
Chapter
4
Heat Transfer in Food Processing
The most common processes found in a food processing plant
involve heating and cooling of foods. In the modern industrialized
food industry, we commonly find unit operations such as refrigera-
tion, freezing, thermal sterilization, drying, and evaporation. These
unit operations involve the transfer of heat between a product and
some heating or cooling medium. Heating and cooling of food pro-
ducts is necessary to prevent microbial and enzymatic degradation.
In addition, desired sensorial properties—color, flavor, texture—are
imparted to foods when they are heated or cooled.
The study of heat transfer is important because it provides a basis for
understanding how various food processes operate. In this chapter, we
will study the fundamentals of heat transfer and learn how they are
related to the design and operation of food processing equipment.
We will begin by studying heat-exchange equipment. We will observe
that there is a wide variety of heat-exchange equipment available for
food applications. This description will identify the need to study prop-
erties of foods that affect the design and operation of heat exchangers.
Thereafter, we will examine various approaches to obtaining thermal
properties of foods. We will consider basic modes of heat transfer such
as conduction, convection, and radiation. Simple mathematical equa-
tions will be developed to allow prediction of heat transfer in solid
as well as liquid foods. These mathematical equations will provide us
with sufficient tools to design and evaluate the performance of simple
heat exchangers. Next, we will consider more complicated situations
arising from heat transfer under unsteady-state conditions, when
temperature changes with time. A good understanding of the various
All icons in this chapter refer to
the author’s web site, which is
independently owned and
operated. Academic Press is not
responsible for the content or
operation of the author’s web site.
Please direct your web site
comments and questions to the
author: Professor R. Paul Singh,
Department of Biological and
Agricultural Engineering,
University of California, Davis,
CA 95616, USA.
Email: [email protected]
265

concepts presented in this chapter is important, since they will be the
basis for topics in the following chapters.
4.1 SYSTEMS FOR HEATING AND
COOLING FOOD PRODUCTS
In a food processing plant, heating and cooling of foods is conducted
in equipment called heat exchangers. As shown in
Figure 4.1, heat
exchangers can be broadly classified into non-contact and contact
types. As the name implies, in non-contact-type heat exchangers, the
product and heating or cooling medium are kept physically sepa-
rated, usually by a thin wall. On the other hand, in contact-type heat
exchangers, there is direct physical contact between the product and
the heating or cooling streams.
For example, in a steam-injection system, steam is directly injected
into the product to be heated. In a plate heat exchanger, a thin metal
plate separates the product stream from the heating or cooling stream
while allowing heat transfer to take place without mixing. We will
discuss some of the commonly used heat exchangers in the food
industry in the following subsections.
4.1.1 Plate Heat Exchanger
The plate heat exchanger invented more than 70 years ago has found
wide application in the dairy and food beverage industry. A sche-
matic of a plate heat exchanger is shown in
Figure 4.2. This heat
exchanger consists of a series of parallel, closely spaced stainless-steel
Heat
exchangers
Noncontact
type
Contact
type
Scraped
surface
Shell and
tube
Tubular Plate
Steam
infusion
Steam
injection
■ Figure 4.1 Classification of commonly used
heat exchangers.
266 CHAPTER 4 Heat Transfer in Food Processing

plates pressed in a frame. Gaskets, made of natural or synthetic rub-
ber, seal the plate edges and ports to prevent intermixing of liquids.
These gaskets help to direct the heating or cooling and the product
streams into the respective alternate gaps. The direction of the prod-
uct stream versus the heating/cooling stream can be either parallel
flow (same direction) or counterflow (opposite direction) to each
other. We will discuss the influence of flow direction on the perfor-
mance of the heat exchanger later in Section 4.4.7.
The plates used in the plate heat exchanger are constructed from
stainless steel: Special patterns are pressed on the plates to cause
increased turbulence in the product stream, thus achieving better
heat transfer. An example of such a pattern is a shallow herringbone-
ribbed design, as shown in
Figure 4.3.
Plate heat exchangers are suitable for low-viscosity (,5 Pa s) liquid
foods. If suspended solids are present, the equivalent diameter of the
(a)
Product
Media
(b)
w
■ Figure 4.2 (a) Plate heat exchanger.
(b) Schematic view of fluid flow between plates.
(Courtesy of Cherry-Burrell Corporation)
2674.1 Systems for Heating and Cooling Food Products

particulates should be less than 0.3 cm. Larger particulates can bridge
across the plate contact points and “burn on” in the heating section.
In industrial-size plate heat exchangers, product flow rates from 5000
to 20,000 kg/h often are obtained. When using plate heat exchangers,
care should be taken to minimize the deposition of solid food mate-
rial such as milk proteins on the surface of the plates. This deposition,
also called fouling, will decrease the heat transfer rate from the heating
medium to the product; in addition, the pressure drop will increase
over a period of time. Eventually, the process is stopped and the plates
are cleaned. For dairy products, which require ultra-high-temperature
applications, the process time is often limited to 34 h. Plate heat
exchangers offer the following advantages:
I The maintenance of these heat exchangers is simple, and they can
be easily and quickly dismantled for product surface inspection.
I The plate heat exchangers have a sanitary design for food
applications.
I Their capacity can easily be increased by adding more plates to
the frame.
I With plate heat exchangers, we can heat or cool product to
within 18C of the adjacent media temperature, with less capital
investment than other non-contact-type heat exchangers.
I Plate heat exchangers offer opportunities for energy conservation
by regeneration.
As shown in a simple schematic in
Figure 4.4, aliquidfoodisheatedto
pasteurization or other desired temperature in the heating section; the
heated fluid then surrenders part of its heat to the incoming raw fluid
in the regeneration section. The cold stream is heated to a temperature
where it requires little additional energy to bring it up to the desired
■ Figure 4.3 Patterns pressed on plates
used on a plate heat exchanger. (Courtesy of
Cherry-Burrell Corporation)
268 CHAPTER 4 Heat Transfer in Food Processing

temperature. For regeneration, additional plates are required; however,
the additional capital cost may be recovered quickly by lowered oper-
ating costs.
An actual two-way regeneration process is shown in Figure 4.5
for pasteurizing grape juice. After the “starter” juice has been heated
to 888C(atlocationA), it is passed through a holding loop and into the
Ice water
cooling
Milk in
Milk out
Hot water
Chilled water
Well water
Milk
Water
cooling
Regenerative
heating/cooling
Heating
External holding cell
w
■ Figure 4.4 A five-stage plate pasteurizer for
processing milk. (Reprinted with permission of
Alfa-Laval AB, Tumba, Sweden, and Alfa-Laval,
Inc., Fort Lee, New Jersey)
Heating section
(hot water at 93°C)
0.5°C
0.5°C
13°C21°C53°C
88°C
88°C73°C38°C
38°C
Juice
54321
Balance
tank
Silo
tank
B
C
E
D
A
1
2
3
4
5
Regeneration section
Cooling with city water
Cooling with chilled water
Cooling with Glycol
w
■ Figure 4.5 A two-way regeneration system
used in processing grape juice. (Courtesy of
APV Equipment, Inc.)
2694.1 Systems for Heating and Cooling Food Products

regenerative section (entering at location B). In this section, the juice
releases its heat to incoming raw juice entering (at location C) into
the exchanger at 388C. The temperature of raw juice increases to 738C(at
location D), and the “starter” juice temperature decreases to 538C(at
location E). In this example, the regeneration is [(7338)/(8838)] 3
100 or 70%, since the incoming raw juice was heated to 70% of its even-
tual pasteurization temperature without the use of an external heating
medium. The juice heated to 738C passes through the heating section,
where its temperature is raised to 888Cbyusing938Chotwaterasthe
heating medium. The heated juice is then pumped to the regeneration
section, where it preheats the incoming raw juice, and the cycle con-
tinues. The cooling of hot pasteurized juice is accomplished by using
city water, chilled water, or recirculated glycol. It should be noted that,
in this example, less heat needs to be removed from the pasteurized
juice, thus decreasing the cooling load by the regeneration process.
4.1.2 Tubular Heat Exchanger
The simplest noncontact-type heat exchanger is a double-pipe heat
exchanger, consisting of a pipe located concentrically inside another
pipe. The two fluid streams flow in the annular space and in the
inner pipe, respectively.
The streams may flow in the same direction (parallel flow) or in the
opposite direction (counterflow).
Figure 4.6 is a schematic diagram
of a counterflow double-pipe heat exchanger.
A slight variation of a double-pipe heat exchanger is a triple-tube
heat exchanger, shown in
Figure 4.7. In this type of heat exchanger,
product flows in the inner annular space, whereas the heating/cooling
medium flows in the inner tube and outer annular space. The
Fluid A in
Fluid B
in
Fluid A out
Fluid B
out
Annular space
w
■ Figure 4.6 Schematic illustration of a tubular
heat exchanger.
270 CHAPTER 4 Heat Transfer in Food Processing

innermost tube may contain specially designed obstructions to create
turbulence and better heat transfer. Some specific industrial applica-
tions of triple-tube heat exchangers include heating single-strength
orange juice from 4 to 938C and then cooling to 48C; cooling cottage
cheese wash water from 46 to 188C with chilled water; and cooling ice
cream mix from 12 to 0.58C with ammonia.
Another common type of heat exchanger used in the food industry
is a shell-and-tube heat exchanger for such applications as heating
liquid foods in evaporation systems. As shown in Figure 4.8, one of
the fluid streams flows inside the tube while the other fluid stream is
pumped over the tubes through the shell. By maintaining the fluid
stream in the shell side to flow over the tubes, rather than parallel to
the tubes, we can achieve higher rates of heat transfer. Baffles located
in the shell side allow the cross-flow pattern. One or more tube
passes can be accomplished, depending on the design. The shell-and-
tube heat exchangers shown in Figure 4.8 are one shell pass with two
tube passes, and two shell passes with four tube passes.
4.1.3 Scraped-Surface Heat Exchanger
In conventional types of tubular heat exchangers, heat transfer to a
fluid stream is affected by hydraulic drag and heat resistance due to
film buildup or fouling on the tube wall. This heat resistance can be
minimized if the inside surface of the tube wall is scraped continu-
ously by some mechanical means. The scraping action allows rapid
heat transfer to a relatively small product volume. A scraped-surface
heat exchanger, used in food processing, is shown schematically in
Figure 4.9.
Heating or
cooling fluid
Heating or
cooling fluid
Product
Heating or
cooling fluid
w
■ Figure 4.7 Schematic illustration of a triple-
tube heat exchanger. (Courtesy of Paul Mueller
Co.)
2714.1 Systems for Heating and Cooling Food Products

Shell fluid
Tube fluid
Shell fluid
Tube fluid
One-shell pass and 2, 4, 6 ... tube passes
Two-shell passes and 4, 8, 12 ... tube passes
w
■ Figure 4.8 A shell-and-tube heat exchanger.
Polished stainless
steel
Scraper blade
Insulation
Media cylinder
Product zone
Product tube
Media zone
w
■ Figure 4.9 A scraped-surface heat exchanger
with a cutaway section illustrating various
components. (Courtesy of Cherry-Burrell
Corporation)
272 CHAPTER 4 Heat Transfer in Food Processing

The food contact areas of a scraped-surface cylinder are fabricated
from stainless steel (type 316), pure nickel, hard chromium-plated
nickel, or other corrosion-resistant material. The inside rotor contains
blades that are covered with plastic laminate or molded plastic
(
Fig. 4.9). The rotor speed varies between 150 and 500 rpm. Although
higher rotation speed allows better heat transfer, it may affect the qual-
ity of the processed product by possible maceration. Thus, we must
carefully select the rotor speed and the annular space between the
rotor and the cylinder for the product being processed.
As seen in Figure 4.9, the cylinder containing the product and the rotor
is enclosed in an outside jacket. The heating/cooling medium is supplied
to this outside jacket. Commonly used media include steam, hot water,
brine, or a refrigerant. Typical temperatures used for processing products
in scraped-surface heat exchangers range from 235 to 1908C.
The constant blending action accomplished in the scraped-surface
heat exchanger is often desirable to enhance the uniformity of prod-
uct flavor, color, aroma, and textural characteristics. In the food pro-
cessing industry, the applications of scraped-surface heat exchangers
include heating, pasteurizing, sterilizing, whipping, gelling, emulsify-
ing, plasticizing, and crystallizing. Liquids with a wide range of vis-
cosities that can be pumped are processed in these heat exchangers;
examples include fruit juices, soups, citrus concentrate, peanut butter,
baked beans, tomato paste, and pie fillings.
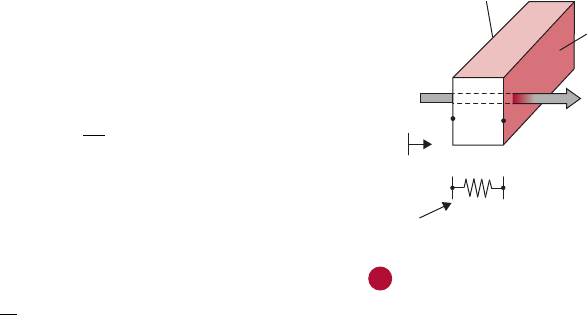
4.1.4 Steam-Infusion Heat Exchanger
A steam-infusion heat exchanger provides a direct contact between
steam and the product. As shown in
Figure 4.10, product in liquid
state is pumped to the top of the heat exchanger and then allowed to
flow in thin sheets in the heating chamber. The viscosity of the liquid
determines the size of the spreaders. Products containing particulates,
such as diced vegetables, meat chunks, and rice, can be handled by
specially designed spreaders. High rates of heat transfer are achieved
when steam contacts tiny droplets of the food. The temperature of
the product rises very rapidly due to steam condensation. The heated
products with condensed steam are released from the chamber at the
bottom. A specific amount of liquid is retained in the bottom of the
chamber to achieve desired cooking.
The temperature difference of the product between the inlet and the
outlet to the heating chamber may be as low as 5.58C, such as for
Product
out
Steam
Product in
w
■ Figure 4.10 A steam-infusion heat
exchanger. (Courtesy of CREPACO, Inc.)
2734.1 Systems for Heating and Cooling Food Products
deodorizing milk (76.7 to 82.28C), or as high as 96.78C, such as for
sterilizing puddings for aseptic packaging (48.9 to 145.68C).
The water added to the product due to steam condensation is some-
times desirable, particularly if the overall process requires addition of
water. Otherwise, the added water of condensation can be “flash ed off”
by pumping the heated liquid into a vacuum cooling system. The
amount of water added due to condensation can be computed by mea-
suring the temperature of the product fed to the heat exchanger and
the temperature of the product discharged from the vacuum cooler.
This type of heat exchanger has applications in cooking and/or
sterilizing a wide variety of products , such as concentrated sou ps,
chocolate, processed cheese, ice cream mixes, puddings, fruit pie
fillings, and milk.
4.1.5 Epilogue
In the preceding subsections, we discussed several types of com-
monly used heat exchangers. It should be evident that a basic under-
standing of the mechanisms of heat transfer, both in the food and
the materials used in construction of the food processing equipment,
is necessary before we can design or evaluate any heat exchange
equipment. A wide variety of food products is processed using heat
exchangers. These products present unique and often complex pro-
blems related to heat transfer. In the following sections, we will
develop quantitative descriptions emphasizing the following:
1. Thermal properties. Properties such as specific heat, thermal
conductivity, and thermal diffusivity of food and equipment
materials (such as metals) play an important role in determin-
ing the rate of heat transfer.
2. Mode of heat transfer. A mathematical description of the actual
mode of heat transfer, such as conduction, convection, and/or
radiation is necessary to determine quantities, such as total
amount of heat transferred from heating or cooling medium
to the product.
3. Steady-state and unsteady-state heat transfer. Calculation procedures
are needed to examine both the unsteady-state and steady-state
phases of heat transfer.
We will develop an analytical approach for cases involving simple
heat transfer. For more complex treatment of heat transfer, such as
274 CHAPTER 4 Heat Transfer in Food Processing

for non-Newtonian liquids, the textbook by Heldman and Singh (1981)
is recommended.
4.2 THERMAL PROPERTIES OF FOODS
4.2.1 Specific Heat
Specific heat is the quantity of heat that is gained or lost by a unit
mass of product to accomplish a unit change in temperature, without
a change in state:
c
p
5
Q
m(ΔT)
ð4:1Þ
where Q is heat gained or lost (kJ), m is mass (kg), ΔT is temperature
change in the material (8C), and c
p
is specific heat (kJ/[kg 8C]).
Specific heat is an essential part of the thermal analysis of food proces-
sing or of the equipment used in heating or cooling of foods. With
food materials, this property is a function of the various components
that constitute a food, its moisture content, temperature, and pressure.
The specific heat of a food increases as the product moisture content
increases. For a gas, the specific heat at constant pressure, c
p
, is greater
than its specific heat at constant volume, c
v
. In most food processing
applications, we use specific heat at constant pressure c
p
, since pressure
is generally kept constant except in high-pressure processing.
For processes where a change of state takes place, such as freezing or
thawing, an apparent specific heat is used. Apparent specific heat
incorporates the heat involved in the change of state in addition to
the sensible heat.
In designing food processes and processing equipment, we need
numerical values for the specific heat of the food and materials to
be used. There are two ways to obtain such values. Published data
are available that provide values of specific heat for some food and
nonfood materials, such as given in Tables A.2.1, A.3.1, and A.3.2
(in the Appendix). Comprehensive databases are also available to
obtain published values (
Singh, 1994). Another way to obtain a spe-
cific heat value is to use a predictive equation. The predictive equa-
tions are empirical expressions, obtained by fitting experimental data
into mathematical models. Typically these mathematical models
are based on one or more constituents of the food. Since water is a
major component of many foods, a number of models are expressed
as a function of water content.
2754.2 Thermal Properties of Foods
One of the earliest models to calculate specific heat was proposed by
Siebel (1892) as,
c
p
5 0:837 1 3:349 X
w
ð4:2Þ
where X
w
is the water content expressed as a fraction. This model
does not show the effect of temperature or other components of a
food product. The influence of product components was expressed in
an empirical equation proposed by
Charm (1978) as
c
p
5 2:093 X
f
1 1:256 X
s
1 4:187 X
w
ð4:3Þ
where X is the mass fraction; and subscripts f is fat, s is nonfat solids,
and w is water. Note that in
Equation (4.3), the coefficients of each
term on the right-hand side are specific heat values of the respective
food constituents. For example, 4.187 is the specific heat of water at
708C, and 2.093 is the specific heat of liquid fat.
Heldman and Singh (1981) proposed the following expression based
on the components of a food product:
c
P
5 1:424 X
h
1 1:549 X
p
1 1:675 X
f
1 0:837 X
a
1 4:187 X
w
ð4:4Þ
where X is the mass fraction; the subscripts on the right-hand side
are h, carbohydrate; p, protein; f, fat; a, ash; and w, moisture.
Note that these equations do not include a dependence on tempera-
ture. However , for processes where temperature changes, we must use
predictive models of specific heat that include temperature dependence.
Choi and Okos (1986) presented a comprehensive model to predict
specific heat based on composition and temperature. Their model is as
follows:
c
p
5
X
n
i51
c
pi
X
i
ð4:5Þ
where X
i
is the fraction of the ith component, n is the total number
of components in a food, and c
pi
is the specific heat of the ith com-
ponent. Table A.2.9 gives the specific heat of pure food components
as a function of temperature. The coefficients in this table may be
programmed in a spreadsheet for predicting specific heat at any
desired temperature, as illustrated in Example 4.1.
276 CHAPTER 4 Heat Transfer in Food Processing

The units for specific heat are
c
p
5
kJ
kg K
Note that these units are equivalent to kJ/(kg 8C), since 18 temperature
change is the same in Celsius or Kelvin scale.
Food composition values may be obtained from Agriculture Handbook
No. 8 (Watt and Merrill, 1975). Values for selected foods are given in
Table A.2.8.
Example 4.1
Predict the specific heat for a model food with the following composition:
carbohydrate 40%, protein 20%, fat 10%, ash 5%, moisture 25%.
Given
X
h
5 0.4 X
p
5 0.2 X
f
5 0.1 X
a
5 0.05 X
m
5 0.25
Approach
Since the product composition is given, Equation (4.4) will be used to predict
specific heat. Furthermore, we will program a spreadsheet with Equation (4.5) to
determine a value for specific heat.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
ABCDEFGHI
Temperature (°C) 20
Water 0.25
Protein 0.2
Fat 0.1
Carbohydrate 0.4
Fiber 0
Ash 0.05
Coefficients
Water 4.1766
Protein 2.0319
Fat 2.0117
Carbohydrate 1.5857
Fiber 1.8807
Ash 1.1289
Eq(4.5)
Water 1.044
Protein 0.406
Fat 0.201
Carbohydrate 0.634
Fiber 0.000
Ash 0.056
Result 2.342
4.17620.000090864*$B$10.0000054731*$B$1^2
2.00820.0012089*$B$10.0000013129*$B$1^2
1.98420.0014733*$B$10.0000048008*$B$1^2
1.54880.0019625*$B$10.0000059399*$B$1^2
1.84590.0018306*$B$10.0000046509*$B$1^2
1.09260.0018896*$B$10.0000036817*$B$1^2
B2*B10
B3*B11
B4*B12
B5*B13
B6*B14
B7*B15
SUM(B18:B23)
■ Figure E4.1 Spreadsheet for data given in Example 4.1.
2774.2 Thermal Properties of Foods

Solution
1. Using Equation (4.4)
c
p
5 (1:424 3 0:4) 1 (1:549 3 0:2) 1 (1:675 3 0:1)
1 (0:837 3 0:05) 1 (4:187 3 0:25)
5 2:14 kJ=(kg 8 C)
2. We can program a spreadsheet using
Equation (4.5) with coefficients
given in Table A.2.9 as shown in Figure E4.1.
3. Specific heat predicted using Equation (4.4) is 2.14 kJ/(kg 8C) whereas
using Equation (4.5) is slightly different as 2.34 kJ/(kg 8C). Equation (4.5)
is preferred since it incorporates information about the temperature.
4.2.2 Thermal Conductivity
The thermal conductivity of a food is an important property used in
calculations involving rate of heat transfer. In quantitative terms, this
property gives the amount of heat that will be conducted per unit
time through a unit thickness of the material if a unit temperature
gradient exists across that thickness.
In SI units, thermal conductivity is
k
J
s m 8C
W
m 8C
ð4:6Þ
Note that W/(m 8C) is same as W/(m K).
There is wide variability in the magnitude of thermal conductivity
values for commonly encountered materials. For example:
I Metals: 50400 W/(m 8C)
I Alloys: 10120 W/(m 8C)
I Water: 0.597 W/(m 8C) (at 208C)
I Air: 0.0251 W/(m 8C) (at 208C)
I Insulating materials: 0.0350.173 W/(m 8C)
Most high-moisture foods have thermal conductivity values closer to
that of water. On the other hand, the thermal conductivity of dried,
porous foods is influenced by the presence of air with its low value.
Tables A.2.2, A.3.1, and A.3.2 show thermal conductivity values
obtained numerically for a number of food and nonfood materials.
In addition to the tabulated values, empirical predictive equations are
useful in process calculations where temperature may be changing.
278 CHAPTER 4 Heat Transfer in Food Processing

For fruits and vegetables with a water content greater than 60%, the
following equation has been proposed (
Sweat, 1974):
k 5 0:148 1 0:493 X
w
ð4:7Þ
where k is thermal conductivity (W/[m 8C]), and X
w
is water content
expressed as a fraction. For meats and fish, temperature 0608C, water
content 6080%, wet basis, Sweat (1975) proposed the following
equation:
k 5 0:08 1 0:52 X
w
ð4:8Þ
Another empirical equation developed by
Sweat (1986) is to fit a set
of 430 data points for solid and liquid foods, as follows:
k 5 0:25 X
h
1 0:155 X
p
1 0:16 X
f
1 0:135 X
a
1 0:58 X
w
ð4:9Þ
where X is the mass fraction, and subscript h is carbohydrate, pis
protein, f is fat, a is ash, and w is water.
The coefficients in
Equation (4.9) are thermal conductivity values of
the pure component. Note that the thermal conductivity of pure water
at 258C is 0.606 W/(m 8C). The coefficient of 0.58 in Equation (4.9)
indicates that there is either a bias in the data set used for regression,
or the effective thermal conductivity of water in a food is different
from that of pure water.
Equations (4.7) to (4.9) are simple expressions to calculate the
thermal conductivity of f oods, however they do not include the
influence of temperature. Choi and Okos (1986) gave the follow-
ing expression that includes the influence of product composition
and temperature:
k 5
X
n
i51
k
i
Y
i
ð4:10Þ
where a food material has n components, k
i
is the thermal conductivity
of the ith component, Y
i
is the volume fraction of the ith component,
obtained as follows:
Y
i
5
X
i
=ρ
i
P
n
i51
ðX
i
=ρ
i
Þ
ð4:11Þ
where X
i
is the weight fraction and ρ
i
is the density (kg/m
3
) of the
ith component.
2794.2 Thermal Properties of Foods

The coefficients for k
i
for pure components are listed in Table A.2.9.
They may be programmed into a spreadsheet, as illustrated later in
Example 4.2.
For the additive models,
Equations (4.10) and (4.11), the food com-
position values may be obtained from Table A.2.8. These equations
predict thermal conductivity of foods within 15% of experimental
values.
In the case of anisotropic foods, the properties of the material
are direction dependent. For example, the presence of fibers in beef
results in different values of thermal conductivi ty when measured
parallel to the fibers (0.476 W/[m 8C]) versus perpendicular to
them (0.431 W/[m 8 C]). Mathematical models to predict the ther-
mal conductivity of anisotropic foods are discussed in
Heldman
and Singh (1981).
4.2.3 Thermal Diffusivity
Thermal diffusivity, a ratio involving thermal conductivity, density,
and specific heat, is given as,
α 5
k
ρc
p
ð4:12Þ
The units of thermal diffusivity are
α
m
2
s
Thermal diffusivity may be calculated by substituting values of
thermal conductivity, density, and specific heat in
Equation (4.12).
Table A.2.3 gives some experimentally determined values of thermal
diffusivity. Choi and Okos (1986) provided the following predictive
equation, obtained by substituting the values of k, ρ, and c
p
in
Equation (4.12):
α 5
X
n
i51
α
i
X
i
ð4:13Þ
where n is the number of components, α
i
is the thermal diffusivity of
the ith component, and X
i
is the mass fraction of each component.
The values of α
i
are obtained from Table A.2.9.
280 CHAPTER 4 Heat Transfer in Food Processing

Example 4.2
Estimate the thermal conductivity of hamburger beef that contains 68.3%
water.
Given
X
m
5 0.683
Approach
We will use Equation (4.8), which is recommended for meats. We will also
program a spreadsheet using Equations (4.10) and (4.11) at 208Ctocalculate
thermal conductivity.
Solution
1. Using Equation (4.8)
k 5 0:08 1 (0:52 3 0:683)
5 0:435 W=(m 8C)
2. Next we will program a spreadsheet as shown in
Figure E4.2 using the com-
position of hamburger beef from Table A.2.8 and coefficients of Equations
(4.10) and (4.11) given in Table A.2.9. We will use a temperature of 208C.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
ABCDEFGHI
Given
Temperature (°C) 20
Water 0.683
Protein 0.207
Fat 0.1
Carbohydrate 0
Fiber 0
Ash 0.01
density coeff Xi/ri Yi
Water 995.739918 0.000686 0.717526
Protein 1319.532 0.000157 0.164102
Fat 917.2386 0.000109 0.114046
Carbohydrate 1592.8908 0.000000 0
Fiber 1304.1822 0.000000 0
Ash 2418.1874 0.000004 0.004326
sum 0.000956
k Coeff
Water 0.6037 0.4331
Protein 0.2016 0.0331
Fat 0.1254 0.0143
Carbohydrate 0.2274 0.0000
Fiber 0.2070 0.0000
Ash 0.3565 0.0015
Result 0.4821
997.180.0031439*$B$30.0037574*$B$3^2
B4/B12
C12/$C$18
0.571090.0017625*$B$30.0000067036*$B$3^2
B21*D12
■ Figure E4.2 Spreadsheet for data given in Example 4.2.
2814.2 Thermal Properties of Foods

3. The thermal conductivity predicted by Equation (4.8) is 0.435 W/(m 8C),
whereas using Equation (4.10) it is 0.4821 W/(m 8C). Although Equation (4.8)
is easier to use, it does not include the influence of temperature.
4.3 MODES OF HEAT TRANSFER
In Chapter 1, we reviewed various forms of energy, such as thermal,
potential, mechanical, kinetic, and electrical. Our focus in this chap-
ter will be on thermal energy, commonly referred to as heat energy
or heat content. As noted in Section 1.19, heat energy is simply the
sensible and latent forms of internal energy. Recall that the heat con-
tent of an object such as a tomato is determined by its mass, specific
heat, and temperature. The equation for calculating heat content is
Q 5 mc
p
ΔT ð4:14Þ
where m is mass (kg), c
p
is specific heat at constant pressure (kJ/[kg K]),
and ΔT is the temperature difference between the object and a reference
temperature (8C). Heat content is always expressed relative to some
other temperature (called a datum or reference temperature).
Although determining heat content is an important calculation,
the knowledge of how heat may transfer from one object to another
or within an object is of even greater practical value. For example, to
thermally sterilize tomato juice, we raise its heat content by transfer-
ring heat from some heating medium such as steam into the juice.
In order to design the sterilization equipment, we need to know how
much heat is necessary to raise the temperature of tomato juice from
the initial to the final sterilization temperature using
Equation (4.14).
Furthermore, we need to know the rate at which heat will transfer
from steam into the juice first passing through the walls of the steril-
izer. Therefore, our concerns in heating calculations are twofold: the
quantity of heat transferred, Q, expressed in the units of joule (J); and
the rate of heat transfer , q, expressed as joule/s (J/s) or watt (W).
We will first review some highlights of the three common modes of
heat transfer—conduction, convection, and radiation—and then exam-
ine selected topics of rates of heat transfer important in the design and
analysis of food processes.
4.3.1 Conductive Heat Transfer
Conduction is the mode of heat transfer in which the transfer of energy
takes place at a molecular level. There are two commonly accepted
282 CHAPTER 4 Heat Transfer in Food Processing

theories that describe conductive heat transfer. According to one theory,
as molecules of a solid material attain additional thermal energy, they
become more energetic and vibrate with increased amplitude of vibra-
tion while confined in their lattice. These vibrations are transmitted
from one molecule to another without actual translatory motion of the
molecules. Heat is thus conducted from regions of higher temperature
to those at lower temperature. The second theory states that conduction
occurs at a molecular level due to the drift of free electrons. These free
electrons are prevalent in metals, and they carry thermal and electrical
energy. For this reason, good conductors of electricity such as silver and
copper are also good conductors of thermal energy.
Note that in conductive mode, there is no physical movement of the
object undergoing heat transfer. Conduction is the common mode of
heat transfer in heating/cooling of opaque solid materials.
From everyday experience, we know that on a hot day, heat transfer
from the outside to the inside through the wall of a room (
Fig. 4.11)
depends on the surface area of the wall (a wall with larger surface area
will conduct more heat), the thermal properties of construction materi-
als (steel will conduct more heat than brick), wall thickness (more heat
transfer through a thin wall than thick), and temperature difference
(more heat transfer will occur when the outside temperature is much
hotter than the inside room temperature). In other words, the rate of
heat transfer through the wall may be expressed as
q ~
ðwall surface areaÞðtemperature differenceÞ
ðwall thicknessÞ
ð4:15Þ
Wall cross-section
Wall thickness
Length
Height
T
o
Ti
Heat transfe
r
Outside
Inside
Temperature
difference
Wall surface
area Length
Height
T
i
■ Figure 4.11 Conductive heat flow in a wall.
2834.3 Modes of Heat Transfer

or
q
x
~
A dT
dx
ð4:16Þ
or, by inserting a constant of proportionality,
q
x
52kA
dT
dx
ð4:17Þ
where q
x
is the rate of heat flow in the direction of heat transfer by con-
duction (W); k is thermal conductivity (W/[m 8C] ); A is area (normal
to the direction of heat transfer) through which heat flows (m
2
); T is
temperature (8C); and x is length (m), avariable.
Equation (4.17) is also called Fourier’s law for heat conduction,
after Joseph Fourier, a French mathematical physicist. According to the
second law of thermodynamics, heat will always conduct from higher
temperature to lower temperature. As shown in Figure 4.12, the gradient
dT/dx is negative, because temperature decreases with increasing values
of x.Therefore, in Equation (4.17), a negative sign is used to obtain a
positive value for heat flow in the direction of decreasing temperature.
Example 4.3
One face of a stainless-steel plate 1 cm thick is maintained at 1108C,
and the other face is at 908C(
Fig. E4.3). Assuming steady-state conditions,
calculate the rate of heat transfer per unit area through the plate. The
thermal conductivity of stainless steel is 17 W/(m 8C).
Given
Thickness of plate 5 1cm5 0.01 m
Temperature of one face 5 1108C
Temperature of other face 5 908C
Thermal conductivity of stainless steel 5 17 W/(m 8C)
Approach
For steady-state heat transfer in rectangular coordinates we will use
Equation (4.17) to compute rate of heat transfer.
Solution
1. From Equation (4.17)
q 52
17 [W=(m 8C)] 3 1[m
2
] 3 (110 2 90) [8C]
(0 2 0:01) [m]
5 34, 000 W
T(x)
ΔT
Δx
dT
dx
Temperature (T )
Distance (x)
■ Figure 4.12 Sign convention for conductive
heat flow.
110°C
90°C
1 cm
x
■ Figure E4.3 Heat flow in a plate.
284 CHAPTER 4 Heat Transfer in Food Processing

2. Rate of heat transfer per unit area is calculated to be 34,000 W. A positive
sign is obtained for the heat transfer, indicating that heat always flows
“downhill” from 1108Cto908C.
4.3.2 Convective Heat Transfer
When a fluid (liquid or gas) comes into contact with a solid body
such as the surface of a wall, heat exchange will occur between the
solid and the fluid whenever there is a temperature difference between
the two. During heating and cooling of gases and liquids the fluid
streams exchange heat with solid surfaces by convection.
The magnitude of the fluid motion plays an important role in con-
vective heat transfer. For example, if air is flowing at a high velocity
past a hot baked potato, the latter will cool down much faster than if
the air velocity was much lower. The complex behavior of fluid flow
next to a solid surface, as seen in velocity profiles for laminar and
turbulent flow conditions in Chapter 2, make the determination of
convective heat transfer a complicated topic.
Depending on whether the flow of the fluid is artificially induced or
natural, there are two types of convective heat transfer: forced con-
vection and free (also called natural) convection. Forced convection
involves the use of some mechanical means, such as a pump or a
fan, to induce movement of the fluid. In contrast, free convection
occurs due to density differences caused by temperature gradients
within the system. Both of these mechanisms may result in either
laminar or turbulent flow of the fluid, although turbulence occurs
more often in forced convection heat transfer.
Consider heat transfer from a heated flat plate, PQRS, exposed to a
flowing fluid, as shown in
Figure 4.13. The surface temperature of
the plate is T
s
, and the temperature of the fluid far away from the
plate surface is T
N
. Because of the viscous properties of the fluid, a
velocity profile is set up within the flowing fluid, with the fluid
velocity decreasing to zero at the solid surface. Overall, we see that
the rate of heat transfer from the solid surface to the flowing fluid is
proportional to the surface area of solid, A, in contact with the fluid,
and the difference between the temperatures T
s
and T
N
.Or,
q ~ AðT
s
2 T
N
Þð4:18Þ
or,
q 5 hAðT
s
2 T
N
Þð4:19Þ
Q
S
R
P
T
s
T∞
q
Fluid flow
w
■ Figure 4.13 Convective heat flow from the
surface of a flat plate.
2854.3 Modes of Heat Transfer

The area is A (m
2
), and h is the convective heat-transfer coefficient
(sometimes called surface heat-transfer coefficient), expressed as
W/(m
2
8C). This equation is also called Newton’s law of cooling.
Note that the convective heat transfer coefficient, h, is not a property
of the solid material. This coefficient, however, depends on a number
of properties of fluid (density, specific heat, viscosity, thermal conduc-
tivity), the velocity of fluid, geometry, and roughness of the surface of
the solid object in contact with the fluid.
Table 4.1 gives some approx-
imate values of h. A high value of h reflects a high rate of heat transfer.
Forced convection offers a higher value of h than free convection. For
example, you feel cooler sitting in a room with a fan blowing air than
in a room with stagnant air.
Example 4.4
The rate of heat transfer per unit area from a metal plate is 1000 W/m
2
.
The surface temperature of the plate is 1208C, and ambient temperature
is 208C(
Fig. E4.4). Estimate the convective heat transfer coefficient.
Given
Plate surface temperature 5 1208C
Ambient temperature 5 208C
Rate of heat transfer per unit area 5 1000 W/m
2
Approach
Since the rate of heat transfer per unit area is known, we will estimate the
convective heat transfer coefficient directly from Newton’s law of cooling,
Equation (4.19).
Table 4.1 Some Approximate Values of Convective Heat-Transfer
Coefficient
Fluid
Convective heat-transfer coefficient
(W/[m
2
K])
Air
Free convection 525
Forced convection 10200
Water
Free convection 20100
Forced convection 5010,000
Boiling water 3000100,000
Condensing water vapor 5000100,000
20°C
120°C
1000 W/m
2
■ Figure E4.4 Convective heat transfer from
a plate.
286 CHAPTER 4 Heat Transfer in Food Processing

Solution
1. From Equation (4.19),
h 5
1000[W=m
2
]
(120 2 20) [8C]
5 10 W=(m
2
8C)
2. The convective heat transfer coefficient is found to be 10 W/(m
2
8C).
4.3.3 Radiation Heat Transfer
Radiation heat transfer occurs between two surfaces by the emission
and later absorption of electromagnetic waves (or photons). In con-
trast to conduction and convection, radiation requires no physical
medium for its propagation—it can even occur in a perfect vacuum,
moving at the speed of light, as we experience everyday solar radia-
tion. Liquids are strong absorbers of radiation. Gases are transparent
to radiation, except that some gases absorb radiation of a particular
wavelength (for example, ozone absorbs ultraviolet radiation). Solids
are opaque to thermal radiation. Therefore, in problems involving
thermal radiation with solid materials, such as with solid foods, our
analysis is concerned primarily with the surface of the material. This
is in contrast to microwave and radio frequency radiation, where the
wave penetration into a solid object is significant.
All objects at a temperature above 0 Absolute emit thermal radiation.
Thermal radiation emitted from an object ’s surface is proportional to
the absolute temperature raised to the fourth power and the surface
characteristics. More specifically, the rate of heat emission (or radia-
tion) from an object of a surface area A is expressed by the following
equation:
q 5 σεAT
4
A
ð4:20Þ
where σ is the StefanBoltzmann
1
constant, equal to 5.669 3
10
28
W/(m
2
K
4
); T
A
is temperature, Absolute; A is the area (m
2
); and
1
Josef Stefan (18351893). An Austrian physicist, Stefan began his academic
career at the University of Vienna as a lecturer. In 1866, he was appointed direc-
tor of the Physical Institute. Using empirical approaches, he derived the law
describing radiant energy from blackbodies. Five years later, another Austrian,
Ludwig Boltzmann, provided the thermodynamic basis of what is now known
as the StefanBoltzmann law.
2874.3 Modes of Heat Transfer

ε is emissivity, which describes the extent to which a surface is similar to
a blackbody. For a blackbody, the value of emissivity is 1. Table A.3.3
gives values of emissivity for selected surfaces.
Example 4.5
Calculate the rate of heat energy emitted by 100 m
2
of a polished iron
surface (emissivity 5 0.06) as shown in
Figure E4.5. The temperature of the
surface is 378C.
Given
Emissivity ε 5 0.06
Area A 5 100 m
2
Temperature 5 378C 5 310 K
Approach
We will use the StefanBoltzmann law, Equation (4.20), to calculate the rate
of heat transfer due to radiation.
Solution
1. From Equation (4.20)
q 5 (5:669 3 10
28
W=[m
2
K
4
])(0:06)(100 m
2
)(310 K)
4
5 3141 W
2. The total energy emitted by the polished iron surface is 3141 W.
4.4 STEADY-STATE HEAT TRANSFER
In problems involving heat transfer, we often deal with steady state
and unsteady state (or transient) conditions. Steady-state conditions
imply that time has no influence on the temperature distribution
within an object, although temperature may be different at different
locations within the object. Under unsteady-state conditions, the
temperature changes with location and time. For example, consider
the wall of a refrigerated warehouse as shown in
Figure 4.14. The
inside wall temperature is maintained at 68C using refrigeration,
while the outside wall temperature changes throughout the day and
night. Assume that for a few hours of the day, the outside wall tem-
perature is constant at 208C, and during that time duration the rate
of heat transfer into the warehouse through the wall will be under
steady-state conditions. The temperature at any location inside the
wall cross-section (e.g., 148C at location A) will remain constant,
q
100 m
2
ε 0.06
37°C
■ Figure E4.5 Heat transfer from a plate.
6°C
8°C
10°C
12°C
14°C
16°C
18°C
20°C
A
Refrigerated
room
Outside
q
■ Figure 4.14 Steady state conductive heat
transfer in a wall.
288 CHAPTER 4 Heat Transfer in Food Processing
although this temperature is different from other locations along
the path of heat transfer within the wall, as shown in Figure 4.14.If,
however, the temperature of the outside wall surface changes (say,
increases above 208C), then the heat transfer through the wall will be
due to unsteady-state conditions, because now the temperature within
the wall will change with time and location. Although true steady-
state conditions are uncommon, their mathematical analysis is much
simpler. Therefore, if appropriate, we assume steady-state conditions
for the analysis of a given problem to obtain useful information for
designing equipment and processes. In certain food processes such as
in heating cans for food sterilization, we cannot use steady-state
conditions, because the duration of interest is when the temperature
is changing rapidly with time, and microbes are being killed. For ana-
lyzing those types of problems, an analysis involving unsteady-state
heat transfer is used, as discussed later in Section 4.5.
Another special case of heat transfer involves change in temperature
inside an object with time but not with location, such as might occur
during heating or cooling of a small aluminum sphere, which has a
high thermal conductivity. This is called a lumped system. We will
discuss this case in more detail in Section 4.5.2.
In the following section, we will examine several applications of
steady-state conduction heat transfer.
4.4.1 Conductive Heat Transfer
in a Rectangular Slab
Consider a slab of constant cross-sectional area, as shown in
Figure 4.15. The temperature, T
1
, on side X is known. We will develop
an equation to determine temperature, T
2
, on the opposite side Y and
at any location inside the slab under steady-state conditions.
This problem is solved by first writing Fourier’s law,
q
x
52kA
dT
dx
ð4:21Þ
The boundary conditions are
x 5 x
1
T 5 T
1
x 5 x
2
T 5 T
2
ð4:22Þ
Separating variables in
Equation (4.21), we get
q
x
A
dx 52kdT ð4:23Þ
Side Y
Side X
x
q
x
x
2
x
1
T
2
T
1
T
1
T
2
R
t
Thermal
resistance circuit
w
■ Figure 4.15 Heat transfer in a wall, also
shown with a thermal resistance circuit.
2894.4 Steady-S tate Heat Transfer

Setting up integration and substituting limits, we have
ð
x
2
x
1
q
x
A
dx 52
ð
T
2
T
1
kdT ð4:24Þ
Since q
x
and A are independent of x, and k is assumed to be indepen-
dent of T,
Equation (4.24) can be rearranged to give
q
x
A
ð
x
2
x
1
dx 52k
ð
T
2
T
1
dT ð4:25Þ
Finally, integrating this equation, we get
q
x
A
ðx
2
2 x
1
Þ52kðT
2
2 T
1
Þð4:26Þ
or
q
x
52kA
ðT
2
2 T
1
Þ
ðx
2
2 x
1
Þ
ð4:27Þ
Temperature on face Y is T
2
; thus, rearranging Equation (4.27),
T
2
5 T
1
2
q
x
kA
ðx
2
2 x
1
Þð4:28Þ
To determine temperature, T, at any location, x, within the slab, we
may replace T
2
and x
2
with unknown T and distance variable x,
respectively, in
Equation (4.28) and obtain,
T 5 T
1
2
q
x
kA
ðx 2 x
1
Þð4:29Þ
4.4.1.1 Thermal Resistance Concept
We noted in Chapter 3 that, according to Ohm’s Law, electrical cur-
rent, I, is directly proportional to the voltage difference, E
V
, and indi-
rectly proportional to the electrical resistance R
E
.Or,
I 5
E
V
R
E
ð4:30Þ
290 CHAPTER 4 Heat Transfer in Food Processing

If we rearrange the terms in Equation (4.27), we obtain
q
x
5
ðT
1
2 T
2
Þ
ðx
2
2 x
1
Þ
kA
ð4:31Þ
or,
q
x
5
T
1
2 T
2
R
t
ð4:32Þ
Comparing
Equations (4.30) and (4.32) , we note an analogy
between rate of heat transfer, q
x
, and electrical current, I, temperature
difference, (T
1
2 T
2
) and electrical voltage, E
v
, and thermal resistance,
R
t
, and electrical resistance, R
E
. From Equations (4.31) and (4.32),
thermal resistance may be expressed as
R
t
5
ðx
2
2 x
1
Þ
kA
ð4:33Þ
A thermal resistance circuit for a rectangular slab is also shown in
Figure 4.15. In solving problems involving conductive heat transfer in
a rectangular slab using this concept, we first obtain thermal resistance
using Equation (4.33) and then substitute it in Equation (4.32).The
rates of heat transfer across the two surfaces of a rectangular slab are
thus obtained. This procedure is illustrated in Example 4.6. The advan-
tage of using the thermal resistance concept will become clear when
we study conduction in multilayer walls. Moreover, the mathematical
computations will be much simpler compared with alter-native proce-
dures used in solving these problems.
Example 4.6
a. Redo Example 4.3 using the thermal resistance concept.
b. Determine the temperature at 0.5 cm from the 1108C temperature face.
Given
See Example 4.3
Location at which temperature is desired 5 0.5 cm 5 0.005 m
Approach
We will use Equation (4.33) to calculate thermal resistance, and then Equation
(4.32) to determine the rate of heat transfer. To determine temperature within the
slab, we will calculate the thermal resistance for the thickness of the slab bounded
2914.4 Steady-S tate Heat Transfer

by 1108C and the unknown temperature (Fig. E4.6). Since the steady-state heat
transfer remains the same throughout the slab, we will use the previously calcu-
lated value of q to determine the unknown temperature using Equation (4.32).
Solution
Part (a)
1. Using
Equation (4.33) , the thermal resistance R
t
is
R
t
5
0:01 [m]
17 [W=(m 8C)] 3 1[m
2
]
R
t
5 5:88 3 10
24
8C=W
2. Using
Equation (4.32) , we obtain rate of heat transfer as
q 5
110 [8C] 2 90 [8 C]
5:88 3 10
24
[8C=W]
or
q 5 34,013 W
Part (b)
3. Using
Equation (4.33) calculate resistance R
t1
R
t1
5
0:005 [m]
17 [W=(m 8C)] 3 1[m
2
]
R
t1
5 2:94 3 10
24
8C=W
4. Rearranging terms in
Equation (4.32) to determine the unknown
temperature T
T 5 T
1
2 (q 3 R
t1
)
T 5 110 [8C] 2 34,013 [W] 3 2:94 3 10
24
[8C=W]
T 5 1008C
5. The temperature at the midplane is 1008C. This temperature was
expected, since the thermal conductivity is constant, and the temperature
profile in the steel slab is linear.
4.4.2 Conductive Heat Transfer through a
Tubular Pipe
Consider a long, hollow cylinder of inner radius r
i
, outer radius r
o
,
and length L, as shown in
Figure 4.16.Lettheinsidewalltemperature
be T
i
and the outside wall temperature be T
o
. We want to calculate the
110°C
90°C
110°C
R
t
90°C
(a)
q
(b)
110°C
R
t1
R
t2
90°C
110°C
90°CT
0.5 cm 0.5 cm
q
■ Figure E4.6 Thermal resistance circuits for
heat transfer through a wall.
292 CHAPTER 4 Heat Transfer in Food Processing

rate of heat transfer along the radial direction in this pipe. Assume ther-
mal conductivity of the metal remains constant with temperature.
Fourier’s law in cylindrical coordinates may be written as
q
r
52kA
dT
dr
ð4:34Þ
where q
r
is the rate of heat transfer in the radial direction.
Substituting for circumferential area of the pipe,
q
r
52kð2πrLÞ
dT
dr
ð4:35Þ
The boundary conditions are
T 5 T
i
r 5 r
i
T 5 T
o
r 5 r
o
ð4:36Þ
Rearranging
Equation (4.35), and setting up the integrals,
q
r
2πL
ð
r
o
r
i
dr
r
52k
ð
T
o
T
i
dT ð4:37Þ
Equation (4.37) gives
q
r
2πL
jln rj
r
o
r
i
52kjTj
T
o
T
i
ð4:38Þ
q
r
5
2πLkðT
i
2 T
o
Þ
lnðr
o
=r
i
Þ
ð4:39Þ
r
i
r
o
r
r
o
r
i
T
i
R
t
T
o
T
i
T
o
q
r
L
w
■ Figure 4.16 Heat transfer in a radial
direction in a pipe, also shown with a thermal
resistance circuit.
2934.4 Steady-S tate Heat Transfer

Again, we can use the electrical resistance analogy to write an expres-
sion for thermal resistance in the case of a cylindrical-shaped object.
Rearranging the terms in
Equation (4.39), we obtain
q
r
5
ðT
i
2 T
o
Þ
lnðr
o
=r
i
Þ
2πLk
ð4:40Þ
Comparing Equation (4.40) with Equation (4.32), we obtain the
thermal resistance in the radial direction for a cylinder as
R
t
5
lnðr
o
=r
i
Þ
2πLk
ð4:41Þ
Figure 4.16 shows a thermal circuit to obtain R
t
. An illustration of
the use of this concept is given in Example 4.7.
Example 4.7
A 2-cm-thick steel pipe (thermal conductivity 5 43 W/[m 8C]) with 6 cm
inside diameter is being used to convey steam from a boiler to process
equipment for a distance of 40 m. The inside pipe surface temperature is
1158C, and the outside pipe surface temperature is 908C(
Fig. E4.7). Calculate
the total heat loss to the surroundings under steady-state conditions.
90°C
115°C
40 m
0.05 m
0.03 m
90°C
115°C
6 cm
10 cm
R
t
115°C90°C
End cross-section
Pipe thickness
■ Figure E4.7 Thermal resistance circuit for
heat transfer through a pipe.
294 CHAPTER 4 Heat Transfer in Food Processing

Given
Thickness of pipe 5 2cm5 0.02 m
Inside diameter 5 6cm5 0.06 m
Thermal conductivity k 5 43 W/(m 8C)
Length L 5 40 m
Inside temperature T
i
5 1158C
Outside temperature T
o
5 908C
Approach
We will determine the thermal resistance in the cross-section of the pipe and
then use it to calculate the rate of heat transfer, using
Equation (4.40) .
Solution
1. Using Equation (4.41)
R
t
5
ln(0:05=0:03)
2π 3 40[m] 3 43[W=(m 8C)]
5 4:727 3 10
25
8C=W
2. From
Equation (4.40)
q 5
115[8C] 2 90[8C]
4:727 3 10
25
[8C=W]
5 528,903 W
3. The total heat loss from the 40 m long pipe is 528,903 W.
4.4.3 Heat Conduction in Multilayered Systems
4.4.3.1 Composite Rectangular Wall (in Series)
We will now consider heat transfer through a composite wall made
of several materials of different thermal conductivities and thicknesses.
An example is a wall of a cold storage, constructed of different layers of
materials of different insulating properties. All materials are arranged in
series in the direction of heat transfer , as shown in
Figure 4.17.
From Fourier’s law,
q 52kA
dT
dx
This may be rewritten as
ΔT 52
qΔx
kA
ð4:42Þ
2954.4 Steady-S tate Heat Transfer

Thus, for materials B, C, and D, we have
ΔT
B
52
qΔx
B
k
B
A
ΔT
C
52
qΔx
C
k
C
A
ΔT
D
52
qΔx
D
k
D
A
ð4:43Þ
From
Figure 4.17,
ΔT 5 T
1
2 T
2
5 ΔT
B
1 ΔT
C
1 ΔT
D
ð4:44Þ
From
Equations (4.42), (4.43), and (4.44),
T
1
2 T
2
52
qΔx
B
k
B
A
1
qΔx
C
k
C
A
1
qΔx
D
k
D
A
ð4:45Þ
or, rearranging the terms,
T
1
2 T
2
52
q
A
Δx
B
k
B
1
Δx
C
k
C
1
Δx
D
k
D
ð4:46Þ
We can rewrite
Equation (4.46) for thermal resistance as
q 5
T
2
2 T
1
Δx
B
k
B
A
1
Δx
C
k
C
A
1
Δx
D
k
D
A
ð4:47Þ
or, using thermal resistance values for each layer, we can write
Equation (4.47) as,
q 5
T
2
2 T
1
R
tB
1 R
tC
1 R
tD
ð4:48Þ
Δx
B
Δx
C
Δx
D
R
tB
R
tC
R
tD
T
1
T
2
q
x
k
B
k
D
k
C
T
1
T
2
Area
A
w
■ Figure 4.17 Conductive heat transfer in a
composite rectangular wall, also shown with
a thermal resistance circuit.
296 CHAPTER 4 Heat Transfer in Food Processing

where
R
tB
5
Δx
B
k
B
A
R
tC
5
Δx
C
k
C
A
R
tD
5
Δx
D
k
D
A
The thermal circuit for a multilayer rectangular system is shown in
Figure 4.17. Example 4.8 illustrates the calculation of heat transfer
through a multilayer wall.
Example 4.8
A cold storage wall (3 m 3 6 m) is constructed of 15-cm-thick concrete
(thermal conductivity 5 1.37 W/[m 8C]). Insulation must be provided to
maintain a heat transfer rate through the wall at or below 500 W (
Fig. E4.8).
If the thermal conductivity of the insulation is 0.04 W/(m 8C), compute the
required thickness of the insulation. The outside surface temperature of
the wall is 388C, and the inside wall temperature is 58C.
Given
Wall dimensions 5 3m3 6m
Thickness of concrete wall 5 15 cm 5 0.15 m
k
concrete
5 1.37 W/(m 8C)
Maximum heat gain permitted, q 5 500 W
k
insulation
5 0.04 W/(m 8C)
Outer wall temperature 5 388C
Inside wall (concrete/insulation) temperature 5 58C
Approach
In this problem we know the two surface temperatures and the rate of heat trans-
fer through the composite wall, therefore, using this information we will first
calculate the thermal resistance in the concrete layer. Then we will calculate the
thermal resistance in the insulation layer, which will yield the thickness value.
Solution
1. Using Equation (4.48)
q 5
(38 2 5)[8 C]
R
t1
1 R
t2
2. Thermal resistance in the concrete layer, R
t2
is
R
t2
5
0:15 [m]
1:37 [W=(m 8C)] 3 18 [m
2
]
R
t2
5 0:00618C=W
38°C
5°C
38°C
5°C
500 W
15 cm
?
R
t1
R
t2
■ Figure E4.8 Heat transfer through a
two-layered wall.
2974.4 Steady-S tate Heat Transfer

3. From step 1,
(38 2 5)[8C]
R
t1
1 0:0061[8C=W]
5 500
or,
R
t1
5
(38 2 5)[8C]
500[W]
5 0:0061[8C=W]
R
t1
5 0:068C=W
4. From
Equation (4.48)
Δx
B
5 R
tB
k
B
A
Thickness of insulation 5 0:06 [8C=W] 3 0:04[W=(m 8C)] 3 18 [m
2
]
5 0:043 m 5 4:3cm
5. An insulation with a thickness of 4.3 cm will ensure that heat loss from
the wall will remain below 500 W. This thickness of insulation allows a
91% reduction in heat loss.
4.4.3.2 Composite Cylindrical Tube (in Series)
Figure 4.18 shows a composite cylindrical tube made of two layers of
materials, A and B. An example is a steel pipe covered with a layer
of insulating material. The rate of heat transfer in this composite
tube can be calculated as follows.
In Section 4.4.2 we found that rate of heat transfer through a single-
wall cylinder is
q
r
5
(T
i
2 T
o
)
ln(r
o
=r
i
)
2πLk
The rate of heat transfer through a composite cylinder using thermal
resistances of the two layers is
q
r
5
(T
1
2 T
3
)
R
tA
1 R
tB
ð4:49Þ
or, substituting the individual thermal resistance values,
q
r
5
(T
1
2 T
3
)
ln(r
2
=r
1
)
2πLk
A
1
ln(r
3
=r
2
)
2πLk
B
ð4:50Þ
298 CHAPTER 4 Heat Transfer in Food Processing

The preceding equatio n is useful in calculating the rate of heat
transfer through a multilayered cylinder. Note that if there were
three laye rs present between the two su rfaces with temperatures T
1
and T
3
, then we just add another thermal resistance term in the
denominator.
Suppose we need to know the temperature at the interface between
two layers, T
2
, as shown in Figure 4.18. First, we calculate the steady-
state rate of heat transfer using Equation (4.50), noting that under
steady-state conditions, q
r
has the same value through each layer of
the composite wall. Then, we can use the following equation, which
represents the thermal resistance between the known temperature, T
1
,
and the unknown temperature, T
2
.
T
2
5 T
1
2 q
ln(r
2
=r
1
)
2πLk
A
ð4:51Þ
This procedure to solve problems for unknown interfacial tempera-
tures is illustrated in Example 4.9.
r
i
r
T
1
r
3
R
tA
R
tB
r
1
r
2
T
3
T
1
T
3
r
1
r
2
r
3
T
2
q
r
B
A
B
A
T
2
k
B
k
A
w
■ Figure 4.18 Conductive heat transfer in concentric cylindrical pipes, also shown with a thermal resistance circuit.
2994.4 Steady-S tate Heat Transfer

Example 4.9
A stainless-steel pipe (thermal conductivity 5 17 W/[m 8C]) is being used
to convey heated oil (
Fig. E4.9). The inside surface temperature is 1308C.
The pipe is 2 cm thick with an inside diameter of 8 cm. The pipe is insu-
lated with 0.04-m-thick insulation (thermal conductivity 5 0.035 W/[m 8C]).
The outer insulation temperature is 258C. Calculate the temperature of the
interface between steel and insulation , assume steady-state conditions.
Given
Thickness of pipe 5 2cm5 0.02 m
Inside diameter 5 8cm5 0.08 m
k
steel
5 17 W/(m 8C)
Thickness of insulation 5 0.04 m
k
insulation
5 0.035 W/(m 8C)
Inside pipe surface temperature 5 1308C
Outside insulation surface temperature 5 258C
Pipe length 5 1 m (assumed)
Approach
We will first calculate the two thermal resistances, inthepipeandtheinsulation.
Then we will obtain the rate of heat transfer through the composite layer. Finally ,
we will use the thermal resistance of the pipe alone to determine the temperature
at the interface between the pipe and insulation.
Solution
1. Thermal resistance in the pipe layer is, from Equation (4.41),
R
t1
5
ln(0:06=0:04)
2π 3 1[m] 3 17 [W=(m 8C)]
5 0:0038 8C=W
10 cm
6 cm
4 cm
130°C
25°C
R
t1
R
t2
r
i
8 cm
12 cm
20 cm
25°C
r
130°C
■ Figure E4.9 Heat transfer through a
multilayered pipe.
300 CHAPTER 4 Heat Transfer in Food Processing

2. Similarly, the thermal resistance in the insulation layer is,
R
t2
5
ln(0:1=0:06)
2π 3 1[m]3 0:035 [W=(m 8C)]
5 2:3229 8C=W
3. Using
Equation (4.49) , the rate of heat transfer is
q 5
(130 2 25)[8C]
0:0038 [8C=W] 1 2:3229 [8C=W]
5 45:13 W
4. Using
Equation (4.40)
45:13 [W] 5
(130 2 T)[8C]
0:0038 [8C=W]
T 5 130 [8C] 2 0:171 [8C]
T 5 129:838C
5. The interfacial temperature is 129.88C. This temperature is very close to
the inside pipe temperature of 1308C, due to the high thermal con-
ductivity of the steel pipe. The interfacial temperature between a hot
surface and insulation must be known to ensure that the insulation
will be able to withstand that temperature.
Example 4.10
A stainless-steel pipe (thermal conductivity 5 15 W/[mK]) is being used to
transport heated oil at 1258C(
Fig. E4.10).Theinsidetemperatureofthepipeis
1208C. The pipe has an inside diameter of 5 cm and is 1 cm thick. Insulation
is necessary to keep the heat loss from the oil below 25 W/m length of the
pipe. Due to space limitations, only 5-cm-thick insulation can be provided. The
outside surface temperature of the insulation must be above 208C(thedew
point temperature of surrounding air) to avoid condensation of water on the
surface of insulation. Calculate the thermal conductivity of insulation that will
result in minimum heat loss while avoiding water condensation on its surface.
Given
Thermal conductivity of steel 5 15 W/(m K)
Inside pipe surface temperature 5 1208C
Inside diameter 5 0.05 m
Pipe thickness 5 0.01 m
Heat loss permitted in 1 m length of pipe 5 25 W
Insulation thickness 5 0.05 m
Outside surface temperature . 208C 5 218C (assumed)
3014.4 Steady-S tate Heat Transfer
Approach
We will first calculate the thermal resistance in the steel layer, and set up an
equation for the thermal resistance in the insulation layer. Then we will sub-
stitute the thermal resistance values into
Equation (4.50). The only unknown,
thermal conductivity, k, will be then calculated.
Solution
1. Thermal resistance in the steel layer is
R
t1
5
ln(3:5=2:5)
2π 3 1[m]3 15 [W=(m 8C)
5 0:0036 8C=W
2. Thermal resistance in the insulation layer is
R
t2
5
ln(8:5=3:5)
2π 3 1[m]3 k[W=(m 8C)
5
0:1412[1=m]
k[W=(m 8C)]
3. Substituting the two thermal resistance values in
Equation (4.50)
25[W] 5
(120 2 21)[8C]
0:0036[8C=W] 1
0:1412 [1=m]
k[W=(m 8C)]
or,
k 5 0:0357 W=(m 8 C)
4. An insulation with a thermal conductivity of 0.0357 W/(m 8C) will ensure
that no condensation will occur on its outer surface.
8.5 cm
3.5 cm
2.5 cm
120°C
20°C
R
t1
R
t2
r
i
5 cm
7 cm
17 cm
20°C
r
120°C
■ Figure E4.10 Heat transfer through a
multilayered pipe.
302 CHAPTER 4 Heat Transfer in Food Processing

4.4.4 Estimation of Convective Heat-Transfer
Coefficient
In Section 4.3.1 on the conduction mode of heat transfer, we observed
that any material undergoing conduction heating or cooling remains
stationary. Conduction is the main mode of heat transfer within solids.
Now we will consider heat transfer between a solid and a surrounding
fluid, a mode of heat transfer called convection. In this case, the material
experiencing heating or cooling (a fluid) also moves. The movement of
fluid may be due to the natural buoyancy effects or caused by artificial
means, such as a pump in the case of a liquid or a blower for air.
Determination of the rate of heat transfer due to convection is compli-
cated because of the presence of fluid motion. In Chapter 2, we noted
that a velocity profile develops when a fluid flows over a solid surface
because of the viscous properties of the fluid material. The fluid next
to the wall does not move but “sticks” to it, with an increasing velocity
away from the wall. A boundary layer develops within the flowing
fluid, with a pronounced influence of viscous properties of the fluid.
This layer moves all the way to the center of a pipe, as was shown
in Figure 2.14. The parabolic velocity profile under laminar flow
conditions indicates that the drag caused by the sticky layer in contact
with the solid surface influences velocity at the pipe center.
Similar to the velocity profile, a temperature profile develops in a
fluid as it flows through a pipe, as sho wn in
Figure 4.19. Sup pose
the temperature of the p ipe surface is kept constant at T
s
, and the
fluid enters with a uniform temperature, T
i
. A temperature profile
develops because the fluid in contact with the pipe surface quickly
reaches the wall temperature, thus setting up a temperature gradient
as shown in the figure. A thermal boundary layer develops. At the
end of the thermal entrance region, the boundary layer extends all
the way to the pipe centerline.
Therefore, when heating or cooling a fluid as it flows through a
pipe, two boundar y layers d eve lop—a hydrodynami c boundary
Thermal entry region
T
s
T
i
Thermally
developed region
x
D
■ Figure 4.19 Thermal entry region in fluid
flowing in a pipe.
3034.4 Steady-S tate Heat Transfer

layer and a thermal boundary layer. These boundary layers have
a major influence on the rate of heat transfer between the pipe
surface and the fluid. The mathematics involved in an analytical
treatment of this subject is complicated and beyond the scope
of this book. However, there is an equal ly useful procedure called
the em pirical approach, which is widely used to determine the rate
of convective heat transfer. A drawback of the empiric al approach
is that it requires a large number of experiments to obtain the
required data. We overcome this problem and keep the data analy-
sis manageable by using dimensionless numbers. To formulate this
approach, first we will identify and review the required di mension-
less numbers: Reynolds number, N
Re
, Nusselt number, N
Nu
, and
Prandtl number, N
Pr
.
The Reynolds number was described in Section 2.3.2. It provides an
indication of the inertial and viscous forces present in a fluid. The
Reynolds number is calculated using Equation (2.20).
The second required dimensionless number for our data analysis is
Nusselt number—the dimensionless form of convective heat transfer
coefficient, h. Consider a fluid layer of thickness l, as shown in
Figure 4.20. The temperature difference between the top and bottom
of the layer is ΔT. If the fluid is stationary, then the rate of heat trans-
fer will be due to conduction, and the rate of heat transfer will be
q
conduction
52kA
ΔT
l
ð4:52Þ
However, if the fluid layer is moving, then the heat transfer will be
due to convection, and the rate of heat transfer using Newton ’s law
of cooling will be
q
convection
5 hAΔT ð4:53Þ
Dividing
Equation (4.53) by (4.52), we get
q
convection
q
conduction
5
hAΔT
kAΔT=l
5
hl
k
N
Nu
ð4:54Þ
Replacing thickness l with a more general term for dimension, the
characteristic dimension d
c
, we get
N
Nu
hd
c
k
ð4:55Þ
T
2
T
1
l
q
ΔT T
2
T
1
■ Figure 4.20 Heat transfer through a
fluid layer.
304 CHAPTER 4 Heat Transfer in Food Processing

Nusselt number may be viewed as an enhancement in the rate of
heat transfer caused by convection over the conduction mode.
Therefore, if N
Nu
5 1, then there is no improvement in the rate of
heat transfer due to convection. However, if N
Nu
5 5, the rate of con-
vective heat transfer due to fluid motion is five times the rate of heat
transfer if the fluid in contact with the solid surface is stagnant. The
fact that by blowing air over a hot surface we can cool it faster is
due to increased Nusselt number and consequently to an increased
rate of heat transfer.
The third required dimensionless number for the empirical approach
to determine convective heat transfer is Prandtl number, N
Pr
, which
describes the thickness of the hydrodynamic boundary layer com-
pared with the thermal boundary layer. It is the ratio between the
molecular diffusivity of momentum to the molecular diffusivity of
heat. Or,
N
Pr
5
molecular diffusivity of momentum
molecular diffusivity of heat
ð4:56Þ
or,
N
Pr
5
kinematic viscosity
thermal diffusivity
5
ν
α
ð4:57Þ
Substituting
Equations (2.11) and (4.12) in Equation (4.57),
N
Pr
5
μc
p
k
ð4:58Þ
If N
Pr
5 1, then the thickness of the hydrodynamic and thermal
boundary layers will be exactly the same. On the other hand, if
N
Pr
{1, the molecular diffusivity of heat will be much larger than that
of momentum. Therefore, the heat will dissipate much faster, as in the
case of a liquid metal flowing in a pipe. For gases, N
Pr
is about 0.7,
and for water it is around 10.
With a basic understanding of these three dimensionless numbers,
we will now plan the following experiment to determine convective
rate of heat transfer. Assume that a fluid is flowing in a heated pipe.
We are interested in determining convective rate of heat transfer from
the inside surface of the heated pipe into the fluid flowing inside the
pipe, as shown in
Figure 4.21. We carry out this experiment by pump-
ing a fluid such as water, entering at a velocity of u
i
at a temperature
of T
i
and flowing parallel to the inside surface of the pipe. The pipe
3054.4 Steady-S tate Heat Transfer

is heated using an electrical heater so that the inside pipe surface is
maintained at temperature T
s
, which is higher than the inlet fluid
temperature, T
i
. We measure the electric current, I, and electrical resis-
tance, R
E
, and calculate the product of the two to determine the rate
of heat transfer, q. The pipe is well insulated so that all the electrically
generated heat transfers into the fluid. Thus, we can experimentally
determine values of q, A, T
i
, u
i
, and T
s
.UsingEquation (4.53), we can
calculate the convective heat transfer coefficient, h.
If we repeat this experiment with a different diameter pipe or differ-
ent temperature of pipe surface, a new value of h will be obtained.
It should become clear that we can perform a series of experiments
to obtain h values that are a function of the operating variables, q, A,
u
i
, T
i
, and T
s
. The disadvantage of this experiment is that a large
amount of experimental data are generated, and organizing these
data for meaningful applications is a daunting task. However, the
data analysis can be greatly simplified if we combine various proper-
ties and operating variables into the three dimensionless numbers,
N
Re
, N
Nu
, and N
Pr
, which will accommodate all the properties and
variables that are important to our experiment.
Thus, for each experimental set, we calculate the respective dimension-
less numbers and, using log-log scale, plot the Nusselt number as a
function of the Reynolds number for different values of the Prandtl
number.
Figure 4.22 shows a typical plot. It has been experimentally
determined that for a given fluid with a fixed Prandtl number, straight
line plots are obtained on the log-log scale, as shown in Figure 4.22.
Fluid inlet
temperature T
i
Velocity u
i
Current I
Surface temperature T
s
■ Figure 4.21 Heating of fluid with electrically
heated pipe surface.
Log N
Re
Log N
Nu
N
Pr1
N
Pr2
N
Pr3
N
Nu
CN
Re
N
Pr
mn
■ Figure 4.22 A plot of Nusselt and Reynolds
numbers on a log-log scale.
306 CHAPTER 4 Heat Transfer in Food Processing

This type of graphical relationship may be conveniently expressed with
an equation as
N
Nu
5 CN
m
Re
N
n
Pr
ð4:59Þ
where C, m, and n are coefficients.
By substituting the experimentally obtained coefficients in
Equation (4. 59), we ob tain empirical correlations specific for a given
condition. Several researchers have determined these empirical cor-
relations for a variety of ope rating conditions, such as fluid flow
inside a pipe, over a pipe, or over a sphere. Different corre lations
are obtained, depending on whether the flow is laminar or
turbulent.
A suggested methodology to solve problems requiring the calculation
of convective heat transfer coefficients using empirical correlations is
as follows:
1. Identify flow geometry. The first step in a calculation involving
convection heat transfer is to clearly identify the geometrical
shape of the solid surface in contact with the fluid and its
dimensions. For example, is it a pipe, sphere, rectangular duct,
or a rectangular plate? Is the fluid flowing inside a pipe or over
the outside surface?
2. Identify the fluid and determine its properties. The second step is
to identify the type of fluid. Is it water, air, or a liquid food?
Determine the average fluid temperature far away from the solid
surface, T
N
. In some cases the average inlet and outlet tempera-
tures may be different, for example, in a heat exchanger; in that
case calculate the average fluid temperature as follows:
T
N
5
T
i
1 T
e
2
ð4:60Þ
where T
i
is the average inlet fluid temperature and T
e
is the
average exit fluid temperature. Use the average fluid tempera-
ture, T
N
, to obtain physical and thermal properties of the fluid,
such as viscosity, density, and thermal conductivity, from appro-
priate tables (such as Table A.4.1 for water, Table A.4.4 for air),
paying careful attention to the units of each property.
3. Calculate the Reynolds number. Using the velocity of the fluid ,
fluid properties and the characteristic dimension of the object
3074.4 Steady-S tate Heat Transfer

in contact with the fluid, calculate the Reynolds number.
The Reynolds number is necessary to determine whether the
flow is laminar, transitional, or turbulent. This information is
required to select an appropriate empirical correlation.
4. Select an appropriate empirical correlation. Using the information
from steps (1) and (3), select an empirical correlation of the
form given in
Equation (4.59) for the conditions and geome-
try of the object that resembles the one being investigated
(as presented later in this section). For example, if the given
problem involves turbulent water flow in a pipe, select the
correlation given in Equation (4.67). Using the selected corre-
lation, calculate Nusselt number and finally the convective
heat transfer coefficient.
The convective heat-transfer coefficient h is predicted from empirical
correlations. The coefficient is influenced by such parameters as type
and velocity of the fluid, physical properties of the fluid, temperature
difference, and geometrical shape of the physical system under
consideration.
The empirical correlations useful in predicting h are presented in the
following sections for both forced and free convection. We will dis-
cuss selected physical systems that are most commonly encountered
in convective heat transfer in food processing. For other situations
refer to handbooks such as
Rotstein et al. (1997) or Heldman and
Lund (1992). All correlations apply to Newtonian fluids only. For
expressions for non-Newtonian fluids, the textbook by Heldman and
Singh (1981) is recommended.
4.4.4.1 Forced Convection
In forced convection, a fluid is forced to move over a solid surface
by external mechanical means, such as an electric fan, pump, or a
stirrer (Fig. 4.23). The general correlation between the dimensionless
numbers is
N
Nu
5 ΦðN
Re
,N
Pr
Þð4:61Þ
where N
Nu
is Nusselt number 5 hd
c
/k; h is convective heat-transfer coef-
ficient (W/[m
2
8C]); d
c
is the characteristic dimension (m); k is thermal
conductivity of fluid (W/[m 8C]); N
Re
is Reynolds number 5 ρud
c
=μ;
ρ is density of fluid (kg/m
3
); u is velocity of fluid (m/s); μ is viscosity
(Pa s); N
Pr
is Prandtl number 5 μc
p
/k; c
p
is specific heat (kJ/[kg 8C]);
and Φ stands for “function of”.
Air flow
directed on
the external
surface of a
pipe
Air flow
directed on
the inside
surface of a
pipe
Fan
Fan
w
■ Figure 4.23 Forced convective heat
transfer from a pipe with flow inside and outside
the pipe.
308 CHAPTER 4 Heat Transfer in Food Processing

Laminar flow in pipes
1. Fully developed conditions with constant surface temperature
of the pipe:
N
Nu
5 3:66 ð4:62Þ
where thermal conductivity of the fluid is obtained at average
fluid temperature, T
N
, andd
c
is the inside diameter of the pipe.
2. Fully developed conditions with uniform surface heat flux:
N
Nu
5 4:36 ð4:63Þ
where thermal conductivity of the fluid is obtained at average
fluid temperature, T
N
, andd
c
is the inside diameter of the pipe.
3. For both entry region and fully developed flow conditions:
N
Nu
5 1:86 N
Re
3 N
Pr
3
d
c
L
0:33
μ
b
μ
w
0:14
ð4:64Þ
where L is the length of pipe (m); characteristic dimension, d
c
,
is the inside diameter of the pipe; all physical properties are
evaluated at the average fluid temperature, T
N
, except μ
w
,
which is evaluated at the surface temperature of the wall.
Transition flow in pipes
For Reynolds numbers between 2100 and 10,000,
N
Nu
5
ðf =8ÞðN
Re
2 1000ÞN
Pr
1 1 12:7ðf =8Þ
1=2
ðN
2=3
Pr
2 1Þ
ð4:65Þ
where all fluid properties are evaluated at the average fluid tempera-
ture, T
N
, d
c
is the inside diameter of the pipe, and the friction factor, f,
is obtained for smooth pipes using the following expression:
f 5
1
ð0:790 ln N
Re
21:64Þ
2
ð4:66Þ
Turbulent flow in pipes
The following equation may be used for Reynolds numbers greater
than 10,000:
N
Nu
5 0:023N
0:8
Re
3 N
0:33
Pr
3
μ
b
μ
w
0:14
ð4:67Þ
3094.4 Steady-S tate Heat Transfer

Fluid properties are evaluated at the average film temperature, T
N
,
except μ
w
, which is evaluated at the wall temperature; d
c
is the inside
diameter of the pipe.
Equation (4.67) is valid both for constant
surface temperature and uniform heat flux conditions.
Convection in noncircular ducts
For noncircular ducts, an equivalent diameter, D
e
, is used for the
characteristic dimension:
D
e
5
4 3 free area
wetted perimeter
ð4:68Þ
Figure 4.24 shows a rectangular duct with sides of length W and H.
The equivalent diameter in this case will be equal to 2 WH/(W 1 H).
Flow past immersed objects
In several applications, the fluid may flow past immersed objects. For
these cases, the heat transfer depends on the geometrical shape of the
object, relative position of the object, proximity of other objects, flow
rate, and fluid properties.
For a flow past a single sphere, when the single sphere may be heated
or cooled, the following equation will apply:
N
Nu
5 2 1 0:60N
0:5
Re
3 N
1=3
Pr
for
1 , N
Re
, 70,000
0:6 , N
Pr
, 400
ð4:69Þ
where the characteristic dimension, d
c
, is the outside diameter of the
sphere. The fluid properties are evaluated at the film temperature T
f
where
T
f
5
T
s
1 T
N
2
For heat transfer in flow past other immersed objects, such as cylinders
and plates, correlations are available in Perry and Chilton (1973).
Example 4.11
Water flowing at a rate of 0.02 kg/s is heated from 20 to 608C in a horizontal
pipe (inside diameter 5 2.5 cm). The inside pipe surface temperature is
908C(
Fig. E4.11). Estimate the convective heat-transfer coefficient if the pipe
is 1 m long.
W
H
■ Figure 4.24 Cross-section of a
rectangular duct.
310 CHAPTER 4 Heat Transfer in Food Processing

Given
Water flow rate 5 0.02 kg/s
Inlet temperature 5 208C
Exit temperature 5 608C
Inside diameter 5 2.5 cm 5 0.025 m
Inside pipe surface temperature 5 908C
Length of pipe 5 1m
Approach
Since water is flowing due to some external means, the problem indicates
forced convective heat transfer. We will first determine if the flow is laminar
by calculating the Reynolds number. If the Reynolds number is less than 2100,
we will use
Equation (4.64) to calculate the Nusselt number. From the Nusselt
number we will calculate the h value.
Solution
1. Physical properties of water are needed to calculate the Reynolds number.
All physical properties except u
w
must be evaluated at average bulk fluid
temperature, (20 1 60)/2 5 408C. From Table A.4.1 at 408C,
Density ρ 5 992.2 kg/m
3
Specific heat c
p
5 4.175 kJ/(kg 8C)
Thermal conductivity k 5 0.633 W/(m 8C)
Viscosity (absolute) μ 5 658.026 3 10
26
Pa s
Prandtl number N
Pr
5 4.3
Thus,
N
Re
5
ρuD
μ
5
4
_
m
πμD
5
(4)(0:02 kg=s)
(π)(658:026 3 10
26
P a s)(0:025 m)
5 1547:9
Note that 1 Pa 5 1kg/(ms
2
). Since the Reynolds number is less than 2100,
the flow is laminar.
2. We select
Equation (4.64), and using μ
w
5 308.909 3 10
26
Pa s at 908C,
N
Nu
5 1:86(1547:9 3 4:3 3 0:025)
0:33
658:016 3 10
26
308:909 3 10
26
0:14
5 11:2
2.5 cm
1 m
90°C
■ Figure E4.11 Convective heat transfer inside
a pipe.
3114.4 Steady-S tate Heat Transfer
3. The convective heat-transfer coefficient can be obtained from the Nusselt
number.
h 5
N
Nu
k
D
5
(11:2)(0:633 W=[m 8C])
(0:025 m)
5 284 W=(m
2
8C)
4. The convective heat-transfer coefficient is estimated to be 284 W/(m
2
8C).
Example 4.12
If the rate of water flow in Example 4.11 is raised to 0.2 kg/s from 0.02 kg/s
while all other conditions are kept the same, calculate the new convective
heat-transfer coefficient.
Given
See Example 4.11
New mass flow rate of water 5 0.2 kg/s
Approach
We will calculate the Reynolds number to find whether the flow is turbulent. If
the flow is turbulent, we will use
Equation (4.67) to compute the Nusselt number.
The surface heat-transfer coefficient will be computed from the Nusselt number.
Solution
1. First, we compute the Reynolds number using some of the properties
obtained in Example 4.11.
N
Re
5
(4)(0:2kg=s)
(π)(658:026 3 10
26
Pa s)(0:025 m)
5 15, 479
Thus, flow is turbulent.
2. For turbulent flow, we select
Equation (4.67) .
N
Nu
5 ð0:023Þð15479Þ
0:8
ð4:3Þ
0:33
658:026 3 10
26
308:909 3 10
26
0
@
1
A
0:14
5 93
3. The convective heat transfer can be computed as
h 5
N
Nu
k
D
5
(93)(0:633 W=[m 8 C])
(0:025 m)
5 2355 W=(m
2
8C)
312 CHAPTER 4 Heat Transfer in Food Processing

4. The convective heat-transfer coefficient for turbulent flow is estimated
to be 2355 W/(m
2
8C). This value is more than eight times higher than
the value of h for laminar flow calculated in Example 4.11.
Example 4.13
What is the expected percent increase in convective heat-transfer coeffi-
cient if the velocity of a fluid is doubled while all other parameters are
kept the same for turbulent flow in a pipe?
Approach
We will use Equation (4.67) to solve this problem.
Solution
1. For turbulent flow in a pipe
N
Nu
5 0:023N
0:8
Re
3 N
0:33
Pr
3
μ
b
μ
w
0:14
We can rewrite this equation as
N
Nu
1
5 f(u
1
)
0:8
N
Nu
2
5 f(u
2
)
0:8
N
Nu
2
N
Nu
1
5
u
2
u
1
0:8
2. Since u
2
5 2u
1
,
N
Nu
2
N
Nu
1
5 (2)
0:8
5 1:74
N
Nu
2
5 1:74N
Nu
1
3. This expression implies that h
2
5 1.74 h
1
. Thus,
%Increase 5
1:74h
1
2 h
1
h
1
3 100 5 74%
4. As expected, velocity has a considerable effect on the convective
heat-transfer coefficient.
3134.4 Steady-S tate Heat Transfer

Example 4.14
Calculate convective heat-transfer coefficient when air at 908C is passed
through a deep bed of green peas. Assume surface temperature of a pea
to be 308C. The diameter of each pea is 0.5 cm. The velocity of air through
the bed is 0.3 m/s.
Given
Diameter of pea 5 0.005 m
Temperature of air 5 908C
Temperature of a pea 5 308C
Velocity of air 5 0.3 m/s
Approach
Since the air flows around a spherically immersed object (green pea), we will
estimate N
Nu
from Equation (4.69). The Nusselt number will give us the value
for h.
Solution
1. The properties of air are evaluated at T
f
, where
T
f
5
T
s
1 T
N
2
5
30 1 90
2
5 608C
From Table A.4.4,
ρ 5 1:025 kg=m
3
c
p
5 1:017 kJ=(kg 8C)
k 5 0:0279 W=(m 8C)
μ 5 19:907 3 10
26
Pa s
N
Pr
5 0:71
2. The Reynolds number is computed as
N
Re
5
(1:025 kg=m
3
)(0:3m=s)(0:005 m)
(19:907 3 10
26
Pa s)
5 77:2
3. From
Equation (4.69) ,
N
Nu
5 2 1 0:6(77:2)
0:5
(0:71)
0:33
5 6:71
314 CHAPTER 4 Heat Transfer in Food Processing

4. Thus
h 5
6:71(0:0279W=[m 8C])
(0:005 m)
5 37 W=(m
2
8C)
5. The convective heat-transfer coefficient is 37 W/(m
2
8C).
4.4.4.2 Free Convection
Free convection occurs because of density differences in fluids as they
come into contact with a heated surface (
Fig. 4.25). The low density
of fluid at a higher temperature causes buoyancy forces, and as a
result, heated fluid moves upward and colder fluid takes its place.
Empirical expressions useful in predicting convective heat-transfer
coefficients are of the following form:
N
Nu
5
hd
c
k
5 aðN
Ra
Þ
m
ð4:70Þ
where a and m are constants; N
Ra
, is the Rayleigh number. Rayleigh
number is a product of two dimensionless numbers, Grashof number
and Prandtl number.
N
Ra
5 N
Gr
3 N
Pr
ð4:71Þ
The Grashof number, N
Gr
, is defined as follows:
N
Gr
5
d
3
c
ρ
2
gβΔT
μ
2
ð4:72Þ
where d
c
is characteristic dimension (m); ρ is density (kg/m
3
); g is accel-
eration due to gravity (9.80665 m/s
2
); β is coefficient of volumetric
expansion (K
21
); ΔT is temperature difference between wall and the
surrounding bulk (8C); and μ is viscosity (Pa s).
A Grashof number is a ratio between the buoyancy forces and
viscous forces. Similar to the Reynolds number, the Grashof number
is useful for determining whether a flow over an object is laminar or
turbulent. For example, a Grashof number greater than 10
9
for fluid
flow over vertical plates signifies a turbulent flow.
In the case of heat transfer due to free convection, physical properties
are evaluated at the film temperature, T
f
5 (T
s
1 T
N
)/2.
Table 4.2 gives various constants that may be used in Equation (4.70)
for natural convection from vertical plates and cylinders, and from
horizontal cylinders and plates.
Air flow
around a pipe
due to a natural
convection
Pipe with a
heated outside
surface
w
■ Figure 4.25 Heat transfer from the outside
of a heated pipe due to natural convection.
3154.4 Steady-S tate Heat Transfer

Table 4.2 Coefficients for Equation (4.70) for Free Convection
Geometry
Characteristic
Length Range of N
Ra
amEquation
Vertical plate L 10
4
10
9
0.59 0.25 N
Nu
5 a(N
Ra
)
m
10
9
10
13
0.1 0.333
L
Inclined plate L Use same equations as vertical plate,
replace g by g cos θ for N
Ra
, 10
9
L
θ
Horizontal plate A/p 10
4
10
7
0.54 0.25 N
Nu
5 a(N
Ra
)
m
Surface area 5 A 10
7
10
11
0.15 0.33
Perimeter 5 p
(a) Upper surface of
a hot plate (or lower
surface of a cold plate)
Hot surface
Horizontal plate A/p 10
5
10
11
0.27 0.25 N
Nu
5 a(N
Ra
)
m
Surface area 5 A
Perimeter 5 p
(b) Lower surface of
a hot plate (or upper
surface of a cold plate)
Hot surface
(Continued)
316 CHAPTER 4 Heat Transfer in Food Processing

Example 4.15
Estima te the convective heat-trans fer coefficient for convective heat
loss from a horizontal 10 cm diameter steam pipe. The surface tempera-
ture of the uninsulated pipe is 1308C, and the air temperature is 308 C
(
Fig. E4.12).
Given
Diameter of pipe 5 10 cm 5 0.1 m
Pipe surface temperature T
w
5 1308C
Ambient temperature T
N
5 308C
Table 4.2 (Continued)
Geometry
Characteristic
Length Range of N
Ra
amEquation
Vertical cylinder
L
L A vertical cylinder can be treated as a
vertical plate when
D $
35L
N
0:25
Gr
Horizontal cylinder
D
D 10
25
10
212
N
Nu
5 0:61
0:387N
1=6
Ra
11
0:559
N
Pr
9=16
"#
8=27
8
>
>
>
>
>
<
>
>
>
>
>
:
9
>
>
>
>
>
=
>
>
>
>
>
;
2
Sphere
D
1
2
πD
N
Ra
# 10
11
(N
Pr
$ 0.7)
N
Nu
5 2 1
0:589N
1=4
Ra
11
0:469
N
Pr
9=16
"#
4=9
3174.4 Steady-S tate Heat Transfer

Approach
Since no mechanical means of moving air are indicated, heat loss from the
horizontal pipe is by free convection. After finding the property values of air
at film temperature, we will calculate the Grashof number. The product of the
Grashof number and Prandtl number will allow determination of a and m
parameters from
Table 4.2; these parameters will be used in Equation (4.72).
We will then compute the surface heat-transfer coefficient from the Nusselt
number.
Solution
1. Since no mechanical means of moving the air are indicated, heat loss
is by free convection.
2. The film temperature is obtained as
T
f
5
T
s
1 T
N
2
5
130 1 30
2
5 808C
3. The properties of air at 808C are obtained from Table A.4.4.
ρ 5 0:968 kg=m
3
β 5 2:83 3 10
23
K
21
c
p
5 1:019 kJ=(kg 8C)
k 5 0:0293 W=(m 8C)
μ 5 20:79 3 10
26
Ns=m
2
N
Pr
5 0:71
g 5 9:81 m=s
2
4. We calculate Rayleigh number, N
Ra
, the product of N
Gr
and N
Pr
. The
characteristic dimension is the outside diameter of the pipe.
N
Gr
5
d
3
c
ρ
2
gβΔT
μ
2
5
(0:1m)
3
(0:968 kg=m
3
)
2
(9:81 m=s
2
)(2:833 10
23
K
21
)(1308C 2 308C)
(20:793 1026Ns=m
2
)
2
5 6:0193 10
6
(Note that 1 N 5 kg m/s
2
.) Thus,
N
Gr
3 N
Pr
5 (6:019 3 10
6
)(0:71) 5 4:27 3 10
6
10 cm
130°C
30°C
■ Figure E4.12 Convective heat transfer from
a horizontal pipe.
318 CHAPTER 4 Heat Transfer in Food Processing

5. From Table 4.2, for horizontal cylinder
N
Nu
5 0:61
0:387(4:27 3 10
6
)
1=6
11
0:559
0:71
9=16
!
8=27
0
B
B
B
B
B
@
1
C
C
C
C
C
A
2
6. N
Nu
5 22
7. Thus
h 5
(22)(0:0293 W=[m 8C])
(0:1m)
5 6:5W=(m
2
8C)
4.4.4.3 Thermal Resistance in Convective Heat Transfer
A thermal resistance term for convective heat transfer may be defined
in a similar manner as in conductive heat transfer (
Section 4.4).
From Equation (4.19), we know that
q 5 hAðT
s
2 T
N
Þð4:73Þ
or, rearranging terms in
Equation (4.73),
q 5
T
s
2 T
N
1
hA
ð4:74Þ
where the thermal resistance due to convection (R
t
)
convection
is
ðR
t
Þ
convection
5
1
hA
ð4:75Þ
In problems involving conduction and convection heat transfer in
series, along the path of heat transfer, the thermal resistance due to
convection is added to the thermal resistance due to conduction
to obtain the total thermal resistance. We will discuss this further
in the context of overall heat transfer involving both conduction and
convection heat transfer.
3194.4 Steady-S tate Heat Transfer

4.4.5 Estimation of Overall Heat-Transfer Coefficient
In many heating/cooling applications, conductive and convective heat
transfer may occur simultaneously. An example shown in
Figure 4.26
involves heat transfer in a pipe that carries a fluid at a temperature
greater than the temperature of the environment surrounding the out-
side of the pipe. In this case, heat must first transfer from the inside
fluid by forced convection to the inside surface of the pipe, then
by conduction through the pipe wall material, and finally by free con-
vection from the outer pipe surface to the surrounding environment.
Thus, heat transfer is through three layers in a series.
Using the approach of thermal resistance values, we can write:
q 5
T
i
2 T
N
R
t
ð4:76Þ
where R
t
is a combination of the thermal resistances in the inside
convective layer, the conductive layer in the pipe material, and the
outside convective layer, or
R
t
5 ðR
t
Þ
inside convection
1 ðR
t
Þ
conduction
1 ðR
t
Þ
outiside convection
ð4:77Þ
where
ðR
t
Þ
inside convection
5
1
h
i
A
i
ð4:78Þ
where h
i
is the inside convective heat transfer coefficient, and A
i
is
the inside surface area of the pipe.
Resistance to heat transfer in the pipe wall is
ðR
t
Þ
conduction
5
ln
r
o
r
i
2πkL
ð4:79Þ
k
T
2
T
1
T
∞
T
i
h
i
h
o
T
i
T
1
T
2
T
∞
Metal
Outside pipe
Inside
pipe
Temperature
Distance from center
■ Figure 4.26 Combined conductive and
convective heat transfer.
320 CHAPTER 4 Heat Transfer in Food Processing

where k is the thermal conductivity of the pipe material (W/[m K]),
r
i
is the inside radius (m), and r
o
is the outside radius (m). Resistance
to heat transfer due to convection at the outside pipe surface is
ðR
t
Þ
outiside convection
5
1
h
o
A
o
ð4:80Þ
where h
o
is the convective heat transfer coefficient at the outside
surface of the pipe (W/[m
2
K]), and A
o
is the outside surface area
of the pipe. Substituting
Equations (4.78), (4.79), and (4.80) in
Equation (4.76), we obtain
q 5
T
i
2 T
N
1
h
i
A
i
1
lnðr
o
=r
i
Þ
2πLk
1
1
h
o
A
o
ð4:81Þ
We can also write an expression for the overall heat transfer for this
example as
q 5 U
i
A
i
ðT
i
2 T
N
Þð4:82Þ
where A
i
is the inside area of the pipe, and U
i
is the overall heat-transfer
coefficient based on the inside area of the pipe. From
Equation (4.82),
q 5
T
i
2 T
N
1
U
i
A
i
ð4:83Þ
From
Equations (4.83) and (4.81) we obtain
1
U
i
A
i
5
1
h
i
A
i
1
ln
r
o
r
i
2πLk
1
1
h
o
A
o
ð4:84Þ
Equation (4.84) is used to calculate the overall heat-transfer coeffi-
cient. The selection of area over which to calculate the overall heat
transfer is quite arbitrary. For example, if U
o
is selected as the overall
heat-transfer coefficient based on outside area of the pipe, then
Equation (4.84) is written as
1
U
o
A
o
5
1
h
i
A
i
1
ln
r
o
r
i
2πLk
1
1
h
o
A
o
ð4:85Þ
3214.4 Steady-S tate Heat Transfer

and Equation (4.82) is modified to
q 5 U
o
A
o
ðT
i
2 T
N
Þð4:86Þ
Both
Equations (4.82) and (4.86) yield the same value of the rate of
heat transfer, q. This is shown in Example 4.16.
Example 4.16
A 2.5-cm inside diameter pipe is being used to convey a liquid food at 808C
(
Fig. E4.13). The inside convective heat transfer coefficient is 10 W/(m
2
8C).
The pipe (0.5 cm thick) is made of steel (thermal conductivity 5 43 W/[m 8C]).
The outside ambient temperature is 208C. The outside convective heat-
transfer coefficient is 100 W/(m
2
8C). Calculate the overall heat transfer coeffi-
cient and the heat loss from 1 m length of the pipe.
Given
Inside diameter of pipe 5 0.025 m
Bulk temperature of liquid food 5 808C
Inside convective heat-transfer coefficient 5 10 W/(m
2
8C)
Outside convective heat-transfer coefficient 5 100 W/(m
2
8C)
k
steel
5 43 W/(m 8C)
Outside ambient temperature 5 208C
Approach
The overall heat-transfer coefficient can be computed by using a basis of
either the inside area of the pipe or the outside area of the pipe. We will use
Equation (4.84) to find U
i
and then use a modification of Equation (4.84) to
find U
o
. We will prove that the computed rate of heat flow will remain the
same regardless of whether U
i
or U
o
is selected.
Solution
1. Calculate the overall heat-transfer coefficient based on inside area using
Equation (4.84):
1
U
i
A
i
5
1
h
i
A
i
1
ln
r
o
r
i
2πLk
1
1
h
o
A
o
2. By canceling area terms and noting that A
i
5 2πr
i
L,
1
U
i
5
1
h
i
1
r
i
ln
r
o
r
i
k
1
r
h
o
r
o
h
o
100 W/m
2
°C
h
i
10W/m
2
°C
20°C
80°C
0.5 cm
2.5 cm
■ Figure E4.13 Overall heat transfer in a pipe.
322 CHAPTER 4 Heat Transfer in Food Processing

3. Substituting,
1
U
i
5
1
10 [W=(m
2
8C)]
1
0:0125 [m] 3 ln
0:0175
0:0125
m
m
43 [W=(m 8C)]
1
0:0125 [m]
100 [W=(m
2
8C)] 3 0:0175 [m]
5 0:1 1 0:0001 1 0:00714 5 0:10724 m
2
8C=W
Thus, U
i
5 9.32 W/(m
2
8C).
4. Heat loss
q 5 U
i
A
i
(80 2 20)
5 9:32[W=ðm
2
8C)] 3 2π 3 1[m]3 0:0125 [m] 3 60 [8C]
5 43:9W
5. Overall heat transfer coefficient based on outside area may be com-
puted as
1
U
o
A
o
5
1
h
i
A
i
1
ln
r
o
r
i
2πkL
1
1
h
o
A
o
6. By canceling area terms and noting that A
o
5 2πr
o
L,
1
U
o
5
r
o
h
i
A
i
1
r
o
ln
r
o
r
i
k
1
1
h
o
Substituting,
1
U
o
5
0:0175 [m]
10 [W=(m
2
8C)] 3 0:0125 [m]
1
0:0175 [m] 3 ln
0:0175
0:0125
m
m
43[W=(m 8C)]
1
1
100 [W=(m
2
8C)]
5 0:14 1 0:00014 1 0:01
5 0:1501 m
2
8C=W
U
o
5 6:66 W=(m
2
8C)
3234.4 Steady-S tate Heat Transfer

7. Heat loss
q 5 U
o
A
o
(80 2 20)
5 6:66[W=m
2
8C] 3 2π 3 0:0175 [m] 3 1[m]3 60 [8C]
5 43:9W
8. As expected, the rate of heat loss remains the same regardless of
which area was selected for computing overall heat-transfer
coefficient.
9. It should be noted from steps (3) and (6) that the resistance offered by
the metal wall is considerably smaller than the resistance offered in
the convective layers.
4.4.6 Fouling of Heat Transfer Surfaces
In heating equipment, when a liquid food comes into contact with a
heated surface, some of its components may deposit on the hot sur-
face, causing an increase in the resistance to heat transfer. This phe-
nomenon of product buildup on the heat transfer surface is called
fouling. A similar phenomenon is observed when a liquid is brought
into contact with subcooled surfaces. Fouling of heat transfer surfaces
not only increases thermal resistance but may also restrict fluid flow.
Furthermore, valuable components of the food are lost to the fouled
layer. Fouling is remedied by cleaning heating surfaces with strong
chemicals that are also environmental pollutants.
Fouling is of major concern in the chemical process industries. Its role is
even more pronounced in the food industry where many heat-sensitive
components of foods can easily deposit on a heat-transfer surface. As a
result, factory operations involving heating or cooling require frequent
cleaning, often on a daily basis. Some of the common types of fouling
and their underlying mechanisms are shown in
Table 4.3.
The fouling layer often has a composition different from the liquid
stream that causes fouling. With milk, which has a protein content of
around 3%, the fouling deposits resulting at temperatures less than
1108C contain 50 to 60% protein and 30 to 35% minerals. About half
of the protein in the fouled layer is β-lactoglobulin. When the tempera-
ture of the milk increases above 70 to 748C, protein denaturation
increases. The protein (β-lactoglobulin) first unfolds and the reactive
sulphydryl groups are exposed. This is followed by polymerization
(or aggregation) of the molecule with itself or other proteins including
α-lactoglobulin.
324 CHAPTER 4 Heat Transfer in Food Processing

Fouling results from a complex series of reactions, and in heating
processes these reactions are accelerated with temperature. To com-
pensate for the reduced rate of heat transfer due to the increased
thermal resistance, a larger heat-transfer surface area is required,
which increases the cost of heat exchange equipment. For an operat-
ing heat exchanger with a fouled surface, the reduced rate of heat
transfer is compensated for by using higher temperature gradients
across the heat transfer medium. Consequently, the energy require-
ments to operate heat exchange equipment increase significantly.
It has been estimated that the annual worldwide cost of fouling to
process industries is several billion dollars.
Let us examine the role of fouling on heat transfer by considering
the rates of heat transfer in a clean pipe and in one that has
been fouled on both the inside and outside surfaces (
Fig. 4.27).
Assuming that the deposited layers are thin, theconvectiveheat
transfer coeffi cient on the inside surface of the fouled pipe , h
fi
, will
be same as that on the inside surface of the clean pipe h
ci
.Thesame
wil l hold true for the convective heat transfer coefficients on the
outside surfaces; that is, h
fo
5 h
co
. Similarly, the inside surface area
of the foule d pipe, A
fi
5 A
ci
5 A
i
, and, for the outside surface area,
A
fo
5 A
co
5 A
o
.
Table 4.3 Common Mechanisms in Fouling of Heat Exchange
Surfaces
Type of fouling Fouling mechanism
Precipitation Precipitation of dissolved substances. Salts such
as CaSO
4
, CaCO
3
cause scaling.
Chemical reaction Surface material acts as a reactant; chemical
reactions of proteins, sugars, and fats.
Particulate Accumulation of fine particles suspended in the
processed fluids on the heat transfer surface.
Biological Attachment of organisms both macro and micro
on heat transfer surface.
Freezing Solidification of liquid components on subcooled
surfaces.
Corrosion Heat transfer surface reacts with ambient and
corrodes.
3254.4 Steady-S tate Heat Transfer

Using Equation (4.85), we write an equation for the overall heat
transfer coefficient based on the outside area for the clean pipe,
1
U
co
A
o
5
1
h
i
A
i
1
ln
r
o
r
i
2πLk
1
1
h
o
A
o
ð4:87Þ
or,
1
U
co
5
A
o
h
i
A
i
1
A
o
ln
r
o
r
i
2πLk
1
1
h
o
ð4:88Þ
Next, we will consider a pipe that is fouled on both the inside
and outside surface. As shown in
Figure 4.27b, the fouling resistance
due to the deposited layer on the inside of the pipe is R
fi
(m
2
8C/W)
and for the outside pipe it is R
fo
(m
2
8C/W). Then for the fouled
pipes,
1
U
fo
A
o
5
1
h
i
A
i
1
R
fi
A
i
1
ln
r
o
r
i
2πLk
1
R
fo
A
o
1
1
h
o
A
o
ð4:89Þ
or,
1
U
fo
5
A
o
h
i
A
i
1
R
fi
A
o
A
i
1
A
o
ln
r
o
r
i
2πLk
1 R
fo
1
1
h
o
ð4:90Þ
Since R
fi
and R
fo
are difficult to determine separately, the two terms
for fouling resistance in
Equations (4.88) are combined to get the
total resistance due to fouling, R
ft
,
R
ft
5
A
o
A
i
R
fi
1 R
fo
ð4:91Þ
Outside
fouling layer
Inside fouling
layer
Pipe wall
(a) Clean pipe (b) Pipe with fouling deposits
h
co
h
ci
h
fi
h
fo
■ Figure 4.27 A pipe with fouling deposits on
the inside and outside surfaces
326 CHAPTER 4 Heat Transfer in Food Processing

Then
1
U
fo
5
A
o
h
i
A
i
1
A
o
ln
r
o
r
i
2πLk
1 R
ft
1
1
h
o
ð4:92Þ
Combining
Equations (4.88) and (4.92), we get
1
U
fo
5
1
U
co
1 R
ft
ð4:93Þ
In industrial practice, a term called Cleaning factor (C
F
) is defined as
a ratio between the two overall heat transfer coefficients, or,
C
F
5
U
fo
U
co
ð4:94Þ
Note that the value of C
F
is less than 1. Substituting Equation (4.93)
in Equation (4.94), and rearranging terms we can write the fouling
resistance in terms of the cleaning factor.
R
ft
5
1
U
co
1
C
F
2 1
ð4:95Þ
In designing heat exchangers, we may want to determine the extra
area required due to expected fouling of the heat transfer area. For
this purpose, we consider the rates of heat transfer in the clean and
fouled surface to be the same. Thus,
q 5 U
co
A
co
ΔT
m
5 U
fo
A
fo
ΔT
m
ð4:96Þ
Then eliminating ΔT
m
in the preceding equation,
U
co
A
co
5 U
fo
A
fo
ð4:97Þ
or,
U
co
U
fo
5
A
fo
A
co
ð4:98Þ
Combining
Equations (4.94), (4.95), and (4.98), and rearranging
terms, we get
R
ft
5
1
U
co
A
fo
A
co
2 1
ð4:99Þ
3274.4 Steady-S tate Heat Transfer

In Equation (4.99) the term A
fo
=A
co
2 1
multiplied by 100 is the
percent extra area required for the fouled surface compared with the
clean surface. In the following example, we will use the previous
derived relations to develop a graph of percent increase in area
required to compensate for fouling for different values of overall
heat transfer coefficients.
Example 4.17
Using a spreadsheet, develop a chart that shows the relationship between
the total fouling resistance as a function of overall heat transfer coeffi-
cients of 1000 to 5000 W/m
2
K for cleanliness factors of 0.8, 0.85, 0.9, 0.95.
Also, develop a chart that demonstrates the increase in surface area
required for fouling resistances of 0.0001, 0.001, 0.01, and 0.05 m
2
K/W for
an overall heat transfer coefficient varying from 1 to 10,000 W/m
2
K.
Given
Part (a)
Overall heat transfer coefficient 5 1000, 2000, 3000, 4000 and 5000 W/m
2
K
Cleanliness factor 5 0.8, 0.85, 0.9, 0.95
Cleanliness Factor
Overall Heat
Transfer
Coefficient
0.80 0.85 0.90 0.95
1000 2.50 1.76 1.11 0.53
2000 1.25 0.88 0.56 0.26
3000 0.83 0.59 0.37 0.18
4000 0.63 0.44 0.28 0.13
5000
3
2
1
ABCDE
4
5
6
7 0.50 0.35 0.22 0.11
0.00
0.50
1.00
1.50
2.00
2.50
3.00
1000 2000 3000 4000 5000
Overall heat transfer coefficient (W/m
2
K)
Total fouling resistance,
(10
4
m
2
K/W)
0.90
0.80
0.85
0.95
1) Enter values as shown in cells A3:A7 and B2:E2
2) Enter(1/$A3)*(1/B$2-1)*10000 in cell B3 and
copy in cells B4:B7 and C3:E7
■ Figure E4.14 Total fouling resistance as a
function of overall heat transfer coefficient for
different cleanliness factors.
328 CHAPTER 4 Heat Transfer in Food Processing

Part (b)
Fouling resistance 5 0.0001, 0.001, 0.01 and 0.05 m
2
K/W
Overall heat transfer coefficient 5 1, 10, 100, 1000, 10,000 W/m
2
K
Approach
We will develop two spreadsheets using Excelt. First, we will calculate the
fouling resistance for Part (a) and the required increase in surface area for
Part (b). Then we will create a plot from the calculated data.
Solution
The first spreadsheet is developed using Equation (4.95) for the fouling resis-
tance R
f
as a function of the overall heat transfer coefficient for a clean pipe.
The second spreadsheet is developed using Equation (4.99), and we will plot
A
fo
A
co
2 1
3 100 against overall heat transfer coefficient on a log-log scale. As
we observe, from Figure E4.14, as the cleanliness factor decreases, the fouling
resistance increases for the same value of clean-pipe overall heat transfer
coefficient. This effect is more pronounced at low values of overall heat
transfer coefficient. Similarly, from Figure E4.15, for a small increase in the
1
10
100
1 10 100 1000 10000
Overall heat transfer coefficient (W/m
2
K)
Increase in surface area due
to fouling (%)
0.0001
0.001
0.01
R
f
0.05
1) Enter values as shown in cells A3:A7 and B2:E2
2) Enter $A3*B$2*100 in cell B3 and
paste in cells B4:B7 and C3:E7
Fouling resistance (m
2
K/W)
Overall Heat
Transfer
Coefficient
0.05
15
10 50
100 500
1000 5000
10000
0.0001
0.01
0.1
1
10
100
0.01
1
10
100
1000
10000
0.001
0.1
1
10
100
1000 50000
3
2
1
ABCDE
4
5
6
7
■ Figure E4.15 Increase in surface area due to
fouling.
3294.4 Steady-S tate Heat Transfer

resistance factor, for the same overall heat transfer coefficient, a much larger
surface area is required. These graphs show that the resistance due to fouling
has a substantial effect on heat transfer, and larger surface areas are required
with increasing fouling.
4.4.7 Design of a Tubular Heat Exchanger
In Section 4.1, we examined a variety of heat exchange equipment
used in the food process industry. Recall that a number of different
geometrical configurations are used in the design of heat exchange
equipment, such as tubular, plate, and scraped surface heat exchan-
gers. The primary objective in using a heat exchanger is to transfer
thermal energy from one fluid to another. In this section we will
develop calculations necessary to design a tubular heat exchanger.
One of the key objectives in calculations involving a heat exchanger
is to determine the required heat transfer area for a given application.
We will use the following assumptions:
1. Heat transfer is under steady-state conditions.
2. The overall heat-transfer coefficient is constant throughout the
length of pipe.
3. There is no axial conduction of heat in the metal pipe.
4. The heat exchanger is well insulated. The heat exchange is
between the two liquid streams flowing in the heat exchanger.
There is negligible heat loss to the surroundings.
Recall from Chapter 1 that change in heat energy in a fluid stream, if
its temperature changes from T
1
to T
2
, is expressed as:
q 5
_
mc
p
ðT
1
2 T
2
Þð4:100Þ
where
_
m is mass flow rate of a fluid (kg/s), c
p
is specific heat of a
fluid (kJ/[kg 8C]), and the temperature change of a fluid is from
some inlet temperature T
1
to an exit temperature T
2
.
Consider a tubular heat exchanger, as shown in
Figure 4.28. A hot
fluid, H, enters the heat exchanger at location (1) and it flows through
the inner pipe, exiting at location (2). Its temperature decreases from
T
H,inlet
to T
H,exit
. The second fluid, C, is a cold fluid that enters the
annular space between the outer and inner pipes of the tubular heat
exchanger at location (1) and exits at location (2). Its temperature
increases from T
C,inlet
to T
C,exit
. The outer pipe of the heat exchanger is
330 CHAPTER 4 Heat Transfer in Food Processing

covered with an insulation to prevent any heat exchange with the sur-
roundings. Because the heat transfer occurs only between fluids H and
C, the decrease in the heat energy of fluid H must equal the increase in
the energy of fluid C. Therefore, conducting an energy balance, the
rate of heat transfer between the fluids is:
q 5
_
m
H
c
pH
ðT
H,inlet
2 T
H,exit
Þ5
_
m
C
c
pC
ðT
C,exit
2 T
C,inlet
Þð4:101Þ
where c
pH
is the specific heat of the hot fluid (kJ/[kg 8C]), c
pC
is the
specific heat of the cold fluid (kJ/[kg 8C]),
_
m
H
is the mass flow
rate of the hot fluid (kg/s), and
_
m
C
is the mass flow rate of the cold
fluid (kg/s).
Equation (4.101) is useful if we are interested in determining the
inlet and exit temperatures of the two fluid streams. Furthermore, we
may use this equation to determine the mass flow rate of either fluid
stream, provided all other conditions are known. But, this equation
does not provide us with any information about the size of the heat
exchanger required for accomplishing a desired rate of heat transfer,
and we cannot use it to determine how much thermal resistance to
heat transfer exists between the two fluid streams. For those ques-
tions, we need to determine heat transfer perpendicular to the flow
of the fluid streams, as discussed in the following.
1
2
Fluid H Fluid H
Fluid C
Fluid C
Insulation
dT
H
dT
C
dq
Temperature
Length or Area
T
H,inlet
T
C,inlet
T
H,exit
T
C,exit
■ Figure 4.28 A concurrent flow heat
exchanger and temperature plots.
3314.4 Steady-S tate Heat Transfer

Consider a thin slice of the heat exchanger, as shown in Figure 4.28.
We want to determine the rate of heat transfer from fluid H to C, per-
pendicular to the direction of the fluid streams. For this thin slice of
the heat exchanger, the rate of heat transfer, dq, from fluid H to fluid
C may be expressed as:
dq 5 U ΔT dA ð4:102Þ
where ΔT is the temperature difference between fluid H and fluid
C. Note that this temperature difference, ΔT, varies from location
(1) to (2) of the heat exchanger. At the inlet of the fluid streams,
location (1), the temperature difference, ΔT, is T
H,inlet
T
C,inlet
and
on the exit side, location (2), it is T
H,exit
T
C,exit
(Fig. 4.28). To
solve Equation (4.102) we can substitute only one value of ΔT, or
its average value that represents the temperature gradient perpendic-
ular to the direction of the flow. Although it may be tempting to
take an arithmetic average of the two ΔT values from locations (1)
and (2), the arithmetic average value will be incorrect because, as
seen in Figure 4.28, the temperature plots are nonlinear. Therefore,
we will develop the following mathematical analysis to determine a
value of ΔT that will correctly identify the “average” temperature
difference between the fluids H and C as they flow through the
heat exchanger.
The temperature difference, ΔT, between the two fluids H and C is
ΔT 5 T
H
2 T
C
ð4:103Þ
where T
H
is the temperature of the hot stream and T
C
is that of the
cold stream. For a small differential ring element as shown in
Figure 4.28, using energy balance for the hot stream H we get
dq 52
_
m
H
c
pH
dT
H
ð4:104Þ
and, for cold stream C in the differential element,
dq 5
_
m
C
c
pC
dT
C
ð4:105Þ
In
Equation (4.104), dT
H
is a negative quantity; therefore, we added
a negative sign to obtain a positive value for dq. Solving for dT
H
and
dT
C
, we obtain
dT
H
52
dq
_
m
H
c
pH
ð4:106Þ
332 CHAPTER 4 Heat Transfer in Food Processing

and
dT
C
52
dq
_
m
C
c
pC
ð4:107Þ
Then, subtracting
Equation (4.107) from Equation (4.106),
dT
H
2 dT
C
5 dðT
H
2 T
C
Þ52dq
1
_
m
H
c
pH
1
1
_
m
C
c
pC
ð4:108Þ
Using
Equations (4.102) and (4.103), and substituting in Equation
(4.108),
dðT
H
2 T
C
Þ
ðT
H
2 T
C
Þ
52U
1
_
m
H
c
pH
1
1
_
m
C
c
pC
dA ð4:109Þ
Integrating
Equation (4.109) from locations (1) to (2) shown in
Figure 4.28,
ln
ðT
H,exit
2 T
C,exit
Þ
ðT
H,inlet
2 T
C,inlet
Þ
52UA
1
_
m
H
c
pH
1
1
_
m
C
c
pC
ð4:110Þ
Noting that
T
H,inlet
2 T
C,inlet
5 ΔT
1
T
H,exit
2 T
C,exit
5 ΔT
2
ð4:111Þ
we get
ln
ΔT
2
ΔT
1
52UA
1
_
m
H
c
pH
1
1
_
m
C
c
pC
ð4:112Þ
Substituting
Equation (4.101) in Equation (4.112),
ln
ΔT
2
ΔT
1
52UA
T
H,inlet
2 T
H,exit
q
1
T
C,exit
2 T
C,inlet
q
ð4:113Þ
Rearranging terms in
Equation (4.113),
ln
ΔT
2
ΔT
1
5
2 UA
q
ðT
H,inlet
2 T
C,inlet
Þ2 ðT
H,exit
2 T
C,exit
Þ
ð4:114Þ
3334.4 Steady-S tate Heat Transfer

Substituting Equation (4.111) in Equation (4.114), we obtain
ln
ΔT
2
ΔT
1
52
UA
q
ðΔT
1
2 ΔT
2
Þð4:115Þ
Rearranging terms,
q 5 UA
ΔT
2
2 ΔT
1
ln
ΔT
2
ΔT
1
ð4:116Þ
q 5 UAðΔT
lm
Þð4:117Þ
where
ΔT
lm
5
ΔT
2
2 ΔT
2
ln
ΔT
2
ΔT
1
ð4:118Þ
ΔT
lm
is called the log mean temperature difference (LMTD).
Equation (4.117) is used to design a heat exchanger and determine
its area and the overall resistance to heat transfer, as illustrated in
Examples 4.18 and 4.19.
Example 4.18
A liquid food (specific heat 5 4.0 kJ/[kg 8C]) flows in the inner pipe of a
double-pipe heat exchanger. The liquid food enters the heat exchanger at
208C and exits at 608C(
Fig. E4.16). The flow rate of the liquid food is
0.5 kg/s. In the annular section, hot water at 908C enters the heat
exchanger and flows countercurrently at a flow rate of 1 kg/s. The average
specific heat of water is 4.18 kJ/(kg 8C). Assume steady-state conditions.
1. Calculate the exit temperature of water.
2. Calculate log-mean temperature difference.
3. If the average overall heat transfer coefficient is 2000 W/(m
2
8C)
and the diameter of the inner pipe is 5 cm, calculate the length of
the heat exchanger.
4. Repeat these calculations for parallel-flow configuration.
Given
Liquid food:
Exit temperature 5 608C
Inlet temperature 5 208C
Length (m)
Temperature (°C)
90°C
60°C
20°C
■ Figure E4.16 A countercurrent flow heat
exchanger with unknown exit temperatures.
334 CHAPTER 4 Heat Transfer in Food Processing

Specific heat 5 4.0 kJ/(kg 8C)
Flow rate 5 0.5 kg/s
Water:
Inlet temperature 5 908C
Specific heat 5 4.18 kJ/(kg 8C)
Flow rate 5 1.0 kg/s
Heat exchanger:
Diameter of inner pipe 5 5cm
Flow 5 countercurrent
Approach
We will first calculate the exit temperature of hot water by using a simple
heat balance equation. Then we will compute log-mean temperature differ-
ence. The length of the heat exchanger will be determined from
Equation
(4.117). The solution will be repeated for parallel-flow configuration to obtain
a new value for log-mean temperature difference and length of the heat
exchanger.
Solution
1. Using a simple heat balance,
q 5
_
m
C
c
pC
ΔT
C
5
_
m
h
c
ph
ΔT
h
5 (0:5kg=s)(4kJ=[kg 8C])(608C 2 20 8C)
5 (1 kg=s)(4:18 kJ=[kg 8C])(908C 2 T
e
8C)
T
e
5 70:98C
2. The exit temperature of water is 70.98C.
3. From
Equation (4.118)
(ΔT)
lm
5
Δ(T)
1
2 Δ(T)
2
ln
Δ(T)
1
Δ(T)
2
2
4
3
5
5
(70:9 2 20) 2 (902 60)
ln
50:9
30
0
@
1
A
5 39:58C
4. The log-mean temperature difference is 39.58C.
5. From
Equation (4.117),
q 5 UA(ΔT)
lm
5 U π D
i
L(ΔT)
lm
where q, from step (1), is
q 5 (0:5kg=s)(4 kJ=[kg 8C])(608C 2 208C) 5 80 kJ=s
3354.4 Steady-S tate Heat Transfer

Thus,
L 5
(80 kJ=s)(1000 J=kJ)
(π)(0:05 m)(39:58C)(2000 W=[m
2
8C])
5 6:45 m
6. The length of the heat exchanger, when operated counter-currently, is
6.5 m.
7. For parallel-flow operation, the system diagram will be as shown in
Figure E4.16 .
8. Assuming that for parallel flow the exit temperature will be the same
as for counterflow, T
e
5 70.98C.
9. Log-mean temperature difference is calculated from
Equation (4.118).
(ΔT)
lm
5
(90 2 20) 2 (70:9 2 60)
ln
90 2 20
70:9 2 60
5 31:88C
10. The log-mean temperature difference for parallel flow is 31.88C, about
88C less than that for the countercurrent flow arrangement.
11. The length can be computed as in step (5).
L 5
(80 kJ=s)(1000 J=kJ)
(π)(0:05 m)(31:88C)(2000 W=[m
2
8C])
5 8m
12. The length of the heat exchanger, when operated as parallel flow, is
8 m. This length of heat exchanger is longer by 1.55 m to obtain the
same exit temperature of hot-water stream as for the counterflow
arrangement.
Example 4.19
Steam with 90% quality, at a pressure of 143.27 kPa, is condensing in the
outer annular space of a 5 m-long double-pipe heat exchanger (
Fig. E.17
and E4.18). Liquid food is flowing at a rate of 0.5 kg/s in the inner pipe.
The inner pipe has an inside diameter of 5 cm. The specific heat of the liq-
uid food is 3.9 kJ/(kg 8C). The inlet temperature of the liquid food is 408C
and the exit temperature is 808C.
a. Calculate the average overall heat-transfer coefficient.
b. If the resistance to conductive heat transfer caused by the inner steel
pipe is negligible, and the convective heat-transfer coefficient on the
steam side is very large (approaches infinity), estimate the convective
heat-transfer coefficient for the liquid food in the inside pipe.
Length (m)
Temperature (°C)
90°C
60°C
20°C
70.9°C
■ Figure E4.17 Temperature plots for a
counterflow heat exchanger.
336 CHAPTER 4 Heat Transfer in Food Processing

Given
Steam pressure 5 143.27 kPa
Length 5 5m
Flow rate of liquid 5 0.5 kg/s
Inside diameter 5 0.05 m
Specific heat 5 3.9 kJ/(kg 8C)
Product inlet temperature 5 408C
Product exit temperature 5 808C
Approach
We will obtain steam temperature from Table A.4.2. We also note that steam
quality has no effect on the steam condensation temperature. We will calcu-
late the heat required to raise the liquid food temperature from 40 to 808C.
Next, we will calculate log-mean temperature difference. Then we will obtain
the overall heat-transfer coefficient by equating the heat gain of the liquid
food and heat transfer across the pipe wall, from steam to the liquid food.
Solution
Part (a)
1. From steam table (Table A.4.2), steam temperature 5 1108C.
2.
q 5
_
mc
p
ΔT
5 (0:5kg=s)(3:9kJ=[kg 8C])(1000 J=kJ)(808C 2 408C)
5 78, 000 J=s
3.
q 5 UA(ΔT)
lm
5
_
mc
p
ΔT
(ΔT)
lm
5
(110 2 40) 2 (110 2 80)
ln
110 2 40
110 2 80
5 47:28C
and
A 5 π(0:05)(5) 5 0:785 m
2
4.
U 5
_
mc
p
ΔT
A(ΔT)
lm
5
(78,800 J=s)
(0:785 m
2
)(47:28C)
5 2105 W=(m
2
8C)
5. Overall heat-transfer coefficient 5 2105 W/(m
2
8C)
Length (m)
Temperature (°C)
110°C
80°C
40°C
110°C
■ Figure E4.18 Temperature plots for a
parallel-flow heat exchanger.
3374.4 Steady-S tate Heat Transfer

Part (b)
The overall heat-transfer equation may be written as follows:
1
U
i
A
i
5
1
h
i
A
i
1
ln
r
o
r
i
2π kL
1
1
h
o
A
o
The second term on the right-hand side of the above equation is zero, since
the resistance offered by steel to conductive heat transfer is considered negli-
gible. Likewise, the third term is zero, because the convective heat-transfer
coefficient on the steam side is very large.
Therefore,
U
i
5 h
i
or
h
i
5 2105 W=(m
2
8C)
4.4.8 The Effectiveness- NTU Method for Designing
Heat Exchangers
In the preceding section, we used the log-mean-temperature-
difference (LMTD) approach to design a heat exchanger. The LMTD
approach works well when we are designing a new heat exchanger,
where the temperatures of the fluid streams at the inlet and exit are
known and we are interested in determining the size of the heat
exchanger (in terms of the heat transfer area, length, and diameter of
the pipe). However, in other situations when the size of the heat
exchanger, and the inlet temperatures of the product and heating/
cooling streams are known but the exit temperatures of the two
streams are unknown, the LMTD approach can be used but the solu-
tion requires iterative procedures and becomes tedious. For this pur-
pose, another calculation technique called the effectiveness-NTU
method is easier to use. This method involves three dimensionless
quantities, namely the heat capacity rate ratio, heat exchanger effec-
tiveness, and number of transfer units (NTU).
4.4.8.1 Heat Capacity Rate Ratio, C*
A heat capacity rate of a liquid stream is obtained as a product of the
mass flow rate and specific heat capacity. Thus, for the hot and cold
338 CHAPTER 4 Heat Transfer in Food Processing

streams the heat capacity rates are, respectively:
C
H
5
_
m
H
c
PH
ð4:119Þ
C
C
5
_
m
C
c
PC
ð4:120Þ
These two quantities are evaluated using the given data in a problem;
the smaller of the two quantities is called C
min
and the larger C
max
.
The heat capacity rate ratio, C*, is defined as
C
5
C
min
C
max
ð4:121Þ
4.4.8.2 Heat Exchanger Effectiveness, ε
E
The heat exchanger effectiveness is a ratio of the actual rate of heat
transfer accomplished and the maximum attainable rate of heat
transfer for a given heat exchanger. The heat exchanger effectiveness,
ε
E
, is defined as
ε
E
5
q
actual
q
max
ð4:122Þ
The actual rate of heat transfer can be determined for both hot and
cold streams as
q
actual
5 C
H
ðT
H,inlet
2 T
H,exit
Þ5 C
C
ðT
C,exit
2 T
C,inlet
Þð4:123Þ
And, the maximum rate of attainable heat transfer is obtained by
observing that in any heat exchanger the maximum possible temper-
ature difference is between the temperatures of hot and cold streams
at the inlet. This temperature difference is multiplied with the mini-
mum heat capacity rate, C
min
, to obtain q
max
:
q
max
5 C
min
ðT
H,inlet
2 T
C,inlet
Þð4:124Þ
Thus, from
Equation (4.122),
q
actual
5 ε
E
q
max
5 ε
E
C
min
ðT
H,inlet
2 T
C,inlet
Þð4:125Þ
3394.4 Steady-S tate Heat Transfer

4.4.8.3 Number of Transfer Units, NTU
The number of transfer units provides a measure of the heat transfer
surface area for a given overall heat transfer coefficient and minimum
heat capacity rate. It is expressed as
NTU 5
UA
C
min
ð4:126Þ
where A is the heat transfer area (m
2
), U is the overall heat transfer
coefficient (W/m
2
8C) based on the selected area (see Section 4.4.5),
and C
min
is the minimum heat capacity rate (W/8C).
Relationships between NTU and effectiveness can be obtained for dif-
ferent types of heat exchangers with prescribed flow conditions (such
as counterflow or concurrent flow). These relationships include the
heat capacity rate ratios. Some of these relationships for commonly
used heat exchangers are shown in
Tables 4.4 and 4.5.InTable 4.4,
the exchanger effectiveness is given in terms of NTU, and in
Table 4.5, the NTU values are given as a function of the exchanger
effectiveness. The steps involved in using the effectiveness-NTU
method are illustrated in the following example.
Table 4.4 Effectiveness-NTU Relations for Heat Exchangers
Type of heat
exchanger Effectiveness relation
Double pipe
Concurrent flow
ε
E
5
1 2 exp½2NTUð1 1 C
Þ
1 1 C
Double pipe
Countercurrent flow
ε
E
5
1 2 exp½2NTUð1 1 C
Þ
1 2 C
exp½2NTUð1 2 C
Þ
Shell and tube:
One-shell pass
2, 4, 6... tube passes
ε
E
5
2
1 1 C
1
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
1 1 C
2
p
1 1 exp 2NTU
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
1 1 C
2
p
hi
1 2 exp 2NTU
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
1 1 C
2
p
hi
Plate heat exchanger
ε
E
5
exp½ð1 2 C
Þ3 NTU 2 1
exp½ð1 2 C
Þ3 NTU 2 C
All heat exchangers,
C* 5 0
ε
E
5 1 2 expð2NTUÞ
340 CHAPTER 4 Heat Transfer in Food Processing

Example 4.20
We will use some of the data from Example 4.18 to show the use of the
effectiveness-NTU method in solving problems when both exit tempera-
tures are unknown. A liquid food (specific heat 5 4.0 kJ/[kg 8C]) flows in
the inner pipe of a double-pipe heat exchanger. The liquid food enters the
heat exchanger at 208C. The flow rate of the liquid food is 0.5 kg/s. In the
annular section, hot water at 908C enters the heat exchanger and flows in
countercurrent direction at a flow rate of 1 kg/s. The average specific heat
of water is 4.18 kJ/(kg 8C). The average overall heat transfer coefficient
based on the inside area is 2000 W/(m
2
8C), and the diameter of the inner
pipe is 5 cm and length is 6.45 m. Assume steady state conditions.
Calculate the exit temperature of liquid food and water.
Given
Liquid food:
Inlet temperature 5 208C
Specific heat 5 4.0 kJ/(kg 8C)
Flow rate 5 0.5 kg/s
Table 4.5 NTU-Effectiveness Relations for Heat Exchangers
Type of heat exchanger NTU relation
Double pipe
Concurrent flow
NTU 52
ln½1 2 ε
E
ð1 1 C
Þ
1 1 C
Double pipe
Countercurrent flow
NTU 5
1
1 2 C
ln
1 2 C
ε
E
1 2 ε
E
2
4
3
5
ðC
,1Þ
NTU 5
ε
E
1 2 ε
E
ðC
5 1Þ
Shell and tube:
One-shell pass
2, 4, 6... tube passes
NTU 5
1
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
1 1 C
2
p
ln
2 2 ε
E
1 1 C
2
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
1 1 C
2
p
hi
2 2 ε
E
1 1 C
1
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
1 1 C
2
p
hi
Plate heat exchanger
NTU 5
ln
ð1 2 C
Þ
ð1 2 ε
E
Þ
ð1 2 C
Þ
All heat exchangers, C* 5 0 NTU 52lnð1 2 ε
E
Þ
3414.4 Steady-S tate Heat Transfer

Water:
Inlet temperature 5 908C
Specific heat 5 4.18 kJ/(kg 8C)
Flow rate 5 1.0 kg/s
Heat exchanger:
Diameter of inner pipe 5 5cm
Length of inner pipe 5 6.45 m
Overall heat transfer coefficient 5 2000 W/m
2
8C
Flow 5 countercurrent
Approach
Since exit temperatures of both streams are unknown (Fig. E4.19), we will use
the effectiveness-NTU method. We will first calculate the maximum and mini-
mum heat capacity rates. The two heat capacity rates will be used to calcu-
late the heat capacity rate ratio, C*. Next, we will obtain NTU from the given
overall heat transfer coefficient, heat exchanger area, and the calculated
value of C
min
. Using the calculated value of NTU, we will use an appropriate
table to determine the exchanger effectiveness. The definition of the
exchanger effectiveness will be used to determine q
actual
, and the unknown
temperatures, T
H,exit
and T
L,exit
.
Solution
1. The heat capacity rates for the hot water and liquid food are,
respectively,
C
H
5
_
m
H
c
pH
5 (1:0kg=s)ð4:18 kJ=[kg 8C])
5 4:18 kW=8C
C
L
5
_
m
L
c
pL
5 (0:5kg=s)ð4kJ=[kg 8C])
5 2kW=8C
Therefore, using the smaller value of C between the above calculated
values C
H
and C
L
,
C
min
5 2kW=8C
Then, C* is obtained as
C
5
2
4:18
5 0:4785
Length (m)
Temperature (°C)
90°C
20°C
■ Figure E4.19 Temperature plots for a
double-pipe heat exchanger.
342 CHAPTER 4 Heat Transfer in Food Processing

2. The NTU value is obtained from Equation (4.126) as
NTU 5
UA
C
min
5
(2000 W=m
2
8C)(π)(0:05 m)(6:45m)
(2 kW=8C)(1000 W=kW)
NTU 5 1:0132
3. From
Table 4.4 we select an expression for ε
E
for tubular heat
exchanger, for counterflow, and substitute known terms as
ε
E
5
1 2 e
(21:0132(120:4785))
1 2 0:4785e
(21:0132(1204785))
ε
E
5 0:5717
According to
Equation (4.124)
q
max
5 (2kW=8C)(90 2 20)(8C) 5 140 kW
Since ε
E
5
q
actual
q
max
q
actual
5 0:5717 3 140(kW) 5 80:038 kW
For hot water stream:
q
actual
5 4:18(kW=8C) 3 (90 2 T
H,exit
)(8C) 5 80:038 kW
T
H,exit
5 90 2 19:15 5 70:858C
Similarly, for liquid food stream:
q
actual
5 2(kW=8C) 3 (T
L,exit
2 20)(8C) 5 80 :038 kW
T
L,exit
5 40:019 1 20 5 608C
The calculated exit temperatures of hot water and product streams are
70.858C and 608C, respectively. These values are comparable to those
given in Example 4.18.
4.4.9 Design of a Plate Heat Exchanger
As discussed in Section 4.1.1, plate heat exchangers are commonly
used in the food industry. The design of a plate heat exchanger
requires certain relationships that are often unique to the plates used
in the heat exchanger. Some of the required information for design
3434.4 Steady-S tate Heat Transfer
purposes is closely guarded by the equipment manufacturers and is
not readily available. We will consider a general design approach
with some of the key relationships required in designing plate heat
exchangers. First, it is important to understand the type of flow pat-
tern present inside a plate heat exchanger.
Figure 4.2 shows an arrangement of plates with two liquid streams,
product and heating/cooling medium, entering and exiting through
their respective ports. The assembly of a plate heat exchanger
involves placing the required number of plates in a frame and tight-
ening the end bolts so that a fixed gap space is created between the
plates. The gaps between the plates create channels through which
the fluid streams flow. The fluid streams enter and exit through the
port holes (Fig. 4.2b).
Each plate contains a gasket that helps orient the direction of the
flow stream through the channel. The assembly of plates with gaskets
is done in such a manner that it allows the product stream to move
in one channel while the heating/cooling stream moves in the neigh-
boring channel. Thus, the flow of product and heating/cooling
streams alternates through the channels and they never come into
direct contact (
Fig. 4.2).
The heat exchange between the two liquid streams in a plate heat
exchanger is across the plates, normal to the direction of the flow.
Therefore, the plates are made as thin as possible to minimize resis-
tance to heat transfer while maintaining the physical integrity of the
plates. The plates are corrugated with a pattern that promotes turbu-
lence in the liquid stream. Several types of patterns are used for the
corrugations stamped on the plates, the most common one being a
herringbone type design called the Chevron design (
Fig. 4.3). The
plates used in food processing applications are made of stainless steel
(ANSI 316), although the thermal conductivity of stainless steel is
not as high as other metals that are used in nonfood applications. In
the past, the material used for gaskets did not permit use of high
temperatures. However, new heat-resistant materials for gaskets have
considerably extended the use of plate heat exchangers for high-
temperature applications.
To determine the rate of heat transfer across the plates, it is necessary
to know the convective heat transfer coefficient on both sides of the
plates. Since the mechanism is forced convection, we use a dimen-
sionless correlation involving Nusselt number, Reynolds number,
and Prandtl number. The dimensionless correlation depends upon
344 CHAPTER 4 Heat Transfer in Food Processing

the design of the corrugations used. An approximate expression
suitable for plate heat exchanger is as follows:
N
Nu
5 0:4N
0:64
Re
N
0:4
Pr
ð4:127Þ
To evaluate the Reynolds number, it is necessary to determine the
velocity of the liquid stream in the channel. This determination is
complex because of the corrugations. We will consider a simplified
method to estimate the fluid velocity.
In a plate heat exchanger, the two end plates do not take part in heat
transfer. Thus, the number of “thermal plates” involved in heat trans-
fer is obtained by subtracting two from the total number of plates in
a heat exchanger.
The flow rate of a liquid stream through each channel is obtained as
_
m
Hc
5
_
m
H
N 1 1
2
ð4:128Þ
and
_
m
Pc
5
2
_
m
P
N 1 1
ð4:129Þ
where
_
m
H
and
_
m
P
are the total mass flow rates of the heating/cooling
stream and the product stream (kg/s), respectively;
_
m
Hc
and
_
m
Pc
are
the channel flow rates for heating/cooling stream and product stream
(kg/s), respectively; and N is the total number of thermal plates.
The cross-sectional area of a channel between two adjacent plates is
obtained as,
A
c
5 bw ð4:130Þ
where b is the gap between two adjacent plates and w is the width of
the plate.
The velocities of the two streams are
u
Pc
5
_
m
Pc
ρ
P
A
c
ð4:131Þ
u
Hc
5
_
m
Hc
ρ
H
A
c
ð4:132Þ
3454.4 Steady-S tate Heat Transfer

The equivalent diameter (or the hydraulic diameter), D
e
, is calculated
from the following expression:
D
e
5
4 3 channel free-flow area for fluid stream
wetted perimeter for the fluid
ð4:133Þ
For simplicity, we use the projected area (disregarding the corruga-
tions) to determine the wetted perimeter as
wetted perimeter for the fluid 5 2ðb 1 wÞð4:134Þ
Channel free-flow area 5 bw ð4:135Þ
Then,
D
e
5
4bw
2ðb 1 wÞ
ð4:136Þ
Since in a plate heat exchanger, b{w, we may neglect b in the
denominator to obtain,
D
e
5 2 3 b ð4:137Þ
The Reynolds number for each stream is obtained as
Product stream:
N
Re,P
5
ρ
P
u
Pc
D
e
μ
P
ð4:138Þ
Heating/cooling stream:
N
Re,H
5
ρ
H
u
Hc
D
e
μ
H
ð4:139Þ
Knowing the Reynolds number, the Nusselt number is calculated for
each stream using
Equation (4.127). The heat transfer coefficient is
obtained as
h
P
5
N
Nu
3 k
P
D
e
ð4:140Þ
h
H
5
N
Nu
3 k
H
D
e
ð4:141Þ
346 CHAPTER 4 Heat Transfer in Food Processing

where k
P
and k
H
are the thermal conductivities of the product and
heating/cooling streams, (W/m 8C), respectively.
The overall heat transfer coefficient is determined from the two
values of the convective heat transfer coefficients, assuming that the
conductive resistance of the thin metal plate is negligible,
1
U
5
1
h
P
1
1
h
H
ð4:142Þ
Once the overall heat transfer coefficient is obtained using
Equation
(4.142), the remaining computations for the design of the plate heat
exchanger are done using the effectiveness-NTU method similar to
the procedure described in Section 4.4.7.2. For a plate heat
exchanger, the relevant expressions between NTU and effectiveness
are given in Tables 4.4 and 4.5. The calculation procedure for design-
ing a plate heat exchanger is illustrated in the following example.
Example 4.21
A c ounterflow plate h eat exc hanger is b eing us ed to heat apple juice
with hot water. The heat exchanger contains 51 plates. Each plate is
1.2 m high and 0.8 m wide. The gap between the plates is 4 mm. The
heating characteristics for this heat ex changer have been pre viously
determined to follow the following relationship, N
Nu
5 0:4N
0:64
Re
N
0:4
Pr
.Hot
water enters the heat exchanger at 958C at a rate of 15 kg/s and apple
juice enters at 158C at a rate of 10 kg/s. It is assumed that the physical
properties of water and apple juice are the sa me. The properties of water
at 558C are density 5 985.7 kg/m
3
, specific heat 5 4179 J/kgK, thermal
cond uctivity 5 0.652 W/m 8C, dynamic viscosity 5 509.946 3 10
26
N
s
/m
2
,
Prandtl Number 5 3.27. Calculate the exit temperatures of water and
apple juice.
Given
Number of plates 5 51
Plate height 5 1.2 m
Plate width 5 0.8 m
Gap between plates 5 4mm5 0.004 m
Inlet temperature of hot water 5 958C
Mass flow rate of hot water 5 15 kg/s
Inlet temperature of apple juice 5 158C
Mass flow rate of apple juice 5 10 kg/s
Properties of water at (95 1 15)/2 5 558C from Table A.4.1
Density 5 985.7 kg/m
3
Specific heat 5 4.179 kJ/kgK
3474.4 Steady-S tate Heat Transfer

Thermal conductivity 5 0.652 W/m 8C
Viscosity 5 509.946 3 10
26
Ns/m
2
Prandtl number 5 3.27
Approach
We will assume that the heat exchanger is operating under steady state condi-
tions. First, we will determine the channel velocity of each fluid stream using
the given flow rates and gap dimensions. Next, we will determine the Reynolds
number, and using the dimensionless correlation, we will calculate Nusselt
number and convective heat transfer coefficient. The convective heat transfer
coefficients from both sides of a plate will be used to determine the overall heat
transfer coefficient. Once the overall heat transfer coefficient is known, then we
will use the effectiveness-NTU method to determine the exit temperatures of
each stream.
Solution
Heat transfer coefficient on the hot water side:
1. Equivalent diameter for the channel created between the plates is
D
e
5 2 3 b 5 2 3 0:004 5 0:008 m
2. Flow rate of hot water in each channel:
Total number of plates 5 51. Since the two end plates do not take
part in heat exchange, the number of thermal plates 5 49. Then
_
m
Hc
5
2 3 15
(491 1)
5 0:60 kg=s
3. The cross-sectional area of each channel is
A
c
5 0:004 3 0:8 5 0:0032 m
2
4. Velocity of hot water in each channel is
u
c
5
0:60 (kg=s)
985:7(kg=m
3
) 3 0:0032 (m
2
)
5 0:19 m=s
5. Reynolds number:
N
Re
5
985:7 3 0:19 3 0:008
509:946 3 10
26
5 2941
6. Nusselt number:
N
Nu
5 0:4 3 2941
0:64
3 3:27
0:4
5 106:6
348 CHAPTER 4 Heat Transfer in Food Processing

Then, heat transfer coefficient on the hot water side is
h
H
5
106:6 3 0:652
0:008
5 8,688 W=m
2
8C
Heat transfer coefficient on the apple juice side:
7. The flow rate of apple juice in each channel is
_
m
A
5
2 3 10
(49 1 1)
5 0:4kg=s
8. The velocity of apple juice in each channel is
u
c
5
0:4 ðkg=s)
985:7(kg=m
3
) 3 0:0032 (m
2
)
5 0:127 m=s
9. The Reynolds number is
N
Re
5
985:7 3 0:127 3 0:008
509:946 3 10
26
5 1961
10. The Nusselt number is
N
Nu
5 0:4 3 1961
0:64
3 3:27
0:4
5 82:2
11. Convective heat transfer coefficient on the apple juice side is
h
H
5
82:2 3 0: 652
0:008
5 6702 W=m
2
8C
12. The overall heat transfer coefficient, U, is
1
U
5
1
8688
1
1
6702
U 5 3784 W=m
2
8C
13. The total area of the heat exchanger is
A
h
5 49 3 1:2 3 0:8 5 47:04 m
2
14. NTU for the heat exchanger is
NTU 5
3784 3 47:04
10 3 4179
5 4:259
15. The capacity rate ratio is
C
5
10
15
5 0:67
3494.4 Steady-S tate Heat Transfer

16. The exchanger effectiveness is obtained from the relationship given in
Table 4.4 as
ε
E
5
exp[(1 2 0:67) 3 4:259] 2 1
exp[(1 2 0:67) 3 4:259] 2 0:67
ε
E
5 0:9
17. The exit temperature of apple juice is determined by noting that
C
juice
5 C
min
, or
q
actual
q
max
5 0:9 5
C
juice
(T
ae
2 15)
C
min
(952 15)
5
(T
ae
2 15)
(95 2 15)
Then, T
ae
5 878C
18. The exit temperature of hot water is determined as
0:9 5
C
water
(95 2 T
we
)
C
min
(95 2 15)
5 1:5
(95 2 T
we
)
(95 2 15)
or, T
we
5 478C
In the given counter flow plate heat exchanger operating under stea-
dy state conditions, the hot water stream will exit at 478C and the
apple juice will be heated to 878C.
4.4.10 Importance of Surface Characteristics in
Radiative Heat Transfer
All materials in the universe emit radiation of an electromagnetic
nature based on their surface temperature. At a temperature of 0K,
the emission of radiation ceases. The characteristics of the radiation
are also dependent on temperature. As temperature increases, the
wavelength decreases. For example, radiation emitted by the sun is
shortwave compared with the longwave radiation emitted by the sur-
face of a hot coffee mug.
When radiation of a given wavelength, as shown in
Figure 4.29, is
incident on an object, some of the incident radiation is reflected,
some transmitted, and some absorbed. The following expression
holds true:
φ 1 χ 1 ψ 5 1 ð4:143Þ
where φ is absorptivity, χ is reflectivity, and ψ is transmissivity. The
absorbed radiation will result in an increase of temperature.
Reflected
Absorbed
Transmitted
Emitted
Incident
■ Figure 4.29 Radiant energy incident on a
semi-opaque object.
350 CHAPTER 4 Heat Transfer in Food Processing

To compare the absorption of radiation for different materials , an
ideal reference called a blackbody is used. For a blackbody, the
absorptivity value is 1.0. Note that nothing in the universe is a true
blackbody; even lampblack has φ 5 0.99 and χ 5 0.01. Regardless,
blackbody is a useful concept for comparing radiative properties of
different materials.
The absolute magnitudes of φ, χ, and ψ depend on the nature of the
incident radiation. Thus, a brick wall of a house is opaque to visible
light but transparent to radio waves.
Energy emitted (also called radiated) and energy reflected must be
clearly distinguished. These are quite different terms. A material,
depending on its surface absorptivity value, will reflect some of the
incident radiation. In addition, based on its own temperature, it will
emit radiation, as shown in
Figure 4.29. The amount of radiation
emitted can be computed from Equation (4.20).
Kirchoff’s law states that the emissivity of a body is equal to its
absorptivity for the same wavelength. Thus, mathematically,
ε 5 φ ð4:144Þ
This identity is discussed in Example 4.22.
Example 4.22
Compare the selection of white paint versus black paint for painting the
rooftop of a warehouse. The objective is to allow minimum heat gain from
the sun during summer.
Given
White paint from Table A.3.3:
ε
shortwave
5 0.18
ε
longwave
5 0.95
Black paint from Table A.3.3:
ε
shortwave
5 0.97
ε
longwave
5 0.96
Approach
From the emissivity values, we will examine the use of white or black paint
for both short- and longwave radiation.
3514.4 Steady-S tate Heat Transfer

Solution
1. White paint: ε
shortwave
5 0.18, therefore from Kirchoff’s law
φ
shortwave
5 0.18, so χ
shortwave
5 1 2 0.18, assuming ψ 5 0, thus
χ
shortwave
5 0.82. Thus, of the total shortwave radiation incident on the
rooftop, 18% is absorbed and 82% reflected.
2. White paint, ε
longwave
5 0.95. The white-painted surface , in terms of
longwave radiation, emits 95% of radiation emitted by a blackbody.
3. Black paint: ε
shortwave
5 0.97, therefore using Kirchoff’sLaw
φ
shortwave
5 0.97. Thus, of the total shortwave radiation incident on the
rooftop, 97% of radiation incident on it is absorbed and 3% is reflected.
4. Black paint: ε
longwave
5 0.96. The black-painted surface, in terms of
longwave radiation, emits 96% of radiation emitted by a blackbody.
5. White paint should be selected, as it absorbs only 18% shortwave (solar)
radiation compared to 97% by black paint. Both black and white paint
are similar in emitting longwave radiation to the surroundings.
4.4.11 Radiative Heat Transfer between Two Objects
The transfer of heat by radiation between two surfaces is dependent
on the emissivity of the radiating surface and the absorptivity of that
same surface. The expression normally used to describe this type of
heat transfer is as follows:
q
122
5 Aσðε
1
T
4
A1
2 φ
122
T
4
A2
Þð4:145Þ
where ε
1
is the emissivity of the radiating surface at temperature T
A1
,
and φ
12
is the absorptivity of the surface for radiation emitted at
temperature T
A2
.
Although the basic expression describing radiative heat transfer is given
by
Equations (4.20) and (4.145), one of the important factors requiring
attention is the shape of the object. The shape factor accounts for the
fraction of the radiation emitted by the high-temperature surface that is
not absorbed by the low-temperature surface. For example, Equation
(4.145) assumes that all radiation emitted at temperature T
A1
is
absorbed by the surface at temperature T
A2
. If both the surfaces are
blackbodies, then the expression describing heat transfer and incorpo-
rating the shape factor would be as follows:
q
122
5 σF
122
A
1
ðT
4
A1
2 T
4
A2
Þð4:146Þ
where F
12
is the shape factor, and it physically represents the frac-
tion of the total radiation leaving the surface A
1
that is intercepted by
352 CHAPTER 4 Heat Transfer in Food Processing

the surface A
2
. The values of shape factors have been tabulated and
presented in the form of curves of the type shown in
Figures 4.30
and 4.31. In the first case, the shape factors deal only with adjacent
rectangles, which are in perpendicular planes. Figure 4.31 can be uti-
lized for various shapes, including disks, squares, and rectangles.
Equation (4.146) does not account for nonblackbodies, and
Equation (4.20) does not account for the shape factor; therefore, an
expression that combines the two must be used. Such an expression
would be
q
122
5 σA
1
ξ
122
ðT
4
A1
2 T
4
A2
Þð4:147Þ
0.50
0.45
0.40
0.35
0.30
0.25
0.20
Factor (F
1–2
)
0.15
0.10
0.05
0
0 0.5 1.0 1.5 2.0 2.5 3.0
8.0
4.0
3.0
2.0
1.6
1.4
1.2
0.9
0.7
0.5
0.3
0.2
z
y
A
2
A
1
Y 6.0
Dimension ratio (Z )
Scale changes here
Asymp-
totes
3.5 4.0 5 6 7 8 9 10
Y 1.0
x
2.5
Y 0.1
0.4
■ Figure 4.30 Shape factors for adjacent rectangles in perpendicular planes. Y (dimension ratio) 5 y/x; Z 5 z/x. (Adapted from Hottel, 1930)
3534.4 Steady-S tate Heat Transfer

where the ξ
12
factor accounts for both shape and emissivity. This
factor can be evaluated by the following expression:
ξ
122
5
1
1
F
122
1
1
ε
1
2 1
1
A
1
A
2
1
ε
2
2 1
ð4:148Þ
Equations (4.147) and (4.148) can be used to compute the net radi-
ant heat transfer between two gray bodies in the presence of radiating
surfaces at uniform temperatures.
Example 4.23
Compute the radiative heat transfer received by a rectangular product mov-
ing through a radiation-type heater (
Fig. E4.20). The radiation source is one
vertical wall of a heater and is held at a constant temperature of 2008C while
the product is moving perpendicular to the radiation source. The product
temperature is 808C with an emissivity of 0.8. The product dimensions are
15 3 20 cm, and the radiation source is 1 3 5m.
Given
Temperature of heater 5 2008C
Product temperature 5 808C
Emissivity of product 5 0.8
Product dimensions 5 0.15 m 3 0.2 m
Heater dimensions 5 1m3 5m
1.0
3
2
1
0.8
0.6
Factor (F
1–2
)
0.4
0.2
0
01234
Ratio
Side or diameter
Distance between planes
567
⎞
⎠
⎛
⎝
■ Figure 4.31 Shape factors for equal and
parallel squares, rectangles, and disks: (1) direct
radiation between disks; (2) direct radiation
between squares; (3) total radiation between
squares or disks connected by nonconducting but
reradiating walls. (Adapted from
Hottel, 1930)
354 CHAPTER 4 Heat Transfer in Food Processing

Approach
To compute the ξ
12
factor from Equation (4.148), we will use Figure 4.30, for
objects perpendicular to each other, for F
12
value. Then we will use Equation
(4.147) to calculate radiative heat received by the rectangular product.
Solution
1. To use Equation (4.147), the ξ factor must be computed from Equation
(4.148).
ξ
122
5
1
1
F
122
1
1
1
2 1
1
5
0:03
1
0:8
2 1
where F
12
must be obtained from Figure 4.30 for z/x 5 5.0 and
y/x 5 0.75.
F
122
5 0:28
Then
ξ
122
5
1
(3:57 1 0 1 41:67)
5
1
45:24
5 0:0221
2. From
Equation (4.147),
q
122
5 (5:669 3 10
28
W=[m
2
K
4
])(0:0221)(5 m
2
)
3 [(473 K)
4
2 (353 K)
4
]
5 216 W
4.5 UNSTEADY-STATE HEAT TRANSFER
Unsteady-state (or transient) heat transfer is that phase of the heating
and cooling process when the temperature changes as a function of
both location and time. By contrast, in steady-state heat transfer, tem-
perature varies only with location. In the initial unsteady-state period,
many important reactions in the food may take place. With thermal
processes, the unsteady-state phase may even dominate the entire pro-
cess; for example, in several pasteurization and food-sterilization pro-
cesses, the unsteady-state period is an important component of the
process. Analysis of temperature variations with time during the
unsteady-state period is essential in designing such a process.
Since temperature is a function of two independent variables, time
and location, the following partial differential equation is the govern-
ing equation for a one-dimensional case:
@T
@t
5
k
ρc
p
r
n
@
@r
r
n
@T
@r
ð4:149Þ
Heater
Product
5 m
1 m
15 cm
20 cm
■ Figure E4.20 Radiation heater.
3554.5 Unsteady-State Heat Transfer

where T is temperature (8C), t is time (s), and r is distance from cen-
ter location (m). We can make this equation specific for a particular
geometrical shape using n 5 0 for a slab, n 5 1 for a cylinder, and
n 5 2 for a sphere. The combination of properties k/ρc
p
is defined as
thermal diffusivity, α. If the rate of heat transfer at the surface of the
object is due to convection, then
k
@T
@r
r5R
5 hðT
a
2 T
s
Þ
ð4:150Þ
where h is the convective heat transfer coefficient (W/[m
2
8C]), T
a
is
the temperature of heating or cooling medium far away from the sur-
face (8C), and T
s
is the temperature at the surface (8C).
The procedure we use to solve the governing equation,
Equation
(4.149), involves the use of advanced mathematics, which is beyond
the scope of this book. Myers (1971) gives the complete derivation
for various types of boundary-value problems encountered in
unsteady-state heat transfer. Due to mathematical complexity, the
analytical solution of Equation (4.149) is possible only for objects of
simplified geometrical shapes, such as a sphere, an infinite cylinder,
or an infinite slab.
An infinite cylinder is a “long” cylinder of radius r, a sphere is of
radius r, an infinite plate is a “large” plane wall of thickness 2z. These
three objects are geometrically and thermally symmetric about their
centerline (for a cylinder), center plane (for a slab), or center point
(for a sphere), as shown in
Figure 4.32.
Consider heating an infinitely long cylindrical object. Initially, the
object is assumed to have a uniform temperature, T
i
. At time t 5 0,
we place this object in a heating medium maintained at a constant
temperature T
a
. A constant heat transfer coefficient, h, describes the
convective heat transfer at the surface of the object. Temperature pro-
files at different time intervals inside the object are shown in
Figure 4.33. At time t 5 0, the temperature is uniform at T
i
. At time,
t 5 t
1
, the temperature along the wall increases, establishing a temper-
ature gradient within the object that promotes heat conduction. At
time t 5 t
2
, the temperature at the center is still at T
i
. However, with
the passage of time, at time t 5 t
3
, the centerline temperature begins
to increase, and eventually at t 5 t
4
the temperature of the cylinder
becomes uniform at T
a
. At this time, the cylinder is in thermal equi-
librium with the surrounding medium, and heat transfer ceases. Note
that there is no heat transfer from the axial ends of the cylinder.
356 CHAPTER 4 Heat Transfer in Food Processing
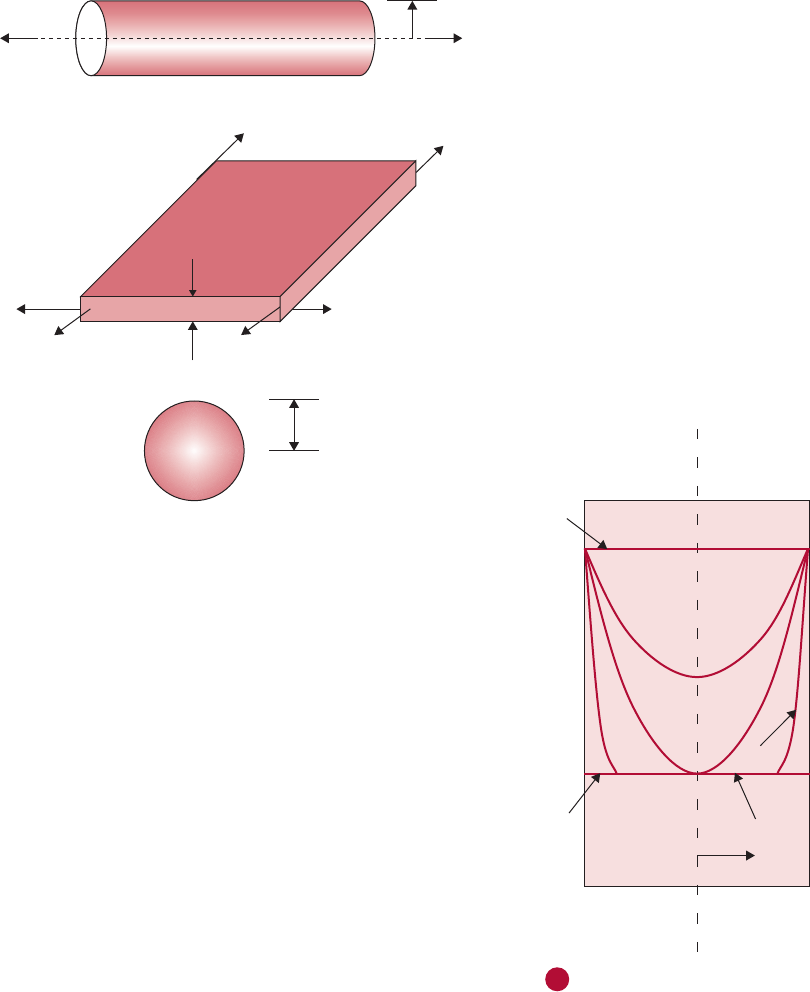
Because the cylinder is infinitely long , the implication of “infinite”
length in this case is that the heat transfer is only in the radial direc-
tion and not axial. Similarly, for an infinite slab of thickness, 2z, the
heat transfer is only from the two faces around the thickness 2z of
the slab. The slab extends to infinity along the other four faces, and
no heat transfer takes place through those sides. We will explore
these concepts in greater detail in the following sections.
4.5.1 Importance of External versus Internal
Resistance to Heat Transfer
In transient heat transfer analysis, one of the first steps is to consider
the relative importance of heat transfer at the surface and interior of
an object undergoing heating or cooling. Consider an object that is
suddenly immersed in a fluid (
Fig. 4.34). If the fluid is at a tempera-
ture different from the initial temperature of the solid, the tempera-
ture inside the solid will increase or decrease until it reaches a value
in equilibrium with the temperature of the fluid.
r
∞∞
(a)
2z
∞
∞
∞∞
∞
∞
(b)
r
(c)
■ Figure 4.32 (a) An infinite cylinder, (b) an
infinite plate, (c) a sphere.
t 0
t t
4
T
i
T
a
t t
1
t t
2
t t
3
r
w
■ Figure 4.33 Temperature profiles as a
function of time in an infinitely long cylinder.
3574.5 Unsteady-State Heat Transfer

During the unsteady-state heating period, the temperature inside the
solid object (initially at a uniform temperature) will vary with loca-
tion and time. Upon immersing the solid in the fluid, the heat trans-
fer from the fluid to the center of the solid will encounter two
resistances: Convective resistance in the fluid layer surrounding the
solid, and conductive resistance inside the solid. The ratio of the
internal resistance to heat transfer in the solid to the external resis-
tance to heat transfer in the fluid is defined as the B
iot
number, N
Bi
.
internal conductive resistance within the body
external convective resistance at the surface of the body
5 N
Bi
ð4:151Þ
or
N
Bi
5
d
c
=k
1=h
ð4:152Þ
or
N
Bi
5
hd
c
k
ð4:153Þ
where d
c
is a characteristic dimension.
According to
Equation (4.151), if the convective resistance at the sur-
face of a body is much smaller than the internal conductive resis-
tance, then the Biot number will be high. For Biot numbers greater
than 40, there is negligible surface resistance to heat transfer. On the
other hand, if internal conductive resistance to heat transfer is small,
then the Biot number will be low. For Biot numbers less than 0.1,
there is negligible internal resistance to heat transfer. Between a Biot
number of 0.1 and 40, there is a finite internal and external resis-
tance to heat transfer. Steam condensing on the surface of a broccoli
stem will result in negligible surface resistance to heat transfer
(N
Bi
$ 40). On the other hand, a metal can containing hot tomato
paste, being cooled in a stream of cold air, will present finite internal
and surface resistance to heat transfer, and a small copper sphere
placed in stagnant heated air will have a Biot number of less than
0.1. In the following subsections, we will consider these three cases
separately.
4.5.2 Negligible Internal Resistance to Heat Transfer
(N
Bi
, 0.1) — A Lumped System Analysis
For a Biot number smaller than 0.1, there is a negligible internal
resistance to heat transfer. This condition will occur in heating and
Solid food
Liquid heating/cooling
medium
■ Figure 4.34 A solid object suddenly exposed
to a heating/cooling medium.
358 CHAPTER 4 Heat Transfer in Food Processing

cooling of most solid metal objects but not with solid foods, because
the thermal conductivity of a solid food is relatively low.
Negligible internal resistance to heat transfer also means that the
temperature is nearly uniform throughout the interior of the object.
For this reason, this case is also referred to as a “lumped” system.
This condition is obtained in objects with high thermal conductivity
when they are placed in a medium that is a poor conductor of heat,
such as motionless air. In these cases heat is transferred instan-
taneously into the object, thus avoiding temperature gradients with
location. Another way to obtain such a condition is a well-stirred liq-
uid food in a container. For this special case, there will be no temper-
ature gradient with location, as the product is well mixed.
A mathematical expression to describe heat transfer for a negligible
internal resistance case may be developed as follows.
Consider an object at low uniform temperature T
i
, immersed in a hot
fluid at temperature T
a
, as shown in Figure 4.34. During the unsteady-
state period, a heat balance across the system boundary gives
q 5 ρc
p
V
dT
dt
5 hAðT
a
2 TÞð4:154Þ
where T
a
is the temperature of the surrounding medium, and A is the
surface area of the object.
By separating variables,
dT
ðT
a
2 TÞ
5
hA dt
ρc
p
V
ð4:155Þ
Integrating, and setting up limits,
ð
T
T
i
dT
T
a
2 T
5
hA
ρc
p
V
ð
t
0
dt ð4:156Þ
2lnðT
a
2 TÞj
T
T
i
5
hA
ρc
p
V
ðt 2 0Þð4:157Þ
2ln
T
a
2 T
T
a
2 T
i
5
hAt
ρc
p
V
ð4:158Þ
3594.5 Unsteady-State Heat Transfer

Rearranging terms,
T
a
2 T
T
a
2 T
i
5 e
2ðhA=ρc
p
V
Þt
ð4:159Þ
Rewriting
Equation (4.159) as
T
a
2 T
T
a
2 T
i
5 e
2bt
ð4:160Þ
where
b 5
hA
ρc
p
V
In
Equation (4.160), the numerator T
a
T on the left-hand side is
the unaccomplished temperature differenc e between the heat trans-
fer medium and the object. The denominator is the maximum tem-
perature differ ence at the start of the heatin g/cooling process. Thus,
the temperature ratio shown on the left-hand side of this equation
is the unaccomplished temperature fraction . At the start of the he at-
ing/cooling process, the unaccomplished temperature fraction is
one, and it decreases with time. The right-hand side of Equation
(4.160) sho ws an exponentially decreasing (or decaying) function.
This implies that with increasing passage of time the unaccom-
plished temperature fraction decreases but it never r eaches a value
of zero, it approaches zero asymptotically. Furthermore, when an
object is being heated, with a higher value of b, the object tempera-
ture increases more rapidly (with a greater decay in temperature dif-
ference). The b value is influenced directly by the convective
conditions on the surface described by h, its thermal properties, and
the size. Small objects with low specific heat take a shorter time to
heat or cool.
Example 4.24
Calculate the temperature of tomato juice (density 5 980 kg/m
3
) in a steam-
jacketed hemispherical kettle after 5 minutes of heating (see
Fig. E4.21). The
radius of the kettle is 0.5 m. The convective heat-transfer coefficient in
the steam jacket is 5000 W/(m
2
8C). The inside surface temperature of the
kettle is 908C. The initial temperature of tomato juice is 208C. Assume spe-
cific heat of tomato juice is 3.95 kJ/(kg 8C).
360 CHAPTER 4 Heat Transfer in Food Processing
Given
Kettle:
Surface temperature T
a
5 908C
Radius of kettle 5 0.5 m
Tomato juice:
Initial temperature T
i
5 208C
Specific heat c
p
5 3.95 kJ/(kg 8C)
Density ρ 5 980 kg/m
3
Time of heating t 5 5 min
Approach
Since the product is well mixed, there are no temperature gradients inside the
vessel, and we have negligible internal resistance to heat transfer. We will use
Equation (4.159) to find the temperature after 5 min.
Solution
1. We will use Equation (4.159). First, the inside surface area and volume
of the hemispherical kettle are computed:
A 5 2πr
2
5 2π(0:5)
2
5 1:57 m
2
V 5
2
3
πr
3
5
2
3
π(0:5)
3
5 0:26 m
3
2. Using Equation (4.159) :
90 2 T
90 2 20
5 exp
2 (5000 W=[m
2
8C])(1:57 m
2
)ð300 s)
(980 kg=m
3
)(3:95 kJ=[kg 8C])(1000 J=kJ)(0:26 m
3
)
90 2 T
90 2 20
5 0:096
T 5 83:38C
3. The product temperature will rise to 83.38C in 5 min of heating.
Example 4.25
An experiment was conducted to determine surface convective heat-
transfer coefficient for peas being frozen in an air-blast freezer. For this
purpose, a metal analog of peas was used. The analog was a solid copper
ball with a diameter of 1 cm. A small hole was drilled to the center of the
copper ball, and a thermocouple junction was located at the center using
a high-conductivity epoxy. The density of copper is 8954 kg/m
3
, and its
Steam
Tomato
juice
■ Figure E4.21 Heating tomato juice in a
hemispherical steam jacketed kettle.
3614.5 Unsteady-State Heat Transfer

specific heat is 3830 J/(kg K). The copper ball (at a uniform initial tempera-
ture of 108C) was hung in the path of air flow (at 2408C) and the center
temperature indicated by the thermocouple was recorded. The following
table lists the temperature at 1-min intervals for 14 min. Determine the
surface heat-transfer coefficient from these data.
Time Temperature
(s) (8C)
0 10.00
60 9.00
120 8.00
180 7.00
240 6.00
300 5.00
360 4.00
420 3.50
480 2.50
540 1.00
600 1.00
660 0.00
720 22.00
780 22.00
840 23.00
Given
Diameter of copper ball D 5 1cm
Density of copper ρ 5 8954 kg/m
3
Specific heat of copper c
p
5 3830 J/(kg K)
Initial temperature of copper T
i
5 108C
Temperature of cold air 5 408C
Approach
We will use a modified form of Equation (4.159) to plot the temperaturetime
data. If these data are plotted on a semilog paper, we can obtain the h value
from the slope. An alternative approach is to use statistical software to
develop a correlation and determine the slope.
Solution
1. Equation (4.159) may be rewritten as follows:
ln(T 2 T
a
) 5 ln(T
i
2 T
a
) 2
hAt
ρc
p
V
2. The tabulated data for temperature and time is converted to ln(T T
a
).
362 CHAPTER 4 Heat Transfer in Food Processing

3. Using a statistical software (e.g., StatViewt), a correlation is obtained
between t and ln(TT
a
). The results are
slope 523:5595 3 10
24
l =s
4. Thus,
hA
ρc
p
V
5 3:5595 3 10
24
5. Surface area of sphere, A 5 4πr
2
Volume of sphere, V 5 4πr
3
/3
6. Substituting given and calculated values in the expression in step (4),
we get
h 5 20 W=(m
2
8C)
7. A pea held in the same place as the copper ball in the blast of air will
experience a convective heat-transfer of 20 W/(m
2
8C).
4.5.3 Finite Internal and Surface Resistance to Heat
Transfer (0.1 # N
Bi
# 40)
As noted previously, the solution of Equation (4.149) is complicated,
and it is available only for well-defined shapes such as sphere, infi-
nite cylinder, and infinite slab. In each case, the solution is an infi-
nite series containing trigonometric and/or transcendental functions.
These solutions are as follows.
Sphere:
T
a
2 T
T
a
2 T
i
5 4
d
c
r
X
N
n51
sin λ
n
2 λ
n
cos λ
n
2λ
n
2 sin 2λ
n
e
2λ
2
n
N
Fo
sin λ
n
r
d
c
ð4:161Þ
With root equation,
1 2 λ
n
cot λ
n
5 N
Bi
ð4:162Þ
Infinite Cylinder:
T
a
2 T
T
a
2 T
i
5 2
X
N
n51
1
λ
n
J
1
ðλ
n
Þ
J
2
0
ðλ
n
Þ1 J
2
1
ðλ
n
Þ
e
2λ
2
n
N
Fo
J
0
λ
n
r
d
c
ð4:163Þ
3634.5 Unsteady-State Heat Transfer

with root equation,
λ
n
J
1
ðλ
n
Þ
J
0
ðλ
n
Þ
5 N
Bi
ð4:164Þ
Infinite Slab:
T
a
2 T
T
a
2 T
i
5 4
X
N
n51
sin λ
n
2λ
n
1 sin 2λ
n
e
2λ
2
n
N
Fo
cos λ
n
x
d
c
ð4:165Þ
With root equation,
λ
n
tan λ
n
5 N
Bi
ð4:166Þ
These analytical solutions with infinite series may be programmed
into a spreadsheet for use on a computer. We will illustrate this pro-
cedure later in this section with an example. These solutions have
also been reduced to simple temperaturetime charts that are rela-
tively easy to use. In constructing a temperaturetime chart for a typ-
ical transient heat transfer problem, the variety of factors can be
numerous: for example, r, t, k, p, c
p
, h, T
i
, and T
a
. However, these fac-
tors may be combined into three dimensionless numbers, making it
convenient to develop charts for universal use regardless of units
used for measuring these factors. Temperaturetime charts developed
for the three geometric shapes (sphere, infinite cylinder, and infinite
slab) are presented in
Figures 4.35, 4.36, and 4.37. These charts are
called Heisler charts, based on the work of Heisler (1944). The three
dimensionless numbers shown on these charts are the unaccom-
plished temperature fraction, (T
a
T)/(T
a
T
i
), Biot number, N
Bi
,
and a dimensionless time expressed as the Fourier
2
number. The
Fourier number is defined as follows:
Fourier number 5 N
Fo
5
k
ρc
p
t
d
2
c
5
αt
d
2
c
ð4:167Þ
where d
c
is a characteristic dimension. The value for d
c
indicates the
shortest distance from the surface to the center of the object. The
characteristic dimension for both a sphere and an infinite cylinder is
the radius; for an infinite slab, it is half the thickness of the slab.
2
Joseph Baron Fourier (17681830) was a French mathematician and highly
regarded Egyptologist. In 1798, he accompanied Napoleon to Egypt and con-
ducted extensive research on Egyptian antiques. From 1798 to 1801 he served
as a Secretary of Institut d’Egypte in Cairo. His work Theorie Analytique de la
Chaleur (The Analytical Theory of Heat) was started in 1807 in Grenoble and
was completed in 1822 in Paris. He developed a mathematical basis for the
conductive heat transfer in solids.
364 CHAPTER 4 Heat Transfer in Food Processing

We can examine the physical significance of the Fourier number, N
Fo
,
by rearranging
Equation (4.167) as follows:
N
Fo
5
αt
d
2
c
5
kð1=d
c
Þd
2
c
ρc
p
d
3
c
=t
5
rate of heat conduction across d
c
in a body of volume d
3
c
ðW=8CÞ
rate of heat storage in a body of volume d
3
c
ðW=8CÞ
For a given volume element, the Fourier number is a measure of the
rate of heat conduction per unit rate of heat storage. Thus, a larger
value of Fourier number indicates deep penetration of heat into the
solid in a given period of time.
1
0.1
(T
a
T)/(T
a
T
i
)
0.01
0.001
012 34510
at/d
c
2
20 30
12 14 1618 20 25 30
40
50
60
70
80
90
100
Sphere
10
9
8
7
6
5
4
3.5
3
2.5
2
1.81.410.6
0.4
0.2
k/(hd
c
)
0
0.1 0.3 0.5 0.8
1.2 1.6
40 50 60 70 80 90 100
w
■ Figure 4.35 Temperature at the geometric center of a sphere of radius d
c
.
3654.5 Unsteady-State Heat Transfer

Note that the Heisler charts shown in Figures 4.35, 4.36, and 4.37
are plotted on a log-linear scale.
4.5.4 Negligible Surface Resistance to Heat Transfer
(N
Bi
$ 40)
For situations where the Biot number is greater than 40, indicating
negligible surface resistance to heat transfer, we can use the charts in
Figures 4.35, 4.36, and 4.37. In these figures, the lines for k/hd
c
5 0
represent negligible surface resistance to heat transfer.
4.5.5 Finite Objects
Myers (1971) has shown mathematically that
T
a
2T
T
a
2T
i
finite cylinder
5
T
a
2T
T
a
2T
i
infinite cylinder
3
T
a
2T
T
a
2T
i
infinite slab
ð4:168Þ
1
0.1
(T
a
T)/(T
a
T
i
)
0.01
0.001
01234510
αt/d
c
2
20 30
1210987654
14
16 18 20
25
30
40
50
60
70
80
90
100
Infinite cylinder
1.6
1.8
2.0
2.5
3.0
3.5
1.4
1.2
0.40.2
k/(hd
c
)
0.05
0.1
0
0.3 0.5 0.6 0.8 1.0
40 50 60 70 80 90 100
■ Figure 4.36 Temperature at the geometric center of an infinitely long cylinder of radius d
c
.
366 CHAPTER 4 Heat Transfer in Food Processing

and
T
a
2T
T
a
2T
i
finite brick shape
5
T
a
2T
T
a
2T
i
infinite slab, width
3
T
a
2T
T
a
2T
i
infinite slab, depth
3
T
a
2T
T
a
2T
i
infinite slab, height
ð4:169Þ
These expressions allow us to determine temperature ratios for
objects of finite geometry, such as a cylindrical can commonly used
in heat sterilization of food. Although the mathematics to prove
Equations (4.168) and (4.169) is beyond the scope of this text,
Figure 4.38 may be studied as a visual aid.
1
0.1
(T
a
T)/(T
a
T
i
)
0.01
0.001
01234
at/d
c
2
435
4
12
30
25
20
18
16
14
40
50
60
70
100
Infinite slab
1.2
1.0
0.8
0.6
0.5
0.4
0.3
1.4
1.8
1.6
2
k
/(hd
c
)
0.05
0.1
0
0.2
678910
5102030405060708090100
■ Figure 4.37 Temperature at the midplane of an infinite slab of thickness 2d
c
.
w
■ Figure 4.38 A finite cylinder considered as
part of an infinite cylinder and an infinite slab.
3674.5 Unsteady-State Heat Transfer
Afinitecylinder(Fig. 4.38) can be visualized as a part of an infinite cylin-
der and a part of an infinite slab. Heat transfer in a radial direction is sim-
ilar to heat transfer for an infinite cylinder. Invoking the infinite cylinder
shape, we mean that heat transfer to the geometric center is only through
the radial direction—through the circumferential area of the cylinder—
whereas the ends of the cylinder are too far to have any measurable influ-
ence on heat transfer. Heat transfer from the two end surfaces is similar
to heat transfer for an infinite slab. Considering the finite cylinder to be
an infinite slab will account for all the heat transfer from the two ends of
the cylindrical can while ignoring heat transfer in the radial direction.
This approach allows us to include heat transfer both from the radial
direction and from the cylinder ends. Similarly, a brick-shape object can
be considered to be constructed from three infinite slabs maintaining
width, depth, and height, respectively, as the finite thickness.
4.5.6 Procedures to Use TemperatureTime Charts
The following steps may be used to determine heat transfer in finite
objects with the use of temperaturetime charts.
Heat transfer to an object of finite cylindrical shape, such as a cylin-
drical can, requires the use of temperaturetime charts for both infi-
nite cylinder and infinite slab. Thus, if the temperature at the
geometric center of the finite cylinder is required at a given time, the
following steps may be used.
For an infinite cylinder:
1. Calculate the Fourier number, using the radius of the cylinder
as the characteristic dimension.
2. Calculate the Biot number, using the radius of the cylinder as
the characteristic dimension. Calculate the inverse of Biot
number for use in the Heisler Charts.
3. Use
Figure 4.36 to find the temperature ratio.
For an infinite slab:
1. Calculate the Fourier number, using the half-height as the
characteristic dimension.
2. Calculate the Biot number, using the half-height as the charac-
teristic dimension. Calculate the inverse of Biot number for
use in the Heisler Charts.
3. Use
Figure 4.37 to determine the temperature ratio.
368 CHAPTER 4 Heat Transfer in Food Processing

The temperature ratio for a finite cylinder is then calculated from
Equation (4.168). We can compute the temperature at the geometric
center if the surrounding medium temperature T
a
and initial temper-
ature T
i
are known.
Steps similar to the preceding may be used to compute temperature
at the geometric center for a finite slab-shaped object (such as a par-
allelepiped or a cube). In the case of a spherical object such as an
orange,
Figure 4.35 for a sphere is used.
A major drawback of the Heisler charts is that they are difficult to use
for situations when the Fourier number is small. For example, with
problems involving transient heat transfer in foods, the Fourier num-
bers are often less than 1 because of the low thermal diffusivity of
foods. In these cases, charts with expanded scales (see Appendix A.8),
based on the work of
Schneider (1963), are helpful. The procedure to
use these expanded-scale charts is exactly the same as with the Heisler
charts. Note that the expanded-scale charts use Biot numbers directly,
without inverting them, and they are plotted on a linear-log scale,
whereas the Heisler charts are plotted on a log-linear scale.
Examples 4.26 to 4.28 illustrate the procedures presented in this section.
Example 4.26
Estimate the time when the temperature at the geometric center of a 6 cm
diameter apple held in a 28C water stream reaches 38C. The initial uniform
temperature of the apple is 158C. The convective heat transfer coefficient in
water surrounding the apple is 50 W/(m
2
8C). The properties of the apple
are thermal conductivity k 5 0.355 W/(m 8C); specific heat c
p
5 3.6 kJ/(kg 8C);
and density ρ 5 820 kg/m
3
.
Given
Diameter of apple 5 0.06 m
Convective heat-transfer coefficient h 5 50 W/(m
2
8C)
Temperature of water stream T
a
5 28C
Initial temperature of apple T
i
5 158C
Final temperature of geometric center T 5 38C
Thermal conductivity k 5 0.355 W/(m 8C)
Specific heat c
p
5 3.6 kJ/(kg 8C)
Density ρ 5 820 kg/m
3
Approach
Considering an apple to be a sphere in shape, we will use Figure 4.35 to find the
Fourier number. The time of cooling will be computed from Equation (4.167).
3694.5 Unsteady-State Heat Transfer

Solution
1. From given temperatures, we first calculate the temperature ratio.
T
a
2 T
T
a
2 T
i
5
2 2 3
2 2 15
5 0:077
2. The Biot number is computed as
N
Bi
5
hd
c
k
5
(50 W=[m
2
8C])(0:03 m)
(0:355 W=[m 8C])
5 4:23
Thus,
1
N
Bi
5 0:237
3. From
Figure 4.35, for a temperature ratio of 0.077 and (1/N
Bi
)of
0.237, the Fourier number can be read as
N
Fo
5 0:5
4. The time is calculated from the Fourier number.
k
ρc
p
t
d
2
c
5 0:5
t 5
(0:5)(820 kg=m
3
)(3:6kJ=[kg 8C])(0:03 m)
2
(1000 J=kJ)
(0:355 W=[m 8C])
5 3742 s
5 1:04 h
Example 4.27
Estimate the temperature at the geometric center of a food product con-
tained in a 303 3 406 can exposed to boiling water at 1008C for 30 minutes.
The product is assumed to heat and cool by conduction. The initial uniform
temperature of the product is 358C. The properties of the food are thermal
conductivity k 5 0.34 W/(m 8C); specific heat cp 5 3.5 kJ/(kg 8C); and density
370 CHAPTER 4 Heat Transfer in Food Processing

ρ 5 900 kg/m
3
. The convective heat transfer coefficient for the boiling water
is estimated to be 2000 W/(m
2
8C).
Given
Can dimensions:
Diameter 5 3
3
16
inches 5 0:081 m
Height 5 4
6
16
inches 5 0:11 m
Convective heat transfer coefficient h 5 2000 W/(m
2
8C)
Temperature of heating media T
a
5 1008C
Initial temperature of food T
i
5 358C
Time of heating 5 30 min 5 1800 s
Properties:
k 5 0:34 W=(m 8C)
c
p
5 3:5kJ=(kg 8C)
ρ 5 900 kg=m
3
Approach
Since a finite cylindrical can may be considered a combination of infinite cyl-
inder and infinite slab, we will use timetemperature figures for both these
shapes to find respective temperature ratios. The temperature ratio for a finite
cylinder will then be calculated from
Equation (4.168).
Solution
1. First, we estimate temperature ratio for an infinite cylinder.
2. Biot number 5 hd
c
/k where d
c
is radius 5 0.081/2 5 0.0405 m
N
Bi
5
(2000 W=[m
2
8C])(0:0405 m)
(0:34 W=[m 8C])
5 238
Thus,
1
N
Bi
5 0:004
3714.5 Unsteady-State Heat Transfer

3. The Fourier number for an infinite cylinder is
N
Fo
5
k
ρc
p
t
d
2
c
5
(0:34 W= [m 8C])(1800 s)
(900 kg=m
3
)(3:5kJ=[kg 8C])(1000 J=kJ)(0:0405 m)
2
5 0:118
4. The temperature ratio can be estimated from
Figure 4.36 for
1/N
Bi
5 0.004 and N
Fo
5 0.118 as
T
a
2T
T
a
2T
i
infinite cylinder
5 0:8
5. Next, we estimate the temperature ratio for an infinite slab.
6. Biot number 5 hd
c
/k where d
c
is half-height 5 0.11/2 5 0.055 m
N
Bi
5
(2000 W=[m
2
8C])(0:055 m)
(0:34 W=[m 8C])
5 323:5
Thus,
1=N
Bi
5 0:003
7. The Fourier number for an infinite slab is
N
Fo
5
kt
ρc
p
d
2
c
5
(0:34 W= [m 8C])(1800 s)
(900 kg=m
3
)(3:5kJ=[kg 8C])(1000 J=kJ)(0:055 m)
2
5 0:064
8. The temperature ratio can be estimated from
Figure 4.37 for
1/N
Bi
5 0.003 and N
Fo
5 0.064 as
T
a
2T
T
a
2T
i
infinite slab
5 0:99
9. The temperature ratio for a finite cylinder is computed using
Equation
(4.168)
T
a
2T
T
a
2T
i
finite cylinder
5 (0:8)(0:99) 5 0:792
372 CHAPTER 4 Heat Transfer in Food Processing

Therefore,
T 5 T
a
2 0:792(T
a
2 T
i
)
5 100 2 0:792(100 2 35)
5 48:48C
10. The temperature at the geometric center of the can after 30 minutes.
will be 48.48C. Note that most of the heat transfers radially; only a
small amount of heat transfers axially, since
T
a
2T
T
a
2T
i
infinite slab
5 0:99
or a value close to 1. If
T
a
2 T
T
a
2 T
i
5 1
then T 5 T
i
; this means that the temperature at the end of the heat-
ing period is still T
i
, the initial temperature, indicating no transfer of
heat. Conversely, if
T
a
2 T
T
a
2 T
i
5 0
then T 5 T
a
, indicating that the temperature of the end-of-heating
period equals that of the surrounding temperature.
Example 4.28
Using Equations (4.161), (4.163), and (4.165), develop spreadsheet pro-
grams, and compare the calculated results with the values obtained from
charts given in Figures 4.35, 4.36, and 4.37.
Approach
We will use spreadsheet EXCEL and program modified forms of Equations
(4.161), (4.163), and (4.165) for negligible surface resistance to heat transfer
(N
Bi
. 40). Since these equations involve series solutions, we will consider the
first 30 terms of each series, which should be sufficiently accurate for our
purposes.
Solution
The spreadsheets written for a sphere, infinite cylinder, and infinite slab are
shown in
Figures E4.22, E4.23, and E4.24, respectively.
3734.5 Unsteady-State Heat Transfer

1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
AB CDEF GH
SPHERE - Negligible surface resistance to heat transfer
Domain: 0 < r < d_c where d_c is characteristic dimension, or radius of sphere)
Terms of series
Fourier number 0.3 n term_n
r/d_c 0.00001 1 1.6265E06
2 2.2572E10
Temperature ratio 0.104 3 8.3965E17
4 8.3722E26
5 2.2376E37
6 1.6031E51
7 3.0784E68
8 1.5846E87
9 2.186E109
10 8.086E134
11 8.015E161
12 2.13E190
13 1.517E222
14 2.896E257
15 1.482E294
16 0
17 0
18 0
19 0
20 0
21 0
22 0
23 0
24 0
25 0
26 0
27 0
28 0
29 0
30 0
((1^(E91))/E9*EXP
(E9*E9*PI()*PI()*$C$8)*
SIN(E9*PI()*$C$9))
Steps:
1) Enter numbers 1 though 30 in cells E9 to E38
2) Enter formula in cell F9, then copy it into cells
F10 to F38.
3) Enter formula in cell C11
4) Enter any Fourier Number in cell C8 and radial
location/(characteristic dimension) in cell C9
For a sphere characteristic dimension is radius
5) If temperature ratio is desired at the center of a
sphere, do not use r 0, instead use a very small
number in cell C9, e.g. 0.00001
6) The result is shown in cell C11
SUM(F9:F38)*2/PI()*(1/$C$9)
■ Figure E4.22 Spreadsheet solution (sphere) for Example 4.26.
374 CHAPTER 4 Heat Transfer in Food Processing

1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
AB CDEF G H I J K L
INFINITE CYLINDER - Negligible Surface Resistance to Heat Transfer
Domain: 0 < r < d_c (where d_c is the characteristic dimension, or radius of infinite cylinder)
Terms of Series J0 for -3 < x < 3 J1 for 3 < x < inf
Fourier Number 0.2 n lambda_n ArgJ0 J0 J1(lambda_n) term_n
r/d_c 0 0 2.4048255577 0 1 0.820682791 0.195379932 0.519147809 0.251944131
1 5.5200781103 0 1 0.802645916 3.23090329 0.340264805 0.00120098.5
Temperature Ratio 0.5015 2 8.6537279129 0 1 0.799856138 6.340621261 0.271452299 1.33199E07
3 11.7915344391 0 1 0.79895277 9.467043934 0.232459829 3.0563E13
4 14.9309177086 0 1 0.798552578 12.5997901 0.206546432 1.4036E20
5 18.0710639679 0 1 0.798341245 15.73559327 0.187728803 1.27219E29
6 21.2116366299 0 1 0.798216311 18.87310399 0.173265895 2.25935E40
7 24.3524715308 0 1 0.798136399 22.01166454 0.161701553 7.82877E53
8 27.4934791320 0 1 0.798082224 25.15091632 0.152181217 5.27858E67
9 30.6346064684 0 1 0.798043816 28.29064728 0.144165981 6.91297E83
Steps:
1) Enter numbers 0 to 9 in cells E9
to E18
2) Enter coefficients as indicated in
cells F9 to F18
3) Enter formulas for cells G9, H9,
I9, J9, K9 and L9, and copy into
cells G10 to G18, H10 to H18,
I10 to I18, J10 to J18, K10 to K18
and L10 to L18, respectively.
4) Enter formula for cell C11
5) Enter any Fourier Number in cell
C8, and r/(characteristic dimension)
in cell C9
6) Results are shown in cell C11
Formulas:
Cell C11 2*SUM(L9:L18)
Cell G9 $C$9*F9
Cell H9 12.2499997*(G9/3)^21.2656208*(G9/3)^40.3163866*(G9/3)^6 0.0444479
*(G9/3)^8 0.0039444*(G9/3)^10 0.00021*(G9/3)^12
Cell I9 0.79788456 0.00000156*(3/F9) 0.01659667*(3/F9)^2 0.00017105*(3/F9)^3
0.00249511*(3/F9)^4 0.00113653*(3/F9)^5 0.00020033*(3/F9)^6
Cell J9 F92.35619449 0.12499612*(3/F9) 0.0000565*(3/F9)^2 0.00637879*(3/F9)^3
0.00074348*(3/F9)^4 0.00079824*(3/F9)^5 0.00029166*(3/F9)^6
Cell K9 F9^(1/2)*I9*COS(J9)
Cell L9 EXP(F9*F9*$C$8)*H9/(F9*K9)
■ Figure E4.23 Spreadsheet solution (infinite cylinder) for Example 4.26.

These spreadsheets also include results for temperature ratios for arbitrarily
selected Fourier numbers. The calculated results for temperature ratios com-
pare favorably with those estimated from
Figures 4.35, 4.36, and 4.37.
4.5.7 Use of f
h
and j Factors in Predicting
Temperature in Transient Heat Transfer
In many problems common to food processing, an unknown tem-
perature is determined after the unaccomplished temperature fraction
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
AB CDEF G H I J
INFINITE SLAB - Negligible Surface Resistance to Heat Transfer
Domain: d_cxd_c where d_c is the characteristic dimension, or half-thickness of a slab
Terms of Series
Fo 1 n lambda_n term_n
x/d_c 0 0 1.570796327 0.107977045
1 4.71238898 9.6289911
Temperature Ratio 0.108 2 7.853981634 4.13498E28
3 10.99557429 5.65531E54
4 14.13716694 2.25317E-88
5 17.27875959 2.5264E131
6 20.42035225 7.8374E183
7 23.5619449 6.6622E243
8 26.70353756 0
9 29.84513021 0
10 32.98672286 0
11 36.12831552 0
12 39.26990817 0
13 42.41150082 0
14 45.55309348 0
15 48.69468613 0
16 51.83627878 0
17 54.97787144 0
18 58.11946409 0
19 61.26105675 0
20 64.4026494 0
21 67.54424205 0
22 70.68583471 0
23 73.82742736 0
24 76.96902001 0
25 80.11061267 0
26 83.25220532 0
27 86.39379797 0
28 89.53539063 0
29 92.67698328 0
30 95.81857593 0
(2*E91)/2*PI()
(2*(1)^E9)*EXP(F9*F9*$C$8)/F9*
COS(F9*$C$9)
SUM(G9:G39)
Steps:
1) Enter numbers 0 to 30
in cells E9 to E39
2) Enter formula in cell F9
3) Copy formula from cell F9
to cells F10 to F39
4) Enter formula in cell G9
5) Copy formula from cell G9
to cells G10 to G39
6) Enter formula in cell C11
7) Result is shown in cell C11
8) Enter any Fourier number in
cell C8 and x/d_c in cell C9
and the results will be shown
in cell C11
■ Figure E4.24 Spreadsheet solution (infinite slab) for Example 4.26.
376 CHAPTER 4 Heat Transfer in Food Processing

has decreased to less than 0.7. In such cases, the series solutions of
the governing partial differential equation (
Eq. (4.149)) is simplified.
Since only the first term of the series is significant, all remaining
terms become small and negligible. This was recognized by Ball
(1923), who developed a mathematical approach to predicting tem-
peratures in foods for calculations of thermal processes. We will con-
sider Ball’s method of thermal process calculations in more detail in
Chapter 5. However, the approach to predict temperature for longer
time durations is presented here.
In Equation (4.160) we noted that the unaccomplished temperature
fraction decays exponentially. Thus, for a general case we may write
T
a
2 T
T
a
2 T
i
5 a
1
e
2b
1
t
1 a
2
e
2b
2
t
1 a
3
e
2b
3
t...
ð4:170Þ
For long time durations, only the first term in the series is significant.
Therefore,
T
a
2 T
T
a
2 T
i
5 a
1
e
2b
1
t
ð4:171Þ
or, rearranging,
ln
ðT
a
2 T)
a
1
ðT
a
2 T
i
)
52b
1
t ð4:172Þ
Ball used two factors to describe heat transfer equation, a time factor
called f
h
and a temperature lag factor called j
c
. To be consistent with
Ball’s method, we will replace symbol a
1
with j
c
, and b
1
with 2.303/
f
h
. The factor 2.303 is due to the conversion of log scale from base
10 to base e. Substituting these symbols in
Equation (4.172),
ln
ðT
a
2 TÞ
j
c
ðT
a
2 T
i
Þ
52
2:303
f
h
t ð4:173Þ
Rearranging,
lnðT
a
2 TÞ52
2:303t
f
h
1 ln j
c
ðT
a
2 T
i
Þ
ð4:174Þ
Converting to log10,
logðT
a
2 TÞ52
t
f
h
1 log j
c
ðT
a
2 T
i
Þ
ð4:175Þ
3774.5 Unsteady-State Heat Transfer

Ball used Equation (4.175) in developing his mathematical approach.
He plotted the unaccomplished temperature (T
a
T) against time t
on a log-linear graph rotated by 1808, as shown in Figure 4.39.From
the plot, he obtained f
h
as the time required for the straight line por-
tion to traverse one log cycle. In other words, f
h
is the time taken for
the unaccomplished temperature to decrease by 90%. The j
c
factor was
obtained by extending the straight line to time 0 to obtain an inter-
cept with the ordinate as T
a
T
A
.Thenj
c
was defined as (T
a
T
A
)/
(T
a
T
i
). A similar procedure, used for a cooling curve, will be illus-
trated with an example at the end of this section.
The exact solutions of the first term of infinite series shown in
Equations (4.161), (4.163), and (4.165) can either be programmed
into a spreadsheet or plotted as originally done by Pflug et al. (1965)
as f
h
vs N
Bi
, j
c
vs N
Bi
, and j
m
vs N
Bi
, shown in Figures 4.40, 4.41 , and
4.42. Factor j
c
is for the temperature lag at the center of an object,
and j
m
is the mean temperature lag for the object. The f
h
value is the
same for either center temperature or mean temperature. It is useful
to know the mean temperature of an object when determining the
0 20406080100
100
10
1
119
20
40
50
60
70
80
90
100
110
111
112
113
114
115
116
117
118
30
Time (minutes)
Temperature (°C)
Retort temperature 120°C
T
i
T
A
f
h
■ Figure 4.39 Heating curve plotted on a semi-log paper rotated 1808.
378 CHAPTER 4 Heat Transfer in Food Processing

0.1
1
10
100
0.1 1 10 100
Biot number, hd
c
/k
fa/dc
2
Sphere
Infinite cylinder
Infinite slab
w
■ Figure 4.40 Heating rate parameter, f
h
, as a function of Biot number.
1
1.1
1.2
1.3
1.4
1.5
1.6
1.7
1.8
1.9
2
2.1
2.2
0.1 1 10 100
Biot number, hd
c
/k
j
c
Sphere
Infinite cylinder
Infinite slab
w
■ Figure 4.41 Lag factor, j
c
, at the geometric center of a sphere, infinite cylinder, and infinite slab as a function of Biot number.
3794.5 Unsteady-State Heat Transfer

heat load required for heating/cooling applications. Using
Figures 4.40, 4.41, or 4.42, the f
h
, j
c
, or j
m
factors are obtained for
simplified geometrical shapes and used in Equation (4.174) to calcu-
late the temperature at any time. If the shape of the object is a finite
cylinder, then we can obtain the f
h
and j
c
factors using the following
relations:
1
f
finite cylinder
5
1
f
infinite cylinder
1
1
f
infinite slab
ð4:176Þ
and
j
c,finite cylinder
5 j
c,infinite cylinder
3 j
c,infinite slab
ð4:177Þ
For a brick-shaped object,
1
f
brick
5
1
f
infinite, slab
1
1
1
f
infinite, slab
2
1
1
f
infinite, slab
3
ð4:178Þ
and
j
c,brick
5 j
c,infinite slab
1
3 j
c,infinite slab
2
3 j
c,infinite slab
3
ð4:179Þ
0.5
0.6
0.7
0.8
0.9
1
1.1
0.1 1 10 100
Biot number, hd
c
/k
j
m
Infinite slab
Infinite cylinder
Sphere
w
■ Figure 4.42 Average lag factor, j
m
of a sphere, infinite cylinder, and infinite slab as a function of Biot number.
380 CHAPTER 4 Heat Transfer in Food Processing

The usefulness of the product rule is limited for shapes with dimen-
sion ratios much greater than 1 (
Pham, 2001). A gross overestimate of
cooling time may occur. In those cases, f
h
and j
c
for finite shapes may
be calculated using empirical methods given by Lin et al. (1996).
Example 4.29
A solid food in a cylindrical container is cooled in a 48C immersion water
cooler. Estimate the f and j factors if the following data were obtained for
temperature at the geometric center of the container.
Time
(minutes)
Temperature
(8C)
058
548
10 40
15 26
20 25
25 19
30 15
35 12
40 10
45 9
50 7.5
55 7
60 6.5
Given
Cooling medium temperature 5 48C
Approach
We will use a two-cycle log paper and create a scale for the y-axis as shown
in
Figure E4.25. The straight line portion will be extended to intersect the
y-axis to determine the pseudo initial temperature. The f
c
factor will be
obtained by determining the time traversed for one log cycle change in
temperature.
Solution
1. As shown in Figure E4.25 the y axis is labeled starting from the bottom
with 1 1 48C 5 58C scale; label the rest of the numbers on the y-axis
according to the log scale.
2. Locate all the points for temperature and time on the graph. Extend
the straight line to intersect the y-axis to determine the pseudo initial
temperature. From the plot, this temperature is 698C.
3814.5 Unsteady-State Heat Transfer

3. Determine the time for the straight line to traverse one log cycle to
obtain f
c
. From the plot we get f
c
5 40 min.
4. The j
c
value is then obtained as
j
c
5
69 2 4
58 2 4
5 1:2
5. For the given data, the f
c
is 40 min and j
c
is 1.2.
Example 4.30
A hot dog is initially at 58C and it is being heated in hot water at 958C. The
convective heat transfer coefficient is 3008 W/(m
2
8C). The dimensions of
the hot dog are 2 cm diameter and 15 cm long. Assuming that the heat
transfer is largely in the radial direction, estimate the product temperature
at the geometric center after 10 minutes. The properties of the hot dog
are as follows: density 5 1100 kg/m
3
, specific heat 5 3.4 kJ/(kg 8C), and
thermal conductivity 5 0.48 W/(m 8C).
Given
Initial temperature 5 58C
Heating medium temperature 5 958C
Heat transfer coefficient 5 300 W/(m
2
8C)
Hot dog length 5 15 cm
Hot dog diameter 5 2cm
Heating time 5 10 min
0 10203040506070
Time (min)
5
6
7
8
14
24
34
44
54
64
104
1
10
100
Temperature (°C)
T T
c
■ Figure E4.25 Cooling curve for data given in
Example 4.27.
382 CHAPTER 4 Heat Transfer in Food Processing

Density 5 1100 kg/m
3
Specific heat 5 3.4 kJ/(kg 8C)
Thermal conductivity 5 0.48 W/(m 8C)
Approach
We will consider Pflug’s charts for solving this problem. First we will calculate
the Biot number and use
Figures 4.40 and 4.41 to obtain f
h
and j
c
factors.
The required temperature will be obtained from Equation (4.176).
Solution
1. Biot number for an infinite cylinder is obtained as
N
Bi
5
300 [W=(m
2
8C)] 3 0:01[m]
0:48 [W=(m 8C)]
N
Bi
5 6:25
2. From
Figure 4.40, we obtain for an infinite cylinder,
f
h
α
d
2
c
5 0:52
f
h
5
0:52 3 (0:01)
2
[m
2
] 3 1100 [kg=m
3
] 3 3400 [J=(kg 8C)]
0:48[W=(m 8C)]
f
h
5 405:17 s
3. From
Figure 4.41, we obtain for an infinite cylinder,
j
c
5 1:53
4. Using
Equation (4.176),
log(95 2 T) 52
10 3 60 [s]
405:17 [s]
1 log(1:53[95 2 5])
T 5 90:458C
5. The temperature at the geometric center after 10 min of heating is
90.458C. The validity of the method may be checked by calculating the
unaccomplished temperature fraction at 10 min. For this example, the
method is valid because the fraction is 0.05{0.7, the assumption used
in this method.
3834.5 Unsteady-State Heat Transfer

4.6 ELECTRICAL CONDUCTIVITY OF FOODS
It is well known that when electrolytes are placed in an electric
field, the ions present within the electrolyte move toward the elec-
trodes with opposite charge. The movement of ions in the electro-
lyte generates heat. Similarly, when a food containing ions is placed
between two electrodes and alternating current or any other wave
form current is passed through the food, the food heats by internal
heat generation.
Electrical conductance, κ
E
, not to be confused with electrical conduc-
tivity, is the reciprocal of electrical resistance, or
κ
E
5
1
R
E
ð4:180Þ
where R
E
is the electrical resistance of the food material (ohms).
From Ohm’s law, we know that
R
E
5
E
V
I
ð4:181Þ
where E
V
is the applied voltage (V), and I is the electric current ( A).
Then, electrical conductance is
κ
E
5
1
E
V
ð4:182Þ
Electrical conductivity, σ
E
, is a measure of a material’s ability to con-
duct electric current. It is equal to electrical conductance measured
between the opposite faces of a 1-meter cube of the material.
σ
E
5
κ
E
L
A
5
IL
E
V
A
ð4:183Þ
where A is the area (m
2
) and L is the length (m). The SI units of elec-
trical conductivity are siemens/m or S/m.
Electrical conductivity of a food material is measured using an electri-
cal conductivity cell, as shown in
Figure 4.43. In this cell, a food
sample is placed between two electrodes and the electrodes are con-
nected to a power supply. Care is taken to ensure that the electrodes
make a firm contact with the food sample.
Plunger
Upper
electrode
Lower
electrode
Food sample
Weight
w
■ Figure 4.43 A setup for measurement of
electrical conductivity of foods. (Adapted from
Mitchell and Alvis, 1989)
384 CHAPTER 4 Heat Transfer in Food Processing

Electrical conductivity of foods increases with temperature in a linear
manner. The following equation may be used to calculate electrical
conductivity of a food:
σ
E
5 σ
o
ð1 1 mvTÞð4:184Þ
where σ
o
is the electrical conductivity at 08C (S/m) , mv is coefficient,
(1/8C); T is temperature, (8C).
If a reference temperature other than 08 C is chosen then an alternate
expression for estimating electrical conductivity is as follows:
σ
E
5 σ
ref
½1 1 KðT 2 T
ref
Þ ð4:185Þ
Values of σ
o
, σ
ref
and coefficients mv and K for different foods are
given in
Table 4.6.
The electrical conductivity of a food is a function of its composi-
tion—the quantity and type of various components present in the
food. Foods containing electrolytes such as salts, acids, certain gums,
and thickeners contain charged groups that have a notable effect on
the value of electrical conductivity. Based on experimental studies,
researchers have reported mathematical relationships to predict
Table 4.6 Coefficients for Equations (4.184) and (4.185) to Estimate
Electrical Conductivity
Product σ
25
(S/m) K (8 C
21
) σ
o
(S/m) mv (8C
21
)
Potato 0.32 0.035 0.04 0.28
Carrot 0.13 0.107 2 0.218 2 0.064
Yam 0.11 0.094 2 0.149 2 0.07
Chicken 0.37 0.019 0.194 0.036
Beef 0.44 0.016 0.264 0.027
Sodium Phosphate
0.025 M
0.189 0.027 0.614 0.083
Sodium Phosphate
0.05 M
0.361 0.022 0.162 0.048
Sodium Phosphate
0.1 M
0.676 0.021 0.321 0.0442
Source: Palaniappan and Sastry (1991)
3854.6 Electrical Conductivity of Foods

electrical conductivity based on selected food constituents. In fruit
juices, the inert suspended solids in the form of pulp or other cellular
material act as insulators and tend to decrease the electrical conduc-
tivity of the liquid media. Sastry and Palaniappan (1991) obtained
the following relationship to describe the effect of solid concentra-
tion on electrical conductivity of orange and tomato juices:
σ
T,tomato
5 0:863½1 1 0:174ðT 2 25Þ2 0:101 3 M
s
ð4:186Þ
σ
T,orange
5 0:567½1 1 0:242ðT 2 25Þ2 0:036 3 M
s
ð4:187Þ
where M
s
is the solid concentration (percent), and T is temperature (8C).
Example 4.31
Estimate the electrical conductivity of 0.1 M Sodium Phosphate solution
at 308C.
Given
Temperature 5 308C
0.1 M Sodium Phosphate solution
Approach
We will use Equation (4.184) to determine the electrical conductivity.
Solution
1. Using Equation (4.184) and appropriate values for electrical conductiv-
ity at the reference temperature of 08C and coefficient mv, we obtain
σ
E
5 0:321(1 1 0:0442 3 30)
σ
E
5 0:746 S=m
2. Note that if we use
Equation (4.185) with appropriate values of electri-
cal conductivity at reference temperature of 258C and coefficient K, we
obtain
σ
E
5 0:676(1 1 0: 021(30 2 25))
σ
E
5 0:747 S=m
As expected, we get the same result using
Equation (4.184) or (4.185).
386 CHAPTER 4 Heat Transfer in Food Processing

4.7 OHMIC HEATING
In ohmic heating, main alternating current is passed directly through
a conductive food, which causes heat generation within the food. Due
to internal heat generation, the heating is rapid and more uniform
than traditional systems used for heating foods where heat must travel
from the outside surface to the inside of the food. The rapid and uni-
form heating of a food is advantageous in retaining many quality
characteristics such as color, flavor, and texture. The efficiency of
ohmic heating is dependent upon how well the electric current can
pass through the food, as determined by its electrical conductivity.
Therefore, the knowledge of electrical conductivity of foods is impor-
tant in designing processes and equipment involving ohmic heating.
As an example of ohmic heating, we will consider heating a liquid
food with Newtonian characteristics when pumped through an
ohmic heater. We assume that the flow conditions through the
tubular-shaped heater are similar to plug flow, and a constant voltage
gradient exists along the heater. In this setup, heat is generated
within the liquid due to ohmic heating, and heat loss from the fluid
is in radial direction to the outside, if the heater pipe is uninsulated.
For this setup, conducting a heat balance we get,
_
mc
p
dT
dt
5 ðjΔVj
2
σ
o
ð1 1 mvTÞÞ
πd
2
c
L
4
2 Uπd
c
LðT 2 T
N
Þð4:188Þ
where jΔVj is voltage gradient along the heater pipe length, (V/m);
σ
o
is the electrical conductivity at 08C; m is the slope obtained from
Equation (4.184); d
c
is the characteristic dimension or diameter of
the heater pipe (m); L is the length of heater pipe (m); U is the over-
all heat transfer coefficient based on the inside area of the heater
pipe, (W/m
2
8C); T
N
is the temperature of the air surrounding the
heater (8C).
The initial condition is
t 5 0, T 5 T
o
Expanding the terms in Equation (4.188) and rearranging, we get
dT
dt
5
απd
c
LT
_
mc
p
1
bπDL
_
mc
p
ð4:189Þ
3874.7 Ohmic Heating

where
a 5
jΔVj
2
d
c
σ
o
mv
4
2 U ð4:190Þ
b 5
d
c
jΔVj
2
σ
o
4
1 UT
N
ð4:191Þ
Integrating
Equation (4.189), we obtain
aT 1 b
aT
o
1 b
5 e
aπd
c
L
_
mc
p
ð4:192Þ
In the following example, we will use
Equation (4.192) to determine
the temperature of a liquid exiting an ohmic heater.
Example 4.32
A liquid food is being pumped through an ohmic heater at 0.5 kg/s. The
inside diameter of the heater pipe is 0.05 m and it is 3 m long. The specific
heat of the liquid food is 4000 J/kg 8C. The applied voltage is 15,000 V. The
overall heat transfer coefficient based on the inside pipe area is 100 W/m
2
8C.
The surrounding temperature of the air is 208C. The liquid food enters the
ohmic heater at 508C. Assume that the properties of the liquid food are similar
to 0.05 M Sodium Phosphate solution. Calculate the temperature at which the
liquid food exits.
Given
Flow rate 5 0.5 kg/s
Inside diameter of ohmic heater 5 0.05 m
Length of ohmic heater 5 3m
Specific heat capacity 5 4000 J/kg 8C
Voltage 5 15000 V
Overall heat transfer coefficient 5 100 W/m
2
8C
Surrounding air temperature 5 208C
Inlet temperature 5 508C
Approach
Using the given information, we will determine the voltage gradient, and
obtain electrical properties using
Table 4.6 for 0.05 M Sodium Phosphate.
Using Equation (4.192) we will calculate the liquid temperature at the exit of
the heater.
388 CHAPTER 4 Heat Transfer in Food Processing

Solution
1. Voltage gradient is obtained from the given information as
jΔVj5
15000
3
5 5000 V=m
2. From
Table 4.6, for 0.05 M Sodium Phosphate,
σ
o
5 0:162 S=m
mv 5 0:048(8 C
21
)
3. Using
Equations (4.190) and (4.191), we obtain a and b values as
follows:
a 5 2330 W=m
2
8C
b 5 52625 W=m
2
8C
4. Substituting a and b values in
Equation (4.192)
2330T 1 52625
2330 3 50 1 52625
5 e
π 3 2330 3 0:05 3 3
0:5 3 4000
Solving for the unknown temperature, T, we get
T 5 1038C
The temperature of the liquid food will increase from 50 to 1038C
when heated in the ohmic heater.
4.8 MICROWAVE HEATING
Electromagnetic radiation is classified by wavelength or frequency.
The electromagnetic spectrum between frequencies of 300 MHz and
300 GHz is represented by microwaves. Since microwaves are used in
radar, navigational equipment, and communication equipment, their
use is regulated by governmental agencies. In the United States, the
Federal Communications Commission (FCC) has set aside two fre-
quencies for industrial, scientific, and medical (ISM) apparatus in the
microwave range, namely 915 6 13 MHz, and 2450 6 50 MHz.
Similar frequencies are regulated worldwide through the International
Telecommunication Union (ITU).
3894.8 Microwave Heating

Microwaves have certain similarities to visible light. Microwaves can
be focused into beams. They can transmit through hollow tubes.
Depending on the dielectric properties of a material, they may be
reflected or absorbed by the material. Microwaves may also transmit
through materials without any absorption. Packaging materials such
as glass, ceramics, and most thermoplastic materials allow micro-
waves to pass through with little or no absorption. When traveling
from one material to another, microwaves may change direction,
similar to the bending of light rays when they pass from air to water.
In contrast to conventional heating systems, microwaves penetrate a
food, and heating extends within the entire food material. The rate of
heating is therefore more rapid. Note that microwaves generate heat
due to their interactions with the food materials. The microwave
radiation itself is nonionizing radiation, distinctly different from ion-
izing radiation such as X-rays and gamma rays. When foods are
exposed to microwave radiation, no known nonthermal effects are
produced in food material (
IFT, 1989; Mertens and Knorr, 1992).
The wavelength, frequency, and velocity of electromagnetic waves are
related by the following expression
λ 5 u=f
0
ð4:193Þ
where, λ is wavelength in meters; f
0
is frequency in hertz; u is the
speed of light (3 3 10
8
m/s).
Using
Equation (4.193), the wavelengths of the permitted ISM fre-
quencies in the microwave range can be calculated as
λ
915
5
3 3 10
8
ðm=sÞ
915 3 10
6
ð1=sÞ
5 0:328 m
and
λ
2450
5
3 3 10
8
ðm=sÞ
2450 3 10
6
ð1=sÞ
5 0:122 m
4.8.1 Mechanisms of Microwave Heating
The absorption of microwaves by a dielectric material results in the
microwaves giving up their energy to the material, with a consequen-
tial rise in temperature. The two important mechanisms that explain
heat generation in a material placed in a microwave field are ionic
polarization and dipole rotation.
390 CHAPTER 4 Heat Transfer in Food Processing

4.8.1.1 Ionic Polarization
When an electrical field is applied to food solutions containing ions,
the ions move at an accelerated pace due to their inherent charge.
The resulting collisions between the ions cause the conversion of
kinetic energy of the moving ions into thermal energy. A solution
with a high concentration of ions would have more frequent ionic
collisions and therefore exhibit an increase in temperature.
4.8.1.2 Dipole Rotation
Food materials contain polar molecules such as water. These mole-
cules generally have a random orientation. However, when an electri-
cal field is applied, the molecules orient themselves according to the
polarity of the field. In a microwave field, the polarity alternates
rapidly (e.g., at the microwave frequency of 2450 MHz, the polarity
changes at 2.45 3 10
9
cycles per second). The polar molecules rotate
to maintain alignment with the rapidly changing polarity (
Fig. 4.44).
Such rotation of molecules leads to friction with the surrounding
medium, and heat is generated. With increasing temperatures, the
molecules try to align more rapidly with the applied field. Several
factors influence the microwave heating of a material, including the
size, shape, state (e.g., water or ice), and properties of the material,
and the processing equipment.
4.8.2 Dielectric Properties
In microwave processing, we are concerned with the electrical proper-
ties of the material being heated. The important electrical properties
are the relative dielectric constant ε
0
and the relative dielectric loss εv.
ve
ve
ve
ve
w
■ Figure 4.44 Movement of a dipole in an electrical field. (From Decareau and Peterson, 1986)
3914.8 Microwave Heating
The term loss implies the conversion (or “loss”) of electrical energy
into heat, and the term relative means relative to free space.
The relative dielectric constant ε
0
expresses the ability of the material
to store electrical energy, and the relative dielectric loss εv denotes the
ability of the material to dissipate the electrical energy. These proper-
ties provide an indication of the electrical insulating ability of the
material. Foods are in fact very poor insulators; therefore, they gener-
ally absorb a large fraction of the energy when placed in a microwave
field, resulting in instantaneous heating (
Mudgett, 1986). The dielec-
tric loss factor for the material, εv, which expresses the degree to
which an externally applied electrical field will be converted to heat,
is given by
εv 5 ε
0
tan δ ð4:194Þ
The loss tangent, tan δ, provides an indication of how well the mate-
rial can be penetrated by an electrical field and how it dissipates elec-
trical energy as heat.
4.8.3 Conversion of Microwave Energy into Heat
Microwave energy in itself is not thermal energy; rather, heating is a
consequence of the interactions between microwave energy and a
dielectric material. The conversion of the microwave energy to heat
can be approximated with the following equation (
Copson, 1975;
Decareau and Peterson, 1986):
P
D
5 55:61 3 10
214
E
2
f
0
ε
0
tan δ ð4:195Þ
where P
D
is the power dissipation (W/cm
3
); E is electrical field
strength (V/cm); f
0
is frequency (Hz); ε
0
is the relative dielectric con-
stant, and tan δ is the loss tangent.
In
Equation (4.195), the dielectric constant ε
0
and the loss tangent
tan δ are the properties of the material, and the electrical field
strength E and frequency f
0
represent the energy source. Thus, there is
a direct relationship between the material being heated and the
microwave system providing the energy for heating. It is evident in
Equation (4.195) that increasing the electrical field strength has a
dramatic effect on the power density, since the relationship involves
a square term.
The governing heat transfer equation presented earlier in this chapter,
Equation (4.149), can be modified for use in predicting heat transfer
392 CHAPTER 4 Heat Transfer in Food Processing

in a material placed in a microwave field. A heat generation term qw
equivalent to the power dissipation obtained from Equation (4.195)
is introduced in Equation (4.149). Thus, for transient heat transfer in
an infinite slab, we can obtain the following expression for a one-
dimensional case:
@
2
T
@x
2
1
qv
0
k
5
ρc
p
@T
k@t
ð4:196Þ
Numerical techniques are used to solve the preceding equation
(
Mudgett, 1986).
4.8.4 Penetration Depth of Microwaves
The energy transfer between microwaves and the material exposed to
the microwave field is influenced by the electrical properties of the
material. The distribution of energy within a material is determined
by the attenuation factor α
0
.
The attenuation factor α
0
is calculated from the values for the loss
tangent, relative dielectric constant, and the frequency of the micro-
wave field:
α
0
5
2π
λ
ε
0
2
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
11tan
2
δ21
p
1=2
ð4:197Þ
The penetration of an electrical field can be calculated from the atten-
uation factor. As shown by
von Hippel (1954), the depth Z below
the surface of the material at which the electrical field strength is 1/e
that of the electrical field in the free space, is the inverse of the atten-
uation factor. Thus,
Z 5
λ
2π
2
ε
0
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
11tan
2
δ
p
21
2
4
3
5
1=2
ð4:198Þ
Noting that frequency and wavelength are inversely related, it is evi-
dent from
Equation (4.198) that microwave energy at 915 MHz
penetrates more deeply than at 2450 MHz.
In addition to the foregoing description of penetration of a micro-
wave field in a material, the depth of penetration for microwave
3934.8 Microwave Heating

power is usually described in two different ways. First, the penetra-
tion depth is the distance from the surface of a dielectric material
where the incident power is decreased to 1/e of the incident power.
Lambert’s expression for power absorption gives
P 5 P
0
e
22α
0
d
ð4:199Þ
where P
0
is the incident power, P is the power at the penetration
depth, d is the penetration depth, and α
0
is the attenuation factor.
If the power is reduced to 1/e of the incident power at depth d, we
have P/P
0
5 1/e. Therefore, from Equation (4.199), 2α
0
d 5 1 and
d 5 1/2α
0
.
The second definition of the penetration depth is stated in terms of
half-power depth (i.e., one-half of the incident power). Therefore, at
half-power depth, P/P
0
5 1/2. From Equation (4.199), e
22
α
0
d 5 1/2,
and solving for d we get d 5 0.347/α
0
.
Example 4.33
In a paper on microwave properties, Mudgett (1986) provides data on
dielectric constants and loss tangents for raw potatoes. For a microwave
frequency of 2450 MHz and at 208C, the dielectric constant is 64 and the
loss tangent is 0.23. Determine the attenuation factor, the field penetration
depth, and the depth below the surface of a potato at which the micro-
wave power is reduced to one-half of the incident power.
Approach
We will use Equations (4.197) and (4.198) to determine the attenuation factor
and the penetration depth for the microwave field, respectively. The distance
from the surface of the material at which the power is reduced to one-half of the
incident power will be calculated using modifications of Equation (4.199).
Solution
1. From Equation (4.197):
α
0
5
2π 3 2450 3 10
6
(1=s)
3 3 10
8
(m=s) 3 100(cm=m)
64
2
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
11(0:23)
2
p
21
2
4
3
5
1=2
α
0
5 0:469 (cm
21
)
2. The penetration depth for the microwave field is the inverse of α
0
as
seen in
Equation (4.198). Therefore,
Field penetration depth 5 Z 5 1=α
0
5 1= 0:469 5 2:13 cm
394 CHAPTER 4 Heat Transfer in Food Processing

3. To obtain the half-power depth of penetration, we use the modifica-
tion of Lambert’s expression,
Equation (4.199) , and solve for d:
d 5
0:347
α
0
5
0:347
0:469
5 0:74 cm
4. The half-power depth for potatoes at 2450 MHz and 208C is calculated
to be 0.74 cm.
4.8.5 Microwave Oven
A typical microwave oven consists of the following major compo-
nents (
Fig. 4.45).
I Power supply. The purpose of the power supply is to draw
electrical power from the line and convert it to the high voltage
required by the magnetron. The magnetron usually requires
several thousand volts of direct current.
I Magnetron or power tube. The magnetron is an oscillator capa-
ble of converting the power supplied into microwave energy.
The magnetron emits high-frequency radiant energy. The polar-
ity of the emitted radiation changes between negative and pos-
itive at high frequencies (e.g., 2.45 3 10
9
cycles per second for
a magnetron operating at a frequency of 2450 MHz, the most
common frequency used for domestic ovens).
I Wave guide or transmission section. The wave guide propa-
gates, radiates, or transfers the generated energy from the mag-
netron to the oven cavity. In a domestic oven , the wave guide
is a few centimeters long, whereas in industrial units it can be
a few meters long. The energy loss in the wave guide is usually
quite small.
I Stirrer. The stirrer is usually a fan-shaped distributor that
rotates and scatters the transmitted energy throughout the
oven. The stirrer disturbs the standing wave patterns, thus
allowing better energy distribution in the oven cavity. This is
particularly important when heating nonhomogenous materi-
als like foods.
I Oven cavity or oven. The oven cavity encloses the food to be
heated within the metallic walls. The distributed energy from
the stirrer is reflected by the walls and intercepted by the food
from many directions with more or less uniform energy density.
The energy impinging on the food is absorbed and converted
into heat. The size of the oven cavity is influenced by the
Wave guide
Stirrer
Magnetron
Oven cavity
w
■ Figure 4.45 Major components of a
microwave oven.
3954.8 Microwave Heating

wavelength. The length of the cavity wall should be greater than
one-half the wavelength and any multiple of a half-wave in the
direction of the wave propagation. The wavelength at
2450 MHz frequency was calculated earlier to be 12.2 cm; there-
fore, the oven cavity wall must be greater than 6.1 cm. The oven
cavity door includes safety controls and seals to retain the
microwave energy within the oven during the heating process.
4.8.6 Microwave Heating of Foods
Heating of foods in a microwave field offers several advantages over
more conventional methods of heating. The following are some of the
important features of microwave heating that merit consideration.
4.8.6.1 Speed of Heating
The speed of heating of a dielectric material is directly proportional
to the power output of the microwave system. In industrial units, the
typical power output may range from 5 to 100 kW. Although high
speed of heating is attainable in the microwave field, many food
applications require good control of the rate at which the foods are
heated. Very high-speed heating may not allow desirable physical
and biochemical reactions to occur. The speed of heating in a micro-
wave is governed by controlling the power output. The power
required for heating is also proportional to the mass of the product.
4.8.6.2 Frozen Foods
The heating behavior of frozen foods is markedly influenced by the
different dielectric properties of ice and water (
Table 4.7). Due to its
low dielectric loss factor, ice is more transparent to microwaves than
water. Thus, ice does not heat as well as water. Therefore, when using
microwaves to temper frozen foods, care is taken to keep the temper-
ature of the frozen food just below the freezing point. If the ice
Table 4.7 Dielectric Properties of Water and Ice at 2450 MHz
Relative
dielectric
constant, ε
0
Relative
dielectric loss
constant, εv
Loss
tangent,
tan δ
Ice 3.2 0.0029 0.0009
Water (at 258C) 78 12.48 0.16
Source: Schiffman (1986)
396 CHAPTER 4 Heat Transfer in Food Processing
melts, runaway heating may occur because the water will heat much
faster due to the high dielectric loss factor of water.
4.8.6.3 Shape and Density of the Material
The shape of the food material is important in obtaining uniformity
of heating. Non-uniform shapes result in local heating; similarly,
sharp edges and corners cause non-uniform heating.
4.8.6.4 Food Composition
The composition of the food material affects how it heats in the
microwave field. The moisture content of food directly affects the
amount of microwave absorption. A higher amount of water in a
food increases the dielectric loss factor εv. In the case of foods of low
moisture content, the influence of specific heat on the heating pro-
cess is more pronounced than that of the dielectric loss factor.
Therefore, due to their low specific heat , foods with low moisture
content also heat at acceptable rates in microwaves. If the food mate-
rial is highly porous with a significant amount of air, then due to
low thermal conductivity of air, the material will act as a good insu-
lator and show good heating rates in microwaves.
Another compositional factor that has a marked influence on heating
rates in microwaves is the presence of salt. As stated previously, an
increased concentration of ions promotes heating in microwaves. Thus,
increasing the salt level in foods increases the rate of heating. Although
oil has a much lower dielectric loss factor than water , oil has a specific
heat less than half that of water. Since a product with high oil content
will require much less heat to increase in temperature, the influence of
specific heat becomes the overriding factor, and oil exhibits a much
higher rate of heating than water (
Ohlsson, 1983). More details on these
and other issues important during the microwave heating of foods are
elaborated by Schiffman (1986) and by Decareau and Peterson (1986).
The industrial applications of microwave processing of foods are
mostly for tempering of frozen foods (increasing the temperature of
frozen foods to 24to2208C), such as meat, fish, butter, and berries;
for drying of pasta, instant tea, herbs, mushrooms , fish protein, bread
crumbs, onions, rice cakes, seaweed, snack foods, and egg yolk; for
cooking of bacon, meat patties, and potatoes; for vacuum drying of
citrus juices, grains, and seeds; for freeze-drying of meat, vegetables,
and fruits; for pasteurization and sterilization of prepared foods; for
baking of bread and doughnuts; and for roasting of nuts, coffee
beans, and cocoa beans (
Decareau, 1992; Giese, 1992 ).
3974.8 Microwave Heating
PROBLEMS
4.1 Calculate the rate of heat transfer per unit area through a
200-mm-thick concrete wall when the temperatures are 208C
and 58C on the two surfaces, respectively. The thermal
conductivity of concrete is 0.935 W/(m 8C).
4.2 A side wall of a storage room is 3 m high, 10 m wide, and
25 cm thick. The thermal conductivity is k 5 0.85 W/(m 8C).
If, during the day, the inner surface temperature of the wall is
228C and the outside surface temperature is 48C:
a. Using the Thermal Resistance Concept, calculate the
resistance to heat transfer for the wall.
b. Calculate the rate of heat transfer through the wall,
assuming steady-state conditions.
4.3 An experiment was conducted to measure thermal
conductivity of a formulated food. The measurement was
made by using a large plane plate of the food material, which
was 5 mm thick. It was found, under steady-state conditions,
that when a temperature difference of 358C was maintained
between the two surfaces of the plate, a heat-transfer rate per
unit area of 4700 W/m
2
was measured near the center of
either surface. Calculate the thermal conductivity of the
product, and list two assumptions used in obtaining the
result.
4.4 Estimate the thermal conductivity of applesauce at 358C.
(Water content 5 78.8% wet basis).
4.5 A 20-cm-diameter cooking pan is placed on a stove. The pan
is made of steel (k 5 15 W/[m 8C]), and it contains water
boiling at 988C. The bottom plate of the pan is 0.4 cm thick.
The inside surface temperature of the bottom plate, in
contact with water, is 1058C.
a. If the rate of heat transfer through the bottom plate is
450 W, determine the outside surface temperature of the
bottom plate exposed to the heating stove.
b. Determine the convective heat-transfer coefficient for
boiling water.
4.6 A 10-m-long pipe has an inside radius of 70 mm, an outside
radius of 80 mm, and is made of stainless steel (k 5 15 W/
[m 8C]). Its inside surface is held at 1508C, and its outside
surface is at 308C. There is no heat generation, and
398 CHAPTER 4 Heat Transfer in Food Processing

steady-state conditions hold. Compute the rate at which heat
is being transferred across the pipe wall.
4.7 In a multilayered rectangular wall, the thermal resistance of
the first layer is 0.0058C/W, the resistance of the second layer
is 0.28C/W, and for the third layer it is 0.18C/W. The overall
temperature gradient in the multilayered wall from one side
to another is 708C.
a. Determine the heat flux through the wall.
b. If the thermal resistance of the second layer is doubled
to 0.48C/W, what will be its influence in % on the heat
flux, assuming the temperature gradient remains the
same?
4.8 A plain piece of insulation board is used to reduce the heat
loss from a hot furnace wall into the room. One surface of
the board is at 1008C and the other surface is at 208C. It is
desired to keep the heat loss down to 120 W/m
2
of the
insulation board. If the thermal conductivity of the board is
0.05 W/(m 8C), calculate the required thickness of the board.
4.9 Consider an ice chest with the following dimensions:
length 5 50 cm, width 5 40 cm, and height 5 30 cm, made of
a 3-cm-thick insulating material (k 5 0.033 W/[m 8C]). The
chest is filled with 30 kg of ice at 08C. The inner wall surface
temperature of the ice chest is assumed to be constant at
08C. The latent heat of fusion of ice is 333.2 kJ/kg. The
outside wall surface temperature of the chest is assumed to
remain constant at 258C. How long would it take to
completely melt the ice? Assume negligible heat transfer
through the bottom surface.
*4.10 Steam with 80% quality is being used to heat a 40% total
solids tomato purée as it flows through a steam injection
heater at a rate of 400 kg/h. The steam is generated at
169.06 kPa and is flowing to the heater at a rate of 50 kg/h.
If the specific heat of the product is 3.2 kJ/(kg K), determine
the temperature of the product leaving the heater when the
initial temperature is 508C. Determine the total solids
content of the product after heating. Assume the specific heat
of the heated purée is 3.5 kJ/(kg 8C).
* Indicates an advanced level of difficulty in solving.
399Problems

4.11 A stainless steel pipe (k 5 15 W/[m 8C]) with a 2.5-cm inner
diameter and 5-cm outer diameter is being used to convey
high-pressure steam. The pipe is covered with a 5-cm layer of
insulation (k 5 0.18 W/[m 8C]). The inside steel pipe surface
temperature is 3008C, and the outside insulation surface
temperature is 908C.
a. Determine the rate of heat transfer per meter length of
the pipe.
b. The insulation selected for the purpose has a melting
temperature of 2208C. Should you be concerned
about the integrity of the insulation for the listed
conditions?
4.12 Air at 258C blows over a heated steel plate with its surface
maintained at 2008C. The plate is 50 3 40 cm and 2.5 cm
thick. The convective heat-transfer coefficient at the top
surface is 20 W/(m
2
K). The thermal conductivity of steel is
45 W/(m K). Calculate the heat loss per hour from the top
surface of the plate.
4.13 A liquid food is being heated in a tubular heat exchanger.
The inside pipe wall temperature is 1108C. The internal
diameter of the pipe is 30 mm. The product flows at
0.5 kg/s. If the initial temperature of the product is 78C,
compute the convective heat-transfer coefficient. The thermal
properties of the product are the following: specific
heat 5 3.7 kJ/(kg 8C), thermal conductivity 5 0.6 W/(m 8C),
product viscosity 5 500 3 10
26
Pa s, density 5 1000 kg/m
2
,
product viscosity at 1108C 5 410 3 10
26
Pa s.
4.14 Compute the convective heat-transfer coefficient for natural
convection from a vertical, 100 mm outside diameter, 0.5 m
long, stainless-steel pipe. The surface temperature of the
uninsulated pipe is 1458C, and the air temperature is 408C.
*4.15 A 30 m long pipe with an external diameter of 75 mm is
being used to convey steam at a rate of 1000 kg/h. The steam
pressure is 198.53 kPa. The steam enters the pipe with a
dryness fraction of 0.98 and must leave the other end of the
pipe with a minimum dryness fraction of 0.95. Insulation
with a thermal conductivity of 0.2 W/(m K) is available.
Determine the minimum thickness of insulation necessary.
* Indicates an advanced level of difficulty in solving.
400 CHAPTER 4 Heat Transfer in Food Processing

The outside surface temperature of insulation is assumed to
be 258C. Neglect the conductive resistance of the pipe
material and assume no pressure drop across the pipe.
4.16 Estimate the convective heat-transfer coefficient for natural
convection from a horizontal steam pipe. The outside surface
temperature of the insulated pipe is 808C. The surrounding
air temperature is 258C. The outside diameter of the
insulated pipe is 10 cm.
4.17 A vertical cylindrical container is being cooled in ambient air
at 258C with no air circulation. If the initial temperature of
the container surface is 1008C, compute the surface heat-
transfer coefficient due to natural convection during the
initial cooling period. The diameter of the container is 1 m,
and it is 2 m high.
4.18 Water at a flow rate of 1 kg/s is flowing in a pipe of internal
diameter 5 cm. If the inside pipe surface temperature is 908C
and mean bulk water temperature is 508C, compute the
convective heat-transfer coefficient.
4.19 A blower is used to move air through a pipe at a rate of
0.01 kg/s. The inside pipe surface temperature is 408C. The
bulk temperature of the air reduces from 808Cto608Casit
passes through a 5-m section of the pipe. The inside
diameter of the pipe is 2 cm. Estimate the convective heat-
transfer coefficient using the appropriate dimensionless
correlation.
4.20 Estimate the convective heat-transfer coefficient on the
outside of oranges (external diameter 5 5 cm) when
submerged in a stream of chilled water pumped around the
orange. The velocity of water around an orange is 0.1 m/s.
The surface temperature of the orange is 208C and the bulk
water temperature is 08C.
*4.21 A flat wall is exposed to an environmental temperature of
388C. The wall is covered with a layer of insulation 2.5 cm
thick whose thermal conductivity is 1.8 W/(m K), and the
temperature of the wall on the inside of the insulation is
3208C. The wall loses heat to the environment by
convection. Compute the value of the convection
* Indicates an advanced level of difficulty in solving.
401Problems
heat-transfer coefficient that must be maintained on the
outer surface of the insulation to ensure that the outer
surface temperature does not exceed 408C.
4.22 Steam at 1508C flows inside a pipe that has an inside radius
of 50 mm and an outside radius of 55 mm. The convective
heat-transfer coefficient between the steam and the inside
pipe wall is 2500 W/(m
2
8C). The outside surface of the pipe
is exposed to ambient air at 208C with a convective heat-
transfer coefficient of 10 W/(m
2
8C). Assuming steady state
and no heat generation, calculate the rate of heat transfer per
meter from the steam to the air across the pipe. Assume
thermal conductivity of stainless steel is 15 W/(m 8C.)
4.23 The outside wall of a refrigerated storage room is 10 m long
and 3 m high and is constructed with 100-mm concrete blocks
(k 5 0.935 W/[m 8C]) and 10 cm of fiber insulation board
(k 5 0.048 W/[m 8C]). The inside of the room is at 2108C
and the convective heat-transfer coefficient is 40 W/(m
2
K);
the outside temperature is 308C with a convective heat-transfer
coefficient of 10 W/(m
2
K) on the outside wall surface.
Calculate the overall heat-transfer coefficient.
4.24 In a food processing plant, a steel pipe (thermal
conductivity 5 17 W/m 8C, internal diameter 5 5 cm;
thickness 5 3 mm) is being used to transport a liquid food.
The inside surface temperature of the pipe is at 958C. A
4-cm-thick insulation (thermal conductivity 5 0.03 W/m 8C)
is wrapped around the pipe. The outside surface temperature
of the insulation is 308C. Calculate the rate of heat transfer
per unit length of the pipe.
4.25 A walk-in freezer of 4 m width, 6 m length, and 3 m height
is being built. The walls and ceiling contain 1.7-mm-thick
stainless steel (k 5 15 W/[m 8C]), 10-cm-thick foam
insulation (k 5 0.036 W/[m 8C]), and some thickness of
corkboard (k 5 0.043 W/[m 8C]) to be established, and
1.27 cm-thickness wood siding (k 5 0.104 W/[m 8C]). The
inside of the freezer is maintained at 2408C. Ambient air
outside the freezer is at 328C. The convective heat-transfer
coefficient is 5 W/(m
2
K) on the wood side of the wall and
2 W/(m
2
K) on the steel side. If the outside air has a dew
point of 298C, calculate the thickness of corkboard
insulation that would prevent condensation of moisture on
402 CHAPTER 4 Heat Transfer in Food Processing

the outside wall of the freezer. Calculate the rate of heat
transfer through the walls and ceiling of this freezer.
4.26 A liquid food is being conveyed through an uninsulated pipe
at 908C. The product flow rate is 0.25 kg/s and has a density
of 1000 kg/m
3
, specific heat of 4 kJ/(kg K), viscosity of
8 3 10
26
Pa s, and thermal conductivity of 0.55 W/(m K).
Assume the viscosity correction is negligible. The internal
pipe diameter is 20 mm with 3 mm thickness made of
stainless steel (k 5 15 W/[m 8C]). The outside temperature is
158C. If the outside convective heat-transfer coefficient is
18 W/(m
2
K), calculate the steady-state heat loss from the
product per unit length of pipe.
*4.27 A liquid food is being pumped in a 1-cm-thick steel pipe.
The inside diameter of the pipe is 5 cm. The bulk
temperature of liquid food is 908C. The inside pipe surface
temperature is 808C. The surface heat-transfer coefficient
inside the pipe is 15 W/(m
2
K). The pipe has a 2-cm-thick
insulation. The outside bulk air temperature is 208C. The
surface heat-transfer coefficient on the outside of insulation
is 3 W/(m
2
K).
a. Calculate the insulation surface temperature exposed to
the outside.
b. If the pipe length is doubled, how would it influence the
insulation surface temperature? Discuss.
4.28 For a metal pipe used to pump tomato paste, the overall heat-
transfer coefficient based on internal area is 2 W/(m
2
K). The
inside diameter of the pipe is 5 cm. The pipe is 2 cm thick.
The thermal conductivity of the metal is 20 W/(m K).
Calculate the outer convective heat-transfer coefficient. The
inside convective heat-transfer coefficient is 5 W/(m
2
K).
4.29 A cold-storage room is maintained at 2188C. The internal
dimensions of the room are 5 m 3 5m3 3 m high. Each wall,
ceiling, and floor consists of an inner layer of 2.5 cm thick
wood with 7 cm thick insulation and an 11 cm brick layer on
the outside. The thermal conductivities of respective materials
are wood 0.104 W/(m K), glass fiber 0.04 W/(m K), and
brick 0.69 W/(m K). The convective heat-transfer coefficient
for wood to still air is 2.5 W/(m
2
K), and from moving air
* Indicates an advanced level of difficulty in solving.
403Problems

to brick is 4 W/(m
2
K). The outside ambient temperature
is 258C. Determine:
a. The overall heat-transfer coefficient.
b. The temperature of the exposed surfaces.
c. The temperatures of the interfaces.
4.30 Steam at 169.60 kPa is condensed inside a pipe (internal
diameter 5 7cm, thickness 5 3 mm). The inside and outside
convective heat-transfer coefficients are 1000 and 10 W/
(m
2
K), respectively. The thermal conductivity of the pipe is
45 W/(m K). Assume that all thermal resistances are based
on the outside diameter of the pipe, and determine the
following:
a. Percentage resistance offered by the pipe, by the steam,
and by the outside.
b. The outer surface temperature of the pipe if the
temperature of the air surrounding the pipe is 258C.
4.31 A steel pipe (outside diameter 100 mm) is covered with two
layers of insulation. The inside layer, 40 mm thick, has a
thermal conductivity of 0.07 W/(m K). The outside layer,
20 mm thick, has a thermal conductivity of 0.15 W/(m K).
The pipe is used to convey steam at a pressure of 700 kPa.
The outside temperature of insulation is 248C. If the pipe is
10 m long, determine the following, assuming the resistance
to conductive heat transfer in steel pipe and convective
resistance on the steam side are negligible:
a. The heat loss per hour.
b. The interface temperature of insulation.
*4.32 A 1-cm-thick steel pipe, 1 m long, with an internal diameter of
5 cm is covered with 4-cm-thick insulation. The inside wall
temperature of the steel pipe is 1008C. The ambient
temperature around the insulated pipe is 208C. The convective
heat-transfer coefficient on the outer insulated surface is
50 W/(m
2
K). Calculate the temperature at the steel insulation
interface. The thermal conductivity of steel is 54 W/(m K), and
the thermal conductivity of insulation is 0.04 W/(m K).
4.33 Calculate the overall heat-transfer coefficient of a steel pipe
based on the inside area. The inside diameter of the pipe is
10 cm, and the pipe is 2 cm thick. The inside convective
* Indicates an advanced level of difficulty in solving.
404 CHAPTER 4 Heat Transfer in Food Processing

heat-transfer coefficient is 350 W/(m
2
8C), the outside
convective heat-transfer coefficient is 25 W/(m
2
8C), the
thermal conductivity of the steel pipe is 15 W/(m 8C). If the
pipe is used to convey steam at a bulk temperature of 1108C
and the outside ambient temperature is 208C, determine the
rate of heat transfer from the pipe.
*4.34 Saturated refrigerant (Freon, R-12) at 2408C flows through a
copper tube of 20 mm inside diameter and wall thickness of
2 mm. The copper tube is covered with 40-mm-thick
insulation (k 5 0.02 W/[m K]). Determine the heat gain per
meter of the pipe. The internal and external convective heat-
transfer coefficients are 500 and 5 W/(m
2
K), respectively.
The ambient air temperature is 258C. Compare the amount
of refrigerant vaporized per hour per meter length of pipe for
insulated versus uninsulated pipe. The latent heat of the
refrigerant at 2408C is 1390 kJ/kg.
4.35 To cool hot edible oil, an engineer has suggested that the oil
be pumped through a pipe submerged in a nearby lake. The
pipe (external diameter 5 15 cm) will be located in a
horizontal direction. The average outside surface temperature
of the pipe will be 1308C. The surrounding water
temperature may be assumed to be constant at 108C. The
pipe is 100 m long. Assume there is no movement of water.
a. Estimate the convective heat-transfer coefficient from the
outside pipe surface into water.
b. Determine the rate of heat transfer from the pipe into
water.
4.36 In a concurrent-flow tubular heat exchanger, a liquid food,
flowing in the inner pipe, is heated from 20 to 408C. In the
outer pipe the heating medium (water) cools from 90 to
508C. The overall heat-transfer coefficient based on the
inside diameter is 2000 W/(m
2
8C). The inside diameter is
5 cm and length of the heat exchanger is 10 m. The average
specific heat of water is 4.181 kJ/(kg 8C). Calculate the mass
flow rate of water in the outer pipe.
*4.37 A countercurrent heat exchanger is being used to heat a
liquid food from 15 to 708C. The heat exchanger has a
23-mm internal diameter and 10 m length with an overall
* Indicates an advanced level of difficulty in solving.
405Problems
heat-transfer coefficient, based on the inside area, of
2000 W/(m
2
K). Water, the heating medium, enters the heat
exchanger at 958C, and leaves at 858C. Determine the flow
rates for product and water that will provide the conditions
described. Use specific heats of 3.7 kJ/(kg K) for product and
4.18 kJ/(kg K) for water.
4.38 A 10-m-long countercurrent-flow heat exchanger is being used
to heat a liquid food from 20 to 808C. The heating medium is
oil, which enters the heat exchanger at 1508C and exits at 608C.
The specific heat of the liquid food is 3.9 kJ/(kg K). The overall
heat-transfer coefficient based on the inside area is 1000 W/
(m
2
K). The inner diameter of the inside pipe is 7 cm.
a. Estimate the flow rate of the liquid food.
b. Determine the flow rate of the liquid food if the heat
exchanger is operated in a concurrent-flow mode for the
same conditions of temperatures at the inlet and exit
from the heat exchanger.
4.39 Calculate the radiative heat gain in watts by a loaf of bread
at a surface temperature of 1008C. The surrounding oven
surface temperature is 10008C. The total surface area of the
bread is 0.15 m
2
and the emissivity of the bread surface is
0.80. Assume the oven is a blackbody radiator.
4.40 It is desired to predict the temperature after 30 min at the
geometric center of a cylindrical can containing a model food.
The dimensions of the can are 5 cm diameter and 3 cm
height. The thermal conductivity of the food is 0.5 W/(m 8C),
specific heat 5 3.9 kJ/(kg 8C), and density 5 950 kg/m
3
.
There is a negligible surface resistance to heat transfer. The
surrounding medium temperature is 1008C and the uniform
initial temperature of food is 208C.
4.41 An 8-m
3
batch of oil with specific heat of 2 kJ/(kg K) and
density of 850 kg/m
3
is being heated in a steam-jacketed,
agitated vessel with 1.5 m
2
of heating surface. The convective
heat-transfer coefficient on the oil side is 500 W/(m
2
K), and
10,000 W/(m
2
K) on the steam side. If the steam
temperature is 1308C and the initial temperature is 208C,
estimate the oil temperature after 10 min.
4.42 Determine if a tubular heat exchanger can operate under the
following conditions: Fluid A enters the heat exchanger at
406 CHAPTER 4 Heat Transfer in Food Processing
1208C and exits at 408C; fluid B enters the heat exchanger at
308C and exits at 708C. Calculate the log mean temperature
difference.
4.43 Milk (c
p
5 3.9 kJ/[kg K]) is cooled in a countercurrent flow
heat exchanger at a rate of 1.5 kg/s from 708Cto308C.
Cooling is done by using chilled water available at 58C with
a flow rate of 2 kg/s. The inside diameter of the inner pipe is
2 cm. The overall heat transfer coefficient is 500 W/(m
2
8C).
Determine the length of the heat exchanger.
4.44 In a double-pipe heat exchanger, made of stainless steel
(k 5 15 W/[m 8C]), the inside pipe has an inner diameter of
2 cm and an outside diameter of 2.5 cm. The outer shell has an
inner diameter of 4 cm. The convective heat transfer coefficient
on the inside surface of the inner pipe is 550 W/(m
2
8C),
whereas on the outside surface of the inner pipe it is
900 W/(m
2
8C). Over continuous use of the heat exchanger,
fouling (depositing of solids from the liquids on the pipe
surfaces) causes additional resistance to heat transfer. It is
determined that the resistance to heat transfer due to fouling
on the inside surface of the inner pipe is 0.00038 m
2
8C/W, and
on the outside surface of the inner pipe is 0.0002 m
2
8C/W .
Calculate:
a. The total thermal resistance of the heat exchanger per unit
length.
b. The overall heat transfer coefficients U
i
and U
o
based on
the inside and outside area of the inner pipe, respectively.
4.45 Water at 58C is being used to cool apples from an initial
temperature of 208 to 88C. The water flow over the surface of
the apple creates a convective heat-transfer coefficient of
10 W/(m
2
K). Assume the apple can be described as a sphere
with an 8-cm diameter and the geometric center is to be
reduced to 88C. The apple properties include thermal
conductivity of 0.4 W/(m K), specific heat of 3.8 kJ/(kg K),
and density of 960 kg/m
3
. Determine the time that the
apples must be exposed to the water.
4.46 A liquid food with density of 1025 kg/m
3
and specific heat
of 3.77 kJ/(kg K) is being heated in a can with 8.5 cm
diameter and 10.5 cm height. The heating will occur in a
retort with temperature at 1158C and convective heat-transfer
coefficients of 50 W/(m
2
K) on the inside of the can and
407Problems

5000 W/(m
2
K) on the outside surface. Determine the
product temperature after 10 minutes if the initial
temperature is 708C. Assume perfect mixing in the can.
4.47 Create a spreadsheet for Example 4.21. Keeping all
conditions the same as given in the example, determine the
exit temperatures of hot water and apple juice if the number
of plates used in the heat exchanger are 21 or 31.
*4.48 A conduction-cooling food product with density of
1000 kg/m
3
, specific heat of 4 kJ/(kg K), and thermal
conductivity of 0.4 W/(m K) has been heated to 808C. The
cooling of the product in a 10 cm high and 8 cm diameter
can is accomplished using cold water with a convective heat-
transfer coefficient of 10 W/(m
2
K) on the can surface.
Determine the water temperature required to reduce the
product temperature at geometric center to 508Cin7h.
Neglect conductive heat resistance through the can wall.
4.49 Cooked mashed potato is cooled on trays in a chilling unit
with refrigerated air at 28C blown over the product surface at
high velocity. The depth of product is 30 mm and the initial
temperature is 958C. The product has a thermal conductivity
of 0.37 W/(m K), specific heat of 3.7 kJ/(kg K), and density
of 1000 kg/m
3
. Assuming negligible resistance to heat
transfer at the surface, calculate product temperature at the
center after 30 minutes.
4.50 Program Example 4.9 on a spreadsheet. Determine the
interfacial temperatures if the following thickness of
insulation are used:
a. 2cm
b. 4cm
c. 6cm
d. 8cm
e. 10 cm
4.51 A liquid food at a flow rate of 0.3 kg/s enters a countercurrent
flow double-pipe heat exchanger at 228C. In the annular
section, hot water at 808C enters at a flow rate of 1.2 kg/s. The
average specific heat of water is 4.18 kJ/(kg 8C). The average
overall heat transfer coefficient based on the inside area
* Indicates an advanced level of difficulty in solving.
408 CHAPTER 4 Heat Transfer in Food Processing
is 500 W/(m
2
8C). The diameter of the inner pipe is 7 cm, and
length is 10 m. Assume steady state conditions. The specific
heat of liquid food is assumed to be 4.1 kJ/[kg 8C]. Calculate
the exit temperature of liquid food and water.
4.52 A pure copper sphere of radius 1 cm is dropped into an
agitated oil bath that has a uniform temperature of 1308C.
The initial temperature of the copper sphere is 208C. Using a
spreadsheet predict the internal temperature of the sphere at
5-min intervals until it reaches 1288C for three different
convective heat-transfer coefficients: 5, 10, and 100 W/(m
2
8C),
respectively. Plot your results as temperature versus time.
4.53 Heated water with a bulk temperature of 908Cisbeing
pumped at a rate of 0.1 kg/s in a metal pipe placed
horizontally in ambient air. The pipe has an internal diameter
of 2.5 cm and it is 1 cm thick. The inside pipe surface
temperature is 858C. The outside surface of the pipe is at 808C
and exposed to the air. The bulk temperature of the air is 208C.
a. Determine the convective heat-transfer coefficient for
water inside the pipe.
b. Determine the convective heat-transfer coefficient for air
outside the pipe.
c. It is desired to double the convective heat-transfer
coefficient inside the pipe. What operating conditions
should be changed? By how much?
4.54 A solid food is being cooled in a cylindrical can of dimensions
12 cm diameter and 3 cm thickness. The cooling medium is
cold water at 28C. The initial temperature of the solid food is
958C. The convective heat-transfer coefficient is 200 W/(m
2
8C).
a. Determine the temperature at the geometric center after
3 h. The thermal properties of the solid food are
k 5 0.36 W/(m 8C), density of 950 kg/m
3
, and specific
heat of 3.9 kJ/(kg 8C).
b. Is it reasonable to assume the cylindrical can to be an
infinite cylinder (or an infinite slab)? Why?
4.55 A three-layered composite pipe with an inside diameter of
1 cm has an internal surface temperature of 1208C. The first
layer, from the inside to the outside, is 2 cm thick with a
thermal conductivity of 15 W/(m 8C), the second layer is
3 cm thick with a thermal conductivity of 0.04 W/(m 8C),
and the third layer is 1 cm thick and has a thermal
409Problems
conductivity of 164 W/(m 8C). The outside surface
temperature of the composite pipe is 608C.
a. Determine the rate of heat transfer through the pipe
under steady-state conditions.
b. Can you suggest an approach that will allow you to
quickly make an estimate for this problem?
4.56 It is known that raw eggs will become hard when heated to
728C. To manufacture diced eggs, trays of liquid egg are
exposed to steam for cooking.
a. How long will it take to cook the eggs given the following
conditions? The tray dimension is 30 cm long, 30 cm
wide, and 2 cm deep. The liquid egg inside the tray is
1 cm deep. The thermal conductivity of liquid egg is
0.45 W/(m 8C); density is 800 kg/m
3
, specific heat
is 3.8 kJ/(kg 8C); surface convective heat transfer coefficient
is 5000 W/(m
2
8C); and the initial temperature of liquid
egg is 28C. Steam is available at 169.06 kPa. Ignore
resistance to heat transfer caused by the metal tray.
b. What rate of steam flow per tray of liquid egg must be
maintained to accomplish this? The latent heat of
vaporization at 169.06 kPa is 2216.5 kJ/kg.
4.57 Determine the time required for the center temperature of a
cube to reach 808C. The cube has a volume of 125 cm
3
.
The thermal conductivity of the material is 0.4 W/(m 8C);
density is 950 kg/m
3
; and specific heat is 3.4 kJ/(kg K). The
initial temperature is 208C. The surrounding temperature is
908C. The cube is immersed in a fluid that results in a
negligible surface resistance to heat transfer.
4.58 A tubular heat exchanger is being used for heating a liquid
food from 308 to 708C. The temperature of the heating
medium decreases from 908 to 608C.
a. Is the flow configuration in the heat exchanger
countercurrent or concurrent flow?
b. Determine the log mean temperature difference.
c. If the heat transfer area is 20 m
2
and the overall heat
transfer coefficient is 100 W/(m
2
8C), determine the rate of
heat transfer from the heating medium to the liquid food.
d. What is the flow rate of the liquid food if the specific
heat of the liquid is 3.9 kJ/(kg 8C)? Assume no heat loss
to the surroundings.
410 CHAPTER 4 Heat Transfer in Food Processing
4.59 What is the flow rate of water in a heat exchanger if it enters
the heat exchanger at 208C and exits at 858C? The heating
medium is oil, where oil enters at 1208C and leaves at 758C.
The overall heat-transfer coefficient is 5 W/(m
2
8C). The area
of the heat exchanger is 30 m
2
.
4.60 A thermocouple is a small temperature sensor used in
measuring temperature in foods. The thermocouple junction,
that senses the temperature, may be approximated as a
sphere. Consider a situation where a thermocouple is
being used to measure temperature of heated air in an oven.
The convective heat-transfer coefficient is 400 W/(m
2
K).
The properties of the thermocouple junction are
k 5 25 W/(m 8C), cp 5 450 J /(kg K), and p 5 8000 kg/m
3
.
The diameter of the junction, considered as a sphere, is
0.0007 m. If the junction is initially at 258C, and it is placed
in the oven where the estimated heated air temperature is
2008C, how long will it take for the junction to reach 1998C?
4.61 In an ohmic heater, the inside diameter of the pipe is 0.07 m
and it is 2 m long. The applied voltage is 15,000 V. A liquid
food is being pumped through the heater at 0.2 kg/s with an
inlet temperature of 308C. The overall heat transfer
coefficient based on the inside pipe area is 200 W/m
2
8C.
The specific heat of the liquid food is 4000 J/kg 8C. The
surrounding temperature of the air is 258C. Assume that the
properties of the liquid food are similar to 0.1 M Sodium
Phosphate solution. Determine the exit temperature of the
liquid food.
4.62 A liquid food at 758C is being conveyed in a steel pipe
(k 5 45 W/[m 8C]). The pipe has an internal diameter of
2.5 cm and it is 1 cm thick. The overall heat-transfer
coefficient based on internal diameter is 40 W/(m
2
K). The
internal convective heat-transfer coefficient is 50 W/(m
2
K).
Calculate the external convective heat-transfer coefficient.
4.63 Set up Example 4.16 on a spreadsheet. Determine the heat loss
from 1 m length of the pipe if the inside diameter of the pipe is
a. 2.5 cm
b. 3.5 cm
c. 4.5 cm
d. 5.5 cm
411Problems

*4.64 A plot of tomato juice density versus temperature was
scanned and digitized from
Choi and Okos (1983). Juice
density versus solids content for several temperatures are
given in the following table.
Solids T 5 308C T 5 408C T 5 508C T 5 608C T 5 708C T 5 808C
0% 997 998 984 985 979 972
4.8% 1018 1018 1012 1006 1003 997
8.3% 1032 1032 1026 1026 1020 1017
13.9% 1070 1067 1064 1061 1058 1048
21.5% 1107 1108 1102 1102 1093 1086
40.0% 1190 1191 1188 1185 1179 1176
60.0% 1290 1294 1288 1289 1286 1276
80.0% 1387 1391 1385 1382 1379 1376
Create a MATLAB
s
script that plots the values of density as a
function of percent solids at a temperature of 408C. Use the
Basic Fitting option under the Tools menu of the Figure win-
dow to fit the data with an appropriate polynomial. Turn in a
copy of your script, plot, and the equation that you determine.
*4.65 A plot of tomato juice density versus temperature was
scanned and digitized by
Choi and Okos (1983). Values of
juice density versus solids content for several temperatures
are shown in a table in Problem 4.64. Write a MATLAB
s
script to evaluate and plot the model for tomato juice
density developed by Choi and Okos.
ρ 5 ρ
s
X
s
1 ρ
w
X
w
ρ
w
5 9:9989 3 10
2
2 6:0334 3 10
22
T 2 3:6710 3 10
23
T
2
ρ
s
5 1:4693 3 10
3
1 5:4667 3 10
21
T 2 6:9646 3 10
23
T
2
ρ 5 density ðkg=m
3
)
ρ
w
5 density of water ðkg=m
3
)
ρ
s
5 density of solids ðkg=m
3
)
T 5 temperature of juice ð8C)
X
s
5 percent solids in juice ð%)
X
w
5 percent water in juice ð%)
Plot this model over the preceding experimental data for 408C.
* Indicates an advanced level of difficulty in solving.
412 CHAPTER 4 Heat Transfer in Food Processing

*4.66 Telis-Romero et al. (1998) presented data for the specific
heat of orange juice as a function of temperature and percent
water. A portion of their data was digitized and is given in
the following table.
X
w
(w/w)
T 5 88C T 5 188C T 5 278C T 5 478C T 5 628C
c
p
(kJ/kg 8C)
c
p
(kJ/kg 8C)
c
p
(kJ/kg 8C)
c
p
(kJ/kg 8C)
c
p
(kJ/kg 8C)
0.34 2.32 2.35 2.38 2.43 2.45
0.40 2.49 2.51 2.53 2.59 2.61
0.44 2.59 2.62 2.64 2.68 2.72
0.50 2.74 2.78 2.80 2.85 2.88
0.55 2.88 2.91 2.93 2.98 3.01
0.59 2.99 3.01 3.03 3.08 3.12
0.63 3.10 3.12 3.14 3.19 3.23
0.69 3.26 3.28 3.30 3.36 3.39
0.73 3.37 3.39 3.41 3.46 3.49
Notice that specific heat is a function of temperature as well
as solids content for all juices.
Two general-purpose empirical equations are often used to
estimate the specific heat for plant material and their juices.
The first equation is known as Siebel’s correlation equation,
and is used to estimate the specific heat of “fat-free fruits and
vegetables, purees, and concentrates of plant origin:”
c
p
5 3:349ðX
w
Þ1 0:8374
The second equation is given from the ASHRAE Handbook—
Fundamentals (2005):
c
p
5 4:187ð0:6X
w
1 0:4Þ
Using MATLAB
s
, create a plot of c
p
vs moisture content using
data given in the table for all temperatures and compare with
calculated values using the two empirical equations.
*4.67 The solution to temperature in an infinite slab with finite
internal and surface resistance to heat transfer is given by
Bergman et al. (2011) as
T 2 T
a
T
i
2 T
a
5
X
N
n51
C
n
expð2β
2
n
N
Fo
Þcosðβ
n
x=d
c
Þ
* Indicates an advanced level of difficulty in solving.
413Problems

C
n
5
4 sinðβ
n
Þ
2β
n
1 sinð2β
n
Þ
β
n
tan β
n
5 N
Bi
They give the first four roots of the transcendental equation for
β for a range of Biot numbers. The first four roots for N
Bi
5 0.5
are β
1
5 0.6533, β
2
5 3.2923, β
3
5 6.3616, and β
4
5 9.4775.
Write a MATLAB
s
script to evaluate the temperature for a
slab with
d
c
5 0:055 ðmÞ
k 5 0:34 ðW=m 8CÞ
c
p
5 3500 ðJ =kg 8CÞ
ρ 5 900 ðkg=m
3
Þ
N
Bi
5 0:5
T
a
5 100 ð8CÞ
T
i
5 35 ð8CÞ
Plot the temperatures T(x,t) over the range of x from 0 to
0.055 m for times of 20, 40, and 60 min.
*4.68 Write a MATLAB
s
script to use the MATLAB
s
function
pdepe to evaluate the temperature for an infinite slab with
d
c
5 0:055 ðmÞ
k 5 0:34 ðW=m 8CÞ
c
p
5 3500 ðJ =kg 8CÞ
ρ 5 900 ðkg=m
3
Þ
N
Bi
5 0:5
T
a
5 100 ð8CÞ
T
i
5 35 ð8CÞ
Plot the temperatures T(x,t) over the range of x from 0 to
0.055 m for times of 20, 40, and 60 min.
LIST OF SYMBOLS
A area (m
2
)
a coefficient in
Equation (4.70)
α thermal diffusivity (m
2
/s)
* Indicates an advanced level of difficulty in solving.
414 CHAPTER 4 Heat Transfer in Food Processing
α
0
attenuation factor (m
21
)
b gap between two adjacent plates (m)
β coefficient of volumetric expansion (K
21
)
C
F
cleaning factor, dimensionless
C
H
heat capacity rate (kJ/[s 8C])
C
min
minimum heat capacity rate (kJ/[s 8C])
C* heat capacity rate ratio, dimensionless
c
p
specific heat at constant pressure (kJ/[kg 8C])
c
v
specific heat at constant volume (kJ/[kg 8C])
χ reflectivity, dimensionless
D diameter (m)
d
c
characteristic dimension (m)
D
e
equivalent diameter (m)
d penetration depth (m)
E electrical field strength (V/cm)
E
V
voltage (V)
ε emissivity, dimensionless
ε
E
heat exchanger effectiveness, dimensionless
ε
0
relative dielectric constant, dimensionless
εv relative dielectric loss constant, dimensionless
F shape factor, dimensionless
f
h
heating rate factor (s)
f friction factor, dimensionless
f
0
frequency (Hz)
g acceleration due to gravity (m/s
2
)
h convective heat transfer coefficient (W/[m
2
K])
I electric current (A)
J
0
Bessel function of zero order
J
1
Bessel function of first order
j
c
temperature lag factor at center
j
m
temperature lag factor, mean
K coefficient in
Equation (4.185)
k thermal conductivity (W/[m K])
κ
E
electrical conductance (siemens)
L length (m)
l thickness of fluid layer (m)
λ wavelength (m)
λ
n
eigenvalue roots
M mass concentration, percent
m mass (kg); coefficient in Equations (4.59) and (4.70)
mv coefficient in Equation (4.184)
_
m mass flow rate (kg/s)
415List of Symbols
μ viscosity (Pa s)
N number of plates
N
Bi
Biot number, dimensionless
N
Fo
Fourier number, dimensionless
N
Gr
Grashoff number, dimensionless
N
Nu
Nusselt number, dimensionless
N
Pr
Prandtl number, dimensionless
N
Re
Reynolds number, dimensionless
N
Ra
Raleigh number, dimensionless
n coefficient in
Equation (4.59)
v kinematic viscosity (m
2
/s)
P power at the penetration depth (W)
P
D
power dissipation (W/cm
3
)
P
o
incident power (W/cm
3
)
φ absorptivity, dimensionless
Φ function
ψ transmissivity, dimensionless
Q heat gained or lost (kJ)
q rate of heat transfer (W)
q
000
rate of heat generation (W/m
3
)
R
E
electrical resistance (Ω)
R
f
fouling resistance, [(m
2
8C]/W)
R
t
thermal resistance (8C/W)
r radius or variable distance in radial direction (m)
r
c
critical radius
ρ density (kg/m
3
)
σ StefanBoltzmann constant (5.669 3 10
-8
W/[m
2
K
4
])
σ
E
electrical conductivity (S/m)
σ
o
electrical conductivity at 08C (S/m)
T temperature (8C)
t time (s)
T
e
exit temperature (8C)
T
A
absolute temperature (K) or pseudo initial temperature
for Ball’s method (8C)
T
a
temperature of surrounding medium (8C)
T
f
film temperature (8C)
T
i
initial or inlet temperature (8C)
T
p
plate surface temperature (8C)
T
s
surface temperature (8C)
T
N
fluid temperature far away from solid surface (8C)
tan δ loss tangent, dimensionless
U overall heat transfer coefficient (W/[m
2
8C])
416 CHAPTER 4 Heat Transfer in Food Processing

u velocity (m/s)
V volume (m
3
)
w width of a plate (m)
X mass fraction, dimensionless
x variable distance in x direction (m)
Y volume fraction, dimensionless
Z depth (m)
z space coordinate
ξ factor to account for shape and emissivity, dimensionless
Subscripts: a, ash; b, bulk; c, channel; ci, inside clean surface; co , outside
clean surface; f , fat; fi, inside fouled surface; fo, outside fouled surface;
h, carbohydrate; i, inside surface; lm, log mean; m, moisture; o, outside surface;
p, protein; r, radial direction; s solid; x, x-direction; w, at wall (or water); H, hot
stream; C, cold stream; P, product stream.
■ BIBLIOGRAPHY
American Society of Heating, Refrigerating and Air Conditioning
Engineers, Inc., 2009. ASHRAE Handbook—Fundamentals.
ASHRAE, Atlanta, Georgia.
Bergman, T.L., Lavine, A.S., Incropera, F.P., DeWitt , D.P., 2011.
Fundamentals of Heat and Mass Transfer, seventh ed. John Wiley
and Sons, inc., New York.
Brennan, J.G., Butters, J.R., Cowell, N.D., Lilly, A.E.V., 1990. Food
Engineering Operations, third ed. Elsevier Science Publishing Co.,
New York.
Cengel, Y.A., Boles, M.A., 2010. Thermodynamics. An Engineering
Approach, seventh ed. McGraw Hill, Boston.
Charm, S.E., 1978. The Fundamentals of Food Engineering, third ed.
AVI Publ. Co., Westport, Connecticut.
Choi, Y., Okos, M.R., 1986. Effects of temperature and composition
on the thermal properties of food. In: Le Maguer, M., Jelen, P.
(Eds.), Food Engineering and Process Applications, Vol. 1.
“Transport Phenomena”. Elsevier Applied Science Publishers,
London,
pp. 93101.
Choi, Y., Okos, M.R., 1983. The thermal properties of tomato juice
concentrates. Trans. ASAE 26 (1), 305315.
Copson, D.A., 1975. Microwave Heating. AVI Publ. Co., Westport,
Connecticut.
Coulsen, J.M., Richardson, J.F., 1999. Chemical Engineering, Vol. 1.
Pergamon Press, Oxford.
417Bibliography
Das, S.K., 2005. Process Heat Transfer. Alpha Science International Ltd.,
Harrow, UK.
Decareau, R.V., 1992. Microwave Foods: New Product Development.
Food and Nutrition Press, Trumbull, Connecticut.
Decareau, R.V., Peterson, R.A., 1986. Microwave Processing and
Engineering. VCH Publ., Deerfield Beach, Florida.
Dickerson Jr., R.W., 1969. Thermal properties of foods. In: Tressler,
D.K., Van Arsdel, W.B., Copley, M.J. (Eds.), The Freezing
Preservation of Foods, fourth ed., Vol. 2. AVI Publ. Co., Westport,
Connecticut, pp. 2651.
Giese, J., 1992. Advances in microwave food processing. Food Tech.
46 (9), 118123.
Green, D.W., Perry, R.H., 2007. Perry ’s Chemical Engineer’s
Handbook, eighth ed. McGraw-Hill, New York.
Heldman, D.R., Lund, D.B., 2007. Handbook of Food Engineering.
CRC Press
, Taylor & Francis Group, Boca Raton, Florida.
Heldman, D.R., Singh, R.P., 1981. Food Process Engineering, second
ed. AVI Publ. Co., Westport, Connecticut.
Holman, J.P., 2009. Heat Transfer, Tenth ed. McGraw-Hill, New York.
Hottel, H.C., 1930. Radiant heat transmission. Mech. Eng. 52 (7), 700.
IFT, 1989. Microwave food processing. A scientific status summary
by the IFT expert panel on food safety and nutrition. Food Tech.
43 (1), 117126.
Kakac, S., Liu, H., 1998. Heat Exchangers. Selection, Rating and
Thermal Design. CRC Press, Boca Raton, Florida.
Kreith, F., 1973. Principles of Heat Transfer. IEP-A Dun-Donnelley
Publisher, New York.
Lin, Z., Cleland, A.C., Sellarach, G.F., Cleland, D.J., 1996. A simple
method for prediction of chilling times: Extension to three-
dimensional irregular shapes. Int. J. Refrigeration 19, 107114,
Erratum, Int. J. Refrigeration. 23, 168.
Mertens, B.
, Knorr, D., 1992. Developments of nonthermal processes
for food preservation. Food Tech. 46 (5), 124133.
Mudgett, R.E., 1986. Microwave properties and heating characteristics
of foods. Food Tech. 40 (6), 8493.
Myers, G.E., 1971. Analytical Methods in Conduction Heat Transfer.
McGraw-Hill, New York.
Ohlsson, T., 1983. Fundamentals of microwave cooking. Microw.
World 4 (2), 4.
Palaniappan, S., Sastry, S.K., 1991. Electrical conductivity of selected
solid foods during ohmic heating. J. Food Proc. Engr. 14, 221236.
418 CHAPTER 4 Heat Transfer in Food Processing
Pflug, I.J., Blaisdell, J.L., Kopelman, J., 1965. Developing
temperature-time curves for objects that can be approximated by
a sphere, infinite plate or infinite cylinder. ASHRAE Trans. 71 (1),
238248.
Pham, Q.T., 2001. Prediction of cooling/freezing time and heat
loads. In: Sun, D.W. (Ed.), Advances in Food Refrigeration.
Leatherhead International, Leatherhead, Surrey, UK.
Rotstein, E., Singh, R.P., Valentas, K.J., 1997. Handbook of Food
Engineering Practice. CRC Press, Boca Raton, Florida.
Schiffman, R.F., 1986. Food product development for microwave
processing. Food Tech. 40 (6), 9498.
Schneider, P.J., 1963. Temperature Response Charts. Wiley, New
York.
Siebel, J.E., 1892. Specific heat of various products. Ice Refrigeration
2, 256.
Singh, R.P., 1994. Food Properties Database. RAR Press, Davis,
California.
Sweat, V.E., 1974. Experimental values of thermal conductivity of
selected fruits and vegetables. J. Food Sci. 39, 1080.
Sweat,
V.E., 1975. Modeling thermal conductivity of meats. Trans.
Am. Soc. Agric. Eng. 18 (1), 564565, 567, 568.
Sweat, V.E., 1986. Thermal properties of foods. In: Rao, M.A., Rizvi,
S.S.H. (Eds.), Engineering Properties of Foods. Marcel Dekker
Inc., New York, pp. 4987.
Telis-Romero, J., Telis, V.R.N., Gabas, A.L., Yamashita, F., 1998.
Thermophysical properties of Brazilian orange juice as affected by
temperature and water content. J. Food Engr. 38, 2740.
von Hippel, A.R., 1954. Dielectrics and Waves. MIT Press,
Cambridge, Massachusetts.
Watt, B.K., Merrill, A.L., 1975. Composition of Foods, Raw,
Processed, Prepared. Agriculture Handbook, Number 8. United
States Department of Agriculture, Washington, D.C.
Whitaker, S., 1977. Fundamental Principles of Heat Transfer. Krieger
Publishing Co., Melbourne, Florida.
419Bibliography
