
Application Bulletin 280/3 e
Automated water content determination with the 874 OSP
Page 1 of
6
Application Bulletin 280/3 e
Automated water content determination with the 874 Oven
Sample Processor
Branch
All branches
Keywords
Titration; Karl Fischer titration; coulometric; gas extraction;
oven technique; automation; 874 Oven Sample Processor;
water standard; sodium tartrate
Summary
In principle the gas extraction or oven technique can be used
with all types of samples which release the contained water
when the samples are heated. The oven technique is
essential whenever direct Karl Fischer titration is impossible
because the sample contains interfering components or, due
to its consistency, is difficult to place in the titration vessel.
This Application Bulletin shows examples from food,
pharmaceutical, plastics and petrochemical industry to
describe automated water content determination using the
oven technique in combination with coulometric KF titration.
Instruments
874 Oven Sample Processor
KF Titrator (coulometric)
Electrodes
Double Pt wire electrode (for coulometry)
Generator electrode with or without diaphragm (for
coulometry)*
* For measurements of samples with absolute water contents
< 100 µg, the generator electrode with diaphragm is recommended.
Reagents
For the coulometric technique, special reagents suitable for
the oven technique are available.
Due to the constant gas flow during measurements, methanol
contained in the reagents evaporates. With the generator
electrode without diaphragm a decreasing methanol content
can lead to false high results, with recoveries of up to 110%.
The methanol loss should therefore be compensated
regularly to avoid too high results.
Coulometric reagents have a limited water capacity. The
capacity is equivalent to the amount of sulfur dioxide.
Decreasing sulfur dioxide concentrations (lower than half of
the initial concentration) lead to longer determination times.
The reagent should be completely replaced. The monitoring
of the reagent capacity and the exchange of the reagent can
be done by the titration system and the software.
Standards
Standards (with different water contents) suitable for the oven
technique are commercially available.
Carrier gas
The carrier gas transports the released water into the titration
vessel. In principle it is not important what gas is used.
However, due to oxidation reactions with oxygen, the thermal
stability of organic substances at higher temperatures is often
poor, when using dry air as carrier gas. Inert gases, usually
nitrogen, offer more flexibility and reliability.
For solids with a water content below 1% we recommend a
gas flow rate of 50 mL/min. Gas flow rates of up to 100
mL/min can be used for solids with higher water contents.
Higher flow rates do usually not lead to shorter determination
times.
Measurements in liquid samples (oil, fuel, solvents,…) should
be carried out using gas flow rates of 80 mL/min or higher.
Furthermore it is recommended that the inlet needle is
immersed in the sample and the gas is allowed to flow
through the sample.
General
The combination of the oven technique with coulometric Karl
Fischer titration is ideal for samples with low water content.
Foodstuff, pharmaceutical products, plastics or mineral oil
products can be analyzed fully automated and accurately.
In accordance with the gas extraction principle the water is
driven out of the heated sample by a stream of dry carrier gas
and transferred to the titration vessel, where the water
content is determined.

Application Bulletin 280/3 e
Automated water content determination with the 874 OSP
Page 2 of
6
Preparation
Sample
The sample is thoroughly mixed. Make sure the sample does
not change its water content during the mixing. The optimal
sample size depends on the water content of the sample.
Unfortunately there is no equation describing the relation
between water content and sample size. But there is a rule of
thumb: the higher the water content, the smaller the sample
size and vice versa. Sample weights that are too low have a
negative effect on the measuring accuracy (balance error).
The upper limit for the sample size is defined by the volume
of the sample vial. Theoretically the maximum sample weight
is also limited by the water capacity of the reagent. Usually
the capacity is approximately 1000 mg H
2
O per 100 mL
reagent (please contact reagent manufacturer for more
information). In other words, with one sample this capacity is
never used.
The absolute amount of water transferred to the titration
vessel is recommended to be in the range of 300 to 5000 µg.
If the absolute amount of water for a sample cannot be
reduced (e.g. smaller sample size …) also larger amounts of
water (> 5000 µg) can be determined using the oven
technique. Please be aware that in such cases the
determination times will increase.
The appropriate amounts of sample are weighed into the
sample vials and the vials are sealed with a septum cap.
Previous to usage, the vials and caps should be conditioned
for at least 24 hours at ambient air.
Table 2 on pages 5 and 6 shows recommended sample
weights for a choice of samples.
Instrument and software
The 874 Oven Sample Processor and the KF Titrator are
connected to a PC. The tiamo™ software is started. After the
instruments are recognized and therefore visible in the device
list, the work position for the conditioning vial, the tower
(sample positions) and the shift position are defined. The
tiamo™ software includes several preprogrammed methods
which can be loaded and used with an 874 system.
Depending on the KF Titrator, the methods need to be
adapted with the right instruments. For the analysis of sample
series, the following sequence of methods is recommended:
• systemprep
• blank value
• water content
The method “systemprep” is run once before a sample series.
This makes sure that the system of tubes is purged and ready
for the sample determinations. Afterwards the blank values of
empty sample vials are determined. Three replicas of the
blank value determination are recommended. Finally the
analysis of the water content of the sample takes place.
Normally a flow rate of the carrier gas (air, nitrogen or a
different inert gas) of 40 to 60 mL/min is sufficient. The flow
rate can be adjusted up to 150 mL/min to make sure that the
gas flow is high enough to transfer the released water as fast
as possible into the titration vessel. Generally the
determination time for liquid samples decreases if the gas
flow is increased. Avoid high gas flow rates when analyzing
solid samples which could swirl up.
The vials for conditioning, system preparation, determination
of the blank and the sample vials are placed on the rack of
the 874 Oven Sample Processor. For coulometric KF
titrations, the titration cell is filled with 150 mL of reagent and
then conditioned.
Temperature gradient
For samples whose temperature behavior is unknown, a so-
called temperature gradient is run (available temperature
range: 50 to 250 °C). The required method “temp gradient” is
a preprogrammed method included in the tiamo™ software.
This method uses a heating rate of 2 °C/min to heat up
samples from 50 to 250 °C. Figure 1 shows a theoretical
temperature gradient where the sample is heated from 50 to
250 °C in 100 min.
Fig. 1: Diagram showing a theoretical temperature gradient.
While a temperature gradient is being run it is possible to
record both, the amount of water released and the drift as a
function of time (see Fig. 2). The software tiamo™ offers the
possibility to correlate the amount of water released and the
drift with the oven temperature. This allows statements about
the kinetics of the water release as a function of the
temperature.

Application Bulletin 280/3 e
Automated water content determination with the 874 OSP
Page 3 of
6
Fig. 2: Temperature gradient (2 °C/min) of sodium tartrate
dihydrate showing the amount of released water and the
associated drift value as a function of the temperature
The temperature gradient of sodium tartrate (Fig. 2) shows
that the surface water and parts of the crystal water are
released starting at 50 °C up to approximately 120 °C. At
120 °C both, the amount of water which is released and the
drift increase again. Up to 140 °C the remaining water of
crystallization is released. After the water of crystallization
has been released, the drift decreases to its basic value of
approximately 10 µg/min. A sign of decomposition is the drift
value, which starts to increase at around 220 °C. Also the
color change of the sodium tartrate after running the
temperature gradient suggests decomposition (see figure 3).
Fig. 3: Sodium tartrate dihydrate before (left) and after (right)
running a temperature gradient.
The temperature curve can be used to determine the optimal
oven temperature for extracting the water from the sample.
This temperature should be high enough for the water to be
extracted completely without any decomposition of the
sample. The determination time should be kept as short as
possible. Therefore the oven temperature should be chosen
as high as possible, but approximately 20 °C below the start
of decomposition. This procedure works for many different
samples and can be used for a fast assessment of the oven
temperature.
However, for sodium tartrate dihydrate the manufacturer
recommends an oven temperature of 160 °C. According to
the temperature gradient in figure 2, an oven temperature of
200°C could be used. The reason for this discrepancy is the
fact that for some samples a heating rate of 2 °C is too high.
In such cases the heating rate may be reduced to find the
optimal oven temperature for the sample analysis. Figure 4
shows the temperature gradient of sodium tartrate dihydrate
using a low heating rate of 0.07 °C/min.
Fig. 4: Temperature gradient (0.07 °C/min) of sodium tartrate
dihydrate.
A closer look at the drift values between 170 and 220 °C
shows that the drift starts to increase already around 190 °C
(see figure 5). Therefore an oven temperature of 160 °C is
used for the analysis of sodium tartrate dihydrate.
Fig. 5: Temperature gradient (0.07 °C/min) of sodium tartrate
dihydrate between 170 and 220 °C.
Please note that the time to record a temperature gradient
increases if the heating rate is reduced. The temperature
gradient in figure 4 took more than 24 hours to finish.
Analysis
Each analysis consists of the following steps:
• Conditioning of the complete titration system (titration
cell and tubing)
Application Bulletin 280/3 e
Automated water content determination with the 874 OSP
Page 4 of
6
• Extraction of the water from the sample
• Transport of the water into the titration cell
• Karl Fischer titration
• Calculation of the result
Conditioning
Conditioning or titrating the titration cell to dryness is carried
out under stirring in the so-called conditioning position. This
conditioning step must be carried out before every
determination. The oven is heated up to the defined
temperature. After the temperature is reached and the
titration system is conditioned, the first measurement can
start.
Carrier gas dried with molecular sieve is used to transport the
released water into the titration cell, where the determination
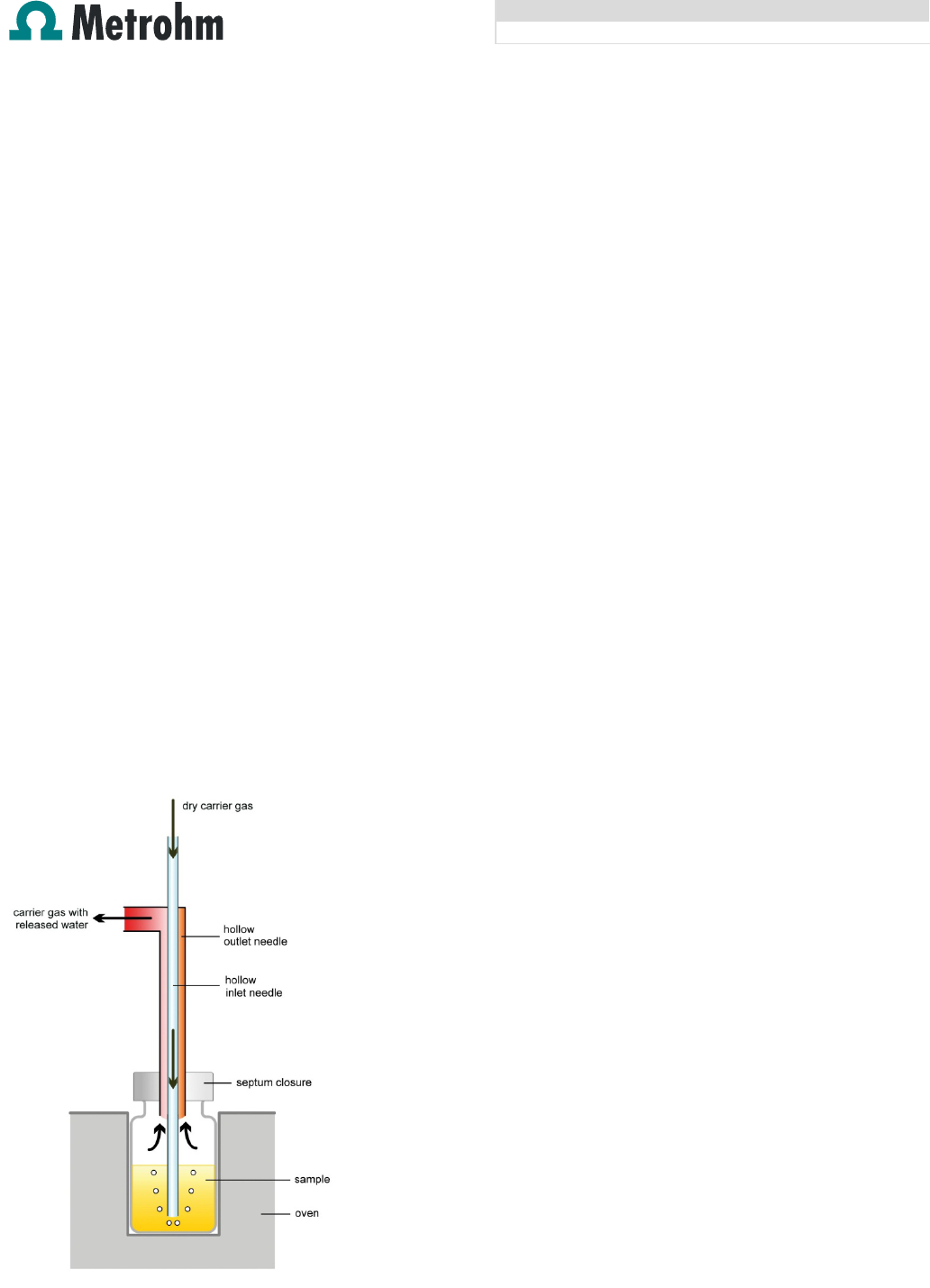
of the water content takes place. By means of a double hollow
needle (figure 6), the gas is led through the sample vial. The
length of the needle can be adjusted depending on the
sample which is analyzed. Generally it is recommended to
use a short immersion depth of the needle for solid samples
(needle holder 6.2049.050). This prevents the needle from
being blocked by the sample. In addition swirling of powdery
samples can be avoided. For liquid samples the needle can
be immersed in the sample (needle holder 6.2049.040). In
this way the gas flows through the sample, which mixes the
sample and leads to faster extraction of the water.
Fig. 6: Principle of the oven technique in liquid samples
Water extraction
The dried carrier gas is passed through the sample vial and
transfers the released water into the titration cell. The
temperature of the oven can be varied according to the
temperature stability of the sample.
It is generally advisable to set an extraction time. During the
extraction time no endpoint is accepted, even if the endpoint
criteria are fulfilled. In this way, there is enough time for the
sample to heat up to the defined temperature. We
recommend a time of 2 to 5 min.
Karl Fischer titration
For a coulometric Karl Fischer titration a KF reagent which
contains iodide is used. During the determination the iodide
is oxidized to iodine at the anode of the generator electrode.
The iodine is used for the KF reaction. The coulometric
technique is an absolute method and there is no titer
determination required. The endpoint indication is bi-
voltametric using a double Pt-wire electrode (indicator
electrode) to which a constant alternating current is applied.
This creates a potential difference (voltage) between the two
platinum wires. If even very small amounts of free iodine are
present, the voltage drops suddenly; which indicates the
endpoint of the titration.
Titration sequence
The water content determined by the gas extraction with
subsequent KF titration is made up as follows:
water content
absolute
=
water content
sample
+ blank value + drift x determination time
During “conditioning” the needle is located in the conditioning
vial, water contained in the system, is removed until a
constant low drift in the range of 1 to 10 µg/min is achieved.
If the automatic drift correction is activated the drift value must
be stable. A stabilizing time can be defined to ensure a stable
drift value. For drift correction, the drift value measured at the
start of the determination is multiplied with the determination
time and subtracted from the found water content at the end
of the determination.
Alternatively the value for drift correction can be determined
using a separate method, which is carried out prior to every
sample series. In a first step, the system is conditioned until
a stable drift is achieved. Depending on the reagent this can
take between approximately 15 min and 2 hours. Then the
consumption of iodine is recorded during 10 min. The
determined mean value is stored and can be used for drift
correction and as stop criterion. It is essential that during the
sample series the system drift does not change (e.g.
interfering additives in sample).

Application Bulletin 280/3 e
Automated water content determination with the 874 OSP
Page 5 of
6
System preparation means that the whole system is adjusted
to the selected conditions. An empty sample vial is treated in
exactly the same way as the following samples, but the value
is not taken into account. We recommend that this step –
which is at the same time used for checking that the analysis
system is working properly – is carried out before every new
sample series.
Apart from the water in the sample, the sample vial also
contains atmospheric humidity; this makes a blank value
determination necessary. A three-fold determination of the
blank value is normally sufficient. The mean value is stored
as a Common Variable and taken into account in the
calculation of the water content (subtracted). If large sample
sizes are used, it might be necessary to correct the blank
value (see KF Application note AN-K-048).
The system preparation and the blank value determination
must be carried out using the same method parameters as
for the analysis of the samples. In order to do this, an empty
system preparation vial and three empty blank value vials are
placed on the rack of the 874 Oven Sample Processor and
analyzed before the samples.
End of titration
The titration and the gas extraction of the sample is stopped
as soon as the drift value (amount of water per time) falls
below a predefined value. Usually the parameter “relative
drift” is used to stop a determination. The stop drift is
calculated by adding the drift at the start of the determination
and the value entered for the “relative drift”. The endpoint is
reached if the actual drift is smaller than the sum of the two
mentioned values. The higher the chosen value for the
“relative drift” the sooner the determination is stopped and the
more water remains in the sample. The “relative drift” value
should not be higher than 5 µg/min. If high accuracy is
required and for small water contents the “relative drift”
should be reduced (e.g. to 2 µg/min).
Parameters
Table 1: Recommended parameters for the oven technique in
combination with coulometry.
Parameter Setting
General parameters I(pol) 10 µA
Generator current
400 mA or
auto*
Control parameters
EP at
50 mV
Dynamics
70 mV
Max. rate
Maximum
µg/min
Min. rate
15 µg/min
Stop criterion rel. drift
Relative stop drift 5 µg/min
Conditioning
parameters
Start drift 10 µg/min
Stabilizing time 60 s
* 400 mA are used with the generator electrode without diaphragm.
The setting auto is chosen for the generator electrode with
diaphragm.
Troubleshooting
Procedure for poor precision (reproducibility):
• Optimize the titration and control parameters.
• Check whether the sample vials are tightly sealed.
• Drift too high: Switch off the gas flow.
If drift value decreases: Check the needle system,
transfer tube.
If drift value does not change: check titration cell,
septum and/or seals leak, molecular sieve exhausted,
poorly conditioned reagent, ensure thorough mixing.
• Clean electrodes according to the electrode leaflet.
• Check the needle system and possibly clean it with
water and methanol. Dry the components afterwards.
• Check the transfer tube and clean it with water and
methanol to remove condensates and dirt.
• KF reagent contaminated/exhausted: change the
solution, possibly use a different batch.
• Check electrical contacts; defective contacts can lead
to an unstable measuring signal.
• Check if chosen oven temperature is suitable for
sample analysis.
• Balance: too inexact, drafts, temperature equilibrium
not reached, sample weight not optimal/too low.
• Possibly carry out a qualification of the analysis system.
Please contact Metrohm Service for further information.
Example applications
The following table 2 provides an overview of samples
analyzed by the oven technique. In each case the Karl
Fischer water determination was carried out in combination
with a Coulometer using N
2
as a carrier gas (flow rate
40 mL/min).

Application Bulletin 280/3 e
Automated water content determination with the 874 OSP
Page 6 of
6
Table 2: Selection of samples which can be analyzed using the
oven technique
Sample
Temp.
[°C]
Sample
weight [g]
Water content
Foodstuff
Lyophilizate 120 0.06 1.4%
Aromas
1
100
0.03–0.08
2.5-5.4%
Maltodextrin
100
0.03–0.08
4.7-8.8%
Lactose
monohydrate
155 0.06 5.2%
Skimmed milk
powder
2
90 0.06 4.4%
Whole milk
powder2
90 0.06 3.9%
Sweet whey
powder
2
90 0.06 4.9%
Glucose
monohydrate
2
90 0.06 8.9%
Maltose
monohydrate
3
120 0.06 5.9%
Garlic powder
110
0.2
3.5%
Mineral mixtures
110
0.3
6.0-7.0%
Plastics
Polypropylene
170
3.0
380 µg/g
Polyethylene 115 3.0 40 µg/g
Olefins 180 3.0 100 µg/g
Polyamide 180 0.3 0.8%
Polyoxy-
methylene
(POM)
170 0.3 0.1%
Polystyrene
120
0.05…0.2
200–500 µg/g
Refinery products
Transformer oil
150
3.0
80 µg/g
Mineral oil
120
1–3
10–100 µg/g
Insulating oil 140 3 5 µg/g
Crude oil 140 2
500-1200
µg/g
Additive 120 0.01…0.03 4.4%
Antimony
dialkyl-
thiocarbamate in
crude oil
50–130 3.0 700 µg/g
Pharmaceutical products
Collagens
160
0.07–0.4
10.6-9.2%
Denture cleaner,
effervescent
tablets
4
70 0.2–1.5 3.8%
Drugs 140 0.04 6.7%
Lyophilizate 150 0.01 5.0%
Others
Sodium tartrate
dihydrate
160 0.02–0.08 15.5%
Potassium
citrate
monohydrate
220 0.03 5.6%
Polyammonium
compounds
220 0.03–0.3 1.0-85.0%
Emulsified fat
compound
220 0.03–0.08 85.0%
Formamidosulfo
nic acid
220 0.2–0.3 < 1.0%
Pigment 100 0.03–0.3 7.9%
Polyol ether 150 0.6–1.3 0.2%
Dibutene
100–
140
0.03 250 µg/g
Lithium cobaltite
100
0.45–1.0
64 µg/g
Building rubble
Surface water
50–60
0.3
0.6%
Bound water
85–140
0.3
1.0%
1
With the 874 Oven Sample Processor the dissolution of the
sample in KF reagent, which is required in a direct Karl Fischer
titration and which frequently cannot be carried out completely , is
no longer necessary
2
With the addition of 4 mL methanol as extraction agent (the water
content of the methanol must be determined and included in the
blank value).
3
With the addition of 4 mL 1,5-pentandiol, extraction time 600 s
(the water content of the 1,5-pentandiol must also be determined
and included in the blank value).
4
Above 70 °C the contained carbonate decomposes.
Reference
• Metrohm Monograph 8.026.5013 - Water Determination
by Karl Fischer Titration
• AN-K-048 – Sample preparation with the oven
technique – relative blank (available on
www.metrohm.com)
Author
Competence Center Titration
Metrohm International Headquarters
