
75
Secretariat of the
Convention on
Biological Diversity
CBD Technical Series
No. 75
An Updated Synthesis
of the Impacts of Ocean
Acidication on Marine
Biodiversity

CBD Technical Series No. 75
AN UPDATED SYNTHESIS OF THE
IMPACTS OF OCEAN ACIDIFICATION
ON MARINE BIODIVERSITY
The designations employed and the presentation of material in this publication do
not imply the expression of any opinion whatsoever on the part of the copyright
holders concerning the legal status of any country, territory, city or area or of its
authorities, or concerning the delimitation of its frontiers or boundaries.
This publication may be reproduced for educational or non-prot purposes
without special permission, provided acknowledgement of the source is made. The
Secretariat of the Convention would appreciate receiving a copy of any publications
that use this document as a source. Reuse of the gures is subject to permission
from the original rights holders.
Published by the Secretariat of the Convention on Biological Diversity.
ISBN 92-9225-527-4 (print version);
ISBN 92-9225-528-2 (web version)
Copyright © 2014, Secretariat of the Convention on Biological Diversity
Citation:
Secretariat of the Convention on Biological Diversity (2014). An Updated Synthesis
of the Impacts of Ocean Acidication on Marine Biodiversity (Eds: S. Hennige,
J.M. Roberts & P. Williamson). Montreal, Technical Series No. 75, 99 pages
For further information, contact:
Secretariat of the Convention on Biological Diversity
World Trade Centre, 413 Rue St. Jacques, Suite 800,
Montréal, Quebec,Canada H2Y 1N9
Tel: +1 (514) 288 2220
Fax: +1 (514) 288 6588
E-mail: [email protected]
Website: www.cbd.int
Cover images, top to bottom:
Katharina Fabricius; N. Bednarsek; IGBP, IOC, SCOR; S.Hennige/J.M. Roberts
Typesetting: Em Dash Design
Acknowledgments
e Secretariat of the Convention on Biological Diversity acknowledges with appreciation the generous
nancial support received from United Kingdom of Great Britain and Northern Ireland for undertak-
ing and coordinating the research for this updated synthesis as well as the European Commission for the
production of this publication. e Secretariat also wishes to thank the following editors, lead authors
and reviewers for their contributions, together with the Secretariat sta who edited the dra report and
coordinated the production of this publication:
Editors
Sebastian Hennige, J. Murray Roberts and Phillip Williamson
Lead authors
Tracy Aze, James Barry, Richard Bellerby, Luke Brander, Maria Byrne, Sam Dupont, Jean-Pierre Gattuso,
Samantha Gibbs, Lina Hansson, Caroline Hattam, Chris Hauton, Jon Havenhand, Jan Helge Fossa,
Christopher Kavanagh, Haruko Kurihara, Richard Matear, Felix Mark, Frank Melzner, Philip Munday,
Barbara Nieho, Paul Pearson, Katrin Rehdanz, Sylvie Tambutte, Carol Turley, Alexander Venn, Michel
Warnau, and Jeremy Young (Additional details given in the Annex on page 99).
Reviewers
e following countries, organizations and individuals are amongst those who kindly provided comments
on an initial dra of this report:
•
Countries: Canada, Colombia, France, Honduras, India, Italy, Japan, Mexico, Nigeria, United Kingdom
of Great Britain and Northern Ireland, United States of America.
•
Organizations: Intergovernmental Oceanographic Commission of the United Nations Educational,
Scientic and Cultural Organization, International Atomic Energy Agency, United Nations Division
for Ocean Aairs and the Law of the Sea.
•
Individual Experts: Jelle Bijma (Germany), Rob Dunbar (USA), Richard Feely (USA), Kunshan
Gao(China), Cli Law (New Zealand), omas Malone (USA), Chou Loke Ming (Singapore); Donna
Roberts (Australia), Rashid Sumaila (Canada), Shirayama Yoshihisa (Japan).

FOREWORD ................................................................................................................................. 6
EXECUTIVE SUMMARY ................................................................................................................... 7
1. BACKGROUND AND INTRODUCTION ................................................................................................11
1.1 Mandate of this review ................................................................................................ 12
1.2 What is ocean acidication? ......................................................................................... 13
1.3 Re-visiting key knowledge gaps identied in the previous CBD review .............................. 18
2. SCIENTIFIC AND POLICY FRAMEWORK .............................................................................................20
2.1 Steps toward global recognition and international scientic collaboration ......................... 20
2.2 Intergovernmental interest in ocean acidication and actions to date ................................ 22
3. GLOBAL STATUS AND FUTURE TRENDS OF OCEAN ACIDIFICATION .........................................................27
3.1 Variability .................................................................................................................. 27
3.2 Modelled simulations of future ocean acidication .......................................................... 29
3.3 Current status of global observations ............................................................................. 32
4. WHAT THE PAST CAN TELL US — PALEO-OCEANOGRAPHIC RESEARCH .....................................................36
4.1 Reconstructing past ocean acidication events ............................................................... 37
4.2 e Paleocene-Eocene ermal Maximum – A natural past “experiment” in ocean
acidication ............................................................................................................... 37
4.3 e impact of past ocean acidication upon calciers ..................................................... 38
4.4 Using the geological record to inform our understanding of ocean acidication .................. 39
4.5 Timescales of ocean acidication .................................................................................. 39
5. PHYSIOLOGICAL RESPONSES TO OCEAN ACIDIFICATION ......................................................................42
5.1 Ocean acidication and cellular processes ..................................................................... 42
5.2 Fertilization, early life and settlement ........................................................................... 44
5.3 Sensory capacity and behaviour ................................................................................... 46
5.4 Immune responses and disease ..................................................................................... 47
6. IMPACTS OF OCEAN ACIDIFICATION ON BENTHIC COMMUNITIES ...........................................................54
6.1 Corals ....................................................................................................................... 55
6.2. Molluscs .................................................................................................................... 58
6.3 Echinoderms .............................................................................................................. 59
6.4 Macroalgae, seagrass and benthic microbiota ................................................................. 59
7. IMPACTS OF OCEAN ACIDIFICATION ON PELAGIC COMMUNITIES ..........................................................65
7.1. Plankton ................................................................................................................... 65
7.2 Fish, squid and cuttlesh ............................................................................................. 70
8. IMPACTS OF OCEAN ACIDIFICATION ON BIOGEOCHEMICAL CYCLES, ECOSYSTEM SERVICES AND LIVELIHOODS ..78
8.1 Ocean biogeochemistry and climate .............................................................................. 78
8.2 Ecosystem services ...................................................................................................... 81
8.3 Economic and livelihood impacts ................................................................................. 83
9. FUTURE CONSIDERATIONS ............................................................................................................88
9.1 Technical challenges: from individuals to ecosystems ...................................................... 88
9.2 eoretical challenges and future priorities .................................................................... 90
9.3 Advances in sensing, monitoring and emerging technologies ........................................... 92
10. CONCLUSIONS ...........................................................................................................................96
ANNEX .....................................................................................................................................99
TABLE OF CONTENTS

6
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
Marine and coastal biodiversity – ecosystems, species and
genetic material – provide enormous benets for human
well-being. Hundreds of millions of people rely directly on
marine biodiversity for their livelihoods. Oceans are criti-
cal to many important global geo-chemical processes, such
as climate regulation and carbon cycling. Ocean ecosystems
provide critical life supporting services to the global popu-
lation and underpin global productivity and well-being.
However, the oceans are facing major threats due to rising
levels of carbon dioxide in the atmosphere. In addition to
driving global climate change, increasing concentrations of
carbon dioxide aect ocean chemistry, impacting marine
ecosystems and compromises the health of the oceans and
their ability to provide important services to the global
community. e impacts of ocean acidication are begin-
ning to be felt in some areas, but future projections indicate
even more broad-reaching deleterious impacts if action is
not taken.
At its ninth meeting, the Conference of the Parties to the CBD
raised concerns about the potential impacts of ocean acid-
ication on marine and coastal biodiversity and requested
the Executive Secretary, in collaboration with Parties, other
Governments, and relevant organizations, to compile and
synthesize available scientic information on ocean acidi-
cation and its impacts on marine biodiversity and habitats.
is resulted in the production of CBD Technical Series No.
46 “Scientic Synthesis of the Impacts of Ocean Acidication
on Marine Biodiversity” in 2009.
Since then, the amount of research on ocean acidication
has grown enormously, as various governments and orga-
nizations around the world expanded their research eorts
to gain an improved understanding of the ecological and
socioeconomic impacts of ocean acidication and means
to address this pressing threat.
In recognition of the need for the most up-to-date infor-
mation in addressing this issue, the COP, in decision XI/18,
requested the Executive Secretary to collaborate with the
Intergovernmental Oceanographic Commission of the United
Nations Educational, Scientic and Cultural Organization,
relevant scientic groups, other relevant organizations, and
indigenous and local communities in the preparation of an
updated systematic review on the impacts of ocean acidica-
tion on biodiversity and ecosystem functions, building upon
CBD Technical Series No. 46, to provide a targeted synthe-
sis of the biodiversity implications of ocean acidication for
marine and coastal systems, including information on the
less-reported paleo-oceanographic research.
is report, CBD Technical Series No. 75, “An updated
synthesis of the impacts of ocean acidication on marine
biodiversity”, represents an enormous scientic eort by
researchers and experts from around the world to synthe-
size the best available and most up-to-date information
on the impacts of changing ocean pH on the health of the
world’s oceans.
Among other ndings, the report notes that ocean acidica-
tion has increased by around 26% since pre-industrial times
and that, based on historical evidence, recovery from such
changes in ocean pH can take many thousands of years. e
report outlines how ocean acidication impacts the physi-
ology, sensory systems and behavior of marine organisms,
and undermines ecosystem health. It, furthermore, shows
that impacts due to ocean acidication are already under-
way in some areas and that future projected impacts could
have drastic irreversible impacts on marine ecosystems.
Despite the growing body of information on ocean acidica-
tion, the report points out key knowledge gaps and, in light
of the many complex interactions related to ocean chemis-
try, stresses the diculty of assessing how future changes
to ocean pH will aect marine ecosystems, food webs and
ecosystems, and the goods and services they provide.
is report, which presents complex scientic information
on ocean acidication in a clear and understandable way,
provides an important reference point for scientists, policy-
makers and anyone else interested in understanding how
ocean acidication aects our oceans and the vital services
they provide. As the need for urgent action to address ocean
acidication becomes ever more pressing, collaboration
among governments and organizations in enhancing and
sharing knowledge through eorts such as this report will
become increasingly important.
Braulio Ferreira de Souza Dias
Executive Secretary
Convention on Biological Diversity
FOREWORD

EXECUTIVE SUMMARY
7
EXECUTIVE SUMMARY
Ocean acidication and awareness of its consequences
1. Ocean acidication has increased by around 26% since pre-industrial times
In the past 200 years, it is estimated that the ocean has absorbed more than a quarter of the carbon
dioxide released by human activity, increasing ocean acidity (hydrogen ion concentration) by a similar
proportion. It is now nearly inevitable that within 50 to 100 years, continued anthropogenic carbon
dioxide emissions will further increase ocean acidity to levels that will have widespread impacts,
mostly deleterious, on marine organisms and ecosystems, and the goods and services they provide.
Marine calcifying organisms seem particularly at risk, since additional energy will be required to
form shells and skeletons, and in many ocean areas, unprotected shells and skeletons will dissolve.
2. International awareness of ocean acidication and its potential consequences is increasing
Many programmes and projects are now investigating the impacts of ocean acidication on marine
biodiversity and its wider implications, with strong international linkages.
e United Nations General
Assembly has urged States to study ocean acidication, minimize its impacts and tackle its causes.
Many United Nations bodies are focusing attention on these issues.
Global status and future trends of ocean acidication
3. Seawater pH shows substantial natural temporal and spatial variability
e acidity of seawater varies naturally on a diurnal and seasonal basis, on a local and regional scale, and
as a function of water depth.
Coastal ecosystems and habitats experience greater variability than those
in the open ocean, due to physical, geochemical and biological processes, and terrestrial inuences.
4. Substantial natural biological variability exists in organisms’ responses to pH changes
Metadata analyses, combining results from many experimental studies, show that there are dier-
ent, but consistent, patterns in the response of dierent taxonomic groups to simulated future ocean
acidication.
ere can also be variability in responses within species, depending on interactions
with other factors.
5. Surface waters in polar seas and upwelling regions are increasingly at risk of becoming
undersaturated with respect to calcium carbonate, dissolving shells and skeletons which are
not protected by an organic layer
In waters where pH is already naturally low (in high latitudes, coastal upwelling regions and on the
shelf slope), widespread undersaturation of the commonest forms of biologically-formed calcium
carbonate, aragonite and calcite, is expected to develop during this century.
Benthic and planktonic
molluscs are amongst the groups likely to be aected, as well as cold-water corals and the structural
integrity of their habitats.
6. International collaboration is underway to improve monitoring of ocean acidication, closely
linked to other global ocean observing systems
A well-integrated global monitoring network for ocean acidication is crucial to improve under-
standing of current variability and to develop models that provide projections of future conditions.
Emerging technologies and sensor development increase the eciency of this evolving network.

8
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
What the past can tell us: paleo-oceanographic research
7. During natural ocean acidication events that occurred in the geological past, many marine
calcifying organisms became extinct
High atmospheric carbon dioxide has caused natural ocean acidication in the past, linked to “coral
reef crises”.
During the Paleo-Eocene ermal Maximum (PETM, ~56 million years ago), the species
extinctions were less severe than earlier events; however, the atmospheric changes that occurred then
were much slower than those happening today.
8. Recovery from a major decrease in ocean pH takes many thousands of years
e paleo-record shows that recovery from ocean acidication can be extremely slow; following the
PETM, for example, this took around 100,000 years.
Impacts of ocean acidication on physiological responses
9. Ocean acidication has implications for acid-base regulation and metabolism for many
marine organisms
When external hydrogen ion levels substantially increase, extra energy may be required to maintain
the internal acid-base balance.
is can lead to reduced protein synthesis and reduction in tness.
Such eects are greatest for sedentary animals, but can be mitigated if food supply is abundant, and
increasing metabolism may oset detrimental eects in some species.
10. Impacts of ocean acidication upon invertebrate fertilization success are highly variable,
indicating the potential for genetic adaptation
Experimental studies on the impact of ocean acidication on fertilization show that some species are
highly sensitive, whilst others are tolerant.
Intra-specic variability indicates the scope for a multi-
generational, evolutionary response.
11. Ocean acidication is potentially detrimental for calcifying larvae
Early life stages of a number of organisms seem to be particularly at risk from ocean acidication, with
impacts including decreased larval size, reduced morphological complexity, and decreased calcication.
12. Ocean acidication can alter sensory systems and behaviour in sh and some invertebrates
Impacts include the loss of ability to discriminate between important chemical cues. Individuals may
become more active and liable to exhibit bolder, riskier behaviour.
Impacts of ocean acidication on benthic communities
13. Around half of benthic species have lower rates of growth and survival under projected future
acidication
For corals, molluscs and echinoderms, many studies show reduction in growth and survival rates with
ocean acidication.
However, these responses are variable, and some species can live at low pH conditions.
14. Many seaweed (macroalgae) and seagrass species can tolerate, or may benet from, future
ocean acidication
Non-calcifying photosynthetic species, which are frequently abundant near natural CO seeps, may
benet from future ocean acidication. Calcifying macroalgae are, however, negatively impacted.
High densities of seagrass and eshy macroalgae can signicantly alter the local carbonate chemis-
try, with potential benet for neighbouring ecosystems.

EXECUTIVE SUMMARY
9
Impacts of ocean acidication on pelagic communities
15.
Many phytoplankton could potentially benet from future ocean acidication
Non-calcifying phytoplankton (e.g., diatoms) can show increased photosynthesis and growth under
high CO conditions.
e response of calcifying phytoplankton (e.g., coccolithophores) is more vari-
able, both between and within species. Mesocosm experiments provide insights into the community
shis that might arise through competitive interactions, as well as the balance between increased photo-
synthesis and decreased calcication. e response of bacterio-plankton to ocean acidication has
not been well studied, but altered decomposition rates would have implications for nutrient cycling.
16. Planktonic foraminifera and pteropods seem likely to experience decreased calcication or
dissolution under projected future conditions
e shells of both of these groups are liable to experience dissolution if calcium carbonate saturation
drops below 1. Decreases in shell thickness and size of planktonic foraminifera may also decrease the
eciency of future carbon transport between the sea surface and the ocean interior.
Impacts of ocean acidication on biogeochemistry
17. Ocean acidication could alter many other aspects of ocean biogeochemistry, with feedbacks
to climatic processes
High CO may alter net primary productivity, trace gas emissions, nitrogen-carbon ratios in food
webs and exported particulate matter, and iron bioavailability. e scale and importance of these
eects are not yet well-understood.
Impacts of ocean acidication on ecosystem services and livelihoods
18. Impacts of ocean acidication on ecosystem services may already be underway
Ocean acidication is apparently already impacting aquaculture in the north-west United States of
America, further decreasing the pH of upwelled water, which has a naturally low saturation state for
calcium carbonate. High mortalities in oyster hatcheries can, however, be mitigated by monitoring
and management measures. Risks to tropical coral reefs are also of great concern, since the livelihoods
of around 400 million people depend on such habitats. Research on the socio-economic impacts of
ocean acidication has only recently started and is growing rapidly.
Resolving uncertainties
19. Existing variability in organism response to ocean acidication needs to be investigated
further, to assess the potential for evolutionary adaptation
Multi-generational studies with calcifying and non-calcifying algal cultures show that adaptation to
high CO is possible for some species.
Such studies are more dicult to conduct for long-lived organ-
isms, and variability in adaptive capacity is likely.
Even with adaptation, community composition and
ecosystem function are still likely to change.
20. Research on ocean acidication increasingly needs to involve other stressors, as will occur
under eld conditions in the future
Acidication may interact with many other changes in the marine environment, local and global;
these “multiple stressors” include temperature, nutrients, and oxygen.
In situ experiments on whole
communities (using natural CO vents or CO enrichment mesocosms) provide a good opportu-
nity to investigate impacts of multiple stressors on communities, to increase our understanding of
future impacts.

10
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
Synthesis
21. Ocean acidication represents a serious threat to marine biodiversity, yet many gaps remain in
our understanding of the complex processes involved and their societal consequences
Ocean acidication is currently occurring at a geologically unprecedented rate, subjecting marine
organisms to an additional, and worsening, environmental stress.
Experimental studies show the
variability of organisms’ responses to simulated future conditions: some are impacted negatively,
some positively, and others are apparently unaected.
Furthermore, responses to ocean acidication
can interact with other stressors and vary over time, with some potential for genetic adaptation.
is
complexity of natural processes makes it extremely challenging to assess how future ocean acidica-
tion will aect natural marine communities, food webs and ecosystems, and the goods and services
they provide.
Nevertheless, substantive environmental perturbations, increased extinction risk for
particularly vulnerable species, and signicant socio-economic consequences all seem highly likely.
Research priorities to reduce the uncertainties relating to future impacts include greater use of natu-
ral high-CO analogues, the geological record, and well-integrated observations, together with large-
scale, long-term and multi-factorial experimental studies.

1. BACKGROUND AND INTRODUCTION
11
Ocean acidication, oen referred to as the “other
CO problem”
[1]
, is a direct result of rising atmo-
spheric carbon dioxide (CO) concentrations due
to the burning of fossil fuels, deforestation, cement
production and other human activities. As atmo-
spheric CO increases, more enters the ocean across
the sea surface. is process has signicant societal
benets: by absorbing around a quarter of the total
human production of CO, the ocean has substan-
tively slowed climate change. But it also has less
desirable consequences, since the dissolved CO
aects seawater chemistry, with a succession of
potentially adverse impacts on marine biodiver-
sity, ecosystem services and human society.
e starting point for such changes is an increase in
seawater acidity, resulting from the release of hydro-
gen ions (H
+
). Acidity is measured on the logarith-
mic pH scale, with H
+
concentrations
*
at pH 7.0
being ten times greater than at pH 8.0. Since pre-
industrial times, the mean pH in the surface ocean
has dropped by 0.1 units, a linear-scale increase in
acidity of ~26%. Unless CO emissions are rapidly
curtailed, mean surface pH is projected – with a
high degree of certainty – to fall by a further ~0.3
units by 2100
[2-4]
, representing an acidity increase
of around 170% compared to pre-industrial levels.
e actual change will depend on future CO emis-
sions, with both regional and local variations in the
oceanic response (Chapter 3).
* pH is dened as the decimal logarithm of the reciprocal of hydrogen
ion activity in a solution. Dierent scales are possible, depending on
buer standards. For seawater, the “total scale” (pH
T
) is now preferred,
and most data given in this report can be assumed to be on that basis.
Very many scientic studies in the past decade have
unequivocally shown that a wide range of marine
organisms are sensitive to pH changes of such magni-
tude, aecting their physiology, tness and survival,
mostly (but not always) in a negative way
[4-6]
. e
consequences of ocean acidication for marine food
webs, ecosystems, biogeochemistry and the human
use of marine resources are, however, much less
certain.
In particular, ocean acidication is not the
only environmental change that organisms will expe-
rience in future, since it will occur in combination
with other stressors (e.g., increasing temperature and
deoxygenation)
[7]
.
e biological eects of multiple
stressors occurring together cannot be assumed to be
additive; instead, due to interactions, their combined
impacts may be amplied (through synergism) or
diminished (antagonism). Furthermore, there is
now evidence that some – but not necessarily all –
organisms may show genetically mediated, adap-
tive responses to ocean acidication
[8]
.
is review provides an updated synthesis of the
impacts of ocean acidication on marine biodiversity
based upon current literature, including emerging
research on the geological history of natural ocean
acidication events, and the projected societal costs
of future acidication. e report takes into consid-
eration comments and feedback submitted by Parties
to the Convention on Biological Diversity, other
Governments and organizations as well as experts
who kindly peer-reviewed the report.
1. BACKGROUND AND INTRODUCTION
KEY MESSAGES
1. Ocean acidication is a process caused by increasing levels of carbon dioxide in the atmosphere
and seawater, with potentially deleterious consequences for marine species and ecosystems
2. The acidity of the surface ocean has increased by ~26% since pre-industrial levels
3. The increased international attention given to ocean acidication, by the CBD and other bodies,
has catalysed research and helped identify knowledge gaps

12
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
1.1 MANDATE OF THIS REVIEW
e Conference of the Parties to the Convention on
Biological Diversity initially raised its concern on
the potential adverse impacts of ocean acidication
at its ninth meeting (COP 9; Bonn, 2008), which
instigated the CBD Secretariat’s rst review on this
topic “Scientic Synthesis of the Impacts of Ocean
Acidication on Marine Biodiversity” (Technical
Series No. 46)
[9]
, carried out jointly with the UNEP
World Conservation Monitoring Centre.
In response
to that review, COP 10 (Nagoya, 2010) recognized
ocean acidication as a new and important issue,
for consideration as an ongoing activity under the
programme of work on marine and coastal biodiver-
sity (decisions X/13 and X/29) and included ocean
acidication in the Strategic Plan for Biodiversity
2011-2020 and the Aichi Biodiversity Targets (Target
10; decision X/2).
In decision X/29, the Conference of the Parties to
the Convention on Biological Diversity established
a series of expert review processes, in collabora-
tion with various relevant organizations, to assess
the impacts of ocean acidication on marine biodi-
versity.
To initiate implementation of the request
in this decision, an Expert Meeting on Ocean
Acidication was convened by the CBD Secretariat,
in collaboration with the Intergovernmental
Oceanographic Commission of the United Nations
Educational, Scientic and Cultural Organization
(IOC-UNESCO), in Montreal in October 2011,
involving representatives from Parties and relevant
organizations.
e Expert Meeting identied gaps
and barriers in existing monitoring and assessment
of ocean acidication in the context of global policy
processes; developed options for addressing those
gaps and barriers; and considered the need for addi-
tional collaborative activities.
e workshop report
[10]
was considered at CBD COP 11 (Hyderabad, 2012),
when Parties decided that a new systematic review
should be prepared as the basis for further policy
action.
COP requested that the updated synthesis – this
document – provide “a targeted synthesis of the
biodiversity implications of ocean acidication for
marine and coastal systems, including information
on the less reported paleo-oceanographic research,
building upon the synthesis provided in CBD
Technical Report Series No 46” (XI/18, para-
graphs 22-24). is updated synthesis document
(UNEP/CBD/SBSTTA/18/INF/6) was considered
by the Subsidiary Body on Scientic, Technical and
Technological Advice, at its 18
th
meeting (Montreal,
23-28 June 2014), which requested the Executive
Secretary to transmit it to the Joint Liaison Group of
the three Rio Conventions and recommended that
the COP request the Executive Secretary to forward
it to Parties, other Governments and relevant orga-
nizations and to transmit it to the Secretariat of the
United Nations Framework Convention on Climate
Change.
In response to a request to Parties to assist in imple-
menting COP 11 decisions, the Government of the
United Kingdom of Great Britain and Northern
Ireland has provided the main nancial support for
preparing the updated synthesis, through the UK
Ocean Acidication research programme, co-funded
by the Natural Environment Research Council, the
Department for Environment, Food and Rural
Aairs, and the Department of Energy and Climate
Change.
e scientic authorship of this review is,
however, fully international, involving contributors
from 12 countries, many of whom also participated
in the 2011 Expert Meeting. In developing the review,
the authors considered “biodiversity implications”
to encompass impacts on marine ecosystems and
wider environmental considerations (i.e., consistent
with the relatively broad denition of biodiversity
in Article 2 of the CBD Convention)
[11]
, rather than
limiting the term to quantied measures of species
richness, heritable variation or habitat diversity.
e increasing international awareness of ocean acid-
ication and its societal implications was demon-
strated at the 14
th
meeting of the UN Open-ended
Informal Consultative Process (ICP) on Oceans and
Law of the Sea (New York, 17-20 June 2013)
[12]
.
At
this meeting, an early dra of this CBD review was
presented and discussed at a side event convened
by CBD Secretariat, in collaboration with the
IOC-UNESCO, and valuable feedback was received.
1. BACKGROUND AND INTRODUCTION
13
e United Nations General Assembly recognized
the attention given to ocean acidication by the
14
th
ICP meeting and committed itself to continue
to pay attention to this important issue, including
taking account of the ongoing work of the recently
established Ocean Acidification International
Coordination Centre of the International Atomic
Energy Agency (A/RES/68/70, para 156; also see
Box 2.1 and Table 2.1 below).
1.2 WHAT IS OCEAN ACIDIFICATION?
Ocean acidication can be dened in relatively
narrow terms, limiting its meaning to a global-
scale, long-term decrease in seawater pH, which
currently is primarily due to the human-driven
increase in atmospheric CO, which will almost
certainly intensify.
e CO-pH relationship has now
been observed at many locations, with the longest
atmospheric CO time series from the Mauna Loa
observatory (Hawaii) and a nearby oceanic time
series (Figure 1.1).
e above denition of ocean acidication focuses
on the reaction of dissolved anthropogenic CO
with water to form carbonic acid (HCO), which
dissociates to form bicarbonate ions (HCO
-
) and
hydrogen ions (H
+
, quantied by the pH scale).
An additional reaction with carbonate ions (CO
2-
;
naturally occurring in seawater) also occurs, reduc-
ing their concentration.
All these reactions are in
dynamic equilibrium (Figure 1.2).
As a result, the
process of ocean acidication can more generally
be considered as changes to the seawater “carbon-
ate system”.
Whilst pH values are of great interest,
it is not straightforward to measure them with high
precision.
Instead, they are oen calculated from
other measured parameters, such as dissolved carbon
dioxide (pCO), total dissolved inorganic carbon
(DIC) and total alkalinity (TA; the combined abun-
dance of proton-acceptors, i.e., negatively charged
ions that react with strong acid).
One further chemical reaction is noteworthy.
A
decline in the abundance of carbonate ions in seawa-
ter aects the stability of calcium carbonate (CaCO)
in solid form, which may be present as bedrock (such
as chalk or limestone), dead shells, or as an exterior
covering or structural component of living organ-
isms – such as molluscs (e.g., mussels, oysters and
sea-snails), echinoderms (e.g., sea urchins), crus-
taceans (e.g., crabs and lobsters), warm and cold-
water corals, and calcifying algae.
Such calcifying
organisms require more energy to produce CaCO
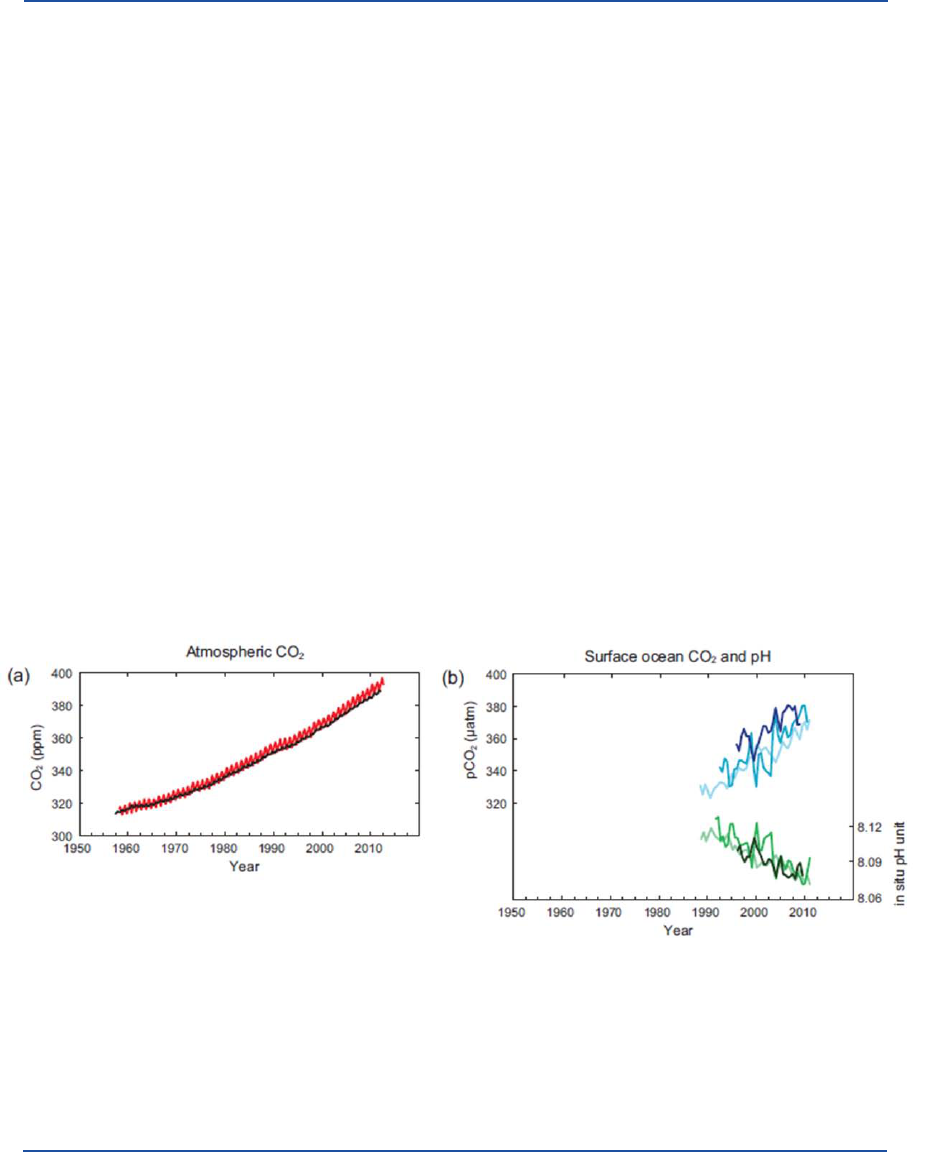
Figure 1.1. Multiple observed indicators of a changing global carbon cycle: (a) atmospheric concentrations of carbon dioxide (CO
2
) from Mauna
Loa (19°32’N, 155°34’W – red) and South Pole (89°59’S, 24°48’W – black) since 1958; (b) partial pressure of dissolved CO
2
at the ocean
surface (blue curves) and in situ pH (green curves), a measure of the acidity of ocean water. Measurements are from three stations from the
Atlantic (29°10’N, 15°30’W – dark blue/dark green; 31°40’N, 64°10’W – blue/green) and the Pacic Oceans (22°45’N, 158°00’W − light blue/
light green). Full details of the datasets shown here are provided in the underlying report of the Contribution of Working Group I to the Fifth
Assessment Report of the Intergovernmental Panel on Climate Change I and the Technical Summary Supplementary Material
[13]
.

14
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
in water with lower pH, but they may also experi-
ence shell dissolution, unless their exoskeletons and
carapaces are protected by an organic layer.
Whether or not such dissolution occurs is deter-
mined by the saturation state () of carbonate,
dened as the ratio between dissolved abundances
of calcium and carbonate ions and their solubility
product constants, the latter being temperature-
specic.
us values need to be greater than 1.0
for unprotected CaCO to be stable, and values
in the range 3.0 - 5.0 are generally considered opti-
mal for bio-calcication to occur.
Currently, the
vast majority of the surface ocean is supersaturated
with respect to CaCO, i.e.
>1.0.
However, most
of the deep ocean (below 1-2 km) is undersatu-
rated, with <1.0, owing to changes in temper-
ature and pressure (increasing solubility product
constants) and the accumulation of biologically
produced CO through decomposition (reducing
carbonate ion abundance).
e depth at which
= 1.0 is the saturation horizon, with most of the
deep ocean below that horizon and therefore corro-
sive to unprotected CaCO.
Some calcareous mate-
rial may be found below that depth if the rate of its
supply from the surface or mid-waters exceeds the
rate of its dissolution; however, it is very unlikely
to be preserved in the fossil record. e few shelled
organisms that survive below the saturation hori-
zon have well-protected shells and/or are limited to
niche habitats, such as hot vents
[17]
.
An additional complication is that there are two
main bio-mineral forms of CaCO, aragonite and
calcite, with the former being slightly more soluble.
us values for aragonite (and aragonite saturation
Figure 1.2.
The chemical reactions that occur when additional carbon dioxide dissolves in seawater, with net effect of increasing
the abundance of hydrogen ions and bicarbonate, whilst reducing carbonate.
Inset graph: Model-based global estimates of the
percentage changes in hydrogen ions, bicarbonate ions and carbonate ions as mean values in the upper ocean as a result of
increases in atmospheric CO
2
of up to 300% on a ~100 year timescale.
This model is relatively unsophisticated (e.g., not allowing
for temperature and circulation effects), and the results should be considered illustrative of the processes occurring in the main
part of this gure.
Images, redrawn from
[14]
and
[15]
; graph based on data in
[16]
.

1. BACKGROUND AND INTRODUCTION
15
horizons) dier slightly from those for calcite, and
the form of the mineral in dierent marine species
aects their sensitivity to ocean acidication.
Due to dierent water mass characteristics, the depth
of saturation horizons varies naturally between ocean
basins. However, it is currently decreasing every-
where, and will continue to do so, as more anthro-
pogenic CO enters the ocean.
By the end of this
century, aragonite saturation horizons are projected
to shallow from >2000 m to ~100 m in the North
Atlantic, from ~150 m to the near-surface in the
North Pacic
[18]
, and to reach the surface in the
Arctic and Southern Ocean
[19]
.
Due to lower temper-
atures in polar regions, the shallowing of satura-
tion horizons is more pronounced there, an eect
described in more detail in Chapter 3.
Aquatic organisms (particularly microbes) have
evolved to survive under a wide range of envi-
ronmental pH conditions, from alkaline lakes to
deep-sea vents.
us, extremophile algae, fungi and
archaea can tolerate pH values as low as 0.5, whilst
bacteria, protists and rotifers can survive at pH
values as high as 10.5
[[20]
.
Nevertheless, all species
have their individual optimal pH ranges and toler-
ance limits that usually closely match the range of
variability naturally encountered in species’ habitats.
As discussed in greater detail in Chapter 3, natural
seawater pH values can vary greatly over seasonal,
daily or annual timescales, and given this vari-
ability, it might be thought that the projected pH
reduction of ~0.3 units during the current century
is unlikely to have substantive biological conse-
quences, at least in coastal waters.
However, an anal-
ogous situation applies to temperature tolerances and
projected global warming. A global surface temper-
ature increase of ~2°C is now generally recognized
as having “dangerous” climatic and ecological conse-
quences, increasing extinction risk for many species
– despite very many organisms experiencing seasonal
(or even daily) temperature ranges that are ve to
ten times greater. It is key to note that it is not just
an absolute value of pH change that is important,
but also the change in potential range and variability.
Figure 1.3. Simplied summary of the impacts of ocean acidication on organisms, ecosystems, ecosystem services and hence
society. Impacts cascade through marine ecosystems, with societal effects including changes to food security, biodiversity, coastal
protection and climate regulation (see Table 5.2 for further detail). DMS, dimethylsulphide; DMSP, dimethylsulphoniopropionate; Ω,
CaCO
3
saturation state.
Based on
[15]
.

16
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
Other important framework considerations relating
to the eects of ocean acidication on biodiversity
include the following:
•
In the same way that global warming is not
limited to temperature change, ocean acidica-
tion is not limited to pH change. Organisms can
respond to changes in any one of the compo-
nents of the carbonate chemistry system (Figure
1.2), and calcication is not the only process that
may be aected. In particular, calcifying algae
demonstrate the potential for opposite responses
to dierent components: if there is sucient light
and nutrients, their photosynthesis (and growth
rates) may benet from higher CO
2
or bicarbon-
ate; however, their calcication may be nega-
tively impacted by decreased pH, occurring at
the same time. Note that decreased calcication
under conditions of ocean acidication is unlikely
to be directly due to the reduced availability of
carbonate, since most calciers take up bicarbon-
ate ions from seawater
[21]
.
•
Even within closely related taxa, not all organ-
isms respond similarly to ocean acidication
under experimental conditions, and dierent
stages in the life cycle may show dierent sensi-
tivities
[5,16]
. ese mixed responses (together with
the complexity of marine ecological interactions)
make it dicult to develop a quantitative, model-
based understanding of the impacts of projected
ocean acidication on communities, food webs,
ecosystems and the services they provide to soci-
ety (Figure 1.3). Nevertheless, recent meta-anal-
yses
[6, 22]
on individuals and taxa have identied
general trends, consistent taxonomic patterns
(Figure 1.4) and life-cycle eects, discussed in
detail in Chapter 5.
•
Ocean acidication has the potential to change
the chemical speciation and solubility of metals
and other elements in seawater. e pH sensitivity
of boron species is noteworthy, aecting the isoto-
pic composition of boron in biominerals, which
can be used in paleo-pH reconstructions (see
Chapter 4). Boron-borate changes can also aect
low-frequency sound transmission, with concerns
Coccolithophores
Diatoms
Fleshy algae
Calcifying algae
Echinoderms
Molluscs
Corals
Photosynthesis - 28%
Abundance
- 80%
Calcification - 32%
Abundance
- 47%
Survival
- 34%
Calcification - 40%
Growth - 17%
Development - 25%
Calcification - 23%
Development - 11%
Growth - 10%
Growth + 17%
Growth + 17%
Photosynthesis + 28%
Figure 1.4. Summary of the main effects of a decrease of 0.5 pH units on taxa
showing greatest sensitivity, based on metadata analysis from 228 experimental
studies. From
[6]

1. BACKGROUND AND INTRODUCTION
17
that future pH reductions would make the ocean
noisier, with biological impacts, for example, on
the behaviour of marine mammals
[23]
. However,
additional physically based analyses indicate that
the problem seems unlikely to be signicant
[24,25]
.
•
Marine organisms are currently subject to many
other environmental changes, in addition to ocean
acidication, with the potential to degrade or
disrupt ecosystems. Most of these drivers are
directly or indirectly due to human activities.
ey can be broadly grouped into local/regional
stressors, for example, due to over-shing, habitat
loss/destruction, pollution, and enhanced nutri-
ent loading (with associated eutrophication and
low oxygen), and global-scale climate-related
impacts that are mostly temperature-driven,
such as changes in stratification, mixing and
other circulation changes, reduced high latitude
surface salinity (due to ice melt and river run-
o), de-oxygenation and increased ultra-violet
(UV) radiation. Key issues relating to the three
main global-scale stressors – acidication, warm-
ing, and de-oxygenation – are summarized in
Table 1.1. Further information on our relatively
limited understanding of the interaction between
ocean acidication and other factors is provided
in subsequent chapters.
Table 1.1. Summary of the causes and impacts of the three main global-scale stressors that will increasingly affect marine
biodiversity, with severity of impacts depending on future emissions of greenhouse gases. Note that there may be reinforcing or
ameliorating interactions (synergies or antagonisms) for biological responses to these stressors, and that there are likely to be
additional interactions with a wide variety of other environmental parameters, at both global and local scales. Based on
[26]
; also see
[7]
.
Stressor Causes Results Direct effects Impacts, including climatic feedback
Acidication Increasing CO
2
in
atmosphere
Some local
contributions
(eutrophication,
industrial emissions)
Change in ocean
pH and carbonate
chemistry
Progressive dissolution
of calcium carbonate
Reduced calcication
and growth in many
species
Reef erosion
Changes in carbon:
nitrogen ratio
Reduced abundance of calcifying
species; other food web changes
Effects on aquaculture and human
food supply
Risk of coral extinctions, with habitat
loss and increased coastal erosion
Reduced ocean uptake of CO
2
Potential warming feedback via DMS
and cloud formation
Warming Increasing greenhouse
gases in atmosphere
Temperature increase
Less ocean mixing
due to increased
stratication
Loss of polar sea ice
More freshwater run-
off in polar regions
(reducing salinity)
Sea-level rise
Reduced solubility of
CO
2
, O
2
and calcium
carbonate
Reduced productivity
where more stratied;
increased productivity
in Arctic
Physiological effects
on organisms
(metabolism, growth
and survival)
Poleward shift of (mobile) species’
ranges
Coral bleaching
Changes in community composition
and food webs
Global reduction in marine productivity
Reduced ocean uptake of CO
2
Reduced carbon export to ocean
interior
De-oxygenation Warming reduces O
2
solubility
Stratication reduces
O
2
supply to ocean
interior
Local causes:
eutrophication
Reduced O
2
availability
for respiration,
especially in productive
regions and mid/deep
water
Slower metabolism
and growth of
zooplankton and sh
Effects on abundances and
distributions
Shift to organisms tolerant to low O
2
(mostly microbial)
Reduced shery yield
Increased marine production
of methane and nitrous oxide
(greenhouse gases)
All three
together
Increasing CO
2
and
other greenhouse
gases
Combined stress of
reduced pH, warming
and low dissolved O
2
Damage to organism
physiology and energy
balance
Disrupted food webs
Major changes to ocean physics,
chemistry and biology
Biodiversity loss, with impacts on
ecosystem services
Risk of multiple positive feedbacks,
increasing rate of future climate change

18
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
1.3 RE-VISITING KEY KNOWLEDGE GAPS IDENTIFIED IN THE PREVIOUS
CBD REVIEW
e concluding chapter (“Uncertainties and other
considerations”) of the 2009 CBD review of ocean
acidication
[9]
identied ve questions to assist in
focussing research eort on important knowledge
gaps.
Table 1.2 below briey revisits these issues,
summarizing relevant progress and the current status
of our understanding of these topic areas. Additional
detail, with supporting scientic citations, is given
in subsequent chapters of this review.
ree generic comments can be made on the 2009
research questions.
Firstly, all ve questions refer
to calcication or calciers, whereas there is now
greater appreciation that a much wider range of
physiological and biogeochemical processes, and
organisms, may be aected (Figures 1.3, 1.4) – and
a recognition that the scale and importance of many
of these additional impacts are still very uncertain.
Second, these questions only indirectly refer, through
adaptation (Q3), to the relevance of genetic and
evolutionary processes in determining the scale of
future acidication impacts. Such issues are now
being given much greater attention
[8]
. ird, none of
the questions explicitly mentions ecosystem services,
societal impacts or possible policy responses.
Whilst
research and understanding in these areas are not
yet well-developed, the current review does include
some consideration of the ‘human dimensions’ of
ocean acidication and its eects on biodiversity.
Table 1.2. Knowledge gaps identied in 2009
[9]
and subsequent relevant research developments.
Research question Summary of recent research progress; other comments
1. How is calcication
affected in organisms at
different stages of their life
cycle?
Signicant progress made on life-cycle experimental studies.
For many species of echinoderms, acidication
slows development of embryos/larvae (hence likely to increase mortality in eld); juveniles may also be
negatively affected, whilst adults are generally more tolerant.
Life-cycle changes in acidication sensitivity not
limited to calciers. Increased awareness that experimental life-cycle studies should be relevant to natural
conditions, with need for ‘realistic’ (yet well-controlled) pH/carbonate system parameters and controlled food
availability. Potential impacts and interactions of multiple stressors (e.g., temperature, nutrients/food, oxygen)
require further study.
2. Why do some calcifying
organisms seem to be less
affected than others?
Increased appreciation that variability of response can be due to: i) different organisms responding to different
aspects of carbonate chemistry (CO
2
, pH, carbonate, bicarbonate and saturation state); ii) non-standard
experimental methods (inter-comparability now much improved through “best practice” protocol development
and improved international liaison); iii) confounding effects of other, non-controlled factors (nutrient/food
availability; light for phytoplankton studies; seasonal cycles affecting physiology and metabolism); and iv)
inherent response variability between strains, species and higher taxonomic groups.
3.
How is adaptation and
survival inuenced by the
different mechanisms
of calcication or other
physiological factors?
This question covers many research topics, not only biological control of the calcication process (that differs
between different groups), but also the scope for genetic adaptation on decadal-to-century timescales. Scope
for adaptation – difcult to determine, but can be informed by paleo-studies – depends on reproductive
strategy, existing genotypic variability (on which selection can operate), and generation time. Such adaptation
may be at cost of reduced tness for other traits, and recent research documents the best approaches for
tackling this challenging issue
[8,27]
. Text on this question in the 2009 report focussed on potential impacts on
pteropods (planktonic molluscs, also known as sea butteries): several new experimental and eld studies on
this group have conrmed their vulnerability to near-future changes in polar water chemistry.
4.
How do other
environmental factors,
such as carbonate
concentration, light levels,
temperature and nutrients,
affect calcication
processes?
There is considerable overlap of this question with the others above, since it addresses the (multi-stressor)
context in which acidication occurs, inuencing not only calcication but other physiological processes.
In the
past 5 years, there have been many two-factor studies (mostly with temperature as second variable), providing
important insights on potential interactions.
However, very few experiments control three or more variables:
whilst such studies are needed, their design, implementation and interpretation are not straightforward.
Mesocosms and natural gradients provide alternative approaches to resolving issues of environmental
complexity.
5. How will communities with
a mixture of calcifying and
non-calcifying organisms
respond to decreasing
calcication rates, and
what impact will this have
on the marine food chain?
Determination of ecosystem-level effects is extremely demanding, and remains an overall goal – taking account
of other processes affected by acidication (Figure 1.3), in addition to calcication.
Model-based approaches
provide scenario-based projections, over a range of spatial and temporal scales, and these can be used for
risk-based policy action; however, models cannot be expected to give single answer, denitive predictions.
In particular, model outputs will necessarily depend on assumptions regarding future CO
2
emissions, as well
as the future scale and inuence of other environmental variables.
Furthermore, models are unable to take
account of factors (e.g., genetic adaptation) that have not yet been well-quantied.

1. BACKGROUND AND INTRODUCTION
19
References
1. Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009)
Ocean acidication: the other CO problem. Annual
Review of Marine Science 1: 169-192.
2. Sabine CL, Feely RA, Gruber N, Key RM, Lee K, et
al. (2004) e oceanic sink for anthropogenic CO.
Science 305: 367-371.
3. Feely RA, Sabine CL, Lee K, Berelson WM, Kleypas
JA, et al. (2004) Impact of anthropogenic CO on the
CaCO system in the oceans. Science 305: 362-366.
4. Gattuso JP, Hansson L (eds) (2011) Ocean
Acidication. Oxford: Oxford University Press, 326 p.
5. Wicks LC, Roberts JM (2012) Benthic invertebrates
in a high-CO world. Oceanography and Marine
Biology: An Annual Review 50: 127-188.
6. Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo
L, et al. (2013) Impacts of ocean acidication on
marine organisms: quantifying sensitivities and
interaction with warming. Global Change Biology 19:
1884-1896.
7. Bijma J, Portner HO, Yesson C, Rogers AD (2013)
Climate change and the oceans - What does the future
hold? Marine Pollution Bulletin 74: 495-505.
8. Sunday JM, Calosi P, Dupont S, Munday PL, Stillman
JH, et al. (2014) Evolution in an acidifying ocean.
Trends in Ecology & Evolution 29: 117-125.
9. Secretariat of the Convention on Biological Diversity.
(2009) Scientic Synthesis of the Impacts of Ocean
Acidication on Marine Biodiversity.
Montreal,. 61 p
10. Convention on Biological Diversity (2012) Report
of the expert meeting to develop a series of joint
expert review processes to monitor and assess
impacts of ocean acidication on marine and coastal
biodiversity. UNEP/CBD/SBSTTA/16/INF/14
11. Convention on Biological Diversity. Convention text
at http://www.cbd.int/convention/text/.
12. Report on the work of the UN Open-ended Informal
Consultative Process on Oceans and Law of the Sea at
its 14th meeting. Letter dated 17 July 2013 from the
Co-Chairs of the Consultative Process addressed to
the President of the General Assembly. http://www.
un.org/Depts/los/consultative_process/consultative_
process.htm; see also http://www.un.org/Depts/los/
general_assembly/general_assembly_reports.htm
13. Intergovernmental Panel on Climate Change (2013)
Summary for Policymakers. In: Climate Change 2013:
e Physical Science Basis. Contribution of Working
Group I to the Fih Assessment Report of the IPCC
(Stocker TF, Qin D, Plattner G-K, Tognor M, Allen
SK, Boschung J, Nauels A, Xia Y, Bex V & Midgley
PM (eds). Cambridge University Press, Cambridge
UK and New York, USA.
14. Ocean Acidication Reference User Group (2010)
Ocean Acidication: Questions Answered. Laoley
D d’A & Baxter JM (eds): European Project on Ocean
Acidication (EPOCA), 24p.
15. Williamson P, Turley C (2012) Ocean acidication in
a geoengineering context. Philosophical Transactions
of the Royal Society A, Mathematical Physical and
Engineering Sciences 370: 4317-4342.
16. e Royal Society (2005) Ocean acidication due
to increasing atmospheric carbon dioxide. London:
Royal Society
17. Tunniclie V, Davies KTA, Buttereld DA, Embley
RW, Rose JM, et al. (2009) Survival of mussels in
extremely acidic waters on a submarine volcano.
Nature Geoscience 2: 344-348.
18. Guinotte JM, Orr JC, Cairns S, Freiwald A, Morgan
L, et al. (2006) Will human-induced changes in
seawater chemistry alter the distribution of deep-
sea scleractinian corals? Frontiers in Ecology and the
Environment 4: 141-146.
19. Feely RA, Sabine CL, Byrne RH, Millero FJ, Dickson
AG, et al. (2012) Decadal changes in the aragonite
and calcite saturation state of the Pacic Ocean.
Global Biogeochemical Cycles 26: GB3001.
20. Rothschild LJ, Mancinelli RL (2001) Life in extreme
environments. Nature 409: 1092-1101.
21. Roleda MY, Boyd PW, Hurd CL (2012) Before ocean
acidication: calcier chemistry lessons. Journal of
Phycology 48: 840-843.
22. Wittmann AC, Portner H-O (2013) Sensitivities of
extant animal taxa to ocean acidication. Nature
Climate Change 3: 995-1001.
23. Ilyina T, Zeebe RE, Brewer PG (2010) Future
ocean increasingly transparent to low-frequency
sound owing to carbon dioxide emissions. Nature
Geoscience 3: 18-22.
24. Reeder DB, Chiu CS (2010) Ocean acidication
and its impact on ocean noise: Phenomenology and
analysis. Journal of the Acoustical Society of America
128: El137-El143.
25. Joseph JE, Chiu CS (2010) A computational
assessment of the sensitivity of ambient noise level to
ocean acidication. Journal of the Acoustical Society
of America 128: El144-El149.
26. Turley CM, Keizer T, Williamson P, Gattuso JP, Ziveri
P, et al.(2013) Hot, Sour and Breathless – Ocean
under Stress. Plymouth Marine Laboratory, UK
Ocean Acidication Research Programme, European
Project on Ocean Acidication, Mediterranean Sea
Acidication in a Changing Climate project, Scripps
Institution of Oceanography at UC San Diego,
OCEANA. 6 p
27. Reusch TB, Boyd PW (2013) Experimental evolution
meets marine phytoplankton. Evolution 67:
1849-1859.

20
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
2.1 STEPS TOWARD GLOBAL RECOGNITION AND INTERNATIONAL
SCIENTIFIC COLLABORATION
Ocean acidication is a relatively young eld of
research. e rst results from laboratory experi-
ments on the eects on marine organisms appeared
in the late 1990s. ese built upon early landmark
studies showing that the uptake of anthropogenic
CO decreased ocean buering capacity
[1]
, and that
this could decrease calcication
[2,3]
by marine organ-
isms. Scientic interest in ocean acidication – not
only by chemists and physiologists, but also by ecol-
ogists, biogeochemists, paleontologists and econo-
mists – has increased exponentially in the past few
years, with a more than twenty-fold increase in the
number of publications from 2004 to 2013, and
a similar increase in numbers of new researchers
entering the eld (Figure 2.1)
[4,5]
.
The prioritization of ocean acidification as a
research topic began around 2003-04, with its
inclusion in the Science Plans of two global-change
research programmes, the Surface Ocean Lower
Atmosphere Study (SOLAS)
[6]
and the Integrated
Marine Biogeochemistry and Ecosystem Research
project (IMBER)
[7]
. In a closely related initiative,
the rst symposium “e Ocean in a High CO
World”, was held in Paris in 2004, convened by the
Scientic Committee on Oceanic Research (SCOR),
the Intergovernmental Oceanographic Commission
of the United Nations Educational, Scientic and
Cultural Organization (IOC-UNESCO) and the
International Geosphere-Biosphere Programme
(IGBP).
2. SCIENTIFIC AND POLICY FRAMEWORK
KEY MESSAGES
1. Research interest in and political awareness of ocean acidication have increased exponentially in
the past few years
2. International cooperation and interdisciplinary research have helped to advance the science of
ocean acidication
3. Many intergovernmental bodies have initiated activities on ocean acidication
Figure 2.1. The annual number of
peer-reviewed publications on ocean
acidication and the number of authors
involved 1900-2013. Data from the
bibliographic database of the IAEA Ocean
Acidication International Coordination
Centre (OA-ICC), updated from Gattuso
and Hansson
[4,5]

2. SCIENTIFIC AND POLICY FRAMEWORK
21
However, wider awareness of ocean acidication
remained extremely low until the Royal Society’s
2005 report “Ocean acidication due to increasing
atmospheric carbon dioxide”
[8]
.
Several other policy-
related publications have subsequently attracted
signicant attention, including:
•
e 2008 Monaco Declaration
[9]
, signed by 155
scientists from 26 countries and endorsed by HSH
Prince Albert II of Monaco. e declaration called
upon policymakers to support initiatives in multi-
disciplinary research, communication and policy
action. It arose from the Second Symposium on
the Ocean in a High-CO World, held in Monaco
and co-organised by the International Atomic
Energy Agency (IAEA).
•
e CBD’s 2009 report “Scientic Synthesis of
the Impacts of Ocean Acidication on Marine
Biodiversity”
[10]
, produced jointly with the World
Conservation Monitoring Centre of the United
Nations Environment Programme (UNEP).
Aspects of that report have already been discussed
in Chapter 1.
•
e 2009 statement on ocean acidication by
the InterAcademy Panel on International Issues
(IAP)
[11]
, endorsed by over 100 academies of
science worldwide.
is called on world leaders
to respond to the emerging threat of ocean acid-
ication by taking action to reduce CO emis-
sions and mitigate damage to marine ecosystems.
•
“Ocean Acidification Summary for Policy-
makers”
[34]
arising from the ird Symposium
on the Ocean in a High-CO World, held in
Monterey, USA in 2012.
•
e 2013 assessment of Arctic Ocean acidication
and its societal implications, carried out by the
Arctic Monitoring and Assessment Programme
(AMAP)
[12]
.
The first large-scale, multi-national project on
ocean acidication was the European Commission’s
“European Project on Ocean Acidification”
(EPOCA
[13]
, 2008-2012.
EPOCA brought together
more than 160 scientists from 32 countries to
address scientic uncertainties on ocean acidica-
tion, including biogeochemical modelling, biological
eects and implications for marine biodiversity.
A
notable output was publication of the book Ocean
Acidication
[5]
in 2011 (Figure 2.2). A second EC
project on ocean acidication has focused on its links
to climate change in the Mediterranean (MedSeA)
[14]
,
2011-2014.
National research eorts, many with close linkages
to international programmes, have included the
German programme Biological Impacts of Ocean
Acidication (BIOACID)
[15]
, that started in 2009,
and is now concluding its second funding phase; US
research support (via NSF and NOAA), mandated
by the 2009 Federal Ocean Acidication Research
and Monitoring (FOARAM) Act
[16]
; the UK Ocean
Acidication Research Programme (UKOA)
[17]
that
began in 2010; and other programmes and, proj-
ects in Australia, China, Japan, Republic of Korea,
Norway and elsewhere. The current breadth of
national involvement in ocean acidication research
is indicated in Figure 2.3.
Linkages between these worldwide research eorts
on ocean acidication have been encouraged at
Figure 2.2. The rst book on ocean acidication, with
international authorship and arising from the European
Commission’s EPOCA project.

22
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
the intergovernmental level (see 2.2 below), as
well as by national funders and non-governmen-
tal science bodies, particularly the SOLAS-IMBER
Ocean Acidication Working Group (SIOA-WG)
[18]
,
which helped to establish the Ocean Acidication
International Coordination Centre (OA-ICC)
[19]
of
the IAEA, based in Monaco.
e OA-ICC became operational in 2012, supported
by IAEA member states; its activities include the
facilitation of global observation and monitoring;
joint-use research platforms and experiments; de-
nition of best practices; data management; capacity
building; dissemination and outreach. OA-ICC liai-
son with policy-makers, the private sector and other
stakeholders is assisted by the Ocean Acidication
international Reference User Group (OA-iRUG).
is body, re-constituted in 2013, was originally
established in 2008 through the EPOCA project; it
involves scientists and research users from indus-
try, government and non-governmental organiza-
tions. OA-iRUG publications
[20]
aim to provide key
policy-relevant messages on ocean acidication to
decision makers.
The most recent initiative to enhance interna-
tional science collaboration has been the develop-
ment of the Global Ocean Acidication Observing
Network (GOA-ON), supported by the OA-ICC,
IOC-UNESCO, the Global Ocean Observing
System (GOOS), the International Ocean Carbon
Coordination Project (IOCCP) and national fund-
ing agencies. Further details are given in section 3.3.
2.2 INTERGOVERNMENTAL INTEREST IN OCEAN ACIDIFICATION AND
ACTIONS TO DATE
Whilst some local and national policy measures
can be taken to address ocean acidication impacts
(e.g., formation of a Marine Resources Advisory
Council by the State of Washington, USA, charged
with safeguarding its shellsh industry against ocean
acidication
[21]
), ocean acidication is essentially
a global problem – requiring a global, intergov-
ernmental policy response.
At the United Nations
Conference on Sustainable Development “Rio+20”
(Rio de Janeiro, June 2012) all stakeholders, includ-
ing UN bodies, intergovernmental organizations
and national governments, were invited to make
commitments to deliver concrete results for sustain-
able development on a voluntary basis. ere was
substantial stakeholder input to the consideration of
ocean acidication resulting in a specic ocean acid-
ication statement (number 166) in the Conference’s
outcome document “e Future We Want”
[22]
.
“We call for support to initiatives that address
ocean acidication and the impacts of climate
change on marine and coastal ecosystems
and resources. In this regard, we reiterate the
need to work collectively to prevent further
ocean acidication, as well as to enhance
the resilience of marine ecosystems and of
the communities whose livelihoods depend
on them, and to support marine scientic
research, monitoring and observation of ocean
acidication and particularly vulnerable
ecosystems, including through enhanced
international cooperation in this regard.”
One of the main outcomes of the Rio+20 Conference
was the agreement by member States to launch a
transparent intergovernmental process to develop a
set of Sustainable Development Goals (SDGs) to be
Figure 2.3. National involvement in ocean acidication research,
based on rst authors’ addresses for peer-reviewed papers
published in 2005-2013 (OA-ICC data)
4
.

2. SCIENTIFIC AND POLICY FRAMEWORK
23
agreed by the General Assembly at its 68th session
(2013 – 2014). e progress report
[23]
of the Open
Working Group of the UN General Assembly tasked
with the development of the SDGs includes mention
of ocean acidication.
Box 2.1 provides relevant text from the 68
th
session
of the UN General Assembly, which recognized
ocean acidication as an issue of concern.
ere
have also been actions by several other intergovern-
mental bodies and organizations, mostly within the
UN system, to inform policy makers and support
policy development
[24]
as summarized in Table 2.1.
Note that no single UN body currently has a desig-
nated lead role for policy development regarding
ocean acidication, and there is ongoing debate
[25,26]
on this issue, particularly with regard to the link-
age to the regulatory framework for CO emission
reductions. CBD’s major role in raising awareness
of ocean acidication and other association actions
has already been covered in Chapter 1 and is only
briey re-presented in Table 2.1.
Box 2.1
Extracts from Resolution 68/70, (http://www.un.org/en/ga/search/view_doc.asp?symbol=A/RES/68/70), of the United Nations General
Assembly (passed on 9 December 2013) giving specic mention to ocean acidication. The General Assembly is the main deliberative,
policy-making and representative organ of the UN.
Paragraph 17
Called upon States and international nancial institutions, including
through bilateral, regional and global cooperation programmes and
technical partnerships, to develop capacity-building activities in and
to transfer to developing countries, in particular least developed
countries and small island developing States, on mutually
agreed terms, and taking into account the Intergovernmental
Oceanographic Commission Criteria and Guidelines on the Transfer
of Marine Technology, environmentally sound technologies to study
and minimize the impacts of ocean acidication
Paragraph 153
Noted the work of the Intergovernmental Panel on Climate Change,
including its recent ndings on the acidication of oceans, and
encouraged States and competent international organizations
and other relevant institutions, individually and in cooperation, to
urgently pursue further research on ocean acidication, especially
programmes of observation and measurement, noting in particular
the continued work of the Convention on Biological Diversity and
paragraph 23 of decision XI/18 adopted at the eleventh meeting
of the Conference of the Parties to the Convention on Biological
Diversity, and to increase national, regional and global efforts to
address levels of ocean acidity and the negative impact of such
acidity on vulnerable marine ecosystems, particularly coral reefs
Paragraph 154
Recalled that, in “The future we want”, States called for support
for initiatives that address ocean acidication and the impacts of
climate change on marine and coastal ecosystems and resources
and, in this regard, reiterated the need to work collectively to
prevent further ocean acidication, as well as to enhance the
resilience of marine ecosystems and of the communities whose
livelihoods depend on them, and to support marine scientic
research, monitoring and observation of ocean acidication and
particularly vulnerable ecosystems, including through enhanced
international cooperation in this regard
Paragraph 155
Noted with concern the approximately 30 per cent increase in
the acidity of ocean surface waters since the beginning of the
industrial era and the wide range of impacts associated with the
continuing and alarming acidication of the world’s oceans, and
urged States to make signicant efforts to tackle the causes of
ocean acidication and to further study and minimize its impacts,
to enhance local, national, regional and global cooperation in this
regard, including the sharing of relevant information, and to take
steps to make marine ecosystems more resilient to the impacts of
ocean acidication
Paragraph 156
Committed itself to continue to pay attention to this important
issue, including by taking into account the rst global integrated
assessment and the ongoing work of the recently established Ocean
Acidication International Coordination Centre of the International
Atomic Energy Agency
Paragraph 217
Recalled that, in “The future we want”, States recognized the
signicant economic, social and environmental contributions of
coral reefs, in particular to islands and other coastal States, as
well as the signicant vulnerability of coral reefs and mangroves
to impacts, including from climate change, ocean acidication,
overshing, destructive shing practices and pollution, and support
international cooperation with a view to conserving coral reef and
mangrove ecosystems and realizing their social, economic and
environmental benets, as well as facilitating technical collaboration
and voluntary information-sharing
Paragraph 218
Encouraged States and relevant international institutions to
improve efforts to address coral bleaching by, inter alia, improving
monitoring to project and identify bleaching events, supporting
and strengthening action taken during such events and improving
strategies

24
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
Table 2.1. Summary of activities of United Nations subsidiary bodies, Conventions and other intergovernmental organizations in
relation to ocean acidication, based on
[24]
.
This list does not claim to be comprehensive.
*Body with government membership but
not part of the United Nations.
Body, subsidiary body/agency or Convention; activities
United Nations Convention
on the Law of the Sea
(UNCLOS)
Treaty that sets out the legal framework within which all
activities in the oceans and seas must be carried out
Part XII of UNCLOS addresses the protection and
preservation of the marine environment
Part XIII of UNCLOS addresses marine scientic
research
United Nations Division for
Ocean Affairs and the Law of
the Sea (DOALOS)
Open-ended Informal
Consultative Process (ICP)
on Oceans and the Law of
the Sea
Forum to facilitate the
annual review by the
General Assembly of
developments in ocean
affairs and the law of
the sea
14th ICP meeting (June 2013) on Impacts of Ocean
Acidication on the Marine Environment; report to
2013 UN General Assembly
[27]
United Nations Environment
Programme
(UNEP)
Coordination of UN environmental activities 2010 publication: “Environmental Consequences of
Ocean Acidication: a Threat to Food Security”
[29]
Co-support of 3rd UN Conference on Sustainable
Development
Lead for Transboundary Waters Assessment Programme
(with IOC-UNESCO and others) that includes
assessment of ocean acidication
United Nations Educational,
Scientic and Cultural
Organization
(UNESCO)
Intergovernmental
Oceanographic
Commission
(IOC)
The UN body for ocean
science, observatories,
data, information
exchange and services
Coordination of OA-relevant chemical and biological
measurements through the Global Ocean Observing
System (GOOS) and the International Ocean Carbon
Coordination Project (IOCCP; co-supported by SCOR);
support for the Global Ocean Acidication Observing
Network (GOA-ON)
Lead for “A Blueprint for Ocean and Coastal
Sustainability”
[28]
(with IMO, FAO and UNDP), including
actions to mitigate and adapt to ocean acidication
Major role in developing the Ocean in a High-CO
2
World symposium series and the associated Ocean
Acidication Summaries for Policymakers (2009 and
2013)
World Meteorological
Organization
(WMO)
Intergovernmental Panel
on Climate Change
(IPCC; created with UNEP,
advises UNFCCC)
Assessments of climate
change and associated
impacts
Ocean acidication included in IPCC 4th Assessment
Report, and in greater detail in 5th Assessment
Report (AR5, Working Groups I, II and III)
[29,30]
. High
condence given to pH decrease of 0.1 in ocean
surface water since the beginning of the industrial era.
International Maritime
Organization
(IMO)
London Convention and
Protocol
Control of marine
pollution through
regulation of waste
disposal
Control of sub sea-bed CO
2
sequestration
Development of regulatory framework (within the
scope of the Convention and Protocol) for research on
ocean fertilization and other marine geoengineering
relevant to ocean acidication
International Atomic Energy
Agency
(IAEA)
Encourage peaceful uses and applications of nuclear
technology
Hosting of Ocean Acidication International
Coordination Centre (OA-ICC) to assist the worldwide
scientic study of ocean acidication
Convening of two workshops (in 2010 and 2012) on
socio-economics of ocean acidication
Development, through GOA-ON, of a global network to
measure changes in ocean carbon chemistry and its
ecological impacts
Improving ocean acidication data management;
capacity building, dissemination and outreach.
United Nations Framework
Convention on Climate
Change
(UNFCCC)
Legal framework for global reduction in CO
2
emissions,
in order to prevent “dangerous anthropogenic
interference with the climate system”.
Limited mention of ocean acidication in UNFCCC
decisions and documents, but discussed as an
“emerging issue” by Subsidiary Body for Scientic and
Technical Advice and by associated research dialogue.
Ocean acidication covered by side-events at UNFCCC
Conference of Parties since 2009

2. SCIENTIFIC AND POLICY FRAMEWORK
25
Body, subsidiary body/agency or Convention; activities
Convention on Biological
Diversity
(CBD)
International treaty to promote conservation and
sustainable use of biological diversity
Concern about ocean acidication raised at the ninth
meeting of the Conference of Parties to the CBD
(COP 9) in 2008
2009 review (with the World Conservation Monitoring
Centre of the United Nations Environment Programme,
UNEP–WCMC)
[10]
At COP 10 (2010), ocean acidication included in CBD
Strategic Plan for Biodiversity (2011-2020) and Aichi
Biodiversity Targets
Expert review process for ocean acidication initiated
by Expert Meeting in 2012 and new review.
Convention for Protection of
the Marine Environment of
the North-East Atlantic*
(OSPAR)
Combines and updates Oslo and Paris Conventions,
covering “all human activities that might adversely
affect the marine environment of the North East
Atlantic”
Concern on ocean acidication expressed in 2012,
resulting in establishment (with ICES) of Study Group
on ocean acidication; reports published in 2013 and
2014
Development of protocols for ocean acidication
monitoring and assessment
[31]
Commission for the
Conservation of Antarctic
Marine Living Resources*
(CCAMLR)
Conservation of Antarctic marine life Expressed concern on potential impacts of ocean
acidication on Antarctic marine life, including effects
on krill
[32]
Joint Group of Experts on the
Scientic Aspects of Marine
Environmental Protection
(GESAMP)
Sponsored by IMO, FAO,
IOC-UNESCO, WMO, IAEA,
UNEP, UNIDO and UNDP
Advises the UN system
on scientic aspects of
marine environmental
protection
CO
2
considered as pollutant; ocean acidication
included in GESAMP contribution to open ocean part of
Transboundary Waters Assessment Programme
Arctic Council* Arctic Monitoring and
Assessment Programme
(AMAP)
Provision of information
on status of Arctic
environment
AMAP Arctic Ocean Acidication Assessment (2013)
[12]
International Union for
Conservation of Nature*
(IUCN)
Aim is to conserve
biodiversity at global and
local level
Work with IOC-UNESCO, Ocean Acidication
international Reference User Group and others to raise
awareness of ocean acidication.
References
1. Revelle R, Suess HE (1957) Carbon dioxide exchange
between atmosphere and ocean and the question
of an increase of atmospheric CO during the past
decades. Tellus 9: 18-27.
2. Broecker WS, Takahash T (1966) Calcium carbonate
precipitation on Bahama Banks. Journal of
Geophysical Research 71: 1575-1602.
3. Smith SV, Pesret F (1974) Processes of carbon dioxide
ux in Fanning-Island lagoon. Pacic Science 28:
225-245.
4. Data from the bibliographic database of the Ocean
Acidication International Coordination Centre
(OA-ICC), updated from ref 5; http://www.iaea.org/
ocean-acidication/page.php?page=2196
5. Gattuso JP, Hansson L (eds) (2011) Ocean
Acidication. Oxford: Oxford University Press. 326 p.
6. IGBP Secretariat. SOLAS (2004) Surface Ocean Lower
Atmosphere Study: Science Plan and Implementation
Strategy. IGBP Report 50. Stockholm
7. IGBP Secretariat. IMBER (2005) Integrated Marine
Biogeochemistry and Ecosystem Study: Science
Plan and Implementation Strategy. IGBP Report 52.
Stockholm. 76 p.
8. e Royal Society. (2005) Ocean acidication due
to increasing atmospheric carbon dioxide. London:
Royal Society
9. Monaco Declaration (2009). Second Symposium on
e Ocean in a High CO World 4 p. Monaco: Prince
Albert II of Monaco Foundation. http://ioc-unesco.
org/index.php?option=com_content&task=view&id=
99&Itemid=112
10. Secretariat of the Convention on Biological Diversity
(2009) Scientic synthesis on the impacts of ocean
acidication on marine biodiversity. Montreal. 61 p.
11. Inter Academy Panel (2009) Statement on
ocean acidication.
http://www.interacademies.
net/10878/13951.aspx

26
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
12. Arctic Monitoring and Assessment Programme
(2013).
AMAP Assessment 2013: Arctic Ocean
Acidication. AMAP, Oslo, Norway. 99p http://www.
amap.no/documents/doc/AMAP-Assessment-2013-
Arctic-Ocean-Acidication/881
13. European Project on Ocean Acidication (EPOCA)
http://www.epoca-project.eu
14. Mediterranean Sea Acidication in a Changing
Climate (MedSeA) http://www.medsea-project.eu
15. Biological Impacts of Ocean Acidication (BIOACID)
http://www.bioacid.de
16. Federal Ocean Acidication Research and Monitoring
(FOARAM) Act http://oceanacidication.noaa.gov/
AboutUs/FOARAMAct.aspx
17. UK Ocean Acidication research programme
(UKOA) http://www.oceanacidication.org.uk
18. Integrated Marine Biogeochemistry and Ecosystem
Research (IMBER) http://www.imber.info/index.php/
Science/Working-Groups/SOLAS-IMBER-Carbon/
Subgroup-3
19. Ocean Acidication International Coordination
Centre (OA-ICC) of the InternationalAtomic
Energy Agency (IAEA) http://www.iaea.org/ocean-
acidication/page.php?page=2181
20. Laoley D d’A, Baxter JM (eds) (2012) (2012)
Ocean acidication: the knowledge base. Updating
what we know about ocean acidication and key
global challenges. European Project on Ocean
Acidication (EPOCA), UK Ocean Acidication
Research Programme (UKOA), Biological Impacts
of Ocean Acidication (BIOACID), Mediterranean
Sea Acidication in a Changing Climate (MedSeA).
8 p. http://www.iaea.org/ocean-acidication/page.
php?page=2198.
21. Feely RA, Sabine CL, Byrne RH, Millero FJ, Dickson
AG, et al. (2012) Decadal changes in the aragonite
and calcite saturation state of the Pacic Ocean.
Global Biogeochemical Cycles 26: GB3001.
22. http://www.uncsd2012.org/thefuturewewant.html
23. http://sustainabledevelopment.un.org/content/
documents/3238summaryallowg.pdf
24. Herr D, Isensee K, Harrould-Kolieb,Turley C
(2014) Ocean acidication: International policy
and governance options. IUCN Gland, Switzerland;
www.iucn.org/about/work/programmes/
marine/?16064/ocean-acidication-policy-guidance
25. Harrould-Kolieb ER, Herr D (2012) Ocean
acidication and climate change: synergies and
challenges of addressing both under the UNFCCC.
Climate Policy 12: 378-389.
26. Kim RE (2012) Is a new multilateral environmental
agreement on ocean acidication necessary?
Review of European Community and International
Environmental Law 21: 243-258.
27. http://www.un.org/depts/los/consultative_process.htm
28. IOC/UNESCO, IMO, FAO, UNDP (2011) A blueprint
for ocean and coastal sustainability.
Paris.http://www.
uncsd2012.org/index.php?page=view&type=510&nr=
180&menu=20
29. Field CB, Barros V, Stocker TF, Qin D, Mach KJ, et al.
(2011) Workshop Report of the Intergovernmental
Panel on Climate Change Workshop on Impacts
of Ocean Acidication on Marine Biology and
Ecosystems. California, USA: Stanford
30. IPCC (2013). Climate Change 2013: e Physical
Science Basis. Working Group I Contribution to the
Fih Assessment Report of the Intergovernmental
Panel on Climate Change. http: www.ipcc.ch
31. Hydes DJ, McGovern E, Walsham P (2013) Chemical
aspects of ocean acidication monitoring in the ICES
marine area. ICES Cooperative Research Report 319:
78 p.
32.
Kawaguchi S, Berli T, King R, Nicol S, Virtue P,
Ishimatsu A (2012) Impacts of ocean acidication on
Antarctic krill biology: preliminary results and future
research directions. CCAMLR document WG-EMM-
12/32; https://www.ccamlr.org/en/wg-emm-12/32
33. Resolution adopted by 66
th
session of UN General
Assembly 27 July 2012.
Paper A/RES/66/288*;
http://www.un.org/ga/search/view_doc.
asp?symbol=A/RES/66/288&Lang=E
34. IGBP, IOC, SCOR (2013). Ocean Acidication
Summary for Policymakers 2013. International
Geosphere-Biosphere Programme. Stockholm,
Sweden.

3. GLOBAL STATUS AND FUTURE TRENDS OF OCEAN ACIDIFICATION
27
3.1 VARIABILITY
Values for pH and other components of the marine
carbon system not only show local and regional natu-
ral spatial variability, but can also change temporally,
on a diurnal to seasonal basis.
Recognition of such
variability, and an understanding of its causes, are
crucial to the valid interpretation of observational
studies and the assessment of anthropogenic ocean
acidication trends
[1]
.
The scale of temporal variability can be habitat-
specic
[2]
(Figure 3.1), whilst strong spatial variabil-
ity can occur both horizontally and vertically in shelf
seas (Figure 3.2).
It is therefore potentially simpler to
detect an ocean acidication signal in the open ocean
than in more variable coastal systems
[1,3-5]
. At many
coastal ocean sites, short-term natural variability expe-
rienced by benthic organisms has a greater range than
the projected pH decline over the next century due
to anthropogenic CO emissions
[3,6]
. For example, the
natural daily variability in pH experienced by warm-
water corals can range between pH 7.8 and 8.3
[7]
.
e following physical, geochemical and biologi-
cal factors may contribute to natural pH variabil-
ity, particularly in shelf and coastal seas:
•
A range of mesoscale hydrodynamic features,
including wind-driven upwelling, which brings
low-pH water to the surface
[8]
; tidal down-well-
ing
[9]
; seasonal sea-ice, aecting CO drawdown;
and localised temperature gradients (due to fron-
tal features and stratication) that directly aect
CO solubility, hence pH and other ocean acidi-
cation parameters.
•
e biological processes of photosynthesis and
respiration/decomposition respectively take up
and release CO; both processes vary with depth
and are generally of greater magnitude and vari-
ability in shallow seas than in the open ocean.
Changes affecting pH occur over day-night
cycles and seasonally
[5,10]
; as well as locally, due
to variable nutrient supply and biological inter-
actions that may promote patchiness of plank-
tonic communities.
•
Land and seaoor boundary conditions, and
riverine inuences, can dier markedly over rela-
tively short distances; all can provide distinct,
geologically derived carbon signatures. River
nutrient inputs, aected by land use and sewage-
derived pollution, can also serve to enhance
biological production (eutrophication). At some
coastal sites (and also at tectonically active deep-
sea locations), vents of CO or methane can cause
dramatic localized pH reductions.
•
Atmospheric inputs of nitrogen and sulphur
compounds produced by the burning of fossil
fuels and by agriculture may also inuence pH
and carbon chemistry close to source regions
[11]
Such dynamic “background” conditions could mean
that organisms from coastal waters and shelf seas
are less susceptible to future ocean acidication
than those from the open ocean, as the former may
already be adapted to tolerate low pH. But it could
also mean that shallow-sea organisms might be
exposed to harmful pH thresholds more quickly. In
3. GLOBAL STATUS AND FUTURE TRENDS OF OCEAN
ACIDIFICATION
KEY MESSAGES
1. Substantial natural temporal and spatial variability occurs in seawater pH, particularly in coastal
waters, due to physical, geochemical and biological processes
2. Polar oceans are expected to experience the impacts of ocean acidication sooner than temperate
or tropical regions, as their saturation horizons are already shallower than at lower latitudes.

28
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
Box 3.1 IPCC scenarios for future CO2 emissions
1.
Main scenarios used by the Intergovernmental Panel on Climate Change (IPCC) for its 4
th
Assess-
ment Report (AR4), (IPCC, 2007).
ese illustrative “families” of pathways were developed in the IPCC
Special Report on Emission Scenarios (SRES)
[
(IPCC, 2000), and are referred to in many ocean acidication
modelling papers published pre-2012.
A1 – Integrated world, rapid economic growth, limited population growth.
ree versions: A1FI (fossil fuel
intensive), A1B (balanced) and A1T (non-fossil energy sources).
A2 – Divided world, regional economic growth and continuous population growth (highest emissions in 2100).
B1 – Integrated world, rapid economic growth, limited population growth with global movement towards eco-
nomic, social and environmental stability (lowest emissions in 2100).
B2 – Divided world, regional economic growth, continuous population growth with regional movement to-
wards economic, social and environmental stability.
2.
Main scenarios used by IPCC for its 5
th
Assessment Report (AR5)
[
(IPCC, 2013), as “Representative
Concentration Pathways” (RCPs)
[26]
.
RCP 2.6 – lowest emissions, atmospheric CO peaks at ~443 ppm in 2050 before declining to ~421 ppm by
2100.
Assumes unspecied “negative emissions” i.e., active CO removal from the atmosphere.
RCP 4.5 – low emissions; atmospheric CO concentrations reach ~538 ppm by 2100.
RCP 6.0 – moderate emissions; atmospheric CO concentrations reach ~670 ppm by 2100.
RCP 8.5 – high emissions; atmospheric CO concentrations reach ~936 ppm by 2100.
Current emissions trend,
hence outcome if no substantive mitigation action is taken (“business as usual”).
IPCC (2007) Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the
Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri, R.K and Reisinger, A.(eds.)]. IPCC, Geneva, Switzerland, 104 pp.
IPCC (2000) IPCC Special Report on Emissions Scenarios. Prepared by Working Group III of the Intergovernmental Panel on Climate
Change, Cambridge University Press, Cambridge, United Kingdom, pp 570.
IPCC (2013) Climate Change 2013: e Physical Science Basis. Contribution of Working Group I to the Fih Assessment Report of the Inter-
governmental Panel on Climate Change [Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M.
Midgley (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 1535 pp.
Figure 3.1. Observed temporal variability in pH at 13 locations in the Pacic and Southern Ocean, each over a period of 10-30 days,
at 0–15 m water depth
[2]
. X-axis denotes measurement days. Note: Figure shows selected graphs from Figure 2 in
[2]
. Not all graphs
from original gure are shown.
Figure 3.2. Spatial variability in pH in surface (left) and bottom water (right), derived from total alkalinity and dissolved inorganic
carbon data for the North Sea in July-August 2011
[13]
.
Many, but not all, of these observed features have been successfully simulated
in high-resolution models of the carbon system in the north-west European shelf
[14,15]

3. GLOBAL STATUS AND FUTURE TRENDS OF OCEAN ACIDIFICATION
29
either case, annual mean values for pH or carbonate
saturation are likely to be poor predictors of impacts;
instead minimum pH levels and/or potential inter-
actions with other stress conditions, including local
pollution,
[12]
could be more important.
Several national and international programmes are
now working to provide high-quality, standard-
ized observations that will lead to key knowledge
of carbon system changes in the marine envi-
ronment, hence improving our understanding of
present-day variability – and our ability to make
reliable projections of future conditions.
Global,
quality-assured datasets of inorganic carbon, total
alkalinity and pCO have now been built through
the Global Ocean Data Analysis Project (GLODAP)
[16]
, CARbon in the Atlantic Ocean (CARINA)
[17]
,
the Surface Carbon CO Atlas (SOCAT)
[18]
, and
the Global Ocean Acidication Observing Network
(GOA-ON).
Additional details on these initiatives
in the context of monitoring ocean acidication are
provided in section 3.3 below.
3.2 MODELLED SIMULATIONS OF FUTURE OCEAN ACIDIFICATION
Future changes in ocean carbon chemistry will neces-
sarily be very closely linked to future increases in
atmospheric CO
[19-23]
, with those increases being
emission-dependent (Box 3.1). Thus under the
lowest current IPCC emission scenario (RCP 2.6),
the projected reduction in global mean surface pH by
2100 would be limited to ~0.1 units; under the high-
est emission scenario (RCP 8.5, the current trajec-
tory), the mean surface pH reduction this century
would be at least 0.3 units (Figure 3.3)
[22-25]
.
ose
lower and higher pH changes represent further
increases in H
+
concentrations of around 25% and
Box 3.1 IPCC scenarios for future CO
2
emissions
1.
Main scenarios used by the Intergovernmental Panel on Climate
Change (IPCC) for its 4
th
Assessment Report (AR4), (IPCC, 2007).
These
illustrative “families” of pathways were developed in the IPCC
Special Report on Emission Scenarios (SRES)
(IPCC, 2000), and
are referred to in many ocean acidication modelling papers
published pre-2012.
A1 – Integrated world, rapid economic growth, limited population
growth.
Three versions: A1FI (fossil fuel intensive), A1B (balanced)
and A1T (non-fossil energy sources).
A2 – Divided world, regional economic growth and continuous
population growth (highest emissions in 2100).
B1 – Integrated world, rapid economic growth, limited population
growth with global movement towards economic, social and
environmental stability (lowest emissions in 2100).
B2 – Divided world, regional economic growth, continuous
population growth with regional movement towards economic, social
and environmental stability.
2.
Main scenarios used by IPCC for its 5
th
Assessment Report (AR5)
(IPCC, 2013), as “Representative Concentration Pathways” (RCPs)
[26]
.
RCP 2.6 – lowest emissions, atmospheric CO
2
peaks at ~443
ppm in 2050 before declining to ~421 ppm by 2100.
Assumes
unspecied “negative emissions” i.e., active CO
2
removal from the
atmosphere.
RCP 4.5 – low emissions; atmospheric CO
2
concentrations reach ~538
ppm by 2100.
RCP 6.0 – moderate emissions; atmospheric CO
2
concentrations reach
~670 ppm by 2100.
RCP 8.5 – high emissions; atmospheric CO
2
concentrations reach
~936 ppm by 2100.
Current emissions trend, hence outcome if no
substantive mitigation action is taken (“business as usual”).
IPCC (2007) Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate
Change [Core Writing Team, Pachauri, R.K and Reisinger, A.(eds.)]. IPCC, Geneva, Switzerland, 104 pp.
IPCC (2000) IPCC Special Report on Emissions Scenarios. Prepared by Working Group III of the Intergovernmental Panel on Climate Change, Cambridge University Press,
Cambridge, United Kingdom, pp 570.
IPCC (2013) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate
Change [Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge, United
Kingdom and New York, NY, USA, 1535 pp.

30
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
Figure 3.3.
Historical and projected changes in global surface ocean pH
over 1870-2100 for the four IPCC AR5 scenarios (see Box 3.1).
Model
means from the Climate Model Intercomparison Project. From
[23]
.
Figure 3.5. Model-derived derived maps of historical (1850, left) and projected (2100, right) aragonite saturation state, with the
latter based on the IPCC RCP 8.5 emissions trajectory. Model projections from Max Planck Institute for Meteorology, map by IGBP/
Globaia, reproduced with permission from
[27]
Figure 3.4. Model-derived maps of historical (1850, left) and projected (2100, right) ocean surface pH, with the latter based on the
IPCC RCP 8.5 emissions trajectory. Model projections from Max Planck Institute for Meteorology, map by IGBP/Globaia, reproduced
with permission from
[27]

3. GLOBAL STATUS AND FUTURE TRENDS OF OCEAN ACIDIFICATION
31
170% respectively, in addition to the increase of
around 25% that has already occurred since the
industrial revolution. e future surface pH change
will not be globally uniform but will vary region-
ally (Figure 3.4), due to latitudinal dierences in
temperature and future warming, aecting CO
solubility, and basin-scale (and more local) circu-
lation patterns and their future changes.
e aragonite saturation horizon, below which arago-
nite (the more soluble form of calcium carbonate)
dissolves, is projected to rise from a few thousand
metres to just a few hundred metres in many temper-
ate and tropical oceans by 2100
[24]
. As a result of
temperature eects on carbon chemistry, high lati-
tude (polar) areas will experience larger declines in
surface pH for any given addition of CO from the
atmosphere. In Southern Ocean surface water, an
atmospheric concentration of about 450 ppm is su-
cient for large areas of this region to be under-satu-
rated with respect to aragonite
[28]
. Similarly, much
of the surface Arctic Ocean is projected to become
undersaturated for aragonite throughout the year
within the next 50 years under most scenarios
[21,23]
(Figure 3.5).
e seasonally variable presence of sea-ice in polar
regions, and its near-certain future decrease, is
an additional confounding inuence that is not
currently well-represented in projections of future
ocean acidication.
Sea-ice can signicantly aect
pH and other components of the carbon system in
many ways, including the following:
•
Under conditions of oshore transport of sea-
ice, the processes of freezing and melting may be
spatially separated, that can result in a net trans-
port of inorganic carbon to the deep ocean
[29]
.
•
Projected future reductions in sea-ice cover
will increase the area of ocean exposed to the
atmosphere, enhancing air-sea CO exchange.
When coupled with the likely freshening of the
surface water (due to melt of land-derived ice)
pH decrease will accelerate in the upper ocean
[30]
.
•
Seasonal sea-ice melt can locally enhance
stratification and primary production, with
indirect eects on pH and other carbon chemis-
try parameters.
To quantify the importance of such factors, high-
resolution ocean carbon models are being developed
for high latitude regions, with current emphasis on
the Arctic.
ese models include improved represen-
tations of climate-driven changes in ice cover, fresh-
water inputs, topographically inuenced circulation
and biogeochemical processes. Two such models
have been developed in association with the Arctic
Monitoring and Assessment Programme (AMAP): a
1-D simulation of carbon transformations and uxes
at an Arctic shelf sea site subject to seasonal sea-ice
cover and strong riverine inuence (Figure 3.6)
[31,32]
,
and a regional model for the Atlantic-Arctic gateway
region, between Greenland, Kingdom of Denmark;
Svalbard, Norway; and the Norwegian mainland
[30]
.
An additional uncertainty for the Arctic relates to
the potential release of methane, and its subsequent
oxidation to CO: locally, this could cause a further
pH reduction of up to 0.25 units by 2100
[33]
.
Figure 3.6. Modelled seasonal changes (January to December) in
temperature (upper), pH (middle) and aragonite saturation state (lower) for
2010 and projected for 2050 under IPCC SRES B1 for a site at 50m water
depth in the Siberian Arctic shelf (Central Laptev Sea).
Note that pH mostly
increases with water depth (unlike the situation in temperate shelf seas,
Fig. 3.2, and the open ocean, Fig. 3.7) and undersaturation is projected
to extend from summer-only to year-round within 40 years under a low
emissions scenario.
From
[30]

32
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
An important factor to recognize is the longev-
ity of ocean acidication: long aer carbon emis-
sions are curtailed, ocean acidication will remain.
Anthropogenic increases in atmospheric CO and
perturbations to ocean chemistry will take tens to
hundreds of thousands of years to return to pre-
industrial values
[34]
, as CO will be slowly buered
by the dissolution of calcium carbonate sediments
and the weathering of silicates to promote the return
of carbon back into geological reservoirs.
3.3 CURRENT STATUS OF GLOBAL OBSERVATIONS
Observations of ocean acidication are not yet on a
fully global scale, not only because of the relatively
short time of awareness of the importance of such
changes, but also due to the high cost of research
expeditions; the inaccessibility of many regions; the
relative unavailability of highly accurate and reliable
pH sensors; and the current limitations of autono-
mous monitoring techniques. ere is also a need
to collect data on other environmental variables for
valid interpretation.
Nevertheless, long time series
do exist on the changing marine carbon system in
the central Pacic (Hawaii Ocean Time series, HOT)
and North Atlantic (Bermuda Atlantic Time-series
Study, BATS; European Station for Time-series in
the Ocean, ESTOC), quantifying surface pH decline
over the last several decades (over the range -0.0016
to -0.0019 yr
-1
)
[35-37]
. e observed decline in surface
pH at these three open-ocean stations is consis-
tent with a surface ocean that is closely tracking the
increase in atmospheric CO levels over the past
three decades
[38]
.
Synthesis products on observations of ocean pCO and
air-sea CO uxes have been developed by the Global
Ocean Data Analysis Project (GLODAP)
[16]
, CARbon
in the Atlantic Ocean (CARINA)
[17]
, the Surface
Carbon CO Atlas (SOCAT)
[18]
, and PACIFICA
[39]
.
ese initiatives include analyses of the penetration
of anthropogenic carbon to the ocean interior, due
to entrainment, mixing, and deep-water formation.
In the North Atlantic and Southern oceans, signals
of decreasing pH have already been observed at the
ocean oor
[40-43]
. Such changes involve more than a
simple shoaling of aragonite and calcite saturation
horizons, since the zone of low pH water may be
extending downwards as well as upwards (Figure
3.7).
In the Pacic and the South Atlantic, signals of
anthropogenic carbon have also been observed in
intermediate waters
[44,45]
. For all ocean basins, model
projections indicate that ocean acidication will occur
throughout the water column by 2100.
Recent international effort has been directed
at extending and complementing these exist-
ing programmes to more explicitly address ocean
acidication and its impacts, with increased atten-
tion to shelf seas and coastal regions.
Relevant
activities are being initiated and implemented at
Figure 3.7. Measured pH proles in the north-east Atlantic along a 3,400 km transect from south-east Greenland to Portugal in
2002 (left) and 2008 (right).
Additional transect data were collected in 1991, 1993, 2004 and 2006, and were fully consistent with
this rapid expansion in the volume of low-pH intermediate water, and associated changes in seaoor conditions. From
[40]
.

3. GLOBAL STATUS AND FUTURE TRENDS OF OCEAN ACIDIFICATION
33
the regional level, for example, through the US
Ocean Margin Ecosystems Group for Acidication
Studies (OMEGAS)
[46]
, and also on a worldwide
basis, through the recently established Global Ocean
Acidication Observing Network (GOA-ON)
[47]
(Figure 3.8).
GOA-ON aims to provide an under-
standing of ocean acidication conditions and the
ecosystem response, as well as to deliver the data
needed to optimize ocean acidication modelling.
Since the potential scope for biological observ-
ing is extremely wide, GOA-ON will build on,
and work in close liaison with, the Global Ocean
Observing System (GOOS) and its Framework for
Ocean Observation. Other bodies contributing to
the development of the network include the IAEA
Ocean Acidication International Coordination
Centre (OA-ICC), IOC-UNESCO, the International
Ocean Carbon Coordination Project (IOCCP), and
a range of national funding agencies. To date, most
ocean acidication observations have been ship-
based.
However, increasing use is expected to be
made of pH sensors on proling oats
[48]
and using
underwater gliders; such issues are also considered
in Chapter 9.
Figure 3.8. Components of the developing Global Ocean Acidication Observing Network (GOA-ON), including moorings, time-series
stations, and ship-based surveys, by voluntary observing ships (VOS), ships of opportunity (SOO) and research vessels.
Status at May
2014 from GOA-ON Requirements and Governance Plan
[47]
.

34
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
References
1. Friedrich T, Timmermann A, Abe-Ouchi A, Bates
NR, Chikamoto MO, et al. (2012) Detecting regional
anthropogenic trends in ocean acidication against
natural variability. Nature Climate Change 2: 167-171.
2. Hofmann GE, Smith JE, Johnson KS, Send U, Levin
LA, et al. (2011) High-frequency dynamics of ocean
pH: A multi-ecosystem comparison. PLoS ONE 6
(12): e28983.
3. Duarte CM, Hendriks IE, Moore TS, Olsen YS,
Steckbauer A, et al. (2013) Is ocean acidication an
open-ocean syndrome? Understanding anthropogenic
impacts on seawater pH. Estuaries and Coasts 36:
221-236.
4. Provoost P, Van Heuven S (2010) Long-term record
of pH in the Dutch coastal zone: a major role for
eutrophication-induced changes. Biogeosciences 7:
4127-4152.
5. Johnson ZI, Wheeler BJ, Blinebry SK, Carlson CM,
Ward CS, et al. (2013) Dramatic variability of the
carbonate system at a temperate coastal ocean site
(Beaufort, North Carolina, USA) is regulated by
physical and biogeochemical processes on multiple
timescales. PLoS ONE 8(12): e85117.
6. Wootton JT, Pster CA (2012) Carbon system
measurements and potential climatic drivers at a
site of rapidly declining ocean pH. PLoS ONE 7(12):
e53396.
7. Shaw EC, McNeil BI, Tilbrook B, Matear R, Bates ML
(2013) Anthropogenic changes to seawater buer
capacity combined with natural reef metabolism
induce extreme future coral reef CO conditions.
Global Change Biology 19: 1632-1641.
8. Feely RA, Sabine CL, Hernandez-Ayon JM, Ianson D,
Hales B (2008) Evidence for upwelling of corrosive
“acidied” water onto the continental shelf. Science
320: 1490-1492.
9. Findlay HS, Artioli Y, Navas JM, Hennige SJ, Wicks
LC, et al. (2013) Tidal downwelling and implications
for the carbon biogeochemistry of cold-water corals
in relation to future ocean acidication and warming.
Global Change Biology 19: 2708-2719.
10. Zhai W-D, Zheng N, Huo C, Xu Y, Zhao H-D, et al.
(2014) Subsurface pH and carbonate saturation state
of aragonite on the Chinese side of the North Yellow
Sea: seasonal variations and controls. Biogeosciences
11: 1103-1123.
11. Doney SC, Mahowald N, Lima I, Feely RA,
Mackenzie FT, et al. (2007) Impact of anthropogenic
atmospheric nitrogen and sulfur deposition on
ocean acidication and the inorganic carbon system.
Proceedings of the National Academy of Sciences of
the United States of America 104: 14580-14585.
12. Roberts DA, Birchenough SNR, Lewis C, Sanders MB,
Bolam T, et al. (2013) Ocean acidication increases
the toxicity of contaminated sediments. Global
Change Biology 19: 340-351.
13. Greenwood N, Pearce D (Figure 2). In: Williamson
P, Turley CM, Brownlee C, Findlay HS, Ridgwell A et
al., Impacts of Ocean Acidication . MCCIP Science
Review 2013: 34-48.
14. Artioli Y, Blackford J, Butenschon M, Holt JT,
Wakelin SL, et al. (2012) e carbonate system of the
NW European shelf: sensitivity and model validation.
Journal of Marine Systems 102-104: 1-13.
15. Artioli Y, Blackford J, Nondal G, Bellerby RGJ,
Wakelin SL, et al. (2014) Heterogeneity of impacts
of high CO on the North Western European Shelf.
Biogeosciences 11: 601-612.
16. Key RM, Kozyr A, Sabine CL, Lee K, Wanninkhof
R, et al. (2004) A global ocean carbon climatology:
Results from Global Data Analysis Project
(GLODAP). Global Biogeochemical Cycles 18:
GB4031.
17. Key RM, Tanhua T, Olsen A, Hoppema M,
Jutterstrom S, et al. (2010) e CARINA data
synthesis project: introduction and overview. Earth
Systems Science Data 2: 105-121.
18. Bakker D.C.E. and 80 others. 2014.
An update to
the Surface Ocean CO Atlas (SOCAT version 2).
Earth System Science Data, 6, 69-90; doi: 10.5194/
essd-6-69-2014
19. Zeebe RE (2012) History of seawater carbonate
chemistry, atmospheric CO, and ocean acidication
Annual Review of Earth and Planetary Sciences, 40:
141-165.
20. Caldeira K, Wickett ME (2005) Ocean model
predictions of chemistry changes from carbon dioxide
emissions to the atmosphere and ocean. Journal of
Geophysical Research-Oceans 110: C09S04.
21. Steinacher M, Joos F, Froelicher TL, Plattner GK,
Doney SC (2009) Imminent ocean acidication in
the Arctic projected with the NCAR global coupled
carbon cycle-climate model. Biogeosciences 6:
515-533.
22. Denman K, Christian JR, Steiner N, Poertner H-O,
Nojiri Y (2011) Potential impacts of future ocean
acidication on marine ecosystems and sheries:
current knowledge and recommendations for
future research. ICES Journal of Marine Science 68:
1019-1029.
23. Bopp L, Resplandy L, Orr JC, Doney SC, Dunne JP,
et al. (2013) Multiple stressors of ocean ecosystems
in the 21st century: projections with CMIP5 models.
Biogeosciences 10: 6225-6245.

3. GLOBAL STATUS AND FUTURE TRENDS OF OCEAN ACIDIFICATION
35
24. Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, et
al. (2005) Anthropogenic ocean acidication over
the twenty-rst century and its impact on calcifying
organisms. Nature 437: 681-686.
25. McNeil BI, Matear RJ (2007) Climate change
feedbacks on future oceanic acidication. Tellus
Series B-Chemical and Physical Meteorology 59:
191-198.
26. Moss RH, Edmonds JA, Hibbard KA, Manning
MR, Rose SK, et al. (2010) e next generation of
scenarios for climate change research and assessment.
Nature 463: 747-756.
27. IGBP, IOC, SCOR (2013). Ocean Acidication
Summary for Policymakers 2013.
International
Geosphere-Biosphere Programme.
Stockholm,
Sweden.
28. McNeil B, Matear R (2008) Southern Ocean
acidication: A tipping point at 450-ppm atmospheric
CO. Proceedings of the National Academy of Science
105: 18860-18864.
29. Loose B, Schlosser P (2011) Sea ice and its eect on
CO ux between the atmosphere and the Southern
Ocean interior. Journal of Geophysical Research-
Oceans 116: C11019.
30. Arctic Monitoring and Assessment Programme
(2013).
AMAP Assessment 2013: Arctic Ocean
Acidication. AMAP, Oslo, Norway. 99p http://www.
amap.no/documents/doc/AMAP-Assessment-2013-
Arctic-Ocean-Acidication/881
31. Wahlstrom I, Omstedt A, Bjork G, Anderson LG
(2013) Modeling the CO dynamics in the Laptev Sea,
Arctic Ocean: Part II. Sensitivity of uxes to changes
in the forcing. Journal of Marine Systems 111: 1-10.
32. Wahlstrom I, Omstedt A, Bjork G, Anderson LG
(2012) Modelling the CO dynamics in the Laptev
Sea, Arctic Ocean: Part I. Journal of Marine Systems
102: 29-38.
33. Biastoch A, Treude T, Rüpke LH, Riebesell U, Roth C,
et al. (2011) Rising Arctic Ocean temperatures cause
gas hydrate destabilization and ocean acidication.
Geophysical Research Letters 38: L08602.
34. Archer D (2005) Fate of fossil fuel CO in geologic
time. Journal of Geophysical Research-Oceans 110:
C09S05.
35. Dore JE, Lukas R, Sadler DW, Church MJ, Karl DM
(2009) Physical and biogeochemical modulation
of ocean acidication in the central North Pacic.
Proceedings of the National Academy of Sciences of
the United States of America 106: 12235-12240.
36. Bates NR, Best MHP, Neely K, Garley R, Dickson AG,
et al. (2012) Detecting anthropogenic carbon dioxide
uptake and ocean acidication in the North Atlantic
Ocean. Biogeosciences 9: 2509-2522.
37. Santana-Casiano JM, Gonzalez-Davila M, Rueda M-J,
Llinas O, Gonzalez-Davila E-F (2007) e interannual
variability of oceanic CO parameters in the northeast
Atlantic subtropical gyre at the ESTOC site. Global
Biogeochemical Cycles 21: GB1015.
38. Intergovernmental Panel on Climate Change (2013)
Summary for Policymakers. In: Climate Change 2013:
e Physical Science Basis. Contribution of Working
Group I to the Fih Assessment Report of the IPCC
(Stocker TF, Qin D, Plattner G-K, Tognor M, Allen
SK, Boschung J, Nauels A, Xia Y, Bex V & Midgley
PM (eds). Cambridge University Press, Cambridge
UK and New York, USA.
39. Suzuki T. and 18 others. (2013) PACIFICA Data
Synthesis Project. ORNL/CDIAC-159, NDP-092.
Carbon Dioxide Information Analysis Center,
Oak Ridge TN, USA; doi: 10.3334/CDIOAC/OTG.
PACIFICA_NDP092.
40. Vazquez-Rodriguez M, Perez FF, Velo A, Rios AF,
Mercier H (2012) Observed acidication trends
in North Atlantic water masses. Biogeosciences 9:
5217-5230.
41. Vazquez-Rodriguez M, Touratier F, Lo Monaco C,
Waugh DW, Padin XA, et al. (2009) Anthropogenic
carbon distributions in the Atlantic Ocean: data-
based estimates from the Arctic to the Antarctic.
Biogeosciences 6: 439-451.
42. Hauck J, Hoppema M, Bellerby RGJ, Voelker C,
Wolf-Gladrow D (2010) Data-based estimation
of anthropogenic carbon and acidication in the
Weddell Sea on a decadal timescale. Journal of
Geophysical Research-Oceans 115: C03004.
43. Olafsson J, Olafsdottir SR, Benoit-Cattin A, Danielsen
M, Arnarson TS, et al. (2009) Rate of Iceland Sea
acidication from time series measurements.
Biogeosciences 6: 2661-2668.
44. Byrne RH, Mecking S, Feely RA, Liu X (2010) Direct
observations of basin-wide acidication of the North
Pacic Ocean. Geophysical Research Letters 37:
L02601.
45. Resplandy L, Bopp L, Orr JC, Dunne JP (2013) Role
of mode and intermediate waters in future ocean
acidication: Analysis of CMIP5 models. Geophysical
Research Letters 40: 3091-3095.
46. Hofmann GE, Evans TG, Kelly MW, Padilla-
Gamiño JL, Blanchette CA et al (2014) Exploring
local adaptation and the ocean acidication seascape
– studies in the California Current Large Marine
Ecosystem. Biogeosciences 11: 1053-1064.
47. GOA-ON http://www.goa-on.org
48. http://www.mbari.org/chemsensor/oatviz.htm

36
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
As well as using models to project climate change, we
can better understand the future impacts of ocean
acidication by studying how biogeochemical cycles
operated in the past, and the impact past events had
on marine ecosystems.
In addition to variations in seawater acidity from
place to place because of circulation patterns, biolog-
ical activity, and other oceanographic processes (see
previous chapter), the average state of the ocean can
also change through time in response to natural
variations in the global carbon cycle. Past changes
in ocean acidity can be studied by chemical analysis
of the skeletons of dead organisms such as molluscs,
foraminifera, corals and algae, or of ocean sedi-
ments, which are accessible by drilling into the sea-
bed. Deep-sea cores commonly contain abundant
fossils of calcifying (carbonate producing) plankton,
such as foraminifera and coccolithophores (Figure
4.1), which are among the groups considered most
at risk in future ocean acidication.
e paleo-record can be used to extend the current
record of acidity changes as it stretches back millions
of years in time. Over the longer term, it contains
evidence of: (1) cyclic changes in ocean chemis-
try associated with glacial / interglacial cycles with
sometimes abrupt transitions; (2) multi-million
4. WHAT THE PAST CAN TELL US
— PALEO-OCEANOGRAPHIC RESEARCH
KEY MESSAGES
1. During a previous period of ocean acidication, which occurred ~56 million years ago and lasted
~6000 years, several deep-sea calcifying organisms became extinct
2. Current ocean acidication is projected to reach similar levels over the next 500 years
3. Ocean acidication may have been a contributing factor in four “coral reef crises” in the last 500
million years
4. The paleo-record conrms that ocean acidication takes many thousands of years to return to original levels
following a CO
2
input event
Figure 4.1. Light microscope (left panel) and scanning electron microscope (middle panel) images of planktonic foraminifera from
Paleocene-Eocene Thermal Maximum (PETM, ~56 million years ago) sediments from Tanzania. Geochemical analysis of foraminifera
shells can provide information about oceanic chemistry millions of years ago. Scale bar 100 μm. The right panel is a well preserved
coccosphere. Source: P. Pearson (foraminifera), P. Bown (coccosphere).

4. WHAT THE PAST CAN TELL US — PALEO-OCEANOGRAPHIC RESEARCH
37
year trends related to global tectonics; and, perhaps
of most interest, (3) past sudden events of similar
scale (if not rate) to the current human-induced
change to the carbon cycle. ese abrupt events
provide us with real-world examples of profound
environmental changes that allow us to study the past
long-term response of marine organisms to ocean
acidication including, for example, their extinc-
tion, migration, assemblage changes, and changes
in calcication style. is information from the past
can be compared with the results of modern eld
and laboratory research.
4.1 RECONSTRUCTING PAST OCEAN ACIDIFICATION EVENTS
To understand the rate and magnitude of past carbon
cycle perturbations and their eect on seawater pH
it is necessary to generate data of various sorts to
help constrain geochemical models. One approach is
the study of the calcium carbonate content of deep-
sea sediments deposited at dierent water depths.
Another valuable tool is to measure the boron isoto-
pic composition (δ
11
B) of marine carbonates which
is inuenced by the pH of the water from which it
was precipitated. Ratios of trace elements to calcium
carbonates and the carbon isotope ratio (δ
13
C) can
also help identify changes in the global carbon cycle.
4.2 THE PALEOCENE-EOCENE THERMAL MAXIMUM – A NATURAL PAST
“EXPERIMENT” IN OCEAN ACIDIFICATION
e Paleogene (23-65 million years ago, compris-
ing the Paleocene, Eocene and Oligocene epochs)
was a period of elevated global temperatures
with high levels of atmospheric CO that at times
exceeded 1000 ppm
[1]
. It was punctuated by a series
of “hyperthermals”, which are geologically short-
lived warming events characterized by evidence of
acidication of the oceans
[1]
. e largest of these was
the Paleocene-Eocene ermal Maximum (PETM)
~56 million years ago,
[2]
which has been proposed as
the closest geological analogue to modern day ocean
acidication due to the volume of carbon released
[3]
.
During this period ~2000-3000 petagrams (also
known as gigatonnes) of carbon were released into
the Earth’s atmosphere over thousands of years
[4,5]
and global temperatures increased by about 5°C
[6]
.
Coincident with this climatic shi was a lowering of
oceanic pH, as evidenced by dissolution of carbon-
ate at the seaoor
[7]
(Figure 4.2).
Figure 4.2. Atlantic Ocean deep-sea core from the Integrated Ocean Drilling Program. Note the brown section of the core, which
represents the dissolution of deep-sea carbonate at the Paleocene-Eocene boundary. This could represent a lack of calciers during
that time period or the dissolution of dead shells. Source: James Zachos.

38
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
4.3 THE IMPACT OF PAST OCEAN ACIDIFICATION UPON CALCIFIERS
Good geological records of corals and calcifying
plankton can be collected due to their structure and
their settlement, respectively. Understanding the
geological history of coral reefs, and whether past
coral reef “crises” were initiated by ocean acidica-
tion is of great importance as we consider the future
fate of coral reefs. Kiessling and Simpson (Table
4.1)
[8]
investigated whether reef crises (declines
in carbonate production) and reef mass extinc-
tions were associated with ocean acidication, and
concluded that it was likely that at least four of the
reef crises in the last 500 million years were partly
caused by ocean acidication whilst also linked to
rapid global warming (Table 4.1).
However, a full understanding of the geological
history of coral reefs may require a combined envi-
ronmental and evolutionary approach.
e modern
Scleractinia (the framework-forming corals that we
know today) appeared in the middle Triassic period,
and two main orders of coral, the Rugosa and the
Tabulata, became extinct before this at the end of
the Permian period. ese corals were believed to be
calcitic, rather than aragonitic like the vast major-
ity of today’s corals. is period was clearly char-
acterized as a time of environmental perturbations
with unusual seawater chemistry
[9,10]
, and thus the
“Sandberg curve”
[11]
which details the dominance
of calcitic and aragonitic biomineralization strat-
egies by marine organisms through time, may be
an important component of future historical coral
research with respect to changing climates.
It appears that not all groups of organisms with
exposed skeletal structures were aected by ocean
acidication in the same way over the last 300 million
years. Some climate and ocean acidication events
are associated with widespread extinction, whereas
others are characterized by evolutionary turnover
[3]
.
For example, during the PETM both planktonic
foraminifera and coccolithophore communities
demonstrated signicant range shis but they were
not subject to mass extinction. Tropical communi-
ties migrated to higher latitudes, coincident with
the appearance of short-lived “excursion taxa”
that appear in the fossil record in lower latitude
Table 4.1.
Assessment of ocean acidication as a probable cause of mass extinctions of corals/other reef-builders and associated
“reef crises” (reduced CaCO
3
production) during the past 500 million years, based on
[8]
.
Geological
period/epoch
Time (million
years ago) Effect on corals and other reef-forming organisms
Evidence for ocean
acidication
Paleocene
– Eocene
55.8
Background extinction, except for benthic foraminifers; coral reef crisis
Strong
Cretaceous
– Paleogene
65.5 Mass depletion of biodiversity and mass extinction, selective against
buffered organisms
Weak
Early Jurassic
183 Modest but selective extinction of corals and other unbuffered organisms;
coral reef crisis
Strong
Triassic – Jurassic
199.6 Mass depletion of biodiversity and mass extinction selective against corals,
sponges and unbuffered organisms; coral reef crisis for all reef types
Strong
Permian - Triassic
251 Mass depletion of biodiversity and mass extinction, especially for
unbuffered organisms; coral reef crisis for all reef types
Strong
Middle - Late
Permian
260.4 Substantial extinction, weakly selective with respect to buffering; coral-
sponge reef crisis only
None
Late Devonian
374.5 Mass depletion of biodiversity. Selective extinction of corals and sponges
over prolonged period of time; reef crisis for corals and sponges
Weak
Late Ordovician
445.6 – 443.7 Mass depletion of biodiversity during double mass extinction. Unselective
with respect to buffering
None

4. WHAT THE PAST CAN TELL US — PALEO-OCEANOGRAPHIC RESEARCH
39
assemblages
[6]
. In contrast, there was a severe extinc-
tion of deep-sea benthic foraminifera with up to 50%
of species lost from the fossil record
[12]
. e extinc-
tion saw the disappearance of long-lived Paleocene
species, and the post-extinction taxa were commonly
smaller and had thinner shells
[12]
.
Recent research has provided detailed information
on biomineralization of the skeletons of pelagic
organisms that are likely sensitive to changes in
surface water chemistry. Analysis of the architecture
of coccolithophores has distinguished impacts on
the skeleton that are associated with cellular func-
tion versus those associated with external carbonate
chemistry of the water they experienced during the
PETM
[13]
. Currently, observed changes suggest that
the impact of ocean acidication across the PETM
was relatively low compared to biogeographic range
changes driven by warming and changes in circula-
tion and the hydrologic cycle.
is does not mean we should not be concerned
for calciers under our current climate regime.
Clearly, communities responded signicantly to
the combined environmental impacts of the PETM,
which like today, consisted of ocean acidication
with additional environmental changes associated
with increased CO, such as changes in tempera-
ture and oxygenation
[14]
. is is particularly the case
for organisms that are unable to migrate in order to
avoid environmental change, such as longer lived,
sessile organisms like oysters and corals. It is espe-
cially important to remember that the changes seen
during the PETM took place over many thousands of
years, at least 10 times slower than anticipated rates
of warming and OA in the century ahead
[14]
. When
the rate of carbon uptake into the ocean outstrips its
capacity to absorb it, a reduction in pH goes hand-
in-hand with a lowering of its saturation state
[15]
. It
is this saturation state (buering capacity) of the
ocean that could impact the functioning of many
calcifying organisms, such as tropical reef-form-
ing corals and planktonic organisms that form the
base of pelagic food webs, especially in the vulner-
able Arctic and Antarctic regions
[14]
.
4.4 USING THE GEOLOGICAL RECORD TO INFORM OUR UNDERSTANDING
OF OCEAN ACIDIFICATION
e geological record provides tangible evidence of
the impacts of ocean acidication on environments
and ecosystems, and provides a unique long-term
perspective. Distinguishing the eects of acidi-
cation from associated environmental variables in
the past is dicult, however, and there is no perfect
geological analogue for modern day ocean acidi-
cation. e PETM, in particular, is widely studied
because it is comparable in magnitude to predicted
anthropogenic CO release, but it diers markedly
in terms of rate of change as it occurred over thou-
sands rather than tens or hundreds of years, as is the
case today. Even so it provides an invaluable test-
bed for studying the overall impact and subsequent
recovery of the Earth system and biotic communi-
ties, as well as potential biotic sensitivity to abrupt
climate change.
4.5 TIMESCALES OF OCEAN ACIDIFICATION
In Earth history, ocean carbonate saturation is
generally well regulated by the simple requirement
that on “long” (>10,000 year) timescales, sources
(weathering) and sinks (shallow- and deep-water
CaCO burial) are kept in balance and regulated
by the position of the calcium carbonate saturation
depths. Only events involving geologically “rapid”
(<10,000 year) CO release will overwhelm the ability
of the ocean and sediments to regulate, producing a
coupled decline in both pH and saturation state
[15]
.
e onset of the PETM occurred over a timescale of
<10,000 years
[16]
and released ~2000-3000 petagrams
of carbon into the Earth’s atmosphere
[17]
(1 petagram
= 1 gigatonne = thousand million tonnes).

40
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
Today’s climate change projections calculate that ~
5000 petagrams of carbon will be released into the
atmosphere over the next 500 years if we follow a
‘business as usual’ scenario
[18,19]
. In Figure 4.3A,
carbon released into the atmosphere during the
PETM, and projected anthropogenic carbon emis-
sions have been overlaid to provide perspective on
the timescales involved. As a result of the carbon
released in 4.3A, the saturation state of calcite
(one of the mineral forms of calcium carbonate)
decreases (Figure 4.3B). An important point to note
is the timescale for the saturation state for calcite to
“recover” to previous levels. Following the PETM,
this took ~100,000 years
[7]
, and it is projected to take
a similar length of time following projected anthro-
pogenic carbon emissions. us we can see that
ocean acidication is not a short-lived problem and
could take many thousands of years to return to pre-
industrial levels even if carbon emissions are curbed.
References
1. Zachos JC, Dickens GR, Zeebe RE (2008) An early
Cenozoic perspective on greenhouse warming and
carbon-cycle dynamics. Nature 451: 279-283.
2. Kennett JP, Stott LD (1991) Abrupt deep-sea
warming, palaeoceanographic changes and benthic
extinctions at the end of the paleocene. Nature 353:
225-229.
3. Hönisch B, Ridgwell A, Schmidt DN, omas E,
Gibbs SJ, et al. (2012) e geological record of
ocean acidication. Science (New York, NY) 335:
1058-1063.
4. Dickens GR (2011) Methane release from gas hydrate
systems during the Paleocene-Eocene thermal
maximum and other past hyperthermal events:
setting appropriate parameters for discussion. Climate
of the Past Discussions 7: 1139-1174.
5. Dickens GR (2011) Down the Rabbit Hole: toward
appropriate discussion of methane release from
gas hydrate systems during the Paleocene-Eocene
thermal maximum and other past hyperthermal
events. Climate of the Past 7: 831-846.
6. McInerney FA, Wing SL (2011) e Paleocene-
Eocene ermal Maximum: A perturbation of carbon
cycle, climate, and biosphere with implications for the
future. In: Jeanloz R, Freeman KH, editors. Annual
Review of Earth and Planetary Sciences, Vol 39.
pp. 489-516.
7. Zachos JC, Rohl U, Schellenberg SA, Sluijs A, Hodell
DA, et al. (2005) Rapid acidication of the ocean
during the Paleocene-Eocene thermal maximum.
Science 308: 1611-1615.
Figure 4.3. Paleocene-Eocene Thermal Maximum (PETM) versus present-day time scales.(A) Carbon emission scenarios as projected
for the future (5000 petagrams of carbon over ~500 years)
[17,18]
and the PETM (3000 petagrams of carbon over ~6000 years). The
onset of the PETM has been aligned with the onset of industrialization. (B) Changes in surface-ocean saturation state of calcite
simulated with the Long-term Ocean-atmosphere-Sediment Carbon Cycle Reservoir (LOSCAR) model in response to the carbon input
shown in (A). Source: Gattuso and Hansson 2011
[20]
© By permission of Oxford University Press.

4. WHAT THE PAST CAN TELL US — PALEO-OCEANOGRAPHIC RESEARCH
41
8. Kiessling W, Simpson C (2011) On the potential for
ocean acidication to be a general cause of ancient
reef crises. Global Change Biology 17: 56-67.
9. Knoll AH, Bambach RK, Caneld DE, Grotzinger JP
(1996) Comparative earth history and Late Permian
mass extinction. Science 273: 452-457.
10. Roberts JM, Wheeler A, Freiwald A, Cairns SD
(2009) Cold-Water Corals: e Biology and Geology
of Deep-Sea Coral Habitats: Cambridge University
Press.
11. Sandberg PA (1983) An oscillating trend in
Phanerozoic non-skeletal carbonate mineralogy.
Nature 305: 19-22.
12. omas E (2007) Cenozoic mass extinctions in the
deep sea: What perturbs the largest habitat on Earth?
Geological Society of America Special Papers 424:
1-23.
13. Gibbs SJ, Poulton AJ, Bown PR, Daniels CJ,
Hopkins J, et al. (2013) Species-specic growth
response of coccolithophores to Palaeocene-Eocene
environmental change. Nature Geoscience 6: 218-222.
14. Bijma J, Portner HO, Yesson C, Rogers AD (2013)
Climate change and the oceans - What does the future
hold? Marine Pollution Bulletin 74: 495-505.
15. Ridgwell A, Schmidt DN (2010) Past constraints
on the vulnerability of marine calciers to massive
carbon dioxide release. Nature Geoscience 3: 196-200.
16. Pearson PN, Nicholas CJ (2014) Layering in the
Paleocene/Eocenr boundary of the Milville core is
drilling disturbance. Proceedings of the National
Academy of Sciences of the United States of America
111: E1064-E1065
17. Dienbaugh NS, Field CB (2013) Changes in
ecologically critical terrestrial climate conditions.
Science 341: 486-492.
18. Zeebe RE, Caldeira K (2008) Close mass balance of
long-term carbon uxes from ice-core CO
2
and ocean
chemistry records. Nature Geoscience 1: 312-315.
19. Zeebe RE, Zachos JC, Caldeira K, Tyrrell T (2008)
Carbon emissions and acidication. Science 321:
51-52.
20. Zeebe RE, Ridgwell A (2011). Past changes in ocean
carbonate chemistry. In: Gattuso JP, Hansson L (eds).
Ocean Acidication. Oxford: Oxford University Press;
p 21-40.

42
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
Ocean acidication will have direct impacts upon
a variety of dierent taxa through dierent mecha-
nisms such as metabolism, pH regulation, calcica-
tion and photosynthesis. ese impacts will inuence
ecosystem dynamics with an end result of potentially
altered ecosystem services. Figure 1.3 summarizes
the interaction between direct eects of CO and
pH (non-comprehensive) and ecosystem services;
in this chapter, physiological responses are consid-
ered in greater detail.
5.1 OCEAN ACIDIFICATION AND CELLULAR PROCESSES
KEY MESSAGES: 5.1
1. Ocean acidication can lead to acid-base imbalance in many marine organisms, such as sh,
invertebrates and sediment fauna
2. Acid-base imbalance can lead to metabolic suppression, reduced protein synthesis and reduction
in long-term tness
3. Some species can modify energetic allocation to compensate for increased energetic costs of
ocean acidication
Most organisms regulate some aspects of their inter-
nal (extra- or intra-cellular) pH, either for calcica-
tion purposes, or because their metabolic activity
requires some level of regulation. is “acid-base
balance” or regulation is an energetic process, so a
disruption caused by changing external CO levels
will require energy to maintain aspects of extra- or
intra-cellular balance. Several studies, for exam-
ple on deep-sea invertebrates
[1]
and sh,
[2,3]
indi-
cate that animals at high pCO require more energy
as compared to those at low pCO, leading to the
hypothesis that additional energy is needed to
maintain the acid-base balance. is implies that,
if a constant total energy budget is assumed, then
increasing energetic investment into acid-base regu-
lation will decrease allocation to other functions,
such as reproduction or growth (Figure 5.1). Some
studies are now demonstrating energetic compen-
sation behaviours
[4]
, but if acid-base balance is not
achieved, metabolism can become depressed as a
short-term response to extend potential tolerance
[5]
.
However, this is not advantageous as it is typically at
the expense of processes such as protein synthesis
[5-7]
.
5. PHYSIOLOGICAL RESPONSES TO OCEAN
ACIDIFICATION
Figure 5.1. Hypothetical energy budget for normal and stressed organisms. M, maintenance costs; R, reproduction; G, growth. In
this hypothetical energy budget, if metabolic depression is also induced by ocean acidication, the total energy budget may also
decrease (hence the smaller pie on the right). From
[8]
in
[9]
. © By permission of Oxford University Press

5. PHYSIOLOGICAL RESPONSES TO OCEAN ACIDIFICATION
43
Many marine organisms produce shells and other
structures composed of calcium carbonate (CaCO).
Future ocean acidication will lower the saturation
state of calcium carbonate (aragonite and calcite) and
if the water is undersaturated, dissolution of unpro-
tected calcium carbonate will occur. e chemistry
of that dissolution process is well-established
[10,11]
.
However, the eects on calcium carbonate formation
– biocalcication – are very much harder to predict.
is is because in most organisms biocalcication
does not occur directly from seawater but rather
in a compartment or space with regulated chemis-
try and biochemistry, which allows controlled crys-
tal formation. Relevant ions have to be transported
into these compartments, and under future ocean
acidication scenarios, these transport mechanisms
may become slower and less ecient; alternatively,
compensation responses may occur. e degree to
which dierent groups of organisms are sensitive
to changes in carbonate chemistry has become a
major focus of ocean acidication research. Here
we give a more in-depth explanation in corals, as
they are one of the key marine calciers that engi-
neer important marine habitats.
In corals, skeletons are laid down in a process
controlled by specialized calcifying cells in an extra-
cellular calcifying medium semi-isolated from the
surrounding seawater environment
[12]
. Since the
growing skeleton is not in direct contact with seawa-
ter, it is not immediately clear why coral calcication
should be aected by ocean acidication occurring
in the exterior seawater
[13]
. Recent research on cellu-
lar processes associated with calcication has started
to identify the pathways that underlie the sensitivity
of corals to ocean acidication.
Firstly, it has been
shown that there is a passage of ions and molecules
from exterior seawater to the calcifying uid
[14]
.
However, the passage of seawater is restrictive, and
coral tissues protect the skeleton from potential
dissolution
[15,16]
. One way for corals to exert biologi-
cal control to buer against the eects of ocean acid-
ication is to increase pH in the calcifying uid
[17]
,
Figure 5.2. Schematic representation of coral calcication and dissolution, showing how the saturation state of the calcifying uid
(shaded dark blue; vertically exaggerated) is affected by external pH and the strength of the H
+
pump.
The latter might be weak,
moderate or strong; for strong H
+
-pumping corals, the rate of gross calcication may initially increase under increased CO
2
levels,
as shown in the inset graph, while net calcication rates may decline owing to dissolution of exposed skeleton. From
[16]
. Reprinted
by permission from Macmillan Publishers Ltd: Nature Climate Change 1: 294-295, © 2011

44
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
eectively increasing Ωaragonite at the site of calci-
cation
[18,19]
. is process (the “H
+
pump”) involves
expelling hydrogen ions into the surrounding seawa-
ter across a concentration gradient. Under ocean
acidication, the gradient is increased; the expul-
sion of H
+
therefore involves greater energetic cost
to the coral
[18-21]
. Gene expression data in corals show
signs that ocean acidication may start to impair the
calcication process when coral acid-base and ion
regulatory systems struggle to maintain homeosta-
sis in the calcifying cells
[21,22]
.
5.2 FERTILIZATION, EARLY LIFE AND SETTLEMENT
KEY MESSAGES: 5.2
1. Impacts of ocean acidication on fertilization success are highly variable and highlight the
potential for genetic adaptation
2. Ocean acidication is generally detrimental for calcifying larvae
Many marine invertebrates have “mixed” life-cycles,
inhabiting benthic and pelagic environments during
dierent developmental stages.
e persistence and
success of these species therefore require that they
can overcome stresses in multiple habitats. Exposure
to stress, even at seemingly mild levels, can result
in negative eects on subsequent stages of the life-
cycle
[23]
. Consequently, a comprehensive under-
standing of the sensitivities of all life-stages, from
planktonic (fertilization, embryos, larvae) to benthic/
pelagic (juveniles, adults) in a changing ocean is vital
if we are to identify vulnerabilities that can threaten
species persistence in the future.
Reported impacts of ocean acidication on fertiliza-
tion success are highly variable, ranging from none to
very negative eects. is variation reects biological
reality – some species are much more tolerant than
others – however, it almost certainly also results from
dierent experimental approaches
[24,25]
; for exam-
ple, relating to dierent source populations
[25,26]
,
the concentration of gametes
[27]
, the number of
parents
[28]
, and the dominance of dierent parental
genotypes in mass spawnings
[29]
. Such variability has
been noted in recent meta-analyses
[30-32]
; in partic-
ular, variability can be enhanced when organisms
are exposed to experimental conditions as part of
a multi-species assemblage, where species-species
interactions and indirect eects also become impor-
tant
[32]
. Importantly, fertilizations using gametes
pooled from multiple parents, mimicking the multi-
ple spawner scenario in the eld, show some resil-
ience to near-future (~pH 7.8) ocean acidication
conditions
[33-35]
, as opposed to single crosses
[36-40]
.
High variability in responses of single crosses to
ocean acidication also highlights the potential for
selection and genetic adaptation, supporting the
concept of winners and losers in the face of chang-
ing ocean conditions
[37-39]
.
e response of isolated sperm to ocean acidica-
tion within the range of near future projections is
also variable. Acidication reduces the percentage
of motile (i.e., moving) sperm (but not swimming
speed) in one species of sea urchin
[38]
, increases
sperm swimming speed in a different echinoid
species
[41]
, has variable and non-linear eects on
Figure 5.3. Sperm and egg of Ascidia mentula. Image courtesy
of Jon Havenhand.

5. PHYSIOLOGICAL RESPONSES TO OCEAN ACIDIFICATION
45
both sperm motility and swimming speed in a poly-
chaete worm
[26]
, and no eect on sperm swimming
speed in an oyster
[37]
. Established theory shows that
reductions in sperm speed and motility would reduce
fertilization success. On the other hand, increases in
temperature have been seen to have a stimulatory
eect on sperm swimming and enhance fertilization
success
[35,42,43]
. Overall, ocean acidication can cause
a reduction in fertilization at low sperm concentra-
tions in some species but not others, and responses
vary markedly among populations.
Prelarval stages - e few studies that have inves-
tigated the eects of ocean acidication on the very
earliest embryos (pregastrula) have not detected any
negative eects at projected near-future levels
[44-46]
.
Additional work is required to illuminate the possi-
bility that acidication-related changes are selecting
a robust subset of progeny that possess pheno-
typic/genetic variation appropriate to future ocean
conditions.
Development of larvae and juveniles - Larval shells
are among the smallest and most fragile shells in the
ocean and are potentially extremely vulnerable to
decreased mineral saturation caused by ocean acidi-
cation. Consequently, most studies have focused on
calcifying larvae
[32,46-51]
. Increased pCO within the
range of near-future projections is in general nega-
tive to calcifying larvae, including mollusc veligers
and sea urchin echinoplutei
[46,52-55]
(Figure 5.4). In
studies where several pH levels were tested, dele-
terious eects (smaller or abnormal larvae, lower
weight juveniles) are evident at pH 7.8 (0.3 to 0.4 pH
units below ambient). One study reported reduced
growth in bivalve larvae with just a slight decrease to
pH 8.0
[53]
.
Oyster larvae may be particularly vulner-
able, with emerging evidence that pH declines of
0.4 to 0.7 units can induce mortality rates of 80 to
>90%
[56]
. Mollusc larvae with unprotected external
skeletons directly exposed to changing ocean chem-
istry may be more sensitive to increasing ocean CO
compared with echinoderm larvae that have internal
skeletons protected by overlying tissue. In the latter,
hypercapnic (increased organism pCO) alteration of
metabolism can also have a negative eect on larval
growth and calcication
[57-59]
. Warming (up to the
thermal limit) may ameliorate the negative eects of
acidication on growth in marine calciers by stim-
ulating growth in addition to changing CO solu-
bility
[55,60-62]
, but such an eect may depend on food
availability and other conditions; for some species,
increased temperatures may exacerbate detrimen-
tal impacts of ocean acidication
[63]
.
Non-calcifying larvae, including coral and some sea
star larvae, are generally more resilient than calcify-
ing larvae to near-future acidication
[64-68]
. However,
some non-calcifying species (e.g., polychaetes) also
show negative responses to acidication
[26]
, and long-
term experiments show that acidication of the
parental environment can lead to impaired larval
growth in species that are “robust” in shorter term
experiments
[65,67]
. Crustacean larvae with poorly
calcied exoskeletons (e.g., amphipods, barnacles,
crabs) appear tolerant to acidication
[61,69-72]
.
There is limited information on the impact of
increased ocean pCO and temperature on the meta-
morphic transition to a benthic life in marine inver-
tebrates and subsequent early juvenile stages. e
transition to the benthos may be aected by the nega-
tive eect of high CO, as shown by reduced coral
larvae settlement
[73]
. Deleterious eects of ocean
acidication (through smaller or lower weight juve-
niles) have been reported for corals, bivalves, poly-
chaetes and echinoderms
[26,74-79]
, with emerging
evidence that current CO values compared to pre-
industrial levels could already have caused a reduc-
tion in some larval sizes
[79]
. Reduced larval size in
a high pCO ocean would have a negative impact
on feeding and swimming ability and make larvae
more vulnerable to predation.
By contrast, no eects of near-future acidication
were evident for juvenile bivalves Mercenaria merce-
naria, well-fed juvenile Mytilus galloprovincialis
[77]
,
or Mytilus edulis
[80]
. Tolerance of these species to
acidication may reect the adaptation to life in low
pH and highly variable environments
[80]
. Juvenile
crustaceans are comparatively tolerant of acidi-
cation
[70,72]
, although again there is variability
[81-83]
.
Understanding how eects at early life-stages can
“carry-over”
[23]
to inuence growth and reproduc-
tion of the adult remains a signicant challenge and
knowledge gap.
46
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
5.3 SENSORY CAPACITY AND BEHAVIOUR
KEY MESSAGES: 5.3
1. Ocean acidication can alter sensory systems and behaviour in sh and some invertebrates
2. Impacts include the loss of ability to discriminate between important chemical cues
Ocean acidication can have signicant direct and
indirect eects on the behaviour of marine organ-
isms. A potentially serious consequence of rising
pCO is that it can aect sensory systems and behav-
iour of marine sh and some invertebrates
[84,85]
. Reef
sh larvae exposed to elevated CO lose their abil-
ity to discriminate between ecologically impor-
tant chemical cues, such as odours from dierent
habitat types, kin and non-kin, the smell of pred-
ators
[86,87,88]
and visual function
[89,90]
. Response to
auditory cues is altered
[91]
, behavioural lateraliza-
tion is lost,
[92]
and sh are no longer able to learn
[93]
.
Impaired ability to discriminate between olfac-
tory and auditory cues, or attraction to inappro-
priate cues, could have serious consequences for
ability of larvae to successfully transition from the
pelagic to benthic environments. Furthermore,
larvae exposed to elevated CO exhibit bolder and
more risky behaviour once they settle to the reef,
potentially leading to higher mortality from pred-
ators
[94,95]
. Behavioural eects are not restricted to
larvae and juveniles. Recent experiments have shown
that adult reef sh also suer impaired olfactory abil-
ity and altered behaviour when exposed to elevated
Figure 5.4. Effect of acidication on larval development (after 5 days) of the sea urchin Tripneustes gratilla; pH 8.15 at 24°C
represents the control. For this species, a 3°C increase in temperature reduced the negative effect of pH on larval growth and
calcication. Feeding effects were not involved, since early larval growth depends on food reserves from the fertilized egg. From
[60]
.

5. PHYSIOLOGICAL RESPONSES TO OCEAN ACIDIFICATION
47
pCO, with potential eects on predator-prey inter-
actions
[96,97]
, habitat selection
[98]
and homing to rest-
ing sites
[99]
. A wide range of reef sh species appear to
be aected
[95]
, including important sheries species
such as the coral trout Plectropomus leopardus
[100]
.
Impaired behaviour at all life stages occurs as a result
of permanent exposure to CO levels ≥ 600-700
µatm CO, well within the range that could occur
in the ocean this century. e ecosystem eects of
impaired sensory behaviour, altered predator-prey
interactions, and changes in behavioural attributes
is unknown, but has the potential to be signicant,
including for functionally and economically impor-
tant species.
Elevated pCO alters behaviour of sh, and possi-
bly of invertebrates, by interfering with brain
neurotransmitter function
[101]
. Sustained exposure
to elevated CO induces acid-base regulatory changes
in sh that could aect the function of GABA-A
receptors, a major inhibitory neurotransmitter. e
GABA-A receptor is an ion-channel with conduc-
tance for chloride (Cl
-
) and bicarbonate (HCO
-
), and
these two ions are also important to acid-base regu-
lation in sh. Given the ubiquity of GABA-A recep-
tors in marine organisms, there is good reason to
suspect that elevated CO levels could cause behav-
ioural abnormalities in a wide range of marine organ-
isms. One example of GABA-A alteration causing
behavioural problems is in the rocksh Sebastes
diploproa
[102]
, which became more anxious under
future ocean acidication conditions. Interestingly,
sensory behavioural “compensation” may partially
reduce detrimental impacts of ocean acidication
with regard to anti-predator responses
[103]
. With
regard to GABA-A receptors, other organisms that
use Cl
-
and/or HCO
-
to maintain their acid-base
balance when exposed to elevated CO may be at
particular risk, and some invertebrates that are weak
acid-base regulators suer metabolic depression
when exposed to high CO
[104,105]
. Reduced meta-
bolic rate could also inuence a wide range of behav-
iours in these species.
A critical question in assessing the impact of behav-
ioural changes in marine organisms is whether indi-
viduals and populations will be able to acclimate
or adapt to rising concentrations of CO. ere is
some hope that adaptation by selection of tolerant
genotypes may occur, because larval damselsh
reared at 700 µatm CO exhibit considerable vari-
ation in responses to olfactory cues, with approxi-
mately half of the larvae responding like unaected
controls
[93]
. ese individuals have much higher
survivorship when exposed to predators compared
with the individuals that are signicantly aected by
700 µatm CO
[106]
. If this variation has a genetic basis,
we might expect rapid selection of tolerant indi-
viduals throughout the population. Understanding
the basis of variation in responses to elevated CO
among individuals will be key to making predic-
tions about the potential for adaptation to rising
CO levels.
5.4 IMMUNE RESPONSES AND DISEASE
KEY MESSAGES: 5.4
1. Impacts of ocean acidication on immune responses and disease is an emerging eld: few studies
have been performed to date
2. Future ocean acidication has the potential to impact immune functions in marine organisms. It
could also affect the virulence and persistence of pathogens
e majority of early research on the eects of ocean
acidication on marine organisms has focussed
on whole organism, or end point measures of
impact – from assessments of increased mortal-
ity to changes in growth rate or calcication. More
recently however, there has been the realization that

48
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
whilst many organisms can acclimate to increases
in environmental pCO at relevant timescales, this
acclimation might take place at a cost to other phys-
iological processes, such as reproductive investment,
immune function, or activity/ecological function.
As a consequence, recent work has considered
impacts of ocean acidication on other physiological
responses, such as the maintenance of immune func-
tion. To date, this work has focussed on commer-
cially important species (crustaceans and molluscs),
which are being increasingly seen as important for
the maintenance of global food security
[107]
.
Elevated pCO can impact the immune system
of marine organisms indirectly, especially if the
changes have a negative impact on protein synthe-
sis rates, thus reducing the synthesis of key immune
enzymes and peptides. Immune system maintenance
has conventionally been regarded as an energeti-
cally expensive constraint on an organism’s energy
budget
[108]
, and it has been speculated that even
chronic moderate reductions in pH
[109]
could be
signicant, especially in resource-limited environ-
ments. However, early published work in this area
has tended to only consider short-term or acute
impacts, which are of limited value in making predic-
tions of the impact of climate relevant increases in
sea water pCO.
Few studies have gone beyond initial acute shock
responses to consider immune impacts once accli-
mation to the modied environment has taken place,
but the limited few have found a signicant impact
upon bivalve haemocyte functionality
[110]
, acido-
sis and phagocyte numbers in echinoderms (vari-
able between species)
[111]
and that over 6 months,
immunity was impaired in sea stars as evidenced
by reduced phagocytic capacity
[112]
. As environ-
mental factors play a signicant role in determin-
ing the course of infection
[7]
, climate change has the
potential to increase susceptibility to disease
[113]
.
From the limited number of examples that are avail-
able, it can be concluded that there is the potential
for future ocean acidication to have an impact on
the immune function of marine organisms, partic-
ularly with reference to commercially important
shellsh. It could be speculated that this will result
in an increased incidence of disease, particularly
when combined with other stresses typically asso-
ciated with aquaculture.
In conclusion, early research using short-term expo-
sure experiments has suggested that there may be
direct and indirect impacts on the immune func-
tion of marine organisms in a future climate. As
this eld matures, it is imperative that more eort
should focus on identifying the long-term (months to
years) impacts of climate-relevant increases in pCO
to immune function in marine invertebrates, espe-
cially in resource or energy-limited environments.
Future eorts should also establish the impacts to
disease resistance using live pathogen infections,
to establish the real endpoint of immune system
perturbation (mortality), whilst acknowledging that
environmental change can simultaneously aect the
virulence and persistence of pathogens
[114]
.
References
1. Seibel BA, Walsh PJ (2001) Potential impacts of CO
injection on deep-sea biota. Science 294: 64-65.
2. Esbaugh AJ, Heuer R, Grosell M (2012) Impacts of
ocean acidication on respiratory gas exchange and
acid-base balance in a marine teleost, Opsanus beta.
Journal of Comparative Physiology B-Biochemical
Systemic and Environmental Physiology 182:
921-934.
3. Strobel A, Bennecke S, Leo E, Mintenbeck K, Pörtner
HO, et al. (2012) Metabolic shis in the Antarctic sh
Notothenia rossii in response to rising temperature
and pCO. Frontiers in Zoology 9: 28.
4. Stumpp M, Hu M, Casties I, Saborowski R, Bleich M,
et al. (2013) Digestion in sea urchin larvae impaired
under ocean acidication. Nature Climate Change 3:
1044-1049.
5. Fabry V, Seibel B, Feely RA (2008) Impacts of
ocean acidication on marine fauna and ecosystem
processes. Journal of Marine Research 65: 414-432.
6. Hand SC (1992) Metabolic dormancy in aquatic
invertebrates. In: Gilles R (ed). Advances in
Comparative and Environmental Physiology.
Heidelberg: Springer-Verlag.

5. PHYSIOLOGICAL RESPONSES TO OCEAN ACIDIFICATION
49
7. Seibel BA, Walsh PJ (2003) Biological impacts of
deep-sea carbon dioxide injection inferred from
indices of physiological performance. Journal of
Experimental Biology 206: 641-650.
8. Barry JP, Widdicombe S, Hall-Spencer JM (2011)
Eects of ocean acidication on marine biodiversity
and ecosystem function. In: Gattuso JP, Hansson L
(eds). Ocean Acidication. Oxford: Oxford University
Press. 326 p
9. Gattuso JP, Hansson L (eds) (2011) Ocean
Acidication. Oxford: Oxford University Press. 326 p
10. Dickson AG (2010) e carbon dioxide system in
seawater: equilibrium chemistry and measurements.
In: Riebesell U, Fabry VJ, L. H, Gattuso JP (eds).
Guide to Best Practices for Ocean Acidifcation
Research and Data Reporting. Luxembourg:
European Union. pp. 17-40.
11. Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, et
al. (2005) Anthropogenic ocean acidication over
the twenty-rst century and its impact on calcifying
organisms. Nature 437: 681-686.
12. Tambutté S, Holcomb M, Ferrier-Pagès C, Reynaud
S, Tambutté É, et al. (2011) Coral biomineralization:
From the gene to the environment. Journal of
Experimental Marine Biology and Ecology 408:
58-78.
13. Erez J, Reynaud S, Silverman J, Schneider K,
Allemand D (2011) In: Dubinsky Z, Stambler N
(eds). Coral Reefs: An Ecosystem in Transition.
SpringerLink.
14. Tambutte E, Tambutte S, Segonds N, Zoccola
D, Venn A, et al. (2012) Calcein labelling and
electrophysiology: insights on coral tissue
permeability and calcication. Proceedings of the
Royal Society B-Biological Sciences 279: 19-27.
15. Rodolfo-Metalpa R, Houlbrèque F, Tambutté É,
Boisson F, Baggini C, et al. (2011) Coral and mollusc
resistance to ocean acidication adversely aected by
warming. Nature Climate Change 1: 1-5.
16. Ries J (2011) Acid ocean cover up. Nature Climate
Change 1: 294-295.
17. Venn A, Tambutte E, Holcomb M, Allemand D,
Tambutte S (2011) Live tissue imaging shows reef
corals elevate pH under their calcifying tissue relative
to seawater. PLoS ONE 6: e20013.
18. McCulloch M, Falter J, Trotter J, Montagna P (2012)
Coral resilience to ocean acidication and global
warming through pH up-regulation. Nature Climate
Change 2: 623-633.
19. Venn AA, Tambutte E, Holcomb M, Laurent J,
Allemand D, et al. (2013) Impact of seawater
acidication on pH at the tissue-skeleton interface
and calcication in reef corals. Proceedings of the
National Academy of Sciences of the United States of
America 110: 1634-1639.
20. Cohen AL, Holcomb M (2009) Why corals care about
ocean acidication: Uncovering the mechanism.
Oceanography 22: 118-127.
21. Vidal-Dupiol J, Zoccola D, Tambutte E, Grunau
C, Cosseau C, et al. (2013) Genes related to
ion-transport and energy production are upregulated
in response to CO-driven pH decrease in corals:
New insights from transcriptome analysis. PloS ONE
8: e58652.
22. Kaniewska P, Campbell PR, Kline DI, Rodriguez-
Lanetty M, Miller DJ, et al. (2012) Major cellular and
physiological impacts of ocean acidication on a reef
building coral. PLoS ONE 7: e34659.
23. Pechenik JA (1999) On the advantages and
disadvantages of larval stages in benthic marine
invertebrate life cycles. Marine Ecology Progress
Series 177: 269-297.
24. Byrne M (2012) Global change ecotoxicology:
Identication of early life history bottlenecks in
marine invertebrates, variable species responses
and variable experimental approaches. Marine
Environmental Research 76: 3-15.
25. Havenhand J, Dupont S, Quinn GP (2010) Designing
ocean acidication experiments to maximise
inference. In: Riebesell U, Fabry VJ, Hansson
L, Gattuso JP (eds). Guide to Best Practices for
Ocean Acidication Research and Data Reporting.
Luxembourg: European Union. pp. 67-80.
26. Lewis C, Clemow K, Holt WV (2013) Metal
contamination increases the sensitivity of larvae but
not gametes to ocean acidication in the polychaete
Pomatoceros lamarckii (Quatrefages). Marine Biology
160: 2089-2101.
27. Reuter K, Lotterhos KE, Crim RN, ompson
CA, Harley CDG (2010) Elevated pCO increases
sperm limitation and risk of polyspermy in the red
sea urchin Strongylocentrotus franciscanus. Global
Change Biology17:163-171
28. Evans JP, Marshall DJ (2005) Male-by-female
interactions inuence fertilization success and
mediate the benets of polyandry in the sea urchin
Heliocidaris erythrogramma. Evolution 59: 106-112.
29. Palumbi SR (1999) All males are not created equal:
Fertility dierences depend on gamete recognition
polymorphisms in sea urchins. Proceedings of the
National Academy of Sciences of the United States of
America 96: 12632-12637.
30. Hendriks IE, Duarte CM, Álvarez M (2010)
Vulnerability of marine biodiversity to ocean
acidication: A meta-analysis. Estuarine, Coastal and
Shelf Science 86: 157-164.
31. Wittmann AC, Portner H-O (2013) Sensitivities of
extant animal taxa to ocean acidication. Nature
Climate Change 3: 995-1001.

50
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
32. Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo
L, et al. (2013) Impacts of ocean acidication on
marine organisms: quantifying sensitivities and
interaction with warming. Global Change Biology 19:
1884-1896.
33. Byrne M, Soars N, Selvakumaraswamy P, Dworjanyn
SA, Davis AR (2010) Sea urchin fertilization in a
warm, acidied and high pCO ocean across a range
of sperm densities. Marine Environmental Research
69: 234-239.
34. Byrne M, Soars NA, Ho MA, Wong E, McElroy D,
et al. (2010) Fertilization in a suite of coastal marine
invertebrates from SE Australia is robust to near-
future ocean warming and acidication. Marine
Biology 157: 2061-2069.
35. Ho MA, Price C, King CK, Virtue P, Byrne M (2013)
Eects of ocean warming and acidication on
fertilization in the Antarctic echinoid Sterechinus
neumayeri across a range of sperm concentrations.
Marine Environmental Research 90: 136-141.
36. Havenhand JN, Buttler F-R, orndyke MC,
Williamson JE (2008) Near-future levels of ocean
acidication reduce fertilization success in a sea
urchin. Current Biology 18: R651-R652.
37. Havenhand JN, Schlegel P (2009) Near-future levels
of ocean acidication do not aect sperm motility
and fertilization kinetics in the oyster Crassostrea
gigas. Biogeosciences 6: 3009-3015.
38. Schlegel P, Havenhand JN, Gillings MR, Williamson
JE (2012) Individual variability in reproductive
success determines winners and losers under ocean
acidication: A case study with sea urchins. PLoS
ONE 7: e53118.
39. Foo SA, Dworjanyn SA, Poore AGB, Byrne M (2012)
Adaptive capacity of the habitat modifying sea urchin
Centrostephanus rodgersii to ocean warming and
ocean acidication: performance of early embryos.
PLoS ONE 7: e42497.
40. Albright R, Mason B (2013) Projected near-future
levels of temperature and pCO reduce coral
fertilization success. PLoS ONE 8: e56468.
41. Caldwell GS, Fitzer S, Gillespie CS, Pickavance G,
Turnbull E, et al. (2011) Ocean acidication takes
sperm back in time. Invertebrate Reproduction &
Development 55: 217-221.
42. Mita M, Hino A, Yasumasu I (1984) Eect of
temperature on interaction between eggs and
spermatozoa of sea-urchin. Biological Bulletin 166:
68-77.
43. Kupriyanova EK, Havenhand JN (2005) Eects
of temperature on sperm swimming behaviour,
respiration and fertilization success in the serpulid
polychaete, Galeolaria caespitosa (Annelida
: Serpulidae). Invertebrate Reproduction &
Development 48: 7-17.
44. Byrne M, Ho M, Selvakumaraswamy P, Nguyen
HD, Dworjanyn SA, et al. (2009) Temperature, but
not pH, compromises sea urchin fertilization and
early development under near-future climate change
scenarios. Proceedings of the Royal Society B -
Biological Sciences 276: 1883-1888.
45. Ericson Ja, Lamare MD, Morley Sa, Barker M (2010)
e response of two ecologically important Antarctic
invertebrates (Sterechinus neumayeri and Parborlasia
corrugatus) to reduced seawater pH: eects on
fertilisation and embryonic development. Marine
Biology 157: 2689-2702.
46. Byrne M, Ho M, Wong E, Soars NA,
Selvakumaraswamy P, et al. (2011) Unshelled abalone
and corrupted urchins: development of marine
calciers in a changing ocean. Proceedings of the
Royal Society B - Biological Sciences 278: 2376-2383.
47. Kurihara H, Ishimatsu A (2008) Eects of high CO
seawater on the copepod (Acartia tsuensis) through
all life stages and subsequent generations. Marine
Pollution Bulletin 56: 1086-1090.
48. Dupont S, Ortega-Martínez O, orndyke MC
(2010) Impact of near-future ocean acidication on
echinoderms. Ecotoxicology 19: 449-462.
49. Gazeau F, Gattuso J-P, Dawber C, Pronker aE, Peene
F, et al. (2010) Eect of ocean acidication on the
early life stages of the blue mussel Mytilus edulis.
Biogeosciences 7: 2051-2060.
50. Hofmann GE, Barry JP, Edmunds PJ, Gates RD,
Hutchins DA, et al. (2010) e Eect of Ocean
Acidication on Calcifying Organisms in Marine
Ecosystems: An Organism-to-Ecosystem Perspective.
Annual Review of Ecology, Evolution, and
Systematics 41: 127-147.
51. Byrne M, Przeslawski R (2013) Multistressor impacts
of warming and acidication of the ocean on
marine invertebrates’ life histories. Integrative and
Comparative Biology 53: 582-596.
52. Dupont S, Havenhand J, orndyke W, Peck L,
orndyke M (2008) Near-future level of CO-driven
ocean acidication radically aects larval survival
and development in the brittlestar Ophiothrix fragilis.
Marine Ecology Progress Series 373: 285-294.
53. Parker LM, Ross P, O’Connor W (2010) Comparing
the eect of elevated pCO and temperature on the
fertilization and early development of two species of
oysters. Marine Biology 11: 2435-2452.
54. Parker LM, Ross PM, O’Connor WA, Borysko L,
Raos DA, et al. (2012) Adult exposure inuences
ospring response to ocean acidication in oysters.
Global Change Biology 18: 82-92.

5. PHYSIOLOGICAL RESPONSES TO OCEAN ACIDIFICATION
51
55. Byrne M (2011) Impact of ocean warming and ocean
acidication on marine invertebrate life history
stages: vulnerabilities and potential for persistence
in a changing ocean. In: Gibson RN, Atkinson RJA,
Gordon JDM (eds). Oceanography and Marine
Biology: an Annual Review, Vol 49. pp. 1-42.
56. Barros P, Sobral P, Range P, Chicharo L, Matias
D (2013) Eects of sea-water acidication on
fertilization and larval development of the oyster
Crassostrea gigas. Journal of Experimental Marine
Biology and Ecology 440: 200-206.
57. Stumpp M, Wren J, Melzner F, orndyke MC,
Dupont ST (2011) CO induced seawater acidication
impacts sea urchin larval development I: Elevated
metabolic rates decrease scope for growth and induce
developmental delay. Comparative Biochemistry and
Physiology A -Molecular & Integrative Physiology
160: 331-340.
58. Stumpp M, Hu MY, Melzner F, Gutowska MA,
Dorey N, et al. (2012) Acidied seawater impacts
sea urchin larvae pH regulatory systems relevant for
calcication. Proceedings of the National Academy
of Sciences of the United States of America 109:
18192-18197.
59. Byrne M, Lamare M, Winter D, Dworjanyn SA,
Uthicke S (2013) e stunting eect of a high CO
ocean on calcication and development in sea urchin
larvae, a synthesis from the tropics to the poles.
Philosophical Transactions of Royal Society B -
Biological Sciences 368: 20120439.
60. Sheppard Brennand H, Soars N, Dworjanyn Sa,
Davis AR, Byrne M (2010) Impact of ocean warming
and ocean acidication on larval development and
calcication in the sea urchin Tripneustes gratilla.
PLoS ONE 5: e11372.
61. Walther K, Sartoris F, Pörtner H (2011) Impacts
of temperature and acidication on larval calcium
incorporation of the spider crab Hyas araneus from
dierent latitudes (54° vs. 79°N). Marine Biology 158:
2043-2053.
62. Byrne M, Ho MA, Koleits L, Price C, King CK, et al.
(2013) Vulnerability of the calcifying larval stage of
the Antarctic sea urchin Sterechinus neumayeri to
near-future ocean acidication and warming. Global
Change Biology 19: 2264-2275.
63. Gianguzza P, Visconti G, Gianguzza F, Vizzini S, Sara
G, et al. (2014) Temperature modulates the response
of the thermophilous sea urchin Arbacia lixula early
life stages to CO-driven acidication. Mar Environ
Res 93: 70-77.
64. Dupont S, Lundve B, orndyke M (2010) Near
future ocean acidication increases growth rate of
the lecithotrophic larvae and juveniles of the sea star
Crossaster papposus. Journal of Experimental Zoology
314B: 382-389.
65. Byrne M, Gonzalez-Bernat M, Doo S, Foo S, Soars
N, et al. (2013) Eects of ocean warming and
acidication on embryos and non-calcifying larvae
of the invasive sea star Patiriella regularis. Marine
Ecology Progress Series 473: 235-246.
66. Chua CM, Leggat W, Moya A, Baird AH (2013)
Temperature aects the early life history stages of
corals more than near future ocean acidication.
Marine Ecology Progress Series 475: 85-92.
67. Gonzalez-Bernat MJ, Lamare M, Barker M (2013)
Eects of reduced seawater pH on fertilisation,
embryogenesis and larval development in the
Antarctic seastar Odontaster validus. Polar Biology 36:
235-247.
68. Nguyen HD, Doo SS, Soars NA, Byrne A (2012)
Noncalcifying larvae in a changing ocean: warming,
not acidication/hypercapnia, is the dominant
stressor on development of the sea star Meridiastra
calcar. Global Change Biology 18: 2466-2476.
69. Walther K, Anger K, Pörtner HO (2010) Eects
of ocean acidication and warming on the larval
development of the spider crab Hyas araneus from
dierent latitudes ( 54 ° vs . 79 ° N ). Marine Ecology
Progress Series 417: 159-170.
70. Pansch C, Schlegel P, Havenhand J (2013) Larval
development of the barnacle Amphibalanus
improvisus responds variably but robustly to near-
future ocean acidication. Ices Journal of Marine
Science 70: 805-811.
71. Pansch C, Nasrolahi A, Appelhans YS, Wahl M (2012)
Impacts of ocean warming and acidication on the
larval development of the barnacle Amphibalanus
improvisus. Journal of Experimental Marine Biology
and Ecology 420: 48-55.
72. Arnberg M, Calosi P, Spicer JI, Tandberg AHS, Nilsen
M, et al. (2013) Elevated temperature elicits greater
eects than decreased pH on the development,
feeding and metabolism of northern shrimp
(Pandalus borealis) larvae. Marine Biology 160:
2037-2048.
73. Doropoulos C, Diaz-Pulido G (2013) High CO
reduces the settlement of a spawning coral on three
common species of crustose coralline algae. Marine
Ecology Progress Series 475: 93-99.
74. Albright R, Mason B, Langdon C (2008) Eect of
aragonite saturation state on settlement and post-
settlement growth of Porites astreoides larvae. Coral
Reefs 27: 485-490.
75. Albright R, Bland C, Gillette P, Serafy JE, Langdon
C, et al. (2012) Juvenile growth of the tropical sea
urchin Lytechinus variegatus exposed to near-future
ocean acidication scenarios. Journal of Experimental
Marine Biology and Ecology 426: 12-17.

52
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
76. Anlauf H, D’Croz L, O’Dea A (2011) A corrosive
concoction: e combined eects of ocean warming
and acidication on the early growth of a stony coral
are multiplicative. Journal of Experimental Marine
Biology and Ecology 397: 13-20.
77. Talmage SC, Gobler CJ (2011) Eects of elevated
temperature and carbon dioxide on the growth and
survival of larvae and juveniles of three species of
northwest Atlantic bivalves. PLoS ONE 6: e26941.
78. Wolfe K, Dworjanyn SA, Byrne M (2013) Eects of
ocean warming and acidication on survival, growth
and skeletal development in the early benthic juvenile
sea urchin (Heliocidaris erythrogramma). Global
Change Biology 19: 2698-2707.
79. Suwa R, Nojiri Y, Ono T, Shirayama Y (2013) Eects
of low pCO conditions on sea urchin larval size.
Marine Ecology 34: 443-450.
80. omsen J, Casties I, Pansch C, Koertzinger A,
Melzner F (2013) Food availability outweighs
ocean acidication eects in juvenile Mytilus edulis:
laboratory and eld experiments. Global Change
Biology 19: 1017-1027.
81. Findlay HS, Kendall MA, Spicer JI, Widdicombe
S (2010) Post-larval development of two intertidal
barnacles at elevated CO and temperature. Marine
Biology 157: 725-735.
82. Findlay HS, Kendall Ma, Spicer JI, Widdicombe S
(2010) Relative inuences of ocean acidication and
temperature on intertidal barnacle post-larvae at
the northern edge of their geographic distribution.
Estuarine, Coastal and Shelf Science 86: 675-682.
83. Poore AGB, Graba-Landry A, Favret M, Brennand
HS, Byrne M, et al. (2013) Direct and indirect
eects of ocean acidication and warming on a
marine plant-herbivore interaction. Oecologia 173:
1113-1124.
84. Bria M, de la Haye K, Munday PL (2012) High CO
and marine animal behaviour: Potential mechanisms
and ecological consequences. Marine Pollution
Bulletin 64: 1519-1528.
85. Munday PL, McCormick MI, Nilsson GE (2012)
Impact of global warming and rising CO levels on
coral reef shes: what hope for the future? Journal of
Experimental Biology 215: 3865-3873.
86. Munday PL, Dixson DL, Donelson JM, Jones GP,
Pratchett MS, et al. (2009) Ocean acidication
impairs olfactory discrimination and homing
ability of a marine sh. Proceedings of the National
Academy of Sciences of the United States of America
106: 1848-1852.
87. Dixson DL, Munday PL, Jones GP (2010) Ocean
acidication disrupts the innate ability of sh to
detect predator olfactory cues. Ecology letters 13:
68-75.
88. Munday PL, Cheal AJ, Dixson DL, Rummer JL,
Fabricius KA (2014) Behavioural impairment in reef
shes caused by ocean acidication at CO seeps.
Nature Climate Change 4: 487-492
89. Knight K (2014) Ocean acidication will interfere
with sh eyes. Journal of Experimental Biology 217:
311-312.
90. Chung WS, Marshall NJ, Watson SA, Munday PL,
Nilsson GE (2014) Ocean acidication slows retinal
function in a damselsh through interference with
GABA(A) receptors. Journal of Experimental Biology
217: 323-326.
91. Simpson SD, Munday PL, Wittenrich ML, Manassa R,
Dixson DL, et al. (2011) Ocean acidication erodes
crucial auditory behaviour in a marine sh. Biology
Letters 7: 917-920.
92. Domenici P, Allan B, McCormick MI, Munday PL
(2012) Elevated carbon dioxide aects behavioural
lateralization in a coral reef sh. Biology Letters 8:
78-81.
93. Ferrari MCO, Manassa RP, Dixson DL, Munday
PL, McCormick MI, et al. (2012) Eects of ocean
acidication on learning in coral reef shes. PLoS
ONE 7: e31478.
94. Munday PL, Dixson DL, McCormick MI, Meekan
M (2010) Replenishment of sh populations is
threatened by ocean acidication. Proceedings of the
National Academy of Science of the United States of
America 107: 12930-12934.
95. Ferrari MCO, Dixson DL, Munday PL, McCormick
MI, Meekan MG, et al. (2011) Intrageneric variation
in antipredator responses of coral reef shes aected
by ocean acidication: implications for climate
change projections on marine communities. Global
Change Biology 17: 2980-2986.
96. Cripps IL, Munday PL, McCormick MI (2011) Ocean
acidication aects prey detection by a predatory reef
sh. PLoS ONE 6: e22736.
97. Ferrari MCO, McCormick MI, Munday PL, Meekan
MG, Dixson DL, et al. (2011) Putting prey and
predator into the CO equation - qualitative and
quantitative eects of ocean acidication on predator-
prey interactions. Ecology Letters 14: 1143-1148.
98. Devine BM, Munday PL (2013) Habitat preferences
of coral-associated shes are altered by short-term
exposure to elevated CO.Marine Biology 160:
1955-1962.
99. Devine BM, Munday PL, Jones GP (2012) Homing
ability of adult cardinalsh is aected by elevated
carbon dioxide. Oecologia 168: 269-276.
100. Munday PL, Pratchett MS, Dixson DL, Donelson
JM, Endo GGK, et al. (2013) Elevated CO aects
the behavior of an ecologically and economically
important coral reef sh. Marine Biology 160:
2137-2144.

5. PHYSIOLOGICAL RESPONSES TO OCEAN ACIDIFICATION
53
101. Nilsson GE, Dixson DL, Domenici P, McCormick
MI, Sorensen C, et al. (2012) Near-future carbon
dioxide levels alter sh behaviour by interfering with
neurotransmitter function. Nature Climate Change 2:
201-204.
102. Hamilton TJ, Holcombe A, Tresguerres M (2014)
CO-induced ocean acidication increases anxiety
in rocksh via alteration of GABAA receptor
functioning. Proceedings of the Royal Society B -
Biological Sciences 281: 20132509.
103. Lonnstedt OM, Munday PL, McCormick MI, Ferrari
MCO, Chivers DP (2013) Ocean acidication and
responses to predators: can sensory redundancy
reduce the apparent impacts of elevated CO on sh?
Ecology and Evolution 3: 3565-3575.
104. Widdicombe S, Spicer J (2008) Predicting the impact
of ocean acidication on benthic biodiversity:
What can animal physiology tell us? Journal of
Experimental Marine Biology and Ecology 366:
187-197.
105. Melzner F, Gutowska MA, Langenbuch M, Dupont
S, Lucassen M, et al. (2009) Physiological basis for
high CO tolerance in marine ectothermic animals:
pre-adaptation through lifestyle and ontogeny?
Biogeosciences 6: 2313-2331.
106. Munday PL, McCormick MI, Meekan M, Dixson
DL, Watson S-A, et al. (2012) Selective mortality
associated with variation in CO tolerance in a
marine sh. Ocean Acidication 1: 1-5.
107. Stentiford GD, Neil DM, Peeler EJ, Shields JD, Small
HJ, et al. (2012) Disease will limit future food supply
from the global crustacean shery and aquaculture
sectors. Journal of Invertebrate Pathology 110:
141-157.
108. Folstad I, Karter AJ (1992) Parasites, bright males,
and the immunocompetence handicap. American
Naturalist 139: 603-622.
109. Li C-C, Chen J-C (2008) e immune response
of white shrimp Litopenaeus vannamei and its
susceptibility to Vibrio alginolyticus under low and
high pH stress. Fish & Shellsh Immunology 25:
701-709.
110. Matozzo V, Chinellato A, Munari M, Finos L, Bressan
M, et al. (2012) First evidence of immunomodulation
in bivalves under seawater acidication and increased
temperature. PLoS ONE 7: e33820.
111. Dupont S, orndyke M (2012) Relationship between
CO-driven changes in extracellular acid-base
balance and cellular immune response in two polar
echinoderm species. Journal of Experimental Marine
Biology and Ecology 424: 32-37.
112. Hernroth B, Baden S, orndyke MC, Dupont S
(2011) Immune suppression of the echinoderm
Asterias rubens (L.) following long-term ocean
acidication. Aquatic Toxicology 103: 222-224.
113. Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson
AP, et al. (2002) Ecology - Climate warming and
disease risks for terrestrial and marine biota. Science
296: 2158-2162.
114. Snieszko SF (1974) Eects of environmental stress on
outbreaks of infectious-diseases of shes. Journal of
Fish Biology 6: 197-208.

54
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
KEY MESSAGES
1. Responses are highly variable, but many benthic species generally have lower growth rates and
survival under projected future acidication
2. For corals, many studies show reduction in growth and increased sensitivity with ocean
acidication, but this response is variable
3. Most adult molluscs are negatively impacted by ocean acidication, but some species can live in
low pH
4. Many macroalgae species are tolerant or may benet from future ocean acidication
Benthic ecosystems comprise some of the key ocean
communities that we rely upon for food and ecosys-
tem services, and occur throughout the world’s
oceans from the splash zones of all shores to the
deepest waters. While none will be able to avoid
future ocean acidication, it remains unclear how
changes in ocean conditions will aect the composi-
tion and function of benthic communities in dier-
ent environments.
Although environmental conditions are less variable
through time in the deep ocean than at the surface,
there is considerable spatial variability, since carbon-
ate chemistry of deep-sea waters is strongly related
to large-scale thermohaline circulation patterns.
Consequently, abyssal pH is ~0.2 pH units lower
in the Pacific than in the Atlantic. Basin-scale
dierences in carbonate saturation are even larger.
Whereas the aragonite saturation boundary (the
depth at which seawater is corrosive to aragonite) is
deeper than 2000 m for much of the North Atlantic,
in the North East Pacic it shoals to ~ 200 m depth.
e most widespread and abundant benthic commu-
nities in the global ocean are those in the deep sea,
and some of these are expected to be particularly
vulnerable to ocean acidication.
Benthic communities will be aected by the direct
and indirect responses of its inhabitants to low pH,
reduced carbonate saturation, or related parame-
ters. Meta-analyses of laboratory and eld experi-
ments
[1,2]
, and observations in naturally high-CO
marine environments
[3,4]
have shown lower rates of
growth, survival, or other performance measures for
many benthic organisms in acidied waters, although
with considerable variability between species and
higher taxonomic groups.
Many other factors and
indirect eects contribute to sensitivity to ocean
acidication
[2]
, including biological processes that
may oset potentially detrimental impacts
[5]
.
A recent meta-analysis
[1]
compared responses of
benthic organisms at different CO concentra-
tions: the commonest response up to around 1000
ppm was a negative impact; at higher concentra-
tions the proportion of negative impacts increased
greatly (Figure 6.1).
Crustaceans appear less sensi-
tive to smaller increases in CO than other groups
(Figure 6.1)
[2,6]
, and may be aected through indi-
rect inuences, such as eects on food palatability
[7]
.
eir tolerance appears to include juvenile as well
as adult growth stages, although there is variability
(see Chapter 5). Further discussion below focuses
on the more sensitive taxa, corals, echinoderms and
molluscs (Figure 6.1), although recognizing that the
responses of benthic plants and microbes can also
be of high ecological importance.
6. IMPACTS OF OCEAN ACIDIFICATION ON BENTHIC
COMMUNITIES

6. IMPACTS OF OCEAN ACIDIFICATION ON BENTHIC COMMUNITIES
55
e sensitivity of entire benthic communities to
ocean acidication is also expected to be linked to
the scale of natural variation in the environment.
Populations inhabiting highly variable habitats,
such as coastal systems, may possess the pheno-
typic and genetic diversity to tolerate and perhaps
thrive across the range of variation in carbonate
parameters. Observations of pH variability from
coastal and open-ocean sites show large dierences
in the magnitude of variation
[8]
, with only small vari-
ation (< 0.1 pH units) in the open ocean over 30
days, but large daily variation (up to 0.8 pH units)
at coastal sites over a single day, driven principally
by the photosynthesis / respiration balance. It is
therefore crucial that future studies expand upon
current research to represent and compare dier-
ent habitats globally.
Organisms that can create substantial calcifying
structures, such as coral reefs, calcifying algae and
polychaete structures, are considered key habitats to
study, as they support substantial associated biodi-
versity and provide other functions such as coastal
protection. Coral reefs are the best studied and one
of the best known examples of calcareous structures,
and as such have received most research attention
to date. However, other structures, such as vermetid
reefs (built by gastropods and coralline algae) could
have impaired recruitment and increased dissolu-
tion under future CO scenarios
[9]
. Considering the
socioeconomic and ecological importance of calcar-
eous structures other than coral reefs, it is also crit-
ical that they undergo further research.
6.1 CORALS
Tropical coral reef ecosystems represent one of the
most biodiverse habitats in the oceans, directly or
indirectly supporting about a third of all marine
species
[10,11]
. Occurring in both cold- and warm-
water environments, stony corals are key engineers
of the coral reef ecosystem, contributing to the reef’s
structural framework and the exchange of nutrients
between several trophic levels
[12]
. In light of their
ecological and economic importance at regional and
global scales, corals are one of the most intensively
studied groups of calciers in terms of their calci-
cation response to ocean acidication.
Cold-water corals, also oen referred to as deep-
water corals, are found in all of the world oceans,
[13-
15]
with new information on their distribution being
updated through national mapping programmes such
as MAREANO in Norway (www.mareano.no), e
Figure 6.1. Sensitivity of animal taxa to ocean acidication. Fractions (%) of corals, echinoderms, molluscs and crustaceans
exhibiting negative, no or positive effects on performance indicators reect individual tness in response to increased CO
2
. Bars
above columns denote count ratios signicantly associated with pCO
2
. Modied from
[1]
. Reprinted by permission from Macmillan
Publishers Ltd: Nature Climate Change 3: 995-1001, © 2013

56
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
Deep Sea Coral Research and Technology Program
(USA), and through European Community projects
including HERMES, HERMIONE and CoralFISH.
Figure 6.2 demonstrates the distribution of frame-
work-forming cold-water corals such as Lophelia
pertusa, but does not represent the occurrence of
the myriad of other cold-water coral species. Many
cold-water coral species require hard substrate for
attachment and growth, and in general they thrive
where there are strong currents that supply them
with food, disperse eggs, sperm and larvae, remove
waste products and keep the surfaces of the coral free
of sediments. is means that they are oen found
on parts of the continental slope or on the summits
of seamounts where currents are strongest. It has
oen been assumed that these deep-water habitats
are relatively stable in terms of their carbonate chem-
istry, but recent evidence suggests that within and
between habitats, a signicant amount of variabil-
ity can exist, even on a daily basis
[16,17]
.
Cold-water coral reef systems are oen structur-
ally complex environments including gorgonians,
stylasterid corals (lace corals), sponges and a vari-
ety of sh and invertebrates in the Arctic and sub-
Arctic
[19,20]
, and are dened as vulnerable marine
ecosystems. Impact or damage to these ecosystems
may lower the local biodiversity and diminish the
possibility for many species to nd shelter and feed-
ing grounds.
Due to the uptake of anthropogenic CO in the
ocean both the aragonite saturation horizon (ASH)
and calcite saturation horizon (CSH) are becoming
shallower. In places, Lophelia pertusa already lives
very close to the ASH, for example in the Gulf of
Mexico
[21]
. By the end of the century, many deep-sea
corals are predicted to be in water undersaturated
with calcium carbonate
[22,23]
. It has been estimated
that >95 % of corals were above the depth of the
ASH in pre-industrial times (year 1765), but by
the end of the century, only ~30 % of coral loca-
tions will be found above this saturation depth
[22]
(Figure 6.3). While gorgonians and stylasterids have
not been well studied with regard to ocean acidi-
cation compared to Lophelia pertusa, their calcium
carbonate and proteinaceous structures also merit
further attention with regard to ocean acidication.
e limited evidence available for how ocean acidi-
cation will impact cold-water corals such as Lophelia
pertusa indicates that in the short term, projected
decreases in pH can decrease metabolism and
growth
[24-26]
, but over 6-12 months, L. pertusa does
Figure 6.2. Global distribution of reef framework–forming cold-water corals. From
[18]
. Reprinted with permission from AAAS.

6. IMPACTS OF OCEAN ACIDIFICATION ON BENTHIC COMMUNITIES
57
not display reductions in growth when subjected to
predicted end of the century CO conditions
[25-27]
.
However, these long-term experiments still do not
account for any impact on future reproduction of
cold-water corals, so the question remains whether
key species such as L. pertusa can merely temporar-
ily tolerate future conditions, or whether they can
thrive under projected future climates. e current
low abundance of cold-water corals below the ASH
suggests not, since the increased energetic demands
for living below the ASH cannot usually be met.
us the long-term survival of cold-water corals
below future calcium carbonate saturation depths
seems unlikely
[28]
.
Although scleractinian corals can up-regulate their
extracellular pH within the calicoblastic layer at
the sites of calcication through energy intensive
processes
[29-32]
, the regulation only applies for coral
skeleton that is covered by living coral tissue. Cold-
water coral reefs are typically composed of a signi-
cant amount of bare, dead skeleton beneath the living
material (Figure 6.4), which would start to dissolve
in undersaturated conditions, and be eroded with
increased eciency by bio-eroding sponges
[33]
. us
future changing conditions could potentially have
large impacts upon current cold-water coral habi-
tats and associated biodiversity
[34]
.
For warm-water corals, many studies demonstrate
a reduction in growth (net calcication rates) in
response to ocean acidication
[29, 35-39]
. However, this
is not a ubiquitous response, with dierent species
exhibiting negative responses
[40]
, no measureable
response
[41]
, or variable responses
[35]
to reduced
pH
[42]
. Examining coral growth rates through time
by analysing coral cores remains dicult, due to
ontogenetic eects and growth variability from coral
age and size
[43]
. Furthermore, responses may be non-
linear, such that there may be no response until a
“tipping point” is reached
[44]
.
Meta-analysis has proved very useful in synthesiz-
ing the data obtained from these multiple studies,
and in identifying the factors that may explain vari-
ation between them
[2,45-47]
. e general conclusion of
these analyses and other reviews
[48-51]
is that warm-
water corals are sensitive to ocean acidication,
Figure 6.3. Modelled depth of aragonite saturation horizon (ASH) in relation to locations
of cold-water corals (green triangles). Left: ASH depth for the year 1995; pCO
2
= 365
ppmv. Right: Projected ASH for the year 2099; pCO
2
= 788 ppmv. Black areas appearing
in Southern Ocean and North Pacic in bottom panel indicate where ASH depth has
reached the surface. Contours indicate diversity for 706 species of azooxanthellate
(without microalgal symbiont) corals. From
[22]
.
Figure 6.4. Image of live Lophelia pertusa with underlying dead
framework (Rockall Bank, NE Atlantic). Source: Heriot-Watt
University/UK Ocean Acidication research programme.
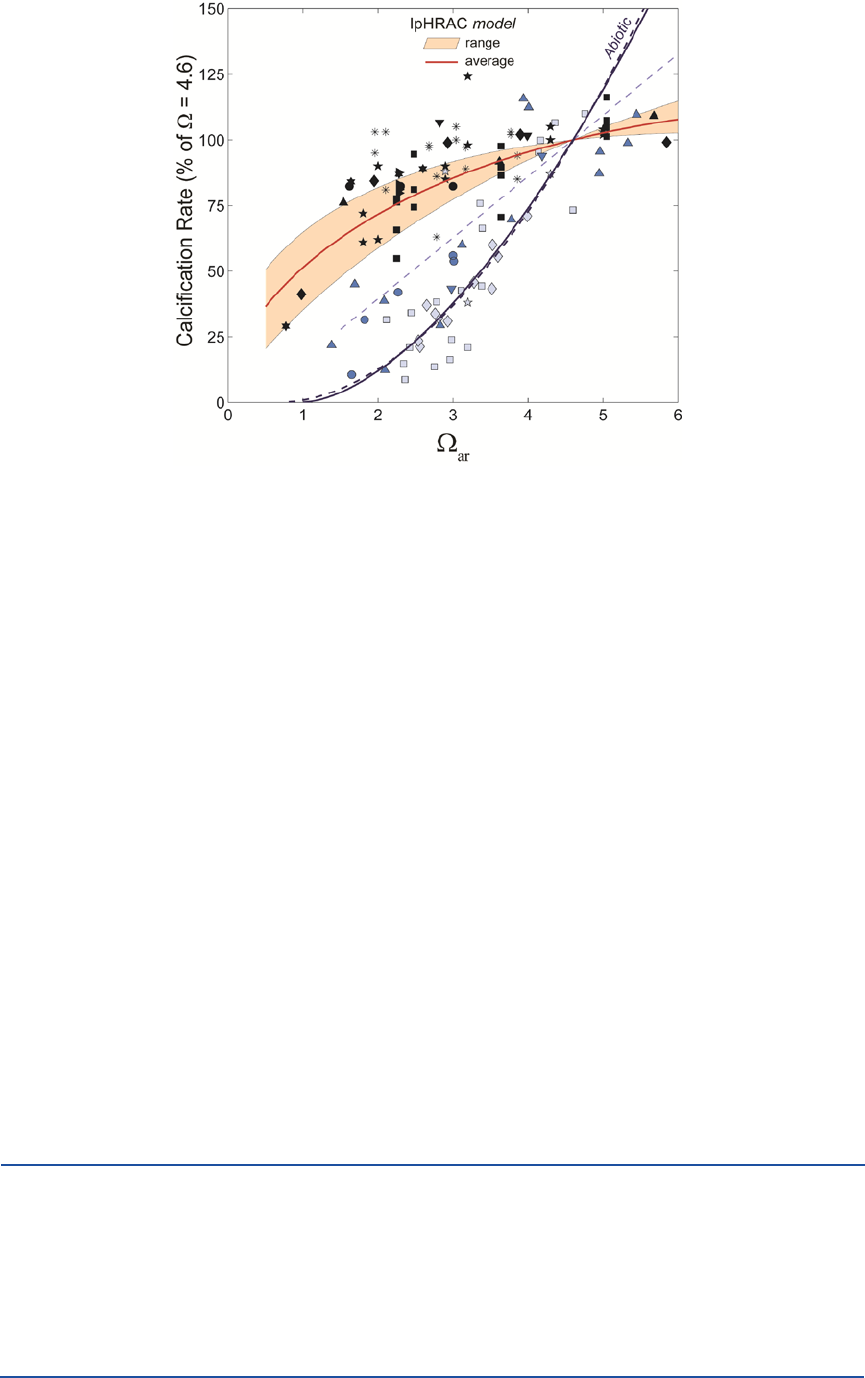
58
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
with declines in coral calcication associated with
declining aragonite saturation state and seawater
pH
[29, 51,52]
. However major questions remain, notably
how and why coral calcication is sensitive to ocean
acidication. is is the subject of recent research
initiatives that investigate the mechanism of calci-
cation, with attention focussing on internal pH
regulation
[29]
(Figure 6.5).
Insight into the physiological mechanisms that corals
use to cope with ocean acidication may explain
inter-species dierences in sensitivity, and may
help to predict winners and losers in a higher CO
ocean. e capacity to regulate ions / pH under
ocean acidication may be a dening physiological
trait that facilitates future survival, with emerging
evidence from coral skeletons that there is strong
inter-specic variability in their ability to up-regu-
late pH control
[53]
. For some species, temperature and
ocean acidication can act synergistically in reduc-
ing calcication rates, i.e., more than the additive
eects of these factors acting alone
[54]
.
us future
studies have to consider multiple stressors to deter-
mine the future fate of these key ecosystems.
Further discussion of the physiological responses
of corals is given in section 5.1, and of the socio-
economic consequences of the loss of warm-water
coral reefs in section 8.2.
6.2 MOLLUSCS
Bivalve molluscs were identied in early ocean
acidication research to be strongly aected by
ocean acidication
[55]
, with many species negatively
impacted by relatively low pH decreases (Figure 6.1).
Reduced growth rates can lead to knock-on eects
such as increased predation for smaller oysters
[56]
.
Nevertheless, while acute experiments without an
acclimation period can result in strong reductions in
calcication
[57]
, adult mytilid mussels can maintain
signicant calcication in longer-term incubations
Figure 6.5. Percentage change in scleractinian coral calcication rates (relative to seawater Ω = 4.6) plotted against aragonite
saturation state, Ω
ar
. Coral species showing low sensitivity (black symbols) are able to regulate their internal pH much more
effectively than those showing high sensitivity (dark blue symbols). Data for calcareous algae (light blue symbols) also included for
comparison. From
[29]
; the IpHRAC model developed by those authors combines information on internal pH regulation of calcifying
uid with abiotic calcication, enabling carbonate precipitation rates to be quantied as a function of seawater saturation state
and temperature; the range of values relates to the variability in species’ ability to regulate their internal pH. Adapted by permission
from Macmillan Publishers Ltd: Nature Climate Change 2: 623-627, © 2012

6. IMPACTS OF OCEAN ACIDIFICATION ON BENTHIC COMMUNITIES
59
with realistic food regimes even when the seawater
is undersaturated with respect to calcium carbon-
ate
[58-60]
. Some mussel species (e.g., Bathymodiolus
brevior) are able to grow close to deep-sea hydro-
thermal vents, at pH values as low as 5.4.
is feature
indicates great biological control over the calcica-
tion process
[61]
, together with a fundamental role
of the external organic cover, the periostracum, to
enable persistence at such extreme locations. A simi-
lar role of the periostracum has been suggested for
coastal Mytilus edulis, which can also calcify at high
rates even when calcium carbonate is undersatu-
rated
[59]
. Results from the western Baltic Sea indicate
that successful settlement and dominance of mytilid
mussels and other calcifying invertebrates is possible
at seawater pCO values similar to those projected
for the end of the century
[59,62]
. Where reductions
in growth and calcication are observed, energy
budget reallocation may be the cause
[63]
, or poten-
tially increased oxidative stress
[64]
. Future research
should thus focus on determining cellular energy
budgets to analyse energetic trade-os.
e impact of ocean acidication to larval stages of
bivalves is potentially of great importance to their
continued survival
[65-67]
. is topic is covered in more
depth in section 5.2.
6.3 ECHINODERMS
Echinoderms have been extensively studied with
respect to sensitivity to simulated ocean acidica-
tion, and in particular with respect to their larval
stages (see section 5.2). While early life stages of
some species react with severely increased mortality
to experimental ocean acidication
[68]
, most species
respond with slight reductions in larval growth
[69,70]
.
When exposed to simulated ocean acidication,
echinoderms experience energy budget reallocation,
with only few examples of increased mortality
[71]
.
Moderately elevated pCO (<1,000 µatm) can
increase feeding and growth rates in intertidal sea
stars
[72]
; however, other studies have shown reduced
investment in growth, calcication, reproduction
or immunity
[73-77]
. Despite studies highlighting the
potential for long-term, trans-generational and
adaptive responses of echinoderms to ocean acid-
ication
[69,70,78,79]
, little research attention has been
devoted to these factors; future studies should reect
this.
6.4 MACROALGAE, SEAGRASS AND BENTHIC MICROBIOTA
Macroalgae and seagrass are important components
of many coastal ecosystems, as primary produc-
ers and habitat engineers. ey generally grow in
relatively shallow coastal waters, where they are
likely to experience wide daily and seasonal vari-
ability in seawater pH
[80]
. e complex interaction
between a wide range of environmental changes in
coastal waters, including eutrophication and hypoxia,
may lead to faster declines in pH with increasing
atmospheric CO
[81]
. e responses of benthic ora
to ocean acidication can be positive or negative,
depending on their distribution and species.
Macroalgae – seaweeds – can be calcareous or non-
calcareous (eshy).
Ocean acidication is gener-
ally detrimental to calcareous algae
[82-84]
, with loss
of structural integrity
[85,86]
and associated changes
in growth forms that can aect competitive inter-
actions
[87]
.
Nevertheless, some calcareous algae may
thrive under naturally low pH conditions (e.g.,
around CO vents), even aer decalcication
[88]
.
Increased productivity has also been observed under
experimental ocean acidication and is likely to be
due to higher CO favouring photosynthesis – with
a similar stimulatory eect also occurring for non-
calcareous species
[4,80,82,89-91]
.
Future impacts do, however, need to be considered
in the context of temperature changes, which may be
highly detrimental to larger non-calcareous macroal-
gae (kelp) in temperate regions
[92]
.
Eects of ocean
acidication on the consumption of seaweeds by

60
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
grazers could also be important. Around CO vents,
invertebrate grazing pressure is reduced; however,
there is also evidence that high CO can decrease
the production of protective phenolic substances
used to deter grazers
[93]
.
is apparent contradic-
tion could be due to grazing itself stimulating the
production of the deterrents, an issue that warrants
further attention.
Seagrasses seem likely to benet from ocean acidi-
cation
[92,94]
and can be abundant around CO seeps
(Figure 6.6).
Elsewhere, they are able to substantially
modify the carbonate chemistry of their environ-
ment through photosynthesis (involving CO uptake)
[95]
, resulting in substantial diel variability in seawa-
ter pH
[96,97]
. In the case of tropical seagrasses, they
could potentially mitigate ocean acidication for
adjacent coral reef systems by elevating pH (by up
to 0.38 pH units) at tidal intervals
[96]
.
e eects of ocean acidication on benthic micro-
biota (including benthic diatoms and dinoagel-
lates, as well as single-celled animals and bacteria)
are not well understood.
Large gradients in pH natu-
rally occur at the seawater-seaoor interface and
in the upper layers of sediment
[98]
; it may there-
fore be thought that future carbonate chemistry
changes would be unlikely to have signicant impact.
Nevertheless, experimental studies on benthic fora-
minifera show functionally important morpholog-
ical changes at 750ppm and 1000ppm CO
[99]
, and
major changes have been found in the abundance
and diversity of benthic microphytobenthos
[100]
,
epilithic bacteria
[101]
and foraminifera
[102,103]
around
natural CO seeps.
Near-future extinctions of benthic
microbiota, similar to those occurring at the PETM
(Chapter 4), are therefore considered likely
[103]
.
References
1. Wittmann AC, Portner H-O (2013) Sensitivities of
extant animal taxa to ocean acidication. Nature
Climate Change 3: 995-1001.
2. Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo
L, et al. (2013) Impacts of ocean acidication on
marine organisms: quantifying sensitivities and
interaction with warming. Global Change Biology 19:
1884-1896.
3. Hall-Spencer JM, Rodolfo-Metalpa R, Martin S,
Ransome E, Fine M, et al. (2008) Volcanic carbon
dioxide vents show ecosystem eects of ocean
acidication. Nature 454: 96-99.
4. Fabricius KE, Langdon C, Uthicke S, Humphrey
C, Noonan S, et al. (2011) Losers and winners in
coral reefs acclimatized to elevated carbon dioxide
concentrations. Nature Climate Change 1: 165-169.
5. Hendriks IE, Duarte C, Álvarez M (2010)
Vulnerability of marine biodiversity to ocean
acidication: A meta-analysis. Estuarine, Coastal and
Shelf Science 86: 157-164.
6. Whiteley NM (2011) Physiological and ecological
responses of crustaceans to ocean acidication.
Marine Ecology Progress Series 430: 257-271.
7. Poore AGB, Graba-Landry A, Favret M, Brennand
HS, Byrne M, et al. (2013) Direct and indirect
eects of ocean acidication and warming on a
marine plant-herbivore interaction. Oecologia 173:
1113-1124.
8. Hofmann GE, Smith JE, Johnson KS, Send U, Levin
LA, et al. (2011) High-frequency dynamics of ocean
pH: A multi-ecosystem comparison. PLoS ONE 6:
e28983.
9. Milazzo M, Rodolfo-Metalpa R, Chan VB, Fine M,
Alessi C, et al. (2014) Ocean acidication impairs
vermetid reef recruitment. Scientic Reports 4: 4189.
10. Reaka-Kudla ML (1997) Global biodiversity of coral
reefs: a comparison with rainforests. In: Reaka-Kudla
ML, Wilson DE (eds). Biodiversity II: Understanding
and Protecting Our Biological Resources: Joseph
Henry Press.
11. Reaka-Kudla ML (2001) Known and unknown
biodiversity, risk of extinction and conservation
strategy in the sea. In: Bendell-Young L, Gallaugher P
(eds). Waters in Peril.
Spinger, p 19-33.
12. Dubinsky Z, Stambler N (eds) (2011) Coral Reefs: An
Ecosystem in Transition. Springer.
Figure 6.6. Seagrass and a natural CO
2
seep. Source: Giorgio
Caramanna.

6. IMPACTS OF OCEAN ACIDIFICATION ON BENTHIC COMMUNITIES
61
13. Rogers AD (1999) e biology of Lophelia pertusa
(Linnaeus 1758) and other deep-water reef-
forming corals and impacts from human activities.
International Review of Hydrobiology 84: 315-406.
14. Freiwald A, Fosså H, Grehan A, Koslow T, Roberts
JM (2004) Cold-water Coral Reefs. Out of Sight - No
Longer out of Mind. UNEP-WCMC, Cambridge, UK
15. Roberts JM, Wheeler A, Freiwald A, Cairns SD
(2009) Cold-Water Corals: e Biology and Geology
of Deep-Sea Coral Habitats: Cambridge University
Press.
16. Findlay HS, Artioli Y, Navas JM, Hennige SJ, Wicks
LC, et al. (2013) Tidal downwelling and implications
for the carbon biogeochemistry of cold-water corals
in relation to future ocean acidication and warming.
Global Change Biology 19: 2708-2719.
17. Findlay HS, Hennige SJ, Wicks LC, Navas JM,
Woodward EMS, et al. (2014) Fine-scale nutrient and
carbonate system dynamics around cold-water coral
reefs in the northeast Atlantic. Scientic Reports 4:
3671.
18. Roberts JM, Wheeler AJ, Freiwald A (2006) Reefs of
the deep: the biology and geology of cold-water coral
ecosystems. Science 312: 543-547.
19. Fosså H, Kutti T (2010) Impacts of human activities
on benthic habitats. Arctic Biodiversity Trends:
Selected Indicators of Change. CAFF International
Secretariat, Akureyri, Iceland..
20. Henry L, Navas JM, Hennige S, Wicks LC, Roberts JM
(2013) Shark spawning grounds on cold-water coral
reefs: a compelling case for protection of vulnerable
marine ecosystems. Biological Conservation 161:
67-70.
21. Lunden JJ, Georgian SE, Cordes EE (2013) Aragonite
saturation states at cold-water coral reefs structured
by Lophelia pertusa in the northern Gulf of Mexico.
Limnology and Oceanography 58: 354-362.
22. Guinotte JM, Orr JC, Cairns S, Freiwald A, Morgan
L, et al. (2006) Will human-induced changes in
seawater chemistry alter the distribution of deep-
sea scleractinian corals? Frontiers in Ecology and the
Environment 4: 141-146.
23. Turley CM, Roberts JM, Guinotte JM (2007) Corals
in deep-water: will the unseen hand of ocean
acidication destroy cold-water ecosystems? Coral
Reefs 26: 445-448.
24. Maier C, Hegeman J, Weinbauer MG (2009)
Calcication of the cold-water coral Lophelia pertusa
under ambient and reduced pH. Biogeosciences 6:
1671-1680.
25. Form AU, Riebesell U (2011) Acclimation to ocean
acidication during long-term CO exposure in the
cold-water coral Lophelia pertusa. Global Change
Biology 18: 843-853.
26. Hennige S, Wicks LC, Keamenos N, Bakker DCE,
Findlay HS, et al. (2014) Short term metabolic and
growth responses of the cold-water coral Lophelia
pertusa to ocean acidication. Deep Sea Research II
99: 27-35.
27. Maier C, Schubert A, Sanchez MMB, Weinbauer MG,
Watremez P, et al. (2013) End of the century pCO
levels do not impact calcication in Mediterranean
cold-water corals. PLoSs ONE 8: e62655.
28. resher RE, Tilbrook B, Fallon S, Wilson NC,
Adkins J (2011) Eects of chronic low carbonate
saturation levels on the distribution, growth and
skeletal chemistry of deep-sea corals and other
seamount megabenthos. Marine Ecology Progress
Series 442: 87-99.
29. McCulloch M, Falter J, Trotter J, Montagna P (2012)
Coral resilience to ocean acidication and global
warming through pH up-regulation. Nature Climate
Change 2: 623-633.
30. Venn A, Tambutté E, Lotto S, D (2009) Imaging
intracellular pH in a reef coral and symbiotic
anemone. Proceedings of the National Academy
of Science of the United States of America 106:
16574-16579.
31. Anagnostou E, Huang KF, You CF, Sikes EL, Sherrell
RM (2012) Evaluation of boron isotope ratio as a pH
proxy in the deep sea coral Desmophyllum dianthus:
Evidence of physiological pH adjustment. Earth and
Planetary Science Letters 349: 251-260.
32. McCulloch M, Trotter J, Montagna P, Falter J, Dunbar
R, et al. (2012) Resilience of cold-water scleractinian
corals to ocean acidication: Boron isotopic
systematics of pH and saturation state up-regulation.
Geochimica et Cosmochimica Acta 87: 21-34.
33. Wisshak M, Schönberg CHL, Form A, Freiwald A
(2012) Ocean acidication accelerates reef bioerosion.
PLoS ONE 7: e45124.
34. Jackson EL, Davies AJ, Howell KL, Kershaw PJ, Hall-
Spencer JM (2014) Future-proong marine protected
area networks for cold water coral reefs.
ICES Journal
of Marine Science, doi: 10.1093/icesjms/fsu099.
35. Gattuso J-P, Frankignoulle M, Bourge I, Romaine S,
Buddemeier RW (1998) Eect of calcium carbonate
saturation of seawater on coral calcication. Global
and Planetary Change 18: 37-46.
36. Kleypas JA, Langdon C (2006) Coral reefs and
changing seawater chemistry. In: Phinney JT, Hoegh-
Guldberg O, Kleypas JA, Skirving W, Strong A
(eds). Coral Reefs and Climate Change: Science
and Management. Washington DC: American
Geophysical Union.
37. Krief S, Hendy EJ, Fine M, Yam R, Meibom A, et
al. (2010) Physiological and isotopic responses
of scleractinian corals to ocean acidication.
Geochimica et Cosmochimica Acta 74: 4988-5001.

62
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
38. Langdon C, Atkinson MJ (2005) Eect of elevated
pCO on photosynthesis and calcication of corals
and interactions with seasonal change in temperature/
irradiance and nutrient enrichment. Journal of
Geophysical Research - Oceans 110: C09S07.
39. Marubini F, Ferrier-Pagès C (2003) Suppression of
skeletal growth in scleractinian corals by decreasing
ambient carbonate-ion concentration: a cross-family
comparison. Proceedings of the Royal Society B:
Biological Sciences 270: 179-184.
40. Ohde S, Hossain M (2004) Eect of CaCO
(aragonite) saturation state of seawater on
calcication of Porites coral. Geochemical Journal 38:
613-621.
41. Reynaud S, Leclercq N, Romaine- S (2003) Interacting
eects of CO partial pressure and temperature on
photosynthesis and calcication in a scleractinian
coral. Global Change Biology 9: 1660-1668.
42. Wicks LC, Roberts JM (2012) Benthic invertebrates
in a high-CO world. Oceanography and Marine
Biology: An Annual Review 50: 127-188.
43. Ridd PV, da Silva ET, Stieglitz T (2013) Have coral
calcication rates slowed in the last twenty years?
Marine Geology 346: 392-399.
44. Ries JB, Cohen AL, McCorkle DC (2010) A nonlinear
calcication response to CO-induced ocean
acidication by the coral Oculina arbuscula. Coral
Reefs 29: 661-674.
45. Hendriks IE, Duarte CM, Álvarez M (2010)
Vulnerability of marine biodiversity to ocean
acidication: A meta-analysis. Estuarine, Coastal and
Shelf Science 86: 157-164.
46. Kroeker KJ, Kordas RL, Crim RN, Singh GG (2010)
Meta-analysis reveals negative yet variable eects of
ocean acidication on marine organisms. Ecology
Letters 13: 1419-1436.
47. Chan NCS, Connolly SR (2013) Sensitivity of coral
calcication to ocean acidication: a meta-analysis.
Global Change Biology 19: 282-290.
48. Erez J, Reynaud S, Silverman J, Schneider K,
Allemand D (2011) In: Dubinsky Z, Stambler N
(eds). Coral Reefs: An Ecosystem in Transition.
SpringerLink.
49. Hoegh-Guldberg O, Mumby P, Hooten A, Steneck
R, Greeneld P, et al. (2007) Coral reefs under rapid
climate change and ocean acidication. Science 318:
1737.
50. Pandol JM, Connolly SRSR, Marshall DJ, Cohen
AL (2011) Projecting coral reef futures under global
warming and ocean acidication. Science 333:
418-418.
51. Andersson AJ, Gledhill D (2013) Ocean acidication
and coral reefs: eects on breakdown, dissolution, and
net ecosystem calcication. Annual Review Marine
Science 5: 321-348.
52. Andersson AJ, Kuner IB, Mackenzie FT, Jokiel PL,
Rodgers KS, et al. (2009) Net Loss of CaCO from
a subtropical calcifying community due to seawater
acidication: mesocosm-scale experimental evidence.
Biogeosciences 6: 1811-1823.
53. McCulloch M, Trotter J, Montagna P, Falter J, Dunbar
R, et al. (2012) Resilience of cold-water scleractinian
corals to ocean acidication: Boron isotopic
systematics of pH and saturation state up-regulation.
Geochimica et Cosmochimica Acta 87: 21-34.
54. Rodolfo-Metalpa R, Houlbrèque F, Tambutté É,
Boisson F, Baggini C, et al. (2011) Coral and mollusc
resistance to ocean acidication adversely aected by
warming. Nature Climate Change 1: 1-5.
55. Gazeau F, Parker LM, Comeau S, Gattuso J-P,
O’Connor WA, et al. (2013) Impacts of ocean
acidication on marine shelled molluscs. Marine
Biology 160: 2207-2245.
56. Sanford E, Gaylord B, Hettinger A, Lenz EA, Meyer
K, et al. (2014) Ocean acidication increases the
vulnerability of native oysters to predation by invasive
snails. Proceedings of the Royal Society B – Biological
Sciences 281: 20132681.
57. Gazeau F, Quiblier C, Jansen J, Gattuso J-P (2007)
Impact of elevated CO on shellsh calcication.
Geophysical Research 34: 101029
58. Michaelidis B, Ouzounis C, Paleras A, Portner HO
(2005) Eects of long-term moderate hypercapnia on
acid-base balance and growth rate in marine mussels
Mytilus galloprovincialis. Marine Ecology Progress
Series 293: 109-118.
59. omsen J, Gutowska Ma (2010) Calcifying
invertebrates succeed in a naturally CO enriched
coastal habitat but are threatened by high levels of
future acidication. Biogeosciences 7: 5119-5156.
60. Melzner F, Stange P, Truebenbach K, omsen J,
Casties I, et al. (2011) Food supply and seawater pCO
impact calcication and internal shell dissolution in
the blue mussel Mytilus edulis. PLoS ONE 6: e24223.
61. Tunniclie V, Davies KTA, Buttereld DA, Embley
RW, Rose JM, et al. (2009) Survival of mussels in
extremely acidic waters on a submarine volcano.
Nature Geoscience 2: 344-348.
62. omsen J, Casties I, Pansch C, Koertzinger A,
Melzner F (2013) Food availability outweighs
ocean acidication eects in juvenile Mytilus edulis:
laboratory and eld experiments. Global Change
Biology 19: 1017-1027.
63. omsen J, Melzner F (2010) Moderate seawater
acidication does not elicit long-term metabolic
depression in the blue mussel Mytilus edulis. Marine
Biology 157: 2667-2676.

6. IMPACTS OF OCEAN ACIDIFICATION ON BENTHIC COMMUNITIES
63
64. Tomanek L, Zuzow MJ, Ivanina AV, Beniash E,
Sokolova IM (2011) Proteomic response to elevated
p-CO level in eastern oysters, Crassostrea virginica:
evidence for oxidative stress. Journal of Experimental
Biology 214: 1836-1844.
65. Talmage SC, Gobler CJ (2011) Eects of elevated
temperature and carbon dioxide on the growth and
survival of larvae and juveniles of three species of
northwest Atlantic bivalves. PLoS ONE 6: e26941.
66. Talmage SC, Gobler CJ (2010) Eects of past, present,
and future ocean carbon dioxide concentrations
on the growth and survival of larval shellsh.
Proceedings of the National Academy of Sciences of
the United States of America 107: 17246-17251.
67. Gazeau F, Gattuso J-P, Greaves M, Eldereld H, Peene
J, et al. (2011) Eect of carbonate chemistry alteration
on the early embryonic development of the Pacic
oyster (Crassostrea gigas). PLoS ONE 6: e23010.
68. Dupont S, Havenhand J, orndyke W, Peck L,
orndyke M (2008) Near-future level of CO-driven
ocean acidication radically aects larval survival
and development in the brittlestar Ophiothrix fragilis.
Marine Ecology Progress Series 373: 285-294.
69. Dupont S, Ortega-Martínez O, orndyke MC
(2010) Impact of near-future ocean acidication on
echinoderms. Ecotoxicology 19: 449-462.
70. Byrne M, Lamare M, Winter D, Dworjanyn SA,
Uthicke S (2013) e stunting eect of a high CO
ocean on calcication and development in sea urchin
larvae, a synthesis from the tropics to the poles.
Philosophical Transactions of Royal Society B -
Biological Sciences 368: 20120439.
71. Shirayama Y, ornton H (2005) Eect of increased
atmospheric CO on shallow water marine benthos.
Journal of Geophysical Research-Oceans 110:
C09S08.
72. Gooding RA, Harley CDG, Tang E (2009) Elevated
water temperature and carbon dioxide concentration
increase the growth of a keystone echinoderm.
Proceedings of the National Academy of Sciences of
the United States of America 106: 9316-9321.
73. Hernroth B, Baden S, orndyke MC, Dupont S
(2011) Immune suppression of the echinoderm
Asterias rubens (L.) following long-term ocean
acidication. Aquatic Toxicology 103: 222-224.
74. Appelhans YS, omsen J, Pansch C, Melzner F, Wahl
M (2012) Sour times: seawater acidication eects
on growth, feeding behaviour and acid-base status of
Asterias rubens and Carcinus maenas. Marine Ecology
Progress Series 459: 85-98.
75. Wood HL, Spicer JI, Widdicombe S (2008) Ocean
acidication may increase calcication rates, but at
a cost. Proceedings of the Royal Society of London
Series B - Biological Sciences 275: 1767-1773.
76. Findlay HS, Wood HL, Kendall MA, Spicer JI,
Twitchett RJ, et al. (2011) Comparing the impact of
high CO on calcium carbonate structures in dierent
marine organisms. Marine Biology Research 7:
565-575.
77. Stumpp M, Trübenbach K, Brennecke D, Hu
MY, Melzner F (2012) Resource allocation and
extracellular acid-base status in the sea urchin
Strongylocentrotus droebachiensis in response to CO
induced seawater acidication. Aquatic toxicology
110-111C: 194-207.
78. Sunday JM, Crim RN, Harley CDG, Hart MW (2011)
Quantifying rates of evolutionary adaptation in
response to ocean acidication. PLoS ONE 6: e22881.
79. Dupont S, Dorey N, Stumpp M, Melzner F,
orndyke M (2013) Long-term and trans-life-cycle
eects of exposure to ocean acidication in the green
sea urchin Strongylocentrotus droebachiensis. Marine
Biology 160: 1835-1843.
80. Porzio L, Buia MC, Hall-Spencer JM (2011) Eects
of ocean acidication on macroalgal communities.
Journal of Experimental Marine Biology and Ecology
400: 278-287.
81. Bauer JE, Cai WJ, Raymond PA, Bianchi TS,
Hopkinson CS, et al. (2013) e changing carbon
cycle of the coastal ocean. Nature 504: 61-70.
82. Gao K, Aruga Y, Asada K, Ishihara T, Akano T, et al.
(1993) Calcication in the articulated coralline alga
Corallina pilulifera, with special reference to the eect
of elevated CO
2
concentration. Marine Biology 117:
129-132.
83. Kuner IB, Andersson AJ, Jokiel PL, Rodgers KuS,
Mackenzie FT (2008) Decreased abundance of
crustose coralline algae due to ocean acidication.
Nature Geoscience 1: 114-117.
84. Martin S, Gattuso J-P (2009) Response of
Mediterranean coralline algae to ocean acidication
and elevated temperature. Global Change Biology 15:
2089-2100.
85. Kamenos NA, Burdett HL, Aloisio E, Findlay HS,
Martin S, et al (2013) Coralline algal structure is more
sensitive to rate, rather than magnitude, of ocean
acidication. Global Change Biology 19: 3621-3628
86. McCoy SJ, Ragazzola F (2014) Skeletal trade-os in
coralline algae in response to ocean acidication.
Nature Climate Change, doi: 10.1038/nclimate2273
87. McCoy SJ, Pster CA (2014) Historical comparisons
reveal altered competitive interactions in a guild of
crustose coralline algae. Ecology Letters 17: 475-483
88. Johnson VR, Russell BD, Fabricius KE, Brownlee
C, Hall-Spencer JM (2012) Temperate and tropical
brown macroalgae thrive, despite decalcication,
along natural CO gradients. Global Change Biology
18: 2792-2803.

64
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
89. Connell SD, Russell BD (2010) e direct eects of
increasing CO and temperature on non-calcifying
organisms: increasing the potential for phase shis
in kelp forests. Proceedings of the Royal Society B:
Biological Sciences 277: 1409-1415.
90. Roleda MY, Morris JN, McGraw CM, Hurd CL (2012)
Ocean acidication and seaweed reproduction:
increased CO ameliorates the negative eect of
lowered pH on meiospore germination in the
giant kelp Macrocystis pyrifera (Laminariales,
Phaeophyceae). Global Change Biology 18: 854-864.
91. Gao K, Aruga Y, Asada K, Ishihara T, Akano T, et al.
(1991) Enhanced growth of the red alga Porphyra-
Yezoensis Ueda in high CO concentrations. Journal
of Applied Phycology 3: 355-362.
92. Brodie J, Williamson CJ, Smale DA, Kamenos
NA, Mieszkowska N et al (2014) e future of the
northeast Atlantic benthic ora in a high CO world.
Ecology and Evolution, doi: 10.1002/ece3.1105
93. Arnold T, Mealey C, Leahey H, Miller AW, Hall-
Spencer JM, et al. (2012) Ocean acidication and the
loss of phenolic substances in marine plants. PLoS
ONE 7: e35107.
94. Koch M, Bowes G, Ross C, Zhang X-H (2013)
Climate change and ocean acidication eects on
seagrasses and marine microalgae. Global Change
Biology 19: 103-132
95. Gao KS, Helbling EW, Hader DP, Hutchins DA
(2012) Responses of marine primary producers
to interactions between ocean acidication, solar
radiation, and warming. Marine Ecology Progress
Series 470: 167-189.
96. Unsworth RKF, Collier CJ, Henderson GM, McKenzie
LJ (2012) Tropical seagrass meadows modify
seawater carbon chemistry: implications for coral
reefs impacted by ocean acidication. Environmental
Research Letters 7: 024026.
97. Hendriks IE, Olsen YS, Ramajo L, Basso L, Steckbauer
A, et al. (2013) Photosynthetic activity buers ocean
acidication in seagrass meadows. Biogeosciences 11:
333-346.
98. Widdicombe S, Spicer JI, Kitidis V (2011) Eects of
ocean acidicatioon on sedident fauna. In: Gattuso
J-P, Hansson L (eds) Ocean Acidication, Oxford:
Oxford Univrersity Press, p 176-191
99. Khanna N, Godbold JA, Austin W, Paterson DM
(2013) the impact of ocean acidication on the
functional morphology of foraminifera. PLoS ONE 8:
e83118
100. Johnson VR, Brownlee C, Rickaby REM, Graziano
M, Milazzo M, Hall-Spencer JM (2013) Responses of
marine benthic microalgae to elevated CO. Marine
Biology 160: 1813-1824
101. Taylor JD, Ellis R, Milazzo M, Hall-Spencer JM,
Cunlie M (2014) Intertidal epilithic bacteria
diversity changes along a naturally occurring carbon
dioxide and pH gradient. FEMS Microbial Ecology,
doi: 10.111/1574-6941.12368
102. Dias BB, Hart MB, Smart CW, Hall-Spencer JM
(2010) Modern seawater acidication: the response
of foraminifera to high-CO
2
conditions in the
Mediterranean Sea. Journal of the Geological Society
167: 843-846.
103. Uthicke S, Momigliano P, Fabricius KE (2013) High
risk of extinction of benthic foraminifera in this
century due to ocean acidication.
Scientic Reports
3: 1769

7. IMPACTS OF OCEAN ACIDIFICATION ON PELAGIC COMMUNITIES
65
7.1. PLANKTON
KEY MESSAGES
1. Non-calcifying phytoplankton may benet from future ocean acidication
2. Calcifying phytoplankton such as coccolithophores exhibit variable responses to future ocean
acidication
3. Mesocosms combining both calcifying and non-calcifying phytoplankton show enhanced net
primary production under elevated CO
2
4. Bacterial responses to ocean acidication are uncertain, but any changes could affect nutrient
cycling
5. Planktonic foraminifera and pteropods are expected to experience decreased calcication rates, or
dissolution under projected future conditions
6. Impacts on foraminifera may decrease efciency of future carbon transport between the sea
surface and seaoor
Plankton – driing organisms – are taxonomically
diverse, comprising phytoplankton (photosynthetic
algae and bacteria), zooplankton (invertebrates and
unicellular animals that spend their whole life in the
water column, as well as larval sh, and the juve-
niles and gametes of many benthic organisms), and
heterotrophic bacteria. ese plankton, calciers
and non-calciers, form a key component of the
marine food chain and also play an important role
in biogeochemical cycling.
Biocalcication (by both phyto- and zooplankton)
aects the ocean carbon cycle by assisting the export
of organic matter from the upper ocean and its burial
in deep-sea sediments. Sedimentologists studying the
ux of particles collected in deep-sea sediment traps
have found that “ballasting” of organic matter aggre-
gates by biominerals may facilitate the ux of organic
carbon from the upper ocean to the seaoor
[1,2]
. If
there is a signicant decrease of biocalcication by
planktonic organisms as a result of ocean acidica-
tion, then a likely secondary eect is reduced export
of organic carbon from the surface ocean and reduc-
tion of the capacity of the ocean to buer the rise in
anthropogenic carbon dioxide (see also section 8.3).
Phytoplankton and bacteria
Non-calcifying phytoplankton. ese organisms
form a signicant proportion of the phytoplankton
and include diatoms, cyanobacteria and dinoagel-
lates, including many harmful algal bloom (HAB)
species. e stimulating eects of increased CO on
photosynthesis and carbon xation have been noted
in all of these groups
[3-6]
. Increased CO could also
aect mitochondrial and photorespiration (which
produce CO); therefore the net eect on primary
production needs to account for both CO xation
and loss
[7,8]
. It is hypothesised that an increase in
CO will be of overall benet to phytoplankton, as
the increased CO in external seawater will reduce
7. IMPACTS OF OCEAN ACIDIFICATION ON
PELAGIC COMMUNITIES

66
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
CO diusion leakage from biological cells (where
the CO is concentrated) to the surrounding seawa-
ter
[3]
. However, photosynthetic mechanisms vary
widely between photosynthetic organisms
[9]
, and
this may lead to a shi in community composition
in the future
[10,11]
. Assessing whether HAB species
will be among those that will benet from future
environmental change remains a key focus for future
research, as there is some evidence suggesting that
the release of toxic compounds could increase
[12,13]
,
or that the lack of carbon concentrating mechanisms
in many HAB species will be of benet to them in
future climates
[14]
.
Calcifying phytoplankton. Of the calcifying algae,
coccolithophores have received most interest.
Coccolithophores are a group of unicellular phyto-
plankton, which produce calcite plates called cocco-
liths (Figure 7.1).
eir cells are typically 5 to 20 µm
across, and can be present in abundances of tens of
thousands to millions per litre in the photic zone.
ey form a major component of the phytoplank-
ton in relatively oligotrophic waters and are biogeo-
chemically important as carbonate producers; they
are also extensively studied by geologists, since chalk
is predominantly composed of fossil coccoliths.
Some species of coccolithophores (such as Emiliania
huxleyi) can readily be grown in laboratory cultures,
and more than 40 research papers on the impact of
ocean acidication on coccolithophores have been
published. Early experimental work with laboratory
cultures and large-scale semi-enclosed eld cultures
(mesocosms), suggested there was a reduction in
calcication with increasing pCO
[15-18]
, with poten-
tial synergistic impacts of increased solar UV radia-
tion due to thinner coccoliths
[19]
. Several ecological
studies have indicated that variations in carbon-
ate saturation state might inuence the population
dynamics and distribution of modern coccolitho-
phores, such as the timing of blooms
[20]
, and absence
of coccolithophores from the Baltic Sea
[21]
and parts
of the Antarctic
[22]
.
It has been suggested that cocco-
lith mass in E. huxleyi and closely related species
is controlled by saturation state in the ocean both
currently and historically
[23]
.
However, the changes in calcication, growth rate
and cell size by dierent strains of E. huxleyi to
elevated pCO conditions can be highly variable,
due to shiing balances in potential positives and
negatives for photosynthesis and calcication
[24]
.
Culture work on coccolithophore species other than
E. huxleyi has also shown variable responses, with
some species apparently unaected by experimen-
tal ocean acidication
[25]
. Importantly, recent studies
indicate that coccolithophores may be able to adapt
to changing conditions, even on the relatively rapid
timescales at which they are occurring
[26,27]
Conicting results have also been found from eld
and geological evidence. Two studies of sediments
from the past 200 years have provided evidence for
increased calcication of coccolithophores over this
time period despite the rise in atmospheric CO,
or even as a (counter-intuitive) response to it
[24,28]
.
Figure 7.1. Left: Drawing of a single cell of Emiliania huxleyi showing coccoliths (black) forming within an intracellular vesicle
before being extruded to form the extracellular coccosphere (image: Peter Westbroek). Right: Scanning electron micrograph of an E.
huxleyi coccosphere.

7. IMPACTS OF OCEAN ACIDIFICATION ON PELAGIC COMMUNITIES
67
Bacteria.
While some planktonic bacteria are photo-
synthetic (e.g., Synechococcus, Prochlorococcus),
and can therefore be considered as phytoplankton,
most are heterotrophic, obtaining nutrition from
organic material.
ey therefore play a key role in
nutrient cycling. ey can either be “free-living”, or
associated with particles, including other compo-
nents of the plankton. A signicant proportion of
the phytoplankton-derived organic carbon ends up
as dissolved organic carbon (DOC), and this can be
taken up by heterotrophic bacteria. e amount and
growth of such bacteria determines the fraction of
DOC that can be re-introduced into the food web
through subsequent grazing
[3]
.
e response of bacteria to projected future changes
is relatively unstudied compared to the calcifying
plankton, but recent studies on surface-living bacte-
ria which form biolms suggests that future changes
will alter bacterial community composition
[31]
. Future
nutrient cycling may also change depending on
whether bacterial communities change signicantly
as pH decreases, which could have direct impacts
upon nutrient cycling between benthic and pelagic
ecosystems
[32]
.
Zooplankton
ere are two main groups of biocalcifying zooplank-
ton, pteropods and foraminifera, both of which have
been the subject of research on the potential eects
of ocean acidication.
Pteropods are a group of planktonic gastropods
(snails) living in the upper layers of the ocean. e
normal gastropod foot is modied into a pair of
swimming wing-like ns, giving them the common
name sea-butteries, and the shell may be elabo-
rately modied
[33]
(Figure 7.2). Pteropods occur
throughout the global ocean but they are most abun-
dant in sub-Arctic and sub-Antarctic to Antarctic
Box 7.1 Svalbard mesocosms case study
Large-scale mesocosms provide invaluable data on how communities of both calcifying and non-calcifying organisms
will fare under future conditions. Mesocosms have been successfully deployed for ocean acidication studies at a
range of locations, including Svalbard, Norway, to assess impacts of high CO
2
over ecologically relevant timescales
under close-to-natural conditions. The Svalbard results indicated that under high CO
2
/ low pH, phytoplankton
community composition changed but the microzooplankton community exhibited high tolerance
[29]
. Importantly, net
carbon uptake by phytoplankton was enhanced, but the systems were pushed towards overall negative effects on
export potential
[30]
.
Experimental mesocosms at Svalbard.
Source: Maike Nicolai, GEOMAR.
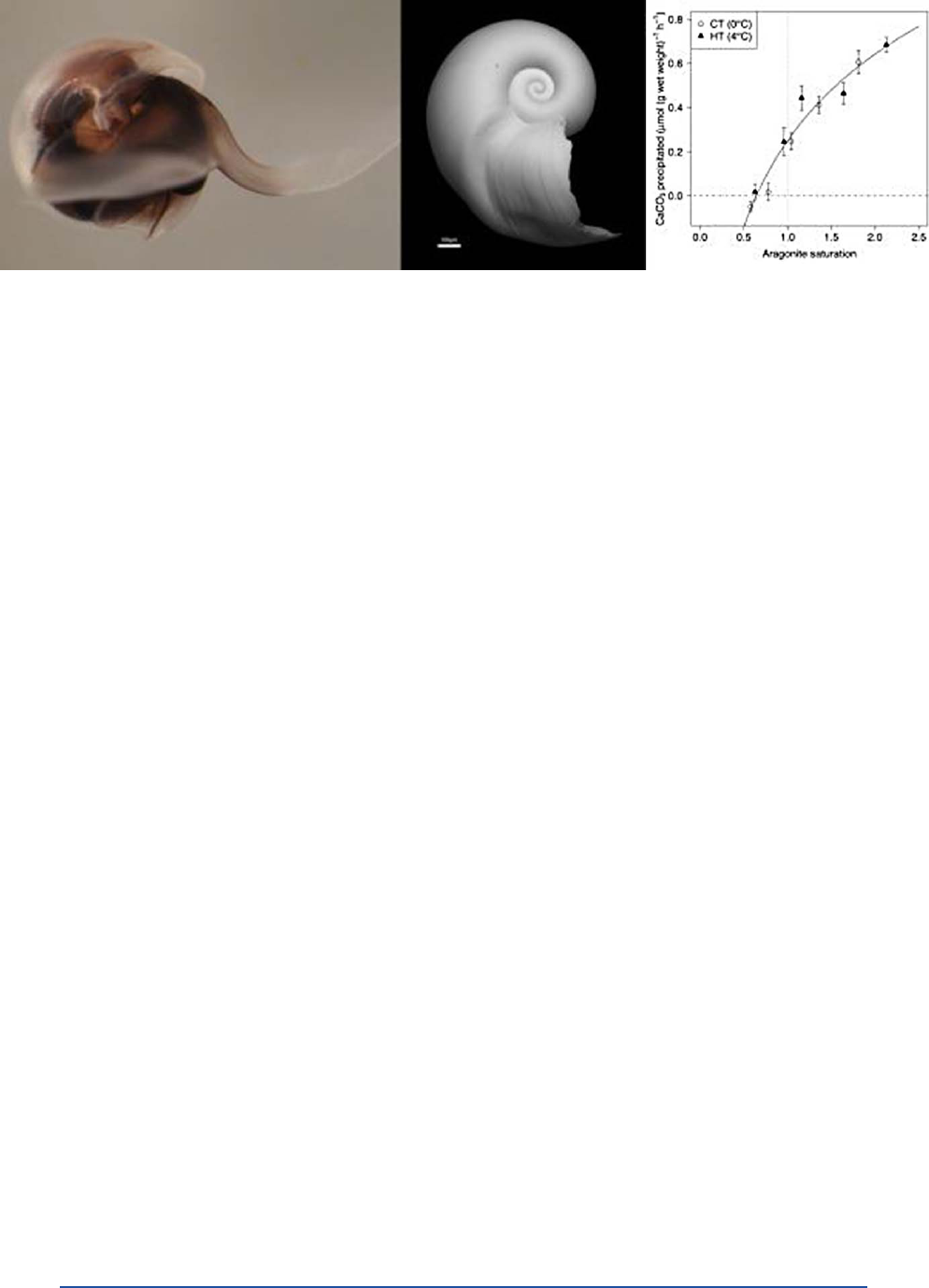
68
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
waters, where they can form a signicant part of
the zooplankton and are important food stocks for
sh and other predators
[34]
. Pteropods have shells
formed of aragonite rather than calcite. e combi-
nation of thin aragonitic shells
[35]
and their abun-
dant occurrence in the Arctic and Southern Oceans
makes them likely to be one of the rst groups of
organisms to be severely aected by ocean acidi-
cation. at is because, as discussed in section 3.2,
undersaturation will rst occur at high latitudes, a
combination of the direct eect of low temperatures
on CO solubility as well as the initially low carbon-
ate ion concentrations
[36]
.
Shipboard incubations have demonstrated that ptero-
pod shell dissolution (erosion) could occur, which
has now been conrmed under eld conditions in
the Antarctic
[36,37]
and northeast Pacic
[38]
at
aragonite
< 1.0. In addition to no calcication occurring when
seawater is undersaturated, it has now also been
demonstrated that calcication may be inhibited
when
aragonite
values are > 1.0
[39-41]
. e vulnerabil-
ity of pteropods to ocean acidication and warm-
ing has been demonstrated for the Arctic pteropod
Limacina helicina, in which shell growth was reduced
and degradation increased at moderately elevated
temperature and pCO (1100 µatm)
[42]
despite some
regulatory capacity to ameliorate these eects
[43]
, and
in the sub-Arctic, where biomass of the dominant
pteropod has decreased notably
[44]
. A modeling study
combined predicted aragonite saturation states for
the end of the century with data on the likely impact
on pteropod calcication, and concluded that “there
appears little future for high-latitude shelled ptero-
pods”
[45]
, which will impact organisms which utilize
these as a food source
[46]
.
Foraminifera are a group of unicellular pelagic (and
benthic) animals forming chambered calcite shells.
e shells are elegant structures typically 0.1 - 0.5
mm across; many species have a halo of delicate
radial spines supporting a mass of protoplasm, gas
bubbles and symbiotic algae (Figure 7.3). Although
they usually only form a minor component of the
total zooplankton, they leave a prolic record of their
existence since their shells sink readily aer death
and form one of the main components of deep-sea
sediments
[47]
. is makes them important contrib-
utors to the ballasting eect, and a group of major
interest to geologists, both as rock-forming organ-
isms and as recorders of ocean chemistry.
Laboratory experiments have shown that carbonate
concentration has signicant impact upon plank-
tonic foraminiferal calcication, with decreases in
shell thickness and weight occurring at levels well
above
calcite
= 1
[48-52]
. Such an eect is also indi-
cated by most, but not all, geological studies
[53-55]
.
Furthermore, eld studies comparing modern plank-
ton from the water column with pre-industrial popu-
lations in the surface sediment have indicated that
marked reductions in shell weight have already
occurred
[54,56]
. Research on tropical benthic fora-
minifera has shown greatest vulnerability to ocean
acidication amongst non-symbiont bearing species
with hyaline or porcelaneous shells
[57]
.
Figure 7.2. Left: A living pteropod from the Arctic (image Vicky Peck, BAS). Centre: The shell of a juvenile pteropod from the South
Atlantic.
Right: Data from laboratory culture experiments on shell growth rate of Limacina helicina incubated under aragonite
saturation states equivalent to those seen in the Arctic at present day (ca 2.0) to the year 2100 (<1.0), from
[41]
.

7. IMPACTS OF OCEAN ACIDIFICATION ON PELAGIC COMMUNITIES
69
e shell mass and thickness of foraminifera are
also controlled by other factors such as tempera-
ture, depth and gametogenic calcite formation, and
to date only a few studies exist on the interaction of
such factors with ocean acidication.
Nevertheless,
the overall evidence strongly suggests that ocean
acidication will have a signicant eect on plank-
tonic foraminifera and hence on their role in ballast-
ing organic carbon uxes.
Copepods.
Direct eects of elevated pCO on cope-
pods have only recently gained attention, and our
knowledge of their response to ocean acidication
remains limited. Copepods are holoplanktonic crus-
taceans that have a maximum size of ~1 cm and
are the most abundant group in marine zooplank-
ton communities worldwide, where they are the
predominant link in pelagic food webs between
primary production and higher trophic levels
[59,60]
.
In controlled experiments, reproductive success (i.e.,
egg production and hatching) decreased at high CO
concentrations (>1000 µatm) compared to low CO
levels
[61-66]
. However, in Arctic mescosm experiments
over 30 days, abundance and stage composition of
Calanus spp., Oithona similis, Acartia longiremis
and Microsetella norvegica did not change with CO
concentrations, indicating that possible eects of
predicted changes in CO were not strong enough to
be reected in the population dynamics
[67]
, although
the grazing rates of Calanus spp. decreased with
increasing CO
[68]
. Other, more sensitive species,
such as Centropages tenuiremis, increased respira-
tion and grazing rates at 1000 µatm, likely to meet
increased energetic demands
[69]
.
On the basis of such results it has been concluded
that direct eects of CO on copepods (and other
non-calciying heterotrophic plankton) may not
be as potentially severe as for calcifying organisms.
However, dierential sensitivity across develop-
mental stages has recently been reported for Acartia
tonsa
[70]
, which needs to be investigated for other
species.
Furthermore, if algal biochemical or species
composition and thus food quality changes due to
increasing pCO, limitations in food quality may
reduce the reproductive success of copepods
[71]
. us,
non-calcifying organisms may also be impacted by
ocean acidication via trophic interactions.
Figure 7.3. Left: Drawing of a modern planktonic foraminifera surrounded by a halo of bubbles and symbiotic algae supported by spines
[58]
.
Centre: Scanning electron micrograph of the shell of juvenile planktonic foraminifera. Right: Laboratory culture data variation in shell wall
thickness in Orbulina universa cultured under bicarbonate conditions equivalent to those from the modern ocean (~ 250µmol kg
-1
) to those
anticipated in 2100 (~ 100 µmolkg
-1
), and under rather more extreme conditions.
From
[50]
.

70
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
7.2 FISH, SQUID AND CUTTLEFISH
KEY MESSAGES
1. Most sh are likely able to maintain sufcient O
2
delivery under future conditions, but cephalopod
metabolism may be reduced
2. Ocean acidication causes sensory and behavioural impairment in many sh species
3. Juvenile life stages appear more susceptible to future ocean acidication
Nektonic (swimming) organisms are those that can
move independently of water currents, as opposed
to plankton, which are more passive. Although sh
represent the majority of nektonic organisms that
have been studied with regard to ocean acidica-
tion, cephalopods are also important in terms of
abundance and economic value.
Fish are generally considered to be more resilient
to direct eects of ocean acidication than many
other marine organisms because they do not have
an extensive skeleton of calcium carbonate, and they
possess well-developed mechanisms for acid-base
regulation
[72]
. Indirect eects of ocean acidication,
such as through “bottom up” changes in the food
web, thus need to be considered in future studies as
well. Fish compensate for acidosis (increased acidity
in blood or tissues) by transport of acid-base rele-
vant ions, mostly across the gills
[73,74]
. In most species
studied to date, almost complete compensation of
acidosis occurs within a few hours or days of expo-
sure to elevated CO
[74-77]
. is tight regulation of
acid-base balance maintains the pH required for e-
cient cellular function in a high CO environment,
but may necessitate additional energy expenditure
[78]
.
One concern is that additional energy expenditure
associated with acid-base regulation, or a decline in
oxygen carrying capacity associated with incom-
plete acid-base regulation, may reduce the scope
for aerobic performance in sh
[79]
. While aerobic
scope in two tropical cardinal sh Ostorhinchus
doederleini declined signicantly at projected future
CO levels
[80]
, Atlantic cod Gadus morhua main-
tained their standard and active metabolic rates,
critical swimming speeds and aerobic scope aer
prolonged exposure (4 and 12 months) to even
higher CO levels
[81]
. Furthermore, studies on fresh-
water and estuarine sh exposed to CO levels many
times greater than end-of-century predictions for
Figure 7.4. Left: the cardinalsh Ostorhinchus doederleini. Right: Atlantic cod Gadus morhua. Image: Goran Nilsson and animalspot.net

7. IMPACTS OF OCEAN ACIDIFICATION ON PELAGIC COMMUNITIES
71
ocean pCO have generally found no eect on oxygen
uptake or swimming performance
[74,78,82]
. ese
results indicate that while sensitivity to elevated
CO varies among species, most sh are probably
able to maintain sucient oxygen delivery at CO
levels predicted to occur in the near-future.
CO eects on cellular energy budgets have scarcely
been studied to date, yet it has been shown for the
Antarctic marbled rockcod Notothenia rossii that
several weeks of exposure to elevated pCO (2000
µatm) can lead to reduced mitochondrial capac-
ities and putative shifts in metabolic pathways
involved in mitochondrial energy metabolism
[76]
.
Increased intracellular levels of bicarbonate, due
to both increased pCO and active pH buering
by bicarbonate uptake
[74]
can lead to competitive
inhibition of enzymes of the Krebs cycle (citrate
synthase, succinate dehydrogenase) and may elicit
transcriptional changes and functional modica-
tions of mitochondrial proteins by activation of a
soluble adenylyl cyclase and subsequent action of
protein kinase A (PKA)
[83]
.
e eects of ocean acidication on development,
growth and survival of marine sh have largely
focused on larval and juvenile stages, because they
are expected to be more sensitive to environmen-
tal stressors, such as elevated pCO, than adults
[78,84]
.
Despite this expectation, recent studies have found
that the early life-history stages of some sh are
resilient to projected future levels of ocean acidi-
cation. Development, growth and survival of larvae
and juveniles of several reef sh species
[85,86]
, the
pelagic cobia Rachycentron canadum
[87]
and wall-
eye pollock eragra chalcogramma
[88]
appear rela-
tively robust to near-future CO levels (≤1000 µatm
CO). In contrast, larval growth declined and mortal-
ity increased in the inland silverside Menidia beryl-
lina, an estuarine species, at similar CO levels
[89]
(Figure 7.5). Tissue development was disrupted
in the Atlantic cod Gadus morhua reared at rela-
tively high CO levels (1,800 and 4,200 µatm)
[90]
,
although the eggs and larval stages did not seem
to be aected
[91]
.
ese studies suggest that the sensitivity of larval
and juvenile sh to rising CO levels is highly vari-
able. Furthermore, there may be trans-generational
eects.
us reduced growth and survival of juve-
nile anemone sh Amphiprion melanopus reared
at high CO levels was reversed when the parents
experienced the same CO conditions as the juve-
niles
[92]
. It may therefore be premature to conclude
that near-future CO levels will have signicant nega-
tive eects on the growth, development or survival
of marine sh until additional studies have included
exposure to high CO during both the parental and
ospring generations.
Preliminary studies on the eects of chronic expo-
sure to high CO on sh reproduction have not
Figure 7.5 Larvae of Menidia beryllina with curved or curled bodies were signicantly more common at increased CO
2
levels (b,c)
when compared with control(a) CO
2
levels. Scale bar=1 mm. From
[89]
, adapted by permission from Macmillan Publishers Ltd: Nature
Climate Change.

72
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
detected substantial impacts, although long-term
consequences in many species remain to be deter-
mined
[93]
. In the short term, reproductive output
can be stimulated by high CO, for example, in the
cinnamon anemonesh Amphiprion melanopus
[93]
.
Sperm motility is arrested by mild increases in
pCO in some atsh
[94]
, but not in the cod, Gadus
morhua
[95]
, or 11 other species from a range of fami-
lies
[94]
. Furthermore, rearing eggs of Atlantic herring
Clupea harengus in acidied water had no detectable
eect on fertilization success, embryonic develop-
ment, hatch rate, length and weight at hatching and
yolk size
[96]
. Sensitivity of sh eggs to elevated CO
varies markedly between species, but species tested
to date typically have 24h LC50 (lethal concentration
resulting in 50% mortality over 24 hours) values well
above 10,000 µatm CO
[78]
, far in excess of projected
end of the century CO levels.
ere are two areas in which consistent eects of
elevated CO have been detected for marine sh.
First, otolith (earbone) size is consistently larger
in larval and juvenile sh reared under elevated
CO. Larger ear bones have been observed in larval
seabass Atractoscion nobilis
[
97]
(Figure 7.6), clown-
sh Amphiprion percula
[98]
, cobia Rachycentron cana-
dum
[87]
and Atlantic cod Gadus morhua
[99]
reared
between 800-1800 µatm CO. While the ecological
signicance of larger otolith size is uncertain, audi-
tory models suggest that larger otoliths could poten-
tially enhance auditory acuity
[100]
. Second, exposure
to elevated CO can cause sensory and behavioural
impairment in a range of marine sh
[101,102]
, including
eects on vision and retinal function
[103, 104, 105]
(see
section 5.3).
While results indicate that most sh are probably able
to maintain sucient oxygen delivery at CO levels
predicted to occur in the near-future, the eect on
squid may be more pronounced. Epipelagic squid
(e.g., Ommastrephidae, Gonatidae, Loliginidae) are
considered to be most severely impacted by the inter-
ference of CO
with oxygen binding at the gills, as
they have a very nely tuned blood oxygen transport
system to maintain high metabolic rates using the
respiratory pigment haemocyanin
[106]
. Haemocyanin
is very sensitive to CO and as such, blood oxygen
transport can be easily disturbed to reduce activ-
ity
[107,108]
as demonstrated in the Pacic jumbo squid
Dosidicus giga, which had signicant reduction of
metabolic rates and activity levels under 1000 µatm
of CO
[109]
. Elevated CO could also aect squid
paralarvae, as demonstrated by abnormal shapes
of aragonite statoliths in the Atlantic Longn squid
Doryteuthis pealeii, which are critical for balance
and detecting movement
[110]
.
The cuttlefish Sepia officinalis, one of the most
common and commercially important cephalopods
in Europe, does not appear detrimentally impacted
by ocean acidication during development and may
even show increased calcium uptake into its cuttle-
bone
[111]
.
However, when reduced pH is combined
with increased temperature, S. ocinalis does display
shorter embryonic periods, lower survival rates and
enhanced premature hatching
[112]
.
Figure 7.6 Dorsal view of sagittal otoliths of 7-day-old white sea bass Atractoscion nobilis grown in seawater at (A) 430, (B) 1000,
and (C) 2500 μatm pCO
2
. Scale bars indicate 10 μm. From
[97]
. Reprinted with permission from AAAS.

7. IMPACTS OF OCEAN ACIDIFICATION ON PELAGIC COMMUNITIES
73
References
1. Armstrong RA, Lee C, Hedges JI, Honjo S, Wakeham
SG (2002) A new, mechanistic model for organic
carbon uxes in the ocean based on the quantitative
association of POC with ballast minerals. Deep-Sea
Research Part II-Topical Studies in Oceanography 49:
219-236.
2. Klaas C, Archer DE (2002) Association of sinking
organic matter with various types of mineral ballast
in the deep sea: Implications for the rain ratio. Global
Biogeochem Cycles 16: 1116.
3. Riebesell U, Tortell PD (2011) Eects of ocean
acidication on pelagic organisms and ecosystems.
In: Gattuso JP, Hansson L (eds). Ocean Acidication.
Oxford: Oxford University Press; p 99-121.
4. Wu Y, Gao K, Riebesell U (2010) CO-induced
seawater acidication aects physiological
performance of the marine diatom Phaeodactylum
tricornutum. Biogeosciences 7: 2915-2923.
5. Rost B, Richter KU, Riebesell U, Hansen PJ
(2006) Inorganic carbon acquisition in red tide
dinoagellates. Plant Cell and Environment 29:
810-822.
6. Kranz SA, Sueltemeyer D, Richter K-U, Rost B
(2009) Carbon acquisition by Trichodesmium: e
eect of pCO and diurnal changes. Limnology and
Oceanography 54: 548-559.
7. Wu YP, Gao KS (2010) Combined eects of solar UV
radiation and CO-induced seawater acidication
on photosynthetic carbon xation of phytoplankton
assemblages in the South China Sea. Chinese Science
Bulletin 55: 3680-3686.
8. Yang GY, Gao KS (2012) Physiological responses
of the marine diatom alassiosira pseudonana
to increased pCO and seawater acidity. Marine
Environmental Research 79: 142-151.
9. Falkowski PG, Katz ME, Knoll AH, Quigg A, Raven
JA, et al. (2004) e evolution of modern eukaryotic
phytoplankton. Science 305: 354-360.
10. Rost B, Zondervan I, Wolf-Gladrow D (2008)
Sensitivity of phytoplankton to future changes in
ocean carbonate chemistry: current knowledge,
contradictions and research directions. Marine
Ecology Progress Series 373: 227-237.
11. Gao KS, Xu JT, Gao G, Li YH, Hutchins DA, et al.
(2012) Rising CO and increased light exposure
synergistically reduce marine primary productivity.
Nature Climate Change 2: 519-523.
12. Lundholm N, Hansen PJ, Kotaki Y (2004) Eect
of pH on growth and domoic acid production by
potentially toxic diatoms of the genera Pseudo-
nitzschia and Nitzschia. Marine Ecology Progress
Series 273: 1-15.
13. Trimborn S, Lundholm N, oms S, Richter KU,
Krock B, et al. (2008) Inorganic carbon acquisition in
potentially toxic and non-toxic diatoms: the eect of
pH-induced changes in seawater carbonate chemistry.
Physiologia Plantarum 133: 92-105.
14. Fu FX, Tatters AO, Hutchins DA (2012) Global
change and the future of harmful algal blooms in the
ocean. Marine Ecology Progress Series 470: 207-233.
15. Riebesell U (2004) Eects of CO enrichment on
marine phytoplankton. Journal of Oceanography 60:
719-729.
16. Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe
RE, et al. (2000) Reduced calcication of marine
plankton in response to increased atmospheric CO.
Nature 407: 364-367.
17. Zondervan I, Rost BÈ, Riebesell U (2002) Eect of
CO concentration on the PIC/POC ratio in the
coccolithophore Emiliania huxleyi grown under light-
limiting conditions and dierent daylengths. Journal
of Experimental Marine Biology and Ecology 272:
55-70.
18. Engel A, Zondervan I, Aerts K, Beaufort L, Benthien
A, et al. (2005) Testing the direct eect of CO
concentration on a bloom of the coccolithophorid
Emiliania huxleyi in mesocosm experiments.
Limnology and Oceanography 50: 493-507.
19. Gao KS, Ruan ZX, Villafane VE, Gattuso JP, Helbling
EW (2009) Ocean acidication exacerbates the eect
of UV radiation on the calcifying phytoplankter
Emiliania huxleyi. Limnology and Oceanography 54:
1855-1862.
20. Merico A, Tyrrell T, Cokacar T (2006) Is there
any relationship between phytoplankton seasonal
dynamics and the carbonate system? Journal of
Marine Systems 59: 120-142.
21. Tyrrell T, Schneider B, Charalampopoulou A,
Riebesell U (2008) Coccolithophores and calcite
saturation state in the Baltic and Black Seas.
Biogeosciences 5: 485-494.
22. Cubillos JC, Wright SW, Nash G, de Salas MF,
Griths B, et al. (2007) Calcication morphotypes
of the coccolithophorid Emiliania huxleyi in the
Southern Ocean: changes in 2001 to 2006 compared
to historical data. Marine Ecology Progress Series
348: 47-54.
23. Beaufort L, Probert I, de Garidel-oron T, Bendif
EM, Ruiz-Pino D, et al. (2011) Sensitivity of
coccolithophores to carbonate chemistry and ocean
acidication. Nature 476: 80-83.
24. Iglesias-Rodriguez MD, Halloran PR, Rickaby
REM, Hall IR, Colmenero-Hidalgo E, et al. (2008)
Phytoplankton calcication in a high-CO world.
Science 320: 336-340.

74
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
25. Langer G, Geisen M, Baumann K-H, Klaes J, Riebesell
U, et al. (2006) Species-specic responses of calcifying
algae to changing seawater carbonate chemistry.
Geochemistry Geophysics Geosystems 7.
26. Lohbeck KT, Riebesell U, Reusch TBH (2012)
Adaptive evolution of a key phytoplankton species to
ocean acidication. Nature Geoscience 5: 346-351.
27. Jin P, Gao KS, Beardall J (2013) Evolutionary
responses of a coccolithophorid Gephyrocapsa
oceanica to ocean acidication. Evolution 67:
1869-1878.
28. Grelaud M, Schimmelmann A, Beaufort L (2009)
Coccolithophore response to climate and surface
hydrography in Santa Barbara Basin, California, AD
1917-2004.Biogeosciences 6: 2025-2039.
29. Aberle N, Schulz KG, Stuhr A, Malzahn AM, Ludwig
A, et al. (2013) High tolerance of microzooplankton
to ocean acidication in an Arctic coastal plankton
community. Biogeosciences 10: 1471-1481.
30. Czerny J, Schulz KG, Boxhammer T, Bellerby RGJ,
Buedenbender J, et al. (2013) Implications of elevated
CO on pelagic carbon uxes in an Arctic mesocosm
study - an elemental mass balance approach.
Biogeosciences 10: 3109-3125.
31. Webster NS, Negri AP, Flores F, Humphrey C, Soo R,
et al. (2013) Near-future ocean acidication causes
dierences in microbial associations within diverse
coral reef taxa. Environmental Microbiology Reports
5: 243-251.
32. Laverock B, Kitidis V, Tait K, Gilbert JA, Osborn AM,
et al. (2013) Bioturbation determines the response of
benthic ammonia-oxidizing microorganisms to ocean
acidication. Philosophical Transactions of Royal
Society London B: Biological Sciences 368: 20120441.
33. Lalii CM, Gilmer RW (1989) Pelagic snails: e
biology of holoplanktonic gastropod mollusks.
Stanford, CA: Stanford University Press.
34. Hunt BPV, Pakhomov EA, Hosie GW, Siegel V,
Ward P, et al. (2008) Pteropods in Southern Ocean
ecosystems. Progress in Oceanography 78: 193-221.
35. Teniswood CMH, Roberts D, Howard WR, Bradby JE
(2013) A quantitative assessment of the mechanical
strength of the polar pteropod Limacina helicina
antarctica shell. ICES Journal of Marine Science 70:
1499-1505.
36. Feely RA, Sabine CL, Lee K, Berelson WM, Kleypas
JA, et al. (2004) Impact of anthropogenic CO on the
CaCO system in the oceans. Science 305: 362.
37. Bednaršek N, Tarling GA, Bakker DCE, Fielding S,
Jones EM, et al. (2012) Extensive dissolution of live
pteropods in the Southern Ocean. Nature Geoscience
5: 881-885.
38. Bednaršek N, Feely RA, Reum JCP, Peterson B,
Menkel J, et al. (2014) Limacina helicina shell
dissolution as an indicator of declining habitat
suitability owing to ocean acidication in the
California Current Ecosystem. Proceedings of the
Royal Society B: Biological Sciences 281: 20140123
39. Comeau S, Gorsky G, Jeree R, Teyssié J-L, Gattuso
J-P (2009) Impact of ocean acidication on a
key Arctic pelagic mollusc (Limacina helicina).
Biogeosciences 6: 1877-1882.
40. Comeau S, Gorsky G, Alliouane S, Gattuso J-P (2010)
Larvae of the pteropod Cavolinia inexa exposed to
aragonite undersaturation are viable but shell-less.
Marine Biology 157: 2341-2345.
41. Comeau S, Jeree R, Teyssié J-L, Gattuso J-P (2010)
Response of the Arctic pteropod Limacina helicina
to projected future environmental conditions. PLoS
ONE 5: e11362.
42. Lischka S, Buedenbender J, Boxhammer T, Riebesell
U (2011) Impact of ocean acidication and elevated
temperatures on early juveniles of the polar shelled
pteropod Limacina helicina: mortality, shell
degradation, and shell growth. Biogeosciences 8:
919-932.
43. Lischka S, Riebesell U (2012) Synergistic eects of
ocean acidication and warming on overwintering
pteropods in the Arctic. Global Change Biology 18:
3517-3528.
44. Mackas DL, Galbraith MD (2012) Pteropod time-
series from the NE Pacic. ICES Journal of Marine
Science 69: 448-459.
45. Comeau S, Gattuso J-P, Nisumaa A-M, Orr J (2012)
Impact of aragonite saturation state changes on
migratory pteropods. Proceedings of the Royal
Society B: Biological Sciences 279: 732-738.
46. Comeau S, Jeree R, Teyssie JL, Gattuso JP (2010)
Response of the Arctic pteropod Limacina helicina
to projected future environmental conditions. PLoS
ONE 5: e11362.
47. Schiebel R (2002) Planktic foraminiferal
sedimentation and the marine calcite budget. Global
Biogeochemical Cycles 16: 1065.
48. Bijma J, Honisch B, Zeebe RE (2002) Impact of the
ocean carbonate chemistry on living foraminiferal
shell weight: Comment on “Carbonate ion
concentration in glacial-age deep waters of the
Caribbean Sea” by WS Broecker and E Clark.
Geochemistry Geophysics Geosystems 3: 1-7.
49. Bjima J, Spero HJ, Lea DW (1999) Reassessing
foraminiferal stable isotope geochemistry: Impact of
the oceanic carbonate system (experimental results).
In: Fisher G (ed). Use of Proxies in Paleoceanography:
Examples from the South Atlantic. New York:
Springer-Verlag.

7. IMPACTS OF OCEAN ACIDIFICATION ON PELAGIC COMMUNITIES
75
50. Russell AD, Honisch B, Spero HJ, Lea DW (2004)
Eects of seawater carbonate ion concentration
and temperature on shell U, Mg, and Sr in
cultured planktonic foraminifera. Geochimica Et
Cosmochimica Acta 68: 4347-4361.
51. Spero HJ, Bijma J, Lea DW, Bemis BE (1997) Eect
of seawater carbonate concentration on foraminiferal
carbon and oxygen isotopes. Nature 390: 497-499.
52. Lombard F, da Rocha RE, Bijma J, Gattuso JP
(2010) Eect of carbonate ion concentration and
irradiance on calcication in planktonic foraminifera.
Biogeosciences 7: 247-255.
53. Barker S, Eldereld H (2002) Foraminiferal
calcication response to glacial-interglacial changes
in atmospheric CO.Science 297: 833-836.
54. de Moel H, Ganssen GM, Peeters FJC, Jung SJA,
Kroon D, et al. (2009) Planktic foraminiferal shell
thinning in the Arabian Sea due to anthropogenic
ocean acidication? Biogeosciences 6: 1917-1925.
55. Davis CV, Badger MPS, Bown PR, Schmidt DN
(2013) Calcication responses to climate change
in the Pliocene. Biogeosciences Discussions 10:
6840-6860.
56. Moy AD, Howard WR, Bray SG, Trull TW (2009)
Reduced calcication in modern Southern Ocean
planktonic foraminifera. Nature Geoscience 2:
276-280.
57. Fujita K, Hikami M, Suzuki A, Kuroyanagi A, Sakai
K, et al. (2011) Eects of ocean acidication on
calcication of symbiont-bearing reef foraminifers.
Biogeosciences 8: 2089-2098.
58. Brady HB (1884) Report on the Foraminifera dredged
by HMS Challenger, during the years 1873-1876,
Reports of the Scientic Results of the Voyage of
HMS Challenger. Zoology 9: 814.
59. Longhurst AR (1985) e structure and evolution of
plankton communities. Progress in Oceanography 15:
1-35.
60. Runge JA (1988) Should we expect a relationship
between primary production and sheries - the role
of copepod dynamics as a lter of trophic variability.
Hydrobiologia 167: 61-71.
61. Kurihara H, Ishimatsu A (2008) Eects of high CO
seawater on the copepod (Acartia tsuensis) through
all life stages and subsequent generations. Marine
Pollution Bulletin 56: 1086-1090.
62. Kurihara H, Shirayama Y (2004) Eects of increased
atmospheric CO on sea urchin early development.
Marine Ecology Progress Series 274: 161-169.
63. Mayor D, Matthews C, Cook K, Zuur A, Hay S (2007)
CO-induced acidication aects hatching success in
Calanus nmarchicus. Marine Ecology Progress Series
350: 91-97.
64. Weydmann A, Soreide JE, Kwasniewski S,
Widdicombe S (2012) Inuence of CO-induced
acidication on the reproduction of a key Arctic
copepod Calanus glacialis. Journal of Experimental
Marine Biology and Ecology 428: 39-42.
65. McConville K, Halsband C, Fileman ES, Somereld
PJ, Findlay HS, et al. (2013) Eects of elevated CO on
the reproduction of two calanoid copepods. Marine
Pollution Bulletin 73: 428-434.
66. Watanabe Y, Yamaguchi A, Ishidai H, Harimoto T,
Suzuki S, et al. (2006) Lethality of increasing CO
levels on deep-sea copepods in the western North
Pacic. Journal of Oceanography 62: 185-196.
67. Nieho B, Schmithuesen T, Knueppel N, Daase M,
Czerny J, et al. (2013) Mesozooplankton community
development at elevated CO concentrations: results
from a mesocosm experiment in an Arctic ord.
Biogeosciences 10: 1391-1406.
68. de Kluijver A, Soetaert K, Czerny J, Schulz KG,
Boxhammer T, et al. (2013) A C-13 labelling study on
carbon uxes in Arctic plankton communities under
elevated CO levels. Biogeosciences 10: 1425-1440.
69. Li W, Gao KS (2012) A marine secondary producer
respires and feeds more in a high CO ocean. Marine
Pollution Bulletin 64: 699-703.
70. Cripps G, Lindeque P, Flynn KJ (2014) Have we been
underestimating the eects of ocean acidication in
zooplankton? Global Change Biology, doi: 10.1111/
gcb.12582
71. Rossoll D, Bermudez R, Hauss H, Schulz KG,
Riebesell U, et al. (2012) Ocean acidication-induced
food quality deterioration constrains trophic transfer.
PLoS ONE 7: e34737.
72. Melzner F, Gutowska MA, Langenbuch M, Dupont
S, Lucassen M, et al. (2009) Physiological basis for
high CO tolerance in marine ectothermic animals:
pre-adaptation through lifestyle and ontogeny?
Biogeosciences 6: 2313-2331.
73. Claiborne JB, Edwards SL, Morrison-Shetlar AI
(2002) Acid-base regulation in shes: Cellular and
molecular mechanisms. Journal of Experimental
Zoology 293: 302-319.
74. Brauner CJ, Baker DW (2009) Patterns of acid-base
regulation during exposure to hypercarbia in shes.
In: Glass ML, Wood SC (eds). Cardio-Respiratory
Control in Vertebrates, Springer p 43-63.
75. Esbaugh AJ, Heuer R, Grosell M (2012) Impacts of
ocean acidication on respiratory gas exchange and
acid-base balance in a marine teleost, Opsanus beta.
Journal of Comparative Physiology B-Biochemical
Systemic and Environmental Physiology 182:
921-934.
76. Strobel A, Bennecke S, Leo E, Mintenbeck K, Pörtner
HO, et al. (2012) Metabolic shis in the Antarctic sh
Notothenia rossii in response to rising temperature
and pCO.Frontiers in Zoology 9: 28.

76
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
77. Michaelidis B, Ouzounis C, Paleras A, Portner HO
(2005) Eects of long-term moderate hypercapnia on
acid-base balance and growth rate in marine mussels
Mytilus galloprovincialis. Marine Ecology Progress
Series 293: 109-118.
78. Ishimatsu A, Hayashi M, Kikkawa T (2008) Fishes in
high-CO, acidied oceans. Marine Ecology Progress
Series 373: 295-302.
79. Pörtner HO, Farrell AP (2008) Physiology and
climate change. Science 322: 690-692.
80. Munday PL, Crawley NE, Nilsson GE (2009)
Interacting eects of elevated temperature and ocean
acidication on the aerobic performance of coral reef
shes. Marine Ecology Progress Series 388: 235-242.
81. Melzner F, Goebel S, Langenbuch M, Gutowska MA,
Pörtner H-O, et al. (2009) Swimming performance
in Atlantic Cod (Gadus morhua) following long-term
(4-12 months) acclimation to elevated seawater pCO.
Aquatic Toxicology 92: 30-37.
82. McKenzie DJ, Piccolella M, Dalla Valle AZ, Taylor
EW, Bolis CL, et al. (2003) Tolerance of chronic
hypercapnia by the European eel Anguilla anguilla.
Journal of Experimental Biology 206: 1717-1726.
83. Tresguerres M, Parks SK, Salazar E, Levin LR,
Goss GG, et al. (2010) Bicarbonate-sensing soluble
adenylyl cyclase is an essential sensor for acid/base
homeostasis. Proceedings of the National Academy
of Sciences of the United States of America 107:
442-447.
84. Melzner F, Gutowska MA, Langenbuch M, Dupont
S, Lucassen M, et al. (2009) Physiological basis for
high CO tolerance in marine ectothermic animals:
pre-adaptation through lifestyle and ontogeny?
Biogeosciences 6: 2313-2331.
85. Munday PL, Donelson JM, Dixson DL, Endo GGK
(2009) Eects of ocean acidication on the early life
history of a tropical marine sh. Proceedings of the
Royal Society B: Biological Sciences 276: 3275-3283.
86. Munday PL, Gagliano M, Donelson JM, Dixson DL,
orrold SR (2011) Ocean acidication does not
aect the early life history development of a tropical
marine sh. Marine Ecology Progress Series 423:
211-221.
87. Bignami S, Sponaugle S, Cowen RK (2013) Response
to ocean acidication in larvae of a large tropical
marine sh, Rachycentron canadum. Global Change
Biology 19: 996-1006.
88. Hurst TP, Fernandez ER, Mathis JT, Miller JA, Stinson
CM, et al. (2012) Resiliency of juvenile walleye
pollock to projected levels of ocean acidication.
Aquatic Biology 17: 247-259.
89. Baumann H, Talmage SC, Gobler CJ (2012) Reduced
early life growth and survival in a sh in direct
response to increased carbon dioxide. Nature Climate
Change 2: 38-41.
90. Frommel AY, Maneja R, Lowe D, Malzahn AM,
Geen AJ, et al. (2012) Severe tissue damage
in Atlantic cod larvae under increasing ocean
acidication. Nature Climate Change 2: 42-46.
91. Frommel AY, Schubert A, Piatkowski U, Clemmesen
C (2013) Egg and early larval stages of Baltic cod,
Gadus morhua, are robust to high levels of ocean
acidication. Marine Biology 160: 1825-1834.
92. Miller GM, Watson S-A, Donelson JM, McCormick
MI, Munday PL (2012) Parental environment
mediates impacts of increased carbon dioxide on a
coral reef sh. Nature Climate Change 2: 858-861.
93. Miller GM, Watson SA, McCormick MI, Munday PL
(2013) Increased CO stimulates reproduction in a
coral reef sh. Global Change Biology 19: 3037-3045.
94. Inaba K, Dreanno C, Cosson J (2003) Control
of atsh sperm motility by CO and carbonic
anhydrase. Cell Motility and the Cytoskeleton 55:
174-187.
95. Frommel AY, Stiebens V, Clemmesen C, Havenhand
J (2010) Eect of ocean acidication on marine sh
sperm (Baltic cod: Gadus morhua). Biogeosciences 7:
3915-3919.
96. Franke A, Clemmesen C (2011) Eect of ocean
acidication on early life stages of Atlantic herring
(Clupea harengus L.). Biogeosciences 8: 3697-3707.
97. Checkley DM, Dickson AG, Takahashi M, Radich
JA, Eisenkolb N, et al. (2009) Elevated CO enhances
otolith growth in young sh. Science 324: 1683.
98. Munday PL, Hernaman V, Dixson DL, orrold
SR (2011) Eect of ocean acidication on otolith
development in larvae of a tropical marine sh.
Biogeosciences 8: 1631-1641.
99. Maneja RH, Frommel AY, Geen AJ, Folkvord
A, Piatkowski U, et al. (2013) Eects of ocean
acidication on the calcication of otoliths of larval
Atlantic cod Gadus morhua. Marine Ecology Progress
Series 477: 251-258.
100. Bignami S, Enochs IC, Manzello DP, Sponaugle S,
Cowen RK (2013) Ocean acidication alters the
otoliths of a pantropical sh species with implications
for sensory function. Proceedings of the National
Academy of Sciences of the United States of America
110: 7366-7370.
101. Munday PL, McCormick MI, Nilsson GE (2012)
Impact of global warming and rising CO levels on
coral reef shes: what hope for the future? Journal of
Experimental Biology 215: 3865-3873.
102. Munday PL, Cheal AJ, Dixson DL, Rummer JL,
Fabricius KA (2014) Behavioural impairment in reef
shes caused by ocean acidication at CO seeps.
Nature Climate Change 4: 487-492
103. Knight K (2014) Ocean acidication will interfere
with sh eyes. Journal of Experimental Biology 217:
311-312.

7. IMPACTS OF OCEAN ACIDIFICATION ON PELAGIC COMMUNITIES
77
104. Chung WS, Marshall NJ, Watson SA, Munday PL,
Nilsson GE (2014) Ocean acidication slows retinal
function in a damselsh through interference with
GABA(A) receptors. Journal of Experimental Biology
217: 323-326.
105. Hamilton TJ, Holcombe A, Tresguerres M (2014)
CO-induced ocean acidication increases anxiety
in rocksh via alteration of GABAA receptor
functioning. Proceedings of the Royal Society B -
Biological Sciences 281: 20132509.
106. Pörtner HO, Zielinski S (1998) Environmental
constraints and the physiology of performance in
squid. South African Journal of Marine Science 20:
207-221.
107. Pörtner HO (1994) Coordination of metabolism,
acid-base regulation and haemocyanin function in
cephalopods. Marine and Freshwater Behaviour and
Physiology 25: 131-148.
108. Pörtner HO, Langenbuch M, Reipschlager A
(2004) Biological impact of elevated ocean CO
concentrations: lessons from animal physiology and
earth history. Journal of Oceanography 60: 705-718.
109. Rosa RA, Seibel BA (2008) Synergistic eects of
climate-related variables suggest future physiological
impairment in a top oceanic predator. Proceedings of
the National Academy of Sciences of the United States
of America 105: 20776.
110. Kaplan MB, Mooney TA, McCorkle DC, Cohen AL
(2013) Adverse eects of ocean acidication on early
development of squid (Doryteuthis pealeii). PLoS
ONE 8: e63714.
111. Dorey N, Melzner F, Martin S, Oberhaensli F, Teyssie
J-L, et al. (2013) Ocean acidication and temperature
rise: eects on calcication during early development
of the cuttlesh Sepia ocinalis. Marine Biology 160:
2007-2022.
112. Rosa R, Truebenbach K, Repolho T, Pimentel M,
Faleiro F, et al. (2013) Lower hypoxia thresholds of
cuttlesh early life stages living in a warm acidied
ocean. Proceedings of the Royal Society B: Biological
Sciences 280.

78
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
KEY MESSAGES
1. Rising CO
2
is expected to affect net primary production and other carbon-cycling processes in the
ocean
2. The net effect of ocean acidication on carbon storage is uncertain
3. Decreased dimethyl sulphide (DMS) production could lead to exacerbated global warming
4. Ocean acidication has already affected provisioning ecosystem services through impacts on
aquaculture
5. Future ocean acidication could also impact regulating, cultural, and supporting services
6. Impacts of unmitigated ocean acidication are estimated to represent a loss to the world economy
of more than US $1 trillion annually by 2100
e previous chapters show how a high CO world
is likely to aect marine biodiversity at the level of
organisms and ecosystems. Here we consider the
wider implications of such changes. In particular,
how ocean acidication might aect other Earth
system process, through ocean biogeochemistry
and climatic feedbacks, and human society. Societal
implications will occur if ocean acidication causes
changes in ecosystem services that depend on the
health, abundance or distribution of marine species
of direct or indirect economic importance
[1]
.
To
date, however, scaling-up the eects of pH change
from individual organisms to populations, commu-
nities and ecosystems has received less attention
[2]
,
although this is changing
[3,4]
.
8.1 OCEAN BIOGEOCHEMISTRY AND CLIMATE
Ocean acidication has the potential to aect major
biogeochemical cycles in several ways
[5]
with feed-
backs to the global climate. Such impacts will not
occur in isolation, but in conjunction with other
global changes (including ocean warming, deoxy-
genation and potentially increased ultra-violet (UV)
radiation), that might signicantly modulate future
consequences
[6]
.
Positive climatic feedback would enhance future
global warming due to increasing greenhouse gases;
negative feedback would reduce future warming.
Biogeochemical processes that can alter ocean
uptake and storage of carbon are of particular
interest, although others may also be important. A
summary is provided in Table 8.1, with discussion
below covering ocean acidication impacts that
may alter either biological production in the photic
zone (where light penetrates), or the remineraliza-
tion (breakdown) of sinking particulate organic and
inorganic carbon.
8. IMPACTS OF OCEAN ACIDIFICATION ON
BIOGEOCHEMICAL CYCLES, ECOSYSTEM SERVICES
AND LIVELIHOODS

8. IMPACTS OF OCEAN ACIDIFICATION ON BIOGEOCHEMICAL CYCLES, ECOSYSTEM SERVICES AND LIVELIHOODS
79
Biological production
Rising CO concentrations in the upper ocean and
associated ocean acidication have the potential
to aect biological production through the follow-
ing processes:
•
Increase net primary productivity and particu-
late organic carbon (POC) production by making
photosynthesis more efficient
[7,8]
. However,
increased vertical stratication of the upper ocean
is likely to reduce nutrient supplies to the eupho-
tic zone, which may counteract potential eects
of rising pCO
2
on phytoplankton production in
the open ocean
[9]
.
•
Alter the stoichiometric nitrogen-to-carbon ratio
in exported particulate organic matter (POM), as
observed in mesocosm experiments
[10,11]
by around
a third (C:N ratio increase from 6.0 at 350 µatm to
8.0 at ~1050 µatm). Assuming sucient supplies of
N and other essential nutrients, this would increase
the storage of carbon in the ocean
[12]
.
•
Aect dinitrogen (N
2
) xation by cyanobacte-
ria, which could also alter primary production in
nitrogen-limited areas. Initial experiments showed
that Trichodesmium may increase its nitrogen xa-
tion under elevated CO
2
[13]
, however, there were
strain-specic dierences
[14]
and other cyanobac-
teria did not respond similarly
[15,16]
. Under real-
istic (low Fe) nutrient levels, low pH may reduce
nitrogen xation by Trichodesmium through
eects on iron uptake
[17]
.
•
Impede the ability of organisms to calcify
[18]
. is
is anticipated to reduce the production of calcium
carbonate.
•
Decrease the bioavailability of dissolved iron (Fe)
to some phytoplankton species. Acidication of
seawater decreases the Fe uptake rate of diatoms
and coccolithophores
[19]
.
Table 8.1. Summary of likely main effects of future ocean acidication on global-scale biogeochemical processes and feedbacks to the climate system
(primarily by increasing or decreasing atmospheric CO
2
) based on Table 12.1 of
[5]
and the ~70 references cited in that paper.
Note that: i) this table focuses
on water column effects in the open ocean; ii) all processes except (1) and (5) involve indirect effects, mediated by marine biota (mostly phytoplankton
and bacteria); iii) information for processes (7) and (8) is based on
[20]
and
[21]
; and iv) information for (9) based on references discussed in text.
Level of
understanding: H, high; M, medium; L, low.
Process Effect of future OA Feedback Magnitude
Level of
under-standing
1.
Ocean’s ability to buffer
atmospheric CO
2
levels
Decreased ocean uptake capacity + ve Large H
2. Photosynthesis Enhanced biological production and organic export from upper ocean – ve Medium M
3. C:N ratio of biomass Increased C:N ratio, affecting food quality and carbon export – ve Small to medium L
4. Calcication Overall decrease in biocalcication (but not all species/strains?) – ve Small to medium L/M
5. Carbonate dissolution Increased CaCO
3
dissolution in particles and sediments, increasing ocean alkalinity – ve Small in short-term;
large in long-term
M
6.
Ballast effect
(sinking particles)
Decreased CaCO
3
production will reduce organic matter export + ve Small to medium L
7. Dimethyl suphide (DMS) Reduced production + ve* Uncertain L
8. Organo-halogens Contradictory evidence: both enhancement and reduction may occur ? Uncertain L
9. Nitrogen xation Contradictory evidence: both enhancement and reduction may occur ? Uncertain L
10. Oxygenation Shallower remineralization increases O
2
demand; expansion of low O
2
regions + ve Medium L
11. Nitrication Reduced ? Small L
12. Nitrous oxide production Decreased O
2
levels will increase N
2
O production + ve Medium L
*feedback via cloud formation

80
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
Remineralization (breakdown) of
particulate material
Dissolution of CaCO is expected, with high
certainty, to increase in response to projected
declines in saturation state
[22]
.
Currently, most of
the exported organic carbon is broken down in the
upper 1000 m, but around 10% continues to the
deep ocean, where it is broken down in the water
column or buried in sediments and sequestered
from the atmosphere on millennial timescales
[23]
.
e analysis of particulate inorganic and organic
carbon (PIC and POC) uxes to water depths greater
than 1000 m suggests a close association between
these uxes
[24]
. Particles rich in CaCO are likely
to act as “ballast” for transporting POC from the
near-surface to deeper waters, thereby increasing its
sinking speed
[25]
. It is hypothesized that the associ-
ation between CaCO and POC might protect the
latter from bacterial degradation. If deep-water POC
uxes are controlled by CaCO, then a decrease in
CaCO production would reduce POC transport to
the deep ocean. POC would break down at shallower
depths, and the overall eciency of the biological
pump would decrease, resulting in reduced carbon
storage in the ocean and seabed, thereby increas-
ing atmospheric CO. ere is also evidence from
dierent regions that bacterial exo-enzyme activity
may increase under elevated CO
[26,27]
. One potential
outcome is an increase in the breakdown of organic
carbon in surface waters, potentially decreasing the
biological pump and carbon storage in the ocean.
Earth system models (ESMs) have been used to
explore the potential consequences of ocean acidi-
cation (and other projected changes) on the marine
biogeochemical processes listed in Table 8.1, and
assess which might be signicantly altered
[28]
. ese
include aragonite and calcite saturation state, export
production, and interior dissolved oxygen concentra-
tions. e general consensus of multi-model climate
projections is a reduction in primary production
and export production with global warming
[29,30]
although there are important regional dierences
between model projections. Where ocean acidica-
tion impacts could be signicant is on the POC and
PIC export from the upper ocean
[28,31]
. is would
aect the ow of energy through ecosystems and
could have signicant impacts on marine ecosys-
tem productivity and biodiversity.
Large changes in PIC and POC export could also
signicantly alter oxygen levels of the ocean inte-
rior. Oceanic oxygen levels are expected to decline
under global warming
[32,33]
, and recent ESMs proj-
ect a small decrease in the total ocean inventory of
dissolved oxygen (2% - 4%) by the end of 2100
[29]
.
However, the projections vary regionally, and the
total volume of hypoxic and suboxic waters remains
relatively unchanged by the end of 2100. e decline
in oxygen with rising CO could also have important
consequences for marine organisms with high meta-
bolic rates. Global warming, lower oxygen and higher
CO levels thus represent physiological stresses for
marine aerobic organisms that may act synergisti-
cally with ocean acidication
[34]
.
Eects of ocean acidication on climatic feedbacks
While CO is the most important greenhouse gas
modulated by the ocean, the air-sea exchanges of
other greenhouses gases may also be altered by ocean
acidication. ese include methane (CH) and
nitrous oxide (NO), as their production in the ocean
is linked to the breakdown of organic matter in low
oxygen water
[35,36]
. Declining oxygen levels should
be associated with increased production of both
these gases
[37,38]
, but it is expected that the impact
of increased production of CH and NO would be
less than the projected impacts of increased CO
[28]
.
However, increased warming could also potentially
destabilise methane hydrates stored in sediments
along continental margins, leading to additional
release of CH
[39]
.
e potential eects of increasing anthropogenic
CO on trace gas production in the oceans are poorly
understood. ese trace gases include climatically
important gases, such as dimethyl sulphide (DMS),
which can alter cloud properties. DMS is a gaseous
sulphur compound produced by marine biota in
surface seawater
[40]
and provides 90% of the biogenic
sulphur in the marine atmosphere
[41]
. Modelling
studies vary substantially in their predictions of
the change in DMS emissions with climate change;
studies for polar waters suggest increases in DMS
emission ranging from 30% to more than 150% by

8. IMPACTS OF OCEAN ACIDIFICATION ON BIOGEOCHEMICAL CYCLES, ECOSYSTEM SERVICES AND LIVELIHOODS
81
2100
[42-44]
, whilst elevated CO predictions in isola-
tion of other environmental change suggest a signif-
icant decrease in future concentration of DMS
[20,
21]
(Figure 8.1). When combined in Earth system
models to simulate future climate change, decreased
DMS production of this magnitude could exacer-
bate global warming
[21]
. However, DMS production
responses measured to date are variable, and the
sensitivity of the climate system to such changes is
uncertain, and may be low
[45]
. us full understand-
ing of the combined global warming and ocean acid-
ication impact on marine DMS and other trace
gas production needs further study to determine
its importance.
8.2 ECOSYSTEM SERVICES
To examine the societal implications of ocean acid-
ication, an ecosystem services framework can be
used. Ecosystem services are the components of
nature that (actively or passively) help create human
well-being and economic wealth
[46]
. ey result from
ecological processes, functions and biodiversity
[47]
,
and society is dependent upon them as a life support
system as well as for enhancing its well-being
[48]
. At a
general level, ecosystem services can be categorised
into four distinct groups
[49]
: provisioning services
(e.g., food and bres); regulating services (e.g., gas
and climate regulation, bioremediation of waste);
cultural services (e.g., education, recreation and
inspiration); and supporting services (e.g., nutri-
ent cycling, primary production and ecosystem resil-
ience) (Figure 8.2).
Figure 8.1. Relationship between dimethyl sulphide (DMS) concentration and pH based on data from mesocosm experiments.
Measurements of DMS and seawater pH are averaged from the mid-phase of Svalbard experiments (orange) and over the entire
experiment (blue). Red denotes measurements from Norwegian mesocosm experiments from three different years. From
[21]
.
Reprinted by permission from Macmillan Publishers Ltd: Nature Climate Change 3: 975-978, © 2013
Figure 8.2 Simplied summary of ecosystem services with
selected examples given
Regulating
Services, e.g.
Climate
regulation
Bioremediation
of waste
ECOSYSTEM SERVICES
Provisioning
Services, e.g.
Food
Materials
Cultural
Services, e.g.
Education
Recreation
Supporting Services, e.g.
Nutrient cycling
Primary Productivity
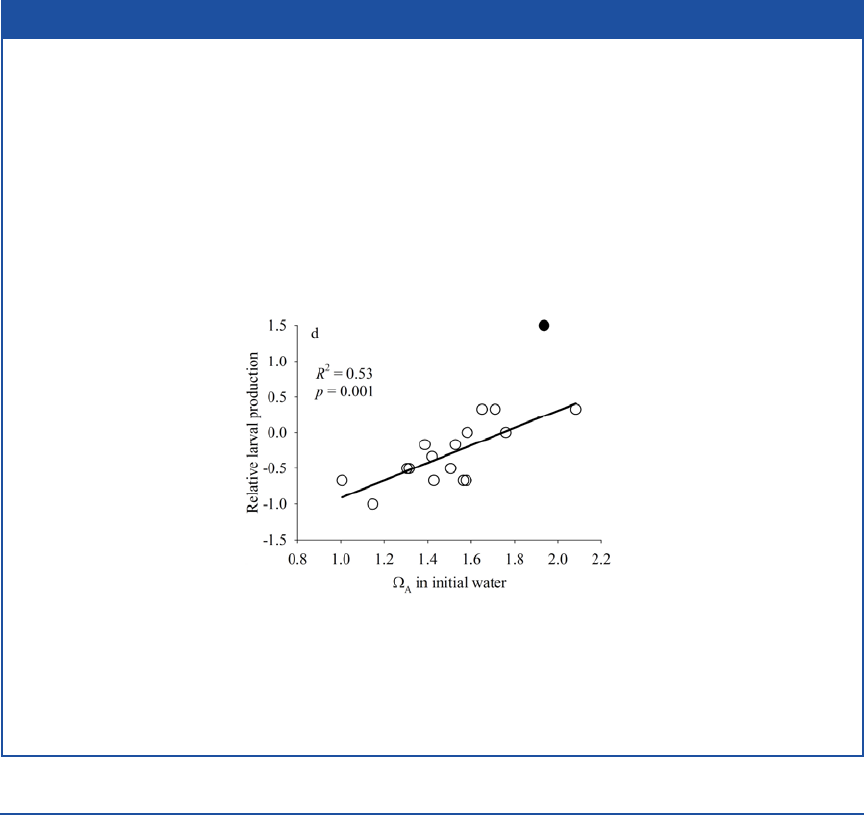
82
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
Supporting services.
ese comprise the processes
and functions that contribute to all other ecosys-
tem services. Any changes in these will have conse-
quences through provisioning, regulating and
cultural services. For example, many species that are
likely to be negatively impacted by pH changes (e.g.,
calciers) are habitat-forming organisms providing
shelter, food and nursery functions to other marine
species, including commercially important sh. ey
also contribute to coastal protection, leisure, recre-
ation and other cultural benets. Nutrient cycling
e.g., changes in N-xation
[50,51]
, or changes in biotur-
bator and bio-irrigator communities, will also change
fundamental processes within ecosystems
[2]
.
Provisioning services.
Ocean acidication can be
expected to aect provisioning services; however,
direct evidence is limited.
Molluscs and crustaceans
harvested for food are likely to be aected as they
have calcareous shells and exoskeletons, with their
sensitivity demonstrated in experimental studies.
Field-based evidence of the impact of ocean acidi-
cation on molluscs has been reported at sites along
the Pacic coast of the United States of America,
where the failure of oyster reproduction in hatch-
eries has been attributed to high levels of CO in
the water that upwells in that region
[52,53]
(Box 8.1).
Impacts such as these may have dierent implications
depending upon their location. For example, small
island developing states that are reliant upon shell-
sh aquaculture for export and for protein intake
could be particularly vulnerable
[54]
, and for both
shellsh and nsh, less developed nations that rely
heavily on artisanal eets will be more exposed to
the direct socio-economic consequences of ocean
acidication
[55]
.
Box 8.1 Impact of ocean acidication on oyster hatcheries
Due to the naturally low and variable pH of upwelled water off the north-west coast of the United States of America,
there is strong evidence that additional acidication due to anthropogenic CO
2
is already having biological impacts
in that region – where carbonate saturation values are now at levels projected for elsewhere 50-100 years in the
future.
Thus established oyster hatcheries in Oregon and Washington have increasingly suffered high larval mortalities
(up to 80%) since 2006, threatening the viability of an industry with total economic value of around $280 million
per year
[56]
.
The variable carbonate chemistry and pH of the hatchery water (due to periodic upwelling events) have
been shown to be major factors affecting the success of larval production and mid-stage growth cohorts of the
Pacic oyster Crassostrea gigas
[53]
. The oyster hatcheries have now adapted their working practices so that they avoid
using very low pH seawater, either by re-circulating their seawater or treating their water during upwelling events. With
these new practices, the north-west coast oyster hatcheries are producing near to full capacity again.
Relationship between aragonite saturation state (Ωaragonite) in incoming seawater and relative larval production at the
Whiskey Creek oyster hatchery, Oregon, United States of America.
Negative production values indicate reduction in total
biomass, due to mortality exceeding growth. Data point given as black lled circle was considered to be a statistical outlier,
and was not used in the regression analysis. From
[53]
. Copyright 2014 by the Association for the Sciences of Limnology and
Oceanography, Inc

8. IMPACTS OF OCEAN ACIDIFICATION ON BIOGEOCHEMICAL CYCLES, ECOSYSTEM SERVICES AND LIVELIHOODS
83
Nevertheless, some commercially important species
may be able to adapt, or may be naturally resilient;
for example, the mussel Mytilus edulis is reportedly
thriving in the naturally CO-enriched waters of
Kiel Fjord
[57]
. Some other species may be indirectly
impacted by ocean acidication through changes
in their food chain and habitat. Examples include
nsh that feed on benthic or pelagic calcifying
organisms (e.g., haddock feeding on echinoderms,
or salmon feeding on pteropods)
[58]
. It is also impor-
tant to consider the national impacts of altered provi-
sioning services.
Regulating services. ese include coastal defense
and carbon storage. Many marine habitats and
ecosystems (e.g., tropical coral reefs, mangroves,
seagrass meadows and bivalve beds) signicantly
dissipate the energy in waves reaching the coast,
increasing sedimentation rates and decreasing coastal
erosion
[59,60]
. Changes in these natural communities
resulting from ocean acidication would therefore
aect their ability to protect the coast. While poten-
tial impacts of ocean acidication on corals and
bivalves may be negative, this may not be true for
seagrasses, which may benet from higher levels of
CO
[61]
in the water and may therefore aord greater
protection of the coast.
Cultural services. e impact of ocean acidication
on cultural ecosystem services is particularly di-
cult to assess. While impacts to tourism, leisure and
recreation can be partially quantied, e.g., through
potential degradation of reefs attracting fewer tour-
ists due to dead coral and through decreased ancil-
lary biodiversity, many cultural services, such as
spiritual enrichment and aesthetic appreciation, are
intangible in nature, and the role of biodiversity in
these services is unclear. Nevertheless, where marine
species and ecosystems are given high inherent
worth (conservation value in developed countries)
or are important to indigenous peoples’ heritage and
identity, their reduction in abundance may result in
signicant cultural loss. Understanding the potential
impacts of ocean acidication on tourism, leisure and
recreation is also challenging, and more research is
required to quantify the scale of such eects.
8.3 ECONOMIC AND LIVELIHOOD IMPACTS
A quantitative assessment of the interactions of
ocean acidification with socio-economics and
human welfare requires that the full impact path-
way is understood and modeled. is requires the
coupling or integration of models that explain each
step in the pathway linking: i) socio-economic activ-
ities responsible for CO emissions, and resultant
changes in water chemistry; ii) impacts on marine
ecosystems; iii) changes in the provision of ecosystem
services; and, nally, iv) impacts on human welfare.
e existing economic literature on ocean acidi-
cation has only assessed a partial set of the poten-
tially impacted ecosystem services, with a focus on
the direct-use values that are most easily addressed.
Of the 13 studies reviewed here (Table 8.2), only
five provide monetary estimates of the costs of
ocean acidication. ree of these are for impacts
on mollusc fisheries (two for the United States
of America and one global estimate); one covers
impacts on sheries and carbon storage; and one is
for impacts on coral reef services. Central estimates
from each study are presented in Table 8.2 and stan-
dardized to annual values in the terminal year of
each analysis in US $ at 2010 price levels. From the
information currently available, impacts to tropical
coral reef services dominate; these are examined in
more detail below.
e economic impacts of ocean acidication on
the fisheries industry are relatively understud-
ied. However, models suggest that there may be
a substantial reduction in sheries catch potential
under future conditions
[62]
, aecting the quantity,
quality and predictability of future harvests
[63]
. It is
also important to consider projected impacts upon
dierent societal components, i.e., indigenous and
local communities as well as global markets. Coastal
indigenous and local peoples catch large quanti-
ties of marine species, which may be consumed,
or traded with inland groups in exchange for other
foods or goods. is may also dier regionally, and

84
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
it could be that coastal communities in the Arctic
are likely to be aected disproportionally by ocean
acidication due to the rapid environmental changes
towards higher latitudes. Further research is thus
needed to understand likely impacts in multiple
coastal communities.
The only study to provide values of the global
economic impact of ocean acidication on tropi-
cal coral reefs estimates the potential annual value
of lost ecosystem services to be up to ~US $1000
billion by 2100
[64]
. e value varies across scenarios
due to i) diering projected rates of CO emissions,
ocean acidication and loss of coral cover; and ii)
diering rates of population and income growth that
determine the value of coral reef services per unit
area of coral cover. e results show that the annual
economic impact (loss of coral reef service value)
escalates rapidly over time, essentially because the
scenarios have high economic growth in countries
with coral reefs, and because demand for coral reef
services increases more than proportionately with
income. Nonetheless, the annual value of foregone
ecosystem services from coral reefs in 2100 is still
only estimated to be a small fraction of total global
income (0.14% or US $870 billion in 2100; 2000
price levels; Special Report on Emissions Scenario
A1B based on rapid and integrated world economic
growth).
e estimated impacts are, however, considered to
be partial since the underlying value data is largely
focused on recreational values and includes limited
information on the value of other services such as
coastal protection or non-use values for biodiver-
sity. Results of a sensitivity analysis show that the
estimated impact is highly uncertain, with a con-
dence interval spanning one order of magnitude. It is
important to note that other threats to the health of
coral reefs and the provision of reef services are not
included (e.g., over-shing, sedimentation, eutro-
phication, sea level and temperature rise)
[75]
.
Table 8.2. Summary of studies that examine the economic impacts of ocean acidication. From
[64]
Study Impacts
Geographic
scope
Emissions
scenario
Period of
analysis
Welfare
measure
*
Annual
value (US $;
billions)**
Armstrong et al.(2012)
[65]
Fisheries
Carbon storage
Norway
Norway
0.5 pH decrease
0.5 pH decrease
2010 – 2110
2010 – 2110
Revenue
Damage Cost
0.01
3
Brander et al. (2012)
[66]
Coral reefs Global SRES A1B 2000 - 2100 Mixed 1,093
Cheung et al. (2011)
[62]
Fish and invertebrates N-E Atlantic SRES A1B 2005 - 2050 - -
Cooley & Doney (2009)
[67]
Molluscs United States IPCC A1F1 2007 - 2060 Revenue 0.07
Cooley et al. (2012)
[54]
Molluscs Global CCSM3 2010 - 2060 - -
Finnoff (2010)
[68]
Fisheries; non-use values Bering Sea - - - -
Harrould-Kolieb et al. (2009)
[69]
Coral reefs; sheries Global SRES A1B 2009 - 2050 - -
Hilmi et al. (2012)
[70]
All Global - - - -
Kite-Powell (2009)
[71]
Coral reefs; sheries Global IS92a - - -
Moore (2011)
[72]
Molluscs United States RCP8.5; RCP6 2010 - 2100 CV 0.31
Narita et al. (2012)
[73]
Molluscs Global IS92a 2000 - 2100 CS, PS 139
Rodrigues et al. (2013)
[74]
Use and non-use values Mediterranean - - - -
Sumaila et al. (2011)
[63]
Capture sheries Global - - - -
*
CV: compensating variation; CS: consumer surplus; PS: producer surplus
**
Impact estimates are standardised to annual values for the terminal year in each analysis (i.e., 2060 for Cooley and Doney
[67]
and 2100 otherwise) in US $2010 price level

8. IMPACTS OF OCEAN ACIDIFICATION ON BIOGEOCHEMICAL CYCLES, ECOSYSTEM SERVICES AND LIVELIHOODS
85
References
1. Cooley SR, Kite-Powell HL, Doney SC (2009)
Ocean acidication’s potential to alter global marine
ecosystem services. Oceanography 22: 172-181.
2. Widdicombe S, Spicer J (2008) Predicting the impact
of ocean acidication on benthic biodiversity:
What can animal physiology tell us? Journal of
Experimental Marine Biology and Ecology 366:
187-197.
3. Hall-Spencer JM, Rodolfo-Metalpa R, Martin S,
Ransome E, Fine M, et al. (2008) Volcanic carbon
dioxide vents show ecosystem eects of ocean
acidication. Nature 454: 96-99.
4. Busch DS, Harvey CJ, McElhany P (2013) Potential
impacts of ocean acidication on the Puget Sound
food web. ICES Journal of Marine Science 70:
823-833.
5. Gehlen M, Gruber N, Gangsto R, Bopp L, Oschlies
A (2011) Biogeochemical consequences of ocean
acidication and feedbacks to the earth system. In:
Gattuso JP, Hansson L (eds). Ocean Acidication.
Oxford: Oxford University Press. pp. 230-248.
6. Brewer PG, Peltzer ET (2009) Limits to marine life.
Science 324: 347-348.
7. Rost B, Zondervan I, Wolf-Gladrow D (2008)
Sensitivity of phytoplankton to future changes in
ocean carbonate chemistry: current knowledge,
contradictions and research directions. Marine
Ecology Progress Series 373: 227-237.
8. Zondervan I, Rost BÈ, Riebesell U (2002) Eect of
CO concentration on the PIC/POC ratio in the
coccolithophore Emiliania huxleyi grown under light-
limiting conditions and dierent daylengths. Journal
of Experimental Marine Biology and Ecology 272:
55-70.
9. Schneider B, Bopp L, Gehlen M, Segschneider
J, Froelicher TL, et al. (2008) Climate-induced
interannual variability of marine primary and export
production in three global coupled climate carbon
cycle models. Biogeosciences 5: 597-614.
10. Riebesell U, Schulz K, Bellerby R, Botros M, P (2007)
Enhanced biological carbon consumption in a high
CO ocean. Nature 450.
11. Bellerby RGJ, Schulz KG, Riebesell U, Neill C, Nondal
G, et al. (2008) Marine ecosystem community carbon
and nutrient uptake stoichiometry under varying
ocean acidication during the PeECE III experiment.
Biogeosciences 5: 1517-1527.
12. Oschlies A, Schulz KG, Riebesell U, Schmittner A
(2008) Simulated 21st century’s increase in oceanic
suboxia by CO-enhanced biotic carbon export.
Global Biogeochemical Cycles 22: GB4008.
13. Hutchins DA, Mulholland MR, Fu F (2009) Nutrient
cycles and marine microbes in a CO-enriched ocean.
Oceanography 22: 128-145.
14. Hutchins DA, Fu FX, Webb EA, Walworth N,
Tagliabue A (2013) Taxon-specic response of
marine nitrogen xers to elevated carbon dioxide
concentrations. Nature Geoscience 6: 790-795.
15. Fu F-X, Mulholland MR, Garcia NS, Beck A,
Bernhardt PW, et al. (2008) Interactions between
changing pCO, N xation, and Fe limitation in the
marine unicellular cyanobacterium Crocosphaera.
Limnology and Oceanography 53: 2472-2484.
16. Law CS, Breitbarth E, Homann LJ, McGraw CM,
Langlois RJ, et al. (2012) No stimulation of nitrogen
xation by non-lamentous diazotrophs under
elevated CO in the South Pacic. Global Change
Biology 18: 3004-3014.
17. Shi DL, Kranz SA, Kim JM, Morel FMM (2012)
Ocean acidication slows nitrogen xation and
growth in the dominant diazotroph Trichodesmium
under low-iron conditions. Proceedings of the
National Academy of Sciences of the United States of
America 109: E3094-E3100.
18. Fabry V, Seibel B, Feely RA (2008) Impacts of
ocean acidication on marine fauna and ecosystem
processes. Journal of Marine Research 65: 414-432.
19. Shi D, Xu Y, Hopkinson BM, Morel FMM (2010)
Eect of ocean acidication on iron availability to
marine phytoplankton. Science 327: 676-679.
20. Hopkins F, Nightingale P, Liss P (2011) Eects
of ocean acidication on the marine source of
atmospherically active trace gases. In: Gattuso JP,
Hansson L (eds). Ocean Acidication. Oxford:
Oxford University Press, p 210-229.
21. Six KD, Kloster S, Ilyina T, Archer SD, Zhang K, et al.
(2013) Global warming amplied by reduced sulphur
uxes as a result of ocean acidication. Nature
Climate Change 3: 975-978.
22. Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, et
al. (2005) Anthropogenic ocean acidication over
the twenty-rst century and its impact on calcifying
organisms. Nature 437: 681-686.
23. Trull TW, Bray SG, Manganini SJ, Honjo S, Francois
R (2001) Moored sediment trap measurements of
carbon export in the Subantarctic and Polar Frontal
Zones of the Southern Ocean, south of Australia.
Journal of Geophysical Research - Oceans 106:
31489-31509.
24. Armstrong RA, Lee C, Hedges JI, Honjo S, Wakeham
SG (2002) A new, mechanistic model for organic
carbon uxes in the ocean based on the quantitative
association of POC with ballast minerals. Deep-Sea
Research Part II-Topical Studies in Oceanography 49:
219-236.
25. Klaas C, Archer DE (2002) Association of sinking
organic matter with various types of mineral ballast
in the deep sea: Implications for the rain ratio. Global
Biogeochem. Cycles 16: 1116

86
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
26. Piontek J, Lunau M, Haendel N, Borchard C,
Wurst M, et al. (2010) Acidication increases
microbial polysaccharide degradation in the ocean.
Biogeosciences 7: 1615-1624.
27. Maas EW, Law CS, Hall JA, Pickmere S, Currie KI,
et al. (2013) Eect of ocean acidication on bacterial
abundance, activity and diversity in the Ross Sea,
Antarctica. Aquatic Microbial Ecology 70: 1-15.
28. Matear R, Lenton A (2014 in press) Quantifying the
impact of ocean acidication on our future climate.
Biogeosciences
29. Cocco V, Joos F, Steinacher M, Froelicher TL, Bopp
L, et al. (2013) Oxygen and indicators of stress
for marine life in multi-model global warming
projections. Biogeosciences 10: 1849-1868.
30. Bopp L, Resplandy L, Orr JC, Doney SC, Dunne JP,
et al. (2013) Multiple stressors of ocean ecosystems
in the 21st century: projections with CMIP5 models.
Biogeosciences 10: 6225-6245.
31. Tagliabue A, Bopp L, Gehlen M (2011) e
response of marine carbon and nutrient cycles to
ocean acidication: Large uncertainties related to
phytoplankton physiological assumptions. Global
Biogeochemical Cycles 25: GB3017.
32. Matear RJ, Hirst AC, McNeil BI (2000) Changes in
dissolved oxygen in the Southern Ocean with climate
change. Geochemistry Geophysics Geosystems 1:
1050.
33. Bopp L, Le Quere C, Heimann M, Manning AC,
Monfray P (2002) Climate-induced oceanic oxygen
uxes: Implications for the contemporary carbon
budget. Global Biogeochemical Cycles 16: 1022.
34. Pörtner HO, Farrell AP (2008) Physiology and climate
change. Science 322: 690-692.
35. Matear RJ, Wang Y-P, Lenton A (2010) Land and
ocean nutrient and carbon cycle interactions. Current
Opinion in Environmental Sustainability 2: 258-263.
36. Gruber N, Galloway JN (2008) An Earth-system
perspective of the global nitrogen cycle. Nature 451:
293-296.
37. Glessmer MS, Eden C, Oschlies A (2009)
Contribution of oxygen minimum zone waters to
the coastal upwelling o Mauritania. Progress in
Oceanography 83: 143-150.
38. Schmittner A, Oschlies A, Matthews HD, Galbraith
ED (2008) Future changes in climate, ocean
circulation, ecosystems, and biogeochemical cycling
simulated for a business-as-usual CO emission
scenario until year 4000 AD. Global Biogeochemical
Cycles 22: GB002953.
39. Biastoch A, Treude T, Rüpke LH, Riebesell U, Roth C,
et al. (2011) Rising Arctic Ocean temperatures cause
gas hydrate destabilization and ocean acidication.
Geophysical Research Letters 38: L08602.
40. Gabric A, Murray N, Stone L, Kohl M (1993)
Modeling the production of dimethylsulde during
a phytoplankton bloom. Journal of Geophysical
Research-Oceans 98: 22805-22816.
41. Arnold HE, Kerrison P, Steinke M (2013) Interacting
eects of ocean acidication and warming on
growth and DMS-production in the haptophyte
coccolithophore Emiliania huxleyi. Global Change
Biology 19: 1007-1016.
42. Cameron-Smith P, Elliott S, Maltrud M, Erickson
D, Wingenter O (2011) Changes in dimethyl
sulde oceanic distribution due to climate change.
Geophysical Research Letters 38: L07704.
43. Kloster S, Six KD, Feichter J, Maier-Reimer E,
Roeckner E, et al. (2007) Response of dimethylsulde
(DMS) in the ocean and atmosphere to global
warming. Journal of Geophysical Research 112:
G03005.
44. Gabric AJ, Qu B, Matrai P, Hirst AC (2005) e
simulated response of dimethylsulde production in
the Arctic Ocean to global warming. Tellus Series B -
Chemical and Physical Meteorology 57: 391-403.
45. Woodhouse MT, Mann GW, Carslaw KS, Boucher
O (2013) Sensitivity of cloud condensation nuclei
to regional changes in dimethyl-sulphide emissions.
Atmospheric Chemistry and Physics 13: 2723-2733
46. Fisher B, Turner RK, Morling P (2009) Dening and
classifying ecosystem services for decision making.
Ecological Economics 68: 643-653.
47. Fisher B, Turner K, Zylstra M, Brouwer R, de Groot
R, et al. (2008) Ecosystem services and economic
theory: integration for policy-relevant research.
Ecological Applications 18: 2050-2067.
48. Turner RK, Paavola J, Cooper P, Farber S, Jessamy
V, et al. (2003) Valuing nature: lessons learned and
future research directions. Ecological Economics 46:
493-510.
49. Millennium Ecosystem Assessment (2003).
Ecosystems and Human Well-being: A Framework for
Assessment. Washington DC
50. Kranz SA, Sueltemeyer D, Richter K-U, Rost B
(2009) Carbon acquisition by Trichodesmium: e
eect of pCO and diurnal changes. Limnology and
Oceanography 54: 548-559.
51. Levitan O, Rosenberg G, Setlik I, Setlikova E, Grigel
J, et al. (2007) Elevated CO enhances nitrogen
xation and growth in the marine cyanobacterium
Trichodesmium. Global Change Biology 13: 531-538.
52. Kerr RA (2010) Ocean acidication unprecedented,
unsettling. Science 328: 1500-1501.
53. Barton A, Hales B, Waldbusser GG, Langdon C, Feely
RA (2012) e Pacic oyster, Crassostrea gigas, shows
negative correlation to naturally elevated carbon
dioxide levels: Implications for near-term ocean
acidication eects. Limnology and Oceanography
57: 698-710.

8. IMPACTS OF OCEAN ACIDIFICATION ON BIOGEOCHEMICAL CYCLES, ECOSYSTEM SERVICES AND LIVELIHOODS
87
54. Cooley SR, Lucey N, Kite-Powell H, Doney SC (2012)
Nutrition and income from molluscs today imply
vulnerability to ocean acidication tomorrow. Fish
and Fisheries 13: 182-215.
55. Hilmi N, Allemand D, Cinar M, Cooley S, Hall-
Spencer JM et al (2014) Exposure of Mediterranean
countries to ocean acidication. Water 6: 1719-1744
56. Pacic Coast Shellsh Growers Association http://
www.pcsga.org/pub/farming/production_stats.pdf
57. omsen J, Gutowska Ma (2010) Calcifying
invertebrates succeed in a naturally CO enriched
coastal habitat but are threatened by high levels of
future acidication. Biogeosciences 7: 5119-5156.
58. Le Quesne WJF, Pinnegar JK (2011) e potential
impacts of ocean acidication: scaling from
physiology to sheries. Fish and Fisheries 13:
333-344.
59. Bos AR, Bouma TJ, de Kort GLJ, van Katwijk MM
(2007) Ecosystem engineering by annual intertidal
seagrass beds: Sediment accretion and modication.
Estuarine Coastal and Shelf Science 74: 344-348.
60. Feagin RA, Lozada-Bernard SM, Ravens TM, Moeller
I, Yeager KM, et al. (2009) Does vegetation prevent
wave erosion of salt marsh edges? Proceedings of the
National Academy of Sciences of the United States of
America 106: 10109-10113.
61. Johnson VR, Russell BD, Fabricius KE, Brownlee
C, Hall-Spencer JM (2012) Temperate and tropical
brown macroalgae thrive, despite decalcication,
along natural CO gradients. Global Change Biology
18: 2792-2803.
62. Cheung WWL, Dunne J, Sarmiento JL, Pauly D
(2011) Integrating ecophysiology and plankton
dynamics into projected maximum sheries catch
potential under climate change in the Northeast
Atlantic. ICES Journal of Marine Science 68:
1008-1018.
63. Sumaila UR, Cheung WWL, Lam VWY, Pauly D,
Herrick S (2011) Climate change impacts on the
biophysics and economics of world sheries. Nature
Climate Change 1: 449-456.
64. Brander L.M., Narita D, Rehdanz K, Tol RSJ (2014)
e economic impact of ocean acidication. In:
Nunes PALD, Kumar P, Dedeurwaerdere (eds).
Handbook on the Economics of Biodiversity and
Ecosystem Services: Edward Elgar, 608 p.
65. Armstrong C., Holen S., Navrud S., (2012) e
Economics of Ocean Acidication – A Scoping Study.
Norway: FRAM Centre
66. Brander LM, Rehdanz K, Tol RSJ, van Beukering PJH.
vB (2012) e economic impact of ocean acidication
on coral reefs. Climate Change Economics 3:
1250002.
67. Cooley SR, Doney SC (2009) Anticipating ocean
acidication’s economic consequences for commercial
sheries. Environmental Research Letters 4: 024007.
68. Finno D (2010) Modeling economic impacts of
climate change and ocean acidication to sheries.
http://yosemite.epa.gov/ee/epa/eerm.nsf/vwAN/
EE-0566-115.pdf/$le/EE-0566-115.pdf
69. Harrould-Kolieb ER, Hirsheld M, Brosius A
(2009) Major Emitters Among Hardest Hit by OA:
An Analysis of the Impacts of Acidication on the
Countries of the World.
Washington DC
70. Hilmi N, Allemand D, Dupont S, Safa A, Haraldsson
G, et al. (2013) Towards improved socio-economic
assessments of ocean acidication’s impacts. Marine
Biology 160: 1773-1787.
71. Kite-Powell H (2009) A global perspective on the
economics of ocean acidication. Journal of Marine
Education 25: 25-29.
72. Moore CC (2011) Welfare impacts of ocean
acidication: An integrated assessment model of the
US mollusk shery. US Environmental Protection
Agency, Working Paper #11-06
73. Narita D, Rehdanz K, Tol RSJ (2012) Economic costs
of ocean acidication: a look into the impacts on
global shellsh production. Climatic Change 113:
1049-1063.
74. Rodrigues LC, van den Bergh JCJM, Ghermandi A
(2013) Socio-economic impacts of ocean acidication
in the Mediterranean Sea. Marine Policy 38: 447-456.
75. Noone KJ, Sumaila UR, Diaz RJ (2013) Managing
Ocean Environments in a Changing Climate:
Sustainability and Economic Perspectives: Elsevier,
376 p.

88
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
KEY MESSAGES
1. A true multidisciplinary approach involving technological advances is needed
2. Research should be oriented toward a quantitative understanding at all levels from chemistry to
socio-economics
3. Research should be solution–oriented, covering the scale from local to global, and should prioritize
the ecosystems and societies most at risk
4. A high density of measurements in space and time is required to identify variability and
anthropogenic ocean acidication
5. Autonomous systems will remove the need for operators and encourage the development of low-
cost, low-power, long-term measurement techniques
9.1 TECHNICAL CHALLENGES: FROM INDIVIDUALS TO ECOSYSTEMS
A large body of scientic information about ocean
acidication has rapidly been generated during
the past few years, contributing to increased polit-
ical and wider societal awareness. e eld evolved
from an exploratory phase leading to several key
proof-of-concepts, toward more hypothesis-driven
research
[1,2]
. Key factors modulating responses of
species, ecosystems and their services have been
identified and include environmental variabil-
ity
[3]
, ecological interactions
[4]
, species potential
for acclimation and adaptation
[5]
and multiple driv-
ers. Ideally, an experiment assessing the impact of
ocean acidication on a given species, community
or ecosystem should include realistic changes for all
environmental drivers (CO, temperature, salinity,
food concentrations, light availability), and be long
term (i.e., several years) to allow for natural variabil-
ity
[6]
and multiple generations of each species under
consideration. Multiple end-points from physiolog-
ical response to biodiversity and socio-economic
impacts should also be considered (Table 9.1). Such
experiments should be replicated several times in
dierent areas to account for spatio-temporal vari-
ability. However, it is obvious that such an approach
is usually unrealistic, and it is impossible to test all
species and ecosystems in the world using such an
experimental design. To address this, future ocean
acidication research should develop and imple-
ment new technology and experimental designs,
and elucidate a greater mechanistic understand-
ing at all levels from chemistry to socio-economics.
Single experimental approaches on single organisms
oen do not capture the true level of complexity of
in situ marine environments, and multi-disciplin-
ary approaches involving technological advance-
ments and development are critically needed. is
includes combining natural variability and moni-
toring with organismal biology
[7]
. Below are some
examples at diering complexity levels of what we
know to date, and of some challenges and focuses for
future research. ese are summarized in Table 9.1.
Ocean acidication at the individual level.
Research
to date has highlighted individual species variability
in response to ocean acidication
[8]
, due in part to
diering organismal capacity to tolerate ocean acid-
ication. e capacity for acid-base regulation is an
important example, and species that show developed
9. FUTURE CONSIDERATIONS

9. FUTURE CONSIDERATIONS
89
capacity are in general expected to be more resil-
ient to ocean acidication
[9,10]
. Other studies have
demonstrated that tolerance to ocean acidication
can even dier between closely related species, or
even within species, as shown in coccolithophores
[11]
.
Variability within species may indicate the potential
for organisms to adapt to ocean acidication, and
indeed adaptation has been documented in meta-
zoan species near natural CO vents
[12]
. Physiological
plasticity of organisms and the potential to adapt to
changing conditions thus remains an important area
for future research
[5]
, supported by observed long-
term acclimatization and adaptation in fast-grow-
ing microalgae in response to ocean acidication
[13]
.
However, it is not feasible to measure this adap-
tation potential in all species due to diering life
history requirements and limitations within labora-
tory experiments. A key focus for future work may
therefore be for taxa and systems involved in ecosys-
tem services, such as coccolithophores
[5]
.
Organism response to ocean acidication may be
dierent for the short term relative to the long term,
or may even dier seasonally as found in one of the
longest experiments to date (542 days)
[3]
. Long-term
eects of ocean acidication may therefore be bu-
ered or exacerbated at dierent times of year. us
while experiments can give us a crucial insight into
how organisms respond to ocean acidication, much
more may be unaccounted for. Other factors that
may lead to variability and hence uncertainty are
projected values for temperature, light, salinity and
nutrients, and even methodological dierences
[8]
.
Light availability is particularly relevant to photo-
synthetic calciers such as tropical coral species, and
food availability has been demonstrated to be impor-
tant in organism response to ocean acidication
[14]
,
Table 9.1. Some key research gaps and challenges for future ocean acidication research
Ocean acidication process Research question(s)
Biogeochemical Will future OA provide signicant feedback to the global carbon cycle and climate change, through global-
scale changes in calcication, ocean productivity, particle sinking in the ocean, and effects on other
climatically active gases, e.g., DMS and N
2
O? Will the ocean become a less important CO
2
sink in the future,
exacerbating atmospheric changes?
Physico-chemical What is the current variability of ocean carbonate chemistry at ecologically signicant temporal and
spatial scales, and how will this change under future climate change scenarios, with associated additional
changes in temperature, oxygen, stratication, ocean circulation, and river inputs? Which areas of the ocean
(e.g., polar regions, upwelling zones, and shelf seas) will experience greatest and most rapid change?
Will chemical changes also impact sound transmission in future oceans, with impacts on organism
communication
[41]
?
Physiological and behavioural What are the unifying mechanisms linking species’ molecular, metabolic and behavioural responses to
ocean acidication? (e.g., based on energy metabolism and acid-base regulation). Does this explain
the high taxonomic variability observed in response to ocean acidication and complex interactions with
other stressors (e.g., temperature, low oxygen and food/nutrient availability, ultraviolet radiation)? How
would different scenarios of ocean acidication affect the immune system resilience of various species to
pathogens?
Genetic How can information from relatively short-term studies (weeks to months) on individual species be applied
to long-term (decadal), multi-generational responses by populations, involving adaptation and evolution?
Does genetic variation confer population resilience? How will this impact marine biodiversity?
Ecological How can experimental studies on ocean acidication impacts be best scaled-up to the ecosystem level
where interacting multi-species communities are subject to other environmental changes, i.e., allowing for
multi-stressor effects, and recognising that negative (or positive) impacts of ocean acidication on one
species may indirectly benet (or disadvantage) another and thus community composition and biodiversity?
How will impacts on one species impact upon others (trophic interactions), and how will this affect food
security through the food chain?
Socio-economic What future socio-economic impacts will arise from ocean acidication? How can we best quantify the
risks to non-market ecosystem services (e.g., storm protection provided by tropical coral reefs) as well
as to aquaculture and sheries? Can adaptation strategies be identied for the most vulnerable people
and industries? How are various types of communities (from indigenous and local communities to global
markets) differentially vulnerable to the impacts of ocean acidication? How can ocean acidication science
best contribute to risk management, the sustainable use of natural resources and national/international
policy development?

90
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
as well-fed organisms might have more energy to
compensate for regulatory changes. Ocean acidi-
cation may alter the behaviour of (and organism
response to) sediment-bound metals by altering their
bioavailability, as demonstrated by DNA damage
and acute toxity in amphipods
[15]
. is could aect
both population and community levels.
Ocean acidication at the population level.
To assess
the potential impacts of ocean acidication at the
population level, it is critical to evaluate dierent
life cycle stages of organisms, such as fertilization,
dispersal larval stages and recruitment. Various stud-
ies have demonstrated that early life stages may be
(but are not always) particularly vulnerable to ocean
acidication. Taking into account that many marine
invertebrates show high mortality rates during plank-
tonic larval stages, detrimental impact at these stages
can mean critical dierences to the population level.
e impact of ocean acidication on gametogene-
sis is a knowledge gap to be addressed across more
species and dierent timescales. Future work capable
of determining the eect of ocean acidication on
several life phases, and of subsequent generations of
the same species combined with population dynamic
models, is therefore required. Building on research
at dierent life stages, evolutionary adaptation could
be assessed at population levels by correlating ecol-
ogy, physiology and taxonomy with evolutionary
capacity, using known population sizes and recom-
bination rates
[5]
.
Ocean acidication at the community level. e
impact of ocean acidication on species interac-
tions remains relatively unstudied, but is a key area
to focus on if whole ecological communities are to
be considered
[16]
. ese interactions include chang-
ing food quality and how this constrains trophic
transfer
[17,18]
, predator-prey relationships
[19-21]
and
feeding rates
[22,23]
; how the presence of one species
(e.g., coralline algae) may directly impact upon the
recruitment or success of another (coral juveniles)
[24-26]
; resource competition
[27,28]
; and how all the
above will have “knock on” eects through compet-
itive interactions
[29]
and food webs. However, quan-
tifying species interactions will be complicated, as
interactions will also be aected by conditioning
time, biotic interactions, and initial community
compositions
[30]
. Embedded within this is the need
to understand the adaptation potential of dier-
ent species, and taxa sensitivity to ocean acidica-
tion
[31]
. A number of major experiments have been
conducted on pelagic communities over several
weeks using mesocosm approaches
[32]
, but there are
few such experiments for benthic systems. While
mesocosm experiments are an extremely valuable
tool for assessing community responses to manipu-
lated variables, there remain scale-dependent chal-
lenges in extrapolating results to ecosystems
[33]
.
Ocean acidication at the ecosystem level.
Natural
volcanic CO vents have provided new insights
on the eects of ocean acidication at the ecosys-
tem level and are a good opportunity to document
species-species and species-environment interactions
under low pH conditions. ese species-environ-
ment interactions are very important to consider, as
simple impacts upon key species may have cascad-
ing eects through the ecosystem
[10]
. For example,
ocean acidication can modify the relationship
between the burrowing shrimp Upogebia deltaura
and ammonia-oxidising microorganisms inhab-
iting their burrows, potentially negating positive
impacts of shrimp bioturbation
[4]
. is could impact
benthic-pelagic nitrogen cycling, which is funda-
mental to the food web and the ecosystem dynam-
ics as a whole
[4,16]
. While these natural systems are
extremely useful in the present, further examina-
tion of the past (see Chapter 4), can also increase
our understanding of how calcifying communities
and ecosystems changed under similar past events.
9.2 THEORETICAL CHALLENGES AND FUTURE PRIORITIES
Whatever technological advances are made, it will
still be impossible to assess the eect of ocean acidi-
cation on all species, all ecosystems and all services in
all parts of the world. us information from specic
studies will still need to be generalised. But there is
currently another fundamental limitation: the lack
of theoretical background regarding the overarching
principles of ocean acidication eects, applicable

9. FUTURE CONSIDERATIONS
91
Box 9.1 Examples of approaches and technical challenges
Laboratory-based perturbation experiments: Understanding of mechanisms in action is needed to improve our predictive
power. This will be possible through a better understanding of the biological responses at molecular and physiological
levels. This is often associated with technological challenges. For example, new in vivo techniques have been developed to
measure extra- and intra-cellular acid-base regulation and digestion in sea urchin larvae
[34,35]
.
Field-based perturbation experiments: Free Ocean CO
2
Enrichment (FOCE) systems have been developed in order
to study the effects of ocean acidication on benthic communities by controlling, for several months, the pH to which
a natural community is exposed. While the original system was designed for a deployment in the deep-sea, worldwide
projects are presently adapting the system to study shallow water areas in temperate and polar environments
[36]
. The
usefulness of this approach was recently demonstrated in a tropical coral reef setting.
Natural CO
2
vents case studies: From studying ecosystems near natural CO
2
vents it is clear that acidication can cause
fundamental changes at the ecosystem level: calcifying communities may shift to algal-dominant ecosystems
[37]
, or
undergo a change in species dominance such as in Papua New Guinea
[38]
, or shift community type
[39]
. A consistent feature
of these studies is that species diversity decreases near CO
2
vents. Importantly, natural CO
2
vent ecosystems include non-
calcifying organisms, which can strongly contribute to species competition and ecosystem function
[40]
.
In vivo measurements of larval stomach pH using ion-selective micro-
electrodes. Adapted from
[34]
. Reprinted by permission from Macmillan
Publishers Ltd: Nature Climate Change 3: 1044-1049, © 2013
The coral proto – free ocean carbon enrichment system
(CP–FOCE) deployed on Heron Island, Great Barrier
Reef, Australia. Source: David Kline, Scripps.
Left to right: healthy coral reef at Papua New Guinea control site, pH 8.1, unaffected by CO
2
seep; seascape showing
moderate seeps, pH 7.8-8.0; and barren seascape showing intense venting of CO
2
and a pH of <7.7, when all coral
growth stops. Images courtesy of Katharina Fabricius.

92
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
across many domains. Conceptual, analytical, and
computational models are invaluable to explain
pattern in nature. e theoretical frameworks and
unifying principles that explain many other topics
and themes in ocean science (e.g., in chemistry, phys-
iology, ecology, evolution, and socio-economics)
need to be much better developed for ocean acidi-
cation, to assist with prediction and anticipation
of its eects, from global to local scales.
Ocean acidication is already underway, and it is
now inevitable that it will, in combination with other
stressors, have signicant eects on marine ecosys-
tems and their services to humankind. Ultimately,
only the reduction of atmospheric CO levels
provides the “solution” to ocean acidication; never-
theless, there may be ways in which ocean acidica-
tion research can become more “solution-oriented”
– rather than only “documenting the disaster”. To
prioritize research eciently, sensitivity should be
assessed at all levels: i) chemistry (e.g., regions expe-
riencing the greatest and fastest changes, such as
polar regions, upwelling zones, and shelf seas); ii)
biology (e.g., sensitive species or ecosystem, biodiver-
sity at risk, etc.), and iii) socio-economics (e.g., less-
developed countries or high dependence on ocean).
By improving our understanding of the impacts of
ocean acidication, we will be able to identify the
organisms and ecosystems most at risk that deserve
the most urgent attention.
Experiments investigating how biota will respond
to ocean acidication have, until recently, largely
focused only on the manipulation of the carbonate
system.
However, marine organisms and ecosystems
are increasingly stressed by other changes in their
physical, chemical and biological environments.
For global variables, there has been considerable
progress in model projections in the past two
years, in conjunction with the preparation of the
5
th
Assessment Report of the Intergovernmental
Panel on Climate Change (AR5). Recent simula-
tions performed in the framework of the Coupled
Model Intercomparison Project 5 have assessed how
several drivers will evolve during the 21st century
[42]
.
For the “business-as-usual” scenario, the model-
mean changes in 2090s (compared to 1990s) for
sea surface temperature, sea surface pH and global
O content amount to +2.7°C, ~-0.33 pH unit, and
-3.5%, respectively. For the “high mitigation eorts
now” scenario, corresponding changes are +0.7°C,
-0.07 pH unit and -1.8%.
Ocean acidication can interact with other variables
synergistically (amplied stress), additively (no addi-
tional stress), or antagonistically (reduced stress)
[43]
.
In 2012, only around a third of the 225 papers that
reported on the biological response to ocean acidi-
cation also manipulated at least one other environ-
mental property. is is a large increase compared
to previous years but knowledge of the impacts of
multiple drivers is still insucient to provide reli-
able projections of biodiversity and ecosystem func-
tion. e challenges associated with conducting more
complex manipulation experiments that include
combined temperature, O stressors, and ultraviolet
radiation in conjunction with ocean acidication are
technological, but also include experimental design
(e.g., replication vs. regression approach, pseudo-
replication, number of treatments for each driver).
However, even then, controlled experiments may not
capture the full complexity of ecosystems. To fully
address the need for multiple-driver approaches,
comparative ecosystem analyses that combine both
experimental observations and models are needed
[44]
.
9.3 ADVANCES IN SENSING, MONITORING AND EMERGING
TECHNOLOGIES
Measuring pH and the other variables of the marine
carbonate system has traditionally been challeng-
ing due to inconsistent pH scales and measurement
routines, as well as non-standardized instrumen-
tation that required skilled technical expertise and
experience. Consequently, inter-comparison exer-
cises between laboratories revealed large discrep-
ancies
[45]
. Over the last 30 years, great strides have
been taken in standardizing our understanding of
what exactly the marine carbonate system is, how to

9. FUTURE CONSIDERATIONS
93
measure it and how to report the results, the avail-
ability of very high quality reference material, and the
standardization of the pH scale for the reporting of
ocean acidication – the total hydrogen ion scale
[46]
.
e majority of established long-term ocean acidi-
cation time series have used standard, shipboard
and laboratory instrumentation for measurements
of the four marine carbonate system variables: pH,
total alkalinity, total inorganic carbon and the partial
pressure (or fugacity) of carbon dioxide in seawa-
ter
[47]
. Using any two of these variables enables the
calculation of the other two, plus the speciation
of the marine carbonate system, calcium carbon-
ate saturation states, buer capacity and the major
contributions to the total alkalinity.
As indicated in Chapter 3, a high density of measure-
ments in both time and space are required if the
fine details of local, regional and global ocean
acidication are to be routinely identied. New
approaches include the adaption of existing tech-
niques, such as ion selective eld eect transistors
(ISFETs)
[48]
and the development of approaches to
enable remote measurements, thus removing the
need for an operator. ese include measurements
from autonomous systems, voluntary observing
ships (or ship of opportunity
[49]
), buoys, proling
oats
[50]
, and wave-riders
[51]
and landers
[52]
(Figure
9.1). Adapting these methods have led to the devel-
opment of novel combinations
[53]
and lower-cost,
low-power, long-term measurement techniques
[54]
that are approaching, and sometimes excelling, the
accuracy of traditional methods. By incorporat-
ing new approaches into the expanding range of
monitoring platforms, through initiatives such as
the Global Ocean Acidication Observing Network
(GOA-ON, see chapters 2 and 3), the current knowl-
edge of natural variability across space and time
will be improved.
Experimental studies can then be
better placed into context (natural variability versus
projected changes), and projections of future condi-
tions will become more accurate and reliable.
References
1. Dupont S, Pörtner H (2013) Get ready for ocean
acidication. Nature 498: 429-429.
2. Dupont S, Pörtner HO (2013) A snapshot of
ocean acidication research. Marine Biology 160:
1765-1771.
3. Godbold JA, Solan M (2013) Long-term eects
of warming and ocean acidication are modied
by seasonal variation in species responses and
environmental conditions. Philosophical Transactions
of the Royal Society B: Biological Sciences 368:
20130186.
4. Laverock B, Kitidis V, Tait K, Gilbert JA, Osborn AM,
et al. (2013) Bioturbation determines the response of
benthic ammonia-oxidizing microorganisms to ocean
acidication. Philosophical Transactions of the Royal
Society B: Biological Sciences 368: 20120441.
5. Sunday JM, Crim RN, Harley CDG, Hart MW (2011)
Quantifying rates of evolutionary adaptation in
response to ocean acidication. PLoS ONE 6: e22881.
6. McElhany P, Busch DS (2013) Appropriate pCO
treatments in ocean acidication experiments. Marine
Biology 160: 1807-1812.
Figure 9.1. A cartoon of various platforms for environmental sensors. These include
autonomous and remotely controlled underwater vehicles, wave gliders, moorings and
benthic landers. Data from these sensors can be used to record physical properties of
ecosystems.
Source: Heriot-Watt University.

94
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
7. Homann D, Evans TG, Kelly MW, Padilla-Gamino
JL, Blanchette CA, et al. (2014) Exploring local
adaptation and the ocean acidication seascape
– studies in the California Current Large Marine
Ecosystem. Biogeosciences 11: 1053-1064.
8. Wicks LC, Roberts JM (2012) Benthic invertebrates
in a high-CO world. Oceanography and Marine
Biology: An Annual Review 50: 127-188.
9. Widdicombe S, Spicer J (2008) Predicting the impact
of ocean acidication on benthic biodiversity:
What can animal physiology tell us? Journal of
Experimental Marine Biology and Ecology 366:
187-197.
10. Melzner F, Gutowska MA, Langenbuch M, Dupont
S, Lucassen M, et al. (2009) Physiological basis for
high CO tolerance in marine ectothermic animals:
pre-adaptation through lifestyle and ontogeny?
Biogeosciences 6: 2313-2331.
11. Hoppe CJM, Langer G, Rost B (2011) Emiliania
huxleyi shows identical responses to elevated pCO in
TA and DIC manipulations. Journal of Experimental
Marine Biology and Ecology 406: 54-62.
12. Calosi P, Rastrick SP, Lombardi C, de Guzman
HJ, Davidson L, et al. (2013) Adaptation and
acclimatization to ocean acidication in marine
ectotherms: an in situ transplant experiment
with polychaetes at a shallow CO vent system.
Philosophical Transactions of the Royal Society B:
Biological Sciences 368: 20120444.
13. Benner I, Diner RE, Lefebvre SC, Li D, Komada T, et
al. (2013) Emiliania huxleyi increases calcication but
not expression of calcication-related genes in long-
term exposure to elevated temperature and pCO.
Philosophical Transactions of the Royal Society B:
Biological Sciences 368: 20130049.
14. Edmunds PJ (2011) Zooplanktivory ameliorates
the eects of ocean acidication on the reef coral.
Limnology 56: 1-11.
15. Roberts DA, Birchenough SNR, Lewis C, Sanders MB,
Bolam T, et al. (2013) Ocean acidication increases
the toxicity of contaminated sediments. Global
Change Biology 19: 340-351.
16. Godbold JA, Calosi P (2013) Ocean acidication and
climate change: advances in ecology and evolution.
Philosophical Transactions of the Royal Society B:
Biological Sciences 368: 20120448.
17. Branch TA, DeJoseph BM, Ray LJ, Wagner CA (2013)
Impacts of ocean acidication on marine seafood.
Trends in Ecology and Evolution 28: 178-186.
18. Rossoll D, Bermudez R, Hauss H, Schulz KG,
Riebesell U, et al. (2012) Ocean acidication-induced
food quality deterioration constrains trophic transfer.
PLoS ONE 7: 34737.
19. Ferrari MCO, Manassa RP, Dixson DL, Munday
PL, McCormick MI, et al. (2012) Eects of ocean
acidication on learning in coral reef shes. PLoS
ONE 7: e31478.
20. Ferrari MCO, McCormick MI, Munday PL, Meekan
MG, Dixson DL, et al. (2011) Putting prey and
predator into the CO equation - qualitative and
quantitative eects of ocean acidication on predator-
prey interactions. Ecology Letters 14: 1143-1148.
21. Cripps IL, Munday PL, McCormick MI (2011) Ocean
acidication aects prey detection by a predatory reef
sh. PLoS ONE 6: e22736
22. Gooding RA, Harley CDG, Tang E (2009) Elevated
water temperature and carbon dioxide concentration
increase the growth of a keystone echinoderm.
Proceedings of the National Academy of Sciences of
the United States of America 106: 9316-9321.
23. Kurihara H, Yin R, Nishihara GN, Soyano K,
Ishimatsu A (2013) Eect of ocean acidication on
growth, gonad development and physiology of the sea
urchin Hemicentrotus pulcherrimus. Aquatic Biology
18: 281-292.
24. Albright R, Langdon C (2011) Ocean acidication
impacts multiple early life history processes of the
Caribbean coral Porites astreoides. Global Change
Biology 17: 2478-2487.
25. Doropoulos C, Diaz-Pulido G (2013) High CO
reduces the settlement of a spawning coral on three
common species of crustose coralline algae. Marine
Ecology Progress Series 475: 93-99.
26. Doropoulos C, Ward S, Diaz-Pulido G, Hoegh-
Guldberg O, Mumby PJ (2012) Ocean acidication
reduces coral recruitment by disrupting intimate
larval-algal settlement interactions. Ecology Letters
15: 338-346.
27. Tatters AO, Roleda MY, Schnetzer A, Fu F, Hurd CL,
et al. (2013) Short- and long-term conditioning of a
temperate marine diatom community to acidication
and warming. Philosophical Transactions of the Royal
Society B: Biological Sciences 368: 20120437.
28. Tatters AO, Schnetzer A, Fu F, Lie AY, Caron DA,
et al. (2013) Short- versus long-term responses to
changing CO
2
in a coastal dinoagellate bloom:
implications for interspecic competitive interactions
and community structure. Evolution 67: 1879-1891.
29. Diaz-Pulido G, Gouezo M, Tilbrook B, Dove S,
Anthony KRN (2011) High CO
2
enhances the
competitive strength of seaweeds over corals. Ecology
Letters 14: 156-162.
30. Eggers SL, Lewandowska AM, Ramos JBE, Blanco-
Ameijeiras S, Gallo F, et al. (2014) Community
composition has greater impact on the functioning
of marine phytoplankton communities than ocean
acidication. Global Change Biology 20: 713-723.

9. FUTURE CONSIDERATIONS
95
31. Wittmann AC, Portner H-O (2013) Sensitivities of
extant animal taxa to ocean acidication. Nature
Climate Change 3: 995-1001.
32. Riebesell U, Czerny J, von Broeckel K, Boxhammer
T, Buedenbender J, et al. (2013) Technical
Note: A mobile sea-going mesocosm system -
new opportunities for ocean change research.
Biogeosciences 10: 1835-1847.
33. Gardner RH, Kemp WM, Kennedy VS, Peterson
JE (eds) (2001) Scaling Relations in Experimental
Ecology. New York: Columbia University Press.
34. Stumpp M, Hu M, Casties I, Saborowski R, Bleich M,
et al. (2013) Digestion in sea urchin larvae impaired
under ocean acidication. Nature Climate Change 3:
1044-1049.
35. Stumpp M, Hu MY, Melzner F, Gutowska MA,
Dorey N, et al. (2012) Acidied seawater impacts
sea urchin larvae pH regulatory systems relevant for
calcication. Proceedings of the National Academy
of Sciences of the United States of America 109:
18192-18197.
36. Free Ocean CO
2
Enrichment (FOCE) http://www.
antarctica.gov.au/about-us/publications/australian-
antarctic-magazine/2011-2015/issue-24-june-2013/
science/building-a-future-ocean
37. Hall-Spencer JM, Rodolfo-Metalpa R, Martin S,
Ransome E, Fine M, et al. (2008) Volcanic carbon
dioxide vents show ecosystem eects of ocean
acidication. Nature 454: 96-99.
38. Fabricius KE, Langdon C, Uthicke S, Humphrey
C, Noonan S, et al. (2011) Losers and winners in
coral reefs acclimatized to elevated carbon dioxide
concentrations. Nature Climate Change 1: 165-169.
39. Inoue S, Kayanne H, Yamamoto S, Kurihara H (2013)
Spatial community shi from hard to so corals in
acidied water. Nature Climate Change 3: 683-687.
40. Connell SD, Kroeker KJ, Fabricius KE, Kline DI,
Russell BD (2013) e other ocean acidication
problem: CO as a resource among competitors for
ecosystem dominance. Philosophical Transactions of
the Royal Society B: Biological Sciences 368: 20442.
41. Ilyina T, Zeebe RE, Brewer PG (2010) Future
ocean increasingly transparent to low-frequency
sound owing to carbon dioxide emissions. Nature
Geoscience 3: 18-22.
42. Bopp L, Resplandy L, Orr JC, Doney SC, Dunne JP,
et al. (2013) Multiple stressors of ocean ecosystems
in the 21st century: projections with CMIP5 models.
Biogeosciences 10: 6225-6245.
43. Byrne M, Przeslawski R (2013) Multistressor impacts
of warming and acidication of the ocean on
marine invertebrates’ life histories. Integrative and
Comparative Biology 53: 582-596.
44. Murawski SA, Steele JH, Taylor P, Fogarty MJ,
Sissenwine MP, et al. (2010) Why compare marine
ecosystems? ICES Journal of Marine Science 67: 1-9.
45. Poisson A, Culkin F, Ridout P (1990) Intercomparison
of CO measurements. Deep-Sea Research Part A:
Oceanographic Research Papers 37: 1647-1650.
46. Dickson AG, Sabine CL, Christian JR (2007) Guide to
Best Practices for Ocean CO Measurements. PICES.
191 p.
47. Schuster U, Hannides A, Mintrop L, Koertzinger A
(2009) Sensors and instruments for oceanic dissolved
carbon measurements. Ocean Science 5: 547-558.
48. Martz TR, Connery JG, Johnson KS (2010) Testing
the Honeywell Durafet for seawater pH applications.
Limnology and Oceanography: Methods 8: 172-184.
49. Hardman-Mountford NJ (2008) An operational
monitoring system to provide indicators of
CO-related variables in the ocean. ICES Journal of
Marine Science 65: 1498-1503.
50. http://www.mbari.org/chemsensor/oatviz.htm
51. Wave Riders http://www.pmel.noaa.gov/co2/story/
Carbon+Wave+Glider
52. Gray SEC, DeGrandpre MD, Langdon C, Corredor
JE (2012) Short-term and seasonal pH, pCO and
saturation state variability in a coral-reef ecosystem.
Global Biogeochemical Cycles 26: GB3012.
53. Easley RA, Patsavas MC, Byrne RH, Liu X, Feely
RA, et al. (2013) Spectrophotometric measurement
of calcium carbonate saturation states in seawater.
Environmental Science & Technology 47: 1468-1477.
54. Rerolle VMC, Floquet CFA, Harris AJK, Mowlem
MC, Bellerby RRGJ, et al. (2013) Development of a
colorimetric microuidic pH sensor for autonomous
seawater measurements. Analytica Chimica Acta 786:
124-131.

96
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
e rate of ocean acidication that we have experi-
enced since pre-industrial times and its projected
continuation are “potentially unparalleled in at least
the last ~300 million years of Earth history”
[1]
.
As
such, current ocean acidication represents a new
and unprecedented chapter of marine ecosystem
change that seems very likely to have a signicant
impact on marine species and ecosystems (including
economically important species), on various indus-
tries and communities, and on global food security.
At the Paleo-Eocene ermal Maximum (56 million
years ago), believed to be the closest historical
analogue to present-day ocean acidication, geolog-
ical records indicate that several deep-sea organisms
became extinct. e speed at which ocean acidi-
cation is currently happening precludes the option
of habitat shis for many benthic species, and may
exceed their ability to adapt.
At current rates, aragonite saturation horizons, below
which aragonite dissolution occurs, are projected
to rise from a few thousand metres to just a few
hundred metres, or to the surface, in many ocean
regions by the end of the century
[2]
. If CO emis-
sions continue on a “business as usual scenario,” it
is projected that by the end of the century global
mean surface pH will further decrease by ~0.33 units
(with H
+
concentrations more than doubling), and
sea surface temperature will increase by 2.7°C
[3]
,
although with considerable regional variability.
Our understanding of ocean acidication and its
consequences has increased tremendously in the past
10 years
[4]
, and research to date, from both labora-
tory and in situ work, has highlighted that organism
responses to ocean acidication can be very mixed,
even between similar species
[5]
. is variability reects
that some species may be better adapted for projected
future conditions than others; it also highlights that
experiment conditions (particularly duration) are
important in assessing future long-term responses.
Some general trends are emerging. Ocean acidication
will have a negative eect on calcication or growth
at dierent life cycle stages in many key organisms,
such as commercial shellsh and corals
[6-10]
, although
adequate food supplies may ameliorate some negative
responses
[11,12]
. Most sh are probably able to main-
tain sucient oxygen delivery under predicted future
CO levels
[13]
, but increased CO can have signicant
impacts upon sh behaviour
[14]
.
Sensitivity to ocean acidication varies at dierent
life stages, so understanding how negative impacts
can “carry-over”
[15]
from larval to adult stages
remains a signicant challenge. Ocean acidica-
tion is generally detrimental to calcifying larvae
[16-18]
;
non-calcifying larvae are more resilient
[19-21]
. e
impacts of ocean acidication on fertilization success
are highly variable highlighting the potential for
selection and genetic adaptation, and supporting
the concept of “winners and losers” in the face of
changing ocean conditions
[22,23]
.
Impacts of ocean acidication will be most keenly and
rapidly experienced in the Arctic and Antarctic envi-
ronments due to their low temperatures, aecting satu-
ration state. e Arctic Monitoring and Assessment
Programme (AMAP) has shown that acidication will
not be uniform across the Arctic Ocean. While impacts
in that region may be positive for some species, other
species may face extinction; furthermore, acidica-
tion may contribute to an alteration in the abundance
of dierent sh species, with potential impact upon
the livelihoods of local communities
[24]
.
When considering how ocean acidication will aect
human society, the response of tropical coral ecosys-
tems is understandably of great concern – since over
400 million people worldwide live within 100 km
of coral reefs, with very many reliant on them for
their livelihoods and food security
[25,26]
. e fact that
over 95% of the world’s calcifying corals currently
occur above the saturation horizon
[27]
, and that coral
growth is much reduced near natural CO vents
[28]
,
indicates that in the long-term, it is unlikely to be
energetically feasible for corals to grow and thrive
below the saturation horizon. Any reduction in coral
growth (tropical or cold-water) in the future will
have repercussions for the communities that directly
or indirectly rely upon them.
10. CONCLUSIONS

10. CONCLUSIONS
97
e economic costs of ocean acidication are only
partially known, with many studies focussing on
local rather than global costs. Nevertheless, the global
cost of ocean acidication impacts on molluscs and
tropical coral reefs is estimated to be over US $1000
billion annually by the end of the century
[29,30]
. ese
calculations are inherently dicult, being based on
what we can currently predict, which largely centre
on loss of earnings and a limited selection of ecosys-
tem services. e actual costs are likely to be in excess
of this gure, particularly when taking account of
potentially compounding factors such as oversh-
ing, sedimentation and temperature rise.
It is important to note that the response to ocean
acidication in coastal regions will be inuenced by
more variable conditions than in the open ocean
[31]
.
Such varying conditions (caused by diel commu-
nity metabolism, local phytoplankton blooms and
watershed processes) could complicate the predic-
tions we can currently make, e.g., by forcing (rela-
tively) rapid selection of tolerant genotypes.
Looking to the immediate future, it is vital to increase
our understanding of how multiple stressors may
aect marine biodiversity and ecosystems
[32]
, as
ocean acidication will be accompanied by, inter
alia, changes in oxygen saturation, temperature
[3]
,
and ultraviolet radiation in surface waters
[33]
. Our
current knowledge of the impacts of multiple driv-
ers is still insucient to provide reliable projections
of biodiversity and ecosystem function; this should
be a priority for future work. Increased monitoring
capacity is also crucial to understand the current
variability in ecosystems and the rate of change they
are experiencing. is should include advances in
autonomous underwater vehicle (AUV) sensing
technology to monitor key benthic and polar ecosys-
tems currently near to aragonite and calcite satura-
tion horizons.
e incorporation of ocean acidication into govern-
mental planning, environmental conservation and
sustainable living has started to accompany growing
awareness of the problem
[34-36]
. is is a very posi-
tive step that has been accompanied by several inter-
national research consortia involved in addressing
key questions to inform policy-making decisions.
However, even if CO emissions are signicantly
curtailed now, anthropogenic ocean acidication
will still last tens of thousands of years. Signicant
ocean ecosystem changes, and the need to learn
to live with those changes, therefore seem certain.
References
1. Honisch B, Ridgwell A, Schmidt DN, omas E,
Gibbs SJ, et al. (2012) e geological record of ocean
acidication. Science 335: 1058-1063.
2. Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, et
al. (2005) Anthropogenic ocean acidication over
the twenty-rst century and its impact on calcifying
organisms. Nature 437: 681-686.
3. Bopp L, Resplandy L, Orr JC, Doney SC, Dunne JP,
et al. (2013) Multiple stressors of ocean ecosystems
in the 21st century: projections with CMIP5 models.
Biogeosciences 10: 6225-6245.
4. Gattuso JP, Hansson L (eds) (2011) Ocean
Acidication. Oxford: Oxford University Press. 326 p
5. Wicks LC, Roberts JM (2012) Benthic invertebrates
in a high-CO world. Oceanography and Marine
Biology: An Annual Review 50: 127-188.
6. Armstrong RA, Lee C, Hedges JI, Honjo S, Wakeham
SG (2002) A new, mechanistic model for organic
carbon uxes in the ocean based on the quantitative
association of POC with ballast minerals. Deep-Sea
Research Part II: Topical Studies in Oceanography 49:
219-236.
7. Klaas C, Archer DE (2002) Association of sinking
organic matter with various types of mineral ballast
in the deep sea: Implications for the rain ratio. Global
Biogeochem Cycles 16: 1116.
8. Erez J, Reynaud S, Silverman J, Schneider K,
Allemand D (2011) In: Dubinsky Z, Stambler N (eds).
Coral Reefs: An Ecosystem in Transition. Springer.
9. Hoegh-Guldberg O, Mumby P, Hooten A, Steneck
R, Greeneld P, et al. (2007) Coral reefs under rapid
climate change and ocean acidication. Science 318:
1737.
10. Gazeau F, Parker LM, Comeau S, Gattuso J-P,
O’Connor WA, et al. (2013) Impacts of ocean
acidication on marine shelled molluscs. Marine
Biology 160: 2207-2245.
11. Edmunds PJ (2011) Zooplanktivory ameliorates
the eects of ocean acidication on the reef coral.
Limnology 56: 1-11.
12. Melzner F, Stange P, Truebenbach K, omsen J,
Casties I, et al. (2011) Food supply and seawater pCO
impact calcication and internal shell dissolution in
the blue mussel Mytilus edulis. PLoS ONE 6: e24223.

98
AN UPDATED SYNTHESIS OF THE IMPACTS OF OCEAN ACIDIFICATION ON MARINE BIODIVERSITY
13. Munday PL, McCormick MI, Nilsson GE (2012)
Impact of global warming and rising CO levels on
coral reef shes: what hope for the future? Journal of
Experimental Biology 215: 3865-3873.
14. Munday PL, Pratchett MS, Dixson DL, Donelson
JM, Endo GGK, et al. (2013) Elevated CO aects
the behavior of an ecologically and economically
important coral reef sh. Marine Biology 160:
2137-2144.
15. Pechenik JA (1999) On the advantages and
disadvantages of larval stages in benthic marine
invertebrate life cycles. Marine Ecology Progress
Series 177: 269-297.
16. Byrne M (2011) Impact of ocean warming and ocean
acidication on marine invertebrate life history
stages: vulnerabilities and potential for persistence
in a changing ocean. In: Gibson RN, Atkinson RJA,
Gordon JDM, editors. Oceanography and Marine
Biology: an Annual Review, Vol 49. pp. 1-42.
17. Dupont S, Havenhand J, orndyke W, Peck L,
orndyke M (2008) Near-future level of CO-driven
ocean acidication radically aects larval survival
and development in the brittlestar Ophiothrix fragilis.
Marine Ecology Progress Series 373: 285-294.
18. Byrne M, Ho M, Wong E, Soars NA,
Selvakumaraswamy P, et al. (2011) Unshelled abalone
and corrupted urchins: development of marine
calciers in a changing ocean. Proceedings of the
Royal Society B - Biological Sciences 278: 2376-2383.
19. Dupont S, Lundve B, orndyke M (2010) Near
future ocean acidication increases growth rate of
the lecithotrophic larvae and juveniles of the sea star
Crossaster papposus. Journal of Experimental Zoology
314B: 382-389.
20. Byrne M, Gonzalez-Bernat M, Doo S, Foo S, Soars
N, et al. (2013) Eects of ocean warming and
acidication on embryos and non-calcifying larvae
of the invasive sea star Patiriella regularis. Marine
Ecology Progress Series 473: 235-246.
21. Chua CM, Leggat W, Moya A, Baird AH (2013)
Temperature aects the early life history stages of
corals more than near future ocean acidication.
Marine Ecology Progress Series 475: 85-92.
22. Foo SA, Dworjanyn SA, Poore AGB, Byrne M (2012)
Adaptive capacity of the habitat modifying sea urchin
Centrostephanus rodgersii to ocean warming and
ocean acidication: performance of early embryos.
PLoS ONE 7: e42497
23. Schlegel P, Havenhand JN, Gillings MR, Williamson
JE (2012) Individual variability in reproductive
success determines winners and losers under ocean
acidication: A case study with sea urchins. PLoS
ONE 7: e53118
24. Arctic Monitoring and Assessment Programme
(2013).
AMAP Assessment 2013: Arctic Ocean
Acidication. AMAP, Oslo, Norway. 99p http://www.
amap.no/documents/doc/AMAP-Assessment-2013-
Arctic-Ocean-Acidication/881
25. Teh LSL, Teh LCL, Sumaila UR (2013) A global
estimate of the number of coral reef shers. PLoS
ONE 8: e65397.
26. Donner SD, Potere D (2007) e inequity of the
global threat to coral reefs. Bioscience 57: 214-215.
27. Guinotte JM, Orr JC, Cairns S, Freiwald A, Morgan
L, et al. (2006) Will human-induced changes in
seawater chemistry alter the distribution of deep-
sea scleractinian corals? Frontiers in Ecology and the
Environment 4: 141-146.
28. Fabricius KE, Langdon C, Uthicke S, Humphrey
C, Noonan S, et al. (2011) Losers and winners in
coral reefs acclimatized to elevated carbon dioxide
concentrations. Nature Climate Change 1: 165-169.
29. Brander L.M., Narita D, Rehdanz K, Tol RSJ (In press)
e economic impact of ocean acidication. In: Paulo
A.L.D., Nunes PALD, P. K, T. D, editors. Economics of
biodiversity and ecosystem services: Edward Elgar.
30. Cooley SR, Lucey N, Kite-Powell H, Doney SC (2012)
Nutrition and income from molluscs today imply
vulnerability to ocean acidication tomorrow. Fish
and Fisheries 13: 182-215.
31. Duarte CM, Hendriks IE, Moore TS, Olsen YS,
Steckbauer A, et al. (2013) Is Ocean Acidication
an Open-Ocean Syndrome? Understanding
Anthropogenic Impacts on Seawater pH. Estuaries
and Coasts 36: 221-236.
32. Noone KJ, Sumaila UR, Diaz RJ (2013) Managing
ocean environments in a changing climate:
sustainability and economic perspectives: Elsevier.
33. IGBP, IOC, SCOR (2013) Ocean Acidication
Summary for Policymakers – ird Symposium
on the Ocean in a High-CO
2
World. International
Geosphere-Biosphere Programme. Stockolm, Sweden
34. Logan C (2010) A Review of Ocean Acidication and
America’s Response. Bioscience 60: 819-828.
35. Fauville G, Saljo R, Dupont S (2013) Impact of ocean
acidication on marine ecosystems: educational
challenges and innovations. Marine Biology 160:
1863-1874.
36. Pope A, Selna E (2013) Communicating Ocean
Acidication. Journal of Museum Educations 38:
279-285.

ANNEX
99
ANNEX
is report was coordinated and edited by the following expert group:
Dr. Sebastian Hennige
Heriot-Watt University, UK
Professor J. Murray Roberts
Heriot-Watt University, UK
Dr. Phillip Williamson
Natural Environment Research Council and
University of East Anglia, UK
Additional expert contributions were provided by:
Dr. Tracy Aze
Cardi University, UK
Dr. James Barry
Monterey Bay Aquarium Research Institute,
USA
Dr. Richard Bellerby
Bjerknes Centre for Climate Research, Norway
Dr. Luke Brander
Environmental Economics, Hong Kong SAR,
China
Professor Maria Byrne
University of Sydney, Australia
Dr. Sam Dupont
University of Gothenburg, Sweden
Professor Jean-Pierre Gattuso
Laboratoire d’Océanographie de Villefranche,
France
Dr. Samantha Gibbs
University of Southampton, UK
Ms. Lina Hansson
International Atomic Energy Agency, Monaco
Dr. Caroline Hattam
Plymouth Marine Laboratory, UK
Dr. Chris Hauton
University of Southampton, UK
Professor Jon Havenhand
University of Gothenburg, Sweden
Dr. Jan Helge Fosså
Institute of Marine Research, Norway
Mr. Christopher Kavanagh
International Atomic Energy Agency, Monaco
Dr. Haruko Kurihara
University of the Ryukyus, Japan
Dr. Richard Matear
Commonwealth Scientic and Industrial
Research Organisation, Australia
Dr. Felix Mark
Alfred Wegener Institute, Germany
Professor Frank Melzner
GEOMAR, Germany
Professor Philip Munday
James Cook University, Australia
Dr. Barbara Nieho
Alfred Wegener Institute, Germany
Professor Paul Pearson
Cardi University, UK
Professor Katrin Rehdanz
Kiel Institute, Germany
Dr. Sylvie Tambutté
Monaco Scientic Centre, Monaco
Dr. Carol Turley
Plymouth Marine Laboratory, UK
Dr. Alexander Venn
Monaco Scientic Centre, Monaco
Dr. Michel Warnau
International Atomic Energy Agency, Monaco
Dr. Jeremy Young
University College London, UK

