
Color analysis for pharmaceutical products using
UV-Visible absorption techniques
Introduction
The collection of reflected light by our eyes leads to the
perception of an object’s color, specifically light in the visible
range of the electromagnetic spectrum (~400 nm – 700 nm).
As our eyes are sensitive to variations in color and brightness,
1
small changes in the color of an object can be easily observed.
In pharmaceutical manufacturing, the color of a drug product
is important to analyze for QA/QC purposes. Not only is it
necessary to minimize batch-to-batch variations for aesthetic
purposes, but changes to the color of a product can have
implications for the quality of the products. Specifically, variations
from the anticipated color could indicate impurities are present
in the product or that the material has degraded.
2–4
This is
particularly important for materials which are easily decomposed,
including light, moisture, and oxygen/air-sensitive substances.
5
Figure 1: Diagram of how the color of an object is perceived.
Qualitatively, a comparison of the color of a finished drug product
with an accepted standard can be used to ensure the material’s
color matches. However, inherently this methodology will introduce
Application note
person-to-person variations.
6
Additionally, environmental effects,
such as the light source or the presence of shadows, can influence
the perceived color. As the color of a material comes from the
reflected visible light, spectroscopic measurements of a material in
the visible spectral range can be used to provide a more rigorous
and quantitative method for assessing color. Consequently, a
UV-Visible spectrophotometer can be used to measure either the
percent of light transmitted (%T) or reflected (%R) across the
visible spectrum for this purpose. As either of these measurement
geometries can be used, this analysis can be applied to both liquid
and solid products.
The American Society for Testing and Materials (ASTM),
7
as well
as USP <1061>,
8
have detailed descriptions of the mathematics
that can be used to assign the sample’s color a coordinate in a
graphical representation of color, also referred to as a color space.
The tristimulus values, calculated through the equations 1 – 3,
are the basis of most other color spaces developed by the
Comission Internationale de l’Eclairage (CIE).
9
These formulas
include the measured reflectance (R(λ)), the spectral power of
an illuminant (S(λ)), a color matching function
(x(λ),y(λ),z(λ))
, and
the normalization factor (k).
(1)
(2)
(3)

As described previously, the color of an object is highly
dependent on environmental factors, such as light source
and the field of view of the object. For example, the intensity
of the light across the visible spectrum can be very different
for various light sources and can lead to differences in how
the color is observed. In the tristimulus equations, this factor
is taken into account through the inclusion of the spectral
power of the illuminant, S(λ). A standardized intensity spectrum
describing the spectral illuminant power as a function of
wavelength was developed to describe a typical intensity
spectrum for common illuminants (e.g., room lights, daylight),
and is included in equations 1 – 3. Additionally, the observer
angle, which defines the field of view of the material, can also
alter the perceived color and is also accounted for in tristimulus
equations through the color-matching functions.
The tristimulus values can condense the measured visible
spectrum of a sample down to a single coordinate, however,
the coordinate space is not uniform.
9
The lack of uniformity
can lead to issues gauging the difference between the
color of a sample and the color of a reference standard. In
pharmaceutical applications, specifically in QA/QC functions,
the ability to compare the sample to an accepted standard, as
well as establish acceptance criteria, is critical. Consequently,
a uniform color space must be used instead. CIE developed
a set of mathematical functions which convert the calculated
tristimulus coordinates into a uniform, cylindrical (CIE L*a*b*)
or spherical (CIE L*C*h*) coordinate system (Figure 2), which is
built on opposing color theory.
Figure 2: CIE L*a*b* and CIE L*C*h* coordinates
Coordinates for the more commonly used CIE L*a*b* color space
are generated through the following mathematical functions,
7, 8
where X, Y, and Z are the calculated tristimulus values and X
n
,
Y
n
, and Z
n
are the tristimulus values of a perfectly reflecting
white diffuser. Here L* describes how light (100) or dark (0)
the materials are, a* represents how red (positive) or green
(negative) the sample is, and b* demonstrates how yellow
(positive) or blue (negative). As this transformation results in a
more uniform color space, a better representation of the color
difference (ΔE*) between the sample and a standard can be
developed. The color difference formula (eq 7) describes how a
color difference is mathematically determined,
where L*
sam
, a*
sam
, and b*
sam
represent the CIE L*a*b* values for
the sample and L*
std
, a*
std
, and b*
std
represent the CIE L*a*b*
values for the standard.
8
As a rule of thumb, two colors are
considered to be indistinguishable from one another by eye if
the color difference between the two substances is less than 3.
The CIE L*C*h* color space uses the same coordinate system
as the CIE Lab system, except it reports the chroma (C
ab
*) and
hue (h
ab
*) of the substance in place of a* and b*. Chroma is
calculated through equation 8,
and describes how colorful a substance is wherein a small
C
ab
* represents a more pale or muted color, while a large C
ab
*
describes a substance with a very vibrant color. Hue describes
the color of the object and is calculated through equation 9.
Color analysis can be a quick and useful tool for assessing the
overall quality of a given product prior to further downstream
processing. Through UV-Visible absorption spectroscopy,
the analysis can be made more rigorous, allowing for a more
accurate measurement of color. Herein, we describe how
color analysis can be applied to both solid and liquid samples
using the Thermo Scientific
™
Evolution
™
Spectrophotometers
and Thermo Scientific
™
Insight
™
Pro Software. Furthermore,
descriptions of the USP requirements for color analysis of samples
are explained in relation to the instrumental analysis method.
(4)
(5)
(6)
(7)
(8)
(9)
Thermo Scientific Evolution Spectrophotometers

Experimental
Materials
USP color-matching solutions were prepared based on
descriptions in USP’s chapter <631>,
10
which includes methods
to analyze and report the color of solution phase samples.
Briefly, three stock solutions were generated:
• 0.27 M CoCl
2
• 6H
2
O (red solution)
• 0.17 M FeCl
3
• 5H
2
O (yellow solution)
• 0.23 M CuSO
4
• 5H
2
O (blue solution)
These solutions were mixed in different proportions to prepare
the color-matching solutions A – T as defined in USP <631>
(see Table 1).
10
Table 1: Proportions of stock color solutions used to prepare color
matching solutions A – T based on USP <631>.
10
Color
Matching
Solution
Volume
CoCl
2
•
6H
2
O (mL)
Volume
FeCl
3
•
5H
2
O (mL)
Volume
CuSO
4
•
5H
2
O (mL)
Volume
H
2
O (mL)
A 0.1 0.4 0.1 4.4
B 0.3 0.9 0.3 3.5
C 0.1 0.6 0.1 4.2
D 0.3 0.6 0.4 3.7
E 0.4 1.2 0.3 3.1
F 0. 1.2 0.0 3.5
G 0.5 1.2 0.2 3.1
H 0.2 1.5 0.0 3.3
I 0.4 2.2 0.1 2.3
J 0.4 3.5 0.1 1.0
K 0.5 4.5 0.0 0.0
L 0.8 3.8 0.1 0.3
M 0.1 2.0 0.1 2.8
N 0.0 4.9 0.1 0.0
O 0.1 4.8 0.1 0.0
P 0.2 0.4 0.1 4.3
Q 0.2 0.3 0.1 4.4
R 0.3 0.4 0.2 4.1
S 0.2 0.1 0.0 4.7
T 0.5 0.5 0.4 3.6
For comparison against a more realistic example, two different
cough syrups were analyzed. One sample was labeled
“Daytime” and the other “Night-time.” Additionally, a set of four
antacid tablets of different colors were analyzed herein. The
tablets were crushed into powders using a mortar and pestle.
Instrument parameters
UV-Visible measurements described herein were collected
using an Evolution One Plus Spectrophotometer. For all
samples, spectral measurements spanning 280 nm and
780 nm were collected using a 1.0 nm spectral bandwidth and
2 nm data interval.
The USP color-matching solutions were measured in transmission
geometry and reported as % Transmission (%T), and the cough
syrup samples were reported in absorption units. For both sample
sets, deionized water was used to establish a 100% transmission
baseline as the blank solution. All USP matching solutions were
measured using a plastic 10 mm cuvette, while the cough syrup
samples were measured in a 10 mm and 1 mm quartz cuvette.
The antacid samples were measured in reflection geometry
using an integrating sphere accessory (ISA-220) with a powder
cell holder. A white Spectrlon
©
disk was used to establish a
100% reflection baseline as the blank. The resulting data was
reported as % Reflectance (%R).
Color analysis parameters
For all samples described herein, the CIE L*a*b* color values were
calculated using Insight Pro Software. The D65 illuminant with a
10˚ observer angle was chosen to reflect the color of all samples.
Color difference measurements were also performed through this
software feature. All calculations performed correspond to the
descriptions outlined in USP <1061>
8
and ASTM-E308.
7
Results and discussion
Analysis of liquid samples—color matching solutions
According to USP <631>, color-matching solutions are to
be used as a comparison point against the produced liquid
product to ensure the product matches the expected color.
As many liquid-based pharmaceutical products are yellow in
hue, the USP monograph includes a procedure for making a
set of standard solutions of varying yellow (Figure 3d).
10
EP has
a different procedure outlined for making color standards and
includes a wider range of colors, including brown, green and
blue, among others.
11
As shown in Figure 3d, some samples appear by eye to be similar
and almost indistinguishable in color. However, as the purpose
of these standards is to serve as different matching solutions,
the variations in the color may be slight and difficult to compare
without instrumental methods like UV-Visible color analysis. To
demonstrate this concept, the percent transmittance of each
matching solution was collected and are shown in Figures 3a – 3c.
a b c d
Figure 3: Absorption spectra of USP color matching solutions (a) A – G, (b) H – N, (c) O – T. (d) An image of the USP color matching solutions.
From these spectra, it is clear there are small differences in the
transmittance, and consequently absorption, of each matching
solution; however, color difference calculations were needed to
rigorously compare the colors. As described previously, the CIE
L*a*b* values were calculated using the Insight Pro Software.
A select set of color-matching standards were chosen for
comparison and are included in Table 2 as these standards
(Soln. A and B, Soln. J and K, and Soln. Q and R) appear similar
enough to each other in color that they are difficult to tell apart.
Table 2: CIE Lab and color difference values for select USP color matching
solutions (A, B, J, K, Q, R). Color difference calculations were carried out
for samples which appear similar by eye.
Solution L* a* b* ΔE*
A 87. 5 0.5 28.5
9.7
B 83.3 2.4 37. 0
J 69.1 12.0 80.0
12.5
K 73.9 12.5 91.5
Q 85.1 2.6 28.3
5.2
R 88.1 2.5 24.0
The color difference values calculated between matching
solutions A and B, J and K, and Q and R are relatively low;
however, a numerical limit is required to put these difference
values into context. In the pharmaceutical industry, different
formulations may require different methods of comparison
against a color-matching standard. For example, one product
may need to have no discernable color (achromatic), while
another must meet a minimum color value. Consequently,
USP has developed a set of criteria which can be used to set
acceptable limits for the calculated color difference from a
standard (Table 3).
There are four main test limits which can be used depending
on the color expectations for the analyzed product. Each test
defines a limit to an acceptable color difference between the
material and a given standard. For a sample which should have
no color, the first test in Table 3 (colorless/achromatic) defines
the necessary color difference limit as ΔE* < 1, where the color-
matching standard is purified water.
For samples where the sample has an expected color, there
are a few different options for analysis. If the color must
match a given standard color exactly, the second test in
Table 3 (Indiscernible from Standard) is required. Here, the
color difference between the product and the color matching
standard is used and must be less than 3. As mentioned
previously, this defines the color difference that is discernable
by the human eye.
10
The last two analyses define maximum
and minimum color limits. Here, a sample can either be more
or less colorful than a given standard. USP defines Δh
ab
*, the
difference in hue between the sample and matching standard
chosen must be less than 15. When setting the maximum or
minimum color limit, instead of comparing the color difference
against a number, two different analyses are required: one
where the color of the standard is compared to the color of
pure water (ΔE
std
*) and one where the color of the product is
compared against pure water (ΔE*).
As the color difference values shown in Table 2 are intended
to determine how similar the color of the two solutions are to
one another, this analysis would follow the “Indiscernible from
Standard” test. The passing criteria would require a calculated
color difference of less than 3. For each set of standards, the
color difference exceeds this limit, indicating they fail this test
and are distinguishable from one another. This result highlights
how small differences in color can be analyzed through the
instrumental method, where it is difficult to perceive visually.
Analysis of liquid samples—cough syrup
The color-matching standards are ideal solutions with
optimized component concentrations to produce a measurable
spectrum in a standard 10 mm cuvette. Real samples may not
be manufactured to produce UV-Visible absorption spectra that
can be easily measured under these conditions. For example,
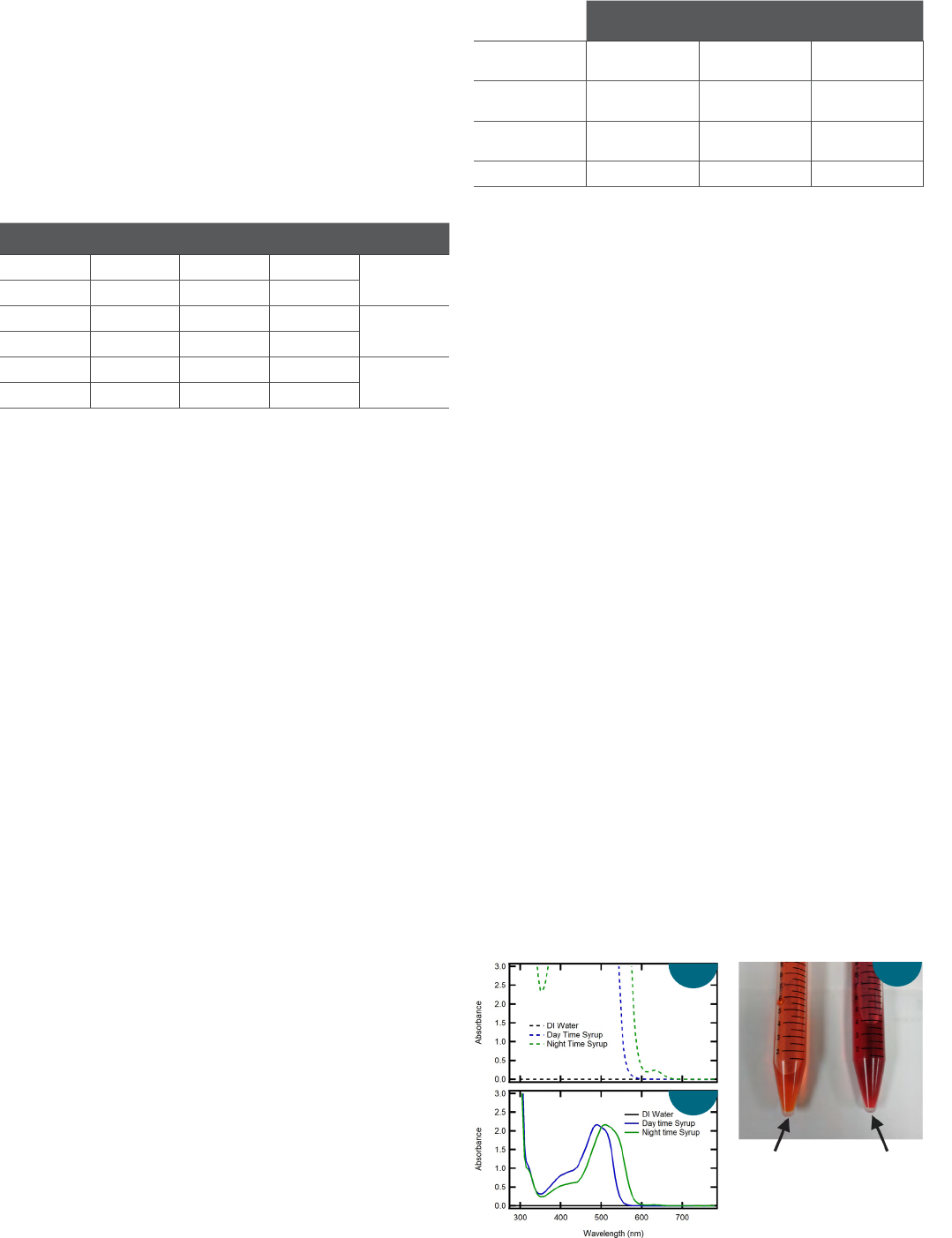
Figure 4a includes the absorption spectra of a “Daytime” and
“Night-time” cough syrup measured in a 10 mm cuvette. By
eye, the “Daytime” syrup appears orange while the “Night-time”
syrup appears red/purple.
As shown, both samples absorb greatly at wavelengths shorter
than 550 nm (A > 3). In UV-Visible absorption measurements,
it is good practice not to use highly absorptive samples for
calculations or quantification, as very little light is allowed to
pass through the sample and be detected by the system. For
example, an absorption of 3 indicates 99.9% of the incident
light is absorbed by the sample, leaving 0.1% of the light
collected by the detector. Consequently, the absorption spectra
in Figure 4a are not ideal for color analysis and result in the
values described in Table 4.
Figure 4: Absorption spectra of "Daytime" and "Night-time" cough syrup
collected using a (a) 10 mm and (b) 1 mm quartz cuvette. (c) An image of the
"Daytime" and "Night-time" cought syrup).
Daytime Night-time
a
b
c
Table 3: Passing criteria for color difference tests from USP <631>.
10
For the
maximum and minimum color difference measurements, ΔE
std
* refers to the
color difference between a matching standard and purified water while ΔE*
refers to the color difference of the sample against purified water.
Test
Color
Standard
Passing
Criteria
1 Colorless
(Achromatic)
Purified Water ΔE* < 1
2 Indiscernible
from Standard
Color Matching
Solution
ΔE* < 3
3 Maximum
Color
Purified Water ΔE* < ΔE
std
*
4 Minimum Color Purified Water ΔE* > ΔE
std
*

Table 4: CIE L*a*b* values for "Daytime" and "Night-time" cough syrup samples. Spectra were measured using a 10 mm and 1 mm path length.
L* a* b*
Sample 10 mm cuvette 1 mm cuvette 10 mm cuvette 1 mm cuvette 10 mm cuvette 1 mm cuvette
Daytime 67. 5 79.7 62.0 40.3 116.2 86.0
Night-time 40.2 62.3 68.5 72.2 69.2 27.6
To avoid issues for highly absorptive samples, instead a short
pathlength cuvette can be used as absorption is directly
proportional to pathlength according to Beer’s law (eq. 10),
where A is the collected absorbance, c is the concentration of
the analyte, l is the path length, and ε is the molar absorptivity
of the analyte. Changing the path length also circumvents the
need to dilute the sample, avoiding some waste of the material.
Herein, both cough syrup samples were measured using a
1 mm cuvette, resulting in the absorption spectra in Figure 4b.
Compared to the spectra shown in Figure 4c, the spectra
collected show much more clearly the absorption features
present in the sample. Included in Table 4 are the resulting
color values based on the spectra collected with a shorter path
length. These reported values are very different from the values
calculated using the spectra collected with a longer path length.
It is important to note that changing the path length not only
changed the perceived lightness/darkness of the sample (L*), but
also how red/green (a*) and how blue/yellow (b*) the samples
appear. This observation further illustrates the importance of
measuring highly absorptive samples in a shorter path length
to avoid significant deviations in the calculated color values. As
good practice, quantification should only be performed when the
highest peak absorption in the spectral region of interest is 1 A
or lower. Given the calculated color values will be sensitive to the
chosen path length, it is important any standard used for color
difference calculations be measured using the same path length.
Analysis of solid samples
USP <631> specifically refers to color analysis procedures
for liquids; however, color analysis can be performed using
solid samples as well, according to USP <1061>.
8,11
For
pharmaceutical analysis, the color of a solid drug product
can also have implications on the quality of the material,
3–6
as
described previously; however, it can also be used to indicate
the dosage of a given product as well as comply with a
company’s branding or marketing needs.
6
For solid materials,
measurements in reflection geometry are appropriate as it is
difficult to pass light through a solid material without scattering
effects. As described in equations 1 – 3, the tristimulus values,
and therefore the CIE L*a*b* values, can be calculated using
reflectance data, allowing for color analysis of solid samples.
a
b
Figure 5 – (a) An image of the four antacid tablets measured. (b)
Reflectance spectra of four antacid tablets (blue—Tablet A, dark
green—Tablet B, brown—Tablet C, and light green —Tablet D) and a white
reflectance standard (Spectralon).
Figure 5b includes the percent reflectance spectra (%R) of
four antacid tablets (Fig. 3a) of varying colors. By eye, Tablets
A – D appear white, yellow, orange, and red, respectively. The
calculated CIE L*a*b* values for each sample are included in
Table 5, along with the color values for a white Spectralon
®
reference material (99% reflectance). Color difference
calculations were then performed to determine how different
each antacid tablet was from the white reference material.
Tablets B – D resulted in very high color differences (between
23 and 27) with respect to the reference standard, as
anticipated as these samples are visually very different from
the white standard. Tablet A, which appears white by eye, is
closer in color to the reference, with a color difference of 8.7
compared with the color difference of the other three tablets,
however as the calculated color difference is greater than 3, it
is distinguishable from the reference standard and would fail a
color matching test.
(10)
Table 5: Calculated CIE L*a*b* color values and color difference values for
antacid tablets. Color Difference Calculations were carried out using the
color values for the Spectralon
®
reference as the standard.
Sample L* a* b* ΔE*
Spectralon
®
Reference
100.0 0.0 0.0 —
Tablet A 92.8 0.3 3.4 7. 9 2
Tablet B 92.8 -5.8 21.7 23.6
Tablet C 88.1 13.7 17. 0 24.9
Tablet D 82.5 19.3 8.7 27. 5

For research use only. Not for use in diagnostic procedures. For current certifications, visit thermofisher.com/certifications
© 2022 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific
and its subsidiaries unless otherwise specified. AN56364_E 11/22M
Learn more at thermofisher.com/evolution
Conclusion
Color analysis can be an effective and quick method for QA/QC
in pharmaceutical manufacturing. As shown in the experiments
described herein, color analysis can be performed using the
Evolution UV-Visible Spectrophotometers to carefully determine a
material’s color without person-to-person variations, allowing for
a quantitative analysis of a produced pharmaceutical. Additionally,
these measurements demonstrate the ability to analyze both liquid
and solid samples following USP color analysis procedures.
References
1. Ng, S.E., Tay, Y.B.; Ho, T.Y.K.; Ankit; Mathews, N., Inorganic Electrochromic
Transistors as Environmentally Adaptable Photodetectors, Nano Energy, 2022, 97,
107142.
2. Zhou, L.; Vogt, F. G.; Overstreet, P. -A.; Dougherty, J. T.; Clawson, J. S.; Kord, A. S.,
A Systematic Method Development Strategy for Quantitative Color Measurement in
Drug Substances, Starting Materials, and Synthetic Intermediates, J. Pharm. Innov.,
2011, 6, 217 – 231.
3. Yamazaki, N.; Taya, K.; Shimokawa, K.-I., Ishii, F., The Most Appropriate Storage
Method in Unit-Dose Package and Correlation between Color Change and
Decomposition Rate of Aspirin Tablets, Int. J. Pharm., 2010, 396, 105 – 110.
4. Oram, P. D.; Strine, J., Color Measurement of a Solid Active Pharmaceutical
Ingredient as an Aid to Identifying Key Process Parameters, J. Pharm. Biomed.
Anal., 2006, 40, 1021 – 1024.
5. Berberich, J., Dee, K.-H., Hayauchi, Y., Pörtner, C., A New Method to Determine
Discoloration Kinetics of Uncoated White Tablets Occurring During Stability
Testing – An Application of Instrumental Color Measurements in the Development
Pharmaceutics, Int. J. Pharm., 2002, 234, 55 – 66.
6. Hetrick, E. M.; Vannoy, J.; Montgomery, L. L.; Pack, B. W., Integrating Tristimulus
Colorimetry into Pharmaceutical Development for Color Selection and Physical
Appearance Control: A Quality-by-Design Approach, J. Pharm. Sci., 2013, 102,
2608 – 2621.
7. ASTM International. Standard Practice for Computing the Color of Objects by Using
the CIE System; ASTM E308-08; West Conshohocken, PA.
8. United States Pharmacopeia and National Formulary. <1061> Color – Instrumental
Measurement. In: USP–NF. Rockville, MD: USP
9. Subert, J.; Cizmarik, J., Application of Instrumental Colour Measurements in
Development and Quality Control of Drugs and Pharmaceutical Excipients,
Pharmazie, 2008, 63, 331 – 336.
10. United States Pharmacopeia and National Formulary. <631> Color and Achromicity.
In: USP–NF. Rockville, MD: USP.
11. European Pharmacopoeia. 2.2.2. Degree of Coloration of Liquids. In: European
Pharmacopoeia. Strasbourg, France: European Pharmacopoeia.
