. (2023), The Greens/EFA (2021)
59
Gundala et al. (2021), Singh & Verma (2017), Paul & Rana (2012), Michaelidou & Hasan (2008), Padel et al.
(2015), Torjusen et al. (2004), Pew Research Centre (2016), IFOAM, BEUC, Special Eurobarometer 520 –
Kantar (2022)
60
European Group on Ethics (EGE) (2021)
61
Directive 2001/18/EC, Regulation (EC) No 1829/2003, Regulation (EC) No 1830/2003, Directive
2009/41/EC.
62
https://bch.cbd.int/protocol/text/

10
In 2018, the Court of Justice of the EU (CJEU) ruled
63
that new mutagenesis techniques are
not exempted from to the requirements of the EU GMO legislation (as is the case of old
mutagenesis techniques) (Annex 8).
In November 2019, the Council noted that, while the Court ruling clarified the scope of the
GMO legislation, concerns remained about the application of the EU legal framework when
products obtained with new mutagenesis techniques are not distinguishable from those
resulting from natural mutations or from conventional breeding. The Council therefore
requested
64
the Commission to prepare a study on the status of NGTs under EU law
(hereinafter referred to as ‘Commission NGT study’).
The Commission 2021 NGT study
65
concluded that, based on the reasoning followed by the
Court, the EU GMO legislation also applies to organisms produced by other NGTs, including
cisgenesis. On the basis of the products in the research and development pipeline, it
considered that NGTs can contribute to the achievement of the Green Deal and Farm to Fork
objectives, as well as to a more competitive economy. The study considered that the current
EU GMO legislation needs adaptation to scientific and technological progress to be suited to
certain NGTs and the diversity of products they can deliver. It also reported on the concerns
expressed by certain stakeholders and concluded that the application of NGTs in agriculture
should not undermine other aspects of food production, e.g., organic agriculture.
In a recent judgment, the Court clarified that organisms obtained by in vitro random
mutagenesis techniques/methods are exempted from the GMO legislation (judgment of 7
February 2023, Case C-688/21 Confédération paysanne and Others)
66
. In vitro random
mutagenesis techniques are not concerned by this policy initiative since they are exempted
from the application of the GMO legislation and are not NGTs.
1.4. International dimension
Globally, NGT organisms
67
and their products are either considered as GMOs or novel foods,
or as conventional products
68
; some countries require a case-by-case consideration of each
product in order to establish the applicable regulatory framework. Various non-EU countries
have adapted, or are in the process of adapting, their legislation in order to specifically
address NGTs; this includes important EU trade partners such as the US, Japan, Argentina,
India and the UK. A recent report by the FAO provides a detailed overview of current
regulatory approaches across the world
69
. China, leading country on research, does not have a
specific regulatory framework on genome edited crops, but has released guidelines for the
safety evaluation of genome edited plants
70
that are intended to provide a more streamlined
approval process than for GMOs. The African Union Agenda 2063
71
aims at utilising genome
editing to improve agricultural productivity and crop resistance. Kenya and Nigeria have
already implemented regulations for a case-by-case review of genome-edited crops
72
. NGTs
could be relevant in low- and middle income countries, which would benefit from adapting
63
Judgment of 25.6.2018 in Case C-528/16 Confédération paysanne and Others. ECLI:EU:C:2018:583
64
Council Decision (EU) 2019/1904
65
European Commission (2021c)
66
ECLI:EU:C:2023:75
67
The term NGT is only used in the EU. Other jurisdictions use other terms such as “genome editing”, “gene-
editing”, “precision breeding” or other variants. These terms may cover different techniques.
68
European Commission (2021c)
69
FAO (2022)
70
USDA (2022)
71
https://au.int/en/agenda2063/overview
72
Buchholzer & Frommer (2023)

11
traditional, local crop species so that they can withstand changing conditions. An enabling
framework in the EU could also support use in those countries.
Switzerland is considering a new authorisation regime
73
for plants that have been obtained
using new breeding technologies and are not transgenic. Canada has a product-based
legislation; products with novel traits are subject to risk assessment, regardless of the
technique used. Other countries, such as New Zealand
74
and South Africa
75
, continue applying
their GMO legislation to NGT organisms.
Actions taken by these third countries thus consist in partial or (most frequently) full
exemption of certain NGT products from GMO authorisation requirements, including specific
GMO labelling and traceability obligations. These exemptions are often linked to the absence
of foreign genetic material and whether a specific product could also have been obtained
naturally or by conventional breeding.
The determination of the status of these products ranges from self-determination by the
developer, voluntary consultation of the competent authority, to compulsory notification or
consultation and decision by a national authority. The evidence to be provided ranges from a
simple documentary declaration to analytical evidence that the product complies with the
exemption criteria.
Third countries that have made adjustments to their regulatory oversight to take into account
the specificities of NGTs are expected to see NGT products increasingly arrive on the market
in the years to come (as shown for example by the 30 NGT plants that have already cleared a
regulatory procedure in the Argentina from 2015 to mid-2022). Emerging evidence from
Argentina and Japan shows that a change in legislation has contributed to the shift in
developers from international to national companies and from multinationals to public
institutions and SMEs.
Currently regulatory developments on NGTs across the world differ to various extents and the
resulting alignment or divergence will impact trade in plants, food and feed, and may lead to
trade frictions as illustrated by the experience with GMOs.
In the context of the WTO’s Sanitary and Phytosanitary Committee, in 2018, 11 countries
76
co-signed an international statement on agricultural applications of precision biotechnology
77
,
acknowledging its role in addressing challenges facing agricultural production and calling for
policy alignment in order to minimise unnecessary trade barriers.
1.5. Scope
The scope of this initiative are plants produced by targeted mutagenesis and cisgenesis
(including intragenesis) and their food and feed products.
The choice of the scope is based on several reasons. There is significant demand in the EU
and globally for NGT plants, in the context of their potential to contribute to current
challenges in the agri-food system. Numerous advanced and early R&D applications concern
plants, and several plant products are already on or very close to the market. Safety data are
mainly available for plants obtained by targeted mutagenesis and cisgenesis (addressed in
expert opinions from EFSA), whereas it is at this stage difficult to draw relevant conclusions
73
Bundesversammlung der Schweizerischen Eidgenossenschaft (2022)
74
Entine et al. (2021)
75
Republic of South Africa (2021)
76
Argentina, Australia, Brazil, Canada, the Dominican Republic, Guatemala, Honduras, Paraguay, Philippines,
the United States of America and Uruguay.
77
WTO (2020)

12
on other techniques and applications in animals and micro-organisms. Furthermore, similar
plants can be obtained in certain cases with conventional breeding and targeted mutagenesis
and cisgenesis, and the appropriateness of subjecting these products to the same regulatory
requirements as transgenic organisms needs to be assessed.
1.6. Interaction with existing legislation and upcoming initiatives
This initiative is framed by the existing legislation on GMOs and has links to other legislation
applicable to plants, food and feed, as well as with planned initiatives implementing the
European Green Deal and the Farm to Fork Strategy. Section 7.3 describes the coherence with
the relevant legislation and upcoming initiatives
78
.
The initiative on NGT plants aims to establish specific rules applicable to the deliberate
release and the placing on the market of these plants and derived food and feed, taking into
account their specificity, while maintaining the objectives of the GMO legislation and
ensuring coherence with it.
The planned initiative on a legislative framework for a sustainable food system (FSFS)
79
covers the entire food system, thus encompassing NGT plants used for food or feed and the
derived food and feed products. The placing on the market and cultivation of plant varieties
derived from NGT plants will also have to comply with EU legislation on the marketing of
seeds and other plant and forest reproductive material (PRM, FRM), which is currently under
revision
80
. In particular, the assessment of the value for cultivation and use (VCU) carried out
under this legislation covers various aspects (e.g. yield, pest resistance, nitrogen-use and
water-use efficiency) which may be linked to traits introduced by NGTs.
NGT plants could be among the tools that contribute to the reduction target on the use and
risk of pesticides set out in the proposal for a regulation on the sustainable use of plant
protection products (‘SUR’)
81
.
EU legislation on organic production and labelling of organic products, the Organic Products
Regulation’
82
bans the use of GMOs and GM food and feed in organic production
83
, and
allows organic operators to rely on the labels and accompanying documents available
pursuant to the GMO legislation. In addition, organic operators have to implement
precautionary measures to avoid the presence of products and substances not authorised for
use in organic production. However, GMOs that are exempted today from the requirements of
the GMO legislation (such as products obtained by random mutagenesis, i.e. old mutagenesis
techniques) are treated as conventional for the purposes of organic production.
Legislation on IPRs will have an impact on the development and marketing of NGT plants.
Two types of IPRs are of particular relevance for NGT plants: biotechnology patents and
plant variety rights. The Biotechnology Directive
84
on the legal protection of biotechnology
inventions provides for the patentability of subject-matter involving biological material which
78
https://agriculture.ec.europa.eu/farming/organic-farming/legislation_en
79
https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13174-Sustainable-EU-food-system-
new-initiative_en
80
https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13083-Plant-and-forest-reproductive-
material-revised-rules-_en
81
European Commission (2022b)
82
Regulation (EU) 2018/848
83
The Organic Products Regulation also prohibits the use of non-food and non-feed products produced from
GMOs or by GMOs in organic food and feed. These categories of products are not subject to the EU legislation
on the deliberate release of GMOs.
84
Directive 98/44/EC

13
is new, involves an inventive step and is susceptible to industrial application. A patent can
cover the biological material as well as the process to produce it, but not plant and animal
varieties and essential biological processes for the production of plants and animals. The latter
exclusion also extends to plants and animals obtained by such essentially biological
processes.
85
Plant variety rights
86
can be granted for plant varieties that are distinct, uniform,
stable and new. Breeders may use protected plant varieties for the purpose of breeding and
marketing new varieties.
1.7. Previous studies on the EU GMO legislation with relevance to NGTs
Two external studies on the EU GMO legislation were carried out for the Commission in
2010 (on GM food and feed)
87
and 2011 (GMO cultivation and placing of GMOs on the
market)
88
. They noted concerns that the legislative framework was only focused on risks and
not suited for the EU to take advantage of new developments in biotechnology. They also
referred to detection challenges resulting from the fact that products of targeted mutagenesis
might not differ from those obtained via conventional breeding. The studies concluded that, as
the rate of innovation in the global biotechnology sector was unlikely to slow down, ensuring
that legislation remained relevant was likely to be an ongoing challenge, especially if the
focus was on the techniques used rather than on the final products. The Commission 2021
NGT study, using the latest available evidence, confirmed that the findings of the previous
studies remain relevant today and that the challenges have grown, especially as regards plants
produced by targeted mutagenesis and cisgenesis. The 2010 and 2011 external studies and the
Commission 2021 NGT study, have provided an analysis of the relevant problems and
evidence. In that context, an additional evaluation of the GMO legislation at this point in time
would not yield further information, in the absence of NGT products on the EU market.
2. Problem definition
2.1. What are the problems?
The current EU GMO legislation is not fit for purpose for NGT plants obtained by targeted
mutagenesis or cisgenesis, and their food and feed products. This was highlighted already in
the 2010/2011 external studies and confirmed by the Commission NGT study in 2021. This is
also the view of most stakeholder groups in the public consultation for this impact
assessment
89
, where 191 (93%) of the respondent academic/research institutions, 102 (84%)
of business associations, 66 (80%) of large enterprises, 70 (72%) of SMEs and 29 (83%) of
public authorities found the existing regulatory provisions inadequate for these plants.
However, other stakeholder groups (47 (58%) of non-governmental organisations (NGO), 16
(80%) of environmental organisations, 3 (60%) of consumer organisations) consider that the
existing legislation is adequate. These results are similar to the outcome of the feedback
received to the inception impact assessment (Annex 2).
The problem is composed of three main components:
85
See Rule 28(2) of the Implementing Regulations to the European Patent Convention; Commission Notice on
certain articles of Directive 98/44/EC of the European Parliament and of the Council on the legal protection of
biotechnological inventions (2016/C 411/03)
86
Regulation (EC) No 2100/94
87
Food Chain Evaluation Consortium (2010)
88
GHK Consulting (2011)
89
https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13119-Legislation-for-plants-
produced-by-certain-new-genomic-techniques/public-consultation_en

14
I) The authorisation procedure and risk assessment requirements of the current EU
GMO legislation are not adapted to the variety of potential plant products that can
be obtained by targeted mutagenesis and cisgenesis, and as a result are
disproportionate or inadequate in certain cases.
Targeted mutagenesis and cisgenesis can produce a diversity of plant products with
different risk profiles. Some are similar to plants occurring naturally, or produced by
conventional breeding, including random mutagenesis techniques, for which there is no
requirement to be authorised or risk assessed as GMOs. Others are similar to plants
obtained by established genomic techniques. However, they are all subject to the same
authorisation and risk assessment requirements in the current GMO legislation. This
results in applying different regulatory oversight and risk assessment requirements to
plant products with similar risk profiles or applying the same regulatory oversight and
risk assessment requirements to plant products with different risk profiles.
The conclusions of scientific bodies in this regard (section 1.1) were shared by the
majority view of stakeholders in the consultation activities. In the public consultation,
829 respondents, 23% of those replying that the legislation is not adequate, indicated as
an underlying reason that the risk assessment approach cannot factor in the diverse risk
profiles of the plants in question
90
; in addition, 61% (1331) of total respondents
supported a risk assessment approach different from the current one
91
. On the other
hand, 22% of respondents (480 responses)
92
were of the view that NGT plants need to be
assessed using the current GMO legislation requirements.
II) The current EU GMO legislation raises implementation and enforcement
challenges for certain plants produced by targeted mutagenesis or cisgenesis.
The EU GMO legislation currently requires applicants to provide an analytical
laboratory method that is specific to the product for which they seek authorisation, i.e. it
can both detect it and differentiate it from other products. The EURL and ENGL have
concluded
93
that, if the genetic alteration is not unique for the relevant product, a specific
detection method cannot be provided. When the same alteration can be introduced by
NGTs or conventional breeding methods, the detection method may be able to detect it,
but will not allow determining whether the product is a GMO subject to the GMO
legislation or not. In such cases, applicants will be unable to comply with an
90
89 (21%) of the Academic/research institutions, 56 (24%) of the Business associations, 68 (25%) of
company/business organisations, 2 (33%) of Consumer organisations, 560 (23%) of EU citizens, 2 (15%) of
Environmental organisations, 19 (24%) of Non-EU citizens, 14 (19%) of Non-governmental organisations, 17
(22%) of Public authorities, 1 (7%) of Trade Unions and 2 (25%) of Other.
91
34% (738) of total respondents believed that risk assessment should have requirements adapted to the
characteristics and risk profile of a plant (41% (84) of the Academic/research institutions, 11% (13) of the
Business associations, 23% (41) of company/business organisations, 20% (4) of Consumer organisations, 37%
(552) of EU citizens, 10% (2) of Environmental organisations, 50% (19) of Non-EU citizens, 10% (8) of Non-
governmental organisations, 49% (17) of Public authorities, 14% (2) of Trade Unions and 20% (1) of Other).
27% (593) believed that risk assessment is not needed when these plants could have been produced through
conventional plant breeding or classical mutagenesis (37% (76) of the Academic/research institutions, 54% (66)
of the Business associations, 42% (75) of company/business organisations, 22% (328) of EU citizens, 10% (2)
of Environmental organisations, 11% (4) of Non-EU citizens, 27% (22) of Non-governmental organisations,
31% (11) of Public authorities, 43% (6) of Trade Unions and 20% (1) of Other).
92
3% (6) of the Academic/research institutions, 16% (20) of the Business associations, 22% (39) of
company/business organisations, 80% (4) of Consumer organisations, 22% (328) of EU citizens, 75% (15) of
Environmental organisations, 18% (7) of Non-EU citizens, 54% (44) of Non-governmental organisations, 17%
(6) of Public authorities, 36% (5) of Trade Unions and 20% (1) of Other.
93
ENGL (2019)

15
authorisation requirement, and other food chain operators and authorities will not be able
to implement or enforce the legislation. This has been confirmed by the fact-finding
studies carried out by the Commission in 2022 in Germany
94
and the Netherlands
95
to
gather information on the implementation of controls on organisms and products
obtained through NGTs.
Furthermore, plants produced by targeted mutagenesis and cisgenesis, which could also
occur naturally or be produced by conventional breeding, need to comply with the
traceability (document-based with specific GMO identifier) and labelling (obligation to
label the products as genetically modified) requirements of the EU GMO legislation.
Consequently, in certain cases, plant products with similar genetic modifications might
be subjected to different labelling and traceability requirements, depending on the
breeding technique that was used to obtain them.
Finally, the emergence of targeted mutagenesis and cisgenesis techniques raises
questions of interpretation of Directive 2001/18/EC (and other GMO legislation). That
legislation lacks definitions of certain key terms or concepts such as ‘mutagenesis’ and
‘genetic material [that] has been altered’. The potential ambiguities can lead to diverging
interpretations, regulatory uncertainty and implementation challenges
96
.
III) The current EU GMO legislation applied to NGTs is not conducive to developing
innovative beneficial products.
NGTs can bring speed and precision to the development of improved plant varieties with
traits that could support a sustainable and resilient EU agri-food system (section 1.1).
However, as a result of the regulatory issues mentioned above, the current EU GMO
legislation applied to NGTs is not conducive to developing these innovative products
and to place them on the market in the EU. In the public consultation, 61% of the total
respondents (1329)
97
believed that maintaining plants produced by targeted mutagenesis
and cisgenesis under the current framework is expected to have short-, medium- or long-
term consequences in their activity or sector. The large majority mentioned negative
consequences, relating to loss of tools to achieve the goals of the Green Deal and the
Farm to Fork Strategy, as well as obstacles to research and development of improved
crops and loss of competitiveness. 19% (424) of the respondents
98
do not expect to
experience any consequence.
Overall, the current GMO legislation meets the objective of safety, albeit with higher
regulatory burden and cost than would be necessary for certain NGT products. It is not
conducive to developing innovative NGT products and to place them on the market in the EU.
Thus, contributions of these products to a sustainable transformation of the food system, and
to the EU’s strategic autonomy and international competitiveness, cannot be harnessed. See
also problem tree (Figure 1 and Annex 9) and Section 3.
94
https://ec.europa.eu/food/audits-analysis/audit-report/details/4543
95
https://ec.europa.eu/food/audits-analysis/audit-report/details/4544
96
European Commission (2021c), section 5.
97
80% of the Academic/research institutions, 89% of the Business Associations, 71% of the Company/business
organisations, 40% of the Consumer Organisations, 55% of EU citizens, 30% of Environmental organisations,
63% on Non-EU citizens, 54% of NGOs, 71% of Public Authorities, 93% of Trade Unions.
98
9% (19) of Academic/research institutions, 9% (11) of Business associations, 22% (40) of Company/business
organisations, 60% (3) of Consumer organisations, 20% (299) of EU citizens, 65% (13) of Environmental
organisations, 21% (8) of Non-EU citizens, 36% (29) of Non-governmental organisations and 6% (2) of Public
authorities.

16
2.2. What is the size of the problem and who is affected?
The problems identified above affect numerous operators across the agri-food system,
especially breeders, the agricultural biotechnology innovation and research sector,
conventional and organic farmers, bio-based industry, consumers, traders, and EU and
national authorities. The overall risk is that the EU would be to a significant extent excluded
from the technological developments and economic, social and environmental benefits
potentially generated by these new technologies, thereby also weakening the EU’s strategic
autonomy. However, since specific regulatory frameworks were introduced in several non-EU
countries only recently and given the limited number of products on the global market (none
in the EU), very few if any data or estimates on economic, social or environmental impacts of
different regulatory regimes for NGT products are available to date. Estimates of the size of
the problems for different types of operators if the current GMO legislation continues to apply
to NGTs thus depend to a large degree on comparison with past developments inside and
outside the EU (e.g. for GMOs obtained by established genomic techniques), expert
assessments, projections, stakeholder expectations and relevant data from the scientific
literature.
In the targeted survey conducted for this impact assessment, respondents active in plant
breeding (45.5%, 56 of 123) pointed to regulatory uncertainty, regulatory costs and time to
market as the most important barriers to developing new plant varieties using targeted
mutagenesis or cisgenesis. Due to uncertainty
99
, the current regulatory regime is seen as
inducing high risk for plant breeders to engage in NGT-related research and product
development, in particularly for start-ups and SMEs. A significant share of companies (40%)
replied that they delayed product development and release due to this regulatory uncertainty.
Large companies respond to this uncertainty by moving R&D on NGTs to non-EU countries,
while SMEs may not have the resources to pursue this strategy (100% of the large companies
vs. 20% SMEs according to a recent survey)
100
. Regulatory developments in third countries
(section 1.4) affect negatively the competitiveness of the EU biotechnology and breeding
sectors, in particular of SMEs, on international markets which is also contributing to
weakening the EU’s strategic autonomy.
In the public consultation, academic/research institutions emphasised the negative impact of
the current situation on the competitiveness of the EU research sector, compared to other
countries with a more enabling environment. Researchers consider the current regulatory
situation as hindering funding decisions by public and private funders and report that, since
the CJEU ruling, several R&D projects were cancelled or shifted abroad. A recent survey
suggests a reduction of 33-40% of R&D activities after the ruling
101
. Young researchers
indicate that the EU has become less attractive for a career in agricultural biotechnology,
leading to a brain drain and recruitment challenges
102
. This could further widen the already
established patenting gap between the EU and US/China in agricultural biotechnology that
emerged since 1998
103
.
99
Regulatory certainty is the predictability that a product fulfilling the requirements is authorised for placing on
market in a given time.
100
Jorasch (2020)
101
Ibid.
102
http;//www.genesproutinitiative.com/about-us/
103
WIPO (2019)

17
Stakeholders from the bio-based economy consider genome-editing as a key enabling
technology
104
. The existing regulatory framework is seen as an obstacle to innovation in this
sector, which will increase the EU’s dependence on technological developments coming from
other parts in the world
105
.
The existing regulatory problems affect also farmers. Farmers are deprived of tools and
products that could contribute to improving the yield/input ratio, to dealing with the
challenges of climate change and to meeting reduction targets for pesticides and fertilisers.
Climate impacts have led to poorer harvests and higher production costs, affecting price,
quantity and the quality of farmed products in parts of the EU. Climate change impacts on
agriculture are projected to lead up to 1% average gross domestic product loss by 2050 but
with large regional differences
106
. Plant breeding is one effective counter measure against
such climate change impacts. Adapting crop varieties to rapid climate change by breeding
requires making rapid incremental changes in the best adapted varieties to keep pace with
changes in the biotic and abiotic environment, to ensure food security
107
and to support the
transition to sustainable agriculture. While conventional breeding has led to a continuous
improvement of a wide range of traits
108
, NGTs are crucial technologies enabling a more rapid
and targeted breeding progress.
Consumers prepared to accept products of biotechnology will have to forego benefits from
products that could be designed to meet their expectations and needs (e.g. improved taste,
improved nutrient profile or reduced allergen content). NGTs can improve the taste of green
leafy vegetables, which are an important source of vitamins, minerals, and phenolic
compounds
109
, or reduce the content of the potential carcinogen acrylamide in wheat
110
.
The current situation has also a negative impact on international trade. In practical terms,
the current regulation is seen as an obstacle for the importation of commodities derived from
NGTs
111
. The current GMO regulation has resulted in lost trade opportunities due to
temporarily closed borders, testing costs, lawsuits, and disputes
112
, whereas asynchronous
authorisations create uncertainty for importers and exporters
113
. The situation for NGTs may
be even more challenging compared to conventional GMOs due to the existing difficulties in
analytical detection possibilities.
National public authorities will face enforcement challenges. For example, if the same
genetic alteration can be introduced by NGTs or conventional breeding methods, the
enforcement authorities, by detecting the alteration, will not be able to assess whether the
product is a GMO subject to the GMO legislation or not.
104
Key enabling technologies are knowledge-intensive technologies that have been identified as drivers of
innovation in different economic sectors and are characterized by a high degree of R&D.
105
Wesseler et al. (2019).
106
EEA (2019) Climate change adaptation in the agriculture sector in Europe.
107
Challinor et al. (2016)
108
Voss-Fels et al. (2019); this paper, importantly, shows that breeding for increased yields does not necessarily
come at the expense of sustainability-related traits. In wheat, over the past 50 years, increased yield was
accompanied by improved disease resistance, nutrient use efficiency and photosynthetic efficiency.
109
Karlson et al. (2022)
110
Raffan et al. (2023).
111
Purnhagen & Wesseler (2021)
112
Smyth (2017)
113
Zimney & Sowa (2021); Eriksson et al. (2019); Purnhagen & Wesseler (2021).

18
Figure 1. Drivers, problems and consequences for plants produced by targeted mutagenesis and cisgenesis.
2.3. What are the problem drivers?
There are two main drivers behind the problems presented above (Annex 9):
I) The current framework is based on genetic modification techniques as understood
in the late 1990s, and lags behind scientific developments. After more than 20 years
of rapid scientific progress in this field, the current legal framework is based on an
outdated understanding of the technical possibilities offered by modern biotechnology,
especially regarding plants produced by targeted mutagenesis and cisgenesis. The
current system has been designed based on the experience with transgenic products, with
requirements fit for products containing foreign genetic material from sexually
incompatible organisms, and which therefore would not generally occur in nature. In
addition, the legislation has limitations in keeping up with developments in this rapidly
evolving field, where new techniques are discovered and applied.
II) The authorisation system in the current framework was designed with safety and the
functioning of internal market as the primary objectives; it is not conducive to
developing innovative NGT products and to placing them on the market, and
therefore, translating their sustainability potential to reality.
2.4. How likely is the problem to persist?
If no action is taken, the problems described will persist and be aggravated. Plants obtained by
targeted mutagenesis and cisgenesis will continue to be regulated under the current EU GMO
legislation, with the current risk assessment, authorisation, traceability and labelling
requirements. As the findings of the consultations suggested, limited research and
development of NGT products in the EU is expected. A recent survey suggests that a
significant share of companies (38%) has delayed product development following the
clarification of the CJEU that these products fall under the current EU GMO legislation
114
.
The cultivation and market uptake of these products would remain limited in the EU. The
high costs and long time needed for authorisation of NGT products – in addition to the
114
Jorasch (2020).
19
potentially high licensing costs for access to these technologies – would make these products
unattractive for SMEs and the existing market structure, with only large companies having the
resources to develop and bring them to the market, would persist. For example, according to
the information from breeders provided in the targeted survey responses and interviews, in
terms of time to market, the length of the current risk assessment for GMOs is estimated to be
on average 6 years for cultivation and 4.5 years for food/feed respectively, making the total
time to market (including R&D) under the current framework 16.5 years on average.
Operators and consumers would face difficulties accessing new products that could meet their
expectations and needs. Obstacles would remain for innovative plant biotechnology to
contribute to the objectives of the European Green Deal and Farm to Fork Strategy.
In some cases, applicants will not be able to meet the detection method requirements as they
stand today and the NGT plants concerned will not be able to obtain authorisation.
At international level, research and development and commercialisation of these products will
increase in most major EU trade partners. With the existing difficulties in analytical detection,
the above developments are expected to further complicate the enforcement and
implementation of the EU GMO legislation, will negatively impact trade and could raise
questions of compatibility with WTO law. More challenging and expensive identity
preservation systems for both NGT and NGT-free products from the EU trade partners would
be required. Regulatory divergence could lead to forced separation of entire agricultural value
chains in and outside the EU, reduced availability of imports due to an unwillingness of trade
partners to comply with labelling and segregation requirements, resulting in higher costs and
input prices. The organic and GM-free value chain will continue to rely on the labelling and
traceability requirements in the existing GMO legislation to build up reliable value chains and
consumer trust for their products, and they consider that the current GMO legislation is the
appropriate legal instrument to deal with these problems. However, they will also be affected
by the challenge of avoiding unintended use of NGT-based inputs such as seed materials,
feed, or food ingredients.
The persistence and aggravation of the problem is also illustrated by some of the megatrends
identified
115
by JRC. In particular, the megatrends climate change, environmental degradation
and aggravating resource scarcity are environmental changes that could create new challenges
in terms of food availability and resilience. NGTs could be one tool contributing to the
necessary adaptation of the food systems at global level.
3. Why should the EU act?
3.1. Legal basis
EU legislation on GMOs is based on Articles 43, 114 and 168(4)(b) of the Treaty on the
Functioning of the European Union (TFEU). These articles provide the legal basis for the EU
to adopt measures which have as their objective to implement the common agricultural policy
(Article 43), and to ensure the good functioning of the internal market (Article 114(1)) while
maintaining a high level of human health protection in the veterinary and phytosanitary fields
115
On 15 February 2022, the JRC (unit I.2, Competence Centre on Foresight) organised a Megatrends Analysis
workshop in the context of this impact assessment. Megatrends are one of the tools used in foresight, i.e. the
structured, systemic and participatory exploration of the future with the aim to better understand and anticipate
possible developments, and to prepare for and shape the future. Out of the 14 megatrends ‘Aggravating resource
scarcity’, ‘Shifting health challenges’, ‘Climate change and environmental degradation’ and ‘Continuing
urbanisation’ were selected as highly relevant for the initiative. Furthermore, ‘Trust’ was identified as a major
element for the future development and application of NGTs.
20
(Article 168(4)(b)). The objectives of this initiative are aligned with these legal bases and, in
addition, this initiative tries to ensure that plants obtained by targeted mutagenesis and
cisgenesis, and food and feed products produced from them contribute to the sustainability
goals of the Green Deal and Farm to Fork strategies. In that regard, the EU’s common
agricultural policy’s objectives of ensuring the rational development of agricultural
production and the optimal use of the factors of production, as well as to assure food security
and the availability of supplies have particular relevance.
3.2. Subsidiarity: Necessity of EU action
Plants obtained by targeted mutagenesis and cisgenesis are living organisms which, as any
other plant, when released into the environment for experimental purposes or as commercial
products, may reproduce in the environment and cross national borders. It is essential to
achieve a harmonised, high level of protection of human and animal health and the
environment in relation to these plants and of food and feed derived from them so that they
may circulate freely within a smooth-functioning internal market. In addition, the EU Farm to
Fork Strategy recognises the potential of NGTs as a possible tool to increase sustainability of
the food system and bring benefits to society as a whole.
The requirements for the deliberate release and the placing on the market of NGT plants and
their derived food and feed are already harmonised at EU level under the existing legal
framework applicable to GMOs but need to be adapted to the specificities of plants obtained
by these new techniques. Carving out NGT plants from the current EU legal framework and
leaving it to Member States to regulate them would likely lead to different regulatory
requirements and levels of protection in the EU Member States. Differing national
requirements for NGT plants would hinder the free movement of these products, fragment the
internal market and lead to uneven competition between economic operators. It would also
impact access of NGT products to the market and thereby limit their potential to contribute to
the international competitiveness, strategic autonomy and sustainability of the EU’s food
system.
3.3. Subsidiarity: Added value of EU action
Compared to individual action by Member States, EU intervention would provide uniform
rules for the development and placing on the market of NGT plants and their food and feed
products. Harmonised EU-wide rules on the marketing of such products would ensure a level-
playing field for operators within the single market and a more predictable and efficient
regulatory oversight. Furthermore, there is an urgent need to ensure availability to farmers,
food operators and consumers of plant varieties that can cope with challenges of a global
nature such as climate change and biodiversity reduction, which have been further aggravated
by the present geopolitical and energy crisis in Europe, and to secure food security in the
future. The Farm to Fork Strategy recognised the role that biotechnology can play in meeting
those global challenges, which need an EU-wide response.
4. Objectives: What is to be achieved?
The intervention logic is presented in Annex 10.
4.1. General objectives
I) Maintain a high level of protection of human and animal health and of the
environment, in accordance with the precautionary principle.
II) Enable the development and placing on the market of plants and plant products
contributing to the innovation and sustainability objectives of the European Green
Deal and of the Farm to Fork and Biodiversity strategies.
21
III) Ensure the effective functioning of the internal market and enhance the
competitiveness of the EU agri-food sector at the EU and global level, providing a
level-playing field for its operators.
Compared to the current GMO legislation, general objectives (I) and partially (III) are the
same, i.e. to protect human and animal health and the environment and to ensure the effective
functioning of the internal market. In line with the European Green Deal and its Farm to Fork
and Biodiversity Strategies, an objective on sustainability is also included (general objective
II). It encompasses the three dimensions of sustainability: economic (e.g., higher yield/input
ratio), social/health (e.g., improved nutrient content) and environmental (e.g., resistance to
pests, less need for fertilisers). An additional objective relates to the competitiveness of the
EU agri-food sector at EU and global level (included in general objective III), which
contributes to food security and EU’s strategic autonomy.
4.2. Specific objectives
I) Procedures for the deliberate release and placing on the market ensure that NGT plants
and derived food/feed products are as safe as their conventional counterparts, while
not entailing unnecessary regulatory burden.
This objective takes into account the diversity of NGT products, based on their risk
profiles, and addresses current implementation and enforcement challenges, in
particular for NGT plants that could also occur in nature or be conventionally bred.
In this respect, the objective addresses specifically problem components (I) and (II)
and drivers (I) and (II).
II) Deliberate release and placing on the market of NGT plants and derived food/feed
products that feature a wide range of plant species and traits by various developers.
This objective is linked to innovation in plant breeding that 1) brings to the market
products that cater for the diversity of needs of farmers and consumers and address
local and regional specificities; 2) facilitates access of new players (such as SMEs,
public institutes) to the market.
In this respect, the objective addresses problem component (III) and driver (II).
III) NGT plants released or placed on the market feature traits that can contribute to a
sustainable agri-food system.
This objective aims to steer plant breeding to plants with traits that have been
identified as being useful in view of sustainability objectives (economic,
environmental, social/health).
In this respect, the objective addresses specifically problem component (III) and driver
(II).
Figure 2 presents the relationship between the general and specific objectives.

22
Figure 2. General and specific objectives.
5. What are the available policy options?
To respond to the specific objectives, this impact assessment considers a range of policy
elements: procedure for the release or placing on the market and risk assessment, traceability,
labelling/transparency, detection method requirements and sustainability. Sustainability is a
novel element, compared to the current GMO legislation, linked to general objective (II). The
others are all elements of the current GMO legislation, and for these, in addition to the status
quo, possible adaptations to the specificity of NGT plants are considered.
The possible choices for each of these policy elements reflect the full range of stakeholders’
views submitted in the context of the Commission 2021 NGT study and the inception impact
assessment as well as other consultation activities carried out in the context of this impact
assessment. They take into account scientific opinions of EFSA and other scientific bodies
and relevant scientific literature. Stakeholders concerned about the safety or other aspects of
GMOs and NGTs in general favour the continued application of the existing GMO legislation
to NGTs. This report takes these views into account by treating the baseline (no change) as a
viable policy option with a full assessment of its impacts. Impacts on specific sectors (organic
and GM-free) that express concerns about NGTs are specifically assessed for each policy
option in section 6.
The key policy choices for each of the policy elements are presented in Figure 3.

23
Figure 3. Policy elements.
The issue of how to steer the development of varieties with traits that contribute to
sustainability is not specific to NGTs. It applies equally to conventionally bred plants and to
GMOs not covered by this initiative. Therefore, the policy choices as regards sustainability
include (in addition to the possibility of specific provisions in this initiative) addressing this
issue in other, horizontal initiatives (FSFS; PRM/FRM) as a feasible option. Assessing such
an option reflects the majority view among stakeholder groups resulting from the public
consultation and the targeted survey that sustainability tests only in the context of the NGT
initiative are not appropriate, and that a holistic assessment of performance – as done in value
for cultivation and use (VCU) testing in the context of the PRM/FRM legislation to which
NGTs are also subject – will be far more useful.

24
Certain of the policy choices above (no risk assessment, notification procedure
116
, no tracing
as GMO) have only been considered as feasible options for a limited range of NGT plants,
namely those which could be obtained naturally or by conventional breeding methods, based
on the risk profile of such NGT plants. Therefore, option 4 (which incorporates this policy
choice) is only assessed as regards such NGT plants. All other options (baseline, options 1 to
3) are assessed as regards all NGT plants under the scope of the initiative (both those that
could or could not occur naturally or be produced by conventional breeding).
The choices for each element have been combined into policy options (see Figure 4). These
represent a range of distinct approaches to the regulation of NGTs. Their impacts including
the trade-offs between elements are analysed in section 6. These options represent
combinations of the different choices for the main elements (risk assessment, regulatory
procedure, etc.) that have been considered feasible and coherent (see section 5.3 for a
discussion on discarded combinations).
Figure 4. Policy options.
The policy instrument considered under all four policy options is a Regulation. The
authorisation procedures in options 1 to 3 as well as the notification system in option 4 are
based on fully harmonised criteria and requirements and procedures that should lead to the
authorisation or acceptance of a notification for the whole EU, ensuring the same level of
protection of health and the environment and the availability of the products concerned across
the EU. A Regulation appears to be the most appropriate legal instrument to embody such
procedures, as well as to achieve a uniform implementation of the policy intervention, which
has an important internal market component. That instrument would be complemented by any
necessary amendments of existing legislation
5.1. What is the baseline from which options are assessed?
The current regulatory framework would remain applicable, meaning that plants obtained by
targeted mutagenesis or cisgenesis, whether cultivated in the EU or imported, and food and
feed produced from them, would require authorisation for their release including placing on
the market. An authorisation would also be required for field trials on such NGT plants
carried out within the EU.
The authorisation procedure for the placing on the market of NGT plants (as such or in food
and feed products) would be conducted at EU level (risk assessment by EFSA and decision by
116
In the legislative proposal, 'notification procedure' is referred to as ‘verification procedure’.
Option 4 - Notification for
certain products
• Notification regime
under new framework
for products that can
also occur naturally or
be obtained by
conventional breeding
• For products meeting
the notification criteria,
no risk assessment,
labelling traceability and
detection method.
• Transparency through
register
• No sustainability
provisions, reliance on
horizontal legislation
Option 3 - Authorisation
with requirements
• Authorisation under
new framework
• Risk assessment
adapted to different risk
profiles
• Traceability and
labelling as a GMO
• Detection method –
only detecting the event
when differentiation not
possible
• Sustainability
requirements
Option 2 - Authorisation
with incentives
• Authorisation under
new framework
• Risk assessment
adapted to different risk
profiles
• Traceability as a GMO
• Sustainability labelling
with GMO labelling/ no
labelling/labelling with
factual statement on
the trait
• Detection method –
only detecting the event
when differentiation not
possible
• Sustainability incentives
Option 1 - Adapted Risk
Assessment
• Authorisation under
current GMO legislation
• Risk assessment
adapted to different risk
profiles
• Traceability and
labelling as a GMO
• Detection method -
only detecting the event
when differentiation not
possible
• No sustainability
provisions, reliance on
horizontal legislation
Baseline
• Authorisation under
current GMO legislation
• Risk assessment of
current GMO legislation
• Traceability and
labelling as a GMO
• Detection method -
detecting and
differentiating the event
• No sustainability
provisions, reliance on
horizontal legislation

25
the Commission), whereas the procedure to authorise the deliberate release of NGT plants for
other purposes (e.g., field trials) would be conducted by Member States (national authority to
which the application is submitted, with the involvement of EFSA in case of objections raised
by the Commission or another Member State).
All current authorisation requirements would apply, i.e. risk assessment to show that the NGT
plant does not present more potential hazards than its conventional counterparts, with current
requirements that contain only limited scope to adapt according to the risk profile of the plant;
the requirement to provide an event-specific detection method (that is, which can both detect
the event and differentiate it from other events); tracing as a GMO (obligation to transmit and
hold information for a period of five years that a product contains or consists of a GMO,
including its unique identifier, and that food or feed is produced from a GMO, at each stage of
their placing on the market); labelling requirement for products destined to consumers that
contain or consist of GMOs and for food and feed produced from GMOs. Authorised products
would be subject to post-market obligations (monitoring for a certain time-period, duty to
submit new information relevant to the assessment of risks, renewal every 10 years).
Sustainability would be addressed by sustainability-related provisions included in the relevant
horizontal and sectoral legislation (e.g. FSFS, PRM
117
, FRM), where applicable.
5.2. Description of the policy options
5.2.1. Option 1: Authorisation with adapted risk assessment and detection
method requirements
The legislation would be adapted to cater for the diverse risk profiles of plants obtained by
targeted mutagenesis and cisgenesis and to address the implementation and enforcement
challenges related to detection.
NGT plants and food and feed produced from them would require (as today) an authorisation
to be used in field trials, cultivated or placed on the market, granted as today if the NGT plant
is shown not to present more potential hazards than its conventionally counterparts. Risk
assessment and detection method requirements, adapted to the diversity of these plants, would
replace the current requirements, while maintaining the general principles and methodologies.
To that end, general principles for risk assessment would be set in the legislation. They would
be accompanied by criteria to inform the adaptation of data requirements to risk profiles. An
indicative list of criteria for an adapted risk assessment
118
is presented in Box 1. They provide
the starting point for the design of an adapted risk assessment in the legislation. Specific data
requirements based on the criteria would be set in tertiary legislation and/or by EFSA.
As regards the authorities competent for the regulatory procedures, as today, the risk
assessment would be carried out by EFSA (for placing on the market, including cultivation,
for food/feed uses) or by national authorities (for field trials and for placing on the market,
including cultivation, for non-food/feed uses). Member States would remain responsible for
granting consent for experimental releases within their territories (field trials). Authorisation
for the placing on the market for cultivation and food and feed uses would be given at EU-
level through an authorisation decision by the Commission.
117
European Commission (2023b) Annex 5, Section 5.
118
EFSA (2022c)

26
Box 1 - Criteria proposed by EFSA for adapted risk assessment of plants obtained by targeted mutagenesis
and cisgenesis
119
:
The first four of the six criteria related to the molecular characterisation of the genetic modification
introduced in the recipient plant. They evaluate:
1. If exogenous DNA sequence is present
2. If yes, whether the sequence is from the breeders’ gene pool (if it is not, the plant is transgenic and
therefore out of the scope of this initiative)
3. How the sequence is integrated, for example is it random or targeted?
4. If any host plant gene was “interrupted” (split) by the newly introduced sequence
Criteria 1-4 are designed to establish if cisgenic and intragenic sequences have altered the host plant’s genes.
If they have not, or if no risk is identified when an exogenous DNA sequence has been introduced, two
further criteria apply:
5. Do the introduced or modified sequence and the associated trait have a history of use?
6. If not, the structure and function of the modified versions of the DNA sequence (“allele”) should be
carefully assessed.
These last two criteria apply also to plants produced by targeted mutagenesis.
The requirement for an event-specific detection method would be waived if the applicant can
justify that developing a detection method that can, not only detect, but also differentiate a
specific NGT product from conventional ones that contain the same modification(s) is not
technically possible. The applicant would still need to submit an analytical method capable of
detecting the product.
Other legal requirements, such as those on traceability, labelling and post-market provisions,
would remain as in the baseline.
Sustainability would be addressed by sustainability-related provisions included in relevant
horizontal and sectoral legislation (e.g. FSFS, PRM, FRM), where applicable.
5.2.2. Option 2: Authorisation with incentives for products containing modified
traits that have the potential to contribute to sustainability
The legislation would be adapted to cater for the diverse risk profiles of NGT plants and to
address the implementation and enforcement challenges related to detection (as in Option 1)
and to incentivise the development and placing on the market of plant products that could
contribute to a sustainable agri-food system.
NGT plants and food and feed produced from them would require an authorisation to be used
in field trials, cultivated or placed on the market, with adapted risk assessment and detection
method requirements (as in Option 1). The competent authorities will be as in option 1.
In addition, regulatory incentives – granted by the authorities competent for the procedure
concerned (at national or EU level) - would be introduced for NGT plants containing traits
that could contribute to sustainability to encourage the development of sustainable products.
The regulatory incentives considered in the impact assessment are: the provision of regulatory
and scientific advice before and during the authorisation procedure (guidance on the overall
development plan and the regulatory procedure, dedicated contact point in EFSA, scientific
advice at key development milestones) and measures to facilitate the authorisation process
119
EFSA (2022d)
27
(waiving of detection method validation fees, faster procedures). During the consultation
activities various stakeholders, in particular breeders, considered waiving labelling as GMO
as the measure that would have greater impact.
Incentives would be linked to the modified trait in the NGT plant and its potential impacts on
sustainability (economic, environmental, and social). The basis for this comparison would be
data provided by the applicant on the characterisation of the modified trait and a pre-defined
list of desirable sustainability impacts to be set out in the legislation. NGT plants would also
be subject to all sustainability-related provisions included in relevant horizontal and sectoral
legislation (e.g. FSFS, PRM, FRM), where applicable.
Traceability and post-market provisions would remain a requirement as in the baseline. With
a view to further steering development towards desirable traits from a sustainability
perspective, in addition to the regulatory incentives, the following sub-options on labelling
have been considered under this option:
A sustainability label (claim) would be added to the GMO label for products that
contribute to sustainability referring to the introduced trait.
A factual statement on the intended purpose of the genetic modification would be added to
the GMO label.
The GMO labelling requirement would be waived for products with a modified trait that is
considered to have the potential to contribute to sustainability.
5.2.3. Option 3: Authorisation with the requirement that products do not contain
modified traits that can be detrimental to sustainability
The legislation would be adapted to cater for the diverse risk profiles of NGT plants and to
address the implementation and enforcement challenges related to detection (as in Option 1)
and to discourage the development of unsustainable products.
NGT plants and food and feed produced from them would require an authorisation to be used
in field trials, cultivated or placed on the market, with adapted risk assessment and detection
method requirements (as in Option 1). The competent authorities will be as in option 1.
A prerequisite for authorisation – verified by the authorities competent for the procedure
concerned (at national or EU level) - would be that applicants show that the introduced trait is
not detrimental to sustainability (economic, environmental, and social). The basis for this
comparison would be data provided by the applicant on the characterisation of the modified
trait and a pre-defined list of undesirable sustainability impacts to be set out in the legislation.
NGT plants would also be subject to any sustainability-related provisions included in relevant
horizontal and sectoral legislation (e.g. FSFS, PRM, FRM), where applicable.
The additional requirement of this option is not linked to potential safety issues of the specific
NGT plant (i.e. risks to health or the environment that result from the genetic modification).
This is addressed by the risk assessment that features in this option (as well as in the baseline
and in options 1 and 2, and by the notification criteria in option 4). Rather, the concrete
sustainability requirement of this option relates to the fact that NGTs could reach the market
featuring traits that, ultimately may have negative effects (e.g. herbicide-tolerant traits that
have been found to in some cases to lead to increased pesticide use or development of
resistance in weeds).
Traceability, labelling and post-marketing provisions would remain as today (baseline).

28
5.2.4. Option 4: Notification of products that could also occur naturally or be
produced by conventional breeding
Option 4 is intended to apply to only a part of the scope of this initiative, i.e. to NGT plants
that could also occur naturally or be produced by conventional breeding. These would be
treated similarly to conventional plants.
This option would introduce a notification procedure for NGT plants based on criteria set out
in the legislation to verify whether a product could also occur naturally or be produced by
conventional breeding. An indicative list of these criteria is presented in Box 2. The criteria
are based on a scientific literature analysis and take into account the work of JRC and EFSA
as well as feedback received in the consultation activities for this impact assessment. A
thorough analysis of the literature confirmed that targeted mutagenesis and cisgenesis
techniques can lead to genetic modifications that are similar to mutations occurring naturally
or resulting from conventional breeding techniques (including random mutagenesis
techniques). These mutations include substitutions, insertions (including translocations and
inversions) and deletions of nucleotides in the DNA. Such mutations are commonly observed
with conventional breeding techniques and span a range of sizes: substitutions and insertions
of limited size as well as deletions of variable dimensions are frequently reported. Larger
translocations, inversions and genome duplications may also occur naturally. Insertions of
cisgenes or part of cisgenes are also possible through natural processes, crossing or
conventional breeding; with natural crossing or conventional breeding, the insertion of a
cisgene (or part of) generally takes place in locations where the gene is naturally occurring in
its breeders’ gene pool. As regards the total number of modifications introduced by
conventional breeding techniques, literature shows that it is also variable depending on the
organism and the method used.
The criteria are product rather than technique-based, reflecting the majority scientific view
(including of EFSA) that the risk profile is not mainly dependent on the technique used but
rather on factors related to the product itself. They are designed to cover the type and extent
of modifications that are also observed with conventional breeding techniques, to ensure that
the NGT plants fulfilling these criteria would not present more potential hazards for human or
animal health and the environment than conventionally bred plants. They are also designed to
be objective to ensure predictability for developers and uniform applicability by the
assessment bodies (EFSA and Member States authorities).
As shown by the scientific literature analysis, conventional breeding techniques can lead to a
high number of modifications. However, certain combinations of modifications would be less
likely to occur by conventional methods. To take this into account, following a conservative
approach and considering the novelty of new genomic techniques, the criteria would be
complemented by thresholds for both size and combination of modifications, also to ensure
that plants obtained by complex modifications are excluded from the notification procedure.
Box 2 - Indicative list of criteria for the notification procedure:
A NGT plant is considered equivalent to conventional plants when it differs from the
recipient/parental plant by no more than a maximum number of genetic modifications of the types
referred to in points 1 to 5, in predictable DNA sequences. A predictable DNA sequence is any
DNA sequence that shares sequence similarity with the targeted site.

29
1. Substitution or insertion of no more than a maximum number of nucleotides
2. Deletion of any number of nucleotides.
3. On the condition that the genetic modification does not result in an intragenic plant:
a. Targeted insertion of a contiguous DNA sequence existing in the breeder’s gene pool
b. Targeted substitution of an endogenous DNA sequence with a contiguous DNA
sequence existing in the breeder’s gene pool
4. Targeted inversion of a sequence of any number of nucleotides
5. Any other targeted modification of any size, on the condition that the resulting DNA
sequences already occur (possibly with modifications as accepted under points 1 and/or
2) in a species from the breeders’ gene pool.
The regulatory status of the product would be subject to a decision based on the notification
criteria. The authority responsible would be:
If the determination of status is sought prior to conducting field trials, the verification
of the criteria and determination of the status would be done by the Member State
where the field trial is intended to be carried out. The national decision would have
EU-wide effects.
If the determination of status is sought prior to the placing on the market of NGT
plants and products for which no field trials have been carried out in the EU, including
imported NGT plants/products, the determination of the status would be done by the
Commission, following verification of the criteria by EFSA.
In both cases, appropriate consultation procedures of Member States, the Commission and
EFSA would be established. The procedure is illustrated in Annex 11.
For products that meet the notification criteria, no authorisation requirement would apply for
the deliberate release, including placing on the market, or to field trials. In addition, there
would be no GMO traceability, labelling and post-market monitoring requirements.
Transparency would be ensured in a public register that would include information on the
product and the modified trait and would link to the notification assessment. The General
Food Law and other relevant legislation on traceability, labelling and monitoring of products
on the market as applicable to conventionally bred plants would apply.
Sustainability would be addressed by sustainability-related provisions included in relevant
horizontal and sectoral legislation (e.g., FSFS, PRM, FRM), where applicable.
There are two sub-options as regards the use in organic production of NGT plants/products
fulfilling the notification criteria. In a first sub-option notified NGT plants/products would
remain subject to the current ban of the use of GMOs in organic production (which applies in
the baseline and options 1 to 3) (see section 1.6.). In a second sub-option, notified NGT
plants/products would be treated as conventional products for the purposes of organic
production (as is the case today for the products of random mutagenesis), and could be used
under certain circumstances (permission to use non-organic agricultural ingredients in
processed organic food and feed, the use of non-organic plant reproductive material). The
Organic Product Regulation does not require coexistence measures for cultivation when it
comes to conventional agriculture. Precautionary measures are used to segregate organic and
non-organic during storage, transport, processing.

30
5.2.5. Combination of option 4 and one of the other options
Options 1, 2 or 3 apply to all types of NGT products. Option 4 applies only to NGT products,
which could occur naturally or be obtained by conventional breeding. Therefore, option 4, if
retained, would in all cases apply in combination with options 1, 2 or 3 or the baseline. The
potential impacts of option 4 and of one of the other options chosen are independent and
therefore can be assessed individually. Relevant cumulative impacts of the possible
combinations, especially as regards specific objective I (ensuring safety while avoiding
unnecessary burden) are discussed in section 7.
5.3. Options discarded at an early stage
An option to initiate a broader legislative action, which would have concerned all NGTs, and
all organisms (plants, animals, micro-organisms) was discarded in the light of the
Commission NGT study. The latter concluded that for other NGTs and for NGT applications
in animals and microorganisms, the necessary scientific knowledge is still limited or lacking,
especially on safety aspects. As a result, when announcing the follow-up to the study, the
Commission indicated that, as regards animals and microorganisms and other NGTs, it
intends to continue to build up the required scientific knowledge, in view of possible further
policy actions
120
.
An even broader action, covering all genetic modification techniques, was also discarded.
This approach would have required an extensive evaluation of the EU GMO legislation, to
analyse issues which were not addressed in the Commission NGT study. In its absence, there
is no substantial evidence to suggest that the current requirements of the GMO legislation are
not suited to the products of established genomic techniques.
The possibility of applying a notification procedure with no risk assessment (as in option 4) to
all NGT plants developed with targeted mutagenesis and cisgenesis was discarded in light of
the conclusion by EFSA and many other scientific bodies, that targeted mutagenesis and
cisgenesis can produce a diversity of plant products with different risk profiles.
An option to exempt targeted mutagenesis from the scope of the GMO legislation entirely, by
adding it to Annex IB of Directive 2001/18/EC, was also discarded, although it is requested
by certain stakeholders given that random mutagenesis is already exempted in that way. This
is linked to the diversity of plant products and risk profiles that can be achieved with all
techniques under the scope of this initiative. On that basis, only options entailing a case-by-
case regulatory procedure (authorisation or notification) were considered.
As explained above, the policy options each address several elements (procedure for the
release or placing on the market and risk assessment, traceability, labelling/transparency,
detection method requirements and sustainability) and, in turn, several policy choices were
considered for each element. While theoretically this could have led to many different
combinations and resulting policy options, policy options 1 to 4 presented above are those that
have been considered feasible and internally coherent.
In this regard, it has been considered that products subject to risk assessment call for policy
elements such as an authorisation procedure and traceability as GMOs, and these have been
packaged together (in options 1, 2 and 3, as in the baseline). Conversely, in the option where
risk assessment is not required (option 4), authorisation, traceability and labelling as GMO are
not required, as the option is based on the logic of treating the products meeting certain
criteria in the same way as the products of conventional breeding.
120
European Commission (2021e)

31
6. What are the impacts of the policy options?
Economic, environmental and social (health) impacts of the options were assessed taking into
consideration scientific literature, expert views, data collection from public and targeted
stakeholder consultations, JRC case studies and quantitative modelling where possible, as
explained in Annex IV on the analytical methods. One important input to the impact analysis
is the development pipeline of NGT products, i.e. applications that are already being
marketed, are at a confirmed pre-market development stage or are at R&D stage but showing
market potential, as mapped by the JRC
121
.
However, inherent limitations remain. Since specific regulatory frameworks were
introduced in several non-EU countries only recently and given the limited number of
products
122
on the global market (none in the EU), very few if any data on economic,
environmental or social (health) impacts of NGT products are available to date. Furthermore,
EU data on cultivation and coexistence with organic
123
/non-GM crops are very limited as
almost no GM crops are cultivated in the EU. To address these issues, the impact estimates
depend to a large degree on comparison with past developments inside and outside the EU
(e.g. for GMOs obtained by established genomic techniques), expert assessments, projections
and stakeholder expectations. Wherever possible, these assessments, projections and
expectations were checked against relevant data from the scientific literature. In addition, the
JRC has conducted three case studies to analyse the potential economic, environmental and
social (health) impacts of selected NGT plants that are in the development pipeline
124
.
Most findings are qualitative, though estimates of costs, especially regulatory, are provided.
Potential benefits are explained and quantified to the extent possible throughout.
A number of fundamental rights enshrined in the Charter of Fundamental Rights of the EU
can be impacted by this initiative. These concern the protection of human health (Article 35),
of the environment (Article 37), of consumers (consumers’ right to information) (Article 38)
and the freedom to conduct business (for the agri-food sector and biotechnology operators and
researchers) (Article 16). Impacts on those rights are addressed under the economic,
environmental, and social impacts analysed below.
1.1. Analysis of the baseline
6.1.1. Economic impacts
Breeders – The current average time to market of an established, single event GMO
(including R&D) developed between 2017 and 2022 is 16.5 years and the average costs are
approximately EUR 121 m
125
. In the EU, authorisations may be for marketing of food and
feed and derived products or for cultivation. Authorisation costs for food and feed uses range
121
Parisi & Rodriguez Cerezo (2021)
122
On the market are a higher oleic-acid soybean of Calyxt in the US (‘Calyno’) (ISAAA, 2019a), a tomato with
a non-proteinogenic amino acid (GABA) commercialised under the name Sicilian Rouge High in Japan (Waltz,
2021), drought-tolerant soybeans in Brazil and Colombia (ISAA, 2023a), a waxy maize in Japan (ISAA, 2023b)
and a non-browning banana in the Philippines (ISAA, 2023c). The commercial farm production of a modified
pennycress (Thlaspi arvense), marketed as CoverCress
TM
,
started in autumn 2022 in the US. The seeds will be
used for large-scale farm trials starting in 2023.
123
See https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Organic_farming_statistics for data on
organic farming in the EU
124
Schneider et al. (2023a, b). Sanchez et al. (2023)
125
AgbioInvestor (2022)

32
from ~EUR 6 m to EUR 20 m (for authorisations of single event GMOs)
126
,
and administrative
costs range from EUR 2 - 2.7 m
127
. For GMOs developed between 2017 and 2022 by
established genomic techniques, the genetic event construction and testing phase represented
55.8% of total costs and 35.6% of the total time. The regulatory phase had the longest
duration of the overall process and accounted for 37.6% of total costs and 51.1% of the total
time
128
. The time to place NGT products on the market would be shorter than for GMOs
obtained by established genomic techniques, because of an accelerated R&D phase: for the
latter, the R&D phase can be more than 10 years, while for NGT products the R&D phase is
around 5 years
129
, thereby reducing the costs of the most expensive phase in product
development.
There is little experience with authorisation costs for cultivation in the EU
130
, and therefore
only approximations are available. Available estimates provided by one company give a range
of costs of EUR 17.5 – EUR 28 m for authorisation plus EUR 0.7 – EUR 1 m per year for
monitoring.
Breeders identified the regulatory uncertainty about a product being authorised after several
years of R&D, regulatory costs and time to market as the main factors making the
development of NGT products under the existing GMO framework unattractive. 54.5% of the
stakeholders
131
in the targeted survey consider that attractiveness of developing plant varieties
using NGTs will decrease and 43.2% consider it will stay the same under this option. Across
all participating stakeholders
132
, there is the expectation to see very few NGT products on the
market for the period 2030-35. Breeders delayed product development and release due to
regulatory uncertainty and high regulatory costs (40% response in targeted survey)
133
.
It is expected that GMOs, including NGT products, mostly of large-scale commodity crops
(e.g., maize, soybean, cotton), would continue to be predominantly developed by large
multinational companies, while the high R&D costs, regulatory uncertainty and long duration
and costs of authorisations will continue to act as a barrier to market entry for SMEs, which
more often focus on niche crops
,
134
. The baseline is thus not conducive to the freedom of
SMEs to conduct business and to their competitiveness by keeping new, innovative
technologies out of their reach.
Academia/Research institutes – While there is only anecdotal evidence and warnings that
academic mobility and migration of EU plant scientists may be affected by policies of
regulating biotechnologies, according to the research sector, in the baseline the EU risks a
brain drain when plant researchers leave Europe for better job opportunities abroad
135
. The
sector expects a negative impact on the competitiveness of EU research, due to competition
from other countries where NGTs are regulated differently, in particular for applied research
126
Annex 7, section 4 in Technopolis Group et al. (2023)
127
AgbioInvestor (2022)
128
Ibid.
129
Lassoued et al. (2019)
130
There has only been one authorisation for cultivation in the EU (maize MON810).
131
47.7% (21) strongly decrease, 4.5% (2) moderate decrease, 2.3% (1) small decrease and 43.2% (19) no
change. Stakeholder's categories are not included in the data.
132
The median is overall 0. Across stakeholders the median is: Academic/research organisation = 0, Business
association = 0, Consumer organisation = 12.5, Large company = 0, NGO = 0, other = 0
133
See also Jorasch (2020)
134
OECD (2018)
135
The main root causes for academic migration to countries outside the EU are better salaries and better career
structures for early-career scientists. See Khan (2021)

33
in plant pre-breeding and breeding. 41% (n=27) of respondents in the targeted survey expect a
decrease in research funding for biotechnology under the baseline option.
Business associations see a negative impact on firm-level innovation in the agricultural
biotech sector, also impacting biotechnology research capabilities in the food and feed
sectors. However, in EU research activities, the use of NGTs is well established and notable
funding is allocated to genomic research and biotechnology in plant and animal breeding.
Public authorities – With no regulatory change, the cost per authorisation (both for food and
feed uses and for cultivation) is expected to remain stable. With very few NGT products
expected to be authorised, it is likely that the overall costs for public authorities remain stable.
National public authorities will face enforcement challenges when the same genetic alteration
can be introduced by NGTs (subject to the GMO legislation), or conventional breeding
methods.
Conventional farmers – Impacts on farmers concern increased imports of more competitively
produced crops from non-EU countries and potential missed opportunities, in particular
regarding reduced costs for inputs (reductions in the amount of pesticides, fertilisers),
improved yield/input ratio, new income opportunities or better prospects for exports.
The shorter development time of NGT plants compared to conventional breeding not being
realised also means that farmers will have to wait longer for new varieties with beneficial
traits or offering new income possibilities if no or very few NGT plants come to the market.
Farmers will have to forego potential improvements of the yield/input ratio, reduced input use
or economic benefits from quality increases of crops. If farmers cultivate NGTs under this
option, they have to implement the nationally applicable coexistence measures (for more
detail see the analysis of coexistence under option 1).
Organic/GM-free farmers and value chain – The EU organic and non-GMO sectors consider
that the baseline option is the one that best protects their sector. If the current situation is
maintained, the labelling and traceability requirements in the existing GMO legislation, as
well as national coexistence measures, will remain as today and continue to support EU value
chains and consumer trust for organic and other GM-free producers. As few NGT products
will reach the market, with very limited cultivation, only a small risk of admixture of NGTs
can be expected.
However, certain negative impacts may also materialise. Increased costs for organic operators
could arise from NGT products that cannot be analytically differentiated from products that
can occur naturally or be produced by conventional breeding, despite the fact that specific
GMO traceability and labelling obligations will apply to such NGT products. More
challenging and expensive identity preservation systems for NGT-free products would be
required, possibly leading to higher prices of GM-free products
136
. Research has shown that
coexistence of GM and non-GM products within food supply chains leads to extra costs for
the non-GM food product. Depending on the segregation strategy undertaken in rapeseed oil
and maize starch supply chains, it was estimated that ensuring coexistence leads to increased
prices for the non-GM product of between 7 and 14%
137
. For NGTs that cannot be
analytically differentiated, these price increases could be higher as further steps might have to
be taken to guarantee the integrity of the supply, such as additional documentation and third-
party verification.
136
IFOAM EU & FiBL (2017)
137
Gabriel & Menrad (2015)

34
Regulatory divergence with non-EU countries (where NGT products may not be subject to
traceability and labelling rules or to any transparency obligations) from which organic or
other GM-free products are imported may also impact the organic sector. For example,
Argentina, where many NGTs are neither traced nor labelled, has been a major exporter of
organic products to the EU (in the range of 10 000 – 50 000 tons per year), in particular of
fruits, wine and vegetables. To continue imports of NGT-free products from countries with a
regulatory framework similar to Argentina’s may require adjustments to supply chain
management, for which no quantitative estimates of costs are available. Under the current EU
regulatory framework, NGTs should not be used in organic products exported to the EU,
therefore, the costs of segregated supply chains in the EU would remain unchanged.
Regulatory divergence may also impact the availability and development of organic varieties.
Under the breeders’ exemption
138
, breeders are allowed to freely use protected varieties as
sources of further variation for the development of new varieties. If protected varieties of
interest for organic breeders originate in non-EU countries that have exempted NGT plants
from GMO labelling and traceability obligations, then organic breeders may have no
guarantee that those are not derived from NGTs. Consequently, sourcing such varieties may
become difficult and in the longer term, organic breeders may thus experience a decrease in
the availability of genetic resources for their breeding programmes, leading to fewer improved
varieties adapted to the needs of organic agriculture
139
.
Trade impacts – International trade with NGT products will be affected by regulatory
divergence with trade partners. Several major trade partners of the EU have introduced
exemptions from their GMO legislation – or are preparing to introduce them – for NGT
products, and this may lead to asynchronous approvals of NGT products. This could lead to a
costly need to separate entire agricultural value chains in and outside the EU
140
. As trade
partners might be unwilling or unable to establish such separate value chains
141
, most targeted
survey respondents (60.7%, 37) expect a decrease of international trade under this option. The
consequences of market disruptions caused by the unintended presence of unauthorised
GMOs can be significant. For example, in 2009, a genetically modified flax from Canada, not
authorised in the EU, was detected in EU food products
142
and the EU immediately stopped
Canadian flax imports. This resulted in an estimated loss of EUR 40 m for the EU flax
processing industry and 600 jobs lost
143
.
Impact on SMEs – The current situation with large, multinational companies dominating the
market for GMOs is likely to apply to NGTs as well. While R&D costs for NGT products will
be considerably lower than for GMOs obtained by established genomic techniques,
authorisation costs would remain in the range of EUR 6 – 20 m, which is a significant part of
the yearly turnover of a SME.
138
Article 15(1)(iii): The breeder’s right shall not extend to (...) (iii) acts done for the purpose of breeding other
varieties, and, except where the provisions of Article 14(5) apply, acts referred to in Article 14(1) to (4) in
respect of such other varieties.
139
IFOAM EU & FiBL (2017)
140
Eriksson et al. (2019)
141
Gabriel & Menrad (2015)
142
The genetically modified flax (“Triffid flax”) was a flax variety that was genetically engineered for resistance
to soil residues of sulfonylurea-based herbicides. To ensure that Triffid flax would not jeopardize future export
markets, the transgenic flax variety was deregistered in 2001. In late 2009, Triffid flax was detected in bakery
goods, cereals, and other products made by companies throughout the EU. See Ryan & Smyth (2019)
143
Ryan & Smyth (2012)

35
Competitiveness – The reduced costs of and time needed for R&D of NGT products could
lead overall to lower costs for the EU breeding and biotech sector
144
. However, breeders in
non-EU countries that exempt NGTs, can save all the costs related to GMO authorisations
(35.8% of the total cost to develop and place on the market a GMO obtained by established
genomic techniques) and place products faster on the market, while the regulatory entry
barriers for NGT products remain higher in the EU. This will give competitors in non-EU
countries a considerable advantage.
6.1.2. Environmental impacts
Among environmental impacts, a distinction should be made between environmental impacts
specifically linked to the genetic modification or the introduced trait(s) and other
environmental impacts that are associated to the use of the plant in the field in relation to its
trait(s). While the first are specific for the NGT plant, the second may be common to other
plants, regardless of the breeding method used, sharing the same trait and use (an example
being the herbicide tolerant plants discussed further below).
As regards potential risks specifically linked to the genetic modification, NGT plants and their
products will only be authorised and released in the environment if the risk assessment
concludes that they are as safe as their conventional counterparts. In addition, NGT products
released or placed on the market will be subject to various monitoring provisions intended to
identify and take measures in response to any potential adverse effect.
As regards other environmental impacts, since no or only very few applications for cultivation
of NGT plants are expected if they remain regulated by the current GMO legislation, the
environmental impacts of these products will be limited. Impacts will be mostly restricted to
missed potential benefits stemming from NGT plants contributing to, for example, lower
pesticide use, lower fertiliser use, reduced need for agricultural land and more space for
nature with higher biodiversity
145
.
The vast majority of survey respondents expect no or moderate (positive or negative) impacts
on non-target organisms (such as pollinators, microbial communities, etc.). Four out of five do
not expect a change of potential impacts on the environment and biodiversity through gene
transfer or accidental consumption during experimental release or placing on the market.
6.1.3. Social, health and safety impacts
Social health and safety impacts – Similarly to environmental impacts, products authorised
for placing on the market and cultivation would be risk assessed (and monitored once released
or placed on the market) to ensure they do not present more potential hazards to health than
their conventional counterparts. The majority of survey respondents does not expect any
change in the safety impacts (for example due to toxicity or allergenicity) of products entering
the market under the current regulatory framework. Potential positive social and health
impacts stemming from NGT products with improved nutritional and functional quality will
be largely missed or delayed.
Impacts on consumers – The current GMO legislation ensures information to consumers
about the use of genetic modification technologies on the product’s label. This is the
preference expressed by a majority of citizens (30%, 733
146
) in the public consultation, as also
144
Lassoued et al. (2019)
145
Phalan (2018), Grass et al. (2019)
146
The second highest preferences, each at 19% (464), were for digital labelling, information on a website/public
database, and no labelling needed if products were produced through conventional plant breeding or classical

36
confirmed by similar findings from research
147
. It guarantees freedom of choice for those not
wishing to use GMOs and would do so in the same way for GMOs obtained by NGTs.
The current label would not inform specifically about NGTs or their applications, and
established consumer attitudes to GMOs would likely extend to NGTs (in the absence of other
sources of awareness and information on NGTs) and have a negative impact on NGT
acceptance and consequently on uptake. This would be reinforced by the fact that NGTs
would remain subject to the GMO legislation as it stands today and applies to transgenic
GMOs (as research
148
suggests that governmental decisions also play a role in shaping
consumer attitudes).
As no or very few NGT products will come on the market under this option, there could be
missed opportunities for consumers willing to consume NGT products. This could be linked
to consumer preferences not fulfilled, but also in some cases to health or other needs not being
addressed (e.g. when NGTs can be used to reduce allergen or toxic content).
Furthermore, given that more costly identity preservation may need to be applied, the price of
some products could rise
149
.
1.2. Analysis of option 1 (Authorisation with adapted risk assessment and
detection method requirements)
6.2.1. Economic impacts
Breeders – Significantly shorter development times and lower development costs for NGT
products and, in some cases, lower costs and time linked to the generation of data for risk
assessment and authorisation, could make the development of certain NGT products more
attractive than GMOs obtained by established genomic techniques. For example, in
conventional potato breeding, the timeline in which breeders operate is approximately 13-15
years
150
. The cost of developing a conventional potato variety is EUR 2-3 m per variety, while
development of NGT potato varieties is expected to cost significantly less money and time,
estimated at EUR 0.5 m per variety and introduction into the market within five years
151
.
Generating the necessary data for risk assessment currently represents the largest share of
authorisation costs. For a risk assessment that is adapted to different risk profiles, the costs of
generating the application will decrease to a variable degree due to potentially reduced data
requirements. In cases where data requirements remain the same as they are today for GMOs,
no cost savings are expected, while maximum cost savings of 85% of this component could
be expected in cases where minimum data requirements apply based on the product’s risk
profile
152
. An adapted risk assessment could also shorten time to market with earlier profit
gains.
Breeders, however, expect only a small improvement concerning the attractiveness to develop
NGT plants as this option would apply the current GMO labelling regime to NGT products
for which they expect only limited consumer acceptance, and because of the perceived
mutagenesis; the rest 11% (269) indicated that transparency is not needed for any plant produced by targeted
mutagenesis and cisgenesis and 2% (49) expressed no opinion.
147
Food Standards Agency (FSA) (2021); YouGov (2022); Lindberg et al (2023)
148
Sendhil et al. (2022).
149
See Gabriel & Menrad (2015)
150
Jansky & Spooner (2018)
151
Annex 5, section 2.3.1. in Technopolis Group et al. (2023)
152
Annex 7, section 4.2. in Technopolis Group et al. (2023)

37
uncertainty about the way the adapted risk assessment would be applied in practice. In this
regard, they expect negligible reductions in administrative costs compared to the baseline.
In the targeted survey, respondents expect on average a small increase in NGT plants on the
market in 2030-35 compared to the baseline.
Academia/Research institutes – This sector expects a limited impact on research funding from
this option both for private and public R&D. Concerns with regard to plant scientists leaving
the EU are similar to the baseline.
Public authorities – Member States competent authorities expect cost decreases for risk
assessment ranging from 8% - 67% (see table 1). The wide range results from the fact that the
adaptation of risk assessment will be case-by-case and is intended to lead to large variability
of data requirements (several scenarios were modelled: the lower value is applicable to a risk
assessment requiring almost the same data as today, and the highest saving is applicable to a
scenario with molecular characterisation and post market monitoring only). There is also a
large variability between the Member States which can in part be explained by different
administrative set-ups.
Table 1. Estimated cost decreases for Member States if data requirements for risk assessment are
adapted to the risk profile of the product (data gathered in the context of Technopolis Group et al.
study).
ESTIMATES
SCENARIOS
SPAIN
NETHERLANDS
GERMANY
BELGIUM
FRANCE
MIN
AVERAGE
MAX
Scenario 1: Full data except for protein
-10%
-8%
-15%
0%
-5%
0%
-8%
-15%
Scenario 2: Molecular characterization
and Safety data on the trait only
-49%
-26%
-35%
0%
-10%
0%
-24%
-49%
Scenario 3: Molecular characterization
and post market monitoring (including
environmental) only
-67%
-60%
-60%
0%
-10%
0%
-39%
-67%
Furthermore, Member States’ authorities will face enforcement challenges in cases where the
same genetic alteration can be introduced by NGTs and conventional breeding methods and
such plants cannot be distinguished by analytical methods.
Conventional farmers – As, under this option, some NGT plants would reach the market,
farmers would be able to benefit from these plants if they feature relevant traits, in particular
regarding reduced costs for inputs (pesticides, fertilisers), improvements of the yield/input
ratio, new income opportunities or better prospects for exports. For example, a case study
153
on cisgenic late blight resistant potatoes and apples, conducted by the JRC, shows that for
potatoes a 50-80% reduction and for apples a 12-58% reduction of fungicide use may be
feasible. This would translate into yearly costs savings ranging from (min-max) EUR 49-576
per hectare for potatoes and EUR 39-712 per hectare for apples (see Annex 7). A gene-edited
pennycress (Thlaspi arvense) is a new oilseed crop (for which large-scale farm trials will take
place starting in 2023 in the US) that can be grown with relatively low greenhouse gas
emissions and without causing detrimental land use changes
154
. In the US, this crop is grown
as a cash cover crop in maize-soybean rotations providing environmental benefits (preventing
soil erosion and nutrient run-off, reduction of pesticide use, carbon sequestration) and
153
Schneider et al. (2023a, b)
154
Jarvis et al. (2021); the plant was developed for the market by a start-up company called CoverCress Inc. In
August 2022, Bayer acquired a majority share of 65% in the company.

38
expected net returns to farmers from biofuel or animal feed uses of EUR 130-180/ha
155
.
Similar efforts are underway in Europe for the establishment of camelina (Camelina sativa) as
a cash cover crop
156
.
In cases where the costs of the authorisation process are considerably reduced because of
significantly lower data requirements, it is likely that SME breeders and farmers can benefit
from this option and that NGT plants from small or neglected crop species will come to the
market under the condition that the license cost for the use of the technologies is not
prohibitive for SMEs and minor crops
157
.
Combining stakeholder and expert estimates of adoption rates of the currently known pipeline
of NGT-applications, as well as expert estimates regarding trait-level impacts, a quantitative
exploratory analysis
158
of impacts shows, for a low adoption rate of NGTs (1-5% of the crop
market), the following estimated mean and, in brackets, range of impacts on key agronomic
parameters for several crop groups (Table 2).
Table 2. Trait-level impacts.
Crop group
yield change/ha
change in
pesticide use/ha
change in
fertiliser use
change in
water use
change in
energy use
Oil and fibre plants
5%
(1% to 16%)
-10%
(-35% to 5%)
-13%
(-8% to -28%)
-3%
(5% to -15%)
-3%
(0% to -10%)
Vegetables
9%
(6% to 24%)
-9%
(-5% to -43%)
-
-
-
Cereals
7%
(3% to 22%)
-14%
(-10% to – 18%)
-10%
(-3% to -17%)
-2%
(1% to -5%)
-6%
((0% to -11%)
Legumes
9%
(6% to 23%)
-9%
(5% to -35%)
-10%
(-5% to -20%)
-5%
(3% o -16%)
-3%
(0% to -10%)
These estimates show that considerable per hectare changes in key agronomic traits are
expected by experts, but also indicate that there is disagreement about the direction of change
in some of those traits, in particular in pesticide and water use, where some experts expect
increased use.
Organic/GM-free farmers and value chain –The labelling and traceability requirements in the
existing GMO legislation will continue to support value chains and consumer trust for organic
and GM-free producers. However, the regulatory divergence with non-EU countries (where
NGT products may not be subject to traceability and labelling rules or to any transparency
obligations) from which organic or other GM-free products are imported and detection
challenges will impact the organic sector as in the baseline. Under the current EU regulatory
framework, NGTs should not be used in organic products exported to the EU, therefore, the
costs of keeping segregated supply chains in the EU would remain unchanged.
Coexistence between conventional/organic/GM-free production and NGT production – As
more NGT plants are expected to be cultivated in this option compared to the baseline, the
risk of admixture will increase and with it the costs for segregation. The purpose of
coexistence measures is to allow consumers and producers a choice between conventional,
organic and GM production. These measures also take into account the wish of some farmers
155
https//:www.covercress.com/farmers.cfm
156
Zanetti et al. (2021)
157
Section 3.3.10. in Technopolis Group et al. (2023)
158
Annex 8, section 1 in Technopolis Group et al. (2023)

39
and operators to ensure that their crops have the lowest possible presence of GMOs,
acknowledging that the potential loss of income for producers of particular agriculture
products such as organic products is not necessarily limited to exceeding the labelling
threshold set out in EU legislation at 0.9% for food and feed. In certain cases, and depending
on market demand and on the respective provisions of national legislations (e.g. some
Member States have developed national standards for different types of ‘GM-free’ labelling),
the presence of traces of GMOs in particular food crops — even at a level below 0.9% — may
cause economic damages to operators who would wish to market them as not-containing any
GMOs
159
.
Article 26a of Directive 2001/18/EC on the deliberate release of GMOs into the environment
allows Member States to take appropriate coexistence measures. A framework for such
measures is defined in the Commission Recommendation of 13 July 2010 on guidelines for
the development of national coexistence measures to avoid the unintended presence of GMOs
in conventional and organic crops
160
. Member States have taken a diversity of approaches –
legislative and non-legislative and a mixture thereof – to implement this recommendation (15
Member States have adopted some form of coexistence measures). Almost all Member States
that have regulated coexistence consider the GMO grower as the operator responsible for the
implementation of the measures. In addition to the coexistence measures set out in the GMO
legislation and the Commission Recommendation, the Organic Products Regulation requires
organic producers to implement precautionary measures to avoid the presence of products and
substances not authorised for use in organic production (Article 28).
The analysis below is based on the assumptions that a certain level of NGT cultivation will
take place, and first considers the impacts on farmers cultivating NGTs and organic farmers,
then the impacts on the respective value chains downstream from the farm.
The SIGMEA project (2004 – 2006), funded under FP6, investigated the costs of measures for
GM-cultivating farmers to ensure coexistence for GM and conventional oilseed rape and
maize in several regions of the EU using spatially explicit simulation models
161
. At the level
of the GM-cultivating farm, two types of coexistence cost are considered: (i) the cost of
compliance with the ex-ante coexistence rules in place, developed to prevent cross-pollination
and/or admixture, (ii) the expenses for ex-post monitoring, because the product has to be
tested for the presence of transgenic DNA. In general, results obtained for different regions
demonstrate that coexistence costs depend on the agricultural context (landscapes, cropping
systems, field shape, climate), the share of GM crops in the Utilised Agricultural Area (UAA)
and the willingness of GM and non-GM farmers to cooperate. The study also concludes that
inflexible coexistence rules (such as standardised large isolation distances) impose a severe
burden on GM cultivation, while flexible, context-dependent measures do not act as a
disincentive to GM cultivation. In the latter case, GM crop adoption is not an issue of costs of
compliance with coexistence measures but rather one of the incentives for adopting or
rejecting the technology.
Example: Oilseed rape in Beauce (France)
Relative to the benchmark scenario, the study simulated six scenarios by varying (i) the GM
crop adoption rate (scenarios 2 and 3), (ii) the share of oilseed rape in the arable area
159
European Coexistence Bureau (2010-2018)
160
OJ C 200, 22.7.2010, p. 1–5
161
The final report is available at https://cordis.europa.eu/docs/results/501/501986/126792601-6_en.pdf

40
(scenarios 4 and 5), and (iii) the isolation distance requirement (scenario 6). Table 3 reports
the estimated average cost for coexistence management.
Table 3. Simulated coexistence management costs of GM and non-GM oil seed rape under alternative
scenarios (averages of 10 simulations varying the spatial allocation of crops in the landscape).
Source: Based on SIGMEA Deliverable 5.2 and 5.3; *costs have been adjusted to take inflation since 2006 into
account.
These data show that coexistence costs for GM-cultivating farmers can decrease with
increasing adoption rate (see scenarios 2 and 3) and that they are highest if large shares of
arable land are cultivated with oilseed rape, if the GM adoption rate is in the middle of the
range and if isolation distances are large (scenario 7).
Example: Maize in Aragón (Spain)
For this case study the small area of Gurrea de Gallego was selected to implement simulations
for coexistence between GM and non-GM maize. The municipality of Gurrea de Gallego has
a total UAA of 964 ha cultivated in 489 fields, which results in an average field size of 1.97
ha. In 2005, maize was cultivated in 47 fields, corresponding to 105 ha or an average of field
size of 2.2 ha.
Scenario
Adoption
Rate
Oilseed
rape
Share
Buffer zone
width
Average cost per
hectare GM
(€/ha)*
1
(Benchmark)
50%
13%
10m
0.47
2
25%
13%
10m
0.75
3
75%
13%
10m
0.23
4
50%
6%
10m
0.33
5
50%
26%
10m
1.13
6
50%
13%
20m
1.21
7
50%
26%
20 m
2.72

41
Table 4. Coexistence costs of buffer zones for different adoption rates and distances in Aragón
(Spain).
GM adoption rate (%)
Buffer zone
Cost per ha GM maize
area (€/ha)*
10%
10m
34.64
20m
62.51
50m
101.71
100m
117.30
30%
10m
29.95
20m
54.23
50m
94.25
100m
116.61
50%
10m
30.64
20m
55.89
50m
97.43
100m
116.89
90%
10m
29.81
20m
54.37
50m
97.15
100m
117.16
*Costs have been adjusted to take inflation since 2006 into account
These data show that, for a given buffer zone size, the coexistence costs for GM farmers are
of moderate size and quite insensitive to the GM adoption rate (Table 4). These findings are in
line with the results of a simulation study of the costs of coexistence measures per hectare of
GM in Switzerland, which finds that the costs amount to 1-3% of total product costs
162
.
Organic operators also have the responsibility to put in place precautionary measures to
avoid admixture with any product that is not authorised in organic production, and to
regularly review these precautionary measures and to adjust these measures if necessary. A
product for which EU law requires GM labelling, e.g. food and feed containing traces of
GMOs above 0.9% (or even below 0.9% if the traces are not adventitious or technically
unavoidable), must not be marketed under the EU organic logo (Art. 30(4) of Regulation
2018/848). For conventional products which are not subject to such labelling requirements,
organic operators must require a vendor declaration to confirm the absence of any GMOs and
derived products. However, certain NGT plants cannot be distinguished from conventionally
bred plants. In such cases, the analytical control supporting the marketing prohibition under
the organic logo might be difficult and in some cases not feasible. Organic farmers would not
have direct economic losses in these cases where NGT presence cannot be established,
however, there is a risk of loss of trust by some consumers if a certain level of NGT
admixture cannot be analytically ruled out. Based on the work of the European Coexistence
Bureau
163
, it is reasonable to assume that risk of such admixture can be considered to be low
for this option with a low to moderate adoption of NGTs, if NGT farmers comply with
coexistence measures and organic farmers with the precautionary measures to avoid
admixture required by the Organic Products Regulation.
There are no quantitative estimates of the costs for GM-cultivating and non-GM farmers in
cases of admixture as these costs depend on the affected product (e.g. its gross margin and
162
Vögeli et al. (2010)
163
European Coexistence Bureau (2010 – 2018)

42
price premium) and (as liability is a component of the costs) on liability regimes, which are
the exclusive competence of Member States
164
. For non-organic farmers, the financial risks of
liability may tip the balance against the decision to cultivate GM crops
165
. The risk of
admixture, and therefore the overall financial risk for organic producers, will depend on the
overall adoption rate of NGTs – the higher the adoption rate, the more likely it is that fields
neighbouring an organically cultivated field will be cultivated with NGTs, thus increasing the
probability of admixture. However, experiences from Spain show that large scale cultivation
of GM maize has not been an obstacle to the expansion of organic cultivation. From 2012 –
2021, the area cultivated with organic maize has increased in three regions with a dominant
presence of Bt maize cultivation: in Aragón, the organic maize area increased from 159 ha to
526 ha, in Cataluña from 30 ha to 351 ha and in Navarra from 11 ha to 44 ha
166
.
Coexistence in the value chain
As the analysis in the baseline shows, additional costs of 7 – 14% can be expected by
organising coexistence between genetically modified and non-GM products in the value chain
from production of farm crops up to the production/processing levels of single supply chains.
These estimates are based on the assumption that GM can be identified by analytical tests. For
NGTs that cannot be analytically differentiated, these price increases could be higher as
further steps might have to be taken to guarantee the integrity of the supply, such as additional
documentation and third-party verification.
Trade impacts – Under this option some NGT plants are expected on the market, but
regulatory divergence with non-EU countries is likely to remain large if this option applies to
all NGT plants, posing challenges for value chain management and identity preservation
systems. Many targeted survey respondents expect a general decrease of international trade of
agricultural products and commodities under this option (31% (21) expect moderate decrease
and 15% (10) expect small decrease), but to a lesser extent than in the baseline (44% (27)
expect moderate decrease and 16% (10) expect small decrease).
Impacts on SMEs – Certain NGTs are considered to be accessible tools for plant breeding due
to their relatively low cost and complexity. The easily adaptable technology used in such
NGTs could lead to a lowering of technological barriers to entry of the plant breeding sector,
benefitting SMEs, provided that access to the technology and costs associated with IP remain
affordable. This has been observed in Argentina after its regulatory shift to excluding certain
NGT products from the GMO definition.
With a maximum of 85% reduction, significant cost reductions for adapted risk assessment,
overall authorisation costs will, in case of the lowest data requirements, be in the approximate
range of EUR 0.9-3 m (15% of costs for food and feed authorisation with full risk
assessment). Administrative costs are not expected to change significantly. The combination
of significantly lower development costs for NGT plants compared to varieties bred
164
Austria, Finland, France, Germany, Hungary, Norway, Poland and Slovakia have specific liability provisions
addressing damages arising from admixture. Compensations may differ substantially between Member States;
they may be calculated on the difference in price between a GM-labelled product and a non-GM labelled
product, while additional costs coming from covering the costs of quality scheme controls or the costs of a new
organic certification process, which depend on the type and size of a farm and are variable between Member
States, are not taken into account.
165
Venus et al. (2017) In Germany, the cost of joint and strict liability for Bt maize (EUR 189/ha) were
estimated to be higher than the average additional revenue (EUR 115/ha) and the additional gross margin (EUR
65/ha). However, farmers planted Bt maize because the GM seed company safeguarded potential economic
damage if the GM farmer complied with the coexistence measures.
166
https://www.mapa.gob.es/es/alimentacion/temas/produccion-eco/default.aspx

43
conventionally or developed with established GM technology (e.g., an estimated EUR 0.5 m
for an NGT potato variety vs. EUR 2-3 m for a conventional variety) and the significant cost
reductions for adapted risk assessment, may make it easier for SMEs in certain circumstances
to compete with larger companies.
Competitiveness – This option will improve the competitiveness of EU operators as costs of
and time needed for R&D of NGT products can be significantly reduced in comparison to the
baseline. Compliance costs related to data requirements for risk assessment will range from
negligible reduction (if a similar set of data, as today, is required) to a reduction of around
85% (if minimum data requirements apply). However, breeders in non-EU countries, which
have exempted certain NGT products from the requirements of their GMO legislation, can
save all the costs related to GMO authorisations for those NGT products, thus having a
competitive advantage over breeders in the EU also under this option. Because of potentially
higher prices for organic/GM-free products, this sector could experience reduced
competitiveness compared to conventional producers
167
.
Simplification – A risk assessment adapted to the diverse risk profiles of NGT plants would be
on a case-by-case basis less burdensome for operators compiling the applications as well as
for the authorities assessing them.
6.2.2. Environmental impacts
As regards potential risks specifically linked to the genetic modification, any NGT product
coming to the market will be risk assessed using criteria and requirements adapted to the
product’s risk profile and designed to ensure environmental protection. As under the current
legislation, potential risks of NGT plants/products will be assessed in comparison with their
conventional counterparts. No change compared to the baseline with regard to safety for the
environment is expected as products will only be authorised for placing on the market and
cultivation if the risk assessment concludes that they do not present more potential hazards
than their conventional counterparts, and post-market monitoring provisions will apply to
identify and take measures in response to any potential adverse effect.
Positive environmental benefits are expected if authorised products contain relevant traits,
such as the reduction in pesticide use as outlined in the case study presented in Annex 7,
resilience to climate change or lower fertiliser input, depending on the number of NGT
products placed on the market. Lower fertiliser input and the development of cash cover crops
could lower greenhouse gas emissions of agriculture.
Regarding pest- or pathogen control, the expected change in pesticide use is highly
controversial, in particular with respect to herbicide-tolerant (HT) crops. The main reasons for
the adoption of HT crops have been improved and simplified weed control, less labour and
fuel cost, no-till planting/planting flexibility, yield increase, extended time window for
spraying, and decreased pesticide input
168
. On the other hand, a recognised negative impact of
HT crops is the evolution of herbicide resistance in weeds, which may lead to higher use of
other selective herbicides, with the attendant environmental and health externalities, and
increased use of tilling and consequently higher GHG emissions
169
. By 2023, 57 weed species
167
Gabriel & Menrad (2015); IFOAM EU & FiBL (2017)
168
Sankula et al. (2015)
169
Schütte et al. (2017); Benbrook (2012); Desquilbet et al. (2019); Lu et al. (2022)

44
have evolved resistance to glyphosate
170
. Herbicide-tolerant weeds also occur in production
systems of herbicide-tolerant crops developed using conventional breeding methods
171
.
HT crops do not represent a strong focus of R&D in the field of NGTs. Among NGT products
in the pre-commercial and advanced R&D stage, herbicide tolerant plants account for 10 out
of 172 products in development
172
. This trait can be developed by established GMO
technology, NGTs and by conventional breeding methods
173
. Impacts of HT crops,
irrespective of the breeding method used for their development, will depend on how they are
used and managed in the field.
Experience with insect resistant GM crops in the EU (Bt maize producing the bacterial protein
Cry1Ab, toxic to insects) has shown that, as of 2021, no decrease in the vulnerability to the
toxic protein had occurred in the relevant pests
174
.
Effects on the diversity of cultivated crops would depend on how much new varieties can
benefit from the lower costs and lower administrative burdens stemming from adapted risk
assessment. From 2002 to 2010, niche crops globally accounted for only 5% of approved
GMOs
175
, although there is considerable technology and valuable traits to be exploited. There
is evidence that this is due in part to regulatory costs of GMO approval
176
, but lack of demand
or market rejection may play a role as well. The case study on chicory (see Annex 7) shows
that NGTs can contribute to the development of new and improved plants and can further
support the use of underutilised, neglected and local crop species
177
, thus leading to increased
agrobiodiversity.
Regarding local production systems and the diversity of local and traditional varieties, some
stakeholders see a danger in the displacement of local production systems by NGT crops,
arguing that increased cultivation of NGT plants would reduce the diversity of crops used in
agriculture, as well as the local knowledge of crop species and varieties suited for the local
climate
178
. However, in cases where adapted risk assessment leads to considerably lowered
regulatory costs, development and cultivation of NGT varieties of niche and locally important
crops may become economically more viable. NGT plants can provide more adaptation to
climate change and thus could lead to the development of plants more suitable to local
climatic conditions and thus maintain or enhance crop diversity
179
.
170
Heap (1993-2023)
171
Wedger et al. (2022)
172
Parisi & Rodriguez-Cerezo (2021), time horizon 2030; in the pre-commercial stage, 7 out of 16 products are
HT-traits; the high proportion of HT-traits in the pre-commercial stage reflects the fact this is a trait well-known
to developers, with which the new gene-editing technologies can be more easily applied and tested than with
new traits. This number may thus not reflect developer priorities (which is evident from the trait distribution in
the early and advanced R&D stage, where HT-traits play a small role) in the medium and longer term.
173
Fried et al. (2022); the following numbers of conventionally bred HT varieties deriving from the Clearfield
technology are listed in the EU Common catalogue of varieties of agricultural species (status in December
2022): turnip rape (Brassica rapa var. silvestris) two out of 39 varieties (5.1%), swede rape (Brassica napus)
116 out of 1333 (8.7%), sunflower (Helianthus annuus) 276 out of 1635 (16.9%) and rice (Oryza sativa) 40 out
of 449 (8.9%).
174
García et al. (2023)
175
Miller & Bradford (2011); however, a later analysis shows that developers from low- and middle-income
countries are starting to enter the commercial with a broader spectrum of crops; see Parisi et al. (2016)
176
Dobres (2008)
177
Venezia & Creasey Krainer (2021); Yaqoob et al. (2023)
178
Catacora-Vargas (2011)
179
Lemmon et al. (2018)

45
60% of respondents in the targeted survey do not see potential risks for the environment and
biodiversity under this option. This view is, however, disputed by certain stakeholders.
Responses mostly coming from citizens, NGOs, consumer organisations and environmental
organisations (18.8% of total participants in the public consultation) stress that the current risk
assessment system has demonstrated its effectiveness.
6.2.3. Social, health and safety impacts
Social, health and safety impacts – Any NGT product coming to the market will be assessed
to consider risks to human and animal health using criteria and requirements adapted to the
product’s risk profile and designed to ensure safety for human and animal health. Therefore,
similarly to potential risks for the environment, no change compared to the baseline with
regard to human and animal health is expected as products will only be authorised for placing
on the market and cultivation if the risk assessment has concluded that they do not present
more potential hazards than their conventional counterparts.
As some NGT products are expected on the market, certain social and health benefits are
expected. Current R&D addresses traits like modified starch, protein and lipid content and
composition or increased functional metabolites and traits enhancing market value (e.g. longer
shelf life)
180
. In oilseed crops, for example, the most preferred target of genome editing is the
production of monounsaturated fatty acids, such as oleic acid, which are generally considered
healthier and more stable, and hence are associated with a longer shelf life. An example of a
trait having an expected direct health effect is low gluten content in wheat produced by
targeted mutagenesis and assessed in one of the JRC case studies (see Annex 7). Such low-
gluten, celiac safe wheat can contribute to making low gluten diets more affordable
181
, to a
more balanced diet and to decreased costs of medical care
182
. In the JRC market study,
modified content is the most common trait addressed in NGT product R&D (see Annex 7).
62% of respondents to the targeted survey expect no change in terms of social impacts under
this option. This view is, however, disputed by certain stakeholders, representing 21% of
respondents and coming especially from NGOs and consumer organisations, expecting
increased potential hazards.
Impacts on consumers – NGT products would be labelled, and similar considerations as in the
baseline apply. Since more NGT products than in the baseline would come on the market, if
they contain relevant traits consumers could benefit from further choice of products with
increased nutritional and functional qualities.
1.3. Analysis of option 2: Authorisation with incentives for products
containing modified traits that have the potential to contribute to
sustainability
6.3.1. Economic impacts
Breeders – With regard to adapted risk assessment and the attendant cost reductions, the
analysis of option 1 applies. This option is judged by breeders to have overall a neutral or
negative impact on time to market or regulatory certainty compared to option 1, for several
180
Ku & Ha (2020)
181
Celiac-safe wheat derived products are expected to be 10 to 30% more expensive than those made from
standard wheat, but still cheaper than gluten-free products, which are on average 200% more expensive than
gluten-containing products.
182
Sánchez et al. (2023)

46
reasons. In case operators decide to apply for incentives, specific costs will result from the
need to comply with a sustainability-related verification and will depend on the criteria and
data requirements necessary for that verification. Apart from the extra costs, breeders consider
that any pre-market data generation may extend the preparation phase for the applicant,
lengthen assessment timelines and diminish the potential benefits of NGTs to speed up plant
breeding. This is also due to the view that the proposed incentives will not necessarily lead to
a significant decrease of administrative burdens. However, if easily implementable criteria to
trigger incentives are designed, stakeholders do see some merit in the sustainability incentives
offered under this option. Data from the cost survey
183
identified potentially significant cost
savings connected to regulatory (EUR 83300 – 833000) and scientific advice (EUR 35700 –
357000) and through the waiving of fees for the validation of the detection method
(EUR 105000, EUR 52500 for SMEs). These cost savings (the estimated savings are
dependent on the future data requirement for the sustainability-related verification) will be
particularly relevant for SMEs, which, as a rule, do not have dedicated regulatory departments
to support the authorisation process. In addition, as the use of incentives is voluntary,
companies are free to choose whether to use this possibility or not based on an economic
analysis of the costs and expected benefits.
65% of respondents in the targeted survey expect no change, while only 4% of respondents
expect a moderate to strong decrease for the attractiveness of developing NGT products
compared to option 1. Given that incentives are voluntary, and breeders can choose to operate
under this option without triggering them, no decrease in attractiveness appears justified. The
option is then equivalent to option 1. If the GMO labelling requirement were to be waived for
products with traits that contribute to sustainability, this could have, according to 40% of
stakeholders from business associations, companies, public authorities and academia/research
organisations a moderate positive impact on the attractiveness to develop such NGT products.
If a sustainability label is introduced, interviews with value chain stakeholders point out that
such a label is, however, not likely to be used in practice if voluntary.
Academia/Research institutes – This sector expects positive impacts in private and public
R&D funding if the GMO label for NGT products with traits that are verified to contribute to
sustainability would be waived. This and other incentives are not specifically aimed at
Academia/Research Institutes but support the regulatory procedures prior to the
commercialisation of products. A regulatory system facilitating the placing on the market of
NGTs will also increase the attractiveness of funding NGT research in academia and the
research sector. This will in turn support the development of the agricultural biotech sector in
the EU. If the GMO label is not waived, no significant positive impact on private or public
funding is expected compared to option 1.
Public authorities – For adapted risk assessment, the analysis of option 1 applies. The
verification how traits can contribute to sustainability is expected to increase the cost of the
authorisation process. The competent authorities’ support of the authorisation process with
incentives for plants with traits that contribute to sustainability (e.g., guidance on overall
development plan and regulatory procedure, dedicated contact point, scientific advice at key
development milestones, fee waivers) is expected to increase costs. Alternatively, cost
decreases are expected in cases when the GMO label is waived.
Conventional farmers – If more NGT products will to come to the market, farmers could
harness their benefits to a larger extent than under option 1.
183
See Annex 7 in Technopolis Group et al. (2023)

47
A quantitative exploratory analysis
184
of impacts shows, for a medium adoption rate of NGTs
(10-23% of the crop market, depending on crop group), the following estimated mean and, in
brackets, range of impacts on key agronomic parameters for several crop groups, showing a
pattern comparable to option 1 (Table 5).
Table 5. Trait-level impacts.
Crop group
yield change/ha
change in
pesticide use/ha
change in
fertiliser use
change in
water use
change in
energy use
Oil and fibre plants
9%
(4% to 20%)
-10%
(5% to -35%)
-13%
(-8% to -28%)
-3%
(5% to -15%)
-3%
(0% to -10%)
Vegetables
9%
(6% - 24%)
-
-
-
-
Cereals
8%
(6% to 22%)
-16%
(-14% to -18%)
-11%
(-5% to -18%)
-5%
(-4% to -7%)
-7%
(-1% to -12%
Legumes
9%
(6% to 23%)
-10%
(5% to -35%)
-10%
(-5% to -20%)
-5%
(3% to -16%)
-3%
(0% to -10%)
The analysis of coexistence measures for conventional farmers cultivating NGTs from option
1 also applies for this option.
Organic/GM free sector – Under the sub-option that maintains a GMO label, the impacts on
the organic/GM-free sector will be similar to option 1. The impacts from the costs of
coexistence will be moderately higher than under option 1, as more NGT products are
expected on the market. As in the baseline, the sectors will be impacted because of the
regulatory divergence with non-EU countries (where NGT products may not be subject to
traceability and labelling rules or to any transparency obligations) from which organic/GM-
free products are imported into the EU. Under the current EU regulatory framework, NGTs
should not be used in organic products exported to the EU, therefore, the costs of keeping
segregated supply chains in the EU would remain unchanged.
The specific impacts of this option relate to labelling options for NGT products featuring
traits that can contribute to sustainability. If a sustainability label is provided for, the focus
group on sustainability
185
conducted in the context of the external contractor’s study, found
that a sustainability claim allowing for higher premiums would be to the disadvantage of
sustainable conventional or organic products.
If NGT products with traits contributing to sustainability would not be labelled, a public
register will enable organic operators to identify varieties developed using NGTs and
traceability would remain as under current GMO legislation. In addition, as the traceability
provisions would apply, the organic/GM-free operators would rely on the business-to-
business obligations to be informed that no NGTs have been used.
Impacts on trade – The impacts on trade will be similar to option 1.
Impacts on SMEs – The impacts of the adapted risk assessment would be the same as in
option 1. Regulatory incentives linked to scientific / regulatory advice or fee waivers would
have the most impact as regards SMEs. Unlike larger companies, SMEs do in general not
have dedicated, specialised staff for authorisations. The incentives may help to lower
administrative burdens and allow more SMEs to benefit from NGTs. Some of the positive
184
Annex 8, section 1 in Technopolis Group et al. (2023)
185
Annex 6, section 1 in Technopolis Group et al. (2023)

48
impacts of incentives could, however, be counteracted by the additional burdens of producing
the data for the verification of the sustainability contribution of the NGT trait.
Competitiveness – Like in option 1, this option will improve the competitiveness of EU
operators as costs of and time needed for R&D of NGT products is reduced compared to the
baseline. Incentives can decrease administrative burdens for SMEs and increase their
competitiveness. Still, breeders in non-EU countries, which have exempted certain NGT
products from the requirements of their GMO legislation, will not have any costs related to
authorisations for such products, thus having a competitive advantage also under this option.
Simplification – Like in option 1, a risk assessment adapted to the diverse risk profiles of
plants obtained by targeted mutagenesis and cisgenesis would lead to a range of reduction in
compliance costs for applicants (from negligible reduction if similar data requirements apply
as today to a maximum reduction of 85% in cases with minimum data requirements) and
national competent authorities (reduction from 8 – 67%). If an applicant seeks to verify the
contribution of the NGT product to sustainability additional procedural steps will be needed,
partially cancelling out the simplifications gains made by adapted risk assessment.
6.3.2. Environmental impacts
As regards potential risks specifically linked to the genetic modification, any NGT product
coming to the market will be risk assessed using criteria and requirements adapted to the
product’s risk profile (as in option 1). Therefore, no change compared to the baseline with
regard to safety for the environment is expected as products will only be authorised for
placing on the market and cultivation if the risk assessment concludes that they do not present
more potential hazards than their conventional counterparts, and post-market monitoring
provisions will apply to identify and take measures in response to any potential adverse effect.
With more NGT products on the market (coupled with incentives steering to certain traits),
certain stakeholder groups expect positive environmental impacts, such as reduced pesticide
and fertiliser use, while others expect negative impacts, such as more large-scale
monocultures and a concomitant increased pesticide use. In the targeted survey, roughly equal
number of stakeholders expect a positive (40%) or a negative (46%) impact. This polarisation
is mostly due to divergent views on the sustainability of HT crops as outlined in section 6.2.1.
The consultations show that stakeholders consider that this option could make a contribution
to pushing the application of NGTs to the breeding of crops more in the direction of
preferential environmental traits; it was primarily supported by respondents from academia
and public authorities and by citizens.
However, this option and option 3 were questioned from the perspective of how the
sustainability contribution of a trait could be verified. A large majority of stakeholders,
representing very different interests (biotech industry and breeders, farmers, NGOs and
organic and GM-free sectors), agree in the public consultation, interviews, the focus group on
sustainability and the targeted survey that the sustainability of a crop does not depend on its
individual traits but on the interplay of the plant with its environment and the farming system
that it is adopted in. There is wide agreement that the holistic, multi-site and multi-year test
for value for cultivation and use (VCU), carried out during variety registration of agricultural
crops under the PRM legislation is far more suitable to assess in a holistic manner the
performance of new varieties
186
. Such an overall performance assessment should not be part
of the procedures for the authorisation of GMOs which are focused on the effect of the
186
Annex 6, section 1 in Technopolis Group et al. (2023)

49
genetic modification only. Moreover, it is considered that, should such a holistic assessment
of the variety be introduced in the NGT authorisation, it would duplicate the VCU assessment
to which NGTs are also subject. These stakeholders consider that sustainability analysis
should be performed using a system approach rather than one at product level. Furthermore,
the discrimination of plants produced by conventional or organic breeding causes concerns
among stakeholders as these plants cannot benefit from a sustainability label.
6.3.3. Social, health and safety impacts
Social, health and safety impacts – Any NGT product coming to the market will be risk
assessed using criteria and requirements adapted to the product’s risk profile (as in option 1).
Therefore, similarly to potential risks for the environment, no change compared to the
baseline with regard to human and animal health is expected as products will only be
authorised for placing on the market and cultivation if the risk assessment has concluded that
they do not present more potential hazards than their conventional counterparts.
If more NGT products will come to the market (coupled with incentives steering to certain
traits), social and health benefits, such as from the low gluten wheat presented in Annex 7, are
expected. NGTs support healthy diets: examples are mustard greens (Brassica juncea) with a
decreased content of pungent compounds
187
or rice lines with lower amylose content, which
may reduce the risk of diabetes
188
.
Impacts on consumers – In the public consultation, a majority of EU and non-EU citizens that
responded supported the view that specific regulatory provisions for sustainability should be
included in this initiative. Survey responses (70%) indicate that a sustainable trait label, also
covering the purpose for which the NGT product was developed, could increase consumers’
willingness to buy NGT products with such a label. Respondents to the targeted survey
189
and
further studies indicate that sustainability-related labelling will increase clarity for the
consumers, allowing them to choose products that contribute to societal benefits and
sustainability, based on their preferences
190
.Available research suggests that consumers may
accept NGTs in particular when they promote societal benefits and sustainability
191
.
In this regard, the sub-option to label NGT products as GM combined with a sustainability
label linked to the introduced trait, would strengthen freedom of choice (by informing about
the technology and its application) and increase consumer and market acceptance. Yet the
focus group on sustainability
192
found that a sustainability claim allowing for higher
premiums would be to the disadvantage of sustainable conventional or organic products.
The sub-option to waive the GMO labelling requirement for NGT plants with traits
contributing to sustainability, but including these products in a publicly accessible database,
makes accessing the relevant information more burdensome for citizens not using modern IT.
This was also highlighted in the public consultation where a majority of the respondent EU
citizens (30%, 733) expressed a preference for physical labelling on the final product. Also,
not labelling products that, because of their characteristics, are made subject to mandatory risk
assessment and authorisation could negatively affect consumer trust.
187
USDA APHIS (2020)
188
Sun et al. (2017)
189
Business associations, large companies, SMEs, some NGOs and academic/research organisations
190
Swedish Gene Technology Advisory Board, Department of Plant biology (SLU), Novus. (2021);
GENEinnovate (2020); Kato-Nitta et al. (2021); Baum et al. (2023)
191
Swedish Gene Technology Advisory Board, Department of Plant biology (SLU), Novus. (2021)
192
Annex 6, section 1 in Technopolis Group et al. (2023)
50
The sub-option to complement the GMO label with a factual statement on the intended
purpose of the genetic modification (e.g. genetically modified for the purpose of improving
drought resistance) could provide relevant information to consumers, without featuring claims
that could be to the disadvantage of sustainable conventional or organic products. This sub-
option could still drive the market demand for products with beneficial traits.
More NGT products than in the baseline would come on the market. If they contain relevant
traits consumers could benefit from further choice of products with increased nutritional and
functional qualities.
1.4. Analysis of option 3: Authorisation with the requirement that products
do not contain modified traits that can be detrimental to sustainability
6.4.1. Economic impacts
Breeders – As for options 1 and 2, significantly shorter development times and lower
development costs for NGT plants and, in some cases, lower costs and time linked to the
generation of data for risk assessment and authorisation, could make the development of
certain NGT plants more attractive than GMOs obtained by established genomic techniques.
Additional, mandatory procedural steps such as the necessity to provide data on the
characterisation of the modified trait to verify its potential impacts on sustainability are
judged by stakeholders to have a mostly negative impact on time to market. More
importantly, the possibility that the NGT product may be refused marketing authorisation
because sustainability requirements are not met will affect regulatory certainty negatively.
Like in option 2, a wide range of stakeholders doubt that the sustainability impact of a single
trait can be reliably verified. The issue of discrimination is also raised for this option, as
conventionally bred plants are not subject to the requirement to demonstrate that the plant is
not detrimental to sustainability. Therefore, the attractiveness of developing NGT plants under
this option is decreased compared to options 1 and 2 because of the introduction of
sustainability requirements. 68% of respondents in the targeted survey expect no change or
decreased attractiveness of developing NGT plants compared to the situation today.
Public authorities – With regard to adapted risk assessment, the analysis of option 1 applies.
In this option, lower savings can be expected as verifying that the modified trait is not
detrimental to sustainability is a requirement for all authorisations. As harmonised standards
and procedures for this verification would have to be put in place, cost increases for the
competent authorities can be expected. However, in the cost survey, Member States’
authorities were not able to provide quantitative estimates of these potential cost increases.
Other sectors – Because of the lower attractiveness of this option for breeders, NGT plants
could achieve a lower market share than under options 1 or 2. Regulatory divergence with
non-EU countries will be larger than under options 1 and 2, and additional requirements will
increase the risk of trade disruptions. The reduction in attractiveness and lower market share
would mean that the potential of NGTs might not be delivered to conventional farmers; at the
same time, as showing that the trait is not detrimental to sustainability is a requirement, all
NGT plants reaching the market will benefit farmers (or consumers). Organic/GM-free
farmers would experience lower risks of admixture and attendant economic losses. Impacts on
academia/research institutes will be similar to option 1.
Impacts on SMEs – While there can be significant cost reductions for adapted risk assessment,
additional data are required to show that the trait is not detrimental to sustainability. Although
no estimates for these additional costs are available, there is the risk that these extra costs
could negate some of the positive impact of adapted risk assessment. Therefore, there is the
51
possibility that SMEs in the EU will have few or no incentives to develop NGT products
under this option. There is furthermore the risk that a NGT product might be refused
authorisation for not meeting the sustainability requirements and this leads to regulatory
uncertainty, which is economically riskier for SMEs than for larger companies.
Competitiveness – Also under this option, costs of and time needed for R&D of NGT products
are reduced in comparison to the baseline (same quantitative estimates as in option 1).
Additional costs and administrative burdens will be imposed on breeders by the requirement
to provide data demonstrating that the NGT trait is not detrimental to sustainability, thereby
cancelling out some of the cost and time savings of option 1. Breeders in non-EU countries,
which have exempted certain NGT products from the requirements of their GMO legislation
and which have no specific requirements for sustainability, can save all the costs related to
GMO authorisations, thus having a competitive advantage also under this option, but more
pronounced than in options 1 and 2.
Simplification – Like in option 1, a risk assessment adapted to the diverse risk profiles of
plants obtained by targeted mutagenesis and cisgenesis would lead in some cases to
significantly reduced administrative costs and burdens for applicants and national competent
authorities. Applicants are obliged to demonstrate, and competent authorities need to verify,
that the NGT trait is not detrimental to sustainability, thereby fully or partially cancelling out
the simplifications gains made by adapted risk assessment.
6.4.2. Environmental impacts
As regards potential risks specifically linked to the genetic modification, any NGT product
coming to the market will be risk assessed using criteria and requirements adapted to the
product’s risk profile (as in option 1). Therefore, no change compared to the baseline with
regard to safety for the environment is expected as products would only be authorised for
placing on the market and cultivation if the risk assessment concludes that they do not present
more potential hazards than their conventional counterparts, and post-market monitoring
provisions will apply to identify and take measures in response to any potential adverse effect.
In addition to refusing authorisation to NGT plants presenting specific risks (as in the baseline
and options 1 and 2), this option would refuse authorisation to NGT plants containing traits
whose use could have negative environmental effects (e.g. increased use of pesticides).
Therefore, in principle, this option would be the most demanding for operators and promising
to deliver on sustainability objectives. However, as explained in section 7.3.2., plants with
traits whose use could have negative environmental effects can be obtained with different
breeding methods, including by conventional means and introducing such a requirement only
for NGT plants would be discriminatory.
Since this option adds administrative burden compared to option 1, it is likely that a lower
number of NGT products than under option 1 will be developed. Therefore, environmental
benefits may be realised to a lower degree than in option 1.
6.4.3. Social, health and safety impacts
For social, health and safety impacts, the same reasoning applies as in section 6.4.2.
Impacts on consumers – NGT products would be labelled as GMO, and similar considerations
as in the baseline and option 1 apply. Although this option does not provide for any
sustainability-related label (as all NGT products would need to meet a minimum requirement
and only the technology would be labelled), if there is widespread information and
understanding of the legal framework and the fact that only NGT products with traits that

52
cannot be detrimental to sustainability can be authorised, consumer acceptance of NGTs could
increase.
As only a small number of NGT products are expected to come on the market, there could be
missed opportunities for consumers willing to consume NGT products with increased
nutritional and functional qualities.
1.5. Analysis of option 4: Notification of products that could also occur
naturally or be produced by conventional breeding
The impacts of this option (intended to apply in combination with one of the other options)
will depend on the share of NGT plants/products that would fall under the notification regime.
It is difficult to anticipate this number, as it cannot be predicted which products in the R&D
pipeline will be pursued for placing on the market and whether they would meet the
notification criteria (data allowing to perform the assessment against the criteria is in most
cases not available for the product pipeline).
A hypothetical scenario for the purposes of the analysis, based on the number of products
expected to come to the market in the next ten years in the JRC study on NGT applications, is
presented in Annex 3.
1.5.1. Economic impacts
Breeders – Under this option, strong effects are expected on the attractiveness to develop and
bring to the market NGT plants with genetic alterations that could also be produced naturally
or by conventional breeding. Apart from an initial verification that the related criteria are met,
such plants would be treated like conventional varieties and therefore the costs for market
authorisation would be similar to conventional varieties
193
, while time to market (development
time + variety registration, which can be expected to be less than 10 years) would be shorter
than for conventionally bred varieties, where variety development alone can take up to 15
years. Additional costs compared to conventional varieties would be due to the necessity to
verify whether a product could also occur naturally or be produced by conventional breeding.
The total cost savings for breeders will depend on the share of NGT products which will
comply with the relevant requirements. As cost and time savings for each variety are
considerable (see section 6.2.1.), there will be strong incentives for breeders to focus their
R&D on varieties which can fulfill the criteria for a notification.
92% of overall stakeholders see an increase of attractiveness under this option. In a recent
survey among European plant breeders, 100% of the larger, 86% of the medium sized and
70% of the small companies would further invest in NGT-related product development if the
products would not be subject to the requirements of the GMO legislation
194
.
Academia/Research institutes – Academia and research
195
expect this option to increase
investments both from large multinational plant breeders as well as SMEs and public sector
research, allowing for both a deeper and broader R&D-base in agricultural biotech due to
lower total aggregate R&D-cost, reduced business risk and shorter time-to-market. This will
increase research competitiveness of the EU and offer research and employment opportunities
for plant scientists, addressing the perceived problem of brain drain.
193
The total annual costs to businesses across the EU27 of registering varieties is estimated to be up to
EUR 73 m with an average cost to businesses per registration of EUR 4434.
194
Jorasch (2020)
195
Section 3.1.23. in Technopolis Group et al. (2023)

53
However, stakeholders from environmental NGOs and the organic sector argue that such
intensified R&D focussed on biotechnology might divert R&D from developing other more
sustainable farming methods, where they already see a lack of investment.
Trade impacts – Unlike the baseline and options 1-3, this option will reduce regulatory
divergence with other jurisdictions. Although different jurisdictions are applying different
criteria and a degree of regulatory divergence will remain, in many third countries the
equivalence to conventional products is the decisive criterion when adapting their regulation.
Public authorities – National authorities can expect significant cost savings per product (as
compared to a situation where the NGT plants concerned would be subject to authorisation).
The total amount of savings will depend on the proportion of NGT products that will comply
with the requirements for the notification procedure. In the most extreme case, that is if all
NGT products brought to market comply with the requirements for the notification procedure,
then competent authorities will only have to bear the compliance costs of verifying that the
product does indeed meet these requirements. However, the number of NGT products that go
through regulatory procedures for the deliberate release including placing on the market could
significantly increase compared to a situation where conventional-like NGT products remain
subject to authorisation (baseline and options 1 to 3).
Conventional farmers – Conventional farmers could maximise benefits of NGTs as set out in
section 6.3.1, for example with regard to pesticide reduction as biotic resistance is the second
largest type of traits addressed in the R&D pipeline (113 of 489) thereby contributing to
resilience and food security. However, as beneficial traits addressing abiotic resistance (water
stress
196
, heat) are generally genetically more complex and the expression of the involved
genes are very dependent on environmental conditions it can be expected that many plant
products incorporating such traits would not meet the criteria to be treated as a product that
could occur naturally or be produced by conventional breeding and be subject to the rules set
out for the baseline option or for options 1, 2 or 3
197
. A quantitative exploratory analysis
198
of
impacts shows, for a high adoption rate of NGTs (10-27% of the crop market, depending on
crop group), the following estimated mean and, in brackets, range of impacts on key
agronomic parameters for several crop groups (Table 6).
Table 6. Trait-level impacts
Crop group
yield change/ha
change in
pesticide use/ha
change in
fertiliser use
change in
water use
change in
energy use
Oil and fibre plants
12%
(9% to 21%)
-10%
(5% to -35%)
-13%
(-8% to -28%)
-3%
(5% to -15%)
-3%
(05 to -10%
Vegetables
9%
(6% to 24%)
-
-
-
-
Cereals
16%
(6% to 33%)
-26%
(-19% to -32%)
-16%
(-10% to -22%)
-11%
(-10% to -11%)
-12%
(-7% to -17%)
Legumes
9%
(6% to 23%)
-10%
(5% to -35%)
-10%
(-5% to -20%)
-5%
(3% to -16%)
-3%
(0% to -10%)
Compared to the estimates for other options, expected gains are particularly pronounced for
yield changes in oil and fibre plants and cereals. This reflects the experts’ expectation that a
196
Van de Wiel et al. (2022)
197
There are some cases where single-gene approaches to abiotic stress-tolerance have been successful in
transgenic GMOs, see for example González et al. (2020)
198
Annex 8, section 1 in Technopolis Group et al. (2023)

54
higher adoption rate means that in these crop groups more NGT plants with traits affecting
yields are expected to come to market than under the other options.
Some farming stakeholders warn that the widespread use of NGT plants could have a negative
impact on them, especially small farmers, and their freedom of choice in the purchase of
seeds, linked to patents in this area and the risk of oligopolistic situations
199
.
Organic/GM free sector – Today, NGT plants are subject to the requirements of the GMO
legislation and are banned in organic production. As noted in section 5.2.4, two sub-options
are considered for NGT plants fulfilling the notification criteria: 1) they are treated like
GMOs for the purposes of organic production and are therefore prohibited; 2) they are treated
like conventional products for the purposes of organic production and can in some instances
be used
200
. This second sub-option applies the logic of the legislation today, where GMO
products produced by random mutagenesis and which are exempt from GMO requirements
are treated as conventional for the purposes of organic production.
In both sub-options, the public register of notified NGT plants and information in the common
catalogues of varieties will enable organic operators to identify varieties developed using
NGTs allowing them to ensure that no such varieties are used on their fields and to keep
supply chains free from them. This transparency would be based on the traceability system of
the supply chain of plant reproductive material, which is based on maintaining a high level of
varietal purity.
In this option, the risk of admixture will be higher than for options 1-3 as the highest number
of NGTs are expected to be placed on the market/cultivated and the costs of segregation for
organic operators will likely increase proportionally to the spread of NGTs. The consequences
of such admixture, should it occur, will depend on the two sub-options.
In the first sub-option, the organic sector will rely on the organic traceability requirements
and the preventive measures provided for in the Organic Products Regulation. In order to
mitigate the impact on organic farming and ensure freedom of choice for farmers, further
measures to support maintaining organic production free from NGTs and consumer trust may
need to be introduced (e.g. the labelling of seeds could be considered). As in other options
(including the baseline), where the admixture cannot be ascertained analytically, and organic
producers are able to show they have complied with all necessary precautionary measures,
there will not be direct economic losses linked to loss of the organic logo. However, the lack
of analytical methods will raise specific challenges in the case of farmers who manage both
organic and conventional production on their holdings
201
. These challenges might erode the
trust of those consumers who are concerned about the adventitious presence of NGTs in
organic products. However, the legal ban on the use of NGTs can also act as reassurance to
consumers that all measures are being taken to avoid NGT presence.
199
CEJA (2022)
200
As there is a lack of organic seeds for many crops, organic farmers are allowed to use non-organic untreated
seeds authorised through derogation requests. For data on the use of non-organic seeds see Solfanelli et al.
(2022). Organic production can also use organisms produced by classical mutagenesis, using irradiation or
chemical mutagens, which are GMOs, but are exempted from the obligations of the GMO legislation. For
example, the cherry variety ‘Sunburst’ was developed by hybridization with mutant variety ‘Stella’ obtained by
irradiation with x-rays. Both these varieties are used in organic cherry production in the EU.
201
Approximately 45% of agricultural holdings with organic production also have non-organically managed
areas; see table 2 in https://ec.europa.eu/eurostat/statistics-
explained/index.php?title=Organic_farming_statistics#Organic_production

55
With regard to supply chains, there are currently a few areas of organic food production,
where there are interfaces to the conventional market
202
. Special precautionary measures for
the organic sector are, in particular, needed when purchasing organic raw materials such as
maize, soya or rape seed from countries where GMO varieties are also produced or from
traders who also trade in GMOs. In order to exclude as far as possible for these origins and
purchasing routes possible admixture with GMO raw materials, appropriate contracts with
producers and/or traders that define the critical points in production, transport and storage to
minimise admixture are concluded. Some suppliers of raw materials or products offer the raw
materials from so-called IP (Identity Preserved) programmes. If more NGTs products come
the market, the number of interfaces of organic food production to the conventional market
may increase, placing more demands on the IP programmes and the precautionary measures
organic operators are obliged to take under the Organic Products Regulation in order to avoid
products not authorised in organic production. This will place additional costs and burdens on
organic operators who will bear segregation costs. There are, however, no quantitative
estimates available.
In this first sub-option, as in all other options, the organic and GM-free sectors can be
impacted because of regulatory divergence with non-EU countries (where NGT products may
not be subject to traceability and labelling rules or to any transparency obligations) from
which organic products are imported into the EU. Under the current EU regulatory
framework, NGTs should not be used in organic products exported to the EU, therefore, the
costs of keeping segregated supply chains in the EU would remain unchanged.
In the second sub-option, notified NGT products could be used in organic production under
the applicable derogations for e.g. non-organic untreated seeds. However, organic producers
would be able to exclude such plants from organic production through the use of information
in the public register of notified NGTs and the precautionary measures provided for in the
Organic Products Regulation.
In this sub-option, the organic sector could be impacted regarding imports of organic
products, in cases where NGTs that have been considered equivalent to conventional varieties
and are prohibited in organic production in third countries.
Moreover, as certain consumers may not accept allowing conventional-like NGTs in organic
production, an erosion of consumer trust in the organic market and EU logo could also
materialise, possibly leading to emergence of private standards. The same reasoning would
apply to the GM-free sector (not regulated under EU law) if it decided to accept notified NGT
products.
This sub-option could deliver positive impacts, as organic farmers would be able to benefit
from the potential of NGTs supporting sustainable farming practices, in particular with regard
to pest resistance and pest tolerance
203
. Organic breeders would also be able to decide to use
notified NGT plants in their breeding programmes, if they see benefits in traits relevant to
organic cultivation.
There are different positions among stakeholders from the organic sector about the use in
organic farming of NGTs if they would be treated as conventional products. Certain
stakeholders consider that NGTs are not compatible with the wider objectives of organic
breeding laid down in Organic Products Regulation, and that these objectives inform
consumer expectations with regard to organic products. Other stakeholders consider that, if
202
IFOAM EU (2014, 2018)
203
Andersen et al. (2015); Crespo-Herrera & Ortiz (2015)
56
certain products of NGTs are treated in regulatory terms as conventional products, this should
equally apply as regards their possible use in organic production.
Impacts on SMEs –This option is the most advantageous for SMEs, other than those involved
in organic and GMO-free production, and supports their freedom to do business, as
administrative and compliance costs will decrease considerably for NGT products to which
the notification procedure is applied. For these NGT products, development costs could be
even lower than for conventional varieties. This option would allow SMEs to use advanced
technologies to strengthen their competitiveness on European and global markets. In turn, an
enabling framework for SMEs could be more conducive to R&D on niche crops and traits.
Competitiveness – A significantly lower regulatory burden will increase investments in R&D,
both from large multinational plant breeders as well as SMEs. For plants meeting the
notification criteria, development costs can be even lower than for conventionally bred
varieties. This will allow operators to compete with operators from non-EU countries which
have exempted conventional-like NGT products from their GMO legislation.
Simplification – This option results in a high degree of simplification and reduction of
administrative burden for applicants and authorities. For plants meeting the notification
criteria, the normal variety registration procedure applies. For agricultural crops undergoing
DUS and VCU trials, these costs amount to an average of EUR 4434 per registration.
1.5.2. Environmental impacts
This option would maintain risk oversight through the case-by-case verification of the criteria
to determine that a NGT plant would not present more potential hazards than plants occurring
naturally or produced by conventional breeding.
The concerns of certain stakeholders about risks to the environment and biodiversity are
intensified under this option due to an expected wider cultivation and the absence of risk
assessment for products fulfilling the notification criteria. 65% of survey respondents do not
see potential risks for the environment and biodiversity under this option, while 32% see
potentially increased risks. However, the majority position in the scientific community and in
an increasing number of jurisdictions globally is that certain NGT plants do not differ from
those occurring naturally or obtained by conventional breeding, including in their level of risk
for human, animal health and the environment. Although contested by certain stakeholders,
this is confirmed by EFSA’s scientific opinions and numerous other scientific reports. This
option would entail verifying, case-by-case and based on predefined criteria (as indicatively
presented in section 5.2.4, Box 2), whether risk assessment is necessary. When the NGT plant
concerned meets these criteria, it would not present more potential hazards than plants
occurring naturally or produced by conventional breeding. The criteria are designed to cover
the type and extent of modifications that are also observed with conventional breeding
techniques. The principle of comparative assessment against the conventional counterpart
remains, therefore, under this option as in the baseline and in the other options. Under this
option, the post-market monitoring provisions of the GMO legislation will not apply to
products fulfilling the notification criteria. However, mechanisms will remain available under
the new framework and in horizontal legislation (e.g. under the General Food Law) to handle
any new information on potential risks.
This option offers the largest opportunities to realise the potential environmental benefits of
NGTs, as it offers the strongest incentive to develop NGT varieties in the EU, although the
development and marketing of NGT products, and the realisation of their potential, depends
also on various factors other than an appropriate regulatory framework. Patents, access of
public organisations and SMEs to the technologies and the market, access and affordability

57
for farmers (particularly small-scale), and factors driving consumer acceptance, will also have
an important impact.
Agrobiodiversity could benefit from this option, as it can become less burdensome and costly
to apply the techniques to small and local crops provided that access to the technology, in
particular for SMEs and the public sector, is not restricted by high licensing costs. A recent
modelling study found that NGT plants, when regulated like conventionally bred crops,
required a 96% smaller potential cultivation area to break even on the financial investment
when compared to established genetic modification
204
. In addition, the FAO reports that NGT
crops are more diverse regarding varieties, traits and institutions developing them (more
public organisations and SMEs) in comparison with first generation GM crops. However,
some NGT crops with traits contributing to sustainability can face significant financial
barriers for development and use
205
. Moreover, if NGT crops are subject to the same
regulatory requirements as GM crops, the cost of regulatory approvals is often prohibitive for
niche crops
206
. Therefore, it is expected that the reduction of burden and costs under this
option would contribute to improve market access to NGTs for public organisations and
SMEs that could in turn bring benefits associated to smaller area crops and/or niche traits.
1.5.3. Social, health and safety impacts
Social, health impacts and safety impacts – As regards risks for human health, the same
considerations as for environmental risks presented in section 6.5.2. apply. When the NGT
plant concerned meets the notification criteria, it would not present more potential hazards to
human health than plants occurring naturally or produced by conventional breeding.
Furthermore, this option offers the largest opportunities to realise the potential health benefits
of NGT products, as it offers the strongest incentive to develop NGT varieties in the EU.
On potential risks for human and animal health such as toxicity or allergenicity, 64% of
respondents expect no change under this option, while 29% expect increased risk.
Impacts on consumers – In a similar way as random mutagenesis today, this option would
treat NGT products that fulfil the notification criteria as conventionally bred products, and
information would not be provided on the product’s label about the technology used when
marketing the product (although all notified products would be entered in a public register). In
this regard, the ability to decide not to consume NGT products would not be ensured by this
option to the same degree as in the baseline and options 1, 2 (where labelling is not waived)
and 3. However, a GM label for products not subject to risk assessment and authorisation as
GMOs, and which could occur in nature or be conventionally bred, could create confusion
about the product’s characteristics and safety profile. In any event, consumers actively
wanting to avoid NGTs would be able to rely on the organic logo (in the sub-option where
notified NGTs would remain banned in organic production).
This option would bring the highest number of products to the market. There could thus be
more opportunities for consumers to access NGT products with increased nutritional and
functional qualities and with other sustainability-relevant traits (e.g. pest resistance) which,
according to available research, rank high among consumers’ concerns.
As this option would entail the lowest costs to access the market, it could result in lower
prices for NGTs compared to other options (see section 7.2 on efficiency).
204
Bullock et al. (2021)
205
Jordan et al. (2022)
206
FAO (2022)
58
7. How do the options compare?
This section compares the expected impacts of the options in terms of their effectiveness to
reach the initiative's objectives, efficiency, coherence and proportionality. This qualitative
assessment of the options is based on the overall analysis of key impacts and the comparison
is with the performance of the baseline. It takes the scoring of the multicriteria analysis
207
into
account, as well as additional evidence gathered during this process. The overall comparison
of the options against the relevant criteria is presented in Table 7.
Table 7. Overall comparison of policy options. Options 1-3 cover all NGT products, option 4 only
products which could also be obtained naturally or by conventional breeding -- N
--
Criteria
Policy Option 1
Policy Option 2
Policy
Option 3
Policy Option 4
Effectiveness: contributing to
achieving the policy objectives
Procedures for the deliberate release
and placing on the market ensure that
NGT plants and derived food/feed
products are as safe as their
conventional counterparts, while not
entailing unnecessary regulatory
burden
+
+
+
+
208
Deliberate release and placing on the
market of NGT plants and derived
food/feed products that feature a wide
range of plant species and traits by
various developers
0/+
+ /++
209
0
++
NGT plants released or placed on the
market feature traits that can contribute
to a sustainable agri-food system.
0/+
+
0/+
++
Effectiveness: specific impacts
Competitiveness
0/+
+
0
++
SMEs
0/+
+
0
++
Social & health
210
impacts
0/+
+
0/+
+ /++
Environmental impacts
211
0/+
+
0 /+
+/++
Impacts on the organic sector
0/-
0/-
0/-
Notified NGTs treated
as GMOs in organic
production: 0/-
Notified NGTs treated
207
Technopolis et al. (2013), section 6, Table 11
208
A combination of option 4 with an adapted risk assessment as envisaged in options 1, 2 and 3 would
maximise this objective (++)
209
The strong positive effect is due to the variant of waiving the GM label
210
Only social and health impacts beyond the impacts specific to the genetic modification considered under the
first policy objective
211
Only environmental impacts beyond the impacts specific to the genetic modification considered under the
first policy objective
59
as conventional in
organic production: -/+
Efficiency
Administrative and compliance costs
0/+
+
212
+/-
++
Savings and benefits
+/++
+
213
+/-
214
++
Coherence
+
+/-
-
Notified NGTs treated
as GMOs in organic
production: -
Notified NGTs treated
as conventional in
organic production: +
Proportionality
+
+
-
++
Overall
0/+
+
0
+/++
* -- strong negative effect, - negative effect, 0 neutral effect, + positive effect, ++ strong positive effect; values
separated by a slash mean that the size of the impacts can take negative, neutral or positive values depending on
choices or alternatives within options.
1.6. Effectiveness
Contributing to achieving the policy objectives
Procedures that ensure safety while avoiding unnecessary administrative burden
The baseline would ensure safety, albeit with unnecessary burden, as it would continue to
apply the current requirements of the GMO legislation to NGT plants, despite the fact that,
according to the conclusions of EFSA and major scientific bodies (reported in section 1.1 and
Annex 6), on a case-by-case basis, a lesser amount of data will be sufficient for the risk
assessment of certain plants produced by these techniques. Certain NGT plants do not present
more potential hazards than plants produced by conventional breeding. The impact analysis
shows that options 1, 2 and 3 (which all feature the same adapted risk assessment to different
levels of risk) would allow achieving safety for all NGT plants, however, with a
disproportionate burden for NGT plants that could have been obtained naturally or by
conventional breeding. Option 4, through the notification procedure, alleviates the burden on
the operators for this type of products, while ensuring safety (but was not considered from the
outset as an option that could deliver on the objective of safety for other NGT plants).
Therefore, this specific objective is best achieved through a combination of option 4 for NGT
plants that could have been obtained naturally or by conventional breeding and the adapted
risk assessment approach of options 1, 2 and 3 for other NGT plants.
All options are based on the application of the precautionary principle. A regulatory oversight
procedure applies in all options to ensure that only NGT plants that are considered as safe as
their conventional counterparts are released or placed on the market.
The regulatory framework for NGTs also needs to be designed in a way that it ensures safety,
when technologies evolve (future-proofness). The baseline does not achieve this objective. It
212
Costs are linked to the voluntary provision of data on sustainability of the trait in order to use incentives
213
Ibid.
214
Costs are linked to the mandatory provision of data demonstrating that the trait is not detrimental to
sustainability
60
entails the continued application of a legal framework that has been assessed not to reflect the
latest scientific developments. It contains no mechanisms to adapt to scientific or
technological progress, or to the wide diversity of NGTs and resulting products, in particular
as regards their different risk profiles.
The adapted risk assessment that features in options 1, 2 and 3 contributes to ensuring safety
when technologies evolve by recognising the diversity of products and risk profiles. Other
aspects of the design of the adapted risk assessment in these options would also contribute to
this: the criteria to adapt the risk assessment are based on the characteristics of the product,
rather than on specified technologies (see section 5.2.1.); provision would be made to allow
for the update by the Commission of the criteria over time based on scientific and technical
progress and experience; specific data requirements based on the criteria would not be laid
down in the basic act but would be developed subsequently in Commission tertiary legislation
and complemented by EFSA guidance.
Future-proofness is embedded in the design of option 4 as well: the criteria to determine
whether an NGT plant could have been obtained naturally or by conventional breeding are
based on the characteristics of the product, rather than on specified technologies (see section
5.2.4); provision would be made to allow for the update by the Commission of the criteria
over time based on scientific and technical progress and experience; the criteria would be
complemented by thresholds concerning the size and combination of modifications, which
would not be laid down in the basic act, but subsequently in Commission legislation and
complemented by EFSA guidance.
Diversity of released and marketed NGT products and of developers
Under the baseline, regulatory costs and time to market will be the same as for GMOs
obtained by established genomic techniques and NGT plants will be marketed as GMOs.
Breeders, and, in particular, SME breeders in the EU will thus have very limited incentives to
develop NGT plants and to go beyond the limited number of traits and crop species to which
established genomic techniques have been applied.
A less burdensome, less costly and faster authorisation procedure can be expected under
option 1 as a result of the adapted risk assessment. However, while the analysis of impacts
shows that the combination of lower R&D costs, faster development time and lower
regulatory costs could lead to a moderate to significant market uptake of NGT products from
a wider diversity of crop species with more diverse traits, breeders expect only a small
increase in the attractiveness to develop NGT products in the EU compared to the baseline.
This is linked to the uncertainty perceived by stakeholders about the adapted risk assessment,
and the concern that it will still be marked by complexity and lack of predictability (as well as
by the GMO label requirement). In any case, the requirement for labelling as GMO under this
option will limit the extent of its potential positive effects.
Options 2 adds the possibility of incentives for applications for NGT plants with traits that can
contribute to sustainability. With easily implementable criteria to trigger regulatory
incentives, stakeholders do see merit in them, especially for SMEs. Incentives would support
the diversity of players, as well as the diversity of traits and crop species on the market. As a
result, option 2 can have a positive impact compared to baseline. If the incentives are not
used, this option is equivalent to option 1 in terms of impacts. Option 2 would considerably
incentivise the development and placing on the market of NGT products if labelling as GMO
is waived for products with a modified trait that is considered to have the potential to
contribute to sustainability. Providing information on the purpose of the trait would increase
transparency and could increase consumer acceptance.
61
The attractiveness of developing diverse NGT products under option 3 is reduced compared to
options 1 and 2 through the introduction of sustainability requirements. The risk that
authorisation could be refused on grounds related to a potential detrimental impact on
sustainability, coupled with the current lack of horizontal definitions and frameworks to
assess sustainability and the uncertainty of assessing sustainability based on a single trait,
would act as a disincentive to apply. These risks are likely to affect SMEs in particular.
Option 4 has by far the strongest positive impact on enabling the development and placing on
the market by SMEs and large companies of NGT products with diverse traits from a wide
range of crop species, as it results in a higher degree of simplification and reduction of
administrative burden and costs for applicants and authorities.
Contribution to sustainable agri-food systems
The ability of all policy options to contribute to this objective depends on the number of NGT
products from diverse crop species, which will come to the market, and on the type of traits
which will be developed (addressed by the previous specific objective). The development
pipeline as described in the JRC report shows that research addresses a large diversity of
crops and that a large share of traits under development could contribute to sustainability, in
particular pest resistance, nutrient-use efficiency, tolerance to abiotic impacts (e.g. droughts
and improved yield/input ratio, yield stability). This research pipeline is answering to the
demand that in turn is also driven by current policy and regulatory developments, such as the
revision of the PRM/FRM legislation (with a stronger sustainability focus) and the
introduction of targets in initiatives such as the SUR, e.g., as regards a reduction of pesticide
use.
Under the baseline, no or only very few NGT plants are expected to be authorised for
cultivation or placing on the market, and therefore positive economic, environmental and
social impacts of NGTs will be very limited, if any materialise at all.
Like the baseline, option 1 does not contain any provision that would specifically steer
towards NGT products containing desirable traits from a sustainability perspective.
Option 2 has an additional, limited positive effect compared to option 1 on the attractiveness
for plant breeders to develop in the EU NGT plants that contribute to sustainability objectives.
Option 3 was designed to deliver on this objective to the largest degree by ensuring that only
plants with no detrimental effects on sustainability reach the market, but the risk that a NGT
product might be refused authorisation for not meeting the sustainability requirements creates
regulatory uncertainty and is a disincentive to develop and apply for authorisation.
An important reason why options 2 and 3 show limited gains (option 2) or negative impact
(option 3) is because they cannot take into account that the sustainability of a crop does not
depend solely on its individual traits but on the interplay of the plant with its environment and
the farming system that it is used in, coupled with the fact that different breeding methods can
be used to reach the same traits. Sustainability incentives, and even more so requirements, for
isolated traits are thus considered not to be efficient. Requirements linked to specific traits but
applicable only when the trait is obtained through biotechnology raise specific discrimination
problems if the same traits can also be obtained by conventional breeding. However, breeders
do see some merit in the regulatory incentives, especially for SMEs which are particularly
vulnerable to regulatory complexity and uncertainty. In turn, incentives with a positive impact
on SME access to the NGT market have the potential to steer R&D towards crops and traits
with sustainability-relevant impacts, beyond the commodity crops with a limited number of
traits developed with established genomic techniques. Also, if incentives as envisaged in
option 2 are combined with a waiver of GMO labelling for products with a modified trait that

62
is considered to have the potential to contribute to sustainability, this objective would be
achieved to a much more considerable extent.
By simplifying the framework for certain NGT plants, and given the traits in the R&D
pipeline as well as the demand and policy pressures for traits that contribute to sustainability,
option 4 will achieve this objective to the largest degree. However, this will only concern
plants that could occur in nature or have been obtained by conventional breeding; this option
on its own will not result in plants with more complex traits that could not be achieved
conventionally.
Digital by default –labelling and traceability, where relevant, are addressed in a way allowing
a wide range of implementations, including purely digital solutions where appropriate. The
risk assessment process managed by EFSA is already largely digitalised in line with the
‘digital by default’ principle. This principle will be also applied for the notification procedure.
Other impacts
Competitiveness and SMEs
Certain NGTs are considered relatively accessible tools for plant breeding compared to
established genomic techniques. In this regard, NGTs could lead to a lowering of
technological barriers to entry of the plant breeding sector, benefitting SMEs in particular.
This has been observed in Argentina after its regulatory decision to exclude certain NGT
products from the GMO framework
215
.
In the baseline, the current situation with large, multinational companies dominating the
market for GMOs will also apply to NGTs, due to the high regulatory burden and costs,
perceived regulatory uncertainty, and the fact that products would be marketed as GMOs.
In terms of the competitiveness of SMEs, under option 1 some improvement for SMEs could
be expected compared to the baseline. SMEs are vulnerable to complex regulatory
requirements. Adapting data requirements to the risk profile of a NGT product will reduce the
complexity, duration and costs of the application for authorisation and this would benefit
SMEs. Clarity and predictability of risk assessment criteria would be particularly relevant for
the access of SMEs.
Options 2 and 3 have the same impacts as option 1 as regards risk assessment. The incentives
envisaged under option 2 could provide some benefits to SMEs by addressing the need for
support measures such as regulatory/scientific advice and for further cost savings such as fee
waivers, while option 3 adds regulatory burden as regards providing mandatory data for
verification of the sustainability contribution of a trait and increases regulatory uncertainty as
products will not be authorised if they ultimately do not meet the sustainability requirement.
Option 4 is by far the most advantageous for SMEs, as administrative and compliance costs
will nearly be eliminated for those NGT products to which the notification procedure is
applicable. A clear definition of objective notification criteria would reduce uncertainties and
render the regulatory process more predictable for SMEs.
However, the ability of SMEs to enter the NGT market will be also dependent on affordable
access to intellectual property. For this reason, some stakeholders consider that their
competitive position will not improve under any of the options, unless patent-related issues
are resolved
216
.
215
Whelan et al. (2020); Goberna et al. (2022)
216
Technopolis Group et al. (2023)
63
In terms of general competitiveness, in the baseline, negative impacts can be expected due to
factors such as reduced access of the EU breeding sector to a global gene pool for plant
breeding. A negative impact on the competitiveness of conventional farming can be expected
due to reduced access to new tools developed through science and innovation efforts, and
increase in input prices (e.g. for feed supply). Option 1 is expected to offer small
improvements as regards competitiveness of breeders and farmers, while under option 2 some
additional improvement is expected in particular if the GMO labelling is waived for products
with traits contributing to sustainability. Option 3 is not expected to bring improvements.
Option 4 has the strongest impact on competitiveness as a lower regulatory burden will enable
investments in R&D and subsequent product development, both from large multinational
plant breeders as well as SMEs. Equally, in terms of international competitiveness, it is option
4 which will have the strongest impact. In major trading partners of the EU (e.g. the United
States or Argentina) NGT plants, and derived food and feed products, that can also result from
conventional breeding are not subject to GMO regimes. The European seed sector is the
largest exporter in the global seed market and using innovative technologies is a prerequisite
for maintaining competitiveness. Option 4 also has the strongest impact on strategic autonomy
and resilience of the EU food system. Under this option, NGTs are expected to be applied to a
large range of crop species and traits by a diverse set of actors.
Social and health impacts
Impacts related to the protection of human and animal health are addressed above in the
section on the specific objective of procedures that ensure safety while avoiding unnecessary
administrative burden.
Under the baseline, potential positive social and health impacts of NGT products with
improved nutritional and functional quality will be largely missed. Under options 1 and 2 and
to a smaller extent under option 3, more NGT products are expected on the market and
therefore certain of the social and health benefits of these products could be realised. This
would be greater in option 2, with some gains resulting from incentives to NGT plants
containing traits with potential positive social and health impacts, and further gains if the
GMO labelling is waived for NGT plants with traits that can contribute to sustainability, as
well as in option 4.
The baseline and options 1 and 3 ensure information to consumers about the use of genetic
modification technologies on the product’s label, according to consumer expectations as they
result from consultation activities and available research. However, this could result in low
levels of consumer acceptance of NGTs, if the negative connotations of the current GM label
extend to NGTs, unless other initiatives are taken to inform and engage consumers about
NGTs. In option 2, the sub-option to label NGTs with traits that can contribute to
sustainability as GM combined with a sustainability label or a factual statement on the
intended purpose of the genetic modification would support freedom of choice and increase
consumer and market acceptance, whereas the sub-option in which the GMO label is waived
if a product contributes to sustainability would make freedom of choice more burdensome and
likely negatively affect trust. The absence of labelling in option 4 would also make freedom
of choice more burdensome, but, on the other hand, a GM label for NGT products which
could occur in nature or be conventionally bred could create confusion.
Opportunities for consumers to access NGT products with increased nutritional and functional
qualities and with other sustainability-relevant traits which, according to available research,
rank high among consumers’ concerns would be greatest in option 4 followed by option 2.
64
Environmental impacts
Impacts related to the protection of the environment are addressed earlier in this section in the
subsection concerning the specific objective of procedures that ensure safety while avoiding
unnecessary administrative burden.
As no or only very few NGT plants are expected to be authorised for cultivation under the
baseline and option 3, the environmental impacts of these plants will be limited. Impacts will
be largely restricted to missed potential environmental benefits coming from NGT plants.
As a certain number of NGT products are expected to be placed on the EU market under
option 1, some environmental benefits are likely to be realised. The same would apply in
option 2, with additional gains resulting from incentives for NGT plants containing traits with
potential positive environmental impacts, and further gains if the GMO labelling is waived for
NGT plants with traits that can contribute to sustainability. This may in particular apply to
plants with biotic resistance, as such traits can be obtained by relatively simple genetic
modifications, leading to lower pesticide use. With more NGT products arriving on the
market, certain stakeholder groups expect positive environmental impacts, while others expect
negative impacts. In the targeted survey, roughly equal number of stakeholders expect a
positive (40%) or a negative (46%) impact. However, this polarisation is primarily due to
divergent views on the effects of herbicide tolerant crops, which, can, however, be developed
by all methods (conventional breeding, established genomic techniques or NGTs), and not to
negative impacts specific to the use of NGTs.
Option 4 offers the largest opportunities to realise the potential environmental benefits of
NGTs. As for options 1 and 2, the expectation is that the most significant environmental
impacts will come through NGT products with traits such as durable pest resistance. The
concerns about risks to the environment and biodiversity are similar to those for options 1 and
2. Agrobiodiversity could benefit from this option, as it can become easier to apply the
techniques to small and local crops.
All options are aligned with the ‘do no harm’ principle and include procedures to ensure that
NGT plants are only released or placed on the market if they are considered to as safe as their
conventional counterparts. Option 3 embeds this principle further by setting as a pre-requisite
for authorisation that the introduced trait is not detrimental to sustainability.
Impacts on the organic and GM-free sectors
The labelling and traceability requirements in the existing GMO legislation, as well as the
national coexistence measures adapted to local circumstances, will continue to apply and
allow separate value chains and consumer trust in these sectors under the baseline as well as
in options 1, 2 (if GMO labelling is not waived) and 3. The risk of admixture during
cultivation, harvest, storage, transport and processing will depend on the adoption rate of
NGTs and thus be moderately higher than the baseline in options 1 and 2 and similar to the
baseline in option 3 and highest in option 4.
Under option 4, the traceability, labelling and coexistence measures of the current GMO
legislation would not apply to notified NGTs. Impacts would differ if the NGTs under
notification are treated for the purposes of organic production as GMOs or as conventional
products. In the first sub-option organic operators would have the responsibility to put in
place precautionary measures, supported by the public register of NGT plants and information
in the common catalogues of plant varieties, to avoid the use of and admixture with any
product that is not authorised in organic production. If NGT products subject to notification
would be treated for the purposes of organic production as conventional products, they could

65
be used in organic production under the applicable derogations (and potentially contribute
benefits). However, there is the risk that consumers would not accept NGTs in organic
production, and a degree of erosion of consumer trust and related economic losses could
materialise.
The labelling sub-options in option 2 would have specific impacts. Under the variant of this
option that maintains a GMO label, the impacts on the organic/GM-free sector will be similar
to option 1. There are concerns that the variant with sustainability labelling of NGTs would be
to the disadvantage of sustainable conventional or organic products, which would not be able
to use such a sustainability label. Such NGT products would in any case still be labelled as
GM and identified as such by operators and consumers. If NGT products with traits
contributing to sustainability would not be labelled, the organic logo would guarantee to
consumers that the product is GM-free.
Under all options, some negative impacts on the organic and GM-free sectors can occur due
to regulatory divergence with non-EU countries from which organic and GM-free products
are imported into the EU, as sourcing of products may become more limited and as more
challenging and expensive identity preservation systems for NGT free products in the EU
trade partners may be required. Under the current EU regulatory framework, NGTs should not
be used in organic products exported to the EU, therefore, the costs of keeping supply chains
in the EU segregated would remain unchanged.
1.7. Efficiency analysis
This section compares the cost-effectiveness of the policy measures in the different options
for important stakeholder groups, based on the data in Annex 3. The wide range of costs and
savings are linked to the potential variability in data requirements, which depend on the
risks/type of products.
Table 8. Current cost for authorisation procedure (baseline). The authorisation cost can be subdivided
in cost for risk assessment, detection method, risk management and administrative cost.
Baseline (in thousands EUR)
Cost for breeders
Cost for
administrations
Cost for farmers and food
businesses
Full authorisation costs
n/a
209
217
n/a
Risk assessment
12 194
n/a
n/a
Detection method
650
n/a
n/a
Risk management
156
218
n/a
Available data not sufficient
for quantification
219
Coexistence and segregation measures
n/a
n/a
Available data not sufficient
for quantification
220
217
For administration, only costs for an authorisation procedure were estimated.
218
For breeders, risk management refers to any post-adoption activities, such as post-market monitoring.
219
Unquantifiable risk management costs for organic farmers are linked to practices and market monitoring of
accidental presence of GM product.
220
Coexistence measures, which are set up at national level, have costs for GM farmers, which are highly
dependent on the crop species, on farm structure and on environmental conditions. In addition, currently only
GM maize MON810 is authorised in the EU for cultivation, and its cultivation is limited to Spain and Portugal.
Therefore, available data on the current costs for co-existence measures and their variability are extremely
limited. The SIGMEA project (2004 – 2006), funded under FP6, mentioned in Section 6 estimates the costs of
potential coexistence measures to GM farmers based on simulations.
Coexistence of GM and non-GM commodity in the value chain creates segregation measures costs for
processing chain and for the food businesses. The current costs cannot be quantified with sufficient certainty as
they vary greatly depending on the GM crop species, its processing and use in the EU. The Co-Extra research
project (2008 – 2009) mentioned in Section 6 estimates the coexistence costs for the food businesses and the
value chain in some products.

66
Assessment of sustainability claim
n/a
n/a
n/a
Administrative cost
4 940
n/a
Available data not sufficient
for quantification
221
Total balance for full authorisation costs
17 940
209
Available data not sufficient
for quantification
Table 9. Costs and savings for authorisation under Option 1 in comparison to the baseline: Negative
amounts correspond to financial savings while positive amounts correspond to costs.
Option 1 (in thousands EUR)
Costs for breeders
Cost for administrations
Cost for farmers and food
businesses
Full authorisation costs
n/a
From 0 to -140
n/a
Risk assessment
From 0 to – 10 365
n/a
n/a
Detection method
No change compared to the
baseline
n/a
n/a
Risk management
No change compared to the
baseline
n/a
No change compared to the
baseline
Coexistence and segregation
measures
n/a
n/a
No change compared to the
baseline
Assessment of sustainability claim
n/a
n/a
n/a
Administrative cost
From 0 to – 1 750
n/a
No change compared to the
baseline
Total balance for full authorisation
costs
From 0 to -12 115
From 0 to -140
No change compared to the
baseline
Table 10. Costs and savings for authorisation under Option 2 in comparison to the baseline: Negative
amounts correspond to financial savings while positive amounts correspond to costs.
Option 2 (in thousands EUR)
Costs for breeders
Costs for administrations
Cost for farmers and food
businesses
Full authorisation costs
n/a
From 0 to -140
n/a
Risk assessment
From 0 to – 10 365
(+)
222
n/a
Detection method
No change compared to the
baseline
n/a
n/a
Risk management
No change compared to the
baseline
n/a
(+) or no change compared to
the baseline
223
Coexistence and segregation measures
n/a
n/a
No change compared to the
baseline
Voluntary assessment of sustainability
claim
From +1 950 to +390
224
(+)
225
n/a
221
Unquantifiable administrative costs for food businesses due to the GM labelling obligation.
222
Potential moderate to significant increases in costs for administrations, due to possible incentives to
encourage the development of sustainable products such as scientific support given to the applicant during
authorisation process.
223
In the first scenario, the GMO labelling would be waived for products with a modified trait that is considered
to have the potential to contribute to sustainability. In that case, an unquantifiable increase in costs is expected
for organic farmers.
In the second scenario, the GMO labelling is maintained, but a possible sustainability labelling can be added to
the GMO labelling. In this case, there is no change in costs compared to the baseline for organic farmers.
224
Voluntary compliance cost. For this analysis, in the absence of reliable information, it is assumed that all
applicants choose to provide data to support such a claim. Therefore, the amount is the same as in the
corresponding entry in Table 11, where these costs are mandatory for all applicants.
225
Potential moderate to significant increase in costs for Member States administrations due to the assessment of
sustainability claim.

67
Administrative cost
From +247 to -1 725
226
n/a
(+) or (-)
227
Total balance for full authorisation costs
From +2 197 to -11 700
228
Available data not
sufficient for quantification
(+) or (-)
229
Table 11. Costs and savings for authorisation under Option 3 in comparison to the baseline: Negative
amounts correspond to financial savings while positive amounts correspond to costs.
Option 3 (in thousands EUR)
Costs for breeders
Costs for administrations
Cost for farmers and food
businesses
Full authorisation costs
From 0 to -140
Risk assessment
From 0 to – 10 365
n/a
n/a
Detection method
No change compared to baseline
n/a
n/a
Risk management
No change compared to the
baseline
(+) or no changes
compared to the
baseline
230
No change compared to the
baseline
Coexistence and segregation measures
n/a
n/a
No change compared to the
baseline
Mandatory assessment of sustainability
claim
From +1950 to +390
231
(+)
232
n/a
Administrative cost
From +247 to -1 725
n/a
No change compared to the
baseline
Total balance for full authorisation costs
From +2 197 to -11 700
Available data not
sufficient for
quantification
No change compared to the
baseline
Table 12. Costs and savings for notification under Option 4 in comparison to the baseline: Negative
amounts correspond to financial savings while positive amounts correspond to costs.
Option 4 (in thousands EUR)
Costs for breeders
Costs for administrations
Costs for farmers and
businesses
Full notification costs
n/a
From 0 to -140
233
n/a
226
The administrative costs of the voluntary assessment of sustainability ranges from +247 000 EUR to +25 000
EUR.
227
In the first scenario, the GMO labelling would be waived for products with modified trait that is considered to
have the potential to contribute to sustainability. In that case, an unquantifiable saving in administrative costs for
food businesses is expected.
In the second scenario, the GMO labelling is maintained, but a possible sustainability labelling can be added to
the GMO labelling. In that case, a limited unquantifiable administrative cost increase is estimated due to the
additional information in the label.
228
The total balance presented in the table takes into account the potential voluntary data produced for the
assessment of sustainability claim.
The total balance in case no assessment of sustainability is performed would range from 0 to -12 115 000 EUR.
229
In the first the scenario, the estimated total would be an unquantifiable increase of costs for organic farmers
and unquantifiable savings for food businesses.
In the second scenario, it is estimated that there is no change compared to the baseline for organic farmers and
limited unquantifiable costs for food businesses.
230
In the first scenario, the GMO labelling would be waived for products with modified trait that is considered to
have the potential to contribute to sustainability. In that case, a slight increase in costs is estimated for the
Member States administrations.
In the second scenario, the GMO labelling is maintained, but a possible sustainability labelling can be added to
the GMO labelling. In that case, Member States competent authorities estimated that costs increase for risk
management, due to the necessity to set up additional control systems, would range from negligeable to high.
231
Mandatory compliance cost.
232
Potential moderate to significant increase in costs for Member States administrations due to the assessment of
sustainability claim.
233
The full notification cost for administrations is to be compared for the authorisation cost in the baseline
scenario.

68
Data for notification
234
From -9 146 to -10 365
n/a
n/a
Detection method
-650
n/a
n/a
Risk management
-156
235
(-)
236
(+)
237
Coexistence and segregation measures
n/a
n/a
(+)
238
Assessment of sustainability claim
n/a
n/a
n/a
Administrative cost
From – 1 615 to – 3 750
n/a
(-)
239
Total balance for full notification costs
From -11 567 to -14 921
From 0 to -140
240
(+) and (-)
241
1.8. Coherence
7.3.1. Coherence with GMO legislation
NGT plants fall under the scope of the current EU legislation on GMOs. The initiative on
NGT plants will propose new measures specifically for NGT plants and food and feed
containing, consisting or produced from such plants. Those measures will share the objectives
of the GMO legislation to ensure a high level of protection of human health and of the
environment in accordance with the precautionary principle and ensure the functioning of the
internal market while addressing the specificity of NGT plants. All policy options are
coherent with the existing framework as regards those objectives. Options 2 and 3 would add
new sustainability elements in the GMO framework only for NGT products.
7.3.2. Coherence with other legislation and initiatives
Plant Reproductive Material (PRM) and Forest Reproductive Material (FRM) legislation –
The objective of the on-going revision of the PRM and FRM legislation is to ensure the
availability of PRM and FRM of high quality and diversity, that contribute to food security,
sustainable production and protection of biodiversity and that are adaptable to climate change.
The objectives of the NGT initiative and the PRM/FRM revision are fully compatible.
While the scope of the NGT initiative covers all plant species, the PRM/FRM revision covers
only the economically most important species for European agriculture, horticulture and
forestry. This is justified by the different rationales of the legislation on NGTs (regulatory
oversight of products obtained by new biotechnologies) and on PRM/FRM (regulatory
oversight to ensure the identity, quality and health of the economically most important
marketed PRM and FRM regardless of the method by which they have been produced).
234
The comparison was done between the estimated costs of the potential data requirements for the notification
of an NGT plant compared to the current cost of data requirements for a GMO application.
235
For breeders, risk management refers to any post-adoption activities, such as post-market monitoring
activities.
236
Significant unquantifiable savings are estimated for administrations in risk management due to the removal of
the traceability and labelling requirements.
237
Potential unquantifiable increase in costs is expected for organic farmers for risk management practices and
market monitoring (for accidental presence of GM/NGT product) due to the uncertainties of potential presence
of notified NGT plants in conventional seeds.
238
Potential unquantifiable cost increase for organic farmers (and others not wishing to use NGTs) due to
potential challenges regarding coexistence as the labelling threshold cannot be effectively checked and enforced.
239
Unquantifiable savings in administrative costs for businesses is expected due to the removal of the traceability
and labelling obligation.
240
This total does not take into account the unquantifiable savings from risk management.
241
Unquantifiable savings are expected for non-organic farmers and businesses while unquantifiable costs are
expected for organic farmers.
69
Possible issues of coherence between the two initiatives are primarily related to the inclusion
of sustainability provisions. Under all options of the PRM/FRM initiative, the assessment of
sustainability characteristics will be extended to all crop groups/FRM categories beyond
agricultural crops on a voluntary or mandatory basis, depending on the option chosen. Both
the current VCU for agricultural crops and vine, and the envisaged extended sustainability
assessment under the PRM initiative, examine the overall performance of new varieties
compared to varieties in the national catalogues in relation to a number of characteristics that
would go beyond one or more introduced traits. The same principle of an overall assessment
of basic material in relation to a number of defined sustainability characteristics holds true for
FRM. As today, the PRM/FRM systems will not provide for the possibility to reject a variety
based on a single trait as inferior characteristics may be discarded if the variety/basic material
as a whole offers clear improvements in comparison with existing varieties/basic material.
In the baseline option and options 1 and 4 no specific sustainability provisions will be
introduced and no issues of coherence with the PRM/FRM revision would need to be
considered.
In Option 2, the specific regulatory incentives considered would not raise a problem of
coherence with the PRM/FRM revision, as they are specific to the authorisation procedure for
NGT products. The sustainability-related procedures of the two systems should not interfere
with each other, because the verification in option 2 relates exclusively to the potential
sustainability impact of an individual trait introduced in a specific plant in comparison to the
same plant without this genetic modification, while the PRM/FRM legislation considers the
overall performance of new agricultural crop varieties. Moreover, assessing during the
authorisation process the potential contribution to sustainability of a trait introduced using
NGTs could trigger incentives in the NGT framework but would not prejudge the outcome of
VCU trials during variety registration. On the other hand, a sustainability claim in the NGT
initiative could raise coherence issues as it would be linked to an individual trait irrespective
of the overall performance of the variety.
Option 3 would not ensure coherence with the PRM/FRM revision as it would lead to having
sustainability as a requirement only for NGT plants, leading to the refusal of the authorisation
to place on the market plants featuring certain traits, while conventionally bred plants
featuring the same traits would not, for that reason alone, be excluded from the market.
Should those plants be subject to VCU examination, the holistic assessment in that context
would also not lead to rejection of a variety containing a trait that could have detrimental
impact on sustainability, if other, superior characteristics are present.
Planned initiative on a legislative framework for a sustainable food system (FSFS) - Both the
FSFS and the NGT initiatives share the objectives of the Green Deal and the Farm to Fork
Strategy and are intended to set out enabling frameworks to achieve these common goals.
Whereas the NGT initiative will set out a framework to encourage the development of NGT
plants that can contribute to those objectives, at the same time ensuring a high level of
protection of human and animal health and of the environment, the FSFS is envisaged as an
enabling framework for an EU sustainable food system. While the main elements of both
initiatives are still being developed, both initiatives will be mutually complementary and work
in synergy to achieve their shared sustainability objectives.
Organic Products Regulation (Regulation (EU) 2018/848) and organic target in the Farm to
Fork Strategy – That regulation bans the use of GMOs in organic production, and considers as
GMOs for the purposes of that Regulation those that are not exempted from the requirements
of the GMO Directive. Conversely, GMOs exempted from the requirements of the GMO
legislation (e.g., plants obtained from random mutagenesis) are treated as conventional for the

70
purposes of organic production and may be used under certain circumstances. In the baseline,
as well as options 1, 2 and 3, NGTs would remain subject to the requirements of the GMO
legislation and would be considered GMOs for the purposes of organic production. If option 4
is retained, the proposal may need to clarify the status of NGT products falling under the
notification in the context of the organic regulation. In any event, all options include
provisions (traceability, labelling or public registries) to allow organic producers to comply
with the Organic Production legislation.
The Farm to Fork Strategy aims for at least 25% of the EU's agricultural land to be under
organic farming by 2030. This aim can be reconciled with increased NGT cultivation through
transparency about NGTs to allow choice in the supply chain in all policy options and the use
of appropriate coexistence/precautionary measures. Should organic operators accept the use of
notified NGT plants with traits relevant to organic production (e.g. pest resistance, tolerance
to drought, nutrient use efficiency, improved yield/input ratio), and if this becomes possible
under the legal framework, this could support efforts towards the 25% target. On the other
hand, this could also lead to the loss of trust of consumers in the organic EU logo and could
impact progress towards the 25% target.
Regulation (EU) 2015/2283 on novel foods (NFR)
242
– This regulation does not apply to food
subject to Regulation (EC) No 1829/2003 on genetically modified food and feed. In option 4,
NGT food and feed products which meet the notification criteria and are not subject to
authorisation as GMOs would no longer be excluded from the NFR. If there would be
significant changes in the composition or structure of food derived from NGT plants affecting
its nutritional value, metabolism or level of undesirable substances, the food would need to be
risk assessed and authorised under the NFR in order to be placed on the market. This would
contribute to the overall goal of ensuring safety.
Legislation on labelling: Regulation (EU) No 1169/2011 on the provision of food information
to consumers (FIC Regulation)
243
, Regulation (EC) No 1924/2006 on nutrition and health
claims, (NHCR)
244
, Regulation (EC) No 767/2009 on the placing on the market and use of
feed
245
and initiative on substantiating green claims
246
– The sustainability label (if
complementary to the existing GMO label) in option 2 for NGT products featuring a modified
trait which contributes to sustainability could raise coherence issues vis à vis current
requirements in the FIC Regulation, the NHCR and the Regulation on marketing of feed as
well as with the green claims initiative. These regulations set out requirements that have to be
met by voluntary information or claims included on the labelling of food intended for the final
consumer. Therefore, any claim regarding the properties of the food derived from a NGT
plant, and in particular on nutrition properties, would have to also comply with those
requirements. As regards environmental claims, the Commission’s green claims initiative
would require companies to substantiate environmental claims about their products with a
standard methodology to assess their impact on the environment. Any environmental claims
made with regard to the modified trait of a NGT plant in option 2 would have to be
242
https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32015R2283
243
https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1169
244
https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1924
245
https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32009R0767
246
Proposal for a Directive of the European Parliament and of the Council on substantiation and communication
of explicit environmental claims (Green Claims Directive). COM/2023/166 final; https://eur-lex.europa.eu/legal-
content/EN/TXT/?uri=COM:2023:0166:FIN
71
substantiated in accordance with the new rules on green claims, once adopted, in order to
ensure coherence.
Directive 98/44/EC on the legal protection of biotechnological inventions, European Patent
Convention and Regulation (EC) No 2100/94 on Community plant variety rights – The
genetic modification made in a plant by means of a NGT is patentable, as well as the
technique used to obtain the modification. Plant varieties obtained by NGTs can be protected
by Community plant variety rights (CPVRs). In this regard, a balanced intellectual property
system, including patent protection, is essential for innovation in agricultural biotechnology,
i.e. by incentivising investments in research and development and promoting the
dissemination of knowledge. At the same time, concerns have been raised by many
stakeholders (farmers, breeders, in particular from the GM-free and organic sectors) on the
possible proliferation of patents on NGT plants in the future and on potential claims being
made by patent holders on conventionally bred plants that cannot be distinguished from NGT
plants, fearing that this scenario could be further exacerbated if NGT plants are not subject to
a GMO authorisation. While from the legal point of view the NGT initiative is independent
from the rules on the protection of intellectual property and does not address matters relating
to the application of the IPR legislation to GMOs, in particular to NGT plants, the
Commission has taken note of the concerns brought forward by certain stakeholders on the
need to ensure in particular the accessibility of farmers to patented seeds and of breeders to
patented genetic material, and will carefully consider them.
1.9. Proportionality
The adapted risk assessment featured in options 1, 2 and 3 will be proportionate as it is
intended that the criteria (and subsequent data requirements based on them) for the risk
assessment of different NGT plants and derived food and feed are not stricter than necessary
to ensure that the potential risks are properly identified and evaluated. This approach of
adapted risk assessment would ensure proportionality for all NGT plants except those that
could have been obtained naturally or by conventional breeding, where a notification regime
as foreseen in option 4 (based on criteria allowing to identify NGT plants equivalent to
conventionally bred plants) would suffice to ensure the objective of safety.
The granting of regulatory incentives in option 2 for applications for NGT plants with
modified traits that can contribute to sustainability would be subject to a requirement to
submit data to substantiate that claim, which would add an administrative and cost burden on
operators wishing to benefit from such incentives. However, since the regulatory incentives
would result in lower application costs (faster procedures, waiving of detection method
validation fees, regulatory and scientific advice during the procedure), such a regime would
not result in an overall increase of the regulatory burden, but a reduction thereof. The degree
to which the burden would be reduced would depend on the amount of data required to obtain
the incentive and on the actual incentives foreseen. Overall, as the incentives regime would be
voluntary and not add an extra burden on operators not wishing to use it, such a measure
would be proportionate.
Option 3 would impose sustainability requirements on NGT products as a condition for their
authorisation and hence for their placing on the market. The fact that under that option NGT
plants containing certain traits may not be allowed on the market for reasons related to the
impact of the trait on sustainability while non-NGT plants containing a similar trait would not
be subject to such a requirement, would render such a measure disproportionate.
Finally, for option 4 the indicative notification criteria (see section 5.2.4 box 2) are designed
to reflect the equivalence of the NGT plant with plants that could occur naturally or be
72
obtained by conventional breeding methods, and (complemented by data requirements linked
to those criteria) should be adequate to ensure proportionality.
8. Preferred Option
8.1. Description of the preferred option
The preferred option is a combination of option 4 for products that could also occur
naturally or be produced by conventional breeding and of option 2 for all other
products. This combination ensures to the largest possible extent that NGT plants and derived
food/feed products are as safe as their conventional counterparts, while not entailing
unnecessary regulatory burden, that NGT plants and derived food/feed products featuring a
wide range of plant species and traits by various developers are placed on the market and that
these plants feature traits that can contribute to a sustainable agri-food system.
Option 4 provides for a notification procedure that would be accessible only to those NGT
plants that could occur naturally or be produced by conventional breeding. It achieves
the objective to maintain safety while ensuring that requirements are proportionate to the risk
involved. The NGT plants concerned, due to their characteristics, would not present more
potential hazards than plants produced by conventional breeding. The notification procedure
includes a case-by-case assessment based on the molecular characteristics of the product
against predefined criteria, and a decision by the relevant authority to establish the status of
products (equivalent to conventional or not).
Option 4 is the best performing option to deliver on the objectives of this initiative and shows
positive impacts in terms of innovation and interest of developers in marketing these products
in the EU, enabling products to contribute to sustainability and food security. This option
offers the largest opportunities to realise the potential benefits of NGTs, also bringing more
diversity regarding species, traits and institutions developing them. This option reduces
regulatory burden and access barriers to SMEs. It is comparable to the approach followed in
an increasing number of third countries and would be the least disruptive of trade.
By treating NGT plants that could have been obtained naturally or by conventional breeding
in a similar way to conventionally bred plants, this option scores best as regards coherence
and proportionality.
Under this option, the NGT products that could occur naturally or be produced by
conventional breeding would not be subject to GMO traceability and labelling but would be
entered into a public register. This would increase transparency compared to the treatment
today, of GMOs exempted from the requirements of the GMO legislation (e.g., the products
of random mutagenesis) for operators (organic, GM-free) and consumers and allow operators
at the beginning of the food chain – from breeding to seed production – to identify products
obtained from NGTs and to avoid them if so wanted. The extent of the impacts of this option
in the organic sector will depend on how notified NGT plants are treated for the purposes of
organic production (either as GMOs or as conventionally bred plants). Considering the
implications of each option as described in this impact assessment as well as the majority
position in the organic sector, the policy choice has been made in the preferred option to treat
such NGT products as GMOs for the purposes of organic production.
Option 4 is not designed to apply to all NGT plants, but only to those that could occur
naturally or be obtained by conventional breeding. Option 2 is the preferred option for NGT
plants that are not similar to conventional products.
The option scores high in terms of ensuring safety and proportionality through requirements
that are adapted to levels of risk of NGT plants that are not similar to conventional products.
73
An adapted risk assessment would bring a moderate improvement concerning attractiveness to
develop NGT plants in the EU. Cost reductions for applicants compared to the baseline range
from low to very substantial, depending on which risk assessment criteria need to be applied
in specific cases. There would be variable but potentially significant cost decreases for risk
assessment. To address concerns expressed about the potential uncertainty and complexity of
this approach, work on the criteria for risk assessment (see section 5.2.1) is intended to ensure
adaptability and predictability.
Regulatory incentives linked to the authorisation of NGT plants under option 2 would bring
moderate positive impacts in terms of steering research and development towards traits with
sustainability potential by facilitating access to and navigation of the regulatory framework,
especially for SMEs, supporting their competitiveness. To avoid pre-empting the development
of horizontal approaches to define and assess contributions to sustainability, and to address
other difficulties of a sustainability assessment in the context of this initiative, option 2 would
be adjusted to link the incentives to the traits conferred by the genetic modification, instead of
linking them to an assessment of sustainability impacts. The incentives would be granted
when the trait appears in a pre-defined list of traits selected because of their potential to
contribute to sustainability objectives (desirable traits).
NGT plants subject to option 2 would also remain subject to traceability, and the related tools
would remain available (as today) to supply chains that do not use GMOs (organic, GM-free
sectors) until the final consumer. The impact of option 2 on the organic and GMO-free sector
has been considered similar to the baseline when labelling is kept.
Regulatory divergence with non-EU countries is likely to remain large for NGT plants subject
to option 2, posing challenges for value chain management and identity preservation systems
in the exporting countries.
The current GM-label is perceived as a warning on possible risks (although only plants as safe
as comparable conventional plants can be placed on the market) and would impact consumer
acceptance, especially of food. It is also a key driver of negative consequences on the
attractiveness to develop NGT plants in the EU. However, there is considerable demand for
labelling and removing the labelling requirement for products subject to the requirements of
risk assessment and authorisation would likely impact consumer trust. NGT plants subject to
option 2 would therefore remain subject to labelling GMOs.
There is equally demand from citizens to use this initiative to actively steer the development
of varieties with traits that support the Farm to Fork objectives, and to be informed about the
sustainability impact of the products they buy. The intended application of the technology
(and whether it delivers benefits to society at large) is relevant information to consumers and
could be a factor influencing acceptability of NGTs. To this end, option 2 included the
possibility to allow sustainability-related claims based on the trait to be mentioned on the
label of the final product. However, a sustainability-related claim for certain NGT products
raised concerns due to difficulty to assess sustainability based on a single trait, differential
treatment vis-à-vis other products and coherence with other legislation on claims. Therefore,
as preferred option, the claim will be replaced with the possibility to add a factual statement
of the trait for NGT products subject to authorisation, i.e. the GM labelling could be
complemented with information on the purpose of the genetic modification (e.g., genetically
modified for the purpose of improving resistance to drought). This should allow operators and
consumers to make informed choices and is expected to drive market demand for products
with beneficial traits.
With the combination of option 2 with option 4, for the most part products subject to
authorisation could be differentiated from plants obtained naturally or produced by
74
conventional breeding techniques. In such cases, applicants for authorisation would be able to
comply with a requirement to submit a specific detection method, the competent authorities
would be able to enforce the legislation and operators would be able to test raw material and
products for traceability purposes.
The preferred option creates an enabling framework to meet the demands of farmers for the
development and commercialisation of new varieties with beneficial traits to respond to the
constraints of their soil and climatic context
247
. For NGT plants subject to notification, the
development of such varieties is also steered by the objectives and provisions of other EU
policy initiatives, in particular the SUR proposal (which has the objective to reduce the use of
pesticides) to which pest-resistant crops can contribute, the PRM/FRM initiative (holistic
assessment of the sustainability characteristics of a variety) and the FSFS initiative (steering
the EU food systems towards sustainability). For NGT products requiring an authorisation,
the preferred option steers developments towards desirable traits by providing regulatory
incentives, which would be helpful in particular for SMEs. The factual label of such products
could impact consumer choice, thus also steering indirectly the development towards
desirable traits.
Consumer acceptance of NGTs is necessary for the benefits of NGTs to materialise. In this
regard, initiatives to inform and engage consumers about NGTs need to be considered to
accompany and explain the transparency measures – label in option 2, registry in option 4 –
provided for in the legislation.
It will also be important to monitor closely the uptake of NGT products and the
accompanying impacts, both positive and negative (see section 9).
The preferred option contributes to three of the four components of food security – food
availability, stability and utilisation. It furthermore contributes to sustainability, the long-term
ability of food systems to provide food security in a way that does not compromise the
economic, social, and environmental bases that generate food security for future generations.
8.2 Simplification and burden reduction, supporting the one-in-one-out
approach
The preferred option presents an important simplification of processes of the current
authorisation procedure, notably through the adapted risk assessment (option 2) and the new
notification procedure (option 4) for products that fulfil the criteria for equivalence to
conventional breeding.
Adjustment costs for the preferred option:
For breeders, the preferred option would entail a significant reduction of adjustment costs.
Based on the hypothetical scenario described in Annex 3 in which breeders would submit 10
notifications and 5 applications for authorisation per year
248
, the total recurrent savings in
adjustment costs per year are estimated to range from a minimum of EUR 99.5 m to a
maximum of EUR 163.5 m
249
.
For national administrations, a potential unquantifiable increase in adjustment costs was
247
CEJA (2022)
248
This estimate is based on the number of products expected to come to the market in the next ten years and on
the assumption that, starting in 2015, 15 products per year will enter the regulatory approval process.
249
The risk assessment will be proportionate to the risks/type of products, it will be variable, which explains the
wide range of savings. This is mainly linked to the potential variability in data requirements as laid out in section
6.2.

75
identified due to the additional support (incentives) for applicants during the authorisation
process for certain NGT products with traits that can contribute to sustainability. This
potential increase in costs would be counterbalanced by the fact that the preferred option
would entail at the same time a significant reduction of the adjustment costs for national
administrations. The total recurrent savings in adjustment costs per year for national
administrations are estimated to range from EUR 0 to EUR 2.1 m.
There is a wide range of savings for the breeders and for national administration as the risk
assessment, which is the main source of adjustment costs for breeder, will be proportionate to
the risks/type of products and the data requirements will vary.
For farmers using NGT varieties, recurrent savings in adjustment costs were estimated to
range from EUR 22 m (for vegetable crops in the most likely scenario) to EUR 2.7 bn (for
cereals in the most likely scenario) due to potential yield improvement of crops. Additional
costs related to segregation (e.g. additional documentation, third-party verification) and
coexistence measures might be added. These could not be quantified.
For organic farmers, the preferred option would entail a potential but unquantifiable increase
in adjustment costs for risk management practices and market monitoring (for accidental
presence of GM/NGT product) due to the uncertainties of potential presence of notified NGT
plants in conventional seeds in the first sub-option, where notified NGTs remain banned in
organic production. In the second sub-option, where notified NGTs would be treated like
conventional products with regard to organic production, this cost increase would not occur.
The second sub-option, though, could lead to the erosion of consumer trust and lead to
economic losses of the organic sector.
Administrative costs for the preferred option
The preferred option would entail an annual overall administrative cost saving for breeders
ranging from EUR 16.15 m to EUR 46.25 m. The range reflects the differences in data
requirements for products with different risk profiles.
For farmers, including organics farmers, no administrative costs are identified.
For food businesses, a limited unquantifiable increase in administrative cost is expected due
to additional information in the GMO label (identification on the label of the purpose of the
genetic modification to the label) and related segregation costs. Recurrent savings in
administrative costs are expected due to the removal of the traceability and labelling
obligation for the notified products; however these could not be quantified.
9. How will actual impacts be monitored and evaluated?
In order to monitor and evaluate the progress made towards the objectives of this initiative
and its economic, environmental and social impacts, indicators have been identified and are
listed in the table below (Table 13). The Commission will review these indicators
periodically.
Data on most indicators will be collected and published annually and will serve as the basis
for regular monitoring reports, that will also be supported by external studies.
The indicators defined will support monitoring of potential risks to health or the environment,
achievement of the initiative’s objectives and impact of NGT plants on environmental,
economic and social sustainability. Indicators are also proposed to monitor impacts on organic
agriculture and on consumers acceptance of NGT products.
A first monitoring report should be presented no sooner than 3 years after the first products

76
have been notified/authorised, to ensure that enough data is available after full
implementation of the new legislation, and at regular intervals thereafter. An evaluation
should be carried out no sooner than 2 years after the first monitoring report has been
published.
Table 13. Monitoring indicators. Indicators that need to be created are marked with ‘*’.
Specific objectives
Indicators
Data
source/frequency
Actors
responsible
for data
collection
S1: Procedures for the deliberate release
and placing on the market ensure that
NGT plants and derived food/feed
products are as safe as their conventional
counterparts, while not entailing
unnecessary regulatory burden
Reported cases demonstrating risk to human
and animal health and the environment due to
genetic modification in authorised or notified
product, and any regulatory action taken.
EC/Annually
EC
Number of products authorised to be placed
on the market
EC /Annually
EC
Number of products notified to be placed on
the market
EC/Annually
EC
Time needed for risk assessment
EFSA and Member
States/ Annually
EC
Time needed for granting
authorisation/notification
EC and Member
States/ Annually
EC
Assessment of encountered hurdles and
barriers in particular for SMEs (qualitative)
External study/ 3
years after the first
products have been
notified/authorised
EC
Costs for the notification/authorisation/risk
assessment
External study/ 3
years after the first
products have been
notified/authorised
EC
S2: Deliberate release and placing on the
market of NGT plants and derived
food/feed products that feature a wide
range of plant species and traits by various
developers
Number of crop-trait combinations (NGTs
and GMOs) in notifications/authorisation
applications
EC/Annually
EC
Number of crop-traits combinations (NGTs
and GMOs) and developers at global level*
External study/
3 years after the first
products have been
notified/authorised
EC
Number and proportion of SMEs/public
institutions applying for field
trials/notifications/authorisation applications*
EC/Annually
EC
Assessment of the impacts of release and
placing on the market of NGT products in the
EU on organic agriculture in view of the 25%
target in F2F* (quantitative / qualitative)
External
study/3years after
the first products
have been
notified/authorised
EC
Consumer acceptance and willingness to buy
NGT products*
Eurobarometer/ 3
years after the
adoption of the new
legislation – to be
repeated every 4
years
EC
S3: NGT plants released or placed on the
market feature traits that can contribute to
a sustainable agri-food system
Impact of NGT plants in the EU on
economic, environmental and social
sustainability, e.g., through pesticide use,
fertiliser user, biodiversity, greenhouse gas
emissions, yield, yield stability, health
benefits *.
External study/
3 years after the first
products have been
notified/authorised
EC
10. List of references
Advisory Committee on Releases to the Environment, 2021. ACRE advice concerning
Defra’s consultation on the regulation of genetic technologies,
https://www.gov.uk/government/publications/acre-advice-the-regulation-of-genetic-
technologies/acre-advice-concerning-defras-consultation-on-the-regulation-of-genetic-
technologies#fn:1
AgbioInvestor, 2022. Time and Cost to Develop a New GM Trait, Cost and Time Required
for the Discovery, Development and Authorisation of a New Plant Biotechnology-Derived
Genetic Trait. A Study on Behalf of Crop Life International, https://croplife.org/wp-
content/uploads/2022/05/AgbioInvestor-Trait-RD-Branded-Report-Final-20220512.pdf
Andersen, M.M., Landes, X., Xiang, W. et al., 2015. Feasibility of new breeding techniques
for organic farming. Trends in Plant Sciences 20, 426-434,
https://doi.org/10.1016/j.tplants.2015.04.011
Babuscio, T., Hill, W., Ryan, C.D. et al. 2016. The Canadian and European Union Impacts
from the Detection of GM Flax. Pp. 167-176 in: Kalaitzandonakes, N., Phillips, P.W.B.,
Wesseler, J. et al. (eds.), The Coexistence of Genetically Modified, Organic and Conventional
Foods. Government Policies and Market Practices. Springer Science+Business Media New
York
Baum, C. M., Kamrath, C., Bröring, S. et al. 2023. Show me the benefits! Determinants of
behavioral intentions towards CRISPR in the United States. Food Quality and Preference,
104842.
Beghin, J. C., & Gustafson, C. R., 2021. Consumer valuation of and attitudes towards novel
foods produced with new plant engineering techniques: A review. Sustainability, 13(20),
11348.
Benbrook, C.M., 2012. Impacts of genetically engineered crops on pesticide use in the U.S.
— the first sixteen years. Environmental Sciences Europe 24, 1–13
Booker, H.M., Lamb, E.G. & Smyth, S.J., 2017. Ex-post assessment of genetically modified,
low-level presence in Canadian flax. Transgenic Research 26, 399-409.
Buchholzer, M. & Frommer, W.B. 2023. An increasing number of countries regulate genome
editing in crops. New Phytologist 237(1), 12-15, DOI: 10.1111/nph.18333
Bullock, D.W., Wilson, W.W. & Neadeau, J., 2021. Gene editing versus genetic modification
in the research and development of new crop traits: An economic comparison. American
Journal of Agricultural Economics 103: 1700–1719; doi:10.1111/ajae.12201
Bundesamt für Naturschutz, 2021. New developments and regulatory issues in plant genetic
engineering. https://www.bfn.de/en/publications/position-paper/new-developments-and-
regulatory-issues-plant-genetic-engineering
Bundesinstitut für Risikobewertung (BfR), 2017. Durchführung von Fokusgruppen zur
Wahrnehmung des Genome Editings (CRISPR/Cas9). - Abschlussbericht - BfR-Wissenschaft
04/2017 (bund.de)
Bundesversammlung der Schweizerischen Eidgenossenschaft, 2022. Bundesgesetz über die
Gentechnik im Ausserhumanbereich (Gentechnikgesetz, GTG). Änderung vom 18. März

2022.
https://www.parlament.ch/centers/eparl/curia/2021/20210049/Schlussabstimmungstext%201
%20NS%20D.pdf
Busch, G., Ryan, E., von Keyserlingk, M. A. et al., 2022. Citizen views on genome editing:
effects of species and purpose. Agriculture and Human Values, 39(1), 151-164
Catacora-Vargas, C., 2011. Genetically Modified Organisms. A Summary of Potential
Adverse Effects Relevant to Sustainable Development. Biosafety Report 2011/02, GenØk -
Centre for Biosafety
Challinor, A.J., Koehler, A.-K., Ramirez-Villegas, J. et al., 2016. Current warming will
reduce yields unless maize breeding and seed systems adapt immediately. Nature Climate
Change 6, 954-958, https://doi.org/10.1038/Nclimate3061
Council of the European Union, 12 April 2023. Conclusions on the opportunities of the
bioeconomy in the light of current challenges with special emphasis on rural areas.
https://data.consilium.europa.eu/doc/document/ST-8194-2023-INIT/en/pdf
Council Decision (EU) 2019/1904 of 8 November 2019 requesting the Commission to submit
a study in light of the Court of Justice’s judgment in Case C-528/16 regarding the status of
novel genomic techniques under Union law, and a proposal, if appropriate in view of the
outcomes of the study (OJ L 293, 14.11.2019, p. 103–104), https://eur-
lex.europa.eu/eli/dec/2019/1904/oj
Crespo-Herrera, L.A. & Ortiz, R., 2015. Plant breeding for organic agriculture: something
new? Agriculture & Food Security 4, 25, https://doi.org/10.1186/s40066-015-0045-1
Desquilbet, M., Bullock, D.S. & D‘Arcangelo, F.M. 2019. A discussion of the market and
policy failures associated with the adoption of herbicide-tolerant crops. International Journal
of Agricultural Sustainability 17, 326-337, https://doi.org/10.1080/14735903.2019.1655191
Directive 98/44/EC of the European Parliament and of the Council of 6 July 1998 on the legal
protection of biotechnological inventions (OJ L 213, 30.7.1998 , p. 13–21); https://eur-
lex.europa.eu/legal-content/EN/TXT/?uri=celex:31998L0044
Directive (EU) 2015/412 of the European Parliament and of the Council of 11 March 2015
amending Directive 2001/18/EC as regards the possibility for the Member States to restrict or
prohibit the cultivation of genetically modified organisms (GMOs) in their territory (OJ L 68,
13.3.2015, p. 1–8); http://data.europa.eu/eli/dir/2015/412/oj
Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on
the deliberate release into the environment of genetically modified organisms and repealing
Council Directive 90/220/EEC - Commission Declaration (OJ L 106, 17.4.2001, p. 1–39),
https://eur-lex.europa.eu/legal-
content/EN/TXT/?uri=CELEX%3A32001L0018&qid=1674643626319
Directive 2009/41/EC of the European Parliament and of the Council of 6 May 2009 on the
contained use of genetically modified micro-organisms (OJ L 125, 21.5.2009, p. 75–97),
https://eur-lex.europa.eu/legal-
content/EN/TXT/?uri=CELEX%3A32009L0041&qid=1674643882006

Dobres, M.S., 2008. Barriers to Genetically Engineered Ornamentals: An Industry
Perspective. Pp. 1-14 in: Teixeira da Silva, J.A. (ed.) Floriculture, Ornamental and Plant
Biotechnology, Vol. V, Global Science Books
EASAC 2017. Genome editing: scientific opportunities, public interests and policy options in
the European Union.
https://easac.eu/fileadmin/PDF_s/reports_statements/Genome_Editing/EASAC_Report_31_o
n_Genome_Editing.pdf
EFSA 2012. EFSA Panel on Genetically Modified Organisms, 2012. Scientific opinion
addressing the safety assessment of plants developed through cisgenesis and intragenesis.
EFSA Journal 2012;10(2):2561.
EFSA 2020. EFSA Panel on Genetically Modified Organisms, ‘Applicability of the EFSA
Opinion on SDNs type 3 for the safety assessment of plants developed using SDNs type 1 and
2 and oligonucleotide-directed mutagenesis’, EFSA Journal 2020;18(11):6299.
https://doi.org/10.2903/j.efsa.2020.6299
EFSA 2021a. EFSA Panel on Genetically Modified Organisms, 2021. Scientific Opinion on
the evaluation of existing guidelines for their adequacy for the molecular characterisation and
environmental risk assessment of genetically modified plants obtained through synthetic
biology. EFSA Journal 2021;19(2):6301, 21 pp. https://doi.org/10.2903/j.efsa.2021.6301
EFSA 2021b. European Food Safety Authority (EFSA). (November 2021). Flash Poll.
https://zenodo.org/record/7081944#.Y5dbs57MKUn
EFSA 2022a. EFSA Panel on Genetically Modified Organisms, 2022. Updated scientific
opinion on plants developed through cisgenesis and intragenesis. EFSA Journal 20(10):7621,
33 pp. https://doi.org/10.2903/j.efsa.2022.7621.
EFSA 2022b. EFSA Panel on Genetically Modified Organisms, 2022. Scientific Opinion on
the evaluation of existing guidelines for their adequacy for the food and feed risk assessment
of genetically modified plants obtained through synthetic biology. EFSA Journal 2022;20
(7):7410, 25 pp. https://doi.org/10.2903/j.efsa.2022.7410
EFSA 2022c. European Food Safety Authority (EFSA). (September 2022). 2022
Eurobarometer on Food Safety in the EU.
https://www.efsa.europa.eu/en/corporate/pub/eurobarometer22
EFSA 2022d. EFSA Panel on Genetically Modified Organisms, 2022. Statement on criteria
for risk assessment of plants produced by targeted mutagenesis, cisgenesis and intragenesis.
EFSA Journal 20(10):7618, 12 pp. https://doi.org/10.2903/j.efsa.2022.7618
ENSSER (2021) Response to the Inception Impact Assessment (IAA) on new Genomic
Techniques. https://ensser.org/publications/2021-publications/enssers-response-to-the-
inception-impact-assessment-iaa-on-new-genomic-techniques/
Entine, J., Felipe, M.S.S., Groenewald, JH. et al., 2021. Regulatory approaches for genome
edited agricultural plants in select countries and jurisdictions around the world. Transgenic
Research 30, 551–584; https://doi.org/10.1007/s11248-021-00257-8
Eriksson, D., Kershen, D., Nepomuceno, A. et al., 2019. A comparison of the EU regulatory
approach to directed mutagenesis with that of other jurisdictions, consequences for

international trade and potential steps forward. New Phytologist 222, 1673-1684;
https://doi.org/10.1111/nph.15627
European Coexistence Bureau (ECoB), 2010-2018. Best practice documents for the
coexistence of genetically modified crops with conventional and organic farming;
https://joint-research-centre.ec.europa.eu/european-coexistence-bureau-ecob-exchange-best-
agricultural-management-practices/ecob-documents/ecob-best-practice-documents_en
European Commission, 2016. Commission Staff Working Document, Genetically modified
commodities in the EU, SWD(2016) 61 final, Brussels,
https://data.consilium.europa.eu/doc/document/ST-6954-2016-INIT/en/pdf
European Commission, 2017. New techniques in agricultural biotechnology. Publications
Office of the European Union, doi:10.2777/17902; https://data.europa.eu/doi/10.2777/574498
European Commission, 2018. A sustainable Bioeconomy for Europe: Strengthening the
connection between economy, society and the environment. COM(2018) 673 final,
https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52018DC0673
European Commission, 2020a. A Farm to Fork Strategy for a fair, healthy and
environmentally-friendly food system. COM(2020) 381 final, https://eur-lex.europa.eu/legal-
content/EN/TXT/?uri=CELEX:52020DC0381
European Commission, 2020b. EU Biodiversity Strategy for 2030. Bringing nature back into
our lives. COM(2020) 380 final, https://eur-lex.europa.eu/legal-
content/EN/TXT/?uri=celex:52020DC0380
European Commission, Joint Research Centre, Broothaerts, W., Jacchia, S., Angers, A. et al.,
2021a. New genomic techniques: state-of-the-art review, Publications Office, 2021,
https://data.europa.eu/doi/10.2760/710056
European Commission 2021b. Trade Policy Review - An Open, Sustainable and Assertive
Trade Policy. COM(2021) 66 final, https://eur-lex.europa.eu/legal-
content/EN/ALL/?uri=COM:2021:66:FIN
European Commission, 2021c. Study on the status of new genomic techniques under Union
law and in light of the Court of Justice ruling in Case C528/16. SWD (2021) 92 final,
https://food.ec.europa.eu/plants/genetically-modified-organisms/new-techniques-
biotechnology/ec-study-new-genomic-techniques_en
European Commission, 2021d. Forging a climate-resilient Europe - the new EU Strategy on
Adaptation to Climate Change, COM(2021)82 final, https://eur-lex.europa.eu/legal-
content/EN/TXT/?uri=CELEX:52021DC0082
European Commission, 2021e. Letter of Maroš Šefčovič, Vice-President of the European
Commission, to Augusto Santos Silva, Minister of Foreign Affairs of Portugal, the Portuguese
Presidency of the Council, https://food.ec.europa.eu/system/files/2021-04/gmo_mod-
bio_ngt_letter.pdf
European Commission, 2022a. Communication from the Commission to the European
Parliament, the European Council, the Council, the European Economic and Social
Committee and the Committee of The Regions, Safeguarding food security and reinforcing
the resilience of food systems. COM (2022) 133 final, https://eur-lex.europa.eu/legal-
content/EN/TXT/?uri=CELEX%3A52022DC0133&qid=1674640431587

European Commission, 2022b. Proposal for a Regulation of the European Parliament and of
the Council on the sustainable use of plant protection products and amending Regulation (EU)
2021/2115, COM (2022)305 final, 2022/0196 (COD), https://eur-lex.europa.eu/legal-
content/EN/TXT/?uri=CELEX%3A52022PC0305&qid=1674645473396
European Commission, 2023a. Drivers of food security, SWD(2023) 4 final,
https://commission.europa.eu/system/files/2023-
01/SWD_2023_4_1_EN_document_travail_service_part1_v2.pdf
European Commission, 2023b. Draft Commission Staff Working Document, Impact
assessment report, Proposal for the revision of the plant and forest reproductive material
legislation.
European Council of Young Farmers (CEJA), 2022. CEJA's position on New Genomic
Techniques (NGTs); https://wordpress.ceja.eu/wp-content/uploads/2022/12/FINAL-Position-
Paper-CEJAs-position-on-New-Genomic-Techniques-NGTs-3.pdf
European Food Safety Authority (EFSA), 2022a. Survey Data on New Genomic Techniques
(Dataset), NGT Flash Poll, https://zenodo.org/record/7081944#.Y5dbs57MKUn
European Food Safety Authority (EFSA), EFSA Panel on Genetically Modified Organisms,
2012. Scientific opinion addressing the safety assessment of plants developed through
cisgenesis and intragenesis. EFSA Journal 10(2):2561;
https://doi.org/10.2903/j.efsa.2012.2561
European Food Safety Authority (EFSA), EFSA Panel on Genetically Modified Organisms,
2020. Applicability of the EFSA Opinion on SDNs type 3 for the safety assessment of plants
developed using SDNs type 1 and 2 and oligonucleotide-directed mutagenesis. EFSA Journal
18(11):6299; https://doi.org/10.2903/j.efsa.2020.6299
European Food Safety Authority (EFSA), EFSA Panel on Genetically Modified Organisms,
2022b. Updated scientific opinion on plants developed through cisgenesis and intragenesis.
EFSA Journal 20(10):7621; https://doi.org/10.2903/j.efsa.2022.7621.
European Food Safety Authority (EFSA), EFSA Panel on Genetically Modified Organisms,
2022c. Criteria for risk assessment of plants produced by targeted mutagenesis, cisgenesis and
intragenesis. EFSA Journal 20(10): 7618; doi: https://doi.org/10.2903/j.efsa.2022.7618
European Food Safety Authority (EFSA). (September 2022). 2022 Eurobarometer on Food
Safety in the EU. https://www.efsa.europa.eu/en/corporate/pub/eurobarometer22
European Group on Ethics (EGE). (2021). Opinion on the Ethics if Genome Editing.
https://op.europa.eu/en/publication-detail/-/publication/6d9879f7-8c55-11eb-b85c-
01aa75ed71a1
European Network of GMO Laboratories (ENGL), 2019. Detection of food and feed plant
products obtained by new mutagenesis techniques. JRC116289, https://gmo-
crl.jrc.ec.europa.eu/doc/JRC116289-GE-reportENGL.pdf
European Network of GMO Laboratories, 2023. Detection of food and feed plant products
obtained by targeted mutagenesis and cisgenesis, Publications Office of the European Union,
Luxembourg, JRC133689, https://data.europa.eu/doi/10.2760/007925

Ferrari, L., Baum, C. M., Banterle, A. et al. 2021. Attitude and labelling preferences towards
gene-edited food: a consumer study amongst millennials and Generation Z. British Food
Journal, 123(3), 1268-1286.
Food and Agriculture Organisation of the United Nations (FAO), 2022. Gene editing and
agrifood systems. Rome. ISBN 978-92-5-137417-7, https://doi.org/10.4060/cc3579en
Food and Agriculture Organisation of the United Nations (FAO), 2023. Gene editing and food
safety – Technical considerations and potential relevance to the work of Codex Alimentarius.
Rome. https://doi.org/10.4060/cc5136en
Food Chain Evaluation Consortium, 2010. Evaluation of the EU legislative framework in the
field of GM food and feed. Final Report. https://food.ec.europa.eu/system/files/2016-
10/gmo_rep-stud_2010_report_eval-gm.pdf
Food Standards Agency, 2021. Consumers’ perception of genome edited food,
https://www.food.gov.uk/sites/default/files/media/document/consumer-perceptions-of-
genome-edited-food.pdf
Fried, G., Le Torre, V., Rakotoson, T. et al., 2022. Impact of new management practices on
arable and field margin plant communities in sunflower, with an emphasis on the abundance
of Ambrosia artemisiifolia (Asteraceae). Weed Research 62, 134-148,
https://doi.org/10.1111/wre.12522
Gabriel, A. & Menrad, K., 2015. Cost of Coexistence of GM and Non-GM Products in the
Food Supply Chains of Rapeseed Oil and Maize Starch in Germany. Agribusiness 31, 472 –
490.
García, M., García-Benítez, C., Ortego, F. et al., 2023. Monitoring Insect Resistance to Bt
Maize in the European Union: Update, Challenges, and Future Prospects. Journal of
Economic Entomology, (in press), https://doi.org/10.1093/jee/toac154
GENEinnovate. 2020. Norwegian consumers’ attitudes toward gene editing in Norwegian
agriculture and aquaculture. https://www.bioteknologiradet.no/filarkiv/2020/04/Report-
consumer-attitudes-to-gene-editing-agri-and-aqua-FINAL.pdf
GHK Consulting, 2011. Evaluation of the EU legislative framework in the field of cultivation
of GMOs under Directive 2001/18/EC and Regulation (EC) No 1829/2003, and the placing on
the market of GMOs as or in products under Directive 2001/18/EC. Final Report.
https://food.ec.europa.eu/system/files/2016-10/gmo_rep-stud_2011_report_cultivation.pdf
Goberna, M. F., Whelan, A. I., Godoy, P. et al., 2022. Genomic Editing: The Evolution in
Regulatory Management Accompanying Scientific Progress. Frontiers in Bioengineering and
Biotechnology 21, https://doi.org/10.3389/fbioe.2022.835378
González, F. G., Rigalli, N., Miranda, P. V. et al., 2020. An Interdisciplinary Approach to
Study the Performance of Second-generation Genetically Modified Crops in Field Trials: A
Case Study With Soybean and Wheat Carrying the Sunflower HaHB4 Transcription Factor.
Frontiers in Plant Science 11, 178. doi:10.3389/fpls.2020.00178
Government of Canada, 2022. Scientific opinion on the regulation of gene-edited plant
products within the context of Division 28 of the Food and Drug Regulations (Novel Foods).
Retrieved from: https://www.canada.ca/en/health-canada/services/food-nutrition/genetically-

modified-foods-other-novel-foods/scientific-opinion-regulation-gene-edited-plant-products-
within-context-division-28-food-drug-regulations.html
Grain Club, 2023. Joint press release, Survey: Consumers open to new biotechnological
methods in plant breeding. https://grain-
club.de/fileadmin/user_upload/Dokumente/Joint_Press_Release__Survey_on_New_Biotechn
ological_Methods_in_Plant_Breeding_and_Position_Paper_17.01.2023__1_.pdf
Grass, I., Batáry P. & Tscharntke, T., 2021. Combining land-sparing and land-sharing in
European landscapes. Advances in Ecological Research 64, 251-303.
https://doi.org/10.1016/bs.aecr.2020.09.002
Gundala, R.R. & Singh A., 2021. What motivates consumers to buy organic foods? Results of
an empirical study in the United States. PLoS One 16(9):e0257288. doi:
10.1371/journal.pone.0257288. PMID: 34506582; PMCID: PMC8432837.
Heap, I., 1993-2023. The International Herbicide-Resistant Weed Database. Accessed April
21, 2023; https:// www.weedscience.org
High Level Panel of Experts on Food Security and Nutrition (HLPE), 2020. Food security and
nutrition: building a global narrative towards 2030. A report by the High Level Panel of
Experts on Food Security and Nutrition of the Committee on World Food Security, Rome,
https://www.fao.org/3/ca9731en/ca9731en.pdf
Hu, Y., House, L. A., & Gao, Z., 2022. How do consumers respond to labels for crispr (gene-
editing)? Food Policy, 112, 102366.
IFOAM EU, 2014. Preventing GMO Contamination.
https://www.organicseurope.bio/content/uploads/2020/06/ifoameu_policy_gmos_dossier_201
412.pdf?dd
IFOAM EU, (2018) Practical guidelines: How to avoid GMOs contaminations.
https://www.organicseurope.bio/content/uploads/2020/06/ifoameu_policy_kgoof_guidelines_
20181205.pdf?dd
IFOAM EU & FiBL, 2017. Socio-economic impacts of GMOs on European Agriculture,
https://www.organicseurope.bio/content/uploads/2020/06/ifoameu_kgof_socioeconomicstudy
_printversion_20180417_web_0.pdf?dd
Ipsos Mori, Food Standards Agency (FSA), 2021. Consumers perception of genome edited
food; https://doi.org/10.46756/sci.fsa.aya629,
https://www.food.gov.uk/sites/default/files/media/document/consumer-perceptions-of-
genome-edited-food.pdf
ISAAA, 2019. Brief 55, Executive Summary, Global Status of Commercialized Biotech/GM
Crops in 2019: Biotech Crops Drive Socio-Economic Development and Sustainable
Environment in the New Frontier,
https://www.isaaa.org/resources/publications/briefs/55/executivesummary/pdf/B55-ExecSum-
English.pdf
ISAA, 2023a. Brazil and Colombia Approve First Drought Tolerant Gene-Edited Soybeans.
https://www.isaaa.org/kc/cropbiotechupdate/ged/article/default.asp?ID=19979

ISAA, 2023b. Japan Gives Nod to Genome-edited Waxy Maize.
https://www.isaaa.org/kc/cropbiotechupdate/article/default.asp?ID=20131
ISAA, 2023c. Tropic's Non-Browning Gene-Edited Banana Determined as Non-GMO in the
Philippines. https://www.isaaa.org/kc/cropbiotechupdate/article/default.asp?ID=20135
Jansky, S.H. & Spooner, D.M., 2018. The evolution of potato breeding. Plant Breeding
Reviews 41, 169-214
Jarvis, B.A., Romsdahl, T.B., McGinn, M.G. et al., 2021. CRISPR/Cas9-induced fad2 and
rod1 mutations stacked with fae1 confer high oleic acid seed oil in pennycress (Thlaspi
arvense L.). Frontiers in Plant Science 12, 652319. https://doi.org/10.3389/fpls.2021.652319
Jorasch, P. 2020. Potential, Challenges, and Threats for the Application of New Breeding
Techniques by the Private Plant Breeding Sector in the EU. Frontiers in Plant Science 11.
https://doi.org/10.3389/fpls.2020.582011
Jordan, N.R., Kuzma, J., Ray, D.K. et al., 2022. Should gene editing be used to develop crops
for continuous-living-cover agriculture? A multi-sector stakeholder assessment using a
cooperative governance approach. Frontiers in Bioengineering and Biotechnology, 10.
https://doi.org/10.3389/fbioe.2022.843093
Karavolias, N.G., Horner, W., Abugu, M.N. & Evanega, S.N., 2021. Application of Gene
Editing for Climate Change in Agriculture. Frontiers in Sustainable Food Systems 5,
https://doi.org/10.3389/fsufs.2021.685801
Karlson, D., Mojica, J.P., Poorten, T.J. et al., 2022. Targeted mutagenesis of the multicopy
Myrosinase gene family in allotetraploid Brassica juncea reduces pungency in fresh leaves
across environments. Plants 11, 2494; https://doi.org/10.3390/plants11192494
Kato-Nitta, N., Tachikawa, M., Inagaki, Y. et al., 2021. Public Perceptions of Risks and
Benefits of Gene-edited Food Crops: An International Comparative Study between the US,
Japan, and Germany. Science, Technology, & Human Values, 01622439221123830.
Kawall, K., 2021. The Generic Risks and the Potential of SDN-1 Applications in Crop Plants.
Plants 10, 2259; https://doi.org/10.3390/plants10112259
Kellerhals, M., Angstl, J., Pfammatter, W. et al., 2004. Portrait des variétés de pommes
résistantes à la tavelure. Revue Suisse Viticulture, Arboriculture et Horticulture 36, 29-36
Khan, J., 2021. European academic brain drain: A meta-synthesis. European Journal of
Education 56, 265–278, https://doi.org/10.1111/ejed.12449
Kock, M.A., 2019. Open Intellectual Property Models for Plant Innovations in the Context of
New Breeding Technologies. Agronomy 11(6), 1218;
https://doi.org/10.3390/agronomy11061218
Ku, H.-K. & Ha, S.-H., 2020. Improving nutritional and functional quality by genome editing
of crops: status and perspectives. Frontiers in Plant Sciemce 11: 577313, doi:
10.3389/fpls.2020.577313
Lassoued, R., Phillips, P.W.B., Smyth, S.J. et al., 2019. Estimating the cost of regulating
genome edited crops: expert judgment and overconfidence, GM Crops & Food 10, 44-62,
DOI: 10.1080/21645698.2019.1612689
Lemarié, S. & Marette, S., 2022. The socio-economic factors affecting the emergence and
impacts of new genomic techniques in agriculture: A scoping review. Trends in Food Science
& Technology 129, 38-48. https://doi.org/10.1016/j.tifs.2022.07.013
Lemmon, Z.H., Reem, N.T., Dalrymple, J. et al., 2018. Rapid improvement of domestication
traits in an orphan crop by genome editing. Nature Plants 4, 766–770.
https://doi.org/10.1038/s41477-018-0259-x
Li, J., Manghwar, H., Sun, L. et al., 2019. Whole genome sequencing reveals rare off-target
mutations and considerable inherent genetic or/and somaclonal variations in CRISPR/Cas9-
edited cotton plants. Plant Biotechnology Journal 17, 858–868. doi: 10.1111/pbi.1302
Lindberg, S. A., Peters, D. J., & Cummings, C. L. 2023. Gene‐Edited Food Adoption
Intentions and Institutional Trust in the United States: Benefits, Acceptance, and Labeling.
Rural Sociology.
Lu, C., Yu, Z., Hennessy, D.A. et al., 2022. Emerging weed resistance increases tillage
intensity and greenhouse gas emissions in the US corn–soybean cropping system. Nature
Food 3, 266-274. https://doi.org/10.1038/s43016-022-00488-w
Mammana I., 2014. The Concentration of Market Power in the EU Seed Market. Study
commissioned by the Greens/EFA Group in the European Parliament, https://www.greens-
efa.eu/files/assets/docs/concentration_of_market_power_in_the_eu_seed_market.pdf
Marangon, F., Troiano, S., Carzedda, M. et al., 2021. Consumers’ acceptance of genome
edited food and the role of information. Italian Review of Agricultural Economics, 76(3), 5-
21.
Michaelidou N. & Hasan, L.M., 2008. The role of health consciousness, food safety concern,
and ethical identity on attitudes and intentions toward organic food. International Journal of
Consumer Studies 32, 163–170.
Miller, J.K. & Bradford, K.J., 2011. The regulatory bottleneck for biotech specialty crops.
Nature Biotechnology 28, 1012-1014.
Modrzejewski, D., Hartung, F., Sprink, T. et al., 2019. What is the available evidence for the
range of applications of genome-editing as a new tool for plant trait modification and the
potential occurrence of associated off-target effects: A systematic map. Environmental
Evidence, 8(1)
National Academies of Sciences, Engineering, and Medicine, 2016. Genetically Engineered
Crops: Experiences and Prospects. The National Academies Press, Washington, DC;
https://doi.org/10.17226/23395
Norwegian Biotechnology Advisory Board 2018. Proposal for relaxation of Norwegian
regulations for deliberate release of genetically modified organisms (GMO), with applicability
also for EU legislation. https://www.bioteknologiradet.no/filarkiv/2019/03/2019-04-16-
Genteknologiloven-komplett-ENGELSK.pdf
Organisation for Economic Co-operation and Development 2018. Concentration in Seed
Markets: Potential Effects and Policy Responses. OECD Publishing, Paris.
https://doi.org/10.1787/9789264308367-en

Parisi, C. & Rodriguez Cerezo, E., 2021. Current and future market applications of new
genomic techniques, EUR 30589 EN, Publications Office of the European Union,
Luxembourg, 2021, ISBN 978-92-76-30206-3, doi:10.2760/02472, JRC123830,
https://publications.jrc.ec.europa.eu/repository/handle/JRC123830
Parisi, C. Tillie, P. & Rodriguez-Cerezo, E., 2016. The global pipeline of GM crops out to
2020. Nature Biotechnology 34, 31-36,
Paul, J. & Rana P., 2012. Consumer behavior and purchase intention for organic food. Journal
of Consumer Marketing 29, 412–422.
Pew Research Centre 2016. Americans’ views about and consumption of organic food. In The
New Food Fights: U.S. Public Divides Over Food Science. Available on-line
https://www.pewresearch.org/internet/wp-
content/uploads/sites/9/2016/11/PS_2016.12.01_Food-Science_FINAL.pdf
Purnhagen, K. & Wesseler, J., 2021. EU Regulation of New Plant Breeding Technologies and
Their Possible Economic Implications for the EU and Beyond. Applied Economic
Perspectives and Policy 43(4), 1621–1637, https://doi.org/10.1002/aepp.13084.
Raffan, S., Oddy, J., Mead, A. et al., 2023. Field assessment of genome edited, low
asparagine wheat: Europe's first CRISPR wheat field trial. Plant Biotechnology Journal.
https://doi.org/10.1111/pbi.14026
Ragonnaud G., 2013. The EU seed and Plant Reproductive Material Market in Perspective: A
Focus on Companies and Market Shares. Policy Department B: Structural and Cohesion
Policies. European Parliament Committee on Agriculture and Rural Development.
Rathenau Instituut, 2023. Editing under provision – Dutch citizens’ views on new genomic
techniques in food crops. Den Haag. Authors: Habets, M., I. Pirson, P. Macnaghten and P.
Verhoef. https://www.rathenau.nl/sites/default/files/2023-
04/Rapport%20Editing%20under%20provision.pdf
Regulation (EC) No 2100/94 of 27 July 1994 on Community plant variety rights (OJ L 227,
01/09/1994 P. 0001 – 0030), https://eur-lex.europa.eu/legal-
content/EN/ALL/?uri=CELEX%3A31994R2100
Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22
September 2003 on GM food and feed (OJ L 268 , 18/10/2003 P. 0001 – 0023), https://eur-
lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32003R1829&qid=1674643411750
Regulation (EC) No 1830/2003 of the European Parliament and of the Council of 22
September 2003 concerning the traceability and labelling of genetically modified organisms
and the traceability of food and feed products produced from genetically modified organisms
and amending Directive 2001/18/EC (OJ L 268, 18.10.2003, p. 24–28), https://eur-
lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32003R1830&qid=1674643740773
Regulation (EC) No 1946/2003 of the European Parliament and of the Council of 15 July
2003 on transboundary movements of genetically modified organisms (OJ L 287, 5.11.2003,
p.1–10), https://eur-lex.europa.eu/legal-
content/EN/TXT/?uri=CELEX%3A32003R1946&qid=1674644039551
Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on
organic production and labelling of organic products and repealing Council Regulation (EC)

No 834/2007 (OJ L 150, 14.6.2018, p. 1–92), https://eur-lex.europa.eu/legal-
content/EN/TXT/?uri=CELEX:32018R0848
Regulation (EU) 2021/1119 of the European Parliament and of the Council of 30 June 2021
establishing the framework for achieving climate neutrality and amending Regulations (EC)
No 401/2009 and (EU) 2018/1999 (OJ L 243, 9.7.2021, p. 1–17), https://eur-
lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R1119&from=EN
Phalan, B.T., 2018. What have we learned from the land sparing-sharing model?
Sustainability 10, 1760, https://doi.org/ 20.3390/su10061760
Republic of South Africa, 2021. Public Notice. South Africa’s approach for new breeding
techniques.
http://www.old.dalrrd.gov.za/doc/Notice%20SA's%20regulatory%20approach%20for%20NB
T's%202021.pdf
Ryan, C. D., & Smyth, S.J., 2012. Economic implications of low-level presence in a zero-
tolerance European import market: the case of Canadian Triffid Flax. AgBioForum 15,21–30.
Ryan, C.D. & Smyth, S.J., 2019. Transgenic Flax and the Triffid Affair. Pp. 249-260 in:
Cullis, C.A. (ed.) Genetics and Genomics of Linum. Springer Nature Switzerland
Saleh, R., Bearth, A., & Siegrist, M. (2021). How chemophobia affects public acceptance of
pesticide use and biotechnology in agriculture. Food Quality and Preference, 91, 104197.
Sánchez, B., Barro, F., Smulders, M. J. M. et al. 2023. Socioeconomic impact of low-gluten,
celiac-safe wheat developed through gene editing, EUR 31380 EN, Publications Office of the
European Union, Luxembourg; https://doi.org/10.2760/280847
Sankula, S., Marmon, G. & Blumenthal, E., 2015. Biotechnology-derived crops planted in
2004 - impacts on US agriculture.
http://ucbiotech.org/biotech_info/PDFs/Sankula_2005_BiotechnologyDerivedCropsPlantedin
2004.pdf
Schneider, K., Barreiro-Hurle, J., Kessel, G. et al., 2023a. Economic and environmental
impacts of disease resistant crops developed with cisgenesis. EUR 31355, Publication Office
of the European Union, Luxembourg; https://doi.org/10.2760/715646
Schneider, K. Barreiro-Hurle, J., Kessel, G. et al., 2023b. Insights on cisgenic plants with
durable disease resistance under the European Green Deal. Trends in Biotechnology.
https://doi.org/10.1016/j.tibtech.2023.02.005 (in press)
Schütte, G., Eckerstorfer, M., Rastelli, V. et al., 2017. Herbicide resistance and biodiversity:
agronomic and environmental aspects of genetically modified herbicide-resistant plants.
Environ. Sci. Eur. 29, 5., https://doi.org/10.1186/s12302-016-0100-y
Sendhil, R., Nyika, J., Yadav, S. et al., P. 2022. Genetically modified foods: Bibliometric
analysis on consumer perception and preference. GM Crops & Food, 13(1), 65-85,
https://doi.org/10.1080/21645698.2022.2038525
Sikkema A. (2021). Citizen’s jury wants gene technology, but subject to conditions. Resource
September 2021. https://www.resource-online.nl/index.php/2021/09/09/citizens-jury-wants-
gene-technology-but-subject-to-conditions/?lang=en

Singh, A. & Verma, P., 2017. Factors influencing Indian consumers’ actual buying behavior
toward organic food products. Journal of Clean Production 167: 473–483
Smyth, S.J., 2017. Genetically modified crops, regulatory delays, and international trade.
Food and Energy Security, 6, 78–86.
Solfanelli, F., Ozturk, E., Zanoli, R. et al., 2021. The state of organic seed in Europe.
https://www.liveseed.eu/wp-content/uploads/2021/03/Booklet2-LIVESEED_2021_web.pdf
Son, E., & Lim, S. S. 2021. Consumer acceptance of gene-edited versus genetically modified
foods in Korea. International journal of environmental research and public health, 18(7),
3805.
Special Eurobarometer 505 – Wave EB93.2 – Kantar, December 2020, Making our food fit
for the future – Citizens’ expectations, https://europa.eu/eurobarometer/surveys/detail/2241
Special Eurobarometer 520 – Kantar, 2022, Europeans, Agriculture and the CAP,
https://agriculture.ec.europa.eu/common-agricultural-policy/cap-overview/cap-
glance/eurobarometer_en
Sprink, T., Wilhelm, R., & Hartung, F. 2022. Genome editing around the globe: An update on
policies and perceptions. Plant Physiology, 190(3), 1579-1587,
https://doi.org/10.1093/plphys/kiac359
Strobbe, S., Wesana, J., van der Straeten, D. et al., 2023. Public acceptance and stakeholder
views of gene edited foods: a global overview. Trends in Biotechnology (in press),
https://doi.org/10.1016/j.tibtech.2022.12.011.
Su, Y., Khaskheli, A., Raza, S. A., & Yousufi, S. Q. 2022. How health consciousness and
social consciousness affect young consumers purchase intention towards organic foods.
Management of Environmental Quality 33, 1249-1270, https://doi.org/10.1108/MEQ-12-
2021-0279
Sun, Y., Jiao, G., Liu, Z. et al., 2017. Generation of High-Amylose Rice through
CRISPR/Cas9-Mediated Targeted Mutagenesis of Starch Branching Enzymes. Frontiers in
Plant Science 8, 298, https:/doi.org/10.3389/fpls.2017.00298
Swedish Gene Technology Advisory Board, 2021. Survey of attitudes among Swedish people
towards applications of genome editing in agriculture. https://www.genteknik.se/wp-
content/uploads/2022/02/Svenskars-installning-till-
genomredigering_2022.pdf?fbclid=IwAR0lgIqrgz-KI-
wH1Vb2YHp6252VP4magr9g22t_O_mx-UHZ0-SfrB-ylBY
Tang, X., Liu, G., Zhou, J. et al., 2018. A large-scale whole-genome sequencing analysis
reveals highly specific genome editing by both Cas9 and Cpf1 (Cas12a) nucleases in rice.
Genome Biology 19, 84. https://doi.org/10.1186/s13059-018-1458-5.
Technopolis Group, Aracadia Int. & Wageningen UR, 2023. Study to support the impact
assessment of legislation for plants produced by certain new genomic techniques. Publication
Office of the European Union, Luxembourg; https://doi.org/10.2875/282347
Testbiotech, 2022. New genomic techniques (NGTs): agriculture, food production and crucial
regulatory issues. https://www.vzbv.de/sites/default/files/2022-11/vzbv-report_final_final.pdf

The Greens/EFA 2021. GMO survey data. http://extranet.greens-
efa.eu/public/media/file/1/6912
Torjusen, H., Sangstad, L., O’Doherty Jensen, K. et al., 2004. European Consumers'
Conceptions of Organic Food: A Review of Available Research. Professional Report 4-2004,
National Institute for Consumer Research, Olso. Available online
https://orgprints.org/id/eprint/2490/1/haccprapport.pdf
United States Department of Agriculture (USDA), Animal and Plant Health Inspection
Service (APHIS), 12 August 2020. Letter of Bernadette Juarez, APHIS Deputy Administrator,
Biotechnology Regulatory Services, USDA, APHIS, to Dan Jenkins, Regulatory Strategy and
Quality Lead. https://www.aphis.usda.gov/biotechnology/downloads/reg_loi/20-168-
07_air_response_signed.pdf.
United States Department of Agriculture Foreign Agricultural Service (USDA), 2022. MARA
Issues First Ever Gene-Editing Guidelines, CH2022-0015,
https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=M
ARA%20Issues%20First%20Ever%20Gene-Editing%20Guidelines_Beijing_China%20-
%20People%27s%20Republic%20of_01-26-2022
Van de Wiel, C.C.M., Bouwman, L.M.S., Kleter, G.A. et al., 2022. An exploration o the
potential contribution of genetic modification and genome editing to the development of
abiotic stress-tolerant crops as compared to conventional breeding. CGM 2022-4
Onderzoeksrapport, https://cogem.net/app/uploads/2022/12/CGM-2022-4-GE-crops-and-
abiotic-stress-tolerance.pdf
Vasquez, O., Hesseln, H., & Smyth, S. J. 2022. Canadian consumer preferences regarding
gene-edited food products. Frontiers in Genome Editing 4,
https://doi.org/10.3389/fgeed.2022.854334
Venezia, M. & Creasey Krainer, K.M., 2021. Current Advancements and Limitations of Gene
Editing in Orphan Crops. Frontiers in Plant Science 12, 742932,
https://doi.org/10.3389/fpls.2021.742932
Venus, T.J., Dillen, K., Punt, M.J. et al. 2017. The costs of coexistence measures for
genetically modified maize in Germany. Journal of Agricultural Economics 68, 407-426.
https://doi.org/10.1111/1477-9552.12178
Vögeli, G.A., Wolf, D. & Lips, M. 2010. Coexistence costs for genetically modified crops in
Switzerland. Food Economics – Acta Agricult. Scand. C 7: 227-233
Voss-Fels, K.P., Stahl, A., Wittkop, B. et al. 2019. Breeding improves wheat productivity
under contrasting agrochemical input levels. Nature Plants 5, 706–714,
https://doi.org/10.1038/s41477-019-0445-5
Waltz, E. 2021. GABA-enriched tomato is first CRISPR-edited food to enter market. Nature
Biotechnology 40, 9–11, https://doi.org/10.1038/d41587-021-00026-2.
Wedger, M.J., Roma-Burgos, N. & Olsen, K.M., 2022. Genomic revolution of US weedy rice
in response to 21st century agricultural technologies. Communications Biology 5, 885,
https://doi.org/10.1038/s42003-022-03803-0
Wesseler, J., Jongeneel, R., & Purnhagen, K., 2019. Bioeconomy economics and policies. In
L. Dries, W. Heijman, R. Jongeneel, K. Purnhagen, & J. Wesseler (Eds.), EU bioeconomy

economics and policies (Vol. I. Palgrave Advances in Bioeconomy: Economics and Policies).
Palgrave Macmillan. https://doi.org/10.1007/978-3-030-28634-7_2
Whelan, A. I., Gutti, P. & Lema, M. A., 2020. Gene Editing Regulation and Innovation
Economics. Frontiers in Bioengineering and Biotechnology 8,
https://doi.org/10.3389/fbioe.2020.00303
WIPO, 2019. World Intellectual Property Report 2019. The Geography of Innovation: Local
Hotspots, Global Networks. Geneva: World Intellectual Property Organization.
https://www.wipo.int/edocs/pubdocs/en/wipo_pub_944_2019.pdf
WTO, 2020. International statement on agricultural applications of precision biotechnology.
G/SPS/GEN/1658/Rev.4
https://docs.wto.org/dol2fe/Pages/SS/directdoc.aspx?filename=q:/G/SPS/GEN1658R4.pdf
Yaqoob, H., Tariq, A., Bhat, B.A. et al., 2023. Integrating genomics and genome editing for
orphan crop improvement: a bridge between orphan crops and modern agriculture system.
GM Crops & Food 14, 1-20, https://doi.org/10.1080/21645698.2022.2146952
YouGov, 2022. Beyond GM. Survey of UK adults preferences on GMOs.
https://docs.cdn.yougov.com/2wqpwz0eid/BeyondGM_Results_221112_W.pdf
Zanetti, F., Alberghini, B., Jeromela, A. M. et al., 2021. Camelina, an ancient oilseed crop
actively contributing to the rural renaissance in Europe. A review. Agronomy for Sustainable
Development 41, 1–18. https://doi.org/10.1007/s13593-020-00663-y.
Zimney, T. & Sowa, S., 2021. Potential effects of asymmetric legal classification of gene
edited plant products in international trade, from the perspective of the EU. EFB Bioeconomy
Journal 1, 100016, https://doi.org/10.1016/j.bioeco.2021.100016

91
ANNEX 1: PROCEDURAL INFORMATION
Lead DG: European Commission, Directorate-General for Health and Food Safety, DG
SANTE
Decide reference: PLAN/2021/11456
The development of this initiative was announced under item 4) in Annex 1 to the
Commission Work Programme 2023
1
.
Organisation and timing
The inception impact assessment (IIA) on the “Legislation for plants produced by certain
new genomic techniques” initiative
2
was available for feedback in the period 24 September
2021 - 22 October 2021. The IIA outlined the initiative’s context and aim, problems and
objectives to address them, policy elements to be considered for the development of policy
options, whilst also discussing expected impacts and presenting the evidence base.
Nine Inter-service Steering Group (ISSG) meetings were held between September 2021 and
February 2023, including participation from the Secretariat General, the Legal Service, the
Joint Research Centre (JRC) and Directorates-General: AGRI, CLIMA, COMP, ENV,
GROW, MARE, RTD and TRADE.
1. Consultation of the RSB
The file benefitted from an upstream meeting with the Regulatory Scrutiny Board (RSB) on
20 May 2022. A first version of this Impact Assessment Report was submitted to the RSB on
15 February 2023, the meeting took place on 15 March 2023 and the RSB written report was
received on 17 March 2023. The Board’s overall opinion was negative based on the
following findings:
(1) The report does not present a clear, consistent, and hierarchical set of general and specific
objectives.
(2) The report does not describe in sufficient detail what the main elements of the options and
the key policy choices are.
(3) The report does not sufficiently assess the impact on consumer trust, the organic sector,
the environment and health. It does not present a comprehensive overview of the costs and
benefits.
(4) The report does not provide a comprehensive assessment of all relevant (combinations of)
options in terms of effectiveness, efficiency and coherence.
The table below lists the changes in response to the recommendations of the RSB in its
opinion. In addition, targeted corrections and amendments have been included in the new
version of the impact assessment report to address the technical comments provided by the
RSB to DG SANTE.
1
https://ec.europa.eu/info/sites/default/files/com_2022_548_1_annexe_en.pdf
2
https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13119-Legislation-for-plants-
produced-by-certain-new-genomic-techniques/feedback_en?p_id=26519622
92
Table 1: Recommendations of the RSB and modifications made in the impact assessment report
RECOMMENDATIONS OF THE RSB
MODIFICATIONS IN THE IMPACT
ASSESSMENT REPORT IN RESPONSE TO
THESE RECOMMENDATIONS
(1) The report should present a more comprehensive
overview of the current context including how NGTs
are being developed at global level, the and the
implications for sustainability and for EU strategic
autonomy and competitiveness. It should better
explain the magnitude of the problems and
consequences identified.
Section 1.1 has been revised to better explain the
scientific context (safety profile of NGT plants and
stakeholder positions). Section 1.2 in the political
context has been revised to include aspects of
sustainability and strategic autonomy and to provide
an overview of recent social science research and
surveys on consumer attitudes towards NGT products.
Section 2.2 has been expanded and revised to explain
better the size of the problem and who is affected.
(2) The report should present a clear, consistent, non
overlapping and hierarchical set of general and
specific objectives. It should more clearly outline
what the substantive key objectives of this initiative
are. In particular, it should explain in more detail the
significance of the sustainability objective, and
whether this is a new objective requiring the revision
of the Directive. The report should clarify to what
extent EU strategic autonomy, including food
security, is a key objective of this initiative based on
the problems identified. The report should further
improve the link between the problems and
objectives. It should, clarify what objectives remain
the same (e.g. human and animal health and
environment in accordance with precautionary
principle) and which ones emerge from the identified
problems.
In section 4, general and specific objectives have been
revised, clarifying which objectives are shared with
the current GMO legislation and which are new, and
explaining further the sustainability objective. Links
between problems and objectives have been revised.
Relationship between general and specific objectives
has been clarified (section 4, figure 2). The
Intervention logic (Annex 10) has been updated.
(3) The report should more fully describe the main
elements of the options and explain who are the actors
determining, implementing, and enforcing them. It
should clearly outline how the notification regime and
adapted risk assessment would work, what parameters
would apply, who would decide, and what follow-up
would be ensured. The report should set out how the
sustainability objective is addressed in each option,
and on whether there are further alternative elements
or combinations of options. It should provide more
detail on what the regulatory incentives and the
different criteria presented would look like in their
final form. It should provide a comprehensive
explanation of why the choice of labelling
requirement differs across the identified options. The
report should be clear on the policy choices and trade-
offs, and to how they are addressed in the policy
options. In addition, the report should clarify the
approach retained in the preferred option as regards
the organic sector.
In Section 5, options have been explained more fully,
including actors involved, how notification and
authorisation criteria and procedures will work,
approaches to sustainability and to labelling in each
option, and concrete details on incentives considered
in option 2.
The different choices for each key element (risk
assessment, traceability, labelling/transparency,
sustainability) have been presented in figure 3 and
policy options in figure 4. Explanation has been
provided on the choice of combined options.
In section 5.2.4, the two policy approaches for the
organic sector for option 4 have been added.
New section 5.2.5 has been added to explain why
option 4 was designed to apply only to certain NGT
products and, if retained, must be combined with
options 1, 2, 3 or the baseline.
In section 5.3 an explanation has been added on why
other combinations have not been pursued.
(4) The report should further develop the assessment
of the impact on health, environment, consumer trust
and the organic sector. Concerning health and
environment, the report should provide a more
balanced analysis accounting for likely environmental
and social benefits as well as possible risks for the
environment and for human and animal health and
how they will be monitored and mitigated. It should
also explain how the impacts on sustainability are
In section 6 impacts on health and environment have
been revised to explain further what the achievement
of the objective means (assessment of options by
comparison to baseline; NGT to be demonstrated to
be as safe as conventional counterpart); further
explanations have been added on the distinction
between the safety aspects associated with the genetic
modification and other potential impacts resulting
from certain traits (irrespectively on the technique
93
assessed. The report should provide further evidence -
coming from recent social science research and
surveys - on consumer attitudes towards NGT
products and assess how consumer trust may impact
uptake of future NGT products. It should discuss the
risk that benefits might not materialise as a result of
lack of consumer trust. The report should further
develop the analysis of the impacts on the organic
sector including quantification of the costs for this
sector.
used).
In sections 6 and 7, the analysis of impacts on the
organic sector has been considerably developed based
on available evidence on coexistence between GMOs
and conventional and organic farming (e.g. the work
of the European Co-existence Bureau established by
the Commission, further literature). The implications
of the two scenarios for the treatment of notified
NGTs (option 4) in organic farming are further
discussed.
The sections “Impacts on consumers” under each
option in section 6 and in section 7 have been revised
to elaborate further on how the different options may
affect consumer uptake and trust.
(5) The report should present a clear and
comprehensive overview of the costs and benefits for
each option. It should better describe the uncertainties
and limitations of the analysis based on hypothetical
scenarios. It should further explain the credibility and
reliability of the wide ranges of estimates presented.
In section 7.2 the cost analysis for each option was
revised to include costs of coexistence and
segregation measures for farmers and food business,
however, due to limited data, those costs were not
quantifiable.
Regarding costs for the preferred option, Section 8.2.
and Annex 3 were further developed to ensure a clear
explanation of the hypothetical scenario for the future
number of authorisations and notifications taken into
consideration for the overall cost calculation.
(6) The report should provide a better comparison of
options, with a consideration of different
combinations of options. It should identify all relevant
combinations upfront and assess and compare them
along the individual options. The effectiveness
analysis should be based on the revised set of specific
objectives, avoiding any double counting. The
comparison summary table should be critically
reviewed to remove overlaps and inconsistencies. The
efficiency analysis should include quantified and
monetised cost and benefit estimates. The used
scoring methodology should be better explained, and
the individual scores better justified. The report
should provide a clear assessment of coherence, in
particular in light of concerns expressed by
stakeholders as regards Farm to Fork and the role of
organic farming.
As regards combinations of options, see (3).
In section 7.1 and 7.2, effectiveness and efficiency
analysis have been revised, making sure, in particular,
that no criteria are double-counted or overlap. The
efficiency analysis includes quantified and monetised
costs and benefits wherever this information is
available. All scores are explained in the detailed
comparison of options in section 7.1. In section 7.3.,
the coherence analysis has been complemented
regarding 25% organic target laid down in the Farm
to Fork Strategy.
A revised version of the Impact Assessment Report was submitted to the RSB on 25 April
2023 for a final opinion. The Board concluded with a positive opinion with reservations on 26
May 2023. The table below lists the changes in response to the recommendations of the RSB
in its second opinion.
Table 2: Recommendation of the RSB and modifications made in the impact assessment report
RECOMMENDATIONS OF THE RSB
MODIFICATIONS IN THE IMPACT
ASSESSMENT REPORT IN RESPONSE TO
THESE RECOMMENDATIONS
(1) The report should provide further information on
the risk assessment via the notification procedure
retained in the preferred option. It should better
explain how the procedure will ensure that the NGTs
covered are plants that could also occur naturally or
be produced by conventional breeding. It should
describe in more detail the key elements of the pre-
The notification criteria in Box 2 in section 5.2. have
been updated and clarified, scientific considerations
underpinning the criteria have been added and a
diagram illustrating how the notification procedure
would work in practice has been added to Annex 11.

94
determined notification criteria, their scientific basis
and their implementation in practice.
(2) As regards the use in organic production of NGT
plants/products fulfilling the notification criteria, the
report should be clear that the two scenarios presented
are in fact policy sub-options. It should indicate the
preferred sub-option as regards use in organic
production and if no preferred sub-option is chosen, it
should clearly state this, together with an explanation
why. The policy choices, implications and impacts of
each sub-option should be explained, including under
what circumstances notified NGT plants/products
could be used or not.
The two scenarios concerning the use of notified NGT
products in organic production are now presented as
sub-options. In section 8.1., the report now clarifies
the preferred sub-option. The implications of the sub-
options have been further explained in section 5.2.4.
(3) As regard the option “authorisation with
incentives for products containing modified traits that
have the potential to contribute to sustainability”, the
report should be clear on the retained sub-options for
labelling. It should also clarify how the label would
identify those NGT as “product of biotechnology”.
Section 8.1. of the report is further clarified as regards
the retained sub-option on labelling.
(4) The report should clearly present the reasons
behind wide ranges such as ‘up to 85%’ cost savings
on the risk assessment, and provide further
explanations of circumstances under which breeders
might receive no savings.
The report now clearly identifies in the impact
analysis (e.g. section 6.2.1.) the situations in which no
or small and large cost savings are expected, and
further explains the reasons for the ranges.
(5) The report should clarify the efficiency analysis.
While the report includes elements of costs
quantification, in particular on coexistence, based on
relevant projects and studies, including the support
study, it should clarify how these available cost
quantifications should be taken into account in the
efficiency analysis of options and in the overview of
benefits and costs, referring, if appropriate, to
uncertainties and data limitations. It should explain
why the aggregate cost for option on “authorisation
with incentives for products containing modified traits
that have the potential to contribute to sustainability”
and option on “authorisation with the requirement that
products do not contain modified traits that can be
detrimental to sustainability” are identical when the
latter introduces an additional ‘trait-specific’
requirement described as the most demanding for
operators. The report should explain why the savings
on incentives are excluded from the efficiency
analysis.
Table 8 and the accompanying footnote 214 in section
7.2., addressing the baseline with a limited extent of
NGT cultivation, now clarifies that available data on
coexistence costs are too limited and uncertain to
derive a quantitative estimate of expected future costs.
The same consideration applies to Tables 9-11. In
Table 12, addressing an option under which more
extensive NGT cultivation is expected, an
unquantified increase in coexistence costs is assumed.
The costs for an assessment of the sustainability
contribution of a trait or of showing that a trait is not
detrimental to sustainability are assumed to be same.
The fact that demonstrating the sustainability
contribution of a trait is voluntary is reflected in
footnote 222, which gives an estimate of the
aggregate cost if the sustainability contribution is not
assessed.
The savings arising from incentives are included in
Annex 3. They are not included in the efficiency
analysis in section 7 as the monetary benefits are
marginal in comparison to the other quantified costs
and benefits.
2. Evidence, sources and quality
External expertise
A consortium led by Technopolis developed a study that contributed to the preparation of
this IA. The study started in March 2022. The consortium provided support in gathering and
analysing evidence, in conducting consultation activities (e.g., targeted consultation of
stakeholders, organisation of focus groups, analysis of stakeholder feedback, interviews of
stakeholders) and a combination of approaches to assess the economic, social and
environmental impacts of the policy options (including case studies, economic modelling,
modelling of environmental impacts and multicriteria analysis).

95
Consultation activities
The IA takes into account the input from a range of stakeholder consultation activities. A
public consultation
3
was held between 29 April and 22 July 2022 (2300 contributions). It
was complemented by a range of targeted consultation activities:
Stakeholder interviews (25) to receive input on the initiative and on the impacts of
policy options, including on costs.
A targeted survey (from 28 June to 05 September 2022) to which 397 stakeholders
and Member State authorities were invited. 123 responded and self-categorised as
business associations (32), NGOs, environmental, consumer and other civil society
organisations (28), public authority/body (23), large company/business (11),
academic/research organisation (9), SMEs (8), other (12).
Two expert focus groups on sustainability and traceability on 22 and 23 September
2022.
Regulatory cost interviews (23) to map regulatory costs of operators developing and
marketing NGT plants as well as to assess costs of risk assessment and enforcement
authorities.
Annex 2 summarises feedback from stakeholder consultations.
Other studies and sources
JRC case studies to analyse the potential economic, environmental and social (health)
impacts of selected NGT plants that are in the development pipeline
4
, conducted for
the purposes of this impact assessment; the impact assessment also relies on the two
JRC reports (on market applications
5
and latest scientific developments relating to
NGTs
6
) supporting the Commission NGT study
7
.
EFSA. Two mandates were given to support this impact assessment (statement on
criteria for risk assessment
8
and update of EFSA’s 2012 opinion on cisgenesis
9
.
3
https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13119-Legislation-for-plants-
produced-by-certain-new-genomic-techniques/public-consultation_en
4
Sánchez, B., Barro, F., Smulders, M. J. M., Gilissen L. J. W. J., Rodríguez Cerezo, E. (2023) Socioeconomic
impact of low-gluten, celiac-safe wheat developed through gene editing, EUR 31380 EN, Publications Office of
the European Union, Luxembourg, Schneider, K.; Barreiro-Hurle, J.; Kessel, G.; Schouten, H.J.; Vossen, J.;
Strassemeyer, J.; Rodriguez-Cerezo, E. (2023). Economic and environmental impacts of disease resistant crops
developed with cisgenesis. EUR 31355, Publication office of the European Union, Luxembourg., Schneider, K.;
Barreiro-Hurle, J.; Vossen, J.; Schouten, H.J.; Kessel, G.; Andreasson, E.; Phuong Lieu, N.; Strassemeyer, J.;
Hristov, J.; Rodriguez-Cerezo, E. (2023). Insights on cisgenic plants with durable disease resistances under the
European Green Deal. Trends in Biotechnology
5
Parisi, C. and Rodriguez Cerezo, E., Current and future market applications of new genomic techniques, EUR
30589 EN, Publications Office of the European Union, Luxembourg, 2021, ISBN 978-92-76-30206-3,
doi:10.2760/02472, JRC123830.
6
Broothaerts, W., Jacchia, S., Angers, A., Petrillo, M., Querci, M., Savini, C., Van den Eede, G. and Emons, H.,
New Genomic Techniques: State-of-the-Art Review, EUR 30430 EN, Publications Office of the European
Union, Luxembourg, 2021, ISBN 978-92-76-24696-1, doi:10.2760/710056, JRC121847 European
Commission, Directorate-General for Research and Innovation, New techniques in agricultural biotechnology,
Publications Office, 2017, https://data.europa.eu/doi/10.2777/574498
7
Study on the status of new genomic techniques under Union law and in light of the Court of Justice ruling in
Case C528/16. SWD (2021) 92 final
8
EFSA Panel on Genetically Modified Organisms, 2022. Statement on criteria for risk assessment of plants
produced by targeted mutagenesis, cisgenesis and intragenesis. EFSA Journal 2022;20(10):7618, 12 pp.
https://doi.org/10.2903/j.efsa.2022.7618

96
Other, previous relevant EFSA opinions (referenced in Annex 6) also underpin tis
impact assessment.
Desk research, including peer-reviewed literature, scientific reports and grey
literature
Position papers
European Commission fact-finding studies on Member States official controls
10
Limitations and robustness of findings
The data collection and analysis carried out have some intrinsic limitations, key among those
the lack of historical data on the cultivation and commercial use of plants produced by
targeted mutagenesis and cisgenesis, as the first of these products have only recently reached
the markets of non-EU countries, and there is no experience within the EU. The impacts of
such limitations on the robustness of findings have been mitigated to a maximum possible
extent utilising a number of analytical approaches such as specific case studies, modelling,
extrapolation from third countries etc.
9
EFSA Panel on Genetically Modified Organisms, 2022. Updated scientific opinion on plants developed
through cisgenesis and intragenesis. EFSA Journal 2022;20(10):7621, 33 pp.,
https://doi.org/10.2903/j.efsa.2022.7621
10
https://ec.europa.eu/food/audits-analysis/audit-report/details/4543
https://ec.europa.eu/food/audits-analysis/audit-report/details/4544

ANNEX 2: STAKEHOLDER CONSULTATION (SYNOPSIS
REPORT)
1. Introduction
This report provides an overview of the consultation activities carried out in the context
of the impact assessment (IA) of legislation for plants produced by certain new genomic
techniques (NGTs). It provides an outline of the consultation strategy, lists the
consultation activities and provides key results for each of them.
The following consultations took place:
Feedback on the Commission’s Inception Impact Assessment
1
(24 September
2021 - 22 October 2021);
Commission’s public consultation (PC) (29 April 2022 - 22 July 2022)
2
;
Targeted stakeholder survey (28 June 2022 – 05 September 2022);
Interviews (June 2022 – December 2022);
Focus groups on sustainability and traceability ((22 and 23 September 2022
respectively).
The first two consultations were carried out by the Commission services (but the external
contractor analysed the replies to the PC); the last three consultations were carried out
entirely in the context of the external study underpinning this IA.
The key objectives of the consultations were:
Ensure that the public and all relevant stakeholders were given the opportunity to
contribute to the policy initiative by providing informed insights.
Obtain relevant information, views and expectations on the problems presented,
the baseline scenario, and on the potential ways forward including the key
components of the policy options (risk assessment, sustainability, labelling,
traceability).
Obtain evidence, views and expectations on the expected key impacts (economic,
social, environmental).
The following key stakeholder groups were identified as targeted audience in the
consultation strategy of the initiative:
The general public (EU and non-EU);
Operators active, from farm to fork, in the agri-food and feed system, including
sectors such as farmers; seed and plant breeders; traders; processors;
manufacturers, retailers and food services; GM-free and organic operators in all of
the above sectors.
Operators of plant and bio-based industries active in sectors other than the agri-
food sector, including ornamental plants, forestry and industrial biotechnology.
Academic and research stakeholders active in the field of biotechnology in
general and agricultural/plant biotechnology in particular.
1
https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13119-Legislation-for-
plants-produced-by-certain-new-genomic-techniques_en
2
https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13119-Legislation-for-
plants-produced-by-certain-new-genomic-techniques/public-consultation_en

98
Civil society/non-governmental organisations with interest in the topic, including
environmental, grass-root farming and consumer organisations.
EU institutions, European Food Safety Authority (EFSA), Member State and third
country authorities
Other stakeholders, such as consultancies and think tanks active or with interest
on the topic.
2. Methodology of the consultation activities
a) Feedback on the Commission’s Inception Impact Assessment (IIA)
The initiative was published on the Commission’s Have Your Say
3
website. The IIA
included the context, problem definition and subsidiarity check, presented the objectives
and the main components of the policy options, contained a preliminary assessment of
expected main impacts and referred to the evidence base and data collection.
70,894 contributions were received overall; 98% (69,414) of the replies were identified as
coming from campaigns. The non-campaign replies amounted to 2% of the total (1,480).
According to self-categorisation, most contributions in the non-campaign replies came
from citizens (70%, 1,030 replies), followed by business organisations/associations, trade
unions (14%, 203), academia/research institutions (8%, 115), NGOs and
consumer/environmental organisations (5%, 81), public authorities (1%, 9) and others
(3%, 42). Contributions (both campaign and non-campaign) originated from 91 countries,
including the 27 Member States and 64 non-EU countries. Top contributions from
Member States were from Germany (32,694), France (25,544), Belgium (2,732),
Netherlands (2,251) and Austria (2,111). Most contributions from non-EU countries
came from Switzerland (782), followed by United Kingdom (759), USA (228), Argentina
(142) and Canada (114). The large number of contributions shows the high interest of
citizens and stakeholders in the Commission’s policy initiative.
b) Public consultation (PC)
The PC was published on the Commission’s Have Your Say
Error! Bookmark not defined.
website. The survey included 11 closed questions with branching sub-questions and open
text sections (with 500-800 characters). Furthermore, 7 questions were included as open
questions (with 1500 characters). These 18 questions in the PC were analysed with
quantitative and qualitative methods.
Overall, 2300 contributions were submitted, out of which 104 were identified as
campaigns and 2196 as individual contributions. The bulk of the contributions came from
23 EU Member States (MS), with three-quarters of total consultation respondents coming
from Germany (599; 27.3%), (Italy 515; 23.5%), France (335; 15.3%), and Spain (194;
8.8%). There were no responses from stakeholders from Cyprus, Luxembourg, Malta or
Slovenia. There were 105 respondents from outside the EU and they account for 4.9% of
the total. They came from Switzerland (33), the United States (17), the United Kingdom
(15), and 40 contributions from 25 other countries. Most respondents self-identified as
‘EU citizen’ (1491; 65.1%), followed by academic/research institutions (206; 9.0 %),
company/business organisations (179; 7.8%) and business associations (122; 5.3%), non-
governmental organisations (NGOs - 81; 3.7%), non-EU citizens (38; 1.7%), public
authorities (35; 1.5%), environmental organisations (20; 0.9%), trade unions (14; 0.6%),
consumer organisations (5; 0.2%), and others (5; 0.2%). Out of the companies/business
3
https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13119-Legislation-for-plants-
produced-by-certain-new-genomic-techniques_en

99
organisations, 30.2% (54) self-identified as large companies and 69.8% (125) as small
and medium sized enterprises (SMEs), comprised of medium companies (28; 15.6%),
small companies (33; 8.4%), and micro-companies (64; 35.8%).
Views from the PC are not statistically representative.
c) Targeted Stakeholder Survey (survey)
The targeted survey
4
aimed to collect information from stakeholders on the performance
of the baseline scenario and on impacts of the policy components. It was designed to
complement the PC. Whereas the PC addressed a wider audience and therefore did not
ask for detailed technical assessments, the targeted survey envisaged detailed
information. The survey was sent to stakeholders and Member States representatives and
included EU as well as non-EU stakeholders, yet with a clear focus on the former.
The survey was designed with a complex branching to include specific questions for all
the categories of the targeted stakeholders. None of the questions was mandatory to be
filled in. A total of 397 were stakeholders invited through Limesurvey These included
academia/research, businesses and their associations (food, feed and non-food operators,
including organic and GM-free ones, across all sectors of activity: plant protection
products/fertilisers, seed/plant breeders, farmers, traders, processors, manufacturers,
retailers/services, bio-based and biotechnology industries, ornamental plants, forestry),
NGOs, environmental, consumer and other civil society organisations, and public
authorities or bodies, including all EU relevant Member state national authorities. A
detailed overview of invited survey participants can be seen in figure 1. Of invited
stakeholders, 123 (31%) provided responses.
Figure 1: Stakeholders’ categories invited to the targeted survey
4
https://food.ec.europa.eu/system/files/2022-09/sc_modif-genet_targeted-survey-questionnaire.pdf

100
A detailed overview of participants that completed the survey, according to their self-
categorisation, can be seen in Figure 2:
Figure 2:Stakeholder categories from the 123 participants that completed the targeted survey.
Concerning their sector of activity, the participants have self-categorised* as can be seen
in Figure 3:
Figure 3: Activity of sectors for the 123 stakeholder participants of the targeted survey
Respondents originated mainly from Belgium (36, 29%), Germany (15, 12%), Italy (8,
7%), France (9, 7%), Netherlands (8, 7%), Spain (6, 5%), Austria (5, 4%), Romania (4,
3%), Denmark (4, 3%), Ireland (3, 2%), Sweden (2, 2%), Greece (2, 2%), Slovenia (2,
2%), Poland (2, 2%), Croatia (2, 2%), Luxembourg (1, 1%), Lithuania (1, 1%), Latvia (1,
1%), Hungary (1, 1%), Finland (1, 1%), Czech Republic (1, 1%), Bulgaria (1, 1%),
Slovakia (1, 1%), Estonia (1, 1%), Cyprus (1, 1%) and Other countries (6, 5%).
d) Interviews
Interviews were devised to collect qualitative and quantitative data and information on
costs, and to obtain insights and views on specific topics relevant to stakeholders.
101
Interviews were conducted by two study team members via MS-Teams. The notes of
each interview were shared with the interview partners for agreement. Relevant
information was coded and analysed. The insights from the interviews fed into the main
study report’s impact section.
For the cost interviews, the stakeholders were briefed about the needs and requirements
of the interview. Dedicated meetings where the relevant cost templates were explained
and discussed have been conducted. Once organisations had provided data, these were
analysed and compared, and used for establishing ranges. The qualitative and quantitative
information was integrated in a dedicated internal data file which was then further used to
obtain robust cost estimates.
There were 25 interviews with key stakeholder organisations at EU-level and 23 cost
interviews at company, national administrative and EU-levels. Several of the general
interviews were also used to enquire about potential collaboration on cost assessments.
For the cost assessment interviews, overall, 23 different organisations throughout the
regulatory trajectory (SMEs, large companies, public authorities, regulatory authorities,
etc.) were approached. There were no one-off interviews but consecutive discussion
rounds with the stakeholders.
Stakeholder categories’ that took part in the general interviews were 20% (5)
Academic/research institutions, 44% (11) business associations, 8% (2) consumer’s
associations, 16% (4) environmental organisations and 12% (3) trade unions. Stakeholder
categories’ that took part in the cost interviews were 13% (3) academic/research
institutions, 30.5% (7) company/business organisations, 43.5% (10) public authorities
and 13% (3) other. Organisations that took part in the general interviews were all based in
Belgium (25, 100%). Organisations that took part in the cost interviews were based in
Austria (1, 4.3%), Belgium (3, 13%), Czech Republic (1, 4.3%), France (1, 4.3%),
Germany (4, 17.5%), Netherlands (1, 4.3%), Portugal (1, 4.3%), Spain (4, 17.5%),
Sweden (3, 13%) and other (4, 17.5%).
e) Focus groups on sustainability and traceability
Two focus groups, on sustainability and traceability respectively, were conducted to
complement the information obtained in interviews and the targeted survey. A concept
note was developed for both focus groups. This included a few high-level questions
which were developed based on results of the targeted stakeholder survey, as well as
insights from the interviews. The focus groups were conducted under a rather strict
protocol where the moderators asked questions and had the focus group members
providing their views. In each group, two study experts joined. The summaries of the
discussions were provided to the participants for validation. Insights from the validated
reports fed into the impact analysis and helped triangulating findings on sustainability
labelling and traceability vs transparency.
In total, 14 stakeholders participated in the two groups. 14% (2) of them were from
academic/research institutions, 29% (4) from business associations, 43% (6) from public
authorities and 14% (2) from trade unions. 42.9% (6) of them were based in Belgium,
7.1% (1) in Denmark, 21.4% (3) in France, 14.3% (2) in Germany, 7.1% (1) in Norway
and 7.1% (1) in Spain.
3 Overview of responses
For a detailed analysis of the feedback received on the IIA see Annex 2A.

102
The consultation activities that followed the IIA were to a large extent complementary
and were triangulated with findings from the literature and experts.
A summary report from the consultation outcome of the PC was published on 16
September 2022
5
.
Key findings from the consultation activities of PC, targeted survey, interviews and focus
groups:
a) Is the current legislation fit for purpose?
Most respondents to consultation activities from Member States authorities, business
organisations/associations, trade unions, academia/research institutions and a few
NGOs/consumer/environmental organisations, welcomed the policy initiative in response
to the IIA. Among the economic sectors, this view was expressed by the large majority of
operators from biotechnology and bio-based industry, farming, feed, ornamental plants,
plant breeding and plant protection and fertilisers, and by the majority of operators from
trade and food processing/manufacture.
The view that the current GMO legislation is fit for NGTs, and therefore does not need
adaptation, was expressed by a large majority of environmental organisations, and by the
majority of NGOs and consumer organisations. Among the economic sectors, this view
was expressed by a large majority of operators in food retail/service, organic, GM-free
and forestry.
Those indicating the legislation is not adequate find the GMO legislation not sufficiently
clear or consider that the risk assessment approach of the GMO legislation cannot factor
in the diverse risk profiles of plants obtained by NGTs. They find the current
authorisation, traceability, and labelling requirements not appropriate, and they also
indicate that the GMO legislation does not consider whether products have the potential
to contribute to sustainability. Those in favour of the current legislation find it
sufficiently flexible and still capable of keeping pace with technological progress. They
also find the GMO legislation sufficiently clear and risk assessment rules, as well as
authorisation, traceability, and labelling requirements appropriate. Sustainability, in their
view, can also be taken into account under the current legislation.
The views of citizens that participated in the two consultation activities open to the public
(feedback to the inception impact assessment and public consultation) varied. 98%
(69,414) of the replies to the inception impact assessment were identified as coming from
campaigns from respondents that self-categorised as citizens and opposed the initiative
calling on the Commission to keep NGT plants and products subject to the current
requirements of the GMO legislation. On the other hand, the majority of responses from
citizens to the inception impact assessment (not coming from campaigns) and to the
public consultation generally supported the adaptation of the legal framework.
b) Risk assessment
In an open question about the main aspects that should be addressed by this initiative
(Question 4 of PC), "risk assessment" was the most frequently mentioned topic. A
majority of respondents consider that the existing GMO requirements need adaptation for
plants produced by targeted mutagenesis or cisgenesis.
5
https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13119-Legislation-for-
plants-produced-by-certain-new-genomic-techniques/public-consultation_en
103
In the PC, 61% (1331) of total respondents supported a risk assessment approach
different from the current one in the GMO framework: 34% (738) of total respondents
believed that risk assessment should have requirements adapted to the characteristics and
risk profile of a plant and 27% (593) believed that risk assessment is not needed when
these plants could have been produced through conventional plant breeding or classical
mutagenesis. The adapted risk assessment approach was the most selected reply by public
authorities, academic/research institutions, EU and non-EU citizens. The approach that
no risk assessment is needed when these plants could have been produced by
conventional plant breeding or classical mutagenesis was supported by the majority of
business associations; it was also the most selected reply of trade unions,
companies/business organisations. This view was expressed by the majority of operators
in ornamental plants and the large majority of the operators in plant protection
products/fertilisers, plant breeding/seeds and biotechnology/bio-based industry; it was
also the most selected reply among the farming, feed and trade sectors. These
respondents emphasise the economic and scientific disadvantage for Europe compared to
non-European countries if the current GMO legislation is applied to NGTs. Also, the
view that the risk assessment should be primarily focused on the final product, rather than
on the process, was expressed by various respondents.
On the other hand, the view that the risk assessment requirements of the current GMO
legislation should be maintained was supported by the majority of NGOs and the large
majority of environmental and consumer organisations. Among the economic sectors, this
view was expressed by the majority of organic and GM-free operators and the large
majority of food retail/services and forestry sectors; it was also the most selected reply of
the food processing/manufacturing sector. These stakeholders, representing 22% (480) of
total respondents in the PC, argue that the application of the current framework has
demonstrated effectiveness regarding risk assessment, authorisation, traceability, and
labelling, and safeguards the freedom of choice.
In the targeted survey, breeders (N = 56) identify regulatory costs (which include costs
for risk assessment) and regulatory uncertainty as the most important factors in deciding
whether to develop NGTs for the EU market in the baseline option. The high costs and
the high perceived regulatory uncertainty mean that the attractiveness of developing
NGTs under the baseline option is very low. A risk assessment adapted to the risk profile
of the NGT is seen by breeders (N = 73) to improve the attractiveness of developing
NGTs (expressed as the expected percentage of NGT varieties on the market in 2030 –
35) to some degree. Treating NGT plants that could also occur naturally or be produced
by conventional breeding like conventional plants is increasing the attractiveness to
develop NGT plants to the strongest degree.
c) Sustainability
Regarding the questions whether a future legislation should contain sustainability
provisions, stakeholders from the farming (including organic and GM-free), breeding,
processing, manufacturing and trading sectors all advocated for a horizontal and coherent
sustainability analysis for all products regardless of the technique. Some consider that
adding that it would be discriminatory to only assess the sustainability of NGT products
and not of products of other breeding methods. Breeding and farming operators
emphasise that the sustainability assessment would increase the administrative and
financial burden. Operators from the breeding sector add that the value for cultivation and
use testing, which most plant varieties have to undergo under the PRM legislation,
considers sustainability. Public authorities, academia and research organisations and a
majority of respondent citizens support, though, the inclusion of sustainability
requirements in the legislation (51% of respondents in the public consultation).
104
In the PC, 41% of the respondents replied that introducing sustainability provisions in
this initiative is unnecessary (replies to Question 5 of PC). This view was predominantly
shared by various stakeholders, including business associations, NGOs, environmental
organisations, trade unions, companies and consumer organisations. Moreover, a wide
range of economic operators, including those in biotechnology/bio-based industry, feed,
food processing/manufacture, food retail/services, GM-free, organic, ornamental plants,
plant breeding/seeds, plant protection products/fertilizers, trade, farming, and forestry
sectors, expressed a similar viewpoint.
PC respondents have evaluated the relevance of specific traits according to their
contribution to sustainability. Traits on the better use of resources (64%), abiotic stress
tolerance (63%), and biotic stress (58%) are considered the most relevant.
Herbicide/insecticide tolerance (21%) and ‘other quality-related characteristics’ (15%)
score the lowest (relatively among all stakeholder groups but consumer organisations)
(Question 6 of PC). The targeted stakeholder survey suggests that the number of NGTs
would be slightly higher with sustainability incentives than under a sustainability
requirement scenario. Under sustainability incentives, plants with traits affecting abiotic
and biotic stress tolerances would have a higher market share from 2030 onwards, than
any other trait.
In the PC, 75% (155 out of 206) of academics and 68% (1014 out of 1491) of citizens are
in favour to provide information about the sustainability contribution to consumers, while
76% (93 out of 122) of businesses, 65% (13 out of 20) of environmental organisations,
68% (55 out of 81) of NGOs, and 60% (3 out of 5) of consumer organisations do not
agree (Question 8 of PC).
47% (28 out of 60) of the targeted survey respondents expect only a moderately higher
willingness to buy NGT products and see only a small increase of clarity for consumers
with an additional sustainability label. 55% (36 out of 66) expect a strong increase in
compliance costs and administrative burden. The organic sector expressed in interviews
that such a label would lead to unfair competition with the organic label.
The focus group on sustainability concluded that sustainability should not be linked to the
plant breeding process but rather a holistic, systemic approach should be put in place. The
group emphasized that sustainability encompasses various dimensions and is context-
dependent, requiring consideration of trade-offs and avoiding discrimination between
different breeding methods. They highlighted that sustainability is a multifaceted concept
encompassing social, health, and economic dimensions, which vary depending on the
specific traits and contextual factors. Balancing trade-offs and avoiding discrimination
between different breeding methods were identified as challenges. They recommended
addressing sustainability through a broader framework, such as the Sustainable Food
Systems Framework, and highlighted the importance of transparency and information for
the organic sector.
d) Traceability and information
There is broad agreement among stakeholders about the need to ensure transparency
about NGTs, but views vary considerably on the means to ensure it. For consumer
organisations, transparency should be provided through labels (80%, 4 out of 5) while
37% (65 out of 177) of the business associations and 32% (80 out of 251) of the
companies do not find transparency necessary for plants produced by targeted
mutagenesis and cisgenesis, when they could have been produced through conventional
plant breeding or classical mutagenesis (Question 12 of PC). 46% (69 out of 149) of plant
breeders and 61% (23 out of 38) of biotechnology/bio-based industries do not see a need
for transparency for such plants, while for 55% (42 out of 77) of the organic, 66% (19 out
105
of 29) of GMO free, 58% (18 out of 31) of food retail and 62% (5 out of 8) of forestry
sectors, a physical label could provide transparency. In interviews, the PC and the focus
groups, the organic sector expressed a need for transparency underlining the importance
of transparency so that organic operators have the freedom to avoid NGTs.
As regards what should be required when reliable analytical methods that can both detect
and differentiate a product cannot be provided for plants produced by targeted
mutagenesis and cisgenesis, responses varied in the PC (Q11). 30% (660 responses)
consider that, in the absence of reliable analytical methods that can both detect and
differentiate a product, operators should “not be allowed to place the product in question
on the market” (a great majority of consumer and environmental organisations and the
majority of NGOs; most selected view among citizens and trade unions; among economic
operators, half of the forestry sector, the majority of the food retail/services sector, as
well as the great majority of the organic and GM-free sector also expressed this view)
Conversely, 27% (599) of respondents considered that operators should “not be asked at
all to provide an analytical method that can both detect and differentiate their product”.
This was the most expressed view among academic/research institutions,
companies/business organisations and non-EU citizens; it was expressed by the great
majority of the plant protection product/fertiliser, plant breeding/seeds and
biotechnology/bio-based industry sectors, and was the most selected response among the
trade, farming and ornamental plant sectors). 20% (431) believe that operators should “be
asked to provide a detection method, but without the need to differentiate, if they can
justify that the latter would be impossible” (the most selected response among public
authorities, as well as half the forestry sector) and 16% (350) respondents consider that
operators should “not be asked to provide an analytical method that can both detect and
differentiate their product, if they can justify that this would be impossible”.
The survey finds that the scenario requiring no labelling and traceability if a product is
also obtainable naturally or by conventional breeding elicits the strongest expectations of
positive impacts on total private R&D funding for plant breeding, for funding for biotech
in academia, for the competitiveness of farming or the SME market share in the breeding
sector.
The focus group on traceability, consisting of experts from various sectors, emphasized
the importance of labelling NGT plant products using existing labelling schemes,
especially if these products are not regulated as GMOs in the future. Certain participants
highlighted that labelling should only be appropriate if there is a distinguishable
difference between NGT plants and those obtained through conventional breeding.
Otherwise, labelling would be misleading. The lack of analytical methods for
identification and differentiation was considered to pose a significant enforcement
challenge. Additionally, traceability requirements for NGT plants should be proportionate
and verifiable by competent authorities through official controls. Document-based
traceability systems, such as those used in the organic sector, can be valuable for
enforcement and traceability, especially in the early stages of the food chain. Introducing
a specific traceability system for NGT plants would be problematic due to the lack of
distinction between NGT and conventional plants in many third countries. Alternative
options such as public registries, leveraging existing databases like the EU Common
Catalogues or national registers, and exploring cost-efficient methods like blockchain for
traceability (not detection) would require international coordination. Some participants
suggested that ruling out analytical methods prematurely may not be appropriate, as
relevant research in this area is still in its early stages. The costs and administrative
106
burden associated with record-keeping systems should be proportionate and manageable,
considering that the majority of NGT developers are SMEs.
e) Coexistence
The majority of conventional farmers emphasise the competitive advantage NGTs can
bring to European farms, e.g. with plants adapted to climatic changes and reduced
environmental impact during cultivation (less fertilizers, water and pesticide uses).
Sharing these views, some organic farmers also see advantages in the use of NGTs, to
enable wider environmental and economic sustainability, as indicated in interviews and
the focus group on sustainability.
However, the majority position expressed by the organic sector is that NGTs are not
compatible with the organic sector. Various points were raised on coexistence matters in
an open question in the PC (Q15), as well as in interviews. Stakeholders from the organic
sector suggest that measures safeguarding coexistence should be strengthened at EU
level, e.g. maintain labelling and traceability, and in addition provide for measures to
guarantee seed purity and protection against contamination, and that measures must
encompass the entire chain from seed production to the finished product. Other
stakeholders expressed the view that conventional-like NGT plants should be treated the
same as conventional plants and therefore be suitable for all kind of agriculture, without
the need of applying any specific coexistence measures.
In position papers submitted to the PC, some public authorities expressed the view that
the current legislation on the traceability and labelling of GMO is key to enable and
guarantee the co-existence with organic and conventional GM-free agriculture as well as
for consumer information and liability issues. They call on the Commission to ensure
research and development of detection methods in this context.
According to the targeted survey, strong and moderate negative impacts on organic
farming are expected predominantly by NGOs (6), SMEs (4), two consumer associations,
one public authority, one business organisation, and one public agency. No impact on the
organic farmers (14) are predominantly expected from business associations (8), public
authorities (2), as well as one NGO and one academic organisation.
For small scale farmers, the picture is very similar: out of the 14 respondents that
expected strong or moderate negative economic effects on small scale farmers were
NGOs (5), SMEs (4), consumer and business associations (each 2), and one ‘Other”
(public agency). In essence, all respondents who saw strong negative economic effects on
organic farmers, also expected them for small scale farmers.
f) Future-proofing
In terms of future-proofing of the legislation, the two most important aspects across all
stakeholder groups (except consumer and environmental organisations, as well as NGOs)
are to improve the legal clarity and to put in place mechanisms that facilitate adaptation
to scientific progress (Question 14 of PC). In addition, an adapted regulatory framework
that is “aligned globally with other commercial areas around the world to minimize
commercial conflict potential and ensure EU competitiveness” and that is “proportionate
and science-based” (Question 18 of PC) were key requirements mentioned by
stakeholders.
Measures to facilitate the uptake of NGTs by SMEs put forward by various stakeholders
during the consultations. According to the PC, “a reliable framework that gives them
certainty concerning their investment” is needed, “the NGTs are suitable for local
contexts”, and that SMEs currently “cannot afford the high costs of lengthy investment
required for R&D and commercialisation of new crops” was shared by business

107
associations and academia while companies/business additional regulatory measures and
citizens and public authorities are divided in their views.
4. Identified campaigns
a) Feedback on the Commission’s Inception Impact Assessment (IIA)
98% (69,414) of the replies were identified as coming from campaigns; campaign
contributors self-categorised as citizens. A user-modifiable, precompiled reply template
was made available on several websites in different EU-countries.
The contributions from the campaign demand to apply the precautionary principle in
accordance with the CJEU ruling of 25 July 2018 in Case C-528/16, Confédération
paysanne and Others
6
. In the view of these respondents, all GMOs, including NGT
products, must fall under the current GMO legislation, and no artificial distinction should
be made between different forms of genetic engineering. A robust, process-based, case-
by-case risk assessment for NGT products should be applied. Moreover, the Commission
should implement policies that are in accordance with the objectives of the Farm to Fork
strategy. More research on unexpected and unwanted effects of NGT plants, including
long-term effects and on interactions of the plant with the environment, is demanded. It is
stressed that sustainability and social criteria of NGTs should be assessed in a systematic
way and the claim that NGTs can contribute to a sustainable food system is discarded.
Campaigners stress the importance of traceability of NGTs and consider that NGTs
should be labelled as GMOs to guarantee freedom of choice. According to the campaigns
respondents, in case of a deregulation, the costs for the organic and the GM-free sector
would drastically increase to avoid contamination of their products. They also express
concerns about the patenting of NGT products, as they would restrict the rights of
farmers and breeders, and only companies would be able to make profits from them.
A small part of campaigners further asks to ban products of and research on genetic
engineering in the EU; some ask that their import should also be prohibited. Some state
that genetic engineering should only be approved in medicine.
More details can be found in the summary report of the feedback from the inception
impact assessment can be found in Annex 2a.
b) Public Consultation (PC)
Similar sets of replies that could potentially constitute campaigns were identified using a
combination of statistical software and manual analysis of responses, based on the
identification of more than ten identical contributions to closed questions and at least one
open question. Overall, five such groups were identified from 109 respondents (4.7% of
all consultation responses, Error! Reference source not found.). Their main messages
were:
Groups 1, 2, 4 and 5: current provisions of the GMO legislation are adequate; risk
assessment using the current GMO legislation requirements; no need for specific
regulatory provisions on sustainability. Groups 1, 2 and 4: effective traceability can be
ensured via documentation, public databases/registries and digital solutions, while
Groups 1, 2 and 5 stated that transparency can be achieved via a physical label.
Group 3: current provisions of the GMO legislation are not adequate; no need for risk
assessment when plants could have been produced through conventional breeding or
7
https://demeter.net/keep-new-gm-food-strictly-regulated-and-labelled/

108
classical mutagenesis; no need for specific regulatory provisions on sustainability;
effective traceability can be ensured via public databases/registries; transparency for
operators and consumers is not necessary, when these plants could have been produced
through conventional breeding or classical mutagenesis.
None of the campaigns identified had a significant weight on the overall outcome of the
consultation, due to the relatively very limited number of respondents involved, ranging
from 0.4% (10) to 2.1% (48) of the overall respondents.
Table 1: Overview of identified campaigns in the PC
Campaign number
Number of
respon
dents
/ shares
*
Stakeholder types
Sectors
Country of origin
Academic /
research
Business
association
Company/bu
siness org.
NGO
EU citizen
Environment
al org.
Non
-EU
citizen
Other
1
13 /
0.6%
2
2
8
1
None
Switzerland: 10,
Germany: 1, Italy: 2
2
18 /
0.8%
4
2
5
3
4
Food
processing/manufact
uring: 1, Plant
breeding/seeds: 1.
Farming: 4, Organic:
4, Trade: 1, Food
processing/manufact
uring; Food
retail/services: 1
Australia: 1, Belgium:
1, Finland:1, France:
1, Germany: 2,
Greece:1, Hungary:1,
Italy:1 Spain: 1,
Sweden: 1,
Switzerland: 1, UK:1,
US: 5
3
20 /
0.9%
5
7
4
4
Farming: 7,
Ornamental Plants: 2,
Plant breeding/seeds:
2, Food
processing/manufact
uring: 2.
Belgium: 3, Estonia 1,
Germany: 2, Italy: 3,
Portugal: 1,
Romania: 9, Sweden:
1
4
48 /
2.1%
1
7
3
4
5
1
Plant breeding/seeds,
Farming: 4. Food
processing/manufact
uring,
Biotechnology/bio-
based industry,
Trade, Organic: 3.
Wine: 1
Austria: 1, Belgium:
4, Denmark: 1,
France: 2, Germany:
4, Hungary: 3,
Poland: 1, Slovakia:
30, Spain: 1, UK: 1
5
10 /
0.4%
1
1
7
1
Organic: 1
Poland: 10
T
O
T
A
L
109 /
4.7%
* Out of total responses: 2 300
5. Ad hoc contributions and outreach
Ad hoc contributions
141 stakeholders (including Member States) attached one or more position papers to the
PC. Following a screening and de-selecting literature already analysed during desk
research, lists of references, and duplicates, 50 papers were analysed in more detail.

109
Error! Reference source not found. presents the analyses of positions papers by
stakeholder type.
Excluding the topics addressed by the questions of the PC, the position papers centred
around the following topics: existing provisions for NGTs; short/medium/long-term
consequences on the sector if no change in the current legislation; risk assessment;
impacts (social / environmental / economic / other impacts) on sustainability; incentives
to encourage sustainable NGTs; traceability of NGTs; on future-proofing of legislation;
measures for coexistence with existing agricultural practices; accessibility to NGT
technologies/resources.
In addition, contributions were received via e-mail. For the PC, these originated from
France, Sweden, the United States of America, Community Plant Variety Office, Union
Fleurs and IGTC, while for the targeted survey these originated from several Member
States (Germany, Austria, Sweden, Poland, Hungary, Lithuania, Romania and the
Netherlands). All contributions were analysed and considered in the analysis.
Figure 2: Position papers by stakeholder type
During the public consultation, an email campaign with 922 contributions from French
citizens was addressed to the Commission. The campaign advocated that the current
GMO-legislation should not be revised.
On 7 February 2023, the Commission received a petition
7
with 420 000 signatures from a
coalition of 55 organisations in 18 Member States, asking the Commission to maintain
risk assessment, traceability, and labelling for organism obtained by NGTs.
Outreach
On 29 November 2021, the Commission organised a high-level event on “New genomic
techniques – the way forward for safe and sustainable innovation in the agri-food
sector”
8
. Three panels, with speakers from the European Parliament, Member States,
academia, NGOs, breeders and farmers, debated on the key elements of the policy action:
7
https://demeter.net/keep-new-gm-food-strictly-regulated-and-labelled/
8
https://commission.europa.eu/events/new-genomic-techniques-way-forward-safe-and-sustainable-
innovation-agri-food-sector-2021-11-29_en

110
sustainability, risk assessment and information to the consumers. The event was followed
by around 1000 people (registered or via webstreaming).
The Commission organised three dedicated meetings with the Member States’ GMO
experts to inform them on the progress on the impact assessment (25 May 2022
9
, 24
October 2022
10
and 9 February 2023
11
). Furthermore, the Commission informed
stakeholders on the progress of the impact assessment in the context of the Advisory
Group on the Food Chain and Animal and Plant Health (6 May 2022
12
) and the Advisory
Group on Sustainability of Food Systems (19 October 2022
13
).
.
9
https://food.ec.europa.eu/system/files/2022-09/sc_modif-genet_20220525_jwg_sum.pdf
10
https://food.ec.europa.eu/system/files/2023-02/sc_modif-genet_20221024_jwg_sum.pdf
11
https://food.ec.europa.eu/system/files/2023-06/sc_modif-genet_20230209_jwg_sum.pdf
12
https://food.ec.europa.eu/system/files/2022-06/adv-grp_plenary_20220506_sum.pdf
13
https://food.ec.europa.eu/system/files/2023-01/adv-grp_plenary_20221019_sum.pdf

111
ANNEX 2A: SUMMARY REPORT ON FEEDBACK RECEIVED
ON INCEPTION IMPACT ASSESSMENT: LEGISLATION
FOR PLANTS PRODUCED BY CERTAIN NEW GENOMIC
TECHNIQUES
The Inception Impact Assessment (IIA) aimed to inform citizens and stakeholders about
the Commission’s policy initiative on plants obtained by targeted mutagenesis and
cisgenesis. Citizens and stakeholders provided feedback from 24 September 2021 to 22
October 2021.
70,894 contributions were received; 98% (69,414) of the replies were identified as
coming from campaigns; contributors self-categorised as citizens. It seems that a user-
modifiable, precompiled reply template was made available on several websites in
different EU-countries.
The non-campaign replies amounted to 2% of the total (1,480). According to self-
categorisation, most contributions were from citizens (70%, 1,030 replies), followed by
business organisations/associations, trade unions (14%, 203), academia/research
institutions (8%, 115), NGOs and consumer/environmental organisations (5%, 81), public
authorities (1%, 9) and others (3%, 42).
Figure 3. Breakdown of non-campaign replies (1,480 total)
Contributions (both campaign and non-campaign) originated from 91 countries, including
the 27 Member States and 64 non-EU countries. Top contributions from Member States
were from Germany (32,694), France (25,544), Belgium (2,732), Netherlands (2,251) and
Austria (2,111). Most contributions from non EU-countries came from Switzerland (782),
followed by United Kingdom (759), USA (228), Argentina (142) and Canada (114).
This large number of campaign and non-campaign contributions shows the interest of
citizens and stakeholders on the Commission’s policy initiative on plants derived from
new genomic techniques (NGTs) and on the topic in general.
The following analysis distinguishes between the non-campaign and campaign
contributions and, for the latter, between different stakeholders groups. It organises the

112
comments according to the following topics: the IIA/policy initiative in general, risk
assessment, sustainability, traceability, information to consumers/awareness, liability/cost
of contamination and intellectual property.
1. NON-CAMPAIGN CONTRIBUTIONS
1.1. Public authorities
Contributions from nine public authorities were received, among them the Member States
Denmark (DK)
14
, Estonia (EE)
15
, Austria (AT)
16
, Spain (ES)
17
, Netherlands (NL)
18
and
France (FR) as well as the Bavarian
19
and Flemish authorities
20
. Furthermore, one reply
was from Argentina
21
.
On IIA/policy initiative
All respondents welcome the policy initiative and support the IIA. ES, EE, FR and NL
stress that an update of the current GMO legislation is needed. ES and NL emphasise that
legal uncertainties must be removed of the GMO legislation. Furthermore, ES states that
duplication of requirements, leading to administrative burden, that are already addressed
in more specific legislation, should be avoided. The Flemish authority criticises that the
IIA does not indicate how the net administrative burden for obtaining an authorisation
will be reduced. FR is not in favour of developing new herbicide resistance varieties,
which has to be considered in the regulatory framework in their point of view. Moreover,
Argentina promotes to harmonise internationally the regulation of NGT products.
Concerning the timing of the policy initiative, the Bavarian authority suggests starting the
public consultation in the 1
st
quarter of 2022. The action is considered urgent, given the
rapid global developments in the field of NGTs. Regarding the scope of the initiative,
DK, ES, NL and EE stress that knowledge of the use of NGTs in microorganisms and
animals has to be built up.
Risk assessment
EE underlines the importance of carrying out a proportionate risk assessment for NGT
products, which should be based on a case-by case approach.
Sustainability
EE and ES support the sustainability assessment of NGT products in the authorisation
process. The Flemish authority states that safety and sustainability assessments must
remain separate. Furthermore, it stresses that sustainability may not become too stringent
so that innovation is possible. Argentina stresses that NGTs could contribute to the
objectives of the European Green Deal and F2F.
Traceability
In the point of view of EE, there should not be excessive focus on traceability, given the
impossibility in certain cases to trace the variety and the fact that plants obtained by new
genomic methods are known to be as safe as those derived from conventional breeding.
14
Ministry of Food, Agriculture and Fisheries; Ministry of Environment
15
Maaeluministeerium
16
Bundesministerium für Soziales, Gesundheit, Pflege und Konsumentenschutz
17
Ministerio de Agricultura, Pesca y Alimentación
18
Ministry of Infrastructure and Water Management
19
Bayerisches Staatsministerium für Umwelt und Verbraucherschutz
20
Interdepartementaal overlegcomité Life Sciences, Vlaamse Overheid
21
Ministerio de Agricultura, Ganaderia y Pesca

113
The Flemish authority stresses that the authorisation should not depend on an analytical
detection method.
Information to consumers/awareness
In the opinion of EE, potential consumer attitudes should be assessed under the economic
impact of the IA. Furthermore, consumers should be better informed about NGTs.
1.2. Business organisations/associations, trade unions
On IIA/policy initiative
Most respondents in this category (167 out of 205, mostly farming, breeding, processing,
manufacturing and trading operators) support a future-proof adaptation of the GMO
legislation to scientific and technological progress, stating that the upcoming proposal
needs proportionate approval requirements, clear definitions and clarification of what
falls under the GMO legislation, and that it should not add administrative complexities.
Some suggest extending Annex 1B of Directive 2001/18/EC to NGT products not
containing foreign DNA. Others propose to regulate phenotypes comparable to those of
natural mutations or conventional breeding by the PRM legislation and not the GMO one.
Some suggest that NGT products without added DNA should only be subject to a
notification procedure like in Japan and stress the importance in their view to harmonise
the EU GMO legislation with other non-EU countries. Some respondents in this group
demand that the inter-institutional negotiation of the proposal should take place during
the current legislative term of the European Parliament, and one suggests an ad hoc
regulation as in the case of the COVID pandemic. Others ask that the scope of the policy
initiative be enlarged to microorganisms and to all genomic techniques, including to
those leading to transgenesis. Furthermore, in the view of some respondents the initiative
needs to address also that the current GMO legislation allows EU countries to opt out of
GMOs.
All organic, GM-free and related operators as well as some farmers’
associations/movements and retailers (38 out of 205) believe that NGT products should
remain subject to the current GMO authorisation procedure, and do not see a legal
uncertainty that justifies the amendment of the current GMO legislation, which should
continue to be applied. They argue that the GMO legislation is not a ban of NGT
products, as research is possible and products of targeted mutagenesis and cisgenesis
could be authorised. Some respondents ask to assess the costs for the GM-free and
organic sector in case of potential contamination by NGT products; they demand that the
impact on biodiversity of NGT products be assessed and that ethical considerations are
included in the decision whether to allow NGTs in the EU.
Risk assessment
Farming, breeding, processing, manufacturing and trading operators for the most part
support a proportionate product-based risk assessment on a case-by-case basis, stressing
that neither risk assessment nor monitoring is needed if NGT plants could have been
produced naturally. They further argue that it should be ensured that SMEs could meet
submission requirements for the risk assessment of NGT products.
Organic, GM-free and related operators as well as some farmers associations/movements
call for a proper comprehensive risk assessment for NGT products. They demand a
process-based, case-by-case risk assessment; some propose that a product/trait-based
assessment should be done additionally.

114
Sustainability
Several farming, breeding, processing, manufacturing and trading operators demand a
consistent and coherent sustainability analysis for all products, adding that it would be
discriminatory to only assess the sustainability of NGT products and not of products of
other breeding methods. Furthermore, the sustainability criteria should be coherent with
the sustainability food system framework. Breeding and farming operators emphasise that
the sustainability assessment would increase the administrative and financial burden.
Breeding operators add that the VCU testing, which most plant varieties have to undergo
under the PRM legislation, considers sustainability. Two respondents of the
biotechnology sector state that sustainability analysis should not play a role in NGT
authorisation, but sustainability related information could be included in a label.
Some organic and GM-free operators support the assessment of the sustainability and
socio-economic impacts of NGT products. However, two respondents of this group
emphasise that the sustainability assessment should not be linked to the risk assessment.
Several farming, breeding, processing, manufacturing and trading operators agree that
NGT products could contribute to the objectives of the Green Deal and the Farm to Fork
Strategy. By contrast, organic and GM-free operators, as well as some farmers’
associations, opposes this idea and stress the importance of traditional farming methods
and agro-ecological practices in this regard.
Traceability
Breeders consider that, in order to ensure traceability, information could be provided in
the EU common catalogue of varieties or the national variety registry. Furthermore, two
respondents stress that product segregation based only on the breeding technique is very
costly.
Organic and GM-free operators as well as respondents of the retail sector call for the
development of detection methods for NGT products. Some organic and GM-free
operators state that detection methods of NGT products must be a mandatory prerequisite
for the approval of NGT products, while other organic and GM-free operators as well as
some farmers’ associations suggest that documentation traceability would be an option in
case no detection methods are available.
Furthermore, three respondents call for the establishment of a transparency register for
NGT products.
Information to consumers/awareness
Organic and GM-free operators as well as respondents of the retail sector want to
maintain labelling of NGT products. They underline that labelling is important to ensure
the freedom of choice of consumers and the organic and GM-free sector. Moreover,
organic and GM-free operators further demand that animal products should be labelled if
the animals were fed GMO feed. One respondent emphasises that the current GMO label
should be used for NGT products, instead of introducing a new label.
Farming, processing, manufacturing and trading operators took the opposing position,
arguing that labelling should be voluntary, underlining that products of traditional
breeding methods would also need labelling, if NGT products are labelled; labelling
similar products differently would breach the principle of non-discrimination and
proportionality. Others add that if a method for production of a variety is not considered
as GMO, it should not be labelled as such. Some emphasise that labelling must be

115
implementable and enforceable or suggest that it could be used to communicate the
benefits of the NGT product.
On the one hand, two respondents stress that a majority of European consumers oppose
genetic engineering in agriculture. On the other hand, one respondent argues that
consumers differentiate between old and new GMOs and that the acceptance increases if
NGT products show personal, health or climate benefits. Four respondents state that
citizens should be better informed about NGTs.
Liability/cost of contamination
Organic, GM-free and related operators call for strict coexistence rules, arguing that even
a partial deregulation of NGT products would make it more difficult for the organic and
GM-free sector to trace them. As there is no “polluter pays principle” in place, the
organic and the GM-free sector would have to bear the cost to avoid the contamination of
their products with NGT products. Furthermore, these respondents stress that lack of
information would also break the trust of the consumer in organic food.
Intellectual property
Especially breeders but also farming, processing, manufacturing and trading operators
support a broad access to breeding technologies and suggest that the Commission should
encourage licensing platforms that provide their members access to patented technology
like the International Licensing Platform (ILP) Vegetable, Corteva’s Open Innovation
Platform and the Agricultural Crops Licensing Platform (ACLP). Some breeders that
support these licensing platforms also acknowledge the importance of plant-related
innovation by patent protection. Two farmers’ associations call for free access to the
entire gene pool and all breeding processes while one other supports access to the
selection method used but the breeder should be allowed to keep confidential
information. Three farmers’ associations demand that patents should be applicable to
plants containing DNA that cannot be found in nature or cannot be obtained by
conventional breeding methods or old and new mutagenesis. Another suggestion is that
the Commission should establish a research institute that will give access to the
technology to all European firms by applying common property principles while the
beneficiary company will pay a reasonable amount for it. One breeder calls for a change
in the intellectual property landscape of plants with a broader interpretation of the
definition of ‘essential biological processes’ in order to cover mutation breeding in both
Directive 98/44 and the European Patent Office (EPO) framework.
Organic and GM-free operators are concerned that patents on NGT products can create
damage to seed availability and lead to the privatisation of modified seeds. Furthermore,
they argue that they would undermine the breeder’s exemption. One farmers’ association
states that the patenting of genetic information contained in the seed would run counter to
the implementation of the rights of farmers on seeds based on the UN Declaration on the
Rights of Peasants and Other People Working in Rural Areas and the International Treaty
on Plant Genetic Resources for Food and Agriculture.
1.3. Academia/research institutions
On IIA/policy initiative
Most respondents of this group demand to modernise the current GMO legislation
according to current knowledge, making it proportionate and future-proof. Legal
uncertainties regarding terms like “mutagenesis”, “conventional use in a number of
applications” and “long safety record” should be removed and harmonised with the
Cartagena protocol. Within this group some are for simplified or product-based

116
authorisation procedures for NGT products. Others ask that NGT products should be
deregulated, or that NGT products should be deregulated if they are not transgenic; others
support their deregulation only when they are also obtainable by conventional breeding.
Suggestions on how to change the GMO legislation were made. These include a pre-
assessment procedure, regulated by a separate piece of legislation, to determine whether
the product falls under the GMO legislation, like in Argentina. Others propose to modify
the GMO definition to exempt NGT products by enlarging the mutagenesis exemption or
introducing a new annex defining the exemptions. In addition, some ask that a future
regulation of NGT plants should not allow individual member states to restrict their use.
Moreover, the international perspective of the GMO legislation and its consequences on
international trade must be considered, e.g. Distinctness, Uniformity and Stability (DUS)
testing is challenging if NGT products are regulated as GMOs in the EU, as this is not the
case in other countries. Regarding the scope of the initiative, some stress that
microorganisms, forest-trees and forest biomass derived products should be included.
Others ask that the impact assessment should consider the human right to benefit from
scientific progress (Art 27 of the 1948 UDHR and Art 15 of ICESCR), as well as and
positive and negative impacts for organic and GM-free agriculture. Moreover, a cost-
benefit analysis of the deployment of NGTs on all the aspects mentioned in the IIA
should be carried out.
Some respondents ask that NGTs continue to be covered by the current GMO legislation
and that it is too early for a proportionate framework, as possible adverse effects of NGT
plants are not reliably known. Furthermore, there is a call for more research on
unintended impacts on environment and human health of NGT products.
Risk assessment
Some respondents call for a product-based risk assessment on a case-by-case basis.
Furthermore, there are proposals for a specific risk assessment for NGT products based
on defined categories of risks. For one respondent of this group the current risk
assessment should apply to all NGT products, as it is too early to assume that they are
safe.
Sustainability
Some respondents agree that NGT products could contribute to the objectives of the
Green Deal, the F2F, the Biodiversity Strategy and the UN SDGs. Moreover, some
propose that not only the risks but also the benefits to environment and to society should
be considered in a legal framework.
Some commenters refer to the Norwegian Gene Technology Act that assesses the
contribution to sustainability, social utility and ethical aspect of GMOs, which could
serve as an example for the EU GMO legislation. Others demand that a sustainability
analysis should not be limited to NGT products but be included in the evaluation of any
new plant variety. Others emphasise that a sustainability analysis would increase the
administrative and economic burden and would not contribute to international
harmonisation.
Traceability
Some respondents highlight the importance of traceability for the GM-free and the
organic sector, as a lack of transparency, traceability and labelling would set the wrong
signal. Moreover, some call for more research on detection methods. As traceability is
not always possible by analytical methods, respondents in this group suggest certification
to trace NGT products. Others stress that the requirements for detection methods should

117
not be barriers in the approval process, or that NGT and conventional products should be
treated equally regarding traceability.
Information to consumers/awareness
As for traceability, some emphasise that labelling is important for the GM-free and
organic sector and needed to ensure the freedom of choice for consumers. Others mention
that the public wants information about the breeding method. Finally, some call for no
labelling of NGT products.
Some respondents ask for constructive public dialogs to better inform consumers about
NGTs, arguing that the public attitude is positive on NGT products if they are beneficial
for environment and society, and stressing that regulating NGTs as GMOs would sow
doubts in the minds of consumers.
Liability/cost of contamination
Some respondents comment that NGT and conventional breeding products are
indistinguishable. Therefore, there is no scientific basis not to use NGT products in
organic farming.
Intellectual property
One respondent notes that, since GMO products seeking for a patent or Community Plant
Variety Rights (CPVR) protection must meet intellectual property rights (IPR) criteria
such as novelty, inventiveness, industrialisation, distinctiveness, stability and uniformity,
by default such a product would also be characterised as non-conventional/traditional,
unknown, unnatural/synthetic and could not legally be described or labelled as natural,
organic, conventional, traditional, having a history of safe use etc. Others ask that NGT
products be excluded from patenting.
1.4. NGOs, consumer/environmental organisations
On IIA/policy initiative
This group of respondents is very critical on the IIA. They argue that the IIA follows the
unverifiable promises of the industry, as it downplays the risks and overemphasises
potential benefits of NGT products, and it does not take into account the benefits for the
food sector, farmers, the environment and the public of the current GMO legislation.
Moreover, according to some of them, the IIA does not follow the CJEU ruling by not
prioritising the precautionary principle and by suggesting that the current GMO
legislation is no longer fit for purpose. Respondents in this group demand that the current
GMO definition is maintained and to not distinguish between different forms of genetic
engineering. They stress that if NGT products were exempt from the GMO legislation,
this would conflict with the EU treaty (Art 169, Art 114,191 TFEU) and the key
objectives of the Biodiversity Strategy. In this group, most respondents demand a
thorough approval procedure for NGT products, rejecting a facilitated authorisation,
suggesting that, like in the pharmaceutical authorisation, an evidence-based benefit
assessment should be needed for the authorisation of a new GMO. Finally, some call for
a general ban of all GMOs in the EU. One NGO claims that apart from the impacts
mentioned in the IIA, the IA should also examine the impact on social justice,
agrobiodiversity, circular economy and ecosystem services. Furthermore, it should
contain a survey on the acceptance by citizens.
A few respondents from this group support an adaptation of the GMO legislation
according to scientific knowledge, as it is not fit for purpose. One respondent supports a

118
product-oriented approach with a case-by-case assessment regardless of the breeding
process.
Risk assessment
Several respondents demand a risk assessment for all GMOs, regardless of the technique
used, supporting a process-based risk assessment on a case-by-case basis; some state that
besides the process also the trait has to be assessed. In addition, one NGO stresses that
also the plant species (e.g. reproduction methods, pollinators involved) has to be
considered. Respondents emphasise that the risk assessment must include new and
additional risks emerging from NGTs and should not be based on defined technique-
based risk levels. They argue that genetic alterations of NGTs go far beyond of those of
conventional breeding techniques.
Sustainability
In general, this group opposes the assumption that NGT products can contribute to
sustainability, as they are part of an industrial agricultural system. Instead, they suggest
that the Commission focuses on agro-ecology and organic agriculture. They argue that
the Commission downplays the dominance of herbicide tolerance in current pre-
commercial NGT crops and call for a ban of pesticide-tolerant varieties.
Within this group, some are in favour of a sustainability analysis for NGT products. They
refer to the Norwegian law, where only GMOs contributing to sustainable development
can be used. Furthermore, they demand the sustainability analysis be based on scientific
evidence, follow strict quality requirements, be comprehensive, and not be limited to a
specific category of GMOs.
Moreover, the importance of the coherence of the sustainability analysis with the policy
action on a sustainable food system framework is emphasised.
Traceability
This group calls for traceability of NGT products to preserve the freedom of choice
throughout the food chain and specifically for the organic and GM-free sectors. It
considers that a weakening of the current traceability rules of NGT products would
contradict the objectives of the F2F, according to which food information should be made
more transparent. Companies should disclose full information about the genome
sequence of their NGT products, and traceability and detection methods should be
mandatory. The need for the development of detection methods was emphasised, asking
in parallel that existing detection methods be used pro-actively in food and feed controls.
Respondents criticise that, according to Commission’s NGTs study, EU member states
spent only 1,6% of their research funding in NGTs for detection methods, monitoring and
risk assessment. If detection methods are not available, some suggest the introduction of
a documentation-based traceability approach like in the organic sector. Finally, some call
for the establishment of a public international registry including all GMO plant varieties.
Information to consumers/awareness
Respondents argue that labelling is important in order to ensure the freedom of choice
throughout the food chain as well as the organic and GM-free sector; a weakening of the
labelling rules would contradict the F2F. Furthermore, they argue that EU consumers are
in favour of labelling of GMOs. This group of respondents opposes a new NGT-label that
would replace the current GMO-label, and the replacement of GMO and NGT labelling
by a sustainability labelling. They further ask for the labelling of animal products, if
animals are fed with genetically modified feed.

119
Liability/cost of contamination
Respondents believe that deregulation of NGTs would make the coexistence of organic
and GM-free impossible. Moreover, the cost to prevent and deal with contamination
would rise dramatically in these sectors. In addition, the seed saving sector already has to
handle the lack of transparency on NGT plants from third countries. Furthermore, it
would contradict the General Food Law if the conventional, organic and GM-free sector
had to account for all administrative and economic burden for testing and segregation
while the potential benefits went to the biotechnology sector. They call for the
introduction of the “polluter pays principle”.
Intellectual property
Respondents of this group stress the negative consequences of patents, like
monopolisation and concentration of the seed market, that apply to GM techniques. They
ask that NGT research, that receives public funding, be free from patents.
1.5. Citizens
On IIA/policy initiative
Most of the respondents (815 out of 1029) believe the GMO legislation is not fit for
purpose and support a future-proof update in accordance with current scientific
knowledge. They ask to remove inconsistences, update definitions, align with global
regulatory approaches and move to a product-based assessment. They argue that each EU
country should not be able to prohibit the commercialisation of a GMO. Moreover, a
separate approval process for NGTs from GMOs is proposed. They suggest including
NGT products not containing foreign DNA in the Annex 1B of the Directive as a first
step, or to consider them in the same way as those of traditional breeding. In the long-
term, the legislation should be amended so that it is product-based. Others propose to
introduce an authorisation for all plant varieties instead of just for GM varieties. Some
further argue that the authorisation of transgenic plants should be simplified. Moreover, a
heavy regulation on NGT products would deter small and medium-sized enterprises
(SMEs) from using them.
A minority of respondents (198 out of 1029) oppose a change of the legislation for
genetically modified organisms (GMOs). According to them, the GMO legislation is fit
for purpose and there is no legal uncertainty that would justify an amendment. They
stress that the ruling of the Court of Justice of the EU (CJEU) of July 2018 and the
precautionary principle have to be respected. They oppose a deregulation of NGT
products in order to prevent the risk of unintended consequences and to control misuse
and propose to authorise NGT products only in the context of industrial and
pharmaceutical biotechnology. Some demand that the EU should not allow the
development of NGTs within its borders.
Some citizens state that societal, environmental and ethical consideration should be
included in the debate on NGTs. Moreover, theological and anthropological research of
the impact of NGTs is needed. Furthermore, the likely impacts on the fundamental right
to freedom of the arts and science as well as the environmental and economic impact of
refraining from biotechnological innovation should be assessed.
Risk assessment
Respondents have different opinions about the risk assessment of NGT products. Some
do not support a change in the risk assessment of NGT products and stress that it should
stay as it is now, but also additionally allow for a more stringent case-by-case assessment
if needed. Some emphasise that no experimental data supports the assumption that NGT
120
products have the same risk profile as conventionally bred ones. They argue that NGTs
differ from conventional breeding methods because of their non-randomness.
Furthermore, they make the whole genome accessible for changes.
Others support a proportionate, product-based, case-by-case risk assessment, suggesting
to introduce a preliminary assessment to determine whether a full risk assessment is
necessary. Others propose to classify genetic engineering methods according to risk
levels, leading to different risk assessment requirements.
Others believe that no risk assessment of NGT products is needed, as they are similar to
conventionally bred ones.
Sustainability
Some citizens think that NGT products could contribute to the objectives of the Green
Deal, the Farm to Fork Strategy (F2F) and the United Nations (UN) Sustainability Goals
(SDGs). Others doubt the sustainability contributions of NGT products, as they are part
of a farming model dependent on chemical use, and F2F aims at circular adapted
agriculture instead of more agro-industry.
Some citizens support a sustainability assessment of NGT plants, stressing however that
such an assessment should not apply only to NGT products. Others oppose the idea of a
sustainability analysis, as they question the robustness and consistency of the approach,
and as they consider that the Value for Cultivation and Use (VCU) testing already
contains a sustainability assessment.
Traceability
Some citizens demand that traceability applies to NGT products, as it is important to
guarantee a GMO-free supply chain. Others state that traceability should not lead to
excessive evaluation costs. If NGT products are difficult or impossible to trace, practical
solutions should be found instead of banning them from the market. Some suggest using
document-based traceability if no detection method is available. Other citizens demand
more research funding for detection methods.
Information to consumers/awareness
Some citizens demand that NGT products have to be labelled to guarantee the freedom of
choice throughout the food chain and the GM-free sector, while others call for labelling
animal products if the animals received GMO feed.
Other citizens suggest to distinguish between NGT and GM products on the label, to
include the benefits of the NGT products on the label or to label every single product,
GMO and non-GMO, with the breeding technique involved. In the point of view of some
respondents, targeted mutagenesis and cisgenesis should not be labelled, or GMO
products should not have to be labelled as they are safe. Some fear that labelling of NGT
products could become a hindrance to their marketing in the EU or would make
necessary imports of some food and feed impossible.
Some citizens call for better communication on NGT and GMO products in the EU.
However, certain respondents also stress that, according to surveys, a majority of the
European population opposes the use of genetic techniques, which has to be respected by
the EU.

121
Liability/cost of contamination
Some citizens fear that a deregulation of NGT products would endanger the coexistence
of organic agriculture, increasing the costs for farmers and processors of the organic and
GM-free sector to ensure no contamination. In their view, the “polluter pays principle”
should be enforced, i.e. that the GMO producer has to prevent and be held accountable
for the contamination of non-GMO materials.
Intellectual property
Some citizens oppose the patenting of NGT products, proposing to make NGTs available
to all public and private research institutions. The absence of patents is also essential in
their view for SMEs to be competitive. They suggest to regulate NGTs according to the
International Union for the Protection of New Varieties of Plants (UPOV) Convention, as
there is no real difference between them and classical breeding technologies. Moreover,
some citizens fear that the introduction of NGT products will create a dependent
agricultural sector.
2. CAMPAIGN CONTRIBUTIONS
On IIA/policy initiative
The contributions from the campaign demand to apply the precautionary principle in
accordance with the CJEU ruling. Therefore, in the view of these respondents, all GMOs,
including NGT products, must fall under the GMO legislation, and no artificial
distinction should be made between different forms of genetic engineering. Moreover, the
Commission should implement policies that are in accordance with the objectives of the
F2F. More research on unexpected and unwanted effects of NGT plants, including long-
term effects and on interactions of the plant with the environment, is demanded. It is
stressed that sustainability and social criteria of NGTs should be assessed in a systematic
way.
A small part of campaigners further asks to ban products of and research on genetic
engineering in the EU; some ask that also their import should be prohibited. Some state
that genetic engineering should only be approved in medicine.
Risk assessment
The campaign demands a robust, process-based, case-by-case risk assessment for NGT
products. Some respondents added that it should be accompanied by a trait-based risk
assessment. An adaptation of the current risk assessment for NGT products would have to
be based on scientific evidence and would have to assess new and additional risks
emerging from NGTs.
Sustainability
The respondents of the campaign oppose the claim that NGTs can contribute to a
sustainable food system. They are part of a farming system relying on massive chemical
use. It is stated that, in countries producing GMOs, they have caused great damage to the
environment and health of the population. The Commission should promote research on
agro-ecology and organic farming as well as the breeding of peasant and farm seeds.
Traceability
The importance of traceability of NGTs is emphasised by the campaign respondents.
They stress that farmers, breeders, food manufacturers, retailers and consumers must be
able to avoid GMOs. According to them, the lack of traceability of NGTs would
endanger the organic and GM-free sector. Moreover, to reach the 25% of organic
agriculture announced in the F2F, it must be possible to avoid NGT products along the

122
food chain. Furthermore, the F2F aims to foster the transparency in the food chain for
consumers. In their view, the traceability of NGT products should be a mandatory
prerequisite for their approval; detection methods should be developed, and existing
methods should be used pro-actively for food and feed controls. Finally, campaign
respondents argue that traceability does not depend on detection methods and call for the
establishment of a public global registry including all GMO plant varieties.
Information to consumers/awareness
The respondents of the campaign consider that NGTs should be labelled as GMOs to
guarantee the freedom of choice. Otherwise, the organic and GM-free sector would be
endangered. Moreover, animal products should be labelled if the animals were fed with
GM feed.
Some stress that a majority of Austrians and Germans are against the cultivation of GMO
plants, even if NGT were used, according to surveys
Liability/cost of contamination
According to the respondents, in case of a deregulation, the costs for the organic and the
GM-free sector would drastically increase to avoid contamination of their products. For
these two sectors, it is essential to know where GMOS are, and call for the introduction
of the “polluter pays principle”.
Intellectual property
Campaign respondents express concerns about the patenting of NGT products as they
would restrict the rights of farmers and breeders and only companies would make profits
from them. Companies would claim property rights on the millennia-long breeding work
of farmers or on natural organisms, which have crossed with GMOs.
123
ANNEX 3: WHO IS AFFECTED AND HOW?
1. Practical implications of the initiative
The proposed measures guarantee safety and have positive implications for EU plant
breeders, academic research, farmers, traders, consumers and regulators, while organic
farmers will face challenges under certain conditions.
For breeders, from large companies and SMEs, NGTs will be a new tool allowing the
rapid, more cost-efficient development of plant varieties with specific characteristics,
thereby responding to the challenges of climate change, contributing to strengthening the
sustainability of the food system and to meet diverse, evolving consumer demands. They
will benefit from simplified and accelerated regulatory processes with regard to adapted
risk assessment and with regard to NGT plants that are equivalent to plants that could
occur naturally or be produced by conventional breeding. Certain NGTs are considered
accessible tools for plant breeding due to their comparatively low cost and complexity. In
this regard, NGTs are technologies, which could lead to a lowering of technological
barriers to entry of the plant breeding sector, benefitting SMEs, in particular.
Academic researchers in the plant sciences expect increased funding from public and
private sources as a result of a regulatory framework that is seen as enabling the marketing
of the products resulting from research, contributing to the EU research base and
innovation capacity in plant biotechnology and allowing researchers to use NGTs to
support environmental and other challenges to the EU and global food system.
Farmers can benefit from varieties with increased resilience, improved pest tolerance and
lower fertiliser needs, while delivering products satisfying the needs of processors,
retailers and consumers. Innovative products can also open up new income sources for
farmers.
Organic farmers will be able to rely on the tools of the GMO legislation available today
as regards NGT plants subject to authorisation (traceability and labelling, coexistence
measures implemented by the farmers cultivating NGT plants) and on transparency
measures (public register, common catalogues of varieties) to identify those subject to
notification as regards NGT plants subject to notification. The risk of admixture will
depend on the adoption rate of NGTs.
Traders of NGT products will be able to offer competitive products and operate in a
system that minimises regulatory divergence with trade partners.
Consumers can have an increased choice of products, including products with increased
beneficial and decreased harmful compounds.
Regulators will benefit from simplified and accelerated regulatory processes.
2. Summary of costs and benefits
The two tables below summarise the benefits and the costs of the preferred option.
The preferred option is a combination of option 4 for products that could also occur
naturally or be produced by conventional breeding and of an option 2 for all other
products. Therefore, in general terms costs and savings for the notification correspond to

124
the costs and savings of option 4, while costs and savings for the authorisation correspond
to the costs and savings of option 2.
However, not all the costs and savings from option 2 are directly applicable to the
preferred option. In the preferred option there will be a list of traits contributing to
sustainability which will not require additional data from the applicant and nor a specific
assessment for administrations. Therefore, those costs, presented in table 5, are not
necessary for the Annex 3.
In addition to calculate the aggregate saving and costs of the preferred option, a
hypothetical scenario in which the breeders would submit 10 notifications and 5
authorisations per year.
The hypothetical scenario is based on the number of products expected to come to the
market in the next ten years in the JRC study on NGT applications attached to the
Commission NGT study. The JRC found 134 products at pre-marketing and advanced
R&D phase, which correspond to products that could potentially reach the market by 2030,
i.e. 27 per year from 2025 when legislation could be in place. We assumed that 55% would
make it to a regulatory approval process, i.e. 15 per year from 2025. This is also consistent
with the Argentina and US figures for the early years of notification-type systems and with
the estimates from the UK 2022 impact assessment
1
for the new legislation on precision
breeding organisms.
Regarding the number of products that would fall under the notification or authorisation,
we were not able to rely on the pipeline data. We relied on the impact assessment
conclusions of the expected attractiveness of each option and assumed that notification
would entail 65% of the total applications and authorisation would entail 35% (10
notifications and 5 authorisations per year).
I. Overview of Benefits (total for all provisions) – Preferred Option
Description
Amount
Comments
Direct benefits
Cost savings for
breeders
Notification: Compared to the baseline breeders are expected to
have a reduction in compliance costs, due to the removal of the
detection method requirement, the removal of the post-market
environmental monitoring and to the change in data requirements
for the notification (data to show compliance with the notification
criteria instead of data for a risk assessment).
The savings for the breeders per notification are estimated to range
from EUR 9 952 000 to EUR 11 171 000.
Authorisation: Compared to the baseline, breeders are expected to
have a reduction in compliance costs linked to the data requirement
for the adapted risk assessment. These savings will be variable as
the adapted risk assessment will not treat all products in the same
way.
The savings for the breeders per authorisation are estimated to
range from EUR 0 to EUR 10 365 000.
Incentives: The waiving of fees for the validation of the detection
methods is considered as a potential incentive would add an extra
saving for breeders in the authorisation procedure which would be
of EUR 105 000 except for SME for which the savings would be of
EUR 52 500
2
.
Total savings for breeders under the preferred option:
Total savings for notification are estimated to range from EUR 99
For the notification, the
estimated savings are dependent
on the future data requirements
for the notification.
For the authorisation, the
estimated savings Are
dependent on the future data
requirements for the risk
assessment and by the type of
NGT
Total savings for breeders under
the preferred option: A
hypothetical scenario was used
in which the breeders would
submit 10 notifications and 5
authorisations per year. These
1
https://publications.parliament.uk/pa/bills/cbill/58-03/0011/GeneticTechnologyBill_IA_0526.pdf
2
Fees for the validation of the detection methods for GMO by the EURL are described in Regulation (EC)
1981/2006. Article 4 of this Regulation currently sets up a 50% reduction of the fees for SMEs.
125
520 000 to 111 710 000 per year.
Total savings for authorisation are estimated to range from EUR 0 to
51 825 000 per year.
are recurrent savings per year.
Cost savings for
administrations
3
Notification: Compared to the baseline scenario, Member States
administrations are expected to have cost reductions due to the
change in data requirements for the notification and to the removal
of the traceability and labelling obligation.
The savings for administrations are estimated to be up to EUR 140
000 for the analysis of the data for notification. In addition, it is
estimated that Member States would have significant savings in the
enforcement, due to the removal of the traceability and labelling
requirement, but these were not quantifiable.
Authorisation: Compared to the baseline scenario, Member States
administrations are expected to have costs reductions due to the
adapted data requirement for the risk assessment.
The savings for administrations are estimated to range from EUR 0
to EUR 140 000.
Total savings for administrations under the preferred option:
Total savings for notification are estimated to be up to EUR 1 400
000 per year.
Total savings for authorisation are estimated to range from EUR 0 to
700 000 per year.
For the notification, no data
were available for the savings as
the notification is linked to new
data requirements. The
monetisation of this saving
corresponds to a hypothetical
scenario in which the only
requirement would be the
current requirements for
molecular characterisation of a
GMO.
Total savings for
administrations under the
preferred option: A hypothetical
scenario was used in which the
breeders would submit 10
notifications and 5
authorisations per year. These
are recurrent savings per year.
Net economic
impact/market value
for farmers
Range of 9% per hectare yield improvement (for oil and fibre crops)
to 16% (for cereals) by 2030-2035. This represents, when including
cost savings from reduced input use, a total annual economic
market value of EUR244 m (for oil and fibre crops) to EUR2.7 bn (for
cereals).
Expected economic benefits to further grow afterwards as more
NGT plants are authorised / accepted under the notification
procedure and more crops are introduced.
Time to market
Breeders: Reduction of the current 4.5-year risk assessment period
for imports (6 years for cultivation). Reduction depends on case-
specific data requirements.
Application of NGTs leads to significant shorter development times
and lower development costs. For example, the introduction into
the market of a NGT potato variety is estimated to take five years, at
a cost of EUR0.5 m instead of 13-15 years for a conventionally bred
variety, at a cost of EUR2-3 m per variety.
Regulatory certainty
(likelihood that a
product is able to be
admitted to the
market after the
R&D-process)
Work on the criteria for risk assessment is intended to ensure
adaptability (requirements proportionate to hazards on a case-by-
case basis depending on the plant’s risk profile), and predictability
(ability of potential applicants to anticipate regulatory
requirements). Work on the equivalence criteria for notification is
intended to ensure predictability (ability of potential applicants to
anticipate whether the requirement for notification would be met)
and based on the product’s molecular characterisation
Trade
The preferred option minimises (compared to the other options)
regulatory divergence with EU trade partners. For example, the
detection of non-authorised GMO Triffid flax in EU food products
and the subsequent import ban on Canadian flax led to a EUR40 m
loss for the EU flax processing industry and 600 jobs lost
Differences in the regulation of
NGTs increase the likelihood of
regulatory asynchronicity.
Environmental
benefits – pesticide
reduction
According to the JRC study of Schneider et al. (2023a, b; see Annex
7):
For cisgenic potatoes: 50-80% reduction of fungicide usage, or 9 kg
per hectare, without impacts on yield or quality.
For cisgenic apples bred with monogenic resistance against scab
disease: reductions between 14% in the Netherlands and 58% in
France could be achieved, the latter equivalent to 15 kg per hectare
less fungicide use.
Environmental
benefits: fertiliser
reduction
Projections 2030-2035 based on the contractor’s study:
A decrease of 0.1% and 4%, depending on crop species and rate of
3
Cost savings for the EU institutions were not considered for this table.

126
adoption of NGT plants
Environmental
benefits: GHG
reduction
Multipurpose use of gene-edited root chicory (production of inulin
and health-beneficial terpenes): reduction of GHG emissions of
around 10% compared to the current inulin production process
when considering the entire value chain.
Use of gene-edited pennycress (Thlaspi arvense) as a cash cover
crop for biofuel production without displacing food crops
Projections 2030-2035 based on the contractor’s study:
A decrease of up to 3.1% depending on crop species and rate of
adoption of NGT plants.
Social benefits –
Health: nutritional
impacts-food
security
Health benefits for consumers would result from increased
beneficial bioactive compounds in food and feed, such as increased
levels of vitamin A, antioxidants, production of monounsaturated
fatty acids and GABA. Moreover, harmful bioactive compounds such
as cyanide, glycoalkaloids, allergens could be removed.
NGTs may affect overall health benefits (in terms of QALYs) in
different ways, including improving the accessibility to products that
might lead to healthier diets.
Such direct and indirect benefits are presented by the JRC study of
Sanchez et al. (2023), described in Annex 7 on low-gluten wheat.
Food security benefits are especially relevant for developing
countries, as Annex 7 demonstrates with the example of Maize
Lethal Necrosis (MLN) resistance, a severe threat to food security in
Eastern Africa.
Social benefits:
consumer variety
and choice
Consumers will experience improved product choice.
Administrative cost savings related to the ‘one in, one out’ approach*
Administrative cost
savings for breeders
Notification:
Reduction in administrative costs related to regulatory support is
expected. The savings for breeders are estimated to range from EUR
83 300 to EUR 833 000.
Reduction in administrative costs related to scientific support. The
savings for breeders are estimated to range from EUR 35 700 to EUR
357 000.
Reduction of the administrative costs as the data requirements for
notification may not require studies to be performed under GLP/ISO
guidelines. The savings for breeders are estimated to range from
EUR 56 000 to EUR 1 120 000.
Reduction of administrative costs as the notified NGT plant will not
require the submission of post-market monitoring. The savings for
breeders are estimated to be EUR 1 200 000.
Reduction in administrative costs as the notified NGT plants will not
be subject to a renewal procedure. The savings for breeders are
estimated to be EUR 240 000.
The total savings per notification is estimated to range from EUR 1
615 000 to EUR 3 750 000
Authorisation:
Reduction in administrative costs related to regulatory support is
expected. The savings for breeders are estimated to range from EUR
0 to EUR 833 000.
Reduction in administrative costs related to scientific support. The
savings for breeders are estimated to range from EUR 0 to EUR 357
000
Reduction of administrative costs as the data requirement in the
adapted risk assessment for authorisation may not require or may
require less studies to be performed under GLP/ISO guidelines. The
savings for breeders are estimated to range from EUR 0 to EUR 560
000.
The total savings per authorisation is estimated to range from EUR 0
to EUR 1 750 000
Total administrative cost savings for breeders under the preferred
option:
Total administrative cost savings for notification are estimated to
For the notification, the
estimated savings are dependent
on the future data requirement
for the notification.
For the authorisation, the
estimated savings are dependent
on the future data requirements
for the risk assessment and by
the type of NGT
Total savings for breeders under
the preferred option: A
hypothetical scenario was used
in which the breeders would
submit 10 notifications and 5
authorisations per year. These
are recurrent savings per year.

127
range from EUR 16 150 000 to 37 500 000 per year.
Total administrative cost savings for authorisation are estimated to
range from EUR 0 to 8 750 000 per year.
Administrative costs
saving for food
businesses
Notification: Unquantifiable recurrent savings are in administrative
costs for food businesses is expected due to the removal of the
traceability and labelling obligation.
II. Overview of costs – Preferred option
Breeders
Administrations
Farmers and food businesses
Notification of a
NGT product
Indirect costs
n/a
n/a
Recurrent costs for organic farmers.
Potential unquantifiable increases
costs risk management practices and
market monitoring (for accidental
presence of GM/NGT product) due to
the uncertainties of potential
presence of notified NGT plants in
conventional seeds.
Recurrent costs for farmers. potential
unquantifiable costs for
segregation/coexistence systems.
Authorisation of a
NGT product
Administrative
costs
n/a
n/a
Recurrent costs for food businesses.
Limited unquantifiable cost increases
due to additional information in the
label (identification on the label of the
purpose of the genetic modification
to the label) and related segregation
costs.

128
Incentive for NGT
products with
traits that can
contribute to
sustainability
Direct
adjustment
costs
n/a
One-off costs: Support
given to the applicant
during authorisation
process due to
sustainability incentive.
Potential unquantifiable
significant increase in cost
for the administrations
n/a
Costs related to the ‘one in, one out’ approach
Total
Direct
adjustment
costs
n/a
n/a
n/a
Indirect
adjustment
costs
n/a
n/a
Unquantifiable increase for farmers.
Administrative
costs (for
offsetting)
None
None
Recurrent costs for food businesses.
Limited unquantifiable cost increases
due to additional information in the
label (identification on the label of the
purpose of the genetic modification
to the label) and related segregation
costs.
3. Relevant sustainable development goals
III. Overview of relevant Sustainable Development Goals – Preferred Option(s)
Relevant SDG
Expected progress towards the Goal
Comments
SDG no. 2 – End
hunger, achieve
food security and
improved nutrition
and promote
sustainable
agriculture
- The initiative facilitates the application of speedy
innovative plant breeding to a wide variety of crops,
including regionally important crops, and traits.
- It contributes to three of the four components of food
security – food availability, stability and utilisation –
by providing a framework to address traits
contributing to pest resistance, yield, yield stability,
pesticide and fertiliser use and resistance to abiotic
factors (e.g. drought, temperature).
- NGTs could be relevant in low- and middle income
countries, which would benefit from adapting
traditional, local crop species so that they can
withstand changing conditions. An enabling
framework in the EU could also support use in those
countries.
Examples are cisgenic
potato and cisgenic
apple as well as a
virus-resistant maize
presented in Annex 7.
SDG no. 3 - Good
Health and Well-
being
- The initiative facilitates the breeding of diverse
nutritious and healthy foods. Health benefits for
consumers could result from increased beneficial
bioactive compounds in food.
One example is the
low-gluten wheat
presented in Annex 7.
SDG no. 9 - Build
resilient
infrastructure,
promote inclusive
and sustainable
industrialization
and foster
innovation
- The initiatives promotes research in plant breeding,
strengthens the research capacity in plant
biotechnology and facilitates the development of
innovative products
- The NGT R&D pipeline contains plants with traits
supporting sustainable farming practices and with
novel functional traits with health benefits for
consumers
One example is the
root chicory presented
in Annex 7.

129
SDG no 12 -
Responsible
consumption and
production.
- NGTs can contribute to the sustainable management
and efficient use of natural resources by addressing
traits improving, for example, nutrient and water use
efficiency.
One example is a
modified maize that is
more resistant to
climate stress, for
which the field trials
are on-going in the
EU
4
.
SDG no. 13 - Take
urgent action to
combat climate
change and its
impacts
- NGTs can contribute to resilience adaptive capacity
to climate-change related impacts by supporting
sustainable farming practices (no tilling), the
development of biofuel crops not requiring land use
change and land saving.
Cover cress (Thlaspi
arvense) is an example
of a cover crop
(thereby not requiring
land use change)
helping to restore soil
fertility and which can
also be used to
produce biofuels
4
https://webgate.ec.europa.eu/fip/GMO_Registers/GMO_Summary.php?NotificationNum=B/BE/22/V3
&Cat=gmp

130
ANNEX 4: ANALYTICAL METHODS
1. JRC CASE STUDIES
Objectives
The JRC conducted three case studies for the purposes of this impact assessment
1
:
Economic and environmental impacts of disease resistant apples and
potatoes developed with cisgenesis
Socio-economic impact of low-gluten celiac-safe wheat developed by gene
editing
Biotic resistance in the context of smallholder farming in East Africa: a
case on gene editing of maize to safeguard food security under the spread
of the Maize Lethal Necrosis
Selection criteria for the JRC case studies
Different criteria were used to select the three JRC case studies included in this staff
working document: economic importance of the crop, objective of the Green Deal to
which it contributes, and type of technology used for its development. In addition, the
product development stage and the information on the traits of the selected case studies
had to be publicly available.
The first criterion was the socioeconomic importance of the crop at European and global
level, as well as any potential contribution of the product developed to the European
Green Deal objectives and the Farm to Fork and the Biodiversity Strategies. The fungal-
resistant potato and apple case was selected because these crops are important for the
European agricultural sector and cuisine. Both crops are currently intensively managed
through pesticides, and the respective pathogens demand the majority of the pesticide
treatments in those crops. The case of low-gluten, celiac-safe wheat was chosen because
wheat is a fundamental crop for human consumption, and it is highly appreciated for its
gluten which enables dough production for baking. However, in turn, gluten is the cause
triggering gluten-related disorders in genetically predisposed individuals worldwide.
Lastly, the virus-resistant maize was selected as case study because maize is a major
staple crop that significantly contributes to the sustenance of millions in East Africa
where the control of the MLN disease is challenging due to economic constraints.
A second criterion corresponded to the technology used. The three case studies use
techniques that are under consideration in this policy initiative and were selected to
include products both of cisgenesis and of targeted mutagenesis. In the case of the fungal-
resistant potato and apple, both crops are notoriously challenging to breed using
conventional approaches. In the case of low-gluten, celiac-safe wheat, it is extremely
difficult to apply conventional breeding techniques to obtain comparable wheat varieties.
Conventional breeding approaches to achieve MLN virus-resistant maize plants are
estimated to take longer time and higher resources than the gene editing ones.
The last criterion was the product development stage and the availability of public
information on the product. Several products in the development pipeline were just in
1
See Annex 7 for details
131
proof-of-concept stage, or data were unavailable due to confidentiality provisions. In the
case of fungal-resistant potato and apple, the developments are beyond a proof-of-
concept with field-trials supporting the evidence collection, and the cisgenic varieties
have been developed by European institutes. Low-gluten, celiac-safe wheat lines have
already been cultivated in greenhouses, and the varieties have been developed by two
main research centres in the EU with published scientific evidence. Virus-resistant maize
plants are currently being tested in greenhouse trials, and the varieties have been
developed by an international research organization in collaboration with an agricultural
research company.
Approach
Economic and environmental impacts of disease resistant crops developed with
cisgenesis
To compute potential hectare-level cost savings from the cultivation of the cisgenic
varieties, a stochastic partial budgeting model was used to simulate changes in expenses
for plant protection products. The model assumes that output and other costs remain
unchanged, which is supported by the field-trial data. Country-specific, hectare-level,
economic data for the years 2014 to 2017 was obtained from the IFM-CAP model and
used to characterize costs and outputs of typical arable crop (fruit) farms with a revenue
share of more than 60 % from potatoes (apples). The model assumes that the share of
fungicides on total pesticide use equals the cost share of fungicides on expenses for plant
protection products. Notably, this approximation is unable to account for potential price
differences between fungicides, insecticides, and herbicides.
To aggregate the simulated hectare-level savings to a country-level across different
scenarios for the adoption rate, the median parameter values for the partial budgeting
model were used and aggregated the hectare-level results to the Member States' area of
production multiplied by a given adoption rate.
To simulate potential sector-wide implications, the Common Agricultural Policy
Regionalised Impact analysis (CAPRI) model was used to shock the cost curve under two
adoption scenarios. For our discussion on the potential contribution to the national HRI
reduction, we used estimates of the employed quantities of active substances in German
potatoes and apples published by the Julius Kühn Institute. Through cross-referencing the
database on the HRI-categorization, JRC derived that majority of the currently used
active substances are in groups two and higher.
To simulated potential changes in greenhouse gas emissions related to agricultural
activities under the adoption scenarios for the cisgenic potato variety, the CAPRI model
was used to calculate N
2
O-emissions and CO
2
-equivalents due to land-use change under
the two adoption scenarios.
To calculate country-specific distributions of minimum distances between fields and
freshwater systems, spatial data from Copernicus and D'Andrimont et al. were used to
calculate the Euclidean distances between land-use pixels and different types of
freshwaters. To calculate changes in fungicide risk in Lower Saxony, the SYNOPS model
was used in conjunction with the field trial fungicide spraying schedules, land-use, soil,
and weather data. In SYNOPS, risk indices are expressed as the Exposure Toxicity Ratio,
calculated as the ratio of the Predicted Environmental Concentration to the toxicity
endpoints half maximum effect concentration, lethal concentration, lethal rate, lethal
dose, and no-effect concentration for specific reference species.
132
Socio-economic impact of low-gluten celiac-safe wheat developed by gene editing
For the impact analysis, it is assumed that the gene-edited low-gluten, celiac-safe wheat
has passed the mandatory regulatory risk assessment and is accepted for EU cultivation
and market. It is assumed that low-gluten, celiac-safe wheat will be a niche product,
likely bought by CD, NCWS and other patients and consumers willing to reduce gluten
consumption. Given the lack of observable data since the product is not yet available on
the market or cultivated in the EU, a literature review on similarities and differences with
standard wheat and with other similar specific products (e.g. gluten-free products) already
existing on the market was carried out. Data collected to be used in the impact analysis
mainly come from scientific articles published in peer-reviewed journals listed in the
Web of Science
TM
, PhD theses and reports produced by different celiac and consumer
associations. Data from the European Commission database on agricultural production
and trade and the EU FADN (farm accountancy data network) were used for the
economic analysis.
To assess the social and health impacts different parameters were calculated including i) a
price comparison between the cost of the current gluten free diet and the current financial
support to cover it, the standard diet cost, and the diet with low-gluten celiac-safe wheat
cost; ii) the potential benefits of low-gluten, celiac-safe wheat from a nutritional
perspective qualitatively measured; and iii) the estimation of costs for the healthcare
system post-diagnosis for celiac disease patients consuming low-gluten, celiac-safe wheat
and, the reduction of related work productivity loss. To assess the economic impacts, an
analysis of whether potential cost fluctuations could be expected for the agricultural
production of low-gluten, celiac-safe wheat in terms of crop yield changes, changes to the
use of fertilisers and plant-protection products and changes to management practices was
carried out. The potential impact of the adoption of the low-gluten, celiac-safe wheat on
gross margins in the EU was calculated by comparing with a standard farm budget for
standard wheat. Lastly, the impact of low-gluten, celiac-safe wheat adoption for the EU
agri-food system was measured as i) potential benefits for the wheat value chain, and ii)
changes on exports and imports depending on three levels of adoption considering the
prevalence of gluten-related pathologies.
Biotic resistance in the context of smallholder farming in East Africa: a case on gene
editing of maize to safeguard food security under the spread of the Maize Lethal Necrosis
For this case study, no original research was carried out. In this case, the JRC team
reached out to the developers at CIMMYT obtain permission to summarize existing
research undertaken at this institution both in terms of technology for development,
comparison with conventional breeding and economic impacts based on modelling. The
original sources are referenced in the case study.
Use
Insights from the case studies were integrated both in the contractor’s study report and in
the section 6 of the Staff Working Document, mainly supporting individual impact areas.
133
2. DOCUMENT REVIEW
Objective
Desk research was carried out to identify relevant reports, opinions, scientific and grey
literature to provide further evidence for the problem analysis, to identify experts for
interviews, focus groups, and surveys, and identify data to support potential quantitative
gaps.
Approach
The document review followed a targeted search on documents related to impacts and
main stakeholder categories. This resulted in a qualitative scanning exercise of 172
documents. A qualitative assessment of the content yielded 31 to 71 documents per
impact category. The documents were processed in the Atlas.ti and coded. The relevant
information per impact was then extracted to serve as information per impact area.
Use
The analysis of the documents helped in the design of interviews and consultations, to
substantiate the various statements put forward in the survey and the identified impact
areas and to analyse impacts. Furthermore, it was used to substantiate the findings
through triangulation.
3. DESCRIPTION OF REGULATORY SITUATION IN OTHER JURISDICTIONS
Objective
The global regulatory situation may impact several aspects considered in the impact
assessment, including trade, enforcement, availability of products, competitiveness. The
objective was to identify countries that have recently adapted the regulatory oversight to
genome edited products or have announced that they are considering it. In addition,
examples were sought also on countries that have decided not to do adaptations.
Approach
For the description of the regulatory situation of NGTs in third countries, the
Commission relied on available information from scientific publications, official
websites, legislation (referenced in section 1.4.). The description covers the US, Japan,
Argentina, India, the UK, China, Kenya, Nigeria, Switzerland, Canada, New Zealand and
South Africa.
Use
The analysis of the documents was used to summarise the approach to regulatory
oversight on genome editing in third countries and to assess impacts linked to regulatory
developments outside the EU (in particular as regards trade and competitiveness).

134
4. STAKEHOLDER CONSULTATION STRATEGY
Objective
In line with Tool #53 of the Better Regulation Toolbox, the consultation strategy
2
was
designed to gather evidence and views from the public and from a broad range of
stakeholders across all levels of the agri-food chain, and beyond, who are impacted by or
interested in the products under the scope of this initiative, and to reach a high degree of
complementarity among the various consultation actions: Inception impact assessment,
public consultation, targeted survey, targeted interviews, focus groups. More particularly,
the consultation activities were designed to allow all interested parties to provide relevant
information on the problems presented, the baseline scenario, and on the potential ways
forward; to assist the Commission in understanding the implications of the possible
policy options for the different stakeholders involved; to enable identifying additional
policy elements and ensure that no impacts are overlooked.
The consultation activities also built on and took into account the information received
during the comprehensive targeted consultation with Member State competent authorities
and EU-level stakeholder associations carried out in the context of the Commission NGT
study.
See detailed analysis of stakeholders’ contributions in Annex 2 – Synopsis report.
Approach
Following the consultation objectives, a broad range of stakeholders were identified in
the consultation strategy. This included operators active, from farm to fork, in the agri-
food and feed system (including farmers, seed and plant breeders, traders, processors,
manufacturers, retailers and food services, GM-free and organic operators), operators of
plant and bio-based industries active in sectors other than the agri-food sector, academic
and research stakeholders, civil society/non-governmental organisations, public
authorities at EU and national level in the EU and third country public authorities. They
represent stakeholder categories directly relevant, i.e. stakeholders affected by the
legislation on GMOs, and stakeholders involved in the implementation or with a stated
interest in the policy.
Stakeholders invited to participate in targeted consultation activities were identified by
the external contractor, based on the objectives and stakeholder categories of the
consultation strategy, and with the aim of ensuring representation of all stakeholder
categories. Concerning the mapping of economic operators, country coverage and size of
enterprises were two important aspects, which does not mean that all EU Member States
would be equally represented but rather that economic characteristics would be taken into
account. In order to capture the large spectrum of economic operators, EU-level
associations were identified.
At national level, public authorities in charge of the implementation of the GMO
legislation and enforcement of the Directive and market surveillance authorities were
addressed in the consultation.
The mapping of the stakeholders was based on:
2
https://food.ec.europa.eu/system/files/2022-09/sc_modif-genet_consultation-strategy-ngts.pdf

135
desk research (see section 2)
the contributions of the Member States and EU-level associations to the
targeted consultation in the context of the 2021 Commission NGT study
the analysis of the respondents of the inception impact assessment
expert contributions
the use of Crunchbase (for companies) and
Scopus, for the identification of academic experts
An internal database of stakeholders was developed to include
Research organisations
Non-governmental organisations
Competent authorities
Private-sector organisations
Stakeholder organisations (associations)
Individual experts
Other
For national authorities, the national competent authorities for the purposes of GMO
legislation were contacted.
The database structured the collected information into name, email, organisation, country,
type of organisation and stakeholder type. It contained more than 650 individuals from
around 570 organisations. In addition, about 1.100 researchers were identified through
Scopus. This list served as a wide reserve in case of a need of academic experts.
Use
The stakeholder database was used as the basis for the selection of the targeted survey
respondents and of the interview partners. Interviewees were asked to suggest individual
organisations which could be invited to the stakeholder survey. The interviewed
stakeholders were equally invited to the stakeholder survey. Cost interview partners were
either addressed following the general interviews or based on further dedicated desk
research. In view of workshops, case studies and focus groups, additional desk research
was conducted to identify relevant experts. The focus groups benefited also from
organisations which had been interviewed in the first phase.

136
Figure 1 Flow diagram of use of stakeholder in consultation
Source: Technopolis Group
5. INTERVIEWS
Objectives
An interview programme was set up by the external contractor. It involved a selection of
the main stakeholder groups (economic operators, research, and non-governmental
organisations, as well as authorities at EU and national levels). The 60 interviews were
divided into three groups:
1. General interviews with key stakeholders.
2. Interviews focused on regulatory cost assessment (see section 4).
3. Interviews for the case studies (see section 8).
The objective of the interviews with (1) key stakeholders was to tap into the knowledge of
the main stakeholder types regarding possible effects and impacts of the policy
components. The second (2) group of interviews focused on current and potential
regulatory and administrative costs. (3) For the case studies, technical experts were
targeted. In case of third countries, relevant associations with the relevant geographic
knowledge were approached.
Approach
General interviews
Through the desk research, document analysis, and exchange with the Commission as well
as the project’s internal experts, the external contractor identified relevant organisations
and potential individual interviewees. They were selected based on their (1)
expertise/knowledge and (2) strategic positioning in the debates on agricultural
biotechnology, and with the aim of ensuring representation of the main stakeholder

137
categories. In addition, the economic operators were selected on the basis of their potential
to act as ‘multipliers’ for the dissemination of the targeted survey.
General interview guideline
Box 1 provides the guideline for the general interviews used by the external contractor.
Box 1. Interview guideline for the stakeholder interviews
The objective of the stakeholder interviews is to collect expert opinions and data in order to
map potential effects and impacts of the different components of the policy scenarios. We
aim to tap into the knowledge of the federations and its members through these interviews.
The objective of the stakeholder interviews differs from the open consultation survey. In the
survey, each stakeholder has the opportunity to provide their position towards the possible
components of the policy scenarios and the current GMO/NGT policy.
With the interviews, we would appreciate a neutral and open conversation regarding
possible effects and impacts in the value chain and wider economy and society.
The interview will follow a semi-structured guide and is based on the Theory of Change
developed during the inception phase of the study. Policy options reflect the public open
consultation conducted by the Commission.
Responses are recorded in an interview report and kept anonymous unless indicated
otherwise by the interviewee.
I Introduction
1. Introduction of interviewee
2. Introduction of the EU-level federation / association and its sectors and main
activities
3. Relevance of NGTs for federation members
a. (Expected) development and use of NGTs in respective section of the value
chain
b. General assessment of current obstacles and enablers of development and
use of NGTs in respective section of the value chain
II Risk assessment and detection requirements
Explain: policy scenario components, implementation effects, value chain and wider impacts
Risk assessment and detection requirements: explanation of main elements
0: Unchanged policy and regulation
A1: Authorisation with risk assessment adapted to risk profile and adapted detection
method requirements
Risk assessment proportionate to the NGT product’s risk profile. Detection method required,
but differentiation of NGT product from conventional product not required if not possible
A2: Pre-notification of products that are also obtainable naturally or by conventional
breeding with decision on regulatory status
Risk assessment not needed if NGT product can also be obtained naturally or by
conventional breeding. Detection not needed if NGT product can also be obtained naturally
or by conventional breeding
Topics to be discussed; probe with developed indicators in area of expertise:
4. How do you expect regulatory product approval costs for applicants to change under
option A1 and option A2?
5. To what degree do you expect the policy options to impact the attractiveness of
introducing new plant varieties on to the market by plant breeders?
6. How will these options impact the availability and attractiveness of using new plant
varieties by farmers?
7. In terms of safety of gene-edited/cisgenic crops and derived products, do you
expect changes in safety under option A1 and A2?
8. Could you assess impacts of the policy options on the wider value chain, including

138
productivity, turnover and value added of economic operators (plant breeders,
farmers, processors, etc.)?
9. Could you assess the impacts of changed risk assessment and detection
requirements on:
EU competitiveness and trade?
Innovation and research?
SME competitiveness?
III Labelling and Traceability Requirements
Explain policy scenario components, implementation effects, value chain and wider impacts
0: Unchanged policy and regulation
B1: Additional sustainability label and traceability
Labelling as today, coupled with additional label for sustainability claims, referring to the
sustainability contribution of the introduced trait. Traceability as today, coupled with
additional traceability to ensure information on the sustainability contribution.
B2: No labelling if sustainable, with traceability and transparency requirements
No specific labelling needed, but inclusion in public registry, if NGT product contributes to
sustainability. Traceability as today, coupled with additional traceability to ensure
information on the sustainability contribution
B3: No labelling and specific traceability requirements if a product obtainable naturally or by
conventional breeding. Transparency requirements
Labelling not needed if NGT product can also be obtained naturally or by conventional
breeding, but inclusion in public registry. Traceability not needed if NGT product can also be
obtained naturally or by conventional breeding
10. Could you assess the most significant impacts of the policy options in terms of
Labelling and traceability costs in the value chain?
Labelling and traceability quality in the value chain?
11. Could you assess the effect of the policy options on the enforcement quality and
transparency?
12. Could you assess the impacts of the policy options on consumer trust and
information rights?
IV Sustainability
Explain policy scenario components, implementation effects, value chain and wider impacts
0: Unchanged policy and regulation
C1: Sustainability incentives for authorisation
Positive regulatory incentives for authorisation: NGT plant products with traits that
contribute to sustainability objectives receive regulatory positive incentives for
authorisation, e.g. regulatory and scientific advice before and during the approval
procedure, measures to facilitate the approval process (waiving of fees, faster procedures),
allowing sustainability-related claims to appear on the final product.
C2: Sustainability requirement: no authorisation if detrimental to sustainability
NGT Plant products with traits that are detrimental to sustainability objectives are not
authorised
13. Do you expect an increase in NGT plant products with traits that contribute to
sustainability objectives in case of regulatory positive incentives for authorisation?
14. Could you assess potential environmental impacts under C1/C2 on:
Biodiversity
Pesticide use
Fertilizer use
Use of natural resources
V Collaboration in study
15. Do you have data on costs and benefits (qualitative and quantitative information)
relevant to the impact assessment and would you be willing to share this

139
Cost interviews
For the cost assessment interviews, 23 different organisations were approached (SMEs,
large companies, public authorities, regulatory authorities, etc.). They were identified
through the initial stakeholder mapping exercise. Following an initial list of suggested
organisations and authorities, a first group of potential organisations were contacted. In
the course of the study, more organisations were approached. The focus here was not on
coverage of all stakeholder groups, but those involved in the regulatory approval process.
Consecutive discussion rounds with public and private entities have been conducted,
where the purpose of the exercise was explained. During the discussions, several public
and private entities dropped out from being prospective data providers. Some considered
that the required level of detail of data was not feasible to provide. This was either due to
time constraints or due to a mismatch of available company cost categories and the level
of breakdowns required.
Overall, a caveat for the cost data was the limited to no experience with GMO
applications in Europe at industry level, the large ranges within estimated cost categories
and difficulties in triangulating rather patchy data with equally patchy evidence from
literature and insights from experts.
Case study interviews
It has been extremely challenging to attract interview partners for the case studies, in
particular those that dealt with third countries. Most cases identified relevant
organisations during the concept-note phase, yet several did not respond when contacted,
even after repeated reminders.
Use
The insights from the general interviews helped greatly to understand the expectations
and concerns of various stakeholders and identify relevant impact areas. Together with
insights from document analysis, interviews were also used to formulate several survey
questions. Interviewees were asked to suggest relevant additional individual stakeholders
(mainly breeders and retailers) which were added to the survey.
The cost interviews and the data gathered (calculations made and qualitative
explanations) provided were the basis for the efficiency sections of the final report.
6. TARGETED STAKEHOLDER SURVEY
Objective
The goals of the targeted survey were primarily
to collect the informed views and information from stakeholders on the
performance of the current baseline scenario as a benchmark to support the
assessment of efficiency
to collect the views, information and assessments on the various impacts of the
policy components
to test the key elements of policy options on the impact indicators
information?

140
to collect information on costs or other pertinent data, e.g., on impacts of the
elements of the policy options and the baseline (current situation).
The survey was envisaged as a targeted survey to complement the (partly in parallel
running) public consultation (PC). Whereas the PC addressed a wider audience and
therefore did not ask for detailed technical assessments, the targeted survey envisaged
detailed information.
Approach
The selection of stakeholder groups to be targeted by the survey followed a further
characterisation of the stakeholders collected in the contractors’ database: all selected
stakeholders either:
had participated in the targeted survey carried out by the Commission in 2020 in
the context of the Commission 2021 NGT study;
had participated in the consultation carried out in the context of the inception
impact assessment; or
were suggested by interview partners - this served the purpose to address in
particular SMEs - such as breeding companies - or retailers.
The majority of the invited participants could not easily be classified with one
characteristic such as “public authority”, “research organisation’, “company”,
‘business/industry association”, or “non-governmental organisation” (NGO) – but they
combined two or more functions/roles. For example, a company could be in breeding, but
also be a manufacturer, or selling. Therefore, the classification of the identified
stakeholders focused on obtaining a reasonable allocation and leaving it up to the survey
respondents to self-classify in the range of stakeholder types. Table 1 provides an
overview of the invited stakeholders. The vast majority of stakeholders are set in Europe
with a clear geographic focus of associations based in Belgium.
The following figures provide information about the invited respondents, using a
classification according to the contractor’s study team.
Almost 400 public and private stakeholders (e.g., associations, authorities,
networks, companies) were targeted to provide their insights and contribute via
the survey.
At EU-level, 101 organisations were invited, the majority (68) being associations
representing different sectors of economic operators. A similar number of national
industry associations were also targeted (63). These tend to be (conventional or
organic) seeds, farmers, breeders, or biotechnology associations.
Private sector organisations – large as well as small and medium-sized companies
were altogether the second largest group. The latter group of SMEs brings
together the range of actors of the value chain from breeders to processors to
retailers. The third largest – and most heterogeneous group – is the one of non-
profit organisations. As indicated above, this includes a range of type of
organisations, including networks (e.g., (organic) seed networks, agroecological
or researchers’ networks), and a broad variety of interest groups (e.g., opposing
GMO or not, consumer and environmental advocacy groups, etc.).
Public sector organisations were distinguished from public authorities/ministries.
These are often independent or sub-ordinate executive entities but have no
political function like ministries.
141
The delineation between research organisation and advisory body is somewhat
artificial. As indicated, some research organisations (public research institutes or
university entities) are equally officially advisory or competent entities for a
public authority.
Table 1 Overview of invited survey participants
Type of stakeholder
EU
national
World
Total
Business/industry association
68
63
6
137
Private-sector organisation
5
66
31
102
Non-profit organisation
19
60
14
93
Political entity
2
2
Public sector organisation
2
2
4
National authority/Ministry
27
1
28
Research organisation
4
11
2
17
Advisory body
1
2
3
Other
9
2
11
Grand Total
101
218
52
397
The contractor’s study team sent out personal invitations to 369 identified stakeholders
through Limesurvey. The Commission forwarded individual survey links to the EU
Member States and EEA countries (in total 28). Those were invited to consolidate
responses per country.
The survey was translated from English and available also in Dutch, French, German,
Italian, Polish, and Spanish.
It was sent to stakeholders and Member State representatives on 28 July 2022 and was
planned to remain open for the stakeholders until 10 August and for Member States until
24 August 2022. Given technical problems and the vacation period hampering the
availability of experts to be consulted, the survey closed on 5 September 2022.
The study team received mails from individual stakeholders who informed that they could
not respond due to time constraints, due to no immediate relevance, or because they found
the survey’s scenario approach problematic.
Some Member States voiced that they had difficulties in filling in the survey without being
able to include explanations; in some cases, they have also submitted an additional
document. Out of the Member States, which did not fill in the survey, one submitted
available relevant information in a dedicated document.
The survey was designed with a complex filtering: all respondents obtained the same
survey but, depending on their self-classification, only specific questions appeared. None
of the questions was mandatory to be filled in. This implies that for the analysis of the
questions, the number of responses varies. Therefore, in the analysis the number of
responses were provided per question. In questions where responses were not provided for
all categories, the minimum number was indicated.
Of the 397 invitations that were sent out, 143 opened the survey and included some
information on the respondent but did not proceed further. These entries were deleted and
not taken into account. Two withdrawals were removed, so that in the end the analysis was
based on 123 responses.
142
Use
The results of the stakeholder survey fed the impact assessment (main study report) by
impact area, indicator, and across the various scenarios.
7. PUBLIC CONSULTATION (PC)
Objectives
Following the Inception impact assessment, the PC was carried out by the Commission
(publication on ‘Have Your Say’ portal) and enquired about the current situation and tested
the general key components of the potential options.
Approach
In line with the Better Regulation Toolbox, a number of small campaigns have been
identified and analysed separately.
The PC replies were analysed by the external contractor with descriptive statistics for the
closed questions by stakeholder group and provided a mixed qualitative/quantitative
approach for the open questions, equally by stakeholder group. A number of position
papers, statements and links to additional references and literature were provided by
respondents. These were classified, analysed and summarised.
Use
A PC as such is not a representative survey and therefore, the results need to be taken as
what they are: a snapshot of views and opinions of individual people and organisations
with a strong interest in the subject matter (self-selection bias). The results from the PC fed
into the main study. They complemented the targeted survey, in particular through
contributions from the research communities and individual citizens. Given the limited
number of questions and their broad level, the PC results fed mainly environmental impact
areas and less so economic or social impacts.
8. FOCUS GROUPS
Objectives
Two focus groups, one on ‘Sustainability’ and the other on ‘Traceability’ were conducted
by the external contractor. They were intended to complement the information obtained in
interviews and the targeted survey. The focus groups aimed at providing specific feedback
and expertise on the topics of:
Traceability and enforcement systems in the agri-food chain (including
tools, registries and procedures used when analytical methods are not available
or not reliable);
Approaches to sustainability analysis in food and agriculture related areas
(including approaches to assess potential positive or negative contributions to
sustainability, and regulatory mechanisms to promote sustainability).
Approach
A concept note was developed for each of the focus groups. This included a few high-level
questions which were developed based on results of the targeted stakeholder survey as well
as insights from the interviews.
143
Relevant potential focus group participants were contacted and invited.
The focus groups’ planning started following the analysis of the targeted stakeholder
survey. In terms of development of key questions, the groups could address, results from
the PC and the targeted survey were used.
Following prior agreement of the participants, the two focus group meetings were
recorded. The recordings were used to inform the summary reports. These were sent for
validation to the members. Suggested amendments were integrated in the reports.
Selection/invitation process
A list of potential participants has been identified on the basis of the stakeholder database
and dedicated desk research. The following selection criteria have been used:
Balance among geographic expertise (different Member State
perspectives);
Balance among different crop/value chains expertise;
Balance among different technical sub-fields expertise.
The list included experts from Member State ministries or authorities, relevant
stakeholders, including operators and academia.
Use
The insights from the focus groups fed into the main report, predominantly in the relevant
impact sections. Arguments raised in the focus group (such as the needed system’s
approach in the sustainability group or the need for transparency in the traceability group)
were included in the analysis.
9. COMPLEMENTARY ANALYSIS
Objectives
Complementary analysis was carried out for specific impacts for which more detailed
information was lacking.
Approach
Three types of cases were envisaged:
A comparative case (geography), looking at third countries where comparable
regulatory changes have already led to economic, environmental, and societal
impacts (Regulatory developments for novel genomic techniques: a case study of
Argentina)
Comparative cases (domain/sector), looking at other sectors and regulatory areas
where similar challenges/problems are present.
Deepening cases, where we develop impacts for specific product/application areas
to arrive at more concrete (e.g., quantitative) estimates relating to the policy
options. (Value chain dynamics: the case of potatoes in the Netherlands; Root
chicory).
The analysis was developed by the external contractor based on desk research and
document review. A short concept note was developed and discussed with one or two of
the technical experts within the team in order to discuss the focus of the case. Ideas on

144
potential interview partners and/or further literature were exchanged and in case of
identified interviewees, they were contacted and in case of agreement, interviewed
eventually.
Use
The insights were integrated in the contractor’s final study report, mainly supporting
individual impact areas.
10. EFFICIENCY /COST BENEFIT ANALYSIS
Objectives
Identification and measurement of current and potential regulatory costs.
Approach
In the absence of NGTs on the market (commercially, neither in Europe nor in the rest of
the world), there is no quantitative data available that allows us to measure directly
regulatory costs. Therefore, an approach was designed that mixes qualitative and
quantitative information and draws from experience with GMO authorisation. Since also
GMO products are not available on the European market, the contractor aimed to address
all stakeholders that have or had relevant experience.
The activities with regulatory cost implications during the lifecycle of a GM crop include
the submissions for field trialing, cultivation, for the authorisation of the use of food and
feed, and the importation into the territory of the European Union.
Identification of stakeholders
Stakeholders covered in the cost assessment: biotech industry/ plant breeders, national
public authorities, European institutions/bodies; processors, food industry, retail, organic
and GMO-free farmers, consumers. For the plant breeders/ plant biotech industry and
national public authorities, costs were monetised while for organic and non-GM farmers,
processors, the food industry, and retail, costs are described qualitatively.
The typology of costs follows the Better Regulation Guidelines, Tool #56. A distinction is
made between monetised costs and those cost categories that remain with a qualitative
description.
Table 2 Mapping of costs (monetised/described)
(1)
Cost typology
Biotech/
Plant
Breeders
Nat’l Public
Authorities
EC &
Agencies
Organic and
GMO -free
farmers
Processors
Retail
DIRECT
Compliance
Administrative costs
Adjustments costs – capital
expenditures
Adjustments costs –
operation and maintenance
costs
Hassle costs

145
ENFORCEMENT
costs
Monitoring; Inspection;
Adjudication/Litigation
INDIRECT
costs
Indirect compliance costs
Transaction costs
Offsetting/substitution costs
Opportunity costs (e.g.
alternative solutions are
neglected
Market Access
Reduced innovation or
investment (research, field
trials relocated outside the
EU)
Other indirect costs –
Competition
Note (1): monetised described
Cost assessment - process
The process is broken down to phases for which costs are estimated (see Better
Regulation Guidelines, tool #56) as indicated below. The process was used for
the BAU and the policy options where applicable and possible.
Table 3 Cost assessment – Phases
Steps
Description
Phase I: Preparatory analysis
1
Identification and classification of information obligations
2
Identification of required actions
3
Classification by regulatory origin
4
Identification of relevant cost parameters
Qualitative assessment of significant burdens
5
Identification of target group(s)
6
Choice of data sources
Phase II: Data capture and standardisation
7
Assessment of the number of entities concerned
8
Assessment of the performance of a ‘normally efficient entity’ in each target group, taking into account cost parameters
identified in step 5
Phase III: Calculation and reporting
9
Extrapolation of validated data to EU level
10
Final reporting and transfer to the database

146
Cost assessment – consultation strategy
Four stakeholder categories were distinguished:
1) Biotech industry and plant breeders: Costs were estimated using inputs from
dedicated interviews (based on detailed cost templates), the targeted
stakeholder survey and desk research.
2) Farmers (GMO free and organic), Processors and Retailers: Costs are
described qualitatively using inputs from interviews, the targeted
stakeholder survey. They were monetised where possible with the combined
inputs of desk research and interviews.
3) Consumers: Costs can only be described qualitatively, based on interviews
and desk research.
The three stakeholder groups were asked on costs for BAU and ‘components’ of the policy
options via the targeted stakeholder survey.
Figure 2 Consultation strategy – per stakeholder type
Use
The results from the cost assessment were directly incorporated in the final report in the
efficiency analysis of the options.
11. MULTICRITERIA ANALYSIS
Objective
Aggregation and analysis of individual impacts into an assessment for each key policy
objective so that policy scenarios and options can be compared more easily and
systematically.
Approach
The approach was based on a multicriteria decision analysis (MCDA), while integrating
some aspects of the social multi criteria evaluation (SMCE). A formal SMCE requires a

147
detailed mapping of values and preferences of each main actor type. However, the
targeted consultations showed that stakeholders have often very idiosyncratic and specific
views regarding the policy options, being in favour of some elements and not others,
highlighting or not specific criteria etc., and therefore it was concluded that a formal
SMCE is not viable. Instead, it was observed that the objectives of the initiative
themselves already provide a clear framework for analysis, as stakeholders were typically
aligned around specific objectives. We thus used the MCDA, which does pay attention to
different stakeholder categories’ views and interests as part of the criteria. It is important
to note that the translation to assessment of policy options itself was carried out based on
a synthesis of these outcomes (see Chapter 6, main study report). The multicriteria
analysis itself was analysed at the level of impact areas (which reflect key stakeholder
perspectives and (parts of) potential subobjectives). Further aggregation (at the level of
dimension or policy option overall) required weights that involved political choices.
Within the impact areas, we therefore assigned equal weighting to each criteria, as a
default option in absence of a credible alternative. As a further step, we concluded a
sensitivity analysis which is described further below.
The full overview of criteria per impact area is presented in Annex 11 of the contractor’s
final report
3
.
The individual scoring of the individual criteria was carried out at the ordinal level, with
a 5-point scale (--, -, 0, +, ++), with the possibility to introduce a range reflecting
uncertainty. The scoring directly mirrored the impact assessment as carried out in the
main study report (Chapter 4) for each impact criterion.
As such, the output is a table with an individual score for each criterion, and an
aggregated score for each impact area, for each policy scenario. We then translated this
back to the policy options, each consisting of a combination of one A-scenario, one B-
scenario and one C-scenario. In order to add up the aggregated score per impact area for
each option, we applied a weighting of 4:2:1 for A:B:C. This weighting was mainly based
on the relative importance of factors for determining the uptake of NGTs as indicated by
stakeholders in the targeted survey (SQ11) and the targeted interviews. The weights do
not reflect any judgement on the importance of related impact areas, but rather on the
degree to which these regulatory components are likely to engender change in economic
behaviour.
Use
The multicriteria analysis contributed to the comparison of the effectiveness of policy
options.
3
Technopolis Group, Arcadia Int. & Wageningen UR (2023)

148
ANNEX 5: THE TECHNIQUES
1. INTRODUCTION
The DNA is the genetic material (genome) of organisms that carries the information for
the development, functioning, growth and reproduction of all known organisms and
many viruses. The information is stored and transmitted to progeny in DNA sequences
based on a four-“letter” code. Changes in these sequences are called mutations. These
can be substitutions/deletions/insertion of “letters” or of longer DNA sequences. These
changes may have no effect or may result in new or altered functions or loss of functions
in the organisms. Mutations are ubiquitous and natural phenomena that form the basis of
plant breeding providing plant variety and food diversity.
Genomic techniques are techniques that can alter the genetic material of an organism. For
the purpose of this document, the term new genomic techniques (NGTs) is used to refer
to technologies that have been developed over the past two decades after adoption of the
current GMO legislation.
New genomic techniques constitute a diverse group of techniques that can be used in
various ways to change the genome of an organism and achieve different results and
products. The objectives of the user and the technique determine the type and magnitude
of the genome modification, which can range from limited changes, that might also occur
in nature or through conventional breeding, to multiple and more extensive
modifications.
Among NGTs, targeted mutagenesis techniques induce mutation(s) in selected target
locations of the genome without insertion of foreign genetic material.
Cisgenesis or intragenesis techniques insert genetic material into a recipient organism
from a donor that is sexually compatible (crossable). The exogenous genetic material can
be introduced without (cisgenesis) or with modifications/rearrangements (intragenesis).
NGTs can also be used in the same way as older, established techniques of genetic
modification, i.e. to insert foreign genetic material into a recipient organism from a donor
organism that is sexually incompatible (transgenesis), e.g. a gene from an insect into a
plant. Transgenesis is however out of the scope of this initiative, which covers only
targeted mutagenesis and cisgenesis (including intragenesis).
More details on mutagenesis, cisgenesis/intragenesis and transgenesis, and the different
technologies that can be used to affect the respective outcomes, are provided below.
2. MUTAGENESIS TECHNIQUES
In plants, substitutions/deletions/insertion of “letters” of the plant’s genome may take
place under natural conditions or by human intervention through different techniques:
a) by conventional breeding techniques
1
:
i) combining, by crossing, the genetic material of different organisms that are
sexually compatible;
1
For a detailed description of conventional breeding techniques see SAM explanatory note on new
techniques in agricultural biotechnology - https://research-and-innovation.ec.europa.eu/knowledge-
publications-tools-and-data/publications/all-publications/new-techniques-agricultural-biotechnology_en

149
ii) by techniques of induced random mutagenesis, i.e. by using chemicals or
radiations that increase the frequency of mutations introduced randomly in the
genome;
b) by new genomic techniques of induced targeted mutagenesis (e.g. type 1 and 2
genome editing, see below, oligonucleotide directed mutagenesis
2
, base editing,
prime editing
3
) that target the mutations to (a) specific region(s) in the genetic
material of an organism.
Figure 1: Mutagenesis under natural conditions or by human intervention
3. CISGENESIS AND INTRAGENESIS TECHNIQUES
In addition, modifications of the genetic material of plants may occur through the
insertion/translocation of longer DNA sequences from the same species or a crossable
species. This may take place under natural conditions or by human intervention
through different techniques:
c) by conventional breeding techniques, e.g. combining, by crossing, the genetic
material of different organisms that are sexually compatible
d) by new genomic techniques of:
i) cisgenesis, inserting an exact copy of a DNA sequence derived from the same
or a crossable species;
ii) intragenesis, inserting a re-arranged copy of a DNA sequence derived from
the same or a crossable species.
In conventional breeding, by crossing, the desired trait is introduced, but together with
other unintended/undesirable traits from the donor organism. Removal of these
unintended/undesirable traits requires several steps of backcrossing (i.e. repeated
2
Oligonucleotide directed mutagenesis (ODM) is based on the use of oligonucleotides for the induction of
targeted mutations in the genome, usually of one or a few adjacent nucleotides.
3
Base editing and prime editing are other targeted mutagenesis techniques introducing specific genetic
changes in a targeted sequence without the use of any template DNA.
150
crossing with the original plant). This can be a long process taking several years (e.g. 8-
10 years for wheat up to 20-25 years for apple
4
).
Cisgenesis and intragenesis techniques are able to introduce the desired trait only,
without other unintended/undesirable traits. The introduction of the trait can be done
through random insertion technologies (which are technologies used for GMOs
authorised so far, such as Agrobacterium-mediated transformation
5
or biolistic
technologies
6
) or targeted insertion technologies (type 3 genome editing, see below).
The process is generally more precise and efficient (taking from 2 to 8 years
7
) compared
to conventional breeding techniques.
Cisgenesis and intragenesis have been included among NGTs since 2007, regardless
of the technology used to produce them (either random or targeted). At that time, a
working group of Member States’ experts was established by the Commission to evaluate
whether certain new techniques result in GMOs subject to the EU GMO legislation. For
this evaluation, the competent authorities provided a list of new techniques that included
cisgenesis and intragenesis. Cisgenesis and intragenesis were described as new breeding
techniques that, while using the same genetic modification technologies as transgenesis,
opened new possibilities through the insertion of genetic material from crossable species.
A similar description of cisgenesis and intragenesis as new breeding techniques was also
provided in the 2011 JRC study “New plant breeding techniques - State-of-the-art and
prospects for commercial development”
8
and in the 2017 SAM explanatory note on new
techniques in agricultural biotechnology
9
.
4. TRANSGENESIS TECHNIQUES
Finally, modifications of the genetic material of plants may also occur through the
insertion of foreign genetic material into a recipient organism from a donor organism that
is sexually incompatible (non-crossable). This generally may only take place by human
intervention through transgenesis techniques. Also in this case, the introduction of the
trait can be done through random insertion technologies or targeted insertion
technologies.
4
JRC case studies.
5
Technologies using Agrobacterium tumefaciens, a bacterium infecting plants, for its natural ability to
transfer DNA to plant cells.
6
Technologies involving microscopic particles coated with the DNA that is to be inserted into the plant.
These particles are then “shot” under high pressure into the plant tissue. The DNA penetrates the cell
where it is sometimes spontaneously incorporated into the genome.
7
JRC case studies.
8
https://publications.jrc.ec.europa.eu/repository/handle/JRC63971, page 24-25
9
https://research-and-innovation.ec.europa.eu/knowledge-publications-tools-and-data/publications/all-
publications/new-techniques-agricultural-biotechnology_en, page 67-68

151
Figure 2: Conventional breeding vs Cisgenesis vs Transgenesis
5. GENOME EDITING
Genome editing is an umbrella term covering several technologies that can be used for
targeted mutagenesis, cisgenesis, intragenesis and transgenesis. Genome editing includes
Site-directed nuclease (SDN) technologies, whose mechanism is based on the use of
enzymes (nucleases) that cut the DNA at specific locations (cleavage sites), as well as
other technologies such as oligonucleotide directed mutagenesis (ODM), base editing,
prime editing, site-directed recombination
10
. These technologies can produce precisely
located alterations to DNA sequences, ranging from 'point mutations' (changes of one or
a few DNA “letters”, which may be either random or non-random) to the insertion of
genes (cisgenes, intragenes, transgenes).
Depending on their application, SDN technologies, which are currently the most widely
used genome editing technologies, can be classified in three types of categories:
1) type 1 (SDN-1): introduction of random mutations in precise locations;
2) type 2 (SDN-2): introduction of non-random mutations in precise locations;
3) type 3 (SDN-3): insertion of large segments (such as genes) in precise locations.
10
Broothaerts et al. (2021)

152
Type 1 and 2 are used for targeted mutagenesis, while type 3 is used for cisgenesis,
intragenesis and transgenesis.
Figure 3: Targeted technologies: Genome editing
Genome editing does not exclude 'off-target' modifications, where a change occurs to a
DNA sequence identical or similar to that in which the change is desired, but in another
location. However, these off-target modifications are fewer than those occurring with
most random mutagenesis techniques. Where they do occur, these changes are of the
same types as those produced by conventional breeding techniques
11
.
6. OVERVIEW OF THE TECHNIQUES
Mutagenesis
Creation of mutation(s)
in an organism without
insertion of foreign
genetic material
Cisgenesis
Insertion of exogenous
genetic material,
without prior
rearrangement, from a
crossable
organism/species
Intragenesis
Insertion of exogenous
genetic material, with
prior rearrangement,
from a crossable
organism/species
Transgenesis
Insertion of foreign
genetic material from
a non-crossable
organism/species
Random
technologies
Irradiation or treatment
with chemicals
Random integration via
Agrobacterium
tumefaciens, gene gun
Random integration via
Agrobacterium
tumefaciens, gene gun
Random integration via
Agrobacterium
tumefaciens, gene gun
Targeted
technologies
SDN (e.g. CRISPR) type 1
or 2, ODM, base editing,
prime editing, etc.
SDN (e.g. CRISPR) type 3
site-directed
recombination
SDN (e.g. CRISPR) type 3
site-directed
recombination
SDN (e.g. CRISPR) type
3 site-directed
recombination
For an extensive description of the techniques, see JRC Technical Report on New
genomic techniques: state-of-the-art review
12
and the Explanatory Note on new
11
EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms), Naegeli H,Bresson J-L, Dalmay
T, Dewhurst IC, Epstein MM, Firbank LG, Guerche P, Hejatko J, Moreno FJ, MullinsE, NogueF,Sanchez
Serrano JJ, Savoini G, Veromann E, Veronesi F, Casacuberta J, Gennaro A,Paraskevopoulos K, Raffaello
T and Rostoks N, 2020. Applicability of the EFSA Opinion on site-directednucleases type 3 for the safety
assessment of plants developed using site-directed nucleases type 1and 2 and oligonucleotide-directed
mutagenesis. EFSA Journal 2020;18(11):6299, 14 pp., https://doi.org/10.2903/j.efsa.2020.6299
12
Broothaerts, W., Jacchia, S., Angers, A., Petrillo, M., Querci, M., Savini, C., Van den Eede, G. and
Emons, H., New Genomic Techniques: State-of-the-Art Review, EUR 30430 EN, Publications Office of
the European Union, Luxembourg, 2021, ISBN 978-92-76-24696-1, doi:10.2760/710056, JRC121847

153
techniques in agricultural biotechnology by the Commission’s Scientific Advice
Mechanism High-Level Group (SAM HLG)
13
. The latter provides also a comparison of
the various techniques, in particular as regards precision, speed and unintended effects as
shown in the figure below (see figure 4 below).
Figure 4: Overview of new and existing breeding techniques (source: SAM HLG)
13
European Commission, Directorate-General for Research and Innovation, New techniques in agricultural
biotechnology, Publications Office, 2017, https://data.europa.eu/doi/10.2777/574498

154
ANNEX 6: OPINIONS OF SCIENTIFIC ADVISORY BODIES
AND SCIENTIFIC ORGANISATIONS ON NGTS
Over the last decade, there have been numerous scientific reports and publications on
NGTs. A non-exhaustive overview of relevant opinions of EU scientific advisory bodies
and scientific organisations is provided below. It reflects a diversity of views, but with
majority positions emerging on relevant scientific issues.
In 2017, the Scientific Advice Mechanism High-Level Group (SAM HLG) issued an
explanatory note on new techniques in agricultural biotechnology applied to plants,
animals and microorganisms
1
. The note describes the nature and characteristics of NGTs
known at that time and their similarities and differences compared to conventional
breeding techniques and established genomic techniques, in particular as regards
precision, efficiency, detectability, cost and speed of product development. The SAM
HLG also issued a statement
2
in 2018 on a “Scientific perspective on the regulatory
status of products derived from gene editing and the implications for the GMO Directive”
where they considered that new scientific knowledge and recent technical developments
have made the GMO legislation no longer fit for purpose.
EASAC, the European Academies' Science Advisory Council, formed by the national
science academies of the EU Member States, Norway, Switzerland and the United
Kingdom, issued a report in 2017 on scientific opportunities, public interests and policy
options in the EU on genome editing
3
.
The Commission Joint Research Centre (JRC) analysed in 2021 the latest scientific
developments
4
relating to NGTs.
The European Food Safety Authority (EFSA) published scientific opinions on NGTs
applied to plants, in particular on SDN type 1 and 2 and oligonucleotide-directed (ODM)
mutagenesis
5
, and on cisgenesis and intragenesis (with a recent opinion updating a
1
European Commission, Directorate-General for Research and Innovation, New techniques in agricultural
biotechnology, Publications Office, 2017, https://data.europa.eu/doi/10.2777/574498
2
European Commission, Directorate-General for Research and Innovation, Group of Chief Scientific
Advisors, A scientific perspective on the regulatory status of products derived from gene editing and the
implications for the GMO Directive : statement by the Group of Chief Scientific Advisors, Publications
Office, 2019, https://data.europa.eu/doi/10.2777/407732
3
EASAC (2017) Genome editing: scientific opportunities, public interests and policy options in the
European Union
https://easac.eu/fileadmin/PDF_s/reports_statements/Genome_Editing/EASAC_Report_31_on_Genome_E
diting.pdf
4
New Genomic Techniques: State-of-the-Art Review https://data.europa.eu/doi/10.2760/710056
5
EFSA Panel on Genetically Modified Organisms, ‘Applicability of the EFSA Opinion on SDNs type 3 for
the safety assessment of plants developed using SDNs type 1 and 2 and oligonucleotide-directed
mutagenesis’, EFSA Journal 2020;18(11):6299. https://doi.org/10.2903/j.efsa.2020.6299

155
previous one from 2012)
6
. EFSA also published scientific opinions on plants obtained
through synthetic biology, where certain applications make use of SDN technologies
7
.
Overall, these opinions and reports recognise the variety of techniques and of different
products with different risk profiles they can generate. They confirm that NGT products
may or may not contain foreign DNA. They also recognise similarities of certain
organisms produced by NGTs with conventionally bred organisms, while others would
resemble organisms produced by established genomic techniques. NGTs may also
introduce into an organism sequences derived from the same species or from other
species, either crossable or uncrossable. In this respect, NGTs may produce
modifications that could or could not be obtained in nature or by conventional breeding.
In the former case, products obtained by NGTs and those occurring naturally or produced
by conventional breeding might be indistinguishable from each other.
The above-mentioned bodies also report on the increasing precision of certain NGTs
compared to conventional breeding approaches. In particular, genome editing enables
small, precise and specific changes, such as point mutations, or makes it possible to
target insertions, resulting in comparatively fewer unintended effects. Variations to the
genome may be entirely novel or may occur already in other individuals of the same or of
another species. When changes are small and directed by similar desirable changes
known in other organisms, the resulting products are expected to display more
predictable characteristics. For these reasons, many NGTs make it possible to shorten the
development time for organisms with desired phenotypes. Also, the assessment of the
characteristics and potential risks might be facilitated on a case-by-case basis.
As regards risks, EFSA concluded that there are no new hazards specifically linked to the
genomic modification produced via SDN-1, SDN-2, ODM as compared with both SDN-
3 and conventional breeding. In assessing different contributions to potential hazards in
cisgenic/intragenic plants, EFSA concluded that no new risks are identified in cisgenic
and intragenic plants compared with plants obtained with conventional breeding or
established genomic techniques. However, intragenic plants may present more hazards
than cisgenic plants.
EFSA also considered that on a case-by-case basis, a lesser amount of data might be
needed for the risk assessment of plants produced by SDN-1, SDN-2, ODM, cisgenesis
and intragenesis and therefore there is a need for flexibility in the data requirements for
risk assessments. EFSA also concluded that in targeted mutagenesis, the potential for
unintended effects, such as off-target effects, may be significantly reduced compared to
random insertions or conventional breeding. Off-target modifications would be fewer
6
EFSA Panel on Genetically Modified Organisms, 2012. Scientific opinion addressing the safety
assessment of plants developed through cisgenesis and intragenesis. EFSA Journal 2012;10(2):2561.
EFSA Panel on Genetically Modified Organisms, 2022. Updated scientific opinion on plants developed
through cisgenesis and intragenesis. EFSA Journal 2022;20(10):7621, 33 pp.
https://doi.org/10.2903/j.efsa.2022.7621.
7
EFSA Panel on Genetically Modified Organisms, 2022. Scientific Opinion on the evaluation of existing
guidelines for their adequacy for the food and feed risk assessment of genetically modified plants obtained
through synthetic biology. EFSA Journal 2022;20 (7):7410, 25 pp. https://doi.org/10.2903/j.efsa.2022.7410
EFSA Panel on Genetically Modified Organisms, 2021. Scientific Opinion on the evaluation of
existing guidelines for their adequacy for the molecular characterisation and environmental risk
assessment of genetically modified plants obtained through synthetic biology. EFSA Journal
2021;19(2):6301, 21 pp. https://doi.org/10.2903/j.efsa.2021.6301
8
Modrzejewski, D., Hartung, F.,
Sprink, T., Krause, D., Kohl, C., Wilhelm, R (2019): What is the available evidence for the range of
applications of genome-editing as a new tool for plant trait modification and the potential occurrence of
associated off-target effects: A systematic map. Environmental Evidence, 8(1):

156
than those occurring with most mutagenesis techniques and, where such changes occur,
they would be of the same types as those produced by conventional breeding techniques.
The scientific literature on off-target effects indicates that there are fewer such effects for
certain targeted mutagenesis methods compared to conventional mutation breeding. Out
of 1328 studies analysed using different genome editing techniques, 252 investigated off-
target mutations. These were detected in around 3% of the analysed potential off-target
sites
8
. Case studies on rice
9
and cotton
10
showed that no off-target sequences were found
with CRISPR/Cas but conventional tissue culture resulted in ~100-250 single nucleotide
variations. Nevertheless, some scientists consider that there are new types of risks
resulting from targeted mutagenesis techniques compared to previous applications of
genetic engineering
11
.
To address the possible need for flexibility in data requirements for risk assessment,
EFSA, upon a request from the Commission, published a statement where six main
criteria are proposed to assist the risk assessment of plants produced by targeted
mutagenesis, cisgenesis and intragenesis
12
(see section 5.2.1, box 1). These criteria relate
to the molecular characterisation of the genetic modification introduced, to the available
knowledge based on the history of use and to the structure and function of the modified
DNA sequence(s). In the statement, EFSA reiterated that for plants in which the newly
modified DNA sequence is successfully targeted there are cases where the potential for
unintended effects, such as off-target effects, is significantly reduced compared to
random insertions or conventional breeding. When that happens, the data requirements
for the risk assessment may be reduced on a case-by-case basis.
The European Union Reference Laboratory for GM food and feed (EURL GMFF) and
the European Network of GMO Laboratories (ENGL) issued in 2019 a report on the
detection of food and feed plant products obtained by new mutagenesis techniques
13
.
They concluded that validation of a specific detection method and its implementation for
market control is feasible only for genome-edited plant products carrying a known DNA
alteration that has been shown to be unique. Under the current circumstances, market
control will fail to detect unknown genome-edited plant products. An updated report by
the EURL-ENGL is expected in mid-2023
14
.
8
Modrzejewski, D., Hartung, F., Sprink, T., Krause, D., Kohl, C., Wilhelm, R (2019): What is the available
evidence for the range of applications of genome-editing as a new tool for plant trait modification and the
potential occurrence of associated off-target effects: A systematic map. Environmental Evidence, 8(1):
9
Tang, X., Liu, G., Zhou, J., Ren, Q., You, Q., Tian, L., et al. (2018). A large-scale whole-genome
sequencing analysis reveals highly specific genome editing by both Cas9 and Cpf1 (Cas12a) nucleases in
rice. Genome Biol. 19, 84. doi:10.1186/s13059-018-1458-5.
10
Li, J., Manghwar, H., Sun, L., Wang, P.,
Wang, G., Sheng, H., et al. (2019): Whole genome sequencing reveals rare off-target mutations and
considerable inherent genetic or/and somaclonal variations in CRISPR/Cas9-edited cotton plants. Plant
Biotechnol. J. 17, 858–868. doi: 10.1111/pbi.1302
10
Li, J., Manghwar, H., Sun, L., Wang, P., Wang, G., Sheng, H., et al. (2019): Whole genome sequencing
reveals rare off-target mutations and considerable inherent genetic or/and somaclonal variations in
CRISPR/Cas9-edited cotton plants. Plant Biotechnol. J. 17, 858–868. doi: 10.1111/pbi.1302
11
Kawall, K. The Generic Risks and the Potential of SDN-1 Applications in Crop Plants. Plants 2021, 10,
2259. https://doi.org/ 10.3390/plants10112259.
12
EFSA Panel on Genetically Modified Organisms, 2022. Statement on criteria for risk assessment of
plants produced by targeted mutagenesis, cisgenesis and intragenesis. EFSA Journal 2022;20(10):7618, 12
pp. https://doi.org/10.2903/j.efsa.2022.7618
13
European Network of GMO Laboratories (ENGL), Detection of food and feed plant products obtained
by new mutagenesis techniques, 26 March 2019 (JRC116289). https://gmo-
crl.jrc.ec.europa.eu/doc/JRC116289-GE-reportENGL.pdf
14
[reference to be added when available]

157
The European Group on Ethics in Science and New Technologies (EGE) published in
2021 an opinion on the ethics of genome editing, which focuses on applications in the
human, animal and plant domains
15
. Among its conclusions on plants, EGE recommends
a systems approach to evaluate costs and benefits (including the impact of continuing
current agricultural practices) in any future use. It also recommends regulation
proportional to the risk: light touch regulation should be used where the change in the
plant could have been achieved naturally, or where genetic material from sexually
compatible plants is introduced. Where genes from non-sexually compatible organisms
or multiple changes are introduced, there should be a comprehensive risk assessment.
Some scientific organisations and agencies, such as the German Federal Agency of
Nature Conservation
16
, the European Network of scientists for social and environmental
responsibility (ENSSER)
17
and TestBiotech
18
, disagree with the above opinions. They
raise the concern that NGTs pose specific risks to the environment and human health.
They consider that NGTs can be used to achieve genomic changes beyond what is known
from conventional breeding and can alter the genome to a much greater extent than with
any previous breeding method. Furthermore, they refer to unintended genetic changes
that, according to them, would be specific to the processes of NGTs and unlikely to occur
via random processes or conventional breeding. Furthermore, these organisations
question that NGTs are precise, controllable and predictable and have a sustainability
potential as claimed.
EFSA has evaluated the scientific literature provided by these organisations in the public
consultations and considered that it does not provide new evidence challenging the
validity of the assessment and conclusions of EFSA scientific opinions
19
.
15
https://ec.europa.eu/info/files/ethics-genome-editing_en
16
https://www.bfn.de/en/publications/position-paper/new-developments-and-regulatory-issues-plant-
genetic-engineering
17
https://ensser.org/publications/2021-publications/enssers-response-to-the-inception-
impact-assessment-iaa-on-new-genomic-techniques/
17
https://ensser.org/publications/2021-publications/enssers-response-to-the-inception-impact-assessment-
iaa-on-new-genomic-techniques/
18
https://www.testbiotech.org/en; https://www.vzbv.de/sites/default/files/2022-11/vzbv-
report_final_final.pdf
19
https://www.efsa.europa.eu/en/news/faq-criteria-risk-assessment-plants-produced-targeted-mutagenesis-
cisgenesis-and-intragenesis

158
ANNEX 7: APPLICATIONS OF NGTS IN PLANTS AND
CASE STUDIES ON POTENTIAL IMPACTS
1. OVERVIEW OF APPLICATIONS OF NGTS IN PLANTS
The Commission Joint Research Centre (JRC) analysed in 2021 the current and future
market applications
1
relating to NGTs.
The JRC report illustrates the wide range of traits and plant species in the focus of NGTs
(see Table 1 and 2 and Figures 1 and 2). Regarding developed traits, disease resistance is
targeted to more types of pathogens and pests. Abiotic stress tolerance is widely explored
and includes resistance to drought, salinity and heat stress. Modified composition goes
beyond starch and oil content, where many crops are in development with improved
nutrition properties (fibres, vitamins content) together with the reduction of potentially
harmful compounds (toxins, allergens, acrylamide, etc.) or gluten. Also, several
applications are dedicated to obtaining higher and more stable yield (in terms of plant
production and/or size of fruits and grains). Regarding targeted plants species, these
cover a wider range of plant groups from cereals to fruits, legumes and vegetables,
compared to GMOs from established genomic techniques, which mainly concern few
cereal (maize, rice) and oil and fibre crops (soybean, cotton, oilseed rape).
Tables 1 and 2 and figures 1, 2 and 3 summarise the plants and traits identified in the
JRC market review and reflect the situation in 2020.
Table 1. Summary of plants identified in the JRC database of NGT products.
Plant groups
Plants included (not exhaustive)
Cereals
Maize, Wheat, Rice, Barley, Sorghum, Millet
Forage and grasses
Alfalfa, Ryegrass, Switchgrass, Setaria viridis
Fruits
Apple, Banana, Orange, Groundcherry, Grapefruit, Grapevine, Kiwifruit,
Melon, Watermelon, Berries, Stone fruits, Avocado
Legumes
Beans, Chickpea, Peanut, Pea, Pigeon Pea
Oil and fibre crops
Soybean, Rapeseed, Cotton, Camelina, Flax, Pennycress, Sunflower,
Mustard, Strawberry
Ornamentals
Chrysanthemum, Dandelion, Orchid, Petunia, Poinsettia, Poppy, Japanese
morning glory, Wishbone flower, Jasmine tobacco
Sugar crops
Sugar beet, Sugarcane
Trees
Poplar, softwood trees
Tubers and root
vegetables
Potato, Sweet potato, Cassava, Beetroot
Vegetable crops
Tomato, Broccoli, Cabbage, Cucumber, Eggplant, Lettuce, Pepper, Chicory
Other plants
Cacao, Coffee, Tobacco, Salvia
1
Parisi, C. and Rodriguez Cerezo, E., Current and future market applications of new genomic techniques,
EUR 30589 EN, Publications Office of the European Union, Luxembourg, 2021, ISBN 978-92-76-30206-
3, doi:10.2760/02472, JRC123830.

159
Figure 1: Distribution of NGT plant entries in the database by plant group and development stage (commercial, pre-
commercial, advanced and early R&D stages)
1
.
Table 2. Trait categories in the JRC NGT plant products database.
Trait category
Description
Biotic Stress Tolerance
Resistance to biotic stressors like, nematodes, fungi, bacteria, viruses and
other pests, pathogens, or parasites
Abiotic stress tolerance
Resistance to abiotic stressors like drought, heat, salt, rain or UV
radiation.
Herbicide tolerance
Tolerance to different types of herbicides
Modified colour/flavour
Modified colour or flavour
Modified composition
Modified content of substances like starch, oil, proteins, vitamins, fibres,
toxic substances, allergens etc. to improve food/feed quality of for a better
industrial use. It includes seedless fruits as a quality characteristic.
Plant yield and architecture
Yield increase (or yield stability) related to higher number of
flowers/seeds/fruits, to fruit size/weight and to photosynthetic efficiency.
It also includes other changes in plant architecture including plant height
and shape, fruit shape, and growth pattern, among others.
Storage performance
Improvement of characteristics like shelf life and storage conditions (e.g.
cold storage), including non-browning and reduced black spot.
Other traits
Remaining traits, not classified in the previous categories, including,
among others, production of molecules of industrial interest, flowering
time for agronomic purposes and nitrogen use.
Breeding tools
Reproductive/flowering characteristics including, among others, induction
of sterility, early flowering, and haploid techniques

160
Figure 2: Distribution of NGT plant entries in the JRC database by trait category and development stage (commercial,
pre-commercial, advanced and early R&D stages)
1
.
Figure 3: Distribution of NGT plant entries in the database by trait category and development stage (commercial, pre-
commercial, advanced R&D), limited to a 2030 horizon.
An overview of applications of NGTs in plants is also provided by the database of
published literature on the use of genome editing in crop plants maintained by the
EU-SAGE network
2
, which contains information on genome-editing research and
provides details on crop plant species and traits addressed and the techniques used and is
kept up to date. Genome editing applications are identified in more than 60 different
crops with the vast majority in rice, tomato, maize, soybean, wheat and potato. The traits
introduced in the improved crops are diverse and relevant for farmers (e.g. agronomic
value) as well as consumers (e.g. nutrition). Table 3 describes the distribution of these
traits for the time-period 1996-2022.
2
https://www.eu-sage.eu/genome-search

161
Table 3. Distribution of genome editing applications according to different trait categories in the period 1996–2022
2
.
Trait category
%
Plant yield and growth
22.2% (144)
Improved food/feed quality
21.9% (142)
Industrial utilisation
14.4% (93)
Biotic Stress Tolerance
8% (117)
Abiotic stress tolerance
7.9% (51)
Herbicide tolerance
7.7% (50)
Modified colour/flavour
5.9% (38)
Storage performance
2% (13)
The Food and Agriculture Organization of the United Nations (FAO) also includes
examples of applications in their recent paper on ‘Gene editing and agrifood systems’
3
,
for all of these trait categories (see Table 4).
Table 4. Applications of genome editing technologies in agrifood systems.
Species
Trait
Research Organisation
Camelina
Improved fatty acid
composition
Department of Plant Sciences and Plant Pathology, Montana State
University, Bozeman, MT 59717, USA
Lettuce
Increased vitamin C
content
State Key Laboratory of Plant Cell and Chromosome
Engineering, Center for Genome Editing, Institute of Genetics
and Developmental Biology, Chinese Academy of Sciences,
Beijing, China
Oilseed rape
Improved fatty acid
composition
National Key Laboratory of Crop Genetic Improvement,
Huazhong Agricultural University, Wuhan, China
Potato
Reduced acrylamide
formation
Cellectis plant sciences Inc., New Brighton, MN, USA
Soybean
Improved fatty acid
composition
Calyxt, Roseville, MN, USA
Tomato
High content of γ-
aminobutyric acid (GABA)
Sanatech Minato-ku, Tokyo, Japan & University of Tsukuba,
Ibaraki, Japan
3
FAO. 2022. Gene editing and agrifood systems. Rome. https://doi.org/10.4060/cc3579en

162
Wheat
Low gluten content
Instituto de Agricultura Sostenible (IAS-CSIC), Córdoba, Spain
Wageningen University, Wageningen, Netherlands
Wild tomato
De novo domestication –
High antioxidant content
Several universities from Brazil, Germany and the USA
Brewer’s
yeast
Flavour improvement in
fermented beverages
Centre of Microbial and Plant Genetics, Leuven, Belgium
Alfalfa
High yield
National Institute of Agricultural Technology, Argentina
Banana
Fungus protection
Queensland University of Technology, Brisbane, Australia
Protection against bacterial
wilt, fusarium wilt and
banana streak virus
International Institute of Tropical Agriculture, Nigeria
Protection against bunchy
top virus
Agricultural Research Council, Pretoria, South Africa
Cacao
Protection against fungal
disease
Pennsylvania State University, USA
Cassava
Reduced cyanide levels
University of California, Berkeley, CA, USA
Virus resistance
Cherry
Virus resistance
Department of Horticulture, Plant Biotechnology Resource
and Outreach Center, Michigan State University, East
Lansing, MI, USA
Citrus
Protection against citrus
canker
Chinese Academy of Sciences, China
Cucumber
Protection against multiple
viruses
Department of Plant Pathology and Weed Research, ARO,
Volcani Center, Bet-Dagan, Israel
Flax
Herbicide tolerance
Cibus, San Diego, CA, USA
Grapevine
Drought tolerance
Stellenbosch University, Stellenbosch, South Africa
Maize
Fungus resistance
DuPont Pioneer, Johnston, IA, USA
Oilseed rape
Herbicide tolerance
Key Laboratory of Plant Functional Genomics of the Ministry of
Education, Yangzhou University, Yangzhou China
Potato and
Sugar beet
Disease-resistant varieties
Russian Academy of Sciences, Russian Federation
Rice
Salt tolerance
National Institute for Plant Biotechnology, New Delhi, India
Fungus protection
Department of Genetics, Development & Cell Biology, Iowa
State University, Ames, Iowa, USA
Salt tolerance
Key Laboratory of Rice Genetic Breeding of Anhui
Province,Rice Research Institute, Anhui Academy of Agricultural
Sciences, Hefei, 230031, China
Sorghum
Increased protein content
University of Queensland, Queensland, Australia
Striga resistance
Kenyatta University, Kenya
Soybean
Nematode resistance
Evogene, Rehovot, Israel & TMG, Cambé, Brazil
Tomato
Bacterial resistance
Department of Plant and Microbial Biology, University of
California, Berkeley, USA
Provitamin D3 enhanced
John Innes Centre, Norwich, United Kingdom

163
Wheat
Fungus protection
State Key Laboratory of Plant Cell and Chromosome
Engineering, Institute of Genetics and Developmental
Biology, Chinese Academy of Sciences, Beijing, China
2. CASE STUDIES
JRC case studies
Very few NGT plants have been commercialised so far and therefore there is limited
knowledge and assessment of their field performance, environmental effects and benefits
for consumers.
For the purposes of this impact assessment, the JRC developed three case studies to
showcase the potential impacts, positive or negative, on the social, environmental and
economical dimensions of sustainability. The studies involved commercially important
plants produced via targeted mutagenesis or cisgenesis that are currently in various stages
of development in the EU or elsewhere and which feature traits with potential to
contribute to sustainability objectives:
disease resistant apples and potatoes (box 1);
low gluten wheat (box 2);
a virus-resistant maize (box 3).
Box 1: Economic and environmental impacts of disease resistant potatoes and apples developed
with cisgenesis
4
A key tool in agricultural management are pesticides, as significant shares of harvests are lost to plant
diseases. To achieve the objectives of the Green Deal, Farm to Fork and the Biodiversity Strategies
which aim at reducing the use of pesticides, the development of disease resistant varieties is crucial.
The case study focuses on fungal resistant potato and apple varieties as both plants are difficult to
breed and because the fungal diseases are severe and can break down resistances. Due to the low
availability of varieties with durable resistances, potatoes and apples are among the most intensively
treated crops in terms of pesticide use. For the potential direct economic impacts, the study focused
on the five EU Member States with the largest average production from 2015 to 2020. For potato
these are France, the Netherlands, Belgium, Germany, Poland and for apple France, Italy, Germany,
Poland, Spain.
The NGT-based potato variety presented here has been developed and extensively tested in multi-
year field trials in the Netherlands. In contrast to the potato variety, the cisgenic apple variety
analysed is still in the early research and development stage. The project did develop a cisgenic apple
as proof of concept, however, only based on an introduction of a single resistance gene. In addition, a
cisgenic construct harbouring three genes against scab was developed and would be readily available.
Both plants are notoriously challenging to breed, making it time consuming to develop pest resistant
varieties. Phytophthora infestans and Venturia inaequalis are key pathogens causing late blight
disease (PLB) in potatoes and scab disease in apple, respectively. In potatoes, the average (maximum)
number of annual fungicide treatments ranges from 3 (17) in Poland, over 7 (18) in Germany, to 13
(21) in the Netherlands, and 14 (23 and 25) in France and Belgium. This corresponds to 1.4 kg of
fungicides per hectare in Poland, 5.2 kg in Germany, 7.1 kg in France, 7.5 kg in the Netherlands, and
11.2 kg in Belgium. In apples, the average (maximum) number of fungicide treatments is over 20 (30)
and the applied quantities range from 2.9 kg in Spain, over 8.7 kg in Poland, to 25.6 kg in France, and
31.7 kg in Germany.
Varieties with durable resistances to these diseases could allow for sizable reductions of fungicide
without affecting yields. In potatoes, a 50-80% reduction of fungicides may be feasible under
commercial conditions. In apples, depending on the region, a reduction of 12-58% is suggested by the
4
Schneider, K., Barreiro-Hurle, J., Kessel, G. et al., 2023. Economic and environmental impacts of disease
resistant crops developed with cisgenesis. EUR 31355, Publication office of the European Union,
Luxembourg.

164
literature. The fungicide reductions may generate cost savings for farmers. In PLB resistant potatoes,
the simulated 90%-confidence-interval of the savings per hectare are EUR350-576 (7.8 to 14.7 % of
total costs) in France, EUR326-535 (6.3-11.2%) in the Netherlands, EUR292-463 (8.2-13.8%) in
Belgium, EUR189-323 (5.9-10.9%) in Germany, and EUR49-99 (3.7-7.9%) in Poland. In scab
resistant apples, the estimates are EUR271-712 (2.7-8.5%) in France, EUR207-556 (2.4-7.3%) in
Italy, EUR151-516 (1.5-5.6%) in Germany, EUR65-234 (2.3-8.7%) in Poland, and EUR39-242 (0.9-
6.2%) in Spain.
At country-level, the rate of adoption of the resistant varieties largely determines the total annual cost
savings. If only the largest farms with more than 20 hectare of potatoes under cultivation were to
adopt the PLB resistant potatoes, the total annual cost savings are estimated at EUR53 million in
France, EUR47 million in Germany, EUR47 million in the Netherlands, EUR8 million in Belgium,
and EUR3 million per year in Poland. If only farms with more than 30 hectare of fruits under
cultivation were to adopt the scab resistant apples, the total annual cost savings are estimated at
EUR8.9 million in France, EUR3.2 million in Germany, EUR2.7 million in Poland, EUR2.3 million
in Italy, and EUR0.9 million in Spain. At EU-level, the PLB resistant potatoes are estimated to
increase the total potato supply by up to 5%, reduce the producer prices by up to 6%, increase the
human consumption by up to 0.8%, increase the use for feed by up to 3.5%, decrease the potato
imports by up to 4.6%, and increase the potato exports by up to 6.2%.
In terms of environmental impacts, in Germany in 2019, the five most used active ingredients in
potatoes belong to three Harmonised Risk Indicator (HRI)-1 groups
5
: group 2
6
(3 substances), group
3
7
(1), and group 4
8
(1). In the case of apples, the five most used active ingredients belong to group 2
(4) and group 3 (1). Therefore, the potential reduction in fungicide use in both crops could have
significant effects on the national risk indicators. The environmental risks of the active ingredients
currently used in both crops are suggested to be strongly related to their presence in freshwater
systems. Fruit orchards less than 1 km away from any freshwater system correspond to approximately
3-15% of the area of production in the different countries. For potato fields in 2018, fields less than 1
km away from freshwater systems corresponded to approximately 3-11% of the countries’ area of
production. No effects from the large-scale adoption of PLB resistant potatoes on the total greenhouse
gas emission related to agricultural activities in the EU were found.
5
Harmonised Risk Indicator-1 is used to estimate the trends in risk from pesticide use. HRI 1 is calculated
by multiplying the quantities of active substances placed on the market in plant protection products by a
weighting factor.
6
Group 2: Active substances approved or deemed to be approved under Regulation (EC) No 1107/2009,
and not falling in other categories, and which are listed in Parts A and B of the Annex to Implementing
Regulation (EU) No 540/2011
7
Group 3: Active substances approved or deemed to be approved under Article 24 of Regulation (EC) No
1107/2009, which are candidates for substitution, and which are listed in Part E of the Annex to
Implementing Regulation (EU) No 540/2011
8
Group 4: Active substances which are not approved under Regulation (EC) No 1107/2009, and therefore
which are not listed in the Annex to Implementing Regulation (EU) No 540/2011

165
Figure 4: Presentation of the JRC case study on cisgenic potato.
Figure 5: Presentation of the JRC case study on cisgenic apple.

166
Box 2: Socioeconomic impact of low-gluten celiac-safe wheat developed by gene editing
9
1 to 2% of the human population (4.5 million in the EU) suffer from coeliac disease (CD), a life-
long autoimmune disorder, triggered by gluten and similar storage proteins in wheat, rye and barley,
with severe underdiagnosis, leading to chronic small intestine inflammation. Next to CD, a large
group of people suffer from non-coeliac wheat sensitivity (NCWS), with overlapping symptoms to
CD but without elevated level of intestinal damage. Current management is through life-long
gluten-free diets, which often lead to unbalanced nutrient intake, due to counterbalancing the lack of
gluten with high salt, fat, and carbohydrates.
Gluten-free products are 200% more expensive than their normal counterparts. The economic aid
offered differs among Member States. Patients and their relatives have as a rule little or no financial
support. Costs for the health care system and productivity are difficult to quantify due to lack of
data. After diagnosis and following a gluten-free diet, quality of life is reported to improve, and
post-diagnosis medical costs reduced by 39%.
It is difficult to generate a low gluten variety via conventional breeding (high copy of genes,
structural complexity).
Researchers from the Institute for Sustainable Agriculture of the Spanish National Research Council
of Córdoba designed a first gene-edited wheat targeting alpha gliadins, which are the most
immunoreactive, to achieve a variety that is not immunogenic for patients with celiac disease and
possibly useful also for those with non-celiac wheat sensitivity. These gene-edited wheat lines
reached a reduction in the alpha-gliadin content from 32% to 82%, while immunoreactivity was
reduced by 85%. These lines have already been cultivated in greenhouses.
In addition, a second gene-edited wheat was obtained targeting gamma- and omega-gliadins, and
achieved a reduction of 70% and 90% in the gamma and omega-gliadins, respectively. These lines
are not yet being tested in greenhouses. Lastly, cross-breeding between gene-edited lines to combine
all gliadin mutations in a single soft wheat genotype was carried out. These lines are currently being
analysed, and the final selection of genotypes containing all mutations is expected to be completed
by mid-2023. After this, clinical trials are planned using the best line.
After diagnosis, outpatient costs are estimated to be reduced by 29% (and total medical cost of care
by 39%) and QALY (quality-adjusted life year) scores are estimated to improve based on adequate
gluten-free diet. Gene edited low-gluten celiac-safe wheat provides a safe alternative to the gluten-
free products for celiac disease patients and reduces possible nutrient deficiencies and imbalance in
gut bacteria often associated with wheat avoidance. In addition, while conventional gluten-free
products are on average 200% more expensive, products based on gene-edited low-gluten non-celiac
wheat are expected to be only 30% more expensive. More affordable safe gluten-free diets will
contribute to reducing medical costs post-diagnosis and improving quality of life.
In addition, the adoption of low-gluten celiac-safe wheat could increase gross margin of farmers per
ha in average by 30% compared to conventional wheat. Finally, the competitiveness of the EU agri-
food system might be enhanced by increasing export volumes (EUR0.5 to 2.6 billion) and lower
import volumes (EUR0.1 to 0.5 billion) of this product, if eventually cultivated in the EU.
9
Sánchez, B., Barro, F., Smulders, M. J. M. et al. 2023. Socioeconomic impact of low-gluten, celiac-safe
wheat developed through gene editing, EUR 31380 EN, Publications Office of the European Union,
Luxembourg.

167
Figure 6: Presentation of the JRC case study on targeted mutagenesis wheat.
Box 3: Biotic resistance in the context of smallholder farming in East Africa: a case on gene
editing of maize to safeguard food security under the spread of the Maize Lethal Necrosis
Maize Lethal Necrosis (MLN) is a complex viral disease, spread recently to East Africa from the
Americas. The disease is highly damaging, putting local food security at risk (majority of calory
intake in the region comes from maize), as well as inflicting critical economic repercussions. MLN
causes immense annual losses (~500 K tonnes in Kenya alone) in the production of maize, which is
a main product on the Sub-Saharan African agricultural market. Maize agriculture provides the
livelihood of 98% of smallholder farmers. With existing preventive measures and if MLN expands
to susceptible countries, the impact of MLN could lead to an additional 122,000 additional people
experiencing severe food insecurity.
Because small farmers in Africa lack the right management tools to handle MLN, resistance is
considered the most economically and sustainably effective way in the battle against this disease
and could save 0.5 Mio people from falling under the poverty line. Given the large variety of
cultivates used in East Africa highly adapted to regional conditions and the faster development
compared to conventional breeding, NGTs provide a valuable tool in fighting MLN and reduce yield
penalty, consequently reducing the number of persons experiencing food insecurity and
safeguarding smallholder farmers’ livelihoods.
The gene-edited virus-resistant maize, currently in development, could prevent a deterioration of the
severe food insecurity in East Africa, as well as deliver economic benefits.
The references are listed in footnote 10.
10
Contribution based on a literature review:
Awata, L. A. O., Beyene, Y., Gowda, M. et al., 2019. Genetic Analysis of QTL for Resistance to Maize
Lethal Necrosis in Multiple Mapping Populations. Genes 11, 32. https://doi.org/10.3390/genes11010032
Awata, L. A. O., Ifie, B. E., Danquah, E.et al., 2021. Introgression of Maize Lethal Necrosis Resistance
Quantitative Trait Loci Into Susceptible Maize Populations and Validation of the Resistance Under Field
Conditions in Naivasha, Kenya. Frontiers in Plant Science 12. https://doi.org/10.3389/fpls.2021.649308
Batchelor, W. D., Suresh, L. M., Zhen, X. et al., 2020. Simulation of Maize Lethal Necrosis (MLN)
Damage Using the CERES-Maize Model. Agronomy 10, 710. https://doi.org/10.3390/agronomy10050710
Beyene, Y., Gowda, M., Suresh, L. M. et al., 2017. Genetic analysis of tropical maize inbred lines for
resistance to maize lethal necrosis disease. Euphytica 213, 224. https://doi.org/10.1007/s10681-017-2012-3

168
Boddupalli, P., Suresh, L. M., Mwatuni, F. et al., 2020. Maize lethal necrosis (MLN): Efforts toward
containing the spread and impact of a devastating transboundary disease in sub-Saharan Africa. Virus
Research 282, 197943. https://doi.org/10.1016/j.virusres.2020.197943
Das, B., Atlin, G. N., Olsen, M., Burgueño, J. et al., 2019. Identification of donors for low-nitrogen stress
with maize lethal necrosis (MLN) tolerance for maize breeding in sub-Saharan Africa. Euphytica 215, 80.
https://doi.org/10.1007/s10681-019-2406-5
De Groote, H., Oloo, F., Tongruksawattana, S., & Das, B., 2016. Community-survey based assessment of
the geographic distribution and impact of maize lethal necrosis (MLN) disease in Kenya. Crop Protection
82, 30–35. https://doi.org/10.1016/j.cropro.2015.12.003
Dhugga, K. S., 2022. Gene Editing to Accelerate Crop Breeding. Frontiers in Plant Science 13.
https://doi.org/10.3389/fpls.2022.889995
FAO, IFAD, UNICEF, WFP and WHO. 2022. The State of Food Security and Nutrition in the World 2022.
Repurposing food and agricultural policies to make healthy diets more affordable. Rome, FAO
Flachowsky, H., Le Roux, P.-M., Peil, A. et al., 2011. Application of a high-speed breeding technology to
apple (Malus × domestica) based on transgenic early flowering plants and marker-assisted selection. New
Phytologist 192, 364–377. https://doi.org/10.1111/j.1469-8137.2011.03813.x
Garrett, K. A., Andersen, K. F., Asche, F. et al., 2017. Resistance Genes in Global Crop Breeding
Networks. Phytopathology 107, 1268–1278. https://doi.org/10.1094/PHYTO-03-17-0082-FI
Isabirye, B. E., & Rwomushana, I., 2016. Current and future potential distribution of maize chlorotic
mottle virus and risk of maize lethal necrosis disease in Africa. Journal of Crop Protection 5, 215–228.
https://doi.org/10.18869/modares.jcp.5.2.215
Jafari Jozani, H., Thiel, M., Abdel-Rahman, E. M. et al., 2022. Investigation of Maize Lethal Necrosis
(MLN) severity and cropping systems mapping in agro-ecological maize systems in Bomet, Kenya
utilizing RapidEye and Landsat-8 Imagery. Geology, Ecology, and Landscapes 6, 125–140.
https://doi.org/10.1080/24749508.2020.1761195
Lee, M., 1998. Genome projects and gene pools: New germplasm for plant breeding? Proceedings of the
National Academy of Sciences 95, 2001–2004. https://doi.org/10.1073/pnas.95.5.2001
Mahuku, G., Lockhart, B. E., Wanjala, B. et al., 2015. Maize Lethal Necrosis (MLN), an Emerging Threat
to Maize-Based Food Security in Sub-Saharan Africa. Phytopathology 105, 956–965.
https://doi.org/10.1094/PHYTO-12-14-0367-FI
Marenya, P. P., Erenstein, O., Prasanna, B., Makumbi, D. et al., 2018. Maize lethal necrosis disease:
Evaluating agronomic and genetic control strategies for Ethiopia and Kenya. Agricultural Systems 162,
220–228. https://doi.org/10.1016/j.agsy.2018.01.016
Murithi, A., Olsen, M. S., Kwemoi, D. B. et al., 2021. Discovery and Validation of a Recessively Inherited
Major-Effect QTL Conferring Resistance to Maize Lethal Necrosis (MLN) Disease. Frontiers in Genetics
12. https://doi.org/10.3389/fgene.2021.767883
Mwatuni, F. M., Nyende, A. B., Njuguna, J. et al., 2020. Occurrence, genetic diversity, and recombination
of maize lethal necrosis disease-causing viruses in Kenya. Virus Research 286, 198081.
https://doi.org/10.1016/j.virusres.2020.198081
Nelson, R., Wiesner-Hanks, T., Wisser, R., & Balint-Kurti, P., 2018. Navigating complexity to breed
disease-resistant crops. Nature Reviews Genetics 19, 21–33. https://doi.org/10.1038/nrg.2017.82
Njeru, F., Mwaura, S., Kusolwa, P. M., & Misinzo, G., 2022 Maize production systems, farmers’
perception and current status of maize lethal necrosis in selected counties in Kenya. All Life 15, 692–705.
https://doi.org/10.1080/26895293.2022.2085815
Pratt, C. F., Constantine, K. L., & Murphy, S. T., 2017. Economic impacts of invasive alien species on
African smallholder livelihoods. Global Food Security 14, 31–37. https://doi.org/10.1016/j.gfs.2017.01.011
Redinbaugh, M. G., & Stewart, L. R., 2018. Maize Lethal Necrosis: An Emerging, Synergistic Viral
Disease. Annual Review of Virology 5, 301–322. https://doi.org/10.1146/annurev-virology-092917-
043413
Regassa, B., Abraham, A., Fininsa, C., & Wegary, D., 2021. Alternate hosts and seed transmission of
maize lethal necrosis in Ethiopia. Journal of Phytopathology 169, 303–315.
https://doi.org/10.1111/jph.12986
Regassa, B., Abraham, A., Fininsa, C. et al., 2020) Distribution of maize lethal necrosis epidemics and its
association with cropping systems and cultural practices in Ethiopia. Crop Protection 134, 105151.
https://doi.org/10.1016/j.cropro.2020.105151
Richard, K., Abdel-Rahman, E. M., Subramanian, S. et al., 2021. Estimating maize lethal necrosis (MLN)
severity in Kenya using multispectral high-resolution data. Applied Geomatics 13, 389–400.
https://doi.org/10.1007/s12518-021-00357-4
Tanksley, S. D., & McCouch, S. R., 1997. Seed Banks and Molecular Maps: Unlocking Genetic Potential
from the Wild. Science 277, 1063–1066. https://doi.org/10.1126/science.277.5329.1063
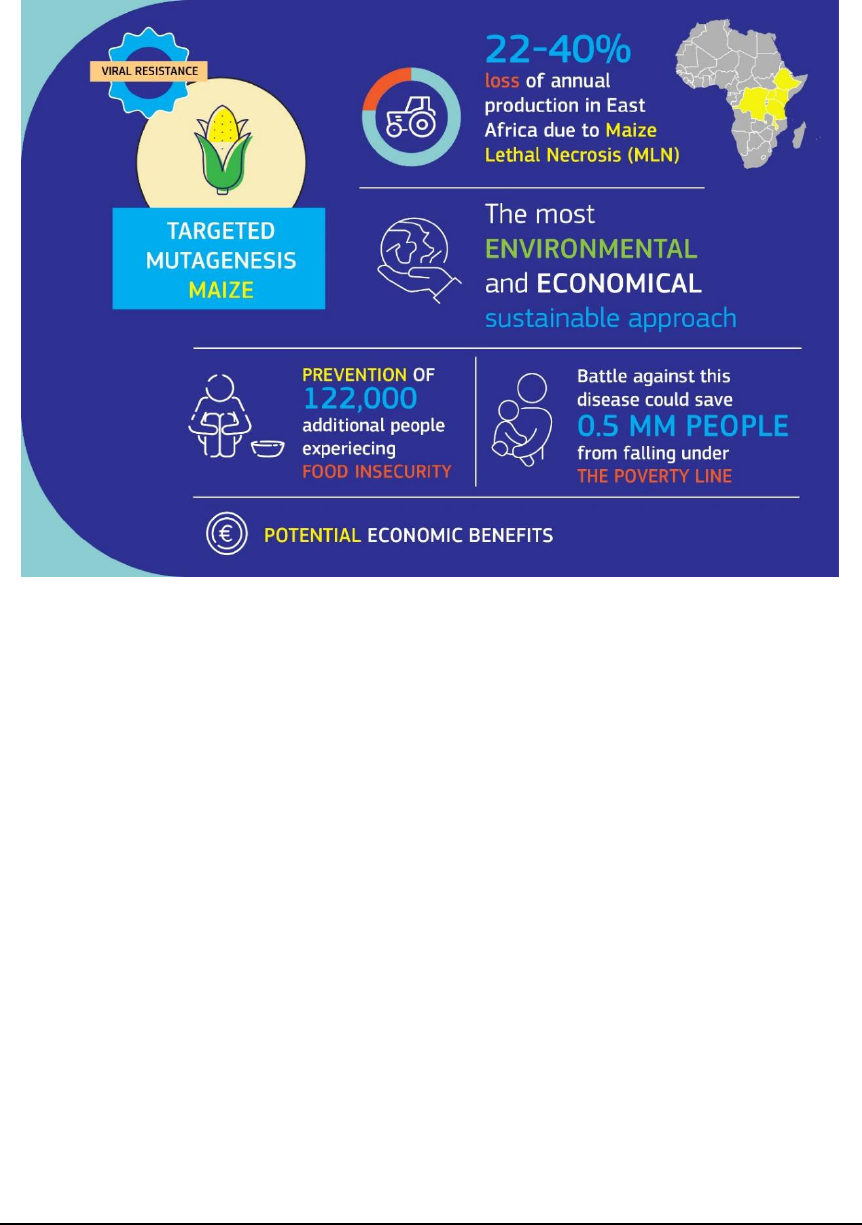
169
Figure 7: Presentation of the JRC case study on targeted mutagenesis maize.
Tracy, W. F., 2004. Breeding: the backcross method. In R. M. Goodman (Ed.), Encyclopedia of Plant and
Crop Science. Routledge. https://doi.org/10.1081/E-EPCS
United Nations (UN), 2022. The Sustainable Development Goals Report 2022. United Nations
Publications, New York.

170
Horizon 2020 projects and other research concerning NGT applications
A Horizon 2020 project has considered an NGT root chicory for inulin production (box
4), and presented an environmental and socio-economic assessment of the whole value
chain of the new chicory variants and their derived products.
Box 4: Root chicory for inulin production (H2020 project)
11
Root chicory (Cichorium intybus) is a specialty crop, nearly exclusively cultivated in Belgium and
the Netherlands where the growing conditions for this crop species are best, and which experiences
increased demands as a source of food ingredients. Root chicory is cultivated for the food fibre
inulin, a chain of fructose molecules – fibre is extracted from the root and included in foods such as
yoghurts and cereal bars as low-calorie sweetener – and for the production of terpenes, which are
able to inhibit, like antibiotics, the growth of fungi or bacteria, or are reported to prevent cancer
cells from growing. The CHIC project
12
employed targeted mutagenesis to develop chicory
varieties that (1) produce more and healthier inulin fibres and (2) produce sufficient amounts of
medicinal terpenes.
A socio-economic and environmental impact assessment, using a multi-regional input-output
(MRIO) analysis and life-cycle assessment (LCA), assumes that the NGT-based root chicory would
not be regulated as a GMO. Three scenarios are modelled:
- A baseline scenario which uses the current situation of chicory yield, cultivation
practices, energy use, and the current levels of inulin and terpene extraction;
- a chicory with a higher inulin content and reduced terpene content;
- a chicory with higher inulin and terpene content.
According to the project’s conclusions, the two scenarios based on new chicory varieties both show
positive socio-economic impacts (production and employment effects) compared to the baseline
scenario. The study found that the higher inulin and terpene content of the NGT chicory varieties
can lead to more jobs, can generate higher added value, can reduce GHG emissions and can reduce
primary energy demand. Despite the market value of inulin, chicory is still an underdeveloped crop
in the EU. NGT varieties of chicory with a higher inulin content and the additional production of
terpenes can increase the competitive value of chicory in comparison to other crop species.
Furthermore, a new Horizon Europe project GeneBEcon
13
, started in September 2022,
will examine the innovation potential of gene editing in enabling a sustainable
bioeconomy in Europe. Through the application of this technology in potato and
microalgae, GeneBEcon intends to promote energy-efficient, low-input, and zero-
pollution agricultural production and clean industrial processing. First, the technical
potential will be explored by applying gene editing to develop 1) a virus-resistant potato
with an industrial tuber starch quality, and 2) microalgae-based production of industrially
relevant mycosporin-like amino acids. Second, the risk-regulatory aspects, economic
incentives, and social perceptions will be investigated. In the latter, comparative analyses
will be enabled by two different production systems: open-field agricultural crop and
contained-system microalgae.
Other national research projects are also investigating the potential of genome editing
(e.g. GenEdit in Belgium
14
). A COST Action (PlantEd, 2019-2023)
15
, involving over 330
experts from 36 European countries and beyond, is aimed at advancing the technology
11
Hingsamer M., Kulmer V., de Roode M. &Kernitzkyi M. (2022) Environmental and socio-economic
impacts of new plant breeding technologies: A case study of root chicory for inulin production. Frontiers
in Genome Editing 4, doi: 10.3389/fgeed.2022.919392
12
https://cordis.europa.eu/project/id/760891/results
13
https://cordis.europa.eu/project/id/101061015
14
https://www.sciensano.be/en/projects/development-and-evaluation-approaches-detection-organisms-
modified-new-genome-editing-techniques
15
https://plantgenomeediting.eu/
171
and assessing its impact, discuss regulatory options, monitor public perceptions and
develop educational and outreach activities.

172
ANNEX 8: EU LEGAL FRAMEWORK ON GMOS
The GMO legal framework has as main aims to protect human and animal health and the
environment in accordance with the precautionary principle and to ensure the effective
functioning of the internal market. It regulates the deliberate release (including the
placing on the market) of GMOs and of food and feed produced from GMOs, the
contained use of GMMs, as well as the export of GMOs to third countries (in application
of the EU’s obligations under the Cartagena Protocol on Biosafety to the UN Convention
on Biological Diversity
1
). The GMO acquis rests on five main pieces of legislation,
namely:
- Directive 2001/18/EC of the European Parliament and of the Council of 12 March
2001 on the deliberate release into the environment of genetically modified
organisms
2
- Directive 2009/41/EC of the European Parliament and of the Council of 6 May
2009 on the contained use of genetically modified micro-organisms
3
- Regulation (EC) No 1829/2003 of the European Parliament and of the Council of
22 September 2003 on genetically modified food and feed
4
- Regulation (EC) No 1830/2003 of the European Parliament and of the Council of
22 September 2003 concerning the traceability and labelling of genetically
modified organisms and the traceability of food and feed products produced from
genetically modified organisms
5
- Regulation (EC) No 1946/2003 of the European Parliament and of the Council of
15 July 2003 on transboundary movements of genetically modified organisms
6
.
The EU GMO legislation applies to GMOs as defined in Article 2(2) of Directive
2001/18/EC, i.e. ‘an organism, with the exception of human beings, in which the genetic
material has been altered in a way that does not occur naturally by mating and/or
natural recombination’. The definition is further refined by a non-exhaustive list of
genetic modification techniques set out in Part 1 of Annex IA to the Directive and by
excluding the techniques listed in Part 2 of that annex, which are not considered to result
in genetic modification under certain conditions. Furthermore, Article 3(1) of the
Directive excludes from the scope of the Directive GMOs that result from the
techniques/methods listed in Annex IB (i.e. mutagenesis and certain types of cell fusion),
provided that they do not involve the use of recombinant nucleic acid molecules or
GMOs.
In 2018, the Court of Justice of the EU clarified the interpretation of the scope of the
mutagenesis exemption (judgment of 25.7.2018, Case C-528/16 Confédération paysanne
and Others
7
). The Court ruled, firstly, that organisms obtained by means of mutagenesis
1
Cartagena Protocol on Biosafety to the Convention on Biological Diversity, adopted on 29 January 2000, concluded
on behalf of the EU by Council Decision 2002/628/EC of 25 June 2002 concerning the conclusion, on behalf of the
European Community, of the Cartagena Protocol on Biosafety, OJ L 201, 31.7.2002, p. 48.
2
OJ L 106, 17.4.2001, p. 1
3
OJ L 125, 21.5.2009, p. 75
4
OJ L 268, 18.10.2003, p. 1
5
OJ L 268, 18.10.2003, p. 24
6
OJ L 287, 5.11.2003, p. 1
7
See footnote 14.

173
techniques/methods constitute GMOs as they meet the definition in Article 2(2) of
Directive 2001/18/EC and are not included in the exhaustive list of techniques not
resulting in genetic modification set out in Part 2 of Annex IA to that directive, and,
secondly, that the mutagenesis exemption in Annex IB to that directive must be
interpreted as only covering organisms obtained by means of mutagenesis
techniques/methods which have conventionally been used in a number of applications
and have a long safety record, and that new techniques/methods that have appeared or
have been mostly developed since Directive 2001/18/EC was adopted are not exempted.
Accordingly, the Court concluded that organisms obtained by means of random
mutagenesis techniques/methods are excluded from the scope of the Directive, whereas
organisms obtained by techniques/methods of targeted mutagenesis are not exempted
(judgment of 25.7.2018, Case C-528/16 Confédération paysanne and Others, paragraphs
48, 51, 54). In a recent judgment, the Court further clarified that organisms obtained by
in vitro random mutagenesis techniques/methods are exempted from the GMO legislation
(judgment of 7 February 2023, Case C-688/21 Confédération paysanne and Others)
8
.
Although the above-mentioned 2018 judgment only concerned the mutagenesis
exemption, its conclusions apply also to organisms produced by means of other NGTs,
such as cisgenesis. Indeed, organisms obtained by those techniques alter the genetic
material in a way that does not occur naturally by mating or natural recombination and,
therefore, constitute GMOs. Since those techniques are neither listed in Part 2 of Annex
IA nor in Annex IB to Directive 2001/18/EC, it must be concluded that organisms
obtained by those techniques are subject to the GMO legislation
9
.
The main elements of the GMO acquis are described in the next paragraphs (see also
diagram in the end of the section).
Directive 2001/18/EC on the deliberate release of genetically modified
organisms
Directive 2001/18/EC requires the relevant national authorities’ prior consent for the
deliberate release of GMOs into the environment. In order to obtain consent, an
application (‘notification’) has to be submitted to the national competent authority,
accompanied inter alia by an environmental risk assessment. The risk assessment must
comply with the general principles and the methodology set out in the Directive and must
draw conclusions for each relevant area of risk. The requirements are different for the
placing on the market of GMOs as or in products (Part C of the Directive) and for other
purposes, such as experimental releases (Part B).
The authorisation procedure for the placing on the market of GMOs other than food and
feed is conducted by Member States. The national competent authority to which the
notification has been submitted must deliver an assessment report on the submitted
notification. In cases where the Commission or another Member State have expressed
objections to the assessment report and no agreement has been reached, the Commission
adopts a decision after obtaining the scientific opinion of EFSA. The national competent
authority that prepared the report rejects the notification or gives written consent for the
placing on the market of the GMO and sets out the conditions, as well as labelling and
monitoring requirements. The consent can be valid for a renewable period of up to 10
years.
8
ECLI:EU:C:2023:75
9
European Commission (2021c), sections 4.2.2, 4.2.3 and 4.2.4.

174
The Directive allows Member States to ban the cultivation of GMOs in their territory or
part thereof based on compelling grounds (such as grounds relating to town and country
planning, land use, etc) other than those concerning the safety of GMOs for health or the
environment. So far this provision has been applied as regards the authorisation for the
cultivation of one GM plant
10
, from which 18 of the 27 Member States
11
excluded all of
part of their territory.
Directive 2009/41/EC on the contained use of genetically modified micro-
organisms (GMMs)
This Directive defines a GMM as ‘a micro-organism in which the genetic material has
been altered in a way that does not occur naturally by mating and/or natural
recombination’ (Article 2(b)). It requires a notification to the national competent
authorities and in some cases their prior consent for the contained use of GMMs. To that
end, the user has to carry out an assessment of the contained uses as regards risks to
human health and the environment.
Directive 2009/41/EC only applies to GMMs. The contained use of other GMOs is
currently not regulated at EU level, but is subject to national legislation in most Member
States.
Regulation (EC) No 1829/2003 on GM food and feed
This Regulation requires authorisation for the placing on the market of food and feed
consisting of, containing or produced from GMOs (‘GM food and feed’). The
authorisation procedure is centralised, providing for a single application to be submitted
to a national competent authority for all the intended uses of the GM food or feed in
question (including cultivation). It is based on an independent risk assessment carried out
by EFSA. Authorisation may be granted only if the risk assessment demonstrates that,
under its intended conditions of use, the product has no adverse effects for human and
animal health and for the environment compared to its conventionl counterparts, does not
mislead the user or the consumer and is not nutritionally disadvantageous compared to
the food or feed it is intended to replace.
Authorisations are granted by the Commission for a renewable period of ten years. They
may impose conditions or restrictions, including post-market monitoring requirements,
and must set out the method for detecting the transformation event, as provided by the
applicant and validated by the EU Reference Laboratory for GM Food and Feed (EURL),
as well as the place from which the reference material may be obtained. The Regulation
also provides for mandatory labelling of authorised GM food and feed, so that final users
and consumers can make an informed choice, but exempts food and feed containing
traces of GMOs or GM material in a proportion no higher than 0.9% of the food/food
ingredient/feed/feed component provided that this presence is adventitious or technically
unavoidable.
10
Commission Implementing Decision (EU) 2016/321 of 3 March 2016 adjusting the geographical scope
of the authorisation for cultivation of genetically modified maize (Zea mays L.) MON 810 (MON-ØØ81Ø-
6) (OJ L60, p 90).
11
AT, BE (Wallonia), BG, CY, DE, DK, EL, FR, HR, HU, IT, LV, LT, LU, MT, NL, PL, SI
175
Regulation (EC) No 1830/2003 on the traceability and labelling of GMOs and
the traceability of food and feed products produced from them
Under this Regulation, operators placing GMOs and GM food and feed on the market
must inform the operators receiving the products, in writing, that the products contain or
consist of GMOs. They must provide an indication of each ingredient/material produced
from GMOs or, for products without an ingredients list, an indication that the product is
produced from GMOs. They must keep that information for five years and be able to
identify the operator(s) by whom and to whom the products have been made available.
Traceability requirements allow for close monitoring of potential effects of the product
on environment and health, and where necessary for the withdrawal of products if an
unexpected risk to human health or to the environment is detected.
Furthermore, the Regulation requires that all products consisting of or containing GMOs
are labelled as GMOs.
The Regulation exempts from the traceability and labelling requirements products
containing traces of GMOs intended for direct use as food, feed or for processing and
food/feed containing traces of GMOs or GM material in a proportion not higher than
0.9% provided that these traces are adventitious or technically unavoidable.
Regulation (EC) No 1946/2003 on transboundary movements of GMOs
At international level, the EU is a party to the Cartagena Protocol on Biosafety. The
purpose of the Protocol, in line with the precautionary approach, is to ensure an adequate
level of protection in the safe transfer, handling and use of GMOs that may have adverse
effects on biodiversity and human health. To that end, it sets out common rules to be
followed in transboundary movements of GMOs and provides for a central database
(Biosafety Clearing House) to allow parties to exchange information on GMOs and help
them to comply with their obligations under the Protocol. The Protocol’s procedures
concerning exports of GMOs are implemented in the EU by Regulation (EC)
No 1946/2003 on transboundary movements of GMOs. In particular, the Regulation
requires EU operators to notify the competent authorities of importing countries of
exports of GMOs intended for deliberate release into the environment and to seek their
consent prior to the first export. It also requires the Commission and Member States to
inform the Biosafety Clearing House of relevant legislation and decisions on GMOs.
.

176

177
ANNEX 9: PROBLEM TREE

178
ANNEX 10: INTERVENTION LOGIC
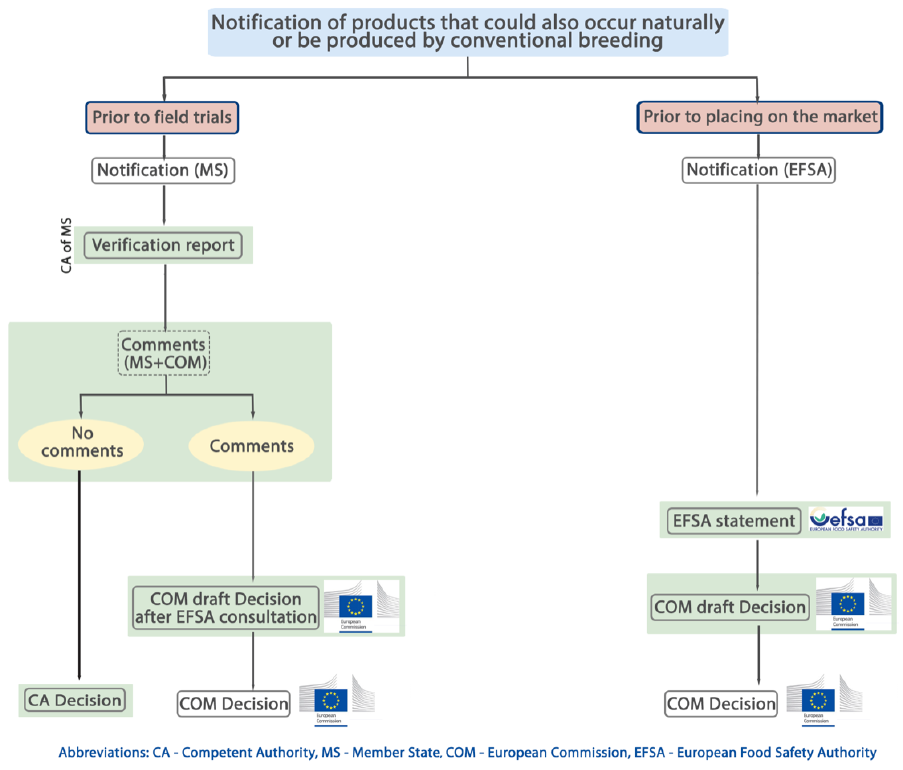
179
ANNEX 11: PROCEDURE FOR NOTIFICATION (OPTION 4)

180
ANNEX 12: SME TEST
Step 1/4: Identification of affected businesses
Key question: To what extent is the initiative relevant for SMEs? (not relevant, relevant, highly
relevant)
Step 2/4: Consultation of SME Stakeholders
SMEs form an important part of the plant breeding sector, with over 90% in some Member States
of plant breeders being SMEs . The majority of EU farmers are small (and micro) enterprises.
Under the current GMO legislation, the SMEs in NGTs and GMO plant breeding seeking
authorisation for the release or placing on the market of products have, though, a very limited
presence. This signals that the current legislation affects them negatively, with the exception of
the organic sector. The latter is dominated by micro and small farmers and small organic breeders.
This initiative is considered relevant for SMEs. Policy objectives of the initiative are aimed at
reducing regulatory costs and regulatory uncertainty, both of which disproportionately affect
SMEs. Moreover, there is an expectation that measures aimed at a level playing field vis à vis
non-EU countries will benefit the internal market players, including SMEs. The initiative also
aims at facilitating the application of NGTs to wider range of traits and of niche or locally
important crop species, which often are the focus of SMEs, thereby opening the market to a
greater diversity of players.
SMEs have been explicitly integrated in the consultation strategy. For the different consultation
methods, the following numbers (shares) of SMEs or associations representing SMEs
participated:
- General Interviews: 11 business associations representing the various value chain
segments which represent SMEs as well as large companies. 3 interviews with farmer
organisations. 2 interviews with organic business stakeholders, mainly representing small
companies (including farmers). Thus 16 out of 25 (64%) of the general interviews concerned
business organisations that represented SMEs.
- Cost-benefit analysis (CBA): the CBA mapped costs for the biotechnology industry,
processors, farmers, and retail. While regulatory costs are the same for all size classes of
companies, SMEs have – for reasons already mentioned above- more difficulties to cover them.
This is covered qualitatively. To understand the situation, out of five interviewed companies for
the CBA, two were small firms.
- Targeted Survey: 8 out 122 respondents (6%) identified as SMEs
- Public consultation (PC): out of 179 companies/business associations 125 (69.8%)
identified as Small and Medium Sized Enterprises (SMEs), comprised of medium companies (28;
15.6%), small companies (33; 8.4%), and micro-companies (64; 35.8%).
- Focus Groups: in two focus groups, six sector and farmer representatives joined (out of 14
participants). In particular, the small farmer’s perspectives were taken into account through the
inclusion of two farmer/organic farmer organisations.
Given that neither SMEs nor larger companies have much experience with GMOs and NGTs in
the EU, most of the quantitative effects are based on expectations and qualitative assessment.

181
Views from the public consultation in response to a question on necessary regulatory measures to facilitate
the uptake of NGTs by SMEs indicate that mainly a stable, reliable, inexpensive framework is needed.
Farmers voice that ‘lower fees and easier and faster procedures will facilitate the uptake by SME’ and it
would be essential to ‘secure a quick and low-cost administrative process’. The plant breeding/seeds sector
indicated that ‘the financial and bureaucratic hurdles should be kept as low as possible in order to grant
SMEs the opportunity to use NGT’.
A potential loss of competitiveness is widely felt among the respondents of the PC in case there is no change
in the current legislation, e.g.: “if we continue GMO regulation, it would mean that we as a small/medium-
sized processing company do not in any way have a chance to use NGT” and that would imply a “loss of
competitiveness with large competitors”. NGTs have the characteristics of a less costly platform technology
that could lead to an opening up of the plant breeding landscape from which a variety of SMEs could
benefit. This has been observed in Argentina after its regulatory shift to excluding certain NGTs from the
GMO definition.
The current GMO legal framework is not fit-for purpose for certain NGTs and their products (Commission
NGT study, 2021). Costs for the required evidence and procedures do not differ between large companies
and SMEs but large companies have different advantages and mitigation measures at hand: SMEs tend to be
more vulnerable to regulatory requirements than larger firms, which benefit from economies of scale, in-
house legal departments, diversification of risks, etc.). Under the current situation, SMEs are more likely to
reduce their NGT-development effort. They are also less likely to shift product development to non-EU
countries and are particularly sensitive to uncertainty. They have fewer financial reserves and cannot spread
their risks over a broad portfolio. Any risks that might result in a lack or delayed market authorisation are
therefore particularly affecting SMEs.
The preferred option is by far the most advantageous for SMEs compared to the current situation, as
administrative and compliance costs will nearly be eliminated for those NGT products to which the
notification procedure is applicable. Furthermore, the clear definition of objective notification criteria would
reduce uncertainties and render the regulatory process more predictable for SMEs. It also has the strongest
impact on competitiveness as a lower regulatory burden will enable investments in R&D and subsequent
product development, both from large multinational plant breeders as well as SMEs. Regulatory incentives
linked to the authorisation of NGT plants under the preferred option would facilitate access to and
navigation of the regulatory framework, especially for SMEs, supporting their competitiveness.
The impact analysis shows that NGTs offer opportunities for small farmers, depending on the developed
traits and plants. With (potentially) less land needed to obtain higher yield, less pesticides used to maintain
healthy crops, less water is needed for irrigation, or crops being more resistant to biotic and abiotic stress,
farmers expect to have economic gains. The gains will be somewhat offset by expected higher seed prices,
but overall a positive economic impact is envisaged. In parallel, positive environmental effects (in particular
on soil, water, air use and quality) would allow farmers to transform current farming practices into more
sustainable ones.
In the preferred option, which is expected to result in the highest number of NGT plants on the EU market
and where notified NGT plants would not be subject to labelling and traceability as GMOs, the highest
negative impact on SMEs from the organic and GM-free sectors in the sector is expected, should notified
NGT plants remain banned in the organic sector.
While from the legal point of view the NGT initiative is independent from the rules on the protection of
intellectual property and does not address matters of application of the IPR legislation to NGT plants, the
Commission has taken note of the concerns brought forward by certain stakeholders on the possible impacts
on SMEs relating to the application of the IPR legislation, and will carefully consider them.
Step 3/4: Assessment of the impact on SMEs

182
The preferred option maximises the positive impacts on SMEs in the breeding sector. The
notification procedure in the preferred option would minimise costs for breeder as they undergo
the same process as conventionally bred plant varieties. SME operators developing plants subject
to the authorisation procedure with adapted risk assessment can benefit from regulatory incentives
such as the additional regulatory advice and guidance or waiving of detection method validation
fees and faster procedures. This particularly relevant for SMEs, which as a rule lack a regulatory
department with dedicated staff. These will help lower barriers to market access and thus support
SMEs competitiveness in the breeding sector.
As regards SMEs in the organic and GM-free sectors, in the case of authorised NGT products, the
current mechanisms of the GMO legislation relied on by organic and GM-free operators will
remain in place (traceability, labelling, national coexistence measures) and will impose obligations
on all actors in the supply chain dealing with NGT plants. As regards notified products, a public
register of NGT plants subject to notification will support the establishment of supply chains free
from NGT products. As regards negative impacts resulting from, if NGT products subject to
notification, which could be produced also by conventional breeding, were accepted in organic
production, this would reduce the burden of establishing NGT free supply chains and the
consequences of possible accidental admixture.
Step 4/4: Minimising negative impacts on SMEs
